5 Steps to Achieve a Successful Drug Product Launch
by Dr. Anna Schamberger, Director Customer Project Management, and Sabrina Schumacher, Team Leader Customer Service | 08/21/2023

Proven Process to Optimize Commercial Readiness
Bringing a new drug product to market can be a major challenge when it comes to guiding the late-stage development through to commercial readiness and launch. In fact, this is often considered one of the most impactful milestones of any drug program, because it culminates years of development work.
It usually is during late-stage development where there is a mindset shift from developing a drug product, to commercializing it. Since timing is a crucial factor, decisive and focused launch management is required and involves a dedicated and experienced team to coordinate all necessary process steps. A successful first commercial production can be achieved in a predetermined timeline by following a thorough step-by-step approach.
The biopharma product launch landscape is very diverse, and as such, the needs of each product to reach the market can vary significantly. To effectively establish a plan for launch management, the Contract Development and Manufacturing Organization (CDMO) partner need to work closely with the customer to decide on the product-specific process.
Part of the launch is designing a tried-and-true methodology to meet the specific needs of each molecule to bring both, a small batch as well as a blockbuster drug to market. Throughout the launch management process, the team is working against the launch date with the intentions to follow the best product implementation strategy for commercial market supply.
Generally, when customers prepare for launch, they have already been collaborating with a CDMO partner and have an assigned project manager who helps guide their drug product through clinical development. When the launch management process begins, customers are paired with a launch manager, in addition to their project manager, to guide them through the journey to commercial production.
At this point, the project manager assumes a new role within the launch team, and the launch manager takes the lead. The customer has access to both, the project and launch teams throughout the journey to commercial readiness. Though the managers will support the seamless step-by-step approach, external factors such as material or API supply must be closely monitored to avoid challenges that may arise and are outside the control of the service provider and customer.
Transparency and open communication on risks and active management of expectations are paramount to make sure all parties involved agree on each process steps. A proven step-by-step approach contributes significant value to achieving market launch on time and allows the customer and partner to keep track of several activities running in parallel, preventing overlooked gaps or missed milestones.

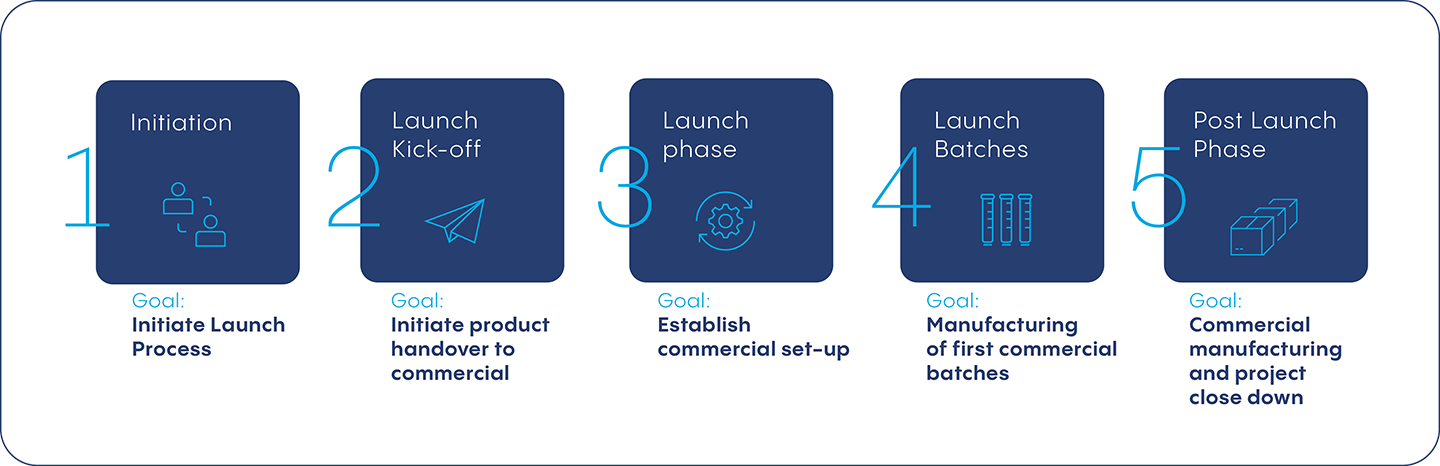
Initiation – Define Launch Process and Timeline
The launch management process is initiated based on the customers product launch plan. This phase allows both parties to define the organizational structure as well as the requirements and expectations for the timeline to bring the product to market. With large teams from various departments such as quality control, demand management and supply chain management involved in the process, it is important to identify the roles and responsibilities of each member. This applies to the filling partner as well as the customer launch teams.
Launch Kickoff – Initiate Product Handover to Commercial Team
The kickoff follows the start of product transfer from project to commercial. This phase includes internal and external kickoff meetings to get all relevant stakeholders involved in the process. During the latter, the service provider introduces the launch management process and provides a full project and product overview to the launch teams. The customer in turn provides a full overview on their final product set up for commercial and their understanding of launch management.
This is also when the project manager, launch manager and customer will align on milestones, common goals, timelines and identify risk factors. Once the project manager hands the lead over to the launch manager, they confirm a joint understanding of the overall approach, the future commercial working frame, and the supply chain management process. Assuming there is agreement on the communication and core team setup, the kickoff is complete.

Launch Phase – Establish Commercial Set-Up
During the launch phase, a filling partner will establish a methodical way of working with the team including both internal and external players. This step also includes gaps and opening actions within a launch checklist developed in collaboration with the customer. Progress made throughout the launch phase is tracked during the regular meetings both internally, within the CDMO, and externally with the customer, to maintain open lines of communication.
It is common during this phase to evaluate both best-case and worst-case forecasts to correspond with the planning of the launch and to develop corresponding scenarios to support flexible handling of the process throughout.
In an effort to conduct commercial setup, the launch management team will allocate process topics e.g. the API allocation, setup primary packaging, management supply, API storage and clean room equipment to match the customer’s forecasting needs and logistics process. Throughout commercial setup, there is an overall awareness of the anticipated timeline and processes, as well as dependencies on both the CDMO and the customer.
As the process goes on and milestones are met, action items on the launch checklist are checked off. If there are any risks, whether related to supply chain, packaging, or otherwise, that might delay launch, they are flagged in this phase of the process.
Launch Batches – Manufacturing First Commercial Batch
This phase marks the completion of product transfer from project to commercial teams and the execution of the first commercial fill within the pre-planned scenario. Most of the defined activities must be completed by the launch tracker such as packaging material availability, personnel training and adherence of correct shipping processes and requirements. Also, all necessary orders are placed, and the availability of crucial components are confirmed.
Post-Launch – Commercial Manufacturing and Project Close Down
Following the successful release of the first launch batch, a formal close-down of the project commences. The CDMO team will collect lessons-learned and feedback from the customer to optimize execution of future launches. Learning occurs on both sides - the customer learns how to better get their products to launch, and the filling partner learns how to further improve its launch management process.
Once a launch batch of the drug product is released, the formal close-down within the system is complete and the project manager and project team are released. At this point, the product has made its way through the launch management process and is now in the hands of the commercial team.
The commercial team will remain responsible for continuous process verification, monitoring key performance indicators and yield data, forecasting and capacity reservation for medication, participating in business review and quality management review meetings, and managing the future lifecycle activities of the product.
Vetter, one of the world’s leading fill and finish CDMOs for injectables, established this 5-step process for maximum efficiency when bringing a drug product to commercialization. This process offers a tried-and-true methodology for getting a small batch as well as blockbuster drug product to market.
Nevertheless, this process requires continued adaptation and adjustment to align with environmental factors. As a result of these external obstacles, Vetter bases success on a plannable and structured process, that provides reliability for customers on the launch management timeline set at the start of the journey.
This reliable step-by-step process has supported customers’ success, big and small, for both startup biotech companies and pharma giants, and will be further adapted to continue doing so in the future.
The article was first published on contractpharma.com
About the authors
Dr. anna schamberger, director customer project management .
Dr. Schamberger has 10+ years of experience in the pharmaceutical industry, primarily working with CDMOs. After joining Vetter in 2016, she began in customer Project Management leading late-stage DP and life cycle projects. Since 2020, Dr. Schamberger has been a director in customer project management leading a team of eight project managers. She is also a program manager for strategic partnerships overlooking and leading product portfolio and supports multiple key strategic and operational activities within Vetter’s customer project management, achieving customer goals to commercialize innovative and life-saving products for a global market.
Sabrina Schumacher, Team Leader Customer Service
Ms. Schumacher has 15 years of experience at Vetter and over seven years of experience as a Customer Relations Manager, leading several launches for various customers in the function of a Launch Manager. She supports customers as a central contact and knowledge carrier within the company and develops solutions to complex challenges throughout the commercial development phase. Since 2020, she has been the Team Lead Customer Service leading a team of nine customer service representatives.

