What Are the Rules for Assigning Oxidation Numbers?
Redox Reactions and Electrochemistry
andriano_cz / Getty Images
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Electrochemical reactions involve the transfer of electrons . Mass and charge are conserved when balancing these reactions, but you need to know which atoms are oxidized and which atoms are reduced during the reaction. Oxidation numbers are used to keep track of how many electrons are lost or gained by each atom. These oxidation numbers are assigned using the following rules.

Rules for Assigning Oxidation Numbers
- The convention is that the cation is written first in a formula, followed by the anion . For example, in NaH, the H is H-; in HCl, the H is H+.
- The oxidation number of a free element is always 0. The atoms in He and N 2 , for example, have oxidation numbers of 0.
- The oxidation number of a monatomic ion equals the charge of the ion. For example, the oxidation number of Na + is +1; the oxidation number of N 3- is -3.
- The usual oxidation number of hydrogen is +1. The oxidation number of hydrogen is -1 in compounds containing elements that are less electronegative than hydrogen, as in CaH 2 .
- The oxidation number of oxygen in compounds is usually -2. Exceptions include OF 2 because F is more electronegative than O, and BaO 2 , due to the structure of the peroxide ion, which is [O-O] 2- .
- The oxidation number of a Group IA element in a compound is +1.
- The oxidation number of a Group IIA element in a compound is +2.
- The oxidation number of a Group VIIA element in a compound is -1, except when that element is combined with one having a higher electronegativity. The oxidation number of Cl is -1 in HCl, but the oxidation number of Cl is +1 in HOCl.
- The sum of the oxidation numbers of all of the atoms in a neutral compound is 0.
- The sum of the oxidation numbers in a polyatomic ion is equal to the charge of the ion. For example, the sum of the oxidation numbers for SO 4 2- is -2.
- Assigning Oxidation States Example Problem
- The Difference Between Oxidation State and Oxidation Number
- How to Balance Net Ionic Equations
- 5 Steps for Balancing Chemical Equations
- Balance Redox Reaction Example Problem
- Oxidation and Reduction Reaction Example Problem
- Ion Definition in Chemistry
- Common Anions Table and Formulas List
- Chemistry Vocabulary Terms You Should Know
- A to Z Chemistry Dictionary
- How to Balance Redox Reactions
- How to Neutralize a Base With an Acid
- Net Ionic Equation Definition
- Reduction Definition in Chemistry
- Learn About Redox Problems (Oxidation and Reduction)
- Oxidation Reduction Reactions—Redox Reactions
4.11: Oxidation Numbers
Chapter 1: introduction: matter and measurement, chapter 2: atoms and elements, chapter 3: molecules, compounds, and chemical equations, chapter 4: chemical quantities and aqueous reactions, chapter 5: gases, chapter 6: thermochemistry, chapter 7: electronic structure of atoms, chapter 8: periodic properties of the elements, chapter 9: chemical bonding: basic concepts, chapter 10: chemical bonding: molecular geometry and bonding theories, chapter 11: liquids, solids, and intermolecular forces, chapter 12: solutions and colloids, chapter 13: chemical kinetics, chapter 14: chemical equilibrium, chapter 15: acids and bases, chapter 16: acid-base and solubility equilibria, chapter 17: thermodynamics, chapter 18: electrochemistry, chapter 19: radioactivity and nuclear chemistry, chapter 20: transition metals and coordination complexes, chapter 21: biochemistry.
The JoVE video player is compatible with HTML5 and Adobe Flash. Older browsers that do not support HTML5 and the H.264 video codec will still use a Flash-based video player. We recommend downloading the newest version of Flash here, but we support all versions 10 and above.
Redox reactions between metals and nonmetals typically involve a complete transfer of electrons to form ionic compounds; hence, they are easy to identify. However, redox reactions involving only nonmetals with a partial transfer of electrons are not as easily identifiable.
Redox reactions are characterized by changes in the oxidation states of the atoms, which indicates electron movement between the atoms.
The oxidation state, or oxidation number, of an atom in a compound is the charge it would have if the shared electrons in each heteronuclear bond were completely transferred to the more electronegative atom. Homonuclear bonds are divided equally.
For instance, in gaseous hydrogen chloride, chlorine is more electronegative. If hydrogen’s electron is transferred completely to chlorine, chlorine gets a 1− charge, corresponding to the −1 oxidation state, and hydrogen gets a 1+ charge, corresponding to the +1 oxidation state.
