Chemistry Learner
It's all about chemistry.
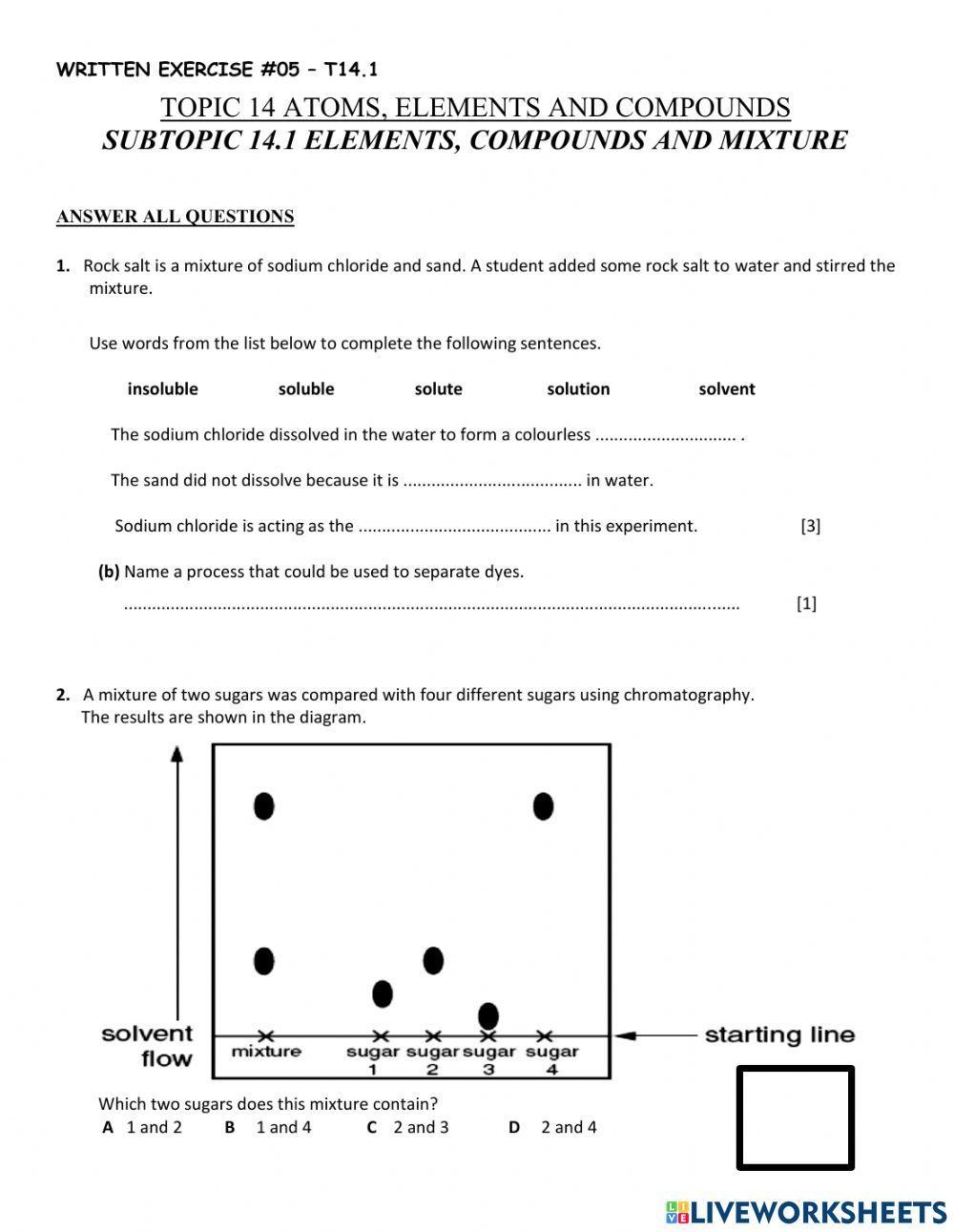
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets

Paper Chromatography
How does paper chromatography work, stationary and mobile phases, paper chromatography experiment, applications.
Paper chromatography is a simple and cost-effective separation technique that separates and identifies different components in a mixture. [1-4]
In paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. The mixture to be analyzed is applied to the paper. As the solvent moves up through capillary action, it carries along the individual components of the mixture at different rates based on their solubility and affinity for the stationary phase.
The principle behind paper chromatography lies in the differential partitioning of compounds between the stationary and mobile phases. The stationary phase typically consists of cellulose fibers embedded in filter paper or thin-layer chromatography plates. These fibers provide an adsorbent surface for compounds to interact with.
Understanding the mechanism behind paper chromatography requires knowledge of several key processes. [1-4]
The first process is capillary action, which refers to the ability of liquids to flow through narrow spaces against gravity. In paper chromatography, capillary action allows the solvent to move up the paper strip due to its attraction to the fibers in the paper. As the solvent moves up, it carries the solutes in the analyzed mixture. This migration of solutes is driven by two main mechanisms: adsorption and partitioning.
Adsorption occurs when solute molecules adhere to the fibers or other surfaces within the paper. It can be influenced by polarity and molecular size, with more polar or larger molecules having stronger interactions with the stationary phase.
Conversely, partitioning involves solute molecules distributing themselves between two immiscible phases – in this case, between the stationary phase (paper) and mobile phase (solvent). The extent of partitioning depends on factors such as solute polarity and affinity for either phase.
As solutes migrate up through capillary action, they may experience different degrees of adsorption and partitioning along their journey. This results in their separation based on their characteristics. By analyzing how far each component migrates on a chromatogram – a visual representation of separated components – scientists can determine properties such as retention factor (R f ) values and identify unknown substances based on known reference compounds.

