Introducing AARP Members Edition: Your source for tips to live well, members-only games, exclusive interviews, recipes and more.
AARP daily Crossword Puzzle
Hotels with AARP discounts
Life Insurance
AARP Dental Insurance Plans
AARP MEMBERSHIP
AARP Membership — $12 for your first year when you sign up for Automatic Renewal
Get instant access to members-only products, hundreds of discounts, a free second membership, and a subscription to AARP the Magazine. Find how much you can save in a year with a membership. Learn more.
- right_container
Work & Jobs
Social Security
AARP en Español
- Membership & Benefits
- Members Edition
AARP Rewards
- AARP Rewards %{points}%
Conditions & Treatments
Drugs & Supplements
Health Care & Coverage
Health Benefits

AARP Hearing Center
Advice on Tinnitus and Hearing Loss

Get Happier
Creating Social Connections

Brain Health Resources
Tools and Explainers on Brain Health

Your Health
8 Major Health Risks for People 50+
Scams & Fraud
Personal Finance
Money Benefits

View and Report Scams in Your Area

AARP Foundation Tax-Aide
Free Tax Preparation Assistance
AARP Money Map
Get Your Finances Back on Track

How to Protect What You Collect
Small Business
Age Discrimination

Flexible Work
Freelance Jobs You Can Do From Home

AARP Skills Builder
Online Courses to Boost Your Career

31 Great Ways to Boost Your Career

ON-DEMAND WEBINARS
Tips to Enhance Your Job Search

Get More out of Your Benefits

When to Start Taking Social Security

10 Top Social Security FAQs

Social Security Benefits Calculator

Medicare Made Easy
Original vs. Medicare Advantage

Enrollment Guide
Step-by-Step Tool for First-Timers

Prescription Drugs
9 Biggest Changes Under New Rx Law

Medicare FAQs
Quick Answers to Your Top Questions
Care at Home
Financial & Legal
Life Balance

LONG-TERM CARE
Understanding Basics of LTC Insurance

State Guides
Assistance and Services in Your Area

Prepare to Care Guides
How to Develop a Caregiving Plan

End of Life
How to Cope With Grief, Loss
Recently Played
Word & Trivia
Atari® & Retro
Members Only
Staying Sharp
Mobile Apps
More About Games

Right Again! Trivia

Right Again! Trivia – Sports

Atari® Video Games

Throwback Thursday Crossword
Travel Tips
Vacation Ideas
Destinations
Travel Benefits

Outdoor Vacation Ideas
Camping Vacations
Plan Ahead for Summer Travel

AARP National Park Guide
Discover Canyonlands National Park

History & Culture
8 Amazing American Pilgrimages
Entertainment & Style
Family & Relationships
Personal Tech
Home & Living
Celebrities
Beauty & Style

Movies for Grownups
Summer Movie Preview

Jon Bon Jovi’s Long Journey Back

Looking Back
50 World Changers Turning 50

Sex & Dating
Spice Up Your Love Life

Friends & Family
How to Host a Fabulous Dessert Party

Home Technology
Caregiver’s Guide to Smart Home Tech

Virtual Community Center
Join Free Tech Help Events

Create a Hygge Haven

Soups to Comfort Your Soul
AARP Solves 25 of Your Problems
Driver Safety
Maintenance & Safety
Trends & Technology

AARP Smart Guide
How to Clean Your Car

We Need To Talk
Assess Your Loved One's Driving Skills

AARP Smart Driver Course

Building Resilience in Difficult Times

Tips for Finding Your Calm

Weight Loss After 50 Challenge

Cautionary Tales of Today's Biggest Scams

7 Top Podcasts for Armchair Travelers

Jean Chatzky: ‘Closing the Savings Gap’

Quick Digest of Today's Top News

AARP Top Tips for Navigating Life

Get Moving With Our Workout Series
You are now leaving AARP.org and going to a website that is not operated by AARP. A different privacy policy and terms of service will apply.
Go to Series Main Page

What is Medicare assignment and how does it work?
Kimberly Lankford,
Because Medicare decides how much to pay providers for covered services, if the provider agrees to the Medicare-approved amount, even if it is less than they usually charge, they’re accepting assignment.
A doctor who accepts assignment agrees to charge you no more than the amount Medicare has approved for that service. By comparison, a doctor who participates in Medicare but doesn’t accept assignment can potentially charge you up to 15 percent more than the Medicare-approved amount.
That’s why it’s important to ask if a provider accepts assignment before you receive care, even if they accept Medicare patients. If a doctor doesn’t accept assignment, you will pay more for that physician’s services compared with one who does.

Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine. Find out how much you could save in a year with a membership. Learn more.
How much do I pay if my doctor accepts assignment?
If your doctor accepts assignment, you will usually pay 20 percent of the Medicare-approved amount for the service, called coinsurance, after you’ve paid the annual deductible. Because Medicare Part B covers doctor and outpatient services, your $240 deductible for Part B in 2024 applies before most coverage begins.
All providers who accept assignment must submit claims directly to Medicare, which pays 80 percent of the approved cost for the service and will bill you the remaining 20 percent. You can get some preventive services and screenings, such as mammograms and colonoscopies , without paying a deductible or coinsurance if the provider accepts assignment.
What if my doctor doesn’t accept assignment?
A doctor who takes Medicare but doesn’t accept assignment can still treat Medicare patients but won’t always accept the Medicare-approved amount as payment in full.
This means they can charge you up to a maximum of 15 percent more than Medicare pays for the service you receive, called “balance billing.” In this case, you’re responsible for the additional charge, plus the regular 20 percent coinsurance, as your share of the cost.
How to cover the extra cost? If you have a Medicare supplement policy , better known as Medigap, it may cover the extra 15 percent, called Medicare Part B excess charges.
All Medigap policies cover Part B’s 20 percent coinsurance in full or in part. The F and G policies cover the 15 percent excess charges from doctors who don’t accept assignment, but Plan F is no longer available to new enrollees, only those eligible for Medicare before Jan. 1, 2020, even if they haven’t enrolled in Medicare yet. However, anyone who is enrolled in original Medicare can apply for Plan G.
Remember that Medigap policies only cover excess charges for doctors who accept Medicare but don’t accept assignment, and they won’t cover costs for doctors who opt out of Medicare entirely.
Good to know. A few states limit the amount of excess fees a doctor can charge Medicare patients. For example, Massachusetts and Ohio prohibit balance billing, requiring doctors who accept Medicare to take the Medicare-approved amount. New York limits excess charges to 5 percent over the Medicare-approved amount for most services, rather than 15 percent.

AARP NEWSLETTERS

%{ newsLetterPromoText }%
%{ description }%
Privacy Policy
ARTICLE CONTINUES AFTER ADVERTISEMENT
How do I find doctors who accept assignment?
Before you start working with a new doctor, ask whether he or she accepts assignment. About 98 percent of providers billing Medicare are participating providers, which means they accept assignment on all Medicare claims, according to KFF.
You can get help finding doctors and other providers in your area who accept assignment by zip code using Medicare’s Physician Compare tool .
Those who accept assignment have this note under the name: “Charges the Medicare-approved amount (so you pay less out of pocket).” However, not all doctors who accept assignment are accepting new Medicare patients.
AARP® Vision Plans from VSP™
Vision insurance plans designed for members and their families
What does it mean if a doctor opts out of Medicare?
Doctors who opt out of Medicare can’t bill Medicare for services you receive. They also aren’t bound by Medicare’s limitations on charges.
In this case, you enter into a private contract with the provider and agree to pay the full bill. Be aware that neither Medicare nor your Medigap plan will reimburse you for these charges.
In 2023, only 1 percent of physicians who aren’t pediatricians opted out of the Medicare program, according to KFF. The percentage is larger for some specialties — 7.7 percent of psychiatrists and 4.2 percent of plastic and reconstructive surgeons have opted out of Medicare.
Keep in mind
These rules apply to original Medicare. Other factors determine costs if you choose to get coverage through a private Medicare Advantage plan . Most Medicare Advantage plans have provider networks, and they may charge more or not cover services from out-of-network providers.
Before choosing a Medicare Advantage plan, find out whether your chosen doctor or provider is covered and identify how much you’ll pay. You can use the Medicare Plan Finder to compare the Medicare Advantage plans and their out-of-pocket costs in your area.
Return to Medicare Q&A main page
Kimberly Lankford is a contributing writer who covers Medicare and personal finance. She wrote about insurance, Medicare, retirement and taxes for more than 20 years at Kiplinger’s Personal Finance and has written for The Washington Post and Boston Globe . She received the personal finance Best in Business award from the Society of American Business Editors and Writers and the New York State Society of CPAs’ excellence in financial journalism award for her guide to Medicare.
Unlock Access to AARP Members Edition
Already a Member? Login

More on Medicare

How Do I Create a Personal Online Medicare Account?
You can do a lot when you decide to look electronically

I Got a Medicare Summary Notice in the Mail. What Is It?
This statement shows what was billed, paid in past 3 months

Understanding Medicare’s Options: Parts A, B, C and D
Making sense of the alphabet soup of health care choices
Recommended for You
AARP Value & Member Benefits

Learn, earn and redeem points for rewards with our free loyalty program

AARP® Dental Insurance Plan administered by Delta Dental Insurance Company
Dental insurance plans for members and their families

The National Hearing Test
Members can take a free hearing test by phone

AARP® Staying Sharp®
Activities, recipes, challenges and more with full access to AARP Staying Sharp®
SAVE MONEY WITH THESE LIMITED-TIME OFFERS

Speak with a Licensed Insurance Agent
- (888) 335-8996
Medicare Assignment
Home / Medicare 101 / Medicare Costs / Medicare Assignment
Summary: If a provider accepts Medicare assignment, they accept the Medicare-approved amount for a covered service. Though most providers accept assignment, not all do. In this article, we’ll explain the differences between participating, non-participating, and opt-out providers. You’ll also learn how to find physicians in your area who accept Medicare assignment. Estimated Read Time: 5 min
What is Medicare Assignment
Medicare assignment is an agreement by your doctor or other healthcare providers to accept the Medicare-approved amount as the full cost for a covered service. Providers who “accept assignment” bill Medicare directly for Part B-covered services and cannot charge you more than the applicable deductible and coinsurance.
Most healthcare providers who opt-in to Medicare accept assignment. In fact, CMS reported in its Medicare Participation for Calendar Year 2024 announcement that 98 percent of Medicare providers accepted assignment in 2023.
Providers who accept Medicare are divided into two groups: Participating providers and non-participating providers. Providers can decide annually whether they want to participate in Medicare assignment, or if they want to be non-participating.
Providers who do not accept Medicare Assignment can charge up to 15% above the Medicare-approved cost for a service. If this is the case, you will be responsible for the entire amount (up to 15%) above what Medicare covers.
Below, we’ll take a closer look at participating, non-participating, and opt-out physicians.
Medicare Participating Providers: Providers Who Accept Medicare Assignment
Healthcare providers who accept Medicare assignment are known as “participating providers”. To participate in Medicare assignment, a provider must enter an agreement with Medicare called the Participating Physician or Supplier Agreement. When a provider signs this agreement, they agree to accept the Medicare-approved charge as the full charge of the service. They cannot charge the beneficiary more than the applicable deductible and coinsurance for covered services.
Each year, providers can decide whether they want to be a participating or non-participating provider. Participating in Medicare assignment is not only beneficial to patients, but to providers as well. Participating providers get paid by Medicare directly, and when a participating provider bills Medicare, Medicare will automatically forward the claim information to Medicare Supplement insurers. This makes the billing process much easier on the provider’s end.
Medicare Non-Participating Providers: Providers Who Don’t Accept Assignment
Healthcare providers who are “non-participating” providers do not agree to accept assignment and can charge up to 15% over the Medicare-approved amount for a service. Non-participating Medicare providers still accept Medicare patients. However they have not agreed to accept the Medicare-approved cost as the full cost for their service.
Doctors who do not sign an assignment agreement with Medicare can still choose to accept assignment on a case-by-case basis. When non-participating providers do add on excess charges , they cannot charge more than 15% over the Medicare-approved amount. It’s worth noting that providers do not have to charge the maximum 15%; they may only charge 5% or 10% over the Medicare-approved amount.
When you receive a Medicare-covered service at a non-participating provider, you may need to pay the full amount at the time of your service; a claim will need to be submitted to Medicare for you to be reimbursed. Prior to receiving care, your provider should give you an Advanced Beneficiary Notice (ABN) to read and sign. This notice will detail the services you are receiving and their costs.
Non-participating providers should include a CMS-approved unassigned claim statement in the additional information section of your Advanced Beneficiary Notice. This statement will read:
“This supplier doesn’t accept payment from Medicare for the item(s) listed in the table above. If I checked Option 1 above, I am responsible for paying the supplier’s charge for the item(s) directly to the supplier. If Medicare does pay, Medicare will pay me the Medicare-approved amount for the item(s), and this payment to me may be less than the supplier’s charge.”
This statement basically summarizes how excess charges work: Medicare will pay the Medicare-approved amount, but you may end up paying more than that.
Your provider should submit a claim to Medicare for any covered services, however, if they refuse to submit a claim, you can do so yourself by using CMS form 1490S .
Opt-Out Providers: What You Need to Know
Opt-out providers are different than non-participating providers because they completely opt out of Medicare. What does this mean for you? If you receive supplies or services from a provider who opted out of Medicare, Medicare will not pay for any of it (except for emergencies).
Physicians who opt-out of Medicare are even harder to find than non-participating providers. According to a report by KFF.org, only 1.1% of physicians opted out of Medicare in 2023. Of those who opted out, most are physicians in specialty fields such as psychiatry, plastic and reconstructive surgery, and neurology.
How to Find A Doctor Who Accepts Medicare Assignment
Finding a doctor who accepts Medicare patients and accepts Medicare assignment is generally easier than finding a provider who doesn’t accept assignment. As we mentioned above, of all the providers who accept Medicare patients, 98 percent accept assignment.
The easiest way to find a doctor or healthcare provider who accepts Medicare assignment is by visiting Medicare.gov and using their Compare Care Near You tool . When you search for providers in your area, the Care Compare tool will let you know whether a provider is a participating or non-participating provider.
If a provider is part of a group practice that involves multiple providers, then all providers in that group must have the same participation status. As an example, we have three doctors, Dr. Smith, Dr. Jones, and Dr. Shoemaker, who are all part of a group practice called “Health Care LLC”. The group decides to accept Medicare assignment and become a participating provider. Dr. Smith decides he does not want to accept assignment, however, because he is part of the “Health Care LLC” group, he must remain a participating provider.
Using Medicare’s Care Compare tool, you can select a group practice and see their participation status. You can then view all providers who are part of that group. This makes finding doctors who accept assignment even easier.
To ensure you don’t end up paying more out-of-pocket costs than you anticipated, it’s always a good idea to check with your provider if they are a participating Medicare provider. If you have questions regarding Medicare assignment or are having trouble determining whether a provider is a participating provider, you can contact Medicare directly at 1-800-633-4227. If you have questions about excess charges or other Medicare costs and would like to speak with a licensed insurance agent, you can contact us at the number above.
Announcement About Medicare Participation for Calendar Year 2024, Centers for Medicare & Medicaid Services. Accessed January 2024
https://www.cms.gov/files/document/medicare-participation-announcement.pdf
Annual Medicare Participation Announcement, CMS.gov. Accessed January 2024
https://www.cms.gov/medicare-participation
Does Your Provider Accept Medicare as Full Payment? Medicare.gov. Accessed January 2024
https://www.medicare.gov/basics/costs/medicare-costs/provider-accept-Medicare

Thomas Liquori

Ashlee Zareczny

- Medicare Eligibility Requirements
- Medicare Enrollment Documents
- Apply for Medicare While Working
- Guaranteed Issue Rights
- Medicare by State
- Web Stories
- Online Guides
- Calculators & Tools
© 2024 Apply for Medicare. All Rights Reserved.
Owned by: Elite Insurance Partners LLC. This website is not connected with the federal government or the federal Medicare program. The purpose of this website is the solicitation of insurance. We do not offer every plan available in your area. Currently we represent 26 organizations which offer 3,740 products in your area. Please contact Medicare.gov or 1-800-MEDICARE or your local State Health Insurance Program to get information on all of your options.
Let us help you find the right Medicare plans today!
Simply enter your zip code below
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
CMS Newsroom
Search cms.gov.
- Physician Fee Schedule
- Local Coverage Determination
- Medically Unlikely Edits
Annual Medicare Participation Announcement
Annual Medicare Participation Open Enrollment Period
Read this year's Announcement (PDF) about the annual Medicare participation open enrollment period.
Every year from mid-November through December 31, providers can decide if they want to participate in Medicare for the upcoming year. In early to mid-November, your MAC will send a post card reminding you about the annual participation open enrollment period.
We’re proud to share that 98% of providers participate in Medicare. As you plan for 2022, this announcement provides information that may help you determine whether you want to continue or become a Medicare participating (PAR) provider.
We pledge to work with you to put patients first. To do this, we must empower patients and providers to work together to make the best health care decisions for patients.
Participating vs. Non-Participating Medicare “participation” means you agree to accept claims assignment for all Medicare-covered services to your patients. By accepting assignment, you agree to accept Medicare-allowed amounts as payment in full. You may not collect more from the patient than the Medicare deductible and coinsurance or copayment .
| Participating Provider or Supplier | Non-Participating Provider or Supplier |
|---|---|
Choose the situation that applies to you to find out what to do between mid-November and December 31 each year.
You don’t need to do anything.
Complete the Medicare Participating Physician or Supplier Agreement (CMS-460) (PDF) and mail it (or a copy) to each MAC to which you’ll send Part B claims.
Submit the Medicare Participating Physician or Supplier Agreement (CMS-460) (PDF) electronically with your enrollment application.
Write to each MAC to which you send Part B claims telling them that you’re terminating your participation in Medicare effective January 1. This written notice must be postmarked before December 31 of the previous effective year.

What You Need to Know About Medicare Assignment
If you are one of the more than 63 million Americans enrolled in Medicare and are on the lookout for a new provider, you may wonder what your options are. A good place to start? Weighing the pros and cons of choosing an Original Medicare plan versus a Medicare Advantage plan—both of which have their upsides.
Let’s say you decide on an Original Medicare plan, which many U.S. doctors accept. In your research, however, you come across the term “Medicare assignment.” Cue the head-scratching. What exactly does that mean, and how might it affect your coverage costs?
What is Medicare Assignment?
It turns out that Medicare assignment is a concept you need to understand before seeing a new doctor. First things first: Ask your doctor if they “accept assignment”—that exact phrasing—which means they have agreed to accept a Medicare-approved amount as full payment for any Medicare-covered service provided to you. If your doctor accepts assignment, that means they’ll send your whole medical bill to Medicare, and then Medicare pays 80% of the cost, while you are responsible for the remaining 20%.
A doctor who doesn’t accept assignment, however, could charge up to 15% more than the Medicare-approved amount for their services, depending on what state you live in, shouldering you with not only that additional cost but also your 20% share of the original cost. Additionally, the doctor is supposed to submit your claim to Medicare, but you may have to pay them on the day of service and then file a reimbursement claim from Medicare after the fact.
Worried that your doctor will not accept assignment? Luckily, 98% of U.S. physicians who accept Medicare patients also accept Medicare assignment, according to the U.S. Centers for Medicare & Medicaid Services (CMS). They are known as assignment providers, participating providers, or Medicare-enrolled providers.
It can be confusing. Here’s how to assess whether your provider accepts Medicare assignment, and what that means for your out-of-pocket costs:
The 3 Types of Original Medicare Providers
1. participating providers, or those who accept medicare assignment.
These providers have an agreement with Medicare to accept the Medicare-approved amount as full payment for their services. You don’t have to pay anything other than a copay or coinsurance (depending on your plan) at the time of your visit. Typically, Medicare pays 80% of the cost, while you are responsible for the remaining 20%, as long as you have met your deductible.
2. Non-participating providers
“Most providers accept Medicare, but a small percentage of doctors are known as non-participating providers,” explains Caitlin Donovan, senior director of public relations at the National Patient Advocate Foundation (NPAF) in Washington D.C. “These may be more expensive,” she adds. Also known as non-par providers, these physicians may accept Medicare patients and insurance, but they have not agreed to take assignment Medicare in all cases. That means they’re not held to the Medicare-approved amount as payment in full. As a reminder, a doctor who doesn’t accept assignment can charge up to 15% more than the Medicare-approved amount, depending on what part of the country you live in, and you will have to pay that additional amount plus your 20% share of the original cost.
What does that mean for you? Besides being charged more than the Medicare-approved amount, you might also be required to do some legwork to get reimbursed by Medicare.
- You may have to pay the entire bill at the time of service and wait to be reimbursed 80% of the Medicare-approved amount. In most cases, the provider will submit the claim for you. But sometimes, you’ll have to submit it yourself.
- Depending on the state you live in, the provider may also charge you as much as 15% more than the Medicare-approved amount. (In New York state, for example, that add-on charge is limited to 5%.) This is called a limiting charge—and the difference, called the balance bill, is your responsibility.
There are some non-par providers, however, who accept Medicare assignment for certain services, on a case-by-case basis. Those may include any of the services—anything from hospital and hospice care to lab tests and surgery—available from any assignment-accepting doctor, with a key exception: If a non-par provider accepts assignment for a particular service, they cannot bill you more than the regular Medicare deductible and coinsurance amount for that specific treatment. Just as it’s important to confirm whether your doctor accepts assignment, it’s also important to confirm which services are included at assignment.
3. Opt-out providers
A small percentage of providers do not participate in Medicare at all. In 2020, for example, only 1% of all non-pediatric physicians nationwide opted out, and of that group, 42% were psychiatrists. “Some doctors opt out of providing Medicare coverage altogether,” notes Donovan.“In that case, the patient would pay privately.” If you were interested in seeing a physician who had opted out of Medicare, you would have to enter a private contract with that provider, and neither you nor the provider would be eligible for reimbursement from Medicare.
How do I know if my doctor accepts Medicare assignment?
The best way to find out whether your provider accepts Medicare assignment is simply to ask. First, confirm whether they are participating or non-participating—and if they are non-participating, ask whether they accept Medicare assignment for certain services.
Also, make sure to ask your provider exactly how they will be billing Medicare and what charges you might expect at the time of your visit so that you’re on the same page from the start.
Is seeing a non-participating provider who accepts Medicare assignment more expensive?
The short answer is yes. There are usually out-of-pocket costs after you’re reimbursed. But it may not cost as much as you think, and it may not be much more than if you see a participating provider. Still, it could be challenging if you’re on a fixed income.
For example, let’s say you’re seeing a physical therapist who accepts Medicare patients but not Medicare assignment. Medicare will pay $95 per visit to the provider; but your provider bills the service at $115. In most states, you’re responsible for a 15% limiting charge above $95. In this case, your bill would be 115% of $95, or $109.25.
Once you get your $95 reimbursement back from Medicare, your cost for the visit—the balance bill—would be $14.25 (plus any deductibles or copays) .
In some states, the maximum cap on the limiting charge is less than 15%. As mentioned earlier, New York state, for instance, allows only a 5% surcharge, which means that physical therapy appointment would cost you just $4.75 extra.
Bottom line: Medicare assignment providers and non-participating providers who agree to accept Medicare assignment are both viable options for patients. So if you want to see a particular provider, don’t rule them out just because they’re non-par.
While seeing a non-participating provider may still be affordable, ultimately, the biggest headache may be keeping track of claims and reimbursements, or simply setting aside the right amount of money to pay for your visit up front.
Before you schedule a visit, be sure to ask how much the service will cost. You can also estimate the payment amount based on Medicare-approved charges. A good place to start is this out-of-pocket expense calculator provided by the CMS.
What if I see a provider who opts out of Medicare altogether?
An opt-out provider will create a private contract with you, underscoring the terms of your agreement. But Medicare will not reimburse either of you for services.
Seeing a provider who does not accept Medicare will likely be more expensive. And your visits won’t count toward your deductible. But you may be able to work out paying reduced fees on a sliding scale for that provider’s services, all of which would be laid out in your contract.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Balance Billing in Health Insurance
- How It Works
- When It Happens
- What to Do If You Get a Bill
- If You Know in Advance
Balance billing happens after you’ve paid your deductible , coinsurance or copayment and your insurance company has also paid everything it’s obligated to pay toward your medical bill. If there is still a balance owed on that bill and the healthcare provider or hospital expects you to pay that balance, you’re being balance billed.
This article will explain how balance billing works, and the rules designed to protect consumers from some instances of balance billing.
Is Balance Billing Legal or Not?
Sometimes it’s legal, and sometimes it isn’t; it depends on the circumstances.
Balance billing is generally illegal :
- When you have Medicare and you’re using a healthcare provider that accepts Medicare assignment .
- When you have Medicaid and your healthcare provider has an agreement with Medicaid.
- When your healthcare provider or hospital has a contract with your health plan and is billing you more than that contract allows.
- In emergencies (with the exception of ground ambulance charges), or situations in which you go to an in-network hospital but unknowingly receive services from an out-of-network provider.
In the first three cases, the agreement between the healthcare provider and Medicare, Medicaid, or your insurance company includes a clause that prohibits balance billing.
For example, when a hospital signs up with Medicare to see Medicare patients, it must agree to accept the Medicare negotiated rate, including your deductible and/or coinsurance payment, as payment in full. This is called accepting Medicare assignment .
And for the fourth case, the No Surprises Act , which took effect in 2022, protects you from "surprise" balance billing.
Balance billing is usually legal :
- When you choose to use a healthcare provider that doesn’t have a relationship or contract with your insurer (including ground ambulance charges, even after implementation of the No Surprises Act).
- When you’re getting services that aren’t covered by your health insurance policy, even if you’re getting those services from a provider that has a contract with your health plan.
The first case (a provider not having an insurer relationship) is common if you choose to seek care outside of your health insurance plan's network.
Depending on how your plan is structured, it may cover some out-of-network costs on your behalf. But the out-of-network provider is not obligated to accept your insurer's payment as payment in full. They can send you a bill for the remainder of the charges, even if it's more than your plan's out-of-network copay or deductible.
(Some health plans, particularly HMOs and EPOs , simply don't cover non-emergency out-of-network services at all, which means they would not cover even a portion of the bill if you choose to go outside the plan's network.)
Getting services that are not covered is a situation that may arise, for example, if you obtain cosmetic procedures that aren’t considered medically necessary, or fill a prescription for a drug that isn't on your health plan's formulary . You’ll be responsible for the entire bill, and your insurer will not require the medical provider to write off any portion of the bill—the claim would simply be rejected.
Prior to 2022, it was common for people to be balance billed in emergencies or by out-of-network providers that worked at in-network hospitals. In some states, state laws protected people from these types of surprise balance billing if they had state-regulated health plans.
But not all states had these protections. And the majority of people with employer-sponsored health insurance are covered under self-insured plans, which are not subject to state regulations. This is why the No Surprises Act was so necessary.
How Balance Billing Works
When you get care from a doctor, hospital, or other healthcare provider that isn’t part of your insurer’s provider network (or, if you have Medicare, from a provider that has opted out of Medicare altogether , which is rare but does apply in some cases ), that healthcare provider can charge you whatever they want to charge you (with the exception of emergencies or situations where you receive services from an out-of-network provider while you're at an in-network hospital).
Since your insurance company hasn’t negotiated any rates with that provider, they aren't bound by a contract with your health plan.
Medicare Limiting Charge
If you have Medicare and your healthcare provider is a nonparticipating provider but hasn't entirely opted out of Medicare, you can be charged up to 15% more than the allowable Medicare amount for the service you receive (some states impose a lower limit).
This 15% cap is known as the limiting charge, and it serves as a restriction on balance billing in some cases. If your healthcare provider has opted out of Medicare entirely, they cannot bill Medicare at all and you'll be responsible for the full cost of your visit.
If your health insurance company agrees to pay a percentage of your out-of-network care, the health plan doesn’t pay a percentage of what’s actually billed . Instead, it pays a percentage of what it says should have been billed, otherwise known as a reasonable and customary amount.
As you might guess, the reasonable and customary amount is usually lower than the amount you’re actually billed. The balance bill comes from the gap between what your insurer says is reasonable and customary, and what the healthcare provider or hospital actually charges.
Let's take a look at an example in which a person's health plan has 20% coinsurance for in-network hospitalization and 40% coinsurance for out-of-network hospitalization. And we're going to assume that the No Surprises Act does not apply (ie, that the person chooses to go to an out-of-network hospital, and it's not an emergency situation).
In this scenario, we'll assume that the person already met their $1,000 in-network deductible and $2,000 out-of-network deductible earlier in the year (so the example is only looking at coinsurance).
And we'll also assume that the health plan has a $6,000 maximum out-of-pocket for in-network care, but no cap on out-of-pocket costs for out-of-network care:
| Coverage | 20% coinsurance with a $6,000 maximum out-of-pocket, including $1,000 deductible that has already been met earlier in the year | 40% coinsurance with no maximum out-of-pocket, (but a deductible that has already been met) with balance bill |
| Hospital charges | $60,000 | $60,000 |
| Insurer negotiates a discounted rate of | $40,000 | There is no discount because this hospital is out-of-network |
| Insurer's reasonable and customary rate | Not applicable, since the insurer has a contract with the hospital | $45,000 |
| Insurer pays | $35,000 (80% of the negotiated rate until the patient hits their maximum out-of-pocket, then the insurer pays 100%) | $27,000 (60% of the $45,000 reasonable and customary rate) |
| You pay coinsurance of | $5,000 (20% of the negotiated rate, until you hit the maximum out-of-pocket of $6,000. This is based on the $1,000 deductible paid earlier in the year, plus the $5,000 from this hospitalization) | $18,000 (40% of $45,000) |
| Balance billed amount | $0 (the hospital is required to write-off the other $20,000 as part of their contract with your insurer) | $15,000 (The hospital's original bill minus insurance and coinsurance payments) |
| When paid in full, you’ve paid | $5,000 (Your maximum out-of-pocket has been met. Keep in mind that you already paid $1,000 earlier in the year for your deductible) | $33,000 (Your coinsurance plus the remaining balance.) |
When Does Balance Billing Happen?
In the United States, balance billing usually happens when you get care from a healthcare provider or hospital that isn’t part of your health insurance company’s provider network or doesn’t accept Medicare or Medicaid rates as payment in full.
If you have Medicare and your healthcare provider has opted out of Medicare entirely, you're responsible for paying the entire bill yourself. But if your healthcare provider hasn't opted out but just doesn't accept assignment with Medicare (ie, doesn't accept the amount Medicare pays as payment in full), you could be balance billed up to 15% more than Medicare's allowable charge, in addition to your regular deductible and/or coinsurance payment.
Surprise Balance Billing
Receiving care from an out-of-network provider can happen unexpectedly, even when you try to stay in-network. This can happen in emergency situations—when you may simply have no say in where you're treated or no time to get to an in-network facility—or when you're treated by out-of-network providers who work at in-network facilities.
For example, you go to an in-network hospital, but the radiologist who reads your X-rays isn’t in-network. The bill from the hospital reflects the in-network rate and isn't subject to balance billing, but the radiologist doesn’t have a contract with your insurer, so they can charge you whatever they want. And prior to 2022, they were allowed to send you a balance bill unless state law prohibited it.
Similar situations could arise with:
- Anesthesiologists
- Pathologists (laboratory doctors)
- Neonatologists (doctors for newborns)
- Intensivists (doctors who specialize in ICU patients)
- Hospitalists (doctors who specialize in hospitalized patients)
- Radiologists (doctors who interpret X-rays and scans)
- Ambulance services to get you to the hospital, especially air ambulance services, where balance billing was frighteningly common
- Durable medical equipment suppliers (companies that provide the crutches, braces, wheelchairs, etc. that people need after a medical procedure)
These "surprise" balance billing situations were particularly infuriating for patients, who tended to believe that as long as they had selected an in-network medical facility, all of their care would be covered under the in-network terms of their health plan.
To address this situation, many states enacted consumer protection rules that limited surprise balance billing prior to 2022. But as noted above, these state rules don't protect people with self-insured employer-sponsored health plans, which cover the majority of people who have employer-sponsored coverage.
There had long been broad bipartisan support for the idea that patients shouldn't have to pay additional, unexpected charges just because they needed emergency care or inadvertently received care from a provider outside their network, despite the fact that they had purposely chosen an in-network medical facility. There was disagreement, however, in terms of how these situations should be handled—should the insurer have to pay more, or should the out-of-network provider have to accept lower payments? This disagreement derailed numerous attempts at federal legislation to address surprise balance billing.
But the Consolidated Appropriations Act, 2021, which was enacted in December 2020, included broad provisions (known as the No Surprises Act) to protect consumers from surprise balance billing as of 2022. The law applies to both self-insured and fully-insured plans, including grandfathered plans, employer-sponsored plans, and individual market plans.
It protects consumers from surprise balance billing charges in nearly all emergency situations and situations when out-of-network providers offer services at in-network facilities, but there's a notable exception for ground ambulance charges.
This is still a concern, as ground ambulances are among the medical providers most likely to balance bill patients and least likely to be in-network, and patients typically have no say in what ambulance provider comes to their rescue in an emergency situation. But other than ground ambulances, patients are no longer subject to surprise balance bills as of 2022.
The No Surprises Act did call for the creation of a committee to study ground ambulance charges and make recommendations for future legislation to protect consumers. The Biden Administration announced the members of that committee in late 2022, and the committee began holding meetings in May 2023.
Balance billing continues to be allowed in other situations (for example, the patient simply chooses to use an out-of-network provider). Balance billing can also still occur when you’re using an in-network provider, but you’re getting a service that isn’t covered by your health insurance. Since an insurer doesn’t negotiate rates for services it doesn’t cover, you’re not protected by that insurer-negotiated discount. The provider can charge whatever they want, and you’re responsible for the entire bill.
It is important to note that while the No Surprises Act prohibits balance bills from out-of-network working at in-network facilities, the final rule for implementation of the law defines facilities as "hospitals, hospital outpatient departments, critical access hospitals, and ambulatory surgical centers." Other medical facilities are not covered by the consumer protections in the No Surprises Act.
Balance billing doesn’t usually happen with in-network providers or providers that accept Medicare assignment . That's because if they balance bill you, they’re violating the terms of their contract with your insurer or Medicare. They could lose the contract, face fines, suffer severe penalties, and even face criminal charges in some cases.
If You Get an Unexpected Balance Bill
Receiving a balance bill is a stressful experience, especially if you weren't expecting it. You've already paid your deductible and coinsurance and then you receive a substantial additional bill—what do you do next?
First, you'll want to try to figure out whether the balance bill is legal or not. If the medical provider is in-network with your insurance company, or you have Medicare or Medicaid and your provider accepts that coverage, it's possible that the balance bill was a mistake (or, in rare cases, outright fraud).
And if your situation is covered under the No Surprises Act (ie, an emergency, or an out-of-network provider who treated you at an in-network facility), you should not be subject to a balance bill. So be sure you understand what charges you're actually responsible for before paying any medical bills.
If you think that the balance bill was an error, contact the medical provider's billing office and ask questions. Keep a record of what they tell you so that you can appeal to your state's insurance department if necessary.
If the medical provider's office clarifies that the balance bill was not an error and that you do indeed owe the money, consider the situation—did you make a mistake and select an out-of-network healthcare provider? Or was the service not covered by your health plan?
If you went to an in-network facility for a non-emergency, did you waive your rights under the No Surprises Act (NSA) and then receive a balance bill from an out-of-network provider? This is still possible in limited circumstances, but you would have had to sign a document indicating that you had waived your NSA protections.
Negotiate With the Medical Office
If you've received a legitimate balance bill, you can ask the medical office to cut you some slack. They may be willing to agree to a payment plan and not send your bill to collections as long as you continue to make payments.
Or they may be willing to reduce your total bill if you agree to pay a certain amount upfront. Be respectful and polite, but explain that the bill caught you off guard. And if it's causing you significant financial hardship, explain that too.
The healthcare provider's office would rather receive at least a portion of the billed amount rather than having to wait while the bill is sent to collections. So the sooner you reach out to them, the better.
Negotiate With Your Insurance Company
You can also negotiate with your insurer. If your insurer has already paid the out-of-network rate on the reasonable and customary charge, you’ll have difficulty filing a formal appeal since the insurer didn’t actually deny your claim . It paid your claim, but at the out-of-network rate.
Instead, request a reconsideration. You want your insurance company to reconsider the decision to cover this as out-of-network care , and instead cover it as in-network care. You’ll have more luck with this approach if you had a compelling medical or logistical reason for choosing an out-of-network provider .
If you feel like you’ve been treated unfairly by your insurance company, follow your health plan’s internal complaint resolution process.
You can get information about your insurer’s complaint resolution process in your benefits handbook or from your human resources department. If this doesn’t resolve the problem, you can complain to your state’s insurance department.
- Learn more about your internal and external appeal rights.
- Find contact information for your Department of Insurance using this resource .
If your health plan is self-funded , meaning your employer is the entity actually paying the medical bills even though an insurance company may administer the plan, then your health plan won't fall under the jurisdiction of your state’s department of insurance.
Self-funded plans are instead regulated by the Department of Labor’s Employee Benefit Services Administration. Get more information from the EBSA’s consumer assistance web page or by calling an EBSA benefits advisor at 1-866-444-3272.
If You Know You’ll Be Legally Balance Billed
If you know in advance that you’ll be using an out-of-network provider or a provider that doesn’t accept Medicare assignment, you have some options. However, none of them are easy and all require some negotiating.
Ask for an estimate of the provider’s charges. Next, ask your insurer what they consider the reasonable and customary charge for this service to be. Getting an answer to this might be tough, but be persistent.
Once you have estimates of what your provider will charge and what your insurance company will pay, you’ll know how far apart the numbers are and what your financial risk is. With this information, you can narrow the gap. There are only two ways to do this: Get your provider to charge less or get your insurer to pay more.
Ask the provider if he or she will accept your insurance company’s reasonable and customary rate as payment in full. If so, get the agreement in writing, including a no-balance-billing clause.
If your provider won’t accept the reasonable and customary rate as payment in full, start working on your insurer. Ask your insurer to increase the amount they’re calling reasonable and customary for this particular case.
Present a convincing argument by pointing out why your case is more complicated, difficult, or time-consuming to treat than the average case the insurer bases its reasonable and customary charge on.
Single-Case Contract
Another option is to ask your insurer to negotiate a single-case contract with your out-of-network provider for this specific service.
A single-case contract is more likely to be approved if the provider is offering specialized services that aren't available from locally-available in-network providers, or if the provider can make a case to the insurer that the services they're providing will end up being less expensive in the long-run for the insurance company.
Sometimes they can agree upon a single-case contract for the amount your insurer usually pays its in-network providers. Sometimes they’ll agree on a single-case contract at the discount rate your healthcare provider accepts from the insurance companies she’s already in-network with.
Or, sometimes they can agree on a single-case contract for a percentage of the provider’s billed charges. Whatever the agreement, make sure it includes a no-balance-billing clause.
Ask for the In-Network Coinsurance Rate
If all of these options fail, you can ask your insurer to cover this out-of-network care using your in-network coinsurance rate. While this won’t prevent balance billing, at least your insurer will be paying a higher percentage of the bill since your coinsurance for in-network care is lower than for out-of-network care.
If you pursue this option, have a convincing argument as to why the insurer should treat this as in-network. For example, there are no local in-network surgeons experienced in your particular surgical procedure, or the complication rates of the in-network surgeons are significantly higher than those of your out-of-network surgeon.
Balance billing refers to the additional bill that an out-of-network medical provider can send to a patient, in addition to the person's normal cost-sharing and the payments (if any) made by their health plan. The No Surprises Act provides broad consumer protections against "surprise" balance billing as of 2022.
A Word From Verywell
Try to prevent balance billing by staying in-network, making sure your insurance company covers the services you’re getting, and complying with any pre-authorization requirements. But rest assured that the No Surprises Act provides broad protections against surprise balance billing.
This means you won't be subject to balance bills in emergencies (except for ground ambulance charges, which can still generate surprise balance bills) or in situations where you go to an in-network hospital but unknowingly receive care from an out-of-network provider.
Congress.gov. H.R.133—Consolidated Appropriations Act, 2021 . Enacted December 27, 2021.
Kona M. The Commonwealth Fund. State balance billing protections . April 20, 2020.
Data.CMS.gov. Opt Out Affidavits .
Chhabra, Karan; Schulman, Kevin A.; Richman, Barak D. Health Affairs. Are Air Ambulances Truly Flying Out Of Reach? Surprise-Billing Policy And The Airline Deregulation Act . October 17, 2019.
Kaiser Family Foundation. 2022 Employer Health Benefits Survey .
Centers for Medicare and Medicaid Services. Members of New Federal Advisory Committee Named to Help Improve Ground Ambulance Disclosure and Billing Practices for Consumers . December 13, 2022.
Centers for Medicare and Medicaid Services. Advisory Committee on Ground Ambulance and Patient Billing (GAPB) .
Internal Revenue Service; Employee Benefits Security Administration; Health and Human Services Department. Requirements Related to Surprise Billing . August 26, 2022.
National Conference of State Legislatures. States Tackling "Balance Billing" Issue . July 2017.
By Elizabeth Davis, RN Elizabeth Davis, RN, is a health insurance expert and patient liaison. She's held board certifications in emergency nursing and infusion nursing.
Medicare Assignment: Understanding How It Works

Medicare assignment is a term used to describe how a healthcare provider agrees to accept the Medicare-approved amount. Depending on how you get your Medicare coverage, it could be essential to understand what it means and how it can affect you.
What is Medicare assignment?
Medicare sets a fixed cost to pay for every benefit they cover. This amount is called Medicare assignment.
You have the largest healthcare provider network with over 800,000 providers nationwide on Original Medicare . You can see any doctor nationwide that accepts Medicare.
Understanding the differences between your cost and the difference between accepting Medicare and accepting Medicare assignment could be worth thousands of dollars.
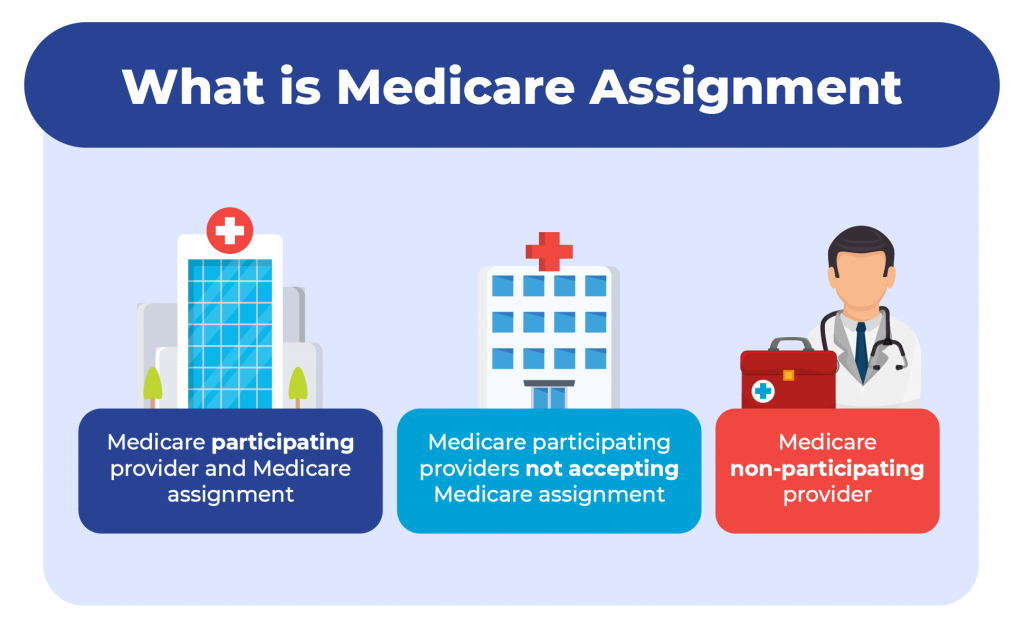
Doctors that accept Medicare
Your healthcare provider can fall into one of three categories:
Medicare participating provider and Medicare assignment
Medicare participating providers not accepting medicare assignment, medicare non-participating provider.
More than 97% of healthcare providers nationwide accept Medicare. Because of this, you can see almost any provider throughout the United States without needing referrals.
Let’s discuss the three categories the healthcare providers fall into.
Participating providers are doctors or healthcare providers who accept assignment. This means they will never charge more than the Medicare-approved amount.
Some non-participating providers accept Medicare but not Medicare assignment. This means you can see them the same way a provider accepts assignment.
You need to understand that since they don’t take the assigned amount, they can charge up to 15% more than the Medicare-approved amount.
Since Medicare will only pay the Medicare-approved amount, you’ll be responsible for these charges. The 15% overcharge is called an excess charge. A few states don’t allow or limit the amount or services of the excess charges. Only about 5% of providers charge excess charges.
Opt-out providers don’t accept Original Medicare, and these healthcare providers are in the minority in the United States. If healthcare providers don’t accept Medicare, they won’t be paid by Medicare.
This means choosing to see a provider that doesn’t accept Medicare will leave you responsible for 100% of what they charge you. These providers may be in-network for a Medicare Advantage plan in some cases.
Avoiding excess charges
Excess charges could be large or small depending on the service and the Medicare-approved amount. Avoiding these is easy. The simplest way is to ask your provider if they accept assignment before service.
If they say yes, they don’t issue excess charges. Or, on Medicare.gov , a provider search tool will allow you to look up your healthcare provider and show if they accept Medicare assignment or not.
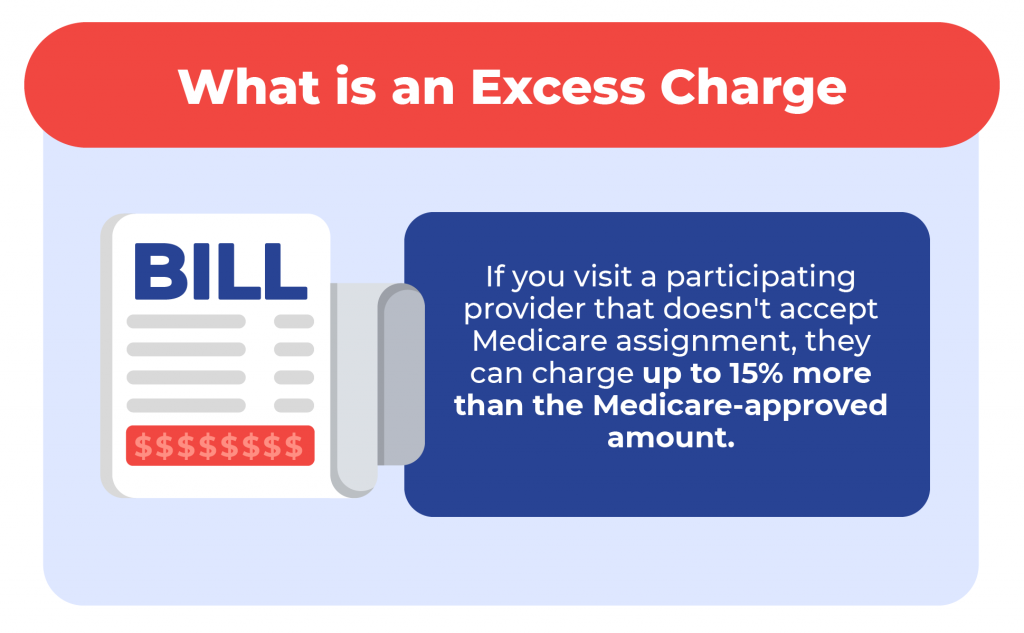
Medicare Supplement and Medicare assignment
Medigap plans are additional insurance that helps cover your Medicare cost-share . If you are on specific plans, they’ll pay any extra costs from healthcare providers that accept Medicare but not Medicare assigned amount. Most Medicare Supplement plans don’t cover the excess charges.
The top three Medicare Supplement plans cover excess charges if you use a provider that accepts Medicare but not Medicare assignment.
Medicare Advantage and Medicare assignment
Medicare assignment does not affect Medicare Advantage plans since Medicare Advantage is just another way to receive your Medicare benefits. Since your Medicare Advantage plan handles your healthcare benefits, they set the terms.
Most Medicare Advantage plans require you to use network providers. If you go out of the network, you may pay more. If you’re on an HMO, you’d be responsible for the entire charge of the provider not being in the network.
Do all doctors accept Medicare Supplement plans?
All doctors that accept Original Medicare accept Medicare Supplement plans. Some doctors don’t accept Medicare. In this case, those doctors won’t accept Medicare Supplements.
Where can I find doctors who accept Medicare assignment?
Medicare has a physician finder tool that will show if a healthcare provider participates in Medicare and accepts Medicare assignments. Most doctors nationwide do accept assignment and therefore don’t charge the Part B excess charges.
Why do some doctors not accept Medicare?
Some doctors are called concierge doctors. These doctors don’t accept any insurance and require cash payments.
What is a Medicare assignment?
Accepting Medicare assignment means that the healthcare provider has agreed only to charge the approved amount for procedures and services.
What does it mean if a doctor does not accept Medicare assignment?
The doctor can change more than the Medicare-approved amount for procedures and services. You could be responsible for up to a 15% excess charge.
How many doctors accept Medicare assignment?
About 97% of doctors agree to accept assignment nationwide.
Is accepting Medicare the same as accepting Medicare assignment?
No. If a doctor accepts Medicare and accepts Medicare assigned amount, they’ll take what Medicare approves as payment in full.
If they accept Medicare but not Medicare assignment, they can charge an excess charge of up to 15% above the Medicare-approved amount. You could be responsible for this excess charge.
What is the Medicare-approved amount?
The Medicare-approved amount is Medicare’s charge as the maximum for any given medical service or procedure. Medicare has set forth an approved amount for every covered item or service.
Can doctors balance bill patients?
Yes, if that doctor is a Medicare participating provider not accepting Medicare assigned amount. The provider may bill up to 15% more than the Medicare-approved amount.
What happens if a doctor does not accept Medicare?
Doctors that don’t accept Medicare will require you to pay their full cost when using their services. Since these providers are non-participating, Medicare will not pay or reimburse for any services rendered.
Get help avoiding Medicare Part B excess charges
Whether it’s Medicare assignment, or anything related to Medicare, we have licensed agents that specialize in this field standing by to assist.
Give us a call, or fill out our online request form . We are happy to help answer questions, review options, and guide you through the process.
Related Articles
- What are Medicare Part B Excess Charges?
- How to File a Medicare Reimbursement Claim?
- Medicare Defined Coinsurance: How it Works?
- Welcome to Medicare Visit
- Guide to the Medicare Program

CALL NOW (833) 972-1339
What Does It Mean for a Doctor to Accept Medicare Assignment?
Written by: Malini Ghoshal, RPh, MS
Reviewed by: Malinda Cannon, Licensed Insurance Agent
Key Takeaways
Doctors who accept Medicare assignment are paid agreed-upon rates for services.
It’s important to verify that your doctor accepts assignment before receiving services to avoid high out-of-pocket costs.
A doctor or clinician may be “non-participating” but can still agree to accept Medicare assignment for some services.
If you visit a doctor or clinician who has opted out (doesn’t accept Medicare), you may have to pay for your entire visit cost unless it’s a medical emergency.
Medigap Supplemental insurance (Medigap) plans won’t pay for service costs from doctors who don’t accept assignment.
One of the things that Original Medicare beneficiaries often enjoy about their coverage is that they can use it anywhere in the country. Unlike plans with provider networks, they can visit doctors either at home or on the road; both are covered the same.
But do all doctors accept Medicare patients?
Truth is, this wide-ranging coverage area only applies to doctors who accept Medicare assignment. Fortunately, most do. If you’re eligible for Medicare, it’s important to visit doctors and clinicians who accept Medicare assignment. This will help keep your out-of-pocket costs within your control. Doctors who agree to accept Medicare assignment sign an agreement that they’re willing to accept payment from Medicare for their services.
If you’re a current beneficiary or nearing enrollment, you may have other questions. Do all doctors accept Medicare Advantage plans? What about Medicare Supplement insurance (Medigap)? Read on to learn how to find doctors that accept Medicare assignment and how this keeps your healthcare costs down.
Let’s find your ideal Medicare Advantage plan.
What Is Medicare Assignment of Benefits?
When you’re eligible for Medicare, you have the option to visit doctors and clinicians who accept assignment. This means they are Medicare-approved providers who agree to receive Medicare reimbursement rates for covered services. This helps save you money.
If you have Original Medicare (Part A and B), your doctor visits are covered by your Part B plan. Inpatient services such as hospital stays and some skilled nursing care are covered by Part A .
In order for a participating doctor (or facility) to bill Medicare and be reimbursed, you must authorize Medicare to reimburse your doctor directly for your covered services. This is called the Medicare assignment of benefits. You transfer your right to receive Medicare payment for a covered service to your doctor or other provider.
Note: If you have a Medicare Supplement insurance ( Medigap ) plan to pay for out-of-pocket costs, you may also need to sign a separate assignment of benefits form for Medigap reimbursement. More on Medigap below.
How Can I Find Doctors Near Me That Accept Medicare?
There are several ways to find doctors and other clinicians who accept Medicare assignment close to you.
First, let’s take a look at the different types of Medicare providers.
They include:
Participating providers: Medicare-participating doctors and providers sign a participation agreement stating they will accept Medicare reimbursement rates for their services.
Non-participating providers: Doctors or providers who are non-participating providers are eligible to accept Medicare assignment but haven’t signed a Medicare agreement. They may choose to accept assignment on a case-by-case basis. If you visit a non-participating provider, make sure to ask if they accept assignment for your particular service. Also get a copy of their fees. They will need to select “yes” on Centers for Medicare & Medicaid Services CMS Form 1500 to accept assignment for the service.
Opt-out providers: Some doctors and other providers choose not to accept Medicare. If they choose to opt out, the period is two years (based on Medicare guidelines). The opt-out automatically renews if the provider doesn’t request a change in their status. You would be responsible for paying all costs for services received from an opt-out provider. You cannot bill Medicare for reimbursement unless the service was an urgent or emergency medical need. According to a report from KFF , roughly 1% of non-pediatric physicians opted out of Medicare in 2023.
Visiting a doctor who doesn’t accept assignment may cost you more. These providers can charge you up to 15% more than the Medicare-approved rate for a given service. This 15% charge is called the limiting charge. Some states limit this extra charge to a certain percent. This may also be called the Part B excess charge.
Here are some tips for finding doctors and providers who accept Medicare assignment:
- The easiest way to find a doctor who accepts Medicare assignment is to contact their office and ask them directly.
- If you’re looking for a new doctor, you can use the Medicare search tool to find clinicians and doctors that accept Medicare assignment.
- You can also ask a state health insurance assistance program (SHIP) representative for help in locating a doctor that accepts Medicare assignment.
- Don’t assume that having a longstanding relationship with your doctor means nothing will ever change. Check in with them to make sure they still accept Medicare assignment and whether they’re planning to opt out.
Note: Your doctor can choose to become a non-participating provider or opt out of participating in Medicare. It’s important to verify they accept Medicare assignment before receiving any services.
Can I bundle multiple benefits into one plan?
Do Doctors Who Accept Medicare Have to Accept Supplement Plans?
If your doctor accepts Medicare assignment and you have Original Medicare (Medicare Part A and Part B) with a Medicare Supplement (Medigap) plan, they will accept the supplemental insurance. Depending on your Medigap plan coverage , it may pay all or part of your out-of-pocket costs such as deductibles, copayments and coinsurance.
However, if you have a Medicare Advantage plan (Part C), you may have a network of covered doctors under the plan. If you visit an out-of-network doctor, you may need to pay all or part of the cost for your services.
Keep in mind that you can’t have a Medigap supplemental plan if you have a Medicare Advantage plan.
If you have questions or want to learn more about different Medicare plans like Original Medicare with Medigap versus Medicare Advantage, GoHealth has licensed insurance agents ready to help. They can shop your different options and offer impartial guidance where you need it.
Do Most Doctors Accept Medicare Advantage Plans?
Many doctors accept Medicare Advantage (Part C) plans, but these plans often use provider networks. These networks are groups of doctors and providers in an area that have agreed to treat an insurance company’s customers. If you have a Part C plan, you may be required to see in-network doctors with few exceptions. However, these types of plans are popular options for all-in-one coverage for your health needs. Plans must offer Part A and B coverage, plus a majority also include Part D , or prescription drug coverage. But whether a doctor accepts a Medicare Advantage plan may depend on where you live and the type of Medicare Advantage plan you have.
There are several types of Medicare Advantage plans including:
- Health Maintenance Organization (HMO): These plans have a network of covered providers, as well as a primary care physician to manage your care. If you visit a doctor outside your plan network, you may have to pay the full cost of your visit.
- Preferred Provider Organization (PPO): You’ll probably still have a primary care physician, but these are more flexible plans that allow you to go out of network in some cases. But you may have to pay more.
- Private Fee for Service (PFFS): You may be able to visit any doctor or provider with these plans, but your costs may be higher.
- Special Needs Plan (SNP): This type of plan is only for certain qualified individuals who either have a specific health condition ( C-SNP ) or who qualify for both Medicaid and Medicare insurance ( D-SNP ).
Find the Medicare Plan that works for you.
What Are Medicare Assignment Codes?
Medicare assignment codes help Medicare pay for covered services. If your doctor or other provider accepts assignment and is a participating provider, they will file for reimbursement for services with a CMS-1500 form and the code will be “assigned.”
But non-participating providers can select “not assigned.” This means they are not accepting Medicare-assigned rates for a given service. They can charge up to 15% over the full Medicare rate for the service.
If you go to a doctor or provider who accepts assignment, you don’t need to file your own claim. Your doctor’s office will directly file with Medicare. Always check to make sure your doctor accepts assignment to avoid excess charges from your visit.
Health Insurance Claim Form . CMS.gov.
Lower costs with assignment . Medicare.gov.
How Many Physicians Have Opted-Out of the Medicare Program? KFF.org.
Joining a plan . Medicare.gov.
This website is operated by GoHealth, LLC., a licensed health insurance company. The website and its contents are for informational and educational purposes; helping people understand Medicare in a simple way. The purpose of this website is the solicitation of insurance. Contact will be made by a licensed insurance agent/producer or insurance company. Medicare Supplement insurance plans are not connected with or endorsed by the U.S. government or the federal Medicare program. Our mission is to help every American get better health insurance and save money. Any information we provide is limited to those plans we do offer in your area. Please contact Medicare.gov or 1-800-MEDICARE to get information on all of your options.
Let's see if you're missing out on Medicare savings.
We just need a few details.
Related Articles

What Is Medicare IRMAA?
What Is an IRMAA in Medicare?

How to Report Medicare Fraud
Medicare Fraud Examples & How to Report Abuse

How to Change Your Address with Medicare
Reporting a Change of Address to Medicare

Can I Get Medicare if I’ve Never Worked?
Can You Get Medicare if You've Never Worked?

Why Are Some Medicare Advantage Plans Free?
Why Are Some Medicare Advantage Plans Free? $0 Premium Plans Explained

What Is Medicare Assignment?

Am I Enrolled in Medicare?

When and How Do I Enroll?
When and How Do I Enroll in Medicare?

Medicare Frequently Asked Questions
Let’s see if you qualify for Medicare savings today!
You are using an outdated browser. Please upgrade your browser to improve your experience.
Article Search
Attorney search, search articles, find attorneys, what you pay your doctor under medicare depends on the doctor.

Medicare Part B recipients must satisfy an annual deductible. Once the deductible has been met, Medicare pays 80 percent of what Medicare considers a "reasonable charge" for the item or service. The beneficiary is responsible for the other 20 percent.
Local Elder Law Attorneys in Your City
Elder Law Attorney
Firm Name City, State
However, in most cases what Medicare calls a "reasonable charge" is less than what a doctor or other medical provider normally charges for a service. Whether a Medicare beneficiary must pay part of the difference between the Medicare-approved charge and the provider's normal charge depends on whether or not the provider has agreed to participate in the Medicare program.
If your doctor participates in Medicare, it means that the doctor "accepts assignment." In other words, the doctor agrees that the total charge for the covered service will be the amount approved by Medicare. Medicare then pays the provider 80 percent of its approved amount, after subtracting any part of your annual deductible that has not already been met. The provider then charges you the remaining 20 percent of the approved "reasonable" charge, plus any part of the deductible that has not been satisfied.
If your doctor does not accept assignment, the rules are different. Non-participating doctors can charge beneficiaries 20 percent of the approved amount plus up to an additional 15 percent more than the Medicare-approved amount. Non-participating doctors can also charge you the entire bill for the care upfront and request that you bill Medicare for reimbursement, while doctors who accept assignment cannot. Note that if you have Medigap plans F and G, they will cover the additional 15 percent charges (however, as of January 1, 2020, plan F is no longer sold).
Doctors can also choose to opt out of Medicare altogether. This means the doctor will not submit any claims to Medicare for reimbursement. If your doctor opts out of Medicare, you will be responsible for the full amount of your bill.
The payment system under Medicare Advantage is different because doctors contract with Advantage’s HMO or PPO plans and agree to the plan’s payment terms. As far as your payment responsibilities go, your plan will require specific copays and deductibles, which will vary plan by plan. Remember that at any time, Medicare Advantage plans can make changes to which doctors they cover, and doctors can choose to join or leave plans, so having a Medicare Advantage plan does not mean your doctor will always be covered. If your doctor leaves your plan’s network and you want to keep your doctor, you may need to switch plans to have your bills covered.
For more information about Medicare, click here .
Related Articles
Where should you put your retirement money.
There are as many opportunities to grow money as there are people with great ideas. ?Here?is a survey of types of investment...
Should You Sell Your Life Insurance Policy?
Older Americans with a life insurance policy that they no longer need have the option to sell the policy to investors. These...
Be on the Lookout for New Medicare Cards (and New Card-Related Scams)
The federal government is issuing new Medicare cards to all Medicare beneficiaries. To prevent fraud and fight identity theft...
Editor's Suggested Articles
Need more information? Subscribe to Elder Law Updates.
Medicaid 101
What medicaid covers.
In addition to nursing home care, Medicaid may cover home care and some care in an assisted living facility. Coverage in your state may depend on waivers of federal rules.
How to Qualify for Medicaid
To be eligible for Medicaid long-term care, recipients must have limited incomes and no more than $2,000 (in most states). Special rules apply for the home and other assets.
Medicaid’s Protections for Spouses
Spouses of Medicaid nursing home residents have special protections to keep them from becoming impoverished.
Medicaid Planning Strategies
Careful planning for potentially devastating long-term care costs can help protect your estate, whether for your spouse or for your children.
Estate Recovery: Can Medicaid Take My House After I’m Gone?
If steps aren't taken to protect the Medicaid recipient's house from the state’s attempts to recover benefits paid, the house may need to be sold.
Help Qualifying and Paying for Medicaid, Or Avoiding Nursing Home Care
There are ways to handle excess income or assets and still qualify for Medicaid long-term care, and programs that deliver care at home rather than in a nursing home.
Are Adult Children Responsible for Their Parents’ Care?
Most states have laws on the books making adult children responsible if their parents can't afford to take care of themselves.
Applying for Medicaid
Applying for Medicaid is a highly technical and complex process, and bad advice can actually make it more difficult to qualify for benefits.
Alternatives to Medicaid
Medicare's coverage of nursing home care is quite limited. For those who can afford it and who can qualify for coverage, long-term care insurance is the best alternative to Medicaid.
Most Recent Medicaid Articles
Elderlaw 101, estate planning.
Distinguish the key concepts in estate planning, including the will, the trust, probate, the power of attorney, and how to avoid estate taxes.
Grandchildren
Learn about grandparents’ visitation rights and how to avoid tax and public benefit issues when making gifts to grandchildren.
Guardianship/Conservatorship
Understand when and how a court appoints a guardian or conservator for an adult who becomes incapacitated, and how to avoid guardianship.
Health Care Decisions
We need to plan for the possibility that we will become unable to make our own medical decisions. This may take the form of a health care proxy, a medical directive, a living will, or a combination of these.
Long-Term Care Insurance
Understand the ins and outs of insurance to cover the high cost of nursing home care, including when to buy it, how much to buy, and which spouse should get the coverage.
Learn who qualifies for Medicare, what the program covers, all about Medicare Advantage, and how to supplement Medicare’s coverage.
Retirement Planning
We explain the five phases of retirement planning, the difference between a 401(k) and an IRA, types of investments, asset diversification, the required minimum distribution rules, and more.
Senior Living
Find out how to choose a nursing home or assisted living facility, when to fight a discharge, the rights of nursing home residents, all about reverse mortgages, and more.
Social Security
Get a solid grounding in Social Security, including who is eligible, how to apply, spousal benefits, the taxation of benefits, how work affects payments, and SSDI and SSI.
Special Needs Planning
Learn how a special needs trust can preserve assets for a person with disabilities without jeopardizing Medicaid and SSI, and how to plan for when caregivers are gone.
Veterans Benefits
Explore benefits for older veterans, including the VA’s disability pension benefit, aid and attendance, and long-term care coverage for veterans and surviving spouses.
Most Recent ElderLaw Articles

What You Need to Know About Medicare Assignment
If you are one of the more than 63 million Americans enrolled in Medicare and are on the lookout for a new provider, you may wonder what your options are. A good place to start? Weighing the pros and cons of choosing an Original Medicare plan versus a Medicare Advantage plan—both of which have their upsides.
Let’s say you decide on an Original Medicare plan, which many U.S. doctors accept. In your research, however, you come across the term “Medicare assignment.” Cue the head-scratching. What exactly does that mean, and how might it affect your coverage costs?
What is Medicare Assignment?
It turns out that Medicare assignment is a concept you need to understand before seeing a new doctor. First things first: Ask your doctor if they “accept assignment”—that exact phrasing—which means they have agreed to accept a Medicare-approved amount as full payment for any Medicare-covered service provided to you. If your doctor accepts assignment, that means they’ll send your whole medical bill to Medicare, and then Medicare pays 80% of the cost, while you are responsible for the remaining 20%.
A doctor who doesn’t accept assignment, however, could charge up to 15% more than the Medicare-approved amount for their services, depending on what state you live in, shouldering you with not only that additional cost but also your 20% share of the original cost. Additionally, the doctor is supposed to submit your claim to Medicare, but you may have to pay them on the day of service and then file a reimbursement claim from Medicare after the fact.
Worried that your doctor will not accept assignment? Luckily, 98% of U.S. physicians who accept Medicare patients also accept Medicare assignment, according to the U.S. Centers for Medicare & Medicaid Services (CMS). They are known as assignment providers, participating providers, or Medicare-enrolled providers.
It can be confusing. Here’s how to assess whether your provider accepts Medicare assignment, and what that means for your out-of-pocket costs:
The 3 Types of Original Medicare Providers
1. participating providers, or those who accept medicare assignment.
These providers have an agreement with Medicare to accept the Medicare-approved amount as full payment for their services. You don’t have to pay anything other than a copay or coinsurance (depending on your plan) at the time of your visit. Typically, Medicare pays 80% of the cost, while you are responsible for the remaining 20%, as long as you have met your deductible.
2. Non-participating providers
“Most providers accept Medicare, but a small percentage of doctors are known as non-participating providers,” explains Caitlin Donovan, senior director of public relations at the National Patient Advocate Foundation (NPAF) in Washington D.C. “These may be more expensive,” she adds. Also known as non-par providers, these physicians may accept Medicare patients and insurance, but they have not agreed to take assignment Medicare in all cases. That means they’re not held to the Medicare-approved amount as payment in full. As a reminder, a doctor who doesn’t accept assignment can charge up to 15% more than the Medicare-approved amount, depending on what part of the country you live in, and you will have to pay that additional amount plus your 20% share of the original cost.
What does that mean for you? Besides being charged more than the Medicare-approved amount, you might also be required to do some legwork to get reimbursed by Medicare.
- You may have to pay the entire bill at the time of service and wait to be reimbursed 80% of the Medicare-approved amount. In most cases, the provider will submit the claim for you. But sometimes, you’ll have to submit it yourself.
- Depending on the state you live in, the provider may also charge you as much as 15% more than the Medicare-approved amount. (In New York state, for example, that add-on charge is limited to 5%.) This is called a limiting charge—and the difference, called the balance bill, is your responsibility.
There are some non-par providers, however, who accept Medicare assignment for certain services, on a case-by-case basis. Those may include any of the services—anything from hospital and hospice care to lab tests and surgery—available from any assignment-accepting doctor, with a key exception: If a non-par provider accepts assignment for a particular service, they cannot bill you more than the regular Medicare deductible and coinsurance amount for that specific treatment. Just as it’s important to confirm whether your doctor accepts assignment, it’s also important to confirm which services are included at assignment.
3. Opt-out providers
A small percentage of providers do not participate in Medicare at all. In 2020, for example, only 1% of all non-pediatric physicians nationwide opted out, and of that group, 42% were psychiatrists. “Some doctors opt out of providing Medicare coverage altogether,” notes Donovan.“In that case, the patient would pay privately.” If you were interested in seeing a physician who had opted out of Medicare, you would have to enter a private contract with that provider, and neither you nor the provider would be eligible for reimbursement from Medicare.
How do I know if my doctor accepts Medicare assignment?
The best way to find out whether your provider accepts Medicare assignment is simply to ask. First, confirm whether they are participating or non-participating—and if they are non-participating, ask whether they accept Medicare assignment for certain services.
Also, make sure to ask your provider exactly how they will be billing Medicare and what charges you might expect at the time of your visit so that you’re on the same page from the start.
Is seeing a non-participating provider who accepts Medicare assignment more expensive?
The short answer is yes. There are usually out-of-pocket costs after you’re reimbursed. But it may not cost as much as you think, and it may not be much more than if you see a participating provider. Still, it could be challenging if you’re on a fixed income.
For example, let’s say you’re seeing a physical therapist who accepts Medicare patients but not Medicare assignment. Medicare will pay $95 per visit to the provider; but your provider bills the service at $115. In most states, you’re responsible for a 15% limiting charge above $95. In this case, your bill would be 115% of $95, or $109.25.
Once you get your $95 reimbursement back from Medicare, your cost for the visit—the balance bill—would be $14.25 (plus any deductibles or copays) .
In some states, the maximum cap on the limiting charge is less than 15%. As mentioned earlier, New York state, for instance, allows only a 5% surcharge, which means that physical therapy appointment would cost you just $4.75 extra.
Bottom line: Medicare assignment providers and non-participating providers who agree to accept Medicare assignment are both viable options for patients. So if you want to see a particular provider, don’t rule them out just because they’re non-par.
While seeing a non-participating provider may still be affordable, ultimately, the biggest headache may be keeping track of claims and reimbursements, or simply setting aside the right amount of money to pay for your visit up front.
Before you schedule a visit, be sure to ask how much the service will cost. You can also estimate the payment amount based on Medicare-approved charges. A good place to start is this out-of-pocket expense calculator provided by the CMS.
What if I see a provider who opts out of Medicare altogether?
An opt-out provider will create a private contract with you, underscoring the terms of your agreement. But Medicare will not reimburse either of you for services.
Seeing a provider who does not accept Medicare will likely be more expensive. And your visits won’t count toward your deductible. But you may be able to work out paying reduced fees on a sliding scale for that provider’s services, all of which would be laid out in your contract.
The independent source for health policy research, polling, and news.
How Many Physicians Have Opted Out of the Medicare Program?
Nancy Ochieng and Gabrielle Clerveau Published: Sep 11, 2023
Medicare provides health insurance coverage to 65 million adults– nearly 20% of the U.S population —and is a major source of revenue for providers, including physicians and other clinicians. In 2022, Medicare spending on Part B services (including physician services, outpatient services, and physician-administered drugs) accounted for nearly half (48%) of total Medicare benefit spending, and this share is expected to grow to more than half (52%) by 2032. Physicians are not required to participate in Medicare, though the vast majority of them choose to do so.
Every year, the Centers for Medicare and Medicaid Services (CMS) updates Medicare payments to physicians under the physician fee schedule through rulemaking, as required under law. Over the years, some have raised concerns that physicians would opt out of Medicare because Medicare payments for physician services are lower, on average, than payments from private insurers , potentially leading to a shortage of physicians willing to treat people with Medicare.
This brief builds on previous KFF analyses by providing the most recent data on the extent to which non-pediatric physicians are opting out of Medicare, by specialty and by state, in 2023, based on data published by CMS as of June 2023. ( See Methods box for details ).
Highlights:
- One percent of all non-pediatric physicians have formally opted-out of the Medicare program in 2023, with the share varying somewhat by specialty type, and highest for psychiatrists (7.7%).
- Psychiatrists account for the largest share (40.2%) of all non-pediatric physicians who have opted out of Medicare in 2023.
- Less than two percent of physicians have opted-out of Medicare in all but four states and the District of Columbia, where the rate is slightly higher: Alaska (3.1%), Colorado (2.3%), Wyoming (2.3%), Idaho (2.1%), and the District of Columbia (2.0%).
Three options for physicians
Currently, physicians and other practitioners choosing to treat patients with Medicare and receive payments from Medicare for these services must enroll in Medicare as a Medicare provider. Physicians may either agree to be a participating provider or non-participating provider. Providers who do not want to enroll in Medicare, treat patients with Medicare, or receive Medicare payments are required to sign an “opt out” agreement with their patients.
- Participating providers agree to accept “assignment” on all Medicare claims for alltheir Medicare patients, which means that they have signed a participation agreement with Medicare, agreeing to accept Medicare’s fee schedule amounts as payment-in-full for all Medicare covered services. Medicare beneficiaries seeing a participating provider can only be liable for the cost sharing required by Medicare. Providers have several incentives to be participating providers, such as being paid higher rates (5% higher) than the rates paid to non-participating providers. The vast majority (98%) of physicians and practitioners billing Medicare are participating providers.
- Non-participating providers accept Medicare patients, but can choose whether to take assignment (i.e., Medicare’s approved amount) on a claim-by-claim basis. Unlike participating providers, who are paid the full Medicare-allowed payment amount, non-participating physicians who take assignment are limited to 95% of the Medicare approved amount. In 2021, 7% of fee schedule claims by non-participating providers were paid on assignment. Physicians who choose to not accept assignment can charge beneficiaries more than the Medicare-approved amount, (“balance bill”) but not exceeding 15% of the fee-schedule allowed amount. Medicare patients are financially liable for this additional amount plus applicable deductibles and coinsurance amounts.
- Opt-out physicians and other practitioners must sign an affidavit to “opt-out” of the Medicare program entirely. These providers enter into private contracts with their Medicare patients, allowing them to bill any amount they determine is appropriate. Providers who have opted-out of the Medicare program must opt-out for all of their Medicare patients. Medicare patients seeing a provider who has opted out of the Medicare program must sign this agreement and agree to be financially responsible for the entire cost of any services received. Neither the provider nor the patient can submit a bill to Medicare for reimbursement.Opt-out agreements last for two consecutive years and are automatically renewed every two years. According to CMS , physicians and other practitioners are not allowed to opt-out of Medicare if they are a Medicare Advantage provider or furnish services covered by traditional Medicare Part B. Providers who have opted-out of the Medicare program must enter a private contract with each of their Medicare patients that states that neither party is allowed to receive payment from Medicare for the services performed.
What Share of Physicians Have Opted Out of Medicare?
1.1 percent of non-pediatric physicians have formally opted-out of the Medicare program. As of June 2023, 11,039 non-pediatric physicians have opted out of Medicare, representing a very small share (1.1%) of the total number active physicians, similar to the shares reported in 2013 and 2022 .
While the overall opt-out rate is 1.1 percent, opt-out rates are somewhat higher for certain specialties, such as psychiatry and plastic and reconstructive surgery. In 2023, 7.7 percent of psychiatrists opted out of Medicare, followed by 4.2 percent of physicians specializing in plastic and reconstructive surgery and 2.8 percent of physicians specializing in neurology (Figure 2).
Psychiatrists are disproportionately represented among the 1.1 percent of active physicians who have opted out of Medicare. Psychiatrists account for the largest share (40%) of opt-out physicians, followed by physicians in family medicine (21%), internal medicine (12.6%), and obstetrics/gynecology (6%) (Figure 3).
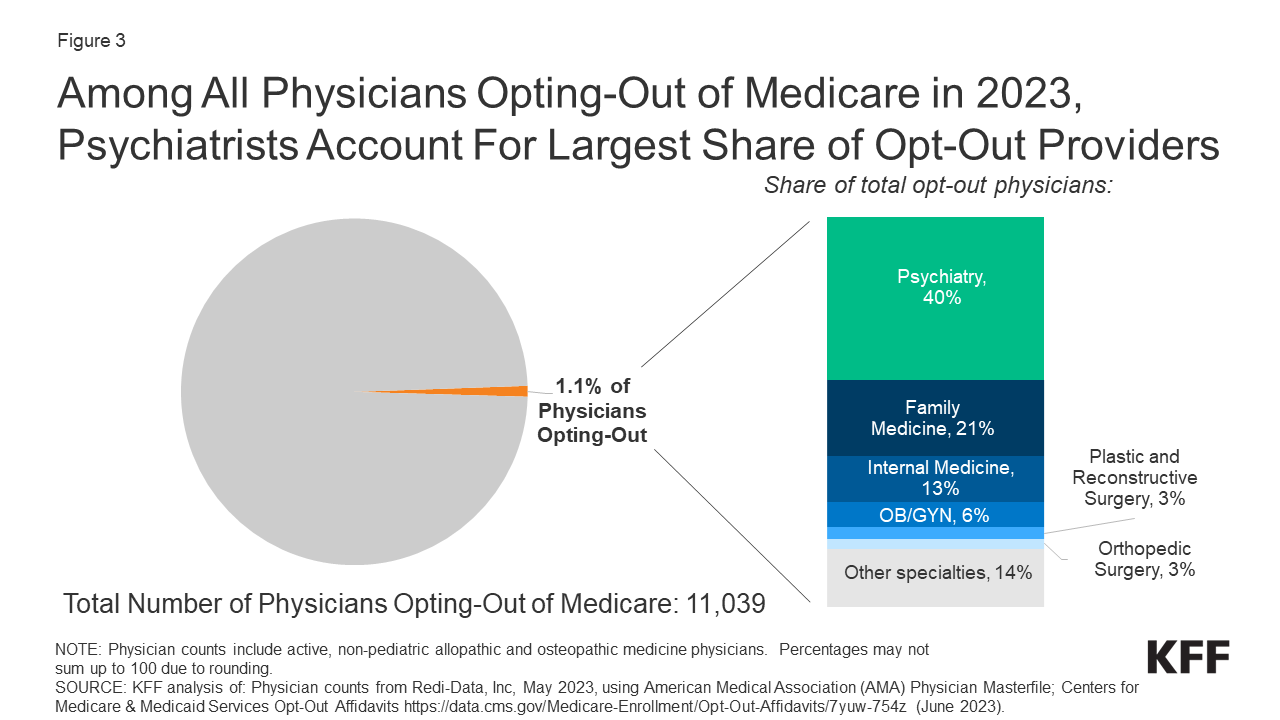
Figure 3: Among All Physicians Opting-Out of Medicare in 2023, Psychiatrists Account For Largest Share of Opt-Out Providers
In addition to physicians, another 4,229 select clinical professionals with doctorate degrees (i.e. oral surgeons, podiatrists, and optometrists) have also opted-out of the Medicare program, with oral surgeons accounting for the vast majority (94%) of this group (Table 1).
Less than two percent of physicians have opted-out of Medicare in all but four states and the District of Columbia. As of June 2023, Alaska (3.1%), Colorado (2.3%), Wyoming (2.3%), Idaho (2.1%), and the District of Columbia (2.0%) have the highest rates of non-pediatric physicians who have opted out of Medicare, though in each case the share is below 4% (Figure 4). Nine states (Wisconsin, Ohio, Mississippi, Iowa, Minnesota, Nebraska, South Dakota, West Virginia, and North Dakota) have less than 0.5% of non-pediatric physicians opting out of Medicare.
This analysis shows that a very small share of non-pediatric physicians are opting-out of Medicare, similar to prior analyses dating back to 2013 . Notably, psychiatrists have the highest opt-out rates and are disproportionately represented among physicians who have opted out of Medicare in 2023. This is consistent with previous analyses that found that psychiatrists are less likely than other physician specialties to accept new patients with Medicare or private insurance, suggesting that psychiatrists may prefer to be paid directly from patients rather than insurers, to avoid the administrative burden and have the flexibility to charge higher fees.
This analysis also finds little state-level variation in the percent of physicians opting-out, with virtually all states having opt out rates below 2%. Despite questions about whether lower fees in Medicare relative to private insurance may discourage physicians from seeing Medicare patients, very few physicians are choosing to opt out of Medicare, which could be explained by several factors. The aging of the U.S population, and consequently, the increase in number of Medicare beneficiaries, means that for many physicians, older adults with Medicare coverage account for a relatively large share of their patient population and revenues. For these physicians, the loss of revenue resulting from opting out of Medicare would be substantial, notwithstanding the difference in payment rates between Medicare and private insurance or self-pay. Other factors, such as physician-level characteristics (e.g., years of practice and age), practice-level characteristics (e.g., solo versus group practices), and patient-level factors (e.g., average income of individuals in an area) may also play a role in physician decision-making.
Nancy Ochieng is with KFF. Gabrielle Clerveau was with KFF at the time this brief was written.
| Methods |
| This analysis uses Medicare opt-out affidavit data from the Centers for Medicare & Medicaid Services (CMS), as of June 2023 ( ). The scope of this analysis was limited to non-pediatric physicians, given its Medicare focus, as well as a select group of other clinicians with doctorates: optometrists, oral surgery, and podiatrists. Therefore, pediatricians and other non-physician specialists, such as certified nurse midwives, clinical social workers, and physician assistants, were excluded from the total number of opt-out physicians. Of note, while some clinicians under the oral surgery specialty group may also hold a medical degree (MD or DO), for the purpose of our analysis, this analysis grouped these physicians in accordance with the primary specialty (oral surgery) associated with their National Provider Identifier (NPI) in CMS’ opt-out file. This analysis obtained data on the number of active allopathic and osteopathic physicians by specialty and state from Redi-data, Inc, which utilizes data from the American Medical Association (AMA) Physician Masterfile. One limitation of this analysis is that due to data source limitations, it was not possible to exclude active physicians in professional activity other than patient care, such as research and administration. The specific physician specialty groups identified in this analysis were selected if they were included in the list of opt-out providers provided by CMS. In order to gain a more complete picture of the distribution of opt-out providers in each specialty category, this analysis grouped some subspecialties under a broader specialty category, consistent with the specialty cross-walk provided by Redi-Data, Inc. Specifically, anesthesiology includes pain management as a subspecialty, obstetrics/gynecology includes reproductive endocrinology, and preventive medicine includes occupational medicine. The specialty group of internal medicine includes the following subspecialties: internal medicine (not otherwise specified), critical care medicine, gastroenterology, hematology, hospice & palliative medicine, infectious disease, nephrology, pulmonary disease, and rheumatology. The “surgery” specialty consists of the following surgical subspecialties: cardiac surgery, colorectal surgery, general surgery, hand surgery, micrographic dermatologic surgery, thoracic surgery, and vascular surgery. The following subspecialties are included in the “other” specialty: addiction medicine, cosmetic surgery anesthetic medicine, Doctor of Medicine, hospitalist, integrative medicine, phlebology, undefined physicians, sleep medicine, osteopathic manipulative medicine, medical toxicology. |
Also of Interest
- What to Know About How Medicare Pays Physicians
- Most Office-Based Physicians Accept New Patients, Including Patients With Medicare and Private Insurance
- FAQs on Mental Health and Substance Use Disorder Coverage in Medicare
Learn About Medicare
What is medicare.
Understand the big picture
When to Enroll
Avoid those painful penalties

Social Security Guide
The benefits & eligibility
Dental, Vision, Hearing
How to get coverage
- Supplemental Plans
Learn about Plan G, N, & More
- Advantage Plans
Learn all about Part C
Rx Plans - Part D
Understand drug coverage
About our Company
How we help.
We walk with you
Client Support
We are a call away
Our Learning Center
We love to educate

- Watch Our Free Workshop
- 6 Tips for Finding Doctors Who Accept Medicare in Your Area

Finding the right Medicare approach for you is only one aspect of covering your healthcare needs in this stage of life. Finding Medicare providers, from primary care to specialists, is no small thing since your medical team will significantly impact the care you receive as you age.
To get you started, we’ve compiled a list of tips to help you choose the Medicare providers and specialists that are right for you:
Tip 1: Know your coverage before seeking a medical provider
Your options for finding a physician who works with your Medicare plan will begin with understanding whether you have Original Medicare with Supplement coverage or a Medicare Advantage plan and the key differences.
How to Find a Medicare Provider with Original Medicare
Suppose you have Medicare Parts A and B, also known as Original Medicare. In this case, you’ll have many options to choose a medical provider who accepts Medicare coverage. Because Medicare is a reliable payor backed by the full faith and credit of the U.S. government, most hospitals, doctors’ offices, medical practices, and other healthcare groups accept it as valid coverage.
In fact, according to the Centers for Medicare and Medicaid Services (CMS), 98 percent of medical service providers accept Medicare . That means your area’s medical services and doctors will likely work with your Original Medicare coverage. The groups most likely not to accept Medicare are private membership clinics that bill based on monthly or yearly fees to the select members that make up their clientele.
Additionally, your Original Medicare coverage won’t cover most vision, hearing, or dental visits.
How to Find a Medicare Provider with Medicare Advantage
Medicare Advantage coverage works similarly to traditional health insurance you would have had while working, where an insurance carrier directs you to their network for coverage and can decline to cover or cap payments for services from out-of-network providers. The functionality of these plans means they might include an allowance for vision, hearing, and dental services, similar to rider plans from traditional insurance plans.
Another distinctive feature of Medicare Advantage networks is that an insurer’s typical network may not be the same as their Advantage plan networks. For instance, an insurance carrier may use a particular doctor in their network. However, beneficiaries of the insurance carrier’s Medicare Advantage coverage may have a slightly different network that doesn’t include that particular doctor.
Looking out for these areas of difference can help you choose a doctor or specialist who can work with your Medicare Advantage plan.
Tip 2: Know What Medical Provider You Need
Before choosing a medical provider who accepts Medicare, consider what you need. Do you have an existing primary care physician who you respect and trust? If that’s the case, you might want to continue seeing that provider even after starting Medicare.
Even with a primary care provider, you’ll likely have other providers you’ll work with as you age. But you may need a new doctor or a specialist. Maybe you’re anticipating needing knee replacements in the future. In that case, you may want to consider how to find the best orthopedic surgeon. Or perhaps you’re curious about getting a primary care doctor better suited for your phase of life.
Knowing what kind of Medicare care provider you’re looking for is essential before you dive wholeheartedly into your search.
Tip 3: Don’t underestimate the importance of location, location, location
Where you live and how far you’re comfortable traveling will also matter since only 12 percent of physicians nationwide operate in rural areas . An orthopedic surgeon who practices in a metropolitan area two hours away may be perfect for surgery. But you likely won’t want to have to drive two hours or book a hotel to be present for a multi-week regimen of outpatient physical therapy.
If you’ve lived in the same area for decades, you’re probably reasonably aware of the small practices, hospital systems, physician groups, and other medical professional arrangements in your area. But if you’re a recent transplant or haven’t used the local medical system much, some internet research may serve you well.
So, before you hunt for a Medicare-approved physician, surgeon, or other medical practitioner, get clarity on what you expect from the practitioner you’re looking for, how often you anticipate seeing them, and what is available in your immediate and surrounding area.

