If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

Unit 3: Chemical reactions and stoichiometry
About this unit.
This unit is part of the Chemistry library. Browse videos, articles, and exercises by topic.
Balancing chemical equations
- Chemical reactions introduction (Opens a modal)
- Balancing chemical equations (Opens a modal)
- Balancing more complex chemical equations (Opens a modal)
- Visually understanding balancing chemical equations (Opens a modal)
- Balancing another combustion reaction (Opens a modal)
- Balancing chemical equation with substitution (Opens a modal)
- Balancing chemical equations 1 Get 3 of 4 questions to level up!
Stoichiometry
- Stoichiometry (Opens a modal)
- Worked example: Calculating amounts of reactants and products (Opens a modal)
- Worked example: Relating reaction stoichiometry and the ideal gas law (Opens a modal)
- Converting moles and mass Get 3 of 4 questions to level up!
- Ideal stoichiometry Get 5 of 7 questions to level up!
Limiting reagent stoichiometry
- Limiting reactant and reaction yields (Opens a modal)
- Worked example: Calculating the amount of product formed from a limiting reactant (Opens a modal)
- Introduction to gravimetric analysis: Volatilization gravimetry (Opens a modal)
- Gravimetric analysis and precipitation gravimetry (Opens a modal)
- 2015 AP Chemistry free response 2a (part 1 of 2) (Opens a modal)
- 2015 AP Chemistry free response 2a (part 2/2) and b (Opens a modal)
- Limiting reagent stoichiometry Get 5 of 7 questions to level up!
Molecular composition
- Empirical, molecular, and structural formulas (Opens a modal)
- Worked example: Calculating mass percent (Opens a modal)
- Worked example: Determining an empirical formula from percent composition data (Opens a modal)
- Worked example: Determining an empirical formula from combustion data (Opens a modal)
Types of chemical reactions
- Oxidation–reduction (redox) reactions (Opens a modal)
- Worked example: Using oxidation numbers to identify oxidation and reduction (Opens a modal)
- Balancing redox equations (Opens a modal)
- Dissolution and precipitation (Opens a modal)
- Precipitation reactions (Opens a modal)
- Double replacement reactions (Opens a modal)
- Single replacement reactions (Opens a modal)
- Molecular, complete ionic, and net ionic equations (Opens a modal)
- 2015 AP Chemistry free response 3a (Opens a modal)
H 2 SO 4 + Na 2 CO 3 g Na 2 SO 4 + H 2 O + CO 2

Chemistry Steps

General Chemistry
Stoichiometry.
This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts.
The links to the corresponding topics are given below.
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Balance the following chemical equations:
a) HCl + O 2 → H 2 O + Cl 2
b) Al(NO 3 ) 3 + NaOH → Al(OH) 3 + NaNO 3
c) H 2 + N 2 → NH 3
d) PCl 5 + H 2 O → H 3 PO 4 + HCl
e) Fe + H 2 SO 4 → Fe 2 (SO 4 ) 3 + H 2
f) CaCl 2 + HNO 3 → Ca(NO 3 ) 2 + HCl
g) KO 2 + H 2 O → KOH + O 2 + H 2 O 2
h) Al + H 2 O → Al 2 O 3 + H 2
i) Fe + Br 2 → FeBr 3
j) Cu + HNO 3 → Cu(NO 3 ) 2 + NO 2 + H 2 O
k) Al(OH) 3 → Al 2 O 3 + H 2 O
l) NH 3 + O 2 → NO + H 2 O
m) Ca(AlO 2 ) 2 + HCl → AlCl 3 + CaCl 2 + H 2 O
n) C 5 H 12 + O 2 → CO 2 + H 2 O
o) P 4 O 10 + H 2 O → H 3 PO 4
p) Na 2 CrO 4 + Pb(NO 3 ) 2 → PbCrO 4 + NaNO 3
q) MgCl 2 + AgNO 3 → AgCl + Mg(NO 3 ) 2
r) KClO 3 → KClO 4 + KCl
s) Ca(OH) 2 + H 3 PO 4 → Ca 3 (PO 4 ) 2 + H 2 O
Consider the balanced equation:
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
Complete the table showing the appropriate number of moles of reactants and products.
