- Research article
- Open access
- Published: 04 June 2021

Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
15k Accesses
28 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
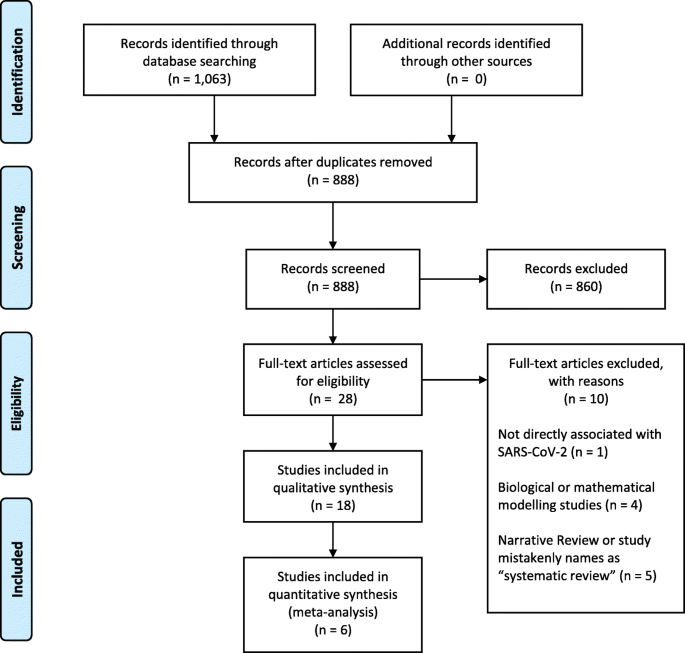
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
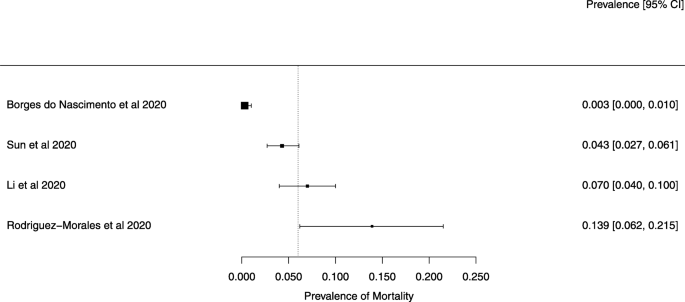
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
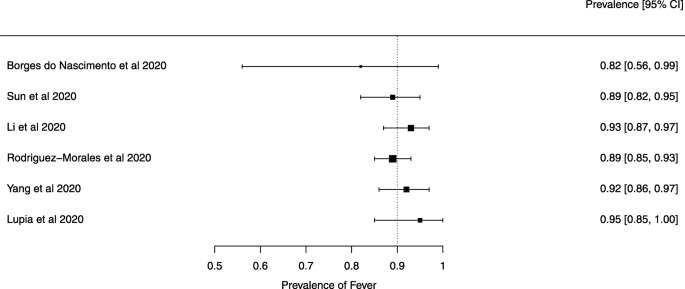
A meta-analysis of the prevalence of fever
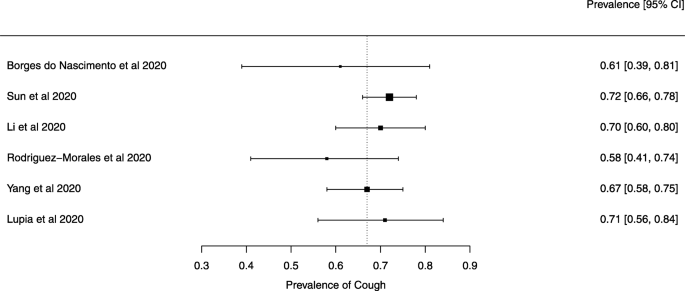
A meta-analysis of the prevalence of cough
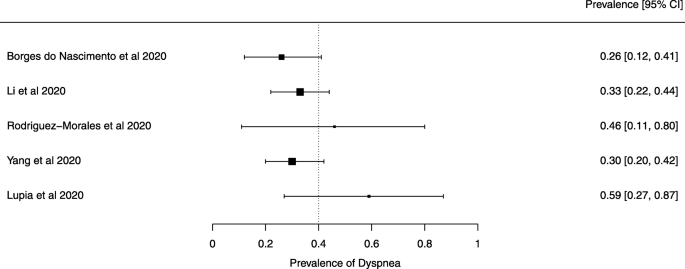
A meta-analysis of the prevalence of dyspnea
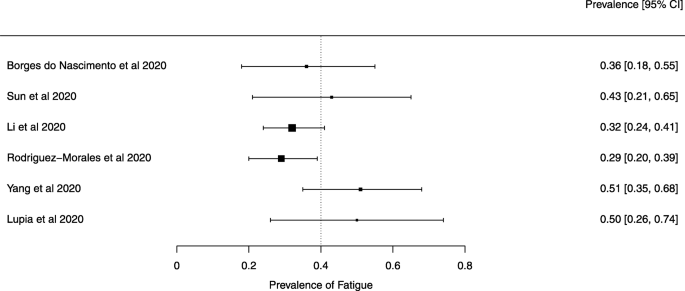
A meta-analysis of the prevalence of fatigue or myalgia
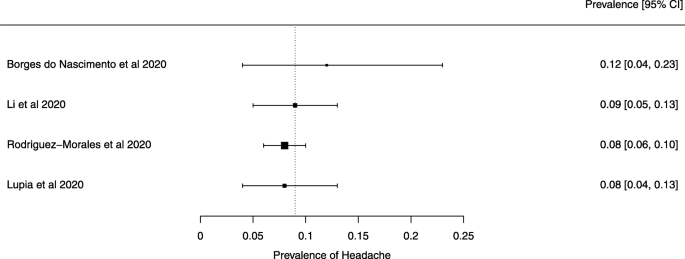
A meta-analysis of the prevalence of headache
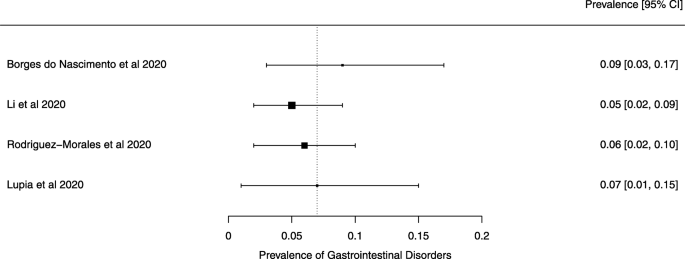
A meta-analysis of the prevalence of gastrointestinal disorders
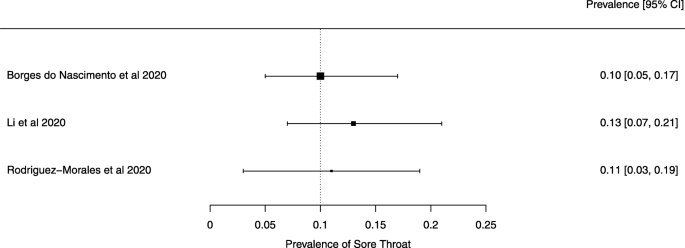
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Contact Tracing
- Pandemic Data Initiative
- Webcasts & Videos
- 30-Minute COVID-19 Briefing
Research Papers
Jhu has stopped collecting data as of.
After three years of around-the-clock tracking of COVID-19 data from...
The Johns Hopkins Coronavirus Resource Center has collected, verified, and published local, regional, national, and international pandemic data since it launched in March 2020. From the beginning, the information has been freely available to all — researchers, institutions, the media, the public, and policymakers. As a result, the CRC and its data have been cited in many published research papers and reports. Here we have gathered publications authored by CRC team members that focus on the CRC or its data.
July 14, 2022
Misaligned Federal and State Covid data limits demographic insights
CDC underreports cases and deaths among African American and Hispanic or Latino individuals.
February 17, 2022
Experts Call for Open Public Health Data
Johns Hopkins team highlighted the urgent need for better COVID data collection.
Unifying Epidemiologists and Economists
Researchers from disparate fields join to chart a new path for formulating policies in response to future pandemics.
Mobility Data Supported Social Distancing
Study found that physical distancing was an effective COVID mitigation strategy.
Johns Hopkins Engineers Build COVID Dashboard
Lancet Infectious Diseases published first paper detailing how the global map was built.
Researchers Identify Disparities in COVID Testing
Johns Hopkins team conducted an analysis of state-published demographic data
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Virology, transmission...
Virology, transmission, and pathogenesis of SARS-CoV-2
Read our latest coverage of the coronavirus outbreak.
- Related content
- Peer review
- Muge Cevik , clinical lecturer 1 2 ,
- Krutika Kuppalli , assistant professor 3 ,
- Jason Kindrachuk , assistant professor of virology 4 ,
- Malik Peiris , professor of virology 5
- 1 Division of Infection and Global Health Research, School of Medicine, University of St Andrews, St Andrews, UK
- 2 Specialist Virology Laboratory, Royal Infirmary of Edinburgh, Edinburgh, UK and Regional Infectious Diseases Unit, Western General Hospital, Edinburgh, UK
- 3 Division of Infectious Diseases, Medical University of South Carolina, Charleston, SC, USA
- 4 Laboratory of Emerging and Re-Emerging Viruses, Department of Medical Microbiology, University of Manitoba, Winnipeg, MB, Canada
- 5 School of Public Health, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China
- Correspondence to M Cevik mc349{at}st-andrews.ac.uk
What you need to know
SARS-CoV-2 is genetically similar to SARS-CoV-1, but characteristics of SARS-CoV-2—eg, structural differences in its surface proteins and viral load kinetics—may help explain its enhanced rate of transmission
In the respiratory tract, peak SARS-CoV-2 load is observed at the time of symptom onset or in the first week of illness, with subsequent decline thereafter, indicating the highest infectiousness potential just before or within the first five days of symptom onset
Reverse transcription polymerase chain reaction (RT-PCR) tests can detect viral SARS-CoV-2 RNA in the upper respiratory tract for a mean of 17 days; however, detection of viral RNA does not necessarily equate to infectiousness, and viral culture from PCR positive upper respiratory tract samples has been rarely positive beyond nine days of illness
Symptomatic and pre-symptomatic transmission (1-2 days before symptom onset), is likely to play a greater role in the spread of SARS-CoV-2 than asymptomatic transmission
A wide range of virus-neutralising antibodies have been reported, and emerging evidence suggests that these may correlate with severity of illness but wane over time
Since the emergence of SARS-CoV-2 in December 2019, there has been an unparalleled global effort to characterise the virus and the clinical course of disease. Coronavirus disease 2019 (covid-19), caused by SARS-CoV-2, follows a biphasic pattern of illness that likely results from the combination of an early viral response phase and an inflammatory second phase. Most clinical presentations are mild, and the typical pattern of covid-19 more resembles an influenza-like illness—which includes fever, cough, malaise, myalgia, headache, and taste and smell disturbance—rather than severe pneumonia (although emerging evidence about long term consequences is yet to be understood in detail). 1 In this review, we provide a broad update on the emerging understanding of SARS-CoV-2 pathophysiology, including virology, transmission dynamics, and the immune response to the virus. Any of the mechanisms and assumptions discussed in the article and in our understanding of covid-19 may be revised as further evidence emerges.
What we know about the virus
SARS-CoV-2 is an enveloped β-coronavirus, with a genetic sequence very similar to SARS-CoV-1 (80%) and bat coronavirus RaTG13 (96.2%). 2 The viral envelope is coated by spike (S) glycoprotein, envelope (E), and membrane (M) proteins ( fig 1 ). Host cell binding and entry are mediated by the S protein. The first step in infection is virus binding to a host cell through its target receptor. The S1 sub-unit of the S protein contains the receptor binding domain that binds to the peptidase domain of angiotensin-converting enzyme 2 (ACE 2). In SARS-CoV-2 the S2 sub-unit is highly preserved and is considered a potential antiviral target. The virus structure and replication cycle are described in figure 1 .

(1) The virus binds to ACE 2 as the host target cell receptor in synergy with the host’s transmembrane serine protease 2 (cell surface protein), which is principally expressed in the airway epithelial cells and vascular endothelial cells. This leads to membrane fusion and releases the viral genome into the host cytoplasm (2). Stages (3-7) show the remaining steps of viral replication, leading to viral assembly, maturation, and virus release
- Download figure
- Open in new tab
- Download powerpoint
Coronaviruses have the capacity for proofreading during replication, and therefore mutation rates are lower than in other RNA viruses. As SARS-CoV-2 has spread globally it has, like other viruses, accumulated some mutations in the viral genome, which contains geographic signatures. Researchers have examined these mutations to study virus characterisation and understand epidemiology and transmission patterns. In general, the mutations have not been attributed to phenotypic changes affecting viral transmissibility or pathogenicity. The G614 variant in the S protein has been postulated to increase infectivity and transmissibility of the virus. 3 Higher viral loads were reported in clinical samples with virus containing G614 than previously circulating variant D614, although no association was made with severity of illness as measured by hospitalisation outcomes. 3 These findings have yet to be confirmed with regards to natural infection.
Why is SARS-CoV-2 more infectious than SARS-CoV-1?
SARS-CoV-2 has a higher reproductive number (R 0 ) than SARS-CoV-1, indicating much more efficient spread. 1 Several characteristics of SARS-CoV-2 may help explain this enhanced transmission. While both SARS-CoV-1 and SARS-CoV-2 preferentially interact with the angiotensin-converting enzyme 2 (ACE 2) receptor, SARS-CoV-2 has structural differences in its surface proteins that enable stronger binding to the ACE 2 receptor 4 and greater efficiency at invading host cells. 1 SARS-CoV-2 also has greater affinity (or bonding) for the upper respiratory tract and conjunctiva, 5 thus can infect the upper respiratory tract and can conduct airways more easily. 6
Viral load dynamics and duration of infectiousness
Viral load kinetics could also explain some of the differences between SARS-CoV-2 and SARS-CoV-1. In the respiratory tract, peak SARS-CoV-2 load is observed at the time of symptom onset or in the first week of illness, with subsequent decline thereafter, which indicates the highest infectiousness potential just before or within the first five days of symptom onset ( fig 2 ). 7 In contrast, in SARS-CoV-1 the highest viral loads were detected in the upper respiratory tract in the second week of illness, which explains its minimal contagiousness in the first week after symptom onset, enabling early case detection in the community. 7

After the initial exposure, patients typically develop symptoms within 5-6 days (incubation period). SARS-CoV-2 generates a diverse range of clinical manifestations, ranging from mild infection to severe disease accompanied by high mortality. In patients with mild infection, initial host immune response is capable of controlling the infection. In severe disease, excessive immune response leads to organ damage, intensive care admission, or death. The viral load peaks in the first week of infection, declines thereafter gradually, while the antibody response gradually increases and is often detectable by day 14 (figure adapted with permission from https://www.sciencedirect.com/science/article/pii/S009286742030475X ; https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30230-7/fulltext )
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) technology can detect viral SARS-CoV-2 RNA in the upper respiratory tract for a mean of 17 days (maximum 83 days) after symptom onset. 7 However, detection of viral RNA by qRT-PCR does not necessarily equate to infectiousness, and viral culture from PCR positive upper respiratory tract samples has been rarely positive beyond nine days of illness. 5 This corresponds to what is known about transmission based on contact tracing studies, which is that transmission capacity is maximal in the first week of illness, and that transmission after this period has not been documented. 8 Severely ill or immune-compromised patients may have relatively prolonged virus shedding, and some patients may have intermittent RNA shedding; however, low level results close to the detection limit may not constitute infectious viral particles. While asymptomatic individuals (those with no symptoms throughout the infection) can transmit the infection, their relative degree of infectiousness seems to be limited. 9 10 11 People with mild symptoms (paucisymptomatic) and those whose symptom have not yet appeared still carry large amounts of virus in the upper respiratory tract, which might contribute to the easy and rapid spread of SARS-CoV-2. 7 Symptomatic and pre-symptomatic transmission (one to two days before symptom onset) is likely to play a greater role in the spread of SARS-CoV-2. 10 12 A combination of preventive measures, such as physical distancing and testing, tracing, and self-isolation, continue to be needed.
Route of transmission and transmission dynamics
Like other coronaviruses, the primary mechanism of transmission of SARS-CoV-2 is via infected respiratory droplets, with viral infection occurring by direct or indirect contact with nasal, conjunctival, or oral mucosa, when respiratory particles are inhaled or deposited on these mucous membranes. 6 Target host receptors are found mainly in the human respiratory tract epithelium, including the oropharynx and upper airway. The conjunctiva and gastrointestinal tracts are also susceptible to infection and may serve as transmission portals. 6
Transmission risk depends on factors such as contact pattern, environment, infectiousness of the host, and socioeconomic factors, as described elsewhere. 12 Most transmission occurs through close range contact (such as 15 minutes face to face and within 2 m), 13 and spread is especially efficient within households and through gatherings of family and friends. 12 Household secondary attack rates (the proportion of susceptible individuals who become infected within a group of susceptible contacts with a primary case) ranges from 4% to 35%. 12 Sleeping in the same room as, or being a spouse of an infected individual increases the risk of infection, but isolation of the infected person away from the family is related to lower risk of infection. 12 Other activities identified as high risk include dining in close proximity with the infected person, sharing food, and taking part in group activities 12 The risk of infection substantially increases in enclosed environments compared with outdoor settings. 12 For example, a systematic review of transmission clusters found that most superspreading events occurred indoors. 11 Aerosol transmission can still factor during prolonged stay in crowded, poorly ventilated indoor settings (meaning transmission could occur at a distance >2 m). 12 14 15 16 17
The role of faecal shedding in SARS-CoV-2 transmission and the extent of fomite (through inanimate surfaces) transmission also remain to be fully understood. Both SARS-CoV-2 and SARS-CoV-1 remain viable for many days on smooth surfaces (stainless steel, plastic, glass) and at lower temperature and humidity (eg, air conditioned environments). 18 19 Thus, transferring infection from contaminated surfaces to the mucosa of eyes, nose, and mouth via unwashed hands is a possible route of transmission. This route of transmission may contribute especially in facilities with communal areas, with increased likelihood of environmental contamination. However, both SARS-CoV-1 and SARS-CoV-2 are readily inactivated by commonly used disinfectants, emphasising the potential value of surface cleaning and handwashing. SARS-CoV-2 RNA has been found in stool samples and RNA shedding often persists for longer than in respiratory samples 7 ; however, virus isolation has rarely been successful from the stool. 5 7 No published reports describe faecal-oral transmission. In SARS-CoV-1, faecal-oral transmission was not considered to occur in most circumstances; but, one explosive outbreak was attributed to aerosolisation and spread of the virus across an apartment block via a faulty sewage system. 20 It remains to be seen if similar transmission may occur with SARS-CoV-2.
Pathogenesis
Viral entry and interaction with target cells.
SARS-CoV-2 binds to ACE 2, the host target cell receptor. 1 Active replication and release of the virus in the lung cells lead to non-specific symptoms such as fever, myalgia, headache, and respiratory symptoms. 1 In an experimental hamster model, the virus causes transient damage to the cells in the olfactory epithelium, leading to olfactory dysfunction, which may explain temporary loss of taste and smell commonly seen in covid-19. 21 The distribution of ACE 2 receptors in different tissues may explain the sites of infection and patient symptoms. For example, the ACE 2 receptor is found on the epithelium of other organs such as the intestine and endothelial cells in the kidney and blood vessels, which may explain gastrointestinal symptoms and cardiovascular complications. 22 Lymphocytic endotheliitis has been observed in postmortem pathology examination of the lung, heart, kidney, and liver as well as liver cell necrosis and myocardial infarction in patients who died of covid-19. 1 23 These findings indicate that the virus directly affects many organs, as was seen in SARS-CoV-1 and influenzae.
Much remains unknown. Are the pathological changes in the respiratory tract or endothelial dysfunction the result of direct viral infection, cytokine dysregulation, coagulopathy, or are they multifactorial? And does direct viral invasion or coagulopathy directly contribute to some of the ischaemic complications such as ischaemic infarcts? These and more, will require further work to elucidate.
Immune response and disease spectrum ( figure 2 )
After viral entry, the initial inflammatory response attracts virus-specific T cells to the site of infection, where the infected cells are eliminated before the virus spreads, leading to recovery in most people. 24 In patients who develop severe disease, SARS-CoV-2 elicits an aberrant host immune response. 24 25 For example, postmortem histology of lung tissues of patients who died of covid-19 have confirmed the inflammatory nature of the injury, with features of bilateral diffuse alveolar damage, hyaline-membrane formation, interstitial mononuclear inflammatory infiltrates, and desquamation consistent with acute respiratory distress syndrome (ARDS), and is similar to the lung pathology seen in severe Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). 26 27 A distinctive feature of covid-19 is the presence of mucus plugs with fibrinous exudate in the respiratory tract, which may explain the severity of covid-19 even in young adults. 28 This is potentially caused by the overproduction of pro-inflammatory cytokines that accumulate in the lungs, eventually damaging the lung parenchyma. 24
Some patients also experience septic shock and multi-organ dysfunction. 24 For example, the cardiovascular system is often involved early in covid-19 disease and is reflected in the release of highly sensitive troponin and natriuretic peptides. 29 Consistent with the clinical context of coagulopathy, focal intra-alveolar haemorrhage and presence of platelet-fibrin thrombi in small arterial vessels is also seen. 27 Cytokines normally mediate and regulate immunity, inflammation, and haematopoiesis; however, further exacerbation of immune reaction and accumulation of cytokines in other organs in some patients may cause extensive tissue damage, or a cytokine release syndrome (cytokine storm), resulting in capillary leak, thrombus formation, and organ dysfunction. 24 30
Mechanisms underlying the diverse clinical outcomes
Clinical outcomes are influenced by host factors such as older age, male sex, and underlying medical conditions, 1 as well as factors related to the virus (such as viral load kinetics), host-immune response, and potential cross-reactive immune memory from previous exposure to seasonal coronaviruses ( box 1 ).
Risk factors associated with the development of severe disease, admission to intensive care unit, and mortality
Underlying condition.
Hypertension
Cardiovascular disease
Chronic obstructive pulmonary disease
Presentation
Higher fever (≥39°C on admission)
Dyspnoea on admission
Higher qSOFA score
Laboratory markers
Neutrophilia/lymphopenia
Raised lactate and lactate dehydrogenase
Raised C reactive protein
Raised ferritin
Raised IL-6
Raised ACE2
D-dimer >1 μg/mL
Sex-related differences in immune response have been reported, revealing that men had higher plasma innate immune cytokines and chemokines at baseline than women. 31 In contrast, women had notably more robust T cell activation than men, and among male participants T cell activation declined with age, which was sustained among female patients. These findings suggest that adaptive immune response may be important in defining the clinical outcome as older age and male sex is associated with increased risk of severe disease and mortality.
Increased levels of pro-inflammatory cytokines correlate with severe pneumonia and increased ground glass opacities within the lungs. 30 32 In people with severe illness, increased plasma concentrations of inflammatory cytokines and biomarkers were observed compared with people with non-severe illness. 30 33 34
Emerging evidence suggests a correlation between viral dynamics, the severity of illness, and disease outcome. 7 Longitudinal characteristics of immune response show a correlation between the severity of illness, viral load, and IFN- α, IFN-γ, and TNF-α response. 34 In the same study many interferons, cytokines, and chemokines were elevated early in disease for patients who had severe disease and higher viral loads. This emphasises that viral load may drive these cytokines and the possible pathological roles associated with the host defence factors. This is in keeping with the pathogenesis of influenza, SARS, and MERS whereby prolonged viral shedding was also associated with severity of illness. 7 35
Given the substantial role of the immune response in determining clinical outcomes, several immunosuppressive therapies aimed at limiting immune-mediated damage are currently in various phases of development ( table 1 ).
Therapeutics currently under investigation
- View inline
Immune response to the virus and its role in protection
Covid-19 leads to an antibody response to a range of viral proteins, but the spike (S) protein and nucleocapsid are those most often used in serological diagnosis. Few antibodies are detectable in the first four days of illness, but patients progressively develop them, with most achieving a detectable response after four weeks. 36 A wide range of virus-neutralising antibodies have been reported, and emerging evidence suggests that these may correlate with severity but wane over time. 37 The duration and protectivity of antibody and T cell responses remain to be defined through studies with longer follow-up. CD-4 T cell responses to endemic human coronaviruses appear to manifest cross-reactivity with SARS-CoV-2, but their role in protection remains unclear. 38
Unanswered questions
Further understanding of the pathogenesis for SARS-CoV-2 will be vital in developing therapeutics, vaccines, and supportive care modalities in the treatment of covid-19. More data are needed to understand the determinants of healthy versus dysfunctional response and immune markers for protection and the severity of disease. Neutralising antibodies are potential correlates of protection, but other protective antibody mechanisms may exist. Similarly, the protective role of T cell immunity and duration of both antibody and T cell responses and the correlates of protection need to be defined. In addition, we need optimal testing systems and technologies to support and inform early detection and clinical management of infection. Greater understanding is needed regarding the long term consequences following acute illness and multisystem inflammatory disease, especially in children.
Education into practice
How would you describe SARS-CoV-2 transmission routes and ways to prevent infection?
How would you describe to a patient why cough, anosmia, and fever occur in covid-19?
Questions for future research
What is the role of the cytokine storm and how could it inform the development of therapeutics, vaccines, and supportive care modalities?
What is the window period when patients are most infectious?
Why do some patients develop severe disease while others, especially children, remain mildly symptomatic or do not develop symptoms?
What are the determinants of healthy versus dysfunctional response, and the biomarkers to define immune correlates of protection and disease severity for the effective triage of patients?
What is the protective role of T cell immunity and duration of both antibody and T cell responses, and how would you define the correlates of protection?
How patients were involved in the creation of this article
No patients were directly involved in the creation of this article.
How this article was created
We searched PubMed from 2000 to 18 September 2020, limited to publications in English. Our search strategy used a combination of key words including “COVID-19,” “SARS-CoV-2,” “SARS”, “MERS,” “Coronavirus,” “Novel Coronavirus,” “Pathogenesis,” “Transmission,” “Cytokine Release,” “immune response,” “antibody response.” These sources were supplemented with systematic reviews. We also reviewed technical documents produced by the Centers for Disease Control and Prevention and World Health Organization technical documents.
Author contributions: MC, KK, JK, MP drafted the first and subsequent versions of the manuscript and all authors provided critical feedback and contributed to the manuscript.
Competing interests The BMJ has judged that there are no disqualifying financial ties to commercial companies. The authors declare the following other interests: none.
Further details of The BMJ policy on financial interests are here: https://www.bmj.com/about-bmj/resources-authors/forms-policies-and-checklists/declaration-competing-interests
Provenance and peer review: commissioned; externally peer reviewed.
This article is made freely available for use in accordance with BMJ's website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
- Bamford CGG ,
- Fischer WM ,
- Gnanakaran S ,
- Sheffield COVID-19 Genomics Group
- Corbett KS ,
- Corman VM ,
- Guggemos W ,
- Cheung MC ,
- Perera RAPM ,
- Cheng H-Y ,
- Taiwan COVID-19 Outbreak Investigation Team
- Meyerowitz E ,
- Richterman A ,
- Nergiz AI ,
- Maraolo AE ,
- Buitrago-Garcia D ,
- Egli-Gany D ,
- Counotte MJ ,
- ↵ European Centre for Disease Prevention and Control. Surveillance definitions for COVID-19. 2020. https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitions .
- Klompas M ,
- ↵ Centers for Disease Control and Prevention. How COVID-19 spreads. 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- ↵ Centers for Disease Control and Prevention. Scientific brief: SARS-CoV-2 and potential airborne transmission. 2020. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html
- Perera MRA ,
- van Doremalen N ,
- Bushmaker T ,
- Morris DH ,
- Monteil V ,
- Flammer AJ ,
- Steiger P ,
- Mangalmurti N ,
- Blanco-Melo D ,
- Nilsson-Payant BE ,
- Carsana L ,
- Sonzogni A ,
- ↵ Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020. https://pubmed.ncbi.nlm.nih.gov/32846427/
- Yale IMPACT Team
- CAP-China Network
- Perera RA ,
- Merrick B ,
- Grifoni A ,
- Weiskopf D ,
- Ramirez SI ,
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
One-year in: COVID-19 research at the international level in CORD-19 data
Roles Conceptualization, Data curation, Formal analysis, Project administration, Writing – original draft
* E-mail: [email protected]
Affiliation John Glenn College of Public Affairs, The Ohio State University, Columbus, Ohio, United States of America
Roles Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing
Affiliation School of Public Affairs, Zhejiang University, Hangzhou, Zhejiang, China
Roles Conceptualization, Formal analysis, Software, Validation, Visualization
Affiliation Australian Artificial Intelligence Institute, University of Technology Sydney, Ultimo, Australia
Roles Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing
Affiliation Shidler College of Business, University of Hawaiʻi at Mānoa, Honolulu, Hawaiʻi, United States of America
- Caroline S. Wagner,
- Xiaojing Cai,
- Yi Zhang,
- Caroline V. Fry

- Published: May 25, 2022
- https://doi.org/10.1371/journal.pone.0261624
- Peer Review
- Reader Comments
The appearance of a novel coronavirus in late 2019 radically changed the community of researchers working on coronaviruses since the 2002 SARS epidemic. In 2020, coronavirus-related publications grew by 20 times over the previous two years, with 130,000 more researchers publishing on related topics. The United States, the United Kingdom and China led dozens of nations working on coronavirus prior to the pandemic, but leadership consolidated among these three nations in 2020, which collectively accounted for 50% of all papers, garnering well more than 60% of citations. China took an early lead on COVID-19 research, but dropped rapidly in production and international participation through the year. Europe showed an opposite pattern, beginning slowly in publications but growing in contributions during the year. The share of internationally collaborative publications dropped from pre-pandemic rates; single-authored publications grew. For all nations, including China, the number of publications about COVID track closely with the outbreak of COVID-19 cases. Lower-income nations participate very little in COVID-19 research in 2020. Topic maps of internationally collaborative work show the rise of patient care and public health clusters—two topics that were largely absent from coronavirus research in the two years prior to 2020. Findings are consistent with global science as a self-organizing system operating on a reputation-based dynamic.
Citation: Wagner CS, Cai X, Zhang Y, Fry CV (2022) One-year in: COVID-19 research at the international level in CORD-19 data. PLoS ONE 17(5): e0261624. https://doi.org/10.1371/journal.pone.0261624
Editor: Alberto Baccini, University of Siena, Italy, ITALY
Received: July 14, 2021; Accepted: December 6, 2021; Published: May 25, 2022
Copyright: © 2022 Wagner et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: https://doi.org/10.6084/m9.figshare.16620274.v1 .
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The COVID-19 pandemic upended many normal practices around the conduct of research and development (R&D); the extent of disruption is revealed across measures of scientific research output [ 1 – 3 ]. This paper revisits the extent to which patterns of international collaboration in coronavirus research during the COVID-19 pandemic depart from ‘normal’ times. We present publication patterns using one full year of publications data from the CORD-19 database, and observations on non-COVID peer-reviewed publications using the Web of Science, to examine national and international publication rates and network patterns. We examine topics of research on COVID-19, and reflect on lessons learned about international collaboration from the disruption. The analysis may be useful to research administrators, international affairs professionals and science studies scholars.
We study the international collaborative linkages as a network. In the absence of a global governing body, international collaborations operate by network dynamics. Scientific connections at the global level reflect collective decisions of hundreds of individuals who seek to connect to each other. Connections are not random; they are influenced by five factors: two personal and three contextual. Personal choices tend towards those previously known or known by reputation or introduction. Contextual choices are 1) resources available, 2) geopolitical factors, and 3) time and attention. Network dynamics emerge from interplay of these factors, although there is little research on how a disaster, such as a pandemic, will affect productivity, collaboration, and topic focus. Moreover, it is difficult to determine expectations of network dynamics in a pandemic because global exogenous disruptions are rare and studies about science in a disaster are sparse. This paper seeks to fill some of these gaps.
This paper is organized to describe the literature supporting our inquiries, and to present hypotheses derived from the literature. We then describe coronavirus research prior to the pandemic, and early policy responses to the crisis. A section on data and methodology presents approaches designed to answer the questions emerging from the hypotheses. A results section describes outcomes of the analyses, followed by limitations of the data and approaches presented here. A discussion section details responses to the hypotheses as well as observations about the research project and avenues for further research. An S1 Appendix provides additional technical details.
Literature review and hypotheses
In the decades preceding the pandemic, R&D spending and output grew rapidly. OECD data shows that, among member nations, R&D spending was 25% higher in 2017 than a decade earlier. The US National Science Foundation (NSF) reports that from 2008–2018 the annual number of citable publications (articles, notes and letters, hereafter, “publications”) worldwide grew by 3.83% per year from 1.8 million to 2.6 million [ 4 ]. Increases in spending, trained practitioners, and publications contributed to an overall growth of the research enterprise in natural sciences and engineering, social sciences, and arts and humanities. Within the research enterprise, among scientifically advanced nations, international collaborative publications grew at a faster rate than national publications, accounting for as much as one-quarter of all publications in 2018, with variations observed across fields, according to the National Science Foundation [ 4 ]. Those fields that rely on large-scale equipment are more highly globalized, but increases in international linkages is observed in most fields, tied, not to funding or equipment, but to the interests of researchers to work together. The size of these teams has grown larger over time [ 5 ].
International collaborative patterns have been dominated by scientifically advanced nations, although, over time, many low-income, emerging and developing nations have become more active, and have partnered with more advanced nations [ 6 ]. Some tendency to collaborate among nations with former colonial ties is observed [ 7 ], but this is likely due to incentivized funding provided by the former colonial power. Political differences do not appear to hinder collaboration, evidenced most notably by the rise of China to be the number one collaborating nation with the United States. Abramo et al. [ 8 ] added to literature on tendency of neighbors to work together, but Choi [ 6 ] shows this tendency to be decreasing over time.
Prior research into collaboration around viral disease events found that, during the 2014 West African Ebola epidemic, collaboration grew between scientists from scientifically advanced nations and the most affected nations [ 9 ], suggesting that connections were made based upon disease location. Ebola outbreaks brought in researchers from scientifically advanced nations to work with local researchers on specific events. Collaborative ties did not persist past the disease event.
A global community of coronavirus researchers predated the advent of the 2019 novel coronavirus; this community formed after the 2002 SARS coronavirus epidemic [ 1 ]. As the new threat emerged in 2019, governments provided emergency R&D funding to encourage targeted research on the novel coronavirus. Most of these funds were committed by governments in scientifically advanced countries and were allocated to national institutions, although the European Union (EU) and the US National Institutes of Health (NIH) fund both national and foreign applicants. National actors receiving funds may then choose, in some instances, to connect to foreign collaborators, creating an international connection. The resulting connections can be studied through coauthorship attributions on paper and interpreted as a self-organizing network of connectivity [ 10 ]. In Fry et al. [ 1 ], we showed that, during the early months of the COVID-19 pandemic, international collaborations in coronavirus research emerged among just a few nations, and, on average, publications had fewer coauthors per paper than pre-pandemic levels. Most nations did not publish on the novel coronavirus in early pandemic research.
We expect that, as funds became available to researchers, and as more knowledge was generated through the first year of the pandemic, cross-national collaborative ties will grow. That said, because of travel limitations and a need for urgent results, we expect the rate of international collaboration and network ties to remain lower than pre-pandemic levels. This expectation is also informed by the research of Rotolo and Frickel [ 11 ] who found that there were fewer ties and smaller teams among researchers just after a hurricane disaster. Further, based upon findings in the wake of the Fukushima disaster [ 12 ] and a survey by Myers et al. [ 2 ] we expect that attention to pandemic-related R&D (including basic science, patient care, and public health) has lessened the output of other scientific research as well as reduced the rate of international collaborations in other fields. In addition to changed collaborative patterns, we expect to see changes in topics throughout the first year of the pandemic with topics becoming more focused as knowledge about events grows, which we explore in a separate article. In Zhang et al. [ 3 ], we showed that, at the beginning of the pandemic, the disrupted knowledge system exhibited very little topic focus. As the pandemic progresses, we expect to see greater topic focus. We further expect to see international collaboration focus on basic science and less on patient care and public health which may have a local, regional, or national characteristics. We expect continued consolidation among leading nations and elite institutions through the pandemic year due to pressures for rapid results and the lack of mobility to begin new collaborations. Further, we expect that geographic distance will mean less during the pandemic because remote collaborators will rely on communications technologies rather than face-to-face consultations.
Science during the COVID-19 pandemic
Coronavirus research predated the COVID-19 crisis, but it was a community of 22,000 researchers working on SARs, MERs, and the porcine diarrhea epidemic [ 1 , 3 ]. Coronavirus research output doubled in number over the decade between 2008–2018, in keeping with numbers in the biological sciences. As the new threat of a novel coronavirus emerged in 2019, governments provided emergency R&D funding to encourage targeted research, which attracted many new researchers from a wide range of fields. More than 156,000 researchers published on COVID-19 in 2020, growing the original community that had worked on coronaviruses by over 130,000.
The United States Government committed the largest amount of funds to the novel coronavirus, through the CARES Act and other legislation, allocating at least $5 billion to basic research, applied research, and development of vaccines, diagnostics, mapping of disease occurrence, analytics, public health, and medicine. The bulk of funds were appropriated by the US Congress to the U.S. National Institutes of Health (NIH), and through them to BARDA, the Biomedical Advanced Research and Development Authority. Other agencies also received additional R&D funds over and above their annual appropriations, including the National Science Foundation ($74 million) and the Department of Energy ($99.5 million). The U.S. government also provided funds to private companies to aid in vaccine development and procurement. For example, Moderna, a pharmaceutical company headquartered in Massachusetts, received $1 billion of R&D funds and in $1.5 billion in advanced purchase agreements.
Germany provided $891 million in R&D funds into coronavirus as well as to vaccine development. The European Commission provided €469 million in R&D funds, along with permission to recipients to reallocate funds originally slotted for other topics. The UK government reports spending £554 million on 3,600 initiatives related to COVID-19. In China, the Ministry of Science and Technology invested $100 million for emergency projects and unknown millions of funds for vaccine development, and the National Natural Science Foundation of China also reallocated approximately $15 million for projects related to COVID-19.
Many research organizations and researchers from various disciplines shifted to focus on aspects of the pandemic and received grant funds to do so. Just as with any other R&D funding, the expectation is that funded research will result in published works, enhanced equipment, and medicines and vaccines. Very early in the pandemic, preprints [ 13 ] (non-peer-reviewed articles) and peer reviewed articles began flooding into publishing venues. The number of scholarly publications related to the crisis grew spectacularly in the early months of the pandemic [ 1 ].
The rush to publish is expected: Zhang et al. [ 14 ] note that historical patterns show that researchers have, in previous cases, responded quickly to public health emergencies with publications, which is the same pattern we see with COVID-19 research. In updating our earlier work [ 15 ], we found that the number of coronavirus publications in CORD-19 grew considerably in the early days of the novel coronavirus, rising at a spectacular rate from a total of 4,875 articles produced on the topic (preprint and peer reviewed) between January and mid-April to an overall sum of 44,013 by mid-July, and accumulated to 87,515 by the start of October 2020. (In comparison, nanoscale science was a rapidly growing field in the 1990s, but it took more than 19 years to go from 4,000 to 90,000 articles [ 16 ]).
The dissemination of publications changed during the pandemic. COVID-19 peer-reviewed and edited publications became available to other researchers through new (CORD-19) and pre-existing (National Library of Medicine) web platforms. COVID-19 publications were much more likely than other works to be published as open access in 2020 [ 17 ]. In 2020, open-access, peer-reviewed publications related to COVID-19 accounted for 76.6% of all publications compared to 51% of all non-COVID publications. Highly cited papers—those in the top 1% most highly cited, with over 500 citations—were more likely than other work to be published in subscription-based journals such as The Lancet , Science , New England Journal of Medicine or Nature but these works were placed into open Web portals for rapid access. The National Library of Medicine served as a repository for most new publications related to COVID-19. The publishing house Elsevier—which publishes many subscription-based journals—created a "Public Health Emergency Collection" to make COVID-19 articles rapidly available regardless of the access status of the original work (subscription or open access). Similarly, CORD-19 (the database which provided data for this article) through Semantic Scholar, made relevant research (including historical work) rapidly and readily available and allowed researchers to deposit work they viewed as relevant.
Researchers from China and the USA increased the rate of collaborative publications on coronavirus in the earliest days of the pandemic Fry et al. [ 1 ]. Liu et al. [ 18 ] showed a surge of what they call ‘parachuting collaborations’–new connections not seen prior to the pandemic–which dramatically increased during the pandemic. Together with the findings in Fry et al. [ 1 ], these findings suggests that search and team formation changed to adapt to the needs of COVID-19 research, a finding also reported by Lee & Haupt [ 19 ]. Liu et al. [ 18 ] found that COVID-19 research papers were less likely to involve international collaboration than non-COVID-19 papers during the same time period, a finding reported by Aviv-Reuven & Rosenfeld [ 20 ] as well, a finding we can confirm.
Several research articles note the absence of emerging and developing nations in early COVID-19 research. Fry et al. [ 1 ] and Lee & Haupt [ 19 ] showed that very few developing nations were involved in the earliest day of the crisis. Zhang et al. [ 3 ] confirmed Fry et al. in finding that the USA, China, and the UK were the three countries with the largest number of articles by mid-year. Several articles report that fewer coauthors appear on article bylines [ 1 , 20]. This is likely due to the need for rapidity in responding to the crisis: fewer coauthors reduces the time needed to communicate, synthesize and submit results.
