Loading metrics
Open Access
Community Page
The Community Page is a forum for organizations and societies to highlight their efforts to enhance the dissemination and value of scientific knowledge.
See all article types »

Open Labware: 3-D Printing Your Own Lab Equipment
* E-mail: [email protected]
Affiliations Werner Reichardt Centre for Integrative Neuroscience (CIN), University of Tübingen, Tübingen, Germany, Bernstein Centre for Computational Neuroscience, University of Tübingen, Tübingen, Germany, Institute for Ophthalmic Research, University of Tübingen, Tübingen, Germany, TReND in Africa gUG, Tübingen, Germany
Affiliations Werner Reichardt Centre for Integrative Neuroscience (CIN), University of Tübingen, Tübingen, Germany, Bernstein Centre for Computational Neuroscience, University of Tübingen, Tübingen, Germany, TReND in Africa gUG, Tübingen, Germany, Hertie Institute for Clinical Brain research, University of Tübingen, Tübingen, Germany, Graduate School of Neural and Behavioural Sciences, International Max Planck Research School, University of Tübingen, Germany
Affiliation Backyard Brains, Ann Arbor, Michigan, United States of America
Affiliations TReND in Africa gUG, Tübingen, Germany, Centre for Integrative Genomics, University of Lausanne, Lausanne, Switzerland
Affiliations Werner Reichardt Centre for Integrative Neuroscience (CIN), University of Tübingen, Tübingen, Germany, Bernstein Centre for Computational Neuroscience, University of Tübingen, Tübingen, Germany, Institute for Ophthalmic Research, University of Tübingen, Tübingen, Germany
- Tom Baden,
- Andre Maia Chagas,
- Greg Gage,
- Timothy Marzullo,
- Lucia L. Prieto-Godino,
- Thomas Euler

Published: March 20, 2015
- https://doi.org/10.1371/journal.pbio.1002086
- Reader Comments
21 May 2015: Baden T, Chagas AM, Gage GJ, Marzullo TC, Prieto-Godino LL, et al. (2015) Correction: Open Labware: 3-D Printing Your Own Lab Equipment. PLOS Biology 13(5): e1002175. https://doi.org/10.1371/journal.pbio.1002175 View correction
The introduction of affordable, consumer-oriented 3-D printers is a milestone in the current “maker movement,” which has been heralded as the next industrial revolution. Combined with free and open sharing of detailed design blueprints and accessible development tools, rapid prototypes of complex products can now be assembled in one’s own garage—a game-changer reminiscent of the early days of personal computing. At the same time, 3-D printing has also allowed the scientific and engineering community to build the “little things” that help a lab get up and running much faster and easier than ever before.
Citation: Baden T, Chagas AM, Gage G, Marzullo T, Prieto-Godino LL, Euler T (2015) Open Labware: 3-D Printing Your Own Lab Equipment. PLoS Biol 13(3): e1002086. https://doi.org/10.1371/journal.pbio.1002086
Copyright: © 2015 Baden et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (Werner Reichardt Centre for Integrative Neuroscience Tübingen, EXC 307 to TE and TB; BA 5283/1-1 to TB) and the U.S. National Institutes of Mental Health Small Business Innovation Research grant #R44 MH093334 to GG and TM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: Authors GG and TM are founders of Backyard Brains ( www.backyardbrains.com ), a company specializing in the design and distribution of Open Labware.
Abbreviations:: ABS, Acrylnitrile butadiene styrene; CNC, computer numerical control; DIY, Do It Yourself; EMG, Electromyogram; ERG, Electroretinogram; FOSSFA, Free Software and Open Source Foundation for Africa; GNU, “GNU is not Unix” (recursive acronym); GPIO, general-purpose input-output; IT, information technology; LED, Light Emitting Diode; NIH, National Institutes of Health; PCB, Printed Circuit Board; PLA, Polylactic acid; TReND, Teaching and Research in Natural Sciences for Development (in Africa); UV, Ultraviolet
Applications of 3-D printing technologies ( Fig. 1A , Box 1 ) have become as diverse as the types of materials that can be used for printing. Replacement parts at the International Space Station may be printed in orbit from durable plastics or metals, while back on Earth the food industry is starting to explore the same basic technology to fold strings of chocolate into custom-shaped confectionary. Also, consumer-oriented laser-cutting technology makes it very easy to cut raw materials such as sheets of plywood, acrylic, or aluminum into complex shapes within seconds. The range of possibilities comes to light when those mechanical parts are combined with off-the-shelf electronics, low-cost microcontrollers like Arduino boards [ 1 ], and single-board computers such as a Beagleboard [ 2 ] or a Raspberry Pi [ 3 ]. After an initial investment of typically less than a thousand dollars (e.g., to set-up a 3-D printer), the only other materials needed to build virtually anything include a few hundred grams of plastic (approximately US$30/kg), cables, and basic electronic components [ 4 , 5 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
A 1 , Components for laboratory tools, such as the base for a micromanipulator [ 18 ] shown here, can be rapidly prototyped using 3-D printing. A 2 , The printed parts can be easily combined with an off-the-shelf continuous rotation servo-motor (bottom) to motorize the main axis. B 1 , A 3-D printable micropipette [ 8 ], designed in OpenSCAD [ 19 ], shown in full (left) and cross-section (right). B 2 , The pipette consists of the printed parts (blue), two biro fillings with the spring, an off-the-shelf piece of tubing to fit the tip, and one screw used as a spacer. B 3 , Assembly is complete with a laboratory glove or balloon spanned between the two main printed parts and sealed with tape to create an airtight bottom chamber continuous with the pipette tip. Accuracy is ±2–10 μl depending on printer precision, and total capacity of the system is easily adjusted using two variables listed in the source code, or accessed via the “Customizer” plugin on the thingiverse link [ 8 ]. See also the first table.
https://doi.org/10.1371/journal.pbio.1002086.g001
Box 1. Glossary
Open source.
A collective license that defines terms of free availability and redistribution of published source material. Terms include free and unrestricted distribution, as well as full access to source code/blueprints/circuit board designs and derived works. For details, see http://opensource.org .
Maker movement
Technology-oriented extension of the traditional “Do-it-Yourself (DIY)” movement, typically denoting specific pursuits in electronics, CNC (computer numerical control) tools such as mills and laser cutters, as well as 3-D printing and related technologies.
3-D printing
Technology to generate three-dimensional objects from raw materials based on computer models. Most consumer-oriented 3-D printers print in plastic by locally melting a strand of raw material at the tip (“hot-end”) and “drawing” a 3-D object in layers. Plastic materials include Acrylnitrile butadiene styrene (ABS) and Polylactic acid (PLA). Many variations of 3-D printers exist, including those based on laser-polymerization or fusion of resins or powdered raw materials (e.g., metal or ceramic printers).
Arduino boards
Inexpensive and consumer-oriented microcontroller boards built around simple processors. These boards offer a variety of interfaces (serial ports, I2C and CAN bus, etc.), μs-timers, and multiple general-purpose input-output (GPIO) pins suitable for running simple, time-precise programs to control custom-built electronics.
Single board computers
Inexpensive single-board computers capable of running a mature operating system with graphical-user interface, such as Linux. Like microcontroller boards, they offer a variety of hardware interfaces and GPIO pins to control custom-built electronics.
It therefore comes as no surprise that these technologies are also routinely used by research scientists and, especially, educators aiming to customize existing lab equipment or even build sophisticated lab equipment from scratch for a mere fraction of what commercial alternatives cost [ 6 ]. Designs for such “Open Labware” include simple mechanical adaptors [ 7 ], micropipettes ( Fig. 1B ) [ 8 ], and an egg-whisk–based centrifuge [ 9 ] as well as more sophisticated equipment such as an extracellular amplifier for neurophysiological experiments [ 10 ], a thermocycler for PCR [ 11 ], or a two-photon microscope [ 12 ]. At the same time, conceptually related approaches are also being pursued in chemistry [ 13 – 15 ] and material sciences [ 16 , 17 ]. See also Table 1 .
https://doi.org/10.1371/journal.pbio.1002086.t001
A Culture of Sharing
Most makers share their designs under an open source license together with detailed assembly instructions in online repositories [ 20 – 22 ] such as the National Institutes of Health (NIH) 3-D print exchange [ 23 ] or in peer-reviewed journals [ 10 , 17 , 24 – 29 ]. As a result, anyone can freely use and modify them. This open culture, which has transformed the world of software engineering over the past decades, offers several advantages over traditional product design. First, designs are not only free, but are directly shaped by people who will actually use the final product. Second, building your own experimental equipment yields a much deeper understanding of the principles underlying its design and a better awareness of its limits. Third, manufacturing is immediate and local—thus empowering laboratories and schools located in difficult-to-reach places. Fourth, the open source movement is a global phenomenon, connecting people worldwide, often to recruit talented builders and coders from outside the traditional scientific establishment. Still, there are drawbacks. For example, a commercial solution may be preferred if the time and cost of in-house development exceeds the benefits of control and instrument knowledge. Nevertheless, and perhaps counter-intuitively, some open designs are published by commercial companies that offer the parts as well as the assembled product with technical support for a fee, while maintaining the source material online under an open source license. Consumers can more freely balance cost against time to build, while companies gain in customer relations and product feedback.
Quality Control and the Evolution of Open Labware Designs
The flagship of the open source movement, the operation system GNU/Linux [ 30 ], is perhaps the best example for the potential of open designs. First available in the 1990s, today it is one of the most widely used system on supercomputers, servers, and mobile phones (Android) and increases its share of home users every year. Critically, Linux is fully open source—anyone can freely access its entire source code, change it, and distribute modifications. Its success flows from the idea that “given enough eyeballs all bugs are shallow” [ 31 ]. In other words, a distributed network of collaborators helps to identify and rectify bugs in the system as they arise. Open Labware works the same way: release of a design sparks feedback and refinements from the community. Although Open Labware’s contributor base is still relatively small, feedback can nonetheless markedly improve product design and expand potential applications over time. For example, free online sharing of the designs for a manually controlled 3-D printed micromanipulator ( Fig. 2A ) [ 18 ] provided the starting point for a high-precision motorized version ( Fig. 2B ) [ 32 ]. Subsequently, and in combination with open designs for smartphone lens-adapters offering portable options for field-microscopy [ 33 ], both designs led to the “Raspberry Pi-scope” [ 34 ]—a self-standing histology microscope based on a Raspberry Pi, equipped with a high-resolution camera module, a low-cost acrylic lens, and the manipulator body to accurately position the sample ( Fig. 2C ). One next step in this evolution may be to add low-cost fluorescence capability through the addition of a ultraviolet light-emitting diode (UV-LED) and the appropriate filters, perhaps inspired by materials used in the less than US$1 fluorescence-capable Foldscope [ 24 ]. Without free-and-open sharing of the complete source materials at all stages of development, this evolution would not have been possible. With educators and researchers increasingly integrating Open Labware approaches into their projects, designs are expected to continuously improve and diversify.
A , A 3-D printable micromanipulator with a slanted Z-axis [ 18 ], here shown amidst commercial alternatives, initially served as the basis for a motorized version with “real” Z axis [ 32 ] (B). B 1,2, the three axes are driven by continuous-rotation micro-servos, controlled by an Arduino fitted with a Joystick-shield and a 9V battery. B 3 , The motorized manipulator offers sufficient precision to target individual hairs on the head of a fruitfly (±5–20 μm during movements, depending on printer precision; <1 μm drift min -1 when stationary). Scale bar 1 mm. C 1 , The same manipulator build was then converted into a microscope-stage to permit accurate placement and focus of histology samples [ 34 ]. The optics are provided by an off-the-shelf, low-power acrylic lens positioned directly above a Raspberry Pi camera module [ 3 ]. C 2 , Image taken with the microscope, showing a slice of mouse brain (hippocampus) stained for cytochrome oxidase C. Scale bar 500 μm.
https://doi.org/10.1371/journal.pbio.1002086.g002
Online Resources for Learning and Problem Solving
Although most Open Labware designs are published with detailed assembly instructions aimed at the non-specialist, a basic understanding of concepts in physics, electronics, and computer programming is certainly helpful. Luckily, freely available online resources facilitate self-learning ( Table 2 ). In addition, many makers hone their skills directly at the workbench, simply by attempting to recreate or modify existing designs. Expert community help can be rapidly found in online forums such as Stack Overflow [ 35 ], returning to the idea that many eyes lead to rapid problem-solving. In addition, online aggregators gather and summarize information on specific topics, serving as a hub for both information seekers and distributors. Some of them are curated in a centralized manner [ 36 ], while others follow the distributed wiki principle [ 37 ].
https://doi.org/10.1371/journal.pbio.1002086.t002
Application in a Resource-Challenged Context
If a commercial alternative is available, it is usually the compromise between time and money spent that determines whether to build or purchase equipment. While Open Labware designs may benefit any research or education setting [ 38 ], one obvious foothold for their possibilities lies in economically deprived schools and universities in the developing world. Here, the introduction of Open Labware may make the crucial difference between having some usable equipment to work with and having none at all. Several benefits of an open model come to light: low cost, local manufacture, and the possibility to customize according to local demands or availability of parts. In sub-Saharan Africa, only a few bases and organizations that offer training in “maker” skills or general promotion of open source principles operate. These include the Free Software and Open Source Foundation for Africa (FOSSFA [ 39 ]), Fundi bots [ 40 ], as well as a few physical spaces such as FabLabs [ 41 ] and Maker- [ 42 ] and Hackerspaces [ 43 ] scattered mostly around the major cities. In addition, companies selling easily transportable 3-D printers and supplies are rapidly establishing their foothold on the African continent, such that local sourcing of the required hardware is no longer an insurmountable obstacle. However, on the whole, Open Labware possibilities remain poorly established in this context, as attested in a survey taken by 89 biomedical researchers from 12 sub-Saharan African countries in August 2014 ( Fig. 3 ). When asked about their software competency, most respondents indicated that while they are comfortable with “basic” IT usage (defined as using office packages or navigating the web), few were routine users of standard open analysis packages such as R [ 44 ], octave [ 45 ], or other Python [ 46 ] based packages, and programming skills were even less prevalent ( Fig. 3A ). Awareness and competency in the use of open hardware approaches were even less developed: over 90% of respondents (83/89) had never used 3-D printing or any form of single-board computers or microcontrollers ( Fig. 3B ).
An online survey was taken by 89 biomedical researchers (MSc. to Professor) at universities in 12 different sub-Saharan African countries in August 2014. Researchers rated their own competency and awareness in aspects of software and hardware usage. A , Software competency rated on a scale of 1 (low) to 10 (high) in “basic usage such as navigating office software or the internet,” “usage of open analysis packages such as R [ 44 ], octave [ 45 ], or similar,” and “programming, e.g., using C++, python, or any other mainstream language.” B , Hardware awareness for possibilities in “3-D printing” (top) and “single board computers/microcontrollers such as Raspberry Pi, Arduino, Beagleboard, or similar” rated in four categories: (i) “I have never heard of this,” (ii) “I have heard of it but I have no access,” (iii) “I have tried using this at least once,” (iv) “I am a competent/routine user.”
https://doi.org/10.1371/journal.pbio.1002086.g003
As part of ongoing efforts to promote scientific education and research in the developing world, we introduced the many possibilities of Open Labware to schools and universities in sub-Saharan Africa (TReND in Africa [ 47 ]) and Latin America (Backyard Brains [ 48 ]). Different formats were used, ranging from three-week intensive neuroscience summer schools aimed at university graduates in Uganda and Tanzania (four events), to multiday MakerSpace workshops aimed at school students and their teachers in Chile and Mexico (12 events), to outreach events and lectures held at scientific conferences, schools, and public spaces on four continents (more than 100 events). Here, we present some experiences and success stories from this work.
In-Depth Exposure during Multiday Training Events
The longer events, such as the three-week neuroscience summer schools in Africa or the multiday MakerSpace workshops in Latin America, afforded participants the opportunity to get hands-on experience using, assembling, and contributing to the development of Open Labware designs. One popular activity was to assemble existing designs (such as a spikerbox, Fig. 4A [ 10 , 49 ]) from off-the-shelf parts. This required participants to study the circuit diagram, note the identity and polarity of simple electronic components such as chips and capacitors and to solder them in place on the printed circuit board (PCB) ( Fig. 4B ). The assembled amplifiers were subsequently used to perform classic neurophysiological experiments such as recording of action potentials from the locust [ 50 ] or cricket [ 51 , 52 ] extensor tibiae ( Fig. 4C ). Although initially daunting especially for the many participants without previous contact with any form of electrical engineering, the experience tended to be very motivating across ages and cultures. In particular, it contributed to take away the fear of experimenting with simple electronic circuits or opening up and attempting to modify or repair existing electronic tools in their daily environment. The low cost of required parts also allowed participants to build their own gear, rather than that of their school or university, making them more invested in its success and maintenance. Critically, building equipment from scratch often resulted in a high level of mechanistic understanding and a curiosity to “play” with the finished product to see if it can be improved or modified to better suit a particular purpose. For example, one undergraduate student linked an electromyogram (EMG)-amplifier to an off-the-shelf “robotic limb” [ 53 ] via an Arduino microcontroller [ 1 ] to remotely control grasping movements by contracting their forearm muscles ( Fig. 4D ). Clearly, introduction of low-cost and open source electronics and mechanical parts has the potential to open up vast possibilities to resourceful people anywhere in the world, independent of financial means or educational background.
A , Each student assembled a spikerbox [ 10 , 49 ], an amplifier for neurophysiological experiments, from its off-the-shelf components. B , Students at a workshop in Dar es Salaam, Tanzania. C , The assembled amplifiers were subsequently used to perform simple neurophysiological experiments [ 51 ]. Image credit for panels B and C: Horst Schneider. D , One student set up a spikerbox via an Arduino to trigger closure of a “gripper-hand,” a low-cost robotic limb, through contractions of their forearm muscles.
https://doi.org/10.1371/journal.pbio.1002086.g004
During the three-week neuroscience summer schools in Uganda and Tanzania [ 54 ] we introduced participants to 3-D printed lab tools such as pipettes [ 8 ], manipulators [ 18 , 32 ], and microscope adapters [ 55 ] and compared the usefulness of self-built versus commercially available solutions for different types of experiments. For example, although printed pipettes [ 8 ] offer lower precision than commercially available ones (±2–10 μl, depending on printer precision) they are nonetheless adequate for many “low precision” tasks such as distributing diluted antibody solutions to different samples or applying mounting media for immunohistochemistry. Similarly, we used different versions of 3-D printed micromanipulators [ 18 , 32 ] to position reference electrodes during neurophysiological experiments, e.g., for Calliphora tangential cell recordings [ 56 ], but maintained the recording tungsten electrode on a more stable commercial manipulator. For some types of recordings, such as Drosophila electroretinograms (ERGs) [ 57 ], the printed manipulators were adequate to hold both electrodes, and the magnification gained by attaching a low-power acrylic lens to a webcam or smartphone easily sufficed for their accurate placement. We also provided access to pre-installed Raspberry Pi computers [ 3 ] running a range of open analysis and office software packages and provided basic training in their use. Hands-on exposure allowed students to judge for themselves which designs would be useful in their own research and teaching activities. In a subsequent survey, students rated the usefulness of every open design described above as at least nine out of ten on average ( n = 33). We believe that the introduction of Open Labware possibilities to the African university system may be a highly effective measure towards fostering excellence in research and education on the continent.
Motivating More High School Graduates to Pursue a Career in Science
One pervasive issue in building a sustainable research infrastructure and scientific culture in resource-challenged countries is a perceived limit on career choices afforded by higher education. Traditionally, three disciplines—medicine, civil engineering, or law—are considered the best choices for reliable income, with few individuals enrolling in a natural science subject and fewer still ending up in active research [ 58 , 59 ]. To encourage students to consider a career in science, we (TReND and Backyard Brains) have participated in more than a hundred science outreach events around (Neuro)science, engaging more than 10,000 students, parents, and teachers. In several science camps (“ChileVA! [ 60 ]”), we gave two-hour lectures to high school students about the brain and how to record from neurons and muscles. The students not only “tolerated” this unusually long-format science lecture without breaks, but swarmed the demonstration booth afterwards. Clearly, low-cost and portable equipment offers the possibility to perform science demonstrations anywhere, independent of local infrastructure.
In TReND’s African activities, student-alumni of our summer workshops have taken over local science outreach by organizing into regional teams to visit schools and universities in their respective home countries [ 61 ]. Many of these young scientists come from similar backgrounds as the students they engage, allowing them to act as powerful role models. Thus, students attending a school that cannot afford the equipment or infrastructure to perform live experiments during science classes are exposed to local scientists with a similar background, handling equipment they know how to build from affordable, local resources. The experience can be very powerful and inspire them to pursue a similar career, as well as offering local teachers ideas to use in future science classes. This approach of “teaching the teachers” also means that the impact of educating a few students at a high level has the potential to trickle-down and achieve a wide impact in the long term.
Policy Recommendations
Clearly, the use and design of Open Labware designs can be a powerful ingredient to foster scientific research, education, and public science engagement. Their evolution spans several disciplines, from computer sciences and mechanical engineering to electronics and biology—thus connecting experts and the wider public across fields and sparking creativity in people of all ages. Their low cost, adaptability and robustness renders designs suitable for a broad range of applications in both teaching and research. Below we present some suggestions for policy implementations to optimize available possibilities.
- Integrate more aspects of design and use of Open Labware into traditional science curricula, ideally at an early age.
- Establish more hands-on training courses in basic hardware design and programming skills for established scientists and educators.
- Establish infrastructural support to afford more students and educators easy and direct access to 3-D printing and related technologies. With 3-D printers starting at a few hundred dollars and their price steadily falling, schools and university departments should not be barred from investing in their own model because of economic reasons.
- Provide incentives for companies to invest in an open model of product design. This move promises to spark a new generation of open companies in more direct dialogue with the end user, towards better, individually tailored, and more affordable product design.
Supporting Information
S1 data. thingiverse files..
Container with all files mentioned in the article that are currently hosted on thingiverse ( www.thingiverse.com ).
https://doi.org/10.1371/journal.pbio.1002086.s001
Acknowledgments
We thank the students and voluntary instructors of the many lectures and workshops mentioned in the manuscript, as well as the TReND outreach team, headed by Mahmoud Bukar Maina and Yunusa Mohammed Garba, for their ongoing efforts in promoting science education in Africa. We thank all sponsors of workshops, most notably including the International Brain Research Organization (IBRO), The Company of Biologists, and the Cambridge Alborada Fund.
- 1. Arduino SA (2015) Arduino. http://www.arduino.cc/ .
- 2. BeagleBoard.org Foundation (2014) Beagleboards. http://beagleboard.org/ .
- 3. Raspberry Pi Foundation (2015) Raspberry Pi. http://www.raspberrypi.org/ .
- 4. Anderson C (2012) Makers: The New Industrial Revolution. New York: Crown Business.
- 5. Levy S (2010) Hackers: Heroes of the Computer Revolution. 25th ed. O’Reilly Media.
- 6. Pearce JM (2013) Open-Source lab. 1st ed. Elsevier.
- View Article
- PubMed/NCBI
- Google Scholar
- 8. Baden T (2014) Biropette: customisable, high precision pipette. http://www.thingiverse.com/thing:255519 .
- 11. Chai Biotechnologies Inc (2014) OpenPCR. http://openpcr.org/ .
- 16. Pearce JM (2013) A Low-Cost Open-Source Metal 3-D Printer. Academia.edu. https://www.academia.edu/5327317/A_Low-Cost_Open-Source_Metal_3-D_Printer .
- 18. Marzullo T (2013) Searcher—3-D printable micromanipulator. https://backyardbrains.com/products/micromanipulator .
- 19. Kintel M (2014) OpenSCAD. http://www.openscad.org/ .
- 20. MakerBot (2015) Thingiverse. http://www.thingiverse.com/ .
- 21. Autodesk, Inc. (2014) Instructables. http://www.instructables.com/ .
- 22. GitHub Inc (2015) GitHub. https://github.com/ .
- 23. National Institutes of Health (2015) NIH 3-D print exchange. http://3-Dprint.nih.gov/ .
- 27. Baker E (2014) Open source data logger for low-cost environmental monitoring. Biodivers data J: e1059.
- 30. XenoForo Ltd (2013) Linux. http://www.linux.org/ .
- 31. Raymond ES (1999) The Cathedral & the Bazaar: Musings on Linux and Open Source by an Accidental Revolutionary. O’Reilly Media.
- 32. Baden T (2014) Parametric 3 axis manipulator—optional servo mounts. http://www.thingiverse.com/thing:239105 .
- 33. Yoshinok. $10 Smartphone to digital microscope conversion. http://www.instructables.com/id/10-Smartphone-to-digital-microscope-conversion/ .
- 34. Baden T (2014) RPi-microscope for histology with focus-drive and XY stage. http://www.thingiverse.com/thing:385308 .
- 35. Stack Exchange Inc (2015) Stack Overflow. http://stackoverflow.com/ .
- 36. Chagas AM (2014) Openeuroscience. http://openeuroscience.wordpress.com/ .
- 37. Appropedia. http://www.appropedia.org/Welcome_to_Appropedia .
- 39. Free Software and Open Source Foundation for Africa (FOSSFA). http://www.fossfa.net/ .
- 40. Fundi Bots (2014) Fundi bots. http://fundibots.org/ .
- 41. Fab Foundation (2014) Fab Labs. http://www.fabfoundation.org/fab-labs/ .
- 42. Google. The Maker Map. http://themakermap.com/ .
- 43. (2013) Hackerspaces. http://hackerspaces.org/wiki/ .
- 44. Institute for Statistics and Mathematics of WU (Wirtschaftsuniversität Wien). R-project. http://www.r-project.org/ .
- 45. Eaton JW (2013) GNU-Octave. https://www.gnu.org/software/octave/ .
- 47. Prieto Godino LL, Yusuf S, Baden T (2014) Teaching and Research in Natural Sciences for Development (TReND) in Africa. www.TReNDinAfrica.org .
- 48. Gage G, Marzullo T (2013) Backyard Brains. www.backyardbrains.com .
- 49. Backyard Brains. (2013) The Electromyogram (EMG) SpikerBox. https://backyardbrains.com/products/emgspikerbox .
- 53. SparkFun Electronics. Robotic Claw. https://www.sparkfun.com/products/11524 .
- 54. Prieto Godino LL, Yusuf S, Baden T (2014) TReND in Africa Neuroscience Summer Schools. http://trendinafrica.org/activities/education/neuroscience-schools/ .
- 55. MakerBot Industries (2015) Universal Smartphone adapter for microscope. http://www.thingiverse.com/thing:78071 .
- 60. Chile VA! Innovacion. http://www.innovacion.gob.cl/etiqueta/chile-va/ .
- 61. Maina MB, Garba YM (2013) TReND in Africa—Science Outreach. http://trendinafrica.org/activities/outreach/ .
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Roger Ware Brockett, 84

