Assign oxidation number to the underlined element in K 2 M n – –– – O 4
Let x e the oxidation number of mn in k 2 m n o 4 we know that the oxidation no. of k = + 1 a n d o = − 2 . so, 2 ( + 1 ) + x + 4 ( − 2 ) = 0 2 + x − 8 = 0 x = + 6 hence, the oxidation no. of mn is +6..

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


22.6: Assigning Oxidation Numbers
- Last updated
- Save as PDF
- Page ID 53960
Moving from studying the element iron to iron compounds, we need to be able to clearly designate the form of the iron ion. An example of this is iron that has been oxidized to form iron oxide during the process of rusting. Although Antoine Lavoisier first began the idea of oxidation as a concept, it was Wendell Latimer (1893-1955) who gave us the modern concept of oxidation numbers. His 1938 book The Oxidation States of the Elements and Their Potentials in Aqueous Solution laid out the concept in detail. Latimer was a well-known chemist who later became a member of the National Academy of Sciences. Not bad for a gentleman who started college planning on being a lawyer.
Assigning Oxidation Numbers
The oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction. In oxidation-reduction processes, the driving force for chemical change is in the exchange of electrons between chemical species. A series of rules have been developed to determine oxidation numbers:
- For free elements (uncombined state), each atom has an oxidation number of zero. \(\ce{H_2}\), \(\ce{Br_2}\), \(\ce{Na}\), \(\ce{Be}\), \(\ce{K}\), \(\ce{O_2}\), \(\ce{P_4}\), all have an oxidation number of 0.
- Monatomic ions have oxidation numbers equal to their charge. \(\ce{Li^+} = +1\), \(\ce{Ba^{2+}} = +2\), \(\ce{Fe^{3+}} = +3\), \(\ce{I^-} = -1\), \(\ce{O^{2-}} = -2\), etc. Alkali metal oxidation numbers \(= +1\). Alkaline earth oxidation numbers \(= +2\). Aluminum \(= +3\) in all of its compounds. Oxygen's oxidation number \(= -2\) except when in hydrogen peroxide \(\left( \ce{H_2O_2} \right)\), or a peroxide ion \(\left( \ce{O_2^{2-}} \right)\) where it is \(-1\).
- Hydrogen's oxidation number is \(+1\), except for when bonded to metals as the hydride ion forming binary compounds. In \(\ce{LiH}\), \(\ce{NaH}\), and \(\ce{CaH_2}\), the oxidation number is \(-1\).
- Fluorine has an oxidation number of \(-1\) in all of its compounds.
- Halogens (\(\ce{Cl}\), \(\ce{Br}\), \(\ce{I}\)) have negative oxidation numbers when they form halide compounds. When combined with oxygen, they have positive numbers. In the chlorate ion \(\left( \ce{ClO_3^-} \right)\), the oxidation number of \(\ce{Cl}\) is \(+5\), and the oxidation number of \(\ce{O}\) is \(-2\).
- In a neutral atom or molecule, the sum of the oxidation numbers must be 0. In a polyatomic ion, the sum of the oxidation numbers of all the atoms in the ion must be equal to the charge on the ion.
Example \(\PageIndex{1}\)
What is the oxidation number for manganese in the compound potassium permanganate \(\left( \ce{KMnO_4} \right)\)?
The oxidation number for \(\ce{K}\) is \(+1\) (rule 2).
The oxidation number for \(\ce{O}\) is \(-2\) (rule 2).
Since this is a compound (there is no charge indicated on the molecule), the net charge on the molecule is zero (rule 6).
So we have:
\[\begin{align*} +1 + \ce{Mn} + 4 \left( -2 \right) &= 0 \\ \ce{Mn} - 7 &= 0 \\ \ce{Mn} &= +7 \end{align*}\nonumber \]
When dealing with oxidation numbers, we must always include the charge on the atom.
Another way to determine the oxidation number of \(\ce{Mn}\) in this compound is to recall that the permanganate anion \(\left( \ce{MnO_4^-} \right)\) has a charge of \(-1\). In this case:
\[\begin{align*} \ce{Mn} + 4 \left( -2 \right) &= -1 \\ \ce{Mn} - 8 &= -1 \\ \ce{Mn} &= +7 \end{align*}\nonumber \]
Example \(\PageIndex{2}\)
What is the oxidation number for iron in \(\ce{Fe_2O_3}\)?
\[\begin{align*} &\ce{O} \: \text{is} \: -2 \: \left( \text{rule 2} \right) \\ &2 \ce{Fe} + 3 \left( -2 \right) = 0 \\ &2 \ce{Fe} = 6 \\ &\ce{Fe} = 3 \end{align*}\nonumber \]
If we have the compound \(\ce{FeO}\), then \(\ce{Fe} + \left( -2 \right) = 0\) and \(\ce{Fe} = 2\). Iron is one of those materials that can have more than one oxidation number.
The halogens (except for fluorine) can also have more than one number. In the compound \(\ce{NaCl}\), we know that \(\ce{Na}\) is \(+1\), so \(\ce{Cl}\) must be \(-1\). But what about \(\ce{Cl}\) in \(\ce{NaClO_3}\)?
\[\begin{align*} \ce{Na} &= 1 \\ \ce{O} &= -2 \\ 1 + \ce{Cl} + 3 \left( -2 \right) &= 0 \\ 1 + \ce{Cl} - 6 &= 0 \\ \ce{Cl} - 5 &= 0 \\ \ce{Cl} &= +5 \end{align*}\nonumber \]
Not quite what we expected, but \(\ce{Cl}\), \(\ce{Br}\), and \(\ce{I}\) will exhibit multiple oxidation numbers in compounds.
- The oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction.
- In oxidation-reduction processes, the driving force for chemical change is in the exchange of electrons between chemical species.
- Six rules for determining oxidation numbers are listed.
- Examples of oxidation number determinations are provided.

