Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Research on drinking water purification technologies for household use by reducing total dissolved solids (TDS)
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Redlands East Valley High School, Redlands, California, United States of America
- Bill B. Wang

- Published: September 28, 2021
- https://doi.org/10.1371/journal.pone.0257865
- Reader Comments
This study, based in San Bernardino County, Southern California, collected and examined tap water samples within the area to explore the feasibility of adopting non-industrial equipment and methods to reduce water hardness and total dissolved solids(TDS). We investigated how water quality could be improved by utilizing water boiling, activated carbon and sodium bicarbonate additives, as well as electrolysis methods. The results show that heating is effective at lower temperatures rather than long boils, as none of the boiling tests were lower than the original value. Activated carbon is unable to lower TDS, because it is unable to bind to any impurities present in the water. This resulted in an overall TDS increase of 3.5%. However, adding small amounts of sodium bicarbonate(NaHCO 3 ) will further eliminate water hardness by reacting with magnesium ions and improve taste, while increasing the pH. When added to room temperature tap water, there is a continuous increase in TDS of 24.8% at the 30 mg/L mark. The new findings presented in this study showed that electrolysis was the most successful method in eliminating TDS, showing an inverse proportion where an increasing electrical current and duration of electrical lowers more amounts of solids. This method created a maximum decrease in TDS by a maximum of 22.7%, with 3 tests resulting in 15.3–16.6% decreases. Furthermore, when water is heated to a temperature around 50°C (122°F), a decrease in TDS of around 16% was also shown. The reduction of these solids will help lower water hardness and improve the taste of tap water. These results will help direct residents to drink more tap water rather than bottled water with similar taste and health benefits for a cheaper price as well as a reduction on plastic usage.
Citation: Wang BB (2021) Research on drinking water purification technologies for household use by reducing total dissolved solids (TDS). PLoS ONE 16(9): e0257865. https://doi.org/10.1371/journal.pone.0257865
Editor: Mahendra Singh Dhaka, Mohanlal Sukhadia University, INDIA
Received: June 22, 2021; Accepted: September 14, 2021; Published: September 28, 2021
Copyright: © 2021 Bill B. Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: The author received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The concentration of total dissolved solids(TDS) present in water is one of the most significant factors in giving water taste and also provides important ions such as calcium, magnesium, potassium, and sodium [ 1 – 3 ]. However, water with high TDS measurements usually indicates contamination by human activities, such as soil and agricultural runoff caused by irrigation, unregulated animal grazing and wildlife impacts, environmentally damaging farming methods such as slash and burn agriculture, and the overuse of nitrate-based fertilizer [ 4 , 5 ], etc. Around tourist areas as well as state parks, these factors will slowly add up over time and influence the water sources nearby [ 5 ]. Water that flows through natural springs and waterways with high concentrations of organic salts within minerals and rocks, or groundwater that originates from wells with high salt concentration will also result in higher particle measurements [ 6 ].
Water sources can be contaminated by substances and ions such as nitrate, lead, arsenic, and copper [ 7 , 8 ] and may cause many health problems related to heavy metal consumption and poisoning. Water reservoirs and treatments plants that do not consider water contamination by motor vehicles, as well as locations that struggle to provide the necessary components required for water treatment will be more prone to indirect contamination [ 9 – 11 ]. Many plants are effective in ensuring the quality and reduction of these contaminants, but often leave out the secondary considerations, The United States Environmental Protection Agency(US EPA)’s secondary regulations recommend that TDS should be below 500 mg/L [ 2 ], which is also supported by the World Health Organization(WHO) recommendation of below 600 mg/L and an absolute maximum of less than 1,000 mg/L [ 3 ]. These substances also form calcium or magnesium scales within water boilers, heaters, and pipes, causing excess buildup and drain problems, and nitrate ions may pose a risk to human health by risking the formation of N -nitroso compounds(NOC) and less public knowledge about such substances [ 12 – 15 ]. Nitrates can pose a non-carcinogenic threat to different communities, but continue to slip past water treatment standards [ 15 ]. Furthermore, most people do not tolerate or prefer water with high hardness or chlorine additives [ 16 ], as the taste changes tremendously and becomes unpreferable. Even so, TDS levels are not accounted for in mandatory water regulations, because the essential removal of harmful toxins and heavy metals is what matters the most in water safety. Some companies indicate risks in certain ions and alkali metals, showing how water hardness is mostly disregarded and is not as well treated as commercial water bottling companies [ 17 , 18 ].
In Southern California, water quality is not as well maintained than the northern counties as most treatment plants in violation of a regulation or standard are located in Central-Southern California [ 19 ], with southern counties having the largest number of people affected [ 20 ]. This study is focused on the Redlands area, which has had no state code violations within the last decade [ 21 ]. A previous study has analyzed TDS concentrations throughout the Santa Ana Basin, and found concentrations ranging from 190–600 ppm as treated wastewater and samples obtained from mountain sites, taking into account the urban runoff and untreated groundwater as reasons for elevated levels of TDS but providing no solution in helping reduce TDS [ 22 ]. Also, samples have not been taken directly through home water supplies, where the consumer is most affected. Other water quality studies in this region have been focused on the elimination of perchlorates in soil and groundwater and distribution of nitrates, but such research on chemicals have ceased for the last decade, demonstrated by safe levels of perchlorates and nitrates in water reports [ 23 , 24 ]. In addition to these studies, despite the improving quality of the local water treatment process, people prefer bottled water instead of tap water because of the taste and hardness of tap water [ 25 ]. Although water quality tests are taken and documented regularly, the taste of the water is not a factor to be accounted for in city water supplies, and neither is the residue left behind after boiling water. The residue can build up over time and cause appliance damage or clogs in drainage pipes.
This study will build upon previous analyses of TDS studies and attempt to raise new solutions to help develop a more efficient method in reducing local TDS levels, as well as compare current measurements to previous analyses to determine the magnitude to which local treatment plants have improved and regulated its treatment processes.
Several methods that lower TDS are reviewed: boiling and heating tap water with and without NaHCO₃, absorption by food-grade activated carbon [ 26 , 27 ], and battery-powered electrolysis [ 28 – 30 ]. By obtaining water samples and determining the difference in TDS before and after the listed experiments, we can determine the effectiveness of lowering TDS. The results of this study will provide options for residents and water treatment plants to find ways to maintain the general taste of the tap water, but also preserve the lifespan of accessories and pipelines. By determining a better way to lower TDS and treat water hardness, water standards can be updated to include TDS levels as a mandatory measurement.
Materials and methods
All experiments utilized tap water sourced from Redlands homes. This water is partially supplied from the Mill Creek (Henry Tate) and Santa Ana (Hinckley) Water Sheds/Treatment Plants, as well as local groundwater pumps. Water sampling and sourcing were done at relatively stable temperatures of 26.9°C (80.42°F) through tap water supplies. The average TDS was measured at 159 ppm, which is slightly lower than the reported 175 ppm by the City of Redlands. Permission is obtained by the author from the San Bernardino Municipal Water Department website to permit the testing procedures and the usage of private water treatment devices for the purpose of lowering water hardness and improving taste and odor. The turbidity was reported as 0.03 Nephelometric Turbidity Units (NTU) post-treatment. Residual nitrate measured at 2.3mg/L in groundwater before treatment and 0.2 mg/L after treatment and perchlorate measured at 0.9 μg/L before treatment, barely staying below the standard of 1 μg/L; it was not detected within post-treatment water. Lead content was not detected at all, while copper was detected at 0.15 mg/L.
For each test, all procedures were done indoors under controlled temperatures, and 20 L of fresh water was retrieved before each test. Water samples were taken before each experimental set and measured for TDS and temperature, and all equipment were cleaned thoroughly with purified water before and after each measurement. TDS consists of inorganic salts and organic material present in solution, and consists mostly of calcium, magnesium, sodium, potassium, carbonate, chloride, nitrate, and sulfate ions. These ions can be drawn out by leaving the water to settle, or binding to added ions and purified by directly separating the water and ions. Equipment include a 50 L container, 1 L beakers for water, a graduated cylinder, a stir rod, a measuring spoon, tweezers, a scale, purified water, and a TDS meter. A standard TDS meter is used, operated by measuring the conductivity of the total amount of ionized solids in the water, and is also cleaned in the same manner as aforementioned equipment. The instrument is also calibrated by 3 pH solutions prior to testing.All results were recorded for and then compiled for graphing and analysis.
Heating/Boiling water for various lengths of time
The heating method was selected because heat is able to break down calcium bicarbonate into calcium carbonate ions that are able to settle to the bottom of the sample. Four flasks of 1 L of tap water were each heated to 40°C, 50°C, 60°C, and 80°C (104–176°F) and observed using a laser thermometer. The heated water was then left to cool and measurements were made using a TDS meter at the 5, 10, 20, 30, and 60-minute marks.
For the boiling experiments, five flasks of 1 L of tap water were heated to boil at 100°C (212°F). Each flask, which was labeled corresponding to its boiling duration, was marked with 2, 4, 6, 10, and 20 minutes. Each flask was boiled for its designated time, left to cool under open air, and measurements were made using a TDS meter at the 5, 10, 20, 30, 60, and 120-minute marks. The reason that the boiling experiment was extended to 120 minutes was to allow the water to cool down to room temperature.
Activated carbon as a water purification additive
This test was performed to see if food-grade, powdered activated carbon had any possibility of binding with and settling out residual particles. Activated carbon was measured using a milligram scale and separated into batches of 1, 2, 4, 5, 10, 30, and 50 mg. Each batch of the activated carbon were added to a separate flask of water and stirred for five minutes, and finally left to settle for another five minutes. TDS measurements were recorded after the water settled.
Baking soda as a water purification additive
To lower scale error and increase experimental accuracy, a concentration of 200 mg/L NaHCO₃ solution was made with purified water and pure NaHCO₃. For each part, an initial TDS measurement was taken before each experiment.
In separate flasks of 1 L tap water, each labeled 1, 2, 4, 5, 10, and 30 mg of NaHCO 3 , a batch was added to each flask appropriately and stirred for 5 minutes to ensure that everything dissolved. Measurements were taken after the water was left to settle for another 5 minutes for any TDS to settle.
Next, 6 flasks of 1 L tap water were labeled, with 5 mg (25 mL solution) of NaHCO₃ added to three flasks and 10 mg (50 mL solution) of NaHCO₃ added to the remaining three. One flask from each concentration of NaHCO₃ was boiled for 2 mins., 4 mins., or 6 mins., and then left to cool. A TDS measurement was taken at the 5, 10, 20, 30, 60, and 120-minute marks after removal from heat.
Electrolysis under low voltages
This test was performed because the ionization of the TDS could be manipulated with electricity to isolate an area of water with lower TDS. For this test, two 10cm long graphite pieces were connected via copper wiring to a group of batteries, with each end of the graphite pieces submerged in a beaker of tap water, ~3 cm apart.
Using groups of 1.5 V double-A batteries, 4 beakers with 40mL of tap water were each treated with either 7.5, 9.0, 10.5, and 12.0 V of current. Electrolysis was observed to be present by the bubbling of the water each test, and measurements were taken at the 3, 5, 7, and 10 minute marks.
Results/Discussion
Heating water to various temperatures until the boiling point.
The goal for this test was to use heat to reduce the amount of dissolved oxygen and carbon dioxide within the water, as shown by this chemical equation: Heat: Ca(HCO 3 ) 2 → CaCO 3 ↓ + H 2 O + CO 2 ↑.
This would decompose ions of calcium bicarbonate down into calcium carbonate and water and carbon dioxide byproducts.
Patterns and trends in decreasing temperatures.
The following trend lines are based on a dataset of changes in temperature obtained from the test results and graphed as Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0257865.g001
To predict the precise temperature measurements of the tap water at 26.9°C, calculations were made based on Fig 1 . The fitting equations are in the format, y = a.e bx . The values for the fitting coefficients a and b, and correlation coefficient R 2 are listed in Table 1 as column a, b and R 2 . The calculated values and the target temperature are listed in Table 1 .
https://doi.org/10.1371/journal.pone.0257865.t001
Fig 2 was obtained by compiling TDS results with different temperatures and times.
https://doi.org/10.1371/journal.pone.0257865.g002
The fitting equations for Fig 2 are also in the format, y = a.e bx . The fitting coefficients a and b, and correlation coefficient R 2 values are listed in Table 2 . Based on the fitting curves in Fig 2 and the duration to the target temperature in Table 1 , We calculated the TDS at 26.9°C as listed in column calculated TDS in Table 2 based on the values we reported on Fig 2 .
https://doi.org/10.1371/journal.pone.0257865.t002
Based on the heating temperature and the calculated TDS with the same target water temperature, we obtained the following heating temperature vs TDS removal trend line and its corresponding fitting curve in Table 2 .
In Fig 1 , a trend in the rate of cooling is seen, where a higher heating temperature creates a steeper curve. During the first five minutes of cooling, the water cools quicker as the absorbed heat is quickly released into the surrounding environment. By the 10-minute mark, the water begins to cool in a linear rate of change. One detail to note is that the 100°C water cools quicker than the 80°C and eventually cools even faster than the 60°C graph. Table 1 supports this observation as the duration to target temperature begins to decrease from a maximum point of 94.8 mins to 80.95 mins after the 80°C mark.
As shown in Fig 2 , all TDS values decrease as the temperature starts to cool to room temperature, demonstrating a proportional relationship where a lower temperature shows lower TDS. This can partially be explained by the ions settling in the flasks. Visible particles can also be observed during experimentation as small white masses on the bottom, as well as a thin ring that forms where the edge of the water contacts the flask. When the water is heated to 40°C and cooled, a 3.8% decrease in TDS is observed. When 50°C is reached, the TDS drops at its fastest rate from an initial value of 202 ppm to 160 ppm after 60 minutes of settling and cooling. The TDS measurements in these experiements reach a maximum of 204 ppm at the 60°C mark. However, an interesting phenomenon to point out is that the water does not hit a new maximum at 100°C. meaning that TDS reaches a plateau at 60°C. Also, the rate of decrease begins to slow down after 20 minutes, showing that an unknown factor is affecting the rate of decrease. It is also hypothesized that the slight increase in TDS between the 5–20 minute range is caused by a disturbance in the settling of the water, where the temperature starts to decrease at a more gradual and constant rate. The unstable and easy formation of CaCO 3 scaling has also been the subject of a study of antiscaling methods, which also supports the result that temperature is a significant influence for scale formation [ 12 ].
In Table 2 , calculations for TDS and the time it takes for each test to cool were made. Using the data, it is determined that the test with 50°C water decreased the most by 16% from the initial measurement of 159ppm. This means that it is most effective when water is heated between temperatures of 40–60°C when it comes to lowering TDS, with a difference of ~7–16%. When water is heated to temperatures greater than 80°C, the water begins to evaporate, increasing the concentration of the ions, causing the TDS to increase substantially when cooled to room temperature.
Finally, in Fig 3 , a line of best fit of function f(x) = -0.0007x 3 + 0.1641x 2 –10.962x + 369.36 is used with R 2 = 0.9341. Using this function, the local minimum of the graph would be reached at 48.4°C.
https://doi.org/10.1371/journal.pone.0257865.g003
This data shows that heating water at low temperatures (i.e. 40–50°C) may be more beneficial than heating water to higher temperatures. This study segment has not been presented in any section within the United States EPA Report on water management for different residual particles/substances. However, warmer water temperatures are more prone to microorganism growth and algal blooms, requiring more intensive treatment in other areas such as chlorine, ozone, and ultraviolet disinfection.
