Cannabis (Marijuana) Research Report Is marijuana safe and effective as medicine?
The potential medicinal properties of marijuana and its components have been the subject of research and heated debate for decades. THC itself has proven medical benefits in particular formulations. The U.S. Food and Drug Administration (FDA) has approved THC-based medications, dronabinol (Marinol ® ) and nabilone (Cesamet ® ), prescribed in pill form for the treatment of nausea in patients undergoing cancer chemotherapy and to stimulate appetite in patients with wasting syndrome due to AIDS.
In addition, several other marijuana-based medications have been approved or are undergoing clinical trials. Nabiximols (Sativex ® ), a mouth spray that is currently available in the United Kingdom, Canada, and several European countries for treating the spasticity and neuropathic pain that may accompany multiple sclerosis, combines THC with another chemical found in marijuana called cannabidiol (CBD).
The FDA also approved a CBD-based liquid medication called Epidiolex ® for the treatment of two forms of severe childhood epilepsy, Dravet syndrome and Lennox-Gastaut syndrome. It’s being delivered to patients in a reliable dosage form and through a reproducible route of delivery to ensure that patients derive the anticipated benefits. CBD does not have the rewarding properties of THC.
Researchers generally consider medications like these, which use purified chemicals derived from or based on those in the marijuana plant, to be more promising therapeutically than use of the whole marijuana plant or its crude extracts. Development of drugs from botanicals such as the marijuana plant poses numerous challenges. Botanicals may contain hundreds of unknown, active chemicals, and it can be difficult to develop a product with accurate and consistent doses of these chemicals. Use of marijuana as medicine also poses other problems such as the adverse health effects of smoking and THC-induced cognitive impairment. Nevertheless, a growing number of states have legalized dispensing of marijuana or its extracts to people with a range of medical conditions.
An additional concern with "medical marijuana" is that little is known about the long-term impact of its use by people with health- and/or age-related vulnerabilities—such as older adults or people with cancer, AIDS, cardiovascular disease, multiple sclerosis, or other neurodegenerative diseases. Further research will be needed to determine whether people whose health has been compromised by disease or its treatment (e.g., chemotherapy) are at greater risk for adverse health outcomes from marijuana use.

Medical Marijuana Laws and Prescription Opioid Use Outcomes
A 2019 analysis, also funded by NIDA, re-examined this relationship using data through 2017. Similar to the findings reported previously, this research team found that opioid overdose mortality rates between 1999-2010 in states allowing medical marijuana use were 21% lower than expected. When the analysis was extended through 2017, however, they found that the trend reversed, such that states with medical cannabis laws experienced an overdose death rate 22.7% higher than expected. 79 The investigators uncovered no evidence that either broader cannabis laws (those allowing recreational use) or more restrictive laws (those only permitting the use of marijuana with low tetrahydrocannabinol concentrations) were associated with changes in opioid overdose mortality rates.
These data, therefore, do not support the interpretation that access to cannabis reduces opioid overdose. Indeed, the authors note that neither study provides evidence of a causal relationship between marijuana access and opioid overdose deaths. Rather, they suggest that the associations are likely due to factors the researchers did not measure, and they caution against drawing conclusions on an individual level from ecological (population-level) data. Research is still needed on the potential medical benefits of cannabis or cannabinoids.
ORIGINAL RESEARCH article
Cannabis for medical use: analysis of recent clinical trials in view of current legislation.

