An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Hum Vaccin Immunother
- v.16(8); 2020

Why vaccines matter: understanding the broader health, economic, and child development benefits of routine vaccination
Arindam nandi.
a Center for Disease Dynamics, Economics & Policy, Washington, DC, USA
b Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Associated Data
- World Health Organization . World Health Organization: 10 facts on immunization ; 2018. [accessed 2019 April9]. https://www.who.int/features/factfiles/immunization/en/ .
The direct benefits of childhood vaccination in reducing the burden of disease morbidity and mortality in a cost-effective manner are well-established. By preventing episodes of vaccine-preventable diseases, vaccination can also help avert associated out-of-pocket medical expenses, healthcare provider costs, and losses in wages of patients and caregivers. Studies have associated vaccines positively with cognition and school attainment, suggesting benefits of long-term improved economic productivity. New evidence suggests that the measles vaccine may improve immunological memory and prevent co-infections, thereby forming a protective shield against other infections, and consequently improving health, cognition, schooling and productivity outcomes well into the adolescence and adulthood in low-income settings. Systematically documenting these broader health, economic, and child development benefits of vaccines is important from a policy perspective, not only in low and middle-income countries where the burden of vaccine-preventable diseases is high and public resources are constrained, but also in high-income settings where the emergence of vaccine hesitancy poses a threat to benefits gained from reducing vaccine-preventable diseases. In this paper, we provide a brief summary of the recent evidence on the benefits of vaccines, and discuss the policy implications of these findings.
Introduction
Childhood vaccines save an estimated 2–3 million lives worldwide every year, which has contributed substantially to the reduction in global infant mortality rate from 65 per 1,000 live births in 1990 to 29 in 2018. 1, 2 Vaccines are found to be the most cost-effective approach for reducing childhood disease burden, especially when compared with interventions such as clean water and improved sanitation which can also reduce disease transmission but require expensive and time-consuming infrastructural investment. 3 Cross-national policy efforts such as the World Health Organization’s (WHO) Expanded Programme on Immunization (EPI) of 1974, and the multi-agency Global Alliance for Vaccines and Immunization (Gavi), established in 1999, have supported several countries with research, logistical planning, supply chain management, and financing of national vaccination programs. In recent times, routine vaccination has been supplemented with additional efforts to optimize community coverage. An example is the government of India’s Mission Indradhanush campaign initiated in 2015, that resulted in an increase of full vaccination coverage in target districts by 10 percentage points in just six months. 4 As a result of these combined in-country and international initiatives, full vaccination rates of children in low-income countries have increased from under 50% to close to 80% during the past two decades. 5
With such improvements in vaccination rates and reduction in child mortality, future changes in the global child health policy can be envisaged in three broad areas. First, as vaccine coverage improves, and there is increasing protection of both vaccinated and unvaccinated populations through the phenomenon of community immunity, we are likely to see fewer vaccine-preventable diseases in the general population. For example, polio has been eliminated from almost all countries and is at the verge of complete global eradication. However, the growing recognition of the importance of health equity has shown that clusters of susceptible populations within vaccinated societies can preempt disease outbreaks, such as the reemergence of diphtheria infections in Bangladesh and India. 6 , 7 Second, the decreasing incidence of vaccine-preventable diseases has diminished the public’s memory of the devastation caused by the diseases, leading to a rise in vaccine hesitancy. Therefore, national programs will have to refocus on maintaining the momentum, although in a world with limited government resources, health policymakers may find it difficult to financially and operationally justify large vaccination programs. Third, the shifting focus from child mortality to morbidity will lead to a greater emphasis on children’s physical, cognitive, and socioemotional development as compared with survival. 8 , 9
Due to changing focus from child survival benefits of vaccines to child development benefits, along with greater reliance on multi-criteria decision-making tools, it is more important than ever before to quantify the broader social and individual benefits of vaccination. In this paper, we discuss evidence from a few key studies, and summarize the benefits of childhood vaccines beyond the intended reduction in disease burden and child mortality.
Economic, equity, and global health benefits of vaccines
Vaccines can have several economic benefits. 3 , 10 One of the most discernible benefits is averted medical expenditure. By preventing an episode of the disease through a vaccine, the economic costs of treatment, such as physician fees, drugs and hospitalization expenses, and associated travel costs and wage loss of caregivers could be averted. This is particularly important for low and middle-income countries (LMICs) where a large part of medical expenditure is out-of-pocket. A clear example is the situation in India, where 65% of health expenditure is private, with extreme costs in some cases, which thrusts 51 million people into poverty every year. 2 , 11 , 12 It is estimated that the measles, rotavirus, and pneumococcal conjugate vaccines could help avert $4.6 billion (2016 US$, adjusted for purchasing power parity) in out-of-pocket medical expenses in 41 Gavi-eligible LMICs during 2016–2030. 13 Vaccines could also reduce the number of people who fall into poverty due to a catastrophic medical expense which is defined as a large proportion (typically, more than 10% to 25%) of household income or expenditure. 12 , 14 - 20
The protection which vaccines provide against the financial risk from a large medical expense can be measured in additional ways. The so-called extended cost-effectiveness (ECEA) studies have estimated large money-metric value of insurance provided by vaccines. 13 , 14 , 16 , 19 The value of insurance is equivalent to risk premium, which is defined as the amount of money one would be willing to pay in order to avoid the financial uncertainty from a vaccine-preventable disease. 21 Paying for vaccines, in this context, is akin to paying for a health insurance premium.
Benefit-cost analysis (BCA) studies of vaccines consider a full range benefits as measured by gains in economic productivity. Several alternative BCA methods exist, including a human capital approach which uses the average annual economic contribution of workers, and a friction cost approach which considers productivity lost during the period when a job position remains unfilled due to sickness. 22 , 23 Mortality and morbidity risk reduction benefits of vaccines have also been measured in terms the value of statistical life year (VSLY). 24 , 25 VSLY is equivalent to the willingness to pay in order to avoid one disability adjusted life year (DALY) from the disease. 26 , 27 It is typically measured as a multiple (approximately 2–4 times) of the per capita national income of a country. 28 - 30 Newer studies such as those commissioned by the Copenhagen Consensus Center have considered a fixed value of either $1,000 or $5,000 per DALY across all countries and contexts. 29 - 31
One of the most comprehensive vaccine BCA studies published recently used the VSLY method and examined the economic benefits of 10 vaccines – for Haemophilus influenzae type b, hepatitis B, human papillomavirus, Japanese encephalitis, measles, Neisseria meningitidis serogroup A, rotavirus, rubella, Streptococcus pneumoniae , and yellow fever – in 73 LMICs. 10 The authors considered averted medical expenses, transportation costs, and productivity gains in their analysis, and estimated that during 2001–2020, the vaccines together would provide a social and economic value of $820 billion (2010 US$). 10 During 2011–2020, the rate of return for investment on these vaccines was estimated to be up to 44 times of the initial cost. 32 Routine vaccination has a positive effect on social and health equity among populations. Infectious disease incidence and mortality are often associated with poverty, and exacerbated by lack of access to clean water, sanitation, and basic hygiene among the poor. Routine childhood vaccinations are, thus, estimated to avert the largest burden of diseases, associated medical expenses, and loss in economic productivity in the poorest segments of the society. 13 , 14 , 17 - 19 , 33 , 34 A recent study in 41 Gavi-eligible LMICs found that universal coverage of the measles, rotavirus, and pneumococcal conjugate vaccines would avert a total of 12.6 million cases of catastrophic health expenditure which might have otherwise propelled patients into poverty. 13 Of those, 75%, 40%, and 22% of cases respectively for the three diseases were from the poorest wealth quintile. 13
New research shows that vaccines can also tackle global health threats such as antimicrobial resistance (AMR). If left unchecked, AMR-related infections are estimated to result in as many as 10 million deaths per year worldwide by 2050, with an associated global economic cost of US$100 trillion. 35 Vaccines could prevent infections – either sensitive or resistant – and also reduce the use of antimicrobials, which in turn could slow the growth of AMR. 36 - 46
Child development benefits of vaccines
Persistent or recurrent infections in early life can lead to poor growth and stunting, which in turn can adversely affect adult health, cognitive capacity, and economic productivity. 47 - 49 The theoretical basis of the long-term benefits of vaccines is anchored in the widely accepted “fetal origins” hypothesis 50 , 51 which links conditions in utero and during early childhood with later life outcomes. 48 , 49 , 52 - 66 Malnutrition, infection, pregnancy and birth complications, and under-stimulation during the first 1000 days of life can have lasting impact on health, cognitive, and economic outcomes well into the old age. In addition to appropriate nutrition and nurturing, health interventions such as routine vaccinations could reduce infectious disease burden in early childhood and thereby help break the intergenerational cycle of poverty, poor health, and low income.
There is a small but growing literature on the potential child development benefits of routine vaccines. The measles vaccine is especially important in this context as episodes of measles could damage protective immune memory for a period of 2–3 years, increasing susceptibility to future measles and non-measles infections. 67 - 69 Using sophisticated techniques, scientists have showed that measles infection in children wipes out preexisting antibodies to different pathogens in the months after the infectious episode, leaving them vulnerable to multiple other infections and possible death. 70 A recent longitudinal study of approximately 2,000 children each in Ethiopia, India, and Vietnam has linked measles vaccination at ages 6–18 months of life with 0.1–0.2 higher anthropometric z-scores, 1.7–4.5 percentage points higher scores on standardized cognition tests, and 0.2–0.3 additional schooling grades at ages 7–8 and 11–12 years. 71 The vaccine has also been associated with 0.2 more schooling grade attainment among South African children and 7.4% higher school enrollment rate among children in Bangladesh. 72 , 73
Similar growth, cognition, and schooling benefits have been observed among Haemophilus influenzae type B (Hib) vaccinated children in India, 74 , 75 and fully vaccinated children in the Philippines. 76 Another study found that exposure to tetanus vaccination in utero increased schooling attainment by 0.3 years for some children in Bangladesh. 77 At the aggregate level, India’s national vaccination program has been associated with 0.3–0.5 higher height-for-age and weight-for-age z-scores at ages 0–4 years, 78 and 0.2 additional schooling grades among adults. 79
Concluding remarks
Childhood vaccines have numerous positive effects beyond disease prevention. The concept of broader benefits of vaccines which would include cognition, schooling, economic productivity, fertility, and related outcomes was first proposed by a key 2005 article. 80 During the present decade, researchers have utilized and expanded this framework across several dimensions and country contexts. 76 , 77 , 81 - 85
A new online database called the Value of Immunization Compendium Evidence (VoICE), created and maintained by the International Vaccine Access Center at the Johns Hopkins University, Bloomberg School of Public Health, now tracks research on the broader benefits of vaccines on health, educational, economic, and equity outcomes worldwide. 86 The Immunization Economics community of research and practice compiles similar and related information. 87 Finally, the World Health Organization is developing an approach for systematically measuring the broader benefits, known as the Full Public Health Value Propositions (FPHVP), in the context of LMICs. 88 , 89 Regardless of income level, countries around the world are facing a crises in the acceptance of the societal benefits of routine vaccines. Going forward, we hope that these new frameworks will be widely used for child health policy globally.
Funding Statement
This work was supported by the Value of Vaccination Research Network (VoVRN) through a grant from the Bill & Melinda Gates Foundation (Grant OPP1158136). The content is solely the responsibility of the authors and does not necessarily reflect the views of the VoVRN or the foundation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 July 2023
Advances in vaccines: revolutionizing disease prevention
- Timir Tripathi ORCID: orcid.org/0000-0001-5559-289X 1
Scientific Reports volume 13 , Article number: 11748 ( 2023 ) Cite this article
4330 Accesses
1 Citations
3 Altmetric
Metrics details
- Biochemistry
- Drug discovery
Vaccines have revolutionized modern medicine by preventing infectious diseases and safeguarding public health. This Collection showcases cutting-edge research on advancements in vaccine development and their impact on disease prevention. The papers presented here report various facets of vaccine efficacy, immunological responses, and design, providing insight into future immunization strategies. I believe this Collection will serve as a catalyst for further advancements in the field of vaccine research.
Vaccines have long been credited as the most effective tool in preventing and managing infectious diseases. They have drastically reduced the global disease burden 1 . Over the years, significant progress has been made in understanding the immune system and developing novel vaccine design and delivery platforms 2 , 3 . From developing mRNA vaccines 4 that offer rapid response to identifying novel antigenic targets for broader protection, we have been at the forefront of innovation. Furthermore, the exploration of advanced adjuvants and delivery systems is enhancing vaccine efficacy and accessibility 5 . These cutting-edge technologies and advancements in vaccine research hold immense potential for tackling infectious diseases and improving global public health. In this Collection, I am delighted to present research articles highlighting the latest advances in vaccine development, shedding light on innovative vaccine design and delivery strategies, novel targets, and promising candidates. These breakthrough articles have the potential to revolutionize the field of vaccines and move us one step closer to a world free from the grip of devastating infectious diseases and outbreaks 6 .
Early strategies for investigating new vaccine targets or developing formulations increasingly rely on sophisticated computational approaches. These approaches help save resources and refine in vitro and in vivo studies. For example, in one of the papers in this Collection, Goodswen et al. 7 present a state-of-the-art methodology for high-throughput in silico vaccine discovery against protozoan parasites, exemplified by discovered candidates for Toxoplasma gondii . Vaccine discovery against protozoan parasites is challenging due to the limited number of current appropriate vaccines compared to the number of protozoal diseases that need one. The group generated a ranked list of T. gondii vaccine candidates and proposed a workflow integrating parasite biology, host immune system defences, and bioinformatics programs to predict vaccine candidates. Although testing in animal models is required to validate these predictions, most of the top-ranked candidates are supported by publications reinforcing the confidence in the approach.
In another paper showcasing the benefits of an in silico approach, Palatnik‐de‐Sousa et al. 8 report the design and development of a multiepitope multivariant vaccine based on highly conserved epitopes of multiple proteins of all SARS-CoV-2 variants. The authors propose that this could offer more long-lasting protection against different strains of SARS-CoV-2 compared with current vaccines. The vaccine was developed based on highly promiscuous and robust HLA binding CD4 + and CD8 + T cell epitopes of the S, M, N, E, ORF1ab, ORF 6 and ORF8 proteins of SARS-CoV-2 variants Alpha to Omicron. The study found that the selected epitopes were 100% conserved among all 10 studied variants, supporting the potential efficacy of the multivariant multiepitope vaccine in generating cross-protection against infections by viruses of different human SARS-CoV-2 clades. The use of immunoinformatics and in silico approaches to design the vaccines in these articles could be a cost-effective and time-efficient method for developing vaccines for other infectious diseases in the future.
The translation of scientific discoveries into practical applications ensures the successful development and evaluation of effective vaccines, such as those reported by Quach et al. 9 and Uddin et al. 10 . Quach et al. 9 report the development of a peptide-based smallpox vaccine by identifying and evaluating immunogenic peptides from vaccinia-derived peptides. They assessed the immunogenicity of these T-cell peptides in both transgenic mouse models and human peripheral blood mononuclear cells. The vaccine, based on four selected peptides, provided 100% protection against a lethal viral challenge and induced a long-term memory T-cell response, highlighting the potential of peptide-based vaccines for infectious diseases. Uddin et al. 10 developed and evaluated a mucosal vaccine against the bovine respiratory pathogen Mannheimia haemolytica using Bacillus subtilis spores as an adjuvant. They found that intranasal immunization of spore-bound antigens generated the best secretory IgA-specific response against both PlpE and LktA in all bronchoalveolar lavage, saliva, and faeces samples. The spore-based vaccine may offer protection in cattle by limiting colonization and subsequent infection, and Spore-MhCP warrants further evaluation in cattle as a mucosal vaccine against M. haemolytica . This technology has potential commercial benefits as production of B. subtilis is well established and has low-cost inputs, and B. subtilis is recognized as a probiotic that has generally regarded as safe status, used commercially in food/feed products for human beings, poultry, cattle, swine, and fish. The use of oral administration of the vaccine would allow for large-scale administration, which is especially important as livestock management strategies, including vaccination, are cost- and ease-of-use dependent. The work highlights innovative approaches to address pressing challenges in vaccine development.
Understanding cellular responses following the administration of vaccines is crucial in assessing their efficacy and safety and in the development of improved vaccine strategies. Gmyrek et al. 11 characterize the B cell response in mice vaccinated with a live-attenuated HSV-1 mutant, 0ΔNLS, and compare it to the parental virus, GFP105. The study found that 0ΔNLS vaccination resulted in a more robust B cell response, including an increase in CD4 + follicular helper T cells, germinal B cells, and class-switched B cells, as well as an elevated titer of HSV-1-specific antibody. The study reports that HSV-1 thymidine kinase and glycoprotein M are likely expendable components in the efficacy of a humoral response to ocular HSV-1 infection. Lunardelli et al. 12 provide a detailed assessment of the immune responses induced after immunization with different regions of the ZIKV envelope protein. The study found that immunization with E ZIKV, EDI/II ZIKV, and EDIII ZIKV proteins induced specific IFNγ-producing cells and polyfunctional CD4 + and CD8 + T cells. The study also identified four peptides present in the envelope protein capable of inducing a cellular immune response to the H-2Kd and H-2Kb haplotypes. The results suggest that the ZIKV envelope glycoprotein is highly immunogenic and could be a potential target for developing a vaccine against ZIKV. A paper by Suryadevara et al. 13 contributes to understanding the molecular signature of CD8 + Trm cells elicited by subunit vaccination and their potential to protect against respiratory infectious diseases. The molecular signature of subunit vaccine-elicited CD8 + Trm cells resembles those elicited by virus infection or vaccination, with distinct molecular signatures distinguishing lung interstitial CD8 + Trm cells from effector memory and splenic memory counterparts. The transcriptome signature of the elicited CD8 + Trm cells provided clues to the basis of their tissue residence and function. Insights into cellular responses, such as those provided by the studies mentioned above, can not only help us understand tissue-specific responses to diseases but also how to harness them to promote resistance or treatment.
The advancements in vaccine research are transforming the landscape of disease prevention. From mRNA vaccines to novel antigenic targets, adjuvants, and delivery systems, these breakthroughs offer new avenues for combating infectious diseases and improving global public health 2 , 3 , 5 , 6 , 14 , 15 . Addressing vaccine hesitancy 16 , 17 and ensuring equitable access to vaccines are also top priorities 18 . Continued investment in research, collaboration, and development is essential to drive innovation and overcome challenges. The Collection highlights the innovative strategies, novel technologies, and cutting-edge research in vaccine technology, formulation, and delivery systems that have revolutionized vaccine development. With these advancements, we are inching closer to a future where the burden of preventable diseases is significantly reduced, paving the way for healthier communities and a safer world.
R Rappuoli CW Mandl S Black E Gregorio De 2011 Vaccines for the twenty-first century society Nat. Rev. Immunol. 11 865 872 https://doi.org/10.1038/nri3085
Article CAS PubMed PubMed Central Google Scholar
JR Mascola AS Fauci 2020 Novel vaccine technologies for the 21st century Nat. Rev. Immunol. 20 87 88 https://doi.org/10.1038/s41577-019-0243-3
Article CAS PubMed Google Scholar
D Riel van E Wit de 2020 Next-generation vaccine platforms for COVID-19 Nat. Mater. 19 810 812 https://doi.org/10.1038/s41563-020-0746-0
Article ADS CAS PubMed Google Scholar
N Pardi MJ Hogan FW Porter D Weissman 2018 mRNA vaccines—a new era in vaccinology Nat. Rev. Drug Discov. 17 261 279 https://doi.org/10.1038/nrd.2017.243
AJ Pollard EM Bijker 2021 A guide to vaccinology: From basic principles to new developments Nat. Rev. Immunol. 21 83 100 https://doi.org/10.1038/s41577-020-00479-7
S Rauch E Jasny KE Schmidt B Petsch 2018 New vaccine technologies to combat outbreak situations Front. Immunol. 9 1963 https://doi.org/10.3389/fimmu.2018.01963
SJ Goodswen PJ Kennedy JT Ellis 2023 A state-of-the-art methodology for high-throughput in silico vaccine discovery against protozoan parasites and exemplified with discovered candidates for Toxoplasma gondii Sci. Rep. 13 8243 https://doi.org/10.1038/s41598-023-34863-9
Article ADS CAS PubMed PubMed Central Google Scholar
I Palatnik-de-Sousa 2022 A novel vaccine based on SARS-CoV-2 CD4+ and CD8+ T cell conserved epitopes from variants Alpha to Omicron Sci. Rep. 12 16731 https://doi.org/10.1038/s41598-022-21207-2
HQ Quach IG Ovsyannikova GA Poland RB Kennedy 2022 Evaluating immunogenicity of pathogen-derived T-cell epitopes to design a peptide-based smallpox vaccine Sci. Rep. 12 15401 https://doi.org/10.1038/s41598-022-19679-3
Uddin, M. S. et al. Development of a spore-based mucosal vaccine against the bovine respiratory pathogen Mannheimia haemolytica. Sci. Rep. https://doi.org/10.1038/s41598-023-29732-4 (2023).
GB Gmyrek AN Berube VH Sjoelund DJJ Carr 2022 HSV-1 0∆NLS vaccine elicits a robust B lymphocyte response and preserves vision without HSV-1 glycoprotein M or thymidine kinase recognition Sci. Rep. 12 15920 https://doi.org/10.1038/s41598-022-20180-0
VAS Lunardelli 2022 ZIKV-envelope proteins induce specific humoral and cellular immunity in distinct mice strains Sci. Rep. 12 15733 https://doi.org/10.1038/s41598-022-20183-x
N Suryadevara 2022 A molecular signature of lung-resident CD8+ T cells elicited by subunit vaccination Sci. Rep. 12 19101 https://doi.org/10.1038/s41598-022-21620-7
J Wallis DP Shenton RC Carlisle 2019 Novel approaches for the design, delivery and administration of vaccine technologies Clin. Exp. Immunol. 196 189 204 https://doi.org/10.1111/cei.13287
P Kalita T Tripathi 2022 Methodological advances in the design of peptide-based vaccines Drug Discov. Today 27 1367 1380 https://doi.org/10.1016/j.drudis.2022.03.004
HJ Larson 2018 The state of vaccine confidence Lancet 392 2244 2246 https://doi.org/10.1016/s0140-6736(18)32608-4
Article PubMed Google Scholar
F DeStefano HM Bodenstab PA Offit 2019 Principal controversies in vaccine safety in the United States Clin. Infect. Dis. 69 726 731 https://doi.org/10.1093/cid/ciz135
Organization, W. H. Access and allocation: How will there be fair and equitable allocation of limited supplies? (2021).
Download references
Acknowledgements
On behalf of all the editors of this Collection, I extend my deepest appreciation to the authors for their invaluable contributions. I appreciate the peer reviewers who generously dedicated their time to evaluate and help improve these articles. I am also grateful to Nature Research and the editorial team at Scientific Reports for extending me an invitation to organize and edit this Collection.
Author information
Authors and affiliations.
Molecular and Structural Biophysics Laboratory, Department of Biochemistry, North-Eastern Hill University, Shillong, 793022, India
Timir Tripathi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Timir Tripathi .
Ethics declarations
Competing interests.
The author declares no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Tripathi, T. Advances in vaccines: revolutionizing disease prevention. Sci Rep 13 , 11748 (2023). https://doi.org/10.1038/s41598-023-38798-z
Download citation
Published : 20 July 2023
DOI : https://doi.org/10.1038/s41598-023-38798-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Open access
- Published: 09 November 2023
To vaccinate or not to vaccinate? The interplay between pro- and against- vaccination reasons
- Marta Caserotti 1 ,
- Paolo Girardi 2 ,
- Roberta Sellaro 1 ,
- Enrico Rubaltelli 1 ,
- Alessandra Tasso 3 ,
- Lorella Lotto 1 &
- Teresa Gavaruzzi 4
BMC Public Health volume 23 , Article number: 2207 ( 2023 ) Cite this article
2292 Accesses
1 Altmetric
Metrics details
By mid 2023, European countries reached 75% of vaccine coverage for COVID-19 and although vaccination rates are quite high, many people are still hesitant. A plethora of studies have investigated factors associated with COVID-19 vaccine hesitancy, however, insufficient attention has been paid to the reasons why people get vaccinated against COVID-19. Our work aims to investigate the role of reasons in the decision to get vaccinated against COVID-19 in a representative sample of 1,689 adult Italians (March–April 2021) balanced in terms of age, gender, educational level and area of residence.
Through an online questionnaire, we asked participants to freely report up to three reasons for and against COVID-19 vaccination, and the weight each had in the decision to get vaccinated. We first investigated the role of emotional competence and COVID-19 risk perception in the generation of both reasons using regression models. Next, we studied the role that the different reasons had in the vaccination decision, considering both the intention to vaccinate (using a beta regression model) and the decision made by the participants who already had the opportunity to get vaccinated (using a logistic regression model). Finally, two different classification tree analyses were carried out to characterize profiles with a low or high willingness to get vaccinated or with a low or high probability to accept/book the vaccine.
High emotional competence positively influences the generation of both reasons (ORs > 1.5), whereas high risk perception increases the generation of positive reasons (ORs > 1.4) while decreasing reasons against vaccination (OR = 0.64). As pro-reasons increase, vaccination acceptance increases, while the opposite happens as against-reasons increase (all p < 0.001). One strong reason in favor of vaccines is enough to unbalance the decision toward acceptance of vaccination, even when reasons against it are also present ( p < 0.001). Protection and absence of distrust are the reasons that mostly drive willingness to be vaccinated and acceptance of an offered vaccine.
Conclusions
Knowing the reasons that drive people’s decision about such an important choice can suggest new communication insights to reduce possible negative reactions toward vaccination and people's hesitancy. Results are discussed considering results of other national and international studies.
Peer Review reports
Introduction
By mid 2023 the European Union reached nearly 75% vaccine coverage for the primary vaccine cycle against COVID-19, with countries such as Croatia, Slovakia, and Poland falling short of 60% and others such as France, Portugal, and Italy close to 90% [ 1 ]. Although vaccination rates are, on average, quite high, many people are still hesitant. Vaccine hesitancy indicates the delay or refusal of a vaccine despite availability in vaccine services [ 2 , 3 ] and is a multidimensional construct, resulting from the interaction between individual, social, and community aspects [ 4 ]. In the last two years, a plethora of studies have investigated factors associated with COVID-19 vaccine hesitancy showing, for example, that vaccine hesitancy is higher in women [ 5 , 6 ], in young people [ 5 , 7 , 8 ], in people with low education [ 8 , 9 ], low trust in authorities [ 10 , 11 ], and strong conspiracy beliefs [ 5 , 12 , 13 ]. However, to the best of our knowledge no one has investigated the interplay that pro- and against- vaccination reasons may play in the choice to get vaccinated, namely what happens when a person has both pro- and against-vaccine considerations. Trying to fill this gap in the literature, our work aims to investigate how different reasons and the importance people place on them are likely to influence the decision to get vaccinated against COVID-19.
In line with the vaccine hesitancy continuum defined by SAGE [ 2 ], while extremely pro-vax people are likely to express only reasons pro-vaccination and extremely no-vax people are likely to express only reasons against vaccination, individuals who fall between the two extreme end-points are likely to feel some doubts. This large number of people offer us the unique opportunity to assess which category of reasons (pro- vs. against- vaccination) is more impactful in driving people's vaccination decisions. As it is reasonable to imagine, among the reasons for choosing to get (or not) vaccinated some reasons are more rational, while others are more related to affect. For example, there are people who rationally recognize the importance of vaccines but at the same time are frightened by the side effects. Thus, the decision to get (or not) vaccinated is the result of a complex process, in which costs and benefits are weighed more or less rationally. Indeed, while several studies have pointed out that the decision to vaccinate is due to cognitive rather than emotional processes [ 14 , 15 , 16 , 17 ], others have highlighted the role of affect and risk perception in the vaccination decision [ 18 , 19 , 20 ]. Thus, the intention to accept the vaccine is driven by emotional and affective feelings as much as by cognitive and rational judgments. Particular attention to what people feel and think about vaccine-preventable diseases and vaccination in general is paid in the model developed by the “Measuring Behavioral and Social Drivers of Vaccination” (BeSD), a global group of experts established by the World Health Organization [ 21 ]. This model encompasses two groups of proximal antecedents of vaccination, namely, what people think and feel (e.g., perceived risk, worry, confidence, trust and safety concerns) and social processes (e.g., provider recommendation, social norms and rumors). Antecedents affect vaccination motivation (i.e., vaccination readiness, willingness, intention, hesitancy), which can then be strengthened or weakened by practical issues (such as vaccine availability, convenience and cost but also requirements and incentives), resulting in acceptance, delay or refusal of vaccination (vaccination behavior).
Although some studies have considered whether the cognitive or affective component has greater weight in determining the intention to vaccinate, no one, to the best of our knowledge, has studied the interplay between pro- and against- vaccination reasons, nor the weight these have in the choice to vaccinate. In addition to the drivers already studied in the literature [ 5 , 6 , 7 , 8 , 11 , 12 ], we believe that the focus on this interaction may be relevant to better understand the complex phenomena related to vaccine hesitancy. Few recent studies have attempted to investigate the complexity of vaccination choice by studying the reasons why people choose to get (or not) vaccinated against COVID-19. Fieselmann and colleagues [ 22 ] highlighted that among the reasons that reduce adherence to vaccination are a low perception of its benefits, a low perception of the risk of contracting COVID-19, health concerns, lack of information, distrust of the system, and spiritual or religious reasons. Another study, instead, shed light on the reasons that encourage hesitant people to consider vaccination, such as protecting themselves, their family, friends and community from COVID-19, and being able to return to normal life [ 23 ].
In the present study we asked the participants to spontaneously come up with their own reasons to get (or not) vaccinated, without limiting or influencing them with a set of predefined options to choose from, thus aiming to obtain more genuine answers that may better capture the intuitive aspect of people’s opinions (for a similar reasoning see [ 24 ]). The procedure we used has been implemented by Moore et al. [ 23 ], the only study, as far as we know, that asked for reasons with an open-ended question. Critically, in their study, participants were asked to report only reasons in favor of vaccination (e.g., "What are your reasons for getting the COVID-19 vaccine?"), excluding reasons against. By contrast, we asked participants to freely report up to three reasons in favor and up to three reasons against COVID-19 vaccination and to rate on a 5-point Likert scale their weight in the decision about getting (or not) vaccinated.
From a theoretical point of view, the reasons pro- and against vaccination may be seen within the framework of prospect theory [ 25 , 26 ] which suggests that people evaluate the outcome of a choice based on a reference point, against which losses and gains are determined: the former below this point, the latter above this point. Importantly, especially in this specific context, losses and negative consequences are weighted more than gains and benefits, making us hypothesize that if a person has one reason for and one reason against the vaccine, which are of equal importance, they will more likely lean toward choosing not to vaccinate. Consistently, it is known that negative experiences have a greater impact than neutral or positive ones (i.e., the negativity bias [ 27 ]).
Besides delving into the reasons that may influence the choice to get (or not) vaccinated, it would be interesting to also look at the individual differences that may determine the reporting of pro- and against- vaccination reasons and their valence. In this regard, the literature suggests that risk perception and emotion regulation can both have a great impact in the decision to get vaccinated. For instance, studies conducted during H1N1 influenza have shown that perception of disease-related risk is one of the strongest predictors of vaccine adherence [ 28 , 29 ]. Additional insights have been provided by more recent studies investigating the role of COVID-19 risk perception in the decision to get vaccinated against COVID-19. Viswanath and colleagues [ 30 ] showed that people are more willing to vaccinate themselves and those under their care to the extent to which they feel more vulnerable to COVID-19 and rate the consequences of a possible infection as severe. Such a relationship between COVID-19 risk perception and intention to vaccinate was confirmed by another study using a cross-sectional design, which focused on the early months of the pandemic [ 31 ]. This study also examined how risk perception changed during the pandemic phases and showed that during the lockdown, compared to the pre-lockdown phase, also those who reported some hesitancy were more likely to get vaccinated when they perceived a strong COVID-19 risk.
