
- Exam Center
- Ticket Center
- Flash Cards
- Heat and Thermal Physics

Heat Practice Problems With Detailed Answers
Check out these heat problems made just for high school students! They’re all solved and ready for you to learn from. A great way to get better at understanding heat.
When heat energy $Q$ causes a change in temperature $\Delta T=T_f-T_i$ in a sample with specific heat capacity $c$ and mass $m$, then we can relate all these physical quantities as following formula \[Q=mc\Delta T=mc(T_f-T_i)\] where $T_f$ and $T_i$ are the initial and final temperatures.
Heat Practice Problems
Problem (1): 5.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.092 cal/g. °C)
Solution : The energy required to change the temperature of a substance of mass $m$ from initial temperature $T_i$ to final temperature $T_f$ is obtained by the formula $Q=mc(T_f-T_i)$, where $c$ is the specific heat of the substance. Thus, we have \begin{align*} Q&=mc\Delta T\\ &= 5\times 0.092\times (80^\circ-20^\circ)\\&= 27.6 \quad {\rm cal} \end{align*} So, it would require 27.6 calories of heat energy to increase the temperature of this substance from 20°C to 80°C.
If this page helped you with homework or anything else, please support me here .
Problem (2): How much heat is absorbed by a 20 g granite boulder as energy from the sun causes its temperature to change from 10°C to 29°C? (Specific heat capacity of granite is 0.1 cal/g.°C)
Solution : to raise the temperature of the granite boulder from 10°C to 29°C, we must add $Q=mc\Delta T$ energy to the granite as below \begin{align*} Q&=mc\Delta T\\ &=20 \times 0.1\times (29^\circ-10^\circ)\\&=38\quad {\rm cal}\end{align*} So, it would require 38 calories of heat energy to increase the temperature of this granite boulder from 10°C to 29°C. This is the amount of energy we must add to the boulder.
In all these example problems, there is no change in the state of the substance. If there were a change in the phase of matter (solid $\Leftrightarrow$ liquid or to liquid$\Leftrightarrow$ gas) read the following page to learn more:
Solved problems on latent heat of fusion
Solved Problems on latent heat of vaporization
Problem (3): How much heat is released when 30 g of water at 96°C cools to 25°C? The specific heat of water is 1 cal/g.°C.
Solution : the amount of energy released is obtained by formula $Q=mc\Delta T$ as below \begin{align*} Q&=mc\Delta T\\&=30\times 1\times (25^\circ-96^\circ)\\&= -2130\quad {\rm cal}\end{align*} The negative sign in the result indicates that the energy is being released from the water. This is because the temperature of the water is decreasing, which means it is losing heat energy.
Therefore, $2130$ calories of heat energy are released from the water when its temperature decreases from 96°C to 25°C. This energy could be transferred to the surrounding environment or used to do work, depending on the specific circumstances.
Were these not helpful? My income comes from your generous donations. Please support me.
Problem (4): If a 3.1 g ring is heated using 10.0 calories, its temperature rises 17.9°C. Calculate the specific heat capacity of the ring.
Solution : Since the given heat causes a change in the temperature of the ring, the amount of heat is obtained by the formula $Q=mc(T_f-T_i)$. By putting known values into it and solving for the unknown value, the specific heat of the ring is calculated as below, \begin{align*} c&=\frac{Q}{m(T_f-T_i)}\\ \\ &=\frac{10}{3.1\times 17.9^\circ}\\ \\&=0.18\quad {\rm cal/g\cdot ^\circ C}\end{align*} So, the specific heat of the ring is calculated to be $0.18\,{\rm cal/g\cdot ^\circ C}$. This value tells us how much heat is required to raise the temperature of $1$ gram of the ring by $1$ degree Celsius. Note that in this problem, the difference between temperatures is given not the initial or final temperatures.
Problem (5): The temperature of a sample of water increases from 20°C to 46.6°C as it absorbs 5650 calories of heat. What is the mass of the sample? (Specific heat of water is 1.0 cal/g.°C)
Solution : As before, using heat formula and solving for mass $m$, we get \begin{align*} m&=\frac{Q}{c\Delta T}\\\\ &=\frac{5650}{1\times (46.6^\circ-20^\circ)}\\ \\&=212.4\quad {\rm g}\end{align*}
Problem (6): The temperature of a sample of iron with a mass of 10.0 g changed from 50.4°C to 25.0°C with the release of 47 calories of heat. What is the specific heat of iron?
Solution : In the specific heat problems , we learned that specific heat is defined as the amount of heat energy required to change the temperature of a sample with mass $m$ by $\Delta T$. Thus, we have \begin{align*} c&=\frac{Q}{m\Delta T}\\ \\&=\frac{47}{10\times (25^\circ-50.4^\circ)}\\ \\&= 0.185\quad {\rm cal/g\cdot ^\circ \! C}\end{align*} Note that the specific heat of materials is a positive quantity, so if you get a negative, as above, you must pick its absolute value.
Problem (7): A 4.50 g coin of copper absorbed 54 calories of heat. What was the final temperature of the copper if the initial temperature was 25°C? The specific heat of copper is 0.092 cal/g.°C.
Solution : Let $T_i$ and $T_f$ be the initial and final temperatures of the copper coin. Again using formula $Q=mc(T_f-T_i)$ and solving for final temperature $T_f$, we have \begin{align*} T_f&=\frac{Q}{mc}+T_i \\ \\ &=\frac{54}{0.092\times 4.5}+25^\circ\\ \\ &=155.43\,{\rm ^\circ C}\end{align*}
Problem (8): A 155 g sample of an unknown substance was heated from 25°C to 40°C. In the process, the substance absorbed 569 calories of energy. What is the specific heat of the substance?
Solution : In the heat formula $Q=mc\Delta T$, the specific heat of any substance is denoted by $c$. Putting known values into this formula and solving for unknown specific heat, we get \begin{align*} c&=\frac{Q}{m\Delta T}\\ \\ &=\frac{569}{155\times (40^\circ-25^\circ)}\\ \\&=0.244\quad {\rm cal/g\cdot^\circ\! C} \end{align*}
Problem (9): What is the specific heat of an unknown substance if a 2.50 g sample releases 12 calories as its temperature changes from 25°C to 20°C?
Solution : same as above, we have \begin{align*} c&=\frac{Q}{m(T_f-T_i)}\\ \\&=\frac{12}{2.5\times (20^\circ-25^\circ)}\\\\&=0.96\quad {\rm cal/g\cdot ^\circ \! C}\end{align*}
Problem (10): When 3 kg of water is cooled from 80°C to 10°C, how much heat energy is lost? (specific heat of water is $c_W=4.179\,{\rm J/g\cdot \!^\circ C}$)
Solution : heat has led to a change in temperature so we must use the formula $Q=mc\Delta T$ to find the lost heat as below \begin{align*} Q&=mc(T_f-T_i)\\&=3000\times 4.179\times (10^\circ-80^\circ)\\&=-877590\quad {\rm J} \\ or &=-877.590\quad {\rm kJ}\end{align*} Note that in above the value of specific heat is given in grams but the weight of water is in kilogram, so first convert them into grams or kilograms and then continue to solve the problem. Here, we converted 3 kg to 3000 g.
The negative shows that the heat is released from the water.
Problem (11): How much heat is needed to raise a 0.30 kg piece of aluminum from 30°C to 150°C? ($c_{Al}=0.9\,{\rm J/g\cdot \!^\circ C}$)
Solution: Let $T_f$ and $T_i$ be the initial and final temperatures of the aluminum so the required heat is computed as below \begin{align*} Q&=mc(T_f-T_i)\\&=0.3\times 900\times (150^\circ-30^\circ)\\&=-32400\quad {\rm J}\\ or &=-32.4\quad {\rm kJ}\end{align*} Here, we converted specific heat in SI units.
Problem (12): Calculate the temperature change when: (a) 10.0 kg of water loses 232 kJ of heat. ($c_W=4.179\,{\rm J/g\cdot \!^\circ C}$) (b) 1.96 kJ of heat is added to 500 g of copper.($c_{Cu}=0.385\,{\rm J/g\cdot \!^\circ C}$)
Solution : In both parts, we use the heat formula when temperature changes, $Q=mc(T_f-T_i)$. (a) Substituting known values $m=10\,{\rm kg}$ and $Q=232\,{\rm kJ}$ into the above equation and solving for the change in temperature $\Delta T=T_f-T_i$, we get \begin{align*} \Delta T&= \frac{Q}{mc}\\ \\&=\frac{-232000}{10\times 4179}\\ \\&=-5.55\,{\rm ^\circ C}\end{align*} Since water loses heat energy (which justify why we inserted a minus sign for Q) so its temperature must be decreasing. In the above, kJ means 1000 J energy.
(b) Heat added to the water so $Q>0$ must be inserted into the formula, \begin{align*}\Delta T&=\frac{Q}{mc}\\ \\&=\frac{1960}{0.5\times 385}\\ \\&=10.18\,{\rm ^\circ C}\end{align*}
Problem (13): When heated, the temperature of a water sample increased from 15°C to 39°C. It absorbed 4300 joules of heat. What is the mass of the sample?
Solution : putting known values into the equation $Q=mc(T_f-T_i)$ and solving for unknown mass, we get \begin{align*} m&=\frac{Q}{c(T_f-T_i)}\\ \\ &=\frac{4300}{4179\times (15^\circ-39^\circ)}\\ \\&=0.0428\quad {\rm kg}\\ \\ or &=42.8\quad {\rm g} \end{align*}
Problem (14): 5.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu?
Solution : the necessary energy is calculated as below, \begin{align*} Q&=mc(T_f-T_i)\\&=5\times 0.385\times (20^\circ-80^\circ)\\&=115.5\quad {\rm J}\end{align*} So, the necessary energy or heat absorbed by the object is calculated to be $\rm 115.5\, J$. This value tells us how much heat energy is required to change the temperature of the $\rm 5\, g$ object by $60$ degrees Celsius. In this problem, we’re given the mass of the object, the specific heat capacity, and the change in temperature, which allows us to calculate the heat energy.
Problem (15): The temperature of a sample of iron with a mass of 10.0 g changed from 50.4°C to 25.0°C with the release of 47 Joules of heat. What is the specific heat of iron?
Solution : the specific heat of iron is determined as below \begin{align*} c&=\frac {Q}{m(T_f-T_i)}\\ \\&=\frac{-47}{10\times (25^\circ-50.4^\circ)}\\ \\&= 0.185\quad {\rm J/g\cdot \! ^\circ C} \end{align*} Since heat is released from the sample so we insert a negative in front of heat to get a positive specific heat of capacity.
Problem (16): The temperature of a sample of water increases from 20°C to 46.6°C as it absorbs 5650 Joules of heat. What is the mass of the sample?
Solution : known values are $T_i={\rm 20^\circ C}$, $T_f={\rm 46.6^\circ C}$ and $Q=5650\,{\rm J}$. Thus, we have \begin{align*} m&=\frac{Q}{c(T_f-T_i)}\\ \\&=\frac{5650}{4179\times (46.6^\circ-20^\circ)}\\ \\ &=0.0508\quad {\rm kg} \\ \\ or &=50.8\quad {\rm g} \end{align*}
Author : Dr. Ali Nemati Page Created: 3/9/2021
© 2015 All rights reserved. by Physexams.com
Free Printable heat transfer and thermal equilibrium worksheets
Explore the fascinating world of heat transfer and thermal equilibrium with our free printable Science worksheets. Discover essential concepts and enhance your students' learning experience in a fun and interactive way.