How many grams of CO 2 and H 2 O are produced from the combustion of 220. g of propane (C 3 H 8 )?
C 3 H 8 (g) + 5O 2 (g) → 3CO 2 (g) + 4H 2 O(g)
How many grams of CaCl 2 can be produced from 65.0 g of Ca(OH) 2 according to the following reaction,
Ca(OH) 2 + 2HCl → CaCl 2 + 2H 2 O
How many moles of oxygen are formed when 75.0 g of Cu(NO 3 ) 2 decomposes according to the following reaction?
2Cu(NO 3 ) 2 → 2CuO + 4NO 2 + O 2
How many grams of MnCl 2 can be prepared from 52.1 grams of MnO 2 ?
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
Determine the mass of oxygen that is formed when an 18.3-g sample of potassium chlorate is decomposed according to the following equation:
2KClO 3 (s) → 2KCl(s) + 3O 2 (g).
How many grams of H 2 O will be formed when 48.0 grams H 2 are mixed with excess hydrogen gas?
2H 2 + O 2 → 2H 2 O
Consider the chlorination reaction of methane (CH4):
CH 4 (g) + 4Cl 2 (g) → CCl 4 (g) + 4HCl(g)
How many moles of CH 4 were used in the reaction if 51.9 g of CCl4 were obtained?
How many grams of Ba(NO 3 ) 2 can be produced by reacting 16.5 g of HNO 3 with an excess of Ba(OH) 2 ?
Ethanol can be obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 g of glucose?
36.0 g of butane (C 4 H 10 ) was burned in an excess of oxygen and the resulting carbon dioxide (CO 2 ) was collected in a sealed vessel.
2C 4 H 10 + 13O 2 → 8CO 2 + 10H 2 O
How many grams of LiOH will be necessary to consume all the CO 2 from the first reaction?
2LiOH + CO 2 → Li 2 CO 3 + H 2 O
13. Which statement about limiting reactant is correct?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more product.
Find the limiting reactant for each initial amount of reactants.
4NH 3 + 5O 2 → 4NO + 6H 2 O
a) 2 mol of NH 3 and 2 mol of O 2
b) 2 mol of NH 3 and 3 mol of O 2
c) 3 mol of NH 3 and 3 mol of O 2
d) 3 mol of NH 3 and 2 mol of O 2
Note: This is not a multiple-choice question. Each row represents a separate question where you need to determine the limiting reactant.
How many g of hydrogen are left over in producing ammonia when 14.0 g of nitrogen is reacted with 8.0 g of hydrogen?
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
How many grams of PCl 3 will be produced if 130.5 g Cl 2 is reacted with 56.4 g P 4 according to the following equation?
6Cl 2 (g) + P 4 (s) → 4PCl 3 (l)
How many grams of sulfur can be obtained if 12.6 g H 2 S is reacted with 14.6 g SO 2 according to the following equation?
2H 2 S(g) + SO 2 (g) → 3S(s) + 2H 2 O(g)
The following equation represents the combustion of octane, C 8 H 18 , a component of gasoline:
2C 8 H 18 (g) + 25O 2 (g) → 16CO 2 (g) + 18H 2 O(g)
Will 356 g of oxygen be enough for the complete combustion of 954 g of octane?
When 140.0 g of AgNO 3 was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected as a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO 3 was added?
AgNO 3 (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq)
Consider the reaction between MnO 2 and HCl:
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
What is the theoretical yield of MnCl 2 in grams when 165 g of MnO 2 is added to a solution containing 94.2 g of HCl?
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 g of a product. What is the percent yield of the product if the theoretical yield was 7.12 g?
When 38.45 g CCl 4 is reacted with an excess of HF, 21.3 g CCl 2 F 2 is obtained. Calculate the theoretical and percent yields of this reaction.