With regard to emotion regulation, the literature suggests that people react differently to affective stimuli [ 32 ] and that their decisions are influenced by their abilities to regulate emotions [ 33 , 34 ]. Recent works investigating the relationship between hesitancy in pediatric vaccinations and the emotional load associated with vaccinations, have shown that a negative affective reaction is one of the factors leading to lower vaccine uptake [ 35 , 36 ]. Specifically, Gavaruzzi and colleagues [ 36 ] showed that concerns about vaccine safety and extreme views against vaccines are associated with vaccine refusal. Interestingly, they also showed that parents' intrapersonal emotional competences, i.e., their ability to manage, identify, and recognize their own emotions, is critical to vaccine acceptance for their children. Therefore, in our study we measured people's risk perception and emotional competencies to assess their possible role in the production of reasons in favor and against vaccination.
As described in Fig. 1 , the relationship between different domains of interest can be hierarchically structured, using a directed acyclic graph, starting from the risk perception and emotion regulation, to the generation of pro- and against- vaccination reasons and their valence, and finally to the vaccination willingness/adherence. With respect to the mentioned structure, we are interested to investigate the following research hypotheses:
The number and weight associated with reasons pro- and against-vaccination should be influenced by individual differences in the ability to regulate emotions;
The number and weight associated with pro-vaccination reasons should be influenced by individual differences in COVID-19 risk perception;
A higher number of strong (i.e., with high weight) reasons pro- (vs. against-) vaccination should correspond to a more (vs. less) likelihood to accept the vaccination.
Generating an equal number of reasons in favor and against vaccination should lead to a weaker likelihood to accept the vaccination.
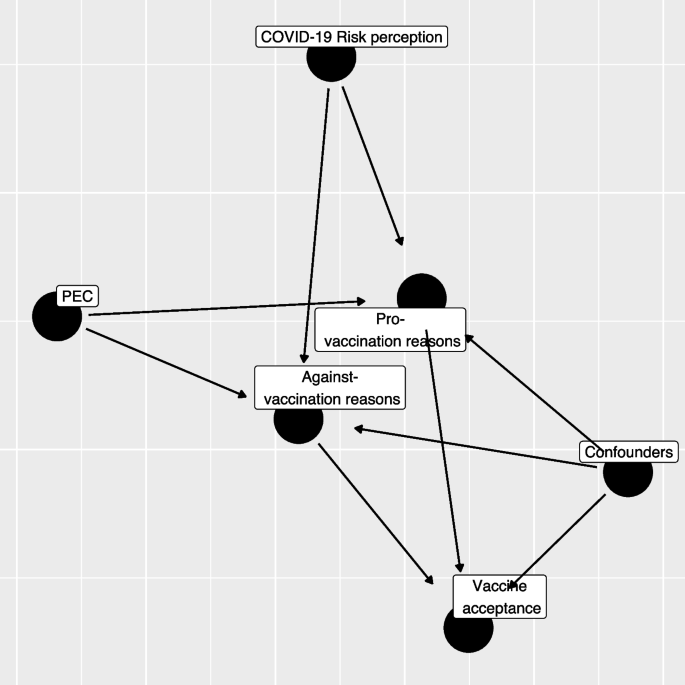
Directed Acyclic Graph (DAG) between variables considered in the study (PEC: Short Profile of Emotional Competence scale)
As we conducted the study between March and April 2021, a time when vaccinations were being progressively rolled out, we decided to consider the role of personal reasons on both the intention to get vaccinated (for those who had not yet had the opportunity to get vaccinated) and the choice already made (e.g., vaccine received or booked vs. refused).
Finally, through a non-parametric classification analysis, we will explore how specific pro- and against-vaccination reasons impact the decision to get (or not) vaccinated. Specifically, we will investigate the role that different categories of reasons play in the choice to vaccinate.
Participants
Data collection was commissioned to a survey and market research agency (Demetra Opinions.net), with the aim of securing a representative sample of the adult (+ 18) Italian population, estimated at 49.8 million [ 37 ]. The sample was balanced in terms of age, gender, educational level (middle school or lower, high school, degree or higher), and area of residence (North, Center, South, and Islands). The agency distributed via email the survey link to its panelists, who freely decided whether to participate in the study in exchange for financial compensation. Out of 1,833 participants who started the questionnaire, 77 (4%) were excluded because they did not complete the survey and 16 (0.9%) were excluded since they reported offensive content in open-ended questions. Finally, 124 (6.8%) participants were excluded because of missing information. Thus, the final sample consisted of 1,689 participants. The project was approved by the ethical committee for Psychology Research of the University of Padova (Italy), with protocol number 3911/2020 and informed consent was obtained for all participants.
We developed an ad-hoc questionnaire including a series of open-ended and closed questions (see Additional file 1 : Appendix 2 for the full material). We first investigated the vaccination status of the participants, asking whether they already had received at least the first dose, whether they had booked it or were still ineligible, and finally whether they had refused the vaccination. Those not yet eligible were asked to rate how likely they would be to get vaccinated at the time they responded (0 = Not at all likely , 100 = Extremely likely ). Then, we asked participants to report a maximum of three reasons both in favor of the COVID-19 vaccine and against it (in counterbalanced order) and to rate how much each of the reported reasons weighed in their choice to vaccinate or not, on a 5-point likert scale (1 = Not at all , 5 = Extremely ). Due to the sparsity on the rate and the number of provided reasons we re-coded the provided information into two semi-quantitative variables, one for pro- and one for against- vaccination reasons, as following: missing/invalid reasons, low average rating (answers 1–3 on the Likert scale) and 1–3 reasons, high rating (answers 4–5 points on the Likert scale) and 1 reason, and high average rating (answer 4–5 points on the Likert scale) and 2–3 reasons.
The questionnaire also included the 20-item Short Profile of Emotional Competence scale (S-PEC; [ 38 ]) to measure intra- and inter-personal emotional competences separately. The intra-personal scale (10 items) refers to emotional competences related to oneself and it includes items such as "In my life I never make decisions based on my emotions'' or "I don't always understand why I react in a certain way". The inter-personal scale (10 items) refers to emotional competences related to other people and it includes items such as “If I wanted, I could easily make someone feel uneasy” or “Most of the time, I understand why the people feel the way they do”. All items are answered on a 7-point likert scale (1 = Not at all agree , 7 = Completely agree ). The internal consistency of the S-PEC scale, measured by means of Cronbach’s α, was adequate (α = 0.81). Further, we measured participants' risk perception of COVID-19 by asking them to indicate how scared they felt of the virus, how serious they think the disease is, how likely they think they are to get sick, and how worried they feel about the various mutations [ 10 , 31 ]. We then asked participants to report their age, gender, educational level, their occupation (health workers, white-collar workers, entrepreneurs, other non-health-related contract forms, and the unemployed), whether they already had COVID-19 (No or don't know, Yes asymptomatic, Yes with few symptoms, and Yes with severe symptoms). The questionnaire was pilot tested by 30 participants who filled the questionnaire first then were asked to discuss and comment on the comprehension of the wording of questions and answer options. Two questions were slightly reworded to improve clarity.
Scoring of reasons
In the first instance, a bottom-up process from reasons to categories was followed by reading a sample of both types of reasons, with the aim of constructing initial categorizing patterns. Examples of pro-vaccination reasons include protection of personal and public health, return to normality, and civic duty; while reasons against vaccination include fears for one's health, sociopolitical perplexity, and distrust of science and institutions (see Additional file 1 : Appendix 1). At this stage, response information was added to the categorizations indicating whether the responses were valid or missing/invalid. Specifically, valid responses had both a reason and the respective weight; missing/invalid responses were those where reason, weight or both were missing or with utterly unrelated concepts or meaningless strings or letters. Finally, by applying a top-down process, we constructed macro categories by merging specific conceptually assimilated categories, so as to avoid the dispersion of data into too many ramifications (see Table S 5 ).
Statistical analysis
Descriptive analysis.
All the analyses were performed only on respondents with no missing observations on the variables of interest (1,681, 92%) excluding also a limited number of those with a non-valid set of pro- or against-vaccination reasons (Table S 1 ; 0.9%). The study variables were summarized in frequency tables and figures (frequency for categorical variables, median and Interquartile Range (IQR) for continuous variables). Kruskal–Wallis tests were computed to compare the distribution of continuous variables across the categories of vaccine status. Categorical variables were compared using chi-squared or Fisher's exact test where expected frequencies in any combination were less than 10. Statistical significance was assumed at the 5% level.
COVID-19 Perceived risk—exploratory factor analysis
An Exploratory Factorial Analysis (EFA) was performed on groups of variables related to COVID-19 perceived risk: scare, severity, contagiousness, and the likelihood of mutation. Since the presence of limited support (0–100 scale) and non-normal marginal distribution, the EFA was performed using a weighted least square mean and variance adjusted (WLSMV) estimator. We extracted from the EFA only the first factor, which explained the highest percentage of variance (Table S 2 ; 61%). The estimated loadings were then used to calculate the regression factor scores. The number and the name of items included, their internal consistency (Cronbach’s α), the estimated loadings, and the proportion of deviance explained are reported in Table S 2 .
Propensity score weighting
At the time of data collection (March–April 2021), the vaccine offer was not opened to the entire population. To adjust the estimates of the following regression models for the propensity to receive the vaccine, we estimated a logistic regression model in which the dependent variable was the response to the question about a previous vaccination offer (Yes/No), while all the factors that can influence the vaccine proposal served as independent variables: age-class (young ≤ 25, young adult 26–45, adult 46–65, elderly 66–84), gender (male, female), occupational status (health worker, not at work, not health worker-employer, not health worker-entrepreneur, not health worker-other), educational level (low = middle school or lower, medium = high school, high = degree or higher), key worker status (yes, no, I don’t know), past COVID-19 contagion (no, yes asymptomatic, yes low symptoms, yes severe symptoms), and familiar status (single/in a relation, married/cohabitant, divorced/separated/other). The predicted probability was used to estimate the weights for the following regression models using a framework based on an inverse probability of treatment weighting (IPTW; for further details, see [ 39 ]).
Regression models
Our research questions can be summarized by trying to describe the relationship exploited by the directed acyclic graph in Fig. 1 . The first step regression model aims to assess how S-PEC scores (inter- and intra-personal) and COVID-19 risk perception influenced the reasons pro- and against-vaccination produced by participants while considering the presence of a set of confounders (age-class, gender, occupational status, educational level, key worker status, and familial status).
Since both the pro- and against-vaccination reasons are formed by a categorical variable with 4 levels (missing/invalid, low 1/2/3 reasons, high 1 reason, high 2/3 reasons), we evaluated whether S-PEC and COVID-19 risk perception scores influenced the distribution of pro- and against-vaccination reasons employing two different multinomial regression models including all the previously mentioned variables (S-PEC, COVID-19 risk perception, and confounders). The overall significance of a variable in the model was tested using an analysis of the variance (ANOVA).
The second step in the analyses was taken to investigate whether the generation of pro- and/or against-vaccination reasons affected the willingness to be vaccinated or the vaccine acceptance. Each participant reported their willingness to get vaccinated on a 0–100 scale or, in case a COVID-19 vaccine had been already offered, their vaccination status (done, booked, or refused). For respondents who had not yet been contacted for booking/getting the vaccination, we evaluated whether pro- and/or against vaccination reasons influenced the willingness to be vaccinated by employing a beta regression model in which the respondent variable scale (0–100) was rescaled to be a relative frequency [ 40 ]. The full models included the semi-quantitative pro- and against-vaccination reasons variables and, even if non-statistically significant, all the confounders in order to adjust for age class, gender, educational level, occupational status, familial status, and key worker status. Beta regression coefficients were estimated using a maximum likelihood estimator (MLE). Results were presented in terms of Odds Ratios (ORs) by exponentiating the estimated coefficients and producing a relative 95% Confidence Interval (95% CI).
A further regression analysis was conducted through a logistic regression model to explain which factors influenced vaccine acceptance (done/booked vs. refused) among those who already received the vaccine offers. The full model included the same variables considered in the previous beta regression model, after recoding the variables related to pro- and against-vaccination reasons into a binary form (missing/invalid vs. presence of at least one valid reason) due to low sample size and the sparsity of the response variable. As a consequence, we tested a simplified version of Hypothesis 3, considering the presence (vs. missing/invalid) of pro- or against-vaccination reasons in order to test their influence on the probability of having accepted/booked the vaccination.
Results were reported employing ORs and relative 95% Confidence Interval (95% CI).
Both the beta regression and logistic regression were weighed using an IPTW scheme to take into account the presence of a different probability of a vaccine offer among respondents.
The presence of an interaction between pro- and against-vaccination reasons was tested by means of a likelihood ratio test. The regression models were estimated through the R 4.0 program (R Core Team, 2021), and for the beta regression we employed the betareg package [ 41 ].
Classification tree analysis
Two different classification tree analyses were carried out to characterize profiles with a low or high willingness to get vaccinated (respondents who had not yet been offered a vaccine) or with a low or high probability to accept/book the vaccine (respondents who had already received a vaccine offer).
Although the dependent variables were non-normally distributed (scale 0–100 or binary 0/1), we considered them continuously distributed adopting a splitting criterion based on the analysis of the variance (ANOVA). We tested the inclusion in the model considering the type of pro- or against-vaccination reasons. A tree pruning strategy was adopted to reduce classification tree overfitting considering the overall determination coefficient (R 2 ) as an indicator and fixing that at each classification step in the tree if the R 2 did not increase by 0.5% the tree should be stopped. Classification tree analysis was performed using the rpart package [ 42 ] on R environment [ 43 ].
The main characteristics of the respondents by vaccination status (received, booked, not yet, and refused) were reported in Table 1 . Among respondents, 23.3% were offered the vaccination and, among them, 13.8% refused it (Fig. S 1 ). Among those not yet eligible, willingness to be vaccinated showed a median value of 80 points (average: 68.7). The distribution of gender was almost equal (51% females, 49% male), and the median age was 47 years old (IQR: 34–57 years). Educational level was low in 41% of the sample, while the most represented employment status was not at work (39%) followed by employed (37%), and entrepreneur (9.8%). A quarter (26%) of respondents classified themselves as key workers during the COVID-19 pandemic. The predominance of respondents (63%) were married or living with a partner, while only 9% had had a COVID-19 infection.
COVID-19 risk perception and the S-PEC score (intra- and inter-personal) were categorized into three categories according to empirical tertiles (low:1 st tertile, medium: 2 nd tertile, high: 3 rd tertile). The level of COVID-19 risk perception differed across vaccination status ( p < 0.001). The reasons pro- and against-vaccination have a different distribution according to COVID-19 vaccination status (Table 2 ). The highest frequency of pro-vaccination reasons was reported by those who received the COVID-19 vaccination; conversely the lowest frequency of pro-vaccination reasons was generated by those who refused the vaccine, whereas, intermediate frequencies were shown by people who were not yet offered the vaccination and those who had booked the vaccine, who reported a comparable distribution of the number of pro-vaccination reasons. A reverse pattern was exhibited for against-vaccination reasons, which were generated with the highest percentage by respondents who refused the vaccine (in particular high and multiple reasons). Conversely those who have booked/done the COVID-19 vaccine showed the lowest frequency of reasons against vaccination, while respondents without a vaccine offer reported an intermediate frequency of reasons against vaccination.
The estimated results of the propensity score model for the vaccine offer are shown in Table S 3 . Respondents older than 65 years exhibited a nearly four-fold increase in the probability to be contacted for the vaccination with respect to the reference age-class (≤ 25 years). All non-health employees showed a high drop in the probability of having received the vaccination offer, while the probability increased as the educational level increased. Being a key worker during pandemic resulted in an increased probability of having received the vaccination proposal while no statistical significant influence was observed for the past COVID-19 contagion or for familial status. The distribution of the propensity score by vaccine status obtained by the model is reported in Fig. S 1 , in which it is shown that the distribution is different by vaccine offer, but the two density functions partially overlap. The discriminant power of the propensity score estimated was only discrete (ROC analysis, AUC: 71.8%).
The results of the multinomial regression models which investigated the effect of emotional competences and risk perception on the generation and the predictors of pro- and against-vaccination reasons with respect to missing/invalid level and the reference categories are presented in Table 3 (see also Fig. 1 ). Compared to the reference category (missing/invalid), high values of S-PEC-self were associated with a higher probability to report pro- and against-vaccination reasons (all ORs > 1.5), while high values of S-PEC-others were associated with a mild probability to report multiple pro-vaccination reasons (all ORs > 1.42). A high (vs. low) COVID-19 risk perception increased the frequency of one strong pro-vaccination reason while it had a null or low decremental effect on the frequency of against weak vaccination reasons. Further, medium (vs. low) COVID-19 risk perception only increased the strong pro-vaccination. Compared to the reference age-class (young), adults and elderly showed a higher probability to generate a strong unique pro-vaccination reason (adults vs. young OR: 1.72, 95%CI: 1.07–2.77); elderly vs. young OR: 2.24, 95%CI: 1.26–4.00), while lower probability to generate against vaccination reasons was observed for elderly compared to young respondents (OR: 0.48, 95%CI: 0.26–0.90). Female participants generated fewer strong pro-vaccination reasons (ORs < 0.73), and also fewer multiple weak against-vaccination reasons (OR: 0.68, 95%CI: 0.51–0.91) compared to male participants. Overall, the occupational status did not affect the generation of pro- and against-vaccination reasons (ANOVA test p > 0.05); however an increased frequency of low 1/2/3 against-vaccination reasons emerged among the category “Other—not health workers” compared to the reference group represented by health workers (OR: 2.52, 95%CI:1.09–5.86). Pro-vaccination reasons are more frequent as the educational level becomes higher, while the relation of the educational level with against- vaccination reasons appears weaker and significantly increased only for the presence of multiple weak reasons against vaccination (High vs. Low educational level, OR: 2.10, 95%CI: 1.45–3.03). Not being a key worker is related to a higher frequency of multiple strong both pro- and against vaccination reasons. The familiar status did not seem to be related to the frequency or the strength of the reasons, except for the status of divorced/separate/other that, with respect to the reference category single/in a relation, showed a twofold increase in the frequency of a strong unique against vaccination reason.
Through a beta regression model we investigated the predictors of willingness to be vaccinated for the participants who had not yet received the vaccination offer. As shown in Table 4 , the generation of pro- and against-vaccination reasons strongly influences the willingness to be vaccinated. The predicted probability from the combination of pro- and against-vaccination reasons is shown in Fig. 2 (and Table S 4 ): respondents who did not report any reasons had an average predicted probability above 60%, while the presence of at least one reason against vaccination decreased the willingness to be vaccinated, in particular in the case of strong multiple against vaccination reasons. On the other hand, the presence of at least one pro-vaccination reason strongly increased the probability. In the end, the presence of both strong multiple pro and against vaccination reasons resulted in a high probability of getting the vaccine. Regression models adjusted by propensity score weighting allowed us to comment the influence of potential confounders: males reported an increased willingness to be vaccinated (vs. females; OR: 1.26, 95%CI: 1.11–1.42), and so did those with a high educational level (vs. low; OR: 1.22, 95%CI: 1.04–1.44) while the opposite was true among no key workers (vs. key workers; OR: 0.85, 95%CI: 0.72–0.99).
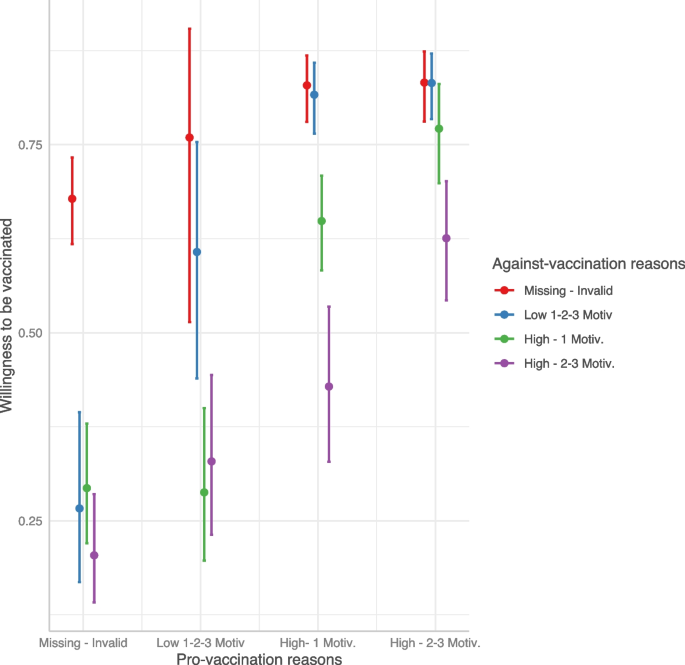
Predicted willingness to get vaccinated by interaction between pro- and against-vaccination reasons
Finally, with a logistic model we investigated the predictors of vaccine acceptance\booking. As shown in Table 5 , people who accepted or booked the COVID-19 vaccine were more likely to show pro-vaccination reasons and less likely to show against-vaccination reasons. Interestingly, when both kinds of reasons were provided, the probability of getting/booking the vaccine remained nevertheless very high (Fig. 3 ). Compared to the age class [46-65], younger age classes reported a strong reduction in the probability to have accepted/booked the vaccine. Male participants (OR: 1.53, 95%CI: 1.10–2.12) and those with a high educational level (OR: 2.65, 95%CI: 1.60–4.54) showed an increased probability of vaccine acceptance/booking when compared to females and participants with medium educational level, respectively. Being a health worker had a strong and positive influence on the probability of getting/booking the vaccine with respect to those employed as no health workers (OR: 6.61, 95%CI: 2.10–30.9).
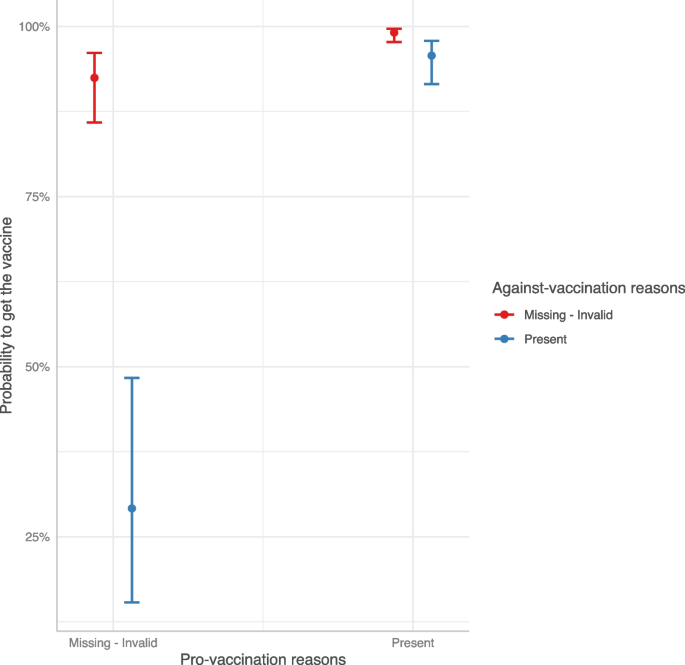
Predicted COVID-19 vaccine acceptance/booking probability by interaction between pro- and against-vaccination reasons
Two regression tree models were estimated separately on the willingness to be vaccinated for those who had not yet received the vaccine offer and on the booking/acceptance of the vaccination in case of vaccine offer. Results are shown in Fig. 4 . Considering the willingness to be vaccinated, the presence of distrust in the vaccination was the most discriminant variable; this latter in conjunction with reasons related to protection, herd immunity, and the absence of no clinical trials guided the willingness to be vaccinated. In particular, the combination of the absence of reasons related to distrust and the presence of protection reasons showed the highest values on the intention to get vaccinated (average value = 83 points, 22% of the sample). On the other side, the presence of at least one reason related to distrust without any positive reasons concerning protection, herd immunity, and trust predicted the lowest willingness to be vaccinated (average value = 29 points, 6% of the sample).
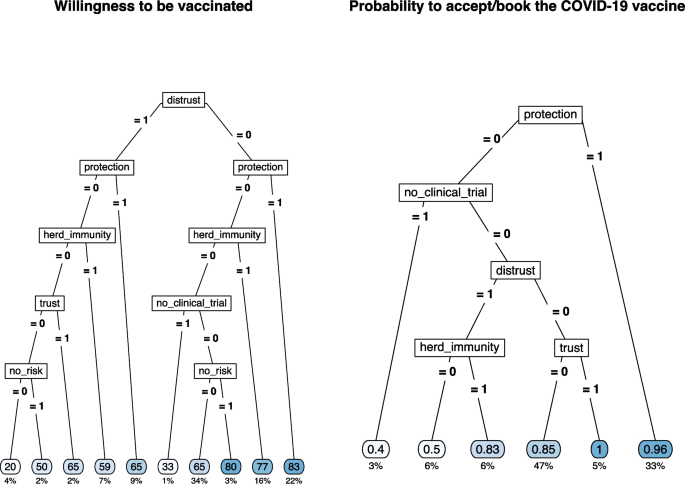
Regression tree for the willingness to be vaccinated (left) and for COVID-19 vaccine acceptance/booking (right) by selected type of pro- and against-vaccination reasons
The sense of protection given by the vaccine or the trust in the vaccination was the main reason for vaccination acceptance/booking (average probability = 0.96 and 1.00, 33% and 5% of the sample, respectively). The combination of the absence of protective reasons and the presence of doubts about the lack of clinical studies results in the lowest likelihood of accepting/booking the vaccination (average probability = 0.40, 3% of the sample). The presence of distrust and the belief in herd immunity were the other discriminant reasons with intermediate results in terms of the probability to accept/book the vaccination.
The frequency of each category of pro- and against-vaccination reasons by COVID-19 vaccine status is shown in Table S 5 .
In the present study we aimed to investigate the reasons behind the decision to get (or not) vaccinated against COVID-19 by asking participants to report up to three reasons in favor and three reasons against the COVID-19 vaccination and to indicate the weight these reasons had in their decision. Although some researchers discourage categorization, the sparsity of the responses related to the number of reasons and their weight implies a semi-quantitative solution since a simple variable multiplication between rating and frequency (recoding to zero in case of zero reasons) is not feasible. In this case, this approach was not satisfactory as such coding would not allow differences underlying identical scores to emerge. For example, only 1 strong motivation (rating 5) would be coded in the same way as three motivations with weights 1, 2, and 2. Instead, we decided to categorize the combination of frequency-weight reasons as categorical variables (missing/invalid, low 1/2/3 reasons, high 1 reason, high 2/3 reasons) in which rating and number of reasons are combined into a single variable. This categorization allows us not only to study the weight that different categories have on the decision to get vaccinated but also to overcome the risk of imputing a specific value for missing responses.
As shown in Fig. 1 , analyses were run in two steps. The first step aimed to assess how emotional competences and risk perception impacted the generation of reasons pro- and against-vaccination (Hypotheses 1A and 1B), whereas the second step investigated how different reasons affected the intention to get vaccinated (Hypotheses 2 and 3). The results support the hypotheses that emotional competences and risk perception play a significant role. Regarding emotional competence as measured by the S-PEC, the results show that high intra-personal emotional competence positively influences the production of stronger and more numerous pro-vaccination and against-vaccination reasons (confirming Hypothesis 1A). This result suggests that greater awareness of one's emotions and of what one is feeling promotes higher fluency in the production of reasons about the vaccination. Research has shown that people can be ambivalent about vaccines and hold both positive and negative reasons [ 2 , 44 ]. It is reasonable to assume that, compared to people with low intra-personal emotional competences, those with high intra-personal emotional competences are more likely to have higher awareness of these contrasting attitudes and to embrace them without suppressing one of the two stances. Furthermore, the results showed that only high inter-personal emotional competences influence the generation of multiple strong reasons in favor of vaccination, and this appears to be related to the perception of vaccines as a public good and a tool to protect others. As for risk perception, a moderate to high perception of risk associated with COVID-19 influences the generation of strong pro-vaccination reasons (confirming Hypothesis 1B). These results are in line with the literature showing that a high perception of risk associated with COVID-19 positively influences the decision to get vaccinated [ 30 , 31 , 45 , 46 , 47 ]. In particular, perceiving a medium/high risk leads to generating a high number of reasons strongly in favor of vaccination, while reducing the number and weight of the reasons against the vaccine. The main premise of the psychological literature examining the relationship between risk perception and affect is that one’s behaviors are affected by rapid and intuitive evaluations, either positive or negative, people make while assessing things happening around them [ 48 , 49 ]. Thus, an event is evaluated not only on the basis of objective information, but also on the basis of the experienced feelings. Emotional competence, which is clearly related to affect, also modulates how we perceive and process the emotional component underlying our judgments [ 36 ].
The results also show that, compared with younger people, those over 45 more frequently produce reasons in favor of vaccines while those over 65 produce fewer reasons against vaccination. These results are in line with the fact that younger people are at lower risk of severe consequences than older people [ 50 ], but can also be explained by considering that age was particularly salient during the period of the data collection, as the vaccination campaign was phased out by age groups, starting from the elderly. As for gender, women produced less strong pro-vaccine and weak-against vaccine reasons than men. These results are congruent with the general findings in the literature on vaccine hesitancy showing that females are more hesitant than males [ 5 , 51 , 52 ]. Furthermore, medium and high educational levels favored the production of both pro- and against-vaccination reasons, whereas not being in a relationship or being divorced/separated increased the generation of a strong reason against vaccination. Consistent with previous work [ 53 ], we confirmed that non-health professionals participants or non-key workers categories showed a lower intention to get vaccinated and a higher likelihood of having refused the vaccine compared to health care and key workers.
Once the role of demographics aspects and individual differences on the generation of reasons pro and/or against vaccination had been established, we ran two additional models to assess the role that those reasons have on the decision to accept the vaccination (see Fig. 1 ). More specifically, we tested the hypothesis that a higher number of pro- (vs. against-) vaccination reasons, connoted by a higher weight, corresponded to a stronger (vs. weaker) acceptance of vaccination (Hypothesis 2). Since data collection took place between March and April 2021, when the vaccination campaign had already started in Italy, we developed two different regression models, with the first investigating the willingness to be vaccinated in participants who were not yet offered the vaccine and the second investigating the likelihood of accepting/booking or refusing the vaccine in those who already received the offer. In particular, thanks to the propensity score weighting technique, we managed to reduce the estimates bias, especially for those factors (age, occupational status, and educational level) that influenced the vaccine offer the most [ 54 ]. The results of the two models are very similar, as the intention to get vaccinated and the likelihood of having accepted/booked the vaccine are predicted by the same factors. Specifically, the production of strong positive reasons increases either the intention to get vaccinated or having accepted/booked the vaccination. In contrast, generating strong negative reasons reduces vaccination intention and predicts the refusal of the vaccination. Hypothesis 2 is thus confirmed.
Results on the interactions between reasons, pro- and against-vaccination, and vaccination intention or vaccination choice are particularly worthy of attention. The third hypothesis was derived from the literature on prospect theory [ 25 , 26 ], suggesting that at equal intensity subjective losses are more important in determining a decision than subjective gains. We therefore expected that negative reasons would count more than positive reasons in deciding whether to get vaccinated or to accept the vaccine. However, in contrast to our hypothesis, the results showed that just the generation of a single positive reason with a strong weight was enough to shift behavior and attitude in favor of the vaccination, regardless of the number and weight of negative reasons. In other words, vaccine refusal is predicted by the absence of any positive strong reasons, while when people generate both positive and negative reasons, the positive ones seem to yield a particularly important role when having a strong weight. According to prospect theory, people evaluate their goals depending on the reference point they focus on. During the pandemic, the vaccination offered an opportunity to be safer, reduced the risk of infection, and more generally appeared as the best way to re-open and get back to life as it was before COVID-19. After a year of pandemic characterized by periods of lockdown and some re-opening attempts, people were likely feeling in a state of loss (e.g., the lost freedom to go out and meet with friends and family, the lost freedom of traveling) and were looking forward to whatever chance available to recover and return to their previous lifestyle and habits. Just as those who gamble are willing to do anything to make up for a loss, so probably those who were not entirely certain about the vaccine were more willing to take risks to recover the loss in quality of life. It follows that the pandemic emergency made people forgo some of their doubts about the vaccine when, at the same time, they had reasons to get their shot. In addition, several studies [ 19 , 55 , 56 ] have highlighted the relationship between anticipated regret and vaccination, showing that anticipated regret is associated with an increased likelihood of adhering, or having one's children adhere, to vaccine offerings. Trusting that the vaccine would work, focusing less on its potential side effects, made sense for people who were looking forward to recovering what was perceived (and was indeed) a loss of quality of life and freedom, because they desired to be back doing the things had ever enjoyed doing (e.g., going to restaurants, movies, etc.). This finding is also interesting from a communicative perspective: providing positive reasons that resonate well with people and have therefore a strong weight for them could offset their doubts, yielding to a greater acceptance of COVID-19 vaccination.
