
Chemistry Steps


General Chemistry
Stoichiometry.
This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts.
The links to the corresponding topics are given below.
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Balance the following chemical equations:
a) HCl + O 2 → H 2 O + Cl 2
b) Al(NO 3 ) 3 + NaOH → Al(OH) 3 + NaNO 3
c) H 2 + N 2 → NH 3
d) PCl 5 + H 2 O → H 3 PO 4 + HCl
e) Fe + H 2 SO 4 → Fe 2 (SO 4 ) 3 + H 2
f) CaCl 2 + HNO 3 → Ca(NO 3 ) 2 + HCl
g) KO 2 + H 2 O → KOH + O 2 + H 2 O 2
h) Al + H 2 O → Al 2 O 3 + H 2
i) Fe + Br 2 → FeBr 3
j) Cu + HNO 3 → Cu(NO 3 ) 2 + NO 2 + H 2 O
k) Al(OH) 3 → Al 2 O 3 + H 2 O
l) NH 3 + O 2 → NO + H 2 O
m) Ca(AlO 2 ) 2 + HCl → AlCl 3 + CaCl 2 + H 2 O
n) C 5 H 12 + O 2 → CO 2 + H 2 O
o) P 4 O 10 + H 2 O → H 3 PO 4
p) Na 2 CrO 4 + Pb(NO 3 ) 2 → PbCrO 4 + NaNO 3
q) MgCl 2 + AgNO 3 → AgCl + Mg(NO 3 ) 2
r) KClO 3 → KClO 4 + KCl
s) Ca(OH) 2 + H 3 PO 4 → Ca 3 (PO 4 ) 2 + H 2 O
Consider the balanced equation:
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
Complete the table showing the appropriate number of moles of reactants and products.
How many grams of CO 2 and H 2 O are produced from the combustion of 220. g of propane (C 3 H 8 )?
C 3 H 8 (g) + 5O 2 (g) → 3CO 2 (g) + 4H 2 O(g)
How many grams of CaCl 2 can be produced from 65.0 g of Ca(OH) 2 according to the following reaction,
Ca(OH) 2 + 2HCl → CaCl 2 + 2H 2 O
How many moles of oxygen are formed when 75.0 g of Cu(NO 3 ) 2 decomposes according to the following reaction?
2Cu(NO 3 ) 2 → 2CuO + 4NO 2 + O 2
How many grams of MnCl 2 can be prepared from 52.1 grams of MnO 2 ?
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
Determine the mass of oxygen that is formed when an 18.3-g sample of potassium chlorate is decomposed according to the following equation:
2KClO 3 (s) → 2KCl(s) + 3O 2 (g).
How many grams of H 2 O will be formed when 48.0 grams H 2 are mixed with excess hydrogen gas?
2H 2 + O 2 → 2H 2 O
Consider the chlorination reaction of methane (CH4):
CH 4 (g) + 4Cl 2 (g) → CCl 4 (g) + 4HCl(g)
How many moles of CH 4 were used in the reaction if 51.9 g of CCl4 were obtained?
How many grams of Ba(NO 3 ) 2 can be produced by reacting 16.5 g of HNO 3 with an excess of Ba(OH) 2 ?
Ethanol can be obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 g of glucose?
36.0 g of butane (C 4 H 10 ) was burned in an excess of oxygen and the resulting carbon dioxide (CO 2 ) was collected in a sealed vessel.
2C 4 H 10 + 13O 2 → 8CO 2 + 10H 2 O
How many grams of LiOH will be necessary to consume all the CO 2 from the first reaction?
2LiOH + CO 2 → Li 2 CO 3 + H 2 O
13. Which statement about limiting reactant is correct?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more product.
Find the limiting reactant for each initial amount of reactants.
4NH 3 + 5O 2 → 4NO + 6H 2 O
a) 2 mol of NH 3 and 2 mol of O 2
b) 2 mol of NH 3 and 3 mol of O 2
c) 3 mol of NH 3 and 3 mol of O 2
d) 3 mol of NH 3 and 2 mol of O 2
Note: This is not a multiple-choice question. Each row represents a separate question where you need to determine the limiting reactant.
How many g of hydrogen are left over in producing ammonia when 14.0 g of nitrogen is reacted with 8.0 g of hydrogen?
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
How many grams of PCl 3 will be produced if 130.5 g Cl 2 is reacted with 56.4 g P 4 according to the following equation?
6Cl 2 (g) + P 4 (s) → 4PCl 3 (l)
How many grams of sulfur can be obtained if 12.6 g H 2 S is reacted with 14.6 g SO 2 according to the following equation?
2H 2 S(g) + SO 2 (g) → 3S(s) + 2H 2 O(g)
The following equation represents the combustion of octane, C 8 H 18 , a component of gasoline:
2C 8 H 18 (g) + 25O 2 (g) → 16CO 2 (g) + 18H 2 O(g)
Will 356 g of oxygen be enough for the complete combustion of 954 g of octane?
When 140.0 g of AgNO 3 was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected as a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO 3 was added?
AgNO 3 (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq)
Consider the reaction between MnO 2 and HCl:
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
What is the theoretical yield of MnCl 2 in grams when 165 g of MnO 2 is added to a solution containing 94.2 g of HCl?
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 g of a product. What is the percent yield of the product if the theoretical yield was 7.12 g?
When 38.45 g CCl 4 is reacted with an excess of HF, 21.3 g CCl 2 F 2 is obtained. Calculate the theoretical and percent yields of this reaction.
CCl 4 + 2HF → CCl 2 F 2 + 2HCl
Iron(III) oxide reacts with carbon monoxide according to the equation:
Fe 2 O 3 ( s ) + 3CO( g ) → 2Fe( s ) + 3CO 2 ( g )
What is the percent yield of this reaction if 623 g of iron oxide produces 341 g of iron?
Determine the percent yield of the reaction if 77.0 g of CO 2 are formed from burning 2.00 moles of C 5 H 12 in 4.00 moles of O 2 .
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
The percent yield for the following reaction was determined to be 84%:
N 2 ( g ) + 2H 2 ( g ) → N 2 H 4 ( l )
How many grams of hydrazine (N 2 H 4 ) can be produced when 38.36 g of nitrogen reacts with 6.68 g of hydrogen?
Silver metal can be prepared by reducing its nitrate, AgNO 3 with copper according to the following equation:
Cu( s ) + 2AgNO 3 ( aq ) → Cu(NO 3 ) 2 ( aq ) + 2Ag( s )
What is the percent yield of the reaction if 71.5 grams of Ag was obtained from 132.5 grams of AgNO 3 ?
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH 3 ( g ) + 5O 2 ( g ) → 4NO( g ) + 6H 2 O( l )
2NO( g ) + O 2 ( g ) → 2NO 2 ( g )
2NO 2 ( g ) + H 2 O( l ) → HNO 3 ( aq ) + HNO 2 ( aq )
Considering that each reaction has an 85% percent yield, how many grams of NH 3 must be used to produce 25.0 kg of HNO 3 by the above procedure?
Aspirin (acetylsalicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acetic anhydride. The chemical equation for aspirin synthesis is shown below:
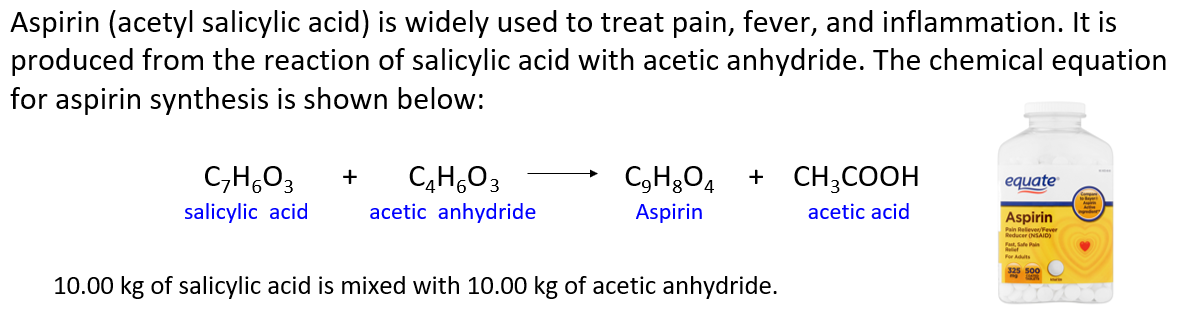
In one container, 10.00 kg of salicylic acid is mixed with 10.00 kg of acetic anhydride.
a) Which reactant is limiting? Which is in excess? b) What mass of excess reactant is left over? c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)? d) What mass of aspirin is formed if the reaction yield is 70.0% ? e) If the actual yield of aspirin is 11.2 kg, what is the percent yield? f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?
3 thoughts on “Stoichiometry Practice Problems”
You forgot the subscript 3 for O in the molecular formula for acetic anhydride and the reaction is not balanced as written. For part F) it’s 18.1 kg and not1.81 kg as written in the final line of the solution.
Thanks for letting me know! Fixed.
You’re welcome!
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.