11th - 12th

10th - 11th

10th - 12th

Explore Worksheets by Grade
Explore worksheets by subjects.
- Social studies
- Social emotional
- Foreign language
- Reading & Writing
Explore printable heat transfer and thermal equilibrium worksheets
Heat transfer and thermal equilibrium worksheets are essential resources for teachers who aim to provide their students with a comprehensive understanding of these fundamental concepts in Science and Chemistry. These worksheets offer a variety of engaging activities, such as labeling diagrams, solving problems, and analyzing real-world scenarios, that cater to different learning styles and help students grasp the principles of heat transfer and thermal equilibrium. By incorporating these worksheets into their lesson plans, teachers can effectively assess students' progress and identify areas that may require additional support or clarification. Furthermore, these resources are designed to align with the grade-specific curriculum standards, ensuring that students are well-prepared for their examinations and future studies in Science and Chemistry. Heat transfer and thermal equilibrium worksheets are, therefore, indispensable tools for teachers who strive to deliver high-quality, targeted instruction to their students.
In addition to heat transfer and thermal equilibrium worksheets, Quizizz offers a wide range of interactive resources that can further enhance teachers' instructional strategies and support student learning. Quizizz provides teachers with access to a vast library of quizzes, games, and other engaging activities that cover various topics in Science, Chemistry, and other subjects. These resources are designed to be compatible with different grade levels and can be easily integrated into the classroom to supplement traditional teaching methods. By utilizing Quizizz's extensive collection of educational materials, teachers can create a dynamic and interactive learning environment that fosters student engagement, promotes critical thinking, and facilitates a deeper understanding of complex concepts. Moreover, Quizizz also offers valuable analytics and reporting tools that enable teachers to monitor student performance, identify areas for improvement, and tailor their instruction to meet the diverse needs of their students. With Quizizz, teachers can confidently deliver effective, data-driven instruction that supports student success in Science, Chemistry, and beyond.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.5: Heat Transfer, Specific Heat, and Calorimetry
- Last updated
- Save as PDF
- Page ID 4347

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
By the end of this section, you will be able to:
- Explain phenomena involving heat as a form of energy transfer
- Solve problems involving heat transfer
We have seen in previous chapters that energy is one of the fundamental concepts of physics. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. As we learned earlier in this chapter, heat transfer is the movement of energy from one place or material to another as a result of a difference in temperature. Heat transfer is fundamental to such everyday activities as home heating and cooking, as well as many industrial processes. It also forms a basis for the topics in the remainder of this chapter.
We also introduce the concept of internal energy, which can be increased or decreased by heat transfer. We discuss another way to change the internal energy of a system, namely doing work on it. Thus, we are beginning the study of the relationship of heat and work, which is the basis of engines and refrigerators and the central topic (and origin of the name) of thermodynamics.
Internal Energy and Heat
A thermal system has internal energy (also called thermal energy ) , which is the sum of the mechanical energies of its molecules. A system’s internal energy is proportional to its temperature. As we saw earlier in this chapter, if two objects at different temperatures are brought into contact with each other, energy is transferred from the hotter to the colder object until the bodies reach thermal equilibrium (that is, they are at the same temperature). No work is done by either object because no force acts through a distance (as we discussed in Work and Kinetic Energy ). These observations reveal that heat is energy transferred spontaneously due to a temperature difference. Figure \(\PageIndex{1}\) shows an example of heat transfer.
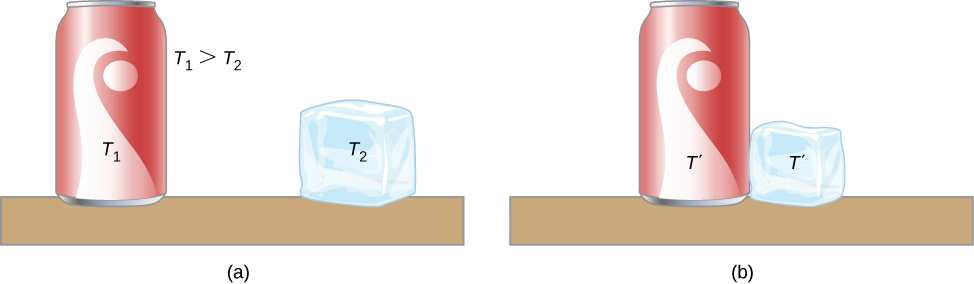
The meaning of “heat” in physics is different from its ordinary meaning. For example, in conversation, we may say “the heat was unbearable,” but in physics, we would say that the temperature was high. Heat is a form of energy flow, whereas temperature is not. Incidentally, humans are sensitive to heat flow rather than to temperature.
Since heat is a form of energy, its SI unit is the joule (J). Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oC\)—specifically, between \(14.5^oC\) and \(15.5^oC\) since there is a slight temperature dependence. Also commonly used is the kilocalorie (kcal), which is the energy needed to change the temperature of 1.00 kg of water by \(1.00^oC\). Since mass is most often specified in kilograms, the kilocalorie is convenient. Confusingly, food calories (sometimes called “big calories,” abbreviated Cal) are actually kilocalories, a fact not easily determined from package labeling.
Mechanical Equivalent of Heat
It is also possible to change the temperature of a substance by doing work, which transfers energy into or out of a system. This realization helped establish that heat is a form of energy. James Prescott Joule (1818–1889) performed many experiments to establish the mechanical equivalent of heat — the work needed to produce the same effects as heat transfer . In the units used for these two quantities, the value for this equivalence is
\[1.000 \, kcal = 4186 \, J.\] We consider this equation to represent the conversion between two units of energy. (Other numbers that you may see refer to calories defined for temperature ranges other than \(14.5^oC\) to \(15.5^oC\).)
Figure \(\PageIndex{2}\) shows one of Joule’s most famous experimental setups for demonstrating that work and heat can produce the same effects and measuring the mechanical equivalent of heat. It helped establish the principle of conservation of energy. Gravitational potential energy ( U ) was converted into kinetic energy ( K ), and then randomized by viscosity and turbulence into increased average kinetic energy of atoms and molecules in the system, producing a temperature increase. Joule’s contributions to thermodynamics were so significant that the SI unit of energy was named after him.
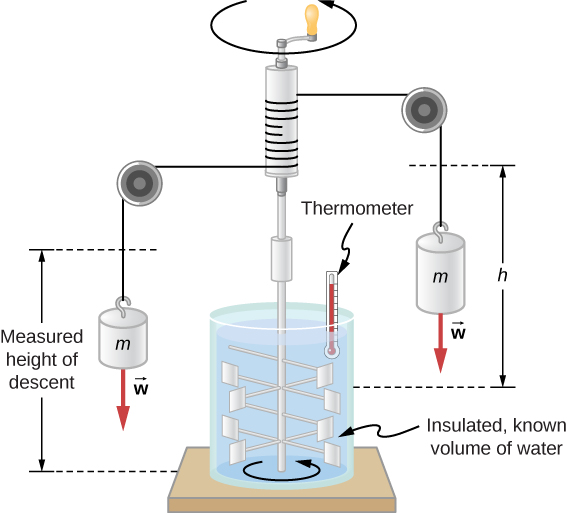
Increasing internal energy by heat transfer gives the same result as increasing it by doing work. Therefore, although a system has a well-defined internal energy, we cannot say that it has a certain “heat content” or “work content.” A well-defined quantity that depends only on the current state of the system, rather than on the history of that system, is known as a state variable . Temperature and internal energy are state variables. To sum up this paragraph, heat and work are not state variables .
Incidentally, increasing the internal energy of a system does not necessarily increase its temperature. As we’ll see in the next section, the temperature does not change when a substance changes from one phase to another. An example is the melting of ice, which can be accomplished by adding heat or by doing frictional work, as when an ice cube is rubbed against a rough surface.
Temperature Change and Heat Capacity
We have noted that heat transfer often causes temperature change. Experiments show that with no phase change and no work done on or by the system, the transferred heat is typically directly proportional to the change in temperature and to the mass of the system, to a good approximation. (Below we show how to handle situations where the approximation is not valid.) The constant of proportionality depends on the substance and its phase, which may be gas, liquid, or solid. We omit discussion of the fourth phase, plasma, because although it is the most common phase in the universe, it is rare and short-lived on Earth.
We can understand the experimental facts by noting that the transferred heat is the change in the internal energy, which is the total energy of the molecules. Under typical conditions, the total kinetic energy of the molecules \(K_{total}\) is a constant fraction of the internal energy (for reasons and with exceptions that we’ll see in the next chapter). The average kinetic energy of a molecule \(K_{ave}\) is proportional to the absolute temperature. Therefore, the change in internal energy of a system is typically proportional to the change in temperature and to the number of molecules, N . Mathematically, \(\Delta U \propto \Delta K_{total} = NK_{ave} \propto N\Delta T\). The dependence on the substance results in large part from the different masses of atoms and molecules. We are considering its heat capacity in terms of its mass, but as we will see in the next chapter, in some cases, heat capacities per molecule are similar for different substances. The dependence on substance and phase also results from differences in the potential energy associated with interactions between atoms and molecules.
Heat Transfer and Temperature Change
A practical approximation for the relationship between heat transfer and temperature change is:
\[Q = mc\Delta T,\]
where \(Q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\Delta T\) is the change in temperature. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The specific heat is numerically equal to the amount of heat necessary to change the temperature of \(1.00 \, kg\) of mass by \(1.00^oC\). The SI unit for specific heat is \(J/(kg \times K)\) or \(J/(kg \times ^oC)\). (Recall that the temperature change \(\Delta T\) is the same in units of kelvin and degrees Celsius.)
Values of specific heat must generally be measured, because there is no simple way to calculate them precisely. Table \(\PageIndex{1}\) lists representative values of specific heat for various substances. We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which means that it takes five times as much heat to raise the temperature of water a given amount as for glass, and 10 times as much as for iron. In fact, water has one of the largest specific heats of any material, which is important for sustaining life on Earth.
The specific heats of gases depend on what is maintained constant during the heating—typically either the volume or the pressure. In the table, the first specific heat value for each gas is measured at constant volume, and the second (in parentheses) is measured at constant pressure. We will return to this topic in the chapter on the kinetic theory of gases.
In general, specific heat also depends on temperature. Thus, a precise definition of c for a substance must be given in terms of an infinitesimal change in temperature. To do this, we note that \(c = \frac{1}{m} \frac{\Delta Q}{\Delta T}\) and replace \(\Delta\) with d:
\[c = \dfrac{1}{m} \dfrac{dQ}{dT}.\]
Except for gases, the temperature and volume dependence of the specific heat of most substances is weak at normal temperatures. Therefore, we will generally take specific heats to be constant at the values given in the table.
Example \(\PageIndex{1}\): Calculating the Required Heat
A 0.500-kg aluminum pan on a stove and 0.250 L of water in it are heated from \(20.0^oC\) to \(80.0^oC\). (a) How much heat is required? What percentage of the heat is used to raise the temperature of (b) the pan and (c) the water?
We can assume that the pan and the water are always at the same temperature. When you put the pan on the stove, the temperature of the water and that of the pan are increased by the same amount. We use the equation for the heat transfer for the given temperature change and mass of water and aluminum. The specific heat values for water and aluminum are given in Table \(\PageIndex{1}\).
- Calculate the temperature difference: \[\Delta t = T_f - T_i = 60.0^oC.\]
- Calculate the mass of water. Because the density of water is \(1000 \, kg/m^3\), 1 L of water has a mass of 1 kg, and the mass of 0.250 L of water is \(m_w = 0.250 \, kg.\)
- Calculate the heat transferred to the water. Use the specific heat of water in Table \(\PageIndex{1}\): \[Q_w = m_wc_w\Delta T = (0.250 \, kg)(4186 \, J/kg ^oC)(60.0 ^oC) = 62.8 \, kJ.\]
- Calculate the heat transferred to the aluminum. Use the specific heat for aluminum in Table \(\PageIndex{1}\): \[Q_{A1} = m_{A1}c_{A1}\Delta T = (0.500 \, kg)(900 \, J/kg^oC)(60.0^oC) = 27.0 \, kJ.\]
- Find the total transferred heat: \[Q_{Total} = Q_W + Q_{A1} = 89.8 \, kJ.\]
Significance
In this example, the heat transferred to the container is a significant fraction of the total transferred heat. Although the mass of the pan is twice that of the water, the specific heat of water is over four times that of aluminum. Therefore, it takes a bit more than twice as much heat to achieve the given temperature change for the water as for the aluminum pan.
Example \(\PageIndex{2}\) illustrates a temperature rise caused by doing work. (The result is the same as if the same amount of energy had been added with a blowtorch instead of mechanically.)
Calculating the Temperature Increase from the Work Done on a Substance.
Truck brakes used to control speed on a downhill run do work, converting gravitational potential energy into increased internal energy (higher temperature) of the brake material (Figure \(\PageIndex{3}\)). This conversion prevents the gravitational potential energy from being converted into kinetic energy of the truck. Since the mass of the truck is much greater than that of the brake material absorbing the energy, the temperature increase may occur too fast for sufficient heat to transfer from the brakes to the environment; in other words, the brakes may overheat.