Providing community support

A change of mind, heart, and soul
A selection of favorite or essential tools used by Harvard scientists.
Photos by Harvard Staff Photographers
Quick, hand me my worm pick
Juan Siliezar
Harvard Staff Writer
Scientists describe cherished tools of the trade
Scientists use a multitude of tools, from advanced quantum machines to chalk and blackboard, to investigate and solve their inquiries. Their equipment varies depending on their subjects, but also from scientist to scientist. The Gazette asked several Harvard researchers for their most treasured or essential pieces of lab, field, or office equipment. The answers range from highly technical to downright quirky.
Photos courtesy of Munazza Alam
Munazza Alam, A.M. ’18, Ph.D. ’21
Graduate researcher, Department of Astronomy Favorite item: Women of NASA Lego set
An astronomer who studies the atmospheres of exoplanets, Alam writes computer programs to process and analyze observations from instruments on NASA’s Hubble Space Telescope. While she’s waiting on these programs, she toys with a piece of office decor: Women of NASA Legos, which feature famed astronauts Sally Ride and Mae Jemison along with computer scientist Margaret Hamilton and astronomer Nancy Grace Roman. “They’re a reminder of the trailblazing women before me,” said Alam, a National Geographic Young Explorer whose favorite is the figure of Roman, considered the mother of the Hubble telescope. “As I’m analyzing Hubble data, to have a little NASA figure of her nearby is very special.”
Photos by Stephanie Mitchell/Harvard Staff Photographer
Oluwaseun Araromi
Postdoctoral researcher, Harvard John A. Paulson School of Engineering and Applied Science Favorite item: Precision laser cutters
When people talk about pinpoint accuracy, Araromi says this is what they are referring to. The laser, which he uses to make soft sensors for wearable robotics, operates at a frequency that can cut through metals with speed and power — and meticulous accuracy. What draws Araromi to the cutter is the seemingly endless possibilities for engineering devices you could make with it.
Photos by Kris Snibbe/Harvard Staff Photographer
Carlos Argüelles-Delgado
Assistant professor, Department of Physics Favorite item: Mechanical keyboard
Argüelles-Delgado’s most cherished tool is his keyboard. Why? For the old-school clicking noise the keys make. “I like the feedback, I like feeling and the sound of typewriters,” he said. “This makes that classic keyboard sound.” It’s not just the clicking; there’s something inherently meta about the act itself as he uses it to develop software for data analysis on neutrino particle events. They are among the fundamental building blocks of nature, observed in the IceCube Neutrino Observatory, near the South Pole in Antarctica: “You’re actually putting science, your knowledge, into the machine, and that is something that I think is neat.”
Photos by Rose Lincoln/Harvard Staff Photographer
Nicole Bush
Graduate researcher, Department of Molecular and Cellular Biology Favorite item: Worm pick
Sometimes it’s the things you make yourself that are the best. That’s the case with Bush, a sixth year Ph.D. student in the molecules, cells and organisms program. She uses a tool called a worm pick to work with C. elegans. “This is a classic tool that every worm lab person needs,” she said. It originated in the 1970s to move worms from plate to plate and has been serving that purpose ever since. Researchers make their own picks and customize them. Bush’s handles are all wrapped in pink, for example. Making the tool involves breaking the end off an old glass Pasteur pipette, inserting a tiny strip of platinum, and melding them. A penny and nickel are then used to hammer the platinum strip, which is also sandpapered so as not to impale the worms. “What I love most about the worm pick is the individuality of each one, and the fact that we make them in our own lab. It’s a huge part of C. elegans lab culture, and is a great way to show your individuality on the bench.”
Postdoctoral fellow, Department of Organismic and Evolutionary Biology Favorite item: Modern sequencing machines
Card relies on modern sequencing machines, which help not only to sequence fragments of a genome but to sequence the entire thing. For a geneticist like Card, who studies traits that seem to show up repeatedly in an organism, these machines are key to figuring out why. There are varieties of them but they are usually desktop- or printer-sized pieces of machinery that scientists can pop DNA fragments into. They’re expensive, futuristic looking, and aesthetically pleasing, Card said. “They’re kind of sexy pieces of equipment.”
Assistant professor, Department of Earth and Planetary Sciences Favorite item: Scotch tape and super glue
Fu uses a novel instrument called a quantum diamond microscope to measure the magnetic history of rocks and meteorites, but his favorite pieces of equipment can be found almost anywhere: double-sided Scotch tape and a bottle of Krazy super glue. “We use the QDM to look at really minute magnetic signatures in rocks,” Fu said. “So we need our samples to be free of contamination — that is free of any kind of iron or magnetic particle.” That’s where the glue and tape come in, which he uses to hold the samples together and in place while under the microscope. “They are really clean magnetically,” he said of his humble favorites. “They are a very reliably pure substance.”
Juliana García-Mejía
Graduate researcher, Center for Astrophysics | Harvard & Smithsonian Favorite item: Opticentric machine
For García-Mejía ’17, who is leading the design and construction of a new telescope camera in the search for signs of extraterrestrial life, the essential lab equipment on hand is an Opticentric machine. It allows scientists to precisely stack lenses on top of each other with just the thinnest of space separating them — about a third the width of a human hair. “I just didn’t even know that there existed a machine that could allow you to get the positioning of something to that level of precision,” said García-Mejía. The Opticentric can also become the lab equivalent of the legendary office water cooler. “The engineers and I often have conversations sitting around it,” she said. “They mentor me and impart knowledge as we joke about the idiosyncrasies of our lives and jobs and discuss intently how we plan to proceed with the project.”
Peter Girguis
Professor, Department of Organismic and Evolutionary Biology Favorite item: Mobile pressure van
Girguis’s favorite piece of equipment is the product of creativity and ingenuity. Dubbed the mobile pressure van, it is a kind of high-pressure aquarium used to keep deep-sea animals alive. The van is believed to be one of two in the world and started as a produce shipping container. “When we bought it, it was specifically hauling lettuce from the Central Valley in California to Massachusetts,” Girguis said. “We’ve taken it and reimagined it as a mobile laboratory, kind of like the International Space Station, but, of course, instead of space, we’re using this to simulate the ice-cold temperatures and high pressures of the deep sea.”
[gz_photo_layout_article_width image=”331095″ credit=”Photos%20by%20Jon%20Chase%2FHarvard%20Staff%20Photographer” /]
Rachel Harris
Postdoctoral researcher, Department of Organismic and Evolutionary Biology Favorite item: Stirred pressure vessel
Hydrothermal vents are fissures on the deep seafloor from which geothermally heated water erupts. Harris’s favorite pieces of equipment mimic that in a lab setting. They are known as stirred pressure vessels and the ones Harris has taken a particular liking to are a suite of four titanium reactors retrofitted for this unique purpose. They allow the deep-sea biologist to study the habitable limits of life on Earth and to explore the potential of other ocean worlds such as Jupiter’s moon Europa and Saturn’s moon Enceladus. “They give me a lot of confidence to be able to explore this new frontier of high-pressure microbiology research,” Harris said.
Shraddha Lall
Graduate researcher, Department of Organismic and Evolutionary Biology Favorite item: MAPLE, the fly-handling robot
For Lall, a third year Ph.D. student, moving a single fly from point A to point B is a repeated everyday task. To do so delicately, efficiently, and without mixing up the flies, she uses a Modular Automated Platform for Large-scale Experiments, or MAPLE. This organism-handling robot can carefully grab one fly at a time, sort flies into different test tube-like wells, and put them back without — it needs to be said — hurting a fly.
Kaitlyn Loftus
Graduate researcher, Department of Earth and Planetary Sciences Favorite item: Textbook
When it comes to her favorite piece of scientific equipment, Loftus is old school. She goes with a textbook. As a theorist who studies how the water cycle works on different planets, the fifth year Ph.D. student says whenever she is working on a project, she almost always keeps a stack of textbooks handy. They can be from the Harvard Library or part of her own collection. “It’s a way to really learn about almost anything that’s been done before,” she said. “[A way of] standing on the shoulders of giants.”
Photos by Kris Snibbe/Harvard file photo; courtesy of Martin Surbeck
Martin Surbeck
Assistant professor, Department of Human Evolutionary Biology Favorite item: Binoculars
For someone who’s always loved observing wild animals, binoculars have been essential. Surbeck remembers as a kid saving all his money to buy binoculars, which can cost around $2,000 for a good pair. “For a long time it was my most expensive possession,” Surbeck said. He’s owned two other pairs since then and they have all come in handy, especially for a primatologist who now spends much of his time observing wild bonobos in the Democratic Republic of the Congo.
Lerato Takana
Undergraduate researcher, Department of Physics Favorite item: Molecular beam epitaxy tool
In his first year in a physics lab, the College senior has been using this ultra-powerful vacuum to grow thin-film oxides. He’s been quite impressed with the machine in part because it gives the operator the ability to synthesize films as thin as 20 nanometers (reminder: a nanometer is one billionth of a meter). The career potential for mastering the tool is incredible, he said. “As my professor usually says: Can you imagine dealing with singular atoms and then actually holding the atoms you grew? Einstein never got this chance.”
Conor Walsh
Paul A. Maeder Professor of Engineering and Applied Sciences and associate faculty member at the Wyss Institute, Harvard John A. Paulson School of Engineering and Applied Sciences Favorite item: 3D motion capture system
Walsh’s 3D motion capture system helps him and his colleagues understand how people move. Set up in a shared space in the new Science and Engineering Complex, the system allows Walsh to carry out many of the biomechanics and physiology experiments crucial to his work. “Essentially, we put reflective markers on various parts of a person’s body … and we use cameras to track those and then are able to use that data combined with a kinematic model to compute how each different part of the body is moving during a walking, running, lifting cycle, or some other type of motion or activity,” he said.
Share this article
You might like.
Memorial Minute — Faculty of Arts and Sciences

Harvard Allston Partnership Fund awards grants to 26 Allston-Brighton nonprofits

Choosing Harvard took LyLena Estabine down an uncertain path. The former student co-president has no regrets.
How old is too old to run?
No such thing, specialist says — but when your body is trying to tell you something, listen
Excited about new diet drug? This procedure seems better choice.
Study finds minimally invasive treatment more cost-effective over time, brings greater weight loss
How far has COVID set back students?
An economist, a policy expert, and a teacher explain why learning losses are worse than many parents realize
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
Students' Level of Knowledge of Laboratory Equipment and Materials
Restiana 1 and Djukri 2
Published under licence by IOP Publishing Ltd Journal of Physics: Conference Series , Volume 1842 , International Conference on Science Education and Technology (ICOSETH) 2020, 24 October 2020, Surakarta, Indonesia Citation Restiana and Djukri 2021 J. Phys.: Conf. Ser. 1842 012022 DOI 10.1088/1742-6596/1842/1/012022
Article metrics
1163 Total downloads
Share this article
Author e-mails.
Author affiliations
1 Biology Education, Postgraduate Program, Yogyakarta State University, Jl. Colombo No. 1, Yogyakarta, 55281, Indonesia
2 Biology Education, Faculty of Mathematics and Natural Science, Yogyakarta State University, Jl. Colombo No. 1, Yogyakarta, 55281, Indonesia
Buy this article in print
Biology Laboratory is an essential variable in determining the quality of secondary school output. Sufficient laboratory equipment suitable for teaching in secondary schools helps improve students' skills. Knowing the equipment and materials in the laboratory supports the students in the learning process in the laboratory. This study aims to identify the level of students' knowledge of laboratory equipment and materials. It used a survey method with a sample of 106 high school students from grade XI in Yogyakarta. The data were collected by testing the students' level of knowledge of laboratory equipment and materials. Then, the data were analyzed by calculating the average percentage and grouped them into a range of category values. The results showed that the level of students' knowledge of the laboratory equipment is quite good; the level of students' knowledge of the function of laboratory equipment is good; and the level of students' knowledge of the procedure to use the laboratory equipment is good. Moreover, the level of students' knowledge of the characteristics of laboratory materials is good and the level of students' knowledge of the group or categorization of the laboratory materials used in practices for certain subjects is poor. The low knowledge of students about laboratory equipment and materials is due to the lack of time allocation for the teacher's explanation about the laboratory equipment and materials and the practicum guidelines used do not summarize the laboratory equipment and materials. Therefore, further research is expected to develop practicum guidelines that contain detailed information on laboratory equipment and materials.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

An Evaluation on the Science Laboratory as Learning Aid for STEM Students

This research paper was done by Nichole Angel M. Teh and Flora Bell Fajardo, students from the Senior High School Department (2019) under the strand of Science, Mathematics, Engineering and Technology(STEM). This is a quantitative research titled the evaluation of the science laboratory as learning aid for the STEM students. This quantitative research was conducted to evaluate the science laboratory as learning aid for STEM students in Mount Carmel School of Maria Aurora(MCSMA), Inc. The objective of this study was to discover if the students are helped by the science laboratory in their studies and if they learn more through experiments in a science laboratory. To gather the data they conducted a survey with a questionnaire that contained 20 questions that was answered by STEM students from grade 12 and 11 who were randomly chosen. There was a total of 79 respondents. The data gathered was analyzed using the percentage formula ( f/nx100). The data collected have been analyzed and interpreted by the researchers and so they came up with a positive result regarding the use of the science laboratory as learning aid for STEM students in Mount Carmel School of Maria Aurora(MCSMA),Inc.
Related Papers
PEDAGOGIK: Jurnal Pendidikan
Alif Mardiana
The science that studies nature and its processes are called Science. When learning about Science, the laboratory has an essential role in growing students' experimentation abilities and increasing students' enthusiasm for learning. This study aims to describe and determine the effectiveness of the use of laboratories in science learning at SMPN 2 Lumajang. This study uses an approach with the interview method. This research was carried out at SMPN 2 Lumajang, which involved subject science teachers at the SMPN. The results showed that the science laboratory at SMPN 2 Lumajang had two rooms: a laboratory room and a storage room. Judging from the aspect of the laboratory space, the science laboratory of SMPN 2 Lumajang still needs to be improved a bit, such as a space to store tools and materials, and there is no preparation room to make preparations before the practicum begins. The existing facilities and infrastructure influence the effectiveness of the use of laboratories ...
Universal Journal of Educational Research
Winda Kuncorowati
Dr. Mool Raj
4th International Scientific Conference on Philosophy of Mind and Cognitive Modelling in Education
Vincentas Lamanauskas
There are some trends currently being monitored in the country: interest in science studies and related professions is decreasing; unsatisfactory results of international student achievement research (PISA, TIMSS). In order to make students more interested in natural sciences and to motivate them to relate their life to STEAM activities, it is appropriate to encourage students to engage in independent research and to discover the joy of discovery. One of the ways to solve this problem is the students' practical experimental activity in the laboratories of University. In this way, students not only get to know the laws of science, new technologies, but also carry out experiments, research and projects. The research analyses the usage of STEAM program “Cognition of Energy and Thermal Processes” for students of ninth (1st Gymnasium) grades in order to deepen and broaden the knowledge of natural science education, develop practical abilities of students and their scientific researcher's competence. Students are advised to do five experimental works in this field. The program engages a basic educational method – inquiry-based learning. The results of the pedagogical experiment and the questionnaire survey are discussed. It can be stated that educational experimental activities are necessary and useful for students. By using these experimental activities, students can be provided with educational material in an attractive form, which stimulates the interest in the subject. Program participants have deepened and expanded their knowledge of energy and thermal processes in nature. Students improved their competence in natural science research. They learned how to plan and perform experiments, acquired the ability to formulate hypotheses, to make assumptions, to analyse and explain results, and to formulate reasoned conclusions. Students acquired practical skills to work properly and safely with devices and tools (computer systems Nova 5000 and Xplorer GLX, temperature, humidity sensors, caliper, scales, etc.) Students liked to be young researchers; they felt the joy of discovery by practically experimenting and independently exploring natural phenomena.
IOER International Multidisciplinary Research Journal
IOER International Multidisciplinary Research Journal ( IIMRJ)
The Philippine K to 12 science curriculum is a learner-centered and inquiry-based discipline that requires learners to utilize learning materials and learning spaces needed for a meaningful understanding of the scientific concepts and for developing their scientific literacy. This is anchored to the constructivism theory that supports 'learning by doing.' A laboratory is an essential place for active learning and science teaching that would provide students with opportunities to think creatively and critically to solve real-world problems. This study assessed the current status of the science laboratory facilities in two public junior high schools in the province of Lanao del Sur. This is to assess the current condition and availability of laboratory facilities and to identify the challenges faced by science teachers. This study employed descriptive case study method, in which the participants were from two selected schools in Lanao del Sur. A researcher-made checklist of laboratory facilities and semi-structured interviews were used to gather the data. Frequency was used as a statistical tool for quantifying the number of available laboratory facilities and equipment. Based on the findings, both schools have inadequate laboratory facilities that hinder the performance of the activities in the science module designed by the Department of Education. The lack of a laboratory room, the inadequacy of laboratory facilities and science equipment, defective laboratory equipment, the inadequacy of learning materials, lack of water supply, lack of electricity are common issues in both schools. Teacher-respondents of this study have difficulty in teaching some science concepts and are not fully equipped on how to use some science equipment. Addressing the identified challenges is recommended to achieve quality education for all.
recsam.edu.my
Lilia Halim
Hakim cerdas
School Science and Mathematics
Lynn Stewart

RELATED PAPERS
Mathematical Inequalities & Applications
Revue Philosophique De Louvain
Robert Franck
Emerging Issues, Challenges, and Opportunities in Urban E-Planning
Mohamed El-Mekawy
Advances in Bioscience and Biotechnology
José María Gómez Gómez
Revista Panorama - Revista de Comunicação Social
Thiago Soares
Markus Matoni
Montan Gonzales Yarik Carlos
Geological Society of America Special Papers
Darrell Henry
International Review of Education
Larissa Jõgi
Konuralp Journal of Mathematics (KJM)
Burak AVŞAR
European Journal of Heart Failure
Vaso Obradovic
Research in Computing Science
luis ernesto mancilla espinoza
Annals of King Edward Medical University
Surgeon Imam baloch
Srdjan Atanasijevic
Chemical Science
Mrinal Bhunia
Journal of Old Turkic Studies
Cement and Concrete Research
Aditya Kumar
Eric Kolotyluk
Case Reports
matthew lucky
Tropical Journal of Pharmaceutical Research
Ercan Ozdemir
Tomislav Bukša
Journal of Interventional Cardiology
Raoul Bonan
International Journal of Mathematical Education in Science and Technology
Arsalan Wares
The Journal of Infectious Diseases
Michael St Louis
Telma Mary Kaneko
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, resuspended freeze-dried nannochloropsis as a model laboratory system for concentrated fresh nannochloropsis in ultrasound cell disruption experiments.