Using the specific heat capacity equation, we can also determine the amount of energy and voltage needed to heat 1 L of water up to 50°C: Q = mcΔT, where c, the specific heat capacity of water, is 4.186 J/g°C, ΔT, the change in temperature from the experimental maximum to room temperature, is 30°C, and m, the mass of the water, is 1000 g. This means that the amount of energy required will be 125580 J, which is 0.035 kWh or 2.1 kW.
After taking all of the different measurements obtained during TDS testing, and compiling the data onto this plot, Fig 4 is created with a corresponding line of best fit:
https://doi.org/10.1371/journal.pone.0257865.g004
In Fig 4 , it can be observed that the relationship between the temperature of the water and its relative TDS value is a downwards facing parabolic graph. As the temperature increases, the TDS begins to decrease after the steep incline at 50–60°C. The line of best fit is represented by the function f(x) = -0.0142x 2 + 2.258x + 105.84. R 2 = 0.6781. Because the R 2 value is less than expected, factors such as the time spent settling and the reaction rate of the ions should be considered. To determine the specifics within this experiment, deeper research and prolonged studies with more highly accurate analyses must be utilized to solve this problem.
Boiling water for various amounts of time
Trend of boiling duration and rate of cooling..
Using the same methods to create the figures and tables for the previous section, Fig 5 depicts how the duration of time spent boiling water affects how fast the water cools.
https://doi.org/10.1371/journal.pone.0257865.g005
As seen in Fig 5 , within the first 10 minutes of the cooling time, the five different graphs are entwined with each other, with all lines following a similar pattern. However, the graph showing 20 minutes of boiling is much steeper than the other graphs, showing a faster rate of cooling. This data continues to support a previous claim in Fig 2 , as this is most likely represented by a relationship a longer the boil creates a faster cooling curve. This also shows that the first 5 minutes of cooling have the largest deviance compared to any other time frame.
The cooling pattern is hypothesized by possible changes in the orderly structure of the hydrogen bonds in the water molecules, or the decreased heat capacity of water due to the increasing concentration of TDS.
Effect on TDS as boiling duration increases.
In Fig 6 , all lines except for the 20-minute line are clustered in the bottom area of the graph. By excluding the last measurement temporarily due to it being an outlier, we have observed that the difference between the initial and final TDS value of each test decreases.
https://doi.org/10.1371/journal.pone.0257865.g006
Despite following a similar trend of an increase in TDS at the start of the tests and a slow decrease overtime, this experiment had an interesting result, with the final test measuring nearly twice the amount of particles compared to any previous tests at 310 ppm, as shown in Fig 6 . It is confirmed that the long boiling time caused a significant amount of water to evaporate, causing the minerals to be more concentrated, thus resulting in a 300 ppm reading. Fig 6 follows the same trend as Fig 2 , except the TDS reading veers away when the boiling duration reaches 20 minutes. Also, with the long duration of heating, the water has developed an unfavorable taste from intense concentrations of CaCO₃. This also causes a buildup of a thin crust of CaCO₃ and other impurities around the container that is difficult to remove entirely. This finding is in accordance with the introductory statement of hot boiling water causing mineral buildups within pipes and appliances [ 9 ]. A TDS reading of 300ppm is still well below federal secondary standards of TDS, and can still even be compared to bottled water, in which companies may fluctuate and contain 335ppm within their water [ 1 , 2 ].
This experiment continues to stupport that the cooling rate of the water increases as the time spent boiling increases. Based on this test, a prediction can be made in which an increased concentration of dissolved solids lowers the total specific heat capacity of the sample, as the total volume of water decreases. This means that a method can be derived to measure TDS using the heat capacity of a tap water mixture and volume, in addition to current methods of using the electrical conductivity of aqueous ions.
Adding food-grade activated carbon to untreated tap water
Fig 7 presents a line graph with little to no change in TDS, with an initial spike from 157 to 163 ppm. The insoluble carbon remains in the water and shows no benefit.
https://doi.org/10.1371/journal.pone.0257865.g007
The food-grade activated carbon proved no benefit to removing TDS from tap water, and instead added around 5–7 ppm extra, which settled down to around +4 ppm at 120 minutes. The carbon, which is not 100% pure from inorganic compounds and materials present in the carbon, can dissolve into the water, adding to the existing concentration of TDS. Furthermore, household tap water has already been treated in processing facilities using a variety of filters, including carbon, so household charcoal filters are not effective in further reducing dissolved solids [ 18 ].
Adding sodium bicarbonate solution to boiled tap water
As seen in Fig 8 , after adding 1 mg of NaHCO 3 in, the TDS rises to 161 ppm, showing a minuscule increase. When 4 mg was added, the TDS drops down to 158 ppm. Then, when 5 mg was added, a sudden spike to 172 ppm was observed. This means that NaHCO 3 is able to ionize some Ca 2+ and Mg 2+ ions, but also adds Na + back into the water. This also means that adding NaHCO 3 has little to no effect on TDS, with 4mg being the upper limit of effectiveness.
https://doi.org/10.1371/journal.pone.0257865.g008
To examine whether or not the temperature plays a role in the effectiveness in adding NaHCO 3 , a boiling experiment was performed, and the data is graphed in Fig 9 .
https://doi.org/10.1371/journal.pone.0257865.g009
Fig 9 presents the relationship between the amount of common baking soda(NaHCO₃) added, the boiling time involved, and the resulting TDS measurements. After boiling each flask for designated amounts of time, the results showed a downward trend line from a spike but does not reach a TDS value significantly lower than the initial sample. It is apparent that the NaHCO₃ has not lowered the TDS of the boiling water, but instead adds smaller quantities of ions, raising the final value. This additive does not contribute to the lowering of the hardness of the tap water. However, tests boiled with 5 mg/L of baking soda maintained a downward pattern as the water was boiled for an increasing amount of time, compared to the seemingly random graphs of boiling with 10 mg/L.
In some households, however, people often add NaHCO₃ to increase the pH for taste and health benefits. However, as shown in the test results, it is not an effective way of reducing TDS levels in the water [ 10 , 16 ], but instead raises the pH, determined by the concentration added. Even under boiling conditions, the water continues to follow the trend of high growth in TDS, of +25–43 ppm right after boiling and the slow drop in TDS (but maintaining a high concentration) as the particles settle to the bottom.
Utilizing the experimental results, we can summarize that after adding small batches of NaHCO3 and waiting up to 5 minutes will reduce water hardness making it less prone to crystallizing within household appliances such as water brewers. Also, this process raises the pH, which is used more within commercial water companies. However, the cost comes at increasing TDS.
Using electrolysis to treat TDS in tap water
Different voltages were passed through the water to observe the change in TDS overtime, with the data being compiled as Fig 10 .
https://doi.org/10.1371/journal.pone.0257865.g010
The process of electrolysis in this experiment was not to and directly remove the existing TDS, but to separate the water sample into three different areas: the anode, cathode, and an area of clean water between the two nodes [ 19 ]. The anions in the water such as OH - , SO 4 2- , HCO 3 - move to the anode, while the cations such as H + , Ca 2+ 、Mg 2+ 、Na + move to the cathode. The middle area would then be left as an area that is more deprived of such ions, with Fig 10 proving this.
As shown in Fig 10 , electrolysis is effective in lower the TDS within tap water. Despite the lines being extremely tangled and unpredictable, the general trend was a larger decrease with a longer duration of time. At 10 minutes, all lines except 10.5 V are approaching the same value, meaning that the deviation was most likely caused by disturbances to the water during measurement from the low volume of water. With each different voltage test, a decrease of 12.7% for 6.0 V, 14.9% for 9.0 V, 22.7% for 10.5 V. and 19.5% for 12.0 V respectfully were observed. In the treatment of wastewate leachate, a study has shown that with 90 minutes of electrical treatment, 34.58% of TDS content were removed, supporting the effectiveness of electricity and its usage in wastewater treatment [ 29 ].
This experiment concludes that electrolysis is effective in lowering TDS, with the possibility to improve this process by further experimentation, development of a water cleaning system utilizing this cathode-anode setup to process water. This system would be a more specific and limited version of a reverse osmosis system by taking away ions through attraction, rather than a filter.
The Southern Californian tap water supply maintains TDS values below the federal regulations. However, crystalline scale buildup in household appliances is a major issue as it is hard to clean and eliminate. To easily improve the taste and quality of tap water at home as well as eliminating the formation of scales, the following methods were demonstrated as viable:
- By heating water to around 50°C (122°F), TDS and water hardness will decrease the most. Also, the boiling process is effective in killing microorganisms and removing contaminants. This process cannot surpass 10 minutes, as the concentration of the ions in the water is too high, which poses human health risks if consumed. These, along with activated carbon and NaHCO₃ additives, are inefficient methods that have minimal effects for lowering TDS.
- Electrolysis is one of the most effective methods of eliminating TDS. Experiments have proven that increased current and duration of time helps lower TDS. However, this method has yet to be implemented into conventional commercial water filtration systems.
Also, some observations made in these experiments could not be explained, and require further research and experimentation to resolve these problems. The first observation is that TDS and increasing water temperature maintain a parabolic relationship, with a maximum being reached at 80°C, followed by a gradual decrease. The second observation is that when water is boiled for an increased duration of time, the rate of cooling also increases.
This experiment utilized non-professional scientific equipment which are prone to mistakes and less precise. These results may deviate from professionally derived data, and will require further study using more advanced equipment to support these findings.
Acknowledgments
The author thanks Tsinghua University Professor and PLOS ONE editor Dr. Huan Li for assisting in experimental setups as well as data processing and treatment. The author also thanks Redlands East Valley High School’s Dr. Melissa Cartagena for her experimental guidance, and Tsinghua University Professor Dr. Cheng Yang for proofreading the manuscript.
- View Article
- Google Scholar
- 2. United States Environmental Protection Agency. 2018 Edition of the Drinking Water Standards and Health Advisories Tables (EPA 822-F-18-001). US EPA, Washington D.C., USA, 2018; pp. 9–19.
- 3. World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. WHO, Geneva, Switzerland, 2017; pp. 7, 219–230, 423.
- PubMed/NCBI
- 6. Chloride, Salinity, and Dissolved Solids. 2019 Mar 1, [cited on 20 September 2020] Available from: https://www.usgs.gov/mission-areas/water-resources/science/chloride-salinity-and-dissolved-solids?qt-science_center_objects=0#qt-science_center_objects .
- 13. Shoukat, Ammara, Hussain, M., Shoukat, Asra. Effects of Temperature on Total dissolved Solid in water. Water Quality Study Conference, Mehran University Sindh, Pakistan, February 2020.
- 17. United States Environmental Protection Agency. Drinking Water Treatment Plant Residuals Management—Technical Report: Summary of Residuals Generation, Treatment, and Disposal at Large Community Water Systems. US EPA, Washington D.C., USA, 2011; pp. 177–182.
- 19. Exceedance and Compliance Status of Public Water Systems. 2012 Feb 1, [cited 22 September 2020] Available from: https://www.arcgis.com/apps/MapJournal/index.html?appid=143794cd74e344a29eb8b96190f4658b# .
- 20. 2019 Water Quality Status Report California. California Water Boards, 2019 July 1, [cited 22 September 2020] Available from: https://gispublic.waterboards.ca.gov/portal/apps/MapJournal/index.html?appid=6cde29ac0afc4d55b0fdaaae6bfc1aa4 .
- 21. City of Redlands—Water Quality Consumer Confidence Reports. City of Redlands, 2010–2020, [cited 23 September 2020] Available from: https://www.cityofredlands.org/post/water-quality .
- 25. Consumers’ Preference For Bottled Water Is Growing And They Want It Available Wherever Drinks Are Sold. 2019 Jan 8, [cited 23 September 2020] Available from: https://www.bottledwater.org/consumers’-preference-bottled-water-growing-and-they-want-it-available-wherever-drinks-are-sold .
- You are here:
- American Chemical Society
- Discover Chemistry
Recent advancements in water treatment
For immediate release, acs news service weekly presspac: january 19, 2022.
Generating clean, safe water is becoming increasingly difficult. Water sources themselves can be contaminated, but in addition, some purification methods can cause unintended harmful byproducts to form. And not all treatment processes are created equal with regard to their ability to remove impurities or pollutants. Below are some recent papers published in ACS journals that report insights into how well water treatment methods work and the quality of the resulting water. Reporters can request free access to these papers by emailing newsroom@acs.org .
“Drivers of Disinfection Byproduct Cytotoxicity in U.S. Drinking Water: Should Other DBPs Be Considered for Regulation?” Environmental Science & Technology Dec.15, 2021
In this paper, researchers surveyed both conventional and advanced disinfection processes in the U.S., testing the quality of their drinking waters. Treatment plants with advanced removal technologies, such as activated carbon, formed fewer types and lower levels of harmful disinfection byproducts (known as DBPs) in their water. Based on the prevalence and cytotoxicity of haloacetonitriles and iodoacetic acids within some of the treated waters, the researchers recommend that these two groups be considered when forming future water quality regulations.
“Complete System to Generate Clean Water from a Contaminated Water Body by a Handmade Flower-like Light Absorber” ACS Omega Dec. 9, 2021 As a step toward a low-cost water purification technology, researchers crocheted a coated black yarn into a flower-like pattern. When the flower was placed in dirty or salty water, the water wicked up the yarn. Sunlight caused the water to evaporate, leaving the contaminants in the yarn, and a clean vapor condensed and was collected. People in rural locations could easily make this material for desalination or cleaning polluted water, the researchers say.
“Data Analytics Determines Co-occurrence of Odorants in Raw Water and Evaluates Drinking Water Treatment Removal Strategies” Environmental Science & Technology Dec. 2, 2021
Sometimes drinking water smells foul or “off,” even after treatment. In this first-of-its-kind study, researchers identified the major odorants in raw water. They also report that treatment plants using a combination of ozonation and activated carbon remove more of the odor compounds responsible for the stink compared to a conventional process. However, both methods generated some odorants not originally present in the water.
“Self-Powered Water Flow-Triggered Piezocatalytic Generation of Reactive Oxygen Species for Water Purification in Simulated Water Drainage” ACS ES&T Engineering Nov. 23, 2021
Here, researchers harvested energy from the movement of water to break down chemical contaminants. As microscopic sheets of molybdenum disulfide (MoS2) swirled inside a spiral tube filled with dirty water, the MoS2 particles generated electric charges. The charges reacted with water and created reactive oxygen species, which decomposed pollutant compounds, including benzotriazole and antibiotics. The researchers say these self-powered catalysts are a “green” energy resource for water purification.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News . ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org .
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Media Contact
ACS Newsroom newsroom@acs.org

Discover Chemistry —Menu
- News Releases
- ACS in the News
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
Nanotechnology: an approach for water purification-review
Rama Sharma 1
Published under licence by IOP Publishing Ltd IOP Conference Series: Materials Science and Engineering , Volume 1116 , International Conference on Futuristic and Sustainable Aspects in Engineering and Technology (FSAET 2020) 18th-19th December 2020, Mathura, India Citation Rama Sharma 2021 IOP Conf. Ser.: Mater. Sci. Eng. 1116 012007 DOI 10.1088/1757-899X/1116/1/012007
Article metrics
1289 Total downloads
Share this article
Author e-mails.
