
- Home
- The Christie Research Publications Repository
- All Christie Publications

Local Links
Show simple item record
Overview of commercial treatment planning systems for targeted radionuclide therapy
Files in this item, this item appears in the following collection(s).
entitlement
Related articles
- BIGDOSE: software for 3D personalized targeted radionuclide therapy dosimetry.
- Authors: Li T, Zhu L, Lu Z, Song N, Lin KH, Mok GSP
- Issue date: 2020 Jan
- Dosimetry in MRT: Our recent experience.
- Authors: Zoglopitou LA
- Issue date: 2023 May-Aug
- Personalized dosimetry of(177)Lu-DOTATATE: a comparison of organ- and voxel-level approaches using open-access images.
- Authors: Carter LM, Ocampo Ramos JC, Kesner AL
- Issue date: 2021 Aug 4
- A single centre intercomparison between commercial treatment planning systems for (90)Y radioembolization using virtual and experimental phantoms.
- Authors: Della Gala G, Santoro M, Rasoatsaratanany GA, Paolani G, Strolin S, Strigari L
- Issue date: 2023 Dec
- Fast beta-emitter Monte Carlo simulations and full patient dose calculations of targeted radionuclide therapy: introducing egs_mird.
- Authors: Martinov MP, Opara C, Thomson RM, Lee TY
- Issue date: 2022 Sep
Export search results
The export option will allow you to export the current search results of the entered query to a file. Different formats are available for download. To export the items, click on the button corresponding with the preferred download format.
By default, clicking on the export buttons will result in a download of the allowed maximum amount of items.
To select a subset of the search results, click "Selective Export" button and make a selection of the items you want to export. The amount of items that can be exported at once is similarly restricted as the full export.
After making a selection, click one of the export format buttons. The amount of items that will be exported is indicated in the bubble next to export format.
Access provided by
Login to your account
If you don't remember your password, you can reset it by entering your email address and clicking the Reset Password button. You will then receive an email that contains a secure link for resetting your password
If the address matches a valid account an email will be sent to __email__ with instructions for resetting your password

Download started.
- Academic & Personal: 24 hour online access
- Corporate R&D Professionals: 24 hour online access
- Add To Online Library Powered By Mendeley
- Add To My Reading List
- Export Citation
- Create Citation Alert
Overview of commercial treatment planning systems for targeted radionuclide therapy
- Giuseppe Della Gala Giuseppe Della Gala Affiliations Department of Medical Physics, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy Search for articles by this author
- Jill Tipping Jill Tipping Affiliations The Christie NHS Foundation Trust, Manchester, UK Search for articles by this author
- Lidia Strigari Lidia Strigari Correspondence Corresponding author. Contact Affiliations Department of Medical Physics, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy Search for articles by this author
- • To implement the European directive a personalized treatment planning needs to be performed.
- • EU Medical Devices Regulation 2017/745 applies also to Treatment Planning Systems (TPSs) for targeted radionuclide therapy (TRT).
- • Several TPS are now commercially available for liver radioembolization and general purpose TRT.
- • An overview of available TPSs is presented based on an ad hoc survey.
Introduction
Materials and methods, conclusions.
- Targeted Radionuclide Therapy
- Treatment planning systems
- Dose calculation
- Dose volume histograms
Abbreviations:
Purchase one-time access:.
- For academic or personal research use, select 'Academic and Personal'
- For corporate R&D use, select 'Corporate R&D Professionals'
- McDevitt M.R.
- Nedrow J.R.
- Scopus (336)
- Google Scholar
- Herrmann K.
- Schwaiger M.
- Solomon S.B.
- McNeil B.J.
- Scopus (130)
- Full Text PDF
- Spyridonidis T.
- Spyridonidis J.
- Papathanasiou N.
- Katsanos K.
- Strigari L.
- Konijnenberg M.
- Gleisner K.S.
- Scopus (156)
Council Directive 2013/59/ EURATOM about Basic Safety Standard, https://eur-lex.europa.eu/eli/dir/2013/59/oj.
Chiesa C, Strigari L, Pacilio M, Richetta E, Cannatà V, Stasi M, et al. Dosimetric optimization of nuclear medicine therapy based on the Council Directive 2013/59/EURATOM and the Italian law N. 101/2020. Position paper and recommendations by the Italian National Associations of Medical Physics (AIFM) and Nuclear Medicine (AIMN). Phys Med. 2021;89:317–26.
- Gabiña P.M.
- Sandström M.
- Scopus (69)
- Kletting P.
- Schimmel S.
- Hänscheid H.
- Fernández M.
- Scopus (33)
- Marcatili S.
- Pettinato C.
- Scopus (53)
- Siantar C.H.
- Wessol D.E.
- Wemple C.A.
- Cogliati J.
- Scopus (31)
- Bouchet L.G.
- Papavasileiou P.
- Flower M.A.
- Scopus (54)
- Sjögreen K.
- Ljungberg M.
- Wingårdh K.
- Strand S.-E.
- Scopus (32)
- Denis-Bacelar A.M.
- Scopus (51)
- Leite Ferreira P.
- Malterre J.
- Verdun F.R.
- Scopus (46)
- Robinson A.P.
- Cullen D.M.
- Hamilton D.
- Scopus (14)
- Kearvell R.
- Scopus (59)
- Lassmann M.
- Werner R.A.
- Scopus (112)
https://www.hermesmedical.com/dosimetry/.
- Dieudonné A.
- Konijnenberg M.W.
- Eckerman K.F.
- Dewaraja Y.K.
- Sjögreen-Gleisner K.
- Scopus (209)
- Roberson P.
- Zanzonico P.B.
- Scopus (275)
- Esquinas P.L.
- Tolhurst S.
- Mora‐Ramirez E.
- Ocampo‐Ramos J.C.
- Pasciak A.S.
- Bourgeois A.C.
- Bradley Y.C.
- Scopus (75)
- Wevrett J.L.
- Garibaldi C.
- Iacoviello G.
- Scopus (57)
- O'Sullivan J.M.
- Howell R.W.
Digital Imaging and Communications in Medicine (DICOM) Supplement 11. Radiotherapy objects.
- Pasquier G.
- Stratakis J.
- Scopus (149)
- Gustafsson J.
- Glatting G.
- Scopus (115)
Article info
Publication history, identification.
DOI: https://doi.org/10.1016/j.ejmp.2021.11.001
ScienceDirect
Related articles.
- Download Hi-res image
- Download .PPT
- Access for Developing Countries
- Articles and Issues
- Articles in Press
- Current Issue
- List of Issues
- Supplements
- For Authors
- About Open Access
- Author Information
- Researcher Academy
- Submit Your Manuscript
- Journal Info
- About the Journal
- Abstracting/Indexing
- Contact Information
- Editorial Biographies
- Editorial Board
- Info for Advertisers
- New Content Alerts
- Article Collections
- Special Issues
- 125 years of X-Ray
- Review Papers
- Editor's Choice
- Free Download Review Papers
- Virtual Issues
- Society Info
- European Federation of Organisations for Medical Physics (EFOMP)
- Associazione Italiana di Fisica Medica e Sanitaria (AIFM)
- Irish Association of Physicists in Medicine
- Société Française de Physique Médicale
- Sociedad Española De Física Médica (SEFM)
- EFOMP Policy statements
The content on this site is intended for healthcare professionals.
- Privacy Policy
- Terms and Conditions
- Accessibility
- Help & Contact

- Reference Manager
- Simple TEXT file
People also looked at
Mini review article, a brief overview of targeted radionuclide therapy trials in 2022.

- 1 California Northstate University College of Medicine, California Northstate University, Sacramento, CA, United States
- 2 Michael G. DeGroote School of Medicine, McMaster University, Hamilton, ON, Canada
- 3 Department of Radiology, University of Arizona, Tucson, AZ, United States
- 4 Departments of Radiology and Medicine, McMaster University, Hamilton, ON, Canada
There is a growing use of radionuclide therapy for the medical care of oncology patients, where radioactive pharmaceuticals are used to target and treat various cancer types. This paper provides a brief overview illustrating the spectrum of ongoing and recently completed radionuclide therapy clinical trials in oncology. The trials selected highlight the potential of radionuclide therapies to provide a promising treatment option across a spectrum of cancer patients, while also discussing the importance of patient selection and monitoring, as well as potential side effects and safety concerns. Ultimately, the results of these trials will be crucial in determining the future use of radionuclide therapies in cancer treatment.