Tip 4: Use the tools at your disposal to choose a Medicare doctor
Once you know what kind of medical care provider you’re looking for and in what area, use a Medicare provider list tool to select the medical professional who is right for you.
If you have Original Medicare, Medicare has a tool — Physician Compare — to help you choose your medical provider by searching for a type of care and proximity to your ZIP code. Medicare Advantage beneficiaries must contact their insurance carrier to select a medical provider from their Advantage-approved network of doctors and hospitals. While many carriers have network searches you can find online, others may require a phone call to verify in-network coverage.
Tip 5: Phone a friend about your Medicare-approved medical provider
If you’re searching for a doctor or hospital, don’t hesitate to ask friends for their recommendations. When choosing primary care physicians, this can be a great way to assess whether your intended doctor fits your values, personality, comfort level, stage of life, or other personal criteria. If you’re choosing a Medicare-compatible specialist like an orthopedic surgeon, word-of-mouth feedback can help determine what you’re looking for in expertise, care, and recovery.
In the age of the internet, “phoning a friend” may also mean searching through patient comments on medical review sites or checking with your state medical board. Online records from the state medical board can indicate a physician’s specialty but also reveal past histories of complaints or questions of conduct that may affect your decision.
Tip 6: Ask your potential Medicare provider if they accept Medicare
If you’re worried about how to find a Medicare provider in a pinch, the quickest way is to ask. This isn’t necessarily the best play for those with Medicare Advantage, thanks to the limited nature of care networks with Advantage programs. However, for those on Original Medicare, finding care may be as simple as calling a hospital or physician and asking, “Do you accept Medicare?” As stated, with 98 percent of medical service providers accepting traditional Medicare, more than nine times out of 10, the answer will be “Yes.”
Find out what to expect from Medicare physician coverage with this video:
Ready to choose a Medicare provider or specialist that’s right for you?
With these tips, you’re ready to find a Medicare-approved doctor for your needs, but if you’re still working to understand your Medicare decisions get one-on-one assistance from a guide at Medicare School. Our online resources are sure to help you choose a Medicare plan with confidence so you can enjoy a worry-free retirement. Learn the Medicare basics in our online workshop or give us a call to discuss your coverage option today!
Recent Posts
- Does Medicare Cover Dental and Vision?
- 7 Medicare Enrollment Mistakes to Avoid
- 7 Common Medicare Myths
- Understanding Medicare Supplement Premiums and This Year’s Costs
- Ancillary Plans
- Featured Articles
- Featured Post
- Medicare 101
- Medicare Daily
- Medicare Enrollment
- Medicare Plans
- Original Medicare (A & B)
- Social Security
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- February 2023
- August 2020
Get It Right The First Time
If you want the best Medicare plans for your retirement, give us a call. We provide the education and award-winning guidance you need to make the right decision.

With so much conflicting and confusing information surrounding Medicare, it’s easy to feel unsure about how to choose the best plan(s). At MedicareSchool.com, we empower you with the knowledge and guidance you need to get the best Medicare plans and live retirement worry-free.
© 2024 MedicareSchool.com. All Rights Reserved. MedicareSchool.com is an EnlightnU USA Company. Medicare has neither reviewed nor endorsed this information. Not connected with or endorsed by the United States government or the federal Medicare program. Calling our phone number will connect you to a licensed broker who is trained and certified to help you review the plan options available in your area. We do not offer every plan available in your area. Any information we provide is limited to those plans we do offer in your area. Please contact medicare(dot)gov or 1-800-medicare to get information on all your options. Privacy Policy | Terms & Conditions | Cookie Policy

Medicare Provider Enrollment Print

What’s Changed?
- Updated the enrollment application fee amount for 2024
- Added marriage and family therapists, mental health counselors, and certain dental specialties to the Part B suppliers list
- Merged Form CMS-855R into the CMS-855I paper enrollment application
- Added new provider specialty code information for dentists
Substantive content changes are in dark red.
Application Fee
Physicians, non-physician practitioners, physician organizations, non-physician organizations, and Medicare Diabetes Prevention Program suppliers don’t pay a Medicare enrollment application fee.
Generally, institutional providers and suppliers like DMEPOS suppliers and opioid treatment programs pay an application fee when enrolling, re-enrolling, revalidating, or adding a new practice location.
Enrollment Application Fee
The 2024 enrollment application fee is $709.
Whether you apply for Medicare enrollment online or use the paper application, you can pay the Medicare application fee online through:
- PECOS: During the application process, PECOS prompts you to pay the application fee
- CMS Paper Application: Go to PECOS Application Fee Information to submit the application fee
A hardship exception exempts you from paying a current application’s fee. If you request a hardship exception, submit a written request and supporting documentation describing the hardship and justifying an exception to paying the application fee with your PECOS or CMS paper application. We grant exceptions on a case-by-case basis.
Medicare Administrative Contractors (MACs) will only process applications with the proper application fee payment or an approved hardship exception.
If you don’t pay the fee or submit a hardship exception request, your MAC will send a letter allowing you 30 days to pay the fee. If you don’t pay the fee on time, the MAC may reject or deny your application or revoke your existing billing privileges, as appropriate.

Providers must enroll in the Medicare Program to get paid for providing covered services to Medicare patients. Determine if you’re eligible to enroll and how to complete enrollment.
We list institutional providers on the Medicare Enrollment Application: Institutional Providers (CMS-855A) , which include:
- Community mental health centers
- Comprehensive outpatient rehabilitation facilities
- Critical access hospitals
- ESRD facilities
- Federally Qualified Health Centers
- Histocompatibility labs
- Home health agencies
- Hospice organizations
- Indian Health Service facilities
- Organ procurement organizations
- Opioid treatment programs
- Outpatient physical therapy, occupational therapy, speech pathology services
- Religious nonmedical health care institutions
- Rural emergency hospitals
- Rural health clinics
- Skilled nursing facilities (SNFs)
Physicians, non-physician practitioners (NPPs), clinics or group practices, and specific suppliers who can enroll as Medicare Part B providers are defined in enrollment forms Medicare Enrollment Application: Physicians and Non-Physician Practitioners (CMS-855I) and Medicare Enrollment Application: Clinics/Group Practices and Other Suppliers (CMS-855B) .
Who’s an NPP?
NPPs include nurse practitioners, clinical nurse specialists, and physician assistants who practice with or under a physician’s supervision.
Physicians, NPPs, & Suppliers (CMS-855I)
- Anesthesiology assistants
- Audiologists
- Certified nurse-midwives
- Certified registered nurse anesthetists
- Clinical nurse specialists
- Clinical psychologists
- Clinical social workers
- Marriage and family therapists
- Mass immunization roster billers (individuals)
- Mental health counselors
- Nurse practitioners
- Occupational or physical therapists in private practice
- Dental anesthesiology
- Dental public health
- Endodontics
- Oral and maxillofacial surgery
- Oral and maxillofacial pathology
- Oral and maxillofacial radiology
- Oral medicine
- Orofacial pain
- Orthodontics and dentofacial orthopedics
- Pediatric dentistry
- Periodontics
- Prosthodontics
- Physician assistants
- Psychologists billing independently
- Registered dietitians or nutrition professionals
- Speech-language pathologists
Clinics, Group Practices, & Specific Suppliers (CMS-855B)
- Ambulatory surgical centers (ASCs)
- Clinics and group practices
- Home infusion therapy suppliers
- Hospital departments
- Independent clinical labs
- Independent diagnostic testing facilities
- Intensive cardiac rehabilitation suppliers
- Mammography centers
- Mass immunization roster billers (entities)
- Physical or occupational therapy groups in private practice
- Portable X-ray suppliers
- Radiation therapy centers
Medicare Diabetes Prevention Program Suppliers
Potential suppliers must use Medicare Enrollment Application: Medicare Diabetes Prevention Program (MDPP) Suppliers (CMS-20134) to enroll in the Medicare Program.
Beginning January 1, 2024, we established new provider specialty codes for dentists.
If you don’t see your provider type listed, contact your MAC’s provider enrollment center before submitting a Medicare enrollment application.
Medicare provider and supplier organizations have business structures, like corporations, partnerships, professional associations, or limited liability companies, which meet the provider and supplier definitions. Provider and supplier organizations don’t include organizations the IRS defines as sole proprietorships.
Provider and supplier organizations include:
- Medical group practices and clinics
You must have a provider or supplier employer identification number (EIN) to enroll in Medicare. An EIN is the same as the provider or supplier organization’s IRS-issued tax identification number (TIN).
Sole Proprietorships & Disregarded Entities
Sections 10.6.4 and 10.6.7.1(D)(5) of Medicare Program Integrity Manual, Chapter 10 have more information about sole proprietorships and disregarded entities.
Medicare participation means you agree to accept claims assignment for all covered patient services. By accepting assignment, you agree to accept Medicare-allowed amounts as payment in full. You can’t collect more from the patient than the deductible and coinsurance or copayment . The Social Security Act says you must submit patient Medicare claims whether or not you participate.
You have 90 days after we send your initial enrollment approval letter to decide if you want to be a participating provider or supplier. To participate as a Medicare Program provider or supplier, submit the Medicare Participating Physician or Supplier Agreement (CMS-460) upon initial enrollment. The only other time you may change your participation status is during the open enrollment period, generally from mid-November–December 31 each year.
Participating Provider or Supplier
- We pay 5% more to participating physicians and other suppliers
- Because these are assigned claims, we pay you directly
- We forward claim information to Medigap (Medicare supplement coverage) insurers
Non-Participating Provider or Supplier
- We pay 5% less to non-participating physicians and other suppliers
- You can’t charge patients more than the limiting charge, 115% of the Medicare Physician Fee Schedule amount
- You may accept assignment on a case-by-case basis
- You have limited appeal rights
Medicare Claims Processing Manual, Chapter 12 has more information.
Step 1: Get an NPI
To enroll in the Medicare Program, get an NPI through:
- Online Application: Get an Identity & Access Management (I&A) System user account. Then apply for an NPI in NPPES .
- Call 1-800-465-3203 (TTY 1-800-692-2326)
- Email [email protected]
- Bulk Enumeration: Apply for Electronic File Interchange access and upload your own CSV or XML files.
Not Sure If You Have an NPI?
Search for your NPI on the NPPES NPI Registry .
CMS Provider Enrollment Systems:
- I&A System
- Electronic Health Record (EHR) Incentive Payments
Multi-Factor Authentication
To better protect your information, we implemented I&A System multi-factor authentication for the provider enrollment systems listed above.
Step 2: Complete Proper Medicare Enrollment Application
After you get an NPI, you can complete Medicare Program enrollment, revalidate your enrollment, or change your enrollment information. Before applying, get the necessary enrollment information , and complete the actions using PECOS or the paper enrollment form.
A. Online PECOS Application
After we approve your I&A System registration, submit your PECOS application.
PECOS offers a scenario-driven application, asking questions to recover the information for your specific enrollment scenario. You can use PECOS to submit all supporting documentation. Follow these instructions:
- Log in to PECOS .
- Continue with an existing enrollment or create a new application.
- When PECOS determines your enrollment scenario and you confirm it’s correct, you’ll see the topics for submitting your application. To complete each topic, enter the necessary information.
- Confirms you entered all necessary data
- Lists MAC documents to submit for review
- Gives the option to electronically sign and certify
- Shows your MAC’s name and mailing address
- Lets you print your enrollment application for your records (don’t submit a paper copy to your MAC)
- Sends the application electronically to your MAC
- Emails you to confirm your MAC got the application
PECOS 2.0 Enhancements
PECOS will have enhanced features to better meet your needs. Watch this 2-minute video or read these FAQs to learn more about:
- A single application for multiple enrollments
- Data pre-population and an application that’s tailored to you
- Enhanced capability to add or delete group members
- Real-time processing checks and status updates
- Revalidation reminders
Visit Introducing PECOS 2.0 for more information.
PECOS Scroll Functionality
PECOS validates that you’ve read and acknowledged certification terms and conditions before you electronically submit your Medicare enrollment application. Review and scroll through each text box with certification requirements before you can select accept on these pages:
- Remote E-sign
Enrolling physicians, NPPs, or other Part B suppliers must choose 1 of the application descriptions below.
- You’re the only owner of a business, set up as a corporation, where you provide health care services
- Your business is legally separate from your personal assets
- You provide all health care services from a facility you own, lease, or rent
- You’re the only owner of a business that provides health care services
- You and your business are legally 1 and the same
- You’re personally responsible for the business’ financial obligations, and you report business income and losses on your personal tax return
- You provide all health care services as an employee of a group practice or clinic
- You arrange with your employer to submit claims and get paid for your services
- Choose Group Member Only if you’re reassigning all your benefits to a group practice or clinic
- You provide health care services as a group practice or clinic employee
- You agree with your employer to submit claims and get paid for your services
- You also provide health care services from a facility that you own, lease, or rent
- Your income through self-employment is part of your personal assets
- Your corporation doesn’t file taxes; instead, you file corporate taxes on your personal tax filing
B. Paper Medicare Enrollment Applications
Submit the appropriate paper enrollment application if you’re unable to use PECOS. Carefully review the paper application instructions to decide which form is right for your practice. The paper enrollment application collects your information, including documentation verifying your Medicare Program enrollment eligibility.
If you submit a paper application, your MAC processes your application and creates a Medicare enrollment record by entering the data into PECOS.
Medicare Enrollment Application: Institutional Providers (CMS-855A) : Institutional providers use this form to begin the Medicare enrollment or revalidation process or to change enrollment information.
Most physicians and NPPs complete the CMS-855I to begin the enrollment process. You can also use the CMS-855I if you reassign your benefits to another entity, like a medical group or group practice that gets paid for your services. We’ve merged the CMS-855R into the CMS-855I paper enrollment application.
- Medicare Enrollment Application: Clinics/Group Practices and Other Suppliers (CMS-855B) : Group practices and other organizational suppliers, except DMEPOS suppliers, use this form to begin the Medicare enrollment or revalidation process or to change enrollment information.
- Medicare Enrollment Application: Enrollment for Eligible Ordering/Certifying Physicians and Other Eligible Professionals (CMS-855O) : Physicians and other eligible NPPs use this form to enroll in Medicare solely to order or certify items or services for Medicare patients. This includes those physicians and other eligible NPPs who don’t send billed services claims to a MAC.
- Medicare Enrollment Application: Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Suppliers (CMS-855S) : DMEPOS suppliers use this form to begin the Medicare enrollment or revalidation process or to change enrollment information.
- Medicare Enrollment Application: Medicare Diabetes Prevention Program (MDPP) Suppliers (CMS-20134) : MDPP suppliers use this form to begin the Medicare enrollment or revalidation process or to change enrollment information.
After you submit an enrollment application and all required supporting documentation to your MAC, they’ll send their recommendations to the State Survey Agency . The State Survey Agency then decides if specific providers meet Medicare enrollment conditions.
After a MAC makes a recommendation, the State Survey Agency or a CMS-recognized accrediting organization conducts a survey. Based on the survey results, the agency or organization recommends that we approve or deny the enrollment (certification of compliance or non-compliance).
Certain institutional provider types may elect voluntary accreditation by a CMS-recognized accrediting organization instead of a State Survey Agency. The accrediting organization will notify the State Survey Agency of their decision.
The State Survey Agency forwards us the survey results. We assign the CMS Certification Number and effective date, sign the provider agreement, and update the certification database. Your MAC will issue your final approval or denial letter.
If approved, you’ll get a fully executed provider agreement.
Electronic Funds Transfer
If enrolling in Medicare, revalidating, or making certain changes to your enrollment, we require you to set up an electronic funds transfer (EFT). Enroll in EFT by completing the PECOS EFT information section. When submitting a PECOS application:
- Complete the EFT information for your organization (if appropriate) or yourself
- Include a copy of a voided check or bank letter that has your individual or business legal name and applicable account and routing numbers
Step 3: Respond to Requests for More Information
MACs pre-screen and verify enrollment applications for completeness. If the MAC needs more information, respond to information requests within 30 days. If you don’t, the MAC may reject your enrollment .
Your MAC won’t fully process your PECOS enrollment application without your electronic or uploaded signature, application fee (if applicable), and necessary supporting documentation. The enrollment application filing date is when the MAC gets your enrollment application.
You can check your PECOS enrollment application status 2 ways:
- Log in to PECOS and select the View Enrollments link. In the Existing Enrollments section, find the application. The system shows the application status.
- To see your enrollment status without logging in, go to PECOS and, under Helpful Links , select Application Status.
When your MAC approves your application, it switches the PECOS record to an approved status and sends you an approval letter.
Provider Enrollment Site Visits
We conduct a site visit verification process using National Site Visit Contractors (NSVCs). A site visit helps prevent questionable providers and suppliers from enrolling or staying enrolled in the Medicare Program.
The NSVCs conduct unannounced site visits for all Medicare Part A and B providers and suppliers, including DMEPOS suppliers. The NSVCs may conduct an observational site visit or a detailed review to verify enrollment-related information and collect other details based on pre-defined CMS checklists and procedures.
During an observational visit, the inspector has minimal contact with the provider or supplier and doesn’t hinder the facility’s daily activities. The inspector will take facility photos as part of the site visit. During a detailed review, the inspector enters the facility, speaks with staff, and collects information to confirm the provider’s or supplier’s compliance with our standards.
Inspectors performing site visits will carry a photo ID and a CMS-issued, signed authorization letter the provider or supplier may review. If the provider or its staff want to verify we ordered a site visit, contact your MAC .
Make your office staff aware of the site visit verification process. An inspector’s inability to perform a site visit may result in denial of your Medicare enrollment application or revocation of your Medicare billing privileges.
Step 4: Use PECOS to Keep Enrollment Information Current
Report a Medicare enrollment change using PECOS. Physicians, NPPs, and physician and NPP organizations must report a change of ownership or control (including a change in authorized or delegated official), a change in practice location, and any final adverse legal actions (like a felony or suspension of a federal or state license) within 30 days of the change and report all other changes within 90 days of the change.
DMEPOS suppliers must report changes in their enrollment application information within 30 days of the change.
Independent diagnostic testing facilities must report changes in ownership, location, general supervision, and adverse legal actions within 30 days of the change and report all other changes within 90 days of the change.
Medicare Diabetes Prevention Program suppliers must report changes in ownership, including AO or Access Manager; location; coach roster; and adverse legal actions within 30 days of the change and report all other changes within 90 days of the change.
PECOS Users
We allow various organizations and users to work in our systems. The type of user depends on their relationship with you and the duties they perform in your practice.
You may choose other users to act for your organization to manage connections and staff, including appointing and approving other system-authorized users. Depending on your professional relationships with other providers, the CMS External User Services Help Desk may ask you for additional validation information.
One Account, Multiple Systems
We use several provider enrollment systems. Organizational providers and suppliers must use the Identity & Access Management (I&A) System to name an AO to work in CMS systems. The I&A System allows you to:
- Use NPPES to apply for and manage NPIs
- Use PECOS to enroll in Medicare or update or revalidate your current enrollment information
- Register to get electronic health record (EHR) incentive payments for eligible professionals and hospitals that adopt, use and upgrade, or show meaningful use of EHR technology
Authorized Officials, Access Managers, Staff End Users, & Surrogates
Organizational providers or suppliers must appoint and authenticate an Authorized Official (AO) through the I&A System to work in PECOS for them. That person must meet the AO regulatory definition. For example, an AO is a chief executive officer, chief financial officer, general partner, chair of the board, or direct owner who can legally enroll in the Medicare Program.
Respond to your employer’s AO invitation or initiate the request yourself. After you’re the confirmed AO, use PECOS for your provider or supplier organization. As an AO, you’re responsible for approving PECOS user system requests to work on behalf of the provider or supplier organization. Regularly check your email and take the requested actions.
AOs may delegate their responsibilities to an Access Manager who can also initiate or accept connections and manage staff for their organizations.
AOs or Access Managers may invite a Staff End User (SEU) or Surrogate to access PECOS for their organization. Once registered, an SEU or Surrogate may log in to access, view, and modify CMS system information, but they can’t represent the practice, manage staff, sign enrollment applications, or initiate or accept connections.
| Role | Represent an Organization | Manage Staff | Approve or Manage Connections | Act on Behalf of Provider in CMS Systems |
|---|---|---|---|---|
| Yes | Yes | Yes | Yes | |
| Yes | Yes | Yes | Yes | |
| Yes | Yes | Yes | Yes | |
| No | No | No | Yes | |
| No | No | No | Yes |
We recommend using the same I&A System-appointed AO and PECOS Access Managers. The assigned AO and Access Managers must have the right to legally bind the company and be responsible for approving the system staff and be CMS-approved in the I&A System.
Only AOs can sign an initial organization enrollment application. An Access Manager can sign changes, updates, and revalidations.
The I&A System Quick Reference Guide has detailed instructions on managing system users.
PECOS Technical Help
Using PECOS may require technical support. The first step toward a solution is knowing which CMS contractor to contact.
Common Problems & Who to Contact
You experience system-generated error messages, have trouble navigating through or accessing PECOS screens, encounter printing problems, or your valid I&A System user ID and password won’t allow PECOS access because of a malfunction (for example, the website operates slowly or not at all or a system-generated error message prevents you from entering data).
A system-generated error message doesn’t include messages created when you enter data incorrectly or ignore system prompts.
Solution: Contact the CMS External User Services Help Desk
The External User Services website has information on common problems and allows you to ask questions, chat live with a support team member, or look up previous support history.
Phone: 1-866-484-8049 (TTY 1-866-523-4759)
Email: [email protected]
EUS Hours of Operation:
- Monday–Friday: 6 am–6 pm CT
- Saturday–Sunday: Closed
Before you log in to PECOS, you need a valid I&A System user ID and password.
Passwords expire every 60 days. The I&A System tells you the number of days until your password expires. If you attempt to log in to PECOS with an expired password, the system redirects you to the I&A System to reset it.
Solution: Access I&A System or Contact I&A System Help
The I&A System website lets you create an I&A System user ID and password, change your password, and recover forgotten login information. You can also access several resources:
- The I&A FAQs helps you resolve common I&A System problems
- The I&A System Quick Reference Guide provides step-by-step instructions, including screenshots, and information about I&A System features and tools
On the I&A System website, select the Help button in the upper right corner of any webpage for more information on that webpage’s topic.
While using PECOS, you may have questions, experience problems enrolling, or need help completing specific PECOS enrollment application sections.
Solution: Contact Your Medicare Enrollment Contractor
Find detailed enrollment contact information in the Medicare Provider Enrollment Contact List . If you have questions, find your MAC’s website .
Solution: Refer to the CMS Provider Enrollment Assistance Guide
If you don’t know who to call for help, refer to the “Who should I call?” CMS Provider Enrollment Assistance Guide .
Find detailed enrollment contact information in the Medicare Provider Enrollment Contact List .
Organizational providers and suppliers must designate a provider enrollment AO to work in CMS systems, including the I&A System , NPPES , and PECOS . The AO may also authorize Access Managers, Surrogates, and SEUs to use PECOS. Individual providers and suppliers don’t require an AO but can authorize Surrogates and SEUs to work in PECOS. Refer to the I&A System Quick Reference Guide and I&A FAQs for more information on registering for an I&A System account or enrolling as an AO.
We use several provider enrollment systems. Specifically, the I&A System allows you to:
- Use PECOS to enroll in Medicare or to update or revalidate your current enrollment information
- Register to get EHR incentive payments for eligible professionals and hospitals that adopt, use and upgrade, or show meaningful use of certified EHR technology
Before completing PECOS enrollment, create an I&A System account. Organizational providers and suppliers must designate an AO to work in these systems.
Use the same information to enroll in Medicare using PECOS as you would for a paper enrollment application.
- If you don’t have an I&A System account, create your username and password
- Use your username and password to log in to NPPES to register for an NPI
- All Medicare provider enrollees must have an active NPI
Based on your provider type, you may also need this information:
- Personal identifying information, including your legal name on file with the Social Security Administration, date of birth, and SSN
- Legal business name of the provider or supplier organization
- Provider or supplier organization’s TIN; if any person or organization has 5% or more partnership interest or ownership (direct or indirect), you must list them on all enrollment records under your TIN
- Professional license information
- School degrees
- Certificates
- W-2 employees and contracted individuals and organizations with managerial control of the provider or supplier
- Accreditation information
- Surety bond information
- Providers self-designate their Medicare specialty on the Medicare enrollment application (CMS 855-I or CMS 855-O) or PECOS when they enroll in the Medicare Program
- Beginning January 1, 2024, we established new provider specialty codes for dentists
- Current medical practice location
- Federal, state, and local (city or county) business and professional licenses, certificates, and registrations specifically required to operate as a health care facility
- Medical record storage information
- Special payment information
- Bank account information
- Suspension, termination, or revocation of a license to provide health care by a state licensing authority or the Medicaid Program
- Conviction of a federal or state felony within 10 years before enrollment, revalidation, or re-enrollment
- Exclusion or debarment from federal or state health care program participation by the Office of Inspector General (OIG) or other federal or state offices with authority to exclude or sanction a provider (or those listed above)
An application is the paper or electronic form you submit for Medicare Program enrollment approval. After the MAC processes the application, PECOS keeps the enrollment record, which includes all your enrollment application data.
You can’t use PECOS to:
- Change your SSN
- Change a provider’s or supplier’s TIN
- Solely owned PA, PC, or LLC can’t be changed to a sole proprietorship
- Sole proprietorship can’t be changed to a PA, a PC, or an LLC
Submit changes noted above using the appropriate paper Medicare enrollment application .
No. All Fee-for-Service (FFS) providers can apply in PECOS.
PECOS is available 24 hours a day, Monday–Saturday, with scheduled downtime on Sunday. We offer technical support daily, 5 am–8 pm CT.
We encourage you to submit your enrollment application through PECOS because it’s faster and easier, but you may complete and mail the appropriate paper Medicare enrollment application to the address on the Medicare Fee-for-Service Provider Enrollment Contact List :
- Parts A and B Providers: Send forms to your Part A or Part B MAC.
- Home Health and Hospice Providers: Send forms to the Home Health and Hospice Contractor.
- DMEPOS Suppliers Send forms to the National Provider Enrollment (NPE) DMEPOS contractor in your region. Find your NPE contractor .
Even if you submit your application on a paper form, your MAC creates an enrollment record in PECOS.
When you electronically submit your Medicare enrollment application, you’ll get a Submission Confirmation page, which will remind you that the individual provider, or the provider or supplier organization AO or Access Manager must electronically sign the application or upload their signature. You’ll be able to see which MAC is processing your application, your unique application tracking number, and real-time information about your application.
PECOS emails the web tracking ID for the submitted application to each address in the Contact Person section of the application. Remember to verify all your completed signatures with either an electronic signature or uploading certification. Mail any required supporting documentation you didn’t upload during submission to the MAC, and include the PECOS tracking ID.
Create a new enrollment:
- If you change your services, like changing specialties
- If you change your location, causing your MAC to need new state surveys and other documentation (your MAC can determine this)
- If you have a change of ownership
- If a provider is creating a new TIN because of a change of ownership
- If you have provider-based vs. freestanding requirements (find your MAC’s website for more information)
Application Fee & Supporting Documentation
Generally, institutional providers and suppliers, like DMEPOS suppliers and opioid treatment programs, pay an application fee when enrolling, re-enrolling, revalidating, or adding a new practice location.
MACs will only process applications with the proper application fee payment or an approved hardship exception.
If you pay the fee during the 30-day period, the MAC processes the application in the usual manner.
No. When you electronically submit the Medicare enrollment application, a page appears that lists the supporting documentation to complete the enrollment. You can submit all this documentation electronically through PECOS.
Yes, either is acceptable. You must send this information electronically (as supporting documentation uploaded into PECOS).
During the PECOS application process, the Penalties for Falsifying Information page has the same text as the paper Medicare enrollment application and lists the consequences for providing false information. These consequences include criminal and civil penalties, fines, civil monetary penalties, exclusion from federal health care programs, and imprisonment, among others. You must acknowledge this page by selecting the Next Page button before continuing the PECOS submission process.
Enrollment Application Issues
First, make sure you entered your correct SSN, legal name, and date of birth. If you believe you entered the correct information but PECOS doesn’t accept this information, contact the Social Security Administration .
You must report an SSN to enroll in Medicare. If you don’t want to report your SSN over the web, use the appropriate paper Medicare enrollment application .
An Invalid Address error indicates the address entered doesn’t comply with the U.S. Postal Service address standards. This page lets you continue by either saving the address you entered or selecting the address PECOS displays.
As a security feature, PECOS will time out if you’re inactive (you don’t hit any keys on your computer keyboard) for 15 minutes. The system warns you of inactivity after 10 minutes. If it gets no response after 5 additional minutes, the system automatically times you out. Save your work if you anticipate inactivity while applying in PECOS. If you don’t save your work and the system times out, you must start from the beginning.
Submitting Reportable Events
No. If you report a change to existing information, check Change , include the effective date of change, and complete the appropriate fields in the impacted sections.
Yes. Following your initial enrollment, report certain changes (reportable events) to your MAC within 30 calendar days of the change. Report all other changes to your MAC within 90 days.
Report a Medicare enrollment change using PECOS. Physicians and NPPs must report a change of ownership or control (including a change in authorized or delegated official), a change in practice location, and any final adverse legal actions (like a felony or suspension of a federal or state license) within 30 days of the change and report all other changes within 90 days of the change.
Since Medicare pays claims by EFT, the Special Payments address should indicate where all other payment information must go (for example, paper remittance notices or special payments).
Providers and suppliers should report most changes using PECOS or the applicable paper Medicare enrollment application .
No. If you have a new business location, complete a new PECOS or paper application. Each DMEPOS enrollment record can only have 1 current business location.
Revalidations
Revalidation means resubmitting and recertifying your enrollment information.
DMEPOS suppliers must revalidate every 3 years, while all other providers and suppliers generally revalidate every 5 years. We can also conduct off-cycle revalidations . You can revalidate using PECOS or by submitting the appropriate paper Medicare enrollment application .
If you’re currently enrolled, check the Medicare Revalidation List to find your revalidation due date. If you see a due date, submit your revalidation before that date. Your MAC will also send you a revalidation notice.
Due dates are:
- Updated in the Medicare Revalidation List every 60 days at the beginning of the month
- Listed up to 7 months in advance or listed as to be determined (TBD) if the due date is more than 7 months away
Yes. Your MAC will send a revalidation notice 90–120 days before your revalidation due date.
If there’s no due date listed on the Medicare Revalidation List or you didn’t get a MAC letter requesting revalidation, don’t submit your revalidation application. Your MAC will return it to you.
However, if you’re within 2 months of the due date listed on the Medicare Revalidation List and didn’t get a MAC notice to revalidate, submit your revalidation application.
Yes. PECOS lets you review information on file and update and electronically submit your revalidation. If you use PECOS, you need to update only changed information.
If you submit your revalidation after its due date, your MAC may place a hold on your Medicare payments or deactivate your Medicare billing privileges. If the MAC requests additional documentation, respond within 30 days. If you don’t, they may deactivate your Medicare billing privileges.
Revalidation ensures all provider enrollment records are accurate and current. Generally, we don’t take administrative action against a provider or supplier for updating their records even though it wasn’t timely. However, we could take administrative actions, including recovering previous Medicare payments, when a provider or supplier that fails to report the change causes their Medicare enrollment to become ineligible.
PECOS users can’t mail documents that require a signature. When submitting your application, be prepared to send an e-signature or upload your signed documents.
Protect Your Identity & Privacy
You can help protect your professional medical identifiers from identity thieves attempting to defraud the Medicare Program.
Keep PECOS Enrollment Information Current
Log in to PECOS and review your Medicare enrollment information several times a year to ensure no unauthorized changes were made.
PECOS Provides Security
Only you, authorized surrogates, authorized CMS officials, and MACs may enter and view your Medicare PECOS enrollment information. CMS officials and MACs get security standards training and must protect your information. We don’t disclose your Medicare enrollment information to anyone, except when authorized or required by law.
Review & Protect Enrollment Information
Review your Medicare enrollment information in PECOS frequently to ensure it’s accurate, current, and unaltered.
Use your I&A System user ID and password to access PECOS. Keep your ID and password secure.
Protect Yourself & CMS Programs from Fraud
Your NPI and TIN are publicly available information. Use extra caution to monitor and protect your professional and personal information to help prevent fraud and abuse. Also ensure your patients’ personal health information is secure. Refer to these resources:
- Medicare Fraud & Abuse: Prevent, Detect, Report
- Office of Inspector General
- Reporting Medicare fraud & abuse
Take these steps to verify your Medicare enrollment information:

If you suspect your PECOS profile is incorrect due to unauthorized account access, contact your MAC, law enforcement authorities, and your bank. Your MAC and bank can flag your respective accounts for possible fraudulent activity, and law enforcement can begin investigating if and how your accounts were compromised.
Additional Privacy Tips
Take these additional actions to protect your Medicare enrollment information:
- Change your password in the I&A System before accessing PECOS the first time. You can’t change your user ID, but you must change your password every 60 days.
- Review your Medicare enrollment information several times a year to ensure no one changed information without your knowledge. Immediately report changes you didn’t submit.
- Maintain your Medicare enrollment record. Report Medicare enrollment changes known as reportable events, including change of ownership or control , change in practice location, banking arrangements, and any final adverse legal actions.
- Store PECOS copies or paper enrollment applications in a secure location. Don’t allow others access to this information as it contains your personal information, including your date of birth and SSN. Don’t leave copies in a public workspace.
- Enroll in electronic Medicare payments, and ensure they deposit directly into your bank account. We require all providers to use electronic funds transfer (EFT) when enrolling in Medicare, revalidating, or making changes to their enrollment. The most efficient way to enroll in EFT is to complete the EFT information section in PECOS and provide the required supporting documentation. Using EFT allows us to send payments directly to your bank account.
DMEPOS Supplier Requirements
Dmepos supplier standards, accreditation, & surety bond.
To enroll or keep your Medicare billing privileges, all DMEPOS suppliers (except certain exempted professionals) must meet supplier and DMEPOS Quality Standards to become accredited. Certain DMEPOS suppliers must also submit a surety bond .
DMEPOS suppliers (except those exempted eligible professionals and other persons) must be accredited by a CMS-approved accrediting organization before submitting a Medicare enrollment application to the National Provider Enrollment (NPE) DMEPOS contractors .
Each enrolled DMEPOS supplier covered under the Health Insurance Portability and Accountability Act (HIPAA) must name each practice location (if it has more than 1) as a sub-part and make sure each sub-part gets its own NPI.
Individual DMEPOS Suppliers (for example, sole proprietorships)
Physicians, NPPs, and DMEPOS suppliers may use their I&A System user ID and password to access PECOS . If you don’t already have an I&A System account, refer to the I&A System User Registration page and enter the information to open an account. For help, refer to the How to Setup Your Account if you are a Sole Owner section in the I&A System Quick Reference Guide .
As an individual DMEPOS supplier, you don’t need an AO or another authorized user.
Organizational DMEPOS Suppliers System Users
A DMEPOS supplier organization must appoint an AO to manage connections and staff, including appointing and approving other authorized PECOS users. The organization must identify the AO in the enrollment application. The AO must have ownership or managing control in the DMEPOS supplier organization.
Providers Who Solely Order or Certify
Physicians and other eligible professionals must enroll in the Medicare Program or have a valid opt-out affidavit on file to solely order or certify Medicare patient items or services.
Those physicians and other eligible professionals enrolled solely as ordering/certifying providers don’t send billed service claims to a MAC.
Ordering/Certifying Terms
Part B claims use the term ordering/certifying provider to identify the professional who orders or certifies an item or service reported in a claim. These are technically correct terms:
- Providers order non-physician patient items or services, like DMEPOS, clinical lab services, or imaging services
- Providers certify patient home health services
The health care industry uses the terms ordered , referred , and certified interchangeably .
Who Are Eligible Ordering/Certifying Providers?
Physicians or eligible professionals who order or certify Part A or Part B services but don’t want to submit Medicare claims are eligible ordering/certifying providers.
A person already enrolled as a Part B provider may submit claims listing themselves as the ordering/certifying provider without re-enrolling using Medicare Enrollment Application: Enrollment for Eligible Ordering/Certifying Physicians and Other Eligible Professionals (CMS-855O) .
Note: Those who enroll as eligible providers using CMS-855O can’t bill Medicare, and we can’t pay for their services because they have no Medicare billing privileges.
Organizational NPIs don’t qualify, and you can’t use them to order or certify.
Eligible providers must meet these basic conditions:
- Have an individual NPI
- Be enrolled in Medicare in either an approved or opt-out status
- Be an eligible specialty type to order or certify
Denial of Ordering/Certifying Claims
If claims lack a valid individual NPI, MACs deny them if they’re from:
- Clinical labs for ordered tests
- Imaging centers for ordered imaging procedures
- DMEPOS suppliers for ordered DMEPOS
- Part A home health agencies that aren’t ordered or certified by a Doctor of Medicine, Osteopathy, or Podiatric Medicine
If you bill a service that needs an eligible provider and they aren’t on the claim, the MAC will deny the claim. The claim must have a valid NPI and the eligible provider’s name as it appears in PECOS.
If a provider who’s on the Preclusion List prescribes a Medicare Part D drug, drug plans will deny it.
Requirement 1: Get an Individual NPI
The 2 types of NPIs are: Type 1 (individual) and Type 2 (organizational). Medicare allows only Type 1 NPIs to solely order items or certify services. Apply for an NPI through:
- Online Application: Get an I&A System user account. Then apply for an NPI in NPPES .
Requirement 2: Enroll in Medicare in an Approved or Opt-Out Status
Once you have an NPI, use PECOS to verify current Medicare enrollment record information, including your NPI and that you’re approved, or go to the Opt Out Affidavits list to check your status. To opt out of Medicare, submit an affidavit expressing your decision to opt out of the program.
Part C and Part D providers don’t have to enroll in Medicare in an approved or opt-out status.
| Verification Option | Enrollment Record Is Current If: |
|---|---|
| Go to the datasets.* | You’re on 1 of these reports. |
| Go to to find your enrollment record. | Your enrollment record displays an approved status. |
| If you submitted an enrollment application as 1 of the eligible provider types on paper (CMS-855O) or using PECOS and want to check the status, go to the and datasets. | Your enrollment application is pending contractor review if you’re on 1 of these reports. |
*We deny certain power mobility device claims if the ordering provider isn’t on our eligible providers list.
Requirement 3: Be Eligible to Order or Certify
The physicians and eligible professionals who may enroll in Medicare solely for ordering or certifying include, but aren’t limited to, physicians and eligible professionals who are:
- Department of Veterans Affairs employees
- Public Health Service employees
- Department of Defense or TRICARE employees
- Indian Health Service or Tribal Organization employees
- Federally Qualified Health Center, Rural Health Clinic, or Critical Access Hospital employees
- Licensed Residents in an approved medical residency program defined in 42 CFR 413.75(b)
- Dentists, including oral surgeons
- Pediatricians
- Retired, licensed physicians
If you’re unsure whether your specific provider specialty qualifies to enroll as an ordering/certifying provider, refer to Section 4 of CMS-855O or find your MAC’s website before submitting a Medicare enrollment application.
Interns & Residents
Claims for items or services ordered or certified by licensed or unlicensed interns and residents must specify a teaching physician’s NPI and name. State-licensed residents may enroll to order or certify and can be listed on claims. If states offer provisional licenses or otherwise permit residents to order/certify, we allow interns and residents to enroll consistent with state law.
Requirement 4: Respond to Requests for More Information
- To see your enrollment status without logging in, go to PECOS and, under Helpful Links , select Application Status .
Requirement 5: Use PECOS to Keep Enrollment Information Current
Report a Medicare enrollment change using PECOS. Providers and suppliers must report a change of ownership or control (including a change in authorized or delegated official), a change in practice location, and any final adverse legal actions (like revocation or suspension of a federal or state license) within 30 days of the change and must report all other changes within 90 days of the change.
Revalidation
Revalidation, or re-submitting and recertifying your enrollment information accuracy, is an important anti-fraud tool. All Medicare-enrolled providers and suppliers must periodically revalidate their enrollment information .
Generally, physicians, including physician organizations, opioid treatment programs, Medicare Diabetes Prevention Program suppliers, and institutional providers, revalidate enrollment every 5 years or when we request it. DMEPOS suppliers must revalidate their enrollment information every 3 years.
PECOS is the most efficient way to revalidate information.
If you’re actively enrolled, go to the Medicare Revalidation List to find your revalidation due date. If you see a due date, submit your revalidation before that date. Your MAC notifies you when it’s time to revalidate. If you submit your revalidation application after the due date, your MAC may hold your Medicare payments or deactivate your billing privileges.
Rebuttal Process
MACs issue Medicare billing privilege deactivations. We permit providers and suppliers to file a rebuttal .
Get more information:
- 42 CFR 424.515
- Provider Enrollment Revalidation Cycle 2 FAQs
- Revalidations (Renewing Your Enrollment)
Large Group Coordination
Groups with more than 200 members can use the Medicare Revalidation List and search by their organization’s name to download group information. Their MAC will send them a letter and spreadsheet that lists the providers linked to their group who must revalidate within 6 months. Large groups should work together to ensure they submit only 1 application from each provider or supplier.
Use these resources to learn how to enroll in the Medicare Program, revalidate your enrollment, or change your enrollment information. Enroll in the Medicare Program to get paid for providing covered patient services. Enroll if you solely order items or certify services.
You can enroll online by using PECOS or the appropriate paper enrollment application you submit to your MAC.
- Get an I&A System user account
- Apply for your NPI in the NPPES
- Enroll in PECOS
| Topic | Title |
|---|---|
| Application fee |
|
| Provider-supplier general information |
| Topic | Title |
|---|---|
| Revalidation due dates | |
| Revalidation overview |
|
| Topic | Title |
|---|---|
| FAQs | |
| Get an NPI |
(video) |
| Online enrollment system | |
| PECOS tutorials | |
| Register for usernames and passwords to access NPPES, PECOS, and the EHR incentive program |
|
| Search NPI records, including the provider’s name, specialty, and practice address | |
| Submit provider NPI applications and update information electronically in NPPES |
| Topic | Contact |
|---|---|
| All other enrollment-related questions | |
| Application for NPI | |
| Institutional and other providers state survey | |
| Navigating and accessing PECOS website |
|
| Paper applications | |
| Provider site visit |
Enrollment Forms
If you enroll using a paper application instead of PECOS , search the CMS Forms List to find the form you need and read on page 1, Who Should Submit This Application .
| Form | Form Number |
|---|---|
| Electronic Funds Transfer (EFT) Authorization Agreement | |
| Health Insurance Benefit Agreement | |
| Medicare Enrollment Application: Clinics/Group Practices and Other Suppliers | |
| Medicare Enrollment Application: Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Suppliers | |
| Medicare Enrollment Application: Enrollment for Eligible Ordering/Certifying Physicians and Other Eligible Professionals | |
| Medicare Enrollment Application: Institutional Providers | |
| Medicare Enrollment Application: Medicare Diabetes Prevention Program (MDPP) Suppliers | |
| Medicare Enrollment Application: Physicians and Non-Physician Practitioners | |
| Medicare Participating Physician or Supplier Agreement | |
| National Provider Identifier (NPI) Application/Update Form |
Commonly Used Terms
View the Medicare Learning Network® Content Disclaimer and Department of Health & Human Services Disclosure .
The Medicare Learning Network®, MLN Connects®, and MLN Matters® are registered trademarks of the U.S. Department of Health & Human Services (HHS).
- Internal Medicine
- Family Practice
- Pediatricians
- Podiatrists
- Obstetrics & Gynecology
- Registered Nurse
- Licensed Practical Nurse
- Nurse Practitioner
- Physician Assistant
- Nursing Homes
- Home Health Care
- Hospice Care
- Dialysis Facilities
- Primary Care Clinics
- Dental Clinics
- Mental Health Clinics
- Psychiatrists
- Clinical Social Workers
- Psychologists
- Marriage Counseling
- Pharmacy and Suppliers
- Pharmacists
- Audiologists
- Chiropractors
- Occupational Therapists
- Optometrists
- Physical Therapists
- Speech-Language Pathologists
- Privacy Policy
Dr Gardner Scott Smith, MD - Medicare General Surgery in Lewistown, MT
Contact information.
| Not Available |
Map and Direction
Physician's Profile
| Full Name | Dr Gardner Scott Smith |
|---|---|
| Gender | Male |
| Speciality | General Surgery |
| Experience | 22 Years |
| Location | 408 Wendell Ave, Lewistown, Montana |
| Accepts Medicare Assignments | Yes. He accepts the Medicare-approved amount; you will not be billed for any more than the Medicare deductible and coinsurance. |
- Dr Gardner Scott Smith attended and graduated from University Of Alabama School Of Medicine in 2002
- NPI Number: 1720182231
- Provider Enumeration Date: 09/13/2006
- Last Update Date: 04/05/2023
- PECOS PAC ID: 0547151888
- Enrollment ID: I20160205002415
Medical Identifiers
| Identifier | Type | State | Issuer |
|---|---|---|---|
| 1720182231 | NPI | - | NPPES |
Medical Taxonomies and Licenses
| Taxonomy | Type | License (State) | Status |
|---|---|---|---|
| 208600000X | Surgery | MED-PHYS-LIC-43906 (Montana) | Primary |
Medical Facilities Affiliation
| Facility Name | Location | Facility Type |
|---|---|---|
| Lewistown, MT | Hospital |
Group Practice Association
| Group Practice Name | Group PECOS PAC ID | No. of Members |
|---|---|---|
| Central Montana Medical Facilities Inc | 5395639793 | 22 |
Medicare Reassignments
| Entity Name | Central Montana Medical Facilities Inc |
|---|---|
| Entity Type | Part B Supplier - Clinic/group Practice |
| Entity Identifiers | 1497868814 5395639793 O20040209000295 |
| Entity Name | Scl Health Medical Group-butte Llc |
|---|---|
| Entity Type | Part B Supplier - Clinic/group Practice |
| Entity Identifiers | 1477869600 2466633102 O20110301000023 |
Medicare Part D Prescriber Enrollment
Mailing address and practice location.
| Mailing Address | Practice Location Address |
|---|---|
| Dr Gardner Scott Smith, MD Ph: (406) 535-1502 | Dr Gardner Scott Smith, MD Ph: (406) 535-1502 |
Reviews and Comments
Surgery doctors in lewistown, mt.
| Accepting Medicare Assignments 310 Wendell Ave, Lewistown, MT 59457 406-535-6262 406-535-6298 | |
| Medicare Enrolled 408 Wendell Ave, Lewistown, MT 59457 406-535-1502 | |
| Accepting Medicare Assignments 310 Wendell Ave, Suite 4, Lewistown, MT 59457 406-538-6262 406-538-6298 |
- Environment
- Science & Technology
- Business & Industry
- Health & Public Welfare
- Topics (CFR Indexing Terms)
- Public Inspection
- Presidential Documents
- Document Search
- Advanced Document Search
- Public Inspection Search
- Reader Aids Home
- Office of the Federal Register Announcements
- Using FederalRegister.Gov
- Understanding the Federal Register
- Recent Site Updates
- Federal Register & CFR Statistics
- Videos & Tutorials
- Developer Resources
- Government Policy and OFR Procedures
- Congressional Review
- My Clipboard
- My Comments
- My Subscriptions
- Sign In / Sign Up
- Site Feedback
- Search the Federal Register
The Federal Register
The daily journal of the united states government.
- Legal Status
This site displays a prototype of a “Web 2.0” version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPO’s govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register (ACFR) issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA's archives.gov.
The OFR/GPO partnership is committed to presenting accurate and reliable regulatory information on FederalRegister.gov with the objective of establishing the XML-based Federal Register as an ACFR-sanctioned publication in the future. While every effort has been made to ensure that the material on FederalRegister.gov is accurately displayed, consistent with the official SGML-based PDF version on govinfo.gov, those relying on it for legal research should verify their results against an official edition of the Federal Register. Until the ACFR grants it official status, the XML rendition of the daily Federal Register on FederalRegister.gov does not provide legal notice to the public or judicial notice to the courts.
Proposed Rule
Medicare program; calendar year (cy) 2025 home health prospective payment system (hh pps) rate update; hh quality reporting program requirements; hh value-based purchasing expanded model requirements; home intravenous immune globulin (ivig) items and services rate update; and other medicare policies.
A Proposed Rule by the Centers for Medicare & Medicaid Services on 07/03/2024
This document has a comment period that ends in 53 days. (08/26/2024) Submit a formal comment
Thank you for taking the time to create a comment. Your input is important.
Once you have filled in the required fields below you can preview and/or submit your comment to the Health and Human Services Department for review. All comments are considered public and will be posted online once the Health and Human Services Department has reviewed them.
You can view alternative ways to comment or you may also comment via Regulations.gov at https://www.regulations.gov/commenton/CMS_FRDOC_0001-3895 .
- What is your comment about?
Note: You can attach your comment as a file and/or attach supporting documents to your comment. Attachment Requirements .
this will NOT be posted on regulations.gov
- Opt to receive email confirmation of submission and tracking number?
- Tell us about yourself! I am... *
- First Name *
- Last Name *
- State Alabama Alaska American Samoa Arizona Arkansas California Colorado Connecticut Delaware District of Columbia Florida Georgia Guam Hawaii Idaho Illinois Indiana Iowa Kansas Kentucky Louisiana Maine Maryland Massachusetts Michigan Minnesota Mississippi Missouri Montana Nebraska Nevada New Hampshire New Jersey New Mexico New York North Carolina North Dakota Ohio Oklahoma Oregon Pennsylvania Puerto Rico Rhode Island South Carolina South Dakota Tennessee Texas Utah Vermont Virgin Islands Virginia Washington West Virginia Wisconsin Wyoming
- Country Afghanistan Åland Islands Albania Algeria American Samoa Andorra Angola Anguilla Antarctica Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bolivia, Plurinational State of Bonaire, Sint Eustatius and Saba Bosnia and Herzegovina Botswana Bouvet Island Brazil British Indian Ocean Territory Brunei Darussalam Bulgaria Burkina Faso Burundi Cambodia Cameroon Canada Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Cocos (Keeling) Islands Colombia Comoros Congo Congo, the Democratic Republic of the Cook Islands Costa Rica Côte d'Ivoire Croatia Cuba Curaçao Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Ethiopia Falkland Islands (Malvinas) Faroe Islands Fiji Finland France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guernsey Guinea Guinea-Bissau Guyana Haiti Heard Island and McDonald Islands Holy See (Vatican City State) Honduras Hong Kong Hungary Iceland India Indonesia Iran, Islamic Republic of Iraq Ireland Isle of Man Israel Italy Jamaica Japan Jersey Jordan Kazakhstan Kenya Kiribati Korea, Democratic People's Republic of Korea, Republic of Kuwait Kyrgyzstan Lao People's Democratic Republic Latvia Lebanon Lesotho Liberia Libya Liechtenstein Lithuania Luxembourg Macao Macedonia, the Former Yugoslav Republic of Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Micronesia, Federated States of Moldova, Republic of Monaco Mongolia Montenegro Montserrat Morocco Mozambique Myanmar Namibia Nauru Nepal Netherlands New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island Northern Mariana Islands Norway Oman Pakistan Palau Palestine, State of Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Poland Portugal Puerto Rico Qatar Réunion Romania Russian Federation Rwanda Saint Barthélemy Saint Helena, Ascension and Tristan da Cunha Saint Kitts and Nevis Saint Lucia Saint Martin (French part) Saint Pierre and Miquelon Saint Vincent and the Grenadines Samoa San Marino Sao Tome and Principe Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Sint Maarten (Dutch part) Slovakia Slovenia Solomon Islands Somalia South Africa South Georgia and the South Sandwich Islands South Sudan Spain Sri Lanka Sudan Suriname Svalbard and Jan Mayen Swaziland Sweden Switzerland Syrian Arab Republic Taiwan, Province of China Tajikistan Tanzania, United Republic of Thailand Timor-Leste Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkmenistan Turks and Caicos Islands Tuvalu Uganda Ukraine United Arab Emirates United Kingdom United States United States Minor Outlying Islands Uruguay Uzbekistan Vanuatu Venezuela, Bolivarian Republic of Viet Nam Virgin Islands, British Virgin Islands, U.S. Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe
- Organization Type * Company Organization Federal State Local Tribal Regional Foreign U.S. House of Representatives U.S. Senate
- Organization Name *
- You are filing a document into an official docket. Any personal information included in your comment text and/or uploaded attachment(s) may be publicly viewable on the web.
- I read and understand the statement above.
- Preview Comment
Document Details
Information about this document as published in the Federal Register .
Document Statistics
Published document.
This document has been published in the Federal Register . Use the PDF linked in the document sidebar for the official electronic format.
Enhanced Content - Table of Contents
This table of contents is a navigational tool, processed from the headings within the legal text of Federal Register documents. This repetition of headings to form internal navigation links has no substantive legal effect.
FOR FURTHER INFORMATION CONTACT:
Supplementary information:, table of contents, i. executive summary, a. purpose and legal authority, 1. home health prospective payment system (hh pps), 2. home health (hh) quality reporting program (qrp), 3. expanded home health value-based purchasing (hhvbp) model, 4. home intravenous immune globulin (ivig) items and services, 5. home health cop changes, 6. provider and supplier enrollment requirements, 7. long-term care (ltc) requirements for acute respiratory illness reporting, b. summary of the provisions of this proposed rule, 2. home health quality reporting program (hh qrp), 3. expanded home health value based purchasing (hhvbp) model, c. summary of costs, transfers, and benefits, ii. home health prospective payment system, a. overview of the home health prospective payment system, 1. statutory background, 2. current system for payment of home health services, b. monitoring the effects of the implementation of pdgm, 1. routine pdgm monitoring, a. utilization, b. analysis of 2022 cost report data for 30-day periods of care, c. clinical groupings and comorbidities, d. admission source and timing, e. functional impairment level, f. therapy visits, g. home health services using telecommunications technology, b. method to annually determine the impact of differences between assumed behavior changes and actual behavior changes on estimated aggregate expenditures, c. calculating permanent and temporary payment adjustments, d. cy 2023 preliminary claims results, e. applying the methodology to cy 2023 data to determine the cy 2025 permanent and temporary adjustments, f. proposed cy 2025 permanent adjustment and temporary adjustment calculations, d. proposed cy 2025 home health low utilization payment adjustment (lupa) thresholds, functional impairment levels, comorbidity sub-groups, case-mix weights, and reassignment of specific icd-10-cm codes under the pdgm, 1. proposed cy 2025 pdgm lupa thresholds, 2. proposed cy 2025 functional impairment levels, 3. proposed cy 2025 comorbidity subgroups, 4. proposed cy 2025 pdgm case-mix weights, 5. suggested reassignment of specific icd-10-cm codes under the pdgm, a. background, b. methodology for icd-10-cm diagnosis code assignments, c. request for icd-10-cm diagnosis code reassignments to a pdgm clinical group or comorbidity subgroup—renal 3 comorbidity subgroup, e. proposed cy 2025 home health payment rate updates, 1. proposed cy 2025 home health market basket update for hhas, 2. proposed adoption of the cbsa delineations for wage index, a. micropolitan statistical areas, b. change to county-equivalents in the state of connecticut, c. urban counties that would become rural, d. rural counties that would become urban, e. urban counties that would move to a different urban cbsa under the revised omb delineations, f. proposed transition period, 3. proposed cy 2025 home health wage index, 4. proposed cy 2025 home health payment update, (b) cy 2025 national, standardized 30-day period payment amount, c. cy 2025 national per-visit rates for 30-day periods of care, d. lupa add-on factors, e. occupational therapy lupa add-on factor, f. payments for high-cost outliers under the hh pps, (1) background, (2) proposed fdl ratio for cy 2025, f. annual rate update for disposable negative pressure wound therapy (dnpwt) device, 1. background, 2. payment policies for dnpwt devices, 3. cy 2025 separate payment amount for dnpwt device, iii. home health quality reporting program (hh qrp), a. background and statutory authority, b. summary of the provision of this proposed rule, c. quality measures currently adopted for the cy 2024 hh qrp, d. proposal to collect four new items as standardized patient assessment data elements and modify one item collected as a standardized patient assessment data element beginning with the cy 2027 hh qrp, 1. definition of standardized patient assessment data, 2. social determinants of health (sdoh) collected as standardized patient assessment data elements.
- 3. Proposal to Collect Four New Items as Standardized Patient Assessment Data Elements Beginning January 1, 2027, for the CY 2027 HH QRP Program Year 31
a. Living Situation
C. utilities, 4. stakeholder input, 5. proposal to modify the transportation item beginning with the cy 2027 hh qrp program year, e. proposal to update oasis all-payer data collection, f. form, manner, and timing of data submission under the hh qrp, 2. proposed reporting schedule for the submission of sdoh assessment items beginning january 1, 2027, with the cy 2027 hh qrp, g. hh qrp quality measure concepts under consideration for future years—request for information (rfi), iv. the expanded home health value-based purchasing (hhvbp) model, a. background, b. request for information on future performance measure concepts for the expanded hhvbp model, c. future approaches to health equity in the expanded hhvbp model, d. social risk factors, e. approaches to a potential health equity adjustment for the expanded hhvbp model, f. other health equity measures, v. medicare home intravenous immune globulin (ivig) items and services, a. general background, 2. overview, 3. legislative summary, 4. demonstration overview, b. scope of expanded ivig benefit, 1. items and services related to the home administration of ivig, 2. home ivig items and services and the relationship to/interaction with home health and home infusion therapy services, c. home ivig administration items and services payment, 1. home ivig administration items and services supplier type, 2. home ivig administration, d. home ivig items and services payment rate, 1. proposed payment rate update for home ivig items and services for cy 2025, vi. home health cop changes and long term (ltc) requirements for acute respiratory illness reporting, a. home health cop changes, 1. background and statutory authority, 2. proposed updates to the home health agency cops to require hhas to establish an acceptance to service policy (§ 484.105(i)), 3. requests for information, a. rfi regarding rehabilitative therapists conducting the initial and comprehensive assessment, b. plan of care development and scope of services home health patients receive, b. long-term care (ltc) requirements for acute respiratory illness reporting, 2. the benefits of and ongoing need for ltc facility respiratory illness and vaccination data, 3. benefits of data collection at the facility and local level, 4. benefits of data collection at the regional and state levels, 5. benefits of data collection at the federal level, 6. proposed continuation of respiratory illness reporting for ltc facilities, 7. proposed collection of additional data elements during a phe, 8. collaboration, vii. provider enrollment—provisional period of enhanced oversight, 1. overview of medicare provider enrollment, 2. legal authorities, b. proposed provisions—provisional period of enhanced oversight (ppeo), viii. collection of information requirements, a. statutory requirement for solicitation of comments, b. information collection requirements (icrs), 1. icrs for hh qrp, start of care, resumption of care, 2. icrs for the expanded hhvbp model, 4. icrs for provider enrollment provisions, 5. icrs related to ltc requirements for acute respiratory illness reporting § 483.80(g), c. submission of pra-related comments, ix. regulatory impact analysis, a. statement of need, 3. expanded hhvbp model, 4. home ivig items and services, 5. hha cop changes: establishing an acceptance to service policy, 6. provider enrollment provisions, 7. ltc requirements for acute respiratory illness reporting, b. overall impact, c. detailed economic analysis, 1. effects of the proposed changes for the cy 2025 hh pps, 2. effects of the proposed changes for the hh qrp for cy 2027, 3. effects of the expanded hh vbp model, 4. impacts of home ivig items and services, 7. effects of the proposed ltc requirements for acute respiratory illness reporting, d. regulatory review cost estimation, e. alternatives considered, 2. home ivig items and services, f. accounting statements and tables, 3. home ivig items and services, g. regulatory flexibility act (rfa), h. unfunded mandates reform act (umra), i. federalism, j. conclusion, x. response to comments, list of subjects, 42 cfr part 424, 42 cfr part 483, 42 cfr part 484, part 424—conditions for medicare payment, part 483—requirements for states and long term care facilities, (1) influenza., ( 1 ) influenza., part 484—home health services, enhanced content - submit public comment.
- Submit a public comment on this document
Enhanced Content - Read Public Comments
- This feature is not available for this document.
Enhanced Content - Sharing
- Email this document to a friend
Enhanced Content - Document Print View
- Print this document
Enhanced Content - Document Tools
These tools are designed to help you understand the official document better and aid in comparing the online edition to the print edition.
These markup elements allow the user to see how the document follows the Document Drafting Handbook that agencies use to create their documents. These can be useful for better understanding how a document is structured but are not part of the published document itself.
Enhanced Content - Developer Tools
This document is available in the following developer friendly formats:.
- JSON: Normalized attributes and metadata
- XML: Original full text XML
- MODS: Government Publishing Office metadata
More information and documentation can be found in our developer tools pages .
Official Content
- View printed version (PDF)
This PDF is the current document as it appeared on Public Inspection on 06/26/2024 at 4:15 pm. It was viewed 0 times while on Public Inspection.
If you are using public inspection listings for legal research, you should verify the contents of the documents against a final, official edition of the Federal Register. Only official editions of the Federal Register provide legal notice of publication to the public and judicial notice to the courts under 44 U.S.C. 1503 & 1507 . Learn more here .
Centers for Medicare & Medicaid Services (CMS), Department of Health and Human Services (HHS).
Proposed rule.
This proposed rule would set forth routine updates to the Medicare home health payment rates; the payment rate for the disposable negative pressure wound therapy (dNPWT) devices; and the intravenous immune globulin (IVIG) items and services payment rate for CY 2025 in accordance with existing statutory and regulatory requirements. In addition, it proposes changes to the Home Health Quality Reporting Program (HH QRP) requirements and provides an update on potential approaches for integrating health equity in the Expanded Health Value Based Purchasing (HHVBP) Model. It also proposes a new standard for acceptance to service policy in the HH conditions of participation (CoPs) and includes requests for information (RFIs) soliciting input on permitting rehabilitative therapists to conduct the initial and comprehensive assessment and the factors that may influence the patient referral and intake processes. Lastly, it proposes updates to provider and supplier enrollment requirements and changes to the long-term care reporting requirements for acute respiratory illnesses.
To be assured consideration, comments must be received at one of the addresses provided in the ADDRESSES section, no later than 5 p.m. EDT on August 26, 2024.
In commenting, please refer to file code CMS-1803-P. Because of staff and resource limitations, we cannot accept comments by facsimile (FAX) transmission. Comments, including mass comment submissions, must be submitted in one of the following three ways (please choose only one of the ways listed):
1. Electronically . You may (and we encourage you to) submit electronic comments on this regulation to https://www.regulations.gov . Follow the instructions under the “submit a comment” tab.
2. By regular mail . You may mail written comments to the following address ONLY:
Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1803-P, P.O. Box 8013, Baltimore, MD 21244-8013.
Please allow sufficient time for mailed comments to be received before the close of the comment period.
3. By express or overnight mail. You may send written comments via express or overnight mail to the following address ONLY:
Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1803-P, Mail Stop C4-26-05, 7500 Security Boulevard, Baltimore, MD 21244-1850.
For information on viewing public comments, we refer readers to the beginning of the SUPPLEMENTARY INFORMATION section.
Brian Slater, (410) 786-5229, for home health and home IVIG payment inquiries.
For general information about the Home Health Prospective Payment System (HH PPS), send your inquiry via email to [email protected] .
For information about the Home Health Quality Reporting Program (HH QRP), send your inquiry via email to [email protected] .
For more information about the expanded Home Health Value-Based Purchasing Model, please visit the Expanded HHVBP Model web page at https://innovation.cms.gov/innovation-models/expanded-home-health-value-based-purchasing-model .
Frank Whelan (410) 786-1302, for Medicare provider and supplier enrollment inquiries.
Mary Rossi-Coajou at [email protected] or Molly Anderson at [email protected] , for more information about the home health conditions of participation (HH CoPs).
Kim Roche ( [email protected] ), for more information about the long-term care requirements for participation.
Inspection of Public Comments: All comments received before the close of the comment period are available for viewing by the public, including any personally identifiable or confidential business information that is included in a comment. We post all comments received before the close of the comment period on the following website as soon as possible after they have been received: https://www.regulations.gov/ . Follow the search instructions on that website to view public comments.
C. Proposed CY 2025 Payment Adjustments Under the HH PPS
IV. The Expanded Home Health Value Based Purchasing (HHVBP) Model
D. Home IVIG Items and Services Payment Rate Start Printed Page 55313
B. Long-term Care (LTC) Requirements for Acute Respiratory Illness Reporting
As required under section 1895(b) of the Social Security Act (the Act), this proposed rule would update the CY 2025 payment rates for home health agencies (HHAs) and the CY 2025 payment rate for the disposable negative pressure wound therapy (dNPWT) device. In this proposed rule, we include analysis on home health utilization, as well as analysis determining the difference between assumed versus actual behavior change on estimated aggregate expenditures for home health payments as result of the change in the unit of payment to 30 days and the implementation of the Patient Driven Groupings Model (PDGM) case-mix adjustment methodology. This rule proposes a crosswalk for mapping the Outcome and Assessment Information Set-D (OASIS-D) data elements to the equivalent OASIS-E data elements for use in the methodology to analyze the difference between assumed versus actual behavior change on estimated aggregate expenditures and proposes a permanent prospective behavior adjustment to the CY 2025 home health payment rate. In addition, this rule proposes to recalibrate the PDGM case-mix weights and to update the low-utilization payment adjustment (LUPA) thresholds, functional impairment levels, and comorbidity adjustment subgroups under section 1895(b)(4)(A)(i) and (b)(4)(B) of the Act for 30-day periods of care in CY 2025; proposes to adopt the most recent Office of Management and Budget (OMB) Core-Based Statistical Area (CBSA) delineations for the home health wage index; and proposes an occupational therapy (OT) LUPA add-on factor and updates to the physical therapy (PT), speech-language pathology (SLP), and skilled nursing (SN) LUPA add-on factors. Additionally, this rule proposes to update the CY 2025 fixed-dollar loss ratio (FDL) for outlier payments (so that outlier payments as a percentage of estimated total payments are projected not to exceed 2.5 percent, as required by section 1895(b)(5)(A) of the Act).
In accordance with the statutory authority at section 1895(b)(3)(B)(v) of the Act, we are proposing updated policies. We are proposing to add four new assessment items and to modify one assessment item on the OASIS, an update to the removal of the suspension of OASIS all-payer data collection, and we are seeking information on future HH QRP quality measure (QM) concepts.
In accordance with the statutory authority at section 1115A of the Act, we are doing the following for the expanded HHVBP Model: (1) providing an update on potential approaches for integrating health equity that are being considered; and (2) including a request for information (RFI) related to future performance measure concepts.
In section V.D.1. of this proposed rule, we propose a rate update for the CY 2025 IVIG items and services payment under the home intravenous immune globulin (IVIG) benefit.
In section VI. A. of this proposed rule, we are proposing to add a new standard at § 484.105(i) that would require HHAs to develop, consistently apply, and maintain an acceptance to service policy, including specified factors, that would govern the process for accepting patients to service. We also propose that HHAs would be required to make specified information about their services and service limitations available to the public. Section VI.B. of this proposed rule includes an RFI to obtain information from stakeholders on whether CMS should shift its longstanding policy and permit rehabilitative therapists to conduct the initial and comprehensive assessment for cases that have both therapy and nursing services ordered as part of the plan of care. In addition, we are seeking public comments on other factors that influence the patient referral and intake processes.
In accordance with section 1866(j)(3)(A) of the Act, we are proposing to revise our requirements in 42 CFR 424.527(a) regarding the application of provisional periods of enhanced oversight (PPEO). Section 1866(j)(3)(A) of the Act states that the Secretary shall establish procedures to provide for a provisional period of between 30 days and 1 year during which new providers and suppliers—as the Secretary determines appropriate, including categories of providers or suppliers—will be subject to enhanced oversight. We are proposing to expand the definition of “new provider or supplier” (solely for purposes of applying a PPEO) to include providers and suppliers that are reactivating their Medicare enrollment and billing privileges.
Sections 1819(d)(3) and 1919(d)(3) of the Act explicitly require that LTC facilities develop and maintain an infection control program that is designed, constructed, equipped, and maintained in a manner to protect the health and safety of residents, personnel, and the general public. In addition, sections 1819(d)(4)(B) and 1919(d)(4)(B) of the Act explicitly authorize the Secretary to issue any regulations he deems necessary to protect the health and safety of residents. As such, we are proposing streamlined weekly data reporting requirements for certain respiratory illnesses. We are also proposing additional, related data elements that could be activated in the event of a future acute respiratory illness public health emergency (PHE).
In section II.B.1. of this proposed rule, we provide monitoring and data analysis on PDGM utilization.
In section II.C.1 of this proposed rule, we propose a permanent adjustment to the base payment rate under the HH PPS. Additionally, we propose a Start Printed Page 55314 crosswalk for mapping the OASIS-D data elements to the equivalent OASIS-E data elements for use in the methodology to analyze the difference between assumed versus actual behavior change on estimated aggregate expenditures.
In section II.D. of this proposed rule, we discuss a proposal to recalibrate the CY 2025 home health LUPA thresholds, case-mix weights, and co-morbidity subgroups. Additionally, we discuss providers' suggestions regarding the reassignment of specific ICD-10-CM diagnosis codes under the PDGM.
In section II.E. of this proposed rule, we propose to update the home health wage index and adopt the new labor market delineations from the July 21, 2023, OMB Bulletin No. 23-01 based on data collected from the 2020 Decennial Census. This section includes the CY 2025 national, standardized 30-day period payment rate update, the updated CY 2025 national per-visit payment amounts by the home health payment update percentage, and the OT LUPA add-on factor and PT, SLP, and SN add-on factor updates. The proposed home health payment update percentage for CY 2025 is 2.5 percent. Additionally, this rule proposes the CY 2025 FDL ratio to ensure that aggregate outlier payments are projected not to exceed 2.5 percent of the total aggregate payments, as required by section 1895(b)(5)(A) of the Act.
In section II.F.4. of this proposed rule, we propose the CY 2025 payment rate update for dNPWT devices.
In section III. of this proposed rule, we are proposing to collect four new items as standardized patient assessment data elements in the social determinants of health (SDOH) category and modify one item collected as a standardized patient assessment data element in the SDOH category beginning with the CY 2027 HH QRP. The four assessment items proposed for collection are: one Living Situation item, two Food items, and one Utilities item. We also propose modifying the current Transportation item beginning with the CY 2027 HH QRP. We are also proposing an update to the removal of the suspension of OASIS all-payer data collection to change all-payer data collection to begin with the start of care OASIS data collection timepoint instead of discharge timepoint. Lastly, we seek input on future HH QRP measure concepts.
In section IV. of this proposed rule, we include an RFI related to future measure concepts for the expanded HHVBP Model. We are also including an update to the RFI, Future Approaches to Health Equity in the Expanded HHVBP Model, that was published in the CY 2023 HH PPS final rule ( 87 FR 66874 , November 4, 2022) and subsequently updated in the CY 2024 HH PPS final rule ( 88 FR 77687 , November 13, 2023).
In section V.D.1. of this proposed rule, we propose a rate update for CY 2025 IVIG items and services payment under the home intravenous immune globulin (IVIG) benefit.
In section VI.A. of this proposed rule, we are proposing to add a new standard at § 484.105(d) that would require HHAs to develop, implement, and maintain an acceptance to service policy that is applied consistently to each prospective patient referred for home health care. We also propose that the policy must address, at minimum, the following criteria related to the HHA's capacity to provide patient care: the anticipated needs of the referred prospective patient, the HHA's case load and case mix, the HHA's staffing levels, and the skills and competencies of the HHA staff. We also propose that HHAs would be required to make specified information available to the public that is reviewed at least annually. Section VI.B. of this proposed rule we include an RFI to obtain information from stakeholders on whether CMS should shift its longstanding policy and permit rehabilitative therapists to conduct the initial and comprehensive assessment for cases that have both therapy and nursing services ordered as part of the plan of care. Specifically, we are seeking information regarding the training and education of rehabilitative therapists that is relevant to conducting the initial and comprehensive assessments and any additional information on any patient health and safety benefits or unintended consequences of expanding the category of clinicians that can conduct the initial and comprehensive assessments. In addition, we are seeking public comments on other factors that influence the patient referral and intake processes.
Section 1866(j)(3)(A) of the Act states that the Secretary shall establish procedures to provide for a provisional period of between 30 days and 1 year during which new providers and suppliers—as the Secretary determines appropriate, including categories of providers or suppliers—will be subject to enhanced oversight. We are proposing to expand the definition of “new provider or supplier” (solely for purposes of applying a PPEO) to include providers and suppliers that are reactivating their Medicare enrollment and billing privileges.
The current LTC requirements for reporting COVID-19 related data expire on December 31, 2024, except for reporting COVID-19 resident and staff vaccination status. Given the utility of LTC facility data, we propose to replace these requirements with streamlined continued data reporting requirements for certain respiratory illnesses. We are also proposing additional, related data elements that could be activated in the event of a future acute respiratory illness PHE.

Section 1895(b)(1) of the Act requires the Secretary to establish a Home Health Prospective Payment System (HH PPS) for all costs of home health services paid under Medicare. Section 1895(b)(2) of the Act requires that, in defining a prospective payment amount, the Secretary will consider an appropriate unit of service and the number, type, and duration of visits provided within that unit, potential changes in the mix of services provided within that unit and their cost, and a general system design that provides for continued access to quality services. In accordance with the statute, as amended by the Balanced Budget Act of 1997 (BBA) ( Pub. L. 105-33 ), we issued a final rule which appeared in the July 3, 2000, Federal Register ( 65 FR 41128 ) to implement the HH PPS legislation.
Section 5201(c) of the Deficit Reduction Act of 2005 (DRA) ( Pub. L. 109-171 , enacted February 8, 2006) added new section 1895(b)(3)(B)(v) to the Act, requiring home health agencies (HHAs) to submit data for purposes of measuring health care quality, and linking the quality data submission to the annual applicable home health payment update percentage increase. This data submission requirement is applicable for CY 2007 and each subsequent year. If an HHA does not submit quality data, the home health market basket percentage increase is reduced by 2 percentage points. In the November 9, 2006, Federal Register ( 71 FR 65935 ), we issued a final rule to implement the pay-for-reporting requirement of the DRA, which was codified at § 484.225(h) and (i) in accordance with the statute. The pay-for-reporting requirement was implemented on January 1, 2007.
Section 51001(a)(1)(B) of the Bipartisan Budget Act of 2018 (BBA of 2018) ( Pub. L. 115-123 ) amended section 1895(b) of the Act to require a change to the home health unit of payment to 30-day periods beginning January 1, 2020. Section 51001(a)(2)(A) of the BBA of 2018 added a new subclause (iv) under section 1895(b)(3)(A) of the Act, requiring the Secretary to calculate a standard prospective payment amount (or amounts) for 30-day units of service furnished that end during the 12-month period beginning January 1, 2020, in a budget neutral manner, such that estimated aggregate expenditures under the HH PPS during CY 2020 are equal to the estimated aggregate expenditures that otherwise would have been made under the HH PPS during CY 2020 in the absence of the change to a 30-day unit of service. Section 1895(b)(3)(A)(iv) of the Act requires that the calculation of the standard prospective payment amount (or amounts) for CY 2020 be made before the application of the annual update to the standard prospective payment amount as required by section 1895(b)(3)(B) of the Act.
Additionally, section 1895(b)(3)(A)(iv) of the Act requires that in calculating the standard prospective payment amount (or amounts), the Secretary must make assumptions about behavior changes that could occur as a result of the implementation of the 30-day unit of service under section 1895(b)(2)(B) of the Act and case-mix adjustment factors established under section 1895(b)(4)(B) of the Act. Section 1895(b)(3)(A)(iv) of the Act further requires the Secretary to provide a description of the behavior assumptions made in notice and comment rulemaking. CMS finalized these behavior assumptions in the CY 2019 HH PPS final rule with comment period ( 83 FR 56461 ).
Section 51001(a)(2)(B) of the BBA of 2018 also added a new subparagraph (D) to section 1895(b)(3) of the Act. Section 1895(b)(3)(D)(i) of the Act requires the Secretary annually to determine the impact of differences between assumed behavior changes, as described in section 1895(b)(3)(A)(iv) of the Act, and actual behavior changes on estimated Start Printed Page 55317 aggregate expenditures under the HH PPS with respect to years beginning with 2020 and ending with 2026. Section 1895(b)(3)(D)(ii) of the Act requires the Secretary, at a time and in a manner determined appropriate, through notice and comment rulemaking, to provide for one or more permanent increases or decreases to the standard prospective payment amount (or amounts) for applicable years, on a prospective basis, to offset for such increases or decreases in estimated aggregate expenditures, as determined under section 1895(b)(3)(D)(i) of the Act. Additionally, section 1895(b)(3)(D)(iii) of the Act requires the Secretary, at a time and in a manner determined appropriate, through notice and comment rulemaking, to provide for one or more temporary increases or decreases to the payment amount for a unit of home health services for applicable years, on a prospective basis, to offset for such increases or decreases in estimated aggregate expenditures, as determined under section 1895(b)(3)(D)(i) of the Act. Such a temporary increase or decrease shall apply only with respect to the year for which such temporary increase or decrease is made, and the Secretary shall not take into account such a temporary increase or decrease in computing the payment amount for a unit of home health services for a subsequent year. Finally, section 51001(a)(3) of the BBA of 2018 amends section 1895(b)(4)(B) of the Act by adding a new clause (ii) to require the Secretary to eliminate the use of therapy thresholds in the case-mix system for CY 2020 and subsequent years.
Division FF, section 4136 of the Consolidated Appropriations Act, 2023 (CAA, 2023) ( Pub. L. 117-328 ) amended section 1834(s)(3)(A) of the Act to require that, beginning with 2024, the separate payment for furnishing negative pressure wound therapy (NPWT) be for just the device and not for nursing and therapy services. Payment for nursing and therapy services are to be included as part of payments under the HH PPS. The separate payment for 2024 was required to be equal to the supply price used to determine the relative value for the service under the Medicare Physician Fee Schedule (as of January 1, 2022) for the applicable disposable device updated by the percentage increase in the Consumer Price Index for All Urban Consumers (CPI-U). The separate payment for 2025 and each subsequent year is to be the payment amount for the previous year updated by the percentage increase in the CPI-U (United States city average) for the 12-month period ending in June of the previous year reduced by the productivity adjustment as described in section 1886(b)(3)(B)(xi)(II) of the Act for such year. The CAA, 2023 also added section 1834(s)(4) of the Act to require that beginning with 2024, as part of submitting claims for the separate payment, the Secretary shall accept and process claims submitted using the type of bill that is most commonly used by home health agencies to bill services under a home health plan of care.
For home health periods of care beginning on or after January 1, 2020, Medicare makes payment under the HH PPS on the basis of a national, standardized 30-day period payment rate that is adjusted for case-mix and area wage differences in accordance with section 51001(a)(1)(B) of the BBA of 2018. The national, standardized 30-day period payment rate includes payment for the six home health disciplines (skilled nursing, home health aide, physical therapy, speech-language pathology, occupational therapy, and medical social services). Payment for non-routine supplies (NRS) is also part of the national, standardized 30-day period rate. Durable medical equipment (DME) provided as a home health service, as defined in section 1861(m) of the Act, is paid the fee schedule amount or is paid through the competitive bidding program and such payment is not included in the national, standardized 30-day period payment amount. Additionally, the 30-day period payment rate does not include payment for certain injectable osteoporosis drugs and disposable negative pressure wound therapy (dNPWT) devices, but such drugs and devices must be billed by the HHA while a patient is under a home health plan of care, as the law requires consolidated billing of osteoporosis drugs and dNPWT devices.
To better align payment with patient care needs and to better ensure that clinically complex and ill beneficiaries have adequate access to home health care, in the CY 2019 HH PPS final rule with comment period ( 83 FR 56406 ), we finalized case-mix methodology refinements through the Patient-Driven Groupings Model (PDGM) for home health periods of care beginning on or after January 1, 2020. The PDGM did not change eligibility or coverage criteria for Medicare home health services, and as long as the individual meets the criteria for home health services as described at 42 CFR 409.42 , the individual can receive Medicare home health services, including therapy services. For more information about the role of therapy services under the PDGM, we refer readers to the Medicare Learning Network (MLN) Matters article SE20005 available at https://www.cms.gov/regulations-and-guidanceguidancetransmittals2020-transmittals/se20005 . To adjust for case-mix for 30-day periods of care beginning on and after January 1, 2020, the HH PPS uses a 432-category case-mix classification system to assign patients to a home health resource group (HHRG) using patient characteristics and other clinical information from Medicare claims and the Outcome and Assessment Information Set (OASIS) assessment instrument. These 432 HHRGs represent the different payment groups based on five main case-mix categories under the PDGM, as shown in figure 1. Each HHRG has an associated case-mix weight that is used in calculating the payment for a 30-day period of care. For periods of care with visits less than the low-utilization payment adjustment (LUPA) threshold for the HHRG, Medicare pays national per-visit rates based on the discipline(s) providing the services. Medicare also adjusts the national standardized 30-day period payment rate for certain intervening events that are subject to a partial payment adjustment. For certain cases that exceed a specific cost threshold, an outlier adjustment may also be available.
Under this case-mix methodology, case-mix weights are generated for each of the different PDGM payment groups by regressing resource use for each of the five categories (admission source, timing, clinical grouping, functional impairment level, and comorbidity adjustment) using a fixed effects model. A detailed description of each of the case-mix variables under the PDGM have been described previously, and we refer readers to the CY 2021 HH PPS final rule ( 85 FR 70303 through 70305 ).

CMS routinely analyzes Medicare home health benefit utilization, including but not limited to, overall total 30-day periods of care and average periods of care per HHA user; distribution of the type of visits in a 30-day period of care; the percentage of periods that receive the LUPA; estimated costs; the percentage of 30-day periods of care by clinical group, comorbidity adjustment, admission source, timing, and functional impairment level; and the proportion of 30-day periods of care with and without any therapy visits, nursing visits, and/or aide/social worker visits. For the monitoring included in this proposed rule, we examine simulated data for CYs 2018 and 2019 and actual data for CYs 2020, 2021, 2022, and 2023 for 30-day periods of care. For CYs 2018 and 2019, because the HH PPS accounted for care in 60-dayepisodes, before the transition to 30-day periods of care beginning in 2020, this actual data was simulated to reflect 30-day periods of care. We refer readers to the CY 2022 HH PPS final rule ( 86 FR 35881 ) for further discussion about simulated data for CYs 2018 and 2019. In this proposed rule, we are also including monitoring of home health visits using telecommunications technology and remote patient monitoring, which we began collecting on claims submitted voluntarily beginning January 1, 2023, and which was required beginning July 1, 2023.
Table 2 shows the overall utilization of home health. The data indicate the average number of 30-day periods of care per unique HHA user is similar per 30-day periods of care between CY 2022 and CY 2023. The data also show a decreasing trend in the overall number of 30-day periods of care between CY 2018 and CY 2023. Table 2 shows utilization of visits per 30-day period of care by home health discipline over time. Table 2 shows the proportion of 30-day periods of care that are LUPAs and the average number of visits per discipline of those LUPA 30-day periods of care over time. The data show a Start Printed Page 55319 decreasing trend in the average number of visits per 30-day period and average number of visits per discipline for LUPA 30-day periods of care between CY 2018 and CY 2023.

In the CY 2024 HH PPS proposed rule ( 88 FR 43664 ), we provided a summary of analysis on FY 2021 HHA cost report data, as this was the most recent and complete cost report data at the time of rulemaking, and CY 2022 claims to estimate 30-day period of care costs. Our analysis showed that the CY 2022 national, standardized 30-day period payment rate of $2,031.64 was approximately 45 percent more than the estimated CY 2022 estimated 30-day period cost of $1,402.27.
Using this same process in this proposed rule to compare home health payment to costs, we examined 2022 HHA Medicare cost reports, as this is the most recent and complete cost report data at the time of rulemaking, and CY 2023 home health claims, to estimate 30-day period of care costs. We excluded LUPAs and visits with partial episode payments (PEPs) when calculating the average number of visits. The 2022 average NRS costs per visit is $4.38. To update the estimated 30-day period of care costs, we begin with the 2022 average costs per visit with NRS for each discipline and multiply that amount by the CY 2023 home health payment update factor of 1.04. That amount for each discipline is then multiplied by the 2023 average number of visits by discipline to determine the 2023 Estimated 30-day Period Costs. Table 5 shows the estimated average costs for 30-day periods of care by discipline with NRS and the total estimated 30-day period of care costs with NRS for CY 2023.

The CY 2023 national standardized 30-day period payment rate was $2,010.69, which is approximately 32 percent more than the estimated CY 2023 30-day period average facility cost of $1,527.23. In its March 2024 Report to Congress, MedPAC assumed costs will increase by only 0.55 percent, the average of the increases in costs per 30-day period for 2021 and 2022. [ 1 ] Furthermore, MedPAC noted that for more than a decade, payments under the HH PPS have significantly exceeded HHAs' costs. MedPAC also noted an increase of 4.0 percent in the costs per 30-day period for freestanding HHAs in 2022, a reversal of the trend for 2021, where costs per 30-day period decreased by 2.9 percent. This increase in 2022 was due to higher costs per visit, but it was offset by a reduction in the number of in-person visits per 30-day period. As shown in table 5 in this proposed rule, HHAs have reduced visits under the PDGM in CY 2022.
Each 30-day period of care is grouped into one of 12 clinical groups, which describe the primary reason for which a patient is receiving home health services under the Medicare home health benefit. The clinical grouping is based on the principal diagnosis reported on the home health claim. Table 6 shows the distribution of the 12 clinical groups over time.

Thirty-day periods of care will receive a comorbidity adjustment category based on the presence of certain secondary diagnoses reported on home health claims. These diagnoses are based on a home health specific list of clinically and statistically significant secondary diagnosis subgroups with similar resource use. We refer readers to section II.B.4.c. of this proposed rule and the CY 2020 final rule with comment period ( 84 FR 60493 ) for further information on the comorbidity adjustment categories. Home health 30-day periods of care can receive a low or a high comorbidity adjustment, or no comorbidity adjustment. Table 7 shows the distribution of 30-day periods of care by comorbidity adjustment category for all 30-day periods.

Each 30-day period of care is classified into one of two admission source categories—community or institutional—depending on what healthcare setting was utilized in the 14 days prior to receiving home health care. Thirty-day periods of care for beneficiaries with any inpatient acute care hospitalizations, inpatient psychiatric facility (IPF) stays, skilled nursing facility (SNF) stays, inpatient rehabilitation facility (IRF) stays, or long-term care hospital (LTCH) stays within 14 days prior to a home health admission will be designated as institutional admissions. The institutional admission source category will also include patients that had an acute care hospital stay during a previous 30-day period of care and within 14 days prior to the subsequent, contiguous 30-day period of care and for which the patient was not discharged from home health and readmitted.
Thirty-day periods of care are classified as “early” or “late” depending on when they occur within a sequence of 30-day periods of care. The first 30-day period of care is classified as early and all subsequent 30-day periods of care in the sequence (second or later) are classified as late. A subsequent 30-day period of care would not be considered early unless there is a gap of more than 60 days between the end of one previous period of care and the start of another. Information regarding the timing of a 30-day period of care comes from Medicare home health claims data and not the OASIS assessment to determine if a 30-day period of care is “early” or “late”. Table 8 shows the distribution of 30-day periods of care by admission source and timing.