Author affiliations
1 Department of Biotechnology, GLA University, Mathura
Buy this article in print
Clean water is the global need and need of life for all the human kinds. But the clean water resources are being contaminated in present time. Nanotechnology is an easy and practical approach to clean waste water by using different methods. Different types of bacteria, toxic chemicals like arsenic, mercury etc., and sediments can be removed by using nanotechnology. Nanomaterial based devices are being used for water purification. Nano filtration method has advantages over other conventional method as low pressure is required to pass the water through filters and these filters can be cleaned easily by back flushing. Smooth interior of carbon nanotubes make them convenient for the removal of almost all types of water contaminants. Because of larger surface area nanostructured materials have advantages over conventional micro structured materials.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Evaluation of commercial importance of endophytes isolated from Argemone mexicana and Papaver rhoeas
- Innovations and Advances in Environmental Sciences & Sustainable Development
- Published: 07 May 2024
Cite this article

- Pooja Singh 1 ,
- Angkita Sharma 1 ,
- Sahana Mukherjee 1 ,
- Manobjyoti Bordoloi 2 &
- Shoma Paul Nandi ORCID: orcid.org/0000-0003-1416-2425 1
32 Accesses
Explore all metrics
The paper industry is a composite one constituting different types of mills, processes, and products. The paper industries consume large amounts of resources, like wood and water. These industries also create huge amounts of waste that have to be treated. In our study, 23 endophytic bacteria were isolated from Argemone mexicana , and 16 endophytic bacteria were isolated from Papaver rhoeas . Seventeen and 15 bacterial endophytes from A. mexicana and P. rhoeas , respectively, showed cellulose-degrading activity. The biochemical and molecular characterization were done for endophytic bacteria with cellulolytic activity. The consortium of cellulose-degrading endophytic bacteria from A. mexicana showed endoglucanase activity (0.462 IU/ml) and FPCase enzyme activity (0.269 IU/ml) and from P. rhoeas gave endoglucanase activity (0.439 IU/ml) and FPCase enzyme activity (0.253 IU/ml). Degraded carboxy methylcellulose and filter paper were further treated by Saccharomyces cerevisiae and bioethanol was produced. Cellulose-degrading endophytic bacteria were also tested for auxin, siderophore production, and phosphate solubilization activities. Individual cellulose-degrading endophytic bacteria with plant growth-promoting activities were used as biofertilizers, tested for plant growth-promoting activities using Basmati Pusa 1121 rice, and plant growth parameters were recorded. The degraded paper enhances the growth of rice plants. Selected bacterial endophytes and their consortia from A. mexicana and P. rhoeas were powerful cellulose degraders, which can be further employed for ethanol production and as significant biofertilizers in agriculture.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
Microbial enzymes: industrial progress in 21st century.

Recent advances in bacterial cellulose: a low-cost effective production media, optimization strategies and applications

Agricultural Waste Management by Production of Second-Generation Bioethanol from Sugarcane Bagasse Using Indigenous Yeast Strain
Data availability.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Alam A, Khan AA (2020) Argemone mexicana L.: a weed with versatile medicinal and pharmacological applications. Ann Phytomed Int J 9: 218–223. https://doi.org/10.21276/ap.2020.9.1.29
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971. https://doi.org/10.3389/fmicb.2017.00971
Article Google Scholar
Bashir A, Nigar S, Yousaf S (2013) Isolation and identification of cellulose degrading bacteria from municipal waste and their screening for potential antimicrobial activity. World Appl Sci J 27:1420–1426. https://doi.org/10.5829/idosi.wasj.2013.27.11.81162
Article CAS Google Scholar
Behera BC, Parida S, Dutta SK, Thatoi HN (2014) Isolation and Identification of cellulose degrading bacteria from mangrove soil of Mahanadi river delta and their cellulase production ability. Am J Microbiol Res 2(1): 41–46. https://doi.org/10.12691/ajmr-2-1-6
Brahmachari G, Gorai D, Roy R (2013) Argemone mexicana : chemical and pharmacological aspects. Rev Bras Farmacogn 23:559–567. https://doi.org/10.1590/S0102-695X2013005000021
Brown DM, Pawlak J, Grunden AM (2021) Bacterial valorization of pulp and paper industry process streams and waste. Appl Microbiol Biotechnol 105(4):1345–1363. https://doi.org/10.1007/s00253-021-11107-2
Chatli AS, Beri V, Sidhu BS (2008) Isolation and characterisation of phosphate solubilising microorganisms from the cold desert habitat of Salix alba Linn. in trans Himalayan region of Himachal Pradesh. Indian J Microbiol 48(2): 267–273. https://doi.org/10.1007/s12088-008-0037-y
Christian M, Steffens B, Schenck D, Burmester S, Böttger M, Lüthen H (2006) How does auxin enhance cell elongation? Roles of auxin-binding proteins and potassium channels in growth control. Plant Biol (stuttg) 8(3):346–352. https://doi.org/10.1055/s-2006-923965
Compant S, Mitter B, Colli-Mull JG, Gangl H, Sessitsch A (2011) Endophytes of grapevine flowers, berries, and seeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb Ecol 62(1):188–197. https://doi.org/10.1007/s00248-011-9883-y
De Almeida MN, Guimarães VM, Bischoff KM et al (2011) Cellulases and hemicellulases from endophytic Acremonium species and its application on sugarcane bagasse hydrolysis. Appl Biochem Biotechnol 165:594–610. https://doi.org/10.1007/s12010-011-9278-z
De Marco ÉG, Heck K, Martos ET, Van der Sand ST (2017) Purification and characterization of a thermostable alkaline cellulase produced by Bacillus licheniformis 380 isolated from compost. Anais Da Acad Brasil De Ciências 89(3):2359–2370. https://doi.org/10.1590/0001-3765201720170408
Dillon J (2004) Dillon VM (2004) The gut bacteria of insects non pathogenic interaction. Annu Rev Entomol 49:71–92. https://doi.org/10.1146/annurev.ento.49.061802.123416
Eida MF, Nagaoka T, Wasaki J, Kouno K (2012) Isolation and characterization of cellulose-decomposing bacteria inhabiting sawdust and coffee residue composts. Microbes Environ 27(3):226–233. https://doi.org/10.1264/jsme2.ME11299
Fadiji AE, Babalola OO (2020) Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol 8:467. https://doi.org/10.3389/fbioe.2020.00467
Fahim S, Nisar N, Ahmad Z, Asghar Z, Said A, Atif S et al (2019) Managing paper and pulp industry by-product waste utilizing sludge as a bio-fertilizer. Pol J Environ Stud 28(1). https://doi.org/10.15244/pjoes/83614
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Ferbiyanto A, Rusmana I, Rafudin R (2015) Characterization and identifcation of cellulolytic bacteria from gut of worker Macrotermes gilvus . HAYATI J Biosci 22:197–200. https://doi.org/10.1016/j.hjb.2015.07.001
Flimban S, Oh SE, Joo JH et al (2019) Characterization and identification of cellulose-degrading bacteria isolated from a microbial fuel cell reactor. Biotechnol Bioproc E 24:622–631. https://doi.org/10.1007/s12257-019-0089-3
Gaur VK, Sharma P, Sirohi R, Awasthi MK, Dussap CG, Pandey A (2020) Assessing the impact of industrial waste on environment and mitigation strategies: a comprehensive review. J Hazard Mater 398:123019. https://doi.org/10.1016/j.jhazmat.2020.123019
Gopal PM, Sivaram NM, Barik D (2019) Paper industry wastes and energy generation from wastes. In Energy from toxic organic waste for heat and power generation. Woodhead Publishing,pp 83–97. https://doi.org/10.1016/B978-0-08-102528-4.00007-9
Gupta P, Samant K, Sahu A (2012) Isolation of cellulose degrading bacteria and determination of their cellulolytic potential. Int J Appl Microbiol Sci 1(2):13–23. https://doi.org/10.1155/2012/578925
Haile A, Gelebo GG, Tesfaye T, Mengie W, Mebrate MA, Abuhay A, Limeneh DY (2021) Pulp and paper mill wastes: utilizations and prospects for high value-added biomaterials. Bioresour Bioprocess 8(1):1–22. https://doi.org/10.1186/s40643-021-00385-3
Hossain MA, Ahammed MA, Sobuj SI, Shifat SK, Somadder PD (2021) Cellulase producing bacteria isolation, screening and media optimization from local soil sample. Am J Microbiol Res 9(3): 62–74 https://doi.org/10.12691/ajmr-9-3-1
Islam F, Roy N (2018) Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res Notes 11:445. https://doi.org/10.1186/s13104-018-3558-4
Jain D, Sanadhya S, Saheewala H, Maheshwari D, Shukwal A, Singh PB, Meena RH, Choudhary R, Mohanti SR, Singh A (2020) Molecular diversity analysis of plant growth promoting rhizobium isolated from groundnut and evaluation of their field efficacy. Curr Microbiol 77:1550–2155. https://doi.org/10.1007/s00284-020-01963-y
Jain D, Ravina BAA, Chauhan S, Rajpurohit D, Mohanty SR (2021) Polyphasic characterization of plant growth promoting cellulose degrading bacteria isolated from organic manures. Curr Microbiol 78(2):739–748. https://doi.org/10.1007/s00284-020-02342-3
Kaur C, Gupta M, Garai S, Mishra SK, Chauhan PS, Sopory S, Singla-Pareek SL, Adlakha N, Pareek A (2022) Microbial methylglyoxal metabolism contributes towards growth promotion and stress tolerance in plants. Environ Microbiol 24(6):2817–2836. https://doi.org/10.1111/1462-2920.15743
Kour R, Jain D, Bhojiya AA, Sukhwal A, Sanadhya S, Saheewala H, Jat G, Singh A, Mohanty SR (2019) Zinc biosorption, biochemical and molecular characterization of plant growth-promoting zinc-tolerant bacteria. 3 Biotech 9(11): 421 https://doi.org/10.1007/s13205-019-1959-2
Krishnan S, Ahmad MF, Zainuddin NA, Din MFM, Rezania S, Chelliapan S et al (2020) Chapter 9: bioethanol production from lignocellulosic biomass (water hyacinth): a biofuel alternative. Bioreactors-Sustainable Design and Industrial Applications in Mitigation of GHG Emissions. Elsevier, pp 123–143. https://doi.org/10.1016/B978-0-12-821264-6.00009-7
Kumar GS, Chandra MS, Sumanth M, Vishnupriya A, Reddy RB, Choi YL (2009) Cellulolytic enzymes from submerged fermentation of different substrates by newly isolated Bacillus spp. FME J Korean Soc Appl Bi 52:17–21. https://doi.org/10.3839/jksabc.2009.003
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol BiolEvol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lo YC, Saratale GD, Chen WM, Bai MD, Chang JS (2009) Isolation of cellulose-hydrolytic bacteria and applications of the cellulolytic enzymes for cellulosic biohydrogen production. Enzyme Microb Technol 44:417–425. https://doi.org/10.1016/j.enzmictec.2009.03.002
Maki ML, Broere M, Leung KT, Qin W (2011) Characterization of some efficient cellulase producing bacteria isolated from paper mill sludges and organic fertilizers. Int J Biochem Mol Biol 2:146–154
CAS Google Scholar
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual, cold spring harbor (N Y: cold spring harbor laboratory), pp 545. https://doi.org/10.1016/0307-4412(83)90068-7
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
N’dayegamiye A, Nyiraneza J, Giroux M, Grenier M, Drapeau A (2013) Manure and paper mill sludge application effects on potato yield, nitrogen efficiency and disease incidence. Agronomy 3(1):43–58. https://doi.org/10.3390/agronomy3010043
Naik SB, Abrar S, Krishnappa M (2019) Industrially important enzymes from fungal endophytes. Rec Adv White Biotechnol Through Fungi 263–280. https://doi.org/10.1007/978-3-030-10480-1_7
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New YorkF
Book Google Scholar
Ng LC, Sariah M, Sariam O, Radziah O, Abidin MA (2012) Rice seed bacterization for promoting germination and seedling growth under aerobic cultivation system. Aust J Crop Sci 6:170–175
Nunes JR, Cabral F, Lopez-Pineiro A (2008) Short-term effects on soil properties and wheat production from secondary paper sludge application on two Mediterranean agricultural soils. Bioresour Technol 99(11):4935–4942. https://doi.org/10.1016/j.biortech.2007.09.016
Pahari A, Pradhan A, Nayak SK, Mishra BB (2017) Bacterial siderophore as a plant growth promoter. In: Patra J, Vishnuprasad C, Das G (eds) Microbial biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-10-6847-8_7
Patten CL, Glick BR (2002) Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can J Microbiol 48(7):635–642. https://doi.org/10.1139/w02-053
Quaye AK, Volk TA, Hafner S, Leopold DJ, Schirmer C (2011) Impacts of paper sludge and manure on soil and biomass production of willow. Biomass bioenerg 35(7):2796–2806. https://doi.org/10.1016/j.biombioe.2011.03.008
Rastogi G, Muppidi GL, Gurram RN, Adhikari A, Bischoff KM, Hughes SR, Apel WA, Bang SS, Dixon DJ, Sani RK (2009) Isolation and characterization of cellulose-degrading bacteria from the deep subsurface of the Homestake gold mine, Lead, South Dakota, USA. J Ind Microbiol Biotechnol 36(4):585–598. https://doi.org/10.1007/s10295-009-0528-9
Ratnaweera PB, de Silva ED, Williams DE, Andersen RJ (2015) Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC Complem Altern M 15: 220 https://doi.org/10.14288/1.0074648
Rawway M, Ali SG, Badawy AS (2017) Isolation and identification of cellulose degrading bacteria from different sources at Assiut governorate Upper Egypt. J Ecol Health Environ 6(1):15–24. https://doi.org/10.18576/jehe/060103
Sadhu S, Maiti TK (2013) Cellulase production by bacteria: a review. Br Microbiol Res J 3(3):235–258. https://doi.org/10.9734/BMRJ/2013/2367
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Saxena S, Bahadur J, Varma A (1993) Cellulose and hemicellulose degrading bacteria from termite gut and mould soils of India. Indian J Microbiol 33:55–60
Google Scholar
Schulz B, Guske S, Dammann U, Boyle C (1998) Endophyte-host interactions II. Defining symbiosis of the endophyte-host interactions. Symbiosis, Philadelphia. Pa (USA) 25:213–227
Sharma S, Prasad RK, Chatterjee S, Sharma A, Vairale MG, Yadav KK (2019) Characterization of Bacillus species with keratinase and cellulase properties isolated from feather dumping soil and cockroach gut. Proc Natl Acad Sci India Sect B Biol Sci 89(3):1079–1086. https://doi.org/10.1007/s40011-018-1026-5
Sharma A, Singh P, Sarmah BM, Nandi SP (2020) Isolation of cellulose-degrading endophyte from Capsicum chinense and determination of its cellulolytic potential. Biointerface Res Appl Chem 10(6):6964–6973. https://doi.org/10.33263/BRIAC106.69646973
Sim CSF, Chen SH, Ting ASY (2019) Endophytes: emerging tools for the bioremediation of pollutants. In Emerging and eco-friendly approaches for waste management. Springer, Singapore, pp 189–217. https://doi.org/10.1007/978-981-10-8669-4_10
Singh S, Singh TD, Singh VP, Pandey VB (2010) Quaternary alkaloids of Argemone mexicana . Pharm Biol 48(2):158–160. https://doi.org/10.3109/13880200903062622
Singh P, Sharma A, Bordoloi M, Nandi SP (2020) Molecular identification of endophytic fungi isolated from medicinal plant. Biointerface Res Appl Chem 10:6436–6443. https://doi.org/10.33263/BRIAC105.64366443
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43(4):777–780. https://doi.org/10.1128/aem.43.4.777-780.1982
Tran TTA, Le TKP, Mai TP, Nguyen DQ (2019) Bioethanol production from lignocellulosic biomass. In: alcohol fuels-current technologies and future prospect. IntechOpen https://doi.org/10.5772/intechopen.86437
Vimal J, Venu A, Joseph J (2016) Isolation and identification of cellulose degrading bacteria and optimization of the cellulase production. Int J Res Biosciences 5(3):58–67. https://doi.org/10.15680/IJIRSET.2015.0408012
Yousef N, Mawad A, Abeed A (2019) Enhancement the cellulase activity induced by endophytic bacteria using calcium nanoparticles. Curr Microbiol 76(3):346–354. https://doi.org/10.1007/s00284-018-1614-x
Download references
Acknowledgements
The authors acknowledge the intellectual support from Prof. Amithabh Bandopadhyay. PS acknowledges a fellowship from UGC.