Stationary and mobile phases play crucial roles in separating components of a mixture. [1-4]
The stationary phase refers to the absorbent material fixed on the chromatography paper. It can be made of cellulose or other materials with high absorbency. The stationary phase acts as a substrate for the sample mixture to interact with during separation.
On the other hand, the mobile phase is the solvent or liquid that moves through the stationary phase, carrying the sample components. The mobile phase must have good solubility with the components of interest. It should be able to flow easily through the paper.
As the mobile phase moves through the stationary phase, it interacts differently with each mixture component based on their solubility and affinity for both phases. This differential interaction leads to separation as different components travel at different rates along the paper.
Choosing an appropriate combination of stationary and mobile phases is important for effective separation in paper chromatography. Factors such as polarity, viscosity, and compatibility between phases must be considered to achieve optimal results.
Performing a paper chromatography experiment involves several essential steps to ensure accurate results. The process begins with preparing samples for paper chromatography, then spotting the sample on the paper strip, and finally, developing the chromatogram. [1-4]
Preparing the samples is crucial in obtaining reliable data. It involves selecting appropriate substances to analyze and ensuring they are suitable for chromatography. Samples can be liquid or solid and must be dissolved or crushed into a solution before application.
Next, spotting the sample on the paper strip is done carefully to ensure accurate separation. A small spot of the prepared sample is placed near one end of a designated area on the filter paper strip. It is essential to use a capillary tube or micropipette for precise and consistent application.
Once all samples are spotted on the filter paper strip, it is time for the development of the chromatogram. This step involves placing one end of the strip into a solvent traveling up through capillary action. The choice of solvent depends on factors such as solubility and desired separation distance.
As the solvent moves up through the filter paper strip, it carries different components in each sample. These components separate based on their affinity for stationary (filter paper) and mobile (solvent) phases. The separation occurs due to differences in molecular size, polarity, or other physical properties.
Throughout this process, it is important to maintain controlled conditions such as temperature and humidity to ensure reproducibility. Further analysis can be conducted once an optimal separation has been achieved, which can take several minutes or hours depending on various factors, including solvent choice and sample composition.
The diverse applications of paper chromatography across various fields are listed below. [1-4]
- It plays a crucial role in forensic analysis by separating and identifying different components in complex mixtures, such as blood or ink samples.
- Aids in the analysis of crime scene evidence, allowing forensic scientists to determine the presence of specific substances and identify potential suspects based on chromatographic patterns
- Enables the separation of different dyes used in food coloring, helping to ensure compliance with regulatory standards and quality control measures
- Determines the authenticity and safety of food products by identifying and quantifying specific components present in complex food matrices
- Separate and identify active ingredients, impurities, and by-products in pharmaceutical formulations.
- Chem.libretexts.org
- Swe.mit.edu
- Chemlab.truman.edu
Trending Topics
© 2024 ( Chemistry Learner )
paper chromatography worksheet
All Formats
Resource types, all resource types.
- Rating Count
- Price (Ascending)
- Price (Descending)
- Most Recent
Paper chromatography worksheet
Paper Chromatography Homework Sheet (Chemistry Revision)

Paper Chromatography with Ink Lab Activity - Matter - Mixtures and Solutions

Paper Chromatography : Simple Lab Exploring Mixtures

Separating Ink Using Paper Chromatography [Practical Experiment]

Middle School Science and Literacy Vocabulary Worksheet - Plants

- Word Document File

Paper Chromatography Pigments and Molecules Lab

AQA GCSE Chemistry: Paper chromatography

Paper Chromatography

LAB - Chemistry Mixtures Separation - Chromatography - Simple Materials

Thanksgiving Science Bundle | Pumpkin Volcano | Lava Lamp | Leaf Chromatography .

Chromatography Lab

Paper Chromatography Word Search Puzzle

Chemical Separations - Chromatography

Chromatography Homework Sheet (Chemistry Revision)

Chromatography Prelab

Physical Methods of Separating Matter

Inquiry Lab with Stations and Activities Involving Properties of Water

Chemistry Laboratory Tools Word Search Puzzle

Fractional distillation and chromatography (GCSE)

Chromatography Workbook

Chromatography KS3 Lesson PowerPoint 7Ed Mixtures & Separation Techniques

Fractional distillation and chromatography Distance learning (GCSE)
- Google Docs™
Living Environment Regents Evidence for Evolution Review Packet
Don't Judge a Leaf by its Color

- We're hiring
- Help & FAQ
- Privacy policy
- Student privacy
- Terms of service
- Tell us what you think

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Chromatography I - Fundamentals (Worksheet)
- Last updated
- Save as PDF
- Page ID 97015
- Thomas Wenzel
- Bates College
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help.
- Draw an idealized plot as greater concentrations of analyte are injected into the chromatographic column.
- Draw what you suspect would really happen.
- What might the peaks look like in the real versus ideal situations?
- What term would we use to describe the movement of a molecule in a liquid stationary phase?
- What is the dominant surface functionality of starch?
- The two other common solid stationary phases are silica gel (SiO 2 ) and alumina (Al 2 O 3 ).What do you think are the surface functionalities of these materials?
FREE K-12 standards-aligned STEM
curriculum for educators everywhere!
Find more at TeachEngineering.org .
- TeachEngineering
- Chromatography Lab
Hands-on Activity Chromatography Lab
Grade Level: 8 (7-9)
Time Required: 45 minutes
Expendable Cost/Group: US $40.00
Group Size: 3
Activity Dependency: Introduction to Water Chemistry
Associated Informal Learning Activity: Chromatography
Subject Areas: Chemistry, Physical Science, Problem Solving, Reasoning and Proof, Science and Technology
NGSS Performance Expectations:

Curriculum in this Unit Units serve as guides to a particular content or subject area. Nested under units are lessons (in purple) and hands-on activities (in blue). Note that not all lessons and activities will exist under a unit, and instead may exist as "standalone" curriculum.
- Thinking Green!
- Water Remediation Lab
- Red Cabbage Chemistry
- Density Column Lab - Part 1
- Density Column Lab - Part 2
TE Newsletter
Engineering connection, learning objectives, materials list, worksheets and attachments, more curriculum like this, introduction/motivation, vocabulary/definitions, troubleshooting tips, activity extensions, user comments & tips.