What is Stoichiometry? Examples and Practice
- The Albert Team
- Last Updated On: March 17, 2024

Have you ever wondered how chemists know exactly how much of each substance to use in a reaction? The answer lies in a fundamental concept called stoichiometry. This crucial aspect of chemistry helps scientists and students alike understand the quantitative relationships in chemical reactions, ensuring that no atom is wasted. In this post, we’ll delve into what stoichiometry is, explore some engaging stoichiometry examples, and provide you with practical stoichiometry practice problems. Whether you’re just getting started or looking to sharpen your skills, this guide will equip you with the knowledge and tools to tackle stoichiometry problems with confidence.
What We Review
What is Stoichiometry?
Stoichiometry is like the recipe for a cake, but instead of baking, we’re dealing with chemical reactions. At its core, stoichiometry is the study of the quantitative relationships between the reactants and products in chemical reactions. When chemists conduct experiments, they need to know how much of each reactant to use and what amount of product to expect. Stoichiometry provides these answers, ensuring that chemical reactions are efficient and effective.
Understanding the Basics
To grasp stoichiometry, you first need to be familiar with a few key concepts:
- Moles: Just like a dozen represents 12 items, a mole is a specific number of molecules (approximately 6.022\times10^{23} molecules, known as Avogadro’s number).
- Molar Mass: This is the weight of one mole of a substance, typically expressed in grams per mole (g/mol). It tells you how much one mole of a substance weighs.
- Mole Ratios: These ratios come from the coefficients of a balanced chemical equation. They tell you the proportion of reactants and products in a reaction.
Real-World Applications

Stoichiometry isn’t just a topic in your chemistry textbook; it has real-world applications. Pharmacists use stoichiometry to mix medications, environmental scientists use it to track pollutant levels, and engineers apply it to design reactors. Understanding stoichiometry means you’re learning a skill that scientists and professionals use daily to make the world work.
Stoichiometry Examples
Understanding stoichiometry becomes clearer with practical examples. Let’s explore a couple of scenarios to illustrate how stoichiometry helps us solve chemical equations.
Example 1: Water Formation
Consider the chemical reaction where hydrogen and oxygen combine to form water:
If we start with 36.0 grams of H_2 and have an excess of O_2 , how many grams of H_2O can we produce? The molar mass of H_2 is 2.02 g/mol, and for H_2O , it’s 18.02 g/mol.
First, convert the mass of H_2 to moles:
Applying the mole ratio from the balanced equation, calculate the moles of H_2O produced:
Then, convert moles of H_2O to grams:
Thus, 36.0 grams of H_2 can produce 321.15 grams of H_2O .
Example 2: Ammonia Synthesis
Now, let’s examine another one of our stoichiometry examples. This problem examines the synthesis of ammonia ( NH_3 ) from nitrogen ( N_2 ) and hydrogen ( H_2 ) in a reaction conducted at STP (standard temperature and pressure):
If we begin with 22.4 liters of N_2 (1 mole at STP) and have an excess of H_2 , how many grams of NH_3 can be produced? The molar mass of NH_3 is 17.03 g/mol.
Using the mole ratio from the chemical equation:
Then, convert moles of NH_3 to grams:
This result shows that 22.4 liters of N_2 at STP can produce 34.06 grams of NH_3 .
Guided Stoichiometry Practice
To master stoichiometry, practice is key. In this section, we’ll explore strategies to approach stoichiometry problems and provide some guided practice examples. These strategies will help you develop a systematic approach to solving stoichiometry questions, enhancing your problem-solving skills.
Understanding the Approach
Identify What’s Given and What’s Unknown: Start by determining what information is provided in the problem and what you need to find. This could be the amount of reactants, products, or even the coefficients in a balanced chemical equation.
Write the Balanced Chemical Equation: Ensure you have the correct balanced chemical equation for the reaction you’re analyzing. This step is crucial as it sets the foundation for the stoichiometric calculations.
Use Mole Ratios: Utilize the coefficients from the balanced equation to establish mole ratios. These ratios are the heart of stoichiometry, allowing you to convert between amounts of reactants and products.
Perform the Calculations: With the mole ratios, you can perform calculations to find the unknown quantities. Remember to keep track of your units, ensuring they are consistent throughout the calculation.
Guided Example
Let’s practice with a guided example. Consider the combustion of propane ( C_3H_8 ) in oxygen to produce carbon dioxide and water:
Suppose you start with 44.1 grams of propane ( C_3H_8 ) and have an excess of oxygen ( O_2 ). How many grams of carbon dioxide ( CO_2 ) are produced?
Given: 44.1 grams of C_3H_8 The molar mass of C_3H_8 is 44.1 g/mol, and for CO_2 , it’s 44.0 g/mol.
Unknown: Grams of CO_2 produced
First, convert the mass of C_3H_8 to moles:
Mole Ratio: From the balanced equation, the mole ratio of C_3H_8 to CO_2 is 1:3.
Calculation:
Now, convert the moles of CO_2 to grams:
So, 44.1 grams of C_3H_8 produces 132.0 grams of CO_2 .
Stoichiometry Practice Problems
Now that you’ve seen stoichiometry in action through examples, it’s time to test your knowledge with some practice problems. Try to solve these problems on your own before checking the solutions provided. This will help you understand stoichiometry better and prepare you for similar problems in the future.
When magnesium burns in the air, it reacts with oxygen to form magnesium oxide. The balanced chemical equation for this reaction is:
If you start with 24.0 grams of magnesium ( Mg ) and have an excess of oxygen ( O_2 ), how many grams of magnesium oxide ( MgO ) will be produced? The molar mass of Mg is 24.3 g/mol, and MgO is 40.3 g/mol.
Hydrochloric acid reacts with sodium hydroxide to produce sodium chloride and water in the following reaction:
If 36.5 grams of hydrochloric acid ( HCl ) react with sufficient sodium hydroxide ( NaOH ), how many grams of sodium chloride ( NaCl ) are produced? Assume the molar mass of HCl is 36.5 g/mol and NaCl is 58.4 g/mol.
Nitrogen gas can react with hydrogen gas to form ammonia through the following reaction:
Calculate the amount of ammonia ( NH_3 ) produced (in grams) when 28.0 liters of nitrogen gas ( N_2 ) react with an excess of hydrogen gas ( H_2 ) at STP. The molar mass of NH_3 is [/latex]17.03[/latex] g/mol.
Stoichiometry Practice Problems Solutions
Here, we’ll go through the solutions to the stoichiometry practice problems we presented earlier. These solutions will help you understand the step-by-step process involved in solving stoichiometry problems.
Solution to Problem 1:
For the reaction 2Mg + O_2 \rightarrow 2MgO , we’re starting with 24.0 grams of Mg . First, convert the mass of Mg to moles:
Using the stoichiometry of the reaction, convert moles of Mg to moles of MgO (the ratio is 1:1):
Now, convert moles of MgO to grams:
So, 24.0 grams of Mg will produce 39.81 grams of MgO .
Solution to Problem 2:
For the reaction HCl + NaOH \rightarrow NaCl + H_2O , we start with 36.5 grams of HCl . Convert the mass of HCl to moles:
The mole ratio of HCl to NaCl is 1:1, so:
Now, convert moles of NaCl to grams:
Thus, 36.5 grams of HCl will produce 58.44 grams of NaCl .
Solution to Problem 3:
For the reaction N_2 + 3H_2 \rightarrow 2NH_3 , starting with 28.0 liters of N_2 at STP (which corresponds to 1.00 mole of N_2 ):
Using the stoichiometry of the reaction (1:2 ratio of N_2 to NH_3 ):
Convert moles of NH_3 to grams:
So, 28.0 liters of N_2 will produce 42.575 grams of NH_3 .
Stoichiometry is a cornerstone concept in chemistry that enables us to predict and quantify the outcomes of chemical reactions. By understanding the relationships between reactants and products, chemists can conduct reactions efficiently and effectively. Throughout this post, we’ve explored what stoichiometry is, provided clear examples, and offered practice problems to help you build your skills.
Remember, the key to mastering stoichiometry lies in practice. The more you work through problems, the more intuitive these concepts will become. Whether you’re balancing equations, calculating molar masses, or determining the amounts of reactants and products, each step you take strengthens your understanding of chemistry.
We encourage you to revisit these examples and practice problems regularly, and don’t hesitate to explore more complex scenarios as you become more comfortable with the basics. Stoichiometry is not just a topic for the classroom; it’s a tool that scientists use to make real-world decisions in industries, research, and environmental science.
So, keep practicing, stay curious, and remember that every chemical equation tells a story of transformation and interaction. With stoichiometry, you’re equipped to understand and narrate these fascinating stories of science.
Interested in a school license?
Popular posts.

AP® Score Calculators
Simulate how different MCQ and FRQ scores translate into AP® scores

AP® Review Guides
The ultimate review guides for AP® subjects to help you plan and structure your prep.

Core Subject Review Guides
Review the most important topics in Physics and Algebra 1 .

SAT® Score Calculator
See how scores on each section impacts your overall SAT® score

ACT® Score Calculator
See how scores on each section impacts your overall ACT® score


Grammar Review Hub
Comprehensive review of grammar skills

AP® Posters
Download updated posters summarizing the main topics and structure for each AP® exam.
Interested in a school license?

Bring Albert to your school and empower all teachers with the world's best question bank for: ➜ SAT® & ACT® ➜ AP® ➜ ELA, Math, Science, & Social Studies aligned to state standards ➜ State assessments Options for teachers, schools, and districts.