- Periodic table of the elements
- Chemistry calculators
- Image gallery
OXIDATION NUMBERS CALCULATOR
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'Calculate' (for example: Ca2+ , HF2^- , Fe4[Fe(CN)6]3 , NH4NO3 , so42- , ch3cooh , cuso4*5h2o ).
The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with the oxidation state. Determining oxidation numbers from the Lewis structure (Figure 1a) is even easier than deducing it from the molecular formula (Figure 1b). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Bonds between atoms of the same element (homonuclear bonds) are always divided equally.
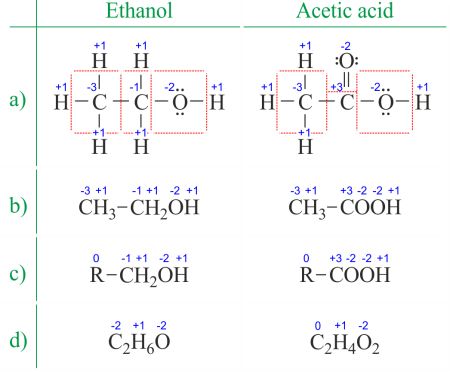
When dealing with organic compounds and formulas with multiple atoms of the same element, it's easier to work with molecular formulas and average oxidation numbers (Figure 1d). Organic compounds can be written in such a way that anything that doesn't change before the first C-C bond is replaced with the abbreviation R (Figure 1c). Unlike radicals in organic molecules, R cannot be hydrogen. Since the electrons between two carbon atoms are evenly spread, the R group does not change the oxidation number of the carbon atom it's attached to. You can find examples of usage on the Divide the redox reaction into two half-reactions page.
Rules for assigning oxidation numbers
- The oxidation number of a free element is always 0.
- The oxidation number of a monatomic ion equals the charge of the ion.
- Fluorine in compounds is always assigned an oxidation number of -1.
- The alkali metals (group I) always have an oxidation number of +1.
- The alkaline earth metals (group II) are always assigned an oxidation number of +2.
- Oxygen almost always has an oxidation number of -2, except in peroxides (H 2 O 2 ) where it is -1 and in compounds with fluorine (OF 2 ) where it is +2.
- Hydrogen has an oxidation number of +1 when combined with non-metals, but it has an oxidation number of -1 when combined with metals.
- The algebraic sum of the oxidation numbers of elements in a compound is zero.
- The algebraic sum of the oxidation states in an ion is equal to the charge on the ion.
Assigning oxidation numbers to organic compounds
- cysteine: HO2CCH(NH2)CH2SH
Back to top ↑
Citing this page:
Generalic, Eni. "Oxidation numbers calculator." EniG. Periodic Table of the Elements . KTF-Split, 18 Jan. 2024. Web. {Date of access} . <https://www.periodni.com/oxidation_numbers_calculator.php>.
- Online calculators
- Scientific calculator
- Preparation of solutions
- Oxidation numbers calculator
- Balancing redox reactions
- Memory game
- Articles and tables
- Chemistry dictionary
- Printable periodic table
- Portable applications
- Chemistry images gallery
- Short form of the periodic table
- Long form of the periodic table
- History of the Periodic table of elements
- Electronic configurations of the elements
- Alphabetical list of chemical elements
- Naming of elements of atomic numbers greater than 100
- ASCII Periodic table
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Labeling of chemical containers
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Find the pairs
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Climate change
- Global warming and mankind
- Story of ozone and ozone holes
- World War 3: Battle for Earth
- The ozone layer is not a shield
- Hexadecimal color codes
- Writing equations on the Web
- Writing chemical equations on the Web
- Character entity references in HTML
- Unicode UTF-8 encoding
- Download PDF Documents
- Download Software
- Download Images
- Periodic table
- Français
- Español
- Search on periodni.com

