- Open access
- Published: 10 October 2019

Food sources, energy and nutrient intakes of adults: 2013 Philippines National Nutrition Survey
- Imelda Angeles-Agdeppa ORCID: orcid.org/0000-0001-9132-7399 1 ,
- Liya Denney 3 ,
- Keith V. Tanda 1 ,
- Royce Ann D. Octavio 1 ,
- Alicia Carriquiry 4 &
- Mario V. Capanzana 1
Nutrition Journal volume 18 , Article number: 59 ( 2019 ) Cite this article
34k Accesses
21 Citations
1 Altmetric
Metrics details
Comprehensive assessment of dietary intakes of foods and nutrients in Filipino adults are lacking. This study evaluated energy and nutrient intakes and food sources of key nutrients consumed by Filipino adults.
The participants were from the 2013 National Nutrition Survey wherein food intake of young adults aged 19–49 years ( n = 12,896) and older adults aged 50 years and above ( n = 7853) were collected using 24-h recalls. Usual nutrient intakes were estimated using PC-SIDE program. The Philippines Dietary Reference Intakes were used to calculate proportions of inadequate intake using Estimated Average Requirement (EAR) and Acceptable Macronutrient Distribution Ranges (AMDR). Energy adequacy was evaluated using the Institute of Medicine (IOM) equation for Estimated Energy Requirements (EER).
The nutrient intakes with the highest prevalence of inadequacy (> 50%) were: iron (97–99%), vitamin C (96–98%), calcium (95–98%), riboflavin (86–91%), folate (89–90%), thiamine (73–89%), energy (67–70%), total fat (55–67%), and vitamin A (54–56%). Refined rice, pork and breads contributed most to daily intakes of energy, protein, carbohydrates, thiamine, riboflavin, and iron. Low intake of vegetables, fruits and dairy was common in both age groups.
Conclusions
This study demonstrated that intakes of many nutrients were markedly inadequate among adults in the Philippines, due to the rice-dominant dietary pattern with few nutrient-dense foods. These results can be used to support the development of specific interventions to improve the shortfalls in nutrient intakes.
Peer Review reports
Suboptimal diet is associated with a range of non-communicable diseases (NCD), and it is potentially a major contributor to NCD mortality worldwide [ 1 ]. In the Philippines, dietary risk is the top risk factor for diseases and is estimated to account for 10.6% of total disability-adjusted life-years [ 2 ]. At present, the Philippines hosts the world’s 12th largest population of about 100 million people, among which 7.3% are aged above 60 years. This percentage is expected to double by 2050 with a predicted increase in life expectancy and decrease in fertility rate [ 3 ]. However, despite being one of the fastest growing economies in Asia, one out of 10 adults suffers from chronic energy deficiency, and a high proportion (70–80%) of adults is not meeting dietary requirements for many key micronutrients [ 4 , 5 , 6 ]. In addition, the prevalence of anemia among older adults is 24% [ 7 ]. On the other hand, similar to what happened in many other developing countries in Asia, Africa, and Latin America [ 8 , 9 ], the Philippines is also experiencing double burden of malnutrition. Three out of 10 adults are overweight or obese [ 4 ], and the number of mortality and disability caused by ischemic heart disease, stroke, diabetes and chronic kidney disease has increased by more than 25% in the past decade [ 10 ].
The aforementioned nutritional issues increase one’s susceptibility particularly among the elderly to various diseases such as fractures, muscle loss, poorer immunity against infections, and other NCDs [ 11 , 12 ]. Nutrition-based intervention strategies are one of the key solutions to improve the health status and quality of life of adult population in the Philippines.
In addition, dietary choices could differ within a population under multiple influences, such as age, gender and social economic status (SES). For instance, younger adults might embrace the nutrition transition towards a more “westernized” diet more than the older adults; and gender has an important impact on the social and biological determinants of health consequences, hence different nutritional needs [ 13 ]. Identifying such needs and differences is crucial to construct nutritional guidelines and solutions that are tailored to different population groups.
The Food and Nutrition Research Institute (FNRI) in the Philippines conducts National Nutrition Surveys (NNS) every 5 years, which are nationally representative and capture the food and beverage consumption of the Filipino population. However, the existing food composition table (FCT) developed in 1997 only included 12 nutrients, thus limiting the nutrient intakes data being reported. In addition, studies on nutrient intakes of Filipino adults by other researchers are limited both in terms of nutrients coverage and population representativeness. In an attempt to comprehensively characterize the diet of Filipino adults, this study expanded the existing FCT from 12 to 27 nutrients, with which we evaluated the usual intakes of energy and nutrients of adults using data from the 2013 NNS. In addition, the influences of age, gender and SES on nutrient intakes and food sources of key nutrients among adults were also investigated in this study.
Study design and population
This study used the data from the 2013 NNS. This is a cross-sectional, population-based survey that characterizes the health and nutritional status, foods consumption and dietary patterns of the Filipino population. The survey used a multi-staged stratified sampling design to represent all 80 provinces of the country covering both urban and rural areas. A total of 8592 sample households were selected from the NNS for the dietary survey with a response rate of 87.7%. Briefly, 20,749 adults were used in this study, comprising specifically 12,896 aged 19–49 years old representing young adults and 7853 aged 50 years and over representing older adults. The age groups are aligned with the Philippine Dietary Reference Intake age grouping (PDRI, 2015). The Ethics Committee of FNRI approved the survey protocol. All surveyed households provided written informed consent prior to participation.
Data collection
Demographic and socio-economic data.
Demographic and socio-economic information were collected from the 2013 NNS survey participants, including age, gender, and area of residence, marital status, education, and the body mass index (BMI). Wealth status of participants was defined by proxy indicators including household possession of vehicles, appliances, materials used for housing construction and sanitation facilities. Scores obtained from principal component analysis were used to define wealth quintiles as poorest, poor, middle, rich and richest. Chronic energy deficiency, overweight and obesity were determined using World Health Organization (WHO) definition [ 14 ].
Dietary data
Twenty four hours dietary recalls were conducted by registered nutritionist-dietitians through face-to-face interviews in households using structured questionnaires. The interviewer recorded all foods and beverages consumed on the previous day from the moment when they woke up until they went to sleep in the evening. The amount of foods and beverages consumed was estimated using household measures (cups, tablespoons and pieces) or through weighting of food samples. The weights of foods were converted to as purchased values using a portion to weight list for common foods compiled by FNRI. If the food was a dish, the interviewee was asked to describe the ingredients of the recipe or name the dish or recipe. The nutrient content of these composite foods were determined by breaking down the different ingredients in the recipe and each was calculated based on INFOODS Guidelines.
A first 24 h recall was collected in all members of all sampled households; and to estimate the day-to-day within-person variability in energy and nutrient intake, a second 24 h recall was carried out among members in 50% of randomly selected households. The repeated 24 h recalls were obtained on non-consecutive days to avoid correlation in nutrient intakes on consecutive days [ 15 ]. The values for the two 24 h recalls were averaged for each person to derive their usual intakes. For the remaining 50% of the respondents with only one 24 h recall, their 1 day recall data were unbiased estimate of their usual intake assuming the measurement error is additive [ 16 ].
Data processing
The estimation of energy and nutrients contents of foods consumed was done through the FNRI-Individual Dietary Evaluation System (IDES) which contains the expanded FCT developed from this project. The FCT was expanded from the original 12 nutrients to 27 nutrients, and it is the first time that these 27 nutrients were analyzed in a nationally representative Filipino population. Details about the development of the expanded FCT will be reported in another paper.
Implausible values of energy and nutrient intakes were identified by a process described below. For the evaluation of energy intake, Estimated Energy Requirement (EER) was calculated for each individual using the Institute of Medicine (IOM) equation [ 17 ] considering age, sex, body weight, height, and physical activity level (PAL) using the WHO STEP instrument [ 18 ]. The ratio of self-reported daily energy intake to the EER was then calculated for each person and each day of reporting. The calculated ratios were then transformed to the logarithmic scale and outliers below and above 3 SDs away from the mean were excluded [ 19 ]. Five hundred fifteen subjects were excluded from this exercise. For micronutrients, excessive intakes were defined as those that exceeded 1.5 times of the 99th percentile of the observed intake distribution in the respective age group. Intakes above this upper limit were substituted by a random value generated from a uniform distribution in the interval with lower bound equal to the 95th percentile of observed intake and an upper bound equal to 1.5 times of the 99th percentile [ 19 ].
To investigate the food sources of energy and nutrients, a list of 87 food groups under 9 major categories (Table 1 ) was created in a similar format to the food categories published by United Nations Food and Agriculture Organization (FAO) [ 20 ] and United States Department of Agriculture (USDA) [ 21 ], while reflecting Filipinos’ frequently consumed foods and traditional way of consumption. All foods, including those less consumed foods, were considered in the analysis.
Statistical analysis
Mean and usual intake distributions of energy and nutrients were estimated using the PC-SIDE software (Software for Intake Distribution Estimation version 1.0, Iowa State University, IA, USA) [ 22 ]. This method developed by Iowa State University could account for the within-person variability of daily intakes across different days, and therefore only reflecting the between-person variability [ 16 ]. To determine if the mean differences of usual nutrient intakes across different age and gender subgroups were statistically significant, Analysis of Covariance (ANCOVA) was used with adjustment for total energy intake.
PDRI was used to evaluate nutrient inadequacies [ 23 ]. Where applicable, the prevalence of inadequacy in a group is estimated as the proportion of individuals with usual intakes below the Estimated Average Requirement (EAR), using the EAR cut-point method [ 24 ]. Due to a skewed distribution of iron intake, a probability approach was used instead to assess the prevalence of inadequate iron intake: the risk of inadequacy of each individual was computed first, and the prevalence of inadequate intake was estimated as the average risk of inadequacy [ 25 ]. Intakes of carbohydrates, fat, and protein were evaluated as percentage of total energy intake, and inadequacy or excessive intake was classified as less than the lower limit or higher than the upper limit of the Acceptable Macronutrient Distribution Ranges (AMDR). Additional file 1 : Table S1 summarizes the EAR and AMDR benchmarks used in this study. Assessment of nutrient adequacy was also computed by gender, age groups, and wealth quintiles, and hypothesis testing comparing two population proportions was used to test the differences in prevalence of inadequacies across various subgroups.
Stata (Stata Statistical Software: Release 15. StataCorp, TX, USA) was used for data management, calculation of summary statistics, and statistical tests of differences. A p -value of < 0.05 was considered significant in all statistical tests. Survey weights were applied in all datasets and calculations to represent national estimates through the complex survey design.
Demographic and socio-economic characteristics, and nutritional status of the study population
Table 2 summarizes the demographic and socio-economic characteristics of the two age groups. Among the young adults and the older adults, respectively 53.8 and 45.1% were males. Approximately half of the study population resided in urban areas, and they were approximately equally distributed across the 5 wealth quintiles. Half of the older adults (51.0%) only attained elementary education, while majority of the younger adults completed high school or higher education (74.8%). The prevalence of chronic energy deficiency among young and older adults was 10.4 and 15.5% respectively, while 27.7 and 28.5% were overweight/obese.
Energy and macronutrient intakes
Table 3 summarizes the mean usual intakes of energy and nutrients by age and gender subgroups. The mean usual energy intake (mean ± standard error) was 1828 ± 6 kcal/day (young adults) and 1527 ± 6 kcal/day (older adults), which was 30.2 and 33.5% lower than the mean estimated EER of 2620 ± 4 kcal/day and 2297 ± 5 kcal/day respectively.
Overall, younger adults consumed significantly more energy and most of the macronutrients than the older adults with the exception of carbohydrates. Males consumed significantly higher energy and many nutrients than females within both age groups. It is also worth noting that the mean consumption of dietary fiber, ranged from 7.6–10.1 g, is far below the recommended nutrient intake of 20–25 g/day for adults.
When examined as percentage of total energy, fat, protein, and carbohydrates contributed to 12.4–16.3%, 13.2–13.5%, and 70.3–73.2% of daily energy intake, respectively. Comparing against the AMDR recommendations, 55–67% of the study population did not consume adequate fat (Table 4 ). The prevalence of inadequate fat intake was significantly higher in older adults, among males (Table 4 ), and in poor and poorest wealth quintiles (Table 5 ).
Protein intake was also evaluated with the EAR in g/day. Unlike when comparing with AMDR, a high prevalence of inadequacy was observed across all age and gender groups, with a more serious situation for older adults, females (Table 4 ), and in poor and poorest wealth quintiles (Table 5 ).
Micronutrient intakes
High prevalence of inadequate micronutrient intakes were found for iron (97–99%), vitamin C (96–98%), calcium (95–98%), folate (89–90%), riboflavin (86–91%), thiamine (73–89%), and vitamin A (54–66%) (Table 4 ). For micronutrients with no established EAR recommendations, including vitamin D, vitamin E, magnesium and potassium, the mean intakes were also far from the adequate intakes.
On average, mean usual intakes of most vitamins and minerals were siginificantly higher in young adults than in older adults. A differing result was observed for vitamin C as the average intake of older adults is higher than that of younger adults, though both were far below the EAsR of 52–60 mg/d. In both age groups, the mean consumption of male adults for most vitamins and minerals was significantly higher than females (Table 3 ).
Corresponding to the differences observed in mean usual intakes, the prevalence of inadequacy increases significantly with age for many micronutrients, in particular thiamine, niacin, vitamin A, vitamin B6, vitamin B12, phosphorus, and zinc. Also, females might be at higher risk of inadequacy for thiamine, niacin, vitamin A, vitamin B6, vitamin B12, folate, iron, calcium and phosphorus than males in both age groups, while males might be at higher risk for vitamin C and zinc inadequacy (Table 4 ).
Higher prevalence of inadequacy was observed among the poorest group for most micronutrients. It is worth noting that the prevalence of inadequacy in vitamin C, folate, iron and calcium remained high across the wealth quintiles, and that more than 50% of adults did not consume adequate vitamin C, folate, riboflavin, thiamine, vitamin A (only for older adults), iron and calcium even in the highest wealth index group (Table 5 ).
Consumption rate and mean consumption per capita of major food groups
Grains, meat and proteins, sweets and vegetables were the top 4 major food groups consumed in both age groups in terms of consumption rate as well as mean intake per capita (Table 6 ). Grains, mainly refined rice, played a dominant role in Filipino’s diet (mean per capita 290.8–350.4 g/day). Only less than 25% of adults consumed fruit, and even fewer consumed milk (9.3–13.4%). The mean consumption per capita of vegetables (66.4–70.1 g/day), fruit (24.4–29.7 g/day) and milk (2.8–3.2 g/day) was far from the recommended 3 servings/day of vegetables, 2–3 servings/day of fruit, and 1 glass/day of milk [ 26 ], and this could explain partially the high prevalence of nutrient inadequacies.
Food sources of energy and nutrients
Figures 1 and 2 depict percentage contribution of the 9 major food groups to energy, macronutrients, and micronutrients with high prevalence of inadequacy (> 50%). The top 3 major food sources of energy were grains (68.8–69.7%), meats and other protein-rich foods (13.3–15.5%), and sweets (7.5–7.6%).
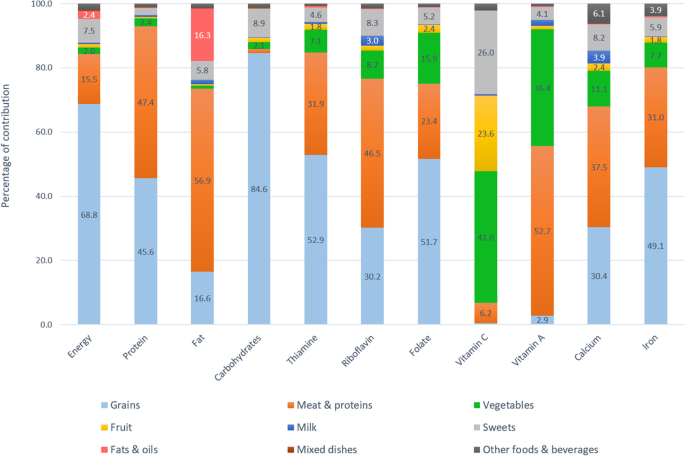
Food sources of energy and key nutrients among adults aged 19 to 49 years
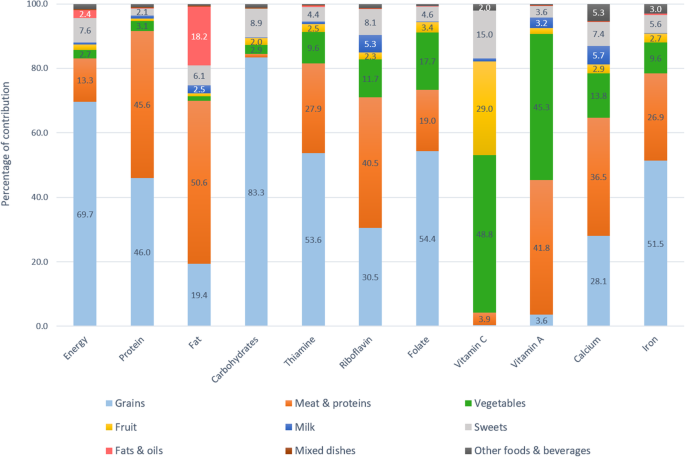
Food sources of energy and key nutrients among older adults aged 50 years and above
Grains contributed nearly 70% of daily energy, more than 80% of carbohydrates, half of thiamine, folate, iron, and protein, and one third of calcium and riboflavin. Meat and other protein-rich foods were the source of half of fat, protein, vitamin A, and riboflavin intakes, one third of thiamine, calcium, and iron intakes, and one fifth of folate intake. Vegetables contributed 40–50% of vitamin C, 40% of vitamin A, and less than 20% of folate, calcium, and 10% of riboflavin, thiamine, and iron. Approximately 20–30% of vitamin C was from fruits and a similar percentage from sweet foods, mainly from fruit-based beverages and fortified sweetened beverages. Milk only contributed 0.4–0.7% of total energy intake due to the very low consumption rate in this population, but being a nutrient-dense food, milk still contributed 3.9–5.7% of calcium, 3–5.3% of riboflavin, and 1.9–3.2% of vitamin A. Fats and oils contributed less than 20% of total fat intake, and minimal amounts to other nutrients. Mixed dishes and other foods and beverages played a little role in the energy and nutrient intakes. The top major sources of energy and nutrients are also available (see Additional file 2 : Table S2, Additional file 3 : Table S3 and Additional file 4 : Table S4.
Our study investigated the usual intakes of energy and nutrients, and their primary food sources among young and older adults in the Philippines. To our knowledge, this is the first study to provide a comprehensive overview of the dietary intakes of 27 macro- and micro-nutrients among Filipino adults with a nationally representative population sample. Our findings provided important insights on the sub-optimal dietary patterns of Filipino adults, and as a result, the large shortfalls of intakes in many nutrients.
The mean energy intake of both young and older adults was approximately 30% lower than the recommended intake, which corroborates with the observation, that 10–15% of them suffer from chronic energy deficiency. In addition, a substantial decline with age in daily energy intakes was observed, which is consistent with many other populations [ 27 , 28 ]. The energy needs decrease as people age, possibly attributable to the decrease in muscle mass, physical activity level, and overall basal metabolic rate [ 28 , 29 ]. On the other hand, the ageing process could mean reduced ability to absorb and metabolize certain nutrients [ 30 ]. It is therefore important for older adults to consume more nutrient-dense foods in order to fulfill their nutritional needs.
The contribution of carbohydrates and protein to energy intake were within the AMDR. However, It is worth noting that the AMDR reference ranges for protein used in Philippines is 10–15% of total energy intake, which is much narrower towards the lower boundary as compared with 10–35% used by the Institute of Medicine [ 17 ]. In addition, the fact that about half of the Filipino adults’ daily protein intake was from grains, mainly refined white rice, suggested a poor quality of dietary protein [ 31 ]. The development of EAR for protein in PDRI has taken into consideration the protein quality in Filipino rice-based diet [ 32 ], and when compared with the protein EAR, 42–62% of adults did not meet the recommendation. On the other hand, the low contribution of fats to energy may pose certain problems on the absorption and utilization of fat-soluble vitamins.
A high prevalence of inadequacy was also observed for many vitamins and minerals crucial for adults’ optimal health: 50–99% of adults did not eat adequate vitamin C, calcium, iron, folate, riboflavin, thiamine, vitamin A, and vitamin B6 (only among older adults), while 25–50% of adults did not meet the EAR for vitamin B6 (young adults), zinc and phosphorus (older adults). In addition, population mean intakes of fiber, vitamin D, vitamin E, magnesium and potassium were far below the adequate intakes. These findings are in general consistent with previous reports in Filipino adults using different dietary intake assessment methods [ 33 , 34 , 35 ]. Compared to the previous NNS conducted in 2008, there was little improvement in the nutritional inadequacies [ 35 ]. Nutrients all play different, yet pivotal roles in the body, and insufficient intakes could increase one’s susceptibility to various diseases. The inadequacy of blood-forming nutrients such as folate, vitamin B6, vitamin B12, and iron may lead to higher susceptibility to anemia [ 36 , 37 , 38 ], increased coronary heart disease risk [ 39 ] or poor cognitive outcomes in older adults [ 40 , 41 ]. Moreover, inadequacy of calcium and vitamin D may increase the risk of osteoporosis and frailty in old age [ 42 , 43 ]. Older people may be more vulnerable to calcium and vitamin D deficiency due to poorer absorption of calcium, reduced vitamin D synthesis in the skin, and decreased ability of the kidney to convert vitamin D to its active form [ 44 ]. The markedly high prevalence of calcium inadequacy (95–98%) in our study population could be explained by the very low intake of dairy products. Many tropical countries still report a considerable proportion of the population having insufficient vitamin D levels due to more time spent indoors and less sunlight exposure [ 45 , 46 , 47 ]. Food fortification with vitamin D has been proposed considering that the natural food sources of vitamin D are not commonly consumed in the studied population [ 43 ].
The Filipino diet is of limited diversity wherein white rice, pork and breads contributed most to daily intake of energy, protein, carbohydrates, thiamine, riboflavin, and iron. Many nutrient-dense food groups such as vegetables, fruit, and dairy were seriously lacking in the diet. Although vegetables and fruits were the top two food sources for vitamin C and folate, less than 70% of the population consumed vegetables daily, and even fewer (less than 25%) consumed fruit, and the amount of consumption was not sufficient to support adults’ nutrition needs. Dairy foods, with only 0.4–0.7% of energy contribution, were the source of 3.9–5.7% of dietary calcium, 3–5.3% of riboflavin, and 1.9–3.2% of vitamin A. Increasing dairy consumption could improve the dietary intake of these key nutrients.
This study also investigated the nutrient intake status across various population subgroups including age, gender, and SES. In general, the prevalence of inadequacy increases with age for most nutrients, in particular thiamine, niacin, vitamin A, vitamin B6, vitamin B12, phosphorus, and zinc. This is due to not only overall reduced food consumption as people age, but also increased nutritional needs because of poorer absorption and metabolism [ 30 ]. Also, in both age groups, females are at higher risk of inadequate for thiamine, niacin, vitamin A, vitamin B6, vitamin B12, folate, iron, calcium and phosphorus than males, while males are at higher risk for vitamin C and zinc inadequacy. Although it has been observed in many developed countries that women were more likely to engage in healthy living and healthy dietary choices [ 48 , 49 ], studies conducted in developing countries as with our study generally reported better nutritional status among the males than females, likely because of the gender differences in social and economic aspects [ 15 ]. Lastly, it was observed that the prevalence of inadequate nutrient intake decreases as wealth status progresses, which was also observed in previous studies [ 50 , 51 ]. However, increasing SES does not necessarily mean better nutritional status [ 52 ]. As demonstrated in the present study, inadequate intake of many key nutrients such as vitamin C, iron, calcium, folate and protein remained high even among the richest wealth quintile. Such inadequacies are likely due to the population-wide dietary pattern with low consumption of nutrient-dense foods including fruit, vegetables, and milk. These results demonstrate that overall nutrient intake and dietary diversity need to be improved, with a special focus on interventions for the elderly, females, and those in low SES and food insecure.
This study has provided a comprehensive summary of the dietary intakes and nutritional status of Filipino adults and older adults. The use of mean intakes provided a general overview of nutrient intake levels of the population, while the EAR cut-point method with the national representative sample allowed an estimate of the prevalence of the population with inadequacy intakes. Detailed segmentation of the studied sample by age, gender and SES is instrumental in constructing future tailored nutritional solutions to meet the needs of specific subgroups of the population. However, our study also has several methodological limitations. Firstly, the use of 24-h recalls to collect dietary intake data relies on the participants’ ability to accurately recall the foods consumed and estimate the portion sizes of consumption. Secondly, information on use of dietary supplements was not captured in this study, which could under-estimate the nutrient intakes. Thirdly, the construction of the Filipino FCT involved matching similar food items with established databases such as USDA, while in reality, the nutritional content could be different for similar foods, due to different breed cultivars, climate conditions, mineral abundance in soil, and national food fortification policies. Therefore, the findings reported in this study could be subject to measurement errors, and it is warranted to, if possible, relate these dietary intake data with nutritional biomarkers and health conditions to facilitate better interpretation.
Our findings provided important insights to the dietary patterns of Filipino adults, and showed that marked nutrient inadequacies exist in the adult population, especially among older adults, females, and people from lower SES. The lack of dietary variety and nutritional quality could explain the large shortfalls of many nutrient intakes. A large proportion of energy intake was from foods with low nutrient density such as refined rice and sweets. Nutrient-dense foods such as vegetables, fruits, and dairy products being the least nutrient contributors as shown in the study, should be greatly encouraged to fulfill the nutritional gaps. Food fortification targeting nutrients that are commonly inadequate in the population should also be considered. Together, the findings can help to support the development of specific interventions to improve nutritional status especially among those more vulnerable to dietary inadequacies.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
Average Macronutrient Distribution Range
Analysis of Covariance
Body Mass Index
Dietary Folate Equivalent
Estimated Average Requirement
Estimated Energy Requirements
Food and Agriculture Organization
Food Composition Table
Food and Nutrition Research Institute
Individual Dietary Evaluation Systems
Institute of Medicine
Monounsaturated Fatty Acids
Niacin Equivalent
National Nutrition Survey
Physical Activity Level
Philippine Dietary Reference Intake
Polyunsaturated Fatty Acids
Retinol Equivalent
Social Economic Status
United States Department of Agriculture
World Health Organization
α-tocopherol equivalent
Global Burden of Disease 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393(10184):1958–72.
Article Google Scholar
Institute for Health Metrics and Evaluation. GBD profile: Philippines. In: Global burden of diseases, injuries, and risk factor s study 2010. Seattle; 2010. https://www.healthypeople.gov/2020/data-source/global-burden-of-diseases-injuries-and-risk-factors-study . Accessed on 22 Aug 2018.
United Nations Department of Economic and Social Affairs Population Division. World population prospects: The 2015 Revision, key findings and advance tables. Working Paper No. ESA/P/WP.241.2015. https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf . Accessed on 5 Dec 2018.
Department of Science and Technology- Food and Nutrition Research Institute (DOST-FNRI). The 8th National Nutrition Survey (2013) results - anthropometric survey; Philippines; 2013.
Google Scholar
Department of Science and Technology- Food and Nutrition Research Institute (DOST-FNRI). The 8th National Nutrition Survey (2013) results - dietary survey; Philippines; 2013.
Risonar MG, Rayco-Solon P, Ribaya-Mercado JD, Solon JA, Cabalda AB, Tengco LW, et al. Physical activity, energy requirements, and adequacy of dietary intakes of older persons in a rural Filipino community. Nutr J. 2009. https://doi.org/10.1186/1475-2891-8-19 .
Department of Science and Technology- Food and Nutrition Research Institute (DOST-FNRI). The 8th National Nutrition Survey (2013) results - biochemical survey; Philippines; 2013.
Haddad L, Cameron L, Barnett I. The double burden of malnutrition in SE Asia and the Pacific: priorities, policies and politics. Health Policy Plan. 2015;30:1193–206.
Steyn NP, Michiza ZJ. Obesity and the nutrition transition in sub-Saharan African. N Y Acad Sci, Ann. 2014;1311:88–101.
Article CAS Google Scholar
Institute for Health Metrics and Evaluation. Global Burden of Disease (GBD) Country Profiles – Philippines. http://www.healthdata.org/sites/default/files/files/country_profiles/GBD/ihme_gbd_country_report_philippines.pdf . Accessed on 22 Aug 2018.
Eggersdorfer M, Akobundu U, Bailey RL, Shlisky J, Beaudreault AR, Bergeron G, et al. Hidden hunger: solutions for America's aging populations. Nutrients. 2018. https://doi.org/10.3390/nu10091210 .
Peter S, Eggersdorfer M, van Asselt D, Buskens E, Detzel P, Freijer K, et al. Selected nutrients and their implications for health and disease across the lifespan: a roadmap. Nutrients. 2014. https://doi.org/10.3390/nu6126076 .
Vlassoff C. Gender differences in determinants and consequences of health and illness. J Health Popul Nutr. 2007;25:47–61.
PubMed PubMed Central Google Scholar
World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: WHO press; 1998.
Hartman AM, Brown CC, Palmgren J, Pietinen P, Verkasalo M, Myer D, et al. Variability in nutrient and food intakes among older middle-aged men. Implications for design of epidemiologic and validation studies using food recording. Am J Epidemiol. 1990;132:999–1012.
Nusser SM, Carriquiry A, Dodd KW, Fuller WA. A semiparametric transformation approach to estimating usual daily intake distributions. J Am Stat Inst. 1991;436:1440–9.
Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids; the. Washington, DC: National Academies Press; 2005. https://doi.org/10.17226/10490pp.1358 .
Book Google Scholar
World Health Organization; STEPwise approach to surveillance: 2018. https://www.who.int/ncds/surveillance/steps/en/ . Accessed 25 Jun 2018
Lopez-Olmedo N, Carriquiry AL, Rodriguez-Ramirez S, Ramirez-Silva I, Espinosa-Montero J, Hernandez-Barrera L, et al. Usual intake of added sugars and saturated fats is high while dietary fiber is low in the Mexican population. J Nutr. 2016. https://doi.org/10.3945/jn.115.218214 .
Food and Agriculture Organization of the United Nations. FAO/INFOODS analytical food composition database version 1.1 – AnFooD1.1; 2016.
United States Department of Agriculture. What we eat in America food categories. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1314/food_category_list.pdf . Accessed 5 Nov 2018.
Iowa State University. Software for intake distribution estimation. http://www.side.stat.iastate.edu/ . Accessed 18 Dec 2018.
Department of Science and Technology- Food and Nutrition Research Institute (DOST-FNRI). Taguig City: Philippine Dietary Reference Intakes 2015; 2017.
Beaton GH. Approaches to analysis of dietary data: relationship between planned analyses and choice of methodology. Am J Clin Nutr. 1994;59. https://doi.org/10.1093/ajcn/59.1.253S .
National Research Council. Nutrient adequacy: assessment using food consumption surveys. Washington, DC: The National Academies Press; 1986. https://doi.org/10.17226/618pp.160 .
Department of Science and Technology- Food and Nutrition Research Institute (DOST-FNRI). Taguig City: Nutritional Guildelines for Filipinos revised 2012; 2015.
Briefel RR, McDowell MA, Alaimo K, Caughman CR, Bischof AL, Carroll MD, et al. Total energy intake of the US population: the third National Health and nutrition examination survey, 1988-1991. Am J Clin Nutr. 1995;62(5):1072S–80S. https://doi.org/10.1093/ajcn/62.5.1072S .
Article CAS PubMed Google Scholar
Wurtman JJ, Lieberman H, Tsay R, Nader T, Chew B. Calorie and nutrient intakes of elderly and young subjects measured under identical conditions. J Gerontol. 1988;43(6):B174–80.
Ahmed T, Haboubi N. Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging. 2010;5:207–16.
Amarya S, Singh K, Sabharwal M. Changes during aging and their association with malnutrition. J Clin Gerontol Geriatr. 2015;6:78–84. https://doi.org/10.1016/j.jcgg.2015.05.003 .
Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. 2015;145:372–9. https://doi.org/10.3945/jn.114.195438 .