1. Introduction
The field of nuclear medicine is ever expanding to include an increasing array of targeted radionuclide therapies (TRTs) capable of treating oncology patients. Although TRT has existed for many years, recently it is gaining attention due to the potential for prolonging patient survival across differing cancer types, often with minimal toxicity. While thyroid cancer, neuroendocrine tumors and prostate cancer remain the most common targeted cancer types, additional malignancies continue to be added to the list. In this paper we provide a short summary of the current spectrum of clinical trials using TRT based on an analysis of those studies listed on clinicaltrials.gov ( 1 ). The studies chosen were based on a search of clinicaltrials.gov up to and including February 14, 2023, for all ongoing and recently completed clinical trials and then selecting those trials that included TRTs across a spectrum of disease. These studies are categorized by the targeted receptor, treatment indication, and clinical trial phase. We also briefly discuss obstacles for TRT research, current gaps in this research and future developments. Trials are summarized in Table 1 .
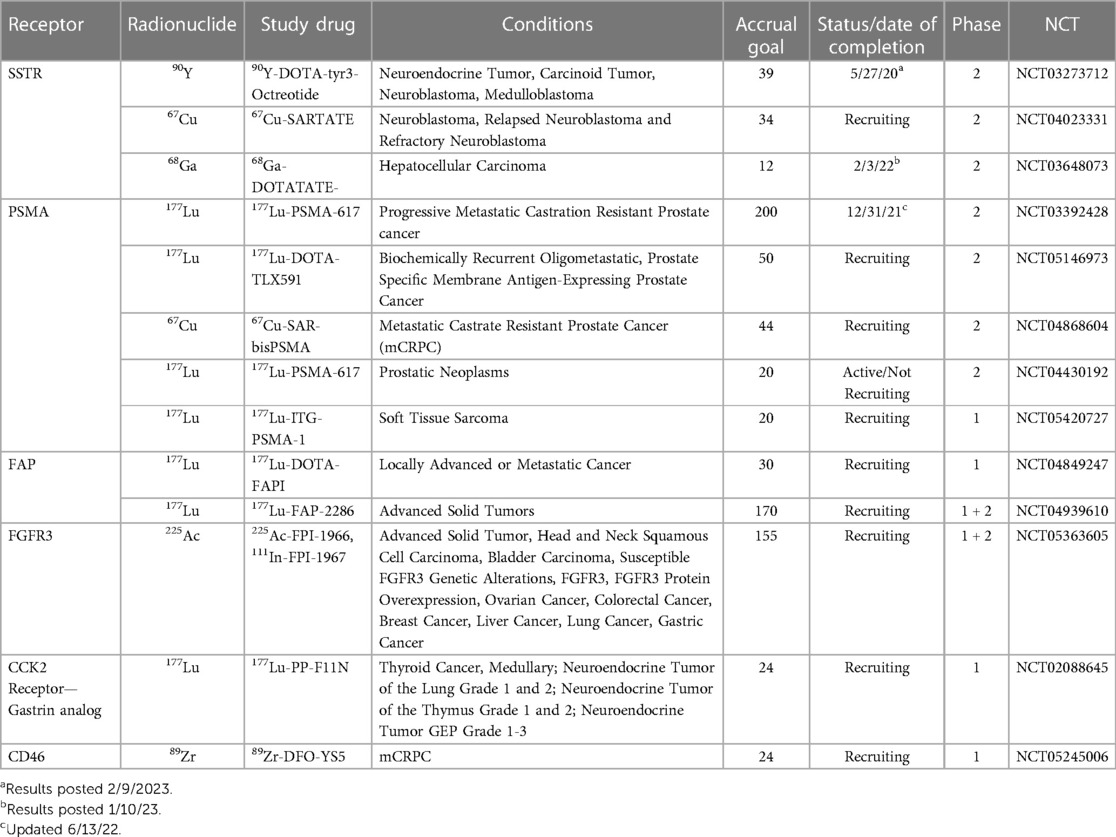
Table 1 . Summary of active or recently completed studies illustrating a Spectrum of radiopharmaceutical advances.
Perhaps one of the most significant obstacles for recent clinical trials including TRT, was the COVID-19 pandemic, that caused many of the studies listed on clinicaltrials.gov to be paused or pre-maturely “completed” because of the associated strain on healthcare resources and shelter-in-place policies that resulted in disruptions to clinical trial activities. Another obstacle inherent in clinical trials including TRT is radiation exposure. While TRTs are designed to target and destroy specific types of cancer cells, they can also damage healthy tissues, leading to adverse effects. One way to reduce the risk of radiation exposure is through pre-therapy imaging and then using this as a tool to optimize the amount of radiopharmaceutical given to provide optimal therapy while minimizing toxicity through exposure to surrounding healthy tissues. In addition, technical advances have enabled the development of increasingly targeted radionuclides with lower off-target exposure. Today, common side-effects associated with TRTs include fatigue, nausea and bone marrow suppression; however, nephrotoxicity, among other side-effects may also be seen ( 2 , 3 ). In certain instances, the co-administration of agents to help reduce side effects and toxicity are given. Example of ongoing trials that have adopted this approach include a Phase 1 trial using 111 In-CP04 to target the CCK2 receptor that includes the co-administration of gelofusine/gelaspan, colloidal plasma substitutes, to minimize nephrotoxic effects and myelosuppression. Another example is a Phase 2 trial using 68 Ga-DOTATOC and 90 Y-DOTATOC that includes the co-administration of lysine and arginine for nephroprotection. Also, in many trials a diagnostic radiopharmaceutical is used to image patients prior to TRT in order to detect patients most likely to benefit and exclude those most likely to experience adverse effects. For example, in patients with hepatocellular carcinoma (HCC), 68 Ga-DOTATATE-PET is done first and only those patients with adequate somatostatin receptor (SSTR) expression on imaging to suggest benefit of the TRT are given 177 Lu-DOTATATE. Another trial includes a diagnostic scan with 68 Ga-DOTA-5G for patients with pancreatic cancer, where only patients with adequate uptake (defined as SUVmax >2-fold above normal lung or liver) are given the TRT, specifically 177 Lu-DOTA-ABM-5G. In another trial 203 Pb-VMT01 and 68 Ga-VMT02 are used for image screening to detect patients with melanoma likely to benefit from 212 Pb-VMT01.
An ongoing and important gap in research involving TRTs is the lack of large-scale clinical trials capable of providing high-quality evidence of the safety and efficacy of these treatments. While there have been many small studies and case reports suggesting promising results and a few larger trials that have impacted clinical practice, there is a need for more large, well-designed clinical trials to validate early phase clinical trial findings and ultimately inform/change clinical practice on a broad scale ( 4 ). In addition, there is a need for further research on radiotracers with improved pharmacokinetic and biodistribution profiles, as well as greater specificity for cancer subtypes. This requires understanding the biology underlying different cancer subtypes, as well as continued advancement in radiopharmaceuticals and imaging techniques to ensure precise targeting of the radiopharmaceutical to the tumor. Another gap in research is determining the best therapy combination to improve overall efficacy and tolerability. For example, cryoablation of disease sites or and/or immunotherapy, among others may have a synergistic effect with TRT.
Finally, most current trials target SSTR and prostate specific membrane antigen (PSMA) receptors with innovations focusing on the development of radiopharmaceuticals that more accurately target these receptors, or that apply already existing TRT to new cancer subtypes. For example, a feasibility study was recently conducted to determine if patients with HCC expressed SSTR adequately to benefit from 177 Lu-DOTATATE treatment. Future developments in the field may increasingly focus on identifying new receptors that can serve as TRT targets; currently, many trials focusing on this are early in the development process. For example, there is a handful of phase 1 trials targeting the fibroblast growth factor receptor 3 (FGFR3), fibroblast activation protein inhibitor (FAPI), cholecystokinin 2 receptor (CCK2), melanocortin sub-type 1 receptor (MC1R) and the chemokine receptor 4 (CXCR4), among others. There are no trials at this time that target the gastrin-releasing peptide receptor (GRP-R) or the integrin αVβ3 or αVβ5 receptors, which have also been identified as potential targets for neuroendocrine tumors ( 5 ). Investigating new receptors has the potential to extend TRT to a variety of cancers subtypes, and could improve the specificity of TRT, which continues to be a barrier for effectiveness.