- Department of Drug Science and Technology, University of Turin, Turin, Italy
Cannabis has long been regarded as a recreational substance in the Western world. The recent marketing authorization of some medicinal products of industrial origin and the introduction onto the market of inflorescences for medical use mean that medical doctors can now prescribe Cannabis -based medicines in those countries which allow it. Nevertheless, there is still considerable controversy on this topic in the scientific community. In particular, this controversy concerns: the plant species to be used; the pathologies that can be treated and consequently the efficacy and safety of use; the routes of administration; the methods of preparation; the type and dosage of cannabinoids to be used; and, the active molecules of interest. As such, although medical Cannabis has been historically used, the results of currently completed and internationally published studies are inconclusive and often discordant. In light of these considerations, the aim of this work is to analyse the current legislation in countries that allow the use of medical Cannabis , in relation to the impact that this legislation has had on clinical trials. First of all, a literature search has been performed (PubMed and SciFinder) on clinical trials which involved the administration of Cannabis for medical use over the last 3 years. Of the numerous studies extrapolated from the literature, only about 43 reported data on clinical trials on medical Cannabis , with these mainly being performed in Australia, Brazil, Canada, Denmark, Germany, Israel, Netherlands, Switzerland, the United Kingdom and the United States of America. Once the reference countries were identified, an evaluation of the legislation in relation to Cannabis for medical use in each was carried out via the consultation of the pertinent scientific literature, but also of official government documentation and that of local regulatory authorities. This analysis provided us with an overview of the different legislation in these countries and, consequently, allowed us to analyse, with greater awareness, the results of the clinical trials published in the last 3 years in order to obtain general interest indications in the prosecution of scientific research in this area.
1 Introduction
Cannabis was widely used in the past for its curative properties. The earliest records of its medicinal use date back to China where Cannabis has been cultivated for millennia for use as a fiber, food, and medicine. Over time, it spread to the whole of Asia, the Middle East, and Africa. In the West, the plant started to attract scientific interest only in the 20th century. However, in the last century, the cultivation, sale, and use of Cannabis was made illegal in the majority of countries ( Lafaye, et al., 2017 ; Pisanti and Bifulco, 2019 ; Romano and Hazekamp, 2019 ; Arias, et al., 2021 ).
In the last few decades, there has been revived support for its decriminalisation, and legalisation for medical uses thanks to new and scientifically founded indications of its potential therapeutic value. This is partly due to the support gained in the media, and to the high expectations for its efficacy, even though these hopes, for many diseases, are not sufficiently supported by scientific research ( Hill, 2015 ; Whiting, et al., 2015 ).
The phytocomplex of Cannabis plants is made up of more than 500 molecules, of which about a hundred belong to the Cannabinoid chemical class. Among these molecules, even small variations in molecular structure can produce significantly different effects. The molecules of greatest interest to pharmacologists are the decarboxylated forms of 9-tetrahydracannabinol (THC) and cannabidiol since these are easily absorbed in the intestine ( Grotenhermen, 2003 ; Gould, 2015 ; Baratta, et al., 2019 ; Baratta, et al., 2021 ).
Recently, Cannabis based industrial medicines have been approved for sale, and medical use inflorescences have been made available. This has given medical doctors, in those countries which allow it, the option to prescribe Cannabis -based products. At present, the most widely available products are: Marinol ® (AbbVie Inc) and Syndros ® (Benuvia Therapeutics) which contain dronabinol, an isomer of delta-9-tetrahydrocannabinol; Cesamet ® based on nabilone (Meda Pharmaceuticals Inc.), another synthetic cannabinoid; Sativex ® (GW Pharma Ltd.), based on an ethanol extraction of Cannabis sativa ; and Epidiolex ® 1 (Greenwich Biosciences), which contains CBD ( Casiraghi, et al., 2018 ).
A variety of pharmaceutical-grade inflorescence products are also available on the market. Usually, the label only indicates the concentrations of THC and CBD. This is a critical point as the phytocomplex of medical Cannabis contains many active molecules which contribute to the “Entourage effect,” a hypothesis postulating a positive synergic action between cannabinoids and terpenes ( Stella, et al., 2021 ; Baratta, et al., 2022 ).
Given the increasing availability of the above products, many countries have introduced specific legislation, regulations, and guidelines regarding the use of medical use Cannabis in the treatment of various pathologies. Nevertheless, debate continues around this subject within the scientific community. The main points of contention are the correct plant varieties to be used, the pathologies to be treated, and, consequently, the efficacy and safety of their use. There are no universally shared indications on the optimum administration route, the preparation methodology, the definitive types of cannabinoids and dosages to recommend, or even the identity of the active molecule of interest. This controversy stems in large part from the findings of the clinical trials conducted till now. Although the number of studies and publications is growing rapidly, for many diseases the results are often contradictory or inconclusive. All too often, these trials were performed on a non-homogeneous population, and utilising diverse plant material, extraction methods, dosages, pharmaceutical forms, and administration routes. Moreover, the trials were often conducted without a control group ( Stella, et al., 2021 ).
In light of all these considerations, the objective of this work is to analyse the current legislation and regulations in a number of countries where medical use Cannabis is permitted in order to evaluate any relationship of these on the design of clinical trials carried out there.
2 Materials and Methods
We carried out a literature search (PubMed and SciFinder) for clinical trials with medical Cannabis published in the last 3 years (2019/01/01–2021/12/15). We excluded literature reviews, non-clinical trials, and articles about non-medical use Cannabis . We also considered published articles about clinical trial protocols to be carried out. The key search terms used were clinical trials, medical Cannabis , and medical use.
After the publications had been selected, the countries of origin were identified in order to perform an evaluation of the current regulations in each regarding medical Cannabis . The scientific literature, and relevant official publications from government and local authorities were consulted for this analysis.
Finally, the characteristics and the results of the clinical studies were analysed to evaluate any possible link to the state legislation where the studies had been carried out.
Of the 400 matches from the literature search, only 10% (43) of the publications reported data from trials or clinical protocols regarding medical Cannabis . The relevant trials were carried out in: Australia, Brazil, Canada, Denmark, Germany, Israel, Netherlands, Switzerland, the United Kingdom, and the United States of America. Given their geographical distribution, these countries can be considered of interest despite the small number of studies available.
For each of the countries in question, the current legislation on medical Cannabis was analysed, and some specific features are reported such as: prescription procedure, indicated pathologies for medical Cannabis , products available for sale, dispensation forms, authorisation to grow Cannabis for medical use, and reimbursement procedure.
3.1 Current Legislation
3.1.1 australia.
Although there are some regulatory differences among the federal states regarding the importation of products, and the qualification required to write a prescription, medical Cannabis may be prescribed after receiving authorisation from the Therapeutic Goods Administration, through the Special Access Scheme for an individual patient, or through the Authorized Prescriber Scheme for a group of patients with the same condition. Products of industrial origin are exempt from these schemes as approval for sale has already been granted (Sativex ® and Epidiolex ® ).
As well as Sativex ® and Epidiolex ® , indicated for the treatment of spasticity in multiple sclerosis and paediatric epilepsy, herbal- Cannabis based products may also be prescribed. The most common conditions are spasticity in multiple sclerosis, nausea or vomiting caused by anti-tumoral chemotherapy, pain or anxiety in patients with terminal diseases, and refractory child epilepsy. The physician may in any case write a prescription for pathologies other than those indicated.
Pharmacies are authorised to dispense medical Cannabis -based products.
The cost of the therapy is not subsidised by the government.
Alcohol and Drug Foundation, 2021 ; Australian Capital Territory Government, 2021 ; Australian Government, 2017a ; Australian Government, 2017b ; Australian Government, 2018 ; Australian Government, 2020 ; Australian Government, 2021 ; Australian Institute of Health and Welfare, 2019 ; Castle, et al., 2019 ; Centre for Medicinal Cannabis Research and Innovation, 2021 ; Health Direct, 2019 ; Mersiades, et al., 2019 ; The Health Products Regulatory Authority, 2017 ; The Office of Drug Control, 2021 )
3.1.2 Brazil
Various products of industrial origin are available such as Epidiolex ® and Sativex ® , and the importation of Cannabis -derived products is generally authorised. However, the importation of the raw plant or parts of the plant is not permitted. Products with a concentration of THC greater than 0.2% may only be prescribed when no alternative therapy is available, and the patient has reached the irreversible or terminal stage of their disease. Prescription is under the responsibility of the prescribing medical doctor. The medication may be taken either orally or by inhalation.
The cost of the treatment is generally high and is completely at the patient’s expense.
The dispensation may take place in a pharmacy, where Cannabis may not be processed, however.
( Crippa, et al., 2018 ; Marketrealist, 2019 ; Ministério da Saúde, 2019 ; Reuters, 2019 ; Brazilian Government, 2021 )
3.1.3 Canada
The situation in Canada is quite different, medical Cannabis (with the exception of approved industrial products) is not considered as a medicine; hence, it is not dispensed in pharmacies. Medical doctors or nurses may prescribe it for individual patients. The patient can then acquire it from a licensed vendor; grow a quantity sufficient for personal use in residence after registering with the Ministry for Health; nominate a grower in their place (a grower can only cultivate for two people); or acquire it from a provincial or area level licensed retailer. The patient is allowed to prepare Cannabis -based products, but the use of organic solvents such as butane, benzene, methyl-chloride, or chlorinated hydrocarbons is forbidden.
Regarding industrial products, Sativex ® is available for sale; it is indicated for the treatment of spasticity in multiple sclerosis. Other recommended uses include additional pain relief for neuropathic pain in adult patients with multiple sclerosis, and additional pain relief for patients with late-stage cancer who experience moderate to serious pain when already undergoing palliative care with the highest tolerable dosages of opioids. Nabilone is approved for treatment of serious nausea and vomiting associated with chemotherapy, while dronabinol is approved for the treatment of AIDS-related anorexia, and for serious nausea and vomiting associated with chemotherapy. Dronabinol was withdrawn for the Canadian market by the producer in February 2012, but not for health risks.
Generally, Cannabis may be used for any symptom without demonstrating the inefficacy of the previous therapies.
The approved industrial products may be reimbursed by health insurance companies, while all the others are non-reimbursable.
( Fischer, et al., 2015 ; Ablin, et al., 2016 ; Health Canada, 2016 ; The Health Products Regulatory Authority, 2017 ; Abuhasira, et al., 2018 ; Conseil fédéral, 2018 ; Government of Canada, 2019 ; Health Canada, 2022 )
3.1.4 Denmark
All medical doctors are authorised to prescribe Cannabis -based products as part of a 4 years pilot project launched in January 2018. As part of this project, a medical doctor may prescribe medicines that are not approved for distribution or sale in Denmark. However, the medical doctor must take full responsibility for the products they prescribe and must determine the proper dosage for each patient. Medical doctors may refer to the guidelines laid out by the Danish Medicines Agency. The imported plant products available for prescription may vary in content, but they must comply with strict standards and regulations governing the cultivation of the plant species, and the production and standardisation of the Cannabis -based product.
Herbal Cannabis is available by prescription only in pharmacies, which may also prepare magistral preparations.
Regarding industrial products, neurologists may prescribe Sativex ® to treat spasticity from multiple sclerosis. In general, medical doctors may prescribe imported Cannabis -derived medicines that have not been approved for sale in Denmark, such as Marinol ® and Cesamet ® on compassionate grounds, but only if the request is approved by the Danish Medicines Agency.
In general, the Danish Medicines Agency indicates that medical Cannabis be considered as a therapy only for the following conditions: painful spasticity in multiple sclerosis, painful spasticity caused by spinal cord damage, chemotherapy-induced nausea, and neuropathic pain. As part of the pilot project, Cannabis may, however, be prescribed to any patient even outside of the guidelines. The use of Cannabis is not recommended for patients under 18 years of age.
The prices of the prescribed products within the pilot project are set freely by the manufacturers. It is possible to obtain a reimbursement as of 01/01/2019 (retroactive for 2018). Patients in the terminal stages of a disease are fully reimbursed, while patients with other illnesses receive a 50% reimbursement, up to annual maximum of 10,000 Danish Krone. The reimbursement is automatically deducted at the time of the purchase in a pharmacy.
For prescriptions that are not part of the pilot project, the medical doctor may request a reimbursement for an individual patient from the Danish Medicines Agency. It will consider the request for those patients with pathologies where Cannabis -based treatment appears to be effective, and for those whom all other treatments with approved medicines have been used without effect.
( The Health Products Regulatory Authority, 2017 ; Abuhasira, et al., 2018 ; Krcevski-Skvarc, et al., 2018 ; Danish Medicines Agency, 2020 ; Gustavsen, et al., 2021 )
3.1.5 Germany
Medical doctors may prescribe medical Cannabis using a specific “narcotics” prescription form. The prescription may be for any condition that has no standard treatment, or the standard treatment cannot be used owing to reactions, or based on the patient’s specific condition. Among the industrial products available is Sativex ® , which is indicated for spasticity in refractory multiple sclerosis. In addition, it is possible to prescribe dronabinol without particular restrictions regarding its indicated use. Nabilone is approved for nausea and vomiting associated with chemotherapy and unresponsive to conventional therapies. Finally, Epidiolex ® and many types of Cannabis inflorescences may also be prescribed. Magisterial preparations may be prescribed, and pharmacies may dispense extracts of Cannabis and inflorescences.
In the past, Cannabis could also be theoretically grown in residence by private individuals if conventional therapies had been inefficacious, no other alternative treatments were available, and/or to reduce the cost of therapy. Actually, this possibility has never been really applied. Since 2019, however, a system of checks on the production and supply of Cannabis has been introduced by the government.
The patients may request a reimbursement from health insurance companies. For this purpose the prescribing medical doctor has the task of certifying the seriousness of the disease, that the standard therapies have been ineffective, or cannot be used due to the patient’s specific condition, or that there is a reasonable likelihood that medical Cannabis will be effective for that subject.
( Grotenhermen and Müller-Vahl, 2012 ; Ablin, et al., 2016 ; The Health Products Regulatory Authority, 2017 ; Abuhasira, et al., 2018 ; Conseil fédéral, 2018 ; Federal Institute for Drugs and Medical Devices, 2018 ; Krcevski-Skvarc, et al., 2018 ; Rasche, et al., 2019 ; Federal Institute for Drugs and Medical Devices, 2022a ; Federal Institute for Drugs and Medical Devices, 2022b ; Federal Institute for Drugs and Medical Devices, 2022c ; Federal Institute for Drugs and Medical Devices, 2022d ; German Institute for Medical Cannabis , 2022 )
3.1.6 Israel
In Israel, patients with a prescription may use a licensed pharmacy to obtain medical Cannabis . There is a list of conditions for which Cannabis may be used, but the medical doctor may also prescribe it for other pathologies: in any case, it may only be used when other therapies have proved ineffective. The list includes neuropathic pain, serious cachexia in AIDS patients, spasticity from multiple sclerosis, pain associated with Parkinson’s disease, Tourette’s syndrome, treatment of metastatic cancer or chemotherapy-induced symptoms, inflammatory intestinal diseases and post-traumatic stress disorders.
In general, the products available are Cannabis inflorescences, Sativex ® and Epidiolex ® . The number of medical Cannabis patients among the Israeli population is one of the highest in the world (on February 2022 about 100,000 Israelis -about 1% of the population-were allowed to consume medical Cannabis ).
Sativex ® is recommended for spasticity from multiple sclerosis unresponsive to other treatments, or as an additional analgesic therapy in adult patients with advanced stage cancer with moderate to severe pain despite being administered the highest tolerable dosage of opioids; Epidiolex ® is used to treat convulsions in Dravet syndrome, and Lennox-Gastaut syndrome.
As for herbal Cannabis , a government-run programme produces and distributes this product. Medical Cannabis is supplied in two forms: as an oil extract for oral administration or sub-lingual deposition, and as the inflorescence which may be smoked or inhaled with vaporisers. The cost of the therapy is reimbursed in part by some private and state health insurance schemes.
( abcNEWS, 2022 ; Ablin, et al., 2016 ; Abuhasira, et al., 2018 ; Krcevski-Skvarc, et al., 2018 ; State of Israel - Minister of Health, 2017 ; State of Israel - Minister of Health, 2022 ; The Health Products Regulatory Authority, 2017 )
3.1.7 Netherlands
In Netherlands, all medical doctors may prescribe medical Cannabis . The pharmacies may also produce extracts using the plant material produced by the Office of Medical Cannabis . These are usually oil extracts to be taken orally or deposited under the tongue. Some types of inflorescences are available for this purpose: the concentration of the active molecules and granulation properties may vary. The inflorescences may also be taken in the decoction form or inhaled through vaporisers.
Sativex ® is approved for the treatment of spasticity from multiple sclerosis refractory to conventional therapies.
Cannabis is indicated for the treatment of pain (multiple sclerosis, or spinal cord injuries), chronic pain, nausea and vomiting (in chemotherapy or radiotherapy, HIV therapies, adverse reactions to hepatitis C medication), palliative care for cancer or AIDS (to increase appetite and alleviate pain, nausea and weight loss), Tourette’s syndrome, and refractory glaucoma, epilepsy and epileptic syndromes (even in children). In addition, its use is indicated in the reduction in symptomology of the following pathologies: Crohn’s disease, ulcerative colitis, itching, migraine, rheumatic conditions, ADHD, post-traumatic stress disorders, agitation in Alzheimer’s disease and cerebral trauma. Medical doctors are in any case authorised to prescribe these therapies for other conditions if they consider it fit. Cannabis -based products must, however, be considered only in cases where authorised medicines have inefficacious or provoked unacceptable adverse reactions.
As concerns the available herbal Cannabis species, Bediol ® (THC 6.3%; CBD 8%) is usually recommended as the first-choice therapy to alleviate pain or as an anti-inflammatory therapy. Bedrocan ® (THC 22%; CBD <1.0%), Bedica ® (THC 14%; CBD <1.0%) and Bedrobinol ® (THC 13.5%; CBD <1.0%) are considered more effective for the treatment of symptoms such as appetite loss, weight loss, nausea, vomiting, anorexia, cachexia, emesis, Tourette’s syndrome, and glaucoma. Bedrolite ® (THC <1.0%; CBD 7.5%) is employed for certain forms of epilepsy.
The healthcare system does not reimburse the cost of Cannabis -based medicines. In some cases, the patient may be able to claim from private insurance schemes.
( The Health Products Regulatory Authority, 2017 ; Abuhasira, et al., 2018 ; Conseil fédéral, 2018 ; Krcevski-Skvarc, et al., 2018 ; Bedrocan, 2021 ; Office of Medicinal Cannabis, 2022 )
3.1.8 Switzerland
The prescription and use of Cannabis -based magistral preparations is authorised for spasticity (multiple sclerosis), chronic pain, appetite loss in AIDS, and nausea, pain, and appetite loss from cancer.
The magistral preparations are prepared in a pharmacy.
Medical doctors may prescribe Cannabis -based medicines only after receiving authorisation from the Federal office of the Public Health System.
The cost of the therapy is not reimbursed systematically, but on a case-by-case basis.
As well as the inflorescence, it is possible to use dronabinol and Epidiolex ® . Sativex ® is also authorised for use and available for treatment of spasticity from multiple sclerosis.
( Abuhasira, et al., 2018 ; Krcevski-Skvarc, et al., 2018 ; Swiss Confederation, Federal Office of Public Health, 2020 ; Swiss Confederation, Federal Office of Public Health, 2021a ; Swiss Confederation, Federal Office of Public Health, 2021b ; Swiss Confederation, Federal Office of Public Health, 2021c )
3.1.9 United Kingdom
In the United Kingdom, medical Cannabis is generally prescribed to adults and children with rare and serious forms of epilepsy, adults suffering from nausea or vomiting from chemotherapy, and adults with muscular stiffness or spasms from multiple sclerosis. This therapy is considered only in cases in which no alternative treatment is available, or other treatments have been inefficacious. The available products are Epidiolex ® , prescribed to patients with Lennox-Gastaut syndrome or Dravet syndrome; nabilone, which is authorised for nausea and vomiting associated with chemotherapy; dronabinol is also available, but it has no marketing authorization; and Sativex ® , which is prescribed for muscular spasms in multiple sclerosis unresponsive to other treatments (even though it is discouraged by NICE in that it is not cost-effective).
The medical Cannabis therapy cannot be obtained from a general practitioner but must be prescribed by a hospital specialist registered with the General Medical Council. The medical doctor may collect data on adverse reactions, which can also be signalled directly by the patient through a yellow card system.
( Department of Health and Social Care, 2018 ; Medicines and healthcare products Regulatory Agency, 2020 ; MS Society, 2021 ; National Health Service, 2021 ; General Medical Council, 2022 ; National Health Service, 2022 ; UK Government, 2022 )
3.1.10 United States of America
There are significant legislative differences among the states concerning Cannabis in the United States. In some states the legislation in force is extremely limiting, in others significantly less restrictive. Therefore, the state laws may not be completely harmonised with federal laws.
Regarding industrial products, the FDA has approved the prescription of dronabinol and nabilone for the treatment of chemotherapy-induced nausea and vomiting. Dronabinol may also be used for the treatment of appetite and weight loss in HIV patients. Epidiolex ® may be prescribed for the treatment of epileptic disorders, Lennox-Gastaut syndrome and Dravet’s syndrome.
Concerning herbal Cannabis , only 36 states have legalised or decriminalised its use. In general, in those states which have authorised the use of medical use Cannabis , there are restrictions on its prescription. Depending to the local laws, therefore, Cannabis may be prescribed for pain, anxiety, epilepsy, glaucoma, appetite and weight loss associated with AIDS, inflammatory intestinal disturbances irritable intestine syndrome, motor disturbances due to Tourette’s syndrome or multiple sclerosis, nausea and vomiting caused by chemotherapy, sleep disorders, posttraumatic stress disorders. Some states allow the addition, at the prescribing medical doctor’s discretion, of pathologies other than those expressly stated.
Generally, medical doctors do not need specific training to prescribe Cannabis , but in many states, it is necessary to register before doing so. In other states, medical doctors must attend a short training course to be able to register. In some states, it is enough that the medical doctor gives advice verbally to take medical Cannabis , or its use may be recommended by a health care professional who is not a medical doctor. On the other hand, in some states, it is necessary that two medical doctors confirm the need for a Cannabis -based treatment for a patient. Depending on the state, Cannabis may be supplied to the patient by licensed dispensaries, or it may be grown at home by the patient or by a caregiver.
Smoking medical Cannabis is prohibited in some states. Similarly even the edible forms are prohibited in some states. Generally, the administration is performed orally or by vaporiser.
Patients are generally registered so that the possession and use of medical Cannabis is not prosecuted.
Abuhasira, et al., 2018 ; Alharbi, 2020 ; Carliner, et al., 2017 ; Choo and Emery, 2017 ; Corroon and Kight, 2018 ; Johnson, et al., 2021 ; Mead, 2017 ; National Conferences of State Legislatures, 2022 ; ProCon, 2022 ; Ryan, et al., 2021 ; The Health Products Regulatory Authority, 2017 )
3.2 Study Protocols and Clinical Trials
There are 43 publications of proposed, or executed, clinical trial protocols in those countries whose legislation has been analysed; eight of these regarded proposed clinical trial protocols.
Hence, 35 publications regarded actual clinical trial data. These were sub-divided into three groups: the first, “positive outcome,” included those studies which demonstrated the efficacy of the preparation administered, or that the actual results were in line with those expected (18). The second group, “negative outcome,” included those studies where the authors reported that the administered product was no more efficacious than the placebo (5). Finally, the third group, “inconclusive outcome,” comprised those studies where the results were not conclusive (12).
The characteristics of the taken into account clinical studies are summarized in Table 1 .
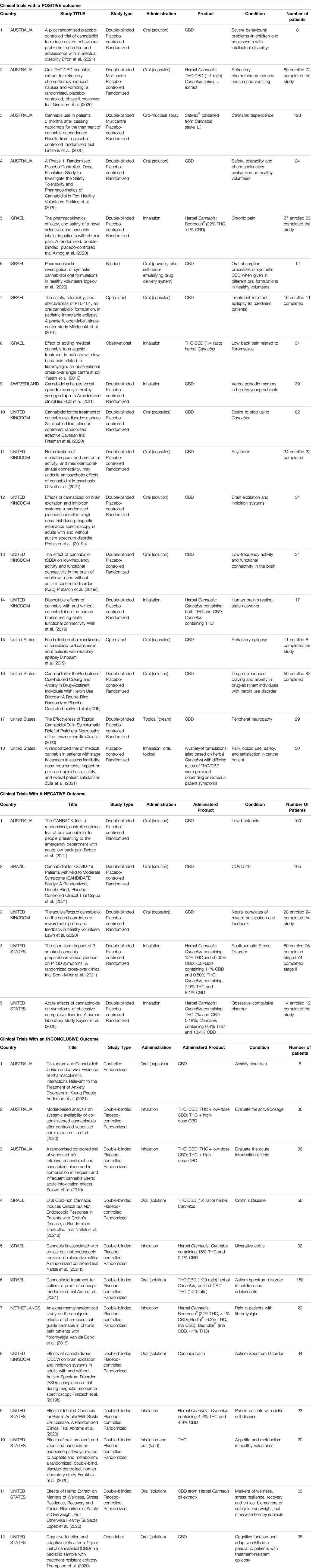
TABLE 1 . Characteristics of the selected clinical trials.
3.2.1 Clinical Trials With a Positive Outcome
Of the 18 studies in this category, 4 were conducted in Australia, 4 in Israel, 1 in Switzerland, 5 in the United Kingdom, and 4 in the United States.
Regarding the study design, 2 were multi-centred, 13 used the double-blind method, 14 had a randomised control design, and 14 used a placebo control group.
The sample size varied greatly, from a minimum of 8 to a maximum of 128 enrolled subjects.
As for the products used in the trials, 12 studies administer CBD, 6 studied herbal Cannabis derivatives.
CBD was administered orally in 10 cases, topically and by inhalation in only one study. The herbal Cannabis derivatives were administered by inhalation in 3 cases, and by the oral route in 2 cases. One study considered products to be administered orally, by inhalation or topically.
In 9 studies, the Cannabis derivatives were administered in addition to a standard therapy.
The most commonly studied conditions were behaviour, cerebral activity, and memory (6), pain (4), addiction or abstinence to drugs (3), epilepsy (2), pharmacokinetic studies, safety, and tolerability (2), and nausea and vomiting (1). Two studies were carried out on a paediatric population.
In general, the studies involving the administration of CBD regarded epilepsy, addiction or abstinence to drugs, behaviour, cerebral activity and memory, peripheral neuropathy, pharmacokinetic studies, and safety and tolerability.
Instead, studies administering herbal Cannabis derivatives focused mainly about pain and then about nausea and vomiting, cerebral activity and Cannabis dependence. In most cases both THC and CBD were administered in different ratios. In some cases, a herbal Cannabis strain was used with a high concentration of THC.
( Almog, et al., 2020 ; Birnbaum, et al., 2019 ; Efron, et al., 2021 ; Freeman, et al., 2020 ; Grimison, et al., 2020 ; Hotz, et al., 2021 ; Hurd, et al., 2019 ; Izgelov, et al., 2020 ; Lintzeris, et al., 2020 ; Mitelpunkt, et al., 2019 ; O'Neill, et al., 2021 ; Perkins, et al., 2020 ; Pretzsch, et al., 2019a ; Pretzsch, et al., 2019c ; Wall, et al., 2019 ; Xu, et al., 2020 ; Yassin, et al., 2019 ; Zylla, et al., 2021 )
3.2.2 Clinical Trials With a Negative Outcome
Five trials had a negative outcome. Two of these were conducted in the United States, 1 in Australia, 1 in Brazil and 1 in the United Kingdom.
All of the trials had a randomised control, used a placebo control group, and a double-blind control. The sample size ranged from 14 to 105 enrolled subjects.
As for the products used, 3 studies administered oral preparations containing CBD. 2 studies were based on the administration of inflorescences by inhalation. 4 studies out of 5 administered the product in addition to a standard therapy.
The conditions studied in these trials with CBD were pain, COVID-19 infection, and the effects on neural correlates of reward anticipation and feedback. Herbal Cannabis , in three different forms and different ratios of THC/CBD), was administered to evaluate its efficacy in the treatment of Obsessive-Compulsive Disorder (OCD) and Post-Traumatic Stress Disorder (PTSD).
None of these studies demonstrated that the administered product was more efficacious than the placebo control.
( Kayser, et al., 2020 ; Lawn, et al., 2020 ; Bebee, et al., 2021 ; Bonn-Miller, et al., 2021 ; Crippa, et al., 2021 )
3.2.3 Clinical Trials With an Inconclusive Outcome
12 studies had an inconclusive outcome: 3 were conducted in Australia, 3 in Israel, 1 in the Netherlands, 1 in the United Kingdom and 4 in the United States.
Regarding study design, 10 included a double-blind system, 11 had a randomised control, and 10 utilised a placebo control group. The sample size ranged from a minimum of 6 subjects to a maximum of 150 individuals. Two of the studies were conducted on paediatric subjects.
Concerning the products used, 2 studies administered CBD alone, one study used THC alone, 1 study administered cannabidivarin, 2 studies administered THC and CBD, both alone and in a mixture, 5 studies administered herbal Cannabis derivatives, and 1 study administered both THC and CBD as well as a herbal Cannabis extract.
CBD and cannabidivarin were administered orally; THC, and the mixtures of THC and CBD were administered by inhalation. THC was also administered orally. The herbal Cannabis derivatives were administered by inhalation in 3 studies, while they were for oral use in 2 studies. 1 study used oral administration of a herbal Cannabis extract or an equivalent mixture of THC and CBD.
Six trials predicted that the administration was additional to standard therapy.
The conditions to be studied for the efficacy of CBD were anxiety and cognitive function in patients suffering from epilepsy. THC and/or CBD were administered to evaluate the active dosage or to study its effects on problems linked to appetite and metabolism, herbal Cannabis derivatives were studied to evaluate their activity in Crohn’s disease, ulcerative colitis, pain, haemolytic anaemia, markers of wellness and clinical biomarkers in obese patients. Trials related to autism were conducted with, as well as cannabidivarin, the administration of a herbal Cannabis extract or an equivalent mixture of THC and CBD.
When herbal Cannabis derivatives were administered, the concentration of THC and CBD, and the ratio of the two varied greatly among the trials. Some used products with a high concentration of THC, while others used products with a high concentration of CBD. In 1 trial, different types of inflorescences were administered to evaluate the most efficacious ratio of THC to CBD concentrations against pain.
( Pretzsch, et al., 2019b ; Solowij, et al., 2019 ; Van de Donk, et al., 2019 ; Abrams, et al., 2020 ; Farokhnia, et al., 2020 ; Liu, et al., 2020 ; Lopez, et al., 2020 ; Thompson, et al., 2020 ; Naftali, et al., 2021a ; Anderson, et al., 2021 ; Aran, et al., 2021 ; Naftali, et al., 2021b )
3.2.4 Study Protocols
There are 8 examples of published protocols that have not yet initiated the clinical trial phase. 4 are in Australia, and 1 each in Denmark, Canada, Germany, and Netherlands. The number of enrolled subjects is between 10 and 180 in total. One study will be carried out among the paediatric population.
Concerning the study design, 3 will be multi-centre studies, 7 use a double-blind system, 8 are randomised, and 7 use a placebo control group.
Regarding the products to be used, 4 protocols will use the oral administration of THC and CBD. The ratio between the components in question varies from study to study. In 2 protocols, the administration of CBD is also foreseen. One protocol foresees the administration of both CBD and a preparation containing a high concentration of THC.
For those studies using THC and CBD mixtures, the pathologies to be studied are, pain, dementia, spasms, and the activation of the immune system in HIV patients. Instead, the CBD alone preparations will be administered for behavioural problems and phobias. The herbal- Cannabis derived product will be administered for chronic tic disorder. The protocol that foresees the administration of both CBD and a preparation with a high concentration of THC will focus on the alleviation of pain.
( Costiniuk, et al., 2019 ; Hendricks, et al., 2019 ; Urbi, et al., 2019 ; Van der Flier, et al., 2019 ; Efron, et al., 2020 ; Hardy, et al., 2020 ; Jakubovski, et al., 2020 ; Timler, et al., 2020 )
4 Discussion
From the analysis of the current legislation in states where clinical trials and proposed protocols on medical Cannabis and derived products have been published in the last 3 years, many significant differences have been found regarding the products available, the indicated pathologies for which it may be prescribed, the production of the raw plant material, as well as its reimbursement and prescription. It was evaluated to consider the studies published in the last 3 years supposing that the researchers have benefited from the latest knowledge on medical Cannabis and to make an overview of the pathologies currently under study.
In particular, regarding industrial products, practically every country, with the exception of the United States, has approved the use of Sativex ® . However, Epidiolex ® , dronabinol.Netherlands, and nabilone are also quite common.
In all the countries, the use of herbal Cannabis is also authorised. The only exception is Brazil, which is certainly the country with the most restrictive legislation. Netherlands is the only country to provide directions for use, which are not binding, but quite strict, regarding the plant strain to be used for a determined pathology based on the concentration of active molecules (THC and CBD). Instead, for the other countries, it must be pointed out that the current legislation provides for the use of inflorescences or herbal Cannabis extracts without providing specific directions concerning the recommended concentration of active molecules to treat a determined condition.
Regarding the pathologies or symptoms associated with the more or less well-defined conditions, the most common are pain, nausea, vomiting, spasticity, and epilepsy followed by spasms, and weight and appetite loss. The less frequently indicated conditions in this case include Tourette’s syndrome, PTSD, and glaucoma. In many countries, additional conditions are considered in more or less detail.
In this regard, it is interesting to note that the country with the greatest number of specifically recommended pathologies not indicated in other countries is the Netherlands: perhaps based on the longstanding use of Cannabis both for medical use and recreational purposes. Although the legislation regarding medical Cannabis is quite comprehensive in all the countries considered, some of them, namely Australia, Canada, Denmark, Germany, Israel, Netherlands, and the United States, also permit the prescription of Cannabis for any therapeutic application at the discretion of the medical doctor. However, in Germany, Netherlands and Israel, this is limited to cases in which other therapies have proved ineffective, excessive adverse reactions to standard treatments have occurred, or valid alternative treatments are not available. Instead, in Australia, Canada, Denmark, and the United States, therapeutic strategies different from those specified are authorised regardless of any prior treatment. The prescription of medical Cannabis for any condition certainly does not conform to the procedures generally in force for other medicinal products, and especially products with a psychoactive effect such as those prepared containing THC.
It is interesting to note that in Canada, and in some states in the United States, the medical inflorescences may be grown directly by the patient, and the treatment may be recommended by a health worker, and not only a medical doctor; in the event that the plant species is not home-grown, it is distributed through a licensed dispensary. In Germany, Israel and Netherlands, herbal Cannabis is grown locally under the supervision of a government agency. This is significant if one considers that, in these three countries, the prescription process is highly deregulated regarding the recommended pathologies to be treated with Cannabis , but the same does not apply to its cultivation.
The normal administration routes are oral or by inhalation. Some countries, such as Israel, authorise smoking Cannabis inflorescences as a route of administration, something that is categorically banned in some states of the United States.
In addition, regarding prescription, it is noteworthy that the United Kingdom is the only country where this must be obtained from a hospital specialist. In some states in the United States, on the other hand, the prescribing medical doctor must be registered to prescribe this therapy and have attended a specific training course. In Australia and Switzerland, medical doctors may write the prescription only after receiving authorisation from a specific agency. Therefore, there is a different focus on the prescription process and hence inhomogeneity in this aspect too. The treatment costs are generally borne by the patient, and no reimbursement is foreseen, unless it is from a private health insurance scheme. This certainly restricts access to this kind of therapy to the more privileged members of society.
Concerning the results of the clinical trials, some interesting observations may be made. In the first place, a greater number of studies have been published in certain countries. These countries are the United States (11) and Australia (9), followed by Israel (7) and the United Kingdom (7). In general, the majority of the studies featured randomisation, the use of a double-blind method, and a placebo control group: these are factors which guarantee the quality of the data gathered. On the other hand, the majority of the studies took place with a small sample size. Moreover, the studies made use of a heterogeneous population: healthy and ill volunteers, adults and children, acute and chronically ill patients, and subjects who had previously used or had never used Cannabis prior to the study. Factors that, being so numerous, make it particularly challenging to draw any conclusive evaluations of the results of these trials, and more in general, the real efficacy of medical Cannabis .
Considering only the studies with a positive outcome, it should be noted that the studied pathologies are coherent with those provided for in current legislation i.e., pain, epilepsy, nausea, and vomiting; on the contrary, psychosis, behavioural problems, memory and cerebral activity represent a novelty. Furthermore, there is a net distinction between the products used based on the different conditions to be treated: the trials on pain, nausea and vomiting with positive outcomes administered herbal Cannabis derivatives in which, in 3 cases out of 4, both THC and CBD are present; the other studies with a positive outcome administered CBD alone. In those trials with a negative outcome, CBD was administered for pain, while herbal Cannabis derivatives were used for conditions such as OCD or PTSD. This consideration supports the use of herbal Cannabis in which both THC and CBD are present for pain, even though it should be stressed that the studies with a positive outcome for this pathology had a maximum of 30 enrolled subjects.
The studies with an inconclusive outcome regarded a variegated list of conditions including anxiety, Crohn’s syndrome, ulcerative colitis, pain, and appetite loss. Many of these are already included in some national regulations although the efficacy of Cannabis in these cases according to the currently available data is not satisfactorily demonstrated.
It is evident that the only pathology present in all three study categories is pain, for which 4 studies had a positive outcome, 1 had a negative outcome, and 1 had an inconclusive outcome.
Among the study protocols to be trialled, pain and spasticity appear again, approved by legislation in most countries and the object of numerous studies, as well as a number of less-investigated conditions such as dementia, phobias, tic disorders and the activation of the immune system in HIV patients.
Based on the research conducted, it is, therefore, possible to stress that in spite of the growing number of recent studies on medical Cannabis , many of which have had a positive outcome while many others have had an inconclusive or negative outcome. The presumed broad spectrum action of Cannabis has led to the initiation of many trials and the preparation of many study protocols for a wide range of pathologies with the enrolment of subjects with diverse characteristics from study to study. This means that there is very little data for each pathology or symptomology.
Another important factor is that the products used are very diverse from each other; consequently, a comparison is extremely difficult to make, especially for the herbal products. All of the trials indicate the precise dosages used in terms of active molecules, but when it comes to inflorescences, or extracts derived from them, the concentration is provided only for the THC and CBD content and not for the other active molecules. Furthermore, the diverse administration routes make a comparison based on pharmacokinetics difficult for the molecules of interest.
Therefore, it is difficult to compare the studies and draw conclusions concerning the efficacy of the protocol for the single pathologies. However, for some, substantial evidence is emerging regarding their efficacy and the suitable products to ensure that. From the analysed data, it is clear that the best pain treatment is herbal Cannabis derivatives containing both THC and CBD, just as the best way to treat epilepsy is to administer CBD.
One interesting point is that for some of the pathologies approved for treatment with medical Cannabis under the current legislation, the data do not paint a definitive picture. This is true for conditions such as anxiety, ulcerative colitis, Crohn’s syndrome, and appetite enhancement.
On the other hand, the current legislation often authorises inflorescences or extracts without indicating the exact concentration of the active molecules. In parallel, many studies use different plant strains or study a small number of subjects, making it difficult to compare and consequently interpret the results. Moreover, in many studies, the Cannabis -based medicines were administered in addition to other treatments making any evaluation of their efficacy it even more complex.
5 Conclusion
Medical Cannabis is often considered as if it were a single active component, but, in fact, there are countless possible variations. Hence, it will be some time before the current list of pathologies that each product may be used for can be updated based on definitive clinical data on the efficacy of the various components. Certainly, the development of standardised industrial products will facilitate the execution of more meaningful trials compared to those that involve the administration of inflorescences or derived extracts prepared using a variety of methods and, thus, highly variable in terms of concentration of the active molecules.
The authors want moreover to put in evidence that, despite legislation authorising the use of medical Cannabis and instituting the national production centre for inflorescences more than 5 years ago, Italy is still among the states where clinical trials have not been conducted. This gap is due to legal restrictions on the approval and conduction of clinical trials in this field, and the difficulty in sourcing the raw plant material, of which there is always a shortage. The result of this is therapies using inflorescences and extracts which have never undergone specific clinical trialling.
In the end, the influence of the media, economic interests, and the demands of associations representing patients affected by these diseases and conditions, for whom Cannabis is a panacea, means that in many countries it is currently possible to use medical Cannabis even though the scientific data do not entirely support the signs of efficacy: certainly this is a special case where the consolidated procedures for the administration of any product in the medical field have been either overlooked or ignored. It is time that the regulatory agencies considered whether this is actually safeguarding the health of patients.
The analysis of the current legislation may not be exhaustive in that it refers only to public texts available online.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
FB and PB performed the conceptualization of the work. FB, IP and LE performed the investigation and took care of the data. FB wrote the manuscript. PB coordinated the project. All authors approved the final version of the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Dr Tom O Byrne for the linguistic revision of the text.
Abbreviations
ADHD, Attention-Deficit/Hyperactivity Disorder; AIDS, Acquired ImmunoDeficiency Syndrome; CBD, CannaBiDiol; COVID-19, COronaVIrus Disease 2019; FDA, Food and Drug Administration; HIV, Human Immunodeficiency Virus; NICE, National Institute for health and Care Excellence; OCD, Obsessive-Compulsive Disorder; PTSD, Post-Traumatic Stress Disorder; THC, delta-9-TetraHydroCannabinol; United States, United States of America.
1 Epidiolex ® has received approval in the European Union under the tradename Epidyolex ® .
abc NEWS (2022). Israeli Bigwigs Eye Profits from Cannabis Legalization. Available at: https://abcnews.go.com/Health/wireStory/israeli-bigwigs-eye-profits-cannabis-legalization-82617899 (Accessed April 26th, 2022).
Google Scholar
Ablin, J., Ste-Marie, P. A., Schäfer, M., Häuser, W., and Fitzcharles, M. A. (2016). Medical Use of Cannabis Products: Lessons to Be Learned from Israel and Canada. Schmerz 30 (1), 3–13. doi:10.1007/s00482-015-0083-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Abrams, D. I., Couey, P., Dixit, N., Sagi, V., Hagar, W., Vichinsky, E., et al. (2020). Effect of Inhaled Cannabis for Pain in Adults with Sickle Cell Disease: A Randomized Clinical Trial. JAMA Netw. Open 3 (7), e2010874. doi:10.1001/jamanetworkopen.2020.10874
Abuhasira, R., Shbiro, L., and Landschaft, Y. (2018). Medical Use of Cannabis and Cannabinoids Containing Products - Regulations in Europe and North America. Eur. J. Intern Med. 49, 2–6. doi:10.1016/j.ejim.2018.01.001
Alcohol and Drug Foundation (2021). Medicinal Cannabis Products FAQs. Available at: https://adf.org.au/talking-about-drugs/medicinal-cannabis-products/medicinal-cannabis-products-faqs/ (Accessed February 15th, 2022).
Alharbi, Y. N. (2020). Current Legal Status of Medical Marijuana and Cannabidiol in the United States. Epilepsy Behav. 112, 107452. doi:10.1016/j.yebeh.2020.107452
Almog, S., Aharon-Peretz, J., Vulfsons, S., Ogintz, M., Abalia, H., Lupo, T., et al. (2020). The Pharmacokinetics, Efficacy, and Safety of a Novel Selective-Dose Cannabis Inhaler in Patients with Chronic Pain: A Randomized, Double-Blinded, Placebo-Controlled Trial. Eur. J. Pain 24 (8), 1505–1516. doi:10.1002/ejp.1605
Anderson, L. L., Doohan, P. T., Oldfield, L., Kevin, R. C., Arnold, J. C., Berger, M., et al. (2021). Citalopram and Cannabidiol: In Vitro and In Vivo Evidence of Pharmacokinetic Interactions Relevant to the Treatment of Anxiety Disorders in Young People. J. Clin. Psychopharmacol. 41 (5), 525–533. doi:10.1097/JCP.0000000000001427
Aran, A., Harel, M., Cassuto, H., Polyansky, L., Schnapp, A., Wattad, N., et al. (2021). Cannabinoid Treatment for Autism: a Proof-Of-Concept Randomized Trial. Mol. Autism 12 (1), 6. doi:10.1186/s13229-021-00420-2
Arias, S., Leon, M., Jaimes, D., and Bustos, R.-H. (2021). Clinical Evidence of Magistral Preparations Based on Medicinal Cannabis. Pharmaceuticals 14 (2), 78. doi:10.3390/ph14020078
Australian Capital Territory Government (2021). Patient Information for Medicinal Cannabis. Available at: https://www.health.act.gov.au/sites/default/files/2018-09/Medicinal%20Cannabis%20-%20Patient%20Information_0.pdf (Accessed February 15th, 2022).
Australian Government, Department of Health (2018). Access to Medicinal Cannabis Products: Frequently Asked Questions (FAQs). Available at: https://www.tga.gov.au/access-medicinal-cannabis-products-frequently-asked-questions-faqs (Accessed February 15th, 2022).
Australian Government, Department of Health (2017a). Guidance for the Use of Medicinal Cannabis in Australia: Overview. Available at: https://www.tga.gov.au/publication/guidance-use-medicinal-cannabis-australia-overview (Accessed February 15th, 2022).
Australian Government, Department of Health (2017b). Guidance for the Use of Medicinal Cannabis in Australia: Patient Information. Available at: https://www.tga.gov.au/publication/guidance-use-medicinal-cannabis-australia-patient-information (Accessed February 15th, 2022).
Australian Government, Department of Health (2020). Medicinal Cannabis: Information for Consumers. Available at: https://www.tga.gov.au/medicinal-cannabis-information-consumers (Accessed February 15th, 2022).
Australian Government, Department of Health (2021). Medicinal Cannabis: Information for Health Professionals. Available at: https://www.tga.gov.au/medicinal-cannabis-information-health-professionals (Accessed February 15th, 2022).
Australian Institute of Health and Welfare (2019). National Drug Strategy Household Survey. Available at: https://www.aihw.gov.au/getmedia/108d1761-b523-492b-81cc-a09db6740e85/aihw-phe-270-Chapter6-Medicinal-cannabis.pdf.aspx#:∼:text=Household%20Survey%202019-,Emerging%20topic%3A%20Medicinal%20cannabis,and%20leaves%20of%20the%20plant (Accessed February 15th, 2022).
Baratta, F., Peira, E., Maza, C., Gallarate, M., and Brusa, P. (2022). Cannabis -Based Oral Emulsion for Medical Purposes to Meet the Needs of Patients: Formulation, Quality and Stability. Pharmaceutics 14, 513. doi:10.3390/pharmaceutics14030513
Baratta, F., Simiele, M., Pignata, I., Ravetto Enri, L., Torta, R., De Luca, A., et al. (2019). Development of Standard Operating Protocols for the Optimization of Cannabis -Based Formulations for Medical Purposes. Front. Pharmacol. 10, 701. doi:10.3389/fphar.2019.00701
Baratta, F., Simiele, M., Pignata, I., Ravetto Enri, L., D’Avolio, A., Torta, R., et al. (2021). Cannabis -Based Oral Formulations for Medical Purposes: Preparation, Quality and Stability. Pharmaceuticals 14 (2), 171. doi:10.3390/ph14020171
Bebee, B., Taylor, D. M., Bourke, E., Pollack, K., Foster, L., Ching, M., et al. (2021). The CANBACK Trial: a Randomised, Controlled Clinical Trial of Oral Cannabidiol for People Presenting to the Emergency Department with Acute Low Back Pain. Med. J. Aust. 214 (8), 370–375. doi:10.5694/mja2.51014
Bedrocan (2021). Products. Available at: https://bedrocan.com/it/prodotti/ (Accessed February 15th, 2022).
Birnbaum, A. K., Karanam, A., Marino, S. E., Barkley, C. M., Remmel, R. P., Roslawski, M., et al. (2019). Food Effect on Pharmacokinetics of Cannabidiol Oral Capsules in Adult Patients with Refractory Epilepsy. Epilepsia 60 (8), 1586–1592. doi:10.1111/epi.16093
Bonn-Miller, M. O., Sisley, S., Riggs, P., Yazar-Klosinski, B., Wang, J. B., Loflin, M. J. E., et al. (2021). The Short-Term Impact of 3 Smoked Cannabis Preparations versus Placebo on PTSD Symptoms: A Randomized Cross-Over Clinical Trial. PloS one 16 (3), e0246990. doi:10.1371/journal.pone.0246990
Brazilian Government (2021). Autorização Sanitária de Produtos de Cannabis. Available at: https://www.gov.br/anvisa/pt-br/assuntos/educacaoepesquisa/webinar/medicamentos/arquivos/perguntas-e-respostas-autorizacao-sanitaria-de-produtos-de-cannabis.pdf (Accessed February 15th, 2022).
Carliner, H., Brown, Q. L., Sarvet, A. L., and Hasin, D. S. (2017). Cannabis Use, Attitudes, and Legal Status in the U.S.: A Review. Prev. Med. 104, 13–23. doi:10.1016/j.ypmed.2017.07.008
Casiraghi, A., Roda, G., Casagni, E., Cristina, C., Musazzi, U. M., Franzè, S., et al. (2018). Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 84 (4), 242–249. doi:10.1055/s-0043-123074
Castle, D. J., Strauss, N., Norman, A., and Bonomo, Y. (2019). Medical Marijuana: The Australian Experience. Mo Med. 116 (4), 270–273.
PubMed Abstract | Google Scholar
Centre for Medicinal Cannabis Research and Innovation (2021). Legal Access to Cannabis Based Medicines. Available at: https://www.medicinalcannabis.nsw.gov.au/__data/assets/pdf_file/0020/2864/Legal-Access-To-Cannabis-Based-Medicines-Fact-Sheet-Medical-Practitioners-FINAL.pdf (Accessed February 15th, 2022).
Choo, E. K., and Emery, S. L. (2017). Clearing the Haze: the Complexities and Challenges of Research on State Marijuana Laws. Ann. N. Y. Acad. Sci. 1394 (1), 55–73. doi:10.1111/nyas.13093
Conseil fédéral (2018). Traiter les personnes gravement malades avec du cannabis. Rapport du Conseil fédéral en réponse à la motion Kessler (14.4164). Available at: https://www.aramis.admin.ch/Default?DocumentID=49994&Load=true (Accessed February 15th, 2022).
Corroon, J., and Kight, R. (2018). Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 3 (1), 190–194. doi:10.1089/can.2018.0030
Costiniuk, C. T., Saneei, Z., Routy, J. P., Margolese, S., Mandarino, E., Singer, J., et al. (2019). Oral Cannabinoids in People Living with HIV on Effective Antiretroviral Therapy: CTN PT028-Study Protocol for a Pilot Randomised Trial to Assess Safety, Tolerability and Effect on Immune Activation. BMJ open 9 (1), e024793. doi:10.1136/bmjopen-2018-024793
Crippa, J. A., Guimarães, F. S., Campos, A. C., and Zuardi, A. W. (2018). Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 9, 2009. doi:10.3389/fimmu.2018.02009
Crippa, J. A. S., Pacheco, J. C., Zuardi, A. W., Guimarães, F. S., Campos, A. C., Osório, F. d. L., et al. (2021). Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE Study): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cannabis Cannabinoid Res. Adv. online Publ. [Epub ahead of print] doi:10.1089/can.2021.0093
CrossRef Full Text | Google Scholar
Danish Medicines Agency (2020). Cannabis-containing Products. Available at: https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis/ (Accessed February 15th, 2022).
Department of Health and Social Care (2018). Cannabis-based Products for Medicinal Use. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/753444/letter-with-guidance-on-cannabis-based-products-for-medicinal-use.pdf (Accessed February 15th, 2022).
Efron, D., Freeman, J. L., Cranswick, N., Payne, J. M., Mulraney, M., Prakash, C., et al. (2021). A Pilot Randomised Placebo-Controlled Trial of Cannabidiol to Reduce Severe Behavioural Problems in Children and Adolescents with Intellectual Disability. Br. J. Clin. Pharmacol. 87 (2), 436–446. doi:10.1111/bcp.14399
Efron, D., Taylor, K., Payne, J. M., Freeman, J. L., Cranswick, N., Mulraney, M., et al. (2020). Does Cannabidiol Reduce Severe Behavioural Problems in Children with Intellectual Disability? Study Protocol for a Pilot Single-Site Phase I/II Randomised Placebo Controlled Trial. BMJ open 10 (3), e034362. doi:10.1136/bmjopen-2019-034362
Farokhnia, M., McDiarmid, G. R., Newmeyer, M. N., Munjal, V., Abulseoud, O. A., Huestis, M. A., et al. (2020). Effects of Oral, Smoked, and Vaporized Cannabis on Endocrine Pathways Related to Appetite and Metabolism: a Randomized, Double-Blind, Placebo-Controlled, Human Laboratory Study. Transl. Psychiatry 10 (1), 71. doi:10.1038/s41398-020-0756-3
Federal Institute for Drugs and Medical Devices (2018). Annual Report 2017/18. Available at: https://www.bfarm.de/SharedDocs/Downloads/EN/BfArM/Publikationen/AnnualReport2017-18.pdf?blob=publicationFile (Accessed February 15th, 2022).
Federal Institute for Drugs and Medical Devices (2022a). BfArM Starts Selling Cannabis for Medical Purposes to Pharmacies. Available at: https://www.bfarm.de/SharedDocs/Pressemitteilungen/DE/2021/pm6-2021.html?nn=471310 (Accessed February 15th, 2022).
Federal Institute for Drugs and Medical Devices (2022b). Cannabis Agency. Available at: https://www.bfarm.de/DE/Bundesopiumstelle/Cannabis-als-Medizin/Cannabisagentur/_artikel.html (Accessed February 15th, 2022).
Federal Institute for Drugs and Medical Devices (2022c). Instructions for Pharmacists. Available at: https://www.bfarm.de/DE/Bundesopiumstelle/Cannabis-als-Medizin/Hinweise-fuer-Apotheker/_node.html (Accessed February 15th, 2022).
Federal Institute for Drugs and Medical Devices (2022d). Notes for Medical Doctors. Available at: https://www.bfarm.de/DE/Bundesopiumstelle/Cannabis-als-Medizin/Hinweise-fuer-Aerzte/_node.html (Accessed February 15th, 2022).
Fischer, B., Kuganesan, S., and Room, R. (2015). Medical Marijuana Programs: Implications for Cannabis Control Policy-Oobservations from Canada. Int. J. Drug Policy 26 (1), 15–19. doi:10.1016/j.drugpo.2014.09.007
Freeman, T. P., Hindocha, C., Baio, G., Shaban, N. D. C., Thomas, E. M., Astbury, D., et al. (2020). Cannabidiol for the Treatment of Cannabis Use Disorder: a Phase 2a, Double-Blind, Placebo-Controlled, Randomised, Adaptive Bayesian Trial. Lancet Psychiatry 7 (10), 865–874. doi:10.1016/S2215-0366(20)30290-X
General Medical Council (2022). Information for Doctors on Cannabis-Based Products for Medicinal Use. Available at: https://www.gmc-uk.org/ethical-guidance/learning-materials/information-for-doctors-on-cannabis-based-products-for-medicinal-use (Accessed February 15th, 2022).
German Institute for Medical Cannabis (2022). Mmedicinal Cannabis. Available at: https://difmc.de/medizinalcannabis/ (Accessed February 15th, 2022).
Gould, J. (2015). The Cannabis Crop. Nature 525 (7570), S2–S3. doi:10.1038/525S2a
Government of Canada (2019). Cannabis for Medical Purposes under the Cannabis Act: Information and Improvements. Available at: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/medical-use-cannabis.html#_Access_to_cannabis (Accessed February 15th, 2022).
Grimison, P., Mersiades, A., Kirby, A., Lintzeris, N., Morton, R., Haber, P., et al. (2020). Oral THC:CBD Cannabis Extract for Refractory Chemotherapy-Induced Nausea and Vomiting: a Randomised, Placebo-Controlled, Phase II Crossover Trial. Ann. Oncol. 31 (11), 1553–1560. doi:10.1016/j.annonc.2020.07.020
Grotenhermen, F., and Müller-Vahl, K. (2012). The Therapeutic Potential of Cannabis and Cannabinoids. Dtsch. Arztebl Int. 109 (29-30), 495–501. doi:10.3238/arztebl.2012.0495
Grotenhermen, F. (2003). Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 42 (4), 327–360. doi:10.2165/00003088-200342040-00003
Hardy, J., Haywood, A., Gogna, G., Martin, J., Yates, P., Greer, R., et al. (2020). Oral Medicinal Cannabinoids to Relieve Symptom Burden in the Palliative Care of Patients with Advanced Cancer: a Double-Blind, Placebo-Controlled, Randomised Clinical Trial of Efficacy and Safety of 1:1 Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD). Trials 21 (1), 611. doi:10.1186/s13063-020-04541-6
Health Canada (2016). Consumer Information—Cannabis. Available at: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/marihuana/info/cons-eng.pdf (Accessed February 15th, 2022).
Health Canada (2022). Marijuana Laws. Available at: https://medicalmarijuana.ca/patients/marijuana-laws/ (Accessed February 15th, 2022).
Health Direct (2019). Medicinal Cannabis. Available at: https://www.healthdirect.gov.au/medicinal-cannabis (Accessed February 15th, 2022).
Hendricks, O., Andersen, T. E., Christiansen, A. A., Primdahl, J., Hauge, E. M., Ellingsen, T., et al. (2019). Efficacy and Safety of Cannabidiol Followed by an Open Label Add-On of Tetrahydrocannabinol for the Treatment of Chronic Pain in Patients with Rheumatoid Arthritis or Ankylosing Spondylitis: Protocol for a Multicentre, Randomised, Placebo-Controlled Study. BMJ open 9 (6), e028197. doi:10.1136/bmjopen-2018-028197
Hill, K. P. (2015). Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems: A Clinical Review. JAMA 313 (24), 2474–2483. doi:10.1001/jama.2015.6199
Hotz, J., Fehlmann, B., Papassotiropoulos, A., de Quervain, D. J., and Schicktanz, N. S. (2021). Cannabidiol Enhances Verbal Episodic Memory in Healthy Young Participants: A Randomized Clinical Trial. J. Psychiatr. Res. 143, 327–333. doi:10.1016/j.jpsychires.2021.09.007
Hurd, Y. L., Spriggs, S., Alishayev, J., Winkel, G., Gurgov, K., Kudrich, C., et al. (2019). Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals with Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Psychiatry 176 (11), 911–922. doi:10.1176/appi.ajp.2019.18101191
Izgelov, D., Davidson, E., Barasch, D., Regev, A., Domb, A. J., and Hoffman, A. (2020). Pharmacokinetic Investigation of Synthetic Cannabidiol Oral Formulations in Healthy Volunteers. Eur. J. Pharm. Biopharm. 154, 108–115. doi:10.1016/j.ejpb.2020.06.021
Jakubovski, E., Pisarenko, A., Fremer, C., Haas, M., May, M., Schumacher, C., et al. (2020). The CANNA-TICS Study Protocol: A Randomized Multi-Center Double-Blind Placebo Controlled Trial to Demonstrate the Efficacy and Safety of Nabiximols in the Treatment of Adults with Chronic Tic Disorders. Front. Psychiatry 11, 575826. doi:10.3389/fpsyt.2020.575826
Johnson, J. K., Johnson, R. M., Hodgkin, D., Jones, A. A., Kritikos, A., Doonan, S. M., et al. (2021). Medical Marijuana Laws (MMLs) and Dispensary Provisions Not Associated with Higher Odds of Adolescent Marijuana or Heavy Marijuana Use: A 46 State Analysis, 1991-2015. Subst. Abus 42 (4), 471–475. doi:10.1080/08897077.2021.1900986
Kayser, R. R., Haney, M., Raskin, M., Arout, C., and Simpson, H. B. (2020). Acute Effects of Cannabinoids on Symptoms of Obsessive-Compulsive Disorder: A Human Laboratory Study. Depress Anxiety 37 (8), 801–811. doi:10.1002/da.23032
Krcevski-Skvarc, N., Wells, C., and Häuser, W. (2018). Availability and Approval of Cannabis-Based Medicines for Chronic Pain Management and Palliative/supportive Care in Europe: A Survey of the Status in the Chapters of the European Pain Federation. Eur. J. Pain 22 (3), 440–454. doi:10.1002/ejp.1147
Lafaye, G., Karila, L., Blecha, L., and Benyamina, A. (2017). Cannabis, Cannabinoids, and Health. Dialogues Clin. Neurosci. 19 (3), 309–316. doi:10.31887/DCNS.2017.19.3/glafaye
Lawn, W., Hill, J., Hindocha, C., Yim, J., Yamamori, Y., Jones, G., et al. (2020). The Acute Effects of Cannabidiol on the Neural Correlates of Reward Anticipation and Feedback in Healthy Volunteers. J. Psychopharmacol. 34 (9), 969–980. doi:10.1177/0269881120944148
Lintzeris, N., Mills, L., Dunlop, A., Copeland, J., Mcgregor, I., Bruno, R., et al. (2020). Amp; Agonist Replacement for Cannabis Dependence Study Group. (Arc-D)Cannabis Use in Patients 3 Months after Ceasing Nabiximols for the Treatment of Cannabis Dependence: Results from a Placebo-Controlled Randomised Trial. Drug alcohol dependence 215, 108220. doi:10.1016/j.drugalcdep.2020.108220
Liu, Z., Galettis, P., Broyd, S. J., van Hell, H., Greenwood, L. M., de Krey, P., et al. (2020). Model-based Analysis on Systemic Availability of Co-administered Cannabinoids after Controlled Vaporised Administration. Intern Med. J. 50 (7), 846–853. doi:10.1111/imj.14415
Lopez, H. L., Cesareo, K. R., Raub, B., Kedia, A. W., Sandrock, J. E., Kerksick, C. M., et al. (2020). Effects of Hemp Extract on Markers of Wellness, Stress Resilience, Recovery and Clinical Biomarkers of Safety in Overweight, but Otherwise Healthy Subjects. J. Diet. Suppl. 17 (5), 561–586. doi:10.1080/19390211.2020.1765941
Marketrealist (2019). Medical Cannabis in Brazil Is Coming. Available at: https://marketrealist.com/2019/12/medical-cannabis-in-brazil-is-coming/ (Accessed February 15th, 2022).
Mead, A. (2017). The Legal Status of Cannabis (Marijuana) and Cannabidiol (CBD) under U.S. Law. Epilepsy Behav. 70 (Pt B), 288–291. doi:10.1016/j.yebeh.2016.11.021
Medicines and healthcare products Regulatory Agency (2020). The Supply, Manufacture, Importation and Distribution of Unlicensed Cannabis-Based Products for Medicinal Use in Humans ‘specials’. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/869284/Cannabis_Guidance__unlicensed_CBPMs__updated_2020.pdf (Accessed February 15th, 2022).
Mersiades, A. J., Stockler, M. R., Olver, I. N., and Grimison, P. (2019). Medicinal Cannabis for Chemotherapy-Induced Nausea and Vomiting: Prescribing with Limited Evidence. Med. J. Aust. 210 (1), 11. doi:10.5694/mja17.01099
Ministério da Saúde (2019). Resolução da Diretoria Colegiada n 327. Available at: https://www.in.gov.br/en/web/dou/-/resolucao-da-diretoria-colegiada-rdc-n-327-de-9-de-dezembro-de-2019-232669072 (Accessed February 15th, 2022).
Mitelpunkt, A., Kramer, U., Hausman Kedem, M., Zilbershot Fink, E., Orbach, R., Chernuha, V., et al. (2019). The Safety, Tolerability, and Effectiveness of PTL-101, an Oral Cannabidiol Formulation, in Pediatric Intractable Epilepsy: A Phase II, Open-Label, Single-Center Study. Epilepsy Behav. 98 (Pt A), 233–237. doi:10.1016/j.yebeh.2019.07.007
MS Society (2021). Sativex (Nabiximols). Available at: https://www.mssociety.org.uk/about-ms/treatments-and-therapies/cannabis/sativex (Accessed February 15th, 2022).
Naftali, T., Bar-Lev Schleider, L., Scklerovsky Benjaminov, F., Konikoff, F. M., Matalon, S. T., and Ringel, Y. (2021b). Cannabis Is Associated with Clinical but Not Endoscopic Remission in Ulcerative Colitis: A Randomized Controlled Trial. PloS one 16 (2), e0246871. doi:10.1371/journal.pone.0246871
Naftali, T., Bar-Lev Schleider, L., Almog, S., Meiri, D., and Konikoff, F. M. (2021a). Oral CBD-Rich Cannabis Induces Clinical but Not Endoscopic Response in Patients with Crohn's Disease, a Randomised Controlled Trial. J. Crohn's colitis 15 (11), 1799–1806. doi:10.1093/ecco-jcc/jjab069
National Conferences of State Legislatures (2022). State Medical Cannabis Laws. Available at: https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (Accessed February 15th, 2022).
National Health Service (2022). Cannabis-based Products for Medicinal Use: Frequently Asked Questions. Available at: https://www.england.nhs.uk/medicines-2/support-for-prescribers/cannabis-based-products-for-medicinal-use/cannabis-based-products-for-medicinal-use-frequently-asked-questions/ (Accessed February 15th, 2022).
National Health Service (2021). Medical Cannabis (And Cannabis Oils). Available at: https://www.nhs.uk/conditions/medical-cannabis/ (Accessed February 15th, 2022).
O'Neill, A., Wilson, R., Blest-Hopley, G., Annibale, L., Colizzi, M., Brammer, M., et al. (2021). Normalization of Mediotemporal and Prefrontal Activity, and Mediotemporal-Striatal Connectivity, May Underlie Antipsychotic Effects of Cannabidiol in Psychosis. Psychol. Med. 51 (4), 596–606. doi:10.1017/S0033291719003519
Office of Medicinal Cannabis (2022). Products and Services. Available at: https://english.cannabisbureau.nl/ (Accessed February 15th, 2022).
Perkins, D., Butler, J., Ong, K., Nguyen, T. H., Cox, S., Francis, B., et al. (2020). A Phase 1, Randomised, Placebo-Controlled, Dose Escalation Study to Investigate the Safety, Tolerability and Pharmacokinetics of Cannabidiol in Fed Healthy Volunteers. Eur. J. Drug Metab. Pharmacokinet. 45 (5), 575–586. doi:10.1007/s13318-020-00624-6
Pisanti, S., and Bifulco, M. (2019). Medical Cannabis: A Plurimillennial History of an Evergreen. J. Cell Physiol. 234 (6), 8342–8351. doi:10.1002/jcp.27725
Pretzsch, C. M., Freyberg, J., Voinescu, B., Lythgoe, D., Horder, J., Mendez, M. A., et al. (2019a). Effects of Cannabidiol on Brain Excitation and Inhibition Systems; a Randomised Placebo-Controlled Single Dose Trial during Magnetic Resonance Spectroscopy in Adults with and without Autism Spectrum Disorder. Neuropsychopharmacol. official Publ. Am. Coll. Neuropsychopharmacol. 44 (8), 1398–1405. doi:10.1038/s41386-019-0333-8
Pretzsch, C. M., Voinescu, B., Lythgoe, D., Horder, J., Mendez, M. A., Wichers, R., et al. (2019b). Effects of Cannabidivarin (CBDV) on Brain Excitation and Inhibition Systems in Adults with and without Autism Spectrum Disorder (ASD): a Single Dose Trial during Magnetic Resonance Spectroscopy. Transl. Psychiatry 9 (1), 313. doi:10.1038/s41398-019-0654-8
Pretzsch, C. M., Voinescu, B., Mendez, M. A., Wichers, R., Ajram, L., Ivin, G., et al. (2019c). The Effect of Cannabidiol (CBD) on Low-Frequency Activity and Functional Connectivity in the Brain of Adults with and without Autism Spectrum Disorder (ASD). J. Psychopharmacol. 33 (9), 1141–1148. doi:10.1177/0269881119858306
ProCon (2022). Legal Medical Marijuana States and DC. Available at: https://medicalmarijuana.procon.org/legal-medical-marijuana-states-and-dc/ (Accessed February 15th, 2022).
Rasche, T., Emmert, D., Radbruch, L., Conrad, R., and Mücke, M. (2019). Cannabis and Cannabinoids in Palliative Care. Bundesgesundheitsblatt Gesundheitsforsch. Gesundheitsschutz 62 (7), 830–835. doi:10.1007/s00103-019-02967-1
Reuters (2019). Brazil Approves Medical Marijuana Rules, Blocks Cannabis Cultivation. Available at: https://www.reuters.com/article/us-brazil-marijuana/brazil-approves-medical-marijuana-rules-blocks-cannabis-cultivation-idUSKBN1Y726W (Accessed February 15th, 2022).
Romano, L., and Hazekamp, A. (2019). An Overview of Galenic Preparation Methods for Medicinal Cannabis. Cbc 15 (2), 174–195. doi:10.2174/1573407214666180612080412
Ryan, J. E., McCabe, S. E., and Boyd, C. J. (2021). Medicinal Cannabis: Policy, Patients, and Providers. Policy, Polit. Nurs. Pract. 22 (2), 126–133. doi:10.1177/1527154421989609
S, G., Hb, S., K, L., R, T., Bs, R., Ps, S., et al. (2021). Safety and Efficacy of Low-Dose Medical Cannabis Oils in Multiple Sclerosis. Mult. Scler. Relat. Disord. 48, 102708. doi:10.1016/j.msard.2020.102708
Solowij, N., Broyd, S., Greenwood, L. M., van Hell, H., Martelozzo, D., Rueb, K., et al. (2019). A Randomised Controlled Trial of Vaporised Δ9-tetrahydrocannabinol and Cannabidiol Alone and in Combination in Frequent and Infrequent Cannabis Users: Acute Intoxication Effects. Eur. Arch. Psychiatry Clin. Neurosci. 269 (1), 17–35. doi:10.1007/s00406-019-00978-2
State of Israel - Minister of Health (2022). Cannabis for Medical Use and for Research. Available at: https://health.gov.il/English/Topics/cannabis/Pages/default.aspx (Accessed February 15th, 2022).
State of Israel - Minister of Health (2017). The First Course for Doctors to Prescribe Medical Cannabis approval Was Completed. 81 Doctors Will Begin to Authorize the Use of Medical Cannabis. Available at: https://health.gov.il/English/News_and_Events/Spokespersons_Messages/Pages/07092017_2.aspx (Accessed February 15th, 2022).
Stella, B., Baratta, F., Della Pepa, C., Arpicco, S., Gastaldi, D., and Dosio, F. (2021). Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 81 (13), 1513–1557. doi:10.1007/s40265-021-01579-x
Swiss Confederation, Federal Office of Public Health (2021a). Cannabidiol (CBD) Containing Products - Overview and Implementation Guide. Available at: https://www.swissmedic.ch/swissmedic/it/home/news/mitteilungen/prodotti-contenenti-cbd--cannabidiol---panoramica.html (Accessed February 15th, 2022).
Swiss Confederation, Federal Office of Public Health (2020). Fact Sheet: Hemp-Based Medicines. Available at: https://www.bag.admin.ch/bag/it/home/medizin-und-forschung/heilmittel/med-anwend-cannabis/gesetzesaenderung-cannabisarzneimittel.html (Accessed February 15th, 2022).
Swiss Confederation, Federal Office of Public Health (2021c). Medical Application of Hemp. Available at: https://www.bag.admin.ch/bag/it/home/medizin-und-forschung/heilmittel/med-anwend-cannabis.html (Accessed February 15th, 2022).
Swiss Confederation, Federal Office of Public Health. (2021b). Hemp-based Medicines: Amendment of the Law. Available at: https://www.bag.admin.ch/bag/it/home/medizin-und-forschung/heilmittel/med-anwend-cannabis/gesetzesaenderung-cannabisarzneimittel.html (Accessed February 15th, 2022).
The Health Products Regulatory Authority (2017). Cannabis for Medical Use - A Scientific Review. Available at: https://www.hpra.ie/docs/default-source/publications-forms/newsletters/cannabis-for-medical-use---a-scientific-review.pdf?sfvrsn=7 (Accessed February 15th, 2022).
The Office of Drug Control (2021). Manufacturers and Suppliers of Medicinal Cannabis Products. https://www.odc.gov.au/manufacturers-and-suppliers-medicinal-cannabis-products (Accessed February 15th, 2022).
Thompson, M. D., Martin, R. C., Grayson, L. P., Ampah, S. B., Cutter, G., Szaflarski, J. P., et al. (2020). Cognitive Function and Adaptive Skills after a One-Year Trial of Cannabidiol (CBD) in a Pediatric Sample with Treatment-Resistant Epilepsy. Epilepsy Behav. 111, 107299. doi:10.1016/j.yebeh.2020.107299
Timler, A., Bulsara, C., Bulsara, M., Vickery, A., Smith, J., and Codde, J. (2020). Use of Cannabinoid-Based Medicine Among Older Residential Care Recipients Diagnosed with Dementia: Study Protocol for a Double-Blind Randomised Crossover Trial. Trials 21 (1), 188. doi:10.1186/s13063-020-4085-x
UK Government (2022). Medicinal Cannabis: Information and Resources. Available at: https://www.gov.uk/government/collections/medicinal-cannabis-information-and-resources (Accessed February 15th, 2022).
Urbi, B., Broadley, S., Bedlack, R., Russo, E., and Sabet, A. (2019). Study Protocol for a Randomised, Double-Blind, Placebo-Controlled Study Evaluating the Efficacy of Cannabis-Based Medicine Extract in Slowing the Disease pRogression of Amyotrophic Lateral Sclerosis or Motor Neurone Disease: the EMERALD Trial. BMJ open 9 (11), e029449. doi:10.1136/bmjopen-2019-029449
Van de Donk, T., Niesters, M., Kowal, M. A., Olofsen, E., Dahan, A., and van Velzen, M. (2019). An Experimental Randomized Study on the Analgesic Effects of Pharmaceutical-Grade Cannabis in Chronic Pain Patients with Fibromyalgia. Pain 160 (4), 860–869. doi:10.1097/j.pain.0000000000001464
Van der Flier, F. E., Kwee, C. M. B., Cath, D. C., Batelaan, N. M., Groenink, L., Duits, P., et al. (2019). Cannabidiol Enhancement of Exposure Therapy in Treatment Refractory Patients with Phobias: Study Protocol of a Randomized Controlled Trial. BMC psychiatry 19 (1), 69. doi:10.1186/s12888-019-2022-x
Wall, M. B., Pope, R., Freeman, T. P., Kowalczyk, O. S., Demetriou, L., Mokrysz, C., et al. (2019). Dissociable Effects of Cannabis with and without Cannabidiol on the Human Brain's Resting-State Functional Connectivity. J. Psychopharmacol. 33 (7), 822–830. doi:10.1177/0269881119841568
Whiting, P. F., Wolff, R. F., Deshpande, S., Di Nisio, M., Duffy, S., Hernandez, A. V., et al. (2015). Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. JAMA 313 (24), 2456–2473. doi:10.1001/jama.2015.6358
Xu, D. H., Cullen, B. D., Tang, M., and Fang, Y. (2020). The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. Curr. Pharm. Biotechnol. 21 (5), 390–402. doi:10.2174/1389201020666191202111534
Yassin, M., Oron, A., and Robinson, D. (2019). Effect of Adding Medical Cannabis to Analgesic Treatment in Patients with Low Back Pain Related to Fibromyalgia: an Observational Cross-Over Single Centre Study. Clin. Exp. Rheumatol. 37, 13–20.
Zylla, D. M., Eklund, J., Gilmore, G., Gavenda, A., Guggisberg, J., VazquezBenitez, G., et al. (2021). A Randomized Trial of Medical Cannabis in Patients with Stage IV Cancers to Assess Feasibility, Dose Requirements, Impact on Pain and Opioid Use, Safety, and Overall Patient Satisfaction. Support Care Cancer 29 (12), 7471–7478. doi:10.1007/s00520-021-06301-x
Keywords: medical Cannabis , clinical trials, study protocols, legislation, law
Citation: Baratta F, Pignata I, Ravetto Enri L and Brusa P (2022) Cannabis for Medical Use: Analysis of Recent Clinical Trials in View of Current Legislation. Front. Pharmacol. 13:888903. doi: 10.3389/fphar.2022.888903
Received: 03 March 2022; Accepted: 09 May 2022; Published: 25 May 2022.
Reviewed by:
Copyright © 2022 Baratta, Pignata, Ravetto Enri and Brusa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. Baratta, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Introduction
- Conclusions
- Article Information
AE indicates adverse event; RCT, randomized controlled trial; and SR, systematic review.
a These excluded reports were screened as full-text articles/reports.
b The number of included RCTS does not sum because some were included in more than 1 indication category.
Odds indicate 30% or greater improvement in pain with cannabinoid compared with placebo, stratified according to cannabinoid. The square data markers indicate odds ratios (ORs) from primary studies, with sizes reflecting the statistical weight of the study using random-effects meta-analysis. The horizontal lines indicate 95% CIs. The blue diamond data markers represent the subtotal and overall OR and 95% CI. The vertical dashed line shows the summary effect estimate, the dotted shows the line of no effect (OR = 1).
The square data markers indicate mean differences from primary studies, with sizes reflecting the statistical weight of the study using random-effects meta-analysis. The horizontal line indicate, 95% CIs. The blue diamond data markers represent the subtotal and overall weighted mean difference and 95% CI. The vertical dashed line shows the summary effect estimate, the solid vertical line shows the line of no effect (mean difference = 0).
The square data markers indicate odds ratios (ORs) from primary studies, with sizes reflecting the statistical weight of the study using random-effects meta-analysis. The horizontal lines indicate 95% CIs. The blue diamond data markers represent the subtotal and overall OR and 95% CI. The vertical dashed line shows the summary effect estimate, the dotted line shows the line of no effect (OR = 1).
eAppendix 1. Review Protocol
eAppendix 2. Search Strategy
eAppendix 3. Nausea and Vomiting Due to Chemotherapy
eAppendix 4. Appetite Stimulation in HIV/AIDS
eAppendix 5. Chronic Pain
eAppendix 6. Spasticity in MS and Paraplegia
eAppendix 7. Depression
eAppendix 8. Anziety Disorder
eAppendix 9. Sleep Disorder
eAppendix 10. Psychosis
eAppendix 11. Glaucoma
eAppendix 12. Tourette Syndrome
eAppendix 13. Risk of Bias in Each Included Study, Grouped by Indication
eReferences
- Medical Marijuana JAMA Editorial June 23, 2015 This Editorial discusses some of the medical and legal considerations surrounding use of medical marijuana and cannabinoid drugs. Deepak Cyril D'Souza, MBBS, MD; Mohini Ranganathan, MD
- Medical Marijuana for Treatment of Chronic Pain and Other Problems JAMA Clinical Crossroads June 23, 2015 This synopsis of a medicine grand rounds conference uses an example of a patient with chronic low back pain as the basis for discussing the use of medical marijuana for treating chronic pain and other disorders. Kevin P. Hill, MD, MHS
- Incorrect Figure Label JAMA Correction August 4, 2015
- Incorrect Values Reported JAMA Correction August 25, 2015
- Medical Use of Cannabinoids JAMA Comment & Response October 27, 2015 Igor Grant, MD
- Medical Use of Cannabinoids JAMA Comment & Response October 27, 2015 Michael E. Schatman, PhD
- Medical Use of Cannabinoids JAMA Comment & Response October 27, 2015 Penny Whiting, PhD; Robert Wolff, MD
- Incorrect Nonproprietary Drug Name and Approved Use JAMA Correction December 1, 2015
- Incorrect Effect Estimate JAMA Correction April 12, 2016
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Whiting PF , Wolff RF , Deshpande S, et al. Cannabinoids for Medical Use : A Systematic Review and Meta-analysis . JAMA. 2015;313(24):2456–2473. doi:10.1001/jama.2015.6358
Manage citations:
© 2024
- Permissions
Cannabinoids for Medical Use : A Systematic Review and Meta-analysis
- 1 School of Social and Community Medicine, University of Bristol, Bristol, United Kingdom
- 2 The National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care West at University Hospitals, Bristol NHS Foundation Trust, Bristol, United Kingdom
- 3 Kleijnen Systematic Reviews Ltd, Escrick, York, United Kingdom
- 4 Department of Medical, Oral, and Biotechnological Sciences, University “G. D'Annunzio” of Chieti-Pescara, Chieti, Italy
- 5 Department of Vascular Medicine, Academic Medical Center, Amsterdam, the Netherlands
- 6 Medical School, Universidad Peruana de Ciencias Aplicadas (UPC), Lima, Peru
- 7 Health Outcomes and Clinical Epidemiology Section, Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio
- 8 Department of Pathology, Radboud University Medical Center, Nijmegen, the Netherlands
- 9 Institut für Epidemiologie und kongenitale Erkrankungen, Cepicon GmbH, Hamburg, Germany
- 10 School for Public Health and Primary Care (CAPHRI), Maastricht University, Maastricht, the Netherlands
- Editorial Medical Marijuana Deepak Cyril D'Souza, MBBS, MD; Mohini Ranganathan, MD JAMA
- Clinical Crossroads Medical Marijuana for Treatment of Chronic Pain and Other Problems Kevin P. Hill, MD, MHS JAMA
- Correction Incorrect Figure Label JAMA
- Correction Incorrect Values Reported JAMA
- Comment & Response Medical Use of Cannabinoids Igor Grant, MD JAMA
- Comment & Response Medical Use of Cannabinoids Michael E. Schatman, PhD JAMA
- Comment & Response Medical Use of Cannabinoids Penny Whiting, PhD; Robert Wolff, MD JAMA
- Correction Incorrect Nonproprietary Drug Name and Approved Use JAMA
- Correction Incorrect Effect Estimate JAMA
Importance Cannabis and cannabinoid drugs are widely used to treat disease or alleviate symptoms, but their efficacy for specific indications is not clear.
Objective To conduct a systematic review of the benefits and adverse events (AEs) of cannabinoids.
Data Sources Twenty-eight databases from inception to April 2015.
Study Selection Randomized clinical trials of cannabinoids for the following indications: nausea and vomiting due to chemotherapy, appetite stimulation in HIV/AIDS, chronic pain, spasticity due to multiple sclerosis or paraplegia, depression, anxiety disorder, sleep disorder, psychosis, glaucoma, or Tourette syndrome.
Data Extraction and Synthesis Study quality was assessed using the Cochrane risk of bias tool. All review stages were conducted independently by 2 reviewers. Where possible, data were pooled using random-effects meta-analysis.
Main Outcomes and Measures Patient-relevant/disease-specific outcomes, activities of daily living, quality of life, global impression of change, and AEs.
Results A total of 79 trials (6462 participants) were included; 4 were judged at low risk of bias. Most trials showed improvement in symptoms associated with cannabinoids but these associations did not reach statistical significance in all trials. Compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete nausea and vomiting response (47% vs 20%; odds ratio [OR], 3.82 [95% CI, 1.55-9.42]; 3 trials), reduction in pain (37% vs 31%; OR, 1.41 [95% CI, 0.99-2.00]; 8 trials), a greater average reduction in numerical rating scale pain assessment (on a 0-10-point scale; weighted mean difference [WMD], −0.46 [95% CI, −0.80 to −0.11]; 6 trials), and average reduction in the Ashworth spasticity scale (WMD, −0.12 [95% CI, −0.24 to 0.01]; 5 trials). There was an increased risk of short-term AEs with cannabinoids, including serious AEs. Common AEs included dizziness, dry mouth, nausea, fatigue, somnolence, euphoria, vomiting, disorientation, drowsiness, confusion, loss of balance, and hallucination.
Conclusions and Relevance There was moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain and spasticity. There was low-quality evidence suggesting that cannabinoids were associated with improvements in nausea and vomiting due to chemotherapy, weight gain in HIV infection, sleep disorders, and Tourette syndrome. Cannabinoids were associated with an increased risk of short-term AEs.
Cannabis is a generic term used for drugs produced from plants belonging to the genus Cannabis . 1 It is one of the most popular recreational drugs; worldwide, an estimated 178 million people aged 15 to 64 years used cannabis at least once in 2012. 2 Cannabis was included as a controlled drug in the United Nations’ Single Convention on Narcotic Drugs, held in 1961, 3 and its use is illegal in most countries.
Medical cannabis refers to the use of cannabis or cannabinoids as medical therapy to treat disease or alleviate symptoms. Cannabinoids can be administered orally, sublingually,or topically; they can be smoked, inhaled, mixed with food, or made into tea. They can be taken in herbal form, extracted naturally from the plant, gained by isomerisation of cannabidiol, or manufactured synthetically. 4 Prescribed cannabinoids include dronabinol capsules, nabilone capsules, and the oromucosal spray nabiximols. 4 Some countries have legalized medicinal-grade cannabis for chronically ill patients. Canada and the Netherlands have government-run programs in which specialized companies supply quality-controlled herbal cannabis. 5 In the United States, 23 states and Washington, DC (May 2015), have introduced laws to permit the medical use of cannabis 6 ; other countries have similar laws. The aim of this systematic review was to evaluate the evidence for the benefits and adverse events (AEs) of medical cannabinoids across a broad range of indications.
This review followed guidance published by the Centre for Reviews and Dissemination and the Cochrane Collaboration. 7 , 8 We established a protocol for the review (eAppendix 1 in Supplement 1 ).
Randomized clinical trials (RCTs) that compared cannabinoids with usual care, placebo, or no treatment in the following indications were eligible: nausea and vomiting due to chemotherapy, appetite stimulation in HIV/AIDS, chronic pain, spasticity due to multiple sclerosis (MS) or paraplegia, depression, anxiety disorder, sleep disorder, psychosis, intraocular pressure in glaucoma, or Tourette syndrome. These indications were prespecified by the project funders, the Swiss Federal Office of Public Health. If no RCTs were available for a particular indication or outcome (eg, long-term AEs such as cancer, psychosis, depression, or suicide), nonrandomized studies including uncontrolled studies (such as case series) with at least 25 patients were eligible.
Twenty-eight databases and gray literature sources were searched from inception to April 2015 without language restriction (Embase search strategy and details of databases searched available in eAppendix 2 in Supplement 2 ). The search strategy was peer reviewed 9 by a second information specialist. Reference lists of included studies were screened. Search results and full-text articles were independently assessed by 2 reviewers; disagreements were resolved through consensus or referral to a third reviewer.
We extracted data about baseline characteristics and outcomes (patient-relevant and disease-specific outcomes, activities of daily living, quality of life, global impression of change, and specified AEs). For dichotomous data such as number of patients with at least 30% improvement in pain, we calculated the odds ratio (OR) and 95% CI. For categorical data, we extracted details about each category assessed and the numbers of patients with an outcome in each category. Continuous data such as the Ashworth spasticity score 10 were extracted as means and SDs at baseline, follow-up, and the change from baseline and used to calculate mean differences with 95% CIs. Results (mean difference, 95% CIs, and P values) from the between-group statistical analyses reported by the study were also extracted. All relevant sources were used for data extraction including full-text journal articles, abstracts, and clinical trial registry entries. Where available, the journal article was used as the primary publication because it had been peer reviewed.
RCTs were assessed for methodological quality using the Cochrane Risk of Bias tool. 11 If at least one of the domains was rated as high, the trial was considered at high risk of bias. If all domains were judged as low, the trial was considered at low risk of bias. Otherwise, the trial was considered as having unclear risk of bias. Data extraction and risk-of-bias assessment were performed independently by 2 reviewers; disagreements were resolved by a third reviewer.
Clinical heterogeneity was assessed by grouping studies by indication, cannabinoid, and outcome. If there were 2 or more trials within a single grouping, data were pooled using random-effects meta-analysis. 12 For continuous outcomes, we analyzed the mean difference in change from baseline; if this was not reported and could not be calculated from other data, we used the mean difference at follow-up. 13 For dichotomous data, we used the OR. In order to avoid double counting, we selected a single data set from each study to contribute to the analysis. For studies evaluating multiple interventions, we selected the intervention or dose that was most similar to the other interventions being evaluated in the same analysis. Heterogeneity was investigated using forest plots and the I 2 statistic. Where data were considered too heterogeneous to pool or not reported in a format suitable for pooling (eg, data reported as medians), we used a narrative synthesis.
Sensitivity analyses were used to assess the statistical effect of trial design. The primary analysis included only parallel-group trials, results from crossover trials were included in an additional analysis. For the analysis of AEs, data for all conditions were combined. We conducted stratified analyses and meta-regression to investigate whether associations varied according to type of cannabinoid, study design (parallel group vs crossover trial), indication (each of the indication categories included in this report), comparator (active vs placebo), and duration of follow-up (<24 hours, 24 hours-1 week, >1 week-4 weeks, >4 weeks) for the outcome of any AE. Statistical analyses were performed using Stata statistical software (version 10).
GRADE (Grading of Recommendations Assessment, Development and Evaluation) was used to rate the overall quality of the evidence for risk of bias, publication bias, imprecision, inconsistency, indirectness, and magnitude of effect. The GRADE ratings of very low–, low-, moderate-, or high-quality evidence reflect the extent to which we are confident that the effect estimates are correct. 14
The searches identified 23 754 hits (records) of which 505 were considered potentially relevant, based on title and abstract screening, and obtained as full-text studies. A total of 79 studies (6462 participants), available as 151 reports, were included; 3 studies (6 reports) were included in multiple indication categories ( Figure 1 ). Thirty-four studies were parallel-group trials (4436 participants), and 45 were crossover trials (2026 participants). Four studies were available only as an abstract, 15 - 18 a further 3 were available only as abstracts 19 - 21 but with additional details available on trial registries including full results in one, 19 and details of 2 trials (including full trial results) were available only as trial registry entries 22 , 23 ; all other trials were reported in full-length journal articles. Where reported, the proportion of participants who were men ranged from 0% to 100% (median, 50% [57 studies]), and the proportion of white participants ranged from 50% to 99% (median, 78% [18 studies]). Publication dates ranged from 1975 to 2015 (median, 2004 [with one-third of trials published before 1990]). Studies were conducted in a wide range of countries. A variety of cannabinoids were evaluated and compared with various different active comparators or placebos; most active comparators were included in the nausea and vomiting indication ( Table 1 ). eAppendices 3 to 12 in Supplement 1 provide an overview of the included studies and their findings.
Four (5%) trials were judged at low risk of bias, 55 (70%) were judged at high risk of bias, and 20 (25%) at unclear risk of bias (eAppendix 13 in Supplement 2 ). The major potential source of bias in the trials was incomplete outcome data. More than 50% of trials reported substantial withdrawals and did not adequately account for this in the analysis. Selective outcome reporting was a potential risk of bias in 16% of trials. These studies did not report data for all outcomes specified in the trial register, protocol, or methods section or changed the primary outcome from that which was prespecified. Most studies reported being double-blinded but only 57% reported that appropriate methods had been used for participant blinding and only 24% reported that outcome assessors had been appropriately blinded.
Full results from included studies are presented in eAppendices 3-12 in Supplement 2 ; pooled results and GRADE ratings are presented in Table 2 .
Nausea and vomiting due to chemotherapy was assessed in 28 studies (37 reports; 1772 participants). 15 , 16 , 24 - 58 Fourteen studies assessed nabilone and there were 3 for dronabinol, 1 for nabiximols, 4 for levonantradol, and 6 for THC. Two studies also included a combination therapy group of dronabinol with ondansetron or prochlorperazine. Eight studies included a placebo control, 3 of these also included an active comparator, and 20 studies included only an active comparator. The most common active comparators were prochlorperazine (15 studies), chlorpromazine (2 studies) and domperidone (2 studies). Other comparators (alizapride, hydroxyzine, metoclopramide and ondansetron) were evaluated in single studies ( Table 1 ). Of all 28 studies, risk of bias was high for 23 or unclear for 5. All studies suggested a greater benefit of cannabinoids compared with both active comparators and placebo, but these did not reach statistical significance in all studies. The average number of patients showing a complete nausea and vomiting response was greater with cannabinoids (dronabinol or nabiximols) than placebo (OR, 3.82 [95% CI, 1.55-9.42]; 3 trials). There was no evidence of heterogeneity for this analysis ( I 2 = 0%) and results were similar for both dronabinol and nabiximols.
Appetite stimulation in HIV/AIDS was assessed in 4 studies (4 reports; 255 participants). 59 - 62 All studies assessed dronabinol, 3 compared with placebo (1 of which also assessed marijuana), and 1 compared with megastrol acetate. All studies were at high risk of bias. There was some evidence that dronabinol is associated with an increase in weight when compared with placebo. More limited evidence suggested that it may also be associated with increased appetite, greater percentage of body fat, reduced nausea, and improved functional status. However, these outcomes were mostly assessed in single studies and associations failed to reach statistical significance. The trial that evaluated marijuana and dronabinol found significantly greater weight gain with both forms of cannabinoid when compared with placebo. 59 The active comparison trial found that megastrol acetate was associated with greater weight gain than dronabinol and that combining dronabinol with megastrol acetate did not lead to additional weight gain. 60
Chronic pain was assessed in 28 studies (63 reports; 2454 participants). 19 , 20 , 22 , 23 , 63 - 120 Thirteen studies evaluated nabiximols, 4 were for smoked THC, 5 for nabilone, 3 for THC oromucosal spray, 2 dronabinol, 1 vaporized cannabis (included 2 doses), 1 for ajuvenic acid capsules, and 1 for oral THC. One trial compared nabilone with amitriptyline 64 ; all other studies were placebo controlled. One of these studies evaluated nabilone as an adjunctive treatment to gabapentin. 121 The conditions causing the chronic pain varied between studies and included neuropathic pain (central, peripheral, or not specified; 12 studies), 3 for cancer pain, 3 for diabetic peripheral neuropathy, 2 for fibromyalgia, 2 for HIV-associated sensory neuropathy, and 1 study for each of the following indications: refractory pain due to MS or other neurological conditions, for rheumatoid arthritis, for noncancer pain (nociceptive and neuropathic), central pain (not specified further), musculoskeletal problems, and chemotherapy-induced pain.
Two studies were at low risk of bias, 9 at unclear risk, and 17 at high risk of bias. Studies generally suggested improvements in pain measures associated with cannabinoids but these did not reach statistical significance in most individual studies.
The average number of patients who reported a reduction in pain of at least 30% was greater with cannabinoids than with placebo (OR, 1.41 [95% CI, 0.99-2.00]; 8 trials; Figure 2 ). One trial assessed smoked THC 77 and reported the greatest beneficial effect (OR, 3.43 [95% CI, 1.03-11.48]), and 7 trials assessed nabiximols ( Figure 2 ). Pain conditions evaluated in these trials were neuropathic pain (OR, 1.38 [95% CI, 0.93-2.03]; 6 trials) and cancer pain (OR, 1.41 [95% CI, 0.99-2.00]; 2 trials), with no clear differences between pain conditions. Nabiximols was also associated with a greater average reduction in the Numerical Rating Scale (NRS; 0-10 scale) assessment of pain (weighted mean difference [WMD], −0.46 [95% CI, −0.80 to −0.11]; 6 trials), brief pain inventory-short form, severity composite index (WMD, −0.17 [95% CI, −0.50 to 0.16]; 3 trials), neuropathic pain scale (WMD, −3.89 [95% CI, −7.32 to −0.47]; 5 trials), and the proportion of patients reporting improvement on a global impression of change score (OR, 2.08 [95% CI, 1.21 to 3.59]; 6 trials) compared with placebo. There was some evidence to support this based on continuous data but this was not consistent across trials. There was no difference in average quality-of-life scores as measured by the EQ-5D health status index (WMD, −0.01 [95% CI, −0.05 to 0.02]; 3 trials) between nabiximols and placebo. Two of the studies included in the meta-analysis for the NRS (0-10 scale) assessed patients with cancer pain, all other studies assessed patients with neuropathic pain. There were no clear differences based on cause of pain in the meta-analysis of NRS. Sensitivity analyses that included crossover trials showed results consistent with those based on parallel-group trials alone.
Fourteen studies (33 reports; 2280 participants) assessed spasticity due to MS or paraplegia. 17 , 19 , 65 , 87 , 91 , 122 - 149 Eleven studies (2138 participants) included patients with MS and 3 included patients with paraplegia (142 participants) caused by spinal cord injury. Six studies assessed nabiximols, 3 for dronabinol, 1 for nabilone, 4 for THC/CBD (2 of these also assessed dronabinol), and 1 each for ECP002A and smoked THC. All studies included a placebo control group; none included an active comparator. Two studies were at low risk of bias, 5 were at unclear risk of bias, and 7 were at high risk of bias. Studies generally suggested that cannabinoids were associated with improvements in spasticity, but this failed to reach statistical significance in most studies. There were no clear differences based on type of cannabinoid. Only studies in MS patients reported sufficient data to allow summary estimates to be generated. Cannabinoids (nabiximols, dronabinol, and THC/CBD) were associated with a greater average improvement on the Ashworth scale for spasticity compared with placebo, although this did not reach statistical significance (WMD, −0.12 [95% CI, −0.24 to 0.01]; 5 trials; Figure 3 ). Cannabinoids (nabilone and nabiximols) were also associated with a greater average improvement in spasticity assessed using numerical rating scales (mean difference, −0.76 [95% CI, −1.38 to −0.14]; 3 trials). There was no evidence of a difference in association according to type of cannabinoid for either analysis. Other measures of spasticity also suggested a greater benefit of cannabinoid but did not reach statistical significance ( Table 2 ). The average number of patients who reported an improvement on a global impression of change score was also greater with nabiximols than placebo (OR, 1.44 [95% CI, 1.07 to 1.94]; 3 trials); this was supported by a further crossover trial of dronabinol and oral THC/CBD that provided continuous data for this outcome. 132 Sensitivity analyses that included crossover trials showed results consistent with those based on parallel group trials alone.
No studies evaluating cannabinoids for the treatment of depression fulfilled inclusion criteria. Five studies included for other indications reported depression as an outcome measure; 4 evaluated chronic pain and 1 evaluated spasticity in MS patients. 67 , 73 , 75 , 80 , 129 One trial assessed dronabinol (2 doses), 3 assessed nabiximols, and 1 assessed nabilone. Two studies were rated as having unclear risk of bias and 3 as having high risk of bias. Three studies suggested no difference between cannabinoids (dronabinol and nabiximols) and placebo in depression outcomes. One parallel-group trial that compared different doses of nabiximols with placebo reported a negative effect of nabiximols for the highest dose (11-14 sprays per day) compared with placebo (mean difference from baseline, 2.50 [95% CI, 0.38 to 4.62]) but no difference between placebo and the 2 lower doses. 67
One small parallel-group trial, judged at high risk of bias, evaluated patients with generalized social anxiety disorder. 150 The trial reported that cannabidiol was associated with a greater improvement on the anxiety factor of a visual analogue mood scale (mean difference from baseline, −16.52; P value = .01)compared with placebo during a simulated public speaking test. Additional data about anxiety outcomes provided by 4 studies (1 parallel group) in patients with chronic pain also suggested a greater benefit of cannabinoids (dronabinol, nabilone, and nabiximols) than placebo but these studies were not restricted to patients with anxiety disorders. 73 - 75 , 80
Two studies (5 reports; 54 participants) evaluated cannabinoids (nabilone) specifically for the treatment of sleep problems. One was a parallel-group trial judged at high risk of bias. This reported a a greater benefit of nabilone compared with placebo on the sleep apnea/hypopnea index (mean difference from baseline, −19.64; P value = .02). The other was a crossover trial judged at low risk of bias in patients with fibromyalgia and compared nabilone with amitriptyline. This suggested that nabilone was associated with improvements in insomnia (mean difference from baseline, −3.25 [95% CI, −5.26 to −1.24]) and with greater sleep restfulness (mean difference from baseline, 0.48 [95% CI, 0.01 to 0.95]). Nineteen placebo-controlled studies included for other indications (chronic pain and MS) also evaluated sleep as an outcome. 22 , 23 , 65 , 67 - 69 , 75 , 76 , 79 - 81 , 87 , 88 , 123 - 125 , 129 - 131 Thirteen studies assessed nabiximols, 1 for nabilone, 1 for dronabinol, 2 for THC/CBD capsules, and two assessed smoked THC (one at various doses). Two of the studies that assessed nabiximols also assessed oral THC and the trial of dronabinol also assessed oral THC/CBD. There was some evidence that cannabinoids may improve sleep in these patient groups. Cannabinoids (mainly nabiximols) were associated with a greater average improvement in sleep quality (WMD, −0.58 [95% CI, −0.87 to −0.29]; 8 trials) and sleep disturbance (WMD, −0.26 [95% CI, −0.52 to 0.00]; 3 trials). One trial assessed THC/CBD, all others assessed nabiximols, results were similar for both cannabinoids.
Psychosis was assessed in 2 studies (9 reports; 71 participants) judged at high risk of bias, which evaluated cannabidiol compared with amisulpride or placebo. 21 , 151 - 158 The trials found no difference in mental health outcomes between treatment groups.
One very small crossover trial (6 participants) 159 judged at unclear risk of bias compared tetrahydrocannabinol (THC; 5 mg), cannabidiol (20 mg), cannabidiol (40 mg) oromucosal spray, and placebo. This trial found no difference between placebo and cannabinoids on measures of intraocular pressure in patients with glaucoma.
Two small placebo-controlled studies (4 reports; 36 participants) 160 - 163 suggested that THC capsules may be associated with a significant improvement in tic severity in patients with Tourette syndrome.
Data about AEs were reported in 62 studies (127 reports). Meta-regression and stratified analysis showed no evidence for a difference in the association of cannabinoids with the incidence of “any AE” based on type of cannabinoid, study design, indication, comparator, or duration of follow-up 15 , 16 , 18 , 22 - 26 , 28 - 31 , 33 - 38 , 41 , 42 , 44 - 47 , 51 , 57 , 58 , 60 , 62 , 64 - 69 , 72 - 85 , 87 , 88 , 123 - 127 , 129 - 131 , 159 , 160 , 162 ; further analyses were conducted for all studies combined. Figure 4 shows the results of the meta-analyses for the number of participants experiencing any AE compared when compared with controls, stratified according to cannabinoid. Cannabinoids were associated with a much greater risk of any AE, serious AE, withdrawals due to AE, and a number of specific AEs ( Table 3 ). No studies evaluating the long-term AEs of cannabinoids were identified, even when searches were extended to lower levels of evidence.
We conducted an extensive systematic review of the benefits and AEs associated with medical cannabinoids across a broad range of conditions. We included 79 RCTs (6462 participants), the majority of which evaluated nausea and vomiting due to chemotherapy or chronic pain and spasticity due to MS and paraplegia. Other patient categories were evaluated in fewer than 5 studies.
Most studies suggested that cannabinoids were associated with improvements in symptoms, but these associations did not reach statistical significance in all studies. Based on the GRADE approach, there was moderate-quality evidence to suggest that cannabinoids may be beneficial for the treatment of chronic neuropathic or cancer pain (smoked THC and nabiximols) and spasticity due to MS (nabiximols, nabilone, THC/CBD capsules, and dronabinol). There was low-quality evidence suggesting that cannabinoids were associated with improvements in nausea and vomiting due to chemotherapy (dronabinol and nabiximols), weight gain in HIV (dronabinol), sleep disorders (nabilone, nabiximols), and Tourette syndrome (THC capsules); and very low-quality evidence for an improvement in anxiety as assessed by a public speaking test (cannabidiol). There was low-quality evidence for no effect on psychosis (cannabidiol) and very low-level evidence for no effect on depression (nabiximols). There was an increased risk of short-term AEs with cannabinoid use, including serious AEs. Common AEs included asthenia, balance problems, confusion, dizziness, disorientation, diarrhea, euphoria, drowsiness, dry mouth, fatigue, hallucination, nausea, somnolence, and vomiting. There was no clear evidence for a difference in association (either beneficial or harmful) based on type of cannabinoids or mode of administration. Only 2 studies evaluated cannabis. 59 , 77 There was no evidence that the effects of cannabis differed from other cannabinoids.
This review followed recommendations for rigorous systematic reviews. 7 , 8 In order to identify as many relevant studies as possible and reduce the risk of publication bias, a highly sensitive search strategy was used and an extensive range of resources were searched including electronic databases, guidelines, and systematic reviews. Both published and unpublished trials were eligible for inclusion. There were no date or language restrictions. In order to minimize bias and errors, the main Embase strategies were peer reviewed by a second independent information specialist 165 and all stages of the review process were performed independently by 2 reviewers. We used the Cochrane risk of bias tool 11 to assess the included RCTs. This highlighted a number of methodological weaknesses in the included trials including failure to appropriately handle withdrawals, selective outcome reporting, and inadequate description of methods of randomization, allocation concealment, and blinding. An additional limitation of many included studies was their very small sample sizes. This was particularly the case for the trial of glaucoma (N = 6), Tourette syndrome (average N = 18), sleep disorder (average N = 27), and anxiety disorder (N = 24), which means these studies may have lacked the power to detect differences between treatment groups.
The synthesis combined a narrative discussion of individual study results with meta-analysis (for studies in which suitable data were available), supplemented by interpretation (following guidance of the GRADE Working Group). 14 The data analysis was complicated by a number of issues. The included studies used a large variety of measures to evaluate outcomes, and even very similar outcomes were often assessed using different measures. Furthermore, a wide range of time points were reported in the included trials, which limited the applicability of the findings of these studies. Multiple different cannabinoids were evaluated in the included studies. We stratified analyses based on type of cannabinoid to investigate whether there were differences in associations based on type of cannabinoid. The majority of the studies were 2-group trials with a placebo control group; however, some studies included active comparisons and multiple groups comparing more than 1 form of cannabinoid, different doses of cannabinoids, or active and placebo comparator groups. This necessitated selecting a single result from each trial to contribute to the meta-analysis to avoid double counting of studies. Where possible, we selected the result for the treatment or dose most similar to the other studies contributing to that meta-analysis and for placebo-controlled comparisons rather than active comparisons. For the short-term AE analysis, we selected the highest-reported cannabinoids dose because we hypothesized that this would be most likely to be associated with AEs—additionally, this analysis would present a worst-case scenario. Studies evaluated various forms of cannabis administered via various routes (oral capsules, smoked, vaporized, oromucosal spray, intramuscular injection) and active comparators differed across trials. These differences in form, combined with the variety of outcome measures and the broad indication groupings considered by this review, resulted in a very heterogeneous set of included studies, which meant that meta-analysis was not always possible or appropriate. Many studies reported insufficient information to allow meta-analysis (eg, reporting only P values for group differences) or no information on the analysis performed. A further difficulty with the continuous data were that even for the same outcomes, some studies reported results as difference between groups at follow-up and others reported results for difference in change from baseline. As advised by the Cochrane Handbook for Systematic Reviews of Interventions , we combined both types of data when estimating summary mean differences. 7 A potential problem with RCTs using crossover designs is the possible unblinding due to strong treatment or AEs. Additionally, studies of this design were rarely analyzed appropriately and none reported the required data accounting for their crossover design to permit appropriate inclusion in meta-analyses. 166 Primary analyses were therefore based on parallel-group studies, with crossover trials included as sensitivity analyses.
Our search identified a number of existing reviews that assessed the use of medical cannabinoids for MS, 167 - 170 nausea and vomiting due to chemotherapy, 171 - 175 pain, 176 - 191 psychosis, 192 - 194 and Tourette syndrome. 195 , 196 Almost all previous reviews focused on single indications and all but one (which evaluated cannabinoids in 4 trials in patients with pain due to rheumatoid arthritis) 188 did not use the GRADE approach to rating the quality of the evidence. As far as we are aware, our review is the first comprehensive review to evaluate the safety and efficacy of cannabinoids across a broad range of indications. A key strength of review was that it allowed us to conduct pooled analysis for the AEs associated with medicinal cannabinoids, adding considerable power to this analysis.
Further large, robust, RCTs are needed to confirm the effects of cannabinoids, particularly on weight gain in patients with HIV/AIDS, depression, sleep disorders, anxiety disorders, psychosis, glaucoma, and Tourette syndrome are required. Further studies evaluating cannabis itself are also required because there is very little evidence on the effects and AEs of cannabis. Future trials should adhere to the CONSORT (Consolidated Standards of Reporting Trials) reporting standards 197 and ensure that appropriate methods are used for randomization, allocation concealment, patient and outcome assessor blinding, handling of withdrawals, and avoiding selective outcome reporting. Future studies should assess patient-relevant outcomes (including disease-specific end points, quality of life, and AEs) using standardized outcome measures at similar time points to ensure inclusion in future meta-analyses.
There was moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain and spasticity. There was low-quality evidence suggesting that cannabinoids were associated with improvements in nausea and vomiting due to chemotherapy, weight gain in HIV, sleep disorders, and Tourette syndrome. Cannabinoids were associated with an increased risk of short-term AEs.
Corresponding Author: Penny Whiting, PhD, NIHR CLAHRC West, University Hospitals Bristol NHS Foundation Trust, Ninth Floor, Whitefriars, Lewins Mead, Bristol BS1 2NT, United Kingdom ( [email protected] ).
Author Contributions: Dr Whiting had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Whiting, Wolff, Misso, Kleijnen.
Acquisition, analysis, or interpretation of data: Whiting, Wolff, Deshpande, Di Nisio, Duffy, Hernandez, Keurentjes, Lang, Misso, Ryder, Schmidlkofer, Westwood.
Drafting of the manuscript: Whiting, Keurentjes, Ryder.
Critical revision of the manuscript for important intellectual content: Whiting, Wolff, Deshpande, Di Nisio, Duffy, Hernandez, Keurentjes, Lang, Misso, Ryder, Schmidlkofer, Westwood, Kleijnen.
Statistical analysis: Whiting, Wolff, Di Nisio, Hernandez, Keurentjes, Schmidlkofer.
Obtained funding: Kleijnen.
Administrative, technical, or material support: Deshpande, Lang, Ryder.
Study supervision: Whiting, Kleijnen.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and declare support from the Swiss Federal Office of Public Health (FOPH) for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr Whiting reports that part of her time on this review was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West at University Hospitals Bristol NHS (National Health Service) Foundation Trust. No additional disclosures were reported.
Funding/Support: This funded by the Swiss Federal Office of Public Health (FOPH) under grant agreement 14.001443/204.0001/-1257.
Role of the Funder/Sponsor: The FOPH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The decision to submit the article for publication was a condition of the funding and was made before any results were available.
Additional Author Contributions: Dr Whiting drafted the article, produced tables and figures and performed the analysis. Drs Whiting, Wolff, and Kleijnen and Ms Misso and Mr Duffy drafted the protocol. Mr Duffy and Ms Misso conducted the literature searches. Drs Whiting, Wolff, and Lang screened searched results and selected full-text studies for inclusion. Drs Whiting, Wolff, Lang, Westwood, Keurentjes, Di Nisio, Hernandez, and Messrs Deshpande and Ryder, and Ms Schmidlkofer performed data extraction and risk-of-bias assessment. Dr Wolff performed the GRADE assessments. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Additional Contributions: We would like to thank Julie Harker (MRes, Kleijnen Systematic Reviews at the time of this project) for help with inclusion screening and data extraction and Gillian Worthy (MSc, Kleijnen Systematic Reviews) for advice on data analysis. Neither of these individuals received additional compensation in association with their work on this article.
Correction: This article was corrected online June 26, 2015, for incorrect axis labeling in Figure 4; on July 13, 2015, for a corrected average reduction to the Ashworth spasticity scale (as reported in the Abstract); on November 5, 2015, for an incorrect nonproprietary name and approved use for a drug in Table 1; and on April 12, 2016, for an incorrect effect estimate.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 10 December 2019
Benefits and harms of medical cannabis: a scoping review of systematic reviews
- Misty Pratt 1 ,
- Adrienne Stevens 1 , 2 ,
- Micere Thuku 1 ,
- Claire Butler 1 , 3 ,
- Becky Skidmore 4 ,
- L. Susan Wieland 5 ,
- Mark Clemons 6 , 7 ,
- Salmaan Kanji 6 , 8 , 9 &
- Brian Hutton ORCID: orcid.org/0000-0001-5662-8647 1 , 6
Systematic Reviews volume 8 , Article number: 320 ( 2019 ) Cite this article
46k Accesses
86 Citations
172 Altmetric
Metrics details
There has been increased interest in the role of cannabis for treating medical conditions. The availability of different cannabis-based products can make the side effects of exposure unpredictable. We sought to conduct a scoping review of systematic reviews assessing benefits and harms of cannabis-based medicines for any condition.
A protocol was followed throughout the conduct of this scoping review. A protocol-guided scoping review conduct. Searches of bibliographic databases (e.g., MEDLINE®, Embase, PsycINFO, the Cochrane Library) and gray literature were performed. Two people selected and charted data from systematic reviews. Categorizations emerged during data synthesis. The reporting of results from systematic reviews was performed at a high level appropriate for a scoping review.
After screening 1975 citations, 72 systematic reviews were included. The reviews covered many conditions, the most common being pain management. Several reviews focused on management of pain as a symptom of conditions such as multiple sclerosis (MS), injury, and cancer. After pain, the most common symptoms treated were spasticity in MS, movement disturbances, nausea/vomiting, and mental health symptoms. An assessment of review findings lends to the understanding that, although in a small number of reviews results showed a benefit for reducing pain, the analysis approach and reporting in other reviews was sub-optimal, making it difficult to know how consistent findings are when considering pain in general. Adverse effects were reported in most reviews comparing cannabis with placebo (49/59, 83%) and in 20/24 (83%) of the reviews comparing cannabis to active drugs. Minor adverse effects (e.g., drowsiness, dizziness) were common and reported in over half of the reviews. Serious harms were not as common, but were reported in 21/59 (36%) reviews that reported on adverse effects. Overall, safety data was generally reported study-by-study, with few reviews synthesizing data. Only one review was rated as high quality, while the remaining were rated of moderate ( n = 36) or low/critically low ( n = 35) quality.
Conclusions
Results from the included reviews were mixed, with most reporting an inability to draw conclusions due to inconsistent findings and a lack of rigorous evidence. Mild harms were frequently reported, and it is possible the harms of cannabis-based medicines may outweigh benefits.
Systematic review registration
The protocol for this scoping review was posted in the Open Access ( https://ruor.uottawa.ca/handle/10393/37247 ).
Peer Review reports
Interest in medical applications of marijuana ( Cannabis sativa ) has increased dramatically during the past 20 years. A 1999 report from the National Academies of Sciences, Engineering, and Medicine supported the use of marijuana in medicine, leading to a number of regulatory medical colleges providing recommendations for its prescription to patients [ 1 ]. An updated report in 2017 called for a national research agenda, improvement of research quality, improvement in data collection and surveillance efforts, and strategies for addressing barriers in advancing the cannabis agenda [ 2 ].
Proponents of medical cannabis support its use for a highly varied range of medical conditions, most notably in the fields of pain management [ 3 ] and multiple sclerosis [ 4 ]. Marijuana can be consumed by patients in a variety of ways including smoking, vaporizing, ingesting, or administering sublingually or rectally. The plant consists of more than 100 known cannabinoids, the main ones of relevance to medical applications being tetrahydrocannabinol (THC) and cannabidiol (CBD) [ 5 ]. Synthetic forms of marijuana such as dronabinol and nabilone are also available as prescriptions in the USA and Canada [ 6 ].
Over the last decade, there has been an increased interest in the use of medical cannabis products in North America. It is estimated that over 3.5 million people in the USA are legally using medical marijuana, and a total of USD$6.7 billion was spent in North America on legal marijuana in 2016 [ 7 ]. The number of Canadian residents with prescriptions to purchase medical marijuana from Health Canada–approved growers tripled from 30,537 in 2015 to near 100,000 in 2016 [ 8 ]. With the legalization of recreational-use marijuana in parts of the USA and in Canada in October 2018, the number of patients using marijuana for therapeutic purposes may become more difficult to track. The likely increase in the numbers of individuals consuming cannabis also necessitates a greater awareness of its potential benefits and harms.
Plant-based and plant-derived cannabis products are not monitored as more traditional medicines are, thereby increasing the uncertainty regarding its potential health risks to patients [ 3 ]. While synthetic forms of cannabis are available by prescription, different cannabis plants and products contain varied concentrations of THC and CBD, making the effects of exposure unpredictable [ 9 ]. While short-lasting side effects including drowsiness, loss of short-term memory, and dizziness are relatively well known and may be considered minor, other possible effects (e.g., psychosis, paranoia, anxiety, infection, withdrawal) may be more harmful to patients.
There remains a considerable degree of clinical equipoise as to the benefits and harms of marijuana use for medical purposes [ 10 , 11 , 12 , 13 ]. To understand the extent of synthesized evidence underlying this issue, we conducted a scoping review [ 14 ] of systematic reviews evaluating the benefits and/or harms of cannabis (plant-based, plant-derived, and synthetic forms) for any medical condition. We located and mapped systematic reviews to summarize research that is available for consideration for practice or policy questions in relation to medical marijuana.
A scoping review protocol was prepared and posted to the University of Ottawa Health Sciences Library’s online repository ( https://ruor.uottawa.ca/handle/10393/37247 ). We used the PRISMA for Scoping Reviews checklist to guide the reporting of this report (see Additional file 1 ) [ 15 ].
Literature search and process of study selection
An experienced medical information specialist developed and tested the search strategy using an iterative process in consultation with the review team. Another senior information specialist peer-reviewed the strategy prior to execution using the PRESS Checklist [ 16 ]. We searched seven Ovid databases: MEDLINE®, including Epub Ahead of Print and In-Process & Other Non-Indexed Citations, Embase, Allied and Complementary Medicine Database, PsycINFO, the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, and the Health Technology Assessment Database. The final peer-reviewed search strategy for MEDLINE was translated to the other databases (see Additional file 2 ). We performed the searches on November 3, 2017.
The search strategy incorporated controlled vocabulary (e.g., “Cannabis,” “Cannabinoids,” “Medical Marijuana”) and keywords (e.g., “marijuana,” “hashish,” “tetrahydrocannabinol”) and applied a broad systematic review filter where applicable. Vocabulary and syntax were adjusted across the databases and where possible animal-only and opinion pieces were removed, from the search results.
Gray literature searching was limited to relevant drug and mental health databases, as well as HTA (Health Technology Assessment) and systematic review databases. Searching was guided by the Canadian Agency for Drugs and Technologies in Health’s (CADTH) checklist for health-related gray literature (see Additional file 3 ). We performed searches between January and February 2018. Reference lists of overviews were searched for relevant systematic reviews, and we searched for full-text publications of abstracts or protocols.
Management of all screening was performed using Distiller SR Software ® (Evidence Partners Inc., Ottawa, Canada). Citations from the literature search were collated and de-duplicated in Reference Manager (Thomson Reuters: Reference Manager 12 [Computer Program]. New York: Thomson Reuters 2011), and then uploaded to Distiller. The review team used Distiller for Levels 1 (titles and abstracts) and 2 (full-text) screening. Pilot testing of screening questions for both levels were completed prior to implementation. All titles and abstracts were screened in duplicate by two independent reviewers (MT and MP) using the liberal accelerated method [ 17 ]. This method requires only one reviewer to assess an abstract as eligible for full-text screening, and requires two reviewers to deem the abstract irrelevant. Two independent reviewers (MT and MP) assessed full-text reports for eligibility. Disagreements during full-text screening were resolved through consensus, or by a third team member (AS). The process of review selection was summarized using a PRISMA flow diagram (Fig. 1 ) [ 18 ].
PRISMA-style flow diagram of the review selection process
Review selection criteria
English-language systematic reviews were included if they reported that they investigated harms and/or benefits of medical or therapeutic use of cannabis for adults and children for any indication. Definitions related to medical cannabis/marijuana are provided in Table 1 . We also included synthetic cannabis products, which are prescribed medicines with specified doses of THC and CBD. Reviews of solely observational designs were included only in relation to adverse effects data, in order to focus on the most robust evidence available. We considered studies to be systematic reviews if at least one database was searched with search dates reported, at least one eligibility criterion was reported, the authors had assessed the quality of included studies, and there was a narrative or quantitative synthesis of the evidence. Reviews assessing multiple interventions (both pharmacological and complementary and alternative medicine (CAM) interventions) were included if the data for marijuana studies was reported separately. Published and unpublished guidelines were included if they conducted a systematic review encompassing the criteria listed above.
We excluded overviews of systematic reviews, reviews in abstract form only, and review protocols. We further excluded systematic reviews focusing on recreational, accidental, acute, or general cannabis use/abuse and interventions such as synthetic cannabinoids not approved for therapeutic use (e.g., K2 or Spice).
Data collection and quality assessment
All data were collected electronically in a pre-developed form using Microsoft Excel software (Microsoft Corporation, Seattle, USA). The form was pilot tested on three included reviews by three people. One reviewer (MP or CB) independently extracted all data, and a second reviewer (MT) verified all of the items collected and checked for any omitted data. Disagreements were resolved by consensus and consultation with a third reviewer if necessary. A data extraction form with the list of included variables is provided in Additional file 4 . All collected data has also been made available in the online supplemental materials associated with this report.
Quality assessment of systematic reviews was performed using the AMSTAR-2 [ 20 ] tool. One reviewer (MP or CB) independently assessed quality, while a second reviewer (MT) verified the assessments. Disagreements were resolved by consensus and consultation with a third reviewer if necessary. The tool consists of 16 items in total, with four critical domains and 12 non-critical domains. The AMSTAR-2 tool is not intended to generate an overall score, and instead allows for an overall rating based on weaknesses in critical domains. Reviews were rated as high (no critical flaws with zero or one non-critical flaw), moderate (no critical flaws with ≥ 1 non-critical flaw), low (one critical flaw with/without non-critical weakness), or critically low (> 1 critical flaw with/without non-critical weakness) quality.
Evidence synthesis
We used a directed content analytic approach [ 21 ] with an initial deductive framework [ 22 ] that allowed flexibility for inductive analysis if refinement or development of new categorization was needed. The framework used to categorize outcome data results is outlined in Table 2 . Where reviews had a mix of narrative and quantitative data, results from meta-analyses were prioritized over count data or study-by-study data. The extraction and reporting of data results was performed at a high level and did not involve an in-depth evaluation, which is appropriate for a scoping review [ 14 ]. Review authors’ conclusions and/or recommendations were extracted and reported narratively.
Changes from the study protocol
For feasibility, we decided to limit the inclusion of systematic reviews of only observational study designs to those that addressed adverse events data. All other steps of the review were performed as planned.
Search findings
The PRISMA flow diagram describing the process of review selection is presented in Fig. 1 . After duplicates were removed, the search identified a total of 1925 titles and abstracts, of which 47 references were located through the gray literature search. Of the total 1925 citations assessed during Level 1 screening, 1285 were deemed irrelevant. We reviewed full-text reports for the 640 reviews of potential relevance, and of these, 567 were subsequently excluded, leaving a total of 72 systematic reviews that were included; the associated data collected are provided in Additional file 5 . A listing of the reports excluded during full-text review is provided in Additional file 6 .
Characteristics of included reviews
There were 63 systematic reviews [ 4 , 19 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 ] and nine guidelines with systematic reviews [ 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 ]. Overall, 27 reviews were performed by researchers in Europe, 16 in the USA, 15 in Canada, eight in Australia, two in Brazil, and one each in Israel, Singapore, South Africa, and China. Funding was not reported in 29 (40%) of the reviews, and the remaining reviews received funding from non-profit or academic ( n = 20; 28%), government ( n = 14; 19%), industry ( n = 3; 4%), and mixed ( n = 1; 1%) sources. Five reviews reported that they did not receive any funding for the systematic review. Tables 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , and 13 provide an overview of the characteristics of the 72 included systematic reviews.
The reviews were published between 2000 and 2018 (median year 2014), and almost half (47%) were focused solely on medical cannabis. Four (6%) reviews covered both medical and other cannabis use (recreational and substance abuse), 19 (26%) reported multiple pharmaceutical interventions (cannabis being one), six (8%) reported various CAM interventions (cannabis being one), and nine (13%) were mixed pharmaceutical and CAM interventions (cannabis being one). Multiple databases were searched by almost all of the reviews (97%), with Medline/PubMed or Embase common to all.
Cannabis use
Figure 2 illustrates the different cannabis-based interventions covered by the included reviews. Plant-based cannabis consists of whole plant products such as marijuana or hashish. Plant-derived cannabinoids are active constituents of the cannabis plant, such as tetrahydrocannabinol (THC), cannabidiol (CBD), or a combination of THC:CBD (also called nabiximols, under the brand name Sativex) [ 3 ]. Synthetic cannabinoids are manufactured rather than extracted from the plant and include drugs such as nabilone and dronabinol.
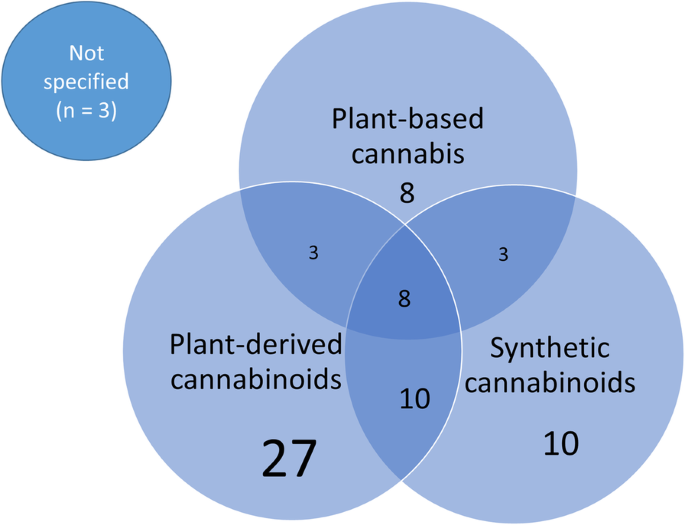
Review coverage of the various cannabis-based interventions
Twenty-seven reviews included solely interventions from plant-derived cannabinoids, 10 studied solely synthetic cannabinoids, and eight included solely studies on plant-based cannabis products. Twenty-four reviews covered a combination of different types of cannabis, and the remaining three systematic reviews did not report which type of cannabinoid was administered in the included studies.
The systematic reviews covered a wide range of conditions and illnesses, the most notable being pain management. Seventeen reviews looked at specific types of pain including neuropathic [ 31 , 42 , 62 , 69 , 85 , 90 ], chronic [ 26 , 32 , 52 , 58 , 80 ], cancer [ 84 , 87 ], non-cancer [ 41 , 68 ], and acute [ 38 ] types of pain (one review covered all types of pain) [ 65 ]. Twenty-seven reviews (38%) also focused on management of pain as a symptom of conditions such as multiple sclerosis (MS) [ 6 , 23 , 27 , 43 , 46 , 52 , 63 , 85 , 92 ], injury [ 29 , 35 , 36 , 69 ], cancer [ 37 , 43 , 65 , 88 ], inflammatory bowel disease (IBD) [ 28 ], rheumatic disease (RD) [ 49 , 51 , 73 ], diabetes [ 68 , 69 , 70 ], and HIV [ 48 , 53 , 67 ]. In Fig. 3 , the types of illnesses addressed by the set of included reviews are graphically represented, with overlap between various conditions and pain. Some systematic reviews covered multiple diseases, and therefore the total number of conditions represented in Fig. 3 is greater than the total number of included reviews.
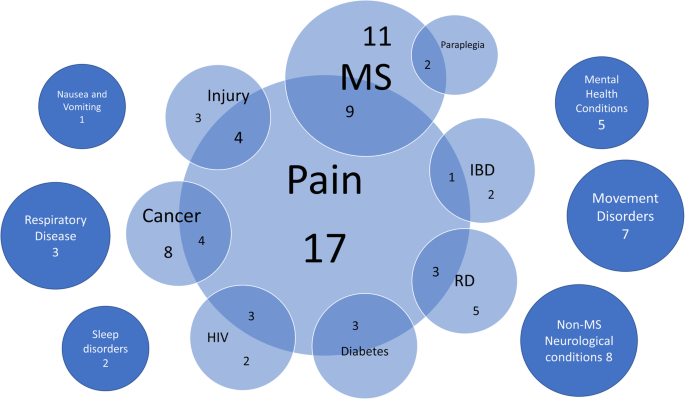
Conditions or symptoms across reviews that were treated with cannabis. IBD inflammatory bowel disease, MS multiple sclerosis, RD rheumatic disease
One review included a pediatric-only population, in the evaluation of marijuana for nausea and vomiting following chemotherapy [ 54 ]. Although trials in both adult and child populations were eligible for thirteen (18%) reviews, only two additional reviews included studies in children; these reviews evaluated cannabis in cancer [ 60 ] and a variety of conditions [ 25 ]. Many of the reviews ( n = 25, 35%) included only adults ≥ 18 years of age. Almost half of the reviews ( n = 33, 46%) did not report a specific population for inclusion.
Cannabis was prescribed for a wide range of medical issues. The indication for cannabis use is illustrated in Fig. 4 . Pain management ( n = 27) was the most common indication for cannabis use. A number of reviews sought to address multiple disease symptoms ( n = 12) or explored a more holistic treatment for the disease itself ( n = 11). After pain, the most common symptoms being treated with cannabis were spasticity in MS, movement disturbances (such as dyskinesia, tics, and spasms), weight or nausea/vomiting, and mental health symptoms.
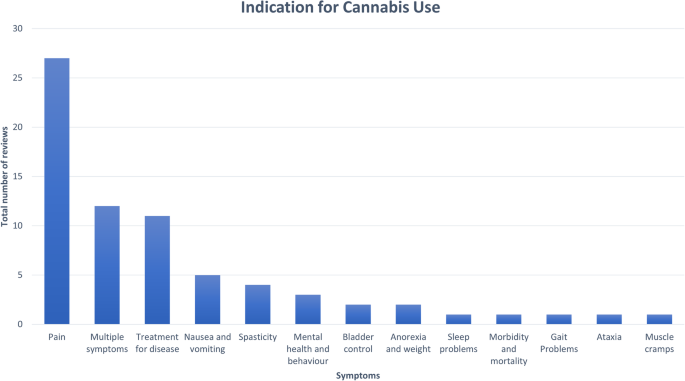
Indications for cannabis use across included reviews
Figure 5 summarizes the breadth of outcomes analyzed in the included reviews. The most commonly addressed outcomes were withdrawal due to adverse effects, “other pain,” neuropathic pain, spasticity, and the global impression of the change in clinical status. Many outcomes were reported using a variety of measures across reviews. For example, spasticity was measured both objectively (using the Ashworth scale) and subjectively (using a visual analog scale [VAS] or numerical rating scale [NRS]). Similarily, outcomes for pain included VAS or NRS scales, reduction in pain, pain relief, analgesia, pain intensity, and patient assessment of change in pain.
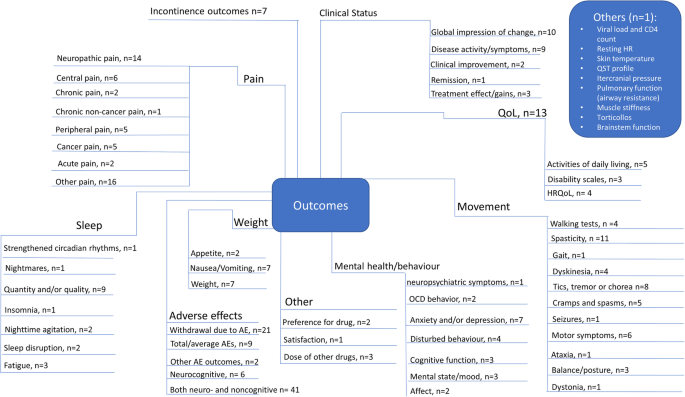
Quality of the systematic reviews
Quality assessments of the included reviews based upon AMSTAR-2 are detailed in Additional file 7 and Additional file 8 . Only one review was rated as high quality [ 45 ]. All other reviews were deemed to be of moderate ( n = 36) or low/critically low ( n = 35) methodological quality. Assessments for the domains deemed of critical importance for determining quality ratings are described below.
Only 20% of reviews used a comprehensive search strategy; another 47% were given a partial score because they had not searched the reference lists of the included reviews, trial registries, gray literature, and/or the search date was older than 2 years. The remaining reviews did not report a comprehensive search strategy.
Over half of the reviews (51%) used a satisfactory technique for assessing risk of bias (ROB) of the individual included studies, while 35% were partially satisfactory because they had not reported whether allocation sequence was truly random and/or they had not assessed selective reporting. The remaining reviews did not report a satisfactory technique for assessing ROB.
Most reviews (71%) could not be assessed for an appropriate statistical method for combining results in a meta-analysis, as they synthesized study data narratively. Approximately 19% of reviews used an appropriate meta-analytical approach, leaving 10% that used inappropriate methods.
The final critical domain for the AMSTAR-2 determines whether review authors accounted for ROB in individual studies when discussing or interpreting the results of the review. The majority of reviews (83%) did so in some capacity.
Mapping results of included systematic reviews
We mapped reviews according to authors’ comparisons, the conditions or symptoms they were evaluating, and the categorization of the results (see Table 2 ). In some cases, reviews contributed to more than one comparison (e.g., cannabis versus placebo or active drug). As pain was the most commonly addressed outcome, we mapped this outcome separately from all other endpoints. This information is shown for all reviews and then restricted to reviews of moderate-to-high quality (as determined using the AMSTAR-2 criteria): cannabis versus placebo (Figs. 6 and 7 ), cannabis versus active drugs (Figs. 8 and 9 ), cannabis versus a combination of placebo and active drug (Figs. 10 and 11 ), one cannabis formulation versus other (Figs. 12 and 13 ), and cannabis analyzed against all other comparators (Fig. 14 ). Details on how to read the figures are provided in the corresponding figure legends. The median number of included studies across reviews was four, and ranged from one to seventy-nine (not shown in figures).
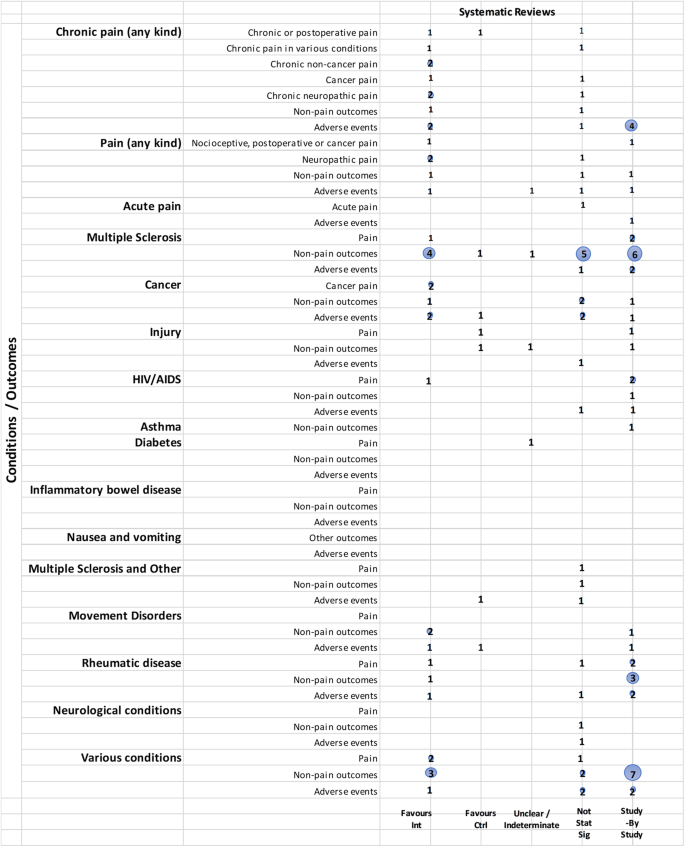
Cannabis vs. placebo. Authors’ presentations of the findings were mapped using the categorization shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
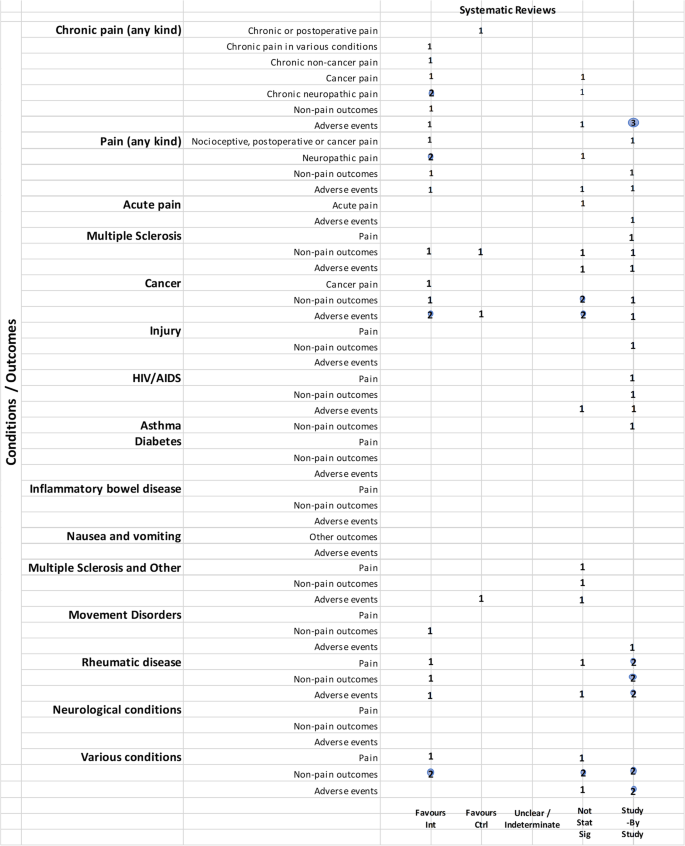
Cannabis vs. placebo, high and moderate quality reviews. Authors’ presentations of the findings were mapped using the categorizations shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
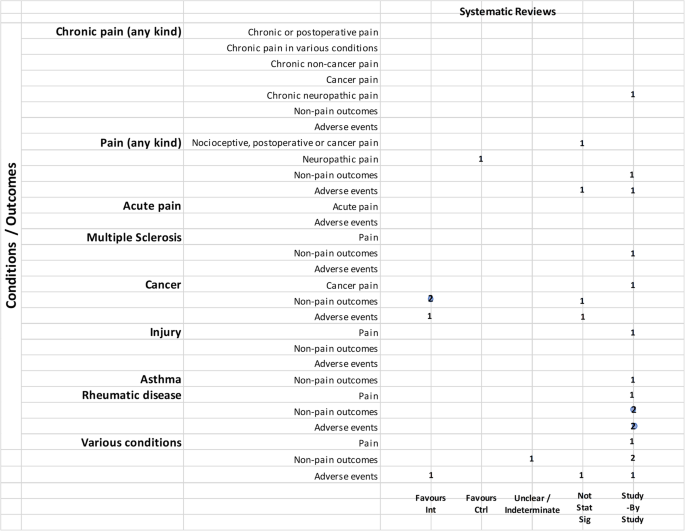
Cannabis vs. active drugs. Authors’ presentations of the findings were mapped using the categorizations shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
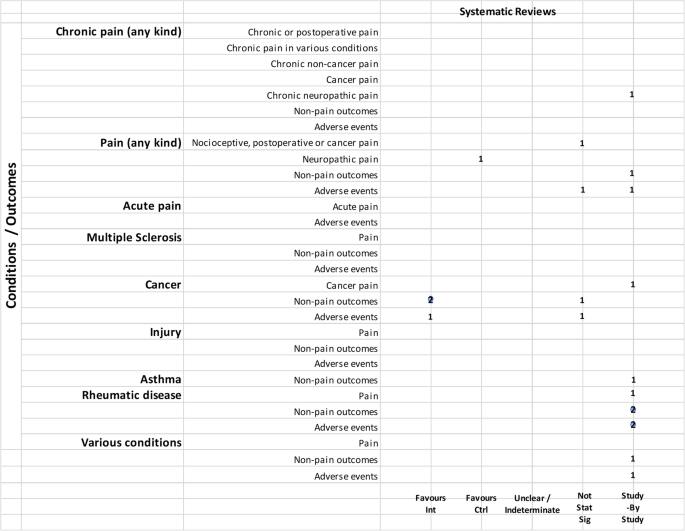
Cannabis vs. active drugs, high and moderate quality reviews. Authors’ presentations of the findings were mapped using the categorizations shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
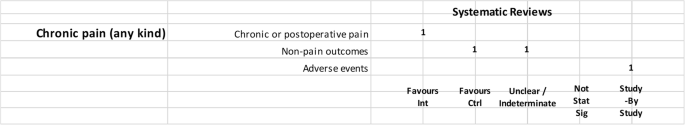
Cannabis vs. placebo + active drug. Authors’ presentations of the findings were mapped using the categorizations shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
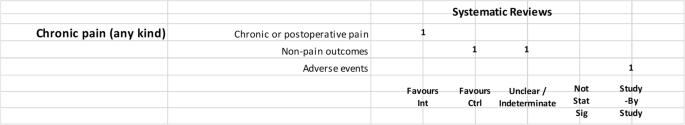
Cannabis vs. placebo + active drug, high and moderate quality reviews. Authors’ presentations of the findings were mapped using the categorizations shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
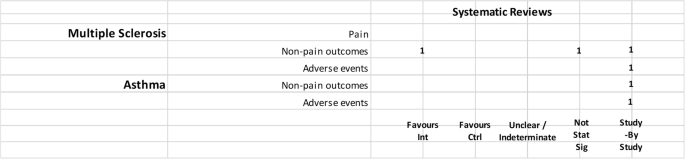
One cannabis formulation vs. other. Authors’ presentations of the findings were mapped using the categorizations shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
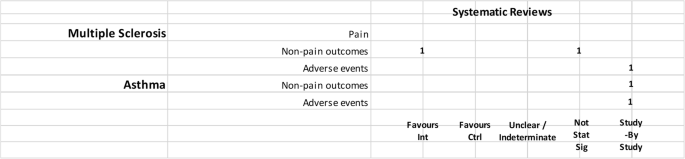
One cannabis formulation vs. other, high and moderate quality reviews. Authors’ presentations of the findings were mapped using the categorizations shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
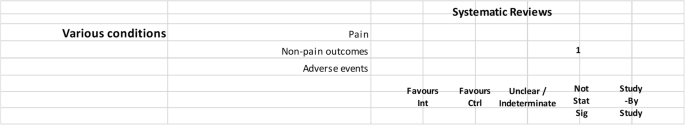
Cannabis vs. all comparators combined. Authors’ presentations of the findings were mapped using the categorizations shown in Table 2 . According to the reviews’ intended scope for the condition being treated, outcomes were mapped into “pain,” “non-pain outcomes,” and “adverse events.” For each condition and outcome pair (i.e., each row in the grid), the number of reviews reporting findings is shown according to the results categorization. For pain, reviews numbered in different categories signal discordant findings across those reviews. For non-pain outcomes, reviews presenting findings in the different categories would signal different results for different outcomes, as well as discordant findings within and across reviews. Adverse events are grouped as a whole and “favors intervention” would be interpreted as a decrease in events with cannabis when compared with the control group. Favors int = favors intervention; Favors Ctrl = favors control; Not stat sig = not statistically significant
Cannabis versus placebo
Most reviews (59/72, 82%) compared cannabis with placebo. Of these reviews, 34 (58%) addressed pain outcomes and 47 (80%) addressed non-pain outcomes, with most outcomes addressed by three reviews or fewer (Fig. 6 ). Some reviews had a mix of quantitative syntheses and study-by-study data reported (13/59, 22%), while another group of reviews (14/59, 24%) only reported results study-by-study. Overall, 24% (14/59) of the cannabis versus placebo reviews had only one included study.
Pain outcomes
Reviews focused on addressing pain across conditions. In most cases, findings were discordant across reviews for the pain outcomes measured. For chronic non-cancer pain, however, two reviews favored cannabis over placebo for decreasing pain. One review assessing acute pain for postoperative pain relief found no difference between various cannabinoid medications and placebo. The distribution of findings was similar when restricting to moderate-to-high-quality reviews.
Reviews focused on treating a condition or family of related conditions . Various results were observed for pain. For MS and HIV/AIDS, one review each reported quantitative results favoring cannabis for decreased pain but with other reviews reporting results study-by-study, it is difficult to know, broadly, how consistent those findings are. For cancer, two reviews reported results favoring cannabis for decreased pain. For rheumatic disease, findings are discordant between two reviews, and another two reviews reported results study-by-study. One review that included studies of MS or paraplegia found no difference in pain between groups. For treating injury, one review showed that the placebo group had less pain and one review reported data study-by-study. No reviews addressed pain in movement disorders, neurological conditions, and IBD.
For those reviews assessing pain as part of a focus on treating a range of conditions, two showed cannabis reduced pain [ 43 , 52 ], but one showed mixed results depending on how pain was measured [ 43 ]. These reviews covered several different conditions, including injury, chronic pain, rheumatoid arthritis, osteoarthritis, fibromyalgia, HIV/AIDS, cancer, and MS or paraplegia.
When restricting to moderate-to-high-quality reviews, only one review each in multiple sclerosis and HIV/AIDS with a study-by-study analysis on pain remained. One review on cancer favored cannabis for pain reduction. Findings remained the same for MS or paraplegia and rheumatic disease. No review for injury and paint outcomes was of higher quality.
Non-pain outcomes
The types of non-pain outcomes included in the reviews varied by condition/illness. The most commonly reported outcomes (see Fig. 5 for overall outcomes) when comparing cannabis to placebo included muscle- or movement-related outcomes ( n = 20), quality of life ( n = 14), and sleep outcomes ( n = 10).
There was no consistent pattern for non-pain outcomes either within or across medical conditions. Many ( n = 24, 33%) reviews assessing non-pain outcomes reported the results of those analyses study-by-study. Conflicting results are observed in some cases due to the use of different measures, such as different ways of quantifying spasticity in patients with multiple sclerosis [ 56 , 91 ]. One review each addressing neurological conditions [ 50 ] (outcome: muscle cramps) and MS/paraplegia [ 27 ] (outcomes: spasticity, spasm, cognitive function, daily activities, motricity, and bladder function) showed no difference between groups.
Adverse effects
Adverse effects were reported in most reviews comparing cannabis with placebo (49/59, 83%). Most adverse events were reported study-by-study, with few reviews ( n = 16/59, 27%) conducting a narrative or quantitative synthesis. Serious adverse effects were reported in 21/59 (36%) reviews, and minor adverse effects were reported in 30/59 (51%) reviews. The remaining reviews did not define the difference between serious and minor adverse events. The most commonly reported serious adverse events included psychotic symptoms ( n = 6), severe dysphoric reactions ( n = 3), seizure ( n = 3), and urinary tract infection ( n = 2). The most commonly reported minor adverse events included somnolence/drowsiness ( n = 28), dizziness ( n = 27), dry mouth ( n = 20), and nausea ( n = 18). Many reviews ( n = 37/59, 63%) comparing cannabis to placebo reported both neurocognitive and non-cognitive adverse effects. Withdrawals due to adverse events were reported in 22 (37%) reviews.
Of the moderate-/high-quality reviews, adverse effect analyses were reported in reviews on pain, multiple sclerosis, cancer, HIV/AIDS, movement disorders, rheumatic disease, and several other conditions. Two reviews on pain showed fewer adverse events with cannabis for euphoria, events linked to alternations in perception, motor function, and cognitive function, withdrawal due to adverse events, sleep, and dizziness or vertigo [ 58 , 90 ]. One review on MS showed that there was no statistically significant difference between cannabis and placebo for adverse effects such as nausea, weakness, somnolence, and fatigue [ 91 ], while another review on MS/paraplegia reported fewer events in the placebo group for dizziness, somnolence, nausea, and dry mouth [ 27 ]. Within cancer reviews, one review found no statistically significant difference between cannabis and placebo for dysphoria or sedation but reported fewer events with placebo for “feeling high,” and fewer events with cannabis for withdrawal due to adverse effects [ 40 ]. In rheumatic disease, one review reported fewer total adverse events with cannabis and found no statistically significant difference between cannabis and placebo for withdrawal due to adverse events [ 51 ].
Cannabis versus other drugs
Relatively fewer reviews compared cannabis with active drugs ( n = 23/72, 32%) (Fig. 8 ). Many of the reviews did not synthesize studies quantitatively, and results were reported study-by-study. The most common conditions in reviews comparing cannabis to active drugs were pain, cancer, and rheumatic disease. Comparators included ibuprofen, codeine, diphenhydramine, amitriptyline, secobarbital, prochlorperazine, domperidone, metoclopramide, amisulpride, neuroleptics, isoproterenol, megestrol acetate, pregabalin, gabapentin, and opioids.
Reviews focused on addressing pain across conditions. When comparing across reviews, a mix of results are observed (see Fig. 8 ), and some were reported study-by-study. One review found no statistically significant difference between cannabinoids and codeine for nociceptive pain, postoperative pain, and cancer pain [ 65 ]. Another review favored “other drugs” (amitriptyline and pregabalin) over cannabinoids for neuropathic pain [ 90 ]. The distribution of findings was similar when restricting to moderate-to-high-quality reviews.
Reviews focused on treating a condition or family of related conditions. One review on cancer compared cannabinoids and codeine or secobarbital and reported pain results study-by-study. Another review on fibromyalgia comparing synthetic cannabinoids with amitriptyline also reported pain data study-by-study [ 39 ].
Two reviews on cancer favored cannabinoids over active drugs (prochlorperazine, domperidone, metoclopramide, and neuroleptics) for patient preference and anti-emetic efficacy [ 40 , 60 ]. Non-pain outcomes were reported study-by-study for the outcome of sleep in neuropathic pain [ 90 ] and rheumatic disease [ 39 , 49 ]. In a review covering various conditions (pain, MS, anorexia, cancer, and immune deficiency), results were unclear or indeterminate for subjective measures of sleep [ 46 ].
Adverse effects were reported in 20/24 (83%) of the reviews comparing cannabis to active drugs, and only 6/20 (30%) reported a narrative or quantitative synthesis. Many reviews that reported narrative data did not specify whether adverse effects could be attributed to a placebo or active drug comparator.
Of the moderate-to-high-quality reviews, two pain reviews found no statistically significant difference for cannabis compared to codeine or amitriptyline for withdrawals due to adverse events [ 65 , 90 ]. Results from one cancer review were mixed, with fewer adverse events for cannabis (compared to prochlorperazine, domperidone, or metoclopramide) or no difference between groups, depending on the type of subgroup analysis that was conducted [ 40 ].
Cannabis + active drugs versus placebo + active drugs
Two reviews compared cannabis with placebo cannabis in combination with an active drug (opioids and gabapentin) (Figs. 10 and 11 ). Both were scored to be of moderate quality. Although one review showed that cannabis plus opioids decreased chronic pain [ 80 ], another review on pain in MS included only a single study [ 81 ], precluding the ability to determine concordance of results. Cannabis displayed varied effects on non-pain outcomes, including superiority of placebo over cannabis for some outcomes. One review reported withdrawal due to adverse events study-by-study and also reported that side effects such as nausea, drowsiness, and dizziness were more frequent with higher doses of cannabinoids (data from two included studies) [ 80 ].
Cannabis versus other cannabis comparisons
Six (8%) reviews compared different cannabis formulations or doses (Figs. 12 and 13 ). Almost all were reported as study-by-study results, with two reviews including only one RCT. One review for PTSD found only observational data [ 33 ] and another review on anxiety and depression combined data from one RCT with cross-sectional study data [ 19 ]. A single review on MS reported a narrative synthesis that found a benefit for spasticity. However, it was unclear if the comparator was placebo or THC alone [ 56 ]. Four reviews reported adverse effects study-by-study, with a single review comparing side effects from different dosages; in this review, combined extracts of THC and CBD were better tolerated than extracts of THC alone [ 56 ].
Cannabis versus all comparators
One review combined all comparators for the evaluation (Fig. 14 ). The review (combining non-users, placebo and ibuprofen) covered a range of medical conditions and was rated as low quality [ 30 ]. No adverse effects were evaluated for this comparison.
Mapping the use of quality assessment and frameworks to interpret the strength of evidence
Although 83% of reviews incorporated risk of bias assessments in their interpretation of the evidence, only 11 (15%) reviews used a framework such as GRADE to evaluate important domains other than risk of bias that would inform the strength of the evidence.
Mapping authors’ conclusions or recommendations
Most reviews (43/72 60%) indicated an inability to draw conclusions, whether due to uncertainty, inconsistent findings, lack of (high quality) evidence, or focusing their conclusion statement on the need for more research. Almost 15% of reviews (10/72) reported recommendations or conclusions that included some uncertainty. One review (1%) provided a statement of the extent of the strength of the evidence, which differed according to outcome.
Eleven reviews provided clearer conclusions (14%). Four indicated that cannabis was not effective or not cost-effective compared to placebo in relation to multiple sclerosis, acute pain, cancer, and injury. Three reviews addressing various conditions provided varying conclusions: one stated cannabis was not effective, one indicated it was modestly safe and effective, and one concluded that cannabis was safe and efficacious as short-term treatment; all reviews were of low quality. The three remaining reviews stated moderate or modest effects for improving chronic pain, compared with placebo or other analgesia; two of those reviews were of medium AMSTAR-2 quality, and one used the GRADE framework for interpreting the strength of the evidence.
The eight remaining included reviews (11%) did not provide a clear conclusion statement or reported only limitations.
Mapping authors’ limitations of the research
Several of the reviews indicated that few studies, small sample sizes, short duration of treatment, and issues related to outcomes (e.g., definition, timing, and types) were drawbacks to the literature. Some reviews noted methodological issues with and heterogeneity among studies as limitations. A few authors stated that restricting eligibility to randomized trials, English-language studies, or full publications may have affected their review results.
With the increasing use of medical cannabis, an understanding of the landscape of available evidence syntheses is needed to support evidence-informed decision-making, policy development, and to inform a research agenda. In this scoping review, we identified 72 systematic reviews evaluating medical cannabis for a range of conditions and illnesses. Half of the reviews were evaluated as being of moderate quality, with only one review scoring high on the AMSTAR-2 assessment tool.
There was disparity in the reported results across reviews, including non-synthesized (study-by-study) data, and many were unable to provide a definitive statement regarding the effectiveness of cannabis (as measured by pain reduction or other relevant outcomes), nor the extent of increased side effects and harms. This is consistent with the limitations declared in general across reviews, such as the small numbers of relevant studies, small sample sizes of individual studies, and methodological weaknesses of available studies. This common theme in review conclusions suggests that while systematic reviews may have been conducted with moderate or high methodological quality, the strength of their conclusions are driven by the availability and quality of the relevant underlying evidence, which was often found to be limited.
Relatively fewer reviews addressed adverse effects associated with cannabis, except to narratively summarize study level data. Although information was provided for placebo-controlled comparisons, none of the comparative effectiveness reviews quantitatively assessed adverse effects data. For the placebo-controlled data, although the majority of adverse effects were mild, the number of reviews reporting serious adverse effects such as psychotic symptoms [ 25 , 42 ] and suicidal ideation [ 68 , 85 ] warrants caution.
A mix of reviews supporting and not supporting the use of cannabis, according to authors’ conclusions, was identified. Readers may wish to consider the quality of the reviews, the use of differing quality assessment tools, additional considerations covered by the GRADE framework, and the potential for spin as possible reasons for these inconsistencies. It is also possible that cannabis has differing effects depending on its type (e.g., synthetic), dose, indication, the type of pain being evaluated (e.g., neuropathic), and the tools used for outcome assessment, which can be dependent on variations in condition. Of potential interest to readers may be a closer examination of the reviews evaluating chronic pain, in order to locate the source(s) of discordance. For example, one review was deemed of moderate quality, used the GRADE framework, and rated the quality of evidence for the effectiveness of cannabis for reducing neuropathic pain as moderate, suggesting that further investigation of cannabis for neuropathic pain may be warranted [ 80 ]. The exploration aspects outlined in this paragraph are beyond the purview of scoping review methodology; a detailed assessment of the reviews, including determining the overlap of included studies among similar reviews, potential reasons for the observed discordance of findings, what re-analysis of study-by-study analyses would yield, and an undertaking of missing GRADE assessments would fall outside the bounds of a scoping review and require the use of overview methodology [ 14 ].
Our findings are consistent with a recently published summary of cannabis-based medicines for chronic pain management [ 3 ]. This report found inconsistent results in systematic reviews of cannabis-based medicines compared to placebo for chronic neuropathic pain, pain management in rheumatic diseases and painful spasms in MS. The authors also concluded that cannabis was not superior to placebo in reducing cancer pain. Four out of eight included reviews scored high on the original AMSTAR tool. The variations between the two tools can be attributed to the differences in our overall assessments. Lastly, the summary report included two reviews that were not located in our original search due to language [ 93 ] and the full-text [ 94 ] of an abstract [ 95 ] that was not located in our search.
This scoping review has identified a plethora of synthesized evidence in relation to medical cannabis. For some conditions, the extent of review replication may be wasteful. Many reviews have stated that additional trials of methodologically robust design and, where possible, of sufficient sample size for precision, are needed to add to the evidence base. This undertaking may require the coordination of multi-center studies to ensure adequate power. Future trials may also help to elucidate the effect of cannabis on different outcomes.
Given authors’ reporting of issues in relation to outcomes, future prospective trials should be guided by a standardized, “core” set of outcomes to strive for consistency across studies and ensure relevance to patient-centered care. Development of those core outcomes should be developed using the Core Outcome Measures in Effectiveness Trials (COMET) methodology [ 96 ], and further consideration will need to be made in relation to what outcomes may be common across all cannabis research and which outcomes are condition-specific. With maturity of the evidence base, future systematic reviews should seek and include non-journal-published (gray literature) reports and ideally evaluate any non-English-language papers; authors should also adequately assess risk of bias and undertake appropriate syntheses of the literature.
The strengths of this scoping review include the use of an a priori protocol, peer-reviewed search strategies, a comprehensive search for reviews, and consideration of observational designs for adverse effects data. For feasibility, we restricted to English-language reviews, and it is unknown how many of the 39 reviews in other languages that we screened would have met our eligibility criteria. The decision to limit the inclusion of reviews of observational data to adverse effects data was made during the process of full-text screening and for pragmatic reasons. We also did not consider a search of the PROSPERO database for ongoing systematic reviews; however, in preparing this report, we performed a search and found that any completed reviews were already considered for eligibility or were not available at the time of our literature search. When charting results, we took a broad perspective, which may be different than if these reviews were more formally assessed during an overview of systematic reviews.
Cannabis-based medicine is a rapidly emerging field of study, with implications for both healthcare practitioners and patients. This scoping review is intended to map and collate evidence on the harms and benefits of medical cannabis. Many reviews were unable to provide firm conclusions on the effectiveness of medical cannabis, and results of reviews were mixed. Mild adverse effects were frequently but inconsistently reported, and it is possible that harms may outweigh benefits. Evidence from longer-term, adequately powered, and methodologically sound RCTs exploring different types of cannabis-based medicines is required for conclusive recommendations.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Abbreviations
Canadian Agency for Drugs and Technologies in Health
Complementary and alternative medicine
Cannabidiol
Grading of Recommendations Assessment, Development and Evaluation
Human immunodeficiency virus
Inflammatory bowel disease
Multiple sclerosis
Numeric rating scale
Randomized controlled trial
Rheumatic disease
Risk of bias
Tetrahydrocannabinol
Visual analog scale
Watson SJ, Benson JA, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57:547–52.
Article CAS PubMed Google Scholar
National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice & Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. US: National Academies Press; 2017.
Google Scholar
Häuser W, Petzke F, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management - an overview of systematic reviews. Eur J Pain Lond Engl. 2018;22:455–70.
Article Google Scholar
Herzog S, et al. Systematic review of the costs and benefits of prescribed cannabis-based medicines for the management of chronic illness: lessons from multiple sclerosis. Pharmacoeconomics. 2017.
Aizpurua-Olaizola O, et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016;79:324–31.
Canadian Agency for Drugs and Technologies in Health. Nabilone for chronic pain management: a review of clinical effectiveness, safety, and guidelines. Rapid Response Rep. 2011.
Number of Legal Medical Marijuana Patients - Medical Marijuana - ProCon.org. https://medicalmarijuana.procon.org/view.resource.php?resourceID=005889 .
Miller, J., December 12, O. C. U. & 2016. Number of Canadians buying legal medical marijuana triples in past year | Ottawa Citizen. https://ottawacitizen.com/news/local-news/number-of-canadians-buying-legal-medical-marijuana-triples-in-just-one-year (2016).
Colizzi M, Bhattacharyya S. Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr Addict Rep. 2017;4:62–74.
Article PubMed PubMed Central Google Scholar
DuPont RL. Examining the debate on the use of medical marijuana. Proc Assoc Am Physicians. 1999;111:166–72.
Cohen PJ. Medical marijuana: the conflict between scientific evidence and political ideology. Part one of two. J Pain Palliat Care Pharmacother. 2009;23:4–25.
Article PubMed Google Scholar
Cohen PJ. Medical marijuana: the conflict between scientific evidence and political ideology. Part two of two. J Pain Palliat Care Pharmacother. 2009;23:120–40.
Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin. Proc. 2012;87:172–86.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
Tricco AC, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–73.
McGowan J, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6.
Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1:10.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64.
Walsh Z, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15–29.
Shea BJ, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Hsief H, Shannon S. Three approaches to qualitative content analysis. Qual Health Res. 2005;15.
Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62:107–15.
Claflin SB, van der Mei IAF, Taylor BV. Complementary and alternative treatments of multiple sclerosis: a review of the evidence from 2001 to 2016. J Neurol Neurosurg Psychiatr. 2017.
Behm K, Morgan P. The effect of symptom-controlling medication on gait outcomes in people with multiple sclerosis: a systematic review. Disabil Rehabil. 2017:1–12.
Lim K, See YM, Lee J. A systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disorders. Clin Psychopharmacol Neurosci Off Sci J Korean Coll Neuropsychopharmacol. 2017;15:301–12.
Article CAS Google Scholar
Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20:E755–96.
CAS PubMed Google Scholar
da Rovare VP, et al. Cannabinoids for spasticity due to multiple sclerosis or paraplegia: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med. 2017;34:170–85.
Norton C, Czuber-Dochan W, Artom M, Sweeney L, Hart A. Systematic review: interventions for abdominal pain management in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:115–25.
Snedecor SJ, et al. Systematic review and comparison of pharmacologic therapies for neuropathic pain associated with spinal cord injury. J Pain Res. 2013;6:539–47.
Article CAS PubMed PubMed Central Google Scholar
Goldenberg M, Reid MW, IsHak WW, Danovitch I. The impact of cannabis and cannabinoids for medical conditions on health-related quality of life: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;174:80–90.
Meng H, Johnston B, Englesakis M, Moulin DE, Bhatia A. Selective cannabinoids for chronic neuropathic pain: a systematic review and meta-analysis. Anesth Analg. 2017;125:1638–52.
Nugent SM, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med. 2017;167:319–31.
O’Neil ME, et al. Benefits and harms of plant-based cannabis for posttraumatic stress disorder: a systematic review. Ann Intern Med. 2017;167:332–40.
Abo Youssef N, et al. Cannabinoids for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis: a systematic review and meta-analysis. BJU Int. 2017;119:515–21.
Mehta S, et al. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97:1381–91.
Fitzcharles M-A, Baerwald C, Ablin J, Häuser W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): a systematic review of randomized controlled trials. Schmerz Berl Ger. 2016;30:47–61.
Tateo S. State of the evidence: Cannabinoids and cancer pain-a systematic review. J Am Assoc Nurse Pract. 2017;29:94–103.
PubMed Google Scholar
Stevens AJ, Higgins MD. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol Scand. 2017;61:268–80.
Walitt B, Klose P, Fitzcharles MA, Phillips T, Hauser W. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7:CD011694.
Smith LA, Azariah F, Lavender VTC, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015:CD009464.
Deshpande A, Mailis-Gagnon A, Zoheiry N, Lakha SF. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: systematic review of randomized controlled trials. Can Fam Physician Med Fam Can. 2015;61:e372–81.
Andreae MH, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain Off J Am Pain Soc. 2015;16:1221–32.
Whiting PF, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–73.
Langhorst J, et al. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J. Crohns Colitis. 2015;9:86–106.
McLoughlin BC, et al. Cannabis and schizophrenia. Cochrane Database Syst Rev. 2014:CD004837.
Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med Rev. 2014;18:477–87.
van den Elsen GAH, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014;14:56–64.
Article PubMed CAS Google Scholar
Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013:CD005175.
Fitzcharles M-A, et al. Efficacy, tolerability, and safety of cannabinoid treatments in the rheumatic diseases: a systematic review of randomized controlled trials. Arthritis Care Res. 2016;68:681–8.
Baldinger R, Katzberg HD, Weber M. Treatment for cramps in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2012:CD004157.
Richards BL, Whittle SL, Buchbinder R. Neuromodulators for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2012;1:CD008921.
Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72:735–44.
Phillips TJC, Cherry CL, Cox S, Marshall SJ, Rice ASC. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PloS One. 2010;5:e14433.
Phillips RS, et al. Antiemetic medication for prevention and treatment of chemotherapy induced nausea and vomiting in childhood. Cochrane Database Syst Rev. 2010:CD007786.
Meyer MJ, et al. Acute management of acquired brain injury part II: an evidence-based review of pharmacological interventions. Brain Inj. 2010;24:706–21.
Lakhan SE, Rowland M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 2009;9:59.
Article PubMed PubMed Central CAS Google Scholar
Curtis A, Clarke CE, Rickards HE. Cannabinoids for Tourette’s syndrome. Cochrane Database Syst Rev. 2009:CD006565.
Martin-Sanchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med Malden Mass. 2009;10:1353–68.
Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. 2009:CD007204.
Machado Rocha FC, Stefano SC, De Cassia Haiek R, Rosa Oliveira LMQ, Da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur J Cancer Care (Engl). 2008;17:431–43.
Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. Can Med Assoc J. 2008;178:1669–78.
Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin. 2007;23:17–24.
Mills RJ, Yap L, Young CA. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst Rev. 2007:CD005029.
Shakespeare DT, Boggild M, Young C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev. 2003:CD001332.
Campbell FA, et al. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001;323:13–6.
Huntley A, Ernst E. Herbal medicines for asthma: a systematic review. Thorax. 2000;55:925–9.
Merlin JS, Bulls HW, Vucovich LA, Edelman EJ, Starrels JL. Pharmacologic and non-pharmacologic treatments for chronic pain in individuals with HIV: a systematic review. AIDS Care Psychol Socio Med Asp AIDSHIV. 2016;28:1506–15.
Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol. 2015;10:293–301.
Finnerup NB, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–73.
Snedecor SJ, et al. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract. 2014;14:167–84.
Kuspinar A, Rodriguez AM, Mayo NE. The effects of clinical interventions on health-related quality of life in multiple sclerosis: a meta-analysis. Mult Scler J. 2012;18:1686–704.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev Online. 2012;6:CD009270.
Macfarlane GJ, et al. Evidence for the efficacy of complementary and alternative medicines in the management of rheumatoid arthritis: a systematic review. Rheumatology. 2011;50:1672–83.
Hanson LC, Ersek M, Gilliam R, Carey TS. Oral feeding options for people with dementia: a systematic review. J Am Geriatr Soc. 2011;59:463–72.
Mestre T, Ferreira J, Coelho MM, Rosa M, Sampaio C. Therapeutic interventions for symptomatic treatment in Huntington’s disease. Cochrane Database Syst Rev. 2009:CD006456.
Wheaton P, Mathias JL, Vink R. Impact of early pharmacological treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J Clin Psychopharmacol. 2009;29:468–77.
Singh BB, et al. Herbal treatments of asthma: a systematic review. J Asthma. 2007;44:685–98.
Chung V, et al. Efficacy and safety of herbal medicines for idiopathic Parkinson’s disease: a systematic review. Mov Disord. 2006;21:1709–15.
Yavuzsen T, Davis MP, Walsh D, LeGrand S, Lagman R. Systematic review of the treatment of cancer-associated anorexia and weight loss. J Clin Oncol. 2005;23:8500–11.
Nielsen S, et al. Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacol. 2017;42:1752–65.
Canadian Agency for Drugs and Technologies in Health. In: Canadian Agency for Drugs and Technologies in Health, editor. Nabilone for non-chemotherapy associated nausea and weight loss due to medical conditions: a review of the clinical effectiveness and guidelines; 2014.
Long-term nabilone use: a review of the clinical effectiveness and safety. Canadian Agency for Drugs and Technologies in Health, 2015.
Staples H, Adcock L. Cannabinoids for behavioural symptoms in adults with dementia: a review of clinical effectiveness and guidelines. CADTH. 2018.
van den Beuken-van Everdingen M, et al. Pharmacological treatment of pain in cancer patients: the role of adjuvant analgesics, a systematic review. Pain Pract Off J World Inst Pain. 2017;17:409–19.
Koppel BS, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556–63.
Pringsheim T, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry Rev Can Psychiatr. 2012;57:133–43.
Scottish Intercollegiate Guidelines Network. Control of pain in adults with cancer: a national clinical guideline. SIGN. 2008.
Paice JA, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:3325–45.
Verleye L, et al. Supportive treatment for cancer - part 2: prevention and treatment of adverse events related to chemotherapy and radiotherapy. Good Clin Pract GCP Bruss Belg Health Care Knowl Cent KCE KCE Reports. 2012;191C.
Centre for Clinical Practice at NICE (UK). In: National Institute for Health and Care Excellence, editor. Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. UK; 2013.
National Clinical Guideline Centre. Multiple sclerosis: management of multiple sclerosis in primary and secondary care. NICE Clinical Guideline. 2014;186.
Yadav V, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology, 2014;82:1083–92.
Mücke M, et al. Cannabinoids in palliative care: systematic review and meta-analysis of efficacy, tolerability and safety. Schmerz Berl Ger. 2016;30:25–36.
Jawahar R, Oh U, Yang S, Lapane KL. A systematic review of pharmacological pain management in multiple sclerosis. Drugs. 2013;73:1711–22.
Jawahar R, Oh U, Yang S, Lapane KL. A systematic review of pharmacological pain management in multiple sclerosis. Database Abstr Rev Eff. 2018;2015.
Williamson PR, et al. The COMET Handbook: version 1.0. Trials. 2017;18:280.
Download references
Acknowledgements
Not applicable.
Research reported in this publication was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under award number R24AT001293. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and affiliations.
Knowledge Synthesis Group, Ottawa Methods Centre, Ottawa Hospital Research Institute, The Ottawa Hospital, General Campus, 501 Smyth Road, Ottawa, Ontario, K1H 8 L6, Canada
Misty Pratt, Adrienne Stevens, Micere Thuku, Claire Butler & Brian Hutton
TRIBE Graduate Program, University of Split School of Medicine, Split, Croatia
Adrienne Stevens
Department of Pharmacology and Therapeutics, McGill University, Montreal, Quebec, H3A 2B4, Canada
Claire Butler
Ottawa, Canada
Becky Skidmore
Center for Integrative Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
L. Susan Wieland
School of Epidemiology and Public Health, University of Ottawa, 451 Smyth Road, Ottawa, Ontario, K1H 8 M5, Canada
Mark Clemons, Salmaan Kanji & Brian Hutton
Division of Medical Oncology and Department of Medicine, University of Ottawa, Ottawa, Canada
Mark Clemons
Department of Pharmacy, The Ottawa Hospital, Ottawa, Canada
Salmaan Kanji
Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Canada
You can also search for this author in PubMed Google Scholar
Contributions
MP, AS, and BH drafted the initial version of the report. BS designed and implemented the literature search. MP, MT, and CB contributed to review of abstracts and full texts as well as data collection. MP, AS, and BH were responsible for analyses. All authors (MP, AS, MT, CB, BS, SW, MC, SK, BH) contributed to interpretation of findings and revision of drafts and approved the final version of the manuscript.
Corresponding author
Correspondence to Brian Hutton .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
BH has previously received honoraria from Cornerstone Research Group for provision of methodologic advice related to the conduct of systematic reviews and meta-analysis. All other authors declare that they have no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
PRISMA Scoping Review Extension Completed Checklist.
Additional file 2.
Literature Search Strategies.
Additional file 3.
Grey Literature Sources.
Additional file 4.
Listing of Data Extraction Items.
Additional file 5.
Data extractions from included studies.
Additional file 6.
Listing of Studies Excluded During Full Text Screening.
Additional file 7.
AMSTAR Scoring Outline.
Additional file 8.
AMSTAR Scores by Review.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Pratt, M., Stevens, A., Thuku, M. et al. Benefits and harms of medical cannabis: a scoping review of systematic reviews. Syst Rev 8 , 320 (2019). https://doi.org/10.1186/s13643-019-1243-x
Download citation
Received : 03 June 2019
Accepted : 24 November 2019
Published : 10 December 2019
DOI : https://doi.org/10.1186/s13643-019-1243-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical marijuana
- Scoping review
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 19 July 2021
Processing and extraction methods of medicinal cannabis: a narrative review
- Masoumeh Pourseyed Lazarjani 1 ,
- Owen Young 2 ,
- Lidya Kebede 1 &
- Ali Seyfoddin ORCID: orcid.org/0000-0003-4343-9905 1
Journal of Cannabis Research volume 3 , Article number: 32 ( 2021 ) Cite this article
102k Accesses
40 Citations
108 Altmetric
Metrics details
Introduction
As the cannabis industry transitions from a black market to a legal market, product development, and methods of extraction have become a focal point. To date, more than thousands of chemical constituents have been identified from the cannabis plant, all of which possess different chemical properties that require different conditions for preservation during drying and extraction. However, scientific publications that explore these areas for the cannabis plant are currently lacking.
This is a narrative review paper which focuses on critiquing drying and extraction methods of Cannabis sativa L. plant. Relevant keywords such as medicinal cannabis, extraction, solvent, cannabinoids, and terpenes have been searched in PubMed, EMBASE, MEDLINE, Google Scholar, and Cochrane Library (Wiley) databases.
To find relevant papers for this narrative review, 93 papers have been reviewed. Among them, 12 irrelevant papers were discarded. The excluded papers were either about hemp seed oil or hemp fiber and protein. Based on this review, solvent extraction is the most common method for cannabis plants. Although solventless and hydrodynamic extraction are known for their high yield and feasibility, more investigation is needed in these areas. Regarding the drying process, hang-drying is the most convenient method; however, it may be substituted by freeze-drying in the near future.
This review analyses various drying and extraction processes to guide the selection of suitable methods for various types of cannabis products and applications. This is done by outlining traditional and modern methods of drying techniques, exploring the importance of solvents for extraction, visiting solventless extraction procedures, and finally comparing conventional and alternative methods of extraction.
In conclusion, based on the current knowledge, using organic solvents is the most convenient method for medicinal cannabis extraction. However, more research is needed for some of the drying and extraction methods. Also, developing a green and sustainable cannabis extraction method should be considered for future studies.
Cannabis is a flowering plant from the Cannabaceae family and genus Cannabis . Cannabis sativa and Cannabis indica are generally well known, while subspecies Cannabis ruderalis is often overlooked due to its limited ability in producing active compounds (Gloss 2015 ). Hybrid species are variable depending on the parent plant; they can be sativa dominant, indica dominant, or balanced. Within the genus, the number of species is disputed, and the traditional nomenclature of sativa and indica may not be correct or useful in determining therapeutic potential. In any case, cannabis is dioicous, meaning it exhibits both male and female reproductive structures in separate individual plants. Female cannabis plants produce more glandular trichomes compared to the male plant. Among all the known compounds in the cannabis plant, cannabinoids and terpenes are the most active compounds with therapeutic potential which largely synthesized in those glandular trichomes. These compounds have shown to have therapeutic effects on a range of conditions such as metabolic disorders, neurodegenerative disorders, movement disorders, anorexia in HIV patients, nausea, and pain after chemotherapy in cancer patients (Namdar et al. 2018 ; Romano and Hazekamp 2013 ) (Table 1 ).
As the cannabis industry transitions from a black market to a legal market, product development, and methods of extraction have become a focal point. Traditionally, the dried cannabis flower has been a popular product for the use of smoking and vaping. However, as the industry expands, the need for cannabis products in different forms and higher potency also increases. Currently available products, medicinal or recreational, come in the forms of topicals, edibles, beverages, and vaporization cartridges. Each product type presents its own set of advantages and disadvantages allowing for customization to serve a particular purpose (Blake and Nahtigal 2019 ). For pharmaceutical and food applications, the extraction and isolation of active components and combinations of identified cannabinoids are critical steps that should be explored (Fathordoobady et al. 2019 ).
The separation of bioactive compounds has recently become rapidly sought after by the pharmaceutical and food industries. This is due to the increased understanding of the dynamic nature and potential of diverse bioactive molecules from natural sources (Azmir et al. 2013 ). To further continue scientific research on the selection, identification, and characterization of bioactive compounds, the selection of a suitable extraction process is imperative (Azmir et al. 2013 ). Failing to designate a fitting method of sample preparation can jeopardize any analytical procedure resulting in unfavorable outcomes. However, the field of extraction is often neglected and is not studied as thoroughly as other processes. This creates a gap in the literature that should be explored more extensively (Smith 2003 ). The process of extraction is commonly employed to obtain target bioactive compounds from complex plant matter, yet it can also be altered to cater for many purposes, for instance, increasing the selectivity and sensitivity of bioassays by increasing the concentration of a target compound, as well as providing a potent and reproducible sample matrix (Smith 2003 ). Valizadehderakhshan et al. ( 2021 ) compared different extraction methods for seed and trichomes in Cannabis sativa L. They also reviewed various parameters that affect cannabinoid transformation after extraction (Valizadehderakhshan et al. 2021 ).
Different methods of extraction will yield varying degrees of extract quality and composition depending on the procedure and substances used (Blake and Nahtigal 2019 ). This review focuses on various drying and extraction methods while comparing conventional and most recent methods. For example, conventional methods of extraction including Soxhlet and dynamic maceration have longer extraction time and large amounts of solvent are required to complete the extraction process (Agarwal et al. 2018 ). Recent methods including ultrasonic-assisted, microwave-assisted, supercritical fluid, and pressurized liquid extraction processes can be considered as an alternative, slightly greener, options as opposed to the conventional methods. These procedures reduce the need for synthetic and organic solvents, cut down on operational time, and produce a better quality extract with a higher yield (Azmir et al. 2013). Solventless methods such as dry sieve and water extraction are particularly known to extract entire trichomes. Hydrocarbon extraction methods can be used to avoid unwanted water and pigments such as chlorophyll. Ethanol can extract flavonoids, while carbon dioxide can be manipulated to extract different compounds depending on the conditions (Blake and Nahtigal 2019 ).
The characteristics of the product must be considered when deciding on a method. For example, depending on the application, cannabinoids can be extracted in either acidic or neutral form. The preservation of acidic cannabinoids requires extraction to be completed at room temperature (Citti et al. 2016 ). To decarboxylate acidic cannabinoids into neutral form, high temperatures are recommended for extraction, although a higher temperature may result in the loss of some terpenes and minor constituents (Fathordoobady et al. 2019 ). Therefore, the selection of an appropriate extraction procedure will benefit future stages of development by minimizing the requirements for refinements (Blake and Nahtigal 2019 ). To further understand the processes and possible outcomes, this review will explore different methods of drying and extraction procedures used for the cannabis plant.
This paper is a narrative review paper which focuses on drying, extraction, and post-extraction methods for Cannabis sativa L. plant. A combination of keywords such as medicinal cannabis, extraction, solvent, and cannabinoids have been searched in databases such as PubMed, EMBASE, MEDLINE, Google Scholar, and Cochrane Library (Wiley) from 1977 to 2021 in English.
The focus of this narrative review was on Cannabis sativa , initially where 93 papers were identified. Papers on various drying and extraction methods specifically for Cannabis sativa L. were included while those for using hemp as fiber and protein sources were excluded. Overall, 12 papers about cannabis seed oil, hemp seed oil, or hemp plant were excluded as this review focuses on the oil coming from flowers. In the end, 81 related papers about various drying, extraction, and post-harvest processes were carefully reviewed.
Influence of external factors on cannabis
External factors such as light duration, oxygen, and harvest time (floral maturity) have been shown to influence the secondary metabolite production in cannabis (Liu et al. 2015 ; Namdar et al. 2019 ). A 4-year study by Lindholst ( 2010 ) found that cannabinoid stability is affected by temperature, light, and air. Three conditions were used to store cannabis resin (hashish slabs) and extract (by the solvent): room temperature and 4 °C both with visible light exposure and darkness, and − 20 °C in darkness. The study identified that in cannabis resin, light exposure can affect the decarboxylation of THCA and the degradation of THC. This is evident as the half-life increased by 40% in darkness. However, it was observed that light was only partially influential. The resin samples that were placed at room temperature, in either light or dark settings, only exhibited little differences in the degradation of neutral THC. The dense color and structure of resin are thought to be the reason behind the reduced light sensitivity of THC. Accordingly, it is suspected that the exposure of light on resin only reaches the cannabinoids on the surface resulting in low degradation levels. This theory is further illustrated when a comparison was done between the degradation levels of both acidic and neutral THC levels in cannabis resin and cannabis extract. It was observed that both the neutral and acidic forms of THC in the cannabis extract degraded significantly more through light exposure. Furthermore, compared to resin, cannabis extract had a 10 times lower half-life (35 days for extract and 330 days for resin), while THCA decreased to nondetectable levels after 140 days. The neutral forms, in the extract, increased during this period, although THC concentrations were reduced to 1.7% after 2 years at room temperature with light exposure. It was also found that extracts stored at 4 °C showed the same pattern, but degradation was slower, while at − 20 °C all measured cannabinoids remained unchanged during the study period (Lindholst 2010 ). Danziger and Bernstein ( 2021a , b ) evaluated the effect of light on three chemovars of cannabis under four different light conditions. In this study, light as the key factor affected the profile and yield of cannabis chemovars. To be precise, using blue to red lights (1:1 and 1:4 ratios) had the highest yield compared to white LED light. In addition, CBGA as a primary cannabinoid and precursor for many cannabinoids increased by using blue light (Danziger and Bernstein 2021a ). The same authors in another study investigated the effect of architectural manipulation of the plant on the cannabinoid’s standardization. Defoliation, removing primary and secondary branches, and pruning have been considered as a part of eight various architectural manipulation treatments in different light intensities. Results showed that plant architectural modulation affects cannabinoid profile while no changes has been reported in the decarboxylation of cannabinoids (Danziger and Bernstein 2021b ). Saloner and Bernstein ( 2021 ) evaluated the effect of nitrogen supply as an environmental factor on cannabinoids and terpenes. Results showed that the concentration of THCA and CBDA decreases by increasing the amount of nitrogen 69% and 63%, respectively. Bernstein et al. ( 2019 ) evaluated the effect of common minerals on the cannabinoid profile by adding humic acid (HA), phosphor (P), nitrogen (N), and potassium (K) to the commercial treatment into irrigation solution for a high THC cannabis chemovar. Each of the supplements affected the cannabinoid concentrations differently based on the organ and its location in the plant. For example, adding NPK supplement increased 71% the amount of CBG in the flower, while it decreased the amount of CBN in the flowers and leaves by 38% and 36%, respectively (Bernstein et al. 2019 ).
For many applications, the dried version of the cannabis herb is required; however, like many plants, cannabis contains approximately 80% water. For this reason, drying is considered an essential step for product development (Hawes and Cohen 2015 ). Drying the plant not only prevents the growth of microorganisms that would otherwise rot plant tissue (based on ASTM D8196-18 which is a standard practice for determination of water activity (aw) in cannabis flower), it would also enable long term storage while maintaining potency, taste, medicinal properties, and efficacy (Hawes and Cohen 2015 ). This is done by maintaining the water activity level between 0.55 and 0.65 aw, minimizing the risk of mold or fungal infection while preserving the quality of the flower (ASTM D8196-18).
Air-drying, also known as hang-drying
Hang-drying or air-drying is considered the oldest way of drying cannabis plants after harvest (Fig. 1 ) that requires no dedicated equipment (Ross and ElSohly 1996 ). Slow-drying includes placing whole plants or separated inflorescence in a cool dark room with a temperature between 18 and 25 °C and humidity between 45 and 55%, either hung from a string or laid out on drying screens (Hawes and Cohen 2015 ). Ross and ElSohly ( 1996 ) applied four treatments for air-drying to evaluate the efficacy of each condition in producing the highest yield of cannabis products. The treatments were extracted immediately, after the flower harvest at room temperature (0.29% yield, w/v) (A), after 1 week of air-drying at room temperature (0.20% yield based on wet material, v/w) (B), after 1 week of air-drying followed by storage for 1 month at room temperature (0.16% yield based on wet material, w/v) (C), and air-drying for 1 week and stored in paper bags for 3 months at room temperature (0.13% yield based on wet material, v/w) (D). From this experiment, it was found that the yield from treatments A to D decreased from 29 to 13%, respectively (Ross and ElSohly 1996 ). Inconveniences of this method include the manual removal of leaves and buds from the stem as well as the time taken to complete the overall process. The separation is crucial as different parts dry at different rates; therefore, a lack of completing this step may result in uneven drying. Consequently, a disadvantage of removing buds from stems is the possibility of producing a product with a harsher taste. Another detriment of this method is the involvement of gravity. The water from the top part of the plant will absorb into the lower parts leading to a slower and uneven drying process. To speed up the procedure, heaters, fans, and dehumidifiers can be used. However, fast-drying can lead to a harsher taste as opposed to slow-drying which produces smoother tasting products. It is also believed that speeding up the drying process can prevent the plant from reaching peak potency in the curing phase (Hawes and Cohen 2015 ). Coffman and Gentner ( 1974 ) evaluated the effect of drying conditions on the cannabinoid profile. They stored the cannabis hang dried leaves in 65, 85, and 105 °C for 1, 4, 16, and 64 h to compare the mean percentage of total cannabinoid content. The results were shown that the percentage of total cannabinoids was decreased by increasing time and temperature. To be precise, the percentage mean weight loss of total cannabinoids increased from 7.5 to 11% in 65 °C after 1 h and 105 °C after 64 h, respectively.