Therefore, it is crucial to consider what kind of reasons drive the decision toward or against vaccination. Allowing participants to openly report their reasons pro- or against- vaccination can facilitate a freer exploration of the concerns and reservations of the most hesitant individuals [ 24 ], thus providing valuable insights for shaping future vaccine-related communications. In fact, thanks to the regression tree on vaccination intention, it emerges that positive attitudes toward vaccines are strongly determined by "Protection" and "Community Protection" reasons. The fact that the sense of individual and collective protection is among the principal determinants of the decision with respect to COVID-19 vaccines suggests that in general vaccination is seen as a means of avoiding nefarious clinical consequences. The effect of the sense of communal protection as the reason favoring vaccination and of other-oriented S-PEC in determining the generation of multiple pro-vaccine motivations confirms previous results suggesting that people often are more willing to get vaccinated primarily to protect their loved ones [ 57 , 58 , 59 ], especially when they have a good understanding of how community immunity works [ 60 , 61 ]. However, it is worth mentioning that, at the time the study was conducted (March–April 2021), there was still uncertainty about whether COVID-19 vaccines could provide sterilizing immunity (i.e., could prevent the transmission of the infection) in addition to protecting the individual. To foster people's willingness to get vaccinated, it is crucial from a public health perspective that people understand that even when vaccines do not yield sterilizing immunity, vaccination can still increase protection of others by reducing the circulation of the virus.
The reasons that influenced the willingness to be vaccinated or the vaccination acceptance/booking were generally in line with the existing literature, although they differed depending on whether respondents had already been offered a vaccine or not: among those who did not received a vaccination offer, the main reasons promoting vaccination acceptance were protection against COVID-19 for oneself, one's family, friends, and community [ 23 ], while among the main reasons that reduced vaccination adherence for those who got the vaccine offer we found the lack of clinical trials [ 62 , 63 ], as well as the distrust of institutions and science [ 22 ]. This latter emerged as the most reported negative reason by those who have refused the vaccine and those who have not yet received the vaccine offer. Thus, effective communication aimed at defusing the perception of risk regarding vaccines themselves should focus on enhancing trust in the scientific process and experimental rigor. Indeed, these reasons were deemed as very important not only by those who refused the vaccination, but also by those who had not yet been offered the vaccine, and even by those who held mixed feelings but eventually chose to get vaccinated. While it is unlikely that individuals firmly against vaccination will be persuaded by simple interventions [ 64 ], we should keep in mind that vaccine hesitancy is a dynamic process. As such, reducing hesitancy or enhancing ambivalence, for example through motivational interviewing (e.g., [ 65 , 66 ]), could potentially lead to small shifts towards greater vaccine acceptance.
Our findings are also in line with the results of other international studies that have used a qualitative approach to examine reasons for and against vaccinations. For example, Hamilton and colleagues [ 67 ] employed a qualitative content analysis to extract the main motivations for and concerns about COVID-19 vaccination from medical records obtained by 102 consults in Australia. The study was conducted in June 2021, and revealed that most consults were driven by doubts about the vaccine available and recommended at that time (i.e., ChAdOx1-S, also known as Vaxzevria), followed by need for further information regarding vaccines and vaccination, also considering specific comorbidities. Notwithstanding the peculiarity of the Australian context in which a very low number of COVID-19 infections was observed, the analysis performed by Hamilton et al. [ 67 ] revealed a set of themes that largely overlaps with the reasons identified in our study. Indeed, among the reason to get vaccinated, 5 themes emerged: a) Protection, b) Occupational or facility responsibility or requirement, c) Trust in primary healthcare physician, d) Autonomy, and e) Civic duty, likewise, concerns about vaccination were mainly in terms of: a) Perceived vaccine risks, b) Perceived vaccine performance, c) Uncertainty, d) Autonomy, and e) Fairness in access. An aspect worth noting is that after the consultation, 81% of participants received the vaccination, 19% did not. Consistent results were observed in another study by Purvis and colleagues [ 68 ] conducted in the USA, which focused specifically on “hesitant adopters”, i.e. those who accepted vaccination but showed some level of hesitancy. To note that in this study the focus was on factors influencing the decision to get the COVID-19 vaccine, not on reasons against it. The authors interviewed 49 participants as a follow up of a larger study ( N = 2022) conducted from mid-September 2021 through mid-October 2021, to explore factors that influenced their decision-making process about COVID-19 vaccination [ 68 ]. Two main themes emerged, each with four subthemes: 1) sociocultural context (political, cultural, health professionals, employment, and media environment) and 2) individual and group influences (attitudes and beliefs related to vaccines, family and social networks, free to return to normal, and COVID-19 outcomes).
As for the Italian context, to the best of our knowledge, only one study (i.e., [ 69 ]) attempted to provide a qualitative examination of the concept associated with vaccination in general, through open-ended and closed questions. Notably, this study was conducted a year later than our own study (April–May 2022) and was administered to a non-representative sample of Italians. The authors used a combination of closed and open-ended questions to assess concepts associated with vaccination in general. Consistent with our findings, Boragno et al. reported that participants who had been vaccinated against COVID-19 (92% of the sample) frequently mentioned concepts related to protection and salvation, whereas those who were not vaccinated frequently mentioned mistrust and ambivalence as concepts associated with vaccination [ 69 ].
This study has some limitations. First, COVID-19 perceived risk score was obtained only with respect to the disease and a similar score should be of interest for the COVID-19 vaccine. Second, data were collected during a vaccine offer limited to a well-defined slice of the population and the investigation on the vaccine acceptance/booking has, as a consequence, a limited sample size. Finally, the lack of a longitudinal perspective does not allow us to evaluate how strong the association is between the willingness to get vaccinated, vaccine acceptance and potential changes in risk perception. Thus, we cannot generalize our results beyond the period of data collection and to other countries or health systems. Since the dynamics have now changed, results may not apply to the decision to get a booster shot or not or an annual shot, however it might be interesting to study what motivations are most relevant now. Likewise, it remains to be established whether our results are generalisable to other populations.
Future studies could consider how the interaction between perceived risk associated with the disease and perceived risk associated with the vaccine influences the choice to get the shot. Furthermore, it would be important to explore how we can harness the reasons that most hold back vaccination in a specific communication strategy for the most hesitant people. Moreover, at the time of data collection, the vaccination campaign was still at an early stage, and only a small portion of the population had already received their shot. Therefore, we believe that it might be of particular interest to know more in detail, with a larger sample, what are the reasons that to date, almost 2 years after the release of the vaccine, still make some people reject the vaccine. Only by knowing these reasons will it be possible to develop appropriate vaccination campaigns.
In conclusion, our work examined pro- and against-vaccination reasons and how these, and their interaction, influence the decision to get vaccinated or not. Specifically, high emotional competence and risk perception influence the generation of pro- and against-vaccination reasons and that the presence of a strong pro-vaccination reason shifts intention toward vaccination. We also highlighted the category of reasons that influence intention to vaccinate. That said, given that the discussion about the next doses is still open and that in any case the next pandemic is a matter of when and not if [ 70 ], it is of paramount importance to know the best way to counteract vaccine hesitancy, fostering more effective communication strategies.
Availability of data and materials
Raw data are available on https://osf.io/dpn2q/?view_only=af05427467634411b471af7a8475ffab .
European Centre for Disease Prevention and Control. Cumulative uptake (%) of the primary course among adults (+18) in EU/EEA countries as of 2023–09–07. https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab . Accessed 12 Sept 2023.
MacDonald NE. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015; https://doi.org/10.1016/j.vaccine.2015.04.036.
Bedford H, Attwell K, Danchin M, Marshall H, Corben P, Leask J. Vaccine hesitancy, refusal and access barriers: the need for clarity in terminology. Vaccine. 2018; https://doi.org/10.1016/j.vaccine.2017.08.004.
Paul KT, Zimmermann BM, Corsico P, Fiske A, Geiger S, Johnson S, Kuiper JM, Lievevrouw E, Marelli L, Prainsack B, Spahl W. Anticipating hopes, fears and expectations towards COVID-19 vaccines: a qualitative interview study in seven European countries. SSM-Qual Res Health. 2022; https://doi.org/10.1016/j.ssmqr.2021.100035.
Murphy J, Vallières F, Bentall RP, Shevlin M, McBride O, Hartman TK, McKay R, Bennett K, Mason L, Gibson-Miller J, Levita L. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat Commun. 2021; https://doi.org/10.1038/s41467-020-20226-9.
Soares P, Rocha JV, Moniz M, Gama A, Laires PA, Pedro AR, Dias S, Leite A, Nunes C. Factors associated with COVID-19 vaccine hesitancy. Vaccines. 2021; https://doi.org/10.3390/vaccines9030300.
Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann Internal Med. 2020; https://doi.org/10.7326/M20-3569.
Robertson E, Reeve KS, Niedzwiedz CL, Moore J, Blake M, Green M, Katikireddi SV, Benzeval MJ. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav Immun. 2021; https://doi.org/10.1016/j.bbi.2021.03.008.
Caserotti M, Gavaruzzi T, Girardi P, Tasso A, Buizza C, Candini V, Zarbo C, Chiarotti F, Brescianini S, Calamandrei G, Starace F. Who is likely to vacillate in their COVID-19 vaccination decision? Free-riding intention and post-positive reluctance. Prev Med. 2022; https://doi.org/10.1016/j.ypmed.2021.106885.
Caserotti M, Girardi P, Tasso A, Rubaltelli E, Lotto L, Gavaruzzi T. Joint analysis of the intention to vaccinate and to use contact tracing app during the COVID-19 pandemic. Sci Rep. 2022; https://doi.org/10.1038/s41598-021-04765-9 .
Pereira B, Fehl AG, Finkelstein SR, Jiga‐Boy GM, Caserotti M. Scarcity in COVID‐19 vaccine supplies reduces perceived vaccination priority and increases vaccine hesitancy. Psychol Mark. 2022; https://doi.org/10.1002/mar.21629 .
Đorđević JM, Mari S, Vdović M, Milošević A. Links between conspiracy beliefs, vaccine knowledge, and trust: Anti-vaccine behavior of Serbian adults. Soc Sci Med. 2021; https://doi.org/10.1016/j.socscimed.2021.113930 .
Candini V, Brescianini S, Chiarotti F, Zarbo C, Zamparini M, Caserotti M, Gavaruzzi T, Girardi P, Lotto L, Tasso A, Starace F. Conspiracy mentality and health-related behaviour during the COVID-19 pandemic: a multi-wave survey in Italy. Public Health. 2023, https://doi.org/10.1016/j.puhe.2022.11.005 .
Asch DA, Baron J, Hershey JC, Kunreuther H, Meszaros J, Ritov I, Spranca M. Omission bias and pertussis vaccination. Medic Decis Mak. 1994; https://doi.org/10.1177/0272989X9401400204 .
Meszaros JR, Asch DA, Baron J, Hershey JC, Kunreuther H, Schwartz-Buzaglo J. Cognitive processes and the decisions of some parents to forego pertussis vaccination for their children. J Clin Epidemiol. 1996; https://doi.org/10.1016/0895-4356(96)00007-8 .
Bell RA, McGlone MS, Dragojevic M. Vicious viruses and vigilant vaccines: Effects of linguistic agency assignment in health policy advocacy. J Health Commun. 2014; https://doi.org/10.1080/10810730.2013.81133 .
Nan X, Madden K. HPV vaccine information in the blogosphere: how positive and negative blogs influence vaccine-related risk perceptions, attitudes, and behavioral intentions. Health Commun. 2012; https://doi.org/10.1080/10410236.2012.661348 .
Christy SM, Winger JG, Raffanello EW, Halpern LF, Danoff-Burg S, Mosher CE. The role of anticipated regret and health beliefs in HPV vaccination intentions among young adults. J Behav Med. 2016; https://doi.org/10.1007/s10865-016-9716-z .
Chapman GB, Coups EJ. Emotions and preventive health behavior: worry, regret, and influenza vaccination. Health Psychol. 2006; https://doi.org/10.1037/0278-6133.25.1.82 .
Klasko-Foster LB, Przybyla S, Orom H, Gage-Bouchard E, Kiviniemi MT. The influence of affect on HPV vaccine decision making in an HPV vaccine naïve college student population. Prev Med Rep. 2020; https://doi.org/10.1016/j.pmedr.2020.101195.
World Health Organization = Organisation mondiale de la Santé. Understanding the behavioural and social drivers of vaccine uptake WHO position paper – May 2022 – Comprendre les facteurs comportementaux et sociaux de l’adoption des vaccins Note de synthèse de l’OMS – mai 2022. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire, 97 (20), 209 - 224. World Health Organization = Organisation mondiale de la Santé. 2022; https://apps.who.int/iris/handle/10665/354460 .
Fieselmann J, Annac K, Erdsiek F, Yilmaz-Aslan Y, Brzoska P. What are the reasons for refusing a COVID-19 vaccine? A qualitative analysis of social media in Germany. BMC Public Health. 2022; https://doi.org/10.1186/s12889-022-13265-y .
Moore R, Purvis RS, Hallgren E, Willis DE, Hall S, Reece S, CarlLee S, Judkins H, McElfish PA. Motivations to vaccinate among hesitant adopters of the COVID-19 vaccine. J Commun Health. 2022; https://doi.org/10.1007/s10900-021-01037-5 .
Cassels TG, Birch SA. Comparisons of an open-ended vs. forced-choice ‘mind reading’task: Implications for measuring perspective-taking and emotion recognition. PLoS One. 2014; https://doi.org/10.1371/journal.pone.0093653 .
Kahneman D, & Tversky A. On the interpretation of intuitive probability: A reply to Jonathan Cohen. 1979; https://doi.org/10.1016/0010-0277(79)90024-6 .
Tversky A, Kahneman D. Advances in prospect theory: Cumulative representation of uncertainty. J Risk Uncertain. 1992; https://doi.org/10.1007/BF00122574 .
Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of general psychology. 2001; https://doi.org/10.1037/1089-2680.5.4.323 .
Chor JS, Ngai KL, Goggins WB, Wong MC, Wong SY, Lee N, Leung TF, Rainer TH, Griffiths S, Chan PK. Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels: two questionnaire surveys. BMJ. 2009; https://doi.org/10.1136/bmj.b3391 .
Pareek M, Clark T, Dillon H, Kumar R, Stephenson I. Willingness of healthcare workers to accept voluntary stockpiled H5N1 vaccine in advance of pandemic activity. Vaccine. 2009; https://doi.org/10.1016/j.vaccine.2008.12.006 .
Viswanath K, Bekalu M, Dhawan D, Pinnamaneni R, Lang J, McLoud R. Individual and social determinants of COVID-19 vaccine uptake. BMC Public Health. 2021; https://doi.org/10.1186/s12889-021-10862-1 .
Caserotti M, Girardi P, Rubaltelli E, Tasso A, Lotto L, Gavaruzzi T. Associations of COVID-19 risk perception with vaccine hesitancy over time for Italian residents. Soc Sci Med. 2021; https://doi.org/10.1016/j.socscimed.2021.113688 .
Finucane ML, Peters E, & Slovic P. (2003). Judgment and decision making: The dance of affect and reason. In: S. L. Schneider & J. Shanteau, editors. Emerging Perspectives on Judgment and Decision Research Cambridge. University Press; 2003. 327–364. https://doi.org/10.1017/CBO9780511609978.012 .
Pittarello A, Conte B, Caserotti M, Scrimin S, Rubaltelli E. Emotional intelligence buffers the effect of physiological arousal on dishonesty. Psychonomic Bull Rev. 2018; https://doi.org/10.3758/s13423-017-1285-9 .
Scrimin S, Rubaltelli E. Dehumanization after terrorism: the role of psychophysiological emotion regulation and trait emotional intelligence. Curr Psychol. 2021; https://doi.org/10.1007/s12144‐019‐00189‐x .
Tomljenovic H, Bubic A, Erceg N. It just doesn’t feel right–the relevance of emotions and intuition for parental vaccine conspiracy beliefs and vaccination uptake. Psychol Health. 2020; https://doi.org/10.1080/08870446.2019.1673894 .
Gavaruzzi T, Caserotti M, Leo I, Tasso A, Speri L, Ferro A, Fretti E, Sannino A, Rubaltelli E, Lotto L. The role of emotional competences in parents’ vaccine hesitancy. Vaccines. 2021; https://doi.org/10.3390/vaccines9030298 .
ISTAT. Resident population on 1st January: By age. http://dati.istat.it/?lang=en&SubSessionId=d7024c9e-239b-455d-924b-df19345a27b2 . Accessed Sept 25, 2023.
Mikolajczak M., Brasseur S, & Fantini-Hauwel C. Measuring intrapersonal and interpersonal EQ: The short profile of emotional competence (S-PEC). Pers Individ Differ. 2014; https://doi.org/10.1016/j.paid.2014.01.023 .
Olmos A, Govindasamy P. A practical guide for using propensity score weighting in R. Pract Assess Res Eval. 2015; https://doi.org/10.7275/jjtm-r398 .
Smithson M, Verkuilen J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychological methods. 2006; https://doi.org/10.1037/1082-989X.11.1.54 .
Ferrari S, Cribari-Neto F. Beta regression for modelling rates and proportions. Journal of applied statistics. 2004; https://doi.org/10.1080/0266476042000214501 .
Thernau T, Atkinson B, Ripley B. Rpart: Recursive Partitioning. R Package 4.1–0. http://CRAN.R-project.org/package=rpart .
RC Team. R Core Team R: A language and environment for statistical computing. R. . Foundation for Statistical Computing. 2014. https://www.r-project.org .
Freeman D, Loe BS, Chadwick A, Vaccari C, Waite F, Rosebrock L, ... & Lambe S. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol Med. 2022; https://doi.org/10.1017/S0033291720005188 .
Caserotti M, Gavaruzzi T, Girardi P, Sellaro R, Rubaltelli E, Tasso A, Lotto L. People’s perspectives about COVID-19 vaccination certificate: Findings from a representative Italian sample. Vaccine. 2022; https://doi.org/10.1016/j.vaccine.2022.08.016 .
MacDonald NE, Comeau J, Dubé È, Graham J, Greenwood M, Harmon S, McElhaney J, Meghan McMurtry C, Middleton A, Steenbeek A, Taddio A. Royal society of Canada COVID-19 report: Enhancing COVID-19 vaccine acceptance in Canada. Facets. 2021; https://doi.org/10.1139/facets-2021-0037 .
Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021; https://doi.org/10.1016/S2468-2667(21)00012-8 .
Slovic P, Finucane M, Peters E, MacGregor DG. Rational actors or rational fools: Implications of the affect heuristic for behavioral economics. J Socio-Econ. 2002; https://doi.org/10.1016/S1053-5357(02)00174-9 .
Slovic P, Finucane ML, Peters E, MacGregor DG. Risk as analysis and risk as feelings: Some thoughts about affect, reason, risk and rationality. In The feeling of risk 2013 Mar 7 (pp. 21–36). Routledge.
Bhopal SS, Bagaria J, Olabi B, Bhopal R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health. 2021; https://doi.org/10.1016/S2352-4642(21)00066-3 .
Lazarus JV, Wyka K, Rauh L, Rabin K, Ratzan S, Gostin LO, Larson HJ, El-Mohandes A. Hesitant or not? The association of age, gender, and education with potential acceptance of a COVID-19 vaccine: a country-level analysis. J Health Commun. 2020; https://doi.org/10.1080/10810730.2020.1868630 .
Seale H, Heywood AE, Leask J, Sheel M, Durrheim DN, Bolsewicz K, Kaur R. Examining Australian public perceptions and behaviors towards a future COVID-19 vaccine. BMC Infect Dis. 2021; https://doi.org/10.1186/s12879-021-05 .
Butter S, McGlinchey E, Berry E, Armour C. Psychological, social, and situational factors associated with COVID‐19 vaccination intentions: A study of UK key workers and non‐key workers. Br J Health Psychol. 2022; https://doi.org/10.1111/bjhp.12530 .
Freedman DA, Berk RA. Weighting regressions by propensity scores. Eval Rev. 2008; https://doi.org/10.1177/0193841X08317586 .
Lagoe C, Farrar KM. Are you willing to risk it? The relationship between risk, regret, and vaccination intent. Psychol Health Med. 2015; https://doi.org/10.1080/13548506.2014.911923 .
Ziarnowski KL, Brewer NT, Weber B. Present choices, future outcomes: anticipated regret and HPV vaccination. Prev Med. 2009; https://doi.org/10.1016/j.ypmed.2008.10.006 .
Betsch C, Böhm R, Korn L, Holtmann C. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017; https://doi.org/10.1038/s41562-017-0056 .
Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021; https://doi.org/10.1038/s41562-021-01056-1 .
Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41(2):85.
Article PubMed Google Scholar
Hakim H, Provencher T, Chambers CT, Driedger SM, Dube E, Gavaruzzi T, ... & Witteman HO. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019; https://doi.org/10.1016/j.vaccine.2018.11.016 .
Hakim H, Bettinger JA, Chambers CT, Driedger SM, Dubé E, Gavaruzzi T, Giguere AMC, Kavanagh É, Leask J, MacDonald SE, Orji R, Parent E, Paquette J, Roberge J, Sander B, Scherer AM, Tremblay-Breault M, Wilson K, Reinharz D, Witteman HO. A Web Application About Herd Immunity Using Personalized Avatars: Development Study. Journal of medical Internet research. 2020; https://doi.org/10.2196/20113 .
Callaghan T, Moghtaderi A, Lueck JA, Hotez P, Strych U, Dor A, Fowler EF, Motta M. Correlates and disparities of intention to vaccinate against COVID-19. Soc Sci Med (1982). 2021; https://doi.org/10.1016/j.socscimed.2020.113638 .
Griffith J, Marani H, Monkman H. COVID-19 vaccine hesitancy in Canada: Content analysis of tweets using the theoretical domains framework. Journal of medical Internet research. 2021; https://doi.org/10.2196/26874 .
Attwell K, Lake J, Sneddon J, Gerrans P, Blyth C, & Lee J. Converting the maybes: Crucial for a successful COVID-19 vaccination strategy. PLoS One. 2021; https://doi.org/10.1371/journal.pone.0245907 .
Breckenridge LA, Burns D, & Nye C. The use of motivational interviewing to overcome COVID‐19 vaccine hesitancy in primary care settings. Public Health Nurs. 2022; https://doi.org/10.1111/phn.13003 .
Gabarda A, & Butterworth SW. Using best practices to address COVID-19 vaccine hesitancy: The case for the motivational interviewing approach. Health Promot Pract, 2021; https://doi.org/10.1177/152483992110164 .
Hamilton EM, Oversby S, Ratsch A, & Kitchener S.COVID-19 vaccination: An exploratory study of the motivations and concerns detailed in the medical records of a regional Australian population. Vaccines. 2022; https://doi.org/10.3390/vaccines10050657 .
Purvis RS, Moore R, Willis DE, Hallgren E, & McElfish PA. Factors influencing COVID-19 vaccine decision-making among hesitant adopters in the United States. Human Vaccines Immunother. 2022; https://doi.org/10.1080/21645515.2022.2114701 .
Boragno P, Fiabane E, Taino I, Maffoni M, Sommovigo V, Setti I, Gabanelli P. Perceptions of COVID-19 Vaccines: Protective Shields or Threatening Risks? A Descriptive Exploratory Study among the Italian Population. Vaccines. 2023; https://doi.org/10.3390/vaccines11030642 .
Centers for Disease Control and Prevention. Why it matters: The pandemic threat. Retrieved December. 2020;1:2020.
Download references
Acknowledgements
Not applicable.
Open access funding provided by Università degli Studi di Padova. The project was developed thanks to institutional research funding of TG and AT.
Author information
Authors and affiliations.
Department of Developmental Psychology and Socialization, University of Padova, Padua, Italy
Marta Caserotti, Roberta Sellaro, Enrico Rubaltelli & Lorella Lotto
Department of Environmental Sciences, Informatics and Statistics, Ca’ Foscari University of Venezia, Venice, Italy
Paolo Girardi
Department of Humanities, University of Ferrara, Ferrara, Italy
Alessandra Tasso
Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
Teresa Gavaruzzi
You can also search for this author in PubMed Google Scholar
Contributions
MC: Conceptualization, Formal analysis, Visualization, Writing—original draft and Writing—review & editing. PG: Conceptualization, Formal analysis, Visualization, Writing—original draft. RS: Conceptualization, Writing—review & editing. ER: Conceptualization, Writing—review & editing. AT: Conceptualization, Writing—review & editing. LL: Conceptualization, Writing—review & editing. TG: Conceptualization, Visualization, Writing—review & editing.
Corresponding author
Correspondence to Marta Caserotti .
Ethics declarations
Ethics approval and consent to participate.
The project was approved by the ethical committee for Psychology Research of the University of Padova (Italy), with protocol number 3911/2020, and informed consent was obtained for all participants.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Scoring for pro- and against-vaccination reasons. Appendix 2. Structure of the questionnaire. Table S1. Selection criteria. Table S2. Number of items, internal consistency (Cronbach’s α), name of the items and their estimated loadings, total deviance explained by the loadings and proportion of variance explained by EFA for COVID-19 perceived risk. Table S3. Odds ratios (ORs) estimated by the logistic model for the propensity score weighting for the COVID-19 vaccine offer. Table S4 . Predicted willingness to get vaccinated by combination of pro- and against-vaccination reasons by category of reference. Table S5. Frequency of reported categories of pro- and against-vaccination reasons overall, and by COVID-19 vaccine status. Figure S1. Distribution of the propensity scores by vaccine offer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Caserotti, M., Girardi, P., Sellaro, R. et al. To vaccinate or not to vaccinate? The interplay between pro- and against- vaccination reasons. BMC Public Health 23 , 2207 (2023). https://doi.org/10.1186/s12889-023-17112-6
Download citation
Received : 12 December 2022
Accepted : 30 October 2023
Published : 09 November 2023
DOI : https://doi.org/10.1186/s12889-023-17112-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pro-and against-reasons
- Vaccination intention
- Risk perception
- Emotional competences
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
MANDATORY VACCINATION: WHY WE STILL GOT TO GET FOLKS TO TAKE THEIR SHOTS
Ben Balding
Class of 2006
April 27, 2006
This paper is submitted in satisfaction of the Food and Drug Law course paper and the Harvard Law School 3L Written Work Requirement
Vaccination is widely considered one of the greatest medical achievements of modern civilization. Childhood diseases that were commonplace less than a generation ago are now increasingly rare because of vaccines. In order to be effective at eliminating communicable diseases, vaccines must be administered to sufficient levels of persons in the community. Because of this, public health officials have mandated vaccination for certain diseases as a condition to school attendance. The overwhelming effectiveness of vaccination programs may lead individuals to ignore the benefits of vaccination and focus more on the risk of side effects. Moreover, some have criticized the coercive nature of these programs. These objections may lead to an unacceptably high number of exemptions, which can compromise vaccination programs and leave the population susceptible to outbreaks.
This paper explores vaccination programs with an eye toward greater public safety without ignoring the reality of a small but committed group of vaccine critics. The paper begins with a discussion of the historical development of mandatory vaccination policies and the issues posed by exemptions. It then addresses some of these issues in the context of vaccine safety. It also seeks solution by framing the discussion in economic terms. It concludes by recommending stricter enforcement of mandatory requirements for most vaccines and greater dissemination of information on the continued importance of vaccination.
TABLE OF CONTENTS
Introduction.
Vaccination is widely considered one of the greatest medical achievements of modern civilization. Childhood diseases that were commonplace less than a generation ago are now increasingly rare because of vaccines. The smallpox vaccine has eradicated a disease that was responsible for centuries of outbreaks and had a 30% fatality rate. [1] Physical handicaps resulting from polio can still be observed on some of those who were children before Jonas Salk developed a vaccine in 1955. Formerly common childhood diseases are now rarely observed. Even ear infections may soon be prevented by vaccination. [2] The widespread success of vaccinations has led one medical report to comment that “[n]ext to clean water, no single intervention has had so profound an effect on reducing mortality from childhood diseases as has the widespread introduction of vaccines.” [3]
The story of modern vaccination begins with Edward Jenner’s development of the vaccine for smallpox, one of the most feared diseases in recent history. At first, vaccination was optional and not everyone chose to vaccinate. [4] In time, states would allow municipalities to mandate vaccination in time of outbreak in order to protect the public from epidemics. [5] A further step was taken when states imposed smallpox vaccination as a prerequisite for attending public schools. [6] These requirements were amended in time as new vaccines were developed. [7] At some point actual outbreaks and epidemics ceased to be the trigger for mandatory vaccination, and prevention became the overriding justification. [8] Most states today require vaccination for a multitude of childhood diseases, including measles, diphtheria, pertussis, polio, and now even chickenpox. [9]
Because of the success and the mandatory nature of vaccination, most people would probably not consider vaccination an optional method of medical treatment. For most parents, the “decision” to vaccinate is equivalent to the “decision” to feed one’s child. [10] Typically, a doctor informs parents of the school vaccination schedule and the parents consent to having their child vaccinated. Since the vaccination schedule usually corresponds to the scheduled doctor visits for infants, full compliance with mandatory vaccination schedules is typically not a problem and can usually be substantially accomplished by age two. [11]
For some parents, however, vaccination is no routine matter. [12] From the time of Jenner’s smallpox vaccine, vaccination has had its critics. [13] In the two centuries since that time, many different types of objections have been raised. Some have questioned the scientific qualifications of mass immunization. [14] Others have focused on the personal liberty interests at stake and have objected to the paternalistic nature of government imposition of what is viewed as a personal medical choice. [15] Still others have opposed vaccination for personal or religious reasons. [16]
Today, some parents raise similar objections. The idea that a potentially harmful substance is being placed directly into the bloodstream raises a red flag for some. Additionally, the decline of many diseases for which vaccination is still mandated may make some parents skeptical of the continued wisdom of subjecting a child to a vaccine, even if the vaccine is considered extremely safe. This skepticism grows when some point to the correlation between vaccinations and conditions such as SIDS and autism. Whether or not such a correlation is scientifically significant, many parents simply wonder if it is wise to vaccinate against a disease unlikely to afflict their child if any chance exists that the vaccine will cause autism, SIDS, or any other side effect. [17]
Since the efficacy of a particular vaccine corresponds directly with the percentage of a given population that has been vaccinated, proponents of mandatory vaccination have sought to convince those with reservations about vaccines that vaccination is the right choice. The Center for Disease Control has attempted to allay possible reservations parents may have with vaccinations by rebutting some of the commonly held fears about vaccines. [18] The CDC has pointed out, for example, that most adverse effects from vaccines are “minor and temporary, such as a sore arm or mild fever.” [19] Because vaccination often involves the introduction of a harmful live (although seriously weakened) organism into the patient, vaccination can never be 100% safe. Serious side effects usually occur only between one per thousands to one per millions of doses, while some serious reactions and death occur so rarely that accurate risk assessment is difficult. [20] The CDC has also responded to many of the other concerns raised about the need for vaccination, and the FDA continually works to ensure vaccine safety and efficacy, but many still harbor reservations toward vaccination.