Barba CV, Cabrera MI. Recommended energy and nutrient intakes for Filipinos 2002. Asia Pac J Clin Nutr. 2008;17(Suppl 2):399–404.
PubMed Google Scholar
Nakatsuka H, Zhang ZW, Agetano MG, Subida RD, Inouguchi N, Watanabe T, et al. Total food duplicate study on nutrient intake of working women in Manila, the Philippines. Tohoku J Exp Med. 1998;184:189–205.
Gibson RS, Cavalli-Sforza T. Using reference nutrient density goals with food balance sheet data to identify likely micronutrient deficits for fortification planning in countries in the Western Pacific region. Food Nutr Bull. 2012;33:S214–20.
Department of Science and Technology-Food and Nutrition Research Institute (FNRI-DOST). The 7th National Nutrition Survey (2008) results - food consumption survey; Philippines; 2008.
Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. https://doi.org/10.1146/annurev.nutr.23.020102.075739 .
Failla ML. Trace elements and host defense: recent advances and continuing challenges. J Nutr. 2003;133:1443s–7s. https://doi.org/10.1093/jn/133.5.1443S .
Haas JD, Brownlie IVT. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–90S. https://doi.org/10.1093/jn/131.2.676S .
Thomas DR. Vitamins in aging, health, and longevity. Clin Interv Aging. 2006;1:81–91.
ter Borg S, Verlaan S, Hemsworth J, Mijnarends DM, Schols JM, Luiking YC, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195–206. https://doi.org/10.1017/s0007114515000203.
Article PubMed PubMed Central Google Scholar
Doets EL, Ueland PM, Tell GS, Vollset SE, Nygard OK, Van't Veer P, et al. Interactions between plasma concentrations of folate and markers of vitamin B(12) status with cognitive performance in elderly people not exposed to folic acid fortification: the Hordaland health study. Br J Nutr. 2014;111:1085–95. https://doi.org/10.1017/s000711451300336x.
Heaney RP. Calcium, dairy products and osteoporosis. J Am Coll Nutr. 2000;19:83s–99s.
Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int. 2015:953241–1. https://doi.org/10.1155/2015/953241 .
Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016;4:16041. https://doi.org/10.1038/boneres.2016.41 .
Robien K, Butler LM, Wang R, Beckman KB, Walek D, Koh WP, et al. Genetic and environmental predictors of serum 25-hydroxyvitamin D concentrations among middle-aged and elderly Chinese in Singapore. Br J Nutr. 2013;109:493–502. https://doi.org/10.1017/S0007114512001675.
Chin KY, Ima-Nirwana S, Ibrahim S, Mohamed IN, Wan Ngah WZ. Vitamin D status in Malaysian men and its associated factors. Nutrients. 2014;6:5419–33. https://doi.org/10.3390/nu6125419 .
Article CAS PubMed PubMed Central Google Scholar
Bi X, Tey SL, Leong C, Quek R, Henry CJ. Prevalence of vitamin D deficiency in Singapore: its implications to cardiovascular risk factors. PLoS One. 2016;11:e0147616. https://doi.org/10.1371/journal.pone.0147616 .
Ek S. Gender differences in health information behaviour: a Finnish population-based survey. Health Promot Int. 2015;30:736–45. https://doi.org/10.1093/heapro/dat063 .
Article PubMed Google Scholar
Vitale M, Masulli M, Cocozza S, Anichini R, Babini AC, Boemi M, et al. Sex differences in food choices, adherence to dietary recommendations and plasma lipid profile in type 2 diabetes - the TOSCA.IT study. Nutr Metab Cardiovasc Dis. 2016;26:879–85. https://doi.org/10.1016/j.numecd.2016.04.006.
Cruz-Gongora V, Martinez-Tapia B, Cuevas-Nasu L, Flores-Aldana M, Shamah-Levy T. Dietary intake and adequacy of energy and nutrients in Mexican older adults: results from two National Health and nutrition surveys. Salud Publica Mex. 2017;59:285–98. https://doi.org/10.21149/7851 .
Nguyen PH, Nguyen H, Gonzalez-Casanova I, Copeland E, Strizich G, Lowe A, et al. Micronutrient intakes among women of reproductive age in Vietnam. PLoS One. 2014;9:e89504. https://doi.org/10.1371/journal.pone.0089504 .
Nikolic M, Glibetic M, Gurinovic M, Milesevic J, Khokhar S, Chillo S, et al. Identifying critical nutrient intake in groups at risk of poverty in Europe: the CHANCE project approach. Nutrients. 2014;6:1374–93. https://doi.org/10.3390/nu6041374 .
Download references
Acknowledgements
The research described here was a collaboration of 2 organizations: The Department of Science and Technology, Food and Nutrition Research Institute (DOST- FNRI), Philippines (data collection and analyses), and Nestlé Research (Nestec S.A.), Switzerland, (funding source and study conceptualization). The authors would like to acknowledge Kristine T. Biona, Regina R. Rodriguez, Glen Melvin Ginorella and Nabil Bosco, Yvonne Lenighan, and Edelwise Sicat for their support in reviewing the manuscript.
The research described here was a collaboration of 2 organizations: The Department of Science and Technology, Food and Nutrition Research Institute (DOST- FNRI), Philippines (data collection and analyses), and Nestlé Research (Nestec S.A.), Switzerland, (funding source and study conceptualization).
Author information
Authors and affiliations.
Department of Science and Technology, Food and Nutrition Research Institute, Bicutan, Taguig, Philippines
Imelda Angeles-Agdeppa, Keith V. Tanda, Royce Ann D. Octavio & Mario V. Capanzana
Nestlé Research, Singapore, Singapore
Nestlé Research, Lausanne, Switzerland
Liya Denney
Iowa State University, Ames, USA
Alicia Carriquiry
You can also search for this author in PubMed Google Scholar
Contributions
IAA, YS, and LD conceptualized and designed the study, interpreted the data, drafted the initial manuscript, and approved the final manuscript as submitted. AC reviewed and confirmed the appropriateness of the statistical design and interpretations. MVC gave advised and technical inputs. KVT did the statistical data processing and analysis, and RAD. Octavio contributed in drafting the initial manuscript. All authors proof-read and approved the manuscript.
Corresponding author
Correspondence to Imelda Angeles-Agdeppa .
Ethics declarations
Ethics approval and consent to participate.
The Ethics Committee of FNRI approved the survey protocol. All surveyed households provided informed consent prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Y.S and L.D. are employees of Nestec S.A., Switzerland. The opinions expressed in the article are those of the authors alone and do not necessarily reflect the views or recommendations of their affiliations.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:.
Dietary reference intakes of nutrients for Filipino adults and older adults. (DOCX 16 kb)
Additional file 2:
Ranking of foods as major sources of energy, protein, total fat, and carbohydrates among adults (19 years and above). (DOCX 18 kb)
Additional file 3:
Ranking of foods as major sources of thiamine, riboflavin, vitamin A, and vitamin C among adults (19 years and above). (DOCX 19 kb)
Additional file 4:
Ranking of foods as major sources of folate, calcium, and iron among adults (19 years and above). (DOCX 16 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Angeles-Agdeppa, I., Sun, Y., Denney, L. et al. Food sources, energy and nutrient intakes of adults: 2013 Philippines National Nutrition Survey. Nutr J 18 , 59 (2019). https://doi.org/10.1186/s12937-019-0481-z
Download citation
Received : 06 March 2019
Accepted : 30 August 2019
Published : 10 October 2019
DOI : https://doi.org/10.1186/s12937-019-0481-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Usual nutrient intake
- Food sources
- Older adults
- The Philippines
Nutrition Journal
ISSN: 1475-2891
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 May 2024
Dietary intake, obesity, and metabolic risk factors among children and adolescents in the SEACO-CH20 cross-sectional study
- Amutha Ramadas 1 ,
- Hussein Rizal 1 , 2 ,
- Sutha Rajakumar 1 , 2 ,
- Jeevitha Mariapun 3 ,
- Mohamed Shajahan Yasin 1 ,
- Miranda E. G. Armstrong 4 na1 &
- Tin Tin Su 1 , 2 na1
Scientific Reports volume 14 , Article number: 11265 ( 2024 ) Cite this article
389 Accesses
1 Altmetric
Metrics details
- Endocrinology
- Risk factors
We investigated the association between dietary intake and metabolic risk factors in children and adolescents within a semi-rural Malaysian community. Using an interviewer-led questionnaire, we surveyed 623 participants aged 7–18 from the South East Asia Community Observatory (SEACO). Anthropometric and blood pressure data were collected from all participants, while a subset (n = 162) provided blood samples for biomarker analysis, including fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Metabolic syndrome was determined using the International Diabetes Federation’s Definition of Metabolic Syndrome in Children and Adolescents. Most participants were Malay (66.8%), with a median household income of MYR1,500 and a balanced sex distribution. Cereals, processed foods, beverages, fruits, and vegetables were commonly consumed. Obesity and abdominal obesity were prevalent, affecting more than a third of participants. Adherence to dietary recommendations was generally poor (ranging from 19.9 to 58.1%) and varied across age, sex, and ethnicity. Notably, some food groups displayed unexpected associations with health markers; for instance, fruit consumption was linked to abdominal obesity in children (abdominal obesity vs. normal: 2.4 servings/day vs. 1.6 servings/day). These findings emphasise the necessity of longitudinal studies to explore the complex relationship between diet and long-term health outcomes, including cardiometabolic diseases, while acknowledging the unique challenges posed by the COVID-19 pandemic on data collection and analysis.
Similar content being viewed by others

Dietary patterns and associations with metabolic risk factors for non-communicable disease
Association of dietary patterns with blood pressure and body adiposity in adolescents: a systematic review
Longitudinal study of dietary patterns and hypertension in adults: China Health and Nutrition Survey 1991–2018
Introduction.
Limited diversity and inadequate consumption of essential food groups, such as fruits and vegetables, often characterise the dietary intake of children and adolescents. Overemphasis on plant-based foods in their diets, alongside a concerning trend of increasing consumption of high-energy snacks and beverages, has been highlighted in the literature 1 . This pattern contributes to a dual burden of malnutrition, with both undernutrition and overnutrition prevalent among this population. Mates et. al. 2 echoed this in their review, identifying urban areas and boys as particularly vulnerable groups with poorer dietary patterns.
Poor diet quality is a common concern, with declining dietary standards observed during the transition from childhood to adolescence 3 . This trend is a major contributor to the rising incidence of non-communicable diseases. Longitudinal studies emphasize the importance of addressing dietary patterns early in life, as childhood dietary behaviours tend to track into adulthood. For instance, Craigie and colleagues 3 have provided compelling evidence supporting this phenomenon. Their research highlights the importance of early intervention to address dietary patterns and habits during childhood and adolescence. By targeting these formative years, interventions have the potential to positively influence long-term health outcomes, mitigating the risk of chronic diseases later in life. This underscores the critical role of early dietary interventions in promoting lifelong health and well-being.
Appanah et al. 4 further highlight the association between poor diet quality and adverse health outcomes among Malaysian adolescents, including cardiometabolic risk factors. However, despite the growing awareness of the importance of good nutrition during childhood and adolescence, there remains a lack of comprehensive studies exploring the impact of diet quality on clinical outcomes in these age groups, particularly in regions such as Malaysia 5 . The existing evidence underscores the critical role of good dietary intake in promoting the health and well-being of children and adolescents, emphasizing the need for targeted interventions and policies to address dietary inadequacies and improve long-term health outcomes in this population.
Metabolic syndrome (MetS) is an emerging area of research among children and adolescents. MetS is characterised by the clustering of risk factors including abdominal obesity, elevated blood pressure (BP), fasting blood glucose (FBG) and triglyceride (TG), and low high-density lipoprotein cholesterol (HDL-C), which increases the risk of developing type 2 diabetes (T2D) and cardiovascular diseases (CVD) later in life 6 . While the assessment of paediatric MetS is still debatable 7 , existing data suggest approximately 3% of children and 5% of adolescents have MetS, with some variation across nations and regions 8 . This translates to more than 25 million children aged 6–12 years and more than 35 million adolescents aged 13–18 years suffering from MetS. The prevalence varies significantly by age, ethnicity and location 9 . This is an emerging public health issue in low and middle-income countries (LMICs) as a recent systematic review reported MetS was found in 4.0% (IDF) 10 , 6.7% (ATP III) 11 and 8.9% (de Ferranti) 12 of children and adolescents in these nations 13 .
Although a recent Malaysian community-based study reported a lower MetS prevalence of 4.8% in children 14 , Wan Mahmud et al. 15 reported a high MetS prevalence of 56% among children with obesity and adolescents among those referred to obesity clinics in tertiary care. The study suggests children with MetS were 14 times more likely to be severely obese. Furthermore, these children exhibited higher odds of having increased FBG, TG and low HDL-C. Earlier studies also reported significantly poorer biochemical profiles, higher body fat percentages and anthropometric measures in overweight and obese children 16 , 17 . Readily available access to calorie-dense, nutrient-poor foods and a lack of physical activity have contributed to a rapid rise in the prevalence of paediatric obesity, which is the primary risk factor for paediatric MetS 15 , 18 , 19 .
Paediatric MetS is strongly associated with T2D and CVD 20 , 21 . The chronic nature of MetS emphasizes the importance of identifying risk factors specific to the population that can be addressed later. Together with the importance of physical activity and adopting a healthier lifestyle, understanding the dietary intake of the community is an essential part of reducing the risk of paediatric MetS and its progression to adulthood complications.
Mohammadi and colleagues (2019) 22 reviewed the association between dietary patterns, physical activity, and metabolic risk factors among Malaysian adolescents. Their research revealed that obese and overweight adolescents exhibited distinct dietary behaviours compared to their normal-weight counterparts, including higher consumption of energy and macronutrients, as well as a greater tendency to skip meals. However, despite these findings, the review highlighted a notable gap in the existing research literature regarding the relationship between dietary habits and other metabolic risk factors such as lipid profile and BP 22 . This gap suggests a need for further investigation into the comprehensive effects of dietary patterns on various metabolic parameters among adolescents, particularly in the Malaysian context. Additional research in this area could provide valuable insights for developing targeted interventions aimed at mitigating metabolic risks and promoting better health outcomes among adolescents.
Hence, we aim to explore the association between dietary intake, obesity and metabolic risk factors among children and adolescents in a semi-rural Malaysian setting.
Study design and participants
South East Asia Community Observatory (SEACO) is a dynamic community observatory cohort of 13,335 households that have been surveyed since 2012 in Segamat, a semi-rural region in the state of Johor Darul Takzim, Malaysia. The assessments conducted in this cohort include questionnaire surveys, blood tests, and physical measurements 23 . The SEACO Health Round Survey 2018 (HR-2018) occurred between July 2018 and August 2019. Subsequently, the SEACO Child Health 2020 (SEACO-CH20) study was conducted to obtain device-measured physical activity and diet measures among Malaysian children and adolescents (aged 7–18) in a subsample of the main SEACO cohort.
The inclusion criteria were children and adolescents aged 7–18 from the SEACO cohort. The participants were sampled from 3 out of the 5 sub-districts of the SEACO cohort—Jabi, Sungai Segamat and Gemereh. Participants were excluded if their parents could not give assent during data collection due to work commitments. Participant information sheets were distributed, and informed consent was taken from parents/guardians on behalf of their child.
The minimum sample size required for this study was determined using Raosoft software 24 with a response distribution of 50% from a population size of 38,712 and a margin of error of 5% with a confidence level of 95%. The recommended sample size is 381 children and adolescents.
Parents of the 728 eligible participants consented to allow their children to participate in the study. Out of these, 623 participants completed the questionnaire, and a subset of 162 provided blood samples (Fig. 1 ). Trained fieldworkers conducted a face-to-face interview from October 2021 to July 2022, during which the participants self-administered the questionnaire. Ethical approval for SEACO-CH20 was obtained from the Monash University Human Research Ethics Committee on 17/03/2020 (Project ID: 23271) prior to data collection. The study was conducted in accordance with the Declaration of Helsinki for experiments involving humans.
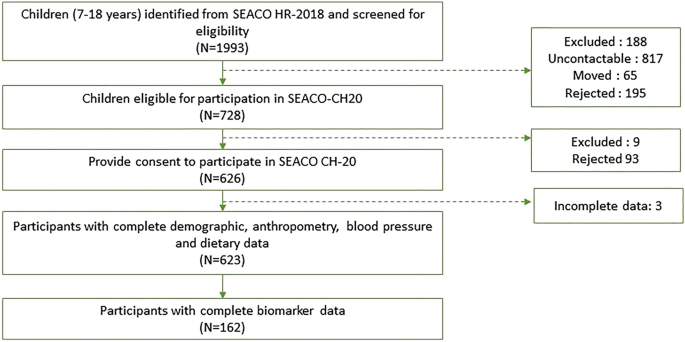
Study flow chart.
Demographic characteristics
The research team extracted information about children’s age, sex, and ethnicity from the SEACO HR 2018 data set. The participants were divided into two age groups: childhood (7–12 years) and adolescence (13–18 years), corresponding to primary and secondary school ages in Malaysia. The study also reported the ethnicity of the children as Malay, Chinese, or Indian. Additionally, the survey reported household income in Ringgit Malaysia (MYR).
Dietary intake
A short self-administered semi-quantitative food frequency questionnaire (SFFQ), adapted from the Malaysian Adults Nutrition Survey (MANS) FFQ 25 was used to collect information on dietary intake. The SFFQ consisted of 45 food items listed in eight food groups. A list of food items according to food groups is presented in Supplementary Table S1 . The SFFQ was administered during the house visits. The participants were asked about the frequency of intake of each food item (per day, week or month), and the number of servings consumed each time they ate a particular food item. Photographs depicting sample serving sizes were also included in the SFFQ to aid with the estimation of serving size. Each food item listed was given a standard serving size based on the Malaysian Dietary Guidelines 2020 26 . Dietary intake of specific food items was estimated based on the following formula: number of servings of food A = (number of servings of food A) × (frequency of intake of food A per day). Subsequently, the number of servings of food items in a particular food group was totalled to give the total number of servings per food group per day. The number of servings is also compared to the recommended servings for the participant’s age and sex according to the Malaysian Dietary Guidelines for Children and Adolescents 2013 27 .
Anthropometric and physical measures
Data collectors measured height and weight using a Transtek digital weighing scale and height gauge and body mass index (BMI) was calculated and converted to age-adjusted standardised z-scores using the WHO 2007 BMI reference for children aged 5–19 (BMI z-score) 28 . Children were classified as underweight, overweight and obese according to the definitions outlined by the WHO 28 , 29 , if the standardised BMI z-score was < − 2, > + 1 and < = 2, and > + 2 standard deviations from the mean, respectively, with the remaining children classified as a healthy weight. Waist circumference (WC) was measured after determining the midpoint between the last rib and the upper edge of the iliac crest on the right-hand side. The WC measurement was measured using Myotape Body Tape Measure (Accufitness, Denver United States). Systolic and diastolic BP were measured three times using the Digital Blood Pressure Monitor (HEM-907) with a 5-min interval between measurements, with the patient having remained seated for more than 5 min. The arterial pressure value was determined from the average of the last two measurements 30 . Both WC and BP were measured by participants’ family members to avoid close contact with data collectors and participants. The research team prepared brochures and videos guiding the family members on measuring WC and BP, and measurements were closely monitored and supervised by the trained data collectors.
Blood sample collection
The blood sample was collected by a privately managed Ministry of Health-certified local laboratory within the study location every Friday and Saturday morning, in accordance with the schedule. Respondents were required to fast from 10 pm on Thursday until the following morning (Friday). Hence, the blood samples were collected from the participants in a fast state and verified by data collectors before being taken. Trained phlebotomists drew intravenous blood samples while using suitable personal protective equipment. The same private laboratory analysed FBG and blood lipid profiles such as total cholesterol (TC), TG, HDL-C and low-density lipoprotein cholesterol (LDL-C). The quality of the samples was maintained via several steps, including visual inspection to detect abnormalities, minimum quantity, proper sealing and absence of leakage or contamination, as well as accurate labeling.
Definition of metabolic syndrome
The five metabolic risk factors (WC, BP, FBG, TG and HDL-C) were screened in the study participants. A total of 162 participants were assessed for the following criteria per the IDF Definition of Metabolic Syndrome in Children and Adolescents by the presence of WC ≥ 90th percentile along with two or more of the following four criteria: FBG ≥ 100 mg/dL; TG ≥ 150 mg/dL; HDL-C ≤ 40 mg/dL; systolic BP ≥ 130 mmHg and/ or diastolic BP ≥ 85 mmHg 31 .
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics for Windows, Version 28.0 (Armonk, NY: IBM Corp). Normality was assessed using visual inspection of the histogram and normality plots. Continuous variables with normal distribution (age, blood pressure, anthropometry, blood biomarkers) were presented as mean and standard deviation (SD), while variables with skewed distribution (daily servings of food intake) were presented as median and interquartile range (IQR). Categorical variables are presented as frequencies and percentages. The Mann–Whitney U or Kruskal–Wallis tests were used to analyse the difference in food intake between demography and metabolic indicators. Pairwise comparisons with Bonferroni corrections were performed if the Kruskal–Wallis test resulted in any significant differences in median servings of food intake. The Chi-square test was performed to assess the association between adherence to guidelines and demographic characteristics. Sub-group analyses were performed by comparing median food intakes between metabolic indicators according to demographic groups. The level of significance was set at p < 0.05.
Characteristics of the study participants
The study included 623 participants, primarily children aged 7–12 and adolescents aged 13–18. Table 1 provides descriptions of overall study participants and according to age groups. The mean age of the study participants was 12.7 + 2.8 years. The majority of participants were Malay, comprising 66.8% of the total. Additionally, there was a nearly equal distribution based on sex, with males accounting for 51% and females for 49%. The median household income was MYR1,500. Demographic characteristics were similar between the age groups.
Participants predominantly consumed cereals and cereal products, processed foods and beverages, and fruits and vegetables. However, a significant portion was overweight or obese, with 17 and 23.3% being overweight and obese, respectively. Abdominal obesity was found in 35.2% of the study participants. Overall, mean BPs, lipid profile, and FBG were normal.
Comparison of dietary intake and adherence according to demography
In our analysis of median daily food group servings by demographics (Table 2 ), children had notably higher median consumption of processed foods and beverages than adolescents (4.8 servings vs. 4.0 servings, p = 0.044). Males consumed more cereals (6.5 servings vs. 4.8 servings, p < 0.001) but fewer vegetables (1.1 servings vs. 1.5 servings, p = 0.015) than females. Among ethnicities, Indians had the lowest intake of cereals, processed foods, and beverages, unlike Malays and Chinese. Chinese participants had the highest intake of vegetables, milk, and dairy, while Malays consumed more fish and seafood.
The adherence to age- and sex-specific dietary recommendations was generally low. Overall, the proportion of participants that adhered to recommended servings was below 50%, except for meat, poultry, and eggs (58.1%). Variations were noted in compliance with dietary guidelines, particularly for cereals and cereal products, fish and seafood, and milk and dairy products, with differences observed based on age, sex, and ethnicity (Figs. 2 , 3 and 4 ).
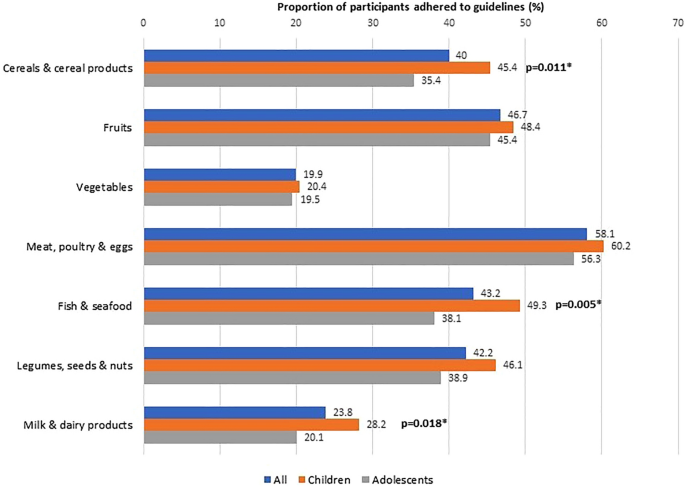
Adherence to dietary guidelines according to age groups (N = 623).
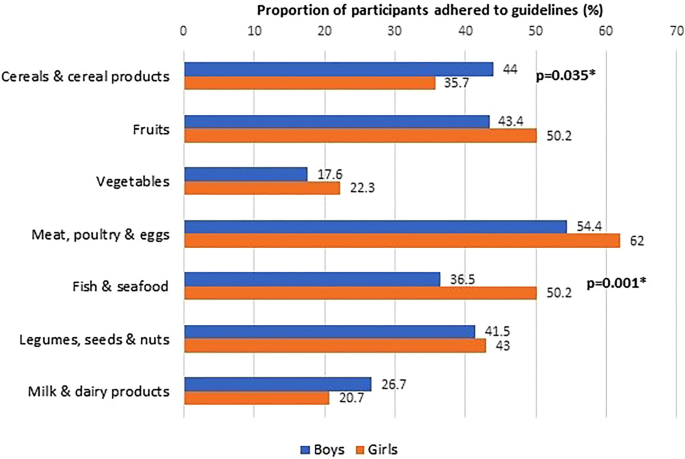
Adherence to dietary guidelines according to sexes (N = 623).
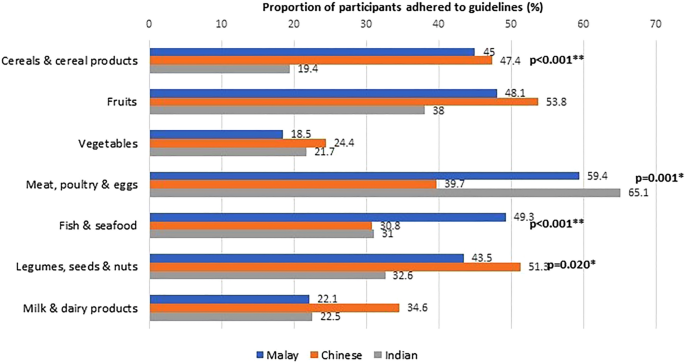
Adherence to dietary guidelines according to ethnicity (N = 623).
Comparison of dietary intake according to metabolic risk factors
Subsequently, we compared the median daily servings of food intake according to metabolic risk factors (Table 3 ) and explored the differences according to demographic sub-groups (Supplementary Tables S2 , S3 and S4 ). Obese participants reportedly consumed more servings of fruits than those with normal weight (2.2 servings/day vs. 1.7 servings/day, p = 0.034). Fruit consumption was also higher among abdominally obese children, males, and Chinese participants (Supplementary Tables S2 , S3 and S4 ).
Overall, participants with elevated BP had a lower intake of milk and dairy products (0.5 servings/day vs. 1.0 servings/day, p = 0.008) and a lower intake of processed foods and beverages (3.3 servings/day vs. 4.4 servings/day, p = 0.003), compared to participants with normal BP levels (Table 3 ). Sub-group analysis showed similar differences persisted among adolescents, males, and Indian participants (Supplementary Tables S2 , S3 and S4 ).
Female participants with elevated TG reported a lower intake of fruits (0.8 servings/day vs. 1.2 servings/day, p = 0.011) but a higher intake of fish and seafood (2.3 servings/day vs. 0.9 servings/day, p = 0.042) (Supplementary Table S3 ), while those with low HDL-C levels consumed more meat, poultry, and eggs (1.7 servings/day vs. 4.0 servings/day, p = 0.011) (Table 3 ). The finding was reaffirmed among adolescents, males, and Indian participants (Supplementary Tables S2 , S3 and S4 ).
Children with elevated FBG levels tended to consume fewer vegetables (0.3 servings/day vs. 1.5 servings/day, p = 0.021), while adolescents consumed more meat, poultry, and eggs (6.1 servings/day vs. 1.7 servings/day, p = 0.040) compared to their counterparts with normal FBG levels (Supplementary Table S2 ). The findings, however, need to be treated with caution due to the small number of participants with elevated FBG.
None of the food groups were associated with overall MetS (Table 3 ). However, the sub-group analyses showed lower consumption of fruits among females with MetS (0.2 servings/day vs. 1.3 servings, p = 0.008) (Supplementary Table S3 ) and higher consumption of meat, poultry, and eggs among Indian participants with MetS (7.4 servings/day vs. 2.0 servings/day, p = 0.011) (Supplementary Table S4 ) than their counterparts without MetS.
Anthropometric and biomarker associations with dietary recommendations (Supplementary Table S5 ) revealed that inadequate consumption of cereals and cereal products was associated with higher median WC (70.0 cm vs. 67.5 cm, p = 0.030), while adherence to fish and seafood intake recommendations was associated with lower median SBP (107 mmHg vs. 110 mmHg, p = 0.001). Conversely, adherence to milk and dairy products was associated with a higher BMI z-score (0.9 vs. 0.4, p = 0.028).
We analysed the dietary data and metabolic indicators of 623 participants enrolled in SEACO-CH20, comprising 285 children (aged 7–12) and 389 adolescents (aged 13–18). Most respondents were Malay, with almost equal proportions of males and females. Cereals and cereal products, processed foods and beverages, and fruits and vegetables were the most consumed food groups. The prevalence of overweight/obesity, and abdominal obesity in this population was worrying, with at least one in three affected. Adherence to dietary guidelines was generally low, except for meat, poultry, and eggs, though we noted differences in food consumption based on age, sex, and ethnicity. Some paradoxical associations between food groups and health markers, such as fruit consumption and obesity were also found.
In our study, children demonstrated higher adherence to dietary guidelines for cereals and cereal products, fish and seafood, and milk and dairy products compared to adolescents. The SEANUTS study 32 further revealed that while younger children (7–9 years) were more likely to meet recommendations for cereals/grains, older children consumed a greater number of servings of cereals/grains, vegetables, meat/poultry, fish, and legumes. Conversely, there was a notable trend among SEACO-CH20 children towards increased consumption of processed foods and beverages, aligning with previous research highlighting their preference for such foods 33 , 34 . Evidence from recent systematic reviews underscores the association between the intake of ultra-processed foods and the risk of obesity and adiposity in children 34 . Additionally, sugars and sweet products emerged as the most favored processed foods among children 35 . These findings emphasize the necessity for early dietary education interventions, particularly given children’s susceptibility to less healthy food choices.
The current study also explored the ethnic-differences in food intake. Children and adolescents of Indian ethnicity reported the lowest intake of cereals and cereal products as well as processed foods and beverages. Indians also reported relatively higher adherence to meat, poultry and eggs recommendations, a finding that contradicted previously reported SEANUTS report 32 . Chinese respondents in SEACO-CH20 reported relatively higher consumption of vegetables, milk, and dairy products. They also more frequently adhered to recommendations for cereals and cereal products, legumes, nuts and seeds. On the other hand, Malays preferred and had higher adherence to fish and seafood. Several studies have explored ethnic variations in adult food intake, though the evidence among children and adolescents is limited. For example, Garba et al. 36 reported ethnicity as a predictor of adolescent diet, especially dietary patterns associated with fruits, vegetables, fats and sugar. Findings from the Malaysian Adolescent Nutrition Survey 2017 also support an association between ethnicity and unhealthy dietary patterns characterised by foods with high sugar content, oil or fat, salt, and processed foods 37 .
We also found a disparity in food intake between the sexes. Male SEACO-CH20 respondents consumed more cereals and cereal products and showed better adherence to the recommended servings. However, they consumed fewer servings of fruits and vegetables than female participants. Female respondents showed higher compliance with guidelines for fish and seafood intake. Several studies have explored sex-related differences in dietary intake. For example, the VYRONAS study 38 reported a preference for cereals and cereal products, especially for breakfast by boys aged 12–17 compared to their female counterparts. This likely reflects the higher need for foods that contribute more energy during rapid growth in boys. In addition, lower fruit and vegetable intake among the boys also corresponds to some previous findings. Boys have been reported to consume fewer fruits and vegetables because they like them less and have a greater liking for energy-dense foods 39 , 40 . However, this is not consistent, as a number of studies did not report a sex difference in vegetable consumption, warranting further exploration 41 .