The work is financially supported by NE-DBT (Grant No: AGRI/2015/48) of SPN.
Author information
Authors and affiliations.
Amity Institute of Biotechnology, Amity University, Noida, Uttar Pradesh, India
Pooja Singh, Angkita Sharma, Sahana Mukherjee & Shoma Paul Nandi
Department of Chemistry, Cotton University, Guwahati, Assam, India
Manobjyoti Bordoloi
You can also search for this author in PubMed Google Scholar
Contributions
SPN, PS, AS, and MJB conceptualized the problem and designed some of the experiments. PS, AS, and SM designed and performed the experiments, and analyzed the data. The manuscript was written by PS which was further edited and modified by SPN. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Shoma Paul Nandi .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Singh, P., Sharma, A., Mukherjee, S. et al. Evaluation of commercial importance of endophytes isolated from Argemone mexicana and Papaver rhoeas . Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33527-z
Download citation
Received : 22 April 2023
Accepted : 27 April 2024
Published : 07 May 2024
DOI : https://doi.org/10.1007/s11356-024-33527-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Papaveraceae
- Cellulose-degrading bacteria
- Endoglucanase
- Plant growth-promoting bacteria
- Agriculture
- Phytohormones
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 July 2020
Public health benefits of water purification using recycled hemodialyzers in developing countries
- Jochen G. Raimann ORCID: orcid.org/0000-0002-8954-2783 1 , 2 , 3 , 4 ,
- Joseph Marfo Boaheng 4 , 5 ,
- Philipp Narh 4 , 6 ,
- Harrison Matti 4 ,
- Seth Johnson 1 , 4 ,
- Linda Donald 1 , 4 ,
- Hongbin Zhang 7 , 8 ,
- Friedrich Port 1 , 9 &
- Nathan W. Levin 1 , 4
Scientific Reports volume 10 , Article number: 11101 ( 2020 ) Cite this article
9847 Accesses
7 Citations
11 Altmetric
Metrics details
- Pathogenesis
In rural regions with limited resources, the provision of clean water remains challenging. The resulting high incidence of diarrhea can lead to acute kidney injury and death, particularly in the young and the old. Membrane filtration using recycled hemodialyzers allows water purification. This study quantifies the public health effects. Between 02/2018 and 12/2018, 4 villages in rural Ghana were provided with a high-volume membrane filtration device (NuFiltration). Household surveys were collected monthly with approval from Ghana Health Services. Incidence rates of diarrhea for 5-month periods before and after implementation of the device were collected and compared to corresponding rates in 4 neighboring villages not yet equipped. Data of 1,130 villagers over 10 months from the studied communities were studied. Incidence rates showed a decline following the implementation of the device from 0.18 to 0.05 cases per person-month (ppm) compared to the control villages (0.11 to 0.08 ppm). The rate ratio of 0.27 for the study villages is revised to 0.38 when considering the non-significant rate reduction in the control villages. Provision of a repurposed hemodialyzer membrane filtration device markedly improves health outcomes as measured by diarrhea incidence within rural communities.
Similar content being viewed by others

Evidence for long-term efficacy of a membrane filtration device in rural villages in Ghana
A critical review of point-of-use drinking water treatment in the United States

Household illness and associated water and sanitation factors in peri-urban Lusaka, Zambia, 2016–2017
Introduction.
Estimates from the World Health Organization and the World Bank place around 1.1 billion people in the world in a position of having to drink unsafe water. Water and sanitation, specifically access to clean water for the world population, were adopted as the Sustainable Development Goal-6 (SDG-6) by all member states of the United Nations. The deserved, widespread attention emphasizes the importance of the issue and the need for more improvement. Industrialized countries have to a large extent solved the problem and a majority of their populations has access to safe drinking water. This is mainly due to the effort of governments, strict laws, regular monitoring, efficient handling and cleaning of sewage, centralized and monitored provision of clean drinking water and lastly to a generally higher level of hygiene (including the use and provision of sanitary facilities). Due to high population growth rates, lack of economic development, and inadequate political efforts this remains a major problem in many countries with limited resources.
Rural areas in developing countries present problems of greatest magnitude. Water is still mainly carried from continually contaminated surface water such as ponds and rivers. Water is often polluted by coliform bacteria and viral pathogens. Factors such as a lack of sanitary facilities, inadequate hygiene practices and substantial flooding during rainy seasons aggravate the problem. Not only surface but also centralized, processed water are at high probability of being contaminated 1 . Wells may also be susceptible to pollution particularly when they are shallow or intermittently overcome by raising water tables. Further, in some low-income countries a flourishing business of sachet water exists, which is assumed to be safe for consumption. However, as shown in work from Nigeria these sachets are also in many cases contaminated due to improper packaging and storage, or inadequate hygiene in the processing. The incidence of diarrhea and its life threatening complications such as dehydration and acute kidney injury correlate with these factors 2 . Non-infectious contaminants in drinking water such as lead and other heavy metals, arsenic, and also organophosphates from pesticides and insecticides contribute to health hazards, problems that are not addressed with our work at present.
Since the first epidemiological studies by the physician John Snow in the nineteenth century, the deleterious effect of microbial pathogens in water has been well established. Estimates of the World Health Organization suggest that 88% of all diarrheal diseases are caused by the consumption of unsafe drinking water and the lack of adequate sanitation facilities 3 . A recent publication of the initiative has identified that a majority of cases of acute kidney injury in the developing world are (in contrast to the most frequently reported pathogenesis in first world countries) are associated with community-acquired disease and to a major part with diarrhea 4 . This is particularly evident in children 2 to 5 years of age in whom mortality is very high 5 . Overall, these data strongly corroborate why it must be a prime goal for the world community to jointly aim to achieve the SDG-6. These data provide a powerful stimulus for widespread joint action by the world community to achieve this goal.
Common approaches to counteract microbial pollution include various filtration devices: Microfiltration, ultrafiltration, nanofiltration and reverse osmosis. Membrane filtration has long been recognized as an effective and likely efficient approach to partly solve the problem in rural regions, however membranes and filtration devices are expensive, and filters are prone to clogging without proper functioning flushing methodologies. The great need that is also building the basis of the SDG-6 of the United Nations, will require an affordable solution to be made available that is not overly prone to malfunction, can sustain functionality over a long period of time and does not require too extensive maintenance in terms of parts and labor. Surface water is often polluted with parasites, bacteria and viruses that can cause serious health issues 6 . Of note, all these pathogens are larger than the pore size of the hemodialyzer that is approximately 0.003 µm. This pore size notably is smaller than most commercially available purification devices, the operation of which has been claimed to be a feasible technique for water purification 2 .
Hemodialysis is a renal replacement therapy modality that uses hemodialyzers in those suffering from renal failure to counteract the consequences of not having kidney function and to ultimately save them from dying. These hemodialyzers are mainly comprised hollow fibers in a plastic casing. This allows, after cannulation of the patient, to pass the patient’s blood inside the fibers, and along the semipermeable membrane of the fiber, until it leaves the hemodialyzer and is returned to the patient. At the same time, dialysis water, containing anions and cations in specifically defined concentrations, passes, in a countercurrent fashion, on the other side of the membrane resulting in gradient-driven diffusion allowing for toxin removal from the blood and by producing a hydrostatic pressure also removes excess water from the patient through volumetric ultrafiltration. These hemodialyzers were commonly being reused after sterilization, a practice that has changed since earlier days of dialysis and current clinical practice commonly uses hemodialyzers only once and discards them after use. Of note, this alone results in approximately 30 kg of annual waste for every (out of approximately 2 million worldwide) dialysis patient 7 . It was shown recently that used and re-sterilized hemodialyzers (a process possible at less than $2 per hemodialyzer) are effective in producing clean water from microbiologically contaminated water when pushed through these hemodialyzers under high hydrostatic pressure.
We, Easy Water for Everyone (EWfE), report here the experience and some preliminary data from the use of this relatively simple technique for preparation of drinking water from polluted river water in rural villages in Ghana that have no electricity. We provided villages with devices containing re-sterilized hemodialyzers uniquely repurposed from their hemodialysis past, which are capable of producing large volumes of water (up to 500 L/h) free of bacteria and viruses for domestic use. Here we report public health outcomes based on prospectively collected self-reported public health information on diarrhea incidence collected before and after implementation of this device in several villages.
Material and methods
Easy Water for Everyone (EWfE) is a 501(c)(3) non-profit, non-governmental organization (NGO) in the United States, Ghana (and with other countries in progress). With the help of local politicians and stakeholders a need for water purification in the estuary of the Volta River in Ghana was identified. For those living in this region the river is the main source for drinking water even though it is known to carry pathogens. Under the supervision of local committees and administrators, EWfE started to install and maintain a device in each of the villages. The chronological order was arbitrary and data collection was commenced on the islands around Ada Foah since 02/2018.

Water purification method
The membrane filtration device (NUF500; NUFiltration, Israel), consists of a set of 8 hollow-fiber hemodialyzers, appropriate tubings and a faucet. These hollow fiber hemodialyzers in this project have been used as hemodialyzers once, then reprocessed and sterilized according to FDA/AAMI standards before installation into the water-purification device. Each hemodialyzer contains around 12,000 capillaries providing a membrane surface area of nearly 2 square meters per hemodialyzer. The membrane pore size is 0.003 µm, notably preventing passage of bacteria, parasites and notably also of pathogenic viruses. The output of pure water can be as high as 500 L/h when actively pumped into the device or up to 250 L/h passed into the device by gravity after being pumped into an overhead tank as used in this study. The pressure by gravity is caused by a height of about 12 feet from which the polluted water enters the eight dialyzers placed in parallel (see Fig. 1 a, b).
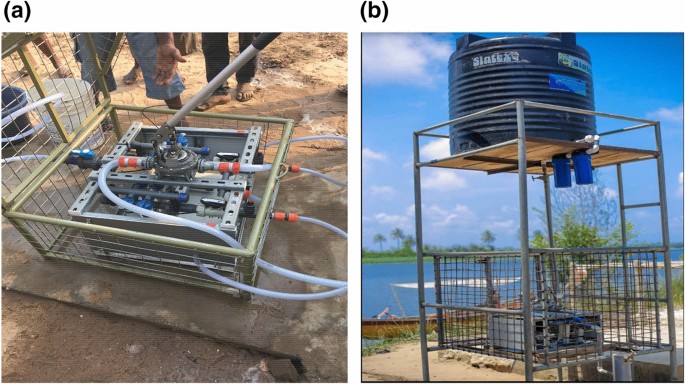
Hemodialyzer membrane filtration device used for our project. Setting with ( a ) a manual pump (up to 500 L/h) and ( b ) gravitational force (up to 250 L/h) for driving the contaminated into the re-sterilized and repurposed hemodialyzer filters.
Contaminated river water enters the inside of the capillaries (“blood” compartment) while clean water collects outside of the capillaries (“dialysate” compartment in clinical hemodialysis). Only water (and dissolved salts) passes through the pores. Organic matter that accumulates on the inside of the capillary fibers needs to be rinsed away by intermittently reversing the pressures and filtering clean water back across the membranes (backwashing) through manual pumping. It takes less than 5 min for the backflow to change from dirty to clean appearance and then regain full efficiency for providing clean water.
Data collection
Following the approval of our research project, embedded in the non-profit endeavor, by Ghana Health Services, we initiated data collection with trained local community members to support our endeavor. Next to demographic data and water results before and after passing through the filter, we collected data monthly from the heads of households on self-reported diarrhea events in 8 villages during the months February through November 2018. This was a subset of villages served by EWfE.
In late June 2018, the hemodialyzer filtration devices became operational in 4 of these villages so that this ongoing monthly data collection started 5 months before the installation. It was concluded 5 months after the installation of the hemodialyzer filtration device. Simultaneously the same data was collected in the 4 villages without the device. For each village and each month, the count of diarrhea events and the number of persons exposed to the data collection were analyzed to estimate the monthly diarrhea incidence rates. Monthly data were summarized for each of the two groups of villages, the control group of 4 villages never exposed to the hemodialyzer water treatment and the group of 4 villages exposed to the water treatment during their second 5 months of the 10-months study period. This approach allowed comparison of the incidence rates during the first and second 5-months periods and incidence rate ratios (second/first 5 months) for the study group and the control group. Having this concomitant data allows us, in a univariate fashion, to use village populations as their own controls and consider the potential confounding effect of seasonality.
The results of water testing showed coliform bacteria at 558 CFU/100 mL in the source water (Volta River) and zero CFU in the filtrate water at the beginning of our installations in the villages of Big Ada. We studied 8 villages (4 were designated control villages and 4 were study villages) in rural Ghana. Table 1 shows the population characteristics of the study arms. Of the village populations studied in this cohort study, 11% and 8% were younger than 5 years of age and notably showed a remarkably high proportion of villagers (96% and 99%) had to resort to open defecation.
Monthly diarrhea incidence rates averaged 0.18 counts per exposure month during the baseline period of the study villages and 0.11 for the same 5 months of the control group. During the first 5 months after the installation of the hemodialyzer filtration device, the rate reduced to 0.05, yielding a rate ratio for the study group of 0.28. For the control group the second 5 months gave an average rate of 0.08, showing modest non-significant reduction from the prior 5 months period with a rate ratio of 0.73 (Table 2 ). Figure 2 a and b show the monthly data for the two periods in both village groups. The control villages of the same region and during the same calendar months allow consideration of a seasonal effect on the diarrhea incidence in the study group. Thus, using the incidence rate ratio for the second 5 months over the first 5 months gives a seasonally adjusted rate ratio of 0.38 (0.28/0.73), which translates to a diarrhea incidence rate that is reduced by 62% following initiation of the hemodialyzer filtration device in the study villages.