CCl 4 + 2HF → CCl 2 F 2 + 2HCl
Iron(III) oxide reacts with carbon monoxide according to the equation:
Fe 2 O 3 ( s ) + 3CO( g ) → 2Fe( s ) + 3CO 2 ( g )
What is the percent yield of this reaction if 623 g of iron oxide produces 341 g of iron?
Determine the percent yield of the reaction if 77.0 g of CO 2 are formed from burning 2.00 moles of C 5 H 12 in 4.00 moles of O 2 .
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
The percent yield for the following reaction was determined to be 84%:
N 2 ( g ) + 2H 2 ( g ) → N 2 H 4 ( l )
How many grams of hydrazine (N 2 H 4 ) can be produced when 38.36 g of nitrogen reacts with 6.68 g of hydrogen?
Silver metal can be prepared by reducing its nitrate, AgNO 3 with copper according to the following equation:
Cu( s ) + 2AgNO 3 ( aq ) → Cu(NO 3 ) 2 ( aq ) + 2Ag( s )
What is the percent yield of the reaction if 71.5 grams of Ag was obtained from 132.5 grams of AgNO 3 ?
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH 3 ( g ) + 5O 2 ( g ) → 4NO( g ) + 6H 2 O( l )
2NO( g ) + O 2 ( g ) → 2NO 2 ( g )
2NO 2 ( g ) + H 2 O( l ) → HNO 3 ( aq ) + HNO 2 ( aq )
Considering that each reaction has an 85% percent yield, how many grams of NH 3 must be used to produce 25.0 kg of HNO 3 by the above procedure?
Aspirin (acetylsalicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acetic anhydride. The chemical equation for aspirin synthesis is shown below:
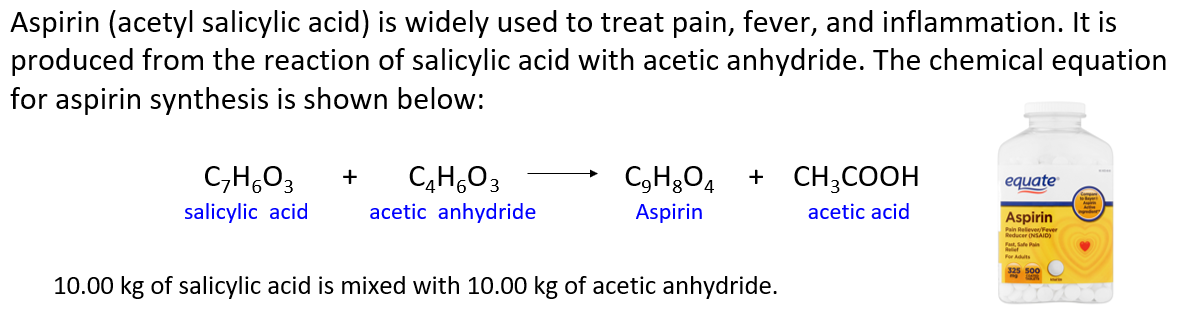
In one container, 10.00 kg of salicylic acid is mixed with 10.00 kg of acetic anhydride.
a) Which reactant is limiting? Which is in excess? b) What mass of excess reactant is left over? c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)? d) What mass of aspirin is formed if the reaction yield is 70.0% ? e) If the actual yield of aspirin is 11.2 kg, what is the percent yield? f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?
3 thoughts on “Stoichiometry Practice Problems”
You forgot the subscript 3 for O in the molecular formula for acetic anhydride and the reaction is not balanced as written. For part F) it’s 18.1 kg and not1.81 kg as written in the final line of the solution.
Thanks for letting me know! Fixed.
You’re welcome!
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4A: Moles & Stoichiometry (Worksheet)
- Last updated
- Save as PDF
- Page ID 81593

- Robert Carter
- University of Massachusetts Boston
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help.
