Conditional gene expression in the respiratory epithelium of the mouse
- Published: February 2002
- Volume 11 , pages 21–29, ( 2002 )

Cite this article

- Anne-Karina T. Perl 1 ,
- Jay W. Tichelaar 1 &
- Jeffrey A. Whitsett 1
1238 Accesses
190 Citations
3 Altmetric
Explore all metrics
Transgenic mouse models mediating conditional temporal and spatial regulation of gene expression to the respiratory epithelium were developed utilizing the reverse tetracycline transactivator (rtTA) expressed under the control of SP-C and CCSP promoters. Luciferase activity was detected in the lungs of fetal and adult double transgenic mice but was not detected in other tissues or in single transgenic mice. In adult mice, maximal luciferase activity was detected 16 h after the administration of doxycycline in the drinking water, or 2 h after the injection of doxycycline. Activation of the transgene was observed after the administration of doxycycline in food pellets. After prolonged exposure to doxycycline, luciferase activity decreased slowly following removal of doxycycline, suggesting the importance of tissue pools which maintained expression of the transgene. In SP-C-rtTA mice, exposure of the pregnant dam to doxycycline induced luciferase activity in fetal lung tissue as early as E10.5. Luciferase activity was maintained in the lung tissue of pups during the period of lactation when the mother received doxycycline in the drinking water. In the CCSP-rtTA mice, luciferase was not detected in the absence of doxycycline. In the SP-C-rtTA mice, luciferase activity was detected in the absence of doxycycline but was enhanced approximately 10-fold by administration of drugs. The SP-C-rtTA and CCSP-rtTA activator mice control the expression of transgenes in the developing and mature respiratory epithelium, and will be useful for the study of gene function in the lung.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Efficient tissue-type specific expression of target genes in a tetracycline-controlled manner from the ubiquitously active Eef1a1 locus

Modulation of Myeloid Cell Function Using Conditional and Inducible Transgenic Approaches
A sox10rtta/+ mouse line allows for inducible gene expression in the auditory and balance organs of the inner ear.
Albanese C, Reutens AT, Bouzahzah B, Fu M, DAmico M, Link T, Nicholson R, Depinho RA and Pestell RG (2000) Sustained mammary gland-directed, ponasterone A-inducible expression in transgenic mice. FAS EB J 14 : 877–884.
Google Scholar
Danielian PS, Muccino D, Rowitch DH, Michael SK and MacMahon AP (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 18 : 1323–1326.
Glasser SW, Burhans MS, Eszterhas SK, Bruno MD, Korfhagen TR (2000) Human SP-C gene sequences that confer lung epithelium-specific expresssion in transgenic mice. Am J Physiol 278 : L933–L945.
Glasser SW, Korfhagen TR, Bruno MD, Dey C, Whitsett JA (1990) Structure and expression of the pulmonary surfactant protein SP-C gene in the mouse. J Biol Chem 265 : 21986–21991.
Gossen M, Freundlieb S, Bender G, Muller G, Hillen W and Bujard H (1995) Transcriptional activation by tetracyclines in mammalian cells. Science 268 : 1766–1769.
Honikel KO, Schmidt U, Woltersdorf W and Leistner L (1978) Effect of storage and processing on tetracycline residues in meat and bones. J Assoc Off Anal Chem 61 : 1222–1227.
Jordan VC and Murphy CS (1990) Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev 11 : 578–610.
Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lübbert H and Bujard H (1996) Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA 93 : 10933–10938.
Korsrud GO, Papich MG, Fesser AC, Salisbury CD and MacNeil JD (1996) Residue depletion in tissues and fluids from swine fed sulfamethazine, chlortetracycline and penicillin G in combination. Food Addit Contam 13 : 287–292.
No D, Yao TP and Evans RM (1996) Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci USA 93 : 3346–3351.
Picard D (1994) Regulation of protein function through expression of chimaeric proteins. Curr Opin Biotechnol 5 : 511–515.
Porter A (1998) Controlling your losses: conditional gene silencing in mammals. Trends Genet 14 : 73–79.
Ray P, Tang W, Wang P, Homer R, Kuhn C III, Flavell RA and Elias JA (1997) Regulated overexpression of interleukin 11 in the lung. Use to dissociate development-dependent and-independent phenotypes. J Clin Invest 100 : 2501–2511.
Saez E, Nelson MC, Eshelman B, Banayo E, Koder A, Cho GJ and Evans RM (2000) Identification of ligands and coligands for the ecdysone-regulated gene switch. Proc Natl Acad Sci USA 97 : 14512–14517.
Schultze N, Burki Y, Lang Y, Certa U and Bluethmann H (1996) Efficient control of gene expression by single step integration of the tetracycline system in transgenic mice. Nat Biotechnol 14 : 499–503.
Spencer DM (1996) Creating conditional mutations in mammals. Trends Genet 12 : 181–187.
Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katayal SL and Whitsett JA (1992) Cis -acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem 267 : 14703–14712.
Tichelaar JW, Lu W and Whitsett JA (2000) Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 275 : 11858–11864.
Tsai SY, O'Malley BW, DeMayo FJ, Wang Y and Chua SS (1998) A novel RU486 inducible system for the activation and repression of genes. Adv Drug Deliv Rev 30 : 23–31.
Wang Y, O'Malley BW Jr, Tsai SY and O'Malley BW (1994) A regulatory system for use in gene transfer. Proc Natl Acad Sci USA 91 : 8180–8184.
Wert SE, Glasser SW, Korfhagen TR and Whitsett JA (1993) Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol 156 : 426–443.
Whitsett JA and Glasser SW (1997) Targeting gene expression to the lung. In: Brigham LA (ed.) Gene Therapy for Diseases of the Lung (for the series: Lenfant C, editor. Lung Biology in Health and Disease). (pp. 193–208) Marcel Dekker, New York.
Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA and Whitsett JA (1994) Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 266 : 1705–1708.
Download references
Author information
Authors and affiliations.
Division of Pulmonary Biology, Children's Hospital Medical Center, Cincinnati, Ohio, USA
Anne-Karina T. Perl, Jay W. Tichelaar & Jeffrey A. Whitsett
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jeffrey A. Whitsett .
Rights and permissions
Reprints and permissions
About this article
Perl, AK.T., Tichelaar, J.W. & Whitsett, J.A. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res 11 , 21–29 (2002). https://doi.org/10.1023/A:1013986627504
Download citation
Issue Date : February 2002
DOI : https://doi.org/10.1023/A:1013986627504
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- clara cell secretory protein promoter
- doxycycline
- pharmacokinetics
- reverse tetracycline transactivator
- surfactant protein C promoter
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- American Journal of Respiratory Cell and Molecular Biology
Conditional Recombination Reveals Distinct Subsets of Epithelial Cells in Trachea, Bronchi, and Alveoli
To identify relationships amongst tracheal and alveolar epithelial cells during lung development, we used conditional systems controlled by the rat CCSP and human SFTPC gene promoters to express Cre-recombinase in the developing mouse lung, thereby permanently labeling cells by expression of alkaline phosphatase or green fluorescent protein. When controlled by the rat CCSP promoter, continuous exposure of the fetus to doxycycline caused widespread recombination in conducting airway epithelial cells, including cells of the trachea, bronchi, and bronchioles before birth, and in both conducting and peripheral airways after birth. Neuroepithelial cells, identified by CGRP staining, were never labeled. Recombination and permanent labeling were observed in both ciliated and nonciliated respiratory epithelial cells, demonstrating their derivation from common progenitor cells during lung morphogenesis. Remarkable dorsal–ventral and cephalo–caudal labeling patterns, established before birth, were identified by recombination controlled by the rat CCSP gene promoter. In the trachea, subsets of epithelial cells labeled by the CCSP promoter were organized horizontally along the dorsal–ventral axis of the trachea, where selective labeling of cells juxtaposed to tracheal and bronchial cartilage was observed. In sharp contrast, recombination controlled by the human SFTPC gene promoter identified related cells that were organized in linear patterns along the cephalo–caudal axis of the conducting airways. Conditional expression of Cre-recombinase in the respiratory epithelium provides a useful model for the study of gene expression and function in the mouse respiratory tract and in the lung.
The lung forms by evagination of endoderm-derived cells from the foregut epithelium, which invade the surrounding splanchnic mesenchyme. The primary conducting airways are established early in lung morphogenesis (embryonic day [E] 9.5–E 11.5). Thereafter, peripheral lung tubules are formed by further budding of the intrapulmonary conducting airways, which leads to formation of the peripheral gas exchange regions, or alveoli, in the postnatal lung ( 1 ). Epithelial cells lining the respiratory tract undergo differentiation to produce the numerous, distinct, epithelial cell types characteristic of the mammalian lung. The numbers and types of cells lining conducting and peripheral airways vary with species, developmental stage, and along the cephalo–caudal axis. Factors controlling epithelial cell differentiation at various sites along the respiratory tract are not fully known, but are likely to be influenced by autocrine and paracrine interactions among epithelial cells, their precursors, and the underlying mesenchymally derived cells, including cartilage, stroma, smooth muscle, vascular, and marrow-derived cell types. Factors controlling the numbers and location of distinct epithelial cell types, including cells of the tracheal glands, ciliated, basal, intermediate, Clara, neuroepithelial, and goblet cells of the conducting airways, and squamous type I and cuboidal type II cells in the alveolar region, are poorly understood ( 2 – 4 ). Recent studies using gene addition and targeted deletion of genes in the respiratory epithelium of the mouse indicate that specification and differentiation of proximal and peripheral respiratory epithelial cells are influenced by multiple signaling pathways, including β-catenin ( 5 ), sonic hedgehog ( 6 – 8 ), BMP's ( 9 , 10 ), Foxa2 ( 11 ), GATA-6 ( 12 , 13 ), TTF-1 ( 14 ), Rb ( 15 ), and others.
In the present study, we used transgenic mice in which both rat CCSP and human SFTPC gene promoters were used to express the reverse tetracycline transactivator (rtTA) ( 16 ), thus placing the expression of Cre-recombinase (CRE) under conditional control of doxycycline during mouse lung morphogenesis. Expression of CRE was used to permanently activate alkaline phosphatase (AP) ( 17 ) or green fluorescent protein (GFP) ( 18 ) in subsets of respiratory epithelial cells in the conducting airways. Each promoter labeled respiratory epithelial cells in stereotypic patterns, either along the cephalo–caudal or dorsal–ventral axis or in relationship to tracheal-bronchial cartilage.
MATERIALS AND METHODS
Transgenic mice were identified using PCR primers specific for each transgene CCSP-rtTA : (5′ CCSP promoter: 5′-ACT GCC CAT TGC CCA AAC AC-3′ and the 3′ primer in rtTA coding sequence (5′-AAA ATC TTG CCA GCT TTC CCC-3′) forward CRE (5′-TGC CAC GAC CAA GTG ACA GCA ATG-3′) and reverse CRE (5′-AGA GAC GGA AAT CCA TCG CTC G-3′). Amplification of PCR products was performed as follows: denaturation at 94°C for 5 min; 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s, followed by a 5-min extension at 72°C. ZAP and ZEG mice were genotyped by positive β-gal staining on tissue.
Animal Use and Doxycycline Administration
Animals were housed in pathogen-free conditions in accordance with institutional guidelines. Animals were mated, and gestational age was determined by detection of the vaginal plug and then correlated with length and weight of each pup at the time of killing. Dams bearing double and triple transgenic pups were maintained on doxycycline containing food (625 mg/kg; Harlan Teklad, Madison, WI) or drinking water (Sigma Chemical Co., St. Louis, MO) at 1 mg/ml for various time spans. The mice were killed by either placing them in a CO 2 chamber or by injection with 0.2–0.3 cc anesthetic (ketamine, xylazine, acepromazine). All experiments were performed with at least five triple transgenic mice from three independent litters.
Timing and Conditional Control of CRE Recombination
Doxycycline was administered to pregnant dams at E 0.5 and maintained until killing. E 0.5 was defined as 12 h after fertilization, as determined by the vaginal plug. To investigate recombination at different periods during embryonic development, doxycycline was administered for shorter time periods by providing the dam doxycycline food at the indicated time points and removing it after 48 h. For postnatal recombination, pregnant and nursing dams were placed on doxycycline food from E 18.5 until PN 9, and recombination was assessed in triple transgenic mice at 3 wk of age. AP staining or detection of fluorescent GFP expression was used to assess recombination. Each experiment represents a group of 3 to 4 pregnant females of a cross resulting in triple transgenic offspring ( CCSP-rtTA/tetOCre/ZAP or CCSP-rtTA/tetOCre/ZEG ).
Lung Histology and In Situ Hybridization
Timed matings were performed to produce litters with triple transgenic offspring. Pregnant dams were administered doxycycline food for times specified in each individual experiment. Before killing, mothers were injected with 0.2–0.3 cc anesthetic (ketamine, xylazine, acepromazine) to prevent pups from breathing. Genotyping was performed on tail DNA. The thorax of both triple transgenic mice and controls were immersion fixed overnight at 4°C with 4% paraformaldehyde in PBS. Lungs from PN5 to PN21 were inflation-fixed at 25 cm of pressure and fixed overnight at 4°C.
For detection of rtTA and CCSP mRNA by in situ hybridization, CCSP-rtTA transgenic pups were obtained from timed matings, lungs were isolated and fixed overnight in 4% paraformaldehyde at 4°C, washed with PBS, dehydrated through a graded series of ethanol solutions, and processed for paraffin embedding. Sections (5 μm) were loaded onto polysine slides. 35 S-UTP labeled sense and antisense riboprobes were generated from a pGEM3z-rtTA and a pGEM4Z-CC10 DNA plasmid (Promega, Madison, WI) and transcribed in vitro with a riboprobe transcription kit (Promega). Conditions and solutions for hybridization were essentially as previously described ( 19 ). Hybridization was performed overnight at 55°C, and the sections were washed under highly stringent conditions. Slides were dipped in Kodak NTB2 emulsion (Fisher Scientific, Pittsburgh, PA), exposed for 7–10 d for detection of rtTA expression in the mouse lung and 10 wk for detection of CCSP expression in both mouse and rat lungs, and developed with Kodak D19.
For visualizing AP activity, samples were fixed in 0.2% glutaraldehyde in PBS, 0.02% Nonident P-40, and 0.01% SDS. Samples were washed with PBS, dehydrated through a graded series of ethanol solutions, and processed for paraffin embedding. Five-micron sections were loaded onto polysine slides. AP staining was performed on tissue sections as previously described ( 17 ). All sections were counterstained with nuclear fast red. Histology was documented with an Optronics digital camera (Optronics, Goleta, CA) attached to a Nikon Microphot FXA (Nikon, Tokyo, Japan). Images were processed with Optronics MagnaFIRE 1.1 and Adobe Photoshop software (Adobe Systems, San Jose, CA).
For studies of GFP expression and co-localization with other markers, samples were fixed in 4% paraformaldehyde in PBS. Images of whole mount trachea and lung parenchyma were acquired with an inverted Olympus microscope (Olympus, Lake Success, NY). For sections, samples were rinsed in PBS, cryoprotected in 30% sucrose in PBS, and infiltrated with a 2:1 mixture of 30% sucrose-OCT, before freezing in OCT. Cryosections were cut at 8 μm, loaded onto silanized slides, and dried at room temperature before storage at −80°C. Colocalization for GFP expression and specific cellular markers was performed by immunohistochemistry. GFP (green) and Alexa Fluor 568 (red) fluorescence were visualized with the appropriate filter sets, and images were acquired with a Zeiss Axioplan 2 Imaging Universal Microscope (Zeiss, Göttingen, Germany) and an Axiocam MRm black and white digital camera (Axiovision Release 4.3; Zeiss) and processed with an Apotome Slider (Zeiss) for pseudoconfocal imaging.
1. Ciliated cells were identified by labeling for β-tubulin IV (1:50, MU178-UC mouse monoclonal antibody OS1A6, Biogenex [San Ramon, CA]; 1:200, Alexa Fluor 568–conjugated donkey anti-mouse IgG1; Molecular Probes [Eugene, OR]).
2. Clara cells were identified by labeling for CCSP (1:500, affinity purified goat anti-rat polyclonal antibody, gift from Dr. Barry Stripp; 1:200, Alexa Fluor 568–conjugated donkey anti-goat IgG; Molecular Probes).
3. Type II cells were identified by labeling for pro–surfactant protein (SP)-C (1:1,000, {"type":"entrez-nucleotide","attrs":{"text":"R09337","term_id":"761260"}} R09337 mono-specific, rabbit anti-mouse polyclonal antibody, in house; 1:200, Alexa Fluor 568–conjugated goat anti-rabbit IgG; Molecular Probes).
4. Neuroendocrine cells were identified by labeling for CGRP (1:500, C8198 polyclonal rabbit anti-rat antibody; Sigma; 1:1,000; 1:200, Alexa Fluor 568–conjugated goat anti rabbit IgG; Molecular Probes).
Expression of rtTA under Control of the Rat CCSP Promoter
Distinct lines of transgenic mice were produced that express rtTA under control of the rat CCSP and human SFTPC gene promoters ( 16 , 20 ). These mice were bred to tetO-CRE mice and either ZEG or ZAP mice ( 17 , 18 ), creating triple transgenic mice (herein designated as CCSPrtTA/tetOCRE/ZEG, CCSPrtTA/tetOCRE/ZAP, SPCrtTA/tetOCRE/ZEG, SPCrtTA/tetOCRE/ZAP ), in which respiratory epithelial cells permanently expressed either AP or GFP after doxycycline-induced recombination ( Figure 1 ). These mice have been bred and maintained for more than 4 yr, with normal lung function and longevity in the vivarium. Sites of CRE expression and recombination in the peripheral lung under control of the SFTPC promoter have been described previously ( 21 ).

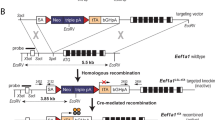
Permanent cell labeling by controlled expression of Cre-recombinase. The rtTA gene is driven by a cell-type specific promoter (rat CCSP ) and followed by a SV40 polyadenylation site. In the presence of doxycycline (Dox), the transactivator ( rtTA ) recognizes its specific DNA target sequence (tetO). Expression of CRE causes recombination at loxP sites. In Z/AP and Z/EG reporter mice β-gal expression changes to alkaline phosphatase (AP) or enhanced green fluorescent protein (GFP). Visualization of AP or GFP expression indicates doxycycline-induced CCSP-dependent recombination in triple transgenic CCSPrtTA/tetOCre/ZAP and CCSPrtTA/tetOCre/ZEG mice.
To identify the sites of rtTA expression in the CCSP-rtTA mouse, in situ hybridization for the reverse tetracycline activator mRNA was performed in mouse lung from E 13.5 to Postnatal Day (PN) 21 ( Figure 2 ). No rtTA mRNA was found in lung epithelium before E 14.5. At E 14.5 and E 16.5, rtTA mRNA was confined to respiratory epithelial cells in the conducting airways (trachea and bronchi) ( Figures 2A and 2B ) and bronchioles. From E 18.5 to PN 21 rtTA mRNA was found in the conducting airways ( Figures 2C–2H ) and in a subset of peripheral epithelial cells, consistent with previous findings demonstrating the sites of expression of the rat CCSP promoter in type II epithelial cells of the adult mouse lung ( 22 , 23 ). In the adult, rtTA mRNA was detected in epithelial cells adjacent to tracheal cartilage rings on the ventral side of both trachea and bronchi. In the dorsal epithelium of the trachea, rtTA mRNA appeared to be random and less frequently expressed ( Figure 2I ). This pattern of rtTA expression, and of subsequent recombination observed in the CCSPrtTA/tetOCRE mice, was distinct from that observed in SPCrtTA/tetOCRE mice, in which recombination was similar on the dorsal and ventral side of the trachea and increased along the proximal to distal axis ( 21 ). In situ hybridization for CCSP mRNA was performed on adult mouse and rat lung tissue to locate cellular expression of endogenous CCSP . In contrast to the mouse, where CCSP mRNA expression was restricted to the conducting airways ( 25 ), rat CCSP was expressed in a subset of alveolar type II cells, as well as in the conducting airways ( Figure 3 ). This finding indicates that the rat CCSP promoter directs transgene expression to cells of both the conducting airways and the alveolar epithelium in transgenic mice ( 22 ). Therefore, rtTA mRNA expression in the mouse is consistent with endogenous rat CCSP gene expression.

Localization of rtTA mRNA in CCSPrtTA transgenic mice. Radioactive in situ hybridization was performed on paraffin sections of lung from CCSP-rtTA transgenic mice killed at E 14.5 ( A ), E 16.5 ( B ), E 18.5 ( C ), PN 5 ( D ), PN 7 ( E ), PN 10 ( F ), PN 14 ( G ), and PN 21 ( H , I ). The CCSP promoter drives rtTA expression in the bronchus and bronchioles as early as E 14.5 and in the alveolar region as early as E 18.5. In the trachea of adult mice, rtTA expression was detected primarily in epithelial cells on the ventral side of the trachea. V, ventral; D, dorsal.