Oxidation states can be assigned to atoms in elemental form and in most ions and compounds using specific rules. The first three rules are always followed. The remaining rules are applied one by one until the first three rules are satisfied.
These rules will now be applied to identify whether the formation reactions of sulfur dioxide and calcium carbonate are redox reactions.
According to rule number 1, elements in the free state have an oxidation number of zero, so elemental sulfur and oxygen are both assigned the oxidation number zero.
According to rule number 3, the sum of the oxidation numbers in a neutral compound is zero so the oxidation numbers of sulfur and oxygen in SO 2 must sum to zero.
In accordance with rule number 6, the oxidation number of each oxygen is −2 in SO 2 . Two oxygen atoms sum to −4. The oxidation number of sulfur is, therefore, +4.
The oxidation number of sulfur increases from zero to +4, so it is oxidized, while the oxidation number of oxygen decreases from zero to −2, so it is reduced. Thus, this is a redox reaction.
In the case of calcium carbonate, the oxidation number of oxygen is −2 in all three compounds, and calcium is +2 in calcium oxide and calcium carbonate. According to rule 3, carbon must be +4 in carbon dioxide and calcium carbonate.
Since there is no change in the oxidation numbers of the atoms during the reaction, this is not a redox reaction.
In redox reactions, the transfer of electrons occurs between reacting species. Electron transfer is described by a hypothetical number called the oxidation number (or oxidation state). It represents the effective charge of an atom or element, which is assigned using a set of rules.
Oxidation Number (Oxidation State)
In the case of an ionic compound, oxidation numbers are assigned based on the number of electrons transferred between reacting species. For example, in the formation of calcium chloride (CaCl 2 ), calcium loses two valence electrons, and the two chlorine atoms gain one electron each. In CaCl 2 , calcium’s oxidation state is +2, and each chlorine’s oxidation state is −1.
In the case of covalent compounds, electrons are not gained or lost but instead are shared between the atoms. The atom with a greater attraction for electrons pulls the shared pair more strongly. Reactions involving covalent compounds are identified as redox by applying the concept of oxidation number to track electron movements. Oxidation states help us easily identify the species being oxidized and reduced in redox reactions.
The Rules for Assigning Oxidation Number
Oxidation numbers can be positive, negative, or zero. They are assigned based on the following rules:
- All free elements have an oxidation number zero. The elements could be monoatomic, diatomic, or polyatomic.
- In a compound, group 1A elements (all alkali metals) have an oxidation number of +1, while group 2A elements (all alkaline earth metals) have an oxidation number of +2.
- Halogens usually have an oxidation number of −1, except in their compounds with oxygen, where they have a positive oxidation state. Fluorine is the most electronegative element. It has a −1 oxidation state in all its compounds.
- For monoatomic ions, the oxidation number is the same as the charge on the ion.
- Oxygen always has an oxidation number of −2, except in peroxides, where its oxidation number is −1.
- Hydrogen has an oxidation state of +1 with nonmetals and −1 with metals.
- The sum of the oxidation number for a neutral compound is zero, while for a polyatomic ion, it is equal to the charge on the ion.
This text is adapted from Openstax, Chemistry 2e, Section 4.2: Classifying Chemical Reactions.
Get cutting-edge science videos from J o VE sent straight to your inbox every month.
mktb-description
We use cookies to enhance your experience on our website.
By continuing to use our website or clicking “Continue”, you are agreeing to accept our cookies.
Note: It has been pointed out to me that there are a handful of obscure compounds of the elements sodium to caesium where the metal forms a negative ion - for example, Na - . That would give an oxidation state of -1.
You can ignore these if you are doing chemistry at A level or its equivalent. The generalisation that Group 1 metals always have an oxidation state of +1 holds good for all the compounds you are likely to meet.
If you are interested in these odd compounds, do an internet search for alkalides .
The reasons for the exceptions
Hydrogen in the metal hydrides
Metal hydrides include compounds like sodium hydride, NaH. In this, the hydrogen is present as a hydride ion, H - . The oxidation state of a simple ion like hydride is equal to the charge on the ion - in this case, -1.
Alternatively, you can think of it that the sum of the oxidation states in a neutral compound is zero. Since Group 1 metals always have an oxidation state of +1 in their compounds, it follows that the hydrogen must have an oxidation state of -1 (+1 -1 = 0).
Oxygen in peroxides
Peroxides include hydrogen peroxide, H 2 O 2 . This is an electrically neutral compound and so the sum of the oxidation states of the hydrogen and oxygen must be zero.