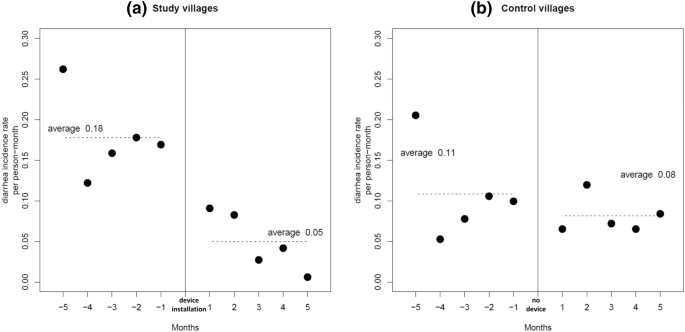
Monthly diarrhea incidence rates between February (Month − 5) and November (Month + 5) 2018 in ( a ) study villages, where the device was installed in late June 2018 and ( b ) control villages with no device installation during the same months.
In many countries microbiologically contaminated water is the underlying cause of gastrointestinal disease, mainly diarrhea, associated with deleterious consequences such as acute kidney injury resulting in a high mortality rate, particularly in weaned children younger than five and the elderly. Our data, collected in 4 rural communities in the Ada-East distric of Greater Accra Region in Ghana, before and after the implementation of a hemodialyzer membrane filtration device to produce clean drinking water, shows a substantially reduced risk (rate) of self-reported diarrhea by 72%. This is a major public health outcome particularly since diarrhea is well known to be associated with deleterious consequences such as acute kidney injury and death, particularly in younger children and the elderly. This finding is striking and the rigorous analytic design where each community serves as their own control allows for drawing solid conclusions. Studying and comparing our data to that of a control group which presented only with modest reduction in the incidence of diarrhea over the same time period, corroborates an effect that can be attributed to implementation of our approach. The only modest reduction of diarrhea incidence in the control villages also reduces concerns of seasonality in the incidence rates confounding our interpretation.
Discussion of our approach in comparison with other approaches
The methods used in the present study have been effective in removing pathogens from consistently polluted river or lake water sources. During the past 3 years the on-site implementation of the hemodialyzer filtration device have allowed us to demonstrate the success of providing clean and pathogen-free drinking water to villages where the source of drinking water had been consistently contaminated. This system works well even in remote areas without requiring electricity or other external power sources. No restrictions on water use need to be imposed and use of clean water can be encouraged also for handwashing with soap. When more water is needed, the filling of the main water tank can be increased from weekly to two to three times a week (or even daily). There are several key elements that contrast our approach to other methods to produce drinking water: (1) Rejection of pathogens is highly effective and includes particles as small as pathogenic viruses, given the pore size of 0.003 µm, (2) no need to add bactericidal agents such as chlorine to kill remaining pathogens in drinking water, (3) the simplicity of this design allows its use in isolated rural villages even in areas that have no electricity, (4) this system becomes almost self-sufficient after a few villagers have been trained to do the thrice daily backwashing, (5) excellent filtration rates have been observed with this setup for over one year, (6) visits by a trained technician once or twice weekly or more frequently when necessary for refilling the large water tanks using a gas-driven pump provide some monitoring of the continued function and service and (7) relatively low cost since the reprocessed hemodialyzers are inexpensive and have shown in our 3-year experience to maintain high output rates of nearly 250 L/h (by gravity feed) for over one year. Furthermore, in circumstances where larger volumes of purified water are needed, an expanded device, employing far more dialyzers could be utilized. It would also be feasible to equip the device with solar panels which would increase water production substantially but would add to the cost.
Comparison of efficacy with other approaches
Attempts to purify water from microbiological contamination have been undertaken in a multitude of studies discussing purification of water from springs, boreholes, and wells, all sources with many opportunities for contamination to occur between sources and point of use. The source water is detoxified and infectious agents are reduced or removed by methods such as chlorination, membrane filtration, flocculation and others. Direct systems include conventional filtration, for example using sand through granular media which removes parasites, bacteria and possibly some viruses. Conventional filtration also includes chemical coagulants such as potassium alum added to source water which produce clots (flocs) which are in turn filtered. These processes are not easy and require expert handling by trained individuals.
Quite commonly reported is household chlorination which is a simple technique with widespread use. It improves water quality and effectively prevent diarrheal diseases. Quantity and acceptance (because of the resultant taste of the water) are downsides of this approach 5 .
With direct filtration, water passes through a medium such as sand or diatomaceous earth, a process which removes giardia lamblia, cryptosporidia, and bacteria from the water. These methods also remove color and turbidity. Filtration bags are warm bags or cartridges containing a filament to strain the water. These bags are however not useful for anything smaller than the giardia. Ceramics may be impregnated with tiny colloidal particles and allows for eradication of most bacteria and protozoan parasites. However, also this method is not adequate for virus removal. Most of these methods however are laborious, require specialized knowledge and infrastructure, and also time.
Membranes are widely used to produce safe drinking water and are the only means available to produce water free of parasites, bacteria and all pathogenic viruses.
Membranes can be divided into groups largely defined by their characteristics in regard to pore sizes. Depending on the degree of pore size, they can also produce water free of many chemical components. In the case of biologically contaminated water some membranes can produce water free of bacteria, parasites and viruses.
Hemodialyzers that are contained in the device we have chosen to implement in village structures have a semi-permeable membrane made of polysulphone and polyethersulphone. The pore size is around 0.003 µm and will not let parasites, bacteria and viruses pass, while still providing an output as large as 500 L/h.
Decreased microbial quantity in drinking water is effective in decreasing diarrhea. Effectiveness does not solely depend on the presence of improved water supplies but will also be affected by the use of sanitization facilities and handwashing with diligent soap procedures. In concert with appropriate education, these interventions will play a powerful role in improving public health outcomes. Also important in the context of effectiveness is the amount of water that is being produced over a defined period of time. In this context it is of note that our approach, even with the use of the gravitational device where water is pumped into an overhead tank and gravitation is being used to transfer contaminated water into the filter, allows for up to 250 L/h.
Household efforts
Household efforts include: improved water storage, chlorination, solar exposure, filtration by filter media in relationship to pore size, combined flocculation and disinfection methods. A combination of efforts including improved water supply and storage, and improved sanitation results in better water supplies thus reducing the risk of developing diarrhea. Various authors provide a range of figures for reduction of diarrhea but overall it is expected that household interventions will provide a risk reduction for diarrhea incidence 8 . The WHO promotes water treatment and safe storage of household water. Affordability, acceptability, sustainability and scale ability are all important factors and these small-scale solutions do provide improvement.
A current technology comparable to our approach are the “Aqua Towers”, an approach that also uses gravitational forces to pass water through the filter. More than 1,000 of these are active in Asia Pacific and Latin America. It utilizes ultrafiltration but the manufacturer does not reveal the membrane type. Activated carbon is used to enhance the quality of the drinking water. In addition, part of the water supply is used for hand washing. The authors claim that viruses larger than 0.01 microns are removed. However, a membrane with pore sizes as large will not exclude the rotavirus (a causative pathogen of diarrhea in up to 40% in some reported populations), and hepatitis B and C viruses, unlike the hollow fiber hemodialyzer membrane as discussed above. Of note, no outcome data have been published for the communities using the “Aqua Towers”, to the best of our knowledge.
Strengths and limitations of our study
Surveys of diarrhea in households may be considered soft data, however the magnitude of a relative 72% reduction in the incidence of diarrhea per monitored population is strikingly large. It is also corroborated by many mothers reporting a sudden virtual absence of diarrhea in their children after availability of the hemodialyzer-filtered water. The marked reduction in the diarrhea incidence may be due to using sterile water instead of river water polluted with known pathogens, such as E. coli , as the main source of drinking water. Additionally, handwashing with clean water may be an important contributor to our observations. While our study cannot prove causation with certainty, the nearly stable rates in the control group suggests a causative role of the change in the water source from river water to filter-sterilized water.
Of note, we decided to not adjust for population characteristics for two reasons: the same population served as their own controls for each household and the groups of villages and secondly the incidence rates during the initial 5 months were similar for the two groups of villages.
Further considerations beyond water purification
The effectiveness of pure drinking water, sanitation and hygiene by the Campbell/Cochrane collaboration showed 66 rigorous evaluations and 71 interventions (accounting for 30,000 children in 35 countries). Point of use water quality was associated with positive outcomes and so did hand-washing with soap. The Cochrane data base of systemic reviews discussed the effect of hand washing promotion for preventing diarrhea induced nutritional deficiency 9 , retarded child development 10 and deaths in low- and middle-income countries. The list of interventions to improve water quality by eliminating or reducing pathogens with the objective of preventing diarrhea is substantial.
Our results on markedly reduced incidence of diarrhea after implementation of the hemodialyzer filtration device agree with prior studies. In Clasen’s data synthesis paper 11 on 42 studies in 21 countries showed that all interventions to improve the microbial quality of drinking water were effective in reducing diarrheal incidents even though variations in design and application of water cleansing systems limit comparability of their cited studies. Results are less consistent for the role of other common environmental interventions (such as sanitation, or instruction in hygiene) 12 .
Our study using monthly surveys of diarrhea in households may be considered soft data, however the magnitude of a relative 72% reduction in the incidence of diarrhea per monitored population is strikingly large. It is also corroborated by many mothers reporting spontaneously a sudden virtual absence of diarrhea in their children after availability of the dialyzer-filtered water. The marked reduction in the diarrhea incidence is likely due to using sterile water instead of using river water polluted with known pathogens, such as E. coli , as the main source of drinking water. It may be expected that combination of installing a membrane filtration device and combining it with WASH initiatives will have a strong amplified effect as compared to clean water provision alone. This however remains to be shown in further prospective research.
The hemodialyzer membrane filtration device used in this study was clearly associated with a substantial reduction in the incidence of self-reported diarrhea compared to the prior period and compared to a control group without the device. Use of repurposed hemodialyzers, that had already saved lives once in their initial purpose in renal replacement therapy, can again serve as an affordable means of water purification to again save lives within entire communities. Our hemodialyzer membrane filtration approach using hollow fibers with pore size as tight as 0.003 µm in the a surface-maximizing configuration used in the technology of the device described in this paper is highly effective and unique. This renders it not only eligible but potentially highly effective to allow the world population to successfully accomplish the United Nations’ Sustainable Development Goal 6.
Kuberan, A. et al. Water and sanitation hygiene knowledge, attitude, and practices among household members living in rural setting of India. J. Nat. Sci. Biol. Med. 6 , S69-74. https://doi.org/10.4103/0976-9668.166090 (2015).
Article PubMed PubMed Central Google Scholar
Peter-Varbanets, M., Zurbrugg, C., Swartz, C. & Pronk, W. Decentralized systems for potable water and the potential of membrane technology. Water Res. 43 , 245–265. https://doi.org/10.1016/j.watres.2008.10.030 (2009).
Article CAS PubMed Google Scholar
Cerda, J. et al. Epidemiology of acute kidney injury. Clin. J. Am. Soc. Nephrol. 3 , 881–886. https://doi.org/10.2215/CJN.04961107 (2008).
Article PubMed Google Scholar
Mehta, R. L. et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet 387 , 2017–2025. https://doi.org/10.1016/S0140-6736(16)30240-9 (2016).
Collaborators, G. B. D. L. R. I. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. Infect. Dis. 18 , 1191–1210. https://doi.org/10.1016/S1473-3099(18)30310-4 (2018).
Article Google Scholar
Piper, J. D. et al. Water, sanitation and hygiene (WASH) interventions: effects on child development in low- and middle-income countries. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD012613 (2017).
McAllister, D. A. et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob. Health 7 , e47–e57. https://doi.org/10.1016/S2214-109X(18)30408-X (2019).
Patrier, L. et al. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional high flux hemodialysis. J. Nephrol. 26 , 342–349. https://doi.org/10.5301/jn.5000150 (2013).
Abdoli, A. & Maspi, N. Commentary: estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the global burden of disease study 2015. Front. Med. 5 , 11. https://doi.org/10.3389/fmed.2018.00011 (2018).
Collaborators, G. L. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. Infect. Dis. 17 , 1133–1161. https://doi.org/10.1016/S1473-3099(17)30396-1 (2017).
Canaud, B., Koehler, K., Bowry, S. & Stuard, S. What is the optimal target convective volume in on-line hemodiafiltration therapy?. Contrib. Nephrol. 189 , 9–16. https://doi.org/10.1159/000450634 (2017).
Bowry, S. K. & Canaud, B. Achieving high convective volumes in on-line hemodiafiltration. Blood Purif. 35 (Suppl 1), 23–28. https://doi.org/10.1159/000346379 (2013).
Download references
Acknowledgments
First and foremost, we would like to thank those who have made this study possible by their generous donations. We further would like to thank all those that supported our work and helped us to get to the point we currently are. Last but certainly not least we would like to thank the village committees and everybody in the studied villages (Adzakeh, Agamakope, Alewusedekope, Amekutsekope, Anazome, Azizakope, Baitlenya and Tornyikope).
Author information
Authors and affiliations.
Easy Water for Everyone, 10 Bank Street, Suite 560, White Plains, NY, 10606, USA
Jochen G. Raimann, Seth Johnson, Linda Donald, Friedrich Port & Nathan W. Levin
Research Division, Renal Research Institute, New York, USA
- Jochen G. Raimann
Katz School at Yeshiva University, New York, USA
Easy Water for Everyone, Accra, Ghana
Jochen G. Raimann, Joseph Marfo Boaheng, Philipp Narh, Harrison Matti, Seth Johnson, Linda Donald & Nathan W. Levin
Department of Field Epidemiology and Applied Biostatistics, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
- Joseph Marfo Boaheng
Ghana Health Services, Big Ada, Ghana
Philipp Narh
Department of Epidemiology and Biostatistics, CUNY Graduate School of Public Health and Health Policy, City University of New York, New York, USA
Hongbin Zhang
CUNY Institute for Implementation Science in Population Health, New York, USA
Departments of Medicine (Nephrology) and Epidemiology, University of Michigan, Ann Arbor, MI, USA
Friedrich Port
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: J. R., S. J., L. D. and N. L.; Data curation: J. R., J. M. B., P. N. and F. P.; Formal analysis: J. R., J. M. B., H. Z. and F. P.; Funding acquisition: L. D. and N. L.; Investigation: J. R., J. M. B., H. Z., F. P. and N. L.; Methodology: J. R., J. M. B., H. Z., F. P. and N. L.; Project administration: P. N., L. D. and N. L.; Resources: J. R., P. N., S. J., H. Z. and N. L.; Software: J. R. and J. M. B.; Supervision: N. L.; Validation: J. R., H. Z., F. P. and N. L.; Visualization: J. R., J. M. B. and F. P.; Writing—original draft: J. R., F. P. and N. L.; Writing—review & editing, J. R., J. M. B., P. N., S. J., L. D., H. Z., F. P. and N. L.
Corresponding author
Correspondence to Jochen G. Raimann .
Ethics declarations
Competing interests.
