- Contact Tracing
- Pandemic Data Initiative
- Events & News

JHU has stopped collecting data as of
After three years of around-the-clock tracking of COVID-19 data from...

What is the JHU CRC Now?
The Johns Hopkins Coronavirus Resource Center established a new standard for infectious disease tracking by publicly providing pandemic data in near real time. It began Jan. 22, 2020 as the COVID-19 Dashboard , operated by the Center for Systems Science and Engineering and the Applied Physics Laboratory . But the map of red dots quickly evolved into the global go-to hub for monitoring a public health catastrophe. By March 3, 2020, Johns Hopkins expanded the site into a comprehensive collection of raw data and independent expert analysis known as the Coronavirus Resource Center (CRC) – an enterprise that harnessed the world-renowned expertise from across Johns Hopkins University & Medicine.
Why did we shut down?
After three years of 24-7 operations, the CRC is ceasing its data collection efforts due to an increasing number of U.S. states slowing their reporting cadences. In addition, the federal government has improved its pandemic data tracking enough to warrant the CRC’s exit. From the start, this effort should have been provided by the U.S. government. This does not mean Johns Hopkins believes the pandemic is over. It is not. The institution remains committed to maintaining a leadership role in providing the public and policymakers with cutting edge insights into COVID-19. See details below.
Ongoing Johns Hopkins COVID-19 Resources
The Hub — the news and information website for Johns Hopkins — publishes the latest updates on COVID-19 research about vaccines, treatments, and public health measures.
The Johns Hopkins Bloomberg School of Public Health maintains the COVID-19 Projects and Initiatives page to share the latest research and practice efforts by Bloomberg faculty.
The Johns Hopkins Center for Health Security has been at the forefront of providing policymakers and the public with vital information on how to mitigate disease spread.
The Johns Hopkins International Vaccine Access Center offers an online, interactive map-based platform for easy navigation of hundreds of research reports into vaccine use and impact.
Johns Hopkins Medicine provides various online portals that provide information about COVID-19 patient care, vaccinations, testing and more.
Accessing past data
Johns Hopkins maintains two data repositories for the information collected by the Coronavirus Resource Center between Jan. 22, 2020 and March 10, 2023. The first features global cases and deaths data as plotted by the Center for Systems Science and Engineering. The second features U.S. and global vaccination data , testing information and demographics that were maintained by Johns Hopkins University’s Bloomberg Center for Government Excellence .
How to use the CSSE GitHub
- Click on csse_covid_19_data to access chronological 'time series' data.
- Click on csse_covid_19_time_series to access daily data for cases and deaths.
- Click on 'confirmed_US' for U.S. cases and “deaths_US” for U.S. fatalities; and “confirmed_global” for international cases and 'deaths_global' for international fatalities.
- Click “View Raw.”
- Left click to “Save As” as spreadsheet.
How to use the GovEx GitHub
- Visit the U.S. 'data dictionary' and the global 'data dictionary' to understand the vaccine, testing and demographic data available for your use.
- Example: Click on either 'us_data' or 'global_data' for vaccine information.
- Click on 'time_series' to access daily reports for either the U.S. or the world.
- Select either 'vaccines' or “doses administered” for the U.S. or the world.
- For either database, click 'view raw' to view the data or to save it to a spreadsheet.
Government Resources
Us cases & deaths.
The U.S. Centers for Disease Control and Prevention maintains a national data tracker.
GLOBAL TRENDS
The World Health Organization provides information about global spread.
The CDC also provides a vaccine data tracker for the U.S., while Our World In Data from Oxford University provides global vaccine information.
HOSPITAL ADMISSIONS
The CDC and the U.S. Department of Health and Human Services have provided hospital admission data in the United States.
THANK YOU TO ALL CONTRIBUTORS TO THE JHU CRC TEAM
Thank you to our partners, thank you to our funders, special thanks to.
Aaron Katz, Adam Lee, Alan Ravitz, Alex Roberts, Alexander Evelo, Amanda Galante, Amina Mahmood, Angel Aliseda Alonso, Anna Yaroslaski, Arman Kalikian, Beatrice Garcia, Breanna Johnson, Cathy Hurley, Christina Pikas, Christopher Watenpool, Cody Meiners, Cory McCarty, Dane Galloway, Daniel Raimi Zlatic, David Zhang, Doug Donovan, Elaine Gehr, Emily Camacho, Emily Pond, Ensheng Dong, Eric Forte, Ethel Wong, Evan Bolt, Fardin Ganjkhanloo, Farzin Ahmadi, Fernando Ortiz-Sacarello, George Cancro, Grant Zhao, Greta Kinsley, Gus Sentementes, Heather Bree, Hongru Du, Ian Price, Jan LaBarge, Jason Williams, Jeff Gara, Jennifer Nuzzo, Jeremy Ratcliff, Jill Rosen, Jim Maguire, John Olson, John Piorkowski, Jordan Wesley, Joseph Duva, Joseph Peterson, Josh Porterfield, Joshua Poplawski, Kailande Cassamajor, Kevin Medina Santiago, Khalil Hijazi, Krushi Shah, Lana Milman, Laura Asher, Laura Murphy, Lauren Kennell, Louis Pang, Mara Blake, Marianne von Nordeck, Marissa Collins, Marlene Caceres, Mary Conway Vaughan, Meg Burke, Melissa Leeds, Michael Moore, Miles Stewart, Miriam McKinney Gray, Mitch Smallwood, Molly Mantus, Nick Brunner, Nishant Gupta, Oren Tirschwell, Paul Nicholas, Phil Graff, Phillip Hilliard, Promise Maswanganye, Raghav Ramachandran, Reina Chano Murray, Roman Wang, Ryan Lau, Samantha Cooley, Sana Talwar, Sara Bertran de Lis, Sarah Prata, Sarthak Bhatnagar, Sayeed Choudury, Shelby Wilson, Sheri Lewis, Steven Borisko, Tamara Goyea, Taylor Martin, Teresa Colella, Tim Gion, Tim Ng, William La Cholter, Xiaoxue Zhou, Yael Weiss
CRC in the Media
Time names crc go-to data source.
TIME Magazine named the Johns Hopkins Coronavirus Resource Center one of its Top 100 Inventions for 2020.
Research!America Praises CRC Work
Research!America awarded the CRC a public health honor for providing reliable real time data about COVID-19.
CRC Earns Award From Fast Company
The Johns Hopkins Coronavirus Resource Center wins Fast Company’s 2021 Innovative Team of the Year award.
Public Service
Lauren Gardner Wins Lasker Award for Service
Lauren Gardner, co-creator of the COVID-19 Dashboard, won the 2022 Lasker-Bloomberg Public Service Award.
- Research article
- Open access
- Published: 04 June 2021
Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
16k Accesses
28 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
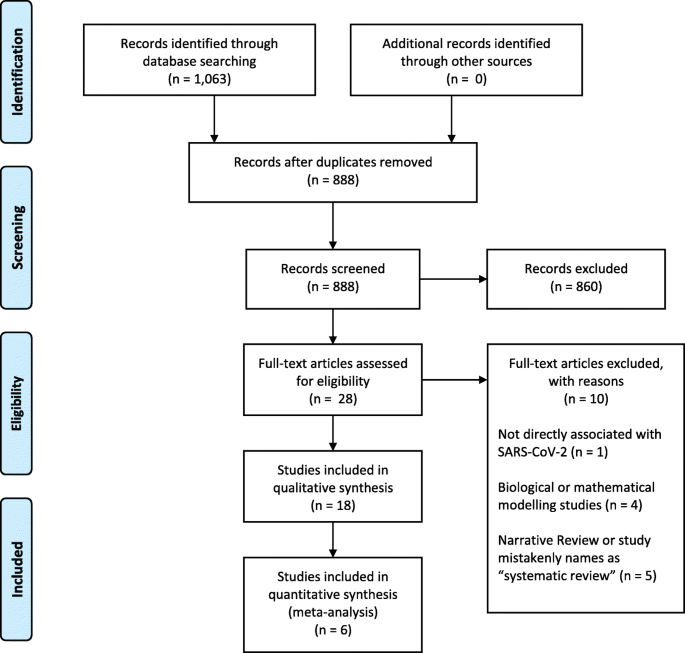
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
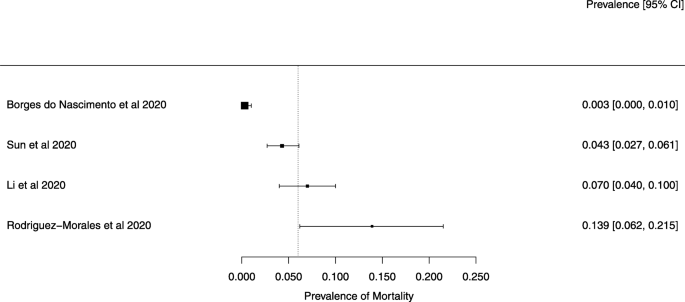
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
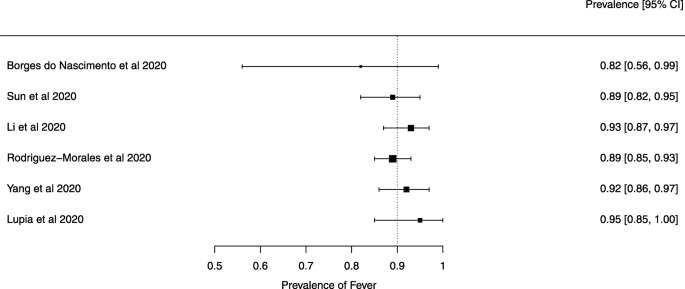
A meta-analysis of the prevalence of fever
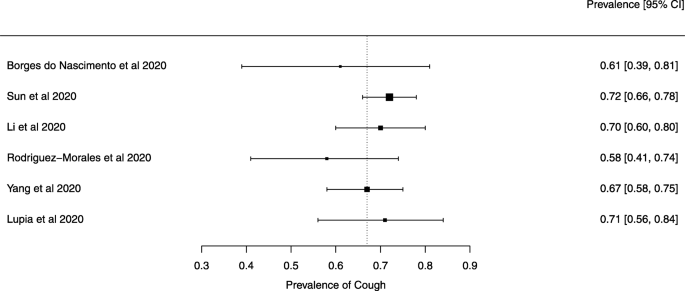
A meta-analysis of the prevalence of cough
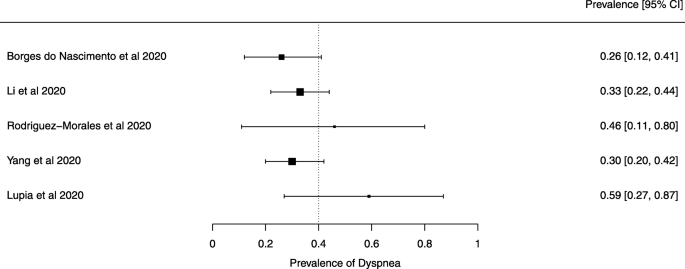
A meta-analysis of the prevalence of dyspnea
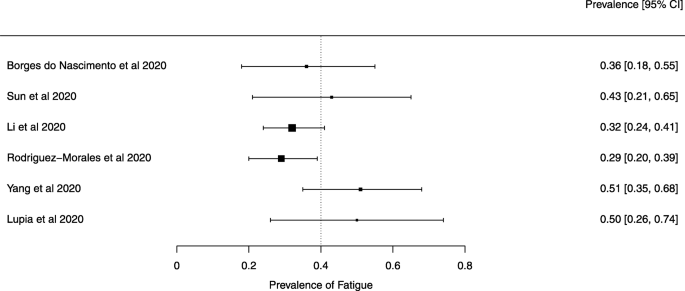
A meta-analysis of the prevalence of fatigue or myalgia
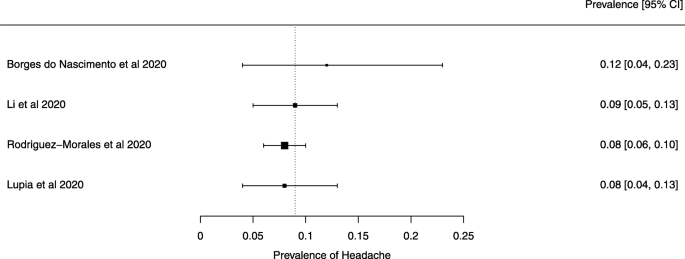
A meta-analysis of the prevalence of headache
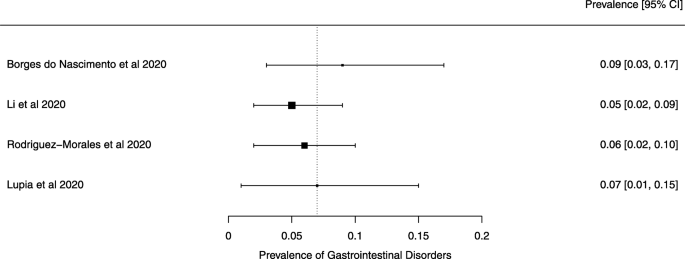
A meta-analysis of the prevalence of gastrointestinal disorders
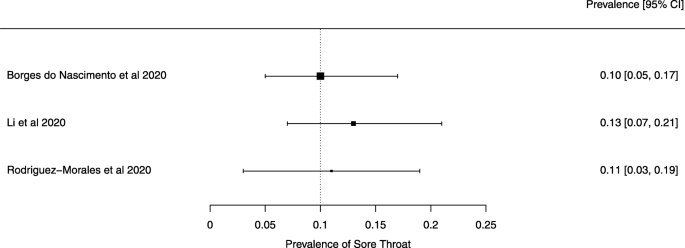
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Read our research on: Gun Policy | International Conflict | Election 2024
Regions & Countries
Coronavirus (covid-19), how americans view the coronavirus, covid-19 vaccines amid declining levels of concern.
Just 20% of the public views the coronavirus as a major threat to the health of the U.S. population and only 10% are very concerned about getting a serious case themselves. In addition, a relatively small share of U.S. adults (28%) say they’ve received an updated COVID-19 vaccine since last fall.
How the Pandemic Has Affected Attendance at U.S. Religious Services
During the pandemic, a stable share of U.S. adults have been participating in religious services in some way – either virtually or in person – but in-person attendance is slightly lower than it was before COVID-19. Among Americans surveyed across several years, the vast majority described their attendance habits in roughly the same way in both 2019 and 2022.
Mental health and the pandemic: What U.S. surveys have found
Here’s a look at what surveys by Pew Research Center and other organizations have found about Americans’ mental health during the pandemic.
All Coronavirus (COVID-19) Publications
Just 20% of the public views the coronavirus as a major threat to the health of the U.S. population and only 10% are very concerned about getting a serious case themselves. In addition, a relatively small share of U.S. adults (28%) say they've received an updated COVID-19 vaccine since last fall.
Online Religious Services Appeal to Many Americans, but Going in Person Remains More Popular
About a quarter of U.S. adults regularly watch religious services online or on TV, and most of them are highly satisfied with the experience. About two-in-ten Americans (21%) use apps or websites to help with reading scripture.
About a third of U.S. workers who can work from home now do so all the time
About a third of workers with jobs that can be done remotely are working from home all the time, according to a new Pew Research Center survey.
Economy Remains the Public’s Top Policy Priority; COVID-19 Concerns Decline Again
Americans now see reducing the budget deficit as a higher priority for the president and Congress to address than in recent years. But strengthening the economy continues to be the public’s top policy priority.
At least four-in-ten U.S. adults have faced high levels of psychological distress during COVID-19 pandemic
58% of those ages 18 to 29 have experienced high levels of psychological distress at least once between March 2020 and September 2022.
Key findings about COVID-19 restrictions that affected religious groups around the world in 2020
Our study analyzes 198 countries and territories and is based on policies and events in 2020, the most recent year for which data is available.
How COVID-19 Restrictions Affected Religious Groups Around the World in 2020
Nearly a quarter of countries used force to prevent religious gatherings during the pandemic; other government restrictions and social hostilities related to religion remained fairly stable.
What Makes Someone a Good Member of Society?
Most in advanced economies say voting, taking steps to reduce climate change and getting a COVID-19 vaccine are ways to be a good member of society; fewer say this about attending religious services.
Refine Your Results
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .
COVID-19 Research
EPA researchers are building on an expansive body of world-class research by applying knowledge to reduce the risk of exposure to SARS-CoV-2, the virus that causes COVID-19. Read an overview of EPA research efforts related to COVID-19 is below, or learn more about EPA's COVID-19 research efforts, including data and results.
- Community Exposure
- Masks and PPE
Research on Covid-19 in the Air
Common modes of transmission of Covid-19 include aerosols or respiratory droplets that are produced when a person coughs, sneezes, sings or talks, particularly in indoor environments with limited ventilation. Aerosols are very tiny particles that linger in the air and move with air currents like smoke or dust. Respiratory droplets are larger and fall, landing on surfaces where they can linger hours to days.
To learn more about how the virus moves in the air, researchers modeled viral aerosols in an office environment. The focus of this research was an “open office” such as a cubicle work environment where there is concern about how aerosols from an infected person, though likely asymptomatic, might affect other workers in other locations in an office. This research will first determine potential levels of exposure and then look at practical office modifications that might reduce exposure to the virus.
Researchers are also looking at technologies that are safe to operate in spaces where there will be people, like offices, subways, restaurants, etc, to reduce the amount of SARS-CoV-2 in the air. These technologies include devices and products like UV-C devices, chemical-based devices, and physical removal devices like filters.
- Read more about EPA's research related to SARS-CoV-2 aerosols.
Research on COVID-19 on Surfaces
Respiratory droplets, which are larger, and fall quickly, can still be a mode of transmission for SARS-CoV-2, the virus that causes Covid-19. First, researchers had to develop a way to quickly detect live virus in surface samples because current testing takes a few days to get results, which would be too late to worry about surface contamination. Researchers developed a rapid analytical method so that they could understand potential surface transmission in a short time.
Areas that are frequently touched by many different people can pose a public health risk during the pandemic, so researchers evaluated commercially available antimicrobial products for potential long-lasting effectiveness against the virus. Currently, EPA-registered products with long-lasting effectiveness claims are limited to those that control odor-causing bacteria on hard, non-porous surfaces; there are no EPA registered products with public health claims that provide long-lasting (e.g., weeks to months) disinfection. The benefits of having a longer-lasting antimicrobial product are important, especially when cleaning and disinfecting a surface or object cannot be accomplished every time someone new touches it.
In addition, ways to apply disinfectants to many different, large surface areas quickly and effectively were needed to reduce the risk of exposure to the virus. Researchers evaluated electrostatic sprayers and foggers to rapidly apply disinfectants over large, complex surface areas.
At the request of some of the countries largest transit agencies, EPA researchers also studied whether UV-C could inactivate the SARS-CoV-2 virus on subways and buses. This results from this research are useful for business owners, school administrators, and others.
Overall, this research will help decision makers determine the best ways to help reduce the risk of exposure to COVID-19 from potential surface transmission.
- Learn more about EPA's research related to COVID-19 and surfaces.
- Review information from CDC about Cleaning and Disinfecting Your Home, workplace and other Facilities.
Determining Community Exposure
With an infectious disease like COVID-19, people may be contagious before they show any symptoms. Some may never show symptoms. To determine the true rate of community infection and to provide information to help public health departments around the country to make the best decisions on directing resources, EPA researchers have developed several ways to monitor exposure.
EPA researchers are analyzing wastewater samples from communities in southwestern Ohio using a molecular approach to look for the genetic marker of SARS-CoV-2. This approach acts as an early warning system to alert public health officials about increasing infection in a community. It can also serve to let the officials know when cases are dropping.
Researchers are also working on a standardized method that could quantify the level of live, or infectious, SARS-CoV-2 detected in raw sewage at wastewater treatment plants.
- Learn more about EPA's research efforts related to COVID-19 and sewage.
EPA researchers have also developed a salivary antibody test that is simple, easy-to-collect, low-cost, and noninvasive. Antibody testing helps identify people who have been exposed to SARS-CoV-2 and have developed an immune response, but who might not have ever developed symptoms. This test can help public health officials determine the rate of infection and provide insights on the true impact of the pandemic in communities across the country.
- Read a summary of EPA's research results related to the creation of SARS-CoV-2 salivary antibody test.
Research on Masks and PPE
Masks and social distancing have been important to reduce the risk of exposure to COVID-19. Researchers studied the effectiveness of different kinds of masks and facial coverings to help people decide which kind of masks to buy and wear to protect themselves and others.
- Learn more about EPA's research related to the effectiveness of masks and facial coverings against COVID-19.
Researchers also evaluated methods to disinfect used PPE and evaluated whether any of the disinfection methods causes damage to the PPE or limited its performance in reducing exposure to COVID-19. This information helps frontline workers such as healthcare staff and emergency responders when PPE are in limited supply. Proper cleaning and disinfection for PPE ensures continued protection from exposure to the disease.
- Read a summary of EPA's research efforts related to proper disinfection of PPE.
- Coronavirus Home
- Disinfectants
- Drinking Water and Wastewater
- Frequent Questions