Solving Stoichiometry Problems

Core Concepts
In this tutorial, you will learn what is stoichiometry and the different types of problems involving it. You will go through several examples to practice and master the content!
Topics Covered in Other Articles
Balancing chemical equations.
- What is a Chemical Reaction?
- Calculating Molar Mass
- Percent Yield Calculation
- How to Calculate Molarity
- What Are Significant Figures
Stoichiometry Definition
What is stoichiometry.
Stoichiometry is math having to do with chemical reactions. There are different types of calculations you can perform; stoichiometry with moles is the most common, but you can also do math with masses and even percentages. Read about the origins of stoichiometry here ! Learn what is mole in chemistry .
Stoichiometric Ratio
A stoichiometric ratio comes into play when talking about the relationships of elements or molecules in specific problems. This is the exact ratio between the coefficients of the reactants and products needed for a reaction to proceed normally. Let’s work through some problems you may see when learning about stoichiometry.

Stoichiometry Problems
A very common type of stoichiometric problem is balancing equations. This is an important chemistry skill to have because you have to have the correct ratio of reactants and products in order for a reaction to proceed; this is also an important foundation for organic chemistry. Although we have a tutorial on balancing equations , let’s look at one example.
Balance the following reaction:
The main idea when balancing equations is that there should be the same number of each element on both sides of the reaction. You can balance the carbons and the hydrogens first, then move onto the oxygen. The balanced equation should look like this:
Example – Using Stoichiometric Ratio (Moles)
Use the equation below to solve the problem.
Example – Using Stoichiometric Ratio (Mass)
Let’s use the same equation as the problem above.
Similar to the previous problem, by using the stoichiometric ratio of reactant to product, you can find the answer. Dimensional analysis is used to go from grams of C 2 H 5 OH to molar mass to mole (stoichiometric) ratio and back to grams.
Stoichiometry Practice Problems
CaCl 2 and AgNO 3 react according to the following equation:
Hexane combusts according to the following reaction:
Stoichiometry Practice Problem Solutions
For more help, view our interactive lecture on introducing stoichiometry problems!
And this video explaining how to solve reaction stoichiometry problems, further reading.
- Percent by Weight Calculation
- Balancing Redox Reactions
- How to Write Net Ionic Equations
- What is Specific Heat?
- Common Ion Effect
- TPC and eLearning
- Read Watch Interact
- What's NEW at TPC?
- Practice Review Test
- Teacher-Tools
- Subscription Selection
- Seat Calculator
- Ad Free Account
- Edit Profile Settings
- Classes (Version 2)
- Student Progress Edit
- Task Properties
- Export Student Progress
- Task, Activities, and Scores
- Metric Conversions Questions
- Metric System Questions
- Metric Estimation Questions
- Significant Digits Questions
- Proportional Reasoning
- Acceleration
- Distance-Displacement
- Dots and Graphs
- Graph That Motion
- Match That Graph
- Name That Motion
- Motion Diagrams
- Pos'n Time Graphs Numerical
- Pos'n Time Graphs Conceptual
- Up And Down - Questions
- Balanced vs. Unbalanced Forces
- Change of State
- Force and Motion
- Mass and Weight
- Match That Free-Body Diagram
- Net Force (and Acceleration) Ranking Tasks
- Newton's Second Law
- Normal Force Card Sort
- Recognizing Forces
- Air Resistance and Skydiving
- Solve It! with Newton's Second Law
- Which One Doesn't Belong?
- Component Addition Questions
- Head-to-Tail Vector Addition
- Projectile Mathematics
- Trajectory - Angle Launched Projectiles
- Trajectory - Horizontally Launched Projectiles
- Vector Addition
- Vector Direction
- Which One Doesn't Belong? Projectile Motion
- Forces in 2-Dimensions
- Being Impulsive About Momentum
- Explosions - Law Breakers
- Hit and Stick Collisions - Law Breakers
- Case Studies: Impulse and Force
- Impulse-Momentum Change Table
- Keeping Track of Momentum - Hit and Stick
- Keeping Track of Momentum - Hit and Bounce
- What's Up (and Down) with KE and PE?
- Energy Conservation Questions
- Energy Dissipation Questions
- Energy Ranking Tasks
- LOL Charts (a.k.a., Energy Bar Charts)
- Match That Bar Chart
- Words and Charts Questions
- Name That Energy
- Stepping Up with PE and KE Questions
- Case Studies - Circular Motion
- Circular Logic
- Forces and Free-Body Diagrams in Circular Motion
- Gravitational Field Strength
- Universal Gravitation
- Angular Position and Displacement
- Linear and Angular Velocity
- Angular Acceleration
- Rotational Inertia
- Balanced vs. Unbalanced Torques
- Getting a Handle on Torque
- Torque-ing About Rotation
- Properties of Matter
- Fluid Pressure
- Buoyant Force
- Sinking, Floating, and Hanging
- Pascal's Principle
- Flow Velocity
- Bernoulli's Principle
- Balloon Interactions
- Charge and Charging
- Charge Interactions
- Charging by Induction
- Conductors and Insulators
- Coulombs Law
- Electric Field
- Electric Field Intensity
- Polarization
- Case Studies: Electric Power
- Know Your Potential
- Light Bulb Anatomy
- I = ∆V/R Equations as a Guide to Thinking
- Parallel Circuits - ∆V = I•R Calculations
- Resistance Ranking Tasks
- Series Circuits - ∆V = I•R Calculations
- Series vs. Parallel Circuits
- Equivalent Resistance
- Period and Frequency of a Pendulum
- Pendulum Motion: Velocity and Force
- Energy of a Pendulum
- Period and Frequency of a Mass on a Spring
- Horizontal Springs: Velocity and Force
- Vertical Springs: Velocity and Force
- Energy of a Mass on a Spring
- Decibel Scale
- Frequency and Period
- Closed-End Air Columns
- Name That Harmonic: Strings
- Rocking the Boat
- Wave Basics
- Matching Pairs: Wave Characteristics
- Wave Interference
- Waves - Case Studies
- Color Addition and Subtraction
- Color Filters
- If This, Then That: Color Subtraction
- Light Intensity
- Color Pigments
- Converging Lenses
- Curved Mirror Images
- Law of Reflection
- Refraction and Lenses
- Total Internal Reflection
- Who Can See Who?
- Formulas and Atom Counting
- Atomic Models
- Bond Polarity
- Entropy Questions
- Cell Voltage Questions
- Heat of Formation Questions
- Reduction Potential Questions
- Oxidation States Questions
- Measuring the Quantity of Heat
- Hess's Law
- Oxidation-Reduction Questions
- Galvanic Cells Questions
- Thermal Stoichiometry
- Molecular Polarity
- Quantum Mechanics
- Balancing Chemical Equations
- Bronsted-Lowry Model of Acids and Bases
- Classification of Matter
- Collision Model of Reaction Rates
- Density Ranking Tasks
- Dissociation Reactions
- Complete Electron Configurations
- Elemental Measures
- Enthalpy Change Questions
- Equilibrium Concept
- Equilibrium Constant Expression
- Equilibrium Calculations - Questions
- Equilibrium ICE Table
- Ionic Bonding
- Lewis Electron Dot Structures
- Limiting Reactants
- Line Spectra Questions
- Mass Stoichiometry
- Measurement and Numbers
- Metals, Nonmetals, and Metalloids
- Metric Estimations
- Metric System
- Molarity Ranking Tasks
- Mole Conversions
- Name That Element
- Names to Formulas
- Names to Formulas 2
- Nuclear Decay
- Particles, Words, and Formulas
- Periodic Trends
- Precipitation Reactions and Net Ionic Equations
- Pressure Concepts
- Pressure-Temperature Gas Law
- Pressure-Volume Gas Law
- Chemical Reaction Types
- Significant Digits and Measurement
- States Of Matter Exercise
- Stoichiometry Law Breakers
- Stoichiometry - Math Relationships
- Subatomic Particles
- Spontaneity and Driving Forces
- Gibbs Free Energy
- Volume-Temperature Gas Law
- Acid-Base Properties
- Energy and Chemical Reactions
- Chemical and Physical Properties
- Valence Shell Electron Pair Repulsion Theory
- Writing Balanced Chemical Equations
- Mission CG1
- Mission CG10
- Mission CG2
- Mission CG3
- Mission CG4
- Mission CG5
- Mission CG6
- Mission CG7
- Mission CG8
- Mission CG9
- Mission EC1
- Mission EC10
- Mission EC11
- Mission EC12
- Mission EC2
- Mission EC3
- Mission EC4
- Mission EC5
- Mission EC6
- Mission EC7
- Mission EC8
- Mission EC9
- Mission RL1
- Mission RL2
- Mission RL3
- Mission RL4
- Mission RL5
- Mission RL6
- Mission KG7
- Mission RL8
- Mission KG9
- Mission RL10
- Mission RL11
- Mission RM1
- Mission RM2
- Mission RM3
- Mission RM4
- Mission RM5
- Mission RM6
- Mission RM8
- Mission RM10
- Mission LC1
- Mission RM11
- Mission LC2
- Mission LC3
- Mission LC4
- Mission LC5
- Mission LC6
- Mission LC8
- Mission SM1
- Mission SM2
- Mission SM3
- Mission SM4
- Mission SM5
- Mission SM6
- Mission SM8
- Mission SM10
- Mission KG10
- Mission SM11
- Mission KG2
- Mission KG3
- Mission KG4
- Mission KG5
- Mission KG6
- Mission KG8
- Mission KG11
- Mission F2D1
- Mission F2D2
- Mission F2D3
- Mission F2D4
- Mission F2D5
- Mission F2D6
- Mission KC1
- Mission KC2
- Mission KC3
- Mission KC4
- Mission KC5
- Mission KC6
- Mission KC7
- Mission KC8
- Mission AAA