This paper will endeavor to discuss some of the most common objections to vaccination programs in general while trying to shed light on the veracity and tenability of these objections. Part I will discuss the nature of mandatory vaccination programs in this country; both scientific and historical issues will play a key part in this discussion. Part II will describe the role of the FDA and other governmental bodies in the overall vaccination picture. Part III will attempt to utilize multiple analytical tools in search of possible solutions to the dangers posed by those who may attempt to opt out of vaccination programs. It will first examine vaccination through the lens of an old television show episode. It will then adopt an economic analytical framework to discuss the balance between individual and general welfare in the context of vaccination. Part IV will conclude with some observations on how the goal of greater public health might be achieved without completely neglecting the concerns of many in the community regarding the prudence of using a medical technique that by definition relies on a degree of coercion.
I. MANDATORY VACCINATION
Historical background.
Jenner’s smallpox vaccine led to the research and development of vaccines for other widespread and epidemic diseases. The twentieth century saw the development of vaccines for such diseases as polio, diphtheria, tetanus, pertussis, measles, and others. [21] As with the smallpox vaccine, many of these vaccines soon found their way into vaccination programs mandated by the government, albeit through a somewhat different pathway.
Mandatory smallpox vaccination programs typically arose through state police power legislation authorizing municipalities to deal with outbreaks. [22] Typically, when a local municipality decided that the threat of outbreak was sufficient to exercise this authority it would require vaccination of everyone in the community (with a possible exception for individuals who could demonstrate uncommonly high health risks from receiving the vaccine, although this exception sometimes applied only to children) and fine and/or quarantine those who refused to be vaccinated. [23] When other diseases became preventable by vaccination, outbreak ceased to be the trigger for mandatory vaccination. Rather, because of their cost-efficiency and their ability to reduce and ultimately eliminate disease, vaccination programs became an important part of general public health policy. [24]
Most of the time, vaccination programs are accomplished through the dual efforts of national entities (which tend to develop and recommend vaccines) and state legislatures and local boards of health (which usually implement these recommended vaccines through vaccination programs). [25] It is not entirely accurate to refer to this as “mandatory vaccination,” as typically individual states will not criminally punish parents for not vaccinating their children or forcefully subject individuals to vaccination. [26] Instead, states typically condition school enrollment on proof of vaccination. [27] Though it may be a high price to pay, home schooling is usually an available means parents have if they wish to bypass these vaccination requirements. Moreover, most states grant exemptions to vaccination requirements for religious reasons and some even grant exemptions for philosophical reasons (in addition, every state exempts from school vaccination requirements individuals who cannot be vaccinated for medical reasons). [28]
The connection between school enrollment and vaccination programs may now seem obvious. Public health officials, faced with a means of protecting the general population from the harmful disease smallpox, realized that mass vaccination could lead to a sufficient level of immunity to eliminate the risk of outbreak, even for those in the community unable to vaccinate (because of medical reasons, for instance). [29] Because of the concept of herd immunity, public health officials considering the proper utilization of vaccines were dealing with a medical procedure quite out of the ordinary. Since vaccination itself does not typically provide 100% immunity to a disease, vaccinated individuals can still contract the disease. [30] Yet because of herd immunity, if a sufficient level of vaccination within a population is attained, the entire population will no longer be susceptible to the disease. In this way, vaccination came to be viewed not only as a personal medical choice but also as a step taken to improve the overall health of the population.
With the rise of public schooling in the mid- to late-nineteenth century, cities decided to condition public school attendance on smallpox vaccination. [31] By the latter part of the century, many states had adopted this practice. [32] Such a policy makes sense when one considers the increased risk of infectious disease in public areas like cities in general and schools in particular. By mandating vaccination for school attendance, of course, the state would eventually have ensured the vaccination of the entire population by the time the initially vaccinated generation became the oldest living one.
These vaccination schemes have faced challenges, both legal and social, throughout their existence. [33] The reasons for such challenges have ranged from personal liberty interests to doubts about the efficacy of vaccines. [34] State courts in the nineteenth century typically upheld both the enactment of mandatory vaccination programs and the delegation of power to local authorities. [35] More importantly for the future of mandatory vaccination policy, two important Supreme Court decisions in the early part of the twentieth century affirmed the power of state governments both to mandate vaccination and to delegate a broad degree of authority to local municipalities and health boards to carry out particular vaccination programs.
Judicial Approval
In 1905 the Court held in Jacobson v. Massachusetts [36] that the general police power of states is broad enough to overcome a Due Process claim brought by an individual who claimed his personal liberty interests were unconstitutionally invaded by the mandatory smallpox vaccination program in question. [37] In an opinion by Justice Harlan, the Court ruled that the constitutional guarantee of liberty “does not import an absolute right in each person, to be, at all times and in all circumstances wholly free from restraint.” [38]
This case still represents the initial constitutional basis of most mandatory vaccination legislation. Many states still provide for the governor or a public health official to mandate vaccination for all in the event of an outbreak. [39] Individuals who cannot vaccinate for health reasons or who refuse to vaccinate may be quarantined in order to protect the population in some states. [40] These laws gained greater relevance following the terrorist attacks of 9/11 and the increased public concerns regarding bioterrorism. For the most part, however, mandatory vaccination laws in the name of outbreak control have given way to vaccination requirements as a prerequisite for school attendance.
The issue of school vaccination came before the Court nearly two decades after Jacobson . In Zucht v. King [41] , the plaintiff challenged a general grant of authority from Texas to local boards of health to condition school entry on proof of vaccination. [42] To differentiate the case from Jacobson , the plaintiff noted that the San Antonio ordinances mandated vaccination even in the absence of evidence of outbreak. [43] The Court, speaking this time through Justice Brandeis, upheld the validity of the ordinances as well as the broad grant of authority to local health boards. [44] On the issue of the state’s power to mandate vaccination, he merely cited Jacobson : “[l]ong before this suit was instituted, Jacobson v. Massachusetts...had settled that it is within the police power of a state to provide for compulsory vaccination.” [45] As for entrusting a broad degree of authority on local health officials, he noted that Jacobson and other cases had affirmed that a state may “delegate to a municipality authority to determine under what conditions health regulations shall become operative.” [46] This delegation includes the permission to vest municipal officials with “broad discretion in matters affecting the application and enforcement of a health law.” [47] In summary, the Court found that these ordinances were valid assignments of “that broad discretion required for the protection of the public health.” [48] The language of the opinion emphasizes the importance of the public health as the key justification for mandatory vaccination.
Zucht , along with Jacobson , thus became the legal foundation for the mandatory vaccination laws of the twentieth century. Modern school vaccination laws and policies have grown from early mandatory smallpox vaccination laws:
The early successes of school vaccination laws against most political, legal, and social challenges helped lay the foundation for modern immunization statutes. Since the introduction of smallpox vaccination policies in the mid-to-late 1800s, states have amended them to include additional diseases as new vaccines become available. [49]
Though various amendments and additions have been made to mandatory vaccination laws throughout their history, the past half century has experienced the true culmination of mandatory vaccination policy. Public health officials have been able to institute a scheme for near-universal vaccination:
Many existing school vaccination laws were enacted in response to the transmission of measles in schools in the 1960s and 1970s. State legislatures at that time were influenced by the significantly lower incidence rates of measles among school children in states that strictly enforced vaccination requirements and school exclusions in outbreak situations without significant community opposition. Rather than having health departments require immunization in emergency conditions, legislatures acted to prevent disease by mandatory immunization as a condition of enrollment or attendance in schools or licensed day care facilities. [50]
Moreover, states have not been completely left to implement the recommended immunization schedule. [51] Though school requirements are still a state matter, national public health officials are typically able to enact their recommendations through federally funded immunization plans. [52] These plans require states to implement and enforce federally recommended immunization requirements before the states can receive federal funds. [53] The current recommended vaccination schedule appears below.
Recommended Childhood and Adolescent Immunization Schedule [54]
Challenges and concessions.
While school vaccination requirements have been credited with bringing about the control and elimination of many devastating childhood diseases, critics have continued to voice concerns and raise legal and political challenges to the entire process of mandatory vaccination.
Personal Liberty Concerns
One key argument against mandatory school vaccination has always focused on government intrusion into what is considered a personal medical choice. [55] Just as the government cannot force a person to have surgery to repair a torn ligament, for example, the government should not be able to force parents to vaccinate their children if the parents believe that vaccination is not the best medical decision. One prominent critic of mandatory vaccination has stated her organization’s goal as simply providing parents with choices: “[w]e believe that health care consumers should have the right to choose the type of preventive health care that they want to use – including choosing whether to use one, ten, or no vaccines.” [56] Other objections along similar bases argue that mandatory vaccination violates the medical ethic of informed consent or even that school district control over mandatory vaccination policies amounts to the unlawful practice of medicine without a license. [57]
The typical counterargument given by the public health officials is to point out that one’s decision to vaccinate, unlike one’s decision whether to undergo surgery, affects the health of others in the community. [58] To allow parents the right to choose not to vaccinate is to infringe on the ability of other parents to raise their children in a society free of certain deadly diseases. From a legal standpoint, Jacobson still seems to have settled the issue that at least under some circumstances, the government may force an individual to receive a vaccination.
Although public health officials have the legal authority to mandate vaccination for the public health under Jacobson, they should be very mindful of the personal liberty concerns just stated. Those with such views often cling to them vigorously. [59] As certain vaccine-preventable diseases decline, such concerns become even stronger. For this reason, it is important for public health officials to support their mandatory vaccination programs with justifiable arguments rather than simply citing legal precedent or historical tradition in support of their exercise of power. Fortunately for public health officials, the benefits provided by vaccination programs can be utilized to justify the existence of such programs.
Safety Accountability Concerns
A variation on the consumer choice challenge to mandatory school vaccination requirements tends to accuse the public health community of conspiring with or at least willfully acquiescing to powerful vaccine manufacturers at the expense of citizens. [60] Mandatory programs, the argument goes, eliminate any accountability from vaccine manufacturers that the free market might otherwise provide. [61] Both the safety and efficacy of vaccines fail to improve because manufacturers do not have to respond to consumer concerns. [62] Mandatory programs thus prevent better vaccines. A prominent critic of these programs has stated that if mandatory vaccination programs are ended, “we will have the ability to put economic pressure on the drug companies and on the health agencies to do a better job with vaccine safety and efficacy.” [63]
The strength of this argument lies in its apparent lack of hostility toward vaccines per se. Given the historical success of vaccination in eradicating smallpox and in reducing or eliminating the risk of other childhood diseases, any critique of mandatory vaccination programs that focuses on the use of vaccines generally is likely to be dismissed by those in the field of public health. By focusing on the economic drawbacks inherent in a mandatory vaccination program and how those drawbacks can negatively affect the quality of vaccines, this argument may gain more traction. Indeed, all sides of this debate claim to desire both safer and more effective vaccines.
The response to this argument, I would imagine, would be to emphasize the drawbacks of opening up the “market” in this case. Because vaccination programs depend on a sufficient percentage of the community being vaccinated, complete consumer choice carries with it problems that might be absent in a standard market. As for vaccine quality, FDA regulation is in place to ensure a sufficient level of safety and efficacy to accomplish the goals of vaccination. [64] The pressure faced by vaccine manufacturers to obtain and maintain FDA approval should provide a check sufficient to guarantee proper vaccine quality. If not, the answer should be to raise FDA standards, rather than to jettison the entire mandatory vaccination process and with it the likelihood of maintaining a sufficient level of immunity among the population.
This response might be unacceptable to those concerned. If the connection between public health officials entrusted with implementing the mandatory vaccination schedule and FDA regulators entrusted with ensuring the safety and efficacy of vaccines is seen as too close, proposing higher FDA standards as a solution may not allay concerns. The independence and integrity of FDA is therefore critical in this arena, just as it is in other areas of public health.
Concern of Unknown Risks
In what may be a combination of the two challenges previously discussed, many individuals challenge vaccine programs because of a lack of information about vaccines. [65] Many people, for example, legitimately question the wisdom of forced vaccination before long-term effects of a vaccine are studied. One website that purports “to provide a wide range of news and views on vaccination and vaccination policy” has summarized this challenge to vaccines simply as opposing the idea of “a parent, any parent, being forced to do something that has even a remote chance of harming their child.” [66] Since long-term (ten or more years down the road) and low-risk (on the order of one-per-million or less, for example) side effects may truly be unknown, this concern does present a challenge for public health officials. [67]
Unfortunately, even the best studies are unable to fully determine all long-term consequences of vaccination. In addition, “[t]here is no such thing as a ‘perfect’ vaccine which protects everyone who receives it AND is entirely safe for everyone.” [68] Therefore, it is true that mandatory vaccination probably forces some parents to inject their children with a substance that will cause some unknown harm.
As with the other objections to mandatory vaccination, however, this objection suffers from a critical flaw. Mandatory school vaccination requirements are not justified solely on the benefit they provide to the recipient. Instead, it is the benefit they provide to the community as a whole by ensuring a sufficient level of vaccination to prevent outbreak that justifies their intrusive nature on individual medical decision-making. [69] For this reason, if public health officials did not enact the mandatory vaccination program, they would be forcing on parents a system that had at least a “remote chance of harming their child.” [70] Because the decision to enact a community-wide vaccination program must be made at the general level if it is to be made at all, and because some children will undoubtedly suffer some health consequences regardless of which policy is chosen, individuals will always be able to raise this argument against mandatory vaccination programs.
A better critique of these programs would focus on whether mandatory vaccination causes more overall harm than a voluntary system; that is, is it better when viewed at the general, rather than the individual, level? Ironically, the very success of vaccination programs in reducing the incidence of once-prominent diseases has led some to ignore the overall and continuing benefit of community vaccination (herd immunity). [71] But for parents to decry the “remote chance” of harm from vaccination while ignoring the very real chance of outbreak in an under-vaccinated population is to reframe the issue entirely.
Other Concerns
Other challenges to vaccination laws have cited strongly held religious or philosophical positions against vaccination in general. Such challenges require a different type of response from public health officials; often the options are limited to overriding such objections and excluding children of parents adhering to such positions from public schools (which is constitutionally permissible under Jacobson and its progeny) or creating exemptions to vaccination requirements (which is detrimental to the overall goals of mandatory vaccination if a sufficient number of exemptors exist). Reactions to such religious and philosophical concerns vary from state to state, with a general trend toward greater accommodation of objectors.
In response to these and other challenges to mandatory vaccination laws, states have enacted various exemptions to vaccination requirements for school entry. Actual enforcement varies by state.
All states provide exemptions for those with medical risks associated with vaccines. [72] If certain contraindications indicate a likelihood of harm from a particular vaccine, the exemption will be allowed. [73] Because such cases are rare and exemptions relatively easy to enforce, there usually is very little risk of compromising the efficacy of the overall vaccination program by granting these exemptions. [74] The ability to grant medical exemptions while still maintaining sufficient levels of vaccination to provide community-wide immunity is one of the great accomplishments of the vaccination system. [75]
In addition to medical exemptions, almost every state grants religious exemptions for those with sincere religious beliefs opposing vaccination. [76] Individual states tend to vary with regard to the level of religious conviction necessary to obtain a religious exemption. Such exemptions reflect the sometimes uneasy balance between mandatory vaccination programs and First Amendment Free Exercise rights, even though the Supreme Court has validated the right of states to mandate vaccination without providing for such exemptions. [77] West Virginia, for example, does not provide religious exemptions. [78]
Some religious exemption statutes have spurred challenges on Establishment Clause grounds by those who claim they favor organized or recognized religions over the sincerely held religious views of others. [79] These challenges, if successful, would lead to the invalidation of many religious exemption statutes. Rather than decrease the number of religious exemptors, however, this may actually lead to more religious exemptors. The political climate of our day, along with the experience of a few states already (such as Arkansas), suggests that legislatures may respond to invalidation of religious exemption statutes that require adherence to an organized religion by drafting more general and expansive religious exemption statutes. [80] By subjugating religion to compulsory vaccination, courts may actually be helping to bring about a system with even more religious exemptors, thereby harming the very vaccination programs to which religious objections had been subordinated. [81]
Philosophical
The possibility that some parents who strongly oppose vaccination for other than religious reasons has led to other means of exempting from mandatory vaccination programs. In some states, people may avoid vaccination requirements by way of philosophical exemptions. [82] In California, for example, a parent need only “submit a letter or affidavit stating that the immunization is contrary to his or her beliefs” to exempt their child from vaccination requirements. [83] “Where available, parents are taking advantage of such exemptions with growing regularity; and in states offering both exemptions, the number of philosophical exemptions far exceeds the number of religious and medical exemptions.” [84]
States without philosophical exemptions, moreover, are often lax with their enforcement of religious exemptions. [85] Because of this, parents in these states can usually submit insincere affidavits purporting to object to vaccination for religious reasons and local health officials, unconcerned with delving into the sincerity of such affidavits, will widely grant exemptions. [86] In most states, therefore, persistent parents can usually find some way to exempt their children from vaccination requirements. If all else fails and vaccination is still regarded as unacceptable to the parent, the option of home schooling may provide a final avenue of evading these school vaccination requirements.
Dangers of Widespread Exemptions
The ease with which non-medical exemptions can typically be obtained has raised concerns among many that the benefits of widespread immunization are being compromised. [87] Because of the nature of medical exemptions, unvaccinated persons in a community with only medical exemptions would be expected to be few and dispersed. Herd immunity can be attained, and protection is ensured for both the vaccinated majority and the unvaccinated few. [88] Broadly granted philosophical and religious exemptions make herd immunity more difficult to attain and increase the risk to the community. This risk is exacerbated by the fact that many of those who apply for such exemptions “will cluster together in one geographic area.” [89] This cluster effect tends to increase the likelihood of serious outbreaks:
Recent studies have shown that clusters of exemptors, who are significantly more susceptible to contracting vaccine preventable illnesses, pose an increased risk of spread of diseases not only to their unimmunized peers, but also to the surrounding, largely vaccinated population. [90]
Given that many childhood diseases seem to be in decline, exemptors may fail to realize the continued value of vaccination. As the mumps outbreak in Iowa makes clear, however, vaccination programs take time and are at risk if vaccination rates fall. Other diseases are still prevalent in other parts of the world, and outbreaks can still occur in this country due to the prevalence of international travel. Ever though measles is rarely observed in the US, for example, the World Health Organization has reported that nearly 900,000 measles-related deaths occurred in developing countries in 1999. [91] Until diseases are eradicated globally, it may be necessary to continue vaccination.
Because many of the aforementioned risks are frequently underappreciated by those who seek exemptions, some have suggested a combination of stricter enforcement of exemption requirements and increased public knowledge of the reasons underlying childhood vaccination requirements. [92] Knowledge is indeed essential to the resolution of this problem. The easier it is to obtain an exemption, the less likely individuals are to understand and appreciate the importance of widespread participation to the success of a vaccination program. Greater public appreciation of the need for such participation (even for diseases that seem to be in retreat), along with greater information on the safety of vaccines can go a long way toward increasing public health in this area. [93]
Partial Exemptors – A Modern Phenomenon
The availability of exemptions has led to other interesting developments in the vaccination debate. Recently, for example, challenges have been raised against the need for mandatory chickenpox and hepatitis B vaccines. Diseases such as these, which are either not greatly feared (chickenpox) or transmitted primarily through voluntary rather than involuntary contact (hepatitis B), do not fit neatly into the typical justification for mandatory vaccination. [94] Nevertheless, public health officials have decided that recently-developed vaccines for these diseases should be placed on the recommended schedule. This has given rise to a significant number of partial exemptors – those who are not opposed to vaccination requirements per se, but who oppose particular vaccines on the schedule. Such a position may not have been comprehended by those who drafted the religious and philosophical exemptions, which seem to assume that a parent’s opposition is to vaccination generally, rather than to a specific vaccine. [95]
Because the religious exemption is usually constructed to apply to those who oppose vaccination generally because of sincere religious beliefs, would-be partial exemptors have difficulty fulfilling their optimal desires. In states without a philosophical objection, parents must choose either to accept the entirety of the recommended schedule of vaccines or to obtain a religious exemption for all vaccinations. [96] Parents who live in states with a philosophical exemption are much more able to tailor their objection to those vaccines with which they disagree. [97]
From the standpoint of a public health official, this presents two possible worlds. In the world with traditional religious exemptors but no philosophical exemptors, overall percentages of vaccinations would be relatively equal from vaccine to vaccine, and higher vaccination rates would be obtained for diseases associated with more objectionable vaccines at the expense of lower vaccination rates for diseases associated with less objectionable vaccines. [98] By contrast, in the world with philosophical exemptors, the public health official would observe higher vaccination rates for the less objectionable vaccines and lower vaccination rates for the more objectionable vaccines. [99]
The difference between these two worlds can have far-reaching implications. If parents are forced to make the all-or-nothing choice, a significant enough number could choose to forego vaccines (including some which they would otherwise accept) that herd immunity is lost, even for less objectionable vaccinations. On the other hand, a significant enough number could accept the more objectionable vaccinations to bring about herd immunity for those diseases. Though the public health official might prefer a world in which neither religious nor philosophical exemptions exist, such a world may not be possible. Therefore, the official should determine which of the two possible worlds provides a greater overall level of safety for the society. In addition, potential public reaction to a vaccine should cause the public health official to consider the ramifications the addition of a vaccine to the schedule will have on those vaccines already on the schedule.
Because partial exemptors have the potential to sway the balance between herd immunity and vulnerability, public health officials must take account of their concerns. Unlike in years past, today the development of a new vaccine presents public health officials with a choice that can affect other vaccines on the recommended schedule. Though the possibility for a chickenpox- and Hepatitis-B-free nation may seem tempting, officials should now consider the possible consequences of mandating such “borderline” vaccines. Parents who might otherwise vaccinate according to the old schedule might have second thoughts about the new vaccines on the schedule and seek means of avoiding the new requirements. If no means exist for avoiding the new vaccines other than complete exemption on religious grounds, parents who would subsequently pursue such exemptions would bring about a lower level of immunization for older diseases.
Studies may be necessary in the above situation to determine whether herd immunity status could be in jeopardy for those diseases for which vaccines are already on the schedule. While one solution might be to provide parents with greater ability to tailor their individual vaccination desires, such a solution would undermine the efficacy of newly scheduled vaccines. In addition, greater levels of flexibility in vaccination choice would undermine public understanding of the community-based nature of vaccination. I think it might be worth sacrificing the efficacy of the newer vaccines in order to maintain that of the more established ones. The public might be willing to suffer the possibility of chickenpox outbreaks, for example, in order to prevent an even minor epidemic of diphtheria or the measles.
Again, information should play a key role in the resolution of this issue. Many of the websites urging parents to carefully consider the vaccination decision do not inform parents that their decision to vaccinate may affect the overall health of the community. [100] The CDC, for its part, does urge parents to take note of this concern. [101] The very persons who most need to know of this concern (those seeking exemptions), however, are often those most likely to distrust CDC publications. For supposed citizen-oriented websites to urge individuals to make vaccination choices without considering how such decisions affect the community is irresponsible, especially given the scientific stability of the concept of herd immunity.
II. THE ROLE OF THE FEDERAL GOVERNMENT
Some of the problems posed to vaccination programs by exemptors and others could be partially solved through greater public awareness of the stringent safety and efficacy testing done on vaccines before they may enter the market. This section summarizes the role of FDA in the context of vaccination programs. In addition, this section will discuss other ways in which the federal government gets involved in the vaccination issue, concluding with a brief synopsis of the no-fault compensation scheme enacted pursuant to the National Childhood Vaccine Injury Act of 1986. [102]
FDA Regulation
Though state governments determine which vaccinations are mandatory for school attendance, the federal government plays a key role in vaccination. Perhaps most importantly, the federal government regulates the safety and effectiveness of all vaccines. The FDA’s Center for Biologics Evaluation and Research (CBER) is charged with this critical task. [103] The role of CBER ranges from pre-approval testing of potential vaccines to facility inspection to continued oversight and sampling after approval. [104] Regulation of vaccines can be more stringent than for other biologics or drugs. [105] Even after a vaccine is licensed, for example, FDA oversight is prevalent. [106] Since vaccines are derived from living organisms and are particularly susceptible to contamination and other environmental factors, manufacturers usually must submit samples of each vaccine lot for testing before release. [107]
Before a vaccine can even be licensed for distribution and use, it must go through an extensive testing process relatively similar to that of drugs and other biologics. [108] First, a new vaccine must be tested for safety on animals. [109] The vaccine manufacturer next must file an Investigational New Drug application (IND) with the FDA. [110] Studies are then undertaken to ensure safety before any human testing takes place. [111] In addition, the IND must describe the studies intended for humans. [112]
Once these initial steps are completed, proposed vaccines must undergo three phases of clinical trials, in which the vaccine is tested on humans. [113] Phase 1 testing looks only for very serious or very common problems. [114] A small number of subjects (usually less than 100) are closely monitored, usually for only a few months. [115] Testing expands in Phase 2 to begin evaluating efficacy, as well as to further test safety. [116] Phase 2 trials can last up to two years and typically include hundreds of subjects. [117] The final stage of testing, Phase 3, further studies safety and effectiveness. [118] Thousands of people may be involved in this stage of testing, and if successful it can lead to application for FDA licensing. [119]
Once the clinical trials are completed, the FDA can examine the results of the tests to determine whether the vaccine is safe and effective enough to be placed on the market. [120] At any point in the process, the FDA may halt ongoing studies if safety concerns require such action. [121] The FDA also reviews the data from the studies and inspects the manufacturing facility. [122] At this point the vaccine may be licensed.
As stated above, the FDA’s role in protecting the safety and effectiveness of vaccines does not end at the licensing stage. [123] Before any vaccines from a particular lot can be released, the manufacturer must typically submit samples for potency, safety, and purity testing. [124] Periodic facility inspections also continue for the duration of the license. [125] Furthermore, formal post-market studies may be conducted in order to identify problems that would not show up in pre-market clinical testing. [126] These tests are referred to as Phase 4 tests and are not mandatory, but can help identify problems that may only occur very infrequently. [127] Post-marketing surveillance programs are important because manufacturers are “never going to be able to do studies big enough to detect risks that might happen at a level of one in 100,000 or one in 1 million.” [128]
The Vaccine Adverse Event Reporting System (VAERS) is another valuable tool in identifying problems with a vaccine once it has been approved for the market. [129] VAERS was developed following Congress’s enactment of the National Childhood Vaccine Injury Act of 1986 and has become a very useful tool for identifying possible adverse effects that would otherwise escape detection. [130] VAERS allows anyone to report a problem that may be associated with any vaccine. [131]
It is important to keep in mind that VAERS is simply a reporting system. Experts and others use the data in VAERS to attempt to determine whether a vaccine actually causes a particular adverse effect, but the events that VAERS documents are not all caused by vaccines. It is therefore easy to understand why VAERS encourages doctors and others to report any adverse event that may be related to a vaccine. “VAERS is designed to detect signals or warnings that there might be a problem rather than to answer questions about what caused the adverse event.” [132] It is important to keep these facts in mind when looking at VAERS data, as many of the adverse effects may be completely unrelated to the vaccine in question. Often the effects are correlated with, but unrelated to, vaccination simply because many of the problems reported are those usually associated with events happening during the vaccination period (the first few years of life). [133]
Used correctly, VAERS can lead to useful studies and the discovery of potentially rare adverse effects. [134] VAERS can also be used to monitor individual lots of a vaccine. [135] Unfortunately, by encouraging individuals to report any adverse effect that may possibly have been caused by a vaccine, VAERS can provide ammunition for those claiming a definite link between a vaccine and a particular adverse effect, even if the data is silent on whether such a link exists. [136] While VAERS is in place to help identify actual risks associated with vaccines, these risks cannot be accurately assessed solely on the basis of reported incidents of adverse effects. [137]
The real value of VAERS lies in the testing and hypotheses that are developed in response to the data that has been reported. Because of the serious adverse effects already occurring during the typical vaccination period, it will often be easy and convenient to point to the correlation between vaccines and reported adverse events. Lost in the picture is the foundational proposition that VAERS is, at its core, a data collection system. To forego scientific inquiry and point instead to simple correlation may be convenient, but it is unwise. [138]
The recent public discussion surrounding the use of thimerosal as a preservative in vaccines helps to illustrate the importance of the FDA and other factors in furthering the goals of vaccine safety and public confidence in the entire safety regulatory process. Thimerosal is a mercury-containing organic compound that for many years has been used as a preservative in vaccines to help prevent contamination with microbes that could potentially be fatal. [139] Recently, fears that mercury at very low levels may be toxic to the brain have raised concern among many in the public about allowing the use of thimerosal in vaccines. [140] Many began to fear a connection between thimerosal and autism. [141] Standard FDA testing of lots, as well as studies measuring the amount of mercury contained in the standard immunization schedule versus accepted safe amounts, did not lead to safety concerns sufficient to pull thimerosal from the market. [142] Though one committee (the Immunization Safety Review Committee, commissioned by the Institute of Medicine) concluded that a theoretical link between thimerosal and autism was biologically plausible, most health experts continue to assert that there simply is no scientific evidence of a link between the two. [143]
During this time period FDA performed additional tests to verify or refute the supposed link between thimerosal and autism. [144] In 1999, FDA performed a comprehensive study and review of thimerosal use in vaccines for children. This review revealed no risk from thimerosal use, other than “local hypersensitivity reactions.” [145] Indeed, none of the standard safety protocols in place suggested or required that FDA pull thimerosal from the market. This is not to say, however, that no risk existed. As is clear from the foregoing summary of FDA vaccine approval, not all adverse effects will be known from clinical trials. [146] It may take years or longer to assess some of the risks of vaccines, including the risk of thimerosal as a preservative. [147]
Continued public concern over the safety of thimerosal caused FDA to begin to work with vaccine manufacturers in order to reduce or eliminate thimerosal from vaccines as a precautionary measure. [148] About this time, the American Academy of Pediatrics and the Public Health Service urged the removal of thimerosal from vaccines. [149] Today, with the exception of the inactivated influenza vaccine, all recommended childhood vaccines are either thimerosal free or contain only trace amounts of the compound. [150] Even though the risk may not have been as great as feared by the public or even existent at all, if the new vaccines are equally effective, the elimination of thimerosal from vaccines can probably be seen as a safety improvement, albeit at the expense of the added research and development needed to create the new thimerosal-free vaccines.
Rather than quell the existing safety concerns, this action led many of those who had decried the use of thimerosal to accuse FDA of participating in a cover-up to protect vaccine manufacturers. [151] Government agencies, for their part, continue to claim that vaccines with thimerosal are as safe as thimerosal-free vaccines, suggesting that the added development may have been superfluous. [152] While this may be so, the availability and now prevalence of thimerosal-free vaccines does provide the scientific and medical community with a new means of assessing the possible autism-causing effects of thimerosal. Namely, since thimerosal is suspected to cause autism within the first few years of life (the routine vaccination calendar), those who were vaccinated in the years since thimerosal-free vaccines have comprised the overwhelming majority of vaccines (that is, those born after 2001) would be expected to experience lower incidences of autism than the groups vaccinated with thimerosal-containing vaccines. [153]
In spite of the potentially costly decision to encourage the development of thimerosal-free vaccines when there is no sufficient safety concern to pull thimerosal from the market, FDA and other government officials have had little success in assuaging the fears and concerns of thimerosal critics. [154] Scientific arguments often fail to persuade, either because they are inconclusive or because of a perceived bias favoring vaccine manufacturers. [155] To back up their own arguments, thimerosal critics rarely point to scientific studies. [156] Instead, their reasoning seems to stem more from anecdotal evidence and comparison of thimerosal (which contains ethyl-mercury) to methyl-mercury-containing fish. [157] Representative Dan Burton (R-Indiana), a key supporter of the fight against thimerosal, explained that his belief in the toxicity of thimerosal stemmed from a personal episode: “[m]y grandson received nine shots in one day, seven of which contained thimerosal, which is 50 percent mercury as you know, and he became autistic a short time later.” [158] Others point to the rise in autism rates in the past twenty years and put the onus on the medical community to prove that this rise is not due to thimerosal. [159]
The response of health officials has been to ask why the burden should be placed on them to disprove a link between thimerosal and autism; cell phones, ultrasound, or diet soda could just as easily be the culprit. [160] Indeed, the typical response to those charging vaccination with causing many of the adverse effects occurring in life’s first few years is to point out that usually such accusations are based on nothing more than the temporal proximity of the vaccine and the illness. Some have suggested that the rates of autism may be on the rise not because of thimerosal, but because of generally more accurate diagnosis of the affliction. [161] In the past, an autistic child may have been wrongfully diagnosed with other mental disorders. [162] Figures showing a correlation between the rise in autism and the drop in other diagnosed mental disorders bolster such assertions, and suggest that vaccination may simply be a convenient scapegoat. [163]
As the thimerosal issue makes clear, vaccines often provoke strong feelings amongst various segments of the population. [164] Proper consideration of public reaction to its actions is a delicate aspect of FDA regulation of vaccine safety. To complicate matters further, one can easily imagine an equally vehement response and similar claims of conspiracy had the FDA not worked to reduce thimerosal from vaccines as a precautionary measure. Indeed, public confidence in the safety of vaccines is often influenced by factors outside the typical FDA calculus. Though FDA must act in the interests of the general safety regardless of public opinion, it may sometimes be necessary for FDA to consider public opinion, at least when exercising discretionary oversight. After all, the entire VAERS system is to a large extent dependant on public cooperation. Nevertheless, when the choice is between FDA popularity and doing what is right for the safety of Americans, the FDA should not allow itself to be swayed by a misinformed public.