Each 30-day period of care is placed into one of three functional impairment levels (low, medium, or high) based on responses to certain OASIS functional items associated with grooming, bathing, dressing, ambulating, transferring, and risk for hospitalization. The specific OASIS items that are used for the functional impairment level are found in table 8 in the CY 2020 HH PPS final rule with comment period ( 84 FR 60490 ). [ 2 ] Responses to these OASIS items are grouped together into response categories with similar resource use and each response category has associated points. A more detailed description as to how these response categories were established can be found in the technical report, “Overview of the Home Health Groupings Model” posted on the HHA web page. [ 3 ] The sum of these points results in a functional Start Printed Page 55323 impairment score used to group 30-day periods of care into a functional impairment level with similar resource use. The scores associated with the functional impairment levels vary by clinical group to account for differences in resource utilization. A patient's functional impairment level will remain the same for the first and second 30-day periods of care unless there is a significant change in condition that warrants an “other follow-up” assessment prior to the second 30-day period of care. For each 30-day period of care, the Medicare claims processing system will look for occurrence code 50 on the claim to correspond to the M0090 date of the applicable assessment. Table 9 shows the distribution of 30-day periods by functional impairment level.

Beginning in CY 2020, section 1895(b)(4)(B)(ii) of the Act eliminated the use of therapy thresholds in calculating payments for CY 2020 and subsequent years. Prior to implementation of the PDGM, HHAs could receive an adjustment to payment based on the number of therapy visits provided during a 60-day episode of care. We examined the proportion of actual 30-day periods of care with and without therapy visits. To be covered as skilled therapy, the services must require the skills of a qualified therapist (that is, PT, OT, or SLP) or qualified therapist assistant and must be reasonable and necessary for the treatment of the patient's illness or injury. [ 4 ] As shown in table 10, we monitor the number of visits per 30-day period of care by each home health discipline. Any 30-day period of care can include both therapy and non-therapy visits. If any 30-day period of care consisted of only visits for PT, OT, and/or SLP, then this 30-day period of care is considered “therapy only”. If any 30-day period of care consisted of only visits for skilled nursing, home health aide, or social worker, then this 30-day period of care is considered “no therapy”. If any 30-day period of care consisted of at least one therapy visit and one non-therapy, then this 30-day period of care is considered “therapy + non-therapy”. Table 10 shows the proportion of 30-day periods of care with only therapy visits, at least one therapy visit and one non-therapy visit, and no therapy visits. Figure 2 shows the proportion of 30-day periods of care by the number of therapy visits (excluding zero) provided during 30-day periods of care.

Both table 10 and figure 2, as previously discussed, indicate there have been changes in the distribution of both therapy and non-therapy visits in CY 2023 compared to CY 2022. For example, the percent of 30-day periods with one through seven therapy visits during a 30-day period increased in CY 2023 compared to CY 2022. Comparing therapy utilization from before the PDGM (CYs 2018 and 2019) to after the implementation of the PDGM (CYs 2020-2023), we have also seen a decline in therapy visits across all clinical groups, as shown in figure 2.

We also examined the proportion of 30-day periods of care with and without skilled nursing, social work, or home health aide visits. Table 12 shows the number of 30-day periods of care with only skilled nursing visits, at least one skilled nursing visit and one other visit type (therapy or non-therapy), and no skilled nursing visits. Table 12 shows the number of 30-day periods of care with and without home health aide and/or social worker visits.

As discussed in the CY 2023 final rule ( 87 FR 66858 ), we began collecting data on the use of telecommunications technology used during a home health period using three new G-codes reported on home health claims. Collecting data on services furnished via telecommunications technology on claims allows CMS to analyze the characteristics of patients using services provided remotely and have a broader understanding of the social determinants that affect who benefits most from these services, including what barriers may potentially exist for certain subsets of patients. The monitoring discussion illustrates which services are most frequently furnished via telecommunication technology and generally how long remote patient monitoring is utilized.
We began collecting this information from HHAs on January 1, 2023, on a voluntary basis and have required this information to be reported on claims starting on July 1, 2023 ( 87 FR 66858 ). The three new G-codes help identify when home health services are furnished using synchronous telemedicine rendered via a real-time two-way audio and video telecommunications system (G320); synchronous telemedicine rendered via telephone or other real-time interactive audio-only telecommunications system (G0321); and the collection of physiologic data digitally stored and/or transmitted by the patient to the home health agency, that is, remote patient monitoring (G0322). We capture the usage and length of remote patient monitoring using the start date of the remote patient monitoring and the number of days of monitoring indicated on the claim. We also looked at the disciplines most often providing remote patient monitoring. We examined the utilization of telecommunications technology device during a home health period and remote patient monitoring by looking at home health claims that included the three G-codes. Tables 14 and 15 shows that the use of telecommunications services reported on CY 2023 home health claims are low (roughly 1 percent of all CY 2023 claims) and are mainly associated with skilled nursing.

We will continue to monitor the provision of home health services, including any changes in the number and duration of home health visits, composition of the disciplines providing such services, telecommunications technology used during home health periods, and overall home health payments to determine if refinements to the case-mix adjustment methodology or other policies may be needed in the future.
1. Proposed Behavior Assumption Adjustments Under the HH PPS
As discussed in section II.A.1. of this proposed rule, starting in CY 2020, the Secretary was statutorily required by section 1895(b)(2)(B) of the Act, to change the unit of payment under the HH PPS from a 60-day episode of care to a 30-day period of care. CMS was also required to make assumptions about behavior changes that could occur as a result of the implementation of the 30-day unit of payment and the case-mix adjustment factors that eliminated the use of therapy thresholds. In the CY 2019 HH PPS final rule with comment period ( 83 FR 56455 ), we finalized three behavior change assumptions which were also described in the CY 2022 and 2023 HH PPS rules ( 86 FR 35890 , 87 FR 37614 , and 87 FR 66795 through 66796 ). In the CY 2020 HH PPS final rule with comment period ( 84 FR 60519 ), we included these behavioral change assumptions in the calculation of the 30-day budget neutral payment amount for CY 2020, finalizing a negative 4.36 percent behavior change assumption adjustment (“assumed behaviors”). We did not propose any changes for CYs 2021 and 2022 relating to the behavior assumptions finalized in the CY 2019 HH PPS final rule with comment period, or to the negative 4.36 percent behavior change assumption adjustment, finalized in the CY 2020 HH PPS final rule with comment period.
In the CY 2023 HH PPS final rule ( 87 FR 66796 ), we stated, based on our annual monitoring at that time, the three Start Printed Page 55328 assumed behavior changes did occur as a result of the implementation of the PDGM and that other behaviors, such as changes in the provision of therapy and changes in functional impairment levels also occurred. We also reminded readers that in the CY 2020 HH PPS final rule with comment period ( 84 FR 60513 ) we stated we interpret actual behavior changes to encompass both behavior changes that were previously outlined as assumed by CMS, and other behavior changes not identified at the time the budget-neutral 30-day payment rate for CY 2020 was established. In the CY 2023 HH PPS final rule ( 87 FR 66796 ) we provided supporting evidence that indicated the number of therapy visits declined in CYs 2020 and 2021, as well as a slight decline in therapy visits beginning in CY 2019 after the finalization of the removal of therapy thresholds, but prior to implementation of the PDGM. In section II.B.1. of this proposed rule, our analysis continues to show overall the actual 30-day periods are similar to the simulated 30-day periods and there continues to be a decline in therapy visits, indicating that HHAs changed their behavior to reduce therapy visits. Although the analysis demonstrates evidence of individual behavior changes (for example, in the volume of visits for LUPAs, therapy sessions, etc.), we use the entirety of the behaviors in order to calculate estimated aggregate expenditures. The law instructs us to ensure that estimated aggregate expenditures under the PDGM are equal to the estimated aggregate expenditures that otherwise would have been made under the prior system.
Section 4142(a) of the CAA, 2023, required CMS to present, to the extent practicable, a description of the actual behavior changes occurring under the HH PPS from CYs 2020-2026. This subsection of the CAA, 2023, also required CMS to provide datasets underlying the simulated 60-day episodes and discuss and provide time for stakeholders to provide input and ask questions on the payment rate development for CY 2023. CMS complied with these requirements by posting online both the supplemental limited data set (LDS) and descriptive files and the description of actual behavior changes that affected CY 2023 payment rate development. Additionally, on March 29, 2023, CMS conducted a webinar entitled Medicare Home Health Prospective Payment System (HH PPS) Calendar Year (CY) 2023 Behavior Change Recap, 60-Day Episode Construction Overview, and Payment Rate Development. The webinar was open to the public and discussed the actual behavior changes that occurred upon implementation of the PDGM, our approach used to construct simulated 60-day episodes using 30-day periods, payment rate development for CY 2023, and information on the supplemental data files containing information on the simulated 60-day episodes and actual 30-day periods used in calculating the permanent adjustment to the payment rate. Materials from the webinar, including the presentation and the CY 2023 descriptive statistics from the supplemental LDS files, containing information on the number of simulated 60-day episodes and actual 30-day periods in CY 2021 that were used to construct the permanent adjustment to the payment rate, as well as information such as the number of episodes and periods by case-mix group, case-mix weights, and simulated payments, can be found on the Home Health Patient-Driven Groupings Model web page at https://www.cms.gov/medicare/medicare-fee-for-service-payment/homehealthpps/hh-pdgm .
In the CY 2023 HH PPS final rule ( 87 FR 66804 ), we finalized the methodology to evaluate the impact of the differences between assumed and actual behavior changes on estimated aggregate expenditures. In the CY 2024 HH PPS final rule ( 88 FR 77687 through 77688 ) we provided an overview of the methodology with detailed instructions for each step. The overall methodology as finalized remains the same for evaluating the impact of behavior changes as required by law; however, due to an update of the Outcome and Assessment Information Set (OASIS) instrument, we need to update two minor technical parts and are proposing to add new assumptions in the first step (creating simulated 60-day episodes from 30-day periods These new assumptions are described in this section.
Section 1895(b)(3)(B)(v) of the Act requires HHAs to report certain quality data. As described in regulation at 42 CFR 484.250(a) , this data is required to be reported using the OASIS instrument. Under the prior 153-group system (and the first three years for assessments associated with the PDGM completed prior to CY 2023), HHAs submitted the OASIS-D version. However, OMB approved an updated version of the OASIS instrument, OASIS-E, on November 30, 2022, effective January 1, 2023. Thus, OASIS-E is the current version of the OASIS instrument used. The valid OMB control number for this information collection is 0938-1279.
There are 13 items from the OASIS-D used in the 153-group system that are included in the OASIS-E; however, the responses for these items are now only recorded at the start of care (SOC) or resumption of care (ROC) assessments in the OASIS-E and not at all for follow-up assessments as shown in the following figure 3.

Three items in the OASIS-E differ slightly from the OASIS-D by incorporating more specific questions and responses than in the OASIS-D. These three items, as shown in figure 4, ask about therapies (M1030), vision (M1200), and the frequency of pain interfering with activity (M1242). Additionally, these three items are only asked at SOC/ROC and not follow-up.

The differences in these three items from what is included in OASIS-E necessitate a mapping methodology to impute the OASIS-D responses using OASIS-E to create simulated 60-day episodes under the 153-group case mix system from 30-day periods under the PDGM. For each of the three items, we considered the clinical relationship between the responses in the OASIS-E items that differ from the OASIS-D items. CMS also considered the response distribution between the OASIS-D and OASIS-E items when creating the mapping of the responses.
CMS believes the following two proposals on assumptions are the most appropriate to address the changes from the OASIS-D to the OASIS-E to continue to create simulated 60-day episodes from 30-day periods.
- If the simulated 60-day episode matches to a SOC or ROC assessment then we are proposing not to impute the 13 items. If the simulated 60-day episode matches to a follow-up assessment, then we are proposing to look back for the most recent 30-day period that is linked to a SOC or ROC assessment and impute the 13 responses for follow-up using the responses at the most recent SOC or ROC assessment. We would limit the look back period to the beginning of the calendar year that precedes the calendar year for the claim. For example, a simulated 60-day episode with a follow-up assessment on June 1, 2023, would have a look-back period for a 30-day period linked to a SOC or ROC assessment that began on or after January 1, 2022. If we cannot find a SOC or ROC assessment in that time period, we are proposing to exclude the claim from analysis because we would not have sufficient timely data to impute responses.
- If the simulated 60-day episode matches to an OASIS-D assessment, then we are proposing to use the OASIS-D for responses. If the simulated 60-day episode matches to an OASIS-E assessment, we are proposing to apply the following mapping for the therapies, vision, and pain items to impute responses as these responses are required for accurate payment calculation under the prior 153-group system. We are also proposing to apply the look-back period as described in the assumption earlier when necessary.

Note, if an OASIS-E assessment has a response of “no” to all three items (O0110H—IV medication, K0520—Parenteral/IV feeding, and K0520—Feeding Tube), as shown in figure 5, then the mapping for M1030 would be a response of “none of the above”.

On the OASIS-D there was one pain item (M1242—Frequency of Pain Interfering with patient's activity or movement) used for payment policy. There are three pain related items on the OASIS-E (J0510—pain effect on sleep, J0520—pain interference with therapy activities, and J0530—pain interference with day-to-day activities) that correspond to the one OASIS-D pain item used for calculating payments. Therefore, we believe using the response from J0510, J0520, or J0530 that reflects the maximum severity would be the most appropriate for mapping back to the OASIS-D. For example, if J0510 (pain effect on sleep) has a response of “rarely”, J0520 (pain interference with therapy activities) has a response of “frequently”, and J0530 has a response of “occasionally”, then we would use the response from J0520 (“frequently”) for mapping as this is the most severe response. Figure 7 shows the proposed mapping based on the maximum severity response for any of the three pain items.

As this overall methodology was previously finalized in the CY 2023 HH PPS final rule ( 87 FR 66804 ) and we are just proposing technical updates based on the updated OASIS instrument, CMS will continue to ensure that estimated aggregate expenditures under the PDGM are equal to the estimated aggregate expenditures that otherwise would have been made under the prior system for assessing behavior changes as required by law. We refer readers to the CY 2024 HH PPS final rule ( 88 FR 77687 through 77688 ) for an overview of the methodology with detailed instructions for each step. We are soliciting comments on these new proposed assumptions related to mapping of the OASIS-E items.
To offset prospectively for such increases or decreases in estimated aggregate expenditures as a result of the impact of differences between assumed behavior changes and actual behavior changes, in any given year, we calculate a permanent prospective adjustment by calculating the percent change between the actual 30-day base payment rate and the recalculated 30-day base payment rate. This percent change is converted into an adjustment factor and applied in the annual rate update process.
To offset retrospectively for such increases or decreases in estimated aggregate expenditures as a result of the impact of differences between assumed behavior changes and actual behavior changes in any given year, we calculate a temporary prospective adjustment by calculating the dollar amount difference between the estimated aggregate expenditures from all 30-day periods using the recalculated 30-day base payment rate, and the aggregate expenditures for all 30-day periods using the actual 30-day base payment rate for the same year. In other words, when determining the temporary retrospective dollar amount, we use the full dataset of actual 30-day periods using both the actual and recalculated 30-day base payment rates to ensure that the utilization and distribution of claims are the same. In accordance with section 1895(b)(3)(D)(iii) of the Act, the temporary adjustment is to be applied on a prospective basis and shall apply only with respect to the year for which such temporary increase or decrease is made. Therefore, after we determine the dollar amount to be reconciled in any given year, we calculate a temporary adjustment factor to be applied to the base payment rate for that year. The temporary adjustment factor is based on an estimated number of 30-day periods in the next year using historical data trends, and as applicable, we control for a permanent adjustment factor, case-mix weight recalibration neutrality factor, wage index budget neutrality factor, and the home health payment update. The temporary adjustment factor is applied last. We refer readers to the CY 2024 HH PPS final rule ( 88 FR 77689 through 77694 ) for analysis for CYs 2020 through 2022 claims. Additionally, at the end of this section we provide a summary table for the permanent adjustment and temporary dollar amounts calculated for each year.
We will continue the practice of using the most recent complete home health claims data available at the time of rulemaking. While the CY 2023 analysis presented in this proposed rule is the most complete data available at the time of this proposed rule, it is considered preliminary and, as more data become available from the latter half of CY 2023, we will update our results in the final rule. The CY 2025 final rule will utilize the CY 2023 finalized data for determining any permanent adjustment needed to the CY 2025 payment rate. However, while the claims data and the Start Printed Page 55333 permanent and temporary adjustment results will be considered complete, any adjustments to future payment rates may be subject to additional considerations such as permanent adjustments taken in previous years.
The claims data used in rulemaking is released twice each year in the HH PPS Limited Data Set (LDS) file, one for the proposed and one for the final. Accordingly, the HH PPS LDS file released with this proposed rule includes two files: the actual CY 2023 30-day periods and the CY 2023 simulated 60-day episodes.
We remind readers a data use agreement (DUA) is required to purchase the CY 2025 proposed HH PPS LDS file. Access will be granted for both the 30-day periods and the simulated 60-day episodes under one DUA. Visit the HH PPS LDS web page for more information. [ 5 ] In addition, the proposed CY 2025 Home Health Descriptive Statistics from the LDS Files spreadsheet is available on the HH PPS Regulations and Notices webpage, [ 6 ] does not require a DUA, and is available at no cost to interested parties. The spreadsheet contains information on the number of simulated 60-day episodes and actual 30-day periods in CY 2023 that were used to determine the adjustments. The spreadsheet also provides information such as the number of episodes and periods by case-mix group, case-mix weights, and simulated payments.
Using the methodology finalized in the CY 2023 HH PPS final rule and described most recently in the CY 2024 HH PPS final rule ( 88 FR 77687 through 77688 ), as well as the two new assumptions related to the OASIS-E mapping, we simulated 60-day episodes using actual CY 2023 30-day periods to determine what the permanent and temporary payment adjustments should be to offset for such increases or decreases in estimated aggregate expenditures as a result of the impact of differences between assumed behavior changes and actual behavior changes.
Using the preliminary CY 2023 dataset, we began with 8,133,377 30-day periods of care and dropped 452,253 30-day periods of care that had claim occurrence code 50 date after October 31, 2023. We also excluded 866,293 30-day periods of care that had claim occurrence code 50 date before January 1, 2023, to ensure the 30-day period would not be part of a simulated 60-day episode that began in CY 2022. Applying the additional exclusions and assumptions as described in the finalized methodology ( 87 FR 66804 ), an additional 12,906 30-day periods were excluded.
Additionally, we excluded 166,441 simulated 60-day episodes of care where no OASIS information was available in the CCW VRDC, a recent SOC/ROC OASIS was not available, or the episode could not be grouped to a HIPPS due to a missing primary diagnosis or other reason. Our simulated 60-day episodes of care produced a distribution of two 30-day periods of care (68.9 percent) and single 30-day periods of care (31.1 percent) that was similar to what we found when we simulated two 30-day periods of care for implementation of the PDGM. After all exclusions and assumptions were applied, the final dataset for this proposed rule included 6,494,947 actual 30-day periods of care and 3,845,954 simulated 60-day episodes of care for CY 2023.
Using the preliminary dataset for CY 2023 (6,494,947 actual 30-day periods which made up the 3,845,954 simulated 60-day episodes) we determined the estimated aggregate expenditures under the pre-PDGM HH PPS were lower than the actual estimated aggregate expenditures under the PDGM HH PPS. This indicates that aggregate expenditures under the PDGM were higher than if the 153-group payment system was still in place in CY 2023 and therefore, we determined the CY 2023 30-day base payment rate should have been $1,873.17 based on actual behavior, as shown in table 16 As stated in the CY 2024 final rule ( 88 FR 77693 ) we determined for CYs 2020 through CY 2022 a total of −5.779 percent permanent adjustment was needed (after accounting for the −3.925 percent applied to the CY 2023 payment rate). In order to determine behavior changes for only CY 2023, we simulated what the CY 2023 base payment rate would have been if the full −5.779 percent adjustment that we determined using CY 2022 claims data had been implemented.
Using the recalculated CY 2022 base payment rate of $1,839.10 ( 88 FR 77693 ), multiplied by the CY 2023 case-mix weights recalibration neutrality factor (0.9904), the CY 2023 wage index budget neutrality factor (1.0001) and the CY 2023 home health payment update factor (1.040), the CY 2023 base payment rate for assumed behavior would have been $1,894.49. For the CY 2023 annual permanent adjustment, we calculated the percent change between the two payment rates for only CY 2023 (assuming the −5.779 percent adjustment was already taken). For the temporary adjustment we calculated the difference in aggregate expenditures in dollars for all CY 2023 PDGM 30-day claims using the actual payment rate ($2,010.69) and recalculated payment ($1,873.17). This difference is shown as the retrospective dollar amount needed to offset payment in a future year. Our results for the CY 2023 annual (single year) permanent and temporary adjustment calculations using CY 2023 preliminary claims data are shown in table 16.

As shown in table 16, a permanent prospective adjustment of −1.125 percent to the CY 2025 30-day payment rate (assuming the −5.779 percent adjustment was already taken) for CY 2023 would be required to offset for such increases in estimated aggregate expenditures in future years. To illustrate this calculation:

Additionally, we determined that our initial estimate of the base payment rate ($2,010.69) resulted in excess expenditures of approximately $966 million in CY 2023. This would require a temporary adjustment, where the dollar amount ($966 million) would be converted to a factor when implemented, to offset for such increases in estimated aggregate expenditures for CY 2023.
In the preceding section we describe how we annually analyzed CY 2023 preliminary data to determine the effects of actual behavior change on estimated aggregate expenditures. Again, that analysis included simulations that assumed that the full payment adjustment (−5.779 percent) was already taken. We note that CMS did not implement the full payment adjustment, so the calculations set forth later in this section reflect the lagging adjustments that are still needed.
That is, the calculation in this section includes any of the remaining adjustments not applied in previous years (that is, CYs 2020 to 2022), as well as the adjustment needed to account for CY 2023 claims. In calculating the full permanent adjustment needed to the CY 2025 30-day payment rate, we compare estimated aggregate expenditures under the PDGM and the prior system. Unlike the annual adjustments described in table 16, we do not assume the full adjustment from prior years had been taken.
As discussed in section II.C.1.d. of this proposed rule, using the preliminary dataset for CY 2023 (6,494,947 actual 30-day periods which made up the 3,845,954 simulated 60-day episodes) we determined the CY 2023 30-day base payment rate should have been $1,873.17 based on actual behavior, rather than the actual CY 2023 30-day base payment rate ($2,010.69) based on assumed behaviors. The percent change, as shown in table 17, between the actual CY 2023 base payment rate of $2,010.69 (based on assumed behaviors and included a −3.925 percent adjustment applied to the CY 2023 payment rate) and the CY 2023 recalculated base payment rate of $1,873.17 (based on actual behaviors) is the total permanent adjustment need for CYs 2020 through 2023.

As shown in table 17, a permanent prospective adjustment of −6.839 percent to the CY 2025 30-day payment rate for CYs 2020 through 2023 would be required to offset for such increases in estimated aggregate expenditures in future years. To illustrate this calculation:

As we stated in the CY 2024 HH PPS final rule ( 88 FR 77697 ), applying a −2.890 percent permanent adjustment to the CY 2024 30-day payment rate would not adjust the rate fully to account for differences in behavior changes on estimated aggregate expenditures in CYs 2020, 2021, and 2022. Using CY 2023 claims data, as shown in table 17, a permanent prospective adjustment of −6.839 percent to the CY 2025 30-day payment rate would be required to offset for such increases in estimated aggregate expenditures for CYs 2020 through 2023. We remind readers adjustment factors are multiplied in this payment system and therefore, individual numbers (that is, percentages) cannot be added or subtracted together to determine the final adjustment. Therefore, we cannot determine the CY 2025 proposed permanent adjustment, which would include estimated aggregate expenditures in CY 2023, by simply subtracting the −2.890 percent applied in CY 2024 from the total permanent adjustment of −6.839 percent.
Instead, we account for the permanent adjustment applied in CY 2024 of −2.890 percent when we calculate the CY 2025 permanent adjustment by solving the following equation (1−0.0289) × (1−χ) = (1−0.06839). To illustrate this calculation we used the following approach.

We are required by law [ 7 ] to annually analyze data from CY 2020 through CY 2026 and offset any increases or decreases in estimated aggregate expenditures at a time and manner determined appropriate. We now have 4 years of claims data under the PDGM, as well as 1 year with a partial permanent adjustment applied. In previous years' rules, we provided the permanent adjustment calculated for each discrete year of claims.
Permanent Adjustments Calculated:
CY 2020 Claims = −6.52% ( 87 FR 66805 )
CY 2021 Claims = −1.42% ( 87 FR 66806 )
CY 2022 Claims = −1.767% ( 88 FR 77692 )
CY 2023 Claims = −1.125% (Table 16)
Permanent Adjustments Applied:
CY 2023 Rate = −3.925% ( 88 FR 66808 )
CY 2024 Rate = 2.890% ( 88 FR 77697 )
Accounting for the previous permanent adjustments applied to the 30-day payment rate in CYs 2023 and 2024, we can simulate the permanent adjustment calculation with the simulated annual permanent adjustment percentage shown previously for CY 2025:
(1−0.0652)(1−0.0142)(1−0.01767)(1−0.01125) = (1−0.03925)(1−0.0289)(1−x).
Solving, x = 4.067%.
In table 18 we provide the base payment rate for assumed behaviors (simulates all prior adjustments were taken), the recalculated base payment rate for actual behaviors, the annual permanent adjustments calculated (assuming prior adjustments had been taken), the cumulative permanent adjustments calculated in each year, the final permanent adjustments implemented in rulemaking, and the temporary adjustment dollar amount based on actual payment rates.

In both the CY 2023 and 2024 final rules ( 87 FR 66790 , 88 FR 77696 ), we acknowledged that the full permanent adjustment may be burdensome for some providers. In those final rules, we finalized only half of the permanent adjustment percentages (−3.925 percent in CY 2023 and −2.890 percent in CY 2024). However, in this proposed rule, we are proposing to apply the full current remaining permanent adjustment of −4.067 percent in CY 2025, as this would satisfy the statutory requirements at section 1895(b)(3)(D) of the Act to offset any increases or decreases on the impact of differences between assumed behavior and actual behavior changes on estimated aggregate expenditures, reduce the need for any future large permanent adjustments, and help slow the accrual of the temporary payment adjustment amount. In addition, we explained in the CY 2023 HH PPS final rule ( 87 FR 66808 ) and the CY 2024 HH PPS final rule ( 88 FR 77697 ) that when we applied a reduced permanent adjustment in CY 2023 and CY 2024, that we would need to continue to implement a reduction in future years to satisfy the statutory requirements. Therefore, we believe that CMS has been clear through notice and Start Printed Page 55337 comment rulemaking that the remainder of these permanent adjustments would be applied, thereby giving HHAs adequate notice to prepare for this year's proposed rate reduction. Accordingly, we are proposing to apply the full remaining permanent adjustment of −4.067 percent to the CY 2025 home health base payment rate, noting that we will update this percentage using more complete claims data in the final rule.
We stated in the CY 2023 HH PPS final rule ( 87 FR 66804 ), the CY 2024 HH PPS proposed rule ( 88 FR 43674 ) and in this proposed rule, that after we determine the total dollar amount to be reconciled, we will calculate a temporary adjustment factor to be applied to the base payment rate for the year in which it is implemented. That is, the temporary adjustment dollar amount (currently estimated at $4.5 billion) will be converted to a factor to be applied to the payment rate in a time and manner determined appropriate. As we noted in the CY 2023 HH PPS proposed rule ( 87 FR 37682 ) and CY 2024 HH PPS proposed rule ( 88 FR 43678 ), we recognize that implementing both the permanent and temporary adjustments in the same year may adversely affect HHAs. Given that the magnitude of both the temporary and permanent adjustments together for CY 2025 rate setting may result in a significant reduction of the payment rate, we are not proposing to take the temporary adjustment in CY 2025. In future year rulemaking, we will propose a temporary adjustment factor to the national, standardized base payment rate in a time and manner determined appropriate. As noted previously, we will update these permanent and temporary adjustments in the final rule to reflect more complete claims data for CY 2023. We solicit comments on the proposal to apply a −4.067 percent permanent adjustment to the CY 2025 base payment rate.
Under the HH PPS, LUPAs are paid when a certain visit threshold for a payment group during a 30-day period of care is not met. In the CY 2019 HH PPS final rule with comment period ( 83 FR 56492 ), we finalized a policy setting the LUPA thresholds at the 10th percentile of visits or two visits, whichever is higher, for each payment group. This means the LUPA threshold for each 30-day period of care varies depending on the PDGM payment group to which it is assigned. If the LUPA threshold for the payment group is met under the PDGM, the 30-day period of care will be paid the full 30-day period case-mix adjusted payment amount (subject to any partial payment adjustment or outlier adjustments). If a 30-day period of care does not meet the PDGM LUPA visit threshold, then payment will be made using the per-visit payment amounts as described in section II.C.4.f.2 of this proposed rule. For example, if the LUPA visit threshold is four, and a 30-day period of care has four or more visits, it is paid the full 30-day period payment amount; if the period of care has three or fewer visits, payment is made using the per-visit payment amounts.
In the CY 2019 HH PPS final rule with comment period ( 83 FR 56492 ), we finalized our policy that the LUPA thresholds for each PDGM payment group would be reevaluated every year based on the most current utilization data available at the time of rulemaking. However, as CY 2020 was the first year of the new case-mix adjustment methodology, we stated in the CY 2021 HH PPS final rule ( 85 FR 70305 , 70306 ) that we would maintain the LUPA thresholds that were finalized and shown in table 17 of the CY 2020 HH PPS final rule with comment period ( 84 FR 60522 ) for CY 2021 payment purposes. We stated that at that time, we did not have sufficient CY 2020 data to reevaluate the LUPA thresholds for CY 2021.
In the CY 2022 HH PPS final rule with comment period ( 86 FR 62249 ), we finalized the proposal to recalibrate the PDGM case-mix weights, functional impairment levels, and comorbidity subgroups while maintaining the LUPA thresholds for CY 2022. We stated that because there are several factors that contribute to how the case-mix weight is set for a particular case-mix group (such as the number of visits, length of visits, types of disciplines providing visits, and non-routine supplies) and the case-mix weight is derived by comparing the average resource use for the case-mix group relative to the average resource use across all groups, we believe the COVID-19 PHE would have impacted utilization within all case-mix groups similarly. Therefore, the impact of any reduction in resource use caused by the PHE on the calculation of the case-mix weight would be minimized since the impact would be accounted for both in the numerator and denominator of the formula used to calculate the case-mix weight. However, in contrast, the LUPA thresholds are based on the number of overall visits in a particular case-mix group (the threshold is the 10th percentile of visits or 2 visits, whichever is greater) instead of a relative value (like what is used to generate the case-mix weight) that would control for the impacts of the COVID-19 PHE. We noted that visit patterns and some of the decrease in overall visits in CY 2020 may not be representative of visit patterns in CY 2022. Therefore, to mitigate any potential future and significant short-term variability in the LUPA thresholds due to the COVID-19 PHE, we finalized the proposal to maintain the LUPA thresholds finalized and displayed in table 17 in the CY 2020 HH PPS final rule with comment period ( 84 FR 60522 ) for CY 2022 payment purposes.
For CY 2024, we proposed to update the LUPA thresholds using CY 2022 Medicare home health claims (as of March 17, 2023) linked to OASIS assessment data. We believed that CY 2022 data will be more indicative of visit patterns in CY 2024 rather than continuing to use the LUPA thresholds derived from the CY 2018 data pre-PDGM. Therefore, we finalized a policy to update the LUPA thresholds for CY 2024 using data from CY 2022.
For CY 2025, we are proposing to update the LUPA thresholds using CY 2023 home health claims utilization data (as of March 19, 2024), in accordance with our policy to annually recalibrate the case-mix weights and update the LUPA thresholds, functional impairment levels and comorbidity subgroups. After reviewing the CY 2023 home health claims utilization data, we determined that LUPA visit patterns in 2023 were similar to visits in 2021. The proposed LUPA thresholds for the CY 2025 PDGM payment groups with the corresponding Health Insurance Prospective Payment System (HIPPS) codes and the case-mix weights are listed in table 20. We solicit public comments on the proposed updates to the LUPA thresholds for CY 2025. The proposed LUPA thresholds will be updated based on more complete CY 2023 claims data in the final rule.
Under the PDGM, the functional impairment level is determined by responses to certain OASIS items associated with activities of daily living and risk of hospitalization; that is, responses to OASIS items M1800-M1860 and M1033. A home health period of care receives points based on Start Printed Page 55338 each of the responses associated with these functional OASIS items, which are then converted into a table of points corresponding to increased resource use. The sum of all these points results in a functional impairment score which is used to group home health periods into a functional level with similar resource use. That is, the higher the points, the more the response is associated with increased resource use, or increased impairment. The three functional impairment levels of low, medium, and high were designed so that approximately one-third of home health periods from each clinical group falls within each level. This means home health periods in the low impairment level have responses for the functional OASIS items that are associated with the lowest resource use, on average. Home health periods in the high impairment level have responses for the functional OASIS items that are associated with the highest resource use on average.
For CY 2025, we propose to use CY 2023 claims data to update the functional points and functional impairment levels by clinical group. The CY 2018 HH PPS proposed rule ( 82 FR 35320 ) and the technical report from December 2016, posted on the Home Health PPS Archive web page located at: https://www.cms.gov/medicare/home-health-pps/home-health-pps-archive , provides a more detailed explanation as to the construction of these functional impairment levels using the OASIS items. We are proposing to use the same methodology previously finalized to update the functional impairment levels for CY 2025. The updated OASIS functional points table and the table of functional impairment levels by clinical group for CY 2025 are listed in tables 20 and 21, respectively. We solicit public comments on the updates to functional points and the functional impairment levels by clinical group.

Thirty-day periods of care receive a comorbidity adjustment category based on the presence of certain secondary diagnoses reported on home health claims. These diagnoses are based on a home-health specific list of clinically and statistically significant secondary diagnosis subgroups with similar resource use, meaning the diagnoses have at least as high as the median resource use and are reported in more than 0.1 percent of 30-day periods of care. Home health 30-day periods of care can receive a comorbidity Start Printed Page 55340 adjustment under the following circumstances:
- High comorbidity adjustment: There are two or more secondary diagnoses on the home health-specific comorbidity subgroup interaction list that are associated with higher resource use when both are reported together compared to when they are reported separately. That is, the two diagnoses may interact with one another, resulting in higher resource use.
- Low comorbidity adjustment: There is a reported secondary diagnosis on the home health-specific comorbidity subgroup list that is associated with higher resource use.
- No comorbidity adjustment: A 30-day period of care receives no comorbidity adjustment if no secondary diagnoses exist or do not meet the criteria for a low or high comorbidity adjustment.
In the CY 2019 HH PPS final rule with comment period ( 83 FR 56406 ), we stated that we would continue to examine the relationship of reported comorbidities on resource utilization and make the appropriate payment refinements to help ensure that payment is in alignment with the actual costs of providing care. For CY 2025, we propose to use the same methodology used to establish the comorbidity subgroups to update the comorbidity subgroups using CY 2023 home health data with linked OASIS data (as of March 19, 2024).
For CY 2025, we propose to update the comorbidity subgroups to include 22 low comorbidity adjustment subgroups as identified in table 22 and 90 high comorbidity adjustment interaction subgroups as identified in table 23. The proposed CY 2025 low comorbidity adjustment subgroups and the high comorbidity adjustment interaction subgroups including those diagnoses within each of these comorbidity adjustments will also be posted on the HHA Center web page at https://www.cms.gov/Center/Provider-Type/Home-Health-Agency-HHA-Center .
We invite comments on the proposed updates to the low comorbidity adjustment subgroups and the high comorbidity adjustment interactions for CY 2025.

As finalized in the CY 2019 HH PPS final rule with comment period ( 83 FR 56502 ), the PDGM places patients into meaningful payment categories based on patient and other characteristics, such as timing, admission source, clinical grouping using the reported principal diagnosis, functional impairment level, and comorbid conditions. The PDGM case-mix methodology results in 432 unique case-mix groups called home health resource groups (HHRGs). We also finalized a policy in the CY 2019 HH PPS final rule with comment period ( 83 FR 56515 ) to annually recalibrate the PDGM case-mix weights using a fixed effects model with the most recent and complete utilization data available at the time of annual rulemaking. Annual recalibration of the PDGM case-mix weights ensures that the case-mix weights reflect, as accurately as possible, current home health resource use and changes in utilization patterns. To generate the proposed recalibrated CY 2025 case-mix weights, we used CY 2023 home health claims data with linked OASIS data (as of March 19, 2024). These data are the most current and complete data available at this time. We believe that recalibrating the case-mix weights using data from CY 2023 would be reflective of PDGM utilization and patient resource use for CY 2025. The proposed recalibrated case-mix weights will be updated based on more complete CY 2023 claims data in the final rule.
The claims data provide visit-level data and data on whether non-routine supplies (NRS) were provided during the period and the total charges of NRS. We determine the case-mix weight for each of the 432 different PDGM payment groups by regressing resource use on a series of indicator variables for each of the categories using a fixed effects model as described in the following steps:
Step 1: Estimate a regression model to assign a functional impairment level to each 30-day period. The regression model estimates the relationship between a 30-day period's resource use and the functional status and risk of hospitalization items included in the PDGM, which are obtained from certain OASIS items. We refer readers to table 18 for further information on the OASIS items used for the functional impairment level under the PDGM. We measure resource use with the cost-per-minute + NRS approach that uses information from 2022 home health cost reports. We use 2022 home health cost report data because it is the most complete cost report data available at the time of rulemaking. Other variables in the regression model include the 30-day period's admission source, clinical group, and 30-day period timing. We also include home health agency level fixed effects in the regression model. After estimating the regression model using 30-day periods, we divide the coefficients that correspond to the functional status and risk of hospitalization items by 10 and round to the nearest whole number. Those rounded numbers are used to compute a functional score for each 30-day period by summing together the rounded numbers for the functional status and risk of hospitalization items that are applicable to each 30-day period. Next, each 30-day period is assigned to a functional impairment level (low, medium, or high) depending on the 30-day period's total functional score. Each clinical group has a separate set of functional thresholds used to assign 30-day periods into a low, medium or high functional impairment level. We set those thresholds so that we assign roughly a third of 30-day periods within each clinical group to each functional impairment level (low, medium, or high).
Step 2: A second regression model estimates the relationship between a 30-day period's resource use and indicator variables for the presence of any of the comorbidities and comorbidity interactions that were originally examined for inclusion in the PDGM. Like the first regression model, this model also includes home health agency level fixed effects and includes control variables for each 30-day period's admission source, clinical group, timing, and functional impairment level. After we estimate the model, we assign comorbidities to the low comorbidity adjustment if any comorbidities have a coefficient that is statistically significant (p-value of 0.05 or less) and which have a coefficient that is larger than the 50th percentile of positive and statistically significant comorbidity coefficients. If two comorbidities in the model and their interaction term have coefficients that sum together to exceed $150 and the interaction term is statistically significant (p-value of 0.05 or less), we assign the two comorbidities together to the high comorbidity adjustment.
Step 3: After Step 2, each 30-day period is assigned to a clinical group, admission source category, episode timing category, functional impairment level, and comorbidity adjustment category. For each combination of those variables (which represent the 432 different payment groups that comprise the PDGM), we then calculate the 10th percentile of visits across all 30-day periods within a particular payment group. If a 30-day period's number of visits is less than the 10th percentile for their payment group, the 30-day period is classified as a Low Utilization Payment Adjustment (LUPA). If a payment group has a 10th percentile of visits that is less than two, we set the LUPA threshold for that payment group to be equal to two. That means if a 30-day period has one visit, it is classified as a LUPA and if it has two or more visits, it is not classified as a LUPA.
Step 4: Take all non-LUPA 30-day periods and regress resource use on the 30-day period's clinical group, admission source category, episode timing category, functional impairment level, and comorbidity adjustment category. The regression includes fixed effects at the level of the home health agency. After we estimate the model, the model coefficients are used to predict each 30-day period's resource use. To create the case-mix weight for each 30-day period, the predicted resource use is divided by the overall resource use of the 30-day periods used to estimate the regression.
The case-mix weight is then used to adjust the base payment rate to determine each 30-day period's payment. Table 24 shows the coefficients of the payment regression used to generate the weights, and the coefficients divided by average resource use.

The case-mix weights proposed for CY 2025 are listed in table 25 and will also be posted on the HHA Center web page [ 8 ] upon display of this proposed rule.

Changes to the PDGM case-mix weights are implemented in a budget neutral manner by multiplying the CY 2025 national standardized 30-day Start Printed Page 55361 period payment rate by a case-mix budget neutrality factor. Typically, the case-mix weight budget neutrality factor is also calculated using the most recent, complete home health claims data available. For CY 2025, we will continue the practice of using the most recent complete home health claims data at the time of rulemaking, which is CY 2023 data. The case-mix budget neutrality factor is calculated as the ratio of 30-day base payment rates such that total payments when the CY 2025 PDGM case-mix weights (developed using CY 2023 home health claims data) are applied to CY 2023 utilization (claims) data are equal to total payments when CY 2024 PDGM case-mix weights (developed using CY 2022 home health claims data) are applied to CY 2023 utilization data. This produces a case-mix budget neutrality factor for CY 2025 of 1.0035.
We invite public comments on the CY 2025 proposed case-mix weights and proposed case-mix weight budget neutrality factor.
The 2009 final rule, “HIPAA Administrative Simplification: Modifications to Medical Data Code Set Standards To Adopt ICD-10-CM and ICD-10-PCS” ( 74 FR 3328 , January 16, 2009), set October 1, 2013, as the compliance date for all covered entities under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) to use the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) and the International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) medical data code sets. The ICD-10-CM diagnosis codes are granular and specific and provide HHAs a better opportunity to report codes that best reflect the patient's conditions that support the need for home health services. However, as stated in the CY 2019 HH PPS final rule with comment period ( 83 FR 56473 ), because the ICD-10-CM is comprehensive, it also contains many codes that may not support the need for home health services. For example, diagnosis codes that indicate death as the outcome are Medicare covered codes but are not relevant to home health. In addition, diagnosis and procedure coding guidelines may specify the sequence of ICD-10-CM coding conventions. For example, the underlying condition must be listed first (for example, Parkinson's disease must be listed prior to Dementia if both codes were listed on a claim). Therefore, not all the ICD-10-CM diagnosis codes are appropriate as principal diagnosis codes for grouping home health periods into clinical groups or to be placed into a comorbidity subgroup when listed as a secondary diagnosis. As such, each ICD-10-CM diagnosis code is assigned, including those diagnosis codes designated as “not assigned” (NA), to a clinical group and comorbidity subgroup within the HH PPS grouper software (HHGS). We reminded readers the ICD-10-CM diagnosis code list is updated each fiscal year with an effective date of October 1st and therefore, the HH PPS is generally subject to a minimum of two HHGS releases, one in October and one in January of each year, to ensure that claims are submitted with the most current code set available. Likewise, there may be new ICD-10-CM diagnosis codes created (for example, codes for emergency use) or a new or revised edit in the Medicare Code Editor (MCE) so an update to the HHGS may occur on the first of each quarter (January, April, July, October). We encourage readers to check the HHGS routinely at these times, as we do not anticipate posting changes to the home health web page.
Although it is not our intent to review all ICD-10-CM diagnosis codes each year, we recognize that occasionally some ICD-10-CM diagnosis codes may require changes to their assigned clinical group and/or comorbidity subgroup. For example, there may be an update to the MCE unacceptable principal diagnosis list, or we receive public comments from interested parties requesting specific changes. Any addition or removal of a specific diagnosis code to the ICD-10-CM code set (for example, three new diagnosis codes, Z28.310, Z28.311 and Z28.39, for reporting COVID-19 vaccination status were effective April 1, 2022) or minor tweaks to a descriptor of an existing ICD-10-CM diagnosis code generally could be implemented as appropriate and may not be discussed in rulemaking.
We rely on the expert opinion of our clinical reviewers (for example, nurse consultants and medical officers) and current ICD-10-CM coding guidelines to determine if the ICD-10-CM diagnosis codes under review for reassignment are significantly similar or different to the existing clinical group and/or comorbidity subgroup assignment. As we stated in the CY 2018 HH PPS proposed rule ( 82 FR 35313 ), the intent of the clinical groups is to reflect the reported principal diagnosis, clinical relevance, and coding guidelines and conventions. Therefore, for the purposes of assignment of ICD-10-CM diagnosis codes into the PDGM clinical groups we would not conduct additional statistical analysis as such decisions are clinically based and the clinical groups are part of the overall case-mix weights.
As we noted in the CY 2019 HH PPS final rule with comment period ( 83 FR 56486 ), the home health-specific comorbidity list is based on the principles of patient assessment by body systems and their associated diseases, conditions, and injuries to develop larger categories of conditions that identified clinically relevant relationships associated with increased resource use, meaning the diagnoses have at least as high as the median resource use and are reported in more than 0.1 percent of 30-day periods of care. If specific ICD-10-CM diagnosis codes are to be reassigned to a different comorbidity subgroup (including NA), we will first evaluate the clinical characteristics (as discussed previously for clinical groups) and if the ICD-10-CM diagnosis code does not meet the clinical criteria, then no reassignment will occur. However, if an ICD-10-CM diagnosis code does meet the clinical criteria for a comorbidity subgroup reassignment, then we will evaluate the resource consumption associated with the ICD-10-CM diagnosis codes, the current assigned comorbidity subgroup, and the proposed (reassigned) comorbidity subgroup. This analysis is to ensure that any reassignment of an ICD-10-CM diagnosis code (if reported as secondary) in any given year would not significantly alter the overall resource use of a specific comorbidity subgroup. For resource consumption, we use non-LUPA 30-day periods to evaluate the total number of 30-day periods for the comorbidity subgroup(s) and the ICD-10-CM diagnosis code, the average number of visits per 30-day periods for the comorbidity subgroup(s) and the ICD-10-CM diagnosis code, and the average resource use for the comorbidity subgroup(s) and the ICD-10-CM diagnosis code. The average resource use measures the costs associated with visits performed during a home health period and was previously described in the CY 2019 HH PPS final rule with comment period ( 83 FR 56450 ). Start Printed Page 55362
We received questions from interested parties regarding the ICD-10-CM diagnosis codes N30.00- (acute cystitis) and the ICD-10-CM diagnosis code N39.0 (urinary tract infection, site not specified). Specifically, CMS received a request to reassign N30.00 to the same clinical and comorbidity group as N39.0. The ICD-10-CM diagnosis codes N30.00- (acute cystitis) are currently assigned to clinical group J (MMTA—Gastrointestinal tract and Genitourinary system) when listed as a primary diagnosis and not assigned to a comorbidity subgroup when listed as a secondary diagnosis. The ICD-10-CM diagnosis code N39.0 (urinary tract infection, site not specified) is currently assigned to clinical group J (MMTA—Gastrointestinal tract and Genitourinary system) when listed as a primary diagnosis and assigned to the renal 3 comorbidity subgroup when listed as a secondary diagnosis.
We reviewed the ICD-10-CM diagnosis codes related to cystitis (N30.-) and determined all 14 of the codes are not assigned to a comorbidity subgroup when listed as a secondary diagnosis. Our clinical reviewers advised that cystitis, including N30.00- (acute cystitis), is to report inflammation of the urinary bladder; whereas N39.0 (urinary tract infection, site not specified) is to report the presence of the infectious microorganisms in the urinary tract system. In addition, we evaluated resource consumption related to the comorbidity subgroup renal 3, as well as diagnosis codes N30.00- (acute cystitis) and N39.0 (urinary tract infection, site not specified) and found that acute cystitis on average has a lower resource use than urinary tract infection. As described earlier, based on clinical review and resources use analysis, the ICD-10-CM diagnosis codes N30.00- (acute cystitis) are currently assigned to the most appropriate comorbidity group, not assigned. Therefore, we are not proposing a reassignment of N30.00- (acute cystitis) at this time.
Section 1895(b)(3)(B) of the Act requires that the standard prospective payment amounts for home health be increased by a factor equal to the applicable home health market basket update for those HHAs that submit quality data as required by the Secretary. In the CY 2024 HH PPS final rule ( 88 FR 77726 ), we finalized a rebasing of the home health market basket to reflect 2021 cost report data. We also finalized a policy for CY 2024 and subsequent years that the labor-related share would be 74.9 percent and the non-labor-related share would be 25.1 percent. A detailed description of how we rebased the HHA market basket and labor-related share is available in the CY 2024 HH PPS final rule ( 88 FR 77726 through 77742 ).
In the CY 2015 HH PPS final rule ( 79 FR 38384 ), we finalized our methodology for calculating and applying the multifactor productivity adjustment. As we explained in that rule, section 1895(b)(3)(B)(vi) of the Act, requires that, in CY 2015 (and in subsequent calendar years, except CY 2018 (under section 411(c) of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) ( Pub. L. 114-10 , enacted April 16, 2015)), the market basket percentage under the HH PPS as described in section 1895(b)(3)(B) of the Act be annually adjusted by changes in economy-wide productivity. Section 1886(b)(3)(B)(xi)(II) of the Act defines the productivity adjustment to be equal to the 10-year moving average of change in annual economy-wide private nonfarm business multifactor productivity (as projected by the Secretary for the 10-year period ending with the applicable fiscal year, calendar year, cost reporting period, or other annual period). The Bureau of Labor Statistics (BLS) publishes the official measures of productivity for the United States economy. We note that previously the productivity measure referenced in section 1886(b)(3)(B)(xi)(II) of the Act was published by BLS as private nonfarm business multifactor productivity. Beginning with the November 18, 2021, release of productivity data, BLS replaced the term “multifactor productivity” with “total factor productivity” (TFP). BLS noted that this is a change in terminology only and will not affect the data or methodology. As a result of the BLS name change, the productivity measure referenced in section 1886(b)(3)(B)(xi)(II) of the Act is now published by BLS as “private nonfarm business total factor productivity”. We refer readers to https://www.bls.gov for the BLS historical published TFP data. A complete description of IGI's TFP projection methodology is available on the CMS website at https://www.cms.gov/data-research/statistics-trends-and-reports/medicare-program-rates-statistics/market-basket-research-and-information .
The proposed home health update percentage for CY 2025 is based on the estimated home health market basket percentage increase, specified at section 1895(b)(3)(B)(iii) of the Act, of 3.0 percent (based on IHS Global Inc.'s first quarter 2024 forecast with historical data through fourth-quarter 2023). The estimated CY 2025 home health market basket percentage increase of 3.0 percent is then reduced by a productivity adjustment, in accordance with section 1895(b)(3)(B)(vi) of the Act. Based on IGI's first quarter 2024 forecast, the proposed productivity adjustment is currently estimated to be 0.5 percentage point for CY 2025. Therefore, the proposed productivity-adjusted CY 2025 home health market basket update is 2.5 percent (3.0 percent market basket percentage increase, reduced by a 0.5 percentage point productivity adjustment). Furthermore, we propose that if more recent data subsequently become available (for example, a more recent estimate of the market basket and/or productivity adjustment), we would use such data, if appropriate, to determine the CY 2025 market basket percentage increase and productivity adjustment in the final rule.
Section 1895(b)(3)(B)(v) of the Act requires that the home health percentage update be decreased by 2 percentage points for those HHAs that do not submit quality data as required by the Secretary. For HHAs that do not submit the required quality data for CY 2025, the proposed home health payment update percentage is 0.5 percent (2.5 percent minus 2 percentage points).
We invite public comment on our proposals for the CY 2025 home health market basket percentage increase and productivity adjustment.
In general, OMB issues major revisions to statistical areas every 10 years, based on the results of the decennial census. However, OMB occasionally issues minor updates and revisions to statistical areas in the years between the decennial censuses.
On February 28, 2013, OMB issued Bulletin No. 13-01, announcing revisions to the delineations of MSAs, Micropolitan Statistical Areas, and CBSAs, and guidance on uses of the delineation of these areas. In the CY 2015 HH PPS final rule ( 79 FR 66085 through 66087 ), we adopted OMB's area delineations using a 1-year transition. Start Printed Page 55363
On August 15, 2017, OMB issued Bulletin No. 17-01 in which it announced that one Micropolitan Statistical Area, Twin Falls, Idaho, now qualifies as a Metropolitan Statistical Area. The new CBSA (46300) comprises the principal city of Twin Falls, Idaho in Jerome County, Idaho and Twin Falls County, Idaho. The CY 2025 HH PPS wage index value for CBSA 46300, Twin Falls, Idaho, will be 0.8555. Bulletin No. 17-01 is available at https://www.whitehouse.gov/wp-content/uploads/legacy_drupal_files/omb/bulletins/2017/b-17-01.pdf .
On April 10, 2018, OMB issued OMB Bulletin No. 18-03, which superseded the August 15, 2017, OMB Bulletin No. 17-01. On September 14, 2018, OMB issued OMB Bulletin No. 18-04 which superseded the April 10, 2018, OMB Bulletin No. 18-03. These bulletins established revised delineations for Metropolitan Statistical Areas, Micropolitan Statistical Areas, and Combined Statistical Areas, and provided guidance on the use of the delineations of these statistical areas. A copy of OMB Bulletin No. 18-04 may be obtained at: https://www.bls.gov/bls/omb-bulletin-18-04-revised-delineations-of-metropolitan-statistical-areas.pdf .
On March 6, 2020, OMB issued Bulletin No. 20-01, which provided updates to and superseded OMB Bulletin No. 18-04 that was issued on September 14, 2018. The attachments to OMB Bulletin No. 20-01 provided detailed information on the update to statistical areas since September 14, 2018, and were based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to Census Bureau population estimates for July 1, 2017, and July 1, 2018. (For a copy of this bulletin, we refer readers to https://www.whitehouse.gov/wp-content/uploads/2020/03/Bulletin-20-01.pdf . ) In OMB Bulletin No. 20-01, OMB announced one new Micropolitan Statistical Area, one new component of an existing Combined Statistical Area and changes to New England City and Town Area (NECTA) delineations. In the CY 2021 HH PPS final rule ( 85 FR 70298 ), we stated that if appropriate, we would propose any updates from OMB Bulletin No. 20-01 in future rulemaking. After reviewing OMB Bulletin No. 20-01, we have determined that the changes in Bulletin 20-01 encompassed delineation changes that would not affect the Medicare home health wage index for CY 2022. Specifically, the updates consisted of changes to NECTA delineations and the re-designation of a single rural county into a newly created Micropolitan Statistical Area. The Medicare home health wage index does not utilize NECTA definitions, and, as most recently discussed in the CY 2021 HH PPS final rule ( 85 FR 70298 ) we include hospitals located in Micropolitan Statistical areas in each State's rural wage index. In other words, these OMB updates did not affect any geographic areas for purposes of the HH PPS wage index calculation.
In the CY 2021 HH PPS final rule ( 85 FR 70298 ), we finalized our proposal to adopt the revised OMB delineations with a 5-percent cap on wage index decreases in CY 2021. As described in the CY 2023 HH PPS final rule ( 87 FR 66851 through 66853 ), we finalized a policy that the CY HH PPS wage index would include a 5-percent cap on wage index decreases for CY 2023 and each subsequent year. Specifically, we finalized for CY 2023 and subsequent years, the application of a permanent 5-percent cap on any decrease to a geographic area's wage index from its wage index in the prior year, regardless of the circumstances causing the decline. That is, we finalized a policy requiring that a geographic area's wage index for CY 2023 would not be less than 95 percent of its final wage index for CY 2022, regardless of whether the geographic area is part of an updated CBSA, and that for subsequent years, a geographic area's wage index would not be less than 95 percent of its wage index calculated in the prior CY. Previously this methodology was applied to all the counties that make up a CBSA or statewide rural area. However, as discussed in section II.E.2. of this proposed rule, if we adopt the proposed revised OMB delineations, we are also proposing that this methodology would also be applied to individual counties.
On July 21, 2023, OMB issued Bulletin No. 23-01, which updates and supersedes OMB Bulletin No. 20-01, issued on March 6, 2020. OMB Bulletin No. 23-01 establishes revised delineations for the MSAs, Micropolitan Statistical Areas, Combined Statistical Areas, and Metropolitan Divisions, collectively referred to as Core Based Statistical Areas (CBSAs). According to OMB, the delineations reflect the 2020 Standards for Delineating Core Based Statistical Areas (CBSAs) (the “2020 Standards”), which appeared in the Federal Register ( 86 FR 37770 through 37778 ) on July 16, 2021, and application of those standards to Census Bureau population and journey-to-work data (for example, 2020 Decennial Census, American Community Survey, and Census Population Estimates Program data). A copy of OMB Bulletin No. 23-01 is available online at: https://www.whitehouse.gov/wp-content/uploads/2023/07/OMB-Bulletin-23-01.pdf .
The July 21, 2023, OMB Bulletin No. 23-01 contains a number of significant changes. For example, there are new CBSAs, urban counties that have become rural, rural counties that have become urban, and existing CBSAs that have been split apart. We believe it is important for the HH PPS wage index to use the latest OMB delineations available in order to maintain a more accurate and up-to-date payment system that reflects the reality of population shifts and labor market conditions. We further believe that using the most current OMB delineations would increase the integrity of the HH PPS wage index by creating a more accurate representation of geographic variation in wage levels. We are proposing to implement the new OMB delineations as described in the July 21, 2023, OMB Bulletin No. 23-01 for the HH PPS wage index effective beginning in CY 2025. This proposal is also consistent with the proposals to adopt the revised OMB delineations in the IPPS and other post-acute care payment systems.
As discussed in the CY 2006 HH PPS proposed rule ( 70 FR 40788 ) and final rule ( 70 FR 68132 ), CMS considered how to use the Micropolitan statistical area definitions in the calculation of the wage index. At the time, OMB defined a “Micropolitan Statistical Area” as a “CBSA” associated with at least one urban cluster that has a population of at least 10,000, but less than 50,000 ( 75 FR 37252 ). We referred to these as Micropolitan Areas. After extensive impact analysis, consistent with the treatment of these areas under the IPPS as discussed in the FY 2005 IPPS final rule ( 69 FR 49029 through 49032 ), we determined the best course of action would be to treat Micropolitan Areas as “rural” and include them in the calculation of each state's home health rural wage index (see 70 FR 40788 and 70 FR 68132 ). Thus, the HH PPS statewide rural wage index is determined using IPPS hospital data from hospitals located in non-Metropolitan Statistical Areas (MSAs). In the CY 2021 HH PPS final rule ( 85 FR 70298 ), we finalized a policy to continue to treat Micropolitan Areas as “rural” and to include Micropolitan Areas in the calculation of each state's rural wage index.
The OMB “2020 Standards” continue to define a “Micropolitan Statistical Area” as a CBSA with at least one urban area that has a population of at least Start Printed Page 55364 10,000, but less than 50,000. The Micropolitan Statistical Area comprises the central county or counties containing the core, plus adjacent outlying counties having a high degree of social and economic integration with the central county, or counties as measured through commuting ( 86 FR 37778 ). Overall, there are the same number of Micropolitan Areas (542) under the new OMB delineations based on the 2020 Census as there were using the 2010 Census. We note, however, that a number of urban counties have switched status and have joined or become Micropolitan Areas, and some counties that once were part of a Micropolitan Area, and thus were treated as rural, have become urban based on the 2020 Decennial Census data. We believe that the best course of action would be to continue our established policy and include Micropolitan Areas in each state's rural wage index as these areas continue to be defined as having relatively small urban cores (populations of 10,000 to 49,999). Therefore, in conjunction with our proposal to implement the new OMB labor market delineations beginning in CY 2025, and consistent with the treatment of Micropolitan Areas under the IPPS, we are also proposing to continue to treat Micropolitan Areas as “rural” and to include Micropolitan Areas in the calculation of each state's rural wage index.
In a June 6, 2022, Notice ( 87 FR 34235-34240 ), the Census Bureau announced that it was implementing the State of Connecticut's request to replace the eight counties in the State with nine new “Planning Regions.” Planning regions are included in OMB Bulletin No. 23-01 and now serve as county-equivalents within the CBSA system. We have evaluated the change and are proposing to adopt the planning regions as county equivalents for wage index purposes. We believe it is necessary to adopt this migration from counties to planning region county-equivalents in order to maintain consistency with our established policy of adopting the most recent OMB updates. We are providing the following crosswalk in table 26 for counties located in Connecticut with the current and proposed Federal Information Processing Series (FIPS) county and county-equivalent codes and CBSA assignments.

Under the revised OMB statistical area delineations (based upon OMB Bulletin No. 23-01), a total of 53 counties (and county equivalents) that are currently considered urban would be considered rural beginning in CY 2025. Table 27 lists the 53 counties that would become rural if we adopt as final our proposal to implement the revised OMB delineations.

Under the revised OMB statistical area delineations (based upon OMB Bulletin No. 23-01), a total of 54 counties (and county equivalents) that are currently located in rural areas would be considered located in urban areas under the revised OMB delineations beginning in CY 2025. Table 28 lists the 54 counties that would be urban if we adopt as final our proposal to implement the revised OMB delineations.

In addition to rural counties becoming urban and urban counties becoming rural, several urban counties would shift from one urban CBSA to a new or existing urban CBSA under our proposal to adopt the revised OMB delineations. In other cases, applying the new OMB delineations would involve a change only in CBSA name or number, while the CBSA would continue to encompass the same constituent counties. For example, CBSA 35154 (New Brunswick-Lakewood, NJ) would experience both a change to its number and its name and become CBSA 29484 (Lakewood-New Brunswick, NJ), while all three of its constituent counties would remain the same. In other cases, only the name of the CBSA would be modified. Table 29 lists CBSAs that would change in name and/or CBSA number only, but the Start Printed Page 55368 constituent counties would not change (except in instances where an urban county became rural or a rural county became urban, as discussed in the previous section).

In some cases, all urban counties from a CY 2024 CBSA would be moved and subsumed by another CBSA in CY 2025. Table 30 lists the CBSAs that, under our proposal to adopt the revised OMB statistical area delineations, would be subsumed by another CBSA.

In other cases, if we adopt the new OMB delineations, some counties would shift between existing and new CBSAs, changing the constituent makeup of the CBSAs. In another type of change, some CBSAs have counties that would split off to become part of, or to form entirely new labor market areas. For example, the District of Columbia, DC, Charles County, MD and Prince Georges County, MD would move from CBSA 47894 (Washington-Arlington-Alexandria, DC-VA-MD-WV) into CBSA 47764 (Washington, DC-MD). Calvert County, MD would move from CBSA 47894 (Washington-Arlington-Alexandria, DC-VA-MD-WV) into CBSA 30500 (Lexington Park, MD). The remaining counties that currently make up 47894 (Washington-Arlington-Alexandria, DC-VA-MD-WV) would move into CBSA 11694 (Arlington-Alexandria-Reston, VA-WV). Finally, in some cases, a CBSA would lose counties to another existing CBSA if we adopt the new OMB delineations. For example, Grainger County, TN would move from CBSA 34100 (Morristown, TN) into CBSA 28940 (Knoxville, TN). Table 31 lists the 73 urban counties that would move from one urban CBSA to a new or modified urban CBSA if we adopt the revised OMB delineations.

In the past we have provided for transition periods when adopting changes that have significant payment implications, particularly large negative impacts, in order to mitigate the potential impacts of proposed home health policies. For example, we have proposed and finalized budget-neutral transition policies to help mitigate negative impacts on HHAs following the adoption of the new CBSA delineations based on the 2010 Decennial Census data in the CY 2015 HH PPS final rule ( 79 FR 66032 ). Specifically, we implemented a 1-year 50/50 blended wage to the new OMB delineations. We applied a blended wage index for 1 year (CY 2015) for all geographic areas that would consist of a 50/50 blend of the wage index values using OMB's old area delineations and the wage index values using OMB's new area delineations. That is, for each county, a blended wage index was calculated equal to 50 percent of the CY 2015 wage index using the old labor market area delineation and 50 percent of the CY 2015 wage index using the new labor market area delineation, which resulted in an average of the two values. Additionally, in the CY 2021 HH PPS final rule ( 85 FR 70312 ), we proposed and finalized a transition policy to apply a 5-percent cap on any decrease in a geographic area's wage index value from the wage index value from the prior CY. This transition allowed the effects of our adoption of the revised CBSA delineations from OMB Bulletin 18-04 to be phased in over 2 years, where the estimated reduction in a geographic area's wage index was capped at five percent in CY 2021 (that is, no cap was applied to the reduction in the wage index for the second year (CY 2022)). We explained that we believed a 5-percent cap on the overall decrease in a geographic area's wage index value would be appropriate for CY 2021, as it provided predictability in payment levels from CY 2020 to CY 2021 and additional transparency because it was administratively simpler than our prior one-year 50/50 blended wage index approach.
In the CY 2023 HH PPS final rule ( 87 FR 66851 through 66853 ), we adopted a permanent 5-percent cap on wage index decreases beginning in CY 2023 and each subsequent year. The policy applies a permanent 5-percent cap on any decrease to a geographic area's wage index from its wage index in the prior year, regardless of the circumstances causing the decline, so that a geographic area's wage index would not be less than 95 percent of its wage index calculated in the prior CY.
For CY 2025, we believe that the permanent 5-percent cap on wage index decreases would be sufficient to mitigate any potential negative impact caused by adopting the revised OMB delineations and that no further transition is necessary. Previously, the 5-percent cap had been applied at the CBSA or statewide rural area level, meaning that all the counties that make up the CBSA or rural area received the 5-percent cap. However, for CY 2025, to mitigate any potential negative impact caused by the adoption of the revised delineations, we propose that in addition to the 5-percent cap being calculated for an entire CBSA or statewide rural, the cap would also be calculated at the county level, so that individual counties moving to a new delineation would not experience more than a 5 percent decrease in wage index from the previous calendar year. Specifically, we are proposing for CY 2025, that the 5-percent cap would also be applied to counties that would move from a CBSA or statewide rural area with a higher wage index value into a new CBSA or rural area with a lower wage index value, so that the county's CY 2025 wage index would not be less than 95 percent of the county's CY 2024 wage index value under the old delineation despite moving into a new delineation with a lower wage index.
Due to the way that we propose to calculate the 5-percent cap for counties that experience an OMB designation change, some CBSAs and statewide rural areas could have more than one wage index value because of the potential for their constituent counties to have different wage index values. Specifically, some counties that change OMB designations would have a wage index value that is different than the wage index value assigned to the other constituent counties that make up the CBSA or statewide rural area that they are moving into because of the application of the 5-percent cap. However, for home health claims processing, each CBSA or statewide rural area can have only one wage index value assigned to that CBSA or statewide rural area.
Therefore, HHAs that serve beneficiaries in a county that would receive the cap would need to use a number other than the CBSA or statewide rural area number to identify the county's appropriate wage index value on home health claims in CY 2025. We are proposing that beginning in CY 2025, counties that have a different wage index value than the CBSA or rural area into which they are designated after the application of the 5-percent cap would use a wage index transition code. These special codes are five digits in length and begin with “50” and the remaining digits are unique for that code. We are using Xs to show how the transition codes could be labeled. The 50XXX [ 9 ] wage index transition codes would be used only in specific counties; counties located in CBSAs and rural areas that do not correspond to a different transition wage index value will still use the CBSA number. For example, FIPS county 13171 Lamar County, GA is currently part of CBSA 12060 Atlanta-Sandy Springs-Alpharetta. However, for CY 2025 we are proposing that Lamar County would be redesignated into the Rural Georgia Code 99911. Because the wage index value of rural Georgia is more than a 5-percent decrease from the wage index value that Lamar County previously received under CBSA 12060, the CY 2025 wage index for Lamar County would be capped at 95 percent of the CY 2024 wage index value for CBSA 12060. Additionally, because rural Georgia can only have one wage index value assigned to code 99911, in order for Lamar County to receive the capped wage index for CY 2025, transition code 50003 would be used on a home health claim instead of rural Georgia code 99911.
We are also proposing that the 5-percent cap would apply to a county that corresponds to a different wage index value than the wage index value in the CBSA or rural area in which they are designated due to a delineation change until the county's new wage index is more than 95 percent of the wage index from the previous calendar year. Therefore, in order to capture the correct wage index value, an HHA would continue to use the assigned 50XXX transition code for the county until the county's wage index value calculated for that calendar year using the new OMB delineations is not less than 95 percent of the county's capped wage index from the previous calendar year. Thus, in the example mentioned earlier, claims for Lamar County would use transition code 50003 until the wage index in its revised designation of Rural Georgia is equal to or more than 95 percent of its wage index value from the previous calendar year. The counties that will require a transition code and the corresponding 50XXX codes are shown in table 32 and will also be shown in the last column of the CY 2025 HH PPS wage index file.

The proposed wage index file applicable to CY 2025 provides a crosswalk between the CY 2025 wage index using the current OMB delineations and the CY 2025 wage index using the proposed revised OMB delineations that would be in effect in CY 2025 if these proposed changes are finalized. This file shows each state and county and its corresponding proposed wage index along with the previous CBSA number, the proposed CBSA number or proposed transition code, and the proposed CBSA name. The proposed HH PPS wage index file applicable for CY 2025 (January 1, 2025, through December 31, 2025) is available on the CMS website at: https://www.cms.gov/medicare/enrollment-renewal/providers-suppliers/home-health-agency-center .
Sections 1895(b)(4)(A)(ii) and (b)(4)(C) of the Act require the Secretary to provide appropriate adjustments to the proportion of the payment amount under the HH PPS that account for area wage differences, using adjustment factors that reflect the relative level of wages and wage-related costs applicable to the furnishing of home health services. Since the inception of the HH PPS, we have used inpatient hospital wage data in developing a wage index to be applied to home health payments. We propose to continue this practice for CY 2025, as it is our belief that, in the absence of home health-specific wage data that accounts for area differences, using inpatient hospital wage data, including any changes made by the Office of Management and Budget (OMB) to Metropolitan Statistical Area (MSA) definitions, is appropriate and reasonable for the HH PPS. The appropriate wage index value is applied to the labor portion of the HH PPS rates based on the site of service for the beneficiary (defined by section 1861(m) of the Act as the beneficiary's place of residence).
For CY 2025, we propose to base the HH PPS wage index on the FY 2025 hospital pre-floor, pre-reclassified wage index for hospital cost reporting periods beginning on or after October 1, 2020, and before October 1, 2021 (FY 2021 cost report data), with the revised OMB delineations. The proposed CY 2025 HH PPS wage index would not take into account any geographic reclassification of hospitals, including those in accordance with section 1886(d)(8)(B) or 1886(d)(10) of the Act but would include the 5-percent cap on wage index decreases.
There exist some geographic areas where there are no hospitals, and thus, no hospital wage data on which to base the calculation of the HH PPS wage index. To address those geographic areas in which there are no inpatient hospitals, and thus, no hospital wage data on which to base the calculation of the CY 2025 HH PPS wage index, we propose to continue to use the same Start Printed Page 55374 methodology discussed in the CY 2007 HH PPS final rule ( 71 FR 65884 ) to address those geographic areas in which there are no inpatient hospitals.
For urban areas without inpatient hospitals, we use the average wage index of all urban areas within the State as a reasonable proxy for the wage index for that CBSA. For CY 2025, the only urban area without inpatient hospital wage data is Hinesville, GA (CBSA 25980). Using the average wage index of all urban areas in Georgia as a proxy, we propose the CY 2025 wage index value for Hinesville, GA, would be 0.8608.
For rural areas that do not have inpatient hospitals, we propose to use the average wage index from all contiguous Core Based Statistical Areas (CBSAs) as a reasonable proxy. The term “contiguous” means sharing a border ( 72 FR 49859 ). For CY 2025, as part of our proposal to adopt the revised OMB delineations discussed further in section III.E.2. of this proposed rule, we are proposing that rural North Dakota would now become a rural area without a hospital from which hospital wage data can be derived. Therefore, in order to calculate the wage index for rural area 99935, North Dakota, we are proposing to use as a proxy, the average pre-floor, pre-reclassified hospital wage data from the contiguous CBSAs: CBSA 13900-Bismark, ND, CBSA 22020-Fargo, ND-MN, CBSA 24220-Grand Forks, ND-MN, and CBSA 33500, Minot, ND, which results in a proposed CY 2025 HH PPS wage index of 0.8334 for rural North Dakota.

Previously, the only rural area without a hospital from which hospital wage data could be derived was in Puerto Rico. However, for rural Puerto Rico, we did not apply this methodology due to the distinct economic circumstances that exist there (for example, due to the proximity of one another of almost all of Puerto Rico's various urban and non-urban areas, this methodology would produce a wage index for rural Puerto Rico that is higher than that in half of its urban areas). Instead, we used the most recent wage index previously available for that area, which was 0.4047. For CY 2025, due to our proposal to adopt the revised OMB delineations discussed previously, there is now a hospital in rural Puerto Rico from which hospital wage data can be derived. Therefore, we are proposing that the wage index for rural Puerto Rico would now be based on the hospital wage data for the area instead of the previously available wage index of 0.4047. The unadjusted CY 2025 proposed wage index for rural Puerto Rico would equal 0.2520. However, because 0.2520 is more than a 5 percent decline in the CY 2024 wage index, the 5-percent cap would be applied. We are proposing that the CY 2025 5-percent cap adjusted wage index for rural Puerto Rico would be set equal to 95 percent of the CY 2024 wage index, which results in a proposed wage index value of 0.3845.
Finally, due to the proposal to adopt the revised OMB delineations, Delaware, which was previously an all-urban state, would now have one rural area with a hospital from which hospital wage data can be derived. As such, the proposed CY 2025 wage index for rural Delaware would be 1.0429.
The complete proposed CY 2025 wage index is available on the CMS website at: https://www.cms.gov/Center/Provider-Type/Home-Health-Agency-HHA-Center .
The HH PPS has been in effect since October 1, 2000. As set forth in the July 3, 2000, final rule ( 65 FR 41128 ), the base unit of payment under the HH PPS was a national, standardized 60-day episode payment rate. As finalized in the CY 2019 HH PPS final rule with comment period ( 83 FR 56406 ), and as described in the CY 2020 HH PPS final rule with comment period ( 84 FR 60478 ), the unit of home health payment changed from a 60-day episode to a 30-day period effective for those 30-day periods beginning on or after January 1, 2020.
As set forth in § 484.220, we adjust the national, standardized prospective payment rates by a case-mix relative weight and a wage index value based on the site of service for the beneficiary. To provide appropriate adjustments to the proportion of the payment amount under the HH PPS to account for area wage differences, we apply the appropriate wage index value to the labor portion of the HH PPS rates. In the CY 2024 HH PPS final rule ( 88 FR 77676 ), we finalized the rebasing of the home health market basket to reflect 2021 Medicare cost report data. We also finalized that for CY 2024 and subsequent years the labor-related share would be 74.9 percent and the non-labor-related share would be 25.1 percent. The following are the steps we take to compute the case-mix and wage-adjusted 30-day period payment amount for CY 2025:
- Multiply the national, standardized 30-day period rate by the patient's applicable case-mix weight.
- Divide the case-mix adjusted amount into a labor (74.9 percent) and a non-labor portion (25.1 percent).
- Multiply the labor portion by the applicable wage index based on the site of service of the beneficiary.
- Add the wage-adjusted portion to the non-labor portion, yielding the case-mix and wage adjusted 30-day period payment amount, subject to any additional applicable adjustments. We provide annual updates of the HH PPS rate in accordance with section 1895(b)(3)(B) of the Act. Section 484.225 sets forth the specific annual percentage update methodology. In accordance with section 1895(b)(3)(B)(v) of the Act Start Printed Page 55375 and § 484.225(i), for an HHA that does not submit home health quality data, as specified by the Secretary, the unadjusted national prospective 30-day period rate is equal to the rate for the previous calendar year increased by the applicable home health payment update percentage, minus 2 percentage points. Any reduction of the percentage change would apply only to the calendar year involved and would not be considered in computing the prospective payment amount for a subsequent calendar year. The final claim that the HHA submits for payment determines the total payment amount for the period and whether we make an applicable adjustment to the 30-day case-mix and wage-adjusted payment amount. The end date of the 30-day period, as reported on the claim, determines which calendar year rates Medicare will use to pay the claim. We may adjust a 30-day case-mix and wage-adjusted payment based on the information submitted on the claim to reflect the following:
- A LUPA is provided on a per-visit basis as set forth in §§ 484.205(d)(1) and 484.230.
- A partial payment adjustment as set forth in §§ 484.205(d)(2) and 484.235.
- An outlier payment as set forth in §§ 484.205(d)(3) and 484.240.
Section 1895(b)(3)(A)(i) of the Act requires that the standard prospective payment rate and other applicable amounts be standardized in a manner that eliminates the effects of variations in relative case-mix and area wage adjustments among different home health agencies in a budget-neutral manner. To determine the CY 2025 national, standardized 30-day period payment rate, we will continue our practice of using the most recent, complete utilization data at the time of rulemaking; that is, we are using CY 2023 claims data for CY 2025 payment rate updates. We apply a permanent adjustment factor, a case-mix weights recalibration budget neutrality factor, a wage index budget neutrality factor, and the home health payment update percentage to update the CY 2025 payment rate. As discussed in section II.C.1. of this proposed rule, we are proposing to implement a permanent adjustment of −4.067 percent to ensure that payments under the PDGM do not exceed what payments would have been under the 153-group payment system as required by law. The proposed permanent adjustment factor is 0.95933. As discussed previously, to ensure the changes to the PDGM case-mix weights are implemented in a budget neutral manner, we apply a case-mix weight budget neutrality factor to the CY 2025 national, standardized 30-day period payment rate. The proposed case-mix weight budget neutrality factor for CY 2025 is 1.0035.
Additionally, we apply a wage index budget neutrality factor to ensure that wage index updates and revisions are implemented in a budget neutral manner. To calculate the wage index budget neutrality factor, we first determine the payment rate needed for non-LUPA 30-day periods using the CY 2025 wage index (with the proposed revised delineations and the 5-percent cap) so those total payments are equivalent to the total payments for non-LUPA 30-day periods using the CY 2024 wage index (with the old delineations and the 5-percent cap) and the CY 2024 national standardized 30-day period payment rate adjusted by the case-mix weights recalibration neutrality factor. Then, by dividing the payment rate for non-LUPA 30-day periods using the CY 2025 wage index (with the proposed revised delineations and a 5-percent cap on wage index decreases) by the payment rate for non-LUPA 30-day periods using the CY 2024 wage index (with the old delineations and a 5-percent cap on wage index decreases), we obtain a wage index budget neutrality factor of 0.9985. We then apply the wage index budget neutrality factor of 0.9985 to the 30-day period payment rate.
Next, we propose to update the 30-day period payment rate by the proposed CY 2025 home health payment update percentage of 2.5 percent. The CY 2025 national standardized 30-day period payment rate is calculated in table 34.

The CY 2025 national standardized 30-day period payment rate for an HHA that does not submit the required quality data is updated by the proposed CY 2025 home health payment update percentage of 0.5 percent (2.5 percent minus 2 percentage points) and is shown in table 35.

The national per-visit rates are used to pay LUPAs and are also used to compute imputed costs in outlier calculations. The per-visit rates are paid by type of visit or home health discipline. The six home health disciplines are as follows:
- Home health aide (HH aide).
- Medical Social Services (MSS).
- Occupational therapy (OT).
- Physical therapy (PT).
- Skilled nursing (SN).
- Speech-language pathology (SLP).
To calculate the proposed CY 2025 national per-visit rates, we started with the CY 2024 national per-visit rates. Then we applied a wage index budget neutrality factor to ensure budget neutrality for LUPA per-visit payments. We calculated the wage index budget neutrality factor by simulating total payments for LUPA 30-day periods of care using the CY 2025 wage index with the new delineations and the 5-percent cap on wage index decreases and comparing it to simulated total payments for LUPA 30-day periods of care using the CY 2024 wage index with the old delineations and the 5-percent cap. By dividing the total payments for LUPA 30-day periods of care using the CY 2025 wage index by the total payments for LUPA 30-day periods of care using the CY 2024 wage index, we obtained a wage index budget neutrality factor of 0.9991. We apply the wage index budget neutrality factor in order to calculate the CY 2025 national per-visit rates.
The LUPA per-visit rates are not calculated using case-mix weights. Therefore, no case-mix weight budget neutrality factor is needed to ensure budget neutrality for LUPA payments. Additionally, we are not applying the permanent adjustment to the per visit payment rates but only to the case-mix adjusted 30-day payment rate. Lastly, the per-visit rates for each discipline are updated by the proposed CY 2025 home health payment update percentage of 2.5 percent. The national per-visit rates are adjusted by the wage index based on the site of service of the beneficiary. The per-visit payments for LUPAs are separate from the LUPA add-on payment amount, which is paid for episodes that occur as the only episode or initial episode in a sequence of adjacent episodes. The CY 2025 national per-visit rates for HHAs that submit the required quality data are updated by the proposed CY 2025 home health payment update percentage of 2.5 percent and are shown in table 36.

The CY 2025 per-visit payment rates for HHAs that do not submit the required quality data are updated by the proposed CY 2025 home health payment update percentage of 2.5 percent minus 2 percentage points and are shown in table 37.