- Mission SM9
- Mission LC7
- Mission LC9
- Mission NL1
- Mission NL2
- Mission NL3
- Mission NL4
- Mission NL5
- Mission NL6
- Mission NL7
- Mission NL8
- Mission NL9
- Mission NL10
- Mission NL11
- Mission NL12
- Mission MC1
- Mission MC10
- Mission MC2
- Mission MC3
- Mission MC4
- Mission MC5
- Mission MC6
- Mission MC7
- Mission MC8
- Mission MC9
- Mission RM7
- Mission RM9
- Mission RL7
- Mission RL9
- Mission SM7
- Mission SE1
- Mission SE10
- Mission SE11
- Mission SE12
- Mission SE2
- Mission SE3
- Mission SE4
- Mission SE5
- Mission SE6
- Mission SE7
- Mission SE8
- Mission SE9
- Mission VP1
- Mission VP10
- Mission VP2
- Mission VP3
- Mission VP4
- Mission VP5
- Mission VP6
- Mission VP7
- Mission VP8
- Mission VP9
- Mission WM1
- Mission WM2
- Mission WM3
- Mission WM4
- Mission WM5
- Mission WM6
- Mission WM7
- Mission WM8
- Mission WE1
- Mission WE10
- Mission WE2
- Mission WE3
- Mission WE4
- Mission WE5
- Mission WE6
- Mission WE7
- Mission WE8
- Mission WE9
- Vector Walk Interactive
- Name That Motion Interactive
- Kinematic Graphing 1 Concept Checker
- Kinematic Graphing 2 Concept Checker
- Graph That Motion Interactive
- Two Stage Rocket Interactive
- Rocket Sled Concept Checker
- Force Concept Checker
- Free-Body Diagrams Concept Checker
- Free-Body Diagrams The Sequel Concept Checker
- Skydiving Concept Checker
- Elevator Ride Concept Checker
- Vector Addition Concept Checker
- Vector Walk in Two Dimensions Interactive
- Name That Vector Interactive
- River Boat Simulator Concept Checker
- Projectile Simulator 2 Concept Checker
- Projectile Simulator 3 Concept Checker
- Hit the Target Interactive
- Turd the Target 1 Interactive
- Turd the Target 2 Interactive
- Balance It Interactive
- Go For The Gold Interactive
- Egg Drop Concept Checker
- Fish Catch Concept Checker
- Exploding Carts Concept Checker
- Collision Carts - Inelastic Collisions Concept Checker
- Its All Uphill Concept Checker
- Stopping Distance Concept Checker
- Chart That Motion Interactive
- Roller Coaster Model Concept Checker
- Uniform Circular Motion Concept Checker
- Horizontal Circle Simulation Concept Checker
- Vertical Circle Simulation Concept Checker
- Race Track Concept Checker
- Gravitational Fields Concept Checker
- Orbital Motion Concept Checker
- Angular Acceleration Concept Checker
- Balance Beam Concept Checker
- Torque Balancer Concept Checker
- Aluminum Can Polarization Concept Checker
- Charging Concept Checker
- Name That Charge Simulation
- Coulomb's Law Concept Checker
- Electric Field Lines Concept Checker
- Put the Charge in the Goal Concept Checker
- Circuit Builder Concept Checker (Series Circuits)
- Circuit Builder Concept Checker (Parallel Circuits)
- Circuit Builder Concept Checker (∆V-I-R)
- Circuit Builder Concept Checker (Voltage Drop)
- Equivalent Resistance Interactive
- Pendulum Motion Simulation Concept Checker
- Mass on a Spring Simulation Concept Checker
- Particle Wave Simulation Concept Checker
- Boundary Behavior Simulation Concept Checker
- Slinky Wave Simulator Concept Checker
- Simple Wave Simulator Concept Checker
- Wave Addition Simulation Concept Checker
- Standing Wave Maker Simulation Concept Checker
- Color Addition Concept Checker
- Painting With CMY Concept Checker
- Stage Lighting Concept Checker
- Filtering Away Concept Checker
- InterferencePatterns Concept Checker
- Young's Experiment Interactive
- Plane Mirror Images Interactive
- Who Can See Who Concept Checker
- Optics Bench (Mirrors) Concept Checker
- Name That Image (Mirrors) Interactive
- Refraction Concept Checker
- Total Internal Reflection Concept Checker
- Optics Bench (Lenses) Concept Checker
- Kinematics Preview
- Velocity Time Graphs Preview
- Moving Cart on an Inclined Plane Preview
- Stopping Distance Preview
- Cart, Bricks, and Bands Preview
- Fan Cart Study Preview
- Friction Preview
- Coffee Filter Lab Preview
- Friction, Speed, and Stopping Distance Preview
- Up and Down Preview
- Projectile Range Preview
- Ballistics Preview
- Juggling Preview
- Marshmallow Launcher Preview
- Air Bag Safety Preview
- Colliding Carts Preview
- Collisions Preview
- Engineering Safer Helmets Preview
- Push the Plow Preview
- Its All Uphill Preview
- Energy on an Incline Preview
- Modeling Roller Coasters Preview
- Hot Wheels Stopping Distance Preview
- Ball Bat Collision Preview
- Energy in Fields Preview
- Weightlessness Training Preview
- Roller Coaster Loops Preview
- Universal Gravitation Preview
- Keplers Laws Preview
- Kepler's Third Law Preview
- Charge Interactions Preview
- Sticky Tape Experiments Preview
- Wire Gauge Preview
- Voltage, Current, and Resistance Preview
- Light Bulb Resistance Preview
- Series and Parallel Circuits Preview
- Thermal Equilibrium Preview
- Linear Expansion Preview
- Heating Curves Preview
- Electricity and Magnetism - Part 1 Preview
- Electricity and Magnetism - Part 2 Preview
- Vibrating Mass on a Spring Preview
- Period of a Pendulum Preview
- Wave Speed Preview
- Slinky-Experiments Preview
- Standing Waves in a Rope Preview
- Sound as a Pressure Wave Preview
- DeciBel Scale Preview
- DeciBels, Phons, and Sones Preview
- Sound of Music Preview
- Shedding Light on Light Bulbs Preview
- Models of Light Preview
- Electromagnetic Radiation Preview
- Electromagnetic Spectrum Preview
- EM Wave Communication Preview
- Digitized Data Preview
- Light Intensity Preview
- Concave Mirrors Preview
- Object Image Relations Preview
- Snells Law Preview
- Reflection vs. Transmission Preview
- Magnification Lab Preview
- Reactivity Preview
- Ions and the Periodic Table Preview
- Periodic Trends Preview
- Intermolecular Forces Preview
- Melting Points and Boiling Points Preview
- Reaction Rates Preview
- Ammonia Factory Preview
- Stoichiometry Preview
- Gaining Teacher Access
- Tasks and Classes
- Tasks - Classic
- Subscription
- Subscription Locator
- 1-D Kinematics
- Newton's Laws
- Vectors - Motion and Forces in Two Dimensions
- Momentum and Its Conservation
- Work and Energy
- Circular Motion and Satellite Motion
- Thermal Physics
- Static Electricity
- Electric Circuits
- Vibrations and Waves
- Sound Waves and Music
- Light and Color
- Reflection and Mirrors
- About the Physics Interactives
- Task Tracker
- Usage Policy
- Newtons Laws
- Vectors and Projectiles
- Forces in 2D
- Momentum and Collisions
- Circular and Satellite Motion
- Balance and Rotation
- Electromagnetism
- Waves and Sound
- Forces in Two Dimensions
- Work, Energy, and Power
- Circular Motion and Gravitation
- Sound Waves
- 1-Dimensional Kinematics
- Circular, Satellite, and Rotational Motion
- Einstein's Theory of Special Relativity
- Waves, Sound and Light
- QuickTime Movies
- About the Concept Builders
- Pricing For Schools
- Directions for Version 2
- Measurement and Units
- Relationships and Graphs
- Rotation and Balance
- Vibrational Motion
- Reflection and Refraction
- Teacher Accounts
- Task Tracker Directions
- Kinematic Concepts
- Kinematic Graphing
- Wave Motion
- Sound and Music
- About CalcPad
- 1D Kinematics
- Vectors and Forces in 2D
- Simple Harmonic Motion
- Rotational Kinematics
- Rotation and Torque
- Rotational Dynamics
- Electric Fields, Potential, and Capacitance
- Transient RC Circuits
- Light Waves
- Units and Measurement
- Stoichiometry
- Molarity and Solutions
- Thermal Chemistry
- Acids and Bases
- Kinetics and Equilibrium
- Solution Equilibria
- Oxidation-Reduction
- Nuclear Chemistry
- NGSS Alignments
- 1D-Kinematics
- Projectiles
- Circular Motion
- Magnetism and Electromagnetism
- Graphing Practice
- About the ACT
- ACT Preparation
- For Teachers
- Other Resources
- Newton's Laws of Motion
- Work and Energy Packet
- Static Electricity Review
- Solutions Guide
- Solutions Guide Digital Download
- Motion in One Dimension
- Work, Energy and Power
- Frequently Asked Questions
- Purchasing the Download
- Purchasing the CD
- Purchasing the Digital Download
- About the NGSS Corner
- NGSS Search
- Force and Motion DCIs - High School
- Energy DCIs - High School
- Wave Applications DCIs - High School
- Force and Motion PEs - High School
- Energy PEs - High School
- Wave Applications PEs - High School
- Crosscutting Concepts
- The Practices
- Physics Topics
- NGSS Corner: Activity List
- NGSS Corner: Infographics
- About the Toolkits
- Position-Velocity-Acceleration
- Position-Time Graphs
- Velocity-Time Graphs
- Newton's First Law
- Newton's Second Law
- Newton's Third Law
- Terminal Velocity
- Projectile Motion
- Forces in 2 Dimensions
- Impulse and Momentum Change
- Momentum Conservation
- Work-Energy Fundamentals
- Work-Energy Relationship
- Roller Coaster Physics
- Satellite Motion
- Electric Fields
- Circuit Concepts
- Series Circuits
- Parallel Circuits
- Describing-Waves
- Wave Behavior Toolkit
- Standing Wave Patterns
- Resonating Air Columns
- Wave Model of Light
- Plane Mirrors
- Curved Mirrors
- Teacher Guide
- Using Lab Notebooks
- Current Electricity
- Light Waves and Color
- Reflection and Ray Model of Light
- Refraction and Ray Model of Light
- Classes (Legacy Version)
- Teacher Resources
- Subscriptions