Data and methodology
Data for this study were extracted in March 2021 from the Covid-19 Open Research Dataset, “CORD-19,” an open resource of scientific papers on COVID-19 and related historical coronavirus research. CORD-19 is designed to facilitate the development of text mining and information retrieval systems for COVID-19 research over its rich collection of metadata and structured full-text papers. It is accessible through the National Library of Medicine, National Institutes of Health, USA. In addition, we accessed the whole of Scopus 2020 data to examine non-COVID publications over the year. To search for evidence of government funding for COVID-19 research, we searched Web of Science, which has a field for funding acknowledgements. (Non-COVID publications were any peer-reviewed, published work that did not include one of the keywords for the COVID search below).
To maintain consistency across our studies, we applied the same search terms as used in Fry et al. [ 1 ], Cai et al. [ 15 ], and Zhang et al. [ 3 ] and limited the search to the dates January 2020 to December 2020 and citation data to March 2021. The following search terms were applied to titles and abstracts to obtain an initial dataset of coronavirus publications:
- coronavirus
- corona virus
- Severe Acute Respiratory Syndrome
- Middle East Respiratory Syndrome
The initial dataset was cleaned to remove the following artifacts: conference papers, preprints, collections of abstracts, symposia results, articles pre-dating 2020, and meeting notes. Preprints were excluded in this report to avoid double-counting in cases where a work is subsequently peer-reviewed and published. The author names, institutional affiliation, and addresses were extracted for analysis. For articles derived from the PubMed Central website, the citation count was extracted up to March 2021. The resulting dataset provided us with 106,993 publications for the calendar year 2020. The final dataset was further divided into four quarters, shown in Table 1 , according to “Published Date”, i.e., the electronic publication dates (if any) or else print publication date: January to March (2020 Q1), April to June (2020 Q2), July to September (2020 Q3), and October to December (2020 Q4). Full counting is used to count the number of publications of a specific country or institution. Among all the publications with at least one author and address, 8,158 (8.9%) are single-author articles, with the rest involving coauthors at the national (78%) or international levels, with 20,203 (22.0%).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0261624.t001
We analyzed the number of authors and coauthors per paper for descriptive statistics of people publishing on the novel coronavirus; we analyzed keyword usage and topics drawn from keywords and abstracts, and we analyzed geographic location of authors to study cooperative patterns at the international level. We collected additional data to answer questions about activities not available in CORD-19 in funding and on non-COVID research publications. We compared the CORD-19 data to a defined dataset of coronavirus research derived from scientific articles on coronavirus-related research on historical data we had earlier extracted from PubMed, Elsevier’s Scopus, and Web of Science (details of the construction of this data can be found in Fry et al. [ 1 ]). The datasets are available at https://figshare.com/articles/dataset/One_Year_of_COVID-19_Int_l_Collaboration/16620274 . For the CORD-19 articles that are also indexed in Clarivate’s Web of Science (WoS), we retrieved funding information to get a rough view about which funding agency is contributing to the coronavirus research in the pandemic period; 35% of CORD-19 articles acknowledged funding. Dimensions database was used to analyze the open access categories.
To test for change in the number of participants in research groups between pre-COVID-19 and COVID-19 periods, we use double-tailed T-tests to compare the average “team” structure between periods. (We employ the word “team” for convenience to describe coauthor groups even though we do not know the mechanism of cooperation among the group.) Statistical significance is assessed at 0.05 level. Team structure is measured as average number of authors per publication, average number of nations per publication, and the percentage share of internationally collaborated articles. We also use regression models to test the relationship between team structure and citation impact. Since the dependent variable, i.e., citations, is a non-negative integer, we apply count-data regression models (i.e., negative binomial regression) that can account for the nature of the data.
To test the relationship between geographic distance and international collaboration among nations, we calculate the geographic distance and collaboration strength between country pairs. The geographic distance between nations is defined as distance between capitals of each nation, based on geographic data about world cities in the R package “map”. Following the normalization approach used in previous research [ 6 , 24 , 25 ], we apply Salton’s cosine measure of international collaboration strength, which takes the publication size of nations into account. It is calculated as the number of collaborative publications divided by the square root of the product of the number of publications of the two collaborating nations (See Appendix Table 2 in S1 Appendix ).
The process of collecting and cleaning the CORD-19 database produced a set of 106,993 publications. The set used for this study is limited to work published in 2020, responding to the search string. We compared the CORD-19 results to Elsevier’s Scopus for 2020: the search of Scopus produced 73,000 COVID-19 publications, so fewer than CORD-19. Scopus limits its indexing of publications to specific journals, while CORD-19 encouraged open deposit of materials, which would include venues not indexed by Scopus—this likely accounts for the differences in numbers among databases.
For all research in 2020, Scopus shows a total of 2,584,701 publications, COVID and non-COVID topics (recall that non-COVID topics are not included in CORD-19). Against expectations, growth in life and health sciences output between 2019 and 2020 is shown in most disciplines of life and health sciences fields, both COVID and other topics. The number of publications on novel coronavirus and resulting disease is about 20 times higher in 2020 than coronavirus research published between 2018 and 2019, when work focused on SARS and MERS—earlier disease events that were not as devastating as COVID-19.
Fig 1 shows the number of coronavirus publications by month compared to the number of reported disease case outbreaks worldwide. Publication numbers grew quickly between February and May 2020 at the same time as the number of COVID-19 cases increased at an alarming rate. Since May 2020, the number of publications has remained stable at over 10,000 publications per month, while the rate of growth in COVID-19 cases declined slightly relative to the earliest months. The surge of publications on COVID-19 clearly result from thousands of ‘new’ researchers from various fields publishing on coronavirus in 2020. In pre-COVID-19 period, coronavirus researchers were drawn mainly from Life Sciences & Biomedical Sciences (e.g., Virology, Infectious Disease) and Natural Sciences (e.g., Multidisciplinary Chemistry and Organic Chemistry). The pandemic calls upon researchers from all research fields, with noticeable increased efforts from Social Sciences (including the authors of this work).
Data on publications and cases are collected from CORD-19 and WHO ( https://covid19.who.int/ ).
https://doi.org/10.1371/journal.pone.0261624.g001
Table 2 shows the top 25 life sciences fields in 2020 from Scopus, with the total number of COVID-19 and non-COVID articles in the same field. Fields that show the highest number of COVID-19 research are medicine, infectious disease, and public health. For all research in life and health sciences, highest growth is seen in surgery, plant science, and psychiatry and mental health. We can assume that the COVID-19 articles were written in 2020, since they are topical—“COVID” was not a keyword in 2019. Moreover, journal editors greatly sped up the processing time for COVID-related review and publication [ 26 , 27 ]. Conversely, the non-COVID articles may represent work conducted years prior, since it takes time to write, review and publish research results [ 28 ]. In fact, peer review in non-COVID related disciplines was delayed in 2020 due to the pandemic [ 26 ] so there may be insufficient time to fully assess the impact of the crisis on non-COVID research of publication output. Data in 2021 will be more telling of the impact of the pandemic year on non-COVID research publication patterns.
Data: Elsevier’s Scopus.
https://doi.org/10.1371/journal.pone.0261624.t002
Contributions by author location
COVID-19 publishing numbers differ considerably by regions of the world. Fig 2 shows regionally aggregated contributions on COVID-19. Asian countries contributed over one-third of world publications in early 2020, but this percentage share dropped in the later months of 2020 as China reduced its output. Europe showed the opposite trend: Europe’s share of world COVID publications increased since April 2020 and the number remained stable through the latter months of 2020. North America’s share of publications increased throughout 2020.
https://doi.org/10.1371/journal.pone.0261624.g002
As expected, scientifically advanced nations, including China, account for the majority of COVID-19 publications. Among all nations publishing related work, USA, China, and UK produced (together and separately) 50% of the coronavirus articles during 2020, shown in Table 3 . As of early 2021, their publications accumulated around 68% of citations made to global publications supporting the expectation of consolidation around expertise and reputation. In the earliest days of the pandemic, three articles from Chinese authors [ 29 – 31 ] contributed key findings that guided much of the ensuing research; each of these articles garnered thousands of citations. Italy, UK, India, and Spain were slower to begin publishing but became more prolific through 2020, and particularly so in the final quarter. Fig 3 shows the rapid growth of monthly publications for selected countries, which also tracks with the trend in national COVID-19 cases; this finding is similar to one found in the 2014 West African Ebola epidemic [ 9 ].
https://doi.org/10.1371/journal.pone.0261624.g003
https://doi.org/10.1371/journal.pone.0261624.t003
International collaborative publication rates in coronavirus research took six months to recover to pre-COVID levels. Collaborative research projects take longer to publish results, so the ‘recovery’ time may simply reflect more communication and production time needed due to the physical distances and time zone differences. As expected, during 2020, international collaborative papers showed fewer nations per paper in the early days, but this number increased through the year. This number stabilizes in the fourth quarter to pre-pandemic levels. Supporting other findings [ 18 ], about 65% of internationally coauthored papers include only two nations—this is a drop from usual patterns.
In earlier work, we noted that developing nations were largely absent from the publication records in the early COVID-19 period. We explored the participation of developing nations in global coronavirus research over the full year, expecting to see some recovery, but it was weak. Pre-COVID-19 coronavirus research in 2018–2019 shows that low-income nations [ 32 ] accounted for 26% of all nations participating in the research, publishing 4% of global articles. This drops during 2020: In the first two quarters of 2020, low-income nations accounted for 21% of active nations and produced 3.4% of global articles (Low-income countries (LIS) are defined by the World Bank, https://data.worldbank.org/country/XM . China, India and Brazil are not low-income countries.). That said, throughout 2020 low-income nations increase their contribution to the coronavirus research, contributing just slightly more in number of publications compared to their participation in pre-COVID-19 period, but much lower than scientifically advanced nations. Against expectations and in contrast to the trend before the pandemic and in the first few months of 2020, we find, by mid-year, Chinese institutions no longer appear in the list of top 10 producing institutions, supporting Liu et al. [ 18 ]. For example, the University of Hong Kong and the Chinese Academy of Agricultural Sciences ranked third and fourth in pre-COVID-19 research but dropped down the list in 2020. This drop tracks with the drop in number of COVID-19 cases in China.
Academic institutions worldwide were responsible for the largest share of publications about coronavirus during the pandemic, although private companies participated in research, usually through coauthorship with academic coauthors. We identified a list of 40,287 institutions involved in coronavirus research with the following rules: (1) we retrieved valid institution names with a list of key strings, such as “hospital”, “univers*”, and “instit*”; and (2) we consolidated variations of the same institutions, such as “MIT” and “Massachusetts Institute of Technology”, and “University of Sydney” and “Sydney University”. Fig 4 shows the institutions making top contributions to COVID-19 cooperation. As expected, the figure shows that highly reputed institutions—Harvard University (Massachusetts, USA), Huazhong University of Science and Technology (Wuhan, China), and the University of California System—produced the largest absolute numbers of publications on coronavirus in 2020. From the CORD-19 data, we find there are 2,232 articles (2.43%) involving authors from private corporations, which is average for corporate participation in Web of Science [ 33 ]. Nevertheless, this percentage is a drop from private sector participation in pre-COVID-19 dataset, where we found that 3.4% of articles involved the private sector, so it was higher than average and dropped to average in 2020.
The percentage shares of publications in global articles of top 10 prolific institutions in COVID-19 (2020) and pre-COVID-19 (2018–2019) are shown.
https://doi.org/10.1371/journal.pone.0261624.g004
Table 4 shows the most frequently acknowledged funding agencies in coronavirus research indexed in Web of Science in 2020. Funders from the USA, China, and UK (or Europe) are the most commonly acknowledged, which is consistent with the publication outputs as shown in Table 3 . The US National Institutes of Health (NIH), the largest scientific organization dedicated to health and medical research, tops the list and is acknowledged in 15.8% of funded articles. The dominant funder in China, National Natural Science Foundation of China (NSFC), ranks second and contributes to 10.4% of publications, despite decreased publication shares in later periods. European funding agencies, including European Commission, UK Research & Innovation (UKRI), and Medical Research Council UK (MRC), also play a vital role as COVID-19 cases and number of publications increase in Europe.
https://doi.org/10.1371/journal.pone.0261624.t004
Co-occurrence network on coronavirus research
Terms retrieved from titles and abstracts of research articles provide useable clues to understand topic focus (Data for network analysis is available at Wagner, Caroline (2021): Figshare_Network files.rar. figshare. Dataset. https://doi.org/10.6084/m9.figshare.16652752.v1 ). The co-occurrence between terms and entities (e.g., funding agencies), and among terms, reveals their semantic connections in research, and may answer questions such as which agencies support which topics. The connection is measured by how many times two terms appear in proximity in the entire dataset, within and across articles. We identified 4,865 terms from a raw set of 1.2 million terms retrieved from titles and abstracts of the 106,993 articles published in 2020 via natural language processing techniques and a term clumping process [ 34 ], with the aid of VantagePoint (VantagePoint is a text mining tool for analyzing bibliometric data. See the link: https://www.thevantagepoint.com/ ). Specifically, the term clumping process facilitated a set of thesauri to remove meaningless terms (e.g., conjunctions, pronouns, and prepositions) and common terms in academic articles (e.g., “method” and “conclusion”), and it then consolidated terms with the same stem (e.g., terms in singular and plural forms).
Fig 5 analyzes the co-occurrence between the top 20 high-frequency terms and the major funding sources, visualized by Circos [ 35 ]. The Chinese agency, National Science Foundation of China (NSFC), was much more likely to fund research related to “Wuhan” while the United States’ National Institutes of Health (NIH) is much more likely to fund research with the term “United States.” Small differences can be observed for “clinical characteristics” (proportionately more from NSFC), and “hospitalization” (proportionately more from NIH) but these two terms are quite similar, so differences may be due to semantics only. Aside from these two differences, it appears that each of the agencies fund similar term portfolios differentiated only in proportion to their contribution, so more focused on basic research, which was our expectation.
https://doi.org/10.1371/journal.pone.0261624.g005
To assess whether international collaborations had different topic focus from domestic collaborations—which we expected—we analyzed co-terms at both levels. Fig 6 shows the co-term network for the internationally collaborative research, and Fig 7 shows the co-term network for domestic-only collaborations. In both networks, one sees the topics that are shown in Fig 5 as focus areas for government funders. With data extracted using Vantagepoint’s Natural Language Processing function ported into VOSViewer, Fig 6 shows international collaboration dominated by three clusters: 1) research on the virus (red, bottom left), 2) on patient care (purple, top right), and 3) on public health (green, bottom left).
Interactive version accessible at https://app.vosviewer.com/?json=https://drive.google.com/uc?id=1MVWE1bsTGi6jJeeU7BNCcjKTd2yjTiv0 .
https://doi.org/10.1371/journal.pone.0261624.g006
Interactive version accessible at https://app.vosviewer.com/?json=https://drive.google.com/uc?id=1RraBpIYbLY5_DfOMC0YJ7IZoq3_sRiKa .
https://doi.org/10.1371/journal.pone.0261624.g007
Fig 7 shows domestic topics, highlighting greater emphasis on patient care and disease characteristics (gold, top center) than seen in Fig 6 . Moreover, a fourth cluster emerges (blue, center) with details about outbreaks, effects, viral loads, and other aspects of health are seen that are not as prevalent at the international level. A table in the S1 Appendix provides more details about the topics. As expected, the domestic publications focus on public health and patient care more than is seen at the international level, where basic science dominates the topics.
Collaboration rate
Table 5 compares collaboration rates in coronavirus publications before the COVID-19 pandemic and in four quarters of 2020. As expected, and in comparison to the number of co-authors in pre-COVID-19 coronavirus research, publications in 2020 show fewer authors, fewer nations per paper, and less frequent international collaboration overall. Team size—represented by the number of authors on a publication—shrank shortly after the outbreak of COVID-19, a finding we highlighted in Fry et al. [ 1 ], but by the end of the year, the number had recovered and risen to just above levels seen in pre-COVID-19 coronavirus research. As expected, the average number of nations per international publication remained at lower levels than pre-COVID-19 levels for USA articles; there was no significant difference in numbers of international partners for Chinese articles in pre-COVID-19 and COVID-19 periods.
https://doi.org/10.1371/journal.pone.0261624.t005
We explored the relationship between team structure as represented by coauthors on papers and the number of citations to COVID-19 publications for publications produced in 2020 (looking at citation records up until March 2021). Table 5 shows the regression results that explore the relationship between citations to publications and international collaborative team structure, for publications with authors from USA, China, and the UK respectively. As expected, a positive correlation is shown between numbers of citations and international collaboration. Further, also meeting expectations, there is a correlation between number of authors and citations, which may reflect an audience affect due to a larger reader network. Also as expected, international teams attracted more citations than domestic-only teams, again, with a possible audience affect. These findings held for all nations except the USA, where international articles are not cited more than domestic-only research when holding constant the number of authors ( Table 6 , column 6).
https://doi.org/10.1371/journal.pone.0261624.t006
Network analysis
Figs 8 and 9 compare internationally collaborative networks in the pre-COVID-19 (2018–2019) and COVID-19 (2020) periods. Recall that these numbers represent about 22% of all COVID research in 2020. Fig 8 shows pre-COVID coronavirus research with two large clusters: one, a European cluster, and two, a global cluster brokered by the USA. The US collaborated closely with China, the UK, France, the Netherlands, and Germany. The dominance of the USA is partly accounted for by the volume of research when compared to the output per nation; the USA leads in publications, citations, and connectivity in coronavirus research prior to the pandemic and remained the leader during the pandemic.
Interactive version accessible at https://app.vosviewer.com/?json=https://drive.google.com/uc?id=1_1ASbt-FheQE6_VQNc5eFhy578jot9co .
https://doi.org/10.1371/journal.pone.0261624.g008
Interactive version accessible at https://app.vosviewer.com/?json=https://drive.google.com/uc?id=1TLdcW-lNUQ1k0kDlpZYOUQL57E8MKNqM .
https://doi.org/10.1371/journal.pone.0261624.g009
Fig 9 shows the COVID-19 collaborative network, where, as expected based upon research by Rotolo & Frickel [ 11 ], we observe more clusters and more brokering hubs than pre-COVID-19. The number of clusters has grown, with four clusters revealing a broader set of countries acting as centralized nodes or hubs, with the UK, Italy, and Germany increasing their bridging role from positions shown in the pre-COVID-19 network. The European research clusters form into two large groups, one with Italy as the brokering hub and one with the UK in a central brokering hub. Italy intensively links to France, the USA, and Switzerland. African nations join the network through the UK connection. Australia is central to a cluster that includes Spain, Brazil, and many smaller nations from South America. As expected, geographic distance between country pairs is negatively related to the collaboration strength of the two countries in all cases (see Appendix Table 2 in S1 Appendix ). However, during COVID-19 period, the negative impact of geographic distance on collaboration strength was weakened as demonstrated by positive and significant coefficient of interaction of COVID and geographic distance. As expected, physical distance was less of a barrier to collaboration than in other scientific research. The result reveals that the pandemic weakened the role of geographic distance in international collaboration (OLS regression analysis of the relationship between geographic distance and collaboration strength).
Table 7 shows the network metrics for the above networks and for four quarters of 2020. (Visuals of the four quarterly networks are shown in the S1 Appendix ) Consistent with the growing number of internationally collaborative articles throughout 2020, the network statistics reveal expanded connections from the first period (January to March) to the third period, cumulative (July to September) in 2020, but then stabilizing.
https://doi.org/10.1371/journal.pone.0261624.t007
The network statistics suggest that COVID-19 research involved many more participants than those who worked on coronavirus in the years prior to the pandemic, as we find by examining the number of unique author names. From the pre-COVID-19 coronavirus network to the COVID-19 network, we see that number of nodes increases from 103 nations before COVID to 173 during COVID. More importantly, the number of links among nations more than triples, suggesting that many more connections were made at the international level than existed prior to the pandemic. Average degree doubles, supporting the observations of many new links. These links likely were forged remotely in a process that Liu et al. [ 18 ] call “parachuting collaborations” that post-date the pandemic. These types of collaborations may have emerged through friend-of-friend connections, since people could not meet face-to-face due to travel restrictions during 2020. Betweenness centrality drops over the year indicating a shift in influence of the initial, dominant hubs to include more participants from more nations over the pandemic year.
Table 8 shows the network metrics for top producers (USA, UK, and China) in the global network. As shown by the average degree of the three countries, the USA was a hub in the network in both periods, more so early in the pandemic, supporting our expectation of consolidation around expertise and reputation, but betweenness centrality drops as researchers from more countries joined into the research. The UK played a much more active role in the COVID network compared to China in the second half of the year. Despite being a hub in the pre-COVID network and in the first months of the pandemic [ 1 ], China played a less prominent role in the network in 2020.
https://doi.org/10.1371/journal.pone.0261624.t008
Limitations of this research
This research project had a number of limitations of data, time, analysis, and scope. Data limitations include constraints of what is measurable in published work (publications, networks, and citations). We decided to use CORD-19 data because it was the most expansive dataset for COVID-19 research, but we may have picked up lower quality work as a result; it is an open dataset with attendant problems. We extracted desired features, but there may be gaps and errors. In order to get a count of open-access publications, we used Dimensions data, but the total number of COVID-19 publications in Dimensions were lower than CORD-19, so open-access publications are likely under-counted. We present the percentages of COVID-19 research that is open access; these calculations are broadly representative, but they cannot be further verified. Moreover, we are unable to show extent of R&D occurring in private research laboratories if it is not published. We hoped to inform policymakers about COVID-19 research trends in a timely manner, which meant we worked with data available at the time (in Spring 2021) rather than waiting until data has been expanded, cleaned or validated. Elsevier and Clarivate databases were also examined; these databases are more carefully curated for quality. We tapped them for comparisons to CORD-19, and especially for non-COVID research. We had planned to test whether the pandemic had an impact on non-COVID research but we were unable to show this outcome: During 2020, peer reviews were delayed [ 36 ], researchers were not able to access labs or other resources, and scholarship was interrupted, but these obstacles are not yet evident in the data. Disruptions to 2020 research activities likely will not be seen in publication data until 2022 and after. We also exclude preprint publications from this analysis, which could present a limitation, given the important role of preprints during the pandemic.
Further, a limitation of this analysis is the reliance on nations as ‘super-nodes’ in a network that consists of individuals with associated cultural contexts that are not captured in network data. This reliance on nations is partly justified in that nations represent the underlying political and social systems that support scientific activities by offering funding, infrastructure, training, and dissemination of results. We acknowledge that the reliance on nations as a unit of measurement is a limitation, imposed on the analyst based upon the ways in which data are collected. Future research will need to ensure cleaner data to validate comparisons presented here. None of these datasets can truly represent the scope of activities that contributed to what is known about COVID-19, and mechanisms to assess knowledge flows are quite limited and time consuming to collect.
A review of one year of research publications about the novel coronavirus that emerged in Wuhan China in late 2019 shows the research community reacting rapidly and robustly to the challenge. The rapidity of response to COVID-19 suggests flexibility in the research system: thousands of researchers from many fields began working on the crisis. Research was disseminated initially in a flood of preprints; rapidly peer-reviewed publications were placed on open data platforms or shared openly on subscription-based platforms. COVID-related, peer-reviewed publications rose sharply in number in early 2020, and these publications were much more likely to be shared on open-access platforms or formats to enable rapid knowledge diffusion than is the norm in scholarly publishing [ 17 ]. The earlist COVID-19 research efforts were conducted by China, the USA, and the UK, and these three nations constitute close to 50% of all COVID publications on the subject in 2020. European nations started off slowly in research publications, but these nations continued to grow their output throughout 2020, as China’s output dropped.
As expected, international collaborations accounted for a smaller percentage of publications than is generally seen in ‘normal’ times, where internationally co-authored articles often account for more than one-quarter of articles [put new footnote here that was added on page 4 and delete this note] We expected the drop-off in international publications because a lack of mobility meant that people were unable to meet and discuss shared insights, or to devise, carry out or compare research results. Remote collaborations involve higher transaction costs and could be expected to slow progress. This possibly explains why international teams were smaller: to cut down on the time needed for communication. Further, the lower rate of international collaboration may be due to topics related to patient care and public health specific to particular regions or nations rendering them less suited to international collaboration [ 3 ]. We also noted that distance was less of a barrier to collaboration than is shown in other studies in times before the COVID-19 crisis.
Interestingly, the number of papers per nation tracks closely with the outbreak of COVID-19 cases in that nation. We expected to find numbers of publications to be more closely correlated to research funding. This may still be the case, but the data is too variable and incomparable to elicit a correlation between funding and output. We surmise that researchers were motived by a desire to be helpful to those suffering with the disease. It may also be the case that local COVID cases provided observational opportunities for researchers, and thus publication opportunities, as well, which produced data that resulted in more national publications.
The low rate of participation by lower-income nations was somewhat unexpected. Lower-income nations had a very low rate of participation in the early days of COVID, and only slowly joined the global publication counts. This may be due to a number of factors, including the need to publish locally to address the crisis, the inability of researchers to access laboratories or data during lock-down periods, the lack of access to one’s office, or the inability of national ministries to provide emergency research funds. People may not have had access to the Internet at home. The lower showing for developing nations is a concern, since these nations need the scientific knowledge to battle viral events just as much or more than advanced nations. This finding clearly requires more research and perhaps policy action.
We expected that the combined rush to work on COVID and the pandemic lock-downs would reduce non-COVID research activities. This may still be the case (reported in a survey by Myer et al. [ 2 ]), but it could not be detected in publication numbers at this writing. Publications in life and health sciences in non-COVID topics increased in number over 2019. As researchers become more comfortable with remote work, they may have persisted in publishing earlier results, however, this does not comport with the findings of Myer et al. [ 2 ]. As the pipeline catches up these activities, it will be worth revisiting the impact on productivity again at the end of 2021.
The stratification and consolidation comport with a model of global science as a reputation-based system creating a social hierarchy: the global network reverts to scientifically advanced nations and elite institutions in a crisis. We expected that the number of papers would align with reputation and resources. The role of reputation is confirmed by the consolidation of actors to fewer, elite institutions in scientifically advanced nations cooperating together more so than prior to the pandemic. The role played by access to resources is unclear—we observe that national output is closely tied to number of COVID-19, which could be due to a desire of scientists to help the effort. This requires more inquiry.
The influence of geopolitical factors also appears to play some role in research output, partnership and productivity. Arguably, Chinese publications initiated most of the COVID-19 research into the nature of the SARS-CoV-2 virus itself [ 29 – 31 ]. Nevertheless, in April 2020, the Chinese government changed requirements for review of articles related to the origins of COVID-19, requiring a more central review for work about the source of the novel coronavirus, which may have reduced the willingness of Chinese authors to cooperate internationally, although this requires more inquiry. Negative political comments made in the United States against China regarding the source of the virus may also have dampened international collaboration, although we did not test for this possibility.
Time and attention may have played a role in the drop in the share of internationally co-authored papers. Transaction costs of distance communications may have hampered some international connections. The drop-off in number of developing nations participating at the start of the pandemic may have contributed to the drop in international collaboration numbers, as well, by lowering the number of potential collaborators.
A remaining question arises around the mechanisms by which people, who had not already worked together before the pandemic, became connected to one another in a year when most people were physically isolated from each other. These connections are what Liu et al. [ 18 ] term “parachuting collaborations.” One would expect that people connect face-to-face: Research shows that the vast majority of collaborative projects start face-to-face or side-by-side. When that cannot take place, it is unclear whether people look for physically proximate partners, choose to work alone, become connected to new people through friend-of-a-friend, through social media, or perhaps just a ‘cold-call’ outreach to someone they do not know. It is clear that many of the connections made around COVID-19 may not have existed prior to the pandemic, so further research is needed to understand how people connected with each other under crisis conditions.
Supporting information
S1 appendix. network visuals..
https://doi.org/10.1371/journal.pone.0261624.s001
Acknowledgments
Thanks go to Clayton E. Tillman and Thomas Collins for help with data collection and formatting.
- View Article
- PubMed/NCBI
- Google Scholar
- 4. National Science Board. The state of U.S. science and engineering. Washington, DC: US Government Printing Office; 2020.
- 7. Glänzel W, Schubert A. Analysing scientific networks through co-authorship. In Handbook of quantitative science and technology research: The Use of Publication and Patent Statistics in Studies of S&T Systems, Moed H., ed., Kluwer Academic Publishers, Amsterdam, 2004. pp. 257–76.
- 13. Preprints contain the results of research activities, but they have not yet been subject to peer review.
- 17. Fry CV, MacGarvie M. Drinking from the firehose: Preprints, Chinese researchers, and the diffusion of knowledge in COVID-19. 2021.
- 18. Liu M, Bu Y, Chen C, Xu J, Li D, Leng Y, et al. Can pandemics transform scientific novelty? Evidence from COVID-19. arXiv. 2020.
- 20. Aviv-Reuven S, Rosenfeld A. Publication patterns’ changes due to the COVID-19 pandemic: A longitudinal and short-term scientometric analysis. arXiv. 2021:02594.
- 26. Horbach SPJM. No time for that now! Qualitative changes in manuscript peer review during the Covid-19 pandemic. Research Evaluation. 2021;(rvaa037).
- 32. DFID. Low-income countries (LIS) as defined by Department for International Development (DFID) of UK [cited 2021]. https://g2lm-lic.iza.org/call-phase-iv/list-of-lic/ .

COVID-19 Research Articles Downloadable Database
March 19, 2020
Updated January 12, 2024
COVID-19 Research Guide Home
- Research Articles Downloadable Database
- COVID-19 Science Updates
- Databases and Journals
- Secondary Data and Statistics
Important announcement:
The CDC Database of COVID-19 Research Articles became a collaboration with the WHO to create the WHO COVID-19 database during the pandemic to make it easier for results to be searched, downloaded, and used by researchers worldwide.
The last version of the CDC COVID-19 database was archived and remain available on this website. Please note that it has stopped updating as of October 9, 2020 and all new articles were integrated into the WHO COVID-19 database . The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
If you have any questions, concerns, or problems accessing the WHO COVID-19 Database please email the CDC Library for assistance.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
Below are options to download the archive of COVID-19 research articles. You can search the database of citations by author, keyword (in title, author, abstract, subject headings fields), journal, or abstract when available. DOI, PMID, and URL links are included when available.
This database was last updated on October 9, 2020 .
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide. The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
- To help inform CDC’s COVID-19 Response, as well as to help CDC staff stay up to date on the latest COVID-19 research, the Response’s Office of the Chief Medical Officer has collaborated with the CDC Office of Library Science to create a series called COVID-19 Science Update . This series, the first of its kind for a CDC emergency response, provides brief summaries of new COVID-19-related studies on many topics, including epidemiology, clinical treatment and management, laboratory science, and modeling. As of December 18, 2021, CDC has stopped production of the weekly COVID-19 Science Update.
Excel download:
- Articles from August until October 9 2020 [XLS – 29 MB]
- Articles from December 2019 through July 2020 [XLS – 45 MB]
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide.
- October 8 in Excel [XLS – 1 MB]
- October 7 in Excel [XLS – 1 MB]
- October 6 in Excel [XLS – 1 MB]
- Note the main Excel file can also be sorted by date added.
Citation Management Software (EndNote, Mendeley, Zotero, Refman, etc.) download:
- Part 1 [ZIP – 38 MB]
- Part 2 [ZIP – 43 MB]
- October 8 in citation management software format [RIS – 2 MB]
- October 7 in citation management software format [RIS – 2 MB]
- October 6 in citation management software format [RIS – 2 MB]
- Note the main RIS file can also be sorted by date added.
The COVID-19 pandemic is a rapidly changing situation. Some of the research included above is preliminary. Materials listed in this database are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
To access the full text, click on the DOI, PMID, or URL links. While most publishers are making their COVID-19 content Open Access, some articles are accessible only to those with a CDC user id and password. Find a library near you that may be able to help you get access to articles by clicking the following links: https://www.worldcat.org/libraries OR https://www.usa.gov/libraries .
CDC users can use EndNote’s Find Full Text feature to attach the full text PDFs within their EndNote Library. CDC users, please email Martha Knuth for an EndNote file of all citations. Once you have your EndNote file downloaded, to get the full-text of journal articles listed in the search results you can do the following steps:
- First, try using EndNote’s “Find Full-Text” feature to attach full-text articles to your EndNote Library.
- Next, check for full-text availability, via the E-Journals list, at: http://sfxhosted.exlibrisgroup.com/cdc/az .
- If you can’t find full-text online, you can request articles via DocExpress, at: https://docexpress.cdc.gov/illiad/
The following databases were searched from Dec. 2019-Oct. 9 2020 for articles related to COVID-19: Medline (Ovid and PubMed), PubMed Central, Embase, CAB Abstracts, Global Health, PsycInfo, Cochrane Library, Scopus, Academic Search Complete, Africa Wide Information, CINAHL, ProQuest Central, SciFinder, the Virtual Health Library, and LitCovid. Selected grey literature sources were searched as well, including the WHO COVID-19 website, CDC COVID-19 website, Eurosurveillance, China CDC Weekly, Homeland Security Digital Library, ClinicalTrials.gov, bioRxiv (preprints), medRxiv (preprints), chemRxiv (preprints), and SSRN (preprints).
Detailed search strings with synonyms used for COVID-19 are below.
Detailed search strategy for gathering COVID-19 articles, updated October 9, 2020 [PDF – 135 KB]
Note on preprints: Preprints have not been peer-reviewed. They should not be regarded as conclusive, guide clinical practice/health-related behavior, or be reported in news media as established information.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings. HHS, PHS, and CDC assume no responsibility for the factual accuracy of the items presented. The selection, omission, or content of items does not imply any endorsement or other position taken by HHS, PHS, and CDC. Opinion, findings, and conclusions expressed by the original authors of items included in these materials, or persons quoted therein, are strictly their own and are in no way meant to represent the opinion or views of HHS, PHS, or CDC. References to publications, news sources, and non-CDC Websites are provided solely for informational purposes and do not imply endorsement by HHS, PHS, or CDC.
To receive the COVID-19 Science Update, please enter your email address to subscribe today.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Emergence of SARS and COVID-19 and preparedness for the next emerging disease X
Affiliations.
- 1 CAS Key Laboratory of Special Pathogens and Biosafety, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan, 430071, China.
- 2 University of Chinese Academy of Sciences, Beijing, 100049, China.
- 3 CAS Key Laboratory of Special Pathogens and Biosafety, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan, 430071, China. [email protected].
- PMID: 38561562
- DOI: 10.1007/s11684-024-1066-6
Severe acute respiratory syndrome (SARS) and Coronavirus disease 2019 (COVID-19) are two human Coronavirus diseases emerging in this century, posing tremendous threats to public health and causing great loss to lives and economy. In this review, we retrospect the studies tracing the molecular evolution of SARS-CoV, and we sort out current research findings about the potential ancestor of SARS-CoV-2. Updated knowledge about SARS-CoV-2-like viruses found in wildlife, the animal susceptibility to SARS-CoV-2, as well as the interspecies transmission risk of SARS-related coronaviruses (SARSr-CoVs) are gathered here. Finally, we discuss the strategies of how to be prepared against future outbreaks of emerging or re-emerging coronaviruses.
Keywords: COVID-19; SARS; coronavirus.
© 2024. Higher Education Press.
Publication types
- Introduction
- Conclusions
- Article Information
Recommendations directly from CDC; regarding bivalent (Omicron) booster, see CDC, 2022. 45 Recommendations directly from ACOG; regarding bivalent (Omicron) booster, see ACOG, 2022. 46
eTable 1. Weighted Demographic Characteristics of 652 Pregnant or Recently Pregnant Respondents From the Vaccine Safety Datalink, Stratified by Self-Reported COVID-19 Vaccination Status, November 2021 to February 2022 (Wave 1)
eTable 2. Weighted Demographic Characteristics of 575 Pregnant or Postpartum Respondents From the Vaccine Safety Datalink, Stratified by Self-Reported COVID-19 Vaccination Status, October 2022 to February 2023 (Wave 2)
eTable 3. Weighted Differences in Attitudes Toward COVID-19 Vaccines Among 652 Pregnant or Postpartum Vaccine Safety Datalink Members, Stratified by Vaccination Status, November 2021 to February 2022 (Wave 1)
eTable 4. Weighted Differences in Attitudes About COVID-19 Vaccines Among 575 Pregnant or Recently Pregnant People in the Vaccine Safety Datalink, Stratified by Vaccination Status, October 2022 to February 2023 (Wave 2)
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing

Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Williams JTB , Kurlandsky K , Breslin K, et al. Attitudes Toward COVID-19 Vaccines Among Pregnant and Recently Pregnant Individuals. JAMA Netw Open. 2024;7(4):e245479. doi:10.1001/jamanetworkopen.2024.5479
Manage citations:
© 2024
- Permissions
Attitudes Toward COVID-19 Vaccines Among Pregnant and Recently Pregnant Individuals
- 1 Ambulatory Care Services, Denver Health and Hospitals, Denver, Colorado
- 2 Department of Pediatrics, University of Colorado School of Medicine, Aurora
- 3 Institute for Health Research, Kaiser Permanente Colorado, Aurora
- 4 Kaiser Permanente Southern California, Pasadena
- 5 Kaiser Permanente Vaccine Study Center, Oakland, California
- 6 Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon
- 7 Health Research Institute, Kaiser Permanente Washington, Seattle
- 8 HealthPartners Institute, Minneapolis, Minnesota
- 9 Marshfield Clinic Research Institute, Marshfield, Wisconsin
- 10 Department of Population Medicine, Harvard Pilgrim Health Care Institute, Boston, Massachusetts
- 11 Immunization Safety Office, US Centers for Disease Control and Prevention, Atlanta, Georgia
Question Do perceptions of COVID-19 vaccines differ among distinct pregnant or recently pregnant respondents from 2 survey waves between November 2021 and February 2023?
Findings In this survey study, 1227 of 2956 people responded. Respondents who received 1 or more COVID-19 vaccines, identified as non-Hispanic White, and preferred the Spanish language had the largest decreases in agreement that COVID-19 vaccines are safe across waves.
Meaning These findings suggest that decreases in perceived COVID-19 vaccine safety among specific groups of insured pregnant and recently pregnant individuals is a public health concern.
Importance Pregnant people and infants are at high risk of severe COVID-19 outcomes. Understanding changes in attitudes toward COVID-19 vaccines among pregnant and recently pregnant people is important for public health messaging.
Objective To assess attitudinal trends regarding COVID-19 vaccines by (1) vaccination status and (2) race, ethnicity, and language among samples of pregnant and recently pregnant Vaccine Safety Datalink (VSD) members from 2021 to 2023.
Design, Setting, and Participants This cross-sectional surveye study included pregnant or recently pregnant members of the VSD, a collaboration of 13 health care systems and the US Centers for Disease Control and Prevention. Unvaccinated, non-Hispanic Black, and Spanish-speaking members were oversampled. Wave 1 took place from October 2021 to February 2022, and wave 2 took place from November 2022 to February 2023. Data were analyzed from May 2022 to September 2023.
Exposures Self-reported or electronic health record (EHR)–derived race, ethnicity, and preferred language.
Main Outcomes and Measures Self-reported vaccination status and attitudes toward monovalent (wave 1) or bivalent Omicron booster (wave 2) COVID-19 vaccines. Sample- and response-weighted analyses assessed attitudes by vaccination status and 3 race, ethnicity, and language groupings of interest.
Results There were 1227 respondents; all identified as female, the mean (SD) age was 31.7 (5.6) years, 356 (29.0%) identified as Black race, 555 (45.2%) identified as Hispanic ethnicity, and 445 (36.3%) preferred the Spanish language. Response rates were 43.5% for wave 1 (652 of 1500 individuals sampled) and 39.5% for wave 2 (575 of 1456 individuals sampled). Respondents were more likely than nonrespondents to be White, non-Hispanic, and vaccinated per EHR. Overall, 76.8% (95% CI, 71.5%-82.2%) reported 1 or more COVID-19 vaccinations; Spanish-speaking Hispanic respondents had the highest weighted proportion of respondents with 1 or more vaccination. Weighted estimates of somewhat or strongly agreeing that COVID-19 vaccines are safe decreased from wave 1 to 2 for respondents who reported 1 or more vaccinations (76% vs 50%; χ 2 1 = 7.8; P < .001), non-Hispanic White respondents (72% vs 43%; χ 2 1 = 5.4; P = .02), and Spanish-speaking Hispanic respondents (76% vs 53%; χ 2 1 = 22.8; P = .002).
Conclusions and Relevance Decreasing confidence in COVID-19 vaccine safety in a large, diverse pregnant and recently pregnant insured population is a public health concern.
Pregnant people infected with SARS-CoV-2 virus have increased risks of morbidity, mortality, and adverse pregnancy outcomes. 1 - 6 While pregnant people were not initially included in COVID-19 vaccination trials, observational data suggest vaccination during pregnancy reduces the risks of severe COVID-19 disease and adverse birth outcomes. 7 - 13 Young children are also at risk of severe disease and death from COVID-19, with infants under 6 months old with the highest rates of severe COVID-19 outcomes. 14 , 15 Early pandemic studies found consistently low levels of vaccination intention or uptake among pregnant people, with low rates in Black and Latino individuals. 16 - 20
As the COVID-19 public health emergency ended, these trends continued. As of July 29, 2023, Vaccine Safety Datalink (VSD) surveillance found just 16.2% of pregnant people aged 18 to 49 years had received a COVID-19 booster vaccine, with only 8.3% of Black pregnant people and 9.6% of Latino pregnant people vaccinated during pregnancy. 21 Assessing attitudes toward COVID-19 vaccines among pregnant and recently pregnant people is critical to public health messaging and clinician counseling. 22 We conducted 2 cross-sectional surveys of distinct samples of pregnant and recently pregnant individuals during the latter portion of the COVID-19 pandemic. Our primary aims were to assess trends in attitudes regarding COVID-19 vaccines by (1) self-reported vaccination status and (2) race, ethnicity, and preferred language.