Chemists are concerned with mass relationships in chemical reactions, usually run on a macroscopic scale (grams, kilograms, etc.). To deal with the very large numbers of atoms and molecules in such samples, chemists developed the unit of the mole (abbreviated mol) and a unit of measure called the molar mass , which has units of g/mol. Next to the atomic theory, the mole concept is the most fundamental unifying idea in all chemistry.
Learning Objective
- Understand the relationship between the mole and Avogadro’s number
- Understand the meaning of molar mass of a substance
- Understand how the mole concept is applied to determining empirical formulas from analytical data
- Understand how the mole concept allows prediction of the mass relationships between reactants and products in a chemical reaction
- Understand the concept of the limiting reagent
Success Criteria
- Convert between numbers of atoms, moles, and mass of sample by using Avogadro’s number and the appropriate molar mass
- Calculate the empirical formula of a compound from percent composition data
- Calculate mass relationships between reactants and products, based on a balanced chemical equation
- Be able to determine the limiting reagent in a chemical reaction and calculate mass relationships for a chemical reaction based on it
Avogadro's Number
One of the most important ideas in chemistry is the mole concept. A mole of substance is that amount that contains as many elementary units (atoms, molecules, or formula units, depending on the nature of the substance) as there are atoms in exactly 12 grams of an isotopically pure sample of 12 C.
12 g 12 C (exactly) = mole 12 C atoms
The number of atoms in such a sample defines Avogadro's number (symbol N A ), which has been experimentally determined to be N A = 6.0221367 x 10 23 . For most of our needs, a value of 6.022 x 10 23 will be sufficiently precise. It follows that if we had a mole of atoms of some other element, that sample would weigh its atomic weight in grams. For example, for aluminum (at. wt. = 26.981538 u), a sample weighing 26.981538 g would contain a mole of aluminum atoms; i.e.,
26.981538 g Al = mole Al atoms
The mass in grams of one mole of substance is called its molar mass . For an element or compound that is composed of molecules, the molar mass in grams is numerically equal to its molecular weight in atomic mass units. The molar mass of a molecular substance contains an Avogadro's number of molecules of the substance. For CO 2 (m.w. = 44.01 u),
mole CO 2 = 44.01 g CO 2 = 6.022 x 10 23 CO 2 molecules
Because each CO 2 molecule is composed of one carbon atom and two oxygen atoms, we could say that a mole of CO 2 contains one mole of carbon atoms and two moles of oxygen atoms. In general, it is useful to think of a mole as just an Avogadro’s number of things. In the case of molecular compounds, that number of molecules has a mass in grams that is numerically equal to the substance’s molecular weight.
For a compound described by an empirical formula (e.g., ionic compound, network solid, empirical formula unit of a molecular compound), the molar mass in grams is numerically equal to the formula weight in atomic mass units. The molar mass based on a formula weight contains an Avogadro's number of formula units of the substance. For NaCl (f.w. = 58.44 u),
mol NaCl = 58.44 g NaCl = 6.022 x 10 23 NaCl formula units
Note: Sodium chloride is an ionic compound so there are no molecules!
The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Why doesn’t a mole of carbon weigh 12 g?
The atomic weight of oxygen is 16.00 u. What is the mass of a mole of O2(g)? How many O2 molecules does a mole of O2(g) contain? How many moles of oxygen atoms does a mole of O2(g) contain?
The mole is sometimes described as “the chemist’s dozen”. How is a mole like a dozen?
Consider a 15.00-g sample of CO 2 (m.w. = 44.01 u). How many moles of CO 2 are there in this sample?
How many CO 2 molecules are there in a 15.00-g sample of carbon dioxide?
How many oxygen atoms are there in a 15.00-g sample of carbon dioxide.
Fluorine consists of a single isotope, 19 F, with a mass of 19.00 u. What is the mass in grams of a single fluorine atom?