We uncovered a surprising and intriguing result where overweight/obese participants consumed more servings of fruits than those with normal weight. Our sub-group analysis revealed higher fruit consumption among abdominally obese children, males, and Chinese participants. This challenges traditional assumptions about the protective effect of fruits and warrants further investigations. Sharma and colleagues 42 suggested the effect of certain types of fruits and the increase in simple sugars as potential factors for the contradictory effects of fruits on obesity.
Participants with elevated BP consumed fewer servings of milk and dairy products. Although the median consumption (1 serving/day) is still below the recommended servings, the protective effect of milk and dairy products corresponds to previous studies. The QUALITY cohort, for example, indicated that high consumption of dairy products has antihypertensive effects on children 43 . However, we discovered another surprising finding with a lower intake of processed foods and beverages with elevated BP. The level of processing of the foods could have influenced the BP. However, the current data did not allow for further subgroup analysis according to the level of processing. Similar to the contradictory effect of fruit-obesity, this paradoxical relationship also should be investigated further.
The SEACO-CH20 finding showing female children and adolescents with elevated TG reported higher fish and seafood consumption, but lower fruit intake is noteworthy. These sex-specific relationships may have implications for managing TG levels and subsequent cardiovascular health. As the evidence has been contradictory 44 , further investigation should explore the type of fish and seafood that influence blood TG levels. Evidence associating fruit intake and TG is also scarce and inconsistent, meriting further exploration 45 .
Low HDL-C levels were associated with a higher intake of animal protein sources, and the effect was specifically seen in adolescents, males and Indian participants of this current study. The negative association between animal protein sources or dietary patterns high in animal sources and low HDL-C in the paediatric population has been previously documented in large-scale surveys 46 , 47 . Higher intake of meat, poultry and eggs was also found among adolescents with elevated levels of FBG in our study.
Although there is an absence of significant associations between food groups and MetS, sub-group analysis showed that females with MetS consumed fewer servings of fruits. In addition, Indian participants with MetS reported higher consumption of meat, poultry and eggs than their counterparts without MetS. Western dietary patterns, which are generally high in animal protein but low in fruits and vegetables, have been associated with the risk for MetS in the past 48 . A meta-analysis suggests a higher consumption of fruits was associated with lower odds for MetS among Asians, although the findings were not specific to adolescents or females 49 . Data from a large nationwide survey in China showed lower fruit intake was associated with some components of metabolic diseases which were more evident among female adolescents. However, the researchers did not associate the fruit intake with MetS itself 50 .
We discovered additional insights into the relationship between dietary intake and metabolic indicators by evaluating adherence to dietary guidelines. Children and adolescents who adhered to the recommended servings of milk and dairy products had higher BMI z-scores. However, the z-score was within the normal range. Hence there is no metabolic risk indicated. Adherence to recommended servings of fish and seafood suggested lower systolic BP, while adherence to cereals and cereal product recommendations suggested lower waist circumference. While optimal intake of fish, seafood, cereals and cereal products may have a positive effect on metabolic health, the impact of the type of foods, including fatty fish and whole grains needs to be investigated further.
The overall dietary intake of the SEACO-CH20 children and adolescents was found to be suboptimal, with less than 50% meeting dietary recommendations across all food groups except for meat, poultry, and eggs (Fig. 2 ). This trend persists when examining the data by sex (Fig. 3 ) and ethnicity (Fig. 4 ). Although this study did not analyze food consumption patterns, there are indications that the participants, particularly the adolescents, may be inclined towards unhealthy dietary habits. Recent local studies have highlighted poor dietary practices and patterns among Malaysian children and adolescents, which have been linked to a poor quality of life 51 , 52 , 53 . Moreover, unhealthy dietary habits established at a young age are associated with an increased risk of cardiometabolic diseases and poor quality of life 52 , 53 , 54 , 55 , 56 . These age-, sex- and ethnic-specific variations in our SEACO-CH20 study highlight the importance of tailoring nutritional interventions to address the unique dietary preferences and cultural contexts of different ethnic communities. Interventions to promote healthy eating habits among children and adolescents, such as school-based nutrition education programs, parental involvement, and policy changes to improve access to nutritious foods in schools’ canteens, are crucial for long-term health outcomes 57 . Cultural beliefs and family dynamics also play a significant role in shaping dietary choices among young individuals, highlighting the need for culturally tailored interventions targeting this age group 58 , 59 .
The current study demonstrates several strengths. We recruited a sizable (N = 623) and diverse paediatric participants comprising an equal number of females and males. This provides a comprehensive representation of the study population in SEACO. In addition, the large sample enhances the generalisability of the findings to the children and adolescent population in Malaysia. Although the number of participants available for the biomarker subset was relatively smaller, we were able to perform sub-group analyses exploring the possible association between dietary intake and metabolic outcomes.
However, this study also exhibits several limitations. The study’s cross-sectional design did not allow for establishing a causal relationship between food intake and metabolic indicators. Future studies should focus on a longitudinal design to provide a clearer understanding of the temporal relationship between these factors. The number of samples available for blood biomarker analysis was significantly lower than the total number of study participants, which could also undermine the strength of the relationship. Owing to the impact of the COVID-19 pandemic, the Malaysian government’s enforcement of movement control measures impeded the recruitment of participants with blood samples to achieve the initially proposed sample size. Consequently, the attained response rate amounted to 23%. This is also a reason why we are not able to control potential confounders. However, we conducted sub-group analyses to explore the effect of these confounders on the association between food intake and metabolic risk factors. We did not correct for multiple testing as the sub-group comparisons were supplementary to the primary hypothesis of assessing the link between demography, metabolic risk factors, and dietary intake (food groups). However, future studies exploring the associations specific to certain demography should consider multiple comparisons in study designs or adjust for multiple testing at the data analysis stage. One limitation of this study is the potential for implausible dietary data reporting among children and adolescents. Dietary data used in this analysis were self-reported and collected using a brief semi-FFQ instrument, which introduces a potential bias that could affect the accuracy of the information. Despite efforts to minimise the bias through standardised methodologies, the possibility of inaccuracies remains. Incorporating dietary biomarkers could be an option for researchers aiming for a more objective assessment of dietary intake in the future. In addition, This study faces another significant limitation stemming from the timing of data collection, which coincided with the COVID-19 pandemic. The pandemic likely influenced participants’ dietary habits and their accuracy in reporting them.
In this paper, we described the dietary intake of a multi-ethnic paediatric population during the recovery period of the COVID-19 pandemic. The study also examined the associations between food intake and metabolic risk factors such as obesity, abdominal obesity, elevated BP, dyslipidaemia and increased FBG. The study resulted in both supportive and contradictory findings in this subject matter. Despite a number of limitations, SEACO-CH20 study’s strengths lie in its large sample size and sub-group analyses. This study contributes valuable knowledge on the complex relationship between diet and metabolic health, especially in an under-studied paediatric population in Malaysia.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files]. Data are available from SEACO by completion of a data application form to: https://www.monash.edu.my/seaco/research-and-training/how-to-collaborate-with-seaco . For the purpose of open access, the author(s) has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.
Ochola, S. & Masibo, P. K. Dietary intake of schoolchildren and adolescents in developing countries. Ann. Nutr. Metab. 64 (2), 24–40. https://doi.org/10.1159/000365125 (2014).
Article CAS PubMed Google Scholar
Mates, E. et al. Nutrition of school-aged children and adolescents in Europe and Central Asia region: A literature and survey review. Food. Nutr. Bull. 44 , 51–61. https://doi.org/10.1177/03795721231163021 (2023).
Article PubMed Google Scholar
Craigie, A. M. et al. Tracking of obesity-related behaviours from childhood to adulthood: A systematic review. Maturitas 70 , 266–284. https://doi.org/10.1016/j.maturitas.2011.08.005 (2011).
Appannah, G. et al. Evaluation of dietary quality using Malaysian healthy eating index and its relationships with cardiometabolic risk factors in Malaysian adolescents. Malays. J. Med. Health Sci. 16 , 46–55 (2020).
Google Scholar
Ramadas, A., Tham, S. M., Lalani, S. A. & Shyam, S. Diet quality of Malaysians across lifespan: A scoping review of evidence in a multi-ethnic population. Nutrients 13 , 1380. https://doi.org/10.3390/nu13041380 (2021).
Article PubMed PubMed Central Google Scholar
Alberti, K. G. et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120 , 1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644 (2009).
Reinehr, T., de Sousa, G., Toschke, A. M. & Andler, W. Comparison of metabolic syndrome prevalence using eight different definitions: A critical approach. Arch. Dis. Child. 92 , 1067–1072. https://doi.org/10.1136/adc.2006.104588 (2007).
Noubiap, J. J. et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child. Adolesc. Health 6 , 158–170. https://doi.org/10.1016/s2352-4642(21)00374-6 (2022).
DeBoer, M. D. Assessing and managing the metabolic syndrome in children and adolescents. Nutrients 11 , 1788. https://doi.org/10.3390/nu1108178 (2019).
Article CAS PubMed PubMed Central Google Scholar
Alberti, K. G. M., Zimmet, P. & Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 366 , 059–062. https://doi.org/10.1016/S0140-6736(05)67402-8 (2005).
Article Google Scholar
Boney, C. M., Verma, A., Tucker, R. & Vohr, B. R. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115 , e290–e296. https://doi.org/10.1542/peds.2004-1808 (2005).
de Ferranti, S. D. et al. Prevalence of the metabolic syndrome in American adolescents: Findings from the third national health and nutrition examination survey. Circulation 110 , 2494–2497. https://doi.org/10.1161/01.CIR.0000145117.40114.C7 (2004).
Bitew, Z. W. et al. Metabolic syndrome among children and adolescents in low and middle income countries: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 12 , 93. https://doi.org/10.1186/s13098-020-00601-8 (2020).
INur Zati Iwani, A. K. et al. Cardiometabolic risk factors among children who are affected by overweight, obesity and severe obesity. Front. Public Health. 11 (1097675), 2023. https://doi.org/10.3389/fpubh.2023.1097675 (2023).
Wan Mahmud Sabri, W. M. N., Mohamed, R. Z., Yaacob, N. M. & Hussain, S. Prevalence of metabolic syndrome and its associated risk factors in pediatric obesity. J. ASEAN Fed. Endocr. Soc. 37 , 24–30. https://doi.org/10.15605/jafes.037.01.05 (2022).
Fadzlina, A. A. et al. Metabolic syndrome among 13 year old adolescents: Prevalence and risk factors. BMC Public Health 14 , S7. https://doi.org/10.1186/1471-2458-14-s3-s7 (2014).
Wee, B. S. et al. Risk of metabolic syndrome among children living in metropolitan Kuala Lumpur: A case control study. BMC Public Health 11 , 1–7. https://doi.org/10.1186/1471-2458-11-333 (2011).
Kelishadi, R. Metabolic syndrome burden in children and adolescents. Lancet Child. Adolesc. Health 6 , 138–139. https://doi.org/10.1016/S2352-4642(21)00401-6 (2022).
Alagappan, M., Rampal, L. & Zalilah, M. S. Prevalence of overweight/obesity and its associated factors among secondary school students in semi urban area in Malaysia. Med. J. Mal. 74 , 513–520 (2019).
CAS Google Scholar
Morrison, J. A., Friedman, L. A., Wang, P. & Glueck, C. J. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 152 , 201–206. https://doi.org/10.1016/j.jpeds.2007.09.010 (2008).
Morrison, J. A., Friedman, L. A. & Gray-McGuire, C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The princeton lipid research clinics follow-up study. Pediatrics 120 , 340–345. https://doi.org/10.1542/peds.2006-1699 (2007).
Mohammadi, S. et al. Dietary and physical activity patterns related to cardio-metabolic health among Malaysian adolescents: A systematic review. BMC Public Health. 19 , 251. https://doi.org/10.1186/s12889-019-6557-z (2019).
Partap, U. et al. HDSS profile: The South East Asia community observatory health and demographic surveillance system (SEACO HDSS). Int. J. Epidemiol. 46 , 1370–1371. https://doi.org/10.1093/ije/dyx113 (2017).
Article MathSciNet PubMed PubMed Central Google Scholar
Raosoft. Raosoft Sample Size Calculator. Raosoft, Inc., Seattle. http://www.raosoft.com/samplesize.html (2004).
Institute for Public Health. National health and morbidity survey 2014: Malaysian Adult Nutrition Survey. Vol. 1: Methodology and General Findings. (Institute for Public Health, 2014).
National Coordinating Committee on Food and Nutrition. Malaysian dietary guidelines 2020. National coordinating committee on food and nutrition. (Ministry of Health Malaysia, 2021).
National Coordinating Committee on Food and Nutrition. Malaysian dietary guidelines for children and adolescents. National coordinating committee on food and nutrition. (Ministry of Health Malaysia, 2013).
World Health Organization. Growth reference 5–19 Years - application tools. (Geneva, 2014).
de Onis, M. et al. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 85 , 660–667. https://doi.org/10.2471/blt.07.043497 (2007).
World Health Organization. WHO STEPS Manual. https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps/manuals (Geneva, 2023).
Zimmet, P. et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr. Diab. 8 , 299–306. https://doi.org/10.1111/j.1399-5448.2007.00271.x (2007).
Koo, H. C. et al. Are Malaysian children achieving dietary guideline recommendations?. Asia Pac. J. Public Health. 28 , 8S-20S. https://doi.org/10.1177/1010539516641504 (2016).
Dogui, D., Doggui, R., Al-Jawaldeh, A., El Ati, J. & El Ati-Hellal, M. Ultra-processed foods are the major sources of total fat, saturated and trans-fatty acids among Tunisian preschool and school children: A cross-sectional study. Children 9 (126), 2022. https://doi.org/10.3390/children9020126 (2022).
Bleiweiss-Sande, R. et al. Processed food consumption is associated with diet quality, but not weight status, in a sample of low-income and ethnically diverse elementary school children. Appetite 151 , 104696. https://doi.org/10.1016/j.appet.2020.104696 (2020).
De Amicis, R. et al. Ultra-processed foods and obesity and adiposity parameters among children and adolescents: A systematic review. Eur. J. Nutr. 61 , 2297–2311. https://doi.org/10.1007/s00394-022-02873-4 (2022).
Garba, J. A., Rampal, L., Hejar, A. R. & Salmiah, M. S. Major dietary patterns and their associations with socio-demographic characteristics and obesity among adolescents in Petaling District, Malaysia. Malays. J. Med. Health Sci. 10 , 13–21 (2014).
Man, C. S. et al. Dietary patterns and associated factors among adolescents in Malaysia: Findings from adolescent nutrition survey 2017. Int. J. Environ. Res. Public Health. 17 , 3431. https://doi.org/10.3390/ijerph17103431 (2020).
Babashahi, M. et al. Systematic review and meta-analysis of the most common processed foods consumed by Iranian children. East. Mediterr. Health J. 27 , 918–930. https://doi.org/10.26719/emhj.21.032 (2021).
Bere, E., Brug, J. & Klepp, K. Why do boys eat less fruit and vegetables than girls?. Public Health Nutr. 11 , 321–325. https://doi.org/10.1017/S1368980007000729 (2008).
Cooke, L. J. & Wardle, J. Age and gender differences in children’s food preferences. Br. J. Nutr. 93 , 741–746. https://doi.org/10.1079/bjn20051389 (2005).
Zeidan, W., Taweel, H., Shalash, A. & Husseini, A. Consumption of fruits and vegetables among adolescents in Arab Countries: A systematic review. Int. J. Behav. Nutr. Phys. Act. 20 , 3. https://doi.org/10.1186/s12966-022-01398-7 (2023).
Sharma, S. P., Chung, H. J., Kim, H. J. & Hong, S. T. Paradoxical effects of fruit on obesity. Nutrients 8 , 633. https://doi.org/10.3390/nu8100633 (2016).
Yuan, W. L. et al. Influence of dairy product consumption on children’s blood pressure: Results from the QUALITY cohort. J. Acad. Nutr. Diet. 113 , 936–941. https://doi.org/10.1016/j.jand.2013.03.010 (2013).
Alhassan, A., Young, J., Lean, M. E. J. & Lara, J. Consumption of fish and vascular risk factors: A systematic review and meta-analysis of intervention studies. Atherosclerosis 266 , 87–94. https://doi.org/10.1016/j.atherosclerosis.2017.09.028 (2017).
Collese, T. S. et al. Role of fruits and vegetables in adolescent cardiovascular health: A systematic review. Nutr. Rev. 75 , 339–349. https://doi.org/10.1093/nutrit/nux002 (2017).
Shi, J. et al. Nutrient patterns and its association and metabolic syndrome among Chinese children and adolescents aged 7–17. Nutrients 15 , 117. https://doi.org/10.3390/nu15010117 (2022).
Shang, X. et al. Dietary pattern and its association with the prevalence of obesity and related cardiometabolic risk factors among Chinese children. PloS One 7 , e43183. https://doi.org/10.1371/journal.pone.0043183 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar
Weiss, R., Bremer, A. A. & Lustig, R. H. What is metabolic syndrome, and why are children getting it?. Ann. N. Y. Acad. Sci. 1281 , 123–140. https://doi.org/10.1111/nyas.12030 (2013).
Tian, Y., Su, L., Wang, J., Duan, X. & Jiang, X. Fruit and vegetable consumption and risk of the metabolic syndrome: A meta-analysis. Public Health Nutr. 21 , 756–765. https://doi.org/10.1017/S136898001700310X (2018).
Liu, J. et al. Association between fruit consumption and lipid profile among children and adolescents: A national cross-sectional study in China. Nutrients 14 , 63. https://doi.org/10.3390/nu14010063 (2021).
Abdullah, N. F., Teo, P. S. & Foo, L. H. Ethnic differences in the food intake patterns and its associated factors of adolescents in Kelantan, Malaysia. Nutrients 8 , 551. https://doi.org/10.3390/nu8090551 (2016).
Appannah, G. et al. The relationships between a dietary pattern linked to cardiometabolic risk factors and life satisfaction in early adolescence. Int. J. Environ. Res. Public Health. 17 , 5489. https://doi.org/10.3390/ijerph17155489 (2020).
Cheah, M. H. J. et al. Factors predicting health-related quality of life of the Malaysian B40 school-aged children living in urban-poor flats in the central region of Malaysia. Asia Pac. J. Clin. Nutr. 31 , 740–747. https://doi.org/10.6133/apjcn.202212_31(4).0015 (2022).
Emi, N. A. et al. Associations of an empirical dietary pattern with cardiometabolic risk factors in Malaysian adolescents. Nutr. Metab. 17 , 28. https://doi.org/10.1186/s12986-020-00447-x (2020).
Article CAS Google Scholar
Kerr, J. A. et al. Diet quality trajectories and cardiovascular phenotypes/metabolic syndrome risk by 11–12 years. Int. J. Obes. 45 , 1392–1403. https://doi.org/10.1038/s41366-021-00800-x (2021).
Codazzi, V., Frontino, G., Galimberti, L., Giustina, A. & Petrelli, A. (Mechanisms and risk factors of metabolic syndrome in children and adolescents. Endocrine https://doi.org/10.1007/s12020-023-03642-x (2023).
Chaudhary, A., Sudzina, F. & Mikkelsen, B. E. Promoting healthy eating among young people-A review of the evidence of the impact of school-based interventions. Nutrients 12 , 2894. https://doi.org/10.3390/nu12092894 (2020).
Scaglioni, S. et al. Factors influencing children’s eating behaviours. Nutrients 10 , 706. https://doi.org/10.3390/nu10060706 (2018).
Islam, M. R. et al. Sociocultural influences on dietary practices and physical activity behaviors of rural adolescents-A qualitative exploration. Nutrients 11 , 2916. https://doi.org/10.3390/nu11122916 (2019).
Download references
Acknowledgements
The Ministry of Higher Education/UK-MY Joint Partnership on Non-Communicable Diseases (2019/MR/T018984/) and the Medical Research Council (MR/T018984/1), both provided funding in support of this research. The SEACO health and demographic surveillance system is supported by Monash University. The study’s funders played no part in the study’s planning, gathering, analyzing, or interpreting data, or in the report’s preparation. The National Institute for Health and Care Research Bristol Biomedical Research Centre also funds the study’s co-authors (MEGA). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The authors also would like to express their appreciation to the SEACO Field Teams and survey participants. The South East Asia Community Observatory (SEACO, https://www.monash.edu.my/seaco ) funded the research detailed in this paper. The authors’ opinions, however, are their own, and there is no real or implied sponsorship from SEACO.
Author information
These authors jointly supervised this work: Miranda E. G. Armstrong and Tin Tin Su.
Authors and Affiliations
Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, 47500, Bandar Sunway, Malaysia
Amutha Ramadas, Hussein Rizal, Sutha Rajakumar, Mohamed Shajahan Yasin & Tin Tin Su
South East Asia Community Observatory (SEACO), Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, 47500, Bandar Sunway, Malaysia
Hussein Rizal, Sutha Rajakumar & Tin Tin Su
Clinical School Johor Bahru, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, 80100, Johor Bahru, Malaysia
Jeevitha Mariapun
Centre for Exercise, Nutrition & Health Sciences, School for Policy Studies, University of Bristol, Bristol, BS8 1TZ, UK
Miranda E. G. Armstrong
You can also search for this author in PubMed Google Scholar
Contributions
A. R. was responsible for the formal analysis and writing of the original and final draft of the manuscript. H. R. contributed towards the writing of the original and final draft of the manuscript. S. R. and J. M. contributed towards the design of methodology, analysis and writing of the final draft of the manuscript. M. S. Y., M. E. G. A. and T. T. S. contributed towards the conceptualisation, methodology, investigation, providing of resources, supervision, project administration, funding acquisition and review of the final manuscript. All authors read and agreed to the final version of this manuscript.
Corresponding author
Correspondence to Amutha Ramadas .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ramadas, A., Rizal, H., Rajakumar, S. et al. Dietary intake, obesity, and metabolic risk factors among children and adolescents in the SEACO-CH20 cross-sectional study. Sci Rep 14 , 11265 (2024). https://doi.org/10.1038/s41598-024-61090-7
Download citation
Received : 04 August 2023
Accepted : 30 April 2024
Published : 17 May 2024
DOI : https://doi.org/10.1038/s41598-024-61090-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Research article
- Open access
- Published: 15 February 2022
Determining intention, fast food consumption and their related factors among university students by using a behavior change theory
- Alireza Didarloo 1 ,
- Surur Khalili 2 ,
- Ahmad Ali Aghapour 2 ,
- Fatemeh Moghaddam-Tabrizi 3 &
- Seyed Mortaza Mousavi 4 , 5
BMC Public Health volume 22 , Article number: 314 ( 2022 ) Cite this article
19k Accesses
6 Citations
Metrics details
Today, with the advancement of science, technology and industry, people’s lifestyles such as the pattern of people’s food, have changed from traditional foods to fast foods. The aim of this survey was to examine and identify factors influencing intent to use fast foods and behavior of fast food intake among students based on the theory of planned behavior (TPB).
A cross-sectional study was conducted among 229 university students. The study sample was selected and entered to the study using stratified random sampling method. Data were collected using a four-part questionnaire including Participants’ characteristics, knowledge, the TPB variables, and fast food consumption behavior. The study data were analyzed in SPSS software (version 16.0) using descriptive statistics (frequencies, Means, and Standard Deviation) and inferential statistics (t-test, Chi-square, correlation coefficient and multiple regressions).
The monthly frequency of fast food consumption among students was reported 2.7 times. The TPB explained 35, 23% variance of intent to use fast food and behavior of fast food intake, respectively. Among the TPB variables, knowledge ( r = .340, p < 0.001) and subjective norm ( r = .318, p < 0.001) were known as important predictors of intention to consume fast foods - In addition, based on regression analyses, intention ( r = .215, p < 0.05), perceived behavioral control ( r = .205, p < 0.05), and knowledge ( r = .127, p < 0.05) were related to fast food consumption, and these relationships were statistically significant.
Conclusions
The current study showed that the TPB is a good theory in predicting intent to use fast food and the actual behavior. It is supposed that health educators use from the present study results in designing appropriate interventions to improve nutritional status of students.
Peer Review reports
Over the past few decades, non-communicable diseases such as eczema, asthma, cancer, type 2 diabetes, obesity, etc. have increased in developed countries [ 1 , 2 ]. Also, these diseases are more prevalent with increasing urbanization in developing countries [ 3 , 4 , 5 ]. The occurrence of many non-communicable diseases is related to diet [ 6 ]. Food habits are rooted from cultural, environmental, economic, social and religious factors. An effective factor in the development of chronic diseases is lifestyle, dietary patterns and habits. Inappropriate food habits and unhealthy environments have increased the incidence of non-communicable diseases in the world [ 7 , 8 ].
Many developing countries with a tendency towards Western dietary culture go away from traditional and local diets [ 6 ]. Healthy foods with nutrients have been replaced by new foods called fast foods [ 9 ]. Fast food is the food prepared and consumed outside and often in fast food restaurants [ 10 ]. Fast food is often highly processed and prepared in an industrial fashion, i.e., with standard ingredients and methodical and standardized cooking and production methods [ 10 ]. In fast food, vitamins, minerals, fiber and amino acids are low or absent but energy is high [ 9 ]. Fast food consumption has increased dramatically in the last 30 years in European and American countries [ 11 ].
Previous studies reported patterns of inappropriate and harmful food consumption in Iranian children and adolescents [ 12 , 13 ]. Most fast food customers are adolescents and youth, as these products are quickly and easily produced and relatively inexpensive [ 14 ]. One Iranian study shows that 51% of children eat inappropriate snacks and drinks over a week [ 15 ]. It is also reported that adults today consume fast food more than previous generations [ 16 ]. Faqih and Anousheh reported that 20% of adolescents and 10% of adults consumed sandwiches 3 or more times a week [ 17 ].
According to two studies, children and adolescents who consume fast food have received more energy, saturated fat, sodium, carbohydrates and more sugar than their peers, but they have less fiber, vitamin A and C, and less fruit and vegetables [ 18 , 19 ]. Also, because of the use of oils to fry these foods at high temperatures, these types of foods may contain toxic and inappropriate substances that threaten the health of consumers [ 20 ].
In a study in the United States on young people between 13 and 17 years old, it was found that there is a significant relationship between weight gain and obesity with pre-prepared foods [ 21 ]. According to the Center for Disease Control and Prevention (2007–2008), 17% of children aged 2 to 19 years and 34% of those aged 20 years and older were obese [ 22 ]. Many Health problems were caused by human health behavior(e.g. exercising regularly, eating a balanced diet, and obtaining necessary inoculations, etc.) and studying behavior change theories/models provides a good insight into the causes and ways of preventing these problems [ 23 ]. One of these theories is the Theory of Planned Behavior (TPB), which is a developed form of the Theory of reasoned action (TRA), and describes a healthy behavior that is not fully under the control of a person [ 24 ]. This theory can successfully predict eating habits and behaviors, and recently this theory has received considerable attention from researchers in identifying norms and beliefs related to the use of fast food [ 25 ].
Based on the TPB, intention to conduct a behavior with following three concepts is controlled: 1. Attitudes (positive and negative evaluation of a behavior), 2. Subjective norms (social pressure received from peers, family, health care providers for doing or not doing a given health behavior), 3. Perceived behavior control (This refers to a person’s perception of the ease or difficulty of performing the behavior of interest.) [ 26 , 27 , 28 ].
The TPB has been tested on different behaviors such as healthy food choice [ 24 ], physical activity [ 29 ], and fast food consumption [ 30 ]. For instance, the study conducted by Seo et al. showed that fast food consumption behavior was significantly associated with behavioral intention and perceived behavioral control. In addition, their findings highlighted that behavioral intention was significantly related to subjective norm and perceived behavioral control [ 28 ].
Given that our study population has cultural diversity and nutritional behaviors different from the societies of other countries and According to the mentioned materials, the researchers decided to test the study with the aim of investigating and explaining the intention and behavior of fast food consumption and their related factors based on the TPB among Urmia University of Medical Sciences students. The results of this study will increase the awareness and knowledge about fast food and, in addition, its results can be used in research, hospitals and healthcare settings.
This cross-sectional study was performed on students of Urmia University of Medical Sciences located in northwest Iran in academic year of 2018–2019. The inclusion criteria for the study are females and males who studied at Urmia University of Medical Sciences, and students’ voluntary participation in the study and obtaining written consent from the students and University principals for the students’ participation in the study. The lack of willingness to continue participating in the study and not signing the informed consent form were considered as exclusion criteria.
According to the results of the study of Yar Mohammadi and et al. [ 31 ], with a 95% confidence interval and an error of 0.05, using the formula for estimating the proportion in society, taking into account the 10% drop rate, sample size was estimated 330students. A randomized stratified sampling method was used to select the study samples. The study sample was randomly selected from each of the strata based on the share of the total sample.
Questionnaire
The data gathering tool in this study was a self-reported questionnaire (Additional file 1 ), which was designed according to the existing measures in scientific literature [ 32 , 33 , 34 ]. The study instrument was translated from English to Persian using a standard forward-backward translation technique [ 35 ]. The original instrument was translated by a bilingual specialist. The Persian version was then retranslated into English by two independent bilingual professionals to assess retention of the original meaning in the source language. Subsequently, translators worked separately in the translation process and then prepared the final version of the Persian translation. Content validity of The Persian version of questionnaire was evaluated by a panel of experts such as 3 nutrition specialists, 3 health education specialists, and 2 instrument designers. After receiving their comments, crucial revisions were conducted in the study tool. Finally, validity of the study instrument was confirmed. The present questionnaire including four following sections:
General characteristics
The first part contains personal information such as age, gender, weight, height, field of study, student education, father’s education, mother’s education, father job, mother’s job, ethnicity, marital status, participating in nutrition educational classes, students’ monthly income, family’s monthly income, housing status, information resource for healthy nutrition.

Constructs of the TPB
The second part contains questions about the constructs of the theory of planned behavior (attitude, subjective norms, perceived behavioral control and behavioral intention). In general, attitudes, subjective norm and perceived behavioral control of students were measured using indirect items. The internal reliability of all subscales of the TPB variables was good, with a Cronbach’s alpha of 0.852.
Attitude toward fast food use
The attitude of the people was evaluated using 28 indirect items (14 items of behavioral beliefs, 14 items of expectations evaluation) based on five-point the Likert scale (from strongly agree to strongly disagree) or (from very important to not at all important), and the score of each item varied from 1 to 5. The minimum and maximum score for the attitude subscale was 14 and 350, respectively. The internal reliability of attitude subscale was good, with a Cronbach’s alpha of 0.778.
Subjective norm
Subjective norms of students were measured by 10 indirect items (5 items of normative beliefs, 5 items of motivation to comply) based on five-point the Likert scale (from strongly agree to strongly disagree) or (from very important to not at all important), and the score of each item varied from 1 to 5. The minimum and maximum score for the subjective norm subscale was 5 and 125, respectively. The internal reliability of subjective norm subscale was good, with a Cronbach’s alpha of 0.726.
Perceived behavioral control
Perceived behavioral control were measured by 18 indirect items (9 items of control beliefs, 9 items of perceive power) based on five-point the Likert scale (from strongly agree to strongly disagree) or (from extremely difficult to extremely easy), and the score of each item varied from 1 to 5. The minimum and maximum score for the perceived behavioral control subscale was 9 and 225, respectively. The internal reliability of subscale of perceived behavioral control was good, with a Cronbach’s alpha of 0.815.
Behavioral intention
Behavioral intention was evaluated by 8 items based on five-point the Likert scale (from strongly agree to strongly disagree), and the score of each item varied from 1 to 5. The minimum and maximum score for the Behavioral intention subscale was 8 and 40, respectively. The internal reliability of behavioral intention subscale was good, with a Cronbach’s alpha of 0.821.
Knowledge of participants
And the third and fourth parts are items related to food knowledge and fast food behavior. Students’ knowledge of fast food was evaluated by 14 items, and the score of each item varied from 0 to 2. The minimum and maximum score for the knowledge subscale was 0 and 28, respectively. The internal reliability of students’ knowledge was good, with a Cronbach’s alpha of 0.783.
Fast food use
Students’ fast food consumption was assessed by frequency of use in a past month. The term “Fast food” was defined as hamburgers, doughnuts, hot dog, snack, pizza, fried chicken and fried potatoes. The frequency of fast food use was analyzed for each food category.