A firm understanding of solutions and mixtures and their components is essential for environmental engineers whose challenge is to prepare solutions to monitor and test groundwater for contaminants, and to develop remediation processes that separate pollutants from their water solutions.
After this activity, students should be able to:
- Describe how chromatography works and what happens during this process to black or colored ink.
- Explain why being able to separate solutions into their components is important to environmental engineering and water quality.
Educational Standards Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards. All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN) , a project of D2L (www.achievementstandards.org). In the ASN, standards are hierarchically structured: first by source; e.g. , by state; within source by type; e.g. , science or mathematics; within type by subtype, then by grade, etc .
Ngss: next generation science standards - science, international technology and engineering educators association - technology.
View aligned curriculum
Do you agree with this alignment? Thanks for your feedback!
State Standards
Missouri - science.
Each group needs:
- Whatman chromatography paper, at least 4 two-inch-wide strips; available at amazon.com
- Sharpie ® black ink marker; can be shared among teams
- Sharpie color markers, of various colors; can be shared among teams
- isopropyl alcohol (rubbing alcohol); higher proof or percent rubbing alcohol is best
- two 500-ml beakers
- Chromatography Lab Worksheet , one per student
Who has heard of chromatography? Chromatography is a way to look at complex mixtures by separating them into their components. The separation of a mixture into its components is a physical process; that is, because the components of the mixture are not chemically combined, they can be separated by physical means. Criminal investigators use this technology to identify substances such as drugs, blood, ink and other fluids.
We have already discussed environmental engineering situations that require water remediation. Can you name some for me? (Listen to student responses.) Yes, examples might include industrial oil spills or chemical leaks into drinking water sources. In order to develop and apply methods of water remediation, environmental engineers, must also be able to separate mixtures to remove the pollutant components.
Here's how chromatography works: Different inks have different properties, such as how much they can be dissolved in solvents. When you place chromatography paper into a solvent, the solvent begins to move up the paper. As the solvent rises, it dissolves the ink on the paper and separates the ink into its components. The farther the ink travels, the more it is attracted to the solvent.
Understanding chemical reactions can also help environment engineers remediate contaminants in water. For example, engineers can use chemical oxidants to degrade certain contaminants; in other words, the contaminant reacts with the treatment chemical to produce a product that is benign, or harmless, unlike the contaminant. Some common indicators of reaction include: changes in odor, temperature, or color, production of gas, or precipitation. However, the only way to be absolutely certain of whether a chemical reaction has occurred is to perform a chemical analysis to determine whether a new chemical is present.
A good understanding of solutions and mixtures and their components, in addition to reaction, is essential for environmental engineers. Most experiments and data gathering done for the purpose of improving groundwater quality involve the preparation of solutions to monitor and test contaminated water.
Before the Activity
- Gather materials and make copies of the Chromatography Lab Worksheet .
- Cut the chromatography paper into strips about two-inches wide. Cut at least four strips for each group.
With the Students
- Divide the class into groups of three students each. Hand out the worksheets.
- Take the strips of chromatography paper and fold both pieces about an inch from the top.
- Use a black Sharpie to draw a horizontal line near the other end of the chromatography paper. Make the line about as high off the bottom as the width of your thumb.
- Use a pencil to suspend the paper in each beaker, making sure the strip does not touch the sides of the beaker.
- Carefully add water to one beaker and alcohol to the other. Add just enough of each liquid so that it touches the bottom of the hanging strip.
- Watch what happens!! Once you see the separation is complete you, get new strips and repeat using ink from a colored Sharpie marker.
- If time permits, have students test other colored Sharpies to see different color separations.
- Have students answer the questions on their worksheets and hand them in for grading.
- Conclude by leading a wrap-up class discussion to compare results and conclusions; see the questions in the Assessment section.
solute: The component of a solution in the smallest amount.
solution: Mixture made of two or more substances.
solvent: The component of a solution in the largest amount.
Pre-Activity Assessment
Predictions: Have students write down predictions for what they think will happen during the activity. Have them identify the components of a solution (that is, solute[s] and solvent). Remind students that we are interested in whether a reaction occurs during the experiment. Review the indicators of reaction (changes in odor, temperature, or color, production of gas, or precipitation.) Ask a few student volunteers to share their answers with the class.
Activity Embedded Assessment
What's Going On? While students are conducting the lab, guided by the Chromatography Lab Worksheet , walk around the room and ask them questions to keep them engaged and on task. Example questions:
- Why doesn't the black Sharpie marker ink separate in water? (Answer: The separation depends on how soluble the ink in the marker is in either water or alcohol. The ink in the black marker is not soluble in water and therefore does not separate. This is also why this type of marker is considered a "permanent" marker—because it is water-insoluble and thus cannot be washed off using water! For more advanced students, explain that the alcohol is a better solvent for chromatographic separation [dissolving the ink] than water due to polar and non-polar interactions.)
- Do you see any of the indicators of reaction? Do you think that a reaction has occurred? (For the teacher: Make sure to discuss the color change indicator with students. Students may think that a reaction has occurred because the ink separates into its color components when immersed in the solvent, so it might appear that the ink has changed color. However, the appearance of the color components is not indicating a chemical reaction, just a separation. One indication that a reaction has not occurred is that no energy change has taken place. All of the indicators of reaction correspond to energy change.)
Post-Activity Assessment
Worksheet: Have students answer the Chromatography Lab Worksheet questions and hand them in for grading (see answers in the Wrap-Up Discussion, below). Review their answers to gauge their comprehension of the material.
Wrap-Up Discussion: At lab end, bring students together as a class and ask them the following questions about how chromatography works, what happens to the ink during this process and its real-world relevance. Make sure everyone understands the answers:
- What were the two solvents used in our lab activity today? (Answer: Water and isopropyl alcohol.)
- Black ink is more attracted to which solvent? How do you know? (Answer: The black ink is more attracted to the isopropyl alcohol. We observed ink separation in the isopropyl alcohol and no ink separation in the water.)
- What colors are present in black ink? (Answer: This varies from group to group, but typically the most common colors are purple, blue and yellow. In addition, black is still visible in the separation.)
- What do these colors represent? (Answer: Each color represents the different solutes or ink components used to make the black ink. We cannot see each of these individual components unless a separation occurs. This separation only occurs when a solvent is used in which the ink is soluble.)
- Why might the ability to separate solutions into their components be important to environmental engineers and water quality? (Answer: Environmental engineers must understand all about solutions and mixtures and their components because most experiments and data gathering done for the purpose of improving groundwater quality involve the preparation of solutions to monitor and test contaminated water. Only if we can separate from water the usually invisible water pollutant chemicals can we design remediation methods to clean the water!)
Safety Issues
When disposing of the isopropyl alcohol, pour the remaining alcohol back into the original bottle to be disposed of as flammable waste.
Make sure students do not draw marker lines too close to the crease in the chromatography paper. If they do, the separation takes a long time because the alcohol must travel further than if ink lines are drawn near the bottom half of the paper.
Students can easily perform this lab activity at home to test different colored markers and different marker types (such as dry erase board markers). Designing inks with different properties and characteristics is a vast chemical engineering industry.

Students investigate different colored pigments in a variety of different colored leaves. By using isopropanol and chromatography paper, students separate the different pigments that make up the color of the leaf. They learn to analyze data by collecting and recording information after assembling an...

Students are presented with examples of the types of problems that environmental engineers solve, specifically focusing on water quality issues. Topics include the importance of clean water, the scarcity of fresh water, tap water contamination sources, and ways environmental engineers treat contamin...