- 1 Research Unit Food and Lipids, KU Leuven Kulak, Kortrijk, Belgium
- 2 Leuven Food Science and Nutrition Research Centre (LFoRCe), KU Leuven, Leuven, Belgium
- 3 Analytical and Circular Chemistry (ACC), Institute for Materials Research (IMO), Hasselt University, Hasselt, Belgium
Microalgae have rigid, complex cell walls hindering direct lipid extraction. Cell disruption techniques are used to rupture these cellular structures to increase lipid extraction. Researchers investigating the downstream processing of microalgae do not always have access to microalgal cultivation systems to generate large amounts of fresh microalgal biomass. Using resuspended freeze-dried microalgal biomass as a model laboratory system for concentrated fresh biomass during cell disruption experiments offers greater flexibility in experimental planning and omits investment costs of microalgal cultivation equipment. So far, it however remains unclear whether freeze-dried resuspended biomass can be used as a model laboratory system to represent concentrated fresh biomass during cell disruption and lipid extraction experiments. This paper thus evaluated the suitability of resuspended freeze-dried Nannochloropsis as a model laboratory system for concentrated fresh Nannochloropsis during cell disruption. Ultrasound assisted cell disruption was used as example cell disruption technique and lipid extraction efficiency and free fatty acid content were investigated. Tap water and 3% sodium chloride are both suitable resuspension media for the resuspension of freeze-dried Nannochloropsis . Resuspension duration should be limited (< 120 min) to prevent the formation of free fatty acids. The condition of the biomass (concentrated fresh, or resuspended freeze-dried) prior to ultrasound assisted cell disruption did not influence the resulting lipid extraction efficiency. Resuspended freeze-dried Nannochloropsis biomass in tap water or 3% sodium chloride can thus be used as a model laboratory system for fresh microalgal biomass during research on ultrasound assisted lipid extraction. The generalization of the results to other cultivation conditions, cell disruption techniques, components of interest or microalgal species should be carefully assessed.
1 Introduction
Microalgae are unicellular organisms able to produce commercially interesting components such as proteins, lipids, carbohydrates, pigments, and vitamins. These microalgae can be used to produce feed, food, and pharmaceuticals either as whole cell biomass or as extracts of these major or minor constituents. One microalgae genus that is extensively studied regarding lipid production is the marine microalga Nannochloropsis ( Barbosa et al., 2023 ). Nannochloropsis can accumulate lipids (up to 60%DM) with a high content of the valuable omega-3 long-chain polyunsaturated fatty acid (n-3 LC-PUFA) eicosapentaenoic acid ( Chua and Schenk, 2017 ; Xu, 2022 ). This n-3 LC-PUFA exhibits health promoting effects as it plays a role in the prevention of cardiovascular diseases ( Calder, 2021 ).
Microalgae, and especially Nannochloropsis , have rigid, complex cell walls hindering direct lipid extraction by organic solvents ( Lee et al., 2012 ; Alhattab et al., 2019 ; de Carvalho et al., 2020 ). To increase lipid extraction efficiency, cell disruption techniques are used to change and/or rupture cellular structures ( Halim et al., 2012 ). The lipids can then either be directly released from the interior of the cells into the bulk extraction solvent or the modified and ruptured cellular structures pose less of a barrier to organic solvent penetration into the cell and diffusion out of the cells ( Halim et al., 2012 ). Altogether, these mechanisms assist in lipid extraction and can result in increased lipid extraction efficiencies. Throughout this paper, the term ‘cell disruption’ is used to describe all these disruption-related phenomena.
Microalgal cell disruption and lipid extraction is subject of extensive laboratory-scale research. Multiple cell disruption techniques such as microwave ( Rezaei Motlagh et al., 2021 ), ultrasound (UAE) ( Yao et al., 2018 ) and enzymatic ( Maffei et al., 2018 ; Mienis et al., 2023 ) treatment and high pressure homogenization ( Balduyck et al., 2018 ; Bernaerts et al., 2019 ) have been studied. These cell disruption techniques require the microalgae to be in suspension ( Halim et al., 2012 ). Resuspended freeze-dried (also referred to as lyophilized) microalgal biomass is often utilized in these laboratory-scale set-ups as a model laboratory system to represent concentrated fresh microalgal biomass during cell disruption and lipid extraction experiments for several reasons. First of all, laboratory-scale cell disruption set-ups involve the optimization of multiple process parameters [e.g., time, temperature, solid to liquid ratio, power input, enzyme concentration ( Zuorro et al., 2016 ; Yao et al., 2018 ; Zhao et al., 2022 )], requiring the use of several to hundreds of grams of microalgae over multiple days. Concentrated fresh microalgal biomass (suspensions of 5-10%DM) however has limited stability and shelf life due to the formation of free fatty acids (FFA) ( Balduyck et al., 2017 ) and fermentation products ( Verspreet et al., 2020 ). Furthermore, the cultivation and harvesting of fresh microalgae requires specific facilities that not all research laboratories have access to, and if microalgae cultivation facilities are present, the total microalgal biomass yield [generally between 0.5 and 5 g/L ( de Carvalho et al., 2020 )] and total volume of the dilute culture is low. The demand for the required amount of concentrated fresh microalgae can therefore often not be met. Compared to concentrated fresh biomass, the use of resuspended freeze-dried microalgae thus requires less investment costs for specific equipment and it allows for flexible planning of large cell disruption optimization experiments executed at different moments in time while using the same batch of biomass. In the present study, the term ‘resuspension’ is used to describe the act of suspending (freeze-)dried microalgal biomass into an aqueous medium. Some authors refer to this as ‘suspension’ or ‘hydration’. As microalgae in their native state are already suspended in aqueous medium, using the term ‘resuspension’ is considered more specific.
Within cell disruption experiments aimed at extracting lipids, the intention is to study the effect of the applied disruption technique on the lipid extraction efficiency. Therefore, it is of importance that the increase in lipid extraction efficiency can be solely attributed to the applied cell disruption and is not caused by any preceding processing steps, sometimes also referred to as pretreatment, such as freeze-drying and subsequent resuspension of biomass. However, in papers using resuspended freeze-dried biomass as a model laboratory system, it is (inexplicitly) assumed that the condition of the biomass (resuspended freeze-dried or concentrated fresh) prior to cell disruption has no influence on the resulting lipid extraction efficiency. For further advancements in the field of microalgal cell disruption and lipid extraction it would be very useful to verify whether resuspended freeze-dried biomass can actually be used to represent concentrated fresh biomass during cell disruption and lipid extraction experiments. To the best of the authors’ knowledge, this assumption has however not been verified. Furthermore, different resuspension media such as demineralized water ( Martínez-Sanz et al., 2020 ; Quesada-Salas et al., 2021 ), tap water, but also growth medium or saline media to mimic saline environment have been used. However, to the best of our knowledge, assessing the effect of resuspension medium and duration on lipid extraction efficiency has also not been studied.
In addition to lipid extraction efficiency, FFA content is another relevant lipid parameter to study during resuspension and cell disruption. FFA content of concentrated fresh Nannochloropsis increased during storage and during cell disruption by high pressure homogenization which was attributed to endogenous lipolytic enzyme activity ( Balduyck et al., 2017 , 2018 ). High FFA content is associated with increased rancidity ( Mannion et al., 2016 ) and can thus be considered a lipid quality indicator. FFA content has however not been extensively studied in previous research regarding resuspension or cell disruption of microalgal biomass.
The overall objective of this study was to assess the suitability of resuspended freeze-dried Nannochloropsis biomass as a model laboratory system for concentrated fresh Nannochloropsis biomass in cell disruption and lipid extraction experiments. The study has been divided into two parts. The first assessed the effect of resuspension medium (tap water or 3% NaCl) and resuspension duration of freeze-dried Nannochloropsis on the lipid extraction efficiency. In the second experiment, UAE was chosen as example cell disruption technique to compare the effectiveness of cell disruption on concentrated fresh and resuspended freeze-dried biomass. In both experiments the FFA content was analyzed to assess the effect on lipid quality.
2 Materials and methods
2.1 materials.
Commercial freeze-dried Nannochloropsis sp. (COM-FD- N sp) (NannoPrime) biomass was obtained from Proviron (Hemiksem, Belgium) and was stored at -80°C until further use. Sodium chloride (≥ 99.5%) and isopropanol (≥ 99.5%, GC/HPLC-grade) were obtained from Sigma-Aldrich (Buch, Switzerland), sodium sulphate (> 99%) and chloroform (≥ 99.9%, HPLC-grade) were obtained from Carl Roth (Karlsruhe, Germany). Ammonium acetate (≥ 98.0, HPLC-grade) was obtained from Merck (Darmstadt, Germany). Methanol (≥ 99.9%, HPLC-grade) and toluene (≥ 99.8%, HPLC-grade) were obtained from Chemlab (Zedelgem, Belgium) and hexane (95%, HPLC-grade) was obtained from Biosolve (Valkenswaard, The Netherlands), Tridecanoic acid (C13:0) was obtained from Sigma-Aldrich (Buch, Switzerland). Myristic acid (C14:0), palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), linolenic acid (C18:3), arachidonic acid (C20:4) and eicosapentaenoic acid (C20:5) were obtained from Nu-Chek Prep (Elysian, USA). All FFA standards had a purity of > 99%.
2.2 Methods
This paper investigated the use of resuspended freeze-dried Nannochloropsis biomass as a model laboratory system to represent concentrated fresh biomass during cell disruption and lipid extraction experiments. Experiment 1 assessed the effect of resuspension medium (tap water or 3% NaCl) and resuspension duration of freeze-dried Nannochloropsis on the lipid extraction efficiency. During experiment 2, UAE was chosen as example cell disruption technique to compare the effectiveness of cell disruption on concentrated fresh and resuspended freeze-dried biomass. The FFA content was analyzed in both experiments to assess the effect of respectively resuspension or UAE on the lipid quality.
Both experimental set-ups ( Figure 1 ) are first discussed separately while the details of the subsequent analyses of total and extractable lipids, lipid extraction efficiency, FFA, ash content, and the statistics are discussed thereafter. An overview of the types of biomasses used in the two experiments and corresponding abbreviations are provided in Table 1 .
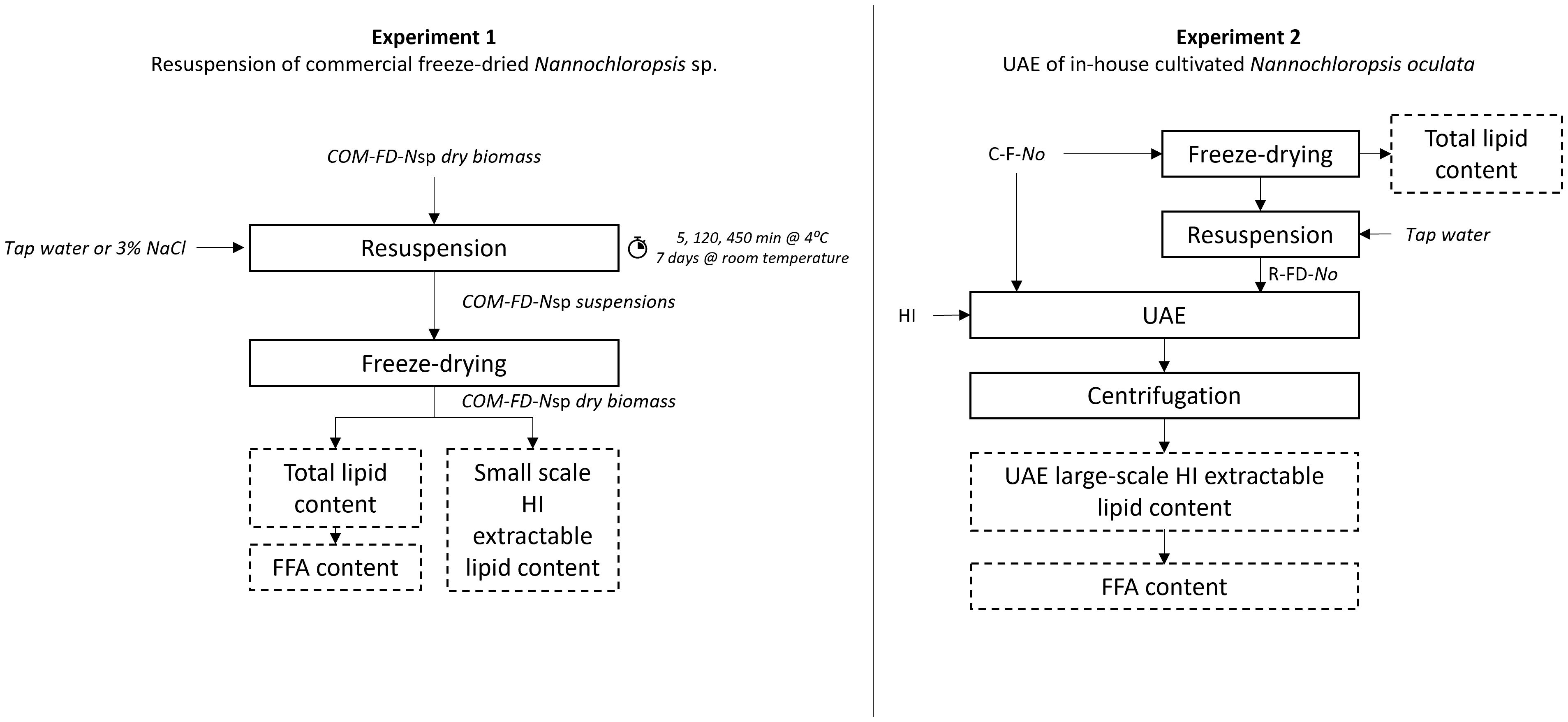
Figure 1 Schematic overview of the experimental set-up of experiment 1 and experiment 2 including the lipid analysis with COM-FD- N sp, Commercial freeze-dried Nannochloropsis sp.; FFA, free fatty acids; HI, hexane/isopropanol; C-F- No , concentrated; fresh Nannochloropsis oculata ; R-FD- No , resuspended freeze-dried Nannochloropsis oculata and UAE, ultrasound assisted lipid extraction.
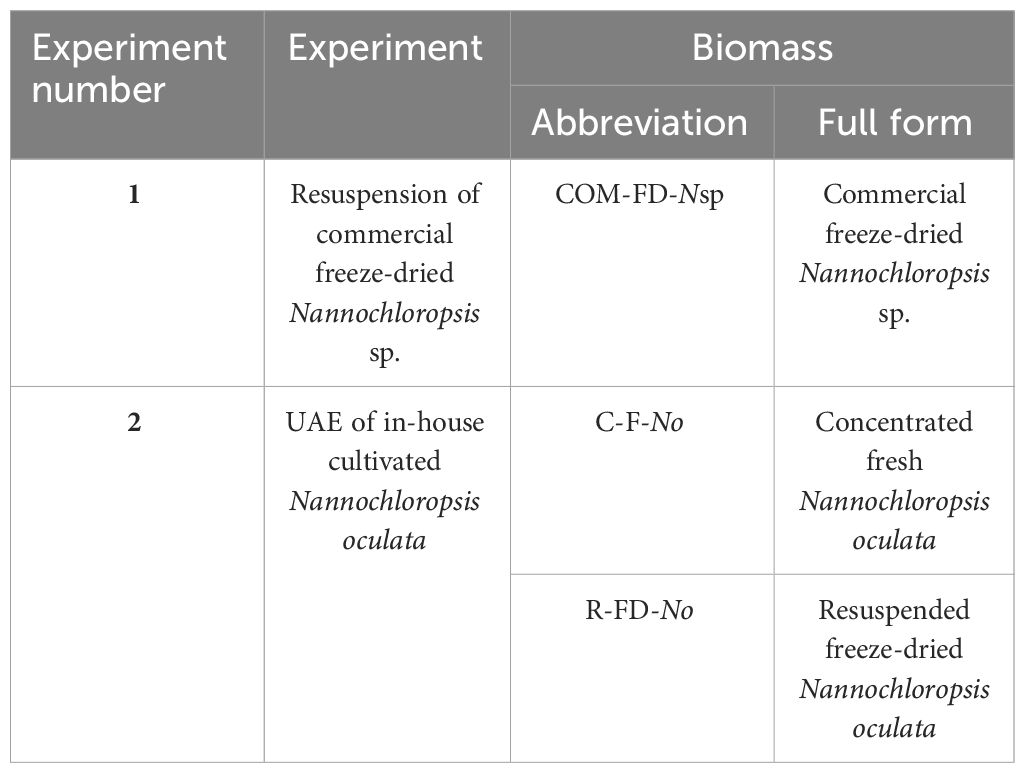
Table 1 Nannochloropsis biomasses and corresponding abbreviations used in the two experimental set-ups.
2.3 Resuspension of commercial freeze-dried Nannochloropsis sp.
In this first experimental set-up, COM-FD- N sp was resuspended, in duplicate, in sterile tap water or sterile 3% w/v sodium chloride (NaCl) solution to a concentration of 11.1% w/v, to mimic a concentrated microalgal suspension, by stirring for 5 min. No demineralized water was used as Nannochloropsis is a marine microalga for which resuspension in demineralized water could cause hypotonic osmotic shock resulting in a small degree of cell disruption ( Halim et al., 2021 ). Instead, 3% NaCl was used to mimic marine cultivation medium. The Nannochloropsis sp. suspensions were stored in closed Falcon tubes in the dark at 4°C for 5, 120 and 450 min. During storage, the suspensions were agitated using a rotary shaking plate at 100 rpm to prevent sedimentation of the microalgal cells. After 450 min, storage continued at room temperature for 7 days as a stress test to study the potential influence of endogenous lipases. After each time interval, the microalgal suspensions were frozen at -80°C, subsequently freeze-dried and stored at -80°C until further analyses.
2.4 UAE of in-house cultivated Nannochloropsis oculata
2.4.1 in-house cultivation and harvesting of nannochloropsis oculata.
Nannochloropsis oculata 38.85 (Sammlung von Algenkulturen, Göttingen, Germany) stock culture was kept in modified Wright’s Cryptophyte (WC) medium ( Guillard & Lorenzen, 1972 ) with double the amount of NaNO 3 (170 mg/L) and to which artificial sea salt (30 g/L) (Homarsel, Zoutman Industries, Roeselare, Belgium) was added. The stock culture was introduced into five 30 L bubble column photobioreactors (cylindrical, 20 cm diameter) containing WC medium with adjusted NaNO 3 concentration of 110 mg/L and artificial sea salt (30 g/L). A pH-stat system set at pH 8.5 was used to control the pH by addition of filtered CO 2 (0.2 μm) to acidify the medium. Each bioreactor was continuously aerated with filtered air (0.2 μm) for biomass mixing and irradiated by two daylight fluorescent tubes (Sylvania GRO-LUX T8 F36W/Gro) in a room with temperature set at 23.5°C. Microalgal growth in each bioreactor was monitored daily by measuring the optical density at 750 nm and showed slight variations indicating biological variation between the cultures within the different bioreactors. The microalgae were harvested and pooled after ten days of cultivation using a disc stack centrifuge (4000 g) with further concentration by centrifugation at 7500 g for 10 min (Sigma 6-16KS, Sigma Laborzentrifugen, Osterode am Harz, Germany).The resulting microalgal paste was diluted with supernatant to obtain a concentrated microalgal suspension (10.89 ± 0.01%DM). Half of the suspension volume was stored overnight at 4°C (for practical reasons) and then directly subjected to UAE (Section 2.4.2), and this is referred to as concentrated fresh N. oculata (C-F- No ) biomass. The remaining biomass was frozen at -80°C, freeze-dried, grinded into a fine powder and stored at -80°C.
The in-house cultivated, freeze-dried N. oculata was resuspended in tap water by stirring for 10 min at 550 rpm to obtain a suspension with dry matter content of 11.05 ± 0.03%, similar to the dry matter content of C-F- No. Tap water was chosen as resuspension medium based on the outcome of the results of the resuspension set-up (Section 3.1) This microalgal suspension is referred to as resuspended freeze-dried N.oculata (R-FD- No ) biomass.
2.4.2 Ultrasound disruption
C-F- No and R-FD- No biomass suspensions were subjected, in duplicate, to five min of UAE in the presence of hexane/isopropanol (3:2 v/v) (HI (3:2)) for simultaneous cell disruption and lipid extraction. A HI (3:2) solvent system was chosen as this solvent is accepted in the food supply chain (EU Directive 2009/32/EC) ( EU, 2009 ), is less toxic compared to other organic solvents, and is able to extract non-polar as well as polar lipids ( Ryckebosch et al., 2014 ). The dry biomass to organic solvent ratio was 1:13.7. A Branson 450 Sonifier with operating frequency of 20 kHz was used. The output control, which determines the amplitude, was set at 6, which corresponded to an initial power of 0.45 W/mL. 50% pulse mode, corresponding to an ultrasound duration of 0.5 second per second, was used. The sonicator tip (13 mm diameter tapped, stepped horn tip) was submerged in the center of the mixture with the probe tip at 2 cm below the liquid surface. After UAE, the biomass-solvent mixture was centrifuged at 3005 g for 10 min and the upper lipid-rich organic solvent layer was filtered (Whatmann n°1) through a sodium sulphate layer, collected in a round-bottom flask and evaporated by rotary evaporation. The lipid yield was gravimetrically determined and is referred to as UAE large-scale HI (3:2) extractable lipid content. Lipid extracts were redissolved in HI (3:2 v/v) and stored in amber vials at -80°C until further analyses.