COVID-19 (2019 Novel Coronavirus) Research Guide
COVID-19 Research Guide Home
- Research Articles Downloadable Database
- COVID-19 Science Updates
- Databases and Journals
- Secondary Data and Statistics
From the CDC’s COVID-19 (2019 Novel Coronavirus) website :
“COVID-19 (coronavirus disease 2019) is a disease caused by a virus named SARS-CoV-2. It can be very contagious and spreads quickly. Over one million people have died from COVID-19 in the United States.
COVID-19 most often causes respiratory symptoms that can feel much like a cold, the flu, or pneumonia. COVID-19 may attack more than your lungs and respiratory system. Other parts of your body may also be affected by the disease. Most people with COVID-19 have mild symptoms, but some people become severely ill.
Some people including those with minor or no symptoms will develop Post-COVID Conditions – also called “Long COVID.”
May 11, 2023, marks the end of the federal COVID-19 PHE declaration . After this date, CDC’s authorizations to collect certain types of public health data will expire.
The latest situation summary updates are available on CDC’s web page for COVID-19 . “
This guide provides resources for researching COVID-19. In this guide you can find the following:
- The CDC Database of COVID-19 Research Articles became a collaboration with the WHO to create the WHO COVID-19 database during the pandemic to make it easier for results to be searched, downloaded, and used by researchers worldwide.
- The last version of the CDC COVID-19 database was archived and remain available on this website. Please note that it has stopped updating as of October 9, 2020 and all new articles were integrated into the WHO COVID-19 database . The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
- COVID-19 Science Updates : To help inform CDC’s COVID-19 Response, as well as to help CDC staff stay up to date on the latest COVID-19 research, the Response’s Office of the Chief Medical Officer has collaborated with the CDC Office of Library Science to create a series called COVID-19 Science Update . This series, the first of its kind for a CDC emergency response, provides brief summaries of new COVID-19-related studies on many topics, including epidemiology, clinical treatment and management, laboratory science, and modeling. As of December 18, 2021, CDC has stopped production of the weekly COVID-19 Science Update.
- Selected scholarly literature databases and journals available to help you find research about COVID-19.
- Search alerts notify you when new research is published on COVID-19.
- Search alerts available for Ovid , PubMed , Scopus , and News sources .
- Selected sources for secondary data and statistics on COVID-19.
- Selected websites and organizations where you can find more information on COVID-19.
Some resources within this guide are accessible only to those with a CDC user ID and password. Find a library near you that may be able to help you access similar resources by clicking the following links: https://www.worldcat.org/libraries OR https://www.usa.gov/libraries .
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings. HHS, PHS, and CDC assume no responsibility for the factual accuracy of the items presented. The selection, omission, or content of items does not imply any endorsement or other position taken by HHS, PHS, and CDC. Opinion, findings, and conclusions expressed by the original authors of items included in these materials, or persons quoted therein, are strictly their own and are in no way meant to represent the opinion or views of HHS, PHS, or CDC. References to publications, news sources, and non-CDC Websites are provided solely for informational purposes and do not imply endorsement by HHS, PHS, or CDC.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
COVID-19 Research
Stanford Medicine scientists have launched dozens of research projects as part of the global response to COVID-19. Some aim to prevent, diagnose and treat the disease; others aim to understand how it spreads and how people’s immune systems respond to it.
Below is a curated selection, including summaries, of the projects.
To participate in research , browse COVID-19 studies . Our research registry also connects people like you with teams conducting research to make advances in health care. If you are eligible for a study, researchers may contact you to provide additional details on how to participate.
By participating in clinical research, you help accelerate medical science by providing valuable insights into potential treatments and methons of prevention.
Stanford COVID-19 Study Directory Stanford Medicine Research Registry
To improve our ability to determine who has COVID-19 and treat those infected.
Transmission
To better prevent and understand the transmission of the coronavirus.
Vaccination and Treatment
To improve our ability to prevent COVID-19 and treat those infected.
Epidemiology
To better understand how the coronavirus is spreading.
Data Science and Modeling
To better predict medical, fiscal and resource-related outcomes of the COVID-19 pandemic.
To better understand immune responses to the coronavirus.
Cardiovascular
To better understand the way the virus affects the cardiovascular system.
To better enable the workforce to achieve its goals during the COVID-19 pandemic.
Miscellaneous
A variety of other research projects related to the COVID-19 pandemic.
The list isn’t comprehensive and instead represents a portion of Stanford Medicine research on COVID-19. If you are a Stanford Medicine scientist and would like to see your research included here, please send a note to: [email protected].
The Stanford Institute for Human-Centered Artificial Intelligence has also created a webpage for COVID-19 research collaborations and other opportunities, such as research positions, internships and funding. If you would like to submit an opening please use the following form and they will post it on their website.
Support Stanford Medicine’s response to COVID-19 by making a gift .
COVID-19 Research Projects