Since each hydrogen has an oxidation state of +1, each oxygen must have an oxidation state of -1 to balance it.
Oxygen in F 2 O
The problem here is that oxygen isn't the most electronegative element. The fluorine is more electronegative and has an oxidation state of -1. In this case, the oxygen has an oxidation state of +2.
Chlorine in compounds with fluorine or oxygen
There are so many different oxidation states that chlorine can have in these, that it is safer to simply remember that the chlorine doesn't have an oxidation state of -1 in them, and work out its actual oxidation state when you need it. You will find an example of this below.
Examples of working out oxidation states
What is the oxidation state of chromium in Cr 2+ ?
What is the oxidation state of chromium in CrCl 3 ?
This is a neutral compound so the sum of the oxidation states is zero. Chlorine has an oxidation state of -1. If the oxidation state of chromium is n :
n + 3(-1) = 0
n = +3 (Again, don't forget the + sign!)
What is the oxidation state of chromium in Cr(H 2 O) 6 3+ ?
This is an ion and so the sum of the oxidation states is equal to the charge on the ion. There is a short-cut for working out oxidation states in complex ions like this where the metal atom is surrounded by electrically neutral molecules like water or ammonia.
What is the oxidation state of chromium in the dichromate ion, Cr 2 O 7 2- ?
The oxidation state of the oxygen is -2, and the sum of the oxidation states is equal to the charge on the ion. Don't forget that there are 2 chromium atoms present.
2n + 7(-2) = -2
Warning: Because these are simple sums it is tempting to try to do them in your head. If it matters (like in an exam) write them down using as many steps as you need so that there is no chance of making careless mistakes. Your examiners aren't going to be impressed by your mental arithmetic - all they want is the right answer!
If you want some more examples to practice on, you will find them in most text books, including my chemistry calculations book .
What is the oxidation state of copper in CuSO 4 ?
Unfortunately, it isn't always possible to work out oxidation states by a simple use of the rules above. The problem in this case is that the compound contains two elements (the copper and the sulphur) whose oxidation states can both change.
The only way around this is to know some simple chemistry! There are two ways you might approach it. (There might be others as well, but I can't think of them at the moment!)
You might recognise this as an ionic compound containing copper ions and sulphate ions, SO 4 2- . To make an electrically neutral compound, the copper must be present as a 2+ ion. The oxidation state is therefore +2.
You might recognise the formula as being copper(II) sulphate. The "(II)" in the name tells you that the oxidation state is 2 (see below).
You will know that it is +2 because you know that metals form positive ions, and the oxidation state will simply be the charge on the ion.
Using oxidation states
In naming compounds
You will have come across names like iron(II) sulphate and iron(III) chloride. The (II) and (III) are the oxidation states of the iron in the two compounds: +2 and +3 respectively. That tells you that they contain Fe 2+ and Fe 3+ ions.
This can also be extended to the negative ion. Iron(II) sulphate is FeSO 4 . There is also a compound FeSO 3 with the old name of iron(II) sulphite. The modern names reflect the oxidation states of the sulphur in the two compounds.
The sulphate ion is SO 4 2- . The oxidation state of the sulphur is +6 (work it out!). The ion is more properly called the sulphate(VI) ion.
The sulphite ion is SO 3 2- . The oxidation state of the sulphur is +4 (work that out as well!). This ion is more properly called the sulphate(IV) ion. The ate ending simply shows that the sulphur is in a negative ion.
So FeSO 4 is properly called iron(II) sulphate(VI), and FeSO 3 is iron(II) sulphate(IV). In fact, because of the easy confusion between these names, the old names sulphate and sulphite are normally still used in introductory chemistry courses.
Note: Even these aren't the full name! The oxygens in the negative ions should also be identified. FeSO 4 is properly called iron(II) tetraoxosulphate(VI). It all gets a bit out of hand for everyday use for common ions.
Using oxidation states to identify what's been oxidised and what's been reduced
This is easily the most common use of oxidation states.
Oxidation involves an increase in oxidation state
Reduction involves a decrease in oxidation state
In each of the following examples, we have to decide whether the reaction involves redox, and if so what has been oxidised and what reduced.
This is the reaction between magnesium and hydrochloric acid or hydrogen chloride gas:
Mg + 2HCl MgCl 2 + H 2
Have the oxidation states of anything changed? Yes they have - you have two elements which are in compounds on one side of the equation and as uncombined elements on the other. Check all the oxidation states to be sure:.