Vaccine Injury Compensation Program
Congressional reaction to safety concerns goes beyond the adverse reporting system VAERS. The National Childhood Vaccine Injury Act of 1986, which created VAERS, also created a no-fault compensation scheme for people injured or killed by vaccines as an alternative to the traditional tort system. [165] This system was intended to efficiently and rapidly compensate those who are actually injured by vaccines while maintaining an environment in which further vaccine research and safety improvement could exist. The situation giving rise to this compensation program sounds remarkably similar to the more recent concerns surrounding thimerosal:
In the early 1980's, reports of harmful side effects following the DTP (diphtheria, tetanus, pertussis) vaccine posed major liability concerns for vaccine companies and health care providers, and caused many to question the safety of the DTP vaccine. Parents began filing many more lawsuits against vaccine companies and health care providers. Vaccination rates among children began to fall and many companies that develop and produce vaccines decided to leave the marketplace, creating significant vaccine shortages and a real threat to the Nation’s health. [166]
Funding for the no-fault compensation scheme initially came from Congressional grants of federal tax dollars totaling $110 million per year. [167] Since October 1, 1988, funding has proceeded from the Vaccine Injury Compensation Trust Fund, which is funded by a $0.75 excise tax on all doses of vaccines covered under the program. [168]
One may wonder what makes vaccines worthy of an alternative dispute resolution system. Perhaps it is the result of the power of the vaccine manufacturing lobby or simply an attempt by Congress to pass some legislation in the face of strong public sentiment. Although these reasons may appear plausible, it seems more likely to me that the Act created this no-fault compensation scheme because of the mandatory nature of vaccination. For those injured by other medical devices or drugs, the traditional tort system or medical insurance seem the proper means of addressing the issue. When people are told to undertake a medical procedure they may not agree with because it helps further a public goal, however, it may make sense to have a system in place whereby they can obtain relief quickly if harmed by the procedure. Moreover, because certain vaccines may be closely associated with particular adverse effects, the efficiency of a no-fault scheme may trump the standard fact-finding processes of the legal system. The government has chosen to enact such a no-fault scheme, and err on the side of compensation.
III. ANALYTICAL MEANS OF ADDRESSING THE ISSUE
The concerns and problems raised in the context of mandatory vaccination programs do not readily suggest a simple answer. In examining the issue, I came across two particularly useful tools for analyzing the problem. The first comes from an old episode of The Andy Griffith Show in which a local farmer refused to accept a vaccination from the local nurse. In addition to providing substantial entertainment to the viewer, the characters can be viewed metaphorically to represent the various parties in the mandatory vaccination debate. The episode’s solution, in turn, sheds some light on the current debate.
This section will also utilize the analytical framework of economic analysis. Though not as enjoyable a topic as The Andy Griffith Show, economic theory helps to reshape the vaccination discussion and greatly facilitates the process of assessing the various positions.
“We got to get folks to take their shots” – Sheriff Andy Taylor [169]
The Andy Griffith Show addressed the concept of popular resistance to universal vaccination over forty years ago. In “The County Nurse,” Sheriff Andy Taylor confronted a local nurse who was trying to bring everyone up to date on their tetanus shots. Not surprisingly, at least to Andy, many of the mountain farmers had not been inoculated. The naïve nurse would soon discover the reason for the low vaccination rate.
Rafe Hollister, one of the leading farmers in Mayberry, had little use for modern medicine or doctors in general. “We don’t need any nurse, nobody gets sick up here.” [170] Thermometers? “I know when I got a fever, I’m hot.” [171] Stethoscopes? “I know my heart’s beating, I’m alive ain’t I?” [172] But his strongest objection was saved for vaccinations: “I ain’t never been jabbed and I ain’t fixin’ to be.” [173] Such were the views that the nurse was up against in her attempt to achieve 100% vaccination rates.
Rafe Hollister
Rafe Hollister’s reasons for opposing vaccination went beyond his desire to avoid getting “jabbed.” He was a farmer who lived off the land, and when he got sick he let his body fight the sickness naturally. His daddy had lived to the age of hundred and he aimed to do the same. [174] The concept of a vaccination was certainly something foreign to him, as was the idea that a health official could force him to do anything. Even in the wake of the nurse’s impassioned plea to accept a shot that could someday save his life, he retorted simply, “I done alright before you come around and I’m doing alright now.” [175]
Although the county nurse was not acting pursuant to a mandatory vaccination program, under the circumstances her attempts to get Rafe inoculated were pretty forceful. The nurse was accompanied by the local sheriff to Rafe’s farm to try to convince him to take the shot, and when he refused, the sheriff and nurse continued to attempt to make him acquiesce. When Deputy Barney Fife heard of Rafe’s stubbornness, he insisted the nurse return to Rafe’s farm with him to force Rafe to take the shot. After all, boasted the deputy, “Rafe Hollister’s like a child and he’s gotta be treated like one...I’ll make him take his shot.” [176] When the deputy arrived at Rafe’s farm yelling that he was forcing Rafe to accept the vaccination, Rafe decided to fight the mandatory vaccination by drawing his rifle and forcing the deputy to leave the farm.
In a classic manifestation of the early spirit of the television series, Sheriff Andy Taylor finally convinced Rafe to take the shot through a little reverse psychology. Andy began by facetiously praising Rafe’s refusal to take the shot as stemming from Rafe’s desire for immortality. Namely, by refusing to take the shot, Rafe was sure to become the impetus for all the other townspeople not to neglect to take their shots. Unfortunately for Rafe, this heroic stature would only be achieved posthumously, as he will have succumbed to a violent and painful death from tetanus. As Andy explained to Rafe, someday, after getting cut by a rusty saw or bitten by an animal, without the shot he’ll “be a cinch to go.” [177] Eschewing the chance to be a dead hero, Rafe finally took the shot.
Sheriff Andy Taylor
Vaccination has changed the modern world. Indeed, it has led to the elimination or significant decline of many diseases that once posed significant and potentially deadly health risks. Public health officials in the United States have managed to institute a program that, though subject to variations on a state by state basis, essentially mandates certain vaccinations as a requirement for school attendance. While these vaccination programs are touted by most public health officials, a significant number of people oppose mandatory vaccination. The County Nurse episode helps illuminate the perspectives of the various sides of the issue, as well as one possible solution.
The nurse herself represents the public health officials. Though she is not implementing a mandatory vaccination program, her stated goal is to inoculate 100% of the population. [178] As mentioned above, she has the assistance of local law enforcement and she is quite persistent. Rafe Hollister, the stubborn farmer, represents those within the community who oppose or resist mandatory vaccination programs. His reasons initially rest on a general reluctance to stray from natural medicine. In this way he represents the contingent of society that scientists and medical researchers will always find difficult to convince of any developments in the medical field. In many ways, he is comparable to the plaintiff in Jacobson . Andy and Barney can be seen as the arms of the state that are entrusted with carrying out the general vaccination plan. Their varying styles can be seen as varying state requirements and enforcement options for vaccination.
Though these comparisons may seem elementary and of little value, the character development that the characters undertake during the episode greatly increases the episode’s usefulness as a surrogate for real world concerns and issues. Rafe resists the shot initially not only because he distrusts medicine in general, but also because he resents the idea that a county nurse can make him do anything. Many who resist mandatory vaccination schemes do so because of personal liberty concerns; they do not want the government to tell them what to do, especially in the context of personal medical decisions. Just as Rafe’s stance becomes more vehement the harder the nurse attempts to convince him, many who oppose mandatory vaccination see the persistence of the medical community as evidence of blind adherence to a potentially dangerous system, or worse yet as an active promotion of the special interests of the vaccine manufacturers. [179] The episode does not paint the nurse in this way at all, however. Rather, after seeing how strongly Rafe opposes vaccination, the nurse passionately pleads with him to reconsider. Her stance truly seems to stem from a genuine concern that he not suffer the potentially terrible effects of the disease. [180] As before, he refuses; this seems to illustrate that the stance of some may be so strong that they will never accept vaccination on the basis of arguments advanced by government officials.
Barney Fife’s insistence that Rafe accept the shot demonstrates the lack of understanding among many in the government and in the general population as to the vehemence with which those opposing mandatory vaccination hold to their views. His paternalistic stand only serves to exacerbate the situation with Rafe. Indeed, Barney Fife helps to illustrate that there cannot be a one-way solution to the issue of mandatory vaccination.
Andy Taylor’s method of convincing, which eventually carried the day, may not be very conducive to real-world implementation. After all, it is unrealistic to think that reverse psychology will convince those currently opposed to vaccination programs to change their minds. What I think is important to notice, however, is the role information can play in this issue. Andy finally convinces Rafe Hollister to take his shot after describing the horrible effects of the disease and how likely Rafe is to contract it. Similarly, any solution to the issue of mandatory vaccination holdouts must rely on increased information dissemination. That the information in the episode came from a trustworthy source may also have been crucial, which seems to imply that public health officials may need to work more closely with local personnel in order to obtain higher vaccination rates.
Because this episode deals with the vaccine for tetanus, a non-communicable disease, the usual community-based arguments in favor of vaccination do not enter the equation. Extra-personal consequences of Rafe’s decision to vaccinate do exist, however. Most importantly, as the unofficial leader of the farming community, his decision will be followed by the other farmers. This is shown both in Andy’s assurances to the nurse that Rafe is the most important of the farmers to convince on the issue and later, after Rafe has decided to get the shot, in his promise to the nurse that all she has to do is come with him and he’ll get all the farmers to take their shots. Perhaps those parents who support vaccination can help bring about higher vaccination rates by being more vocal and persistent with their neighbors who oppose vaccination programs.
Economic Analysis
Economic analysis [181] provides a useful theoretical basis for evaluating the competing sides of the vaccination debate. Arguments regarding the wisdom of the current vaccination policy can often be recast as economic questions involving a cost-benefit analysis.
When an epidemic breaks out, for example, the benefits of vaccination (protection from the disease both for the individual and for society through herd immunity) seem more clearly to outweigh the costs (potential side effects of the vaccine, decreased ability of the immune system to defend the body from variant strands of the disease, or personal or religious objection). Vaccination rates would, therefore, be expected to be highest during such epidemics. Consequently, those few who continue to oppose vaccination during such epidemics would be expected to do so for only the strongest reasons. This is due to the fact that in economic terms, the opponent of vaccination would have to believe that the benefits of vaccination still do not outweigh the costs, even during an epidemic. This might stem from a relative undervaluation of the benefits of vaccination (perhaps due to a belief that contracting the disease would not be so bad) or a relative overvaluation of the costs of vaccination (possibly due to the greater cost to the conscience of the personal or religious opponent of vaccination) or some combination of both. Medical exemptions directly illustrate this cost-benefit analysis: for a person likely to suffer serious side effects from a vaccine, the cost of vaccination is much greater than the cost to the average individual. Even in a time of epidemic, therefore, vaccination might not be rational for such an individual.
This economic analysis of vaccination is well illustrated by the facts of Jacobson v. Massachusetts [182] , the first Supreme Court case addressing the constitutionality of mandatory vaccination legislation. The case involved a Massachusetts statute allowing local authorities to mandate vaccination for smallpox if necessary for the public health and safety. [183] Subsequently, and upon a determination that smallpox was “prevalent to some extent” and “continues to increase,” the city of Cambridge passed a mandatory vaccination ordinance. [184] This ordinance represented the economic determination that the benefit of mandatory vaccination outweighed the cost of supplying vaccines, finding and prosecuting holdouts (such as Jacobson), and the decreased liberty of individuals to be permitted to decide whether to vaccinate.
Jacobson subsequently challenged his prosecution under the ordinance by claiming it to be an unconstitutional denial of his liberty under the 14th Amendment (as well as in violation of the Preamble and the “spirit” of the Constitution, arguments that were summarily dismissed). [185] In economic terms, this may simply indicate that he viewed the cost of accepting a forced vaccination (perhaps of any kind, in any circumstance) as greater than any possible benefit. A closer look at his arguments, however, suggests that he may have performed a more detailed cost-benefit analysis. One can easily convert the various arguments he attempted to advance into economic costs. Among these arguments were the likelihood of vaccination to bring about “serious and permanent injury” and occasional death, the inability of an individual to assess the risk of vaccination in a particular case, and the potential impurity of vaccines and inability to test such impurity, among others. [186] At the very least, it would appear that Jacobson attributed a greater than average cost to vaccination.
The statute also provided that ordinances mandating vaccination provide an exception for “children who present a certificate, signed by a registered physician, that they are unfit subjects for vaccination.” [187] This reflects the state’s determination that the cost of forcing vaccination upon those more likely to suffer adverse side effects outweighed the benefit of completely universal vaccination. Given the determination that near-universal vaccination was required to provide the desired benefit, one would expect that the state expected to grant relatively few medical exemptions (or at least few enough not to seriously compromise the goal of providing protection against smallpox through vaccination).
In rejecting Jacobson’s liberty challenge to the ordinance, the Court endorsed the concept that the State’s cost-benefit analysis can supersede that of the individual, at least in the area of public health. The Court’s decision, in fact, makes irrelevant any individual cost-benefit analysis in the face of a comprehensive mandatory vaccination program.
Various vaccination-related developments in the century since Jacobson can also be cast in an economic analytical framework. Certainly the benefit from vaccination disappears when a disease has been eradicated, which explains why the smallpox vaccine is no longer mandated. Any cost greater than zero (the likely benefit of smallpox vaccination at this point, barring of course a reintroduction of the disease using laboratory samples) will suffice to outweigh this benefit. [188] The success of vaccination policies, however, may lead to an undervaluation of the benefit of continuing to vaccinate due to the lack of visible instances of the disease. [189] This problem may be compounded when vaccines are mandated for diseases which are not associated with high mortality rates, such as chickenpox. A further complication to the cost-benefit analysis arises when assessing vaccination policy for diseases such as Hepatitis B, which is spread typically through voluntary contact. In such a case, an individual who feels highly unlikely to engage in the behavior giving rise to the risk of the disease might rationally see very minimal benefit from vaccination, while the state may view widespread vaccination as the most cost-effective method of dealing with the disease. [190]
Altruism and Free Riding
Given the continuing policy of vaccinating for diseases that have become relatively rare in recent decades, one might expect individual cost-benefit analyses to increasingly come into conflict with the societal policy. Several factors, however, serve to counteract this possibility. Perhaps most significantly, it is likely that many parents defer on the question of vaccination and accept the cost-benefit analysis of the state (communicated to the individual through the vaccination schedule and through doctor’s recommendations) as their own. Along the same lines, many individuals might not strongly consider the pros and cons involved in vaccinating; if the possibility exists for contracting a disease, and a vaccination is available, the decision may already be made. [191] A third possibility implicates a factor that I have not yet mentioned in relation to the individual cost-benefit analysis: altruism.
Some have proposed that altruism may bridge the gap between incompatible cost-benefit analyses of states and individuals. [192] Whereas typical medical decisions affect only the patient making the decision, it is pointed out, medical decisions regarding vaccine-preventable diseases usually implicate outside interests. [193] A patient thinking only of his own interests may forego vaccination if he feels the risk from vaccination outweighs the personal benefit. Altruism, it is argued, may present a separate benefit for such an individual. [194] Though the individual may not consider the risk of contracting the disease high enough by itself to justify vaccination, he may still vaccinate in order to help accomplish the public goal of eliminating the threat of an epidemic. Public health officials hope that comprehensive vaccination will produce herd immunity. [195] Thus the individual who may otherwise forego vaccination might undertake it in order to “do his part” for the community at large. Individuals who cannot vaccinate are particularly dependent on this sort of altruistic behavior, as they often have no other protection from the disease. [196]
Working against this altruistic behavior is the temptation of individuals to enjoy the benefit conferred on them by herd immunity without undertaking the cost of being vaccinated personally. [197] This is widely referred to as “free riding,” and greatly undermines the goal of comprehensive vaccination. Since herd immunity is supposed to create a level of protection sufficient for even those few who are not vaccinated, a small number of free riders might not pose a significant problem. As described earlier in this paper, comprehensive vaccination programs are designed to work even though some members of society cannot be vaccinated. [198] The problem arises when the number of free riders becomes sufficiently high to compromise the ability of the society to achieve herd immunity. Since the average citizen (one with no greater reason to avoid vaccination than any other member of society) could always choose to free ride if immunization were voluntary, herd immunity might never be achieved. This is one of the key arguments advanced in support of government mandated vaccinations. [199]
Ex Ante Versus Ex Post
The concepts of altruism and especially free riding emphasize the importance of ex ante (before the fact) versus ex post (after the fact) decision making in the context of vaccination. One of the main benefits of economic analysis is that it requires decisions to be justified ex ante. Public health officials, for example, are faced with the decision of whether to mandate vaccination for a particular disease at a time when all adverse effects cannot be known. They must weigh the possible consequences of allowing a disease to continue against the possible known and unknown adverse effects of a vaccine that may have just entered the market. When this decision is made properly, the benefit of the vaccination program will have outweighed the cost. The benefit is manifested in lower or no occurrences of the disease, while the cost is seen most directly in those children who have actually experienced adverse effects as a result of the vaccine. If the benefit is greater than the cost from an ex ante perspective, to the economist there should be no second-guessing of the vaccination program. [200]
The economist, of course, is not the parent. Parents who decry mandatory vaccination as the cause of their child’s adverse reaction are typically viewing the situation ex post. That the program has been implemented assumes that the sum of these adverse reactions was an acceptable alternative to non-implementation, and should therefore not be allowed to undermine public confidence in the program. When one surveys the landscape of the vaccination issue, however, objections are usually of the ex post variety. Since it is harder to appreciate the absence of an epidemic than the presence of a child suffering a vaccine-related injury, it is easy to look at the issue solely ex post. In the interests of public safety, such reasoning should be avoided.
This is not to imply that all critics of mandatory vaccination are on unsound theoretical footing. In fact, those whose objections are marked by a distrust of the government authorities in charge of implementing vaccination programs can be seen as questioning only the ex ante judgment of the officials. If this is so, they are actually on firmer ground than those who object to the programs because they feel their child was harmed by the vaccine. Ex ante critiques are valuable because they can bring about change in the system at a time when it can still prove useful.
The National Vaccine Injury Compensation Program represents a theoretically sound program under these criteria. Economically, it represents the idea that some of the costs of mandatory vaccination programs known only ex post will be compensated by all those who share the benefits ex ante. The excise tax, paid ex ante by all who receive the vaccine, is used to compensate anyone who experiences certain adverse effects ex post. This is simply an example of the government distributing the costs of the vaccination program across the spectrum of those who receive the benefit, rather than an ex post complaint by those on whom the costs have fallen.
Other Issues
The modern trend toward more widely-granted exemptions represents government acquiescence toward a certain degree of free riding. Should such exemptions proliferate too widely, herd immunity may indeed be lost and a recalculation of the cost-benefit analysis of individuals will be necessary. In the face of a greater potential to contract disease, the benefit of vaccination grows significantly, while the cost of accepting the vaccine remains the same. Likewise, from the standpoint of the government, the cost of allowing widespread exemptions will eventually overtake the benefit of permitting such exemptions if that cost suddenly includes serious risk of epidemic.
The risks associated with non-vaccination can be illustrated through a rather simplified mathematical example. [201] Suppose a school with 1,000 students is exposed to a measles outbreak. 990 of the students have received all of their measles shots, and so are fully immunized. Suppose further that the measles vaccine is 99% effective; that is, it produces complete immunity in 99% of patients. [202] Therefore, 10 out of the 990 who have been fully immunized will be susceptible to the disease. In addition, all 10 of the 1,000 students who had not been fully immunized will be susceptible to measles. Therefore, 20 out of 1,000 students will get the disease. Although the number of infected students who were vaccinated is equal to the number who were not, this example demonstrates that vaccination can be very effective even if it sometimes does not produce immunity in an individual. If no one had been vaccinated, 980 more students would probably have caught the measles. It is also important to note that this example assumes an epidemic; in reality, herd immunity would probably be attained at this level of inoculation and none of the 1,000 students would have caught the disease.
IV. CONCLUSION
Vaccines have immeasurably improved our quality of life. They have led to the eradication of deadly diseases like smallpox and the near elimination of diseases such as diphtheria, polio, and measles. Outbreaks of vaccine-preventable diseases, such as mumps, are infrequent and are also quite newsworthy on the rare occasion that they do occur. And people like Rafe Hollister can survive a run-in with a rusty saw or an animal bite.
The life-saving benefits of vaccination often overshadow the vast economic and personal benefits it has helped provide. Jonas Salk’s cure for polio has spared generations from a life hindered by the devastating physical handicaps of that terrible affliction. Children no longer must miss vast stretches of school to overcome a debilitating battle with pertussis (although there is no doubt that some children lament this decline in excused absences from school). Parents no longer have to spend restless hours worrying as their children suffer the body’s natural response to disease. In economic terms, this translates directly into fewer missed hours of work and less administrative difficulty, leading to a generally more productive society.
For all the benefits of vaccines, of course, it is important not to ignore the costs. The National Vaccine Injury Compensation Program is one way of dealing with the economic costs of vaccination, but this may provide little solace to the parent of a child who has been injured by a vaccine for a disease that is seemingly in decline. Side effects with very low probability will sometimes occur; though from a community-wide view this possibility is acceptable, for the individual who experiences the adverse effect the vaccination may not have been the best medical decision. Many who view natural immunity as a rite of passage for children might not desire a means of bypassing the disease entirely.
Some may accuse public health officials of dreaming for an unreachable day when all diseases are controlled by vaccination. Zeal on the part of public health officials, however, should not overshadow the actual benefits of vaccination generally. Soon may come the day when diphtheria, like smallpox, will be eradicated globally. At that point, it can be removed from the vaccination schedule and future generations will reap the benefits of vaccination while undertaking none of the costs.
This prospect, I think, sheds light on the ultimate solution to vaccination issues that have been discussed in this paper. Highly communicable and especially terrible diseases should continue on the vaccination schedule until they are virtually eliminated. The eventual elimination of these scourges will someday make vaccination unnecessary, and the costs of vaccination will drop to zero. Until that time, officials should seek stricter enforcement of the mandatory vaccination laws and should tighten down on non-medical exemptions. At the same time, information campaigns should be considered in the interest of reminding the public of the continued importance and relevance of vaccine programs. Though risks are unavoidable when dealing with vaccines, parents should constantly be reminded that immunity depends on a high level of cooperation. This will hopefully keep immunization rates high, at least for the most harmful diseases.
Meanwhile, public health officials may be wise to consider an alternate stance toward somewhat less-important vaccines such as Hepatitis B and varicella (chickenpox). [203] With such diseases it may be worthwhile to wait longer before placing the vaccines on the recommended schedule. This will undoubtedly make herd immunity more difficult if not impossible to attain, while simultaneously announcing to parents that undertaking the vaccine in question is a personal medical decision. Most of those who choose to vaccinate (and accept the risk of adverse effects from these newer vaccines) will still acquire immunity. Without a mandatory program in place, however, one would still expect to see regular occurrences of the disease. Given the relatively high likelihood of outbreak under these circumstances, a percentage of those who vaccinate will probably get the disease. They will likely turn to those who did not vaccinate at all and see them as the cause of the outbreak. In time, social pressures may lead to greater vaccination rates, and the time may be ripe for greater acceptance of mandatory vaccination for the disease.
One significant benefit to this approach lies in its natural tendency to point out to parents the importance of receiving the more important vaccines. When some vaccines are mandatory and others are not, the distinction between the two types of vaccines is impossible to neglect. It would hopefully make parents think more carefully before attempting to gain an insincere exemption. This approach would fail to satisfy those who want parents to have the option to choose “one, ten, or no vaccines,” [204] but it would at least allow an element of choice for some vaccines while hopefully maintaining a sufficient level of immunization for the more important vaccines. It is also important to remember that parents with serious reservations about any vaccines will usually have the option of home schooling. Overall, this approach might have the advantage of winning over those who only partially object to the vaccination schedule, thus helping bring about a greater chance of herd immunity for diseases associated with less objectionable vaccines.
Vaccination certainly is unique among medical treatments, both for its incredible potential and its coercive nature. It is unfortunate that questionable evidence has led many concerned parents to question the wisdom of vaccination programs that still serve important goals. Given the importance of public support for the achievement of these goals, however, public health officials must account for sometimes questionable concerns in determining vaccination policy. Greater information dissemination, combined with more sharply drawn (and potentially vaccine-specific) guidelines, can hopefully further the important goals of vaccination policy.
[1] Center for Disease Control, “Smallpox Disease Overview,” at http://www.bt.cdc.gov/agent/smallpox/overview/disease-facts.asp (last visited April 27, 2006).
[2] GlaxoSmithKline is currently developing an ear infection vaccine and plans to seek regulatory approval shortly. Jessica Said, “Vaccine Could End Children’s Ear Infections,” CNN online article, March 3, 2006 (on file with author).
[3] Institute of Medicine. CP Howson, et al. eds. Adverse Effects of Pertussis and Rubella Vaccines. Washington, DC: National Academy Press; 1991, at 1.
[4] James G. Hodge, Jr. and Lawrence O. Gostin, School Vaccination Requirements: Historical, Social, and Legal Perspectives , 90 Ky. L. J. 831, 867 (2001).
[5] See, e.g., Jacobson v. Massachusetts , 197 U.S. 11 (1905).
[6] Hodge and Gostin, supra note 4, at 867.
[7] Id. at 868.
[8] “Rather than having health departments require immunization in emergency conditions, legislatures acted to prevent disease by mandatory immunization as a condition of enrollment or attendance in schools or licensed day care facilities.” Id.
[9] See id. ; see also infra Part I (chart describing the current recommended vaccination schedule).
[10] The Center for Disease Control has gone so far as to suggest that “to have a medical intervention as effective as vaccination in preventing disease not use it would be unconscionable.” Center for Disease Control, National Immunization Program publication, “Six Common Misconceptions About Vaccination and How to Respond to Them,” at http://www.cdc.gov/nip/publications/6mishome.htm (last visited April 27, 2006) (hereinafter “Six Common Misconceptions”).
[11] Center for Disease Control, National Immunization Program publication, “Ten Things You Need to Know about Immunizations,” at http://www.cdc.gov/nip/publications/fs/gen/shouldknow.htm (last visited April 27, 2006).
[12] This is not to imply that parents who vaccinate without carefully considering the pros and cons of vaccination are in the wrong. The health and safety of a child is of paramount importance to most parents, and every parent must make decisions that affect the welfare of the child. Most parents approach such decisions with a sincere desire to promote the child’s best interests, and this desire is no different in the context of vaccination.
[13] “Despite its utility, vaccination has provoked popular resistance from the beginning.” Hodge and Gostin, supra note 4, at 834.
[14] “Some opponents express valid scientific objections about effectiveness or need for mass vaccinations; some fear harmful effects arising from the introduction of foreign particles into the human body; and others worry that vaccination actually transmits, rather than prevents, disease, or weakens the immune system.” Id.
[15] See, e.g. , Jacobson v. Massachusetts , 197 U.S. 11 (1905) (constitutional challenge to government mandated smallpox vaccination); “Six Common Misconceptions,” supra note 10 (“[s]ome see mandatory vaccination as interference by the government into what they believe should be a personal choice”).
[16] “Six Common Misconceptions,” supra note 10.
[17] A more detailed explanation of this subject appears in Part I of this paper.
[21] See, e.g. , “Ten Things You Need to Know about Immunizations,” supra note 11.
[22] Angie A. Welborn, “Mandatory Vaccinations: Precedent and Current Laws,” CRS Report for Congress, at http://www.fas.org/sgp/crs/RS21414.pdf (last updated Jan. 18, 2005).
[23] For a typical scenario of public health response to outbreak, see the facts of Jacobson v. Massachusetts , 197 U.S. 11 (1905).
[24] Hodge and Gostin, supra note 4, at 833-34.
[25] Id. at 867-68.
[26] Id. at 833.
[29] This level of immunity is often referred to as “herd immunity,” the concept that not everyone in a population must be vaccinated in order for the entire population to be protected. Abi Berger, “How Does Herd Immunity Work?” 319 BMJ 1466 (1999). “As long as a sufficient number of children are immunised against each disease for which there is a vaccine, protection against that disease will be conferred on everybody.” Id. Also, the level of vaccination necessary to attain herd immunity increases as the infectivity of the disease increases. Id. Highly infectious diseases, therefore, require higher levels of immunity for herd immunity to occur. Id. The concept of herd immunity will arise throughout this paper, with particular emphasis in Part III.
[30] This is evidenced by the fact that in time of outbreak, the vaccinated population can still be susceptible to the disease, although usually the vaccinated population is far less susceptible to the disease than the unvaccinated population. Vaccines typically produce the desired antibody in an individual around 90% of the time, with actual percentages varying from vaccine to vaccine. Some vaccines, moreover, lose their efficacy and require boosters. These concepts will be further developed throughout this paper.
[31] Hodge and Gostin, supra note 4, at 850-51.
[32] Id. at 851.
[33] Id. at 834.
[34] Id. at 834-35.
[35] See, e.g. , Duffield v. Sch. Dist. , 29 A. 742 (Penn. 1894).
[36] 197 U.S. 11 (1905).
[38] Id. at 26.
[39] Welborn, supra note 22.
[41] 260 U.S. 174 (1922).
[42] Id. at 175.
[43] Id. (“[t]he bill charges that there was then no occasion for requiring vaccination” and that the ordinances “in effect, mak[e] vaccination compulsory”).
[45] Id. at 176.
[48] Id. at 177.
[49] Hodge and Gostin, supra note 4, at 867-68.
[50] Id. at 868.
[51] The schedule of immunizations is published by the Center for Disease Control, and follows the recommendations of the Advisory Committee on Immunizations Practices, the American Academy of Pediatrics’ Committee on Infectious Diseases, and the American Academy of Family Physicians. Id.
[52] Id. at 869.
[54] Based on chart publicized by Center for Disease Control, approved by Advisory Committee on Immunization Practices, American Academy of Pediatrics, American Academy of Family Physicians, available at http://www.cispimmunize.org/IZSchedule_2006.pdf (last visited April 27, 2006).
[55] Indeed, the law in Jacobson was challenged for this reason.
[56] Statement of Barbara Fisher, founder of National Vaccine Information Center, quoted in Neenyah Ostrom, “First Do No Harm,” at http://www.chronicillnet.org/online/Fisher.html (last visited April 27, 2006).
[57] K.N.O.W. Vaccines, Vaccine Awareness of Florida fact sheet, at http://www.know-vaccines.org/vaccine_fact.html (last visited April 27, 2006).
[58] The most direct way in which this occurs surrounds the concept of herd immunity, as discussed elsewhere throughout this paper. If a sufficient number of persons in the community does not vaccinate, herd immunity may be unattainable and others may be put at risk.