Let expect oxidation number of Mn is x.
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} Oxidation\text{ }number\text{ }of\text{ }K\text{ }=\text{ }+1 \\ ~ \\ Oxidation\text{ }number\text{ }of\text{ }O\text{ }=\text{ }-\text{ }2 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f60a41ef6136d6defbeb2beb89533b17_l3.png)
Then, at that point, we have:
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} 2\left( +1 \right)\text{ }+\text{ }1\left( x \right)\text{ }+\text{ }4\text{ }\left( -\text{ }2 \right)\text{ }=\text{ }0 \\ ~ \\ =\text{ }2\text{ }+\text{ }x\text{ }-\text{ }8\text{ }=\text{ }0 \\ ~ \\ =\text{ }x\text{ }-\text{ }6\text{ }=\text{ }0 \\ ~ \\ =\text{ }x\text{ }=\text{ }+6 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c8bfa865150b70a2b511f66f4470c805_l3.png)
Thus, Oxidation number of Mn is +6
More Material
Write chromyl chloride test with equation, what do you understand by lanthanide contraction, what are lanthanide elements, explain oxidization properties of potassium permanganate in acidic medium., show that the lines, compute the shortest distance between the lines, find the shortest distance between the given lines..
Book a Trial With Our Experts
Hey there! We receieved your request
Stay Tuned as we are going to contact you within 1 Hour
Thank you for registering.
One of our academic counsellors will contact you within 1 working day.

Click to Chat
- 1800-5470-145
- +91 7353221155
- Login | Register
- My Classroom
- My Self Study Packages
- Batch Discussion
- My Forum Activity
- Refer a Friend
- Edit Profile
- Add Question
- Add Paragraph
- Search Coupon
Use Coupon: CART20 and get 20% off on all online Study Material
Complete Your Registration (Step 2 of 2 )

Register Now and Win Upto 25% Scholorship for a Full Academic Year !
Enter your details.