2. Examples of clinical trials targeting SSTR
Somatostatin receptors are overexpressed in a variety of neuroendocrine tumors (NETs), making them attractive targets for radionuclide therapy. Radiolabeled somatostatin analogs such as octreotide and DOTATATE have been developed to target SSTRs for imaging and therapy by delivering targeted radiation directly to tumor cells. The use of radiolabeled somatostatin analogs for therapy has shown promise in clinical trials. Specifically, a phase 3 clinical trial showed that 177 Lu-DOTATATE improved progression-free survival in patients with midgut NETs compared to standard therapy ( 6 ). A phase 3 clinical trial found that 177 Lu-DOTATOC, a radiolabeled somatostatin analog, improved progression-free survival in patients with gastroenteropancreatic NETs ( 7 ). In addition, 177 Lu and 90 Y-labeled PRRT have been used in clinical trials showing promise in treating gastroenteropancreatic and bronchial NETs ( 2 ). Research is ongoing in an effort to optimize somatostatin receptor-targeted therapy, including determining the optimal amount of TRT to be effective across a spectrum of patient populations, selecting patients most likely to benefit and using existing TRTs in new cancer subtypes. A few examples illustrating the spectrum of ongoing clinical trials is given below.
NCT03273712 is a trial designed to evaluate the safety and efficacy of Dosimetry-Guided, Peptide Receptor Radiotherapy (PRRT) with 90 Y-DOTA-tyr3-Octreotide ( 90 Y-DOTATOC) in patients with inoperable, somatostatin receptor-positive neuroendocrine tumors. This trial will use dosimetry to determine an individualized radiation dose for each patient, with a maximum of 4 treatment cycles given at 8–12 week intervals ( 8 ).
NCT04023331 aims to evaluate the safety and efficacy of 67 Cu-SARTATE in pediatric patients with high-risk neuroblastoma through an adaptive and personalized design. The trial consists of two phases, a dose escalation phase, and a cohort expansion phase. Dose escalation will use a modified 3 + 3 study design with up to 4 cohorts of increasing doses monitoring pre-defined Dose Limiting Toxicities for 6 weeks post administration of one therapy cycle of 67 Cu-SARTATE. Patients who benefit may be offered additional therapy cycles, up to a maximum of 4. Once the Maximum Tolerated Dose (MTD) is established, or Cohort 4 is completed, the study will be expanded to enroll an additional 10 subjects who will receive at least 2 therapy cycles of 67 Cu-SARTATE at the MTD dose level ( 9 ).
NCT03648073 aims to use 68 Ga-DOTATATE to determine HCCs expressing SSTR levels deemed sufficiently high to benefit from targeted radionuclide therapy with 177 Lu-DOTATATE. If successful, this approach may offer a new therapeutic option for HCC patients who are not candidates for other therapies ( 10 ).
3. Example of clinical trials targeting PSMA
Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein that is highly expressed in prostate cancer cells. PSMA has been the focus of much research in recent years as a target for radionuclide therapy in men with prostate cancer. In the case of PSMA based TRT, the radioactive isotopes are attached to PSMA-targeting molecules that bind to PSMA on the surface of prostate cancer cells. One of the most ubiquitous radionuclides for PSMA-targeted therapy is 177 Lu. Clinical trials have shown that 177 Lu-PSMA therapy improves progression-free survival and overall survival in men with metastatic castration resistant prostate cancer (mCRPC) who have failed other therapies ( 11 , 12 ) and this TRT was approved for use by the FDA in 2022. Other radionuclides, such as 225 Ac and 227 Th, are currently being investigated for PSMA-targeted therapy, with promising preclinical results ( 13 ). The examples below illustrate the spectrum of ongoing trials focusing on expanding earlier promising results of TRT in men prostate cancer, assessing combination therapy with TRT, assaying novel TRT agents, evaluating where in the spectrum of disease TRT has the best effect and assaying known TRT agents in novel cancer types.
NCT03392428 aims to evaluate the efficacy of 177 Lu-PSMA-617 in men with metastatic prostate cancer who have progressed despite hormonal therapy and chemotherapy. The trial compares the effects of 177 Lu-PSMA radionuclide therapy with cabazitaxel chemotherapy ( 14 ). This study will recruit 200 participants from sites across Australia to expand on the previous randomized phase 2 trial (TheraP) that showed 177 Lu-PSMA-617 led to a higher PSA response and fewer grade 3 or 4 adverse events compared with cabazitaxel published in 2022, suggesting TRT could be a good alternative to cabazitaxel ( 15 ).
NCT05146973 aims to assess the effectiveness of 177 Lu-TLX591, a radiolabelled PSMA-targeting antibody, in combination with external beam radiation therapy (EBRT) for the treatment of biochemically recurrent, oligometastatic, PSMA-expressing prostate cancer. The therapeutic potential of TLX591 lies in its ability to target PSMA-expressing tumors by radiolabeling it with a therapeutic radioactive isotope ( 16 ).
NCT04868604 is a phase 1/2 clinical trial that aims to evaluate the safety, dosimetry, and therapeutic potential of two copper-labeled PSMA-targeting agents, 64 Cu-SAR-bisPSMA and 67 Cu-SAR-bisPSMA, for identifying and treating PSMA-expressing metastatic castration-resistant prostate cancer ( 17 ).
NCT04430192 assesses the safety, effectiveness, and appropriate dosage of 177 Lu-PSMA in men with high PSMA-expressing high-risk localized or locoregional advanced prostate cancer undergoing radical prostatectomy and pelvic lymph node dissection. The trial evaluates the radiation dose absorbed, imaging response, biochemical response, pathological response, adverse effects, surgical safety, and quality of life. Patients will receive one or two cycles of 177 Lu-PSMA before surgery ( 18 ).
NCT05420727 evaluates the use of 68 Ga-PSMA-11 PET/CT for imaging and 177 Lu-ITG-PSMA-1 treatment, in patients with soft tissue sarcomas ( 19 ).
4. Examples of clinical trials targeting novel receptors
There are early phase trials targeting a host of receptors. For example, there are several types of Fibroblast Growth Factor Receptors (FGFR), transmembrane receptors associated with cell growth and invasiveness. Mutations in this receptor have been associated with a spectrum of tumor types. Radioimmunotherapy (RIT) is a type of targeted therapy that involves using a radioactive isotope conjugated to a monoclonal antibody (mAb) to selectively deliver radiation and immunotherapy to cancer cells expressing the targeted protein. Studies have suggested that FGFR3 is associated with bladder cancer and multiple myeloma ( 20 ). Research is ongoing to optimize the clinical application, dosing schedule and combination of therapies including FGFR3-targeted therapy. NCT05363605 is an example of an early phase trial evaluating safety, tolerability, and distribution of 225 Ac-FPI-1966, 111 In-FPI-1967, and vofatamab (anti-FGFR3 antibody) in patients with FGFR3-expressing solid tumors. The study includes 5 dose escalation cohorts, and a subsequent expansion cohort of two tumor-specific cohorts and one basket cohort. The study evaluate the impact of vofatamab given prior to TRT on the dosimetry and tolerability of 225 Ac-FPI-1966 and 111 In-FPI-1967 ( 21 ).
Several tumor types are characterized by a strong desmoplastic reaction resulting in cancer-associated fibrosis and many of these fibroblasts differ from normal fibroblasts through their expression of a Fibroblast Activation Protein (FAP). NCT04849247 is an early phase trial investigating the safety and efficacy of 68 Ga-DOTA-FAPI and 177 Lu-DOTA-FAPI, to diagnose and treat a spectrum of advanced or metastatic cancers. A baseline PET/CT with 68 Ga-DOTA-FAPI is used to identify patients eligible for 177 Lu-DOTA-FAPI therapy, which is then administered in escalating doses to determine the Recommended Phase 2 Dose (RP2D) of the treatment ( 22 ). NCT04939610 is a similar study that investigates the safety and efficacy of 68 Ga-DOTA-FAPI and 177 Lu-DOTA-FAPI in patients with locally advanced or metastatic cancer. This study also uses a 68 Ga-DOTA-FAPI PET/CT scan to identify patients eligible for 177 Lu-DOTA-FAPI therapy, which is administered in escalating doses to obtain the RP2D of 177 Lu-DOTA-FAPI ( 23 ).