Localization of endogenous CCSP mRNA in rat and mouse lung. Radioactive in situ hybridization was performed on paraffin sections of lung from adult rat ( A , C ) and mouse ( B ). Endogenous CCSP mRNA expression was detected in the bronchus, bronchioles, and a subset of alveolar type II cells in the rat ( A ). In the mouse, CCSP mRNA was detected in conducting airways but not in the alveoli ( B ). Hybridization with the sense RNA is shown on rat tissue ( C ). Size bars = 200 μm.
Mild but consistent enlargement of alveoli was noted in the lungs of all CCSPrtTA mice in the presence or absence of doxycycline or additional transgenes.
Conditional Recombination in the Postnatal Lung Controlled by CCSPrtTA
Exposure of the adult triple transgenic CCSPrtTA/tetOCRE/ZAP mice to doxycycline for 3 wk caused expression of AP in the conducting airways and in a subset of type II epithelial cells in the lung periphery ( Figures 4A and 4B ), demonstrating the sites and extent of CRE-mediated recombination. In the absence of doxycycline, very few cells in the bronchioles of adult and 3-wk-old mice were labeled ( Figure 4A ). Doxycycline-independent recombination was never found in the alveolar region ( 21 ) ( Figures 4A, 4E, and 4F ). AP staining was never found in double transgenic tetOCRE/ZAP mice ( Figures 4C and 4D ). When triple transgenic mice were exposed to doxycycline from E 18.5 to PN 9, clusters of AP-labeled epithelial cells were observed lining the bronchioles ( Figure 4G ). After this time of prolonged doxycycline exposure, a subset of type II cells was labeled ( Figure 5H ), consistent with the timing and extent of expression of the rtTA mRNA as detected by in situ hybridization ( Figure 2 ). Labeling of alveolar type I cells was not observed in the CCSPrtTA/tetOCRE/ZAP mice. In contrast, both alveolar type I and type II cells were extensively labeled in SPCrtTA/tetOCRE/ZAP mice. This finding suggests that in the CCSPrtTA transgenic mice this subset AP labeled of type II cells does not differentiate into type I cells and, therefore, represents a distinct subset of type II cells from those targeted in SPCrtTA mice ( 21 ).

CCSPrtTA mediated recombination in adult and E 18.5 to PN 9 mouse lung. Lung sections of adult ( A , B ) and PN21 ( C–H ) double and triple transgenic mice were analyzed for AP expression. In the absence of doxycycline, rare recombination in the conducting airways was observed in lung sections of adult ( arrows in A ) and PN 21 ( arrowhead in E , F ) triple transgenic CCSPrtTA/tetOCRE/ZAP mice. No AP-positive cells were found in the lung periphery ( A , F ). No AP-positive cells were detected in double transgenic control lungs ( C , D ). After 3 wk of doxycycline treatment, recombination was found in ciliated cells, Clara cells ( arrows in B ), and a subset of type II cells ( arrowheads in B ) in triple transgenic adult CCSPrtTA/tetOCRE/ZAP mouse lung. After doxycycline exposure from E 18.5 to PN 9, most nonciliated and ciliated cells in the bronchioles were positive for AP staining in triple transgenic CCSPrtTA/tetOCRE/ZAP PN 18 lungs. Cil, ciliated cells; Cla, Clara cells. Size bars: A , B = 50 μm; C–H = 10 μm.

Recombination in embryonic lungs of CCSPrtTA/tetOCRE/ZAP mice after continuous exposure to doxycycline. Dams were treated with doxycycline from E 6.5 until killing. Mice were killed on E 14.5 ( A ), E 15.5 ( B ), E 16.5 ( C ), and at birth ( D ). Rare AP-positive cells were detected in conducting airways as early as E 14.5 ( arrowhead ), and in the lung parenchyma as early as E 16.5 ( arrowhead ). Numbers of labeled epithelial cells increased from E 14.5 until birth. Size bar = 50 μm.
Timing of Conditional Recombination in the Embryonic Respiratory Epithelium with the Rat CCSP Promoter
When the CCSPrtTA dams were exposed to doxycycline from E 6.5 and thereafter, labeling of conducting airway epithelial cells was first detected at E 14.5 ( Figure 5 ). The numbers of labeled epithelial cells in the conducting airways increased between E 15.5–16.5 and thereafter. Most bronchiolar cells were labeled during this period of doxycycline exposure. Alveolar cells were first labeled at E 16.5 and thereafter ( Figure 5 ), in contrast to findings in the SPCrtTA mice, where labeling of peripheral progenitor cells occurred very early in lung morphogenesis ( 21 ). Endogenous CCSP mRNA expression was detected in mouse bronchioles at E 16.5 ( 22 ), and protein expression was detected by E 17.5 ( 24 ). E 16.5 defines the end of the pseudoglandular stage, a time when specific cellular markers of the proximal and peripheral epithelium are increasingly expressed. Dams were exposed to doxycycline for 48-h periods to label progenitor cells at precise intervals ( Figure 6 ). Lungs of triple transgenic CCSPrtTA/tetOCRE/ZAP offspring were analyzed at E18.5. When exposed to doxycycline from E 12.5–E 14.5 ( Figures 6A, 6D, and 6G ), infrequent tracheal and bronchial cells were labeled, but no cells in the bronchioles or peripheral saccules were labeled. Between E 14.5 and E 16.5 ( Figures 6B, 6E, and 6H ), extensive labeling was observed in trachea, bronchi and bronchioles, and frequently in the peripheral saccules. Between E 16.5 and E 18.5 ( Figures 6C, 6F, and 6I ), recombination was detected in virtually all cells lining the bronchioles, and in a subset of epithelial cells in the lung periphery ( Figure 6 ). In contrast to SPCrtTA mice ( 21 ), thyroid and thymus were never labeled in the CCSPrtTA mice.

Timing of recombination in the prenatal period. Dams were treated with doxycycline for 48-h periods. AP staining was assessed in triple transgenic CCSPrtTA/tetOCRE/ZAP lungs at E 18.5. When exposed to doxycycline from E 12.5–14.5, few cells were labeled in the bronchioles ( A , D , G ). After exposure from E 14.5–16.5, extensive labeling was observed in bronchioles and bronchiolar-alveolar portals, with a subset of cells labeled in peripheral saccules ( B , E , H ). After exposure from E 16.5–18.5, recombination was detected in most cells in the proximal and terminal bronchioles, but was rarely detected in peripheral lung saccules ( C , F , I ). Labeling was not detected in thymus or thyroid. Size bar = 100 μm.
Distinct Patterns of Recombination in Conducting Airways
ZEG mice, expressing GFP following recombination, were used to more precisely image the spatial organization of labeled epithelial cells in the lung. Dams were provided doxycycline from conception throughout pregnancy. Triple transgenic CCSPrtTA/tetOCRE/ZEG and SPCrtTA/tetOCRE/ZEG mice were analyzed on PN 7 ( Figure 7 ). Continuous exposure of the dams to doxycycline caused a stereotypic pattern of recombination in subsets of conducting airway cells. CCSPrtTA/tetOCRE/ZEG , GFP expressing cells were observed in both cephalic and caudal regions of the trachea. GFP was expressed in a horizontal banding pattern along the ventral side of the trachea. Epithelial cells overlying the cartilage rings were labeled, while GFP labeling was rare in inter-cartilaginous regions ( Figure 7A ). This pattern of discontinuous labeling was observed from the larynx to the end of the cartilaginous region in the main stem and lobar bronchi. On the membranous, or dorsal, side of the trachea, GFP-labeled cells were distributed randomly ( Figures 7C , ,7E, 7E , ,7G, 7G , ,8A, 8A , and and8B). 8B ). The same dorsal ventral pattern was found by in situ hybridization for rtTA mRNA in the trachea ( Figure 2I ). The pattern of labeling in the CCSPrtTA/tetOCRE/ZEG was distinct from that observed in SPCrtTA/tetOCRE/ZEG mice, in which epithelial cells of the trachea were labeled with increasing frequency in a proximal to distal gradient. Distal trachea and mainstem bronchi of SPCrtTA/tetOCRE/ZEG mice contained labeled cells forming longitudinal stripes that were similar on both dorsal and ventral surfaces ( Figures 7D, 7F, and 7H ).

Pattern of recombination from the trachea to the alveolar epithelium. Dams were treated with doxycycline from E 6.5 to PN 7. Tracheas of triple transgenic CCSPrtTA/tetOCRE/ZEG ( A , C , E , G , I , K ) and SPCrtTA/tetOCRE/ZEG ( B , D , F , H , J , L ) mice were visualized using an inverted microscope with fluorescence optics. Whole mount of the proximal trachea is shown in A and B . The main stem bronchi are shown in C and D . Inserts in A and C demonstrate the ventral ( A ) and dorsal ( C ) aspects of the trachea at 4× higher magnification. In CCSPrtTA/tetOCRE/ZEG tracheas, labeled cells were present in a dorsal–ventral pattern with increased density of labeled cells overlaying the cartilage rings. In the SPCrtTA/tetOCRE/ZEG tracheas, labeled cells formed longitudinal stripes with increasing numbers of labeled cells observed from the proximal to distal region. In the mainstem bronchi ( E , G ), labeled cells where found in a random pattern in the lungs of CCSPrtTA/tetOCRE/ZEG mice. Longitudinal stripes were observed in the bronchi of SPCrtTA/tetOCRE/ZEG mice ( F , H ). Recombination was frequent in epithelial cells of the bronchioles, as well as in the alveoli of both transgenic lines ( I–L ). Size bars: A , B = 400 μm; E , F = 500 μm; G , H , I , J = 250 μm; K , L = 400 μm. Note: autofluorescence can be seen in all tissue. V, ventral; D, dorsal.

Colocalization of GFP with β-tubulin and endogenous CCSP in the trachea. Dams were treated with doxycycline from E 6.5 to PN 7. Frozen sections of trachea ( A , B ) of triple transgenic CCSPrtTA/tetOCRE/ZEG mice were visualized with an upright microscope using fluorescence optics. Numerous GFP-positive cells were detected all along the ventral or cartilaginous side of the trachea ( arrows in A ) from the larynx to the mainstem bronchi ( A , B ). Tracheal glands lacked GFP staining ( arrows in B ). Frozen sections of CCSPrtTA/tetOCRE/ZEG trachea were stained for β-tubulin ( C , D ; red ) or CCSP ( E , F ; red ) and visualized for dual fluorescence with GFP ( green ). Ciliated cells on the dorsal side of the trachea were predominantly GFP-negative ( C , arrows ). The majority of ciliated cells (β-tubulin) on the ventral side of the trachea were labeled with GFP ( D , arrow ), although some ciliated cells were GFP-negative ( C , D ; arrowhead ). CCSP did not colocalize with GFP anywhere in the trachea ( E , F ; arrowhead ). Note: The yellow color on F results from close proximity of the red CCSP signal and the green GFP signal, but does not reflect colocalization. Arrows in all panels show GFP-negative cells that express differentiation markers; arrowheads demonstrate colocalization. Size bars: A , B = 500 μm; C , E = 200 μm; D , F = 50 μm.
Conditional Labeling in the Peripheral Lung
After continuous doxycycline treatment during embryonic development, GFP-positive cells lined small airways and bronchioles of both CCSPrtTA/tetOCRE/ZEG and SPCrtTA/tetOCRE/ZEG lungs, and GFP-positive cells were found in the alveolar regions ( Figures 7I , ,7J, 7J , ,9A, 9A , and and9B). 9B ). GFP-positive alveolar type I and type II cells were found in SPCrtTA/tetOCRE/ZEG lungs. GFP-positive alveolar type II cells, but not type I cells, were found in the lungs of CCSPrtTA/tetOCRE/ZEG mice.