Sign up for our "CDMO Insights" newsletter
And tap into deep insights on market trends, challenges, best practices, and more.
Strategy & Planning for Commercial Launch Success in Pharma
By Kurt Arco
Course Details
What you will learn, expert faculty, who is this course for, recommendations by your peers, dates - locations - fees, registration details, related courses, save this course.
Your company only gets ONE chance to launch a new pharmaceutical.
Yet many launches fail to achieve their maximum potential.
Commercial launch success requires a set of competencies to be embedded across the organisation.
A well-established foundation of effective strategic thinking, decision-making processes, and optimal organisational structure must be firmly established well in advance of the product launch.
In addition, cross-functional alignment is a pre-requisite in today’s multi-channel and multi-stakeholder world.
This course will teach you all concepts and evidence-based tools for effective strategic decision-making and planning in the process up to commercial launch.
You will not only be able to ask your specific questions to a true expert, but also have the unique opportunity to share experiences and have in-depth discussions with your international peers.
Great course, lots of details! I highly valued the templates we got, the case studies and the real-world examples. Thank you!
Michael Kuderka, Executive Director Established Brands Marketing - United States - December 2023
The 4 launch types that are based on the company/brand’s profile, and the strategic implications thereof.
A roadmap with timelines, milestones and best practice requirements to prepare a successful global launch across all functions.
The market and stakeholder insights that are crucial for defining the global launch strategy.
How to crystallise a launch strategy based on segmentation, targeting, and positioning.
How to prepare the cross-functional global launch team .
A fairly intensive and concise course that gives a complete overview of the critical points when organising a complex drug launch.
Aleksandr Lifanov, Brand Lead - Italy - March 2024
To see the detailed agenda, please submit your details in the form below the table.
To see the detailed agenda, please submit your details in the form below:
As an independent consultant, Kurt Arco advises on commercial and launch excellence, drawing from his 20+ years of international experience within big and small pharma/biotech companies.
Formerly, he was Global Launch Excellence Expert with Trilations, a strategic consultancy.
Prior to that, Kurt led several major brand launches at MSD (Merck & Co), internationally and across several therapeutic areas.
When responding to questions from the audience, Kurt effortlessly leverages his extensive expertise in pharma launches, bringing theory to life by providing real-world examples.
A cross-functional launch team typically consists of executives from Marketing, Sales, Medical, Market Access, External Affairs and Business Intelligence. This course is a must-attend for all members of any cross-functional launch team that needs to launch a brand in the coming 1-3 years. In particular, all executives responsible for building, executing and measuring a launch plan.
If you are a consultant, please contact Annelies Swaan , who will check with the faculty whether you can attend.
Would you like to receive a list of anonymised participant profiles from previous sessions?
Email [email protected]
The average Recommendation Score from participants of the last session was
Read this selection of testimonials from recent participants:
I think the most valuable take-aways were all the real-life examples that Kurt gave. You can tell that he has seen a lot and gained a lot of experience which he did not hesitate to share with us. The group interaction was also very pleasant, and it was valuable to share experiences and work together in the exercises.
goetzpartners
Melanie Hesslinger, Consultant - Germany - March 2024
Highly valuable course with clear content and a complete 360 vision. The real-life cases were very much appreciated.
HRA Pharma Rare Disease
Tamara Nasser, International Product Manager - France - March 2024
Very clear and well-structured course full of highly valuable information!
Abacus Medicine Pharma Services
Tristan Hollyer, Product Manager - Denmark - December 2023
The course was very well structured and the expert was the real added value, bringing real-life experience to the table!
Iacopo Vanzetto, New Products Lead, Respiratory - Italy - December 2023
The strategy and planning process theory was put into context with many real life examples. This course helps people become more competent and gives many useable formats that can be adopted and contextualised into one's own company, therapy area, or brand. This course assists anyone who is tasked with an upcoming launch, or is part of a global or local brand team, to work in a strategic, structured way aiming to ensure launch excellence.
Eduardo de Moura Correia, Marketing Manager EU & RoW - Switzerland - December 2022
The course was very valuable and insightful. All the tools we have been provided with can be useful in everyday work.
Nives Šimac, Portfolio & Launch Manager - Croatia - December 2022
Very good content to understand the key to success in terms of Launch. I also learnt a lot in the discussions with other participants. Thanks!
Agnese Volfa, Strategic Marketing Director - Latvia - June 2022
Great insights, practical tips, concrete examples, actionable frameworks, experience-sharing among participants…all this with a very good and experienced speaker! Excellent content, adapted format and great organisation for a fantastic e-learning experience! Everything was there within these two days - this will clearly be useful for our upcoming launch!
Cyril Martin, Global Brand Director Hematology - France - June 2022
I truly recommend participating in CELforPharma’s course ‘Strategy & Planning for Commercial Launch Success in Pharma’. By attending, you get a very well structured, clear set of information, and a great summary of key tools and info supporting overall success of launches, establishing Launch Excellence. Kurt also gives real-life examples and there are many valuable discussions! Professional!
Katalin Egyed, Head of International Specialty Marketing Division - Hungary - June 2022
Flen Health
Tom Ryssaert, OTC/DTC Product Manager - Belgium - June 2022
Kurt covered the launch process in great detail. I learned a lot from the experiences he shared with us and the content he provided. This alongside the group exercise made this course a great learning experience. Great job CELforPharma, thank you.
Alexion - AstraZeneca Rare Disease
Matthias Durrer, Commercial Analyst - Switzerland - December 2021
Great course, professionally delivered. I would highly recommend.
Rosemont Pharmaceuticals
Caroline Husic, Senior Marketing Manager - United Kingdom - December 2021
The course was very useful for someone from Medical Affairs. It was interesting to learn all the steps and important aspects of the launch process from the Marketing point of view, which for sure will help me generate an appropriate launch strategy for MA in the future. The content was clear and nicely explained by Kurt. Thanks Kurt and thanks CELforPharma!
The Medical Affairs Company
Cecilia Soledad Tomni, Medical Science Liaison - Germany - December 2021
If you want a broad view of the commercial launch process from a true expert with useful insights, tools and materials, this course is definitely for you. Thanks CELforPharma!
Diego Zappalà, Critical Care Strategy & Execution Lead, Global Marketing - Italy - December 2021
I found the course very structured and hands-on, with a nice approach to pre-launch preparations, not just theory. The expert trainer gave us step-by-step guidance, shared valuable business case discussions and gave us a launch toolkit we can use after the course. CELforPharma were very nice and pro-active with their communication and gave clear instructions to make sure we all had what we needed. Many thanks!
WÖRWAG Pharma
Larissa Sauter, Global Brand Manager - Germany - October 2021
An ideal course for those that are beginning to prepare for their first product launch within the pharma industry; this course comes with a comprehensive strategic launch toolset that I can easily tailor to my individual product's launch and implement throughout my organisation.
Cook Myosite
Liam McNamara, Product Manager - United States - October 2021
I really feel that this training is very useful for my daily job and very applicable to my reality.
CSL Behring
Nadia Castillo, International Sr. Marketing Manager Immunology & Neurology - Switzerland - October 2021
This course gives a great framework to work with, and includes a wealth of ideas to challenge your own beliefs and look at things from a different perspective. I loved the interactivity throughout, the practical approach to each topic and the general process overview which was very valuable. Overall, a great course with excellent hosting from CELforPharma!
José Miguel Sánchez Toll, Strategy Readiness Advisor - Spain - March 2021
A very insightful course with excellent topics and exercises which were enhanced with real life examples. I really enjoyed learning about the importance of strategic planning and gaining a new outlook for launching products. Kurt and Leen were great and the service from CELforPharma was better than I have experienced with any other online courses. Thanks very much to all!
Klavs Petersons, Market Research Specialist - Latvia - March 2021
The course was very intense and exact in strategical launch planning. I especially enjoyed the different launch types, the omnichannel explanation and the timeline of key events including the market environment. The interaction within the groups was very nice and insightful. Thank you for a perfectly organised course. I believe I will really benefit from the course and I am very grateful for the knowledge and expertise shared during the 2 days.
Maja Hurwits, Marketing Manager - Slovenia - March 2021
A really nice course that was well balanced between theory and practice. I especially valued the topics focused on segmentation and reviewing the 4 launch types as well as the use of real data from the experts’ own desk research and surveys. The service from CELforPharma was great both before and during the course, thank you!
Svitlana Orlynska, Senior Marketing Manager - Austria - March 2021
Very practical course with good content that set clear objectives and KPI’s that I can put into action in my company. The speakers showed great expertise and presentation skills throughout. I feel I now have a better understanding of how to think critically when reviewing data and insights during a launch process and enjoyed learning about how to set up a launch team and the pitfalls that can occur along the way. Thank you for putting together a great course!
Stephen van Vucht, Sales & Marketing Manager - The Netherlands - February 2021
This course gives a profound overview and broad scope of the Commercial Launch process, enriched with many years of experience/examples by the experts which helped to understand the topics better. Thanks!
Carmen Jacob, Strategy & Operations Manager - Switzerland - February 2021
Highly informative course, puts the whole launch in perspective and gives tools to apply to your own launch.
Daniëlle de Ridder, Medical Science Liaison - The Netherlands - February 2021
The course is full of useful information regarding launches and helps to systematise your knowledge with lots of excellent examples. I liked the interaction and open discussion between the group throughout the 2 days. Very enjoyable course that I would highly recommend to others to attend!
Ekaterina Shustova, BGM Product Manager - Russian Federation - February 2021
The expert course leaders were highly informative and clearly had both experience and relevant examples to add to the course content, which brought the theory to life and made the course highly engaging and enjoyable to attend. There were many aspects I valued from this course, including Understanding the 4 Launch types & how they shape strategy, Segmentation, targeting & profiling and also Developing a Launch Roadmap & Critical Success Factors. Great course and great course leaders, thank you!
Kyowa Kirin
Sue Kemp, Commercial Manager - United Kingdom - February 2021
19-20 November 2024 , Live online
Early bird € 2 670
(Full fee € 2 870)
(until 18/10/2024)
Important information:
- Our courses typically take place between 9 AM and 5 PM CET (Brussels, Amsterdam, Paris).
- Live online sessions will be hosted in Zoom.
- Face-to-face courses will take place at a four-star business hotel close to the airport.
- You will receive all practical details after your registration.
For any questions related to the above, please do not hesitate to email Lisa Causero or contact her by phone at +32 484 70 91 97.
How to Register
Click the green "Register" button on the course session of your preference.
The registration wizard will guide you through the personal and company data that we need to process your registration.
You have the choice between 2 payment options:
Payment by credit card. You will not be required to pay immediately, but you will receive a secured payment link together with the invoice.
Payment via bank transfer
Click "Register Now". You will be sent an automatic email confirming your registration followed by a personal email containing an invoice and further payment instructions.
For assistance with your registration, please email Annelies Swaan or contact her by phone at +32 486 98 52 49.
Included in the Registration Fee:
For live online courses:
Access to a virtual small-class (max 20 participants), expert led course in real-time, using an interactive platform for break-out rooms, whiteboard exercises, polling, plenary discussions and chats.
Course material will be provided to you in both digital format (by email) and in hard copy (by post, so you can make notes during the course).
Certificate of attendance signed by the expert and CELforPharma.
For face-to-face courses:
Participation in a small-class (max 20 participants), expert led face-to-face course with interactive group exercises and plenary discussions. You will have the opportunity to meet the expert and discuss your own projects/issues during the breaks.
Course material will be provided to you in both digital format (by email) and in hard copy (so you can make notes during the course).
Opportunities for networking and informal discussions during coffee/tea breaks and lunch.
A group dinner in a nearby restaurant on the evening of day 1.
Certificate of attendance signed by the expert and CELforPharma.
VAT Information
The prices on our website are excluding VAT. Depending on the format of the course (face-to-face in Brussels or live online or self-study programme) and your location, we are obliged by Belgian law to apply VAT.
Further information about Belgian VAT rules for training courses:
We are obliged by Belgian law to apply VAT in the following cases:
Face-to-Face public course organised in Belgium
Article 53 of the European Union VAT Directive 2006/112 states that : “The place of supply of services in respect of admission to cultural, artistic, sporting, scientific, educational, entertainment or similar events, such as fairs and exhibitions, and of ancillary services related to the admission, supplied to a taxable person, shall be the place where those events actually take place.” If the course that you register for takes place in Belgium, we are obliged to charge 21% Belgian VAT. However, you can recover this Belgian VAT through the refund procedure. Note that this procedure differs depending on the country where you are located. It is best to contact your local VAT administration for this.
Online Public courses - live webinar
Webinars where the participants can communicate among themselves or ask questions to the speaker(s) (be it verbally, via a chat function or similar), are considered as ‘online interactive webinars’ by the Belgian VAT administration. According to Article 53 of the European Union VAT Directive 2006/112, these online interactive webinars take place where the organisation of the seminar takes place, which in this case is Belgium. So, if you register for one of our live online courses (organised from our offices in Belgium), we are obliged to charge 21% Belgian VAT. However, you can recover this Belgian VAT through the refund procedure. Note that this procedure differs depending on the country where you are located. It is best to contact your local VAT administration for this.
Self-Study Programme:
A self-study programme is a service provided electronically. It is the period of self-study that is the most important in our self-study Basics of Health Economics and not the kickoff and closing webinars. As a result, this self-study program follows a different VAT application and the place of the service is where the customer is established. Therefore, we follow the VAT rules applicable to the country of your invoicing address.
Group Discounts
Team discounts can be offered to 2 or more delegates from the same company.
Email Annelies Swaan , Director Business Operations, for more details.
Transfer & Cancellation Policy
Flexible Transfer
If a registrant cannot attend the scheduled course, he/she can avoid any cancellation charge by sending a suitable replacement participant.
Alternatively, the registrant can transfer once on a “space available” basis at no extra cost, until 3 weeks prior to the event, to another course of the same value held within one year of the original course date.
Registration Cancellation
Less than 14 days prior to the course date: registrant liable to pay the full invoiced registration fee.
Between 15 and 30 days prior to the course date: registrant liable to pay 50% of the invoiced registration fee.
More than 30 days prior to the course date: registrant liable to pay a cancellation fee of € 450 per registrant.
If a registrant postpones his/her participation to a future course, and cancels again, no refund can be claimed for registration fees.
What are the benefits of, respectively, the face-to-face and the live online course formats?
Benefits of CELforPharma’s face-to-face courses:
You have the unique opportunity to interact 1:1 with the expert(s).
Plenty of opportunity for informal 1:1 and group conversations with your international peers to exchange experiences and ideas, to discuss issues and to network.
Face-to-face interactions allow for more depth during Q&As and group work.
You can step away from your daily routine in the office and enjoy a more focused learning experience in a comfortable business hotel environment.
Benefits of CELforPharma’s live online courses:
The formal programme is as impactful as a face-to-face course thanks to the highly interactive format (using breakouts, polls, quizzes …).
No travel & accommodation expenses
You can attend the course from the comfort of your own office or home.
We send you the course materials by post so that you can take notes and fully engage with the course content.
If I choose credit card payment, do I have to pay immediately?
No, if you choose credit card payment, we will send you a secure payment link together with the invoice.
Can I benefit from a group/volume discount?
Team discounts can be offered for 2 or more registrations confirmed at the same time. The level of discount will depend on the number of registrations and the availability of seats.
Email Annelies Swaan , Director Business Operations, for more details.
Will VAT be applied to my registration fee?
Depending on the format of the course (face-to-face in Brussels or live online or self-study programme) and your location, we are obliged by Belgian law to apply VAT in the following cases:
Can I book a room at the hotel where the face-to-face courses are held?
Our face-to-face courses in Brussels take place in four-star business hotels close to the airport. As part of our strong relationship with these hotels, CELforPharma has secured a discount on the daily room rate for our delegates who book their accommodation via us. Upon your registration, we will assist you to make your hotel booking. For any assistance in this matter please do not hesitate to email Lisa Causero , Programme Coordinator, or contact her by phone at +32 484 70 91 97.
Are live online course sessions recorded?
We do not record our live online public courses as we want to offer a safe environment for all attendees to share their experiences and freely ask questions.
This way, by participating in our courses, you do not only learn from our expert faculty but also from your international peers in the room.
Can I buy the course materials?
No, we don't sell the course materials as a stand-alone. Without the expert's explanation, the exercises and the discussions with other course participants, there is very little value in the slides.
I do not know which course would be best for me.
Email Inge Cornelis , partner with CELforPharma, to help you choose the course that is best suited.
Can CELforPharma help me train my team?
We have helped many managers with setting up an in-house training programme for their team.
Email Inge Cornelis , partner with CELforPharma, to discuss your team training needs.
Do you have any other questions? Email Annelies Swaan or call +32 486 98 52 49.
People attending this course are typically also interested in:

By clicking on the below button, you will save this course to MY COURSES in the upper right corner of the website. This allows you to quickly retrieve it later. Don’t forget to make a personal account using the Login button in the upper right corner of the website to keep your list of saved courses for your next visit.
Dates & Locations
19-20 November 2024
Live online
Upcoming Session
(until 18/10/2024) Prices are excl. VAT
Keep me informed
Subscribe to the waiting list, create profile.
Passwords do not match! Please try again.
Pharmaceutical Product Pre-Launch Activities: How to Engage Key Audiences in Advance
Pre pharmaceutical product launch, medical and commercial teams have a lot on their plate. Their prime objective: ensure the messaging they’ve crafted resonates with each key stakeholder audience, including patients, HCPs, payors, and potentially many more (or several segments within each category).
Despite being so critical to the success of the drug—and the reputation of the company— budgetary pressures and competing priorities can make it difficult to engage these audiences as early as you ought.
When engagement comes too late (or in extreme cases, not at all), you can find yourself in the unenviable position of having to build relationships with KOLs and educate their markets about a new product too close to (or even after ) launch. This can directly undermine the entire commercial strategy in the crucial early days of the product.
Any delays in prescribing efficacious new treatments can mean an opportunity cost of millions of dollars for the business and serious ramifications for patients in need of the therapy.
This is why it’s critical to engage and educate all of these groups early enough in the pre-launch phase . The earlier teams can build these relationships, the faster those relationships can bear fruit for both the company and patients.
No matter how big or small the company is—or whether it’s the first or fiftieth product it's bringing to market—the salesforce ought to have the proven messaging and established relationships it needs to hit the ground running immediately following approval and launch.
This, as we just briefly mentioned, is easier said than done. So, let’s linger on the challenge of early audience engagement for just a moment before outlining some solutions.

The Patient Centricity Playbook
Seven strategies pharmaceutical commercial teams are using to better engage patients—and how to deploy them yourself
Get a copy (PDF) »
The biggest obstacle to early KOL engagement
One of the most frequent obstacles we’ve seen impede companies from engaging these audiences early enough is a prolonged focus on the earlier clinical aspects of the product’s R&D when that focus ought to shift—quite decisively—to commercialization .
Given how much time, effort, attention, and resources are invested in clinical studies, it’s not surprising that pharma teams find themselves tacitly fixated on exciting clinical details at the tail end of their research.
But in the pre-launch phases of the product lifecycle, it’s important to shift the focus and prioritize readiness across several areas that demand thoughtful, organized strategy and execution. These areas include:
- manufacturing;
- prescribing;
- insurance coverage, and
- patient awareness.
To orchestrate these critical activities early enough, it’s useful to ask what specific steps you need to take to ensure preparing stakeholders is a priority.
- Some companies may consider a single advisory board meeting enough to get the word out about their new product, but most products will need much more engagement across several audiences.
- A single meeting may not be sufficient to convince a group of advisors that your solution is a viable option to explore, and may be easily forgotten by the time the product is approved by the FDA.
- By the same token, this approach does not offer ample time to answer, for example, all physicians’ questions or address concerns about potential risks from the treatment.
Virtual advisory boards offer a distinct advantage in these areas, as they can enable more frequent, economical engagements, allowing you to take your time in building relationships with stakeholders. With a technology platform purpose-built for facilitating and better managing these specific types of engagements, you can generate awareness, answer more questions, and even have the opportunity to test messaging to see if it resonates long before launch.
How to engage pharma stakeholders with pre-launch activities
For many companies, roughly one year prior to getting FDA approval is the optimal time to begin preparing commercial advisory boards and other engagement vehicles as the company will likely be putting together its plans for building its sales teams and MSLs, and starting to bring an outreach strategy to life.
A series of convenient, cost-effective virtual ad boards with each group of stakeholders can be a more efficient and fruitful alternative to traditional, in-person ad boards that might occur once or twice a year.
💡 Here’s some high-level guidance on what such a process entails:
⟶ To begin the planning process, create three small groups of stakeholders segmented by community: 1) patients, 2) payors, and 3) HCPs.
⟶ Next, create a plan for reaching out to these groups at regular intervals, such as by hosting an ad board each quarter.
⟶ Consider how you could restructure your contracts to accommodate a digital engagement format that replaces one or two physical engagements a year with fewer, more convenient, and insightful digital engagements using asynchronous discussion forums, surveys, and other convenient formats that enable individuals to engage with content on their own time and provide space for thoughtful answers.
⟶ By systematizing this outreach process, you can also save time by contracting for the entire period, instead of creating a new contract each quarter.
📄 Read our other guide, How to Run an Effective Advisory Board Meeting in 2023 , to zoom in on this process and get practical, specific advice on shifting to a virtual ad board model.
The biggest benefit of regular, planned engagements
This segmented, higher-frequency approach offers a huge advantage that holistically and efficiently helps you achieve your end goal of refining your messaging and building connections with stakeholders for a smooth and rapid market entrance.
In short, it creates opportunities for stakeholders to regularly learn from their peers and compare experiences—bridging the gap between research and clinical practitioners, which may be a unique and valuable opportunity for them.
Regular engagements facilitated through a convenient digital platform can quickly build a sense of community, trust, and familiarity both within groups and between them.
The company, in turn, can identify patterns , such as common concerns about their product or obstacles KOLs have to overcome. Sometimes, you may even uncover common obstacles for one of your groups regarding another group. Since you can host more frequent interactions, you have the opportunity to discuss these at the next meeting and between them.
This process allows stakeholders to begin to drive their concerns as you identify new priorities for your company. For example, if you find that there is concern among physicians about getting electrocardiograms approved by insurers at the rate that is necessary, you might discuss this in your next meeting with the payors group.
In following this process, the company can uncover both data and insights that can inform messaging, as ExtendMed’s Amy Ravi explains below.
“It's not only gathering all this data, but also the testimonials almost, the understanding of all the constituents in a way that is relevant. So you have both the data and the safety and the efficacy of a product and how you've done that in different clinical trials with the right audiences and right patient groups and so forth. But then you also have the people who have expressed that this is a need: ‘20% of my patient population fits into this group, and we're not currently treating them.’ You can have some of the evidence that you've collected with these different target groups, who are going to be your future community. That can be beneficial in how you reflect your responses back to the FDA and to your time, you know, your future sales opportunities.”