Converting Analytical Data into Empirical Formulae
The elemental composition of a compound can be determined experimentally by a variety of techniques. The results of chemical analysis are usually expressed in terms of weight percentages of each element in the compound, which can be converted into masses of each element for a given sample. The masses of each element can be used to calculate the numbers of moles of each element, from which the lowest whole number ratios between the moles of elements can be determined. These ratios are the same as the ratios between the numbers of individual atoms of each elements in the empirical formula. The strategy for converting analytical data into an empirical formula generally uses the following steps:
- Convert weight percentages into grams of each element. Often it is helpful to assume a sample size of exactly 100 grams; then the given percentages are numerically equal to the number of grams of each element.
- Convert grams of each element into moles of each element, using atomic weights.
- Find the lowest whole number ratios among the moles of elements. To do this, start by dividing the smallest number of moles into each of the numbers of moles of elements (i.e., set the smallest number to 1). This may yield integers, or it may yield decimal results that correspond closely to rational fractions. For example,\[ 1.25 : 2.75 = 1¼ : 2¾ = 5 : 11 \nonumber \]
- Write the empirical formula, using the same whole-number ratios between atoms of each element as the ratios among moles of elements.
- If the molecular weight is known, divide the formula weight for the empirical formula into the molecular weight to determine the number of formula units in the molecular formula. Using this integer factor, multiply all the subscripts (including any implied 1's) in the empirical formula to obtain the molecular formula.
If you have data for the percent composition of a compound, element by element, do you need to know the size of the sample in order to figure out the empirical formula? Why or why not?
How is the molecular formula of a molecular compound related to its empirical formula?
A compound is found to contain 54.52% C, 9.17% H, and 36.31% O. What is the empirical formula of the compound? If the compound is found to have molecular weight of 88.12 u, what is the molecular formula?
What is the empirical formula of an oxide of nitrogen whose composition is 25.94% nitrogen?
Combustion Analysis
One experimental method for determining the composition of organic compounds is combustion analysis , in which a weighed sample of the compound is burned in excess oxygen. In all cases all the carbon in the compound is converted to CO 2 , and all the hydrogen is converted to H 2 O, which can be separated from each other and weighed. The masses of carbon and hydrogen in the original sample can be calculated from the weights of CO 2 and H 2 O. If the compound also contains oxygen, its amount can be obtained by subtracting the found masses of carbon and hydrogen from the total mass of the sample. These masses can then be converted into moles, from which the empirical formula can be obtained.
A 2.554-g sample of a certain hydrocarbon is burned in excess oxygen, producing 8.635 g CO 2 ( g ) and 1.768 g H 2 O( l ). If the molecular weight of the hydrocarbon is found to be 78.11 u, what is its molecular formula? [m.w. CO 2 = 44.01 u; m.w. H 2 O = 18.02 u]
Stoichiometry and Yields
For the burning (combustion) of propane gas the balanced equation is
\[C_3H_8(g) + 5 O_2(g) \rightarrow 3 CO_2(g) + 4 H_2O(l) \nonumber \]
When we first encountered reaction equations, we thought of this in terms of ratios among reactant and product species; e.g.,
“For every molecule of \(C_3H_8(g)\), five molecules of \(O_2(g)\) are required to produce three molecules of \(CO_2(g)\) and four molecules of \(H_2O(l)\).”
The relationships between individual reactant and product species and between moles of those species is multiplication by the constant Avogadro’s number. Therefore, the ratios among moles of reactants and products are the same as between individual reactant and product species; e.g.,
“For every mole of \(C_3H_8(g)\), five moles of \(O_2(g)\) are required to produce three moles of \(CO_2(g)\) and four moles of \(H_2O(l)\).”
Thus, we can routinely interpret balanced chemical equations in terms of mole relationships. Once we know the numbers of moles, we can use the relationships between moles and molar masses of the various species to calculate masses of reactants and/or products, as needed. These mass relationships, made through moles, are called stoichiometry (Gk stoicheon , element + -metry , measure).