2.5 Analyses
For the total lipid content, small-scale hexane/isopropanol (3:2) extractable lipid content and the ash content determination all samples are required to be dry. The suspensions obtained in the resuspension experiment (Section 2.3) were thus freeze-dried before all three analyses. Likewise, the suspensions of C-F- No and R-FD- N o obtained in the UAE experiment (Section 2.4.1) were also freeze-dried prior to ash content determination and total lipid content determination (only for C-F- No) .
2.5.1 Total lipid content of Nannochloropsis biomass
The total lipid content of all samples was determined using chloroform/methanol (1:1 v/v) according to EN 17908:2023 CEN (2023) and Ryckebosch et al. (2012) . Briefly, 4 mL methanol, 2 mL chloroform and 0.4 mL demineralized water were added to 100 mg freeze-dried biomass. The sample was vortexed followed by addition of 2 mL of chloroform and 2 mL of demineralized water. The samples were then vortexed again and centrifuged at 750 g for 10 min. The upper layer was discarded, and the bottom organic solvent layer was collected. The pellet was re-extracted with 4 mL chloroform/methanol (1:1 v/v), vortexed and centrifuged. The organic solvent was pooled with the previously collected solvent. These steps were repeated on the remaining pellet and the organic solvent was pooled with the previously collected organic solvent. The organic solvent was filtered (Whatmann n°1) through a sodium sulfate layer, the filter was rinsed with chloroform/methanol (1:1 v/v), and the filtered solvent was removed by rotary evaporation. The mass of the extracted lipids after solvent evaporation was gravimetrically determined. The total lipid content of all samples was determined minimally in duplicate.
2.5.2 Small scale hexane/isopropanol (3:2) extractable lipid content
The small scale HI (3:2) extractable lipid content of samples obtained during the resuspension experiment was determined using the method described by Ryckebosch et al. (2013) with modifications. In brief, 6 mL of HI (3:2) was added to 100 mg of freeze-dried biomass and homogenized. Subsequently, the samples were centrifuged for 10 min at 750 g, and the organic solvent layer collected. The remaining pellet was re-extracted three more times using the same procedure. Solvent layers were pooled, filtered (Whatmann n°1) and evaporated to obtain the extracted lipids. The small-scale HI (3:2) extractable lipid content was determined minimally in duplicate.
2.5.3 Dry matter and ash content
The dry matter content of C-F- No and R-FD- No was determined at least in triplicate by comparing the weight pre- and post- freeze-drying. The ash content of samples was determined at least in duplicate according to the NREL protocol ‘Determination of Total Solids and Ash in Algal Biomass’ ( Van Wychen & Laurens, 2015 ), with slight modifications. In brief, crucibles were pre-conditioned at 575 ± 25°C. 100 mg freeze-dried biomass was prepared in pre-weighted crucibles. Samples were dried in an oven at 60 ± 10°C for at least 18 hours until constant weight. The dried samples were ashed in a muffle furnace at 575 ± 25°C for 24 ± 6 hours. The weight of the ashed sample was recorded until constant weight. The ash content was used to determine the lipid extraction efficiency on ash-free dry weight in the resuspension experiment by expressing both the small-scale HI (3:2) extractable lipid content and total lipid content on ash-free dry weight (Section 2.5) to correct for the contribution of added NaCl.
2.5.4 Free fatty acid content
The FFA content of the total lipid extracts obtained in the resuspension experiment and of UAE large-scale HI (3:2) lipid extracts obtained during the UAE experiments were determined according to a method based on Van Meulebroek et al. (2017) with modifications and described previously by Van Wayenbergh et al. (2023) for one FFA (C13:0)). For each experiment, a separate calibration curve was constructed for C14:0, C16:0, C16:1, C18:0, C18:1, C18:2, C18:3, C20:4 and C20:5 in toluene/methanol (1:2) ( Table 2 ). Each lipid sample was standardized to a final lipid concentration of 500 ng/µL in toluene/methanol (1:2). Tridecanoic acid was used as internal standard in the samples and in each level of the calibration curve.
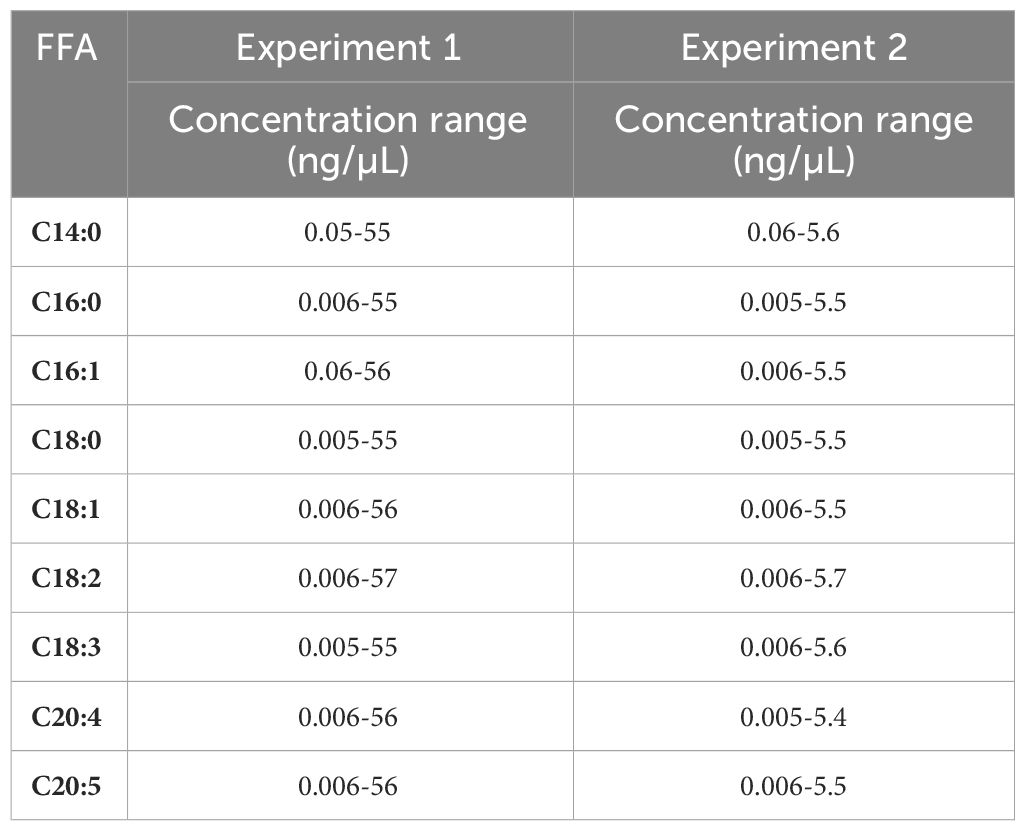
Table 2 FFA concentration range (ng/µL) of each FFA used for the construction of the calibration curves in experiment 1 and 2.
LC-MS was used for the quantitative analysis of the nine FFAs. FFA separation was achieved by UHPLC on an Vanquish Flex UHPLC system operated at 40°C with an Accucore 80A-C18 reverse phase column with a universal guard column (Uniguard, Thermo Fischer, holder for 2.1/3.0MM). A binary pump system was used for mixing two mobile phases: 20 mM ammonium acetate in MilliQ water (mobile phase A) and 20 mM ammonium acetate in methanol (mobile phase B). Gradient elution was applied (expressed in % of mobile phase B) for a total run time of 25 min with mobile phase flow of 0.3 mL/min:
- 0-1 min: isocratic 75%
- 1-2 min: linear 75-90%
- 2-6 min: linear 90-98%
- 6-15 min: linear 98-100%
- 15-22 min: isocratic 100%
- 22-25 min: linear 75%
5 µL of sample was injected for analysis. Q Exactive MS with electrospray ionization (ESI) and orbitrap detector was used to detect the FFA. The MS was run in negative ion mode for detection of FFA as ionization occurs due to proton loss. The scan range was 153.4 – 2300 m/z with sheath, auxiliary and sweep gas flow rate of 60, 20 and 2 arbitrary units, respectively. Capillary temperature was 285°C and the auxiliary gas heater temperature was 370°C. The mass resolution was 70 000 full-width half maximum (fwhm, 1 Hz). The ESI spray voltage was 3 kV, and the S-Lens radio frequency level was 70.
Data analysis was performed using TraceFinder 5.1 (Thermo Fisher Scientific). Identification of the FFAs of interest was based on the m/z value of the molecular ion with maximum m/z variation of 3 ppm. The peak areas of FFA in the calibration curves were integrated and corrected for IS. Linear calibration curves were created for each FFA based on the calibration levels. Peak areas of the FFA in the samples were corrected for IS and quantitative determination of the FFAs was achieved using the calibration curves. Total FFA content within the samples was determined by summation of the content of the individual detected FFA (C14:0, C16:0, C16:1, C18:1, C18:2, C20:4 and C20:5).
2.6 Lipid extraction efficiency
In the resuspension experiment (Section 2.3) the lipid extraction efficiency was determined according to Equation 1 . The small-scale HI (3:2) extractable lipid content (Section 2.5.2) was divided by the total lipid content as determined according to Section 2.5.1. Both were expressed on ash free dry weight.
The lipid extraction efficiency in the UAE experiment was determined by dividing the UAE large-scale HI (3:2) extractable lipid content (Section 2.4.2) by the total lipid content as determined according to Section 2.5.1 ( Equation 2 ). The lipid extraction efficiency of the initial, undisrupted in-house cultivated N. oculata prior to UAE was determined by Equation 1 .
2.7 Statistics
Statistical analysis was performed using JMP Pro 16.2.0 (SAS Institute Inc, Cary, USA) with a significance level of 0.05. For the resuspension experiment, a two-way analysis of variance (ANOVA) was conducted to assess the impact of resuspension duration (5, 120 and 450 minutes) and resuspension medium (tap water or 3% NaCl) on the lipid extraction efficiency and the FFA content. The presence of interaction effects was tested by using a multiplicative model. When no significant interaction effects were present, an additive model was applied. In case of a significant effect, a post-hoc Tukey-HSD test was performed. A one-way ANOVA was conducted to study the effect of resuspension treatment (no resuspension, in tap water or 3% NaCl) on the lipid extraction efficiency and FFA content. A two-way ANOVA was performed to study the effect of resuspension treatment (450 minutes at 4°C or 7 days at room temperature) and resuspension medium (tap water or 3% NaCl) on the FFA content.
For UAE, t-tests were conducted to assess the effect of the condition of the biomass (concentrated fresh or resuspended freeze-dried) on the UAE large-scale HI (3:2) extractable lipid content and FFA content in the HI-extractable lipids.
3.1 Experiment 1: resuspension of commercial freeze-dried Nannochloropsis sp.
3.1.1 lipid extraction efficiency.
The lipid extraction efficiency (according to Equation 1 ) of initial, not-resuspended COM-FD- N sp and COM-FD- N sp after resuspension (5, 120 and 450 min) in tap water or 3% NaCl was determined ( Figure 2 ). The total lipid content of the initial, not resuspended COM-FD- N sp was 30.5 ± 0.2% on ash free dry weight. The total lipid content of the resuspended biomass was similar. The two-way ANOVA indicated that there was no significant impact of resuspension medium nor resuspension duration on the lipid extraction efficiency. The lipid extraction efficiency of initial not-resuspended COM-FD- N sp was 32.4 ± 0.7% on ash free dry weight and was compared to five minutes of resuspension in tap water or 3% NaCl (respectively 38.5 ± 2.3 and 40.3 ± 1.4% on ash free dry weight) by a one-way ANOVA. No significant effect of the resuspension treatment (not resuspended, in tap water or 3% NaCl) was found on the lipid extraction efficiency. In summary, no effects for resuspension itself nor for resuspension medium (tap water or 3% NaCl) or resuspension duration on lipid extraction efficiency were observed.
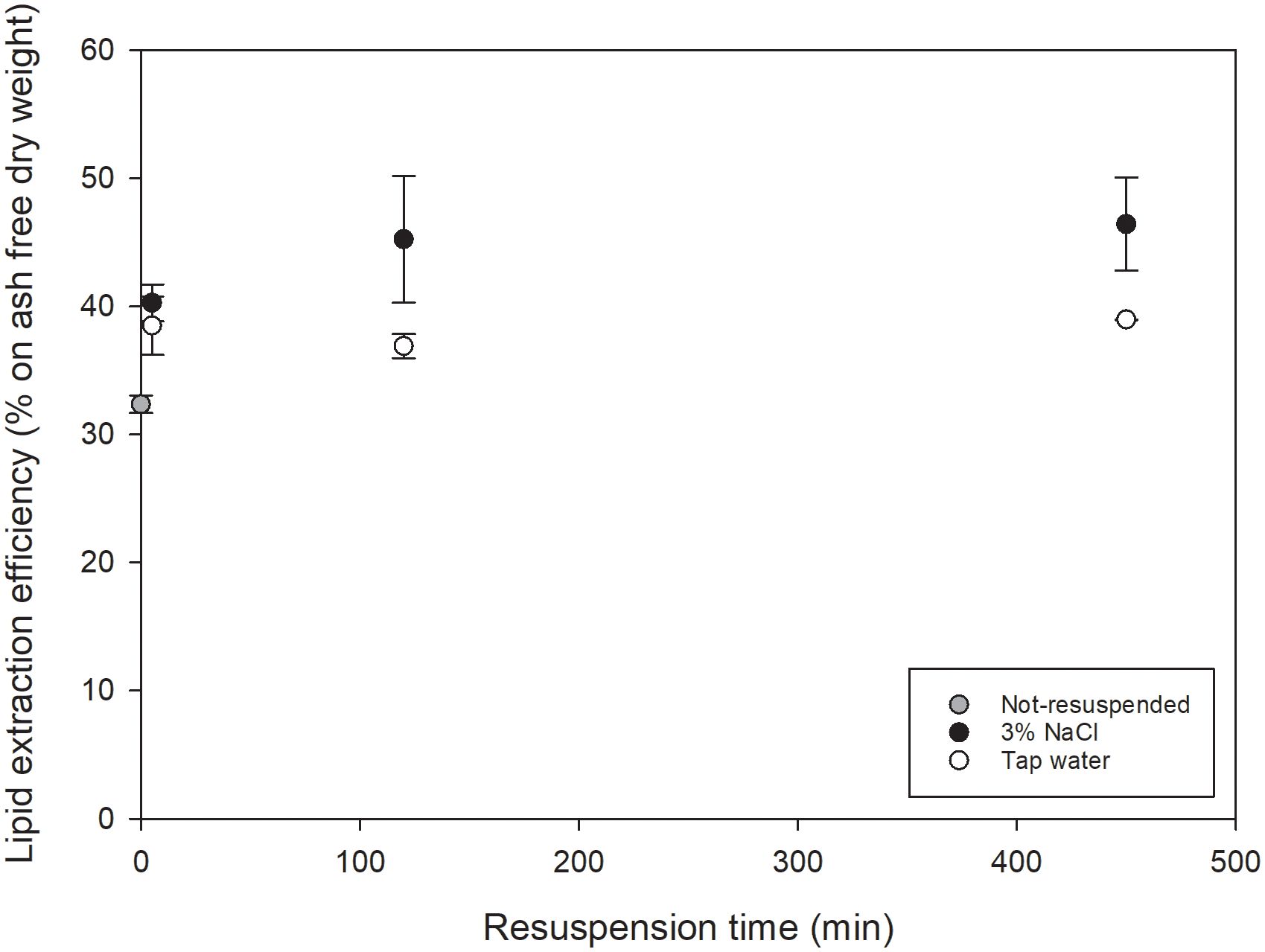
Figure 2 Lipid extraction efficiency (% on ash free dry weight) as function of resuspension time (min) for COM-FD- N sp that was not resuspended (gray symbol), resuspended in 3% NaCl (black symbols) or resuspended in tap water (white symbols) reported as mean +/- st.dev with n=2*2 for resuspended biomass and n=4 for not-resuspended biomass. No significant differences (α=0.05) were observed for the effect of resuspension, nor of resuspension duration nor resuspension medium.
3.1.2 FFA content
The FFA content of initial, not-resuspended COM-FD- N sp and COM-FD- N sp after resuspension (5, 120, 450 min, and 7 days) in tap water or 3% NaCl was quantified by LC-MS ( Figure 3 ). Based on the two-way ANOVA, no significant interaction effect between resuspension medium and resuspension duration was found. The resuspension duration had a significant effect on the FFA content (p<0.0001), where 120 and 450 minutes of resuspension each led to a significant increase in FFA content compared to the preceding time point. No significant influence of resuspension medium was found. The FFA content of initial not-resuspended COM-FD- N sp was 7.3 ± 0.2% of total lipids. Compared to not-resuspended COM-FD- N sp, resuspension for 5 minutes did not significantly increase the FFA content, independent of resuspension medium ( Figure 3 ).
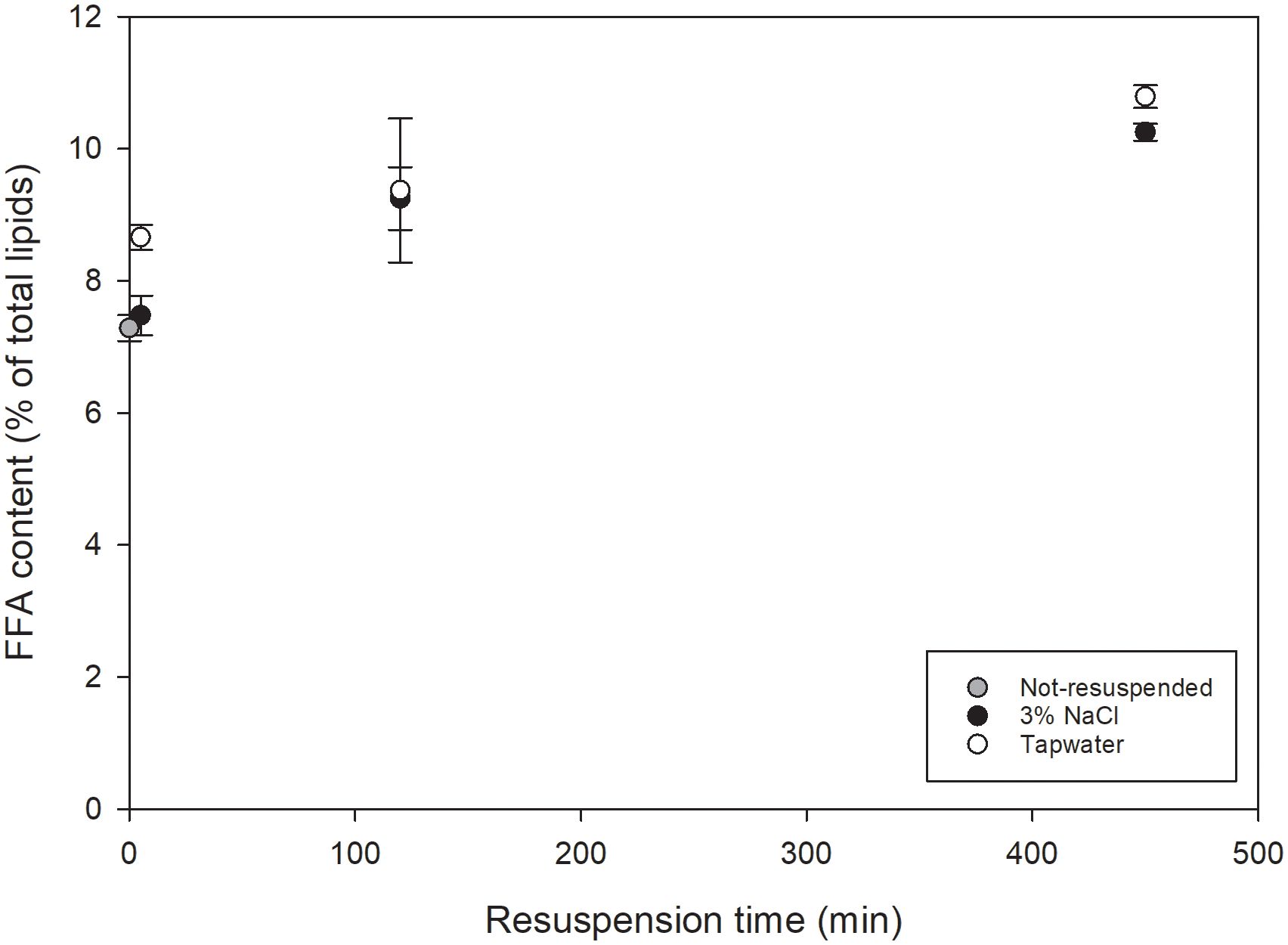
Figure 3 FFA content expressed as % of total lipids as function of resuspension time (min) for COM-FD- N sp that was not resuspended (gray symbols), resuspended in 3% NaCl (black symbols) or resuspended in tap water (white symbols) reported as mean +/- st.dev with n=2*2 for resuspended biomass and n=4 for not-resuspended biomass. A significant effect of resuspension duration was observed. Neither resuspension medium nor the resuspension itself had a significant effect.
The activity of endogenous lipases in COM-FD- N sp was assessed by a stress test. After 450 minutes of resuspension at 4°C, storage of the resuspended samples was continued at room temperature in the dark for 7 days. This change in storage temperature was deliberately chosen to create circumstances that have shown to extensively activate endogenous lipases in concentrated fresh N. oculata resulting in an increased FFA content ( Balduyck et al., 2017 ). Unlike Balduyck et al. (2017) , this paper utilized resuspended freeze-dried Nannochloropsis sp. to verify its suitability as a model laboratory system for concentrated fresh biomass, but it was uncertain whether the endogenous lipases in COM-FD- N sp retained their lipolytic activity after undergoing freeze-drying. The FFA content of COM-FD- N sp resuspended in tap water or 3% NaCl after seven days of storage at 20°C was 32.0 ± 0.3 and 28.9 ± 2.9% of total lipids, respectively. Based on a two-way ANOVA, the prolonged storage of 7 days at room temperature significantly increased (p = 0.001) the FFA content compared to resuspension for 450 min at 4°C confirming the presence of active endogenous lipases in COM-FD- N sp. Exogenous lipase activity was excluded as autoclaved resuspension media were used. No effect of resuspension medium was found.
3.2 Experiment 2: UAE of in-house cultivated Nannochloropsis oculata
Based on the results in Section 3.1, freeze-dried in-house cultivated N. oculata was resuspended in tap water for 10 min. The total lipid content of this in-house cultivated batch of N. oculata was 25.6 ± 0.7%. The lipid extraction efficiency ( Equation 2 ) of C-F- No and R-FD- No suspensions after UAE was not statistically different ( Table 3 ). Unlike in Section 3.1.1, lipid extraction efficiency was not expressed on ash free dry weight, as the ash content of C-F- No and R-FD- No suspensions was similar (16.8 ± 0.1% and 16.6 ± 0.2%, respectively) and correction for different ash content of the biomass was thus not required. The FFA content in the HI (3:2) lipid extract obtained after UAE was (marginally) significantly higher (p=0.044) for R-FD- No compared to C-F- No ( Table 3 ). These results indicated that the condition of the N. oculata biomass, either as concentrated fresh or resuspended freeze-dried biomass before UAE has no influence on the obtained lipid extraction efficiency. However, there was a (marginally) significant effect of the condition of the biomass on the FFA content in the obtained HI (3:2) lipid extract.
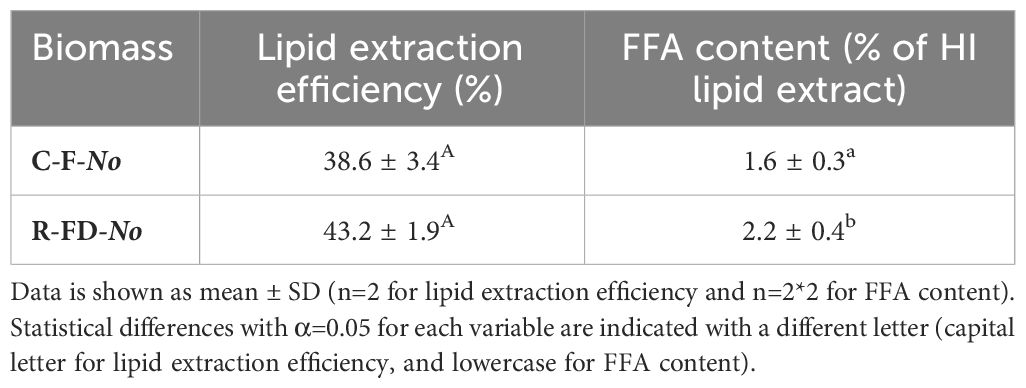
Table 3 Lipid extraction efficiency (%) and FFA content (% of HI lipid extract) of C-F- No and R-FD- No after UAE for 5 min (experiment 2).
4 Discussion
Resuspended freeze-dried microalgal biomass is often used as model laboratory system to represent concentrated fresh microalgal biomass during cell disruption and lipid extraction experiments. For correct interpretation of the effect of cell disruption on the increase in lipid extraction efficiency, it is important that the increase in lipid extraction efficiency is exclusively caused by the applied cell disruption technique, and not by any preceding processing steps. Such a preceding processing step, the resuspension of COM-FD- N sp in tap water or 3% NaCl and subsequent freeze-drying was evaluated in this study. The resuspension and subsequent freeze-drying will be referred to as ‘resuspension’ to increase readability in the remainder of the text. Resuspension itself did not influence the lipid extraction efficiency nor FFA content independent of resuspension medium. In addition, resuspension medium (tap water or 3% NaCl) and resuspension duration (5, 120 or 450 min) did not significantly increase the lipid extraction efficiency. In contrast, the FFA content used as lipid quality indicator in this study, increased significantly for longer resuspension duration (120, 450 min and 7 days), confirming the presence of endogenous lipases. This implies that tap water or 3% NaCl can both be used to resuspend freeze-dried Nannochloropsis , but the resuspension duration should be short (< 120 min) to avoid lipid degradation.