— Amy Ravi, CEO, ExtendMed
By the time you’re ready to launch, the company already has stakeholders who are educated about your product, know the need, and have had a chance to test that with some of their peers.
They’ve had the opportunity to discuss the best use cases, how to mitigate risk, and other concerns, so that they are comfortable not only with thinking about and using their product for their own patients but spreading the word with their peers.
How ExtendMed helps pharmaceutical companies engage KOLs earlier
From the very first step of identifying diverse groups of people you want to work with, ExtendMed helps you shift your contracting to:
- engage them across the entire year—or however long your engagement period is;
- pull together different communities of people for different purposes throughout the year, and
- provide all the tools you need to solicit rich insights from them .
Teams using ExtendMed build out their advisory communities for each particular objective and systematize the process of contracting and communicating with them through live, virtual meetings and over time through a suite of ongoing engagement tools like discussion boards, surveys, and more.
With ExtendMed , you can:
- Host a virtual ad board, a steering committee, or a clinical study update meeting for participating sites around the globe.
- Engage asynchronously with virtual discussion boards in the same platform as synchronous engagements
- Engage HCPs and other stakeholders via surveys, quizzes, and assessments
- Leverage educational content across regions, professions, and specialties. Manage faculty, curricula, multimedia, content, and certification in one platform.
- Share information with stakeholders in different formats to suit each individual’s content consumption preferences.
- Track how many participants have engaged in each of your activities in your portal through a simple reporting dashboard.
(The list keeps going here .)
Ready for better engagement pre-launch? Let’s start the conversation.
Our goal is to help you have richer and more frequent engagements with each of your stakeholders, at a lower cost.
Visit pages.extendmed.com/solutions for a quick look at our solutions, and how we empower teams to have richer engagements with each of their stakeholders. Want to get in touch with questions or schedule a free platform demo? Contact us .
📄 Want some more food for thought? Check out our free white paper below and get seven strategies pharmaceutical commercial teams are using to better engage patients—and tips on how to deploy them yourself.
KOL Management in Pharma: A Complete Guide

How to Run an Effective Advisory Board Meeting in 2024

Pharma Advisory Boards: 5 Best Practices for Better Engagement in 2024

Drug Rehabilitation Business Plan Template
Written by Dave Lavinsky

Drug Rehabilitation Business Plan
Over the past 20+ years, we have helped over 1,000 entrepreneurs and business owners create business plans to start and grow their drug rehabilitation companies. We have the experience, resources, and knowledge to help you create a great business plan.
In this article, you will learn some background information on why business planning is important. Then, you will learn how to write a drug rehabilitation business plan step-by-step so you can create your plan today.
Download our Ultimate Business Plan Template here >
What is a Drug Rehabilitation Business Plan?
A business plan provides a snapshot of your drug rehabilitation business as it stands today, and lays out your growth plan for the next five years. It explains your business goals and your strategies for reaching them. It also includes market research to support your plans.
Why You Need a Business Plan for a Drug Rehab Center
If you’re looking to start a drug rehabilitation business or grow your existing drug rehabilitation company, you need a business plan. A business plan will help you raise funding, if needed, and plan out the growth of your drug rehabilitation business to improve your chances of success. Your drug rehabilitation business plan is a living document that should be updated annually as your company grows and changes.
Sources of Funding for Drug Rehabilitation Businesses
With regards to funding, the main sources of funding for a drug rehabilitation business are personal savings, credit cards, bank loans, and angel investors. When it comes to bank loans, banks will want to review your business plan and gain confidence that you will be able to repay your loan and interest. To acquire this confidence, the loan officer will not only want to ensure that your financials are reasonable, but they will also want to see a professional plan. Such a plan will give them the confidence that you can successfully and professionally operate a business. Personal savings and bank loans are the most common funding paths for drug rehabilitation companies.
Finish Your Business Plan Today!
How to write a business plan for a drug rehabilitation business.
If you want to start a drug rehabilitation business or expand your current one, you need a business plan. The business plan outline below details the necessary information for how to write each essential component of your drug rehabilitation business plan.
Executive Summary
Your executive summary provides an introduction to your business plan, but it is normally the last section you write because it provides a summary of each key section of your plan.
The goal of your executive summary is to quickly engage the reader. Explain to them the kind of drug rehabilitation business you are running and the status. For example, are you a startup, do you have a drug rehabilitation business that you would like to grow, or are you operating a chain of drug rehabilitation businesses?
Next, provide an overview of each of the subsequent sections of your plan.
- Give a brief overview of the drug rehabilitation industry.
- Discuss the type of drug rehabilitation business you are operating.
- Detail your direct competitors. Give an overview of your target customers.
- Provide a snapshot of your marketing strategy. Identify the key members of your team.
- Offer an overview of your financial plan.
Company Overview
In your company overview, you will detail the type of drug rehabilitation business you are operating.
For example, you might specialize in one of the following types of drug rehabilitation businesses:
- Medical detox/symptom management drug rehabilitation: this type of drug rehabilitation focuses on medical assistance while individuals are in withdrawal, along with the management of symptoms the individual may have during recovery.
- Substance abuse treatment center: Commonly seen as highly-effective, substance abuse treatment centers focus on one or a combination of the following: counseling, group therapy, medically-assisted treatment therapy (MAT) and other forms of assistance that support holistic health and well-being.
- Self-help drug rehabilitation: this type of therapy is designed for someone who prefers to work on an individual basis with one counselor, commonly a professional certified in alcohol and drug rehabilitation.
In addition to explaining the type of drug rehabilitation business you will operate, the company overview needs to provide background on the business.
Include answers to questions such as:
- When and why did you start the business?
- What milestones have you achieved to date? Milestones could include the number of patients served, the number of cases with positive outcomes, reaching X number of contracted medical providers who refer directly to your company, etc.
- Your legal business Are you incorporated as an S-Corp? An LLC? A sole proprietorship? Explain your legal structure here.
Industry Analysis
In your industry or market analysis, you need to provide an overview of the drug rehabilitation industry.
While this may seem unnecessary, it serves multiple purposes.
First, researching the drug rehabilitation industry educates you. It helps you understand the market in which you are operating.
Secondly, market research can improve your marketing strategy, particularly if your analysis identifies market trends.
The third reason is to prove to readers that you are an expert in your industry. By conducting the research and presenting it in your plan, you achieve just that.
The following questions should be answered in the industry analysis section of your drug rehabilitation business plan:
- How big is the drug rehabilitation industry (in dollars)?
- Is the market declining or increasing?
- Who are the key competitors in the market?
- Who are the key suppliers in the market?
- What trends are affecting the industry?
- What is the industry’s growth forecast over the next 5 – 10 years?
- What is the relevant market size? That is, how big is the potential target market for your drug rehabilitation business? You can extrapolate such a figure by assessing the size of the market in the entire country and then applying that figure to your local population.
Customer Analysis
The customer analysis section of your drug rehabilitation business plan must detail the customers you serve and/or expect to serve.
The following are examples of customer segments: individuals, family members of addicts, physicians and licensed therapists.
As you can imagine, the customer segment(s) you choose will have a great impact on the type of drug rehabilitation business you operate. Clearly, individuals would respond to different marketing promotions than medical personnel, for example.
Try to break out your target customers in terms of their demographic and psychographic profiles. With regards to demographics, including a discussion of the ages, genders, locations, and income levels of the potential customers you seek to serve.
Psychographic profiles explain the wants and needs of your target customers. The more you can recognize and define these needs, the better you will do in attracting and retaining your customers.
Finish Your Drug Rehabilitation Business Plan in 1 Day!
Don’t you wish there was a faster, easier way to finish your business plan?
With Growthink’s Ultimate Business Plan Template you can finish your plan in just 8 hours or less!
Competitive Analysis
Your competitive analysis should identify the indirect and direct competitors your business faces and then focus on the latter.
Direct competitors are other drug rehabilitation businesses.
Indirect competitors are other options that customers have to purchase from that aren’t directly competing with your product or service. This includes psychiatrists, other healthcare providers, or members of the clergy. You need to mention direct competition, as well.
For each direct competitor, provide an overview of their business and document their strengths and weaknesses. Unless you once worked at your competitors’ businesses, it will be impossible to know everything about them. But you should be able to find out key things about them such as
- What types of customers do they serve; in-patient or out-patient?
- What type of drug rehabilitation business are they?
- What is their pricing (premium, low, etc.)?
- What are they good at?
- What are their weaknesses?
With regards to the last two questions, think about your answers from the customers’ perspective. And don’t be afraid to ask your competitors’ customers what they like most and least about them.
The final part of your competitive analysis section is to document your areas of competitive advantage. For example:
- Will you provide options for the uninsured?
- Will you offer 24/7 care services that your competition doesn’t?
- Will you provide a one-to-one ratio of therapist- to-addict service?
- Will you provide certified personnel in every department of your business?
Think about ways you will outperform your competition and document them in this section of your plan.
Marketing Plan
Traditionally, a marketing plan includes the four P’s: Product, Price, Place, and Promotion. For a drug rehabilitation business plan, your marketing strategy should include the following:
Product : In the product section, you should reiterate the type of drug rehabilitation company that you documented in your company overview. Then, detail the specific products or services you will be offering. For example, will you provide therapy that includes in-patient care coupled with therapy sessions for management of addiction?
Price : Document the prices you will offer and how they compare to your competitors. Essentially in the product and price sub-sections of your plan, you are presenting the products and/or services you offer and their prices.
Place : Place refers to the site of your drug rehabilitation company. Document where your company is situated and mention how the site will impact your success. For example, is your drug rehabilitation business located near a hospital, in a clinic in a pleasant neighborhood, or is it in a stand-alone building? Discuss how your site might be the ideal location for your customers.
Promotions : The final part of your drug rehabilitation marketing plan is where you will document how you will drive potential customers to your location(s). The following are some promotional methods you might consider:
- Advertise in local papers, radio stations and/or magazines
- Reach out to websites
- Distribute flyers to area medical and therapist personnel
- Engage in email marketing
- Advertise on social media platforms
- Improve the SEO (search engine optimization) on your website for targeted keywords
Operations Plan
While the earlier sections of your business plan explained your goals, your operations plan describes how you will meet them. Your operations plan should have two distinct sections as follows.
Everyday short-term processes include all of the tasks involved in running your drug rehabilitation business, including answering calls, overseeing clinic or substance abuse treatment center practices, providing daily care, billing insurance, etc.
Long-term goals are the milestones you hope to achieve. These could include the dates when you expect to book your Xth substance abuse treatment center patient, or when you hope to reach $X in revenue. It could also be when you expect to expand your drug rehabilitation business to a new city.
Management Team
To demonstrate your drug rehabilitation business’ potential to succeed, a strong management team is essential. Highlight your key players’ backgrounds, emphasizing those skills and experiences that prove their ability to grow a company.
Ideally, you and/or your team members have direct experience in managing drug rehabilitation businesses. If so, highlight this experience and expertise. But also highlight any experience that you think will help your business succeed.
If your team is lacking, consider assembling an advisory board. An advisory board would include 2 to 8 individuals who would act as mentors to your business. They would help answer questions and provide strategic guidance. If needed, look for advisory board members with experience in managing a drug rehabilitation business or successfully running a therapist practice.
Financial Plan
Your financial plan should include your 5-year financial statement broken out both monthly or quarterly for the first year and then annually. Your financial statements include your income statement, balance sheet, and cash flow statements.
Income Statement
An income statement is more commonly called a Profit and Loss statement or P&L. It shows your revenue and then subtracts your costs to show whether you turned a profit or not.
In developing your income statement, you need to devise assumptions. For example, will you admit 10 new patients per day, and/or offer group therapy sessions? And will sales grow by 2% or 10% per year? As you can imagine, your choice of assumptions will greatly impact the financial forecasts for your business. As much as possible, conduct research to try to root your assumptions in reality.
Balance Sheets
Balance sheets show your assets and liabilities. While balance sheets can include much information, try to simplify them to the key items you need to know about. For instance, if you spend $50,000 on building out your drug rehabilitation business, this will not give you immediate profits. Rather it is an asset that will hopefully help you generate profits for years to come. Likewise, if a lender writes you a check for $50,000, you don’t need to pay it back immediately. Rather, that is a liability you will pay back over time.