CCK2 receptors have been shown to be overexpressed in various cancers, including medullary thyroid cancer (MTC). Radiolabeled peptides, such as 177 Lu-DOTA-CCK, have been assayed in the treatment of MTC ( 24 ). Also, use of 177 Lu-DOTA-CCK in combination with other treatments, such as chemotherapy, may have promise in improving overall survival rates in patients with advanced MTC. NCT02088645 aims to investigate 177 Lu-PP-F11N, a gastrin analog, for imaging and therapy in patients with advanced medullary thyroid carcinoma (MTC), as well as gastroenteropancreatic-neuroendocrine tumors (GEP-NET) and NETs of the lung or thymus. The trial consists of two phases: a pilot study to evaluate tumor detection and a dose escalation phase to determine the maximum tolerated dose of 177 Lu-PP-F11N in patients with MTC. The study will also assess the correlation between dose and treatment response, as well as organ radiation exposure and the maximum tolerated dose, in order to develop individualized therapy planning ( 25 ).
CD46 is a membrane-bound complement regulator protein that is overexpressed in various cancer cells. CD46-targeted radionuclide therapy has shown promising results in preclinical studies, with significant tumor growth inhibition observed in various tumor models ( 26 ). NCT05245006 is an early phase study that aims to investigate the feasibility and safety of targeting CD46 in mCRPC using an imaging biomarker and an antibody-drug conjugate ( 27 ).
Melanocortin sub-type 1 receptor (MC1R) is a G protein-coupled receptor that plays a critical role in skin pigmentation and is involved in the regulation of cell proliferation and differentiation. MC1R has been identified as a potential target for radionuclide therapy in melanoma, as its expression is highly upregulated in melanoma cells compared to normal skin cells. Preclinical studies have investigated the use of radiolabeled alpha-melanocyte-stimulating hormone (α-MSH), a natural ligand of MC1R, for targeted radionuclide therapy of melanoma. NCT04904120 aims to evaluate the safety and feasibility of using the agents ( 203 Pb-VMT01 and 68 Ga-VMT02) to image melanoma tumors expressing the melanocortin sub-type 1 receptor (MC1R). The study has a cross-over design in which participants with stage IV or inoperable stage III metastatic melanoma serve as their own comparators. The primary outcome measure is the safety and tolerability of the imaging agents, and the results will be used to guide the development of imaging and dosing for future trials of 212 Pb-VMT01 in the treatment of metastatic melanoma ( 28 ).
5. Conclusion
The field of nuclear medicine is growing field and the use of TRT for imaging and therapy is becoming increasingly ubiquitous in clinical trials and routine clinical practice. Obstacle include the risk of radiation exposure and associated adverse events. Often side effects from these therapies include fatigue, nausea and myelosuppression. Several ongoing trials aim to reduce toxicity and enhance efficacy by combining TRT with pre-medication or other therapies. Larger clinical trials are needed to provide high-quality evidence on optimal dosing and timing of when to include TRT in the algorithm of patient care along the course of their disease. A handful of early phase trials illustrate the continued search for novel TRT with improved specificity for cancer cells and identification of new targets beyond SSTR and PSMA.
We hope this brief overview inspires interest in the area of TRT and showcases the current state of radionuclide therapies in clinical trials, and emerging field with very promising new treatment options for our cancer patients.

Author contributions
All authors have contributed to the preparation, drafting, and revision of this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
PK is a consultant and/or speaker for Amgen, Bayer, Blue Earth Diagnostics, Chimerix, Eisai, Fusion Pharma, General Electric Healthcare, Invicro, Novartis, Radionetics, and UroToday. He is a recipient of research grants from Blue Earth Diagnostics and General Electric Healthcare. KZ is a consultant for Invicro and Fusion Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) KZ declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ClinicalTrials.gov. Available at: https://clinicaltrials.gov (Accessed June 3, 2023)
2. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O’Dorisio TM, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging . (2013) 40(5):800–16. doi: 10.1007/s00259-012-2330-6
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Duan H, Iagaru A, Aparici CM. Radiotheranostics—precision medicine in nuclear medicine and molecular imaging. Nanotheranostics . (2022) 6(1):103–17. doi: 10.7150/ntno.64141
4. Fortunati E, Bonazzi N, Zanoni L, Fanti S, Ambrosini V. Molecular imaging theranostics of neuroendocrine tumors. Semin Nucl Med . (2023) 53(4):539–554. doi: 10.1053/j.semnuclmed.2022.12.00736623974
5. Baratto L, Jadvar H, Iagaru A. Prostate cancer theranostics targeting gastrin-releasing peptide receptors. Mol Imaging Biol . (2018) 20(4):501–9. doi: 10.1007/s11307-017-1151-1
6. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177 Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med . (2017) 376(2):125–35. doi: 10.1056/NEJMoa1607427
7. Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol . (2020) 31(6):844–60. doi: 10.1016/j.annonc.2020.03.304
8. O’Dorisio S. Dosimetry-Guided, Peptide Receptor Radiotherapy (PRRT) With 90Y-DOTA- tyr3-Octreotide (90Y-DOTATOC). Clinicaltrials.gov (2017). Available at: https://clinicaltrials.gov/ct2/show/NCT03273712 (Accessed February 15, 2023)
9. Clarity Pharmaceuticals Ltd. 67Cu-SARTATE™ Peptide Receptor Radionuclide Therapy Administered to Pediatric Patients With High-Risk, Relapsed, Refractory Neuroblastoma. Clinicaltrials.gov (2019). Available at: https://clinicaltrials.gov/ct2/show/NCT04023331 (Accessed February 15, 2023)
10. Galgano S. [68Ga]DOTATATE-PET/MRI in Hepatocellular Carcinoma (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03648073 (Accessed February 15, 2023)
11. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol . (2018) 19(6):825–33. doi: 10.1016/S1470-2045(18)30198-0
12. Rahbar K, Afshar-Oromieh A, Jadvar H, Ahmadzadehfar H. PSMA theranostics: current status and future directions. Mol Imaging . (2018) 17:1536012118776068. doi: 10.1177/1536012118776068
13. Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. Ac-225-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med . (2016) 57(12):1941–4. doi: 10.2967/jnumed.116.178673
14. Australian and New Zealand Urogenital and Prostate Cancer Trials Group. A Trial of 177Lu-PSMA617 Theranostic Versus Cabazitaxel in Progressive Metastatic Castration Resistant Prostate Cancer (TheraP) (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03392428 (Accessed February 15, 2023)
15. Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet . (2021) 397(10276):797–804. doi: 10.1016/S0140-6736(21)00237-3
16. External Beam Therapy With Theranostic Radioligand Therapy for Oligometastatic Prostate Cancer (ProstACT TARGET)—Full Text View—ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT05146973 (Cited February 14, 2023) (Accessed February 15, 2023)
17. Clarity Pharmaceuticals Ltd. A Phase I/IIa Theranostic Study of 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA for Identification and Treatment of PSMA-expressing Metastatic Castrate Resistant Prostate Cancer. clinicaltrials.gov. Report No.: NCT04868604 (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT04868604 (Cited February 13, 2023) (Accessed February 15, 2023)
18. Peter MacCallum Cancer Centre, Australia. Study of the Dosimetry, Safety and Potential Benefit of 177Lu-PSMA-617 Radionuclide Therapy Prior to Radical Prostatectomy in Men With High-risk Localised Prostate Cancer. clinicaltrials.gov. Report No.: NCT04430192 (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT04430192 (Cited February 13, 2023) (Accessed February 15, 2023)
19. Prior JO. Theranostics in Soft Tissue Sarcoma Using a Vascular Disruption Approach. clinicaltrials.gov. Report No.: NCT05420727 (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT05420727 (Cited February 13, 2023) (Accessed February 15, 2023)
20. Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol . (2007) 213(1):91–8. doi: 10.1002/path.2207
21. Fusion Pharmaceuticals Inc. A Phase 1/2 Study of [225Ac]-FPI-1966, [111In]-FPI-1967, and Vofatamab in Participants With FGFR3-expressing Advanced, Inoperable, Metastatic and/or Recurrent Solid Tumours. clinicaltrials.gov. Report No.: NCT05363605 (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05363605 (Cited February 13, 2023) (Accessed February 15, 2023)
22. The First Affiliated Hospital of Xiamen University. 68 Ga-DOTA-FAPI and 177Lu-DOTA-FAPI Theranostic Pair in Patients With Various Types of Cancer (Locally Advanced or Metastatic Cancer). clinicaltrials.gov. Report No.: NCT04849247 (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04849247 (Cited February 13, 2023) (Accessed February 15, 2023)
23. Clovis Oncology, Inc. LuMIERE: A Phase 1/2, Multicenter, Open-label, Non-randomized Study to Investigate Safety and Tolerability, Pharmacokinetics, Dosimetry, and Preliminary Activity of 177Lu-FAP-2286 in Patients With an Advanced Solid Tumor. clinicaltrials.gov. Report No.: NCT04939610 (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT04939610 (Cited February 13, 2023) (Accessed February 15, 2023)
24. Salavati A, Puranik A. Peptide receptor radionuclide therapy (PRRT) of medullary and nonmedullary thyroid cancer using radiolabeled somatostatin analogues. Semin Nucl Med . (2016) 46(3):215–24. doi: 10.1053/j.semnuclmed.2016.01.010
25. University Hospital, Basel, Switzerland. 177Lu-PP-F11N for Receptor Targeted Therapy and Imaging (Theranostics) of Metastatic Medullary Thyroid Cancer—a Pilot and a Phase I Study. clinicaltrials.gov. Report No.: NCT02088645 (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT02088645 (Cited February 13, 2023) (Accessed February 15, 2023)
26. Elvington M, Liszewski MK, Atkinson JP. CD46 and oncologic interactions: friendly fire against cancer. Antibodies (Basel) . (2020) 9(4):59. doi: 10.3390/antib9040059
27. Flavell R. A First-in-Human, Pilot PET Imaging Study of 89Zr-DFO-YS5, an immunoPET Agent for Detecting CD46 Positive Malignancy in Men With Prostate Cancer. clinicaltrials.gov. Report No.: NCT05245006 (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05245006 (Cited February 13, 2023) (Accessed February 15, 2023)
Google Scholar
28. Viewpoint Molecular Targeting. A Phase 1 Cross-over Biodistribution Study of [203Pb]VMT01 for Single Photon Emission Computed Tomography (SPECT) Imaging and [68Ga]VMT02 for Positron Emission Tomography (PET) Imaging of Stage IV Metastatic Melanoma. clinicaltrials.gov. Report No.: NCT04904120 (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT04904120 (Cited February 13, 2023) (Accessed February 15, 2023)
Keywords: targeted radionuclide therapy, clinical trials, somatostatin receptors, prostate specific membrane antigen, nuclear medicine
Citation: Healy A, Ho E, Kuo P and Zukotynski K (2023) A brief overview of targeted radionuclide therapy trials in 2022. Front. Nucl. Med. 3:1169650. doi: 10.3389/fnume.2023.1169650
Received: 19 February 2023; Accepted: 26 May 2023; Published: 23 June 2023.