Using mole and mass relationships, we can calculate the mass of product that should be produced from a given amount of reactant when it is completely consumed in the reaction. This calculated amount of product is called the theoretical yield. In running real chemical reactions, it often occurs that less product is obtained than expected, for a variety of reasons. The amount obtained is the actual yield . A comparison of the actual yield to the theoretical yield, expressed as a percentage, is a statement of the percent yield ; i.e.,
\[ \% \, \text{yield} = \dfrac{\text{actual yield}}{\text{theoretical yield}} \times 100\% \nonumber \]
In the complete combustion of propane, how many moles of \(CO_2(g)\) are produced per mole of \(O_2(g)\)?
In the complete combustion of propane, how many moles of \(H_2O(l)\) are produced per mole of \(O_2(g)\)?
In the complete combustion of propane, how many moles of \(H_2O(l)\) are produced per mole of \(CO_2(g)\)?
A 1.638-g sample of propane is burned in excess oxygen. What are the theoretical yields (in grams) of \(CO_2(g)\) and \(H_2O(l)\) expected from the reaction? [m.w. \(C_3H_8\) = 44.09 u, m.w. \(CO_2\) = 44.01 u, m.w. \(H_2O\) = 18.02 u)
If 4.750 g of \(CO_2(g)\) was obtained from the combustion of 1.638 g of propane, what was the percent yield?
Non-Stoichiometric Reactions (Limiting Reagents)
Very often when we run a reaction between two or more substances, the amounts of reactants are not present in precisely the stoichiometric ratio indicated by the balanced chemical equation. In such cases, one reactant may be present in short supply, while other reactants may be present in abundance. Assuming complete reaction, the reactant in shortest supply will be completely consumed, but some amounts of the other reagents will be left over after the reaction is finished. In such cases, the amount of product obtained is limited by the reactant in shortest supply, which is called the limiting reagent . It is important to realize that the limiting reagent is present in shortest supply on the basis of the stoichiometry of the balanced chemical equation in moles; i.e., the mole ratios implied by the balanced equation. In some cases, the limiting reagent may be the substance present with larger absolute amount (either in grams or moles), but used in greater quantity in the balanced equation. In any case, the theoretical yield of product always will be limited by the stoichiometric relationship between the limiting reagent and products. Therefore, in any case where amounts of reactants are specified, determine the moles of each present, and then determine which reactant is the limiting reagent. All calculations of the theoretical yield for the reaction (or any other stoichiometric calculations) must be based on the amount of the limiting reagent, using the stoichiometric relationships in the balanced chemical equation.
How do we know which of two or more reactants is limiting? There are a number of ways to determine this. One of the most efficient is to see the amounts of each reagent in terms stoichiometric units, or what we might call “sets”(for want of a better term). For example, suppose we were building toy wagons and had 24 wheels and 15 wagon bodies, We would take the wheels in sets of four and the bodies in sets of one to build each wagon. Therefore we have 24/4 = 6 sets of wheels and 15/1 = 15 sets of bodies. As we assemble wagons the wheels will run out (the “limiting reagent”) before the bodies. Based on the wheels as the “limiting reagent” and their “stoichiometric” relationship to completed wagons (4 wheels/wagon), we could make only six wagons. In doing this, we would use six bodies, and we would have 15 - 6 = 9 bodies left over. Applying this approach to chemical reactions, if we take the number of moles of each reagent and divide that by its stoichiometric coefficient in the balanced equation, we will have a number for each that represents its number of reaction “sets”. The reagent that has the smallest number by this calculation is the limiting reagent; any other reagent is an excess reagent. We then use the number of moles of the limiting reagent (not its calculated number of “sets”) as the basis for all our further calculations, such as theoretical yield or amount of non-limiting reagent used. In short, all calculations are based on the moles of the limiting reagent and the stoichiometric relationships implied by the balanced chemical equation.
Define what is meant by the terms limiting reagent and excess reagent.
In the reaction \(2 A + 3 B \rightarrow products\), if you have 0.500 mol A and 0.500 mol B, which is the limiting reagent? How much of the excess reagent will be left over, if compete reaction takes place?