Calculate the temperature increase of 10 kg of brake material with an average specific heat of \(800 \, J/kg \cdot ^C\) if the material retains 10% of the energy from a 10,000-kg truck descending 75.0 m (in vertical displacement) at a constant speed.
We calculate the gravitational potential energy ( Mgh ) that the entire truck loses in its descent, equate it to the increase in the brakes’ internal energy, and then find the temperature increase produced in the brake material alone.
First we calculate the change in gravitational potential energy as the truck goes downhill:
\[Mgh = (10,000 \, kg)(9.80 \, m/s^2)(75.0 \, m) = 7.35 \times 10^6 \, J. \nonumber\]
Because the kinetic energy of the truck does not change, conservation of energy tells us the lost potential energy is dissipated, and we assume that 10% of it is transferred to internal energy of the brakes, so take \(Q = Mgh/10\). Then we calculate the temperature change from the heat transferred, using
\[\Delta T = \dfrac{7.35 \times 10^5 \, J}{(10 \, kg)(800 \, J/kg^oC)} = 92^oC. \nonumber\]
If the truck had been traveling for some time, then just before the descent, the brake temperature would probably be higher than the ambient temperature. The temperature increase in the descent would likely raise the temperature of the brake material very high, so this technique is not practical. Instead, the truck would use the technique of engine braking. A different idea underlies the recent technology of hybrid and electric cars, where mechanical energy (kinetic and gravitational potential energy) is converted by the brakes into electrical energy in the battery, a process called regenerative braking.
In a common kind of problem, objects at different temperatures are placed in contact with each other but isolated from everything else, and they are allowed to come into equilibrium. A container that prevents heat transfer in or out is called a calorimeter , and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is called calorimetry .
We will use the term “calorimetry problem” to refer to any problem in which the objects concerned are thermally isolated from their surroundings. An important idea in solving calorimetry problems is that during a heat transfer between objects isolated from their surroundings, the heat gained by the colder object must equal the heat lost by the hotter object, due to conservation of energy:
\[Q_{cold} + Q_{hot} = 0.\]
We express this idea by writing that the sum of the heats equals zero because the heat gained is usually considered positive; the heat lost, negative.
Calculating the Final Temperature in Calorimetry
Suppose you pour 0.250 kg of \(20.0^oC\) water (about a cup) into a 0.500-kg aluminum pan off the stove with a temperature of \(150^oC\). Assume no heat transfer takes place to anything else: The pan is placed on an insulated pad, and heat transfer to the air is neglected in the short time needed to reach equilibrium. Thus, this is a calorimetry problem, even though no isolating container is specified. Also assume that a negligible amount of water boils off. What is the temperature when the water and pan reach thermal equilibrium?
Originally, the pan and water are not in thermal equilibrium: The pan is at a higher temperature than the water. Heat transfer restores thermal equilibrium once the water and pan are in contact; it stops once thermal equilibrium between the pan and the water is achieved. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry.
- Use the equation for heat transfer \(Q = mc\Delta T\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan, and the final temperature: \[Q_{hot} = m_{A1}c_{A1}(T_f - 150^oC). \nonumber\]
- Express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature: \[Q_{cold} = m_wc_w(T_f - 20.0^oC). \nonumber\]
- Note that \(Q_{hot} <0\) and \(Q_{cold} > 0 \) and that as stated above, they must sum to zero: \[Q_{cold} + Q_{hot} = 0\]\[Q_{cold} = -Q_{hot}\]\[m_wc_w(T_f - 20.0 ^C) = -m_{A1}c_{A1} (T_f - 150^oC). \nonumber\]
- This a linear equation for the unknown final temperature, \(T_f\). Solving for \(T_f\), \[T_f = \dfrac{m_{A1}c_{A1}(150^oC) + m_wc_w(20.0^oC)}{m_{A1}c_{A1} + m_wc_w}, \nonumber\] and insert the numerical values: \[T_f = \dfrac{(0.500 \, kg)(900 \, J/kg^oC)(150^oC) + (0.250 \, kg)(4186 \, J/kg^oC)(20.0^oC)}{(0.500 \, kg)(900 \, J/kg^oC) + (0.250 \, kg)(4186 \, J/kg^oC)} = 59.1 \, ^oC. \nonumber\]
Significance Why is the final temperature so much closer to \(20.0^oC\) than to \(150^oC\)? The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. A large body of water, such as a lake, requires a large amount of heat to increase its temperature appreciably. This explains why the temperature of a lake stays relatively constant during the day even when the temperature change of the air is large. However, the water temperature does change over longer times (e.g., summer to winter).
Exercise \(\PageIndex{3}\)
If 25 kJ is necessary to raise the temperature of a rock from \(25^oC\) to \(30^oC\), how much heat is necessary to heat the rock from \(45^oC\) to \(50^oC\)?
To a good approximation, the heat transfer depends only on the temperature difference. Since the temperature differences are the same in both cases, the same 25 kJ is necessary in the second case. (As we will see in the next section, the answer would have been different if the object had been made of some substance that changes phase anywhere between \(30^oC\) and \(50^oC\).)
Temperature-Dependent Heat Capacity
At low temperatures, the specific heats of solids are typically proportional to \(T^3\). The first understanding of this behavior was due to the Dutch physicist Peter Debye , who in 1912, treated atomic oscillations with the quantum theory that Max Planck had recently used for radiation. For instance, a good approximation for the specific heat of salt, NaCl, is \(c = 3.33 \times 10^4 \frac{J}{kg \cdot k}\left(\frac{T}{321 \, K}\right)^3\). The constant 321 K is called the Debye temperature of NaCl, \(\Theta_D\) and the formula works well when \(T < 0.04 \Theta_D\). Using this formula, how much heat is required to raise the temperature of 24.0 g of NaCl from 5 K to 15 K?
Because the heat capacity depends on the temperature, we need to use the equation \[c = \dfrac{1}{m} \dfrac{dQ}{dT}.\]
We solve this equation for Q by integrating both sides: \(Q = m \int_{T_1}^{T_2} cdT\).
Then we substitute the given values in and evaluate the integral:
\[Q = (0.024 \, kg) \int_{T1}^{T2} 333 \times 10^4 \dfrac{J}{kg \cdot K}\left(\dfrac{T}{321 \, K}\right)^3 dT = \left( 6.04 \times 10^{-4} \dfrac{J}{K^4}\right) T^4 |_{5 \, K}^{15 \, K} = 30.2 \, J.\]
Significance If we had used the equation \(Q = mc\Delta T\) and the room-temperature specific heat of salt, \(880 \, J/kg \cdot K\), we would have gotten a very different value.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
UP Class 7th Science
Course: up class 7th science > unit 5.
- Activity: Conduction
- Explanation: Conduction
- Activity: Convection
- Explanation: Convection
Methods of heat transfer
- Engineering & Technology
- Mechanical Engineering
- Heat Transfer
Heat Transfer/ Specific Heat Problems Worksheet