Preparing for the next health crisis: COVID-19 showed the importance of community-engaged research
Research Associate, Health Sciences, Simon Fraser University
Assistant Professor, Faculty of Health Sciences, Simon Fraser University
Disclosure statement
Julia Smith receives funding from the Canadian Institutes of Health Research and Health Research BC.
Simran Purewal does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Simon Fraser University provides funding as a member of The Conversation CA.
Simon Fraser University provides funding as a member of The Conversation CA-FR.
View all partners
Community-engaged research involves the active and meaningful involvement of people directly affected by a research problem. Like most activities that require personal interaction, this type of research was disrupted by the restrictions of the COVID-19 pandemic.
This research approach is intended to build trustworthy relationships and yield mutual benefits. Community-engaged projects have garnered attention over the past two decades as they focus on tackling inequities , which often arise during public health crises.
Conducting community-engaged research has several benefits, like increasing the relevancy of studies , incorporating lived experience , and supporting the sharing of findings back to affected communities. Community engagement also plays an important role in emergency responses. Community-engaged emergency responses can promote the uptake of public health interventions and bolster advocacy efforts.
Community-engaged research during the pandemic
Researchers’ ability to engage with communities was impacted by the COVID-19 pandemic. Public health measures focused on preventing the spread of COVID-19 (such as limits on in-person gatherings) halted traditional forms of fieldwork. For example, researchers could not safely host in-person interviews. They were forced to quickly adapt to unfamiliar virtual teaching and learning platforms.
At the same time, civil society organizations (CSOs), non-profit agencies operating separately from government and business, were stretched thin as their demand soared. These organizations are sought after as community research partners since they are embedded in the communities they serve and provide crucial services to community members. Examples include the United Way BC and Sources Community Resource Centre , which provide direct services, support and relief to communities across B.C.
In 2023, the Pacific Institute on Pathogens, Pandemics, and Society ( PIPPS ) hosted a roundtable with community-engaged researchers at Simon Fraser University to learn about their experiences engaging with communities amid the pandemic. Findings from our roundtable, supplementing this article, have also been included in our Community-Engaged Research during Health Crises: Engaging with Civil Society Organizations handbook published by PIPPS and SFU Community Engaged Research Initiative .
Barriers to conducting community-engaged research
Roundtable attendees first discussed the challenges of conducting research remotely, with one noting how their research plans were put on pause for more than three months because of pandemic-related restrictions. Attendees also highlighted how managing multiple forms of online communication disrupted their work-life balance.
Several attendees found it difficult to recruit research participants; they discussed the challenges of the digital divide , referring to the gap between communities’ access to information and communication technologies.
Researchers faced challenges with ethics review boards, which did not consider the risks and unique considerations of engaging communities in a public health crisis. While ethics applications were expedited, researchers felt they lacked guidance for community-engaged research during the pandemic.
Researchers also found it hard to maintain relationships with communities amidst the pandemic. Some key ways researchers connect with communities are through sharing findings and hosting food-sharing events, such as lunch and learns. These opportunities were not available during the pandemic. In addition, many of their long-standing relationships with CSOs were strained as they experienced layoffs and increased demand. Researchers did not want to impede on CSOs’ frontline pandemic-related efforts.
Opportunities emerging from the pandemic

Despite the challenges they faced, researchers identified a range of opportunities that emerged as a result of the pandemic. They noted how they could increase the scope of their projects since virtual tools, like Zoom, allowed them to reach rural and remote communities. These platforms also provided low-barrier forms of participation for participants with accommodation needs.
Researchers also discussed how the pandemic forced the “professional veneers to slip away.” Over time, researchers connected on a more vulnerable level with their community partners, as they all attempted to get through the pandemic. Collectively, they showed up in their most authentic way and practised humility in their partnerships. Moreover, research teams emphasized the importance of building community, which reinforced their commitment to mutual benefit .
Through their community-engaged work, some researchers hired people with lived experience of the research problem of interest. This opportunity emerged during the pandemic. Compensating members of the research team for their knowledge strengthened the relevancy of their findings as they directly learned how the pandemic was impacting distinct groups.
Lessons learned: Conducting community-engaged research in future crises
Participants were asked what they would do differently in future health crises. Some discussed the significance of holding informal check-ins with their teams to openly discuss professional and personal challenges. Others pointed to the need for knowledge and resource sharing with other community-engaged researchers, to break down silos.
Additionally, attendees underscored the benefits of interdisciplinary research teams , bringing together diverse skills and expertise. In health crises, they aim to work collaboratively with academics and service providers from CSOs.

Based on the key themes of the roundtable, three recommendations emerged to support community-engaged research in future public health crises:
1) Post-secondary institutions should develop guidance for community-engaged research in health emergencies
Since post-secondary institutions increasingly recognize the importance of community-university partnerships , institutions should create protocols to support community-engaged research in public health crises. Attention should be paid to crisis-related considerations, including funding sources, resource challenges and ethics.
2) Develop targeted funding opportunities for community-engaged research partnerships
During the pandemic, research unrelated to COVID-19 faced funding drawbacks and resource constraints . This may have secondary effects in the “ post-pandemic era ,” especially for projects addressing health inequities. To avoid these unintended consequences, partnerships should be proactively supported by post-secondary institutions and funding agencies, to provide research partners with honoraria for their time and insights shared, and to help academic researchers build networks for engagement.
3) Prioritize capacity-building in partnerships
Mutual benefit is a guiding principle of community-engaged research . In partnerships, academic researchers often benefit through career advancement and a sense of fulfillment . Yet, community partners are not always assured the same benefits. To ensure mutual benefit, capacity building , referring to the process of building skills, abilities and resources, should guide community-engaged research partnerships. Both academic researchers and community partners bring significant assets to projects; these assets and training gaps should be uncovered at the outset of projects and considered throughout the partnership.
In addition to these recommendations, a repeated theme for participants was how community-engaged research should be viewed as a fundamental component of their work, as opposed to an afterthought. Rather than treating community-engaged research as a “ peripheral activity ,” the principles of community engagement should be embedded in research, teaching and learning.
- Public health
- Online communication
- Community engagement
- Public health crisis
- Civil society organizations
- Pandemic restrictions

Senior Research Development Coordinator

Audience Development Coordinator (fixed-term maternity cover)

Lecturer (Hindi-Urdu)