What is the theoretical yield of \(Ca_3(PO_4)_2(s)\) by the reaction
\[\ce{3 Ca(OH)2(s) + 2 H3PO4(l) \rightarrow Ca3(PO4)2(s) + 3 H2O(l)} \nonumber \]
when 10.00 g \(\ce{Ca(OH)2}\) and 10.00 g \(\ce{H3PO4}\) are mixed? [f.w. \(\ce{Ca(OH)2}\) = 74.10 u; m.w. \(\ce{H3PO4}\) = 97.99 u; f.w. \(\ce{Ca3(PO4)2}\) = 310.18 u]

COMMENTS
Solution Stoichiometry Worksheet. Solve the following solutions Stoichiometry problems: 1. How many grams of silver chromate will precipitate when 150. mL of 0.500 M silver nitrate are added to 100. mL of 0.400 M potassium chromate? 2 AgNO3(aq) + K2CrO4(aq) Ag2CrO4(s) + 2 KNO3(aq) 0.150 L AgNO3. 0.500 moles AgNO3.
How many moles of water are produced when 57 moles of nitrogen are made? 3. Calculate the mass of aluminum oxide produced when 3.75 moles of aluminum burn in oxygen. Answers: 1A. 30 mol Ag 1B. 30 mol AgNO3. 1C. 20 mol H2O 1D. 10 mol NO. 2A. 38 mol N2H4 2B. 19 mol N2O4. 2C. 76 mol H2O.
The molar concentration can also be expressed as the following: 1.00MNaCl = 1.00 mol NaCl 1L NaCl solution. and. 1.50MPb(NO 3) 2 = 1.50 mol Pb(NO 3) 2 1 LPb(NO 3) 2solution. First, we must examine the reaction stoichiometry in the balanced reaction (Equation 13.8.1 ). In this reaction, one mole of Pb(NO 3) 2 reacts with two moles of NaCl to ...
Stoichiometry with Solutions Name _____ 1. H3PO4 + 3 NaOH --> Na3PO4 + 3 H2O How much 0.20 M H3PO4 is needed to react with 100 ml. of 0.10 M NaOH? 2. 2 HCl + Zn --> ZnCl2 + H2 When you use 25 ml. of 4.0 M HCl to produce H2 gas, how many grams of zinc does it react with? What volume of H2 gas is produced at STP? 3.
6/22/2017 B . Solution Stoichiometry . Name_____ CHEMISTRY 110 . last first . 1] How many grams of calcium phosphate can be produced from the reaction of 2.50 L of 0.250 M Calcium chloride with and excess of phosphoric acid?
Q4. Given the following reaction: H2SO4 + Na2CO3 → Na2SO4 +H2O + CO2 H 2 S O 4 + N a 2 C O 3 → N a 2 S O 4 + H 2 O + C O 2. Calculate the molarity of the H2SO4 H 2 S O 4 solution if it takes 40.0 mL of H2SO4 H 2 S O 4 to neutralize 46.7 mL of a 0.364 M Na2CO3 N a 2 C O 3 solution.
Additional resources for Solution Stoichiometry PRACTICE PROBLEMS AND ACTIVITIES (82) When 75.0 mL of a 0.100 M lead(II) nitrate solution is mixed with 100.0 mL of a 0.190 M potassium iodide solu-...
Solution. First we need to determine the number of moles of Na 2 C 2 O 4 that reacted. We will convert the volume to liters and then use the concentration of the solution as a conversion factor: 9.04 mL × 1 L 1000mL × 0.1074molNa2C2O4 L = 0.000971molNa2C2O4 9.04 m L × 1 L 1000 m L × 0.1074 m o l N a 2 C 2 O 4 L = 0.000971 m o l N a 2 C 2 O 4.
Solution Stoichiometry Name ________________ Chem Worksheet 15-6. The molarity of a solution is a ratio of the moles of solute per liters of solution. The units for molarity are written as mol/L or M. This measurement is used to perform stoichiometric calculations. The strategy used for. solving these solution stoichiometry problems is to set ...