The condition of N. oculata biomass (concentrated fresh or resuspended freeze-dried) prior to cell disruption by UAE did not influence the lipid extraction efficiency. However, the FFA content in the lipid extract of resuspended freeze-dried biomass was only slightly, yet statistically significant, higher compared to concentrated fresh biomass (2.2 ± 0.4 and 1.6 ± 0.3% of HI lipid extract, respectively). However, as the total amount of FFA detected is low, the increase in FFA with respect to the total amount of lipids is low, the sample size of each treatment is small, the p-value is only marginally significant, and the lipid extraction efficiency is unaffected, this statistical significance should not be used to disprove the suitability of resuspended freeze-dried biomass as a model laboratory system for concentrated fresh biomass.
The lipid extraction efficiency of initial, undisrupted in-house cultivated N. oculata used in the UAE experiment and calculated using Equation 1 was 11.0 ± 0.6%. A different extraction method with different contact time between organic solvent and biomass, presence or absence of cell disruption technique and different organic solvent to biomass ratio and water content, was used after the UAE experiment to calculate the lipid extraction efficiency, resulting in two different equations ( Equations 1 , 2 ). Due to these different methods and equations, a direct statistical comparison between the lipid extraction efficiency prior to and post UAE cannot be made. However, the results indicated that the UAE increased the lipid extraction efficiency (lipid extraction efficiency of 11% prior to and ~40% post UAE). These results are in correspondence with literature, where an increase in lipid extraction efficiency is observed after ultrasound treatment of Nannochloropsis ( Bermúdez Menéndez et al., 2014 ; Yao et al., 2018 ; Figueiredo et al., 2019 ) The selection of UAE as example cell disruption technique was thus appropriate.
The lipid extraction efficiencies and FFA content of the two Nannochloropsis biomasses used in the two experimental set-ups discussed in this paper differ. Comparison of the absolute values regarding lipid extraction efficiency and FFA content obtained from different batches of microalgae, even on species level, should always be performed with caution as strain and cultivation, harvesting and storage conditions can have an influence on the properties (e.g. cell wall thickness, activation of lipases) of the microalgae ( Beacham et al. (2014) ; Balduyck et al., 2017 . In turn, this can influence the susceptibility to cell disruption and resulting lipid extraction efficiency and FFA content ( Halim et al., 2019 ). Due to these variations, trends (e.g., increases or decreases) should be considered instead.
In a similar fashion, extrapolation of the results obtained in this paper (in tap water resuspended, freeze-dried Nannochloropsis can be used as a model laboratory system for concentrated fresh Nannochloropsis during cell disruption, more specifically UAE) to other microalgae, cultivation conditions, cell disruption techniques and release of other components of interest should be done with caution. Nannochloropsis is a marine microalgae with a complex cell wall consisting of algeanan and (hemi)cellulose ( Scholz et al., 2014 ) and a small size (2-5 µm) which greatly differs from the cell wall composition and size of other (freshwater) microalgae ( de Carvalho et al., 2020 ). The different size and composition of cell walls, or lack thereof, can influence the response of the microalgal cell to resuspension medium and duration, the interaction with lipid extraction solvent and the susceptibility to cell disruption ( Natarajan et al. (2014) . In addition, the proposed underlying mechanism of cell disruption techniques (e.g. weakening of the cell wall, increased temperature, large shear forces, or particle-size reduction) varies depending on disruption technique and microalgal species will likely respond differently to these techniques ( Zhang et al., 2019 ). Further investigation is needed to verify if the results can be transferred to other cell disruption techniques, (lipid) extraction systems, and biorefinery products for Nannochloropsis and microalgae in general. Nevertheless, the results discussed in this paper are highly relevant for research involving the resuspension and ultrasound cell disruption of (freeze-dried ) Nannochloropsis for the extraction of lipids.
5 Conclusion
The suitability of resuspended freeze-dried Nannochloropsis biomass as a model laboratory system for concentrated fresh biomass during ultrasound cell disruption and lipid extraction experiments was assessed. Tap water as well as 3% NaCl can be used as resuspension medium for a resuspension duration up to 450 min. If FFA content is a relevant quality indicator, the resuspension duration should be minimized (< 120 min) to avoid lipid degradation. The lipid extraction efficiency after UAE was not affected by the condition of the biomass (concentrated fresh or resuspended freeze-dried). Resuspended freeze-dried microalgal biomass is thus a versatile alternative for fresh biomass during ultrasound cell disruption. Generalization of the results to other cultivation conditions, cell disruption techniques, components of interest or microalgal species should be carefully assessed. Nevertheless, the findings presented in this paper hold significant relevance for researchers applying (ultrasound) cell disruption techniques to resuspended freeze-dried Nannochloropsis and other microalgae.
Data availability statement
The data presented in the study are deposited in the KU Leuven Research Data Repository repository, accession number doi:10.48804/C3YCPZ.
Author contributions
EM: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. DV: Conceptualization, Funding acquisition, Writing – review & editing. IF: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Flanders’ Food and funded by Flanders Innovation and Entrepreneurship (VLAIO) through the cSBR project EffSep (Grant number HBC.2019.0012).
Acknowledgments
The authors wish to thank Dr. Jonas Blockx for proofreading the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
n-3 LC-PUFA, Omega-3 long-chain polyunsaturated fatty acid; FFA, free fatty acids; HI, Hexane/isopropanol; COM-FD- N sp, Commercial freeze-dried Nannochloropsis sp.; C-F- No , Concentrated fresh Nannochloropsis oculata ; R-FD- No , Resuspended freeze-dried Nannochloropsis oculata .
Alhattab M., Kermanshahi-Pour A., Brooks M. S.-L. (2019). Microalgae disruption techniques for product recovery: influence of cell wall composition. J. Appl. Phycol. 31, 61–88. doi: 10.1007/s10811-018-1560-9
CrossRef Full Text | Google Scholar
Balduyck L., Bruneel C., Goiris K., Dejonghe C., Foubert I. (2018). Influence of high pressure homogenization on free fatty acid formation in nannochloropsis sp. Eur. J. Lipid Sci. Technol. 120, 1700436. doi: 10.1002/ejlt.201700436
Balduyck L., Stock T., Bijttebier S., Bruneel C., Jacobs G., Voorspoels S., et al. (2017). Integrity of the microalgal cell plays a major role in the lipolytic stability during wet storage. Algal Res. 25, 516–524. doi: 10.1016/j.algal.2017.06.013
Barbosa M. J., Janssen M., Südfeld C., D’Adamo S., Wijffels R. H. (2023). Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 41, 452–471. doi: 10.1016/j.tibtech.2022.12.017
Beacham T. A., Bradley C., White D. A., Bond P., Ali S. T. (2014). Lipid productivity and cell wall ultrastructure of six strains of Nannochloropsis : Implications for biofuel production and downstream processing. Algal Res. 6, 64–69. doi: 10.1016/j.algal.2014.09.003
Bermúdez Menéndez J. M., Arenillas A., Menéndez Díaz J.Á., Boffa L., Mantegna S., Binello A., et al. (2014). Optimization of microalgae oil extraction under ultrasound and microwave iradiation. J. Chem. Technol. Biotechnol. 89, 1779–1784. doi: 10.1002/jctb.4272
Bernaerts T. M. M., Gheysen L., Foubert I., Hendrickx M. E., Van Loey A. M. (2019). Evaluating microalgal cell disruption upon ultra high pressure homogenization. Algal Res. 42, 101616. doi: 10.1016/j.algal.2019.101616
Calder P. C. (2021). “Chapter 2 - Health benefits of omega-3 fatty acids,” in Omega-3 delivery systems . Eds. García-Moreno P. J., Jacobsen C., Sørensen A.-D. M., Yesiltas B. (London, United Kingdom: Academic Press), 25–53. doi: 10.1016/b978-0-12-821391-9.00006-5
CEN (2023). The European Committee for Standardization .
Google Scholar
Chua E. T., Schenk P. M. (2017). A biorefinery for Nannochloropsis : Induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour. Technol. 244, 1416–1424. doi: 10.1016/j.biortech.2017.05.124
de Carvalho J. C., Magalhães A. I., de Melo Pereira G. V., Medeiros A. B. P., Bittencourt Sydney E., Rodrigues C., et al. (2020). Microalgal biomass pretreatment for integrated processing into biofuels, food, and feed. Bioresour. Technol. 300, 122719. doi: 10.1016/j.biortech.2019.122719
EU (2009). Directive 2009/32/EC. Commision Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients (Official Journal of the European Union). L 141/3 = L 141/11.
Figueiredo A. R. P., da Costa E., Silva J., Domingues M. R., Domingues P. (2019). The effects of different extraction methods of lipids from Nannochloropsis oceanica on the contents of omega-3 fatty acids. Algal Res. 41, 101556. doi: 10.1016/j.algal.2019.101556
Guillard R. R. L., Lorenzen C. J. (1972). Yellow-green algae with chlorophyllide. C. J.Phycol. 8, 10–14. doi: 10.1111/j.1529-8817.1972.tb03995.x
Halim R., Danquah M. K., Webley P. A. (2012). Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 30, 709–732. doi: 10.1016/j.biotechadv.2012.01.001
Halim R., Hill D. R. A., Hanssen E., Webley P. A., Blackburn S., Grossman A. R., et al. (2019). Towards sustainable microalgal biomass processing: Anaerobic induction of autolytic cell-wall self-ingestion in lipid-rich: Nannochloropsis slurries. Green Chem. 21, 2967–2982. doi: 10.1039/C8GC03186J
Halim R., Papachristou I., Kubisch C., Nazarova N., Wüstner R., Steinbach D., et al. (2021). Hypotonic osmotic shock treatment to enhance lipid and protein recoveries from concentrated saltwater Nannochloropsis slurries. Fuel 287, 119442. doi: 10.1016/j.fuel.2020.119442
Lee A. K., Lewis D. M., Ashman P. J. (2012). Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenergy 46, 89–101. doi: 10.1016/j.biombioe.2012.06.034
Maffei G., Bracciale M. P., Broggi A., Zuorro A., Santarelli M. L., Lavecchia R. (2018). Effect of an enzymatic treatment with cellulase and mannanase on the structural properties of Nannochloropsis microalgae. Bioresour. Technol. 249, 592–598. doi: 10.1016/j.biortech.2017.10.062
Mannion D. T., Furey A., Kilcawley K. N. (2016). Free fatty acids quantification in dairy products. Int. J. Dairy Technol. 69, 1–12. doi: 10.1111/1471-0307.12301
Martínez-Sanz M., Garrido-Fernández A., Mijlkovic A., Krona A., Martínez-Abad A., Coll-Marqués J. M., et al. (2020). Composition and rheological properties of microalgae suspensions: Impact of ultrasound processing. Algal Res. 49, 101960. doi: 10.1016/j.algal.2020.101960
Mienis E., Vandamme D., Foubert I. (2023). Enzyme-assisted disruption of oleaginous microalgae to increase the extraction of lipids: Nannochloropsis as a case study. Curr. Opin. Food Sci. 51, 101034. doi: 10.1016/j.cofs.2023.101034
Natarajan R., Ang W. M. R., Chen X., Voigtmann M., Lau R. (2014). Lipid releasing characteristics of microalgae species through continuous ultrasonication. Bioresour. Technol. 158, 7–11. doi: 10.1016/j.biortech.2014.01.146
Quesada-Salas M. C., Delfau-Bonnet G., Willig G., Préat N., Allais F., Ioannou I. (2021). Optimization and comparison of three cell disruption processes on lipid extraction from microalgae. Processes 9, 1–20. doi: 10.3390/pr9020369
Rezaei Motlagh S., Harun R., Awang Biak D. R., Hussain S. A., Omar R., Khezri R., et al. (2021). Ionic liquid-based microwave-assisted extraction of lipid and eicosapentaenoic acid from Nannochloropsis oceanica biomass: experimental optimization approach. J. Appl. Phycol. 33, 2015–2029. doi: 10.1007/s10811-021-02437-9
Ryckebosch E., Bermúdez S. P. C., Termote-Verhalle R., Bruneel C., Muylaert K., Parra-Saldivar R., et al. (2014). Influence of extraction solvent system on the extractability of lipid components from the biomass of Nannochloropsis gaditana . J. Appl. Phycol. 26, 1501–1510. doi: 10.1007/s10811-013-0189-y
Ryckebosch E., Bruneel C., Termote-Verhalle R., Lemahieu C., Muylaert K., Van Durme J., et al. (2013). Stability of omega-3 LC-PUFA-rich photoautotrophic microalgal oils compared to commercially available omega-3 LC-PUFA oils. J. Agric. Food Chem. 61, 10145–10155. doi: 10.1021/jf402296s
Ryckebosch E., Muylaert K., Foubert I. (2012). Optimization of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc 89, 189–198. doi: 10.1007/s11746-011-1903-z
Scholz M. J., Weiss T. L., Jinkerson R. E., Jing J., Roth R., Goodenough U., et al. (2014). Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell 13, 1450–1464. doi: 10.1128/EC.00183-14
Van Meulebroek L., De Paepe E., Vercruysse V., Pomian B., Bos S., Lapauw B., et al. (2017). Holistic lipidomics of the human gut phenotype using validated ultra-high-performance liquid chromatography coupled to hybrid orbitrap mass spectrometry. Analytical Chem. 89, 12502–12510. doi: 10.1021/acs.analchem.7b03606
Van Wayenbergh E., Blockx J., Langenaeken N. A., Foubert I., Courtin C. M. (2023). Conversion of retinyl palmitate to retinol by wheat bran endogenous lipase reduces vitamin A stability. Foods 13, 80. doi: 10.3390/foods13010080
Van Wychen S., Laurens L. M. L. (2015) Determination of Total Solids and Ash in Algal Biomass. Tech. Rep. NREL/TP-5100-60956. Available online at: http://www.osti.gov/servlets/purl/1118077/ .
Verspreet J., Kreps S., Bastiaens L. (2020). Evaluation of microbial load, formation of odorous metabolites and lipid stability during wet preservation of Nannochloropsis gaditana concentrates. Appl. Sci. 10, 3419. doi: 10.3390/app10103419
Xu Y. (2022). Biochemistry and biotechnology of lipid accumulation in the microalga nannochloropsis oceanica . J. Agric. Food Chem. 70, 11500–11509. doi: 10.1021/acs.jafc.2c05309
Yao S., Mettu S., Law S. Q. K., Ashokkumar M., Martin G. J. O. (2018). The effect of high-intensity ultrasound on cell disruption and lipid extraction from high-solids viscous slurries of Nannochloropsis sp. biomass. Algal Res. 35, 341–348. doi: 10.1016/j.algal.2018.09.004
Zhang R., Parniakov O., Grimi N., Lebovka N., Marchal L., Vorobiev E. (2019). Emerging techniques for cell disruption and extraction of valuable bio-molecules of microalgae Nannochloropsis sp. Bioprocess Biosyst. Eng. 42, 173–186. doi: 10.1007/s00449-018-2038-5
Zhao K., Zhang L., Zhang M., Tian H., He D., Zheng J. (2022). Response surface optimization of enzyme pretreatment improves yield of ethanol-extracted lipids from nannochloropsis oceanica . Eur. J. Lipid Sci. Technol. 124, 2100200. doi: 10.1002/ejlt.202100200
Zuorro A., Miglietta S., Familiari G., Lavecchia R. (2016). Enhanced lipid recovery from Nannochloropsis microalgae by treatment with optimized cell wall degrading enzyme mixtures. Bioresour. Technol. 212, 35–41. doi: 10.1016/j.biortech.2016.04.025
Keywords: microalgae, Nannochloropsis , disruption, resuspension, lipid extraction efficiency, free fatty acid, ultrasonication
Citation: Mienis E, Vandamme D and Foubert I (2024) Resuspended freeze-dried Nannochloropsis as a model laboratory system for concentrated fresh Nannochloropsis in ultrasound cell disruption experiments. Front. Mar. Sci. 11:1359090. doi: 10.3389/fmars.2024.1359090
Received: 20 December 2023; Accepted: 22 April 2024; Published: 03 May 2024.
Reviewed by:
Copyright © 2024 Mienis, Vandamme and Foubert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imogen Foubert, [email protected]
This article is part of the Research Topic
The Biorefineries and Application of Green Technologies for Recovering Bioactive Compounds from Microalgae
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Welcome to the Purdue Online Writing Lab