Contributors
Supporting program, acknowledgements.
This curriculum was developed with support from National Science Foundation GK-12 grant number DGE 0538541. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: August 30, 2019
- Topic Specification

Chromatography Revision
Try it yourself.
- Example Questions
- Worksheets 1

Supercharge your learning
Filter by Level
Filter by tier, filter by exam board, chromatography, chromatography .
Chromatography can be used to separate mixtures into their component compounds. It can also be used to identify the different compounds present in a mixture. By calculating \text{R}_f values, paper chromatography can be used to identify different chemicals from a mixture.
Chromatographic Methods
Chromatography refers to a group of methods that can be used to separate mixtures of chemicals into their component parts . There are lots of different methods of chromatography but all methods have three components:
- The analyte : The mixture of compounds being separated during the chromatography experiment.
- The stationary phase : A polar substance (often solid) through which the mobile phase moves.
- The mobile phase : A solvent used to dissolve the analyte . The mobile phase moves through the stationary phase , carrying the analyte with it.
During a chromatography experiment, the analyte moves through the stationary phase which will attract the components of the mixture to itself. The strength of this attraction will depend on the polarity of the stationary phase and the different polarities of the analyte components. The speed with which a compound moves through the stationary phase is dependent on the strength of its attraction to the stationary phase.
Compounds with a strong attraction to the stationary phase will be slowed down by this attraction and so will take a long time to move through the system. Compounds that experience a weak attraction to the stationary phase will move through the system more quickly .
The difference in the time taken for compounds to move through the stationary phase will lead to their separation .
Paper Chromatography
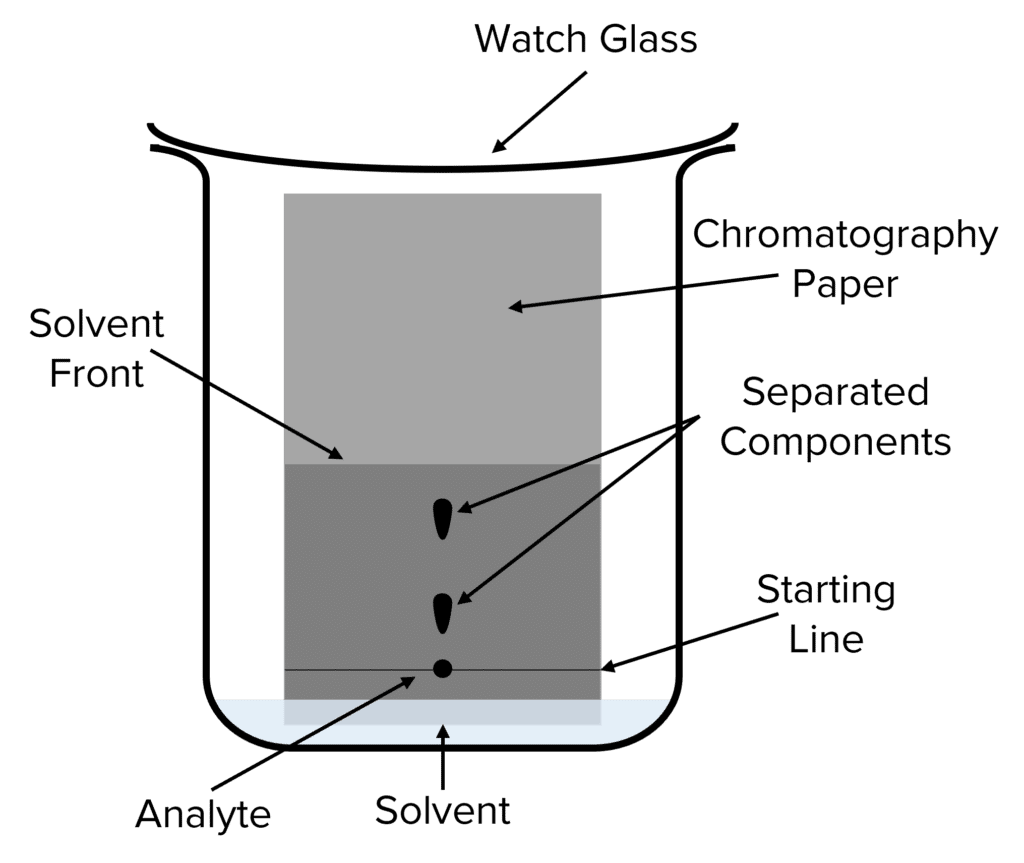
Paper chromatography is a type of chromatography that is often used to quickly analyse mixtures in chemistry. This method employs chromatography paper as the stationary phase and a solvent as the mobile phase . During paper chromatography the solvent soaks into the chromatography paper creating a line called the solvent front . This solvent front moves up the length of the chromatography paper, pulling the analyte up along with it.
As the analyte moves up the paper, the attractions between its components and the stationary chromatography paper separates them out. Compounds that are more polar (i.e. experience stronger electrostatic attractions) will move a small distance up the paper before they become stuck, compounds with a weaker attraction will move a greater distance .
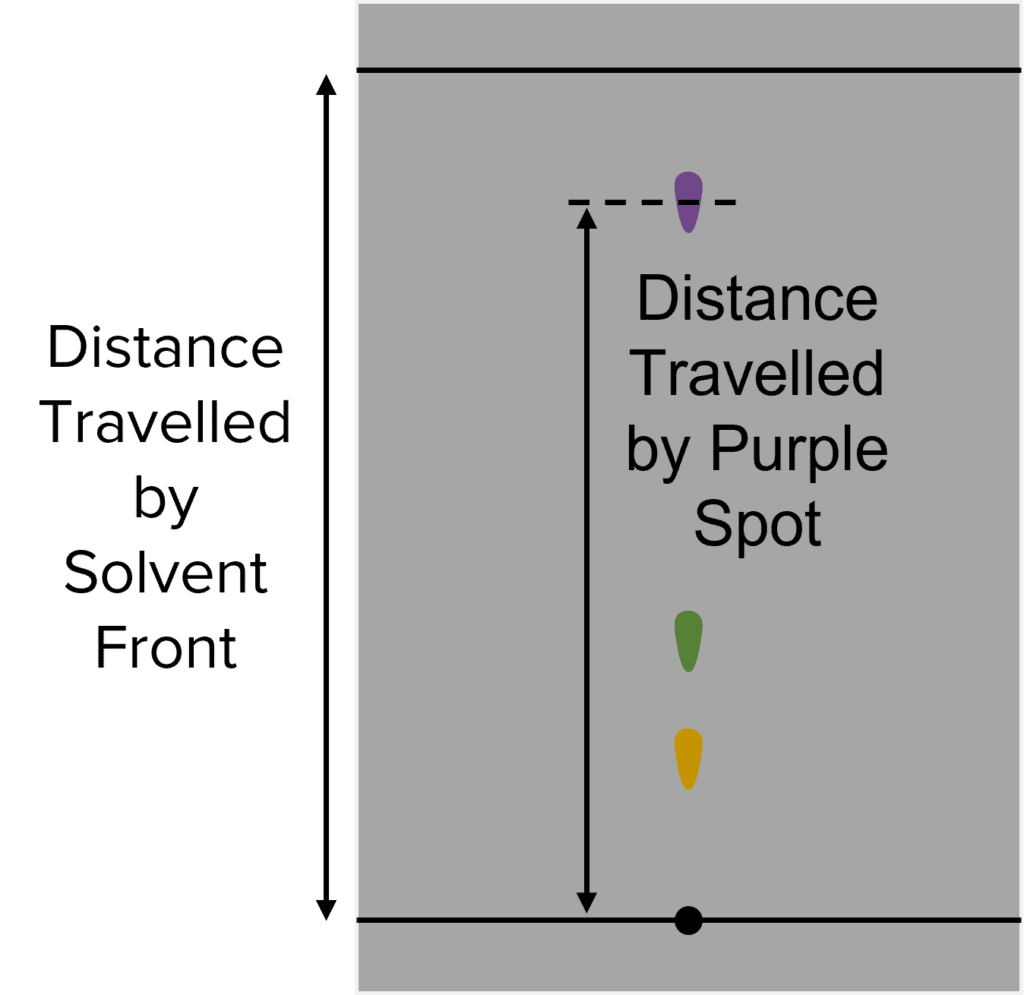
The distance that components move up the chromatography paper can be quantified by calculating \text{R}_f value. This value is calculated by diving the distance traveled by a component by the distance traveled by the solvent:
\text{R}_f=\frac{\text{Distance Traveled by Component}}{\text{Distance Traveled by Solvent}}
The \text{R}_f value of a given compound remains constant , it will move the same distance up the chromatography paper relative to the solvent . We can identify compounds in mixtures by calculating their \text{R}_f values.
Required Practical
Chromatography paper.
Paper chromatography can be used to show the different components that make up black ink, usually a mixture of different coloured chemicals.
- With a ruler and pencil , draw a straight line across a rectangle of a chromatography paper 2\text{ cm} in from the short edge of the rectangle. Note, you must use a pencil for this as a line drawn in ink will dissolve in the solvent.
- Pour solvent into a beaker to a depth of 1\text{ cm} .
- Using a pipette drop a sample of ink on to the middle of the pencil line and draw a circle around it in pencil.
- Place the chromatography paper into the beaker so that the starting line is just above the solvent. Ensure that the ink spot does not fall below the solvent surface.
- Place a watch glass over the top of the beaker and let the solvent front move up the chromatography paper. When it is about 1\text{ cm} from the top of the paper, remove the paper from the beaker.
- Using a pencil , draw a line across the solvent front and circle the spots left by the separating ink.
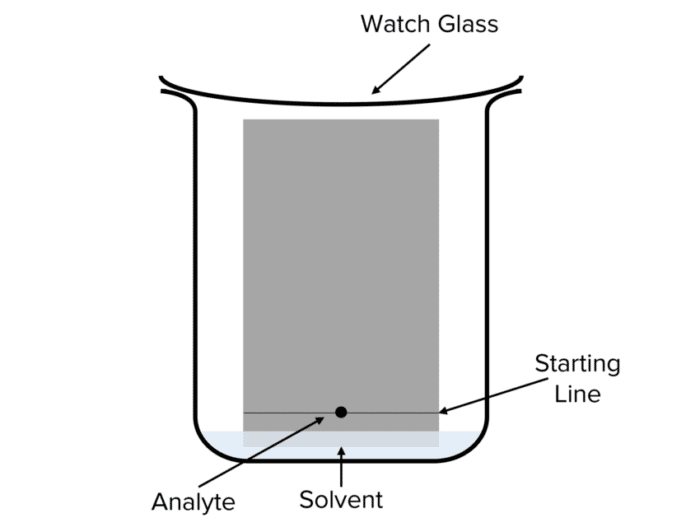
- Using a ruler, measure the distance between the starting line of the experiment and the solvent front .
- Measure the distances that each ink spot has traveled from the starting line .
- Calculate the \text{R}_f values for each spot by dividing the distance traveled by the spot by the distance traveled by the solvent front .
Note: when measuring the distance traveled by a spot across the paper, remember to measure from the centre of the spot.