Name: Stoichiometry WorkSheet #1: Worked Solutions. Answer the following questions on your own paper. Show all work. Circle the final. answer, giving units and the correct number of significant figures. 1. Based on the following equation, how many moles of each product are produced when 5.9 moles of Zn(OH)2 are reacted with H3PO4?
7 PRACTICE PROBLEM. Consider the following solutions that are combined in the following steps: Step 1: Initial solution contains 200.0 mL of 0.150 M Ca (OH) 2. Step 2: 100.0 mL of 0.150 M AgClO 4 is added to the solution. Step 3: 100.0 mL of 0.150 M H 2 CO 3 is added to the solution.
Worksheet. The Atom (Simplified) 9m. Subatomic Particles (Simplified) 12m. Isotopes. 17m. Ions (Simplified) 22m. Atomic Mass (Simplified) 17m. ... Solution Stoichiometry deals with stoichiometric calculations in solutions that involve volume and molarity. Solution Stoichiometry. 1. concept. Solution Stoichiometry.
Scanned Document. Solution Stoichiometry Name Chem Worksheet 15-6 The molarity of a solution is a ratio of the moles of solute per liters of solution. The units for molarity are written as mol/L or M. This measurement is used to perform stoichiometric calculations. The strategy used for solving these solution stoichiometry problems is to set up ...
Worksheet 4.7: Solutions Solution stoichiometry Page 1 © Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2008. This page from the ...
Solution Stoichiometry Worksheet. Solve the following solutions Stoichiometry problems: 1. How many grams of silver chromate will precipitate when 150. mL of 0.500 M silver nitrate are added to 100. mL of 0.400 M potassium chromate? 2 AgNO3(aq) + K2CrO4(aq) Î Ag2CrO4(s) + 2 KNO3(aq) 2.
Types of chemical reactions. Oxidation-reduction (redox) reactions. Worked example: Using oxidation numbers to identify oxidation and reduction. Balancing redox equations. Dissolution and precipitation. Precipitation reactions. Double replacement reactions. Single replacement reactions. Molecular, complete ionic, and net ionic equations.
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support ...
Worksheet. Stoichiometry (using solutions) 1. Given the following reaction: (hint: balance the equation first) H2SO4 + NaOH g Na2SO4 + H2O. If 43.2 mL of 0.236 M NaOH reacts with 36.7 mL of H2SO4, what is the concentration of the H2SO4 solution? answer. 2. Given the following equation: NaOH + HCl g H2O + NaCl.
Chemistry: Stoichiometry - Problem Sheet 2 Directions: Solve each of the following problems. Show your work, including proper units, to earn full credit. 1. ___ CaCl 2 + ___ AgNO 3 ___ Ca(NO 3) 2 + ___ AgCl How many grams of silver chloride are produced when 45 g of calcium chloride react with excess silver nitrate? 116 g AgCl 1 mol AgCl
A 0.5895-g sample of impure magnesium hydroxide is dissolved in 100.0 mL of 0.2050 M HCl solution. The excess acid then needs 19.85 mL of 0.1020 M NaOH for neutralization. Calculate the percentage by mass of magnesium hydroxide in the sample, assuming that it is the only substance reacting with the HCl solution. 1124.
Stoichiometry Practice Problems. This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts. The links to the corresponding topics are given below. The Mole and Molar Mass.
NAME: Question. Answer. Determine the amount (in mol) of barium sulfate that will be precipitated when 200.0 cm3 of 0.450 mol dm-3 barium nitrate solution is added to an excess of sodium sulfate solution, given that the equation for the reaction is: Ba(NO3)2(aq) + Na2SO4(aq) BaSO4(s) + 2NaNO3(aq) 2. Liquid hydrazine reacts explosively with ...
This page titled 4A: Moles & Stoichiometry (Worksheet) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Robert Carter. Chemists are concerned with mass relationships in chemical reactions, usually run on a macroscopic scale (grams, kilograms, etc.).