Prior to the implementation of the 30-day unit of payment, LUPA episodes were eligible for a LUPA add-on payment if the episode of care was the first or only episode in a sequence of adjacent episodes. As stated in the CY 2008 HH PPS final rule, the average visit lengths in these initial LUPAs are 16 to 18 percent higher than the average visit lengths in initial non-LUPA episodes ( 72 FR 49848 ). LUPA episodes that occur as the only episode or as an initial episode in a sequence of adjacent episodes are adjusted by applying an additional amount to the LUPA payment before adjusting for area wage differences.
In the CY 2014 HH PPS final rule ( 78 FR 72305 ), we changed the methodology for calculating the LUPA add-on amount, whereby we finalized the approach of multiplying the per-visit payment amount for the first skilled nursing (SN), physical therapy (PT), or speech language pathology (SLP) visit in LUPA episodes that occur as the only episode or an initial episode in a sequence of adjacent episodes by 1 + the proportional increase in minutes for an initial visit over non-initial visits. Specifically, we updated the analysis using 100 percent of LUPA episodes and a 20 percent sample of non-LUPA first episodes from CY 2012 claims data. The analysis showed that the average excess of minutes for the first visit in LUPA episodes that were the only episode or an initial LUPA in a sequence of adjacent episodes are 37.27 minutes for the first visit if SN, 31.69 minutes for the first visit if PT, and 31.56 minutes for the first visit if SLP. The average minutes for all non-first visits in non-LUPA episodes were 44.10 minutes for SN, 47.30 minutes for PT, and 50.37 minutes for SLP. To determine the final LUPA add-on factors for each discipline, we calculated the ratio of the average excess minutes for the first visits in LUPA claims to the average minutes for all non-first visits in non-LUPA claims. (Of note, the average excess minutes for the first visit in LUPA add-on claims equal, for each discipline, is equal to the average minutes for the first visit in LUPA add-on claims minus the average minutes for non-first visits in LUPA add-on claims.) We then added one to these ratios to obtain the respective finalized add on factors: 1.8451 for SN; 1.6700 for PT; and 1.6266 for SLP. In the CY 2019 HH PPS final rule with comment period ( 83 FR 56440 ), in addition to finalizing a 30-day unit of payment, we finalized our policy of continuing to multiply the per-visit payment amount for the first SN, PT, or SLP visit in LUPA periods that occur as the only period of care or the initial 30-day period of care in a sequence of adjacent 30-day periods of care by the appropriate add-on factor (using the already established LUPA add-on factors of 1.8451 for SN, 1.6700 for PT, and 1.6266 for SLP) to determine the LUPA add-on payment amount for 30-day periods of care under the PDGM.
At this time, in an effort to enhance the accuracy and relevance of LUPA add-on factors to reflect current healthcare practices and costs, CMS is proposing to update the LUPA add-on factors for PT, SN, and SLP, which have not been revised since the CY 2014 HH PPS final rule, during which CY 2012 data was used. For this proposed rule, we are proposing to use the same methodology used to establish the LUPA add-on amount for CY 2014, using updated claims data.
Specifically, we are proposing to update the LUPA add-on factors by using 100 percent of LUPA periods and a 100 percent sample of non-LUPA first periods from CY 2023 claims data. In doing so, the analysis demonstrates that the average excess of minutes for the first visit in LUPA periods that were the only period or an initial LUPA in a sequence of adjacent periods are 30.00 minutes for the first visit if SN, 28.18 minutes for the first visit if PT, and 31.59 minutes for the first visit if SLP. The average minutes for all non-first visits in non-LUPA episodes are 41.51 minutes for SN, 45.11 minutes for PT, and 47.13 minutes for SLP. The following table 38 shows the average excess minutes for the first visit in LUPA periods, the average minutes for all non-first visits in non-LUPA episodes, as well as the current LUPA add-on factors, the proposed LUPA add-on factors, and the percent change between the current and the proposed LUPA add-on factors. This table also shows the proposed OT LUPA add-on factor outlined in section II.4.e. of this proposed rule as follows:

To determine the LUPA add-on factors for each discipline in relation to the aforementioned proposed LUPA add-on factor updates, we calculate the ratio of the average excess minutes for the first visits in LUPA claims to the average minutes for all non-first visits in non-LUPA claims. We then add one to these ratios to obtain the proposed add on factors: 1.7227 for SN; 1.6247 for PT; and 1.6703 for SLP. As an example of the application of the proposed add-on factors for CY 2025, for LUPA periods that occur as the only episode or an initial period in a sequence of adjacent periods, if the first skilled visit is SN, the payment for that visit would be $297.03 (1.7227 multiplied by $172.42). The proposed LUPA add-on factors will be updated based on more complete CY 2023 claims data in the final rule. As such, we solicit comments on the proposals to update the LUPA factors using the 2014 methodology and based on these updated numbers, re-price the LUPA payment amounts.
In order to implement Division CC, section 115, of the Consolidation Appropriations Act (CAA), 2021, CMS finalized changes to regulations at § 484.55(a)(2) and (b)(3) that allowed occupational therapists to conduct initial and comprehensive assessments for all Medicare beneficiaries under the home health benefit when the plan of care does not initially include skilled nursing care, but included OT, as well as either PT or SLP ( 86 FR 62351 ). This change necessitated the establishment of a LUPA add-on factor for calculating the LUPA add-on payment amount for the first skilled OT visit in LUPA periods that occurs as the only period of care or the initial 30-day period of care in a sequence of adjacent 30-day periods of care. However, at the time of the implementation, as we stated in the CY 2022 HH PPS final rule ( 86 FR 62289 ), there was not sufficient data regarding the average excess of minutes for the first visit in LUPA periods when the initial and comprehensive assessments are conducted by occupational therapists. Therefore, we finalized that we would use the PT LUPA add-on factor of 1.6700 as a proxy. We also stated in the CY 2022 final rule that we would use the PT LUPA add-on factor as a proxy until we have CY 2022 data to establish a more accurate OT add-on factor for the LUPA add-on payment amounts ( 86 FR 62289 ). Ultimately, we refrained from using CY 2022 data (and instead utilized the PT LUPA add-on factor as a proxy for the OT LUPA add-on factor), as we marked the first year that occupational therapists were permitted to conduct the initial assessment. Therefore, we wanted to extend our analysis to ensure we had sufficient data to reflect OT time spent conducting initial assessments to establish a discrete OT LUPA add-on factor ( 86 FR 62240 ). Accordingly, we continued analyzing claims data and have opted to utilize CY 2023 data to make this proposal.
With sufficient recent claims data available, and to establish equitable compensation for all home health services, CMS is now proposing to establish a definitive OT-specific LUPA add-on factor and discontinue the temporary use of the PT LUPA add-on factor as a proxy. For this proposal, we are using the same methodology used to establish the LUPA add-on amount for CY 2014, as also described previously for the SN, PT and SLP add-on factors. Specifically, we are updating the analysis using 100 percent of LUPA periods and a 100 percent sample of non-LUPA first periods from CY 2023 claims data. The analysis shows that the average excess of minutes for the first OT visit in LUPA periods that were the only period or an initial LUPA in a sequence of adjacent periods is 33.40 minutes for the first visit. The average number of minutes for all non-first visits in non-LUPA periods is 45.97 minutes for OT.
To determine the LUPA add-on factors for OT to adequately adjust LUPA payments to account for the excess minutes during the first visit in a LUPA period, we are proposing to calculate the ratio of the average excess minutes for the first visits in LUPA claims to the average minutes for all non-first visits in non-LUPA claims. We are proposing to then add one to this ratio to obtain the proposed add on factor: 1.7266 for OT. As an example of the application of the proposed add-on factor, for LUPA periods that occur as the only period or as an initial period in a sequence of adjacent periods, if the first skilled visit is OT, the payment for that visit will be $327.62 (1.7266 multiplied by $189.75). Table 38 shows the current LUPA add-on factors and the proposed LUPA add-on factors. The proposed OT LUPA add-on factor will be updated based on more complete CY 2023 claims data in the final rule. As such, we solicit comments on the proposed use of OT data to determine the OT LUPA add-on factor, as well as the proposed methodology to determine this OT LUPA add-on factor.
Section 1895(b)(5) of the Act allows for the provision of an addition or adjustment to the home health payment amount otherwise made in the case of outliers because of unusual variations in the type or amount of medically necessary care. Under the HH PPS and the previous unit of payment (that is, 60-day episodes), outlier payments were made for 60-day episodes whose estimated costs exceed a threshold amount for each HHRG. The episode's estimated cost was established as the sum of the national wage-adjusted per visit payment amounts delivered during the episode. The outlier threshold for each case-mix group or PEP adjustment defined as the 60-day episode payment or PEP adjustment for that group plus a fixed-dollar loss (FDL) amount. For the purposes of the HH PPS, the FDL amount is calculated by multiplying the home health FDL ratio by a case's wage-adjusted national, standardized 60-day episode payment rate, which yields an FDL dollar amount for the case. The outlier threshold amount is the sum of Start Printed Page 55379 the wage and case-mix adjusted PPS episode amount and wage-adjusted FDL amount. The outlier payment is defined to be a proportion of the wage-adjusted estimated cost that surpasses the wage-adjusted threshold. The proportion of additional costs over the outlier threshold amount paid as outlier payments is referred to as the loss-sharing ratio.
As we noted in the CY 2011 HH PPS final rule ( 75 FR 70397 through 70399 ), section 3131(b)(1) of the Affordable Care Act amended section 1895(b)(3)(C) of the Act to require that the Secretary reduce the HH PPS payment rates such that aggregate HH PPS payments were reduced by 5 percent. In addition, section 3131(b)(2) of the Affordable Care Act amended section 1895(b)(5) of the Act by redesignating the existing language as section 1895(b)(5)(A) of the Act and revised the language to state that the total amount of the additional payments or payment adjustments for outlier episodes could not exceed 2.5 percent of the estimated total HH PPS payments for that year. Section 3131(b)(2)(C) of the Affordable Care Act also added section 1895(b)(5)(B) of the Act, which capped outlier payments as a percent of total payments for each HHA for each year at 10 percent.
As such, beginning in CY 2011, we reduced payment rates by 5 percent and targeted up to 2.5 percent of total estimated HH PPS payments to be paid as outliers. To do so, we first returned the 2.5 percent held for the target CY 2010 outlier pool to the national, standardized 60-day episode rates, the national per visit rates, the LUPA add-on payment amount, and the NRS conversion factor for CY 2010. We then reduced the rates by 5 percent as required by section 1895(b)(3)(C) of the Act, as amended by section 3131(b)(1) of the Affordable Care Act. For CY 2011 and subsequent calendar years we targeted up to 2.5 percent of estimated total payments to be paid as outlier payments, and apply a 10-percent agency-level outlier cap.
In the CY 2017 HH PPS proposed and final rules ( 81 FR 43737 through 43742 and 81 FR 76702 ), we described our concerns regarding patterns observed in home health outlier episodes. Specifically, we noted the methodology for calculating home health outlier payments may have created a financial incentive for providers to increase the number of visits during an episode of care in order to surpass the outlier threshold; and simultaneously created a disincentive for providers to treat medically complex beneficiaries who require fewer but longer visits. Given these concerns, in the CY 2017 HH PPS final rule ( 81 FR 76702 ), we finalized changes to the methodology used to calculate outlier payments, using a cost-per-unit approach rather than a cost-per-visit approach. This change in methodology allows for more accurate payment for outlier episodes, accounting for both the number of visits during an episode of care and the length of the visits provided. Using this approach, we now convert the national per-visit rates into per 15-minute unit rates. These per 15-minute unit rates are used to calculate the estimated cost of an episode to determine whether the claim will receive an outlier payment and the amount of payment for an episode of care. In conjunction with our finalized policy to change to a cost-per-unit approach to estimate episode costs and determine whether an outlier episode should receive outlier payments, in the CY 2017 HH PPS final rule we also finalized the implementation of a cap on the amount of time per day that would be counted toward the estimation of an episode's costs for outlier calculation purposes ( 81 FR 76725 ). Specifically, we limit the amount of time per day (summed across the six disciplines of care) to 8 hours (32 units) per day when estimating the cost of an episode for outlier calculation purposes.
In the CY 2017 HH PPS final rule ( 81 FR 76724 ), we stated that we did not plan to re-estimate the average minutes per visit by discipline every year. Additionally, the per unit rates used to estimate an episode's cost were updated by the home health update percentage each year, meaning we would start with the national per visit amounts for the same calendar year when calculating the cost-per-unit used to determine the cost of an episode of care ( 81 FR 76727 ). We will continue to monitor the visit length by discipline as more recent data becomes available and may propose to update the rates as needed in the future.
In the CY 2019 HH PPS final rule with comment period ( 83 FR 56521 ), we finalized a policy to maintain the current methodology for payment of high-cost outliers upon implementation of PDGM beginning in CY 2020 and calculated payment for high-cost outliers based upon 30-day period of care. Upon implementation of the PDGM and 30-day unit of payment, we finalized the FDL ratio of 0.56 for 30-day periods of care in CY 2020. Given that CY 2020 was the first year of the PDGM and the change to a 30-day unit of payment, we finalized maintaining the same FDL ratio of 0.56 in CY 2021 as we did not have sufficient CY 2020 data at the time of CY 2021 rulemaking to propose a change to the FDL ratio for CY 2021. In the CY 2022 HH PPS final rule with comment period ( 86 FR 62292 ), we estimated that outlier payments would be approximately 1.8 percent of total HH PPS final rule payments if we maintained an FDL of 0.56 in CY 2022. Therefore, in order to pay up to, but no more than, 2.5 percent of total payments as outlier payments we finalized an FDL of 0.40 for CY 2022. In the CY 2023 HH PPS final rule ( 87 FR 66875 ), using CY 2021 claims utilization data, we finalized an FDL of 0.35 in order to pay up to, but no more than, 2.5 percent of the total payment as outlier payments in CY 2023. In the CY 2024 HH PPS final rule ( 88 FR 77749 ), using CY 2022 claims utilization data, we finalized an FDL of 0.27 for CY 2024.
For a given level of outlier payments, there is a trade-off between the values selected for the FDL ratio and the loss-sharing ratio. A high FDL ratio reduces the number of periods that can receive outlier payments but makes it possible to select a higher loss-sharing ratio, and therefore, increase outlier payments for qualifying outlier periods. Alternatively, a lower FDL ratio means that more periods can qualify for outlier payments, but outlier payments per period must be lower.
The FDL ratio and the loss-sharing ratio are selected so that the estimated total outlier payments do not exceed the 2.5 percent aggregate level (as required by section 1895(b)(5)(A) of the Act). Historically, we have used a value of 0.80 for the loss-sharing ratio, which, we believe, preserves incentives for agencies to attempt to provide care efficiently for outlier cases. With a loss-sharing ratio of 0.80, Medicare pays 80 percent of the additional estimated costs that exceed the outlier threshold amount. Using CY 2023 claims data (as of March 19, 2024) and given the statutory requirement that total outlier payments do not exceed 2.5 percent of the total payments estimated to be made under the HH PPS, we are proposing an FDL ratio of 0.38 for CY 2025 which is higher than the finalized CY 2024 FDL of 0.27. CMS will update the FDL, if needed, in the final rule once we have more complete CY 2023 claims data.
Negative pressure wound therapy (NPWT) is a medical procedure in which a vacuum dressing is used to enhance and promote healing in acute, chronic, and burn wounds. The therapy Start Printed Page 55380 involves using a sealed wound dressing attached to a pump to create a negative pressure environment in the wound. The therapy can be administered using the conventional NPWT system, classified as durable medical equipment (DME), or can be administered using a disposable device. A disposable NPWT (dNPWT) device is a single-use integrated system that consists of a non-manual vacuum pump, a receptacle for collecting exudate, and wound dressings. Unlike conventional NPWT systems classified as DME, dNPWT devices have preset continuous negative pressure, no intermittent setting, are pocket-sized and easily transportable, and are generally battery-operated with disposable batteries. In order for a beneficiary to receive dNPWT under the home health benefit, the beneficiary must qualify for the home health benefit in accordance with existing eligibility requirements.
Prior to CY 2024, the separate payment amount for dNPWT included the furnishing of services as well as the dNPWT device. The separate payment amount was set equal to the amount of the payment that would be made under the Medicare Hospital Outpatient Prospective Payment System (OPPS) using the CPT codes 97607 and 97608. Payment for visits where the sole purpose of a home health visit was to furnish dNPWT was not made under the HH PPS. Therefore, visits performed solely for the purpose of furnishing a new dNPWT device were not reported on the HH PPS claim (TOB 32x), instead HHAs submitted these claims on a TOB 34x. However, if a home health visit included the provision of other home health services in addition to, and separate from, furnishing dNPWT, the HHA submitted both a TOB 32x and TOB 34x—the TOB 32x for other home health services and the TOB 34x for furnishing NPWT using a disposable device.
Beginning in CY 2024, Division FF, section 4136 of the CAA, 2023 ( Pub. L. 117-328 ) amended section 1834 of the Act ( 42 U.S.C. 1395m(s) ) and mandated several amendments to the Medicare separate payment for dNPWT. These changes included—
- For CY 2024, the separate payment amount for an applicable dNPWT device was set equal to the supply price used to determine the relative value for the service under the Physician Fee Schedule (PFS) under section 1848 as of January 1, 2022 (CY 2022), updated by the percent increase in the CPI-U for the 12-month period ending with June of the preceding year reduced by m the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act for such year;
- For 2025 and each subsequent year, the separate payment amount was to be set equal to the payment amount established for the device in the previous year, updated by the percent increase in the CPI-U for the 12-month period ending with June of the preceding year reduced by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) for such year.
- The separate payment amount for applicable devices furnished on or after January 1, 2024, would no longer include payment for nursing or therapy services described in section 1861(m) of the Act so that payment for such nursing or therapy services are now made under the HH PPS, and is no longer separately billable.
- Claims for the separate payment amount of an applicable dNPWT device are now accepted and processed on claims submitted using the type of bill (TOB) 32X.
In the CY 2024 HH PPS final rule ( 88 FR 77676 ), we finalized our proposal to codify these changes to dNPWT payments mandated by the CAA, 2023. Beginning January 1, 2024, the separate payment for a dNPWT device is made to an HHA for an individual who is under a home health plan of care using Healthcare Common Procedure Coding System (HCPCS) code A9272. The code HCPCS A9272 is defined as a wound suction, disposable, includes dressing, all accessories and components, any type, each. The HHA reports the HCPCS code A9272 for the device only on the home health TOB 32X. The services related to the application of the device are included in the home health payment and are excluded from the separate payment amount for the device. The CY 2024 single payment amount for a dNPWT device for individuals under a home health plan of care was set equal to $270.09, which equaled the supply price of an applicable device under the Medicare PFS (as of January 1, 2022) of $263.25 updated by the 2.6 percent increase in the CPI-U for the 12-month period ending in June of 2023, minus the productivity adjustment.
For CY 2025, we are proposing that the separate payment amount for a dNPWT device would be set equal to the CY 2024 payment amount of $270.09 updated by the CPI-U for June 2024, minus the productivity adjustment, as mandated by the CAA, 2023. The application of the productivity adjustment may result in a net update that may be less than 0.0 for a year and may result in the separate payment amount for an applicable device for a year being less than such separate payment amount for such device for the preceding year. We note that the CPI-U for the 12-month period ending in June of 2024 is not available at the time of this proposed rulemaking. Therefore, the CY 2025 payment amount, as well as the CPI-U for the 12-month period ending in June of 2024, and the corresponding productivity adjustment will be updated in the final rule.
For CY 2026 and subsequent years, if CMS does not intend to propose changes to its established methodology for calculating dNPWT payments, payment rates will be updated using CMS's established methodology via the Home Health Prospective Payment System Rate Update Change Request and posted on the HHA Center website at https://www.cms.gov/medicare/enrollment-renewal/providers-suppliers/home-health-agency-center . For more in-depth information regarding the finalized policies associated with the scope of the payment for dNPWT and conditions for payment, we refer readers to the CY 2024 HH PPS final rule ( 88 FR 77749 through 77752 ).
The HH QRP is authorized by section 1895(b)(3)(B)(v) of the Act. Section 1895(b)(3)(B)(v)(II) of the Act requires that, for 2007 and subsequent years, each home health agency (HHA) submit to the Secretary in a form and manner, and at a time, specified by the Secretary, such data that the Secretary determines are appropriate for the measurement of health care quality. To the extent that an HHA does not submit data in accordance with this clause, the Secretary shall reduce the home health market basket percentage increase applicable to the HHA for such year by 2 percentage points. As provided at section 1895(b)(3)(B)(vi) of the Act, depending on the market basket percentage increase applicable for a particular year, as further reduced by the productivity adjustment (except in 2018 and 2020) described in section 1886(b)(3)(B)(xi)(II) of the Act, the reduction of that increase by 2 percentage points for failure to comply with the requirements of the HH QRP may result in the home health market basket percentage increase being less than 0.0 percent for a year, and may result in payment rates under the Home Start Printed Page 55381 Health PPS for a year being less than payment rates for the preceding year. Section 1890A of the Act requires that the Secretary establish and follow a pre-rulemaking process, in coordination with the consensus-based entity (CBE) with a contract under section 1890 of the Act, to solicit input from certain groups regarding the selection of quality and efficiency measures for the HH QRP. The HH QRP regulations can be found at 42 CFR 484.245 and 484.250 .
Based on feedback from patients and stakeholders, CMS has launched an effort to update and shorten the Home Health Consumer Assessment of Healthcare Providers and Systems (HHCAHPS) survey. In 2023 CMS tested a shortened survey across a variety of different types of HHAs. We are reviewing the findings of the field test and plan to propose in the future updates to the survey with the intent to shorten it.
In this proposed rule, we are proposing to add four new items and to modify one assessment item on the OASIS. Second, we propose an update to the removal of the suspension of OASIS all-payer data collection. Third, we are seeking information on future HH QRP quality measure concepts. These proposals are further specified in the following sections.
For a detailed discussion of the considerations, we historically use for measure selection for the HH QRP quality, resource use, and other measures, we refer readers to the CY 2016 HH PPS final rule ( 80 FR 68695 through 68696 ). In the CY 2019 HH PPS final rule with comment period ( 83 FR 56548 through 56550 ) we finalized the factors we consider for removing previously adopted HH QRP measures.
The HH QRP currently includes 21 measures for the CY 2024 program year, as described in table 39.

In this proposed rule, we are proposing to add four new items [ 10 ] to be collected as standardized patient assessment data elements under the social determinants of health (SDOH) category HH QRP: Living Situation (one item); Food (two items); and Utilities (one item). We are also proposing to modify one of the current items collected as standardized patient assessment data under the SDOH category (the Transportation item) as described in section III.D.5. of this proposed rule.
Section 1895(b)(3)(B)(v) of the Act requires that for CY 2007 and subsequent years, HHAs submit quality data to the Secretary. Section 1899B(a)(1)(C) of the Act requires, in part, the Secretary to modify the post-acute care (PAC) assessment instruments for PAC providers, including HHAs, to submit standardized patient assessment data under the Medicare program. Section 1899B(b)(1)(A) of the Act requires PAC providers to submit standardized patient assessment data under applicable reporting provisions (which, for HHAs, is the HH QRP) for the admission (start and resumption of care) and discharge of an individual (and more frequently as the Secretary deems appropriate). Section1899B(b)(1)(B) of the Act defines standardized patient assessment data as data required for at least the quality measures described in section 1899B(c)(1) of the Act and that is concerning the following categories: (1) functional status, such as mobility and self-care at admission to a PAC provider and before discharge from a PAC provider; (2) cognitive function, such as ability to express ideas and to understand, and mental status, such as depression and dementia; (3) special services, treatments, and interventions, such as need for ventilator use, dialysis, chemotherapy, central line placement, and total parenteral nutrition; (4) medical conditions and comorbidities, such as diabetes, congestive heart failure, and pressure ulcers; (5) impairments, such as incontinence and an impaired ability to hear, see, or swallow, and (6) other categories deemed necessary and appropriate by the Secretary.
Section 1899B(b)(1)(B)(vi) of the Act authorizes the Secretary to collect standardized patient assessment data elements with respect to other categories deemed necessary and appropriate. Accordingly, we finalized the creation of the SDOH category of standardized patient assessment data elements in the CY 2020 HH PPS final rule ( 84 FR 60597 through 60608 ). SDOH are the socioeconomic, cultural, and environmental circumstances in which individuals live that impact their health. [ 11 ] According to the World Health Organization research shows that the SDOH can be more important than health care or lifestyle choices in influencing health, accounting for between 30-55% of health outcomes. [ 12 ] This is a part of a growing body of research that highlights the importance of SDOH on health outcomes. Subsequent to the CY 2020 HH PPS final rule, we expanded our definition of SDOH: SDOH are the conditions in the environments where people are born, live, learn, work, play, worship and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks. [ 13 14 15 ] This expanded definition aligns our definition of SDOH with the definition used by HHS agencies, including OASH, the Centers for Disease Control and Prevention (CDC) and the White House Office of Science and Technology Policy. [ 16 17 ] We currently collect seven items in this SDOH category of standardized patient assessment data elements: ethnicity, race, preferred language, interpreter services, health literacy, transportation, and social isolation ( 84 FR 60597 through 60608 ). [ 18 ] In accordance with our authority under section 1899B(b)(1)(B)(vi) of the Act, we similarly finalized the creation of the SDOH category of standardized patient assessment data elements for skilled nursing facilities (SNFs) in the FY 2020 SNF PPS final rule ( 84 FR 38805 through 38817 ), for Inpatient Rehabilitation Facilities (IRFs) in the FY 2020 IRF PPS final rule ( 84 FR 39149 through 39161 ), and for Long Term Acute Hospitals (LTCHs) in the FY 2020 LTCH PPS final rule ( 84 FR 42577 through 42579 ). We also collect the same seven SDOH items in these PAC providers' respective patient/resident assessment instruments ( 84 FR 38817 , 39161 , and 42577 , respectively).
Adding access to standardized data relating to SDOH on a national level permits us to conduct periodic analyses, and to assess their appropriateness as risk adjustors or in future quality measures. Our ability to perform these analyses and to make adjustments relies on existing data collection of SDOH items from PAC settings. We adopted these SDOH items using common standards and definitions across the four PAC providers to promote interoperable exchange of longitudinal information among these PAC providers, including HHAs, and other providers. We believe this information may facilitate coordinated care, improve patient focused care planning, and allow for continuity of the discharge planning process from PAC settings.
We noted in our CY 2020 HH PPS final rule that each of the items was identified in the 2016 National Academies of Sciences, Engineering, and Medicine (NASEM) report as impacting care use, cost, and outcomes for Medicare beneficiaries ( 84 FR 60598 through 60602 ). At that time, we acknowledged that other items may also be useful to understand. The SDOH items we are proposing to collect as standardized patient assessment data elements under the SDOH category in this proposed rule were also identified Start Printed Page 55384 in the 2016 NASEM report [ 19 ] or the 2020 NASEM report [ 20 ] as impacting care use, cost and outcomes for Medicare beneficiaries. These items have the potential to affect treatment preferences and goals of patients and their caregivers. Identification of the SDOH items may also help HHAs be in a position to offer assistance, by connecting patients and their caregivers with these associated needs to social support programs, as well as inform our understanding of patient complexity.
Health-related social needs (HRSNs) are the resulting effects of SDOH, which are individual-level, adverse social conditions that negatively impact a person's health or health care. [ 21 ] Examples of HRSN include lack of access to food, housing, or transportation, and these have been associated with poorer health outcomes, greater use of emergency departments and hospitals, and higher health care costs. [ 22 23 ] Certain HRSNs can lead to unmet social needs that directly influence an individual's physical, psychosocial, and functional status. [ 24 ] This is particularly true for food security, housing stability, utilities security, and access to transportation. [ 25 ] Evidence supports the positive impact on health outcomes of interventions aimed at addressing HRSNs. [ 26 ]
We are proposing to require HHAs collect and submit four new items in the OASIS as standardized patient assessment data elements under the SDOH category because these items would collect information not already captured by the current SDOH items. Specifically, we believe the ongoing identification of SDOH would have three significant benefits. First, promoting screening for SDOH could serve as evidence-based building blocks for supporting healthcare providers in actualizing their commitment to address disparities that disproportionately impact underserved communities. Second, screening for SDOH advances health equity through identifying potential social needs so the HHA may address those with the patient, their caregivers, and community partners during the home health episode and discharge planning process, if indicated. [ 27 ] Third, these SDOH items would support ongoing HH QRP initiatives by providing data with which to stratify HHAs' performance on current and future quality measures to improve care quality across different populations.
Additional collection of SDOH items would permit us to continue developing the statistical tools necessary to maximize the value of Medicare data and improve the quality of care for all beneficiaries. For example, we recently developed and released the Health Equity Confidential Feedback Reports, which provided data to HHAs on whether differences in quality measure outcomes are present for their patients by dual-enrollment status and race and ethnicity. [ 28 ] We note that advancing health equity by addressing the health disparities that underlie the country's health system is one of our strategic pillars [ 29 ] and a Biden-Harris Administration priority. [ 30 ]
3. Proposal to Collect Four New Items as Standardized Patient Assessment Data Elements Beginning January 1, 2027, for the CY 2027 HH QRP Program Year [ 31 ]
We are proposing to require HHAs collect four new items as standardized patient assessment data elements under the SDOH category using the OASIS: one item for living situation, as described in III.D.3.a. of this proposed rule; two items for food, as described in section III.D.3.b. of this proposed rule; and one item for utilities, as described in section III.D.3.c of this proposed rule.
We selected the proposed SDOH items from the Accountable Health Communities (AHC) HRSN Screening Tool developed for the AHC Model. The AHC HRSN Screening Tool is a universal, comprehensive screening for HRSNs that was developed by a technical expert panel (TEP) in July 2016 to discuss opportunities and challenges involved in screening for HRSNs, consider and pare down CMS' list of evidence-based screening questions, and recommend a short list of questions for inclusion in the final tool. [ 32 33 ] The TEP agreed to prioritize the inclusion of five SDOH domains as follows: (1) housing instability (for example, homelessness, poor housing quality); (2) food insecurity; (3) transportation difficulties; (4) utility assistance needs; and (5) interpersonal safety concerns (for example, intimate- Start Printed Page 55385 partner violence, elder abuse, child maltreatment). [ 34 ]
We believe that requiring HHAs to report new items that are currently included in the AHC HRSN Screening Tools would further standardize the screening of SDOH across patient assessment instruments and the various quality reporting programs. For example, our proposal would align, in part, with the requirements of the Hospital Inpatient Quality Reporting (IQR) Program and the Inpatient Psychiatric Facility Quality Reporting (IPFQR) Program. As of January 2024, hospitals are required to report whether they have screened patients for the standardized SDOH categories of housing stability, food security, and access to transportation to meet the Hospital IQR Program requirements. [ 35 ] Beginning January 2025, IPFs will also be required to report whether they have screened patients for the same set of SDOH categories. [ 36 ] As we continue to standardize data collection across PAC settings, we believe using common standards and definitions for new items is important to ensure the interoperable exchange of longitudinal information between HHAs and other providers to facilitate coordinated care, continuity in care planning, and the discharge planning process.
In the following section we describe each of the four proposed items in more detail.
Healthy People 2030 prioritizes economic stability as a key SDOH, of which housing stability is a component. [ 37 38 ] Lack of housing stability encompasses several challenges, such as having trouble paying rent, overcrowding, moving frequently, or spending the bulk of household income on housing. [ 39 ] These experiences may negatively affect physical health and make it harder to access health care. Lack of housing stability can also lead to homelessness, which is housing deprivation in its most severe form. [ 40 ] On a single night in 2023, roughly 653,100 people, or 20 out of every 10,000 people in the United States, were experiencing homelessness.” [ 41 ] Rates of chronic disease and premature mortality are higher among the unsheltered homeless relative to the sheltered. [ 42 ] Older adults (aged 65 years and older) have lower rates of experiencing any housing instability compared to younger people (8.8% versus 18.7%), but low-income older adults may be more at risk for housing instability if they lack the resources necessary to secure and/or maintain structurally sound housing. [ 43 ] Adults (aged 18-64 years) with disabilities experience challenges to securing stable housing including affordability and accessibility. [ 44 ] We believe that HHAs can use information obtained from the Living Situation assessment item during a patient's initial assessment as well as in discharge planning. For example, HH social workers can work with patients experiencing housing instability to ensure patients are referred to available community resources, such as supportive housing programs. HHAs could work in partnership with community care hubs and community-based organizations to establish new care transition workflows, including referral pathways, contracting mechanisms, data sharing strategies, and implementation training that can track both health and social needs outcomes to ensure unmet needs, such as housing, are successfully addressed through closed loop referrals and follow-up. [ 45 ] HHAs could also take action to help alleviate a patient's other related costs of living, like food, by referring patients to community-based organizations that would allow patients' additional resources to be allocated towards housing without sacrificing other needs. [ 46 ] Finally, HHAs could use the information obtained from the Living Situation assessment item to better coordinate with other PAC facilities and agencies during transitions of care, so that referrals to address a patient's housing stability are not lost during vulnerable transition periods.
Due to the potential negative impacts housing instability can have on a patient's health, we are proposing to adopt the Living Situation assessment item as a new standardized patient assessment data element under the SDOH category. This Living Situation assessment item is currently collected in the AHC HRSN Screening Tool [ 47 48 ] and was adapted from the Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences (PRAPARE) tool. [ 49 ] The proposed Living Situation item asks, “What is your living situation today?” The proposed response options are: (1) I have a steady place to live; (2) I have a place to live today, but I am worried about losing it in the future; (3) I do not have a steady place to live; (4) Patient unable to respond; and (5) Patient declines to respond. A draft of the proposed Living Situation item can be found in the Downloads section of the HH QRP Quality Measures web page at https:// Start Printed Page 55386 www.cms.gov/medicare/quality/home-health/home-health-quality-measures .
The U.S. Department of Agriculture (USDA), Economic Research Service defines a lack of food security as a household-level economic and social condition of limited or uncertain access to adequate food. [ 50 ] Adults who are food insecure may be at an increased risk for a variety of negative health outcomes and health disparities. For example, a study found that food-insecure adults may be at an increased risk for obesity. [ 51 ] Nutrition security is also an important component that builds on and complements long standing efforts to advance food security. The USDA defines nutrition security as “consistent and equitable access to healthy, safe, affordable foods essential to optimal health and well-being.” [ 52 ] While having enough food is one of many predictors for health outcomes, a diet low in nutritious foods is also a factor. [ 53 ] Studies have shown that older adults struggling with food security consume fewer calories and nutrients and have lower overall dietary quality than those who are food secure, which can put them at nutritional risk. Older adults are also at a higher risk of developing malnutrition, which is considered a state of deficit, excess, or imbalance in protein, energy, or other nutrients that adversely impacts an individual's own body form, function, and clinical outcomes. About 50% of older adults are affected by malnutrition, which is further aggravated by a lack of food security and poverty. [ 54 ] We believe that adopting items to collect and analyze information about a patient's food security at home could provide additional insight into their health complexity and help facilitate coordination with other healthcare providers, facilities, and agencies during transitions of care, so that referrals to address a patient's food security are not lost during vulnerable transition periods. For example, an HHA's registered nurse (RN) or other clinically qualified nutrition professional could work with the patient to plan healthy, affordable food choices prior to discharge. [ 55 ] HHAs could also refer any patient that indicates lack of food security to government initiatives such as home delivered meals programs provided by Area Agencies on Aging, [ 56 ] the Supplemental Nutrition Assistance Program (SNAP), and food pharmacies (programs to increase access to healthful foods by making them affordable), initiatives that have been associated with lower health care costs and reduced hospitalization and emergency department visits. [ 57 ]
We are proposing to adopt two new food-related standardized patient assessment data elements under the SDOH category. These proposed items are based on the Food data elements currently collected in the AHC Screening Tool and were adapted from the U.S. Department of Agriculture 18-item Household Food Security Survey (HFSS). [ 58 ] The first proposed Food item states, “Within the past 12 months, you worried that your food would run out before you got money to buy more.” The second proposed Food item states, “Within the past 12 months, the food you bought just didn't last and you didn't have money to get more.” We propose the same response options for both items: (1) Often true; (2) Sometimes true; (3) Never True; (4) Patient declines to respond; and (5) Patient unable to respond. A draft of the proposed Food items to be adopted as standardized patient assessment data elements under the SDOH category can be found in the Downloads section of the HH QRP Quality Measures web page at https://www.cms.gov/medicare/quality/home-health/home-health-quality-measures .
A lack of energy (utility) security can be defined as an inability to adequately meet basic household energy needs. [ 59 ] According to the Department of Energy, one in three households in the U.S. are unable to adequately meet basic household energy needs. [ 60 ] The median energy burden for rural households of older adults is considerably higher than that for households without older adults. [ 61 ] The consequences associated with a lack of utility security are represented by three primary dimensions: economic, physical, and behavioral. Patients with low incomes are disproportionately affected by high energy costs, and they may be forced to prioritize paying for housing and food over utilities. Among older adults, food insecurity and high energy costs together are prevalent. [ 62 ] Some patients with low incomes may face limited housing options and be at increased risk of living in lower-quality physical conditions with malfunctioning heating and cooling systems, poor lighting, and outdated plumbing and electrical systems. Finally, patients with a lack of utility security may use concerning behavioral approaches to cope, such as using stoves and space heaters for heat. [ 63 ] In addition, data from the Department of Energy's U.S. Energy Information Administration confirm that a lack of energy security disproportionately affects certain populations, such as low-income and African American households. [ 64 ] The effects of a lack of utility security include vulnerability to environmental exposures such as dampness, mold, and thermal discomfort in the home, which Start Printed Page 55387 have direct effect on patients' health. [ 65 ] For example, research has shown associations between a lack of energy security and respiratory conditions as well as mental health-related disparities and poor sleep quality in vulnerable populations such as the elderly, children, the socioeconomically disadvantaged, and the medically vulnerable. [ 66 ] We believe adopting an item to collect information about a patient's utility security upon start or resumption of care in HHAs would facilitate the identification of patients who may not have utility security and who may benefit from engagement efforts. For example, HHAs could use the information on utility security to help connect identified patients in need, such as older adults, to programs that can help pay for home energy (heating/cooling) costs, like the Low-Income Home Energy Assistance Program (LIHEAP) [ 67 ] or receive broadband internet service through the Affordable Connectivity Program. [ 68 ] HHAs can also partner with community care hubs and community-based organizations to assist patients in applying for these and other local utility assistance programs, as well as helping them navigate the enrollment process. [ 69 ]
We are proposing to adopt a new item, Utilities, as a new standardized patient assessment data element under the SDOH category. This proposed item is based on the Utilities item currently collected in the AHC HRSN Screening Tool and was adapted from the Children's Sentinel Nutrition Assessment Program (C-SNAP) survey. [ 70 ] The proposed Utilities item asks, “In the past 12 months, has the electric, gas, oil, or water company threatened to shut off services in your home?” The proposed response options are: (1) Yes; (2) No; (3) Already shut off; (4) Patient unable to respond; and (5) Patient declines to respond. A draft of the proposed Utilities item to be adopted as a standardized patient assessment data element under the SDOH category can be found in the downloads section of the HH QRP Quality Measures web page at https://www.cms.gov/medicare/quality/home-health/home-health-quality-measures .
We developed our proposal after considering the feedback we received when we proposed the creation of the SDOH category of standardized patient assessment data elements in the CY 2020 HH PPS proposed rule ( 84 FR 34677 through 34684 ). Commenters were generally in favor of the concept of collecting SDOH data elements and stated that if implemented appropriately the data could be useful in identifying and addressing health care disparities, as well as refining the risk adjustment of outcome measures. We incorporated this input into the development of this proposal.
We invite comment on the proposal to adopt four new items as standardized patient assessment data elements under the SDOH category beginning with the CY 2027 HH QRP: one living situation item; two food items; and one utilities item.
Beginning January 1, 2023, HHAs began collecting seven standardized patient assessment data elements under the SDOH category on the OASIS Version E. One of these items, A1250. Transportation collects data on whether a lack of transportation has kept a patient from getting to and from medical appointments, meetings, work, or from getting things they need for daily living. This item was adopted as a standardized patient assessment data element under the SDOH category in the CY 2020 HH PPS final rule ( 84 FR 60478 ). As we discussed in the CY 2020 HH PPS final rule, we continue to believe that access to transportation for ongoing health care and medication access needs, particularly for those with chronic diseases, is essential to successful chronic disease management and the collection of a Transportation item would facilitate the connection to programs that can address identified needs.
As part of our routine item and measure monitoring work, we continue to assess the implementation of the new SDOH items. We have identified an opportunity to improve the data collection for A1250. Transportation by aligning it with the Transportation category collected in our other programs. Specifically, we are proposing to modify the current Transportation item so that it aligns with a Transportation item collected on the AHC HRSN Screening Tool available to the IPFQR and IQR Programs. A1250. Transportation currently collected in the OASIS asks, “Has lack of transportation kept you from medical appointments, meetings, work, or from getting things needed for daily living?” The response options are: (A) Yes, it has kept me from medical appointments or from getting any medications; (B) Yes, it has kept me from non-medical meetings, appointments, work, or from getting things that I need; (C) No; (X) Patient unable to respond; and (Y) Patient declines to respond. The Transportation item collected in the AHC HRSN Screening Tool asks, “In the past 12 months, has lack of reliable transportation kept you from medical appointments, meetings, work or from getting things needed for daily living?” The two response options are: (1) Yes; and (2) No. Consistent with the AHC HRSN Screening Tool, we are proposing to modify the A1250. Transportation item currently collected in the OASIS in two ways: (1) revise the look-back period for when the patient experienced lack of reliable transportation; and (2) simplify the response options.
While the current Transportation assessment item uses a look-back period of six to 12 months, we believe the distinction of a 12-month lookback period will reduce ambiguity for both patients and clinicians, and therefore improve the validity of the data collected. Second, we are proposing to simplify the response options. Currently, HHAs separately collect information on whether a lack of reliable transportation has kept the patient from medical appointments or from getting medications, and whether a lack of transportation has kept the patient from non-medical meetings, appointments, work, or from getting things they need. Although transportation barriers can directly affect a person's ability to attend medical appointments and obtain medications, a lack of transportation can also affect a person's health in other Start Printed Page 55388 ways, including accessing goods and services, obtaining adequate food and clothing, and social activities. [ 71 ] The proposed modified Transportation item would collect information on whether a lack of reliable transportation has kept the patient from medical appointments, meetings, work or from getting things needed for daily living, rather than collecting the information separately. As discussed previously, we believe reliable transportation services are fundamental to a person's overall health, and as a result, the burden of collecting this information separately outweighs its potential benefit.
For the reasons stated, we are proposing to modify A1250. Transportation based on the Transportation item adopted for use in the AHC HRSN Screening Tool and adapted from the PRAPARE tool. The proposed Transportation item asks, “In the past 12 months, has a lack of reliable transportation kept you from medical appointments, meetings, work or from getting things needed for daily living?” The proposed response options are: (0) Yes; (1) No; (7) Patient declines to respond; and (8) Patient unable to respond. A draft of the proposed Transportation item to be adopted as a standardized patient assessment data element under the SDOH category can be found on the HH QRP Quality Measures web page at https://www.cms.gov/medicare/quality/home-health/home-health-quality-measures/downloads .
We invite comment on this proposal to modify the current Transportation item previously adopted as a standardized patient assessment data element under the SDOH category beginning January 1, 2027, with the CY 2027 HH QRP.
In the CY 2023 HH PPS final rule CMS finalized the end of the temporary suspension of OASIS data collection on non-Medicare/non-Medicaid HHA patients and the requirement for HHAs to submit all-payer OASIS data for purposes of the HH QRP, beginning with the CY 2027 Program Year ( 87 FR 66862 through 66865 ). Consistent with the two-quarter phase-in that we typically use when changing data submission items or requirements, HHAs will have an opportunity to begin submitting this data for patients discharged between January 1, 2025, through June 30, 2025, but we will not use that phase-in data to make a compliance determination. We noted that the new all-payer OASIS data reporting will be required beginning with the CY 2027 program year, with data for that program year required for patients discharged between July 1, 2025, and June 30, 2026. For HHAs to operationalize this requirement, CMS determined that further details would be needed to clarify OASIS data collection and submission for non-Medicare/non-Medicaid patients. The CY23 final rule referenced discharge as the time point to identify when all-payer data collection would start but did not address the other data collection time points.
To clarify expectations around the start of OASIS all-payer data collection we are proposing to establish a change from data collection beginning with the OASIS discharge time point to using the start of care (SOC) time point. The SOC is the first assessment that can be submitted for a non-Medicare/non-Medicaid patient, either on or after January 1, 2025, for the phase-in (voluntary) period or on or after July 1, 2025, for the mandatory period. We will use the M0090 Date Assessment Completed date of the SOC assessment to identify non-Medicare/non-Medicaid patient assessments in the phase-in and mandatory periods.
Using the SOC time point ensures agency demographics (for example, Agency's CMS Certification Number (CCN), State and Branch ID#s) and patient demographics (for example, patient name, State, zip code, Social Security number (SSN), gender, date of birth (DOB), payment source) are collected for each non-Medicare/non-Medicaid patient assessment at the start of all-payer OASIS data collection. After these are collected and submitted with the SOC assessment, they are resubmitted with each subsequent OASIS submission (that is, ROC, recert, other follow up, transfer, discharge, death at home). Using the SOC time point would ensure that baseline data is available for use in calculating or risk-adjusting quality measures, and in linking to prior OASIS assessments. The data would also be available for matching purposes to support use of the current quality assessments only (QAO) metric used in the annual payment update (APU) calculation.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 ( Pub. L. 108-173 ; December 8, 2003) finalized the temporary suspension of OASIS requirements for collection of data on non-Medicare/non-Medicaid patients. [ 72 ] The CY 2023 HH PPS final rule ends this temporary suspension of OASIS data collection for non-Medicare/non-Medicaid patients.
CMS is providing a voluntary phase-in period for HHAs to begin OASIS data collection and submission for all non-Medicare/non-Medicaid patients.
- Prior to January 1, 2025—Per the HH CoPs and OASIS guidance, HHAs are required to collect and submit OASIS assessments for all skilled Medicare and/or Medicaid patients, with some exemptions. OASIS assessment time points include start of care, resumption of care, recertification, other follow-up, transfer, discharge, and death at home. The criteria for patients exempt from OASIS data collection are not changing and will continue to include patients under 18, patients receiving maternity services, and patients receiving only personal care, housekeeping or chore services.
- January 1, 2025, through June 30, 2025—For non-Medicare/non-Medicaid patients who are not exempt from OASIS data collection, and who begin receiving home health care services with an OASIS SOC M0090 date from January 1, 2025, through June 30, 2025, OASIS data collection and submission are voluntary. When OASIS data collection and submission are started for a non-Medicare/non-Medicaid patient with the SOC OASIS assessment, HHAs may but are not required to complete all subsequent OASIS time point assessments related to the patient's home health stay (that is, resumption of care, recertification, other follow up, transfer, discharge, and death at home) including assessments completed on or after July 1, 2025.
- Beginning July 1, 2025—For patients with any pay source who are not exempt from OASIS data collection, and who begin receiving home health care services with an OASIS SOC M0090 date on or after July 1, 2025, OASIS data collection and submission to the internet Quality Improvement Evaluation System (iQIES) are required. This includes the SOC OASIS as well as any subsequent OASIS time point assessments relevant to the patient's home health stay (that is, resumption of care, recertification, other follow up, transfer, discharge, and death at home).
We invite comment on this proposal to update requirements for OASIS all-payer data collection beginning January 1, 2025. Start Printed Page 55389
We refer readers to the regulatory text at § 484.45 for information regarding the current policies for reporting HH QRP data.
As discussed in section III.D.3. of this proposed rule, we are proposing to adopt four new items as standardized patient assessment data elements in the SDOH category: one living situation item, two food items, and one utilities item, and to modify the transportation item in section III.D.5. of this proposed rule beginning January 1, 2027, with the CY 2027 HH QRP.
We are proposing that HHAs would be required to report these new assessment items using the OASIS beginning with patients admitted on January 1, 2027, for purposes of the CY 2027 HH QRP program year. Starting in CY 2027, HHAs would be required to submit data for the entire calendar year, corresponding to the CY 2028 HH QRP program year with respect to OASIS submission requirements.
We are also proposing that HHAs that submit the living situation, food, utilities, and transportation items with respect to start or resumption of care would be deemed to have submitted those assessment items with respect to both start or resumption of care and discharge, because it is unlikely that the assessment of those items at start or resumption of care will differ from the assessment of the same item at discharge. A draft of the proposed assessment items is available in the Downloads section of the HH QRP Quality Measures web page at https://www.cms.gov/medicare/quality/home-health/home-health-quality-measures . As we noted in section III.D.5 of this proposed rule, we continue to assess the implementation of the new items in the SDOH category, including A1250. Transportation, as part of our routine assessment item and measure monitoring work. We analyzed the data home health agencies reported from January 1, 2023, through September 30, 2023 (Q1 2023-Q3 2023) and found that home health patient responses do not significantly change from admission to discharge. Specifically, the proportion of patients who responded “Yes” to the A1250 transportation item at start of care or resumption of care (8.87 percent) versus at discharge to community (5.71 percent) differed by only 3.16 percentage points during this period. We find these results convincing, and therefore are proposing to require HHAs to submit the proposed item, transportation, at the start and resumption of care only.
We invite public comment on our proposal to collect data on the following items in the SDOH category start or resumption of care beginning January 1, 2027 with the CY 2027 HH QRP program year: one Living Situation item as described in section III.D.3.a of this proposed rule; two Food items, as described in section III.D.3.b of this proposed rule; one Utilities item as described in section III.D.3.c of this proposed rule; and one Transportation item as described in section III.D.5 of this proposed rule.
We are seeking input on the importance, relevance, appropriateness, and applicability of each of the following concepts under consideration for future years in the HH QRP: vaccinations, depression, pain management, and substance use disorders. In the CY 2024 HH PPS proposed rule ( 88FR 43738 through 43740 ), we published a request for information (RFI) on a set of principles for selecting and prioritizing HH QRP measures, identifying measurement gaps, and suitable measures for filling these gaps. Within this proposed rule, we also sought input on data available to develop measures, approaches for data collection, perceived challenges or barriers, and approaches for addressing identified challenges. We refer readers to the CY 2024 HH PPS final rule ( 88 FR 77772 through 77774 ) for a summary of the public comments we received in response to the RFI.
Subsequently, our measure development contractor convened a TEP on December 15, 2023 to obtain input on the future measure concepts that could fill the measurement gaps identified in our CY 2024 RFI. [ 73 ] The TEP discussed the alignment of PAC and Hospice measures with CMS' “Universal Foundation” of quality measures. [ 74 ] The Universal Foundation aims to focus provider attention, reduce burden, identify disparities in care, prioritize development of interoperable, digital quality measures, allow for comparisons across programs, and help identify measurement gaps.
In consideration of the feedback, we received from interested parties through these activities, we are seeking input on four concepts for the HH QRP. One is a composite of vaccinations, [ 75 ] which could represent overall immunization status of patients such as the Adult Immunization Status measure [ 76 ] in the Universal Foundation. A second concept on which we are seeking feedback is the concept of depression for the HH QRP, similar to the Clinical Screening for Depression and Follow-up measure [ 77 ] in the Universal Foundation. Third, we are seeking feedback on the concept of pain management. Finally, we seek input on a measure concept relating to substance use disorders, such as the Initiation and Engagement of Substance Use Disorder Treatment measure [ 78 ] included in the Universal Foundation of Quality Measures.
While we will not be responding to specific comments in response to this RFI in the CY 2025 HH PPS final rule, we invite public comment on these four measure concepts and intend to use this input to inform our future measure development efforts.
As authorized by section 1115A of the Act and finalized in the CY 2016 HH PPS final rule ( 80 FR 68624 ), the Center for Medicare and Medicaid Innovation (Innovation Center) implemented the Home Health Value-Based Purchasing (HHVBP) Model (“original Model”) in nine states on January 1, 2016. The design of the original HHVBP Model leveraged the successes and lessons learned from other CMS value-based purchasing programs and demonstrations to shift from volume-based payments to a model designed to Start Printed Page 55390 promote the delivery of higher quality care to Medicare beneficiaries. The specific goals of the original HHVBP Model were to—
- Provide higher incentives for better quality care with greater efficiency;
- Study new potential quality and efficiency measures for appropriateness in the home health setting; and
- Enhance the current public reporting process.
The original HHVBP Model resulted in an average 4.6 percent improvement in HHAs' total performance scores (TPS) and an average annual savings of $141 million to Medicare without evidence of adverse risks. [ 79 ] The evaluation of the original Model also found reductions in unplanned acute care hospitalizations and skilled nursing facility (SNF) stays, resulting in reductions in inpatient and SNF spending. The U.S. Secretary of Health and Human Services determined that expansion of the original HHVBP Model would further reduce Medicare spending and improve the quality of care. In October 2020, the CMS Chief Actuary certified that expansion of the HHVBP Model would produce Medicare savings if expanded to all States. [ 80 ]
On January 8, 2021, CMS announced the certification of the HHVBP Model for expansion nationwide, as well as the intent to expand the Model through notice and comment rulemaking. [ 81 ]
In the CY 2022 HH PPS final rule ( 86 FR 62292 through 62336 ) we finalized the decision to expand the HHVBP Model to all Medicare certified HHAs in the 50 States, territories, and District of Columbia beginning January 1, 2022. CY 2022 was a pre-implementation year. The first payment year is CY 2025 based on the first performance year which was CY 2023. Our codified policies for the expanded HHVBP Model can be found in our regulations at 42 CFR part 484, subpart F , §§ 484.300 through 484.375.
The expanded HHVBP Model provides an opportunity to examine a broad array of quality measures that address critical gaps in care. A comprehensive review of the Value-Based Purchasing (VBP) experience, conducted by the Office of the Assistant Secretary for Planning and Evaluation (ASPE), identified several objectives for HHVBP measures. [ 82 ] The recommended objectives emphasize measuring patient outcomes and functional status; appropriateness of care; and incentives for providers to build infrastructure to facilitate measurement within the quality framework. The study identified the following seven objectives which served as guiding principles for the development of performance measures used in the original HHVBP Model:
- Use a broad measure set that captures the complexity of the HHA service provided.
- Incorporate the flexibility to include Improving Medicare Post-Acute Care Transformation (IMPACT) Act of 2014 measures that are cross-cutting amongst post-acute care settings.
- Develop second-generation measures of patient outcomes, health and functional status, shared decision making, and patient activation.
- Include a balance of process, outcome, and patient experience measures.
- Advance the ability to measure cost and value.
- Add measures for appropriateness or overuse.
- Promote infrastructure investments.
A central driver of the process used to select measures for the original HHVBP Model was incorporating innovative thinking from the field while simultaneously drawing on evidence-based literature and documented best practices. Broadly, measures were selected based on their impact on care delivery and to support the goal of improving health outcomes, quality, safety, efficiency, and experience of care for patients.
As we continue to leverage our value-based purchasing initiatives to improve the quality of care furnished across healthcare settings, we are interested in considering new performance measures for inclusion in the expanded HHVBP Model. We specifically request public comments on several specific performance measures as well as general comments on other future model concepts that may be considered for inclusion in the expanded HHVBP Model. These measures are based on input from the HHVBP Technical Expert Panel (TEP), which met in Fall 2023. The TEP included experts from the home health setting specializing in quality assurance, patient advocacy, clinical work, and measure development. The meeting included a discussion of potential measures for inclusion in the expanded HHVBP Model. These include a combination of new measure concepts (for example, family caregiver measure), already developed measures that are not currently in the measure set for the expanded HHVBP Model (for example, Medicare Spending per Beneficiary (MSPB)), and new OASIS-based and claims-based measures.
- Family caregiver measure: Generally, TEP members were very supportive of future development of a family caregiver measure. One TEP member encouraged CMS to “think outside the box” to find ways of including the caregiver's voice in quality reporting. The TEP discussed OASIS items that provide information related to the patient's caregiver status. While acknowledging that the focus of the Medicare home health benefit is the patient, not the caregiver, they recommended that CMS consider the caregiver as a partner and measure caregivers' needs and not just the needs as they relate to the beneficiary. The TEP noted that the caregivers are often the reason patients are even able to be at home (vs. receiving care in the more costly nursing home setting). CMS intends to develop a patient-reported outcome performance measure (PRO-PM) to assess caregiver burden in the Guiding an Improved Dementia Experience (GUIDE) Model that may be a useful example for caregiver measures that may be developed for HHVBP. [ 83 ] Creating one or more measures based on an HHA's ability to meet caregiver needs would permit measurement of changes in caregiver quality-of-life.
- Falls with injury (claims-based): Several TEP members suggested that CMS explore a claims-based measure of falls with injury. One TEP member noted an Office of Inspector General (OIG) study that found that HHAs failed to report 55 percent of falls leading to major injuries and hospitalizations on their OASIS data. [ 84 ] While it may not be possible to identify all falls from claims data, a claims-based measure may be more accurate, although, as with other claims-based measures, data would only be available for Fee for Service patients. Due to the high rate of non-reporting, the OASIS-based falls measure may not Start Printed Page 55391 provide accurate information about the incidence of these falls.
- Medicare spending per Beneficiary: The TEP also discussed potentially adding the MSPB measure to the HHVBP applicable measure set. This cross-setting measure is part of the Home Health Quality Reporting Program and is currently publicly reported on Care Compare. MSPB may be a valid tool for measuring the value of the care that HHAs provide that may be appropriate for use in the expanded HHVBP Model. The measure would provide information on the efficiency of home health providers, as measured by Medicare payments for their patients.
- Function measures to complement existing cross-setting Discharge (DC) Function measure: Several TEP members raised a concern that the measure does not include the full self-care/activities of daily living elements (for example, bathing, dressing), which they noted as critically important for home health patients and caregivers. Another TEP member indicated that patients often already have capacity to do things like roll and sit up when they enter home health care but may not be able to bathe or get dressed without assistance. The TEP emphasized the importance of functional cognition, which is included in OASIS item GG0100 as part of prior functional status but is not included as part of the current DC Function measure.
As we continue to explore refinements to the expanded HHVBP Model, we request comments related to adding the potential performance measures described previously to the HHVBP Measure Set. We also request comments about other potential performance measures that we should consider for the expanded HHVBP Model.
In alignment with the President's Executive orders [ 85 ] to support underserved communities, CMS is working to advance health equity by designing, implementing, and operationalizing policies and programs that support health for all the people served by our programs, eliminating avoidable differences in health outcomes experienced by people who are disadvantaged or underserved, and providing the care and support that our enrollees need to thrive. As we continue to leverage our value-based purchasing initiatives to improve the quality of care furnished across healthcare settings, we are interested in exploring the role of health equity in creating better health outcomes for all populations in our programs and models. In the CY 2023 HH PPS final rule, we stated that we are committed to achieving equity in health care outcomes for beneficiaries by supporting providers in quality improvement activities to reduce health disparities, enabling beneficiaries to make more informed decisions, and promoting provider accountability for health care disparities. [ 86 ]
The CY 2023 HH PPS rule ( 87 FR 66874 through 66876 ) included an RFI, “Future Approaches to Health Equity in the expanded HHVBP Model.” The RFI requested feedback on policy changes that we should consider on the topic of health equity and specific actions the expanded HHVBP Model should take to address healthcare disparities and advance health equity. We specifically requested comments on whether we should consider incorporating adjustments into the expanded HHVBP Model to reflect the varied patient populations that HHAs serve around the country and tie health equity outcomes to the payment adjustments we make based on HHA performance under the Model. One possible approach is to make adjustments at the measure level such as stratification by which additional points are provided to HHAs that provide care to underserved communities (for example, based on dual status or other metrics). [ 87 ] Payment adjustments could also be incorporated at the scoring level in forms such as modified benchmarks, points adjustments, or modified payment adjustment percentages (for example, peer comparison groups based on whether the HHA includes a high proportion of dual eligible beneficiaries). We requested commenters' views on which of these adjustments, if any, would be most effective for the expanded HHVBP Model. Commenters shared that relevant data collection and appropriate stratification are very important in addressing any health equity gaps. While not suggesting specific approaches, these commenters noted that CMS should consider potential stratification of health outcomes. Stakeholders, including providers, also shared their strategies for addressing health disparities, noting that this was an important commitment for many health provider organizations.
Several previous studies have found that historically underserved communities, including Medicare beneficiaries who are dually enrolled in Medicaid, live in a low-income neighborhood, or are Black, receive lower quality home health care relative to communities not historically underserved. [ 88 ] Previous studies have found that patients from underserved communities have higher rates of hospital readmissions, are more likely to be discharged without functional improvement, [ 89 ] are less likely to receive care from high-quality HHAs, and have worse patient-reported care experiences. Improving the quality of care for these underserved communities is an important quality improvement goal under the expanded HHVBP Model.
Disparities in health care outcomes may result from differences within HHAs (for example, patients from underserved communities within certain HHAs service areas are less likely to have good outcomes, such as functional improvement, discharge to community, and avoiding readmission to a hospital). These disparities may also result from differences across HHAs. That is, patients from underserved communities are less likely than other patients to receive care from good quality HHAs and thus at higher risk of poor outcomes. [ 90 ] The literature is mixed on the sources of these disparities. One study found that differences in readmission rates for underserved communities were primarily within, rather than across, HHAs. [ 91 ] Another study found that Start Printed Page 55392 differences both within and across HHAs contribute to the overall disparities in patients' functional improvement. [ 92 ] This same study found that roughly half of observed individual-level disparities in the use of high-quality home health agencies was attributable to neighborhood-level factors. [ 93 ] Differences in care experience for underserved communities were explained by differences both within and across HHAs, but the within-HHA variations more often accounted for a greater proportion of the differences. [ 94 ]
We have been exploring several potential approaches for integrating health equity concepts into the expanded HHVBP Model. Considerations for evaluating these approaches include the following:
- Effectiveness: Does the approach further the model test? What would its impact on underserved communities be?
- Feasibility: How long would it take to implement the approach? Are the necessary data currently being collected? How many HHAs would be included?
- Reliability: Does the approach allow for reliable measurement of health equity within HHAs?
- Alignment: Is this approach aligned with other Medicare quality and VBP Programs?
As part of our work developing potential equity measures, we are exploring potential definitions to use for defining historically underserved communities. Building on feedback from the CY 2024 SNF VBP proposals, our analyses have focused on three potential social risk factors Dual eligible status (DES), Area Deprivation Index (ADI), and Medicaid as sole payment source that can serve as a proxy to identify the underserved. Note that we also examined low-income subsidy (LIS) as a potential measure of equity but did not include it in further analyses, because the correlation for the Dual Eligible Status (DES) proportion and the LIS eligibility proportion is above 0.98. have not yet examined low-income subsidy (LIS) eligibility. We also plan to assess disparities between rural and urban home health providers and patients when analyzing social risk factors, perhaps measuring rurality using the rural-urban commuting area (RUCA) codes, which classify U.S. census tracts using measures of population density, urbanization, and daily commuting.
One of the approaches that we have explored is the Health Equity Adjustment (HEA) that will begin in the Skilled Nursing Facility (SNF) VBP starting with the FY 2027 program year. The HEA is calculated using a methodology that considers a SNF's performance on the SNF VBP quality measures and the proportion of the SNF's residents with DES. Under the HEA, SNFs that perform well on the SNF VBP quality measures and serve a higher proportion of residents with DES will earn HEA bonus points are added to normalized sum of all points a SNF is awarded for each measure. That sum is then the final SNF Performance Score. More information on the HEA can be found in the FY 2024 SNF PPS final rule ( 88 FR 53304 ).
We used the HEA methodology that was finalized for the SNF VBP to simulate how that methodology would impact the expanded HHVBP Model, using the current measure set for the Model and July 2023 Interim Performance Report (IPR) data. A limitation of using the July 2023 IPR data for these analyses is that the TPS for the July 2023 IPRs was mainly based on achievement points—there are no improvement points for the claims-based and HHCAHPS measures (due to lags in the data for these measures) and only small improvement points for the OASIS-based measures. This may distort results of the equity implications of the HEA methodology, but we believe that using the more current data is preferable to using earlier data from prior to the public health emergency. We used data on the proportion of HHA patients who are dually eligible at any point during the performance year. The HEA methodology is fully described in the FY 2024 Skilled Nursing Facility Prospective Payment System final rule ( 88 FR 53307 through 53316 ) that included—
- Determine number of measures for which HHA is a top tier performer;
- Calculate measure performance scaler;
- Calculate underserved multiplier;
- Calculate HEA Bonus Points; and
- Add HEA Bonus Points to the Normalized Sum of all Points Awarded for Each Measure.
Using the original TPS and a TPS measure that includes the HEA bonus points), we simulated payment adjustment amounts with and without the HEA. We examined the change in payment adjustment percentage for HHAs based on their dual eligibility status (for example, decile in terms of percentage of dual eligible patients) and HEA bonus points.
Of the 10,218 active HHAs in the July 2023 quarterly monitoring analytic file, 9,591 (93.9 percent) have information on the number of beneficiaries with dual eligible status (DES) that were served by the HHA in the performance year. Of these HHAs, a TPS was calculated for 7,556. Because the HEA operates by adding points to the TPS, it is only possible to calculate a TPS including the HEA for these 7,556 HHAs that had a valid TPS.
We found that the average TPS was higher for HHAs in the highest decile in terms of share of beneficiaries with DES than for HHAs in any other decile, before applying the HEA. Applying the HEA primarily increased TPS for these HHAs that were already high performing, which increased the gap in the average payment adjustment for these HHAs and the average payment adjustment for HHAs serving a lower share of beneficiaries with DES. As a result, we concluded that the HEA using DES as the proxy for the underserved, as designed for SNF VBP, may not the best approach for the home health setting. In contrast, the average TPS was higher for HHAs with a relatively low share of beneficiaries living in a neighborhood with a high ADI.
We also plan to consider how changes to the definition of the underserved population, as codified in the SNF VBP reg text at § 413.338(a) would alter the effects of the HEA. In contrast to the results for dual eligibility, we have found that average TPS was lower for HHAs serving a high share of beneficiaries living in a neighborhood with a high ADI. We also found that HHAs in the highest ADI quintile and highest DES quintile had lower average TPS than other groups. These results suggest that defining the underserved population using ADI or a combination of ADI and DES would alter the effects of the HEA. We are also examining measures of the underserved population that are based on the percentage of Start Printed Page 55393 patients with Medicaid as the only payment source.
We are also exploring other health equity measures that would more directly focus on certain disparities. These could be structured in several different ways:
- Measure(s) for particular underserved communities: Performance on one or more measures for specific underserved communities (for example, based on DES).
- Measure(s) based on within-provider differences in performance for underserved communities (for example, based on DES): This type of measure could be based on a single outcome or multiple outcomes (that is, a composite measure).
- Measure(s) based on the worst performing group: Calculate performance scores for multiple patient groups and set the measure performance equal to the score for the worst performing group.
We have examined the reportability of these other health equity measures and have found that several HHAs will not have a sufficient number of DES beneficiaries for these measures to be calculated. Our analyses of data used for the July 2023 IPRs found that, overall, 25.4 percent of HHAs served fewer than 12 beneficiaries with DES. This suggests that roughly one-fourth of HHAs may not serve enough beneficiaries with DES to calculate a performance measure using only beneficiaries with DES. The percentage of HHAs that served fewer than 12 beneficiaries with DES or fewer than 12 beneficiaries without DES was 36.5 percent. Although the reportability for these measures do exclude some smaller HHAs that serve fewer underserved patients, the reportability level will be closely aligned to the current SNF VBP HEA. As the 25.4 percent proportion that are not reported is not that much more than is currently being excluded on the SNF VBP HEA where SNFs in the bottom 20 percent of proportion duals are excluded. The impact or reportability of a potential HHVBP HEA needs more analysis for future consideration.
Looking forward, we recognize that the exact structure of the current SNF VBP HEA may not be the most efficient approach for the unique attributes of care being provided in the home versus care in the SNF. However, CMS is committed to and working towards the establishment of an HHVBP HEA that rewards HHAs that provide high quality care to underserved communities. We will continue to explore the addition of other measures, using other proxies for identifying the underserved and possibly adjusting the scoring mechanism to be more effective at addressing the issue.
As a reminder, we stated in the CY 2024 HH PPS final rule ( 88 FR 77790 ), we will gather at least 2 years of performance data, and study effects of the expanded Model on health equity outcomes before incorporating any potential changes to the expanded Model regarding health equity.
Division FF, section 4134 of the CAA, 2023 added coverage and payment of items and services related to administration of IVIG in a patient's home of a patient with a diagnosed primary immune deficiency disease furnished on or after January 1, 2024. Division FF, section 4134(a) of the CAA, 2023 amended the existing IVIG benefit category at section 1861(s)(2)(Z) of the Act by adding coverage for IVIG administration items and services in a patient's home of a patient with a diagnosed primary immune deficiency disease. This benefit covers items and services related to administration of IVIG in a patient's home of a patient with a diagnosed primary immune deficiency disease. In addition, section 4134(b) of Division FF of the CAA, 2023 amended section 1842(o) of the Act by adding a new paragraph (8) that established the payment for IVIG administration items and services. Under the CAA, 2023 provision, payment for these IVIG administration items and services is required to be a bundled payment separate from the payment for the IVIG product, made to a supplier for all items and services related to administration of IVIG furnished in the home during a calendar day.
Primary immune deficiency diseases (PIDD) are conditions triggered by genetic defects that cause a lack of and/or impairment in antibody function, resulting in the body's immune system not being able to function in a normal way. Immune globulin (Ig) therapy is used to temporarily replace some of the antibodies (that is, immunoglobulins) that are missing or not functioning properly in people with PIDD. [ 95 ] The goal of Ig therapy is to use Ig obtained from normal donor plasma to maintain a sufficient level of antibodies in the blood of individuals with PIDD to fight off bacteria and viruses. Ig is formulated for both intravenous and subcutaneous administration (SCIg). Clinicians can prescribe either product to the beneficiary with PIDD according to clinical need and preference, and beneficiaries can switch between intravenous and subcutaneous administration of Ig.
Section 642 of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (Pu Law 108-173) amended section 1861 of the Act to provide Medicare Part B coverage of the IVIG product for the treatment of PIDD in the home, but not the items and services involved with administration.
Section 101 of the Medicare IVIG Access and Strengthening Medicare and Repaying Taxpayers Act of 2012 (Medicare IVIG Access Act) ( Pub. L. 112-242 ) mandated the establishment, implementation, and evaluation of a 3-year Medicare Intravenous Immune Globulin (IVIG) Demonstration Project (the Demonstration) under Part B of title XVIII of the Act. The Demonstration was implemented to evaluate the benefits of providing coverage and payment for items and services needed for the home administration of IVIG for the treatment of PIDD, and to determine if it would improve access to home IVIG therapy for patients with PIDD. The Medicare IVIG Access Act mandated that Medicare would establish a per visit payment amount for the items and services necessary for the home administration of IVIG therapy for beneficiaries with specific PIDD diagnoses. The Demonstration did not include Medicare payment for the IVIG product which continues to be paid under Part B in accordance with sections 1842(o) and 1847(A) of the Act. The Demonstration covered and paid a per visit payment amount for the items and services needed for the administration of IVIG in the home. Items may include infusion set and tubing, and services include nursing services to complete an infusion of IVIG lasting on average three to five hours. [ 96 ]
On September 28, 2017, Congress passed the Disaster Tax Relief and Airport and Airway Extension Act of 2017 ( Pub. L. 115-63 ). Section 302 of Public Law 115-63 extended the Start Printed Page 55394 Demonstration through December 31, 2020.
Division CC, section 104, of the Consolidated Appropriations Act, 2021 (CAA, 2021) ( Pub. L. 116-260 ), further extended the Demonstration for another 3 years through December 31, 2023.
Division FF, section 4134 of the CAA, 2023 (CAA, 2023) ( Pub. L. 117-328 ) mandated that CMS establish permanent coverage and payment for items and services related to administration of IVIG in a patient's home of a patient with PIDD. The permanent home IVIG items and services payment is effective for home IVIG administration furnished on or after January 1, 2024. Payment for these items and services is required to be a separate bundled payment made to a supplier for all administration items and services furnished in the home during a calendar day. The statute provides that payment amount may be based on the amount established under the Demonstration. The standard Part B coinsurance and the Part B deductible is required to apply. In addition, that statute states that the separate bundled payment for these IVIG administration items and services does not apply for individuals receiving services under the Medicare home health benefit. The CAA, 2023 provision clarifies that a supplier who furnishes these services meet the requirements of a supplier of medical equipment and supplies.
Under the Demonstration, Medicare provided a bundled payment under Part B, that is separate from the IVIG product, for items and services that are necessary to administer IVIG in the home to enrolled beneficiaries who are not otherwise homebound and receiving services under the home health benefit. The Demonstration only applied to situations where the beneficiary required IVIG for the treatment of certain PIDD diagnoses or was receiving SCIg to treat PIDD and wished to switch to IVIG.
Services covered under the Demonstration were required to be provided and billed by specialty pharmacies, enrolled as durable medical equipment (DME) suppliers, that provided the Medicare Part B-covered Ig. The covered items and services under the Demonstration were paid as a single bundle and subject to coinsurance and deductible in the same manner as other Part B services. HHAs were not eligible to bill for services covered under the Demonstration but could bill for services related to the administration of IVIG if the patient was receiving services under a home health episode of care, in which case the home health payment covered the items and services.
In order to participate in the Demonstration, beneficiaries must have met the following requirements:
- Be eligible to have the IVIG paid for at home under Part B FFS.
- Have a diagnosis of PIDD.
- Not be enrolled in a Medicare Advantage plan.
- Cannot be in a home health episode of care on the date of service (in such circumstances, the home health payment covers the services).
- Must receive the service in their home or a setting that is “home like”.
To participate in the Demonstration, the beneficiary was required to submit an application, signed by their physician.
DME suppliers billing for the items and services covered under the Demonstration must have met the following requirements:
- Meet all Medicare, as well as other national, state, and local standards and regulations applicable to the provision of services related to home infusion of IVIG.
- Be enrolled and current with the National Supplier Clearinghouse.
- Be able to bill the DME Medicare Administrative Contractors (MACs).
CMS implemented a bundled per visit payment amount under the Demonstration, statutorily required to be based on the national per visit low-utilization payment adjustment (LUPA) for skilled nursing services used under the Medicare HH PPS established under section 1895 of the Act. The payment amount was subject to coinsurance and deductible.
For billing under the Demonstration, CMS established a “Q” code for services, supplies, and accessories used in the home:
- Q2052—(Long Description)—Services, supplies, and accessories used in the home under Medicare Intravenous immune globulin (IVIG) Demonstration.
- Q2052—(Short Description)—IVIG demo, services/supplies.
Suppliers billed Q2052 as a separate claim line on the same claim for the IVIG product.
As discussed previously, Division FF, section 4134 of the CAA, 2023, added coverage of items and services related to the administration of IVIG in a patient's home, to the existing IVIG benefit category at section 1861(s)(2)(Z) of the Act, effective January 1, 2024. IVIG is covered in the home under Part B if all the following criteria are met:
- It is an approved pooled plasma derivative for the treatment of primary immune deficiency disease.
- The patient has a diagnosis of primary immune deficiency disease.
- The IVIG is administered in the home.
- The treating practitioner has determined that administration of the IVIG in the patient's home is medically appropriate.
Therefore, as section 4134(a)(1) of the CAA, 2023, adds the items and services (furnished on or after January 1, 2024) related to the administration of IVIG to the benefit category defined under section 1861(s)(2)(Z) of the Act (the Social Security Act provision requiring coverage of the IVIG product in the home), the same beneficiary eligibility requirements for the IVIG product apply for the IVIG administration items and services. Subpart B of part 410 of the regulations set out the medical and other health services requirements under Part B. The regulations at § 410.10 identify the services that are subject to the conditions and limitations specified in this subpart. Section 410.10(y) includes intravenous immune globulin administered in the home for the treatment of primary immune deficiency diseases. Section 410.12 outlines general basic conditions and limitations for coverage of medical and other health services under Part B, as identified in § 410.10. Section 410.12(a) includes the conditions that must be met for these services to be covered, and include the following:
- When the services must be furnished. The services must be furnished while the individual is in a period of entitlement.
- By whom the services must be furnished. The services must be furnished by a facility or other entity as specified in §§ 410.14 through 410.69.
- Physician certification and recertification requirements. If the services are subject to physician certification requirements, they must be certified as being medically necessary, and as meeting other applicable requirements, in accordance with subpart B of part 424.
As the definition of IVIG at section 1861(zz) of the Act now includes the items and services necessary to administer IVIG in the home, in the CY 2024 HH PPS final rule ( 88 FR 77793 ), we finalized the amendment to the regulation at § 410.10(y) to add “items and services”. Furthermore, sub-regulatory guidance documents (that is, IVIG LCD (33610) [ 97 ] and IVIG Policy Start Printed Page 55395 Article (A52509) [ 98 ] ) provide direction on coding and coverage for the IVIG product at home. Through the Local Coverage Determination (LCD) for Intravenous Immune Globulin (L33610), [ 99 ] the Durable Medical Equipment Medicare administrative contractors (DME MACs) specify the Healthcare Common Procedure Coding System (HCPCS) codes for which IVIG derivatives are covered under this benefit. Therefore, a beneficiary must be receiving one of the IVIG derivatives specified under the LCD for IVIG to qualify to receive the items and services covered under section 1861(s)(2)(Z) of the Act. Furthermore, for any item (including IVIG) to be covered by Medicare, it must (1) be eligible for a defined Medicare benefit category, (2) be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member, and (3) meet all other applicable Medicare statutory and regulatory requirements. Policy guidance for the LCD for IVIG [ 100 ] identifies the ICD-10-CM codes that support medical necessity for the provision of IVIG in the home. These diagnosis codes are listed in table 40.