Registration done!
Sit and relax as our customer representative will contact you within 1 business day
Mobile Verification
OTP to be sent to Change
- Junior Hacker

- Junior Hacker New
- Self Study Packages
- JEE Advanced Coaching
- 1 Year Study Plan
- Rank Predictor
- Paper Pattern
- Important Books
- Sample Papers
- Past Papers
- Preparation Tips
- Latest News
- JEE Main Exams
- Online Coaching
- Branch Predictor
- JEE Main Syllabus
- Past Year Papers
- Math Preparation Tips
- IIT JEE Exam Details
- JEE Syllabus
- IIT JEE Toppers Tips
- IIT JEE Preparation Tips
- IIT JEE Preparation Tips for Class 11
- IIT JEE Preparation Tips for Class 9
- IIT JEE Preparation Tips for Class 8
- IIT JEE Preparation Time Table
- IIT JEE Online Coaching
- Correspondence Course For IIT JEE
- IIT JEE Coaching after 10th
- IIT JEE Coaching For Foundation Classes
- JEE Coaching Institutes
- IIT JEE Coaching in Kota
- IIT JEE Coaching Institutes In Kota
- BITSAT Examination
- View complete IIT JEE Section
- View All Engineering Exams
- Top Engineering Colleges
- Top Engineering Branches
- Engineering Exam Calendar
- NEET Entrance Exam
- NEET Online Coaching
- NEET Preparation Tips
- Participating States
- AIIMS Examination
- AIIMS Online Coaching
- View all Medical Exams
- Top Medical Colleges
- Medical Exam Coaching
- Best Medical Coaching In Kota
- Medical Exam Calendar
- NTSE Examination
- Notifications
- Application
- Important Dates
- Eligibility
- Study Material
- KVPY Examination
- Olympiads Examination
- Indian National Mathematics Olympiad
- Physics Olympiad
- Chemistry Olympiad
- Biology Olympiad
- Olympiads Sample Papers
- INMO Papers
- CBSE School Exams
- Solutions for Board Exam
- JEE Advanced
- Karnataka CET
- Manipal UGET
- NCERT Class 12 Solutions
- NCERT Class 11 Solutions
- NCERT Class 10 Solutions
- NCERT Class 9 Solutions
- NCERT Class 8 Solutions
- NCERT Class 7 Solutions
- NCERT Class 6 Solutions
- List of JEE Main & JEE Advanced Books
- R.D. Sharma Solutions PDF
- Concepts of Physics by HC Verma for JEE
- HC Verma Solutions Part 1
- HC Verma Solutions Part 2
- Most Scoring Topics in IIT JEE
- IIT JEE Entrance Exam
- Discuss with Colleagues and IITians
- Engineering Entrance Exams
- Branch Ranking of IIT
- Discuss with Askiitians Tutors
- NEET (AIPMT)
- Marks and Rank in IIT JEE
- Top Engineering Colleges in India
- AIEEE Entrance Exam
- Electric Current
- Wave Motion
- Modern Physics
- Thermal Physics
- Electromagnetic Induction
- General Physics
- Electrostatics
- Wave Optics
- Physical Chemistry
- Organic Chemistry
- Inorganic Chemistry
- Trigonometry
- Analytical Geometry
- Differential Calculus
- Integral Calculus
- Magical Mathematics
- Online Tutoring
- View complete NRI Section
- View Complete Study Material
- View Complete Revision Notes
- Ahmadi (FAIPS)
- Khaitan (Carmel School)
IIT JEE Courses

One Year IIT Programme
- Super Premium LIVE Classes
- Top IITian Faculties
- 955+ hrs of Prep
- Test Series & Analysis

Two Year IIT Programme
- 1,835+ hrs of Prep

Crash Course
- LIVE + Pre Recorded Sessions
- 300+ hrs of Prep
NEET Courses

One Year NEET Programme
- Top IITian & Medical Faculties
- 900+ hrs of Prep

Two Year NEET Programme
- 1,820+ hrs of Prep

- LIVE 1-1 Classes
- Personalized Sessions
- Design your own Courses
- Personalized Study Materials