The Colorado Multiple Institutional Review Board (COMIRB) approved this survey study; participating sites’ institutional review boards ceded oversight to COMIRB. COMIRB granted a waiver of written consent for study participation because the survey’s introduction language made it clear a respondent’s choice to complete the survey indicated consent to participate. This work was part of a larger project assessing vaccination attitudes and status among pregnant and nonpregnant VSD members over time. 21 Distinct cohorts of pregnant and recently pregnant members were sampled over time, with the first survey administered from November 1, 2021, to February 1, 2022, and the second survey administered from October 1, 2022, to February 1, 2023. Respondents were asked to report their COVID-19 vaccination status and share their attitudes toward COVID-19 infection and monovalent COVID-19 vaccines (wave 1) or bivalent Omicron booster vaccines (wave 2). Self-reported vaccination status was considered the criterion standard, as with prior attitudinal surveys in the VSD. 23 We followed the American Association for Public Opinion Research ( AAPOR ) reporting guideline for survey studies, applying Response Rate Definition 6. 24
The VSD is a collaboration between the US Centers for Disease Control and Prevention (CDC) and 13 integrated health care systems (called sites). 25 Vaccination data were derived from the electronic health record (EHR) and reconciled routinely with state immunization information systems. 26 VSD members represent over 3% of the total US population. 27 Eight VSD sites contributed data: Denver Health (Colorado), Kaiser Permanente Colorado, Marshfield Clinic (Wisconsin), HealthPartners (Minnesota), Kaiser Permanente Washington, Kaiser Permanente Northwest (Oregon), Kaiser Permanente Northern California, and Kaiser Permanente Southern California.
The VSD has a validated algorithm to identify pregnancies in near real-time with a high degree of accuracy. 28 - 30 We used this algorithm to identify adults aged 18 to 49 years who were pregnant any time between December 11, 2020, and August 31, 2021 (wave 1), or January 1, 2022, and August 1, 2022 (wave 2). As time elapsed between sampling and surveys going into the field, respondents included currently and recently pregnant people. Eligible individuals had continuous health insurance enrollment during the same periods for each wave, except at Denver Health, which uses empanelment as a proxy for enrollment. 31 We excluded people with an International Statistical Classification of Diseases, Tenth Revision, Clinical Modification code for adverse pregnancy outcomes (eg, spontaneous abortion, anencephaly), those with possible data errors (eg, simultaneous administration of multiple COVID-19 vaccines), and individuals who had opted out of research.
Within eligible cohorts, we conducted stratified sampling at each VSD site using 6 mutually exclusive strata defined by the following EHR factors: COVID-19 vaccination (unvaccinated or ≥1 vaccines), race (Black or other race and ethnicity, which included American Indian or Alaskan Native, Asian, Native Hawaiian or Pacific Islander, and White), and preferred language (English or Spanish). We oversampled individuals roughly 2:1 whose EHR indicated they were unvaccinated or self-identified as Black race or preferred Spanish language, as prior surveys of pregnant VSD members have shown lower response rates in these groups. 23 Spanish-speaking participants were sampled from Denver Health and Kaiser Permanente Southern California. 32 Separate samples were created for each wave.
The target sample size for each survey wave was determined by a related project outcome: the accuracy of EHR vaccination data vs self-reported vaccination. Assuming variable response rates by vaccination status and race, 23 a 60% negative predictive value (EHR-unvaccinated people reporting they were unvaccinated), and a sample of 907 unvaccinated individuals and 593 vaccinated individuals for each survey wave, the study was powered to achieve a 2.0% CI around the vaccination confirmation rate and an 8.0% CI around the nonvaccination confirmation rate in each wave. Estimated CIs were corrected for the anticipated response rate.
Survey instruments aligned with the Health Belief Model. 33 - 36 We based our survey questions on previously published instruments, 23 , 37 - 39 using wording from COVID-19 studies when possible. 40 - 43 One question probed perceptions of trusted information sources, and as response categories changed between waves, we have presented data for this question from wave 2 only. We included questions about race, ethnicity, household income, household size, and highest educational attainment. For respondents, self-reported race and ethnicity were the reference standards 44 ; EHR data were used when survey data were missing. Race was assessed in this study because vaccination rates have been found to be lower for Black and Latino individuals. 16 - 20
Draft surveys underwent a first round of revisions from VSD site content experts. Afterwards, the survey was translated into Spanish by a certified bilingual translator. It was pilot tested at Denver Health with 20 pregnant people (10 in English, 10 in Spanish). Interviewees were recruited from pediatric and obstetric waiting rooms, expressed verbal consent, and received a $25 gift card. A second round of revisions incorporated interviewees’ feedback for English and Spanish versions. Surveys were reviewed a third time by VSD sites and CDC experts (J.T.B.W., K.K., K.B., L.H., J.A.S., L.M.R., M.F.D., B.J.L., K.G., M.L.H., J.C.N., G.V.B., K.E.H., C.C.F., E.S.W., M.M.M., and S.J.H.) and finalized.
Due to the timing of survey administration and official vaccine recommendations, question wording regarding attitudes toward COVID-19 vaccines changed between waves. In wave 1, questions about COVID-19 vaccines referred generally to “COVID-19 vaccines” (ie, original monovalent mRNA and adenoviral vector vaccines). In wave 2, questions referred specifically to the “COVID-19 Omicron booster vaccines,” and question instructions noted that these vaccines were also called “bivalent booster vaccines.” Questions about COVID-19 booster vaccines also differed. In wave 1, we asked, “Have you received one or more COVID-19 booster vaccines?” In wave 2, we asked, “Have you received a COVID-19 Omicron booster vaccine?” The Figure illustrates changes in recommendations over time. 45 , 46
Prospective participants received up to 10 survey invitations via postal mail, e-mail, and telephone calls, per tailored survey design best practices. 47 Survey administration was consistent across VSD sites, except 1 site required participants to receive a presurvey letter with an opportunity to opt out and prohibited contact by email or phone. Outreach stopped after survey completion or if a person opted out. Surveys were hosted online via Research Electronic Data Capture Software. 48 Respondents received a $25 gift card. We obtained a waiver of written consent, but participants could opt out by email, in writing, or by phone.
We defined respondents as people who completed the first survey question: “Have you received a COVID-19 vaccine?” We used the Pearson χ 2 test to compare survey respondents to nonrespondents on sociodemographic variables available via EHR using 2-sided tests, considering a P value less than .05 significant. Among respondents, we calculated weighted descriptive statistics for any self-reported COVID-19 vaccination (ie, ≥1 dose ever), self-reported monovalent booster vaccination among those who had received at least 1 prior COVID-19 vaccine (wave 1), and self-reported Omicron booster vaccination among those who had received at least 1 prior COVID-19 vaccine (wave 2). For attitudinal and sociodemographic measures, we compared respondents by self-reported vaccination status (unvaccinated vs receipt of ≥1 dose) and by 3 mutually exclusive race, ethnicity, and language groups (non-Hispanic White, non-Hispanic Black, and Spanish-speaking Hispanic of any race).
Missingness in the dataset was low (<10%), and unknown or missing categories were included in analyses. We used the Rao-Scott χ 2 test for weighted tables using 2-sided hypothesis testing, considering a P value less than .05 significant. We included a finite population correction and incorporated inverse probability weighting to account for sampling and response probability by VSD site, vaccination status, and oversampling of non-Hispanic Black and Spanish-speaking Hispanic people. When considering both waves, individual survey weights were adjusted to reflect an average annual population. 49 Analyses were conducted using SAS version 9.4 (SAS Institute). Data were analyzed from May 2022 to September 2023.
In wave 1, 123 655 pregnant and recently pregnant people were eligible; 1500 (1.2%) were sampled. By EHR, 877 of these were unvaccinated, 551 identified as non-Hispanic Black, and 510 preferred Spanish language. In wave 2, 123 690 people were eligible and 1456 (1.2%) were sampled. By EHR, 826 of these were unvaccinated, 542 identified as non-Hispanic Black, and 476 preferred Spanish language. There were 652 respondents in wave 1 (652 of 1500 participants [43.5%]) and 575 respondents in wave 2 (575 of 1456 participants [39.5%]).
Of the 1227 total respondents, all identified as female, the mean (SD) age was 31.7 (5.6) years, 356 (29.0%) identified as Black race, 555 (45.2%) identified as Hispanic ethnicity, and 445 (36.3%) preferred the Spanish language. Eight individuals were sampled twice and completed both surveys; otherwise, respondents in wave 1 differed from respondents in wave 2. Table 1 provides EHR-derived vaccination status (unvaccinated or ≥1 doses received) and demographic information for respondents and nonrespondents by wave. Overall, people whose EHR-recorded race was Black or who were unvaccinated against COVID-19 were less likely to respond across both waves, whereas those with an EHR-recorded ethnicity of Hispanic were more likely to respond ( Table 1 ). Respondents in wave 2 were less likely than wave 1 respondents to identify as Black (154 [26.9%] vs 202 [31.0%]; χ 2 6 = 13.5; P = .04) or non-Hispanic (295 [51.4%] vs 377 [57.8%]; χ 2 1 = 5.0; P = .03) and prefer the English language (340 [59.2%] vs 442 [67.8%]; χ 2 1 = 9.5; P = .002).
Overall, 76.8% (95% CI, 71.5%-82.2%) of respondents reported receiving at least 1 COVID-19 vaccine. Of the 3 racial, ethnic, and language groups of interest, Spanish-speaking Hispanic individuals had the highest self-reported rate of any COVID-19 vaccination, 86.9% (95% CI, 82.0%-91.8%) in wave 1 and 84.2% (95% CI, 80.4%-88.1%) in wave 2; non-Hispanic Black respondents had the lowest vaccination rates, with 68.0% (95% CI, 57.9%-78.1%) in wave 1 and 69.7% (95% CI, 61.2%-78.2%) in wave 2. Of all vaccinees, 24.0% (95% CI, 15.4%-32.7%) reported 1 or more monovalent booster vaccinations in wave 1, and 25.4% (95% CI, 10.4%-40.5%) reported 1 or more Omicron booster vaccinations in wave 2 (χ 2 1 = 0.02; P = .88). Only 11.5% (95% CI, 6.3%-16.7%) of Spanish-speaking Hispanic respondents reported having received an Omicron booster vaccine in wave 2, whereas 40.2% (95% CI, 11.5%-68.9%) of Non-Hispanic White respondents self-reported 1 or more Omicron booster vaccinations in wave 2.
Table 2 compares the weighted estimates for self-reported sociodemographic characteristics in ever-vaccinated respondents and unvaccinated respondents across waves. Generally, sociodemographic characteristics did not change among vaccinees from wave 1 to wave 2, but unvaccinated individuals in wave 2 were more likely to be older, identify as White or other races, have an associate degree (or higher), and have household income above 200% of the federal poverty level ( Table 2 ). Ever-vaccinated respondents differed from unvaccinated respondents in many respects in wave 1 (eTable 1 in Supplement 1 ) but only by educational attainment by wave 2 (eTable 2 in Supplement 1 ).
Table 3 provides weighted estimates of COVID-19 vaccine safety perceptions across waves, stratified by vaccination status. Among those reporting 1 or more COVID-19 vaccination, we observed a 34% relative decrease in the proportion in agreement that COVID-19 vaccines are safe for pregnant people and a 32% relative decrease in the proportion agreeing that COVID-19 vaccines are safe for a pregnant person’s baby ( Table 3 ). There was a 41% relative decrease (χ 2 1 = 10.3; P < .01) in the proportion of those ever-vaccinated agreeing that most pregnant people should get a COVID-19 vaccine. Among unvaccinated respondents, attitudes toward COVID-19 vaccines were largely unfavorable and did not change across waves ( Table 3 ). Attitudes toward COVID-19 vaccines differed by vaccination status in both waves (eTable 3 and eTable 4 in Supplement 1 ). In wave 1, desiring to wait until after pregnancy and perceived vaccine adverse effects were the top reported reasons for being unvaccinated (eTable 3 in Supplement 1 ).
Table 4 summarizes weighted estimates of COVID-19 vaccine safety perceptions across waves by 3 mutually exclusive ethnic, racial, and linguistic groups of interest. The greatest decreases in perceived COVID-19 vaccine safety were observed among non-Hispanic White respondents, both for themselves (relative difference, −41%; χ 2 1 = 5.4; P = .02) and their babies (relative difference, −40%; χ 2 1 = 3.4; P = .04). Hispanic Spanish-speaking respondents had the greatest decrease in the proportion of respondents “not at all” or “not too” hesitant about receiving a COVID-19 vaccine (relative difference, −42%; χ 2 1 = 33.9; P < .01), and non-Hispanic White respondents had the greatest decrease in agreement that most pregnant people should get a COVID-19 vaccine (relative difference, −43%; χ 2 1 = 3.5; P = .04). In comparison, COVID-19 vaccine attitudes did not change significantly across waves for Non-Hispanic Black respondents ( Table 4 ).
In the second survey wave, respondents’ most trusted sources for information about COVID-19 and COVID-19 vaccines varied by vaccination status (eTable 4 in Supplement 1 ). Among ever-vaccinated participants, 34.4% (95% CI, 18.5%-50.3%) trusted the CDC the most for this information, and a similar proportion, 34.4% (95% CI, 18.5%-50.2%), chose their physician. Of unvaccinated participants, 9.2% (95% CI, 0%-19.0%) reported trusting the CDC most, with 11.0% (95% CI, 4.5%-17.5%) reporting their physician. Among all Non-Hispanic Black respondents, 32.5% (95% CI, 22.6%-42.4%) reported trusting the CDC most and 18.7% (95% CI, 11.1%-26.3%) their physicians. Conversely, Spanish-speaking Hispanic participants had a lower proportion trusting the CDC (26.1%; 95% CI, 19.6%-32.6%), but a higher proportion trusting their physicians (35.1%; 95% CI, 28.1%-42.0%). Among Non-Hispanic White participants, 26.6% (95% CI, 5.7%-47.6%) trusted the CDC most while 38.2% (95% CI, 14.5%-62.0%) trusted their physicians most.
Among diverse pregnant and recently pregnant VSD members during the latter part of the COVID-19 public health emergency, we observed significant changes in perceptions of COVID-19 vaccine safety over time. First, we observed a significant change over time in respondent groups’ perceptions of COVID-19 vaccine safety for pregnant persons and their infants. While we did not survey the same individuals at 2 time points, the general trends we observed among those who had received at least 1 COVID-19 vaccine and among racially, ethnically, and linguistically diverse groups are concerning. Substantial evidence continues to accrue supporting COVID-19 vaccine safety for pregnant people, 10 , 50 - 52 but the concomitant spread of misinformation 53 , 54 may partially explain our declines in perceived safety. A Kaiser Family Foundation survey ending in June 2023 found that 27% of respondents believed COVID-19 vaccines have been shown to cause infertility; 68% of respondents were uncertain whether the claim was definitely true or definitely false. 55 Interestingly, 94% of adults in the Kaiser survey reported “a great deal” or “fair amount” of trust in their physician to make the appropriate vaccination recommendations for them, with the next most highly trusted information source being the CDC. 55 Our data suggest more modest levels of trust in the CDC and physicians with notable differences by race, ethnicity, and language preference. Future work could study perceived safety of COVID-19 vaccines as a function of message tailoring for diverse groups.
Weighted rates of receipt of 1 or more COVID-19 vaccines were highest for Spanish-speaking Hispanic respondents in both waves. This finding contrasts with early and late pandemic studies identifying low COVID-19 vaccination rates and delayed first doses among Hispanic individuals. 16 - 19 , 21 , 56 - 58 The cultural phenomenon of respeto , by which Spanish-speaking families have been observed to subjugate personal concerns to clinical expertise, 59 may explain this early difference. However, by wave 2, Spanish-speaking Hispanic respondents became less confident in COVID-19 vaccine safety, were less in agreement that pregnant people should get a COVID-19 vaccine, and had significantly lower rates of Omicron booster vaccine receipt. Culturally tailored COVID-19 vaccine interventions for Spanish-speaking Hispanic communities exist 60 and should be carefully implemented in diverse contexts. We suggest they engage health care practitioners, which were most highly trusted for information by Spanish speaking Hispanic participants in our survey.
Perceptions in non-Hispanic Black and non-Hispanic White respondents are also concerning. Non-Hispanic Black respondents were most concerned about safety of COVID-19 vaccines in both waves. Meanwhile, Non-Hispanic White respondents became significantly more hesitant about COVID-19 vaccines, less sure of COVID-19 vaccine safety, and more uncertain that pregnant people should get a COVID-19 vaccine. These trends in non-Hispanic White individuals, who were early adopters of COVID-19 vaccines, 16 , 17 , 19 , 20 are an important finding and deserve attention. Persistently more unfavorable perceptions of COVID-19 vaccine safety in Black respondents underline the importance of continually attending to safety concerns in historically marginalized communities.
This study has limitations. Survey nonresponse may have influenced our results, as nonrespondents differed from respondents, respondents differed across waves, response rates decreased as the pandemic evolved (as others have noted), and increasing nonresponse may itself indicate decreasing confidence in COVID-19 vaccines. 61 , 62 Second, we sampled Spanish-speaking Hispanic members at only 2 VSD sites; findings may not be generalizable to Spanish-speaking Hispanic individuals in other areas. Third, we had low numbers of respondents from non-Hispanic Asian, Pacific Islander, and Indigenous groups, which caused significant uncertainty in their attitudinal estimates; studies have shown the highest COVID-19 vaccination rates in individuals who identify as non-Hispanic Asian. 20 , 54 Question wording about COVID-19 vaccines and COVID booster vaccines differed between survey waves due to changing federal recommendations. Additionally, we did not ask respondents if they were pregnant at the time of survey completion, which could have provided additional insights.
During the latter portion of the public health emergency, we observed significant changes in COVID-19 vaccine hesitancy and perceptions of COVID-19 vaccine safety among insured pregnant and recently pregnant people. These differences, despite accruing evidence of COVID-19 vaccine safety in this high-risk group, are concerning for clinicians and public health officials.
Accepted for Publication: February 9, 2024.
Published: April 8, 2024. doi:10.1001/jamanetworkopen.2024.5479
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Williams JTB et al. JAMA Network Open .
Corresponding Author: Joshua T. B. Williams, MD, Wellington Webb Clinic, 301 W 6th Ave, MC 1911, Denver, CO 80207 ( [email protected] ).
Author Contributions: Drs Williams and Stein had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Williams, Kurlandsky, Reifler, Daley, Vazquez-Benitez, Weintraub, Hambidge.
Acquisition, analysis, or interpretation of data: Williams, Kurlandsky, Breslin, Durfee, Stein, Hurley, Shoup, Reifler, Daley, Lewin, Goddard, Henninger, Nelson, Nelson, McNeil, Fuller, Hanson, Hambidge.
Drafting of the manuscript: Williams, Kurlandsky, Stein.
Critical review of the manuscript for important intellectual content: Williams, Kurlandsky, Breslin, Durfee, Hurley, Shoup, Reifler, Daley, Lewin, Goddard, Henninger, Nelson, Vazquez-Benitez, McNeil, Fuller, Hanson, Weintraub, Hambidge.
Statistical analysis: Breslin, Durfee, Stein, Vazquez-Benitez.
Obtained funding: Williams, Daley, Goddard, Nelson, Hanson, Hambidge.
Administrative, technical, or material support: Williams, Kurlandsky, Shoup, Daley, Goddard, Henninger, Nelson, McNeil, Hanson, Weintraub, Hambidge.
Supervision: Williams, Hurley, McNeil, Weintraub, Hambidge.
Conflict of Interest Disclosures: Dr Reifler reported a family member owning stock in Johnson and Johnson. Dr Daley reported serving as a voting member of the Advisory Committee on Immunization Practices, which provides advice to the Centers for Disease Control and Prevention (CDC) regarding vaccine policy matters. Dr Nelson reported receiving personal fees from Harvard Pilgrim Health Care for statistical consulting services outside the submitted work. Dr Vazquez-Benitez reported receiving grants from Abvvie Research and Sanofi Research outside the submitted work. Dr Fuller reported that her institution has received funding for research from Pfizer and Johnson and Johnson, which is unrelated to this work. No other disclosures were reported.
Funding/Support: This study was supported by the CDC, contract number 200-2012-53581-0011. This activity was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy. See, for example, 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Role of the Funder/Sponsor: The study sponsor, CDC, participated as a coinvestigator and contributed to protocol development, conduct of the study, interpretation of the data, review and revision of the manuscript, approval of the manuscript through official CDC scientific clearance processes, and the decision to submit the manuscript for publication. CDC authors must receive approval through the CDC scientific clearance process to submit an article for publication. Final decision to submit rested with the first author. The study sponsor did not have the right to direct the submission to a particular journal.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We thank VSD site data managers who reviewed our sampling program and procured population estimates at each site for weighting purposes. We also thank VSD site project managers who assisted with institutional review board ceding processes and coordination with site principal investigators.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Study links key gene to protection from severe illness and death from COVID infections in men under age 75
A certain variant of a key anti-inflammatory gene protects men under age 75 from severe illness and death when hospitalized from Covid-19, a genetic analysis of their blood shows.
According to the study authors, the protective gene in question, an interleukin-1 receptor antagonist (IL1RN) variant, appears to tamp down inflammation. Inflammation is the body's normal reaction to infection, but when unchecked, inflammation can go too far and damage tissues as part of many diseases, including in severe cases of infection with the pandemic virus SARS-CoV-2.
Published as a "major article" in The Journal of Infectious Diseases online March 13, the study showed that 124 men between the ages of 19 and 74 who possessed the IL1RN variant, called rs419598, were less likely to become severely ill after hospitalization for Covid-19, and 80% less likely to die from the disease.
IL1RN is expressed naturally in the body. Different types of interleukin genes are known to dial inflammation up or down in the context of arthritis, and researchers say the results of the current study suggest that a similar dynamic influences the interleukin-1-related inflammation seen in Covid-19 patients.
The findings, from researchers at NYU Grossman School of Medicine, stand out because historically more men than women are known to die from Covid-19, and the IL1RN rs419598 variant appears to selectively protect only men up to age 74, but not beyond that as age-related chronic illnesses unfold.
The research team used sequencing technologies for the study to determine the presence of specific genes or variations in the letter code that makes up genes in blood samples from 2,589 men and women hospitalized for Covid-19 at NYU Langone's Tisch Hospital in Manhattan from March 2020 to March 2021.
More than half of the men and women in the study were older than age 60 and obese, factors that are known to increase the risk of death from the viral infection. Overall, more men than women (240 men, at 60.5%; and 157 women, at 39.5%) died from their disease, with women 20% less likely to die than men.
"Our study results show that among hospitalized patients, while women are still overall less likely than men to die from Covid-19, those men age 74 and younger who possess the IL1RN gene variant rs419598 are much less likely to suffer the severe inflammation tied to SARS-CoV-2 infection and less likely to die from the disease," said study colead investigator and molecular biologist Mukundan Attur, PhD. Attur is an associate professor in the Department of Medicine at NYU Langone Health.
Among the study's other findings was that average blood levels of the anti-inflammatory protein IL-1Ra, coded by IL1RN, were 14 times higher in 181 hospitalized men than in healthy male study controls from the general population, and 10 times as high in 178 hospitalized women than in healthy females. However, researchers say the increased levels of IL-1Ra in women did not result in any statistically significant reductions in death.
"Our analysis offers substantial evidence of the biological link between the severe inflammation seen in SARS-CoV-2 and that which occurs in rheumatoid arthritis," said study senior investigator Steven Abramson, MD, the Frederick H. King Professor of Internal Medicine at NYU Langone.
Abramson, a rheumatologist who also serves as chair of the Department of Medicine and chief academic officer at NYU Langone, says previous research has shown that such rheumatoid inflammation is lower in people who possessed one of the three IL1RN variants analyzed in the study.
More importantly, Abramson says, the new research suggests that restraining the interleukin-1 biological pathway, which is in part tamped down by the anti-inflammatory protein IL-1Ra, could help prevent the severe inflammation seen in SARS-CoV-2 infection. Further research, he says, is warranted into whether IL-1-inhibiting therapies, such as the IL1 receptor antagonists anakinra, canakinumab, and rilonacept, are effective against Covid infection.
Abramson already has plans to investigate if the IL-1 pathway plays a role in long Covid, when people experience new or lingering symptoms, such as fatigue and "brain fog," months after recuperating from their initial infection.
Abramson points out that the new study adds to the growing scientific evidence about the biological factors that contribute to gender differences seen in deaths from Covid-19, which are known to vary widely across the United States.
Funding support for this study was provided by National Institutes of Health grants P30CA016087 and R21AR078466.
Besides Abramson and Attur, other NYU Langone researchers involved in this study are co-lead investigators Christopher Petrilli, MD, and Samrachana Adhikari, PhD, and study co-investigators Eduardo Iturrate, MD; Xiyue Li, MS; Stephanie Tuminello, MPH; Nan Hu, MS; Aravinda Chakravarti, PhD; and David Beck, MD, PhD.
- COVID and SARS
- Men's Health
- Infectious Diseases
- Healthy Aging
- Crohn's Disease
- Diseases and Conditions
- Sudden infant death syndrome
- Programmed cell death
- Ischaemic heart disease
Story Source:
Materials provided by NYU Langone Health / NYU Grossman School of Medicine . Note: Content may be edited for style and length.
Journal Reference :
- Mukundan Attur, Christopher Petrilli, Samrachana Adhikari, Eduardo Iturrate, Xiyue Li, Stephanie Tuminello, Nan Hu, Aravinda Chakravarti, David Beck, Steven B Abramson. Interleukin-1 Receptor Antagonist Gene (IL1RN) Variants Modulate the Cytokine Release Syndrome and Mortality of COVID-19 . The Journal of Infectious Diseases , 2024; 10.1093/infdis/jiae031 [ abstract ]
Cite This Page :
Explore More
- Soft, Flexible 'Skeletons' for 'Muscular' Robots
- Toothed Whale Echolocation and Jaw Muscles
- Friendly Pat On the Back: Free Throws
- How the Moon Turned Itself Inside Out
- A Welcome Hug Is Good for Your Health
- Climate Change Threatens Antarctic Meteorites
- Precise Measurement of Our Expanding Universe
- Little Research On 'Polycrisis' Humanity Faces
- Prebiotic Molecular Kitchen
- A Neurodegenerative Disease Triggered by Virus
Trending Topics
Strange & offbeat.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 08 April 2024
Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease
- Felicity Liew 1 na1 ,
- Claudia Efstathiou ORCID: orcid.org/0000-0001-6125-8126 1 na1 ,
- Sara Fontanella 1 ,
- Matthew Richardson 2 ,
- Ruth Saunders 2 ,
- Dawid Swieboda 1 ,
- Jasmin K. Sidhu 1 ,
- Stephanie Ascough 1 ,
- Shona C. Moore ORCID: orcid.org/0000-0001-8610-2806 3 ,
- Noura Mohamed 4 ,
- Jose Nunag ORCID: orcid.org/0000-0002-4218-0500 5 ,
- Clara King 5 ,
- Olivia C. Leavy 2 , 6 ,
- Omer Elneima 2 ,
- Hamish J. C. McAuley 2 ,
- Aarti Shikotra 7 ,
- Amisha Singapuri ORCID: orcid.org/0009-0002-4711-7516 2 ,
- Marco Sereno ORCID: orcid.org/0000-0003-4573-9303 2 ,
- Victoria C. Harris 2 ,
- Linzy Houchen-Wolloff ORCID: orcid.org/0000-0003-4940-8835 8 ,
- Neil J. Greening ORCID: orcid.org/0000-0003-0453-7529 2 ,
- Nazir I. Lone ORCID: orcid.org/0000-0003-2707-2779 9 ,
- Matthew Thorpe 10 ,
- A. A. Roger Thompson ORCID: orcid.org/0000-0002-0717-4551 11 ,
- Sarah L. Rowland-Jones 11 ,
- Annemarie B. Docherty ORCID: orcid.org/0000-0001-8277-420X 10 ,
- James D. Chalmers 12 ,
- Ling-Pei Ho ORCID: orcid.org/0000-0001-8319-301X 13 ,
- Alexander Horsley ORCID: orcid.org/0000-0003-1828-0058 14 ,
- Betty Raman 15 ,
- Krisnah Poinasamy 16 ,
- Michael Marks 17 , 18 , 19 ,
- Onn Min Kon 1 ,
- Luke S. Howard ORCID: orcid.org/0000-0003-2822-210X 1 ,
- Daniel G. Wootton 3 ,
- Jennifer K. Quint 1 ,
- Thushan I. de Silva ORCID: orcid.org/0000-0002-6498-9212 11 ,
- Antonia Ho 20 ,
- Christopher Chiu ORCID: orcid.org/0000-0003-0914-920X 1 ,
- Ewen M. Harrison ORCID: orcid.org/0000-0002-5018-3066 10 ,
- William Greenhalf 21 ,
- J. Kenneth Baillie ORCID: orcid.org/0000-0001-5258-793X 10 , 22 , 23 ,
- Malcolm G. Semple ORCID: orcid.org/0000-0001-9700-0418 3 , 24 ,
- Lance Turtle 3 , 24 ,
- Rachael A. Evans ORCID: orcid.org/0000-0002-1667-868X 2 ,
- Louise V. Wain 2 , 6 ,
- Christopher Brightling 2 ,
- Ryan S. Thwaites ORCID: orcid.org/0000-0003-3052-2793 1 na1 ,
- Peter J. M. Openshaw ORCID: orcid.org/0000-0002-7220-2555 1 na1 ,
- PHOSP-COVID collaborative group &
ISARIC investigators
Nature Immunology ( 2024 ) Cite this article
Metrics details
- Inflammasome
- Inflammation
- Innate immunity
One in ten severe acute respiratory syndrome coronavirus 2 infections result in prolonged symptoms termed long coronavirus disease (COVID), yet disease phenotypes and mechanisms are poorly understood 1 . Here we profiled 368 plasma proteins in 657 participants ≥3 months following hospitalization. Of these, 426 had at least one long COVID symptom and 233 had fully recovered. Elevated markers of myeloid inflammation and complement activation were associated with long COVID. IL-1R2, MATN2 and COLEC12 were associated with cardiorespiratory symptoms, fatigue and anxiety/depression; MATN2, CSF3 and C1QA were elevated in gastrointestinal symptoms and C1QA was elevated in cognitive impairment. Additional markers of alterations in nerve tissue repair (SPON-1 and NFASC) were elevated in those with cognitive impairment and SCG3, suggestive of brain–gut axis disturbance, was elevated in gastrointestinal symptoms. Severe acute respiratory syndrome coronavirus 2-specific immunoglobulin G (IgG) was persistently elevated in some individuals with long COVID, but virus was not detected in sputum. Analysis of inflammatory markers in nasal fluids showed no association with symptoms. Our study aimed to understand inflammatory processes that underlie long COVID and was not designed for biomarker discovery. Our findings suggest that specific inflammatory pathways related to tissue damage are implicated in subtypes of long COVID, which might be targeted in future therapeutic trials.
One in ten severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections results in post-acute sequelae of coronavirus disease 2019 (PASC) or long coronavirus disease (COVID), which affects 65 million people worldwide 1 . Long COVID (LC) remains common, even after mild acute infection with recent variants 2 , and it is likely LC will continue to cause substantial long-term ill health, requiring targeted management based on an understanding of how disease phenotypes relate to underlying mechanisms. Persistent inflammation has been reported in adults with LC 1 , 3 , but studies have been limited in size, timing of samples or breadth of immune mediators measured, leading to inconsistent or absent associations with symptoms. Markers of oxidative stress, metabolic disturbance, vasculoproliferative processes and IFN-, NF-κB- or monocyte-related inflammation have been suggested 3 , 4 , 5 , 6 .
The PHOSP-COVID study, a multicenter United Kingdom study of patients previously hospitalized with COVID-19, has reported inflammatory profiles in 626 adults with health impairment after COVID-19, identified through clustering. Elevated IL-6 and markers of mucosal inflammation were observed in those with severe impairment compared with individuals with milder impairment 7 . However, LC is a heterogeneous condition that may be a distinct form of health impairment after COVID-19, and it remains unclear whether there are inflammatory changes specific to LC symptom subtypes. Determining whether activated inflammatory pathways underlie all cases of LC or if mechanisms differ according to clinical presentation is essential for developing effective therapies and has been highlighted as a top research priority by patients and clinicians 8 .
In this Letter, in a prospective multicenter study, we measured 368 plasma proteins in 657 adults previously hospitalized for COVID-19 (Fig. 1a and Table 1 ). Individuals in our cohort experienced a range of acute COVID-19 severities based on World Health Organization (WHO) progression scores 9 ; WHO 3–4 (no oxygen support, n = 133 and median age of 55 years), WHO 5–6 (oxygen support, n = 353 and median age of 59 years) and WHO 7–9 (critical care, n = 171 and median age of 57 years). Participants were hospitalized for COVID-19 ≥3 months before sample collection (median 6.1 months, interquartile range (IQR) 5.1–6.8 months and range 3.0–8.3 months) and confirmed clinically ( n = 36/657) or by PCR ( n = 621/657). Symptom data indicated 233/657 (35%) felt fully recovered at 6 months (hereafter ‘recovered’) and the remaining 424 (65%) reported symptoms consistent with the WHO definition for LC (symptoms ≥3 months post infection 10 ). Given the diversity of LC presentations, patients were grouped according to symptom type (Fig. 1b ). Groups were defined using symptoms and health deficits that have been commonly reported in the literature 1 ( Methods ). A multivariate penalized logistic regression model (PLR) was used to explore associations of clinical covariates and immune mediators at 6 months between recovered patients ( n = 233) and each LC group (cardiorespiratory symptoms, cardioresp, n = 398, Fig. 1c ; fatigue, n = 384, Fig. 1d ; affective symptoms, anxiety/depression, n = 202, Fig. 1e ; gastrointestinal symptoms, GI, n = 132, Fig. 1f ; and cognitive impairment, cognitive, n = 61, Fig. 1g ). Women ( n = 239) were more likely to experience CardioResp (odds ratio (OR 1.14), Fatigue (OR 1.22), GI (OR 1.13) and Cognitive (OR 1.03) outcomes (Fig. 1c,d,f,g ). Repeated cross-validation was used to optimize and assess model performance ( Methods and Extended Data Fig. 1 ). Pre-existing conditions, such as chronic lung disease, neurological disease and cardiovascular disease (Supplementary Table 1 ), were associated with all LC groups (Fig. 1c–g ). Age, C-reactive protein (CRP) and acute disease severity were not associated with any LC group (Table 1 ).
a , Distribution of time from COVID-19 hospitalization at sample collection. All samples were cross-sectional. The vertical red line indicates the 3 month cutoff used to define our final cohort and samples collected before 3 months were excluded. b , An UpSet plot describing pooled LC groups. The horizontal colored bars represent the number of patients in each symptom group: cardiorespiratory (Cardio_Resp), fatigue, cognitive, GI and anxiety/depression (Anx_Dep). Vertical black bars represent the number of patients in each symptom combination group. To prevent patient identification, where less than five patients belong to a combination group, this has been represented as ‘<5’. The recovered group ( n = 233) were used as controls. c – g , Forest plots of Olink protein concentrations (NPX) associated with Cardio_Resp ( n = 365) ( c ), fatigue (n = 314) ( d ), Anx_Dep ( n = 202) ( e ), GI ( n = 124) ( f ) and cognitive ( n = 60) ( g ). Neuro_Psych, neuropsychiatric. The error bars represent the median accuracy of the model. h , i , Distribution of Olink values (NPX) for IL-1R2 ( h ) and MATN2, neurofascin and sCD58 ( i ) measured between symptomatic and recovered individuals in recovered ( n = 233), Cardio_Resp ( n = 365), fatigue ( n = 314) and Anx_Dep ( n = 202) groups ( h ) and MATN2 in GI ( n = 124), neurofascin in cognitive ( n = 60) and sCD58 in Cardio_Resp and recovered groups ( i ). The box plot center line represents the median, the boundaries represent IQR and the whisker length represents 1.5× IQR. The median values were compared between groups using two-sided Wilcoxon signed-rank test, * P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001.
To study the association of peripheral inflammation with symptoms, we analyzed cross-sectional data collected approximately 6 months after hospitalizations. We measured 368 immune mediators from plasma collected contemporaneously with symptom data. Mediators suggestive of myeloid inflammation were associated with all symptoms (Fig. 1c–h ). Elevated IL-1R2, an IL-1 receptor expressed by monocytes and macrophages modulating inflammation 11 and MATN2, an extracellular matrix protein that modulates tissue inflammation through recruitment of innate immune cells 12 , were associated with cardioresp (IL-1R2 OR 1.14, Fig. 1c,h ), fatigue (IL-1R2 OR 1.45, Fig. 1d,h ), anxiety/depression (IL-1R2 OR 1.34. Fig. 1e,h ) and GI (MATN2 OR 1.08, Fig. 1f ). IL-3RA, an IL-3 receptor, was associated with cardioresp (OR 1.07, Fig. 1c ), fatigue (OR 1.21, Fig. 1d ), anxiety/depression (OR 1.12, Fig. 1e ) and GI (OR 1.06, Fig. 1f ) groups, while CSF3, a cytokine promoting neutrophilic inflammation 13 , was elevated in cardioresp (OR 1.06, Fig. 1c ), fatigue (OR 1.12, Fig. 1d ) and GI (OR 1.08, Fig. 1f ).
Elevated COLEC12, which initiates inflammation in tissues by activating the alternative complement pathway 14 , associated with cardioresp (OR 1.09, Fig. 1c ), fatigue (OR 1.19, Fig. 1d ) and anxiety/depression (OR 1.11, Fig. 1e ), but not with GI (Fig. 1f ) and only weakly with cognitive (OR 1.02, Fig. 1g ). C1QA, a degradation product released by complement activation 15 was associated with GI (OR 1.08, Fig. 1f ) and cognitive (OR 1.03, Fig. 1g ). C1QA, which is known to mediate dementia-related neuroinflammation 16 , had the third strongest association with cognitive (Fig. 1g ). These observations indicated that myeloid inflammation and complement activation were associated with LC.
Increased expression of DPP10 and SCG3 was observed in the GI group compared with recovered (DPP10 OR 1.07 and SCG3 OR 1.08, Fig. 1f ). DPP10 is a membrane protein that modulates tissue inflammation, and increased DPP10 expression is associated with inflammatory bowel disease 17 , 18 , suggesting that GI symptoms may result from enteric inflammation. Elevated SCG3, a multifunctional protein that has been associated with irritable bowel syndrome 19 , suggested that noninflammatory disturbance of the brain–gut axis or dysbiosis, may occur in the GI group. The cognitive group was associated with elevated CTSO (OR 1.04), NFASC (OR 1.03) and SPON-1 (OR 1.02, Fig. 1g,i ). NFASC and SPON-1 regulate neural growth 20 , 21 , while CTSO is a cysteine proteinase supporting tissue turnover 22 . The increased expression of these three proteins as well as C1QA and DPP10 in the cognitive group (Fig. 1g ) suggested neuroinflammation and alterations in nerve tissue repair, possibly resulting in neurodegeneration. Together, our findings indicated that complement activation and myeloid inflammation were common to all LC groups, but subtle differences were observed in the GI and cognitive groups, which may have mechanistic importance. Acutely elevated fibrinogen during hospitalization has been reported to be predictive of LC cognitive deficits 23 . We found elevated fibrinogen in LC relative to recovered (Extended Data Fig. 2a ; P = 0.0077), although this was not significant when restricted to the cognitive group ( P = 0.074), supporting our observation of complement pathway activation in LC and in keeping with reports that complement dysregulation and thrombosis drive severe COVID-19 (ref. 24 ).
Elevated sCD58 was associated with lower odds of all LC symptoms and was most pronounced in cardioresp (OR 0.85, Fig. 1c,i ), fatigue (OR 0.80, Fig. 1d ) and anxiety/depression (OR 0.83, Fig. 1e ). IL-2 was negatively associated with the cardioresp (Fig. 1c , OR 0.87), fatigue (Fig. 1d , OR 0.80), anxiety/depression (Fig. 1e , OR 0.84) and cognitive (Fig. 1g , OR 0.96) groups. Both IL-2 and sCD58 have immunoregulatory functions 25 , 26 . Specifically, sCD58 suppresses IL-1- or IL-6-dependent interactions between CD2 + monocytes and CD58 + T or natural killer cells 26 . The association of sCD58 with recovered suggests a central role of dysregulated myeloid inflammation in LC. Elevated markers of tissue repair, IDS and DNER 27 , 28 , were also associated with recovered relative to all LC groups (Fig. 1c–g ). Taken together, our data suggest that suppression of myeloid inflammation and enhanced tissue repair were associated with recovered, supporting the use of immunomodulatory agents in therapeutic trials 29 (Supplementary Table 2 ).