Cash Flow Statement
Your cash flow statement will help determine how much money you need to start or grow your business, and ensure you never run out of money. What most entrepreneurs and business owners don’t realize is that you can turn a profit but run out of money and go bankrupt.
When creating your Income Statement and Balance Sheets be sure to include several of the key costs needed in starting or growing a drug rehabilitation business:
- Cost of equipment, furnishings, and housing
- Payroll or salaries paid to staff
- Business insurance
- Other start-up expenses (if you’re a new business) like legal expenses, permits, computer software, and equipment
Attach your full financial projections in the appendix of your plan along with any supporting documents that make your plan more compelling. For example, you might include your office location mortgage or a list of contracted providers who refer individuals to your substance abuse treatment center.
Writing a business plan for your drug rehabilitation business is a worthwhile endeavor. If you follow the template above, by the time you are done, you will truly be an expert. You will understand the drug rehabilitation industry, your competition, and your customers. You will develop a marketing strategy and will understand what it takes to launch and grow a successful drug rehabilitation business.
Drug Rehabilitation Business Plan FAQs
What is the easiest way to complete my drug rehabilitation business plan.
Growthink's Ultimate Business Plan Template allows you to quickly and easily write your drug rehabilitation business plan.
How Do You Start a Drug Rehabilitation Business?
Starting a Drug Rehabilitation business is easy with these 14 steps:
- Choose the Name for Your Drug Rehabilitation Business
- Create Your Drug Rehabilitation Business Plan
- Choose the Legal Structure for Your Drug Rehabilitation Business
- Secure Startup Funding for Your Drug Rehabilitation Business (If Needed)
- Secure a Location for Your Business
- Register Your Drug Rehabilitation Business with the IRS
- Open a Business Bank Account
- Get a Business Credit Card
- Get the Required Business Licenses and Permits
- Get Business Insurance for Your Drug Rehabilitation Business
- Buy or Lease the Right Drug Rehabilitation Business Equipment
- Develop Your Drug Rehabilitation Business Marketing Materials
- Purchase and Setup the Software Needed to Run Your Drug Rehabilitation Business
- Open for Business
Where Can I Download a Free Business Plan Template PDF?
Click here to download the pdf version of our basic business plan template.
Our free business plan template pdf allows you to see the key sections to complete in your plan and the key questions that each must answer. The business plan pdf will definitely get you started in the right direction.
We do offer a premium version of our business plan template. Click here to learn more about it. The premium version includes numerous features allowing you to quickly and easily create a professional business plan. Its most touted feature is its financial projections template which allows you to simply enter your estimated sales and growth rates, and it automatically calculates your complete five-year financial projections including income statements, balance sheets, and cash flow statements. Here’s the link to our Ultimate Business Plan Template.
Don’t you wish there was a faster, easier way to finish your Drug Rehabilitation business plan?
OR, Let Us Develop Your Plan For You
Since 1999, Growthink has developed business plans for thousands of companies who have gone on to achieve tremendous success. Click here to learn about Growthink’s business plan writing services .
Other Helpful Business Plan Articles & Templates

Need a business plan? Call now:
Talk to our experts:
- Business Plan for Investors
- Bank/SBA Business Plan
- Operational/Strategic Planning
- L1 Visa Business Plan
- E1 Treaty Trader Visa Business Plan
- E2 Treaty Investor Visa Business Plan
- EB1 Business Plan
- EB2 Visa Business Plan
- EB5 Business Plan
- Innovator Founder Visa Business Plan
- UK Start-Up Visa Business Plan
- UK Expansion Worker Visa Business Plan
- Manitoba MPNP Visa Business Plan
- Start-Up Visa Business Plan
- Nova Scotia NSNP Visa Business Plan
- British Columbia BC PNP Visa Business Plan
- Self-Employed Visa Business Plan
- OINP Entrepreneur Stream Business Plan
- LMIA Owner Operator Business Plan
- ICT Work Permit Business Plan
- LMIA Mobility Program – C11 Entrepreneur Business Plan
- USMCA (ex-NAFTA) Business Plan
- Franchise Business Planning
- Landlord Business Plan
- Nonprofit Start-Up Business Plan
- USDA Business Plan
- Cannabis business plan
- eCommerce business plan
- Online Boutique Business Plan
- Mobile Application Business Plan
- Daycare business plan
- Restaurant business plan
- Food Delivery Business Plan
- Real Estate Business Plan
- Business Continuity Plan
- Buy Side Due Diligence Services
- ICO whitepaper
- ICO consulting services
- Confidential Information Memorandum
- Private Placement Memorandum
- Feasibility study
- Fractional CFO
- How it works
- Business Plan Examples
Pharmacy Business Plan Sample
Apr.29, 2017
Average rating 4.5 / 5. Vote count: 4
No votes so far! Be the first to rate this post.