Related documents

Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer
11.2 Heat, Specific Heat, and Heat Transfer
Section learning objectives.
By the end of this section, you will be able to do the following:
- Explain heat, heat capacity, and specific heat
- Distinguish between conduction, convection, and radiation
- Solve problems involving specific heat and heat transfer
Teacher Support
The learning objectives in this section will help your students master the following standards:
- (F) contrast and give examples of different processes of thermal energy transfer, including conduction, convection, and radiation.
Section Key Terms
[BL] [OL] [AL] Review concepts of heat, temperature, and mass.
[AL] Check prior knowledge of conduction and convection.
Heat Transfer, Specific Heat, and Heat Capacity
We learned in the previous section that temperature is proportional to the average kinetic energy of atoms and molecules in a substance, and that the average internal kinetic energy of a substance is higher when the substance’s temperature is higher.
If two objects at different temperatures are brought in contact with each other, energy is transferred from the hotter object (that is, the object with the greater temperature) to the colder (lower temperature) object, until both objects are at the same temperature. There is no net heat transfer once the temperatures are equal because the amount of heat transferred from one object to the other is the same as the amount of heat returned. One of the major effects of heat transfer is temperature change: Heating increases the temperature while cooling decreases it. Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance’s temperature, the mass of the substance, and certain physical properties related to the phase of the substance.
The equation for heat transfer Q is
where m is the mass of the substance and Δ T is the change in its temperature, in units of Celsius or Kelvin. The symbol c stands for specific heat , and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºC. The specific heat c is a property of the substance; its SI unit is J/(kg ⋅ ⋅ K) or J/(kg ⋅ ⋅ °C °C ). The temperature change ( Δ T Δ T ) is the same in units of kelvins and degrees Celsius (but not degrees Fahrenheit). Specific heat is closely related to the concept of heat capacity . Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °C °C . In equation form, heat capacity C is C = m c C = m c , where m is mass and c is specific heat. Note that heat capacity is the same as specific heat, but without any dependence on mass. Consequently, two objects made up of the same material but with different masses will have different heat capacities. This is because the heat capacity is a property of an object, but specific heat is a property of any object made of the same material.
Values of specific heat must be looked up in tables, because there is no simple way to calculate them. Table 11.2 gives the values of specific heat for a few substances as a handy reference. We see from this table that the specific heat of water is five times that of glass, which means that it takes five times as much heat to raise the temperature of 1 kg of water than to raise the temperature of 1 kg of glass by the same number of degrees.
[BL] [OL] [AL] Explain that this formula only works when there is no change in phase of the substance. The transfer of thermal energy, heat, and phase change will be covered later in the chapter.
Misconception Alert
The units of specific heat are J/(kg ⋅ °C ⋅ °C ) and J/(kg ⋅ ⋅ K). However, degrees Celsius and Kelvins are not always interchangeable. The formula for specific heat uses a difference in temperature and not absolute temperature. This is the reason that degrees Celsius may be used in place of Kelvins.
Temperature Change of Land and Water
What heats faster, land or water? You will answer this question by taking measurements to study differences in specific heat capacity.
- Open flame—Tie back all loose hair and clothing before igniting an open flame. Follow all of your teacher's instructions on how to ignite the flame. Never leave an open flame unattended. Know the location of fire safety equipment in the laboratory.
- Sand or soil
- Oven or heat lamp
- Two small jars
- Two thermometers
Instructions
- Place equal masses of dry sand (or soil) and water at the same temperature into two small jars. (The average density of soil or sand is about 1.6 times that of water, so you can get equal masses by using 50 percent more water by volume.)
- Heat both substances (using an oven or a heat lamp) for the same amount of time.
- Record the final temperatures of the two masses.
- Now bring both jars to the same temperature by heating for a longer period of time.
- Remove the jars from the heat source and measure their temperature every 5 minutes for about 30 minutes.
- The pond will reach 0 °C first because of water’s greater specific heat.
- The field will reach 0 °C first because of soil’s lower specific heat.
- They will reach 0° C at the same time because they are exposed to the same weather.
- The water will take longer to heat as well as to cool. This tells us that the specific heat of water is greater than that of land.
Conduction, Convection, and Radiation
Whenever there is a temperature difference, heat transfer occurs. Heat transfer may happen rapidly, such as through a cooking pan, or slowly, such as through the walls of an insulated cooler.
There are three different heat transfer methods: conduction , convection , and radiation . At times, all three may happen simultaneously. See Figure 11.3 .
Conduction is heat transfer through direct physical contact. Heat transferred between the electric burner of a stove and the bottom of a pan is transferred by conduction. Sometimes, we try to control the conduction of heat to make ourselves more comfortable. Since the rate of heat transfer is different for different materials, we choose fabrics, such as a thick wool sweater, that slow down the transfer of heat away from our bodies in winter.
As you walk barefoot across the living room carpet, your feet feel relatively comfortable…until you step onto the kitchen’s tile floor. Since the carpet and tile floor are both at the same temperature, why does one feel colder than the other? This is explained by different rates of heat transfer: The tile material removes heat from your skin at a greater rate than the carpeting, which makes it feel colder.
[BL] [OL] [AL] Ask students what the current temperature in the classroom is. Ask them if all the objects in the room are at the same temperature. Once this is established, ask them to place their hand on their desk or on a metal object. Does it feel colder? Why? If their desk is Formica laminate, then it will feel cool to their hand because the laminate is a good conductor of heat and draws heat from their hand creating a sensation of “cold” due to heat leaving the body.
Some materials simply conduct thermal energy faster than others. In general, metals (like copper, aluminum, gold, and silver) are good heat conductors, whereas materials like wood, plastic, and rubber are poor heat conductors.
Figure 11.4 shows particles (either atoms or molecules) in two bodies at different temperatures. The (average) kinetic energy of a particle in the hot body is higher than in the colder body. If two particles collide, energy transfers from the particle with greater kinetic energy to the particle with less kinetic energy. When two bodies are in contact, many particle collisions occur, resulting in a net flux of heat from the higher-temperature body to the lower-temperature body. The heat flux depends on the temperature difference Δ T = T hot − T cold Δ T = T hot − T cold . Therefore, you will get a more severe burn from boiling water than from hot tap water.
Convection is heat transfer by the movement of a fluid. This type of heat transfer happens, for example, in a pot boiling on the stove, or in thunderstorms, where hot air rises up to the base of the clouds.
Tips For Success
In everyday language, the term fluid is usually taken to mean liquid. For example, when you are sick and the doctor tells you to “push fluids,” that only means to drink more beverages—not to breath more air. However, in physics, fluid means a liquid or a gas . Fluids move differently than solid material, and even have their own branch of physics, known as fluid dynamics , that studies how they move.
As the temperature of fluids increase, they expand and become less dense. For example, Figure 11.4 could represent the wall of a balloon with different temperature gases inside the balloon than outside in the environment. The hotter and thus faster moving gas particles inside the balloon strike the surface with more force than the cooler air outside, causing the balloon to expand. This decrease in density relative to its environment creates buoyancy (the tendency to rise). Convection is driven by buoyancy—hot air rises because it is less dense than the surrounding air.
Sometimes, we control the temperature of our homes or ourselves by controlling air movement. Sealing leaks around doors with weather stripping keeps out the cold wind in winter. The house in Figure 11.5 and the pot of water on the stove in Figure 11.6 are both examples of convection and buoyancy by human design. Ocean currents and large-scale atmospheric circulation transfer energy from one part of the globe to another, and are examples of natural convection.
Radiation is a form of heat transfer that occurs when electromagnetic radiation is emitted or absorbed. Electromagnetic radiation includes radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays, all of which have different wavelengths and amounts of energy (shorter wavelengths have higher frequency and more energy).
[BL] [OL] Electromagnetic waves are also often referred to as EM waves. We perceive EM waves of different frequencies differently. Just as we are able to see certain frequencies as visible light, we perceive certain others as heat.
You can feel the heat transfer from a fire and from the sun. Similarly, you can sometimes tell that the oven is hot without touching its door or looking inside—it may just warm you as you walk by. Another example is thermal radiation from the human body; people are constantly emitting infrared radiation, which is not visible to the human eye, but is felt as heat.
Radiation is the only method of heat transfer where no medium is required, meaning that the heat doesn’t need to come into direct contact with or be transported by any matter. The space between Earth and the sun is largely empty, without any possibility of heat transfer by convection or conduction. Instead, heat is transferred by radiation, and Earth is warmed as it absorbs electromagnetic radiation emitted by the sun.
All objects absorb and emit electromagnetic radiation (see Figure 11.7 ). The rate of heat transfer by radiation depends mainly on the color of the object. Black is the most effective absorber and radiator, and white is the least effective. People living in hot climates generally avoid wearing black clothing, for instance. Similarly, black asphalt in a parking lot will be hotter than adjacent patches of grass on a summer day, because black absorbs better than green. The reverse is also true—black radiates better than green. On a clear summer night, the black asphalt will be colder than the green patch of grass, because black radiates energy faster than green. In contrast, white is a poor absorber and also a poor radiator. A white object reflects nearly all radiation, like a mirror.
Ask students to give examples of conduction, convection, and radiation.
Virtual Physics
Energy forms and changes.
In this animation, you will explore heat transfer with different materials. Experiment with heating and cooling the iron, brick, and water. This is done by dragging and dropping the object onto the pedestal and then holding the lever either to Heat or Cool. Drag a thermometer beside each object to measure its temperature—you can watch how quickly it heats or cools in real time.
Now let’s try transferring heat between objects. Heat the brick and then place it in the cool water. Now heat the brick again, but then place it on top of the iron. What do you notice?
Selecting the fast forward option lets you speed up the heat transfers, to save time.
- Water will take the longest, and iron will take the shortest time to heat, as well as to cool. Objects with greater specific heat would be desirable for insulation. For instance, woolen clothes with large specific heat would prevent heat loss from the body.
- Water will take the shortest, and iron will take the longest time to heat, as well as to cool. Objects with greater specific heat would be desirable for insulation. For instance, woolen clothes with large specific heat would prevent heat loss from the body.
- Brick will take shortest and iron will take longest time to heat up as well as to cool down. Objects with greater specific heat would be desirable for insulation. For instance, woolen clothes with large specific heat would prevent heat loss from the body.
- Water will take shortest and brick will take longest time to heat up as well as to cool down. Objects with greater specific heat would be desirable for insulation. For instance, woolen clothes with large specific heat would prevent heat loss from the body.