The magnesium's oxidation state has increased - it has been oxidised. The hydrogen's oxidation state has fallen - it has been reduced. The chlorine is in the same oxidation state on both sides of the equation - it hasn't been oxidised or reduced.
The reaction between sodium hydroxide and hydrochloric acid is:
NaOH + HCl NaCl + H 2 O
Checking all the oxidation states:
Nothing has changed. This isn't a redox reaction.
This is a sneaky one! The reaction between chlorine and cold dilute sodium hydroxide solution is:
2NaOH + Cl 2 NaCl + NaClO + H 2 O
Obviously the chlorine has changed oxidation state because it has ended up in compounds starting from the original element. Checking all the oxidation states shows:
The chlorine is the only thing to have changed oxidation state. Has it been oxidised or reduced? Yes! Both! One atom has been reduced because its oxidation state has fallen. The other has been oxidised.
This is a good example of a disproportionation reaction. A disproportionation reaction is one in which a single substance is both oxidised and reduced.
Using oxidation states to identify the oxidising and reducing agent
This is just a minor addition to the last section. If you know what has been oxidised and what has been reduced, then you can easily work out what the oxidising agent and reducing agent are.
This is the reaction between chromium(III) ions and zinc metal:
2Cr 3+ + Zn 2Cr 2+ + Zn 2+
The chromium has gone from the +3 to the +2 oxidation state, and so has been reduced. The zinc has gone from the zero oxidation state in the element to +2. It has been oxidised.
So what is doing the reducing? It is the zinc - the zinc is giving electrons to the chromium (III) ions. So zinc is the reducing agent.
Similarly, you can work out that the oxidising agent has to be the chromium(III) ions, because they are taking electrons from the zinc.
This is the equation for the reaction between manganate(VII) ions and iron(II) ions under acidic conditions. This is worked out further down the page.
Looking at it quickly, it is obvious that the iron(II) ions have been oxidised to iron(III) ions. They have each lost an electron, and their oxidation state has increased from +2 to +3.
The hydrogen is still in its +1 oxidation state before and after the reaction, but the manganate(VII) ions have clearly changed. If you work out the oxidation state of the manganese, it has fallen from +7 to +2 - a reduction.
So the iron(II) ions have been oxidised, and the manganate(VII) ions reduced.
What has reduced the manganate(VII) ions - clearly it is the iron(II) ions. Iron is the only other thing that has a changed oxidation state. So the iron(II) ions are the reducing agent.
Similarly, the manganate(VII) ions must be the oxidising agent.
Using oxidation states to work out reacting proportions
This is sometimes useful where you have to work out reacting proportions for use in titration reactions where you don't have enough information to work out the complete ionic equation.
Remember that each time an oxidation state changes by one unit, one electron has been transferred. If one substance's oxidation state in a reaction falls by 2, that means that it has gained 2 electrons.
Something else in the reaction must be losing those electrons. Any oxidation state fall by one substance must be accompanied by an equal oxidation state increase by something else.
This example is based on information in an old AQA A' level question.
Ions containing cerium in the +4 oxidation state are oxidising agents. (They are more complicated than just Ce 4+ .) They can oxidise ions containing molybdenum from the +2 to the +6 oxidation state (from Mo 2+ to MoO 4 2- ). In the process the cerium is reduced to the +3 oxidation state (Ce 3+ ). What are the reacting proportions?
The oxidation state of the molybdenum is increasing by 4. That means that the oxidation state of the cerium must fall by 4 to compensate.
But the oxidation state of the cerium in each of its ions only falls from +4 to +3 - a fall of 1. So there must obviously be 4 cerium ions involved for each molybdenum ion.
The reacting proportions are 4 cerium-containing ions to 1 molybdenum ion.
Or to take a more common example involving iron(II) ions and manganate(VII) ions . . .
A solution of potassium manganate(VII), KMnO 4 , acidified with dilute sulphuric acid oxidises iron(II) ions to iron(III) ions. In the process, the manganate(VII) ions are reduced to manganese(II) ions. Use oxidation states to work out the equation for the reaction.
The oxidation state of the manganese in the manganate(VII) ion is +7. The name tells you that, but work it out again just for the practice!
In going to manganese(II) ions, the oxidation state of manganese has fallen by 5. Every iron(II) ion that reacts, increases its oxidation state by 1. That means that there must be five iron(II) ions reacting for every one manganate(VII) ion.