Air-drying (hang-drying) of the cannabis plant
Oven-drying
A faster direct method of drying is the oven-drying approach (Mujumdar 2006 ). This method can be carried out in either a vacuum chamber, vacuum desiccator, or in a drying oven with or without air circulation (Hawes and Cohen 2015 ). To illustrate the outcomes of the process, an early study tested out four different oven conditions to compare the end products. Inflorescences were dried for 1, 4, 16, and 64 h at 65, 85, and 105 °C. After extraction with ethanol, gas chromatography showed that the yield of CBD and THC decreased as the temperature and time of drying increased. It was also observed that at temperature 105 °C, the thermal degradation of THC increased the CBN content (Coffman and Gentner 1974 ). CBN is considered a less potent psychoactive and mild analgesic; therefore, conversion of THC to CBN will decrease the therapeutic potential (Citti et al. 2016 ).
Additionally, using high temperatures and excessive drying can result in the loss of key components (Hawes and Cohen 2015 ). This statement can be the reason for the lack of information about using oven dying in the cannabis industry. This was highlighted in a study that compared the ratio of cannabinoid and by-product produced during vaporization. The cannabis material was placed in the desiccator for 5 days to dry out, while the smoke condensate and vaporized condensate trapped in the organic solvent were dried with a rotary evaporator at 40 °C. These approaches had produced intense fragrance which is indicative of the loss of terpenoids and other volatile components (Pomahacova et al. 2009 ).
Freeze-drying
Freeze-drying (also known as lyophilization) has become a popular option due to the increasing demand for high-quality medicinal cannabis. The freeze-drying method holds the cannabis plant at temperatures far below those of air or oven, while removing the water content, in the form of vapor, via sublimation in a vacuum chamber (Mujumdar 2006 ). The nascent legal cannabis industry claims that freeze-drying preserves the volatile compounds and acidic form of cannabinoids (Tambunan et al. 2001 ). It is generally agreed that the end products of freeze-drying are considered high quality compared to other methods of drying. This is due to the structural rigidity found on the surface of frozen materials where sublimation occurs, preventing the disintegration of the solid matrix and resulting in a porous, unaltered structure (Mujumdar 2006 ). When assessing the end product produced by freeze-drying, it was found that the composition is largely unaffected from that found in the plant (Tambunan et al. 2001 ). A disadvantage of freeze-drying is the cost of operation. This procedure requires an intense amount of energy to maintain such temperatures, vacuum, and long-running time (Mujumdar 2006 ).
Comparing the different drying methods, we can safely state that the approach elected will affect the yield and cannabinoid profiles in the extracts. Therefore, the selection of a drying procedure will largely alter the outcomes (Coffman and Gentner 1974 ). The process of hang-drying cannabis was found to be time-consuming as it can take several days, while the main factors that increase the rate of drying were determined to be moving air and low humidity (Ross and ElSohly 1996 ). In contrast, the oven-drying method was observed to be faster, but readily volatile compounds and neutral forms of cannabinoids decreased in extracts to almost non-detectable concentrations, affecting therapeutic potential (Coffman and Gentner 1974 ). To address this issue, freeze-drying is thought to be the preferred method. Freeze-drying enables the preservation of flavor qualities in many foods, themselves often due to the presence of volatile compounds (Tambunan et al. 2001 ).
In all the drying methods mentioned above, humidity, temperature, ventilation rate, and time are the most important parameters to be optimized. Incorrect drying conditions may cause decarboxylation of acidic cannabinoids and loss of terpenes. The presence of light, oxygen, and heat may also cause degradation in cannabinoids and terpenes and can affect the taste (Jin and Chen 2019 ).
Curing is the final post-harvest procedure that allows for the development of the maximum flavor in the cannabis plant (Vogel 2018 ). Jin et al. ( 2019 ) believed that the best temperature and humidity for curing are at 18 °C and 60% RH for 14 days. Green et al. ( 2018 ) suggested keeping the trimmed flowers in a can for up to 4 weeks in a dark cupboard while opening the lid every day for about 6 h is the best method for curing (Jin and Chen 2019 ). At temperatures between 15–21 °C and 45–55% humidity, enzymes and aerobic bacteria will be in the optimum condition to breakdown undesired sugars and degrade minerals. Curing can reduce the harsh smell and the sense of throat burning during smoking or vaping as well as increasing the shelf life by minimizing mold growth. It is also believed that curing can increase cannabis potency as the number of cannabinoids such as THC and CBN will increase by curing. Although curing is one of the most significant post-harvest stages for the cannabis plant, there are not enough academic investigations around this area.
Extraction methods
Cannabis extraction can be used to concentrate target components for product development. There are important parameters that can affect the yield of the cannabis extract such as mean particle size, size distribution, temperature, rate of agitation, and extraction time (Fathordoobady et al. 2019 ). Solventless, solvent-based, convention, and alternative methods of extraction are explored concerning cannabis extraction.
Solventless extraction
Long-established solventless methods such as dry-sieving, water extraction, and rosin press extraction lack coverage in literature due to outdated techniques and difficulty in scaling despite having simple procedures. Dry sieve extraction produces a powder-like Kief with a potency of approximately 35–50% THC. The process of dry-sieving begins by beating dried cannabis against a mesh screen and forcing the trichomes to separate and fall off. The final product can either be pressed further into hashish or mixed with dried flowers. This simple procedure is time-consuming and labor-intensive, therefore, not popular for the industrial level. Water extraction produces roughly the same potency of THC as the dry sieve method, although it also depends on the potency of the starting material. The procedure begins by placing the cannabis plant in a mesh bag immersing it in ice water and finally stirring it to knock the trichome off. The trichome is further filtered through a series of screens then allowed to settle before collecting and drying the final product, commonly known as water hash or bubble hash. Similarly, to dry sieving, this process is difficult to upscale as well as limited control of potency (Blake and Nahtigal 2019 ).
Solventless extraction exploits the fact that cannabinoids are semi-liquid and can be extracted by suitable heating and pressure. Rosin extraction uses compression and heat to obtain oils and rosin. Rosin extraction can be as simple as using a hair straightener for recreational extractions. For more commercial medicinal applications, a modified hat press is adopted. For both methods, high pressure at low temperatures is not achievable; therefore, the retention of terpenes is limited (analytical cannabis.com) (Lamy et al. 2018 ). To prevent high-temperature changes, a typical pneumatic press can be used, exerting some lower temperatures and preserving the terpenes. Pressures up to 137.8 MPa can be generated in some pneumatic presses.
Solvent-based extraction
Solvent-based extraction methods such as Soxhlet, maceration both static and dynamic, ultrasonic-assisted extraction, and microwave-assisted extraction require a solvent to complete the extraction process. A variety of solvents can be used to extract cannabinoids including ethanol, butane, propane, hexane, petroleum ether, methyl tertbutyl ether, diethyl ether, carbon dioxide (CO 2 ), and olive oil (Dussy et al. 2005 ; Lehmann and Brenneisen 1992 ; Romano and Hazekamp 2013 ; Rovetto and Aieta 2017 ). Gaseous solvents such as butane and propane can also be used for extraction purposes (Raber et al. 2015 ). Gas solvent extractions start in the gas phase at room temperature and are either cooled or pressurized into a liquid state as they run through the sample material (Rovetto and Aieta 2017 ). The extracted sample is collected, and the solvent is evaporated (Chan et al. 2017 ). The process of pressurizing these flammable and potentially explosive gases poses safety hazards (Jensen et al. 2015 ). In addition, the gases used in cannabis extractions are often industrial grade and contain impurities that end up in the cannabis extracts. Moreover, the solvents themselves may become a residue in the final extract (Raber et al. 2015 ).
The differing solubilities of individual cannabinoids and other phytochemicals are thought to be an important factor that needs to be considered when selecting a solvent. The stickiness and viscosity of cannabis oil result in binding to solvents; therefore, it is important to consider the toxicity, affinity, and temperature profile of the solvents being used (Fathordoobady et al. 2019 ). The efficiency of conventional methods of extraction is presented to be heavily dependent on the solvent of choice. Solubility, molecular affinity, mass transfer, co-solvent, toxicity, and environmental safety are major factors that should also be considered during the solvent selection process (Azmir et al. 2013 ). Commonly used solvents to extract cannabis can be divided into three groups, low molecular mass organic solvents, vegetable fats (oils), and supercritical fluids, notably supercritical carbon dioxide (Reichardt and Welton 2011 ).