School Board
Live online classes, class 11 & 12.
- Class 11 Engineering
- Class 11 Medical
Class 9 & 10
Class 6, 7 & 8, test series, jee test series.
- 2 Year Jee Test Series
- 1 Year Jee Test Series
NEET test series
- 2 Year NEET Test Series
- 1 Year NEET Test Series
C.B.S.E test series
- 11 Engineering
- 12 Engineering
Complete Self Study Packages
Full course.
- 2 year NEET
- Chemistry 11th & 12th
- Maths 11th & 12th
- Physics 11th & 12th
- Biology 11th & 12th
- View Complete List
For class 12th
- Chemistry class 12th
- Maths class 12th
- Physics class 12th
- Biology class 12 th
For class 11th
- Chemistry class 11th
- Maths class 11th
- Physics class 11th
- Biology class 11th
- Assign oxidation number to the underlined...
Assign oxidation number to the underlined elements in each of the following species: (a) NaH2PO4 (b) NaHSO4 (c) H4P2O7 (d) K2MnO4 (e) CaO2 (f) NaBH4 (g) H2S2O7 (h) KAl(SO4)2.12 H2O ?
FOLLOW QUESTION
We will notify on your mail & mobile when someone answers this question., think you can provide a better answer , other related questions on physical chemistry.

- During the labaratory preparation of hydrogen at room temperature, zinc metal is reacted with... Answer & Earn Cool Goodies
- PART B QN 1 PLS SOLVE . Oxidation of K [Fe(CN),] by an oxidising agent is taking place to yield... Answer & Earn Cool Goodies
- important question of chapter 3 for classe for exam please 1 Answer(s) Available
- Find the Effective Atomic Number of the compound [Ni(CO) 4 ] . 2 Answer(s) Available
- Plz give me answer for the following attachment................................................... 1 Answer(s) Available
ASK QUESTION
Get your questions answered by the expert for free

Your Question has been posted!
You will get reply from our expert in sometime.
We will notify you when Our expert answers your question. To View your Question
POST QUESTION
Select the tag for question.

Answer and earn gift vouchers
Win Gift vouchers upto Rs 500/-
View courses by askIITians

Design classes One-on-One in your own way with Top IITians/Medical Professionals

Complete Self Study Package designed by Industry Leading Experts

Live 1-1 coding classes to unleash the Creator in your Child

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Register now & win upto 25% scholorship.
Register Yourself for a FREE Demo Class by Top IITians & Medical Experts Today !
BOOK A FREE TRIAL
Your answer has been successfully posted.

Snapsolve any problem by taking a picture. Try it in the Numerade app?
What are the oxidation numbers of the underlined elements in each of the following and how do you rationalise your results? K I – 3 H 2 S – – 4 O 6 F e – – – 3 O 4 C – – H 3 C H 2 O H C – – H 3 C – – O O H
K i – 3 in k i 3 , the oxidation number (o.n.) of k is + 1. hence, the average oxidation number of i is − 1 3 . however, o.n. cannot be fractional. therefore, we will have to consider the structure of k i 3 to find the oxidation states. in a k i 3 molecule, and atom of iodine forms a coordinate covalent bond with an iodine molecule. + 1 k + [ 0 i − 0 i ← − i i ] hence, in a ki3 molecule, the o.n. of the two i atoms forming the i 2 molecule is 0, whereas the o.n. of the i atom forming the coordinate bond is –1. h 2 s – – 4 o 6 + 1 h 2 x s o 4 − 2 o 6 now, 2 ( + 1 ) + 4 ( x ) + 6 ( − 2 ) = 0 ⇒ 2 + 4 x − 12 = 0 ⇒ 4 x = 10 ⇒ x = + 2 1 2 however, o.n. cannot be fractional. hence, s must be present in different oxidation states in the molecule. the o.n. of two of the four s atoms is +5 and the o.n. of the other two s atoms is 0. f e – – – 3 o 4 on taking the o.n. of o as –2, the o.n. of fe is found to be + 2 2 3 . however, o.n. cannot be fractional. here, one of the three fe atoms exhibits the o.n. of +2 and the other two fe atoms exhibit the o.n. of +3. + 2 f e o , + 3 f e 2 o 3 c – – h 3 c h 2 o h x c 2 + 1 h 6 − 2 o 2 ( x ) + 4 ( + 1 ) + 1 ( − 2 ) = 0 ⇒ 2 x + 6 − 2 = 0 ⇒ x = − 2 hence, the o.n. of c is - 2. x c 2 + 1 h 4 2 o 1 2 ( x ) + 4 ( + 1 ) + 2 ( − 2 ) = 0 ⇒ 2 x + 4 − 4 = 0 ⇒ x = 0 however, 0 is average o.n. of c. the two carbon atoms present in this molecule are present in different environments. hence, they cannot have the same oxidation number. thus, c exhibits the oxidation states of +2 and –2 in c h 3 c o o h ..