In accordance with this guidance, a beneficiary must be diagnosed with one of the primary immune deficiencies identified by the ICD-10-CM codes, set out in table 40 and as updated in subregulatory guidance, to qualify to receive the items and services covered under section 1861(s)(2)(Z) of the Act. This policy guidance is revised as needed by the DME MACs. And finally, to qualify to receive IVIG in the home, section 1861(zz) of the Act requires that a treating practitioner must have determined that administration of the IVIG in the patient's home is medically appropriate. Accordingly, we updated the sub-regulatory guidance pursuant to the CAA, 2023 to reflect the expansion of the benefit to the items and services related to the home administration of IVIG. Leveraging the existing regulations and sub-regulatory guidance maintains one set of standards across the entire IVIG benefit (that is, for the product and for the related items and services needed for home administration).
Section 101(c) of the Medicare IVIG Access Act established coverage for items and services needed for the in-home administration of IVIG for the treatment of primary immunodeficiencies under a Medicare demonstration program. In the CY 2024 HH PPS final rule, we stated that we interpreted section 4134 of the CAA, 2023 to make permanent coverage of the same items and services under the existing IVIG Demonstration to promote Start Printed Page 55396 continuous and comprehensive coverage for beneficiaries who choose to receive home IVIG therapy ( 88 FR 77794 ). Under the Demonstration, the bundled payment for the items and services necessary to administer the drug intravenously in the home included the infusion set and tubing, and nursing services to complete an infusion of IVIG lasting on average three to five hours. [ 101 ] Although “items and services” are not explicitly defined under section 4134 of the CAA, 2023, we stated in the CY 2024 HH PPS proposed rule ( 88 FR 43755 ) that we believed the items and services covered under the Demonstration are inherently the same items and services that would be covered under the payment added to the benefit category at section 1861(s)(2)(Z) of the Act. We also did not enumerate a list of services that must be included in the separate bundled payment; however, we stated that we anticipated the nursing services would include such professional services as IVIG administration, assessment and site care, and education ( 88 FR 43755 ). Moreover, we stated that it is up to the provider to determine the services and supplies that are appropriate and necessary to administer the IVIG for each individual, and this may or may not include the use of a pump. Because IVIG does not have to be administered through a pump (although it can be), external infusion pumps are not covered under the DME benefit for the administration of IVIG. An external infusion pump is only covered under the DME benefit if the infusion pump is necessary to safely administer the drug. The Local Coverage Determination (LCD) for External Infusion Pumps identify the drugs and biologicals that the DME Medicare Administrative Contractors (MACs) have determined require the use of such pumps and cannot be administered via a disposable elastomeric pump or the gravity drip method. [ 102 ] As such, under the IVIG Demonstration, coverage did not extend to the DME pump, and thereby, is not covered separately under the home IVIG items and services payment.
Prior to enactment of the CAA, 2023, IVIG administration items and services were explicitly excluded from coverage under the Part B IVIG benefit. However, if a beneficiary was considered homebound and qualified for the home health benefit, the items and services needed to administer IVIG in the home could be covered as home health services. Section 4134(b) of the CAA, 2023 excludes the IVIG items and services bundled payment in the case of an individual receiving home health services under section 1895 of the Act. Therefore, we clarified in the CY 2024 HH PPS final rule that a beneficiary does not have to be considered confined to the home (that is, homebound) in order to be eligible for the home IVIG benefit; however, homebound beneficiaries requiring items and services related to the administration of home IVIG, and who are receiving services under a home health plan of care, may continue to receive services related to the administration of home IVIG as covered home health services ( 88 FR 77794 ). We also clarified that the items and services related to the administration of IVIG in the home, and as identified on the home health plan of care, would be included in the payment for the 30-day home health period payment. HHAs must provide home health items and services included on the plan of care either directly or under arrangement and must bill and be paid under the HH PPS for such covered home health services. If an HHA is unable to furnish the items and services related to the administration of IVIG (as indicated in the plan of care) in the home, they are responsible for arranging these services (including arranging for services in an outpatient facility) and are required to bill these services as home health services under the HH PPS ( 88 FR 77795 ).
Regarding the home infusion therapy (HIT) services benefit, we reminded readers that Medicare payment for home infusion therapy services is for services furnished in coordination with the furnishing of intravenous and subcutaneous infusion drugs and biologicals specified on the DME LCD for External Infusion Pumps (L33794), [ 103 ] with the exception of insulin pump systems and certain drugs and biologicals on a self-administered drug exclusion list ( 88 FR 77794 ). For the drugs and biologicals to be covered under the Part B DME benefit they must require infusion through an external infusion pump. If the drug or biological can be infused through a disposable pump or by a gravity drip, it does not meet this criterion. IVIG does not require an external infusion pump for administration purposes and therefore, is explicitly excluded from the DME LCD for External Infusion Pumps. However, subcutaneous immunoglobulin (SCIg) is covered under the DME LCD for External Infusion Pumps, and items and services for administration of SCIg in the home are covered under the HIT services benefit. While a DME supplier and a HIT supplier (or a DME supplier also enrolled as a HIT supplier) could not furnish services related to the administration of immunoglobulin (either IVIG or SCIg) to the same beneficiary on the same day, a beneficiary could potentially receive services under both benefits for services related to the infusion of different drugs. For example, a DME supplier also accredited and enrolled as a HIT supplier, could furnish HIT services to a beneficiary receiving intravenous acyclovir as well as IVIG, and bill both the IVIG and the HIT services benefits on the same date of service. We also recognize that a beneficiary may, on occasion, switch from receiving immunoglobulin subcutaneously to intravenously and vice versa, and as such, utilize both the HIT services and the IVIG benefits within the same month.
Section 101 of the Medicare IVIG Access Act established the authority for a Demonstration providing payment for items and services needed for the in-home administration of IVIG. In the CY 2024 HH PPS final rule, we stated that we believed the provisions established under that law serve as the basis for the conditions for payment with respect to the requirements that must be met for Medicare payment to be made to suppliers for the items and services covered under section 1861(s)(2)(Z) of the Act and clarified that the relevant regulations and subregulatory guidance also apply.
Section 4134(b) of the CAA, 2023 amends section 1842(o) of the Act by adding a new paragraph (8) that establishes a separate bundled payment to the supplier for all items and services related to the administration of such intravenous immune globulin, described in section 1861(s)(2)(Z) of the Act to such individual in the patient's home during a calendar day. Section 4134(c) of the CAA, 2023 amends section Start Printed Page 55397 1834(j)(5) of the Act, which are a requirement for supplier of medical equipment and supplies, by adding a new subparagraph (E), clarifying with respect to payment, that items and services related to the administration of intravenous immune globulin furnished on or after January 1, 2024, as described in section 1861(zz) of the Act, are included in the definition of medical equipment and supplies. This means that suppliers that furnish IVIG administration items and services must meet the existing DMEPOS supplier requirement for payment purposes under this benefit. Suppliers of IVIG administration items and services must enroll as a DMEPOS supplier and comply with the Medicare program's DMEPOS supplier standards (found at 42 CFR 424.57(c) ) and DMEPOS quality standards to become accredited for furnishing medical equipment and supplies. Further, to receive payment for home IVIG items and services, the supplier must also meet the requirements under subpart A of part 424, Conditions for Medicare Payment. The DMEPOS supplier may subcontract with a provider to meet the professional services identified in section V.B.1. of this proposed rule. All professionals who furnish services directly, under an individual contract, or under arrangement with a DMEPOS supplier to furnish services related to the administration of IVIG in the home, must be legally authorized (licensed, certified, or registered) in accordance with applicable Federal, State, and local laws, and must act only within the scope of their State license or State certification, or registration. A supplier may not contract with any entity that is currently excluded from the Medicare program, any State health care programs or from any other Federal procurement or non-procurement programs.
Section 1861(s)(2)(Z) of the Act defines benefit coverage of intravenous immune globulin for the treatment of primary immune deficiency diseases in the home. Under the IVIG Demonstration, beneficiaries are eligible to participate if they receive IVIG services in “their home or a setting that is `home like' ”. [ 104 ] Section 410.12(b) identifies the supplier types who can furnish the services identified at § 410.10. Section 410.38 provides the conditions for payment for DME suppliers and identifies the institutions that may not qualify as the patient's home. As such, the home administration of IVIG items and services must be furnished in the patient's home, defined as a place of residence used as the home of an individual, including an institution that is used as a home. An institution that is used as a home may not be a hospital, critical access hospital (CAH), or SNF as defined in § 410.38(b).
Section 1842(o) of the Act provides the authority for the development of a separate bundled payment for Medicare-covered items and services related to the administration of intravenous immune globulin to an individual in the patient's home during a calendar day, in an amount that the Secretary determines to be appropriate. This section of the Act also states payment may be based on the payment established pursuant to section 101(d) of the Medicare IVIG Access Act. Section 4134(d) of the CAA, 2023, amends section 1833(a)(1) of the Act to provide that, with respect to items and services related to the administration of IVIG furnished on or after January 1, 2024, as described in section 1861(zz) of the Act, the amounts paid shall be the lesser of the 80 percent of the actual charge or the payment amount established under section 1842(o)(8) of the Act.
In accordance with section 101(d) of the Medicare IVIG Access Act, the Secretary established a per visit Demonstration payment amount for the items and services needed for the in-home administration of IVIG based on the national per visit low-utilization payment amount (LUPA) under the prospective payment system for home health services established under section 1895 of the Social Security Act. Under the Demonstration, the bundled payment amount for services needed for the home administration of IVIG included infusion services provided by a skilled nurse. Therefore, the bundled payment was based on the LUPA amount for skilled nursing, based on an average 4-hour infusion. The initial payment rate for the first year of the Demonstration, was based on the full skilled nursing LUPA for the first 90 minutes of the infusion and 50 percent of the LUPA for each hour thereafter for an additional 3 hours. Thereafter, the payment rate was annually updated based on the nursing LUPA rate for such year. The service was subject to coinsurance and deductibles similar to other Part B services.
We stated in the CY 2024 HH PPS proposed rule ( 88 FR 43755 ), we believed payment under section 1861(s)(2)(Z) of the Act covers the same items and services covered under the IVIG Demonstration. We also agreed that the professional services needed to safely administer IVIG in the home would be services furnished by a registered nurse ( 88 FR 43756 ). Therefore, we stated that setting the CY 2024 payment rate for the home IVIG items and services under section 1861(s)(2)(Z) of the Act, based on the CY 2023 payment amount established under the Demonstration was appropriate. However, we noted the Demonstration used the LUPA rate, which is annually adjusted by the wage index budget neutrality factor, as well as the home health payment rate update percentage, and stated that we believed it was appropriate to update the CY 2023 IVIG services Demonstration rate by only the CY 2024 home health payment rate update percentage. We stated that we would not include the wage index budget neutrality factor, as the IVIG items and services payment rate is not statutorily required to be geographically wage adjusted. Further, although section 1842(o) of the Act states that payment is for the items and services furnished to an individual in the patient's home during a calendar day, we stated that, as the statute aligns the payment amount with such amount determined under the Demonstration, we believed the best reading of “calendar day” is “per visit.” Additionally, we stated that we would expect a supplier to furnish only one visit per calendar day ( 88 FR 43756 ).
In the CY 2024 HH PPS final rule, we established a new subpart R under the regulations at 42 CFR part 414 to incorporate payment provisions for the implementation of the IVIG items and services payment in accordance with section 1842(o) of the Act for home IVIG items and services furnished on or after January 1, 2024. We finalized a policy at § 414.1700(a), that a single payment amount is made for items and services furnished by a DMEPOS supplier per visit. We finalized a policy at § 414.1700(b), setting the initial payment amount equivalent to the CY 2023 “Services, Supplies, and Accessories Used in the home under the Medicare IVIG Demonstration” payment amount, updated by the CY 2024 home health update percentage of 3.0 percent. We also finalized a policy at § 414.1700(c) to annually update the CY 2025 home IVIG items and services payment rate and subsequent years, by Start Printed Page 55398 the home health payment rate update percentage for such year. Therefore, the proposed CY 2025 home IVIG items and services payment rate would be the CY 2024 IVIG items and services payment rate of $420.48 updated by the proposed home health payment update percentage of 2.5 percent ($420.48 * 1.025 = $430.99).
The updated home intravenous immune globulin items and services payment rate will be posted in the Billing and Rates section of the CMS' Home Infusion Therapy (HIT) web page (found at https://www.cms.gov/medicare/payment/fee-for-service-providers/home-infusion-therapy ) once this rate is finalized. In subsequent years, if CMS does not intend to propose changes to its established methodology for calculating the IVIG items and services payment, this payment rate will be updated using CMS' established methodology via the Home Health Prospective Payment System Rate Update Change Request and posted on the CMS HIT/Home IVIG Services web page. [ 105 ] For more in-depth information regarding the finalized policies associated with the scope of the home IVIG items and services payment, we refer readers to the CY 2024 HH PPS final rule ( 88 FR 77791 ).
CMS has broad statutory authority to establish health and safety standards for most Medicare- and Medicaid-participating provider and supplier types. The Secretary gives CMS the authority to enact regulations that are necessary in the interest of the health and safety of individuals who are furnished services in an institution, while other laws, as outlined later, give CMS the authority to prescribe regulations as may be necessary to carry out the administration of the program. Sections 1861(o) and 1891 of the Act authorize the Secretary to establish the requirements that an HHA must meet to participate in the Medicare Program, and these conditions of participation (CoPs) are set forth in regulations at 42 CFR part 484 .
The CoPs apply to the HHA as an entity, as well as to the services furnished to each individual patient under the care of the HHA. In accordance with section 1861(o) of the Act, the Secretary is responsible for establishing additional CoPs besides those set out in the statute that are adequate to protect the health and safety of the individuals under HHA care. Section 1891(c)(2) of the Act establishes the requirements for surveying HHAs to determine whether they meet the CoPs.
Admission to HHA services is a critical step in the process of patients receiving timely, appropriate care to meet their needs. In accordance with the requirements of § 484.105(f)(1), each HHA must furnish skilled nursing services and at least one other therapeutic service (physical therapy, speech-language pathology, occupational therapy, medical social services, or home health aide services) on a visiting basis and in a place of residence that is used as a patient's home. As such, the services provided by each HHA vary, creating challenges for individuals seeking to find the right HHA to meet their unique care needs. Likewise, the unique mix of services provided by an HHA also necessitates an HHA-specific approach to accepting referrals for care to ensure that the HHA is capable of meeting the needs of the referred patient, in accordance with the requirements of § 484.60. This CoP states that patients are accepted for treatment on the reasonable expectation that an HHA can meet the patient's needs in his or her place of residence. Thus, a timely, appropriate admission process serves both prospective patients seeking care and ensures that HHAs accept for treatment only those patients for whom there is a reasonable expectation of being able to meet the patient's care needs.
Researchers have found that timely admission to home health, and in turn the initiation of services are key to good home health patient outcomes. Timely initiation of home health care lowers the risk of 30-day hospital readmissions. [ 107 ] According to one study published in 2021, when the initiation of home health services is significantly delayed (that is, from 8 to14 days after discharge), the odds of rehospitalization for diabetic patients were four times greater than among patients receiving home health service initiation within 2 days. [ 106 ] Yet the rate of timely initiation of home health care varies significantly, indicating that the referral and acceptance process is in need of improvement. In a study of initiation of home health care for individuals diagnosed with Alzheimer's disease and related dementia, [ 107 ] only 57.3 percent of patients discharged from the hospital began home health services within 48 hours of discharge, while 21.6 percent of patients had care initiated between 3 and 7 days post-discharge, another 8.4 percent had care initiated between 8-10 days post-discharge, and 12.8 percent experienced a delay of 11 to 14 days between discharge and home health care initiation. The 42.7 percent of patients in the study who waited 3 or more days after discharge for initiation of home health services were more likely to be dually enrolled in Medicare and Medicaid, live in rural areas, have experienced longer hospital stays, experienced a hospital acquired condition, or experienced an intensive care unit stay. Additionally, this population was more likely to have been discharged with a lower functional status and were more likely to live alone. In another study of Medicare beneficiaries, [ 108 ] only 54 percent of patients discharged from the hospital to home health care received home health care services within 14 days of discharge. The rate of patients receiving home health services within 14 days of discharge with a home health referral was even lower among Black and Hispanic patients, those who were dually enrolled in both Medicare and Medicaid, and patients who lived in high-poverty, high unemployment zip codes. This research brings attention to vulnerable populations at risk of poor outcomes associated with delays in the timely initiation of home health care services. [ 109 ]
Timely initiation of home health care services is also intrinsically linked to the home health referral process, whereby connections are made between referral sources and HHAs. Patient referral sources are varied, with some patients and caregivers conducting their own searches for care, known as self-referrals, while others are referred by a community-based practitioner or an acute care provider such as a hospital. Patients that begin HHA care without an Start Printed Page 55399 immediate prior hospitalization, those who are self-referrals or referred by a community-based practitioner, tend to be Medicaid recipients, have cognitive impairments, and are more socially vulnerable (defined as the gap between patient needs and the patient's available resources) than patients admitted from acute care. Additionally, they tend to have received 80 or more hours per month of family caregiver assistance prior to their acceptance to HHA services. [ 110 ] This population has unique needs and circumstances needs that may make finding the right HHA a challenging process, and they may not have access to information needed to target their search for an HHA in an effective and efficient manner. Given the significant delays in home health care initiation described earlier and the role that this information plays in facilitating care initiation, we are concerned about this lack of public transparency and whether referral sources, including patients and caregivers searching for HHA services, currently have access to sufficient and timely information necessary to locate an HHA that is capable of meeting each specific patient's needs. Without such information, care delays are likely to occur, placing patients at higher risk of poor outcomes.
In addition to the challenges of finding the right HHA and resultant potential delays in the timely initiation of home health care, we are also concerned that HHAs are at higher risk of overextending their available resources when accepting new patients to HHA services. Delays in service initiation may indicate not only that referral sources have difficulty locating an appropriate HHA, but also that HHAs are accepting patients when and for whom they are not capable of delivering timely care. We are aware of anecdotal reports of home care agencies not providing care to meet patient needs [ 111 112 ] and reports by agencies of challenges maintaining appropriate staff caseloads to continue delivering care to patients that have been accepted for service. We acknowledge that these challenges may be related to workforce shortages. HHAs are expected to discharge patients for whom the HHA is unable to deliver care to meet patient needs, and to adhere to the HHA discharge requirements at § 484.58. Such discharges create transition of care burdens for patients and their caregivers that may be prevented by consistently applying an admission to service policy that includes the elements proposed in this rule to ensure the correct match of an HHA's available patient care resources and the anticipated needs of the patients accepted for service by that HHA. In line with this HHA proposal, CMS recently published a final rule titled “Ensuring Access to Medicaid Services” ( 89 FR 40542 , May 10, 2024), which requires States to report how they establish and maintain Home and Community Based Services (HCBS) wait lists, assess wait times, and report on quality measures. That final rule aims to increase transparency and accountability and standardize data and monitoring, with the goal of improving access to care.
To address these dual concerns regarding the referral and acceptance process and their implications for prospective and current patients, we propose to add a new standard at § 484.105(i) that would require HHAs to develop, implement, and maintain an acceptance to service policy that is applied consistently to each prospective patient referred for home health care. We propose to require that the policy be reviewed annually and address, at minimum, the following criteria related to the HHA's capacity to provide patient care: the anticipated needs of the referred prospective patient, the HHA's case load and case mix, the HHA's staffing levels, and the skills and competencies of the HHA staff. It is our understanding, based on information provided by HHA accrediting organizations and the largest HHA trade association, that HHAs typically have acceptance to service policies that are categorical in nature, meaning that the policies address entire categories of diagnosis or service types that they are or are not capable of providing care for. This proposed rule would not prevent HHAs from maintaining these existing policies and is intended to complement them. We also understand that an HHA's case load, case mix, and staffing levels may change over time, and that an HHA may choose to pre-establish methodologies that take into account such fluctuations as part of their acceptance to service policy to ensure consistency and minimize administrative efforts in maintaining the policy.
We propose, at § 484.105(i)(1)(i) through (iv), that HHAs would be required to include information regarding the HHA's case load and case mix (that is, the volume and complexity of the patients currently receiving care from the HHA), anticipated needs of the referred prospective patient, the HHA's current staffing levels, and the skills and competencies of the HHA staff. These proposed elements are designed to inform an HHA's assessment of its capacity and determine its suitability to meet the anticipated needs of the prospective patient that has been referred for HHA services. While all of a prospective patient's needs may not be known at the time of referral, general information regarding the patient's diagnosis and recent hospitalization (as appropriate), and specific orders from the patient's medical provider would provide a reasonable basis for HHAs to anticipate the overall needs of the patient and determine whether, in light of the described factors, the prospective patient is or is not appropriate for the HHA to accept for service. In accordance with § 484.60, HHAs may only accept those patients for whom there is a reasonable expectation that the HHA will be able to meet the patient's needs.
We therefore propose that the patient acceptance to service policy be applied consistently to ensure that HHAs only accept those patients for whom there is a reasonable expectation that the HHA can meet the referred patient's needs. Not only would consistent application of the acceptance to service policy help to ensure that HHAs only accept referrals for care that they can deliver, it would also ensure that HHAs apply their acceptance policy based on clinical and operational factors (those criteria included in proposed § 484.105(i)(1)(ii) through (iv)) that impact patient health and safety. While Medicare-participating HHAs may choose to accept other, non-Medicare sources of payment, we expect that HHAs would apply their acceptance to service policy consistently in a manner that is neutral to the source(s) of payment for a referral. That is, if an HHA accepts payment from both Medicare and another payment source, “source X,” the HHA's referral policy should be applied consistently to referrals for patients having Medicare or “source X” as a payment source. It is our position that HHAs should accept or decline patient referrals based solely on clinical considerations and the capacity of the HHA to safely and effectively deliver care to meet patient needs, Start Printed Page 55400 rather than on financial factors related to the perceived adequacy of the payment rate that the HHA has already voluntarily agreed to accept upon establishment of relationships with its payment sources.
We also propose, at § 484.105(i)(2), that HHAs make public accurate information regarding the services offered by the HHA and any limitations related to the types of specialty services, service duration, or service frequency, and that HHAs review that information annually or as necessary. As previously discussed, HHAs have the flexibility to choose the services that they provide and the geographic areas that they serve. As such, each HHA may provide a different mix of services or offer different specialty services. Likewise, each HHA has different geographical boundaries for its service area. Knowing which areas are served by an HHA and which services an HHA does and does not provide would assist referral sources and self-referrals alike in identifying HHAs that provide the services needed by the patient. Likewise, each HHA has unique staffing levels and staffing competencies affecting its capacity to deliver patient care, and those may change over time. To the extent that these variations in staffing impact the capacity of an HHA to provide its typically offered services, we would require that HHAs make public such limitations on specialty services, service duration, and service frequency to further inform the search efforts of all referral sources.
In short, making information regarding the services offered by the HHA and any limitations related to the types of specialty services, service duration, or service frequency available to the public, such as sharing it on the HHA's website and providing the same information upon request for those without access to the website, would facilitate the search for an HHA to meet a patient's needs, both from clinical referral sources such as hospitals and physician offices, and from patients and caregivers directly seeking care. The goal of this proposal is to reduce the delay between the time when a patient is identified as needing home health care and the time when the patient begins receiving such care by making key information readily available, thus improving identification of HHAs capable of meeting patient needs. Reducing the time delay would improve patient outcomes, as longer delays between referral and the initiation of HHA care are more likely to result in 30-day rehospitalizations and may place vulnerable populations at risk for various other adverse outcomes.
We request public comment on these proposals. Specifically, we request comment on alternative ways to address the delay of home health care initiation, barriers for patients with complex needs to find and access HHAs, and other opportunities to improve transparency regarding home health patient acceptance policies to better inform referral sources. We also request public comment regarding other ways to improve the referral process for referral sources, patients, and HHAs.
The current CoPs at § 484.55(a)(1) require the registered nurse to conduct an initial assessment visit to determine the immediate care and support needs of the patient within 48 hours of referral, within 48 hours of the patient's return home, or on the physician allowed practitioner-ordered start of care date. The initial assessment establishes a patient's eligibility for coverage under the Medicare home health benefit. The clinician conducting the initial visit should determine the patient's homebound status, primary physician, and skilled services required. Section 484.55(b) further requires that the comprehensive assessment must be completed in a timely manner by a registered nurse, but no later than 5 calendar days after the start of care. However, when therapy services are the sole services ordered by the physician or allowed practitioner, the initial and comprehensive assessments can be conducted by rehabilitation professionals (specifically occupational therapists (OT), physical therapists (PT), or speech-language pathologists (SLP)), subject to certain limitations, as specified by § 484.55(a)(2) and (b)(3).
Section 484.55(c) establishes the minimum content of the comprehensive assessment, which must accurately reflect the patient's status and include the patient's current health, psychosocial, functional, and cognitive status. The comprehensive assessment must also reflect the patient's strengths, goals, and care preferences, including information that may be used to demonstrate the patient's progress towards the achievement of the goals identified by the patient and the measurable outcomes identified by the HHA. Additionally, the comprehensive assessment must include a determination of the patient's continuing need for home care, and their medical, nursing, rehabilitative, social, and discharge planning needs. Further, the comprehensive assessment must also include a review of the patient's medication and identify the patient's primary caregiver(s) or patient representative. Lastly, the comprehensive assessment must incorporate the current version of the Outcome and Assessment Information Set (OASIS) items. HHAs must complete data collection for the comprehensive assessment within 5 days of the start of care.
At the beginning of the COVID-19 Public Health Emergency (PHE), CMS waived the requirements at § 484.55(a)(2) and (b)(3) and thus permitted rehabilitation professionals to perform the initial and comprehensive assessment in instances when both nursing and therapy services are ordered. This temporary blanket waiver reflected the unique circumstances of the PHE, with its acute pressures on the nursing workforce, and allowed rehabilitation professionals to perform the initial and comprehensive assessment for patients receiving therapy services as part of the broader nursing and therapy care plan, to the extent permitted under State law, regardless of whether the therapy service established patient eligibility to receive home care.
Subsequently, Division CC, section 115 of the Consolidated Appropriations Act (CAA) of 2021 ( Pub. L. 116-260 ), permitted OTs to conduct the initial and comprehensive assessments only when OT is on the home health plan of care with either PT or speech therapy, and skilled nursing services are not initially on the plan of care. CMS proposed changes to § 484.55(a)(3) and (b)(2) in the CY 2022 Home Health PPS proposed rule ( 86 FR 35874 ), and finalized the changes in the CY 2022 Home Health PPS final rule ( 86 FR 62240 ).
However, some groups continue to advocate for CMS to permanently allow therapists to perform the initial and comprehensive assessment in the home health setting when both therapy and nursing services are ordered. While CMS received limited feedback during the CY 2022 Home Health PPS proposed rule from several commenters supporting a change of this type, we are interested in obtaining additional feedback on this specific potential change.
The three types of rehabilitative therapists (OT, PT, and SLP) have different education requirements for entry into to practice. Currently, entry-level education for OT is either a Master's degree or Doctorate of Occupational Therapy. Education programs are accredited by the Accreditation Council for Occupational Start Printed Page 55401 Therapy Education (ACOTE) of the American Occupational Therapy Association (AOTA). The ACOTE establishes, approves, and administers educational standards to evaluate occupational therapy educational programs. Graduates of ACOTE-accredited programs are eligible to take the National Board for Certification in Occupational Therapy (NBCOT) certification exam and apply for State licensure.
Physical therapy entry-level education requires a Doctor of Physical Therapy degree. The Commission on Accreditation in Physical Therapy Education (CAPTE) of American Physical Therapy Association (APTA) accredits entry-level physical therapist education programs. Graduates of these programs are then eligible to take the National Physical Therapy Examination and apply for State licensure.
Speech Language Pathologists must obtain a Certificate of Clinical Competence in Speech-Language Pathology (CCC-SLP) as well as state licensure. This requires graduation from a program accredited by the Council on Academic Accreditation in Audiology and Speech-Language Pathology (CAA) of the American Speech-Language-Hearing Association (ASHA). Individuals applying for certification in speech-language pathology must have been awarded a master's, doctoral, or other recognized post-baccalaureate degree. Once students complete all academic coursework and a graduate student clinical practicum, they must also complete a clinical fellowship under the supervision of a SLP mentor. The clinical fellowship requires working at least 36 weeks and 1,260 hours and is intended to transition the fellow from a student enrolled in a communication sciences and disorders (CSD) program to an independent provider of speech-language pathology clinical services.
The APTA has indicated that the accreditation standards for entry-level certification programs for physical therapy have evolved over time, with major changes occurring between 1996 and 2024, and that contemporary PT education programs are now required to include content for students on broader health care issues to promote team-based and interdisciplinary practice. The association states that the current criteria reflect that PT education has shifted significantly since the initial and comprehensive assessments were codified into the home health CoPs, preparing any PT to perform these assessments safely. APTA further contends that education standards have shifted from an initial focus on physical sciences to expressly incorporate interdisciplinary care topics, including pharmacology and psychosocial, and clinical educational experience in practice settings common to PTs (for example, HHAs, skilled nursing facilities (SNFs), and inpatient rehabilitation facility (IRFs)). The curricular requirements include the general clinical skills required to conduct the initial and comprehensive assessments, both in the identification of immediate care and support needs, as well as the assessment of the patient's general health, psychosocial, functional, cognitive, and pharmacological status.
Given recent input from stakeholders, including therapy professional organizations, we seek public comments regarding whether CMS should shift its longstanding policy and permit all classes of rehabilitative therapists (PTs, SLPs, and OTs) to conduct the initial assessment and comprehensive assessment for cases that have both therapy and nursing services ordered as part of the plan of care. We ask the public for data, detailed analysis, academic studies, or any other information to support their comments that provide a direct link to patient health and safety. Specifically, we solicit comment regarding the following:
- What types of mentorships, preceptorship, or training do these disciplines have qualifying them to conduct the initial assessment and comprehensive assessment?
- How do HHAs currently assign staff to conduct the initial assessment and comprehensive assessment? Do HHAs implement specific skill and competency requirements?
- Do the education requirements for entry-level rehabilitative therapist provide them with the skills to perform both the initial assessment and comprehensive assessment? Is this consistent across all the therapy disciplines? How does this compare with entry-level education for nursing staff?
- What, if any, potential education or skills gaps may exist for rehabilitative therapists in conducting the initial assessment and comprehensive assessment?
- What challenges did HHAs and therapists that conducted these assessments under the PHE waiver experience that may have impacted the quality of these assessments?
- For the HHAs and therapists that conducted the initial assessment and comprehensive assessment under the PHE waiver, what were the benefits and were there any unintended consequences of this on patient health and safety?
- What challenges, barriers, or other factors, such as workforce shortages, particularly in rural areas, impact rehabilitative therapists and nurses in meeting the needs of patients at the start of care and early in the plan of care?
In an effort to improve the HHA referral process, ensure the timely delivery of home health care, and ensure that home health care is delivered in a manner that meets patient needs and achieves the measurable outcomes and goals set forth in each patient's individualized plan of care, we are requesting public comment on policies related to these goals. Anecdotally, CMS has received an increasing number of beneficiary complaints related to the difficulty of finding a HHA to accept them for service. Beneficiaries complain that in some instances, HHA services are being altered or diminished from the original plan of care without an accompanying reduction in patient needs or achievement of the measurable outcomes and goals set forth in the plan of care. We seek to better understand these issues to inform future policy decisions, consistent with our statutory authority to ensure the health and safety of home health patients.
In CY 2022 Home Health PPS proposed rule, we solicited comments seeking information about the adequacy of aide staffing ( 86 FR 35874 , July 7, 2021). However, only a few commenters provided information specific to the questions we posed in our request. This information was not sufficient to gain insight to the factors that may be impacting the decline in aide services. No further development action was taken due to the lack of substantive data and response from stakeholders.
The number of referrals to HHAs continue to increase. Patient acuity is also increasing [ 113 114 ] with the evolving practice of direct discharge of intensive care unit (ICU) patients, a practice borne out of resource constrained health care infrastructure. [ 115 ] Compared to 2019 averages, patients are 6 percent more acute at discharge. Patients with higher acuity typically have more complex care needs and a higher risk of Start Printed Page 55402 complications. [ 116 117 ] The overall aging of the U.S. population contributes to overall higher patient acuity. The U.S. Census Bureau estimated that by 2023 73 million baby boomers in the U.S. will be 65 or older. Chronic conditions are more common in older adults and can contribute to higher acuity. [ 118 119 ] In addition, procedures that were once traditionally done in hospitals are migrating to the outpatient setting and patients are being discharged on the same day as the procedure. Specifically, surgical procedures within the U.S. are increasingly shifting to outpatient or non-hospital locations, as seen in the expected 4 percent annual expansion rate of the ambulatory surgery center (ASC) market over the 10-year period from 2017 to 2027. [ 120 ] For example, the incidence of total knee arthroplasty (TKA) surgery performed in the outpatient setting has increased as a result of improved perioperative recovery protocols and challenges brought by the COVID-19 pandemic on health systems. [ 121 ] Further, literature shows that factors like the pandemic and “acuity creep” have resulted in HHAs accepting for service much more complicated patients. “As the demand for home-based care continues to rise, so does the need for more intensive care plans as patients continue to be sicker and more complex.” [ 122 ]
We acknowledge that there may be additional factors, such as the shortage of health care practitioners (nurses and aides) across various health care sectors, and HHA business operations and practices that also influence an HHA's care planning and delivery of services. We seek to understand the changes in practice that have occurred since publication of the January 13, 2017 “Medicare and Medicaid Program: Conditions of Participation for Home Health Agencies” final rule that revised the home health agency CoPs ( 82 FR 4504 ) and review any potential CoP revisions that should be considered. In order to protect the health and safety of all HHA patients, we seek to understand how the services offered and business operations of the HHA may influence the development and implementation of care plans. We are also seeking additional information on how HHAs communicate with patients' ordering physicians and allowed practitioners regarding the frequency and duration of services.
We are seeking public comments on factors that influence the services HHAs provide, the referral process, limitations on patients being able to obtain HHA service, such as rural location and availability of staff, plan of care development, and the HHA's communication with patients' ordering physicians and allowed practitioners. We ask the public for data, detailed analysis, academic studies, or any other information to support their comments that provide a direct link to patient health and safety. Specifically, we solicit comment regarding the following questions:
- What factors influence an HHA's decision on what services to offer as part of its business model and how often do HHAs change the service mix?
- What are the common reasons for an HHA to not accept a referral?
- How do physicians and allowed practitioners use their role in establishing and reviewing the plan of care to ensure patients are receiving the right mix, duration, and frequency of services to meet the measurable outcomes and goals identified by the HHA and the patient?
- To what extent do physicians rely on HHA clinician evaluations and reports in establishing the mix of services, service frequency, and service duration included in the plan of care?
- What are the patient and caregiver experiences in receiving nursing, aide, and therapy services when under the care of a home health agency?
- What additional evidence is available regarding negative outcomes or adverse events that may be attributable to the mix, duration, and service frequency provided by HHAs, including, but not limited to, avoidable hospitalizations?
- In what ways can referring providers and HHAs improve the referral process?
- What other factors may influence the provision of services that impact the timeliness of services and service initiation?
- What additional areas should CMS consider to address HHA patient health and safety concerns?
Under sections 1866 and 1902 of the Act, providers of services seeking to participate in the Medicare or Medicaid program, respectively, must enter into an agreement with the Secretary or the State Medicaid agency, as appropriate. Long-term care (LTC) facilities seeking to be Medicare and Medicaid providers of services must be certified as meeting Federal participation requirements. LTC facilities include skilled nursing facilities (SNFs) for Medicare and nursing facilities (NFs) for Medicaid. The Federal participation requirements for SNFs, NFs, and dually certified facilities, are set forth in sections 1819 and 1919 of the Act and codified in the implementing regulations at 42 CFR part 483, subpart B .
Sections 1819(d)(3) and 1919(d)(3) of the Act explicitly require that LTC facilities develop and maintain an infection control program that is designed, constructed, equipped, and maintained in a manner to protect the health and safety of residents, personnel, and the general public. In addition, sections 1819(d)(4)(B) and 1919(d)(4)(B) of the Act explicitly authorize the Secretary to issue any regulations he deems necessary to protect the health and safety of residents. Continuous and systematic collection of data is an essential component of any infection control program, as the data provides information about potential health threats and enables prevention planning to mitigate severe health outcomes. LTC residents are vulnerable to infection from SARS-CoV-2 because of chronic health conditions, immunosenesence, and residence in a communal living setting. Vaccination provides protection against infection but does not eliminate the risk of acquiring SARS-CoV-2. Epidemiologic data from the CDC's National Healthcare Safety Network (NHSN) indicate that weekly COVID-19 cases continue to follow the general surge patterns of 2020 to 2023, despite the vaccination status of the nursing home population. Additionally, the U.S. population remains at risk of increased infection incidence and adverse outcomes as additional SARS-CoV-2 strains continue to emerge, and immunity induced by COVID-19 vaccines wane. As such, in alignment with the sections 1819(d)(3), 1919(d)(3), 1819(d)(4)(B), and 1919(d)(4)(B) of the Start Printed Page 55403 Act, the policy proposed in this regulation to establish the ongoing collection of the proposed set of data elements is necessary to quickly identify threats to resident health and safety and initiate requisite responses. The data proposed in this regulation for ongoing collection would support facility, State, and Federal-level public health actions that protect the health and safety of residents and ongoing response efforts. In addition, the data collected would continue to be supplied directly to LTC facilities, State health departments, the CDC, and CMS to detect infection outbreaks, monitor the impact of infection prevention strategies, and vaccination uptake (sections VI.B.2. through B.5. of this proposed rule outline specific benefits because of the proposed data collections).
Infection prevention and control in LTC facilities was especially important during the COVID-19 PHE. Under the explicit instructions of Congress, existing regulations at § 483.80 require facilities to, among other things, establish and maintain an infection prevention and control program (IPCP) designed to provide a safe, sanitary, and comfortable environment and to help prevent the development and transmission of communicable diseases and infections. The COVID-19 PHE placed enormous strain on the Nation's healthcare systems, requiring LTC facilities nationwide to take extraordinary measures in the face of staff shortages, and the scarcity of personal protective equipment (PPE) and critical supplies. Protecting residents in these circumstances demanded that we have better visibility and data on the spread and impact of COVID-19 in the Nation's LTC facilities. In response, CMS issued an evolving series of requirements to obtain those data through several interim final rules with comment period (IFC) during the height of the PHE and subsequent final rules to support ongoing efforts to monitor and protect residents against COVID-19. When the CDC started collecting COVID-19 case data on a national scale in LTC facilities we began to understand the epidemiological trends of COVID-19 disease in LTC residents. The data highlighted how LTC facilities played a large role in viral transmission and that LTC residents were disproportionally impacted by COVID-19 compared to community dwelling adults. Even after the end of the PHE, national data collected in LTC facilities has shown that LTC residents continue to be impacted by COVID-19 at higher rates than older adults in the community and are more likely to develop severe outcomes. Continuing to understand trends of COVID-19 and other significant respiratory diseases (for example, RSV, Influenza) in the LTC population is critical to understanding the burden of respiratory viruses on the country.
First, on May 8, 2020, we issued a IFC titled “Medicare and Medicaid Programs, Basic Health Program, and Exchanges; Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency and Delay of Certain Reporting Requirements for the Skilled Nursing Facility Quality Reporting Program” ( 85 FR 27550 ), which revised the infection prevention and control requirements for LTC facilities to more effectively respond to the specific challenges posed by the COVID-19 pandemic. Specifically, this May 2020 IFC added provisions to require facilities to electronically report information related to confirmed or suspected COVID-19 cases to the Centers for Disease Control and Prevention (CDC) and required facilities to inform residents and their representatives of confirmed or suspected COVID-19 cases in the facility among residents and staff.
Second, on September 2, 2020, we issued a IFC titled “Medicare and Medicaid Programs, Clinical Laboratory Improvement Amendments (CLIA), and Patient Protection and Affordable Care Act, Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency” ( 85 FR 54873 ). This September 2020 IFC set out provisions regarding testing for COVID-19 in LTC facilities, including documentation requirements and protocols specifying actions to be taken if a resident or staff member tests positive. On May 13, 2021, we issued another IFC titled “Medicare and Medicaid Programs; COVID-19 Vaccine Requirements for Long-Term Care (LTC) Facilities and Intermediate Care Facilities for Individuals with Intellectual Disabilities (ICFs-IID) Residents, Clients, and Staff” ( 86 FR 26306 ), which further revised the infection control requirements that LTC facilities and intermediate care facilities for individuals with intellectual disabilities (ICFs-IID) must meet to participate in the Medicare and Medicaid programs. This May 2021 IFC aimed to reduce the spread of SARS-CoV-2 infections, the virus that causes COVID-19, by requiring education about COVID-19 vaccines for LTC facility residents, ICF-IID clients, and staff serving both populations, and by requiring that such vaccines, when available, be offered to all residents, clients, and staff. It also required LTC facilities to report COVID-19 vaccination status of residents and staff to CDC.
To retain the data reporting requirements after the end of the PHE, on November 9, 2021, we subsequently published a final rule titled “CY 2022 Home Health Prospective Payment System Rate Update; Home Health Value-Based Purchasing Model Requirements and Model Expansion; Home Health and Other Quality Reporting Program Requirements; Home Infusion Therapy Services Requirements; Survey and Enforcement Requirements for Hospice Programs; Medicare Provider Enrollment Requirements; and COVID-19 Reporting Requirements for Long-Term Care Facilities” ( 86 FR 62421 ), which finalized the COVID-19 data reporting requirements from the May 2020 and May 2021 IFCs. Specifically, in this November 2021 final rule, we revised the requirements at § 483.80(g)(1)(i) through (ix), to reduce the burden on the LTC facilities by allowing for a reduced frequency of reporting (weekly unless the Secretary specifies a lesser frequency) and modified the specific data elements to be reported. The rule states that until December 31, 2024, facilities must electronically report, in a standardized format specified by the Secretary, information on suspected and confirmed COVID-19 infections among residents and staff, including residents previously treated for COVID-19, total deaths and COVID-19 deaths among residents and staff, personal protective equipment and hand hygiene supplies in the facility, ventilator capacity and supplies available in the facility, resident beds and census, access to COVID-19 testing while the resident is in the facility, and staffing shortages. In addition, on an ongoing basis with no sunset date, facilities are required to report information on resident and staff vaccination status for COVID-19.
Finally, on June 5, 2023, we issued a final rule titled “Medicare and Medicaid Programs' Policy and Regulatory Changes to the Omnibus COVID-19 Health Care Staff Vaccination Requirements; Additional Policy and Regulatory Changes to the Requirements for LTC Facilities and ICF-IIDs to Provide COVID-19 Vaccine Education and Offer Vaccinations to Residents, Clients, and Staff; Policy and Regulatory Changes to the LTC Facility COVID-19 Testing Requirements” ( 88 FR 36485 ). [ 123 ] This June 2023 final rule Start Printed Page 55404 removed expired language addressing COVID-19 testing requirements issued in the September 2020 IFC, withdrew requirements mandating COVID-19 vaccinations for staff (see 86 FR 61555 for details regarding the IFC that issued the requirements), [ 124 ] and finalized requirements issued in the May 2021 IFC for facilities to provide education about vaccines and to offer COVID-19 vaccines to residents and staff.
There are over 1.3 million older adults aged 65 years and older living in LTC facilities in the United States; and while LTC facility residents make up less than 0.5 percent of the population in the U.S., they were estimated to account for between 23 percent and 40 percent of deaths due to COVID-19 in the first two years of the COVID-19 PHE. [ 125 126 ] Older residents are at greater risk for both developing COVID-19 and other respiratory illnesses (for example, influenza, RSV) and for developing a protracted course of disease. [ 127 ] Age-associated changes in immune function (that is, immunosenecense) can increase susceptibility to infection and decrease response to vaccination. Additionally, older adults often have multiple co-morbidities leading to increased morbidity and mortality when coupled with a respiratory tract infection. [ 128 ] The congregate setting of LTC facilities can also increase risk of disease transmission given the proximity of residents. In addition, providing care for residents often involves close-contact activities (for example, dressing, bathing) and the same health care personnel provide care to residents across different rooms and shared spaces. This readily facilitates transmission of respiratory viruses in this setting. [ 129 ] Furthermore, LTC facility staffing shortages and consistent staff turnover, that are ever-present, but were greatly exacerbated during the COVID-19 PHE, make it even more challenging to provide quality care and to implement infection practices effectively and consistently, demonstrating the need for timely and actionable surveillance. [ 130 ]
The COVID-19 PHE highlighted the value and potential utility of greater integration between public health and health care, particularly when data are available to direct collaborative actions that support patient, resident, and public health and safety. Data from health care providers, including LTC facilities, remain a key driver to identify and respond to patient, resident, and public health threats, yet health care and public health data systems have long persisted on separate, often poorly compatible tracks. [ 131 ] The COVID-19 PHE also highlighted the importance of taking a broader view of patient and resident safety—one that recognizes patient and resident safety is determined not only by what is happening at the bedside, but also what is happening, in the facility as a whole, in neighboring facilities (for example, individuals moving between hospitals and LTC facilities and health care providers working in multiple facilities), and across the region, State, and county. The value of this broader view was particularly evident from the experience of LTC facilities, where systematic communicable disease and vaccination surveillance had never been integrated.
For the first time, during the COVID-19 PHE, the nation had a real-time comprehensive picture of a disease, its vaccine, and its impact in the nearly 16,000 U.S. LTC facilities because of data reported to the CDC's NHSN application. Ultimately, access to this information proved critical to providing resources and supporting coordinated action by facilities, health systems, communities and jurisdictions in responding to the PHE and protecting the health, safety and lives of LTC facility residents.
The resources made available during the PHE response helped build resilience in some parts of the health care system, but the pandemic also exacerbated sources of fragility that continue to leave the United States underprepared to respond to surges—even relatively typical ones. Efforts to support the LTC community and facility infrastructure include the CMS final rule titled “Medicare and Medicaid Programs; Minimum Staffing Standards for Long-Term Care Facilities and Medicaid Institutional Payment Transparency Reporting,” published on May 10, 2024 ( 89 FR 40876 ). [ 132 ] This final rule established a consistent floor (baseline) for nurse staffing across all LTC facilities in an effort to reduce the variability in nurse staffing. The final rule policies aim to advance equitable, safe, and quality care for all residents receiving care from the Nation's Medicare and Medicaid participating LTC facilities. The finalized minimum staffing standards coupled with the respiratory illness data reporting requirements proposed in this rule would support targeted high-quality care for residents. For example, timely and actionable surveillance at the facility and local level would support efforts to identify and allocate resources to maintain the appropriate care needed to keep residents safe.
Data collected from LTC facilities is used by local health departments to provide specific outreach to individual facilities. This can include interventions such as site visits from health departments, providing additional supplies such as PPE and/or testing supplies, recommendations for testing protocols and individualized advice for infection prevention and control practices to protect the health and safety of residents within individual facilities. LTC facilities care for some of the most vulnerable older adults who are disproportionally impacted by respiratory viruses and severe outcomes, such as hospitalizations and death. Ongoing data collection as part of a facility infection prevention and control program helps each facility to promptly identify a respiratory viral outbreak so that containment and important interventions, such as early anti-viral treatment (SARS-CoV-2, Influenza) and anti-viral prophylaxis (Influenza), can minimize the severity of an outbreak and protect residents' health and safety. Identifying strategies to provide early Start Printed Page 55405 antiviral treatments for COVID-19 and influenza may also help prevent more serious outcomes in individual residents.
Like other settings where health care is delivered, LTC facilities are part of an ecosystem caring for individuals in their community. This interdependency is especially highlighted during times of health care system strain. Insight into LTC facility capacity helps ensure capabilities are available to meet health care needs with quality care through enhanced planning, technical assistance, resource allocation, and coordination. [ 133 ] Health care coalitions (HCCs) are one example of local health care partners working together to increase local health care resilience during respiratory illness surges and more. [ 134 ] HCCs plan and respond together, sharing real-time information and providing technical assistance to support their partners. [ 135 ] Collaborative, data-driven approaches have helped to inform and direct action throughout the health care ecosystem, ultimately improving resident care and outcomes.
Data from LTC facilities is an important component to understanding potential bottlenecks in the health care ecosystem and ways to address them. During the COVID-19 PHE, hospitals struggled with being able to discharge patients to post-acute care, specifically LTC facilities. The availability of LTC facility capacity data helped to inform their response by monitoring triggers for patient load balancing, allocations of scarce resources, and requests for additional resources or mutual aid. [ 136 ] LTC facilities, hospitals, and other health care partners also use the information for planning purposes, identifying how their facility may be impacted and preparing accordingly. [ 137 ] Information sharing across the health care ecosystem helps the health care community to prepare for, and effectively respond to, respiratory illness surges in ways that maintain the safety and availability of critical care services.
Additionally, since the start of the PHE, data reported under our requirements at § 483.80(g)(1) through (3) have been used by LTC facilities and their local health systems to take actions aimed at protecting residents. Facilities can view all reported data and generate reports within the CDC's NHSN application. This allows facilities to review their data in real time and implement any applicable mitigation strategies/infection control practices, based on the counts they are seeing to help reduce outbreak occurrences. LTC facilities have used the CDC's NHSN dashboards and reports to track new cases and up-to-date vaccination status of residents in the facility and take action by identifying areas where they need to strengthen infection and control practices, explore vaccination progress, and consider targeted quality improvement activities as part of their Quality Assurance and Performance Improvement (QAPI) initiatives. LTC facilities can use NHSN to create custom data reports and analyses, tailoring the information for purposes and improvements that best meet their needs for protecting the residents in their care. [ 138 ]
COVID-19 and other respiratory illness case, hospitalization, and vaccination data together provide critical situational awareness for regional and State leadership to inform a national strategy in response to the ongoing public health threat that respiratory illnesses including COVID-19 pose to residents. At the State and regional levels, public health departments and Quality Improvement Organizations (QIOs) have used these data to provide outreach and technical support directly to LTC facilities with high case and hospitalization counts and offer additional resources and support. QIOs use COVID-19 case and vaccination data to identify LTC facility outbreaks, provide 1:1 infection control assistance, and direct any other COVID-19 reduction assistance requested by those nursing homes. These data will continue to be critical to support the ongoing work of the QIOs. They will plan and provide technical assistance and training to LTC facilities identified by CMS for performance improvement based on quality measurement and enforcement data. The QIOs will also work on strengthening the quality management systems in LTC facilities, leadership and governance, culture of safety, workforce planning and focused clinical outcomes. Additionally, resident vaccination data direct the education and assistance efforts of partners like the QIOs and LTC associations to improve vaccination uptake in facilities with the lowest up-to-date vaccination rates among residents and staff. For example, the Nursing Home Command Center, in charge of directing QIOs, reviews NHSN COVID-19 case and vaccination data daily and considers NHSN data to be the best source to identify nursing homes in need of assistance to improve resident outcomes. [ 139 ]
In July 2020, the Federal Government launched a strike team initiative to address COVID-19 outbreaks in LTC facilities. This initiative relied upon data reported by LTC facilities to focus response efforts on the facilities with the highest number of cases. The Federal strike team initiative highlighted significant challenges faced by facilities and was foundational in identifying areas of infection prevention and control need, such as education for front line nursing staff, staffing shortages, and coordination among Federal State and local entities. [ 140 ] These efforts further emphasized that without data to direct assistance to places with the greatest need, response efforts and the limited resources, especially in non-emergency times of typical disease transmission, would be dispersed and far less effective. Building upon the 2020 Federal strike team efforts, a total of $500 million, was made available in 2021, through Sections 9402 and 9818 of the American Rescue Plan (ARP) Act of 2021, Public Law 117-2 , to State and local health departments through the CDC's Epidemiology and Laboratory Capacity (ELC) Cooperative Agreement (CK19-1904), as the “Nursing Home & Long-term Care Facility Strike Team and Infrastructure Project.” This funding allowed States to continue dedicated support to LTC facilities and was used to build and maintain the infection prevention infrastructure necessary to Start Printed Page 55406 support resident, visitor, and facility healthcare personnel safety. State and local strike teams illustrated the power of public health and healthcare stakeholders working together to share data and information and collaborate effectively respond to respiratory illness surges. Between August 2021 and July 2022, health departments conducted over 26,000 Nursing Home COVID-19 responses, including more than 5,000 onsite assessments and more than 5,000 investigations that included staff supported specifically by Strike Team funding. For example, States like Massachusetts have lauded the value of the strike team investments and asserted that improved State and Federal data infrastructure is needed to respond to future outbreaks and protect nursing home residents. [ 141 ]
At the Federal level, the CDC actively uses weekly NHSN data reports to provide direct outreach to LTC facilities with >8 COVID-19 hospitalizations and >20 cases. These weekly reports are sent to State health departments to provide actionable data including confirmed COVID-19 cases among residents and staff, COVID-19 related deaths among residents, COVID-19 hospitalizations among residents, vaccine coverage among residents and staff, and COVID-19 potential outbreak alerts among other data elements. CDC also monitors downloads of these reports and provides ongoing support to States and facilities with these data, showing that the data are actively being used and are found to be valuable to direct response and vaccination efforts to the LTC facilities that most need support and intervention. For example, vaccination data are critical for decision making, targeting outreach for vaccination campaigns efforts, insights into vaccination disparities [ 142 ] and for vaccine effectiveness studies. [ 143 ] The availability of vaccination data from LTC facilities, not only provides a window into national efforts for improving access to vaccines for the LTC industry, but also can indicate the effectiveness of vaccination training and education efforts with residents and families, that promote the benefits of vaccination to help ensure that residents will achieve the best outcome possible if infected with the SARS-CoV-2 virus.
NHSN data has also been used by the CDC and QIOs to contact facilities with high vaccination coverage to understand the successful strategies they employed and promote these strategies to other nursing homes via webinars and the development of tools and resources. Information from this outreach was used to identify and respond to vaccination barriers by creating tools and resources, such as the Healthcare Provider Toolkit, to help nursing homes educate their staff, residents, and families to remove barriers to vaccination.
Furthermore, COVID-19, influenza, and RSV vaccination data continue to be used for establishing policies that promote better protection for residents and staff. These data continue to serve as supporting evidence to make and revise recommendations regarding vaccination to improve the safety of residents and staff while balancing the burden to facilities to report. For example, early in the PHE, increasing staff vaccination rates was associated with lower incidence of COVID-19 cases and deaths among residents and staff in LTC facilities. However, as newer, more infectious, and transmissible variants of the virus emerged, increasing staff vaccination rates of the original 2-dose regimen of the COVID-19 vaccine as recommended in December 2020, was no longer associated with lower rates of adverse COVID-19 outcomes in nursing homes, resulting in updated recommendations to the public. [ 144 ]
Given the value of respiratory illness and vaccination reporting during the COVID-19 PHE in supporting resident health and safety, we are considering the continued utility of LTC facility respiratory illness data to monitor and protect residents against respiratory illnesses and the ongoing need for such data in the “new normal” of diverse respiratory disease threats. While the COVID-19 PHE has ended, SARS-CoV-2 continues to circulate throughout the globe and although epidemic waves are less severe than those of 2020 through early 2022, there was no epidemiologic bright line associated with the end of the PHE. While COVID-19 hospital admissions were modestly lower in January 2024 than they were at the July 2022 or December 2022 peaks, [ 145 ] adults 65 years and older represented more than half of COVID-19 hospitalizations during October 2023 to December 2023. [ 146 ] Additionally, during the 2023-2024 fall/winter respiratory virus season, COVID-19-associated hospitalizations among LTC facility residents peaked at a weekly rate that was more than eight times higher than the peak weekly rate among all U.S. adults aged ≥70 years. [ 147 ] At the same time, other respiratory viruses have also seen a resurgence, and the moderate COVID-19 burden coinciding with resurgent influenza and RSV has led to an overall hospitalization burden larger than observed during severe influenza and RSV seasons prior to the COVID-19 pandemic. [ 148 ]
The elevated risks of respiratory viruses in the post-PHE era present ongoing threats, both direct and indirect, to resident health and safety. The result of this “new normal” will be more burdensome respiratory virus seasons for the foreseeable future, which promises to threaten the health and safety of LTC facility residents across the Nation. [ 149 ] In response to this changed landscape, public health agencies, such as the CDC, have shifted prevention and control strategies from a focus on specific viruses to an approach that addresses the threats presented by the broader respiratory virus season, including focused efforts to mitigate impacts on nursing home residents and staff. [ 150 ] Likewise, we believe it is vital Start Printed Page 55407 to maintain national surveillance of these emerging and evolving respiratory illnesses as a means of guiding infection control interventions to keep residents safe. As such, we propose to continue some of the reporting requirements finalized in November 2021 and set to expire in December 2024. Specifically, we propose to revise the infection prevention and control requirements for LTC facilities to extend reporting in NHSN for a limited subset of the current COVID-19 elements and also require reporting for data related to influenza and RSV.
Specifically, we propose to replace the existing reporting requirements for LTC facilities at § 483.80(g)(1)(i) through (ix) and (g)(2) with new requirements to report information addressing respiratory illnesses. Beginning on January 1, 2025, facilities would be required to electronically report information about COVID-19, influenza, and RSV in a standardized format and frequency specified by the Secretary. Currently, we propose to continue weekly reporting through the CDC's NHSN. To the extent to be determined by the Secretary, through this rulemaking cycle, we propose that the data elements for which reporting would be required include all of the following:
- Facility census (defined as the total number of residents occupying a bed at this facility for at least 24 hours during the week of data collection).
- Resident vaccination status for a limited set of respiratory illnesses including but not limited to COVID-19, influenza, and RSV.
- Confirmed, resident cases of a limited set of respiratory illnesses including but not limited to COVID-19, influenza, and RSV (overall and by vaccination status).
- Hospitalized residents with confirmed cases of a limited set of respiratory illnesses including but not limited to COVID-19, influenza, and RSV (overall and by vaccination status).
These proposals are scaled back and tailored from the current post-COVID-19 PHE requirements, continuing the collection of the minimal necessary data to maintain a level of situational awareness that would protect resident health and safety in LTC facilities across the country while reducing reporting burden on those facilities. We are also interested in the utility of additional reporting on limited demographic data and solicit public comment on whether the collection of data regarding race, ethnicity, and socioeconomic status should be explicitly included as part of these proposed requirements for ongoing reporting beginning on January 1, 2025. We are particularly interested in comments that address the ways these additional data elements could be used to better protect resident and community health and safety both during and outside of a declared PHE. In addition, we are interested in comments on how to protect resident privacy within demographic groups and how to best use the data to inform public health efforts without stigmatizing demographic groups. [ 151 ] Lastly, we welcome comments that address system readiness and capacity to collect and report these data.
In determining the data elements to propose for ongoing reporting, we considered the data elements that proved most actionable and informative over the course of the COVID-19 PHE, with evidence of protecting health and safety, as well as more recent lessons that have emerged during the 2023-2024 respiratory virus response. [ 152 153 ] We also considered ways to balance the burden of reporting on LTC facilities with the need to maintain a level of situational awareness that will benefit residents, their families, and their communities.
In the absence of a declared national PHE for an acute respiratory illness, we propose that LTC facilities would continue to report these data on a weekly basis through a format specified by the Secretary with continued reporting through the CDC's NHSN. Sustained data collection and reporting outside of emergencies would help ensure that LTC facilities maintain a functional reporting capacity that could be mobilized quickly when a new threat emerges to inform and direct response efforts (for example, resource allocations) that protect residents and their communities. These data collections would also provide the baseline information necessary to forecast, detect, quantify and, ultimately, direct responses to signals of strain within regions and LTC facilities.
Unlike the previous and sunsetting LTC reporting requirements, the requirements proposed in this rule are not tied to a specific PHE declaration. PHE declarations are valuable tools for marshalling nimble and fast emergency responses. However, there are many respiratory disease threats to LTC facility operations and resident safety that would not necessarily be subject to a PHE declaration nor have significant potential to become a PHE. In those instances, routine data about cases and hospitalizations due to respiratory viruses like COVID-19, influenza, and RSV are critical to inform technical assistance, infection prevention and control support, and resource allocations to support LTC facilities and safeguard their residents.
We welcome public comments on our proposals, and on ways that reporting burden can be minimized while still providing adequate data. We also welcome feedback on any challenges of collecting and reporting these data; ways that CMS could reduce reporting burden for facilities; and alternative reporting mechanisms or quality reporting programs through which CMS could instead effectively and sustainably incentivize reporting. Finally, we welcome comments on the value of these data in protecting the health and safety of individuals receiving care and treatment and working in LTC facilities.
The COVID-19 PHE strained the healthcare system substantially, introducing new safety risks and negatively impacting patient and resident safety in the normal delivery of care. Data from the pandemic showed that the incidence of healthcare-associated infections would increase when COVID-19 hospitalizations were high, [ 154 ] a feedback loop between increased stress on hospitals, LTC facilities, illness in the community, and patient and resident health and safety. Start Printed Page 55408 Degradation in other measures of resident safety, including pressure ulcers and falls, further demonstrate how the strains associated with surge response adversely affect routine safety practices. [ 155 ] [ 156 ] Specifically in LTC facilities, the significant adverse health impacts on residents caused by COVID-19 went far beyond the direct effects of COVID-19 morbidity and mortality. [ 157 ] Given the unprecedented impacts of, and learnings derived from, the COVID-19 PHE, we believe that it is imperative to enhance preparedness and resiliency to improve health system responses to future threats, including pandemics that pose catastrophic risks to resident safety. As such, we propose additional data reporting that would be required in the event of an acute respiratory illness PHE, or after the Secretary's determination that a significant threat of one exists.
Accordingly, we propose that during a declared national, State, or local PHE for a respiratory infectious disease (or if the Secretary determines a significant threat for one exists) the Secretary may require facilities to report:
- Data up to a daily frequency without additional notice and comment rulemaking.
- Additional or modified data elements relevant to the PHE, including relevant confirmed infections among staff, supply inventory shortages, staffing shortages, and relevant medical countermeasures and therapeutic inventories, usage, or both.
- If the Secretary determines that an event is significantly likely to become a PHE for an infectious disease, the Secretary may require LTC facilities to report additional or modified data elements without notice and comment rulemaking.
We invite comments on if, during a PHE, there should be limits to the data the Secretary can require without notice and comment rulemaking, such as limits on the duration of additional reporting or the scope of the jurisdiction of reporting (that is, State or local PHEs). We also seek comments on whether and how the Secretary should still seek stakeholder feedback on additional elements during a PHE without notice and comment rulemaking and how HHS should notify LTC facilities of new required infectious disease data. Furthermore, we invite comments on the evidence HHS should provide to demonstrate that—(1) an event is “significantly likely to become a PHE”; or (2) the increased scope of required data will be used to protect resident and community health and safety. We also invite comments on the utility and burden of specifically staffing and supply shortage data we propose to collect during national, State, or local PHE for a respiratory infectious disease (or if the Secretary determines a significant threat for one exists). Based on LTC facilities experience with the COVID-19 PHE, how could HHS collect this data specifically in a way that would be beneficial to LTC facilities?
To further reduce burden in the short term, we are working with the CDC to ensure LTC facilities can continue to use existing, established systems to report data in the interim. CDC will continue increasing the automation capabilities of the surveillance systems like NHSN and its ability to connect with other data submission techniques, vendors, and systems. [ 158 ] CDC is collaborating with LTC partner organizations and State health departments to pilot projects aimed at streamlining and modernizing vaccination data reporting. This includes efforts to automate reporting of LTC facility vaccination data from electronic health records to NHSN and to connect person-level vaccination data in NHSN to State Immunization Information Systems (IIS). These modernization efforts should reduce the reporting burden on facilities over time. In addition, CDC provides users with technical assistance, targeted data quality outreach and webinars, and continues to actively collaborate with users and partners to improve system design and functionality. For example, the development of the NHSN person-level vaccination forms allowed for complex definitions that change over time (for example, up to date with COVID-19 vaccines) to be applied automatically to and aggregate resident-level data.
CMS, CDC, and the Administration for Strategic Preparedness and Response (ASPR) recognize the immense value of partnerships with LTC facilities, State, Tribal, Local, and Territorial (STLT) health systems, associations, and other partners. Throughout the COVID-19 PHE, partners at all levels worked alongside CMS, CDC, and ASPR to provide additional context, insight, and feedback based on conditions on the ground. This context helped data collections be more effective and helped provide a fuller picture than data alone. CMS, CDC, and ASPR are grateful for the many collaborations with partners on data and beyond. CDC, ASPR, and the Office of the National Coordinator for Health Information Technology (ONC) will explore opportunities to codify continued partnerships to prepare for and respond to incidents such as respiratory illnesses more effectively. We welcome public comment on ways that all public agencies involved in these types of data collections can be good partners.
Section 1866(j)(1)(A) of the Act requires the Secretary to establish a process for the enrollment of providers and suppliers into the Medicare program. The overarching purpose of the enrollment process is to help confirm that providers and suppliers seeking to bill Medicare for services and items furnished to Medicare beneficiaries meet all applicable Federal and State requirements to do so. The process is, to an extent, a “gatekeeper” that prevents unqualified and potentially fraudulent individuals and entities from entering and inappropriately billing Medicare. Since 2006, we have undertaken rulemaking efforts to outline our enrollment procedures. These regulations are generally codified in 42 CFR part 424, subpart P (currently §§ 424.500 through 424.575 and hereafter occasionally referenced as subpart P). They address, among other things, requirements that providers and suppliers must meet to obtain and maintain Medicare billing privileges.
As outlined in § 424.510, one such requirement is that the provider or supplier must complete, sign, and submit to its assigned Medicare Administrative Contractor (MAC) the appropriate enrollment form, typically the Form CMS-855 (OMB Control No. 0938-0685). The Form CMS-855, which can be submitted via paper or electronically through the internet-based Provider Enrollment, Chain, and Ownership System (PECOS) process (System of Records notice (SORN): 09-70-0532, PECOS), collects important information about the provider or supplier. Such data includes, but is not limited to, general identifying Start Printed Page 55409 information (for example, legal business name), licensure and/or certification data, ownership information, and practice locations. The application is used for a variety of provider enrollment transactions, including the following:
- Initial enrollment—The provider or supplier is—(1) enrolling in Medicare for the first time; (2) enrolling in another Medicare contractor's jurisdiction; or (3) seeking to enroll in Medicare after having previously been enrolled.
- Change of ownership—The provider or supplier is reporting a change in its ownership.
- Revalidation—The provider or supplier is revalidating its Medicare enrollment information in accordance with § 424.515. (Suppliers of durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) must revalidate their enrollment every 3 years; all other providers and suppliers must do so every 5 years.)
- Reactivation—The provider or supplier is seeking to reactivate its Medicare enrollment and billing privileges after it was deactivated in accordance with § 424.540.
- Change of information—The provider or supplier is reporting a change in its existing enrollment information in accordance with § 424.516.
After receiving the provider's or supplier's initial enrollment application, CMS or the MAC reviews and confirms the information thereon and determines whether the provider or supplier meets all applicable Medicare requirements. We believe this screening process has greatly assisted CMS in executing its responsibility to prevent Medicare fraud, waste, and abuse.
As previously discussed, over the years we have issued various final rules pertaining to provider enrollment. These rules were intended not only to clarify or strengthen certain components of the enrollment process but also to enable us to take action against providers and suppliers: (1) engaging (or potentially engaging) in fraudulent or abusive behavior; (2) presenting a risk of harm to Medicare beneficiaries or the Medicare Trust Funds; or (3) that are otherwise unqualified to furnish Medicare services or items. Consistent with this, and as we discuss in section VIII.B. of this proposed rule, we are proposing a change to our existing Medicare provider enrollment regulations.
There are two principal categories of legal authorities for our proposed Medicare provider enrollment provisions:
- Section 1866(j) of the Act furnishes specific authority regarding the enrollment process for providers and suppliers.
- Sections 1102 and 1871 of the Act provide general authority for the Secretary to prescribe regulations for the efficient administration of the Medicare program.
Section 1866(j)(3)(A) of the Act states that the Secretary shall establish procedures to provide for a provisional period of between 30 days and 1 year during which new providers and suppliers—as the Secretary determines appropriate, including categories of providers or suppliers—will be subject to enhanced oversight. (Per section 1866(j)(3)(A) of the Act, such oversight can include, but is not limited to, prepayment review and payment caps.) As authorized by section 1866(j)(3)(B) of the Act, we previously implemented such procedures through subregulatory guidance with respect to newly enrolling HHAs' requests for anticipated payments (RAP). [ 159 ] More recently, in July 2023 we began placing new hospices located in Arizona, California, Nevada, and Texas in a provisional period of enhanced oversight. (See https://www.cms.gov/files/document/mln7867599-period-enhanced-oversight-new-hospices-arizona-california-nevada-texas.pdf for more information.)
During the PPEO involving HHA RAPs, CMS received several stakeholder requests for clarification regarding the PPEO's scope. One of these concerned the meaning of the term “new” for purposes of applying a PPEO. While section 1866(j)(3)(B) of the Act states that we may implement procedures by program instruction, we finalized new § 424.527(a) in the CY 2024 HH PPS final rule to address this issue. Specifically, new § 424.527(a)(1) through (3) defined a “new” provider or supplier (again, exclusively for purposes of our PPEO authority under section 1866(j)(3) of the Act) as any of the following:
- A newly enrolling Medicare provider or supplier. (This includes providers that must enroll as a new provider per the change in majority ownership provisions in § 424.550(b).)
- A certified provider or certified supplier undergoing a change of ownership consistent with the principles of 42 CFR 489.18 . (This includes providers that qualify under § 424.550(b)(2) for an exception from the change in majority ownership requirements in § 424.550(b)(1) but which are undergoing a change of ownership under 42 CFR 489.18 .)
- A provider or supplier (including an HHA or hospice) undergoing a 100 percent change of ownership via a change of information request under § 424.516.
We included these transactions within this definition because they have historically involved the effective establishment of a new provider or supplier for purposes of Medicare enrollment. For this reason, we have also received recent inquiries as to whether a reactivation should fall within the scope of § 424.527(a).
Under § 424.540 and the definition of “deactivate” in § 424.502, a deactivated provider's or supplier's enrollment and billing privileges are “stopped but can be restored upon the submission of updated information.” This restoration, or reactivation, generally involves: (1) the completion of a full Form CMS-855 application; and (2) a CMS or MAC determination as to whether the provider or supplier meets all enrollment requirements. These two steps generally mirror what occurs with the initial and change of ownership applications referenced in § 424.527(a). Although a deactivation does not rise to the level of a revocation of Medicare enrollment and billing privileges under § 424.535—for a revocation bars the provider or supplier from reenrolling in Medicare for a period of 1 to 10 years (with certain exceptions)—a deactivated provider or supplier cannot resume billing Medicare until the requirements for reactivation are met. It has, in effect, been blocked from the Medicare program. Indeed, as with a provider or supplier that voluntarily terminated its Medicare enrollment and now seeks to rejoin the program via an initial, new enrollment application, a reactivating provider, too, is requesting to rejoin the program. Described otherwise, a reactivating provider or supplier is resuming its involvement in the Medicare program after a stoppage (which, at least for practical and operational purposes, amounts to a loss) of Medicare enrollment and billing privileges. From this standpoint, we thus believe that a reactivating provider or supplier is no less “new” (for provider enrollment purposes) than one that is initially enrolling or undergoing a change of ownership.
Our interpretation is also supported by the fact that a significant number of our grounds for deactivation under Start Printed Page 55410 § 424.540(a) involve conduct or inaction in which the provider or supplier—as with a revocation—is not adhering to Medicare enrollment requirements. These include, for example, the provider or supplier—
- Failing to report a change to the information supplied on the enrollment application within the required timeframe (§ 424.540(a)(2));
- Failing to timely respond to a revalidation request (§ 424.540(a)(3));
- Failing to maintain compliance with all enrollment requirements (§ 424.540(a)(4)); and
- Having a non-operational or otherwise invalid practice location (§ 424.540(a)(5)).
The provider or supplier can also be revoked under § 424.535(a) on any of these bases (for instance, under § 424.535(a)(1) relating to noncompliance). Because these bases are overlapping, it is CMS' principled view that reactivating providers and suppliers that were deactivated for any of these reasons should be subject to the same PPEO scrutiny. CMS has a legitimate oversight interest that the prior non-compliance has been corrected and that adherence will continue after their reactivation, which would be satisfied through the post-enrollment monitoring the PPEO affords.
Concerning our other deactivation grounds, § 424.540(a)(1) permits CMS to deactivate a provider or supplier that has not billed Medicare for 6 consecutive months. We recognize that there may be a legitimate reason for which a provider or supplier ceases billing Medicare for an extended period. (For example, a provider enrolls in Medicare strictly to enroll in and bill another health care program.) At the same time, a reactivation request after months of billing inactivity raises questions as to whether—
- The provider or supplier is and will remain compliant with Medicare enrollment requirements once reactivated following such a period of non-billing;
- Another party has compromised the provider's or supplier's deactivated enrollment and billing privileges and seeks to fraudulently bill Medicare via the latter's reactivated enrollment; or
- The provider or supplier had secured multiple billing numbers, one of which was revoked for improper activity, another was deactivated for non-billing, and the provider or supplier now seeks to reactivate the latter number to bill for services that were previously furnished under the revoked number.
CMS has indeed identified such scenarios in its program integrity oversight activities. We believe that using a PPEO to closely monitor reactivated providers or suppliers that had been deactivated under § 424.540(a)(1) would help prevent improper activity and help ensure program integrity where the PPEO applies.
Deactivation can also occur under § 424.540 if: (1) the provider or supplier is voluntarily withdrawing from the Medicare program (that is, voluntarily terminating its Medicare enrollment) (§ 424.540(a)(7)); (2) the provider is the seller (and is hence leaving the Medicare program) in an HHA change in majority ownership under § 424.550(b) (§ 424.540(a)(8)); or (3) an individual provider or supplier is deceased (§ 424.540(a)(6)). The same concerns we expressed regarding reactivations following a § 424.540(a)(1) deactivation apply to these three deactivation bases. If a reactivation request arrives after the provider or supplier was deactivated upon departing the Medicare program, the provider's or supplier's former enrollment may have been compromised by an unscrupulous party. Even if no improper conduct is involved and the voluntarily terminated provider or supplier simply wishes to reenter and resume billing Medicare, they are effectively returning to the program as a new provider or supplier after having departed. This situation is not appreciably different from that where the provider or supplier is enrolling in Medicare for the first time. Given this, we believe that providers and suppliers that are reactivating their enrollment after having left the Medicare program should be subject to the same PPEO analysis as other providers and suppliers who are treated as an initial enrollee for Medicare provider enrollment purposes, for consistency and uniformity.
For all the foregoing reasons, we propose to add a new paragraph (a)(4) to § 424.527 that includes providers and suppliers that are reactivating their enrollment and billing privileges under § 424.540(b). We have elected to address this issue via rulemaking in proposed § 424.527(a)(4). However, we retain the authority under section 1866(j)(3)(B) of the Act to establish and implement PPEO procedures via subregulatory guidance.
Under the Paperwork Reduction Act of 1995, we are required to provide a 60-day notice in the Federal Register and solicit public comment before a collection of information requirement is submitted to the Office of Management and Budget (OMB) for review and approval. In order to fairly evaluate whether an information collection should be approved by OMB, section 3506(c)(2)(A) of the Paperwork Reduction Act of 1995 requires that we solicit comment on the following issues:
- The need for the information collection and its usefulness in carrying out the proper functions of our agency.
- The accuracy of our estimate of the information collection burden.
- The quality, utility, and clarity of the information to be collected.
- Recommendations to minimize the information collection burden on the affected public, including automated collection techniques.
In the CY 2024 HH PPS rule, we solicited public comment on each of these issues for the following sections of this document that contain information collection requirements (ICRs).
As discussed in section III.D.3. of this proposed rule, we are proposing to collect four additional items as standardized patient assessment data elements and replace one item collected as a standardized patient assessment data element beginning with the CY 2027 HH QRP. The four assessment items proposed for collection are (1) Living Situation, (2) Food Runs Out, (3) Food Doesn't Last, and (4) Utilities. We also propose replacing the current Access to Transportation item with a revised Transportation (Access to Transportation) item beginning with the CY 2027 HH QRP as outlined in section III.D.5. of this proposed rule. All elements discussed will be collected at the start of care timepoint. We assumed the Living Situation and Utilities data elements require 0.3 minutes each of clinician time to complete. We assume the Food Runs Out and Food Doesn't Last data elements require 0.15 minutes each of clinician time to complete. We assume the replacement of the current Access to Transportation item with a revised Transportation will not result in a change in burden. Therefore, we estimated that there will be an increase Start Printed Page 55411 in clinician burden per OASIS assessment of 0.9 minutes at start of care.
As stated in section III.E. of this proposed rule, CMS is also proposing an update to the removal of the suspension of OASIS all-payer data collection to change all-payer data collection beginning with the start of care OASIS data collection timepoint instead of discharge timepoint. There is no associated change in burden resulting from this proposal as burden for collection of for non-Medicare/non-Medicaid patients at all OASIS data collection timepoints was estimated in the CY 2023 HH PPS final rule.
The net effect of these proposals is an increase in four data elements collected at the start of care for the OASIS implemented on January 1, 2027.
For purposes of calculating the costs associated with the information collection requirements, we obtained median hourly wages for these from the U.S. Bureau of Labor Statistics' May 2023 National Occupational Employment and Wage Estimates ( https://www.bls.gov/oes/current/oes_nat.htm ). To account for other indirect costs such as overhead and fringe benefits (100 percent), we have doubled the hourly wage. These amounts are detailed in table 41.

The OASIS is completed by RNs or PTs, or very occasionally by occupational therapists (OT) or speech language pathologists (SLP/ST). Data from 2021 show that the SOC/ROC OASIS is completed by RNs (approximately 77.14 percent of the time), PTs (approximately 22.16 percent of the time), and other therapists, including OTs and SLP/STs (approximately 0.7 percent of the time). Based on this analysis, we estimated a weighted clinician average hourly wage of $85.73, inclusive of fringe benefits, using the hourly wage data in table 41 0.7714 × 82.76+0.2216 × 95.98 + 0.007 × 89.26 = 85.74. Individual providers determine the staffing resources necessary.
For purposes of estimating burden, we compare the item-level burden estimates for the OASIS that will be released on January 1, 2027, to the OASIS-E1 as anticipated for implementation as of January 1, 2025, and finalized in CY2024 HH PPS Final Rule. The first component needed to calculate burden is the total estimated assessments for each year in question. Table 42 shows the total number of OASIS assessments that HHAs completed in CY 2023 at start of care and resumption of care. It also outlines the estimated assessments that are expected to be collected in 2025 based on a thirty percent increase in completed assessments required for all payer data submission requirements for (CY23 assessment total + CY23 assessment total *0.3 = Estimated CY25 Assessment total based on all payer data collection).

The totals from table 42 are used to calculate the hourly burden estimates in table B3 based on the following calculations:
Estimated time spent per each 2025 OASIS-E1 SOC Assessment/Patient = 56.4 clinician minutes
200 data elements × (range of 0.15 to 0.3) minutes per data element = 56.4 minutes of clinical time spent to complete data entry for the OASIS-E1 SOC assessment.
- 21 data elements counted as 0.15 minutes/data element (3.15 minutes)
- 9 data elements counted as 0.25 minutes/data element (2.25 minutes)
- 170 data elements counted as 0.30 minutes/data element (51 minutes)
Clinician Estimated hourly burden for all HHAs (11,904) for 2025 OASIS-E1 SOC assessments = 8,099,309 hours
56.4 clinician minutes per SOC assessment × 8,616,286 assessments = 485,958,530 minutes/60 minutes per hour = 8,099,309 hours for all HHAs
Estimated time spent per each 2027 OASIS SOC Assessment/Patient = 57.3 clinician minutes
204 data elements × (range of 0.15 to 0.3) minutes per data element = 57.3 minutes of clinical time spent to complete data entry for the OASIS SOC assessment.
- 23 data elements counted as 0.15 minutes/data element (3.45 minutes)
- 172 data elements counted as 0.30 minutes/data element (51.6 minutes)
Clinician Estimated hourly burden for all HHAs (11,904) for 2027 OASIS SOC assessments = 8,228,553 hours
57.3 clinician minutes per SOC assessment × 8,616,286 assessments = 493,713,188 = minutes/60 minutes per hour = 8,228,553 hours for all HHAs
Estimated time spent per each 2025 OASIS-E1 ROC Assessment/Patient = 47.1 minutes
169 data elements × (range of 0.15 to 0.3) minutes per data element = 47.1 minutes of clinical time spent to complete data entry for the OASIS-E1 ROC assessment
- 19 data elements counted as 0.15 minute/data element (2.85 minutes)
- 9 data elements counted as 0.25 minute/data element (2.25 minutes)
- 140 data elements counted as 0.30 minute/data element (42 minutes)
Clinician Estimated Hourly Burden for all HHAs for 2025 OASIS-E1 ROC assessments = 823,310 hours
47.1 clinician minutes per ROC assessment × 1,184,618 ROC assessments = 55,795,508 minutes/60 minutes = 929,925hours for all HHAs
Estimated time spent per each 2027 OASIS ROC Assessment/Patient = 48 minutes
173 data elements × (range of 0.15 to 0.3) minutes per data element = 48 minutes of clinical time spent to complete data entry for the OASIS ROC assessment
- 21 data elements counted as 0.15 minute/data element (3.15 minutes)
- 142 data elements counted as 0.30 minute/data element (42.6 minutes)
Clinician Estimated Hourly Burden for all HHAs for 2027 OASIS ROC assessments = 947,694 hours
48 clinician minutes per ROC assessment × 1,184,618 ROC assessments = 56,861,664 minutes/60 minutes = 947,694 hours for all HHAs
Table 43 summarizes the estimated clinician hourly burden for the OASIS that will be implemented in 2027 with this proposed rule's changes of an increase in four data elements at start of care and resumption of care compared to the anticipated 2025 OASIS-E1 burden. This is calculated by multiplying the total number of assessments by the increase in assessment time required. We calculate the 2025 and 2027 burden estimate in minutes and then calculate an hourly burden shown in table 43. We estimated a net increase of 147,013 hours of clinician burden across all HHAs or 12.35 hours (147,013/11,904) for each of the 11,904 active HHAs.

Table 44 summarizes the estimated clinician costs for the 2025 OASIS-E1 and the 2027 OASIS with the net addition of four data elements at start of care using CY 2023 BLS wage inputs. Total clinician cost for 2025 and 2027 is estimated by multiplying total hourly burden for each year as reported in table 43 by the weighted clinician average hourly wage of $85.74. We then calculate the difference in clinician estimated costs between 2027 and 2025. This calculates the estimated increase in costs associated with adding the four data elements at start of care and resumption of care. We estimate an increase in clinician costs $12,604,894.62 between 2027 and 2025 related to the implementation of the proposals outlined in this proposed rule across all HHAs or a $1,058.88 increase (12,604,894.62/11,904) for each of the 11,904 active HHAs. This increase in burden will begin with the January 1, 2027, OASIS assessments.

The RFI and the health equity update for the expanded HHVBP Model included in section IV. of this proposed rule do not result in an increase in costs to HHAs. Section 1115A(d)(3) of the Act exempts Innovation Center model tests and expansions, which include the expanded HHVBP Model, from the provisions of the PRA. Specifically, this section provides that the provisions of the PRA do not apply to the testing and evaluation of Innovation Center models or to the expansion of such models.
3. ICRs Related to Conditions of Participation (CoPs): Organization and Administration of Services (§ 484.105)
In section VII.A. of this proposed rule, we discuss our proposal to add a new standard at § 484.105(i), which would set forth a requirement for HHAs to establish an “acceptance to service” policy. This new standard would require the HHA to develop, implement, and maintain through an annual review a patient acceptance to service policy that addressed criteria related to the HHA's capacity to provide patient care, including, but not limited to, anticipated needs of the referred prospective patient, case load and case mix of the HHA, staffing levels of the HHA, and competencies and skills of the HHA staff. In addition, we propose the HHA would have to make public accurate information about the services offered by the HHA and any limitations related to the types of specialty services, service duration, and service frequency. We believe that most HHAs already have a policy related to the admission to service. The burden associated with this requirement is the burden required to develop, implement, and maintain an updated policy that would meet the requirements of this proposed rule, and the burden associated with making specified information available to the public.
Section 1861(o)(2) of the Act requires HHAs to have policies established by a group of professional personnel (associated with the agency or organization), including one or more physicians and one or more registered professional nurses. Therefore, we expect the HHA to utilize a physician and nurse to create and update the HHA's policies. We estimate there are 9,565 Medicare-certified HHAs and that this proposed new requirement would take 1 hour each of a physician and a registered nurse's time on a one-time basis, for an HHA to develop an acceptance to service policy at a cost of $321.84 per HHA ($82.76 + $239.08) and $3,078,400 for all HHA's ($791,599 + $2,286,800). We also estimate the HHA nurse would review the acceptance to service policy on an annual basis. This annual review would take 5 minutes for an HHA nurse at a cost of $7 per HHA ($82.76 × 5/60 minute = $6.90) or $65,999 for all HHAs ($6.90 × 9,565 = $65,999) to fulfill this requirement.

In addition, we estimate this proposed new requirement would take 15 minutes on a one-time basis for an HHA to the specified information public at a cost of $10.43 per HHA or $99,763 for all HHA's, based on the assumption that the HHA administrative professional will process this task. The average hourly rate for an administrative employee is $41.70, therefore it is $10.43 per HHA ($41.70 hour × 15/60 minutes = $10.43) or $99,763 for all HHA's ($10.43 × 9,565) to fulfill the requirement. We also estimate the HHA administrative professional would review this website annually to assure the continued accuracy of the posted information. This annual review would take 5 minutes at a cost of $3.48 per HHA ($41.70 × 5/60 minute = $3.48) or $33,286 for all HHA's (3.48 × 9,565 = $33,286) to fulfill this requirement.
Administrative professional: https://www.bls.gov/oes/current/oes436013.htm .
Section 1866(j)(3)(A) of the Act states that the Secretary shall establish procedures to provide for a provisional period of between 30 days and 1 year during which new providers and Start Printed Page 55414 suppliers—as the Secretary determines appropriate, including categories of providers or suppliers—will be subject to enhanced oversight. These procedures have been codified in § 424.527. As explained in section VII. of this proposed rule, we are proposing to expand the definition of “new provider or supplier” in § 424.527(a) (solely for purposes of applying a provisional period of enhanced oversight (PPEO)) to include providers and suppliers that are reactivating their Medicare enrollment and billing privileges under § 424.540(b). We do not anticipate any ICR burdens associated with this provision, for we are merely expanding an existing regulatory definition.
The ICR burden currently associated with § 483.80(g) is included under OMB control number 0938-1363; expiration date: April 30, 2026.
In section VII.B. of this proposed rule we discuss our proposals related to LTC requirements for acute respiratory illness reporting. At § 483.80(g)(1)(i) through (ix) and (g)(2), we propose to replace the existing reporting requirements for LTC facilities with new requirements to report information addressing respiratory illnesses. Beginning on January 1, 2025, facilities would be required to electronically report information about COVID-19, influenza, and RSV in a standardized format and frequency specified by the Secretary. To the extent to be determined by the Secretary, through this rulemaking cycle, we propose that the data elements for which reporting would be required include—
- Facility census;
- Resident vaccination status for a limited set of respiratory illnesses including but not limited to COVID-19, influenza, and RSV;
- Confirmed, resident cases of a limited set of respiratory illnesses including but not limited to COVID-19, influenza, and RSV (overall and by vaccination status); and
- Hospitalized residents with confirmed cases of a limited set of respiratory illnesses including but not limited to COVID-19, influenza, and RSV (overall and by vaccination status.).
In the absence of a declared national PHE for an acute respiratory illness, we propose that LTC facilities would continue to report these data on a weekly basis through a format specified by the Secretary and specifically we intend to continue reporting through the CDC's NHSN. There may be instances in which the Secretary may determine a need to change reporting frequency, such as during a future PHE, and we would provide appropriate notice and guidance at that time.
These proposals are scaled back and tailored from the current post-COVID-19 PHE requirements, continuing the collection of the minimal necessary data to maintain a level of situational awareness that would protect resident health and safety in LTC facilities across the country while reducing reporting burden on those facilities. However, during a declared Federal, state, or local PHE for a respiratory infectious disease we also propose that the Secretary may require facilities to report:
- Additional or modified data elements relevant to the PHE, including relevant confirmed infections among staff, supply inventory shortages, and relevant medical countermeasures and therapeutics inventories, usage, or both, and additional demographic factors.
Since the infection prevention and control program (IPCP) is the responsibility of the infection preventionist (IP), we anticipate that the IP would be responsible for reviewing and updating the policies and procedures for the facility's IPCP to comply with these new proposals. We estimate that it would require 2 hours of the IP's time to update the facility's policies and procedures to ensure that they reflect the proposed requirements. In analyzing the ICRs related to this proposal we obtained salary information from the May 2023 National Occupational Employment and Wage Estimates, BLS at https://www.bls.gov/oes/current/oes_nat.htm . We have calculated the estimated hourly rate for an IP using the occupation code for a registered nurse (29-1141) based on the national mean salary increased by 100 percent to account for overhead costs and fringe benefits ($45.42 × 2= $90.84 (rounded to $91). According to CMS, there are currently 14,926 LTC facilities as of April 2024. [ 160 ]
Based on this salary information and facility data, we estimate that total annual burden hours for all LTC facilities to review and update their current policies and procedures would be 29,852 hours (2 hours × 14,926 facilities) at a cost of $2,716,532 (29,852 × $91) or $182 ($91 × 2 hours) per facility annually.
In addition, LTC facilities will need to continue locating the required information and electronically reporting in the frequency specified to the NHSN. Currently, the ICR associated with this reporting requirement under OMB control #0938-1363 estimates a total burden cost of $55,972,800 (1 hour × 52 weeks × $69 (IP 2022 salary) × 15,600 LTC facilities as of 2022) based on weekly reporting. While the number of required data elements for ongoing reporting have decreased from the current post-COVID-19 PHE reporting requirements set to expire December 2024, we acknowledge that the data elements and reporting frequency could increase or decrease due to what the Secretary deems necessary based on changes in circumstance or given another PHE and these changes would impact this burden estimate. For instance, weekly data reporting could be decreased to bi-weekly reporting or the increased reporting of additional data elements during a PHE could be activated and remain active for less than or more than a year depending on the circumstances. Since we cannot predict with certainty how often the Secretary would require data reporting for a future PHE, we are including two burden estimates to cover a range in frequency of reporting. The lower range is based on weekly reporting and the higher range is based on daily reporting.
Based on the assumption of a weekly reporting frequency and 1 hour of the IP's time to locate and electronically report the information, we estimate that total annual burden hours for all LTC facilities to comply would be 776,152 hours (1 hour × 52 weeks × 14,926 facilities) at a cost of $70,629,832 (776,152 total hours × $91) or $4,732 ($91 × 1 hour × 52 weeks) per facility annually.
Based on the assumption of a daily reporting frequency, we estimate that total annual burden hours for all LTC facilities to comply would be 5,447,990 hours (1 hour × 365 days a year × 14,926 facilities) at a cost of $495,767,090 (5,447,990 total hours × $91) or $33,215 ($91 × 1 hour × 365 days a year) per facility annually.
In summary the total annual burden for all LTC facilities for these proposed ICRs is 806,004 to 5,477,842 hours at an estimated cost of $73,346,364 to $498,483,622 or 54 to 367 hours at an estimated cost of $4,914 to $33,397 per Start Printed Page 55415 facility annually. We will submit the revised information collection request to OMB for approval under OMB control number 0938-1363.

We welcome public comments on our ICR burden estimates, and on ways that reporting burden can be minimized while still providing adequate data. We also welcome feedback on any challenges of collecting and reporting these data; ways that CMS could reduce reporting burden for facilities; and alternative reporting mechanisms or quality reporting programs through which CMS could instead effectively and sustainably incentivize reporting. Lastly, we welcome comments that address system readiness and capacity to collect and report these data.
We have submitted a copy of this final rule to OMB for its review of the rule's information collection requirements. The requirements are not effective until they have been approved by OMB.
To obtain copies of the supporting statement and any related forms for the proposed collections, as previously discussed, please visit the CMS website at https://www.cms.hhs.gov/PaperworkReductionActof1995 , or call the Reports Clearance Office at 410-786-1326.
We invite public comments on these potential information collection requirements.
Section 1895(b)(1) of the Act requires the Secretary to establish a HH PPS for all costs of home health services paid under Medicare. In addition, section 1895(b) of the Act requires: (1) the computation of a standard prospective payment amount include all costs for home health services covered and paid for on a reasonable cost basis and that such amounts be initially based on the most recent audited cost report data available to the Secretary; (2) the prospective payment amount under the HH PPS to be an appropriate unit of service based on the number, type, and duration of visits provided within that unit; and (3) the standardized prospective payment amount be adjusted to account for the effects of case-mix and wage levels among HHAs. Section 1895(b)(3)(B) of the Act addresses the annual update to the standard prospective payment amounts by the home health applicable percentage increase. Section 1895(b)(4) of the Act governs the payment computation. Sections 1895(b)(4)(A)(i) and (b)(4)(A)(ii) of the Act requires the standard prospective payment amount be adjusted for case-mix and geographic differences in wage levels. Section 1895(b)(4)(B) of the Act requires the establishment of appropriate case-mix adjustment factors for significant variation in costs among different units of services. Lastly, section 1895(b)(4)(C) of the Act requires the establishment of wage adjustment factors that reflect the relative level of wages, and wage-related costs applicable to home health services furnished in a geographic area compared to the applicable national average level.
Section 1895(b)(3)(B)(iv) of the Act provides the Secretary with the authority to implement adjustments to the standard prospective payment amount (or amounts) for subsequent years to eliminate the effect of changes in aggregate payments during a previous year or years that were the result of changes in the coding or classification of different units of services that do not reflect real changes in case-mix. Section 1895(b)(5) of the Act provides the Secretary with the option to make changes to the payment amount otherwise paid in the case of outliers because of unusual variations in the type or amount of medically necessary care. Section 1895(b)(3)(B)(v) of the Act requires HHAs to submit data for purposes of measuring health care quality and links the quality data submission to the annual applicable percentage increase.
Sections 1895(b)(2) and 1895(b)(3)(A) of the Act, as amended by sections 51001(a)(1) and 51001(a)(2) of the BBA of 2018 respectively, required the Secretary to implement a 30-day unit of service, for 30-day periods beginning on and after January 1, 2020. Section 1895(b)(3)(D)(i) of the Act, as added by section 51001(a)(2)(B) of the BBA of 2018, requires the Secretary to annually determine the impact of differences between assumed behavior changes, as described in section 1895(b)(3)(A)(iv) of the Act, and actual behavior changes on estimated aggregate expenditures under the HH PPS with respect to years beginning with 2020 and ending with 2026. Section 1895(b)(3)(D)(ii) of the Act requires the Secretary, at a time and in a manner determined appropriate, through notice and comment rulemaking, to provide for one or more permanent increases or decreases to the standard prospective payment amount (or amounts) for applicable years, on a prospective basis, to offset for such increases or decreases in estimated aggregate expenditures, as determined under section 1895(b)(3)(D)(i) of the Act. Additionally, 1895(b)(3)(D)(iii) of the Act requires the Secretary, at a time and in a manner determined appropriate, through notice and comment rulemaking, to provide for one or more temporary increases or decreases to the payment amount for a unit of home health services for applicable years, on a prospective basis, to offset for such increases or decreases in estimated aggregate expenditures, as determined under section 1895(b)(3)(D)(i) of the Act. The HH PPS wage index utilizes the wage adjustment factors used by the Secretary for purposes of sections 1895(b)(4)(A)(ii) and (b)(4)(C) of the Act for hospital wage adjustments.
Section 1895(b)(3)(B)(v) of the Act authorizes the HH QRP, which requires Start Printed Page 55416 HHAs to submit data in accordance with the requirements specified by CMS. Failure to submit data required under section 1895(b)(3)(B)(v) of the Act with respect to a program year will result in the reduction of the annual home health market basket percentage increase otherwise applicable to an HHA for the corresponding calendar year by 2 percentage points.
In the CY 2022 HH PPS final rule ( 86 FR 62292 through 62336 ) and codified at 42 CFR part 484, subpart F , we finalized our policy to expand the HHVBP Model to all Medicare certified HHAs in the 50 States, territories, and District of Columbia beginning January 1, 2022. CY 2022 was a pre-implementation year. CY 2023 was the first performance year in which HHAs individual performance on the applicable measures will affect their Medicare payments in CY 2025. In this proposed rule, we include a request for information (RFI) related to the future measure concepts for the expanded HHVBP Model. We also provide an update on potential future approaches for integrating health equity that are being considered for the expanded HHVBP Model.
Division FF, section 4134 of the CAA, 2023 ( Pub. L. 117-328 ) mandated that CMS establish a permanent, bundled payment for items and services related to administration of IVIG in a patient's home. The permanent, bundled home IVIG items and services payment is effective for home IVIG infusions furnished on or after January 1, 2024. Payment for these items and services is required to be a separate bundled payment made to a supplier for all items and services furnished in the home during a calendar day. This payment amount may be based on the amount established under the Demonstration. The standard Part B coinsurance and the Part B deductible apply. The separate bundled payment does not apply for individuals receiving services under the Medicare home health benefit. The CAA, 2023 provision clarifies that a supplier who furnishes these services meet the requirements of a supplier of medical equipment and supplies.
In sections 1861(o) and 1891 of the Act, the Secretary has established in regulations the requirements that an HHA must meet to participate in the Medicare program. These requirements are set forth in regulations at 42 CFR part 484 , Home Health Services, and regulations at 42 CFR 440.70(d) specify that HHAs participating in the Medicaid program must also meet the Medicare Conditions of Participation (CoPs). Section 1861(o)(6) of the Act requires that an HHA must meet the CoPs specified in section 1891(a) of the Act, and other CoPs as the Secretary finds necessary in the interest of the health and safety of patients. The CoPs for HHAs protect all individuals under the HHA's care, unless a requirement is specifically limited to Medicare beneficiaries. As explained in section VI.A. of this proposed rule, we are proposing to add a new standard at § 484.105(i) that would require HHAs to develop, consistently apply, and maintain an acceptance to service policy, including specified factors, that would govern the process for accepting patients to service. We also propose that HHAs would be required to make specified information about their services and service limitations available to the public. In this proposed rule, we include a request for information (RFI) to obtain information from stakeholders on whether CMS should shift its longstanding policy and permit rehabilitative therapists to conduct the initial and comprehensive assessment for cases that have both therapy and nursing services ordered as part of the plan of care. In addition, we are seeking public comments on other factors that influence the patient referral and intake processes.
Section 1866(j)(3)(A) of the Act states that the Secretary shall establish procedures to provide for a provisional period of between 30 days and 1 year during which new providers and suppliers—as the Secretary determines appropriate, including categories of providers or suppliers—will be subject to enhanced oversight. These procedures have been codified in 42 CFR 424.527 . As explained in section VII. of this proposed rule, we are proposing to expand the definition of “new provider or supplier” in § 424.527(a) (solely for purposes of applying a provisional period of enhanced oversight (PPEO) to include providers and suppliers that are reactivating their Medicare enrollment and billing privileges under § 424.540(b).
Sections 1819(d)(3) and 1919(d)(3) of the Act explicitly require that LTC facilities develop and maintain an infection control program that is designed, constructed, equipped, and maintained in a manner to protect the health and safety of residents, personnel, and the general public. In addition, sections 1819(d)(4)(B) and 1919(d)(4)(B) of the Act explicitly authorize the Secretary to issue any regulations he deems necessary to protect the health and safety of residents. As such, we are proposing streamlined weekly data reporting requirements for certain respiratory illnesses. We are also proposing additional, related data elements that could be activated in the event of a future acute respiratory illness PHE.
We have examined the impacts of this proposed rule as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (January 18, 2011), Executive Order 14094 on Modernizing Regulatory Review (April 6, 2023), the Regulatory Flexibility Act (RFA) (September 19, 1980, Pub. L. 96 354), section 1102(b) of the Act, section 202 of the Unfunded Mandates Reform Act of 1995 (March 22, 1995; Pub. L. 104-4 ), Executive Order 13132 on Federalism (August 4, 1999), and the Congressional Review Act ( 5 U.S.C. 804(2) ).
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). Executive Order 14094 amends section 3(f) of Executive Order 12866 to define a “significant regulatory action” as an action that is likely to result in a rule: (1) having an annual effect on the economy of $200 million or more in any 1 year, or adversely affect in a material way the economy, productivity, competition, jobs, the environment, public health or safety, or State, local, or Tribal governments or communities; (2) creating a serious inconsistency or otherwise interfering with an action taken or planned by another agency; (3) materially altering the budgetary impacts of entitlement grants, user fees, or loan programs or the rights and obligations of recipients thereof; or (4) raising legal or policy issues for which centralized review would meaningfully further the President's priorities or the principles set forth in this Executive order.
A regulatory impact analysis (RIA) must be prepared for significant rules. Start Printed Page 55417 Based on our estimates, OMB'S Office of Information and Regulatory Affairs has determined this rulemaking is significant per section 3(f)(1) as measured by the $200 million or more in any 1 year. Accordingly, we have prepared a regulatory impact analysis that to the best of our ability presents the costs and benefits of the rulemaking. Therefore, OMB has reviewed this proposed rule, and the Departments have provided the following assessment of their impact. We solicit comments on the regulatory impact analysis provided.
This rule proposes to update Medicare payments under the HH PPS for CY 2025. The net transfer impact related to the changes in payments under the HH PPS for CY 2025 is estimated to be −$280 million (−1.7 percent). The $280 million decrease in estimated payments for CY 2025 reflects the effects of the proposed CY 2025 home health payment update percentage of 2.5 percent ($415 million increase), an estimated 3.6 percent decrease that reflects the effects of the permanent adjustment ($595 million decrease), and an estimated 0.6 percent decrease that reflects the effects of an updated FDL ($100 million decrease).
We use the latest data and analysis available. However, we do not adjust for future changes in such variables as number of visits or case-mix. This analysis incorporates the latest estimates of growth in service use and payments under the Medicare home health benefit, based primarily on Medicare claims data for periods that ended on or before December 31, 2023. We note that certain events may combine to limit the scope or accuracy of our impact analysis, because such an analysis is future-oriented and, thus, susceptible to errors resulting from other changes in the impact time period assessed. Some examples of such possible events are newly-legislated general Medicare program funding changes made by the Congress or changes specifically related to HHAs. In addition, changes to the Medicare program may continue to be made as a result of new statutory provisions. Although these changes may not be specific to the HH PPS, the nature of the Medicare program is such that the changes may interact, and the complexity of the interaction of these changes could make it difficult to predict accurately the full scope of the impact upon HHAs.
Table 48 represents how HHA revenues are likely to be affected by the proposed policy changes for CY 2025. For this analysis, we used an analytic file with linked CY 2023 OASIS assessments and home health claims data for dates of service that ended on or before December 31, 2023. The first column of table 48 classifies HHAs according to a number of characteristics including provider type, geographic region, and urban and rural locations. The second column shows the number of facilities in the impact analysis. The third column shows the payment effects of the permanent assumption adjustment on all payments. The aggregate impact of the permanent adjustment reflected in the third column does not equal the proposed −4.067 percent permanent adjustment because the adjustment only applies to the national, standardized 30-day period payments and does not impact payments for 30-day periods which are LUPAs. The fourth column shows the payment effects of the recalibration of the case-mix weights offset by the case-mix weights budget neutrality factor. The fifth column shows the payment effects of updating the CY 2025 wage index (that is, the FY 2025 hospital pre-floor, pre-reclassified wage index for hospital cost reporting periods beginning on or after October 1, 2020, and before October 1, 2021 (FY 2021 cost report data)) with the revised OMB delineations and a 5-percent cap on wage index decreases. The aggregate impact of the changes in the fifth column is zero percent, due to the wage index budget neutrality factor. The sixth column shows the payment impacts of the proposed update to the LUPA add-on factors. The seventh column shows the payment effects of the proposed CY 2025 home health payment update percentage. The eighth column shows the payment effects of the revised FDL, and the last column shows the combined effects of all the proposed provisions.
Overall, it is projected that aggregate payments in CY 2025 would decrease by 1.7 percent which reflects the 3.6 percent decrease from the permanent adjustment, the 2.5 payment update percentage increase, and the 0.6 percent decrease from increasing the FDL. As illustrated in table 48, the combined effects of all changes vary by specific types of providers and by location. We note that some individual HHAs within the same group may experience different impacts on payments than others due to the distributional impact of the CY 2025 wage index, the percentage of total HH PPS payments that were subject to the LUPA or paid as outlier payments, and the degree of Medicare utilization.