Low molecular mass organic solvents
Low molecular mass organic solvents are hydrocarbon-based with limited polarity due to the presence of oxygen. Halogen substituted hydrocarbons are also included in this group.
These solvents are known for their ability to dissolve generally nonpolar compounds, following the chemistry adage: like dissolves like. Inspection of cannabinoids in Table 2 shows that they are dominated by carbon and hydrogen, making them generally nonpolar. However, the presence of alcohol and acid groups requires some polarity in extraction solvents and solvent mixtures.
Table 2 shows some of the properties of the most popular organic solvents in cannabis extraction. Notably absent from this popular group are dichloromethane and chloroform, both halogenated hydrocarbons are commonly used in analytical fat/oil extraction from plant and animal tissue. These solvents are observed to have low boiling points and high volatility, indicating their ability to be easily separated from the extract at low temperatures after the extraction process (Reichardt and Welton 2011 ).
To illustrate how different solvents can affect the yield of compounds from the source material, consider the example of phenolic extraction from grape pomace and elderberry. Phenols are nominally water soluble. The solvent combinations ethanol–water and acetone–water mixtures had a higher yield than ethyl acetate-water mixture (Vatai et al. 2009 ). In another example, isopropanol-hexane, chloroform–methanol, and hexane were used as solvents for crude fat extraction from insect, egg yolk, and krill powders in one-step organic solvent extraction. The highest fat yield was achieved with a chloroform–methanol mixture (Rose 2019 ). Thus, with a mixture of cannabinoids, terpenes, chlorophyll, carotenoids, and other fat-soluble classes in cannabis flowers, different extraction efficiencies can be confidently predicted. If seeds have matured, the fats (triacylglycerols) that comprise the energy stored in seeds will also be extractable to some extent.
Namdar et al. ( 2018 ) reported that for cannabis plant extraction, the ratio and the nature of the solvents can determine the evaporation time after extraction, which should be minimized. A mixture of polar and non-polar solvents achieved the highest yield for all the compounds in the cannabis plant (Namdar et al. 2018 ).
Vegetable fats (oils)
Vegetable oils are routinely extracted from seeds or fruits such as rapeseed, sunflower, or olive, and even brans, making them an inexpensive option. These oils are considered lipophilic due to their nonpolar characteristic, which enables selective dissolving properties. Approximately, 95 to 98% of vegetable oils consist of triglycerols whose composition is dominated by six fatty acids (Yara-Varón et al. 2017 ). Figure 2 shows the major fatty acids in different vegetable oils (Yara-Varón et al. 2017 ). Each of these has a degree of emulsifying capacity that may play a role in cannabinoid extraction. Interestingly, apart from olive oil, some specialized oils, nearly all commercial oils, are refined to eliminate the minor components. Whether this could affect cannabinoid extraction is unknown.
Vegetable oils composition by fatty acid profile, inspired by Yara-Varón et al. ( 2017 )
Olive oil is a well-known solvent in the cannabis extraction field. It is also one of the least refined oils with characteristically high oleic acid content. Terpenes can be preserved during extraction with olive oil due to their low volatile nature. Romano and Hazekamp ( 2013 ) used two different protocols with olive oil for cannabis extraction. In the first experiment, 5 g cannabis with 20 ml olive oil and 50 ml water were mixed and heated up to 60 min. In the second experiment, 10 g cannabis with 100 ml olive oil were mixed and heated for up to 120 min. The extract concentration to the solvent ratio for the first and second protocols was 5 g/20 ml and 10 g/100 ml, respectively. The high yield of terpenes obtained from using olive oil as a solvent is thought to be due to its efficient capabilities in solubilizing and limiting loss of product by protecting the compounds from evaporation (Romano and Hazekamp 2013 ).
Supercritical carbon dioxide (CO 2 )
In common with other solvents, CO 2 —which is nominally a polar gas—enters a so-called supercritical state at a defined temperature and pressure. In a supercritical state, distinct liquid and gas phases do not exist. In the case of CO 2 , the critical temperature is 31.06 °C, the critical pressure is 73.83 bar, and the critical density is 0.460 g/cm 3 (Raventós et al. 2002 ). Supercritical CO 2 behaves like a non-polar solvent, capable of extracting a broad range of non-polar solutes, cannabinoids included. In comparison, strongly polar water becomes supercritical and useful as a non-polar solvent but at a much higher temperature and pressure, 647 K and 22.1 MPa (Fig. 3 ). Therefore, CO 2 is the solvent of choice due to low critical temperature and pressure. It is also non-flammable, non-toxic, inert, renewable, easy to remove, abundant, and relatively low-cost. As an example, consider supercritical extraction of linalyl acetate from lavender oil compared with its extraction by conventional steam distillation (Reverchon et al. 1995 ). The yields for supercritical extraction were 34.7% compared with 12.1% for the conventional steam distillation. The reason proposed was that the higher temperature of steam distillation caused the undesirable hydrolysis of the linalyl acetate to linalool and acetic acid.
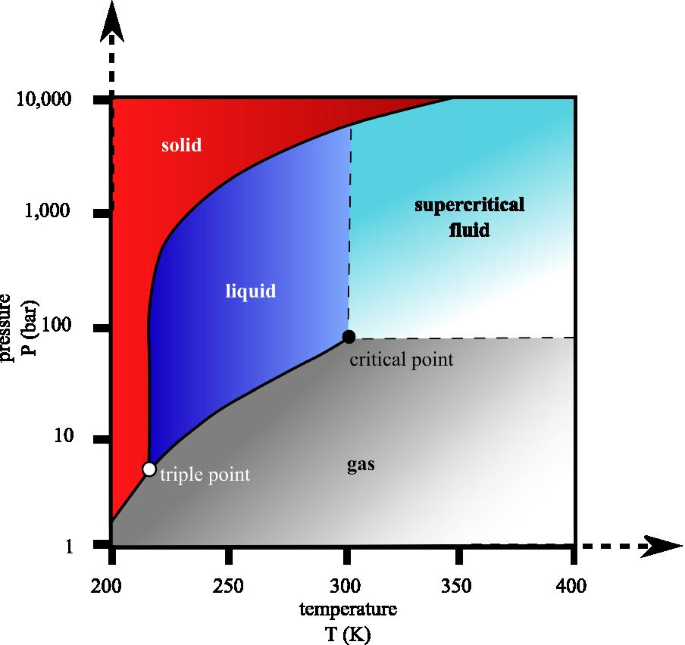
CO 2 pressure–temperature phase diagram, the critical temperature is 304.13 K or 31.0 °C or 87.8°F, and the critical pressure is 7.3773 MPa or 72.8 atm or 1070 psi or 73.8 bar. (Adopted from Wikimedia commons URL: https://upload.wikimedia.org/wikipedia/commons/1/13/Carbon_dioxide_pressure-temperature_phase_diagram.svg )
Thus, the low base temperature of supercritical CO 2 is probably an intrinsic advantage (Reverchon et al. 1995 ).
Conventional methods of extraction
Soxhlet extraction.
Soxhlet extraction was first proposed by Franz Ritter Von Soxhlet, a German chemist, as a method of extraction of, primarily, lipids. However, over the years, this procedure has become widely employed for various extraction purposes, commonly used for the separation of bioactive compounds from plant matter. Soxhlet is also extensively used as a model for the comparison and development of alternative methods of separation (Azmir et al. 2013 ). The process begins by placing a small amount of the dried sample in a thimble that is then transferred to a distillation flask containing a particular solvent. When the overflow level is reached by the solution, a siphon is used to aspirate the solute and unload it into the distillation flask with the extracted analyte carried along into the bulk liquid. This procedure is repeated several times until total extraction is complete (Luque de Castro and Garcı́a-Ayuso 1998 ). For cannabis extractions using the Soxhlet apparatus, Lewis-Bakker et al. ( 2019 ) compared different types of organic solvents for the procedure and found ethanol had exhibited the highest yields of cannabinoids (Lewis-Bakker et al. 2019 ). As commonly witnessed by other conventional processes, the long-running time and the large amount of solvent required are limitations that not only increase the cost of operation but also cause environmental complications (Luque de Castro and Garcı́a-Ayuso 1998 ). These drawbacks were demonstrated by a study conducted by Wianowska et al. ( 2015 ) that compared the extraction profiles of THCA and THC using the Soxhlet extraction procedure. It was clear that the long-lasting high temperature accentuated the degradation pathway from THCA to THC and finally to CBN, resulting in high levels of THC and CBN (Wianowska et al. 2015 ).
The simplicity in methodology alongside the ease of system optimization can result in high sample throughput and yield. The minimal requirement for a trained personal for process operation is also considered advantageous when compared to recently developed methods of extraction. Soxhlet methods can be manual or automatic, and the latter is less hazardous and allows multiple treatments to be examined simultaneously to optimize solvent composition, solvent to plant ratio, and extraction time (Luque de Castro and Garcı́a-Ayuso 1998 ).
Dynamic maceration (DM)
Dynamic maceration is a conventional solid-lipid extraction procedure that is based on soaking a sample in organic solvents (solvent varies depending on the polarity of the target compound) for a specific time at a specific temperature and followed by agitation (Fathordoobady et al. 2019 ). This process of separation is inexpensive and a popular method used to obtain essential oils and bioactive compounds (Azmir et al. 2013 ). Recently, the use of vegetable oils (e.g., olive oil) as maceration extraction solvents was found to be more useful for extracting higher amounts of terpenes than alcoholic solvents, notably when using extended heating time. However, vegetable oils are not volatile and are difficult to remove from extracted isolates (Romano and Hazekamp 2013 ). Alternatively, ethanol is suggested as a preferred solvent for cannabinoid extraction. A study conducted by Fathordoobady et al. ( 2019 ) demonstrated that there was no significant difference between other organic solvents (n-hexane, acetone, methanol) and ethanol when used for neutral cannabinoid recovery. However, when the recovery of acidic cannabinoids was tested, ethanol had the highest yield. The use of ethanol for maceration extraction of cannabinoids was found to produce the highest yield when used twice compared to other methods of extractions, for instance, ultrasonic-assisted extraction (UAE) or supercritical fluid extraction (SFE) (Fathordoobady et al. 2019 ).
Romano and Hazekamp ( 2013 ) compared five different solvents (naphtha, petroleum ether, ethanol, olive oil + water, and olive oil) using DM (Table 3 ). Except for naphtha, other extracts contained a small amount of THC and THCA around 5–10%. Naphtha was an exception which had 33% THC plus THCA. With ethanol as solvent, unwanted chlorophyll was extracted along with the cannabinoids. The unwanted chlorophyll not only added an unpleasant flavor and a green tinge to the end product, but it also demonstrated accounts of interference with gas chromatography–mass spectrometry analysis, hence removal is considered necessary (Ciolino et al. 2018 ). To eliminate unwanted chlorophyll, the ethanol extract can be treated with activated charcoal. However, the use of activated charcoal can result in the reduction of cannabinoid content by approximately 50%. Consequently, although yields are high with ethanol, the removal of unwanted chlorophyll with charcoal comes at the expense of cannabinoid loss. In respect of toxicity, Romano and Hazekamp ( 2013 ) found significant amounts of petroleum hydrocarbon residues in the extracts obtained with naphtha and petroleum ether, indicating that special attention must be paid to ensure safe residual concentrations (Romano and Hazekamp 2013 ).
In the same study, when compared to other solvents, the olive oil extract was shown to contain the largest number of terpenes, making it a superior crude extract. Olive oil is a cost-effective nonflammable solvent that is considered nontoxic when applied topically or consumed orally, and not through the lungs. As an added benefit, Citti et al. ( 2016 ) recognized that olive oil-based cannabis extracts maintained their cannabinoid concentration longer than ethanol-based extracts. A disadvantage associated with olive oil extracts, however, is that extracts cannot be concentrated by evaporation. This means that larger volumes of olive oil extracts need to be consumed to have the same therapeutic effects as other extracts (Romano and Hazekamp 2013 ). In another study by Hazekamp et al. ( 2009 ), hexane—the usual form of petroleum ether—was used as a solvent for the maceration method in fiber and drug varieties of cannabis. The yields of cannabinoids were discovered to be 3% and 17%, respectively. For this study, hexane was particularly used as it does not extract chlorophyll and is easily evaporated after extraction (Hazekamp et al. 2009 ).
Methods to extract chlorophyll from plants generally required acetone as the preferred solvent; however, as acetone is considered carcinogenic, it is not recommended to be used in cannabinoid extraction. Namdar et al. ( 2018 ) extracted cannabinoids with ethanol (partly polar) and hexane (non-polar), and their mixture. The highest yield was achieved with the mixture, but for cannabinoids, the polar solvent was best (Namdar et al. 2018 ). Likewise, Brighenti et al. ( 2017 ) concluded that dynamic maceration with ethanol for 45 min at ambient temperature was the best way of extracting non-psychoactive cannabinoids especially the acidic forms compared to more elaborate methods like ultrasonic-assisted extraction (UAE) (Brighenti et al. 2017 ).
Alternative methods of extraction
Ultrasonic-assisted extraction (uae).
Ultrasound technology is widely adopted in the food and chemical industry for its ability to significantly influence the rate of various processes (Chemat et al. 2008 ). The main feature that sets ultrasonic-assisted extraction (UAE) apart from other processes is the use of sound waves, commonly with frequencies between 20 to 100 kHz. This enables the penetration of solvents into a sample matrix to extract the compounds of interest. This is done during the process of cavitation. Cavitation is described as the formation, expansion, and collapse of bubbles within the solution that allows for intense mass transfer and accelerated solvent access into cell material (Azmir et al. 2013 ). The effective mixing ability of the UAE can be explained by the faster energy transfer, micro-mixing, and reduced extraction temperature (Otles 2016 ). Factors such as moisture content of a sample, particle size, milling degree, solvent, temperature, pressure, and time of sonication must be considered and manipulated to achieve efficient extractions (Azmir et al. 2013 ). A study that employed the ultrasonication method to leach and hydrolyze phenolic compounds presented evidence of low analyte decomposition during the extraction procedure when compared to other methods such as subcritical water, and microwave-assisted and solid–liquid extractions. After assessing the degradation of phenolic compounds, the decrease in decomposition was found to be due to the low energy type produced by the sonication mechanism and the short duration time. However, this was only evident when the exposure time to ultrasound was less than 10 min (Herrera et al. 2005 ).
De Vita et al. ( 2018 ) compared different methods for the extraction of commercially available hemp and medicinal cannabis to evaluate the changes in cannabinoid composition. The experimentation demonstrated the optimal conditions for the highest yield of cannabinoids using ultrasonication to be 50 min at 60 °C with ethanol as a solvent. Despite the optimal conditions, the total amounts of THC and CBD extracted were slightly lower when compared to the controls, which were obtained under reflux at 90 °C for 50 min in ethanol. Although low yield was obtained, the ultrasonication procedure had provided extracts using lower temperatures in an environmentally friendly, safe, and energy-efficient way. This study also found that ethanol extract yield was 3 to 4 times higher than olive oil extract (De Vita et al. 2020 ). To further explore the concept of solvent influence in UAE, Lewis-Bakker et al. ( 2019 ) conducted an extraction procedure with the following parameters: UAE in 80 W of ultrasonic bath power, 63 W of heating power, at 40 kHz for 5 min. A mix of ethanol, hexane, and isopropanol: hexanes (1:1) were used as solvents. The results showed that the yield for ethanol and hexane was almost the same, and isopropanol: hexanes achieved the highest yield of the extract. However, an HPLC analysis showed a reverse relationship between the extract yield and cannabinoids: the isopropanol: hexanes product had the lowest cannabinoid content, due to coextracted non-cannabinoid content. The authors also indicated that the acidic forms of cannabinoids (four shown in Fig. 2 ) were almost intact with UAE extraction compared to other methods (Lewis-Bakker et al. 2019 ). To optimize the extraction of target cannabis compounds, it is suggested to use UAE as a conditioning step for conventional extraction methods. For example, it was found that using UAE before a Soxhlet extraction improved the crude lipid yield by more than 24% without affecting the quality of extract (Fathordoobady et al. 2019 ).
Microwave-assisted extraction (MAE)
In 1980, the increasing demand for environmentally friendly and sustainable industrial processes had provoked the development of the Microwave-assisted extraction procedure (Otles 2016 ). The electromagnetic energy provided in the form of microwaves, with frequencies between 300 MHz and 300 GHz, is used to produce rapid heating following ionic conduction and dipole rotation (Azmir et al. 2013 ). This procedure directly exposes each molecule to a microwave field which is converted to kinetic energy that can break cell walls and release their contents into a liquid phase. The enhanced performance of this green extraction process can be attributed to improved solubility, efficient mass transfer, and increased surface equilibrium. These factors result in a system that uses less energy with fast processes requiring less solvent consumption but also producing a final product with high purity (Fig. 4 ) (Ani et al. 2012 ). De Vita et al. ( 2018 ) used MAE to explore time, temperature, ramping time, and solvent as variables. The study demonstrated that the extraction yield of CBD increased with increasing temperature and duration by at least 4 times when compared to the reference sample, which was prepared by ethanol reflux at 90 °C for 50 min. It was also noted that olive oil had superior properties when compared to ethanol during an MAE (De Vita et al. 2020 ).
MAE process where the flask is housed in the microwave oven (Krishnan and Rajan 2017 ). Placing the flask containing the sample in the microwave, attached to a condenser outside of microwave to capture the solution of interest compounds after distillation
Neutral phytocannabinoids have been established as important for their medicinal properties; therefore, using extraction procedures to obtain these compounds is considered essential. Methods used for the extraction of neutral cannabinoids can be explored by investigating their decarboxylation efficiencies of phytocannabinoid acids. For example, Lewis-Bakker et al. ( 2019 ) had studied the processes of different isolation methods and found MAE to be superior in terms of yielding high neutral cannabinoids. The study had found high temperature (> 130 °C) led to decarboxylation of more than 99% of acidic cannabinoids during MAE. To further promote the decarboxylation of acidic phytocannabinoids, MAE was used for 10 min at 150 °C with extracts from prior Soxhlet, UAE, and SFE extractions. However, only the isolates from the Soxhlet method had completely decarboxylated. Although prolonging the duration time to 30 min in MAE, extracts yielded 0.6% CBN. As CBN is produced from the oxidation changes of THC, this can be due to a radical-mediated or oxidation during MAE (Lewis-Bakker et al. 2019 ).
Pressurized liquid extraction (PLE)
Pressurized liquid extraction (PLE), also known as accelerated solvent extraction (ASE) (Duarte et al. 2014 ), is documented to be a highly efficient and rapid method of compound extraction. In this approach, high pressures facilitate the extraction while the high temperatures promote solubility and mass transfer to increase analyte solubility, as well as reduce solvent viscosity and surface tension (Azmir et al. 2013 ). Accordingly, altering temperature and pressure enables influence over the solubility of the compound of interest (Wianowska et al. 2015 ). This procedure also does not require a filtration step as the insoluble matrix components are contained inside the extraction cell. This feature allows for the process automation for continuous operation (Fathordoobady et al. 2019 ). Figure 5 visualizes the PLE process.
PLE process using organic solvent as extracting solvent coupled with supercritical antisolvent (SAS) precipitation process (1) heat exchanger for cooling, (2) pump, (3) heat exchanger for heating, (4) extractor, (5) T-mixer, (6) precipitation vessels, and (7) filter (Santos and Meireles 2015 )
When comparing PLE to conventional methods such as Soxhlet, features such as shorter duration, reduced solvent consumption, and decreased sample handling are observed (Rodrigues et al. 2016). To demonstrate this, Wianowska et al. ( 2015 ) compared the amount of THCA, THC, and CBN obtained from a Soxhlet and PLE process with two types of extractants, methanol, and n-hexane. Employing methanol as an extractant, the first set of results had indicated, even in high temperatures, the concentration of THC was lower than THCA using the PLE method. The Soxhlet process had contrasting results as the concentration of THC was much higher than THCA. The data obtained illustrates the influence of parameters such as time and pressure have on the end product. The high pressure applied enables the use of temperatures above the boiling point of the extractant. This increases the penetration ability of the selected solvent into the plant matrix in a short time. The high temperature used in PLE does not avoid the transformation of THCA and THC to CBN; however, the degree at which this occurs is found to be much lower than that demonstrated by the Soxhlet extraction (Wianowska et al. 2015 ).
For the extraction of cannabis constituents, Fathordoobady ( 2019 ) demonstrated that by using methanol and acetone/methanol (50:50) as solvents with PLE parameters of 1250 bar at 60 °C temperature, 17 various compounds, and three cannabinoids (Δ9-THC and its metabolites 11-nor-9-carboxy-THC and 11-hydroxy-THC) were identified from the cannabis plant (Fathordoobady et al. 2019 ).
Supercritical fluid extraction
Green approaches, such as supercritical fluid extraction (SFE), are used to displace conventional methods of pressing and organic solvent extractions. These procedures decrease environmental impacts and reduce toxic residue on products by using supercritical fluids (Aladić et al. 2015 ). The process behind SFE can be condensed into two steps: (1) the plant material of interest is solubilized in a supercritical solvent of choice, commonly CO + , to extract the desired compound. (2) Those compounds are then recovered from the solvent to produce the end product. The use of supercritical fluids is advantageous as at room temperature they are in a gaseous, allowing for recovery of extract via simple evaporation (Santos and Meireles 2015 ). The differing solubilities of different solvents allow for selective extraction, as small variations to pressure and/or temperature can allow for selectivity (Perrotin-Brunel 2011 ). The employment of low temperatures is also considered advantageous as it results in low energy consumption as well as allowing for the preservation of thermosensitive compounds, such as cannabinoids (Aladić et al. 2015 ).
Under conditions except for supercritical, CO 2 behaves as a polar compound. In instances where supercritical CO 2 is not sufficiently polar to act as a solvent, polarity modifiers, such as alcohols, water, and acids, can be used as co-solvents (Rovetto and Aieta 2017 ). However, CBD and THC are soluble in supercritical CO 2 because they are dominantly nonpolar, making this the solvent of an appropriate choice (Grijó et al. 2018 ). Rovetto and Aieta ( 2017 ) evaluated the effect of pressure and the use of ethanol as a co-solvent on cannabinoid extraction. Extractions were run at 17, 24, and 34 MPa pressure. The yields increased almost linearly to 34 MPa, 0.185 g/g of cannabis at this pressure, compared with yield from a traditional ethanol extraction of 0.132 g/g. Increased pressure can increase the solvation power but decreases the selectivity of the extraction, so a higher pressure may not be the ideal condition. Ethanol was indicated to be useful as a co-solvent: When added in pulses, it can increase the rate of supercritical CO 2 extraction of cannabinoids (Rovetto and Aieta 2017 ). Omar et al. ( 2013 ) also demonstrated that using a co-solvent can increase the yield (Omar et al. 2013 ). The optimum yield of these cannabinoids was achieved by using ethanol as co-solvent at 55 °C and 34 MPa (Fathordoobady et al. 2019 ). However, when comparing SFE with other methods of extraction, Brighenti et al. ( 2017 ) revealed that the lowest amount of CBDA, CBD, and CBG was obtained (Brighenti et al. 2017 ). Figure 6 visualizes the supercritical fluid extraction process.
Diagram of a supercritical fluid extraction (Adopted from Wikiwand.com URL: https://www.wikiwand.com/en/Supercritical_fluid_extraction# )
Hydrodynamic cannabis extraction
Hydrodynamic cannabis extraction is a recent development within the cannabis industry that can be used to produce full-spectrum cannabis extracts with high bioavailability. There have been accounts of companies, such as IASO (Incline Village, Nevada), claiming to have developed a unique extraction system that produces products with high yield and increased potency. This alternative method involves freezing fresh plant material and converting it into a nanoemulsion in water by ultrasonication. Hydrodynamic force is then used to break the cell wall and release its contents. This is followed by liquid–liquid extraction using solvents, centrifugal separation, and finally low-temperature drying. The initial step of freezing the plant matter helps preserve the volatile compounds as well as acidic cannabinoids during the following steps. Hydrodynamic extraction is claimed to exceed conventional methods mainly due to the lack of high temperatures, short contact distillation, and low organic solvent consumption (admin, n.d.). Ishida and Chapman ( 2012 ) used this technique to extract carotenoids from tomatoes and found that the extractable lycopene, other carotenoids, and accessibility of carotenoids significantly improved (Ishida and Chapman 2012 ). However, to this date, there has been no scientific publication that explores this method of extraction. Therefore, to fully understand the efficacy of this method, more research is required.
Traditionally, the dried cannabis flower was the product of choice; however, as the industry expands, the demand for various products with distinct properties also increases. Therefore, multiple factors should be considered when selecting a drying technique or an extraction method to produce a specific product. Among different drying methods for post-harvest processing, freeze-drying is considered more appropriate when compared to other methods; however, there is currently a lack of academic research and evidence to support this. Hang-drying as a traditional technique is still the most convenient way to reduce the prevalence of mold and bacteria during storage before extraction. Solventless extraction and hydrodynamic extraction are of interest due to their high yield, easy, and fast process but lack the scientific publication to promote their employment for large-scale production. According to cannabinoids’ lipophilic or hydrophobic properties, slightly polar solvents are recommended for extraction. Although for terpenes with more than 15 carbons, non-polar solvents are suggested. Soxhlet and dynamic maceration are being used as traditional methods which are time- and solvent-consuming but accurate enough to be compared with modern techniques. Among modern methods, SFE, MAE, and UAE are well recognized as feasible and convenient techniques.
In this narrative review paper, the advantages and disadvantages of various drying and extraction methods have been discussed. The best methods for industries based on the final products have been reviewed and suggested. Some gaps are found in this review paper including the lack of information and knowledge about using freeze dryer for drying plant material after harvest, hydrodynamic extraction method, and a developed green extraction technique in the cannabis research area as well as cannabis industry which needs more investigations in the future studies.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
(-)-Trans-Δ 9 -tetrahydrocannabinol
(-)-Trans-Δ 9 -tetrahydrocannabinolic acid A
(-)-Trans-Δ 8 -tetrahydrocannabinol
Cannabidiol
Cannabidiolic acid
Cannabigerol
Cannabigerolic acid
Cannabichromene
Cannabichromenic acid
Endocannabinoid system
Ultrasound-assisted extraction
Microwave-assisted extraction
Dynamic maceration
High-performance liquid chromatography
Pressurized liquid extraction
Agarwal C, et al. Ultrasound-assisted extraction of cannabinoids from cannabis sativa L. optimized by response surface methodology. J Food Sci. 2018;83(3):700–10.
Aladić K, et al. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind Crops Prod. 2015;76:472–8.
Al-Ani RA, et al. Antibacterial activity of clove, cinnamon, and datura extracts against Erwinia carotovora subsp. atroseptica causative agent of black stem and soft rot on potato. J Med Plants Res. 2012;6(10):1891–5.
Azmir J, et al. Techniques for extraction of bioactive compounds from plant materials: A review. J food Eng. 2013;117(4):426–36.
Bernstein N, et al. Impact of N, P, K, and humic acid supplementation on the chemical profile of medical cannabis (Cannabis sativa L). Front Plant Sci. 2019;10:736.
Article Google Scholar
Blake A, Nahtigal I. The evolving landscape of cannabis edibles. Curr Opin Food Sci. 2019;28:25–31.
Brighenti V, Pellati F, Steinbach M, Maran D, Benvenuti S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J Pharm Biomed Anal. 2017;143:228–36.
Article CAS Google Scholar
Chan GCK, Hall W, Freeman TP, Ferris J, Kelly AB, Winstock A. User characteristics and effect profile of Butane Hash Oil: an extremely high-potency cannabis concentrate. Drug Alcohol Depend. 2017;178:32–8. https://doi.org/10.1016/j.drugalcdep.2017.04.014 .
Article CAS PubMed Google Scholar
Chemat F, et al. Ultrasound-assisted extraction in food analysis. Handb food Anal Instrum. 2008;85–103.
Ciolino LA, Ranieri TL, Taylor AM. Commercial cannabis consumer products part 1: GC-MS qualitative analysis of cannabis cannabinoids. Forensic Sci Int. 2018;289:429–37. https://doi.org/10.1016/j.forsciint.2018.05.032 .
Citti C, Ciccarella G, Braghiroli D, Parenti C, Vandelli MA, Cannazza G. Medicinal cannabis: principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J Pharm Biomed Anal. 2016;128:201–9. https://doi.org/10.1016/j.jpba.2016.05.033 .
Coffman C, Gentner W. Cannabis sativa L.: effect of drying time and temperature on cannabinoid profile of stored leaf tissue. Bull Narc. 1974;26(1):68–70.
Google Scholar
Danziger N, Bernstein N. Light matters: effect of light spectra on cannabinoid profile and plant development of medical cannabis (Cannabis sativa L.). Ind Crops Prod. 2021a;164:113351.
Danziger N, Bernstein N. Plant architecture manipulation increases cannabinoid standardization in ‘drug-type’medical cannabis. Ind Crops Prod. 2021b;167:113528.
De Castro ML, Garcıa-Ayuso L. Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal Chim Acta. 1998;369(1-2):1–10.
De Vita D, Madia VN, Tudino V, Saccoliti F, De Leo A, Messore A, Roscilli P, Botto A, Pindinello I, Santilli G, Scipione L, Costi R, Di Santo R. Comparison of different methods for the extraction of cannabinoids from cannabis. Nat Prod Res. 2020;34:20:2952–8. https://doi.org/10.1080/14786419.2019.1601194 .
De Vita MJ, et al. Association of cannabinoid administration with experimental pain in healthy adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75(11):1118–27.
Duarte K, Justino C, Gomes A, Rocha-Santos T, Duarte A. Chapter 4—Green analytical methodologies for preparation of extracts and analysis of bioactive compounds. In: Teresa RS, Armando CD, editors. Comprehensive analytical chemistry, vol. 65. Amsterdam: Elsevier; 2014. p. 59–78.
Dussy FE, Hamberg C, Luginbuhl M, Schwerzmann T, Briellmann TA. Isolation of Delta9-THCA-A from hemp and analytical aspects concerning the determination of Delta9-THC in cannabis products. Forensic Sci Int. 2005;149(1):3–10. https://doi.org/10.1016/j.forsciint.2004.05.015 .
Fathordoobady F, Singh A, Kitts DD, Pratap Singh A. Hemp (Cannabis Sativa L.) extract: anti-microbial properties, methods of extraction, and potential oral delivery. Food Rev Int. 2019;35(7):664–84. https://doi.org/10.1080/87559129.2019.1600539 .
Gloss D. An overview of products and bias in research. Neurother. 2015;12(4):731–4.
Green G, et al. The cannabis grow bible, Greg Green. 2001.
Grijó DR, Vieitez Osorio IA, Cardozo-Filho L. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers. J CO2 Util. 2018;28:174–80. https://doi.org/10.1016/j.jcou.2018.09.022 .
Hawes MD, Cohen MR. Method of drying cannabis materials, Google Patents. 2015.
Hazekamp A, Simons R, Peltenburg-Looman A, Sengers M, van Zweden R, Verpoorte R. Preparative isolation of cannabinoids from Cannabis sativa by centrifugal partition chromatography. J Liq Chromatogr Relat Technol. 2009;27(15):2421–39. https://doi.org/10.1081/jlc-200028170 .
Herrera M, De Castro ML. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. J Chromatogr A. 2005;1100(1):1–7.
Ishida BK, Chapman MH. Effects of a hydrodynamic process on extraction of carotenoids from tomato. Food Chem. 2012;132(3):1156–60.
Jensen G, Bertelotti R, Greenhalgh D, Palmieri T, Maguina P. Honey oil burns: a growing problem. J Burn Care Res. 2015;36(2):e34-37. https://doi.org/10.1097/BCR.0000000000000067 .
Article PubMed Google Scholar
Jin D, et al. Cannabis indoor growing conditions, management practices, and post-harvest treatment: a review. Am J Plant Sci. 2019;10(06):925.
Jin S, Chen J. Cannabis indoor growing conditions, management practices, and post-harvest treatment: a review. Am J Plant Sci. 2019;10(06):925.
Krishnan RY, Rajan K. Influence of microwave irradiation on kinetics and thermodynamics of extraction of flavonoids from Phyllanthus emblica. Braz J Chem Eng. 2017;34(3):885–99.
Lamy FR, et al. You got to love rosin: Solventless dabs, pure, clean, natural medicine. Exploring Twitter data on emerging trends in Rosin Tech marijuana concentrates. Drug Alcohol Depend. 2018;183:248–52.
Lehmann T, Brenneisen R. A new chromatographic method for the isolation of (-)-A’-(trans) tetrahydrocannabinolic acid A. Phytochem Anal. 1992;3:88–90.
Lewis-Bakker MM, Yang Y, Vyawahare R, Kotra LP. Extractions of medical cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis Cannabinoid Res. 2019;4(3):183–94.
Lindholst C. Long term stability of cannabis resin and cannabis extracts. Aust J Forensic Sci. 2010;42(3):181–90.
Liu M, Fernando D, Daniel G, Madsen B, Meyer AS, Ale MT, Thygesen A. Effect of harvest time and field retting duration on the chemical composition, morphology and mechanical properties of hemp fibers. Ind Crops Prod. 2015;69:29–39.
Mujumdar AS. Principles, classification, and selection of dryers. Handb Ind Drying. 2006;3:3–32.
Namdar D, Charuvi D, Ajjampura V, Mazuz M, Ion A, Kamara I, Koltai H. LED lighting affects the composition and biological activity of Cannabis sativa secondary metabolites. Ind Crops Prod. 2019;132:177–85.
Namdar D, Mazuz M, Ion A, Koltai H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind Crops Prod. 2018;113:376–82.
Omar J, Olivares M, Alzaga M, Etxebarria N. Optimisation and characterisation of marihuana extracts obtained by supercritical fluid extraction and focused ultrasound extraction and retention time locking GC-MS. J Sep Sci. 2013;36(8):1397–404. https://doi.org/10.1002/jssc.201201103 .
Ötles S, Kartal C. Solid-Phase Extraction (SPE): Principles and applications in food samples. Acta Scientiarum Polonorum Technologia Alimentaria. 2016;15(1):5–15.
Perrotin-Brunel H. Sustainable production of cannabinoids with supercritical carbon dioxide technologies. 2011.
Pomahacova B, et al. Cannabis smoke condensate III: the cannabinoid content of vaporised Cannabis sativa. Inhal Toxicol. 2009;21(13):1108–12.
Raber JC, Elzinga S, Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J Toxicol Sci. 2015;40(6):797–803.
Raventós M, et al. Application and possibilities of supercritical CO2 extraction in food processing industry: an overview. Food Sci Technol Int. 2002;8(5):269–84.
Reichardt C, Welton T. Solvents and solvent effects in organic chemistry, John Wiley & Sons. 2011.
Reverchon E, Porta GD, Senatore F. Supercritical CO 2 extraction and fractionation of lavender essential oil and waxes. J Agric Food Chem. 1995;43(6):1654–8.
Romano LL, Hazekamp A. Cannabis oil: chemical evaluation of an upcoming cannabis-based medicine. Cannabinoids. 2013;1(1):1–11.
Rose A. Characterization of lipids and the protein co-products from various food sources using a one-step organic solvent extraction process. 2019.
Book Google Scholar
Ross SA, ElSohly MA. The volatile oil composition of fresh and air-dried buds of Cannabis sativa. J Nat Prod. 1996;59(1):49–51.
Rovetto LJ, Aieta NV. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J Supercrit Fluids. 2017;129:16–27. https://doi.org/10.1016/j.supflu.2017.03.014 .
Saloner A, Bernstein N. Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Ind Crops Prod. 2021;167:113516.
Santos DT, Meireles MADA. Developing novel one-step processes for obtaining food-grade O/W emulsions from pressurized fluid extracts: processes description, state of the art and perspectives. Food Sci Technol. 2015;35(4):579–87.
Smith RM. Before the injection—modern methods of sample preparation for separation techniques. J Chromatogr A. 2003;1000(1-2):3–27.
Tambunan A, Yudistira K, Hernani. Freeze drying characteristics of medicinal herbs. Dry Technol. 2001;19(2):325–31.
Valizadehderakhshan M, et al. Extraction of cannabinoids from Cannabis sativa L. (Hemp). Agriculture. 2021;11(5):384.
Vatai T, Škerget M, Knez Ž. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J Food Eng. 2009;90(2):246–54.
Vogel A. Commercial cultivation of cannabis In: Cannabis: a clinician’s guide. 2018. p. 95.
Wianowska D, Dawidowicz A, Kowalczyk M. Transformations of tetrahydrocannabinol, tetrahydrocannabinolic acid and cannabinol during their extraction from Cannabis sativa L. J Anal Chem. 2015;70(8):920–5.
Yara-Varón E, Li Y, Balcells M, Canela-Garayoa R, Fabiano-Tixier A-S, Chemat F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules. 2017;22(9):1474.
Download references
Acknowledgements
Not applicable.
This study has not had any funds.
Author information
Authors and affiliations.
Drug Delivery Research Group, School of Science, Faculty of Health and Environmental Sciences, Auckland University of Technology, Auckland, New Zealand
Masoumeh Pourseyed Lazarjani, Lidya Kebede & Ali Seyfoddin
School of Science, Faculty of Health and Environmental Sciences, Auckland University of Technology, Auckland, New Zealand
You can also search for this author in PubMed Google Scholar
Contributions
Masoumeh Pourseyed Lazarjani has written the first version of this paper. Lidya Kebede added some parts to it and then Masoumeh edited different drafts which were revised by Ali Seyfoddin and Owen Young. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Ali Seyfoddin .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lazarjani, M.P., Young, O., Kebede, L. et al. Processing and extraction methods of medicinal cannabis: a narrative review. J Cannabis Res 3 , 32 (2021). https://doi.org/10.1186/s42238-021-00087-9
Download citation
Received : 17 July 2020
Accepted : 29 June 2021
Published : 19 July 2021
DOI : https://doi.org/10.1186/s42238-021-00087-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cannabinoids
Journal of Cannabis Research
ISSN: 2522-5782
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Research explores liberalization of medical marijuana and mental health in the US
T he approval of marijuana for medical use has had little effect on the mental health of the general population in the US. But legalization for therapeutic purposes does benefit those for whom it is intended. This is the conclusion of a study by researchers at the University of Basel. The research is published in the journal Health Economics, Policy and Law .
In the US, access to marijuana has been facilitated in most states since the mid-1990s—whether through medical clearance or through decriminalization of recreational use. However, liberalization is still controversial, and the effects on the well-being of specific groups and the therapeutic value of marijuana remain debated.
While some fear negative consequences from addiction, others highlight the potential medical benefits for people suffering from chronic pain, nausea or convulsions.
In a new study, researchers from Basel have now investigated whether medical cannabis legislation in the U.S. is improving the situation for sick people and whether it has a negative impact on the mental health of the overall population.
Probability-based analysis
For their analysis, the researchers combined two large datasets. They used data from almost eight million people who took part in telephone surveys between 1993 and 2018 as part of the Behavioral Risk Factor Surveillance System, which collects data about mental well-being, among other things. But they also used data from the National Survey on Drug Use and Health, which collects information on health-related issues such as drug use in the United States.
The researchers formed different groups using statistical assignment. They include individuals who are highly likely to abstain from using marijuana, to use marijuana as a recreational drug or to use it for medical reasons. It was also possible to identify individuals with a high probability of chronic pain. Mental health was measured using self-assessment, in which respondents reported the number of days they had had mental health problems in the previous month.
Positive effects of therapeutic use
Using statistical methods, the researchers were able to estimate the impact of the legal approval of marijuana for medical use. The result: Easier access improves the mental health of individuals who use marijuana for medical reasons. The same applies to people who are very likely to suffer from pain. The study authors estimate that these two groups spend 0.3 days less per month in poor mental health due to the change in the law.
At the same time, the researchers found no effect on the mental health of recreational users or on younger populations.
"Overall, our results show that medical cannabis legislation in the U.S. benefits the people it is intended for without harming other groups," summarizes the study leader, Prof. Alois Stutzer from the University of Basel.
More information: Jörg Kalbfuss et al, Medical marijuana laws and mental health in the United States, Health Economics, Policy and Law (2024). DOI: 10.1017/S1744133124000033
Provided by University of Basel
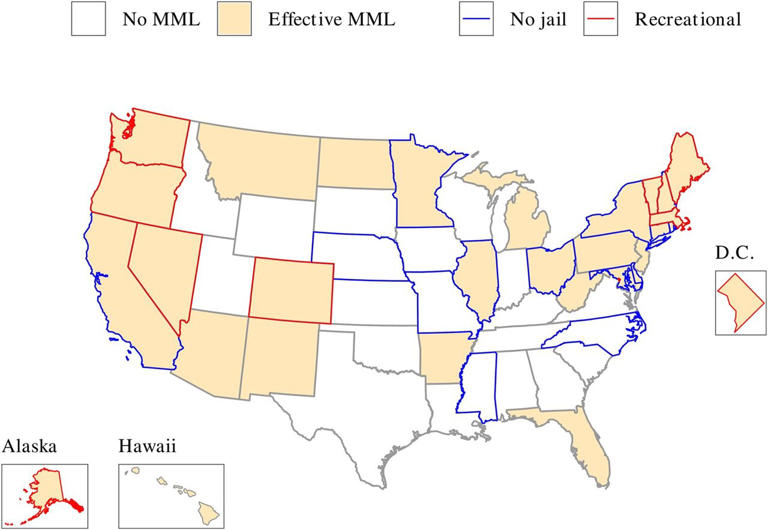
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 5, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
Researchers find antipsychotics help ease episodes of marijuana-induced psychosis
by Ernie Mundell