Table of Content
When someone is found doing workouts at home how to start a pharmacy, he must need relevant information, charts, table of content, independent pharmacy business plans, pharmacy business plan as well as few sample templates for pharmacy business branding. So, the entire business plans for pharmacy must include the most important points which must be well covered to start pharmacy business in full fledged.
Pharmacies must have stock of the best medications, and valuable healthcare accessories including sanitary pads, contraceptive pills and many more. Be modern and try to design a good pharmacy marketing plan before going for investment of financial resources in the industry. Get more new pharmacy business plan ideas from different sources.
Diseases, infections and virus attacks are very dangerous to shorten up the span of life of a guy. So, medications are needed to remove injuries, reinforce the immune system and increase the life expectancy to a great extent. For this reason, pharmaceutical agencies and drug manufacturing companies supply new drugs to people to resist the diseases in advance. Pharmacy business is really lucrative and profitable to an entrepreneur. Drugs are needed in hospitals, clinics, healthcare centers and in residential houses for preventive care.
Drugs are used to cure feeble patients. Children need a number of boosters and antibiotic shots to overtake a number of complicated health hazards. In large scale, medications and potions for patients are required in hospitals. Therefore, pharmacies have the jobs of selling drugs to customers. As an entrepreneur you can also start a small pharmacy or drug store in your locality. Sample pharmacy business plans and template will guide you. We, at OGSCapital, help you with this. Our executives have all the expertise for helping you to develop an appropriate strategy to fulfill your all objectives. If you wish to initiate the process, you just need to fill a contact form.
Choose Top Pharmacy Business Plans to Start Pharmacy Successfully
Well, are you a pharmacist to have licenses for running pharmacies in any town? This question is asked by many enthusiasts who are eager to know whether anyone has the permission to open a local drug store in the vicinity. Certainly, opening local drug stores, you must have an experienced pharmacist to check the prescribed drug lists. Pharmacists have the ability to read the prescriptions and identify the drugs at a first glance. His expertise in prescription reading, drug list checking and familiarity with new drugs helps the vendor to sell drugs comfortably.
If you are not a pharmacist, you need a specialist to hire in the case of inaugurating pharmacy in any popular area. He will help you to read prescriptions, handpick particular medications and understand the medical terms. Drug manufacturing companies in America have to sell only FDA approved medications. They must get authentic papers, citations and drug selling licenses from FDA as well American government.
Therefore, be familiar with local rules to starting a retail pharmacy business plan commercially. Go through a top sample pharmacy business plan and free template for more information.
Basic Requirements to Start Pharmacy Business
- A complete retail pharmacy business plan
- A preliminary financial budget
- Specific site for posting retail pharmacy business plans
- A specialist/experienced pharmacy
- Good stock of new drugs in
- Healthcare accessories like sanitary pads
- Basic amenities like refrigerators, electricity, water and good air vents
- Pharmacy needs to have a site for industry promotion
- Site map with a sample Pharma business plan as well as template
- Information brochures
- Medical aids kits to supply
- Initial affordable drug selling packages to attract customers to have drugs at low price
- Free quotes to do comprehensive studies for opening up a pharmacy.
Understand the Innovation in Pharmacy Business
Pharmacies must not be dirty and poorly managed. Expensive drugs should be stored in safer place which is much more eco-friendly. Heat, fire, rain water and bacteria destroy life saving drugs. The drug store should have excellent air ventilation, clean ambience, least toxins inside the shop and soothing ambience. Refrigerators keep stored medications in good condition.
Internet browsing is not harmful. So, regular data checking must stand you in good stead. The more you research the more you will get new ideas to launch a compact pharmacy business project with bright expectation to have excellent returns. Initially, as a newbie, your ability to design a pharmacy business plan must be limited. You are an educated person with enthusiasm to stand resilient financially. You went for getting advices from seniors to open pharmacies.
Maybe you have had dream of becoming a good pharmacist with your own outlet to sell qualitative drugs. Fake and spurious medications, potions and medical aids are destructive. These fake drug dealers must be punished. In the open market, low quality, sub-standard medications and expired healthcare tonics are sold at low prices. Therefore, every year, the death toll increases due to the exposure to the drug adulteration. Perhaps you have the setback or previous caustic experience. Someone might have died due to consumption of bad drugs. Your dream must be fulfilled.
Online planners in pharmaceutical industry have pre-designed samples, fact sheets, research papers and documents to train newcomers. Novice financers have no industry expansion ideas. Especially pharmacies are different projects to highlight. Drug is the most valuable product as it saves lives. So, definitely lot of care is needed to run pharmacies or drug stores in the city. What type of pharmacy business do you need to opt for? Basically, drug retailers, individual sellers and small size company partners like to have free consultation before establishing a start-up drug store in any known area. The advantages of opening drug stores or local pharmacies in the residential areas include
- Known ambience
- Good familiarity with local citizens
- Be accustomed to local administration
- Good relationship with doctors, patients and oldies
- Easy to find customers
- Better options to enlarge pharmacy business in the home town
However, most probably, you are trapped because you must struggle to find more genuine options to expand your drug stores to have lot of money. So, geographical barrier must not be a problem. Often, it is a lucrative business for you in case you have the plan and templates to take your drug store to backcountry. There are not many drug stores. Competition is low. You can buy and sell expensive drugs to rich persons. Poor people will get affordable medications from your shop.
So, it is good business to manage in the rural areas. However, drug stores in cities are always dazzling in vanity. The buying strength of local people is obviously higher. City based pharmacies are equipped with modern infrastructures. In villages, poor people are not able to buy costly drugs. The availability of sumptuous medical aids packages is also not good in rural belts. Urban areas are developed. Therefore, locate the place and start evaluation. Pros and cons must be checked before deciding to deal with pharmaceutical industry. Retail pharmacy business plan writers are also helpful to people to have guidance in designing the independent plan for starting retail drug store business plan .
Brand medications are not cheaper. These drugs have qualitative components used by manufacturers to produce standard medications in the market. The effect of intake of brand medications is really awesome to help patients to avoid disaster. Doctors prescribe brand medications. Great. Well, one of the best ways to sell brand medications is to find the high profile class. Online ventures must bring a new customer to you. This is a different industry for entrepreneur as there is little chance to meet vendors face to face.
Customers hit the online sites to check the pharmacies for buying the branded drugs recommended by experts. All are not financially sound to buy brand drugs. Pack of Viagra (branded) costs a customer around $1000. Or a pack of 60 pills of Soma is equivalent to $400 inclusive of overnight shipment cost. So, economical buyers find generic medications which are affordable to some extent. Therefore, your drug stores must have both branded medications for the rich and generic medications for the economical class.
It will be a strategy to win customers by providing alternative medication buying option. Generic medications are cheap and components used in manufacturing these affordable drugs are not low in quality. The impact of consumption of generic medications is equal to the effect experienced by a person who takes brand medications. Retail pharmacy business plan writer has many ideas/ template to precise the process of industry inauguration smoothly.
Discount Pharmacies
In the pharmaceutical industry, scientific probing, research and deep analysis are inseparable. Scientists in the medical arena are trying to invent more cost efficient products which must be vehicles for economical customers to save money. Pharmacists will emulate new technologies to increase the cost effectiveness and quality of the drugs. So, doctors, scientists and pharmacists are closely connected for making a convenient customer support portal for better drug supply at low cost. Discount pharmacies are byproduct of the innovative thoughts nurtured by experts.
The collaborative venture in the pharmaceutical industry has made a strong platform for pharmacy business owners to sell affordable medications at discounts. Customers get promotional codes on different types of generic medications. It is much more cost efficient. It is much affordable to a young guy. Result is also same. So as a pharmacist, offer your customers what you have in stock. Discount pharmacies are the places for buying cheap generic medications. Brick and mortar discount drug stores reduce the cost of buying valuable medications.
Well, think whether you are fitted to drug selling industry. A discount drug store needs to have only cheap medications which must be good. Customers will get their drugs by showcasing prescriptions. Now, tailor a business plan identifying the trend in the market. You have handful options to use. As a local guy, you can apply for loan for industry running. Banks will give you support to invest in the pharmaceutical industry.
Next steps include the easy refinancing, recruitment of competent employees, assistants , the availability of good medications at discounts and drug safety programs to minimize the threat. People need proper medical assistance from a vendor. As being a professional pharmacist, it is your concern to prioritize the table work/ground work to inaugurate the drug store in your residential area. Innovation can’t be stopped. Americans are habituated to consume sleeping pills, anti-anxiety drugs and self-boosting capsules to have energy in excess.
They are fast, sophisticated and crazy to travel for exploration. Well, most of American customers are seen buying drugs from online pharmacies which offer discounts. These online discount pharmacies assist busy Americans to have affordable qualitative medications at considerably cheap prices. So, you must have a compatible optimized online shop with an inventory storing only new drugs/ prescribed medications/non prescription medications at discounts. The local stores for drug selling are not permitted to sell non prescription drugs.
They need prescriptions. However, comparatively online pharmacies have good options to help customers to purchase non prescription drugs. Customers don’t need to send the scanned photocopies of prescriptions to pharmacists to buy packet of Soma or Viagra. Buy medications from vendors at any point of time. So, if you want to modernize your retail drug store, design an affordable business plan to start selling generic medications at discount drug stores without putting a band of legal obligation. The online pharmacy is not a local departmental store.
Customers don’t need to visit the store for product purchasing. The virtual shopping cart or pharmacy is actually run through internet. Its customers place orders at the shopping cart and complete transactions instantly. Vendors are liable to ship products to the customers. Now you must have a site which supports mobile phones, computers and different smart devices. Consumers will cross check list of drugs available in their areas. You have a group of employees who manage customers online. Usually, online pharmacies have no go-down or small warehouses.
They are professional and hire moving companies for product shipment. It is a chain for drug supplying. No manual paperwork is conducted. Nor is there any option for meeting customers physically. So, the whole transaction is done via internet. E-commerce infrastructure is easy to operate. This marketing strategy is extensive to help local traders to go for vast venture to reach million customers on a single go. Around $829 billion is invested in global pharmacy business. So, this opportunity is also open to you to have scum of the profits to upgrade your own life.
Online discount pharmacy is open regularly round the clock. Customers from different locations hit the online drug store and buy medications. Prices of these lifesaving drugs, sanitary pads, healthcare pills and antibiotic pills are affordable. Discounts of these medications are attractive to impress economical class. Like Rite Aid, you can also have a chain meds service to bring more fluent drug delivery options to customers. Give individualized service by keeping personal records of patients after clearing transactions.
In future, if the customers have allergies or infections, try to avoid supplying medications which are not prescribed. Besides, help them to choose the prefect meds from the inventory. Unlike local drug store, online discount pharmacies have multi channels to get customers. If you have licenses or permit to export medications to market abroad, strategy vastly. Then, you are also a good exporter with permits to deal with overseas clients. Online platform for medication selling is now modernized.
The shopping carts online must have fast content management, instant credit checking while making payment, quick transactions and free registration. The prescription refill process must be fast and easy. On the other side, the non prescription generic or brand medications should be supplied to have more positive visitors to hit your e-commerce website.
So, you will also have lot of sources to track the best retail pharmacy business plan/ templates, pharmacy start up business plan, pharmacy business plan pdf and innovative pharmaceutical sales business plans. Go through ins and outs of pharmaceutical business plan, pharmaceutical business plans sample, and retail pharmacy business plan including new templates.
Product Quality – Must
In the pharmacy business, the product quality is a must. A vendor or pharmacist should not earn money by delivering expired meds to patients. He should have social obligations to maintain for fairness in pharmacy business. Same way, he is also responsible to process the orders. If meds are delivered late then patients can die. If it is urgent, then shipping process must be fast. Overnight drug supply is not cost effective. Extra processing charges are borne by the buyer.
When your pharmacy business blooms, you must have some better options to make your customers happy. Your online pharmaceutical has few exceptional features which are unbeaten to take your pharmaceutical to million customers outperforming rivals. Pharmaceutical companies must facilitate customers to have their drugs at discounts. Quality of the drugs must not be low. For this reason, there must be analysts and experts to make their comments based on the products meant for sale. Customers will talk to consultants for getting pharma template.
They will go through reports, comments and reviews to have ideas about the quality of meds kept for being sold. Your online med supply stores should be compatible with multiple devices including smart phones. Customers are interested to cross check regular information and updates on their mobile phones. Your e-commerce portal is connected with vast network to support such a sophisticated device. Online customer management center assists new customers to buy products.
For this reason, people feel free to go for buying expensive brand meds from the best pharmacy online. Jot down pros of running a discount pharmaceutical online. Why do people want to buy products online? What is the difference between a local med store and the pharmaceutical on internet? What type of med is sold online? What are the most important pros of buying meds from online pharmacies? Is it cost effective or less time consuming to make deals with online vendors? Well, there are more such questions which can be highlighted for discussion.
Basically, internet based online med stores don’t need manual paperwork. Nor is there any need to entertain customers at the shop. So, investors are not required to buy the land for constructing a big set-up to sell packets of life saving meds, boosters, and health tonics. Online meds stores are operated by pharmacists through broadband. It is a different setting to attract people for business related transaction. Many people don’t understand how to buy meds online. They must be educated.
Well, your pharmacy business promoting campaigns should encourage customers to know about the good aspects of online transactions. First of all, post few blogs, photos, slide shows and of course glossy video clips on home screen to lure newbie customers to have a fast look at the site. After visiting the site, they will be energized to read content, check photos, videos, slide shows and navigate in the site. Online free start-up pharma template will make you more confident.
Site accessibility, information delivery, transaction method, registration, product buying as well as packing for shipment, prices of medications, and way of billing must be innovated. Therefore, ask your website designers to upgrade the site nicely to make it much more compatible with the latest i-devices. Ask them to assist you to change the old policy. Crazy customers in America, Canada, the UK and other parts in Europe have tendencies to buy non-prescription drugs which are not recommended or prescribed by doctors.
They have to pay higher prices to get packets of sedatives or anti-anxiety pills from the local market. Often they are addicted to spurious drugs which enhance the scope of faster deterioration in the health management. They don’t need prescribed drugs as they are not permitted to consume multiple sedatives within 24 hours. They are drug addicted and therefore proper counseling is needed. However, when they hit the online sites or med stores on internet, the surprising gifts are waiting for them.
At a time, it is possible to buy different meds from stores without providing prescriptions. Secondly, they have facilities to deal with a number of drug stores in this online drugstore industry. Promotional offers and discounts are offered to customers as well. At first, when you establish a small size pharmacy, you require the base to stand. It is the ground for you to start building up a strong long lasting structure. It is a dynamic policy to enhance the much faster business promoting.
New Policy for Starting Pharmaceutical Company
Minimum investment is needed to run a pharmacy business. So, the product selling base is required first to ensure the good prospect in this meds supply industry. How to achieve success depends on the strength, resilience, willing force, and lot of energy to do the hard work out. Maybe, it is time consuming but success will come through devotion, hard struggles, and meticulous research to find the best opportunities to be successful entrepreneur with a solid pharmacy business structure.
Invest in the best market where there is excellent ambience to gear up the money earning. It is one of the best things for you to locate the area where you will open the small drug store with innovated structure, and other facilities to tempt customers. It stands to reason; you require lot of plans to design. When you have the dream projects to implement for the business expansion, you must have someone to take you to the last resort successfully.
Many of start-up entrepreneurs are not well organized due to the lack of experience in pharmacy business planning as well as endorsement policy. Well, in this case, a professional business planer needs to be hired for innovating the business plans before investing in the pharmacy industry. Money is needed but you should have excellent projects which have lot of information, data, template, strategy, table of content and resources to let the pharmacy business run smoothly.
Research, probing, intuition and self-discovery study are important to aspiring pharmacy entrepreneurs. To become an experienced entrepreneur, you will have to have strong desires to probe deeply. This research oriented mind will give you a booster to scale up in the industry with success. That’s why, when you have new innovative commercial management plans, policy, programs and glossy projects to implement, you must not stop. The start-up pharmacy business must be expanded.
The profits must be generated. The mobility in the business promotion should be uninterrupted. The longevity of the pharmacy business should be surprisingly longer. Therefore, concentrate on different aspects of commercial management. Go to professional business consultants, experts and business analyzers to have new guidelines/instructions/ plans for extensive analysis.
Brand Business
Brand name of your business works as a booster. It is the workforce. It is the vehicle with a new strategy for you to start the business smoothly. Who will track your business? Brand name is the sign of identification. People will be familiar with your company through the brand name. So, choose the most suitable name for your pharmacy. Well, maybe you have lot of confusion how to configure the brand name and logo.
Really it is much important to you. A cumbersome brand name is not easy to understand. If the name of the pharmacy is long and technically intricate, people are not able to read the brand name easily. So, select the relevant short and attractive words to name your med store. Obviously you need a strategy to conduct more productive business branding.
Invest Money in Promoting Products
Million dollars are overspent in the business promotion campaigns. Giant entrepreneurs have lot of money and they spend financial resources to promote business. Gifts, discounts, and promo codes are offered by these multinational companies. Well, you are a small entrepreneur and you need to earn more money. In the beginning, you have few attractive projects to inaugurate the small business. Well, strategically, you must advertise your products. Advertisement agencies can make your dream productive through lot of ground work, plans, programs and tips.
Hire the top notch advertisers who will make a brief-up covering the most important areas of pharmacy business. Your online advertisements, ads and video clips showcase the med store to impress customers. Certainly, you must have some awesome product endorsement publicizing projects. How to promote the pharmacy business? The objective of your promotional expedition lies in the expansion of the customer management platform with more opportunities to build up the foundation for rejuvenating the business.
Business branding through internet is the weapon for you to convey the best message to people. Your strategy to run business must be effective. What type of medication do you need to sell? Are you a drug retailer? Are you able to sell cheap generic meds? What sort of med do you have in stock? People need better price tags. They are economical. They have the least interest to buy brand meds. Well, your discount drug pharmacy is very much modernized with the stock of high caliber generic medications, life saving meds and qualitative drugs at discounts.
Next step is to have all legal papers, and documents. The paperwork must be done step-wise. First of all, you have to apply for a license. It is the passport for your pharmacy to run. Certainly, it is not easy. Drug licenses are approved only after several surveys, cross verifications and probing. You can’t damage one’s precious life by supplying low quality spurious meds. Right now, government and FDI are not flexible to issue drug licenses easily. Drug addiction is now accelerating to make young generation incompetent.
They have lot of drug buying options. Fake dealers and spurious drug suppliers are seen tempting customers. That’s why; fake licenses and wrong documentation are severely increasing to threaten up people to a great extent. Therefore, you must be fair and honest to have your licenses legally. In this connection, meet an attorney who will advise you how to get the drug license from the superior authority. Smartly speaking, it is not entirely difficult. The legal power will assist you to convince the concerned authority to have the original drug license.
In the beginning, it is the most unavoidable assignment to contact a group of legal experts to ensure the advertising project completion successfully. Pharmacy business must not be dried up after few weeks. You should not backtrack in shame. Bold and strong entrepreneurs must have energy to prioritize the collection of important components to format a new strategic version of business plan to nourish the pharmacy outlet dynamically.
It is a must to maximize the vast media exposure, business branding, meticulous analysis and study to probe deep with good motif to locate the most fruitful niche to speed up the business promotion. Your decision must not be obscure because of the disorders created by your subordinates. Therefore, organize your team to finalize the business branding program and innovative commercial management strategy with the resolution of giving thousand horsepower to your small med store to gain speed.
Tailor Futuristic Advertising Projects
Design futuristic advertising projects. Calculate how much fund is required to establish a single compartment as a small street outlet for selling generic meds. Money you need must be available. If you are not so lucky to arrange fund immediately to overtake hurdle, you must not be found being in lethargic state. Business loans, short term financial aids, and good financial support from different agencies are obtained without complicated paperwork. So, search for this type of secured/unsecured business loan to finance the med store. Financial assistance must be required to buy packets of brands drugs, install computers, and other tools to decorate the drug store.
Basic Components of Discount Pharmacy Business Plan
- Basic indoor furniture pieces like table, chair, desks, small file storing cabinets
- Cash registering systems
- Front counter
- Computer terminal
- Printer for billing
- Electrical goods
- Small data storage server
- Shipping accessories
- Good insurance coverage
- Storing bins
Important Facts to Remember
Customers are different in nature. They don’t have similar mindsets. Nor are they on same strings. Well, study and then evaluate the mindsets of customers. Choose the area where you will get positive customers to sell drugs. Reinvent new strategy to promote pharma. Specific groups of customers need to make walk-in visits to the outlet to purchase meds. This sort of customer has the habit to go to the local med store to buy meds. They are literate.
However, many laymen in the lower middle class are interested to buy prescribed meds from the pharmacies. Secondly, there is another group of people. They are online buyers. They have money to go to the online pharmacy to purchase drugs. Basically, generic medications, and different types of stress management pills they buy from online stores. Prescriptions are not a must for them to buy Viagra, Cialis, Soma or any cheap generic medication to consume.
Apart from this economical class, the enriched and affluent high profile buyers opt for brand meds. Now these medications are very expensive. Even vendors of publicizing portal are not capable of storing different types of brand medications for sale. So, tailor a compact financial budget which must include the overall cost of storing brand, generic and local meds at discounts. The product sale must be fast. Drugs which stored in your refrigerator must be qualitative.
Top Strategies to Expand Pharma
Strategies are applicable to the drug selling. Promotional campaigns are conducive to the faster development of the business. However, forecast is needed to evaluate the vision of yours in the long run. In the first year, estimate the profit percentage. Then you must compare whether you are a gainer or loser. Moderate revenue collection is also good as you are a novice entrepreneur. Well, you need to improve by hook or by crook. Commercial management is not a new thing. Even local traders and informal entrepreneurs know this term.
When your pharmacy business will be launched, your target must concentrate on the store management. Employees should be duty bound. Your pharmacist must be responsible. Drugs kept in your refrigerator must be hygienic. Temperature inside the drug store must be eco-friendly. Apart from this, documentation, billing, registration process and other paper work must be carried out properly. At present, computer application is very urgent. Your tiny med store must be renovated with a set of computers, laser printer and printable papers.
Customers will get bills and invoices through automated bill generated machine. So innovation in the decoration of the med store is certainly essential. Commercial management is also done via internet. Online documentation is undoubtedly smooth. Contact customers on online chatting platform. Talk to them over phone. Make video calls to have advices from experts if required. Permit your employees to use cell phones to send emails to your inbox. Oracle based commercial management portal must be installed into your pharmacy store for taking care of back office jobs smoothly.
Operational and Strategic Planning
Understand competition and reset your marketing strategy.
You are not alone in the pharmacy advertising portal. Regularly minimum 1 million visitors check over thousand sites to buy medications in the world. Billion dollars are hovering in the med manufacturing trade. The advancement in the med field is the turning point and slowly people are showcasing their interest in buying drugs from the online pharmacies. Rivalry, competition, and struggles are basic things for a trader. To make your pharmacy business/ marketing profitable, be proactive to be much more competent.
Emulate new strategies. Borrow much innovative marketing plans and imitate successful sample business templates to bring innovation to the pharmacy business/ pharma marketing. Who is your rival in the market? Well, rivalry doesn’t mean hostility. You must not have impatience to kill someone. Nor are you a militant to spray venom for terminating the city. So, don’t be misled. Competition means the fair rivalry in advertising trade. You should upgrade your trade structure.
You should be efficient to sell drugs at more affordable prices. You should have dozens of futuristic plans to enlarge the trade faster. The cost efficiency is the objective of a newbie to reduce the cost of drugs. Similarly he will not violate the FDI law to sell drugs at lower prices. Competition is needed for the betterment of the service. Fancy how to bring a result oriented business promotion strategy to expect a sound financial structure by operating a small pharmacy store.
Be a creative person. You are budding pharmacy entrepreneurs. So, recycle your raw energy embedded in mind. Go through periodicals, brochures, and magazines to have authentic information. Research materials need to be gathered for making a giant project to enhance the superb commercial management. Therefore, create a different business plan for pharmacy. It is your draft. You have quality to express what you need. You have bundles of new concepts, and ideas to use.
So in draft assignment, feel free to describe the goals, objectives, business starting plans, budget related issues, sources of arranging fund and basic requirements and marketing to start the drug store. Well, the finishing touch needs to be added to the draft. An experienced pharmacy business planner has over hundred samples. He is a professional business plan writer for pharmacy.
He has solved complicated problems. He is one of the most successful planners in the field. So he can give you a summary of the business plan. Even his instant business plan writing backup must be fruitful to you. Do more probing in the modernized med manufacturing and pharmacy company.
Upgrade your basic knowledge for the sake of good familiarity with the pharmacy field/ marketing. Online library, data and million tons of samples boost up novice traders to track their loopholes in upgrading futuristic strategy. Through compact SWOT analysis, he is able to find the negative factors which are not helpful to him to run the small size drug store. Strength of the entrepreneurs must be identified. It is also applicable to you as well. Track where are going? If you have no vision, the area will not be resilient. You should find the niche in which you will cultivate the land for flourishing the pharmacy business dynamically.
Install Plan Software
Conventional planning needs to be replaced. Computers minimize the jobs of taking care of customers. Well, few years back, drug stores have to keep large space for entertaining customers who were found standing for buying medications. Separate counters for billing were open to all consumers. Drugs were not sold through a single counter during emergency. So, there must be extra space for drugs storing, supplying and customer management. Besides, lighting fixtures, indoor furniture pieces and other important accessories must be installed in the drug store.
Simultaneously, change the traditional billing system. Commercial management/ marketing process must undergo vast innovation. Compact highly advanced SAP software for management/ marketing software is on display in Google. Experts have reset this SAP model to help entrepreneurs to complete handful jobs perfectly. Billing, customer care, marketing, data management, invoice processing, and company analysis are conducted. You have the fast software to take decision within short span of time.
Your marketing trade must be perfectly integrated into a unique platform. Monthly you must analyze the trend. Is your pharmacy running fast or any downtime is likely to happen? Profit ratio, and revenues collected for the particular year must be cross checked. Industry remodeling must be done. However, it is really urgent to go deep and find more facts to compare. Is your drug store compatible with new technology? For instance, virtual pharmacy industry is now much sought-after.
Many American guys buy drugs from online med stores. However, in South Africa, Nigeria, and many countries located in Asia are not perfectly tuned up to run online med stores. Mail order delivery system is not innovative in the remote places as well. So, before opening a new drug store online, once again study to understand the mindsets of local people. However, online mail order marketing system is a vast business expansion portal. You will get more customers in this pharma industry.
Even if you have authentic permits and licenses to export expensive meds to overseas customers, you are a successful entrepreneur with good prospect. Drugs are not useless things. Patients must need medications to save their lives. So, the importance of online pharmacy business will not go down even after a millennium. So, slowly you must have command over the pharmacy commercial management/ marketing. Newbie entrepreneurs should do experiments to be much acquainted with the latest industry operating ethics.
It is obviously time consuming but with technical innovation, and advancement in the commercial management, it is wonderfully much more convenient for a newcomer to learn how to open a good pharmacy store in this specific industry. Starting a pharmacy business plan designed by experts is basically a tool for a newcomer to get a roadmap to innovate the pharmacy in much more dynamic way.
In this connection, OGS capital consultants online are experts to guide newcomers how to chalk out futuristic pharmacy business projects fantastically. Take valuable business promoting tips, modern strategy and free advices from these consultants. Chat with OGS capital business consultants to have new strategy, and ideas to remodel the entire corporate portal successfully.
Download Pharmacy Business Plan Sample in pdf
OGS capital writers specialize in business plan themes such as massage business plan , medical clinic business plan , cannabis dispensary business plan , nursing business plan ideas , transportation corporation health services business plan , reiki business plan and many others.
OGSCapital’s team has assisted thousands of entrepreneurs with top-rate business plan development, consultancy and analysis. They’ve helped thousands of SME owners secure more than $1.5 billion in funding, and they can do the same for you.