What are the oxidation numbers of the underlined elements in each of the following and how do you rationalise your results?
(a) K I 3 (b) H 2 S 4 O 6 (c) Fe 3 O 4 (d) C H 3 C H 2 OH (e) C H 3 C OOH
Assign oxidation numbers to the underlined elements in each of the following species: N a H 2 P O 4
N a H S – – O 4
H 4 P – – 2 O 7
K 2 M n – –– – O 4
C a O – – 2
N a B – – H 4
H 2 S – – 2 O 7
K A l S – – O 4 ) 2 .12 H 2 O


IMAGES
VIDEO
COMMENTS
Click here👆to get an answer to your question ️ Assign oxidation number to the underlined element in K2 MnO4. Solve Study Textbooks Guides. Join / Login >> Class 11 >> Chemistry >> Redox Reactions >> Oxidation Number ... Assign oxidation number to the underlined element in N a H 2 ...
Assign oxidation numbers to the underlined elements in each of the following species:a NaH 2 PO 4 b NaHSO 4 c H 4 P 2 O 7 d K 2 MnO 4e CaO 2 f Na B H 4 g H 2 S 2 O 7 h KAl S O 42· 12 H 2 O. ... Assign oxidation number to the underlined elements in each of the following species. C a O ...
To find the correct oxidation state of Mn in K2MnO4 (Potassium manganate), and each element in the compound, we use a few rules and some simple math.First, s...
The oxidation number for O O is −2 − 2 (rule 2). Since this is a compound (there is no charge indicated on the molecule), the net charge on the molecule is zero (rule 6). So we have: +1 + Mn + 4(−2) Mn − 7 Mn = 0 = 0 = +7 + 1 + Mn + 4 ( − 2) = 0 Mn − 7 = 0 Mn = + 7. When dealing with oxidation numbers, we must always include the ...
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'Calculate' (for example: Ca2+, HF2^-, Fe4 [Fe (CN)6]3, NH4NO3, so42-, ch3cooh, cuso4*5h2o ). The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with ...
2K2MnO4 = 4K + Mn2 + 4O2 is a redox reaction where O is oxidized and K, Mn are reduced. K 2 MnO 4 are reducing agents (i.e. they lost electrons) and K 2 MnO 4, K 2 MnO 4 are oxidizing agents (i.e. they gained electrons). Balance Using Half-Reaction (aka Ion-Electron) Method. Balance Using Oxidation Number Change Method.
K2MnO4 Let the oxidation number ON ofMnbex We know that The ON of K1 The ON of O2 Then we have 211x420 2x80 x6 Hence the ON of Mn6 . ...
Solution: K2MnO4 Let expect oxidation number of Mn is x. Then, at that point, we have: Thus, Oxidation number of Mn is +6
Assign oxidation number to the underlined elements in each of the following species:(a) \( \mathrm{NaH}_{2} \underline{\mathrm{PO}}_{4} \)(b) \( \mathrm{NaH}...
Assign oxidation number to the underlined elements in each of the following species: (a) NaH2PO4 (b) NaHSO4 (c) H4P2O7 (d) K2MnO4 (e) CaO2 (f) NaBH4 (g) H2S2O7
The oxidation number of K is + 1; The oxidation number of O is -2; The only element left is Mn, and we are unsure of its oxidation number. Assume that Mn has an oxidation number of x. Given that the molecule in K 2 MnO 4 has a neutral charge, The entire oxidation number must thus be 0. This means, + 2 (K) + x (Mn) + (4) (O) = 0 + 2 (1) + x (Mn ...