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue. Students, members of the community, and users worldwide will find information to assist with many writing projects. Teachers and trainers may use this material for in-class and out-of-class instruction.
The Purdue On-Campus Writing Lab and Purdue Online Writing Lab assist clients in their development as writers—no matter what their skill level—with on-campus consultations, online participation, and community engagement. The Purdue Writing Lab serves the Purdue, West Lafayette, campus and coordinates with local literacy initiatives. The Purdue OWL offers global support through online reference materials and services.
A Message From the Assistant Director of Content Development
The Purdue OWL® is committed to supporting students, instructors, and writers by offering a wide range of resources that are developed and revised with them in mind. To do this, the OWL team is always exploring possibilties for a better design, allowing accessibility and user experience to guide our process. As the OWL undergoes some changes, we welcome your feedback and suggestions by email at any time.
Please don't hesitate to contact us via our contact page if you have any questions or comments.
All the best,
Social Media
Facebook twitter.
- Work & Careers
- Life & Arts
Become an FT subscriber
Try unlimited access Only $1 for 4 weeks
Then $75 per month. Complete digital access to quality FT journalism on any device. Cancel anytime during your trial.
- Global news & analysis
- Expert opinion
- Special features
- FirstFT newsletter
- Videos & Podcasts
- Android & iOS app
- FT Edit app
- 10 gift articles per month
Explore more offers.
Standard digital.
- FT Digital Edition
Premium Digital
Print + premium digital, weekend print + standard digital, weekend print + premium digital.
Essential digital access to quality FT journalism on any device. Pay a year upfront and save 20%.
- Global news & analysis
- Exclusive FT analysis
- FT App on Android & iOS
- FirstFT: the day's biggest stories
- 20+ curated newsletters
- Follow topics & set alerts with myFT
- FT Videos & Podcasts
- 20 monthly gift articles to share
- Lex: FT's flagship investment column
- 15+ Premium newsletters by leading experts
- FT Digital Edition: our digitised print edition
- Weekday Print Edition
- Videos & Podcasts
- Premium newsletters
- 10 additional gift articles per month
- FT Weekend Print delivery
- Everything in Standard Digital
- Everything in Premium Digital
Complete digital access to quality FT journalism with expert analysis from industry leaders. Pay a year upfront and save 20%.
- 10 monthly gift articles to share
- Everything in Print
Terms & Conditions apply
Explore our full range of subscriptions.
Why the ft.
See why over a million readers pay to read the Financial Times.
International Edition