Statistical analyses
All statistical analyzes were performed using SPSS 16.0 software. Descriptive statistics methods such as frequencies, means and standard deviations were used along with independent t and χ2 tests. Pearson correlation test was used to investigate the relationship between TPB variables with intent to use fast food and the real use of fast food. Multiple regressions were used for further analysis.
Descriptives
A total of 330 students were selected and recruited to the study, but some subjects (31 samples) were excluded from the study due to incomplete questionnaires (21cases), and no return of questionnaires (10 cases). Statistical analyses were performed on 229 students. Of these, 28.4% of the students were males and 71.6% were females. The results of the study showed that the average age for all the students was 22.10 ± 3.30 (the average age for male and female sexes were 22.66 ± 4.47 and 21.84 ± 2.50, respectively). The two sexes differed in terms of BMI, so that the mean of BMI was higher in boy students than in girls, and this difference was statistically significant. Almost more than 72% of the students had normal weight, and 28% of subjects were in other weights. Approximately 20.51, 54.50, 79.77% of the students reported the professional doctoral degree, Azeri ethnicity and single.
In addition, findings revealed that 64.90% of the participants lived in the dormitory, and 35.10% of them lived in personal or rental housing. The most common level of education for father (37.10%) and mother (44.10%) of students was diploma. Nearly, 46.50% of students gained food information (especially fast food) from health care providers, while 53.50% of them received their food information from other sources. Most students had zero monthly income, but 61.61% of the students reported their family’s monthly income more than 50 million Rials and 38.39% of their family had income lower than the mentioned amount. Table 1 provides detailed information on students’ characteristics.
Main analysis
Table 2 presents the mean score of knowledge and variables of the study-related theoretical framework. As the mean score of subjective norm, perceived behavioral control and behavioral intention in male students compared to female students was high, but those were not significant statistically( p > 0.05).
Some variables of the TPB were significantly correlated with each other ( P < 0.01, Table 3 ). In particular, fast food consumption behavior was highly ( r = 0.382) correlated with behavioral intention. Multiple regression analyses were conducted to determine the relative importance of the variables of the TPB to behavioral intention and fast food consumption behavior (Tables 4 and 5 ). In these analyzes, when the attitude toward behavior, subjective norms, and perceived control was regressed to behavioral intention, the model was very significant ( P = 0.000) and explained 0.347 of variance of behavioral intention. While attitude and perceived behavioral control were not significant, the subjective norms and students’ knowledge were significantly related to the intention to eat fast food. It seems that subjective norms and students’ knowledge to be the most important predictors of behavioral intent. Table 4 shows more information about predictors of behavioral intention.
The second model, using fast food consumption as a dependent variable, was also very significant ( P = 0.000), and explained nearly a quarter of the variance (0.231) of fast food consumption. Both behavioral intention and perceived behavioral control were significantly associated with fast food consumption, of which behavioral intention appeared to be more important. Table 5 presents more information about predictors of fast food consumption.
This investigation was conducted on a sample of university students to assess the status of their fast-food consumption. It also examined the factors affecting behavioral intent and fast food consumption by applying the TPB. The results of the present study showed that students consumed fast food at an average of 2.7 times a month. Fast food in male students was often reported more than female students. A study on fast food consumption among students at Daejeon School reported monthly frequencies of fast food types: 2.7 for burgers, 2.1 for French fries, 1.8 for chicken [ 24 ]. Results of Kim study and other similar researches [ 31 , 36 ] approximately were in line with findings of the present study.
Given that most men do not have the time and skill to make traditional foods, and because of a lot of work, they prefer to turn to fast-foods, and so they are more likely to use fast foods. Meanwhile, the results of some studies indicate that most women are not very happy from high weight and are more likely to reduce their weight [ 37 ]. Therefore women do not have a positive attitude toward obesogenic foods compared to men [ 38 ], which can be a reason for consuming less fast food among women. Instead, the results of a study done by Seo et al. In Korea indicated that fast food consumption among high school students was 4.05 times a month and this consumption was reported among boys more than girls [ 28 ]. The results of the Korean study were contrary to the results of the study, meaning that fast food in Korean samples was more than Iranian. The reason for this difference can be traced to factors such as sample size, cultural, social, and economic characteristics of the samples.
Performing and not performing the behavior by a person is a function of several factors based on the theory of planned behavior. One of these factors is the person’s intention and desire to do the behavior. Behavioral intention itself is also affected by factors such as attitude, students’ knowledge, social pressure, and perceived behavioral control. In the present study, based on linear regression analysis, students’ knowledge and social pressure were both related to their intention and consume fast foods. That is, students who had the necessary information about nutrition, especially fast foods, had a high intent to choose and consume foods.
Several studies have examined the relationship between knowledge of foods and their contents and attitudes toward fast foods and processed foods or relationship between attitudes toward food additives and food choice behavior [ 39 , 40 , 41 , 42 ]. Aoki et al. [ 39 ] found that information about food and its contents positively or negatively affects attitudes and intentions towards food. They pointed out that food information was important for consumers in choosing food. Back and Lee [ 43 ] found that consumers had inadequate and incorrect information about foods, which could affect their attitudes or intent. These studies suggest that providing more information about foods and their compounds can help them to improve their attitude towards foods. Therefore, training on the performance, benefits and safety of foods, including positive and negative sides, should prevent misunderstandings about food supplements and reduce food safety concerns.
The findings of the present investigation showed that subjective norms of students were effective on intent to use fast foods. Friends had the most impact on the plan to eat fast foods, as expected. In addition, the normative beliefs of students were also more positive for friends than family and teachers. This conclusion suggests that most training programs should focus on their friends as a critical group that may affect intent to use fast foods.
Results of some previous studies were similar to findings of the current study. One study conducted by Mirkarimi et al. highlighted that subjective norms had the main role on students’ intent to use fast foods [ 44 ]. In the other words, they found that behavioral intention was affected by subjective norms. In addition, the study of Yarmohammadi and et al. showed that subjective norms predict intention and behavior [ 31 ].
In this study, TPB demonstrated to be a sound conceptual framework for explaining closely35% of the variance in students’ behavioral intention to consume fast-food. Among the TPB variables, subjective norm and knowledge of students were the most important predictors of intention to use fast foods. These findings are consistent with other results that identify that subjective norms have a significant effect on consuming fruits and vegetables [ 45 ]. In study of Lynn Fudge, Path analysis highlighted that TPB explained adolescent fast-food behavioral intention to consume fast food. The model identified subjective norms had the strongest relationship with adolescent behavioral intention to consume fast food [ 46 ].
The results of this study showed that the attitude toward fast food behavior did not predict intent and the behavior. However, some studies have reported contradictory findings with the study. For example, the findings of Stefanie and Chery’s study showed that attitude was a predictor for intent to use healthy nutrition [ 47 ]. Yarmohammadi and colleagues stated in their study that attitude was the most important predictor of behavioral intent [ 31 ]. In the study of determinants of fast food intake, Dunn et al. has identified attitude as a predictor of the intent of fast food consumption [ 32 ]. The results of studies by Seo et al., Ebadi et al., along with the findings of this study, showed that attitude toward fast food consumption is not significantly related to behavioral intention [ 28 , 48 ]. Based on the findings of the current study, fast-food consumption of students was also influenced by some the TPB variables. Multiple linear regression analyses revealed that the constructs of the TPB explained fast food use behaviors with R-squared (R 2 ) of 0.23. In these analyses, intention, perceived behavioral control, and knowledge were known as effective factors on fast-food consumption. Among the TPB constructs, behavioral intention was the most important predictor of fast-food consumption. The intention plays a fundamental role in the theory of planned behavior. The intentions include motivational factors that influence behavior and show how much people want to behave and how hard they try to do the behavior [ 49 ]. In study Ebadi et al., regression analysis showed the intention as a predictor of fast food consumption behavior [ 48 ]. In studies of Stefanie et al. and Seo et al., has reported intention as correlate of the behavior [ 28 , 47 ]. All these studies confirmed and supported this part of our study findings. In addition, the results indicated that perceived behavioral control directly influenced the behavior of fast-food consumption. Some investigations confirmed this portion of our results. For instance, the results of Dunn et al. showed that perceived behavioral control (PBC) and intent predicted the behavior of fast food consumption [ 32 ]. Also, in the study of Seo et al., regression analysis showed that fast food consumption behavior was correlated with perceived behavioral control [ 28 ]. Yarmohammadi et al. found that in predicting behavior, perceived behavioral control along with intention could predict 6% of behavior [ 31 ]. Although this study provides valuable knowledge regarding the relationships between behavioral intent and TPB variables, this study, like other studies, has a number of limitations. First, a cross-sectional study was used to examine the relationship between the variables. Due to the fact that in cross-sectional studies, all data are collected in a period of time, as a result, these studies do not have the necessary ability to examine the cause-and-effect relationships between variables. Second, the results of this type of study can only be generalized to populations with similar characteristics and have no generalizability beyond that. Third, since the data of this study were collected using the self-report questionnaire, the respondents may have errors and bias in completing the questionnaire and this can affect the results of the study.
In sum, this study was conducted to identify factors influencing intention and behavior of fast-food consumption among students by using the theory of planned behavior. The findings revealed that changeability of students’ intention to use fast food and their real behavior is dependent on the TPB variables. As this theoretical framework explained 35, 23% of intent to consume fast-foods and fast-food consumption, respectively. Among the TPB constructs, knowledge and subjective norm were known as the most important predictors of intention to use fast foods. In addition, the results indicated that intention and perceived behavioral control were the most important factors influencing consumption of fast foods among participants. It is imperative that health educators and promoters use these results in designing suitable educational interventions to improve people’s nutritional behavior.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available due to confidentiality of data and subsequent research, but are available from the corresponding author on reasonable request.
Abbreviations
Theory of Planned Behavior
Theory of Reasoned Action
Statistical Package for Social Sciences
Body Mass Index
ISAAC Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhino conjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–32.
Google Scholar
Anonymous. Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community respiratory health survey (ECRHS). Eur Respir J. 1996;9:687–95.
Hijazi N, Abalkhail B, Seaton A. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax. 2000;55:775–9.
CAS PubMed PubMed Central Google Scholar
Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43.
PubMed Google Scholar
Beaglehole R, Bonita R, Horton R, et al. Priority actions for the non-communicable disease crisis. Lancet. 2011;377:1438–47.
Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–74.
CAS PubMed Google Scholar
Nazari B, Asgari S, Sarrafzadegan N, et al. Evaluation and types of fatty acids in some of the most consumed foods in Iran. J Isfahan Med School. 2010;27(99):526–34.
Word Health Organization (WHO). Diet, nutrition and the prevention of chronic diseases report of a joint WHO/FAO expert consultation. Geneva: WHO.2003. Available at: http://whqlibdoc.who.int/publications/9241590416.pdf . [Accessed Jun 21, 2011].
Ashakiran S, Deepthi R. Fast foods and their impact on health. JKIMSU. 2012;1(2):7–15.
Vaida N. Prevalence of fast food intake among urban adolescent students. IJES. 2013;2(1):353–9.
Bowman SA, Vinyard BT. Fast food consumption of US adults: impact on energy and nutrient intakes and overweight status. J Am Coll Nutr. 2004;23(2):163–8.
Abdollahi M, Amini M, Kianfar H, et al. Qualitative study on nutritional knowledge of primary-school children and mothers in Tehran. EMHJ-Eastern Mediterranean Health Journal. 2008;14(1):82–9.
Shahanjarini A, Shojaezadeh D, Majdzadeh R, et al. Application of an integrative approach to identify determinants of junk food consumption among female adolescents. Iran J Nutr Sci Food Technol. 2009;4(2):61–70.
Lee JS. A comparative study on fast food consumption patterns classified by age in Busan. Korean J Commun Nutr. 2007;12(5):534–44.
Dehdari T, Mergen T. A survey of factors associated with soft drink consumption among secondary school students in Farooj city, 2010. J Jahrom Univ Med Sci. 2012;9(4):33–9.
Brownell KD. Does a" toxic" environment make obesity inevitable? Obssity Manage. 2005;1(2):52–5.
Faghih A, Anousheh M. Evaluating some of the feeding behaviors in obese patients visiting affiliating health centers. Hormozgan Med J. 2008;12(1):53–60.
Paeratakul S, Ferdinand DP, Champagne CM, et al. Fast-food consumption among US adults and children: dietary and nutrient intake profile. J Am Diet Assoc. 2003;103(10):1332–8.
Timperio AF, Ball K, Roberts R, et al. Children’s takeaway and fast-food intakes: associations with the neighbourhood food environment. Public Health Nutr. 2009;12(10):1960–4.
Pour Mahmoudi A, Akbar TabarTuri M, Pour Samad A, et al. Determination of peroxide in the oil consumed in restaurants and snack bar Yasuj. J Knowledge. 2008;13(1):116–23 [In Persian].
SadrizadehYeganeh H, AlaviNaein A, DorostiMotlagh A, et al. Obesity is associated with certain feeding behaviors in high school girls in Kerman. Payesh Quarterly Summer. 2007;6(3):193–9 [In Persian].
Greger N, Edwin CM. Obesity: a pediatric epidemic. Pediatr Ann. 2001;30(11):694–700.
Ghaffari M, Gharghani ZG, Mehrabi Y, et al. Premarital sexual intercourse-related individual factors among Iranian adolescents: a qualitative study. Iran Red Crescent Med J. 2016;18(2):e21220.
PubMed PubMed Central Google Scholar
Kim KW, Ahn Y, Kim HM. Fast food consumption and related factors among university students in Daejeon. Korean J Commun Nutr. 2004;9(1):47–57.
Harris KM, Gordon-Larsen P, Chantala K, et al. Longitudinal trends in race/ethnic disparities in leading health indicators from adolescence to young adulthood. Arch Pediatr Adolesc Med. 2006;160(1):74–81.
Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179–211.
Branscum P, Sharma M. Using the theory of planned behavior to predict two types of snack food consumption among Midwestern upper elementary children: implications for practice. Int Quarterly Commun Health Educ. 2011;32(1):41–55.
Seo H-s, Lee S-K, Nam S. Factors influencing fast food consumption behaviors of middle-school students in Seoul: an application of theory of planned behaviors. Nutr Res Pract. 2011;5(2):169–78.
Hewitt AM, Stephens C. Healthy eating among 10-13-year-old New Zealand children: understanding choice using the theory of planned behavior and the role of parental influence. Psychol Health Med. 2007;12:526–35.
Didarloo A, Shojaeizadeh D, EftekharArdebili H, et al. Factors influencing physical activity behavior among Iranian women with type 2 diabetes using the extended theory of reasoned action. Diabetes Metab J. 2011;35(5):513–22.
Yarmohammai P, Sharirad GH, Azadbakht L, et al. Assessing predictors of behavior of high school students in Isfahan on fast food consumption using theory of planned behavior. Journal of Health Syst Res. 2011;7(4):449–59.
Dunn K, Mohr P, Wilson C, et al. Determinants of fast food consumption: an application of the theory of planned behavior. Appetite. 2011;23(57):349–57.
Dunn KI, Mohr PB, Wilson CJ, Wittert GA. Beliefs about fast food in Australia: a qualitative analysis. Appetite. 2008;51(2):331–4.
Denney-Wilson E, Crawford D, Dobbins T, Hardy L, Okely AD. Influences on consumption of soft drinks and fast foods in adolescents. Asia Pac J Clin Nutr. 2009;18(3):447–52.
Brisling RW. The wording and translation of research instruments. In: Loner WJ, Berry JW, editors. Field Methods in Cross-cultural Research. Beverly Hills, CA: Sage; 1986. p. 134–64.
Sanaye S, Azarghashb A, Derisi M, et al. A survey on knowledge and attitude of students of ShahidBeheshti University of medical sciences toward fast food. Scientific J Med Council Islamic Republic Iran. 2016;34(1):23–30.
Driskell JA, Meckna BR, Scales NE. Differences exist in the eating habits of university men and women at fast-food restaurants. J Nutr. 2006;26(10):524–30.
CAS Google Scholar
Morse KL, Driskell JA. Observed sex differences in fast-food consumption and nutrition self-assessments and beliefs of college students. Sci Direct J Nutr Res. 2009;29(3):173–9.
Aoki K, Shen J, Saijo T. Consumer reaction to information on food additives: evidence from an eating experiment and a field survey. J Econ Behav Organ. 2010;73:433–8.
Stern T, Haas R, Meixner O. Consumer acceptance of wood-based food additives. Br Food J. 2009;11:179–95.
Kim H, Kim M. Consumers' awareness of the risk elements associated with foods and information search behavior regarding food safety. J East Asian Soc Diet Life. 2009;19:116–29.
Seo S, Kim OY, Shim S. Using the theory of planned behavior to determine factors influencing processed foods consumption behavior. Nutr Res Pract. 2014;8(3):327–35.
Back BS, Lee YH. Consumer's awareness and policies directions on food additives-focusing on consumer information. J Consum Stud. 2006;17:133–50.
Mirkarimi K, Mansourian M, Kabir MJ, et al. Fast food consumption behaviors in high-school students based on the theory of planned behavior (TPB). Int J Pediatr. 2016;4(7):2131–42.
Murnaghan DA, Blanchard CM, Rodgers WM, et al. Predictors of physical activity, healthy eating and being smoke-free in teens: a theory of planned behavior approach. Psychol Health. 2010;25:925–41. https://doi.org/10.1080/08870440902866894 .
Article PubMed Google Scholar
Julie Lynn Fudge. Explaining adolescent behavior intention to consume fast food using the theory of planned behavior. Dissertation Submitted to the Graduate Faculty Of the North Dakota State University Of Agriculture and Applied Science. lib.ndsu.nodak.edu. 2013.
Stefanie A, Chery S. Applying the theory of planned behavior to healthy eating behaviors in urban native American youth. Int J Behav Nutr Phys Act. 2006;30(3):1–10.
Ebadi L, Rakhshanderou S, Ghaffari M. Determinants of fast food consumption among students of Tehran: application of planned behavior theory. Int J Pediatr. 2018;6(10):8307–16.
Pender NJ, Murdaugh C, Parsons MA. Health promotion in nursing practice. 4th edition. Upper Saddle River, NJ: Prentice-Hall Health Inc; 2002. p. 250–5.
Download references
Acknowledgements
The article authors hereby express their gratitude to Vice Chancellors for Research of Urmia University of Medical Sciences and Education Department for supporting this study.
This study is supported by Urmia University of Medical Science, grant number(No: 2017–2323) .
Author information
Authors and affiliations.
Social Determinants of Health Research Center, Clinical Research Institute, Urmia University of Medical Sciences, The Province of Western Azarbaijan, Urmia, 5756115198, Iran
Alireza Didarloo
Faculty of Health, Urmia University of Medical Sciences, the Province of Western Azarbaijan, Urmia, 5756115198, Iran
Surur Khalili & Ahmad Ali Aghapour
Reproductive Health Research Center, Urmia University of Medical Sciences, the Province of Western Azarbaijan, Urmia, 5756115198, Iran
Fatemeh Moghaddam-Tabrizi
Faculty of Paramedical Sciences, Urmia University of Medical Sciences, the Province of Western Azarbaijan, Urmia, 5756115198, Iran
Seyed Mortaza Mousavi
Department of Paramedical Science, School of Paramedical Sciences, Urmia University of Medical Sciences, Urmia, Iran
You can also search for this author in PubMed Google Scholar
Contributions
All authors contribute in conceive, design of this study. A.D, S.K, A.A,FTM and S.M contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. All authors revised the manuscript critically for important intellectual content and read and approved the final manuscript.
Corresponding author
Correspondence to Seyed Mortaza Mousavi .
Ethics declarations
Ethics approval and consent to participate.
Research has been presented in the ethics committee of Urmia University of Medical Sciences and has received the code of ethics (IR. UMSU.REC.1397.43). written informed consent was obtained from all participants in this study, and all provisions of the Helsinki Statement on Research Ethics were considered.
Consent for publication
Not applicable.
Competing interests
The authors declared no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
The questionnire used in the study to collect the data. The first part of the questionnaire included General characteristics. The second part of the questionnaire consisted of the Constructs of TPB. The third part consisted of knowledge of participants. The fourth part consisted of Fast food use.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Didarloo, A., Khalili, S., Aghapour, A.A. et al. Determining intention, fast food consumption and their related factors among university students by using a behavior change theory. BMC Public Health 22 , 314 (2022). https://doi.org/10.1186/s12889-022-12696-x
Download citation
Received : 07 December 2020
Accepted : 02 February 2022
Published : 15 February 2022
DOI : https://doi.org/10.1186/s12889-022-12696-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Theory of planned behavior
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
Food and Fluid Intake Research Paper

View sample food and fluid intake research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
Ingestive behaviors play primary roles in the maintenance of fluid homeostasis and energy balance. Feeding and drinking are intermittent behaviors that both renew and anticipate depletions. Their controls are complex and redundant. Early views of homeostasis focused on physiological mechanisms in the body for maintaining constant internal states. Claude Bernard (1859) and Walter Cannon (1932) put forth the concept of homeostasis as the maintenance of the internal milieu within fixed limits through the coordination of controlled physiological processes. Although Bernard and Cannon recognized a role for behavior in these processes, Curt Richter (1943) expanded the view of physiological defenses of the internal milieu to include behavior as a major regulating factor. For Richter, the role of behavioral regulators in homeostasis was broadly conceived and studied. A focus on the behavior itself has revealed that both food intake and water intake have multiple levels of control that interact to ensure that the body has adequate hydration and energy stores.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% off with 24start discount code.
In the following sections we focus on the major physiological systems involved in the controls of food and fluid intake, identifythesystemsthatmonitoravailablenutrientandhydrationalstores,discusssystemsthatrespondtotheconsequences of food and fluid intake, and identify how interactions among these systems produce behavioral outcomes that result in adequate and appropriate food and fluid intake.
Food Intake and Energy Balance
What and how much we eat depend on a wide variety of factors. These include factors related to palatability or taste, learning, social and cultural influences, environmental factors, and physiological controls. The relative contributions of these many factors to feeding control vary across species and testing situations. We concentrate on the roles of three interacting systems important in feeding control. These are systems that mediate (a) signals related to metabolic state, especially to the degree of adiposity; (b) affective signals related to taste and nutritional consequences that serve to reinforce aspects of ingestive behavior; and (c) signals that arise within an individual meal and produce satiety. We also identify the important interactions among these systems that permit the overall regulation of energy balance.
Metabolic Signals and Their Mediation
Arole for signals related to the availability of energy stores in the control of food intake has long been postulated. Depletionrepletion models tied to carbohydrate availability (Mayer, 1953) and fat stores (Kennedy, 1953) have been proposed. Although neither of these individual models is sufficient to explain the multiple variations in food intake that occur throughout the life cycle, evidence for food intake controls that depend upon monitoring fuel availability and utilization is strong. Administration of metabolic inhibitors that act on differing metabolic pathways stimulate food intake. For example, treatment with either 2-deoxy-D-methyl glucose (2-DG), which inhibits glucose utilization, or methyl palmoxirate, which suppresses fatty acid oxidation, stimulates food intake in satiated animals. Sites of action in both the liver and brain have been identified (Ji, Graczyk-Milbrandt, & Friedman, 2000; Ritter, Dinh, & Zhang, 2000).
Alterations in circulating glucose have been tied to meal initiation in both rats and humans. Campfield and colleagues have shown that transient declines and partial restorations in blood glucose levels reliably predict meal initiation (Campfield & Smith, 1986; Melanson, Westerterp-Plantenga, Saris, Smith, & Campfield, 1999). Demonstrations that experimentally induced declines in blood glucose can result in meal initiation suggest that the relationship may be more than correlational (Smith & Campfield, 1993).
Studies of genetic obesity models had long suggested the importance of circulating factors in overall body weight control. Having identified two different mutations in mice that led to obesity, Coleman (1973) conducted parabiotic experiments involving two strains of obese (obese, ob/ob, and diabetic, db/db) and normal mice in which the blood supply between the two mice in a parabiotic pair was shared. The results showed that when paired with db/db mice, ob/ob mice became hypoglycemic, lost weight, and eventually died—asimilar response to that seen in normal mice combined with db/db mice. In contrast, when combined with normal mice, ob/ob mice gained less weight than they otherwise would but were fully viable. The results led Coleman to conclude that the ob/ob mouse lacked a circulating satiety factor that, in its absence, results in hyperphagia and obesity whereas the db/db mouse produced the factor but lacked the ability to respond appropriately to it.
The positional cloning of leptin as the product of the ob gene (Zhang et al., 1994) and subsequent identification of the leptin receptor as the product of the db gene (Chua et al., 1996; Tartaglia et al., 1995) has provided the basis for Coleman’s observations. Leptin not only normalizes food intake and body weight in ob/ob mice but also reduces food intake in normal mice and rats (Campfield, Smith, Guisez, Devos, & Burn, 1995; Seeley et al., 1996). Leptin is produced in white adipose tissue, and circulating leptin levels correlate positively with adipose mass as animals and humans become obese (Maffei et al., 1995). Thus, leptin signals the availability of body energy stores.
Leptin is currently viewed as the major adiposity factor important for the long-term control of energy balance. Leptin receptors are members of the cytokine-receptor superfamily. Multiple leptin receptor isoforms that arise from differential splicing have been identified. The predominant form of the leptin receptor is the short form (Ob-Ra), which is widely expressed in multiple areas including the choroid plexus and brain microvasculature (Bjorbaek et al., 1998). These binding sites are likely to function as a part of a saturable transport system for circulating leptin to gain access into the brain.Thelong form of the leptin receptor (Ob-Rb) can activate Janus kinase (JAK) signal transduction andsignal transducers and activators of transcription (STAT) elements to mediate leptin’s cellular actions (Bjorbaek, Uotoni, da Silva, & Flier, 1997). Ob-Rb is highly expressed within hypothalamic nuclei with identified roles in energy balance. Highest concentrations of the long form of the leptin receptor are found within the arcuate, paraventricular, and dorsomedial hypothalamic nuclei as well as within the lateral hypothalamus (LH; Elmquist, Bjorbaek, Ahima,Flier,&Saper,1998).InteractionsofleptinwithOb-Rb receptors within these hypothalamic nuclei result in the activation or inactivation of hypothalamic pathways containing various orexigenic and anorexigenic peptides (M. W. Schwartz, Seeley, Campfield, Burn, & Baskin, 1996).
Hypothalamic Systems Involved in Food Intake
The role of the hypothalamus in food intake control was established through the classic experiments of Heatherington and Ranson (1940) and Anand and Brobesck (1951). Using stereotaxically placed lesions, they demonstrated that bilateral lesions of the medial hypothalamus resulted in hyperphagia and obesity whereas lesions of the LH produced profound anorexia and weight loss. Subsequent work demonstrated that stimulation of these hypothalamic regions had the opposite effects. Medial hypothalamic stimulation inhibited food intake whereas stimulation of the LH produced food intake. Results such as these led Stellar (1954) to propose the classic dual center hypothesis for the role of the hypothalamus in food intake. The ventromedial region (VMH) was viewed as a satiety center, and the LH was viewed as a feeding center.
The roles of various hypothalamic nuclei in food intake are now much better understood, and many of the peptide neurotransmitters through which these actions are mediated have been identified. Table 11.1 depicts the variety of the hypothalamic peptides that have effects on food intake. These may be broadly classified as falling into two categories: orexigenic, or those that stimulate or increase food intake, and anorexigenic, or those that decrease food intake.
Among the orexigenic peptides, the one that has received the most attention is neuropeptide Y (NPY). Intracerebroventricular or direct hypothalamic injection of NPY potently stimulates feeding (Clark, Kalra, Crowley, & Kalra, 1984; Morley, Levine, Gosnell, Kneip, & Grace, 1987; Stanley, Krykouli, Lampert, & Leibowitz, 1986), and repeated or chronic NPY administration results in obesity (Stanley et al., 1986; Zarjevski, Cusin, Vetter, Rohner-Jeanrenaud, & Jeanrenaud, 1993). Hypothalamic NPY gene expression and secretion increase in response to food deprivation (Kalra, Dube, Sahu, Phelps, & Kalra, 1991; White & Kershaw, 1989) or exercise (Lewis et al., 1993) and decrease in response to overconsumption of a highly palatable high-energy diet (Widdowson et al., 1999). Cell bodies of neurons expressing NPY are found in multiple hypothalamic nuclei including the arcuate and dorsomedial hypothalamic nuclei (Chornwall et al., 1985). Important projection sites for these neurons in mediating the feeding stimulatory actions of NPY are the paraventricular nucleus andperifornicalregionoftheLH(Stanley&Leibowitz,1985; Stanley, Magdalin, Seirafi, Thomas, & Leibowitz, 1993). Whereas chronic treatment with NPY results in obesity, absence of NPYor its receptors does not result in the absence of food intake or wasting. Murine knockout models that do not express NPY or NPY receptors are viable (Erikson, Clegg, & Palmiter, 1996; Marsh, Hollopeter, Kafer, & Palmiter, 1998; Pedrazzini et al., 1998). Rather than suggesting that NPYdoes notplayaroleinfoodintakecontrolandenergybalance,these results should be interpreted as suggesting that there are multiple redundant systems available for stimulating food intake and that the absence of one is not sufficient to block this critical behavior significantly.
Other hypothalamic orexigenic peptides have been identified and their roles in food intake investigated. These include galanin, hypocretin 1 and 2 (also known as orexin A and B) and melanin concentrating hormone (MCH). Galanin stimulates food intake following either intraventricular or hypothalamic administration (Crawley et al., 1990; Kyrkouli, Stanley, & Leibowitz, 1986). Galanin levels and mRNA expression are elevated in rats consuming a high-fat diet but do not appear to be affected by food deprivation (Beck, Burlet, Nicolas, & Burlet, 1993; Mercer, Lawrence, & Atkinson, 1996). Whereas galanin antagonists block the actions of exogenous galanin on food intake, little effect of the antagonists alone have been demonstrated (Crawley, 1999).
Hypocretin 1 and 2 (i.e., orexin Aand B) are recently identified peptides that are coded from same prepro-mRNA (Sakurai et al., 1998). Both compounds increase food intake when centrally administered, but orexin A is much more potent (Sakurai et al., 1998; Sweet, Levine, Billington, & Kotz, 1999). Orexin-containing neurons are found in the perifornical area of the hypothalamus and project throughout the hypothalamus (Peyron et al., 1998). Prepro-orexin expression is increased in response to deprivation (Lopez et al., 2000), and administration of an orexin A antagonist has been demonstrated to inhibit food intake, suggesting a role for endogenous orexin A in food intake control (Haynes et al., 2000).
Intraventricular MCH administration increases food intake in a dose-related fashion in short-term tests but does not alter 24-hr food intake, and chronic administration does not result in significant weight gain (Rossi et al., 1997). MCH expression is increased in obesity, and levels are modulated by fasting (Qu et al., 1996). MCH neurons in the LH are a distinct population from those expressing hypocretin/orexin; like orexin neurons, however, they are innervated by arcuate nucleus NPY-containing fibers (Broberger, DeLecea, Sutcliffe, & Hokfelt, 1998).
Endogenous melanocortins have both feeding-stimulatory and feeding-inhibitory actions. Pro-opiomelanocortin (POMC) is the precursor for a variety of peptides. Among these is the anorexigenic peptide alpha-melanocyte stimulating hormone (-MSH). Central administration of -MSH or synthetic melanocortin agonists potently inhibits food intake (Benoit et al., 2000; Fan, Boston, Kesterson, Hruby, & Cone, 1997). The feeding inhibitory actions of central melanocortins are mediated primarily through interactions with the melanocortin-4 (MC-4) receptors. Within the hypothalamus, POMC expression is limited to the arcuate nucleus. Arcuate POMC expression decreases with food deprivation (Kim, Welch, Grace, Billington, & Levine, 1996) and increases with overfeeding (Hagan et al., 1999), suggesting a regulatory role for this peptide in feeding control. Important roles for melanocortin signaling in energy balance are demonstrated in experiments examining the effects of POMC (Yaswen, Diehl, Brennan, & Hochgeschwender, 1999) or MC-4 receptor (Huszar et al., 1997) knockouts. Unlike many other peptide-signaling systems that affect food intake, the melanocortin system has an endogenous receptor antagonist that is orexigenic. Agouti-related protein (AgRP) is localized to the arcuate nucleus, and its expression is up-regulated by fasting (Hahn, Breininger, Baskin, & Schwartz, 1998). AgRP or synthetic melanocortin antagonists increase food intake when administered centrally, and their effects are long lasting (Fan et al., 1997).