We next sought to validate the experimental and analytical approaches used. Although Olink has been validated against other immunoassay platforms, showing superior sensitivity and specificity 30 , 31 , we confirmed the performance of Olink against chemiluminescent immunoassays within our cohort. We performed chemiluminescent immunoassays on plasma from a subgroup of 58 participants (recovered n = 13 and LC n = 45). There were good correlations between results from Olink (normalized protein expression (NPX)) and chemiluminescent immunoassays (pg ml −1 ) for CSF3, IL-1R2, IL-3RA, TNF and TFF2 (Extended Data Fig. 3 ). Most samples did not have concentrations of IL-2 detectable using a mesoscale discovery chemiluminescent assay, limiting this analysis to 14 samples (recovered n = 4, LC n = 10, R = 0.55 and P = 0.053, Extended Data Fig. 3 ). We next repeated our analysis using alternative definitions of LC. The Centers for Disease Control and Prevention and National Institute for Health and Care Excellence definitions for LC include symptoms occurring 1 month post infection 32 , 33 . Using the 1 month post-infection definition included 62 additional participants to our analysis (recovered n = 21, 3 females and median age 61 years and LC n = 41, 15 females and median age 60 years, Extended Data Fig. 2c ) and found that inflammatory associations with each LC group were consistent with our analysis based on the WHO definition (Extended Data Fig. 2d–h ). Finally, to validate the analytical approach (PLR) we examined the distribution of data, prioritizing proteins that were most strongly associated with each LC/recovered group (IL-1R2, MATN2, NFASC and sCD58). Each protein was significantly elevated in the LC group compared with recovered (Fig. 1h,i and Extended Data Fig. 4 ), consistent with the PLR. Alternative regression approaches (unadjusted regression models and partial least squares, PLS) reported results consistent with the original analysis of protein associations and LC outcome in the WHO-defined cohort (Fig. 1c–g , Supplementary Table 3 and Extended Data Figs. 5 and 6 ). The standard errors of PLS estimates were wide (Extended Data Fig. 6 ), consistent with previous demonstrations that PLR is the optimal method to analyze high-dimensional data where variables may have combined effects 34 . As inflammatory proteins are often colinear, working in-tandem to mediate effects, we prioritized PLR results to draw conclusions.
To explore the relationship between inflammatory mediators associated with different LC symptoms, we performed a network analysis of Olink mediators highlighted by PLR within each LC group. COLEC12 and markers of endothelial and mucosal inflammation (MATN2, PCDH1, ROBO1, ISM1, ANGPTL2, TGF-α and TFF2) were highly correlated within the cardioresp, fatigue and anxiety/depression groups (Fig. 2 and Extended Data Fig. 7 ). Elevated PCDH1, an adhesion protein modulating airway inflammation 35 , was highly correlated with other inflammatory proteins associated with the cardioresp group (Fig. 2 ), suggesting that systemic inflammation may arise from the lung in these individuals. This was supported by increased expression of IL-3RA, which regulates innate immune responses in the lung through interactions with circulating IL-3 (ref. 36 ), in fatigue (Figs. 1d and 2 ), which correlated with markers of tissue inflammation, including PCDH1 (Fig. 2 ). MATN2 and ISM1, mucosal proteins that enhance inflammation 37 , 38 , were highly correlated in the GI group (Fig. 2 ), highlighting the role of tissue-specific inflammation in different LC groups. SCG3 correlated less closely with mediators in the GI group (Fig. 2 ), suggesting that the brain–gut axis may contribute separately to some GI symptoms. SPON-1, which regulates neural growth 21 , was the most highly correlated mediator in the cognitive group (Fig. 2 and Extended Data Fig. 7 ), highlighting that processes within nerve tissue may underlie this group. These observations suggested that inflammation might arise from mucosal tissues and that additional mechanisms may contribute to pathophysiology underlying the GI and cognitive groups.
Network analysis of Olink mediators associated with cardioresp ( n = 365), fatigue ( n = 314), anxiety/depression ( n = 202), GI ( n = 124) and cognitive groups ( n = 60). Each node corresponds to a protein mediator identified by PLR. The edges (blue lines) were weighted according to the size of Spearman’s rank correlation coefficient between proteins. All edges represent positive and significant correlations ( P < 0.05) after FDR adjustment.
Women were more likely to experience LC (Table 1 ), as found in previous studies 1 . As estrogen can influence immunological responses 39 , we investigated whether hormonal differences between men and women with LC in our cohort explained this trend. We grouped men and women with LC symptoms into two age groups (those younger than 50 years and those 50 years and older, using age as a proxy for menopause status in women) and compared mediator levels between men and women in each age group, prioritizing those identified by PLR to be higher in LC compared with recovered. As we aimed to understand whether women with LC had stronger inflammatory responses than men with LC, we did not assess differences in men and women in the recovered group. IL-1R2 and MATN2 were significantly higher in women ≥50 years than men ≥50 years in the cardioresp group (Fig. 3a , IL-1R2 and MATN2) and the fatigue group (Fig. 3b ). In the GI group, CSF3 was higher in women ≥50 years compared with men ≥50 years (Fig. 3c ), indicating that the inflammatory markers observed in women were not likely to be estrogen-dependent. Women have been reported to have stronger innate immune responses to infection and to be at greater risk of autoimmunity 39 , possibly explaining why some women in the ≥50 years group had higher inflammatory proteins than men the same group. Proteins associated with the anxiety/depression (IL-1R2 P = 0.11 and MATN2 P = 0.61, Extended Data Fig. 8a ) and cognitive groups (CTSO P = 0.64 and NFASC P = 0.41, Extended Data Fig. 8b ) were not different between men and women in either age group, consistent with the absent/weak association between sex and these outcomes identified by PLR (Fig. 1e,g ). Though our findings suggested that nonhormonal differences in inflammatory responses may explain why some women are more likely to have LC, they require confirmation in adequately powered studies.
a – c , Olink-measured plasma protein levels (NPX) of IL-1R2 and MATN2 ( a and b ) and CSF3 ( c ) between LC men and LC women divided by age (<50 or ≥50 years) in the cardiorespiratory group (<50 years n = 8 and ≥50 years n = 270) ( a ), fatigue group (<50 years n = 81 and ≥50 years n = 227) ( b ) and GI group (<50 years n = 34 and ≥50 years n = 82) ( c ). the median values were compared between men and women using two-sided Wilcoxon signed-rank test, * P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001. The box plot center line represents the median, the boundaries represent IQR and the whisker length represents 1.5× IQR.
To test whether local respiratory tract inflammation persisted after COVID-19, we compared nasosorption samples from 89 participants (recovered, n = 31; LC, n = 33; and healthy SARS-CoV-2 naive controls, n = 25, Supplementary Tables 4 and 5 ). Several inflammatory markers were elevated in the upper respiratory tract post COVID (including IL-1α, CXCL10, CXCL11, TNF, VEGF and TFF2) when compared with naive controls, but similar between recovered and LC (Fig. 4a ). In the cardioresp group ( n = 29), inflammatory mediators elevated in plasma (for example, IL-6, APO-2, TGF-α and TFF2) were not elevated in the upper respiratory tract (Extended Data Fig. 9a ) and there was no correlation between plasma and nasal mediator levels (Extended Data Fig. 9b ). This exploratory analysis suggested upper respiratory tract inflammation post COVID was not specifically associated with cardiorespiratory symptoms.
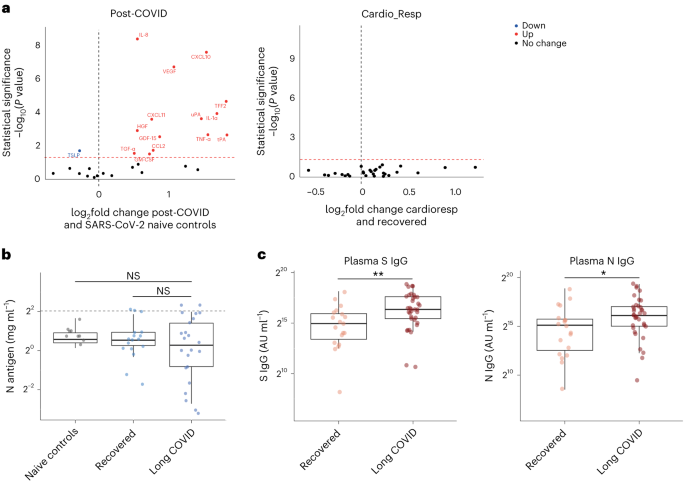
a , Nasal cytokines measured by immunoassay in post-COVID participants ( n = 64) compared with healthy SARS-CoV-2 naive controls ( n = 25), and between the the cardioresp group ( n = 29) and the recovered group ( n = 31). The red values indicate significantly increased cytokine levels after FDR adjustment ( P < 0.05) using two-tailed Wilcoxon signed-rank test. b , SARS-CoV-2 N antigen measured in sputum by electrochemiluminescence from recovered ( n = 17) and pooled LC ( n = 23) groups, compared with BALF from SARS-CoV-2 naive controls ( n = 9). The horizontal dashed line indicates the lower limit of detection of the assay. c , Plasma S- and N-specific IgG responses measured by electrochemiluminescence in the LC ( n = 35) and recovered ( n = 19) groups. The median values were compared using two-sided Wilcoxon signed-rank tests, NS P > 0.05, * P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001. The box plot center lines represent the median, the boundaries represent IQR and the whisker length represents 1.5× IQR.
To explore whether SARS-CoV-2 persistence might explain the inflammatory profiles observed in the cardioresp group, we measured SARS-CoV-2 nucleocapsid (N) antigen in sputum from 40 participants (recovered n = 17 and LC n = 23) collected approximately 6 months post hospitalization (Supplementary Table 6 ). All samples were compared with prepandemic bronchoalveolar lavage fluid ( n = 9, Supplementary Table 4 ). Only four samples (recovered n = 2 and LC n = 2) had N antigen above the assay’s lower limit of detection, and there was no difference in N antigen concentrations between LC and recovered (Fig. 4b , P = 0.78). These observations did not exclude viral persistence, which might require tissues samples for detection 40 , 41 . On the basis of the hypothesis that persistent viral antigen might prevent a decline in antibody levels over time, we examined the titers of SARS-CoV-2-specific antibodies in unvaccinated individuals (recovered n = 19 and LC n = 35). SARS-CoV-2 N-specific ( P = 0.023) and spike (S)-specific ( P = 0.0040) immunoglobulin G (IgG) levels were elevated in LC compared with recovered (Fig. 4c ).
Overall, we identified myeloid inflammation and complement activation in the cardioresp, fatigue, anxiety/depression, cognitive and GI groups 6 months after hospitalization (Extended Data Fig. 10 ). Our findings build on results of smaller studies 5 , 6 , 42 and are consistent with a genome-wide association study that identified an independent association between LC and FOXP4 , which modulates neutrophilic inflammation and immune cell function 43 , 44 . In addition, we identified tissue-specific inflammatory elements, indicating that myeloid disturbance in different tissues may result in distinct symptoms. Multiple mechanisms for LC have been suggested, including autoimmunity, thrombosis, vascular dysfunction, SARS-CoV-2 persistence and latent virus reactivation 1 . All these processes involve myeloid inflammation and complement activation 45 . Complement activation in LC has been suggested in a proteomic study in 97 mostly nonhospitalized COVID-19 cases 42 and a study of 48 LC patients, of which one-third experienced severe acute disease 46 . As components of the complement system are known to have a short half-life 47 , ongoing complement activation suggests active inflammation rather than past tissue damage from acute infection.
Despite the heterogeneity of LC and the likelihood of coexisting or multiple etiologies, our work suggests some common pathways that might be targeted therapeutically and supports the rationale for several drugs currently under trial. Our finding of increased sCD58 levels (associated with suppression of monocyte–lymphocyte interactions 26 ) in the recovered group, strengthens our conclusion that myeloid inflammation is central to the biology of LC and that trials of steroids, IL-1 antagonists, JAK inhibitors, naltrexone and colchicine are justified. Although anticoagulants such as apixaban might prevent thrombosis downstream of complement dysregulation, they can also increase the risk of serious bleeding when given after COVID-19 hospitalization 48 . Thus, clinical trials, already underway, need to carefully assess the risks and benefits of anticoagulants (Supplementary Table 2 ).
Our finding of elevated S- and N-specific IgG in LC could suggest viral persistence, as found in other studies 6 , 42 , 49 . Our network analysis indicated that inflammatory proteins in the cardioresp group interacted strongly with ISM1 and ROBO1, which are expressed during respiratory tract infection and regulate lung inflammation 50 , 51 . Although we were unable to find SARS-CoV-2 antigen in sputum from our LC cases, we did not test for viral persistence in GI tract and lung tissue 40 , 41 or in plasma 52 . Evidence of SARS-CoV-2 persistence would justify trials of antiviral drugs (singly or in combination) in LC. It is also possible that autoimmune processes could result in an innate inflammatory profile in LC. Autoreactive B cells have been identified in LC patients with higher SARS-CoV-2-specific antibody titers in a study of mostly mild acute COVID cases (59% WHO 2–3) 42 , a different population from our study of hospitalized cases.
Our observations of distinct protein profiles in GI and cognitive groups support previous reports on distinct associations between Epstein–Barr virus reactivation and neurological symptoms, or autoantibodies and GI symptoms relative to other forms of LC 49 , 53 . We did not assess autoantibody induction but found evidence of brain–gut axis disturbance (SCG3) in the GI group, which occurs in many autoimmune diseases 54 . We found signatures suggestive of neuroinflammation (C1QA) in the cognitive group, consistent with findings of brain abnormalities on magnetic resonance imaging after COVID-19 hospitalization 55 , as well as findings of microglial activation in mice after COVID-19 (ref. 56 ). Proinflammatory signatures dominated in the cardioresp, fatigue and anxiety/depression groups and were consistent with those seen in non-COVID depression, suggesting shared mechanisms 57 . The association between markers of myeloid inflammation, including IL-3RA, and symptoms was greatest for fatigue. Whilst membrane-bound IL-3RA facilitates IL-3 signaling upstream of myelopoesis 36 its soluble form (measured in plasma) can bind IL-3 and can act as a decoy receptor, preventing monocyte maturation and enhancing immunopathology 58 . Monocytes from individuals with post-COVID fatigue are reported to have abnormal expression profiles (including reduced CXCR2), suggestive of altered maturation and migration 5 , 59 . Lung-specific inflammation was suggested by the association between PCDH1 (an airway epithelial adhesion molecule 35 ) and cardioresp symptoms.
Our observations do not align with all published observations on LC. One proteomic study of 55 LC cases after generally mild (WHO 2–3) acute disease found that TNF and IFN signatures were elevated in LC 3 . Vasculoproliferative processes and metabolic disturbance have been reported in LC 4 , 60 , but these studies used uninfected healthy individuals for comparison and cannot distinguish between LC-specific phenomena and residual post-COVID inflammation. A study of 63 adults (LC, n = 50 and recovered, n = 13) reported no association between immune cell activation and LC 3 months after infection 61 , though myeloid inflammation was not directly measured, and 3 months post infection may be too early to detect subtle differences between LC and recovered cases due to residual acute inflammation.
Our study has limitations. We designed the study to identify inflammatory markers identifying pathways underlying LC subgroups rather than diagnostic biomarkers. The ORs we report are small, but associations were consistent across alternative methods of analysis and when using different LC definitions. Small effect sizes can be expected when using PLR, which shrinks correlated mediator coefficients to reflect combined effects and prevent colinear inflation 62 , and could also result from measurement of plasma mediators that may underestimate tissue inflammation. Although our LC cohort is large compared with most other published studies, some of our subgroups are small (only 60 cases were designated cognitive). Though the performance of the cognitive PLR model was adequate, our findings should be validated in larger studies. It should be noted that our cohort of hospitalized cases may not represent all types of LC, especially those occurring after mild infection. We looked for an effect of acute disease severity within our study and did not find it, and are reassured that the inflammatory profiles we observed were consistent with those seen in smaller studies including nonhospitalized cases 42 , 46 . Studies of posthospital LC may be confounded by ‘posthospital syndrome’, which encompasses general and nonspecific effects of hospitalization (particularly intensive care) 63 .
In conclusion, we found markers of myeloid inflammation and complement activation in our large prospective posthospital cohort of patients with LC, in addition to distinct inflammatory patterns in patients with cognitive impairment or gastrointestinal symptoms. These findings show the need to consider subphenotypes in managing patients with LC and support the use of antiviral or immunomodulatory agents in controlled therapeutic trials.
Study design and ethics
After hospitalization for COVID-19, adults who had no comorbidity resulting in a prognosis of less than 6 months were recruited to the PHOSP-COVID study ( n = 719). Patients hospitalized between February 2020 and January 2021 were recruited. Both sexes were recruited and gender was self-reported (female, n = 257 and male, n = 462). Written informed consent was obtained from all patients. Ethical approvals for the PHOSP-COVID study were given by Leeds West Research Ethics Committee (20/YH/0225).
Symptom data and samples were prospectively collected from individuals approximately 6 months (IQR 5.1–6.8 months and range 3.0–8.3 months) post hospitalization (Fig. 1a ), via the PHOSP-COVID multicenter United Kingdom study 64 . Data relating to patient demographics and acute admission were collected via the International Severe Acute Respiratory and Emerging Infection Consortium World Health Organization Clinical Characterisation Protocol United Kingdom (ISARIC4C study; IRAS260007/IRAS126600) (ref. 65 ). Adults hospitalized during the SARS-CoV-2 pandemic were systematically recruited into ISARIC4C. Written informed consent was obtained from all patients. Ethical approval was given by the South Central–Oxford C Research Ethics Committee in England (reference 13:/SC/0149), Scotland A Research Ethics Committee (20/SS/0028) and WHO Ethics Review Committee (RPC571 and RPC572l, 25 April 2013).
Data were collected to account for variables affecting symptom outcome, via hospital records and self-reporting. Acute disease severity was classified according to the WHO clinical progression score: WHO class 3–4: no oxygen therapy; class 5: oxygen therapy; class 6: noninvasive ventilation or high-flow nasal oxygen; and class 7–9: managed in critical care 9 . Clinical data were used to place patients into six categories: ‘recovered’, ‘GI’, ‘cardiorespiratory’, ‘fatigue’, ‘cognitive impairment’ and ‘anxiety/depression’ (Supplementary Table 7 ). Patient-reported symptoms and validated clinical scores were used when feasible, including Medical Research Council (MRC) breathlessness score, dyspnea-12 score, Functional Assessment of Chronic Illness Therapy (FACIT) score, Patient Health Questionnaire (PHQ)-9 and Generalized Anxiety Disorder (GAD)-7. Cognitive impairment was defined as a Montreal Cognitive Assessment score <26. GI symptoms were defined as answering ‘Yes’ to the presence of at least two of the listed symptoms. ‘Recovered’ was defined by self-reporting. Patients were placed in multiple groups if they experienced a combination of symptoms.
Matched nasal fluid and sputum samples were prospectively collected from a subgroup of convalescent patients approximately 6 months after hospitalization via the PHOSP-COVID study. Nasal and bronchoalveolar lavage fluid (BALF) collected from healthy volunteers before the COVID-19 pandemic were used as controls (Supplementary Table 4 ). Written consent was obtained for all individuals and ethical approvals were given by London–Harrow Research Ethics Committee (13/LO/1899) for the collection of nasal samples and the Health Research Authority London–Fulham Research Ethics Committee (IRAS project ID 154109; references 14/LO/1023, 10/H0711/94 and 11/LO/1826) for BALF samples.
Ethylenediaminetetraacetic acid plasma was collected from whole blood taken by venepuncture and frozen at −80 °C as previously described 7 , 66 . Nasal fluid was collected using a NasosorptionTM FX·I device (Hunt Developments), which uses a synthetic absorptive matrix to collect concentrated nasal fluid. Samples were eluted and stored as previously described 67 . Sputum samples were collected via passive expectoration and frozen at −80 °C without the addition of buffers. Sputum samples from convalescent individuals were compared with BALF from healthy SARS-CoV-2-naive controls, collected before the pandemic. BALF samples were used to act as a comparison for lower respiratory tract samples since passively expectorated sputum from healthy SARS-CoV-2-naive individuals was not available. BALF samples were obtained by instillation and recovery of up to 240 ml of normal saline via a fiberoptic bronchoscope. BALF was filtered through 100 µM strainers into sterile 50 ml Falcon tubes, then centrifuged for 10 min at 400 g at 4 °C. The resulting supernatant was transferred into sterile 50 ml Falcon tubes and frozen at −80 °C until use. The full methods for BALF collection and processing have been described previously 68 , 69 .
Immunoassays
To determine inflammatory signatures that associated with symptom outcomes, plasma samples were analyzed on an Olink Explore 384 Inflammation panel 70 . Supplementary Table 8 (Appendix 1 ) lists all the analytes measured. To ensure the validity of results, samples were run in a single batch with the use of negative controls, plate controls in triplicate and repeated measurement of patient samples between plates in duplicate. Samples were randomized between plates according to site and sample collection date. Randomization between plates was blind to LC/recovered outcome. Data were first normalized to an internal extension control that was included in each sample well. Plates were standardized by normalizing to interplate controls, run in triplicate on each plate. Each plate contained a minimum of four patient samples, which were duplicates on another plate; these duplicate pairs allowed any plate to be linked to any other through the duplicates. Data were then intensity normalized across all cohort samples. Finally, Olink results underwent quality control processing and samples or analytes that did not reach quality control standards were excluded. Final normalized relative protein quantities were reported as log 2 NPX values.
To further validate our findings, we performed conventional electrochemiluminescence (ECL) assays and enzyme-linked immunosorbent assay for Olink mediators that were associated with symptom outcome ( Supplementary Methods ). Contemporaneously collected plasma samples were available from 58 individuals. Like most omics platforms, Olink measures relative quantities, so perfect agreement with conventional assays that measure absolute concentrations is not expected.
Sputum samples were thawed before analysis and sputum plugs were extracted with the addition of 0.1% dithiothreitol creating a one in two sample dilution, as previously described 71 . SARS-CoV-2 S and N proteins were measured by ECL S-plex assay at a fixed dilution of one in two (Mesoscale Diagnostics), as per the manufacturers protocol 72 . Control BALF samples were thawed and measured on the same plate, neat. The S-plex assay is highly sensitive in detecting viral antigen in respiratory tract samples 73 .
Nasal cytokines were measured by ECL (mesoscale discovery) and Luminex bead multiplex assays (Biotechne). The full methods and list of analytes are detailed in Supplementary Methods .
Statistics and reproducibility
Clinical data was collected via the PHOSP REDCap database, to which access is available under reasonable request as per the data sharing statement in the manuscript. All analyses were performed within the Outbreak Data Analysis Platform (ODAP). All data and code can be accessed using information in the ‘Data sharing’ and ‘Code sharing’ statements at the end of the manuscript. No statistical method was used to predetermine sample size. Data distribution was assumed to be normal but this was not formally tested. Olink assays and immunoassays were randomized and investigators were blinded to outcomes.
To determine protein signatures that associated with each symptom outcome, a ridge PLR was used. PLR shrinks coefficients to account for combined effects within high-dimensional data, preventing false discovery while managing multicollinearity 34 . Thus, PLR was chosen a priori as the most appropriate model to assess associations between a large number of explanatory variables (that may work together to mediate effects) and symptom outcome 34 , 62 , 70 , 74 . In keeping with our aim to perform an unbiased exploration of inflammatory process, the model alpha was set to zero, facilitating regularization without complete penalization of any mediator. This enabled review of all possible mediators that might associate with LC 62 .
A 50 repeats tenfold nested cross-validation was used to select the optimal lambda for each model and assess its accuracy (Extended Data Fig. 1 ). The performance of the cognitive impairment model was influenced by the imbalance in size of the symptom group ( n = 60) relative to recovered ( n = 250). The model was weighted to account for this imbalance resulting in a sensitivity of 0.98, indicating its validity. We have expanded on the model performance and validation approaches in Supplementary Information .
Age, sex, acute disease severity and preexisting comorbidities were included as covariates in the PLR analysis (Supplementary Tables 1 and 3 ). Covariates were selected a priori using features reported to influence the risk of LC and inflammatory responses 1 , 39 , 64 , 75 . Ethnicity was not included since it has been shown not to predict symptom outcome in this cohort 64 . Individuals with missing data were excluded from the regression analysis. Each symptom group was compared with the ‘recovered’ group. The model coefficients of each covariate were converted into ORs for each outcome and visualized in a forest plot, after removing variables associated with regularized OR between 0.98 and 1.02 or in cases where most variables fell outside of this range, using mediators associated with the highest decile of coefficients either side of this range. This enabled exclusion of mediators with effect sizes that were unlikely to have clinical or mechanistic importance since the ridge PLR shrinks and orders coefficients according to their relative importance rather than making estimates with standard error. Thus, confidence intervals cannot be appropriately derived from PLR, and forest plot error bars were calculated using the median accuracy of the model generated by the nested cross-validation. To verify observations made through PLR analysis, we also performed an unadjusted PLR, an unadjusted logistic regression and a PLS analysis. Univariate analyses using Wilcoxon signed-rank test was also performed (Supplementary Table 8 , Appendix 1 ). Analyses were performed in R version 4.2.0 using ‘data.table v1.14.2’, ‘EnvStats v2.7.0’ ‘tidyverse v1.3.2’, ‘lme4 v1.1-32’, ‘caret v6.0-93’, ‘glmnet v4.1-6’, ‘mdatools v0.14.0’, ‘ggpubbr v0.4.0’ and ‘ggplot2 v3.3.6’ packages.
To further investigate the relationship between proteins elevated in each symptom group, we performed a correlation network analysis using Spearman’s rank correlation coefficient and false discovery rate (FDR) thresholding. The mediators visualized in the PLR forest plots, which were associated with cardiorespiratory symptoms, fatigue, anxiety/depression GI symptoms and cognitive impairment were used, respectively. Analyses were performed in R version 4.2.0 using ‘bootnet v1.5.6 ’ and ‘qgraph v1.9.8 ’ packages.
To determine whether differences in protein levels between men and women related to hormonal differences, we divided each symptom group into premenopausal and postmenopausal groups using an age cutoff of 50 years old. Differences between sexes in each group were determined using the Wilcoxon signed-rank test. To understand whether antigen persistence contributed to inflammation in adults with LC, the median viral antigen concentration from sputum/BALF samples and cytokine concentrations from nasal samples were compared using the Wilcoxon signed-rank test. All tests were two-tailed and statistical significance was defined as a P value < 0.05 after adjustment for FDR ( q -value of 0.05). Analyses were performed in R version 4.2.0 using ‘bootnet v1.5.6’ and ‘qgraph v1.9.8’ packages.
Extended Data Fig. 10 was made using Biorender, accessed at www.biorender.com .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
This is an open access article under the CC BY 4.0 license.
The PHOSP-COVID protocol, consent form, definition and derivation of clinical characteristics and outcomes, training materials, regulatory documents, information about requests for data access, and other relevant study materials are available online at ref. 76 . Access to these materials can be granted by contacting [email protected] and [email protected].
The ISARIC4C protocol, data sharing and publication policy are available at https://isaric4c.net . ISARIC4C’s Independent Data and Material Access Committee welcomes applications for access to data and materials ( https://isaric4c.net ).
The datasets used in the study contain extensive clinical information at an individual level that prevent them from being deposited in an public depository due to data protection policies of the study. Study data can only be accessed via the ODAP, a protected research environment. All data used in this study are available within ODAP and accessible under reasonable request. Data access criteria and information about how to request access is available online at ref. 76 . If criteria are met and a request is made, access can be gained by signing the eDRIS user agreement.
Code availability
Code was written within the ODAP, using R v4.2.0 and publicly available packages (‘data.table v1.14.2’, ‘EnvStats v2.7.0’, ‘tidyverse v1.3.2’, ‘lme4 v1.1-32’, ‘caret v6.0-93’, ‘glmnet v4.1-6’, ‘mdatools v0.14.0’, ‘ggpubbr v0.4.0’, ‘ggplot2 v3.3.6’, ‘bootnet v1.5.6’ and ‘qgraph v1.9.8’ packages). No new algorithms or functions were created and code used in-built functions in listed packages available on CRAN. The code used to generate data and to analyze data is publicly available at https://github.com/isaric4c/wiki/wiki/ISARIC ; https://github.com/SurgicalInformatics/cocin_cc and https://github.com/ClaudiaEfstath/PHOSP_Olink_NatImm .
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21 , 133–146 (2023).
CAS PubMed PubMed Central Google Scholar
Antonelli, M., Pujol, J. C., Spector, T. D., Ourselin, S. & Steves, C. J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 399 , 2263–2264 (2022).
Talla, A. et al. Persistent serum protein signatures define an inflammatory subcategory of long COVID. Nat. Commun. 14 , 3417 (2023).
Captur, G. et al. Plasma proteomic signature predicts who will get persistent symptoms following SARS-CoV-2 infection. EBioMedicine 85 , 104293 (2022).
Scott, N. A. et al. Monocyte migration profiles define disease severity in acute COVID-19 and unique features of long COVID. Eur. Respir. J. https://doi.org/10.1183/13993003.02226-2022 (2023).
Klein, J. et al. Distinguishing features of Long COVID identified through immune profiling. Nature https://doi.org/10.1038/s41586-023-06651-y (2023).
Article PubMed PubMed Central Google Scholar
Evans, R. A. et al. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir. Med . 10 , 761–775 (2022).
CAS Google Scholar
Houchen-Wolloff, L. et al. Joint patient and clinician priority setting to identify 10 key research questions regarding the long-term sequelae of COVID-19. Thorax 77 , 717–720 (2022).
PubMed Google Scholar
Marshall, J. C. et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 20 , e192–e197 (2020).
Post COVID-19 condition (long COVID). World Health Organization https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition#:~:text=Definition,months%20with%20no%20other%20explanation (2022).
Peters, V. A., Joesting, J. J. & Freund, G. G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 32 , 1–8 (2013).
CAS PubMed Google Scholar
Luo, Z. et al. Monocytes augment inflammatory responses in human aortic valve interstitial cells via β2-integrin/ICAM-1-mediated signaling. Inflamm. Res. 71 , 681–694 (2022).
Bendall, L. J. & Bradstock, K. F. G-CSF: from granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 25 , 355–367 (2014).
Ma, Y. J. et al. Soluble collectin-12 (CL-12) is a pattern recognition molecule initiating complement activation via the alternative pathway. J. Immunol. 195 , 3365–3373 (2015).
Laursen, N. S. et al. Functional and structural characterization of a potent C1q inhibitor targeting the classical pathway of the complement system. Front. Immunol. 11 , 1504 (2020).
Dejanovic, B. et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2 , 837–850 (2022).
Xue, G., Hua, L., Zhou, N. & Li, J. Characteristics of immune cell infiltration and associated diagnostic biomarkers in ulcerative colitis: results from bioinformatics analysis. Bioengineered 12 , 252–265 (2021).
He, T. et al. Integrative computational approach identifies immune‐relevant biomarkers in ulcerative colitis. FEBS Open Bio. 12 , 500–515 (2022).
Sundin, J. et al. Fecal chromogranins and secretogranins are linked to the fecal and mucosal intestinal bacterial composition of IBS patients and healthy subjects. Sci. Rep. 8 , 16821 (2018).
PubMed PubMed Central Google Scholar
Kriebel, M., Wuchter, J., Trinks, S. & Volkmer, H. Neurofascin: a switch between neuronal plasticity and stability. Int. J. Biochem. Cell Biol. 44 , 694–697 (2012).
Woo, W.-M. et al. The C. elegans F-spondin family protein SPON-1 maintains cell adhesion in neural and non-neural tissues. Development 135 , 2747–2756 (2008).
Yadati, T., Houben, T., Bitorina, A. & Shiri-Sverdlov, R. The ins and outs of cathepsins: physiological function and role in disease management. Cells 9 , 1679 (2020).
Taquet, M. et al. Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID-19 hospitalization. Nat. Med. https://doi.org/10.1038/s41591-023-02525-y (2023).
Siggins, M. K. et al. Alternative pathway dysregulation in tissues drives sustained complement activation and predicts outcome across the disease course in COVID‐19. Immunology 168 , 473–492 (2023).
Pol, J. G., Caudana, P., Paillet, J., Piaggio, E. & Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 217 , e20191247 (2020).
Zhang, Y., Liu, Q., Yang, S. & Liao, Q. CD58 immunobiology at a glance. Front. Immunol. 12 , 705260 (2021).
Demydchuk, M. et al. Insights into Hunter syndrome from the structure of iduronate-2-sulfatase. Nat. Commun. 8 , 15786 (2017).
Wang, Z. et al. DNER promotes epithelial–mesenchymal transition and prevents chemosensitivity through the Wnt/β-catenin pathway in breast cancer. Cell Death Dis. 11 , 642 (2020).
Bonilla, H. et al. Therapeutic trials for long COVID-19: a call to action from the interventions taskforce of the RECOVER initiative. Front. Immunol. 14 , 1129459 (2023).
Wik, L. et al. Proximity extension assay in combination with next-generation sequencing for high-throughput proteome-wide analysis. Mol. Cell. Proteomics 20 , 100168 (2021).
Measuring protein biomarkers with Olink—technical comparisons and orthogonal validation. Olink Proteomics https://www.olink.com/content/uploads/2021/09/olink-technical-comparisons-and-orthogonal-validation-1118-v2.0.pdf (2021).
COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN) and Royal College of General Practitioners (RCGP) https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 (2022).
Long COVID or post-COVID conditions. Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html#:~:text=Long%20COVID%20is%20broadly%20defined,after%20acute%20COVID%2D19%20infection (2023).
Firinguetti, L., Kibria, G. & Araya, R. Study of partial least squares and ridge regression methods. Commun. Stat. Simul. Comput 46 , 6631–6644 (2017).
Google Scholar
Mortensen, L. J., Kreiner-Moller, E., Hakonarson, H., Bonnelykke, K. & Bisgaard, H. The PCDH1 gene and asthma in early childhood. Eur. Respir. J. 43 , 792–800 (2014).
Tong, Y. et al. The RNFT2/IL-3Rα axis regulates IL-3 signaling and innate immunity. JCI Insight 5 , e133652 (2020).
Wu, Y. et al. Effect of ISM1 on the immune microenvironment and epithelial-mesenchymal transition in colorectal cancer. Front. Cell Dev. Biol. 9 , 681240 (2021).
Luo, G. G. & Ou, J. J. Oncogenic viruses and cancer. Virol. Sin. 30 , 83–84 (2015).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16 , 626–638 (2016).
Gaebler, C. et al. Evolution of antibody immunity to SARS-CoV-2. Nature 591 , 639–644 (2021).
Bussani, R. et al. Persistent SARS‐CoV‐2 infection in patients seemingly recovered from COVID‐19. J. Pathol. 259 , 254–263 (2023).
Woodruff, M. C. et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat. Commun. 14 , 4201 (2023).
Lammi, V. et al. Genome-wide association study of long COVID. Preprint at medRxiv https://doi.org/10.1101/2023.06.29.23292056 (2023).
Ismailova, A. et al. Identification of a forkhead box protein transcriptional network induced in human neutrophils in response to inflammatory stimuli. Front. Immunol. 14 , 1123344 (2023).
Beurskens, F. J., van Schaarenburg, R. A. & Trouw, L. A. C1q, antibodies and anti-C1q autoantibodies. Mol. Immunol. 68 , 6–13 (2015).
Cervia-Hasler, C. et al. Persistent complement dysregulation with signs of thromboinflammation in active long Covid. Science 383 , eadg7942 (2024).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14 , 857–877 (2015).
Toshner, M. R. et al. Apixaban following discharge in hospitalised adults with COVID-19: preliminary results from a multicentre, open-label, randomised controlled platform clinical trial. Preprint at medRxiv , https://doi.org/10.1101/2022.12.07.22283175 (2022).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185 , 881–895.e20 (2022).
Branchfield, K. et al. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351 , 707–710 (2016).
Rivera-Torruco, G. et al. Isthmin 1 identifies a subset of lung hematopoietic stem cells and it is associated with systemic inflammation. J. Immunol. 202 , 118.18 (2019).
Swank, Z. et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 76 , e487–e490 (2023).
Peluso, M. J. et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J. Clin. Invest. 133 , e163669 (2023).
Bellocchi, C. et al. The interplay between autonomic nervous system and inflammation across systemic autoimmune diseases. Int. J. Mol. Sci. 23 , 2449 (2022).
Raman, B. et al. Multiorgan MRI findings after hospitalisation with COVID-19 in the UK (C-MORE): a prospective, multicentre, observational cohort study. Lancet Respir. Med 11 , 1003–1019 (2023).
Fernández-Castañeda, A. et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185 , 2452–2468.e16 (2022).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9 , 46–56 (2008).
Broughton, S. E. et al. Dual mechanism of interleukin-3 receptor blockade by an anti-cancer antibody. Cell Rep. 8 , 410–419 (2014).
Ley, K., Miller, Y. I. & Hedrick, C. C. Monocyte and macrophage dynamics during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 31 , 1506–1516 (2011).
Iosef, C. et al. Plasma proteome of long-COVID patients indicates HIF-mediated vasculo-proliferative disease with impact on brain and heart function. J. Transl. Med. 21 , 377 (2023).
Santopaolo, M. et al. Prolonged T-cell activation and long COVID symptoms independently associate with severe COVID-19 at 3 months. eLife 12 , e85009 (2023).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33 , 1–22 (2010).
Voiriot, G. et al. Chronic critical illness and post-intensive care syndrome: from pathophysiology to clinical challenges. Ann. Intensive Care 12 , 58 (2022).
Evans, R. A. et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir. Med 9 , 1275–1287 (2021).
Docherty, A. B. et al. Features of 20,133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ https://doi.org/10.1136/bmj.m1985 (2020).
Elneima, O. et al. Cohort profile: post-hospitalisation COVID-19 study (PHOSP-COVID). Preprint at medRxiv https://doi.org/10.1101/2023.05.08.23289442 (2023).
Liew, F. et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. EBioMedicine 87 , 104402 (2023).
Ascough, S. et al. Divergent age-related humoral correlates of protection against respiratory syncytial virus infection in older and young adults: a pilot, controlled, human infection challenge model. Lancet Healthy Longev. 3 , e405–e416 (2022).
Guvenel, A. et al. Epitope-specific airway-resident CD4+ T cell dynamics during experimental human RSV infection. J. Clin. Invest. 130 , 523–538 (2019).
PubMed Central Google Scholar
Greenwood, C. J. et al. A comparison of penalised regression methods for informing the selection of predictive markers. PLoS ONE 15 , e0242730 (2020).
Higham, A. et al. Leukotriene B4 levels in sputum from asthma patients. ERJ Open Res. 2 , 00088–02015 (2016).
SARS-CoV-2 spike kit. MSD https://www.mesoscale.com/~/media/files/product%20inserts/s-plex%20sars-cov-2%20spike%20kit%20product%20insert.pdf (2023).
Ren, A. et al. Ultrasensitive assay for saliva-based SARS-CoV-2 antigen detection. Clin. Chem. Lab. Med. 60 , 771–777 (2022).
Breheny, P. & Huang, J. Penalized methods for bi-level variable selection. Stat. Interface 2 , 369–380 (2009).
Thwaites, R. S. et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci. Immunol. 6 , eabg9873 (2021).
Resources. PHOSP-COVID https://phosp.org/resource/ (2022).