Director, Defence and Security

Opportunities with the new CIEHF
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Coronavirus disease 2019 (COVID-19): A literature review
Harapan harapan.
a Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
b Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
c Department of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
d Division of Infectious Diseases, AichiCancer Center Hospital, Chikusa-ku Nagoya, Japan
Amanda Yufika
e Department of Family Medicine, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
Wira Winardi
f Department of Pulmonology and Respiratory Medicine, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
g School of Medicine, The University of Western Australia, Perth, Australia
Haypheng Te
h Siem Reap Provincial Health Department, Ministry of Health, Siem Reap, Cambodia
Dewi Megawati
i Department of Microbiology and Parasitology, Faculty of Medicine and Health Sciences, Warmadewa University, Denpasar, Indonesia
j Department of Medical Microbiology and Immunology, University of California, Davis, CA, USA
Zinatul Hayati
k Department of Clinical Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
Abram L. Wagner
l Department of Epidemiology, University of Michigan, Ann Arbor, Michigan, MI 48109, USA
Mudatsir Mudatsir
In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern. As of February 14, 2020, 49,053 laboratory-confirmed and 1,381 deaths have been reported globally. Perceived risk of acquiring disease has led many governments to institute a variety of control measures. We conducted a literature review of publicly available information to summarize knowledge about the pathogen and the current epidemic. In this literature review, the causative agent, pathogenesis and immune responses, epidemiology, diagnosis, treatment and management of the disease, control and preventions strategies are all reviewed.
On December 31, 2019, the China Health Authority alerted the World Health Organization (WHO) to several cases of pneumonia of unknown aetiology in Wuhan City in Hubei Province in central China. The cases had been reported since December 8, 2019, and many patients worked at or lived around the local Huanan Seafood Wholesale Market although other early cases had no exposure to this market [1] . On January 7, a novel coronavirus, originally abbreviated as 2019-nCoV by WHO, was identified from the throat swab sample of a patient [2] . This pathogen was later renamed as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the Coronavirus Study Group [3] and the disease was named coronavirus disease 2019 (COVID-19) by the WHO. As of January 30, 7736 confirmed and 12,167 suspected cases had been reported in China and 82 confirmed cases had been detected in 18 other countries [4] . In the same day, WHO declared the SARS-CoV-2 outbreak as a Public Health Emergency of International Concern (PHEIC) [4] .
According to the National Health Commission of China, the mortality rate among confirmed cased in China was 2.1% as of February 4 [5] and the mortality rate was 0.2% among cases outside China [6] . Among patients admitted to hospitals, the mortality rate ranged between 11% and 15% [7] , [8] . COVID-19 is moderately infectious with a relatively high mortality rate, but the information available in public reports and published literature is rapidly increasing. The aim of this review is to summarize the current understanding of COVID-19 including causative agent, pathogenesis of the disease, diagnosis and treatment of the cases, as well as control and prevention strategies.
The virus: classification and origin
SARS-CoV-2 is a member of the family Coronaviridae and order Nidovirales. The family consists of two subfamilies, Coronavirinae and Torovirinae and members of the subfamily Coronavirinae are subdivided into four genera: (a) Alphacoronavirus contains the human coronavirus (HCoV)-229E and HCoV-NL63; (b) Betacoronavirus includes HCoV-OC43, Severe Acute Respiratory Syndrome human coronavirus (SARS-HCoV), HCoV-HKU1, and Middle Eastern respiratory syndrome coronavirus (MERS-CoV); (c) Gammacoronavirus includes viruses of whales and birds and; (d) Deltacoronavirus includes viruses isolated from pigs and birds [9] . SARS-CoV-2 belongs to Betacoronavirus together with two highly pathogenic viruses, SARS-CoV and MERS-CoV. SARS-CoV-2 is an enveloped and positive-sense single-stranded RNA (+ssRNA) virus [16] .
SARS-CoV-2 is considered a novel human-infecting Betacoronavirus [10] . Phylogenetic analysis of the SARS-CoV-2 genome indicates that the virus is closely related (with 88% identity) to two bat-derived SARS-like coronaviruses collected in 2018 in eastern China (bat-SL-CoVZC45 and bat-SL-CoVZXC21) and genetically distinct from SARS-CoV (with about 79% similarity) and MERS-CoV [10] . Using the genome sequences of SARS-CoV-2, RaTG13, and SARS-CoV [11] , a further study found that the virus is more related to BatCoV RaTG13, a bat coronavirus that was previously detected in Rhinolophus affinis from Yunnan Province, with 96.2% overall genome sequence identity [11] . A study found that no evidence of recombination events detected in the genome of SARS-CoV-2 from other viruses originating from bats such as BatCoV RaTG13, SARS-CoV and SARSr-CoVs [11] . Altogether, these findings suggest that bats might be the original host of this virus [10] , [11] .
However, a study is needed to elucidate whether any intermediate hosts have facilitated the transmission of the virus to humans. Bats are unlikely to be the animal that is directly responsible for transmission of the virus to humans for several reasons [10] : (1) there were various non-aquatic animals (including mammals) available for purchase in Huanan Seafood Wholesale Market but no bats were sold or found; (2) SARS-CoV-2 and its close relatives, bat-SL-CoVZC45 and bat-SL-CoVZXC21, have a relatively long branch (sequence identity of less than 90%), suggesting those viruses are not direct ancestors of SARS-CoV-2; and (3) in other coronaviruses where bat is the natural reservoir such as SARS-CoV and MERS-CoV, other animals have acted as the intermediate host (civets and possibly camels, respectively). Nevertheless, bats do not always need an intermediary host to transmit viruses to humans. For example, Nipah virus in Bangladesh is transmitted through bats shedding into raw date palm sap [12] .
Transmission
The role of the Huanan Seafood Wholesale Market in propagating disease is unclear. Many initial COVID-19 cases were linked to this market suggesting that SARS-CoV-2 was transmitted from animals to humans [13] . However, a genomic study has provided evidence that the virus was introduced from another, yet unknown location, into the market where it spread more rapidly, although human-to-human transmission may have occurred earlier [14] . Clusters of infected family members and medical workers have confirmed the presence of person-to-person transmission [15] . After January 1, less than 10% of patients had market exposure and more than 70% patients had no exposure to the market [13] . Person-to-person transmission is thought to occur among close contacts mainly via respiratory droplets produced when an infected person coughs or sneezes. Fomites may be a large source of transmission, as SARS-CoV has been found to persist on surfaces up to 96 h [16] and other coronaviruses for up to 9 days [17] .
Whether or not there is asymptomatic transmission of disease is controversial. One initial study published on January 30 reported asymptomatic transmission [18] , but later it was found that the researchers had not directly interviewed the patient, who did in fact have symptoms prior to transmitting disease [19] . A more recent study published on February 21 also purported asymptomatic transmission [20] , but any such study could be limited by errors in self-reported symptoms or contact with other cases and fomites.
Findings about disease characteristics are rapidly changing and subject to selection bias. A study indicated the mean incubation period was 5.2 days (95% confidence interval [95%CI]: 4.1–7.0) [13] . The incubation period has been found to be as long as 19 or 24 days [21] , [22] , although case definitions typically rely on a 14 day window [23] .
The basic reproductive number ( R 0 ) has been estimated with varying results and interpretations. R 0 measures the average number of infections that could result from one infected individual in a fully susceptible population [24] . Studies from previous outbreaks found R 0 to be 2.7 for SARS [25] and 2.4 for 2009 pandemic H1N1 influenza [26] . One study estimated that that basic reproductive number ( R 0 ) was 2.2 (95% CI: 1.4–3.9) [13] . However, later in a further analysis of 12 available studies found that R 0 was 3.28 [27] . Because R 0 represents an average value it is also important to consider the role of super spreaders, who may be hugely responsible for outbreaks within large clusters but who would not largely influence the value of R 0 [28] . During the acute phase of an outbreak or prepandemic, R 0 may be unstable [24] .
In pregnancy, a study of nine pregnancy women who developed COVID-19 in late pregnancy suggested COVID-19 did not lead to substantially worse symptoms than in nonpregnant persons and there is no evidence for intrauterine infection caused by vertical transmission [29] .
In hospital setting, a study involving 138 COVID-19 suggested that hospital-associated transmission of SARS-CoV-2 occurred in 41% of patients [30] . Moreover, another study on 425 patients found that the proportion of cases in health care workers gradually increased by time [13] . These cases likely reflect exposure to a higher concentration of virus from sustained contact in close quarters.
Outside China, as of February 12, 2020, there were 441 confirmed COVID-19 cases reported in 24 countries [6] of which the first imported case was reported in Thailand on January 13, 2020 [6] , [31] . Among those countries, 11 countries have reported local transmission with the highest number of cases reported in Singapore with 47 confirmed cases [6] .
Risk factors
The incidence of SARS-CoV-2 infection is seen most often in adult male patients with the median age of the patients was between 34 and 59 years [20] , [30] , [7] , [32] . SARS-CoV-2 is also more likely to infect people with chronic comorbidities such as cardiovascular and cerebrovascular diseases and diabetes [8] . The highest proportion of severe cases occurs in adults ≥60 years of age, and in those with certain underlying conditions, such as cardiovascular and cerebrovascular diseases and diabetes [20] , [30] . Severe manifestations maybe also associated with coinfections of bacteria and fungi [8] .
Fewer COVID-19 cases have been reported in children less than 15 years [20] , [30] , [7] , [32] . In a study of 425 COVID-19 patients in Wuhan, published on January 29, there were no cases in children under 15 years of age [13] , [33] . Nevertheless, 28 paediatric patients have been reported by January 2020 [34] . The clinical features of infected paediatric patients vary, but most have had mild symptoms with no fever or pneumonia, and have a good prognosis [34] . Another study found that although a child had radiological ground-glass lung opacities, the patient was asymptomatic [35] . In summary, children might be less likely to be infected or, if infected, present milder manifestations than adults; therefore, it is possible that their parents will not seek out treatment leading to underestimates of COVID-19 incidence in this age group.
Pathogenesis and immune response
Like most other members of the coronavirus family, Betacoronavirus exhibit high species specificity, but subtle genetic changes can significantly alter their tissue tropism, host range, and pathogenicity. A striking example of the adaptability of these viruses is the emergence of deadly zoonotic diseases in human history caused by SARS-CoV [36] and MERS-CoV [37] . In both viruses, bats served as the natural reservoir and humans were the terminal host, with the palm civet and dromedary camel the intermediary host for SARS-CoV and MERS-CoV, respectively [38] , [39] . Intermediate hosts clearly play a critical role in cross species transmission as they can facilitate increased contact between a virus and a new host and enable further adaptation necessary for an effective replication in the new host [40] . Because of the pandemic potential of SARS-CoV-2, careful surveillance is immensely important to monitor its future host adaptation, viral evolution, infectivity, transmissibility, and pathogenicity.
The host range of a virus is governed by multiple molecular interactions, including receptor interaction. The envelope spike (S) protein receptor binding domain of SARS-CoV-2 was shown structurally similar to that of SARS-CoV, despite amino acid variation at some key residues [10] . Further extensive structural analysis strongly suggests that SARS-CoV-2 may use host receptor angiotensin-converting enzyme 2 (ACE2) to enter the cells [41] , the same receptor facilitating SARS-CoV to infect the airway epithelium and alveolar type 2 (AT2) pneumocytes, pulmonary cells that synthesize pulmonary surfactant [42] . In general, the spike protein of coronavirus is divided into the S1 and S2 domain, in which S1 is responsible for receptor binding and S2 domain is responsible for cell membrane fusion [10] . The S1 domain of SARS-CoV and SARS-CoV-2 share around 50 conserved amino acids, whereas most of the bat-derived viruses showed more variation [10] . In addition, identification of several key residues (Gln493 and Asn501) that govern the binding of SARS-CoV-2 receptor binding domain with ACE2 further support that SARS-CoV-2 has acquired capacity for person-to-person transmission [41] . Although, the spike protein sequence of receptor binding SARS-CoV-2 is more similar to that of SARS-CoV, at the whole genome level SARS-CoV-2 is more closely related to bat-SL-CoVZC45 and bat-SL-CoVZXC21 [10] .
However, receptor recognition is not the only determinant of species specificity. Immediately after binding to their receptive receptor, SARS-CoV-2 enters host cells where they encounter the innate immune response. In order to productively infect the new host, SARS-CoV-2 must be able to inhibit or evade host innate immune signalling. However, it is largely unknown how SARS-CoV-2 manages to evade immune response and drive pathogenesis. Given that COVID-19 and SARS have similar clinical features [7] , SARS-CoV-2 may have a similar pathogenesis mechanism as SARS-CoV. In response to SARS-CoV infections, the type I interferon (IFN) system induces the expression of IFN-stimulated genes (ISGs) to inhibit viral replication. To overcome this antiviral activity, SARS-CoV encodes at least 8 viral antagonists that modulate induction of IFN and cytokines and evade ISG effector function [43] .
The host immune system response to viral infection by mediating inflammation and cellular antiviral activity is critical to inhibit viral replication and dissemination. However, excessive immune responses together with lytic effects of the virus on host cells will result in pathogenesis. Studies have shown patients suffering from severe pneumonia, with fever and dry cough as common symptoms at onset of illness [7] , [8] . Some patients progressed rapidly with Acute Respiratory Stress Syndrome (ARDS) and septic shock, which was eventually followed by multiple organ failure and about 10% of patients have died [8] . ARDS progression and extensive lung damage in COVID-19 are further indications that ACE2 might be a route of entry for the SARS-CoV-2 as ACE2 is known abundantly present on ciliated cells of the airway epithelium and alveolar type II (cells (pulmonary cells that synthesize pulmonary surfactant) in humans [44] .
Patients with SARS and COVID-19 have similar patterns of inflammatory damage. In serum from patients diagnosed with SARS, there is increased levels of proinflammatory cytokines (e.g. interleukin (IL)-1, IL6, IL12, interferon gamma (IFNγ), IFN-γ-induced protein 10 (IP10), macrophage inflammatory proteins 1A (MIP1A) and monocyte chemoattractant protein-1 (MCP1)), which are associated with pulmonary inflammation and severe lung damage [45] . Likewise, patients infected with SARS-CoV-2 are reported to have higher plasma levels of proinflammatory cytokines including IL1β, IL-2, IL7, TNF-α, GSCF, MCP1 than healthy adults [7] . Importantly, patients in the intensive care unit (ICU) have a significantly higher level of GSCF, IP10, MCP1, and TNF-α than those non-ICU patients, suggesting that a cytokine storm might be an underlying cause of disease severity [7] . Unexpectedly, anti-inflammatory cytokines such as IL10 and IL4 were also increased in those patients [7] , which was uncommon phenomenon for an acute phase viral infection. Another interesting finding, as explained before, was that SARS-CoV-2 has shown to preferentially infect older adult males with rare cases reported in children [7] , [8] . The same trend was observed in primate models of SARS-CoV where the virus was found more likely to infect aged Cynomolgus macaque than young adults [46] . Further studies are necessary to identify the virulence factors and the host genes of SARS-CoV-2 that allows the virus to cross the species-specific barrier and cause lethal disease in humans.
Clinical manifestations
Clinical manifestations of 2019-nCoV infection have similarities with SARS-CoV where the most common symptoms include fever, dry cough, dyspnoea, chest pain, fatigue and myalgia [7] , [30] , [47] . Less common symptoms include headache, dizziness, abdominal pain, diarrhoea, nausea, and vomiting [7] , [30] . Based on the report of the first 425 confirmed cases in Wuhan, the common symptoms include fever, dry cough, myalgia and fatigue with less common are sputum production, headache, haemoptysis, abdominal pain, and diarrhoea [13] . Approximately 75% patients had bilateral pneumonia [8] . Different from SARS-CoV and MERS-CoV infections, however, is that very few COVID-19 patients show prominent upper respiratory tract signs and symptoms such as rhinorrhoea, sneezing, or sore throat, suggesting that the virus might have greater preference for infecting the lower respiratory tract [7] . Pregnant and non-pregnant women have similar characteristics [48] . The common clinical presentation of 2019-nCoV infection are presented in Table 1 .
Clinical symptoms of patients with 2019-nCoV infection.
Severe complications such as hypoxaemia, acute ARDS, arrythmia, shock, acute cardiac injury, and acute kidney injury have been reported among COVID-19 patients [7] , [8] . A study among 99 patients found that approximately 17% patients developed ARDS and, among them, 11% died of multiple organ failure [8] . The median duration from first symptoms to ARDS was 8 days [30] .
Efforts to control spread of COVID-19, institute quarantine and isolation measures, and appropriately clinically manage patients all require useful screening and diagnostic tools. While SARS-CoV-2 is spreading, other respiratory infections may be more common in a local community. The WHO has released a guideline on case surveillance of COVID-19 on January 31, 2020 [23] . For a person who meets certain criteria, WHO recommends to first screen for more common causes of respiratory illness given the season and location. If a negative result is found, the sample should be sent to referral laboratory for SARS-CoV-2 detection.
Case definitions can vary by country and will evolve over time as the epidemiological circumstances change in a given location. In China, a confirmed case from January 15, 2020 required an epidemiological linkage to Wuhan within 2 weeks and clinical features such as fever, pneumonia, and low white blood cell count. On January 18, 2020 the epidemiological criterion was expanded to include contact with anyone who had been in Wuhan in the past 2 weeks [50] . Later, the case definitions removed the epidemiological linkage.
The WHO has put forward case definitions [23] . Suspected cases of COVID-19 are persons (a) with severe acute respiratory infections (history of fever and cough requiring admission to hospital) and with no other aetiology that fully explains the clinical presentation and a history of travel to or residence in China during the 14 days prior to symptom onset; or (b) a patient with any acute respiratory illness and at least one of the following during the 14 days prior to symptom onset: contact with a confirmed or probable case of SARS-CoV-2 infection or worked in or attended a health care facility where patients with confirmed or probable SARS-CoV-2 acute respiratory disease patients were being treated. Probable cases are those for whom testing for SARS-CoV-2 is inconclusive or who test positive using a pan-coronavirus assay and without laboratory evidence of other respiratory pathogens. A confirmed case is one with a laboratory confirmation of SARS-CoV-2 infection, irrespective of clinical signs and symptoms.
For patients who meet diagnostic criteria for SARS-CoV-2 testing, the CDC recommends collection of specimens from the upper respiratory tract (nasopharyngeal and oropharyngeal swab) and, if possible, the lower respiratory tract (sputum, tracheal aspirate, or bronchoalveolar lavage) [51] . In each country, the tests are performed by laboratories designated by the government.
Laboratory findings
Among COVID-19 patients, common laboratory abnormalities include lymphopenia [8] , [20] , [30] , prolonged prothrombin time, and elevated lactate dehydrogenase [30] . ICU-admitted patients had more laboratory abnormalities compared with non-ICU patients [30] , [7] . Some patients had elevated aspartate aminotransferase, creatine kinase, creatinine, and C-reactive protein [20] , [7] , [35] . Most patients have shown normal serum procalcitonin levels [20] , [30] , [7] .
COVID-19 patients have high level of IL1β, IFN-γ, IP10, and MCP1 [7] . ICU-admitted patients tend to have higher concentration of granulocyte-colony stimulating factor (GCSF), IP10, MCP1A, MIP1A, and TNF-α [7] .
Radiology findings
Radiology finding may vary with patients age, disease progression, immunity status, comorbidity, and initial medical intervention [52] . In a study describing 41 of the initial cases of 2019-nCoV infection, all 41 patients had pneumonia with abnormal findings on chest computed tomography (CT-scan) [7] . Abnormalities on chest CT-scan were also seen in another study of 6 cases, in which all of them showed multifocal patchy ground-glass opacities notably nearby the peripheral sections of the lungs [35] . Data from studies indicate that the typical of chest CT-scan findings are bilateral pulmonary parenchymal ground-glass and consolidative pulmonary opacities [7] , [8] , [20] , [30] , [32] , [53] . The consolidated lung lesions among patients five or more days from disease onset and those 50 years old or older compared to 4 or fewer days and those 50 years or younger, respectively [47] .
As the disease course continue, mild to moderate progression of disease were noted in some cases which manifested by extension and increasing density of lung opacities [49] . Bilateral multiple lobular and subsegmental areas of consolidation are typical findings on chest CT-scan of ICU-admitted patients [7] . A study among 99 patients, one patient had pneumothorax in an imaging examination [8] .
Similar to MERS-CoV and SARS-CoV, there is still no specific antiviral treatment for COVID-19 [54] . Isolation and supportive care including oxygen therapy, fluid management, and antibiotics treatment for secondary bacterial infections is recommended [55] . Some COVID-19 patients progressed rapidly to ARDS and septic shock, which was eventually followed by multiple organ failure [7] , [8] . Therefore, the effort on initial management of COVID-19 must be addressed to the early recognition of the suspect and contain the disease spread by immediate isolation and infection control measures [56] .
Currently, no vaccination is available, but even if one was available, uptake might be suboptimal. A study of intention to vaccinate during the H1N1 pandemic in the United States was around 50% at the start of the pandemic in May 2009 but had decreased to 16% by January 2010 [57] .
Neither is a treatment available. Therefore, the management of the disease has been mostly supportive referring to the disease severity which has been introduced by WHO. If sepsis is identified, empiric antibiotic should be administered based on clinical diagnosis and local epidemiology and susceptibility information. Routine glucocorticoids administration are not recommended to use unless there are another indication [58] . Clinical evidence also does not support corticosteroid treatment [59] . Use of intravenous immunoglobulin might help for severely ill patients [8] .
Drugs are being evaluated in line with past investigations into therapeutic treatments for SARS and MERS [60] . Overall, there is not robust evidence that these antivirals can significantly improve clinical outcomes A. Antiviral drugs such as oseltamivir combined with empirical antibiotic treatment have also been used to treat COVID-19 patients [7] . Remdesivir which was developed for Ebola virus, has been used to treat imported COVID-19 cases in US [61] . A brief report of treatment combination of Lopinavir/Ritonavir, Arbidol, and Shufeng Jiedu Capsule (SFJDC), a traditional Chinese medicine, showed a clinical benefit to three of four COVID-19 patients [62] . There is an ongoing clinical trial evaluating the safety and efficacy of lopinavir-ritonavir and interferon-α 2b in patients with COVID-19 [55] . Ramsedivir, a broad spectrum antivirus has demonstrated in vitro and in vivo efficacy against SARS-CoV-2 and has also initiated its clinical trial [63] , [64] . In addition, other potential drugs from existing antiviral agent have also been proposed [65] , [66] .
Control and prevention strategies
COVID-19 is clearly a serious disease of international concern. By some estimates it has a higher reproductive number than SARS [27] , and more people have been reported to have been infected or died from it than SARS [67] . Similar to SARS-CoV and MERS-CoV, disrupting the chain of transmission is considered key to stopping the spread of disease [68] . Different strategies should be implemented in health care settings and at the local and global levels.
Health care settings can unfortunately be an important source of viral transmission. As shown in the model for SARS, applying triage, following correct infection control measures, isolating the cases and contact tracing are key to limit the further spreading of the virus in clinics and hospitals [68] . Suspected cases presenting at healthcare facilities with symptoms of respiratory infections (e.g. runny nose, fever and cough) must wear a face mask to contain the virus and strictly adhere triage procedure. They should not be permitted to wait with other patients seeking medical care at the facilities. They should be placed in a separated, fully ventilated room and approximately 2 m away from other patients with convenient access to respiratory hygiene supplies [69] . In addition, if a confirmed COVID-19 case require hospitalization, they must be placed in a single patient room with negative air pressure – a minimum of six air changes per hour. Exhausted air has to be filtered through high efficiency particulate air (HEPA) and medical personnel entering the room should wear personal protective equipment (PPE) such as gloves, gown, disposable N95, and eye protection. Once the cases are recovered and discharged, the room should be decontaminated or disinfected and personnel entering the room need to wear PPE particularly facemask, gown, eye protection [69] .
In a community setting, isolating infected people are the primary measure to interrupt the transmission. For example, immediate actions taken by Chinese health authorities included isolating the infected people and quarantining of suspected people and their close contacts [70] . Also, as there are still conflicting assumptions regarding the animal origins of the virus (i.e. some studies linked the virus to bat [71] , [72] while others associated the virus with snake [73] ), contacts with these animal fluids or tissues or consumption of wild caught animal meet should be avoided. Moreover, educating the public to recognize unusual symptoms such as chronic cough or shortness of breath is essential therefore that they could seek medical care for early detection of the virus. If large-scale community transmission occurs, mitigating social gatherings, temporary school closure, home isolation, close monitoring of symptomatic individual, provision of life supports (e.g. oxygen supply, mechanical ventilator), personal hand hygiene, and wearing personal protective equipment such as facemask should also be enforced [74] .
In global setting, locking down Wuhan city was one of the immediate measure taken by Chinese authorities and hence had slowed the global spread of COVID-19 [74] . Air travel should be limited for the cases unless severe medical attentions are required. Setting up temperature check or scanning is mandatory at airport and border to identify the suspected cases. Continued research into the virus is critical to trace the source of the outbreak and provide evidence for future outbreak [74] .
Conclusions
The current COVID-19 pandemic is clearly an international public health problem. There have been rapid advances in what we know about the pathogen, how it infects cells and causes disease, and clinical characteristics of disease. Due to rapid transmission, countries around the world should increase attention into disease surveillance systems and scale up country readiness and response operations including establishing rapid response teams and improving the capacity of the national laboratory system.
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not required.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 9, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
written by researcher(s)
Preparing for the next health crisis: COVID-19 showed the importance of community-engaged research
by Simran Purewal and Julia Smith, The Conversation