Colocalization of GFP with epithelial cell markers in the peripheral lung. Dams were treated with doxycycline from E 6.5 to PN 7. Most bronchiolar cells ( A ) and some alveolar type II cells ( B ) expressed GFP. At PN 7 frozen sections of CCSPrtTA/tetOCRE/ZEG lungs were stained for β-tubulin, CCSP, pro–SP-C, or CGRP ( red ) and visualized for dual fluorescence with GFP ( green ). Some ciliated (β-tubulin–positive) cells expressed GFP ( arrowhead in C ). In the bronchioles, some Clara cells (CCSP-positive) expressed GFP ( arrowhead in D ), although not all nonciliated, GFP-expressing cells expressed CCSP ( arrows in D ). A subset of alveolar type II cells (pro–SP-C) expressed GFP ( arrowhead in E ). GFP was not detected in squamous type I epithelial cells ( E ). GFP expression ( green ) was not colocalized with CGRP ( arrows in F ). Note: arrows in all panels show GFP-negative cells that do express differentiation markers; arrowheads show colocalization. Size bars: A = 500 μm; B = 125 μm; C–F ( left ) = 50 μm; C–F ( right ) = 10 μm.
Correlations between Airway Epithelial Differentiation and Recombination
GFP expression in the tracheal epithelium of CCSPrtTA/tetOCRE/ZEG mice was correlated with sites of immunohistochemical staining for β-tubulin (ciliated cells), CCSP (Clara cells), pro–SP-C (alveolar type II cells), and CGRP (neuroepithelial cells). Ciliated cells overlying tracheal cartilage on the ventral surfaces of the trachea and bronchi expressed GFP. In contrast, ciliated cells on the dorsal side of the trachea or in the bronchioles were GFP-negative ( Figures 8C , ,8D, 8D , and and9C). 9C ). Since the CCSP promoter is not active in ciliated cells, these findings suggest that expression of the rat CCSPrtTA transgene occurred in progenitor cells that were labeled before ciliated cell differentiation. Exposure of the dams to doxycycline for 48-h periods, starting at E 12.5, E 14.5, or E 16.5, revealed that recombination in the tracheal epithelium occurred during doxycycline exposure from E 12.5 to E 14.5 and did not occur when the dam was exposed to doxycycline after E 16.5 (data not shown). Since targeting of tracheal epithelial cells occurred early in development, nontargeted ciliated cells are likely derived from progenitor cells that are distinct form those of GFP-positive ciliated cells on the ventral side of the trachea. Surprisingly, epithelial cells expressing endogenous mouse CCSP in the adult trachea did not express GFP. Thus, the rat CCSP promoter induced recombination in a subset of cells that have differentiated later and no longer express endogenous CCSP. Furthermore, differentiated CCSP-expressing cells in the trachea of postnatal mice are not susceptible to recombination.
Some, but not all bronchiolar epithelial cells co-expressed CCSP and GFP, indicating that the rat CCSP promoter targeted a subset of bronchiolar Clara cells in this transgenic mouse line ( Figure 9D ). In the peripheral lung, GFP was colocalized with pro–SP-C in a subset of alveolar type II cells ( Figure 9E ). This site of recombination is consistent with the expression of the rtTA mRNA under the control of the rat CCSPrtTA promoter and with expression of the endogenous CCSP gene in the rat. In both CCSPrtTA and SPCrtTA transgenic mice ( 21 ), GFP was never detected in CGRP-reactive cells, indicating that CCSP lineage did not overlap with that of the neuroepithelial cells identified by CGRP ( Figure 9F ). Cells of the tracheal glands ( Figure 8B ), which are located at the proximal end of the trachea, were not labeled by expression of doxycycline-induced CRE under the control of either the rat CCSP or human SPC promoter.
The CCSPrtTA and SPCrtTA transgenic lines have been used to conditionally express or delete expression of various genes from the developing and adult respiratory epithelium ( 5 , 7 , 11 , 26 , 27 ). By using either the SPCrtTA or CCSPrtTA transgenic mice, genes can be readily activated or inactivated at specific times during development. The sites and extent of gene activation or deletion can be influenced by the promoter, as well as timing of exposure to doxycycline. The pattern of rtTA expression controls time-dependent CRE activation and recombination that is useful for inducing gene addition or deletion in defined subsets of cells at specific times. The CCSP rtTA conditional system is particularly useful for alteration of gene expression in the conducting airways and for conditional regulation of genes that may be lethal if altered before birth. The present study compares the sites and timing of recombination, using the CCSPrtTA inducible system with previous studies using the SPCrtTA -inducible system ( 21 ). Because these mice are used by many investigators, knowledge of the utility and limitations of the models should be useful in the interpretation of experiments designed to discern gene function using this system.
Timing of Gene Expression and Recombination in CCSPrtTA versus SPCrtTA
CRE-mediated SPCrtTA -controlled recombination occurs before formation of definitive lung buds and mediates recombination throughout the intrapulmonary respiratory epithelium ( 21 ). In contrast, recombination with the CCSPrtTA transgene occurs after E 14.5 and targets subsets of epithelial cells, which are distinct from those targeted in the SPCrtTA mice. Since gene addition or deletion with the SPCrtTA line may occur throughout the epithelium, gene alterations may limit perinatal survival. The CCSPrtTA system targets subsets of epithelial cells later in lung morphogenesis and can be used to bypass potentially lethal gene effects. In the postnatal period, recombination with the CCSPrtTA line occurs more frequently than with the SPCrtTA mouse line ( 21 ), making the CCSPrtTA line more suitable for studies in which recombination is used to study the adult lung.
Cellular Localization of Recombination
Consistent and reproducible labeling patterns were found in the trachea and lung with both SPCrtTA/tetOCRE and CCSPrtTA/tetOCRE recombination systems. Analysis of both models indicated that extensive and widespread random migration or “shuffling” of cells does not occur during lung morphogenesis. In the SPCrtTA transgenic mice, exposure to doxycycline before E 9–10 targets only a few precursor cells that results in labeling of virtually all intrapulmonary epithelial cells ( 21 ). Thus, all intraparenchymal respiratory epithelial cells, except the neuroepithelial cells, are derived from a pool of common endodermal precursor cells identified by recombination with the SP-C promoter.
A rare subset of tracheal epithelial cells can be targeted with the SPCrtTA system in early lung development, providing a useful model for studying paracrine signaling between epithelial and mesenchymal cells of the trachea. For example, expression of Fgf18 ( 28 ) or deletion of Shh ( 7 ) in the tracheal epithelium from E 11.5–12.5 using the SPCrtTA system, resulted in aberrant cartilage ring formation in the trachea close to the laryngeal region. Later in development, SPCrtTA-induced gene expression or deletion in the respiratory epithelium adjacent to cartilaginous structures had no effect on cartilage ring formation, indicating that tracheal epithelial cells influence the sites and extent of tracheal-bronchial cartilage formation only during early developmental stages. Likewise, the labeling pattern of distinct subsets of related cells identified by CCSPrtTA -induced recombination supports the concept that cartilage-related cells provide paracrine signals that influence cell type patterning along the ventral side of the trachea and bronchi. Most GFP-positive cells were found ventrally, overlying the cartilage rings. Previous scanning electron microscopy studies of the rat trachea demonstrate that ciliated cells are concentrated in the tracheal ligament between cartilage rings, which may represent specialized regions of active mucous clearance ( 29 ). In the mouse, ciliated cells in the tracheal ligament were not extensively labeled using the CCSPrtTA mouse line, which suggests that the progenitor cells of these specialized ciliated cells were not targeted with the CCSPrtTA system. A subset of GFP-positive tracheal cells did not express the differentiation markers β-tubulin or CCSP, and may represent cells that have the potential for self renewal after injury, as described by Plopper and coworkers ( 30 ). In the bronchioles, a subset of CCSP-positive cells were also GFP-positive. Their random and scattered location, however, makes them distinct from the label retaining subset of Clara Cells found in association with neuroepithelial bodies in the airway ( 31 ). In addition, the rat CCSP promoter directed gene expression to a subset of Clara cells in contrast to the murine CCSP promoter, which labeled all of the Clara cells ( 31 ). Whether this subset of GFP-positive Clara Cells serves as a source of progenitor cells in the airway remains to be investigated.
Sites of rtTA Expression and Recombination Do Not Correlate in Adult Lung
In the rat, endogenous CCSP is expressed in a subset of alveolar type II cells, whereas in the mouse lung CCSP expression is restricted to Clara cells in the conducting airways. Transgenes driven by the rat CCSP promoter in the mouse were expressed in both conducting airways and in alveolar type II cells. In the postnatal period, rtTA expression directed by the rat CCSP promoter was detected at high frequency on the ventral side of the trachea. In the postnatal period recombination did not occur in Clara cells of the conducting airways, even though these cells do express endogenous CCSP. Thus, in the postnatal lung, rat CCSP promoter–driven rtTA expression and CRE-mediated recombination do not entirely overlap with sites and extent of endogenous mouse CCSP expression, indicating that either labeling occurs in precursor cells that differentiate into various cell types or that labeling occurs only in a subset of differentiated cells. Similarly in the alveolar region of the adult lung, only a subset of alveolar type II cells, which expresses CRE controlled by CCSPrtTA or SPCrtTA, undergoes recombination. In contrast to the paucity of doxycycline-induced recombination observed in type II cells in adult SPCrtTA mice, recombination occurred frequently in the adult CCSPrtTA mouse lung. Furthermore, labeling in alveolar type I cells was observed in SPCrtTA but not CCSPrtTA transgenic mice. Based on these results, we postulate that the CCSPrtTA and the SPCrtTA transgenes target distinct subsets of alveolar type II cells. The present SPCrtTA and CCSPrtTA systems are being widely used; therefore, characterization of the sites and timing of expression will be useful for design and interpretation of experiments. Because the sites and extent of gene expression can be controlled by the timing and duration of doxycycline treatment, the SPCrtTA and CCSPrtTA systems can be used to control differential gene-expression in subsets of lung epithelial cells. CCSPrtTA and SPCrtTA, when used with tetOCRE and other Tet operator–driven genes have been useful for conditional control of gene expression or for permanently altering gene expression in various subsets of respiratory epithelial cells in both fetal and postnatal mice. Moreover, SPCrtTA and CCSPrtTA transgenic mice can be used to manipulate gene expression during well-defined periods of development to target subsets of alveolar progenitor cells by the administration of doxycycline.
The present labeling experiments demonstrated an unexpected diversity of labeling and gene expression amongst morphologically indistinguishable cells. In the trachea, distinct dorsal–ventral and horizontal patterning of labeled cells was observed in the CCSPrtTA transgenic mouse line, while a proximal–distal gradient of longitudinal stripes was observed in the SPCrtTA transgenic mice ( 21 ). In the lung parenchyma, subpopulations of both ciliated and Clara cells were identified that were not directly lineage related. Likewise, sites and extent of cell types labeled with the CCSPrtTA or the SPCrtTA system were distinct. We conclude that the CCSPrtTA system is most useful for targeting gene expression or deletion later in development and in the adult lung. The SPCrtTA system is well suited for alterations of gene expression during embryonic lung development.
Acknowledgments
The authors thank Andreas Nagy, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Canada for providing the tetOCre transgenic mice. They also thank Corrinne G. Lobe, Sunnybrook and Women's College Health Science Centre, Toronto, Ontario, Canada for providing the ZAP and ZEG reporter mice.
This work was supported by the National Institutes of Health HL 56387 and the Research and Development Program of the Cystic Fibrosis Foundation.
Conflict of Interest Statement : None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 September 2018
Characterization of a lung epithelium specific E-cadherin knock-out model: Implications for obstructive lung pathology
- S. Post 1 , 2 , 4 na1 ,
- I. H. Heijink 1 , 2 , 3 na1 ,
- L. Hesse 1 , 2 ,
- H. K. Koo 4 ,
- F. Shaheen 4 ,
- M. Fouadi 4 ,
- V. N. S. Kuchibhotla 1 , 2 ,
- B. N. Lambrecht 5 , 6 , 7 ,
- A. J. M. Van Oosterhout 1 , 2 ,
- T. L. Hackett 4 na2 &
- M. C. Nawijn 1 , 2 na2
Scientific Reports volume 8 , Article number: 13275 ( 2018 ) Cite this article
4284 Accesses
38 Citations
4 Altmetric
Metrics details
- Molecular biology
The airway epithelium regulates responses to aeroallergens, acting as a physical and immunological barrier. In asthma, epithelial barrier function and the expression of adherens junction protein E-cadherin is compromised, but it is unknown whether this is cause or consequence of the disease. We hypothesized that airway epithelial loss of E-cadherin is a critical step in the development of manifestations of asthma. We generated a transgenic mouse model with conditional loss of E-cadherin in lung epithelial cells at birth and onwards. We observed normal lung development at the time of birth in mice lacking E-cadherin in the lung epithelium. However, E-cadherin deficiency led to progressive epithelial damage in mice growing into adulthood, as evidenced by airway epithelial denudation, decreased zonula occludens (ZO)-1 expression, loss of ciliated cells, and enlarged alveolar spaces. In addition, spontaneous goblet cell metaplasia with mucus production was observed. These epithelial changes were accompanied by elevated levels of the epithelial-derived chemokine CCL17, infiltration of eosinophils and dendritic cells, and mucus production. In conclusion, loss of E-cadherin induces features in the lung reminiscent of those observed in asthma, indicating that the disruption of E-cadherin-mediated cell-cell contacts may play a key role in the development of asthma manifestations.
Similar content being viewed by others
Loss of E-cadherin is causal to pathologic changes in chronic lung disease
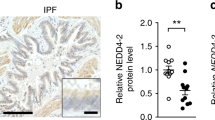
Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice
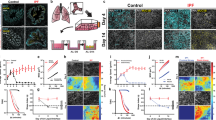
Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional
Introduction.
The airway epithelium forms a structural and immunological barrier against environmental insults, such as inhaled allergens, viruses and particular matter. The pseudostratified airway epithelial layer that lines the conducting airways is composed of basal/progenitor epithelial cells, columnar ciliated cells and mucus secreting cells, of which the latter two are responsible for mucociliary removal of inhaled environmental particulates. Epithelial barrier function is maintained by formation of tight and adherens junctions. Tight junctions are comprised of proteins such as occludin, zonula occludens (ZO)-1 and claudins, and maintain a size- and ion selective barrier, regulating the permeability of the epithelium 1 , 2 . Adherens junctions, which contain the transmembrane protein E-cadherin, are critical for maintaining apical-basolateral polarization and adhesion to neighboring cells 3 . E-cadherin-mediated contacts are thought to provide the architecture required to form the other junctional complexes 4 . Additionally, E-cadherin has been shown to suppress intracellular signaling pathways, regulating epithelial activation, proliferation and differentiation 3 . We have previously shown that siRNA down-regulation of E-cadherin increases epidermal growth factor receptor (EGFR) activation, inducing expression of the pro-allergic C-C motif ligand 17 (CCL17) and thymic stromal lymphopoietin (TSLP) in human bronchial epithelial cells 5 .
In asthma, the airway epithelial barrier is often compromised, with epithelial denudation, goblet cell metaplasia, ciliary dysfunction and reduced expression of E-cadherin and ZO-1 1 , 6 , 7 , 8 , 9 , 10 . This compromised airway epithelial barrier function has already been observed in children with asthma 11 . Asthma is a chronic inflammatory disease of the airways, characterized by eosinophilia, goblet cell metaplasia, airway hyperreactivity and airway remodeling including damage of the airway epithelium. Asthma susceptibility has a genetic component, and the disease is triggered by a hypersensitivity reaction following the interaction of genetic and specific environmental factors, such as aeroallergens, leading to a type-2 immune response.
Aeroallergens are known to directly and indirectly cause disruption of E-cadherin-mediated epithelial junctions 3 . We previously reported that asthma-derived as well as transforming growth factor-beta (TGF)-β-treated airway epithelial cells 12 , 13 are more prone to house dust mite (HDM)-induced barrier dysfunction. We further observed that the ability of HDM to induce barrier dysfunction is associated with its ability to induce allergic sensitization and manifestations of asthma in vivo 14 . Intranasal HDM exposure has been reported to induce E-cadherin loss in vivo and lead to epithelial-to-mesenchymal transition (EMT), a process involved in tissue repair that has been implicated in airway remodeling in asthma 15 , 16 . It is currently unknown whether the loss of epithelial barrier function in asthma patients is a consequence or cause of the disease.
Since E-cadherin regulates airway epithelial structure, barrier function and innate immune responses 3 , 5 , 12 , 15 , 16 , we hypothesized that loss of airway epithelial loss of E-cadherin by itself is a critical step leading to the development of asthma manifestations. To test our hypothesis, we generated lung epithelial specific, conditional E-cadherin deficient mice, as germ-line E-cadherin loss has previously been shown to be lethal 17 . We used sftpc-rtTA/ (tetO) 7 -Cre mice, where pregnant dams were maintained on doxycycline to allow recombination of the conditional allele throughout the conducting airways and parenchyma, as all epithelial cells express surfactant protein C (SFTPC) during early development. Within the airways, SFTPC positive non-ciliated secretory cells called Club cells act as progenitor cells for the goblet and ciliated cells that are responsible for mucociliary clearance 18 , 19 , 20 . Within the alveolar structures, SFTPC positive alveolar type II (ATII) cells serve as progenitors for the alveolar type I (ATI) cells that are responsible for gas exchange 21 . We investigated whether loss of E-cadherin in SFTPC expressing cells during early stages of life is accompanied by an altered airway epithelial phenotype and increased airway inflammation and remodeling later on in life.
Generation of E-cadherin knockout (Cdh1−/−) mice
Conditional E-cadherin knock-out mice ( Cdh1 fl/fl , B6.129-Cdh1tm2Kem/J), backcrossed onto the C57Bl/6 J background were purchased from Jackson Laboratory (Bar Harbor, ME). The compound transgenic sftpc-rtTA/ (tetO) 7 -Cre mice that express the reverse tetracycline trans-activator (rtTA) under control of the rat SFTPC promoter were kindly provided by Prof. Geoffrey Whittset. The SFTPC promoter is expressed as early as day 10 of gestation in epithelial cells of the primordial lung buds 22 , 23 , causing all lung epithelial cells to express Cre recombinase under control of the tet operator (tetO) 23 . Cdh1 fl/fl were crossed for two generations with sftpc-rtTA/ (tetO) 7 -Cre mice to obtain both homozygous Cdh1 fl/fl Cre + mice ( sftpc-rtTA/(tetO-) 7 Cre + /Cdh1 fl/fl ) and Cdh1 fl/fl Cre − ( sftpc-rtTA/(tetO) 7 -Cre − /Cdh1 fl/fl ) mice as littermate controls. Pregnant dams were maintained on doxycycline 24 , to allow recombination of the conditional allele in all lung epithelial cells from the earliest developmental stages onward in Cre + progeny. Genotyping was performed as described in the online supplementary information.
Mice were kept under specific pathogen-free conditions and maintained on a 12-hour light-dark cycle, with food and water ad libitum . Cdh1 fl/fl Cre + and Cdh1 fl/fl Cre − animals were mated and pregnant dams were fed doxycycline-containing chow (200 mg/kg; Bio-Serv, Frenchtown, NJ) until the end of the experiment. Mice were killed by anesthetizing the animals with isoflurane/oxygen (Nicholas Piramal India Ltd., London, UK) at the indicated time points (n = 5–7 mice per group), and bleeding them before removing the lungs. All animal experiments were reviewed and approved by The Institutional Animal Care and Use Committee of the University of Groningen (The Netherlands) and Ghent (Belgium). All experiments were performed in accordance with relevant guidelines and regulations.
Measurement of airspace enlargement
The mean linear intercept (Lm) was used as morphometric parameter for quantifying airspace size. Histological lung sections, stained with hematoxylin, were imaged using Aperio Scanscope XT and representative samples (three per histological section) were obtained using the non-biased, Systematic Uniform Random Sampling (SURS) method as further detailed in the online supplementary information.
Immunochemistry, scanning electron microscopy (SEM), flow cytometry and cytokine assays in mouse lung tissue
Lungs were collected for morphometry analysis at day (D)0, week (W)2, W4 and W10 and processed for immunohistochemistry, flow cytometry (only for W2 and W4), see figure S1 in the online data supplement for the gating strategy), SEM, PCR or ELISA (only for W4) as described in the online supplementary information. For the immunohistochemical analysis, staining and number of total airway epithelial cells per length of basement membrane was quantified using Image-Pro Plus. Cells per 500 µm basement membrane (5–25 counts per lung fragment) were counted. Mucus production was assessed by alcian blue staining and goblet cells were stained by Periodic-acid Schiff (PAS).
The non-parametric Mann Whitney U test was performed to assess for significant differences between the Cdh1 fl/fl Cre + and Cdh1 fl/fl Cre − mice for all analyses. P < 0.05 was considered statistically significant.
E-cadherin loss in the airway epithelium causes reduced airway epithelial cell numbers and epithelial denudation
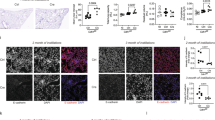
To assess how the loss of E-cadherin within the lung epithelium affects lung development, mice were euthanized on the day of birth (Day (D)0) and at week 4 and 10 of age (W4;W10). At birth, histologically the Cdh1 fl/fl Cre + mice displayed normal lung development and the loss of E-cadherin expression in the airway epithelium had no major effect on the development of the bronchi and bronchioles of the lung at this point. Staining for E-cadherin showed presence of E-cadherin in the airway epithelium of Cdh1 fl/fl Cre − control littermates at birth, while E-cadherin was significantly reduced and only present in a few scattered airway epithelial cells of the Cdh1 fl/fl Cre + mice (Fig. 1A,B ). At 4 W, airway epithelial E-cadherin expression remained significantly reduced (Fig. 1A,C ), while E-cadherin expression was restored at W10 in the few remaining cells lining the airways (Fig. 1A,D ). Accordingly, we observed that E-cadherin mRNA expression was reduced in lung homogenates of Cdh1 fl/fl Cre + mice at W4 (Fig. 1E ), but not at W10 (Fig. 1F ), while we were unable to collect lung tissue for RNA isolation at D0 because of the small size of the lungs.
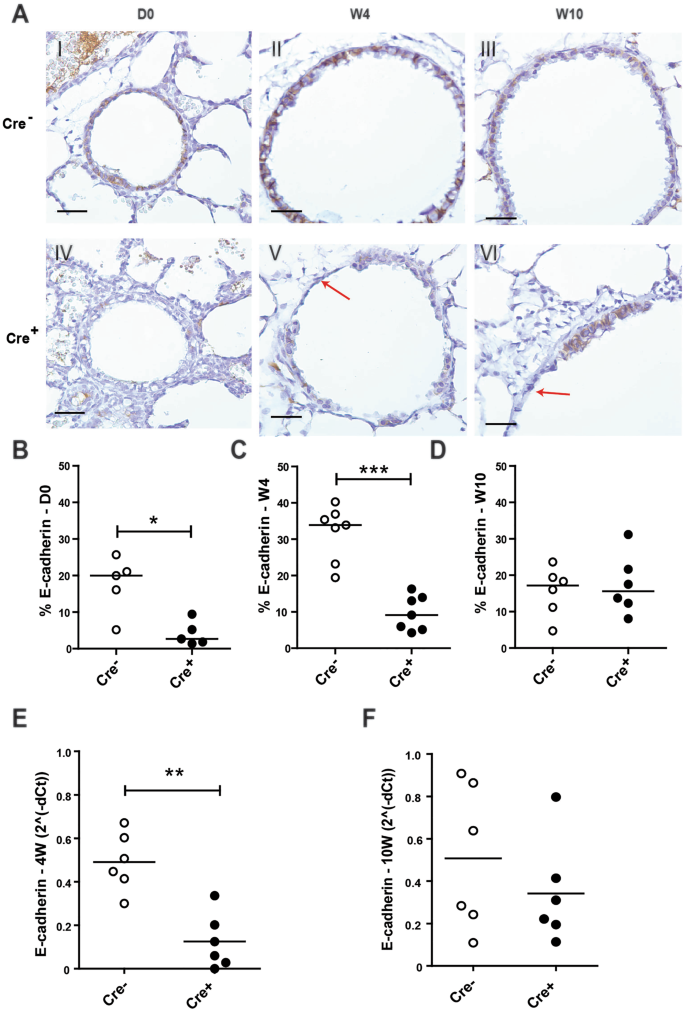
E-cadherin expression in the lungs of E-cadherin knockout (Cdh1 fl/fl Cre + ) and wild type (Cdh1 fl/fl Cre − ) mice (n = 5–7 per group). ( A ) E-cadherin staining of airway epithelium at day (D)0 (I,IV), week (W) 4 (II,V) and W10 (III,VI) of Cdh1 fl/fl Cre − (I-III)/Cre + (IV-VI) mice. Red arrows indicate epithelial denudation areas. Scale bars: 10 μm. Percentage of E-cadherin positive cell numbers as analyzed by Image-Pro Plus at ( B ) D0, ( C ) W4 and ( D ) W10. mRNA expression of E-cadherin ( cdh1 ) in lung homogenates at ( E ) W4 and ( F ) W10. E-cadherin levels were related to the housekeeping genes hprt1 and pgk1 and expressed as 2 −ΔCt . Medians are indicated. *p < 0.05, **p < 0.01 and ***p < 0.001 as assessed by the Mann Whitney U test.
Interestingly, from the age of W4 onward we observed a damaged airway epithelial layer throughout the lung of the Cdh1 fl/fl Cre + mice, with areas showing complete epithelial denudation (Fig. 1A ).
We also assessed the effect of E-cadherin loss on tight junction formation by the expression of ZO-1 within the airway epithelium. While there was widespread expression of ZO-1 at D0, by W4 the percentage of positive staining for ZO-1 in Cdh1 fl/fl Cre + mice was significantly lower compared to control littermates. At W10, ZO-1 expression was no longer significantly decreased in Cdh1 fl/fl Cre + mice (Fig S1 in the online data supplement).
Quantification of the number of total airway epithelial cells per length of basement membrane revealed that Cdh1 fl/fl Cre + mice were in fact born (D0) with significantly reduced airway epithelial cell numbers compared to their control littermates (Fig. 2A ), and that the airways of these mice became more denuded at W4 and W10 (Fig. 2B-C ). The severity of the epithelial denudation was illustrated by SEM analysis, which revealed almost complete loss of epithelial lining in large regions of the airway lumen in the Cdh1 fl/fl Cre + mice compared to their control littermates already at the age of W4 (Fig. 2D ).

Characterization of airway epithelium in E-cadherin knockout (Cdh1 fl/fl Cre + ) and wild type (Cdh1 fl/fl Cre − ) mice. Analysis of percent total cells in the epithelium of Cdh1 fl/fl Cre − /Cre + mice at ( A ) day (D)0, ( B ) W4 and ( C ) W10, where the total cell count per 500 µm basement membrane (5–25 counts per lung fragment) per mouse is presented as percentage of the group average. Medians are indicated. ( D ) Electron microscopy images at W4, magnifications are as indicated. White arrows indicate epithelial denudation areas. ( E ) Hematoxylin staining in lung tissue of Cdh1 fl/fl Cre − /Cre + mice at W8, magnifications are as indicated. *p < 0.05, **p < 0.01 and ***p < 0.001 between the Cdh1 fl/fl Cre + and Cdh1 fl/fl Cre − mice (n = 5–7 per group) as assessed by the Mann Whitney U test.
Furthermore, when investigating lung morphology by hematoxylin staining, we observed striking infiltration of inflammatory cells (Fig. 2E ).
E-cadherin deficiency in alveolar type II epithelium leads to increased mean airspace size
In addition to the loss of E-cadherin in airway epithelium, we observed E-cadherin loss in ATII cells, which normally express E-cadherin at each time point 25 . This demonstrates that loss of E-cadherin was induced throughout the lung epithelium. Microscopic evaluation of the lung parenchyma revealed that Cdh1 fl/fl Cre + mice displayed increased mean airspace size (Lm) at W4 and W10 compared to their control littermates (Fig. 3A ). Cdh1 fl/fl Cre + mice demonstrated loss of E-cadherin in all intrapulmonary epithelial cells, including ATII cells (Fig. 3A ). Histology analysis and measurement of mean airspace size (Lm) showed that, although Cdh1 fl/fl Cre + mice had a similar Lm to control Cdh1 fl/fl Cre − littermates at the time of birth (Fig. 3B ), Lm significantly increased in the Cdh1 fl/fl Cre + mice at W4 and W10 compared to control Cdh1 fl/fl Cre − littermates (Fig. 3C-D ) as further illustrated by HE staining (Fig. 3E ). These results indicate that the loss of E-cadherin in ATII cells, the only alveolar cell type to express E-cadherin 25 , affects the overall lung structure inducing emphysematous lesions.