Reviewed by:
© 2023 Healy, Ho, Kuo and Zukotynski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine Zukotynski [email protected]
† These authors have contributed equally to this work
Advertisement

- Previous Article
- Next Article
Introduction
Immunomodulatory effects of external beam irradiation, immunomodulatory effects of trt, preclinical studies on combined trt and ici, clinical studies on combined trt and ici, discussion and future perspectives, authors' disclosures, acknowledgments, combining targeted radionuclide therapy and immune checkpoint inhibition for cancer treatment.
Clin Cancer Res 2022;28:3652–7
- Funder(s): Dutch Cancer Society
- Award Id(s): 12567
- Funder(s): Dutch Research Council
- Award Id(s): 09150172010054

- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Version of Record September 1 2022
- Proof May 9 2022
- Accepted Manuscript April 26 2022
Simone C. Kleinendorst , Egbert Oosterwijk , Johan Bussink , Harm Westdorp , Mark W. Konijnenberg , Sandra Heskamp; Combining Targeted Radionuclide Therapy and Immune Checkpoint Inhibition for Cancer Treatment. Clin Cancer Res 1 September 2022; 28 (17): 3652–3657. https://doi.org/10.1158/1078-0432.CCR-21-4332
Download citation file:
- Ris (Zotero)
- Reference Manager
The development of immunotherapy, in particular immune checkpoint inhibitors (ICI), has revolutionized cancer treatment in the past decades. However, its efficacy is still limited to subgroups of patients with cancer. Therefore, effective treatment combination strategies are needed. Here, radiotherapy is highly promising, as it can induce immunogenic cell death, triggering the release of pro-inflammatory cytokines, thereby creating an immunogenic phenotype and sensitizing tumors to ICI. Recently, targeted radionuclide therapy (TRT) has attained significant interest for cancer treatment. In this approach, a tumor-targeting radiopharmaceutical is used to specifically deliver a therapeutic radiation dose to all tumor cells, including distant metastatic lesions, while limiting radiation exposure to healthy tissue. However, fundamental differences between TRT and conventional radiotherapy make it impossible to directly extrapolate the biological effects from conventional radiotherapy to TRT. In this review, we present a comprehensive overview of studies investigating the immunomodulatory effects of TRT and the efficacy of combined TRT-ICI treatment. Preclinical studies have evaluated a variety of murine cancer models in which α- or β-emitting radionuclides were directed to a diverse set of targets. In addition, clinical trials are ongoing to assess safety and efficacy of combined TRT-ICI in patients with cancer. Taken together, research indicates that combining TRT and ICI might improve therapeutic response in patients with cancer. Future research has to disclose what the optimal conditions are in terms of dose and treatment schedule to maximize the efficacy of this combined approach.
The development of immune checkpoint inhibitors (ICI) revolutionized cancer treatment ( 1 ). Key immune checkpoints are inhibitory T-cell regulators cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1; refs. 2, 3 ). Antibodies blocking these checkpoints, such as ipilimumab (anti–CTLA-4), pembrolizumab and nivolumab (anti–PD-1), atezolizumab and durvalumab (anti–PD-L1), have shown remarkable efficacy in subsets of patients with cancer ( 1 ). However, a significant number of patients do not respond because they fail to generate an effective antitumor immune response ( 4 ). To increase the efficacy in nonresponding patients, combinations with other therapies that create a more immunogenic tumor microenvironment (TME) and thereby sensitize tumors to ICI, are needed.
There is considerable evidence that ionizing radiation can boost antitumor immunity, mostly originating from studies with external beam radiation therapy (EBRT). However, with EBRT only a limited number of tumor lesions are irradiated. In targeted radionuclide therapy (TRT), a radionuclide is either linked to a carrier molecule, such as a small molecule or a monoclonal antibody directed towards a TME-associated antigen, or accumulates in lesions of interest by physiologic uptake ( 5 ). Therefore, TRT results in specific irradiation of all tumor lesions, regardless of location, while sparing healthy tissue. This makes TRT an attractive approach to treat patients with metastatic disease or tumors present in close proximity to radiosensitive organs, to which EBRT would cause severe toxicity ( 6 ). TRT has grown significantly over recent years, exemplified by the NETTER-1 trial ( 7 ) and subsequent clinical approval of lutetium-177-DOTATATE ([ 177 Lu]Lu-DOTATATE) for the treatment of neuroendocrine tumors, the phase III VISION trial with [ 177 Lu]Lu-PSMA-617 for prostate cancer treatment (NCT03511664), and the development of agents directed to TME-associated targets (e.g., fibroblast activation protein), which are not limited to treat one specific cancer type ( 8, 9 ).
The biological effects of radiation depend on many factors including total absorbed dose, absorbed dose heterogeneity, dose rate, and fractionation, which are different between EBRT and TRT and also differ for various types of TRT ( 6 ). Definition of these and other common radiation terms are included in Table 1 ( 10 ). EBRT involves a homogeneous beam of X-rays with a low linear energy transfer (LET). EBRT is given to the tumor at a high dose rate often in a fractionated manner. In TRT the absorbed dose rate is 100 to 1,000 times lower, but the exposure time of the tumor is much longer. Furthermore, in TRT, the radiation exposure is heterogeneous, consisting of α-, β-, or Auger particles accompanied or not by X or γ rays. These particles have variable LET, path-length, and half-life, all depending on the radionuclide. β-particles generally have a multicellular range (1–10 mm) and high energy (0.1–1 MeV), α-particles have a cellular range (50–80 μm), and very high energy (5–8 MeV), and Auger electrons have a very short, subcellular range (1–1,000 nm) and low energy (<25 keV; refs. 5, 8, 11 ). Due to these different physical properties, findings regarding immunologic effects of radiation cannot be directly extrapolated to TRT ( 12 ).
Definition of radiation terms.
Note: Data from ICRU report 96 (ref. 10 ).
This review aims to ( 1 ) describe the immunomodulatory effects of TRT ( 2 ), present an overview of the studies on combined TRT and ICI to date, and ( 3 ) provide directions for future research.