Download references
Acknowledgements
This research used data assets made available by ODAP as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (grant ref. MC_PC_20058). This work is supported by the following grants: the PHOSP-COVD study is jointly funded by UK Research and Innovation and National Institute of Health and Care Research (NIHR; grant references MR/V027859/1 and COV0319). ISARIC4C is supported by grants from the National Institute for Health and Care Research (award CO-CIN-01) and the MRC (grant MC_PC_19059) Liverpool Experimental Cancer Medicine Centre provided infrastructure support for this research (grant reference C18616/A25153). Other grants that have supported this work include the UK Coronavirus Immunology Consortium (funder reference 1257927), the Imperial Biomedical Research Centre (NIHR Imperial BRC, grant IS-BRC-1215-20013), the Health Protection Research Unit in Respiratory Infections at Imperial College London and NIHR Health Protection Research Unit in Emerging and Zoonotic Infections at University of Liverpool, both in partnership with Public Health England, (NIHR award 200907), Wellcome Trust and Department for International Development (215091/Z/18/Z), Health Data Research UK (grant code 2021.0155), MRC (grant code MC_UU_12014/12) and NIHR Clinical Research Network for providing infrastructure support for this research. We also acknowledge the support of the MRC EMINENT Network (MR/R502121/1), which is cofunded by GSK, the Comprehensive Local Research Networks, the MRC HIC-Vac network (MR/R005982/1) and the RSV Consortium in Europe Horizon 2020 Framework Grant 116019. F.L. is supported by an MRC clinical training fellowship (award MR/W000970/1). C.E. is funded by NIHR (grant P91258-4). L.-P.H. is supported by Oxford NIHR Biomedical Research Centre. A.A.R.T. is supported by a British Heart Foundation (BHF) Intermediate Clinical Fellowship (FS/18/13/33281). S.L.R.-J. receives support from UK Research and Innovation (UKRI), Global Challenges Research Fund (GCRF), Rosetrees Trust, British HIV association (BHIVA), European & Developing Countries Clinical Trials Partnership (EDCTP) and Globvac. J.D.C. has grants from AstraZeneca, Boehringer Ingelheim, GSK, Gilead Sciences, Grifols, Novartis and Insmed. R.A.E. holds a NIHR Clinician Scientist Fellowship (CS-2016-16-020). A. Horsley is currently supported by UK Research and Innovation, NIHR and NIHR Manchester BRC. B.R. receives support from BHF Oxford Centre of Research Excellence, NIHR Oxford BRC and MRC. D.G.W. is supported by an NIHR Advanced Fellowship. A. Ho has received support from MRC and for the Coronavirus Immunology Consortium (MR/V028448/1). L.T. is supported by the US Food and Drug Administration Medical Countermeasures Initiative contract 75F40120C00085 and the National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections (NIHR200907) at the University of Liverpool in partnership with UK Health Security Agency (UK-HSA), in collaboration with Liverpool School of Tropical Medicine and the University of Oxford. L.V.W. has received support from UKRI, GSK/Asthma and Lung UK and NIHR for this study. M.G.S. has received support from NIHR UK, MRC UK and Health Protection Research Unit in Emerging and Zoonotic Infections, University of Liverpool. J.K.B. is supported by the Wellcome Trust (223164/Z/21/Z) and UKRI (MC_PC_20004, MC_PC_19025, MC_PC_1905, MRNO2995X/1 and MC_PC_20029). The funders were not involved in the study design, interpretation of data or writing of this manuscript. The views expressed are those of the authors and not necessarily those of the Department of Health and Social Care (DHSC), the Department for International Development (DID), NIHR, MRC, the Wellcome Trust, UK-HSA, the National Health Service or the Department of Health. P.J.M.O. is supported by a NIHR Senior Investigator Award (award 201385). We thank all the participants and their families. We thank the many research administrators, health-care and social-care professionals who contributed to setting up and delivering the PHOSP-COVID study at all of the 65 NHS trusts/health boards and 25 research institutions across the United Kingdom, as well as those who contributed to setting up and delivering the ISARIC4C study at 305 NHS trusts/health boards. We also thank all the supporting staff at the NIHR Clinical Research Network, Health Research Authority, Research Ethics Committee, Department of Health and Social Care, Public Health Scotland and Public Health England. We thank K. Holmes at the NIHR Office for Clinical Research Infrastructure for her support in coordinating the charities group. The PHOSP-COVID industry framework was formed to provide advice and support in commercial discussions, and we thank the Association of the British Pharmaceutical Industry as well the NIHR Office for Clinical Research Infrastructure for coordinating this. We are very grateful to all the charities that have provided insight to the study: Action Pulmonary Fibrosis, Alzheimer’s Research UK, Asthma and Lung UK, British Heart Foundation, Diabetes UK, Cystic Fibrosis Trust, Kidney Research UK, MQ Mental Health, Muscular Dystrophy UK, Stroke Association Blood Cancer UK, McPin Foundations and Versus Arthritis. We thank the NIHR Leicester Biomedical Research Centre patient and public involvement group and Long Covid Support. We also thank G. Khandaker and D. C. Newcomb who provided valuable feedback on this work. Extended Data Fig. 10 was created using Biorender.
Author information
These authors contributed equally: Felicity Liew, Claudia Efstathiou, Ryan S. Thwaites, Peter J. M. Openshaw.
Authors and Affiliations
National Heart and Lung Institute, Imperial College London, London, UK
Felicity Liew, Claudia Efstathiou, Sara Fontanella, Dawid Swieboda, Jasmin K. Sidhu, Stephanie Ascough, Onn Min Kon, Luke S. Howard, Jennifer K. Quint, Christopher Chiu, Ryan S. Thwaites, Peter J. M. Openshaw, Jake Dunning & Peter J. M. Openshaw
Institute for Lung Health, Leicester NIHR Biomedical Research Centre, University of Leicester, Leicester, UK
Matthew Richardson, Ruth Saunders, Olivia C. Leavy, Omer Elneima, Hamish J. C. McAuley, Amisha Singapuri, Marco Sereno, Victoria C. Harris, Neil J. Greening, Rachael A. Evans, Louise V. Wain, Christopher Brightling & Ananga Singapuri
NIHR Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, UK
Shona C. Moore, Daniel G. Wootton, Malcolm G. Semple, Lance Turtle, William A. Paxton & Georgios Pollakis
The Imperial Clinical Respiratory Research Unit, Imperial College NHS Trust, London, UK
Noura Mohamed
Cardiovascular Research Team, Imperial College Healthcare NHS Trust, London, UK
Jose Nunag & Clara King
Department of Population Health Sciences, University of Leicester, Leicester, UK
Olivia C. Leavy, Louise V. Wain & Beatriz Guillen-Guio
NIHR Leicester Biomedical Research Centre, University of Leicester, Leicester, UK
Aarti Shikotra
Centre for Exercise and Rehabilitation Science, NIHR Leicester Biomedical Research Centre-Respiratory, University of Leicester, Leicester, UK
Linzy Houchen-Wolloff
Usher Institute, University of Edinburgh, Edinburgh, UK
Nazir I. Lone, Luke Daines, Annemarie B. Docherty, Nazir I. Lone, Matthew Thorpe, Annemarie B. Docherty, Thomas M. Drake, Cameron J. Fairfield, Ewen M. Harrison, Stephen R. Knight, Kenneth A. Mclean, Derek Murphy, Lisa Norman, Riinu Pius & Catherine A. Shaw
Centre for Medical Informatics, The Usher Institute, University of Edinburgh, Edinburgh, UK
Matthew Thorpe, Annemarie B. Docherty, Ewen M. Harrison, J. Kenneth Baillie, Sarah L. Rowland-Jones, A. A. Roger Thompson & Thushan de Silva
Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, UK
A. A. Roger Thompson, Sarah L. Rowland-Jones, Thushan I. de Silva & James D. Chalmers
University of Dundee, Ninewells Hospital and Medical School, Dundee, UK
James D. Chalmers & Ling-Pei Ho
MRC Human Immunology Unit, University of Oxford, Oxford, UK
Ling-Pei Ho & Alexander Horsley
Division of Infection, Immunity and Respiratory Medicine, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK
Alexander Horsley & Betty Raman
Radcliffe Department of Medicine, University of Oxford, Oxford, UK
Betty Raman & Krisnah Poinasamy
Asthma + Lung UK, London, UK
Krisnah Poinasamy & Michael Marks
Department of Clinical Research, London School of Hygiene and Tropical Medicine, London, UK
Michael Marks
Hospital for Tropical Diseases, University College London Hospital, London, UK
Division of Infection and Immunity, University College London, London, UK
Michael Marks & Mahdad Noursadeghi
MRC Centre for Virus Research, School of Infection and Immunity, University of Glasgow, Glasgow, UK
Antonia Ho & William Greenhalf
Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK
William Greenhalf & J. Kenneth Baillie
The Roslin Institute, University of Edinburgh, Edinburgh, UK
J. Kenneth Baillie, J. Kenneth Baillie, Sara Clohisey, Fiona Griffiths, Ross Hendry, Andrew Law & Wilna Oosthuyzen
Pandemic Science Hub, University of Edinburgh, Edinburgh, UK
J. Kenneth Baillie
The Pandemic Institute, University of Liverpool, Liverpool, UK
Malcolm G. Semple & Lance Turtle
University of Manchester, Manchester, UK
Kathryn Abel, Perdita Barran, H. Chinoy, Bill Deakin, M. Harvie, C. A. Miller, Stefan Stanel & Drupad Trivedi
Intensive Care Unit, Royal Infirmary of Edinburgh, Edinburgh, UK
Kathryn Abel & J. Kenneth Baillie
North Bristol NHS Trust and University of Bristol, Bristol, UK
H. Adamali, David Arnold, Shaney Barratt, A. Dipper, Sarah Dunn, Nick Maskell, Anna Morley, Leigh Morrison, Louise Stadon, Samuel Waterson & H. Welch
University of Edinburgh, Manchester, UK
Davies Adeloye, D. E. Newby, Riinu Pius, Igor Rudan, Manu Shankar-Hari, Catherine Sudlow, Sarah Walmsley & Bang Zheng
King’s College Hospital NHS Foundation Trust and King’s College London, London, UK
Oluwaseun Adeyemi, Rita Adrego, Hosanna Assefa-Kebede, Jonathon Breeze, S. Byrne, Pearl Dulawan, Amy Hoare, Caroline Jolley, Abigail Knighton, M. Malim, Sheetal Patale, Ida Peralta, Natassia Powell, Albert Ramos, K. Shevket, Fabio Speranza & Amelie Te
Guy’s and St Thomas’ NHS Foundation Trust, London, UK
Laura Aguilar Jimenez, Gill Arbane, Sarah Betts, Karen Bisnauthsing, A. Dewar, Nicholas Hart, G. Kaltsakas, Helen Kerslake, Murphy Magtoto, Philip Marino, L. M. Martinez, Marlies Ostermann, Jennifer Rossdale & Teresa Solano
Royal Free London NHS Foundation Trust, London, UK
Shanaz Ahmad, Simon Brill, John Hurst, Hannah Jarvis, C. Laing, Lai Lim, S. Mandal, Darwin Matila, Olaoluwa Olaosebikan & Claire Singh
University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK
N. Ahmad Haider, Catherine Atkin, Rhiannon Baggott, Michelle Bates, A. Botkai, Anna Casey, B. Cooper, Joanne Dasgin, Camilla Dawson, Katharine Draxlbauer, N. Gautam, J. Hazeldine, T. Hiwot, Sophie Holden, Karen Isaacs, T. Jackson, Vicky Kamwa, D. Lewis, Janet Lord, S. Madathil, C. McGee, K. Mcgee, Aoife Neal, Alex Newton-Cox, Joseph Nyaboko, Dhruv Parekh, Z. Peterkin, H. Qureshi, Liz Ratcliffe, Elizabeth Sapey, J. Short, Tracy Soulsby, J. Stockley, Zehra Suleiman, Tamika Thompson, Maximina Ventura, Sinead Walder, Carly Welch, Daisy Wilson, S. Yasmin & Kay Por Yip
Stroke Association, London, UK
Rubina Ahmed & Richard Francis
University College London Hospital and University College London, London, UK
Nyarko Ahwireng, Dongchun Bang, Donna Basire, Jeremy Brown, Rachel Chambers, A. Checkley, R. Evans, M. Heightman, T. Hillman, Joseph Jacob, Roman Jastrub, M. Lipman, S. Logan, D. Lomas, Marta Merida Morillas, Hannah Plant, Joanna Porter, K. Roy & E. Wall
Oxford University Hospitals NHS Foundation Trust and University of Oxford, Oxford, UK
Mark Ainsworth, Asma Alamoudi, Angela Bloss, Penny Carter, M. Cassar, Jin Chen, Florence Conneh, T. Dong, Ranuromanana Evans, V. Ferreira, Emily Fraser, John Geddes, F. Gleeson, Paul Harrison, May Havinden-Williams, P. Jezzard, Ivan Koychev, Prathiba Kurupati, H. McShane, Clare Megson, Stefan Neubauer, Debby Nicoll, C. Nikolaidou, G. Ogg, Edmund Pacpaco, M. Pavlides, Yanchun Peng, Nayia Petousi, John Pimm, Najib Rahman, M. J. Rowland, Kathryn Saunders, Michael Sharpe, Nick Talbot, E. M. Tunnicliffe & C. Xie
St George’s University Hospitals NHS Foundation Trust, London, UK
Mariam Ali, Raminder Aul, A. Dunleavy, D. Forton, Mark Mencias, N. Msimanga, T. Samakomva, Sulman Siddique, Vera Tavoukjian & J. Teixeira
University Hospitals of Leicester NHS Trust and University of Leicester, Leicester, UK
M. Aljaroof, Natalie Armstrong, H. Arnold, Hnin Aung, Majda Bakali, M. Bakau, E. Baldry, Molly Baldwin, Charlotte Bourne, Michelle Bourne, Nigel Brunskill, P. Cairns, Liesel Carr, Amanda Charalambou, C. Christie, Melanie Davies, Enya Daynes, Sarah Diver, Rachael Dowling, Sarah Edwards, C. Edwardson, H. Evans, J. Finch, Sarah Glover, Nicola Goodman, Bibek Gooptu, Kate Hadley, Pranab Haldar, Beverley Hargadon, W. Ibrahim, L. Ingram, Kamlesh Khunti, A. Lea, D. Lee, Gerry McCann, P. McCourt, Teresa Mcnally, George Mills, Will Monteiro, Manish Pareek, S. Parker, Anne Prickett, I. N. Qureshi, A. Rowland, Richard Russell, Salman Siddiqui, Sally Singh, J. Skeemer, M. Soares, E. Stringer, T. Thornton, Martin Tobin, T. J. C. Ward, F. Woodhead, Tom Yates & A. J. Yousuf
University of Exeter, Exeter, UK
Louise Allan, Clive Ballard & Andrew McGovern
University of Leicester, Leicester, UK
Richard Allen, Michelle Bingham, Terry Brugha, Selina Finney, Rob Free, Don Jones, Claire Lawson, Daniel Lozano-Rojas, Gardiner Lucy, Alistair Moss, Elizabeta Mukaetova-Ladinska, Petr Novotny, Kimon Ntotsis, Charlotte Overton, John Pearl, Tatiana Plekhanova, M. Richardson, Nilesh Samani, Jack Sargant, Ruth Saunders, M. Sharma, Mike Steiner, Chris Taylor, Sarah Terry, C. Tong, E. Turner, J. Wormleighton & Bang Zhao
Liverpool University Hospitals NHS Foundation Trust and University of Liverpool, Liverpool, UK
Lisa Allerton, Ann Marie Allt, M. Beadsworth, Anthony Berridge, Jo Brown, Shirley Cooper, Andy Cross, Sylviane Defres, S. L. Dobson, Joanne Earley, N. French, Kera Hainey, Hayley Hardwick, Jenny Hawkes, Victoria Highett, Sabina Kaprowska, Angela Key, Lara Lavelle-Langham, N. Lewis-Burke, Gladys Madzamba, Flora Malein, Sophie Marsh, Chloe Mears, Lucy Melling, Matthew Noonan, L. Poll, James Pratt, Emma Richardson, Anna Rowe, Victoria Shaw, K. A. Tripp, Lilian Wajero, S. A. Williams-Howard, Dan Wootton & J. Wyles
Sherwood Forest Hospitals NHS Foundation Trust, Nottingham, UK
Lynne Allsop, Kaytie Bennett, Phil Buckley, Margaret Flynn, Mandy Gill, Camelia Goodwin, M. Greatorex, Heidi Gregory, Cheryl Heeley, Leah Holloway, Megan Holmes, John Hutchinson, Jill Kirk, Wayne Lovegrove, Terri Ann Sewell, Sarah Shelton, D. Sissons, Katie Slack, Susan Smith, D. Sowter, Sarah Turner, V. Whitworth & Inez Wynter
Nottingham University Hospitals NHS Trust and University of Nottingham, London, UK
Paula Almeida, Akram Hosseini, Robert Needham & Karen Shaw
Manchester University NHS Foundation Trust and University of Manchester, London, UK
Bashar Al-Sheklly, Cristina Avram, John Blaikely, M. Buch, N. Choudhury, David Faluyi, T. Felton, T. Gorsuch, Neil Hanley, Tracy Hussell, Zunaira Kausar, Natasha Odell, Rebecca Osbourne, Karen Piper Hanley, K. Radhakrishnan & Sue Stockdale
Imperial College London, London, UK
Danny Altmann, Anew Frankel, Luke S. Howard, Desmond Johnston, Liz Lightstone, Anne Lingford-Hughes, William Man, Steve McAdoo, Jane Mitchell, Philip Molyneaux, Christos Nicolaou, D. P. O’Regan, L. Price, Jennifer K. Quint, David Smith, Jonathon Valabhji, Simon Walsh, Martin Wilkins & Michelle Willicombe
Hampshire Hospitals NHS Foundation Trust, Basingstoke, UK
Maria Alvarez Corral, Ava Maria Arias, Emily Bevan, Denise Griffin, Jane Martin, J. Owen, Sheila Payne, A. Prabhu, Annabel Reed, Will Storrar, Nick Williams & Caroline Wrey Brown
British Heart Foundation, Birmingham, UK
Shannon Amoils
NHS Greater Glasgow and Clyde Health Board and University of Glasgow, Glasgow, UK
David Anderson, Neil Basu, Hannah Bayes, Colin Berry, Ammani Brown, Andrew Dougherty, K. Fallon, L. Gilmour, D. Grieve, K. Mangion, I. B. McInnes, A. Morrow, Kathryn Scott & R. Sykes
University of Oxford, Oxford, UK
Charalambos Antoniades, A. Bates, M. Beggs, Kamaldeep Bhui, Katie Breeze, K. M. Channon, David Clark, X. Fu, Masud Husain, Lucy Kingham, Paul Klenerman, Hanan Lamlum, X. Li, E. Lukaschuk, Celeste McCracken, K. McGlynn, R. Menke, K. Motohashi, T. E. Nichols, Godwin Ogbole, S. Piechnik, I. Propescu, J. Propescu, A. A. Samat, Z. B. Sanders, Louise Sigfrid & M. Webster
Belfast Health and Social Care Trust and Queen’s University Belfast, Belfast, UK
Cherie Armour, Vanessa Brown, John Busby, Bronwen Connolly, Thelma Craig, Stephen Drain, Liam Heaney, Bernie King, Nick Magee, E. Major, Danny McAulay, Lorcan McGarvey, Jade McGinness, Tunde Peto & Roisin Stone
Airedale NHS Foundation Trust, Keighley, UK
Lisa Armstrong, Brigid Hairsine, Helen Henson, Claire Kurasz, Alison Shaw & Liz Shenton
Wrightington Wigan and Leigh NHS Trust, Wigan, UK
A. Ashish, Josh Cooper & Emma Robinson
Leeds Teaching Hospitals and University of Leeds, Leeds, UK
Andrew Ashworth, Paul Beirne, Jude Clarke, C. Coupland, Matthhew Dalton, Clair Favager, Jodie Glossop, John Greenwood, Lucy Hall, Tim Hardy, Amy Humphries, Jennifer Murira, Dan Peckham, S. Plein, Jade Rangeley, Gwen Saalmink, Ai Lyn Tan, Elaine Wade, Beverley Whittam, Nicola Window & Janet Woods
University of Liverpool, Liverpool, UK
M. Ashworth, D. Cuthbertson, G. Kemp, Anne McArdle, Benedict Michael, Will Reynolds, Lisa Spencer, Ben Vinson, Katie A. Ahmed, Jane A. Armstrong, Milton Ashworth, Innocent G. Asiimwe, Siddharth Bakshi, Samantha L. Barlow, Laura Booth, Benjamin Brennan, Katie Bullock, Nicola Carlucci, Emily Cass, Benjamin W. A. Catterall, Jordan J. Clark, Emily A. Clarke, Sarah Cole, Louise Cooper, Helen Cox, Christopher Davis, Oslem Dincarslan, Alejandra Doce Carracedo, Chris Dunn, Philip Dyer, Angela Elliott, Anthony Evans, Lorna Finch, Lewis W. S. Fisher, Lisa Flaherty, Terry Foster, Isabel Garcia-Dorival, Philip Gunning, Catherine Hartley, Karl Holden, Anthony Holmes, Rebecca L. Jensen, Christopher B. Jones, Trevor R. Jones, Shadia Khandaker, Katharine King, Robyn T. Kiy, Chrysa Koukorava, Annette Lake, Suzannah Lant, Diane Latawiec, Lara Lavelle-Langham, Daniella Lefteri, Lauren Lett, Lucia A. Livoti, Maria Mancini, Hannah Massey, Nicole Maziere, Sarah McDonald, Laurence McEvoy, John McLauchlan, Soeren Metelmann, Nahida S. Miah, Joanna Middleton, Joyce Mitchell, Ellen G. Murphy, Rebekah Penrice-Randal, Jack Pilgrim, Tessa Prince, P. Matthew Ridley, Debby Sales, Rebecca K. Shears, Benjamin Small, Krishanthi S. Subramaniam, Agnieska Szemiel, Aislynn Taggart, Jolanta Tanianis-Hughes, Jordan Thomas, Erwan Trochu, Libby van Tonder, Eve Wilcock & J. Eunice Zhang
University College London, London, UK
Shahab Aslani, Amita Banerjee, R. Batterham, Gabrielle Baxter, Robert Bell, Anthony David, Emma Denneny, Alun Hughes, W. Lilaonitkul, P. Mehta, Ashkan Pakzad, Bojidar Rangelov, B. Williams, James Willoughby & Moucheng Xu
Hull University Teaching Hospitals NHS Trust and University of Hull, Hull, UK
Paul Atkin, K. Brindle, Michael Crooks, Katie Drury, Nicholas Easom, Rachel Flockton, L. Holdsworth, A. Richards, D. L. Sykes, Susannah Thackray-Nocera & C. Wright
East Kent Hospitals University NHS Foundation Trust, Canterbury, UK
Liam Austin, Eva Beranova, Tracey Cosier, Joanne Deery, Tracy Hazelton, Carly Price, Hazel Ramos, Reanne Solly, Sharon Turney & Heather Weston
Baillie Gifford Pandemic Science Hub, Centre for Inflammation Research, The Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Nikos Avramidis, J. Kenneth Baillie, Erola Pairo-Castineira & Konrad Rawlik
Roslin Institute, University of Edinburgh, Edinburgh, UK
Nikos Avramidis, J. Kenneth Baillie & Erola Pairo-Castineira
Newcastle upon Tyne Hospitals NHS Foundation Trust and University of Newcastle, Newcastle upon Tyne, UK
A. Ayoub, J. Brown, G. Burns, Gareth Davies, Anthony De Soyza, Carlos Echevarria, Helen Fisher, C. Francis, Alan Greenhalgh, Philip Hogarth, Joan Hughes, Kasim Jiwa, G. Jones, G. MacGowan, D. Price, Avan Sayer, John Simpson, H. Tedd, S. Thomas, Sophie West, M. Witham, S. Wright & A. Young
East Cheshire NHS Trust, Macclesfield, UK
Marta Babores, Maureen Holland, Natalie Keenan, Sharlene Shashaa & Helen Wassall
Sheffield Teaching NHS Foundation Trust and University of Sheffield, Sheffield, UK
J. Bagshaw, M. Begum, K. Birchall, Robyn Butcher, H. Carborn, Flora Chan, Kerry Chapman, Yutung Cheng, Luke Chetham, Cameron Clark, Zach Coburn, Joby Cole, Myles Dixon, Alexandra Fairman, J. Finnigan, H. Foot, David Foote, Amber Ford, Rebecca Gregory, Kate Harrington, L. Haslam, L. Hesselden, J. Hockridge, Ailsa Holbourn, B. Holroyd-Hind, L. Holt, Alice Howell, E. Hurditch, F. Ilyas, Claire Jarman, Allan Lawrie, Ju Hee Lee, Elvina Lee, Rebecca Lenagh, Alison Lye, Irene Macharia, M. Marshall, Angeline Mbuyisa, J. McNeill, Sharon Megson, J. Meiring, L. Milner, S. Misra, Helen Newell, Tom Newman, C. Norman, Lorenza Nwafor, Dibya Pattenadk, Megan Plowright, Julie Porter, Phillip Ravencroft, C. Roddis, J. Rodger, Peter Saunders, J. Sidebottom, Jacqui Smith, Laurie Smith, N. Steele, G. Stephens, R. Stimpson, B. Thamu, N. Tinker, Kim Turner, Helena Turton, Phillip Wade, S. Walker, James Watson, Imogen Wilson & Amira Zawia
University of Nottingham, Nottingham, UK
David Baguley, Chris Coleman, E. Cox, Laura Fabbri, Susan Francis, Ian Hall, E. Hufton, Simon Johnson, Fasih Khan, Paaig Kitterick, Richard Morriss, Nick Selby, Iain Stewart & Louise Wright
Wirral University Teaching Hospital, Wirral, UK
Elisabeth Bailey, Anne Reddington & Andrew Wight
MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh, Western General Hospital, Edinburgh, UK
University of Swansea, Swansea, UK
University of Southampton, London, UK
David Baldwin, P. C. Calder, Nathan Huneke & Gemma Simons
Royal Brompton and Harefield Clinical Group, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
R. E. Barker, Daniele Cristiano, N. Dormand, P. George, Mahitha Gummadi, S. Kon, Kamal Liyanage, C. M. Nolan, B. Patel, Suhani Patel, Oliver Polgar, L. Price, P. Shah, Suver Singh & J. A. Walsh
York and Scarborough NHS Foundation Trust, York, UK
Laura Barman, Claire Brookes, K. Elliott, L. Griffiths, Zoe Guy, Kate Howard, Diana Ionita, Heidi Redfearn, Carol Sarginson & Alison Turnbull
NHS Highland, Inverness, UK
Fiona Barrett, A. Donaldson & Beth Sage
Royal Papworth Hospital NHS Foundation Trust, Cambridge, UK
Helen Baxendale, Lucie Garner, C. Johnson, J. Mackie, Alice Michael, J. Newman, Jamie Pack, K. Paques, H. Parfrey, J. Parmar & A. Reddy
University Hospitals of Derby and Burton, Derby, UK
Paul Beckett, Caroline Dickens & Uttam Nanda
NHS Lanarkshire, Hamilton, UK
Murdina Bell, Angela Brown, M. Brown, R. Hamil, Karen Leitch, L. Macliver, Manish Patel, Jackie Quigley, Andrew Smith & B. Welsh
Cambridge University Hospitals NHS Foundation Trust, NIHR Cambridge Clinical Research Facility and University of Cambridge, Cambridge, UK
Areti Bermperi, Isabel Cruz, K. Dempsey, Anne Elmer, Jonathon Fuld, H. Jones, Sherly Jose, Stefan Marciniak, M. Parkes, Carla Ribeiro, Jessica Taylor, Mark Toshner, L. Watson & J. Worsley
Loughborough University, Loughborough, UK
Lettie Bishop & David Stensel
Betsi Cadwallader University Health Board, Bangor, UK
Annette Bolger, Ffyon Davies, Ahmed Haggar, Joanne Lewis, Arwel Lloyd, R. Manley, Emma McIvor, Daniel Menzies, K. Roberts, W. Saxon, David Southern, Christian Subbe & Victoria Whitehead
Nottingham University Hospitals NHS Trust and University of Nottingham, Nottingham, UK
Charlotte Bolton, J. Bonnington, Melanie Chrystal, Catherine Dupont, Paul Greenhaff, Ayushman Gupta, W. Jang, S. Linford, Laura Matthews, Athanasios Nikolaidis, Sabrina Prosper & Andrew Thomas
King’s College London, London, UK
Kate Bramham, M. Brown, Khalida Ismail, Tim Nicholson, Carmen Pariante, Claire Sharpe, Simon Wessely & J. Whitney
Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK
Lucy Brear, Karen Regan, Dinesh Saralaya & Kim Storton
South London and Maudsley NHS Foundation Trust and King’s College London, London, UK
G. Breen & M. Hotopf
London School of Hygiene and Tropical Medicine, London, UK
Andrew Briggs
Whittington Health NHS Trust, London, UK
E. Bright, P. Crisp, Ruvini Dharmagunawardena & M. Stern
Cardiff and Vale University Health Board, Cardiff, UK
Lauren Broad, Teriann Evans, Matthew Haynes, L. Jones, Lucy Knibbs, Alison McQueen, Catherine Oliver, Kerry Paradowski, Ramsey Sabit & Jenny Williams
Yeovil District Hospital NHS Foundation Trust, Yeovil, UK
Andrew Broadley
University of Birmingham, Birmingham, UK
Mattew Broome, Paul McArdle, Paul Moss, David Thickett, Rachel Upthegrove, Dan Wilkinson, David Wraith & Erin L. Aldera
BHF Centre for Cardiovascular Science, University of Edinburgh, Edinburgh, UK
Anda Bularga
University of Cambridge, Cambridge, UK
Ed Bullmore, Jonathon Heeney, Claudia Langenberg, William Schwaeble, Charlotte Summers & J. Weir McCall
NIHR Leicester Biomedical Research Centre–Respiratory Patient and Public Involvement Group, Leicester, UK
Jenny Bunker, Rhyan Gill & Rashmita Nathu
Imperial College Healthcare NHS Trust and Imperial College London, London, UK
L. Burden, Ellen Calvelo, Bethany Card, Caitlin Carr, Edwin Chilvers, Donna Copeland, P. Cullinan, Patrick Daly, Lynsey Evison, Tamanah Fayzan, Hussain Gordon, Sulaimaan Haq, Gisli Jenkins, Clara King, Onn Min Kon, Katherine March, Myril Mariveles, Laura McLeavey, Silvia Moriera, Unber Munawar, Uchechi Nwanguma, Lorna Orriss-Dib, Alexandra Ross, Maura Roy, Emily Russell, Katherine Samuel, J. Schronce, Neil Simpson, Lawrence Tarusan, David Thomas, Chloe Wood & Najira Yasmin
Harrogate and District NHD Foundation Trust, Harrogate, UK
Tracy Burdett, James Featherstone, Cathy Lawson, Alison Layton, Clare Mills & Lorraine Stephenson
Newcastle University/Chair of NIHR Dementia TRC, Newcastle, UK
Oxford University Hospitals NHS Foundation Trust, Oxford, UK
A. Burns & N. Kanellakis
Tameside and Glossop Integrated Care NHS Foundation Trust, Ashton-under-Lyne, UK
Al-Tahoor Butt, Martina Coulding, Heather Jones, Susan Kilroy, Jacqueline McCormick, Jerome McIntosh, Heather Savill, Victoria Turner & Joanne Vere
University of Oxford, Nuffield Department of Medicine, Oxford, UK
University of Glasgow, Glasgow, UK
Jonathon Cavanagh, S. MacDonald, Kate O’Donnell, John Petrie, Naveed Sattar & Mark Spears
United Lincolnshire Hospitals NHS Trust, Grantham, UK
Manish Chablani & Lynn Osborne
Department of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK
Trudie Chalder
University Hospital of South Manchester NHS Foundation Trust, Manchester, UK
N. Chaudhuri
University Hospital Southampton NHS Foundation Trust and University of Southampton, Southampton, UK
Caroline Childs, R. Djukanovic, S. Fletcher, Matt Harvey, Mark Jones, Elizabeth Marouzet, B. Marshall, Reena Samuel, T. Sass, Tim Wallis & Helen Wheeler
King’s College Hospital/Guy’s and St Thomas’ NHS FT, London, UK
A. Chiribiri & C. O’Brien
Barts Health NHS Trust, London, UK
K. Chong-James, C. David, W. Y. James, Paul Pfeffer & O. Zongo
NHS Lothian and University of Edinburgh, Edinburgh, UK
Gaunab Choudhury, S. Clohisey, Andrew Deans, J. Furniss, Ewen Harrison, S. Kelly & Aziz Sheikh
School of Cardiovascular Medicine and Sciences. King’s College London, London, UK
Phillip Chowienczyk
Lewisham and Greenwich NHS Trust, London, UK
Hywel Dda University Health Board, Haverfordwest, UK
S. Coetzee, Kim Davies, Rachel Ann Hughes, Ronda Loosley, Heather McGuinness, Abdelrahman Mohamed, Linda O’Brien, Zohra Omar, Emma Perkins, Janet Phipps, Gavin Ross, Abigail Taylor, Helen Tench & Rebecca Wolf-Roberts
NHS Tayside and University of Dundee, Dundee, UK
David Connell, C. Deas, Anne Elliott, J. George, S. Mohammed, J. Rowland, A. R. Solstice, Debbie Sutherland & Caroline Tee
Swansea Bay University Health Board, Port Talbot, UK
Lynda Connor, Amanda Cook, Gwyneth Davies, Tabitha Rees, Favas Thaivalappil & Caradog Thomas
Faculty of Medicine, Nursing and Health Sciences, School of Biomedical Sciences, Monash University, Melbourne, Victoria, Australia
Eamon Coughlan
Rotherham NHS Foundation Trust, Rotherham, UK
Alison Daniels, Anil Hormis, Julie Ingham & Lisa Zeidan
Salford Royal NHS Foundation Trust, Salford, UK
P. Dark, Nawar Diar-Bakerly, D. Evans, E. Hardy, Alice Harvey, D. Holgate, Sean Knight, N. Mairs, N. Majeed, L. McMorrow, J. Oxton, Jessica Pendlebury, C. Summersgill, R. Ugwuoke & S. Whittaker
Cwm Taf Morgannwg University Health Board, Mountain Ash, UK
Ellie Davies, Cerys Evenden, Alyson Hancock, Kia Hancock, Ceri Lynch, Meryl Rees, Lisa Roche, Natalie Stroud & T. Thomas-Woods
Borders General Hospital, NHS Borders, Melrose, UK
Joy Dawson, Hosni El-Taweel & Leanne Robinson
Aneurin Bevan University Health Board, Caerleon, UK
Amanda Dell, Sara Fairbairn, Nancy Hawkings, Jill Haworth, Michaela Hoare, Victoria Lewis, Alice Lucey, Georgia Mallison, Heeah Nassa, Chris Pennington, Andrea Price, Claire Price, Andrew Storrie, Gemma Willis & Susan Young
University of Exeter Medical School, Exeter, UK
London North West University Healthcare NHS Trust, London, UK
Shalin Diwanji, Sambasivarao Gurram, Padmasayee Papineni, Sheena Quaid, Gerlynn Tiongson & Ekaterina Watson
Alzheimer’s Research UK, Cambridge, UK
Hannah Dobson
Health and Care Research Wales, Cardiff, UK
Yvette Ellis
University of Bristol, Bristol, UK
Jonathon Evans
University of Sheffield, Sheffield, UK
L. Finnigan, Laura Saunders & James Wild
Great Western Hospital Foundation Trust, Swindon, UK
Eva Fraile & Jacinta Ugoji
Royal Devon and Exeter NHS Trust, Barnstaple, UK
Michael Gibbons
Kettering General Hospital NHS Trust, Kettering, UK
Anne-Marie Guerdette, Melanie Hewitt, R. Reddy, Katie Warwick & Sonia White
NIHR Leicester Biomedical Research Centre, Leicester, UK
Beatriz Guillen-Guio
University of Leeds, Leeds, UK
Elspeth Guthrie & Max Henderson
Royal Surrey NHS Foundation Trust, Cranleigh, UK
Mark Halling-Brown & Katherine McCullough
Chesterfield Royal Hospital NHS Trust, Calow, UK
Edward Harris & Claire Sampson
Long Covid Support, London, UK
Claire Hastie, Natalie Rogers & Nikki Smith
King’s College Hospital, NHS Foundation Trust and King’s College London, London, UK
Department of Oncology and Metabolism, University of Sheffield, Sheffield, UK
Simon Heller
NIHR Office for Clinical Research Infrastructure, London, UK
Katie Holmes
Asthma UK and British Lung Foundation Partnership, London, UK
Ian Jarrold & Samantha Walker
North Middlesex University Hospital NHS Trust, London, UK
Bhagy Jayaraman & Tessa Light
Action for Pulmonary Fibrosis, Peterborough, UK
Cardiff University, National Centre for Mental Health, Cardiff, UK
McPin Foundation, London, UK
Thomas Kabir
Roslin Institute, The University of Edinburgh, Edinburgh, UK
Steven Kerr
The Hillingdon Hospitals NHS Foundation Trust, London, UK
Samantha Kon, G. Landers, Harpreet Lota, Mariam Nasseri & Sofiya Portukhay
Queen Mary University of London, London, UK
Ania Korszun
Swansea University, Swansea Welsh Network, Hywel Dda University Health Board, Swansea, UK
Royal Infirmary of Edinburgh, NHS Lothian, Edinburgh, UK
Nazir I. Lone
Barts Heart Centre, London, UK
Barts Health NHS Trust and Queen Mary University of London, London, UK
Adrian Martineau
Salisbury NHS Foundation Trust, Salisbury, UK
Wadzanai Matimba-Mupaya & Sophia Strong-Sheldrake
University of Newcastle, Newcastle, UK
Hamish McAllister-Williams, Stella-Maria Paddick, Anthony Rostron & John Paul Taylor
Gateshead NHS Trust, Gateshead, UK
W. McCormick, Lorraine Pearce, S. Pugmire, Wendy Stoker & Ann Wilson
Manchester Centre for Clinical Neurosciences, Salford Royal NHS Foundation Trust, Manchester, UK
Katherine McIvor
Kidney Research UK, Peterborough, UK
Aisling McMahon
NHS Dumfries and Galloway, Dumfries, UK
Michael McMahon & Paula Neill
Swansea University, Swansea, UK
MQ Mental Health Research, London, UK
Lea Milligan
BHF Centre for Cardiovascular Science, Usher Institute of Population Health Sciences and Informatics, University of Edinburgh, Edinburgh, UK
Nicholas Mills
Shropshire Community Health NHS Trust, Shropshire, UK
Sharon Painter, Johanne Tomlinson & Louise Warburton
Somerset NHS Foundation Trust, Taunton, UK
Sue Palmer, Dawn Redwood, Jo Tilley, Carinna Vickers & Tania Wainwright
Francis Crick Institute, London, UK
Markus Ralser
Manchester University NHD Foundation Trust, Manchester, UK
Pilar Rivera-Ortega
Diabetes UK, University of Glasgow, Glasgow, UK
Elizabeth Robertson
Barnsley Hospital NHS Foundation Trust, Barnsley, UK
Amy Sanderson
MRC–University of Glasgow Centre for Virus Research, Glasgow, UK
Janet Scott
Diabetes UK, London, UK
Kamini Shah
British Heart Foundation Centre, King’s College London, London, UK
King’s College Hospital NHS Foundation Trust, London, UK
University Hospitals Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK
Institute of Cardiovascular and Medical Sciences, BHF Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, UK
University College London NHS Foundation Trust, London and Barts Health NHS Trust, London, UK
Northumbria University, Newcastle upon Tyne, UK
Ioannis Vogiatzis
Swansea University and Swansea Welsh Network, Swansea, UK
N. Williams
DUK | NHS Digital, Salford Royal Foundation Trust, Salford, UK
Queen Alexandra Hospital, Portsmouth, UK
- Kayode Adeniji
Princess Royal Hospital, Haywards Heath, UK
Daniel Agranoff & Chi Eziefula
Bassetlaw Hospital, Bassetlaw, UK
Darent Valley Hospital, Dartford, UK
Queen Elizabeth the Queen Mother Hospital, Margate, UK
Ana Alegria
School of Informatics, University of Edinburgh, Edinburgh, UK
Beatrice Alex, Benjamin Bach & James Scott-Brown
North East and North Cumbria Ingerated, Newcastle upon Tyne, UK
Section of Biomolecular Medicine, Division of Systems Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Petros Andrikopoulos, Kanta Chechi, Marc-Emmanuel Dumas, Julian Griffin, Sonia Liggi & Zoltan Takats
Section of Genomic and Environmental Medicine, Respiratory Division, National Heart and Lung Institute, Imperial College London, London, UK
Petros Andrikopoulos, Marc-Emmanuel Dumas, Michael Olanipekun & Anthonia Osagie
John Radcliffe Hospital, Oxford, UK
Brian Angus
Royal Albert Edward Infirmary, Wigan, UK
Abdul Ashish
Manchester Royal Infirmary, Manchester, UK
Dougal Atkinson
MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh, Western General Hospital, Crewe Road, Edinburgh, UK
Section of Molecular Virology, Imperial College London, London, UK
Wendy S. Barclay
Furness General Hospital, Barrow-in-Furness, UK
Shahedal Bari
Hull University Teaching Hospital Trust, Kingston upon Hull, UK
Gavin Barlow
Hillingdon Hospital, Hillingdon, UK
Stella Barnass
St Thomas’ Hospital, London, UK
Nicholas Barrett
Coventry and Warwickshire, Coventry, UK
Christopher Bassford
St Michael’s Hospital, Bristol, UK
Sneha Basude
Stepping Hill Hospital, Stockport, UK
David Baxter
Royal Liverpool University Hospital, Liverpool, UK
Michael Beadsworth
Bristol Royal Hospital Children’s, Bristol, UK
Jolanta Bernatoniene
Scarborough Hospital, Scarborough, UK
John Berridge
Golden Jubilee National Hospital, Clydebank, UK
Colin Berry
Liverpool Heart and Chest Hospital, Liverpool, UK
Nicola Best
Centre for Inflammation Research, The Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Debby Bogaert & Clark D. Russell
James Paget University Hospital, Great Yarmouth, UK
Pieter Bothma & Darell Tupper-Carey
Aberdeen Royal Infirmary, Aberdeen, UK
Robin Brittain-Long
Adamson Hospital, Cupar, UK
Naomi Bulteel
Royal Devon and Exeter Hospital, Exeter, UK
Worcestershire Royal Hospital, Worcester, UK
Andrew Burtenshaw
ISARIC Global Support Centre, Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Gail Carson, Laura Merson & Louise Sigfrid
Conquest Hospital, Hastings, UK
Vikki Caruth
The James Cook University Hospital, Middlesbrough, UK
David Chadwick
Dorset County Hospital, Dorchester, UK
Duncan Chambler
Antimicrobial Resistance and Hospital Acquired Infection Department, Public Health England, London, UK
Meera Chand
Department of Epidemiology and Biostatistics, School of Public Health, Faculty of Medicine, Imperial College London, London, UK
Kanta Chechi
Royal Bournemouth General Hospital, Bournemouth, UK
Harrogate Hospital, Harrogate, UK
Jenny Child
Royal Blackburn Teaching Hospital, Blackburn, UK
Srikanth Chukkambotla
Edinburgh Clinical Research Facility, University of Edinburgh, Edinburgh, UK
Richard Clark, Audrey Coutts, Lorna Donelly, Angie Fawkes, Tammy Gilchrist, Katarzyna Hafezi, Louise MacGillivray, Alan Maclean, Sarah McCafferty, Kirstie Morrice, Lee Murphy & Nicola Wrobel
Torbay Hospital, Torquay, UK
Northern General Hospital, Sheffield, UK
Paul Collini, Cariad Evans & Gary Mills
Liverpool Clinical Trials Centre, University of Liverpool, Liverpool, UK
Marie Connor, Jo Dalton, Chloe Donohue, Carrol Gamble, Michelle Girvan, Sophie Halpin, Janet Harrison, Clare Jackson, Laura Marsh, Stephanie Roberts & Egle Saviciute
Department of Infectious Disease, Imperial College London, London, UK
Graham S. Cooke & Shiranee Sriskandan
St Georges Hospital (Tooting), London, UK
Catherine Cosgrove
Blackpool Victoria Hospital, Blackpool, UK
Jason Cupitt & Joanne Howard
The Royal London Hospital, London, UK
Maria-Teresa Cutino-Moguel
MRC-University of Glasgow Centre for Virus Research, Glasgow, UK
Ana da Silva Filipe, Antonia Y. W. Ho, Sarah E. McDonald, Massimo Palmarini, David L. Robertson, Janet T. Scott & Emma C. Thomson
Salford Royal Hospital, Salford, UK
University Hospital of North Durham, Durham, UK
Chris Dawson
Norfolk and Norwich University Hospital, Norwich, UK
Samir Dervisevic
Intensive Care Unit, Royal Infirmary Edinburgh, Edinburgh, UK
Annemarie B. Docherty & Seán Keating
Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, UK
Cara Donegan & Rebecca G. Spencer
Salisbury District Hospital, Salisbury, UK
Phil Donnison
National Phenome Centre, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Gonçalo dos Santos Correia, Matthew Lewis, Lynn Maslen, Caroline Sands, Zoltan Takats & Panteleimon Takis
Section of Bioanalytical Chemistry, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Gonçalo dos Santos Correia, Matthew Lewis, Lynn Maslen, Caroline Sands & Panteleimon Takis
Guy’s and St Thomas’, NHS Foundation Trust, London, UK
Sam Douthwaite, Michael MacMahon, Marlies Ostermann & Manu Shankar-Hari
The Royal Oldham Hospital, Oldham, UK
Andrew Drummond
European Genomic Institute for Diabetes, Institut Pasteur de Lille, Lille University Hospital, University of Lille, Lille, France
Marc-Emmanuel Dumas