Community-engaged research involves the active and meaningful involvement of people directly affected by a research problem. Like most activities that require personal interaction, this type of research was disrupted by the restrictions of the COVID-19 pandemic.
This research approach is intended to build trustworthy relationships and yield mutual benefits. Community-engaged projects have garnered attention over the past two decades as they focus on tackling inequities , which often arise during public health crises.
Conducting community-engaged research has several benefits, like increasing the relevancy of studies , incorporating lived experience , and supporting the sharing of findings back to affected communities. Community engagement also plays an important role in emergency responses. Community-engaged emergency responses can promote the uptake of public health interventions and bolster advocacy efforts.
Community-engaged research during the pandemic
Researchers' ability to engage with communities was impacted by the COVID-19 pandemic. Public health measures focused on preventing the spread of COVID-19 (such as limits on in-person gatherings) halted traditional forms of fieldwork. For example, researchers could not safely host in-person interviews. They were forced to quickly adapt to unfamiliar virtual teaching and learning platforms.
At the same time, civil society organizations (CSOs), non-profit agencies operating separately from government and business, were stretched thin as their demand soared. These organizations are sought after as community research partners since they are embedded in the communities they serve and provide crucial services to community members. Examples include the United Way BC and Sources Community Resource Center , which provide direct services, support and relief to communities across B.C.
In 2023, the Pacific Institute on Pathogens, Pandemics, and Society ( PIPPS ) hosted a roundtable with community-engaged researchers at Simon Fraser University to learn about their experiences engaging with communities amid the pandemic. Findings from our roundtable, supplementing this article, have also been included in our Community-Engaged Research during Health Crises: Engaging with Civil Society Organizations handbook published by PIPPS and SFU Community Engaged Research Initiative .
Barriers to conducting community-engaged research
Roundtable attendees first discussed the challenges of conducting research remotely, with one noting how their research plans were put on pause for more than three months because of pandemic-related restrictions. Attendees also highlighted how managing multiple forms of online communication disrupted their work-life balance.
Several attendees found it difficult to recruit research participants; they discussed the challenges of the digital divide , referring to the gap between communities' access to information and communication technologies.
Researchers faced challenges with ethics review boards, which did not consider the risks and unique considerations of engaging communities in a public health crisis. While ethics applications were expedited, researchers felt they lacked guidance for community-engaged research during the pandemic.
Researchers also found it hard to maintain relationships with communities amidst the pandemic. Some key ways researchers connect with communities are through sharing findings and hosting food-sharing events, such as lunch and learns. These opportunities were not available during the pandemic. In addition, many of their long-standing relationships with CSOs were strained as they experienced layoffs and increased demand. Researchers did not want to impede on CSOs' frontline pandemic-related efforts.
Opportunities emerging from the pandemic
Despite the challenges they faced, researchers identified a range of opportunities that emerged as a result of the pandemic. They noted how they could increase the scope of their projects since virtual tools, like Zoom, allowed them to reach rural and remote communities. These platforms also provided low-barrier forms of participation for participants with accommodation needs.
Researchers also discussed how the pandemic forced the "professional veneers to slip away." Over time, researchers connected on a more vulnerable level with their community partners, as they all attempted to get through the pandemic. Collectively, they showed up in their most authentic way and practiced humility in their partnerships. Moreover, research teams emphasized the importance of building community, which reinforced their commitment to mutual benefit .
Through their community-engaged work, some researchers hired people with lived experience of the research problem of interest. This opportunity emerged during the pandemic. Compensating members of the research team for their knowledge strengthened the relevancy of their findings as they directly learned how the pandemic was impacting distinct groups.
Lessons learned: Conducting community-engaged research in future crises
Participants were asked what they would do differently in future health crises. Some discussed the significance of holding informal check-ins with their teams to openly discuss professional and personal challenges. Others pointed to the need for knowledge and resource sharing with other community-engaged researchers, to break down silos.
Additionally, attendees underscored the benefits of interdisciplinary research teams , bringing together diverse skills and expertise. In health crises, they aim to work collaboratively with academics and service providers from CSOs.
Based on the key themes of the roundtable, three recommendations emerged to support community-engaged research in future public health crises:
1. Post-secondary institutions should develop guidance for community-engaged research in health emergencies
Since post-secondary institutions increasingly recognize the importance of community-university partnerships , institutions should create protocols to support community-engaged research in public health crises . Attention should be paid to crisis-related considerations, including funding sources, resource challenges and ethics.
2. Develop targeted funding opportunities for community-engaged research partnerships
During the pandemic, research unrelated to COVID-19 faced funding drawbacks and resource constraints . This may have secondary effects in the " post-pandemic era ," especially for projects addressing health inequities. To avoid these unintended consequences, partnerships should be proactively supported by post-secondary institutions and funding agencies, to provide research partners with honoraria for their time and insights shared, and to help academic researchers build networks for engagement.
3. Prioritize capacity-building in partnerships
Mutual benefit is a guiding principle of community-engaged research . In partnerships, academic researchers often benefit through career advancement and a sense of fulfillment . Yet, community partners are not always assured the same benefits. To ensure mutual benefit, capacity building , referring to the process of building skills, abilities and resources, should guide community-engaged research partnerships. Both academic researchers and community partners bring significant assets to projects; these assets and training gaps should be uncovered at the outset of projects and considered throughout the partnership.
In addition to these recommendations, a repeated theme for participants was how community-engaged research should be viewed as a fundamental component of their work, as opposed to an afterthought. Rather than treating community-engaged research as a " peripheral activity ," the principles of community engagement should be embedded in research, teaching and learning.
Explore further
Feedback to editors