Have students consider the differences in the interactive exercise results if different materials were used. For example, ask them whether the temperature change would be greater or smaller if the brick were replaced with a block of iron with the same mass as the brick. Ask students to consider identical masses of the metals aluminum, gold, and copper. After they have stated whether the temperature change is greater or less for each metal, have them refer to Table 11.2 and check whether their predictions were correct.
Solving Heat Transfer Problems
Worked example, calculating the required heat: heating water in an aluminum pan.
A 0.500 kg aluminum pan on a stove is used to heat 0.250 L of water from 20.0 °C °C to 80.0 °C °C . (a) How much heat is required? What percentage of the heat is used to raise the temperature of (b) the pan and (c) the water?
The pan and the water are always at the same temperature. When you put the pan on the stove, the temperature of the water and the pan is increased by the same amount. We use the equation for heat transfer for the given temperature change and masses of water and aluminum. The specific heat values for water and aluminum are given in the previous table.
Because the water is in thermal contact with the aluminum, the pan and the water are at the same temperature.
- Calculate the temperature difference. Δ T = T f − T i = 60.0 °C Δ T = T f − T i = 60.0 °C 11.8
- Calculate the mass of water using the relationship between density, mass, and volume. Density is mass per unit volume, or ρ = m V ρ = m V . Rearranging this equation, solve for the mass of water. m w = ρ ⋅ V = 1000 kg/m 3 × ( 0 .250 L× 0 .001 m 3 1 L ) =0 .250 kg m w = ρ ⋅ V = 1000 kg/m 3 × ( 0 .250 L× 0 .001 m 3 1 L ) =0 .250 kg 11.9
- Calculate the heat transferred to the water. Use the specific heat of water in the previous table. Q w = m w c w Δ T = ( 0.250 kg ) ( 4186 J/kg °C ) ( 60 .0 °C ) = 62 .8 kJ Q w = m w c w Δ T = ( 0.250 kg ) ( 4186 J/kg °C ) ( 60 .0 °C ) = 62 .8 kJ 11.10
- Calculate the heat transferred to the aluminum. Use the specific heat for aluminum in the previous table. Q A l = m A l c A l Δ T = ( 0.500 kg ) ( 900 J/kg °C ) ( 60 .0 °C ) = 27 .0 ×10 3 J = 27 .0 kJ Q A l = m A l c A l Δ T = ( 0.500 kg ) ( 900 J/kg °C ) ( 60 .0 °C ) = 27 .0 ×10 3 J = 27 .0 kJ 11.11
- Find the total transferred heat. Q T o t a l = Q w + Q A l = 62 .8 kJ + 27 .0 kJ = 89 .8 kJ Q T o t a l = Q w + Q A l = 62 .8 kJ + 27 .0 kJ = 89 .8 kJ 11.12
The percentage of heat going into heating the pan is
The percentage of heat going into heating the water is
In this example, most of the total heat transferred is used to heat the water, even though the pan has twice as much mass. This is because the specific heat of water is over four times greater than the specific heat of aluminum. Therefore, it takes a bit more than twice as much heat to achieve the given temperature change for the water than for the aluminum pan.
Water can absorb a tremendous amount of energy with very little resulting temperature change. This property of water allows for life on Earth because it stabilizes temperatures. Other planets are less habitable because wild temperature swings make for a harsh environment. You may have noticed that climates closer to large bodies of water, such as oceans, are milder than climates landlocked in the middle of a large continent. This is due to the climate-moderating effect of water’s large heat capacity—water stores large amounts of heat during hot weather and releases heat gradually when it’s cold outside.
Calculating Temperature Increase: Truck Brakes Overheat on Downhill Runs
When a truck headed downhill brakes, the brakes must do work to convert the gravitational potential energy of the truck to internal energy of the brakes. This conversion prevents the gravitational potential energy from being converted into kinetic energy of the truck, and keeps the truck from speeding up and losing control. The increased internal energy of the brakes raises their temperature. When the hill is especially steep, the temperature increase may happen too quickly and cause the brakes to overheat.
Calculate the temperature increase of 100 kg of brake material with an average specific heat of 800 J/kg ⋅ °C ⋅ °C from a 10,000 kg truck descending 75.0 m (in vertical displacement) at a constant speed.
We first calculate the gravitational potential energy ( Mgh ) of the truck, and then find the temperature increase produced in the brakes.
- Calculate the change in gravitational potential energy as the truck goes downhill. M g h = ( 10 , 000 kg ) (9 .80 m/s 2 ) ( 75 .0 m ) = 7.35 × 10 6 J M g h = ( 10 , 000 kg ) (9 .80 m/s 2 ) ( 75 .0 m ) = 7.35 × 10 6 J 11.15
where m is the mass of the brake material (not the entire truck). Insert the values Q = 7.35×10 6 J (since the heat transfer is equal to the change in gravitational potential energy), m = = 100 kg, and c = = 800 J/kg ⋅ ⋅ °C °C to find
This temperature is close to the boiling point of water. If the truck had been traveling for some time, then just before the descent, the brake temperature would likely be higher than the ambient temperature. The temperature increase in the descent would likely raise the temperature of the brake material above the boiling point of water, which would be hard on the brakes. This is why truck drivers sometimes use a different technique for called “engine braking” to avoid burning their brakes during steep descents. Engine braking is using the slowing forces of an engine in low gear rather than brakes to slow down.
Practice Problems
How much heat does it take to raise the temperature of 10.0 kg of water by 1.0 °C ?
Calculate the change in temperature of 1.0 kg of water that is initially at room temperature if 3.0 kJ of heat is added.
Check Your Understanding
Use these questions to assess student achievement of the section’s learning objectives. If students are struggling with a specific objective, these questions will help identify which and direct students to the relevant content.
- The mass difference between two objects causes heat transfer.
- The density difference between two objects causes heat transfer.
- The temperature difference between two systems causes heat transfer.
- The pressure difference between two objects causes heat transfer.
- The overall direction of heat transfer is from the higher-temperature object to the lower-temperature object.
- The overall direction of heat transfer is from the lower-temperature object to the higher-temperature object.
- The direction of heat transfer is first from the lower-temperature object to the higher-temperature object, then back again to the lower-temperature object, and so-forth, until the objects are in thermal equilibrium.
- The direction of heat transfer is first from the higher-temperature object to the lower-temperature object, then back again to the higher-temperature object, and so-forth, until the objects are in thermal equilibrium.
- conduction, radiation, and reflection
- conduction, reflection, and convection
- convection, radiation, and reflection
- conduction, radiation, and convection
True or false—Conduction and convection cannot happen simultaneously
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute Texas Education Agency (TEA). The original material is available at: https://www.texasgateway.org/book/tea-physics . Changes were made to the original material, including updates to art, structure, and other content updates.
Access for free at https://openstax.org/books/physics/pages/1-introduction
- Authors: Paul Peter Urone, Roger Hinrichs
- Publisher/website: OpenStax
- Book title: Physics
- Publication date: Mar 26, 2020
- Location: Houston, Texas
- Book URL: https://openstax.org/books/physics/pages/1-introduction
- Section URL: https://openstax.org/books/physics/pages/11-2-heat-specific-heat-and-heat-transfer
© Jan 19, 2024 Texas Education Agency (TEA). The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.5.1: Practice Problems- Calorimetry
- Last updated
- Save as PDF
- Page ID 217300
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
PROBLEM \(\PageIndex{1}\)
A 500-mL bottle of water at room temperature and a 2-L bottle of water at the same temperature were placed in a refrigerator. After 30 minutes, the 500-mL bottle of water had cooled to the temperature of the refrigerator. An hour later, the 2-L of water had cooled to the same temperature. When asked which sample of water lost the most heat, Student A replied that both bottles lost the same amount of heat because they started at the same temperature and finished at the same temperature. Student B thought that the 2-L bottle of water lost more heat because there was more water. A third student believed that the 500-mL bottle of water lost more heat because it cooled more quickly. A fourth student thought that it was not possible to tell because we do not know the initial temperature and the final temperature of the water. Indicate which of these answers is correct and describe the error in each of the other answers.
Student A is incorrect because the mass of water in both containers is not the same.
Student C is incorrect because the bottle cooled quicker due to less mass of water.
Student D is incorrect because it doesn't matter what the change in temperature is as long as it is the same for both bottles.
Student B is correct: if the change in temperature is the same, the one with the more mass (the 2L bottle) had more heat loss. We could prove this using \(q=c×m×ΔT=c×m×(T_\ce{final}−T_\ce{initial})\) from Section 8.1 .
PROBLEM \(\PageIndex{2}\)
How many milliliters of water at 23 °C with a density of 1.00 g/mL must be mixed with 180 mL (about 6 oz) of coffee at 95 °C so that the resulting combination will have a temperature of 60 °C? Assume that coffee and water have the same density and the same specific heat (4.184 J/g °C).
*The section number changed after this video was made*
PROBLEM \(\PageIndex{3}\)
How much will the temperature of a cup (180 g) of coffee at 95 °C be reduced when a 45 g silver spoon (specific heat 0.24 J/g °C) at 25 °C is placed in the coffee and the two are allowed to reach the same temperature? Assume that the coffee has the same density and specific heat as water.
The temperature of the coffee will drop 1 degree.
PROBLEM \(\PageIndex{4}\)
A 45-g aluminum spoon (specific heat 0.88 J/g °C) at 24 °C is placed in 180 mL (180 g) of coffee at 85 °C and the temperature of the two become equal.
- What is the final temperature when the two become equal? Assume that coffee has the same specific heat as water.
- The first time a student solved this problem she got an answer of 88 °C. Explain why this is clearly an incorrect answer.
81.95 °C
This temperature is higher than the starting temperature of the coffee, which is impossible.
PROBLEM \(\PageIndex{5}\)
The temperature of the cooling water as it leaves the hot engine of an automobile is 240 °F. After it passes through the radiator it has a temperature of 175 °F. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184 J/g °C.
\(5.7 \times 10^2\; kJ\)
PROBLEM \(\PageIndex{6}\)
When 50.0 g of 0.200 M NaCl( aq ) at 24.1 °C is added to 100.0 g of 0.100 M AgNO 3 ( aq ) at 24.1 °C in a calorimeter, the temperature increases to 25.2 °C as AgCl( s ) forms. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat in joules produced.
PROBLEM \(\PageIndex{7}\)
The addition of 3.15 g of Ba(OH) 2 •8H 2 O to a solution of 1.52 g of NH 4 SCN in 100 g of water in a calorimeter caused the temperature to fall by 3.1 °C. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the following equation:
\[Ba(OH)_2 \cdot 8H_2O_{(s)} + 2NH_4SCN_{(aq)} \rightarrow Ba(SCN)_{2(aq)} + 2NH_{3(aq)} + 10H_2O_{(l)}\]
PROBLEM \(\PageIndex{8}\)
When 1.0 g of fructose, C 6 H 12 O 6 ( s ), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the calorimeter increases by 1.58 °C. If the heat capacity of the calorimeter and its contents is 9.90 kJ/°C, what is q for this combustion?
PROBLEM \(\PageIndex{9}\)
One method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. To determine the rate at which coal is to be fed into the burner in this type of plant, the heat of combustion per ton of coal must be determined using a bomb calorimeter. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °C. If the heat capacity of the calorimeter is 21.6 kJ/°C, determine the heat produced by combustion of a ton of coal (2000 pounds). Remember 1 kg = 2.2 pounds
2.91 x 10 7 kJ
PROBLEM \(\PageIndex{10}\)
A teaspoon of the carbohydrate sucrose (common sugar) contains 16 Calories (16 kcal). What is the mass of one teaspoon of sucrose if the average number of Calories for carbohydrates is 4.1 Calories/g?
*This problem was renumbered after the video was made*
PROBLEM \(\PageIndex{11}\)
What is the maximum mass of carbohydrate in a 6-oz serving of diet soda that contains less than 1 Calorie per can if the average number of Calories for carbohydrates is 4.1 Calories/g?
PROBLEM \(\PageIndex{12}\)
A pint of premium ice cream can contain 1100 Calories. What mass of fat, in grams and pounds, must be produced in the body to store an extra 1.1 × 10 3 Calories if the average number of Calories for fat is 9.1 Calories/g? Remember 1 kg = 2.2 pounds
120.87 g or 1.2 x 10 2 g with 2 significant figures
0.266 lbs or 0.27 lbs with 2 significant figures
PROBLEM \(\PageIndex{13}\)
A serving of a breakfast cereal contains 3 g of protein, 18 g of carbohydrates, and 6 g of fat. What is the Calorie content of a serving of this cereal if the average number of Calories for fat is 9.1 Calories/g, for carbohydrates is 4.1 Calories/g, and for protein is 4.1 Calories/g?
1.4 × 10 2 Calories
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/[email protected] ).
- Adelaide Clark, Oregon Institute of Technology