Failure to submit HH QRP data required under section 1895(b)(3)(B)(v) of the Act with respect to a program year will result in the reduction of the annual home health market basket percentage increase otherwise applicable to an HHA for the corresponding calendar year by 2 percentage points. For the CY 2023 program year, 820 of the 11,549 active Medicare-certified HHAs, or approximately 7.1 percent, did not receive the full annual percentage increase because they did not meet assessment submission requirements. The 820 HHAs that did not satisfy the reporting requirements of the HH QRP for the CY 2023 program year represent $149 million in home health claims payment dollars during the reporting period out of a total $16.4 billion for all HHAs.
This proposed rule proposes to collect four additional items as standardized patient assessment data elements and replace one item collected as a standardized patient assessment data element beginning with the CY 2027 HH QRP. The four assessment items proposed for collection are (1) Living Situation, (2) Food Runs Out, (3) Food Doesn't Last, and (4) Utilities. We also propose replacing the current Access to Transportation item with a revised Transportation (Access to Transportation) item beginning with the CY 2027 HH QRP. CMS is also proposing an update to the removal of the suspension of OASIS all-payer data collection to change all-payer data collection beginning with the start of care OASIS data collection timepoint instead of discharge timepoint. The net effect of these proposals is an increase of four data elements at the start of care time point and a net increase in burden.
Section VIII.B.1. of this proposed rule provides a detailed description of the net increase in burdens associated with the proposed changes. We proposed that additions of data elements associated with the HH QRP proposals would begin with January 1, 2027, discharges. The cost impact of these proposed changes was estimated to be a net increase of $12,604,894.62 in annualized cost to HHAs, discounted at 2 percent relative to year 2023, over a perpetual time horizon beginning in CY 2027. We described the estimated burden and cost reductions for these measures in section VIII. of this proposed rule. In summary, the implementation of proposals outlined in this proposed rule for the HH QRP is estimated to increase the burden on HHAs by $1,058.88 per HHA annually, or $12,604,894.62 for all HHAs annually.
There are no proposed changes to the expanded HHVBP Model for CY 2025. Therefore, we assume there are no impacts resulting from this proposed rule. Furthermore, the public comments received related to the Request for Information on Future Performance Measure Concepts for the Expanded HHVBP Model and the update on Future Approaches to Health Equity in the Expanded HHVBP Model, included in section IV. of this proposed rule, will be summarized in the final rule and may inform proposals through future rulemaking.
The following analysis applies to the home IVIG items and services payment rate as set forth in section V.D.1. of this proposed rule as added by section 4134 of the CAA, 2023 and accordingly, describes the impact for CY 2025 only. Table 49 represents the estimated aggregate costs of home IVIG users for CY 2025. We used CY 2023 data to identify beneficiaries actively enrolled in the IVIG demonstration (that is, beneficiaries with Part B claims that contain the Q2052 HCPCS code) to estimate the number of potential CY 2025 active enrollees in the new benefit, which are shown in column 2. In column 3, CY 2023 claims for IVIG visits under the Demonstration were again used to estimate potential utilization under the new benefit in CY 2025. Column 4 shows the proposed CY 2025 home IVIG items and services rate. The fifth column estimates the cost to Medicare for CY 2025 ($9,435,233). Table 50 represents the estimated impacts of the home IVIG items and services payment for CY 2025 by census region.

We propose to add a new standard § 484.105(i), which sets forth a requirement for HHAs to establish an acceptance to service policy. All cost associated with this policy are located in the section VIII. of this proposed rule (Collection of Information). There are no transfers associated with this requirement.
For purposes of applying a PPEO, we are proposing to expand the definition of “new provider or supplier” in § 424.527(a) to include providers and suppliers that are reactivating their Medicare enrollment and billing privileges under § 424.540(b). However, we are unable to establish an estimate of any potential burden associated with this provision for two main reasons. First, we do not have sufficient data upon which we can formulate a burden projection. Second, we cannot predict the scope, extent, and length of any future PPEO or the provider or supplier type(s) to which it may apply. Accordingly, we solicit public comment from stakeholders on the potential burden of our expansion of § 424.527(a).
We propose to update the requirements related to reporting acute respiratory illnesses for LTC facilities at § 483.80(g). All cost associated with this policy are located in the section IX. of this proposed rule (Collection of Information). There are no transfers associated with this requirement. We welcome public comments on our estimates, and on ways that reporting burden can be minimized while still providing adequate data. We also welcome feedback on any challenges of collecting and reporting these data; ways that CMS could reduce reporting burden for facilities; and alternative reporting mechanisms or quality reporting programs through which CMS could instead effectively and sustainably incentivize reporting. Lastly, we welcome comments that address system readiness and capacity to collect and report these data.
If regulations impose administrative costs on private entities, such as the time needed to read and interpret this proposed rule, we should estimate the cost associated with regulatory review. Due to the uncertainty involved with accurately quantifying the number of entities that will review the rule, we assume that the total number of unique commenters on last year's proposed rule will be the number of reviewers of this proposed rule. We acknowledge that this assumption may understate or overstate the costs of reviewing this proposed rule. It is possible that not all commenters reviewed last year's rule in detail, and it is also possible that some reviewers chose not to comment on the proposed rule. For these reasons we thought that the number of past commenters would be a fair estimate of the number of reviewers of this proposed rule. We welcome any comments on the approach used in estimating the number of entities reviewing this proposed rule.
We recognize that different types of entities are in many cases affected by mutually exclusive sections of this proposed rule. Therefore, for the purposes of our estimate we assume that each reviewer reads approximately 50 percent of the rule. Finally, in our estimates, we have used the 948 number of timely pieces of correspondence on the CY 2024 HH PPS proposed rule as our estimate for the number of reviewers of this proposed rule. We continue to acknowledge the uncertainty involved with using this number, but we believe it is a fair estimate due to the variety of entities affected and the likelihood that some of them choose to rely (in full or in part) on press releases, newsletters, fact sheets, or other sources rather than the comprehensive review of preamble and regulatory text. We seek comments on this assumption. Using the median hourly wage information from the BLS for medical and health service managers (Code 11-9111), we estimate that the cost of reviewing the proposed rule is $106.42 per hour, including overhead and fringe benefits ( https://www.bls.gov/oes/current/oes_nat.htm ). Assuming an average reading speed, we estimate that Start Printed Page 55422 it would take approximately 2.77 hours for the staff to review half of this proposed rule. For each entity that reviews this proposed rule, the estimated cost is $294.78 (2.77 hours × $106.42). Therefore, we estimate that the total cost of reviewing this proposed rule is $279,451 ($294.78 × 948 reviewers).
For the CY 2025 HH PPS proposed rule, we considered alternatives to the provisions articulated in section II.C. of this proposed rule. As described in section II.C.1.b. of this proposed rule, we proposed a mapping of three OASIS items (therapies, vision, and pain) in order to impute the responses from the OASIS-E to the OASIS-D to create simulated 60-day episodes from 30-day periods. We considered not proposing the mapping methodology; however, to continue with the best reading of the law and the finalized methodology for assessing behavior changes we proposed the OASIS mapping.
As described in section II.C.1.g. of this proposed rule, to achieve appropriate payments, we calculated a permanent adjustment by determining what the 30-day base payment amount should have been in CYs 2020, 2021, 2022, and 2023 in order to achieve the same estimated aggregate expenditures as obtained from the simulated 60-day episodes. One alternative to the proposed −4.067 percent permanent adjustment included halving the proposed permanent adjustment similar to how we finalized the permanent adjustment for CY 2024. Another alternative would be a phase-in approach, where we could reduce the permanent adjustment, by spreading out the CY 2025 permanent adjustment over a specified period of years, rather than halving the adjustment in CY 2025. Another alternative would be to delay the permanent adjustment to a future year. However, we believe that a reduction, a phase-in approach, or delay in the permanent adjustment would not be appropriate, as reducing, phasing in, or delaying the permanent adjustment would further impact budget neutrality and likely lead to a compounding effect creating the need for a larger reduction to the payment rate in future years.
We also considered proposing to implement the one-time temporary adjustment to reconcile retrospective overpayments in CYs 2020, 2021, 2022, and 2023. However, as stated previously in this proposed rule, we believe that implementing both the permanent and temporary adjustments to the CY 2025 payment rate may adversely affect HHAs given the magnitude of the adjustment to the payment rate in a single year. Likewise, section 1895(b)(3)(D)(iii) of the Act gives CMS the authority to make any temporary adjustment in a time and manner appropriate though notice and comment rulemaking. Therefore, we believe it is best to propose only the implementation of the permanent decrease of 4.067 percent to the CY 2025 base payment rate.
Finally, we considered not proposing to adopt the OMB delineations listed in OMB Bulletin 23-01. However, we have historically adopted the latest OMB delineations in subsequent rulemaking after a new OMB Bulletin is released.
For the CY 2025 HH PPS proposed rule, we did not consider alternatives to implementing the home IVIG items and services payment for CY 2025 because section 1842(o)(8) of the Act requires the Secretary to establish a separate bundled payment to the supplier for all items and services related to the administration of intravenous immune globulin to an individual in the patient's home during a calendar day effective January 1, 2024.
As required by OMB Circular A-4 (available at https://www.whitehouse.gov/wp-content/uploads/legacy_drupal_files/omb/circulars/A4/a-4.pdf ), in table 51, we have prepared an accounting statement showing the classification of the transfers and benefits associated with the CY 2025 HH PPS provisions of this proposed rule.

As required by OMB Circular A-4 (available at https://www.whitehouse.gov/wp-content/uploads/legacy_drupal_files/omb/circulars/A4/a-4.pdf ), in table 52, we have prepared an accounting statement showing the classification of the costs associated with the ICRs for the proposed HH QRP provisions in CY 2027.

As required by OMB Circular A-4 (available at https://www.whitehouse.gov/wp-content/uploads/legacy_drupal_files/omb/circulars/A4/a-4.pdf ), in table 53, we have prepared an accounting statement showing the classification of the transfers and benefits associated with the CY 2025 IVIG provisions of this proposed rule.

The RFA requires agencies to analyze options for regulatory relief of small entities, if a rule has a significant impact on a substantial number of small entities. For purposes of the RFA, small entities include small businesses, nonprofit organizations, and small governmental jurisdictions. In addition, HHAs are small entities, as that is the term used in the RFA. Individuals and States are not included in the definition of a small entity.
The North American Industry Classification System (NAICS) was adopted in 1997 and is the current standard used by the Federal statistical agencies related to the U.S. business economy. We utilized the NAICS U.S. industry title “Home Health Care Services” and corresponding NAICS code 621610 in determining impacts for small entities. The NAICS code 621610 has a size standard of $19 million [ 161 ] and approximately 96 percent of HHAs are considered small entities. Table 54 shows the number of firms, revenue, and estimated impact per home health care service category.

The economic impact assessment is based on estimated Medicare payments (revenues) and HHS's practice in interpreting the RFA is to consider effects economically “significant” only if greater than 5 percent of providers reach a threshold of 3 to 5 percent or more of total revenue or total costs. The majority of HHAs' visits are Medicare paid visits and therefore the majority of HHAs' revenue consists of Medicare payments. Based on our analysis, we conclude that the policies proposed in this rule would result in an estimated total impact of 3 to 5 percent or more on Medicare revenue for greater than 5 percent of HHAs. Therefore, the Secretary has determined that this HH PPS proposed rule will have significant economic impact on a substantial number of small entities. Specifically, we estimate that the net impact of the policies in this proposed rule will have a significant impact on hospices in the Start Printed Page 55424 New England, Mid Atlantic, and Pacific regions, which is reflected in the last column in table 48 as a greater than 33 percent decrease in expenditures when comparing CY 2025 payments to estimated CY 2024 payments. The reason for the net decrease in CY 2025 is mostly driven by the impact of the permanent adjustment reflected in the third column of table 48. Further detail is presented in table 48, by HHA type and location.
Regarding options for regulatory relief, we note that section 1895(b)(3)(D)(i) of the Act requires CMS to annually determine the impact of differences between the assumed behavior changes finalized in the CY 2019 HH PPS final rule with comment period ( 83 FR 56455 ) and actual behavior changes on estimated aggregate expenditures under the HH PPS with respect to years beginning with 2020 and ending with 2026. Additionally, section 1895(b)(3)(D)(ii) and (iii) of the Act requires us to make permanent and temporary adjustments to the payment rate to offset for such increases or decreases in estimated aggregate expenditures through notice and comment rulemaking. While we find that the −4.067 percent permanent adjustment, described in section II.C.1.g. of this proposed rule, is necessary to offset the increase in estimated aggregate expenditures for CYs 2020 through 2023 based on the impact of the differences between assumed behavior changes and actual behavior changes, we will also continue to reprice claims, per the finalized methodology, and make any additional adjustments at a time and manner deemed appropriate in future rulemaking. As discussed previously, we also explored alternatives to the proposed −4.067 percent permanent adjustment including a phase-in approach, where we could reduce the permanent adjustment, by spreading out the CY 2025 permanent adjustment over a period of years. Another alternative would be to delay the permanent adjustment to a future year. However, we believe that a reduction to the permanent adjustment, a phase-in approach, or delay in the permanent adjustment would not be appropriate, as reducing, phasing in, or delaying the permanent adjustment would further impact budget neutrality and likely lead to a compounding effect creating the need for a larger reduction to the payment rate in future years. We also considered proposing to implement the one-time temporary adjustment to reconcile retrospective overpayments in CYs 2020, 2021, 2022, and 2023. However, as stated previously in this proposed rule, we recognize that applying the full permanent and temporary adjustments to the CY 2025 payment rate may adversely affect HHAs, including small entities. We are soliciting comments on the overall HH PPS RFA analysis.
Among the over 7,500 HHAs that are estimated to qualify to compete in the expanded HHVBP Model, we estimate that the percent payment adjustment resulting from this proposed rule would be larger than 3 percent, in magnitude, for about 28 percent of competing HHAs (estimated by applying the proposed 5-percent maximum payment adjustment under the expanded Model to CY 2019 data). As a result, more than the RFA threshold of 5-percent of HHA providers nationally would be significantly impacted. We refer readers to tables 43 and 44 in the CY 2022 HH PPS final rule ( 86 FR 62407 through 62410 ) for our analysis of payment adjustment distributions by State, HHA characteristics, HHA size, and percentiles.
In addition, section 1102(b) of the Act requires us to prepare an RIA if a rule may have a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 603 of RFA. For purposes of section 1102(b) of the Act, we define a small rural hospital as a hospital that is located outside of a metropolitan statistical area and has fewer than 100 beds. This proposed rule is not applicable to hospitals. Therefore, the Secretary has certified that this proposed rule would not have a significant economic impact on the operations of small rural hospitals.
Section 202 of UMRA of 1995 UMRA also requires that agencies assess anticipated costs and benefits before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. In 2024, that threshold is approximately $183 million. This proposed rule would not impose a mandate that will result in the expenditure by State, local, and Tribal governments, in the aggregate, or by the private sector, of more than $183 million in any one year.
Executive Order 13132 establishes certain requirements that an agency must meet when it promulgates a proposed rule (and subsequent final rule) that imposes substantial direct requirement costs on State and local governments, preempts State law, or otherwise has federalism implications. We have reviewed this proposed rule under these criteria of Executive Order 13132 and have determined that it would not impose substantial direct costs on State or local governments.
In conclusion, we estimate that the provisions in this proposed rule will result in an estimated net decrease in home health payments of 1.7 percent for CY 2025 (−$280 million). The $280 million decrease in estimated payments for CY 2025 reflects the effects of the proposed CY 2025 home health payment update percentage increase of 2.5 percent ($415 million increase), a 0.6 percent decrease in payments due to the new higher FDL ratio, which will decrease outlier payments in order to target to pay no more than 2.5 percent of total payments as outlier payments ($100 million decrease), and an estimated 3.6 percent decrease in payments that reflects the effects of the permanent behavior adjustment ($595 million decrease). In addition, the estimated aggregate impacts of the home IVIG items and services payment for CY 2025 is $9.4 million. Lastly, the implementation of the HH QRP proposed policy is estimated to increase the costs to HHAs by $1,058.88 per HHA annually, or $12,604,894.62 in the aggregate for HHAs annually.
Because of the large number of public comments we normally receive on Federal Register documents, we are not able to acknowledge or respond to them individually. We will consider all comments we receive by the date and time specified in the DATES section of this preamble, and, when we proceed with a subsequent document, we will respond to the comments in the preamble to that document.
Chiquita Brooks-LaSure, Administrator of the Centers for Medicare & Medicaid Services, approved this document on June 12, 2024.
- Emergency medical services
- Health facilities
- Health professions
- Reporting and recordkeeping requirements
- Grant programs—health
- Health records
For the reasons set forth in the preamble, the Centers for Medicare & Medicaid Services proposes to amend 42 CFR chapter IV as follows:
1. The authority for part 424 continues to read as follows:
Authority: 42 U.S.C. 1302 and 1395hh .
2. Section 424.527 is amended by adding paragraph (a)(4) to read as follows:
(4) A provider or supplier reactivating the provider's or supplier's Medicare enrollment and billing privileges in accordance with § 424.540(b).
3. The authority citation for part 483 continues to read as follows:
Authority: 42 U.S.C. 1302 , 1320a-7 , 1395i , 1395hh and 1396r .
4. Section 483.80 is amended by revising paragraph (g) to read as follows:
(g) Respiratory illness reporting —(1) Ongoing reporting. The facility must electronically report information on acute respiratory illnesses, including influenza, SARS-CoV-2/COVID-19, and RSV.
(i) The report must be in a standardized format and frequency specified by the Secretary.
(ii) To the extent as required by the Secretary, this report must include all of the following data elements:
(A) Facility census (defined as the total number of residents occupying a bed at this facility for at least 24 hours during the week of data collection).
(B) Resident vaccination status for a limited set of respiratory illnesses, including but not limited to the following:
( 2 ) SARS-CoV-2/COVID-19.
(C) Confirmed, resident cases of a limited set of respiratory illnesses, including but not limited to the following:
(D) Hospitalized residents with confirmed cases of a limited set of respiratory illnesses, including but not limited to the following:
(2) Public health emergency (PHE) reporting. In the event that the Secretary has declared a national, State, or local PHE for an acute infectious illness or determined that a significant threat for one exists, the facility must also electronically report all of the following data elements in a standardized format and frequency specified by the Secretary:
(i) Relevant confirmed infections for staff.
(ii) Supply inventory shortages.
(iii) Staffing shortages.
(iv) Relevant medical countermeasures and therapeutic inventories, usage, or both.
5. The authority citation for part 484 continues to read as follows:
6. Section 484.105 is amended by adding paragraph (i) to read as follows:
(i) HHA acceptance to service. An HHA must do both of the following:
(1) Develop, implement, and maintain through an annual review, a patient acceptance to service policy that is applied consistently to each prospective patient referred for home health care, which addresses criteria related to the HHA's capacity to provide patient care, including, but not limited to, all of the following:
(i) Anticipated needs of the referred prospective patient.
(ii) Case load and case mix of the HHA.
(iii) Staffing levels of the HHA.
(iv) Skills and competencies of the HHA staff.
(2) Make available to the public accurate information regarding the services offered by the HHA and any limitations related to types of specialty services, service duration, or service frequency. The information is reviewed at least annually.
Xavier Becerra,
Secretary, Department of Health and Human Services.
1. Report to Congress, Medicare Payment Policy. Home Health Care Services, Chapter 7. MedPAC. March 2024 ( https://www.medpac.gov/wp-content/uploads/2024/03/Ch7_Mar24_MedPAC_Report_To_Congress_SEC.pdf ).
2. CMS continues to use the M1800-1860 items to determine functional impairment level for case mix purposes while we continue to analyze the relationship between the analogous GG items (required as standardized patient assessment data) and the M1800 items used for payment.
3. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/HH-PDGM .
4. Medicare Benefit Policy Manual, Chapter 7 Home Health Services, Section 40.2 Skilled Therapy Services ( https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c07.pdf ).
5. https://www.cms.gov/research-statistics-data-and-systems/files-for-order/limiteddatasets/home_health_pps_lds .
6. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/Home-Health-Prospective-Payment-System-Regulations-and-Notices .
7. Sections 1895(b)(3)(D)(i) and 1895(b)(3)(D)(ii) of the Act.
8. HHA Center web page: https://www.cms.gov/Center/Provider-Type/Home-Health-Agency-HHA-Center .
9. The remaining 3 characters of the code to be determined if finalized.
10. Items may also be referred to as “data elements.”
11. Office of the Assistant Secretary for Planning and Evaluation (ASPE). Second Report to Congress on Social Risk and Medicare's Value-Based Purchasing Programs. June 28, 2020. Available at: https://aspe.hhs.gov/reports/second-report-congress-social-risk-medicares-value-based-purchasing-programs .
12. World health Organization. Social determinants of health. Available at: https://www.who.inte/health-topics/social-determinants-of-health#tab=tab_1 .
13. Using Z Codes: The Social Determinants of Health (SDOH). Data Journey to Better Outcomes.
14. Improving the Collection of Social Determinants of Health (SDOH) Data with ICD-10-CM Z Codes. https://www.cms.gov/files/document/cms-2023-omh-z-code-resource.pdf .
15. CMS.gov Measures Management System (MMS). CMS Focus on Health Equity. Health Equity Terminology and Quality Measures. https://mmshub.cms.gov/about-qulaity-quality-at-CMS/goals/cms-focus-on-health-equity/health-equity-terminology .
16. Centers for Disease Control and Prevention. Social Determinants of Health (SDOH) and PLACES Data.
17. “U.S. Playbook to Address Social Determinants of Health” from the White House Office Of Science And Technology Policy (November 2023).
18. These SDOH data are also collected for purposes outlined in section 2(d)(2)(B) of the Improving Medicare Post-Acute Care Transitions Act (IMPACT Act). For a detailed discussion on SDOH data collection under section 2(d)(2)(B) of the IMPACT Act, see the CY 2020 HH PPS final rule ( 84 FR 60597 through 60608 ).
19. Social Determinants of Health. Healthy People 2020. https://www.healthypeople.gov/2020/gopics-objectives/topic/social-determinnats-of-health . February 2019.
20. National Academies of Sciences, Engineering, and Medicine. 2020. Leading Health Indicators 2030: Advancing Health, Equity, and Well-Being. Washington, DC: The National Academies Press. https://doi.org/1017226/25682 .
21. Centers for Medicare & Medicaid Services. “A Guide to Using the Accountable Health Communities Health-Related Social Needs Screening Tool: Promising Practices and Key Insights.” August 2022. Available at https://www.cms.gov/priorities/innovation/media/document/ahcm-screeningtool-companion .
22. Berkowitz, S.A., T.P. Baggett, and S.T. Edwards, “Addressing Health-Related Social Needs: Value-Based Care or Values-Based Care?” Journal of General Internal Medicine, vol. 34, no. 9, 2019, pp. 1916-1918, https://doi.org/10.1007/s11606-019-05087-3 .
23. Whitman A, De Lew N, Chappel A, Aysola V, Zuckerman R, & Sommers B D. Addressing social determinants of health: Examples of successful evidence-based strategies and current federal efforts. ASPE (Assistant Secretary for Planning and Evaluation) Office of Health Policy. Report HP-2022-12 April 1, 2022. SDOH-Evidence-Review.pdf (hhs.gov). Accessed 3/1/2024.
24. Hugh Alderwick and Laura M. Gottlieb, “Meanings and Misunderstandings: A Social Determinants of Health Lexicon for Health Care Systems: Milbank Quarterly,” Milbank Memorial Fund, November 18, 2019, https://www.milbank.org/quarterly/articles/meanings-and-misunderstandings-a-social-determinants-of-health-lexicon-for-health-care-systems/ .
25. Hugh Alderwick and Laura M. Gottlieb, “Meanings and Misunderstandings: A Social Determinants of Health Lexicon for Health Care Systems: Milbank Quarterly,” Milbank Memorial Fund, November 18, 2019, https://www.milbank.org/quarterly/articles/meanings-and-misunderstandings-a-social-determinants-of-health-lexicon-for-health-care-systems/ .
26. Whitman A, De Lew N, Chappel A, Aysola V, Zuckerman R, & Sommers B D. Addressing social determinants of health: Examples of successful evidence-based strategies and current federal efforts. ASPE (Assistant Secretary for Planning and Evaluation) Office of Health Policy. Report HP-2022-12 April 1, 2022. SDOH-Evidence-Review.pdf ( hhs.gov ). Accessed 5/29/2024.
27. American Hospital Association (2020). Health Equity, Diversity & Inclusion Measures for Hospitals and Health System Dashboards. December 2020. Accessed: January 18, 2022. Available at: https://ifdhe.aha.org/system/files/media/file/2020/12/ifdhe_inclusion_dashboard.pdf .
28. In October 2023, we released two new annual Health Equity Confidential Feedback Reports to HHAs: The Discharge to Community (DTC) Health Equity Confidential Feedback Report and the Medicare Spending Per Beneficiary (MSPB) Health Equity Confidential Feedback Report. The PAC Health Equity Confidential Feedback Reports stratified the DTC and MSPB measures by dual-enrollment status and race/ethnicity. For more information on the Health Equity Confidential Feedback Reports, please refer to the Education and Outreach materials available here: https://www.cms.gov/medicare/quality/snf-quality-reporting-program/training .
29. Brooks-LaSure, C. (2021). My First 100 Days and Where We Go from Here: A Strategic Vision for CMS. Centers for Medicare & Medicaid. Available at: https://www.cms.gov/blog/my-first-100-days-and-where-we-go-here-strategic-vision-cms .
30. The White House. The Biden-Harris Administration Immediate Priorities. https://www.whitehouse.gov/priorities/ .
31. Per the authority for the OASIS assessment instrument under 1891(d)(1), Home Health Conditions of Participation [ 42 U.S.C. 1395bbb ].
32. Centers for Medicare & Medicaid Services. “A Guide to Using the Accountable Health Communities Health-Related Social Needs Screening Tool: Promising Practices and Key Insights.” August 2022. Available at https://www.cms.gov/priorities/innovation/media/document/ahcm-screeningtool-companion .
33. Billioux, A., K. Verlander, S. Anthony, and D. Alley. 2017. Standardized screening for health-related social needs in clinical settings: The accountable health communities screening tool. Discussion Paper, National Academy of Medicine, Washington, DC. https://nam.edu/wp-content/uploads/2017/05/Standardized-Screening-for-Health-Related-Social-Needsin-Clinical-Settings.pdf .
34. More information about the AHC HRSN Screening Tool is available on the website at https://innovation.cms.gov/Files/worksheets/ahcm-screeningtool.pdf .
35. Centers for Medicare & Medicaid Services, FY2023 IPPS/LTCH PPS final rule ( 87 FR 49191 through 49194 ).
36. Centers for Medicare & Medicaid Services, FY 2024 Inpatient Psychiatric Prospective Payment System—Rate Update ( 88 FR 51107 through 51121 ).
37. https://health.gov/healthypeople/priority-areas/social-determinants-health .
38. Healthy People 2030 is a long-term, evidence-based effort led by the U.S. Department of Health and Human Services (HHS) that aims to identify nationwide health improvement priorities and improve the health of all Americans.
39. Kushel, M. B., Gupta, R., Gee, L., & Haas, J.S. (2006). Housing instability and food insecurity as barriers to health care among low-income Americans. Journal of General Internal Medicine, 21 (1), 71-77. doi: 10.1111/j.1525-1497.2005.00278.x
40. Homelessness is defined as “lacking a regular nighttime residence or having a primary nighttime residence that is a temporary shelter or other place not designed for sleeping.” Crowley, S. (2003). The affordable housing crisis: Residential mobility of poor families and school mobility of poor children. Journal of Negro Education, 72(1), 22-38. doi: 10.2307/3211288.
41. The 2023 Annual Homeless Assessment Report (AHAR) to Congress. The U.S. Department of Housing and Urban Development 2023. https://www.huduser.gov/portal/sites/default/files/pdf/2023-AHAR-Part-1.pdf .
42. Richards J, & Kuhn R. Unsheltered homelessness and health: A Literature Review. AJPM focus 2023; 2(1):100043. American Journal of Preventive Medicine. Unsheltered Homelessness and Health: A Literature Review ( sciencedirectassets.com ). Accessed 3/1/2024.
43. Bhat, Aarti C., David M. Almeida, Andrew Fenelon, and Alexis R. Santos-Lozada. “A longitudinal analysis of the relationship between housing insecurity and physical health among midlife and aging adults in the United States.” SSM-Population Health 18 (2022): 101128.
44. Popkin SJ, Hermans A, Oneto AD, Farrell L, Connery M, & Cannington A. 2022. People with Disabilities Living in the US Face Urgent Barriers to Housing: Federal Programs are not Meeting the Housing Needs of Disabled People. Urban Institute. People with Disabilities Living in the US Face Urgent Barriers to Housing_0.pdf ( urban.org ). Accessed 5/29/2024.
45. U.S. Department of Health & Human Services (HHS), Call to Action, “Addressing Health Related Social Needs in Communities Across the Nation.” November 2023. https://aspe.hhs.gov/sites/default/files/documents/3e2f6140d0087435cc6832bf8cf32618/hhs-call-to-action-health-related-social-needs.pdf .
46. Henderson, K.A., Manian, N., Rog, D.J., Robison, E., Jorge, E., AlAbdulmunem, M. “Addressing Homelessness Among Older Adults” (Final Report). Washington, DC: Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. October 26, 2023.
47. More information about the AHC HRSN Screening Tool is available on the website at https://innovation.cms.gov/Files/worksheets/ahcm-screeningtool.pdf .
48. The AHC HRSN Screening Tool Living Situation item includes two questions. In an effort to limit HHA burden, we are only proposing the first question.
49. National Association of Community Health Centers and Partners, National Association of Community Health Centers, Association of Asian Pacific Community Health Organizations, Association OPC, Institute for Alternative Futures. “PRAPARE.” 2017. https://prapare.org/the-prapare-screening-tool/ .
50. U.S. Department of Agriculture, Economic Research Service (n.d.). Definitions of food security. Retrieved March 10, 2022, from https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-u-s/definitions-of-food-security/ .
51. Hernandez, D. C., Reesor, L. M., & Murillo, R. (2017). Food insecurity and adult overweight/obesity: Gender and race/ethnic disparities. Appetite, 117, 373-378.
52. Food and Nutrition Security (n.d.). USDA. https://www.usda.gov/nutrition-security .
53. National Center for Health Statistics. (2022, September 6). Exercise or Physical Activity. Retrieved from Centers for Disease Control and Prevention: https://www.cdc.gov/nchs/fastats/exercise.htm .
54. Food Research & Action Center (FRAC). “Hunger is a Health Issue for Older Adults: Food Security, Health, and the Federal Nutrition Programs.” December 2019. https://frac.org/wp-content/uploads/hunger-is-a-health-issue-for-older-adults-1.pdf .
55. Schroeder K, Smaldone A. Food Insecurity: A Concept Analysis. Nurse Forum. 2015 Oct-Dec;50(4):274-84. doi: 10.1111/nuf.12118. Epub 2015 Jan 21. PMID: 25612146; PMCID: PMC4510041.
56. Administration for Community Living. Nutrition Services. Last updated 02/02/2024. Accessed 04/19/2024. https://acl.gov/programs/health-wellness/nutrition-services .
57. Tsega M, Lewis C, McCarthy D, Shah T, Coutts K. Review of Evidence for Health-Related Social Needs Interventions. July 2019. The Commonwealth Fund. https://www.commwealthfund.org/sites/default/files/2019-07/ROI-EVIDENCE-REVIEW-FINAL-VERSION.pdf .
58. More information about the HFSS tool can be found at https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-u-s/survey-tools/ .
59. Hernández D. Understanding 'energy insecurity' and why it matters to health. Soc Sci Med. 2016 Oct; 167:1-10. doi: 10.1016/j.socscimed.2016.08.029. Epub 2016 Aug 21. PMID: 27592003; PMCID: PMC5114037.
60. U.S. Energy Information Administration. “One in Three U.S. Households Faced Challenges in Paying Energy Bills in 2015.” 2017 Oct 13. https://www.eia.gov/consumption/residential/reports/2015/energybills/ .
61. Simes, Miranda, Farzana Khan, and Diana Hernández. “Energy Insecurity and Social Determinants of Health.” In Handbook of Social Sciences and Global Public Health, pp. 2119-2137. Cham: Springer International Publishing, 2023.
62. Simes, Miranda, Farzana Khan, and Diana Hernández. “Energy Insecurity and Social Determinants of Health.” In Handbook of Social Sciences and Global Public Health, pp. 2119-2137. Cham: Springer International Publishing, 2023.
63. Hernández D. “What `Merle' Taught Me About Energy Insecurity and Health.” Health Affairs, VOL.37, NO.3: Advancing Health Equity Narrative Matters. March 2018. https://doi.org/10.1377/hlthaff.2017.1413 .
64. U.S. Energy Information Administration. “One in Three U.S. Households Faced Challenges in Paying Energy Bills in 2015.” 2017 Oct 13. https://www.eia.gov/consumption/residential/reports/2015/energybills/ .
65. Shahrestanaki, S.K., Rafii, F., Najafi Ghezeljeh, T. et al. Patient safety in home health care: a grounded theory study. BMC Health Serv Res 23, 467 (2023). https://doi.org/10.1186/s12913-023-09458-9 .
66. Siegel, Eva Laura, Kathryn Lane, Ariel Yuan, Lauren A. Smalls-Mantey, Jennifer Laird, Carolyn Olson, and Diana Hernández. “Energy Insecurity Indicators Associated With Increased Odds Of Respiratory, Mental Health, And Cardiovascular Conditions: Study examines energy insecurity and health conditions.” Health Affairs 43, no. 2 (2024): 260-268.
67. Low Income Home Energy Assistance Program (LIHEAP) | The Administration for Children and Families ( hhs.gov ) ( https://www.acf.hhs.gov/ocs/programs/liheap ).
68. https://www.fcc.gov/broadbandbenefit .
69. National Council on Aging (NCOA). “How to Make It Easier for Older Adults to Get Energy and Utility Assistance.” Promising Practices Clearinghouse for Professionals. Jan 13, 2022. https://www.ncoa.org/article/how-to-make-it-easier-for-older-adults-to-get-energy-and-utility-assistance .
70. This validated survey was developed as a clinical indicator of household energy security among pediatric caregivers. Cook, J.T., D.A. Frank., P.H. Casey, R. Rose-Jacobs, M.M. Black, M. Chilton, S. Ettinger de Cuba, et al. “A Brief Indicator of Household Energy Security: Associations with Food Security, Child Health, and Child Development in US Infants and Toddlers.” Pediatrics, vol. 122, no. 4, 2008, pp. e874-e875. https://doi.org/10.1542/peds.2008-0286 .
71. Victoria Transport Policy Institute (2016, August 25). Basic access and basic mobility: Meeting society's most important transportation needs. Retrieved from http://www.vtpi.org/tdm/tdm103.htm .
72. E:\PUBLAW\PUBL173.108 ( congress.gov ).
73. The Post-Acute Care (PAC) and Hospice Quality Reporting Program Cross-Setting TEP summary report will be published in early summer or as soon as technically feasible. IRFs can monitor the Partnership for Quality Measurement website at https://mmshub.cms.gov/get-involved/technical-expert-panel/updates for updates.
74. Centers for Medicare & Medicaid Services. Aligning Quality Measures Across CMS—the Universal Foundation. November 17, 2023. https://www.cms.gov/aligning-quality-measures-across-cms-universal-foundation .
75. A composite measure can summarize multiple measures through the use of one value or piece of information. More information can be found at https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/composite-measures.pdf .
76. CMS Measures Inventory Tool. Adult immunization status measure found at https://cmit.cms.gov/cmit/#/FamilyView?familyId=26 .
77. Preventative Care and Screening: Screening for Depression and Follow Up measure found at https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-Measures/2023_Measure_134_MIPSCQM.pdf .
78. Initiation and Engagement of Substance Use Disorder Treatment measure found at https://ecqi.healthit.gov/ecqm/ec/2023/cms0137v11 .
79. https://innovation.cms.gov/data-and-reports/2020/hhvbp-thirdann-rpt .
80. https://www.cms.gov/files/document/certificationhome-health-value-based-purchasing-hhvbpmodel.pdf .
81. https://www.cms.gov/newsroom/press-releases/cms-takes-action-improve-home-health-care-seniors-announces-intent-expand-home-health-value-based .
82. U.S. Department of Health and Human Services. Office of the Assistant Secretary for Planning and Evaluation (ASPE) (2014). Measuring Success in Health Care Value-Based Purchasing Programs. Cheryl L. Damberg et al. on behalf of RAND Health.
83. For more details on the GUIDE Model, see the Model web page ( https://www.cms.gov/priorities/innovation/innovation-models/guide ). For more details on the caregiver measures being developed for GUIDE, see the Request for Applications ( https://www.cms.gov/files/document/guide-rfa.pdf ).
84. https://oig.hhs.gov/oei/reports/OEI-05-22-00290.asp .
85. Executive Orders 13985, “Advancing Racial Equity and Support for Underserved Communities Through the Federal Government,” and 14091, “Executive Order on Further Advancing Racial Equity and Support for Underserved Communities Through The Federal Government.”
86. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/CMS-Quality-Strategy.pdf .
87. CMS defines an “underserved community” as “individuals who share a particular characteristic—demographic, geographic (urban or rural), or other factor—that results in them being systemically denied full opportunity to participate in aspects of economic, social, and civic life. (Source: https://www.cms.gov/priorities/innovation/key-concepts/health-equity ).
88. Joynt Maddox, K.E., Chen, L.M., Zuckerman, R., & Epstein, A.M. (2018). Association Between Race, Neighborhood, and Medicaid Enrollment and Outcomes in Medicare Home Health Care. Journal of the American Geriatrics Society, 66(2), 239-246. https://doi.org/10.1111/jgs.15082 .
89. Fashaw-Walters, S.A., Rahman, M., Jarrín, O.F., Gee, G., Mor, V., Nkimbeng, M., & Thomas, K.S. (2023). Getting to the root: Examining within and between home health agency inequities in functional improvement. Health Services Research. https://doi.org/10.1111/1475-6773.14194 .
90. Fashaw-Walters, S.A., Rahman, M., Gee, G., Mor, V., White, M., & Thomas, K.S. (2022). Out Of Reach: Inequities in the Use of High-Quality Home Health Agencies. Health Affairs (Project Hope), 41(2), 247-255. https://doi.org/10.1377/hlthaff.2021.01408 .
91. Joynt Maddox, K.E., Chen, L.M., Zuckerman, R., & Epstein, A.M. (2018). Association Between Race, Neighborhood, and Medicaid Enrollment and Outcomes in Medicare Home Health Care. Journal of the American Geriatrics Society, 66(2), 239-246. https://doi.org/10.1111/jgs.15082 .
92. Fashaw-Walters, S.A., Rahman, M., Jarrín, O.F., Gee, G., Mor, V., Nkimbeng, M., & Thomas, K.S. (2023). Getting to the root: Examining within and between home health agency inequities in functional improvement. Health Services Research. https://doi.org/10.1111/1475-6773.14194 .
93. Fashaw-Walters SA, Rahman M, Gee G, Mor V, White M, Thomas KS. Out Of Reach: Inequities In The Use Of High-Quality Home Health Agencies. Health Aff (Millwood). 2022 Feb;41(2):247-255. doi: 10.1377/hlthaff.2021.01408. PMID: 35130066; PMCID: PMC8883595.
94. Joynt Maddox, K.E., Chen, L.M., Zuckerman, R. and Epstein, A.M. (2018), Association Between Race, Neighborhood, and Medicaid Enrollment and Outcomes in Medicare Home Health Care. J Am Geriatr Soc, 66: 239-246. https://doi.org/10.1111/jgs.15082 .
95. Perez EE, Orange JS, Bonilla F, et al. (2017) Update on the use of immunoglobulin in human disease: A review of evidence; Journal Allergy Clin Immunol. 139(3S): S1-S46.
96. Updated Interim Report to Congress: Evaluation of the Medicare Patient Intravenous Immunoglobulin Demonstration Project, 2022: https://innovation.cms.gov/data-and-reports/2022/ivig-updatedintrtc .
97. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=33610 .
98. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleId=52509 .
99. Local Coverage Determination (LCD): IVIG (L33610) https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=33610&ContrId=389 .
100. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleId=52509 .
101. Updated Interim Report to Congress: Evaluation of the Medicare Patient Intravenous Immunoglobulin Demonstration Project, August 2022 found at: https://innovation.cms.gov/data-and-reports/2022/ivig-updatedintrtc .
102. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=33794 .
103. Local Coverage Determination (LCD): External Infusion Pumps (L33794) https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=33794 .
104. Intravenous Immune Globulin Demonstration MLN Fact Sheet: https://www.cms.gov/files/document/mln3191598-intravenous-immune-globulin-demonstration.pdf .
105. https://www.cms.gov/medicare/payment/fee-for-service-providers/home-infusion-therapy .
106. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8197411/ , date accessed 5-7-24.
107. Amol M. Karmarkar, Indrakshi Roy, Taylor Lane, Stefany Shaibi, Julie A. Baldwin & Amit Kumar (2023), Home health services for minorities in urban and rural areas with Alzheimer's and related dementia, Home Health Care Services Quarterly, 42:4, 265-281, DOI: 10.1080/01621424.2023.2206368.
108. Assessment of Receipt of the First Home Health Care Visit After Hospital Discharge Among Older Adults. Jun Li, Ph.D., Mingyu Qi, MS, and Rachel M. Werner, Ph.D., MD. JAMA Netw Open. 2020 Sep; 3(9): e2015470. doi: 10.1001/jamanetworkopen.2020.15470:10.1001/jamanetworkopen.2020.15470.
109. https://alz-journals.onlinelibrary.wiley.com/doi/full/10.1002/alz.13139 , date accessed 5-08-24.
110. Social Vulnerability and Medical Complexity Among Medicare Beneficiaries Receiving Home Health Without Prior Hospitalization, Julia G. Burgdorf, Ph.D., Tracy M. Mroz, OTR/L, Ph.D., and Jennifer L. Wolff, Ph.D. Innovation in Aging, 2020, Vol. 4, No. 6, 1-9 doi:10.1093/geroni/igaa049.
111. Pandemic-Fueled Shortages of Home Health Workers Strand Patients Without Necessary Care, February 3, 2022, KFF Health News. https://kffhealthnews.org/news/article/pandemic-fueled-home-health-care-shortages-strand-patients/ . Accessed March 12, 2024.
112. Caregiver Needed: How the Nation's Workforce Shortages Make it Harder to Age Well at Home, September 2022, USAging. https://www.usaging.org/Files/Workforce-Issues_508.pdf . Accessed March 14, 2024.
113. The evolution of care: An annual care delivery report, from CarePort, powered by WellSky, 2023.
114. https://www.fiercehealthcare.com/providers/hospitals-struggling-discharge-patients-post-acute-care-settings-wellsky-report#:~:text=Patients%20in%20the%20hospital%20are,post%2Dacute%20settings%20more%20difficult .
115. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9750104/ .
116. The evolution of care: An annual care delivery report, from CarePort, powered by WellSky, 2023.
117. https://www.fiercehealthcare.com/providers/hospitals-struggling-discharge-patients-post-acute-care-settings-wellsky-report#:~:text=Patients%20in%20the%20hospital%20are,post%2Dacute%20settings%20more%20difficult .
118. https://www.fiercehealthcare.com/providers/hospitals-struggling-discharge-patients-post-acute-care-settings-wellsky-report#:~:text=Patients%20in%20the%20hospital%20are,post%2Dacute%20settings%20more%20difficult .
119. https://www.census.gov/library/stories/2019/12/by-2030-all-baby-boomers-will-be-age-65-or-older.html .
120. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10562071/#:~:text=Surgical%20procedures%20within%20the%20United,In%202018%2C%20Young%20et%20al .
121. https://josr-online.biomedcentral.com/articles/10.1186/s13018-023-03750-4 .
122. https://homehealthcarenews.com/2024/01/home-health-agencies-grapple-with-acuity-creep-as-patient-needs-become-more-complex/ .
123. June 2023 Final Rule. https://www.govinfo.gov/content/pkg/FR-2023-06-05/pdf/2023-11449.pdf .
124. COVID-19 Health Care Staff Vaccination Interim Final Rule. https://www.federalregister.gov/documents/2021/11/05/2021-23831/medicare-and-medicaid-programs-omnibus-covid-19-health-care-staff-vaccination .
125. Grabowski DC, Mor V. Nursing Home Care in Crisis in the Wake of COVID-19. JAMA. 2020;324(1):23. doi:10.1001/jama.2020.8524.
126. Chidambaram P. Over 200,000 Residents and Staff in Long-Term Care Facilities Have Died From COVID-19. Kaiser Family Foundation. Published online February 3, 2022. https://www.kff.org/policy-watch/over-200000-residents-and-staff-in-long-term-care-facilities-have-died-from-covid-19/ .
127. The New York Times. Nearly One-Third of U.S. Coronavirus Deaths Are Linked to Nursing Homes. https://www.nytimes.com/interactive/2020/us/coronavirus-nursing-homes.html . Published June 1, 2021.
128. Vital and Health Statistics, Series 3, Number 47 ( cdc.gov ) ( https://www.cdc.gov/nchs/data/series/sr_03/sr03-047.pdf ).
129. MMWR, Rates of COVID-19 Among Residents and Staff Members in Nursing Homes—United States, May 25-November 22, 2020 ( cdc.gov ) ( https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7002e2-H.pdf ).
130. Infection prevention and control in nursing homes during COVID-19: An environmental scan—PMC ( nih.gov ) ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8810224/ ).
131. Vital and Health Statistics, Series 3, Number 47 ( cdc.gov ) ( https://www.cdc.gov/nchs/data/series/sr_03/sr03-047.pdf ).
132. https://www.govinfo.gov/content/pkg/FR-2024-05-10/pdf/FR-2024-05-10.pdf .
133. https://aspr.hhs.gov/HealthCareReadiness/StoriesfromtheField/Pages/Stories/Kentucky-Collaborates-Community.aspx .
134. https://aspr.hhs.gov/HealthCareReadiness/HealthCareReadinessNearYou/Documents/HCC-FactSheet-April2021-508.pdf .
135. https://aspr.hhs.gov/HealthCareReadiness/HealthCareReadinessNearYou/Documents/HCC-FactSheet-April2021-508.pdf .
136. Mitchell SH, Rigler J, Baum K. Regional Transfer Coordination and Hospital Load Balancing During COVID-19 Surges. JAMA Health Forum. 2022;3(2):e215048. doi:10.1001/jamahealthforum.2021.5048. https://aspr.hhs.gov/HealthCareReadiness/StoriesfromtheField/Pages/Stories/HCC-Regional-Approach-Illinois.aspx .
137. https://aspr.hhs.gov/HealthCareReadiness/StoriesfromtheField/Pages/Stories/Maryland-HCC-covid19.aspx .
138. Coverage with Influenza, Respiratory Syncytial Virus, and Updated COVID-19 Vaccines Among Nursing Home Residents—National Healthcare Safety Network, United States, December 2023 | MMWR ( cdc.gov ) ( https://www.cdc.gov/mmwr/volumes/72/wr/mm7251a3.htm ).
139. Report to Congress, November 2023, for Fiscal Year 2022, The Administration, Cost, and Impact of the Quality Improvement Organization Program for Medicare Beneficiaries ( https://www.cms.gov/files/document/final-fy-2022-qio-rtc.pdf ).
140. Protecting Nursing Home Residents from Covid-19: Federal Strike Team Findings and Lessons Learned | NEJM Catalyst ( https://catalyst.nejm.org/doi/full/10.1056/CAT.21.0144 ).
141. https://doi.org/10.1111/jgs.18402 .
142. Haanschoten E, Dubendris H, Reses HE, Barbre K, Meng L, Benin A, Bell JM. Disparities in COVID-19 Vaccination Status Among Long-Term Care Facility Residents—United States, October 31, 2022-May 7, 2023. MMWR Morb Mortal Wkly Rep. 2023 Oct 6;72(40):1095-1098. doi: 10.15585/mmwr.mm7240a4. PMID: 37796756; PMCID: PMC10564329.
143. Wong E, Barbre K, Wiegand RE, Reses HE, Dubendris H, Wallace M, Dollard P, Edwards J, Soe M, Meng L, Benin A, Bell JM. Effectiveness of Up-to-Date COVID-19 Vaccination in Preventing SARS-CoV-2 Infection Among Nursing Home Residents—United States, November 20, 2022-January 8, 2023. MMWR Morb Mortal Wkly Rep. 2023 Jun 23;72(25):690-693. doi: 10.15585/mmwr.mm7225a4. PMID: 37347711; PMCID: PMC10328477.
144. Sinha S, Konetzka RT. Association of COVID-19 Vaccination Rates of Staff and COVID-19 Illness and Death Among Residents and Staff in US Nursing Homes. JAMA Netw Open. 2022;5(12):e2249002. doi:10.1001/jamanetworkopen.2022.49002.
145. https://covid.cdc.gov/covid-data-tracker/#trends_weeklyhospitaladmissions_select_00 .
146. CDC COVID Data Tracker: Hospital Admissions ( https://covid.cdc.gov/covid-data-tracker/#datatracker-home ).
147. Franklin D, Barbre K, Rowe TA, Reses HE, Massey J, Meng L, Dollard P, Dubendris H, Stillions M, Robinson L, Clerville JW, Jacobs Slifka K, Benin A, Bell JM. COVID-19 vaccination coverage and rates of SARS-CoV-2 infection and COVID-19-associated hospitalization among residents in nursing homes. MMWR Morb Mortal Wkly Rep 2024;73:339-344. DOI: http://dx.doi.org/10.15585/mmwr.mm7315a3 .
148. Respiratory Disease Season Outlook ( cdc.gov ) ( https://www.cdc.gov/forecast-outbreak-analytics/about/season-outlook.html ).
149. Respiratory Disease Season Outlook ( cdc.gov ) ( https://www.cdc.gov/forecast-outbreak-analytics/about/season-outlook.html ).
150. See https://www.cdc.gov/respiratory-viruses/index.html and data summaries of respiratory virus burden at https://www.cdc.gov/respiratory-viruses/data-research/dashboard/snapshot.html and https://www.cdc.gov/respiratory-viruses/whats-new/track-hospital-capacity.html .
151. Landers S, Kapadia F, Tarantola D. Monkeypox, After HIV/AIDS and COVID-19: Suggestions for Collective Action and a Public Health of Consequence, November 2022. Am J Public Health. 2022 Nov;112(11):1564-1566. doi: 10.2105/AJPH.2022.307100. PMID: 36223580; PMCID: PMC9558195. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9558195/ .
152. https://emergency.cdc.gov/han/2023/han00503.asp , https://emergency.cdc.gov/han/2023/han00498.asp .
153. Coverage with Influenza, Respiratory Syncytial Virus, and Updated COVID-19 Vaccines Among Nursing Home Residents—National Healthcare Safety Network, United States, December 2023 | MMWR ( cdc.gov ) ( https://www.cdc.gov/mmwr/volumes/72/wr/mm7251a3.htm#F1_down ).
154. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic | Infection Control & Hospital Epidemiology | Cambridge Core; https://www.nejm.org/doi/full/10.1056/NEJMp2118285 ; The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network—PubMed ( nih.gov ) ( https://pubmed.ncbi.nlm.nih.gov/34473013/ ); Impact of COVID-19 pandemic on central-line-associated bloodstream infections during the early months of 2020, National Healthcare Safety Network—PubMed ( nih.gov ) ( https://pubmed.ncbi.nlm.nih.gov/33719981/ ).
155. Falls Risk in Long-Term Care Residents With Cognitive Impairment: Effects of COVID-19 Pandemic—PubMed ( nih.gov ) ( https://pubmed.ncbi.nlm.nih.gov/38104633/ ).
156. https://www.nejm.org/doi/full/10.1056/NEJMp2118285 .
157. The Adverse Effects of the COVID-19 Pandemic on Nursing Home Resident Well-Being—PMC ( nih.gov ) ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7980137/ ).
158. For more information about USCDI+ https://www.healthit.gov/topic/interoperability/uscdi-plus .
159. CMS eliminated the use of RAPs for HHAs; beginning January 1, 2022, CMS replaced RAP submissions with a Notice of Admission.
160. https://qcor.cms.gov/active_nh.jsp?which=0&report=active_nh.jsp , report ran 4/24/2024.
161. https://www.sba.gov/sites/sbagov/files/2023-03/Table%20of%20Size%20Standards_Effective%20March%2017%2C%202023.xlsx .
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4210-01-P
[ FR Doc. 2024-14254 Filed 6-26-24; 4:15 pm]
- Executive Orders
Reader Aids
Information.
- About This Site
- Accessibility
- No Fear Act
- Continuity Information
- Quick Links
- Make An Appointment
- Our Services
- Price Estimate
- Price Transparency
- Pay Your Bill
- Patient Experience
- Careers at UH
Schedule an appointment today