Characterization of alveoli in E-cadherin knockout (Cdh1 fl/fl Cre + ) and wild type (Cdh1 fl/fl Cre − ) mice. ( A ) E-cadherin staining of alveoli at day (D)0 (I,IV), week (W)4 (II,V) and W10 (III,VI) of Cdh1 fl/fl Cre − (I-III)/Cre + (IV-VI) mice. Scale bars: 10 μm. Measurement of mean linear intercept (Lm) in the lung tissue at ( B ) D0, ( C ) W4 and ( D ) W10. ( E ) Hematoxylin staining in lung tissue of Cdh1 fl/fl Cre − /Cre + mice at D0, W4 and W10. Scale bars, 100 μm. An average Lm was calculated from 3 randomly selected regions within each lung. Lm = Number of lines × Length of test line/Number of intersections. A greater Lm value therefore indicates increased air-space size.Medians are indicated. *p < 0.05, **p < 0.001 and ***p < 0.0001 between the Cdh1 fl/fl Cre + and Cdh1 fl/fl Cre − mice (n = 5–7 per group) as assessed by the Mann Whitney U test.
E-cadherin loss leads to spontaneous loss of ciliated cells and mucus hypersecretion indicating development of goblet cell metaplasia
To assess the effect of E-cadherin loss on airway epithelial differentiation, we performed immunohistochemistry staining using the cilia marker acetylated α-tubulin. We observed that acetylated α-tubulin expression was significantly reduced upon E-cadherin loss of in the airways of Cdh1 fl/fl Cre + mice at W4 and W10 but not at D0, compared to their Cdh1 fl/fl Cre − control littermates (Fig. 4A–C ). This was supported by scanning EM microscopy, revealing that E-cadherin deficiency induced loss of ciliated cell organization within the airways, with remaining ciliated cells scattered in a disorganized fashion along the airways, contrasting the rows of ciliated cells placed at regular intervals along the wild-type control airways (Fig. 4D ). In contrast to the observed depletion of ciliated airway epithelial cells, we observed an increase in mucus production, as measured by increased alcian blue staining in Cdh1 fl/fl Cre + mice at W8 (Fig. 4F–H ).
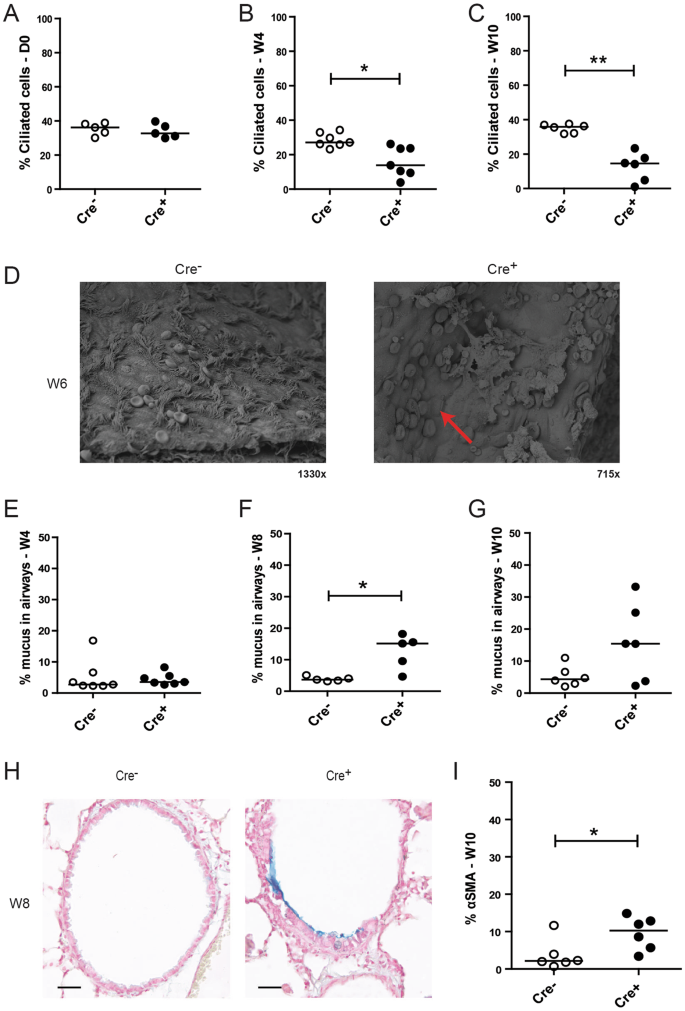
Loss of ciliated cells and increased mucus production in E-cadherin knockout (Cdh1 fl/fl Cre + ) and wild type (Cdh1 fl/fl Cre − ) mice. Analysis of percent ciliated cells at ( A ) day (D)0, ( B ) week (W)4 and ( C ) W10, where the ciliated cell count per mouse was calculated per 500 µm basement membrane (5–25 counts per lung fragment) as percentage of the group average. ( D ) Electron microscopy images at Week W6. Red arrow indicates loss of ciliated cells. Measurement of percentage alcian blue staining in the airways of Cdh1 fl/fl Cre − /Cre + mice at E) W4, ( F ) W8 and ( G ) W10, ( H ) Representative image of alcian blue staining of Cdh1 fl/fl Cre − /Cre + mice at W8. Scale bars, 10 μm. ( I ) Percentage of alpha-smooth muscle actin (α-SMA) positive cell numbers as analyzed by Image-Pro Plus in the airways of Cdh1 fl/fl Cre − /Cre + mice at W10. Medians are indicated. *p < 0.05 and **p < p0.01 between the Cdh1 fl/fl Cre + and Cdh1 fl/fl Cre − mice (n = 5–7 per group) as assessed by the Mann Whitney U test.
In addition, we assessed the expression of alpha-smooth muscle actin (α-SMA) within the airway epithelium to study if E-cadherin loss induced a more mesenchymal phenotype. We observed an increased expression of α-SMA in E-cadherin deficient airway epithelial cells at age W10, which was not present in the airways of Cre − control littermates (Figure 4 J I).
Loss of E-cadherin results in a pro-inflammatory epithelial phenotype and innate immune activatio
As we hypothesized that loss of E-cadherin expression leads to increased production of pro-inflammatory cytokines and activation of an innate immune response, we next investigated the levels of epithelial derived chemokines and recruitment of inflammatory cells into the lung. We observed a significant increase in type-2 T cell attractant CCL17 in Cdh1 fl/fl Cre + mice compared to Cdh1 fl/fl Cre − mice when mice reached the age of W4 (Fig. 5A ), while levels of TSLP, C-C motif chemokine 11 (CCL11) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were not different between the groups (Fig. 5B–D ).
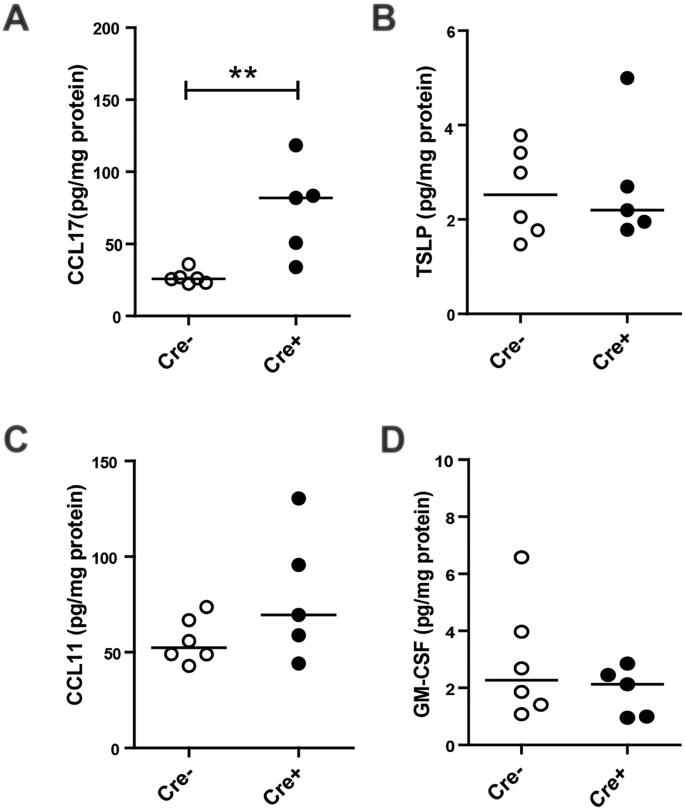
Cytokine responses in E-cadherin knockout (Cdh1 fl/fl /Cre + ) and wild type (Cdh1 fl/fl Cre − ) mice.Enzyme-linked immunosorbent assay (ELISA) analysis for ( A ) Chemokine (C-C motif) ligand 17 (CCL17), ( B ) Thymic stromal lymphopoietin (TSLP), ( C ) C-C motif chemokine 11 (CCL11) and ( D ) Granulocyte-macrophage colony-stimulating factor (GM-CSF) levels in whole lung homogenates at week (W)4. Medians are indicated. **p < 0.01 between the Cdh1 fl/fl Cre + and Cdh1 fl/fl Cre − mice (n = 5–7 per group) as assessed by the Mann Whitney U test.
Next, we performed a detailed flow cytometric characterization of the infiltrated inflammatory cells observed by hematoxylin staining (Fig. 2E ) in the lung tissue at W2 and W4 (Fig. 6A–H ). We found that eosinophil numbers increase as early as at W2 in the lungs of Cdh1 fl/fl Cre + mice (Fig. 6A ) compared to Cdh1 fl/fl Cre − mice. This increase reached statistical significance at W4 (Fig. 6E ). Additionally, we observed that the total dendritic cell (DC) population was significantly increased in the lungs of the Cdh1 fl/fl Cre + mice compared to their control littermates at W2 (Fig. 6B ), but no longer at W4 (Fig. 6F ). Interestingly, phenotyping the DC subpopulations at W2 showed that the CD103 + conventional (c)DC population was significantly elevated in Cdh1 fl/fl Cre + lungs compared to Cdh1 fl/fl Cre − lungs (Fig. 6C ), while the inflammatory monocyte-derived DCs (moDCs) were significantly elevated in the Cdh1 fl/fl Cre + lungs at W4 (Fig. 6G ). On the other hand, no effect was observed for CD11b + cDCs at either 2 W or 4 W (Fig. 6C,G ). Furthermore, total lymphocyte, neutrophil and macrophage numbers in the lungs were not affected by E-cadherin deficiency at any age (Fig. 6A,D,E,H ). Together, these data show that E-cadherin deficiency leads to pro-inflammatory activation of the lung epithelium that specifically attracts eosinophilic granulocytes and inflammatory DCs into the airways.

Inflammatory responses in E-cadherin knockout (Cdh1 fl/fl Cre + ) mice) and wild type (Cdh1 fl/fl Cre − ) mice. Flow cytometry analysis for inflammatory cells in whole lungs of Cdh1 fl/fl Cre − /Cre + mice at ( A ) week (W)2 and ( E ) W4. Dendritic cell (DC) populations at ( B ) W2 and F ) W4 and DC sub-populations (CD103 + conventional (c ) DCs, CD11b + DCs and monocyte-derived (mo)DCs) at ( C ) W2 and ( G ) W4. Alveolar macrophages at ( D ) 2 W and ( H ) 4 W. Medians are indicated. *p < 0.05, **p < 0.01 or p value is as indicated between the Cdh1 fl/fl Cre + and Cdh1 fl/fl Cre − mice (n = 5–7 per group) as assessed by the Mann Whitney U test.
We show for the first time that conditional loss of E-cadherin results in airway epithelial denudation, loss of ciliated cells, spontaneous induction of mucus hypersecretion indicating goblet cell metaplasia and eosinophilic inflammation, all characteristics of asthma. Conditional loss of E-cadherin in the lung epithelium did not affect lung development up to birth. However, as the Cre + mice aged, they developed airspace enlargement characteristic of emphysematous lesions associated with loss of E-cadherin in ATII cells.
Our current study supports the hypothesis that loss of epithelial E-cadherin as observed in asthma has important consequences, contributing to the pathogenesis of asthma, integrating structural and immunological regulatory functions within the airway epithelium 3 . Although E-cadherin is known to be critical for organogenesis of several epithelial tissues 17 , 26 , we observed a roughly normal lung anatomy at the time of birth in the Cdh1 fl/fl Cre + mice, with only very few epithelial cells expressing E-cadherin. This might be explained by the timing of E-cadherin loss in our model. A previous study showed that SFTPC-CAT expression at high levels was first detected as early as day 10 of gestation (E10) in epithelial cells (both airway epithelial cells and ATII cells) of the primordial lung buds 23 . Therefore, we anticipate that E-cadherin deficiency would not be introduced until the second week of gestation (~E10), when the primary lung epithelium has already formed, allowing normal development of the lung. Nonetheless, SFTPC-driven Cre expression during embryonic development has previously been shown to induce recombination in all epithelial cells contributing to lung development, and was passed on to all lung epithelial cell into adulthood 23 . These data may explain our observation of normal lung development at birth, even with almost complete E-cadherin loss in the lung epithelium.
The lung morphology markedly changed when Cdh1 fl/fl Cre + mice reached an adult age, confirming that E-cadherin is a central regulator of lung structure and inflammation. Its loss resulted in epithelial denudation, decreased ZO-1 expression, loss of ciliated cell numbers and organization, goblet cell metaplasia, and inflammatory cell infiltration in the conducting airways as well as enlarged airspace size. These data are in line with a recent study demonstrating that E-cadherin is necessary for the differentiation of Club cells 27 , acting as progenitor cells for ciliated cells 28 . Postnatal inactivation of E-cadherin in mice impaired the repair of the conducting airway epithelium after site-specific Club cell injury 27 . We anticipate that a repair response is provoked in Cre + mice upon the loss of E-cadherin, requiring proliferation and re-differentiation of Club cell s progenitors to reconstruct a polarized, functionally intact ciliated epithelial layer. Lack of this process in Cdh1 fl/fl Cre + mice could be a consequence of E-cadherin down-regulation in Club cells. Of note, basal side population cells that express breast cancer resistance protein (BCRP1), cytokeratin (CK)5 and p63 have been proposed as a major airway epithelial stem cell involved in repair of the conducting airways 8 . These BCRP1 + CK5 + p63 + cells are also capable of generating Club cell progenitors, but do not express transcription factors such as SFPTC, which are turned on later during development or regeneration. Therefore, these side population cells may be responsible for the recurrence of E-cadherin expressing cells in the bronchial epithelial lining of Cdh1 fl/fl Cre + mice at adult age, since these and their daughter cells are not affected by doxycycline-induced E-cadherin deficiency. Additionally, epithelial injury has been shown to induce Club cell metaplasia into mucus-producing cells in order to restore the damage 20 . Our data suggest that E-cadherin deficiency results in spontaneous goblet cell metaplasia, indicative of mucus hypersecretion by Club cells. In addition, we observed α-SMA expression in the epithelial layer, which could reflect a repair mechanism involving the transition to a more mesenchymal phenotype (e.g. EMT). In line, postnatal inactivation of E-cadherin was previously shown to induce WNT/β-catenin signaling, an important component of EMT, and bronchiolar metaplasia 27 . Expression of activated β-catenin in airway epithelium may not only result in EMT-like features, but has also been shown to result in goblet cell metaplasia through a mechanism involving down-regulation of mucus repressor Foxa2 29 . Thus, the spontaneous goblet cell metaplasia in the Cdh1 fl/fl Cre + mice could be explained by dysregulated β-catenin signalling and abnormal repair responses. Another mechanism that could be involved in the observed mucus hypersecretion upon E-cadherin deficiency may be an increase in EGFR signalling, as we previously observed that loss of E-cadherin expression by airway epithelium in vitro leads to increased EGFR signalling 30 , which has been implicated in mucus production 31 . E-cadherin deficiency also resulted in decreased tight junctional protein ZO-1, which is in line with previous studies that have shown that loss of E-cadherin impairs formation of tight junctions, leading to barrier dysfunction and reduced expression of ZO-1 3 . These data along with studies in asthmatic airway epithelial biopsies that have previously shown decreased E-cadherin and ZO-1 expression 6 further support the role of E-cadherin loss in compromised airway epithelial barrier function in asthma.
Alongside the airway epithelial changes, lungs of Cdh1 fl/fl Cre + mice showed enlarged airspaces with thinned and damaged septa, which became more pronounced with age. While asthma is considered mainly as an airway obstructive lung disease, a limited number of studies demonstrated parenchymal abnormalities in asthma patients, including centrilobular micronodules, mosaic perfusion and increased percent lower attenuation areas related to emphysematous changes 32 , 33 . In the parenchyma, specifically ATII cells express SFTPC, thus the SFTPC promotor ensured E-cadherin deficiency in ATII cells in our model. ATII cells function as a progenitor for alveolar type I epithelium in rodents and humans 34 , 35 . In addition, ATII cells are responsible for the production of pulmonary surfactant, which is required for epithelial integrity, adapting to breathing after birth by reducing surface tension in the alveolus 36 . Disruption of E-cadherin in mature ATII cells was previously shown to lead to diffuse hyperplasia and airspace enlargement 37 . Similarly, expression of activated β-catenin and of Foxa2 expression in mouse lungs resulted in aberrant ATII differentiation and airspace enlargement 29 , 38 . No formal study has investigated whether the mechanism underlying the emphysematous changes in E-cadherin deficient mice involves loss of SFTPC + ATII cells or deregulated β-catenin signaling, needing further investigation.
In addition to direct effects on airway epithelium, we observed a striking pro-inflammatory response of the airway epithelium, as evidenced by the production of CCL17, finally resulting in airway inflammation characterized by eosinophils and inflammatory dendritic cells. We have previously shown that down-regulation of E-cadherin expression in bronchial epithelial cells results in increased EGFR-induced expression of CCL17 and TSLP 39 . Additionally, E-cadherin loss may lead to activation of the nuclear factor-kappa B (NF-κB) and MAPK signaling pathways 40 . Both EGFR and NF-κB-mediated signaling can induce secretion of pro-inflammatory cytokines and chemokines, including CCL17, suggesting that the induction of E-cadherin loss by itself is sufficient to induce pro-inflammatory activity of the airway epithelium 12 . In this way, loss of E-cadherin could lead to type-2 T cell-mediated eosinophilia. Alternatively, type-2 innate lymphocyte cells could be responsible for the observed eosinophils 41 , but this requires further investigation and will be subject of future studies. Of interest, loss of Foxa2 expression in lung epithelium in a mouse model not only induced goblet cell metaplasia, but also spontaneous pulmonary eosinophilic inflammation, recruitment of mDCs and type-2 T cells and increased levels of various chemokines, including CCL17 42 . As Foxa2 is known to activate the E-cadherin promoter 43 , loss of E-cadherin might play a central role in this phenotype given the striking similarities with our model. In addition, to the lung epithelium, CCL17 can be produced by CD103 + cDC and moDCs populations, which play a predominant pro-inflammatory role in the development of asthma 44 . We observed significantly elevated levels of both CD103 (α E ß 7 integrin) + cDC and moDCs in the lungs of the young adult Cdh1 fl/fl Cre + mice compared to their littermate controls. This may contribute to innate immune activation in the E-cadherin-deficient mice.
Our results in this novel mouse model of engineered loss of epithelial integrity strongly suggest that E-cadherin delocalization by itself may be sufficient for the development of airway inflammation. In line, structural changes in the airway epithelium as well as airway eosinophilic inflammation have been observed in the lungs of children before allergic asthma was even diagnosed 45 . In addition, a study of Ierodiakonou et al . showed that CDH1 gene polymorphisms are associated with airway remodeling and inflammation as well as lung functions in asthmatic patients 46 , but only in patients using inhaled corticosteroids. Collectively, these studies and our data suggest that disruption of epithelial cell-cell contacts with junctional loss of E-cadherin are a crucial event in the development of asthma. In future studies, it will be of interest to assess whether the loss of E-cadherin also increases allergic sensitization.
Taken together, our data show that loss of E-cadherin in lung epithelia induces spontaneous changes that have remarkable characteristics of an asthmatic phenotype, including progressive loss of airway epithelial cells, spontaneously mucus hypersecretion and eosinophilic airway inflammation. This indicates that loss of E-cadherin in the airway epithelium is not merely a consequence of disease, but actively contributes to the pathogenesis of asthma, identifying E-cadherin as a novel target for future therapeutic strategies.
Dataset Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Goto, Y. et al . Dislocation of E-cadherin in the airway epithelium during an antigen-induced asthmatic response. Am. J. Respir. Cell Mol. Biol. 23 , 712–718 (2000).
Article PubMed CAS Google Scholar
Tam, A., Wadsworth, S., Dorscheid, D., Man, S. F. & Sin, D. D. The airway epithelium: more than just a structural barrier. Ther. Adv. Respir. Dis. 5 , 255–273 (2011).
Article PubMed Google Scholar
Nawijn, M. C., Hackett, T. L., Postma, D. S., Van Oosterhout, A. J. & Heijink, I. H. E-cadherin: gatekeeper of airway mucosa and allergic sensitization. Trends Immunol. 32 , 248–255 (2011).
Tunggal, J. A. et al . E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 24 , 1146–56 (2005).
Article PubMed PubMed Central CAS Google Scholar
Heijink, I. H. et al . Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J. Immunol. (Baltimore, Md. 1950) 178 , 7678–7685 (2007).
Article CAS Google Scholar
de Boer, W. I. et al . Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can. J. Physiol. Pharmacol. 86 , 105–112 (2008).
Hackett, T. L. et al . Characterization of side population cells from human airway epithelium. Stem Cells 26 , 2576–2585 (2008).
Article PubMed PubMed Central Google Scholar
Thomas, B. et al . Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J. Allergy Clin. Immunol . 126 (2010).
Hackett, N. R. et al . The human airway epithelial Basal cell transcriptome. PLoS One 6 , e18378 (2011).
Article ADS PubMed PubMed Central CAS Google Scholar
Xiao, C. et al . Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 128 , 512–549 (2011).
Looi, K. et al . Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin. Exp. Allergy . https://doi.org/10.1111/cea.13097 (2018).
Post, S. et al . House dust mite-induced calcium signaling instigates epithelial barrier dysfunction and CCL20 production. Allergy 68 , 1117–1125 (2013).
PubMed CAS Google Scholar
Heijink, I. H., Postma, D. S., Noordhoek, J. A., Broekema, M. & Kapus, A. House dust mite-promoted epithelial-to-mesenchymal transition in human bronchial epithelium. Am. J. Respir. Cell Mol. Biol. 42 , 69–79 (2010).
Post, S. et al . The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax 67 , 488–495 (2012).
Hackett, T. L. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr. Opin. Allergy Clin. Immunol. 12 , 53–59 (2012).
Johnson, J. R., Roos, A., Berg, T., Nord, M. & Fuxe, J. Chronic respiratory aeroallergen exposure in mice induces epithelial-mesenchymal transition in the large airways. PLoS One 6 , e16175 (2011).
Larue, L., Ohsugi, M., Hirchenhain, J. & Kemler, R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. USA 91 , 8263–8267 (1994).
Article ADS PubMed CAS Google Scholar
Holgate, S. T. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol. Rev. 242 , 205–219 (2011).
Irwin, R. S. et al . Spread the word about the journal in 2013: from citation manipulation to invalidation of patient-reported outcomes measures to renaming the Clara cell to new journal features. Chest 143 , 1–4 (2013).
Reynolds, S. D. & Malkinson, A. M. Clara cell: progenitor for the bronchiolar epithelium. Int. J. Biochem. Cell Biol. 42 , 1–4 (2010).
Rackley, C. R. & Stripp, B. R. Building and maintaining the epithelium of the lung. J. Clin. Invest. 122 , 2724–2730 (2012).
Perl, A. K., Tichelaar, J. W. & Whitsett, J. A. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 11 , 21–29 (2002).
Wert, S. E., Glasser, S. W., Korfhagen, T. R. & Whitsett, J. A. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev. Biol. 156 , 426–443 (1993).
Perl, A. K., Wert, S. E., Nagy, A., Lobe, C. G. & Whitsett, J. A. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. USA 99 , 10482–10487 (2002).
Kasper, M., Behrens, J., Schuh, D. & Muller, M. Distribution of E-cadherin and Ep-CAM in the human lung during development and after injury. Histochem. Cell Biol. 103 , 281–286 (1995).
Takeichi, M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 102 , 639–655 (1988).
Ceteci, F. et al . E-cadherin Controls Bronchiolar Progenitor Cells and Onset of Preneoplastic Lesions in Mice. Neoplasia 14 , 1164–1177 (2012).
Rawlins, E. L. et al . The role of Scgb1a1 + Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4 , 525–534 (2009).
Mucenski, M. L. et al . Beta-catenin regulates differentiation of respiratory epithelial cells in vivo . Am. J. Physiol. Lung Cell. Mol. Physiol. 289 , L971–L979 (2005).
Heijink, I. H., van Oosterhout, A. & Kapus, A. EGFR signaling contributes to house dust mite-induced epithelial barrier dysfunction. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physio , https://doi.org/10.1183/09031936.00125809 (2010).
Nadel, J. A. & Burgel, P. R. The role of epidermal growth factor in mucus production. Curr. Opin. Pharmacol. 1 , 254–258 (2001).
Nakano, Y., Van Tho, N., Yamada, H., Osawa, M. & Nagao, T. Radiological approach to asthma and COPD–the role of computed tomography. Allergol. Int. 58 , 323–31 (2009).
Mitsunobu, F. et al . Complexity of terminal airspace geometry assessed by computed tomography in asthma. Am. J. Respir. Crit. Care Med. 167 , 411–417 (2003).
Hoffman, A. M. & Ingenito, E. P. Alveolar epithelial stem and progenitor cells: emerging evidence for their role in lung regeneration. Curr. Med. Chem . (2012).
Galambos, C. & Demello, D. E. Regulation of alveologenesis: clinical implications of impaired growth. Pathology 40 , 124–140 (2008).
Perl, A. K., Riethmacher, D. & Whitsett, J. A. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am. J. Respir. Crit. Care Med. 183 , 511–521 (2011).
Ceteci, F. et al . Disruption of Tumor Cell Adhesion Promotes Angiogenic Switch and Progression to Micrometastasis in RAF-Driven Murine Lung Cancer. Cancer Cell 12 , 145–159 (2007).
Wan, H. et al . Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 131 , 953–964 (2004).
Heijink, I. H. et al . Der p, IL-4, and TGF-beta cooperatively induce EGFR-dependent TARC expression in airway epithelium. Am. J. Respir. Cell Mol. Biol. 36 , 351–359 (2007).
Cowell, C. F. et al . Loss of cell-cell contacts induces NF-kappaB via RhoA-mediated activation of protein kinase D1. J. Cell. Biochem. 106 , 714–728 (2009).
Lambrecht, B. N. & Hammad, H. The immunology of asthma. Nat. Immunol . 16 (2014).
Chen, G. et al . Foxa2 Programs Th2 Cell-Mediated Innate Immunity in the Developing Lung. J. Immunol. 184 , 6133–6141 (2010).
Zhang, Z. et al . FOXA2 attenuates the epithelial to mesenchymal transition by regulating the transcription of E-cadherin and ZEB2 in human breast cancer. Cancer Lett. 361 , 240–250 (2015).
Plantinga, M. et al . Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38 , 322–335 (2013).
Barbato, A. et al . Epithelial damage and angiogenesis in the airways of children with asthma. Am. J. Respir. Crit. Care Med. 174 , 975–981 (2006).
Ierodiakonou, D. et al . E-cadherin gene polymorphisms in asthma patients using inhaled corticosteroids. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 38 , 1044–1052 (2011).
CAS Google Scholar
Download references
Acknowledgements
The authors would like to thank Uilke Brouwer and Arjen Petersen from the UMCG for performing the genotyping of the mice and assistance with the mouse experiments, respectively. Furthermore, we would like to thank Justine Van Moorleghem and Manon Vanheerswynghels for their assistance with the mouse experiments at the Inflammation Research Centre. Additionally, we would like to thank Anneke Kremer, Saskia Lippens, Sonia Bartunkova and Dr. Christopher Guérin from the Bio Imaging Core (Flanders Institute for Biotechnology) for their assistance with the sample preparation and electron microscopy pictures. Lastly, we would like to thank Amrit Samra from the Centre for Heart and Lung Innovation for her histological expertise.
Author information
S. Post and I. H. Heijink contributed equally.
T.L. Hackett and M.C. Nawijn jointly supervised this work.
Authors and Affiliations
University of Groningen, University Medical Center Groningen, Department of Pathology & Medical Biology, laboratory of Experimental Pulmonology and Inflammation Research (EXPIRE), Groningen, The Netherlands
S. Post, I. H. Heijink, L. Hesse, V. N. S. Kuchibhotla, A. J. M. Van Oosterhout & M. C. Nawijn
University of Groningen, University Medical Center Groningen, GRIAC Research Institute, Groningen, The Netherlands
University of Groningen, University Medical Center Groningen, Department of Pulmonology, Groningen, The Netherlands
I. H. Heijink
University of British Columbia, Centre for Heart and Lung Innovation, Department of Anesthesiology, Pharmacology and Therapeutics, St. Paul’s Hospital, Vancouver, British Columbia, Canada
S. Post, H. K. Koo, F. Shaheen, M. Fouadi & T. L. Hackett
Laboratory of Immunoregulation and Mucosal Immunology, Department for Molecular Biomedical Research, Inflammation Research Centre (IRC), Ghent, Belgium
B. N. Lambrecht
Department of Pulmonary Medicine, Ghent University, Ghent, Belgium
Department of Pulmonary Medicine, Erasmus University Medical Center Rotterdam, Rotterdam, The Netherlands
You can also search for this author in PubMed Google Scholar
Contributions
S.P., I.H.H., A.J.M.v.O., T.L.H., B.N.L. and M.C.N. conceived and designed the experiments S.P., L.H., H.K.K., F.S., M.F. and V.N.S.K. performed the experiments. S.P., I.H.H., T.L.H. and M.C.N. analyzed the data. S.P., I.H.H., T.L.H. and M.C.N. drafted the paper. All authors critically read and revised the paper.
Corresponding author
Correspondence to I. H. Heijink .
Ethics declarations
Competing interests.
This study was funded by unrestricted research grants from the Dutch Lung Foundation (Longfonds; 3.2.07.019), Stichting Astma Bestrijding (2014/008), European Respiratory Society (LTRF-118-2012) and the Canadian Institutes for Health Research (260046). We have no non-financial competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Post, S., Heijink, I.H., Hesse, L. et al. Characterization of a lung epithelium specific E-cadherin knock-out model: Implications for obstructive lung pathology. Sci Rep 8 , 13275 (2018). https://doi.org/10.1038/s41598-018-31500-8
Download citation
Received : 29 January 2018
Accepted : 13 August 2018
Published : 05 September 2018
DOI : https://doi.org/10.1038/s41598-018-31500-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The relation between age and airway epithelial barrier function.
- M. de Vries
- K. O. Nwozor
- C. A. Brandsma
Respiratory Research (2022)
Epithelial coxsackievirus adenovirus receptor promotes house dust mite-induced lung inflammation
- Elena Ortiz-Zapater
- Dustin C. Bagley
- Maddy Parsons
Nature Communications (2022)
- Baishakhi Ghosh
- Jeffrey Loube
- Venkataramana K. Sidhaye
Communications Biology (2022)
Tight Junctions, the Epithelial Barrier, and Toll-like Receptor-4 During Lung Injury
- Nachiket M. Godbole
- Asif Alam Chowdhury
- Shanjana Awasthi
Inflammation (2022)
Loss of E-cadherin due to road dust PM2.5 activates the EGFR in human pharyngeal epithelial cells
- Nguyen Thanh Tung
- Hsiao-Chi Chuang
Environmental Science and Pollution Research (2021)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung
Affiliation.
- 1 Laboratory of Genetics, University of Wisconsin-Madison, Madison, Wisconsin 53706, USA.
- PMID: 19618463
- PMCID: PMC3538083
- DOI: 10.1002/dvdy.22032
Fibroblast growth factor 10 (FGF10) signaling through FGF receptor 2 (FGFR2) is required for lung initiation. While studies indicate that Fgf10 and Fgfr2 are also important at later stages of lung development, their roles in early branching events remain unclear. We addressed this question through conditional inactivation of both genes in mouse subsequent to lung initiation. Inactivation of Fgf10 in lung mesenchyme resulted in smaller lobes with a reduced number of branches. Inactivation of Fgfr2 in lung epithelium resulted in disruption of lobes and small epithelial outgrowths that arose arbitrarily along the main bronchi. In both mutants, there was an increase in cell death. Also, the expression patterns of key signaling molecules implicated in branching morphogenesis were altered and a proximal lung marker was expanded distally. Our results indicate that both Fgf10 and Fgfr2 are required for a normal branching program and for proper proximal-distal patterning of the lung.
Copyright (c) 2009 Wiley-Liss, Inc.

Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Base Sequence
- Body Patterning / genetics
- Body Patterning / physiology
- Cell Death / genetics
- Cell Death / physiology
- Cell Survival / genetics
- Cell Survival / physiology
- DNA Primers / genetics
- Epithelium / embryology
- Epithelium / physiology
- Fibroblast Growth Factor 10 / deficiency
- Fibroblast Growth Factor 10 / genetics
- Fibroblast Growth Factor 10 / physiology*
- Gene Expression Regulation, Developmental
- Gene Targeting
- Lung / abnormalities
- Lung / embryology*
- Lung / physiology
- Mesoderm / embryology
- Mesoderm / physiology
- Mice, Knockout
- Mice, Mutant Strains
- Mice, Transgenic
- Receptor, Fibroblast Growth Factor, Type 2 / deficiency
- Receptor, Fibroblast Growth Factor, Type 2 / genetics
- Receptor, Fibroblast Growth Factor, Type 2 / physiology*
- SOX9 Transcription Factor / genetics
- SOX9 Transcription Factor / physiology
- SOXB1 Transcription Factors / genetics
- SOXB1 Transcription Factors / physiology
- Signal Transduction
- DNA Primers
- Fgf10 protein, mouse
- Fibroblast Growth Factor 10
- SOX9 Transcription Factor
- SOXB1 Transcription Factors
- Sox2 protein, mouse
- Sox9 protein, mouse
- Fgfr2 protein, mouse
- Receptor, Fibroblast Growth Factor, Type 2
Grants and funding
- R01DC005608/DC/NIDCD NIH HHS/United States
- R01 HD045522/HD/NICHD NIH HHS/United States
- R01 DC005608/DC/NIDCD NIH HHS/United States
- R21 DK082888/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01DC004185/DC/NIDCD NIH HHS/United States
- Open access
- Published: 18 May 2024
Statin administration or blocking PCSK9 alleviates airway hyperresponsiveness and lung fibrosis in high-fat diet-induced obese mice
- Lin Liang 1 , 2 ,
- Sook In Chung 2 ,
- Tae-Eun Guon 2 ,
- Kyung Hee Park 2 , 3 ,
- Jae-Hyun Lee 2 , 3 &
- Jung-Won Park 2 , 3
Respiratory Research volume 25 , Article number: 213 ( 2024 ) Cite this article
Metrics details
Obesity is associated with airway hyperresponsiveness and lung fibrosis, which may reduce the effectiveness of standard asthma treatment in individuals suffering from both conditions. Statins and proprotein convertase subtilisin/kexin-9 inhibitors not only reduce serum cholesterol, free fatty acids but also diminish renin-angiotensin system activity and exhibit anti-inflammatory effects. These mechanisms may play a role in mitigating lung pathologies associated with obesity.
Male C57BL/6 mice were induced to develop obesity through high-fat diet for 16 weeks. Conditional TGF-β1 transgenic mice were fed a normal diet. These mice were given either atorvastatin or proprotein convertase subtilisin/kexin-9 inhibitor (alirocumab), and the impact on airway hyperresponsiveness and lung pathologies was assessed.
High-fat diet-induced obesity enhanced airway hyperresponsiveness, lung fibrosis, macrophages in bronchoalveolar lavage fluid, and pro-inflammatory mediators in the lung. These lipid-lowering agents attenuated airway hyperresponsiveness, macrophages in BALF, lung fibrosis, serum leptin, free fatty acids, TGF-β1, IL-1β, IL-6, and IL-17a in the lung. Furthermore, the increased RAS, NLRP3 inflammasome, and cholecystokinin in lung tissue of obese mice were reduced with statin or alirocumab. These agents also suppressed the pro-inflammatory immune responses and lung fibrosis in TGF-β1 over-expressed transgenic mice with normal diet.
Conclusions
Lipid-lowering treatment has the potential to alleviate obesity-induced airway hyperresponsiveness and lung fibrosis by inhibiting the NLRP3 inflammasome, RAS and cholecystokinin activity.
Graphical abstract
Introduction
Obesity correlates with metabolic dysfunction and exacerbates asthma incidence and severity [ 1 ]. According to the Global Initiative for Asthma 2022, asthma with obesity is now recognized as a distinct phenotype, often associated with heightened symptoms, resistance to corticosteroids, and non-eosinophilic airway inflammation [ 2 ].
Animal studies highlight that a high-fat diet (HFD) induces obesity in mice, resulting in airway hyperresponsiveness (AHR) and pulmonary fibrosis [ 3 ]. Elevated systemic pro-inflammatory markers like C-reactive protein, TNF-α, TGF-β, leptin, and IL-6 are linked to the obesity [ 4 ].
The NLRP3 inflammasome pathway, activated by metabolic damage-related factors such as excessive glucose, reactive oxygen species (ROS), oxidized lipids, and cholesterol crystals, contributes to inflammatory responses, insulin resistance, and metabolic syndrome [ 5 ]. In obesity-related asthma, heightened oxidative stress and NLRP3 inflammasome activation in airways are implicated in symptom manifestation [ 6 , 7 ]. Increased renin and angiotensin II levels in obese mice suggest a potential link between the renin-angiotensin system (RAS) and fibrosis development in various organs, including the lungs [ 8 , 9 ]. Cross-talk between RAS and TGF-β1 signaling pathways, facilitated by ROS-NLRP3 pathway, contributes to fibrosis [ 10 ]. Consequently, inhibiting RAS activation, NLRP3, and TGF-β1 expression could offer novel therapeutic avenues for managing asthma in patients with both obesity and asthma.
Furthermore, in obese mice, elevated free fatty acid (FFA) levels stimulate cholecystokinin (CCK) secretion, potentially contributing to AHR via enhanced CCK receptor expression in lung smooth muscles. Blocking CCK receptors has shown promise in mitigating AHR in asthma with obesity patients [ 11 ].
Statins and PCSK9 inhibitors, known for lowering LDL-cholesterol, exhibit anti-inflammatory effects. PCSK9 binds to LDL receptor on cell surface and induce intracellular degradation of LDL receptors, and cause hypercholesterolemia. Subsequently, human monoclonal antibodies that target PCSK-9 decrease LDL cholesterol level [ 12 ]. PCSK9’s role in LDL receptor degradation correlates with heightened PCSK9 levels in obesity [ 13 , 14 ], while TGF-β1 induces PCSK9 secretion [ 15 ]. Studies suggest PCSK9 knockout suppresses NLRP3 inflammatory pathways [ 16 ], and statins exhibit inhibitory effects on NLRP3 and TLR signaling pathways, potentially beneficial for airway inflammatory diseases [ 17 , 18 , 19 , 20 ].
This study aims to assess the impact of statins or PCSK9 inhibitors on AHR and lung fibrosis in an HFD-induced obesity model of mouse. It seeks to unravel mechanisms of these lipid lowering agents involving RAS activation, NLRP3 inflammasome, pro-inflammatory cytokines, and CCK expression.
Materials and methods
Study scheme and animals.
Male C57BL/6 mice were fed a normal diet (ND) or HFD for 16 weeks. The diets consisted of an isocaloric control diet (fat comprised 10% of calories; D12450; Research Diets Inc.) or an HFD (fat comprised 60% of calories; D12492; Research Diets Inc.). The mice were weighed every week. Administration of the anti-PCSK9 monoclonal antibody alirocumab (Biorbyt, Cambridge, UK) or atorvastatin (Cayman Chemical, Ann Arbor, MI, USA) began at week 5. Alirocumab was injected (3 or 10 mg/kg) weekly for 16 weeks. Atorvastatin was orally administered five times per week for the same 16-week period at a dose of 10 mg/kg (Fig. 1 A). Lee and colleagues kindly provided the triple-transgenic TGF-β1 mice for this study [ 21 ]. Male and female transgene + mice, and transgene − littermates aged 6–8 weeks were fed 0.5 mg/ml doxycycline in water ad libitum for 4 weeks with intraperitoneal injection of alirocumab (10 mg/kg) weekly and oral administration of atorvastatin (10 mg/kg) five times per week (Fig. 8A). All animal procedures were performed according to the Institutional Animal Care and Use Committee (IACUC) regulations of Yonsei University College of Medicine (Seoul, Korea), which has been fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (IACUC approval number: 2021 − 0256).
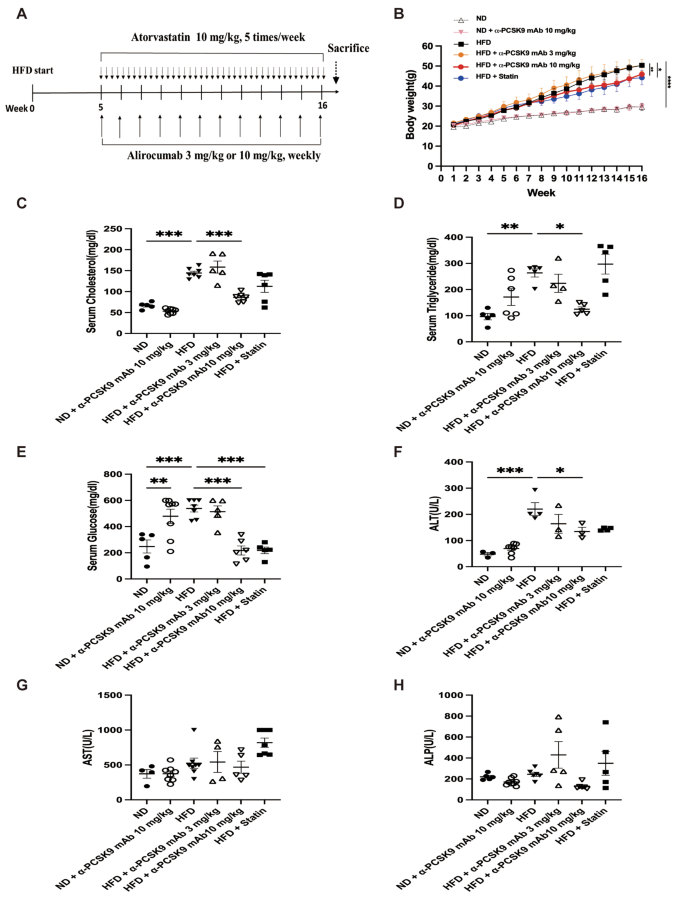
Effects of PCSK9 inhibition or statin administration on body weight and serum biochemical marker levels in HFD-induced obese mice. ( A ) Experimental scheme of PCSK9 inhibition or statin treatment in HFD or normal chow-fed mice. ( B ) Body weight in mice on the ND or HFD. ( C ) Cholesterol, ( D ) triglyceride, ( E ) glucose, ( F ) Alanine aminotransferase (ALT), ( G ) Aspartate aminotransferase (AST), and ( H ) Alkaline phosphatase (ALP) levels were measured in serum. The results are expressed as the mean ± SEM ( n = 6 per group). Statistical analysis of body weight changes was performed using repeated-measures ANOVA, and other analyses were performed with one-way ANOVA with Bonferroni correction. *: P < 0.05, **: P < 0.01, and ***: P < 0.001. ND: normal diet; HFD: high-fat diet
In vitro Bronchial Epithelial Cell Stimulation with TGF-β1
Human bronchial epithelial BEAS-2B cells were grown and maintained in bronchoepithelial basal medium (Lonza, Basel, Swiss) with supplements in 6-well plates. Following a 24-hour serum starvation, cells were treated with alirocumab (10 µg/ml) or atorvastatin (20 µM) for 24 h and then stimulated with TGF-β1 (10 ng/ml) for 10 min. Real-time PCR analysis was conducted.
Measurement of AHR
AHR was assessed using the FlexiVent system (SCIREQ, Montreal, QC, Canada). To determine the baseline airway resistance (Rrs), mice were exposed to nebulized phosphate-buffered saline for 3 min followed by progressive exposure to 6.25, 12.5, 25, 50, and 100 mg/ml nebulized methacholine (MCh; Sigma-Aldrich, St. Louis, MO, USA) using an ultrasonic nebulizer (DeVilbiss, Somerset, PA, USA). Each test lasted for 3 min, and average Rrs values were calculated for each MCh concentration.
Bronchoalveolar lavage
Prior to tracheostomy, mice were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg; Hanlim Pharma Co., Seoul, Korea). A 23-gauge needle was used to insert a silicone tube into the mouse trachea, which was connected to an 800-µl tuberculin injector to deliver 1 ml of Hank’s balanced salt solution (HBSS; Thermo Fisher Scientific, Waltham, MA, USA) to the lungs. The recovered bronchoalveolar lavage fluid (BALF) was centrifuged for 3 min at 10,000 rpm at 4 °C, and the supernatant was stored at − 70 °C. Whole cells were re-suspended in HBSS, and BALF cell smears were prepared using cytocentrifugation (Thermo Shandon Cytospin 3, Marshall Scientific, Hampton, NH, USA) and then stained with Diff-Quick (Sysmax, Kobe, Japan). The percentages of macrophages, eosinophils, lymphocytes, and neutrophils in BALF were determined by counting 500 leukocytes in randomly selected fields under a light microscope.
Serum biochemical assays
Serum was harvested following centrifugation of clotted blood samples and examined for the following biochemical parameters using an automated clinical chemistry analyzer (Dri-Chem 4000i, Fujifilm, Japan): serum cholesterol, triglyceride, glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP).
Enzyme-linked immunosorbent assays (ELISAs)
To analyze cytokine levels, 100 mg of right lung tissue was lysed using a tissue homogenizer (Biospec Products, Bartlesville, OK, USA) with RIPA buffer (Thermo Fisher Scientific Inc., Rockford, IL, USA). After incubation on ice for 30 min, the homogenates were centrifuged at 10,000 rpm for 10 min. Lung homogenate supernatants were collected, passed through a 0.45-µm filter (Gelman Science, Ann Arbor, MI, USA), and stored at − 80℃ to measure cytokines, angiotensin II, and angiotensin II receptor type 1 levels. The measured cytokine levels were adjusted to the lung tissue weight. IL-1β, IL-6, IL-17a, TGF-β1, TNF-α, and leptin concentrations in lung homogenate or serum were measured using commercial ELISA kits (R&D Systems, Inc. Minneapolis, MN, USA).
To evaluate the degree of oxidative stress, the concentration of malondialdehyde (MDA) in lung homogenate or serum was measured using an ELISA (DoGenBio, Seoul, Korea). CCK was detected in lung homogenate or serum using the CCK Enzyme Immunoassay Kit (Biorbyt). An FFA Assay Kit (DoGenBio) was used to detect FFAs in lung homogenate or serum. The procedures were performed following the recommended manufacturer’s protocols.
RNA purification, reverse transcription, and real-time PCR amplification
Total RNA was isolated from extracted lungs using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was then synthesized using an RNA to cDNA EcoDry premix kit (Takara Bio, Kusatsu, Japan) following the manufacturer’s recommended protocol. PCR master mix (Power SYBR Green PCR Master Mix, Applied Biosystems, Warrington, UK) was used to perform quantitative RT-PCR with a StepOnePlus ™ PCR System (Applied Biosystems). The relative expression levels of target genes were normalized to the β-actin expression levels. The primer sequences are shown in Table 1 .
Histopathology and immunohistochemistry
The left lung was perfused with 4% paraformaldehyde solution and then embedded in paraffin. Hematoxylin and eosin (H&E) staining was performed on lung sections to evaluate tissue inflammation, and periodic acid–Schiff (PAS) staining was employed to detect goblet cell hyperplasia and submucosal gland hypertrophy. In addition, Masson’s trichrome (MT) staining was conducted to assess fibrosis.
For NLRP3 and caspase-1 immunohistochemical staining (IHS), lung sections from each paraffin block were deparaffinized with xylene and rehydrated in ethanol. Antigen retrieval was conducted by autoclaving the sections at 120 °C for 15 min in citrate buffer (pH 6.0). Then, the sections were incubated in 3% hydrogen peroxide for 15 min to inactivate endogenous catalase. Next, the sections were incubated with anti-NLRP3 (1:100, SAB, USA), anti-caspase-1 (1:200, Abcam, Cambridge, UK), and anti-alpha smooth muscle actin (α-SMA) (1:100, Abcam) antibodies overnight at 4 °C. Finally, the sections were incubated with streptavidin horseradish peroxidase, and the percentage of positively-stained area was calculated using ImageJ software.
Statistical analysis
Data were analyzed using Prism software (GraphPad Inc., San Diego, CA, USA). Changes in AHR and body weight were evaluated by repeated-measures analysis of variance (ANOVA) and a Bonferroni post-hoc test, and differences between the other variables were compared by one-way ANOVA and a Bonferroni post-hoc test. A value of P < 0.05 was considered statistically significant.
PCSK9 inhibition or statin administration induced weight loss and altered serum biochemical marker levels in HFD-induced obese mice
The HFD resulted in significant weight gain compared with the standard chow diet (Fig. 1 B). Among the HFD groups, administration of 10 mg/kg alirocumab ( P = 0.005) or statin ( P = 0.024) significantly reduced body weight, but 3 mg/kg alirocumab did not exhibit the same effect. The HFD notably influenced serum cholesterol ( P < 0.001), triglyceride ( P = 0.002), glucose ( P < 0.001), and ALT ( P < 0.001) levels. Treatment with alirocumab at 10 mg/kg reduced these levels to those observed in the ND group, whereas 3 mg/kg alirocumab did not yield a similar effect (Fig. 1 C–H). However, in normal diet (ND)-fed mice, alirocumab treatment resulted in increased serum glucose levels ( P = 0.009). Additionally, the statin displayed a trend in reducing blood lipid levels and demonstrated a statistically significant decrease in serum glucose level ( P < 0.001).
PCSK9 inhibition or statin administration suppressed AHR, monocytosis in BALF, and systemic pro-inflammatory mediators in the lungs of HFD mice
The HFD group exhibited significantly increased AHR compared to the ND group ( P = 0.005). Treatment with either alirocumab (3 mg/kg: P = 0.018 and 10 mg/kg: P = 0.003) or the statin ( P = 0.022) alleviated AHR in the HFD group (Fig. 2 A). BALF analysis revealed higher total cell number ( P < 0.001) and macrophage count ( P < 0.001) in the HFD compared to the ND group, with no notable increase in eosinophils, neutrophils, and lymphocytes due to the HFD. PCSK9 inhibition or statin administration reduced the total cell and macrophage counts in the HFD group (Fig. 2 B).
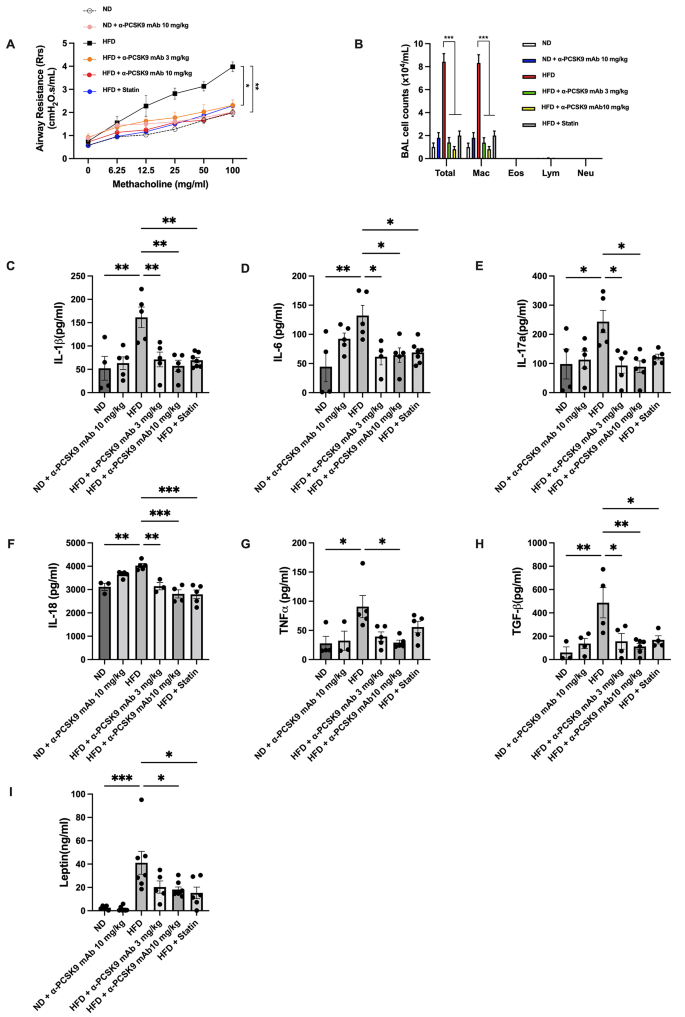
Effects of PCSK9 inhibition or statin administration on AHR and airway inflammation in HFD-induced obese mice. ( A ) AHR was measured as the Rrs at 24 h after the final treatment. ( B ) Effect of the HFD on cell counts in BALF. Mice were sacrificed 24 h after the final treatment, and BALF cells were isolated. ( C – H ) IL-1β ( C ), IL-6 ( D ), IL-17a ( E ), IL-18 ( F ), TNF-α ( G ), and TGF-β ( H ) levels in lung homogenates and leptin ( I ) levels in serum were evaluated by ELISAs. The results are expressed as the mean ± SEM ( n = 6 per group). Statistical analysis of AHR was performed using repeated-measures ANOVA, and other analyses were performed using one-way ANOVA with Bonferroni correction. *: P < 0.05, **: P < 0.01, and ***: P < 0.001
The lungs of HFD mice showed significantly increased expression of IL-1β, IL-6, IL-17a, IL-18, TGF-β, and TNF-α, along with elevated serum leptin compared to the ND group. Alirocumab (10 mg/kg) notably attenuated these pro-inflammatory cytokines (Fig. 2 C–I). Statin administration also attenuated these parameters, except for IL-17a and TNF-α.
PCSK9 inhibition or statin administration attenuated fibrosis and epithelial-mesenchymal transition (EMT) markers in HFD mouse lungs
Histopathological examination of lung tissue revealed that HFD mice lacked eosinophilic inflammation or goblet cell proliferation (Fig. 3 A). However, MT staining indicated increased peribronchial and perivascular fibrosis in the HFD group, a condition mitigated by alirocumab or statin administration (Fig. 3 A and B). This observation was corroborated by HIS of α-SMA, showing heightened expression in the peribronchial and perivascular regions of HFD mice, which was effectively reduced by either alirocumab (3 and 10 mg/kg) or statin administration (Fig. 3 A and C).
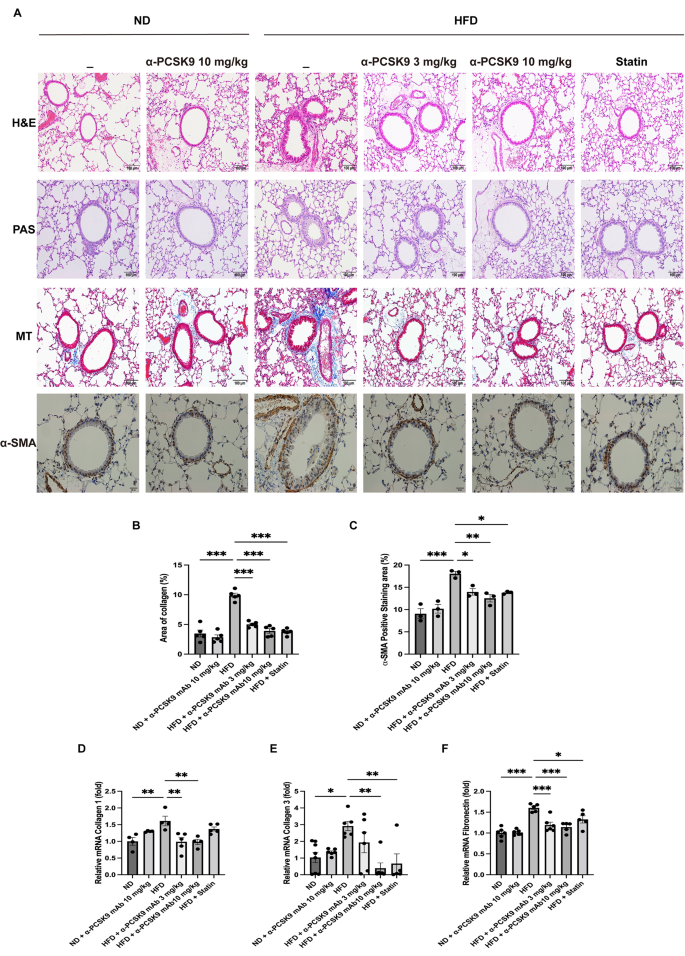
Effects of PCSK9 inhibition or statin administration on fibrosis and EMT markers in HFD mouse lungs. ( A ) Paraffin-embedded lung tissue sections underwent H&E, PAS, MT (original magnification: 100×), and α-SMA IHS (original magnification: 50×) staining. ( B-C ) Quantitative analyses of the fibrosis and peribronchial α-SMA-positive staining areas were performed using an image analysis system. Quantification of EMT markers in the lungs was performed using mRNA expression of collagen 1 ( D ), collagen 3 ( E ), and fibronectin ( F ). The results are expressed as the mean ± SEM ( n = 6 per group). Statistical analysis was performed using one-way ANOVA with Bonferroni correction. *: P < 0.05, **: P < 0.01, and ***: P < 0.001. EMT: epithelial–mesenchymal transition, H&E: hematoxylin and eosin; IHS: immunohistochemical staining; MT: Masson-trichrome; PAS: periodic acid–Schiff; α-SMA: alpha-smooth muscle actin
To confirm these histological findings, we assessed mRNA expression of collagen 1, collagen 3, and fibronectin in lung homogenates (Fig. 3 D–F). The HFD resulted in increased expression of collagen 1 ( P = 0.008), collagen 3 ( P = 0.017), and fibronectin ( P < 0.001) mRNA levels, all of which were consistently diminished by alirocumab (3 and 10 mg/kg) or statin administration.
PCSK9 inhibition or statin administration suppressed RAS activation in HFD mouse lungs
Our subsequent investigation aimed to determine if PCSK9 inhibition or statin administration could impede the activation of RAS activity in HFD mice. Comparatively, the HFD group displayed heightened levels of angiotensin II ( P = 0.024) and angiotensin II receptor type 1 ( P = 0.002) compared with those in the ND group. Notably, alirocumab administration (3 and 10 mg/kg) mitigated these elevated expression levels. However, the effect of statin on RAS activity in the HFD group was not as pronounced as that of alirocumab (Fig. 4 A and B).
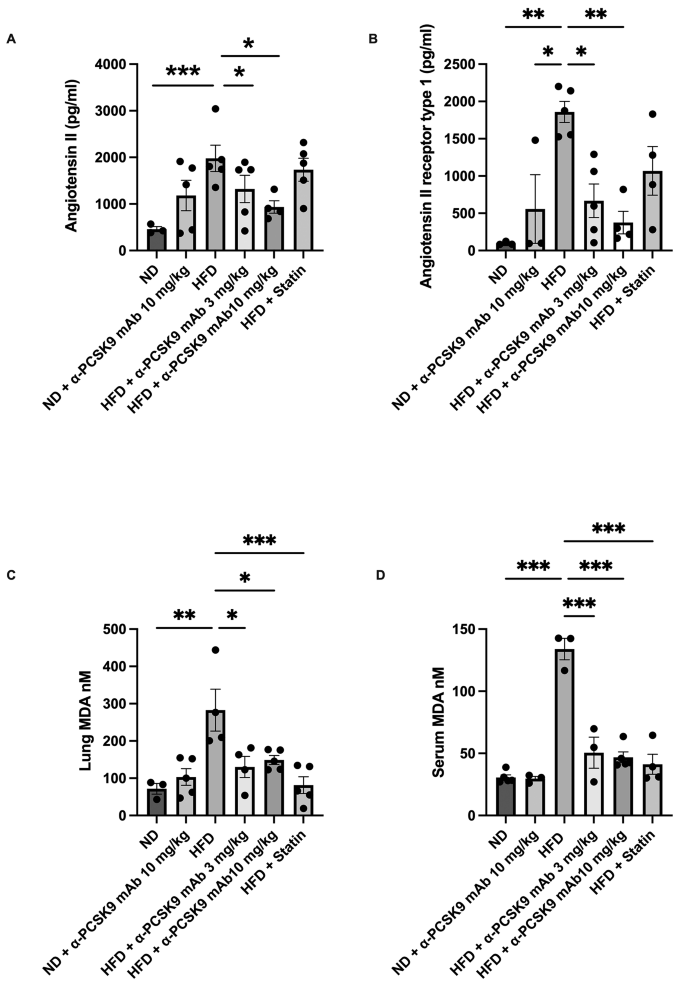
Effects of PCSK9 inhibition or statin administration on RAS activation in HFD mouse lungs. Protein expression of angiotensin II ( A ), angiotensin II receptor type 1 ( B ), and MDA levels ( C ) in lung homogenates and MDA ( D ) in serum were measured by ELISAs. The results are expressed as the mean ± SEM ( n = 6 per group). Statistical analysis was performed using one-way ANOVA with Bonferroni correction. *: P < 0.05, **: P < 0.01, and ***: P < 0.001. MDA: malondialdehyde
Moreover, the oxidative stress biomarker MDA significantly higher level in both the serum ( P < 0.001) and lung homogenate ( P = 0.002) of the HFD group compared to those in the ND group. Interestingly, administration of either alirocumab (3 and 10 mg/kg) or statin significantly reduced MDA levels, both locally and systemically, in the HFD mice (Fig. 4 C and D).
PCSK9 inhibition or statin administration decreased CCK expression and FFA levels in HFD mouse lungs
Obesity correlates with elevated circulating FFAs. In our study, we noted heightened FFAs and CCK levels in serum and lungs of the HFD group compared to those in the ND group. Notably, administration of alirocumab (3 and 10 mg/kg) or statin resulted in significant reduction in FFA and CCK levels in the lungs (Fig. 5 A–D).
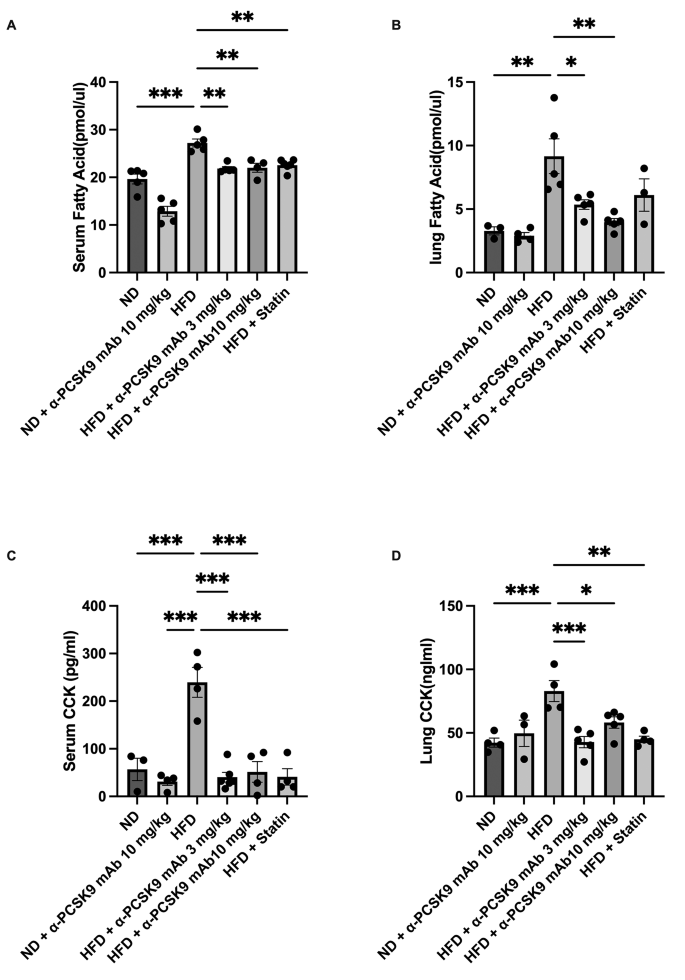
Effects of PCSK9 inhibition or statin administration on CCK expression and FFA levels in HFD mouse lungs. FFA ( A – B ) and CCK ( C – D ) protein expression in both lungs and serum were measured by ELISAs. The results are expressed as the mean ± SEM ( n = 6 per group). Statistical analysis was performed using one-way ANOVA with Bonferroni correction. *: P < 0.05, **: P < 0.01, and ***: P < 0.001. FFA: free fatty acid, CCK: cholecystokinin
PCSK9 inhibition or statin administration decreased NLRP3 inflammasome activity in HFD mouse lungs
IHS for NLRP3 and caspase-1 revealed heightened expression in the respiratory epithelium of HFD mice, a phenomenon effectively mitigated by alirocumab (3 and 10 mg/kg) or statin administration (Fig. 6 A–C). To further validate these histological findings, we assessed the mRNA expression of NLRP3 in the lungs.
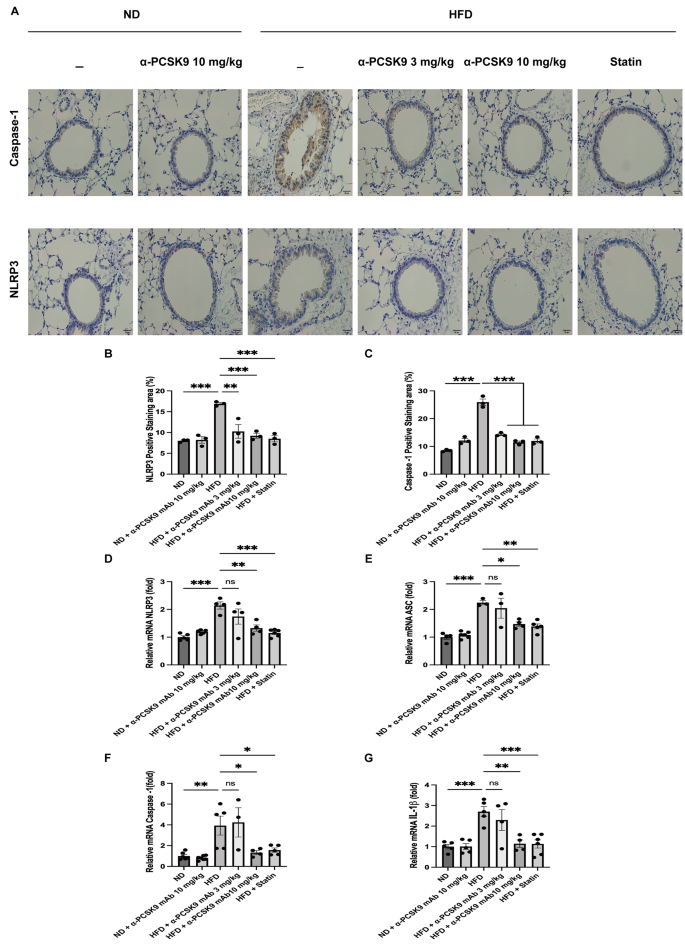
Effects of PCSK9 inhibition or statin administration on NLRP3 activity in HFD mouse lungs. Representative photomicrographs of caspase-1- and NLRP3- positive areas ( A ) in lung sections from mice of the different treatment groups are shown (50×). Quantification of NLRP3- ( B ) and caspase-1-( C ) positive areas was performed using an image analysis system. Quantitative RT-PCR measurement of NLRP3 ( D ), ASC ( E ), caspase-1 ( F ), and IL-1β ( G ) mRNA expression in the lungs is shown. The results are expressed as the mean ± SEM ( n = 6 per group). Statistical analysis was performed using one-way ANOVA with Bonferroni correction. *: P < 0.05, **: P < 0.01, and ***: P < 0.001
Consistent with the staining results, HFD mice exhibited increased mRNA expression of NLRP3 ( P < 0.001), caspase-1 ( P = 0.004), ASC ( P < 0.001), and IL-1β ( P < 0.001). Both statin and alirocumab administration were effective in attenuating these elevated level (Fig. 6 D–G).
PCSK9 inhibition or statin administration decreased NLRP3 production of respiratory epithelium and improved lung fibrosis in TGF-β1 overexpressing transgenic mice .
Multiple studies have demonstrated that TGF-β1 enhanced NLRP3 expression, subsequently triggering fibrosis in major organs [ 22 , 23 ]. So, we initiated stimulation of BEAS-2B cells with TGF-β1 gauge the mRNA expression of NLRP3 related mediators. As a result, the mRNA expression of NLRP3 ( P < 0.001), caspase-1 ( P < 0.001), and IL-1β ( P < 0.001) were notably elevated upon TGF-β1 stimulation. Upon administration of alirocumab, there was a reduction in mRNA expression of NLRP3 ( P = 0.020), caspase 1 ( P < 0.001), and IL-1β ( P = 0.001). Statin treatment resulted in a decreased expression of NLRP3 and caspase-1. But, it concurrently led to an increased secretion of IL-1β. (Fig. 7 A–C).