The left-hand side of the equation will therefore be:
MnO 4 - + 5Fe 2+ + ?
The right-hand side will be:
Mn 2+ + 5Fe 3+ + ?
After that you will have to make guesses as to how to balance the remaining atoms and the charges. In this case, for example, it is quite likely that the oxygen will end up in water. That means that you need some hydrogen from somewhere.
That isn't a problem because you have the reaction in acid solution, so the hydrogens could well come from hydrogen ions.
Eventually, you will end up with this:
Personally, I would much rather work out these equations from electron-half-equations!
Where would you like to go now?
To the Redox menu . . .
To the Inorganic Chemistry menu . . .
To Main Menu . . .
© Jim Clark 2002 (last modified November 2021)
Update 2020-11: The Adobe Shockwave is no longer supported but I have received requests to access this interactive tutorial. So now I have made this interative tutorial available as an executable for Windows .
Click here to download the executable. Remember where you download the file to. After it has finished downloading, double-click on the .exe file to run it. It does not require Internet access to run.
Please email me if you need an executable for MacOS.
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History
... and beyond
- Socratic Meta
- Featured Answers

- Oxidation Numbers
Key Questions
The valence electrons determine how many electrons an atom is willing to give up or how many spaces need to be filled in order to satisfy the rule of octet.
Lithium (Li), Sodium (Na) and Potassium (K) all have an electron configuration that ends as #s^1# . Each of these atoms would readily release this electron to have a filled valence shell and become stable as #Li^+1# , #Na^+1# and #K^+1# . Each element having an oxidation state of +1.
Oxygen (O) and Sulfur (S) all have an electron configuration that ends as #s^2 p^4# . Each of these atoms would readily take on two electrons to have a filled valence shell and become stable as #O^-2# , and #S^-2# . Each element having an oxidation state of -2.
There are exceptions to the rules and the transition metals usually have more than one oxidation state.
I hope this was helpful. SMARTERTEACHER

And so we can interpret a given chemical reaction with the use of oxidation numbers. Consider the oxidation of ammonia to give nitrate ion..........in terms of formal oxidation state this is the transition, #stackrel(-III)N# to #stackrel(+V)N# , an 8 electron oxidation, which we formally represent in the equation.......
#NH_3(aq) +3H_2O rarr NO_3^(-) +9H^(+) + 8e^(-)# #(i)#
Is this balanced with respect to mass and charge? It must be if we purport to represent physical reality.
And, inevitably, something must be reduced to effect the oxidation; let's say it is oxygen.
#stackrel(0)O_2+4e^(-) rarr2O^(2-)# #(ii)#
We add the individual redox equations together in a way to eliminate the electrons, which are particles of convenience......And so we take #(i) + 2xx(ii)# :
#NH_3(aq) +3H_2O +2O_2+8e^(-)rarr NO_3^(-) +underbrace(9H^(+) +4O^(2-))_(4H_2O+H^+) + 8e^(-)#
And so we cancel out what we can.....
#NH_3 +cancel(3H_2O) +2O_2+cancel(8e^(-))rarr NO_3^(-) +underbrace(9H^(+) +4O^(2-))_(cancel(4)H_2O+H^+) + cancel(8e^(-))#
....to give finally.........
#NH_3 +2O_2rarr NO_3^(-) +H_2O+H^+#
....or........
#stackrel(-III)NH_3 +2stackrel(0)O_2rarr Hstackrel(+V)NO_3 +H_2stackrel(-II)O#
I acknowledge that is a lot of pfaff.......but your question was rather open-ended.

The oxidation number of an atom in an ion or compound can be determined using the above rules. Let us look at a few examples [1-6] .
1. Sulfuric Acid (H 2 SO 4 )
The oxidation number of hydrogen (H) and oxygen (O) are +1 and -2, respectively. Sulfuric acid is a neutral compound. Let x be the oxidation number of sulfur (S). Therefore,
(+1) x 2 + x + (-2) x 4 = 0
Or, 2 + x – 8 = 0
2. Nitric Acid (HNO 3 )
The oxidation numbers of hydrogen (H) and oxygen (O) are +1 and -2, respectively. Nitric acid is a neutral compound. Let x be the oxidation number of nitrogen (N). Therefore,
+1 + x + (-2) x 3 = 0
Or, +1 + x – 6 = 0

3. Potassium Permanganate (KMnO 4 )
The oxidation numbers of potassium (K) is +1 and oxygen (O) is -2. KMnO 4 is a neutral compound. Let x be the oxidation number of magnesium (Mn). Therefore,
+1 + x + (-2) x 4 = 0
Or, +1 + x – 8 = 0
4. Dichromate Ion (Cr 2 O 7 2- )
Dichromate is a complex ion. The oxidation number of oxygen (O) is -2. The charge of Cr 2 O 7 2- is -2. Let x be the oxidation number of chromium (Cr).