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Heat Transfer – Conduction, Convection, Radiation

Heat transfer occurs when thermal energy moves from one place to another. Atoms and molecules inherently have kinetic and thermal energy, so all matter participates in heat transfer. There are three main types of heat transfer, plus other processes that move energy from high temperature to low temperature.

What Is Heat Transfer?
Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The energy transfer is always from higher temperature to lower temperature, due to the second law of thermodynamics . The units of heat transfer are the joule (J), calorie (cal), and kilocalorie (kcal). The unit for the rate of heat transfer is the kilowatt (KW).
The Three Types of Heat Transfer With Examples
The three types of heat transfer differ according to the nature of the medium that transmits heat:
- Conduction requires contact.
- Convection requires fluid flow.
- Radiation does not require any medium.
- Conduction is heat transfer directly between neighboring atoms or molecules. Usually, it is heat transfer through a solid. For example, the metal handle of a pan on a stove becomes hot due to convection. Touching the hot pan conducts heat to your hand.
- Convection is heat transfer via the movement of a fluid, such as air or water. Heating water on a stove is a good example. The water at the top of the pot becomes hot because water near the heat source rises. Another example is the movement of air around a campfire. Hot air rises, transferring heat upward. Meanwhile, the partial vacuum left by this movement draws in cool outside air that feeds the fire with fresh oxygen.
- Radiation is the emission of electromagnetic radiation. While it occurs through a medium, it does not require one. For example, it’s warm outside on a sunny day because solar radiation crosses space and heats the atmosphere. The burner element of a stove also emits radiation. However, some heat from a burner comes from conduction between the hot element and a metal pan. Most real-life processes involve multiple forms of heat transfer.
Conduction requires that molecules touch each other, making it a slower process than convection or radiation. Atoms and molecules with a lot of energy have more kinetic energy and engage in more collisions with other matter. They are “hot.” When hot matter interacts with cold matter, some energy gets transferred during the collision. This drives conduction. Forms of matter that readily conduct heat are called thermal conductors .
Examples of Conduction
Conduction is a common process in everyday life. For example:
- Holding an ice cube immediately makes your hands feel cold. Meanwhile, the heat transferred from your skin to the ice melts it into liquid water.
- Walking barefoot on a hot road or sunny beach burns your feet because the solid material transmits heat into your foot.
- Iron clothes transfers heat from the iron to the fabric.
- The handle of a coffee cup filled with hot coffee becomes warm or even hot via conduction through the mug material.
Conduction Equation
One equation for conduction calculates heat transfer per unit of time from thermal conductivity, area, thickness of the material, and the temperature difference between two regions:
Q = [K ∙ A ∙ (T hot – T cold )] / d
- Q is heat transfer per unit time
- K is the coefficient of thermal conductivity of the substance
- A is the area of heat transfer
- T hot is the temperature of the hot region
- T cold is the temperature of the cold region
- d is the thickness of the body
Convection is the movement of fluid molecules from higher temperature to lower temperature regions. Changing the temperature of a fluid affects its density, producing convection currents. If the volume of a fluid increases, than its density decreases and it becomes buoyant.
Examples of Convection
Convection is a familiar process on Earth, primarily involving air or water. However, it applies to other fluids, such as refrigeration gases and magma. Examples of convection include:
- Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules.
- Hot air rises and cooler air sinks and replaces it.
- Convection drives global circulation in the oceans between the equators and poles.
- A convection oven circulates hot air and cooks more evenly than one that only uses heating elements or a gas flame.
Convection Equation
The equation for the rate of convection relates area and the difference between the fluid temperature and surface temperature:
Q = h c ∙ A ∙ (T s – T f )
- Q is the heat transfer per unit time
- h c is the coefficient of convective heat transfer
- T s is the surface temperature
- T f is the fluid temperature
Radiation is the release of electromagnetic energy. Another name for thermal radiation is radiant heat. Unlike conduction or convection, radiation requires no medium for heat transfer. So, radiation occurs both within a medium (solid, liquid, gas) or through a vacuum.
Examples of Radiation
There are many examples of radiation:
- A microwave oven emits microwave radiation, which increases the thermal energy in food
- The Sun emits light (including ultraviolet radiation) and heat
- Uranium-238 emits alpha radiation as it decays into thorium-234
Radiation Equation
The Stephan-Boltzmann law describes relationship between the power and temperature of thermal radiation:
P = e ∙ σ ∙ A· (Tr – Tc) 4
- P is the net power of radiation
- A is the area of radiation
- Tr is the radiator temperature
- Tc is the surrounding temperature
- e is emissivity
- σ is Stefan’s constant (σ = 5.67 × 10 -8 Wm -2 K -4 )
More Heat Transfer – Chemical Bonds and Phase Transitions
While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. For example, atoms release energy when chemical bonds break and absorb energy in order to form bonds. Releasing energy is an exergonic process, while absorbing energy is an endergonic process. Sometimes the energy is light or sound, but most of the time it’s heat, making these processes exothermic and endothermic .
Phase transitions between the states of matter also involve the absorption or release of energy. A great example of this is evaporative cooling, where the phase transition from a liquid into a vapor absorbs thermal energy from the environment.
- Faghri, Amir; Zhang, Yuwen; Howell, John (2010). Advanced Heat and Mass Transfer . Columbia, MO: Global Digital Press. ISBN 978-0-9842760-0-4.
- Geankoplis, Christie John (2003). Transport Processes and Separation Principles (4th ed.). Prentice Hall. ISBN 0-13-101367-X.
- Peng, Z.; Doroodchi, E.; Moghtaderi, B. (2020). “Heat transfer modelling in Discrete Element Method (DEM)-based simulations of thermal processes: Theory and model development”. Progress in Energy and Combustion Science . 79: 100847. doi: 10.1016/j.pecs.2020.100847
- Welty, James R.; Wicks, Charles E.; Wilson, Robert Elliott (1976). Fundamentals of Momentum, Heat, and Mass Transfer (2nd ed.). New York: Wiley. ISBN 978-0-471-93354-0.
Related Posts
- Chemistry Concept Questions and Answers
Heat Transfer Questions
Heat can be transferred via any matter composed of atoms and molecules. At any given time, atoms are moving in a variety of ways. The motion of molecules and atoms produces heat or thermal energy, and this energy is present in all matter. The heat energy will increase as the number of molecules moves. However, when it comes to heat transfer, it simply refers to the movement of heat from a high-temperature body to a low-temperature one.
Heat Transfer Chemistry Questions with Solutions
Q1: How is Heat Transferred?
Heat can flow from one place to another in many ways. The following are many heat transfer modes:
Meanwhile, if the two systems have a temperature difference, heat will find a way to pass from the higher to the lower system.
Q2: What is Convection of Heat? Give an example.
Heat is transferred from a higher temperature region to a lower temperature region in liquids and gases during this process. Convection heat transfer occurs partly due to molecular movement and partly as a result of mass transfer.
For example. Heating of milk in a pan.
Q3: Name the general mode of heat transfer in
b) Liquids and gases.
(a) The general mode of heat transfer in solids is conduction.
(b) The general mode of heat transfer in liquids and gases is convection.
Q4: State Fourier’s law of conduction.
The rate of heat conduction is proportional to the area evaluated normal to the direction of heat flow and to the temperature difference in that direction.
Q = −kAdT / dx
Q5: Define Radiation. Give an example.
It is the method of transferring heat from one body to another without engaging the medium’s molecules. Radiation heat transfer does not rely on the medium.
For example: In a microwave, the substances are heated directly without any heating medium.
Q6: Heat transfer takes place according to which of the following law?
a) Newton’s second law of motion
b) First law of thermodynamics
c) Second law of thermodynamics
d) Newton’s law of cooling
Answer: c) Second law of thermodynamics
Explanation: According to the second law of thermodynamics, the total entropy of an isolated system (the thermal energy per unit temperature that is unavailable for doing meaningful work) can never decrease.
Q7: Which of the following is the rate of heat transfer unit?
Answer: d) Watt
Explanation: The unit of heat transfer is the joule, and the rate of heat transfer is measured in joules per second, or watts.
Q8: Which way is heat transfer believed to occur in a long, hollow cylinder kept at consistent but varied temperatures on its inner and outer surfaces?
a) Unpredictable
b) Radial only
c) No heat transfer takes place
d) Axial only
Answer: b) Radial only
Explanation: The ambient temperature is uniform on the cylinder’s periphery, and the temperature is uniform. As a result, it only happens in the radial direction.
Q9: A person prefers to sit by a fire during the cold winter months. Which of the following heat transfer types gives him the most heat?
a) Radiation will provide quick warmth
b) Convection and radiation together
c) If it is near the fire, convection sounds good
d) Conduction from the fire
Answer: a) Radiation will provide quick warmth
Explanation: Heat transfer via radiation can occur between two bodies even when separated by a medium that is colder than both of them.
Q10: For conduction heat transfer, the heat energy propagation will be minimal for __________
Answer: b) Air
Explanation: Because of all the possibilities, air has the lowest heat conductivity.
Q11: Define fins or extended surfaces.
Fins are surfaces that extend from an object to increase the heat transfer rate to or from the environment by enhancing convection in the study of heat transfer. The amount of heat transferred by an object is determined by its conduction, convection, or radiation.
There are four types of fins which are Straight fin, Radial fin, Annular fin, and Pin fin.
Q12: The non-dimensional parameter known as Stanton number is used in which of the following heat transfer?
a) Natural convection heat transfer
b) Unsteady state heat transfer
c) Condensation heat transfer
d) Forced convection heat transfer
Answer: d) Forced convection heat transfer
Explanation: It is the ratio of the heat transfer coefficient to the heat flow per unit temperature increase due to fluid velocity. It can only be used to transfer heat by forced convection.
Q13: Define a Black Surface.
Three characteristics describe a black surface:
- It traps all radiation that strikes it.
- According to Planck’s rule, it emits the most energy feasible for a given temperature and radiation wavelength.
- A blackbody’s radiation does not have a directed nature (it is a diffuse emitter).
The optimal emitter and absorber of radiation is a black surface. It’s an idealistic concept (no surface is perfectly black), and real-world properties are compared to an ideal black surface.
Q14: A greenhouse has an enclosure with high transmissivity at short wavelengths and a very low transmissivity (almost opaque) for high wavelengths. Why does a greenhouse get warmer than the surrounding air during clear days? Will it have a similar effect during clear nights?
Solar radiation is discriminatory toward the shorter wavelengths. The greenhouse’s glass allows a considerable part of the incident radiation to pass through on a clear day. The various surfaces (plants, for example) inside the greenhouse reflect the radiation, but the reflected radiation is spectrally different, with a higher proportion of long wavelengths. As a result, the reflected radiation does not pass through the glass wall and is reflected back into the greenhouse. Because of the ‘trapped’ radiation, the interior heats up. Because there is no sun radiation on a clear night, the same effect will not be visible.
Q15: Define fin efficiency and fin effectiveness.
The ratio of actual heat transfer to the maximum possible heat transfer by a fin is known as its efficiency.
Ƞfin = Q fin / Q max
Fin effectiveness is the ratio of heat transfer with fin to that without fin.
Fin effectiveness = Q with fin/ Q without fin .
Practise Questions on Heat Transfer
Q1: What is internal energy generation? Give examples where internal energy generation occurs.
Q2: What is the difference between diffusion and radiation heat transfer?
Q3: Which of the following has the highest overall heat transfer coefficient value?
b) Feedwater heaters
c) Steam condensers
d) Alcohol condensers
Q4: How is natural convection different from forced convection?
Q5: What is heat generation in solids? Give examples.
Click the PDF to check the answers for Practice Questions. Download PDF
Recommended Videos
Thermal properties of matter class 11: radiation (ch-11) | neet physics | neet 2022 prep | byju’s.

Convection Currents And It’s Examples
Class 6-10 – Visualizing Heat

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


Chemistry Steps
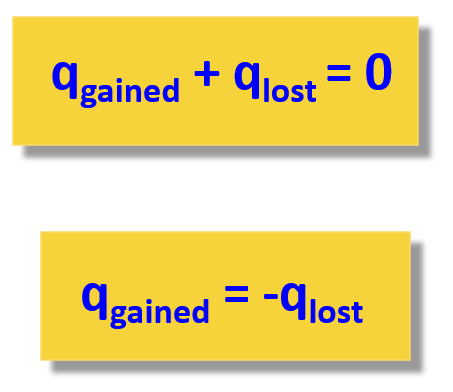
General Chemistry
Thermochemistry.
In the previous post , we talked about heat capacity, specific heat, and the formula for the relationship between these and the change in temperature.
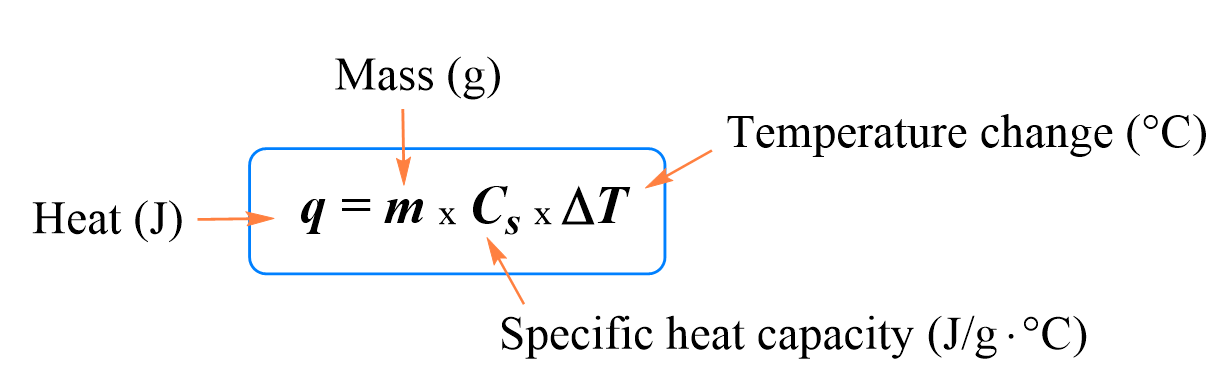
Below are some additional practice examples of heat capacity problems.
When solving a problem related to heat capacity and heat transfer, remember that most of the time, it is assumed the heat is not lost, and it only flows from the object with a higher temperature to the colder one:

The second expression adding the lost and gained heat is easier to use because you do not need to remember which one has a negative sign.
How much heat does it take to increase the temperature of a 540.6-g sample of Fe from 20.0 °C to 84.3 °C? The specific heat of iron = 0.450 J/g °C.
Calculate the specific heat capacity of a metal if a 17.0 g sample requires 481 J to change the temperature of the metal from 25.0 °C to 67.0 °C?
Calculate the energy of combustion for one mole of butane if burning a 0.367 g sample of butane (C 4 H 10 ) has increased the temperature of a bomb calorimeter by 7.73 °C. The heat capacity of the bomb calorimeter is 2.36 kJ/ °C.
How many joules of energy is required to melt 40.0 g of ice at 0 °C? The heat of fusion (Δ H fus ) for ice is 334.0 J/g.
How many kJ of energy does it take to change 36.0 g of ice at -15.0 °C to water at 0. °C ? The specific heat of ice is 2.10 J/g°C and the heat of fusion (Δ H fus ) for ice is 334.0 J/g.
- Energy Related to Heat and Work
- Endothermic and Exothermic Processes
- Heat Capacity and Specific Heat
- What is Enthalpy
- Constant-Pressure Calorimetry
- Bomb calorimeter – Constant Volume Calorimetry
- Stoichiometry and Enthalpy of Chemical Reactions
- Hess’s Law and Enthalpy of Reaction
- Hess’s Law Practice Problems
- Standard Enthalpies of Formation
- Enthalpy of Reaction from Enthalpies of Formation
- Thermochemistry Practice Problems
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.
- School Guide
- Class 11 Syllabus
- Class 11 Revision Notes
- Maths Notes Class 11
- Physics Notes Class 11
- Chemistry Notes Class 11
- Biology Notes Class 11
- NCERT Solutions Class 11 Maths
- RD Sharma Solutions Class 11
- Math Formulas Class 11
- CBSE Class 11 Physics Notes
Chapter 1: Physical World
- What is Physics? Definition, History, Importance, Scope
- How is Physics related to Other Sciences?
- Fundamental Forces
Chapter 2: Units and Measurement
- System of Units
- Length Measurement
- Measurement of Area, Volume and Density
- Rounding Numbers
- Dimensional Analysis
- Significant Figures
- Errors in Measurement
Chapter 3: Motion in a Straight Line
- What is Motion?
- Distance and Displacement
- Speed and Velocity
- Acceleration
- Uniform Acceleration
- Sample Problems on Equation of Motion
- Solving Problems Based on Free Fall
- Relative Motion
- Relative Motion in One Dimension
- Relative Motion in Two Dimension
- Calculating Stopping Distance and Reaction Time
Chapter 4: Motion in a Plane
- Scalar and Vector
- Vector Operations
- Product of Vectors
- Scalar Product of Vectors
- Dot and Cross Products on Vectors
- Position and Displacement Vectors
- Average Velocity
- Motion in Two Dimension
- Projectile Motion
- Uniform Circular Motion
- Centripetal Acceleration
- Motion in Three Dimensions
Chapter 5: Laws of Motion
- Contact and Non Contact Forces
- Inertia Meaning
- Law of Inertia
- What is Impulse?
- Solving Problems in Mechanics
- Linear Momentum of a System of Particles
- Newton's Second Law of Motion: Definition, Formula, Derivation, and Applications
- Laws of Conservation of Momentum
- What is Equilibrium? - Definition, Types, Laws, Effects
- Law of Action and Reaction
- Types of Friction - Definition, Static, Kinetic, Rolling and Fluid Friction
- Increasing and Reducing Friction
- Factors Affecting Friction
- Motion Along a Rough Inclined Plane
- Problems on Friction Formula
- Centripetal and Centrifugal Force
- Solved Examples on Dynamics of Circular Motion
- Dynamics of Circular Motion
- Motion in a Vertical Circle
Chapter 6: Work, Energy and Power
- Work Energy Theorem
- Practice Problems on Kinetic Energy
- Work Done by a Variable Force
- What is Potential Energy?
- Potential Energy of a Spring
- Practice Problems on Potential Energy
- Law of Conservation of Energy
- Difference Between Work and Energy
- Types of Collisions
- Collisions in One Dimension
- Collisions in Two Dimensions
Chapter 7: Systems of Particles and Rotational Motion
- Rigid Body - Definition, Rotation, Angular Velocity, Momentum
- Motion of a Rigid Body
- Centre of Mass
- Center of Mass of Different Objects
- Motion of Center of Mass
- Torque and Angular Momentum
- What are Couples? Definition, Moment of Couple, Applications
- What is the Principle of Moments?
- Centre of Gravity
- Moment of Inertia
- Kinematics of Rotational Motion
- Dynamics of Rotational Motion
- Angular Momentum in Case of Rotation About a Fixed Axis
- Rolling Motion
- Relation between Angular Velocity and Linear Velocity
Chapter 8: Gravitation
- Kepler's Laws of Planetary Motion
- Universal Law of Gravitation
- Factors affecting Acceleration due to Gravity
- Variation in Acceleration due to Gravity
- Potential Energy
- Escape Velocity
- Binding Energy of Satellites
- Weightlessness
Chapter 9: Mechanical Properties of Solids
- Elastic Behavior of Materials
- Elasticity and Plasticity
- Stress and Strain
- Hooke's Law
- Stress-Strain Curve
- Young's Modulus
- Shear Modulus and Bulk Modulus
- Poisson's Ratio
- Elastic Potential Energy
- Stress, Strain and Elastic Potential Energy
Chapter 10: Mechanical Properties of Fluids
- Fluid Pressure
- Pascal's Law
- Variation of Pressure With Depth
- How to calculate Atmospheric Pressure?
- Hydraulic Machines
- Streamline Flow
- Bernoulli's Principle
- Bernoulli's Equation
- What is Viscosity?
- Stoke's Law
- Reynolds Number
- Surface Tension
Chapter 11: Thermal Properties of Matter
- Difference between Heat and Temperature
- Temperature Scales
- Ideal Gas Law
- Thermal Expansion
- Heat Capacity
- Calorimetry
- Change of State of Matter
- Latent Heat
- Thermal Conduction
Sample Problems on Heat Conduction
- What is Radiation - Types, Scource, Ionizing and Non-Ionizing Radiation
- Greenhouse Effect
- Newton's Law of Cooling
Chapter 12: Thermodynamics
- Thermodynamics
- Zeroth Law of Thermodynamics
- Heat, Internal Energy and Work
- First Law of Thermodynamics
- Specific Heat Capacity
- Thermodynamic State Variables and Equation of State
- Thermodynamic Processes
- Second Law of Thermodynamics
- Reversible and Irreversible Processes
Chapter 13: Kinetic Theory
- Behavior of Gas Molecules - Kinetic Theory, Boyle's Law, Charles's Law
- Molecular Nature of Matter - Definition, States, Types, Examples
- Kinetic Theory of Gases
- Mean Free Path - Definition, Formula, Derivation, Examples
Chapter 14: Oscillations
- Oscillatory and Periodic Motion
- Simple Harmonic Motion
- Force Law for Simple Harmonic Motion
- Displacement in Simple Harmonic Motion
- Velocity and Acceleration in Simple Harmonic Motion
- Energy in Simple Harmonic Motion
- Some Systems executing Simple Harmonic Motion
Chapter 15: Waves
- Introduction to Waves - Definition, Types, Properties
- Speed of a Travelling Wave
- Reflection of Waves
- Properties of Waves
- Principle of Superposition of Waves
- Energy in Wave Motion
- Doppler Effect - Definition, Formula, Examples
Heat may be transferred via any substance made up of atoms and molecules. At any one time, the atoms are in many states of motion. Heat or thermal energy is produced by the motion of molecules and atoms, and it is present in all matter.
The more molecules move, the more heat energy is released. When it comes to heat transfer, however, it simply refers to the act of transferring heat from a high-temperature body to a low-temperature body. Heat may move from one location to another in a variety of ways. Meanwhile, if the two systems have a temperature difference, heat will find a method to flow from the upper to the lower system.
The following are the heat transmission modes: Conduction, Convection and Radiation.
What is Conduction?
The process of heat transfer from things with higher temperatures to items with lower temperatures is known as conduction.
Thermal energy is transferred from a higher kinetic energy area to a lower kinetic energy area. When high-speed particles collide with slow-moving particles, the kinetic energy of the slow-moving particles increases. Conduction can occur in solids, liquids and gases.
When heat is transmitted from one molecule to another by conduction, the heat energy is generally transported from one molecule to another because they are in direct touch. The location of the molecules, however, remains unchanged. They just resonate with one another.
Conduction Equation
When it comes to conduction, the coefficient of thermal conductivity reveals that a metal body transmits heat better.
The following equation can be used to calculate the rate of conduction:
q = Q ⁄ t = K A (T h – T c ) ⁄ d where K is the thermal conductivity, q is heat transfer rate, t is the time of transfer, Q is the amount of heat transfer,A is the area of surfaced is the thickness of the body, T h is the temperature of the hot region and T c is the temperature of the cold region.
Sample Problems
Problem 1: A 10 cm thick block of ice with a temperature of 0 °C lies on the upper surface of 2400 cm 2 slab of stone. The slab is steam-exposed on the lower surface at a temperature of 100 °C. Find the heat conductivity of stone if 4000 g of ice is melted in one hour given that the latent heat of fusion of ice is 80 cal ⁄ gm.
Given: Area of slab, A = 2400 cm 2 Thickness of ice, d = 10 cm Temperature difference, T h – T c = 100 °C – 0 °C = 100 °C Time of heat transfer, t = 1 hr = 3600 s Amount of heat transfer, Q = m L = 4000 × 80 = 320000 cal Heat transfer rate, q = Q ⁄ t = 320000 cal ⁄ 3600 s = 89 cal ⁄ s The formula for heat transfer rate is given as: q = K A (T h – T c ) ⁄ d Rearrange the above formula in terms of K. K = q d ⁄ A (T h – T c ) = (89 × 10) ⁄ (2400 × 100) cal ⁄ cm s °C = 3.7 × 10 -3 cal ⁄ cm s °C Hence, the thermal conductivity of stone is 3.7 × 10 -3 cal ⁄ cm s °C .
Problem 2: A metal rod 0.4 m long & 0.04 m in diameter has one end at 373 K & another end at 273 K. Calculate the total amount of heat conducted in 1 minute. (Given K = 385 J ⁄ m s °C)
Given: Thermal conductivity, K = 385 J ⁄ m s °C Length of rod, d = 0.4 m Diameter of rod, D = 0.04 m Area of slab, A = π D 2 ⁄ 4 = 0.001256 m 2 Temperature difference, T h – T c = 373 K – 273 K = 100 K Time of heat transfer, t = 1 min = 60 s The formula for heat transfer rate is given as: Q ⁄ t = K A (T h – T c ) ⁄ d Q = K A t (T h – T c ) ⁄ d = (385 × 0.001256 × 60 × 100) ⁄ 0.4 J = 7.25 × 10 3 J Hence, the total amount of heat transfer is 7.25 × 10 3 J .
Problem 3: An aluminium rod and a copper rod of equal length 2.0 m and cross-sectional area 2 cm 2 are welded together in parallel. One end is kept at a temperature of 10 °C and the other at 30 °C . Calculate the amount of heat taken out per second from the hot end . (Thermal conductivity of aluminium is 200 W ⁄ m °C and of copper is 390 W ⁄ m °C).
Given: Thermal conductivity of aluminium, K Al = 200 W ⁄ m °C Thermal conductivity of aluminium, K Cu = 390 W ⁄ m °C Combined thermal conductivity for parallel combination, K = 200 W ⁄ m °C + 390 W ⁄ m °C = 590 W ⁄ m °C Length of rod, d = 2 m Area of rod, A = 2 cm 2 = 2 × 10 -4 m 2 Temperature difference, T h – T c = 30 °C – 10 °C = 20 °C The formula for heat transfer rate is given as: q = K A (T h – T c ) ⁄ d = (590 × 2 × 10 -4 × 20) ⁄ 2 W = 1.18 W Hence, the total amount of heat transfer is 1.18 W .
Problem 4: The average rate at which energy is conducted outward through the ground surface at a place is 50.0 mW ⁄ m 2 , and the average thermal conductivity of the near-surface rocks is 2.00 W ⁄ m K. Assuming surface temperature of 20.0 °C, find the temperature at a depth of 25.0 km.
Given: Average thermal conductivity, K = 2.00 W ⁄ m K Depth, d = 25.0 km = 2.50 × 10 4 m Surface temperature, T c = 20.0 °C = (20 + 273) K = 293 K Heat transfer rate per unit area, q ⁄ A = 50.0 mW ⁄ m 2 = 50.0 × 10 -3 W ⁄ m 2 The formula for heat transfer rate is given as: q = K A (T h – T c ) ⁄ d Rearrange the above formula in terms of T h . T h = q d ⁄ KA + T c = ((50.0 × 10 -3 × 2.00 × 10 4 ) ⁄ 2.00) + 293 = (500 + 293) K = 893 – 273 K = 520 °C Hence, the temperature at depth of 25.0 km is 520 °C .
Problem 5: The energy lost from a 10 cm thick slab of steel is 50 W. Assuming the temperature difference of 10.0 K, find the area of the slab. (Thermal conductivity of steel = 45 W ⁄ m K).
Given: Thermal conductivity, K = 45 W ⁄ m K Thickness of slab, d = 10 cm = 0.1 m Temperature difference, T h – T c = 10.0 K Energy lost per sec, q = 50 W The formula for heat transfer rate is given as: q = K A (T h – T c ) ⁄ d Rearrange the above formula in terms of A. A = q d ⁄ K (T h – T c ) = (50 × 0.1) ⁄ (45 × 10.0) m 2 = 0.011 m 2 Hence, the area of the slab is 0.011 m 2 .
Problem 6: One face of an aluminium cube of edge 5 meters is maintained at 60 ºC and the other end is maintained at 0 ºC. All other surfaces are covered by adiabatic walls. Find the amount of heat flowing through the cube in 2 seconds. (Thermal conductivity of aluminium is 209 W ⁄ m ºC).
Given: Edge length of cube, d = 5 m Surface area of cube, A = d 2 = (5 m) 2 = 25 m 2 Temperature difference, T h – T c = 60 ºC – 0 ºC = 60 ºC Thermal conductivity, K = 209 W ⁄ m ºC Heat transfer time, t = 2 sec The formula for heat transfer rate is given as: q = K A (T h – T c ) ⁄ d = (209 × 25 × 60) ⁄ 5 J = 62700 J = 62.7 KJ Hence, the amount of heat that flows through the cube is 62.7 KJ .
Problem 7: An aluminium rod and a copper rod of equal length 2.0 m and cross-sectional area 2 cm 2 are welded together in series. One end is kept at a temperature of 10 °C and the other at 30 °C . Calculate the amount of heat taken out per second from the hot end . (Thermal conductivity of aluminium is 200 W ⁄ m °C and of copper is 390 W ⁄ m °C).
Given: Thermal conductivity of aluminium, K Al = 200 W ⁄ m °C Thermal conductivity of aluminium, K Cu = 390 W ⁄ m °C Combined thermal conductivity for parallel combination, 1 ⁄ K = 1 ⁄ 200 W ⁄ m °C + 1 ⁄ 390 W ⁄ m °C K = (200 × 390) ⁄ (200 + 390) W ⁄ m °C = 132.2 W ⁄ m °C Length of rod, d = 2 m Area of rod, A = 2 cm 2 = 2 × 10 -4 m 2 Temperature difference, T h – T c = 30 °C – 10 °C = 20 °C The formula for heat transfer rate is given as: q = K A (T h – T c ) ⁄ d = (132.2 × 2 × 10 -4 × 20) ⁄ 2 W = 0.2644 W Hence, the total amount of heat transfer is 0.2644 W .
Please Login to comment...
Similar reads.
- Physics-Class-11
- School Learning
- School Physics
Improve your Coding Skills with Practice
What kind of Experience do you want to share?