[59] See, e.g. , the discussion in Part III involving The Andy Griffith Show.
[60] See Statement of Barbara Fisher, quoted in Ostrom, supra note 56. See also “Autism and Vaccines: Activists Wage a Nasty Campaign to Silence Scientists,” Wall Street Journal, February 16, 2004, at http://www.opinionjournal.com/forms/printThis.html?id=110004700 (last visited April 27, 2006) (citing vaccination critics who had accused the vaccination-defending writers of “having an ‘industry profit promoting agenda’”).
[61] See Statement of Barbara Fisher, quoted in Ostrom, supra note 56.
[64] See the discussion in Part II regarding vaccine safety.
[65] See, e.g. , “Six Common Misconceptions,” supra note 10.
[66] Mission Statement of Vaccination News website, at http://www.vaccinationnews.com (last visited April 27, 2006).
[67] As the discussion in Part II on vaccine safety demonstrates, pre-licensing testing for very rare adverse effects cannot take place if vaccines are ever to reach the market. Phase 4 post-licensing testing does exist, but may take years to discover extremely rare adverse effects.
[68] World Health Organization Immunization Safety page, “Adverse Events Following Immunization,” at http://www.who.int/immunization_safety/aefi/en/ (last visited April 27, 2006).
[69] As the recent mumps outbreak in Iowa demonstrates, not everyone who receives a vaccine develops immunity to the disease. For this reason, the success of vaccination depends on a sufficient level of vaccination in the community. When a significant percentage of the population has not received the vaccine, an outbreak can occur and even threaten some of those who have been vaccinated. See David Pitt, “Iowa Mumps Epidemic Continues to Broaden,” Associated Press, April 13, 2006, at http://www.breitbart.com/news/2006/04/13/D8GVGL600.html (last visited April 27, 2006). See also the above discussion of the history of vaccination.
[70] Mission Statement of Vaccination News website, supra note 66.
[71] See, e.g. , Ross D. Silverman, “No More Kidding Around: Restructuring Non-Medical Childhood Immunization Exemptions to Ensure Public Health Protection,” 12 Annals Health L. 277, 278-79 (2003).
[A]s risks of contracting many deadly and crippling diseases continue to decline to near negligible levels, and rates of childhood immunization continue to reach record levels, the public today places greater attention on the relative weaknesses and dangers of immunizations, and the systems through which they are administered.
[72] Hodge and Gostin, supra note 4, at 874.
[73] Usually this requires physician certification. Id.
[74] Indeed, the CDC itself presupposes the existence of medical exemptors in any broad mandatory vaccination program. See “Six Common Misconceptions,” supra note 10 (noting that the mandatory vaccination program can work to protect even those few who cannot vaccinate because of the possibility of adverse medical reactions).
[76] Hodge and Gostin, supra note 4, at 874.
[77] Jacobson v. Massachusetts , 197 U.S. 11 (1905). See also Employment Division v. Smith , 494 U.S. 872 (1990) (permitting neutral laws of general applicability that incidentally affect religion); Boone v. Boozman , 217 F.Supp.2d 938 (E.D. Ark. 2002) (“constitutionally-protected free exercise of religion does not excuse an individual from compulsory immunization...the right to free exercise of religion and parental rights are subordinated to society’s interest in protecting against the spread of disease”).
[78] W. Va. Code Sec. 16-3-4 (2004).
[79] See, e.g. , Boone v. Boozman , 217 F.Supp.2d 938 (E.D. Ark. 2002). The challenged Arkansas immunization statute exempted “individuals for whom ‘immunization conflicts with the religious tenets and practices of a recognized church or religious denomination of which [they are] an adherent or member.’” The statute was struck down under the Establishment Clause using the test laid out in Lemon v. Kurtzman , 403 U.S. 602 (1971). 217 F.Supp.2d at 950. The Arkansas legislature subsequently amended the exemption generally to allow for religious or philosophical objections without regard to recognized churches. Ark. Code Sec. 6-18-702(d).
[80] See Silverman, supra note 71, at 290-93.
[81] See id.
[82] Hodge and Gostin, supra note 4, at 874.
[83] Cal. Health and Safety Code Sec. 120365 (2003).
[84] Silverman, supra note 71, at 284.
[85] Id. at 285.
[87] See id.
[88] Recall that for those unable to vaccinate for medical reasons, herd immunity provides the only protection from the disease. See “Six Common Misconceptions,” supra note 10.
[89] Silverman, supra note 71, at 285.
[90] Id. The recent mumps outbreak may directly demonstrate this. Officials have pointed out that vaccination only confers immunity on 95% of patients, and of those affected in the recent outbreak, 25% have been vaccinated. See Pitt, supra note 69. The strong implication is that the 75% of those inflicted who were not vaccinated have put the entire community at risk.
[91] Center for Disease Control, National Immunization Program publication, “What Would Happen If We Stopped Vaccinations?” at http://www.cdc.gov/nip/publications/fs/gen/WhatIfStop.htm (last visited April 27, 2006).
[92] Silverman, supra note 71, at 293.
[93] Silverman suggests that eliminating philosophical and religious exemptions would do more harm than good. This approach, he believes, “would exacerbate feelings of animosity and skepticism toward vaccination and the public health system in general.” Id. at 293. On this score he is probably correct, and I agree that wider knowledge, at the very least, is a better initial response to this problem.
[94] Incidentally, it is worth mentioning that of the more longstanding vaccines, the tetanus vaccine stands out as unique. Tetanus is a very harmful disease with about a 20% fatality rate. “What Would Happen If We Stopped Vaccinations?” supra note 91. What makes it unique in the vaccine schedule is that tetanus is not contagious. That is, herd immunity is not attainable and cannot be used to justify mandatory tetanus vaccination. The reason for the general acceptance of the tetanus vaccine seems to stem both from the high risk of the disease and the fact that tetanus can only be prevented by immunization. In addition, the tetanus vaccine for infants has been combined with the vaccines for diphtheria and pertussis. On strictly public health grounds, however, the status of the tetanus shot on the compulsory vaccination schedule comes closest to government fiat of individual health decisions.
[95] Because medical risks may vary from vaccine to vaccine, and thus the justification for such exemptions remains even if the risk is to some but not all vaccines, medical exemptions are somewhat outside the scope of this discussion.
[96] Sean Coletti, Taking Account of Partial Exemptors in Vaccination Law, Policy, and Practice , 36 Conn. L. Rev. 1341, 1344 (2004).
[98] This follows directly from the all-or-nothing nature of the vaccination decision in this world.
[99] Again, this follows directly from the nature of the decision.
[100] See, e.g. , National Vaccine Information Center, at http://www.nvic.org (last visited April 27, 2006) (urging parents to consider eight questions before vaccinating, none of which inform parents of the effect their decision may have on others).
[101] See “Six Common Misconceptions,” supra note 10.
[102] 42 U.S.C. §§ 300aa-1 to 300aa-34.
[103] U.S. Food and Drug Administration, Center for Biologics Evaluation and Research, “Vaccine Product Approval Process,” updated July 27, 2002, at http://www.fda.gov/cber/vaccine/vacappr.htm (last visited April 27, 2006) (hereinafter “Vaccine Product Approval Process”).
[104] See id.
[105] See, e.g. , Isadora Stehlin, “How FDA Works to Ensure Vaccine Safety,” FDA Consumer magazine (December 1995), at http://www.fda.gov/fdac/features/095_vacc.html (last visited April 27, 2006).
[106] “Licensing of a vaccine is only the beginning of FDA’s oversight.” Id.
[109] “Vaccine Product Approval Process,” supra note 103.
[112] Stehlin, supra note 105.
[115] Id. ; “Vaccine Product Approval Process,” supra note 103.
[116] Stehlin, supra note 105.
[118] “Vaccine Product Approval Process,” supra note 103.
[121] Id. ; Stehlin, supra note 105.
[123] Indeed, the National Immunization Program has confidently pointed to the FDA’s role in continued oversight of vaccines:
FDA would recall a lot of vaccine at the first sign of problems. There is no benefit to either the FDA or the manufacturer in allowing unsafe vaccine to remain on the market. The American public would not tolerate vaccines if they did not have to conform to the most rigorous safety standards. The mere fact that a vaccine lot [is] still in distribution says that the FDA considers it safe.
“Six Common Misconceptions,” supra note 10.
[124] “Vaccine Product Approval Process,” supra note 103.
[127] Stehlin, supra note 105.
[128] So states Susan Ellenberg, Ph.D., director of CBER’s division of biostatistics and epidemiology. Id.
[129] “Vaccine Product Approval Process,” supra note 103.
[130] Stehlin, supra note 105.
[136] See, e.g. , “Six Common Misconceptions,” supra note 10 (“[o]nly some of the reported health conditions are side effects related to vaccines. A certain number of VAERS reports of serious illnesses or death do occur by chance alone among persons who have been recently vaccinated”).
[137] “VAERS reports have many limitations since they often lack important information, such as laboratory results, used to establish a true association with the vaccine.” Id.
[138] “In summary, scientists are not able to identify a problem...based on VAERS reports alone without scientific analysis of other factors and data.” Id.
[139] U.S. Food and Drug Administration, Center for Biologics Evaluation and Research, “Thimerosal in Vaccines,” at http://www.fda.gov/Cber/vaccine/thimerosal.htm (last updated Sept. 6, 2005).
[141] See, e.g. , Gardiner Harris and Anahad O’Connor, “On Autism’s Cause, It’s Parents vs. Research,” New York Times, June 25, 2005, at http://www.nytimes.com/2005/06/25/science/25autism.html (last visited April 27, 2006) (reporting the ongoing tension between parents of autistic children and the medical community over the use of thimerosal in vaccines).
[142] See, e.g. , Center for Disease Control, National Immunization Program publication, “Mercury and Vaccines (Thimerosal),” at http://www.cdc.gov/nip/vacsafe/concerns/thimerosal/default.htm (last visited April 27, 2006) (studies have failed to find any association between exposure to thimerosal in vaccines and autism); “On Autism’s Cause, It’s Parents vs. Research,” supra (noting that the amount of ethyl mercury in each childhood vaccine was once about the same as the amount of methyl mercury, a more toxic compound, found in an average tuna sandwich).
[143] “Thimerosal in Vaccines,” supra note 139.
[146] Stehlin, supra note 105.
[149] “On Autism’s Cause, It’s Parents vs. Research,” supra note 141.
[150] “Thimerosal in Vaccines,” supra note 139; see also “On Autism’s Cause, It’s Parents vs. Research,” supra note 141 (“[b]y 2001, no vaccine routinely administered to children in the United States had more than half a microgram of mercury – about what is found in an infant’s daily supply of breast milk”).
[151] “Autism and Vaccines: Activists Wage a Nasty Campaign to Silence Scientists,” Wall Street Journal editorial, February 16, 2004, at http://www.opinionjournal.com/forms/printThis.html?id=110004700 (last visited April 27, 2006).
[152] “On Autism’s Cause, It’s Parents vs. Research,” supra note 141.
[153] Indeed, one recent study has suggested that neurological disorders have decreased with the removal of thimerosal from most vaccines. See David A. Geier and Mark R. Geier, “Early Downward Trends in Neurodevelopmental Disorders Following Removal of Thimerosal-Containing Vaccines,” 11 J. Am. Physicians and Surgeons 8 (2006). This study should be taken with a grain of salt, however, as the Geiers are widely known thimerosal critics. Years before this study, Dr. Mark Geier called thimerosal use in vaccines the world’s “greatest catastrophe that’s ever happened, regardless of cause.” “On Autism’s Cause, It’s Parents vs. Research,” supra note 141. A witness in many vaccine cases, a judge once ruled that he was “a professional witness in areas for which he has no training, expertise and experience.” Id. Scientists have criticized his prior studies and even called his methods “voodoo science.” Id.
[155] See id.
[156] See “The Politics of Autism: Lawsuits and Emotion vs. Science and Childhood Vaccines,” Wall Street Journal editorial, Dec. 29, 2003, at http://www.opinionjournal.com/forms/printThis.html?id=110004487 (last visited April 27, 2006) (characterizing the position of thimerosal critics as “scientifically untenable”).
[157] See generally “On Autism’s Cause, It’s Parents vs. Research,” supra note 141.
[161] “The Politics of Autism,” supra note 156.
[162] Id. ; “Study: Autism Rise from Labeling, Not Epidemic,” April 3, 2006, at http://www.cnn.com/2006/EDUCATION/04/03/health.autism.reut/index.html (last visited April 27, 2006) (noting rise in diagnosed cases of autism since 1994 is correlated with fall in diagnosed cases of mental retardation and learning disabilities).
[163] The Politics of Autism,” supra note 156.
[164] See, e.g. , “Six Common Misconceptions,” supra note 10 (noting that many anti-vaccine publications claim vaccines are unsafe on the basis of sheer numbers of reports to VAERS without noting that many of them may not represent actual vaccine side-effects).
[165] National Vaccine Information Center, “The Vaccine Injury Compensation Program,” at http://www.909shot.com/Issues/Comp_Summary.htm (last visited April 27, 2006).
[166] Center for Disease Control, National Vaccine Program Office, Vaccine Fact Sheets, “National Vaccine Injury Compensation Program,” at http://www.hhs.gov/nvpo/factsheets/fs_tableIV_doc1.htm (last visited April 27, 2006).
[167] See National Vaccine Injury Compensation Program, at http://www.hrsa.gov/vaccinecompensation/ (last visited April 27, 2006).
[169] The Andy Griffith Show: The County Nurse (CBS television broadcast, March 19, 1962).
[177] Id. That is, there will be a high probability of death.
[179] See the discussion above in Part I of this paper.
[180] For example, she begs Rafe to consider his family and what his decision could mean to them. She literally appears to be on the verge of tears as he refuses.
[181] In utilizing the theoretical framework of economic analysis, it is useful to keep in mind a few foundational concepts. First, a policy or program (in this case mandatory vaccination) is desirable if the overall benefit to society as a whole outweighs the cost of the program, where benefits and costs include both monetary and non-monetary factors. Second, individuals making rational choices regarding vaccination will vaccinate when the benefits of vaccination outweigh the risks or costs of non-vaccination to the individual. This decision-making process can be skewed by externalities, such as an unforeseeable decrease in the effectiveness of a vaccine due to a reduction in vaccination by others unknown to the individual at the time of the decision.
[182] 197 U.S. 11 (1905).
[183] Id. at 12.
[185] Id. at 13, 22.
[186] Id. at 36.
[187] Id. at 12.
[188] As the CDC itself explains, “[e]ven one serious adverse effect in a million doses of vaccine cannot be justified if there is no benefit from the vaccination.” “Six Common Misconceptions,” supra note 10.
[189] In Japan in the 1970s, for instance, pertussis vaccination coverage fell from 80% to 20%, leading to an outbreak in 1979 resulting in 13,000 cases and 41 deaths. “What Would Happen If We Stopped Vaccinations?” supra note 91.
[190] Judge Richard Posner has suggested that this difference between sexually transmitted diseases and air- and water-borne diseases may imply a lesser imperative to eliminate sexually transmitted diseases:
[T]he externality created by sexually transmitted diseases is smaller than in the case of other contagious diseases. Sexually transmitted disease is spread primarily by voluntary contact, implying (to the economist) that a person is compensated...for assuming the risk of contracting the disease. Hence the number of cases of sexually transmitted diseases may be closer to the optimum than in the usual air-borne or water-borne or insect-borne epidemics.
Posner, Economic Analysis of Law 162. (6th Ed. 2003).
[191] Additionally, if vaccination rates are high, these individuals may assume that those in society who have already made the choice to vaccinate have performed a similar cost-benefit analysis. These individuals choose to vaccinate based simply on vaccination rates in the community. See John C. Hershey et al., The Roles of Altruism, Free Riding, and Bandwagoning in Vaccination Decisions , 59 Organizational Behavior and Human Processes 177, 178 (1994).
[192] See, e.g. , id. (behavioral survey studying various factors individuals use to make vaccination decisions).
[194] See id. at 178 (“[i]f a patient believes vaccination is in his own best interests, then he has two reasons to vaccinate. One is selfish, in that he will improve his own well being. The other is altruistic, in that he can improve the health prospects of those around him who might otherwise become infected if he is not vaccinated himself”).
[195] The concept of herd immunity is discussed in Part I. Note that “[i]n economic terms, herd immunity is a positive externality of vaccination. Altruistic individuals who recognize and value this externality may undergo vaccination partly to help others in addition to themselves.” Id. See also Berger, supra note 29 (“‘[h]erd immunity’...is the concept that not everybody in a population has to be immunised to protect everyone in that population. As long as a sufficient number of children are immunised against each disease for which there is a vaccine, protection against that disease will be conferred on everybody”).
[196] The CDC has pointed to this as one of the two most important reasons to vaccinate:
There is a small number of people who cannot be vaccinated (because of severe allergies to vaccine components, for example), and a small percentage of people don’t respond to vaccines. These people are susceptible to disease, and their only hope of protection is that people around them are immune and cannot pass disease along to them. A successful vaccination program, like a successful society, depends on the cooperation of every individual to ensure the good of all.
[197] In economic terms, “[w]idening vaccine use decreases each individual’s benefit from being vaccinated, but leaves unchanged each individual’s risk from the vaccination itself.” Hershey, supra note 191, at 178.
[198] “Six Common Misconceptions, supra note 10.
[199] Hershey, supra note 191, at 178.
[200] Suppose, for sake of example, that a vaccination program, if implemented, would save ten lives out of a thousand that would otherwise have perished without the program. Unfortunately, the vaccine will randomly cause death to five persons out of a thousand. From an ex ante perspective, the vaccination program should be implemented as it will save five lives overall. Concerns or complaints from those five persons who die (or their estates) represent ex post objections, and, though unfortunate, should not affect evaluations of the soundness of the program.
[201] This mathematical explanation is a slight variation of that found at CDC, “Six Common Misconceptions,” supra note __.
[202] Note that no vaccine is 100% effective, and vaccination efficacy rates for most childhood vaccinations range from 85 to 95%. Id. As stated in an earlier section, herd immunity is relied upon to protect those who do not develop full immunity from the vaccine.
[203] Given that these particular vaccines are already on the schedule, I think it would be unwise to remove them now. My analysis applies to comparable vaccines that may arise in the future – vaccines for those communicable diseases that do not pose relatively significant health risks. The definition of such diseases, of course, would be a matter of debate. Vaccines for noncommunicable diseases like ear infections would also fall within this rubric.
[204] Statement of Barbara Fisher, quoted in Ostrom, supra note 56.
Answers to All Your Questions About Getting Vaccinated for Covid-19
By The New York Times Updated October 18, 2021
- Share full article

With the rollout of new coronavirus vaccines in the United States, an end to the pandemic is finally in sight. Health and science reporters from The Times have answered many of your questions about getting the vaccine , booster shots , children and schools , safety and side effects , fertility and pregnancy , medical concerns , how the vaccines work and what happens after vaccination .
What do you want to know about the coronavirus vaccine?
Try this experimental tool, which relies on machine learning , to find the answers you are looking for from this guide. if we can’t answer your specific question, we will do our best to address it in the future., getting the vaccine, which vaccine is best.
All three vaccines used in the United States — made by Pfizer-BioNtech, Moderna and Johnson & Johnson — remain highly effective at preventing serious illness, hospitalization and death from Covid-19. But some recent studies have shown differences in efficacy. The findings don’t translate to a meaningful difference in protection in the real world, because all of the vaccines work well. But the research could influence decisions about the timing of booster shots.
One study evaluated the real-world effectiveness of the Pfizer and Moderna vaccines at preventing symptomatic illness in about 5,000 health care workers in 25 states. The study found that the Moderna vaccine had an effectiveness of 96.3 percent, compared with Pfizer’s 88.8 percent. A study conducted by the Centers for Disease Control and Prevention found that Moderna’s protection against hospitalization didn’t wane after four months, but Pfizer’s fell to 77 percent from 91 percent.
In a different study, the effectiveness of the Johnson & Johnson vaccine against hospitalization remained steady five months after vaccination, at around 81 percent. —Apoorva Mandavilli
Read More Moderna vs. Pfizer: Both Knockouts, but One Seems to Have the Edge
Are the vaccines free?
Yes. You should never have to pay anything out of pocket to get a Covid vaccine, although you will be asked for insurance information. If you don’t have insurance, you can still get the vaccine at no charge. If you get your vaccine from a doctor’s office or an urgent care clinic, ask about potential hidden charges. To be sure you won’t get a surprise bill, the best bet is to get your vaccine at a health department vaccination site or a local pharmacy. —Sarah Kliff
Read More The Vaccines Are Supposed to Be Free. Surprise Bills Could Happen Anyway.
How do I get a shot?
Shots are given at local pharmacies, state health department vaccination centers and doctors’ offices. Check the websites of your state’s health department or local pharmacy chains for more details. You can schedule an appointment online or, in some cases, just drop in, although the recent approval of booster shots for eligible people could increase the wait time.
A Covid vaccine is free, but bring your insurance card if you have one. You may be asked for identification, but in most cases you won’t be turned away if you don’t have it. Call ahead to be sure. Plan to spend about 30 minutes at an appointment, including a 15- to 30-minute waiting period after you get a shot for observation. You’ll be given a vaccine card at the end of the appointment that will include the date to return for your second dose, if required. —Tara Parker-Pope
Will it hurt?
The jab of the needle feels like any other vaccination. Sore arms after the shot are common. Many who have received the vaccine likened the arm pain following the injection to that of a flu shot; for others, it was considerably worse. The New York Times interviewed several dozen of the newly vaccinated about how they felt in the days afterward. They recounted a wide spectrum of responses, from no reaction at all to symptoms like uncontrolled shivering and “brain fog.” —Amy Harmon
Read More What the Vaccine Side Effects Feel Like, According to Those Who've Gotten It
Can I choose which vaccine I get?
The online scheduling systems should tell you which vaccine you’re signing up for, or you can call ahead to make sure the vaccination site is dispensing the one you want. While the major pharmacies are offering all three vaccines, most locations will have only one type available at a specific site. —Tara Parker-Pope
Why do I have to wait around after I get the shot?
Everyone who gets the vaccine will be asked to stick around for about 15 minutes after getting the shot. This will allow health workers to monitor you for any signs of an allergic reaction, which are rare. A person with a history of severe allergies may be asked to stay for 30 minutes. Make sure you bring a quality mask to your vaccination appointment to wear while you wait. Even though you just received the vaccine it will take a few weeks after your final dose before you are protected. —Tara Parker-Pope
What if I can’t get the second dose on time, or I forget to go?
Both the vaccines from Pfizer-BioNTech and from Moderna have two doses. Pfizer-BioNTech’s second dose comes three weeks after the first, and Moderna’s comes four weeks later. The second dose provides a potent boost that gives people strong, long-lasting immunity. You should try to stick as closely as possible to the prescribed schedule. If scheduling conflicts prevent you from coming back for the booster shot on the exact day it’s due, federal health officials say it's okay to get the second dose up to four days early or up to six weeks after the first dose. If you miss this window, you should still come back for the second dose as soon as you can. —Carl Zimmer, Tara Parker-Pope
How long will it take for the vaccine to start working?
The U.S. Centers for Disease Control has said a person is fully vaccinated two weeks after receiving the final dose of whatever vaccine you’ve been given. If your vaccine requires only one dose, it will take about two weeks for your body to build a strong immune response. If your vaccine requires two doses, the peak vaccine response won’t be reached until two weeks after the second dose. —Carl Zimmer and Denise Grady
Read More Pfizer’s Vaccine Offers Strong Protection After First Dose
Booster Shots
Who is eligible for a booster shot.
The Food and Drug Administration authorized booster shots for a select group of people who received their second doses of the Pfizer-BioNTech vaccine at least six months ago. Eligible people include Pfizer recipients who are 65 or older or who live in long-term care facilities. The agency also authorized boosters for adults who are at high risk of severe Covid-19 because of an underlying medical condition, as well as for health care workers and others whose jobs put them at risk.
People with weakened immune systems who received the Pfizer or Moderna vaccines are also eligible for a third shot at least four weeks after their second doses. For these patients, the third shot is not considered a booster dose — it is now part of the recommended immunization schedule for those with compromised immune systems who don’t generate a robust response after just two shots. —Tara Parker-Pope
Read More Answers to Your Questions About Booster Shots
Will people who received Moderna or Johnson & Johnson vaccines be eligible for booster shots?
Regulators are expected to authorize booster shots for recipients of the Moderna and Johnson & Johnson vaccines soon. An F.D.A. advisory panel has voted in favor of emergency authorization of a half-dose booster of Moderna vaccine, at least six months after the second dose. Those eligible would include Moderna recipients over 65 and other adults considered at high risk — the same groups eligible for a Pfizer-BioNTech booster. The same expert committee voted unanimously in favor of authorizing booster shots for all adults (roughly 15 million people) who received the single-dose Johnson & Johnson vaccine. The second J&J dose would be given at least two months after the first dose. —Carl Zimmer
Read More An extra J. & J. shot substantially boosts protection against Covid, the company reports.
What underlying medical conditions qualify for a booster shot?
The C.D.C. has said the conditions that qualify a person for a booster shot include:
- Hypertension and heart disease
- Diabetes or obesity
- Cancer or blood disorders
- Weakened immune system
- Chronic lung, kidney or liver disease
- Dementia and certain disabilities
Pregnant women and current and former smokers are also eligible. —Tara Parker-Pope
Should everyone who is eligible get a booster shot?
No. The C.D.C. has recommended booster shots only for people 65 or older, those in long-term care facilities, and people 50 or older who are at high risk.
The agency has not given advice to others who are eligible, saying only that at-risk adults 18 to 49 or those in frontline jobs should evaluate their individual risk, or discuss the issue with a health care provider, before getting a booster shot. —Tara Parker-Pope
What occupations are eligible for boosters?
The F.D.A. authorized booster doses for workers whose jobs put them at high risk of exposure to potentially infectious people. The C.D.C. says that group includes:
- Emergency medical workers (health care workers, firefighters, police officers, employees at congregate care facilities including those who work in homeless shelters)
- Education workers (teachers, support staff workers, day care workers)
- Food and agriculture workers
- Manufacturing workers
- Corrections workers
- U.S. Postal Service workers
- Public transit workers
- Grocery store workers
The C.D.C. has said the list may change over time. —Tara Parker-Pope
How and when can I get a booster if I qualify?
Health departments, pharmacies and doctors’ offices will dispense boosters much the same way that they administered the first and second doses. Call ahead to find out about scheduling, and bring your vaccine card. Proof of an underlying medical condition won’t be required.
You will be able to find more information about getting a booster shot on your state’s health department website or pharmacy websites. You can also talk to a health care provider about whether you should get a third shot. Since the F.D.A. fully approved the Pfizer-BioNTech vaccine as a two-dose regimen this past summer, physicians have had broad latitude to prescribe a third dose to people they deem in need of one. —Tara Parker-Pope
What if it has been less than six months since I was vaccinated?
While people who are severely immune-compromised can get a third shot sooner, everyone else who qualifies should wait until at least six months after getting a second shot. Getting a booster too soon is probably a waste of a dose and may not increase your antibodies in a meaningful way; there is also a lack of data on the safety of booster shots given early. —Tara Parker-Pope
What are the side effects of booster shots?
While data is limited, so far reactions reported after the third mRNA dose from Pfizer or Moderna are similar to those experienced after the two-dose series: Fatigue and pain at the injection site are the most commonly reported side effects, and, overall, most symptoms are mild to moderate, the C.D.C. says. A survey from Israel, where booster shots are already being given, found that 88 percent of Pfizer vaccine recipients said that in the days after the third dose they felt “similar or better” to how they felt after the second shot. About a third of respondents reported some side effects, with the most common being soreness at the injection site, and 1 percent said they sought medical treatment because of one or more side effects. —Tara Parker-Pope
Can I mix Covid vaccines?
It is not recommended yet, although new guidance is expected soon. The F.D.A. is reviewing preliminary data suggesting that Johnson & Johnson recipients may be better off with a booster shot from Moderna or Pfizer. —Tara Parker-Pope

Will a third dose ever be available to the general public?
While the Biden administration has said it supports booster shots for everyone who is eight months post-vaccination, the plan has been rejected by F.D.A. scientists. But the recommendation could change in the coming weeks or months as more data becomes available on the durability of vaccine antibodies over time. The good news is that the consensus in the scientific community is that all of the vaccines continue to provide strong protection against severe illness, hospitalization and death from Covid-19. —Tara Parker-Pope
Can I get a flu shot at the same time as a Covid vaccine or booster shot?
Yes. The C.D.C. says the Covid vaccine may be administered without regard to the timing of other vaccines, and many pharmacy sites are allowing people to schedule a flu shot at the same time as a booster dose. Side effects are generally similar when the vaccines are given simultaneously as when they are administered separately. If you do get a flu shot and a Covid vaccine at the same time, experts advise using different arms to avoid soreness, or at least spacing the injection site for each shot by at least one inch. —Christine Hauser
Read More It's Time to Get a Flu Shot
Children and Schools
What vaccines have been authorized for children.
The Food and Drug Administration so far has given emergency authorization only to the Pfizer-BioNTech vaccine for young people aged 12 to 17. Moderna and Johnson & Johnson are expected to seek emergency authorization for children in the coming months. —Tara Parker-Pope
When will children younger than 12 be eligible?
The date for when a vaccine will be approved for children ages 5 to 11 has been something of a moving target. Pfizer has asked the F.D.A. for authorization to use its vaccine in this younger age group. If the regulatory review follows a timeline similar to the ones for older children and adults, it’s possible that millions of elementary school students could be inoculated before Halloween. But public health officials have said it may take longer to review the data, with estimates for authorization ranging from late fall to the end of 2021.
Vaccines for very young children, ages 6 months to 4 years old, aren’t expected until 2022. Trial results for children younger than 5 are not expected until the fourth quarter of this year at the earliest, according to Dr. Bill Gruber, a senior vice president at Pfizer and a pediatrician. —Tara Parker-Pope and Apoorva Mandavilli
Is the amount of vaccine given to children any different than for adults?
The dosage of Pfizer vaccine authorized for 12- to 17-year-olds is identical to the dosage given to adults: two doses of 30 micrograms given three weeks apart.
The dose for children ages 5 to 11 is expected to be just 10 micrograms per shot — a third of the dose given to older children and adults. The children who got the lower dose of vaccine produced a response comparable to the levels of antibodies seen in the earlier trials of participants ages 16 to 25. In children younger than 5, just three micrograms — one-tenth of the adult dose — is being tested in trials.
Moderna is studying different dosing strategies for its vaccine in 6,750 healthy children in the United States and Canada. In adults, the standard dose is 100 micrograms given four weeks apart. In Moderna’s study of children ages 2 to 11, the company is testing doses of either 50 or 100 micrograms. In children under 2, Moderna is studying shots of 25, 50 or 100 micrograms. —Apoorva Mandavilli
Where can I have my child vaccinated?
Children who are eligible will be able to receive a shot from their pediatricians, local pharmacies or school-based clinics. Check your local health department’s website or ask your pediatrician for details. Some sites may require appointments, while others will offer shots on a walk-in basis. —Tara Parker-Pope
Can a child who recently got other vaccinations get the Covid shot?
Yes. The C.D.C. says Covid vaccines and other vaccines can be given without regard to timing. The agency says that if multiple vaccines are administered during a single visit, the injections may be given in different parts of the body.
“Experience with other vaccines has shown that the way our bodies develop protection, known as an immune response, after getting vaccinated and possible side effects of vaccines are generally the same when given alone or with other vaccines,” the C.D.C. says. —Tara Parker-Pope
Are the side effects different for children and adults?
Detailed side effect data for children ages 5 to 11 hasn’t been released. In studies of 12- to 15-year-olds, fevers were slightly more common in children compared with adults. But in general, the side effects reported in children have been similar to those seen in older people. The F.D.A. said that the most commonly reported side effects in the adolescent clinical trial participants were pain at the injection site, tiredness, headache, chills, muscle pain, fever and joint pain. Side effects typically lasted one to three days. Although pain at the injection site was common after both shots, more adolescents reported side effects after their second doses. Younger people tend to have a more powerful immune response than older people because they have more robust immune systems. It’s possible that children may experience more side effects than their parents did from the same shot.