Jochen Raimann and Seth Johnson are employees of Renal Research Institute/Fresenius Medical Care. All other authors have no financial disclosure.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Raimann, J.G., Boaheng, J.M., Narh, P. et al. Public health benefits of water purification using recycled hemodialyzers in developing countries. Sci Rep 10 , 11101 (2020). https://doi.org/10.1038/s41598-020-68408-1
Download citation
Received : 18 October 2019
Accepted : 30 April 2020
Published : 06 July 2020
DOI : https://doi.org/10.1038/s41598-020-68408-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Nathan W. Levin
Scientific Reports (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Research Note
- Open access
- Published: 09 May 2024
Sub-microscopic Plasmodium falciparum infections and multiple drug resistant single nucleotide polymorphic alleles in pregnant women from southwestern Nigeria
- Agatha N. Ibekpobaoku 1 ,
- Mary A. Oboh 2 , 3 , 4 ,
- Fatou Faal 2 ,
- Elizabeth Adeniji 2 ,
- Olusola Ajibaye 5 ,
- Emmanuel T. Idowu 1 &
- Alfred Amambua-Ngwa 2
BMC Research Notes volume 17 , Article number: 129 ( 2024 ) Cite this article
Metrics details
The study evaluated sub-microscopic malaria infections in pregnancy using two malaria Rapid Diagnostic Tests (mRDTs), microscopy and RT-PCR and characterized Plasmodium falciparum dihydrofolate reductase ( Pf dhfr) and Plasmodium falciparum dihydropteroate synthase ( Pf dhps) drug resistant markers in positive samples.
This was a cross sectional survey of 121 pregnant women. Participants were finger pricked, blood drops were collected for rapid diagnosis with P. falciparum histidine-rich protein 11 rapid diagnostic test kit and the ultra-sensitive Alere Pf malaria RDT, Blood smears for microscopy and dried blood spots on Whatman filter paper for molecular analysis were made. Real time PCR targeting the var acidic terminal sequence (varATS) gene of P. falciparum was carried out on a CFX 96 real time system thermocycler (BioRad) in discriminating malaria infections. For each run, laboratory strain of P. falciparum 3D7 and nuclease free water were used as positive and negative controls respectively. Additionally, High resolution melt analyses was employed for genotyping of the different drug resistance markers.
Out of one hundred and twenty-one pregnant women sampled, the SD Bioline™ Malaria Ag P.f HRP2-based malaria rapid diagnostic test (mRDT) detected eight (0.06%) cases, the ultra-sensitive Alere™ malaria Ag P.f rapid diagnostic test mRDT had similar outcome in the same samples as detected by the HRP2-based mRDT. Microscopy and RT-PCR confirmed four out of the eight infections detected by both rapid diagnostic tests as true positive and RT-PCR further detected three false negative samples by the two mRDTs providing a sub-microscopic malaria prevalence of 3.3%. Single nucleotide polymorphism in Pf dhps gene associated with sulphadoxine resistance revealed the presence of S613 mutant genotypes in three of the seven positive isolates and isolates with mixed wild/mutant genotype at codon A613S. Furthermore, four mixed genotypes at the A581G codon were also recorded while the other Pf dhps codons (A436G, A437G and K540E) showed the presence of wild type alleles. In the Pf dhfr gene, there were mutations in 28.6%, 28.6%, and 85.7% at the I51, R59 and N108 codons respectively. Mixed wild and mutant type genotypes were also observed in 28.6% each of the N51I, and C59R codons. For the Pf crt, two haplotypes CVMNK and CVIET were observed. The SVMNT was altogether absent. Triple mutant CVIET 1(14.3%) and triple mutant + wild genotype CVIET + CVMNK 1(14.3%) were observed. The Pf mdr1 haplotypes were single mutants Y YND 1(14.3%); N F ND 1(14.3%) and double mutants YF ND 4(57.1%); Y Y D D 1(14.3%).
Peer Review reports
Introduction
Nigeria contributes about 27% to the global malaria burden and 24% to malaria death globally [ 1 ]. Of the 33.2 million pregnant women in 2019, 35% (11.8 million) were exposed to malaria infection in 33 moderate to high transmission countries located in the World Health Organisation (WHO) Africa Region [ 1 ]. Pregnant women are among the high risk groups vulnerable to malaria infection due to their temporarily compromised immune system [ 2 ]. Pregnancy associated malaria (PAM) is a frequent occurrence in women living in malaria endemic countries especially in tropical and sub-tropical regions of the world such as Africa [ 3 , 4 ]. PAM is defined as the detection of asexual parasite stages in peripheral or sequestered blood cells in the placenta of a pregnant woman [ 3 ]. Plasmodium falciparum is the most prevalent and implicated species in PAM and causes complications both in the mother and the foetus [ 5 , 6 ]., sometimes leading to maternal anaemia, spontaneous abortion, stillbirths, premature and low birth weight [ 3 , 6 , 7 , 8 , 9 , 10 ]. As a result of the presence of sub-microscopic infections in pregnant women, detection of malaria infections requires high sensitive diagnostic tools that can detect low parasite density [ 11 ]. Traditionally, microscopy is the gold standard, but due to the various challenges (inadequate training/experienced of microscopy readers, deficiency in personnels, sub-standard or inadequate equipment, lack of power supply etc.) that surrounds it, [ 12 , 13 ] rapid diagnostic tool such as P. falciparum histidine rich protein II (HRP-2) malaria RDTs are deployed in many endemic areas where microscopy is unavailable. It requires less training, cost effective and produces timely result [ 14 , 15 , 16 ]. However, the circulation of parasite with deleted PfHRP2 gene makes parasite detection with mRDT difficult [ 17 , 18 ] Therefore, more sensitive mRDT would be effective in providing timely results [ 19 – 20 ] WHO treatment policy for uncomplicated malaria in the general population is the use of artemisinin based combination therapy while in pregnant women, at least 2 doses of Intermittent Preventive Treatment with sulfadoxine-pyrimethamine (IPTp-SP) after quickening. In sub-Saharan Africa, IPTp-SP has been shown to reduce adverse infant and maternal outcomes such as maternal anaemia, low birth weight, placental malaria and perinatal mortality [ 21 , 22 ], and neonatal mortality [ 23 ]. The implementation and effectiveness of this approach has been largely riddled by the development of P. falciparum resistance to SP [ 24 , 25 ]. Single nucleotide polymorphisms in the P. falciparum dihydrofolate reductase ( Pfdhfr ) and the P. falciparum dihydropteroate synthase ( Pfdhps ) genes have been associated with resistance to pyrimethamine and sulfadoxine respectively. Substitutions at the Pfdhfr 51, 59, 108, 164, and Pfdhps 437, 540, 581 and 613 codons have been evaluated and detected in various populations in different endemic settings [ 26 , 27 ]. Mutations at the P. falciparum multi-drug resistant gene 1 Pfmdr1 ) has been associated with various artemisinin partner drugs. For instance, substitutions in codons N86, 184 F and D1246 of Pfmdr1 have been associated with resistance to lumefantrine and amodiaquine while the Pfmdr1 86Y allele has been strongly linked with chloroquine and amodiaquine resistance [ 44 , 46 ]. Moreover, 1246Y alleles have also been shown to increase P. falciparum susceptibility to mefloquine, halofantrine and artemisinin [ 45 ]. While the resistance of P. falciparum kelch 13 propeller domain was uncommon, there were fixation in the prevalence of Pfcrt at codons 74–76 and 86Y and high prevalence of Pfdhfr and Pfdhps among Nigeria populations [ 47 ]. The NFD haplotype (86 N-184 F– 1246D) have been reported to significantly associate with treatment failure among children under five [ 26 ] in Nigeria. However, there is paucity of data of these resistant associated mutations in pregnant women from Lagos State, Nigeria. Therefore, this study was designed to i) evaluate sub-microscopic malaria infections in pregnant women using two mRDTs, microscopy and RT-PCR; ii). Characterize Pfdfr , Pfdhps , Pfcrt and Pfmdr1 drug resistant markers in positive isolates.
Materials and methods
Study design, sites and participants.
This was a cross-sectional study that involved pregnant women attending antenatal clinic in Epe and Ogudu Primary Health Centres, in Lagos State. The study sites are located in the south– western part of Nigeria and have high malaria transmission. The entomological inoculation rate as reported from a previous study [ 28 ] is 8.4 infective bites per person per month (ib/p/m) by human bait and 5.45 ib/p/m by pyrethrum sprays catch. The time frame for the collection of the pregnant women samples was from November, 2019 to March, 2021.
Written and verbal consents were obtained from all the participating pregnant women before the commencement of the study. Only consenting pregnant women with no pregnancy-related complications were enrolled into the study. Pregnant women not meeting study criteria were excluded.
After sensitisation and consenting, one hundred and twenty- one pregnant women of were recruited into the study.
Blood collection
Participants were finger -pricked and blood drops collected for rapid diagnosis with P. falciparum histidine-rich protein II rapid diagnostic test kit and the ultra-sensitive Alere Pf malaria RDT, blood smears for microscopy and dried blood spots (DBS) on Whatman filter paper for molecular analysis were also collected. DBS were air-dried and kept in sealed plastic bags containing desiccants until use.
For pregnant women that tested positive, the attention of their attending obstetrician and gynecologist were brought to the situation and care thereafter were outside the purview of the study.
DNA extraction and real time PCR (RT-PCR) for malaria molecular diagnosis
Genomic DNA of all samples was extracted from three punches of 3 mm dried blood spot using the QIAamp DNA Blood Mini Kit (Qiagen®, Hilden, Germany), eluted in a 100 µL final volume and stored at − 20 °C until use.
To accurately detect the presence of malaria parasites from these gDNA samples, RT-PCR targeting the var acidic terminal sequence ( va rATS) gene of P. falciparum was carried out as detailed elsewhere [ 29 , 30 ]. Briefly, 5 µL of template gDNA was added to a master mix containing 1 µL of nuclease-free water, 10 µL of 2x Taqman Universal PCR Mastermix (Applied Biosystems, New Jersey, USA), 1.6 µL of 10 µM forward and reverse primer each and 0.8 µL of 10 µM probe. The master mix together with the template gDNA was run on a CFX 96 real-time system thermocycler (BioRad). For each run, laboratory strain of P. falciparum 3D7 and nuclease free water was used as positive and negative controls respectively.
High Resolution Melting (HRM) for P. Falciparum drug genotyping
Evaluation of single nucleotide polymorphism associated with resistance to pyrimethamine Pfdhfr (codons 51, 59, 108,164), sulphadoxine - Pfdhps (codons 436, 437, 540, 581, 613), artemisinin-partner drug such as lumefantrine and amodiaquine - Pfmdr (86, 184, 1042, 1246), and chloroquine Pfcrt (72–76) were done using the Qiagen TypeIT master mix. First, the primers were reconstituted and diluted to 10X, from this, 0.7µM and 1X of Qiagen master mix, 3.3 Μl of nuclease free water and 1 µL of gDNA (Supplementary Table 1 ) was used for the HRM drug assay as described earlier [ 31 ] For each drug target, mutant and wild type strains of laboratory cultured adapted parasites and nuclease free water were used as controls.
Statistical analysis
Data was entered in excel and exported to Statistical Package for Social Sciences (SPSS) version 21.0 (SPSS, Inc. Chicago IL, USA) for analyses. The performance of each diagnostic tool was evaluated as per their sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with RT- PCR as the gold standard. Agreement between pairs of evaluation tools was tested based on Cohen Kappa’s statistics where ≤ 0 indicates no agreement, 0.01–0.40 - slight to an average agreement, 0.41–0.80– moderate-stable agreement, and 0.81-1.00 perfect agreement [ 48 ].
Results of the drug resistant assay were scored using the Light-cycler software supplied with the machine after adjusting the sliding window to the appropriate melt curve. Samples with the same curve profiles with either the mutant or wild type controls were scored accordingly (wild or mutant or mixed in cases where it has the curves of both strain).
Further, amino acid mutation of the single nucleotide polymorphisms at each codon of each target molecular marker ( Pfcrt , Pfdhfr , Pfdhps and Pfmdr1 ) were used in constructing the different haplotype per specific gene.
Participants background information and parasite diagnosis
Majority of the pregnant women sampled was within the age brackets 30–34 (33.1%) and 25–29(33.1%). This was followed by the age brackets 20–24(21.5%) and 35–39(10.7%). Only one participant each falls within the age brackets 15–19 (0.8%) and 40–44 (0.8%). There was more Multigravida − 86(71.1%) than Primigravida– 35(28.9%) women in the study. In terms of pregnancy terms, the highest participants were on their second trimester (51.2%) followed by the third trimester (48.0%), while the least were the first trimester (0.8%). With regards to the use of IPTp with sulphadoxine pyremethamine, surprisingly, majority (59.5%) of them did not receive the preventive drug prior to the day that they were enrolled in the study, and only 24 (19.8%) of them had taken one dose, 18(14.9%) had taken two doses, while only 7(5.8%) of them had used three doses (Table 1 below).
One hundred and twenty-one (52 from Kosofe and 69 from Epe) pregnant women were recruited into this study. Of this, rapid diagnosis with P. falciparum HRP2-mRDT detected malaria infections in eight (0.06%) of them, the ultra-sensitive Alere™ Pf malaria RDT also gave similar outcome in the same samples as detected by the mRDT (Fig. 1 ). In contrast, microscopy and RT-PCR confirmed only four of the eight infections detected by both rapid diagnostic tests to be true positive and RT-PCR detected additional three samples not shown to be positive by either of the mRDT. Thus, employing RT-PCR as the comparator (gold standard), PfHRP2 and uRDT both showed high specificity (96.9% ), but their sensitivities were low (Table 2 ). However, the sensitivity and specificity of microscopy with regards to RT-PCR was almost perfect (96.69% and 96.58% respectively). For all further drug genotyping assay, only the seven samples positive by both microscopy and RT-PCR were included.
Showing the observed number of positives and negatives across the four diagnostic tools
Drug resistant genotypes of Pfdhps and Pfdhfr from the study participants
Single nucleotide polymorphism in Pfdhps gene associated with sulphadoxine resistant revealed the presence of S 613 mutant genotypes in three of the seven positive isolates and isolates with mixed wild/mutant genotype (A613S) at this codon. In addition, four mixed genotypes at the A581G codon was also recorded while the other Pfdhps codons showed the presence of wild type alleles.
In the Pfdhfr gene associated with pyrimethamine resistant, we observed mutations in 28.6%, 28.6%, 85.7% at the I 51 , R 59 and N 108 codons respectively. Mixed wild and mutant type genotypes were also observed in 28.6% at each of the N51I, and C59R codons respectively (Fig. 2 ).
Bar chart of frequencies of wild, mutant and mixed alleleic infections in Pfdhps and Pfdhfr gene
Profiles of malaria resistant markers of Pfmdr1 and pfcrt from the study participants
The P. falciparum multi-drug resistant gene 1 which has been associated with reduced parasite tolerance to amodiaquine and lumefantrine was also genotyped. Of the seven samples assayed, 85.7%, 71.4% and 14.3% harboured parasites that are resistant at the 86Y, 184 F and 1042D codons respectively. All parasites were of the wild type allele for the D1246 codon (Fig. 3 ). The Pfcrt mutant haplotype (CVIET) was observed in 14.3% of the isolates and a mixed mutant/wild genotype (14.3%) at these codons (72–76) was also observed (Table 3 ).