A new screening protocol can detect aggressive prostate cancers more selectively
1 minute ago
How a new drug prototype regenerates lung tissue
19 minutes ago

Why some people with rheumatoid arthritis have pain without inflammation

Researchers show chemical found naturally in cannabis may reduce anxiety-inducing effects of THC
'Virtual biopsy' lets clinicians analyze skin noninvasively
2 hours ago
Research team discovers new way to generate human cartilage

Filling in genomic blanks for disease studies works better for some groups than others

Researchers find new origin of deep brain waves
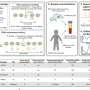
Study suggests liquid biopsy could detect and monitor aggressive small-cell lung cancer

Analysis finds mixed diets balance nutrition and reduce carbon footprints
Related stories.

Study identifies successful methods to recruit South Asian women for breast cancer research
Mar 11, 2024

Closing the clinical trials disparity gap
Feb 22, 2024

New study examines ethics of community-engaged research from the perspective of community partners
Apr 21, 2022

How to strengthen community resilience in a world plagued by crises
Jan 15, 2024
Research group releases new white paper on molecular HIV epidemiology
May 26, 2023

Building trust and saving lives: A community approach to genetic education
Jan 12, 2024
Recommended for you
Study highlights impact of aldehydes on DNA damage and aging
11 hours ago
Cockayne syndrome: New insights into cellular DNA repair mechanism
5 hours ago

Serious flu damage prevented by compound that blocks unnecessary cell death

Sepsis signature shifts speed of diagnosis
4 hours ago

Scientists flag previously overlooked type of immune cell as suspected source of severe COVID-19
6 hours ago

Researchers advance understanding of Parkinson's disease
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Fight coronavirus (COVID-19) transmission at home
Used properly, most common household disinfectants will kill the virus that causes COVID-19.
Getting your COVID-19 vaccine is the best way to lower your risk of serious illness. Getting the vaccine also prevents the spread of the COVID-19 virus.
In addition to the vaccine there are other actions you can take to keep this coronavirus from spreading in your home. Wash your hands well and often to lower your risk of infection. Improving air flow in your home and filtering the air can help. And for the coronavirus and other germs, clean those high-touch surfaces in your home and disinfect them when needed.
What's the difference between cleaning and disinfecting?
Cleaning removes most germs and dust or dirt. Disinfecting kills most germs. If someone in your home has COVID-19, clean first, then disinfect.
How is COVID-19 spread?
The virus that causes COVID-19 spreads mainly from person to person. It can spread from people who are infected but have no symptoms. When people with COVID-19 cough, sneeze, breathe, sing or talk, the virus carried on their breath can land on the faces of people nearby. The virus spreads when other people breathe in infected droplets or when the droplets land in their eyes, noses or mouths.
The COVID-19 virus also can spread if people touch their eyes, noses or mouths after touching a surface with the virus on it. Without cleaning and disinfection, the COVID-19 virus may stay on surfaces from hours to days. But the risk of COVID-19 through contact with infected surfaces seems low.
How can I clean and disinfect my home?
You can lower the risk of spreading of the COVID-19 virus by focusing on surfaces that are touched often. Examples are tables, doorknobs, light switches, handles, counters, desks, toilets, faucets and sinks. Clean these things with soap and water or with a product made for the specific surface. Follow the instructions on the product label.
Most often, cleaning is enough to lower the risk. Clean more often if someone in your home is at higher risk of severe illness from COVID-19.
If someone who is sick with COVID-19 lives with you or has been in your home within the last 24 hours, disinfect surfaces right after cleaning them. Disinfecting helps kill germs that are left.
Disinfecting uses strong chemicals.
Read product labels before use and follow instructions carefully. Many disinfectants need to stay on surfaces for some time to work. This is called the contact time, and the label will tell you that length of time.
Put on gloves before disinfecting. Disposable gloves are best because you can throw them away when you're done. If you have only gloves that you reuse, don't use them for anything else. Wash your hands well with soap and water for 20 seconds right after cleaning and disinfecting.
Keep doors or windows open and use a fan to help increase air flow while disinfecting your home.
What disinfectants kill COVID-19?
The U.S. Environmental Protection Agency (EPA) has a list of disinfectants for use against COVID-19. Look for products with active ingredients such as ethanol, hydrogen peroxide or quaternary ammonium. In the U.S., check labels for EPA registration numbers.
Does bleach work against COVID-19?
Yes. You can make a disinfecting solution by mixing 4 teaspoons (about 20 milliliters) of household bleach and 1 quart (a bit less than 1 liter) of water.
Read and follow instructions on your bottle of bleach. It's also important to wear gloves and make sure there's good airflow in the room. Don't mix bleach with ammonia or any other cleanser because mixing can cause toxic fumes.
How can I disinfect phones and other electronics?
Cellphones are high-touch devices that can carry COVID-19 germs. Follow makers' advice for cleaning and disinfecting them and other electronics. You also may consider covering your phone or shared electronics, such as a keyboard, with a product that can be easily disinfected.
Protect yourself every day
As you touch people, surfaces and objects throughout the day, you get germs on your hands. You can infect yourself with these germs by touching your eyes, nose or mouth.
To protect yourself, wash your hands often with soap and water for at least 20 seconds. If there's no soap and water, use an alcohol-based hand sanitizer with at least 60% alcohol.
- When and how to clean and disinfect your home. Centers for Disease Control and Prevention. https://www.cdc.gov/hygiene/cleaning/cleaning-your-home.html. Accessed Feb. 28, 2024.
- McIntosh K. COVID-19: Epidemiology, virology, and prevention. https://www.uptodate.com/contents/search. Accessed Feb. 28, 2024.
- Smith BA. COVID-19: Infection prevention for persons with SARS-CoV-2 infection. https://www.uptodate.com/contents/search. Accessed Feb. 28, 2024.
- COVID-19: How to protect yourself and others. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. Accessed Feb. 28, 2024.
- Cleaning and disinfecting: Best practices during the COVID-19 pandemic. Environmental Protection Agency. https://www.epa.gov/coronavirus/cleaning-and-disinfecting-best-practices-during-covid-19-pandemic. Accessed Feb. 28, 2024.
- List N advanced search page: Disinfectants for coronavirus (COVID-19). https://www.epa.gov/coronavirus/list-n-advanced-search-page-disinfectants-coronavirus-covid-19. Accessed Feb. 28, 2024.
Products and Services
- A Book: Endemic - A Post-Pandemic Playbook
- Begin Exploring Women's Health Solutions at Mayo Clinic Store
- A Book: Future Care
- Antibiotics: Are you misusing them?
- COVID-19 and vitamin D
- Convalescent plasma therapy
- Coronavirus disease 2019 (COVID-19)
- COVID-19: How can I protect myself?
- Herd immunity and coronavirus
- COVID-19 and pets
- COVID-19 and your mental health
- COVID-19 antibody testing
- COVID-19, cold, allergies and the flu
- COVID-19 drugs: Are there any that work?
- Long-term effects of COVID-19
- COVID-19 tests
- COVID-19 in babies and children
- Coronavirus infection by race
- COVID-19 travel advice
- COVID-19 vaccine: Should I reschedule my mammogram?
- COVID-19 vaccines for kids: What you need to know
- COVID-19 vaccines
- COVID-19 variant
- COVID-19 vs. flu: Similarities and differences
- COVID-19: Who's at higher risk of serious symptoms?
- Debunking coronavirus myths
- Different COVID-19 vaccines
- Extracorporeal membrane oxygenation (ECMO)
- Fever: First aid
- Fever treatment: Quick guide to treating a fever
- Honey: An effective cough remedy?
- How do COVID-19 antibody tests differ from diagnostic tests?
- How to take your pulse
- How to measure your respiratory rate
- How to take your temperature
- How well do face masks protect against COVID-19?
- Is hydroxychloroquine a treatment for COVID-19?
- Loss of smell
- Mayo Clinic Minute: You're washing your hands all wrong
- Mayo Clinic Minute: How dirty are common surfaces?
- Multisystem inflammatory syndrome in children (MIS-C)
- Nausea and vomiting
- Pregnancy and COVID-19
- Safe outdoor activities during the COVID-19 pandemic
- Safety tips for attending school during COVID-19
- Sex and COVID-19
- Shortness of breath
- Thermometers: Understand the options
- Treating COVID-19 at home
- Unusual symptoms of coronavirus
- Vaccine guidance from Mayo Clinic
- Watery eyes
Related information
- COVID-19 vaccines: Get the facts
- How well do face masks protect against coronavirus?
- Post-COVID Recovery
News on coronavirus disease 2019 (COVID-19)
Learn the latest medical news about COVID-19 on Mayo Clinic News Network.
- Fight coronavirus COVID-19 transmission at home
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
12-year-old describes 4-year battle with long COVID
Theo Huot de Saint-Albin was just 9 when he first contracted COVID in 2020.
Theo Huot de Saint-Albin was a 9-year-old elementary school student when he first contracted COVID-19 in July 2020, near the start of the coronavirus pandemic .
Four years later, as much of the world has moved on from the pandemic and resumed normal life, Theo, now nearly a teenager, is still battling the effects of long COVID.
"What happened directly after COVID-19 was worse than my actual COVID-19," Theo, now in seventh grade, told " Good Morning America ." For me, I have chronic migraines ... it doesn't mean the migraine is terrible every day. It's very unpredictable. It goes in waves. But it's always there. It never leaves."
Over the past four years, Theo has contracted COVID-19 a total of three times, and each case has been mild. It's what happens in the weeks and months after the COVID-19 diagnosis that he says has impacted his ability to learn, go to school and play with friends.
In addition to battling chronic migraines, Theo was also diagnosed with postural orthostatic tachycardia syndrome, or POTS, a blood circulation disorder that can cause dizziness, lightheadedness and a rapid heartbeat, according to the U.S. National Institute of Neurological Disorders and Stroke .