McGill University and Genome Quebec Innovation Centre, Montreal, Qeubec, Canada
National Infection Service, Public Health England, London, UK
Jake Dunning & Maria Zambon
Hereford Count Hospital, Hereford, UK
Ingrid DuRand
Southampton General Hospital, Southampton, UK
Ahilanadan Dushianthan
Northampton General Hospital, Northampton, UK
Tristan Dyer
University Hospital of Wales, Cardiff, UK
Chrisopher Fegan
University Hospitals Bristol NHS Foundation Trust, Bristol, UK
Liverpool School of Tropical Medicine, Liverpool, UK
Tom Fletcher
Leighton Hospital, Crewe, UK
Duncan Fullerton & Elijah Matovu
Manor Hospital, Walsall, UK
Scunthorpe Hospital, Scunthorpe, UK
Sanjeev Garg
Cambridge University Hospital, Cambridge, UK
Effrossyni Gkrania-Klotsas
West Suffolk NHS Foundation Trust, Bury St Edmunds, UK
Basingstoke and North Hampshire Hospital, Basingstoke, UK
Arthur Goldsmith
North Cumberland Infirmary, Carlisle, UK
Clive Graham
Paediatric Liver, GI and Nutrition Centre and MowatLabs, King’s College Hospital, London, UK
Tassos Grammatikopoulos
Institute of Liver Studies, King’s College London, London, UK
Institute of Microbiology and Infection, University of Birmingham, Birmingham, UK
Christopher A. Green
Department of Molecular and Clinical Cancer Medicine, University of Liverpool, Liverpool, UK
William Greenhalf
Institute for Global Health, University College London, London, UK
Rishi K. Gupta
NIHR Health Protection Research Unit, Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, UK
Hayley Hardwick, Malcolm G. Semple, Tom Solomon & Lance C. W. Turtle
Warwick Hospital, Warwick, UK
Elaine Hardy
Birmingham Children’s Hospital, Birmingham, UK
Stuart Hartshorn
Nottingham City Hospital, Nottingham, UK
Daniel Harvey
Glangwili Hospital Child Health Section, Carmarthen, UK
Peter Havalda
Alder Hey Children’s Hospital, Liverpool, UK
Daniel B. Hawcutt
Department of Infectious Diseases, Queen Elizabeth University Hospital, Glasgow, UK
Antonia Y. W. Ho
Bronglais General Hospital, Aberystwyth, UK
Maria Hobrok
Worthing Hospital, Worthing, UK
Luke Hodgson
Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Peter W. Horby
Rotheram District General Hospital, Rotheram, UK
Anil Hormis
Virology Reference Department, National Infection Service, Public Health England, Colindale Avenue, London, UK
Samreen Ijaz
Royal Free Hospital, London, UK
Michael Jacobs & Padmasayee Papineni
Homerton Hospital, London, UK
Airedale Hospital, Airedale, UK
Paul Jennings
Basildon Hospital, Basildon, UK
Agilan Kaliappan
The Christie NHS Foundation Trust, Manchester, UK
Vidya Kasipandian
University Hospital Lewisham, London, UK
Stephen Kegg
The Whittington Hospital, London, UK
Michael Kelsey
Southmead Hospital, Bristol, UK
Jason Kendall
Sheffield Childrens Hospital, Sheffield, UK
Caroline Kerrison
Royal United Hospital, Bath, UK
Ian Kerslake
Department of Pharmacology, University of Liverpool, Liverpool, UK
Nuffield Department of Medicine, Peter Medawar Building for Pathogen Research, University of Oxford, Oxford, UK
Paul Klenerman
Translational Gastroenterology Unit, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Public Health Scotland, Edinburgh, UK
Susan Knight, Eva Lahnsteiner & Sarah Tait
Western General Hospital, Edinburgh, UK
Oliver Koch
Southend University Hospital NHS Foundation Trust, Southend-on-Sea, UK
Gouri Koduri
Hinchingbrooke Hospital, Huntingdon, UK
George Koshy & Tamas Leiner
Royal Preston Hospital, Fulwood, UK
Shondipon Laha
University Hospital (Coventry), Coventry, UK
Steven Laird
The Walton Centre, Liverpool, UK
Susan Larkin
ISARIC, Global Support Centre, COVID-19 Clinical Research Resources, Epidemic diseases Research Group, Oxford (ERGO), University of Oxford, Oxford, UK
James Lee & Daniel Plotkin
Centre for Health Informatics, Division of Informatics, Imaging and Data Science, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK
Gary Leeming
Hull Royal Infirmary, Hull, UK
Patrick Lillie
Nottingham University Hospitals NHS Trust:, Nottingham, UK
Wei Shen Lim
Darlington Memorial Hospital, Darlington, UK
Queen Elizabeth Hospital (Gateshead), Gateshead, UK
Vanessa Linnett
Warrington Hospital, Warrington, UK
Jeff Little
Bristol Royal Hospital for Children, Bristol, UK
Mark Lyttle
St Mary’s Hospital (Isle of Wight), Isle of Wight, UK
Emily MacNaughton
The Tunbridge Wells Hospital, Royal Tunbridge Wells, UK
Ravish Mankregod
Huddersfield Royal, Huddersfield, UK
Countess of Chester Hospital, Liverpool, UK
Ruth McEwen & Lawrence Wilson
Frimley Park Hospital, Frimley, UK
Manjula Meda
Nuffield Department of Medicine, John Radcliffe Hospital, Oxford, UK
Alexander J. Mentzer
Department of Microbiology/Infectious Diseases, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford, UK
MRC Human Genetics Unit, MRC Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK
Alison M. Meynert & Murray Wham
St James University Hospital, Leeds, UK
Jane Minton
Arrowe Park Hospital, Birkenhead, UK
Kavya Mohandas
Great Ormond Street Hospital, London, UK
Royal Shrewsbury Hospital, Shrewsbury, UK
Addenbrookes Hospital, Cambridge, UK
Elinoor Moore
Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, UK
Shona C. Moore, William A. Paxton & Georgios Pollakis
East Surrey Hospital (Redhill), Redhill, UK
Patrick Morgan
Burton Hospital, Burton, UK
Craig Morris & Tim Reynolds
Peterborough City Hospital, Peterborough, UK
Katherine Mortimore
Kent and Canterbury Hospital, Canterbury, UK
Samuel Moses
Weston Area General Trust, Bristol, UK
Mbiye Mpenge
Bedfordshire Hospital, Bedfordshire, UK
Rohinton Mulla
Glasgow Royal Infirmary, Glasgow, UK
Michael Murphy
Macclesfield General Hospital, Macclesfield, UK
Thapas Nagarajan
Derbyshire Healthcare, Derbyshire, UK
Megan Nagel
Chelsea and Westminster Hospital, London, UK
Mark Nelson & Matthew K. O’Shea
Watford General Hospital, Watford, UK
Lillian Norris & Tom Stambach
EPCC, University of Edinburgh, Edinburgh, UK
Lucy Norris
Section of Biomolecular Medicine, Division of Systems Medicine, Department of Metabolism, Digestion and Reproduction, London, UK
Michael Olanipekun
Imperial College Healthcare NHS Trust: London, London, UK
Peter J. M. Openshaw
Division of Systems Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Anthonia Osagie
Prince Philip Hospital, Llanelli, UK
Igor Otahal & Andrew Workman
George Eliot Hospital – Acute Services, Nuneaton, UK
Molecular and Clinical Cancer Medicine, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK
Carlo Palmieri
Clatterbridge Cancer Centre NHS Foundation Trust, Liverpool, UK
Kettering General Hospital, Kettering, UK
Selva Panchatsharam
University Hospitals of North Midlands NHS Trust, North Midlands, UK
Danai Papakonstantinou
Russells Hall Hospital, Dudley, UK
Hassan Paraiso
Harefield Hospital, Harefield, UK
Lister Hospital, Lister, UK
Natalie Pattison
Musgrove Park Hospital, Taunton, UK
Justin Pepperell
Kingston Hospital, Kingston, UK
Mark Peters
Queen’s Hospital, Romford, UK
Mandeep Phull
Southport and Formby District General Hospital, Southport, UK
Stefania Pintus
St George’s University of London, London, UK
Tim Planche
King’s College Hospital (Denmark Hill), London, UK
Centre for Clinical Infection and Diagnostics Research, Department of Infectious Diseases, School of Immunology and Microbial Sciences, King’s College London, London, UK
Nicholas Price
Department of Infectious Diseases, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
The Clatterbridge Cancer Centre NHS Foundation, Bebington, UK
David Price
The Great Western Hospital, Swindon, UK
Rachel Prout
Ninewells Hospital, Dundee, UK
Nikolas Rae
Institute of Evolutionary Biology, University of Edinburgh, Edinburgh, UK
Andrew Rambaut
Poole Hospital NHS Trust, Poole, UK
Henrik Reschreiter
William Harvey Hospital, Ashford, UK
Neil Richardson
King’s Mill Hospital, Sutton-in-Ashfield, UK
Mark Roberts
Liverpool Women’s Hospital, Liverpool, UK
Devender Roberts
Pinderfields Hospital, Wakefield, UK
Alistair Rose
North Devon District Hospital, Barnstaple, UK
Guy Rousseau
Queen Elizabeth Hospital, Birmingham, UK
Tameside General Hospital, Ashton-under-Lyne, UK
Brendan Ryan
City Hospital (Birmingham), Birmingham, UK
Taranprit Saluja
Department of Pediatrics and Virology, St Mary’s Medical School Bldg, Imperial College London, London, UK
Vanessa Sancho-Shimizu
The Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne, UK
Matthias Schmid
NHS Greater Glasgow and Clyde, Glasgow, UK
Janet T. Scott
Respiratory Medicine, Institute in The Park, University of Liverpool, Alder Hey Children’s Hospital, Liverpool, UK
Malcolm G. Semple
Broomfield Hospital, Broomfield, UK
Stoke Mandeville, UK
Prad Shanmuga
University Hospital of North Tees, Stockton-on-Tees, UK
Anil Sharma
Institute of Translational Medicine, University of, Liverpool, Merseyside, UK
Victoria E. Shaw
Royal Manchester Children’s Hospital, Manchester, UK
Anna Shawcross
New Cross Hospital, Wolverhampton, UK
Jagtur Singh Pooni
Bedford Hospital, Bedford, UK
Jeremy Sizer
Colchester General Hospital, Colchester, UK
Richard Smith
University Hospital Birmingham NHS Foundation Trust, Birmingham, UK
Catherine Snelson & Tony Whitehouse
Walton Centre NHS Foundation Trust, Liverpool, UK
Tom Solomon
Chesterfield Royal Hospital, Calow, UK
Nick Spittle
MRC Centre for Molecular Bacteriology and Infection, Imperial College London, London, UK
Shiranee Sriskandan
Princess Alexandra Hospital, Harlow, UK
Nikki Staines & Shico Visuvanathan
Milton Keynes Hospital, Eaglestone, UK
Richard Stewart
Division of Structural Biology, The Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK
David Stuart
Royal Bolton Hopital, Farnworth, UK
Pradeep Subudhi
Department of Medicine, University of Cambridge, Cambridge, UK
Charlotte Summers
Department of Child Life and Health, University of Edinburgh, Edinburgh, UK
Olivia V. Swann
Royal Gwent (Newport), Newport, UK
Tamas Szakmany
The Royal Marsden Hospital (London), London, UK
Kate Tatham
Blood Borne Virus Unit, Virus Reference Department, National Infection Service, Public Health England, London, UK
Richard S. Tedder
Transfusion Microbiology, National Health Service Blood and Transplant, London, UK
Department of Medicine, Imperial College London, London, UK
Queen Victoria Hospital (East Grinstead), East Grinstead, UK
Leeds Teaching Hospitals NHS Trust, Leeds, UK
Robert Thompson
Royal Stoke University Hospital, Stoke-on-Trent, UK
Chris Thompson
Whiston Hospital, Rainhill, UK
Ascanio Tridente
Tropical and Infectious Disease Unit, Royal Liverpool University Hospital, Liverpool, UK
Lance C. W. Turtle
Croydon University Hospital, Thornton Heath, UK
Mary Twagira
Gloucester Royal, Gloucester, UK
Nick Vallotton
West Hertfordshire Teaching Hospitals NHS Trust, Hertfordshire, UK
Rama Vancheeswaran
North Middlesex Hospital, London, UK
Rachel Vincent
Medway Maritime Hospital, Gillingham, UK
Lisa Vincent-Smith
Royal Papworth Hospital Everard, Cambridge, UK
Alan Vuylsteke
Derriford (Plymouth), Plymouth, UK
St Helier Hospital, Sutton, UK
Rachel Wake
Royal Berkshire Hospital, Reading, UK
Andrew Walden
Royal Liverpool Hospital, Liverpool, UK
Ingeborg Welters
Bradford Royal infirmary, Bradford, UK
Paul Whittaker
Central Middlesex, London, UK
Ashley Whittington
Royal Cornwall Hospital (Tresliske), Truro, UK
Meme Wijesinghe
North Bristol NHS Trust, Bristol, UK
Martin Williams
St. Peter’s Hospital, Runnymede, UK
Stephen Winchester
Leicester Royal Infirmary, Leicester, UK
Martin Wiselka
Grantham and District Hospital, Grantham, UK
Adam Wolverson
Aintree University Hospital, Liverpool, UK
Daniel G. Wootton
North Tyneside General Hospital, North Shields, UK
Bryan Yates
Queen Elizabeth Hospital, King’s Lynn, UK
Peter Young
You can also search for this author in PubMed Google Scholar
PHOSP-COVID collaborative group
- Kathryn Abel
- , H. Adamali
- , Davies Adeloye
- , Oluwaseun Adeyemi
- , Rita Adrego
- , Laura Aguilar Jimenez
- , Shanaz Ahmad
- , N. Ahmad Haider
- , Rubina Ahmed
- , Nyarko Ahwireng
- , Mark Ainsworth
- , Asma Alamoudi
- , Mariam Ali
- , M. Aljaroof
- , Louise Allan
- , Richard Allen
- , Lisa Allerton
- , Lynne Allsop
- , Ann Marie Allt
- , Paula Almeida
- , Bashar Al-Sheklly
- , Danny Altmann
- , Maria Alvarez Corral
- , Shannon Amoils
- , David Anderson
- , Charalambos Antoniades
- , Gill Arbane
- , Ava Maria Arias
- , Cherie Armour
- , Lisa Armstrong
- , Natalie Armstrong
- , David Arnold
- , H. Arnold
- , A. Ashish
- , Andrew Ashworth
- , M. Ashworth
- , Shahab Aslani
- , Hosanna Assefa-Kebede
- , Paul Atkin
- , Catherine Atkin
- , Raminder Aul
- , Hnin Aung
- , Liam Austin
- , Cristina Avram
- , Nikos Avramidis
- , Marta Babores
- , Rhiannon Baggott
- , J. Bagshaw
- , David Baguley
- , Elisabeth Bailey
- , J. Kenneth Baillie
- , Steve Bain
- , Majda Bakali
- , E. Baldry
- , Molly Baldwin
- , David Baldwin
- , Clive Ballard
- , Amita Banerjee
- , Dongchun Bang
- , R. E. Barker
- , Laura Barman
- , Perdita Barran
- , Shaney Barratt
- , Fiona Barrett
- , Donna Basire
- , Neil Basu
- , Michelle Bates
- , R. Batterham
- , Helen Baxendale
- , Gabrielle Baxter
- , Hannah Bayes
- , M. Beadsworth
- , Paul Beckett
- , Paul Beirne
- , Murdina Bell
- , Robert Bell
- , Kaytie Bennett
- , Eva Beranova
- , Areti Bermperi
- , Anthony Berridge
- , Colin Berry
- , Sarah Betts
- , Emily Bevan
- , Kamaldeep Bhui
- , Michelle Bingham
- , K. Birchall
- , Lettie Bishop
- , Karen Bisnauthsing
- , John Blaikely
- , Angela Bloss
- , Annette Bolger
- , Charlotte Bolton
- , J. Bonnington
- , A. Botkai
- , Charlotte Bourne
- , Michelle Bourne
- , Kate Bramham
- , Lucy Brear
- , Jonathon Breeze
- , Katie Breeze
- , Andrew Briggs
- , E. Bright
- , Christopher Brightling
- , Simon Brill
- , K. Brindle
- , Lauren Broad
- , Andrew Broadley
- , Claire Brookes
- , Mattew Broome
- , Vanessa Brown
- , Ammani Brown
- , Angela Brown
- , Jeremy Brown
- , Terry Brugha
- , Nigel Brunskill
- , Phil Buckley
- , Anda Bularga
- , Ed Bullmore
- , Jenny Bunker
- , L. Burden
- , Tracy Burdett
- , David Burn
- , John Busby
- , Robyn Butcher
- , Al-Tahoor Butt
- , P. Cairns
- , P. C. Calder
- , Ellen Calvelo
- , H. Carborn
- , Bethany Card
- , Caitlin Carr
- , Liesel Carr
- , G. Carson
- , Penny Carter
- , Anna Casey
- , M. Cassar
- , Jonathon Cavanagh
- , Manish Chablani
- , Trudie Chalder
- , James D. Chalmers
- , Rachel Chambers
- , Flora Chan
- , K. M. Channon
- , Kerry Chapman
- , Amanda Charalambou
- , N. Chaudhuri
- , A. Checkley
- , Yutung Cheng
- , Luke Chetham
- , Caroline Childs
- , Edwin Chilvers
- , H. Chinoy
- , A. Chiribiri
- , K. Chong-James
- , N. Choudhury
- , Gaunab Choudhury
- , Phillip Chowienczyk
- , C. Christie
- , Melanie Chrystal
- , Cameron Clark
- , David Clark
- , Jude Clarke
- , S. Clohisey
- , G. Coakley
- , Zach Coburn
- , S. Coetzee
- , Joby Cole
- , Chris Coleman
- , Florence Conneh
- , David Connell
- , Bronwen Connolly
- , Lynda Connor
- , Amanda Cook
- , Shirley Cooper
- , B. Cooper
- , Josh Cooper
- , Donna Copeland
- , Tracey Cosier
- , Eamon Coughlan
- , Martina Coulding
- , C. Coupland
- , Thelma Craig
- , Daniele Cristiano
- , Michael Crooks
- , Andy Cross
- , Isabel Cruz
- , P. Cullinan
- , D. Cuthbertson
- , Luke Daines
- , Matthhew Dalton
- , Patrick Daly
- , Alison Daniels
- , Joanne Dasgin
- , Anthony David
- , Ffyon Davies
- , Ellie Davies
- , Kim Davies
- , Gareth Davies
- , Gwyneth Davies
- , Melanie Davies
- , Joy Dawson
- , Camilla Dawson
- , Enya Daynes
- , Anthony De Soyza
- , Bill Deakin
- , Andrew Deans
- , Joanne Deery
- , Sylviane Defres
- , Amanda Dell
- , K. Dempsey
- , Emma Denneny
- , J. Dennis
- , Ruvini Dharmagunawardena
- , Nawar Diar-Bakerly
- , Caroline Dickens
- , A. Dipper
- , Sarah Diver
- , Shalin Diwanji
- , Myles Dixon
- , R. Djukanovic
- , Hannah Dobson
- , S. L. Dobson
- , Annemarie B. Docherty
- , A. Donaldson
- , N. Dormand
- , Andrew Dougherty
- , Rachael Dowling
- , Stephen Drain
- , Katharine Draxlbauer
- , Katie Drury
- , Pearl Dulawan
- , A. Dunleavy
- , Sarah Dunn
- , Catherine Dupont
- , Joanne Earley
- , Nicholas Easom
- , Carlos Echevarria
- , Sarah Edwards
- , C. Edwardson
- , Claudia Efstathiou
- , Anne Elliott
- , K. Elliott
- , Yvette Ellis
- , Anne Elmer
- , Omer Elneima
- , Hosni El-Taweel
- , Teriann Evans
- , Ranuromanana Evans
- , Rachael A. Evans
- , Jonathon Evans
- , Cerys Evenden
- , Lynsey Evison
- , Laura Fabbri
- , Sara Fairbairn
- , Alexandra Fairman
- , K. Fallon
- , David Faluyi
- , Clair Favager
- , Tamanah Fayzan
- , James Featherstone
- , T. Felton
- , V. Ferreira
- , Selina Finney
- , J. Finnigan
- , L. Finnigan
- , Helen Fisher
- , S. Fletcher
- , Rachel Flockton
- , Margaret Flynn
- , David Foote
- , Amber Ford
- , D. Forton
- , Eva Fraile
- , C. Francis
- , Richard Francis
- , Susan Francis
- , Anew Frankel
- , Emily Fraser
- , N. French
- , Jonathon Fuld
- , J. Furniss
- , Lucie Garner
- , N. Gautam
- , John Geddes
- , J. George
- , P. George
- , Michael Gibbons
- , Rhyan Gill
- , Mandy Gill
- , L. Gilmour
- , F. Gleeson
- , Jodie Glossop
- , Sarah Glover
- , Nicola Goodman
- , Camelia Goodwin
- , Bibek Gooptu
- , Hussain Gordon
- , T. Gorsuch
- , M. Greatorex
- , Paul Greenhaff
- , William Greenhalf
- , Alan Greenhalgh
- , Neil J. Greening
- , John Greenwood
- , Rebecca Gregory
- , Heidi Gregory
- , D. Grieve
- , Denise Griffin
- , L. Griffiths
- , Anne-Marie Guerdette
- , Beatriz Guillen-Guio
- , Mahitha Gummadi
- , Ayushman Gupta
- , Sambasivarao Gurram
- , Elspeth Guthrie
- , Kate Hadley
- , Ahmed Haggar
- , Kera Hainey
- , Brigid Hairsine
- , Pranab Haldar
- , Lucy Hall
- , Mark Halling-Brown
- , Alyson Hancock
- , Kia Hancock
- , Neil Hanley
- , Sulaimaan Haq
- , Hayley Hardwick
- , Tim Hardy
- , Beverley Hargadon
- , Kate Harrington
- , Edward Harris
- , Victoria C. Harris
- , Ewen Harrison
- , Paul Harrison
- , Nicholas Hart
- , Alice Harvey
- , Matt Harvey
- , M. Harvie
- , L. Haslam
- , Claire Hastie
- , May Havinden-Williams
- , Jenny Hawkes
- , Nancy Hawkings
- , Jill Haworth
- , A. Hayday
- , Matthew Haynes
- , J. Hazeldine
- , Tracy Hazelton
- , Liam Heaney
- , Cheryl Heeley
- , Jonathon Heeney
- , M. Heightman
- , Simon Heller
- , Max Henderson
- , Helen Henson
- , L. Hesselden
- , Melanie Hewitt
- , Victoria Highett
- , T. Hillman
- , Ling-Pei Ho
- , Michaela Hoare
- , Amy Hoare
- , J. Hockridge
- , Philip Hogarth
- , Ailsa Holbourn
- , Sophie Holden
- , L. Holdsworth
- , D. Holgate
- , Maureen Holland
- , Leah Holloway
- , Katie Holmes
- , Megan Holmes
- , B. Holroyd-Hind
- , Anil Hormis
- , Alexander Horsley
- , Akram Hosseini
- , M. Hotopf
- , Linzy Houchen-Wolloff
- , Luke S. Howard
- , Kate Howard
- , Alice Howell
- , E. Hufton
- , Rachel Ann Hughes
- , Joan Hughes
- , Alun Hughes
- , Amy Humphries
- , Nathan Huneke
- , E. Hurditch
- , John Hurst
- , Masud Husain
- , Tracy Hussell
- , John Hutchinson
- , W. Ibrahim
- , Julie Ingham
- , L. Ingram
- , Diana Ionita
- , Karen Isaacs
- , Khalida Ismail
- , T. Jackson
- , Joseph Jacob
- , W. Y. James
- , Claire Jarman
- , Ian Jarrold
- , Hannah Jarvis
- , Roman Jastrub
- , Bhagy Jayaraman
- , Gisli Jenkins
- , P. Jezzard
- , Kasim Jiwa
- , C. Johnson
- , Simon Johnson
- , Desmond Johnston
- , Caroline Jolley
- , Ian Jones
- , Heather Jones
- , Mark Jones
- , Don Jones
- , Sherly Jose
- , Thomas Kabir
- , G. Kaltsakas
- , Vicky Kamwa
- , N. Kanellakis
- , Sabina Kaprowska
- , Zunaira Kausar
- , Natalie Keenan
- , Steven Kerr
- , Helen Kerslake
- , Angela Key
- , Fasih Khan
- , Kamlesh Khunti
- , Susan Kilroy
- , Bernie King
- , Clara King
- , Lucy Kingham
- , Jill Kirk
- , Paaig Kitterick
- , Paul Klenerman
- , Lucy Knibbs
- , Sean Knight
- , Abigail Knighton
- , Onn Min Kon
- , Samantha Kon
- , Ania Korszun
- , Ivan Koychev
- , Claire Kurasz
- , Prathiba Kurupati
- , Hanan Lamlum
- , G. Landers
- , Claudia Langenberg
- , Lara Lavelle-Langham
- , Allan Lawrie
- , Cathy Lawson
- , Claire Lawson
- , Alison Layton
- , Olivia C. Leavy
- , Ju Hee Lee
- , Elvina Lee
- , Karen Leitch
- , Rebecca Lenagh
- , Victoria Lewis
- , Joanne Lewis
- , Keir Lewis
- , N. Lewis-Burke
- , Felicity Liew
- , Tessa Light
- , Liz Lightstone
- , W. Lilaonitkul
- , S. Linford
- , Anne Lingford-Hughes
- , M. Lipman
- , Kamal Liyanage
- , Arwel Lloyd
- , Nazir I. Lone
- , Ronda Loosley
- , Janet Lord
- , Harpreet Lota
- , Wayne Lovegrove
- , Daniel Lozano-Rojas
- , Alice Lucey
- , Gardiner Lucy
- , E. Lukaschuk
- , Alison Lye
- , Ceri Lynch
- , S. MacDonald
- , G. MacGowan
- , Irene Macharia
- , J. Mackie
- , L. Macliver
- , S. Madathil
- , Gladys Madzamba
- , Nick Magee
- , Murphy Magtoto
- , N. Majeed
- , Flora Malein
- , Georgia Mallison
- , William Man
- , S. Mandal
- , K. Mangion
- , C. Manisty
- , R. Manley
- , Katherine March
- , Stefan Marciniak
- , Philip Marino
- , Myril Mariveles
- , Michael Marks
- , Elizabeth Marouzet
- , Sophie Marsh
- , M. Marshall
- , B. Marshall
- , Jane Martin
- , Adrian Martineau
- , L. M. Martinez
- , Nick Maskell
- , Darwin Matila
- , Wadzanai Matimba-Mupaya
- , Laura Matthews
- , Angeline Mbuyisa
- , Steve McAdoo
- , Hamish McAllister-Williams
- , Paul McArdle
- , Anne McArdle
- , Danny McAulay
- , Hamish J. C. McAuley
- , Gerry McCann
- , W. McCormick
- , Jacqueline McCormick
- , P. McCourt
- , Celeste McCracken
- , Lorcan McGarvey
- , Jade McGinness
- , K. McGlynn
- , Andrew McGovern
- , Heather McGuinness
- , I. B. McInnes
- , Jerome McIntosh
- , Emma McIvor
- , Katherine McIvor
- , Laura McLeavey
- , Aisling McMahon
- , Michael McMahon
- , L. McMorrow
- , Teresa Mcnally
- , M. McNarry
- , J. McNeill
- , Alison McQueen
- , H. McShane
- , Chloe Mears
- , Clare Megson
- , Sharon Megson
- , J. Meiring
- , Lucy Melling
- , Mark Mencias
- , Daniel Menzies
- , Marta Merida Morillas
- , Alice Michael
- , Benedict Michael
- , C. A. Miller
- , Lea Milligan
- , Nicholas Mills
- , Clare Mills
- , George Mills
- , L. Milner
- , Jane Mitchell
- , Abdelrahman Mohamed
- , Noura Mohamed
- , S. Mohammed
- , Philip Molyneaux
- , Will Monteiro
- , Silvia Moriera
- , Anna Morley
- , Leigh Morrison
- , Richard Morriss
- , A. Morrow
- , Paul Moss
- , Alistair Moss
- , K. Motohashi
- , N. Msimanga
- , Elizabeta Mukaetova-Ladinska
- , Unber Munawar
- , Jennifer Murira
- , Uttam Nanda
- , Heeah Nassa
- , Mariam Nasseri
- , Rashmita Nathu
- , Aoife Neal
- , Robert Needham
- , Paula Neill
- , Stefan Neubauer
- , D. E. Newby
- , Helen Newell
- , J. Newman
- , Tom Newman
- , Alex Newton-Cox
- , T. E. Nichols
- , Tim Nicholson
- , Christos Nicolaou
- , Debby Nicoll
- , Athanasios Nikolaidis
- , C. Nikolaidou
- , C. M. Nolan
- , Matthew Noonan
- , C. Norman
- , Petr Novotny
- , Kimon Ntotsis
- , Jose Nunag
- , Lorenza Nwafor
- , Uchechi Nwanguma
- , Joseph Nyaboko
- , Linda O’Brien
- , C. O’Brien
- , Natasha Odell
- , Kate O’Donnell
- , Godwin Ogbole
- , Olaoluwa Olaosebikan
- , Catherine Oliver
- , Zohra Omar
- , Peter J. M. Openshaw
- , D. P. O’Regan
- , Lorna Orriss-Dib
- , Lynn Osborne
- , Rebecca Osbourne
- , Marlies Ostermann
- , Charlotte Overton
- , Jamie Pack
- , Edmund Pacpaco
- , Stella-Maria Paddick
- , Sharon Painter
- , Erola Pairo-Castineira
- , Ashkan Pakzad
- , Sue Palmer
- , Padmasayee Papineni
- , K. Paques
- , Kerry Paradowski
- , Manish Pareek
- , Dhruv Parekh
- , H. Parfrey
- , Carmen Pariante
- , S. Parker
- , M. Parkes
- , J. Parmar
- , Sheetal Patale
- , Manish Patel
- , Suhani Patel
- , Dibya Pattenadk
- , M. Pavlides
- , Sheila Payne
- , Lorraine Pearce
- , John Pearl
- , Dan Peckham
- , Jessica Pendlebury
- , Yanchun Peng
- , Chris Pennington
- , Ida Peralta
- , Emma Perkins
- , Z. Peterkin
- , Tunde Peto
- , Nayia Petousi
- , John Petrie
- , Paul Pfeffer
- , Janet Phipps
- , S. Piechnik
- , John Pimm
- , Karen Piper Hanley
- , Riinu Pius
- , Hannah Plant
- , Tatiana Plekhanova
- , Megan Plowright
- , Krisnah Poinasamy
- , Oliver Polgar
- , Julie Porter
- , Joanna Porter
- , Sofiya Portukhay
- , Natassia Powell
- , A. Prabhu
- , James Pratt
- , Andrea Price
- , Claire Price
- , Carly Price
- , Anne Prickett
- , I. Propescu
- , J. Propescu
- , Sabrina Prosper
- , S. Pugmire
- , Sheena Quaid
- , Jackie Quigley
- , Jennifer K. Quint
- , H. Qureshi
- , I. N. Qureshi
- , K. Radhakrishnan
- , Najib Rahman
- , Markus Ralser
- , Betty Raman
- , Hazel Ramos
- , Albert Ramos
- , Jade Rangeley
- , Bojidar Rangelov
- , Liz Ratcliffe
- , Phillip Ravencroft
- , Konrad Rawlik
- , Anne Reddington
- , Heidi Redfearn
- , Dawn Redwood
- , Annabel Reed
- , Meryl Rees
- , Tabitha Rees
- , Karen Regan
- , Will Reynolds
- , Carla Ribeiro
- , A. Richards
- , Emma Richardson
- , M. Richardson
- , Pilar Rivera-Ortega
- , K. Roberts
- , Elizabeth Robertson
- , Leanne Robinson
- , Emma Robinson
- , Lisa Roche
- , C. Roddis
- , J. Rodger
- , Natalie Rogers
- , Gavin Ross
- , Alexandra Ross
- , Jennifer Rossdale
- , Anthony Rostron
- , Anna Rowe
- , J. Rowland
- , M. J. Rowland
- , A. Rowland
- , Sarah L. Rowland-Jones
- , Maura Roy
- , Igor Rudan
- , Richard Russell
- , Emily Russell
- , Gwen Saalmink
- , Ramsey Sabit
- , Beth Sage
- , T. Samakomva
- , Nilesh Samani
- , A. A. Samat
- , Claire Sampson
- , Katherine Samuel
- , Reena Samuel
- , Z. B. Sanders
- , Amy Sanderson
- , Elizabeth Sapey
- , Dinesh Saralaya
- , Jack Sargant
- , Carol Sarginson
- , Naveed Sattar
- , Kathryn Saunders
- , Peter Saunders
- , Ruth Saunders
- , Laura Saunders
- , Heather Savill
- , Avan Sayer
- , J. Schronce
- , William Schwaeble
- , Janet Scott
- , Kathryn Scott
- , Nick Selby
- , Malcolm G. Semple
- , Marco Sereno
- , Terri Ann Sewell
- , Kamini Shah
- , Ajay Shah
- , Manu Shankar-Hari
- , M. Sharma
- , Claire Sharpe
- , Michael Sharpe
- , Sharlene Shashaa
- , Alison Shaw
- , Victoria Shaw
- , Karen Shaw
- , Aziz Sheikh
- , Sarah Shelton
- , Liz Shenton
- , K. Shevket
- , Aarti Shikotra
- , Sulman Siddique
- , Salman Siddiqui
- , J. Sidebottom
- , Louise Sigfrid
- , Gemma Simons
- , Neil Simpson
- , John Simpson
- , Ananga Singapuri
- , Suver Singh
- , Claire Singh
- , Sally Singh
- , D. Sissons
- , J. Skeemer
- , Katie Slack
- , David Smith
- , Nikki Smith
- , Andrew Smith
- , Jacqui Smith
- , Laurie Smith
- , Susan Smith
- , M. Soares
- , Teresa Solano
- , Reanne Solly
- , A. R. Solstice
- , Tracy Soulsby
- , David Southern
- , D. Sowter
- , Mark Spears
- , Lisa Spencer
- , Fabio Speranza
- , Louise Stadon
- , Stefan Stanel
- , R. Steeds
- , N. Steele
- , Mike Steiner
- , David Stensel
- , G. Stephens
- , Lorraine Stephenson
- , Iain Stewart
- , R. Stimpson
- , Sue Stockdale
- , J. Stockley
- , Wendy Stoker
- , Roisin Stone
- , Will Storrar
- , Andrew Storrie
- , Kim Storton
- , E. Stringer
- , Sophia Strong-Sheldrake
- , Natalie Stroud
- , Christian Subbe
- , Catherine Sudlow
- , Zehra Suleiman
- , Charlotte Summers
- , C. Summersgill
- , Debbie Sutherland
- , D. L. Sykes
- , Nick Talbot
- , Ai Lyn Tan
- , Lawrence Tarusan
- , Vera Tavoukjian
- , Jessica Taylor
- , Abigail Taylor
- , Chris Taylor
- , John Paul Taylor
- , Amelie Te
- , Caroline Tee
- , J. Teixeira
- , Helen Tench
- , Sarah Terry
- , Susannah Thackray-Nocera
- , Favas Thaivalappil
- , David Thickett
- , David Thomas
- , S. Thomas
- , Caradog Thomas
- , Andrew Thomas
- , T. Thomas-Woods
- , A. A. Roger Thompson
- , Tamika Thompson
- , T. Thornton
- , Matthew Thorpe
- , Ryan S. Thwaites
- , Jo Tilley
- , N. Tinker
- , Gerlynn Tiongson
- , Martin Tobin
- , Johanne Tomlinson
- , Mark Toshner
- , T. Treibel
- , K. A. Tripp
- , Drupad Trivedi
- , E. M. Tunnicliffe
- , Alison Turnbull
- , Kim Turner
- , Sarah Turner
- , Victoria Turner
- , E. Turner
- , Sharon Turney
- , Lance Turtle
- , Helena Turton
- , Jacinta Ugoji
- , R. Ugwuoke
- , Rachel Upthegrove
- , Jonathon Valabhji
- , Maximina Ventura
- , Joanne Vere
- , Carinna Vickers
- , Ben Vinson
- , Ioannis Vogiatzis
- , Elaine Wade
- , Phillip Wade
- , Louise V. Wain
- , Tania Wainwright
- , Lilian Wajero
- , Sinead Walder
- , Samantha Walker
- , S. Walker
- , Tim Wallis
- , Sarah Walmsley
- , Simon Walsh
- , J. A. Walsh
- , Louise Warburton
- , T. J. C. Ward
- , Katie Warwick
- , Helen Wassall
- , Samuel Waterson
- , L. Watson
- , Ekaterina Watson
- , James Watson
- , M. Webster
- , J. Weir McCall
- , Carly Welch
- , Simon Wessely
- , Sophie West
- , Heather Weston
- , Helen Wheeler
- , Sonia White
- , Victoria Whitehead
- , J. Whitney
- , S. Whittaker
- , Beverley Whittam
- , V. Whitworth
- , Andrew Wight
- , James Wild
- , Martin Wilkins
- , Dan Wilkinson
- , Nick Williams
- , N. Williams
- , B. Williams
- , Jenny Williams
- , S. A. Williams-Howard
- , Michelle Willicombe
- , Gemma Willis
- , James Willoughby
- , Ann Wilson
- , Imogen Wilson
- , Daisy Wilson
- , Nicola Window
- , M. Witham
- , Rebecca Wolf-Roberts
- , Chloe Wood
- , F. Woodhead
- , Janet Woods
- , Dan Wootton
- , J. Wormleighton
- , J. Worsley
- , David Wraith
- , Caroline Wrey Brown
- , C. Wright
- , S. Wright
- , Louise Wright
- , Inez Wynter
- , Moucheng Xu
- , Najira Yasmin
- , S. Yasmin
- , Tom Yates
- , Kay Por Yip
- , Susan Young
- , Bob Young
- , A. J. Yousuf
- , Amira Zawia
- , Lisa Zeidan
- , Bang Zhao
- , Bang Zheng
- & O. Zongo
- , Daniel Agranoff
- , Ken Agwuh
- , Katie A. Ahmed
- , Dhiraj Ail
- , Erin L. Aldera
- , Ana Alegria
- , Beatrice Alex
- , Sam Allen
- , Petros Andrikopoulos
- , Brian Angus
- , Jane A. Armstrong
- , Abdul Ashish
- , Milton Ashworth
- , Innocent G. Asiimwe
- , Dougal Atkinson
- , Benjamin Bach
- , Siddharth Bakshi
- , Wendy S. Barclay
- , Shahedal Bari
- , Gavin Barlow
- , Samantha L. Barlow
- , Stella Barnass
- , Nicholas Barrett
- , Christopher Bassford
- , Sneha Basude
- , David Baxter
- , Michael Beadsworth
- , Jolanta Bernatoniene
- , John Berridge
- , Nicola Best
- , Debby Bogaert
- , Laura Booth
- , Pieter Bothma
- , Benjamin Brennan
- , Robin Brittain-Long
- , Katie Bullock
- , Naomi Bulteel
- , Tom Burden
- , Andrew Burtenshaw
- , Nicola Carlucci
- , Gail Carson
- , Vikki Caruth
- , Emily Cass
- , Benjamin W. A. Catterall
- , David Chadwick
- , Duncan Chambler
- , Meera Chand
- , Kanta Chechi
- , Nigel Chee
- , Jenny Child
- , Srikanth Chukkambotla
- , Richard Clark
- , Tom Clark
- , Jordan J. Clark
- , Emily A. Clarke
- , Sara Clohisey
- , Sarah Cole
- , Paul Collini
- , Marie Connor
- , Graham S. Cooke
- , Louise Cooper
- , Catherine Cosgrove
- , Audrey Coutts
- , Helen Cox
- , Jason Cupitt
- , Maria-Teresa Cutino-Moguel
- , Ana da Silva Filipe
- , Jo Dalton
- , Paul Dark
- , Christopher Davis
- , Chris Dawson
- , Thushan de Silva
- , Samir Dervisevic
- , Oslem Dincarslan
- , Alejandra Doce Carracedo
- , Cara Donegan
- , Lorna Donelly
- , Phil Donnison
- , Chloe Donohue
- , Gonçalo dos Santos Correia
- , Sam Douthwaite
- , Thomas M. Drake
- , Andrew Drummond
- , Marc-Emmanuel Dumas
- , Chris Dunn
- , Jake Dunning
- , Ingrid DuRand
- , Ahilanadan Dushianthan
- , Tristan Dyer
- , Philip Dyer
- , Angela Elliott
- , Cariad Evans
- , Anthony Evans
- , Chi Eziefula
- , Cameron J. Fairfield
- , Angie Fawkes
- , Chrisopher Fegan
- , Lorna Finch
- , Adam Finn
- , Lewis W. S. Fisher
- , Lisa Flaherty
- , Tom Fletcher
- , Terry Foster
- , Duncan Fullerton
- , Carrol Gamble
- , Isabel Garcia-Dorival
- , Atul Garg
- , Sanjeev Garg
- , Tammy Gilchrist
- , Michelle Girvan
- , Effrossyni Gkrania-Klotsas
- , Jo Godden
- , Arthur Goldsmith
- , Clive Graham
- , Tassos Grammatikopoulos
- , Christopher A. Green
- , Julian Griffin
- , Fiona Griffiths
- , Philip Gunning
- , Rishi K. Gupta
- , Katarzyna Hafezi
- , Sophie Halpin
- , Elaine Hardy
- , Ewen M. Harrison
- , Janet Harrison
- , Catherine Hartley
- , Stuart Hartshorn
- , Daniel Harvey
- , Peter Havalda
- , Daniel B. Hawcutt
- , Ross Hendry
- , Antonia Y. W. Ho
- , Maria Hobrok
- , Luke Hodgson
- , Karl Holden
- , Anthony Holmes
- , Peter W. Horby
- , Joanne Howard
- , Samreen Ijaz
- , Clare Jackson
- , Michael Jacobs
- , Susan Jain
- , Paul Jennings
- , Rebecca L. Jensen
- , Christopher B. Jones
- , Trevor R. Jones
- , Agilan Kaliappan
- , Vidya Kasipandian
- , Seán Keating
- , Stephen Kegg
- , Michael Kelsey
- , Jason Kendall
- , Caroline Kerrison
- , Ian Kerslake
- , Shadia Khandaker
- , Katharine King
- , Robyn T. Kiy
- , Stephen R. Knight
- , Susan Knight
- , Oliver Koch
- , Gouri Koduri
- , George Koshy
- , Chrysa Koukorava
- , Shondipon Laha
- , Eva Lahnsteiner
- , Steven Laird
- , Annette Lake
- , Suzannah Lant
- , Susan Larkin
- , Diane Latawiec
- , Andrew Law
- , James Lee
- , Gary Leeming
- , Daniella Lefteri
- , Tamas Leiner
- , Lauren Lett
- , Matthew Lewis
- , Sonia Liggi
- , Patrick Lillie
- , Wei Shen Lim
- , James Limb
- , Vanessa Linnett
- , Jeff Little
- , Lucia A. Livoti
- , Mark Lyttle
- , Louise MacGillivray
- , Alan Maclean
- , Michael MacMahon
- , Emily MacNaughton
- , Maria Mancini
- , Ravish Mankregod
- , Laura Marsh
- , Lynn Maslen
- , Hannah Massey
- , Huw Masson
- , Elijah Matovu
- , Nicole Maziere
- , Sarah McCafferty
- , Katherine McCullough
- , Sarah E. McDonald
- , Sarah McDonald
- , Laurence McEvoy
- , Ruth McEwen
- , John McLauchlan
- , Kenneth A. Mclean
- , Manjula Meda
- , Alexander J. Mentzer
- , Laura Merson
- , Soeren Metelmann
- , Alison M. Meynert
- , Nahida S. Miah
- , Joanna Middleton
- , Gary Mills
- , Jane Minton
- , Joyce Mitchell
- , Kavya Mohandas
- , James Moon
- , Elinoor Moore
- , Shona C. Moore
- , Patrick Morgan
- , Kirstie Morrice
- , Craig Morris
- , Katherine Mortimore
- , Samuel Moses
- , Mbiye Mpenge
- , Rohinton Mulla
- , Derek Murphy
- , Lee Murphy
- , Michael Murphy
- , Ellen G. Murphy
- , Thapas Nagarajan
- , Megan Nagel
- , Mark Nelson
- , Lisa Norman
- , Lillian Norris
- , Lucy Norris
- , Mahdad Noursadeghi
- , Michael Olanipekun
- , Wilna Oosthuyzen
- , Anthonia Osagie
- , Matthew K. O’Shea
- , Igor Otahal
- , Mark Pais
- , Massimo Palmarini
- , Carlo Palmieri
- , Selva Panchatsharam
- , Danai Papakonstantinou
- , Hassan Paraiso
- , Brij Patel
- , Natalie Pattison
- , William A. Paxton
- , Rebekah Penrice-Randal
- , Justin Pepperell
- , Mark Peters
- , Mandeep Phull
- , Jack Pilgrim
- , Stefania Pintus
- , Tim Planche
- , Daniel Plotkin
- , Georgios Pollakis
- , Frank Post
- , Nicholas Price
- , David Price
- , Tessa Prince
- , Rachel Prout
- , Nikolas Rae
- , Andrew Rambaut
- , Henrik Reschreiter
- , Tim Reynolds
- , Neil Richardson
- , P. Matthew Ridley
- , Mark Roberts
- , Stephanie Roberts
- , Devender Roberts
- , David L. Robertson
- , Alistair Rose
- , Guy Rousseau
- , Bobby Ruge
- , Clark D. Russell
- , Brendan Ryan
- , Debby Sales
- , Taranprit Saluja
- , Vanessa Sancho-Shimizu
- , Caroline Sands
- , Egle Saviciute
- , Matthias Schmid
- , Janet T. Scott
- , James Scott-Brown
- , Aarti Shah
- , Prad Shanmuga
- , Anil Sharma
- , Catherine A. Shaw
- , Victoria E. Shaw
- , Anna Shawcross
- , Rebecca K. Shears
- , Jagtur Singh Pooni
- , Jeremy Sizer
- , Benjamin Small
- , Richard Smith
- , Catherine Snelson
- , Tom Solomon
- , Rebecca G. Spencer
- , Nick Spittle
- , Shiranee Sriskandan
- , Nikki Staines
- , Tom Stambach
- , Richard Stewart
- , David Stuart
- , Krishanthi S. Subramaniam
- , Pradeep Subudhi
- , Olivia V. Swann
- , Tamas Szakmany
- , Agnieska Szemiel
- , Aislynn Taggart
- , Sarah Tait
- , Zoltan Takats
- , Panteleimon Takis
- , Jolanta Tanianis-Hughes
- , Kate Tatham
- , Richard S. Tedder
- , Jo Thomas
- , Jordan Thomas
- , Robert Thompson
- , Chris Thompson
- , Emma C. Thomson
- , Ascanio Tridente
- , Erwan Trochu
- , Darell Tupper-Carey
- , Lance C. W. Turtle
- , Mary Twagira
- , Nick Vallotton
- , Libby van Tonder
- , Rama Vancheeswaran
- , Rachel Vincent
- , Lisa Vincent-Smith
- , Shico Visuvanathan
- , Alan Vuylsteke
- , Sam Waddy
- , Rachel Wake
- , Andrew Walden
- , Ingeborg Welters
- , Murray Wham
- , Tony Whitehouse
- , Paul Whittaker
- , Ashley Whittington
- , Meme Wijesinghe
- , Eve Wilcock
- , Martin Williams
- , Lawrence Wilson
- , Stephen Winchester
- , Martin Wiselka
- , Adam Wolverson
- , Daniel G. Wootton
- , Andrew Workman
- , Nicola Wrobel
- , Bryan Yates
- , Peter Young
- , Maria Zambon
- & J. Eunice Zhang
Contributions
F.L. recruited participants, acquired clinical samples, analyzed and interpreted data and cowrote the manuscript, including all drafting and revisions. C.E. analyzed and interpreted data and cowrote this manuscript, including all drafting and revisions. S.F. and M.R. supported the analysis and interpretation of data as well as drafting and revisions. D.S., J.K.S., S.C.M., S.A., N.M., J.N., C.K., O.C.L., O.E., H.J.C.M., A. Shikotra, A. Singapuri, M.S., V.C.H., M.T., N.J.G., N.I.L. and C.C. contributed to acquisition of data underlying this study. L.H.-W., A.A.R.T., S.L.R.-J., L.S.H., O.M.K., D.G.W., T.I.d.S. and A. Ho made substantial contributions to conception/design and implementation of this work and/or acquisition of clinical samples for this work. They have supported drafting and revisions of the manuscript. E.M.H., J.K.Q. and A.B.D. made substantial contributions to the study design as well as data access, linkage and analysis. They have supported drafting and revisions of this work. J.D.C., L.-P.H., A. Horsley, B.R., K.P., M.M. and W.G. made substantial contributions to the conception and design of this work and have supported drafting and revisions of this work. J.K.B. obtained funding for ISARIC4C, is ISARIC4C consortium co-lead, has made substantial contributions to conception and design of this work and has supported drafting and revisions of this work. M.G.S. obtained funding for ISARIC4C, is ISARIC4C consortium co-lead, sponsor/protocol chief investigator, has made substantial contributions to conception and design of this work and has supported drafting and revisions of this work. R.A.E. and L.V.W. are co-leads of PHOSP-COVID, made substantial contributions to conception and design of this work, the acquisition and analysis of data, and have supported drafting and revisions of this work. C.B. is the chief investigator of PHOSP-COVID and has made substantial contributions to conception and design of this work. R.S.T. and L.T. made substantial contributions to the acquisition, analysis and interpretation of the data underlying this study and have contributed to drafting and revisions of this work. P.J.M.O. obtained funding for ISARIC4C, is ISARIC4C consortium co-lead, sponsor/protocol chief investigator and has made substantial contributions to conception and design of this work. R.S.T. and P.J.M.O. have also made key contributions to interpretation of data and have co-written this manuscript. All authors have read and approve the final version to be published. All authors agree to accountability for all aspects of this work. All investigators within ISARIC4C and the PHOSP-COVID consortia have made substantial contributions to the conception or design of this study and/or acquisition of data for this study. The full list of authors within these groups is available in Supplementary Information .