Your 2024 Revision Partner
Chromatography Example Questions
Question 1 : Define the term ‘stationary phase’.
A polar solid substance through which the mobile phase moves .

Save your answers with

Gold Standard Education
Question 2: A student carries out a paper chromatography experiment. Below is an image of their setup:
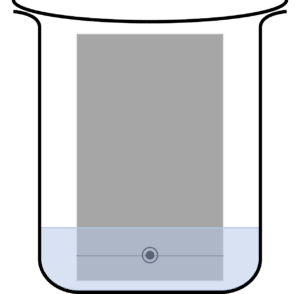
State an issue with the students set up.
Too much mobile phase has been added, submerging the ink spot .
This will cause the ink spot do dissolve into the mobile phase before the experiment is completed.
Question 3: In another experiment a student produces the following chromatography paper:
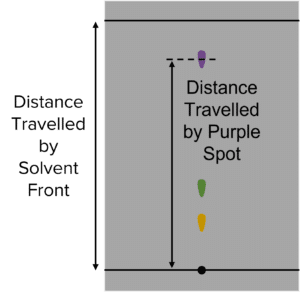
Calculate the \text{R}_f value of the purple spot.
\text{R}_f=\frac{\text{Distance Travelled by Purple Spot}}{\text{Distance Travelled by Mobile Phase}}\\\text{}\\=\frac{4.67}{5.23}\\\text{}\\=0.89
Chromatography Worksheet and Example Questions
Purity, Formulations and Chromatography Questions
Mme premium membership.
Learn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Where next?
Previous gcse chemistry topic, pure substances and formulations, gcse chemistry revision home, go back to the main topic list for gcse chemistry, next gcse chemistry topic, chemical tests.
By clicking continue and using our website you are consenting to our use of cookies in accordance with our Cookie Policy
MME Revision Challenge

Report a Question
You must be logged in to vote for this question..
Your personal data will be used to support your experience throughout this website, to manage access to your account, and for other purposes described in our privacy policy .
Paper Chromatography
Related Topics: More Lessons for IGCSE Chemistry Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry). Paper chromatography is a good way of separating coloured substances mixed together in a solution. During chromatography, the solvent soaks up the paper past the spot of the mixture and the different substances move upwards too. They move different distances depending on what kind of substance they are, what the solvent is and how far the solvent itself travels. The method allows us to compare an unknown substance with a known one. If two substances travel the same distance in the same experiment, they are probably the same substance. In this experiment, we will show how to separate the ink from different coloured markers using paper chromatography.
- Take a piece of chromatography paper. Draw a pencil line across it about 1 cm from one end and mark small crosses on the line.
- Using coloured markers, place small spots of colour on the crosses.
- Put water in the beaker to a depth of about 0.5 cm.
- Hang the chromatography paper in the beaker so that it dips into the water but with the spots of colour above the water level.
- Allow the water to rise up the paper until it is almost to the top.
- Remove the paper, mark where the water reached (solvent front) and hang it up to dry.
- When dry, measure the distances moved by the spots and the solvent front.
- Why should the line at the bottom of the paper be drawn in pencil?
- What would happen if the level of the solvent came above the level of the spots at the start?
- Why should the solvent not be run off the top of the paper?
- We use a pencil because it does not dissolve or smear when the solvent rises past the line.
- If the solvent started above the samples, the sample spots will simply dissolve and will not move up the paper.
- If the solvent ran off the top of the paper, we will not be able to measure the solvent front which is needed to calculate the R f values.
How to use paper chromatography to separate soluble substances and identify an unknown compound?