Effects of PCSK9 inhibition or statin administration on the NLRP3 inflammasome activity in respiratory epithelial cells stimulated by TGF-β1. The mRNA expression of NLRP3 ( A ), caspase-1 ( B ) and IL-1β ( C ) are shown. The respiratory epithelium was stimulated with 10 ng/ml of TGF-β1. Statistical analysis was performed using one-way ANOVA with Bonferroni correction. *: P < 0.05, **: P < 0.01, and ***: P < 0.001
Subsequent to this, we investigated whether administration of alirocumab or statin could alleviate NLRP3 activity, ultimately ameliorating lung fibrosis in TGF-β1 overexpressing transgenic mice. TGF-β1 overexpression increased the mRNA levels of NLRP3 ( P < 0.001), caspase-1 ( P < 0.001), ASC ( P < 0.001), and IL-1β ( P = 0.002), which were notably suppressed upon alirocumab or statin administration (Fig. 8 B–E).
MT staining showed an increase in peribronchial and perivascular fibrosis in the TGF-β1 overexpression group, which was mitigated by alirocumab or statin treatment (Fig. 8 F and G). Moreover, mRNA of the EMT markers collagen 1, collagen 3, and fibronectin exhibited a decrease following alirocumab or statin treatment (Fig. 8 H–J).
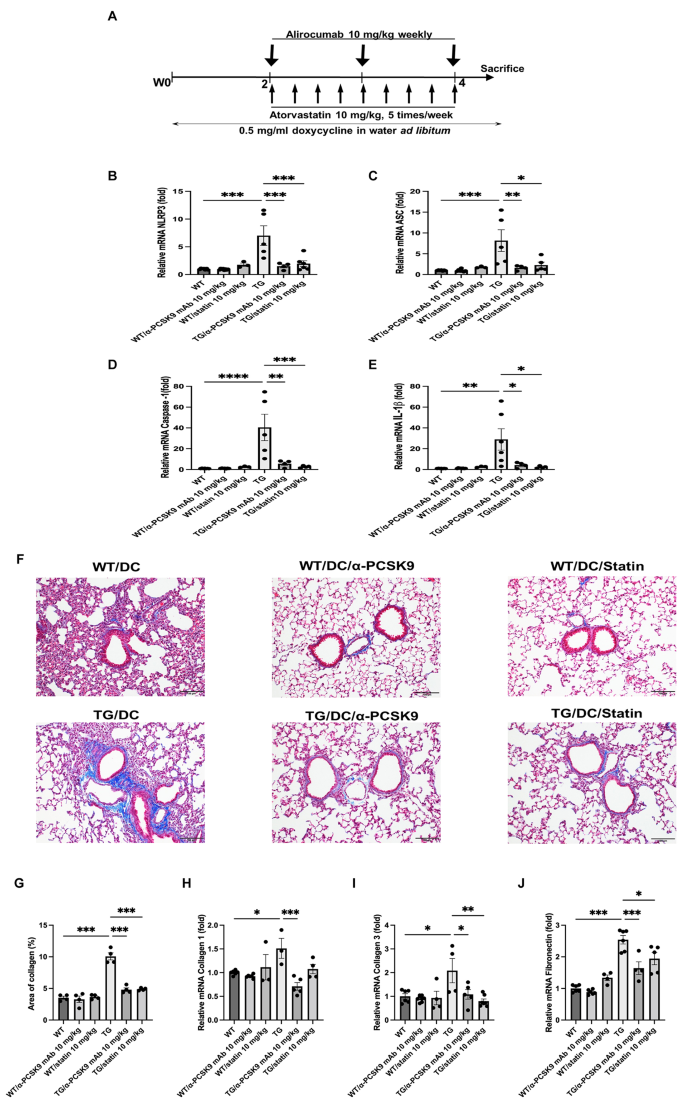
Effects of PCSK9 inhibition or statin administration on TGF-β1 overexpressing transgenic mice. ( A ) Experimental scheme of the TGF-β1 transgenic mouse study. Male and female transgene + mice and transgene − littermates aged 6–8 weeks were fed 0.5 mg/ml doxycycline in water ad libitum for 4 weeks, with intraperitoneal injection of alirocumab (10 mg/kg) weekly and oral administration of atorvastatin (10 mg/kg) five times per week. Quantitative RT-PCR measurement of NLRP3 ( B ), ASC ( C ), caspase-1 ( D ), and IL-1β ( E ) mRNA expression in the lungs is shown. ( F ) MT staining also showed inhibition of peribronchial and perivascular fibrosis by PCSK9 inhibition or statin administration in transgenic mice (original magnification: 100×). ( G ) Quantitative analyses of the fibrosis area were performed using an image analysis system. Quantification of EMT markers in the lungs was performed using mRNA expression of collagen 1 ( H ), collagen 3 ( I ), and fibronectin ( J ). The results are expressed as the mean ± SEM ( n = 6 per group). Statistical analysis was performed using one-way ANOVA with Bonferroni correction. *: P < 0.05, **: P < 0.01, and ***: P < 0.001. DC: doxycycline; WT: wild-type
Our study underscores the potential advantages of cholesterol-lowering medications such as alirocumab and statins in addressing asthma among patients with obesity by mitigating airway hyperresponsiveness (AHR) and lung fibrosis. In contrast to the typical Th2 high inflammation, a pro-inflammatory environment marked by monocytosis in the lungs may contribute to AHR and fibrosis in asthma patient associated with obesity. Our findings further indicate that these lipid-lowering agents may mitigate this pro-inflammatory state and monocytosis, thus offering beneficial effects for asthma patients with obesity who exhibit inadequate responses to conventional therapies. Further studies are required for the effect of lipid lowering agents using allergic asthma model with HFD induced obesity model.
Obesity often correlates with heightened levels of systemic pro-inflammatory markers like C-reactive protein, TNF-α, TGF-β, leptin, and IL-6 [ 24 , 25 ], and we have shown that reducing TNF-α and TGF-β1 levels improved AHR and fibrosis in the same HFD-induced obesity model [ 3 , 25 ]. Factors such as lipopolysaccharide, oxidized LDL, TNF-α, and IL-1β have been noted for inducing PCSK9 secretion in various organs, contributing to conditions like hyperlipidemia, atherosclerosis, diabetes, and hypertension [ 15 ]. Studies have highlighted how cholesterol accumulation or cholesterol crystal deposits inside or outside cells, triggers the NLRP3 inflammasome in myeloid cells and macrophages, ultimately playing a pivotal role in generating inflammatory lesions within atherosclerosis plaques [ 26 , 27 ].
In our HFD-induced model, there was an increase solely in macrophages in the BALF, which was reduced by both lipid-lowering agents. These observations suggest that the anti-inflammatory characteristics of these drugs may involve suppressing NLRP3 activity in macrophages. Our prior research demonstrated that reducing lung macrophages mitigated AHR in a high-fat diet-induced obesity model [ 25 ], affirming the critical role of lung macrophages in AHR development.
Numerous clinical studies have highlighted a significant correlation between serum PCSK9 level and pro-inflammatory cytokines like IL-6, IL-1β, TNFα, and hsCRP [ 28 , 29 , 30 ]. The activation of TLRs and the NLRP3 inflammasome serves as intermediate steps leading to the production and release of PCSK9 [ 31 ]. However, given that obesity is a systemic disease and pro-inflammatory markers were heightened in serum, determining the precise contribution of inflammation - whether systemic, local lung-based, or a blend of both - to lung fibrosis and AHR development remains an open question.
Furthermore, the administration of 10 mg/kg of alirocumab or statin resulted in a modest yet statistically significant reduction in body weight compared to the high-fat diet (HFD) group. It is important to note that the improvement in airway hyperresponsiveness (AHR) observed after treatment with statins or anti-PCSK9 may be attributed to mechanical changes resulting from weight loss. Previous research has established a causal link between obesity-related mechanical alterations, decreased lung function, and AHR, suggesting that this relationship may not solely depend on airway inflammation [ 32 ]. However, we posit that the modest weight loss observed in the HFD mice treated with alirocumab or statin might not have been sufficient to induce substantial mechanical changes in this study.
The NLRP3 inflammasome and RAS play crucial roles in the pathogenesis of pulmonary fibrosis [ 8 , 33 ]. NLRP3 triggers the release of pro-inflammatory cytokines like IL-1β and IL-18. In pulmonary fibrosis, the NLRP3 inflammasome is implicated in perpetuating inflammation within the lungs. The RAS is a hormone system that regulates blood pressure and fluid balance in the body. Beyond its cardiovascular roles, components of the RAS, such as angiotensin II, have been found to be involved in pulmonary fibrosis. Angiotensin II, a key player in the RAS, is known to promote inflammation, fibroblast activation, and collagen deposition in lung tissues, contributing to the development and progression of pulmonary fibrosis.
These two pathways, the NLRP3 inflammasome and the RAS, interact and contribute to a cascade of events leading to chronic inflammation, tissue injury, and ultimately fibrosis [ 34 ]. Angiotensin II is known to stimulate the production of ROS, and this ROS production has been linked to the activation of the NLRP3 inflammasome. When NLRP3 is activated by ROS, it initiates a cascade of events leading to the secretion of TGF-β from mouse cardiac fibroblasts [ 35 ]. Our earlier investigations revealed that HFD-induced obesity markedly stimulates the RAS and insulin resistance, amplifying the signaling of TGF-β1. These interconnected pathways collectively contributed to the development of fibrosis in the lungs of mice [ 3 ]. Consequently, this study suggests that systematically reducing certain biochemical elements by statin or anti-PCSK-9 can attenuate the initiation or stimulation of the inflammasome, potentially improving pulmonary function. These findings align with a recently published study demonstrating that direct inhibition of NLRP3 with MCC950, targeting the NATCH domain, mitigates airway hyperresponsiveness (AHR) and inflammatory cell recruitment in an obesity-induced mode [ 36 ]. Understanding and targeting these pathways present promising avenues for developing treatments that could mitigate or even arrest the progression of pulmonary fibrosis. An animal study demonstrated that the elimination of PCSK9 specifically in cardio-myocytes led to the suppression of NLRP3 inflammasome signaling [ 16 ]. In our study, the lipid-lowering agents exhibited inhibition of collagen 1, collagen 3, fibronectin mRNA, and SMA protein expression. Consequently, we propose that administering alirocumab or statins might prevent lung fibrosis induced by the overexpression of the TGF-β/Smad signaling pathway.
Remarkably, our in vitro respiratory cell study revealed that TGF-β1 also amplifies NLRP3 expression in downstream pathway, suggesting the autocrine activation of TGF-β1 in NLRP3 pathway in the obesity model, and this phenomenon can also be attenuated through PCSK9 inhibition or statin administration. These findings were supported by our investigation using conditional transgenic TGF-β1 overexpressing mice, which exhibited lung fibrosis and heightened levels of NLRP3, caspase-1, ASC, and IL-1β mRNA, even without concurrent body weight increase. However, these effects were mitigated upon administration of alirocumab or statins. Consequently, we posit that alirocumab or statin administration holds promise in preventing lung fibrosis by suppressing pro-inflammatory cytokines, the RAS, the NLRP3 inflammasome, and subsequently, TGF-β1 signaling, independent of obesity.
Nonetheless, this study was subject to certain limitations, notably its concentration on the NLRP3 inflammasome and downstream signaling pathways in transgenic mice with TGF-β1 overexpression. Future research utilizing the TGF-β overexpressing mouse model should delve deeper into the precise mechanisms through which TGF-β affects both the priming pathway and the secondary activation of the NLRP3 inflammasome.
The elevation of FFAs is a well-documented occurrence in individuals with obesity, attributed to increased FFAs release from adipose tissue or impaired clearance mechanisms [ 37 ]. FFAs have the capacity to induce mitochondrial ROS, potentially contributing to inflammation and endothelial dysfunction [ 38 ]. Notably, in an HFD-induced obesity model, one study revealed an autocrine stimulatory loop involving CCK-activated, CCKA receptor-mediated airway smooth muscle contraction, a process potentially exacerbated by elevated FFAs [ 11 ]. Our observations in HFD mice indicated the increases of both FFAs and CCK in the serum and lungs. Additionally, a research has shown that angiotensin II can upregulate CCK expression at both mRNA and protein levels in cardio-myocytes [ 39 ]. Consequently, heightened lung CCK levels in the context of obesity are likely to impact lung function and exacerbate AHR.
Moreover, our findings demonstrated that both alirocumab and statin administration significantly reduced FFAs and subsequently CCK levels in the lungs and serum of HFD mice. This suggests that inhibiting the FFAs-CCK pathway via lipid-lowering agents could represent a promising strategy for treating individuals with asthma and obesity.
In summary, our data indicate that in the HFD-induced obesity model, there are heightened activation of RAS and NLRP3 inflammasome signaling, alongside increased CCK activity. These phenomena are likely due to elevated cytoplasmic LDL-cholesterol and FFAs. These alterations contribute to amplified the expressions of pro-inflammatory cytokines and TGF-β1, culminating in lung fibrosis and AHR. Alirocumab and statins might exert pleiotropic effects in halting these cascades and averting the onset of lung fibrosis and AHR within this obesity model. Further investigations utilizing specific inhibitors of these molecules are imperative to ascertain the precise mechanisms. Lastly, we propose that lipid-lowering agents could represent a viable strategy in treating individuals with asthma with obesity, especially those with inadequate responses to standard asthma medications. Nevertheless, substantiating the clinical efficacy of this approach demands real-world or epidemiological studies involving large sample sizes and long-term randomized clinical trials.
Our findings suggest that the serum lipid-lowering treatments may alleviate obesity-induced AHR and lung fibrosis through anti-inflammatory responses by inhibition of RAS and NLRP3 inflammasome, and CCK activity. Lipid-lowering strategies may prove beneficial in treating asthma patients with obesity who exhibit poor response to typical asthma medications.
Data availability
The datasets in this study are available from the corresponding author on reasonable request.
Abbreviations
Airway hyperresponsiveness
Proprotein convertase subtilisin/kexin-9
Free fatty acids
Renin-angiotensin system
High-fat diet
Reactive oxygen species
Cholecystokinin
NOD-, LRR- and pyrin domain-containing protein 3
Epithelial transition markers
Bronchoalveolar lavage fluid
Alanine aminotransferase
Aspartate aminotransferase
Alkaline phosphatase
Periodic Acid-Schiff
Hematoxylin and eosin
Masson-trichrome
Alpha-smooth muscle actin
Immunohistochemical staining
Toll-like receptors
Transforming growth factor beta
Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–6.
Article PubMed PubMed Central Google Scholar
Levy ML, Bacharier LB, Bateman E, Boulet LP, Brightling C, Buhl R, et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. NPJ Prim Care Respir Med. 2023;33:7.
Park YH, Oh EY, Han H, Yang M, Park HJ, Park KH, et al. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-beta1 pathway. Exp Mol Med. 2019;51:1–12.
PubMed PubMed Central Google Scholar
van Huisstede A, Rudolphus A, Cabezas MC, Biter LU, van de Geijn GJ, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70:659–67.
Article PubMed Google Scholar
Wani K, AlHarthi H, Alghamdi A, Sabico S, Al-Daghri NM. Role of NLRP3 inflammasome activation in obesity-mediated metabolic disorders. Int J Environ Res Public Health 2021, 18.
Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res 2007, 8.
Wood LG, Li Q, Scott HA, Rutting S, Berthon BS, Gibson PG, et al. Saturated fatty acids, obesity, and the nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome in asthmatic patients. J Allergy Clin Immunol. 2019;143:305–15.
Article CAS PubMed Google Scholar
Uhal BD, Li XP, Piasecki CC, Molina-Molina M. Angiotensin signalling in pulmonary fibrosis. Int J Biochem Cell Biol. 2012;44:465–8.
Murphy AM, Wong AL, Bezuhly M. Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenesis Tissue Repair. 2015;8:7.
Adamcova M, Kawano I, Simko F. The impact of microRNAs in Renin-Angiotensin-System-Induced Cardiac Remodelling. Int J Mol Sci 2021, 22.
Panganiban RAM, Yang Z, Sun M, Park CY, Kasahara DI, Schaible N, et al. Antagonizing cholecystokinin A receptor in the lung attenuates obesity-induced airway hyperresponsiveness. Nat Commun. 2023;14:47.
Article CAS PubMed PubMed Central Google Scholar
Rosenson RS, Hegele RA, Fazio S, Cannon CP. The evolving future of PCSK9 inhibitors. J Am Coll Cardiol. 2018;72:314–29.
Weitz JI, Fazio S. Overview of therapeutic approaches for cholesterol lowering and attenuation of thrombosis for Prevention of Atherothrombosis. Circ Res. 2019;124:351–3.
Shapiro MD, Tavori H, Fazio S. PCSK9: from Basic Science discoveries to clinical trials. Circ Res. 2018;122:1420–38.
Ding Z, Pothineni NVK, Goel A, Luscher TF, Mehta JL. PCSK9 and inflammation: role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc Res. 2020;116:908–15.
Zou Y, Chen Z, Zhang X, Yu J, Xu H, Cui J, et al. Targeting PCSK9 ameliorates graft vascular disease in mice by inhibiting NLRP3 inflammasome activation in vascular smooth muscle cells. Front Immunol. 2022;13:894789.
Xu L, Dong XW, Shen LL, Li FF, Jiang JX, Cao R, et al. Simvastatin delivery via inhalation attenuates airway inflammation in a murine model of asthma. Int Immunopharmacol. 2012;12:556–64.
Zeki AA, Oldham J, Wilson M, Fortenko O, Goyal V, Last M et al. Statin use and asthma control in patients with severe asthma. Bmj Open 2013, 3.
Naing C, Ni H. Statins for asthma. Cochrane Database Syst Rev 2020.
Koushki K, Shahbaz SK, Mashayekhi K, Sadeghi M, Zayeri ZD, Taba MY, et al. Anti-inflammatory action of statins in Cardiovascular Disease: the role of Inflammasome and Toll-Like receptor pathways. Clin Rev Allergy Immunol. 2021;60:175–99.
Lee CG, Cho SJ, Kang MJ, Chapoval SR, Lee PJ, Noble PW, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor β-induced pulmonary fibrosis. J Exp Med. 2004;200:377–89.
Zhang K, Fan C, Cai D, Zhang Y, Zuo R, Zhu L, et al. Contribution of TGF-Beta-mediated NLRP3-HMGB1 activation to Tubulointerstitial Fibrosis in Rat with Angiotensin II-Induced chronic kidney disease. Front Cell Dev Biol. 2020;8:1.
Kang H, Seo E, Oh YS, Jun HS. TGF-β activates NLRP3 inflammasome by an autocrine production of TGF-β in LX-2 human hepatic stellate cells. Mol Cell Biochem. 2022;477:1329–38.
Leiria LO, Martins MA, Saad MJ. Obesity and asthma: beyond T(H)2 inflammation. Metabolism. 2015;64:172–81.
Kim JY, Sohn JH, Lee JH, Park JW. Obesity increases airway hyperresponsiveness via the TNF-α pathway and treating obesity induces recovery. PLoS ONE. 2015;10:e0116540.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61.
Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM, et al. Cholesterol efflux pathways suppress Inflammasome activation, NETosis, and Atherogenesis. Circulation. 2018;138:898–912.
Ding ZF, Liu SJ, Wang XW, Deng XY, Fan YB, Shahanawaz J, et al. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc Res. 2015;107:556–67.
Giunzioni I, Tavori H, Covarrubias R, Major AS, Ding L, Zhang YM, et al. Local effects of human PCSK9 on the atherosclerotic lesion. J Pathol. 2016;238:52–62.
Ricci C, Ruscica M, Camera M, Rossetti L, Macchi C, Colciago A et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci Rep 2018, 8.
Liu S, Deng X, Zhang P, Wang X, Fan Y, Zhou S, et al. Blood flow patterns regulate PCSK9 secretion via MyD88-mediated pro-inflammatory cytokines. Cardiovasc Res. 2020;116:1721–32.
Tashiro H, Kurihara Y, Kuwahara Y, Takahashi K. Impact of obesity in asthma: possible future therapies. Allergol Int. 2024;73:48–57.
Wang J, Chen L, Chen B, Meliton A, Liu SQ, Shi Y, et al. Chronic activation of the renin-angiotensin system induces lung fibrosis. Sci Rep. 2015;5:15561.
Espitia-Corredor JA, Boza P, Espinoza-Perez C, Lillo JM, Rimassa-Tare C, Machuca V, et al. Angiotensin II triggers NLRP3 inflammasome activation by a ca(2+) signaling-dependent pathway in Rat Cardiac Fibroblast Ang-II by a ca(2+)-Dependent mechanism triggers NLRP3 inflammasome in CF. Inflammation. 2022;45:2498–512.
Gao X, He X, Luo B, Peng L, Lin J, Zuo Z. Angiotensin II increases collagen I expression via transforming growth factor-beta1 and extracellular signal-regulated kinase in cardiac fibroblasts. Eur J Pharmacol. 2009;606:115–20.
Pinkerton JW, Kim RY, Brown AC, Rae BE, Donovan C, Mayall JR, et al. Relationship between type 2 cytokine and inflammasome responses in obesity-associated asthma. J Allergy Clin Immunol. 2022;149:1270–80.
Legrand-Poels S, Esser N, L’homme L, Scheen A, Paquot N, Piette J. Free fatty acids as modulators of the NLRP3 inflammasome in obesity/type 2 diabetes. Biochem Pharmacol. 2014;92:131–41.
Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci 2017, 24.
Wang C, Yu H, Wei LM, Zhang JQ, Hong MY, Chen L, et al. Protective effect of cholecystokinin octapeptide on angiotensin II-induced apoptosis in H9c2 cardiomyoblast cells. J Cell Biochem. 2020;121:3560–9.
Download references
Acknowledgements
Not applicable.
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1324).
Author information
Authors and affiliations.
Graduate School of Medicine, Yonsei University College of Medicine, Seoul, Korea
Institute of Allergy, Yonsei University College of Medicine, Seoul, Korea
Lin Liang, Sook In Chung, Tae-Eun Guon, Kyung Hee Park, Jae-Hyun Lee & Jung-Won Park
Division of Allergy and Immunology, Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul, 03722, Korea
Kyung Hee Park, Jae-Hyun Lee & Jung-Won Park
You can also search for this author in PubMed Google Scholar
Contributions
LL: Investigation and methodology, analysis of data, writing original draft; CSI & GTE: Investigation and methodology; PKH & LJH: Analysis of data, critical revision of draft; PJW: Conceptualization of study, analysis of data, writing original draft, funding acquisition. All the authors discussed the results and approved the final version of the manuscript.
Corresponding author
Correspondence to Jung-Won Park .
Ethics declarations
Ethics approval.
All animal procedures were performed according to the Institutional Animal Care and Use Committee (IACUC) regulations of Yonsei University College of Medicine (approval number: 2021 − 0256).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liang, L., Chung, S.I., Guon, TE. et al. Statin administration or blocking PCSK9 alleviates airway hyperresponsiveness and lung fibrosis in high-fat diet-induced obese mice. Respir Res 25 , 213 (2024). https://doi.org/10.1186/s12931-024-02842-x
Download citation
Received : 28 March 2024
Accepted : 07 May 2024
Published : 18 May 2024
DOI : https://doi.org/10.1186/s12931-024-02842-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (44,081,970 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap.
Author information, orcids linked to this article.
- Perl AK | 0000-0002-8445-4565
American Journal of Respiratory Cell and Molecular Biology , 01 Jan 2009 , 40(1): 1-3 https://doi.org/10.1165/rcmb.2008-0011ed PMID: 19075182 PMCID: PMC2720111
Free full text in Europe PMC
Abstract
Free full text , conditional expression of genes in the respiratory epithelium in transgenic mice.
In vitro and in vivo studies have repeatedly demonstrated the limitations and potential toxicity of various genes (proteins) used for both labeling cells (e.g., with green fluorescent protein [GFP], β-galactosidase, and luciferase) or for the deletion/addition of mutation of genes (e.g., reverse tetracycline transactivator protein [rtTA], tetracycline transactivator protein [tTA], Cre-recombinase [Cre], or Cre ER ). High levels of the introduced protein can cause endoplasmic reticulum stress, genetic instability, immunologic recognition, and/or disrupt cellular homeostasis. Resultant cell injury, death, or other off-target effects on gene expression may be caused by expression of the transgene. A number of systems for gene addition and deletion in the respiratory epithelium have been developed and widely used for the study of gene function, lung morphogenesis, and function. The Scgb1a1 (Clara Cell Secretory Protein or CCSP) and SFTPC (Surfactant Protein C or SP-C) promoters have been used by our laboratory and others ( 1 , 2 ). These promoters are highly cell specific and generate robust levels of gene expression. To conditionally express genes in the lung, transgenic mice were produced expressing the reverse tetracycline transactivator that is active when doxycycline is provided to the mouse ( 3 , 4 ). To target proximal airways, the 2.3-kb rat Scgb1a1 promoter was used to drive the reverse tetracycline activator (CCSP-rtTA transgenic mice). To target distal lung structures, we used the 3.7-kb human SFTPC promoter (SP-C-rtTA transgenic mice). Airspace enlargement unrelated to the effects of the transgene were observed in various mouse strains bearing the CCSP-rtTA transgene (line 1) ( 5 – 7 ). The potential for toxicity related to the reverse tetracycline activator, doxycycline, and Cre-recombinase was reviewed previously ( 7 ). Such overt rtTA toxicity was not seen in many experiments with the initial 3.7-kb human SP-C-rtTA transgenic mice that have been used in numerous studies.
Subsequently, both CCSP-rtTA and SP-C-rtTA mice were bred to a line of (otet) 7 CMV-Cre mice that enables doxycycline-regulated expression of Cre-recombinase in the respiratory epithelium in vivo ( 6 , 8 ). When mated to mice bearing floxed alleles, the addition of doxycycline to chow or drinking water ( 3 ) causes excision and recombination of DNA sequences located between engineered loxP recognition sites, causing deletion, mutation, or activation of the appropriately engineered floxed gene in the mouse lung ( 6 – 8 ). This system has been useful in lineage analysis, the study of gene function, and lung development. Initial studies failed to reveal significant misexpression of Cre-recombinase or overt histologic or biological toxicity.
As our laboratory has bred the SP-C-rtTA, (otet) 7 CMV-Cre mice (primarily maintained in FVBN strain) with other strains bearing floxed alleles and other mouse genetic backgrounds, we have observed off-target effects influencing both lung morphogenesis and perinatal survival. In generating mice in which GP130 receptor, mediating the activation of STAT3, was deleted ( 9 ), initial F 1 mice were bred, in line, with resultant production of two distinct strains that ( 1 ) lacked discernable phenotype, unless injured ( 9 ), or ( 2 ) died of severe lung pathology at birth. Severe morphologic effects were observed when doxycycline was provided to the dam from approximately Embryonic Day (E)6 to birth. Toxicity was not dependent on the presence of the floxed allele, but on the presence of both SP-C-rtTA and (otet) 7 CMV-Cre, indicating that toxicity was likely dependent on the expression of Cre-recombinase or the combined expression of rtTA and Cre-recombinase in this strain. Changes in lung morphogenesis and perinatal survival have not been seen if doxycycline was limited to E6.5–14.5 in a number of experiments with SP-C-rtTA, (otet) 7 CMV-Cre mice bred to a number of mouse strains in our laboratory. The distinct mouse lines produced from the initial SP-C-rtTA, (otet) 7 CMV-Cre, GP130 loxP founders maintained these distinct features, indicating the potential for the presence of inherited genes that modify susceptibility or resistance to this pulmonary toxicity. As previously noted ( 4 ), SP-C-rtTA mice (line 1) cannot be maintained in homozygous state, indicating the potential for a gene dose effect on survival. SP-C-rtTA line 1 mice express high levels of rtTA, and the activity increases in the perinatal period, consistent with the expression of endogenous Sftpc gene expression in the lung ( 3 ). Taken together, these observations demonstrate the potential for variable and strain-dependent off-target toxicity of rtTA, Cre-recombinase, and doxycycline. Because doxycycline is stored in tissues, prolonged exposure to doxycycline can result in its continued release from tissue pools. To avoid repeated or prolonged doxycycline exposure to the dams or pups, we now routinely limit the period of doxycycline treatment. The timing and duration of treatment needed to target subsets of epithelial cells have been described ( 6 , 8 ). It is sufficient to expose dams to doxycycline from E8.5 to E14.5 (SP-C-rtTA mice) and E14.5 to E18.5 (CCSP-rtTA mice) to permanently target floxed genes. To control for phenotypes not related to the activation/inactivation of the gene of interest, compound mutant mice should be tested in the absence of doxycycline. Similarly, heterozygous compound mutant mice bearing the floxed allele should be tested after doxycycline treatment. Littermate controls are used when possible to minimize strain or age related variability.
- BUILDING A BETTER MOUSE TRAP: NEW SP-C-rtTA AND CCSP-rtTA TRANSGENIC LINES
SP-C-rtTA (Line 2)
Because of high levels of non–doxycycline-dependent expression ( 3 ) and Cre-related toxicity seen when double transgenic SP-C-rtTA/otet-Cre mice were exposed to doxycycline in late gestation, we have produced a new SP-C-rtTA line (SP-C-rtTA [line 2]) and bred it to (otet) 7 CMV-Cre mice. These mice can be made homozygous for the SP-C-rtTA transgene, and are doxycycline responsive with less non–doxycycline-dependent expression (“leak”) ( 10 ). When crossed into the (otet) 7 CMV-Cre line, Cre is expressed throughout the alveolar region and peripheral bronchioles at lower levels per cell than in SP-C-rtTA/(otet) 7 CMV-Cre (line 1) ( Figure 1 ). We have not seen lung pathology or dysfunction in experiments when used to delete several genes with these mice, but our experience is not as extensive as with SP-C-rtTA (line 1). Gene deletion in the respiratory epithelium was widespread and perinatal toxicity in the absence of floxed alleles has not been seen to date. Thus, this new SP-C-rtTA (line 2) may have features that have advantages compared with SP-C-rtTA (line 1).