2x + (-2) x 7 = -2
Or, 2x -14 = -2
5. Carbonate (CO 3 2- )
The oxidation number of oxygen (O) is -2 and the charge on CO 3 2- is -2. Let x be the oxidation number of carbon (O). Therefore,
x + (-2) x 3 = -2
Or, x – 6 = -2
6. Phosphite (PO 3 3- )
The oxidation number of oxygen (O) is -2 and the charge of PO 3 3- is -3. Let x be the oxidation number of phosphorous (P). Therefore,
x + (-2) x 3 = -3
Or, x – 6 = -3
7. Potassium Perchlorate (KClO 4 )
The oxidation numbers of potassium (K) and oxygen (O) are +1 and -2, respectively. Let x be the oxidation number of chlorine (Cl). KClO 4 is a neutral compound. Therefore,
Or, 1 + x – 8 = 0
8. Potassium Nitrate (KNO 3 )
The oxidation number of potassium (K) and oxygen (O) are +1 and -2, respectively. KNO 3 is a neutral compound. Let x be the oxidation number of nitrogen (N). Therefore,
Or, 1 + x – 6 = 0
The following image shows a chart consisting of the oxidation numbers of the periodic table elements [7].

Ans. The difference between valency and oxidation number is that valency is the maximum number of electrons an atom can donate, accept, or share to become stable. In contrast, the oxidation number is the number of electrons an atom can donate or accept to form a bond with another atom.
Ans. The d-block or translational elements have incomplete d- and s-subshells. The valence electrons are present in both these subshells. It is for this reason that they can form variable oxidation states.
- Chem.libretexts.org
- Chemistrytalk.org
- Khanacademy.org
- Mccord.cm.utexas.edu
- Courses.lumenlearning.com
- Chemguide.co.uk
- Chemed.chem.purdue.edu
Related Articles

Sublimation

Graham’s Law

Crystal Field Theory

Van der Waals Equation
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Trending Topics
© 2024 ( Chemistry Learner )

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

11.16: Oxidation Numbers and Redox Reactions
- Last updated
- Save as PDF
- Page ID 49538

- Ed Vitz, John W. Moore, Justin Shorb, Xavier Prat-Resina, Tim Wendorff, & Adam Hahn
- Chemical Education Digital Library (ChemEd DL)
Redox reactions may involve proton transfers and other bond-breaking and bond-making processes, as well as electron transfers, and therefore the equations involved are much more difficult to deal with than those describing acid-base reactions . In order to be able to recognize redox reactions, we need a method for keeping a careful account of all the electrons. This is done by assigning oxidation numbers to each atom before and after the reaction.
For example, in NO 3 – the nitrogen is assigned an oxidation number of +5 and each oxygen an oxidation number of –2. This arbitrary assignment corresponds to the nitrogen’s having lost its original five valence electrons to the electronegative oxygens. In NO 2 , on the other hand, the nitrogen has an oxidation number of + 4 and may be thought of as having one valence electron for itself, that is, one more electron than it had in NO 3 – .
This arbitrarily assigned gain of one electron corresponds to reduction of the nitrogen atom on going from NO 3 – to NO 2 . As a general rule, reduction corresponds to a lowering of the oxidation number of some atom. Oxidation corresponds to increasing the oxidation number of some atom. Applying the oxidation number rules to the following equation, we have
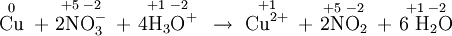
Since the oxidation number of copper increased from 0 to +2, we say that copper was oxidized and lost two negatively charged electrons. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Each nitrogen gained one electron, so 2 e – were needed for the 2 NO 3 – . The nitrogen was reduced by electrons donated by copper, and so copper was the reducing agent. Copper was oxidized because its electrons were accepted by an oxidizing agent, nitrogen (or nitrate ion).