Department of Agricultural, Food, and Resource Economics Innovation Lab for Food Security Policy, Research, Capacity and Influence

Exploring Cooperation in Sustainable Agriculture and Value Addition in BIMSTEC Region
May 8, 2024 - Mustafizur Rahman, Muhammad Nafis Shahriar Farabi, Aleen Mukherjee, Satish Y. Deodhar, Sushil Kumar Saxena, Mohammad Jahangir Alam, Md Mazadul Hoque, Ismat Ara Begum, U.M. Aruna Kumara
The Bay of Bengal Initiative for Multi-Sectoral Technical and Economic Cooperation (BIMSTEC) has emerged as one of the fastest-growing regions of the world. After 27 years of its existence, the region is rapidly ‘catching up’ with its peers in the immediate neighborhood of South East Asia, reflecting development convergence in Asia. This progress is further buoyed by increasing trade among them and with others in the region, driven by price competitiveness. The objective of highlighting these developments is to present that BIMSTEC is a vibrant and economically active region, where growth convergence is taking place due to the rapid growth of the region. This may get further acceleration as graduation of LDCs moves forward. The collective goal of these member states is to foster cooperation and collaboration across various sectors, and agriculture emerges as a critical driver for economic development within this regional framework. As the global agricultural sector grapples with challenges arising from climate change, pollution, lack of nutritional security, decreasing genetic diversity, and evolving protectionist trade regimes, the BIMSTEC nations are not insulated from these challenges. RIS has been keenly focusing on examining the prospects and role of agriculture and its specific sub-sectors in fostering the process of intraregional trade in the BIMSTEC region. RIS, along with the International Food Policy Research Institute (IFPRI) has brought forth this report that highlights the importance of investigating potential avenues for regional cooperation among all members of BIMSTEC in areas of agricultural trade. To stabilize prices, promote regional food security, increase production and productivity, and establish resilient value chains, the chapters urge the BIMSTEC member states to work more closely together.
DOWNLOAD FILE
Tags: prci research paper
new - method size: 1 - Random key: 0, method: personalized - key: 0
You Might Also Be Interested In
STAAARS+ RFP webinar Sept 14 2022
Published on September 15, 2022
PRCI STAAARS+ Teams Presentation Video 2022
Published on July 26, 2022