Immunohistochemistry for Cre protein expression is shown after 48 hours of doxycycline treatment of adult transgenic mice. SP-C-rtTA (line 1) and SP-C-rtTA (line 2) otet-Cre mice express Cre in conducting airways ( open arrowheads ) and alveolar type II cells ( arrowheads in A and B ). ( C ) CCSP-rtTA (line 1) mice express Cre at high levels in a subset of nonciliated bronchiolar and alveolar cells. ( D ) With the CCSP-rtTA (line 2) mice, Cre was readily detected at high levels in most non-ciliated bronchiolar epithelial cells and staining was rarely seen in alveolar type II cells.
CCSP-rtTA (Line 2)
CCSP-rtTA mice (line 1) commonly develop postnatal airspace enlargement ( 5 , 6 , 11 , 12 ). The presence and severity of emphysema varies with mouse strain, occurs postnatally, and is not dependent upon doxycycline. In CCSP-rtTA (line 1), rtTA expression is directed to subsets of conducting airway epithelial cells, as well as to a subset of alveolar type II cells, consistent with the site of expression of the endogenous CCSP in the rat lung ( 1 , 6 ). Because of these limitations, we have generated a new doxycycline-inducible Clara cell–specific rtTA transgenic mouse line (CCSP-rtTA [line 2]) by oocyte injection of the initial 2.3-kb rat Scgb1a1 -rtTA construct. Seventeen new CCSP-rtTA transgenic founder lines were produced. The extent and specificity of Clara cell targeting was verified by screening for doxycycline-inducible Cre expression. In the new line, CCSP-rtTA (line 2), conditional gene expression was doxycycline dependent and specific to conducting airway cells, with little expression in the alveolus. Minimal focal airspace enlargement was observed in the adult mice. When bred to the (otet)-Cre mice, Cre was conditionally expressed when mice were exposed to doxycycline from E14.5-birth, as well after short-term (48 h) exposure to doxycycline in adults. Expression of Cre-recombinase in the absence of doxycycline was not detected in the conducting airways. After 48 to 72 hours of doxycycline treatment, Cre expression was detected in most nonciliated respiratory epithelial cells and rarely in alveolar type II cells. When mated to ZEG mice (bearing a lox-stop construct expressing GFP after recombination) and exposed to doxycycline from E14.5 to birth, extensive GFP expression was noted in the adult lung, indicating the long-term survival of cells expressing GFP in this model. Thus, this new transgenic line (CCSP-rtTA [line 2]) has advantages of specificity and lack of overt off-target toxicity compared with the previous mouse (CCSP-rtTA [line 1]). Sites and intensity of Cre expression in the new and previous mouse lines are compared in Figure 1 ( 6 , 8 , 10 ). It should be noted that both the SP-C-rtTA and the CCSP-rtTA promoters may be influenced by the genes being conditionally regulated, thereby influencing the sites of transgene expression.
- SUGGESTED GUIDELINES FOR USE OF SP-C-ttTA/tetO-CRE AND CCSP-rtTA/tetO-CRE MICE
Many systems for conditional gene addition, mutation, and deletion have been developed, and all have potential problems. Nevertheless, these systems are useful for the study of gene function and are applicable to many areas of lung biology. These mice have been widely provided to the research community and experience with this system has increased. We can suggest some guidelines for their use that include breeding strategies, limitations of the timing and duration of exposure to doxycycline, and the distinct features of the mouse lines that we have produced to date. Critical to the avoidance or minimization of off-target effects are the recognition that rtTA, Cre-recombinase, and doxycycline are potentially toxic, and that toxicity may be combinatorial and may vary among various mouse strains. Considerations for timing of doxycycline to target gene expression and recombination to specific subsets of cells have been described previously ( 6 , 8 ). We hope that this information will minimize off-target errors and conclusions derived from the use of previous and new transgenic lines that we have made widely available to the research community.
This work was supported by National Institutes of Health HL090156 and HL085610 (J.A.W.).
Full text links
Read article at publisher's site: https://doi.org/10.1165/rcmb.2008-0011ed
Free after 12 months at intl-ajrcmb.atsjournals.org http://intl-ajrcmb.atsjournals.org/cgi/reprint/40/1/1.pdf
Free to read at intl-ajrcmb.atsjournals.org http://intl-ajrcmb.atsjournals.org/cgi/content/abstract/40/1/1
Free after 12 months at intl-ajrcmb.atsjournals.org http://intl-ajrcmb.atsjournals.org/cgi/content/full/40/1/1
Citations & impact
Impact metrics, citations of article over time, article citations, alveolar repair following lps-induced injury requires cell-ecm interactions..
Sucre JM , Bock F , Negretti NM , Benjamin JT , Gulleman PM , Dong X , Ferguson KT , Jetter CS , Han W , Liu Y , Kook S , Gokey JJ , Guttentag SH , Kropski JA , Blackwell TS , Zent R , Plosa EJ
JCI Insight , 8(14):e167211, 24 Jul 2023
Cited by: 2 articles | PMID: 37279065 | PMCID: PMC10443799
Lentiviral vectors for inducible, transactivator-free advanced therapy medicinal products: Application to CAR-T cells.
Tristán-Manzano M , Maldonado-Pérez N , Justicia-Lirio P , Cortijo-Gutierréz M , Tristán-Ramos P , Blanco-Benítez C , Pavlovic K , Aguilar-González A , Muñoz P , Molina-Estevez FJ , Griesche V , Marchal JA , Heras SR , Benabdellah K , Martin F
Mol Ther Nucleic Acids , 32:322-339, 28 Mar 2023
Cited by: 0 articles | PMID: 37125150 | PMCID: PMC10141506
Simultaneous isolation of proximal and distal lung progenitor cells from individual mice using a 3D printed guide reduces proximal cell contamination of distal lung epithelial cell isolations.
Alsafadi HN , Stegmayr J , Ptasinski V , Silva I , Mittendorfer M , Murray LA , Wagner DE
Stem Cell Reports , 17(12):2718-2731, 01 Dec 2022
Cited by: 0 articles | PMID: 36460000 | PMCID: PMC9768627
RNA-Binding Protein HuR Promotes Airway Inflammation in a House Dust Mite-Induced Allergic Asthma Model.
Herjan T , Xiao J , Dziendziel Kolanek M
J Interferon Cytokine Res , 42(1):29-38, 01 Jan 2022
Cited by: 1 article | PMID: 35041516 | PMCID: PMC8787712
A mouse model for the study of anti-tumor T cell responses in Kras-driven lung adenocarcinoma.
Fitzgerald B , Connolly KA , Cui C , Fagerberg E , Mariuzza DL , Hornick NI , Foster GG , William I , Cheung JF , Joshi NS
Cell Rep Methods , 1(5):100080, 16 Sep 2021
Cited by: 10 articles | PMID: 34632444 | PMCID: PMC8500377
Data that cites the article
This data has been provided by curated databases and other sources that have cited the article.
Mouse Genome Informatics (MGI)
- http://www.informatics.jax.org/reference/19075182
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Conditional gene expression in the respiratory epithelium of the mouse.
Perl AK , Tichelaar JW , Whitsett JA
Transgenic Res , 11(1):21-29, 01 Feb 2002
Cited by: 173 articles | PMID: 11874100
Human SP-C gene sequences that confer lung epithelium-specific expression in transgenic mice.
Glasser SW , Burhans MS , Eszterhas SK , Bruno MD , Korfhagen TR
Am J Physiol Lung Cell Mol Physiol , 278(5):L933-45, 01 May 2000
Cited by: 33 articles | PMID: 10781423
Secretion-competent mouse tracheal epithelial cell culture from the genetically altered mouse: pathway analysis via gene array.
Martin LD , Adler KB , Akley NJ , Crews A , Sharova L
Chest , 121(3 suppl):79S, 01 Mar 2002
Cited by: 1 article | PMID: 11893699
Cell-, age-, and phenotype-dependent differences in the control of gene expression.
Stenmark KR
Am J Physiol Lung Cell Mol Physiol , 281(4):L762-5, 01 Oct 2001
Cited by: 2 articles | PMID: 11557579
Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion.
Adler KB , Li Y
Am J Respir Cell Mol Biol , 25(4):397-400, 01 Oct 2001
Cited by: 36 articles | PMID: 11694442
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: HL085610
9 publication s
Grant ID: HL090156
19 publication s
Europe PMC is part of the ELIXIR infrastructure