Overuse of marijuana is increasingly being linked to dangerous bouts of psychosis, and a new study finds that antipsychotics may be needed to keep such patients out of the hospital.
Psychotic episodes involve a dangerous psychiatric state in which people lose their connection with reality. These episodes can get so out of control that people may need hospitalization.
However, new research finds that people who overuse marijuana and then experience their first psychotic episode may be helped by the quick use of injected antipsychotics.
"These findings encourage the early use of second-generation, long-acting injectables as an important secondary prevention strategy to reduce rates of hospitalization" in such patients, reports a team led by Dr. Alexander Denissoff, of the University of Turku in Finland.
His team tracked outcomes for 1,820 people who had a first psychotic episode and also had cannabis use disorder between 2006 and 2021.
Just over 1,100 of these patients ended up being hospitalized due to "psychotic relapse," according to the American Psychiatric Association news release. However, folks who had received any antipsychotic med were a third less likely to require hospitalization due to relapse, compared to those who hadn't gotten these drugs.
Comparing the effectiveness of various antipsychotics , risperidone came out on top, cutting the odds for relapse-linked hospitalization by 60%, the researchers found, followed by aripiprazole (58% reduction), oral clozapine (57%), and paliperidone (54%).
Denissoff's team stressed the odds that a person with cannabis use disorder experiences multiple psychotic episodes rises if they use marijuana after experiencing a first episode and/or do not take antipsychotic medicines as prescribed.
For those who were hospitalized due to any substance use disorder , clozapine seemed to work best, with an 86% lower risk of re-hospitalization due to any substance use, followed by risperidone (67%) and paliperidone (63%), the researchers said.
The findings were published recently in the journal Schizophrenia Bulletin .
© 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