Vegetable Farming Business Plan

Trading Business Plan

How To Write A Textile Manufacturing Business Plan

Start a Vending Machine Business in 2024: A Detailed Guide

Oil and Gas Business Plan

What Is Strategic Planning: Definition and Process

Any questions? Get in Touch!
We have been mentioned in the press:
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Search the site:
Teva Pharm to launch Humira biosimilar as Q1 profit misses estimates
- Medium Text

- Company Abbvie Inc Follow
- Company Alvotech SA Follow
- Company Cigna Group Follow
Sign up here.
Reporting by Steven Scheer; Additional reporting by Patrick Wingrove in New York; editing by Jason Neely and Michael Erman
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Business Chevron

Wall Street closes up, another weekly gain ahead of inflation data
U.S. stocks eked out modest gains on Friday and all three indexes posted another weekly advance as investors parsed comments from Federal Reserve officials and looked ahead to crucial inflation data next week.


Times of San Diego
Local News and Opinion for San Diego
Opinion: South County Stands United Against an Unnecessary and Unjust Landfill Plan

Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to print (Opens in new window)

The Tijuana River is the environmental and social justice crisis of our lifetime in San Diego. Despite recent successes, things could soon get much worse.
That’s because a pair of private developers is trying to build a new landfill in East Otay Mesa, within the Tijuana River watershed. On land that drains directly into the contaminated river. Near vulnerable and underserved communities that already suffer the greatest pollution burden in the entire state.

It’s the last place on earth we should ever put a new landfill.
South County stands united on this. I’m proud to have joined with a broad coalition of elected officials, environmental groups, community advocates and local residents to raise awareness of this dangerous project that can only prolong our region’s pollution nightmare.
And for us, it’s personal.
South County beaches near the Tijuana Slough have been closed continuously since Dec. 8, 2021, due to unsafe conditions. That’s more than 800 straight days that local families have been denied the ability to enjoy our shore.
Now this Tijuana River Landfill could drain even more pollution into the Pacific Ocean, causing more beach closures and sick surfers and swimmers. This, in a state where coastal access is paramount, and in a region where our sunny beaches fuel an economy that relies heavily on tourist dollars.
The Tijuana River Landfill also poses a significant threat to our South County environment. It will bulldoze hundreds of acres in the Otay Foothills, eliminating critical habitat for numerous endangered, threatened and sensitive species. It will sever a key wildlife movement corridor used by animals migrating between the two countries as well. If this landfill is constructed, the only movement corridors will be lines of trash trucks heading to and from the dump.
Plus, the existing pollution crisis has degraded the Tijuana River Estuary and our coastal ecosystem to the brink of collapse. We cannot risk further damage to these wetlands from even the best planned and mitigated landfill.
But more than anything, the Tijuana River Landfill is bad for South County’s working families. It will be located near one of the most polluted and underserved areas in all of California. This is where so many of our region’s essential workers call home – our nurses, janitors, construction crews and cooks. These mainly black and brown communities already bear the brunt from decades of incompatible land uses that harm our health and quality of life. An injustice against them is an injustice against all of South County.
There’s already one landfill in South County just a few miles away and it has plenty of room left. But here they go again trying to burden us with another. It is morally wrong to build a new landfill in this area and subject the next several generations to the same history of environmental injustice. Simply put, they would never try this in other parts of the County.
As an elected official, I’m equally troubled by the approval process for this landfill. The developers are using a rare procedure that bypasses all local environmental review. This would be the only landfill in California approved in this manner. As it stands today, we risk the project breaking ground without a single meaningful public hearing or a single elected representative having a say in the matter. That’s a dangerous precedent that will effectively silence disadvantaged communities throughout the state moving forward.
And to make matters worse, we don’t even need another landfill! Studies show San Diego County has enough capacity in existing landfills to last more than 30 years. We won’t need another one until the 2050s, and likely longer as we continue to reduce waste and increase recycling. As a result, there is absolutely no reason to risk all this environmental and social harm.
Despite this, developers are fast-tracking the project and could break ground as soon as next year.
The good news is that a solution is in the works. State Sen. Steve Padilla has introduced legislation, Senate Bill 1208 , that would prohibit the construction of new landfills within the Tijuana River watershed. This important bill would help break the cycle of environmental injustice and avoid an unnecessary burden on those who can least afford it. It sends a clear message that South County and our border region face unique challenges, and that bold action is necessary to protect our communities from further harm.
We thank Senator Padilla and all of our region’s leaders who have stepped up to address the Tijuana River pollution crisis. Let’s not move backwards by dumping a landfill into the mix.
Ron Morrison is mayor of National City. For more information on the No Tijuana River Landfill Coalition, visit notjriverlandfill.org .
- Share full article
For more audio journalism and storytelling, download New York Times Audio , a new iOS app available for news subscribers.

- May 10, 2024 • 27:42 Stormy Daniels Takes the Stand
- May 9, 2024 • 34:42 One Strongman, One Billion Voters, and the Future of India
- May 8, 2024 • 28:28 A Plan to Remake the Middle East
- May 7, 2024 • 27:43 How Changing Ocean Temperatures Could Upend Life on Earth
- May 6, 2024 • 29:23 R.F.K. Jr.’s Battle to Get on the Ballot
- May 3, 2024 • 25:33 The Protesters and the President
- May 2, 2024 • 29:13 Biden Loosens Up on Weed
- May 1, 2024 • 35:16 The New Abortion Fight Before the Supreme Court
- April 30, 2024 • 27:40 The Secret Push That Could Ban TikTok
- April 29, 2024 • 47:53 Trump 2.0: What a Second Trump Presidency Would Bring
- April 26, 2024 • 21:50 Harvey Weinstein Conviction Thrown Out
- April 25, 2024 • 40:33 The Crackdown on Student Protesters
Stormy Daniels Takes the Stand
The porn star testified for eight hours at donald trump’s hush-money trial. this is how it went..
Hosted by Michael Barbaro
Featuring Jonah E. Bromwich
Produced by Olivia Natt and Michael Simon Johnson
Edited by Lexie Diao
With Paige Cowett
Original music by Will Reid and Marion Lozano
Engineered by Alyssa Moxley
Listen and follow The Daily Apple Podcasts | Spotify | Amazon Music | YouTube
This episode contains descriptions of an alleged sexual liaison.
What happened when Stormy Daniels took the stand for eight hours in the first criminal trial of former President Donald J. Trump?
Jonah Bromwich, one of the lead reporters covering the trial for The Times, was in the room.
On today’s episode

Jonah E. Bromwich , who covers criminal justice in New York for The New York Times.

Background reading
In a second day of cross-examination, Stormy Daniels resisted the implication she had tried to shake down Donald J. Trump by selling her story of a sexual liaison.
Here are six takeaways from Ms. Daniels’s earlier testimony.
There are a lot of ways to listen to The Daily. Here’s how.
We aim to make transcripts available the next workday after an episode’s publication. You can find them at the top of the page.
The Daily is made by Rachel Quester, Lynsea Garrison, Clare Toeniskoetter, Paige Cowett, Michael Simon Johnson, Brad Fisher, Chris Wood, Jessica Cheung, Stella Tan, Alexandra Leigh Young, Lisa Chow, Eric Krupke, Marc Georges, Luke Vander Ploeg, M.J. Davis Lin, Dan Powell, Sydney Harper, Mike Benoist, Liz O. Baylen, Asthaa Chaturvedi, Rachelle Bonja, Diana Nguyen, Marion Lozano, Corey Schreppel, Rob Szypko, Elisheba Ittoop, Mooj Zadie, Patricia Willens, Rowan Niemisto, Jody Becker, Rikki Novetsky, John Ketchum, Nina Feldman, Will Reid, Carlos Prieto, Ben Calhoun, Susan Lee, Lexie Diao, Mary Wilson, Alex Stern, Dan Farrell, Sophia Lanman, Shannon Lin, Diane Wong, Devon Taylor, Alyssa Moxley, Summer Thomad, Olivia Natt, Daniel Ramirez and Brendan Klinkenberg.
Our theme music is by Jim Brunberg and Ben Landsverk of Wonderly. Special thanks to Sam Dolnick, Paula Szuchman, Lisa Tobin, Larissa Anderson, Julia Simon, Sofia Milan, Mahima Chablani, Elizabeth Davis-Moorer, Jeffrey Miranda, Renan Borelli, Maddy Masiello, Isabella Anderson and Nina Lassam.
Jonah E. Bromwich covers criminal justice in New York, with a focus on the Manhattan district attorney’s office and state criminal courts in Manhattan. More about Jonah E. Bromwich
Advertisement
- Personal Finance
- Today's Paper
- Partner Content
- Entertainment
- Social Viral
- Pro Kabaddi League
Cipla, Glenmark recall drugs from US over manufacturing issues: USFDA
As per the latest enforcement report issued by the usfda, a new jersey-based subsidiary of cipla is recalling 59,244 packs of ipratropium bromide and albuterol sulfate.
)
Drug makers Cipla and Glenmark are recalling products from the American market due to manufacturing issues, according to the US health regulator.
Listen to This Article
Warning letter for pithampur sez facility remains an overhang for cipla, glenmark cuts diabetes drug cost by 70% with first indian biosimilar launch, cipla's pithampur unit gets usfda warning; analysts remain bullish, cipla receives approval from drug regulator cdsco for uti drug, cipla's net profit jumps 32% to rs 1,055.9 crore in third quarter, us tech industry can't survive without indians: svc chamber of commerce ceo, trudeau upholds canada's rule-of-law post indians' arrest in nijjar killing, russia lists zelenskyy as 'wanted'; ukraine calls it act of 'desperation', bank of england's rate cut plan set to differ from federal reserve's, a post-buffett berkshire hathaway is omaha's focus after munger's passing.
(Only the headline and picture of this report may have been reworked by the Business Standard staff; the rest of the content is auto-generated from a syndicated feed.)
Don't miss the most important news and views of the day. Get them on our Telegram channel
First Published: May 05 2024 | 11:28 AM IST
Explore News
- Suzlon Energy Share Price Adani Enterprises Share Price Adani Power Share Price IRFC Share Price Tata Motors Share Price Tata Steel Share Price Yes Bank Share Price Infosys Share Price SBI Share Price Tata Power Share Price
- Latest News Company News Market News India News Politics News Cricket News Personal Finance Technology News World News Industry News Education News Opinion Shows Economy News Lifestyle News Health News
- Today's Paper About Us T&C Privacy Policy Cookie Policy Disclaimer Investor Communication GST registration number List Compliance Contact Us Advertise with Us Sitemap Subscribe Careers BS Apps
- Budget 2024 Lok Sabha Election 2024 IPL 2024 Pro Kabaddi League IPL Points Table 2024
- Election 2024
- Entertainment
- Newsletters
- Photography
- Personal Finance
- AP Investigations
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election Results
- Delegate Tracker
- AP & Elections
- Auto Racing
- 2024 Paris Olympic Games
- Movie reviews
- Book reviews
- Personal finance
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
US poised to ease restrictions on marijuana in historic shift, but it’ll remain controlled substance
The U.S. Drug Enforcement Administration will move to reclassify marijuana as a less dangerous drug, a historic shift to generations of American drug policy that could have wide ripple effects across the country.
FILE - In this Friday, March 22, 2019, file photo, a marijuana plant is visible at Compassionate Care Foundation’s medical marijuana dispensary in Egg Harbor Township, N.J. The U.S. Drug Enforcement Administration will move to reclassify marijuana as a less dangerous drug, a historic shift to generations of American drug policy that could have wide ripple-effects across the country. The DEA’s proposal still must be reviewed by the White House Office of Management and Budget. (AP Photo/Julio Cortez, File)
- Copy Link copied
WASHINGTON (AP) — The U.S. Drug Enforcement Administration will move to reclassify marijuana as a less dangerous drug, The Associated Press has learned, a historic shift to generations of American drug policy that could have wide ripple effects across the country.
The proposal, which still must be reviewed by the White House Office of Management and Budget, would recognize the medical uses of cannabis and acknowledge it has less potential for abuse than some of the nation’s most dangerous drugs. However, it would not legalize marijuana outright for recreational use.
The agency’s move, confirmed to the AP on Tuesday by five people familiar with the matter who spoke on the condition of anonymity to discuss the sensitive regulatory review, clears the last significant regulatory hurdle before the agency’s biggest policy change in more than 50 years can take effect.
Once OMB signs off, the DEA will take public comment on the plan to move marijuana from its current classification as a Schedule I drug, alongside heroin and LSD. It moves pot to Schedule III, alongside ketamine and some anabolic steroids, following a recommendation from the federal Health and Human Services Department. After the public comment period and a review by an administrative judge, the agency would eventually publish the final rule.
“Today, the Attorney General circulated a proposal to reclassify marijuana from Schedule I to Schedule III,” Justice Department director of public affairs Xochitl Hinojosa said in a statement. The DEA is a component of the Department of Justice. “Once published by the Federal Register, it will initiate a formal rulemaking process as prescribed by Congress in the Controlled Substances Act.”
Attorney General Merrick Garland’s signature throws the full weight of the Justice Department behind the move and appears to signal its importance to the Biden administration.
It comes after President Joe Biden called for a review of federal marijuana law in October 2022 and moved to pardon thousands of Americans convicted federally of simple possession of the drug. He has also called on governors and local leaders to take similar steps to erase marijuana convictions.
“Criminal records for marijuana use and possession have imposed needless barriers to employment, housing, and educational opportunities,” Biden said in December. “Too many lives have been upended because of our failed approach to marijuana. It’s time that we right these wrongs.”
The election year announcement could help Biden, a Democrat, boost flagging support, particularly among younger voters .
Biden and a growing number of lawmakers from both major political parties have been pushing for the DEA decision as marijuana has become increasingly decriminalized and accepted, particularly by younger people. A Gallup poll last fall found 70% of adults support legalization, the highest level yet recorded by the polling firm and more than double the roughly 30% who backed it in 2000.
The DEA didn’t respond to repeated requests for comment.
Schedule III drugs are still controlled substances and subject to rules and regulations, and people who traffic in them without permission could still face federal criminal prosecution.
Some critics argue the DEA shouldn’t change course on marijuana, saying rescheduling isn’t necessary and could lead to harmful side effects.
Jack Riley, a former deputy administrator of the DEA, said he had concerns about the proposed change because he thinks marijuana remains a possible “gateway drug,” one that may lead to the use of other drugs.
“But in terms of us getting clear to use our resources to combat other major drugs, that’s a positive,” Riley said, noting that fentanyl alone accounts for more than 100,000 deaths in the U.S. a year.
On the other end of the spectrum, others argue marijuana should be treated the way alcohol is.
“While this rescheduling announcement is a historic step forward, I remain strongly committed to continuing to work on legislation like the SAFER Banking Act as well as the Cannabis Administration and Opportunity Act, which federally deschedules cannabis by removing it from the Controlled Substances Act,” Senate Majority Leader Sen. Chuck Schumer of New York said in a statement. “Congress must do everything we can to end the federal prohibition on cannabis and address longstanding harms caused by the War on Drugs.”
Federal drug policy has lagged behind many states in recent years, with 38 having already legalized medical marijuana and 24 legalizing its recreational use .
That’s helped fuel fast growth in the marijuana industry, with an estimated worth of nearly $30 billion. Easing federal regulations could reduce the tax burden that can be 70% or more for businesses, according to industry groups. It could also make it easier to research marijuana, since it’s very difficult to conduct authorized clinical studies on Schedule I substances.
The immediate effect of rescheduling on the nation’s criminal justice system would likely be more muted, since federal prosecutions for simple possession have been fairly rare in recent years.
But loosening restrictions could carry a host of unintended consequences in the drug war and beyond.
Critics point out that as a Schedule III drug, marijuana would remain regulated by the DEA. That means the roughly 15,000 cannabis dispensaries in the U.S. would have to register with the DEA like regular pharmacies and fulfill strict reporting requirements, something that they are loath to do and that the DEA is ill equipped to handle.
Then there’s the United States’ international treaty obligations, chief among them the 1961 Single Convention on Narcotic Drugs, which requires the criminalization of cannabis. In 2016, during the Obama administration, the DEA cited the U.S.’ international obligations and the findings of a federal court of appeals in Washington in denying a similar request to reschedule marijuana.
Goodman reported from Miami, Mustian from New Orleans. AP writer Colleen Long contributed.