- Newton's Laws
- Einstein's Theory of Special Relativity
- About Concept Checkers
- School Pricing
- Newton's Laws of Motion
- Newton's First Law
- Newton's Third Law
Chemistry: Stoichiometry of Reactions

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.3: Stoichiometry Calculations
- Last updated
- Save as PDF
- Page ID 98551
Learning Objectives
- To balance equations that describe reactions in solution.
- To calculate the quantities of compounds produced or consumed in a chemical reaction.
- To solve quantitative problems involving the stoichiometry of reactions in solution.
A balanced chemical equation gives the identity of the reactants and the products as well as the accurate number of molecules or moles of each that are consumed or produced. Stoichiometry is a collective term for the quantitative relationships between the masses, the numbers of moles, and the numbers of particles (atoms, molecules, and ions) of the reactants and the products in a balanced chemical equation. A stoichiometric quantity is the amount of product or reactant specified by the coefficients in a balanced chemical equation. This section describes how to use the stoichiometry of a reaction to answer questions like the following: How much oxygen is needed to ensure complete combustion of a given amount of isooctane? (This information is crucial to the design of nonpolluting and efficient automobile engines.) How many grams of pure gold can be obtained from a ton of low-grade gold ore? (The answer determines whether the ore deposit is worth mining.) If an industrial plant must produce a certain number of tons of sulfuric acid per week, how much elemental sulfur must arrive by rail each week?
All these questions can be answered using the concepts of the mole, molar and formula masses, and solution concentrations, along with the coefficients in the appropriate balanced chemical equation.
Stoichiometry Problems
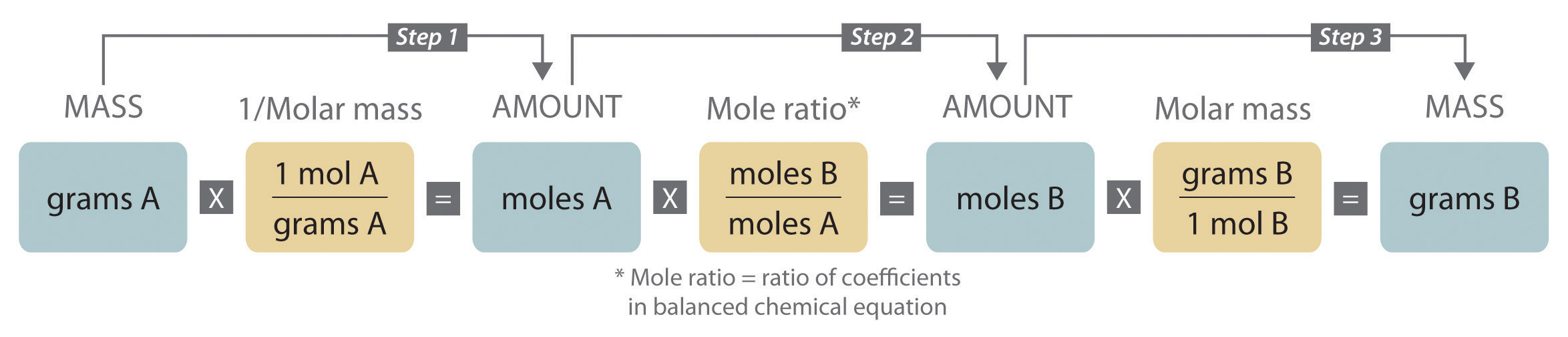
When carrying out a reaction in either an industrial setting or a laboratory, it is easier to work with masses of substances than with the numbers of molecules or moles. The general method for converting from the mass of any reactant or product to the mass of any other reactant or product using a balanced chemical equation is outlined in and described in the following text.
Steps in Converting between Masses of Reactant and Product
- Convert the mass of one substance (substance A) to the corresponding number of moles using its molar mass.
- From the balanced chemical equation, obtain the number of moles of another substance (B) from the number of moles of substance A using the appropriate mole ratio (the ratio of their coefficients).
- Convert the number of moles of substance B to mass using its molar mass. It is important to remember that some species are present in excess by virtue of the reaction conditions. For example, if a substance reacts with the oxygen in air, then oxygen is in obvious (but unstated) excess.
Converting amounts of substances to moles—and vice versa—is the key to all stoichiometry problems, whether the amounts are given in units of mass (grams or kilograms), weight (pounds or tons), or volume (liters or gallons).
To illustrate this procedure, consider the combustion of glucose. Glucose reacts with oxygen to produce carbon dioxide and water:
\[ C_6H_{12}O_6 (s) + 6 O_2 (g) \rightarrow 6 CO_2 (g) + 6 H_2O (l) \label{3.6.1} \]
Just before a chemistry exam, suppose a friend reminds you that glucose is the major fuel used by the human brain. You therefore decide to eat a candy bar to make sure that your brain does not run out of energy during the exam (even though there is no direct evidence that consumption of candy bars improves performance on chemistry exams). If a typical 2 oz candy bar contains the equivalent of 45.3 g of glucose and the glucose is completely converted to carbon dioxide during the exam, how many grams of carbon dioxide will you produce and exhale into the exam room?
The initial step in solving a problem of this type is to write the balanced chemical equation for the reaction. Inspection shows that it is balanced as written, so the strategy outlined above can be adapted as follows:
1. Use the molar mass of glucose (to one decimal place, 180.2 g/mol) to determine the number of moles of glucose in the candy bar:
\[ moles \, glucose = 45.3 \, g \, glucose \times {1 \, mol \, glucose \over 180.2 \, g \, glucose } = 0.251 \, mol \, glucose \nonumber \]
2. According to the balanced chemical equation, 6 mol of CO 2 is produced per mole of glucose; the mole ratio of CO 2 to glucose is therefore 6:1. The number of moles of CO 2 produced is thus
\[ moles \, CO_2 = mol \, glucose \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose } \nonumber \]
\[ = 0.251 \, mol \, glucose \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose } \nonumber \]
\[ = 1.51 \, mol \, CO_2 \nonumber \]
3. Use the molar mass of CO 2 (44.010 g/mol) to calculate the mass of CO 2 corresponding to 1.51 mol of CO 2 :
\[ mass\, of\, CO_2 = 1.51 \, mol \, CO_2 \times {44.010 \, g \, CO_2 \over 1 \, mol \, CO_2} = 66.5 \, g \, CO_2 \nonumber \]
These operations can be summarized as follows:
\[ 45.3 \, g \, glucose \times {1 \, mol \, glucose \over 180.2 \, g \, glucose} \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose} \times {44.010 \, g \, CO_2 \over 1 \, mol \, CO_2} = 66.4 \, g \, CO_2 \nonumber \]
Discrepancies between the two values are attributed to rounding errors resulting from using stepwise calculations in steps 1–3. (Remember that you should generally carry extra significant digits through a multistep calculation to the end to avoid this!) This amount of gaseous carbon dioxide occupies an enormous volume—more than 33 L. Similar methods can be used to calculate the amount of oxygen consumed or the amount of water produced.
The balanced chemical equation was used to calculate the mass of product that is formed from a certain amount of reactant. It can also be used to determine the masses of reactants that are necessary to form a certain amount of product or, as shown in Example \(\PageIndex{1}\), the mass of one reactant that is required to consume a given mass of another reactant.
Example \(\PageIndex{1}\): The US Space Shuttle
The combustion of hydrogen with oxygen to produce gaseous water is extremely vigorous, producing one of the hottest flames known. Because so much energy is released for a given mass of hydrogen or oxygen, this reaction was used to fuel the NASA (National Aeronautics and Space Administration) space shuttles, which have recently been retired from service. NASA engineers calculated the exact amount of each reactant needed for the flight to make sure that the shuttles did not carry excess fuel into orbit. Calculate how many tons of hydrogen a space shuttle needed to carry for each 1.00 tn of oxygen (1 tn = 2000 lb).