He said he also suffers from symptoms like muscle pain, extreme fatigue and brain fog, and takes around two dozen medications and supplements each day to help manage his symptoms.
"There's no real way to tell how I'm going to feel," Theo said, noting that some days he is able to go to school for only a half-day, while other days he feels closer to his pre-COVID-19 self, and still others he can't move beyond the couch all day.
"It's especially hard because it's 'invisible,'" Theo said of his long COVID diagnosis. "Sometimes it's hard to get people to believe you as well because they can empathize with something they know is there. 'Oh, you have a broken leg, I can see that. Wow, you can't walk. That must be tough.' But, your head hurts? 'I don't see a big bulge on your head. I don't see bandages wrapped around it.'"
An 'invisible' condition impacting potentially millions of kids
While battling long COVID can seem lonely, Theo is far from alone, data shows.
An analysis published in February in the journal Pediatrics reported that as many as 5.8 million children in the United States have developed long COVID, or between 10% to 20% of children who have contracted COVID-19.
Figures from the Centers for Disease Control and Prevention show a smaller but still noteworthy estimate of 1.3% of children in the U.S. having had long COVID as of 2022.
Long COVID is diagnosed when patients still have symptoms at least four weeks after they have cleared the infection, according to the CDC . In some cases, like Theo's, symptoms can be present for months or years.
It's not clear if long COVID symptoms last a lifetime. Many people eventually recover, but scientists are still working to understand who is most affected, and why.
MORE: 4 years later, experts are just beginning to 'scratch the surface' of understanding long COVID
Symptoms vary and can include fatigue, difficulty breathing, headaches, dizziness brain fog, joint and muscle pain and continued loss of taste and smell, according to the CDC.
In kids, symptoms of long COVID can also include rashes, diarrhea, heart issues and diabetes, according to the research published in Pediatrics.
Part of the complication with long COVID is there is no single test or bloodwork to diagnose it. Instead, doctors have to rule out other conditions and rely on patients to describe and track their symptoms, which can be difficult with kids.
For Theo, it took nearly two years for him to be diagnosed with long COVID, according to his mom Meredith Eubanks.
Eubanks said she was told "no" by doctors when she would ask if her son might have long COVID, and faced misdiagnoses along the way, like Lyme disease. Both she and Theo struggled to answer when asked roughly how many doctors he had seen over the past four years.
In April 2022, Eubanks said Theo was diagnosed with long COVID by an infectious disease group at a local children's hospital in Atlanta, where the family lives. But the hospital, according to Eubanks, had no answer to her question of "Now what?" in terms of treatments and rehabilitation.

For that, the family traveled over 600 miles to Baltimore, where Dr. Laura Malone , a pediatric neurologist, had established the Pediatric Post-COVID-19 Rehabilitation Clinic at the Kennedy Krieger Institute, a pediatric-focused nonprofit health organization affiliated with Johns Hopkins Medicine.

"They were the first place we got to where they were like, 'Here's a list of symptoms, and did you have any pre-COVID, and what did you have post-COVID?'" Eubanks said, recalling how Theo checked nearly all of the symptoms on the list. "I just remember that was such relief. It was just like, 'Oh, you know, they're recognizing this and it's official, and Theo is not alone.'"
Related Stories

Google reveals top destinations for summer 2024
- Apr 9, 4:07 PM

Woman wins $1M lottery jackpot by mistake
- Apr 9, 12:33 PM

Jessica Alba steps down as The Honest Company CCO
- Apr 10, 10:50 AM
Malone said she and her team at the Kennedy Krieger Institute established the clinic in the summer of 2020 as they saw reports of adults developing long COVID. As the pandemic continued, demand began to grow.
"Everybody was, early in the pandemic, very focused on hospitalized cases, and the sequela after people get care in the ICU or are very critically ill with the acute infection, and that's not generally what we see in pediatrics," Malone said. "Most children can have a relatively mild infection and then go on to develop long-term sequela. So, that took a little bit of time to recognize and for patients to seek care, both from their primary care doctors and then also from clinics like us."

As long COVID became more recognized, Malone said the clinic has seen steady demand from pediatric patients across the country, while she said other patients may go undiagnosed.
"You have to look at a lot of behavioral changes, especially in younger children, to say, 'Something seems off'.' So I do think that [long COVID] is probably a little bit under-recognized still," Malone said. "We do see that there can be a lot of resiliency in children, and so despite them sometimes maybe having the symptoms, they may not always bring it up to family members or doctors but rather just try and manage the symptoms, and it's only when it gets to be intolerable that sometimes it will present to more medical care."
Helping kids return to 'normal' life
Along with there being no diagnostic test for long COVID, there is also no cure for the condition. Much of what can be done for patients is symptom management, according to Malone.
For kids, she said that means helping them manage their symptoms so they can, at least to some degree, return to school and social activities.
"Participation in life and all the activities, including education, that kids are designed to be participating in is really important," Malone said. "One of our big focuses is to try to provide accommodations to get kids back into school, but meet them where they are, because they may not be able to do a full course load, or they may not be able to make it through the full day of school, but there is still benefit if they can go for an hour, and gradually increase that over time to getting them back into that sort of routine, and getting them back into the social aspects of school and the educational aspects."

Patients at the clinic see not only medical doctors like Malone, but a team of experts including behavioral and neuropsychologists, social workers, pain specialists and physical therapists.
Ellen Henning, Ph.D., a pediatric psychologist at the Kennedy Krieger Institute, said patients often struggle with anxiety and depression due to long COVID. She said new research is also suggesting that long COVID itself could be influencing mental health symptoms due to factors like inflammation in the brain and lower levels of serotonin.
"We learn new things constantly and we adjust as we as we go," Henning said. "We try to provide the best supports that we can and then we all are always integrating new knowledge and adjusting things as we need to."
MORE: New long COVID study uncovers high inflammation in patients as Senate calls for more research on 'crisis'
In October, the clinic received a $5 million grant from the Department of Health and Human Services that it is using to help train school nurses and other community health care providers to identify long COVID in students and provide accommodations for students already diagnosed with the condition.
"We have a lot of families and children that say that they have to educate, sometimes, their providers and tell their doctors at home about what's going on and about long COVID," Malone said. "That can just be really exhausting for kid, so that's a big thing that we've been working on, and we're really proud of that."
Theo said while his long COVID symptoms continue, he has felt more at ease since receiving his diagnosis and as the condition becomes more recognized.

With the help of Malone and the team at the Kennedy Krieger Institute, Theo is back in school for periods of time and working on catching up with his classmates.
"We have a lot of hope," he said. "I know I'm going to finish school at some point. Maybe a little later than most people, but who knows. And I think I'm going to get better. With all the research that's going to come out, hopefully, something will help me more than anything else."