Bar chart showing frequency of wild, mutant and mixed allele infections in Pfmdr1 and Pfcrt
Haplotypic frequency and prevalence of the different drug resistant genes
Parasites with the Pfdhps single mutant SAKA S (with mutation at the 613 codon) were the most common occurring in 42.9% of the isolates while the single mutant Pfdhfr NC N I was the most common observed in these pregnant women, followed by the triple mutant IRN L (28.6%). In the Pfmdr1 gene, double mutant ( YF ND) was the most prevalent (57.1%) while the other single mutants Y YND, N F ND and Y Y D D occurred in similar proportion (14.3%). The CVIET triple codon mutation and the triple mutant + wild type has similar proportion (14.3%) (Table 3 above).
Malaria in pregnancy remains a public health challenge especially in malaria endemic areas such as Nigeria, and as a result of the growing evolution of malaria drug resistance, effective treatment of pregnant women with malaria is now more than ever threatened. The study evaluated four malaria diagnostic tools among pregnant women suspected of malaria infections and characterized Pfdhfr, Pfdhps , Pfmdr1 , and Pfcrt drug resistant markers in malaria positive isolates. One hundred and twenty-one women were enrolled into this study and majority of them were in the age brackets 20–39 years. In addition, majority of the women had had multiple pregnancies before (multigravida), and unfortunately many of the recruited women had not taken IPTp-SP which poses a disturbing scenario, as this will result in the continuous transmission of P. falciparum with detrimental outcome to both the pregnant woman and the foetus. The study showed concordant results for both the Pf HRP2 and the ultra-sensitive Alere mRDTs in terms of sensitivity and specificity. This is similar to the findings of Unwin et al., 2020 [ 19 ]. Both of them showed high specificity and low sensitivity. In contrary, Briand et al., 2020 [ 32 ] reported that the ultra-sensitive rapid diagnostic test - uRDT specificity was slightly lower than that for conventional mRDT among Beninese pregnant women. The sensitivity from their study was high particularly among those in their first trimester, the multigravidae and asymptomatic. In addition, a Colombian study by Vasquez et al., 2018 also demonstrated a non-significant higher sensitivity of uRDT than Standard Bioline (sdRDT) [ 33 ]. Differences in transmission dynamics, endemicity and parasite density in these study areas could have been responsible for the variation in the performance of the mRDT tools.
Our microscopy and real-time PCR (RT-PCR) confirmed only four out of the eight infections detected by both mRDTs to be truly positive and further detected three samples also classified as negative to be positive for falciparium malaria. Misclassification of results as false negative by any of these mRDTs has serious implications to maternal and child health on one hand, and continuous malaria transmission on the other hand. The importance of diagnosis cannot be overemphasized as it is a prerequisite for treatment. Therefore, in order not to miss submicroscopic infections in pregnant women and avoid the deleterious effects of PAM, more sensitive tools should be employed. uRDT has been considered to be better in terms of sensitivity and specificity, however varying low sensitive and specificity results are presented from different studies and so there is need for re-evaluation of the efficiency of the uRDT. In addition to this it is very expensive when compared with the conventional mRDT.
Drug resistant typing was carried out on codons A613S, A581G, G436S, G437 and K540E for Pfdhps gene; N51,I C59R S108N, and I164L for Pfdhfr gene; 72–76 for Pfcrt and codons N86Y, Y184F, N1042D and D1246Y for Pfmdr 1. Haplotypic distribution and prevalence of the different drug resistant genes were also estimated. Single nucleotide polymorphism (SNP) data from our study showed high prevalence of single mutant N108Y (57.1%), triple mutant N51I, C59R, S108N (28.6%) Pfdhfr alleles and single mutant A613S/T (42.9%) Pfdhps allele.
The single mutant haplotype- SAKA S in Pfdhps was the most prevalent haplotype (42.9%) while single mutation (NC N I) in Pfdhfr also was the most prevalent haplotype (57.1%) from our study. Our finding is dissimilar to that of Lucchi et al., 2015 [ 34 ] which noted that prevalence of these haplotypes in West Africa are generally high. Although, the prevalence observed here is low compared to a previous study conducted elsewhere in Nigeria; [ 26 ] in Equatorial Guinea [ 35 ] and in Democratic Republic of Congo [ 36 ]. This could be due to the small number of sample size used for the drug resistant assay in the present study. However, lower prevalence (26.5– 56.25%) has also been reported in other West African countries such as in Senegal [ 29 ] and in Bukina Faso [ 37 ]. In our study, wild type alleles were recorded for S436, A437 and K540 of Pfdhps and I164 of the Pfdhfr. Mixed wild and mutant alleles were recorded for A581G of the Pfdhps as well. As per the WHO recommendation [ 38 ]for the discontinuation of IPT-SP in areas where K540E mutation prevalence is > 95% and A581G > 10% constant surveillance should be taken seriously in the study areas to be able to identify and track any change in the distribution of mutation that would inform policy on the IPT-SP use.
In our study, polygenomic infection was observed in A581G, and A613S/T of the Pfdhps alleles and N51I, C59R and S108N of the Pfdhfr alleles. The mutations in these two ( Pfdhfr and Pfdhps ) alleles were high. This is in agreement with the findings of Adegbola et al., 2023 [ 47 ] which reported a relatively high prevalence of SNPs from both Pfdhps and Pfdhfr genes. They also reported a relatively high prevalence of SNPs from both Pfdhps and Pfdhfr genes. They also reported that sextuple and septuple contributed to about 25.0% of the P. falciparum isolates in their study and opined that their presence might pose a high probability of the malaria parasite becoming extensively resistant to SP in Nigeria.
Quadruple[ [ 26 , 27 ] (reported earlier in Nigeria), quintuple and sextuple [ 31 , 40 , 41 ](reported in South and East Africa) mutant haplotypes which had been linked with both in vivo and in vitro SP resistance were not observed in our study [ 39 , 40 , 41 ].
For the Pfmdr1 , out of the seven samples assayed, 85.7%, 71.4% and 14.3% harboured parasites with resistant alleles at the 86Y, 184 F and 1042D codons respectively. This is similar to the findings of Issa et al., 2022 and Adamu et al., 2020 [ 41 , 42 ] where Pfmdr1 184 F and 86Y predominated in their studies in Niger Republic and Northern part of Nigeria respectively. The high prevalence of Pfmdr1 86Y alleles has been associated with chloroquine resistance. This might be an indication of the risk in the efficacy of Arthemeter Lumefanterine (AL). Prevalence of Pfmdr1 N86 allele seen in our study might be suggestive of possible AL pressure in the population. The Pfmdr1 double mutant 86Y/184F was found in 57.1% of our study. This is in line with the findings of Issa et al., 2022 and Tuedom et al., 2021 [ 42 , 43 ]. While the S1034C and D1246Y mutations were detected in our study, they were not found in the study by Issa et al., 2022 [ 42 ]. For the Pfcrt , it has been established that the CVIET haplotype is widely prevalent in Nigeria from various studies [ 44 , 45 , 46 ]. Our study reported 14.3% CVIET haplotype distribution which is lower than what Issa et al., 2022 reported [ 42 ]. They reported a higher 33.07% isolates from their study that harboured the CVIET mutant haplotype.
Although, this study is limited in the number of sample size included in the study and the geographical spread of the samples, however, it emphasize the importance of the use of high sensitive tools for the diagnosis of malaria especially because of the submicroscopic infections that might not be detected with less sensitive tools. Relying solely on the outcome of RDT should be done with caution since misclassification of results as false negative by the mRDTs is possible as evident in this study. This has implication to the maternal, neonatal and child health and ultimately will perpetuate the continuous transmission of malaria. The presence of polygenomic infection which is indicative of high parasite recombination events and the possibility of spread of mutant parasite strains due to high transmission has the potential of jeopardizing the use of SP- IPT P in the study area. Therefore, continuous monitoring should be done so as to identify presence of mutation and take appropriate and prompt action especially considering the fact that SP is the only available preventive treatment for the pregnant women.
Data availability
The datasets generated and analyzed in this study have been included in this manuscript.
Abbreviations
Deoxyribonucleic Acid
Histidine - Rich Protein 2
Intermittent Preventive Treatment in Pregnancy with Sulphadoxine Pyrimethamine
Malaria Rapid Diagnostic Test
Negative Predicative Value
London School of Hygiene & Tropical Medicine
Medical Research Council
Nigerian Institute of Medical Research
Pregnancy Associated Malaria
Plasmodium falciparum Chloroquine Resistant Transporter
Plasmodium falciparum dihydrofolate reductase
Plasmodium falciparum dihydropteroate synthase
Plasmodium falciparum Multidrug Resistance
Positive Predicative Value
Real Time Polymerase Chain Reaction
Ultra Sensitive Rapid Diagnostic Test
var Acid Terminal Segment
World Health Organization
WHO. 2020. World malaria report 2019. Geneva, World Health Organization, 2019.
Mendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;11:179–83.
Google Scholar
Moya-Alvarez V, Abellana R, Cot M. Pregnancy-associated malaria and malaria in infants: an old problem with present consequences. Malar J. 2014;13:271.
Article PubMed PubMed Central Google Scholar
World Health Organisation. WHO policy brief for the implementation of intermittent preventive of malaria in pregnancy using sulfadoxin-pyrimethamine (IPTp-SP). WHO Global Malaria Programme; 2014.
Rogerson SJ, Mwapasa V, Meshnick SR. Malaria in pregnancy: linking immunity and pathogenesis to prevention. Am J Trop Med Hyg. 2007;77:14–22.
Article PubMed Google Scholar
Le Hesran J-Y, Cot M, Personne P, Fievet N, Dubois B, Beyeme M, Boudin C, Deloron P. Maternal placental infection with Plasmodium Falciparum and malaria morbidity during the first 2 years of life. Am J Epidemiol. 1997;146:826–31.
Brabin B. The risk and severity of Malaria in pregnant women. World Health Organisation; 1991.
Thompson JM, Eick SM, Dailey C, Dale AP, Mehta M, Nair A, Cordero JF, Welton M. Relationship between pregnancy-Associated Malaria and adverse pregnancy outcomes: a systematic review and Meta-analysis. J Trop Pediatr. 2020;66:327–38.
Umbers AJ, Aitken EH, Rogerson SJ. Malaria in pregnancy: small babies, big problem. Trends Parasitol. 2011;27:168–75.
Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hygiene. 2001;64:28–35.
Article CAS Google Scholar
World Health Organisation. 2015. Guidelines for the Treatment of Malaria, 3rd edition. Geneva, Switzerland:WHO.
Ohrt C, Purnomo M, Sutamihardja MA, Tang D, Kain KC. Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J Infect Dis. 2002;186:540–6.
Payne D. Use and limitations of light-microscopy for diagnosing malaria at the primary health-care level. Bulleting World Health Organisation. 1988;66:621–6.
CAS Google Scholar
Mathison BA, Pritt BS. Update on malaria diagnostics and test utilization. J Clin Microbiol. 2017;55:2009–17.
Article CAS PubMed PubMed Central Google Scholar
Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in endemic settings. Clin Microbiol Infect. 2013;19:399–407.
Article CAS PubMed Google Scholar
Gerstl S, Dunkley S, Mukhtar A, De Smet M, Baker S, Maikere J. Assessment of two malaria rapid diagnostic tests in children under five years of age, with follow-up of false-positive pLDH test results, in a hyperendemic falciparum malaria area, Sierra Leone. Malar J. 2010;9:28.
World Health Organisation. Response plan to Pf hrp2 gene deletions. Geneva: World Health Organisation; 2019.
Bosco AB, Nankabirwa JI, Yeka A, Nsobya S, Gresty K, Anderson K, Mbaka P, Prosser C, Smith D, Opigo J, Namubiru R, Arinaitwe E, Kissa J, Gonahasa S, Won S, Lee B, Lim CS, Karamagi C, Cheng Q, Nakaaga JK, Kama MR. Limitations of rapid diagnostic tests in malaria surveys in areas with varied transmission intensity in Uganda 2017–2019: implications for selection and use of HRP2 RDTs. PLoS ONE. 2020;15(12):e0244457.
Unwin VT, Ahmed R, Noviyanti R, Puspitasari AM, Utami RAS, Trianty L, Lukito T, Syafruddin D, Poespoprodjo JR, Santana-Morales MA, Kuile FOT, Adams ER. Use of a highly–sensitive rapid diagnostic test to screen for malaria in pregnancy in Indonesia. Malar J. 2020;19:28.
Acquah FK, Donu D, Obboh EK, Bredu D, Mawuli B, Amponsah JA, Quarte J, Amoah LE. Diagnostic performance of an ultrasensitive HRP2–based malaria rapid diagnostic test kit used in surveys of afebrile people living in Southern Ghana. Malar J. 2021;20:125.
Kayentao K, Garner P, van Eijk AM. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604.
Oyibo WA, Agomo CO. Scaling up of intermittent preventive treatment of malaria in pregnancy using sulphadoxine-pyrimethamine: prospects and challenges. Maternal Child Health J. 2011;15:542e552.
Article Google Scholar
Menéndez C, Bardají A, Sigauque B, Sanz S, Aponte JJ, Mabunda S. (2010) Malaria prevention with IPTp during pregnancy reduces neonatal mortality. PLoS ONE. 5:e9438. practices among mothers delivering in an urban hospital in south west Nigeria. Journal of Vector Borne Diseases 45:217– 24.
Happi CT, Gbotosho GO, Folarin OA, Akinboye DO, Yusuf BO, Ebong OO. Polymorphisms in Plasmodium Falciparum dhfr and dhps genes and age related in vivo sulfadoxine–pyrimethamine resistance in malariainfected patients from Nigeria. Acta Trop. 2005;95:183–93.
Mockenhaupt FP, Teun Bousema J, Eggelte TA, Schreiber J, Ehrhardt S, Wassilew N, Otchwemah RN, Sauerwein RW, Bienzle U. Plasmodium Falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Trop Med Int Health. 2005;10(9):901–8.
Kayode AT, Ajogbasile FV, Akano K, Uwanibe JN, Oluniyi PE, Eromon PJ, Folarin OA, Sowunmi A, Wirth DF, Happi CT. Polymorphisms in Plasmodium Falciparum dihydropteroate synthetase and dihydrofolate reductase genes in Nigerian children with uncomplicated malaria using high–resolution melting technique. Nat Res. 2021;11:471.
Oguike MC, Falade CO, Shu E, Enato IG, Watila I, Baba ES, Bruce J, Webster J, Hamade P, Meek S, Chandramohan D, Sutherland CJ, Warhurst D, Roper C. Molecular determinants of sulfadoxine-pyrimethamine resistance in Plasmodium Falciparum in Nigeria and the regional emergence of dhps 431V. Int J Parasitology: Drugs Drug Resist. 2016;6:220–9.
Okwa OO, Akinmolayan FI, Carter V, Hurd H. Transmission dynamics of malaria in four selected ecological zones of Nigeria in the rainy season. Ann Afr Med. 2009;8(1):1–9.
Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of Multi-copy Subtelomeric targets. PLoS Med. 2015;12(3):e1001788.
Ndiaye YD, Diedhiou CK, Bei AK, Dieye B, Mbaye A, Mze NP, Daniels RF, Ndiaye IM, Deme AB, Gaye A, Sy M, Ndiaye T, Badiane AS, Ndiaye M, Premji Z, Wirth DF, Mboup S, Krogstad D, Volkman SK, Ahouidi AD, Ndiaye D. High-resolution melting: a useful field-deployable method to measure dhfr and dhps drug resistance in both highly and lowly endemic plasmodium populations. Malar J. 2017;16:153.