EBRT generates local tumor control through DNA damage–induced cell death, but in rare clinical cases regression of distant unirradiated lesions has been observed: the “abscopal effect” ( 13, 14 ). Preclinical studies have shown the role of the adaptive immune system in these abscopal responses. An important mechanism involved is immunogenic cell death (ICD). ICD can be triggered by reactive oxygen species, which are formed upon ionizing radiation ( 15, 16 ). To induce ICD-based antitumor immune responses, four steps are required. First, radiation increases the presence of neoantigens and tumor-associated antigens resulting in a so-called in situ vaccination effect ( 17 ). Second, cytokines and DAMP, such as ATP, high mobility group box 1 (HMGB1), calreticulin, and annexin-A1 are released. In addition, the cytoplasmic DNA-sensing cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway can be activated by cytosolic DNA ( 18–20 ). The resulting type I interferon response is essential for dendritic cell (DC) function and is therefore a central player in activation of adaptive immune responses ( 21–23 ). Third, professional antigen-presenting cells (APC), such as DCs are recruited into the tumor ( 24, 25 ). Fourth, tumor-specific effector T cells are activated and infiltrate the tumor, resulting in a long-lasting antitumor immune response ( 26 ). Opposed to these immunostimulatory effects, radiation also induces suppressive signaling, directly or indirectly through activation of the cGAS-STING-IFN1 axis ( 27 ). This is characterized by the recruitment of immunosuppressive cells, like regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC; refs. 28, 29 ), and PD-L1 upregulation ( 28, 30 ). In addition, immune cells are inherently radiosensitive, thus radiation can also deplete tumor-infiltrating lymphocytes. Whether radiation induces immunostimulatory or -suppressive effects can be dose-dependent. Taken together, the irradiated TME can be chronically inflamed, but also strongly immunosuppressive ( Fig. 1 ). In this regard, a combination of EBRT and ICI could improve treatment efficacy. Various preclinical studies have shown that the combination of EBRT with ICI can improve treatment outcome. A large phase III trial in patients with unresectable stage III non–small cell lung cancer (PACIFIC) demonstrated improved progression-free survival and overall survival ( 31 ) and many clinical studies are ongoing [reviewed in ( 16, 32, 33 )]. Nevertheless, the response to EBRT-ICI combination therapy highly depends on tumor type and many open questions remain about the optimal dose, fractionation, and treatment schedule for EBRT-ICI combinations ( 16 ).

Immunostimulatory (left) and immunosuppressive (right) effects of radiation. ICD results in the release of DAMPs such as ATP, HMGB1, calreticulin (CALR), and annexin A1 (ANXA1). This release promotes cross-presentation of tumor antigens by DCs, resulting in expansion of CD8 + T cells. The diversity of the T-cell receptor (TCR) repertoire on CD8 + T cells and expression of MHC on tumor cells are increased. The presence of cytosolic DNA triggers cGAS-STING signaling, resulting in IFN1-induced inflammatory remodeling. Release of chemokine (C-X-C motif) ligand 9 (CXCL9), CXCL10, and CXCL16 by tumor cells and DCs promotes T-cell infiltration. On the other hand, tumor cells release immunosuppressive C-C motif chemokine 5 (CCL5) and CCL2, which promote infiltration of Tregs and myeloid-derived suppressor cells (MDSC). Production of TGFβ inhibits DC maturation and T-cell cytotoxicity. PD-L1 overexpression on tumor cells results in T-cell inhibition.
TRT with β -emitting radionuclides
Several immunomodulatory effects of irradiation described for EBRT have also been observed for β-emitting TRT. In a murine melanoma model, treatment with iodine-131-anti-melanin ([ 131 I]I-anti-melanin) increased the presence of DAMPs annexin-A1 and calreticulin ( 34 ), demonstrating that β-irradiation may induce ICD. In two recent preclinical studies with yttrium-90-NM600 ([ 90 Y]Y-NM600) activation of the STING-IFN1 pathway and production of pro-inflammatory cytokines were observed ( 35, 36 ). Modulation of the immune system by β-emitter TRT is also exemplified by changes in immune-cell infiltration. Several studies have observed an increased infiltration of immunostimulatory immune cells into the TME after TRT, for example CD4 + and/or CD8 + T cells ( 34, 36–39 ), APCs, natural killer cells ( 36, 40 ), and other innate immune cells ( 34, 36 ). In addition, the amount of immunosuppressive immune cells, like Tregs or macrophages, was reported to decrease after TRT ( 37, 39 ). However, these events are not always consistent between all treatments or models. In a recent study using [ 177 Lu]Lu-albumin the numbers of CD4 + and CD8 + T cells in the TME were reduced and fewer circulating B cells and DCs were found ( 39 ). β-irradiation can also trigger immunosuppressive features: two independent studies with [ 177 Lu]Lu-anti–PD-L1 and [ 177 Lu]Lu-anti–integrin-α v ß 3 have shown upregulation of PD-L1 and increased infiltration of PD-L1 + immune cells to the TME ( 38, 41 ). In addition, increased expression of Treg-regulated genes and genes involved in immune tolerance were found after [ 131 I]I-anti-melanin ( 34 ). So far, evidence for abscopal effects of radionuclide therapy is limited to one case, where 90 Y-radioembolization resulted in complete regression of an untargeted lesion ( 42 ).
TRT with α -emitting radionuclides
Although the number of in-depth studies with α-emitter TRT is relatively limited, there is substantial evidence that α-irradiation can induce ICD. Radium-223-dichloride ([ 223 Ra]Ra-dichloride) treatment resulted in calreticulin upregulation and in vitro activation of CD8 + T cells ( 43 ). Similarly, studies with thorium-227 ( 227 Th)-conjugates targeted to mesothelin ( 44, 45 ) and bismuth-213-albumin ([ 213 Bi]Bi-albumin; ref. 46 ) observed upregulation of DAMPs and DC activation in vitro . When mice were vaccinated with [ 213 Bi]Bi-albumin-irradiated cancer cells prior to injection of non-irradiated cancer cells, immunocompetent but not immunodeficient mice were protected from tumor growth for at least two months ( 46 ). A similar protective effect after vaccination was found with lead-212 ( 212 Pb) targeted to the melanocortin 1 receptor ( 47 ) and radium-224 ( 224 Ra; ref. 48 ). In the latter study, the protective effect was more pronounced in a highly immunogenic tumor model compared with a weakly immunogenic model. In addition, changes in immune-cell infiltration upon α-irradiation have been reported. One preclinical study showed that melanocortin 1 receptor-targeted 212 Pb-TRT increased the number of tumor-infiltrating lymphocytes ( 47 ) and increased neutrophil blood counts were found after astatine-211-anti-PARP ([ 211 At]At-anti-PARP) therapy ( 49 ). However, a decrease of CD8 + T cells in the TME initially decreased lymphocyte blood counts, and increased infiltration of macrophages and CD4 + T cells were also observed in the latter study ( 49 ). In patients with prostate cancer, 223 Ra-treatment induced changes in circulating immune cells and immune checkpoint expression. For example, 223 Ra can reduce the number of PD-1 expressing CTLs, increase CTLs expression of co-stimulatory or inhibitory (PD-L1, PD-1, and TIM-3) checkpoint molecules, and increase expression of PD-L1 on plasma-derived exosomes ( 50–52 ). In addition, solid tumor biopsies of 223 Ra-treated patients showed PD-L1 upregulation ( 52 ).
Combination of β -emitter TRT with ICI
Various preclinical studies have examined combination therapy of 177 Lu-TRT with ICI ( 38, 39, 41, 53–56 ). For example, Chen and colleagues evaluated a combination of [ 177 Lu]Lu-EB-RGD, targeting integrin α v ß 3 , with anti–PD-L1 in a colorectal cancer model ( 41 ). Mice were responsive to both TRT and ICI monotherapy, but combination therapy was superior in delaying tumor growth and prolonging survival. After combined treatment tumors demonstrated reduced glucose metabolism, a lower vascular density, increased apoptosis, earlier necrosis, and reduced tumor cell proliferation, compared with monotherapy. Furthermore, the number of tumor-infiltrating CD8 + T cells significantly increased, while the number of Tregs did not change. Finally, combination therapy generated immunologic memory, as recovered mice rechallenged with cancer cells, rejected the tumors. Other studies have confirmed the superior efficacy of 177 Lu-TRT-ICI combination therapy in various tumor models and for different types of ICIs ( 38, 39, 53–55 ). Occasionally, efficacy could be explained by increased infiltration of T cells or decreased infiltration of immunosuppressive cells into the TME ( 38, 39 ), while others did not observe these changes ( 54 ). Furthermore, immunosuppressive features like enhanced PD-L1 expression were reported ( 38 ). Efficacy of TRT-ICI combination therapy has also been reported for the β-emitters 131 I and 90 Y in various tumor models ( 34–36, 57 ). Again, the immunologic effects of combined treatment were diverse and sometimes contradictory.