Momofuku responds to 'chile crunch' backlash
- Apr 10, 7:26 AM

Bill Murray, son celebrate after championship win
- Apr 9, 2:40 PM
ABC News Live
24/7 coverage of breaking news and live events
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 15 February 2023
Coronavirus research: knowledge gaps and research priorities
- Stanley Perlman ORCID: orcid.org/0000-0003-4213-2354 1 &
- Malik Peiris ORCID: orcid.org/0000-0001-8217-5995 2 , 3
Nature Reviews Microbiology volume 21 , pages 125–126 ( 2023 ) Cite this article
5769 Accesses
4 Citations
16 Altmetric
Metrics details
- Policy and public health in microbiology
Decades of coronavirus research and intense studies of SARS-CoV-2 since the beginning of the COVID-19 pandemic have led to an unprecedented level of knowledge of coronavirus biology and pathogenesis, yet many outstanding questions remain. Here, we discuss knowledge gaps and research priorities in the field.
Introduction
The COVID-19 pandemic showed that, based on previous research efforts, we understood many aspects of coronavirus biology and pathogenesis, but also that there was much we did not know. In 2019, the worldwide number of coronavirus investigators was small, having increased after the severe acute respiratory syndrome coronavirus (SARS-CoV) outbreak in 2003 but decreasing thereafter. The influx of scientists with diverse expertise into the field after the pandemic onset contributed to an increased understanding of coronavirus replication, epidemiology, SARS-CoV-2 pathogenesis and immune responses in humans, to the development and characterization of experimentally infected animal models for COVID-19, and to SARS-CoV-2 vaccine and antiviral drug development. Here, as investigators who have studied coronaviruses for decades, we outline some of the outstanding research questions that we think need to be addressed.
SARS-CoV-2 emergence
Where did SARS-CoV-2 originate and how did it evolve to infect humans? The emergence of SARS-CoV-2 continues to be an area of controversy and has been, and is being, investigated by many national and international organizations, including the WHO (World Health Organization). It is almost certain that the virus originated in bats and crossed species to humans either directly or indirectly via intermediary hosts. There remains debate on whether the virus first infected humans from a zoonotic source or from a research laboratory, but, no matter what the answer to this question is, it is clear to us that in order to be prepared for the next pandemic, we need to further delineate the panoply of coronaviruses present in bats and possible intermediary hosts 1 . We need to better understand coronavirus circulation in hotspots, such as parts of China and Southeast Asia, where humans, wildlife gathered for food or medicinal purposes and bats are in close proximity. These investigations should include surveillance (virological and serological) of humans in close contact with bats and the game animal trade, with or without respiratory disease, for evidence of coronavirus infection. A related question, discussed below, is why coronaviruses are especially good at jumping species, to humans and other animals.
Zoonotic risk
Once coronaviruses in animal reservoirs are identified, can they be better risk assessed for threats for human spillover? Surveillance of bat reservoirs of sarbecoviruses (Sarbecovirus is the subgenus to which SARS-CoV-2 belongs) had previously found evidence of viruses with a capacity for infecting human cells using the angiotensin converting enzyme 2 (ACE2) receptor (reminiscent of SARS-CoV) 2 . Serological evidence of viral spillover to humans was demonstrated before the emergence of SARS-CoV-2 (ref. 3 ). Arguably, these signals together should have been triggers for action to develop countermeasures with greater urgency. The availability of human organoid cultures and ex vivo cultures of human respiratory tissue may enable the use of physiologically relevant systems for a more systematic risk assessment of animal coronaviruses in the future, analogous to ongoing risk assessments being carried out for animal influenza viruses 4 .
SARS-CoV-2 transmissibility
What explains the high transmissibility of SARS-CoV-2 compared with SARS-CoV or Middle East respiratory syndrome coronavirus (MERS-CoV)? A critical factor leading to the COVID-19 pandemic was the ability of SARS-CoV-2 to grow to high levels in the upper respiratory tract and therefore to readily transmit to other humans. Titres of SARS-CoV and MERS-CoV in the upper respiratory tract peak at later times after infection 5 , consistent with the ability to interrupt transmission with relevant public health infection-prevention methods. A second, related question is why SARS-CoV and a common cold coronavirus, HCoV-NL63, which both use the same receptor as SARS-CoV-2 (ACE2) 6 , have such different patterns of infection within the human respiratory tract. HCoV-NL63 rarely infects the lower respiratory tract, whereas SARS-CoV preferentially causes pneumonia. These different patterns of infection most likely relate to differences in cell entry, including differences in co-receptor usage, host protease usage or fusogenicity of the spike protein, but there are other possibilities. Understanding these differences will provide information on which coronavirus might be expected to be transmissible and to identify additional targets for therapeutic interventions. Further elucidation of the factors that contribute to virus spread will require additional experimental animal models of coronavirus transmission.
The SARS-CoV-2 outbreak also highlighted the lack of evidence-based data on the transmission of coronaviruses, or indeed respiratory viruses in in general, and on which non-pharmaceutical countermeasures (for example, social distancing and masks (surgical versus N99/FFP3 masks)) are effective or not. The SARS-CoV-2 outbreak demonstrated that the only effective control options available in the first months of the pandemic were non-pharmaceutical, but our understanding of the efficacy of specific measures is limited.
Coronavirus genome complexity
Why do coronavirus genomes encode so many more proteins than other RNA viruses? Coronavirus genomes are bigger than those of any other RNA virus, apart from those of related members of the Nidovirales order. The genomes are so large that they require genomic proofreading activity to avoid error catastrophe 7 . A large genome size may contribute to enhanced cross-species transmission, but, at present, this notion is speculative. In any case, an important question is to understand the function of the many non-structural proteins involved in virus replication. Development of a cell-free or entirely in vitro replication system would facilitate detailed probing of the role of individual proteins in replication and transmission. Efforts to develop such cell-free systems were initiated 40 years ago, but it is only in the past few years with the advent of cryo-electron microscopy and new biochemical approaches that progress has been made. These efforts are expected to complement studies in intact cells, which use high-resolution microscopy and related techniques to analyse macromolecular interactions and function at the subcellular level.
Related to the previous question, why do coronaviruses encode so many proteins with apparent immunoevasive function? Coronaviruses encode a variable number of accessory proteins, the genes of which are intermingled within the structural protein genes located at the 3′ end of the genome. For example, SARS-CoV-2 encodes at least six such proteins, with several other putative open reading frames in the genome hypothesized to be expressed and have immunoevasive properties 8 . Confusingly, these genes are often deleted in viruses isolated from infected animals, without apparent loss of virulence. This was shown most clearly in the case of MERS-CoV, in which diverse deletions and insertions in accessory genes were detected in some isolates obtained in Africa from camels, the primary host of the virus 9 . These genetic changes may have unpredictable consequences for virus transmissibility or pathogenesis. Deletion of these genes occasionally leads to increased virulence 10 . The variable and sometimes unexpectedly high numbers of these proteins suggest that they have redundant and, perhaps, additional functions. Such redundancy could contribute to cross-species transmission. The genetic instability of MERS-CoV camels in Africa therefore needs to be monitored and evidence for human spillover needs to be continually assessed.
Predictive evolution
Can coronavirus evolution in infected human or other animal hosts be predicted? Coronaviruses readily mutate and recombine as they adapt to a new host. This is well illustrated by the COVID-19 pandemic, in which ancestral strains of SARS-CoV-2 initially mutated to better infect humans, and later evolved to evade the human immune response, generating a series of variants of concern. Several studies have modelled SARS-CoV-2 evolution but so far it has not been possible to predict how the virus will evolve in the future. Such predictive modelling is recognized to be difficult, but would be very useful in the present pandemic as well as in future coronavirus outbreaks or pandemics for vaccine development, for anticipating clinical disease and pathogenesis, and for risk assessment of animal viruses with zoonotic potential.
Keusch, G. T. et al. Pandemic origins and a One Health approach to preparedness and prevention: solutions based on SARS-CoV-2 and other RNA viruses. Proc. Natl Acad. Sci. USA 119 , e2202871119 (2022).
Article CAS PubMed PubMed Central Google Scholar
Menachery, V. D. et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 21 , 1508–1513 (2015).
Li, H. et al. Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China. Biosaf. Health 1 , 84–90 (2019).
Article PubMed PubMed Central Google Scholar
Cox, N. J., Trock, S. C. & Burke, S. A. Pandemic preparedness and the Influenza Risk Assessment Tool (IRAT). Curr. Top. Microbiol. Immunol. 385 , 119–136 (2014).
PubMed Google Scholar
Cevik, M. et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2 , e13–e22 (2021).
Article CAS PubMed Google Scholar
Hofmann, H. et al. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl Acad. Sci. USA 102 , 7988–7993 (2005).
Eckerle, L. D., Lu, X., Sperry, S. M., Choi, L. & Denison, M. R. High fidelity of murine hepatitis virus replication is decreased in nsp14 exoribonuclease mutants. J. Virol. 81 , 12135–12144 (2007).
Lowery, S. A., Sariol, A. & Perlman, S. Innate immune and inflammatory responses to SARS-CoV-2: implications for COVID-19. Cell Host Microbe 29 , 1052–1062 (2021).
Chu, D. K. W. et al. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc. Natl Acad. Sci. USA 115 , 3144–3149 (2018).
Gutierrez-Alvarez, J. et al. Middle East respiratory syndrome coronavirus gene 5 modulates pathogenesis in mice. J. Virol. 95 , e01172-20 (2021).
Download references
Author information
Authors and affiliations.
Department of Microbiology and Immunology, University of Iowa, Iowa City, IA, USA
Stanley Perlman
HKU-Pasteur Research Pole, The University of Hong Kong (HKU), Hong Kong Special Administrative Region, People’s Republic of China
Malik Peiris
School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU), Hong Kong Special Administrative Region, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stanley Perlman .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Perlman, S., Peiris, M. Coronavirus research: knowledge gaps and research priorities. Nat Rev Microbiol 21 , 125–126 (2023). https://doi.org/10.1038/s41579-022-00837-3
Download citation
Published : 15 February 2023
Issue Date : March 2023
DOI : https://doi.org/10.1038/s41579-022-00837-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Sars-cov-2 and innate immunity: the good, the bad, and the “goldilocks”.
- Benjamin L. Sievers
- Mark T. K. Cheng
- Ravindra K. Gupta
Cellular & Molecular Immunology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

COMMENTS
Learn the innate and adaptive immune responses to SARS-CoV-2 with this COVID-19 wallchart. Explore the life cycle of and immune responses to COVID-19.
Find COVID-19 datasets, data tools, and publications to use in research. EXPLORE COVID-19 DATA. Learn how NIH is supporting research in COVID-19 testing, treatments, and vaccines.
Yet well into the fourth year of the Covid-19 pandemic, intense political and scientific debates about its origins continue. The two major hypotheses are a natural zoonotic spillover, most likely ...
The WHO Covid-19 Research Database is a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued. The WHO evidence retrieval sub-group has begun ...
SARS-CoV-2 is a positive-sense single-stranded RNA virus. It is contagious in humans and is the cause of the coronavirus disease 2019 (COVID-19). A trial that took mobile health services to rural ...
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
The 25 most downloaded Nature Communications articles* on COVID-19 published in 2021 illustrate the collaborative efforts of the international community to combat the ongoing pandemic.These papers ...
The Johns Hopkins Coronavirus Resource Center established a new standard for infectious disease tracking by publicly providing pandemic data in near real time. It began Jan. 22, 2020 as the COVID-19 Dashboard, operated by the Center for Systems Science and Engineering and the Applied Physics Laboratory. But the map of red dots quickly evolved ...
Explore a collection of articles and other resources on the Coronavirus (Covid-19) outbreak, including clinical reports, management guidelines, and commentary.
In Fig. 2, the overview of the global COVID-19 vaccine landscape in clinical development depicts that there are seven major types of vaccine candidates for COVID-19 is illustrated as (inactivated, non-replicating viral vectors, replicating viral vectors, protein subunit, nucleic acid-based, and virus-like particles [VLP]), showing the percentage of candidate vaccines that are currently under ...
Abstract. The outbreak of COVID-19 has proven to be an unprecedented disaster for the whole world. The virus has inflicted billion of lives across the globe in all aspects—physically, psychologically, as well as socially. Compared to the previous strains of β-CoV genera- MERS and SARS, SARS-CoV-2 has significantly higher transmissibility and ...
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ].
Just 20% of the public views the coronavirus as a major threat to the health of the U.S. population and only 10% are very concerned about getting a serious case themselves. In addition, a relatively small share of U.S. adults (28%) say they've received an updated COVID-19 vaccine since last fall. report | Mar 28, 2023.
COVID-19 pandemic has severely impacted the crude, stock market, gold and metals and almost all areas of the global market [ 1 ]. Large research laboratories and corporate houses are working with a high speed to develop medicines and vaccines for the prevention and treatment of this dreaded disease. To deal with these current health management ...
COVID-19 Resource Centre. As of January 2024, changes have been made to our COVID-19 Resource Centre. A COVID-19 Collection is available where you can continue to explore and access The Lancet Group's COVID-19 research, reviews, commentary, news, and analysis as it is published.
Research on COVID-19 on Surfaces. Respiratory droplets, which are larger, and fall quickly, can still be a mode of transmission for SARS-CoV-2, the virus that causes Covid-19. First, researchers had to develop a way to quickly detect live virus in surface samples because current testing takes a few days to get results, which would be too late ...
The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
Andrew McGuire at the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues collected blood from ten people who had recovered from COVID-19; they collected additional ...
Stanford Medicine scientists have launched dozens of research projects as part of the global response to COVID-19. Some aim to prevent, diagnose and treat the disease; others aim to understand how it spreads and how people's immune systems respond to it. Below is a curated selection, including summaries, of the projects.
In this phase 2-3 trial, we randomly assigned adults who had confirmed Covid-19 with symptom onset within the past 5 days in a 1:1 ratio to receive nirmatrelvir-ritonavir or placebo every 12 ...
Community-engaged research was disrupted by COVID-19 restrictions, meaning researchers faced serious challenges when their results were most needed: during a public health crisis.
Abstract. In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern.
During the pandemic, research unrelated to COVID-19 faced funding drawbacks and resource constraints. This may have secondary effects in the " post-pandemic era ," especially for projects ...
Research findings could explain why young kids rarely get very sick from COVID-19. Children's noses pack a punch that could help explain COVID-19's typically mild course in young kids. Researchers hope to parlay that 'nasal magic' into increased protections for adults.
The COVID-19 virus also can spread if people touch their eyes, noses or mouths after touching a surface with the virus on it. Without cleaning and disinfection, the COVID-19 virus may stay on surfaces from hours to days. But the risk of COVID-19 through contact with infected surfaces seems low.
Theo Huot de Saint-Albin was a 9-year-old elementary school student when he first contracted COVID-19 in July 2020, near the start of the coronavirus pandemic.. Four years later, as much of the ...
Cellular & Molecular Immunology (2023) Decades of coronavirus research and intense studies of SARS-CoV-2 since the beginning of the COVID-19 pandemic have led to an unprecedented level of ...
"The fact is that the bat coronavirus research conducted by EcoHealth Alliance and the Wuhan Institute of Virology could not have started the COVID-19 pandemic," the spokesperson said.