Other hypothalamic anorexigenic peptides have been identified. These include corticotrophin-releasing hormone (CRH), urocortin, and cocaine and amphetamine regulated transcript (CART). Central administration of each of these peptides decreases food intake. The expression of each is decreased in response to food deprivation and increased in states of positive energy balance.
A number of these hypothalamic orexigenic and anorexigenic peptides have been implicated in the actions of leptin. Thus, a primary site of leptin action is within the arcuate nucleus. Ob-Rb receptors are localized to two distinct arcuate nucleus neuronal populations. Within the medial arcuate, Ob-Rb is expressed in cells that also express the orexigenic peptides NPY and AgRP. In more lateral aspects of the arcuate, Ob-Rb is expressed in cells containing the anorexigenic peptides CART and the anorexigenic peptide precursor POMC. Leptin up-regulates POMC- and CART-containing neurons and down-regulates NPY- and AgRP-containing neurons resulting in increased anorexigenic and decreased orexigenic activity (Kristensen et al., 1998; M. J. Schwartz et al., 1996, 1997). Leptin also affects the activity of MCH (Sahu, 1998), orexins (Beck & Richy, 1999) and CRH (van Dijk et al., 1999), down-regulating the expression of the orexigenic peptides and up-regulating the activity of CRH. Thus, as shown in Table 11.2, many of these hypothalamic signaling pathways are responsive to the overall level of adiposity as reflected by circulating leptin levels.

Although leptin is the adiposity signal that has received the most attention, insulin also acts in the hypothalamus as an adiposity signal. Insulin is secreted from pancreatic beta cells rather than adipose tissue. However, insulin levels increase with increased adiposity in response to growing insulin resistance. Insulin is transported from the circulation into the brain, and insulin receptors are localized to the hypothalamus with a high concentration in the arcuate nucleus (Corp et al., 1986). Central insulin inhibits food intake (Woods, Lotter, McKay, & Porte, 1979) and decreases NPY mRNA expression (M. W. Schwartz et al., 1992).
Whereas the hypothalamus has been the main focus of study for anorexigenic and orexigenic peptides, a number of these also have ingestive effects when delivered to the dorsal hindbrain. Thus, fourth-cerebroventricular administration of NPY (Corp, Melville, Greenberg, Gibbs, & Smith, 1990) or a melanocortin antagonist (Grill, Ginsburg, Seeley, & Kaplan, 1998) potently increases food intake, whereas a melanocortin agonist (Grill et al., 1998), CART (Aja, Sahandy, Ladenheim, Schwartz, & Moran, 2001), and urocortin (Grill, Markison, Ginsberg, & Kaplan, 2000) inhibit food intake when administered at this site. These hindbrain actions suggest that the central feeding regulatory system is a distributed one. How these hindbrain and hypothalamic systems interact with one another remains to be determined.
The Role of Reward in Food Intake Control
Taste and palatability play major roles in dietary choices and in the amount of a particular food that is consumed. The effects of taste on ingestion are best demonstrated under conditions in which the feedback effects of postingestional consequences are minimized. A number of paradigms that specifically assess the effects of palatability on ingestion have been commonly used. The first of these is the sham feeding paradigm in which animals have an esophageal or gastric fistula so that consumed liquid nutrients drain out of the fistula and do not accumulate in the stomach. Such a preparation was first employed by Pavlov (1910). Pavlov demonstrated that dogs with open esophageal fistulas did not develop satiety but continued to eat for hours. The sham feeding paradigm has clearly demonstrated the important role of orosensory stimuli in ingestion. Increasing the concentration of saccharide solutions or oil emulsions increases the amount consumed in a linear fashion over extensive concentration ranges (Grill & Kaplan, 1992; Mook, 1963; Weingarten & Watson, 1982).
A second method for assessing the effects of palatability on ingestion involves examining rates of ingestion when access is limited to a brief time period or examining ingestion rates at the very beginning of an ingestive bout. Both of these allow ingestion to be monitored at times during which the inhibitory effects of postoral feedback are minimized. In such tests, increasing concentrations of sugars or adding saccharin to sugar solutions can be shown to produce more rapid rates of licking in rats (Breslin, Davis, & Rosenak, 1996; Davis & Levine, 1977).
The effects of palatability on ingestion have both opioid and dopaminergic mediations. It has long been known that opiate agonists can increase feeding whereas antagonists decrease food intake. The effects of opioid ligands on ingestion appear to occur through alterations in palatability. Morphine enhances the intake of preferred over nonpreferred diets (Gosnell, Krahn, & Majchrzak, 1990) and enhances hedonic responses to sweet solutions as measured in taste reactivity tests (Doyle, Berridge, & Gosnell, 1993). In contrast, administration of the opiate antagonist naloxone specifically reduces the intake of a preferred diet while not affecting the intake of a nonpreferred diet in a choice paradigm in 24-hr deprived rats (Glass et al., 1996).
Although there has been significant interest in the hypothesis that opioids specifically affect the intake of fats deriving from studies demonstrating specific increases or decreases in fat intake with morphine or naloxone in nutrient selfselection paradigms (Marks-Kaufman & Kanarek, 1990), analyses of baseline nutrient preferences have indicated that morphine stimulates fat intake in fat-preferring rats and carbohydrate intake in carbohydrate-preferring rats (Gosnell et al., 1990). Such results have led to the conclusion that opioid effects on macronutrient selection and overall food intake are mediated through their actions in modulating food reward (Glass, Billington, & Levine, 2000).
Dopaminergic mediation of aspects of palatability has also been documented. Low doses of dopamine agonists increase food intake (Sills & Vaccarino, 1996), and animals with severe neurotoxin-induced dopamine depletions (Ungerstedt, 1971) or dopamine deficiency through gene knockout (Zhou & Palmiter, 1995) fail to consume food independently. Feeding increases extracellular dopamine within the nucleus accumbens, and the increase is greater with the consumption of a highly palatable food (Martel & Fantino, 1996), suggesting a specific role for mesolimbic dopamine in mediating food reward. A specific role for dopamine in signaling the incentive value of foods is also supported by work with dopamine antagonists. Dopamine antagonists potently reduce the sham intake of palatable diets. An ID 50 dose of the dopamine 2 (D2) antagonist raclopride produces the same effect on both overall intake and the microstructure of licking as does halving the sucrose concentration (Schneider, Davis, Watson, & Smith, 1990). The consumption of 10% sucrose with raclopride resembles the consumption of 5% sucrose without antagonist pretreatment. Such data have been interpreted as suggesting that dopamine plays a critical role in the hedonic processing of orosensory stimuli. However, unlike opioids that can be shown to shift the hedonic response to ingestants in taste reactivity tests, dopamine antagonists suppress both hedonic and aversive responses (Pecina, Berridge, & Parker, 1997), suggesting alterations in intake through a change in sensorimotor responses rather than through a shift in taste palatability. Such data have been interpreted to suggest that dopaminergic antagonists reduce ingestion of palatable diets by affecting the incentive salience rather than by shifting the hedonic value of palatable diets.
The nutrient consequences of ingestion can also serve to reinforce dietary choice. This is best demonstrated in experiments that pair a novel taste with an intragastric nutrient infusion. Rats prefer the taste that has been associated with intragastric nutrient (Bolles, Hayward, & Crandall, 1981; Sclafani, 1991). Such nutrient conditioning has been demonstrated with simple and complex carbohydrates (Elizalde & Sclafani, 1990; Perez, Lucas, & Sclafani, 1998), proteins (Perez, Ackroff, & Sclafani, 1996), and fats (Lucas & Sclafani, 1989).Although the phenomena of flavor conditioning are well documented, the neural mediation is not well understood. There is some evidence that such preference conditioning can alter the taste responses to the paired flavor at the level of the nucleus of the solitary tract (Giza, Ackroff, McCaughey, Sclafani, & Scott, 1997). However, these effects are relatively weak. Potential opioid mediation of nutrient conditioning has also been investigated. The opioid antagonist naltrexone fails to block either the acquisition or the expression of a flavor preference conditioned by intragastric nutrients (Azzara, Bodnar, Delameter, & Sclafani, 2000), arguing for a nonopioid mediation of nutrient conditioning.
Satiety Signaling
In many species, including humans, food intake occurs in distinct bouts or meals. Meal initiation is determined by a variety of factors, especially food availability. During a meal, ingested nutrients contact a variety of receptors within the oral cavity and gastrointestinal tract, resulting in neural and hormonal signals that contribute to the determination of meal size. Meal size can be highly variable, and alterations in meal size appear to be a major determinant of overall food intake.
Taste plays an important role in determining meal size. Palatable or good-tasting substances are consumed more rapidly and in greater amounts than are unpalatable foods. Analyses of patterns of sham feeding not only have demonstrated effects of palatability on ingestion but also have revealed pregastric contributions to satiety. Sham feeding does eventually stop, and a number of processes have been proposed to contribute to the cessation of sham feeding, including oral metering (Mook, 1990), habituation (Swithers & Hall, 1994), and sensory-specific satiety (decreasing pleasantness of a specific food as more is ingested; B. J. Rolls, 1986). The amount that is sham fed depends also on the experience of the animal with the sham feeding paradigm. Although the rats’intakes double the first time that they sham feed, continued experience with sham feeding significantly increases intake over the next three or four tests. These data demonstrate the presence of a conditioned inhibition on food intake that is due to an association of the oral stimulation with postingestive negative feedback. Only with continued experience is this conditioned inhibition on intake overcome (Davis & Smith, 1990; Weingarten & Kulikovsky, 1989).
In normal ingestion, consumed nutrients contact mechanoand chemosensitive receptors that provide feedback information to the brain that is important to the control of meal size. The potential range of feedback mechanism that could be operating to lead to meal termination is dependent on the distribution of ingested nutrients during the meal. Kaplan, Spector, and Grill (1992) demonstrated in the rat that when the stomach is filled at rates mimicking normal ingestion rates, gastric emptying during the period of gastric fill is much more rapid than following fill, occurs at a constant rate for the duration of the fill period, and is not affected by nutrient concentration. Similar results were found whether the meal was infused or ingested by the rat (Kaplan, Seimers, & Grill, 1997). These data demonstrate that a significant portion of ingested nutrients (in the rat as much as 30%) enters the duodenum, contacts duodenal receptors, and is available for absorption. Similar dynamics of gastric emptying during fill have been demonstrated in rhesus monkeys; however, although volume is a main determinant, nutrient concentration also plays a significant role (Moran, Knipp, & Schwartz, 1999). Thus, the stomach and a significant proportion of the upper intestine are potential sites for within-meal generation of feedback signals.
The vagus nerve (Xth cranial nerve) is the major neuroanatomical link between the gastrointestinal tract and the brain. Vagal afferent fibers with cell bodies in the nodose ganglion arise from the digestive organs and project to the nucleus of the solitary tract (NTS) with a rough viscerotopic representation of the alimentary canal (Altschuler, Bao, Bieger, Hopkins, & Miselis, 1989). The response properties of vagal afferents depend in part on the target organ from which they arise. Although there are also significant spinal gut neural connections, the response properties of this system have not been well characterized.
Mechanosensitive gastric vagal afferents increase their firing in response to increasing gastric load volume. Slowly adapting mechanoreceptive fibers increase their response rate with increasing gastric volume (Andrews, Grundy, & Scratcherd, 1980). The fibers remain active while load volume is retained and show an off response in which activity briefly drops below baseline levels when the load volume is removed. Individual afferents are differentially tuned such that there are differences in their dynamic ranges (G. J. Schwartz, McHugh, & Moran, 1993). Some afferents reach their maximal activity at small intragastric volumes, whereas others do not begin to respond until a significant gastric load is present. Gastric mechanoreceptive vagal afferents do not respond directly to the chemical character of the gastric load. Response rate is similarly increased by nutrient and nonnutrient load volumes that are restricted to the stomach by a pyloric noose (Mathis, Moran, & Schwartz, 1998).
Duodenal vagal afferents are activated by both intraluminal load volume and nutrient character. Slowly adapting mechanoreceptive fibers in the duodenum have been identified. Similar to gastric mechanoreceptive fibers, activity increases with increases in load volume. Duodenal vagal afferents are also directly responsive to nutrient character. For example, both intestinal casein (Eastwood, Maubach, Kirkup, & Grundy, 1998) or lipid infusions (Randich et al., 2000) increase vagal afferent activity. Although gastric vagal activity is not directly responsive to intragastric nutrient character, gastric afferent responsivity can be altered by duodenal nutrient (G. J. Schwartz & Moran, 1998). Thus, gastric vagal afferent activity is modulated in the presence of duodenal nutrients.
These alterations in vagal afferent activity may reflect the actions of duodenal nutrient-induced release of gastrointestinal (GI) peptides. For example, the brain-gut peptide cholecystokinin (CCK) is released by the duodenal presence of nutrient digestion products. Local arterial CCK administration results in increases in vagal gastric mechanoreceptive afferent activity similar to those produced by intragastric load (G. J. Schwartz, McHugh, & Moran, 1991). Combinations of gastric load and CCK produce greater degrees of activity than either alone (G. J. Schwartz et al., 1991). CCK also modifies responses to subsequent intragastric load such that load volume results in greater degrees of activity following CCK administration than prior to it even at times when the initial response has disappeared (G. J. Schwartz et al., 1993). Duodenal vagal afferents also are activated by CCK; combinations of load and CCK combine to produce greater duodenal vagal afferent activity than either alone, and CCK affects the response to subsequent load volumes (G. J. Schwartz, Tougas, & Moran, 1995). CCK also plays a role in the response of duodenal afferents to nutrients. Administration of a CCK antagonist blocks the increase in vagal afferent activity produced by intraduodenal casein (Eastwood et al., 1998).
CCK-induced changes in gastric vagal afferent activity appear to result from a direct action of the peptide on the vagal afferent fibers. Vagal afferents contain CCK receptors (Moran, Norgren, Crosby, & McHugh, 1990), and CCK induces decreases in intragastric pressure that would not be expected to result in a secondary increase in vagal afferent activity (G. J. Schwartz, Moran, White, & Ladenheim, 1997). In contrast, gastrin-releasing peptide (GRP) induced increases in gastric vagal activity appear to be secondary to local peptide-induced changes in gastric motility. GRP increases gastric wall tension and intragastric pressure, and the increases in vagal afferent activity are correlated with these changes. In addition, GRP receptors are not found on vagal afferents (G. J. Schwartz et al., 1997).
Elimination of aspects of vagal afferent or peptideinduced feedback can result in significant alterations in the way that rats pattern their food intake. Surgical vagal deafferentation results in alterations in meal patterns in rats maintained on liquid diet in that such rats consume larger, less frequent meals than do sham-operated controls (G. J. Schwartz, Salorio, Skoglund, & Moran, 1999). Meal frequency is reduced in response to these increases in meal size such that overall food intake is unchanged. Similar alterations in meal size have been reported in response to capsaicin-induced chemical deafferentation. Following capsaicin treatment, rats consume larger meals on a novel diet (Chavez, Kelly, York, & Berthoud, 1997) or with calorically dilute sucrose access (Kelly, Morales, Smith, & Berthoud, 2000). These data demonstrate a role for vagal afferent feedback in the controls of meal size.
Peripheral Peptide Satiety Signaling
A number of peripherally acting peptides with roles in the controls of food intake have been identified. The best characterized of these is the brain-gut peptide CCK. Exogenously administered CCK was originally demonstrated to decrease food intake in rats (Gibbs, Young, & Smith, 1973). This feeding-inhibitory action of CCK and CCK agonists has been demonstrated in a variety of species including humans and nonhuman primates (Moran & McHugh, 1982; Pi-Sunyer et al., 1982). Exogenously administered CCK reduces meal size and results in an earlier appearance of a behavioral satiety sequence (Antin, Gibbs, Holt, Young, & Smith, 1975). A role for CCK in the control of the size of individual meals was confirmed by experiments examining the effects of repeated, meal-contingent CCK administration. CCK consistently reduced meal size without producing a significant change in overall daily food intake (West, Fey, & Woods, 1984).
The satiety actions of CCK depend on interactions with multiple receptor sites. CCK-A receptors, the receptor subtype through which the satiety actions of CCK are mediated, are found on vagal afferent fibers and on circular muscle cells within the pyloric sphincter. As discussed earlier, CCK activates vagal afferents. Surgical or chemical disruption of subdiaphragmatic vagal afferent innervation significantly affects the ability of CCK to inhibit food intake (Ritter & Ladenheim, 1985; Smith, Jerome, & Norgren, 1985; Moran, Baldessarini, Solorio, Lowerry, & Schwartz, 1997). The nature of this disruption is a reduction in CCK’s potency. Low doses of CCK that inhibit food intake in intact rats are ineffective following vagal afferent lesions. Higher doses inhibit intake but to a smaller degree. In contrast, surgical removal of the pyloric sphincter does not affect the ability of low doses of CCK to inhibit intake but truncates the dose-effect curve such that the additional suppression that normally accompanies higher CCK doses is eliminated (Moran, Shnayder, Hostetler, & McHugh, 1988). Results such as these have led to the proposal that the satiety actions of CCK are multifaceted and are, in part, secondary to its local gastrointestinal effects (Moran & McHugh, 1992).
A role for endogenous CCK in satiety is supported by data demonstrating that administration of CCK antagonists with specificity for the CCK-Areceptor results in increases in food intake (Moran, Ameglio, Peyton, Schwartz, & McHugh, 1993; Reidelberger & O’Rourke, 1989). In the primate, the effects have been demonstrated to be dose related with a maximum increase of around 40% in daily food intake in monkeys with 4-hr daily food access. This increase is almost completely accounted for by an increase in the size of their first meal (Moran et al., 1993). Alterations in meal patterns are also evident in rats lacking CCK-A receptors. Otsuka Long Evans Tokushima Fatty (OLETF) rats have been demonstrated to have approximately a 6-kb (kilobase) deletion in the CCK-A receptor gene spanning the promotor region and the first and second exons. This deletion prevents protein expression resulting in a CCK-A receptor knockout rat (Takiguchi et al., 1997). OLETF rats are obese and hyperphagic. Characterization of their spontaneous solid food intake has revealed a 35% increase in daily food intake resulting from a 78% increase in meal size combined with an insufficient decrease in meal frequency. Similar results are obtained when OLETF rats are maintained on liquid diet. Meal size, expressed as the number of licks, is increased by 93% (Moran, Katz, Plata-Salaman, & Schwartz, 1998).
Satiety actions have also been demonstrated for the mammalian bombesin-like peptides GRP and neuromedin-B (NMB). These peptides reduce food intake following peripheral exogenous administration (Gibbs, Fauser, Rowe, Rolls, & Maddison, 1979; Ladenheim, Taylor, Coy, & Moran, 1994; Stein & Woods, 1982). Bombesin is the most potent—an effect that can be best explained by its high affinity for both GRP and NMB receptors. Bombesin activates both mammalian pathways and produces an effect similar in magnitude to combined GRP and NMB administration (Ladenheim, Wirth, & Moran, 1996). Both vagal and spinal afferents contribute to the mediation of the satiety actions of abdominal bombesin-like peptides. Either combined vagotomy, dorsal rhizotomy, and cord section or neonatal capsaicin administration are necessary to abolish the effects of bombesin on food intake (Stuckey, Gibbs, & Smith, 1985; Michaud, Anisman, & Merali,1999). Bombesin-like peptides also inhibit food intake following central administration, and the site of action for this effect is within the caudal hindbrain (F. W. Flynn, 1989; Ladenheim & Ritter, 1988). There does appear to be a relationship between the central and peripheral actions of these peptides because central antagonist administration can block the effect of peripherally administered peptides (Ladenheim, Taylor, Coy, Moore, & Moran, 1996). Such results suggest the possibility that peripherally administered bombesin-like peptides may exert some of their actions through a central site.
Arole for endogenous mammalian bombesin-like peptides in satiety is supported by data demonstrating increases in food intake following antagonist administration. Central GRP (F. W. Flynn, 1992; Merali, Moody, & Coy, 1993) and NMB receptor antagonists (Ladenheim et al., 1997) have been demonstrated to increase food intake in a variety of feeding paradigms.These data provide further support for a central site of action as being important for the feeding effects of bombesin-like peptides and are consistent with a role for endogenous bombesin-like peptides in the controls of meal size.
Satiety actions for the pancreatic peptides glucagon and amylin have also been demonstrated. Rapidly eating elicits an increase in pancreatic glucagon secretion (Langhans, Pantel, Muller-Schell, Effengerger, & Scharrer, 1984). Because an increase in plasma glucagon is also stimulated by sham feeding, this appears to be a cephalic phase response (Nilsson & Uvnas-Wallenstien, 1977). Glucagon is rapidly cleared from the circulation by the liver (Langhans et al., 1984),which appears to be the site of glucagon’s satietyaction (Geary, 1998). Hepatic-portal infusion of glucagon at meal onset elicits a dose-related reduction in meal size (Geary & Smith, 1982a), and glucagon’s satiety actions have been demonstrated in human subjects (Geary, Kissileff, Pi-Sunyer, & Hinton, 1992). Glucagon’s satiety action requires the presence of other forms of ingestional consequences because glucagon does not affect sham feeding (Geary & Smith, 1982b).Arole for endogenous glucagon in the control of meal size is supported by data demonstrating the ability of hepatic portal infusions of glucagon antibody to increase meal size (LeSauter, Noh, & Geary, 1991).
Amylin inhibits feeding in a dose-dependent and behaviorally specific manner following either peripheral or central administration (Lutz, Geary, Szabady, Del Prete, & Scharrer, 1995; Lutz, Rossi, Althaus, Del Prete, & Scharrer, 1998). Although meal-related amylin release has not been specifically shown, amylin is obligatorily cosecreted with insulin by pancreatic beta cells (Cooper, 1994). Thus, amylin levels rise rapidly with meal onset and remain elevated for a significant period of time during and following meals. Amylin’s site of action is within the area postrema, a hindbrain structure lacking a blood-brain barrier. The area postrema contains amylin receptors, and lesions of the area postrema block the feedinginhibitory actions of peripherally administered amylin (Lutz, Senn, et al., 1998). A physiological role for endogenous amylin in feeding controls is supported by experiments demonstrating increases in food intake in response to administration of amylin antagonists (Rushing et al., 2001).
Unlike these peptides that play roles in limiting food intake, ghrelin, a brain-gut peptide that is primarily synthesized in the stomach, has recently been shown to stimulate food intake following peripheral or central administration (Tschop, Smiley, & Heiman, 2000; Wren et al., 2000). Ghrelin synthesis and plasma ghrelin levels are increased by food deprivation and reduced by refeeding (Tschop et al., 2000). Systemic and central ghrelin administration produce c-fos activation within the arcuate nucleus (Hewson & Dickson, 2000; Nakazato et al., 2001), and central ghrelin administration increases arcuate NPY expression (Shintani et al., 2001), suggesting a hypothalamic site of action. Aphysiological role for ghrelin in feeding initiation or maintenance is supported by data demonstrating that ghrelin antibodies suppress food intake (Nakazato et al., 2001). Together, these data suggest a novel action for a gastric peptide in stimulating food intake.
Interactions Among Control Systems
With food intake being influenced by these seemingly separate neural systems, the question of how they interact with one another is important. A number of the clearest demonstrations of interactions involve the adiposity signal leptin. As noted earlier, leptin circulates in direct relation to the degree of adiposity serving as a feedback signal for the overall regulation of energy balance. Both peripheral and central leptin administration reduce food intake, and a number of experiments have demonstrated that leptin’s effects on feeding are specific to reducing meal size without changing meal frequency (Eckel et al., 1998; M. C. Flynn, Scott, Pritchard, & Plata-Salaman, 1998; Kahler et al., 1998).
How does a signal that is critically involved in regulating hypothalamic pathways involved in energy balance result in reductions in the size of individual meals? Recent experiments have suggested multiple mechanisms through which leptin may affect food intake. Leptin’s actions may depend in part on its interactions with within-meal signals. For example, central administration of leptin at doses that are subthreshold for inhibiting feeding when administered alone enhance the satiating potential of peripheral CCK or an intragastric preload (Emond, Schwartz, Ladenheim, & Moran, 2001; Emond, Schwartz, & Moran, 1999). This action of leptin appears to depend on its ability to enhance the degree of NTS neural activation produced by these peripheral manipulations. That is, leptin enhances the dorsal hindbrain representation of ascending vagal afferent feedback signals arising from CCK or gastrointestinal stimulation induced by gastric preload.
Reducing leptin levels through food deprivation has the opposite result: The satiating potency of CCK is reduced (Billington, Levine, & Morley, 1983; McMinn, Sindelar, Havel, & Schwartz, 2000). This effect may be mediated through enhanced NPY signaling because NPY administration has the opposite effect to that of leptin on both the behavioral and neural activation potencies of CCK. NPY reduces the degree of NTS activation in response to CCK (McMinn et al., 2000).
Leptin also may result in reductions in meal size through its direct actions on taste sensitivity. Leptin specifically reduces chorda tympani and glossopharyngeal sensitivity to sweet stimuli without altering responses to other tastants (Kawai, Sugimoto, Nakashima, Mura, & Ninomiya, 2000). This appears to be a direct effect at the level of the taste bud because leptin hyperpolarizes the taste cell. Finally, leptin may decrease meal size by altering the reinforcing effects of ingestion. Leptin reduces the rewarding efficacy of electrical brain stimulation (Fulton, Woodside, & Shizgal, 2000). Thus, a signal derived from fat stores serving as a long-term regulator of energy balance has multiple actions. Many of these may contribute to its reductions in food intake in ways that enhance the negative feedback effects of ingestion while also reducing the positive feedback effects. Together these actions result in consistent reductions in meal sizes that over the long term serve to constrain energy intake and contribute to overall energy balance.
Satiety signals can also affect the efficacy of adiposity signals. For example, not only doesleptin enhance the potency of CCK within an individual meal situation, but also CCK enhances the leptin’s ability to reduce food intake and decrease body weight over the longer term (Matson, Reid, Cannon, & Ritter, 2000; Matson & Ritter, 1999). A dose of CCK that alone has no effect on 24-hr food intake or body weight significantly increases leptin’s effects on food intake and body weight. The site of action for this effect is yet to be determined,butitmaybehypothalamicbecauseinashort-termtest CCK significantly enhances the leptin-induced neural activation within the paraventricular nucleus (Emond, Schwartz, & Moran, 1998).
Other kinds of interactions have also been demonstrated. As ingestion continues, the perceived pleasantness or palatability of foods can change. That is, feedback signals arising from ingestion or ingestive consequences can alter aspects of taste processing. This may occur at multiple levels of the neural axis. For example, continued consumption of a single food results in that food’s being perceived as less pleasant in comparison to other nonconsumed foods. Such a phenomenon is referred to a sensory-specific satiety (B. J. Rolls, 1986). These changes are rapid and do not depend on the nutritional value of the consumed food, indicating that they likely arise from the sensory properties of the food, or on cognitive processes involved in assessing that enough of a particular type of food has been consumed. Sensory-specific satiety has been proposed to be an important mechanism for ensuring that a variety of foods are consumed, increasing the likelihood that an organism will maintain nutritional balance (B. J. Rolls, 1986). Sensory-specific satiety has a neurophysiological basis in that LH neurons that have ceased to respond to the taste of one food will respond to a different food (E. T. Rolls, Murzi, Yaxley, Thorpe, & Simpson, 1986).
Perceived pleasantness or palatability can also be reduced by gastrointestinal nutrient stimulation—a phenomena that has been termed alliesthesia (Cabanac, 1971). Thus, human subjects rate a sweet solution as less pleasant following a gastric glucose load (Cabanac & Fantino, 1977). Similar findings have been obtained in rats, using orofacial responses as a measure of the perceived pleasantness of taste stimuli (see Grill & Norgren, 1978). Gastric or intestinal nutrient infusions reduce the incidence of positive orofacial responses and increase the incidence of negative responses to an oral sucrose infusion (Cabanac & LaFrance, 1992). Similar results are produced by exogenous CCK, and the phenomenon is blocked by vagotomy (Cabanac & Zhao, 1994). Thus, one of the ways that within-meal negative feedback signaling affects ingestion is through a change in the perceived pleasantness of ingestive stimuli. In primates, the orbitofrontal cortex appears to be the likely neural site where such effects are mediated. Taste-evoked activity in the orbitofrontal cortex is suppressed by gastrointestinal nutrients (Scott, Yan, & Rolls, 1995).
A final example of interactions among signaling systems suggests a role for central reinforcing pathways in mediating the feeding actions of hypothalamic signaling systems. The opiate antagonist naloxone blocks the feeding stimulatory action of NPY (Kotz, Grace, Briggs, Levine, & Billington, 1995). The site of action for naloxone for this effect is within the medial subnucleus of the NTS (Kotz, Glass, Levine, & Billington, 2000). The site of action for the interaction appears to be within the amygdala. Naloxone does not affect NPY’s ability to induce c-fos within the hypothalamic paraventricular nucleus, but both NPY and naloxone induce c-fos within the central nucleus of the amygdala but do so in different cellular population (Pomonis, Levine, & Billington, 1997). Together, these data suggest that neural systems normally involved in palatability-induced feeding stimulation also play a modulatory role in the feeding induced by the hypothalamic signaling system’s response to adiposity stores.
The body contains multiple systems for regulating overall energy balance. These systems derive from and control different aspects of ingestive behavior and its consequences. Although adiposity, satiety, and reinforcement signaling have different primary sites of mediation within the brain, they are interacting systems that together ensure that the organism consumes an adequate amount and variety of nutrients. Although such interactions can now be demonstrated, little is known about the underlying cellular mechanisms through which they are mediated. Furthermore, how these interactions at the level of individual neurons are translated into behavioral outcomes remains to be determined. These are the two major issues currently facing investigators involved in research on the controls of food intake.
Over the past 10 years our knowledge of the brain sites and signaling systems involved in energy balance has grown exponentially. This has provided multiple targets for potential treatment development in obesity and eating disorders. A more complete understanding of how these systems respond under multiple metabolic states and interact with one another will be necessary to provide a rational base for such eventual treatment development.
Water Intake and Fluid Balance
The amount of water that we drink, like the amount of food that we eat, depends on a rich variety of factors that include homeostatic controls, learning and experience, and environmental social and cultural influences. Although there is ample evidence that the contribution of each of these factors is neurally mediated in the control of water intake, we concentrate here on the role of three relatively well-characterized systems that interact among themselves and that are important in the control of water balance. These three systems include, respectively, (a) neural and hormonal signals related to the detection of plasma osmolality and extracellular fluid volume that influence the initiation of bouts of ingestion, (b) neural and hormonal signals related to myriad factors that lead to satiety and thus terminate bouts of drinking, and (c) brain sites that receive and integrate these signals and that elicit appropriate physiological and behavioral responses. We also discuss the important interactions among these systems that permit overall regulation of body fluid balance.
Osmotic and Hypovolemic Signals That Stimulate Water Intake
All physiological processes occur in one or another internal sea consisting of mild salt solutions, and maintenance of the appropriate volume and concentration of the various fluid compartments in the body is essential for these processes to occur. Regulation occurs at the cellular level, enabling normal intracellular processes to occur, as well as at the level of the fluids that interconnect the cells, such that the formation and maintenance of extracellular fluid is a high priority. In this regard, maintenance of adequate blood volume is particularly essential for the delivery of nutrients to tissues and for the removal of metabolites for excretion. Thus, when body fluid balance is compromised, both physiological and behavioral responses are initiated to defend further aberrations in body fluid balance and to replenish lost body fluid stores. This could occur when fluid is shifted between compartments within the body (as in edema), when excess fluid is lost from the body (as occurs following hemorrhage or extreme vomiting), or when insufficient water and minerals are available for consumption. If water or sodium is lacking, the antidiuretic hormone arginine vasopressin (AVP) and the antinatriuretic hormone aldosterone work together to promote renal conservation of both water and sodium, thus preventing further body fluid depletion and maintaining the best possible level of osmolality. The behavioral responses of thirst and sodium appetite can also be engaged to restore lost water or salt because this is the only mechanism by which the lost fluids and electrolytes can be replaced. Both the physiological and the behavioral responses to perturbations of body fluid balance are under tight control by the brain. Although the careful balance of ingesting both water and salt is necessary for maintenance of extracellular fluid volume and concentration, this research paper focuses on the endocrine and neural controls of water intake.