Stopping aspirin 1 month after coronary stenting significantly reduces bleeding complications in heart attack patients
16 hours ago

Oral vaccine for UTI is potential alternative to antibiotics, finds 9-year study
Apr 6, 2024

Study: Epilepsy patients benefit from structured 'seizure action plans'

Screening with a PSA test has a small impact on prostate cancer deaths but leads to overdiagnosis, finds study

Clinical trial: First cardiac bioimplants for treatment of myocardial infarction using umbilical cord stem cells
Apr 5, 2024

Research team builds first tandem repeat expansions genetic reference maps
Human neuron model paves the way for new Alzheimer's therapies

A deep dive into the genetics of alcohol consumption

First atlas of the human ovary with cell-level resolution is a step toward artificial ovary
Pig hearts kept alive outside the body for more than 24 hours offers hope for many humans needing a transplant
Related stories.
Antipsychotic injections linked to a sharp drop in hospital readmissions
Jan 30, 2024

Study: Cannabis use disorder increasing among veterans with psychiatric disorders
Nov 29, 2023

Bipolar disorder, unipolar depression up with cannabis use disorder
May 25, 2023
MRI may predict who'll respond best to schizophrenia treatment
Mar 15, 2024

Many with psychosis have long-term functional morbidity
Nov 22, 2022

Effectiveness of antipsychotics dips for women after age 45 years, says study
Oct 24, 2022
Recommended for you

How do wildfires affect mental health? A new study examines the connection

Study finds lonely women experience increased activation in regions of the brain associated with food cravings
Apr 4, 2024

Running style may be linked to personality type, study suggests
Body mapping links responses to music with degree of uncertainty and surprise
Study questions effectiveness of brain stimulation for memory enhancement
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Read our research on: Gun Policy | International Conflict | Election 2024
Regions & Countries
Most americans favor legalizing marijuana for medical, recreational use, legalizing recreational marijuana viewed as good for local economies; mixed views of impact on drug use, community safety.
Pew Research Center conducted this study to understand the public’s views about the legalization of marijuana in the United States. For this analysis, we surveyed 5,140 adults from Jan. 16 to Jan. 21, 2024. Everyone who took part in this survey is a member of the Center’s American Trends Panel (ATP), an online survey panel that is recruited through national, random sampling of residential addresses. This way nearly all U.S. adults have a chance of selection. The survey is weighted to be representative of the U.S. adult population by gender, race, ethnicity, partisan affiliation, education and other categories. Read more about the ATP’s methodology .
Here are the questions used for the report and its methodology .
As more states pass laws legalizing marijuana for recreational use , Americans continue to favor legalization of both medical and recreational use of the drug.
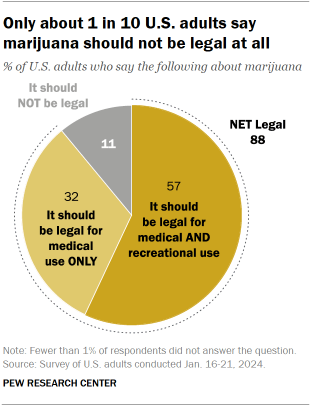
An overwhelming share of U.S. adults (88%) say marijuana should be legal for medical or recreational use.
Nearly six-in-ten Americans (57%) say that marijuana should be legal for medical and recreational purposes, while roughly a third (32%) say that marijuana should be legal for medical use only.
Just 11% of Americans say that the drug should not be legal at all.
Opinions about marijuana legalization have changed little over the past five years, according to the Pew Research Center survey, conducted Jan. 16-21, 2024, among 5,14o adults.
The impact of legalizing marijuana for recreational use
While a majority of Americans continue to say marijuana should be legal , there are varying views about the impacts of recreational legalization.
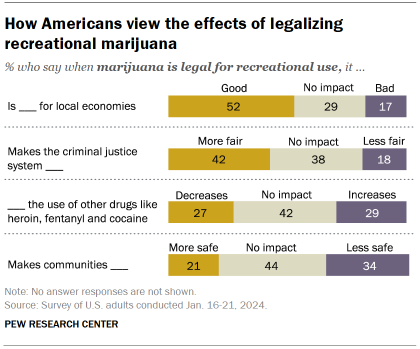
About half of Americans (52%) say that legalizing the recreational use of marijuana is good for local economies; just 17% think it is bad and 29% say it has no impact.
More adults also say legalizing marijuana for recreational use makes the criminal justice system more fair (42%) than less fair (18%); 38% say it has no impact.
However, Americans have mixed views on the impact of legalizing marijuana for recreational use on:
- Use of other drugs: About as many say it increases (29%) as say it decreases (27%) the use of other drugs, like heroin, fentanyl and cocaine (42% say it has no impact).
- Community safety: More Americans say legalizing recreational marijuana makes communities less safe (34%) than say it makes them safer (21%); 44% say it has no impact.
Partisan differences on impact of recreational use of marijuana
There are deep partisan divisions regarding the impact of marijuana legalization for recreational use.
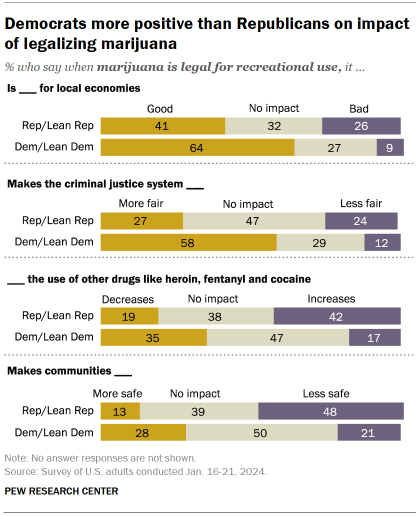
Majorities of Democrats and Democratic-leaning independents say legalizing recreational marijuana is good for local economies (64% say this) and makes the criminal justice system fairer (58%).
Fewer Republicans and Republican leaners say legalization for recreational use has a positive effect on local economies (41%) and the criminal justice system (27%).
Republicans are more likely than Democrats to cite downsides from legalizing recreational marijuana:
- 42% of Republicans say it increases the use of other drugs, like heroin, fentanyl and cocaine, compared with just 17% of Democrats.
- 48% of Republicans say it makes communities less safe, more than double the share of Democrats (21%) who say this.
Demographic, partisan differences in views of marijuana legalization
Sizable age and partisan differences persist on the issue of marijuana legalization though small shares of adults across demographic groups are completely opposed to it.
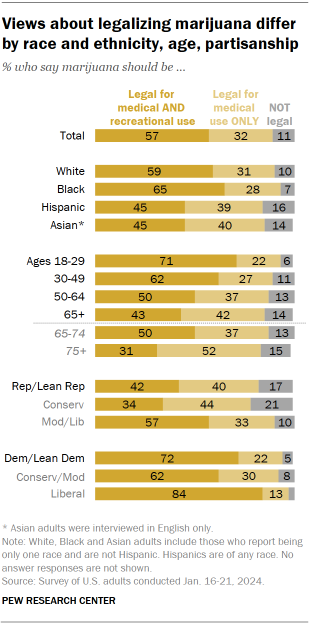
Older adults are far less likely than younger adults to favor marijuana legalization.
This is particularly the case among adults ages 75 and older: 31% say marijuana should be legal for both medical and recreational use.
By comparison, half of adults between the ages of 65 and 74 say marijuana should be legal for medical and recreational use, and larger shares in younger age groups say the same.
Republicans continue to be less supportive than Democrats of legalizing marijuana for both legal and recreational use: 42% of Republicans favor legalizing marijuana for both purposes, compared with 72% of Democrats.
There continue to be ideological differences within each party:
- 34% of conservative Republicans say marijuana should be legal for medical and recreational use, compared with a 57% majority of moderate and liberal Republicans.
- 62% of conservative and moderate Democrats say marijuana should be legal for medical and recreational use, while an overwhelming majority of liberal Democrats (84%) say this.
Views of marijuana legalization vary by age within both parties
Along with differences by party and age, there are also age differences within each party on the issue.
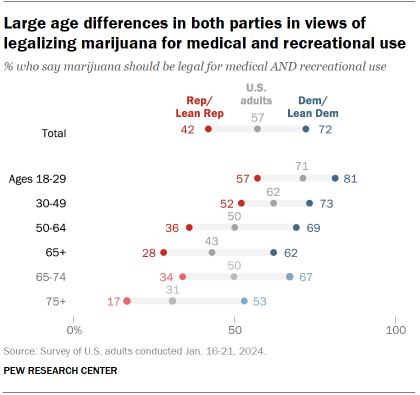
A 57% majority of Republicans ages 18 to 29 favor making marijuana legal for medical and recreational use, compared with 52% among those ages 30 to 49 and much smaller shares of older Republicans.
Still, wide majorities of Republicans in all age groups favor legalizing marijuana at least for medical use. Among those ages 65 and older, just 20% say marijuana should not be legal even for medical purposes.
While majorities of Democrats across all age groups support legalizing marijuana for medical and recreational use, older Democrats are less likely to say this.
About half of Democrats ages 75 and older (53%) say marijuana should be legal for both purposes, but much larger shares of younger Democrats say the same (including 81% of Democrats ages 18 to 29). Still, only 7% of Democrats ages 65 and older think marijuana should not be legalized even for medical use, similar to the share of all other Democrats who say this.
Views of the effects of legalizing recreational marijuana among racial and ethnic groups
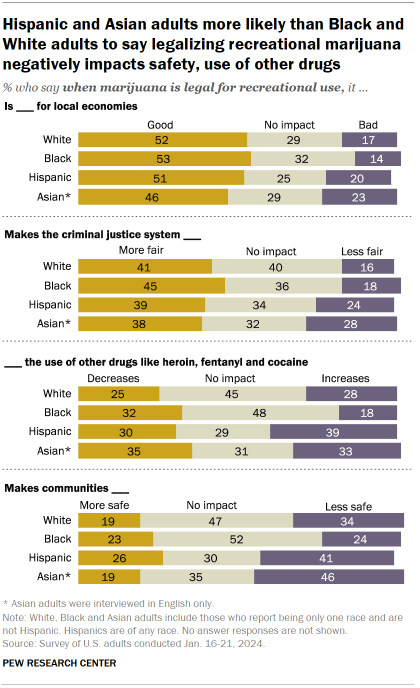
Substantial shares of Americans across racial and ethnic groups say when marijuana is legal for recreational use, it has a more positive than negative impact on the economy and criminal justice system.
About half of White (52%), Black (53%) and Hispanic (51%) adults say legalizing recreational marijuana is good for local economies. A slightly smaller share of Asian adults (46%) say the same.
Criminal justice
Across racial and ethnic groups, about four-in-ten say that recreational marijuana being legal makes the criminal justice system fairer, with smaller shares saying it would make it less fair.
However, there are wider racial differences on questions regarding the impact of recreational marijuana on the use of other drugs and the safety of communities.
Use of other drugs
Nearly half of Black adults (48%) say recreational marijuana legalization doesn’t have an effect on the use of drugs like heroin, fentanyl and cocaine. Another 32% in this group say it decreases the use of these drugs and 18% say it increases their use.
In contrast, Hispanic adults are slightly more likely to say legal marijuana increases the use of these other drugs (39%) than to say it decreases this use (30%); 29% say it has no impact.
Among White adults, the balance of opinion is mixed: 28% say marijuana legalization increases the use of other drugs and 25% say it decreases their use (45% say it has no impact). Views among Asian adults are also mixed, though a smaller share (31%) say legalization has no impact on the use of other drugs.
Community safety
Hispanic and Asian adults also are more likely to say marijuana’s legalization makes communities less safe: 41% of Hispanic adults and 46% of Asian adults say this, compared with 34% of White adults and 24% of Black adults.
Wide age gap on views of impact of legalizing recreational marijuana
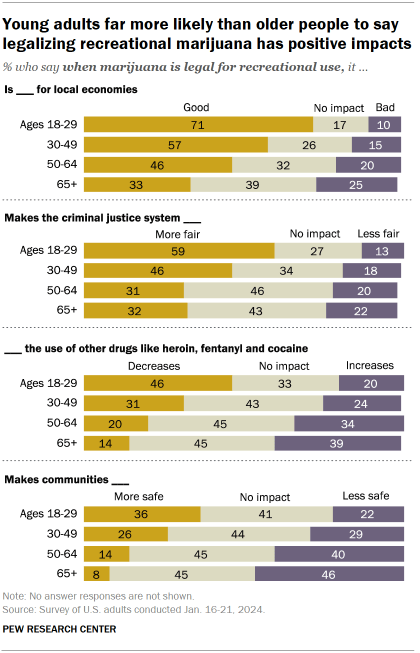
Young Americans view the legalization of marijuana for recreational use in more positive terms compared with their older counterparts.
Clear majorities of adults under 30 say it is good for local economies (71%) and that it makes the criminal justice system fairer (59%).
By comparison, a third of Americans ages 65 and older say legalizing the recreational use of marijuana is good for local economies; about as many (32%) say it makes the criminal justice system more fair.
There also are sizable differences in opinion by age about how legalizing recreational marijuana affects the use of other drugs and the safety of communities.
Facts are more important than ever
In times of uncertainty, good decisions demand good data. Please support our research with a financial contribution.
Report Materials
Table of contents, most americans now live in a legal marijuana state – and most have at least one dispensary in their county, 7 facts about americans and marijuana, americans overwhelmingly say marijuana should be legal for medical or recreational use, clear majorities of black americans favor marijuana legalization, easing of criminal penalties, religious americans are less likely to endorse legal marijuana for recreational use, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Topics › Drug Policy
Marijuana legalization is not associated with increases in youth suicide rates
Youth suicide rates actually tend to drop following legal access to recreational and medical marijuana..

The Journal of the American Academy of Child & Adolescent Psychiatry (JAACAP) just published a paper that found that new marijuana legalization laws correlated with increases in suicide among youth. Although the authors likely did their math correctly, they may have chosen a modeling strategy that produced inaccurate results. The results also don’t explain how marijuana laws are connected to these suicide rate increases among youth when youth marijuana use did not increase after marijuana markets were established at the state level.
In the new publication, “ Association Between Marijuana Laws and Suicide Among 12- to 25-Year-Olds in the United States From 2000 to 2019 ”, Christopher J. Hammond, J. Madison Hyer, Anne E. Boustead, Mary A. Fristad, Danielle L. Steelesmith, Guy N. Brock, Deborah S. Hasin, and Cynthia A. Fontanella compared suicide mortality data to state-level marijuana legalization efforts, such as permitting regulated medical and recreational markets. The authors found that recreational marijuana laws were associated with a 9% increase in suicide rates among all youth ages 14 to 16. They also found that medical and recreational laws were associated with 10% and 16% increases in suicide rates among female youth ages 12 to 25, respectively.
My preprint study with Robert Capodilupo, Michael Schemenaur, and Jeffrey A. Singer found the opposite results among similar age groups—marijuana laws were associated with a decline in suicide rates. So why the difference?
The difference is due to technical concerns of statistical analysis. Concisely, the authors chose an inappropriate model given the data distribution. The methods in the Hammond study exaggerate the effect of marijuana laws on suicide rates in states with relatively few suicides. States that had not yet or never did legalize marijuana were more likely to have near-zero suicide rates, because suicide rates have increased across all states over time. Because of the model the authors selected, the JAACAP study shows that states with regulated marijuana markets have higher suicide rates, regardless of whether suicides have risen at faster rates in those states after their marijuana laws went into effect.
The new JAACAP study employed a negative binomial regression , which assumes that the plurality of states had zero suicides to begin with and then examines changes in the number of suicides in each state. In reality, suicides occur in every state, and the average state-level suicide rate is approximately 14 deaths per 100,000 population. Therefore, state-level suicide rates don’t follow a negative binomial distribution.
For example, consider the distributions of state-level suicide rates among males ages 15 to 19 in Figure 1 below, which we analyzed in our study. The raw data (on the left) almost follow a normal distribution in which observations are closely grouped around a median value. A negative binomial distribution, however, assumes the modal observation is a zero value. Many statistical models exist to help researchers match their approach to the data and, in theory, the distribution curve associated with any model should closely fit the shape of the data. In Figure 1, we superimpose the data shape implied by the two models over the data as a red line. In our preferred model (on the right), we have taken a natural log of the data and then treated it as a normal distribution. The data fits the normal distribution shape implied by the red line much better than the model used by the Hammond team. This means that a basic linear regression on log-transformed data is more appropriate for this type of analysis, lending greater credibility to our analysis and conclusions.
After transforming the data and controlling for various sociodemographic factors, such as personal income and unemployment, we found that suicide rates on average generally drop among female and male youth after states allow both medical and recreational marijuana access, although these results weren’t statistically significant for all of our robustness checks. Therefore, suicide rates tend to drop with greater marijuana access, but we can’t conclude that the policies themselves were the cause of the drop. However, suicide rates do predictively drop about 5.4% for males ages 30 to 39 following medical marijuana access, which drove reductions in the total male suicide rate ( table of our full results). To be clear, our results are all associations and should not necessarily be interpreted as causal, but the data show that suicide rates don’t increase after state laws allow for greater marijuana access.
Actual marijuana use by teens hasn’t changed with legalization, another strike against the Hammond study’s conclusion. If marijuana legalization led to changes in suicide rates among youth, you’d expect it to be because youth are using marijuana more often. However, our analysis also shows that rates of marijuana use among youth remained stable after states adopted recreational marijuana markets (Figure 2).
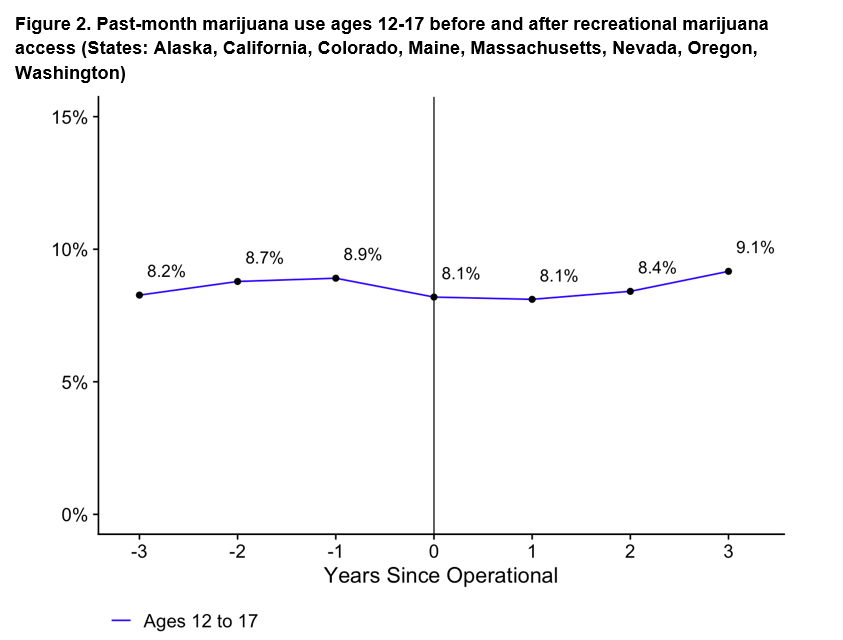
Inappropriate model selection is why conflicting results appear throughout the public health literature. In the case of suicide rates, our study rigorously shows that they tend to drop among youth following both recreational and medical marijuana access, which supports the original findings by Anderson et al. (2014) in the American Journal of Public Health .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Medical Marijuana and Marijuana Legalization
Rosalie liccardo pacula.
1 RAND Corporation, Santa Monica, California 90407; gro.dnar@alucap , gro.dnar@tramsr
2 National Bureau of Economic Research, Cambridge, Massachusetts 02138
Rosanna Smart
State-level marijuana liberalization policies have been evolving for the past five decades, and yet the overall scientific evidence of the impact of these policies is widely believed to be inconclusive. In this review we summarize some of the key limitations of the studies evaluating the effects of decriminalization and medical marijuana laws on marijuana use, highlighting their inconsistencies in terms of the heterogeneity of policies, the timing of the evaluations, and the measures of use being considered. We suggest that the heterogeneity in the responsiveness of different populations to particular laws is important for interpreting the mixed findings from the literature, and we highlight the limitations of the existing literature in providing clear insights into the probable effects of marijuana legalization.
INTRODUCTION
Although the federal law has prohibited the use and distribution of marijuana in the United States since 1937, for the past five decades states have been experimenting with marijuana liberalization polices. State decriminalization policies were first passed in the 1970s, patient medical access laws began to get adopted in the 1990s, and more recently states have been experimenting with legalization of recreational markets. This has resulted in a spectrum of marijuana liberalization policies across the United States that is often not fully recognized or considered when conducting evaluations of recent policy changes. Consider for example the state of marijuana policies in the United States at a single point of time. As shown in Figure 1 , as of January 1, 2016, 21 states 1 have decriminalized certain marijuana possession offenses ( NCSL 2016a ), 26 states have legalized medical marijuana use, and another 16 states have adopted cannabidiol (CBD)-only laws ( NCSL 2016b ) that protect only certain strains of marijuana to be used for medicinal purposes. However, there is tremendous overlap because some states have implemented combinations of each of these policies, as shown by the fact that the five states currently legalizing recreational marijuana use (Alaska, Colorado, Oregon, Washington, and the District of Columbia) all initially decriminalized marijuana and then passed medical marijuana allowances before passing their legalization policies. Thus, the vast majority of US states have moved away from a strict prohibition position toward marijuana well before they started considering outright legalization.