In contrast, several studies do not support the potential efficacy of β-TRT-ICI. For example, combined [ 90 Y]Y-NM600-ICI therapy could not control primary tumor growth in a Lewis lung carcinoma model, while in various other cancer models it could ( 57 ). In addition, melanin-targeted 177 Lu-TRT combined with anti–PD-1 or anti–PD-L1 in a melanoma model did not improve therapeutic efficacy compared with ICI monotherapy, while combination with anti–CTLA-4 therapy was successful ( 34 ). The authors suggest that immunogenic tolerance dominates the observed immune escape after TRT, as T-cell exhaustion was absent. Finally, one study even reported a negative effect of combining TRT with anti–PD-1 in a breast cancer model, where VEGF-targeted 177 Lu-TRT monotherapy was very effective, but the addition of anti–PD-1 diminished the therapeutic effect ( 56 ).
Combination of α -emitter TRT with ICI
Combined α-emitter TRT and ICI has shown diverse results. For example, [ 212 Pb]Pb-VMT01 targeting melanocortin 1 receptor induced immunogenicity in an otherwise immunotolerant murine melanoma model ( 47 ). In addition, when cancer cells were irradiated in vitro before injection, the tumor was sensitized to ICI treatment. Also, combined [ 212 Pb]Pb-VMT01 and ICI (anti–CTLA-4 and anti–PD-1) more effectively inhibited tumor growth compared with TRT or ICI monotherapy. Rechallenge of mice that showed complete response with cancer cells resulted in very slow or absent tumor growth, indicating the presence of adaptive antitumor immunity. Superior efficacy of combined α-emitter TRT and ICI has also been reported for 211 At, 213 Bi, 225 Ac, 227Th , and 223 Ra directed to various targets ( 45, 49, 52, 58, 59 ) and in the latter studies, T-cell activation was observed ( 45, 52 ).
In contrast to these findings, melanin-targeted or PD-L1–targeted 225 Ac-TRT-ICI combination therapy was not superior to monotherapies in a melanoma ( 54 ) and breast cancer model ( 60 ), respectively. In the latter study, combination therapy even reduced survival significantly compared with monotherapies, although it was not reported whether this effect was due to progressive tumor growth or treatment toxicity ( 60 ). In addition, the therapeutic efficacy of combined [ 213 Bi]Bi-anti-melanin and anti–PD-1 in murine melanoma was dependent on the treatment schedule, with the most effective growth inhibition when ICI was sandwiched between two doses of TRT or when TRT was administered after ICI ( 58 ). Finally, combined [ 213 Bi]Bi-anti-melanin and anti–CTLA-4 therapy did not improve TRT monotherapy in a metastatic melanoma model ( 61 ).
Several trials with combined TRT and ICI are currently ongoing (NCT03658447, NCT03805594, NCT04261855, NCT03457948, NCT03325816). So far, results are only available from the [ 177 Lu]Lu-DOTATATE (Luthathera) phase I study, which showed that combined Lutathera and nivolumab treatment was safe and led to antitumor activity in some patients ( 62 ). In addition, two recent case studies with [ 177 Lu]Lu-DOTATATE-ICI therapy in a patient with an aggressive pituitary tumor and [ 177 Lu]Lu-DOTATOC-ICI in a patient with metastatic Merkel cell carcinoma showed safety and antitumor activity ( 63, 64 ). Finally, two phase II trials with [ 177 Lu]Lu-TLX250 in combination with ICI for the treatment of metastatic clear cell renal cell carcinoma, are awaited (STARLITE-1 and STARLITE-2).
Several preclinical studies provide proof-of-concept that TRT-ICI combination therapy can initiate powerful antitumor immune responses. On the other hand, a number of studies have reported negative or contradictory findings. Therefore, a better understanding of radiobiological and immunologic effects is crucial to optimize TRT-ICI combination therapy for clinical translation.
Absorbed dose to the tumor
How cancer cells die and modulate the TME in response to radiation depends on the radiation dose ( 22 ). With TRT, the tumor absorbed dose is determined by the administered activity and tracer accumulation and retention in the tumor. The latter is determined by several factors, such as target expression and tumor perfusion. Dosimetry can be used to accurately estimate the absorbed dose in the tumor. However, the absence of dosimetry in most of the published studies makes it impossible to directly relate tumor dose to immune effects and to compare findings between different studies. Furthermore, the extent of immune modulation may depend on the characteristics of the radionuclide used. For example, α-particles contain much higher energies than β-particles and deposit this energy over a much shorter range at different dose rates. In-depth studies investigating the relation between the physical properties of the radionuclide and the radiobiological and immunologic effects are lacking. Therefore, future preclinical TRT studies should include dosimetry to assess dose-effect relationships to elucidate which dose, particle type, or dose rate is preferred for immune activation.
Tumor characteristics
Various characteristics of the tumor and its microenvironment determine the response or resistance to TRT, including tumor solidity, intra-tumor heterogeneity, and mutations of specific cellular mechanisms such as apoptotic pathways ( 65 ). First, the intrinsic radiosensitivity of cancer cells is highly heterogeneous both within and between tumor types and depends on the genetic background ( 66, 67 ). This affects the type of cell death induced by radiation and, therefore, the extent of ICD and tumor-associated antigen release ( 15 ). Second, TME characteristics, such as tumor metabolism, perfusion, hypoxia, and immunogenicity determine responsiveness to both radiation and immunotherapy, and therefore, most likely also affect radiation-induced immune modulation ( 22, 68 ). With regards to TRT, hypoxia is of particular interest as the effects of α-radiation are less dependent on oxygen, which potentially makes α-TRT a fitting treatment for hypoxic tumors that are otherwise resistant to conventional EBRT and ICI ( 5 ). Taken together, resistance to TRT is determined by various tumor characteristics, which most likely also affect TRTs immunologic effects.
Finally, it remains to be elucidated which types of targets are preferred to enhance immune responses. For example, targeting vasculature did not synergize with ICI therapy ( 56 ), and further research should examine how targeting tumor stroma or other components of the TME would impact immunomodulation. Future research should investigate the relation between tumor characteristics and immunologic response to TRT, as these insights are essential to understand which tumor types will benefit from TRT-ICI combination therapies in the clinical setting. Elaborate tumor characterization and careful selection of tumor model would, therefore, improve preclinical studies on TRT-ICI combination therapy.
Treatment schedule
Mechanistically, sequential TRT followed by ICI is expected to be most effective, as TRT will induce ICD resulting in an immunogenic TME followed by ICI to release the brakes of the immune system. Notably, TRT generally exposes the tumor to a low dose rate over a long period, which may result in irradiation of infiltrating immune cells potentially hampering the antitumor immune response. Comparison of different treatment schedules within one study showed that concurrent administration was more effective than sequential administration ( 41, 45, 58 ), but only a limited number of studies included this comparison and elaborated on the underlying mechanism. In all studies, different readouts to measure immune effects were used, such as cytokine release and IHC analysis of tumor tissue, but selecting the appropriate time-window for these methods is challenging. In vivo molecular imaging of immune cells and other relevant immune-related targets could help to elucidate the immune effects of TRT in living animals or patients. In addition, only one study examined the effect of fractionation and found that the efficacy of combined TRT-ICI was decreased ( 63 ). Future studies are warranted to directly compare treatment schedules and fractionation regimens and to investigate the underlying mechanisms ( 16 ). Finally, whether hematologic toxicity upon TRT might limit ICI efficacy remains unexplored. Therefore, ICI dose-finding for TRT combination strategies remains an important issue for future research to reduce ICI toxicity without compromising efficacy ( 69, 70 ).
TRT is a rapidly expanding field aiming to treat patients with metastatic cancer and its ionizing effects can potentially boost antitumor immunity. This review provides an up-to-date overview of the immunomodulatory effects of TRT and its efficacy in combination with ICI. However, the results discussed in this review are dispersed and the optimal conditions for clinical translation remain largely unknown. Therefore, in-depth preclinical studies are warranted, to elucidate how absorbed dose, fractionation, and tumor characteristics are related to neoantigen release, ICD induction, and long-lasting antitumor immune responses. These studies will have essential implications for the design of future clinical trials on combined TRT and ICI.