Contemporary understanding of the physiology of water intake began with Andersson’s (1953; Andersson & Wyrwicka, 1957) report of the elicitation of drinking following the administration of hyperosmotic solutions to the brain of goats. Although the conscious goats had no apparent interest in water under basal conditions, they drank avidly when stimulated briefly within the anterior hypothalamus by small volumes of hypertonic saline. In later experiments drinking was elicited by weak electrical currents applied to the same anatomical sites. These reports demonstrated that water intake could be elicited by direct stimulation of the brain, and they thereby challenged the prevailing view that water intake was merely a sensation or a reflexive response to reduced salivary flow produced by dehydration (Cannon, 1918). In addition, these studies heralded the modern investigation of water intake by exploring its central neural basis and its instinctive (Lashley, 1938) and motivated (Stellar, 1954) origins. In the mid 1950s and early 1960s research focused on investigations of the water intake that accompanies cellular dehydration. This concept was initially proposed by Wettendorff (1901) and then established as a mechanism of
water intake by Gilman’s (1937) well-known experiments demonstrating that the administration of solutes that are excluded from cells (such as sodium) elicit cellular dehydration and are consequently highly effective dipsogens.
The more molar context of current research on the neural mechanisms of water intake was not achieved until Fitzsimons (1961) established hypovolemia (reduced blood volume) as an independent stimulus for thirst. He accomplished this by eliciting water intake in rats using several experimental manipulations that all resulted in reduced blood volume (e.g., hemorrhage, ligation of the inferior vena cava, hyperoncotic colloid dialysis). It is important that all of these paradigms resulted in reduced blood volume with no change of osmolarity of the remaining plasma, and hence with no change of cell volume. This essential point has been confirmed more clearly by the work of Tang (1976), who found (a) that these treatments reduce the plasma volume of rats without altering serum electrolytes or osmolarity and (b) that drinking is suppressed if the reduction in intravascular volume is prevented by intravenous infusion of an isotonic plasma substitute. Although earlier research had suggested that the causes of thirst are necessarily complex and that changes in extracellular volume, among others, must be considered (e.g., Adolph, Barker, & Hoy, 1954), the concept that hypovolemia is a second and potent cause of water intake— that hypovolemia operates under normal conditions of dehydration and has an independent sensory system utilizing detectors of reduced blood volume—was not considered. Rather, these concepts were elaborated by Fitzsimons (see Fitzsimons, 1979, for a full review) along with the subsequent proof of concept by Stricker (1968). Together, their work demonstrated that hypovolemia lowers the threshold for the initiation of drinking, that the water intake that is generated is a function of the magnitude of the reduction in blood volume, and that hypovolemia elicits drinking with the expected properties of motivation. In addition, the pioneering studies of hypovolemia-induced water intake also revealed the role of the renin angiotensin hormone system as an important systemic system that accesses the brain and stimulates water intake (Fitzsimons, 1969). Ultimately, the demonstration that cellular dehydration and extracellular volume loss can independently elicit water intake was suggested by the double depletion hypothesis of thirst (Epstein, Kissileff, & Stellar, 1973). Over a lengthy series of experiments, it was demonstrated that water intake in many naturalistic situations, and especially water deprivation, could be precisely predicted by this hypothesis. The bottom line is that cellular dehydration locally in the brain and systemic hypovolemia combine to produce the urge to drink, and the concurrent restoration of each deficit results in a summative suppression of drinking. The nature of the two depletions, the portions of the brain devoted to their regulation, and the manner of their joint function in the control of spontaneous drinking behavior directed much of the subsequent research on the physiology of water intake.
Cellular Dehydration and Brain Osmosensors
Cellular dehydration-induced water intake requires that the brain somehow detect water loss from osmosensitive or volume-sensitive cells and to generate a signal that leads to drinking. Studies in the early 1970s focused on cells within the brain that could be sensors that arouse drinking as a consequence of cellular water loss. Experiments by Peck and Novin (1971) and Blass and Epstein (1971) demonstrated that cells in the lateral preoptic area of the hypothalamus contained a large concentration of osmosensitive cells. When hyperosmotic solutions were applied locally in the vicinity of these cells, water intake was elicited. Conversely, when the cell group in the lateral preoptic area was selectively lesioned, the animals demonstrated impaired drinking stimulated by sudden increases in the osmolarity of the blood reaching the brain, whereas drinking elicited by hypovolemia remained intact.
Although more recent data have continued to support a role of the lateral preoptic area as a major osmosensitive area in the brain that elicits drinking, there remains considerable uncertainty about the location of the specific osmosensitive neurons that stimulate vasopressin secretion to promote water retention by the kidney. Candidate brain areas for these osmoreceptors are other subnuclei of the hypothalamus (including the lamina terminalis and the supraoptic nucleus) and the circumventricular organs (CVOs). The latter are implicated because they lack a blood-brain barrier and hence are sensitive to both plasma and brain interstitial osmotic influences. Further, they have axonal connections to areas that control drinking behavior.
Circumventricular Organs and Hypovolemic Water Intake
The demonstration that hypovolemia-induced intake is independent of cellular dehydration-induced intake arose from experiments in which rats drank water in response to an isotonic reduction of blood volume (Fitzsimons, 1961; Stricker, 1969; Tang, 1976). Because the osmolarity of the plasma is not increased as a function of reduced blood volume per se, the water intake cannot be attributed to dehydration of cells. The discovery of a hormonal control over this kind of drinking came from Fitzsimons’s (1964, 1969) demonstrations that the kidneys must be attached to the general circulation in order for hypovolemic treatments to have their full dipsogenic effects. This was demonstrated most clearly in following caval ligation, a procedure in which the inferior vena cava is occluded, preventing the return of the blood from the abdomen and lower limbs and thus reducing cardiac output by approximately 40%. The ensuing water intake that develops is dependent on access of the kidneys to the circulation. Because nephrectomy reduces caval ligation–induced intake, Fitzsimons reasoned not only that the kidney is necessary for eliciting hypovolemia-induced water intake but also that it does so as an endocrine rather than as an exocrine organ. The subsequent identification of renin, a peptide hormone produced by the kidney, and the demonstration that its levels are the rate-limiting step in the renin-angiotensin cascade that produces a powerful dipsogenic action, completed the story (Fitzsimons & Simons, 1969). It was subsequently found that renin acts as an enzyme that causes the formation of the peptide angiotensin II in the blood and that angiotensin II in turn gains access to the brain and stimulates drinking by acting on receptors in the CVOs.
The CVO that was initially observed to be particularly sensitive to the local application of angiotensin II in terms of eliciting a dipsogenic response was the subfornical organ (SFO; Simpson & Routtenberg, 1974). Using novel neuropharmacological application techniques, Simpson and his colleagues subsequently demonstrated that the SFO is exquisitely sensitive to the dipsogenic actions of angiotensin II as well as to other known dipsogenic agents such as the cholinomimetic carbachol. That group also found that lesions of the SFO rendered animals less responsive to hypovolemic stimuli as well as to intravenously administered angiotensin II, while still being responsive to cellular dehydration– induced stimuli (Simpson, Epstein, & Camardo, 1978). Subsequently, receptors that specifically bind angiotensin II have been localized in high concentrations in the SFO as well as in other brain areas. The distribution of angiotensin II receptors in the brain is of interest because many of the brain sites that contain high concentrations of these receptors receive direct projections from the SFO and are in other areas that lack a blood-brain barrier (Mendelsohn, Quirion, Saavedra, Aguiler, & Catt, 1984; Miselis, 1981).
The activation of these additional brain sites by angiotensin II is thought to occur by endogenous angiotensin II that is centrally generated because all of the components that are required to produce angiotensin II are present within the brain (Ganten, Hutchinson, Schelling, Ganten, & Fischer, 1975). Subsequent pharmacological studies have now revealed that there are at least two subtypes of angiotensin II receptors, designated angiotensin AT1 and AT2 receptors.
Although both receptor subtypes bind the native ligand angiotensin II with equal affinity, they differ in their amino acid sequences by over 70%. Based on this, the synthesis of nonpeptidergic ligands for each receptor subtype has become possible, and it is now recognized that the two receptors differ in binding affinity for these novel ligands and engage different second-messenger signaling systems once activated. Due in part to the widespread interest in these receptor subtypes in the control of various physiological functions, both AT1 and AT2 receptors have been cloned and sequenced. Subsequent research utilizing specific antisense oligodeoxynucleotide sequences has allowed both in vitro and in vivo receptor knockdown of each angiotensin receptor subtype. The bottom line from many experiments is that over 95% of the biological actions of angiotensin II appear to be mediated through its binding at the AT1 receptor. The physiological role of activation of the AT2 receptor subtype remains unclear. In sum, the SFO is a major site of action for peripherally generated angiotensin II in response to hypovolemia. The stimulation of the SFO, an area that contains high concentrations of angiotensin AT1 receptors, by systemic angiotensin II may also trigger the central angiotensin system to stimulate drinking as well as other physiological responses (such as the release of vasopressin) to maintain fluid homeostasis in response to hypovolemia.
Satiety Signals for Water Intake
The intake of water, like the intake of food, is under the control of diverse signals, some of which initiate the behavior and others of which stop it (i.e., satiety signals ). Unlike the well-described satiety signals that terminate feeding, however, the satiety signals that terminate drinking are much less clear. A thirsty animal allowed the opportunity to drink water will rapidly consume sufficient water to restore the lost fluids. Although the animal may ingest a large quantity of fluid, satiation generally occurs several minutes prior to the time that substantial water is absorbed from the digestive system (Ramsay, Rolls, & Wood, 1977). Thus, the possibility that some sort of oral metering of ingested fluids provides a least one level of input to the satiation of thirst has been considered. Support for this concept derives from the data of Nicolaidis (1968), who demonstrated that infusions of water into the oral cavity of dehydrated rats produced rapid decreases in plasma vasopressin prior to any substantial absorption of the fluid by the digestive system. Although receptors in the mouth and throat can be demonstrated to influence the amount of water an animal ingests, receptors in the stomach, small intestine, and liver are also critically involved in the normal satiation of drinking. That is, preloads of water given by gastric gavage (thereby bypassing oral stimulation) also reduce drinking. Unlike the signals that lead to satiation of food intake, there is no clear evidence that receptors in the duodenum are involved in satiation of water intake.
The site of integration of the satiety signals for drinking is also unclear, although recent data have implicated the lateral parabrachial nucleus in the caudal brain stem as being important. This nucleus receives gustatory input from the tongue and appears to be an important site for the integration of signals that control fluid intake. Data from Menani and colleagues have demonstrated that this brain area may be producing a tonic serotonergic inhibitory tone on fluid intake (Menani, Colombari, Beltz, Thunhorst, & Johnson, 1998). During episodes of hypovolemia, parabrachial serotonergic tone is decreased, thus allowing the expression of drinking. Other neurotransmitters have also been found to inhibit fluid intake, including oxytocin, which is generated in forebrain areas and is projected to caudal brain sites to inhibit fluid ingestion. The identification of satiety signals for drinking awaits future research.
Interactions Among Other Control Systems
Besides being mediated by both osmotic and hypovolemic signals, the controls of water intake interact with other homeostatic control systems as well. Sodium homeostasis and its behavioral counterpart, sodium appetite, provide an important example of how the controls of water intake interact with other systems. Recall that for adequate reestablishment of extracellular fluid volume, electrolytes that act as osmotic agents are essential for maintaining water within the extracellular fluid compartment. There is ample evidence that the angiotensin II that is secreted in response to hypovolemic signals also stimulates a specific appetite for sodium as well as for water (Weisinger, Blair-West, Burns, Denton, & Tarjan, 1997). In addition, many of the same brain areas (SFO, other CVOs, several hypothalamic nuclei) at which the actions of angiotensin II regulate water intake also alter sodium appetite. For example, the expression of angiotensin II receptors can be differentially regulated by circulating levels of adrenal steroids such as aldosterone in sodiumdepleted rats, and sex steroids such as estrogen can modulate the dipsogenic potency of angiotensin II in the normally cycling female rat (Kisley, Sakai, & Fluharty, 1999).
In summary, we have reviewed the multiple mechanisms known to influence the elicitation and cessation of drinking. Because the maintenance of blood volume and osmotic pressure is so critical to the functioning of every organ system, and because even small deviations from the ideal can soon incapacitate an organism, the control system is exquisitely sensitive and fast to respond. In an ideal world, water and electrolytes would be consumed in the right volumes and concentrations to preclude having to monitor and adjust their levels constantly, and at one level of control this is what actually happens. Most individuals, when they are able, consume sufficient electrolytes and water with their food to ensure adequate regulation. In fact, estimates of the percentage of total daily water that is consumed when food is being eaten (i.e., at meal times) under conditions of ad libitum access range from 70% to 90% or more. Any excess water or electrolytes that are consumed during meals are rapidly and efficiently excreted from the body in the urine.
Unfortunately, few organisms live in such luxury and thus cannot rely on prandial consumption of sufficient water and electrolytes. As a result, they fall back on the control systems described in this research paper. In this process, the brain relies primarily on osmotic and volumetric signals arising in key sensory receptors in strategic locations in the body, as well as in the brain itself, to determine body fluid status. When deviations from the ideal are detected, the brain has a complex armamentarium of responses on which it can draw to reverse the problem and preclude its worsening. Hence, the brain can engage specific neurohormonal systems such as the reninangiotensin system to restore fluid balance.
As with food intake, there are signals that stimulate drinking, as well as signals that terminate drinking; the two interacting types of signals maximize the likelihood of consuming adequate amounts of water and electrolytes. The normal integration of these stimuli ensures that behavioral and physiological responses occur, in many cases, in anticipation of need states such that the individual is protected from large demands to defend homeostatic processes. That is, in a predictable environment, when an inadequate supply of water and electrolytes is inevitable, animals learn to activate the appropriate regulatory responses in anticipation of the situation and hence circumvent problems of fluid balance before they arise. These vital and complex regulatory processes are controlled, in many cases, utilizing redundant systems such that even in the case of disease or injury the individual is still able to function and respond normally.
One area in fluid balance that is not yet well understood is the nature of the controls involved in prandial drinking (drinking in association with meals). We do not know whether prandial drinking is elicited by the osmotic load of the meal or if the drinking occurs in anticipation of the osmotic load. We also do not yet appreciate whether the neurotransmitters and hormones that we normally associate with controlling water intake specific to fluid balance are involved in prandial drinking or whether this represents a unique situation. These issues remain to be investigated.
The investigation of water and sodium ingestion has provided insights into how the brain controls motivated behavior. A number of points are obvious. The first is that the controls over fluid balance in the body parallel in many ways those involved in energy regulation. Just as the body monitors key parameters such as blood glucose and body adiposity, it tracks osmolarity and blood volume. The second is that the central control over all homeostatically regulated systems, including fluid balance, is integrated such that water and electrolyte intake and excretion do not occur in a vacuum. Rather, the brain takes into account all of the key systems, compromises where necessary, and ensures the long-term survival of the organism. The study of water intake has also provided a model system to examine how peptide and steroid hormones interact with neural signals in the control of behavior. Specific brain areas that are critical in the control of these behaviors, as well as specific chemical signals that mediate this control (hormones and neurotransmitters), have been identified through the incorporation of modern biochemical and molecular biological tools. Because of the explosion of new techniques available in the last decade, great advances into how this complex behavior is governed have been forthcoming.As we look to the future, studies examining the interactions among the controls over caloric, thermal, and fluid needs, including the various neurochemical systems that mediate them, will be more clearly examined. Finally and most important, given the increasing knowledge of the controls of ingestive behavior, we hope to begin to use this information to develop rational and viable treatments for common human disorders such as obesity and hypertension.
Bibliography:
- Adolph, E. F., Barker, J. P., & Hoy, P. A. (1954). Multiple factors in thirst. American Journal of Physiology, 178, 538–562.
- Aja, S., Sahandy, S., Ladenheim, E. E., Schwartz, G. J., & Moran, T. H. (2001). Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. American Journal of Physiology, 281, R1862–R1867.
- Altschuler, S. M., Bao, X., Bieger, D., Hopkins, D. A., & Miselis, R. R. (1989). Viscerotopic representation of the upper gastrointestinal tract in the rat: Sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. Journal of Comparative Neurology, 243, 248–268.
- Anand, B. K., & Brobesck, J. R. (1951). Localization of a feeding center in the hypothalamus of the rat. Proceedings of the Society for Experimental Biology and Medicine, 77, 323–324.
- Andersson, B. (1953). The effect of injections of hypertonic NaClsolutions in different parts of the hypothalamus of goats. Acta Physiologica Scandinavica, 28, 188–201.
- Andersson, B., & Wyrwicka, W. (1957). The elicitation of a drinking motor conditioned reaction by electrical stimulation of the hypothalamic “drinking area.” Acta Physiologica Scandinavica, 41, 194–198.
- Antin, J., Gibbs, J., Holt, J., Young, R. C., & Smith, G. P. (1975). Cholecystokinin elicits the complete behavioral satiety sequence in rats. Journal of Comparative and Physiological Psychology, 89, 784–790.
- Azzara, A. V., Bodnar, R. J., Delameter, A. R., & Sclafani, A. (2000). Naltrexone fails to block the acquisition or expression of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacology, Biochemistry, and Behavior, 67, 545– 557.
- Beck, B., Burlet, A., Nicolas, J. P., & Burlet, C. (1993). Galanin in the hypothalamus of fed and fasted lean and obese Zucker rats. Brain Research, 623, 124–130.
- Beck, B., & Richy, S. (1999). Hypothalamic hypocretin/orexin and neuropeptide Y: Divergent interaction with energy depletion and leptin. Biochemical Biophysical Research Communications, 258, 119–122.
- Benoit, S. C., Schwartz, M. W., Lachey, J. L., Hagan, M. M., Rushing, P. A., Blake, K. A., Yagaloff, K. A., Kurylko, G., Franco, L., Danhoo, W., & Seeley, R. J. (2000). Anovel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. Journal of Neuroscience, 20, 3442–3448.
- Bernard, C. (1859). Lecons sur les proprietes physiologiques et les alterations pathologiques de l’organisme. Paris: Baillers.
- Billington, C. J., Levine, A. S., & Morley, J. E. (1983). Are peptides truly satiety agents? A method for testing for neurohumoral satiety effects. American Journal of Physiology, 245, R920– R926.
- Bjorbaek, C., Elmquist, J. K., Michl, P., Ahima, R. S., van Buer, A., McCall, A. L., & Flier, J. S. (1998). Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology, 139, 3485–3491.
- Bjorbaek, C., Uotoni, S., da Silva, B., & Flier, J. S. (1997). Divergent signaling capacities of the long and short isoforms of the leptin receptor. Journal of Biochemistry, 272, 32686–32695.
- Blass, E. M., & Epstein, A. N. (1971). A lateral preoptic osmosensitive zone for thirst in the rat. Journal of Comparative and Physiological Psychology, 76, 378–394.
- Bolles, R. C., Harward, L., & Crandall, C. (1981). Conditioned taste preferences based on caloric density. Journal of Experimental Psychology (Animal Behavior Processes), 7, 59–69.
- Breslin, P. A. S., Davis, J. D., & Rosenak, R. (1996). Saccharin increase the effectiveness of glucose in stimulating ingestion in rats but has little effect on negative feedback. Physiology and Behavior, 60, 411–416.
- Broberger, C., DeLecea, L., Sutcliffe, J. G., & Hokfelt, T. (1998). Hypocretin/orexin and melanin concentrating hormone expressing cells form distinct populations in the rodent lateral hypothalamus: Relationship to neuropeptide Y and agouti gene related protein systems. Journal of Comparative Neurology, 402, 460–474.
- Cabanac, M. (1971). Physiological role of pleasure. Science, 173, 1103–1107.
- Cabanac, M., & Fantino, M. (1977). Origin of olfacto-gustatory alliesthesia: Intestinal sensitivity to carbohydrate concentration? Physiology and Behavior, 18, 1039–1045.
- Cabanac, M., & LaFrance, L. (1992). Duodenal preabsorptive origin of gustatory alliesthesia in rats. American Journal of Physiology, 263, R1013–R1017.
- Cabanac, M., & Zhao, C. (1994). Postingestive alliesthesia produced by exogenous choloecystokinin and blocked by abdominal vagotomy. American Journal of Physiology, 266, R633–R637.
- Campfield, L.A., & Smith, F. J. (1986). Functional coupling between transient declines in blood glucose and feeding behavior; Temporal relationships. Brain Research Bulletin, 17, 427–433.
- Campfield, L. A., Smith, F. A., Guisez, Y., Devos, R., & Burn, P. (1996). Recombinant mouse ob protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science, 271, 994–996.
- Cannon, W. B. (1918). The physiological basis of thirst. Proceedings of the Royal Society, London, 90B, 283–301.
- Cannon, W. B. (1932). The wisdom of the body. New York: Norton.
- Chavez, M., Kelly, L., York, D. A., & Berthoud, H. R. (1997). Chemical lesion of visceral afferents causes transient overconsumption of unfamiliar high-fat diets in rats. American Journal of Physiology, 272, R1657–R1673.
- Chornwall, B. M., Di Maggio, D. A., Massari, V. J., Pickel, S. M., Ruggiero, D. A., & O’Donohue, T. L. (1985). The anatomy of neuropeptide Y containing neurons in the rat brain. Neuroscience, 15, 1159–1181.
- Chua, S. C., Chung, W. K., Wu-Peng, X. S., Zhang, Y., Liu, S. M., Tartaglia, L., & Liebel, R. L. (1996). Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science, 271, 994–996.
- Clark, J. T., Kalra, P. S., Crowley, W. R., & Kalra, S. P. (1984). Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology, 115, 427–429.
- Coleman, D. L. (1973). Effects of parabiosis of obese and diabetes and normal mice. Diabetologia, 9, 294–298.
- Cooper, G. J. (1994). Amylin compared with calcitonin gene-related peptide: Structure, biology and relevance to metabolic disease. Endocrine Review, 15, 163–201.
- Corp, E. S., Melville, L. D., Greenberg, D., Gibbs, J., & Smith, G. P. (1990). Effect of 4th ventricular neuropeptide Y and peptide YY on ingestive and other behaviors. American Journal of Physiology, 259, R317–R323.
- Corp, E. S., Woods, S. C., Porte, D., Jr., Dorsa, D. M., Figlewicz, D. P., & Baskin, D. G. (1986). Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neuroscience Letters, 70, 17–22.
- Crawley, J. N. (1999). The role of galanin in feeding behavior. Neuropeptides, 33, 369–375.
- Crawley, J. N., Austin, M. C., Fiske, S. M., Martin, B., Consolo, S., Berthold, M., Langel, U., Fisone, G., & Bartfai, T. (1990). Activity of centrally administered galanin fragments on stimulation of feeding behavior and on galanin receptor binding in the rat hypothalamus. Journal of Neuroscience, 10, 3695–3700.
- Davis, J. D., & Levine, M. (1977). A model for the control of ingestion. Psychological Review, 84, 379–412.
- Davis, J. D., & Smith, G. P. (1990). Learning to sham feed: Behavioral adjustments to loss of physiological postingestive stimuli. American Journal of Physiology, 259, R1228–R1235.
- Doyle, T. G., Berridge, K. C., & Gosnell, B. A. (1993). Morphine enhances hedonic taste palatability in rats. Pharmacology, Biochemistry, and Behavior, 46, 745–749.
- Eastwood, C., Maubach, K., Kirkup, A. J., & Grundy, D. (1998). The role of endogenous cholecystokinin in the sensory transduction of luminal nutrient signaling in the rat jejunum. Neuroscience Letters, 254, 145–153.
- Eckel, L. A., Langhans, W., Kahler, A., Campfield, L. A., Smith, F. J., & Geary, N. (1998). Chronic administration of OB protein decreases food intake by selectively meal size in female rats. American Journal of Physiology, 275, R186–R189.
- Elizalde, G., & Sclafani, A. (1990). Flavor preferences conditioned by intragastric polycose; A detailed analysis using an electronic esophagus preparation. Physiology and Behavior, 47, 63–67.
- Elmquist, J. K., Bjorbaek, C., Ahima, R. S., Flier, J. S., & Saper, C. B. (1998). Distribution of leptin receptor mRNA isoforms in the rat brain. Journal of Comparative Anatomy, 395, 535–547.
- Epstein, A. N., Kissileff, H. R., & Stellar, E. (Eds.). (1973). The neuropsychology of thirst. Washington, DC: V. H. Winston & Sons.
- Erikson, J. C., Clegg, K. E., & Palmiter, R. D. (1996). Sensitivity to leptin and susceptibility to seizures in mice lacking neuropeptude Y. Nature, 381, 415–418.
- Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J., & Cone, R. D. (1997). Role of melanocortinergic neurons in feeding and agouti obesity syndrome. Nature, 385, 165–168.
- Fitzsimons, J. T. (1961). Drinking by rats depleted of body fluid without increase in osmotic pressure. Journal of Physiology (London), 159, 297–309.
- Fitzsimons, J. T. (1964). Drinking caused by constriction of the inferior vena cava in the rat. Nature, 204, 479–480.
- Fitzsimons, J. T. (1969). The role of renal thirst factor in drinking induced by extracellular stimuli. Journal of Physiology (London), 201, 349–369.
- Fitzsimons, J. T. (1979). The physiology of thirst and sodium appetite. Cambridge, UK: Cambridge University Press.
- Fitzsimons, J. T., & Simons, B. J. (1969). The effect on drinking in the rat of intravenous infusion of angiotensin, given alone or in combination with other stimuli of thirst. Journal of Physiology (London), 203, 45–57.
- Flynn, F. W. (1989). Fourth ventricle bombesin injection suppresses ingestive behavior in rats. American Journal of Physiology, 256, R590–R596.
- Flynn, F. W. (1992). Fourth ventricular injection of selective bombesin receptor antagonists facilitates feeding in rats. American Journal of Physiology, 264, R218–R221.
- Flynn, M. C., Scott, T. R., Pritchard, T. C., & Plata-Salaman, C. R. (1998). Mode of action of OB protein (leptin) on feeding. American Journal of Physiology, 275, R174–R179.
- Fulton, S., Woodside, B., & Shizgal, P. (2000). Modulation of brain reward circuitry by leptin. Science, 287, 125–128.
- Ganten, D., Hutchinson, J. S., Schelling, P., Ganten, U., & Fischer, H. (1975). The isorenin angiotensin systems in extrarenal tissue. Clinical Experimental Pharmacology and Physiology, 2, 127–151.
- Geary, N. (1998). Glucagon and the control of meal size. In G. P. Smith (Ed.), Satiation: From gut to brain (pp. 164–197). New York: Oxford University Press.
- Geary, N., Kissileff, H. R., Pi-Sunyer, F. X., & Hinton, V. (1992). Individual, but not simultaneous, glucagon and cholecystokinin infusion inhibit feeding in men. American Journal of Physiology, 262, R975–R980.
- Geary, N., & Smith, G. P. (1982a). Pancreatic glucagon and postprandial satiety in the rat. Physiology and Behavior, 28, 313– 322.
- Geary, N., & Smith, G. P. (1982b). Pancreatic glucagon fails to inhibit sham feeding in the rat. Peptides, 3, 163–166.
- Gibbs, J., Fauser, D. J., Rowe, E. A., Rolls, E. T., & Maddison, S. P. (1979). Bombesin suppresses feeding in rats. Nature, 245, 323– 325.
- Gibbs, J., Young, R. C., & Smith, G. P. (1973). Cholecystokinin decreases food intake in rats. Journal of Comparative and Physiological Psychology, 84, 488–495.
- Gilman, A. (1937). The relation between blood osmotic pressure, fluid distribution and voluntary water intake. American Journal of Physiology, 120, 323–328.
- Giza, A. K., Ackroff, K., McCaughey, S. A., Sclafani, A., & Scott, T. R. (1997). Preference conditioning alters taste responses in the nucleus of the solitary tract. American Journal of Physiology, 273, R1230–R1240.
- Glass, M. J., Billington, C. J., & Levine, A. S. (2000). Opioids, food reward and macronutrient selection. In H.-R. Berthoud & R. J. Seeley (Eds.), Neural and metabolic control of macronutrient intake (pp. 407–423). Boca Raton, FL: CRC Press.
- Glass, M. J., Grace, M., Cleary, J. P., Billington, C. J., & Levine, A. S. (1996). Potency of naloxone’s anorectic effect in rats in dependent on diet preference. American Journal of Physiology, 271, R217–R221.
- Gosnell, B. A., Krahn, D. D., & Majchrzak, M. J. (1990). The effects of morphine on diet selection are dependent on baseline diet preferences. Pharmacology, Biochemistry, and Behavior, 37, 207–212.
- Grill, H. J., Ginsburg, A. B., Seeley, R. J., & Kaplan, J. M. (1998). Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. Journal of Neuroscience, 18, 10128–10135.
- Grill, H. J., & Kaplan, J. M. (1992). Sham feeding in intact and chronic decerebrate rats. American Journal of Physiology, 262, R1070–R1074.
- Grill, H. J., Markison, S., Ginsberg, A., & Kaplan, J. M. (2000). Long term effects on feeding and body weight after stimulation of forebrain or hindbrain CRH receptors with urocortin. Brain Research, 867, 19–28.
- Grill, H. J., & Norgren, (1978). The taste reactivity test: I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research, 143, 263–279.
- Hagan, M. M., Rushing, P. A., Schwartz, M. W., Yagaloff, K. A., Burn, P., Woods, S. C., & Seeley, R. J. (1999). Role of CNS melanocortin system in the response to overfeeding. Journal of Neuroscience, 19, 2362–2367.
- Hahn, T., Breininger, J., Baskin, D., & Schwartz, M. (1998). Coexpression of AgRP and NPY in fasting activated hypothalamic neurons. Nature Neuroscience, 1, 271–272.
- Haynes, A. C., Jackson, B., Chapman, H., Tadyyon, M., Johns, A., Porter, R. A., & Arch, J. R. (2000). A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regulatory Peptides, 96, 45–51.
- Heatherington, A. W., & Ranson, S. W. (1940). Hypothalamic lesions and adiposity in the rat. Anatomical Record, 78, 149–172.
- Hewson, A. K., & Dickson, S. L. (2000). Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamus arcuate nucleus of fasted and fed rats. Journal of Endocrinology, 12, 1047–1049.
- Huszar, D., Lynch, C. A., Fairchild-Huntress, V., Dunmore, J. H., Fang, Q., Berlemeier, L. R., Gu, W., Kesterson, R. A., Boston, B. A., Cone, R. D., Smith, F. J., Campfield, L. A., Burn, P., & Lee, F. (1997). Targeted disruption of the melanocortin-4 receptor results in obesity. Cell, 88, 131–141.
- Ji, H., Graczyk-Milbrandt, G., & Friedman, M. I. (2000). Metabolic inhibitors synergistically decrease hepatic energy status and increase food intake. American Journal of Physiology, 278, R1579–R1582.
- Kahler, A., Geary, N., Eckel, L. A., Campfield, L. A., Smith, F. J., & Langhans, W. (1998). Chronic administration of OB protein decreases food intake by selectively meal size in male rats. American Journal of Physiology, 275, R180–R185.
- Kalra, S. P., Dube, M. G., Sahu,A., Phelps, C. P., & Kalra, P. (1991). Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proceedings of the National Academy of Sciences, 88, 10931–10935.
- Kaplan, J. M., Siemers, W. H., & Grill, H. J. (1997). Effect of oral versus gastric delivery on gastric emptying of corn oil emulsions. American Journal of Physiology, 273, R1263–R1270.
- Kaplan, J. M., Spector, A. C., & Grill, H. J. (1992). Dynamics of liquid gastric emptying during and after stomach fill. American Journal of Physiology, 263, R813–R819.
- Kawai, K., Sugimoto, K., Nakashima, K., Mura, H., & Ninomiya, Y. (2000). Leptin as a modulator of sweet taste sensitivities in mice. Proceedings of the National Academy of Sciences, 97, 11044–11049.