Children should not get the Pfizer vaccine if they have a history of severe allergic reaction to any ingredient (such as polyethylene glycol) in that vaccine. Allergies to the vaccine ingredients are rare. The vaccine does not contain eggs, preservatives or latex. If you have doubts or aren’t sure, talk to your pediatrician before having your child vaccinated. If your child has severe allergies to anything else (medications, foods, bees), remain at the vaccination site for 30 minutes after the injection, instead of the 15 minutes that the general population is recommended to wait. —Tara Parker-Pope
Can schools require Covid vaccines for students?
The answer depends on whether your child attends public or private school. Private schools, day care centers and camps can decide whether to require students to be vaccinated against Covid-19 as a condition of returning to school or the facility. So far more than 800 colleges and universities have adopted campus-wide vaccine mandates.
For public K-12 schools, vaccination requirements are largely left up to the states. All 50 states have legislation requiring specified vaccines for students, although no state currently requires children to receive the Covid-19 vaccine as a condition of returning to school, according to the National Conference of State Legislatures. Exemptions to school immunization requirements vary, but all states grant exemptions to children for medical reasons. Children of parents who have religious objections to immunizations can receive a religious exemption in 44 states and Washington, D.C. And 15 states allow philosophical exemptions for children whose parents object to immunizations because of personal, moral or other beliefs.
Los Angeles is the first major school district in the United States to mandate coronavirus vaccines for students 12 and older who are attending class in person, but legal challenges are expected. —Tara Parker-Pope
Read More Los Angeles Mandates Vaccines for Students 12 and Older
How many children were studied in the clinical trials and for how long?
Pfizer’s trial included 2,268 children ages 5 to 11, two-thirds of whom received two doses of the vaccine three weeks apart; the rest were injected with two doses of saltwater placebo. Given how rarely children become severely ill, the trial was not big enough to draw meaningful conclusions about the vaccine’s ability to prevent Covid or hospitalization. Instead, the researchers relied on measurements of the children's immune response, on the assumption that the protective levels of antibodies seen in older people would be as protective in younger children.
The Pfizer study for older children enrolled 2,260 participants ages 12 to 15. Of those children, 1,131 received the vaccine (two shots, given three weeks apart) and 1,129 received saline placebo shots. The vaccine worked even better in children than it did in adults. No children in the vaccine group got sick with Covid-19, while 18 children in the placebo group became ill. The company is still gathering information, including testing the trial participants every two weeks for the coronavirus.
Moderna recently released the results of its trial testing the vaccine in 3,732 people ages 12 to 17, two-thirds of whom received two vaccine doses. There were no cases of symptomatic Covid-19 in fully vaccinated adolescents, the company reported.
The F.D.A. has asked the companies to include 3,000 children in tests of 5- to 11-year-olds. Beyond the clinical trials, health officials are continuing to gather information on the 12.7 million young people ages 12 to 17 in the United States who have received at least one dose of the Pfizer shot, according to C.D.C. data. —Tara Parker-Pope
What do we know about long-term effects of these vaccines on growing bodies?
Given that the vaccine’s mRNA molecule mimics a natural human process, experts say they are confident that the vaccines are safe for growing bodies. Dr. Paul Offit, director of the vaccine education center at Children’s Hospital of Philadelphia and a member of the Food and Drug Administration’s vaccine advisory panel, notes that while mRNA vaccines are new, mRNA molecules occur naturally throughout the human body.
“Every child in their cells has about 200,000 copies of messenger RNA,” said Dr. Offit. “Every cell in your body has these molecules which are making proteins and enzymes so you can continue to live. Although the technology is new for a vaccine, it’s not like it’s a molecule we haven’t seen before. I understand the anxiety, but it’s no different than when you make insulin or hemoglobin or albumin or any of the other proteins your body makes.”
One reassuring fact about the mRNA vaccines is that the molecule is destroyed by the cell once it completes its mission, so it doesn’t stay in the body. Another common worry among parents is the effect of a new drug or vaccine on brain development. Dr. Offit noted that the body has a blood-brain barrier that prevents most proteins from entering the brain. “Your brain is an immunologically protected site,” he said.
Although mRNA technology has been studied for about 15 years, this is the first time it has been used in a vaccine. It’s also being studied to treat cancer, muscular dystrophy and other diseases. —Tara Parker-Pope
Given the low risk of Covid to children, why not wait for more data to get my child vaccinated?
While children are less likely to develop severe illness from Covid-19, they are still at risk. In early September, children accounted for nearly 30 percent of coronavirus cases, and the highly contagious Delta variant has sent more children into hospitals and intensive care units in the past few weeks than at any other time during the pandemic. This past winter, doctors reported growing numbers of patients with Multisystem Inflammatory Syndrome in Children, or MIS-C, a condition linked to Covid which can affect multiple organs, including the heart.
Unvaccinated children, even if they do not become ill, can spread the virus to family members, teachers and others they interact with regularly, including grandparents or others who are at higher risk for severe disease or death. —Tara Parker-Pope
Read More Hospitalizations for Children Sharply Increase as Delta Surges
Vaccine Safety and Side Effects
What are the side effects.
The stab of the needle into your arm won’t feel different than any other vaccine that requires an injection. But the rate of short-lived side effects from Covid-19 vaccines does appear higher than that of a flu shot. Sore arms are common. Millions of people have already received the vaccines, and the overwhelming majority have not reported any serious health problems. (Severe allergic reactions have occurred in a fraction of cases. You can read more in the next question.) Side effects, which sometimes can resemble the symptoms of Covid-19, last about a day and appear more likely after the second dose. Early reports suggest some people feel lousy and might need to take a day off from work after receiving the second dose. In the Pfizer study, about half developed fatigue. Other side effects occurred in at least 25 to 33 percent of patients, sometimes more, including headaches, chills and muscle pain.
While these experiences aren’t pleasant, they are a good sign that your own immune system is mounting a potent response to the vaccine that will provide long-lasting immunity. The New York Times interviewed several dozen of the newly vaccinated in the days afterward. They recounted a wide spectrum of responses, from no reaction at all to symptoms like uncontrolled shivering and “brain fog.” As vaccines go, experts have agreed, the two Covid vaccines being distributed now elicit more reactions than most. "We call them ‘side' effects, but it’s really just an effect,” said Dr. Paul Offit, who is a member of the Food and Drug Administration’s vaccine advisory panel. “This is what your immune response does when it’s responding to an infection.” —Abby Goodnough and Amy Harmon
If I have allergies, should I be concerned about the vaccine?
While severe allergic reactions, called anaphylaxis, have occurred in the minutes following an injection with the new vaccines, it’s an extremely rare event. The rate of anaphylaxis has been 11.1 cases per million doses, as of December. By comparison, the rate of severe allergic reaction to the flu shot is about 1.35 cases per million doses. While those statistics may sound scary, severe allergic reactions are a risk with many drugs. Dr. Aaron Carroll, a professor of pediatrics at Indiana University School of Medicine, notes that the risk of anaphylaxis from penicillin drugs is even higher — between one in 2,500 and one in 5,000 — but that doesn’t stop doctors from prescribing them. You can read more from Dr. Carroll about the risks of vaccines here.
All the patients who experienced severe reactions to the vaccines were treated and have recovered. Most of them had a history of anaphylaxis or allergies, but some patients had no known allergies. Every vaccination site is required to have epinephrine and other emergency supplies in case a patient has an allergic reaction. (If you normally carry an EpiPen, bring it to your vaccination.) If you have a history of anaphylaxis, you’ll be monitored for 30 minutes after the shot, instead of the standard 15 minutes.
If you’ve ever had anaphylaxis for any reason, or an allergic reaction to a vaccination, you should talk to your doctor about how to safely get the vaccine and what precautions to take. You may be advised to schedule your shot in a hospital or close to a health care facility. If you had an allergic reaction to your first dose of Covid vaccine, you’ll be advised to skip the second dose. People who have had allergic reactions to either of two ingredients — polyethylene glycol or polysorbate — are also being warned not to receive a vaccine. —Tara Parker-Pope
Read More Here’s What People With Allergies Should Know About Covid Vaccines
How long would it take side effects to show up?
Side effects like fatigue, headaches and muscle pain should show up within one to three days after vaccination, and resolve one to three days after they start. Sore arms and fevers are common. Contact your doctor if the redness or tenderness where you got the shot increases after 24 hours. You should also call your doctor if your side effects are causing you worry, or they do not seem to be going away after a few days.
Dr. Sylvia Owusu-Ansah, an emergency physician in Pittsburgh, chronicled her vaccine side effects on Facebook . She experienced mild muscles aches and a sore left arm on Day 1 but was still experiencing nasal congestion and mild headache on Days 3 through 5. Along with a card reminding you to get the necessary second dose, vaccine recipients are handed information on how to report side effects to the Centers for Disease Control and Prevention through an app called V-Safe , a smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins after you receive a Covid-19 vaccination. —Dani Blum and Amy Harmon
I’ve been hearing that the side effects after the second shot are far worse than the first shot. Is that true?
Short-lived side effects like fatigue, headache, muscle aches and fever are more common after the second dose of both the Pfizer-BioNTech and the Moderna vaccines, which each require two shots. (The Johnson & Johnson vaccine requires only a single shot.) Patients who experience unpleasant side effects after the second dose often describe feeling as if they have a bad flu and use phrases like “it flattened me” or “I was useless for two days.” During vaccine studies, patients were advised to schedule a few days off work after the second dose just in case they needed to spend a day or two in bed.
Data collected from v-safe, the smartphone-based tool everyone is encouraged to use to track side effects after vaccination, also show an increase in reported side effects after the second dose. For instance, about 29 percent of people reported fatigue after the first Pfizer-BioNTech shot, but that jumped to 50 percent after the second dose. Muscle pain rose from 17 percent after the first shot to 42 percent after the second. While only about 7 percent of people got chills and fever after the first dose, that increased to about 26 percent after the second dose. —Tara Parker-Pope
Read More What the Vaccine Side Effects Feel Like, According to Those Who’ve Gotten It
I’ve heard that taking a pain reliever after getting a Covid-vaccine could blunt its effectiveness. Is that true?
Most experts agree it’s safe to take a pain reliever or fever reducer like acetaminophen or ibuprofen to relieve discomfort after you get vaccinated. You shouldn’t try to stave off discomfort by taking a pain reliever before getting the shot.
The concern about whether pain relievers might dampen the effect of the vaccine stems from research in pediatric patients. Parents sometimes give children pain relievers like acetaminophen or ibuprofen before and after they get vaccinated to reduce fevers and aches that might occur following childhood vaccinations. Because fevers and other side effects are also a sign that the body is mounting a strong immune response, some researchers have questioned whether giving a child a pain reliever or fever reducer before or after a shot might blunt the effectiveness of the vaccine.
A review of studies of more than 5,000 children compared antibody levels in children who took pain relievers before and after vaccinations and those who did not. They found that pain relievers did not have a meaningful impact on immune response, and that children in both groups generated adequate levels of antibodies after their shots. Another study looked specifically at giving 142 children acetaminophen, ibuprofen or a placebo after a flu shot. The vaccine response was not significantly different in patients taking pain relievers or the placebo.
Neither Pfizer nor Moderna offers guidance about taking pain relievers to treat side effects. A tip sheet from the Centers for Disease Control and Prevention suggests talking with a doctor before taking an over-the-counter pain reliever after your vaccine.
An after-care guide from the British Columbia Centre for Disease Control is more specific about treating discomfort with over-the-counter pain relievers. “You may feel unwell for a day or two,” the guide states. “If you are unable to carry on with your regular activities because of these symptoms, you can take medication such as acetaminophen or ibuprofen. Check with your health care provider if you need advice about medication.”
Several medical and health groups, including the Henry Ford Health System and UCI Health , advise against taking prophylactic pain relievers before your shot, but they agree it’s fine to take an over-the-counter pain reliever for discomfort after getting the vaccine.
“Taking over-the-counter medications such as acetaminophen and ibuprofen before receiving a vaccine may reduce its ability to work and blunt your immune response to the vaccine,” advises UCI Health. “After the vaccination, don’t hesitate to take an over-the-counter medication if you have symptoms that make you uncomfortable." —Tara Parker-Pope
I haven’t had any side effects after the vaccine. Does that mean it’s not working?
Just as some people experience side effects from medications and some don’t, people have varied reactions to vaccines. While we tend to hear only about the unpleasant reactions after the vaccine, a lot of people experience only mild discomfort or no symptoms at all after getting the shot.
In the Pfizer trial, for instance, about half the participants developed fatigue. Other side effects occurred in at least 25 to 33 percent of patients, including headaches, chills and muscle pain. That means that half or more of the participants did not have those side effects, and yet the overall efficacy of the vaccine was 95 percent, suggesting that a lack of side effects does not mean a vaccine isn’t working. We also know that older people tended to report fewer side effects than younger people, probably because aging immune systems aren’t as strong. As people age, bodily defenses against pathogens weaken, and the response to vaccines also falters. But in the Pfizer and Covid vaccine trials, older people still produced adequate levels of antibodies, indicating a strong immune response after the vaccine. If you don’t have side effects after your shot, be glad you are one of the lucky ones and don’t worry. —Tara Parker-Pope
Do women have more side effects after vaccination than men?
An analysis from the first 13.7 million Covid-19 vaccine doses given to Americans found that side effects were more common in women. And while severe reactions to the Covid vaccine are rare, nearly all the cases of anaphylaxis, or life-threatening allergic reactions, occurred in women.
The finding that women are more likely to report and experience unpleasant side effects to the Covid vaccine is consistent with other vaccines as well. Women and girls can produce up to twice as many antibodies after receiving flu shots and vaccines for measles, mumps and rubella (M.M.R.) and hepatitis A and B. One study found that over nearly three decades, women accounted for 80 percent of all adult anaphylactic reactions to vaccines.
While it’s true that women may be more likely to report side effects than men, the higher rate of side effects in women also has a biological explanation. Estrogen can stimulate an immune response, whereas testosterone can blunt it. In addition, many immune-related genes are on the X chromosome, of which women have two copies and men have only one. These differences may help explain why far more women than men are afflicted with autoimmune disease, which occurs when a robust immune response attacks the body’s healthy tissue. —Tara Parker-Pope
Read More The Most Common Questions About Vaccination Side Effects, Answered
Is it true that cosmetic injections (like those used to plump lips or smooth out wrinkles) can cause an allergic reaction to the vaccine?
A rare side effect of the vaccine has been seen in a few people who have previously been injected with dermal fillers, also called “wrinkle fillers,” which are gel-like substances used to smooth wrinkles and facial lines around the nose and mouth, plump lips and restore volume to sunken cheeks.
In a few cases, people have developed swelling in the parts of the face that had been treated with the fillers. One to two days after getting the vaccine during the Moderna clinical trials, three women (out of 15,184 people who received at least one dose of the vaccine) developed swelling where they had previously been injected with cosmetic fillers. A 29-year-old woman developed swelling in her lips two days after the vaccine, and reported she had previously had a similar reaction to the flu shot.
The American Society for Dermatologic Surgery said the side effect also has been seen after viral and bacterial illnesses, other vaccinations and dental procedures. The group said people with dermal fillers should not delay or avoid the Covid vaccine. The side effect is rare, temporary and responds to treatments such as oral corticosteroids and an enzyme called hyaluronidase. The swelling also can resolve without treatment. The side effect has not been seen with wrinkle-relaxing injections like Botox or Dysport. If you’re concerned or not sure what type of injection you’ve gotten in the past, check with the doctor who gave you the cosmetic treatment. —Tara Parker-Pope
If I have been allergic to other vaccines because of egg allergens or preservatives, can I take the new Covid vaccines?
The Pfizer, Moderna, Johnson & Johnson and AstraZeneca vaccines don’t contain egg or any preservatives found in common vaccines. The vial stoppers are not made with natural rubber latex, so the vaccines are safe for people with latex allergy, according to the Allergy & Asthma Network. If you’ve ever had an allergic reaction to a vaccine, check with your doctor, but in most cases, you’ll still be encouraged to get the vaccine. As new Covid-19 vaccines come on the market, you should double check ingredient lists if you have had allergic reactions in the past. The Allergy & Asthma Network has published a chart showing all the ingredients in the Pfizer and Moderna vaccines.
The list of people who should not get vaccinated is very short. You should not get a second dose of the Pfizer or Moderna vaccine if you had a severe allergic reaction (anaphylaxis) to your first dose. Check with your doctor about whether you’re a candidate for another type of Covid vaccine when it becomes available. The Centers for Disease Control and Prevention also says you should not get the vaccine if you have a rare allergy to polyethylene glycol (PEG), a compound derived from petroleum and found in both vaccines. PEG is used in everyday products such as toothpaste and shampoo as “thickeners, solvents, softeners and moisture carriers,” and are also found in laxatives, according to Science magazine .You should not get the Pfizer, Moderna or Johhnson & Johnson vaccines if you are allergic to polysorbate, which is an ingredient in the Johnson & Johnson vaccine and closely related to PEG. Allergies to the ingredients are rare. —Tara Parker-Pope
Is there a risk of developing Covid-19 from any of the vaccines?
No. The Pfizer, Moderna and Johnson & Johnson vaccines approved in the United States do not contain any live virus, weakened virus, dead virus or any infectious element, so there is no way for the vaccine to give you Covid-19. The best way to understand the mRNA vaccines is that they carry a set of instructions to teach your body’s immune system how to attack the coronavirus. The Johnson & Johnson vaccine uses a different technology to send similar instructions. —Tara Parker-Pope
Read More How 9 Covid-19 Vaccines Work
Do vaccines cause blood clots?
Two vaccines, Johnson & Johnson and AstraZeneca, have been linked to extremely rare clotting disorders. In the United States, federal health regulators have told Johnson & Johnson to add a warning to its label to note the potential risk of rare blood clots. European regulators who concluded that a vaccine made by AstraZeneca may also be the cause of a similar, extremely rare clotting disorder. In both cases, the risk is iinfinitesimal, and health officials have said the shot's benefits outwieigh the risk. The F.D.A. has not found a higher risk of clotting disorders in people who have received the Moderna or Pfizer-BioNTech vaccines. —Emily Anthes, Carl Zimmer and Noah Weiland
Read More The F.D.A. Ended Its Pause on the J&J Vaccine
Do the vaccines cause myocarditis?
Federal officials are reviewing hundreds of cases of rare heart problems following immunization with the coronavirus vaccines made by Pfizer-BioNTech and Moderna. The conditions are myocarditis, inflammation of the heart muscle, and pericarditis, inflammation of the membrane surrounding the heart. A study from Israel confirmed that the Pfizer-BioNTech Covid-19 vaccine is associated with an increased risk of myocarditis. But the side effect remains rare, and Covid-19 is more likely to cause myocarditis than the vaccine is, scientists say. —Emily Anthes, Noah Weiland and Apoorva Mandavilli
Read More Heart Problem More Common After Covid-19 Than After Vaccination, Study Finds
Fertility and Pregnancy
Is the vaccine safe for women who are pregnant or breastfeeding.
The C.D.C. has recommended that coronavirus vaccines be made available to pregnant women, though it also suggests that they consult with their doctors when making a decision about vaccination. Covid-19 poses serious risks during pregnancy. Pregnant women who develop symptoms of the disease are more likely to become seriously ill, and more likely to die, than nonpregnant women with symptoms.
In an early analysis of coronavirus vaccine safety data, C.D.C. researchers have found no evidence that the Pfizer-BioNTech or Moderna vaccines pose serious risks during pregnancy. The findings are preliminary and cover just the first 11 weeks of the U.S. vaccination program. But the study, which included self-reported data on more than 35,000 people who received one of the vaccines during or shortly before pregnancy, is the largest yet on the safety of the coronavirus vaccines in pregnant people. After vaccination, pregnant participants reported the same general pattern of side effects that nonpregnant ones did, the researchers found: pain at the injection site, fatigue, headaches and muscle pain. Women who were pregnant were slightly more likely to report injection site pain than women who were not, but less likely to report the other side effects. They were also slightly more likely to report nausea or vomiting after the second dose. —Emily Anthes
Read More No evidence that Pfizer or Moderna vaccines are unsafe during pregnancy, a preliminary study says.
I’ve seen rumors online about the vaccines and fertility. Are they true?
A false claim has been circulating online that the new vaccine will threaten women’s fertility by harming the placenta. Here’s why it’s not true.
The claim stems from the fact that the vaccines from Pfizer and Moderna cause our immune systems to make antibodies to something called a “spike” protein on the coronavirus. The false claim about fertility risk is based on the unfounded concern that these antibodies could also attack a similar protein that is made in the placenta during pregnancy, called syncytin. In reality, the spike protein and syncytin are similar only in one very small region, and there’s no reason to believe antibodies that can grab onto spike proteins would lock onto syncytin.
What’s more, the human body generates its own supply of spike antibodies when it fights off the coronavirus, and there’s been no sign that these antibodies attack the placenta in pregnant women who become sick with Covid-19. If they did, you’d expect that women who got Covid-19 would suffer miscarriages. But a number of studies show that Covid-19 does not trigger miscarriages. —Carl Zimmer
Should I wait to conceive until after I get the vaccine?
Obstetricians recommend being up-to-date on all vaccines before pregnancy, so it’s a good idea to get the Covid-19 vaccine as soon as you are eligible and can get an appointment. Covid-19 poses an especially high risk to pregnant women, so ideally you should get vaccinated before you become pregnant. “It’s really good if you can be protected from Covid before pregnancy,” said Dr. Denise Jamieson, an obstetrician at Emory University in Atlanta and a member of the American College of Obstetricians and Gynecologists committee on Covid vaccines.
The challenge for women of childbearing age is that most are not in the priority age groups scheduled to get the vaccine first. If the timing of your pregnancy does not matter to you, then it’s up to you if you prefer to get vaccinated first. But lack of access to the vaccine now should not be a reason to delay your pregnancy, experts say.
“Timing pregnancy is not necessarily as easy as we would like it to be, meaning it often takes women/couples time to conceive,” said Dr. Geeta Swamy, an obstetrician at Duke University in Durham, N.C., and a member of the ACOG vaccine committee. “Additionally, it is unclear when nonpregnant women will be eligible for vaccination if they are not in a high-risk category to get vaccinated. So given all of these unpredictable aspects combined with the fact that we have no concerns about vaccination impacting conception/early pregnancy, ACOG and others do not recommend delaying pregnancy until after vaccination.” —Dani Blum
Will the vaccine affect my fertility treatment schedule?
Fertility patients who are scheduled for procedures like egg retrieval, embryo transfer or intrauterine insemination are advised to avoid getting a Covid vaccine within three days before and three days after the procedure, according to the American Society for Reproductive Medicine. That’s because patients undergoing surgical procedures could develop vaccine-related side effects like fever or chills that might make it difficult for doctors to know if a post-surgical infection is brewing. In addition, many medical providers may not allow a patient who is experiencing Covid-like symptoms into their facility, even if it’s likely that the symptoms are from a vaccine and their Covid-19 test is negative. —Christina Caron
Read More What Women Need to Know About the Covid Vaccine
Will the vaccine affect my mammogram?
Coronavirus vaccinations can cause enlarged lymph nodes in the armpit that will show up as white blobs on mammograms. This type of swelling is a normal reaction to the vaccine and will typically occur on the same side as the arm where the shot was given, said Dr. Geeta Swamy, a maternal-fetal medicine specialist and a member of the American College of Obstetricians and Gynecologists’s Covid vaccine group. It usually only lasts for a few weeks. But the vaccine’s effect on mammograms can be concerning to radiologists, she added, because “if someone had breast cancer we might see enlarged lymph nodes as well.” Because this type of swelling could be mistaken as a sign of cancer, the Society of Breast Imaging recommends trying to schedule your routine mammogram before your first Covid-19 vaccine dose or at least one month after your second vaccine dose. The guidance is only for women getting routine mammograms. If you are getting a mammogram because of a suspicious lump or other symptoms, don’t delay. You should keep your current mammogram appointment as well as your vaccination appointment, and tell your radiologist the date that you received the vaccine. —Christina Caron
Will the vaccine affect my menstrual cycle?
Some women say they have observed changes in the flow or timing of their period after getting vaccinated. So far, there’s no data linking the vaccines to changes in menstruation. Even if there is a connection, one unusual period is no cause for alarm. There is a long list of triggers that can cause changes to the menstrual cycle, including stress, illness and changes in diet and physical activity. Although more study is needed, there is a link between menstruation and the immune system. Both the thickening and thinning of the uterine lining are facilitated by different teams of immune cells and signals moving in and out of the reproductive tract; one wave helps to build, others help to dismantle. The process of shedding this lining during menstruation is in part an inflammatory response, which is why women often experience cramping and pain during this stage.
Since the cycle is supported by the immune system at every turn, it is possible that the vaccines, which are designed to ignite an immune response, could temporarily change the normal course of events. For example, an activated immune system might interfere with the usual balance of immune cells and molecules in the uterus. These types of disturbances have been found in studies to contribute to changes in periods, including heavy menstrual flows. But no one can say whether this may explain potential post-vaccine disruptions to the menstrual cycle. To find out, we would need a controlled study with a placebo group. Clinical trials, including those for vaccines, typically omit the tracking of menstrual cycles, so we lack the evidence required to put these reports in context. If you have questions about your menstrual cycle, be sure to speak with your doctor. —Alice Lu-Culligan and Randi Hutter Epstein
Read More No, We Don’t Know if Vaccines Change Your Period
Medical Concerns
Do i need to get the vaccine if i’ve already had covid-19.
Even if you’ve had Covid-19, you still will get stronger immunity from vaccination. A person’s immune response to a natural infection is highly variable. Some people may produce few antibodies, and some variants seem to dodge natural antibodies more easily than stronger vaccine-generated antibodies. While it’s not clear how much extra benefit a recovered Covid patient gets from two doses, versus a single dose, you need a second dose to provide proof of full vaccination, should you need it for travel or for work. People who have had Covid-19 in the past are advised to wait about 90 days after infection before getting vaccinated if they were treated with convalescent plasma or monoclonal antibodies. If you get Covid-19 after your first dose, you may need to adjust your vaccination schedule until you are fully recovered and no longer need to isolate. Check with your doctor about the best timing if you’re not sure. —Tara Parker-Pope
Read More ‘Natural Immunity’ From Covid Is Not Safer Than a Vaccine
What do we know about how the vaccines work in people with compromised immune systems?
People with weakened immune systems don't generate as robust a response to the vaccines as others. The C.D.C. has advised people with weakened immune systems to get a third dose of the Pfizer or Moderna vaccine four weeks after the second shot. This extra shot is not technically a booster shot, but is now considered part of the regular immunication schedule for this group of patients. About 3 percent of Americans have weakened immune systems for a variety of reasons, including organ transplants, a history of cancer or use of medications like steroids that suppress the immune system. —Noah Weiland and Sharon LaFraniere
Read More The F.D.A. authorized a third dose of Covid vaccines for immunocompromised people.
Were cancer patients studied in the vaccine trials? How does a cancer patient safely get the vaccine?
Patients in active treatment should consult with their medical team about how and when to get the vaccine. Some patients may be advised to time the vaccine, if possible, between rounds of chemo — when their white blood cell counts are highest — to optimize their immune response. Ideally, cancer patients in active treatment should receive vaccinations under the care of a doctor, or in a cancer center, where they can be closely monitored and are likely to encounter fewer people than they would at a mass distribution site. The state-by-state nature of vaccination rules can complicate vaccination for cancer patients. For instance, if you live in one state and get cancer treatment in another, your cancer center may not be allowed to give the vaccine to an out-of-state patient.
Patients with a history of cancer or undergoing active treatment may be advised to follow the three-dose immunization schedule recommended for patients with weakened immune systems. —Dani Blum
Read More In the Vaccine Scramble, Cancer Patients Are Left Behind
I have an autoimmune disease. Will the vaccine work for me?
An estimated 8% of Americans have an autoimmune disease such as rheumatoid arthritis or lupus, which occur when the immune system mistakenly attacks normal body tissues. Although people with autoimmune conditions were allowed to enroll in the Covid vaccine trials, patients were excluded if they were taking the type of immune-suppressing drugs used to treat autoimmune disorders. As a result, doctors believe the vaccine is safe for people with autoimmune conditions, but they don’t have specific data showing how well the vaccine works in these patients. The National Institutes of Health has announced a new study to determine whether people with autoimmune conditions should receive a third dose of Covid vaccine, similar to the immunization schedule approved for patients with weakened immune systems. The study will also investigate whether pausing immunosuppressive therapy for autoimmune disease improves the antibody response to an extra dose of a COVID-19 vaccine. In the meantime, patients with autoimmune conditions should consult with their physicians about getting the Covid vaccine. —Dani Blum
If I have the virus but don’t know it, will the vaccine still work?
While there’s not yet a lot of data about this scenario, vaccine researchers say there’s no cause for additional worry if you find out you were infected at the time of vaccination. “You won’t feel very well, but that’s due to the Covid, not the Covid vaccine,” said Dr. Helen Talbot, a member of a panel advising the Centers for Disease Control and Prevention and an infectious disease specialist at Vanderbilt University.
It’s unlikely the first dose of vaccine has had enough time to help your body fight the infection, but the vaccine should still spur your body to produce a lasting immune response. Once you’ve recovered, you should plan to get your second dose as planned. “If you unknowingly have the virus and are immunized, the vaccine will not prevent disease but will likely help in the overall development of immune response,” said Dr. Talbot. “You would then get your second immunization once fully recovered — likely after the usual 21 or 28 days. No need to start the vaccine series over." —Dani Blum
Will it be safe for people with Guillain-Barré Syndrome?
Guillain-Barré syndrome is a rare and serious condition that occurs when the body’s immune system attacks the nerves, causing muscle weakness and sometimes paralysis. Nobody knows exactly what causes it, but most patients report that they had recently recovered from a respiratory or gastrointestinal infection. Guillain-Barré syndrome also has been pinpointed as a possible complication of Covid-19.
Although regulators have found that the chances of developing the condition are low, they appear to be three to five times higher among recipients of the Johnson & Johnson vaccine than among the general population in the United States. The F.D.A. has now attached a warning to the Johnson & Johnson shot about the increased risk of developing Guillain-Barré after vaccination.
For people who have had Guillain-Barré in the past, the guidance has been confusing. The official guidance from the Centers for Disease Control and Prevention is that people with a past history of Guillain-Barré should get the Covid vaccine. But Dr. Anthony S. Fauci, the nation’s leading infectious disease expert, has said that he does not recommend that people with a history of Guillain-Barré get the vaccine. “We recommend those people do not get vaccinated because you might trigger a similar serious response,” Dr. Fauci said.
But not everybody agrees with that advice. Members of the GBS/CIDP International Foundation, an advocacy group for people with Guillain-Barré, wrote an open letter to Dr. Fauci urging people with the syndrome to still get the vaccine. “At this time, there is no reason that those who had GBS in the past cannot get the current Covid vaccines,” they wrote. “If they have concerns, they should speak to their local health care professionals.” —Dani Blum
Read More F.D.A. Attaches Warning of Rare Nerve Syndrome to Johnson & Johnson Vaccine
Do we know if these vaccines will be safe for people with H.I.V.?
Trials studying the Covid-19 vaccine have included people with H.I.V., albeit in small numbers. The Pfizer trial included 120 people with H.I.V., and the Moderna trial included 176 people with H.I.V., according to Poz , a news site for people living with and those affected by H.I.V./AIDS. Although the numbers are too small to draw meaningful conclusions, no unusual safety concerns were reported for people with H.I.V. Because the Pfizer and Moderna vaccines do not contain weakened or inactivated virus, they are believed to be a safe option for people with H.I.V. and AIDS. Like other people with weakened immune systems, people with H.I.V. and AIDS who received the Pfizer or Moderna vaccines now are eligible for a third shot four weeks after the second dose. —Tara Parker-Pope
Understanding the Vaccine
How do these new genetic vaccines work.