- Diabetes & Endocrinology
- UH CEO Dr. Cliff Megerian's Blog
- Emergency Medicine
- Gastroenterology & GI Surgery
- Geriatric Medicine
- Heart & Vascular
- Integrative Health Network
- Neurology & Neurological Surgery
- Obstetrics & Gynecology
- Orthopaedics
- Otolaryngology - Head and Neck Surgery
- Pulmonology, Critical Care & Sleep Medicine
- Rheumatology
- Roe Green Center for Travel Medicine & Global Health
- UH Clinical Leaders Blog
- UH Drusinsky Sports Medicine Institute
- Medical Articles
- Medical Education
- Pediatric Orthopaedic Update
- UH Alumni Association e-Newsletter
- UH Clinical Update
- UH CMC Update
- UH Innovations
- UH OBGYN Clinical Update
- UH Pediatric Clinical Update
- UH Research & Education Update
What the Latest Medicare News Means for University Hospitals
August 29, 2022
UH Clinical Update | August 2022
By Cliff A. Megerian, MD, FACS, Chief Executive Officer; Jane and Henry Meyer Chief Executive Officer Distinguished Chair
You may have read the recent positive news about the changes in Medicare prescription coverage: beginning in 2025, Medicare recipients with prescription drug coverage will see significant savings, as their cost for medication will not exceed $2,000 annually.
This change was made to help people on Medicare who have been overwhelmed by the high cost of prescriptions, and are often unable to pay when just one prescription can cost thousands of dollars each month. Not infrequently, people then forgo the drugs, with dire results for their health.
As detailed in the New York Times, nearly 49 million people, most of them older Americans, get prescription drug coverage through Medicare, yet many find that it does not go far when it comes to paying for the medication they need. While low-income people qualify for subsidies, people in the middle class are hit hard.
Now, with the healthcare bill passed, many on Medicare can save hundreds, or likely thousands, on those prescriptions each year. That’s because the bill allows Medicare to negotiate prices with drug makers to drive down the costs, to the benefit of those who rely on those medications.
There are other positives, too. Starting next year, insulin co-payments for Medicare recipients will not exceed $35 a month. And in 2024, beneficiaries who have costs high enough to qualify for “catastrophic coverage” would no longer have to pay up to 5 percent of the cost of every prescription.
All of this is tremendous news for many of our patients, and we are happy and relieved for all Medicare recipients who will benefit from this.
As physicians, it’s also something to keep in mind as we counsel our patients. We want to make sure that they take their medications as prescribed, and also to remind them how important it is that they avail themselves of the annual or age-related screenings that Medicare requires, including those for cancer, heart disease and diabetes. We have seen the metrics on how effective these screenings are and what a big difference they make in population health.
This is also a good time to note that UH provides prescription cost assistance in another way, which is through our 340B savings program. This program was created 30 years ago as a safety net for low-income patients with or without insurance, but also for other patients who would never be able to afford the medicines they need when they are ill (such as extremely expensive cancer drugs) or when they are suffering from a chronic condition. The program’s name comes from Section 340B of the U.S. Public Health Service Act of 1992.
It works by requiring pharmaceutical manufacturers that participate in Medicaid to sell outpatient drugs at discounted prices to healthcare organizations that care for many uninsured and low-income patients. The savings in those prices allows UH to benefit the community, to reach more eligible patients and provide more comprehensive care, including pharmacist staff for services used by patients with such chronic illnesses as diabetic hypertension, dyslipidemia, asthma and COPD.
In 2021, the UH Specialty Pharmacy team, which is funded by the savings from the 340B program, helped secure $42 million in funding to support patients getting access to medications that they could not afford.
And yes, while the new health bill means that many patients who are on Medicare will benefit from the upcoming prescription savings, for healthcare systems like ours, there is a different story.
Here’s what is happening. While you may have seen news headlines about hospital reimbursement rate increases from the Centers for Medicaid and Medicare Services (CMS), the fact is there also were cuts mandated earlier in the year that offset those increases. So the net increase is very modest and not nearly what is needed to keep pace with rising inflation and our supply costs.
Medicare is the No. 1 provider of reimbursement to hospitals, and nearly 60 percent of our revenue comes from Medicare and Medicaid reimbursement. So these cuts are being made when we already are in challenging economic times.
Workforce shortages are also at critical levels across many positions at American hospitals, including those of nurses, hospitalists, intensivists, lab technicians, nursing assistants, and environmental service workers. In some cases, this leads to a delay in patients getting needed care.
Hospitals are hurting already, and then facing a cut in reimbursement by the biggest payer – all this during a time of once-in-a-generation inflation.
Why is Medicare cutting funding? They see the overall cost of healthcare continuing to increase and Medicare has only so much money in its trust fund, so Congress and the Administration have pushed to reduce spending. That’s understandable. Our UH Government Relations team is doing what it does best and because of its strong relationships with government officials, we are working with our political leaders, encouraging them to put forward legislation that can reverse these cuts.
The Government Relations team has ensured that I meet regularly with members of Ohio’s congressional delegation. Also, this past spring, we hosted CMS Administrator Chiquita Brooks LaSure for a healthcare roundtable at UH Cleveland Medical Center, which gave us the opportunity to build strong relationships with the Biden administration’s leading health policy makers.
We are working hard to ensure that our elected leaders know the significance of the financial and staffing struggles that health systems are facing and that we need them as a partner.
It is one of the most crucial issues to address now, and we will remain persistent.
Tags: UH Clinical Update August 2022
LOPER BRIGHT ENTERPRISES v. RAIMONDO
- Supreme Court
LOPER BRIGHT ENTERPRISES v. RAIMONDO No. 22–451, 45 F. 4th 359 & No. 22–1219, 62 F. 4th 621, vacated and remanded.
- Syllabus [Syllabus] [PDF]
- Opinion , Roberts [Roberts Opinion] [PDF]
- Concurrence , Thomas [Thomas Concurrence] [PDF]
- Concurrence , Gorsuch [Gorsuch Concurrence] [PDF]
- Dissent , Kagan [Kagan Dissent] [PDF]
NOTE: Where it is feasible, a syllabus (headnote) will be released, as is being done in connection with this case, at the time the opinion is issued. The syllabus constitutes no part of the opinion of the Court but has been prepared by the Reporter of Decisions for the convenience of the reader. See United States v. Detroit Timber & Lumber Co., 200 U. S. 321 , 337.
SUPREME COURT OF THE UNITED STATES
LOPER BRIGHT ENTERPRISES et al. v . RAIMONDO, SECRETARY OF COMMERCE, et al.
certiorari to the united states court of appeals for the district of columbia circuit
The Court granted certiorari in these cases limited to the question whether Chevron U. S. A. Inc. v. Natural Resources Defense Council, Inc. , 467 U. S. 837 , should be overruled or clarified. Under the Chevron doctrine, courts have sometimes been required to defer to “permissible” agency interpretations of the statutes those agencies administer—even when a reviewing court reads the statute differently. Id. , at 843. In each case below, the reviewing courts applied Chevron ’s framework to resolve in favor of the Government challenges by petitioners to a rule promulgated by the National Marine Fisheries Service pursuant to the Magnuson-Stevens Act, 16 U. S. C. §1801 et seq. , which incorporates the Administrative Procedure Act (APA), 5 U. S. C. §551 et seq .
Held : The Administrative Procedure Act requires courts to exercise their independent judgment in deciding whether an agency has acted within its statutory authority, and courts may not defer to an agency interpretation of the law simply because a statute is ambiguous; Chevron is overruled. Pp. 7–35.
(a) Article III of the Constitution assigns to the Federal Judiciary the responsibility and power to adjudicate “Cases” and “Controversies”—concrete disputes with consequences for the parties involved. The Framers appreciated that the laws judges would necessarily apply in resolving those disputes would not always be clear, but envisioned that the final “interpretation of the laws” would be “the proper and peculiar province of the courts.” The Federalist No. 78, p. 525 (A. Hamilton). As Chief Justice Marshall declared in the foundational decision of Marbury v. Madison , “[i]t is emphatically the province and duty of the judicial department to say what the law is.” 1 Cranch 137, 177. In the decades following Marbury , when the meaning of a statute was at issue, the judicial role was to “interpret the act of Congress, in order to ascertain the rights of the parties.” Decatur v. Paulding , 14 Pet. 497, 515.
The Court recognized from the outset, though, that exercising independent judgment often included according due respect to Executive Branch interpretations of federal statutes. Such respect was thought especially warranted when an Executive Branch interpretation was issued roughly contemporaneously with enactment of the statute and remained consistent over time. The Court also gave “the most respectful consideration” to Executive Branch interpretations simply because “[t]he officers concerned [were] usually able men, and masters of the subject,” who may well have drafted the laws at issue. United States v. Moore , 95 U. S. 760 , 763. “Respect,” though, was just that. The views of the Executive Branch could inform the judgment of the Judiciary, but did not supersede it. “[I]n cases where [a court’s] own judgment . . . differ[ed] from that of other high functionaries,” the court was “not at liberty to surrender, or to waive it.” United States v. Dickson , 15 Pet. 141, 162 .
During the “rapid expansion of the administrative process” that took place during the New Deal era, United States v. Morton Salt Co. , 338 U. S. 632 , 644, the Court often treated agency determinations of fact as binding on the courts, provided that there was “evidence to support the findings,” St. Joseph Stock Yards Co. v. United States , 298 U. S. 38 , 51. But the Court did not extend similar deference to agency resolutions of questions of law . “The interpretation of the meaning of statutes, as applied to justiciable controversies,” remained “exclusively a judicial function.” United States v. American Trucking Assns., Inc. , 310 U. S. 534 , 544. The Court also continued to note that the informed judgment of the Executive Branch could be entitled to “great weight.” Id. , at 549. “The weight of such a judgment in a particular case,” the Court observed, would “depend upon the thoroughness evident in its consideration, the validity of its reasoning, its consistency with earlier and later pronouncements, and all those factors which give it power to persuade, if lacking power to control.” Skidmore v. Swift & Co. , 323 U. S. 134 , 140.
Occasionally during this period, the Court applied deferential review after concluding that a particular statute empowered an agency to decide how a broad statutory term applied to specific facts found by the agency. See Gray v. Powell , 314 U. S. 402 ; NLRB v. Hearst Publications, Inc. , 322 U. S. 111 . But such deferential review, which the Court was far from consistent in applying, was cabined to factbound determinations. And the Court did not purport to refashion the longstanding judicial approach to questions of law. It instead proclaimed that “[u]ndoubtedly questions of statutory interpretation . . . are for the courts to resolve, giving appropriate weight to the judgment of those whose special duty is to administer the questioned statute.” Id. , at 130–131. Nothing in the New Deal era or before it thus resembled the deference rule the Court would begin applying decades later to all varieties of agency interpretations of statutes under Chevron . Pp. 7–13.
(b) Congress in 1946 enacted the APA “as a check upon administrators whose zeal might otherwise have carried them to excesses not contemplated in legislation creating their offices.” Morton Salt , 338 U. S., at 644. The APA prescribes procedures for agency action and delineates the basic contours of judicial review of such action. And it codifies for agency cases the unremarkable, yet elemental proposition reflected by judicial practice dating back to Marbury : that courts decide legal questions by applying their own judgment. As relevant here, the APA specifies that courts, not agencies, will decide “ all relevant questions of law” arising on review of agency action, 5 U. S. C. §706 (emphasis added)—even those involving ambiguous laws. It prescribes no deferential standard for courts to employ in answering those legal questions, despite mandating deferential judicial review of agency policymaking and factfinding. See §§706(2)(A), (E). And by directing courts to “interpret constitutional and statutory provisions” without differentiating between the two, §706, it makes clear that agency interpretations of statutes—like agency interpretations of the Constitution—are not entitled to deference. The APA’s history and the contemporaneous views of various respected commentators underscore the plain meaning of its text.
Courts exercising independent judgment in determining the meaning of statutory provisions, consistent with the APA, may—as they have from the start—seek aid from the interpretations of those responsible for implementing particular statutes. See Skidmore , 323 U. S., at 140. And when the best reading of a statute is that it delegates discretionary authority to an agency, the role of the reviewing court under the APA is, as always, to independently interpret the statute and effectuate the will of Congress subject to constitutional limits. The court fulfills that role by recognizing constitutional delegations, fixing the boundaries of the delegated authority, and ensuring the agency has engaged in “ ‘reasoned decisionmaking’ ” within those boundaries. Michigan v. EPA , 576 U. S. 743 , 750 (quoting Allentown Mack Sales & Service, Inc. v. NLRB , 522 U. S. 359 , 374). By doing so, a court upholds the traditional conception of the judicial function that the APA adopts. Pp. 13–18.
(c) The deference that Chevron requires of courts reviewing agency action cannot be squared with the APA. Pp. 18–29.
(1) Chevron , decided in 1984 by a bare quorum of six Justices, triggered a marked departure from the traditional judicial approach of independently examining each statute to determine its meaning. The question in the case was whether an Environmental Protection Agency (EPA) regulation was consistent with the term “stationary source” as used in the Clean Air Act. 467 U. S., at 840. To answer that question, the Court articulated and employed a now familiar two-step approach broadly applicable to review of agency action. The first step was to discern “whether Congress ha[d] directly spoken to the precise question at issue.” Id. , at 842. The Court explained that “[i]f the intent of Congress is clear, that is the end of the matter,” ibid. , and courts were therefore to “reject administrative constructions which are contrary to clear congressional intent,” id. , at 843, n. 9. But in a case in which “the statute [was] silent or ambiguous with respect to the specific issue” at hand, a reviewing court could not “simply impose its own construction on the statute, as would be necessary in the absence of an administrative interpretation.” Id. , at 843 (footnote omitted) . Instead, at Chevron ’s second step, a court had to defer to the agency if it had offered “a permissible construction of the statute,” ibid. , even if not “the reading the court would have reached if the question initially had arisen in a judicial proceeding,” ibid. , n. 11. Employing this new test, the Court concluded that Congress had not addressed the question at issue with the necessary “level of specificity” and that EPA’s interpretation was “entitled to deference.” Id. , at 865.
Although the Court did not at first treat Chevron as the watershed decision it was fated to become, the Court and the courts of appeals were soon routinely invoking its framework as the governing standard in cases involving statutory questions of agency authority. The Court eventually decided that Chevron rested on “a presumption that Congress, when it left ambiguity in a statute meant for implementation by an agency, understood that the ambiguity would be resolved, first and foremost, by the agency, and desired the agency (rather than the courts) to possess whatever degree of discretion the ambiguity allows.” Smiley v. Citibank (South Dakota), N. A. , 517 U. S. 735 , 740–741. Pp. 18–20.
(2) Neither Chevron nor any subsequent decision of the Court attempted to reconcile its framework with the APA. Chevron defies the command of the APA that “the reviewing court”—not the agency whose action it reviews—is to “decide all relevant questions of law” and “interpret . . . statutory provisions.” §706 (emphasis added). It requires a court to ignore , not follow, “the reading the court would have reached” had it exercised its independent judgment as required by the APA. Chevron , 467 U. S., at 843, n. 11. Chevron insists on more than the “respect” historically given to Executive Branch interpretations; it demands that courts mechanically afford binding deference to agency interpretations, including those that have been inconsistent over time, see id. , at 863, and even when a pre-existing judicial precedent holds that an ambiguous statute means something else, National Cable & Telecommunications Assn. v. Brand X Internet Services , 545 U. S. 967 , 982. That regime is the antithesis of the time honored approach the APA prescribes.
Chevron cannot be reconciled with the APA by presuming that statutory ambiguities are implicit delegations to agencies. That presumption does not approximate reality. A statutory ambiguity does not necessarily reflect a congressional intent that an agency, as opposed to a court, resolve the resulting interpretive question. Many or perhaps most statutory ambiguities may be unintentional. And when courts confront statutory ambiguities in cases that do not involve agency interpretations or delegations of authority, they are not somehow relieved of their obligation to independently interpret the statutes. Instead of declaring a particular party’s reading “permissible” in such a case, courts use every tool at their disposal to determine the best reading of the statute and resolve the ambiguity. But in an agency case as in any other, there is a best reading all the same—“the reading the court would have reached” if no agency were involved. Chevron , 467 U. S., at 843, n. 11. It therefore makes no sense to speak of a “permissible” interpretation that is not the one the court, after applying all relevant interpretive tools, concludes is best.
Perhaps most fundamentally, Chevron ’s presumption is misguided because agencies have no special competence in resolving statutory ambiguities. Courts do. The Framers anticipated that courts would often confront statutory ambiguities and expected that courts would resolve them by exercising independent legal judgment. Chevron gravely erred in concluding that the inquiry is fundamentally different just because an administrative interpretation is in play. The very point of the traditional tools of statutory construction is to resolve statutory ambiguities. That is no less true when the ambiguity is about the scope of an agency’s own power—perhaps the occasion on which abdication in favor of the agency is least appropriate. Pp. 21–23.
(3) The Government responds that Congress must generally intend for agencies to resolve statutory ambiguities because agencies have subject matter expertise regarding the statutes they administer; because deferring to agencies purportedly promotes the uniform construction of federal law; and because resolving statutory ambiguities can involve policymaking best left to political actors, rather than courts. See Brief for Respondents in No. 22–1219, pp. 16–19. But none of these considerations justifies Chevron ’s sweeping presumption of congressional intent.
As the Court recently noted, interpretive issues arising in connection with a regulatory scheme “may fall more naturally into a judge’s bailiwick” than an agency’s. Kisor v. Wilkie , 588 U. S. 558 , 578. Under Chevron ’s broad rule of deference, though, ambiguities of all stripes trigger deference, even in cases having little to do with an agency’s technical subject matter expertise. And even when an ambiguity happens to implicate a technical matter, it does not follow that Congress has taken the power to authoritatively interpret the statute from the courts and given it to the agency. Congress expects courts to handle technical statutory questions, and courts did so without issue in agency cases before Chevron . After all, in an agency case in particular, the reviewing court will go about its task with the agency’s “body of experience and informed judgment,” among other information, at its disposal. Skidmore , 323 U. S., at 140. An agency’s interpretation of a statute “cannot bind a court,” but may be especially informative “to the extent it rests on factual premises within [the agency’s] expertise.” Bureau of Alcohol, Tobacco and Firearms v. FLRA , 464 U. S. 89 , 98, n. 8. Delegating ultimate interpretive authority to agencies is simply not necessary to ensure that the resolution of statutory ambiguities is well informed by subject matter expertise.
Nor does a desire for the uniform construction of federal law justify Chevron . It is unclear how much the Chevron doctrine as a whole actually promotes such uniformity, and in any event, we see no reason to presume that Congress prefers uniformity for uniformity’s sake over the correct interpretation of the laws it enacts.
Finally, the view that interpretation of ambiguous statutory provisions amounts to policymaking suited for political actors rather than courts is especially mistaken because it rests on a profound misconception of the judicial role. Resolution of statutory ambiguities involves legal interpretation, and that task does not suddenly become policymaking just because a court has an “agency to fall back on.” Kisor , 588 U. S., at 575. Courts interpret statutes, no matter the context, based on the traditional tools of statutory construction, not individual policy preferences. To stay out of discretionary policymaking left to the political branches, judges need only fulfill their obligations under the APA to independently identify and respect such delegations of authority, police the outer statutory boundaries of those delegations, and ensure that agencies exercise their discretion consistent with the APA. By forcing courts to instead pretend that ambiguities are necessarily delegations, Chevron prevents judges from judging. Pp. 23–26.
(4) Because Chevron ’s justifying presumption is, as Members of the Court have often recognized, a fiction, the Court has spent the better part of four decades imposing one limitation on Chevron after another. Confronted with the byzantine set of preconditions and exceptions that has resulted, some courts have simply bypassed Chevron or failed to heed its various steps and nuances. The Court, for its part, has not deferred to an agency interpretation under Chevron since 2016. But because Chevron remains on the books, litigants must continue to wrestle with it, and lower courts—bound by even the Court’s crumbling precedents—understandably continue to apply it. At best, Chevron has been a distraction from the question that matters: Does the statute authorize the challenged agency action? And at worst, it has required courts to violate the APA by yielding to an agency the express responsibility, vested in “the reviewing court ,” to “decide all relevant questions of law” and “interpret . . . statutory provisions.” §706 (emphasis added). Pp. 26–29.
(d) Stare decisis , the doctrine governing judicial adherence to precedent, does not require the Court to persist in the Chevron project. The stare decisis considerations most relevant here—“the quality of [the precedent’s] reasoning, the workability of the rule it established, . . . and reliance on the decision,” Knick v. Township of Scott , 588 U. S. 180 , 203 (quoting Janus v. State, County, and Municipal Employees , 585 U. S. 878 , 917)—all weigh in favor of letting Chevron go.
Chevron has proved to be fundamentally misguided. It reshaped judicial review of agency action without grappling with the APA, the statute that lays out how such review works. And its flaws were apparent from the start, prompting the Court to revise its foundations and continually limit its application.
Experience has also shown that Chevron is unworkable. The defining feature of its framework is the identification of statutory ambiguity, but the concept of ambiguity has always evaded meaningful definition. Such an impressionistic and malleable concept “cannot stand as an every-day test for allocating” interpretive authority between courts and agencies. Swift & Co. v. Wickham , 382 U. S. 111 , 125. The Court has also been forced to clarify the doctrine again and again, only adding to Chevron ’s unworkability, and the doctrine continues to spawn difficult threshold questions that promise to further complicate the inquiry should Chevron be retained. And its continuing import is far from clear, as courts have often declined to engage with the doctrine, saying it makes no difference.
Nor has Chevron fostered meaningful reliance. Given the Court’s constant tinkering with and eventual turn away from Chevron , it is hard to see how anyone could reasonably expect a court to rely on Chevron in any particular case or expect it to produce readily foreseeable outcomes. And rather than safeguarding reliance interests, Chevron affirmatively destroys them by allowing agencies to change course even when Congress has given them no power to do so.
The only way to “ensure that the law will not merely change erratically, but will develop in a principled and intelligible fashion,” Vasquez v. Hillery , 474 U. S. 254 , 265, is for the Court to leave Chevron behind. By overruling Chevron , though, the Court does not call into question prior cases that relied on the Chevron framework. The holdings of those cases that specific agency actions are lawful—including the Clean Air Act holding of Chevron itself—are still subject to statutory stare decisis despite the Court’s change in interpretive methodology. See CBOCS West, Inc. v. Humphries , 553 U. S. 442 , 457. Mere reliance on Chevron cannot constitute a “ ‘special justification’ ” for overruling such a holding. Halliburton Co. v. Erica P. John Fund, Inc. , 573 U. S. 258 , 266 (quoting Dickerson v. United States , 530 U. S. 428 , 443). Pp. 29–35.
No. 22–451, 45 F. 4th 359 & No. 22–1219, 62 F. 4th 621, vacated and remanded.
Roberts , C. J., delivered the opinion of the Court, in which Thomas , Alito , Gorsuch , Kavanaugh , and Barrett , JJ., joined. Thomas , J., and Gorsuch , J., filed concurring opinions. Kagan , J., filed a dissenting opinion, in which Sotomayor , J., joined, and in which Jackson , J., joined as it applies to No. 22–1219. Jackson , J., took no part in the consideration or decision of the case in No. 22–451.
1 *Together with No. 22–1219, Relentless , Inc. , et al. v. Department of Commerce , et al. , on certiorari to the United States Court of Appeals for the First Circuit.
NOTICE: This opinion is subject to formal revision before publication in the United States Reports. Readers are requested to notify the Reporter of Decisions, Supreme Court of the United States, Washington, D. C. 20543, [email protected], of any typographical or other formal errors.
_________________
Nos. 22–451 and 22–1219
LOPER BRIGHT ENTERPRISES, et al. , PETITIONERS
GINA RAIMONDO, SECRETARY OF COMMERCE, et al .
on writ of certiorari to the united states court of appeals for the district of columbia circuit
RELENTLESS, INC., et al ., PETITIONERS
22–1219 v.
DEPARTMENT OF COMMERCE, et al .
on writ of certiorari to the united states court of appeals for the first circuit
Chief Justice Roberts delivered the opinion of the Court.
Since our decision in Chevron U. S. A. Inc. v. Natural Resources Defense Council, Inc. , 467 U. S. 837 (1984) , we have sometimes required courts to defer to “permissible” agency interpretations of the statutes those agencies administer—even when a reviewing court reads the statute differently. In these cases we consider whether that doctrine should be overruled.
Our Chevron doctrine requires courts to use a two-step framework to interpret statutes administered by federal agencies. After determining that a case satisfies the various preconditions we have set for Chevron to apply, a reviewing court must first assess “whether Congress has directly spoken to the precise question at issue.” Id. , at 842. If, and only if, congressional intent is “clear,” that is the end of the inquiry. Ibid. But if the court determines that “the statute is silent or ambiguous with respect to the specific issue” at hand, the court must, at Chevron ’s second step, defer to the agency’s interpretation if it “is based on a permissible construction of the statute.” Id. , at 843. The reviewing courts in each of the cases before us applied Chevron ’s framework to resolve in favor of the Government challenges to the same agency rule.
Before 1976, unregulated foreign vessels dominated fishing in the international waters off the U. S. coast, which began just 12 nautical miles offshore. See, e.g. , S. Rep. No. 94–459, pp. 2–3 (1975). Recognizing the resultant overfishing and the need for sound management of fishery resources, Congress enacted the Magnuson-Stevens Fishery Conservation and Management Act (MSA). See 90 Stat. 331 (codified as amended at 16 U. S. C. §1801 et seq .). The MSA and subsequent amendments extended the jurisdiction of the United States to 200 nautical miles beyond the U. S. territorial sea and claimed “exclusive fishery management authority over all fish” within that area, known as the “exclusive economic zone.” §1811(a); see Presidential Proclamation No. 5030, 3 CFR 22 (1983 Comp.); §§101, 102, 90 Stat. 336 . The National Marine Fisheries Service (NMFS) administers the MSA under a delegation from the Secretary of Commerce.
The MSA established eight regional fishery management councils composed of representatives from the coastal States, fishery stakeholders, and NMFS. See 16 U. S. C. §§1852(a), (b). The councils develop fishery management plans, which NMFS approves and promulgates as final regulations. See §§1852(h), 1854(a). In service of the statute’s fishery conservation and management goals, see §1851(a), the MSA requires that certain provisions—such as “a mechanism for specifying annual catch limits . . . at a level such that overfishing does not occur,” §1853(a)(15)—be included in these plans, see §1853(a). The plans may also include additional discretionary provisions. See §1853(b). For example, plans may “prohibit, limit, condition, or require the use of specified types and quantities of fishing gear, fishing vessels, or equipment,” §1853(b)(4); “reserve a portion of the allowable biological catch of the fishery for use in scientific research,” §1853(b)(11); and “prescribe such other measures, requirements, or conditions and restrictions as are determined to be necessary and appropriate for the conservation and management of the fishery,” §1853(b)(14).
Relevant here, a plan may also require that “one or more observers be carried on board” domestic vessels “for the purpose of collecting data necessary for the conservation and management of the fishery.” §1853(b)(8). The MSA specifies three groups that must cover costs associated with observers: (1) foreign fishing vessels operating within the exclusive economic zone (which must carry observers), see §§1821(h)(1)(A), (h)(4), (h)(6); (2) vessels participating in certain limited access privilege programs, which impose quotas permitting fishermen to harvest only specific quantities of a fishery’s total allowable catch, see §§1802(26), 1853a(c)(1)(H), (e)(2), 1854(d)(2); and (3) vessels within the jurisdiction of the North Pacific Council, where many of the largest and most successful commercial fishing enterprises in the Nation operate, see §1862(a). In the latter two cases, the MSA expressly caps the relevant fees at two or three percent of the value of fish harvested on the vessels. See §§1854(d)(2)(B), 1862(b)(2)(E). And in general, it author izes the Secretary to impose “sanctions” when “any payment required for observer services provided to or contracted by an owner or operator . . . has not been paid.” §1858(g)(1)(D).
The MSA does not contain similar terms addressing whether Atlantic herring fishermen may be required to bear costs associated with any observers a plan may mandate. And at one point, NMFS fully funded the observer coverage the New England Fishery Management Council required in its plan for the Atlantic herring fishery. See 79 Fed. Reg. 8792 (2014). In 2013, however, the council proposed amending its fishery management plans to empower it to require fishermen to pay for observers if federal funding became unavailable. Several years later, NMFS promulgated a rule approving the amendment. See 85 Fed. Reg. 7414 (2020).
With respect to the Atlantic herring fishery, the Rule created an industry funded program that aims to ensure observer coverage on 50 percent of trips undertaken by vessels with certain types of permits. Under that program, vessel representatives must “declare into” a fishery before beginning a trip by notifying NMFS of the trip and announcing the species the vessel intends to harvest. If NMFS determines that an observer is required, but declines to assign a Government-paid one, the vessel must contract with and pay for a Government-certified third-party observer. NMFS estimated that the cost of such an observer would be up to $710 per day, reducing annual returns to the vessel owner by up to 20 percent. See id. , at 7417–7418.
Petitioners Loper Bright Enterprises, Inc., H&L Axelsson, Inc., Lund Marr Trawlers LLC, and Scombrus One LLC are family businesses that operate in the Atlantic herring fishery. In February 2020, they challenged the Rule under the MSA, 16 U. S. C. §1855 (f ), which incorporates the Administrative Procedure Act (APA), 5 U. S. C. §551 et seq. In relevant part, they argued that the MSA does not authorize NMFS to mandate that they pay for observers required by a fishery management plan. The District Court granted summary judgment to the Government. It concluded that the MSA authorized the Rule, but noted that even if these petitioners’ “arguments were enough to raise an ambiguity in the statutory text,” deference to the agency’s interpretation would be warranted under Chevron . 544 F. Supp. 3d 82, 107 (DC 2021); see id. , at 103–107.
A divided panel of the D. C. Circuit affirmed. See 45 F. 4th 359 (2022). The majority addressed various provisions of the MSA and concluded that it was not “wholly unambiguous” whether NMFS may require Atlantic herring fishermen to pay for observers. Id. , at 366. Because there remained “some question” as to Congress’s intent, id. , at 369, the court proceeded to Chevron ’s second step and deferred to the agency’s interpretation as a “reasonable” construction of the MSA, 45 F. 4th, at 370. In dissent, Judge Walker concluded that Congress’s silence on industry funded observers for the Atlantic herring fishery—coupled with the express provision for such observers in other fisheries and on foreign vessels—unambiguously indicated that NMFS lacked the authority to “require [Atlantic herring] fishermen to pay the wages of at-sea monitors.” Id. , at 375.
Petitioners Relentless Inc., Huntress Inc., and Seafreeze Fleet LLC own two vessels that operate in the Atlantic herring fishery: the F/V Relentless and the F/V Persistence . 1 These vessels use small-mesh bottom-trawl gear and can freeze fish at sea, so they can catch more species of fish and take longer trips than other vessels (about 10 to 14 days, as opposed to the more typical 2 to 4). As a result, they generally declare into multiple fisheries per trip so they can catch whatever the ocean offers up. If the vessels declare into the Atlantic herring fishery for a particular trip, they must carry an observer for that trip if NMFS selects the trip for coverage, even if they end up harvesting fewer herring than other vessels—or no herring at all.
This set of petitioners, like those in the D. C. Circuit case, filed a suit challenging the Rule as unauthorized by the MSA. The District Court, like the D. C. Circuit, deferred to NMFS’s contrary interpretation under Chevron and thus granted summary judgment to the Government. See 561 F. Supp. 3d 226, 234–238 (RI 2021).
The First Circuit affirmed. See 62 F. 4th 621 (2023). It relied on a “default norm” that regulated entities must bear compliance costs, as well as the MSA’s sanctions provision, Section 1858(g)(1)(D). See id. , at 629–631. And it rejected petitioners’ argument that the express statutory authorization of three industry funding programs demonstrated that NMFS lacked the broad implicit authority it asserted to impose such a program for the Atlantic herring fishery. See id. , at 631–633. The court ultimately concluded that the “[a]gency’s interpretation of its authority to require at-sea monitors who are paid for by owners of regulated vessels does not ‘exceed[ ] the bounds of the permissible.’ ” Id. , at 633–634 (quoting Barnhart v. Walton , 535 U. S. 212 , 218 (2002); alteration in original). In reaching that conclusion, the First Circuit stated that it was applying Chevron ’s two-step framework. 62 F. 4th, at 628. But it did not explain which aspects of its analysis were relevant to which of Chevron ’s two steps. Similarly, it declined to decide whether the result was “a product of Chevron step one or step two.” Id. , at 634.
We granted certiorari in both cases, limited to the question whether Chevron should be overruled or clarified. See 601 U. S. ___ (2023); 598 U. S. ___ (2023). 2
Article III of the Constitution assigns to the Federal Judiciary the responsibility and power to adjudicate “Cases” and “Controversies”—concrete disputes with consequences for the parties involved. The Framers appreciated that the laws judges would necessarily apply in resolving those disputes would not always be clear. Cognizant of the limits of human language and foresight, they anticipated that “[a]ll new laws, though penned with the greatest technical skill, and passed on the fullest and most mature deliberation,” would be “more or less obscure and equivocal, until their meaning” was settled “by a series of particular discussions and adjudications.” The Federalist No. 37, p. 236 (J. Cooke ed. 1961) (J. Madison).
The Framers also envisioned that the final “interpretation of the laws” would be “the proper and peculiar province of the courts.” Id. , No. 78, at 525 (A. Hamilton). Unlike the political branches, the courts would by design exercise “neither Force nor Will, but merely judgment.” Id. , at 523. To ensure the “steady, upright and impartial administration of the laws,” the Framers structured the Constitution to allow judges to exercise that judgment independent of influence from the political branches. Id. , at 522; see id. , at 522–524; Stern v. Marshall , 564 U. S. 462 , 484 (2011).
This Court embraced the Framers’ understanding of the judicial function early on. In the foundational decision of Marbury v. Madison , Chief Justice Marshall famously declared that “[i]t is emphatically the province and duty of the judicial department to say what the law is.” 1 Cranch 137, 177 (1803). And in the following decades, the Court understood “interpret[ing] the laws, in the last resort,” to be a “solemn duty” of the Judiciary. United States v. Dickson , 15 Pet. 141, 162 (1841) (Story, J., for the Court). When the meaning of a statute was at issue, the judicial role was to “interpret the act of Congress, in order to ascertain the rights of the parties.” Decatur v. Paulding , 14 Pet. 497, 515 (1840).
The Court also recognized from the outset, though, that exercising independent judgment often included according due respect to Executive Branch interpretations of federal statutes. For example, in Edwards’ Lessee v. Darby , 12 Wheat. 206 (1827), the Court explained that “[i]n the construction of a doubtful and ambiguous law, the contemporaneous construction of those who were called upon to act under the law, and were appointed to carry its provisions into effect, is entitled to very great respect.” Id. , at 210; see also United States v. Vowell , 5 Cranch 368, 372 (1809) (Marshall, C. J., for the Court).
Such respect was thought especially warranted when an Executive Branch interpretation was issued roughly contemporaneously with enactment of the statute and remained consistent over time. See Dickson , 15 Pet., at 161; United States v. Alabama Great Southern R. Co. , 142 U. S. 615 , 621 (1892); National Lead Co. v. United States , 252 U. S. 140 , 145–146 (1920). That is because “the longstanding ‘practice of the government’ ”—like any other interpretive aid—“can inform [a court’s] determination of ‘what the law is.’ ” NLRB v. Noel Canning , 573 U. S. 513 , 525 (2014) (first quoting McCulloch v. Maryland , 4 Wheat. 316, 401 (1819); then quoting Marbury , 1 Cranch, at 177). The Court also gave “the most respectful consideration” to Executive Branch interpretations simply because “[t]he officers concerned [were] usually able men, and masters of the subject,” who were “[n]ot unfrequently . . . the draftsmen of the laws they [were] afterwards called upon to interpret.” United States v. Moore , 95 U. S. 760 , 763 (1878); see also Jacobs v. Prichard , 223 U. S. 200 , 214 (1912).
“Respect,” though, was just that. The views of the Executive Branch could inform the judgment of the Judiciary, but did not supersede it. Whatever respect an Executive Branch interpretation was due, a judge “certainly would not be bound to adopt the construction given by the head of a department.” Decatur , 14 Pet., at 515; see also Burnet v. Chicago Portrait Co. , 285 U. S. 1 , 16 (1932). Otherwise, judicial judgment would not be independent at all. As Justice Story put it, “in cases where [a court’s] own judgment . . . differ[ed] from that of other high functionaries,” the court was “not at liberty to surrender, or to waive it.” Dickson , 15 Pet., at 162.
The New Deal ushered in a “rapid expansion of the administrative process.” United States v. Morton Salt Co. , 338 U. S. 632 , 644 (1950). But as new agencies with new powers proliferated, the Court continued to adhere to the traditional understanding that questions of law were for courts to decide, exercising independent judgment.
During this period, the Court often treated agency determinations of fact as binding on the courts, provided that there was “evidence to support the findings.” St. Joseph Stock Yards Co. v. United States , 298 U. S. 38 , 51 (1936). “When the legislature itself acts within the broad field of legislative discretion,” the Court reasoned, “its determinations are conclusive.” Ibid. Congress could therefore “appoint[ ] an agent to act within that sphere of legislative authority” and “endow the agent with power to make findings of fact which are conclusive, provided the requirements of due process which are specially applicable to such an agency are met, as in according a fair hearing and acting upon evidence and not arbitrarily.” Ibid. (emphasis added).
But the Court did not extend similar deference to agency resolutions of questions of law . It instead made clear, repeatedly, that “[t]he interpretation of the meaning of statutes, as applied to justiciable controversies,” was “exclusively a judicial function.” United States v. American Trucking Assns., Inc. , 310 U. S. 534 , 544 (1940); see also Social Security Bd. v. Nierotko , 327 U. S. 358 , 369 (1946); Medo Photo Supply Corp. v. NLRB , 321 U. S. 678 , 681–682, n. 1 (1944). The Court understood, in the words of Justice Brandeis, that “[t]he supremacy of law demands that there shall be opportunity to have some court decide whether an erroneous rule of law was applied.” St. Joseph Stock Yards , 298 U. S., at 84 (concurring opinion). It also continued to note, as it long had, that the informed judgment of the Executive Branch—especially in the form of an interpretation issued contemporaneously with the enactment of the statute—could be entitled to “great weight.” American Trucking Assns. , 310 U. S., at 549.
Perhaps most notably along those lines, in Skidmore v. Swift & Co. , 323 U. S. 134 (1944) , the Court explained that the “interpretations and opinions” of the relevant agency, “made in pursuance of official duty” and “based upon . . . specialized experience,” “constitute[d] a body of experience and informed judgment to which courts and litigants [could] properly resort for guidance,” even on legal questions. Id. , at 139–140. “The weight of such a judgment in a particular case,” the Court observed, would “depend upon the thoroughness evident in its consideration, the validity of its reasoning, its consistency with earlier and later pronouncements, and all those factors which give it power to persuade, if lacking power to control.” Id. , at 140.
On occasion, to be sure, the Court applied deferential review upon concluding that a particular statute empowered an agency to decide how a broad statutory term applied to specific facts found by the agency. For example, in Gray v. Powell , 314 U. S. 402 (1941) , the Court deferred to an administrative conclusion that a coal-burning railroad that had arrangements with several coal mines was not a coal “producer” under the Bituminous Coal Act of 1937. Congress had “specifically” granted the agency the authority to make that determination. Id. , at 411. The Court thus reasoned that “[w]here, as here, a determination has been left to an administrative body, this delegation will be respected and the administrative conclusion left untouched” so long as the agency’s decision constituted “a sensible exercise of judgment.” Id. , at 412–413. Similarly, in NLRB v. Hearst Publications, Inc. , 322 U. S. 111 (1944) , the Court deferred to the determination of the National Labor Relations Board that newsboys were “employee[s]” within the meaning of the National Labor Relations Act. The Act had, in the Court’s judgment, “assigned primarily” to the Board the task of marking a “definitive limitation around the term ‘employee.’ ” Id. , at 130. The Court accordingly viewed its own role as “limited” to assessing whether the Board’s determination had a “ ‘warrant in the record’ and a reasonable basis in law.” Id. , at 131.
Such deferential review, though, was cabined to factbound determinations like those at issue in Gray and Hearst . Neither Gray nor Hearst purported to refashion the longstanding judicial approach to questions of law. In Gray , after deferring to the agency’s determination that a particular entity was not a “producer” of coal, the Court went on to discern, based on its own reading of the text, whether another statutory term—“other disposal” of coal—encompassed a transaction lacking a transfer of title. See 314 U. S., at 416–417. The Court evidently perceived no basis for deference to the agency with respect to that pure legal question. And in Hearst , the Court proclaimed that “[u]ndoubtedly questions of statutory interpretation . . . are for the courts to resolve, giving appropriate weight to the judgment of those whose special duty is to administer the questioned statute.” 322 U. S., at 130–131. At least with respect to questions it regarded as involving “statutory interpretation,” the Court thus did not disturb the traditional rule. It merely thought that a different approach should apply where application of a statutory term was sufficiently intertwined with the agency’s factfinding.
In any event, the Court was far from consistent in reviewing deferentially even such factbound statutory determinations. Often the Court simply interpreted and applied the statute before it. See K. Davis, Administrative Law §248, p. 893 (1951) (“The one statement that can be made with confidence about applicability of the doctrine of Gray v. Powell is that sometimes the Supreme Court applies it and sometimes it does not.”); B. Schwartz, Gray vs. Powell and the Scope of Review, 54 Mich. L. Rev. 1, 68 (1955) (noting an “embarrassingly large number of Supreme Court decisions that do not adhere to the doctrine of Gray v. Powell ”). In one illustrative example, the Court rejected the U. S. Price Administrator’s determination that a particular warehouse was a “public utility” entitled to an exemption from the Administrator’s General Maximum Price Regulation. Despite the striking resemblance of that administrative determination to those that triggered deference in Gray and Hearst , the Court declined to “accept the Administrator’s view in deference to administrative construction.” Davies Warehouse Co. v. Bowles , 321 U. S. 144 , 156 (1944). The Administrator’s view, the Court explained, had “hardly seasoned or broadened into a settled administrative practice,” and thus did not “overweigh the considerations” the Court had “set forth as to the proper construction of the statute.” Ibid.
Nothing in the New Deal era or before it thus resembled the deference rule the Court would begin applying decades later to all varieties of agency interpretations of statutes. Instead, just five years after Gray and two after Hearst , Congress codified the opposite rule: the traditional understanding that courts must “decide all relevant questions of law.” 5 U. S. C. §706 . 3
Congress in 1946 enacted the APA “as a check upon administrators whose zeal might otherwise have carried them to excesses not contemplated in legislation creating their offices.” Morton Salt , 338 U. S., at 644. It was the culmination of a “comprehensive rethinking of the place of administrative agencies in a regime of separate and divided powers.” Bowen v. Michigan Academy of Family Physicians , 476 U. S. 667 , 670–671 (1986).
In addition to prescribing procedures for agency action, the APA delineates the basic contours of judicial review of such action. As relevant here, Section 706 directs that “[t]o the extent necessary to decision and when presented, the reviewing court shall decide all relevant questions of law, interpret constitutional and statutory provisions, and determine the meaning or applicability of the terms of an agency action.” 5 U. S. C. §706 . It further requires courts to “hold unlawful and set aside agency action, findings, and conclusions found to be . . . not in accordance with law.” §706(2)(A).
The APA thus codifies for agency cases the unremarkable, yet elemental proposition reflected by judicial practice dating back to Marbury : that courts decide legal questions by applying their own judgment. It specifies that courts, not agencies, will decide “ all relevant questions of law” arising on review of agency action, §706 (emphasis added)—even those involving ambiguous laws—and set aside any such action inconsistent with the law as they interpret it. And it prescribes no deferential standard for courts to employ in answering those legal questions. That omission is telling, because Section 706 does mandate that judicial review of agency policymaking and factfinding be deferential. See §706(2)(A) (agency action to be set aside if “arbitrary, capricious, [or] an abuse of discretion”); §706(2)(E) (agency factfinding in formal proceedings to be set aside if “unsupported by substantial evidence”).
In a statute designed to “serve as the fundamental charter of the administrative state,” Kisor v. Wilkie , 588 U. S. 558 , 580 (2019) (plurality opinion) (internal quotation marks omitted), Congress surely would have articulated a similarly deferential standard applicable to questions of law had it intended to depart from the settled pre-APA understanding that deciding such questions was “exclusively a judicial function,” American Trucking Assns. , 310 U. S., at 544. But nothing in the APA hints at such a dramatic departure. On the contrary, by directing courts to “interpret constitutional and statutory provisions” without differentiating between the two, Section 706 makes clear that agency interpretations of statutes—like agency interpretations of the Constitution—are not entitled to deference. Under the APA, it thus “remains the responsibility of the court to decide whether the law means what the agency says.” Perez v. Mortgage Bankers Assn. , 575 U. S. 92 , 109 (2015) (Scalia, J., concurring in judgment). 4
The text of the APA means what it says. And a look at its history if anything only underscores that plain meaning. According to both the House and Senate Reports on the legislation, Section 706 “provide[d] that questions of law are for courts rather than agencies to decide in the last analysis.” H. R. Rep. No. 1980, 79th Cong., 2d Sess., 44 (1946) (emphasis added); accord, S. Rep. No. 752, 79th Cong., 1st Sess., 28 (1945). Some of the legislation’s most prominent supporters articulated the same view. See 92 Cong. Rec. 5654 (1946) (statement of Rep. Walter); P. McCarran, Improving “Administrative Justice”: Hearings and Evidence; Scope of Judicial Review, 32 A. B. A. J. 827, 831 (1946). Even the Department of Justice—an agency with every incentive to endorse a view of the APA favorable to the Executive Branch—opined after its enactment that Section 706 merely “restate[d] the present law as to the scope of judicial review.” Dept. of Justice, Attorney General’s Manual on the Administrative Procedure Act 108 (1947); see also Kisor , 588 U. S., at 582 (plurality opinion) (same). That “present law,” as we have described, adhered to the traditional conception of the judicial function. See supra , at 9–13.
Various respected commentators contemporaneously maintained that the APA required reviewing courts to exercise independent judgment on questions of law. Professor John Dickinson, for example, read the APA to “impose a clear mandate that all [questions of law] shall be decided by the reviewing Court itself, and in the exercise of its own independent judgment.” Administrative Procedure Act: Scope and Grounds of Broadened Judicial Review, 33 A. B. A. J. 434, 516 (1947). Professor Bernard Schwartz noted that §706 “would seem . . . to be merely a legislative restatement of the familiar review principle that questions of law are for the reviewing court, at the same time leaving to the courts the task of determining in each case what are questions of law.” Mixed Questions of Law and Fact and the Administrative Procedure Act, 19 Ford. L. Rev. 73, 84–85 (1950). And Professor Louis Jaffe, who had served in several agencies at the advent of the New Deal, thought that §706 leaves it up to the reviewing “court” to “decide as a ‘question of law’ whether there is ‘discretion’ in the premises”—that is, whether the statute at issue delegates particular discretionary authority to an agency. Judicial Control of Administrative Action 570 (1965).
The APA, in short, incorporates the traditional understanding of the judicial function, under which courts must exercise independent judgment in determining the meaning of statutory provisions. In exercising such judgment, though, courts may—as they have from the start—seek aid from the interpretations of those responsible for implementing particular statutes. Such interpretations “constitute a body of experience and informed judgment to which courts and litigants may properly resort for guidance” consistent with the APA. Skidmore , 323 U. S., at 140. And interpretations issued contemporaneously with the statute at issue, and which have remained consistent over time, may be especially useful in determining the statute’s meaning. See ibid. ; American Trucking Assns. , 310 U. S., at 549.
In a case involving an agency, of course, the statute’s meaning may well be that the agency is authorized to exercise a degree of discretion. Congress has often enacted such statutes. For example, some statutes “expressly delegate[ ]” to an agency the authority to give meaning to a particular statutory term. Batterton v. Francis , 432 U. S. 416 , 425 (1977) (emphasis deleted). 5 Others empower an agency to prescribe rules to “fill up the details” of a statutory scheme, Wayman v. Southard , 10 Wheat. 1, 43 (1825), or to regulate subject to the limits imposed by a term or phrase that “leaves agencies with flexibility,” Michigan v. EPA , 576 U. S. 743 , 752 (2015), such as “appropriate” or “reasonable.” 6
When the best reading of a statute is that it delegates discretionary authority to an agency, the role of the reviewing court under the APA is, as always, to independently interpret the statute and effectuate the will of Congress subject to constitutional limits. The court fulfills that role by recognizing constitutional delegations, “fix[ing] the boundaries of [the] delegated authority,” H. Monaghan, Marbury and the Administrative State, 83 Colum. L. Rev. 1, 27 (1983), and ensuring the agency has engaged in “ ‘reasoned decisionmaking’ ” within those boundaries, Michigan , 576 U. S., at 750 (quoting Allentown Mack Sales & Service, Inc. v. NLRB , 522 U. S. 359 , 374 (1998)); see also Motor Vehicle Mfrs. Assn. of United States, Inc. v. State Farm Mut. Automobile Ins. Co. , 463 U. S. 29 (1983) . By doing so, a court upholds the traditional conception of the judicial function that the APA adopts.
The deference that Chevron requires of courts reviewing agency action cannot be squared with the APA.
In the decades between the enactment of the APA and this Court’s decision in Chevron , courts generally continued to review agency interpretations of the statutes they administer by independently examining each statute to determine its meaning. Cf. T. Merrill, Judicial Deference to Executive Precedent, 101 Yale L. J. 969, 972–975 (1992). As an early proponent (and later critic) of Chevron recounted, courts during this period thus identified delegations of discretionary authority to agencies on a “statute-by-statute basis.” A. Scalia, Judicial Deference to Administrative Interpretations of Law, 1989 Duke L. J. 511, 516.
Chevron , decided in 1984 by a bare quorum of six Justices, triggered a marked departure from the traditional approach. The question in the case was whether an EPA regulation “allow[ing] States to treat all of the pollution- emitting devices within the same industrial grouping as though they were encased within a single ‘bubble’ ” was consistent with the term “stationary source” as used in the Clean Air Act. 467 U. S., at 840. To answer that question of statutory interpretation, the Court articulated and employed a now familiar two-step approach broadly applicable to review of agency action.
The first step was to discern “whether Congress ha[d] directly spoken to the precise question at issue.” Id. , at 842. The Court explained that “[i]f the intent of Congress is clear, that is the end of the matter,” ibid. , and courts were therefore to “reject administrative constructions which are contrary to clear congressional intent,” id. , at 843, n. 9. To discern such intent, the Court noted, a reviewing court was to “employ[ ] traditional tools of statutory construction.” Ibid.
Without mentioning the APA, or acknowledging any doctrinal shift, the Court articulated a second step applicable when “Congress ha[d] not directly addressed the precise question at issue.” Id. , at 843. In such a case—that is, a case in which “the statute [was] silent or ambiguous with respect to the specific issue” at hand — a reviewing court could not “simply impose its own construction on the statute, as would be necessary in the absence of an administrative interpretation.” Ibid. (footnote omitted). A court instead had to set aside the traditional interpretive tools and defer to the agency if it had offered “a permissible construction of the statute,” ibid. , even if not “the reading the court would have reached if the question initially had arisen in a judicial proceeding,” ibid. , n. 11. That directive was justified, according to the Court, by the understanding that administering statutes “requires the formulation of policy” to fill statutory “gap[s]”; by the long judicial tradition of according “considerable weight” to Executive Branch interpretations; and by a host of other considerations, including the complexity of the regulatory scheme, EPA’s “detailed and reasoned” consideration, the policy-laden nature of the judgment supposedly required, and the agency’s indirect accountability to the people through the President. Id. , at 843, 844, and n. 14, 865.
Employing this new test, the Court concluded that Congress had not addressed the question at issue with the necessary “level of specificity” and that EPA’s interpretation was “entitled to deference.” Id. , at 865. It did not matter why Congress, as the Court saw it, had not squarely addressed the question, see ibid. , or that “the agency ha[d] from time to time changed its interpretation,” id. , at 863. The latest EPA interpretation was a permissible reading of the Clean Air Act, so under the Court’s new rule, that reading controlled.
Initially, Chevron “seemed destined to obscurity.” T. Merrill, The Story of Chevron : The Making of an Accidental Landmark, 66 Admin. L. Rev. 253, 276 (2014). The Court did not at first treat it as the watershed decision it was fated to become; it was hardly cited in cases involving statutory questions of agency authority. See ibid. But within a few years, both this Court and the courts of appeals were routinely invoking its two-step framework as the governing standard in such cases. See id. , at 276–277. As the Court did so, it revisited the doctrine’s justifications. Eventually, the Court decided that Chevron rested on “a presumption that Congress, when it left ambiguity in a statute meant for implementation by an agency, understood that the ambiguity would be resolved, first and foremost, by the agency, and desired the agency (rather than the courts) to possess whatever degree of discretion the ambiguity allows.” Smiley v. Citibank (South Dakota), N. A. , 517 U. S. 735 , 740–741 (1996); see also, e.g. , Cuozzo Speed Technologies, LLC v. Lee , 579 U. S. 261 , 276–277 (2016); Utility Air Regulatory Group v. EPA , 573 U. S. 302 , 315 (2014); National Cable & Telecommunications Assn. v. Brand X Internet Services , 545 U. S. 967 , 982 (2005).
Neither Chevron nor any subsequent decision of this Court attempted to reconcile its framework with the APA. The “law of deference” that this Court has built on the foundation laid in Chevron has instead been “[h]eedless of the original design” of the APA. Perez , 575 U. S., at 109 (Scalia, J., concurring in judgment).
Chevron defies the command of the APA that “the reviewing court”—not the agency whose action it reviews—is to “decide all relevant questions of law” and “interpret . . . statutory provisions.” §706 (emphasis added). It requires a court to ignore , not follow, “the reading the court would have reached” had it exercised its independent judgment as required by the APA. Chevron , 467 U. S., at 843, n. 11. And although exercising independent judgment is consistent with the “respect” historically given to Executive Branch interpretations, see, e.g. , Edwards’ Lessee , 12 Wheat., at 210; Skidmore , 323 U. S., at 140, Chevron insists on much more. It demands that courts mechanically afford binding deference to agency interpretations, including those that have been inconsistent over time. See 467 U. S., at 863. Still worse, it forces courts to do so even when a pre-existing judicial precedent holds that the statute means something else—unless the prior court happened to also say that the statute is “unambiguous.” Brand X , 545 U. S., at 982. That regime is the antithesis of the time honored approach the APA prescribes. In fretting over the prospect of “allow[ing]” a judicial interpretation of a statute “to override an agency’s” in a dispute before a court, ibid. , Chevron turns the statutory scheme for judicial review of agency action upside down.
Chevron cannot be reconciled with the APA, as the Government and the dissent contend, by presuming that statutory ambiguities are implicit delegations to agencies. See Brief for Respondents in No. 22–1219, pp. 13, 37–38; post , at 4–15 (opinion of Kagan , J.). Presumptions have their place in statutory interpretation, but only to the extent that they approximate reality. Chevron ’s presumption does not, because “[a]n ambiguity is simply not a delegation of law-interpreting power. Chevron confuses the two.” C. Sunstein, Interpreting Statutes in the Regulatory State, 103 Harv. L. Rev. 405, 445 (1989). As Chevron itself noted, ambiguities may result from an inability on the part of Congress to squarely answer the question at hand, or from a failure to even “consider the question” with the requisite precision. 467 U. S., at 865. In neither case does an ambiguity necessarily reflect a congressional intent that an agency, as opposed to a court, resolve the resulting interpretive question. And many or perhaps most statutory ambiguities may be unintentional. As the Framers recognized, ambiguities will inevitably follow from “the complexity of objects, . . . the imperfection of the human faculties,” and the simple fact that “no language is so copious as to supply words and phrases for every complex idea.” The Federalist No. 37, at 236.
Courts, after all, routinely confront statutory ambiguities in cases having nothing to do with Chevron —cases that do not involve agency interpretations or delegations of authority. Of course, when faced with a statutory ambiguity in such a case, the ambiguity is not a delegation to anybody, and a court is not somehow relieved of its obligation to independently interpret the statute. Courts in that situation do not throw up their hands because “Congress’s instructions have” supposedly “run out,” leaving a statutory “gap.” Post , at 2 (opinion of Kagan , J.). Courts instead understand that such statutes, no matter how impenetrable, do—in fact, must—have a single, best meaning. That is the whole point of having written statutes; “every statute’s meaning is fixed at the time of enactment.” Wisconsin Central Ltd. v. United States , 585 U. S. 274 , 284 (2018) (emphasis deleted). So instead of declaring a particular party’s reading “permissible” in such a case, courts use every tool at their disposal to determine the best reading of the statute and resolve the ambiguity.
In an agency case as in any other, though, even if some judges might (or might not) consider the statute ambiguous, there is a best reading all the same—“the reading the court would have reached” if no agency were involved. Chevron , 467 U. S., at 843, n. 11. It therefore makes no sense to speak of a “permissible” interpretation that is not the one the court, after applying all relevant interpretive tools, concludes is best. In the business of statutory interpretation, if it is not the best, it is not permissible.
Perhaps most fundamentally, Chevron ’s presumption is misguided because agencies have no special competence in resolving statutory ambiguities. Courts do. The Framers, as noted, anticipated that courts would often confront statutory ambiguities and expected that courts would resolve them by exercising independent legal judgment. And even Chevron itself reaffirmed that “[t]he judiciary is the final authority on issues of statutory construction” and recognized that “in the absence of an administrative interpretation,” it is “necessary” for a court to “impose its own construction on the statute.” Id. , at 843, and n. 9. Chevron gravely erred, though, in concluding that the inquiry is fundamentally different just because an administrative interpretation is in play. The very point of the traditional tools of statutory construction—the tools courts use every day—is to resolve statutory ambiguities. That is no less true when the ambiguity is about the scope of an agency’s own power—perhaps the occasion on which abdication in favor of the agency is least appropriate.
The Government responds that Congress must generally intend for agencies to resolve statutory ambiguities because agencies have subject matter expertise regarding the statutes they administer; because deferring to agencies purportedly promotes the uniform construction of federal law; and because resolving statutory ambiguities can involve policymaking best left to political actors, rather than courts. See Brief for Respondents in No. 22–1219, pp. 16–19. The dissent offers more of the same. See post , at 9–14. But none of these considerations justifies Chevron ’s sweeping presumption of congressional intent.
Beginning with expertise, we recently noted that interpretive issues arising in connection with a regulatory scheme often “may fall more naturally into a judge’s bailiwick” than an agency’s. Kisor , 588 U. S., at 578 (opinion of the Court). We thus observed that “[w]hen the agency has no comparative expertise in resolving a regulatory ambiguity, Congress presumably would not grant it that authority.” Ibid. Chevron ’s broad rule of deference, though, demands that courts presume just the opposite. Under that rule, ambiguities of all stripes trigger deference. Indeed, the Government and, seemingly, the dissent continue to defend the proposition that Chevron applies even in cases having little to do with an agency’s technical subject matter expertise. See Brief for Respondents in No. 22–1219, p. 17; post , at 10.
But even when an ambiguity happens to implicate a technical matter, it does not follow that Congress has taken the power to authoritatively interpret the statute from the courts and given it to the agency. Congress expects courts to handle technical statutory questions. “[M]any statutory cases” call upon “courts [to] interpret the mass of technical detail that is the ordinary diet of the law,” Egelhoff v. Egelhoff , 532 U. S. 141 , 161 (2001) (Breyer, J., dissenting), and courts did so without issue in agency cases before Chevron , see post , at 30 ( Gorsuch, J., concurring). Courts, after all, do not decide such questions blindly. The parties and amici in such cases are steeped in the subject matter, and reviewing courts have the benefit of their perspectives. In an agency case in particular, the court will go about its task with the agency’s “body of experience and informed judgment,” among other information, at its disposal. Skidmore , 323 U. S., at 140. And although an agency’s interpretation of a statute “cannot bind a court,” it may be especially informative “to the extent it rests on factual premises within [the agency’s] expertise.” Bureau of Alcohol, Tobacco and Firearms v. FLRA , 464 U. S. 89 , 98, n. 8 (1983). Such expertise has always been one of the factors which may give an Executive Branch interpretation particular “power to persuade, if lacking power to control.” Skidmore , 323 U. S., at 140; see, e.g. , County of Maui v. Hawaii Wildlife Fund , 590 U. S. 165 , 180 (2020); Moore , 95 U. S., at 763.
For those reasons, delegating ultimate interpretive authority to agencies is simply not necessary to ensure that the resolution of statutory ambiguities is well informed by subject matter expertise. The better presumption is therefore that Congress expects courts to do their ordinary job of interpreting statutes, with due respect for the views of the Executive Branch. And to the extent that Congress and the Executive Branch may disagree with how the courts have performed that job in a particular case, they are of course always free to act by revising the statute.
Nor does a desire for the uniform construction of federal law justify Chevron . Given inconsistencies in how judges apply Chevron , see infra , at 30–33, it is unclear how much the doctrine as a whole (as opposed to its highly deferential second step) actually promotes such uniformity. In any event, there is little value in imposing a uniform interpretation of a statute if that interpretation is wrong. We see no reason to presume that Congress prefers uniformity for uniformity’s sake over the correct interpretation of the laws it enacts.
The view that interpretation of ambiguous statutory provisions amounts to policymaking suited for political actors rather than courts is especially mistaken, for it rests on a profound misconception of the judicial role. It is reasonable to assume that Congress intends to leave policymaking to political actors. But resolution of statutory ambiguities involves legal interpretation. That task does not suddenly become policymaking just because a court has an “agency to fall back on.” Kisor , 588 U. S., at 575 (opinion of the Court). Courts interpret statutes, no matter the context, based on the traditional tools of statutory construction, not individual policy preferences. Indeed, the Framers crafted the Constitution to ensure that federal judges could exercise judgment free from the influence of the political branches. See The Federalist, No. 78, at 522–525. They were to construe the law with “[c]lear heads . . . and honest hearts,” not with an eye to policy preferences that had not made it into the statute. 1 Works of James Wilson 363 (J. Andrews ed. 1896).
That is not to say that Congress cannot or does not confer discretionary authority on agencies. Congress may do so, subject to constitutional limits, and it often has. But to stay out of discretionary policymaking left to the political branches, judges need only fulfill their obligations under the APA to independently identify and respect such delegations of authority, police the outer statutory boundaries of those delegations, and ensure that agencies exercise their discretion consistent with the APA. By forcing courts to instead pretend that ambiguities are necessarily delegations, Chevron does not prevent judges from making policy. It prevents them from judging.
In truth, Chevron ’s justifying presumption is, as Members of this Court have often recognized, a fiction. See Buffington v. McDonough , 598 U. S. ___, ___ (2022) ( Gorsuch , J., dissenting from denial of certiorari) (slip op., at 11); Cuozzo , 579 U. S., at 286 ( Thomas , J., concurring); Scalia, 1989 Duke L. J., at 517; see also post , at 15 (opinion of Kagan , J.). So we have spent the better part of four decades imposing one limitation on Chevron after another, pruning its presumption on the understanding that “where it is in doubt that Congress actually intended to delegate particular interpretive authority to an agency, Chevron is ‘inapplicable.’ ” United States v. Mead Corp. , 533 U. S. 218 , 230 (2001) (quoting Christensen v. Harris County , 529 U. S. 576 , 597 (2000) (Breyer, J., dissenting)); see also Adams Fruit Co. v. Barrett , 494 U. S. 638 , 649 (1990).
Consider the many refinements we have made in an effort to match Chevron ’s presumption to reality. We have said that Chevron applies only “when it appears that Congress delegated authority to the agency generally to make rules carrying the force of law, and that the agency interpretation claiming deference was promulgated in the exercise of that authority.” Mead , 533 U. S., at 226–227. In practice, that threshold requirement—sometimes called Chevron “step zero”—largely limits Chevron to “the fruits of notice-and-comment rulemaking or formal adjudication.” 533 U. S., at 230. But even when those processes are used, deference is still not warranted “where the regulation is ‘procedurally defective’—that is, where the agency errs by failing to follow the correct procedures in issuing the regulation.” Encino Motorcars, LLC v. Navarro , 579 U. S. 211 , 220 (2016) (quoting Mead , 533 U. S., at 227).
Even where those procedural hurdles are cleared, substantive ones remain. Most notably, Chevron does not apply if the question at issue is one of “deep ‘economic and political significance.’ ” King v. Burwell , 576 U. S. 473 , 486 (2015). We have instead expected Congress to delegate such authority “expressly” if at all, ibid. , for “[e]xtraordinary grants of regulatory authority are rarely accomplished through ‘modest words,’ ‘vague terms,’ or ‘subtle device[s],’ ” West Virginia v. EPA , 597 U. S. 697 , 723 (2022) (quoting Whitman v. American Trucking Assns., Inc. , 531 U. S. 457 , 468 (2001); alteration in original). Nor have we applied Chevron to agency interpretations of judicial review provisions, see Adams Fruit Co. , 494 U. S., at 649–650, or to statutory schemes not administered by the agency seeking deference, see Epic Systems Corp. v. Lewis , 584 U. S. 497 , 519–520 (2018). And we have sent mixed signals on whether Chevron applies when a statute has criminal applications. Compare Abramski v. United States , 573 U. S. 169 , 191 (2014), with Babbitt v. Sweet Home Chapter, Communities for Great Ore. , 515 U. S. 687 , 704, n. 18 (1995).
Confronted with this byzantine set of preconditions and exceptions, some courts have simply bypassed Chevron , saying it makes no difference for one reason or another. 7 And even when they do invoke Chevron , courts do not always heed the various steps and nuances of that evolving doctrine. In one of the cases before us today, for example, the First Circuit both skipped “step zero,” see 62 F. 4th, at 628, and refused to “classify [its] conclusion as a product of Chevron step one or step two”—though it ultimately appears to have deferred under step two, id. , at 634.
This Court, for its part, has not deferred to an agency interpretation under Chevron since 2016. See Cuozzo , 579 U. S., at 280 (most recent occasion). But Chevron remains on the books. So litigants must continue to wrestle with it, and lower courts—bound by even our crumbling precedents, see Agostini v. Felton , 521 U. S. 203 , 238 (1997)—understandably continue to apply it.
The experience of the last 40 years has thus done little to rehabilitate Chevron . It has only made clear that Chevron ’s fictional presumption of congressional intent was always unmoored from the APA’s demand that courts exercise independent judgment in construing statutes administered by agencies. At best, our intricate Chevron doctrine has been nothing more than a distraction from the question that matters: Does the statute authorize the challenged agency action? And at worst, it has required courts to violate the APA by yielding to an agency the express responsibility, vested in “the reviewing court ,” to “decide all relevant questions of law” and “interpret . . . statutory provisions.” §706 (emphasis added).
The only question left is whether stare decisis , the doctrine governing judicial adherence to precedent, requires us to persist in the Chevron project. It does not. Stare decisis is not an “inexorable command,” Payne v. Tennessee , 501 U. S. 808 , 828 (1991), and the stare decisis considerations most relevant here—“the quality of [the precedent’s] reasoning, the workability of the rule it established, . . . and reliance on the decision,” Knick v. Township of Scott , 588 U. S. 180 , 203 (2019) (quoting Janus v. State, County, and Municipal Employees , 585 U. S. 878 , 917 (2018))—all weigh in favor of letting Chevron go.
Chevron has proved to be fundamentally misguided. Despite reshaping judicial review of agency action, neither it nor any case of ours applying it grappled with the APA— the statute that lays out how such review works. Its flaws were nonetheless apparent from the start, prompting this Court to revise its foundations and continually limit its application. It has launched and sustained a cottage industry of scholars attempting to decipher its basis and meaning. And Members of this Court have long questioned its premises. See, e . g ., Pereira v. Sessions , 585 U. S. 198 , 219–221 (2018) (Kennedy, J., concurring); Michigan , 576 U. S., at 760–764 ( Thomas , J., concurring); Buffington , 598 U. S. ___ (opinion of Gorsuch , J.); B. Kavanaugh, Fixing Statutory Interpretation, 129 Harv. L. Rev. 2118, 2150–2154 (2016). Even Justice Scalia, an early champion of Chevron , came to seriously doubt whether it could be reconciled with the APA. See Perez , 575 U. S., at 109–110 (opinion concurring in judgment). For its entire existence, Chevron has been a “rule in search of a justification,” Knick , 588 U. S., at 204, if it was ever coherent enough to be called a rule at all.
Experience has also shown that Chevron is unworkable. The defining feature of its framework is the identification of statutory ambiguity, which requires deference at the doctrine’s second step. But the concept of ambiguity has always evaded meaningful definition. As Justice Scalia put the dilemma just five years after Chevron was decided: “How clear is clear?” 1989 Duke L. J., at 521.
We are no closer to an answer to that question than we were four decades ago. “ ‘[A]mbiguity’ is a term that may have different meanings for different judges.” Exxon Mobil Corp. v. Allapattah Services, Inc. , 545 U. S. 546 , 572 (2005) (Stevens, J., dissenting). One judge might see ambiguity everywhere; another might never encounter it. Compare L. Silberman, Chevron—The Intersection of Law & Policy, 58 Geo. Wash. L. Rev. 821, 822 (1990), with R. Kethledge, Ambiguities and Agency Cases: Reflections After (Almost) Ten Years on the Bench, 70 Vand. L. Rev. En Banc 315, 323 (2017). A rule of law that is so wholly “in the eye of the beholder,” Exxon Mobil Corp. , 545 U. S., at 572 (Stevens, J., dissenting), invites different results in like cases and is therefore “arbitrary in practice,” Gulfstream Aerospace Corp. v. Mayacamas Corp. , 485 U. S. 271 , 283 (1988). Such an impressionistic and malleable concept “cannot stand as an every-day test for allocating” interpretive authority between courts and agencies. Swift & Co. v. Wickham , 382 U. S. 111 , 125 (1965).
The dissent proves the point. It tells us that a court should reach Chevron ’s second step when it finds, “at the end of its interpretive work,” that “Congress has left an ambiguity or gap.” Post , at 1–2. (The Government offers a similar test. See Brief for Respondents in No. 22–1219, pp. 7, 10, 14; Tr. of Oral Arg. 113–114, 116.) That is no guide at all. Once more, the basic nature and meaning of a statute does not change when an agency happens to be involved. Nor does it change just because the agency has happened to offer its interpretation through the sort of procedures necessary to obtain deference, or because the other preconditions for Chevron happen to be satisfied. The statute still has a best meaning, necessarily discernible by a court deploying its full interpretive toolkit. So for the dissent’s test to have any meaning, it must think that in an agency case (unlike in any other), a court should give up on its “interpretive work” before it has identified that best meaning. But how does a court know when to do so? On that point, the dissent leaves a gap of its own. It protests only that some other interpretive tools—all with pedigrees more robust than Chevron ’s, and all designed to help courts identify the meaning of a text rather than allow the Executive Branch to displace it—also apply to ambiguous texts. See post , at 27. That this is all the dissent can come up with, after four decades of judicial experience attempting to identify ambiguity under Chevron , reveals the futility of the exercise. 8
Because Chevron in its original, two-step form was so indeterminate and sweeping, we have instead been forced to clarify the doctrine again and again. Our attempts to do so have only added to Chevron ’s unworkability, transforming the original two-step into a dizzying breakdance. See Adams Fruit Co. , 494 U. S., at 649–650; Mead , 533 U. S., at 226–227; King , 576 U. S., at 486; Encino Motorcars , 579 U. S., at 220; Epic Systems , 584 U. S., at 519–520; on and on. And the doctrine continues to spawn difficult threshold questions that promise to further complicate the inquiry should Chevron be retained. See, e . g ., Cargill v. Garland , 57 F. 4th 447, 465–468 (CA5 2023) (plurality opinion) (May the Government waive reliance on Chevron ? Does Chevron apply to agency interpretations of statutes imposing criminal penalties? Does Chevron displace the rule of lenity?), aff ’d, 602 U. S. ___ (2024).
Four decades after its inception, Chevron has thus become an impediment, rather than an aid, to accomplishing the basic judicial task of “say[ing] what the law is.” Marbury , 1 Cranch, at 177. And its continuing import is far from clear. Courts have often declined to engage with the doctrine, saying it makes no difference. See n. 7, supra . And as noted, we have avoided deferring under Chevron since 2016. That trend is nothing new; for decades, we have often declined to invoke Chevron even in those cases where it might appear to be applicable. See W. Eskridge & L. Baer, The Continuum of Deference: Supreme Court Treatment of Agency Statutory Interpretations From Chevron to Hamdan , 96 Geo. L. J. 1083, 1125 (2008). At this point, all that remains of Chevron is a decaying husk with bold pretensions.
Nor has Chevron been the sort of “ ‘stable background’ rule” that fosters meaningful reliance. Post , at 8, n. 1 (opinion of Kagan, J. ) (quoting Morrison v. National Australia Bank Ltd. , 561 U. S. 247 , 261 (2010)). Given our constant tinkering with and eventual turn away from Chevron , and its inconsistent application by the lower courts, it instead is hard to see how anyone—Congress included—could reasonably expect a court to rely on Chevron in any particular case. And even if it were possible to predict accurately when courts will apply Chevron , the doctrine “does not provide ‘a clear or easily applicable standard, so arguments for reliance based on its clarity are misplaced.’ ” Janus , 585 U. S., at 927 (quoting South Dakota v. Wayfair, Inc. , 585 U. S. 162 , 186 (2018)). To plan on Chevron yielding a particular result is to gamble not only that the doctrine will be invoked, but also that it will produce readily foreseeable outcomes and the stability that comes with them. History has proved neither bet to be a winning proposition.
Rather than safeguarding reliance interests, Chevron affirmatively destroys them. Under Chevron , a statutory ambiguity, no matter why it is there, becomes a license authorizing an agency to change positions as much as it likes, with “[u]nexplained inconsistency” being “at most . . . a reason for holding an interpretation to be . . . arbitrary and capricious.” Brand X , 545 U. S., at 981. But statutory ambiguity, as we have explained, is not a reliable indicator of actual delegation of discretionary authority to agencies. Chevron thus allows agencies to change course even when Congress has given them no power to do so. By its sheer breadth, Chevron fosters unwarranted instability in the law, leaving those attempting to plan around agency action in an eternal fog of uncertainty.
Chevron accordingly has undermined the very “rule of law” values that stare decisis exists to secure. Michigan v. Bay Mills Indian Community , 572 U. S. 782 , 798 (2014). And it cannot be constrained by admonishing courts to be extra careful, or by tacking on a new batch of conditions. We would need to once again “revis[e] its theoretical basis . . . in order to cure its practical deficiencies.” Montejo v. Louisiana , 556 U. S. 778 , 792 (2009). Stare decisis does not require us to do so, especially because any refinements we might make would only point courts back to their duties under the APA to “decide all relevant questions of law” and “interpret . . . statutory provisions.” §706. Nor is there any reason to wait helplessly for Congress to correct our mistake. The Court has jettisoned many precedents that Congress likewise could have legislatively overruled. See, e.g. , Patterson v. McLean Credit Union , 485 U. S. 617 , 618 (1988) ( per curiam ) (collecting cases). And part of “judicial humility,” post , at 3, 25 (opinion of Kagan , J.,), is admitting and in certain cases correcting our own mistakes, especially when those mistakes are serious, see post , at 8–9 (opinion of Gorsuch , J.).
This is one of those cases. Chevron was a judicial invention that required judges to disregard their statutory duties. And the only way to “ensure that the law will not merely change erratically, but will develop in a principled and intelligible fashion,” Vasquez v. Hillery , 474 U. S. 254 , 265 (1986), is for us to leave Chevron behind.
By doing so, however, we do not call into question prior cases that relied on the Chevron framework. The holdings of those cases that specific agency actions are lawful—including the Clean Air Act holding of Chevron itself—are still subject to statutory stare decisis despite our change in interpretive methodology. See CBOCS West, Inc. v. Humphries , 553 U. S. 442 , 457 (2008). Mere reliance on Chevron cannot constitute a “ ‘special justification’ ” for overruling such a holding, because to say a precedent relied on Chevron is, at best, “just an argument that the precedent was wrongly decided.” Halliburton Co. v. Erica P. John Fund, Inc. , 573 U. S. 258 , 266 (2014) (quoting Dickerson v. United States , 530 U. S. 428 , 443 (2000)). That is not enough to justify overruling a statutory precedent.
The dissent ends by quoting Chevron : “ ‘Judges are not experts in the field.’ ” Post , at 31 (quoting 467 U. S., at 865). That depends, of course, on what the “field” is. If it is legal interpretation, that has been, “emphatically,” “the province and duty of the judicial department” for at least 221 years. Marbury , 1 Cranch, at 177. The rest of the dissent’s selected epigraph is that judges “ ‘are not part of either political branch.’ ” Post , at 31 (quoting Chevron , 467 U. S., at 865). Indeed. Judges have always been expected to apply their “judgment” independent of the political branches when interpreting the laws those branches enact. The Federalist No. 78, at 523. And one of those laws, the APA, bars judges from disregarding that responsibility just because an Executive Branch agency views a statute differently.
Chevron is overruled. Courts must exercise their independent judgment in deciding whether an agency has acted within its statutory authority, as the APA requires. Careful attention to the judgment of the Executive Branch may help inform that inquiry. And when a particular statute delegates authority to an agency consistent with constitutional limits, courts must respect the delegation, while ensuring that the agency acts within it. But courts need not and under the APA may not defer to an agency interpretation of the law simply because a statute is ambiguous.
Because the D. C. and First Circuits relied on Chevron in deciding whether to uphold the Rule, their judgments are vacated, and the cases are remanded for further proceedings consistent with this opinion.
It is so ordered.
1 For any landlubbers, “F/V” is simply the designation for a fishing vessel.
2 Both petitions also presented questions regarding the consistency of the Rule with the MSA. See Pet. for Cert. in No. 22–451, p. i; Pet. for Cert. in No. 22–1219, p. ii. We did not grant certiorari with respect to those questions and thus do not reach them.
3 The dissent plucks out Gray , Hearst , and—to “gild the lily,” in its telling—three more 1940s decisions, claiming they reflect the relevant historical tradition of judicial review. Post , at 21–22, and n. 6 (opinion of Kagan , J.). But it has no substantial response to the fact that Gray and Hearst themselves endorsed, implicitly in one case and explicitly in the next, the traditional rule that “questions of statutory interpretation . . . are for the courts to resolve, giving appropriate weight”—not outright deference—“to the judgment of those whose special duty is to administer the questioned statute.” Hearst , 322 U. S., at 130–131. And it fails to recognize the deep roots that this rule has in our Nation’s judicial tradition, to the limited extent it engages with that tradition at all. See post , at 20–21, n. 5. Instead, like the Government, it strains to equate the “respect” or “weight” traditionally afforded to Executive Branch interpretations with binding deference. See ibid. ; Brief for Respondents in No. 22–1219, pp. 21–24. That supposed equivalence is a fiction. The dissent’s cases establish that a “ contemporaneous construction” shared by “not only . . . the courts” but also “the departments” could be “controlling,” Schell’s Executors v. Fauché , 138 U. S. 562 , 572 (1891) (emphasis added), and that courts might “lean in favor” of a “contemporaneous” and “continued” construction of the Executive Branch as strong evidence of a statute’s meaning, United States v. Alabama Great Southern R. Co. , 142 U. S. 615 , 621 (1892). They do not establish that Executive Branch interpretations of ambiguous statutes—no matter how inconsistent, late breaking, or flawed—always bound the courts. In reality, a judge was never “bound to adopt the construction given by the head of a department.” Decatur v. Paulding , 14 Pet. 497, 515 (1840).
4 The dissent observes that Section 706 does not say expressly that courts are to decide legal questions using “a de novo standard of review.” Post , at 16. That much is true. But statutes can be sensibly understood only “by reviewing text in context.” Pulsifer v. United States , 601 U. S. 124 , 133 (2024). Since the start of our Republic, courts have “decide[d] . . . questions of law” and “interpret[ed] constitutional and statutory provisions” by applying their own legal judgment. §706. Setting aside its misplaced reliance on Gray and Hearst , the dissent does not and could not deny that tradition. But it nonetheless insists that to codify that tradition, Congress needed to expressly reject a sort of deference the courts had never before applied—and would not apply for several decades to come. It did not. “The notion that some things ‘go without saying’ applies to legislation just as it does to everyday life.” Bond v. United States , 572 U. S. 844 , 857 (2014).
5 See, e.g. , 29 U. S. C. §213(a)(15) (exempting from provisions of the Fair Labor Standards Act “any employee employed on a casual basis in domestic service employment to provide companionship services for individuals who (because of age or infirmity) are unable to care for themselves ( as such terms are defined and delimited by regulations of the Secretary )” (emphasis added)); 42 U. S. C. §5846(a)(2) (requiring notification to Nuclear Regulatory Commission when a facility or activity licensed or regulated pursuant to the Atomic Energy Act “contains a defect which could create a substantial safety hazard, as defined by regulations which the Commission shall promulgate ” (emphasis added)).
6 See, e.g. , 33 U. S. C. §1312(a) (requiring establishment of effluent limitations “[w]henever, in the judgment of the [Environmental Protection Agency (EPA)] Administrator . . . , discharges of pollutants from a point source or group of point sources . . . would interfere with the attainment or maintenance of that water quality . . . which shall assure” various outcomes, such as the “protection of public health” and “public water supplies”); 42 U. S. C. §7412(n)(1)(A) (directing EPA to regulate power plants “if the Administrator finds such regulation is appropriate and necessary”).
7 See, e . g ., Guedes v. Bureau of Alcohol, Tobacco, Firearms and Explosives , 45 F. 4th 306, 313–314 (CADC 2022), abrogated by Garland v. Cargill , 602 U. S. ___ (2024); County of Amador v. United States Dept. of Interior , 872 F. 3d 1012 , 1021–1022 (CA9 2017); Estrada-Rodriguez v. Lynch , 825 F. 3d 397 , 403–404 (CA8 2016); Nielsen v. AECOM Tech. Corp. , 762 F. 3d 214 , 220 (CA2 2014); Alaska Stock, LLC v. Houghton Mifflin Harcourt Publishing Co. , 747 F. 3d 673 , 685, n. 52 (CA9 2014); Jurado-Delgado v. Attorney Gen. of U. S. , 498 Fed. Appx. 107, 117 (CA3 2009); see also D. Brookins, Confusion in the Circuit Courts: How the Circuit Courts Are Solving the Mead -Puzzle by Avoiding It Altogether, 85 Geo. Wash. L. Rev. 1484, 1496–1499 (2017) (documenting Chevron avoidance by the lower courts); A. Vermeule, Our Schmittian Administrative Law, 122 Harv. L. Rev. 1095, 1127–1129 (2009) (same); L. Bressman, How Mead Has Muddled Judicial Review of Agency Action, 58 Vand. L. Rev. 1443, 1464–1466 (2005) (same).
8 Citing an empirical study, the dissent adds that Chevron “fosters agreement among judges.” Post , at 28. It is hardly surprising that a study might find as much; Chevron ’s second step is supposed to be hospitable to agency interpretations. So when judges get there, they tend to agree that the agency wins. That proves nothing about the supposed ease or predictability of identifying ambiguity in the first place.
Concurrence
Justice Thomas , concurring.
I join the Court’s opinion in full because it correctly concludes that Chevron U. S. A. Inc. v. Natural Resources Defense Council, Inc. , 467 U. S. 837 (1984) , must finally be overruled. Under Chevron , a judge was required to adopt an agency’s interpretation of an ambiguous statute, so long as the agency had a “permissible construction of the statute.” See id. , at 843. As the Court explains, that deference does not comport with the Administrative Procedure Act, which requires judges to decide “all relevant questions of law” and “interpret constitutional and statutory provisions” when reviewing an agency action. 5 U. S. C. §706 ; see also ante, at 18–23; Baldwin v. United States , 589 U. S. ___, ___–___ (2020) ( Thomas, J. , dissenting from denial of certiorari) (slip op., at 4–5).
I write separately to underscore a more fundamental problem: Chevron deference also violates our Constitution’s separation of powers, as I have previously explained at length. See Baldwin , 589 U. S., at ___–___ (dissenting opinion) (slip op., at 2–4); Michigan v. EPA , 576 U. S. 743 , 761–763 (2015) (concurring opinion); see also Perez v. Mortgage Bankers Assn. , 575 U. S. 92 , 115–118 (2015) (opinion concurring in judgment). And, I agree with Justice Gorsuch that we should not overlook Chevron ’s constitutional defects in overruling it. 1 * Post , at 15–20 (concurring opinion). To provide “practical and real protections for individual liberty,” the Framers drafted a Constitution that divides the legislative, executive, and judicial powers between three branches of Government. Perez , 575 U. S., at 118 (opinion of Thomas, J. ). Chevron deference compromises this separation of powers in two ways. It curbs the judicial power afforded to courts, and simultaneously expands agencies’ executive power beyond constitutional limits.
Chevron compels judges to abdicate their Article III “judicial Power.” §1. “[T]he judicial power, as originally understood, requires a court to exercise its independent judgment in interpreting and expounding upon the laws.” Perez , 575 U. S., at 119 (opinion of Thomas, J. ); accord, post , at 17–18 (opinion of Gorsuch, J. ). The Framers understood that “legal texts . . . often contain ambiguities,” and that the judicial power included “the power to resolve these ambiguities over time.” Perez , 575 U. S., at 119 (opinion of Thomas, J. ); accord, ante, at 7–9. But, under Chevron , a judge must accept an agency’s interpretation of an ambiguous law, even if he thinks another interpretation is correct. Ante , at 19. Chevron deference thus prevents judges from exercising their independent judgment to resolve ambiguities. Baldwin , 589 U. S., at ___ (opinion of Thomas, J. ) (slip op., at 3); see also Michigan , 576 U. S., at 761 (opinion of Thomas, J. ); see also Perez , 575 U. S., at 123 (opinion of Thomas, J. ). By tying a judge’s hands, Chevron prevents the Judiciary from serving as a constitutional check on the Executive. It allows “the Executive . . . to dictate the outcome of cases through erroneous interpretations.” Baldwin , 589 U. S., at ___ (opinion of Thomas, J. ) (slip op., at 4); Michigan , 576 U. S., at 763, n. 1 (opinion of Thomas, J. ); see also Perez , 575 U. S., at 124 (opinion of Thomas, J. ). Because the judicial power requires judges to exercise their independent judgment, the deference that Chevron requires contravenes Article III’s mandate.
Chevron deference also permits the Executive Branch to exercise powers not given to it. “When the Government is called upon to perform a function that requires an exercise of legislative, executive, or judicial power, only the vested recipient of that power can perform it.” Department of Transportation v. Association of American Railroads , 575 U. S. 43 , 68 (2015) ( Thomas, J. , concurring in judgment). Because the Constitution gives the Executive Branch only “[t]he executive Power,” executive agencies may constitutionally exercise only that power. Art. II, §1, cl. 1. But, Chevron gives agencies license to exercise judicial power. By allowing agencies to definitively interpret laws so long as they are ambiguous, Chevron “transfer[s]” the Judiciary’s “interpretive judgment to the agency.” Perez , 575 U. S., at 124 (opinion of Thomas, J. ); see also Baldwin , 589 U. S., at ___ (opinion of Thomas, J. ) (slip op., at 4); Michigan , 576 U. S., at 761–762 (opinion of Thomas, J. ); post , at 18 ( Gorsuch, J. , concurring).
Chevron deference “cannot be salvaged” by recasting it as deference to an agency’s “formulation of policy.” Baldwin , 589 U. S., at ___ (opinion of Thomas, J. ) (internal quotation marks omitted) (slip op., at 3). If that were true, Chevron would mean that “agencies are unconstitutionally exercising ‘legislative Powers’ vested in Congress.” Baldwin , 589 U. S., at ___ (opinion of Thomas , J.) (slip op., at 3) (quoting Art. I, §1). By “giv[ing] the force of law to agency pronouncements on matters of private conduct as to which Congress did not actually have an intent,” Chevron “permit[s] a body other than Congress to perform a function that requires an exercise of legislative power.” Michigan , 576 U. S., at 762 (opinion of Thomas, J. ) (internal quotation marks omitted). No matter the gloss put on it, Chevron expands agencies’ power beyond the bounds of Article II by permitting them to exercise powers reserved to another branch of Government.
Chevron deference was “not a harmless transfer of power.” Baldwin , 589 U. S., at ___ (opinion of Thomas, J. ) (slip op., at 3). “The Constitution carefully imposes structural constraints on all three branches, and the exercise of power free of those accompanying restraints subverts the design of the Constitution’s ratifiers.” Ibid . In particular, the Founders envisioned that “the courts [would] check the Executive by applying the correct interpretation of the law.” Id ., at ___ (slip op., at 4). Chevron was thus a fundamental disruption of our separation of powers. It improperly strips courts of judicial power by simultaneously increasing the power of executive agencies. By overruling Chevron , we restore this aspect of our separation of powers. To safeguard individual liberty, “[s]tructure is everything.” A. Scalia, Foreword: The Importance of Structure in Constitutional Interpretation, 83 Notre Dame L. Rev. 1417, 1418 (2008). Although the Court finally ends our 40-year misadventure with Chevron deference, its more profound problems should not be overlooked. Regardless of what a statute says, the type of deference required by Chevron violates the Constitution.
1 *There is much to be commended in Justice Gorsuch ’s careful consideration from first principles of the weight we should afford to our precedent. I agree with the lion’s share of his concurrence. See generally Gamble v. United States , 587 U. S. 678 , 710 (2019) ( Thomas, J. , concurring).
Justice Gorsuch , concurring.
In disputes between individuals and the government about the meaning of a federal law, federal courts have traditionally sought to offer independent judgments about “what the law is” without favor to either side. Marbury v. Madison , 1 Cranch 137, 177 (1803). Beginning in the mid-1980s, however, this Court experimented with a radically different approach. Applying Chevron deference, judges began deferring to the views of executive agency officials about the meaning of federal statutes. See Chevron U. S. A. Inc. v. Natural Resources Defense Council, Inc. , 467 U. S. 837 (1984) . With time, the error of this approach became widely appreciated. So much so that this Court has refused to apply Chevron deference since 2016. Today, the Court places a tombstone on Chevron no one can miss. In doing so, the Court returns judges to interpretive rules that have guided federal courts since the Nation’s founding. I write separately to address why the proper application of the doctrine of stare decisis supports that course.
Today, the phrase “common law judge” may call to mind a judicial titan of the past who brilliantly devised new legal rules on his own. The phrase “ stare decisis ” might conjure up a sense that judges who come later in time are strictly bound to follow the work of their predecessors. But neither of those intuitions fairly describes the traditional common-law understanding of the judge’s role or the doctrine of stare decisis .
At common law, a judge’s charge to decide cases was not usually understood as a license to make new law. For much of England’s early history, different rulers and different legal systems prevailed in different regions. As England consolidated into a single kingdom governed by a single legal system, the judge’s task was to examine those pre-existing legal traditions and apply in the disputes that came to him those legal rules that were “common to the whole land and to all Englishmen.” F. Maitland, Equity, Also the Forms of Action at Common Law 2 (1929). That was “common law” judging.
This view of the judge’s role had consequences for the authority due judicial decisions. Because a judge’s job was to find and apply the law, not make it, the “opinion of the judge” and “the law” were not considered “one and the same thing.” 1 W. Blackstone, Commentaries on the Laws of England 71 (1765) (Blackstone) (emphasis deleted). A judge’s decision might bind the parties to the case at hand. M. Hale, The History and Analysis of the Common Law of England 68 (1713) (Hale). But none of that meant the judge had the power to “make a Law properly so called” for society at large, “for that only the King and Parliament can do.” Ibid.
Other consequences followed for the role precedent played in future judicial proceedings. Because past decisions represented something “less than a Law,” they did not bind future judges. Ibid. At the same time, as Matthew Hale put it, a future judge could give a past decision “Weight” as “Evidence” of the law. Ibid. Expressing the same idea, William Blackstone conceived of judicial precedents as “evidence” of “the common law.” 1 Blackstone 69, 71. And much like other forms of evidence, precedents at common law were thought to vary in the weight due them. Some past decisions might supply future courts with considerable guidance. But others might be entitled to lesser weight, not least because judges are no less prone to error than anyone else and they may sometimes “mistake” what the law demands. Id. , at 71 (emphasis deleted). In cases like that, both men thought, a future judge should not rotely repeat a past mistake but instead “vindicate” the law “from misrepresentation.” Id ., at 70 .
When examining past decisions as evidence of the law, common law judges did not, broadly speaking, afford overwhelming weight to any “single precedent.” J. Baker, An Introduction to English Legal History 209–210 (5th ed. 2019). Instead, a prior decision’s persuasive force depended in large measure on its “Consonancy and Congruity with Resolutions and Decisions of former Times.” Hale 68. An individual decision might reflect the views of one court at one moment in time, but a consistent line of decisions representing the wisdom of many minds across many generations was generally considered stronger evidence of the law’s meaning. Ibid.
With this conception of precedent in mind, Lord Mansfield cautioned against elevating “particular cases” above the “general principles” that “run through the cases, and govern the decision of them.” Rust v. Cooper , 2 Cowp. 629, 632, 98 Eng. Rep. 1277, 1279 (K. B. 1777). By discarding aberrational rulings and pursuing instead the mainstream of past decisions, he observed, the common law tended over time to “wor[k] itself pure.” Omychund v. Barker , 1 Atk. 22, 33, 26 Eng. Rep. 15, 23 (Ch. 1744) (emphasis deleted). Reflecting similar thinking, Edmund Burke offered five principles for the evaluation of past judicial decisions: “They ought to be shewn; first, to be numerous and not scattered here and there;—secondly, concurrent and not contradictory and mutually destructive;—thirdly, to be made in good and constitutional times;—fourthly, not to be made to serve an occasion;—and fifthly, to be agreeable to the general tenor of legal principles.” Speech of Dec. 23, 1790, in 3 The Speeches of the Right Honourable Edmund Burke 513 (1816).
Not only did different decisions carry different weight, so did different language within a decision. An opinion’s holding and the reasoning essential to it (the ratio decidendi ) merited careful attention. Dicta, stray remarks, and digressions warranted less weight. See N. Duxbury, The Intricacies of Dicta and Dissent 19–24 (2021) (Duxbury). These were no more than “the vapours and fumes of law.” F. Bacon, The Lord Keeper’s Speech in the Exchequer (1617), in 2 The Works of Francis Bacon 478 (B. Montagu ed. 1887) (Bacon).
That is not to say those “vapours” were worthless. Often dicta might provide the parties to a particular dispute a “fuller understanding of the court’s decisional path or related areas of concern.” B. Garner et al., The Law of Judicial Precedent 65 (2016) (Precedent). Dicta might also provide future courts with a source of “thoughtful advice.” Ibid. But future courts had to be careful not to treat every “hasty expression . . . as a serious and deliberate opinion.” Steel v. Houghton , 1 Bl. H. 51, 53, 126 Eng. Rep. 32, 33 (C. P. 1788). To do so would work an “injustice to [the] memory” of their predecessors who could not expect judicial remarks issued in one context to apply perfectly in others, perhaps especially ones they could not foresee. Ibid. Also, the limits of the adversarial process, a distinctive feature of English law, had to be borne in mind. When a single judge or a small panel reached a decision in a case, they did so based on the factual record and legal arguments the parties at hand have chosen to develop. Attuned to those constraints, future judges had to proceed with an open mind to the possibility that different facts and different legal arguments might dictate different outcomes in later disputes. See Duxbury 19–24.
Necessarily, this represents just a quick sketch of traditional common-law understandings of the judge’s role and the place of precedent in it. It focuses, too, on the horizontal, not vertical, force of judicial precedents. But there are good reasons to think that the common law’s understandings of judges and precedent outlined above crossed the Atlantic and informed the nature of the “judicial Power” the Constitution vests in federal courts. Art. III, §1.
Not only was the Constitution adopted against the backdrop of these understandings and, in light of that alone, they may provide evidence of what the framers meant when they spoke of the “judicial Power.” Many other, more specific provisions in the Constitution reflect much the same distinction between lawmaking and lawfinding functions the common law did. The Constitution provides that its terms may be amended only through certain prescribed democratic processes. Art. V. It vests the power to enact federal legislation exclusively in the people’s elected representatives in Congress. Art. I, §1. Meanwhile, the Constitution describes the judicial power as the power to resolve cases and controversies. Art. III, §2, cl. 1. As well, it delegates that authority to life-tenured judges, see §1, an assignment that would have made little sense if judges could usurp lawmaking powers vested in periodically elected representatives. But one that makes perfect sense if what is sought is a neutral party “to interpret and apply” the law without fear or favor in a dispute between others. 2 The Works of James Wilson 161 (J. Andrews ed. 1896) (Wilson); see Osborn v. Bank of United States , 9 Wheat. 738, 866 (1824).
The constrained view of the judicial power that runs through our Constitution carries with it familiar implications, ones the framers readily acknowledged. James Madison, for example, proclaimed that it would be a “fallacy” to suggest that judges or their precedents could “repeal or alter” the Constitution or the laws of the United States. Letter to N. Trist (Dec. 1831), in 9 The Writings of James Madison 477 (G. Hunt ed. 1910). A court’s opinion, James Wilson added, may be thought of as “effective la[w]” “[a]s to the parties.” Wilson 160–161. But as in England, Wilson said, a prior judicial decision could serve in a future dispute only as “evidence” of the law’s proper construction. Id ., at 160; accord, 1 J. Kent, Commentaries on American Law 442–443 (1826).
The framers also recognized that the judicial power described in our Constitution implies, as the judicial power did in England, a power (and duty) of discrimination when it comes to assessing the “evidence” embodied in past decisions. So, for example, Madison observed that judicial rulings “ repeatedly confirmed ” may supply better evidence of the law’s meaning than isolated or aberrant ones. Letter to C. Ingersoll (June 1831), in 4 Letters and Other Writings of James Madison 184 (1867) (emphasis added). Extending the thought, Thomas Jefferson believed it would often take “numerous decisions” for the meaning of new statutes to become truly “settled.” Letter to S. Jones (July 1809), in 12 The Writings of Thomas Jefferson 299 (A. Bergh ed. 1907).
From the start, too, American courts recognized that not everything found in a prior decision was entitled to equal weight. As Chief Justice Marshall warned, “It is a maxim not to be disregarded, that general expressions, in every opinion, are to be taken in connection with the case in which those expressions are used.” Cohens v. Virginia , 6 Wheat. 264, 399 (1821). To the extent a past court offered views “beyond the case,” those expressions “may be respected” in a later case “but ought not to control the judgment.” Ibid. One “obvious” reason for this, Marshall continued, had to do with the limits of the adversarial process we inherited from England: Only “[t]he question actually before the Court is investigated with care, and considered in its full extent. Other principles which may serve to illustrate it, are considered in their relation to the case decided, but their possible bearing on all other cases is seldom completely investigated.” Id. , at 399–400.
Abraham Lincoln championed these traditional understandings in his debates with Stephen Douglas. Douglas took the view that a single decision of this Court—no matter how flawed—could definitively resolve a contested issue for everyone and all time. Those who thought otherwise, he said, “aim[ed] a deadly blow to our whole Republican system of government.” Speech at Springfield, Ill. (June 26, 1857), in 2 The Collected Works of Abraham Lincoln 401 (R. Basler ed. 1953) (Lincoln Speech). But Lincoln knew better. While accepting that judicial decisions “absolutely determine” the rights of the parties to a court’s judgment, he refused to accept that any single judicial decision could “fully settl[e]” an issue, particularly when that decision departs from the Constitution. Id ., at 400–401. In cases such as these, Lincoln explained, “it is not resistance, it is not factious, it is not even disrespectful, to treat [the decision] as not having yet quite established a settled doctrine for the country.” Id. , at 401.
After the Civil War, the Court echoed some of these same points. It stressed that every statement in a judicial opin ion “must be taken in connection with its immediate context,” In re Ayers , 123 U. S. 443 , 488 (1887), and stray “remarks” must not be elevated above the written law, see The Belfast , 7 Wall. 624, 641 (1869); see also, e . g ., Trebilcock v. Wilson , 12 Wall. 687, 692–693 (1872); Mason v. Eldred , 6 Wall. 231, 236–238 (1868). During Chief Justice Chase’s tenure, it seems a Justice writing the Court’s majority opinion would generally work alone and present his work orally and in summary form to his colleagues at conference, which meant that other Justices often did not even review the opinion prior to publication. 6 C. Fairman, History of the Supreme Court of the United States 69–70 (1971). The Court could proceed in this way because it understood that a single judicial opinion may resolve a “case or controversy,” and in so doing it may make “effective law” for the parties, but it does not legislate for the whole of the country and is not to be confused with laws that do.
From all this, I see at least three lessons about the doctrine of stare decisis relevant to the decision before us today. Each concerns a form of judicial humility.
First , a past decision may bind the parties to a dispute, but it provides this Court no authority in future cases to depart from what the Constitution or laws of the United States ordain. Instead, the Constitution promises, the American people are sovereign and they alone may, through democratically responsive processes, amend our foundational charter or revise federal legislation. Unelected judges enjoy no such power. Part I–B, supra .
Recognizing as much, this Court has often said that stare decisis is not an “ ‘inexorable command.’ ” State Oil Co. v. Khan , 522 U. S. 3 , 20 (1997). And from time to time it has found it necessary to correct its past mistakes. When it comes to correcting errors of constitutional interpretation, the Court has stressed the importance of doing so, for they can be corrected otherwise only through the amendment process. See, e.g. , Franchise Tax Bd. of Cal. v. Hyatt , 587 U. S. 230 , 248 (2019). When it comes to fixing errors of statutory interpretation, the Court has proceeded perhaps more circumspectly. But in that field, too, it has overruled even longstanding but “flawed” decisions. See, e.g. , Leegin Creative Leather Products, Inc. v. PSKS, Inc. , 551 U. S. 877 , 904, 907 (2007).
Recent history illustrates all this. During the tenures of Chief Justices Warren and Burger, it seems this Court overruled an average of around three cases per Term, including roughly 50 statutory precedents between the 1960s and 1980s alone. See W. Eskridge, Overruling Statutory Precedents, 76 Geo. L. J. 1361, 1427–1434 (1988) (collecting cases). Many of these decisions came in settings no less consequential than today’s. In recent years, we have not approached the pace set by our predecessors, overruling an average of just one or two prior decisions each Term. 1 But the point remains: Judicial decisions inconsistent with the written law do not inexorably control.
Second , another lesson tempers the first. While judicial decisions may not supersede or revise the Constitution or federal statutory law, they merit our “respect as embodying the considered views of those who have come before.” Ramos v. Louisiana , 590 U. S. 83 , 105 (2020). As a matter of professional responsibility, a judge must not only avoid confusing his writings with the law. When a case comes before him, he must also weigh his view of what the law demands against the thoughtful views of his predecessors. After all, “[p]recedent is a way of accumulating and passing down the learning of past generations, a font of established wisdom richer than what can be found in any single judge or panel of judges.” Precedent 9.
Doubtless, past judicial decisions may, as they always have, command “greater or less authority as precedents, according to circumstances.” Lincoln Speech 401. But, like English judges before us, we have long turned to familiar considerations to guide our assessment of the weight due a past decision. So, for example, as this Court has put it, the weight due a precedent may depend on the quality of its reasoning, its consistency with related decisions, its workability, and reliance interests that have formed around it. See Ramos , 590 U. S., at 106. The first factor recognizes that the primary power of any precedent lies in its power to persuade—and poorly reasoned decisions may not provide reliable evidence of the law’s meaning. The second factor reflects the fact that a precedent is more likely to be correct and worthy of respect when it reflects the time-tested wisdom of generations than when it sits “unmoored” from surrounding law. Ibid. The remaining factors, like workability and reliance, do not often supply reason enough on their own to abide a flawed decision, for almost any past decision is likely to benefit some group eager to keep things as they are and content with how things work. See, e.g. , id ., at 108. But these factors can sometimes serve functions similar to the others, by pointing to clues that may suggest a past decision is right in ways not immediately obvious to the individual judge.
When asking whether to follow or depart from a precedent, some judges deploy adverbs. They speak of whether or not a precedent qualifies as “demonstrably erroneous,” Gamble v. United States , 587 U. S. 678 , 711 (2019) ( Thomas, J. , concurring), or “egregiously wrong,” Ramos , 590 U. S., at 121 ( Kavanaugh, J. , concurring in part). But the emphasis the adverb imparts is not meant for dramatic effect. It seeks to serve instead as a reminder of a more substantive lesson. The lesson that, in assessing the weight due a past decision, a judge is not to be guided by his own impression alone, but must self-consciously test his views against those who have come before, open to the possibility that a precedent might be correct in ways not initially apparent to him.
Third , it would be a mistake to read judicial opinions like statutes. Adopted through a robust and democratic process, statutes often apply in all their particulars to all persons. By contrast, when judges reach a decision in our adversarial system, they render a judgment based only on the factual record and legal arguments the parties at hand have chosen to develop. A later court assessing a past decision must therefore appreciate the possibility that different facts and different legal arguments may dictate a different outcome. They must appreciate, too, that, like anyone else, judges are “innately digressive,” and their opinions may sometimes offer stray asides about a wider topic that may sound nearly like legislative commands. Duxbury 4. Often, enterprising counsel seek to exploit such statements to maximum effect. See id ., at 25. But while these digressions may sometimes contain valuable counsel, they remain “vapours and fumes of law,” Bacon 478, and cannot “control the judgment in a subsequent suit,” Cohens , 6 Wheat., at 399.
These principles, too, have long guided this Court and others. As Judge Easterbrook has put it, an “opinion is not a comprehensive code; it is just an explanation for the Court’s disposition. Judicial opinions must not be confused with statutes, and general expressions must be read in light of the subject under consideration.” United States v. Skoien , 614 F. 3d 638 , 640 (CA7 2010) (en banc); see also Reiter v. Sonotone Corp. , 442 U. S. 330 , 341 (1979) (stressing that an opinion is not “a statute,” and its language should not “be parsed” as if it were); Nevada v. Hicks , 533 U. S. 353 , 372 (2001) (same). If stare decisis counsels respect for the thinking of those who have come before, it also counsels against doing an “injustice to [their] memory” by overreliance on their every word. Steel , 1 Bl. H., at 53, 126 Eng. Rep., at 33. As judges, “[w]e neither expect nor hope that our successors will comb” through our opinions, searching for delphic answers to matters we never fully explored. Brown v. Davenport , 596 U. S. 118 , 141 (2022). To proceed otherwise risks “turn[ing] stare decisis from a tool of judicial humility into one of judicial hubris.” Ibid.
Turning now directly to the question what stare decisis effect Chevron deference warrants, each of these lessons seem to me to weigh firmly in favor of the course the Court charts today: Lesson 1, because Chevron deference contravenes the law Congress prescribed in the Administrative Procedure Act. Lesson 2, because Chevron deference runs against mainstream currents in our law regarding the separation of powers, due process, and centuries-old interpretive rules that fortify those constitutional commitments. And Lesson 3, because to hold otherwise would effectively require us to endow stray statements in Chevron with the authority of statutory language, all while ignoring more considered language in that same decision and the teachings of experience.
Start with Lesson 1. The Administrative Procedure Act of 1946 (APA) directs a “reviewing court” to “decide all relevant questions of law” and “interpret” relevant “constitutional and statutory provisions.” 5 U. S. C. §706 . When applying Chevron deference, reviewing courts do not interpret all relevant statutory provisions and decide all relevant questions of law. Instead, judges abdicate a large measure of that responsibility in favor of agency officials. Their interpretations of “ambiguous” laws control even when those interpretations are at odds with the fairest reading of the law an independent “reviewing court” can muster. Agency officials, too, may change their minds about the law’s meaning at any time, even when Congress has not amended the relevant statutory language in any way. National Cable & Telecommunications Assn. v. Brand X Internet Services , 545 U. S. 967 , 982–983 (2005). And those officials may even disagree with and effectively overrule not only their own past interpretations of a law but a court’s past interpretation as well. Ibid. None of that is consistent with the APA’s clear mandate.
The hard fact is Chevron “did not even bother to cite” the APA, let alone seek to apply its terms. United States v. Mead Corp. , 533 U. S. 218 , 241 (2001) (Scalia, J., dissenting). Instead, as even its most ardent defenders have conceded, Chevron deference rests upon a “ fictionalized statement of legislative desire,” namely, a judicial supposition that Congress implicitly wishes judges to defer to executive agencies’ interpretations of the law even when it has said nothing of the kind. D. Barron & E. Kagan, Chevron’s Nondelegation Doctrine, 2001 S. Ct. Rev. 201, 212 (Kagan) (emphasis added). As proponents see it, that fiction represents a “policy judgmen[t] about what . . . make[s] for good government.” Ibid. 2 But in our democracy unelected judges possess no authority to elevate their own fictions over the laws adopted by the Nation’s elected representatives. Some might think the legal directive Congress provided in the APA unwise; some might think a different arrangement preferable. See, e.g. , post , at 9–11 ( Kagan, J. , dissenting). But it is Congress’s view of “good government,” not ours, that controls.
Much more could be said about Chevron ’s inconsistency with the APA. But I have said it in the past. See Buffington v. McDonough , 598 U. S. ___, ___–___ (2022) (opinion dissenting from denial of certiorari) (slip op., at 5–6); Gutierrez-Brizuela v. Lynch , 834 F. 3d 1142 , 1151–1153 (CA10 2016) (concurring opinion). And the Court makes many of the same points at length today. See ante , at 18–22. For present purposes, the short of it is that continuing to abide Chevron deference would require us to transgress the first lesson of stare decisis —the humility required of judges to recognize that our decisions must yield to the laws adopted by the people’s elected representatives. 3
Lesson 2 cannot rescue Chevron deference. If stare decisis calls for judicial humility in the face of the written law, it also cautions us to test our present conclusions carefully against the work of our predecessors. At the same time and as we have seen, this second form of humility counsels us to remember that precedents that have won the endorsement of judges across many generations, demonstrated coherence with our broader law, and weathered the tests of time and experience are entitled to greater consideration than those that have not. See Part I, supra . Viewed by each of these lights, the case for Chevron deference only grows weaker still.
Start with a look to how our predecessors traditionally understood the judicial role in disputes over a law’s meaning. From the Nation’s founding, they considered “[t]he interpretation of the laws” in cases and controversies “the proper and peculiar province of the courts.” The Federalist No. 78, p. 467 (C. Rossiter ed. 1961) (A. Hamilton). Perhaps the Court’s most famous early decision reflected exactly that view. There, Chief Justice Marshall declared it “emphatically the province and duty of the judicial department to say what the law is.” Marbury , 1 Cranch, at 177. For judges “have neither FORCE nor WILL but merely judgment”—and an obligation to exercise that judgment independently. The Federalist No. 78, at 465. No matter how “disagreeable that duty may be,” this Court has said, a judge “is not at liberty to surrender, or to waive it.” United States v. Dickson , 15 Pet. 141, 162 (1841) (Story, J.). This duty of independent judgment is perhaps “the defining characteristi[c] of Article III judges.” Stern v. Marshall , 564 U. S. 462 , 483 (2011).
To be sure, this Court has also long extended “great respect” to the “contemporaneous” and consistent views of the coordinate branches about the meaning of a statute’s terms. Edwards’ Lessee v. Darby , 12 Wheat. 206, 210 (1827); see also McCulloch v. Maryland , 4 Wheat. 316, 401 (1819); Stuart v. Laird , 1 Cranch 299, 309 (1803). 4 But traditionally, that did not mean a court had to “defer” to any “reasonable” construction of an “ambiguous” law that an executive agency might offer. It did not mean that the government could propound a “reasonable” view of the law’s meaning one day, a different one the next, and bind the judiciary always to its latest word. Nor did it mean the executive could displace a pre-existing judicial construction of a statute’s terms, replace it with its own, and effectively overrule a judicial precedent in the process. Put simply, this Court was “not bound” by any and all reasonable “administrative construction[s]” of ambiguous statutes when resolving cases and controversies. Burnet v. Chicago Portrait Co. , 285 U. S. 1 , 16 (1932). While the executive’s consistent and contemporaneous views warranted respect, they “by no means control[led] the action or the opinion of this court in expounding the law with reference to the rights of parties litigant before them.” Irvine v. Marshall , 20 How. 558, 567 (1858); see also A. Bamzai, The Origins of Judicial Deference to Executive Interpretation, 126 Yale L. J. 908, 987 (2017).
Sensing how jarringly inconsistent Chevron is with this Court’s many longstanding precedents discussing the nature of the judicial role in disputes over the law’s meaning, the government and dissent struggle for a response. The best they can muster is a handful of cases from the early 1940s in which, they say, this Court first “put [deference] principles into action.” Post , at 21 ( Kagan, J. , dissenting). And, admittedly, for a period this Court toyed with a form of deference akin to Chevron , at least for so-called mixed questions of law and fact. See, e.g. , Gray v. Powell , 314 U. S. 402 , 411–412 (1941); NLRB v. Hearst Publications, Inc. , 322 U. S. 111 , 131 (1944). But, as the Court details, even that limited experiment did not last. See ante , at 10–12 . Justice Roberts, in his Gray dissent, decried these decisions for “abdicat[ing our] function as a court of review” and “complete[ly] revers[ing] . . . the normal and usual method of construing a statute.” 314 U. S., at 420–421. And just a few years later, in Skidmore v. Swift & Co. , 323 U. S. 134 (1944), the Court returned to its time-worn path.
Echoing themes that had run throughout our law from its start, Justice Robert H. Jackson wrote for the Court in Skidmore . There, he said, courts may extend respectful consideration to another branch’s interpretation of the law, but the weight due those interpretations must always “depend upon the[ir] thoroughness . . . , the validity of [their] reasoning, [their] consistency with earlier and later pronouncements, and all those factors which give [them] power to persuade.” Id. , at 140. In another case the same year, and again writing for the Court, Justice Jackson expressly rejected a call for a judge-made doctrine of deference much like Chevron , offering that, “[i]f Congress had deemed it necessary or even appropriate” for courts to “defe[r] to administrative construction[,] . . . it would not have been at a loss for words to say so.” Davies Warehouse Co. v. Bowles , 321 U. S. 144 , 156 (1944).
To the extent proper respect for precedent demands, as it always has, special respect for longstanding and mainstream decisions, Chevron scores badly. It represented not a continuation of a long line of decisions but a break from them. Worse, it did not merely depart from our precedents. More nearly, Chevron defied them.
Consider next how uneasily Chevron deference sits alongside so many other settled aspects of our law. Having witnessed first-hand King George’s efforts to gain influence and control over colonial judges, see Declaration of Independence ¶ 11, the framers made a considered judgment to build judicial independence into the Constitution’s design. They vested the judicial power in decisionmakers with life tenure. Art. III, §1. They placed the judicial salary beyond political control during a judge’s tenure. Ibid. And they rejected any proposal that would subject judicial decisions to review by political actors. The Federalist No. 81, at 482; United States v. Hansen , 599 U. S. 762 , 786–791 (2023) ( Thomas, J. , concurring). All of this served to ensure the same thing: “A fair trial in a fair tribunal.” In re Murchison , 349 U. S. 133 , 136 (1955). One in which impartial judges, not those currently wielding power in the political branches, would “say what the law is” in cases coming to court. Marbury , 1 Cranch, at 177.
Chevron deference undermines all that. It precludes courts from exercising the judicial power vested in them by Article III to say what the law is. It forces judges to abandon the best reading of the law in favor of views of those presently holding the reins of the Executive Branch. It requires judges to change, and change again, their interpretations of the law as and when the government demands. And that transfer of power has exactly the sort of consequences one might expect. Rather than insulate adjudication from power and politics to ensure a fair hearing “without respect to persons” as the federal judicial oath demands, 28 U. S. C. §453 , Chevron deference requires courts to “place a finger on the scales of justice in favor of the most powerful of litigants, the federal government.” Buffington , 598 U. S., at ___ (slip op., at 9). Along the way, Chevron deference guarantees “systematic bias” in favor of whichever political party currently holds the levers of executive power. P. Hamburger, Chevron Bias, 84 Geo. Wash. L. Rev. 1187, 1212 (2016).
Chevron deference undermines other aspects of our settled law, too. In this country, we often boast that the Constitution’s promise of due process of law, see Amdts. 5, 14, means that “ ‘no man can be a judge in his own case.’ ” Williams v. Pennsylvania , 579 U. S. 1 , 8–9 (2016); Calder v. Bull , 3 Dall. 386, 388 (1798) (opinion of Chase, J.). That principle, of course, has even deeper roots, tracing far back into the common law where it was known by the Latin maxim nemo iudex in causa sua . See 1 E. Coke, Institutes of the Laws of England §212, *141a. Yet, under the Chevron regime, all that means little, for executive agencies may effectively judge the scope of their own lawful powers. See, e.g., Arlington v. FCC , 569 U. S. 290 , 296–297 (2013).
Traditionally, as well, courts have sought to construe statutes as a reasonable reader would “when the law was made.” Blackstone 59; see United States v. Fisher , 2 Cranch 358, 386 (1805). Today, some call this “textualism.” But really it’s a very old idea, one that constrains judges to a lawfinding rather than lawmaking role by focusing their work on the statutory text, its linguistic context, and various canons of construction. In that way, textualism serves as an essential guardian of the due process promise of fair notice. If a judge could discard an old meaning and assign a new one to a law’s terms, all without any legislative revision, how could people ever be sure of the rules that bind them? New Prime Inc. v. Oliveira , 586 U. S. 105 , 113 (2019). Were the rules otherwise, Blackstone warned, the people would be rendered “slaves to their magistrates.” 4 Blackstone 371.
Yet, replace “magistrates” with “bureaucrats,” and Blackstone’s fear becomes reality when courts employ Chevron deference. Whenever we confront an ambiguity in the law, judges do not seek to resolve it impartially according to the best evidence of the law’s original meaning. Instead, we resort to a far cruder heuristic: “The reasonable bureaucrat always wins.” And because the reasonable bureaucrat may change his mind year-to-year and election-to-election, the people can never know with certainty what new “interpretations” might be used against them. This “fluid” approach to statutory interpretation is “as much a trap for the innocent as the ancient laws of Caligula,” which were posted so high up on the walls and in print so small that ordinary people could never be sure what they required. United States v. Cardiff , 344 U. S. 174 , 176 (1952).
The ancient rule of lenity is still another of Chevron ’s victims. Since the founding, American courts have construed ambiguities in penal laws against the government and with lenity toward affected persons. Wooden v. United States , 595 U. S. 360 , 388–390 (2022) ( Gorsuch, J. , concurring in judgment). That principle upholds due process by safeguarding individual liberty in the face of ambiguous laws. Ibid. And it fortifies the separation of powers by keeping the power of punishment firmly “ ‘in the legislative, not in the judicial department.’ ” Id. , at 391 (quoting United States v. Wiltberger , 5 Wheat. 76, 95 (1820)). But power begets power. And pressing Chevron deference as far as it can go, the government has sometimes managed to leverage “ambiguities” in the written law to penalize conduct Congress never clearly proscribed. Compare Guedes v. ATF , 920 F. 3d 1 , 27–28, 31 (CADC 2019), with Garland v. Cargill , 602 U. S. 604 (2024) .
In all these ways, Chevron ’s fiction has led us to a strange place. One where authorities long thought reserved for Article III are transferred to Article II, where the scales of justice are tilted systematically in favor of the most powerful, where legal demands can change with every election even though the laws do not, and where the people are left to guess about their legal rights and responsibilities. So much tension with so many foundational features of our legal order is surely one more sign that we have “taken a wrong turn along the way.” Kisor v. Wilkie , 588 U. S. 558 , 607 (2019) ( Gorsuch, J., concurring in judgment). 5
Finally, consider workability and reliance. If, as I have sought to suggest, these factors may sometimes serve as useful proxies for the question whether a precedent comports with the historic tide of judicial practice or represents an aberrational mistake, see Part I–C, supra , they certainly do here.
Take Chevron ’s “workability.” Throughout its short life, this Court has been forced to supplement and revise Chevron so many times that no one can agree on how many “steps” it requires, nor even what each of those “steps” entails. Some suggest that the analysis begins with “step zero” (perhaps itself a tell), an innovation that traces to United States v. Mead Corp. , 533 U. S. 218 . Mead held that, before even considering whether Chevron applies, a court must determine whether Congress meant to delegate to the agency authority to interpret the law in a given field. 533 U. S., at 226–227. But that exercise faces an immediate challenge: Because Chevron depends on a judicially implied, rather than a legislatively expressed, delegation of interpretive authority to an executive agency, Part II–A, supra , when should the fiction apply and when not? Mead fashioned a multifactor test for judges to use. 533 U. S., at 229–231. But that test has proved as indeterminate in application as it was contrived in origin. Perhaps for these reasons, perhaps for others, this Court has sometimes applied Mead and often ignored it. See Brand X , 545 U. S., at 1014, n. 8 (Scalia, J., dissenting).
Things do not improve as we move up the Chevron ladder. At “step one,” a judge must defer to an executive official’s interpretation when the statute at hand is “ambiguous.” But even today, Chevron ’s principal beneficiary—the federal government—still cannot say when a statute is sufficiently ambiguous to trigger deference. See, e.g. , Tr. of Oral Arg. in American Hospital Assn. v. Becerra , O. T. 2021, No. 20–1114, pp. 71–72. Perhaps thanks to this particular confusion, the search for ambiguity has devolved into a sort of Snark hunt: Some judges claim to spot it almost everywhere, while other equally fine judges claim never to have seen it. Compare L. Silberman, Chevron —The Intersection of Law & Policy, 58 Geo. Wash. L. Rev. 821, 826 (1990), with R. Kethledge, Ambiguities and Agency Cases: Reflections After (Almost) Ten Years on the Bench, 70 Vand. L. Rev. En Banc 315, 323 (2017).
Nor do courts agree when it comes to “step two.” There, a judge must assess whether an executive agency’s interpretation of an ambiguous statute is “reasonable.” But what does that inquiry demand? Some courts engage in a comparatively searching review; others almost reflexively defer to an agency’s views. Here again, courts have pursued “wildly different” approaches and reached wildly different conclusions in similar cases. See B. Kavanaugh, Fixing Statutory Interpretation, 129 Harv. L. Rev. 2118, 2152 (2016) (Kavanaugh).
Today’s cases exemplify some of these problems. We have before us two circuit decisions, three opinions, and at least as many interpretive options on the Chevron menu. On the one hand, we have the D. C. Circuit majority, which deemed the Magnuson-Stevens Act “ambiguous” and upheld the agency’s regulation as “ ‘permissible.’ ” 45 F. 4th 359, 365 (2022). On the other hand, we have the D. C. Circuit dissent, which argues the statute is “unambiguou[s]” and that it plainly forecloses the agency’s new rule. Id. , at 372 (opinion of Walker, J.). And on yet a third hand, we have the First Circuit, which claimed to have identified “clear textual support” for the regulation, yet refused to say whether it would “classify [its] conclusion as a product of Chevron step one or step two.” 62 F. 4th 621, 631, 634 (2023). As these cases illustrate, Chevron has turned statutory interpretation into a game of bingo under blindfold, with parties guessing at how many boxes there are and which one their case might ultimately fall in.
Turn now from workability to reliance. Far from engendering reliance interests, the whole point of Chevron deference is to upset them. Under Chevron , executive officials can replace one “reasonable” interpretation with another at any time, all without any change in the law itself. The result: Affected individuals “can never be sure of their legal rights and duties.” Buffington , 598 U. S., at ___ (slip op., at 12).
How bad is the problem? Take just one example. Brand X concerned a law regulating broadband internet services. There, the Court upheld an agency rule adopted by the administration of President George W. Bush because it was premised on a “reasonable” interpretation of the statute. Later, President Barack Obama’s administration rescinded the rule and replaced it with another. Later still, during President Donald J. Trump’s administration, officials replaced that rule with a different one, all before President Joseph R. Biden, Jr.’s administration declared its intention to reverse course for yet a fourth time. See Safeguarding and Securing the Open Internet, 88 Fed. Reg. 76048 (2023); Brand X , 545 U. S., at 981–982. Each time, the government claimed its new rule was just as “reasonable” as the last. Rather than promoting reliance by fixing the meaning of the law, Chevron deference engenders constant uncertainty and convulsive change even when the statute at issue itself remains unchanged.
Nor are these antireliance harms distributed equally. Sophisticated entities and their lawyers may be able to keep pace with rule changes affecting their rights and responsibilities. They may be able to lobby for new “ ‘reasonable’ ” agency interpretations and even capture the agencies that issue them. Buffington , 598 U. S., at ___, ___ (slip op., at 8, 13). But ordinary people can do none of those things. They are the ones who suffer the worst kind of regulatory whiplash Chevron invites.
Consider a couple of examples. Thomas Buffington, a veteran of the U. S. Air Force, was injured in the line of duty. For a time after he left the Air Force, the Department of Veterans Affairs (VA) paid disability benefits due him by law. But later the government called on Mr. Buffington to reenter active service. During that period, everyone agreed, the VA could (as it did) suspend his disability payments. After he left active service for a second time, however, the VA turned his patriotism against him. By law, Congress permitted the VA to suspend disability pay only “for any period for which [a servicemember] receives active service pay.” 38 U. S. C. §5304(c) . But the VA had adopted a self-serving regulation requiring veterans to file a form asking for the resumption of their disability pay after a second (or subsequent) stint in active service. 38 CFR §3.654(b)(2) (2021). Unaware of the regulation, Mr. Buffington failed to reapply immediately. When he finally figured out what had happened and reapplied, the VA agreed to resume payments going forward but refused to give Mr. Buffington all of the past disability payments it had withheld. Buffington , 598 U. S., at ___–___ (slip op., at 1–4).
Mr. Buffington challenged the agency’s action as inconsistent with Congress’s direction that the VA may suspend disability payments only for those periods when a veteran returns to active service. But armed with Chevron , the agency defeated Mr. Buffington’s claim. Maybe the self-serving regulation the VA cited as justification for its action was not premised on the best reading of the law, courts said, but it represented a “ ‘permissible’ ” one. 598 U. S., at ___ (slip op., at 7). In that way, the Executive Branch was able to evade Congress’s promises to someone who took the field repeatedly in the Nation’s defense.
In another case, one which I heard as a court of appeals judge, De Niz Robles v. Lynch , 803 F. 3d 1165 (CA10 2015), the Board of Immigration Appeals invoked Chevron to overrule a judicial precedent on which many immigrants had relied, see In re Briones , 24 I. & N. Dec. 355, 370 (BIA 2007) (purporting to overrule Padilla–Caldera v. Gonzales , 426 F. 3d 1294 (CA10 2005)). The agency then sought to apply its new interpretation retroactively to punish those immigrants—including Alfonzo De Niz Robles, who had relied on that judicial precedent as authority to remain in this country with his U. S. wife and four children. See 803 F. 3d, at 1168 –1169. Our court ruled that this retrospective application of the BIA’s new interpretation of the law violated Mr. De Niz Robles’s due process rights. Id. , at 1172. But as a lower court, we could treat only the symptom, not the disease. So Chevron permitted the agency going forward to overrule a judicial decision about the best reading of the law with its own different “reasonable” one and in that way deny relief to countless future immigrants.
Those are just two stories among so many that federal judges could tell (and have told) about what Chevron deference has meant for ordinary people interacting with the federal government. See, e.g. , Lambert v. Saul , 980 F. 3d 1266 , 1268–1276 (CA9 2020); Valent v. Commissioner of Social Security , 918 F. 3d 516 , 525–527 (CA6 2019) (Kethledge, J., dissenting); Gonzalez v. United States Atty. Gen. , 820 F. 3d 399 , 402–405 (CA11 2016) ( per curiam ).
What does the federal government have to say about this? It acknowledges that Chevron sits as a heavy weight on the scale in favor of the government, “oppositional” to many “categories of individuals.” Tr. of Oral Arg. in No. 22–1219, p. 133 (Relentless Tr.). But, according to the government, Chevron deference is too important an innovation to undo. In its brief reign, the government says, it has become a “fundamenta[l] . . . ground rul[e] for how all three branches of the government are operating together.” Relentless Tr. 102. But, in truth, the Constitution, the APA, and our longstanding precedents set those ground rules some time ago. And under them, agencies cannot invoke a judge-made fiction to unsettle our Nation’s promise to individuals that they are entitled to make their arguments about the law’s demands on them in a fair hearing, one in which they stand on equal footing with the government before an independent judge.
How could a Court, guided for 200 years by Chief Justice Marshall’s example, come to embrace a counter- Marbury revolution, one at war with the APA, time honored precedents, and so much surrounding law? To answer these questions, turn to Lesson 3 and witness the temptation to endow a stray passage in a judicial decision with extraordinary authority. Call it “power quoting.”
Chevron was an unlikely place for a revolution to begin. The case concerned the Clean Air Act’s requirement that States regulate “stationary sources” of air pollution in their borders. See 42 U. S. C. §7401 et seq . At the time, it was an open question whether entire industrial plants or their constituent polluting parts counted as “stationary sources.” The Environmental Protection Agency had defined entire plants as sources, an approach that allowed companies to replace individual plant parts without automatically triggering the permitting requirements that apply to new sources. Chevron , 467 U. S., at 840.
This Court upheld the EPA’s definition as consistent with the governing statute. Id ., at 866. The decision, issued by a bare quorum of the Court, without concurrence or dissent, purported to apply “well-settled principles.” Id ., at 845. “If a court, employing traditional tools of statutory construction, ascertains that Congress had an intention on the precise question at issue,” Chevron provided, then “that intention is the law and must be given effect.” Id ., at 843, n. 9. Many of the cases Chevron cited to support its judgment stood for the traditional proposition that courts afford respectful consideration, not deference, to executive interpretations of the law. See, e.g. , Burnet , 285 U. S., at 16; United States v. Moore , 95 U. S. 760 , 763 (1878). And the decision’s sole citation to legal scholarship was to Roscoe Pound, who long championed de novo judicial review. 467 U. S., at 843, n. 10; see R. Pound, The Place of the Judiciary in a Democratic Polity, 27 A. B. A. J. 133, 136–137 (1941).
At the same time, of course, the opinion contained bits and pieces that spoke differently. The decision also said that, “if [a] statute is silent or ambiguous with respect to [a] specific issue, the question for the court is whether the agency’s answer is based on a permissible construction of the statute.” 467 U. S., at 843. But it seems the government didn’t advance this formulation in its brief, so there was no adversarial engagement on it. T. Merrill, The Story of Chevron : The Making of an Accidental Landmark, 66 Admin. L. Rev. 253, 268 (2014) (Merrill). As we have seen, too, the Court did not pause to consider (or even mention) the APA. See Part II–A, supra. It did not discuss contrary precedents issued by the Court since the founding, let alone purport to overrule any of them. See Part II–B–1, supra. Nor did the Court seek to address how its novel rule of deference might be squared with so much surrounding law. See Part II–B–2, supra . As even its defenders have acknowledged, “ Chevron barely bothered to justify its rule of deference, and the few brief passages on this matter pointed in disparate directions.” Kagan 212–213. “[T]he quality of the reasoning,” they acknowledge, “was not high,” C. Sunstein, Chevron as Law, 107 Geo. L. J. 1613, 1669 (2019).
If Chevron meant to usher in a revolution in how judges interpret laws, no one appears to have realized it at the time. Chevron ’s author, Justice Stevens, characterized the decision as a “simpl[e] . . . restatement of existing law, nothing more or less.” Merrill 255, 275. In the “19 argued cases” in the following Term “that presented some kind of question about whether the Court should defer to an agency interpretation of statutory law,” this Court cited Chevron just once. Merrill 276. By some accounts, the decision seemed “destined to obscurity.” Ibid .
It was only three years later when Justice Scalia wrote a concurrence that a revolution began to take shape. Buffington , 598 U. S., at ___ (slip op., at 8). There, he argued for a new rule requiring courts to defer to executive agency interpretations of the law whenever a “ ‘statute is silent or ambiguous.’ ” NLRB v. Food & Commercial Workers , 484 U. S. 112 , 133–134 (1987) (opinion of Scalia, J.). Eventually, a majority of the Court followed his lead. Buffington , 598 U. S., at ___ (slip op., at 8). But from the start, Justice Scalia made no secret about the scope of his ambitions. See Judicial Deference to Administrative Interpretations of Law, 1989 Duke L. J. 511, 521 (1989) (Scalia). The rule he advocated for represented such a sharp break from prior practice, he explained, that many judges of his day didn’t yet “understand” the “old criteria” were “no longer relevant.” Ibid . Still, he said, overthrowing the past was worth it because a new deferential rule would be “easier to follow.” Ibid.
Events proved otherwise. As the years wore on and the Court’s new and aggressive reading of Chevron gradually exposed itself as unworkable, unfair, and at odds with our separation of powers, Justice Scalia could have doubled down on the project. But he didn’t. He appreciated that stare decisis is not a rule of “if I thought it yesterday, I must think it tomorrow.” And rather than cling to the pride of personal precedent, the Justice began to express doubts over the very project that he had worked to build. See Perez v. Mortgage Bankers Assn. , 575 U. S. 92 , 109–110 (2015) (opinion concurring in judgment); cf. Decker v. Northwest Environmental Defense Center , 568 U. S. 597 , 617–618, 621 (2013) (opinion concurring in part and dissenting in part). If Chevron ’s ascent is a testament to the Justice’s ingenuity, its demise is an even greater tribute to his humility. 6
Justice Scalia was not alone in his reconsideration. After years spent laboring under Chevron , trying to make sense of it and make it work, Member after Member of this Court came to question the project. See, e.g. , Pereira v. Sessions , 585 U. S. 198 , 219–221 (2018) (Kennedy, J., concurring); Michigan v. EPA , 576 U. S. 743 , 760–764 (2015) ( Thomas , J., concurring); Kisor , 588 U. S., at 591 ( Roberts , C. J., concurring in part); Gutierrez-Brizuela , 834 F. 3d, at 1153 ; Buffington , 598 U. S., at ___–___ (slip op., at 14–15); Kavanaugh 2150–2154. Ultimately, the Court gave up. Despite repeated invitations, it has not applied Chevron deference since 2016. Relentless Tr. 81; App. to Brief for Respondents in No. 22–1219, p. 68a. So an experiment that began only in the mid-1980s effectively ended eight years ago. Along the way, an unusually large number of federal appellate judges voiced their own thoughtful and extensive criticisms of Chevron . Buffington , 598 U. S., at ___–___ (slip op., at 14–15) (collecting examples). A number of state courts did, too, refusing to import Chevron deference into their own administrative law jurisprudence. See 598 U. S., at ___ (slip op., at 15).
Even if all that and everything else laid out above is true, the government suggests we should retain Chevron deference because judges simply cannot live without it; some statutes are just too “technical” for courts to interpret “intelligently.” Post , at 9, 32 (dissenting opinion). But that objection is no answer to Chevron ’s inconsistency with Congress’s directions in the APA, so much surrounding law, or the challenges its multistep regime have posed in practice. Nor does history counsel such defeatism. Surely, it would be a mistake to suggest our predecessors before Chevron ’s rise in the mid-1980s were unable to make their way intelligently through technical statutory disputes. Following their lead, over the past eight years this Court has managed to resolve even highly complex cases without Chevron deference, and done so even when the government sought deference. Nor, as far as I am aware, did any Member of the Court suggest Chevron deference was necessary to an intelligent resolution of any of those matters. 7 If anything, by affording Chevron deference a period of repose before addressing whether it should be retained, the Court has enabled its Members to test the propriety of that precedent and reflect more deeply on how well it fits into the broader architecture of our law. Others may see things differently, see post , at 26–27 (dissenting opinion), but the caution the Court has exhibited before overruling Chevron may illustrate one of the reasons why the current Court has been slower to overrule precedents than some of its predecessors, see Part I–C, supra .
None of this, of course, discharges any Member of this Court from the task of deciding for himself or herself today whether Chevron deference itself warrants deference. But when so many past and current judicial colleagues in this Court and across the country tell us our doctrine is misguided, and when we ourselves managed without Chevron for centuries and manage to do so today, the humility at the core of stare decisis compels us to pause and reflect carefully on the wisdom embodied in that experience. And, in the end, to my mind the lessons of experience counsel wisely against continued reliance on Chevron ’s stray and unconsidered digression. This Court’s opinions fill over 500 volumes, and perhaps “some printed judicial word may be found to support almost any plausible proposition.” R. Jackson, Decisional Law and Stare Decisis, 30 A. B. A. J. 334 (1944). It is not for us to pick and choose passages we happen to like and demand total obedience to them in perpetuity. That would turn stare decisis from a doctrine of humility into a tool for judicial opportunism. Brown , 596 U. S., at 141.
Proper respect for precedent helps “keep the scale of justice even and steady,” by reinforcing decisional rules consistent with the law upon which all can rely. 1 Blackstone 69. But that respect does not require, nor does it readily tolerate, a steadfast refusal to correct mistakes. As early as 1810, this Court had already overruled one of its cases. See Hudson v. Guestier , 6 Cranch 281, 284 (overruling Rose v. Himely , 4 Cranch 241 (1808)). In recent years, the Court may have overruled precedents less frequently than it did during the Warren and Burger Courts. See Part I–C, supra . But the job of reconsidering past decisions remains one every Member of this Court faces from time to time. 8
Justice William O. Douglas served longer on this Court than any other person in the Nation’s history. During his tenure, he observed how a new colleague might be inclined initially to “revere” every word written in an opinion issued before he arrived. W. Douglas, Stare Decisis, 49 Colum. L. Rev. 735, 736 (1949). But, over time, Justice Douglas reflected, his new colleague would “remembe[r] . . . that it is the Constitution which he swore to support and defend, not the gloss which his predecessors may have put on it.” Ibid. And “[s]o he [would] com[e] to formulate his own views, rejecting some earlier ones as false and embracing others.” Ibid. This process of reexamination, Justice Douglas explained, is a “necessary consequence of our system” in which each judge takes an oath—both “personal” and binding—to discern the law’s meaning for himself and apply it faithfully in the cases that come before him. Id. , at 736–737.
Justice Douglas saw, too, how appeals to precedent could be overstated and sometimes even overwrought. Judges, he reflected, would sometimes first issue “new and startling decision[s],” and then later spin around and “acquire an acute conservatism” in their aggressive defense of “their new status quo .” Id ., at 737. In that way, even the most novel and unlikely decisions became “coveted anchorage[s],” defended heatedly, if ironically, under the banner of “ stare decisis .” Ibid. ; see also Edwards v. Vannoy , 593 U. S. 255 , 294, n. 7 (2021) ( Gorsuch, J. , concurring).
That is Chevron ’s story: A revolution masquerading as the status quo. And the defense of it follows the same course Justice Douglas described. Though our dissenting colleagues have not hesitated to question other precedents in the past, they today manifest what Justice Douglas called an “acute conservatism” for Chevron ’s “startling” development, insisting that if this “coveted anchorage” is abandoned the heavens will fall. But the Nation managed to live with busy executive agencies of all sorts long before the Chevron revolution began to take shape in the mid-1980s. And all today’s decision means is that, going forward, federal courts will do exactly as this Court has since 2016, exactly as it did before the mid-1980s, and exactly as it had done since the founding: resolve cases and controversies without any systemic bias in the government’s favor.
Proper respect for precedent does not begin to suggest otherwise. Instead, it counsels respect for the written law, adherence to consistent teachings over aberrations, and resistance to the temptation of treating our own stray remarks as if they were statutes. And each of those lessons points toward the same conclusion today: Chevron deference is inconsistent with the directions Congress gave us in the APA. It represents a grave anomaly when viewed against the sweep of historic judicial practice. The decision undermines core rule-of-law values ranging from the promise of fair notice to the promise of a fair hearing. Even on its own terms, it has proved unworkable and operated to undermine rather than advance reliance interests, often to the detriment of ordinary Americans. And from the start, the whole project has relied on the overaggressive use of snippets and stray remarks from an opinion that carried mixed messages. Stare decisis ’s true lesson today is not that we are bound to respect Chevron ’s “startling development,” but bound to inter it.
1 For relevant databases of decisions, see Congressional Research Service, Table of Supreme Court Decisions Overruled by Subsequent Decisions, Constitution Annotated, https://constitution.congress.gov/resources/ decisions-overruled/; see also H. Spaeth et al., 2023 Supreme Court Database, http://supremecourtdatabase.org.
2 See also A. Scalia, Judicial Deference to Administrative Interpretations of Law, 1989 Duke L. J. 511, 516–517 (1989) (describing Chevron ’s theory that Congress “delegat[ed]” interpretive authority to agencies as “fictional”); S. Breyer, Judicial Review of Questions of Law and Policy, 38 Admin. L. Rev. 363, 370 (1986) (describing the notion that there exists a “ ‘legislative intent to delegate the law-interpreting function’ as a kind of legal fiction”).
3 The dissent suggests that we need not take the APA’s directions quite so seriously because the “finest administrative law scholars” from Harvard claim to see in them some wiggle room. Post , at 18 (opinion of Kagan, J .). But nothing in the APA commands deference to the views of professors any more than it does the government. Nor is the dissent’s list of Harvard’s finest administrative law scholars entirely complete. See S. Breyer et al., Administrative Law and Regulatory Policy 288 (7th ed. 2011) (acknowledging that Chevron deference “seems in conflict with . . . the apparently contrary language of 706”); Kagan 212 (likewise acknowledging Chevron deference rests upon a “fictionalized statement of legislative desire”).
4 Accord, National Lead Co. v. United States , 252 U. S. 140 , 145–146 (1920) (affording “great weight” to a “contemporaneous construction” by the executive that had “been long continued”); Jacobs v. Prichard , 223 U. S. 200 , 214 (1912) (“find[ing] no ambiguity in the act” but also finding “strength” for the Court’s interpretation in the executive’s “immediate and continued construction of the act”); Schell’s Executors v. Fauché , 138 U. S. 562 , 572 (1891) (treating as “controlling” a “contemporaneous construction” of a law endorsed “not only [by] the courts but [also by] the departments”).
5 The dissent suggests that Chevron deference bears at least something in common with surrounding law because it resembles a presumption or traditional canon of construction, and both “are common.” Post , at 8, n. 1, 28–29 (opinion of Kagan, J. ). But even that thin reed wavers at a glance. Many of the presumptions and interpretive canons the dissent cites—including lenity, contra proferentem , and others besides—“ ‘embod[y] . . . legal doctrine[s] centuries older than our Republic.’ ” Opati v. Republic of Sudan , 590 U. S. 418 , 425 (2020). Chevron deference can make no such boast. Many of the presumptions and canons the dissent cites also serve the Constitution, protecting the lines of authority it draws. Take just two examples: The federalism canon tells courts to presume federal statutes do not preempt state laws because of the sovereignty States enjoy under the Constitution. Bond v. United States , 572 U. S. 844 , 858 (2014). The presumption against retroactivity serves as guardian of the Constitution’s promise of due process and its ban on ex post facto laws, Landgraf v. USI Film Products , 511 U. S. 244 , 265 (1994). Once more, however, Chevron deference can make no similar claim. Rather than serve the Constitution’s usual rule that litigants are entitled to have an independent judge interpret disputed legal terms, Chevron deference works to undermine that promise. As explored above, too, Chevron deference sits in tension with many traditional legal presumptions and interpretive principles, representing nearly the inverse of the rules of lenity, nemo iudex , and contra proferentem .
6 It should be recalled that, when Justice Scalia launched the Chevron revolution, there were many judges who “abhor[red] . . . ‘plain meaning’ ” and preferred instead to elevate “legislative history” and their own curated accounts of a law’s “purpose[s]” over enacted statutory text. Scalia 515, 521. Chevron , he predicted, would provide a new guardrail against that practice. Scalia 515, 521 . As the Justice’s later writings show, he had the right diagnosis, just the wrong cure. The answer for judges eliding statutory terms is not deference to agencies that may seek to do the same, but a demand that all return to a more faithful adherence to the written law. That was, of course, another project Justice Scalia championed. And as we like to say, “we’re all textualists now.”
7 See, e.g., Becerra v. Empire Health Foundation, for Valley Hospital Medical Center , 597 U. S. 424 , 434 (2022) (resolving intricate Medicare dispute by reference solely to “text,” “context,” and “structure”); see also Sackett v. EPA , 598 U. S. 651 (2023) (same in a complex Clean Water Act dispute); Johnson v. Guzman Chavez , 594 U. S. 523 (2021) (same in technical immigration case).
8 Today’s dissenters are no exceptions. They have voted to overrule precedents that they consider “wrong,” Hurst v. Florida , 577 U. S. 92 , 101 (2016) (opinion for the Court by Sotomayor , J., joined by, inter alios , Kagan , J.); Obergefell v. Hodges , 576 U. S. 644 , 665, 675 (2015) (opinion for the Court, joined by, inter alios , Sotomayor and Kagan , JJ.); that conflict with the Constitution’s “original meaning,” Alleyne v. United States , 570 U. S. 99 , 118 (2013) ( Sotomayor , J., joined by, inter alias , Kagan , J., concurring); and that have proved “unworkable,” Johnson v. United States , 576 U. S. 591 , 605 (2015) (opinion for the Court, joined by, inter alios , Sotomayor and Kagan , JJ.); see also Erlinger v. United States , 602 U. S. ___, ___ (2024) ( Jackson, J. , dissenting) (slip op., at 1) (arguing Apprendi v. New Jersey , 530 U. S. 466 (2000) , and the many cases applying it were all “wrongly decided”).
Justice Kagan , with whom Justice Sotomayor and Justice Jackson join, 1 * dissenting.
For 40 years, Chevron U. S. A. Inc. v. Natural Resources Defense Council, Inc. , 467 U. S. 837 (1984) , has served as a cornerstone of administrative law, allocating responsibility for statutory construction between courts and agencies. Under Chevron , a court uses all its normal interpretive tools to determine whether Congress has spoken to an issue. If the court finds Congress has done so, that is the end of the matter; the agency’s views make no difference. But if the court finds, at the end of its interpretive work, that Congress has left an ambiguity or gap, then a choice must be made. Who should give content to a statute when Congress’s instructions have run out? Should it be a court? Or should it be the agency Congress has charged with administering the statute? The answer Chevron gives is that it should usually be the agency, within the bounds of reasonableness. That rule has formed the backdrop against which Congress, courts, and agencies—as well as regulated parties and the public—all have operated for decades. It has been applied in thousands of judicial decisions. It has become part of the warp and woof of modern government, supporting regulatory efforts of all kinds—to name a few, keeping air and water clean, food and drugs safe, and financial markets honest.
And the rule is right. This Court has long understood Chevron deference to reflect what Congress would want, and so to be rooted in a presumption of legislative intent. Congress knows that it does not—in fact cannot—write perfectly complete regulatory statutes. It knows that those statutes will inevitably contain ambiguities that some other actor will have to resolve, and gaps that some other actor will have to fill. And it would usually prefer that actor to be the responsible agency, not a court. Some interpretive issues arising in the regulatory context involve scientific or technical subject matter. Agencies have expertise in those areas; courts do not. Some demand a detailed understanding of complex and interdependent regulatory programs. Agencies know those programs inside-out; again, courts do not. And some present policy choices, including trade-offs between competing goods. Agencies report to a President, who in turn answers to the public for his policy calls; courts have no such accountability and no proper basis for making policy. And of course Congress has conferred on that expert, experienced, and politically accountable agency the authority to administer—to make rules about and otherwise implement—the statute giving rise to the ambiguity or gap. Put all that together and deference to the agency is the almost obvious choice, based on an implicit congressional delegation of interpretive authority. We defer, the Court has explained, “because of a presumption that Congress” would have “desired the agency (rather than the courts)” to exercise “whatever degree of discretion” the statute allows. Smiley v. Citibank (South Dakota), N. A. , 517 U. S. 735 , 740–741 (1996).
Today, the Court flips the script: It is now “the courts (rather than the agency)” that will wield power when Congress has left an area of interpretive discretion. A rule of judicial humility gives way to a rule of judicial hubris. In recent years, this Court has too often taken for itself decision-making authority Congress assigned to agencies. The Court has substituted its own judgment on workplace health for that of the Occupational Safety and Health Administration; its own judgment on climate change for that of the Environmental Protection Agency; and its own judgment on student loans for that of the Department of Education. See, e.g. , National Federation of Independent Business v. OSHA , 595 U. S. 109 (2022) ; West Virginia v. EPA , 597 U. S. 697 (2022) ; Biden v. Nebraska , 600 U. S. 477 (2023) . But evidently that was, for this Court, all too piecemeal. In one fell swoop, the majority today gives itself exclusive power over every open issue—no matter how expertise-driven or policy-laden—involving the meaning of regulatory law. As if it did not have enough on its plate, the majority turns itself into the country’s administrative czar. It defends that move as one (suddenly) required by the (nearly 80-year-old) Administrative Procedure Act. But the Act makes no such demand. Today’s decision is not one Congress directed. It is entirely the majority’s choice.
And the majority cannot destroy one doctrine of judicial humility without making a laughing-stock of a second. (If opinions had titles, a good candidate for today’s would be Hubris Squared.) Stare decisis is, among other things, a way to remind judges that wisdom often lies in what prior judges have done. It is a brake on the urge to convert “every new judge’s opinion” into a new legal rule or regime. Dobbs v. Jackson Women’s Health Organization , 597 U. S. 215 , 388 (2022) (joint opinion of Breyer, Sotomayor , and Kagan , JJ., dissenting) (quoting 1 W. Blackstone, Commentaries on the Laws of England 69 (7th ed. 1775)). Chevron is entrenched precedent, entitled to the protection of stare decisis , as even the majority acknowledges. In fact, Chevron is entitled to the supercharged version of that doctrine because Congress could always overrule the decision, and because so many governmental and private actors have relied on it for so long. Because that is so, the majority needs a “particularly special justification” for its action. Kisor v. Wilkie , 588 U. S. 558 , 588 (2019) (opinion of the Court). But the majority has nothing that would qualify. It barely tries to advance the usual factors this Court invokes for overruling precedent. Its justification comes down, in the end, to this: Courts must have more say over regulation—over the provision of health care, the protection of the environment, the safety of consumer products, the efficacy of transportation systems, and so on. A longstanding precedent at the crux of administrative governance thus falls victim to a bald assertion of judicial authority. The majority disdains restraint, and grasps for power.
Begin with the problem that gave rise to Chevron (and also to its older precursors): The regulatory statutes Congress passes often contain ambiguities and gaps. Sometimes they are intentional. Perhaps Congress “consciously desired” the administering agency to fill in aspects of the legislative scheme, believing that regulatory experts would be “in a better position” than legislators to do so. Chevron , 467 U. S., at 865. Or “perhaps Congress was unable to forge a coalition on either side” of a question, and the contending parties “decided to take their chances with” the agency’s resolution. Ibid. Sometimes, though, the gaps or ambiguities are what might be thought of as predictable accidents. They may be the result of sloppy drafting, a not infrequent legislative occurrence. Or they may arise from the well-known limits of language or foresight. Accord, ante , at 7, 22. “The subject matter” of a statutory provision may be too “specialized and varying” to “capture in its every detail.” Kisor , 588 U. S., at 566 (plurality opinion). Or the provision may give rise, years or decades down the road, to an issue the enacting Congress could not have anticipated. Whichever the case—whatever the reason—the result is to create uncertainty about some aspect of a provision’s meaning.
Consider a few examples from the caselaw. They will help show what a typical Chevron question looks like—or really, what a typical Chevron question is . Because when choosing whether to send some class of questions mainly to a court, or mainly to an agency, abstract analysis can only go so far; indeed, it may obscure what matters most. So I begin with the concrete:
Under the Public Health Service Act, the Food and Drug Administration (FDA) regulates “biological product[s],” including “protein[s].” 42 U. S. C. §262(i)(1) . When does an alpha amino acid polymer qualify as such a “protein”? Must it have a specific, defined sequence of amino acids? See Teva Pharmaceuticals USA, Inc. v. FDA , 514 F. Supp. 3d 66, 79–80, 93–106 (DC 2020).
Under the Endangered Species Act, the Fish and Wildlife Service must designate endangered “vertebrate fish or wildlife” species, including “distinct population segment[s]” of those species. 16 U. S. C. §1532(16) ; see §1533. What makes one population segment “distinct” from another? Must the Service treat the Washington State population of western gray squirrels as “distinct”
because it is geographically separated from other western gray squirrels? Or can the Service take into account that the genetic makeup of the Washington population does not differ markedly from the rest? See Northwest Ecosystem Alliance v. United States Fish and Wildlife Serv. , 475 F. 3d 1136 , 1140–1145, 1149 (CA9 2007).
Under the Medicare program, reimbursements to hospitals are adjusted to reflect “differences in hospital wage levels” across “geographic area[s].” 42 U. S. C. §1395ww(d)(3)(E)(i) . How should the Department of Health and Human Services measure a “geographic area”? By city? By county? By metropolitan area? See Bellevue Hospital Center v. Leavitt , 443 F. 3d 163 , 174–176 (CA2 2006).
Congress directed the Department of the Interior and the Federal Aviation Administration to reduce noise from aircraft flying over Grand Canyon National Park—specifically, to “provide for substantial restoration of the natural quiet.” §3(b)(1), 101 Stat. 676 ; see §3(b)(2). How much noise is consistent with “the natural quiet”? And how much of the park, for how many hours a day, must be that quiet for the “substantial restoration” requirement to be met? See Grand Canyon Air Tour Coalition v. FAA , 154 F. 3d 455 , 466–467, 474–475 (CADC 1998).
Or take Chevron itself. In amendments to the Clean Air Act, Congress told States to require permits for modifying or constructing “stationary sources” of air pollution. 42 U. S. C. §7502(c)(5) . Does the term “stationary source[ ]” refer to each pollution-emitting piece of equipment within a plant? Or does it refer to the entire plant, and thus allow escape from the permitting requirement when increased emissions from one piece of equipment are offset by reductions from another? See 467 U. S., at 857, 859.
In each case, a statutory phrase has more than one reasonable reading. And Congress has not chosen among them: It has not, in any real-world sense, “fixed” the “single, best meaning” at “the time of enactment” (to use the majority’s phrase). Ante , at 22. A question thus arises: Who decides which of the possible readings should govern?
This Court has long thought that the choice should usually fall to agencies, with courts broadly deferring to their judgments. For the last 40 years, that doctrine has gone by the name of Chevron deference, after the 1984 decision that formalized and canonized it. In Chevron , the Court set out a simple two-part framework for reviewing an agency’s interpretation of a statute that it administers. First, the reviewing court must determine whether Congress has “directly spoken to the precise question at issue.” 467 U. S., at 842. That inquiry is rigorous: A court must exhaust all the “traditional tools of statutory construction” to divine statutory meaning. Id. , at 843, n. 9. And when it can find that meaning—a “single right answer”—that is “the end of the matter”: The court cannot defer because it “must give effect to the unambiguously expressed intent of Congress.” Kisor , 588 U. S., at 575 (opinion of the Court); Chevron , 467 U. S., at 842–843. But if the court, after using its whole legal toolkit, concludes that “the statute is silent or ambiguous with respect to the specific issue” in dispute—for any of the not-uncommon reasons discussed above—then the court must cede the primary interpretive role. Ibid. ; see supra , at 4–5. At that second step, the court asks only whether the agency construction is within the sphere of “reasonable” readings. Chevron , 467 U. S., at 844. If it is, the agency’s interpretation of the statute that it every day implements will control.
That rule, the Court has long explained, rests on a presumption about legislative intent—about what Congress wants when a statute it has charged an agency with implementing contains an ambiguity or a gap. See id. , at 843– 845; Smiley , 517 U. S., at 740–741. An enacting Congress, as noted above, knows those uncertainties will arise, even if it does not know what they will turn out to be. See supra , at 4–5. And every once in a while, Congress provides an explicit instruction for dealing with that contingency—assigning primary responsibility to the courts, or else to an agency. But much more often, Congress does not say. Thus arises the need for a presumption—really, a default rule—for what should happen in that event. Does a statutory silence or ambiguity then go to a court for resolution? Or to an agency? This Court has long thought Congress would choose an agency, with courts serving only as a backstop to make sure the agency makes a reasonable choice among the possible readings. Or said otherwise, Congress would select the agency it has put in control of a regulatory scheme to exercise the “degree of discretion” that the statute’s lack of clarity or completeness allows. Smiley , 517 U. S., at 741. Of course, Congress can always refute that presumptive choice—can say that, really, it would prefer courts to wield that discretionary power. But until then, the presumption cuts in the agency’s favor. 2 The next question is why.
For one, because agencies often know things about a statute’s subject matter that courts could not hope to. The point is especially stark when the statute is of a “scientific or technical nature.” Kisor , 588 U. S., at 571 (plurality opinion). Agencies are staffed with “experts in the field” who can bring their training and knowledge to bear on open statutory questions. Chevron , 467 U. S., at 865. Consider, for example, the first bulleted case above. When does an alpha amino acid polymer qualify as a “protein”? See supra , at 5. I don’t know many judges who would feel confident resolving that issue. (First question: What even is an alpha amino acid polymer?) But the FDA likely has scores of scientists on staff who can think intelligently about it, maybe collaborate with each other on its finer points, and arrive at a sensible answer. Or take the perhaps more accessible-sounding second case, involving the Endangered Species Act. See supra , at 5–6. Deciding when one squirrel population is “distinct” from another (and thus warrants protection) requires knowing about species more than it does consulting a dictionary. How much variation of what kind—geographic, genetic, morphological, or behavioral—should be required? A court could, if forced to, muddle through that issue and announce a result. But wouldn’t the Fish and Wildlife Service, with all its specialized expertise, do a better job of the task—of saying what, in the context of species protection, the open-ended term “distinct” means? One idea behind the Chevron presumption is that Congress— the same Congress that charged the Service with implementing the Act—would answer that question with a resounding “yes.”
A second idea is that Congress would value the agency’s experience with how a complex regulatory regime functions, and with what is needed to make it effective. Let’s stick with squirrels for a moment, except broaden the lens. In construing a term like “distinct” in a case about squirrels, the Service likely would benefit from its “historical familiarity” with how the term has covered the population segments of other species. Martin v. Occupational Safety and Health Review Comm’n , 499 U. S. 144 , 153 (1991); see, e.g. , Center for Biological Diversity v. Zinke , 900 F. 3d 1053 , 1060–1062 (CA9 2018) (arctic grayling); Center for Biological Diversity v. Zinke , 868 F. 3d 1054 , 1056 (CA9 2017) (desert eagle). Just as a common-law court makes better decisions as it sees multiple variations on a theme, an agency’s construction of a statutory term benefits from its unique exposure to all the related ways the term comes into play. Or consider, for another way regulatory familiarity matters, the example about adjusting Medicare reimbursement for geographic wage differences. See supra , at 6. According to a dictionary, the term “geographic area” could be as large as a multi-state region or as small as a census tract. How to choose? It would make sense to gather hard information about what reimbursement levels each approach will produce, to explore the ease of administering each on a nationwide basis, to survey how regulators have dealt with similar questions in the past, and to confer with the hospitals themselves about what makes sense. See Kisor , 588 U. S., at 571 (plurality opinion) (noting that agencies are able to “conduct factual investigations” and “consult with affected parties”). Congress knows the Department of Health and Human Services can do all those things—and that courts cannot.
Still more, Chevron ’s presumption reflects that resolving statutory ambiguities, as Congress well knows, is “often more a question of policy than of law.” Pauley v. BethEnergy Mines, Inc. , 501 U. S. 680 , 696 (1991). The task is less one of construing a text than of balancing competing goals and values. Consider the statutory directive to achieve “substantial restoration of the [Grand Canyon’s] natural quiet.” See supra , at 6. Someone is going to have to decide exactly what that statute means for air traffic over the canyon. How many flights, in what places and at what times, are consistent with restoring enough natural quiet on the ground? That is a policy trade-off of a kind familiar to agencies—but peculiarly unsuited to judges. Or consider Chevron itself. As the Court there understood, the choice between defining a “stationary source” as a whole plant or as a pollution-emitting device is a choice about how to “reconcile” two “manifestly competing interests.” 467 U. S., at 865. The plantwide definition relaxes the permitting requirement in the interest of promoting economic growth; the device-specific definition strengthens that requirement to better reduce air pollution. See id. , at 851, 863, 866. Again, that is a choice a judge should not be making, but one an agency properly can. Agencies are “subject to the supervision of the President, who in turn answers to the public.” Kisor , 588 U. S., at 571–572 (plurality opinion). So when faced with a statutory ambiguity, “an agency to which Congress has delegated policymaking responsibilities” may rely on an accountable actor’s “views of wise policy to inform its judgments.” Chevron , 467 U. S., at 865.
None of this is to say that deference to agencies is always appropriate. The Court over time has fine-tuned the Chevron regime to deny deference in classes of cases in which Congress has no reason to prefer an agency to a court. The majority treats those “refinements” as a flaw in the scheme, ante , at 27, but they are anything but. Consider the rule that an agency gets no deference when construing a statute it is not responsible for administering. See Epic Systems Corp. v. Lewis , 584 U. S. 497 , 519–520 (2018). Well, of course not—if Congress has not put an agency in charge of implementing a statute, Congress would not have given the agency a special role in its construction. Or take the rule that an agency will not receive deference if it has reached its decision without using—or without using properly—its rulemaking or adjudicatory authority. See United States v. Mead Corp. , 533 U. S. 218 , 226–227 (2001); Encino Motorcars, LLC v. Navarro , 579 U. S. 211 , 220 (2016). Again, that should not be surprising: Congress expects that authoritative pronouncements on a law’s meaning will come from the procedures it has enacted to foster “fairness and deliberation” in agency decision-making. Mead , 533 U. S., at 230. Or finally, think of the “extraordinary cases” involving questions of vast “economic and political significance” in which the Court has declined to defer. King v. Burwell , 576 U. S. 473 , 485–486 (2015). The theory is that Congress would not have left matters of such import to an agency, but would instead have insisted on maintaining control. So the Chevron refinements proceed from the same place as the original doctrine. Taken together, they give interpretive primacy to the agency when—but only when—it is acting, as Congress specified, in the heartland of its delegated authority.
That carefully calibrated framework “reflects a sensitivity to the proper roles of the political and judicial branches.” Pauley , 501 U. S., at 696. Where Congress has spoken, Congress has spoken; only its judgments matter. And courts alone determine when that has happened: Using all their normal interpretive tools, they decide whether Congress has addressed a given issue. But when courts have decided that Congress has not done so, a choice arises. Absent a legislative directive, either the administering agency or a court must take the lead. And the matter is more fit for the agency. The decision is likely to involve the agency’s subject-matter expertise; to fall within its sphere of regulatory experience; and to involve policy choices, including cost-benefit assessments and trade-offs between conflicting values. So a court without relevant expertise or experience, and without warrant to make policy calls, appropriately steps back. The court still has a role to play: It polices the agency to ensure that it acts within the zone of reasonable options. But the court does not insert itself into an agency’s expertise-driven, policy-laden functions. That is the arrangement best suited to keep every actor in its proper lane. And it is the one best suited to ensure that Congress’s statutes work in the way Congress intended.
The majority makes two points in reply, neither convincing. First, it insists that “agencies have no special competence” in filling gaps or resolving ambiguities in regulatory statutes; rather, “[c]ourts do.” Ante , at 23. Score one for self-confidence; maybe not so high for self-reflection or -knowledge. Of course courts often construe legal texts, hopefully well. And Chevron ’s first step takes full advantage of that talent: There, a court tries to divine what Congress meant, even in the most complicated or abstruse statutory schemes. The deference comes in only if the court cannot do so—if the court must admit that standard legal tools will not avail to fill a statutory silence or give content to an ambiguous term. That is when the issues look like the ones I started off with: When does an alpha amino acid polymer qualify as a “protein”? How distinct is “distinct” for squirrel populations? What size “geographic area” will ensure appropriate hospital reimbursement? As between two equally feasible understandings of “stationary source,” should one choose the one more protective of the environment or the one more favorable to economic growth? The idea that courts have “special competence” in deciding such questions whereas agencies have “no[ne]” is, if I may say, malarkey. Answering those questions right does not mainly demand the interpretive skills courts possess. Instead, it demands one or more of: subject-matter expertise, long engagement with a regulatory scheme, and policy choice. It is courts (not agencies) that “have no special competence”—or even legitimacy—when those are the things a decision calls for.
Second, the majority complains that an ambiguity or gap does not “necessarily reflect a congressional intent that an agency” should have primary interpretive authority. Ante , at 22. On that score, I’ll agree with the premise: It doesn’t “necessarily” do so. Chevron is built on a presumption . The decision does not maintain that Congress in every case wants the agency, rather than a court, to fill in gaps. The decision maintains that when Congress does not expressly pick one or the other, we need a default rule; and the best default rule—agency or court?—is the one we think Congress would generally want. As to why Congress would generally want the agency: The answer lies in everything said above about Congress’s delegation of regulatory power to the agency and the agency’s special competencies. See supra , at 9–11. The majority appears to think it is a showstopping rejoinder to note that many statutory gaps and ambiguities are “unintentional.” Ante , at 22. But to begin, many are not; the ratio between the two is uncertain. See supra , at 4–5. And to end, why should that matter in any event? Congress may not have deliberately introduced a gap or ambiguity into the statute; but it knows that pretty much everything it drafts will someday be found to contain such a “flaw.” Given that knowledge, Chevron asks, what would Congress want? The presumed answer is again the same (for the same reasons): The agency. And as with any default rule, if Congress decides otherwise, all it need do is say.
In that respect, the proof really is in the pudding: Congress basically never says otherwise, suggesting that Chevron chose the presumption aligning with legislative intent (or, in the majority’s words, “approximat[ing] reality,” ante , at 22). Over the last four decades, Congress has authorized or reauthorized hundreds of statutes. The drafters of those statutes knew all about Chevron . See A. Gluck & L. Bressman, Statutory Interpretation From the Inside—An Empirical Study of Congressional Drafting, Delegation, and the Canons: Part I, 65 Stan. L. Rev. 901, 928 (fig. 2), 994 (2013). So if they had wanted a different assignment of interpretive responsibility, they would have inserted a provision to that effect. With just a pair of exceptions I know of, they did not. See 12 U. S. C. §25b(b)(5)(A) (exception #1); 15 U. S. C. §8302(c)(3)(A) (exception #2). Similarly, Congress has declined to enact proposed legislation that would abolish Chevron across the board. See S. 909, 116th Cong., 1st Sess., §2 (2019) (still a bill, not a law); H. R. 5, 115th Cong., 1st Sess., §202 (2017) (same). So to the extent the majority is worried that the Chevron presumption is “fiction[al],” ante , at 26—as all legal presumptions in some sense are—it has gotten less and less so every day for 40 years. The congressional reaction shows as well as anything could that the Chevron Court read Congress right.
The majority’s principal arguments are in a different vein. Around 80 years after the APA was enacted and 40 years after Chevron , the majority has decided that the former precludes the latter. The APA’s Section 706, the majority says, “makes clear” that agency interpretations of statutes “are not entitled to deference.” Ante , at 14–15 (emphasis in original). And that provision, the majority continues, codified the contemporaneous law, which likewise did not allow for deference. See ante, at 9–13, 15–16. But neither the APA nor the pre-APA state of the law does the work that the majority claims. Both are perfectly compatible with Chevron deference.
Section 706, enacted with the rest of the APA in 1946, provides for judicial review of agency action. It states: “To the extent necessary to decision and when presented, the reviewing court shall decide all relevant questions of law, interpret constitutional and statutory provisions, and determine the meaning or applicability of the terms of an agency action.” 5 U. S. C. §706 .
That text, contra the majority, “does not resolve the Chevron question.” C. Sunstein, Chevron As Law, 107 Geo. L. J. 1613, 1642 (2019) (Sunstein). Or said a bit differently, Section 706 is “generally indeterminate” on the matter of deference. A. Vermeule, Judging Under Uncertainty 207 (2006) (Vermeule). The majority highlights the phrase “decide all relevant questions of law” (italicizing the “all”), and notes that the provision “prescribes no deferential standard” for answering those questions. Ante , at 14. But just as the provision does not prescribe a deferential standard of review, so too it does not prescribe a de novo standard of review (in which the court starts from scratch, without giving deference). In point of fact, Section 706 does not specify any standard of review for construing statutes. See Kisor , 588 U. S., at 581 (plurality opinion). And when a court uses a deferential standard—here, by deciding whether an agency reading is reasonable—it just as much “decide[s]” a “relevant question[ ] of law” as when it uses a de novo standard. §706. The deferring court then conforms to Section 706 “by determining whether the agency has stayed within the bounds of its assigned discretion—that is, whether the agency has construed [the statute it administers] reasonably.” J. Manning, Chevron and the Reasonable Legislator, 128 Harv. L. Rev. 457, 459 (2014); see Arlington v. FCC , 569 U. S. 290 , 317 (2013) ( Roberts , C. J., dissenting) (“We do not ignore [Section 706’s] command when we afford an agency’s statutory interpretation Chevron deference; we respect it”). 3
Section 706’s references to standards of review in other contexts only further undercut the majority’s argument. The majority notes that Section 706 requires deferential review for agency fact-finding and policy-making (under, respectively, a substantial-evidence standard and an arbitrary-and-capricious standard). See ante , at 14. Congress, the majority claims, “surely would have articulated a similarly deferential standard applicable to questions of law had it intended to depart” from de novo review. Ibid. Surely? In another part of Section 706, Congress explicitly referred to de novo review. §706(2)(F). With all those references to standards of review—both deferential and not—running around Section 706, what is “telling” ( ante , at 14) is the absence of any standard for reviewing an agency’s statutory constructions. That silence left the matter, as noted above, “generally indeterminate”: Section 706 neither mandates nor forbids Chevron -style deference. Vermeule 207. 4
And contra the majority, most “respected commentators” understood Section 706 in that way—as allowing, even if not requiring, deference. Ante , at 16. The finest administrative law scholars of the time (call them that generation’s Manning, Sunstein, and Vermeule) certainly did. Professor Louis Jaffe described something very like the Chevron two-step as the preferred method of reviewing agency interpretations under the APA. A court, he said, first “must decide as a ‘question of law’ whether there is ‘discretion’ in the premises.” Judicial Control of Administrative Action 570 (1965). That is akin to step 1: Did Congress speak to the issue, or did it leave openness? And if the latter, Jaffe continued, the agency’s view “if ‘reasonable’ is free of control.” Ibid. That of course looks like step 2: defer if reasonable. And just in case that description was too complicated, Jaffe conveyed his main point this way: The argument that courts “must decide all questions of law”—as if there were no agency in the picture—“is, in my opinion, unsound.” Id. , at 569. Similarly, Professor Kenneth Culp Davis, author of the then-preeminent treatise on administrative law, noted with approval that “reasonableness” review of agency interpretations—in which courts “refused to substitute judgment”—had “survived the APA.” Administrative Law 880, 883, 885 (1951) (Davis). Other contemporaneous scholars and experts agreed. See R. Levin, The APA and the Assault on Deference, 106 Minn. L. Rev. 125, 181–183 (2021) (Levin) (listing many of them). They did not see in their own time what the majority finds there today. 5
Nor, evidently, did the Supreme Court. In the years after the APA was enacted, the Court “never indicated that section 706 rejected the idea that courts might defer to agency interpretations of law.” Sunstein 1654. Indeed, not a single Justice so much as floated that view of the APA. To the contrary, the Court issued a number of decisions in those years deferring to an agency’s statutory interpretation. See, e.g. , Unemployment Compensation Comm’n of Alaska v. Aragon , 329 U. S. 143 , 153–154 (1946); NLRB v. E. C. Atkins & Co. , 331 U. S. 398 , 403 (1947); Cardillo v. Liberty Mut. Ins. Co. , 330 U. S. 469 , 478–479 (1947). And that continued right up until Chevron . See, e.g. , Mitchell v. Budd , 350 U. S. 473 , 480 (1956); Zenith Radio Corp. v. United States , 437 U. S. 443 , 450 (1978). To be clear: Deference in those years was not always given to interpretations that would receive it under Chevron . The practice then was more inconsistent and less fully elaborated than it later became. The point here is only that the Court came nowhere close to accepting the majority’s view of the APA. Take the language from Section 706 that the majority most relies on: “decide all relevant questions of law.” See ante , at 14. In the decade after the APA’s enactment, those words were used only four times in Supreme Court opinions (all in footnotes)—and never to suggest that courts could not defer to agency interpretations. See Sunstein 1656.
The majority’s view of Section 706 likewise gets no support from how judicial review operated in the years leading up to the APA. That prior history matters: As the majority recognizes, Section 706 was generally understood to “restate[ ] the present law as to the scope of judicial review.” Dept. of Justice, Attorney General’s Manual on the Administrative Procedure Act 108 (1947); ante , at 15–16. The problem for the majority is that in the years preceding the APA, courts became ever more deferential to agencies. New Deal administrative programs had by that point come into their own. And this Court and others, in a fairly short time, had abandoned their initial resistance and gotten on board. Justice Breyer, wearing his administrative-law-scholar hat, characterized the pre-APA period this way: “[J]udicial review of administrative action was curtailed, and particular agency decisions were frequently sustained with judicial obeisance to the mysteries of administrative expertise.” S. Breyer et al., Administrative Law and Regulatory Policy 21 (7th ed. 2011). And that description extends to review of an agency’s statutory constructions. An influential study of administrative practice, published five years before the APA’s enactment, described the state of play: Judicial “review may, in some instances at least, be limited to the inquiry whether the administrative construction is a permissible one.” Final Report of Attorney General’s Committee on Administrative Procedure (1941), reprinted in Administrative Procedure in Government Agencies, S. Doc. No. 8, 77th Cong., 1st Sess., 78 (1941). Or again: “[W]here the statute is reasonably susceptible of more than one interpretation, the court may accept that of the administrative body.” Id. , at 90–91. 6
Two prominent Supreme Court decisions of the 1940s put those principles into action. Gray v. Powell , 314 U. S. 402 (1941) , was then widely understood as “the leading case” on review of agency interpretations. Davis 882; see ibid. (noting that it “establish[ed] what is known as ‘the doctrine of Gray v. Powell’ ”). There, the Court deferred to an agency construction of the term “producer” as used in a statutory exemption from price controls. Congress, the Court explained, had committed the scope of the exemption to the agency because its “experience in [the] field gave promise of a better informed, more equitable, adjustment of the conflicting interests.” Gray , 314 U. S., at 412. Accordingly, the Court concluded that it was “not the province of a court” to “substitute its judgment” for the agency’s. Ibid. Three years later, the Court decided NLRB v. Hearst Publications, Inc. , 322 U. S. 111 (1944) , another acknowledged “leading case.” Davis 882; see id. , at 884. The Court again deferred, this time to an agency’s construction of the term “employee” in the National Labor Relations Act. The scope of that term, the Court explained, “belong[ed] to” the agency to answer based on its “[e]veryday experience in the administration of the statute.” Hearst , 322 U. S., at 130. The Court therefore “limited” its review to whether the agency’s reading had “warrant in the record and a reasonable basis in law.” Id. , at 131. 7 Recall here that even the majority accepts that Section 706 was meant to “restate[ ] the present law” as to judicial review. See ante , at 15–16; supra , at 19–20. Well then? It sure would seem that the provision allows a deference regime.
The majority has no way around those two noteworthy decisions. It first appears to distinguish between “pure legal question[s]” and the so-called mixed questions in Gray and Hearst , involving the application of a legal standard to a set of facts. Ante , at 11. If in drawing that distinction, the majority intends to confine its holding to the pure type of legal issue—thus enabling courts to defer when law and facts are entwined—I’d be glad. But I suspect the majority has no such intent, because that approach would preserve Chevron in a substantial part of its current domain. Cf. Wilkinson v. Garland , 601 U. S. 209 , 230 (2024) ( Alito, J., dissenting) (noting, in the immigration context, that the universe of mixed questions swamps that of pure legal ones). It is frequently in the consideration of mixed questions that the scope of statutory terms is established and their meaning defined. See H. Monaghan, Marbury and the Administrative State, 83 Colum. L. Rev. 1, 29 (1983) (“Administrative application of law is administrative formulation of law whenever it involves elaboration of the statutory norm”). How does a statutory interpreter decide, as in Hearst , what an “employee” is? In large part through cases asking whether the term covers people performing specific jobs, like (in that case) “newsboys.” 322 U. S., at 120. Or consider one of the examples I offered above. How does an interpreter decide when one population segment of a species is “distinct” from another? Often by considering that requirement with respect to particular species, like western gray squirrels. So the distinction the majority offers makes no real-world (or even theoretical) sense. If the Hearst Court was deferring to an agency on whether the term “employee” covered newsboys, it was deferring to the agency on the scope and meaning of the term “employee.”
The majority’s next rejoinder—that “the Court was far from consistent” in deferring—falls equally flat. Ante , at 12. I am perfectly ready to acknowledge that in the pre-APA period, a deference regime had not yet taken complete hold. I’ll go even further: Let’s assume that deference was then an on-again, off-again function (as the majority seems to suggest, see ante , at 11–12, and 13, n. 3). Even on that assumption, the majority’s main argument—that Section 706 prohibited deferential review—collapses. Once again, the majority agrees that Section 706 was not meant to change the then-prevailing law. See ante , at 15–16. And even if inconsistent, that law cannot possibly be thought to have prohibited deference. Or otherwise said: “If Section 706 did not change the law of judicial review (as we have long recognized), then it did not proscribe a deferential standard then known and in use.” Kisor , 588 U. S., at 583 (plurality opinion).
The majority’s whole argument for overturning Chevron relies on Section 706. But the text of Section 706 does not support that result. And neither does the contemporaneous practice, which that text was supposed to reflect. So today’s decision has no basis in the only law the majority deems relevant. It is grounded on air.
And still there is worse, because abandoning Chevron subverts every known principle of stare decisis . Of course, respecting precedent is not an “inexorable command.” Payne v. Tennessee , 501 U. S. 808 , 828 (1991). But overthrowing it requires far more than the majority has offered up here. Chevron is entitled to stare decisis ’s strongest form of protection. The majority thus needs an exceptionally strong reason to overturn the decision, above and beyond thinking it wrong. And it has nothing approaching such a justification, proposing only a bewildering theory about Chevron ’s “unworkability.” Ante , at 32. Just five years ago, this Court in Kisor rejected a plea to overrule Auer v. Robbins , 519 U. S. 452 (1997) , which requires judicial deference to agencies’ interpretations of their own regulations. See 588 U. S., at 586–589 (opinion of the Court). The case against overruling Chevron is at least as strong. In particular, the majority’s decision today will cause a massive shock to the legal system, “cast[ing] doubt on many settled constructions” of statutes and threatening the interests of many parties who have relied on them for years. 588 U. S., at 587 (opinion of the Court).
Adherence to precedent is “a foundation stone of the rule of law.” Michigan v. Bay Mills Indian Community , 572 U. S. 782 , 798 (2014). Stare decisis “promotes the evenhanded, predictable, and consistent development of legal principles.” Payne , 501 U. S., at 827. It enables people to order their lives in reliance on judicial decisions. And it “contributes to the actual and perceived integrity of the judicial process,” by ensuring that those decisions are founded in the law, and not in the “personal preferences” of judges. Id. , at 828; Dobbs , 597 U. S., at 388 (dissenting opinion). Perhaps above all else, stare decisis is a “doctrine of judicial modesty.” Id. , at 363. In that, it shares something important with Chevron . Both tell judges that they do not know everything, and would do well to attend to the views of others. So today, the majority rejects what judicial humility counsels not just once but twice over.
And Chevron is entitled to a particularly strong form of stare decisis , for two separate reasons. First, it matters that “Congress remains free to alter what we have done.” Patterson v. McLean Credit Union , 491 U. S. 164 , 173 (1989); see Kisor , 588 U. S., at 587 (opinion of the Court) (making the same point for Auer deference). In a constitutional case, the Court alone can correct an error. But that is not so here. “Our deference decisions are balls tossed into Congress’s court, for acceptance or not as that branch elects.” 588 U. S., at 587–588 (opinion of the Court). And for generations now, Congress has chosen acceptance. Throughout those years, Congress could have abolished Chevron across the board, most easily by amending the APA. Or it could have eliminated deferential review in discrete areas, by amending old laws or drafting new laws to include an anti- Chevron provision. Instead, Congress has “spurned multiple opportunities” to do a comprehensive rejection of Chevron , and has hardly ever done a targeted one. Kimble v. Marvel Entertainment, LLC , 576 U. S. 446 , 456 (2015); see supra , at 14–15. Or to put the point more affirmatively, Congress has kept Chevron as is for 40 years. It maintained that position even as Members of this Court began to call Chevron into question. See ante , at 30. From all it appears, Congress has not agreed with the view of some Justices that they and other judges should have more power.
Second, Chevron is by now much more than a single decision. This Court alone, acting as Chevron allows, has upheld an agency’s reasonable interpretation of a statute at least 70 times. See Brief for United States in No. 22–1219, p. 27; App. to id ., at 68a–72a (collecting cases). Lower courts have applied the Chevron framework on thousands upon thousands of occasions. See K. Barnett & C. Walker, Chevron and Stare Decisis, 31 Geo. Mason L. Rev. 475, 477, and n. 11 (2024) (noting that at last count, Chevron was cited in more than 18,000 federal-court decisions). The Kisor Court observed, when upholding Auer , that “[d]eference to reasonable agency interpretations of ambiguous rules pervades the whole corpus of administrative law.” 588 U. S., at 587 (opinion of the Court). So too does deference to reasonable agency interpretations of ambiguous statutes—except more so. Chevron is as embedded as embedded gets in the law.
The majority says differently, because this Court has ignored Chevron lately; all that is left of the decision is a “decaying husk with bold pretensions.” Ante , at 33. Tell that to the D. C. Circuit, the court that reviews a large share of agency interpretations, where Chevron remains alive and well. See, e.g. , Lissack v. Commissioner , 68 F. 4th 1312, 1321–1322 (2023); Solar Energy Industries Assn. v. FERC , 59 F. 4th 1287, 1291–1294 (2023). But more to the point: The majority’s argument is a bootstrap. This Court has “avoided deferring under Chevron since 2016” ( ante , at 32) because it has been preparing to overrule Chevron since around that time. That kind of self-help on the way to reversing precedent has become almost routine at this Court. Stop applying a decision where one should; “throw some gratuitous criticisms into a couple of opinions”; issue a few separate writings “question[ing the decision’s] premises” ( ante , at 30); give the whole process a few years . . . and voila!—you have a justification for overruling the decision. Janus v. State, County, and Municipal Employees , 585 U. S. 878 , 950 (2018) ( Kagan, J. , dissenting) (discussing the overruling of Abood v. Detroit Bd. of Ed. , 431 U. S. 209 (1977) ); see also, e.g. , Kennedy v. Bremerton School Dist. , 597 U. S. 507 , 571–572 (2022) ( Sotomayor, J. , dissenting) (similar for Lemon v. Kurtzman , 403 U. S. 602 (1971) ); Shelby County v. Holder , 570 U. S. 529 , 587–588 (2013) (Ginsburg , J. , dissenting) (similar for South Carolina v. Katzenbach , 383 U. S. 301 (1966) ). I once remarked that this overruling-through-enfeeblement technique “mock[ed] stare decisis .” Janus , 585 U. S., at 950 (dissenting opinion). I have seen no reason to change my mind.
The majority does no better in its main justification for overruling Chevron —that the decision is “unworkable.” Ante , at 30. The majority’s first theory on that score is that there is no single “answer” about what “ambiguity” means: Some judges turn out to see more of it than others do, leading to “different results.” Ante , at 30–31. But even if so, the legal system has for many years, in many contexts, dealt perfectly well with that variation. Take contract law. It is hornbook stuff that when (but only when) a contract is ambiguous, a court interpreting it can consult extrinsic evidence. See CNH Industrial N.V. v. Reese , 583 U. S. 133 , 139 (2018) ( per curiam ). And when all interpretive tools still leave ambiguity, the contract is construed against the drafter. See Lamps Plus, Inc. v. Varela , 587 U. S. 176 , 186–187 (2019). So I guess the contract rules of the 50 States are unworkable now. Or look closer to home, to doctrines this Court regularly applies. In deciding whether a government has waived sovereign immunity, we construe “[a]ny ambiguities in the statutory language” in “favor of immunity.” FAA v. Cooper , 566 U. S. 284 , 290 (2012). Similarly, the rule of lenity tells us to construe ambiguous statutes in favor of criminal defendants. See United States v. Castleman , 572 U. S. 157 , 172–173 (2014). And the canon of constitutional avoidance instructs us to construe ambiguous laws to avoid difficult constitutional questions. See United States v. Oakland Cannabis Buyers’ Cooperative , 532 U. S. 483 , 494 (2001). I could go on, but the point is made. There are ambiguity triggers all over the law. Somehow everyone seems to get by.
And Chevron is an especially puzzling decision to criticize on the ground of generating too much judicial divergence. There’s good empirical—meaning, non-impressionistic—evidence on exactly that subject. And it shows that, as compared with de novo review, use of the Chevron two-step framework fosters agreement among judges. See K. Barnett, C. Boyd, & C. Walker, Administrative Law’s Political Dynamics, 71 Vand. L. Rev. 1463, 1502 (2018) (Barnett). More particularly, Chevron has a “powerful constraining effect on partisanship in judicial decisionmaking.” Barnett 1463 (italics deleted); see Sunstein 1672 (“[A] predictable effect of overruling Chevron would be to ensure a far greater role for judicial policy preferences in statutory interpretation and far more common splits along ideological lines”). So if consistency among judges is the majority’s lodestar, then the Court should not overrule Chevron , but return to using it.
The majority’s second theory on workability is likewise a makeweight. Chevron , the majority complains, has some exceptions, which (so the majority says) are “difficult” and “complicate[d]” to apply. Ante , at 32. Recall that courts are not supposed to defer when the agency construing a statute (1) has not been charged with administering that law; (2) has not used deliberative procedures— i.e., notice-and-comment rulemaking or adjudication; or (3) is intervening in a “major question,” of great economic and political significance. See supra , at 11–12; ante , at 27–28. As I’ve explained, those exceptions—the majority also aptly calls them “refinements”—fit with Chevron ’s rationale: They define circumstances in which Congress is unlikely to have wanted agency views to govern. Ante , at 27; see supra , at 11–12. And on the difficulty scale, they are nothing much. Has Congress put the agency in charge of administering the statute? In 99 of 100 cases, everyone will agree on the answer with scarcely a moment’s thought. Did the agency use notice-and-comment or an adjudication before rendering an interpretation? Once again, I could stretch my mind and think up a few edge cases, but for the most part, the answer is an easy yes or no. The major questions exception is, I acknowledge, different: There, many judges have indeed disputed its nature and scope. Compare, e . g ., West Virginia , 597 U. S., at 721–724, with id ., at 764–770 ( Kagan , J., dissenting). But that disagreement concerns, on everyone’s view, a tiny subset of all agency interpretations. For the most part, the exceptions that so upset the majority require merely a rote, check-the-box inquiry. If that is the majority’s idea of a “dizzying breakdance,” ante , at 32, the majority needs to get out more.
And anyway, difficult as compared to what? The majority’s prescribed way of proceeding is no walk in the park. First, the majority makes clear that what is usually called Skidmore deference continues to apply. See ante , at 16–17. Under that decision, agency interpretations “constitute a body of experience and informed judgment” that may be “entitled to respect.” Skidmore v. Swift & Co. , 323 U. S. 134 , 140 (1944). If the majority thinks that the same judges who argue today about where “ambiguity” resides (see ante , at 30) are not going to argue tomorrow about what “respect” requires, I fear it will be gravely disappointed. Second, the majority directs courts to comply with the varied ways in which Congress in fact “delegates discretionary authority” to agencies. Ante , at 17–18. For example, Congress may authorize an agency to “define[ ]” or “delimit[ ]” statutory terms or concepts, or to “fill up the details” of a statutory scheme. Ante , at 17, and n. 5. Or Congress may use, in describing an agency’s regulatory authority, inherently “flexib[le]” language like “appropriate” or “reasonable.” Ante , at 17, and n. 6. Attending to every such delegation, as the majority says, is necessary in a world without Chevron . But that task involves complexities of its own. Indeed, one reason Justice Scalia supported Chevron was that it re placed such a “statute-by-statute evaluation (which was assuredly a font of uncertainty and litigation) with an across-the-board presumption.” A. Scalia, Judicial Deference to Administrative Interpretations of Law, 1989 Duke L. J. 511, 516. As a lover of the predictability that rules create, Justice Scalia thought the latter “unquestionably better.” Id. , at 517.
On the other side of the balance, the most important stare decisis factor—call it the “jolt to the legal system” issue—weighs heavily against overruling Chevron . Dobbs , 597 U. S., at 357 ( Roberts, C. J. , concurring in judgment). Congress and agencies alike have relied on Chevron —have assumed its existence—in much of their work for the last 40 years. Statutes passed during that time reflect the expectation that Chevron would allocate interpretive authority between agencies and courts. Rules issued during the period likewise presuppose that statutory ambiguities were the agencies’ to (reasonably) resolve. Those agency interpretations may have benefited regulated entities; or they may have protected members of the broader public. Either way, private parties have ordered their affairs—their business and financial decisions, their health-care decisions, their educational decisions—around agency actions that are suddenly now subject to challenge. In Kisor , this Court refused to overrule Auer because doing so would “cast doubt on” many longstanding constructions of rules, and thereby upset settled expectations. 588 U. S., at 587 (opinion of the Court). Overruling Chevron , and thus raising new doubts about agency constructions of statutes, will be far more disruptive.
The majority tries to alleviate concerns about a piece of that problem: It states that judicial decisions that have upheld agency action as reasonable under Chevron should not be overruled on that account alone. See ante , at 34–35. That is all to the good: There are thousands of such decisions, many settled for decades. See supra , at 26. But first, reasonable reliance need not be predicated on a prior judicial decision. Some agency interpretations never challenged under Chevron now will be; expectations formed around those constructions thus could be upset, in a way the majority’s assurance does not touch. And anyway, how good is that assurance, really? The majority says that a decision’s “[m]ere reliance on Chevron ” is not enough to counter the force of stare decisis ; a challenger will need an additional “special justification.” Ante , at 34. The majority is sanguine; I am not so much. Courts motivated to overrule an old Chevron -based decision can always come up with something to label a “special justification.” Maybe a court will say “the quality of [the precedent’s] reasoning” was poor. Ante , at 29. Or maybe the court will discover something “unworkable” in the decision—like some exception that has to be applied. Ante , at 30. All a court need do is look to today’s opinion to see how it is done.
Judges are not experts in the field, and are not part of either political branch of the Government.
— Chevron U. S. A. Inc. v. Natural ResourcesDefense Council, Inc. , 467 U. S. 837 , 865 (1984)
Those were the days, when we knew what we are not. When we knew that as between courts and agencies, Congress would usually think agencies the better choice to resolve the ambiguities and fill the gaps in regulatory statutes. Because agencies are “experts in the field.” And because they are part of a political branch, with a claim to making interstitial policy. And because Congress has charged them, not us, with administering the statutes containing the open questions. At its core, Chevron is about respecting that allocation of responsibility—the conferral of primary authority over regulatory matters to agencies, not courts.
Today, the majority does not respect that judgment. It gives courts the power to make all manner of scientific and technical judgments. It gives courts the power to make all manner of policy calls, including about how to weigh competing goods and values. (See Chevron itself.) It puts courts at the apex of the administrative process as to every conceivable subject—because there are always gaps and ambiguities in regulatory statutes, and often of great import. What actions can be taken to address climate change or other environmental challenges? What will the Nation’s health-care system look like in the coming decades? Or the financial or transportation systems? What rules are going to constrain the development of A.I.? In every sphere of current or future federal regulation, expect courts from now on to play a commanding role. It is not a role Congress has given to them, in the APA or any other statute. It is a role this Court has now claimed for itself, as well as for other judges.
And that claim requires disrespecting, too, this Court’s precedent. There are no special reasons, of the kind usually invoked for overturning precedent, to eliminate Chevron deference. And given Chevron ’s pervasiveness, the decision to do so is likely to produce large-scale disruption. All that backs today’s decision is the majority’s belief that Chevron was wrong—that it gave agencies too much power and courts not enough. But shifting views about the worth of regulatory actors and their work do not justify overhauling a cornerstone of administrative law. In that sense too, today’s majority has lost sight of its proper role.
And it is impossible to pretend that today’s decision is a one-off, in either its treatment of agencies or its treatment of precedent. As to the first, this very Term presents yet another example of the Court’s resolve to roll back agency authority, despite congressional direction to the contrary. See SEC v. Jarkesy , 603 U. S. ___ (2024); see also supra , at 3. As to the second, just my own defenses of stare decisis — my own dissents to this Court’s reversals of settled law—by now fill a small volume. See Dobbs , 597 U. S., at 363–364 (joint opinion of Breyer, Sotomayor , and Kagan , JJ.); Edwards v. Vannoy , 593 U. S. 255 , 296–297 (2021); Knick v. Township of Scott , 588 U. S. 180 , 207–208 (2019); Janus , 585 U. S. , at 931–932. Once again, with respect, I dissent.
1 * Justice Jackson did not participate in the consideration or decision of the case in No. 22–451 and joins this opinion only as it applies to the case in No. 22–1219.
2 Note that presumptions of this kind are common in the law. In other contexts, too, the Court responds to a congressional lack of direction by adopting a presumption about what Congress wants, rather than trying to figure that out in every case. And then Congress can legislate, with “predictable effects,” against that “stable background” rule. Morrison v. National Australia Bank Ltd. , 561 U. S. 247 , 261 (2010). Take the presumption against extraterritoriality: The Court assumes Congress means for its statutes to apply only within the United States, absent a “clear indication” to the contrary. Id. , at 255. Or the presumption against retroactivity: The Court assumes Congress wants its laws to apply only prospectively, unless it “unambiguously instruct[s]” something different. Vartelas v. Holder , 566 U. S. 257 , 266 (2012). Or the presumption against repeal of statutes by implication: The Court assumes Congress does not intend a later statute to displace an earlier one unless it makes that intention “clear and manifest.” Epic Systems Corp. v. Lewis , 584 U. S. 497 , 510 (2018). Or the (so far unnamed) presumption against treating a procedural requirement as “jurisdictional” unless “Congress clearly states that it is.” Boechler v. Commissioner , 596 U. S. 199 , 203 (2022). I could continue, except that this footnote is long enough. The Chevron deference rule is to the same effect: The Court generally assumes that Congress intends to confer discretion on agencies to handle statutory ambiguities or gaps, absent a direction to the contrary. The majority calls that presumption a “fiction,” ante , at 26, but it is no more so than any of the presumptions listed above. They all are best guesses—and usually quite good guesses—by courts about congressional intent.
3 The majority tries to buttress its argument with a stray sentence or two from the APA’s legislative history, but the same response holds. As the majority notes, see ante , at 15, the House and Senate Reports each stated that Section 706 “provid[ed] that questions of law are for courts rather than agencies to decide in the last analysis.” H. R. Rep. No. 1980, 79th Cong., 2d Sess., 44 (1946); S. Rep. No. 752, 79th Cong., 1st Sess., 28 (1945). But that statement also does not address the standard of review that courts should then use. When a court defers under Chevron , it reviews the agency’s construction for reasonableness “in the last analysis.” The views of Representative Walter, which the majority also cites, further demonstrate my point. He stated that the APA would require courts to “determine independently all relevant questions of law,” but he also stated that courts would be required to “exercise . . . independent judgment” in applying the substantial-evidence standard (a deferential standard if ever there were one). 92 Cong. Rec. 5654 (1946). He therefore did not equate “independent” review with de novo review; he thought that a court could conduct independent review of agency action using a deferential standard.
4 In a footnote responding to the last two paragraphs, the majority raises the white flag on Section 706’s text. See ante , at 15, n. 4. Yes, it finally concedes, Section 706 does not say that de novo review is required for an agency’s statutory construction. Rather, the majority says, “some things go without saying,” and de novo review is such a thing. See ibid. But why? What extra-textual considerations force us to read Section 706 the majority’s way? In its footnote, the majority repairs only to history. But as I will explain below, the majority also gets wrong the most relevant history, pertaining to how judicial review of agency interpretations operated in the years before the APA was enacted. See infra , at 19–23.
5 I concede one exception (whose view was “almost completely isolated,” Levin 181), but his comments on Section 706 refute a different aspect of the majority’s argument. Professor John Dickinson, as the majority notes, thought that Section 706 precluded courts from deferring to agency interpretations. See Administrative Procedure Act: Scope and Grounds of Broadened Judicial Review, 33 A. B. A. J. 434, 516 (1947) (Dickinson); ante , at 16. But unlike the majority, he viewed that bar as “a change” to, not a restatement of, pre-APA law. Compare Dickinson 516 with ante , at 15–16. So if the majority really wants to rely on Professor Dickinson, it will have to give up the claim, which I address below, that the law before the APA forbade deference. See infra , at 19–23.
6 Because the APA was meant to “restate[ ] the present law,” the judicial review practices of the 1940s are more important to understanding the statute than is any earlier tradition (such as the majority dwells on). But before I expand on those APA-contemporaneous practices, I pause to note that they were “not built on sand.” Kisor v. Wilkie , 588 U. S. 558 , 568–569 (2019) (plurality opinion). Since the early days of the Republic, this Court has given significant weight to official interpretations of “ambiguous law[s].” Edwards’ Lessee v. Darby , 12 Wheat. 206, 210 (1827). With the passage of time—and the growth of the administrative sphere—those “judicial expressions of deference increased.” H. Monaghan, Marbury and the Administrative State, 83 Colum. L. Rev. 1, 15 (1983). By the early 20th century, the Court stated that it would afford “great weight” to an agency construction in the face of statutory “uncertainty or ambiguity.” National Lead Co. v. United States , 252 U. S. 140 , 145 (1920); see Schell’s Executors v. Fauché , 138 U. S. 562 , 572 (1891) (“controlling” weight in “all cases of ambiguity”); United States v. Alabama Great Southern R. Co. , 142 U. S. 615 , 621 (1892) (“decisive” weight “in case of ambiguity”); Jacobs v. Prichard , 223 U. S. 200 , 214 (1912) (referring to the “rule which gives strength” to official interpretations if “ambiguity exist[s]”). So even before the New Deal, a strand of this Court’s cases exemplified deference to executive constructions of ambiguous statutes. And then, as I show in the text, the New Deal arrived and deference surged—creating the “present law” that the APA “restated.”
7 The majority says that I have “pluck[ed] out” Gray and Hearst , impliedly from a vast number of not-so-helpful cases. Ante , at 13, n. 3. It would make as much sense to say that a judge “plucked out” Universal Camera Corp. v. NLRB , 340 U. S. 474 (1951) , to discuss substantial-evidence review or “plucked out” Motor Vehicle Mfrs. Assn. of United States, Inc. v. State Farm Mut. Automobile Ins. Co. , 463 U. S. 29 (1983) , to discuss arbitrary-and-capricious review. Gray and Hearst , as noted above, were the leading cases about agency interpretations in the years before the APA’s enactment. But just to gild the lily, here are a number of other Supreme Court decisions from the five years prior to the APA’s enactment that were of a piece: United States v. Pierce Auto Freight Lines, Inc. , 327 U. S. 515 , 536 (1946); ICC v. Parker , 326 U. S. 60 , 65 (1945); Federal Security Administrator v. Quaker Oats Co. , 318 U. S. 218 , 227–228 (1943). The real “pluck[ing]” offense is the majority’s—for taking a stray sentence from Hearst ( ante , at 13, n. 3) to suggest that both Hearst and Gray stand for the opposite of what they actually do.