We are asked to assign oxidation numbers to each element. We'll talk about the rules that we're using along the way. Oxygen has an oxidation number of negative two if it's in a peroxide, which is the first element I want to Get 5 free video unlocks on our app with code GOMOBILE Invite sent! Login; Sign up; Textbooks; Ace - AI ...
Assign oxidation numbers to the underlined elements in each of the following species:(a)NaH2PO4(b)NaHSO4(c)H4P2O7(d)K2MnO4(e)CaO2(f) NaBH4(g)H2S2O7(h)KAl(SO4)2.12 H2O. Select Goal & City. Select Goal. Search for Colleges, Exams, Courses and More.. Write a Review Get Upto ₹500* Explore. ... > assign oxidation numbers to the underlined element;
Assign oxidation numbers to the underlined elements in each of the following species:N aH 2 P O 4NaH S O 4H 4 P 2 O 7K 2 M n O 4Ca O 2NaB H 4H 2 S 2 O 7.KAl SO 42 .12 H 2 O ... Assign oxidation numbers to the underlined elements in each of the following species: (a) NaH 2 PO 4 (b) NaHSO 4 (c) H 4 P 2 O 7 (d) K 2 MnO 4 (e) CaO 2 (f) NaBH 4 (g) H ...
Question: Assign oxidation numbers to the elements who atoms are bold and underlined in each of the following species: Enter your answer with the sign in front of the number. 1) CrO42- = 2) H2C2O4 = 3) CH3OH = 4) Na2S2O3 =. Assign oxidation numbers to the elements who atoms are bold and underlined in each of the following species:
Assign oxidation number to the underlined element in each of the following specie: K 2 Mn O 4. Solve with us. K2MnO4 Let assume oxidation number of Mn is x Oxidation number of K1 Oxidation number of O2 Then we have 211x4202x80x60x6 Hence Oxidation number of Mn is 6.
What are the oxidation numbers of the underlined elements in each of the following and how do you rationalise your results? K I 3H 2 S 4 O 6Fe 3 O 4C H 3 CH 2 OHC H 3 C OOH. Login. ... Assign oxidation numbers to the underlined elements in each of the following species: N a H 2 P O 4.
Chemistry questions and answers. Exercise: Assign oxidation numbers to the underlined element in each of the following compounds. Remember an oxidation number is for one atom of the element. FeQ 6. Cuzo 1. 2. Al4C3 7. SnBr4 3. Cul 8.
Let the oxidation number of P be x. We know that, Oxidation number of Na = +1 . Oxidation number of H = +1 . Oxidation number of O = -2 . Na +1 H +1 2 P x O-2 4. Then, we have . Hence, the oxidation number of P is +5. (b) Na +1 H +1 S x O-2 4. Then, we have . Hence, the oxidation number of S is + 6. (c) H +1 4 P x 2 O-2 7. Then, we have ...
Using the oxidation number rules, assign oxidation numbers to the elements underlined in each molecule: a} HCl b) NaHCO 3. H 3 PO 4 d) Na 2 CrO 4. CH 4 f) CHCl 3. For each of the following reactions, indicate whether the underlined element has been oxidized, reduced, or neither: a) 2Ca + O 2---à 2CaO b) 2Na + 2H 2 O ----à 2NaOH + H 2
Let the oxidation number ofMnbex Oxygen is assigned an oxidation state of2in most of its compounds except peroxides and super oxides Alkali metals exhibits an oxidation state of1in all their compounds and hence the oxidation state ofKis1inK2MnO4 In a neutral molecule likeK2MnO4the sum of oxidation states of all the atoms is equal to zero ...