Although these are described as “genetic” vaccines, the new Covid vaccines don’t alter your genes in any way. Instead, the Pfizer-BioNTech and Moderna vaccines use a genetic molecule to prime the immune system. That molecule is known as mRNA — the “m” stands for messenger. Think of this molecule like a set of instructions. While a traditional vaccine uses a weakened or inactivated germ to trigger an immune response in our bodies, the mRNA vaccines carry a set of instructions to teach our cells how to make a protein that will trigger an immune response and produce antibodies to the virus.
Here’s how it works. When you get your shot in the arm, the injection includes the messenger molecule, which is packaged in an oily bubble that fuses to a cell. The cell then uses the mRNA molecule as a set of instructions to make something called a “spike protein.” (The surface of the coronavirus is covered with similar spikes.)
Your immune system quickly recognizes that the spike protein is a foreign invader, and begins attacking it. The vaccine has essentially trained your immune system to recognize and attack the spike. Now, if you ever come into contact with the actual coronavirus, your immune system has learned how to handle it. You can learn more about mRNA vaccines from the Centers for Disease Control and Prevention website. —Tara Parker-Pope
Read More How the Pfizer-BioNTech Vaccine Works
Does the mRNA molecule in the new vaccines alter my genes?
No! The messenger RNA used in the in the Pfizer and Moderna vaccines never enters the nucleus of your cells and has no effect on your DNA. The mRNA molecule survives long enough for your cells to copy the instructions and create the spike protein that is used to train your immune system against coronavirus. So what happens to the molecule after it delivers these instructions? It lasts a few days, and then the cell shreds it and gets rid of it using special enzymes. It’s important to understand that the vaccine mimics a natural process that goes on in your body every day. At any moment, each of our cells may contain hundreds of thousands of mRNA molecules, which they produce in order to make a wide variety of proteins of their own. —Tara Parker-Pope and Carl Zimmer
How does the Johnson & Johnson vaccine work compared to the Pfizer and Moderna vaccines?
Like the Pfizer and Moderna vaccines, the Johnson & Johnson vaccine gives the body a set of instructions to build a spike protein that can train the immune system to ward off a coronavirus infection. While the Pfizer and Moderna vaccines use a genetic molecule called mRNA to provide the instructions, the Johnson & Johnson vaccine uses DNA to give the message, and the DNA is carried by a so-called viral vector, Adenovirus 26. Adenoviruses are common viruses that typically cause colds or flu-like symptoms. The Johnson & Johnson team used a modified adenovirus that can enter cells to deliver the instructions, but can’t replicate inside them or cause illness.
Johnson & Johnson’s vaccine comes out of decades of research on adenovirus-based vaccines. In July, the first one was approved for general use — a vaccine for Ebola, also made by Johnson & Johnson. The company is also running trials on adenovirus-based vaccines for other diseases, including H.I.V. and Zika. Some other coronavirus vaccines are also based on adenoviruses, such as the one developed by the University of Oxford and AstraZeneca, using a chimpanzee adenovirus.
Adenovirus-based vaccines for Covid-19 are more rugged than mRNA vaccines from Pfizer and Moderna. DNA is not as fragile as RNA, and the adenovirus’s tough protein coat helps protect the genetic material inside. As a result, the Johnson & Johnson vaccine can be stored using traditional refrigeration methods for up to three months. —Carl Zimmer and Tara Parker-Pope
Read More How the Johnson & Johnson Vaccine Works
What is the status of the AstraZeneca vaccine in the United States?
While more than 70 countries have authorized the vaccine, the United States has not. AstraZeneca has not yet applied to the Food and Drug Administration for authorization. —Denise Grady and Rebecca Robbins
Why not take my chances with Covid-19 rather than get a vaccine?
Covid-19 is by far the more dangerous option. Covid vaccines carry little known risk. But the perils of Covid-19 have been well documented. About 20 percent of people who come down with Covid-19 symptoms develop serious, potentially life-threatening illness. Although people who are older, obese or have other health problems are at highest risk for complications from Covid-19, younger people can become severely ill, too. In a study of more than 3,000 people ages 18 to 34 who were hospitalized for Covid, 20 percent required intensive care and 3 percent died.
The long-term health complications associated with Covid-19 are a serious concern. As many as one in three people who recover from Covid have chronic complaints for months afterward, including exhaustion, a racing heart, blood clots and loss of sense of smell or taste. —Apoorva Mandavilli
Will the vaccines work against the new variants that have emerged around the world?
While the rise of more infectious variants, including the highly-infectious Delta variant, has caused cases of Covid-19 to surge around the world, the risk is primarily to the unvaccinated, for whom there is great concern. But for the vaccinated, the outlook is much more hopeful. While it’s true that the vaccines have different success rates against different variants, the perception that they don’t work against variants at all is incorrect. In fact, the available vaccines have worked remarkably well at preventing serious illness and hospitalization, even as new variants circulate around the globe.
The variants are “all the more reason to get vaccinated,” said Dr. Anthony S. Fauci, the nation’s top infectious disease specialist. “The bottom line is the vaccines we are using very well protect against the most dominant variant we have right now, and to varying degrees protect against serious disease among several of the other variants.” —Tara Parker-Pope
Read More Can the Covid Vaccine Protect Me Against Virus Variants?
I’ve heard rumors and jokes about microchips in the new vaccines. What is that about?
The false conspiracy theory about microchips emerged after Bill Gates, the founder of Microsoft, made a comment about “digital certificates” that might one day be used to show a person had been tested or vaccinated for Covid-19. The reference prompted conspiracy theories to circulate online speculating that a tracking microchip would be planted by the government to surveil the movements of Americans. For months, widely shared videos and viral posts on social media have baselessly claimed that such technologies could find their way into syringes delivering shots. None of the rumors are true.
The Pfizer and Moderna vaccines have one active ingredient: a molecule called messenger RNA, or mRNA, which contains genetic instructions for a coronavirus protein called spike. Once injected, the mRNA will instruct human cells to manufacture spike, exposing the immune system to a highly recognizable feature of the virus. The remaining ingredients are lipids, including cholesterol, that form a fatty bubble around the fragile mRNA molecule, as well as sucrose (sugar) and various salts. —Katherine J. Wu and Tara Parker-Pope
Read More No, There Are No Microchips in Coronavirus Vaccines
What is the difference between emergency use authorization vs. normal approval of a vaccine?
Of the three vaccines approved for use in the United States, only the Pfizer vaccine has received formal approval from the F.D.A. The other two vaccines -- Moderna and Johnson & Johnson -- have been authorized for emergency use. An emergency use authorization is surprisingly similar to a standard approval. It includes the same basic steps — preclinical testing, Phase 1 safety trials, Phase 2 expanded trials and Phase 3 efficacy trials — that would be required in the traditional approval process. The main difference is that, in an emergency, the F.D.A. gives the application priority and speeds up its own review of the research. One way to do that is to solve logistical concerns early, while waiting for clinical trials to finish. For instance, the F.D.A. worked with the vaccine companies to solve manufacturing and distribution issues before the firms had completed their clinical trials or submitted applications for emergency use. In an interview with Scientific American magazine, the former F.D.A. commissioner Dr. Stephen Hahn said that in an emergency, the agency can prioritize an application over other demands to reduce the four-to-six-month review process to just several weeks.
Once vaccine makers win an emergency use authorization, they continue collecting information on the safety and efficacy of the vaccine and apply for a license, which is the final step in the approval process. Additional data will be collected on special patient populations like children, pregnant women and immune-compromised patients who weren’t studied in the first round of research. —Tara Parker-Pope and Carl Zimmer
Read More The Vaccine Testing Process
What about the billions of people who live outside the United States? How will the rest of the planet get vaccinated?
Vaccination efforts against Covid-19 have revealed an extraordinary gap in access to the vaccines around the world. Rich nations like the United States and Britain have cut deals with multiple drug manufacturers and secured enough doses of vaccines likely to come on the market this year to immunize their citizens multiple times over . China and Russia have developed their own vaccines and begun mass immunization programs. In stark contrast, most poor nations rely on a complex global vaccine-sharing initiative called Covax, and are likely to receive only enough doses to vaccinate at most 25 percent of their populations this year. Run by the World Health Organization and two global nonprofits, Covax relies on financial assistance and other support from wealthy nations. It wasn’t until this month that the U.S. agreed to participate and provide funding.
Worldwide, 81 percent of shots that have been administered have been in high- and upper-middle-income countries, according to the Our World in Data project at the University of Oxford. Only 0.4 percent of doses have been administered in low-income countries. —Megan Twohey, Keith Collins and Katie Thomas
Read More Rich countries have first dibs on vaccines, while poor nations struggle to get enough.
After Vaccination
What's the risk of getting covid-19 after vaccination.
There’s no one-size-fits-all answer to those questions because risk changes from one individual to the next, depending on a person’s overall health, where they live and those they spend time with. While most people who get vaccinated will not experience a breakthrough infection, some people will. Taking reasonable precautions, like wearing a mask in public indoor spaces when you don't know the vaccination status of others and avoiding crowded gatherings, will lower your risk. The vaccines remain highly protective against serious illness. A recent study in Los Angeles County showed that while breakthrough infections can happen, the unvaccinated are 29 times as likely to end up hospitalized from Covid-19 as a vaccinated person. —Tara Parker-Pope
Read More What Vaccinated People Need to Know About Breakthrough Infections
What’s the chance of a vaccinated person spreading Covid-19?
While unvaccinated people are by far at highest risk for catching and spreading Covid-19, it’s also possible for a vaccinated person to become infected and transmit the illness to others. A recent outbreak in Provincetown, Mass., where thousands of people gathered in bars and restaurants, showed that vaccinated people can sometimes spread the virus.Even so, many experts believe the risk of getting infected from a vaccinated person is still relatively low. A study from Singapore looked at vaccinated and unvaccinated people infected with the Delta variant. The researchers found that while viral loads in vaccinated and unvaccinated workers are similar at the onset of illness, the amount of virus declines more rapidly in the vaccinated after the first week, suggesting vaccinated people are infectious for a shorter period of time. —Tara Parker-Pope
What happens if I contract the virus between the first and second dose?
If your vaccine requires two doses, you're not fully protected against Covid-19 while you’re waiting for your second shot. There have been a few reports of people appearing to become infected with the virus after receiving their first shot. In most cases, it’s not known whether the patients already had the virus when they were given the vaccine, or whether they were exposed after vaccination but before their bodies had built up enough immunity to fight off the virus. You should still plan on getting the second dose on schedule, but check with your doctor first. And remember, even after two doses, no vaccine offers 100 percent protection. But even if you do catch the virus after vaccination, it's likely that you will experience mild illness because your body, has antibodies ready to fight off the virus. —Katherine J. Wu
If I’ve been vaccinated, why do I still need to wear a mask?
While being fully vaccinated protects against serious illness and hospitalization from Covid-19, no vaccine offers 100 percent protection. As long as large numbers of people remain unvaccinated and continue to spread coronavirus, vaccinated people will be exposed to the Delta variant, and a small percentage of them will develop so-called breakthrough infections.
It's a good idea to wear a mask when you don't know the vaccination status of those around you, particularly when you're in indoor public spaces. The risk is lower if you live in a community where vaccination rates are high and overall case rates are low and dropping. Most public health experts agree it's safe for fully vaccinated people to spend time indoors and unmasked with vaccinated friends and family members. But vaccinated people who have been traveling or recently attended a large gathering may want to get tested or avoid spending time unmasked with others until they are sure they weren't infected. —Tara Parker-Pope
Read More Should Vaccinated People Start Wearing Masks Again?
Will my employer require a Covid vaccination?
Employers do have the right to compel their workers to be vaccinated once a vaccine is formally approved. Many hospital systems, for example, require annual flu shots. But employees can seek exemptions based on medical reasons or religious beliefs. In such cases, employers are supposed to provide a “reasonable accommodation” — with a coronavirus vaccine, for example, a worker might be allowed to work if they wear a mask, or to work from home.
The Biden administration has mandated that all companies with more than 100 workers require vaccination or weekly testing. Mr. Biden also moved to mandate shots for health care workers, federal contractors and the vast majority of federal workers, who could face disciplinary measures if they refuse. —Abby Goodnough
Read More Employers Can Require Workers to Get Covid-19 Vaccine, U.S. Says
Will I be required to provide proof of vaccination to travel?
Some countries now require travellers to provide proof of vaccination as well as a negative Covid test before being allowed to board flights. Before traveling internationally, be sure to check the U.S. State Department’s detailed COVID-19 travel information at Travel.State.gov because travel restrictions can change quickly. And beginning in early November, all adult foreign nationals will be required to be fully vaccinated and show proof of vaccination against COVID-19 prior to boarding flights to the United States. —Sarah Firshein
Read More What Will I Need to Show When Traveling and Where?
What if I lose my vaccine card?
If you got your shot at a pharmacy, an employee can print out a new card from your electronic records. Vaccinations are also tracked by state health departments, so you can reach out to your state’s agency to get a replacement card.
Read More What You Need to Know About Your Covid-19 Vaccine Card
If you still have questions after reading this guide, tell us what else you want to know using this form.
Design and production by Rebecca Lieberman and Jaspal Riyait. Additional production by Dani Blum, Tara Parker-Pope and Karen Barrow.
Illustration by Timo Lenzen.
Research & Development contributions by Jack Cook and Amelia Pisapia.
Reporting was contributed by Tara Parker-Pope, Dani Blum, Keith Collins, David Gelles, Abby Goodnough, Amy Harmon, Dana Goldstein, Denise Grady, Sarah Kliff, Sharon LaFraniere, Apoorva Mandavilli, Donald G. McNeil Jr., Amelia Nierenberg, Adam Pasick, Natasha Singer, Sheryl Gay Stolberg, Katie Thomas, Lucy Tompkins, Megan Twohey, Carl Zimmer, Noah Weilan and Katherine J. Wu.
Advertisement
Intermittent Fasting
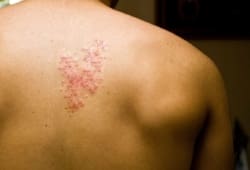
Pre-Exposure Prophylaxis (PrEP)
The importance of vaccinations.
Last Updated August 2023 | This article was created by familydoctor.org editorial staff and reviewed by Deepak S. Patel, MD, FAAFP, FACSM
Related Topics
Childhood vaccines: what they are and why your child needs them, immunization schedules, preventive services for healthy living.
Name (required)
Mail (will not be published) (required)
Gimme a site! Just a username, please.
Remember Me
There has been confusion and misunderstandings about vaccines. But vaccinations are an important part of family and public health. Vaccines prevent the spread of contagious, dangerous, and deadly diseases. These include measles, polio, mumps, chicken pox, whooping cough, diphtheria, HPV, and COVID-19.
The first vaccine discovered was the smallpox vaccine. Smallpox was a deadly illness. It killed 300 million to 500 million people around the world in the last century. After the vaccine was given to people, the disease was eventually erased. It’s the only disease to be completely destroyed. There are now others close to that point, including polio.
When vaccination rates decline, cases of preventable diseases go up. This has been happening in recent years with measles. As of July 7, 2023, the Centers for Disease Control has been notified of 18 confirmed cases in 12 U.S. jurisdictions. That may not seem like a lot but compare it with just 3 cases during the same time in 2022. By the end of 2022, there were 121 cases. Almost all those cases could have been prevented with vaccines.
What are vaccines?
A vaccine (or immunization) is a way to build your body’s natural immunity to a disease before you get sick. This keeps you from getting and spreading the disease.
For some vaccines, a weakened form of the disease germ is injected into your body. This is usually done with a shot in the leg or arm. Your body detects the invading germs (antigens) and produces antibodies to fight them. Those antibodies then stay in your body for a long time. In many cases, they stay for the rest of your life. If you’re ever exposed to the disease again, your body will fight it off without you ever getting the disease.
Some illnesses, like strains of cold viruses, are fairly mild. But some, like COVID-19, smallpox or polio, can cause life-altering changes. They can even result in death. That’s why preventing your body from contracting these illnesses is very important.
How does immunity work?
Your body builds a defense system to fight foreign germs that could make you sick or hurt you. It’s called your immune system. To build up your immune system, your body must be exposed to different germs. When your body is exposed to a germ for the first time, it produces antibodies to fight it. But that takes time, and you usually get sick before the antibodies have built up. But once you have antibodies, they stay in your body. So, the next time you’re exposed to that germ, the antibodies will attack it, and you won’t get sick.
Path to improved health
Everyone needs vaccines. They are recommended for infants, children, teenagers, and adults. There are widely accepted immunization schedules available. They list what vaccines are needed, and at what age they should be given. Most vaccines are given to children. It’s recommended they receive 12 different vaccines by their 6th birthday. Some of these come in a series of shots. Some vaccines are combined so they can be given together with fewer shots.
The American Academy of Family Physicians (AAFP) believes that immunization is essential to preventing the spread of contagious diseases. Vaccines are especially important for at-risk populations such as young children and older adults. The AAFP offers vaccination recommendations, immunization schedules , and information on disease-specific vaccines.
Being up to date on vaccines is especially important as children head back to school. During the 2021 school year, state-required vaccines among kindergarteners dropped from 95% to 94%. In the 2021-2022 year it fell again to 93%. Part of this was due to disruptions from the COVID-19 pandemic.
Is there anyone who can’t get vaccines?
Some people with certain immune system diseases should not receive some types of vaccines and should speak with their health care providers first. There is also a small number of people who don’t respond to a particular vaccine. Because these people can’t be vaccinated, it’s very important everyone else gets vaccinated. This helps preserve the “herd immunity” for the vast majority of people. This means that if most people are immune to a disease because of vaccinations, it will stop spreading.
Are there side effects to vaccines?
There can be side effects after you or your child get a vaccine. They are usually mild. They include redness or swelling at the injection site. Sometimes children develop a low-grade fever. These symptoms usually go away in a day or two. More serious side effects have been reported but are rare.
Typically, it takes years of development and testing before a vaccine is approved as safe and effective. However, in cases affecting a global, public health crisis or pandemic, it is possible to advance research, development, and production of a vaccine for emergency needs. Scientists and doctors at the U.S. Food and Drug Administration (FDA) study the research before approving a vaccine. They also inspect places where the vaccines are produced to make sure all rules are being followed. After the vaccine is released to the public, the FDA continues to monitor its use. It makes sure there are no safety issues.
The benefits of their use far outweigh any risks of side effects.
What would happen if we stopped vaccinating children and adults?
If we stopped vaccinating, the diseases would start coming back. Aside from smallpox, all other diseases are still active in some part of the world. If we don’t stay vaccinated, the diseases will come back. There would be epidemics, just like there used to be.
This happened in Japan in the 1970s. They had a good vaccination program for pertussis (whooping cough). Around 80% of Japanese children received a vaccination. In 1974, there were 393 cases of whooping cough and no deaths. Then rumors began that the vaccine was unsafe and wasn’t needed. By 1976, the vaccination rate was 10%. In 1979, there was a pertussis epidemic, with more than 13,000 cases and 41 deaths. Soon after, vaccination rates improved, and the number of cases went back down.
Things to consider
There have been many misunderstandings about vaccines. There are myths and misleading statements that spread on the internet and social media about vaccines. Here are answers to 5 of the most common questions/misconceptions about vaccines.
Vaccines do NOT cause autism.
Though multiple studies have been conducted, none have shown a link between autism and vaccines. The initial paper that started the rumor has since been discredited.
Vaccines are NOT too much for an infant’s immune system to handle.
Infants’ immune systems can handle much more than what vaccines give them. They are exposed to hundreds of bacteria and viruses every day. Adding a few more with a vaccine doesn’t add to what their immune systems are capable of handling.
Vaccines do NOT contain toxins that will harm you.
Some vaccines contain trace amounts of substances that could be harmful in a large dose. These include formaldehyde, aluminum, and mercury. But the amount used in the vaccines is so small that the vaccines are completely safe. For example, over the course of all vaccinations by the age of 2, a child will take in 4mg of aluminum. A breast-fed baby will take in 10mg in 6 months. Soy-based formula delivers 120mg in 6 months. In addition, infants have 10 times as much formaldehyde naturally occurring in their bodies than what is contained in a vaccine. And the toxic form of mercury has never been used in vaccines.
Vaccines do NOT cause the diseases they are meant to prevent.
This is a common misconception, especially about the flu vaccine. Many people think they get sick after getting a flu shot. But flu shots contain dead viruses—it’s impossible to get sick from the shot but mild symptoms can occur because the vaccine may trigger an immune response, which is normal. Even with vaccines that use weakened live viruses, you could experience mild symptoms similar to the illness. But you don’t actually have the disease.
We DO still need vaccines in the U.S., even though infection rates are low.
Many diseases are uncommon in the U.S. because of our high vaccination rate. But they haven’t been eliminated from other areas of the world. If a traveler from another country brings a disease to the U.S., anyone who isn’t vaccinated is at risk of getting that disease. The only way to keep infection rates low is to keep vaccinating.
Questions to ask your doctor
- Why does my child need to be vaccinated?
- What are the possible side effects of the vaccination?
- What do I do if my child experiences a side effect from the vaccine?
- What happens if my child doesn’t get all doses of the recommended vaccines? Will he or she be able to go to daycare or school?
- We missed a vaccination. Can my child still get it late?
- Are there new vaccines that aren’t on the immunization schedules for kids?
- What should I do if I don’t have health insurance, or my insurance doesn’t cover vaccinations?
- What vaccinations do I need as an adult?
- Why do some people insist they became sick after getting the flu vaccine?
Centers for Disease Control and Prevention: Vaccines & Immunizations
Last Updated: August 10, 2023
This article was contributed by familydoctor.org editorial staff.
Copyright © American Academy of Family Physicians
This information provides a general overview and may not apply to everyone. Talk to your family doctor to find out if this information applies to you and to get more information on this subject.
Related Articles
Most schools and daycares require certain childhood vaccines, but they’re also good for your child’s health.
Certain immunizations are important at every age to reduce illness, hospitalization, or death.
A preventive service might be a test, an immunization or vaccine, or advice from your doctor. These services can…
Advertisement

familydoctor.org is powered by

Visit our interactive symptom checker
We use cookies to enhance our website for you. Proceed if you agree to this policy or learn more about it.
- Essay Database >
- Essays Samples >
- Essay Types >
- Term Paper Example
Vaccination Term Papers Samples For Students
50 samples of this type
Do you feel the need to check out some previously written Term Papers on Vaccination before you get down to writing an own piece? In this free collection of Vaccination Term Paper examples, you are granted an exciting opportunity to examine meaningful topics, content structuring techniques, text flow, formatting styles, and other academically acclaimed writing practices. Implementing them while crafting your own Vaccination Term Paper will surely allow you to complete the piece faster.
Presenting superb samples isn't the only way our free essays service can help students in their writing endeavors – our authors can also create from point zero a fully customized Term Paper on Vaccination that would make a genuine basis for your own academic work.
Good Example Of Tuberculosis Term Paper
(City, State)
Introduction
Free term paper on medical ethics: vaccinations for children of undocumented immigrants, surgical infection term paper examples.
Don't waste your time searching for a sample.
Get your term paper done by professional writers!
Just from $10/page
Example Of Term Paper On The Respiratory System
Historical foundations and achievements in public health term paper samples, vaccinationsname, free term paper on pertussis vaccine and supplementing health policy, the problem term paper example, term paper on firstname lastname, immunotherapy of malignant melanoma using dendritic cells vaccines, term paper on artificial immune system, infection control term paper examples.
There are a lot of ways to tackle an existing or a relatively new disease and one of the most effective ones is by proper conduct of infection control procedures. Infections are most common in hospitals where even the health workers (what more the patients) could be affected. According to an article published by Medline (2011), a lot of people die inside hospitals simply because of poor infection control SOPs. A result of poor infection control often results in a spread of infection.
Objectives of Infection Control
Asa college: term paper you might want to emulate, childhood immunizations, free mandatory vaccinations for children term paper example, vaccination for children, good signs and symptoms term paper example, influenza injection program, chikungunya term paper sample, analysis of diseases.
Overview Chikungunya (CHIK) refers to a re-emerging arboviral disease transmitted by the mosquitos Aedes aegypty and Aedes albopictus (Pialoux 318). The virus belongs to the genus Alphavirus and family Togaviridae. The word chikungunya derives from Makonde language (spoken in Tanzania and northern Mozambique), where it means “to walk bent over” (Halstead 557). This name refers to the main feature of both chronic and acute phase of the disease a change in posture causes by the arthritis and arthralgia (Charrel, Lamballerie and Raoult 769).
The Prevention Of Catheter-Associated Urinary Tract Infections Term Paper Example
Ecpi medical careers institute, theory application group project/paper: a sample term paper for inspiration & mimicking, phase 3 – behavioral and environmental diagnosis of vaccinating children, good example of hepatitis- a review of viral disease term paper, hepatitis- a review of viral disease, developing a business in vietnam term paper to use for practical writing help, the history of vietnam, draw topic & writing ideas from this term paper on colon cancer and its treatment, brief information, risk factors and symptoms, good term paper on the population of india: past, present, and future, guillain- barre syndrome term papers example, pathophysiology, youre name term papers example, pandemic plan, term paper on environmental health promotion, good example of nursing process papers, application of the nursing process, good term paper on health politics and policy, difference between entitlement program and block grants, free term paper about world history, the black death: europe's most notorious plague, biochemistry of depression term paper example, extrinsic asthma and its effect on the respiratory system term paper, sjogren syndrome: pathophysiology, diagnosis, and treatment term paper examples, aging term papers example, free malaria term paper example, imogene thompson, good example of term paper on nutrition.
(Author Full Name) (Professor Name)
Good Example Of The Nazi Medical Experiments Term Paper
Assignment number:, operation of different divisions term paper samples.
.University.. .professor..
Overview of Novartis
Novartis, founded in 1996, is one of the leading multinational pharmaceutical companies of the world. It is a healthcare company with leading positions in pharmaceuticals, eye care, generic medicines, vaccines and diagnostics, and consumer health. It is focused on innovating to meet the new patient needs, increasing its presence in new and emerging markets, and raising productivity to generate future and increase shareholder return. According to IMS, the company ranked no. 1 in top 20 global corporations of 2012 by sales with USD 50.76bn in sales.
Approach To Care Term Paper Example
Facilitator:, example of the aids crisis in africa term paper, cancer disease term paper samples, good polycystic kidney disease term paper example, introduction, good term paper about stress at work, free performance appraisal methods term paper sample, complementary and traditional interventions: example term paper by an expert writer to follow, complementary and traditional interventions, type 1 diabetes: islet-cells transplant term paper sample, pathophysiology: nrs-410v, good example of a lawyers ethical dilemma term paper.
[Class Title]
Good Term Paper On Disorders Of The Cardiovascular System
Analyzing impact of a news on a publicly traded company course term paper examples, executive summary, good term paper on da vinci surgical system, audience analysis, ageism term paper examples, cancer psychology and health issues term paper example, term paper on alzheimers disease effects on the caregivers, homelessness term paper, homelessness.
Password recovery email has been sent to [email protected]
Use your new password to log in
You are not register!
By clicking Register, you agree to our Terms of Service and that you have read our Privacy Policy .
Now you can download documents directly to your device!
Check your email! An email with your password has already been sent to you! Now you can download documents directly to your device.
or Use the QR code to Save this Paper to Your Phone
The sample is NOT original!
Short on a deadline?
Don't waste time. Get help with 11% off using code - GETWOWED
No, thanks! I'm fine with missing my deadline

COMMENTS
Economic, equity, and global health benefits of vaccines. Vaccines can have several economic benefits. 3, 10 One of the most discernible benefits is averted medical expenditure. By preventing an episode of the disease through a vaccine, the economic costs of treatment, such as physician fees, drugs and hospitalization expenses, and associated travel costs and wage loss of caregivers could be ...
Frontline health care personnel (HCP) were among the first to receive the COVID-19 vaccines. Physicians, nurses, allied health professionals, and others with direct patient contact or who handle biological materials are at high risk of exposure and illness and have duties of care and protection to patients, coworkers, and communities. Yet well into 2021, some HCP remain vaccine hesitant, an ...
The vaccine, based on four selected peptides, provided 100% protection against a lethal viral challenge and induced a long-term memory T-cell response, highlighting the potential of peptide-based ...
DRAFT Prepared by the SAGE Working Group on COVID-19 Vaccines 22 December 2020 5 . even death. Several occupational risks for health workers emerged or were amplified by the COVID-
of vaccines despite availability" (Macdonald, 2015, p. 34). After a thorough literature review, evidence reveals that there is a gap between perceived vaccine importance and perceived vaccine safety in developed nations as many survey respondents believe in the efficacy and importance of vaccines but lack confidence in the safety of vaccines.
Background By mid 2023, European countries reached 75% of vaccine coverage for COVID-19 and although vaccination rates are quite high, many people are still hesitant. A plethora of studies have investigated factors associated with COVID-19 vaccine hesitancy, however, insufficient attention has been paid to the reasons why people get vaccinated against COVID-19. Our work aims to investigate the ...
Vaccines and immunization. Immunization is a global health and development success story, saving millions of lives every year. Vaccines reduce risks of getting a disease by working with your body's natural defences to build protection. When you get a vaccine, your immune system responds. We now have vaccines to prevent more than 20 life ...
Vaccination is widely considered one of the greatest medical achievements of modern civilization. Childhood diseases that were commonplace less than a generation ago are now increasingly rare because of vaccines. In order to be effective at eliminating communicable diseases, vaccines must be administered to sufficient levels of persons in the community.
A plasma-derived inactivated vaccine is approved for commercial use from 1981 to 1990, and a genetically engineered (or DNA recombinant) vaccine, developed in 1986, is still in use today. The measles vaccine (1963) is combined with the recently developed vaccines against mumps (1967) and rubella (1969) into a single vaccination (MMR).
Vaccination is safe and side effects from a vaccine are usually minor and temporary, such as a sore arm or mild fever. More serious side effects are possible, but extremely rare. Any licensed vaccine is rigorously tested across multiple phases of trials before it is approved for use, and regularly reassessed once it is introduced. Scientists ...
3.1 Identify and partner with trusted COVID-19 vaccine messengers. 3.2 Create accurate, transparent, and truthful vaccine messages. 3.3 Frame vaccine acceptance as a social norm. 3.4 Use behavioral nudges. 3.5 Enact value-concordant messaging that is sensitive to the emotional state of the audience.
The dosage of Pfizer vaccine authorized for 12- to 17-year-olds is identical to the dosage given to adults: two doses of 30 micrograms given three weeks apart. The dose for children ages 5 to 11 ...
A vaccine can confer active immunity against a specific harmful agent by stimulating the immune system to attack the agent. Once stimulated by a vaccine, the antibody-producing cells, called B cells (or B lymphocytes), remain sensitized and ready to respond to the agent should it ever gain entry to the body.A vaccine may also confer passive immunity by providing antibodies or lymphocytes ...
vaccine-induced passive immunity, fears of harm secondary to vaccination loom. In the 21st Century, many people are unaware of diseases that still occur in undeveloped countries due to herd immunity through a majority vaccinated pop - ulation. The diseases, however, are only a plane ride away. Due to globalization, vaccine preventable diseases ...
Vaccines prevent the spread of contagious, dangerous, and deadly diseases. These include measles, polio, mumps, chicken pox, whooping cough, diphtheria, HPV, and COVID-19. The first vaccine discovered was the smallpox vaccine. Smallpox was a deadly illness. It killed 300 million to 500 million people around the world in the last century.
Vaccination. production of antibodies and provide immunity against one or several diseases, prepared from the causative agent of a disease, its products, or a synthetic substitute, treated to act as an antigen without inducing the disease. What is the purpose of Vaccinations? To produce immunity.
In this free collection of Vaccination Term Paper examples, you are granted an exciting opportunity to examine meaningful topics, content structuring techniques, text flow, formatting styles, and other academically acclaimed writing practices. Implementing them while crafting your own Vaccination Term Paper will surely allow you to complete the ...