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
- Analytical Chemistry
Paper Chromatography
What is paper chromatography.
Chromatography technique that uses paper sheets or strips as the adsorbent being the stationary phase through which a solution is made to pass is called paper chromatography. It is an inexpensive method of separating dissolved chemical substances by their different migration rates across the sheets of paper. It is a powerful analytical tool that uses very small quantities of material. Paper chromatography was discovered by Synge and Martin in the year 1943.
Table of Contents
Paper chromatography principle, paper chromatography diagram, paper chromatography procedure, paper chromatography applications.
- Types of Paper Chromatography
- Frequently Asked Questions – FAQs
The principle involved can be partition chromatography or adsorption chromatography. Partition chromatography because the substances are partitioned or distributed between liquid phases. The two phases are water held in pores of the filter paper and the other phase is a mobile phase which passes through the paper. When the mobile phase moves, the separation of the mixture takes place. The compounds in the mixture separate themselves based on the differences in their affinity towards stationary and mobile phase solvents under the capillary action of pores in the paper. Adsorption chromatography between solid and liquid phases, wherein the solid surface of the paper is the stationary phase and the liquid phase is the mobile phase.
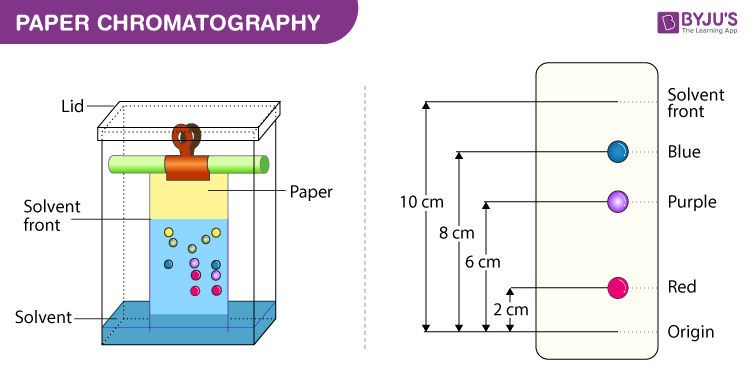
Below we have explained the procedure to conduct Paper Chromatography Experiment for easy understanding of students.
- Selecting a suitable type of development: It is decided based on the complexity of the solvent, paper, mixture, etc. Usually ascending type or radial paper chromatography is used as they are easy to perform. Also, it is easy to handle, the chromatogram obtained is faster and the process is less time-consuming.
- Selecting a suitable filter paper : Selection of filter paper is done based on the size of the pores and the sample quality.
- Prepare the sample: Sample preparation includes the dissolution of the sample in a suitable solvent (inert with the sample under analysis) used in making the mobile phase.
- Spot the sample on the paper: Samples should be spotted at a proper position on the paper by using a capillary tube.
- Chromatogram development: Chromatogram development is spotted by immersing the paper in the mobile phase. Due to the capillary action of paper, the mobile phase moves over the sample on the paper.
- Paper drying and compound detection : Once the chromatogram is developed, the paper is dried using an air drier. Also, detecting solution can be sprayed on the chromatogram developed paper and dried to identify the sample chromatogram spots.
There are various applications of paper chromatography . Some of the uses of Paper Chromatography in different fields are discussed below:
- To study the process of fermentation and ripening.
- To check the purity of pharmaceuticals.
- To inspect cosmetics.
- To detect the adulterants.
- To detect the contaminants in drinks and foods.
- To examine the reaction mixtures in biochemical laboratories.
- To determine dopes and drugs in humans and animals.
Types of paper chromatography:
- Ascending Paper Chromatography – The techniques goes with its name as the solvent moves in an upward direction.
- Descending Paper Chromatography – The movement of the flow of solvent due to gravitational pull and capillary action is downwards, hence the name descending paper chromatography.
- Ascending – Descending Paper Chromatography – In this version of paper chromatography, movement of solvent occurs in two directions after a particular point. Initially, the solvent travels upwards on the paper which is folded over a rod and after crossing the rod it continues with its travel in the downward direction.
- Radial or Circular Paper Chromatography – The sample is deposited at the centre of the circular filter paper. Once the spot is dried, the filter paper is tied horizontally on a Petri dish which contains the solvent.
- Two Dimensional Paper Chromatography – Substances which have the same r f values can be resolved with the help of two-dimensional paper chromatography.
Frequently Asked Questions – FAQs
What are the advantages of paper chromatography.
Paper Chromatography Has Many Benefits Simple and rapid Paper chromatography necessitates a minimal amount of quantitative material. Paper chromatography is less expensive than other chromatography methods. The paper chromatography method can identify both unknown inorganic and organic compounds. Paper chromatography takes up little space when compared to other analytical methods or equipment. Outstanding resolving power
Why water is not used in paper chromatography?
It is preferable to use a less polar solvent, such as ethanol, so that the non-polar compounds will travel up the paper while the polar compounds will stick to the paper, separating them.
What are the limitations of Paper Chromatography?
Limitations of Paper Chromatography are as follows- Paper chromatography cannot handle large amounts of sample. Paper chromatography is ineffective in quantitative analysis. Paper chromatography cannot separate complex mixtures. Less Accurate than HPLC or HPTLC
What is the importance of paper chromatography?
Paper chromatography has traditionally been used to analyse food colours in ice creams, sweets, drinks and beverages, jams and jellies. Only edible colours are permitted for use to ensure that no non-permitted colouring agents are added to the foods. This is where quantification and identification come into play.
Is paper chromatography partition or adsorption?
A type of partition chromatography is paper chromatography.
To learn more about the different types of paper chromatography from the experts, register with BYJU’S now!
Other important links:

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
It is so easy to understand by students Explained with applications also.
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- International
- Schools directory
- Resources Jobs Schools directory News Search

Chromatography
Subject: Chemistry
Age range: 11-14
Resource type: Worksheet/Activity
Last updated
12 October 2018
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