State marijuana policies as of January 1, 2016. Data from the RAND Marijuana Policy Database ( Pacula et al. 2015 ) and NCSL (2016a , b) with permission. Abbreviation: CBD, cannabidiol.
A number of factors have driven the policy changes observed over the past several decades, including rising state budgetary costs associated with arresting and incarcerating nonviolent drug offenders ( Raphael & Stoll 2013 , Reuter et al. 2001 ), growing scientific evidence of the therapeutic benefits of cannabinoids found in the marijuana plant ( Hill 2015 , Koppel et al. 2014 ), and strained state budgets that have caused legislatures to look for new sources of tax revenue ( Caulkins et al. 2015 , Kilmer et al. 2010 ).
The tremendous policy variation over time and across states would appear to give researchers ample opportunities to quantitatively assess the effect of marijuana liberalization policies on a variety of health and social outcomes. However, the scientific literature has been slow to develop, and what exists in the literature offers generally mixed and largely insignificant findings. This has led many to conclude that the previous liberalization policies must be harmless and that ongoing legalization would similarly generate very little harm to society. Indeed, recent surveys of people’s attitudes about marijuana show a clear shift in favor of legalization ( Caulkins et al. 2015 ).
As we will argue throughout this article, however, at least three reasons suggest that we use caution in drawing conclusions from the mixed empirical evidence or, more importantly, in assuming that a change to legally protected commercial markets would result in outcomes similar to those of the previous experiments. First, the literature has largely treated both decriminalization and medical marijuana policies as if they were simple dichotomous choices, implemented similarly across states. Such a treatment ignores the significant heterogeneity in these policies that can differentially influence harms and benefits and also contributes to what appear to be mixed results from evaluations. Second, the vast majority of policy evaluations conducted thus far examine the effect of the policy in terms of changes in prevalence rates in the general population, which assumes that the proportion of casual and heavy users, who are pooled together in these simple prevalence rates, remains stable even as the policy changes. Finally, research has been slow to consider the extent to which these changes in policies influence the method by which the typical user consumes marijuana. The potential acute harm associated with smoking a joint is different from that associated with consuming an edible or dabbing wax, particularly given that the average potency of the product typically differs and the body’s rate of absorption of THC varies by method ( Huestis 2007 ).
In this article, we review the existing literature on the effects of decriminalization and medical marijuana laws on marijuana use and marijuana use disorders in light of these limitations. Unlike other reviews, our goal is not to summarize all the existing literature on the effects of decriminalization and medicalization. Rather, the purpose of this review is to provide a better understanding of what can be gleaned from the literature when more consideration is given to the complexities of these policies, the populations examined, and the measures of use considered. Doing so allows us to convey the need for more research, in terms of measurement and analysis, before we can truly understand the impacts of marijuana liberalization policies.
WHAT IS MEANT BY HETEROGENEOUS MARIJUANA POLICIES
Defining the policies.
It is important for any discussion of the literature to begin by defining the policies being considered. For the purposes of this review, we define four specific marijuana policies (prohibition, decriminalization, medical marijuana, and legalization) in terms of their legal definitions rather than their implementation in local communities, as the latter is often a function of the level of enforcement, which is difficult to measure in a systematic and analytic way. Prohibition, therefore, can be defined as a law that maintains the criminal status of any action related to marijuana possession, use, cultivation, sale, or distribution. The level of crime may be statutorily defined as either a misdemeanor (incurring relatively lower criminal penalties that may or may not include jail time) or a felony (entailing much more serious charges, tougher sanctions, and certain prison time), and the charge may be a function of the amount of marijuana involved or simply of the nature of the activity (e.g., sale to minors). Regardless, the emphasis is on the criminal status of the related offenses, not the degree to which local law enforcement chooses to enforce it. The US federal government, for example, retains its prohibition on all marijuana activities (possession, use, cultivation, distribution, processing, and sale) as do cities like San Francisco, although San Francisco has adopted a policy of low-priority enforcement ( Ross & Walker 2017 ).
Decriminalization is a policy that was first defined by the 1972 Shaffer Commission (also known as the National Commission on Marihuana and Drug Abuse), and it describes policies that do not define possession for personal use or casual (nonmonetary) distribution as a criminal offense. The Shaffer Commission clearly stated that policies that simply lowered the penalties without removing the criminal status of the offense were not technically decriminalized, because they maintained the substantial social harm of the associated criminal convictions ( Natl. Comm. Marihuana Drug Abus. 1972 ). This distinction between policies that simply lower penalties and those that actually change the legal status of the offense is important, and yet it is not widely understood by many researchers evaluating even the early policies. At least 2 of the 11 widely recognized decriminalized states from the 1970s and 1980s, California and North Carolina, did not remove the criminal status of the offense ( Pacula et al. 2003 , Reuter & MacCoun 1995 ). Instead, these states merely reduced the penalties associated with possession and/or use of marijuana, a policy generally known as depenalization ( MacCoun & Reuter 2001 , Pacula et al. 2005 ). Yet, individuals in depenalization jurisdictions can still face significant barriers to access work, student loans, and public assistance if caught in possession of marijuana, even if they are only charged with a small fine, because they can still get a criminal charge on their record.
Medical marijuana laws (MMLs) remove state penalties for the use of marijuana for medicinal purposes under specified conditions. Although the federal government continues to retain the 1970 classification of marijuana as a Schedule I substance with high potential for abuse and no accepted medical value (Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970, P.L. 91–513, October 27, 1970, 84 Stat. 1242, 21 U.S.C. 801, et seq.), states have employed a number of regulatory approaches aimed at increasing access to marijuana for medicinal purposes since the 1970s. Early initiatives through the 1980s aimed to encourage study of the therapeutic value of marijuana, but they had little practical significance due to their heavy reliance on federal cooperation and the failure to establish a legitimate supply channel for patients ( Pacula et al. 2002 ). Initiatives passed since the 1990s have been far more comprehensive, establishing allowances for the use, possession, and supply of high (>3%) Δ 9 -tetrahydrocannabinol (THC) products for qualifying patients and their caregivers or providers. These modern MMLs have become the most commonly evaluated policies in comparative alcohol and drugs policy analysis ( Ritter et al. 2016 ), but incomplete consideration of widespread variation in how these laws have been designed and implemented has resulted in inconclusive and often contradictory findings ( Hunt & Miles 2015 ; Pacula et al. 2014a , 2015 ).
Legalization removes criminal and monetary penalties for the possession, use, and supply of marijuana for recreational purposes. Whereas decriminalized countries such as the Netherlands have histories of de facto legalization, and medical marijuana programs are often regarded as thinly veiled recreational legalization ( Fischer et al. 2015 , Haney & Evins 2016 ), de jure legalization is a relatively new phenomenon. The November 2012 ballot initiatives passed by voters in Colorado and Washington marked the first time that any jurisdiction worldwide has legally regulated marijuana. Much attention has been given to the recently created retail markets for legal marijuana in these two states, but the commercial model is but one regulatory option for legal production, and a number of alternative strategies are available ( Caulkins et al. 2015 ). Research has not yet assessed the consequences of legalization, but the effects on the prevalence of marijuana use and use disorders will depend largely on the specific state-level regulations adopted as well as the response of the federal government.
Establishing clear definitions for decriminalized, medicalized, and legalized states is not merely a semantic exercise; rather, it highlights the different mechanisms through which policies may influence use, including changes in perceptions of risk or social disapproval, changes in product availability and variety, and changes in production methods or costs that reduce prices. Although it is tempting to use evaluations of decriminalization and medical marijuana policies to shed light on the likely consequences of legalization, the experiences of these states may not fully reflect the changes in price, potency, and product variety that will likely result from increased commercialization and promotion under legalization ( Caulkins et al. 2012 ). Additionally, prior research on decriminalization and MMLs has suffered from serious limitations due to an overreliance on crude indicators that do not account for the complex and varied ways in which states have designed and implemented their policies ( Pacula & Sevigny 2014a , b ; Pacula et al. 2005 ). Although the existing literature may be limited in answering how legalization will affect marijuana use and associated outcomes, it offers significant insights into how we should evaluate the effects of marijuana policy changes in a rapidly evolving and multilayered policy environment.
Decriminalization and Definitional Problems
As stated previously, much of the scientific research evaluating the impacts of decriminalization in the United States has ignored the legal definition provided by the Shaffer Commission. In an examination of the original 11 statutes passed shortly after the Shaffer Commission, Pacula and colleagues (2003) discovered that 2 of the 11 widely recognized decriminalized states (California and North Carolina) retained the criminal status of marijuana possession offenses. Moreover, the reduced penalties in 4 of the original 11 states (Minnesota, Mississippi, Nebraska, and North Carolina) only applied to first-time offenders, a distinction not consistent with the spirit of the Shaffer Commission definition. A comparison of state statutory penalties in so-called nondecriminalized states and in decriminalized states reveals that it is not possible to uniquely distinguish the two groups ( Pacula et al. 2003 , 2005 ). As early as 2001, there were 7 so-called nondecriminalized states that had removed the criminal status of all marijuana possession offenses and another 13 states that allowed for the reduced penalties and expungement of the criminal offense for first-time offenders ( Pacula et al. 2005 ). Yet, research continued to use the decriminalization variable to identify differences in state marijuana policies that were not truly based on the criminal status or level of penalties.
Given that most US studies have made use of a single dichotomous measure that cannot uniquely differentiate states with lower penalties and reduced criminal status, it is not surprising that they had mixed results. Even early studies examining immediate changes in laws using data from the 1970s and 1980s did not generate consistent findings. Although several studies making use of population survey data found no statistically significant impact of decriminalization on general prevalence rates of marijuana use ( Johnston et al. 1981 , Maloff 1981 , Single 1989 ), one study looking at emergency room episodes found that cities in states that had decriminalized had higher marijuana-involved episodes than cities in nondecriminalized states ( Model 1993 ). More recent studies that analytically relied on cross-sectional variation in decriminalization status in the late 1980s and 1990s also produced mixed findings. For example, studies examining self-reported use among youth and young adults that only included the single dichotomous measure for marijuana decriminalization found no statistical association with measures of past-year or past-month use ( DiNardo & Lemieux 2001 , Pacula 1998 , Thies & Register 1993 ). Yet analyses of the adult household population ( Saffer & Chaloupka 1999 ) and studies examining youth but incorporating other measures of legal risk ( DeSimone & Farrelly 2003 , Pacula et al. 2003 ) did find evidence of a positive association between decriminalization status and prevalence of use. MacCoun et al. (2009) note that the fact that the state decriminalization indicator remains positive and significant in analyses that also include additional controls for the statutory penalties for these offenses suggests that this measure is picking up something other than a signal related to a reduction in the legal risk. Hypotheses offered include a proxy of broader social acceptance of marijuana use and an advertising effect of the reduced policies.
Even beyond the problem of policy measurement, results from US studies evaluating the impact of marijuana decriminalization need to be interpreted with caution for several reasons. First, in many studies, marijuana possession penalties do not vary substantially over time, which analytically confounds the effects of unobserved state characteristics (e.g., tough-on-crime lawmakers) with differences observed in the level of penalties. Second, because there is no comprehensive data source reporting the actual penalties incurred by offenders, these studies have all relied on proxies, such as maximum or median fines as indicated by statutory laws. These statutory penalties may or may not accurately reflect the true severity of the penalties imposed in a jurisdiction. Last, evidence has shown that citizens have relatively limited knowledge as to the statutory penalties and policies for marijuana possession in their states ( MacCoun et al. 2009 ), which makes it difficult to interpret evidence showing that removal of such penalties has a significant causal effect on marijuana consumption.
Medical Marijuana Laws in a Complex and Dynamic Policy Environment
In 1996, California became the first state to pass what is now commonly recognized as an MML. As of January 2016, 25 additional states have passed similar legislation. Empirical evidence consistently shows a strong correlation between MMLs and the prevalence of marijuana use and marijuana use disorders ( Cerdá et al. 2012 , Wall et al. 2011 ), but studies have not consistently supported a causal interpretation ( Anderson et al. 2015 , Hasin et al. 2015b , Lynne-Landsman et al. 2013 , Wen et al. 2015 ).
One explanation for the inconsistent findings from causal studies is that the specific provisions of state MMLs have varied widely both among states and within any given state over time ( Pacula et al. 2014a , b ). The use of a single dichotomous indicator for the initial passage of an MML in policy evaluation obscures both types of variation. Because the effects of any policy will depend on the specific statutory provisions and their implementation, studies examining outcome data covering different time frames are in fact evaluating the effects of very different policies. Further confounding comparison of prior estimates is the fact that the federal enforcement position has changed over time, and state MML provisions have adapted alongside changes in the federal stance.
When one takes a historical look at how MMLs have evolved since the passage of California’s law in 1996, it becomes easy to understand how a single dichotomous measure falls short of describing these policies within a state and across states over time. We broadly categorize state policies into three waves, each initiated by an important political change: the ballot era (1996–2000), the early legislative era (2000–2009), and the late legislative era (2009–present).
The ballot era states are the first seven states that enacted policies through ballot initiatives (whether subsequently contested by state courts or not). These early laws aimed to protect the rights of patients who used medical marijuana and their caregivers who assisted in that use. Federal opposition to these policies was explicit, and one month after Proposition 215 passed in California, then-drug czar Barry McCaffrey threatened to arrest any physician who recommended cannabis to a patient ( Pertwee 2014 ). The threat of federal enforcement created an important barrier to establishing clearly defined legal access to medical marijuana. Early MMLs during the ballot era were often vague, defining medical use broadly to include consumption, home cultivation, production, transportation, and acquisition. Most of the laws were ambiguous as to the legality of group growing or storefront dispensaries, resulting in confusion among law enforcement, patients, and caregivers as to what constituted legal participation in the medical marijuana market. Furthermore, the uncertainty of the federal response to these state experiments meant that ballot era policies rarely mandated patients to register with a state authority, making it even more difficult for law enforcement to differentiate legitimate medical users from recreational users. It is thus unsurprising that research examining the effects of the early state ballot laws on marijuana use has found insignificant effects ( Gorman & Huber 2007 , Khatapoush & Hallfors 2004 ).
With the passage of S.B. 862 in 2000, Hawaii became the first state to pass an MML through the state legislature rather than by ballot initiative. Learning from the frustrating experiences of patients and law enforcement under the earlier state policies, states that passed laws during this early legislative era (2000–2009) made more explicit allowances regarding the supply chain. Most laws passed during this period included patient registry provisions, allowances for home cultivation, and limits on the amount of marijuana that patients or caregivers could possess and grow. In addition, many states that had initially passed laws through ballot initiatives (e.g., California and Oregon) made further policy changes through their state legislatures during this period in an attempt to clarify issues and address tensions that had emerged.
Although MMLs during this early legislative era established clearer definitions of what constituted legal supply, uncertainty about the federal response to these policies inhibited a formal state regulation of producers. For instance, Colorado’s 2001 law did not explicitly sanction cooperative growing, but the ambiguity of the law allowed for its de facto operation. Through S.B. 420, California amended its initial MML to explicitly allow for cooperative cultivation, but regulatory discretion was left to local governments. New Mexico was the only state in the early legislative era to establish legal provisions for state-licensed dispensaries in its initial legislation in July 2007, but threats of federal prosecution led to indefinite delays in licensing ( Baker 2007 ).
Protracted legal disputes about the legitimacy of retail outlets under state law combined with tremendous uncertainty about the federal response led to the slow development of medical marijuana markets throughout many states during the early legislative era, which helps explain why many studies evaluating MMLs from this period find insignificant effects on prevalence of marijuana use ( Anderson et al. 2012 , 2015 ; Harper et al. 2012 ; Lynne-Landsman et al. 2013 ; Pacula et al. 2015 ). Whereas norms may have been changing in response to these laws, direct access through markets was not necessarily increasing ( Smart 2016 ). Yet, two studies making use of data from only this time period find a significant positive effect of MML enactment on use among specific high-risk populations ( Chu 2014 , Pacula et al. 2010 ). Making use of quarterly data from the 2000–2003 Arrestee Drug Abuse Monitoring (ADAM), Pacula et al. (2010) find a positive association between MML and self-reported marijuana use (confirmed through urine samples) among adult male arrestees. Chu (2014) similarly found significant positive effects of MML policies on marijuana possession arrests and marijuana-related treatment admissions, though the results are sensitive to model specification. These studies may indicate that increased medical marijuana supply in an uncertain policy environment primarily affected marijuana consumption among an at-risk population of heavy users. However, the results are also consistent with endogenous responses by police enforcement or treatment facilities and may not reflect actual changes in use.
In 2009, the uncertainty about the federal government’s response was seemingly resolved. Shortly following the inauguration of President Barack Obama, Attorney General Eric Holder issued a statement that federal authorities would cease interfering with medical marijuana dispensaries operating in compliance with state law ( Johnston & Lewis 2009 ). On October 19, 2009, Deputy Attorney General David Ogden formalized this policy of federal nonenforcement with a memorandum stating that federal prosecutors “should not focus federal resources … on individuals who are in clear and unambiguous compliance with existing state laws providing for the medical use of marijuana” ( Ogden 2009 , pp. 1–2).
The clarification of the federal position dramatically changed the regulatory structure of state medical marijuana supply channels. State MMLs passed during the late legislative era (2009–present) established far more comprehensive and explicit regulations regarding medical marijuana distribution, often requiring elaborate systems that would take years to fully implement. Several early-enacting states (e.g., Oregon and Maine) amended their laws to formally allow and regulate state-licensed dispensaries. State regulatory authorities became more prominently involved in the production and distribution of marijuana by overseeing the dispensing, manufacturing, and labeling of cannabis-derived products.
Following the Ogden Memo, requirements for the registration of patients and caregivers became far more standard in state policies, and the participation of both increased dramatically in state medical marijuana programs ( Fairman 2015 , Sevigny 2014 ). States that had delayed the implementation of formal supply channels (e.g., New Mexico) moved quickly to license dispensaries, and other states began to resolve legislative disputes about what constituted legally protected sources of supply. Alongside this expansion of medical marijuana markets during this period, media attention toward the issue of legal marijuana also increased markedly ( Schuermeyer et al. 2014 , Stringer & Maggard 2016 ).
Compared to earlier time periods, in the late legislative era marijuana use might respond more significantly to changes in policy as the availability and potency of the drug evolved with the changing structure and size of medical marijuana markets ( Sevigny et al. 2014 ). Indeed, the one study to evaluate the effects of MML passage using only policies enacted in the early and late legislative eras ( Wen et al. 2015 ) found a significant positive effect of MML enactment on the probability of recent marijuana use (14%), daily marijuana use (15%), and marijuana use disorders (10%). More studies focused on these later laws are needed to assess if these findings are robust.
Perhaps because of the federal permission for states to regulate medical marijuana more directly, medical marijuana policies adopted by states for the first time during this postlegislative era (e.g., by New York, Massachusetts, Illinois) contain a variety of features that differ considerably from those of the laws of early adopting states. For example, all MMLs passed after 2009 have established a state-licensed dispensary system and do not allow personal cultivation by patients or their caregivers, except under narrowly defined circumstances. Moreover, since 2010, states have adopted medical marijuana policies that are more consistent with traditional medical care and pharmaceutical regulation ( Williams et al. 2016 ). For example, all require testing and labeling of marijuana cannabinoid profiles in addition to a bona-fide clinical doctor-patient relationship requiring the ongoing management of the condition.
Evidence that MML statutes are continuing to move in a more medicalized direction is evident by the growing number of high CBD-only laws since 2014. CBD is a naturally occurring nonpsychoactive compound in cannabis that has been demonstrated in a variety of clinical studies not only to have therapeutic effects but also to counter the intoxicating effects of THC ( Koppel et al. 2014 , Russo et al. 2007 , Whiting et al. 2015 ). These new laws allow qualifying patients to use CBD extract, mostly in oil form, with minimal THC content, and its use is generally only allowed for a narrow range of medical conditions. Sixteen states have passed CBD laws since 2014, but these policies have been largely ignored by advocacy groups, and no research is studying their impacts ( NCSL 2016b ). With some exceptions, there is still limited regulation on potency (THC concentration) and other cannabinoids, medical product testing, and methods of consumption.
Considering Heterogeneous Implementation of Legalization
As of July 2016, five states have policies legalizing the possession of specified quantities of marijuana by adults aged 21 and older for recreational purposes. 2 Voters in Colorado and Washington approved legalization initiatives in November 2012, and additional policies were passed in Alaska, Oregon, and the District of Columbia in November 2014. The current regulatory environment is complex and dynamic, as state and local governments are continually adapting legislation to evolve along with the industry ( Subritzky et al. 2016 ). The effects of these policies on marijuana use and use disorders will be determined by how the design and implementation of the legal regulatory framework influence market structure, price and availability, and perceptions of risk and social approval. As research moves forward in evaluating the effects of recreational legalization, consideration needs to be given to differences and similarities in the regulatory frameworks established by each state.
The District of Columbia is the only legalized jurisdiction in the United States that does not allow the sale of marijuana for recreational use. Under DC’s law, adults can legally grow up to six plants (of which no more than three can be mature) in their primary residence and transfer up to 1 ounce of marijuana to another adult aged 21 and older if there is no remuneration. Sale of any amount of marijuana remains a criminal offense, punishable by up to six months in jail and a fine of $1,000 ( Marijuana Work. Group 2016 ). In contrast, policies in Colorado, Washington, Oregon, and Alaska establish commercialized models of marijuana regulation. Retail sales in Colorado and Washington began respectively in January and July 2014, and Oregon began allowing sales for recreational use from medical marijuana dispensaries in October 2015. Alaska began licensing retail and product manufacturers in September 2016 ( Hall & Lynskey 2016 ). Relative to the home cultivation model of the District of Columbia, commercialization is expected to substantially reduce production costs and generate incentives for legal suppliers to promote heavy consumption ( Caulkins & Kilmer 2016 ).
However, the commercial model of legalization also offers increased scope for regulation, and each state has crafted its own collection of regulatory guidelines and legal provisions that could have important implications for the markets that develop within them. For example, whereas all states require separate licenses for cultivators, manufacturers or processors, and retailers, as well as licensing or certification for testing facilities, Washington alone has adopted regulations restricting the number of licenses a single firm can own. Moreover, Washington prohibits license holders from being involved in both production and retail, in an effort to forbid vertical integration and the efficiencies in production and distribution that can come with it. Washington has further limited the number of retail store licenses available to avoid issues related to overproduction; the other states have not. However, all states except Alaska restrict the size of cultivation facilities, and Washington has an additional cap on total statewide production. In addition to this policy heterogeneity at the state level, local municipalities have some discretion in determining the number of establishments permitted, the strictness of zoning requirements, and the time and manner in which businesses are allowed to operate. These differences in the structure of the market should theoretically influence the availability and cost of marijuana in each state, for reasons described in greater detail below.
Other important legal differences exist across states in terms of the allowance for a nonretail market. Washington is the only state that requires all marijuana for recreational use to be purchased through state-licensed retailers; no home cultivation is allowed. The other three states permit home cultivation by adults subject to specified plant limits (as in the District of Columbia). There are also different approaches to taxation. Currently, the three states with operating retail markets (Colorado, Washington, and Oregon) have instituted ad valorem taxes specific to marijuana, ranging from 17% in Oregon to 37% in Washington. In contrast, Alaska’s policy establishes a tax on cultivation, imposing a $50 per ounce tax on marijuana bud (i.e., flowers) and a $15 per ounce tax on other parts of the plant (stems and leaves).
Differences in how state and local governments regulate the commercial market will generate heterogeneous effects on the retail price of marijuana, which will have important consequences for both the extensive and intensive margins of use and abuse ( Pacula & Lundberg 2014 , Pacula et al. 2014b ). Moreover, because marijuana is involved in a variety of forms and potencies, choices about the tax level, base, and point of collection can also influence the products and potencies available to consumers and the prices they face ( Caulkins et al. 2015 ). Currently, retail stores are allowed to offer marijuana flowers, concentrates, and infused products in solid and liquid form. The original legalization measures in Colorado and Washington did not explicitly distinguish between product types when establishing consumer purchase limits. As marijuana concentrates and infused products have captured an increasing share of legal retail sales, regulations have had to expand. Effective October 2016, adult residents in Colorado are limited to purchasing 1 ounce of marijuana flower, 8 g of concentrates, or 80 10-mg servings of THC in infused product form. In Washington and Alaska, consumers can purchase 1 ounce of marijuana flower, 7 g of marijuana concentrates, 16 ounces of infused product in solid form, or 72 ounces in beverage form. Oregon’s regulations are similar, except for a stricter limit of 5 g for marijuana concentrates. Alaska’s rules also limit buyers to 5,600 mg of THC in a single purchase.
Due to concerns regarding accidental ingestion of edibles by children, states have further regulated marijuana-infused products by implementing stricter packaging and labeling requirements and designating potency limits for individual serving sizes. Washington and Colorado designate individual serving sizes of 10 mg of THC and 100 mg total for an individually wrapped package. In Colorado, products that cannot be stamped, such as drinks or granola, must contain no more than a designated individual serving, effectively banning many of the high-potency marijuana-infused beverages currently sold. Oregon and Alaska have more conservative requirements, designating individual serving sizes of 5 mg of THC and 50 mg total for an individually wrapped package. Still, no state has capped the potency of marijuana products. A measure to limit the THC content of all marijuana products sold at retail stores in Colorado to 16% (Initiative 139) was withdrawn by the Healthy Colorado Coalition in 2016 due to the emergence of a well-funded opposition campaign ( Armbrister 2016 ). In Alaska, a proposal to cap marijuana product potency at 76% THC was also voted down. The lack of restrictions on potency enables the marketing of products with very high (and often uncertain) levels of THC.
Increased marketing has been an important concern under the commercial model, because advertising can be used to promote harmful use and has been shown to influence adolescent marijuana use and intention to use ( D’Amico et al. 2015 ). Colorado’s regulations prohibit Internet pop-up advertisements and advertisements that target children. Washington allows retailers to have only two signs (not to exceed 1,600 square inches) at their place of business, but the signs cannot contain marijuana-themed imagery nor can marijuana-related imagery be featured in window displays. Alaska and Oregon continue to revise rules for marijuana marketing. The strictness of state regulations for advertising and the way they are enforced can partly mediate the extent to which legalization influences perceptions and consumption behaviors among legal consumers as well as adolescents. However, these potential benefits of advertising restrictions must be balanced against potential efficiency costs resulting from information asymmetries between suppliers and consumers.
As was the case with decriminalization and MMLs, legalization is not a binary policy variable. The home cultivation model of the District of Columbia will have very different implications for supply than the commercialized models of Colorado, Washington, Oregon, and Alaska. Within commercialized states, heterogeneity in how production and price are regulated will lead to different consequences for consumption by legal adult users and spillovers to adolescent markets. Restrictions placed on advertising could limit youth exposure to messaging that could encourage experimentation, but only if the regulations are enforced. The way in which product availability and potency are regulated will have important effects on the total quantity of marijuana consumed by users and their level of intoxication, which will in turn influence the prevalence of marijuana use disorders. Legalized states have chosen different ways of regulating, and this policy heterogeneity will need to be considered in future work when assessing the effects of legalization on use.
WHAT IS MEANT BY HETEROGENEOUS POPULATIONS
The previous section focused on the heterogeneity of the policies being implemented. However, the effects of these diverse policies may well vary depending on the population group studied. Heterogeneous effects across population subgroups may be driven by differences in budget constraints ( Markowitz & Taurus 2009 ), price elasticities ( Pacula & Lundberg 2014 ), preferences for risk ( Fox & Tannenbaum 2011 ), or search costs ( Galenianos et al. 2012 , Pacula et al. 2010 ), to name a few. Mixed findings in the current literature with respect to the impact of prior liberalization policies may thus reflect legitimate differences in the populations being studied.
Past research has generally attempted to accommodate this potential heterogeneity by stratifying analyses by age (e.g., adolescents, young adults, older adults) and, to a lesser extent, frequency of use (number of times used in the past month/year or near-daily use). The potential effects on youth consumption have been of particular concern in the literature, because evidence suggests that use of marijuana during early adolescence predicts increased risk of dependence, lower educational attainment, and cognitive impairment ( Hall 2009 , 2015 ). Limiting the analysis to adolescents, research shows that MML enactment has largely insignificant or even negative effects on youth marijuana use measures ( Anderson et al. 2015 , Choo et al. 2014 , Gorman & Huber 2007 , Harper et al. 2012 , Hasin et al. 2015b , Lynne-Landsman et al. 2013 ), with only Wen et al. (2015) finding a significant increase in the probability of past-year initiation among youths aged 12–20. The results of the few studies that have focused on changes in marijuana consumption among adults have been more mixed, with some showing no effect of MML passage on measures of use ( Gorman & Huber 2007 , Harper et al. 2012 ) and others finding significant positive effects ( Chu 2014 , Wen et al. 2015 ).
Yet, as noted above, the use of a dichotomous MML variable misses important variations in the specific implementation of supply channels, which may be particularly important in determining the extent to which medical marijuana is diverted to adolescent markets ( Boyd et al. 2015 , Nussbaum et al. 2015 , Salomonsen-Sautel et al. 2012 ). When studies focus on the effects of dispensary legalization, there is some evidence of a significant increase in youth consumption ( Pacula et al. 2015 , Wen et al. 2015 ), though other studies find no effect ( Hasin et al. 2015b ). Even within the same study, estimated effects switch sign depending on whether consumption is measured by past-month use, frequency of use, or dependence ( Pacula et al. 2015 , Wen et al. 2015 ). Similar inconsistencies exist in studies of the effects of specific dimensions of MML policy on measures of marijuana use in the general population ( Anderson & Rees 2014 , Choi 2014 , Pacula et al. 2015 ). Thus, age alone is clearly not an adequate way of capturing population heterogeneity.
Perhaps a more relevant dimension of population heterogeneity pertains to differentiating casual or light users from high-risk consumers, often identified in this literature as arrestees ( Chu 2014 , Pacula et al. 2010 ), polysubstance users ( Wen et al. 2015 , Williams & Mahmoudi 2004 ), or those admitted to treatment ( Pacula et al. 2015 ). Only a few studies have focused on high-risk users, but those that have tend to find more consistent evidence that marijuana liberalization significantly increases use ( Chu 2014 ; Model 1993 ; Pacula et al. 2010 , 2015 ; Wen et al. 2015 ). The response of high-risk users to marijuana policy changes will likely differ from that of casual users or nonusers due to differences in price sensitivity ( Pacula & Lundberg 2014 , Sumnall et al. 2004 ), knowledge of the policy environment ( MacCoun et al. 2009 ), engagement with drug markets ( Pacula et al. 2010 ), and perceived social or physical harms from use ( Haardörfer et al. 2016 , Kilmer et al. 2007 ). By examining how marijuana liberalization policy affects the prevalence of marijuana use, many past evaluations have conflated changes in the consumption of casual users with changes in the consumption of regular or heavy users. Because casual users represent a larger proportion of the total number of users, such analyses will discount the behaviors of heavy users, who account for a larger proportion of the total quantity of marijuana consumed ( Burns et al. 2013 , Davenport & Caulkins 2016 ).
The overreliance on using prevalence measures as the outcome of interest in past work is largely a consequence of limited data availability, but as legal markets for marijuana develop, there is an urgent need to assess the alternative measures of use that are more relevant for understanding potential harms. Nationally representative data show that the number of daily or near-daily (DND) users has increased approximately sevenfold since 1992 ( Burns et al. 2013 ), and the prevalence of marijuana use disorders has almost doubled since 2001 ( Hasin et al. 2015a ). Simultaneous use of marijuana with other substances (e.g., tobacco and alcohol) is common and has been shown to be associated with increased risk of adverse consequences ( Subbaraman & Kerr 2015 , Terry-McElrath et al. 2014 ). Currently, we have little evidence to indicate how marijuana liberalization policies will affect these outcomes ( Wen et al. 2015 ). Moving forward, it will be important to develop more comprehensive data collection and sampling designs to assess how marijuana liberalization policies affect populations at risk for problematic use as well as the use of particularly dangerous products or methods of consumption.
WHAT IS MEANT BY HETEROGENEOUS PRODUCTS
Past research has generally focused on how liberalization affects the prevalence of marijuana use and has paid less attention to how liberalization affects the type of marijuana used or the way in which it is consumed. But marijuana is not a uniform product. The cannabis plant itself can develop in a number of different ways, depending on the genetic variety, temperature, culture condition, and lighting it receives. The potency of the consumable product, typically measured by concentration or level of THC, will vary by strain, cultivation technique, and method of processing. There are also a variety of ways to consume marijuana, with the most common methods including smoking, vaporization, and ingestion of edible products ( Schauer et al. 2016 ).
Both potency and methods of consumption have evolved over time. Decriminalization occurred during a time when marijuana was largely smoked, which facilitated comparisons of marijuana use rates between decriminalized and nondecriminalized states. Medical marijuana brought with it new products (e.g., oils and edibles), new methods for consuming it (e.g., dabbing, vaping), and new techniques for controlling potency ( Pacula et al. 2016 , Rendon 2013 ). Legalization only extends these new products to even more users. It is difficult to predict the extent to which legalization will increase product innovation, as growth in the industry will promote the development of new methods for extracting and synthesizing the hundreds of chemicals in the cannabis plant, of which relatively little is known ( Caulkins et al. 2015 ).
Systematic data collection on methods of use and potency is limited, but available evidence indicates that marijuana users in states with medical or recreational legalization consume a different product mix than users in other states. Individuals living in MML states, particularly in states with greater access to dispensaries, have significantly higher likelihood of vaporizing or ingesting marijuana products compared to individuals in states without MMLs ( Borodovsky et al. 2016 ). Evidence also suggests that states that legally permit medical marijuana dispensaries experience significant increases in average marijuana potency ( Sevigny et al. 2014 ). Within states with legalized dispensaries, adults who use marijuana for medicinal purposes are significantly more likely to vaporize it or consume edibles than individuals who use it for recreational purposes ( Pacula et al. 2016 ).
It is complicated to assess the impact of policy on use if the product being consumed or the method of consumption changes in line with the policy. Outcomes such as level of intoxication or dependency may well vary according to the type and method of marijuana consumption, and simply comparing use in legalized states to use in nonlegalized states will not reflect these differences. Changes in product variety will not threaten the identification of changes on the extensive margin of use (meaning any use or prevalence), because existing survey measures can provide information on the number of people who transition from nonusers to users and those who continue using rather than quitting. However, most of the adverse physical and behavioral consequences associated with marijuana use come from heavy users ( Gordon et al. 2013 , Hall 2015 , Volkow et al. 2014 ). Proper evaluation of the public health consequences of legalization relies on the ability of research to estimate the effects of marijuana policy changes on the intensive margin of use.
Data on quantity of marijuana used are surprisingly limited, and researchers have yet to construct a standardized measure for the unit of marijuana consumption (as exists with alcohol). Prior research has examined changes on the intensive margin through self-reported data on frequency of use, measured by days of use in the past month or past year. The implicit assumption has been that more days of use accurately proxies for higher intensity of use ( Temple et al. 2011 ). Yet, marijuana consumption among DND users can vary from smoking a single low-THC joint each day to using high-THC products multiple times per day via multiple delivery methods ( Hughes et al. 2014 , Zeisser et al. 2012 ). Given the variety of delivery devices, strains, and cannabinoid concentrations that become available as the legal industry expands, measuring changes in days of use will fail to capture a number of individuals who transition from occasional to heavy users.
Heterogeneity of marijuana products presents further problems for understanding how medical and recreational legalization affect marijuana use disorders. Previous research examining patterns of use and the development of dependence may not generalize to a legal environment in which there is greater social acceptance, fewer perceived risks and harms, and a wider variety of product types and potencies ( Asbridge et al. 2014 ). Although the definition of marijuana use disorder is evolving ( Compton & Baler 2016 , Hasin et al. 2013 ), there has been little clinical assessment of whether the use of different marijuana products carries different risks of dependence or harms. Some evidence suggests that vaporizing hash oil or dabbing is more positively associated with tolerance and withdrawal among adults compared to smoking marijuana ( Loflin & Earleywine 2014 ), but there may be differential effects for adolescents. As marijuana product diversity expands, there is a need for a more comprehensive understanding and analysis of consumption to accurately evaluate changes in use prevalence, intensity of use, and risk for marijuana use disorder.
AN ALTERNATIVE PERSPECTIVE FOR EVALUATING THE EFFECTS OF MEDICAL MARIJUANA LAWS AND LEGALIZATION
In light of the substantial variation underlying the policies being evaluated, the populations considered, and the products consumed, it is not surprising that the scientific literature evaluating the impact of these policies is inconclusive. The decisions made by researchers to focus on specific time periods, states, populations, and/or outcome measures have often been driven by what data were available and not by a careful consideration of the mechanisms by which these policies are expected to influence marijuana use or use disorders among various populations. As this article has established, these decisions can influence the likelihood of finding—or not finding—specific effects because of the heterogeneity of these policies and of the markets that are emerging in light of them.
The program evaluation literature has widely recognized the time it takes between the passing of new policies and their full implementation as a problematic issue ( Hunt & Miles 2015 , King & Behrman 2009 ). A common empirical strategy for accommodating delays in implementation is the inclusion of lagged policy variables, and this approach has been explored in a few articles from the medical marijuana literature ( Anderson et al. 2013 , Bachhuber et al. 2014 , Chu 2014 ). However, assuming a constant allowance for lagged effects obscures the fact that these delays are not random but are correlated with the specific provisions established by state law, the broader federal policy environment, and the setting in which the policy change occurs.
The relationship between state policy heterogeneity and variation in how long it takes for markets to emerge is something that is just beginning to receive the attention it deserves in the literature ( Collett et al. 2013 , Smart 2016 ). As explained by Smart (2016) , patient registration rates do a better job than simple dichotomous policy variables at capturing the extent to which medical marijuana markets are operating throughout a state. Smart notes that despite the adoption of early policies by many states, the relative size of the associated markets, as measured by registered patients, remained small in most states until federal enforcement policy was clarified in 2009, at which time markets in all states grew substantially faster. In an analysis that explicitly accounts for changes in the size of medical marijuana markets, Smart (2016) finds statistically more robust and consistent evidence of the impacts of these markets on various measures of consumption across users from all age groups.
The consideration of the relative size of these markets across states highlights the necessity to consider the issue of dynamics. Whereas some aspects of medical marijuana and legalization policies can have immediate impacts (e.g., on the criminalization of marijuana use or the ability to grow it at home), other effects of these policies take time to occur or disseminate. In the case of markets, for example, it takes time for regulations to develop regarding how many businesses are allowed, who is allowed to operate a business, and where those businesses are allowed to operate. It takes even longer once those rules are passed for businesses to obtain permits and begin distribution. Thus, it should not be surprising that after the passing of marijuana legalization measures in Colorado and Washington in November 2012, it took at least 18–20 months for retail stores to open. Data on the consequences of the opening of these stores beyond sales and tax revenues are just beginning to become available, which is why rigorous scientific evaluations of the impact of these policies have been slow to develop.
What that means is that researchers working in this space need to pay far greater attention to the specific mechanisms that different types of policies are likely to influence and to consider them within the proper timeframe when assessing impacts on specific populations. We show in Figure 2 some of the primary mechanisms discussed in the literature through which these changes in policies might impact use (i.e., perceived harm, disapproval of regular use, legal risk of use, ease of access and price) as well as the hypothesized effects of various types of policies on each. For simplicity, we consider each mechanism separately, though it is important to note that these are likely not independently determined (e.g., changes in legal risk may influence perceived harms, or changes in ease of access may influence disapproval). A small, medium, or large arrow (pointing up or down) in each cell indicates the relative magnitude and direction of the hypothesized effect. Shading represents the availability of empirical evidence to support the theoretical prediction, with white indicating an absence of existing studies and darker shades representing greater and more consistent support for the hypothesized effect. We provide three simplified versions of a medical marijuana policy and a legal recreational market to illustrate a wider range of policies that would to varying degrees influence the general size of the associated markets (in terms of both users and sellers).