Launches in oncology: The elements of success
Our experience shows that companies who launch oncology drugs successfully excel in four areas:
- Agility, speed, and competitive mind-set: They operate in an agile governing model allowing for competitive entry and faster approvals.
- Distinctive product strategy: They have a clearly defined product strategy underscored by strong product planning.
- Mastering market access: They know how to demonstrate value, are braced for increasingly difficult access negotiations, and effectively manage the evolving stakeholder landscape.
- Go-to-market models: They develop new approaches to maximize their launch opportunity.
Area 1: Agility, speed, and competitive mind-set
Agility and the right organization principles form the foundation of successful oncology launches given the complexity and intensity of the market environment.
As background, given the pace of innovation within this therapeutic area, companies must often respond to shortened approval time lines, rapid follow-on competitive entries, varying results on market access negotiations as well as success and failure in follow-on clinical programs. For example, oncology drug development programs between 2006 and 2015 accounted for 31 percent of the 9,985 efforts in total. This underscores a continuously large and continuously moving therapeutic area that through its relatively lower approval rate (5.1 percent Phase I to approval versus 11.9 percent for non-oncology) mandates agility and adaptability from its players. 1 1. “Clinical Development Success Rates 2006–2015,” BIO, Biomedtracker, Amplion 2016.
Additionally, time to approval has remained steady at 8.8 years between 2010 and 2015, however, oncology has seen the fastest filing to approval across all therapeutic areas. While neurology drugs took the longest to approve on average, at two years, oncology drugs were approved almost twice as fast at 1.1 years. 2 2. “Clinical Development Success Rates 2006–2015,” BIO, Biomedtracker, Amplion 2016. Many oncology drugs for unmet medical need may have benefited from expedited approval pathways from the FDA that include Breakthrough Therapy Designation and Accelerated Approval. 3 3. Richard K. Harrison, “Phase II and phase III failures: 2013-2015,” Nature Reviews Drug Discovery, December 15, 2016, nature.com. In FDA trials from 2013–15, nearly half of the drugs were approved by Phase II driven by breakthrough designation status. 4 4. Richard K. Harrison, “Phase II and phase III failures: 2013-2015,” Nature Reviews Drug Discovery, December 15, 2016, nature.com. Recent product approvals (for example, Keytruda, Tagrisso) suggest even more compressed timelines. This means a few years of additional data collection and launch preparation are simply not available any longer, requiring an agile launch set-up.
Finally, product life cycles are shortening generally, but particularly in oncology, with the number of years until competitive follow-on launches compressed dramatically. Time without competition may now be as short as one to two years (Mekinist, Keytruda, and Lynparza all had two years until competition), whereas products like Herceptin and Avastin enjoyed six to seven years on the market before entry of other compounds with similar modes of action even if not in the same tumor type or line of therapy (for example, Tykerb, Zaltrap) (see exhibit 2). There is an increasing focus on subsegments of drugs, where generally more competitive freedom can be seen than for a larger indication. The race around first-to-market entry into the immuno-oncology space is the most striking example of parallel development at this time. Achieving launch product potential in oncology therefore is increasingly difficult. 5 5. PharmaProjects.
For pharmaceuticals in this area to not become a victim of the situation, but proactively drive excellence, “agile” organizational principles must be maintained.
Generally, being agile requires an organization to make more informed decisions earlier to avoid a string of iterations, as well as being able to autocorrect quickly when it does happen. In oncology with fast innovation cycles and large data this is even more critical.
This requires two things. First, advances in data and advanced analytics need to be leveraged to enable faster and more focused decision making, allowing the organization to be more agile at launch. Second, pharmaceuticals need to keep complexity to a minimum in oncology launch situations. They can ensure this by having launch leaders effectively engage across functional boundaries and by remaining flexible enough to rapidly steer-correct their launch especially in the first six months.
Would you like to learn more about our Life Sciences Practice ?
A different organization of the launch team is needed to ensure agility: while a project management team ensures completeness as a stable backbone, dynamic capabilities are needed to solve tough challenges quickly and effectively. Cross-functional teams can contribute to solving critical decisions more dynamically, while short timelines allow for arriving at a minimum viable product quickly to prevent “better becoming the enemy of good enough.” This can be effectively achieved by running teams in four-to-six week “sprint” work cycles that must produce concrete outputs and workable products rather than partial answers. A senior leadership team with focus on high-priority deliverables ensures arriving at a “working answer” quickly, which can then be refined continuously.
Leadership therefore is critical, not only to appropriately deal with biases but also to effectively coordinate the teams and bridge organizational siloes. A vision, the ability to identify what really matters paired with decisiveness under uncertainty and a drive for focused execution are characteristics of seasoned leaders required in launches. Given the high value at stake and the importance of “first time right,” targeted development of “launch leaders” comes at a significant return on investment.
Governance mechanisms like a control tower/situation room have been implemented by most companies and are deemed best practice today in allowing to proactively manage the launch and course corrections quickly. Innovation here has come mostly in the tools that are becoming available to allow members of the launch to drive a more effective launch, for example advanced analytics employed on customer data support for example, live market tests and A/B testing at launch. Exploiting the potential of CRM data for quick and robust decision making supports maximizing the launch curves against the background of shortening product life cycles is one of those tools.
The latest oncology launches will be measured by how well they launch and how agile they are in their set-up to master this critical stage.
Area 2: Distinctive product strategy
In oncology, a set of key decisions around the launch and the product strategy in general can determine a high share of the value, because competition has stiffened and timelines have been compressed. Thus, launch decisions need to be considered in the broader context of a product strategy, which needs to be distinctive.
A set of key questions from early planning stages to leadership and implementation needs to be mastered. To create a distinctive product strategy, you will need to answer a set of strategic questions and plan accordingly. They are as follows:
Do you understand healthcare providers and patient journeys, and does your launch strategy lead to unmatched experiences?
Are the brand access and scientific stories compelling to the respective audiences? Are they mutually consistent and reinforcing?
Do you have a clear, outcome-based value proposition to anticipate payer constraints?
Do you use agile teams for make-or-break topics, and combine them with a reliable “machine” to execute standard launch plans?
Do you prepare launch leaders at all levels for the challenges they will face?
Do you incentivize these leaders in alignment with the overall strategy, and reward them adequately?
Do you de-bias the critical launch decision?
Do you leverage advanced analytics to develop hypotheses and design your strategy?
Do you prepare an adequate data monitoring to be able to steer correctively after the launch?
Perhaps even more important than what is how you answer them. For example, to take decisions increasing the odds for a successful launch, a solid fact-based foundation can be built leveraging new analytics tools. For improved clinical trial enrollment, predictive algorithms can forecast site-level patient recruitment and through site optimization reduce enrollment time by 10–30 percent. 6 6. McKinsey Quantum Black. Such data-driven approaches allow choosing sites within regulatory boundaries while optimizing expected performance. During trials, real-time performance monitoring augmented with scenario tools to plan interventions can leverage information to sense and counter quality issues early.
Area 3: Mastering market access
As outlined in the first chapter, faster access is central for a successful oncology launch and to maximize the product life cycle. However, faster access comes at the cost of increased uncertainty and complexity. With best practice data generation both aspects can be tackled, achieving faster approval while mitigating uncertainty risks. Merck (Keytruda) and AstraZeneca (Tagrisso) have shown that a well-executed seamless expansion of a single trial can lead quickly to launch. This faster access in turn makes postlaunch data generation ever more important for two reasons, to strengthen the early data and to potentially expand the label. In both cases, additional data generates a competitive edge compared to future entrants. Janssen has successfully employed this strategy with Darzalex and Pfizer with Ibrance, for which the vast amount of clinical trials was performed post launch. This increases the need for (field) medical to be able to coordinate the large number of investigator-initiated studies and other post authorization studies.
Complexity is added in this space as patient populations get fragmented and the treatment paradigms become increasingly complex, with various drugs used in different lines, for different subtypes and indications. This increases the need for subtype and indication specific data to guide payers and physicians to bring the best treatment to the patients. Decisions are becoming more difficult as complexity continues to increase, and simplifying the story become a must have.
In addition to the complexities of data for a single product, the rise of combination therapies for which multiple drugs, potentially from competitors, are combined, further add to the increasing complexity. The main driver for combination therapies are immuno-oncology drugs, whose market is expected to grow from about $5 billion today to over $35 billion by 2022. 7 7. "Immunotherapy’s cancer remit widens," nature , nature.com, May 28, 2013. However, given that the reimbursement of combination therapies at monotherapy price is difficult (see below), this trend demands better clinical trial data. With precise outcome data, the added value of a combination compared to the monotherapy can be proven and arguments for reimbursement are strengthened, enabling a successful launch. Further, this data can serve as the foundation for the value split negotiations with the partner company and hence further improves the likelihood of success.

Pursuing breakthroughs in cancer-drug development
As oncology drugs command some of the highest prices in the industry, health authorities are further scrutinizing them. Three themes of payer behavior to reduce oncology costs have emerged over the last years that need to be addressed in anticipation of launch, namely:
- Higher HTA bar in Europe. Increasing rates of negative/restrictive decisions in health technology assessments, limiting target population or the number of incremental therapies that are reimbursed.
- Increased pressure. As companies have expanded the patient pools with additional clinical trials, so has the scrutiny increased.
- Shifting outcomes risk to pharmacos. Drugs only receive a conditional reimbursement during which additional real-world evidence or clinical data must be collected to prove the additional benefit of the treatment and individual patient costs are only reimbursed in case of treatment success.
This increased pressure poses challenges for future oncology drug launches, but it also presents an opportunity for pharmaceutical companies and payers to work more closely together. To realize this opportunity, companies need to excel at understanding payers’ needs and the available opportunities, as well as on generating additional real-word evidence, to provide the underlying data for innovative pricing and contracting models, such as risk-sharing or outcome-based contracts. Hence, pharmaceutical companies that excel at oncology launches tend to invest into generating such evidence in the real world for several years before negotiating with payers.
As such, outcome-focused payer collaboration models will require an oncology player to adapt a holistic view of the patient journey and the payers’ interests. A seven-step process can be employed to uncover common ground for collaboration: 8 8. McKinsey analysis.
- Map end-to-end disease pathways
- Prioritize untapped value pools for health systems
- Identify and prioritize solutions ideas to improve outcomes
- Assess potential of solution ideas in countries
- Determine implication for data generation
- Determine directional pricing and business case
- Create an implementation plan
This approach can generate win-win outcomes that benefit both sides.
Area 4: Go-to-market models
The go-to-market models employed by pharmaceutical companies evolved significantly over the past years and the rapidly changing oncology market makes go-to-market models for oncology drugs particularly important.
Plus, oncology differs as a therapeutic area in that its stakeholders are cross-cutting across other disease areas. A prescriber will not only be an oncologist, depending on the type of cancer, but could for example be a urologist or gynecologist. For example, 88 percent of the key prescribers for ovarian cancer are at the same time also key lung prescribers, creating commercial synergies between the prescriber bases. Yet, these groups have been deproritized in many launches we have observed, which can lead to underwhelming uptake. To avoid this, oncology players need to use the full range of channels available in innovative ways (see below). By leveraging the dynamically evolving mix of channels based on customer preferences and availability of new media, pharmaceuticals can set themselves up for launch success in oncology.
Especially two aspects are critical for success in future oncology launches:
- Channel mix. Shorter life cycles, complex products and an evolving stakeholder landscape increase the need for targeted, high-quality communications. A sophisticated channel mix of new, digital engagements and traditional sales-rep-based detailing is therefore at the heart of every successfully executed launch.
- Partnering for combination indications. Launch and marketing of combination therapies demands some form of collaboration between the involved companies. Finding the right framework for the partnership is therefore key to ensure success.
A successful channel strategy needs to leverage a diverse mix of channels, find the right balance for interactions and coordinate the activities across all channels. The most sophisticated players excel across these factors. From the bouquet of alternative channels, (for an overview, see exhibit 4) the appropriate ones are selected for each stakeholder and deployed in innovate ways.
For example, e-detailing activities differentiate between different levels of physicians. HCPs that are also visited in person get additional material and personalized invites following up on the visit, while lower-tier physicians are targeted to participate in online webinars where the information is shared with a broader audience. Further, the frequency of interactions with each stakeholder is adjusted to its preferred level through opt-ins and advanced analytics. Interactions are being tracked and success is regularly monitored with KPIs. Further, the different departments with key stakeholder interactions, that is, marketing, sales, medical and access, coordinate their activities within the legal framework to ensure a seamless and coherent experience.
Combination indications, in which the combined drugs are from different companies are the second field, for which go-to-market models have the highest impact. The two companies need to find a partner agreement that optimizes for both of their assets. Given divergent long-term strategies and potential competition in other fields, this can be a challenging task. Specifically, two key questions need to be answered in these cases. First, how should the two companies work together on a day-to-day basis and on a functional level, and second, how should the value between the two companies be split.
To resolve the former question a dedicated agreement is necessary based on a strong partner management. In this agreement, incentives and guidelines should be placed that ensure the appropriate marketing of the combination as well as the monotherapy, for each indication to maximize patient benefit. Further, the roles, responsibilities and accountabilities for the marketing, sales and medical departments of both companies should be specified, for example, territories for sales and MSL should be defined and the ownership of medical information answering and pharmacovigilance reporting and liability should be assigned. Different degrees of separation/sharing between the two companies are possible. However, learnings from other industries suggest that clear and simple rules minimize distraction and maximize overall value.