IMAGES
VIDEO
COMMENTS
In each of the following situations, identify the method of heat transfer taking place (conduction, convection, radiation). More than one process may be occurring. 1. Hot coffee is stirred with a spoon, the spoon gets hot due to _____. 2. A chair is placed several feet from a fire in a fireplace. The fireplace has a glass screen. The
In this problem, we're given the mass of the object, the specific heat capacity, and the change in temperature, which allows us to calculate the heat energy. Problem (15): The temperature of a sample of iron with a mass of 10.0 g changed from 50.4°C to 25.0°C with the release of 47 Joules of heat.
Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚C. Every substance has its own specific heat depending on the bonds and forces it has. 1. At the park, why do you tend to steer clear of metal benches and prefer wooden picnic benches? Which has a lower specific heat? 2.
Heat is transferred in three ways: radiation, conduction, and convection. Radiation is the direct transfer of energy. The direct transfer of heat from one substance to another substance that it is touching is called conduction. Conduction works well in some solids, but not as well in fluids (liquids and gases).
engine is made of steel with a specific heat of 0.45 kJ/kg˚C and has a mass of 300 kg. The antifreeze is 50% water/50% ethylene glycol with a specific heat of 3.33 kJ/kg˚C and a density of 1,050 kg/m3. The initial temperature of the antifreeze is 20˚C. Calculate the heat lost by the engine: Q. lost = m. engine. C. p,steel (T. i, engine - T ...
A. Heat transfers from a heat source through a solid. B. Infrared heat waves like in the Electromagnetic spectrum. C. Heated gas or liquid particles rise. Label each example with the appropriate type of heat transfer: radiation, convection, or conduction.
Heat transfer and thermal equilibrium worksheets are essential resources for teachers who aim to provide their students with a comprehensive understanding of these fundamental concepts in Science and Chemistry. These worksheets offer a variety of engaging activities, such as labeling diagrams, solving problems, and analyzing real-world ...
The thickness of each wall of the cooler is 15 mm, with a side length of 1 m. If it is 40°C outside, how long will 2 kg of ice last in the cooler? Assume that during the melting process, the temperature inside the cooler remains at 0°C and that no heat enters from the bottom of the cooler. Note that the latent heat of fusion for water is 334 ...
14.6: Convection. 20. One way to make a fireplace more energy efficient is to have an external air supply for the combustion of its fuel. Another is to have room air circulate around the outside of the fire box and back into the room. Detail the methods of heat transfer involved in each. 21.
1 PRACTICE PROBLEM. A food container has a total area of 0.242 m2. A warm liquid at 42°C is placed in the container. The temperature of the outer wall after thermal equilibrium has been established is found to be 35°C. The container has a thickness of 2.4 mm, and it loses heat to the surroundings at a rate of 191 W.
A practical approximation for the relationship between heat transfer and temperature change is: Q = mcΔT, where Q is the symbol for heat transfer ("quantity of heat"), m is the mass of the substance, and ΔT is the change in temperature. The symbol c stands for the specific heat (also called " specific heat capacity ") and depends on ...
Heat transfer in solids happen by. Heat transfer in liquids happen by. Report a problem. Loading... Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
Worksheet. Introduction to Units. 23m. Unit Conversions. 18m. Solving Density Problems. 13m. ... Conceptual Problems with Position-Time Graphs. 22m. Velocity-Time Graphs & Acceleration. 5m. Calculating Displacement from Velocity-Time Graphs. 15m. ... Heat Transfer L1 p4 - Conduction Rate Equation - Fourier's Law. Ron Hugo. 154.
Heat Transfer/ Specific Heat Problems Worksheet Solving For Heat (q) 1. How many joules of heat are required to raise the temperature of 550 g of water from 12.0 oC to 18.0 oC? 2. How much heat is lost when a 64 g piece of copper cools from 375 oC, to 26 oC? (The specific heat of copper is 0.38452 J/g x oC). Place your answer in kJ. 3.
rmgallagher. A small review on Unit 5 Target 3: I can describe the direction of heat flow and relate it to experimental design and analysis & 4: I can explain the concept of specific heat and solve associated problems. Make sure to go over the sections in your packet and look over the labs. The temprature change of a substance depends on 3 things:
where m is the mass of the substance and ΔT is the change in its temperature, in units of Celsius or Kelvin.The symbol c stands for specific heat, and depends on the material and phase.The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºC. The specific heat c is a property of the substance; its SI unit is J/(kg ⋅ ⋅ K) or J/(kg ⋅ ⋅ ...
PROBLEM 8.5.1.6 8.5.1. 6. When 50.0 g of 0.200 M NaCl ( aq) at 24.1 °C is added to 100.0 g of 0.100 M AgNO 3 ( aq) at 24.1 °C in a calorimeter, the temperature increases to 25.2 °C as AgCl ( s) forms. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat in joules produced. Answer.
The three types of heat transfer differ according to the nature of the medium that transmits heat: Conduction requires contact. Convection requires fluid flow. Radiation does not require any medium. Conduction is heat transfer directly between neighboring atoms or molecules. Usually, it is heat transfer through a solid.
The blood plays an important role in removing heat from the body by bringing this energy directly to the surface where it can radiate away. Nevertheless, this heat must still travel through the skin before it can radiate away. Assume that the blood is brought to the bottom layer of skin at 37.0°C and that the outer surface of the skin is at 30 ...
Now, with expert-verified solutions from Heat Transfer 10th Edition, you'll learn how to solve your toughest homework problems. Our resource for Heat Transfer includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can ...
For example. Heating of milk in a pan. Q3: Name the general mode of heat transfer in. a) Solids. b) Liquids and gases. Answer: (a) The general mode of heat transfer in solids is conduction. (b) The general mode of heat transfer in liquids and gases is convection. Q4: State Fourier's law of conduction.
In the previous post, we talked about heat capacity, specific heat, and the formula for the relationship between these and the change in temperature.. Below are some additional practice examples of heat capacity problems. When solving a problem related to heat capacity and heat transfer, remember that most of the time, it is assumed the heat is not lost, and it only flows from the object with ...
Hence, the thermal conductivity of stone is 3.7 × 10-3 cal ⁄ cm s °C. Problem 2: A metal rod 0.4 m long & 0.04 m in diameter has one end at 373 K & another end at 273 K. Calculate the total amount of heat conducted in 1 minute. (Given K = 385 J ⁄ m s °C) Solution: Given: Thermal conductivity, K = 385 J ⁄ m s °C.