Although they are useful and necessary for recognizing redox reactions, oxidation numbers are a highly artificial device. The nitrogen atom in NO 3 – does not really have a +5 charge which can be reduced to +4 in NO 2 . Instead, there are covalent bonds and electron-pair sharing between nitrogen and oxygen in both species, and nitrogen has certainly not lost its valence electrons entirely to oxygen. Even though this may (and indeed should) make you suspicious of the validity of oxidation numbers, they are undoubtedly a useful tool for spotting electron-transfer processes. So long as they are used for that purpose only, and not taken to mean that atoms in covalent species actually have the large charges oxidation numbers often imply, their use is quite valid.
The general rules for oxidation numbers are seen below, taken from the following page in the Analytical Chemistry Core Textbook: Oxidation States
Determining Oxidation States
Counting the number of electrons transferred is an inefficient and time-consuming way of determining oxidation states.These rules provide a simpler method:
- The oxidation state of an uncombined element is zero. This applies regardless of the structure of the element: Xe, Cl 2 , S 8 , and large structures of carbon or silicon each have an oxidation state of zero.
- The sum of the oxidation states of all the atoms or ions in a neutral compound is zero.
- The sum of the oxidation states of all the atoms in an ion is equal to the charge on the ion.
- The more electronegative element in a substance is assigned a negative oxidation state. The less electronegative element is assigned a positive oxidation state. Remember that electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left.
- Some elements almost always have the same oxidation states in their compounds:
Exceptions:
Hydrogen in the metal hydrides : Metal hydrides include compounds like sodium hydride, NaH. Here the hydrogen exists as a hydride ion, H - . The oxidation state of a simple ion like hydride is equal to the charge on the ion—in this case, -1.
Alternatively, the sum of the oxidation states in a neutral compound is zero. Because Group 1 metals always have an oxidation state of +1 in their compounds, it follows that the hydrogen must have an oxidation state of -1 (+1 -1 = 0).
Oxygen in peroxides : Peroxides include hydrogen peroxide, H 2 O 2 . This is an electrically neutral compound, so the sum of the oxidation states of the hydrogen and oxygen must be zero.
Because each hydrogen has an oxidation state of +1, each oxygen must have an oxidation state of -1 to balance it.
Oxygen in F 2 O : The deviation here stems from the fact that oxygen is less electronegative than fluorine; the fluorine takes priority with an oxidation state of -1. Because the compound is neutral, the oxygen has an oxidation state of +2.
Chlorine in compounds with fluorine or oxygen : Because chlorine adopts such a wide variety of oxidation states in these compounds, it is safer to simply remember that its oxidation state is not -1, and work the correct state out using fluorine or oxygen as a reference.
Example \(\PageIndex{1}\) : Redox Reactions
Identify the redox reactions and the reducing and oxidizing agents from the following:
- \(\ce{2MnO4^{–} + 5SO2 + 6H2O -> 5SO4^{2–} + 2Mn^{2+} + 4H3O^{+}}\)
- \(\ce{NH4^+ + PO4^{3–} -> NH3 + PO4^{2–}}\)
- \(\ce{HClO + H2S -> H3O^+ + Cl^{–} + S}\)
a) The appropriate oxidation numbers are
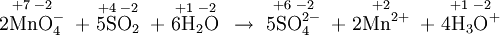
b) The oxidation numbers
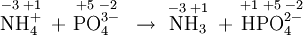
show that no redox has occurred. This is an acid-base reaction because a proton, but no electrons, has been transferred.
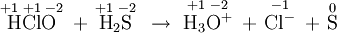
H 2 S has been oxidized, losing two electrons to form elemental S. Since H 2 S donates electrons, it is the reducing agent. HClO accepts these electrons and is reduced to Cl – . Since it accepts electrons, HClO is the oxidizing agent.

IMAGES
VIDEO
COMMENTS
Assigning Oxidation Numbers. The oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction. In oxidation-reduction processes, the driving force for chemical change is in the exchange of electrons between chemical species. A series of rules have been developed to determine ...
Write the oxidation number with the sign of the charge followed by its value. For example, write +1 and -3 rather than 1+ and 3-. The latter form is used to indicate oxidation state. The oxidation number of a free element or neutral molecule is 0. For example, the oxidation number of C, Ne, O 3, N 2, and Cl 2 is 0.
The convention is that the cation is written first in a formula, followed by the anion. For example, in NaH, the H is H-; in HCl, the H is H+. The oxidation number of a free element is always 0. The atoms in He and N 2, for example, have oxidation numbers of 0. The oxidation number of a monatomic ion equals the charge of the ion.