Subscribe to the Shortlist
McKinsey’s new weekly newsletter, featuring must-read content on a range of topics, every Friday
For the latter question, other industries can serve as learning models. These suggest three main models. In the first “flexible solution” model players are free to decide on how to interact and mainly outside market forces define the efficiency of the solutions, for example, in case of monetary caps such as a patient flat fee, a deal might fail as both parties are better off separately, leading to lower patient benefit. In the second and third more regulated models such as industry “guidelines” or “fixed formulas,” both can lead to better outcomes, for example, a third party can own the revenue split formula and each party has the right to opt out. Applied to the budget-cap situation the third party could find a revenue maximizing strategy that when split appropriately, benefits both players more as when they would market the monotherapy only.
In general, four key learnings can be drawn from other industries. First, simple metrics are difficult to find but reduce the complexity and increase the stability of the partnership on the long run. Second, collaboration models can reduce the control for each company, but can be very successful (for example, IATA). Third, price negotiations should be separated from the revenue split mechanism and fourth, most collaboration models are evolutions from simpler systems and needed time to find the right balance.
The number of annual cancer cases is expected to rise by almost 70 percent within the next two decades, making launch excellence the fundament of success for oncology players. As such, launch success in oncology is driven by four main elements: an agile government model, a focus on data, a clear value demonstration with strong access capabilities, and a deep understanding of the changing stakeholder landscape and a tailored go-to-market model:
- The level of agility exhibited by a pharmaceutical’s government model is determined by five organizational principles: leveraging of advances in data and advanced analytics, an evolution of medical-access commercial models, a strong product positioning with sufficient real-world evidence, the use of cross-functional global teams, and the reduction of complexity.
- The oncology drug space is facing increasing complexity due to shorter time to market, higher activity with niche focus, and the rise of combination therapies. To overcome these complexities and to successfully launch companies must focus on data generation to support their access and value claims and partnership negotiations.
- To demonstrate value and ensure access in light of increasing cost pressure from payers in oncology, pharmaceuticals have to collaborate more closely with payers, excel at tendering and truly understand their stakeholders.
- Two main factors should be considered for the go-to-market model: the channel mix should enable a tailored offering to each stakeholder with a coordinated and coherent message from sales, marketing, and medical and for drug combinations a suitable partnership model with clear governance should be established.
Björn Albrecht is a partner in McKinsey’s London office, Jan Ascher is a partner in the Zurich office, where Martin Peters is an associate partner and Lena Stiehl is a consultant; Philippe Menu is an associate partner in the Geneva office.
Explore a career with us
Related articles.

The future of healthcare: Finding the opportunities that lie beneath the uncertainty

The next wave of innovation in oncology

Blackstone LaunchPad Student Start-Ups Place in the Finals of the 2024 New York Business Plan Competition
Two Syracuse University Libraries’ Blackstone LaunchPad (LaunchPad) student start-up teams placed in the finals of the New York Business Plan Competition (NYBPC) , powered by Upstate Capital, held in Albany on April 25.

Motolani Oladitan ’24 (College of Arts and Sciences), left, founder of Tá Beautie, and Natasha Brao ’22 (College of Visual and Performing Arts) G’23, G’24 (Whitman School of Management), founder of Shooka Sauce.
Natasha Brao ’22, (College of Visual and Performing Arts) G’23, G’24 (Whitman School of Management), founder of Shooka Sauce, won the 3 rd place prize of $1,000 in the food and agriculture track. Shooka Sauce is a Mediterranean-spiced tomato sauce based on the dish Shakshuka, inspired by mixing and melding cultural flavors to promote creative cooking.
Motolani Oladitan ’24 (College of Arts and Sciences), founder of Tá Beautie, was awarded the Concept Stage Award of $500 in the software and services track. Tá Beautie is a virtual marketplace connecting African beauty and wellness brands with the diaspora, making it easier for consumers to discover and purchase high-quality, authentic African products.
Five Launchpad student start-up teams attended the 2024 New York Business Plan Competition. Other student teams to reach the finals include Frank Marin ’24 (Marhold Space Systems), Adya Parida ’25 (Scale Sense), and Dylan Bardsley ’26 and Mark Leaf ’27 (Clarity).
The NYBPC attracts some of New York state’s best student entrepreneurs. The competition promotes entrepreneurial opportunities for college students from across the state to pitch their business plans to seasoned investors. They also receive the opportunity to engage with mentors and judges from the business community. The finals event connects students with business professionals, provides experiential learning opportunities through competitions, connects entrepreneurs with resources at the Entrepreneurship Expo and awards up to $100,000 in cash prizes to help seed new ventures.
Cristina Hatem
- Sei Whale Death Sparks Conversations About Ecology, Marine Safety Friday, May 10, 2024, By Daryl Lovell
- 5 Questions for Commencement Speaker Dario Nardella, Mayor of Florence, Italy Thursday, May 9, 2024, By News Staff
- Commencement 2024 by the Numbers Thursday, May 9, 2024, By Christine Grabowski
- In Memoriam: Life Trustee Michael ‘Mike’ Falcone ’57 Thursday, May 9, 2024, By Eileen Korey
- In Memoriam: Life Trustee Bernard ‘Bernie’ Kossar ’53, L’55 Thursday, May 9, 2024, By Eileen Korey
More In Campus & Community
Graduate aims to bring visibility to indigenous community through fashion.
Growing up, Yegunahareeta (Hareeta) Printup ’24 was immersed in the tradition and beauty of Indigenous culture. Printup, a fashion design major in the College of Visual and Performing Arts (VPA), a 2024 VPA Scholar, a Haudenosaunee Promise Scholar and a…
Student Leaders Leondra Tyler ’24 and Omnia Shedid L’24 Make Their Mark on Campus, Plan for the Future (Podcast)
This weekend’s Syracuse University Commencement marks a time to reflect and celebrate the end of a long journey for students. Two decorated student leaders, Leondra Tyler’24 and Omnia Shedid L’24, share their stories and their paths to Syracuse University on…
5 Questions for Commencement Speaker Dario Nardella, Mayor of Florence, Italy
Dario Nardella, mayor of Florence, Italy, will share some words of wisdom with graduating students at Sunday’s Commencement in the JMA Wireless Dome. But what was one of the best pieces of advice he received as a young person? SU…
Commencement 2024 by the Numbers
As the various celebrations begin for the Class of 2024’s Commencement weekend, there are many important details that the graduates and their families and friends need to know. But have you ever wondered what goes into the behind-the-scenes details that…
In Memoriam: Life Trustee Michael ‘Mike’ Falcone ’57
Michael “Mike” Falcone ’57 often said he was born into a family of entrepreneurs, and when he passed away on April 10, 2024, accolades poured in for the man who helped develop millions of square feet of office buildings, shopping…
Subscribe to SU Today
If you need help with your subscription, contact [email protected] .
Connect With Us
For the media.

COMMENTS
The Bain & Company study examined the performance of each launch across 20 launch activities. Our findings show that companies with successful launches do three things right: They differentiate their drug through messaging, post-launch data and services. They create broad customer advocacy via a superior customer experience.
This article is drawn from Beyond the storm: Launch excellence in the new normal (PDF-2.8 MB), a compendium from McKinsey's pharmaceuticals and medical products practice that provides details on how to tackle each of these tasks and further insight on how to approach them effectively. Hemant Ahlawat is a principal in McKinsey's Brussels ...
Traditionally, a marketing plan includes the four P's: Product, Price, Place, and Promotion. For a pharmaceutical company, your marketing strategy should include the following: Product: In the product section, you should reiterate the type of pharmaceutical business that you documented in your company overview.
Executive summary. WITH the growing costs of developing new drugs, increasing competition, and shortening times to peak sales, the importance of getting a drug launch right has never been greater.As payer influence grows and new therapies target smaller patient populations with complex needs, developing and executing a winning launch strategy becomes increasingly difficult.
Shaping how your drug's target disease is perceived by medical professionals and the public can make a big difference to the success of your launch. Jan Adams, Chinmay Bhatt, and Brent Hooper ... inconsistent execution of the launch plan. To get the fundamentals right, companies need to focus on: Developing a launch roadmap. Most companies ...
For instance, among the executives taking part in a recent launch roundtable, more than a third had no prior launch experience. 1 McKinsey roundtable for executives from emerging biopharma companies, September 2020. And the same applies to companies; many promising biotechs have yet to launch a drug themselves.
evidence-generation plan in place 18 months before the launch to generate a steady stream of data after the launch that supports the drug's effi cacy. Take the ex-ample of Celgene, which produced almost twice as many postlaunch studies in Europe for its multiple myeloma drug Revlimid as compared with its closest competitor.
Five levers for successful launch. 1. Early preparation. A successful launch requires evidence generation for reimbursement, the identification and development of a new set of advocates, and/or the identification of a suitable patient group. The planning process should start approximately three years before launch, though this may not always be ...
Specifically, we measured how the following eight key performance drivers affect launch performance: level of unmet need in the disease, order of entry, efficacy, safety, convenience, product novelty, marketing spend across channels and payer influence. The pharmaceutical industry has long sought a crystal ball that can accurately predict ...
Micro-battle approach. Head of launch reports to medical marketing. Head of launch team reports to CEO. 90% of work takes place in functional silos. Launch team empower to work across functions. Functions control launch budget. Head of launch team controls budget. Team focused on functional checklists.
Launch Phase - Establish Commercial Set-Up. During the launch phase, a filling partner will establish a methodical way of working with the team including both internal and external players. This step also includes gaps and opening actions within a launch checklist developed in collaboration with the customer. Progress made throughout the ...
19-20 November 2024, Live online. Early bird € 2 670. (Full fee € 2 870) (until 18/10/2024) Register. Your company only gets ONE chance to launch a new pharmaceutical. Yet many launches fail to achieve their maximum potential. Commercial launch success requires a set of competencies to be embedded across the organisation.
Pre pharmaceutical product launch, medical and commercial teams have a lot on their plate. Their prime objective: ensure the messaging they've crafted resonates with each key stakeholder audience, including patients, HCPs, payors, and potentially many more (or several segments within each category). Despite being so critical to the success of the drug—and the reputation of the company ...
Drug Rehabilitation Business Plan. Over the past 20+ years, we have helped over 1,000 entrepreneurs and business owners create business plans to start and grow their drug rehabilitation companies. We have the experience, resources, and knowledge to help you create a great business plan. In this article, you will learn some background ...
As an entrepreneur you can also start a small pharmacy or drug store in your locality. Sample pharmacy business plans and template will guide you. We, at OGSCapital, help you with this. Our executives have all the expertise for helping you to develop an appropriate strategy to fulfill your all objectives.
Three converging factors are swelling the ranks of first-time launchers of pharma products—the business cycle, the availability of talent, and the proliferation of vendors: Business cycle. For much of the past five years, small drug companies found it relatively easy to raise money. Economic growth was strong, interest rates were low, and ...
Teva plans to launch six biosimilar drugs by 2027, including Simlandi and psoriasis treatment Selarsdi, a copycat of Johnson & Johnson's blockbuster drug Stelara. It developed both drugs with ...
The founder and chairman of San Antonio-based WellMed, Dr. George Rapier, is expanding his business portfolio to include a high-end hospitality venture in Tuscany.
Nearly 1 in 8 adults in the United States has used a GLP-1 drug, such as Ozempic or Mounjaro, according to new survey data from healthcare policy think tank KFF.. According to the survey, 12% of ...
Takeda scored three new drug approvals from the U.S. Food and Drug Administration in the 2023 fiscal year. This year, Takeda plans to have up to six programs in late-stage development.
South County beaches near the Tijuana Slough have been closed continuously since Dec. 8, 2021, due to unsafe conditions. That's more than 800 straight days that local families have been denied ...
An upcoming change in federal regulations could save Maryland cannabis businesses millions of dollars in taxes. The Drug Enforcement Administration is moving to reclassify cannabis as a Schedule ...
Even before the COVID-19 outbreak, launching a new drug was far from straightforward. Forty percent of worldwide drug launches between 2009 and 2017 failed to meet their two-year sales forecasts. 1 McKinsey analysis of pharmaceutical-industry data from Evaluate, August 2020.
This episode contains descriptions of an alleged sexual liaison. What happened when Stormy Daniels took the stand for eight hours in the first criminal trial of former President Donald J. Trump?
Some lessons learned that produced commercial momentum include establishment of a launch business unit where all new products are grown until they reach a certain size; weekly management meetings to review launch progress (e.g., Apple); 20% to 30% of every employee's incentive linked to the success of critical launches (e.g., Google+); data ...
Drug makers Cipla and Glenmark are recalling products from the American market due to manufacturing issues, according to the US health regulator. As per the latest Enforcement Report issued by the US Food and Drug Administration (USFDA), a New Jersey-based subsidiary of Cipla is recalling 59,244 ...
WASHINGTON (AP) — The U.S. Drug Enforcement Administration will move to reclassify marijuana as a less dangerous drug, The Associated Press has learned, a historic shift to generations of American drug policy that could have wide ripple effects across the country. The proposal, which still must be reviewed by the White House Office of Management and Budget, would recognize the medical uses ...
Our experience shows that companies who launch oncology drugs successfully excel in four areas:. Agility, speed, and competitive mind-set: They operate in an agile governing model allowing for competitive entry and faster approvals. Distinctive product strategy: They have a clearly defined product strategy underscored by strong product planning. Mastering market access: They know how to ...
Natasha Brao '22, (College of Visual and Performing Arts) G'23, G'24 (Whitman School of Management), founder of Shooka Sauce, won the 3 rd place prize of $1,000 in the food and agriculture track. Shooka Sauce is a Mediterranean-spiced tomato sauce based on the dish Shakshuka, inspired by mixing and melding cultural flavors to promote creative cooking.