Corresponding authors
Correspondence to Ryan S. Thwaites or Peter J. M. Openshaw .
Ethics declarations
Competing interests.
F.L., C.E., D.S., J.K.S., S.C.M., C.D., C.K., N.M., L.N., E.M.H., A.B.D., J.K.Q., L.-P.H., K.P., L.S.H., O.M.K., S.F., T.I.d.S., D.G.W., R.S.T. and J.K.B. have no conflicts of interest. A.A.R.T. receives speaker fees and support to attend meetings from Janssen Pharmaceuticals. S.L.R.-J. is on the data safety monitoring board for Bexero trial in HIV+ adults in Kenya. J.D.C. is the deputy chief editor of the European Respiratory Journal and receives consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Insmed, Janssen, Novartis, Pfizer and Zambon. A. Horsley is deputy chair of NIHR Translational Research Collaboration (unpaid role). B.R. receives honoraria from Axcella therapeutics. R.A.E. is co-lead of PHOSP-COVID and receives fees from AstraZenaca/Evidera for consultancy on LC and from AstraZenaca for consultancy on digital health. R.A.E. has received speaker fees from Boehringer in June 2021 and has held a role as European Respiratory Society Assembly 01.02 Pulmonary Rehabilitation secretary. R.A.E. is on the American Thoracic Society Pulmonary Rehabilitation Assembly program committee. L.V.W. also receives funding from Orion pharma and GSK and holds contracts with Genentech and AstraZenaca. L.V.W. has received consulting fees from Galapagos and Boehringer, is on the data advisory board for Galapagos and is Associate Editor for the European Respiratory Journal . A. Ho is a member of NIHR Urgent Public Health Group (June 2020–March 2021). M.M. is an applicant on the PHOSP study funded by NIHR/DHSC. M.G.S. acts as an independent external and nonremunerated member of Pfizer’s External Data Monitoring Committee for their mRNA vaccine program(s), is Chair of Infectious Disease Scientific Advisory Board of Integrum Scientific LLC, and is director of MedEx Solutions Ltd. and majority owner of MedEx Solutions Ltd. and minority owner of Integrum Scientific LLC. M.G.S.’s institution has been in receipt of gifts from Chiesi Farmaceutici S.p.A. of Clinical Trial Investigational Medicinal Product without encumbrance and distribution of same to trial sites. M.G.S. is a nonrenumerated member of HMG UK New Emerging Respiratory Virus Threats Advisory Group and has previously been a nonrenumerated member of the Scientific Advisory Group for Emergencies (SAGE). C.B. has received consulting fees and/or grants from GSK, AstraZeneca, Genentech, Roche, Novartis, Sanofi, Regeneron, Chiesi, Mologic and 4DPharma. L.T. has received consulting fees from MHRA, AstraZeneca and Synairgen and speakers’ fees from Eisai Ltd., and support for conference attendance from AstraZeneca. L.T. has a patent pending with ZikaVac. P.J.M.O. reports grants from the EU Innovative Medicines Initiative 2 Joint Undertaking during the submitted work; grants from UK Medical Research Council, GSK, Wellcome Trust, EU Innovative Medicines Initiative, UK National Institute for Health Research and UK Research and Innovation–Department for Business, Energy and Industrial Strategy; and personal fees from Pfizer, Janssen and Seqirus, outside the submitted work.
Peer review
Peer review information.
Nature Immunology thanks Ziyad Al-Aly and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Ioana Staicu was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 penalized logistic regression performance..
Graphs show classification error and Area under curve (AUC) from the 50 repeats tenfold nested cross-validation used to optimise and assess the performance of PLR testing associations with each LC outcome relative to Recovered (n = 233): Cardio_Resp (n = 398), Fatigue (n = 384), Anxiety/Depression (n = 202), GI (n = 132), ( e ) Cognitive (n = 6). The distributions of classification error and area under curve (AUC) from the nested cross-validation are shown. Box plot centre line represents the Median and boundaries of the box represent interquartile range (IQR), the whisker length represent 1.5xIQR.
Extended Data Fig. 2 Associations with long COVID symptoms in full study cohort.
( a ) Fibrinogen levels at 6 months were compared between pooled LC cases (n = 295) and Recovered (n = 233) and between the Cognitive group (n = 41) and Recovered (n = 233). Box plot centre line represent the Median and boundaries of the box represent interquartile range (IQR), the whisker length represents 1.5xIQR, any outliers beyond the whisker range are shown as individual dots. Median differences were compared using two-sided Wilcoxon signed-rank test *= p < 0·05, **= p < 0·01, ***= p < 0·001, ****= p < 0·0001. Unadjusted p-values are reported. b ) Distribution of time from COVID-19 hospitalisation at sample collection applying CDC and NICE definitions of LC (n = 719) ( c ) Upset plot of symptom groups. Horizontal coloured bars represent the number of patients in each symptom group: Cardiorespiratory (Cardio_Resp), Fatigue, Cognitive, Gastrointestinal (GI) and Anxiety/Depression (Anx_Dep). Vertical black bars represent the number of patients in each symptom combination group. To prevent patient identification, where less than 5 patients belong to a combination group, this has been represented as ‘<5’. The Recovered group (n = 250) were used as controls. Forest plots show Olink protein concentrations (NPX) associated with ( d ) Cardio_Resp (n = 398), ( e ) Fatigue (n = 342), ( f ) Anx_Dep (n = 219), ( g ) GI (n = 134), and ( h ) Cognitive (n = 65). Error bars represent the median accuracy of the model.
Extended Data Fig. 3 Validation of olink measurements using conventional assays in plasma.
Olink measured protein (NPX) were compared to chemiluminescence assays (ECL or ELISA, log2[pg/mL]) to validate our findings, where contemporaneously collected plasma samples were available (n = 58). Results from key mediators associated with LC groups were validated: CSF3, IL1R2, IL2, IL3RA, TNFa, TFF2. R = spearman rank correlation coefficient and shaded areas indicated the 95% confidence interval. Samples that fell below the lower limit of detection for a given assay were excluded and the ‘n’ value on each panel indicates the number of samples above this limit.
Extended Data Fig. 4 Univariate analysis of proteins associated with each symptom.
Olink measured plasma protein levels (NPX) compared between LC groups (Cardio_Resp, n = 398, Fatigue n = 384, Anxiety/Depression, n = 202, GI, n = 132 and Cognitive, n = 60) and Recovered (n = 233). Proteins identified by PLR were compared between groups. Median differences were compared using two-sided Wilcoxon signed-rank test. * = p < 0·05, ** = p < 0·01, *** = p < 0·001, ****= p < 0·0001 after FDR adjustment. Box plot centre line represent the Median and boundaries of the box represent interquartile range (IQR), the whisker length represents 1.5xIQR, any outliers beyond the whisker range are shown as individual dots.
Extended Data Fig. 5 Unadjusted Penalised Logistic Regression.
Olink measured proteins (NPX) and their association with Cardio_Resp (n = 398), Fatigue (n = 342), Anx_Dep (n = 219), GI (n = 134), and Cognitive (n = 65). Forest plots show odds of each LC outcome vs Recovered (n = 233), using PLR without adjusting for clinical co-variates. Error bars represent the median accuracy of the model.
Extended Data Fig. 6 Partial Least Squares analysis.
Olink measured proteins (NPX) and their association with Cardio_Resp (n = 398), Fatigue (n = 342), Anx_Dep (n = 219), GI (n = 134), and Cognitive (n = 65) groups. Forest plots show odds of LC outcome vs Recovered (n = 233), using PLS analysis. Error bars represent the standard error of the coefficient estimate.
Extended Data Fig. 7 Network analysis centrality.
Each graph shows the centrality score for each Olink measured protein (NPX) found to have significant associations with other proteins that were elevated in the Cardio_Resp (n = 398), Fatigue (n = 342), Anx_Dep (n = 219), GI (n = 134), and Cognitive (n = 65) groups relative to Recovered (n = 233).
Extended Data Fig. 8 Inflammation in men and women with long COVID.
Olink measured plasma protein levels (NPX) between men and women with symptoms, divided by age (<50 or >=50years): (a) shows IL1R2 and MATN2 in the Anxiety/Depression group (<50 n = 55, >=50 n = 133), (b) shows CTSO and NFASC in the Cognitive group (<50 n = 11, >=50 n = 50). Median values were compared between men and women using two-sided Wilcoxon signed-rank test. Box plot centre line represent the Median and boundaries represent interquartile range (IQR), the whisker length represents 1.5xIQR.
Extended Data Fig. 9 Inflammation in the upper respiratory tract.
Nasal cytokines measured by immunoassay in the CardioResp Group (n = 29) and Recovered (n = 31): ( a ) shows IL1a, IL1b, IL-6, APO-2, TGFa, TFF2. Median differences were compared using two-sided Wilcoxon signed-rank test. Box plot centre line represents the Median and boundaries of the box represent interquartile range (IQR), the whisker length represent 1.5xIQR. ( b ) Shows cytokines measured by immunoassay in paired plasma and nasal (n = 70). Correlations between IL1a, IL1b, IL-6, APO-2, TGFa and TFF2 in nasal and plasma samples were compared using Spearman’s rank correlation coefficient ( R ). Shaded areas indicated the 95% confidence interval of R.
Extended Data Fig. 10 Graphical abstract.
Summary of interpretation of key findings from Olink measured proteins and their association with CardioResp (n = 398), Fatigue (n = 342), Anx/Dep (n = 219), GI (n = 134), and Cognitive (n = 65) groups relative to Recovered (n = 233).
Supplementary information
Supplementary information.
Supplementary Methods, Statistics and reproducibility statement, Supplementary Results, Supplementary Tables 1–7, Extended data figure legends, Appendix 1 (Supplementary Table 8), Appendix 2 (PHOSP-COVID author list) and Appendix 3 (ISARIC4C author list).
Reporting Summary
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Liew, F., Efstathiou, C., Fontanella, S. et al. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat Immunol (2024). https://doi.org/10.1038/s41590-024-01778-0
Download citation
Received : 11 August 2023
Accepted : 06 February 2024
Published : 08 April 2024
DOI : https://doi.org/10.1038/s41590-024-01778-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 12 December 2023
Examining the role of community resilience and social capital on mental health in public health emergency and disaster response: a scoping review
- C. E. Hall 1 , 2 ,
- H. Wehling 1 ,
- J. Stansfield 3 ,
- J. South 3 ,
- S. K. Brooks 2 ,
- N. Greenberg 2 , 4 ,
- R. Amlôt 1 &
- D. Weston 1
BMC Public Health volume 23 , Article number: 2482 ( 2023 ) Cite this article
1772 Accesses
21 Altmetric
Metrics details
The ability of the public to remain psychologically resilient in the face of public health emergencies and disasters (such as the COVID-19 pandemic) is a key factor in the effectiveness of a national response to such events. Community resilience and social capital are often perceived as beneficial and ensuring that a community is socially and psychologically resilient may aid emergency response and recovery. This review presents a synthesis of literature which answers the following research questions: How are community resilience and social capital quantified in research?; What is the impact of community resilience on mental wellbeing?; What is the impact of infectious disease outbreaks, disasters and emergencies on community resilience and social capital?; and, What types of interventions enhance community resilience and social capital?
A scoping review procedure was followed. Searches were run across Medline, PsycInfo, and EMBASE, with search terms covering both community resilience and social capital, public health emergencies, and mental health. 26 papers met the inclusion criteria.
The majority of retained papers originated in the USA, used a survey methodology to collect data, and involved a natural disaster. There was no common method for measuring community resilience or social capital. The association between community resilience and social capital with mental health was regarded as positive in most cases. However, we found that community resilience, and social capital, were initially negatively impacted by public health emergencies and enhanced by social group activities.
Several key recommendations are proposed based on the outcomes from the review, which include: the need for a standardised and validated approach to measuring both community resilience and social capital; that there should be enhanced effort to improve preparedness to public health emergencies in communities by gauging current levels of community resilience and social capital; that community resilience and social capital should be bolstered if areas are at risk of disasters or public health emergencies; the need to ensure that suitable short-term support is provided to communities with high resilience in the immediate aftermath of a public health emergency or disaster; the importance of conducting robust evaluation of community resilience initiatives deployed during the COVID-19 pandemic.
Peer Review reports
For the general population, public health emergencies and disasters (e.g., natural disasters; infectious disease outbreaks; Chemical, Biological, Radiological or Nuclear incidents) can give rise to a plethora of negative outcomes relating to both health (e.g. increased mental health problems [ 1 , 2 , 3 , 4 ]) and the economy (e.g., increased unemployment and decreased levels of tourism [ 4 , 5 , 6 ]). COVID-19 is a current, and ongoing, example of a public health emergency which has affected over 421 million individuals worldwide [ 7 ]. The long term implications of COVID-19 are not yet known, but there are likely to be repercussions for physical health, mental health, and other non-health related outcomes for a substantial time to come [ 8 , 9 ]. As a result, it is critical to establish methods which may inform approaches to alleviate the longer-term negative consequences that are likely to emerge in the aftermath of both COVID-19 and any future public health emergency.
The definition of resilience often differs within the literature, but ultimately resilience is considered a dynamic process of adaptation. It is related to processes and capabilities at the individual, community and system level that result in good health and social outcomes, in spite of negative events, serious threats and hazards [ 10 ]. Furthermore, Ziglio [ 10 ] refers to four key types of resilience capacity: adaptive, the ability to withstand and adjust to unfavourable conditions and shocks; absorptive, the ability to withstand but also to recover and manage using available assets and skills; anticipatory, the ability to predict and minimize vulnerability; and transformative, transformative change so that systems better cope with new conditions.
There is no one settled definition of community resilience (CR). However, it generally relates to the ability of a community to withstand, adapt and permit growth in adverse circumstances due to social structures, networks and interdependencies within the community [ 11 ]. Social capital (SC) is considered a major determinant of CR [ 12 , 13 ], and reflects strength of a social network, community reciprocity, and trust in people and institutions [ 14 ]. These aspects of community are usually conceptualised primarily as protective factors that enable communities to cope and adapt collectively to threats. SC is often broken down into further categories [ 15 ], for example: cognitive SC (i.e. perceptions of community relations, such as trust, mutual help and attachment) and structural SC (i.e. what actually happens within the community, such as participation, socialising) [ 16 ]; or, bonding SC (i.e. connections among individuals who are emotionally close, and result in bonds to a particular group [ 17 ]) and bridging SC (i.e. acquaintances or individuals loosely connected that span different social groups [ 18 ]). Generally, CR is perceived to be primarily beneficial for multiple reasons (e.g. increased social support [ 18 , 19 ], protection of mental health [ 20 , 21 ]), and strengthening community resilience is a stated health goal of the World Health Organisation [ 22 ] when aiming to alleviate health inequalities and protect wellbeing. This is also reflected by organisations such as Public Health England (now split into the UK Health Security Agency and the Office for Health Improvement and Disparities) [ 23 ] and more recently, CR has been targeted through the endorsement of Community Champions (who are volunteers trained to support and to help improve health and wellbeing. Community Champions also reflect their local communities in terms of population demographics for example age, ethnicity and gender) as part of the COVID-19 response in the UK (e.g. [ 24 , 25 ]).
Despite the vested interest in bolstering communities, the research base establishing: how to understand and measure CR and SC; the effect of CR and SC, both during and following a public health emergency (such as the COVID-19 pandemic); and which types of CR or SC are the most effective to engage, is relatively small. Given the importance of ensuring resilience against, and swift recovery from, public health emergencies, it is critically important to establish and understand the evidence base for these approaches. As a result, the current review sought to answer the following research questions: (1) How are CR and SC quantified in research?; (2) What is the impact of community resilience on mental wellbeing?; (3) What is the impact of infectious disease outbreaks, disasters and emergencies on community resilience and social capital?; and, (4) What types of interventions enhance community resilience and social capital?
By collating research in order to answer these research questions, the authors have been able to propose several key recommendations that could be used to both enhance and evaluate CR and SC effectively to facilitate the long-term recovery from COVID-19, and also to inform the use of CR and SC in any future public health disasters and emergencies.
A scoping review methodology was followed due to the ease of summarising literature on a given topic for policy makers and practitioners [ 26 ], and is detailed in the following sections.
Identification of relevant studies
An initial search strategy was developed by authors CH and DW and included terms which related to: CR and SC, given the absence of a consistent definition of CR, and the link between CR and SC, the review focuses on both CR and SC to identify as much relevant literature as possible (adapted for purpose from Annex 1: [ 27 ], as well as through consultation with review commissioners); public health emergencies and disasters [ 28 , 29 , 30 , 31 ], and psychological wellbeing and recovery (derived a priori from literature). To ensure a focus on both public health and psychological research, the final search was carried across Medline, PsycInfo, and EMBASE using OVID. The final search took place on the 18th of May 2020, the search strategy used for all three databases can be found in Supplementary file 1 .
Selection criteria
The inclusion and exclusion criteria were developed alongside the search strategy. Initially the criteria were relatively inclusive and were subject to iterative development to reflect the authors’ familiarisation with the literature. For example, the decision was taken to exclude research which focused exclusively on social support and did not mention communities as an initial title/abstract search suggested that the majority of this literature did not meet the requirements of our research question.
The full and final inclusion and exclusion criteria used can be found in Supplementary file 2 . In summary, authors decided to focus on the general population (i.e., non-specialist, e.g. non-healthcare worker or government official) to allow the review to remain community focused. The research must also have assessed the impact of CR and/or SC on mental health and wellbeing, resilience, and recovery during and following public health emergencies and infectious disease outbreaks which affect communities (to ensure the research is relevant to the review aims), have conducted primary research, and have a full text available or provided by the first author when contacted.
Charting the data
All papers were first title and abstract screened by CH or DW. Papers then were full text reviewed by CH to ensure each paper met the required eligibility criteria, if unsure about a paper it was also full text reviewed by DW. All papers that were retained post full-text review were subjected to a standardised data extraction procedure. A table was made for the purpose of extracting the following data: title, authors, origin, year of publication, study design, aim, disaster type, sample size and characteristics, variables examined, results, restrictions/limitations, and recommendations. Supplementary file 3 details the charting the data process.
Analytical method
Data was synthesised using a Framework approach [ 32 ], a common method for analysing qualitative research. This method was chosen as it was originally used for large-scale social policy research [ 33 ] as it seeks to identify: what works, for whom, in what conditions, and why [ 34 ]. This approach is also useful for identifying commonalities and differences in qualitative data and potential relationships between different parts of the data [ 33 ]. An a priori framework was established by CH and DW. Extracted data was synthesised in relation to each research question, and the process was iterative to ensure maximum saturation using the available data.
Study selection
The final search strategy yielded 3584 records. Following the removal of duplicates, 2191 records remained and were included in title and abstract screening. A PRISMA flow diagram is presented in Fig. 1 .
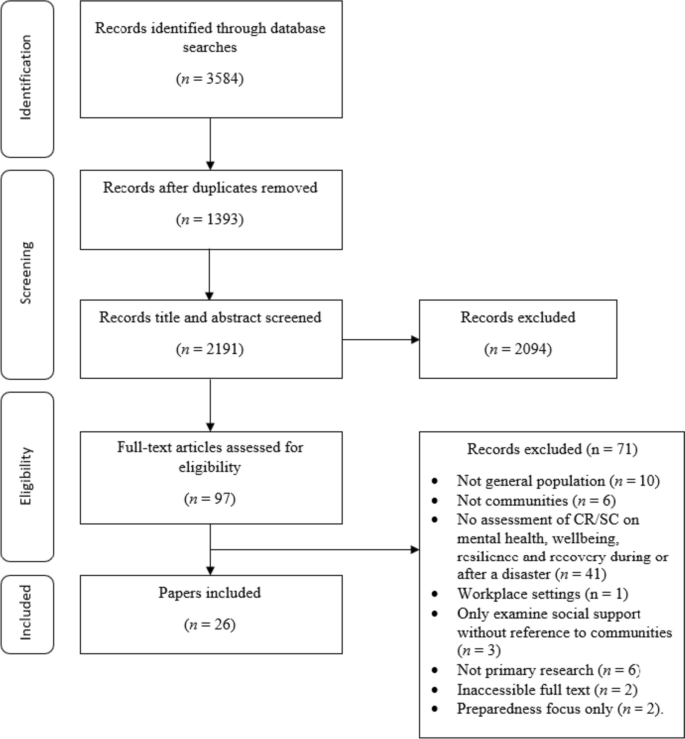
PRISMA flow diagram
At the title and abstract screening stage, the process became more iterative as the inclusion criteria were developed and refined. For the first iteration of screening, CH or DW sorted all records into ‘include,’ ‘exclude,’ and ‘unsure’. All ‘unsure’ papers were re-assessed by CH, and a random selection of ~ 20% of these were also assessed by DW. Where there was disagreement between authors the records were retained, and full text screened. The remaining papers were reviewed by CH, and all records were categorised into ‘include’ and ‘exclude’. Following full-text screening, 26 papers were retained for use in the review.
Study characteristics
This section of the review addresses study characteristics of those which met the inclusion criteria, which comprises: date of publication, country of origin, study design, study location, disaster, and variables examined.
Date of publication
Publication dates across the 26 papers spanned from 2008 to 2020 (see Fig. 2 ). The number of papers published was relatively low and consistent across this timescale (i.e. 1–2 per year, except 2010 and 2013 when none were published) up until 2017 where the number of papers peaked at 5. From 2017 to 2020 there were 15 papers published in total. The amount of papers published in recent years suggests a shift in research and interest towards CR and SC in a disaster/ public health emergency context.
Graph to show retained papers date of publication
Country of origin
The locations of the first authors’ institutes at the time of publication were extracted to provide a geographical spread of the retained papers. The majority originated from the USA [ 35 , 36 , 37 , 38 , 39 , 40 , 41 ], followed by China [ 42 , 43 , 44 , 45 , 46 ], Japan [ 47 , 48 , 49 , 50 ], Australia [ 51 , 52 , 53 ], The Netherlands [ 54 , 55 ], New Zealand [ 56 ], Peru [ 57 ], Iran [ 58 ], Austria [ 59 ], and Croatia [ 60 ].
There were multiple methodological approaches carried out across retained papers. The most common formats included surveys or questionnaires [ 36 , 37 , 38 , 42 , 46 , 47 , 48 , 49 , 50 , 53 , 54 , 55 , 57 , 59 ], followed by interviews [ 39 , 40 , 43 , 51 , 52 , 60 ]. Four papers used both surveys and interviews [ 35 , 41 , 45 , 58 ], and two papers conducted data analysis (one using open access data from a Social Survey [ 44 ] and one using a Primary Health Organisations Register [ 56 ]).
Study location
The majority of the studies were carried out in Japan [ 36 , 42 , 44 , 47 , 48 , 49 , 50 ], followed by the USA [ 35 , 37 , 38 , 39 , 40 , 41 ], China [ 43 , 45 , 46 , 53 ], Australia [ 51 , 52 ], and the UK [ 54 , 55 ]. The remaining studies were carried out in Croatia [ 60 ], Peru [ 57 ], Austria [ 59 ], New Zealand [ 56 ] and Iran [ 58 ].
Multiple different types of disaster were researched across the retained papers. Earthquakes were the most common type of disaster examined [ 45 , 47 , 49 , 50 , 53 , 56 , 57 , 58 ], followed by research which assessed the impact of two disastrous events which had happened in the same area (e.g. Hurricane Katrina and the Deepwater Horizon oil spill in Mississippi, and the Great East Japan earthquake and Tsunami; [ 36 , 37 , 38 , 42 , 44 , 48 ]). Other disaster types included: flooding [ 51 , 54 , 55 , 59 , 60 ], hurricanes [ 35 , 39 , 41 ], infectious disease outbreaks [ 43 , 46 ], oil spillage [ 40 ], and drought [ 52 ].
Variables of interest examined
Across the 26 retained papers: eight referred to examining the impact of SC [ 35 , 37 , 39 , 41 , 46 , 49 , 55 , 60 ]; eight examined the impact of cognitive and structural SC as separate entities [ 40 , 42 , 45 , 48 , 50 , 54 , 57 , 59 ]; one examined bridging and bonding SC as separate entities [ 58 ]; two examined the impact of CR [ 38 , 56 ]; and two employed a qualitative methodology but drew findings in relation to bonding and bridging SC, and SC generally [ 51 , 52 ]. Additionally, five papers examined the impact of the following variables: ‘community social cohesion’ [ 36 ], ‘neighbourhood connectedness’ [ 44 ], ‘social support at the community level’ [ 47 ], ‘community connectedness’ [ 43 ] and ‘sense of community’ [ 53 ]. Table 1 provides additional details on this.
How is CR and SC measured or quantified in research?
The measures used to examine CR and SC are presented Table 1 . It is apparent that there is no uniformity in how SC or CR is measured across the research. Multiple measures are used throughout the retained studies, and nearly all are unique. Additionally, SC was examined at multiple different levels (e.g. cognitive and structural, bonding and bridging), and in multiple different forms (e.g. community connectedness, community cohesion).
What is the association between CR and SC on mental wellbeing?
To best compare research, the following section reports on CR, and facets of SC separately. Please see Supplementary file 4 for additional information on retained papers methods of measuring mental wellbeing.
- Community resilience
CR relates to the ability of a community to withstand, adapt and permit growth in adverse circumstances due to social structures, networks and interdependencies within the community [ 11 ].
The impact of CR on mental wellbeing was consistently positive. For example, research indicated that there was a positive association between CR and number of common mental health (i.e. anxiety and mood) treatments post-disaster [ 56 ]. Similarly, other research suggests that CR is positively related to psychological resilience, which is inversely related to depressive symptoms) [ 37 ]. The same research also concluded that CR is protective of psychological resilience and is therefore protective of depressive symptoms [ 37 ].
- Social capital
SC reflects the strength of a social network, community reciprocity, and trust in people and institutions [ 14 ]. These aspects of community are usually conceptualised primarily as protective factors that enable communities to cope and adapt collectively to threats.
There were inconsistencies across research which examined the impact of abstract SC (i.e. not refined into bonding/bridging or structural/cognitive) on mental wellbeing. However, for the majority of cases, research deems SC to be beneficial. For example, research has concluded that, SC is protective against post-traumatic stress disorder [ 55 ], anxiety [ 46 ], psychological distress [ 50 ], and stress [ 46 ]. Additionally, SC has been found to facilitate post-traumatic growth [ 38 ], and also to be useful to be drawn upon in times of stress [ 52 ], both of which could be protective of mental health. Similarly, research has also found that emotional recovery following a disaster is more difficult for those who report to have low levels of SC [ 51 ].
Conversely, however, research has also concluded that when other situational factors (e.g. personal resources) were controlled for, a positive relationship between community resources and life satisfaction was no longer significant [ 60 ]. Furthermore, some research has concluded that a high level of SC can result in a community facing greater stress immediately post disaster. Indeed, one retained paper found that high levels of SC correlate with higher levels of post-traumatic stress immediately following a disaster [ 39 ]. However, in the later stages following a disaster, this relationship can reverse, with SC subsequently providing an aid to recovery [ 41 ]. By way of explanation, some researchers have suggested that communities with stronger SC carry the greatest load in terms of helping others (i.e. family, friends and neighbours) as well as themselves immediately following the disaster, but then as time passes the communities recover at a faster rate as they are able to rely on their social networks for support [ 41 ].
Cognitive and structural social capital
Cognitive SC refers to perceptions of community relations, such as trust, mutual help and attachment, and structural SC refers to what actually happens within the community, such as participation, socialising [ 16 ].
Cognitive SC has been found to be protective [ 49 ] against PTSD [ 54 , 57 ], depression [ 40 , 54 ]) mild mood disorder; [ 48 ]), anxiety [ 48 , 54 ] and increase self-efficacy [ 59 ].
For structural SC, research is again inconsistent. On the one hand, structural SC has been found to: increase perceived self-efficacy, be protective of depression [ 40 ], buffer the impact of housing damage on cognitive decline [ 42 ] and provide support during disasters and over the recovery period [ 59 ]. However, on the other hand, it has been found to have no association with PTSD [ 54 , 57 ] or depression, and is also associated with a higher prevalence of anxiety [ 54 ]. Similarly, it is also suggested by additional research that structural SC can harm women’s mental health, either due to the pressure of expectations to help and support others or feelings of isolation [ 49 ].
Bonding and bridging social capital
Bonding SC refers to connections among individuals who are emotionally close, and result in bonds to a particular group [ 17 ], and bridging SC refers to acquaintances or individuals loosely connected that span different social groups [ 18 ].
One research study concluded that both bonding and bridging SC were protective against post-traumatic stress disorder symptoms [ 58 ]. Bridging capital was deemed to be around twice as effective in buffering against post-traumatic stress disorder than bonding SC [ 58 ].
Other community variables
Community social cohesion was significantly associated with a lower risk of post-traumatic stress disorder symptom development [ 35 ], and this was apparent even whilst controlling for depressive symptoms at baseline and disaster impact variables (e.g. loss of family member or housing damage) [ 36 ]. Similarly, sense of community, community connectedness, social support at the community level and neighbourhood connectedness all provided protective benefits for a range of mental health, wellbeing and recovery variables, including: depression [ 53 ], subjective wellbeing (in older adults only) [ 43 ], psychological distress [ 47 ], happiness [ 44 ] and life satisfaction [ 53 ].
Research has also concluded that community level social support is protective against mild mood and anxiety disorder, but only for individuals who have had no previous disaster experience [ 48 ]. Additionally, a study which separated SC into social cohesion and social participation concluded that at a community level, social cohesion is protective against depression [ 49 ] whereas social participation at community level is associated with an increased risk of depression amongst women [ 49 ].
What is the impact of Infectious disease outbreaks / disasters and emergencies on community resilience?
From a cross-sectional perspective, research has indicated that disasters and emergencies can have a negative effect on certain types of SC. Specifically, cognitive SC has been found to be impacted by disaster impact, whereas structural SC has gone unaffected [ 45 ]. Disaster impact has also been shown to have a negative effect on community relationships more generally [ 52 ].