- Kelly, L., Morales, S., Smith, B. K., & Berthoud, H. R. (2000). Capsaicin-treated rats permanently overingest low but not high concentration sucrose solutions. American Journal of Physiology, 279, R1805–R1812.
- Kennedy, G. C. (1953). The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society, London, 140, 579–592.
- Kim, E. M., Welch, C. C., Grace, M. K., Billington, C. J., & Levine, A. S. (1996). Chronic food restriction and acute food deprivation decrease mRNA levels of opioid peptides in the arcuate nucleus. American Journal of Physiology, 270, R1019–R1024.
- Kisley, L. R., Sakai, R. R., Fluharty, S. J. (1999). Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Research, 844 (1-2), 34–42.
- Kotz, C. M., Glass, M. J., Levine, A. S., & Billington, C. J. (2000). Regional effect of naltrexone in the nucleus of the solitary tract in blockade of NPY-induced feeding. American Journal of Physiology, 278, R499–R503.
- Kotz, C. M., Grace, M. K., Briggs, J. E., Levine, A. S., & Billington, C. J. (1995). Effects of opiate antagonists naloxone and naltrexone on neuropeptide Y induced feeding and brown fat thermoregulation in the rat: Neural site of action. Journal of Clinical Investigation, 96, 163–170.
- Kristensen, P., Judge, M. E., Thim, L., Ribel, U., Christjansan, K. N., Wulff, B. S., Clausen, J. T., Jensen, P. B., Madsen, O. D., Vrang, N., Larsen, P. J., & Hastrup, S. (1998). Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature, 393, 72–76.
- Ladenheim, E. E., & Ritter, R. C. (1988). Low-dose 4th ventricular bombesin selectively suppresses food intake. American Journal of Physiology, 255, R988–R992.
- Ladenheim, E. E., Taylor, J. E., Coy, D. H., Carrigan, T. S., Wohn, A., & Moran, T. H. (1997). Caudal hindbrain neuromedin Bpreferring receptors participate in the control of food intake. American Journal of Physiology, 272, R433–R437.
- Ladenheim, E. E., Taylor, J. E., Coy, D. H., Moore, K. A., & Moran, T. H. (1996). Hindbrain GRP receptor blockade antagonizes feeding suppression by peripherally administered GRP. American Journal of Physiology, 271, R180–R184.
- Ladenheim, E. E., Taylor, J. E., Coy, D. H., & Moran, T. H. (1994). Blockade of feeding inhibition by neuromedin B using a selective receptor antagonist. European Journal of Pharmacology, 271, R7–R9.
- Ladenheim, E. E., Wirth, K., & Moran, T. H. (1996). Receptor subtype mediation of the feeding suppression by bombesin-like peptides. Pharmacology, Biochemistry, and Behavior, 54, 705–711.
- Langhans, W., Pantel, K., Muller-Schell, W., Effengerger, E., & Scharrer, E. (1984). Hepatic handling of pancreatic glucagon and glucose during meals in rats. American Journal of Physiology, 247, R827–R832.
- Lashley, K. S. (1938). The experimental analysis of instinctive behavior. Psychological Review, 45, 445–471.
- LeSauter, J., Noh, U., & Geary, N. (1991). Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. American Journal of Physiology, 261, R154–R161.
- Lewis, D. E., Shellard, L., Koeslag, D. C., Boer, D. E., McCarthy, H. D., McKibbin, P. E., Russell, J. C., & Williams, G. (1993). Intense exercise and food deprivation cause similar hypothalamic neuropeptide Y increases in rats. American Journal of Physiology, 264, E279–E284.
- Lopez, M., Seoane, L., del Carmen Garcia, M., Lago, F., Casanueva, F. F., Senaris, R., & Dieguez, C. (2000). Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochemical and Biophysical Research Communications, 269, 41–45.
- Lucas, F., & Sclafani, A. (1989). Flavor preferences conditioned by intragastric fat infusions in the rat. Physiology and Behavior, 46, 403–412.
- Lutz, T. A., Geary, N., Szabady, M. M., Del Prete, E., & Scharrer, E. (1995). Amylin deceases meal size in rats. Physiology and Behavior, 58, 1197–1202.
- Lutz, T. A., Rossi, R., Althaus, J., Del Prete, E., & Sharrer, E. (1998). Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides, 19, 1533–1540.
- Lutz, T. A., Senn, M., Althaus, J., Del Prete, E., Ehrensperger, E., & Scharrer, E. (1998). Lesion of the area postema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene related peptide (CGRP) in rats. Peptides, 19, 309–317.
- Maffei, M., Halaas, E., Ravussin, E., Pratley, R. E., Lee, G. H., Zhang, Y., Fei, H., Kim, S., Lallone, R., Ranganathan, S., Kern, P.A., & Friedman, J. M. (1995). Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight reduced subjects. Nature Medicine, 1, 1155–1161.
- Marks-Kaufman, R., & Kanarek, R. B. (1990). Diet selection following chronic morphine and naloxone regimen. Pharmacology, Biochemistry, and Behavior, 35, 665–669.
- Marsh, D. J., Hollopeter, G., Kafer, K. E., & Palmiter, R. D. (1998). Role of Y5 neuropeptide Y receptor in feeding and obesity. Nature Medicine, 4, 718–721.
- Martel, P., & Fantinio, M. (1996). Mesolimbic dopaminergic system activity as a function of food reward: A microdialysis study. Pharmacology, Biochemistry, and Behavior, 53, 221–226.
- Mathis, C., Moran, T. H., & Schwartz, G. J. (1998). Load sensitive rat gastric vagal afferents encode volume but not gastric nutrients. American Journal of Physiology, 274, R280–R286.
- Matson, C. A., Reid, D. F., Cannon, T. A., & Ritter, R. C. (2000). Cholecystokinin and leptin act synergistically to reduce body weight. American Journal of Physiology, 278, R882–R890.
- Matson, C. A., & Ritter, R. C. (1999). Long term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. American Journal of Physiology, 276, R1038–R1045.
- Mayer, J. (1953). Glucostatic mechanisms of regulation of food intake. New England Journal of Medicine, 249, 13–16.
- McMinn, J. E., Sindelar, D. K., Havel, P. J., & Schwartz, M. W. (2000). Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology, 141, 4442–4448.
- Melanson, K. J., Westerterp-Plantenga, M. S., Saris, W. H., Smith, F. J., & Campfield, L. A. (1999). Blood glucose patterns and appetite in time blinded humans: Carbohydrate vs fat. American Journal of Physiology, 277, R337–R345.
- Menani, J. V., Colombari, D. S. A., Beltz, T. G., Thunhorst, R. L., & Johnson, A. K. (1998). Salt appetite: Interaction of forebrain angiotensinergic and hindbrain serotonergic mechanisms. Brain Research, 801 (1-2), 29–35.
- Mendelsohn, F. A. O., Quirion, R., Saavedra, J. M., Aguiler, G., & Catt, K. J. (1984). Autoradiographic localization of angiotensin II receptors in rat brain. Proceeding of the National Academy of Sciences, USA, 81, 1575–1579.
- Merali, Z., Moody, T. W., & Coy, D. (1993). Blockade of brain bombesin/GRP receptors increases food intake in sated rats. American Journal of Physiology, 264, R1031–R1034.
- Mercer, J. G., Lawrence, C. B., & Atkinson, T. (1996). Regulation of galanin gene expression in the hypothalamic paraventricular nucleus of the obese Zucker rat by manipulation of dietary macronutrient. Molecular Brain Research, 43, 202–208.
- Michaud, D., Anisman, H., & Merali, Z. (1999). Capsaicin sensitive fibers are required for the anorexic action of systemic but not central bombesin. American Journal of Physiology, 276, R1617– R1622.
- Miselis, R. R. (1981). The efferent projections of the subfornical organ of the rat: A circumventricular organ within a neural network subserving water balance. Brain Research, 230, 1–23.
- Mook, D. G. (1963). Oral and postingestional determinants of the intake of various solutions in rats with esophagela fistulas. Journal of Comparative and Physiological Psychology, 56, 645– 659.
- Mook, D. G. (1990). Satiety, specifications and stop rules: Feeding as a voluntary act. In A. N. Epstein & A. R. Morrison (Eds.), Progress in psychobiology and physiological psychology: Vol. 14. (pp. 1–65). New York: Academic Press.
- Moran, T. H., Ameglio, P. J., Peyton, H. J., Schwartz, G. J., & McHugh, P. R. (1993). Blockade of type A, but not type B, CCK receptors postpones satiety in rhesus monkeys. American Journal of Physiology, 265, R620–R624.
- Moran, T. H., Baldessarini, A. R., Solorio, C. F., Lowerry, T., & Schwartz, G. J. (1997). Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. American Journal of Physiology, 272, R1245–R1251.
- Moran, T. H., Katz, L. F., Plata-Salaman, C. R., & Schwartz, G. J. (1998). Disordered food intake and obesity in rats lacking CCKA receptors. American Journal of Physiology, 274, R618–R625.
- Moran, T. H., Knipp, S., & Schwartz, G. J. (1999). Gastric and duodenal features of meals mediate controls of liquid gastric emptying during fill in rhesus monkeys. American Journal of Physiology, 277, R1282–R1290.
- Moran, T. H., & McHugh, P. R. (1982). Cholecystokinin decreases food intake by inhibiting gastric emptying. American Journal of Physiology, 242, R491–R497.
- Moran, T. H., & McHugh, P. R. (1992). Gastric mechanisms in CCK satiety. In C. T. Dourish, S. J. Cooper, S. D. Iversen, & L. L. Iversen (Eds.), Multiple cholecystokinin receptors in the CNS (pp. 183–205). Oxford: Oxford University Press.
- Moran, T. H., Norgren, R., Crosby, R. J., & McHugh, P. R. (1990). Central and peripheral vagal transport of CCK binding sites occurs in afferent fibers. Brain Research, 526, 95–102.
- Moran, T. H., Shnayder, L., Hostetler, A. M., & McHugh, P. R. (1988). Pylorectomy reduces the satiety actions of cholecystokinin. American Journal of Physiology, 255, R1059– R1063.
- Morley, J. E., Levine, A. S., Gosnell, B. A., Kneip, J., & Grace, M. (1987). Effect of neuropeptide Y on ingestive behaviors in the rat. American Journal of Physiology, 252, R599–R609.
- Nakazato, M., Murakami, N., Date, Y., Kojima, M., Matuso, H., Kanagawa, K., & Matsukara, S. (2001). A role for ghrelin in the central regulation of feeding. Nature, 409, 194–198.
- Nilsson, G., & Uvnas-Wallenstein, K. (1977). Effect of teasing and sham feeding on plasma glucagon concentration in dogs. Acta Physiologica Scandinavia, 100, 298–302.
- Pavlov, I. P. (1910). The work of the digestive glands (2nd ed.). London: Charles Griffin.
- Pecina, S., Berridge, K. C., & Parker, L. A. (1997). Pimozide does not shift palatability: Separation of anhedonia from sensorimotor suppression of taste reactivity. Pharmacology, Biochemistry, and Behavior, 58, 801–811.
- Peck, J. W., & Novin, D. (1971). Evidence that osmoreceptors mediating drinking in rabbits are in the lateral preoptic area. Journal of Comparative and Physiological Psychology, 74, 134–147.
- Pedrazzini, T., Seydoux, J., Kunster, P., Aubert, J.-F., Grouzmann, E., Beerman, F., & Brunner, H.-R. (1998). Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nature Medicine, 4, 722–726.
- Perez, C., Ackroff, K., & Sclafani, A. (1996). Carbohydrate and protein conditioned flavor preferences: Effects of nutrient preloads. Physiology and Behavior, 59, 467–474.
- Perez, C., Lucas, F., & Sclafani, A. (1998). Increased flavor acceptance and preference conditioned by the postingestive actions of glucose. Physiology and Behavior, 64, 483–492.
- Peyron, C., Tighe, D. K., van den Pol, A. N., de Leces, L., Heller, H. C., Sutcliffe, J. G., & Kilduff, T. S. (1998). Neurons containing hypocretin (orexin) project to multiple neural systems. Journal of Neuroscience, 18, 9996–10015.
- Pomonis, J. D., Levine, A. S., & Billington, C. J. (1997). Interaction of hypothalamic paraventricular nucleus and central nucleus of the amygdala in naloxone blockade of neuropeptide Y induced feeding revealed by c-fos expression. Journal of Neuroscience, 17, 5175–5182.
- Qu, D., Ludwig, D. S., Gammeltolft, S., Piper, M., Pellymounter, M. A., Cullen, M. J., Mathes, W. F., Przypek, J., Kanarek, R., & Maratos-Flier, E. (1996). A role for melanin concentrating hormone in the central regulation of feeding behavior. Nature, 380, 243–247.
- Ramsay, D. J., Rolls, B. J., & Wood, R, J. (1977). Thirst following water deprivation in dogs. American Journal of Physiology, 232, R93–R100.
- Randich, A., Tyler, W. J., Cox, J. E., Meller, S. T., Kelm, G. R., & Bharaj, S. S. (2000). Responses of celiac and cervical vagal afferents to infusions of lipids in the jejunum or ileum of the rat. American Journal of Physiology, 278, R34–R43.
- Reidelberger, R. D., & O’Rourke, M. F. (1989). Potent cholecystokinin antagonist L-364,718 stimulates food intake in rats. American Journal of Physiology, 257, R1512–R1518.
- Richter, C. P. (1943). Total self regulatory functions in animals and human beings. Harvey Lectures, 38, 63–103.
- Ritter, R. C., & Ladenhiem, E. E. (1985). Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. American Journal of Physiology, 248, R501–R504.
- Ritter, S., Dinh, T. T., & Zhang, Y. (2000). Localization of hindbrain glucoreceptors sites controlling food intake and blood glucose. Brain Research, 856, 37–47.
- Rolls, B. J. (1986). Sensory-specific satiety. Nutrition Reviews, 44, 93–101.
- Rolls, E. T., Murzi, E., Yaxley, S., Thorpe, S. J., & Simpson, S. J. (1986). Sensory specific satiety: Food specific reduction in responsiveness of ventral forebrain neurons after feeding in the monkey. Brain Research, 368, 79–86.
- Rossi, M., Choi, S. J., O’Shea, D., Miyoshi, T., Ghatei, M. A., & Bloom, S. R. (1997). Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology, 138, 351–355.
- Rushing, P. A., Haga, M. M., Seeley, R. J., Lutz, T. A., D’Alessio, D. A., Air, E. L., & Woods, S. C. (2001). Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology, 142, 5035–5038.
- Sahu, A. (1999). Evidence suggesting that galanin, melanin concentrating hormone, neurotensin, proopiomelanocortin and neuropeptide Y are targets of leptin signaling in the hypothalamus. Endocrinology, 139, 795–798.
- Sakurai, T., Amemiya, A., Ishii, M., Matszaki, I., Chemelli, R. M., Tanaka, H., Williams, S. C., Richardson, J. A., Kozlowski, G.P.,Wilson,S.,Arch,J.R.S.,Buckingham,R.E.,Haynes,A.C., Carr, S.A.,Annan, R. S., McNulty, D. E., Liu, W.-S., Terett, J.A., Elshourbagy, N. A., Bergsma, D. J., & Yanagisawa, M. (1998). Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein coupled receptors that regulate feeding behavior. Cell, 92, 573–585.
- Schneider, L. H., Davis, J. D., Watson, C. W., & Smith, G. P. (1990). Similar effects of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. European Journal of Pharmacology, 186, 61–70.
- Schwartz, G. J., McHugh, P. R., & Moran, T. H. (1991). Integration of vagal afferent responses to gastric load and CCK in rats. American Journal of Physiology, 261, R64–R69.
- Schwartz, G. J., McHugh, P. R., & Moran, T. H. (1993). Gastric loads and CCK synergistically stimulate rat gastric vagal afferents. American Journal of Physiology, 265, R872–R876.
- Schwartz, G. J., & Moran, T. H. (1998). Duodenal nutrient exposure elicits nutrient specific gut motility and vagal afferent signals in rats. American Journal of Physiology, 274, R1236–R1242.
- Schwartz, G. J., Moran, T. H., White, W. O., & Ladenheim, E. E. (1997). Relationship between gastric motility and gastric vagal afferent responses to CCK and GRP in rats. American Journal of Physiology, 272, R1726–R1733.
- Schwartz, G. J., Solorio, C. F., Skoglund, C., & Moran, T. H. (1999). Gut vagal afferent lesions increase meal size but do not block gastric preload induced feeding suppression. American Journal of Physiology, 276, R1629–R1999.
- Schwartz, G. J., Tougas, G., & Moran, T. H. (1995). Integration of vagal afferent responses to duodenal loads and exogenous CCK. Peptides, 16, 707– 711.
- Schwartz, M. W., Seeley, R. J., Campfield, L.A., Burn, P., & Baskin, D. G. (1996). Identification of targets of leptin action in rat hypothalamus. Journal of Clinical Investigation, 98, 1101–1106.
- Schwartz, M. W., Seeley, R. J., Woods, S. C., Weigle, D. S., Campfield, L. A., Burn, P., & Baskin, D. G. (1997). Leptin increases hypothalamic proopiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes, 46, 2119–2123.
- Schwartz, M. W., Sipols, A. J., Marks, J. L., Sanacora, G., White, J. D., Scheurink, A., Kahn, S. E., Baskin, D. G., Woods, S. C., Figlewicz, D. P., & Porte, D. J., Jr. (1992). Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology, 130, 3608–3616.
- Sclafani, A. (1991). Conditioned food preferences. Bulletin of the Psychonomic Society, 29, 256–260.
- Scott, T. R., Yan, J., & Rolls, E. T. (1995). Brain mechanisms of satiety and taste in macaques. Neurobiology, 3, 281–292.
- Seeley, R. J., van Dijk, G., Campfield, L. A., Smith, F. J., Burn, , Nelligan, J. A., Baskin, D. G., & Woods, S. C. (1996). Intraventricular leptin reduces food intake and body weight in lean rats but not obese Zucker rats. Hormone and Metabolism Research, 28, 664–668.
- Shintani,M.,Ogawa,Y.,Ebihara,K.,Aizawa-Abe,M.,Miyanaga,F., Takaya, K., Hayashi, T., Inoue, G., Hosada, K., Kojima, M., Kanagawa, K., & Nakao, K. (2001). Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptideY/Y1 receptor pathway. Diabetes, 50, 227–232.
- Sills, T. L., & Vaccarino, F. J. (1996). Individual differences in sugar consumption following systemic or intraccumbens administration of low doses of amphetamine in deprived rats. Pharmacology, Biochemistry and Behavior, 54, 665–670.
- Simpson, J. B., Epstein, A. N., & Camardo, J. S. (1978). The localization of receptors for the dipsogenic action of angiotensin II in the subfornical organ. Journal of Comparative and Physiological Psychology, 92, 581–608.
- Simpson, J. B., & Routtenberg, A. (1974). Subfornical organ: Acetylcholine application elicits drinking. Brain Research, 79, 157–164.
- Smith, F. J., & Campfield, L. A. (1993). Meal initiation occurs after experimental induction of transient declines in blood glucose. American Journal of Physiology, 265, R1423–R1429.
- Smith, G. P., Jerome, C., & Norgren, R. (1985). Afferent axons in abdominal vagus mediate the satiety effect of cholecystokinin. American Journal of Physiology, 249, R638–R641.
- Stanley, B. G., Kyrkouli, S. E., Lampert, S., & Leibowitz, S. F. (1986). Neuropeptide Y chronically injected into the hypothalamus: A powerful neurochemical inducer of hyperphagia and obesity. Peptides, 7, 1189–1192.
- Stanley, B. G., & Leibowitz, S. F. (1985). Neuropeptide Y injected into the paraventricular hypothalamus: A powerful stimulant of feeding behavior. Proceedings of the National Academy of Sciences, 82, 3940–3943.
- Stanley, B. G., Magdalin, W., Seirafi, A., Thomas, W. J., & Leibowitz, S. F. (1993). The perifornical area: The major focus of a patchily distributed hypothalamic neuropeptide Y sensitive feeding system(s). Brain Research, 604, 304–317.
- Stein, L. J., & Woods, S. C. (1982). Gastrin releasing peptide reduces meal size in rats. Peptides, 3, 833–835.
- Stellar, E. (1954). The physiology of motivation. Psychological Review, 61, 5–22.
- Stricker, E. M. (1968). Some physiological and motivational properties of the hypovolemic stimulus for thirst. Physiology and Behavior, 3, 379–385.
- Stricker, E. M. (1969). Osmoregulation and volume regulation in rats: Inhibition of hypovolemic thirst by water. American Journal of Physiology, 217, 98–105.
- Stuckey, J. A., Gibbs, J., & Smith, G. P. (1985). Neural disconnection of the gut from brain blocks bombesin-induced satiety. Peptides, 6, 1249–1252.
- Sweet, D. C., Levine, A. S., Billington, C. J., & Kotz, C. M. (1999). Feeding responses to central orexins. Brain Research, 821, 535–538.
- Swithers, S. E., & Hall, W. G. (1994). Does oral experience terminate ingestion? Appetite, 23, 113–138.
- Takiguchi, S., Takata, T., Funakoshi, K., Miyasaka, K., Kataoka, K., Fujimura, Y., Goto, T., & Kono, A. (1997). Disrupted cholecystokinin type-A receptor (CCK-AR) gene in OLETF rats. Gene, 197, 169–175.
- Tang, M. (1976). Dependence of polyethylene glycol-induced dipsogenesis of intravascular fluid volume depletion. Physiology and Behavior, 17, 811–816.
- Tartaglia, L. A., Demski, M., Weng, X., Deng, N., Culpepper, J., Devos, R., Richards, G. J., Campfield, L. A., Clarck, F. T., Deeds, J., Muir, C., Sanker, S., Moriarity, A., Moore, K. J., Smutko, J. S., Mays, G. G., Wolfe, E. A., Monroe, C. A., & Tepper, R. I. (1995). Identification and expression of a leptin receptor, OB-R. Cell, 83, 1–20.
- Tschop, M., Smiley, D. L., & Heiman, M. L. (2000). Ghrelin induces adiposity in rodents. Nature, 407, 908–913.
- van Dijk, G., Seeley, R. J., Theile, T. E., Freidman, M. I., Ji, H., Wikinson, C. W., Burn, P., Campfield, L. A., Tenenbaum, R., Baskin, D. G., Woods, S. C., & Schwartz, M. W. (1999). Metabolic, gastrointestinal and CNS neuropeptide effects of brain leptin administration in the rat. American Journal of Physiology, 276, R1425–R1433.
- Weingarten, H. P., & Kulikovsky, O. T. (1989). Taste-to-postingestive consequence conditioning: Is the rise in sham feeding with repeated experience a learning phenomenon? Physiology and Behavior, 45, 471–476.
- Weingarten, H. P., & Watson, S. D. (1982). Sham feeding as a procedure for assessing the influence of diet palatability on food intake. Physiology and Behavior, 28, 401–407.
- Weisinger, R. S., Blair-West, J. R., Burns, P., Denton, D. A., & Tarjan, E. (1997). Role of Brain angiotensin in thirst and sodium appetite of rats. Peptides, 18, 977–984.
- West, D. B., Fey, D., & Woods, S. C. (1984). Cholecystokinin persistently suppresses meal size but not food intake in free feeding rats. American Journal of Physiology, 246, R776–R787.
- Wettendorff, H. (1901). Modifications de sang sous l’influence de la privation d’eau: Contribution a l’etude de la soif. Travaux du Laboratoire de Physiologie, Institut de Physiologie, Instituts, Solvay, 4, 353–384.
- White, J. D., & Kershaw, M. (1989). Increased neuropeptide Y expression following food deprivation. Molecular and Cellular Neuroscience, 1, 41–48.
- Widdowson, P. S., Henderson, L., Pickavance, L., Buckingham, R., Tadayyon, M., Arch, J. R. S., & Williams, G. (1999). Hypothalamic NPY status during positive energy balance and the effects of the NPY antagonist, BW1229U91, on the consumption of highly palatable energy rich diet. Peptides, 20, 367–372.
- Woods, S. C., Lotter, E. C., McKay, L. D., & Porte, D., Jr. (1979). Chronic intracerebroventricular infusion of insulin reduces food intake and body weight in baboons. Nature, 282, 503–505.
- Wren, A. M., Small, C. J., Ward, H. L., Murphey, K. G., Dakin, C. L., Taheri, S., Kennedy, A. R., Roberts, G. H., Morgan, D. G., Ghatei, M. A., & Bloom, S. R. (2000). The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology, 141, 4325–4328.
- Yaswen, J., Diehl, N., Brennan, M. B., & Hochgeschwender, U. (1999). Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nature Medicine, 5, 1066–1070.
- Zarjevski, N., Cusin, I., Vetter, R., Rohner-Jeanrenaud, F., & Jeanrenaud, B. (1993). Chronic intracerebroventricular NPY administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology, 133, 1753–1758.
- Zhang, Y., Porcina, R., Maffei, M., Barone, M., Leopold, L., & Freidman, J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372, 425–432.
- Zhou, Q. Y., & Palmiter, R. D. (1995). Dopamine-deficient mice are severely hypoactive, adipsic and aphagic. Cell, 83, 1197– 1209.
ORDER HIGH QUALITY CUSTOM PAPER

Gluten: A Benefit or Harm to the Body?

The quick answer is that it can be either, but it all depends on the individual.
What is Gluten?
Gluten is a protein naturally found in some grains including wheat, barley, and rye. It acts like a binder, holding food together and adding a “stretchy” quality—think of a pizza maker tossing and stretching out a ball of dough. Without gluten, the dough would rip easily.
Other grains that contain gluten are wheat berries, spelt, durum, emmer, semolina, farina, farro, graham, khorasan wheat, einkorn, and triticale (a blend of wheat and rye). Oats—though naturally gluten free—often contain gluten from cross-contamination when they are grown near, or processed in the same facilities as the grains listed above. Gluten is also sold as wheat gluten, or seitan, a popular vegan high-protein food. Less obvious sources of gluten include soy sauce and modified food starch, however gluten-free options of these products are available and labeled as such to comply with the U.S. Food and Drug Administration’s gluten-free labeling rule.
Gluten and Health Benefits
Gluten is most often associated with wheat and wheat-containing foods that are abundant in our food supply. Negative media attention on wheat and gluten has caused some people to doubt its place in a healthful diet. There is little published research to support these claims; in fact published research suggests the opposite.
In a 2017 study of over 100,000 participants without celiac disease, researchers found no association between long-term dietary gluten consumption and heart disease risk. [1] In fact, the findings also suggested that non-celiac individuals who avoid gluten may increase their risk of heart disease, due to the potential for reduced consumption of whole grains.
- Many studies have linked whole grain consumption with improved health outcomes. For example, groups with the highest intakes of whole grains including wheat (2-3 servings daily) compared with groups eating the lowest amounts (less than 2 servings daily) were found to have significantly lower rates of heart disease and stroke, development of type 2 diabetes, and deaths from all causes. [2-5]
Gluten may also act as a prebiotic, feeding the “good” bacteria in our bodies. Arabinoxylan oligosaccharide is a prebiotic carbohydrate derived from wheat bran that has been shown to stimulate the activity of bifidobacteria in the colon. These bacteria are normally found in a healthy human gut. Changes in their amount or activity have been associated with gastrointestinal diseases including inflammatory bowel disease, colorectal cancer, and irritable bowel syndrome. [6,7]
When Gluten Is a Problem
What’s not great about gluten is that it can cause serious side effects in certain individuals. Some people react differently to gluten, where the body senses it as a toxin, causing one’s immune cells to overreact and attack it. If an unknowingly sensitive person continues to eat gluten, this creates a kind of battle ground resulting in inflammation. The side effects can range from mild (fatigue, bloating, alternating constipation and diarrhea) to severe (unintentional weight loss, malnutrition, intestinal damage) as seen in the autoimmune disorder celiac disease . Estimates suggest that 1 in 133 Americans has celiac disease, or about 1% of the population, but about 83% of them are undiagnosed or misdiagnosed with other conditions. [8,9] Research shows that people with celiac disease also have a slightly higher risk of osteoporosis and anemia (due to malabsorption of calcium and iron, respectively); infertility; nerve disorders; and in rare cases cancer. [10] The good news is that removing gluten from the diet may reverse the damage. A gluten-free diet is the primary medical treatment for celiac disease. However, understanding and following a strict gluten-free diet can be challenging, possibly requiring the guidance of a registered dietitian to learn which foods contain gluten and to ensure that adequate nutrients are obtained from gluten-free alternatives. Other conditions that may require the reduction or elimination of gluten in the diet include:
- Non-celiac gluten sensitivity, also referred to as gluten sensitive enteropathy (GSE) or gluten intolerance —An intolerance to gluten with similar symptoms as seen with celiac disease, but without the accompanying elevated levels of antibodies and intestinal damage. There is not a diagnostic test for GSE but is determined by persistent symptoms and a negative diagnostic celiac test.
- Wheat allergy —An allergy to one or more of the proteins (albumin, gluten, gliadin, globulin) found in wheat, diagnosed with positive immunoglobulin E blood tests and a food challenge. Compare this with celiac disease, which is a single intolerance to gluten. Symptoms range from mild to severe and may include swelling or itching of the mouth or throat, hives, itchy eyes, shortness of breath, nausea, diarrhea, cramps, and anaphylaxis. People who test negative for this condition may still have gluten sensitivity. This condition is most often seen in children, which most outgrow by adulthood.
- Dermatitis herpetiformis (DH) —A skin rash that results from eating gluten. It is an autoimmune response that exhibits itself as a persistent red itchy skin rash that may produce blisters and bumps. Although people with celiac disease may have DH, the reverse is not always true.
It is important to note that gluten is a problem only for those who react negatively to it, or test positive for celiac disease. Most people can and have eaten gluten most of their lives, without any adverse side effects.
Does gluten cause brain fog?
But does this side effect occur in people without a true gluten intolerance, and can the reverse be suggested in that the avoidance of gluten might sharpen the mind? A large cohort study disagrees. Almost 13,500 middle-aged women from the Nurses’ Health Study II without celiac disease were followed for 28 years to observe any potential links between gluten intake and mental ability. [15] No significant differences were found in cognitive scores (measuring reaction time, attention, memory, etc.) comparing women with the highest and lowest gluten intakes. The lack of association remained even after excluding women with a dementia or cancer diagnosis.
Unless a person has diagnosed celiac disease, a wheat allergy, or a gluten sensitivity, current evidence does not support that eating gluten increases inflammation in the brain or negatively affects brain health.
What Is a “Gluten-Free Diet”?
This is essentially a diet that removes all foods containing or contaminated with gluten . However, since gluten-containing whole grains contain fiber and nutrients including B vitamins , magnesium , and iron , it’s important to make up for these missing nutrients. Along with consuming naturally gluten-free foods in their whole form like fruits , vegetables , legumes, nuts , seeds, fish, eggs , and poultry, the following whole grains are also inherently gluten-free:
- Brown, black, or red rice
- Gluten-free oats
It’s also key not to rely on processed gluten-free foods that may be high in calories, sugar, saturated fat, and sodium and low in nutrients, such as gluten-free cookies, chips, and other snack foods. Often, these foods are made with processed unfortified rice, tapioca, corn, or potato flours.
The gluten-free food industry has grown 136% from 2013 to 2015 with almost $12 billion in sales in 2015. Interestingly, studies show that people who do not have celiac disease are the biggest purchasers of gluten-free products. [11] Consumer surveys show that the top three reasons people select gluten-free foods are for “no reason,” because they are a “healthier option,” and for “digestive health.” [12] For those who are not gluten-intolerant, there is no data to show a specific benefit in following a gluten-free diet, particularly if processed gluten-free products become the mainstay of the diet. In fact, research following patients with celiac disease who change to a gluten-free diet shows an increased risk of obesity and metabolic syndrome. This could be partly due to improved intestinal absorption, but speculation has also focused on the low nutritional quality of processed gluten-free foods that may contain refined sugars and saturated fats and have a higher glycemic index. [13,14]
- Diet Review: Gluten-Free for Weight Loss
- Whole Grains
- Lebwohl B, Cao Y, Zong G, Hu FB, Green PHR, Neugut AI, Rimm EB, Sampson L, Dougherty L, Giovannucci E, Willett WC, Sun Q, Chan AT. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. BMJ . 2017 May 2;357:j1892.