The US space shuttle Discovery during liftoff . The large cylinder in the middle contains the oxygen and hydrogen that fueled the shuttle’s main engine .
Given : reactants, products, and mass of one reactant
Asked for : mass of other reactant
- Write the balanced chemical equation for the reaction.
- Convert mass of oxygen to moles. From the mole ratio in the balanced chemical equation, determine the number of moles of hydrogen required. Then convert the moles of hydrogen to the equivalent mass in tons.
We use the same general strategy for solving stoichiometric calculations as in the preceding example. Because the amount of oxygen is given in tons rather than grams, however, we also need to convert tons to units of mass in grams. Another conversion is needed at the end to report the final answer in tons.
A We first use the information given to write a balanced chemical equation. Because we know the identity of both the reactants and the product, we can write the reaction as follows:
\[ H_2 (g) + O_2 (g) \rightarrow H_2O (g) \nonumber \]
This equation is not balanced because there are two oxygen atoms on the left side and only one on the right. Assigning a coefficient of 2 to both H 2 O and H 2 gives the balanced chemical equation:
\[ 2 H_2 (g) + O_2 (g) \rightarrow 2 H_2O (g) \nonumber \]
Thus 2 mol of H 2 react with 1 mol of O 2 to produce 2 mol of H 2 O.
1. B To convert tons of oxygen to units of mass in grams, we multiply by the appropriate conversion factors:
\[ mass \, of \, O_2 = 1.00 \, tn \times { 2000 \, lb \over tn} \times {453.6 \, g \over lb} = 9.07 \times 10^5 \, g \, O_2 \nonumber \]
Using the molar mass of O 2 (32.00 g/mol, to four significant figures), we can calculate the number of moles of O 2 contained in this mass of O 2 :
\[ mol \, O_2 = 9.07 \times 10^5 \, g \, O_2 \times {1 \, mol \, O_2 \over 32.00 \, g \, O_2} = 2.83 \times 10^4 \, mol \, O_2 \nonumber \]
2. Now use the coefficients in the balanced chemical equation to obtain the number of moles of H 2 needed to react with this number of moles of O 2 :
\[ mol \, H_2 = mol \, O_2 \times {2 \, mol \, H_2 \over 1 \, mol \, O_2} \nonumber \]
\[ = 2.83 \times 10^4 \, mol \, O_2 \times {2 \, mol \, H_2 \over 1 \, mol \, O_2} = 5.66 \times 10^4 \, mol \, H_2 \nonumber \]
3. The molar mass of H 2 (2.016 g/mol) allows us to calculate the corresponding mass of H 2 :
\[mass \, of \, H_2 = 5.66 \times 10^4 \, mol \, H_2 \times {2.016 \, g \, H_2 \over mol \, H_2} = 1.14 \times 10^5 \, g \, H_2 \nonumber \]
Finally, convert the mass of H2 to the desired units (tons) by using the appropriate conversion factors:
\[ tons \, H_2 = 1.14 \times 10^5 \, g \, H_2 \times {1 \, lb \over 453.6 \, g} \times {1 \, tn \over 2000 \, lb} = 0.126 \, tn \, H_2 \nonumber \]
The space shuttle had to be designed to carry 0.126 tn of H 2 for each 1.00 tn of O 2 . Even though 2 mol of H 2 are needed to react with each mole of O 2 , the molar mass of H 2 is so much smaller than that of O 2 that only a relatively small mass of H 2 is needed compared to the mass of O 2 .
Exercise \(\PageIndex{1}\): Roasting Cinnabar
Cinnabar, (or Cinnabarite) \(HgS\) is the common ore of mercury. Because of its mercury content, cinnabar can be toxic to human beings; however, because of its red color, it has also been used since ancient times as a pigment.

Alchemists produced elemental mercury by roasting cinnabar ore in air:
\[ HgS (s) + O_2 (g) \rightarrow Hg (l) + SO_2 (g) \nonumber \]
The volatility and toxicity of mercury make this a hazardous procedure, which likely shortened the life span of many alchemists. Given 100 g of cinnabar, how much elemental mercury can be produced from this reaction?
Calculating Moles from Volume
Quantitative calculations involving reactions in solution are carried out with masses , however, volumes of solutions of known concentration are used to determine the number of moles of reactants. Whether dealing with volumes of solutions of reactants or masses of reactants, the coefficients in the balanced chemical equation give the number of moles of each reactant needed and the number of moles of each product that can be produced. An expanded version of the flowchart for stoichiometric calculations is shown in Figure \(\PageIndex{2}\). The balanced chemical equation for the reaction and either the masses of solid reactants and products or the volumes of solutions of reactants and products can be used to determine the amounts of other species, as illustrated in the following examples.
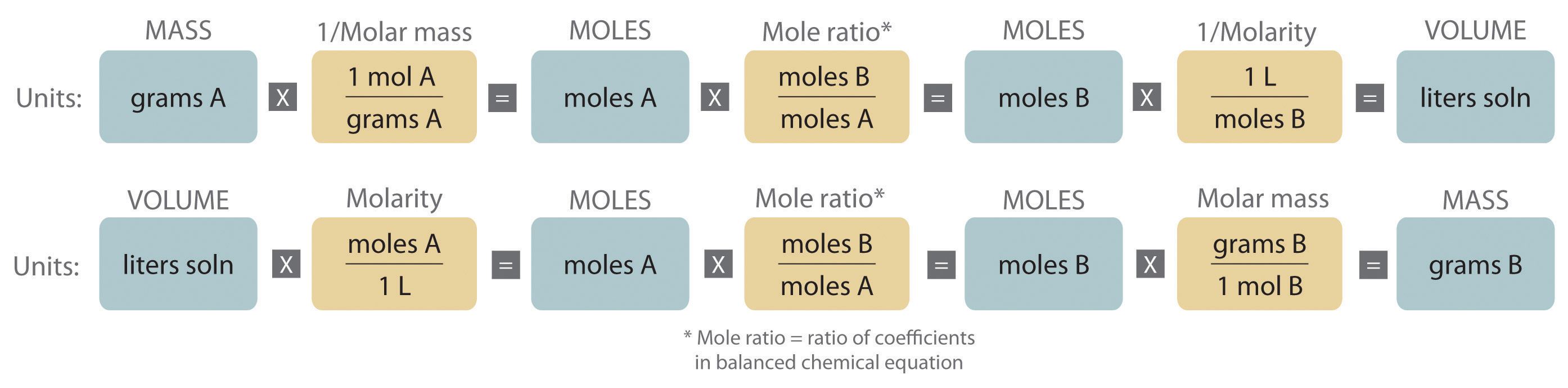
The balanced chemical equation for a reaction and either the masses of solid reactants and products or the volumes of solutions of reactants and products can be used in stoichiometric calculations.
Example \(\PageIndex{2}\) : Extraction of Gold
Gold is extracted from its ores by treatment with an aqueous cyanide solution, which causes a reaction that forms the soluble [Au(CN) 2 ] − ion. Gold is then recovered by reduction with metallic zinc according to the following equation:
\[ Zn(s) + 2[Au(CN)_2]^-(aq) \rightarrow [Zn(CN)_4]^{2-}(aq) + 2Au(s) \nonumber \]
What mass of gold can be recovered from 400.0 L of a 3.30 × 10 −4 M solution of [Au(CN) 2 ] − ?
Given: chemical equation and molarity and volume of reactant
Asked for: mass of product
- Check the chemical equation to make sure it is balanced as written; balance if necessary. Then calculate the number of moles of [Au(CN) 2 ] − present by multiplying the volume of the solution by its concentration.
- From the balanced chemical equation, use a mole ratio to calculate the number of moles of gold that can be obtained from the reaction. To calculate the mass of gold recovered, multiply the number of moles of gold by its molar mass.
A The equation is balanced as written; proceed to the stoichiometric calculation. Figure \(\PageIndex{2}\) is adapted for this particular problem as follows:
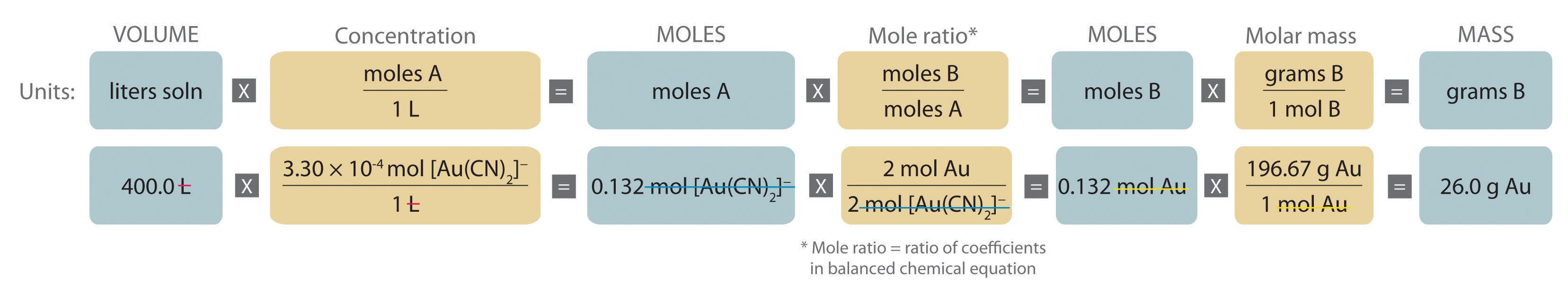
As indicated in the strategy, start by calculating the number of moles of [Au(CN) 2 ] − present in the solution from the volume and concentration of the [Au(CN) 2 ] − solution:
\( \begin{align} moles\: [Au(CN)_2 ]^- & = V_L M_{mol/L} \\ & = 400 .0\: \cancel{L} \left( \dfrac{3 .30 \times 10^{4-}\: mol\: [Au(CN)_2 ]^-} {1\: \cancel{L}} \right) = 0 .132\: mol\: [Au(CN)_2 ]^- \end{align} \)
B Because the coefficients of gold and the [Au(CN) 2 ] − ion are the same in the balanced chemical equation, assuming that Zn(s) is present in excess, the number of moles of gold produced is the same as the number of moles of [Au(CN) 2 ] − (i.e., 0.132 mol of Au). The problem asks for the mass of gold that can be obtained, so the number of moles of gold must be converted to the corresponding mass using the molar mass of gold:
\( \begin{align} mass\: of\: Au &= (moles\: Au)(molar\: mass\: Au) \\ &= 0 .132\: \cancel{mol\: Au} \left( \dfrac{196 .97\: g\: Au} {1\: \cancel{mol\: Au}} \right) = 26 .0\: g\: Au \end{align}\)
At a 2011 market price of over $1400 per troy ounce (31.10 g), this amount of gold is worth $1170.
\( 26 .0\: \cancel{g\: Au} \times \dfrac{1\: \cancel{troy\: oz}} {31 .10\: \cancel{g}} \times \dfrac{\$1400} {1\: \cancel{troy\: oz\: Au}} = \$1170 \)
Exercise \(\PageIndex{2}\) : Lanthanum Oxalate
What mass of solid lanthanum(III) oxalate nonahydrate [La 2 (C 2 O 4 ) 3 •9H 2 O] can be obtained from 650 mL of a 0.0170 M aqueous solution of LaCl 3 by adding a stoichiometric amount of sodium oxalate?
Finding Mols and Masses of Reactants and Products Using Stoichiometric Factors (Mol Ratios): Finding Mols and Masses of Reactants and Products Using Stoichiometric Factors, YouTube(opens in new window) [youtu.be]
Either the masses or the volumes of solutions of reactants and products can be used to determine the amounts of other species in the balanced chemical equation. Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration instead of masses of reactants or products. The coefficients in the balanced chemical equation tell how many moles of reactants are needed and how many moles of product can be produced.
Stoichiometry & Limiting Reagents Quiz
This online quiz is intended to give you extra practice in performing stoichiometric conversions, including limiting reagent and percent yield problems. This quiz aligns with the following NGSS standard(s): HS-PS1-7
Select your preferences below and click 'Start' to give it a try!