IMAGES
VIDEO
COMMENTS
If your doctor, provider, or supplier doesn't accept assignment: You might have to pay the full amount at the time of service. They should submit a claim to Medicare for any Medicare-covered services they give you, and they can't charge you for submitting a claim. If they refuse to submit a Medicare claim, you can submit your own claim to ...
Yes, MD Anderson consists of hospitals that accept Medicare assignment, meaning you can use your benefits at its 13 hospital systems throughout 11 states. What you need to realize is that while benefits for Original Medicare and Medigap are accepted, MD Anderson's Medicare Advantage options are quite limited. Furthermore, it is advised that ...
Find hospitals near me. Find and compare information about the quality of care at over 4,000 Medicare-certified hospitals, including over 130 Veterans Administration (VA) medical centers and over 50 military hospitals, across the country. My Location Enter street, ZIP code, city, or state.
All providers who accept assignment must submit claims directly to Medicare, which pays 80 percent of the approved cost for the service and will bill you the remaining 20 percent. You can get some preventive services and screenings, such as mammograms and colonoscopies, without paying a deductible or coinsurance if the provider accepts assignment.
Most doctors, providers, and suppliers accept assignment, but you should always check to make sure. Participating providers have signed an agreement to accept assignment for all Medicare-covered services. NPI Number: The National Provider Identifier (NPI) is a unique identification number for covered health care providers. The NPI must be used ...
Providers who accept assignment are also known as Medicare participating providers. Non-participating providers can charge patients 115% of the Medicare approved amount, less Medicare's payment. Medigap Plans F and G cover these amounts, ... All Advantage plans must include an adequate number of providers and hospitals in their networks. If ...
Summary: Medicare Assignment is an agreement between healthcare providers and Medicare, where providers accept the Medicare-approved amount as full payment, preventing them from charging beneficiaries extra. This benefits Medicare beneficiaries by controlling their costs and ensuring they only pay deductibles and copayments.
What is Medicare Assignment. Medicare assignment is an agreement by your doctor or other healthcare providers to accept the Medicare-approved amount as the full cost for a covered service. Providers who "accept assignment" bill Medicare directly for Part B-covered services and cannot charge you more than the applicable deductible and ...
The majority of doctors accept assignment. Participating health providers have an agreement with Medicare to accept assignment for all Medicare-covered services. If the doctor accepts assignment ...
Medicare "participation" means you agree to accept claims assignment for all Medicare-covered services to your patients. By accepting assignment, you agree to accept Medicare-allowed amounts as payment in full. You may not collect more from the patient than the Medicare deductible and coinsurance or copayment. Participating Provider or ...
or not to accept assignment. When they accept assignment, Medicare makes the payment directly to the physician and collects the 20 percent coinsurance from the patient, but the physician cannot collect the full limiting charge amount. For unassigned claims, Medicare reimburses the patient and the physician collects the entire limiting charge
If your doctor accepts assignment, that means they'll send your whole medical bill to Medicare, and then Medicare pays 80% of the cost, while you are responsible for the remaining 20%. A doctor who doesn't accept assignment, however, could charge up to 15% more than the Medicare-approved amount for their services, depending on what state ...
Original Medicare is the fee-for-service plan that includes Medicare Part A, which covers hospital costs. And it also includes Medicare Part B, which pays for other healthcare services, ... If the therapist accepts the Medicare assignment, they will charge you $100 and bill Medicare. After Medicare pays $100, you'll owe 20%, or $20 for ...
For example, when a hospital signs up with Medicare to see Medicare patients, it must agree to accept the Medicare negotiated rate, including your deductible and/or coinsurance payment, as payment in full. ... But if your healthcare provider hasn't opted out but just doesn't accept assignment with Medicare (ie, doesn't accept the amount ...
Medicare sets a fixed cost to pay for every benefit they cover. This amount is called Medicare assignment. You have the largest healthcare provider network with over 800,000 providers nationwide on Original Medicare. You can see any doctor nationwide that accepts Medicare. Understanding the differences between your cost and the difference ...
Medicare assignment codes help Medicare pay for covered services. If your doctor or other provider accepts assignment and is a participating provider, they will file for reimbursement for services with a CMS-1500 form and the code will be "assigned.". But non-participating providers can select "not assigned.".
The provider then charges you the remaining 20 percent of the approved "reasonable" charge, plus any part of the deductible that has not been satisfied. If your doctor does not accept assignment, the rules are different. Non-participating doctors can charge beneficiaries 20 percent of the approved amount plus up to an additional 15 percent more ...
There are some non-par providers, however, who accept Medicare assignment for certain services, on a case-by-case basis. Those may include any of the services—anything from hospital and hospice care to lab tests and surgery—available from any assignment-accepting doctor, with a key exception: If a non-par provider accepts assignment for a ...
Accepting assignment entails two conditions: agreeing to accept Medicare's fee-schedule amount as payment-in-full for a given service and collecting Medicare's portion directly from Medicare ...
Less than two percent of physicians have opted-out of Medicare in all but four states and the District of Columbia. As of June 2023, Alaska (3.1%), Colorado (2.3%), Wyoming (2.3%), Idaho (2.1% ...
Providers that fully accept assignment are known as participating providers. They agree to accept all of Medicare's predetermined prices for all procedures and tests that are provided under Medicare coverage. This means that no matter what a hospital normally charges for a procedure, they agree to only charge Medicare recipients a set price.
Welcome! You can use this tool to find and compare different types of Medicare providers (like physicians, hospitals, nursing homes, and others). Use our maps and filters to help you identify providers that are right for you. Find Medicare-approved providers near you & compare care quality for nursing homes, doctors, hospitals, hospice centers ...
Nonassignment of Benefits. The second reimbursement method a physician/supplier has is choosing to not accept assignment of benefits. Under this method, a non-participating provider is the only provider that can file a claim as non-assigned. When the provider does not accept assignment, the Medicare payment will be made directly to the beneficiary.
Location: 1701 Veterans Drive, Florence, Alabama 35630. Ratings: Phone: (256) 768-8400. Mizell Memorial Hospital. Acute Care Hospital (Medicare Certified) Location: 702 N Main St, Opp, Alabama 36467. Ratings: Phone: (334) 493-3541. Crenshaw Community Hospital. Acute Care Hospital (Medicare Certified)
Because Medicare is a reliable payor backed by the full faith and credit of the U.S. government, most hospitals, doctors' offices, medical practices, and other healthcare groups accept it as valid coverage. In fact, according to the Centers for Medicare and Medicaid Services (CMS), 98 percent of medical service providers accept Medicare. That ...
Medicare participation means you agree to accept claims assignment for all covered patient services. By accepting assignment, you agree to accept Medicare-allowed amounts as payment in full. You can't collect more from the patient than the deductible and coinsurance or copayment.The Social Security Act says you must submit patient Medicare claims whether or not you participate.
Dr Gardner Scott Smith is licensed to practice in Montana (license number MED-PHYS-LIC-43906) and he also participates in the medicare program. He accepts medicare assignments (which means he accepts the Medicare-approved amount; you will not be billed for any more than the Medicare deductible and coinsurance) and his NPI Number is 1720182231.
The Medicare home health wage index does not utilize NECTA definitions, and, as most recently discussed in the CY 2021 HH PPS final rule (85 FR 70298) we include hospitals located in Micropolitan Statistical areas in each State's rural wage index. In other words, these OMB updates did not affect any geographic areas for purposes of the HH PPS ...
I Accept. Schedule an appointment today. Call to Schedule: 1-866-UH4-CARE. ... You may have read the recent positive news about the changes in Medicare prescription coverage: beginning in 2025, Medicare recipients with prescription drug coverage will see significant savings, as their cost for medication will not exceed $2,000 annually ...
The Court granted certiorari in these cases limited to the question whether Chevron U. S. A. Inc. v. Natural Resources Defense Council, Inc., 467 U. S. 837, should be overruled or clarified.Under the Chevron doctrine, courts have sometimes been required to defer to "permissible" agency interpretations of the statutes those agencies administer—even when a reviewing court reads the statute ...