A detailed, engaging and informative lesson presentation (50 slides) and accompanying worksheets that looks at the commonly misunderstood topic of chromatography. This lesson goes through paper, thin-layer and gas chromatography so that students can analyse and interpret the results that would be found on a chromatogram. The lesson begins by challenging the students to recall details of this separation method when they met it at KS3. Students will meet the two chemical phases, mobile and stationary, and begin to understand that this method relies on the distribution of substances between these two phases. Students will meet the calculation for retention factor and be shown how to tackle questions on this topic before trying themselves. Time is taken to go over the details of gas chromatography, in a step by step guide format, as this is a poorly understood topic. There are progress checks throughout the lesson, which include mark schemes and detailed explanations, so that students can assess their understanding and address any misconceptions that could arise.
This lesson has been written for GCSE students but could be used with A-level students
Creative Commons "Sharealike"
Get this resource as part of a bundle and save up to 44%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Topic C1b: Elements, compounds and mixtures (Edexcel iGCSE Chemistry)
This bundle of 7 lessons covers the majority of the content in Topic C1b (Elements, compounds and mixtures) of the Edexcel iGCSE Chemistry specification. The topics and specification points covered within these lessons include: * Understand how to classify a substance as an element, compound and mixture * Understand that a pure substance has a fixed melting and boiling point * Separating mixtures by simple distillation * Separating mixtures by fractional distillation * Separating mixtures by filtration and crystallisation * Separating mixtures by paper chromatography * Interpreting and analysing chromatograms All of these lesson presentations and accompanying resources are detailed and engaging and contain regular progress checks to allow the students to constantly assess their understanding.
Topic C2: Elements, compounds and mixtures (OCR Gateway A GCSE Chemistry)
This bundle of 19 lessons covers the majority of the content in Topic C2 (Elements, compounds and mixtures) of the OCR Gateway A GCSE Chemistry specification. The topics covered within these lessons include: Relative formula mass Empirical formula Pure and impure substances Filtration and crystallisation Distillation Chromatography Metals and non metals Electronic structure Forming ions Ionic compounds Simple molecules Giant covalent structures Polymer molecules Metallic bonding Allotropes of carbon Graphene and the fullerenes Changing state Nanoparticles All of these lesson presentations and accompanying resources are detailed and engaging and contain regular progress checks to allow the students to constantly assess their understanding.
Topic C2: Elements, compounds and mixtures (OCR Gateway A GCSE Combined Science)
This bundle of 18 lessons covers the majority of the content in Topic C2 (Elements, compounds and mixtures) of the OCR Gateway A GCSE Combined Science specification. The topics covered within these lessons include: Elements Electron configurations Compounds Chemical formula of ionic compounds Ionic compounds Covalent substances Simple molecules Polymers Metallic bonding Diamond and graphite Graphene and the fullerenes Changing states Pure and impure substances Distillation Filtration and crystallisation Chromatography Interpreting chromatograms Relative formula masses Empirical formula All of these lesson presentations and accompanying resources are detailed and engaging and contain regular progress checks to allow the students to constantly assess their understanding.
Topic C1: Atomic structure and the Periodic Table (AQA Trilogy GCSE Combined Science)
This bundle of 16 lessons covers the majority of the content in Topic C1 (Atomic structure and the Periodic Table) of the AQA Trilogy GCSE Combined Science specification. The topics covered within these lessons include: Atoms Elements Compounds Chemical equations Chromatography Separation methods Development of the atomic model Electronic structure Development of the Periodic Table Metals and non-metals The alkali metals The halogens The Noble gases All of these lesson presentations and accompanying resources are detailed and engaging and contain regular progress checks to allow the students to constantly assess their understanding.
Topic C2.1: Purity and separating mixtures (OCR Gateway A GCSE Combined Science)
This bundle of 10 lessons covers all of the content in the sub-topic C2.1 (Purity and separating mixtures) of the OCR Gateway A GCSE Combined Science specification. The topics covered within these lessons include: * Explain what is meant by the purity of a substance and use melting point to distinguish pure from impure * Calculate the relative formula mass separately and in a balanced symbol equation * Deduce the empirical formula of a compound * Explain that many useful materials are formulations of mixtures * Describe and explain the processes of filtration, crystallisation, simple distillation and fractional distillation * Describe the processes of paper and thin-layer chromatography * Recall that chromatography involves a mobile and stationary phase * Interpret chromatograms All of these lesson presentations and accompanying resources are detailed and engaging and contain regular progress checks to allow the students to constantly assess their understanding.
Topic C2: Experimental techniques (Cambridge iGCSE Science Double Award)
This bundle of 5 lessons covers the majority of the content in Topic C2 (Experimental techniques) of the core and supplement sections of the Cambridge iGCSE Science Double Award specification. The topics and specification points covered within these lessons include: Understand the use of paper chromatography Interpreting paper chromatograms Pure and impure substances Separation methods including filtration, crystallisation, distillation, fractional distillation and paper chromatography All of these lesson presentations and accompanying resources are detailed and engaging and contain regular progress checks to allow the students to constantly assess their understanding
Topic C2: States of matter and mixtures (Edexcel GCSE Combined Science & Chemistry)
This bundle of 6 lessons covers the majority of the content in Topic C2 (States of matter and mixtures) of the Edexcel GCSE Combined Science & GCSE Chemistry specifications. The topics covered within these lessons include: Particle arrangement in the states of matter Physical and chemical changes Pure and impure substances Separation methods Paper chromatography Interpreting a chromatogram All of these lesson presentations and accompanying resources are detailed and engaging and contain regular progress checks to allow the students to constantly assess their understanding.
Topic C4.2: Identifying the products of chemical reactions (OCR Gateway A GCSE Chemistry)
This bundle of 4 lessons covers all of the content in the sub-topic C4.2 (Identifying the products of chemical reactions) of the OCR Gateway A GCSE Chemistry specification. The topics covered within these lessons include: Detecting gases Detecting cations Detecting anions Instrumental methods of analysis All of these lesson presentations and accompanying resources are detailed and engaging and contain regular progress checks to allow the students to constantly assess their understanding.
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
roystonalfie
Just what I was looking for, thanks for sharing and for free
GJHeducation
Empty reply does not make any sense for the end user
Veronicaconway
Thank you made it very easy to teach, very good resource.
Fantastic resource - really clear and broken down perfectly in order to guide students through the understanding of the three types of chromatography. Thank you!!
Excellent afl throughout the lesson
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:
Chromatography
Loading ad...
Cikgu_Husna_
CHromatography
- Google Classroom
- Microsoft Teams
- Download PDF