H 2 + O 2 ---> H 2 O
2H 2 + O 2 ---> 2H 2 O
54.0 g / 32.0 g/mol = 1.6875 mol of O 2 Note the use of 32.0 and not 16.0. The chemical substance is O 2 . Students have been known to sometimes forget to write the subscript of 2 on a diatomic element (H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , I 2 )
H 2 ––– O 2
2 mol H 2 ––––––– 1 mol O 2
x ––––––––––– 1.6875 mol O 2 No unit is attached to the unknown 'x.' Note also that I did not round off. I'll do that at the end.
2 mol H 2 x ––––––– = ––––––––––– 1 mol O 2 1.6875 mol O 2 x = 3.375 mol of H 2 required
(3.375 mol) (2.016 g/mol) = 6.80 g (to three sig figs)
54.0 g O 2 1 mol O 2 2 mol H 2 2.016 g H 2 ––––––– x ––––––– x ––– x ––––––– = 6.80 g H 2 32.0 g O 2 1 mol O 2 1 mol H 2 When doing DA, it is very helpful to identify each unit as being one chemical or the other. This will help cut down on confusion when you are placing values, deciding if a given value goes in the numerator or the denominator.
105.0 g / 18.015 g/mol = 5.82848 mol of H 2 O I rounded off some, but I made sure to keep more digits than what I will round off to at the end.
H 2 –––– H 2 O
2 mol H 2 ––––––––– 2 mol H 2 O
x ––––––––––––––– 5.82848 mol H 2 O
2 mol H 2 x ––––––––– = –––––––––––––– 2 mol H 2 O 5.82848 mol H 2 O x = 5.82848 mol of H 2 required
(5.82848 mol) (2.016 g/mol) = 11.75 g of H 2 (to four sig figs)
105.0 g H 2 O 1 mol H 2 O 2 mol H 2 2.016 g H 2 –––––––––– x ––––––––––– x –––––––––– x ––––––– = 11.75 g H 2 18.015 g H 2 O 2 mol H 2 O 1 mol H 2 Remember, in dimensional analysis, you cancel units on the diagonal, never up and down. This is why you write the substance in the unit. 'mol H 2 ' and 'mol H 2 O' do not cancel.
N 2 + H 2 ---> NH 3
N 2 + 3H 2 ---> 2NH 3
85.2 g / 17.0307 g/mol = 5.00273 mol
H 2 –––– NH 3
3 x ––– = ––––––– 2 5.00273 x = 7.504095 mol of H 2
(7.504095 mol) (2.016 g/mol) = 15.1 g (to three sig figs)
85.2 g NH 3 1 mol NH 3 3 mol H 2 2.016 g H 2 ––––––––– x –––––––––––– x ––––––––– x ––––––––– = 15.1 g H 2 17.0307 g NH 3 2 mol NH 3 1 mol H 2
AuCl 3 ---> Au + Cl 2
2AuCl 3 ---> 2Au + 3Cl 2
Let x = the moles of AuCl 3 64.00 g x = –––––––––––– 303.32 g/mol x = 0.210998 mol of AuCl 3 The ChemTeam has heard many variations of this: "But how did you know to convert grams of AuCl 3 to moles?"
AuCl 3 ––––– Cl 2
2 mol AuCl 3 0.210998 mol AuCl 3 –––––––––– = –––––––––––––––– 3 mol Cl 2 x x = 0.316497 mol of Cl 2
(0.316497 mol) (70.906 g/mol) = 22.44 g (to four sig figs)
64.00 g AuCl 3 1 mol AuCl 3 3 mol Cl 2 70.906 g Cl 2 ––––––––– x –––––––––––– x ––––––––– x ––––––––– = 22.44 g Cl 2 303.32 g AuCl 3 2 mol AuCl 3 1 mol Cl 2
3AgNO 3 + AlCl 3 ---> 3AgCl + Al(NO 3 ) 3
200. g –––––––––––– = 1.499914 mol of AlCl 3 133.341 g/mol I picked AlCl 3 because it was the substance has a gram amount associated with it in the problem.
AgCl ––––– AlCl 3 3 mol AgCl x ––––––––– = –––––––––––– 1 mol AlCl 3 1.499914 mol AlCl 3 x = 4.499742 mol of AgCl The 'x' in the right-hand ratio is associated with the substance we are trying to calculate an amount for (the AgCl). Look for phrases like "Calculate the mass of . . ." or "Determine the mass of . . . " in the problem statement.
(4.499742 mol) (143.323 g/mol) = 645 g (to three sig figs)
1 mol AlCl 3 1.499914 mol AlCl 3 –––––––––– = ––––––––––––––– 3 mol AgCl x
200. g AuCl 3 1 mol AlCl 3 3 mol AgCl 143.323 g AgCl ––––––––––– x –––––––––––– x ––––––––– x –––––––––––– = 645 g AgCl 133.341 g AlCl 3 1 mol AlCl 3 1 mol AgCl
2KI + Pb(NO 3 ) 2 ---> PbI 2 + 2KNO 3
30.0 g –––––––––––– = 0.180725 mol of KI 165.998 g/mol
This ratio: 2 ––– 1
0.180725 –––––––– x
2 0.180725 –– = –––––––– 1 x
(0.0903625 mol) (461.01 g/mol) = 41.6 g (to three sig figs)
Al(NO 3 ) 3 + Mg ---> Mg(NO 3 ) 2 + Al
2Al(NO 3 ) 3 + 3Mg ---> 3Mg(NO 3 ) 2 + 2Al
92.0 g –––––––––– = 3.4099 mol of Al 26.98 g/mol
Al –––––––– Al(NO 3 ) 3
2 3.4099 –– = ––––––– 2 x x = 3.4099 mol 3 ) 3 , NOT moles of Al
(3.4099 mol) (212.994 g/mol) = 726 g (to three sig figs)
2Au + 3Cl 2 ---> 2AuCl 3
100.0 g –––––––––– = 1.41032 mol of Cl 2 70.906 g/mol
3 1.41032 mol –– = ––––––––––– 2 x x = 0.940213 mol
(0.940213 mol) (303.329 g/mol) = 285 g
volume of Al foil ---> (1.00 cm) (1.00 cm) (0.0540 cm) = 0.0540 cm 3 Note the change of mm to cm.
Note the use of the density of aluminum.
2Al + 3Br 2 ---> 2AlBr 3 The Al to Br 2 molar ratio of 2:3 will be used to answer (a). The Al to AlBr 3 molar ratio of 2:2 will be used to answer (b).
2 0.0054037 mol –––– = ––––––––––––– 3 x x = 0.00810555 mol (of Br 2 )
(0.00810555 mol) (159.808 g/mol) = 1.30 g (to three sig figs)
2 0.0054037 mol –––– = ––––––––––––– 2 x x = 0.0054037 mol (of AlBr 3 )
(0.0054037 mol) (266.694 g/mol) = 1.44 g (to three sig figs)
66.0 g / 40.078 g/mol = 1.6468 mol
3 8 ––––––––– = ––– 1.6368 mol x x = 4.3648 mol
(4.3648 mol) (16.00 g/mol) = 69.8 g
2LiOH + CO 2 ---> Li 2 CO 3 + H 2 O
2Li + 2H 2 O ---> 2LiOH + H 2
2Li + CO 2 + H 2 O ---> Li 2 CO 3 + H 2 Note that two LiOH and one H 2 O cancel out. This third reaction gives me the Li to CO 2 as 2 to 1, so I am now ready to continue on.
1000 g / 6.941 g/mol = 144.07 mol
2 144.07 mol ––– = ––––––––– 1 x x = 72.035 mol (of CO 2 )
(72.035 mol) (44.009 g/mol) = 3170 g
PV = nRT (1.00 atm) (V) = (72.035 mol) (0.08206 L atm / mol K) (273.15 K) V = 1614.6 L (to three sig figs, this would be 1610 L)
(22.414 L/mol) (72.035 mol) = 1614.6 L (1610 L to three sig figs)