Scoping Study of Agriculture Development Strategy of Nepal (ADS) (Five-year achievements)
Published on February 1, 2023
Sugarcane Production and Food Security in Uganda
Published on September 1, 2023
Institutional Arrangements Between Sugarcane Growers and Millers in Uganda and Implications for Grower Productivity and Profitability

Rwanda Natural Forest Cover Dynamics between 2015 and 2020
Published on June 19, 2023
Accessibility Questions:
For questions about accessibility and/or if you need additional accommodations for a specific document, please send an email to ANR Communications & Marketing at [email protected] .
- prci research paper,
- innovation lab for food security policy research capacity & influence

COMMENTS
This study aims to identify the level of students' knowledge of laboratory equipment and materials. It used a survey method with a sample of 106 high school students from grade XI in Yogyakarta ...
In the CLSI QMS13-A guideline, laboratory equipment can be classified into two major categories: general laboratory equipment and laboratory instrumentation. General laboratory equipment is that which can be used in various laboratory settings or methods, while laboratory instrumentation is used to produce measurements in a specific examination ...
Interestingly, in the few papers presenting laboratory work with an emphasis on more student-centred approaches, Puttick et al. (Citation 2015) found that the research designs frequently covered a whole series of lessons. It is therefore reasonable to conclude that implementation of the open inquiry approach in science teaching is demanding and ...
Full Length Research Effects of laboratory equipment on secondary school students' performance and attitude change to biology learning in federal capital territory, Abuja, Nigeria Katcha, M. A. and Wushishi, D. I.* 1Department of Science and Environmental Education, Faculty of Education, University of Abuja
The data shows that the actual number of monitors for quality control testing in 2020 is 502 units, 496 units have passed the initial inspection, and 502 units have passed the maintenance, which shows that the maintenance and quality control of medical equipment based on information fusion technology is effective. 1.
It is noticeable from Table 1 that the majority of equipment items that researchers would consider as the most expensive in their laboratory are categorised at level 5. Higher grade 6 and 7 items are a rarity in a biological research laboratory. Whilst mid-range level 5 automation items undoubtably increase the efficiency of laboratory research, they are designed for specific subtasks in a ...
Examples of open 3-D printed laboratory tools. A1, Components for laboratory tools, such as the base for a micromanipulator [ 18] shown here, can be rapidly prototyped using 3-D printing. A2, The printed parts can be easily combined with an off-the-shelf continuous rotation servo-motor (bottom) to motorize the main axis.
A laboratory is an essential place for active learning and science teaching that would provide students with opportunities to think creatively and critically to solve real-world problems. This study assessed the current status of the science laboratory facilities in two public junior high schools in the province of Lanao del Sur.
Equipment features comprise several characteristics designed for the equipment and exist since the unit is manufactured. One of the parameters used to assess the medical equipment condition is age (19, 20, 22-24, 26, 27, 29-31). The equipment age reflects the overall condition of the equipment.
Their equipment varies depending on their subjects, but also from scientist to scientist. The Gazette asked several Harvard researchers for their most treasured or essential pieces of lab, field, or office equipment. The answers range from highly technical to downright quirky. Photos courtesy of Munazza Alam.
High quality laboratory equipment in Physic are a must for the proper research and scientific studies ... The paper described to determine physics laboratory equipment in the subject area and the article cover more information on physics laboratory equipment like Electrical equipment, Magnetic equipment, Optical equipment, Gravity related ...
Knowing the equipment and materials in the laboratory supports the students in the learning process in the laboratory. This study aims to identify the level of students' knowledge of laboratory equipment and materials. It used a survey method with a sample of 106 high school students from grade XI in Yogyakarta.
Utilization of science laboratory equipment refers to the number of time that the available science laboratory equipment are used during classes or laboratory practical lessons. According to Lawal (2013), laboratory equipment influences practical learning, making the classroom real, lively and more meaningful.
4 The Foundation of Firms Based on Scientific Research. With the invention of the laboratory, instrument making was internalized within science. As science and the need for research equipment grew, instrument production began to be externalized and made into an industry. Scientific instrument making is an early source of firm formation linked ...
This research paper was done by Nichole Angel M. Teh and Flora Bell Fajardo, students from the Senior High School Department (2019) under the strand of Science, Mathematics, Engineering and Technology(STEM). This is a quantitative research titled the ... According to a research, laboratory equipment helps students to easily visualize and ...
As regard to laboratory services, 75 (35.2%) laboratory professionals believed that their laboratories did not provide quality of laboratory services as per the standards and 74 (34.7%) respondents indicated that their laboratories did not provide diagnostic services for all requested tests, in addition to this, 111 (52.1%) of respondents also ...
Adequate. The average mean score in the adequacy of the instructional tools and equipment used in teaching Module 2 in Cookery is 3.36. On the other hand the utilization of mixing tools in Module 2 in Cookery has mean score of 3.52 verbally interpreted as Much Utilized and equipment has mean score of 1.38 verbally interpreted as Not Utilized.
This paper thus evaluated the suitability of resuspended freeze-dried Nannochloropsis as a model laboratory system for concentrated fresh Nannochloropsis during cell disruption. Ultrasound assisted cell disruption was used as example cell disruption technique and lipid extraction efficiency and free fatty acid content were investigated.
The science laboratory has a direct effect on both students' ... should be equipped with laboratories or equipment for science and mathematical activities. The absence of such facilities ... The position paper brought out by NCERT on teaching of science (NCERT, 2005b) suggested a 2-fold approach to
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue. Students, members of the community, and users worldwide will find information to assist with many writing projects.
According to a Financial Times analysis of hundreds of LinkedIn profiles as well as public job postings and research papers, the $2.7tn company has undertaken a hiring spree over recent years to ...
RIS, along with the International Food Policy Research Institute (IFPRI) has brought forth this report that highlights the importance of investigating potential avenues for regional cooperation among all members of BIMSTEC in areas of agricultural trade. ... prci research paper, innovation lab for food security policy research capacity ...