This chemistry tutorial discusses how to assign oxidation numbers and includes examples of how to determine the oxidation numbers in a compound following som...
By assigning oxidation numbers to the atoms of each element in a redox equation, we can determine which element is oxidized and which element is reduced during the reaction. In this video, we'll use this method to identify the oxidized and reduced elements in the reaction that occurs between I⁻ and MnO₄⁻ in basic solution. Created by Sal ...
Oxidation numbers can be assigned using the set of rules outlined below. ... When assigning oxidation numbers, you do so for each individual atom. In the above example, the oxidation number of sulfur could also have been determined by looking at just the thiosulfate ion, \(\ce{S_2O_3^{2-}}\).
Oxidation-reduction reactions, commonly known as redox reactions, are reactions that involve the transfer of electrons from one species to another. The species that loses electrons is said to be oxidized, while the species that gains electrons is said to be reduced. We can identify redox reactions using oxidation numbers, which are assigned ...
The Rules for Assigning Oxidation Number. Oxidation numbers can be positive, negative, or zero. They are assigned based on the following rules: All free elements have an oxidation number zero. The elements could be monoatomic, diatomic, or polyatomic. In a compound, group 1A elements (all alkali metals) have an oxidation number of +1, while ...
Let x equal the oxidation number for sulfur; set the sum of the charges equal to zero since the compound is neutral. Follow the order of operations and use an inverse operation to isolate x. (+1)(2) + x + (-2)(4) = 0 Sum of the charges = 0. 2 + x - 8 = 0 Multiply from left to right. x - 6 = 0 Add.
The (II) and (III) are the oxidation states of the iron in the two compounds: +2 and +3 respectively. That tells you that they contain Fe 2+ and Fe 3+ ions. This can also be extended to the negative ion. Iron (II) sulphate is FeSO 4. There is also a compound FeSO 3 with the old name of iron (II) sulphite.
7 years ago. We assign oxidation numbers (ONs) to elements using these rules: Rule 1: The ON of an element in its free state is zero — examples are Al, Zn, H₂, O₂, N₂. Rule 2: The ON of a monatomic ion is the same as its charge — examples are Na⁺ = +1; S²⁻ = -2. Rule 3: The sum of all ONs in a neutral compound is zero.
Assigning Oxidation Numbers - General Chemistry: Rules for Assigning Oxidation Numbers: Basic Rules. Any atom in its elemental form (Na, O 2, Mg, Cl 2, ...) has and oxidation number of zero. For monoatomic ions, the oxidation number equals the ion's charge. For polyatomic ions the sum of the constituent atoms' oxidation numbers is equal to ...
Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Assigning these numbers involves several rules: Free atoms (H2) usually have an oxidation number of 0, monoatomic ions (Cl-) are usually equal to their charge, and polyatomic ions have several governing principles.
Chad begins a chapter on Electrochemistry with a lesson on How to Assign Oxidation Numbers (i.e. Oxidation States). Six rules for determining oxidation numb...
By definition, the oxidation number of an atom is the charge that atom would have if the compound was composed of ions. 1. The oxidation number of an atom is zero in a neutral substance that contains atoms of only one element. Thus, the atoms in O 2, O 3, P 4, S 8 , and aluminum metal all have an oxidation number of 0.
In order to assign oxidation numbers to atoms, we need to follow a set of rules. 1. The oxidation number of an element in its free state is zero. Example: The oxidation number of Zn, Al, H 2, O 2, and Cl 2 is zero. 2. The oxidation number of a monatomic ion is the same as the charge on the ion.
Using a list of simple rules you'll learn how to find the oxidation numbers for elements and compounds. More oxidation help at https://www.Breslyn.org/uploa...
There are seven elements that can achieve +8 (Ru, Xe, Os, Ir, Pu, Cm and Hs). Since the results for the +9 oxidation state in Ir were first published just a few weeks ago (October 23, 2014) most sources you see will say that +8 is the highest oxidation state. The lowest known oxidation state is −4, which only Group 14 elements are known to ...
a) The appropriate oxidation numbers are. The only atoms which change are Mn, from +7 to +2, a reduction, and S, from +4 to +6, an oxidation. The reaction is a redox process. SO 2 has been oxidized by MnO 4-, and so MnO 4- is the oxidizing agent. MnO 4- has been reduced by SO 2, and so SO 2 is the reducing agent. b) The oxidation numbers.
This chemistry video tutorial provides a basic introduction on how to calculate oxidation numbers. It discusses how to find the oxidation states of elements...