IMAGES
VIDEO
COMMENTS
Abstract. Transgenic mouse models mediating conditional temporal and spatial regulation of gene expression to the respiratory epithelium were developed utilizing the reverse tetracycline transactivator (rtTA) expressed under the control of SP-C and CCSP promoters. Luciferase activity was detected in the lungs of fetal and adult double ...
A number of systems for gene addition and deletion in the respiratory epithelium have been developed and widely used for the study of gene function, lung morphogenesis, and function. The Scgb1a1 (Clara Cell Secretory Protein or CCSP) and SFTPC (Surfactant Protein C or SP-C) promoters have been used by our laboratory and others ( 1 , 2 ).
Transgenic mouse models mediating conditional temporal and spatial regulation of gene expression to the respiratory epithelium were developed utilizing the reverse tetracycline transactivator (rtTA) expressed under the control of SP-C and CCSP promoters, and will be useful for the study of gene function in the lung. Transgenic mouse models mediating conditional temporal and spatial regulation ...
Conditional gene expression in the respiratory epithelium of the mouse Anne-Karina T. Perl1, Jay W. Tichelaar1 & Jeffrey A. Whitsett1,∗ 1Children's Hospital Medical Center, Division of Pulmonary Biology, Cincinnati, Ohio, USA Received 1 March 2001; revised 8 May 2001; accepted 16 May 2001
Resultant cell injury, death, or other off-target effects on gene expression may be caused by expression of the transgene. A number of systems for gene addition and deletion in the respiratory epithelium have been developed and widely used for the study of gene function, lung morphogenesis, and function.
Summary. In summary, a number of transgenic systems have been devel-oped that enable conditional control of gene expression in vivo and in vitro. These systems have been adapted for use in respira-tory epithelial cells. As in all experiments, each variable that is introduced into the system may influence the outcome and interpretation of ...
This website requires cookies, and the limited processing of your personal data in order to function. By using the site you are agreeing to this as outlined in our privacy notice and cookie policy.
The SP-C-rtTA and CCSP-rtTA activator mice control the expression of transgenes in the developing and mature respiratory epithelium, and will be useful for the study of gene function in the lung ...
Transgenic mouse models mediating conditional temporal and spatial regulation of gene expression to the respiratory epithelium were developed utilizing the reverse tetracycline transactivator (rtTA) expressed under the control of SP-C and CCSP promoters, and will be useful for the study of gene function in the lung. ...
Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Am J Respir Cell Mol Biol 2009; 40 :1-3. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Transgenic mouse models mediating conditional temporal and spatial regulation of gene expression to the respiratory epithelium were developed utilizing the reverse tetracycline transactivator (rtTA) expressed under the control of SP-C and CCSP promoters. Luciferase activity was detected in the lungs of fetal and adult double transgenic mice but was not detected in other tissues or in single ...
In such studies, standard gene-targeting techniques are used to produce a mouse in which an essential region of a gene of interest is floxed, so that tissue-specific cre expression results in the ...
To identify relationships amongst tracheal and alveolar epithelial cells during lung development, we used conditional systems controlled by the rat CCSP and human SFTPC gene promoters to express Cre-recombinase in the developing mouse lung, thereby permanently labeling cells by expression of alkaline phosphatase or green fluorescent protein. When controlled by the rat CCSP promoter, continuous ...
In summary, a number of transgenic systems have been developed that enable conditional control of gene expression in vivo and in vitro. These systems have been adapted for use in respiratory epithelial cells. As in all experiments, each variable that is introduced into the system may influence the outcome and interpretation of experiments.
Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Am J Respir Cell Mol Biol 2009 ;40: 1 - 3 . Abstract , Medline , Google Scholar
We generated a transgenic mouse model with conditional loss of E-cadherin in lung epithelial cells at birth and onwards. ... J. W. & Whitsett, J. A. Conditional gene expression in the respiratory ...
Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung Dev Dyn. 2009 Aug;238(8) :1999-2013. ... the expression patterns of key signaling molecules implicated in branching morphogenesis were altered and a proximal lung marker was expanded distally. Our results ...
Conditional expression of Cre-recombinase in the respiratory epithelium provides a useful model for the study of gene expression and function in the mouse respiratory tract and in the lung. View ...
DOI: 10.1165/RCMB.F310 Corpus ID: 24120943; Conditional control of gene expression in the respiratory epithelium: A cautionary note. @article{Whitsett2006ConditionalCO, title={Conditional control of gene expression in the respiratory epithelium: A cautionary note.}, author={Jeffrey A. Whitsett and Anne-Karina T. Perl}, journal={American journal of respiratory cell and molecular biology}, year ...
IHS for NLRP3 and caspase-1 revealed heightened expression in the respiratory epithelium of HFD mice, a phenomenon effectively mitigated by alirocumab (3 and 10 mg/kg) or statin administration (Fig. 6A-C). To further validate these histological findings, we assessed the mRNA expression of NLRP3 in the lungs.
Peroxiredoxin 1 (Prx1) is an antioxidant protein that may promote the carcinogenesis in oral leukoplakia (OLK). To investigate the effect of Prx1 on the oral mucosal epithelium of OLK, we generated a Prx1 conditional knockout (cKO) mouse model. The mRNA and gRNA were generated using the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9 ...
The epithelial cell adhesion molecule (EpCAM) is a single transmembrane protein on the cell surface. Given its strong expression on epithelial cells and epithelial cell-derived tumors, EpCAM has been identified as a biomarker for circulating tumor cells (CTCs) and exosomes and a target for cancer therapy. As a cell adhesion molecule, EpCAM has a crystal structure that indicates that it forms a ...
Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Sign in | Create an account. https://orcid.org. Europe PMC. Menu ...
Uncovering the function of understudied G protein-coupled receptors (GPCRs) provides a wealth of untapped therapeutic potential. The poorly understood adhesion GPCR Gpr126 (Adgrg6) is widely expressed in developing kidneys. In adulthood, Gpr126 expression is enriched in parietal epithelial cells (PECs) and epithelial cells of the collecting duct and urothelium. Whether Gpr126 plays a role in ...