Daniels R, Ndaye D, Wall M, McKinney J, Sene PD, Sabeti PC, Volkman SK, Mboup S, Wirth DF. Rapid, Field-Deployable Method for genotyping and Discovery of single-nucleotide polymorphisms Associated with Drug Resistance in Plasmodium Falciparum . Antimicrob Agents Chemother. 2012;56(6):2976–86.
Briand V, Cottrell G, Ndam NT, Vendrell XM, Vianou B, Mama A, Kouwaye B, Houzé S, Bailly J, Gbaguidi E, Sossou D, Massougbodji A, Accrombessi M, Mayor A, Ding XC, Fievet N. Prevalence and clinical impact of malaria infections detected with a highly sensitive HRP2 rapid diagnostic test in Beninese pregnant women. Malar J. 2020;19:188.
Vasquez AM, Medina AC, Tobon-Castano A, Posada M, Velez GJ, Campillo A, Gonzalez IJ, Ding X. Performance of a highly sensitive rapid diagnostic test (HS-RDT) for detecting malaria in peripheral and placental blood samples from pregnant women in Colombia. PLoS ONE. 2018;13(8):e0201769.
Lucchi NW, Okoth SA, Komino F, Onyona P, Goldman IF, Ljolje D, Shi YP, Barnwell JW, Udhayakumar V, Kariuki S. (2015). Increasing prevalence of a novel triple-mutant dihydropteroate synthase genotype in Plasmodium falciparum in western Kenya. Antimicrob. Agents Chemother 59(7), 3995–4002.Maltha J, Gillet P and Jacobs J.(2013) Malaria rapid diagnostic tests in endemic settings. Clinical Microbiology & Infection 19:399–407.
Jiang T, Chen J, Fu H, Wu K, Yao Y, Eyi JUM, Matesa RA, Obono MMO, Du W, Tan H, Lin M, Li J. High prevalence of pfdhfr – pfdhps quadruple mutations associated with sulfadoxine–pyrimethamine resistance in Plasmodium Falciparum isolates from Bioko Island, Equatorial Guinea. Malar J. 2019;18:101.
Mobula L, Lilley B, Tshefu AK, Rosenthal PJ. Resistance-mediating polymorphisms in Plasmodium falciparum infections in Kinshasa, Democratic Republic of the Congo. Am J Trop Med Hygiene. 2009;80:555–8.
Tahita MC, Tinto H, Erhart A, Kazienga A, Fitzehenry R, VanOvermeir C, Rosanas-Urgell, Ouedraogo J, Guiguemde RT, Vangeertruyden JP, D’Alessandro U. Prevalence of the dhfr and dhps mutations among pregnant women in rural Burkina Faso five years after the introduction of intermittent preventive treatment with sulfadoxine-pyrimethamine. PLoS ONE. 2015;10(9):e0137440–0137440.
World Health Organisation. WHO Policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). Geneva: World Health Organization; 2013.
Naidoo I, Roper C. Drug resistance maps to guide intermittent preventive treatment of malaria in African infants. Parasitology. 2011;138:1469–79.
Bwijo B, Kaneko A, Takechi M, Zungu IL, Moriyama Y, Lum K, Tsukahara T, Mita T, Takahashi N, Berggvist Y, Bjorkman A, Kobayakawa T. High prevalence of quintuple mutant dhps/dhfr genes in Plasmodium falciparum infections seven years after introduction of sulfadoxine and pyrimethamine as first line treatment in Malawi. Acta Trop. 2003;85:363–73.
Roper C, Pearce R, Nair S, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124.
Issa I, Lamine MM, Hubert V, Ilagouma A, Adehossi E, Mahamadou A, Loobo NF, Sarr D, Shollenberger LM, Sandrine H, Jambou R, Laminou IM. Prevalence of mutations in the Pfdhfr, Pfdhps, and Pfmdr1 genes of Malarial parasites isolated from symptomatic patients in Dogondoutchi, Niger. Trop Med Infect Dis. 2022;7:155.
Tuedom AGB, Sarah-Matio EM, Moukoko CEE, Feufack-Donfack BL, Maffo CN, Bayibeki AN, Awono-Ambene HP, Ayong L, Berry A, Abate L, Morlais I, Nsango SE. Antimalarial drug resistance in the Central and Adamawa regions of Cameroon: prevalence of mutations in P. Falciparum Crt, Pfmdr1, Pfdhfr and pfdhps genes. PLoS ONE. 2021;16(8):e0256343. https://doi.org/10.1371/journal .
Adams R, Mukhtar MM, Abubakar UF, Damudi HA, Muhammad A, Ibrahim SS. Polymorphism analysis of Pf mdr1 and Pf crt from Plasmodium Falciparum isolates in Northwestern Nigeria revealed the Major Markers Associated with Antimalarial Resistance. Diseases. 2021;9:6.
Gbotosho GO, Folarin OA, Bustamante C, Pereira da Silva LH, Mesquita E, Sowunmi A, Zalis MG, Oduola AMJ, Happi CT. Different patterns of Pf crt and Pf mdr1 polymorphisms in P. Falciparum isolates from Nigeria and Brazil: the potential role of Antimalarial Drug Selection pressure. Am J Trop Med Hygiene. 2012;88(2):211–3.
Ikegbulam MN, Nkonganyi CN, Thomas AN, Esimone CO, Velavan TP, Ojurongbe O. Analysis of Plasmodium Falciparum Pfcrt and Pfmdr1 genes in parasite isolates from asymptomatic individuals in Southeast Nigeria 11 years after withdrawal of chloroquine. Malar J. 2019;18:343.
Adegbola AJ, Ijarotimi OA, Ubom AE, Adesoji BA, Babalola OE, Hocke EF, Hansson H, Mousa A, Bolaji OO, Alifrangis M, Roper C. A snapshot of the prevalence of dihydropteroate synthase-431V mutation and other sulfadoxine-pyrimethamine resistance markers in Plasmodium Falciparum isolates in Nigeria. Malar J. 2023;22:71.
McHugh ML. Interrater reliability: the kappa statistic. Biochemia Med. 2012;22(3):276–82.
Download references
Acknowledgements
We thank all the pregnant participants for their willingness to enroll in the study. We thank the Pharmanews and Emzor Pharmaceuticals for providing Sulfadoxine-Pyrimethamine (Maldox) for the participating health facilities for pregnant women. We also thank the Director, Medical Services and Disease control, Lagos State Primary Health care board, the laboratory scientists and the Medical Officers of health at Ogudu and Epe Primary Health Centres.
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The research work was self-sponsored.
Author information
Authors and affiliations.
University of Lagos, Akoka, Nigeria
Agatha N. Ibekpobaoku & Emmanuel T. Idowu
Medical Research Council The Gambia Unit (MRC), Banjul, Gambia
Mary A. Oboh, Fatou Faal, Elizabeth Adeniji & Alfred Amambua-Ngwa
Rochester Institute of Technology, Rochester, USA
Mary A. Oboh
University of Medical Sciences, Ondo, Nigeria
Nigerian Institute of Medical Research (NIMR), Yaba, Lagos, Nigeria
Olusola Ajibaye
You can also search for this author in PubMed Google Scholar
Contributions
A. I and M. O carried out the study design and manuscript writing. A. I collected blood samples. M.O. did data analysis. F.F and E.A. carried out laboratory experiment. O.A., E.I and A, N reviewed and critiqued the manuscript.
Corresponding author
Correspondence to Mary A. Oboh .
Ethics declarations
Ethical approval and consent to participate.
The study was conducted in accordance with the Declaration of Helsinki, and the ethical approval (IRB/17/048) was obtained from the ethical review board of the Nigerian Institute of Medical Research (NIMR), Yaba, Lagos. Verbal and written informed consent was obtained from all pregnant women included in this study after detailed explanation of the study objectives to each of them. Pregnant women included in this study are those that consented with no pregnancy-related complications.
Consent for publication
All the authors have given consent for the publication of this work.
Consent for publication from participants
Not applicable.
Limitations of study
This study is limited in the number of sample size included in the study and the geographical spread of the samples because it was self-funded.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ibekpobaoku, A.N., Oboh, M.A., Faal, F. et al. Sub-microscopic Plasmodium falciparum infections and multiple drug resistant single nucleotide polymorphic alleles in pregnant women from southwestern Nigeria. BMC Res Notes 17 , 129 (2024). https://doi.org/10.1186/s13104-024-06763-2
Download citation
Received : 13 June 2023
Accepted : 02 April 2024
Published : 09 May 2024
DOI : https://doi.org/10.1186/s13104-024-06763-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- P. falciparum
- Malaria in pregnancy
- Malaria RDTs
- Sulphadoxine Pyrimethamine
- Single nucleotide polymorphism
BMC Research Notes
ISSN: 1756-0500
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
This study, based in San Bernardino County, Southern California, collected and examined tap water samples within the area to explore the feasibility of adopting non-industrial equipment and methods to reduce water hardness and total dissolved solids(TDS). We investigated how water quality could be improved by utilizing water boiling, activated carbon and sodium bicarbonate additives, as well ...
This review paper focuses on the various types of Filtration techniques. available and whic h tec hnique is most suitable for which t ype of region. W e. will study the t ypes of natural ...
The area of water purification has been one of the most dynamic research fields in recent years with strong public policy implications with over 39,000 papers. Similarly, the areas of nanomaterials and nanoprocesses have been one of the most dynamic research fields in recent years with significant public policy implications with over 1,000,000 ...
This requires investment in water purification technologies. World Water Day offers an opportunity to discuss whether such investment will help achieve this laudable goal. ... Chemical Research in ...
In 2019, about 6% of public water utilities in the U.S. had a health-based violation. Due to the high risk of exposure to various contaminants in drinking water, point-of-use (POU) drinking water ...
The need for research into water purification is pressing. In an extensive Review Article, Mark Shannon et al. highlight the developing technologies that — it is hoped — can provide our ...
The aim of this study was to find out whether domestic water purification systems could eliminate the essential materials such as fluoride besides filtrating the heavy ions and other unwanted particles out of water. Materials and Method. In this study, 6 frequently used commercial brands of water purifiers in Ahwaz were compared. ...
Agricultural development, extensive industrialization, and rapid growth of the global population have inadvertently been accompanied by environmental pollution. Water pollution is exacerbated by the decreasing ability of traditional treatment methods to comply with tightening environmental standards. This review provides a comprehensive description of the principles and applications of ...
Several treatment technologies, as well as water treatment materials, have now been developed. Nevertheless, the practical application value in water treatment is often ignored. This is not consistent with the sustainable develop-ment of water resources. Many studies focus on developing something new, like "to write new versus with a false ...
Dec.15, 2021. In this paper, researchers surveyed both conventional and advanced disinfection processes in the U.S., testing the quality of their drinking waters. Treatment plants with advanced removal technologies, such as activated carbon, formed fewer types and lower levels of harmful disinfection byproducts (known as DBPs) in their water.
Sustainable and affordable supply of clean, safe, and adequate water is one of the most challenging issues facing the world. Membrane separation technology is one of the most cost-effective and widely applied technologies for water purification. Polymeric membranes such as cellulose-based (CA) membranes and thin-film composite (TFC) membranes have dominated the industry since 1980.
The paper proposes the study of the environmental impact caused by the wastewater discharges in the Portoviejo River basin, through the evaluation of its self-purification capacity through the use ...
There are number of water purification techniques but the adsorption is one of the most simplest, effective and economical method for wastewater purification. ... adsorbents has been used for the removal of pollutants from water and their mechanisms have been discussed in number of research and review papers. Agricultural waste peels, ...
In drinking water treatment, filtration plays an important role in the multi-barrier approach employed for the removal of pathogens. The presence of suspended solids and other particulate matter in water increases the resistance of most microbes to disinfection. Therefore, high performance in the removal of particles achieved by granular filtration can increase the disinfection efficiency ...
The design of a solar-powered water purification system is based totally on the thermal. method by using the thermal heating system principle which converts sunlight rays into heat. The most vital ...
In a News & Views in June 2023 4 we highlighted a paper studying the fundamental aspects of water transport in reverse osmosis membranes 5. Among the papers we published, a clear example is the ...
The researchers showed that this technology can efficiently remove chemicals, oils, metals, and pathogens from the water. Since the device uses passive, gravity-based filtration, the only energy required for it to work comes from the sun. Additionally, this device is able to purify water much faster than similar existing technologies.
Water scarcity is one of the major problems in the world and millions of people have no access to freshwater. Untreated wastewater is widely used for agriculture in many countries. This is one of the world-leading serious environmental and public health concerns. Instead of using untreated wastewater, treated wastewater has been found more applicable and ecofriendly option. Moreover ...
Nanotechnology is an easy and practical approach to clean waste water by using different methods. Different types of bacteria, toxic chemicals like arsenic, mercury etc., and sediments can be removed by using nanotechnology. Nanomaterial based devices are being used for water purification. Nano filtration method has advantages over other ...
This special issue WPP (Water: From Pollution to Purification) of Environmental Science and Pollution Research presents se-lected papers presented at the second International conference on Water: From Pollution to Purification (ICW2016) conducted during Dec.12-15, 2016, in Kottayam (Kerala, India) hosted jointly by Inter University ...
The results of this study will provide options for residents and water treatment plants to find ways to maintain the general taste of the tap water, but also preserve the lifespan of accessories and pipelines. ... Water Research, 2002, 36 (14); pp. 3647-3653, doi: 10.1016/s0043-1354(02)00049- [Google Scholar] 25. Consumers ...
Everyday activity incurs carbon footprints, which are classified as personal, production, organizational and national, and may be assessed by input-output analysis (IOA), life-cycle assessment (LCA), or the combination of LCA and IOA methods. Notwithstanding international standards, like ISO 14064 and Publicly Available Specification (PAS) released for standardization, carbon footprint ...
The large area filter paper made it possible to fabricate a prototype of a solar-thermal water purification system (Fig. 3a). For this purpose, a freestanding 30 μm thick filter paper with ...
@article{Peng2024ResearchOT, title={Research on the Coordinated Relationship between Sewage Resource Utilization and Economic Research on the Coordinated Relationship between "Dual-cycle" Wastewater Resource Utilization and Economic Development}, author={Hui Peng and Haigang Li and Zhao Zhang}, journal={Desalination and Water Treatment}, year ...
The paper industry is a composite one constituting different types of mills, processes, and products. The paper industries consume large amounts of resources, like wood and water. These industries also create huge amounts of waste that have to be treated. In our study, 23 endophytic bacteria were isolated from Argemone mexicana, and 16 endophytic bacteria were isolated from Papaver rhoeas ...
Membrane filtration using recycled hemodialyzers allows water purification. This study quantifies the public health effects. ... In Clasen's data synthesis paper 11 on 42 studies in 21 countries ...
The study evaluated sub-microscopic malaria infections in pregnancy using two malaria Rapid Diagnostic Tests (mRDTs), microscopy and RT-PCR and characterized Plasmodium falciparum dihydrofolate reductase (Pfdhfr) and Plasmodium falciparum dihydropteroate synthase (Pfdhps) drug resistant markers in positive samples. This was a cross sectional survey of 121 pregnant women.