- Liu S, Stampfer MJ, Hu FB, et al. Whole-grain consumption and risk of coronary heart disease: results from the Nurses’ Health Study. Am J Clin Nutr . 1999;70:412-9.
- Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis . 2008;18:283-90.
- de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med . 2007;4:e261.
- Johnsen, N.F., et al. Whole-grain products and whole-grain types are associated with lower all-cause and cause-specific mortality in the Scandinavian HELGA cohort. British Journal of Nutrition , 114(4), 608-23.
- Neyrinck, A.M., et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes . 2012 Jan; 2(1): e28.
- Tojo, R., et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol . 2014 Nov 7;20(41):15163-76.
- Beyond Celiac. Celiac Disease: Fast Facts https://www.beyondceliac.org/celiac-disease/facts-and-figures/ Accessed 4/1/2017.
- Riddle, M.S., Murray, J.A., Porter, C.K. The Incidence and Risk of Celiac Disease in a Healthy US Adult Population. Am J Gastroenterol . 2012;107(8):1248-1255.
- N., Freeman, H.J., Thomson, A.B.R. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol . 2012 Nov 14; 18(42): 6036–6059.
- Topper A. Non-celiacs Drive Gluten-Free Market Growth. Mintel Group Ltd. Web. http://www.mintel.com/blog/food-market-news/gluten-free-consumption-trends . Accessed Mar 27, 2017.
- Reilly, N.R. The Gluten-Free Diet: Recognizing Fact, Fiction, and Fad. The Journal of Pediatrics. Volume 175, August 2016, pages 206–210.
- Tortora, R., et al. Metabolic syndrome in patients with celiac disease on a gluten-free diet. Aliment Pharmacol Ther . 2015 Feb;41(4):352-9.
- Kabbani, T.A., et al. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment Pharmacol Ther . 2012 Mar;35(6):723-9.
- Wang Y, Lebwohl B, Mehta R, Cao Y, Green PHR, Grodstein F, Jovani M, Lochhead P, Okereke OI, Sampson L, Willett WC, Sun Q, Chan AT. Long-term Intake of Gluten and Cognitive Function Among US Women. JAMA Netw Open. 2021 May 3;4(5):e2113020. Disclosures: B Lebwohl reported receiving personal fees from Takeda and Kanyos outside the submitted work. OI Okereke reported receiving royalties from Springer Publishing outside the submitted work and receiving honoraria from the AARP for participation at the Global Council on Brain Health meetings. AT Chan reported receiving personal fees from Pfizer, Boehringer Ingelheim, Bayer Pharma, and Zoe Global outside the submitted work.
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cambridge Open

Consumption of ultra-processed foods and health status: a systematic review and meta-analysis
1 Department of Experimental and Clinical Medicine, University of Florence, 50134 Florence, Italy
2 Unit of Clinical Nutrition, Careggi University Hospital, 50134 Florence, Italy
M. P. Madarena
M. bonaccio.
3 Department of Epidemiology and Prevention, IRCCS Neuromed, Pozzilli, 86077 Isernia, Italy
L. Iacoviello
4 Department of Medicine and Surgery, Research Center in Epidemiology and Preventive Medicine (EPIMED), University of Insubria, 21100 Varese, Italy
Associated Data
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0007114520002688.
Increasing evidence suggests that high consumption of ultra-processed foods (UPF) is associated with an increase in non-communicable diseases, overweight and obesity. The present study systematically reviewed all observational studies that investigated the association between UPF consumption and health status. A comprehensive search of MEDLINE, Embase, Scopus, Web of Science and Google Scholar was conducted, and reference lists of included articles were checked. Only cross-sectional and prospective cohort studies were included. At the end of the selection process, twenty-three studies (ten cross-sectional and thirteen prospective cohort studies) were included in the systematic review. As regards the cross-sectional studies, the highest UPF consumption was associated with a significant increase in the risk of overweight/obesity (+39 %), high waist circumference (+39 %), low HDL-cholesterol levels (+102 %) and the metabolic syndrome (+79 %), while no significant associations with hypertension, hyperglycaemia or hypertriacylglycerolaemia were observed. For prospective cohort studies evaluating a total population of 183 491 participants followed for a period ranging from 3·5 to 19 years, highest UPF consumption was found to be associated with increased risk of all-cause mortality in five studies (risk ratio (RR) 1·25, 95 % CI 1·14, 1·37; P < 0·00001), increased risk of CVD in three studies (RR 1·29, 95 % CI 1·12, 1·48; P = 0·0003), cerebrovascular disease in two studies (RR 1·34, 95 % CI 1·07, 1·68; P = 0·01) and depression in two studies (RR 1·20, 95 % CI 1·03, 1·40; P = 0·02). In conclusion, increased UPF consumption was associated, although in a limited number of studies, with a worse cardiometabolic risk profile and a higher risk of CVD, cerebrovascular disease, depression and all-cause mortality.
Ultra-processed foods (UPF) are, according to the NOVA classification, ‘formulations of ingredients, mostly for industrial use only, derived from a series of industrial processes’ ( 1 ) . Examples of UPF are breakfast cereals, savoury snacks, reconstituted meat products, frankfurters, pre-packaged frozen dishes, soft and/or sweetened drinks, distilled alcoholic beverages and supplements.
UPF represents an important and growing part of the world’s food supply. Recent studies have reported that these foods account for a significant percentage of about 50–60 % of the energy content in the usual diet of the average US, Canadian or British consumer ( 2 – 4 ) . The increase in the volume of industrially processed products in the global food supply has coincided with an increasing prevalence of obesity and non-communicable diseases in many countries ( 5 ) , suggesting a possible association between UPF consumption and obesity risk, but studies on the potential health effects of UPF are limited.
Some cross-sectional studies have reported a significant association between UPF consumption, obesity ( 6 – 9 ) and the metabolic syndrome ( 10 ) , while others have shown no association ( 11 , 12 ) . In addition, results from a large French prospective cohort study, the NutriNet-Santé study, found that high UPF consumption led to a significant increase in the risk of CVD ( 13 ) , diabetes ( 14 ) , depressive symptoms ( 15 ) and cancer ( 16 ) . To date, despite great interest in the subject in both scientific and lay communities, there is a lack of consensus in terms of evaluation and impact of UPF on health and no systematic reviews, and meta-analyses were conducted so far on adults. Our study aimed to assess the relationship between UPF consumption as defined by NOVA and health status by conducting a comprehensive systematic review with meta-analysis of all the cross-sectional and cohort studies published so far.
Search strategy and selection of studies
The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines ( 17 ) . The protocol was registered at www.crd.york.ac./PROSPERO/uk as CRD42020165495. Two authors (G. P. and M. D.) independently performed systematic literature searches in MEDLINE, Embase, Scopus, Web of Science and Google Scholar databases, from inception to June 2020. Further studies were searched by checking the references of the identified articles. The keywords ‘ultra processed’ or ‘ultraprocessed’ or ‘ultra-processed’ or ‘NOVA’, and ‘food’ or ‘foods’ were used in combination as medical subject heading (MeSH) terms and text words. No language limitations were applied. Missing data or additional information were requested from the corresponding authors of the articles.
Two investigators (G. P. and M. D.) independently assessed articles potentially relevant for eligibility. Observational studies that reported a measure of association (risk estimates with CI or standard errors or sufficient data to calculate them) between the UPF consumption – defined by the NOVA Food Classification System ( 18 , 19 ) and evaluated by dietary recalls, food records or questionnaires – and health indicators were considered eligible for inclusion. The decision to include the studies was based on the study title, abstract and full-text screening. The inclusion and exclusion criteria are summarised in online Supplementary Table S1 , following the PECOS (Population, Exposure, Comparison, Outcome, Study) design format. Eligible studies were included if they met the inclusion criteria for the study population (clinically healthy subjects aged ≥18 years), exposure (high UPF consumption), reference group (low UPF consumption), outcome (any health indicator), study design (cross-sectional and prospective cohort studies) and statistics (sufficient data to allow calculation of differences between subjects consuming high UPF levels and those consuming low UPF levels). Case–control studies were excluded to minimise bias in recall and selection. Review articles, letters to the editor, comments, case reports and randomised controlled trials were also excluded. Discrepancies were resolved through consensus and discussion with a third investigator (M. P. M.) if consensus could not be reached.
Data extraction
Data extraction was carried out in duplicate by two investigators (M. D. and G. P.) using a standardised form. Disagreements were resolved by consensus or by a third investigator (M. P. M.) if consensus could not be reached. The following data were extracted from the original articles: main author, year of publication, country of study population, cohort, number of participants evaluated and events, length of follow-up (years), age of the population at baseline, sex, definition of outcome of interest, method used to assess UPF intake, comparison, measures of effect size and CI, and details of adjustment for confounding factors in the multivariate model. If the results were reported separately for men and women, they were included in the analysis as separate cohorts.
Assessment of methodological quality
Two investigators (G. P. and M. D.) independently assessed the methodological quality of each included study using the National Institutes of Health study quality assessment tool for observational cohort and cross-sectional studies ( 20 ) . This tool has fourteen items in total, with an overall rating based on weaknesses in critical domains (see online Supplementary Table S2 ). The critical domains were the following: research question, exposure assessed prior to outcome measurement, exposure measurements and evaluation, outcome measurements, and statistical analysis. The final results lead to an overall methodological evaluation of good, fair or poor. Disagreements were resolved by consensus or by a third investigator (M. P. M.) if consensus could not be reached.
Statistical analysis
All data were analysed using Review Manager (RevMan; version 5.3 for Macintosh). A random-effects model (DerSimonian and Laird method) was applied to combine multivariable-adjusted risk ratios (RR) or OR of the highest v . the lowest category of UPF consumption. The pooled results were reported as RR and were presented with 95 % CI with two-sided P values. Meta-analysis was conducted if ≥2 studies were available for an outcome. Outcomes expressed in β -coefficient or prevalence ratio have been excluded from the meta-analysis. The statistical heterogeneity between studies was estimated using the χ 2 Cochran’s Q-test with the I 2 statistic, which provides an estimate of the amount of variance between studies due to the heterogeneity rather than sampling error ( 21 ) . Where I 2 exceeded 50 %, heterogeneity was considered substantial and subgroup analyses were performed to explore the source of the heterogeneity ( 22 ) . The robustness of the results was established by eliminating each study one by one from the meta-analysis and recalculating the summary estimate (the ‘leave one out’ approach). If ≥5 studies were available, the possibility of publication bias was explored by visual inspection of funnel plot of the effect size against standard error. A P <0·05 was considered statistically significant.
Search results
The selection process is shown in Fig. 1 , according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. The search produced 2619 articles. After title and abstract screening, sixty-three articles were selected for the evaluation of the full text. At the end of the selection process, twenty-three articles were included in the qualitative analysis and nineteen in the quantitative analysis.

Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram for search strategy. RCT, randomised controlled trials.
Selected cross-sectional studies ( n 10 studies) examined the association between the UPF consumption and the following health outcomes: overweight/obesity ( n 5), high waist circumference or abdominal obesity ( n 5), BMI gain ( n 3), hypertension ( n 3), low HDL-cholesterol ( n 3), the metabolic syndrome ( n 3), hypertriacylglycerolaemia ( n 3), hyperglycaemia ( n 3), waist circumference gain ( n 2), irritable bowel syndrome ( n 1), and C-reactive protein levels ( n 1). Selected prospective cohort studies ( n 13) examined the association between the UPF consumption and all-cause mortality ( n 5), CVD risk/mortality ( n 3), overweight/obesity ( n 2), depression ( n 2), IHD/cerebrovascular risk/mortality ( n 2), waist circumference gain ( n 1), hypertension ( n 1), frailty ( n 1), CHD ( n 1), overall cancer risk/mortality ( n 1), breast cancer ( n 1), prostate cancer ( n 1) and colorectal cancer ( n 1).
Cross-sectional studies
Table 1 presents the main characteristics of the ten cross-sectional studies included in the systematic review. The overall analysis comprised 113 753 participants. Three studies were conducted in Brazil ( 6 , 8 , 23 ) , two in Canada ( 9 , 24 ) , two in the USA ( 7 , 10 ) , one in France ( 25 ) , one in the UK ( 26 ) and one in Lebanon ( 12 ) . The evaluation of UPF consumption was conducted through 24-h dietary recall in five studies ( 7 , 9 , 10 , 24 , 25 ) , 24-h food records in one study ( 6 ) , 4-d food records in one study ( 26 ) and FFQ in the remaining three studies ( 8 , 12 , 23 ) . Exposure was assessed through the total energy contribution from UPF. The methodological quality score was fair in six studies ( 6 – 8 , 10 , 25 , 26 ) and poor in four studies ( 9 , 12 , 23 , 24 ) .
Characteristics of cross-sectional studies evaluating ultra-processed food (UPF) consumption and different health outcomes
CCHS, Canadian Community Health Survey; CRP, C-reactive protein; E, energy; ELSA, Brazilian Longitudinal Study of Adult Health; F, female; FGID, four functional gastrointestinal disorders; IBS, irritable bowel syndrome; NHANES, National Health and Nutrition Examination Survey; M, male; mPNNS-GS, Modified Programme National Nutrition Santé Guideline Score; NDNS, UK National Diet and Nutrition Survey; TE, total energy; WC, waist circumference.
Meta-analytic pooling under a random-effects model indicated a significant association between the highest UPF consumption and increased risk of overweight/obesity in five studies ( 6 – 9 , 26 ) with a total population of 73 169 subjects (OR 1·39, 95 % CI 1·29, 1·50; P < 0·00001), without any evidence of statistical heterogeneity between studies ( I 2 = 0 %; P = 0·47) ( Fig. 2 ). Similarly, a statistically significant association was found between highest UPF consumption and increased risk of high waist circumference or abdominal obesity in four studies ( 7 , 8 , 24 , 26 ) with a population of 31 908 (OR 1·39, 95 % CI 1·16, 1·67; P = 0·0003), without any statistical heterogeneity between studies ( I 2 = 49 %; P = 0·12). In addition, highest UPF intake was associated with increased risk of the metabolic syndrome (OR 1·79, 95 % CI 1·10, 2·90; P = 0·02) and reduced HDL-cholesterol levels (OR 2·02, 95 % CI 1·27, 3·21; P = 0·003), with no evidence of statistical heterogeneity between studies ( I 2 = 0 %; P = 0·49 and I 2 = 0 %; P = 0·86, respectively) in two studies ( 12 , 24 ) involving a limited population of 1113 subjects. On the other hand, no statistically significant associations emerged between highest consumption of UPF and hypertension ( 12 , 24 ) (OR 1·31, 95 % CI 0·50, 3·43; P = 0·58), hyperglycaemia ( 12 , 24 ) (OR 1·10, 95 % CI 0·34, 3·52; P = 0·87) or hypertriacylglycerolaemia ( 12 , 24 ) (OR 0·95, 95 % CI 0·60, 1·50; P = 0·82).

Forest plot of cross-sectional studies investigating the association between ultra-processed foods consumption and different health outcomes. P value is for Z test of no overall association between exposure and outcome; P het is for test of no differences in association measure among studies; I 2 estimates from heterogeneity rather than sampling error. WC, waist circumference.
Prospective cohort studies
Table 2 presents the main characteristics of the thirteen prospective cohort studies included in the systematic review. The overall analysis comprised 183 491 participants followed over a period ranging from 3·5 to 19 years. Two cohorts were based in Spain ( 27 – 32 ) , one in France ( 13 , 15 , 16 , 33 ) , one in Brazil ( 34 ) , one in Italy ( 35 ) and one in the USA ( 36 ) . The evaluation of UPF consumption was conducted through 24-h dietary recalls, FFQ and dietary history. Exposure is extremely variable and ranges from the contribution of the total energy of UPF to servings per d or daily intake. The methodological quality score was good in all studies but one ( 32 ) .
Characteristics of prospective cohort studies evaluating ultra-processed food (UPF) consumption and different health outcomes
CDS, Cognitive Difficulties Scale; CES-D, Center for Epidemiologic Studies Depression Scale; CV, cerebrovascular; ELSA, Brazilian Longitudinal Study of Adult Health; ENRICA, Study on Nutrition and Cardiovascular risk factors in Spain; F, female; F-up, follow-up; M, male; mPNNS-GS, Modified Programme National Nutrition Santé Guideline Score; NA, not available; NHANES, National Health and Nutrition Examination Survey; RR, risk ratio; TV, television; SUN, University of Navarra Follow-Up Project; WC, waist circumference.
The results of the pooled analysis for all included studies are shown in Fig. 3 . The highest consumption of UPF was found to be associated with an increased risk of all-cause mortality in five studies ( 29 , 31 , 33 , 35 , 36 ) involving 111 056 subjects and 4687 deaths (RR 1·25, 95 % CI 1·14, 1·37; P < 0·00001), with no statistical heterogeneity between studies ( I 2 = 2 %; P = 0·40). In addition, highest UPF intake showed a significant association with increased risk of CVD incidence and/or mortality in three studies ( 13 , 35 , 36 ) with 2501 cases (RR 1·29, 95 % CI 1·12, 1·48; P = 0·0003; I 2 = 7 %, P = 0·34), cerebrovascular disease incidence and/or mortality in two studies ( 13 , 35 ) with 1150 cases (RR 1·34, 95 % CI 1·07, 1·68; P = 0·01; I 2 = 32 %, P = 0·22) and depression in two studies ( 15 , 30 ) with 2995 cases (RR 1·20, 95 % CI 1·03, 1·40; P = 0·02; I 2 = 42 %, P = 0·19). The statistically significant association was also found for overweight/obesity in two studies ( 27 , 34 ) with 2911 cases, (RR 1·23, 95 % CI 1·11, 1·36; P < 0·0001) and no evidence of heterogeneity between studies ( I 2 = 0 %, P = 0·64).

Forest plot of prospective cohort studies investigating the association between ultra-processed foods consumption and different health outcomes. P value is for Z test of no overall association between exposure and outcome; P het is for test of no differences in association measure among studies; I 2 estimates from heterogeneity rather than sampling error. CV, cerebrovascular.
Sensitivity analysis and publication bias
A leave-one-out sensitivity analysis was performed by iteratively removing one study at a time to confirm that our results were not determined by a single study. There were few changes in the quantitative measurements of OR, RR and the 95 % CI, without any study affecting the results for almost all of the outcomes investigated. The only exceptions were found in cross-sectional study analyses for the metabolic syndrome, low HDL-cholesterol levels and hyperglycaemia. For the metabolic syndrome and low HDL-cholesterol levels, the removal of the study by Lavigne-Robichaud et al . ( 24 ) changed the relative effect from significant (in the main analysis) to non-significant in the sensitivity analysis (OR 1·11, 95 % CI 0·26, 4·65; P = 0·89 and OR 1·82, 95 % CI 0·52, 6·42; P = 0·35). Conversely, for hyperglycaemia, the removal of the study by Nasreddine et al . ( 12 ) changed the relative effect from non-significant in the main analysis to significant in the sensitivity analysis (OR 1·76, 95 % CI 1·04, 2·97; P = 0·03).
The publication bias was evaluated for all-cause mortality (online Supplementary Fig. S1 ). The shape of the funnel plot did not show any evident asymmetry, suggesting the absence of possible publication biases.
The present study is the first systematic review with meta-analysis that evaluated all available observational studies that assessed the association between UPF consumption and health status. By comparing the highest v . the lowest UPF consumption, the pooled analysis of cross-sectional studies, carried out for each result in a limited number of studies, showed a possible increase in the risk of overweight/obesity, high waist circumference, reduced levels of HDL-cholesterol and the metabolic syndrome. Similarly, for prospective cohort studies, the increased UPF consumption was associated with an increased risk of all-cause mortality in five studies, CVD in three studies, cerebrovascular disease and depression in only two studies, and confirmed the significant association found for overweight/obesity, but only in two studies. These results, although reporting interesting and useful data to formulate a hypothesis, must be carefully interpreted due to the low number of subjects and studies investigated.
In recent years, the global food system has undergone a profound transformation in terms of technology and food processing. The food profile of the world’s countries has changed significantly in favour of the consumption of highly processed industrial products for reasons of economic convenience, industrial competition and attractiveness to the consumer ( 37 ) . For all these reasons, the availability and consumption of UPF have increased significantly in all countries, regardless of economic level ( 38 ) . These foods are defined based on a classification system called NOVA , which classifies foods into four groups according to the industrial processing used in their production ( 1 ) . The NOVA classification is a simple classification based on the food technology and does not provide any indications on the nutritional content of the food. According to this classification, UPF are defined as products ‘created mostly or entirely from substances extracted from food or derived from food constituents with little or no intact food’. Since Monteiro coined the term UPF, there have been an increasing number of studies that have associated UPF consumption with negative health outcomes in adult subjects ( 3 , 39 ) , including cardiometabolic risk factors ( 40 ) , CVD ( 35 ) , cancer ( 16 ) and many other outcomes ( 15 , 25 , 32 ) .
In the present study, we have thoroughly evaluated all the observational studies that investigated the possible association between UPF consumption and health status, and we made a quantitative assessment of the association through the meta-analytical procedure. The number of studies included was limited, especially for individual outcomes, and does not allow us to be sure that the results obtained are completely reliable but is sufficient to hypothesise the nature of the association that must then be tested in intervention studies for validation. The analysis of the ten cross-sectional studies showed an increased risk of overweight/obesity, high waist circumference, reduced HDL-cholesterol levels and the metabolic syndrome but not of the other outcomes such as hypertension, hyperglycaemia or hypertriacylglycerolaemia in adults who consume high levels of UPF compared with those who consume less. The analysis of prospective cohort studies confirmed the significant increase in the risk of overweight/obesity and documented a 29 % increase in the risk of CVD incidence and/or mortality, a 34 % increase in the risk of cerebrovascular disease and a 20 % increase in the risk of depression.
The explanations for the possible harmful effects of UPF on health are different and may lie in the fact that these foods are, de facto , indicators of poor food quality, containing high amounts of free or added sugars, fats, low levels of fibre and high energy density ( 41 ) . These characteristics can reasonably explain the negative effect of these products on cardiovascular and cardiometabolic risk factors, as well as the risk of overweight/obesity. However, beyond the nutritional composition, UPF could also explain their harmful effects through other mechanisms, such as the presence of compounds that are formed during the processing of the food, and therefore more present in UPF. For example, both acrylamide – a contaminant present in heat-treated processed food products – and acrolein – a compound formed during fat heating – have been associated with an increased risk of CVD ( 42 , 43 ) . In addition, bisphenol A – an industrial chemical used in some UPF plastic packaging – has been found associated with an increased risk of cardiometabolic disorders ( 44 ) . Although bisphenol A is banned for use in food packaging in many countries, it has now been replaced by other components such as bisphenol S, which also has endocrine-disrupting properties, and is suspected to be absorbed more orally than bisphenol A ( 45 ) . Recent studies have confirmed that UPF consumption is associated with increased exposure to endocrine-disrupting chemicals and phthalates used in industrial plastic packaging ( 45 , 46 ) . Another possible explanation for the harmful effects of UPF on health status is related to their organoleptic characteristics, which have led to an increase in the eating rate and delayed satiety signalling, leading to higher overall food intake. Hall et al . recently ( 47 ) conducted a randomised controlled trial on twenty weight-stable adults who were randomised to receive either ultra-processed or unprocessed ad libitum diets for 2 weeks. At the end of the study, a significant increase in body weight was reported along with an overall increase in energy intake only after the UPF-rich diet. In addition, it was also hypothesised that UPF can adversely affect health by modifying the gut microbiome in such a way that it disturbs the energy balance and promotes the selection of microbes that promote inflammation-related diseases such as CVD and metabolic diseases and even depression ( 48 , 49 ) .
The present study has several limitations that should be addressed. First, the included studies evaluated UPF consumption through self-reported tools (FFQ, food records and 24-h recalls), which are generally accepted, but which are susceptible to recall bias, and which are not specifically designed to collect UPF data as described by the NOVA classification. This may result in an over- or underestimation of the UPF intake level. Indeed, the application of the National Institutes of Health study quality assessment tool suggested that the methodological quality of all the cross-sectional studies included was fair or poor, mainly due to the lack of details on the validity and reliability of the questionnaires used to assess UPF consumption. Secondly, the overall analyses for each different outcome were carried out in a limited number of studies, thus reducing the statistical power of the analysis. Third, only a limited number of studies included total energy intake as a confounding variable in the multivariable models, thus introducing a possible limitation in the interpretation of the results. However, it should be noted that total energy intake can also be part of the causal pathway of UPF intake; therefore, this aspect is not necessarily a study limitation. In addition, it is well known that unhealthy eating habits (i.e. high consumption of UPF) are commonly associated with other unhealthy lifestyle behaviours, such as sedentary habits, which in turn are associated with adverse health outcomes. Thus, the results of the present meta-analysis should be interpreted with caution, since not all the included studies considered unhealthy lifestyle behaviours as confounding factors in the multivariable models. On the other hand, the study presents also some strengths such as a rigorous search and selection strategy that identified all available cross-sectional and prospective cohort studies examining the relationship between UPF consumption and health status, and the fact that all but one of the included cohort studies had good methodological quality, with an adequate follow-up, and high participation rates.
In conclusion, we reported for the first time in a systematic review with meta-analysis the possible association between high UPF consumption, worse cardiometabolic risk profile (reported mainly by an increased risk of overweight/obesity, elevated waist circumference, reduced HDL-cholesterol levels and increased risk of the metabolic syndrome), and greater risk of all-cause mortality, CVD, cerebrovascular disease and depression. The available literature still has several limitations and the methods used to classify these foods need careful review, so reducing the applicability and transferability of these results to the general population. However, these findings have important public health implications, especially for food policymakers who should discourage the consumption of UPF and promote fresh and minimally processed foods to improve health status.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
M. B., L. I., F. S. designed the research; G. P. and M. D. conducted the systematic literature search, performed the quality assessment and the data extraction; G. P. performed the statistical analysis; M. D., G. P., M. P. M. wrote the paper; M. B., L. I., F. S. critically reviewed the manuscript and had primary responsibility for final content; all authors contributed to writing and reviewing the manuscript and read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material

IMAGES
VIDEO
COMMENTS
Introduction. Eating a variety of foods—in a single meal or across days—is one factor that has been shown to increase food intake (), with seminal experimental research on this effect (in human participants) being published by Barbara Rolls and colleagues in 1981 ().As "food variety" is generally considered a good indicator of nutritional adequacy, this effect of variety can be ...
Research suggests that "healthy" food choices such as eating fruits and vegetables have not only physical but also mental health benefits and might be a long-term investment in future well-being.
4. Diet and Culture for Healthy and Long Life. What elevates food to become diet and a meal is the manner and the context in which that food is consumed [].Numerous traditional and socio-cultural facets of dietary habits can be even more significant than their molecular, biochemical, and physiological concerns regarding their nutritional ingredients and composition.
2.1. Daily Quantity. The current international Recommended Dietary Allowance (RDA) for protein is 0.8 g per kg of body weight (bw), regardless of age [28,29].In the UK, the Reference Nutrient Intake (RNI) is 0.75 g/kg/bw [].These recommendations are derived as a minimum amount to maintain nitrogen balance and are not optimised for physical activity level (PAL).
The biological factors that control food intake can be tempered by learning, experience, or be altered through disease states. ... of the paper, revisions, and reviewed and approved the final draft of the manuscript. A version of this paper was presented at the International Consultation on Sustainable and Healthy Diets, FAO headquarters, Rome ...
Approach to Obesity, Calories, and Energy Balance. obesity has remained a substantial and increasing contributor to the global burden of disease, with current prevalence estimates of 5% in children and 12% in adults, representing more than a twofold increase since 1980 ().In the United States, over 66% adults are overweight, 33% are obese, and the proportion of very obese is growing rapidly ().
Hence, dietary pattern analysis is considered a technology complementary to the study of single nutrients or food. In the past few decades, statistical methods have emerged that make full use of dietary information collected across populations to create dietary patterns [ 2, 4, 8 ]. In nutritional epidemiology studies, regardless of the ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... satiety and food intake in ...
Whereas the consumption of white potatoes is associated with lower incomes, the consumption of salad leafy greens is much higher among higher income groups. A recent paper modeled the frequency of salad consumption using residential property values as a predictor of dietary behavior (Drewnowski, Buszkiewicz, and Aggarwal Citation 2019). These ...
The outcome of the search strategy and application of the eligibility criteria to yield the final, retrieved papers were illustrated in Fig. 1 as recommended within the PRISMA guidelines. After duplicates were removed, 1671 papers were identified as potentially relevant, after titles were screened, 262 articles remained and after full text screening 22 papers were retained and an additional ...
Therefore, research to progressively identify the challenges and improve these methods of data collection is highly warranted [2]. Dietary data collection tools are many and their usages in clinical, public health as well as in research contexts are different. ... paper based food record: yes: no: Energy intake and participants preferences ...
2. Components of a Healthy Diet and Their Benefits. A healthy diet is one in which macronutrients are consumed in appropriate proportions to support energetic and physiologic needs without excess intake while also providing sufficient micronutrients and hydration to meet the physiologic needs of the body [].Macronutrients (i.e., carbohydrates, proteins, and fats) provide the energy necessary ...
Background Comprehensive assessment of dietary intakes of foods and nutrients in Filipino adults are lacking. This study evaluated energy and nutrient intakes and food sources of key nutrients consumed by Filipino adults. Methods The participants were from the 2013 National Nutrition Survey wherein food intake of young adults aged 19-49 years (n = 12,896) and older adults aged 50 years and ...
The overall dietary intake of the SEACO-CH20 children and adolescents was found to be suboptimal, with less than 50% meeting dietary recommendations across all food groups except for meat, poultry ...
98.00% had breakfast and 75.00% had at least four meals a day. High intake of grains and simple sugars, dairy products, and meats; low. intake of fish and fibers. 91.25% needed to make dietary ...
This paper presents a systematic review of the use of technology for food intake detection, focusing on the different sensors and methodologies used. The search was performed with a Natural Language Processing (NLP) framework that helps screen irrelevant studies while following the PRISMA methodology.
Frequent eating out, energy-dense snack food and fast-food consumption among adolescents were evidence of high energy, fat and low micronutrient intake associated with a higher risk of overweight ...
A food record is a comprehensive recording of all foods, beverages, and dietary supplements that a participant in a research study consumed within a designated period of time. Usually 3-4 days of intake are recorded as participant burden generally causes a decline in the quality of information recorded if more days are recorded.
Background Today, with the advancement of science, technology and industry, people's lifestyles such as the pattern of people's food, have changed from traditional foods to fast foods. The aim of this survey was to examine and identify factors influencing intent to use fast foods and behavior of fast food intake among students based on the theory of planned behavior (TPB). Methods A cross ...
Understanding the relationship between the intake of sugars and diet quality can inform public health recommendations. This systematic review synthesized recent literature on associations between sugar intake and diet quality in generally healthy populations aged 2 years or older. We searched databases from 2010 to 2022 for studies of any design examining associations between quantified sugar ...
Consumption of fast foods t wo times or more per. week has been associa ted with 31% highe r. prevalen ce of moderate abdominal obesity in men. and 25% higher preval ence in women 70. Obesity is ...
View sample food and fluid intake research paper. Browse research paper examples for more inspiration. If you need a psychology research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance.
Gianfredi and colleagues published a systematic review and meta-analysis to explore the association between dietary fibre and risk of CRC through a comparison between individuals with the highest and lowest dietary fibre intake. Of 376 papers, 25 datasets were included in the analysis, which revealed a protective role of dietary fibre intake on ...
There is little published research to support these claims; in fact published research suggests the opposite. ... ' Health Study II without celiac disease were followed for 28 years to observe any potential links between gluten intake and mental ability. ... The gluten-free food industry has grown 136% from 2013 to 2015 with almost $12 ...
Increasing evidence suggests that high consumption of ultra-processed foods (UPF) is associated with an increase in non-communicable diseases, overweight and obesity. The present study systematically reviewed all observational studies that investigated the association between UPF consumption and health status. A comprehensive search of MEDLINE ...