IMAGES
VIDEO
COMMENTS
Stoichiometry Practice Problems. This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts. The links to the corresponding topics are given below. The Mole and Molar Mass.
PROBLEM 5.2.1.1 5.2.1. 1. Write the balanced equation and determine the information requested. Don't worry about state symbols in these reactions. The number of moles and the mass (in grams) of chlorine, Cl 2, required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl. The number of moles and the mass (in milligrams) of ...
Stoichiometry Practice Problems. Now that you've seen stoichiometry in action through examples, it's time to test your knowledge with some practice problems. Try to solve these problems on your own before checking the solutions provided. This will help you understand stoichiometry better and prepare you for similar problems in the future ...
Ideal stoichiometry. Google Classroom. You might need: Calculator, Periodic table. Given the following reaction: Zn + CuCl A 2 ZnCl A 2 + Cu. How many moles of ZnCl A 2 will be produced from 23.0 g of Zn , assuming CuCl A 2 is available in excess? moles (round to three significant figures) Show Calculator. Show Periodic Table.
Stoichiometry questions. Google Classroom. One type of anaerobic respiration converts glucose ( C 6 H 12 O 6 ) to ethanol ( C 2 H 5 O H ) and carbon dioxide. If the molecular weight of glucose is 180 grams/mol and the molar mass of ethanol is 46 g/mol, how many grams of carbon dioxide are produced when 1 mol of glucose is digested via respiration?
How many moles of water are produced when 57 moles of nitrogen are made? 3. Calculate the mass of aluminum oxide produced when 3.75 moles of aluminum burn in oxygen. Answers: 1A. 30 mol Ag 1B. 30 mol AgNO3. 1C. 20 mol H2O 1D. 10 mol NO. 2A. 38 mol N2H4 2B. 19 mol N2O4. 2C. 76 mol H2O.
Question 10. Identify the energy graphs A and B as being for an endothermic or exothermic reaction. A. B. 4.7: Unit 4 Practice Problems is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.
Stoichiometry - AP level. Stoichiometry - AP level All Examples and Problems. Problems #1-10. Problems #11-25. Problems #26-50. Return to Stoichiometry Menu. Ten Examples. Example #1: A mixture of Mg and Zn with a combined mass of 1.0875 g was burned in oxygen producing MgO and ZnO with a combined mass of 1.4090 g.
Step 1: Convert known reactant mass to moles. In order to relate the amounts H A 2 SO A 4 and NaOH using a mole ratio, we first need to know the quantity of H A 2 SO A 4 in moles. We can convert the 3.10 grams of H A 2 SO A 4 to moles using the molar mass of H A 2 SO A 4 ( 98.08 g / mol ):
20 PRACTICE PROBLEM. A metal from group 2A forms a bromide with the formula MBr2 and chloride with the formula MCl2. The bromide can be converted into chloride when reacted with chlorine gas. MBr2 + Cl2 → MCl2 + Br2. When a 0.2554 g sample of MBr2 is reacted with excess Cl2, 0.1321 g of MCl2 is produced.
More complex stoichiometry problems using balanced chemical reactions can also use concentrations as conversion factors. For example, suppose the following equation represents a chemical reaction: ... A student takes a precisely measured sample, called an aliquot, of 10.00 mL of a solution of FeCl 3. The student carefully adds 0.1074 M Na 2 C 2 ...
I2 is produced by the reaction of 0.4235. The reaction between potassium chlorate and red phosphorus. Stoichiometry is based on the law of. The coefficients in a balanced chemical equation always can express the ratio of. List the four steps to solve stoichiometric problems.
Similar to the previous problem, by using the stoichiometric ratio of reactant to product, you can find the answer. Dimensional analysis is used to go from grams of C 2 H 5 OH to molar mass to mole (stoichiometric) ratio and back to grams. Stoichiometry Practice Problems. Problem 1
What volume of H2 gas is produced at STP? 3. CaCO3 + 2 HCl --> CaCl2 + H2O + CO2 How much 0.80 M HCl would be needed to dissolve a CaCO3 pearl which weighs 4.0 grams? 4. 3 Fe + 2 Au(NO3)3 --> 3 Fe(NO3)2 + 2 Au Throwing some scrap iron in a gold nitrate solution causes the gold metal to precipitate.
Problem Set ST4: Mole-to-Mole-to-Mass Stoichiometry 2. To perform two-step conversions to determine the mass of a reactant or product from knowledge of the number of moles of a reactant or product involved in the reaction (or vice versa). Includes 6 problems. Problem Set ST5: Introduction to Mass-to-Mass Stoichiometry.
Converting amounts of substances to moles—and vice versa—is the key to all stoichiometry problems, whether the amounts are given in units of mass (grams or kilograms), weight (pounds or tons), or volume (liters or gallons). Figure \(\PageIndex{1}\): A Flowchart for Stoichiometric Calculations Involving Pure Substances.
Stoichiometry. A student conducts an experiment to determine the molarity of a K A 2 CO A 3 ( a q) solution of unknown concentration. The student mixes 100. mL of the solution with excess CaCl A 2 ( a q) , causing CaCO A 3 ( s) to precipitate. The student then filters and dries the precipitate and records the data in the table below.
Oxygen on hand ⇒ 10.0 g / 31.9988 g/mol = 0.3125 mol. Since the oxygen required is greater than that on hand, it will run out before the sucrose. Oxygen is the limiting reagent. Solution path #2: 1) Calculate moles: sucrose ⇒ 0.0292146 mol oxygen ⇒ 0.3125 mol. 2) Divide by coefficients of balanced equation:
Stoichiometry & Limiting Reagents Quiz. This online quiz is intended to give you extra practice in performing stoichiometric conversions, including limiting reagent and percent yield problems. This quiz aligns with the following NGSS standard (s): HS-PS1-7. Select your preferences below and click 'Start' to give it a try! Number of problems: 1. 5.
But, they don't have to be. Here is an example of a mass-mass stoichiometric problem based on the relationships within one chemical substance. Solution: 1) Determine moles of calcium: 66.0 g / 40.078 g/mol = 1.6468 mol. 2) Determine moles of oxygen in the sample, based on a 3:8 ratio between Ca and O: 3. 8.
Study with Quizlet and memorize flashcards containing terms like N₂ + H₂ → NH₃ How many moles of ammonia are produced when 6 moles of hydrogen gas react with nitrogen gas?, KClO₃ → KCl + O₂ How many moles of potassium chlorate are needed to produce 15 moles of oxygen?, Zn + HCl → ZnCl₂ + H₂ Calculate the moles of HCl needed to completely react with 8.25 moles of zinc. and more.
Ideal Stoichiometry vs limiting-reagent stoichiometry…Stoichiometry…clear & simple (with practice problems)…Mole-to-Mass, Mass-to-Mole, Mass-to-Mass, Mole-to...
Limiting reagent stoichiometry. Google Classroom. You might need: Calculator, Periodic table. Given the following reaction: Cu + 2 AgNO 3 → 2 Ag + Cu (NO 3) 2. How many grams of Ag will be produced from 5.00 g of Cu and 1 .00 g of AgNO 3 ?
Champs : Course on Advanced Problems in Physical Chemistry - JEE 2024. Brijesh Jindal. In this course, Brijesh Jindal Sir will provide in-depth knowledge of Physical Chemistry. The course will be helpful for aspirants preparing for IIT JEE. Learners at any stage of their preparation will be benefi...