COMMENTS
It links chromatography with particle theory and develops the tools of analogy and modelling. ... Chromatography worksheet PDF, Size 0.12 mb; No comments. Level. 11-14 years; 14-16 years; Use. Handout; Download; Category. ... 2.10 Interpret a paper chromatogram: to distinguish between pure and impure substances; to identify substances by ...
Paper Chromatography. This technique is used to separate substances that have different solubilities in a given solvent (e.g. different coloured inks that have been mixed to make black ink) A pencil line is drawn on chromatography paper and spots of the sample are placed on it. Pencil is used for this as ink would run into the chromatogram ...
Figure 1: Completed paper chromatography containing only 1 dye. In this experiment, students will measure the values of several dyes in 3 different solvent systems. Students will also analyze an unknown mixture of dyes in order to identify the dyes present in the mixture. The three different solvent systems are 1) laboratory water, 2) an ...
More resources. Investigate the separation of inks using the Paper chromatography practical video with full teacher and technician notes, student worksheets and PowerPoint slides to support and consolidate live practical work.; Master your teaching of paper chromatography with these five teacher-tested tips and build on learners' prior knowledge from 11-14.
4. Place the chromatography paper (aka. Paper towel) inside the cup and make sure that the solvent Does Not reach where the ink is (there should be a gap between ink and solvent). Also, make sure that the chromatography paper doesn't touch the bottom of the cup. Tape the other end of chromatography paper to a pencil and place it on the cup. 5.
Paper chromatography is a simple and cost-effective separation technique that separates and identifies different components in a mixture. [1-4] Principle. In paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. The mixture to be analyzed is applied to the paper.
Chromatography Lab activity — Worksheet. 1. Procedure. 1. Take the strips of chromatography paper and fold both pieces about an inch from the top. 2. Using the black Sharpie, draw a horizontal line near the other end of the chromatography paper. Make the line about as high off the bottom as the length of your thumb. 3.
This Chemistry Worksheet comes with Answers and covers Paper Chromatography in Chemical Analysis. Key words include: Solvent, Solute, Stationary Phase, Mobile, Baseline, Inks, Mixtures, Dyes, Rf Value, Insoluble and Purity.Who is this for?This homework is intended for upper middle school and high school students.Files 1 worksheet 1 answer sheetThese worksheets come as 2 separate PDF files in a ...
Chromatography Worksheet. 1) Do you think that chromatography would be useful for separating a very large quantity of a mixture? Explain why or why not. 2) If the mobile phase in a chromatographic experiment moved 15 centimeters and the Rf value of one of the compounds in the mixture was 0.85, how far would the compound move on the paper?
Activity Guide: Chromatography Museum Connection: Diorama halls have excellent examples of art work. Overview of activity: In this activity, we will learn about paper chromatography and along the way we'll also make a chromatography "flower" Grade Level: Grades 1-5 Main Idea: Understand that chromatography is the chemistry of taking things apart through the
pdf, 309.58 KB. A revision homework or class worksheet with answers that covers Paper Chromatography in C8 GCSE Chemistry. Topics include Practical Methods, Apparatus/Equipment Setup, Separation, Solvents, Rf Value Calculations and Mobile/Stationary Phase with a variety of questions in a PDF format. Get 20% off this resource with the discount ...
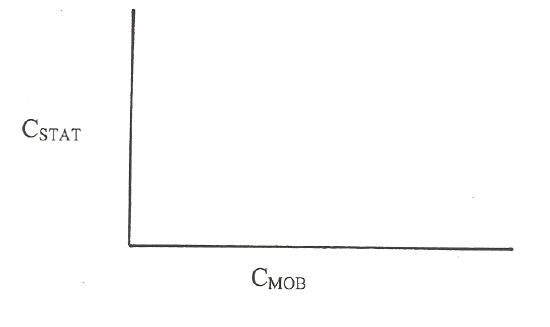
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help. Consider a plot that has the concentration of analyte in the stationary phase on the Y-axis and the concentration of analyte in the mobile phase on the X-axis.
Hand out the worksheets. Take the strips of chromatography paper and fold both pieces about an inch from the top. Use a black Sharpie to draw a horizontal line near the other end of the chromatography paper. Make the line about as high off the bottom as the width of your thumb.
Student worksheet 04SW Chromatography Page 1 of 2 Chromatography 1. Use some of the following information and what you know about particles to put together an explanation as to why different colour dyes travel different distances on chromatography paper. Here the term 'sticky' refers to how strongly the dye particles stick to the paper. a.
Paper chromatography is a type of chromatography that is often used to quickly analyse mixtures in chemistry. This method employs chromatography paper as the stationary phase and a solvent as the mobile phase.During paper chromatography the solvent soaks into the chromatography paper creating a line called the solvent front.This solvent front moves up the length of the chromatography paper ...
Generic Simple Paper Chromatography Worksheet. A double sided worksheet which can be used for most simple paper chromatography experiments, including food dyes, inks, pens, amino acids, plant pigments etc. There are spaces for: an apparatus / equipment list, a labelled diagram (beaker / jar only so students can customise according to the ...
Method. Take a piece of chromatography paper. Draw a pencil line across it about 1 cm from one end and mark small crosses on the line. Using coloured markers, place small spots of colour on the crosses. Put water in the beaker to a depth of about 0.5 cm. Hang the chromatography paper in the beaker so that it dips into the water but with the ...
All chromatography techniques use two phases called the mobile phase and the stationary phase In paper chromatography: The mobile phase is the solvent in which the sample molecules can move, which in paper chromatography is liquid e.g. water or ethanol The stationary phase in paper chromatography is the actual chromatography paper itself The substances which are more soluble in the solvent ...
Paper Chromatography Principle. The principle involved can be partition chromatography or adsorption chromatography. Partition chromatography because the substances are partitioned or distributed between liquid phases. The two phases are water held in pores of the filter paper and the other phase is a mobile phase which passes through the paper.
Paper chromatography bundle. A bundle of 4 resources suitable for the new AQA GCSE Chemistry syllabus. Resources on paper chromatography include a knowledge organiser containing all the facts on chromatography that students need to know, a differentiated 1-9 worksheet featuring exam style questions and a plenary chromatography traffic light quiz (useful for assessment for learning at the end ...
Liveworksheets transforms your traditional printable worksheets into self-correcting interactive exercises that the students can do online and send to the teacher. ... Main content: Chromatography (1899320) Chromatography. Loading ad... Share / Print Worksheet. Google Classroom Microsoft Teams Facebook Pinterest Twitter ...
Chromatography. A detailed, engaging and informative lesson presentation (50 slides) and accompanying worksheets that looks at the commonly misunderstood topic of chromatography. This lesson goes through paper, thin-layer and gas chromatography so that students can analyse and interpret the results that would be found on a chromatogram.
CHromatography Liveworksheets transforms your traditional printable worksheets into self-correcting interactive exercises that the students can do online and send to the teacher. Chromatography worksheet | Live Worksheets