S. Heskamp reports grants from Dutch Cancer Society (12567), Dutch Research Council (09150172010054), and Telix Pharma during the conduct of the study. No disclosures were reported by the other authors.
This project was supported by grants from the Dutch Cancer Society (12567; to S. Heskamp) and grants from Dutch Research Council (NWO; 09150172010054; to S. Heskamp).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Citing articles via
Email alerts.
- Online First
- Collections
- Online ISSN 1557-3265
- Print ISSN 1078-0432
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Targeted radionuclide therapy--an overview
Affiliation.
- 1 Radiopharmaceuticals Division, Bhabha Atomic Research Centre, Mumbai 400 085, India. [email protected].
- PMID: 24059327
- DOI: 10.2174/18744710113066660023
Radionuclide therapy (RNT) based on the concept of delivering cytotoxic levels of radiation to disease sites is one of the rapidly growing fields of nuclear medicine. Unlike conventional external beam therapy, RNT targets diseases at the cellular level rather than on a gross anatomical level. This concept is a blend of a tracer moiety that mediates a site specific accumulation followed by induction of cytotoxicity with the short-range biological effectiveness of particulate radiations. Knowledge of the biochemical reactions taking place at cellular levels has stimulated the development of sophisticated molecular carriers, catalyzing a shift towards using more specific targeting radiolabelled agents. There is also improved understanding of factors of importance for choice of appropriate radionuclides based on availability, the types of emissions, linear energy transfer (LET), and physical half-life. This article discusses the applications of radionuclide therapy for treatment of cancer as well as other diseases. The primary objective of this review is to provide an overview on the role of radionuclide therapy in the treatment of different diseases such as polycythaemia, thyroid malignancies, metastatic bone pain, radiation synovectomy, hepatocellular carcinoma (HCC), neuroendocrine tumors (NETs), non-Hodgkin's lymphoma (NHL) and others. In addition, recent developments on the systematic approach in designing treatment regimens as well as recent progress, challenges and future perspectives are discussed. An examination of the progress of radionuclide therapy indicates that although a rapid stride has been made for treating hematological tumors, the development for treating solid tumors has, so far, been limited. However, the emergence of novel tumor-specific targeting agents coupled with successful characterization of new target structures would be expected to pave the way for future treatment for such tumors.
Publication types
- Research Support, U.S. Gov't, Non-P.H.S.
- Bone Diseases / radiotherapy
- Linear Energy Transfer
- Nanoparticles / chemistry
- Neoplasms / radiotherapy*
- Neuroendocrine Tumors / radiotherapy
- Nuclear Medicine / methods*
- Pain Management
- Polycythemia / radiotherapy
- Radioimmunotherapy / methods
- Radioisotopes / therapeutic use*
- Radiopharmaceuticals / chemistry*
- Radiopharmaceuticals / therapeutic use*
- Radiotherapy / methods*
- Radioisotopes
- Radiopharmaceuticals

IMAGES
VIDEO
COMMENTS
Abstract. Introduction: Targeted Radionuclide Therapy (TRT) is a branch of cancer medicine dealing with the therapeutic use of radioisotopes associated with biological vectors accumulating in the tumors/targets, indicated as Molecular Radiotherapy (MRT), or directly injected into the arteries that supply blood to liver tumour vasculature ...
EU Medical Devices Regulation 2017/745 applies also to Treatment Planning Systems (TPSs) for targeted radionuclide therapy (TRT). •. Several TPS are now commercially available for liver radioembolization and general purpose TRT. •. An overview of available TPSs is presented based on an ad hoc survey.
Targeted Radionuclide Therapy (TRT) is a branch of cancer medicine dealing with the use of radionuclides for a therapeutic purpose. Overview of commercial treatment planning systems for targeted radionuclide therapy - Physica Medica: European Journal of Medical Physics
Targeted Radionuclide Therapy (TRT) is a branch of cancer medicine dealing with the therapeutic use of radioisotopes associated with biological vectors accumulating in the tumors/targets, indicated as Molecular Radiotherapy (MRT), or directly injected into the arteries that supply blood to liver tumour vasculature, indicated as Selective RT ...
Request PDF | Overview of commercial treatment planning systems for targeted radionuclide therapy | Introduction Targeted Radionuclide Therapy (TRT) is a branch of cancer medicine dealing with the ...
DOI: 10.1016/j.ejmp.2021.11.001 Corpus ID: 244862341; Overview of commercial treatment planning systems for targeted radionuclide therapy. @article{DellaGala2021OverviewOC, title={Overview of commercial treatment planning systems for targeted radionuclide therapy.}, author={Giuseppe Della Gala and Manuel Bardi{\`e}s and Jill Tipping and Lidia Strigari}, journal={Physica medica : PM : an ...
Targeted radionuclide therapy has become increasingly prominent as a nuclear medicine subspecialty. For many decades, treatment with radionuclides has been mainly restricted to the use of iodine-131 in thyroid disorders. Currently, radiopharmaceuticals, consisting of a radionuclide coupled to a vector that binds to a desired biological target with high specificity, are being developed. The ...
Della Gala G, Bardiès M, Tipping J, Strigari L. Overview of commercial treatment planning systems for targeted radionuclide therapy Vol. 92, Physica Medica. Elsevier BV; 2021. p. 52-61. en
Targeted Radionuclide Therapy Treatment planning systems Dose calculation Kernel Dose volume histograms ABSTRACT Introduction: Targeted Radionuclide Therapy (TRT) is a branch of cancer medicine dealing with the therapeutic use of radioisotopes associated with biological vectors accumulating in the tumors/targets, indicated as Mo-
There is a growing use of radionuclide therapy for the medical care of oncology patients, where radioactive pharmaceuticals are used to target and treat various cancer types. This paper provides a brief overview illustrating the spectrum of ongoing and recently completed radionuclide therapy clinical trials in oncology. The trials selected highlight the potential of radionuclide therapies to ...
OBJECTIVE. This article reviews recent developments in targeted radionuclide therapy (TRT) approaches directed to malignant liver lesions, bone metastases, neuroendocrine tumors, and castrate-resistant metastatic prostate cancer and discusses challenges and opportunities in this field. CONCLUSION. TRT has been employed since the first radioiodine thyroid treatment almost 75 years ago. Progress ...
Abstract. The development of immunotherapy, in particular immune checkpoint inhibitors (ICI), has revolutionized cancer treatment in the past decades. However, its efficacy is still limited to subgroups of patients with cancer. Therefore, effective treatment combination strategies are needed. Here, radiotherapy is highly promising, as it can induce immunogenic cell death, triggering the ...
Targeted radionuclide therapy has become increasingly prominent as a nuclear medicine subspecialty. For many decades, treatment with radionuclides has been mainly restricted to the use of iodine-131 in thyroid disorders. Currently, radiopharmaceuticals, consisting of a radionuclide coupled to a vector that binds to a desired biological target ...
Abstract. Radionuclide therapy (RNT) based on the concept of delivering cytotoxic levels of radiation to disease sites is one of the rapidly growing fields of nuclear medicine. Unlike conventional external beam therapy, RNT targets diseases at the cellular level rather than on a gross anatomical level. This concept is a blend of a tracer moiety ...
Some AI technologies have already been clinically implemented by commercial systems. In this article, we will provide an overview to methods involved with treatment planning in radiation therapy. In particular, we will review the recent research and literature related to automation of the treatment planning process, leading to potentially ...
Radionuclide therapy (RNT) based on the concept of delivering cytotoxic levels of radiation to disease sites is one of the rapidly growing fields of nuclear medicine. Unlike conventional external ...
In comparison to external-beam radiation therapy, which involves personalized treatment regimens, most RPT administers a fixed activity. ... The impact of PET and SPECT on dosimetry for targeted radionuclide therapy. Z Med Phys. 2006; 16: 47 ... Overview of commercial treatment planning systems for targeted radionuclide therapy. Phys Med. 2021 ...
Tumor infiltration is directly responsible for the pain phenomenon. Targeted radionuclide therapy can offer a significant therapeutic alternative to relieve pain for the patients [138]. Increased osteoblastic activity under the effect of metastatic tumor cells is responsible for the hyperfixation of the radiotracer.