Mechanisms through which marijuana policies might affect marijuana use and use disorders. This simple illustration shows that even within a single policy area (e.g., medical marijuana), the different variations of the policy can differentially influence each of the mechanisms related to use. For example, we hypothesize that medical marijuana policies will ceteris paribus have a larger impact on people’s perceptions about the drug (perceived harm and disapproval of regular use) than they will have on the legal risk and ease of access to marijuana regardless of policy, assuming that only medical users are provided access and legal protections. Relatedly, because these markets serve a relatively smaller group of users, the overall impacts on price are presumed to be small, although they might increase with the third type of MML, which could allow for competitive forces among suppliers to start influencing price ( Anderson et al. 2013 , Humphreys 2016 , Pacula et al. 2010 ) and potency ( Sevigny et al. 2014 ) in these markets. The existing evidence generally suggests that the passage of any type of MML significantly lowers perceived harms among adults ( Choi 2014 , Khatapoush & Hallfors 2004 ) but not among adolescents ( Choi 2014 , Keyes et al. 2016 ). However, the expansion of commercial medical marijuana markets and increased exposure to medical marijuana after 2009 have been associated with significant reductions in adolescent perceptions of harm or disapproval associated with marijuana use ( Miech et al. 2015 , Schuermeyer et al. 2014 , Sobesky & Gorgens 2016 , Thurstone et al. 2011 ).
Of course, under a policy of legalization, the hypothesized effects on some of the mechanisms (perceptions and legal risk) are larger and more immediate. Preliminary evidence from Colorado and Washington shows that commercial legalization has significantly reduced perceived harms and disapproval of marijuana use ( Kosterman et al. 2016 , Sobesky & Gorgens 2016 ), and marijuana-related arrests have plummeted ( Gettman 2015a , b ). Access and prices, however, will likely still be differentially influenced by the regulations that shape the market structure and the level of competition in the market ( Caulkins et al. 2015 , Smart 2016 ). The overall impact on consumption, then, would depend on ( a ) the relative importance of perceptions and legal risk vis-à-vis access and price for the specific population being evaluated, and ( b ) whether one is evaluating an immediate (short-run) response to the policy or a long-run effect that is inclusive of market mechanisms.
Another important consideration for interpreting findings when evaluating legalization effects is the baseline policy in place prior to legalization. Because most careful evaluations are done based on marginal changes over time, the baseline policy in the states that subsequently legalize will determine the extent to which a particular mechanism is impacted by the change in formal policy. States like Washington and Colorado, for example, which moved to legalization from a medical marijuana policy that already provided broad access and loose regulation of dispensaries, will likely experience far less of an impact on perceptions and access than states starting from a more restrictive medical marijuana policy or no law at all. Generalization of findings from these two state experiences, therefore, would not necessarily apply to states that may be considering a move to legalization without first allowing medical marijuana markets.
Thus far we have discussed heterogeneous policies, populations, and products as limitations that complicate the evaluation of how marijuana liberalization policies affect marijuana use and marijuana use disorders. However, Figure 2 suggests that this rich variation also offers unique opportunities for future research. By carefully considering the specific aspects of legalization statutes in the context of existing state policies, researchers have increased the scope for determining the mechanisms that are most important for influencing marijuana use among different populations. As more comprehensive data on marijuana prices and products become available, future work can examine not only whether liberalization affects marijuana use, but also whether it affects who uses marijuana, what products are used, and how these products are consumed. The literature has shown that not all marijuana liberalization policies are created equal, but by exploiting this variation we will be able to better evaluate which policy designs will maximize the potential benefits of legalization while minimizing potential harms.
The variety of marijuana liberalization policies across the US states is often ignored or inadequately considered when assessing the impacts of further policy reform. Despite the widespread state experimentation with alternative marijuana policies since the 1970s, our knowledge of the impact of these liberalization policies on the consumption of marijuana, and its benefits and harms, is far less developed than one would expect. There are a number of reasons for this, including, particularly, lack of attention to the heterogeneity of existing policies, the specificity of the populations examined, and modes of consumption.
Although findings tend to be mixed when we look at the literature as a whole, some consistent themes seem to emerge when we consider the literature with an eye toward differences between policies and populations. For example, studies that are attentive to the development of medical marijuana markets (e.g., through measures of the presence of active dispensaries or the size of the market) seem to consistently show a positive correlation of liberalization policies with use among high-risk users (arrestees, people in need of treatment, and polysubstance users). Similarly, many studies have shown a positive association with adult use of marijuana, whereas most have found no association with youth prevalence or frequency of use in general school populations. The extent to which these findings can be drawn on to make inferences about the potential impact of legalization on these same populations is not clear. Just as it took time for researchers to pay more careful attention to the differential effects of policy elements over time ( Hasin et al. 2015b , Pacula et al. 2015 , Smart 2016 , Wen et al. 2015 ), as well as possible heterogeneous responses by different types of users ( Pacula et al. 2015 , Wen et al. 2015 ), it will take time for research to emerge that fully reconsiders these associations in light of the full policy dynamics (i.e., changes in a policy within a single state over time and duration of exposure of a population to a given policy type). As more studies account for and consider these heterogeneous effects and dynamics, we may get better clarity regarding the margins on which particular types of policies do or do not influence behavior, and for whom.
Because legal markets will continue to evolve before these questions are fully answered, the real work that lies ahead relies on obtaining more accurate information on the amount and type of products that various people are consuming. Imagine trying to communicate to the public health field the health benefits or harms of alcohol consumption without being able to indicate specific levels or amounts that translate into impairment in well-understood dose-response relationships. Or imagine trying to assess the harmful effects of smoking without being able to differentiate an experimental or occasional smoker from someone who smokes a pack a day. Yet, that is exactly where the science is today in terms of our measurement of marijuana consumption. Precise data on things such as a standardized dose, regular versus experimental use, heavy use, episodic impairment, or even simultaneous use of marijuana and alcohol are not yet captured in most of the data tracking systems used to evaluate the impact of these policies, and they are desperately needed. If marijuana is anything like alcohol, little harm will come from casual, occasional use by mature adults, and indeed such use might generate considerable benefits. Moreover, it is also possible that marijuana, like alcohol, generates positive benefits for one population (mature adults) while also causing negative harms for another population (youth and young adults). Scientific research needs to be mindful of this heterogeneity.
SUMMARY POINTS
- State policies legalizing marijuana are part of the evolution of state liberalization policies that has taken place since the 1970s.
- Existing studies evaluating the impacts of prior state experimentation have generated inconclusive findings, and only recently has research attempted to understand the reasons for these mixed results.
- One should be cautious when interpreting the evidence from all studies pooled together, because studies are not equivalent in their attention to policy heterogeneity, policy dynamics, and population heterogeneity.
- The literature has largely treated both decriminalization and medical marijuana policies as if they were simple dichotomous choices, when in fact there can be substantial variation in the implementation of these policies that influences how adults or youth respond.
- Relatively few studies evaluating the impact of MMLs give adequate consideration to the fact that some aspects of liberalizations policies are realized immediately (e.g., ability to grow one’s own), whereas other aspects may take time to evolve (e.g., opening of a market) or change in response to future state and federal policies.
- Studies that focus on how marijuana liberalization policies influence past-month or past-year prevalence conflate changes in consumption among light and casual users with changes in consumption among regular and heavy users.
- Although relatively few in number, studies that focus on high-risk users (arrestees, poly-substance users, heavy users) tend to find more consistent evidence that medical marijuana policies increase use, suggesting that this segment of the population is particularly sensitive to policy changes.
FUTURE ISSUES
- As legal markets for marijuana develop, there is an urgent need to assess the consequences of liberalization on alternative measures of use that are relevant for understanding potential harms; this requires developing better measures of standardized dose, heavy use, episodic impairment, and simultaneous use.
- Research needs to pay more attention to the influence of these policies on the types of products consumed, the amount of THC being consumed in different products, and product development.
- Future work also needs to give stronger consideration of the baseline from which new state policies are being evaluated. For example, legalization is likely to generate smaller population changes in medical marijuana states that already have active dispensaries than in states with no prior medical marijuana stores.
- Researchers need to pay far greater attention to the specific mechanisms different types of policies are likely to influence and to consider them within the proper timeframe when assessing impacts on specific populations because not all users will respond in the same ways.
ACKNOWLEDGMENTS
This article was supported by a grant from the National Institute on Drug Abuse to the RAND Corporation (R01DA032693). The article benefited from research assistance provided by Anne Boustead, Ervant Maksabedian, and Gabriel Weinberger. We should also give credit to several of our DPRC colleagues whom we have been fortunate enough to conduct research with and who have influenced our thinking on this literature, including Jonathan Caulkins, Beau Kilmer, Mark Kleiman, Mireille Jacobson, Priscillia Hunt, David Powell, Paul Heaton, Eric Sevigny, Peter Reuter, and Rob MacCoun. All errors in the article are our own.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
1 For simplicity, this article refers to the District of Columbia (DC) as a state.
2 Uruguay also legalized recreational marijuana in 2013, and Canada’s prime minister is working on a formal proposal expected to be delivered to the Canadian Parliament in April 2017. We are focusing on the US experience here because no formal stores are open in either Uruguay or Canada at this time.
LITERATURE CITED
- Anderson DM, Hansen B, Rees DI. 2012. Medical marijuana laws and teen marijuana use . Disc. Pap. 6592, IZA, Bonn, Ger. [ Google Scholar ]
- Anderson DM, Hansen B, Rees DI. 2013. Medical marijuana laws, traffic fatalities, and alcohol consumption . J. Law Econ 56 :333–69 [ Google Scholar ]
- Anderson DM, Hansen B, Rees DI. 2015. Medical marijuana laws and teen marijuana use . Am. Law Econ. Rev 17 ( 2 ):495–528 [ Google Scholar ]
- Anderson DM, Rees DI. 2014. The role of dispensaries: The devil is in the details . J. Policy Anal. Manag 33 ( 1 ):235–40 [ PubMed ] [ Google Scholar ]
- Armbrister M 2016. Colorado pot potency ballot initiative is withdrawn . Denver Bus. J , July 8 [ Google Scholar ]
- Asbridge M, Duff C, Marsh DC, Erickson PG. 2014. Problems with the identification of “problematic” cannabis use: examining the issues of frequency, quantity, and drug use environment . Eur. Addict. Res 20 :254–67 [ PubMed ] [ Google Scholar ]
- Bachhuber MA, Saloner B, Cunningham CO, Barry CL. 2014. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010 . JAMA Intern. Med 174 ( 10 ):1668–73 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Baker D 2007. N.M.:Won’t oversee marijuana production . Assoc. Press , August 16 [ Google Scholar ]
- Borodovsky JT, Crosier BS, Lee DC, Sargent JD, Budney AJ. 2016. Smoking, vaping, eating: Is legalization impacting the way people use cannabis? Int. J. Drug Policy 36 :141–47 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boyd CJ, Veliz PT, McCabe SE. 2015. Adolescents’ use of medical marijuana: a secondary analysis of Monitoring the Future data . J. Adolesc. Health 57 ( 2 ):241–44 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Burns RM, Caulkins JP, Everingham SS, Kilmer B. 2013. Statistics on cannabis users skew perceptions of cannabis use . Front. Psychiatry 4 ( 138 ):1–10 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Caulkins JP, Hawken A, Kilmer B, Kleiman M. 2012. Marijuana Legalization: What Everyone Needs to Know . New York: Oxford Univ. Press [ Google Scholar ]
- Caulkins JP, Kilmer B. 2016. Considering marijuana legalization carefully: insights for other jurisdictions from analysis for Vermont . Addiction 111 ( 12 ):2082–89 [ PubMed ] [ Google Scholar ]
- Caulkins JP, Kilmer B, Kleiman MAR, MacCoun RJ, Midgette G, et al. 2015. Considering Marijuana Legalization: Insights for Vermont and Other Jurisdictions . Santa Monica, CA: RAND Corp. [ Google Scholar ]
- Cerdà M, Wall M, Keyes KM, Galea S, Hasin DS. 2012. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence . Drug Alcohol Depend . 120 :22–27 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Choi A 2014. The impact of medical marijuana laws on marijuana use and other risky health behaviors . Presented at ASHE Conf., 5th, Los Angeles [ Google Scholar ]
- Choo EK, Benz M, Zaller N, Warren O, Rising KL, McConnell KJ. 2014. The impact of state medical marijuana legislation on adolescent marijuana use . J. Adolesc. Health 55 ( 2 ):160–66 [ PubMed ] [ Google Scholar ]
- Chu YWL. 2014. The effects of medical marijuana laws on illegal marijuana use . J. Health Econ . 38 :43–61 [ PubMed ] [ Google Scholar ]
- Collett SC, Gariffo T, Hernandez-Morgan M. 2013. Evaluation of the Medical Marijuana Program in Washington, D.C . Los Angeles: UCLA [ Google Scholar ]
- Compton WM, Baler R. 2016. The epidemiology ofDSM-5 cannabis use disorders among U.S. adults: science to inform clinicians working in a shifting social landscape . Am. J. Psychiatry 173 ( 6 ):551–53 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- D’Amico EJ, Miles JNV, Tucker JS. 2015. Gateway to curiosity: medical marijuana ads and intention and use during middle school . Psychol. Addict. Behav 29 ( 3 ):613–19 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Davenport SS, Caulkins JP. 2016. Evolution of the United States: marijuana market in the decade of liberalization before full legalization . J. Drug Issues 46 ( 4 ):411–27 [ Google Scholar ]
- DeSimone J, Farrelly MC. 2003. Price and enforcement effects on cocaine and marijuana demand . Econ. Inquiry 41 :98–115 [ Google Scholar ]
- Dinardo J, Lemieux T. 2001. Alcohol, marijuana, and American youth: the unintended consequences of government regulation . J. Health Econ 20 ( 6 ):991–1010 [ PubMed ] [ Google Scholar ]
- Fairman BJ. 2015. Trends in registered medical marijuana participation rates across 13 US states and District of Columbia . Drug Alcohol Depend . 159 :72–79 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fischer B, Kuganesan S, Room R. 2015. Medical marijuana programs: implications for cannabis control policy—observations from Canada . Int. J. Drug Policy 26 ( 1 ):15–19 [ PubMed ] [ Google Scholar ]
- Fox CR, Tannenbaum D. 2011. The elusive search for stable risk preferences . Front. Psychol 2 :298. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Galenianos M, Pacula RL, Persico N. 2012. A search-theoretic model of the retail market for illicit drugs . Rev. Econ. Studies 79 :1239–69 [ Google Scholar ]
- Gettman J 2015a. Marijuana Arrests in Colorado After the Passage of Amendment 64 . New York: Drug Policy Alliance [ Google Scholar ]
- Gettman J 2015b. Status Report: Marijuana Legalization in Washington After 1 Year of Retail Sales and 2.5 Years of Legal Possession . New York: Drug Policy Alliance [ Google Scholar ]
- Gordon AJ, Conley JW, Gordon JM. 2013. Medical consequences of marijuana use: a review of the current literature . Curr. Psychiatry Rep 15 ( 12 ):419. [ PubMed ] [ Google Scholar ]
- Gorman DM, Huber J. 2007. Do medical cannabis laws encourage cannabis use? Int. J. Drug Policy 18 ( 3 ):160–67 [ PubMed ] [ Google Scholar ]
- Haardörfer R, Berg CJ, Lewis M, Payne J, Pillai D, et al. 2016. Polytobacco, marijuana, and alcohol use patterns in college students: a latent class analysis . Addict. Behav 59 :58–64 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hall W 2009. The adverse health effects of cannabis use: What are they, and what are their implications for policy? Int. J. Drug Policy 20 ( 6 ):458–66 [ PubMed ] [ Google Scholar ]
- Hall W 2015. What has research over the past two decades revealed about the adverse effects of cannabis use? Addiction 110 ( 1 ):19–35 [ PubMed ] [ Google Scholar ]
- Hall W, Lynskey M. 2016. Evaluating the public health impacts of legalizing recreational cannabis use in the United States . Addiction 111 ( 10 ):1764–73 [ PubMed ] [ Google Scholar ]
- Haney M, Evins AE. 2016. Does cannabis cause, exacerbate, or ameliorate psychiatric disorders? An oversimplified debate discussed . Neuropsychopharmacology 41 ( 2 ):393–401 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Harper S, Strumpf EC, Kaufman JS. 2012. Do medical marijuana laws increase marijuana use? Replication study and extension . Ann. Epidemiol 22 ( 3 ):207–12 [ PubMed ] [ Google Scholar ]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, et al. 2013. DSM-5 criteria for substance use disorders: recommendations and rationale . Am. J. Psychiatry 170 :834–51 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, et al. 2015a. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013 . JAMA Psychiatry 72 ( 12 ):1235–42 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hasin DS, Wall M, Keyes KM, Cerdà M, Schulenberg J, et al. 2015b Medical marijuana laws and adolescent marijuana use in the USA from 1991–2014: results from annual, repeated cross-sectional surveys . Lancet Psychiatry 2 ( 7 ):601–8 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hill KP. 2015. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review . JAMA 313 ( 24 ):2474–83 [ PubMed ] [ Google Scholar ]
- Huestis MA. 2007. Human cannabinoid pharmacokinetics . Chem. Biodivers 4 ( 8 ):1770–804 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hughes JR, Fingar JR, Budney AJ, Naud S, Helzer JE. 2014. Marijuana use and intoxication among daily users: an intensive longitudinal study . Addict. Behav 39 ( 10 ):1464–70 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Humphreys K 2016. So, something interesting happens to weed after it’s legal. The Washington Post Wonkblog , May 4. https://www.washingtonpost.com/news/wonk/wp/2016/05/04/the-priceof-legal-pot-is-collapsing/
- Hunt PE, Miles J. 2015. The impact of legalizing and regulating weed: issues with study design and emerging findings in the USA . Curr. Topics Behav. Neurosci 10.1007/7854_2015_423 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Johnston D, Lewis NA. 2009. Ending raids of dispensers of marijuana for patients . New York: Times, March 18, p. A20 [ Google Scholar ]
- Johnston LD, O’Malley PM, Bachman JG. 1981. Marijuana decriminalization: the impact on youth 1975–1980 Monitoring the Future Occas . Pap. 13, Inst. Soc. Res., Univ. Mich., Ann Arbor [ Google Scholar ]
- Keyes KM, Wall M, Cerdà M, Schulenberg J, O’Malley PM, et al. 2016. How does state marijuana policy affect US youth? Medical marijuana laws, marijuana use and perceived harmfulness: 1991–2014 . Addiction 111 :2187–95. 10.1111/add.13523 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khatapoush S, Hallfors D. 2004. “Sending the wrong message”: Did medical marijuana legalization in California change attitudes about and use of marijuana? J. Drug Issues 34 :741–70 [ Google Scholar ]
- Kilmer B, Caulkins JP, Pacula RL, MacCoun RJ, Reuter P. 2010. Altered State? Assessing How Marijuana Legalization in California Could Influence Marijuana Consumption and Public Budgets . Santa Monica, CA: RAND Corp. [ Google Scholar ]
- Kilmer JR, Hunt SB, Lee CM, Neighbors C. 2007. Marijuana use, risk perception, and consequences: Is perceived risk congruent with reality? Addict. Behav 32 :3026–33 [ PubMed ] [ Google Scholar ]
- King E, Behrman J. 2009. Timing and duration of exposure in evaluations of social programs . World Bank Econ. Rev 22 :539–66 [ Google Scholar ]
- Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, et al. 2014. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Report of the Guideline Development Subcommittee of the American Academy of Neurology . Neurology 82 ( 17 ):1556–63 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kosterman R, Bailey JA, Guttmannova K, Jones TM, Eisenberg N, et al. 2016. Marijuana legalization and parents’ attitudes, use, and parenting in Washington State . J. Adolesc. Health 59 ( 4 ):450–56 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Loflin M, Earleywine M. 2014. A new method of cannabis ingestion: the dangers of dabs? Addict. Behav 39 ( 10 ):1430–33 [ PubMed ] [ Google Scholar ]
- Lynne-Landsman SD, Livingston MD, Wagenaar AC. 2013. Effects of state medical marijuana laws on adolescent marijuana use . Am. J. Public Health 103 ( 8 ):1500–6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- MacCoun R, Pacula RL, Chriqui JF, Harris K, Reuter P. 2009. Do citizens know whether their state has decriminalized marijuana? Assessing the perceptual component of deterrence theory . Rev. Law Econ . 5 :347–71 [ Google Scholar ]
- MacCoun R, Reuter P. 2001. Evaluating alternative cannabis regimes . Br. J. Psychiatry 178 :123–28 [ PubMed ] [ Google Scholar ]
- Maloff D 1981. A review of the effects of the decriminalization of marijuana . Contemp. Drug Probl . 10 :307–22 [ Google Scholar ]
- Marijuana Work. Group. 2016. Initiative 71: Marijuana Working Group Status Report . Washington, DC: Gov. D.C. [ Google Scholar ]
- Markowitz S, Tauras J. 2009. Substance use among adolescent students with consideration of budget constraints . Rev. Econ. Househ 7 :423–46 [ Google Scholar ]
- Miech RA, Johnston L, O’Malley PM, Bachman JG, Schulenberg J, Patrick ME. 2015. Trends in use of marijuana and attitudes toward marijuana among youth before and after decriminalization: the case of California 2007–2013 . Int. J. Drug Policy 26 :336–44 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Model K 1993. The effect of marijuana decriminalization on hospital emergency room episodes: 1975–1978 . J. Am. Stat. Assoc 88 ( 423 ):737–47 [ Google Scholar ]
- Natl. Comm. Marihuana Drug Abus. 1972. Marihuana: a signal of misunderstanding First Rep. Natl. Comm. Marihuana Drug Abus , US Gov. Print. Off., Washington, DC [ Google Scholar ]
- NCSL (Natl. Conf. State Legis.). 2016a. Marijuana overview , Aug. 2. http://www.ncsl.org/research/civil-and-criminal-justice/marijuana-overview.aspx
- NCSL (Natl. Conf. State Legis.). 2016b. State medical marijuana laws , Jul. 20. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
- Nussbaum AM, Thurstone C, McGarry L, Walker B, Sabel AL. 2015. Use and diversion of medical marijuana among adults admitted to inpatient psychiatry . Am. J. Drug Alcohol Abus . 41 ( 2 ):166–72 [ PubMed ] [ Google Scholar ]
- Ogden DW.2009. Investigations and Prosecutions in States Authorizing the Medical Use of Marijuana: Memorandum for Selected United States Attorneys . Washington, DC: US Dep. Justice, Off. Deputy Atty. [ Google Scholar ]
- Pacula RL. 1998. Does increasing the beer tax reduce marijuana consumption? J. Health Econ 17 ( 5 ):557–86 [ PubMed ] [ Google Scholar ]
- Pacula RL, Boustead A, Hunt P. 2014a Words can be deceiving: a review of variation among legally effective medical marijuana laws in the United States . J. Drug Policy Anal 7 ( 1 ):1–19 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pacula RL, Chriqui JF, King J. 2003. Decriminalization in the United States: What does it mean? Work. Pap. 9690, NBER, Cambridge, MA [ Google Scholar ]
- Pacula RL, Chriqui JF, Reichmann DA, Terry-McElrath YM. 2002. State medical marijuana laws: understanding the laws and their limitations . J. Public Health Policy 23 ( 4 ):413–39 [ PubMed ] [ Google Scholar ]
- Pacula RL, Heaton P, Powell D, Sevigny EL. 2015. Assessing the effects of medical marijuana laws on marijuana use: The devil is in the details . J. Policy Anal. Manag 34 ( 1 ):7–31 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pacula RL, Jacobson M, Maksabedian EJ. 2016. In the weeds: a baseline view of cannabis use among legalizing states and their neighbors . Addiction 111 :973–80 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pacula RL, Kilmer B, Grossman M, Chaloupka FJ. 2010. Risks and prices: the role of user sanctions in marijuana markets . B. E. J. Econ. Anal. Policy 10 ( 1 ):1–36 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pacula RL, Kilmer B, Wagenaar AC, Chaloupka FJ, Caulkins JP. 2014b Developing public health regulations for marijuana: lessons from alcohol and tobacco . Am. J. Public Health 104 ( 6 ):1021–28 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pacula RL, Lundberg R. 2014. Why changes in price matter when thinking about marijuana policy: a review of the literature on the elasticity of demand . Public Health Rev . 35 ( 2 ):1–18 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pacula RL, MacCoun R, Reuter P, Chriqui J, Kilmer B, et al. 2005. What does it mean to decriminalize marijuana? A cross-national empirical examination In Substance Abuse: Individual Behaviour, Social Interactions, Markets and Politics , Vol. 16 , ed. Grossman M, Lindgren B, pp. 347–70. Amsterdam: Elsevier [ PubMed ] [ Google Scholar ]
- Pacula RL, Powell D, Heaton P, Sevigny E. 2015. Assessing the effects of medical marijuana laws on marijuana: The devil is in the details . J. Public Policy Anal. Manag 34 :7–31 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pacula RL, Sevigny EL. 2014a Marijuana liberalization policies: why we can’t learn much from policy still in motion . J. Policy Anal. Manag 33 ( 1 ):212–21 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pacula RL, Sevigny EL. 2014b. Natural experiments in a complex and dynamic environment: the need for measured assessment of the evidence . J. Policy Anal. Manag 33 ( 1 ):232–35 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pertwee R 2014. Handbook of Cannabis . Oxford, UK: Oxford Univ. Press [ Google Scholar ]
- Raphael S, Stoll MA. 2013. Why Are So Many Americans in Prison? New York: Russell Sage Found. [ Google Scholar ]
- Rendon J 2013. Super-Charged: How Outlaws, Hippies, and Scientists Reinvented Marijuana . Portland, OR: Timber Press [ Google Scholar ]
- Reuter P, Hirschfield P, Davies C. 2001. Assessing the Crackdown on Marijuana in Maryland . Baltimore, MD: Abell Found. [ Google Scholar ]
- Reuter P, MacCoun RJ. 1995. Assessing the legalization debate In Policies and Strategies to Combat Drugs in Europe , ed. Estievenart G, pp. 39–49. Amsterdam: Kluwer [ Google Scholar ]
- Ritter A, Livingston M, Chalmers J, Berends L, Reuter P. 2016. Comparative policy analysis for alcohol and drugs: current state of the field . Int. J. Drug Policy 31 :39–50 [ PubMed ] [ Google Scholar ]
- Ross A, Walker A. 2017. The impact of low-priority laws on criminal activity: evidence from California . Contemp. Econ. Policy In press 10.1111/coep.12179 [ CrossRef ] [ Google Scholar ]
- Russo EB, Guy GW, Robson PJ. 2007. Cannabis, pain, and sleep: lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine . Chem. Biodivers 4 ( 8 ):1729–43 [ PubMed ] [ Google Scholar ]
- Saffer H, Chaloupka FJ. 1999. The demand for illicit drugs . Econ. Inq 37 ( 3 ):401–11 [ Google Scholar ]
- Salomonsen-Sautel S, Sakai JT, Thurstone C, Corley R, Hopfer C. 2012. Medical marijuana use among adolescents in substance abuse treatment . J. Am. Acad. Child Adolesc. Psychiatry 51 ( 7 ):694–702 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. 2016. Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014 . Am. J. Prev. Med 50 ( 1 ):1–8 [ PubMed ] [ Google Scholar ]
- Schuermeyer J, Salomonsen-Sautel S, Price RK, Balan S, Thurstone C, et al. 2014. Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003–2011 . Drug Alcohol Depend . 140 :145–55 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sevigny EL. 2014. Medical marijuana by the numbers Presented at Annu. Conf. Int. Soc. Study Drug Policy , 8th, Rome [ Google Scholar ]
- Sevigny EL, Pacula RL, Heaton P. 2014. The effects of medical marijuana laws on potency . Int. J. Drug Policy 25 :308–19 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Single E 1989. The impact of marijuana decriminalization: an update . J. Public Health Policy 10 :456–66 [ PubMed ] [ Google Scholar ]
- Smart R 2016. Essays on the effects of medical marijuana laws PhD Thesis , Univ. Calif., Los Angeles [ Google Scholar ]
- Sobesky M, Gorgens K. 2016. Cannabis and adolescents: exploring the substance misuse treatment provider experience in a climate of legalization . Int. J. Drug Policy 33 :66–74 [ PubMed ] [ Google Scholar ]
- Stringer RJ, Maggard SR. 2016. Reefer madness to marijuana legalization: media exposure and American attitudes toward marijuana (1975–2012) . J. Drug Issues 46 ( 4 ):428–45 [ Google Scholar ]
- Subbaraman MS, Kerr WC. 2015. Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey . Alcohol. Clin. Exp. Res 39 ( 5 ):872–79 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Subritzky T, Pettigrew S, Lenton S. 2016. Issues in the implementation and evolution of the commercial recreational cannabis market in Colorado . Int. J. Drug Policy 27 :1–12 [ PubMed ] [ Google Scholar ]
- Sumnall HR, Tyler E, Wagstaff GF, Cole JC. 2004. A behavioural economic analysis of alcohol, amphetamine, cocaine and ecstasy purchases by polysubstance misusers . Drug Alcohol Depend . 76 ( 1 ):93–99 [ PubMed ] [ Google Scholar ]
- Temple EC, Brown RF, Hine DW. 2011. The “grass ceiling”: Limitations in the literature hinder our understanding of cannabis use and its consequences . Addiction 106 :238–44 [ PubMed ] [ Google Scholar ]
- Terry-McElrath YM, O’Malley PM, Johnston LD. 2014. Alcohol and marijuana use patterns associated with unsafe driving among U.S. high school seniors: high use frequency, concurrent use, and simultaneous use . J. Stud. Alcohol Drugs 75 ( 3 ):378–89 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thies CF, Register CA. 1993. Decriminalization of marijuana and the demand for alcohol, marijuana and cocaine . Soc. Sci. J 30 ( 4 ):385–99 [ Google Scholar ]
- Thurstone C, Lieberman SA, Schmiege SJ. 2011. Medical marijuana diversion and associated problems in adolescent substance treatment . Drug Alcohol Depend . 118 ( 2–3 ):489–92 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Volkow ND, Baler RD, Compton WM, Weiss SRB. 2014. Adverse health effects of marijuana use . N. Engl. J. Med 370 :2219–27 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wall MM, Poh E, Cerdà M, Keyes KM, Galea S, Hasin DS. 2011. Adolescent marijuana use from 2002 to 2008: higher in states with medical marijuana laws, cause still unclear . Ann. Epidemiol 21 ( 9 ):714–16 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wen H, Hockenberry JM, Cummings JR. 2015. The effect of medical marijuana laws on adolescent and adult use of marijuana, alcohol, and other substances . J. Health Econ . 42 :64–80 [ PubMed ] [ Google Scholar ]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, et al. 2015. Cannabinoids for medical use: a systematic review and meta-analysis . JAMA 313 ( 24 ):2456–73 [ PubMed ] [ Google Scholar ]
- Williams AR, Olfson M, Kim JH, Martins SS, Kleber HD. 2016. Older, less regulated medical marijuana programs have much greater enrollment rates than newer “medicalized” programs . Health Aff . 35 ( 3 ):480–88 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Williams J, Mahmoudi P. 2004. Economic relationship between alcohol and cannabis revisited . Econ. Rec 80 ( 248 ):36–48 [ Google Scholar ]
- Zeisser C, Thompson K, Stockwell T, Duff C, Chow C, et al. 2012. A “standard joint”? The role of quantity in predicting cannabis-related problems . Addict. Res. Theory 20 ( 1 ):82–92 [ Google Scholar ]
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Mental health care is hard to find, especially for people with Medicare or Medicaid
Rhitu Chatterjee

With rates of suicide and opioid deaths rising in the past decade and children's mental health declared a national emergency , the United States faces an unprecedented mental health crisis. But access to mental health care for a significant portion of Americans — including some of the most vulnerable populations — is extremely limited, according to a new government report released Wednesday.
The report, from the Department of Health and Human Services' Office of Inspector General, finds that Medicare and Medicaid have a dire shortage of mental health care providers.
The report looked at 20 counties with people on Medicaid, traditional Medicare and Medicare Advantage plans, which together serve more than 130 million enrollees — more than 40% of the U.S. population, says Meridith Seife , the deputy regional inspector general and the lead author of the report.
Medicaid serves people on low incomes, and Medicare is mainly for people 65 years or older and those who are younger with chronic disabilities.
The report found fewer than five active mental health care providers for every 1,000 enrollees. On average, Medicare Advantage has 4.7 providers per 1,000 enrollees, whereas traditional Medicare has 2.9 providers and Medicaid has 3.1 providers for the same number of enrollees. Some counties fare even worse, with not even a single provider for every 1,000 enrollees.
"When you have so few providers available to see this many enrollees, patients start running into significant problems finding care," says Seife.
The findings are especially troubling given the level of need for mental health care in this population, she says.
"On Medicare, you have 1 in 4 Medicare enrollees who are living with a mental illness," she says. "Yet less than half of those people are receiving treatment."
Among people on Medicaid, 1 in 3 have a mental illness, and 1 in 5 have a substance use disorder. "So the need is tremendous."
The results are "scary" but "not very surprising," says Deborah Steinberg , senior health policy attorney at the nonprofit Legal Action Center. "We know that people in Medicare and Medicaid are often underserved populations, and this is especially true for mental health and substance use disorder care."
Among those individuals able to find and connect with a provider, many see their provider several times a year, according to the report. And many have to drive a long way for their appointments.
"We have roughly 1 in 4 patients that had to travel more than an hour to their appointments, and 1 in 10 had to travel more than an hour and a half each way," notes Seife. Some patients traveled two hours each way for mental health care, she says.
Mental illnesses and substance use disorders are chronic conditions that people need ongoing care for, says Steinberg. "And when they have to travel an hour, more than an hour, for an appointment throughout the year, that becomes unreasonable. It becomes untenable."
"We know that behavioral health workforce shortages are widespread," says Heather Saunders , a senior research manager on the Medicaid team at KFF, the health policy research organization. "This is across all payers, all populations, with about half of the U.S. population living in a workforce shortage."
But as the report found, that's not the whole story for Medicare and Medicaid. Only about a third of mental health care providers in the counties studied see Medicare and Medicaid patients. That means a majority of the workforce doesn't participate in these programs.
This has been well documented in Medicaid, notes Saunders. "Only a fraction" of providers in provider directories see Medicaid patients, she says. "And when they do see Medicaid patients, they often only see a few."
Lower reimbursement rates and a high administrative burden prevent more providers from participating in Medicaid and Medicare, the report notes.
"In the Medicare program, they set a physician fee rate," explains Steinberg. "Then for certain providers, which includes clinical social workers, mental health counselors and marriage and family therapists, they get reimbursed at 75% of that rate."
Medicaid reimbursements for psychiatric services are even lower when compared with Medicare , says Ellen Weber , senior vice president for health initiatives at the Legal Action Center.
"They're baking in those discriminatory standards when they are setting those rates," says Steinberg.
The new report recommends that the Centers for Medicare & Medicaid Services (CMS) take steps to increase payments to providers and lower administrative requirements. In a statement, CMS said it has responded to those recommendations within the report.
According to research by Saunders and her colleagues at KFF, many states have already started to take action on these fronts to improve participation in Medicaid.
Several have upped their payments to mental health providers. "But the scale of those increases ranged widely across states," says Saunders, "with some states limiting the increase to one provider type or one type of service, but other states having rate increases that were more across the board."
Some states have also tried to simplify and streamline paperwork, she adds. "Making it less complex, making it easier to understand," says Saunders.
But it's too soon to know whether those efforts have made a significant impact on improving access to providers.
CMS has also taken steps to address provider shortages, says Steinberg.
"CMS has tried to increase some of the reimbursement rates without actually fixing that structural problem," says Steinberg. "Trying to add a little bit here and there, but it's not enough, especially when they're only adding a percent to the total rate. It's a really small increase."
The agency has also started covering treatments and providers it didn't use to cover before.
"In 2020, Medicare started covering opioid treatment programs, which is where a lot of folks can go to get medications for their substance use disorder," says Steinberg.
And starting this year, Medicare also covers "mental health counselors, which includes addiction counselors, as well as marriage and family therapists," she adds.
While noteworthy and important, a lot more needs to be done, says Steinberg. "For example, in the substance use disorder space, a lot of addiction counselors do not have a master's degree. And that's one of their requirements to be a counselor in the Medicare program right now."
Removing those stringent requirements and adding other kinds of providers, like peer support specialists, is key to improving access. And the cost of not accessing care is high, she adds.
"Over the past two decades, [in] the older adult population, the number of overdose deaths has increased fourfold — quadrupled," says Steinberg. "So this is affecting people. It is causing deaths. It is causing people to go to the hospital. It increases [health care] costs."
- Centers for Medicare & Medicaid Services
- mental health
Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19
Affiliations.
- 1 Clinical Research Department, The Federal Budget Institute of Science "Central Research Institute for Epidemiology" of The Federal Service on Customers' Rights Protection and Human Well-being Surveillance Moscow, Russia.
- 2 Medical Center "Eco-safety" Saint-Petersburg, Russia.
- 3 Medical Center "Group of Companies "MEDSI" JSC Moscow, Russia.
- 4 Clinical Pharmacology Department, "Clinical Hospital of Zhukovsky" Zhukovsky, Russia.
- 5 Medical Center "Neuroprofi" LLC Korolev, Russia.
- 6 L.A. Vorokhobov City Clinical Hospital No. 67 of The Moscow City Healthcare Department Moscow, Russia.
- 7 City Clinical Hospital No. 52 of The Moscow City Healthcare Department Moscow, Russia.
- 8 Infectious Clinical Hospital No. 1 of The Moscow City Healthcare Department Moscow, Russia.
- 9 Voronezh Regional Clinical Hospital No. 1 Voronezh, Russia.
- 10 City Hospital No. 40 of The Kurortny District Sestroretsk, Russia.
- 11 N.I. Pirogov National Medical and Surgical Center of The Ministry of Health of The Russian Federation Moscow, Russia.
- 12 N.N. Burdenko National Medical Research Centr of Neurosurgery of The Ministry of Health of The Russian Federation Moscow, Russia.
- 13 R-Pharm Group of Companies Moscow, Russia.
- PMID: 34956474
- PMCID: PMC8661194
Favipiravir has demonstrated efficacy against the SARS-CoV-2 virus in several preliminary studies. This study aimed to evaluate the efficacy and safety of favipiravir for treatment of mild to moderate COVID-19 in outpatients and hospitalized patients. We conducted an open-label, randomized, active-controlled trial of a generic form of favipiravir in patients with COVID-19 confirmed by PCR-test. Eligible patients (18-60 years) after stratification were randomly assigned (in a 2:1 ratio) to receive either favipiravir (1800 mg BID on day 1, followed by 800 mg BID for up to 9 days), or standard of care (SOC) treatment (umifenovir + intranasal interferon alpha-2b, or hydroxychloroquine) for up to 10 days. The co-primary outcomes were the time to clinical improvement and the time to viral clearance. Among 190 patients assessed for eligibility 168 were randomized to favipiravir (n=112) or to SOC (n=56) group. The median time to clinical improvement was 6.0 days (IQR 4.0; 9.3) in the favipiravir group and 10.0 (IQR 5.0; 21.0) days in the SOC group; the median difference was 4 days (HR 1.63; 95% CI 1.14-2.34; P=0.007). The statistically significant difference in the median time to viral clearance was observed only for hospitalized patients: 3.0 (IQR 3.0; 3.0) days in the favipiravir group vs. 5.0 (IQR 4.5; 5.5) days in the SOC group (HR 2.11; 95% CI 1.04-4.31; P=0.038). The rate of viral elimination on Day 5 in the favipiravir group was significantly higher than in SOC group: 81.2% vs. 67.9% (RR 1.22; 05% CI 1.00-1.48; P=0.022). The rate of clinical improvement on Day 7 in the favipiravir group was 1.5-fold higher than in SOC group: 52.7% vs. 35.8% (RR 1.50; 95% CI 1.02-2.22; P=0.020). Favipiravir was well-tolerated and the most common adverse reactions were asymptomatic hyperuricemia, transient elevation of ALT & AST, and mild gastrointestinal disorders. Favipiravir was superior to the SOC in shortening the time to clinical improvement in patients with mild to moderate COVID-19.
Keywords: COVID-19; SARS-CoV-2; coronavirus; favipiravir.
AJTR Copyright © 2021.
Columbia University in the City of New York
Miriam and ira d. wallach art gallery.
- Visitor Information
- Exhibitions
- Publications
Moscow City, Spectacle, Capital of Photography
Nadia Michoustina Wallach Art Gallery, 2003 8 x 10", 88 pp., 46 b&w illus. ISBN 1-884919-13-8, Paper, $25
The history of photography, more than of the city, is traced through 34 monochrome works by photographers who lived and worked in Moscow from the 1920s to the present. These photographs are from the collection of the Cultural Center Dom, Moscow, and were exhibited at Columbia University April through June 2003. An essay, interview, and biographies are included.

IMAGES
VIDEO
COMMENTS
Background. Interest in medical applications of marijuana (Cannabis sativa) has increased dramatically during the past 20 years.A 1999 report from the National Academies of Sciences, Engineering, and Medicine supported the use of marijuana in medicine, leading to a number of regulatory medical colleges providing recommendations for its prescription to patients [].
INTRODUCTION. Medicinal cannabis, or medicinal marijuana, is a therapy that has garnered much national attention in recent years. Controversies surrounding legal, ethical, and societal implications associated with use; safe administration, packaging, and dispensing; adverse health consequences and deaths attributed to marijuana intoxication; and therapeutic indications based on limited ...
Further research is warranted, particularly regarding products similar to those currently available in dispensaries. Because medical marijuana lacks quality standards and FDA regulation, available products have shown significant inconsistencies, with one study revealing that only 17% of edible cannabis products were accurately labeled . It is ...
Cannabis has been used since as early as 100 ce for its potential therapeutic and medicinal properties from its multiple compounds, particularly Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Over the past 25 years, attitudes toward the recreational and medicinal use of cannabis have rapidly evolved in the United States from illicit to decriminalized to legalized at the state level ...
Objective To assess whether patients using medical cannabis report improvements in health-related quality of life over time. Design, Setting, and Participants This retrospective case series study was conducted at a network of specialist medical clinics (Emerald Clinics) located across Australia. Participants were patients who received treatment ...
The cannabis plant has been used in traditional medicine for centuries, and within the last few decades it has generated considerable attention among the general population, modern medical community and regulatory bodies for its potential medicinal capabilities (Alsherbiny and Li 2018).The effects of cannabis are due to the action of cannabinoids, a diverse group of chemical compounds found in ...
A 2014 analysis found that US state laws permitting medical cannabis "are associated with significantly lower state-level opioid overdose mortality rates." 3 In a 2019 commentary, Thomas Clark, PhD, a professor of biology at Indiana University South Bend, cited evidence that moderate use of marijuana can help to reduce reliance on ...
The potential medicinal properties of marijuana and its components have been the subject of research and heated debate for decades. THC itself has proven medical benefits in particular formulations. The U.S. Food and Drug Administration (FDA) has approved THC-based medications, dronabinol (Marinol) and nabilone (Cesamet), prescribed in pill form for the treatment of nausea in patients ...
The Journal of Cannabis Research is an international, fully open access, peer-reviewed journal covering all topics pertaining to cannabis, including original research, perspectives, commentaries and protocols. Our goal is to provide an accessible outlet for expert interdisciplinary discourse on cannabis research. Read Aims & Scope.
Despite this growth in evidence, the findings and conclusions of these studies have been inconsistent. In this paper, we outline the current evidence for medical cannabis and cannabis-based medicines in the treatment and management of chronic non-cancer pain. ... Medical Marijuana Grant support 1119992/National Health and Medical Research ...
Significant support exists in the United States for legalization of marijuana/cannabis. As of 2021, 36 states and four territories approved the legalization of medical cannabis via medical marijuana laws (MMLs), and 15 states and District of Columbia (DC) have adopted recreational marijuana laws (RMLs).
Medical Cannabis Real World Evidence [ 44 - 46] A Canadian, prospective, non-interventional, observational study led by the University Health Network in Toronto. It aims to explore the benefits of medical cannabis in an observational setting for adults with conditions such as chronic pain, anxiety or depression.
Department of Drug Science and Technology, University of Turin, Turin, Italy; Cannabis has long been regarded as a recreational substance in the Western world. The recent marketing authorization of some medicinal products of industrial origin and the introduction onto the market of inflorescences for medical use mean that medical doctors can now prescribe Cannabis-based medicines in those ...
The average number of patients showing a complete nausea and vomiting response was greater with cannabinoids (dronabinol or nabiximols) than placebo (OR, 3.82 [95% CI, 1.55-9.42]; 3 trials). There was no evidence of heterogeneity for this analysis ( I2 = 0%) and results were similar for both dronabinol and nabiximols.
Interest in medical applications of marijuana (Cannabis sativa) has increased dramatically during the past 20 years.A 1999 report from the National Academies of Sciences, Engineering, and Medicine supported the use of marijuana in medicine, leading to a number of regulatory medical colleges providing recommendations for its prescription to patients [].
The focus of this narrative review was on Cannabis sativa, initially where 93 papers were identified.Papers on various drying and extraction methods specifically for Cannabis sativa L. were included while those for using hemp as fiber and protein sources were excluded. Overall, 12 papers about cannabis seed oil, hemp seed oil, or hemp plant were excluded as this review focuses on the oil ...
Using statistical methods, the researchers were able to estimate the impact of the legal approval of marijuana for medical use. The result: Easier access improves the mental health of individuals ...
However, new research finds that people who overuse marijuana and then experience their first psychotic episode may be helped by the quick use of injected antipsychotics. "These findings encourage ...
ABSTRACT: Cannabis, or marijuana, has potential therapeutic and medicinal properties related to multiple compounds, particularly Δ-9-tetrahydrocannabinol and cannabidiol. Over the past 25 years, attitudes toward cannabis have evolved rapidly, with expanding legalization of medical and recreational use at the state level in the United States and
3 Department of Human Anatomy, the First Moscow State Medical University (Sechenov University), ... 6 Research Institute of Human Morphology, 3-Tsyurupy Street, Moscow, 117418, Russian Federation. PMID: 35272590 DOI: 10.2174/1570159X20666220310121441 Abstract The cerebellum is a well-established primary brain center in charge of controlling ...
A 57% majority of Republicans ages 18 to 29 favor making marijuana legal for medical and recreational use, compared with 52% among those ages 30 to 49 and much smaller shares of older Republicans. Still, wide majorities of Republicans in all age groups favor legalizing marijuana at least for medical use. Among those ages 65 and older, just 20% ...
Introduction. Medical cannabis is available to patients by physician order in 33 states and territories in the USA as of 2020. However, at the federal level, cannabis remains classified as a schedule I controlled substance, which limits efficacy and safety investigations [].Collectively, "medical cannabis" encompasses various terms used in reference to medical marijuana, cannabis-derived ...
The authors found that recreational marijuana laws were associated with a 9% increase in suicide rates among all youth ages 14 to 16. They also found that medical and recreational laws were associated with 10% and 16% increases in suicide rates among female youth ages 12 to 25, respectively. My preprint study with Robert Capodilupo, Michael ...
Affiliations 1 Children's City Clinical Hospital named after Z. A. Bashlyaeva of the Moscow City Health Department, 125373, Moscow, Russian Federation.; 2 Pirogov Russian National Research Medical University, 117997, Moscow, Russian Federation.; 3 Russian Medical Academy of Continuous Professional Education of the Ministry of Healthcare of the Russian Federation, 125993, Moscow, Russian ...
Abstract. State-level marijuana liberalization policies have been evolving for the past five decades, and yet the overall scientific evidence of the impact of these policies is widely believed to be inconclusive. In this review we summarize some of the key limitations of the studies evaluating the effects of decriminalization and medical ...
A report from the Department of Health and Human Services' inspector general finds a dire shortage of mental health care providers in Medicaid and Medicare, which together serve some 40% of Americans.
Affiliations 1 Clinical Research Department, The Federal Budget Institute of Science "Central Research Institute for Epidemiology" of The Federal Service on Customers' Rights Protection and Human Well-being Surveillance Moscow, Russia.; 2 Medical Center "Eco-safety" Saint-Petersburg, Russia.; 3 Medical Center "Group of Companies "MEDSI" JSC Moscow, Russia.
ISBN 1-884919-13-8, Paper, $25. The history of photography, more than of the city, is traced through 34 monochrome works by photographers who lived and worked in Moscow from the 1920s to the present. These photographs are from the collection of the Cultural Center Dom, Moscow, and were exhibited at Columbia University April through June 2003 ...