Additionally, of the eight studies which collected data at multiple time points [ 35 , 36 , 41 , 42 , 47 , 49 , 56 , 60 ], three reported the effect of a disaster on the level of SC within a community [ 40 , 42 , 49 ]. All three of these studies concluded that disasters may have a negative impact on the levels of SC within a community. The first study found that the Deepwater Horizon oil spill had a negative effect on SC and social support, and this in turn explained an overall increase in the levels of depression within the community [ 40 ]. A possible explanation for the negative effect lays in ‘corrosive communities’, known for increased social conflict and reduced social support, that are sometimes created following oil spills [ 40 ]. It is proposed that corrosive communities often emerge due to a loss of natural resources that bring social groups together (e.g., for recreational activities), as well as social disparity (e.g., due to unequal distribution of economic impact) becoming apparent in the community following disaster [ 40 ]. The second study found that SC (in the form of social cohesion, informal socialising and social participation) decreased after the 2011 earthquake and tsunami in Japan; it was suggested that this change correlated with incidence of cognitive decline [ 42 ]. However, the third study reported more mixed effects based on physical circumstances of the communities’ natural environment: Following an earthquake, those who lived in mountainous areas with an initial high level of pre-community SC saw a decrease in SC post disaster [ 49 ]. However, communities in flat areas (which were home to younger residents and had a higher population density) saw an increase in SC [ 49 ]. It was proposed that this difference could be due to the need for those who lived in mountainous areas to seek prolonged refuge due to subsequent landslides [ 49 ].
What types of intervention enhance CR and SC and protect survivors?
There were mixed effects across the 26 retained papers when examining the effect of CR and SC on mental wellbeing. However, there is evidence that an increase in SC [ 56 , 57 ], with a focus on cognitive SC [ 57 ], namely by: building social networks [ 45 , 51 , 53 ], enhancing feelings of social cohesion [ 35 , 36 ] and promoting a sense of community [ 53 ], can result in an increase in CR and potentially protect survivors’ wellbeing and mental health following a disaster. An increase in SC may also aid in decreasing the need for individual psychological interventions in the aftermath of a disaster [ 55 ]. As a result, recommendations and suggested methods to bolster CR and SC from the retained papers have been extracted and separated into general methods, preparedness and policy level implementation.
General methods
Suggested methods to build SC included organising recreational activity-based groups [ 44 ] to broaden [ 51 , 53 ] and preserve current social networks [ 42 ], introducing initiatives to increase social cohesion and trust [ 51 ], and volunteering to increase the number of social ties between residents [ 59 ]. Research also notes that it is important to take a ‘no one left behind approach’ when organising recreational and social community events, as failure to do so could induce feelings of isolation for some members of the community [ 49 ]. Furthermore, gender differences should also be considered as research indicates that males and females may react differently to community level SC (as evidence suggests males are instead more impacted by individual level SC; in comparison to women who have larger and more diverse social networks [ 49 ]). Therefore, interventions which aim to raise community level social participation, with the aim of expanding social connections and gaining support, may be beneficial [ 42 , 47 ].
Preparedness
In order to prepare for disasters, it may be beneficial to introduce community-targeted methods or interventions to increase levels of SC and CR as these may aid in ameliorating the consequences of a public health emergency or disaster [ 57 ]. To indicate which communities have low levels of SC, one study suggests implementing a 3-item scale of social cohesion to map areas and target interventions [ 42 ].
It is important to consider that communities with a high level of SC may have a lower level of risk perception, due to the established connections and supportive network they have with those around them [ 61 ]. However, for the purpose of preparedness, this is not ideal as perception of risk is a key factor when seeking to encourage behavioural adherence. This could be overcome by introducing communication strategies which emphasise the necessity of social support, but also highlights the need for additional measures to reduce residual risk [ 59 ]. Furthermore, support in the form of financial assistance to foster current community initiatives may prove beneficial to rural areas, for example through the use of an asset-based community development framework [ 52 ].
Policy level
At a policy level, the included papers suggest a range of ways that CR and SC could be bolstered and used. These include: providing financial support for community initiatives and collective coping strategies, (e.g. using asset-based community development [ 52 ]); ensuring policies for long-term recovery focus on community sustainable development (e.g. community festival and community centre activities) [ 44 ]; and development of a network amongst cooperative corporations formed for reconstruction and to organise self-help recovery sessions among residents of adjacent areas [ 58 ].
This scoping review sought to synthesise literature concerning the role of SC and CR during public health emergencies and disasters. Specifically, in this review we have examined: the methods used to measure CR and SC; the impact of CR and SC on mental wellbeing during disasters and emergencies; the impact of disasters and emergencies on CR and SC; and the types of interventions which can be used to enhance CR. To do this, data was extracted from 26 peer-reviewed journal articles. From this synthesis, several key themes have been identified, which can be used to develop guidelines and recommendations for deploying CR and SC in a public health emergency or disaster context. These key themes and resulting recommendations are summarised below.
Firstly, this review established that there is no consistent or standardised approach to measuring CR or SC within the general population. This finding is consistent with a review conducted by the World Health Organization which concludes that despite there being a number of frameworks that contain indicators across different determinants of health, there is a lack of consensus on priority areas for measurement and no widely accepted indicator [ 27 ]. As a result, there are many measures of CR and SC apparent within the literature (e.g., [ 62 , 63 ]), an example of a developed and validated measure is provided by Sherrieb, Norris and Galea [ 64 ]. Similarly, the definitions of CR and SC differ widely between researchers, which created a barrier to comparing and summarising information. Therefore, future research could seek to compare various interpretations of CR and to identify any overlapping concepts. However, a previous systemic review conducted by Patel et al. (2017) concludes that there are nine core elements of CR (local knowledge, community networks and relationships, communication, health, governance and leadership, resources, economic investment, preparedness, and mental outlook), with 19 further sub-elements therein [ 30 ]. Therefore, as CR is a multi-dimensional construct, the implications from the findings are that multiple aspects of social infrastructure may need to be considered.
Secondly, our synthesis of research concerning the role of CR and SC for ensuring mental health and wellbeing during, or following, a public health emergency or disaster revealed mixed effects. Much of the research indicates either a generally protective effect on mental health and wellbeing, or no effect; however, the literature demonstrates some potential for a high level of CR/SC to backfire and result in a negative effect for populations during, or following, a public health emergency or disaster. Considered together, our synthesis indicates that cognitive SC is the only facet of SC which was perceived as universally protective across all retained papers. This is consistent with a systematic review which also concludes that: (a) community level cognitive SC is associated with a lower risk of common mental disorders, while; (b) community level structural SC had inconsistent effects [ 65 ].
Further examination of additional data extracted from studies which found that CR/SC had a negative effect on mental health and wellbeing revealed no commonalities that might explain these effects (Please see Supplementary file 5 for additional information)
One potential explanation may come from a retained paper which found that high levels of SC result in an increase in stress level immediately post disaster [ 41 ]. This was suggested to be due to individuals having greater burdens due to wishing to help and support their wide networks as well as themselves. However, as time passes the levels of SC allow the community to come together and recover at a faster rate [ 41 ]. As this was the only retained paper which produced this finding, it would be beneficial for future research to examine boundary conditions for the positive effects of CR/SC; that is, to explore circumstances under which CR/SC may be more likely to put communities at greater risk. This further research should also include additional longitudinal research to validate the conclusions drawn by [ 41 ] as resilience is a dynamic process of adaption.
Thirdly, disasters and emergencies were generally found to have a negative effect on levels of SC. One retained paper found a mixed effect of SC in relation to an earthquake, however this paper separated participants by area in which they lived (i.e., mountainous vs. flat), which explains this inconsistent effect [ 49 ]. Dangerous areas (i.e. mountainous) saw a decrease in community SC in comparison to safer areas following the earthquake (an effect the authors attributed to the need to seek prolonged refuge), whereas participants from the safer areas (which are home to younger residents with a higher population density) saw an increase in SC [ 49 ]. This is consistent with the idea that being able to participate socially is a key element of SC [ 12 ]. Overall, however, this was the only retained paper which produced a variable finding in relation to the effect of disaster on levels of CR/SC.
Finally, research identified through our synthesis promotes the idea of bolstering SC (particularly cognitive SC) and cohesion in communities likely to be affected by disaster to improve levels of CR. This finding provides further understanding of the relationship between CR and SC; an association that has been reported in various articles seeking to provide conceptual frameworks (e.g., [ 66 , 67 ]) as well as indicator/measurement frameworks [ 27 ]. Therefore, this could be done by creating and promoting initiatives which foster SC and create bonds within the community. Papers included in the current review suggest that recreational-based activity groups and volunteering are potential methods for fostering SC and creating community bonds [ 44 , 51 , 59 ]. Similarly, further research demonstrates that feelings of social cohesion are enhanced by general social activities (e.g. fairs and parades [ 18 ]). Also, actively encouraging activities, programs and interventions which enhance connectedness and SC have been reported to be desirable to increase CR [ 68 ]. This suggestion is supported by a recent scoping review of literature [ 67 ] examined community champion approaches for the COVID-19 pandemic response and recovery and established that creating and promoting SC focused initiatives within the community during pandemic response is highly beneficial [ 67 ]. In terms of preparedness, research states that it may be beneficial for levels of SC and CR in communities at risk to be assessed, to allow targeted interventions where the population may be at most risk following an incident [ 42 , 44 ]. Additionally, from a more critical perspective, we acknowledge that ‘resilience’ can often be perceived as a focus on individual capacity to adapt to adversity rather than changing or mitigating the causes of adverse conditions [ 69 , 70 ]. Therefore, CR requires an integrated system approach across individual, community and structural levels [ 17 ]. Also, it is important that community members are engaged in defining and agreeing how community resilience is measured [ 27 ] rather than it being imposed by system leads or decision-makers.
In the aftermath of the pandemic, is it expected that there will be long-term repercussions both from an economic [ 8 ] and a mental health perspective [ 71 ]. Furthermore, the findings from this review suggest that although those in areas with high levels of SC may be negatively affected in the acute stage, as time passes, they have potential to rebound at a faster rate than those with lower levels of SC. Ongoing evaluation of the effectiveness of current initiatives as the COVID-19 pandemic progresses into a recovery phase will be invaluable for supplementing the evidence base identified through this review.
- Recommendations
As a result of this review, a number of recommendations are suggested for policy and practice during public health emergencies and recovery.
Future research should seek to establish a standardised and validated approach to measuring and defining CR and SC within communities. There are ongoing efforts in this area, for example [ 72 ]. Additionally, community members should be involved in the process of defining how CR is measured.
There should be an enhanced effort to improve preparedness for public health emergencies and disasters in local communities by gauging current levels of SC and CR within communities using a standardised measure. This approach could support specific targeting of populations with low levels of CR/SC in case of a disaster or public health emergency, whilst also allowing for consideration of support for those with high levels of CR (as these populations can be heavily impacted initially following a disaster). By distinguishing levels of SC and CR, tailored community-centred approaches could be implemented, such as those listed in a guide released by PHE in 2015 [ 73 ].
CR and SC (specifically cognitive SC) should be bolstered if communities are at risk of experiencing a disaster or public health emergency. This can be achieved by using interventions which aim to increase a sense of community and create new social ties (e.g., recreational group activities, volunteering). Additionally, when aiming to achieve this, it is important to be mindful of the risk of increased levels of CR/SC to backfire, as well as seeking to advocate an integrated system approach across individual, community and structural levels.
It is necessary to be aware that although communities with high existing levels of resilience / SC may experience short-term negative consequences following a disaster, over time these communities might be able to recover at a faster rate. It is therefore important to ensure that suitable short-term support is provided to these communities in the immediate aftermath of a public health emergency or disaster.
Robust evaluation of the community resilience initiatives deployed during the COVID-19 pandemic response is essential to inform the evidence base concerning the effectiveness of CR/ SC. These evaluations should continue through the response phase and into the recovery phase to help develop our understanding of the long-term consequences of such interventions.
Limitations
Despite this review being the first in this specific topic area, there are limitations that must be considered. Firstly, it is necessary to note that communities are generally highly diverse and the term ‘community’ in academic literature is a subject of much debate (see: [ 74 ]), therefore this must be considered when comparing and collating research involving communities. Additionally, the measures of CR and SC differ substantially across research, including across the 26 retained papers used in the current review. This makes the act of comparing and collating research findings very difficult. This issue is highlighted as a key outcome from this review, and suggestions for how to overcome this in future research are provided. Additionally, we acknowledge that there will be a relationship between CR & SC even where studies measure only at individual or community level. A review [ 75 ] on articulating a hypothesis of the link to health inequalities suggests that wider structural determinants of health need to be accounted for. Secondly, despite the final search strategy encompassing terms for both CR and SC, only one retained paper directly measured CR; thus, making the research findings more relevant to SC. Future research could seek to focus on CR to allow for a comparison of findings. Thirdly, the review was conducted early in the COVID-19 pandemic and so does not include more recent publications focusing on resilience specifically in the context of COVID-19. Regardless of this fact, the synthesis of, and recommendations drawn from, the reviewed studies are agnostic to time and specific incident and contain critical elements necessary to address as the pandemic moves from response to recovery. Further research should review the effectiveness of specific interventions during the COVID-19 pandemic for collation in a subsequent update to this current paper. Fourthly, the current review synthesises findings from countries with individualistic and collectivistic cultures, which may account for some variation in the findings. Lastly, despite choosing a scoping review method for ease of synthesising a wide literature base for use by public health emergency researchers in a relatively tight timeframe, there are disadvantages of a scoping review approach to consider: (1) quality appraisal of retained studies was not carried out; (2) due to the broad nature of a scoping review, more refined and targeted reviews of literature (e.g., systematic reviews) may be able to provide more detailed research outcomes. Therefore, future research should seek to use alternative methods (e.g., empirical research, systematic reviews of literature) to add to the evidence base on CR and SC impact and use in public health practice.
This review sought to establish: (1) How CR and SC are quantified in research?; (2) The impact of community resilience on mental wellbeing?; (3) The impact of infectious disease outbreaks, disasters and emergencies on community resilience and social capital?; and, (4) What types of interventions enhance community resilience and social capital?. The chosen search strategy yielded 26 relevant papers from which we were able extract information relating to the aims of this review.
Results from the review revealed that CR and SC are not measured consistently across research. The impact of CR / SC on mental health and wellbeing during emergencies and disasters is mixed (with some potential for backlash), however the literature does identify cognitive SC as particularly protective. Although only a small number of papers compared CR or SC before and after a disaster, the findings were relatively consistent: SC or CR is negatively impacted by a disaster. Methods suggested to bolster SC in communities were centred around social activities, such as recreational group activities and volunteering. Recommendations for both research and practice (with a particular focus on the ongoing COVID-19 pandemic) are also presented.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Social Capital
Zortea TC, Brenna CT, Joyce M, McClelland H, Tippett M, Tran MM, et al. The impact of infectious disease-related public health emergencies on suicide, suicidal behavior, and suicidal thoughts. Crisis. 2020;42(6):474–87.
Article PubMed PubMed Central Google Scholar
Davis JR, Wilson S, Brock-Martin A, Glover S, Svendsen ER. The impact of disasters on populations with health and health care disparities. Disaster Med Pub Health Prep. 2010;4(1):30.
Article Google Scholar
Francescutti LH, Sauve M, Prasad AS. Natural disasters and healthcare: lessons to be learned. Healthc Manage Forum. 2017;30(1):53–5.
Article PubMed Google Scholar
Jones L, Palumbo D, Brown D. Coronavirus: How the pandemic has changed the world economy. BBC News; 2021. Accessible at: https://www.bbc.co.uk/news/business-51706225 .
Below R, Wallemacq P. Annual disaster statistical review 2017. Brussels: CRED, Centre for Research on the Epidemiology of Disasters; 2018.
Google Scholar
Qiu W, Chu C, Mao A, Wu J. The impacts on health, society, and economy of SARS and H7N9 outbreaks in China: a case comparison study. J Environ Public Health. 2018;2018:2710185.
Worldometer. COVID-19 coronavirus pandemic. 2021.
Harari D, Keep M. Coronavirus: economic impact house of commons library. Briefing Paper (Number 8866); 2021. Accessible at: https://commonslibrary.parliament.uk/research-briefings/cbp-8866/ .
Nabavi N. Covid-19: pandemic will cast a long shadow on mental health, warns England’s CMO. BMJ. 2021;373:n1655.
Ziglio E. Strengthening resilience: a priority shared by health 2020 and the sustainable development goals. No. WHO/EURO: 2017-6509-46275-66939. World Health Organization; Regional Office for Europe; 2017.
Asadzadeh A, Kotter T, Salehi P, Birkmann J. Operationalizing a concept: the systematic review of composite indicator building for measuring community disaster resilience. Int J Disaster Risk Reduct. 2017;25:147.
Sherrieb K, Norris F, Galea S. Measuring capacities for community resilience. Soc Indicators Res. 2010;99(2):227.
Poortinga W. Community resilience and health: the role of bonding, bridging, and linking aspects of social capital. Health Place. 2011;18(2):286–95.
Ferlander S. The importance of different forms of social capital for health. Acta Sociol. 2007;50(2):115–28.
Nakagawa Y, Shaw R. Social capital: a missing link to disaster recovery. Int J Mass Emerge Disasters. 2004;22(1):5–34.
Grootaert C, Narayan D, Jones VN, Woolcock M. Measuring social capital: an integrated questionnaire. Washington, DC: World Bank Working Paper, No. 18; 2004.
Adler PS, Kwon SW. Social capital: prospects for a new concept. Acad Manage Rev. 2002;27(1):17–40.
Aldrich DP, Meyer MA. Social capital and community resilience. Am Behav Sci. 2015;59(2):254–69.
Rodriguez-Llanes JM, Vos F, Guha-Sapir D. Measuring psychological resilience to disasters: are evidence-based indicators an achievable goal? Environ Health. 2013;12(1):115.
De Silva MJ, McKenzie K, Harpham T, Huttly SR. Social capital and mental Illness: a systematic review. J Epidemiol Community Health. 2005;59(8):619–27.
Bonanno GA, Galea S, Bucciarelli A, Vlahov D. Psychological resilience after disaster: New York City in the aftermath of the september 11th terrorist attack. Psychol Sci. 2006;17(3):181.
World Health Organization. Health 2020: a European policy framework and strategy for the 21st century. World Health Organization. Regional Office for Europe; 2013.
Public Health England. Community-Centred Public Health: Taking a Whole System Approach. 2020.
SPI-B. The role of Community Champion networks to increase engagement in the context of COVID19: Evidence and best practice. 2021.
Public Health England. Community champions: A rapid scoping review of community champion approaches for the pandemic response and recovery. 2021.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
World Health Organisation. WHO health evidence network synthesis report: what quantitative and qualitative methods have been developed to measure health-related community resilience at a national and local level. 2018.
Hall C, Williams N, Gauntlett L, Carter H, Amlôt R, Peterson L et al. Findings from systematic review of public perceptions and responses. PROACTIVE EU. Deliverable 1.1. 2019. Accessible at: https://proactive-h2020.eu/wp-content/uploads/2021/04/PROACTIVE_20210312_D1.1_V5_PHE_Systematic-Review-of-Public-Perceptions-and-Responses_revised.pdf .
Weston D, Ip A, Amlôt R. Examining the application of behaviour change theories in the context of Infectious disease outbreaks and emergency response: a review of reviews. BMC Public Health. 2020;20(1):1483.
Article PubMed PubMed Central CAS Google Scholar
Patel SS, Rogers MB, Amlôt R, Rubin GJ. What do we mean by ‘community resilience’? A systematic literature review of how it is defined in the literature. PLoS Curr. 2017;9:ecurrents.dis.db775aff25efc5ac4f0660ad9c9f7db2.
PubMed PubMed Central Google Scholar
Brooks SK, Weston D, Wessely S, Greenberg N. Effectiveness and acceptability of brief psychoeducational interventions after potentially traumatic events: a systematic review. Eur J Psychotraumatology. 2021;12(1):1923110.
Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review-a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy. 2005;10(1_suppl):21–34.
Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:1–8.
Bearman M, Dawson P. Qualitative synthesis and systematic review in health professions education. Med Educ. 2013;47(3):252–60.
Heid AR, Pruchno R, Cartwright FP, Wilson-Genderson M. Exposure to Hurricane Sandy, neighborhood collective efficacy, and post-traumatic stress symptoms in older adults. Aging Ment Health. 2017;21(7):742–50.
Hikichi H, Aida J, Tsuboya T, Kondo K, Kawachi I. Can community social cohesion prevent posttraumatic stress disorder in the aftermath of a disaster? A natural experiment from the 2011 Tohoku Earthquake and tsunami. Am J Epidemiol. 2016;183(10):902–10.
Lee J, Blackmon BJ, Cochran DM, Kar B, Rehner TA, Gunnell MS. Community resilience, psychological resilience, and depressive symptoms: an examination of the Mississippi Gulf Coast 10 years after Hurricane Katrina and 5 years after the Deepwater Horizon oil spill. Disaster med. 2018;12(2):241–8.
Lee J, Blackmon BJ, Lee JY, Cochran DM Jr, Rehner TA. An exploration of posttraumatic growth, loneliness, depression, resilience, and social capital among survivors of Hurricane Katrina and the deepwater Horizon oil spill. J Community Psychol. 2019;47(2):356–70.
Lowe SR, Sampson L, Gruebner O, Galea S. Psychological resilience after Hurricane Sandy: the influence of individual- and community-level factors on mental health after a large-scale natural disaster. PLoS One. 2015;10(5):e0125761.
Rung AL, Gaston S, Robinson WT, Trapido EJ, Peters ES. Untangling the disaster-depression knot: the role of social ties after deepwater Horizon. Soc Sci Med. 2017;177:19–26.
Weil F, Lee MR, Shihadeh ES. The burdens of social capital: how socially-involved people dealt with stress after Hurricane Katrina. Soc Sci Res. 2012;41(1):110–9.
Hikichi H, Aida J, Matsuyama Y, Tsuboya T, Kondo K, Kawachi I. Community-level social capital and cognitive decline after a Natural Disaster: a natural experiment from the 2011 Great East Japan Earthquake and Tsunami. Soc Sci Med. 2018;257:111981.
Lau AL, Chi I, Cummins RA, Lee TM, Chou KL, Chung LW. The SARS (severe acute respiratory syndrome) pandemic in Hong Kong: effects on the subjective wellbeing of elderly and younger people. Aging Ment Health. 2008;12(6):746–60.
Sun Y, Yan T. The use of public health indicators to assess individual happiness in post-disaster recovery. Int J Environ Res Public Health. 2019;16(21):4101.
Wong H, Huang Y, Fu Y, Zhang Y. Impacts of structural social capital and cognitive social capital on the psychological status of survivors of the yaan Earthquake. Appl Res Qual Life. 2018;14:1411–33.
Xiao H, Zhang Y, Kong D, Li S, Yang N. Social capital and sleep quality in individuals who self-isolated for 14 days during the coronavirus disease 2019 (COVID-19) outbreak in January 2020 in China. Med Sci Monit. 2020;26:e923921.
PubMed PubMed Central CAS Google Scholar
Matsuyama Y, Aida J, Hase A, Sato Y, Koyama S, Tsuboya T, et al. Do community- and individual-level social relationships contribute to the mental health of disaster survivors? A multilevel prospective study after the great East Japan earthquake. Soc Sci Med. 2016;151:187–95.
Ozaki A, Horiuchi S, Kobayashi Y, Inoue M, Aida J, Leppold C, Yamaoka K. Beneficial roles of social support for mental health vary in the Japanese population depending on disaster experience: a nationwide cross-sectional study. Tohoku J Exp Med. 2018;246(4):213–23.
Sato K, Amemiya A, Haseda M, Takagi D, Kanamori M, Kondo K, et al. Post-disaster changes in Social Capital and Mental Health: a natural experiment from the 2016 Kumamoto Earthquake. Am J Epidemiol. 2020;189(9):910–21.
Tsuchiya N, Nakaya N, Nakamura T, Narita A, Kogure M, Aida J, Tsuji I, Hozawa A, Tomita H. Impact of social capital on psychological distress and interaction with house destruction and displacement after the great East Japan earthquake of 2011. J Neuropsychiatry Clin Neurosci. 2017;71(1):52–60.
Brockie L, Miller E. Understanding older adults’ resilience during the Brisbane floods: social capital, life experience, and optimism. Disaster Med Pub Health Prep. 2017;11(1):72–9.
Caldwell K, Boyd CP. Coping and resilience in farming families affected by drought. Rural Remote Health. 2009;9(2):1088.
PubMed Google Scholar
Huang Y, Tan NT, Liu J. Support, sense of community, and psychological status in the survivors of the Yaan earthquake. J Community Psychol. 2016;44(7):919–36.
Wind T, Fordham M, Komproe H. Social capital and post-disaster mental health. Glob Health Action. 2011;4(1):6351.
Wind T, Komproe IH. The mechanisms that associate community social capital with post-disaster mental health: a multilevel model. Soc Sci Med. 2012;75(9):1715–20.
Hogg D, Kingham S, Wilson TM, Ardagh M. The effects of spatially varying earthquake impacts on mood and anxiety symptom treatments among long-term Christchurch residents following the 2010/11 Canterbury Earthquakes, New Zealand. Health Place. 2016;41:78–88.
Flores EC, Carnero AM, Bayer AM. Social capital and chronic post-traumatic stress disorder among survivors of the 2007 earthquake in Pisco, Peru. Soc Sci Med. 2014;101:9–17.
Rafiey H, Alipour F, LeBeau R, Salimi Y, Ahmadi S. Exploring the buffering role of social capital in the development of posttraumatic stress symptoms among Iranian earthquake survivors. Psychol Trauma. 2019;14(6):1040–6.
Babcicky P, Seebauer S. The two faces of social capital in private Flood mitigation: opposing effects on risk perception, self-efficacy and coping capacity. J Risk Res. 2017;20(8):1017–37.
Bakic H, Ajdukovic D. Stability and change post-disaster: dynamic relations between individual, interpersonal and community resources and psychosocial functioning. Eur J Psychotraumatol. 2019;10(1):1614821.
Rogers RW. A protection motivation theory of fear appeals and attitude change. J Psychol. 1975;91(1):93–114.
Lindberg K, Swearingen T. A reflective thrive-oriented community resilience scale. Am J Community Psychol. 2020;65(3–4):467–78.
Leykin D, Lahad M, Cohen O, Goldberg A, Aharonson-Daniel L. Conjoint community resiliency assessment measure-28/10 items (CCRAM28 and CCRAM10): a self-report tool for assessing community resilience. Am J Community Psychol. 2013;52:313–23.
Sherrieb K, Norris FH, Galea S. Measuring capacities for community resilience. Soc Indic Res. 2010;99:227–47.
Ehsan AM, De Silva MJ. Social capital and common mental disorder: a systematic review. J Epidemiol Community Health. 2015;69(10):1021–8.
Pfefferbaum B, Van Horn RL, Pfefferbaum RL. A conceptual framework to enhance community resilience using social capital. Clin Soc Work J. 2017;45(2):102–10.
Carmen E, Fazey I, Ross H, Bedinger M, Smith FM, Prager K, et al. Building community resilience in a context of climate change: the role of social capital. Ambio. 2022;51(6):1371–87.
Humbert C, Joseph J. Introduction: the politics of resilience: problematising current approaches. Resilience. 2019;7(3):215–23.
Tanner T, Bahadur A, Moench M. Challenges for resilience policy and practice. Working paper: 519. 2017.
Vadivel R, Shoib S, El Halabi S, El Hayek S, Essam L, Bytyçi DG. Mental health in the post-COVID-19 era: challenges and the way forward. Gen Psychiatry. 2021;34(1):e100424.
Article CAS Google Scholar
Pryor M. Social Capital Harmonised Standard. London: Government Statistical Service. 2021. Accessible at: https://gss.civilservice.gov.uk/policystore/social-capital/ .
Public Health England NE. A guide to community-centred approaches for health and wellbeing. 2015.
Hawe P. Capturing the meaning of ‘community’ in community intervention evaluation: some contributions from community psychology. Health Promot Int. 1994;9(3):199–210.
Uphoff EP, Pickett KE, Cabieses B, Small N, Wright J. A systematic review of the relationships between social capital and socioeconomic inequalities in health: a contribution to understanding the psychosocial pathway of health inequalities. Int J Equity Health. 2013;12:1–12.
Download references
Acknowledgements
Not applicable.
This study was supported by the National Institute for Health Research Research Unit (NIHR HPRU) in Emergency Preparedness and Response, a partnership between Public Health England, King’s College London and the University of East Anglia. The views expressed are those of the author(s) and not necessarily those of the NIHR, Public Health England, the UK Health Security Agency or the Department of Health and Social Care [Grant number: NIHR20008900]. Part of this work has been funded by the Office for Health Improvement and Disparities, Department of Health and Social Care, as part of a Collaborative Agreement with Leeds Beckett University.
Author information
Authors and affiliations.
Behavioural Science and Insights Unit, Evaluation & Translation Directorate, Science Group, UK Health Security Agency, Porton Down, Salisbury, SP4 0JG, UK
C. E. Hall, H. Wehling, R. Amlôt & D. Weston
Health Protection Research Unit, Institute of Psychology, Psychiatry and Neuroscience, King’s College London, 10 Cutcombe Road, London, SE5 9RJ, UK
C. E. Hall, S. K. Brooks & N. Greenberg
School of Health and Community Studies, Leeds Beckett University, Portland Building, PD519, Portland Place, Leeds, LS1 3HE, UK
J. Stansfield & J. South
King’s Centre for Military Health Research, Institute of Psychology, Psychiatry and Neuroscience, King’s College London, 10 Cutcombe Road, London, SE5 9RJ, UK
N. Greenberg
You can also search for this author in PubMed Google Scholar
Contributions
DW, JSo and JSt had the main idea for the review. The search strategy and eligibility criteria were devised by CH, DW, JSo and JSt. CH conducted the database searches. CH and DW conducted duplicate, title and abstract and full text screening in accordance with inclusion criteria. CH conducted data extraction, CH and DW carried out the analysis and drafted the initial manuscript. All authors provided critical revision of intellectual content. All authors approved the final manuscript.
Authors’ information
Corresponding author.
Correspondence to D. Weston .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., additional file 2., additional file 3., additional file 4., additional file 5., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hall, C.E., Wehling, H., Stansfield, J. et al. Examining the role of community resilience and social capital on mental health in public health emergency and disaster response: a scoping review. BMC Public Health 23 , 2482 (2023). https://doi.org/10.1186/s12889-023-17242-x
Download citation
Received : 04 April 2022
Accepted : 16 November 2023
Published : 12 December 2023
DOI : https://doi.org/10.1186/s12889-023-17242-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mental health
- Community cohesion
- Public health emergency
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Fight coronavirus (COVID-19) transmission at home
Used properly, most common household disinfectants will kill the virus that causes COVID-19.
Getting your COVID-19 vaccine is the best way to lower your risk of serious illness. Getting the vaccine also prevents the spread of the COVID-19 virus.
In addition to the vaccine there are other actions you can take to keep this coronavirus from spreading in your home. Wash your hands well and often to lower your risk of infection. Improving air flow in your home and filtering the air can help. And for the coronavirus and other germs, clean those high-touch surfaces in your home and disinfect them when needed.
What's the difference between cleaning and disinfecting?
Cleaning removes most germs and dust or dirt. Disinfecting kills most germs. If someone in your home has COVID-19, clean first, then disinfect.
How is COVID-19 spread?
The virus that causes COVID-19 spreads mainly from person to person. It can spread from people who are infected but have no symptoms. When people with COVID-19 cough, sneeze, breathe, sing or talk, the virus carried on their breath can land on the faces of people nearby. The virus spreads when other people breathe in infected droplets or when the droplets land in their eyes, noses or mouths.
The COVID-19 virus also can spread if people touch their eyes, noses or mouths after touching a surface with the virus on it. Without cleaning and disinfection, the COVID-19 virus may stay on surfaces from hours to days. But the risk of COVID-19 through contact with infected surfaces seems low.
How can I clean and disinfect my home?
You can lower the risk of spreading of the COVID-19 virus by focusing on surfaces that are touched often. Examples are tables, doorknobs, light switches, handles, counters, desks, toilets, faucets and sinks. Clean these things with soap and water or with a product made for the specific surface. Follow the instructions on the product label.
Most often, cleaning is enough to lower the risk. Clean more often if someone in your home is at higher risk of severe illness from COVID-19.
If someone who is sick with COVID-19 lives with you or has been in your home within the last 24 hours, disinfect surfaces right after cleaning them. Disinfecting helps kill germs that are left.
Disinfecting uses strong chemicals.
Read product labels before use and follow instructions carefully. Many disinfectants need to stay on surfaces for some time to work. This is called the contact time, and the label will tell you that length of time.
Put on gloves before disinfecting. Disposable gloves are best because you can throw them away when you're done. If you have only gloves that you reuse, don't use them for anything else. Wash your hands well with soap and water for 20 seconds right after cleaning and disinfecting.
Keep doors or windows open and use a fan to help increase air flow while disinfecting your home.
What disinfectants kill COVID-19?
The U.S. Environmental Protection Agency (EPA) has a list of disinfectants for use against COVID-19. Look for products with active ingredients such as ethanol, hydrogen peroxide or quaternary ammonium. In the U.S., check labels for EPA registration numbers.
Does bleach work against COVID-19?
Yes. You can make a disinfecting solution by mixing 4 teaspoons (about 20 milliliters) of household bleach and 1 quart (a bit less than 1 liter) of water.
Read and follow instructions on your bottle of bleach. It's also important to wear gloves and make sure there's good airflow in the room. Don't mix bleach with ammonia or any other cleanser because mixing can cause toxic fumes.
How can I disinfect phones and other electronics?
Cellphones are high-touch devices that can carry COVID-19 germs. Follow makers' advice for cleaning and disinfecting them and other electronics. You also may consider covering your phone or shared electronics, such as a keyboard, with a product that can be easily disinfected.
Protect yourself every day
As you touch people, surfaces and objects throughout the day, you get germs on your hands. You can infect yourself with these germs by touching your eyes, nose or mouth.
To protect yourself, wash your hands often with soap and water for at least 20 seconds. If there's no soap and water, use an alcohol-based hand sanitizer with at least 60% alcohol.
- When and how to clean and disinfect your home. Centers for Disease Control and Prevention. https://www.cdc.gov/hygiene/cleaning/cleaning-your-home.html. Accessed Feb. 28, 2024.
- McIntosh K. COVID-19: Epidemiology, virology, and prevention. https://www.uptodate.com/contents/search. Accessed Feb. 28, 2024.
- Smith BA. COVID-19: Infection prevention for persons with SARS-CoV-2 infection. https://www.uptodate.com/contents/search. Accessed Feb. 28, 2024.
- COVID-19: How to protect yourself and others. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. Accessed Feb. 28, 2024.
- Cleaning and disinfecting: Best practices during the COVID-19 pandemic. Environmental Protection Agency. https://www.epa.gov/coronavirus/cleaning-and-disinfecting-best-practices-during-covid-19-pandemic. Accessed Feb. 28, 2024.
- List N advanced search page: Disinfectants for coronavirus (COVID-19). https://www.epa.gov/coronavirus/list-n-advanced-search-page-disinfectants-coronavirus-covid-19. Accessed Feb. 28, 2024.
Products and Services
- A Book: Endemic - A Post-Pandemic Playbook
- Begin Exploring Women's Health Solutions at Mayo Clinic Store
- A Book: Future Care
- Antibiotics: Are you misusing them?
- COVID-19 and vitamin D
- Convalescent plasma therapy
- Coronavirus disease 2019 (COVID-19)
- COVID-19: How can I protect myself?
- Herd immunity and coronavirus
- COVID-19 and pets
- COVID-19 and your mental health
- COVID-19 antibody testing
- COVID-19, cold, allergies and the flu
- COVID-19 drugs: Are there any that work?
- Long-term effects of COVID-19
- COVID-19 tests
- COVID-19 in babies and children
- Coronavirus infection by race
- COVID-19 travel advice
- COVID-19 vaccine: Should I reschedule my mammogram?
- COVID-19 vaccines for kids: What you need to know
- COVID-19 vaccines
- COVID-19 variant
- COVID-19 vs. flu: Similarities and differences
- COVID-19: Who's at higher risk of serious symptoms?
- Debunking coronavirus myths
- Different COVID-19 vaccines
- Extracorporeal membrane oxygenation (ECMO)
- Fever: First aid
- Fever treatment: Quick guide to treating a fever
- Honey: An effective cough remedy?
- How do COVID-19 antibody tests differ from diagnostic tests?
- How to take your pulse
- How to measure your respiratory rate
- How to take your temperature
- How well do face masks protect against COVID-19?
- Is hydroxychloroquine a treatment for COVID-19?
- Loss of smell
- Mayo Clinic Minute: You're washing your hands all wrong
- Mayo Clinic Minute: How dirty are common surfaces?
- Multisystem inflammatory syndrome in children (MIS-C)
- Nausea and vomiting
- Pregnancy and COVID-19
- Safe outdoor activities during the COVID-19 pandemic
- Safety tips for attending school during COVID-19
- Sex and COVID-19
- Shortness of breath
- Thermometers: Understand the options
- Treating COVID-19 at home
- Unusual symptoms of coronavirus
- Vaccine guidance from Mayo Clinic
- Watery eyes
Related information
- COVID-19 vaccines: Get the facts
- How well do face masks protect against coronavirus?
- Post-COVID Recovery
News on coronavirus disease 2019 (COVID-19)
Learn the latest medical news about COVID-19 on Mayo Clinic News Network.
- Fight coronavirus COVID-19 transmission at home
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

IMAGES
COMMENTS
Coronavirus disease 2019 (COVID-19) is a highly contagious viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has had a catastrophic effect on the world, resulting in more than 6 million deaths worldwide. After the first cases of this predominantly respiratory viral illness were reported in Wuhan, Hubei Province, China, in late December 2019, SARS ...
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
From online data collection to identification of disease mechanisms: the IL-1ß, IL-6 and TNF-α cytokine triad is associated with post-acute sequelae of COVID-19 in a digital research cohort ...
These papers highlight valuable research into the biology of coronavirus infection, its detection, treatment as well as into vaccine development and the epidemiology of the disease. Browse all Top ...
Long-term effects of coronavirus disease 2019 (COVID-19). The meta-analysis of the studies included an estimate for one symptom or more reported that 80% of the patients with COVID-19 have long ...
The Johns Hopkins Coronavirus Resource Center has collected, verified, and published local, regional, national and international pandemic data since it launched in March 2020. From the beginning, the information has been freely available to all — researchers, institutions, the media, the public, and policymakers. As a result, the CRC and its data have been cited in many published research ...
### What you need to know Since the emergence of SARS-CoV-2 in December 2019, there has been an unparalleled global effort to characterise the virus and the clinical course of disease. Coronavirus disease 2019 (covid-19), caused by SARS-CoV-2, follows a biphasic pattern of illness that likely results from the combination of an early viral response phase and an inflammatory second phase. Most ...
The WHO Covid-19 Research Database is a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued. The WHO evidence retrieval sub-group has begun ...
Yet well into the fourth year of the Covid-19 pandemic, intense political and scientific debates about its origins continue. The two major hypotheses are a natural zoonotic spillover, most likely ...
Introduction. The COVID-19 pandemic upended many normal practices around the conduct of research and development (R&D); the extent of disruption is revealed across measures of scientific research output [1-3].This paper revisits the extent to which patterns of international collaboration in coronavirus research during the COVID-19 pandemic depart from 'normal' times.
Across the world, there are multiple variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (Covid-19).
As reported by the CDC, from February 12 to April 2, 2020, of 149,760 cases of confirmed COVID-19 in the United States, 2572 (1.7%) were children aged <18 years, similar to published rates in ...
The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued. ... The Centers for Disease Control and Prevention (CDC ...
Although coronavirus disease 2019 (Covid-19) can be mild or asymptomatic, 1,2 severe cases can lead to hospitalization and are associated with substantial morbidity and mortality. 3,4 Older ...
Abstract. Severe acute respiratory syndrome (SARS) and Coronavirus disease 2019 (COVID-19) are two human Coronavirus diseases emerging in this century, posing tremendous threats to public health and causing great loss to lives and economy. In this review, we retrospect the studies tracing the molecular evolution of SARS-CoV, and we sort out ...
Introduction. During the Coronavirus disease 2019 (COVID-19) outbreak, problems have not been limited to respiratory illness, but have also included multi-organ failure, intensive care, and geriatric issues, necessitating various care approaches [Citation 1, Citation 2].Hospitalists have been defined as physicians who provide comprehensive acute care services for inpatients [Citation 3 ...
The process of SARS-CoV-2 infection, responsible for the COVID-19 pandemic, is carried out through different steps, with the interaction between ACE2 and Spike protein (S) being crucial. Besides of that, the acidic environment of endosomes seems to play a relevant role in the virus uptake into cells and its intracellular replication. Patients affected by two rare genetic tubulopathies ...
U-shaped structure was used in medical image segmentation tasks, but information was lost between the downsampling and upsampling stages, resulting in incomplete and inadequate segmentation results. To solve these challenges, this paper designed a dual-path information enhanced pyramid Unet network (DIEP-Unet) for COVID-19 CT image segmentation.
Pregnant people infected with SARS-CoV-2 virus have increased risks of morbidity, mortality, and adverse pregnancy outcomes. 1-6 While pregnant people were not initially included in COVID-19 vaccination trials, observational data suggest vaccination during pregnancy reduces the risks of severe COVID-19 disease and adverse birth outcomes. 7-13 ...
Published as a "major article" in The Journal of Infectious Diseases online March 13, the study showed that 124 men between the ages of 19 and 74 who possessed the IL1RN variant, called rs419598 ...
Background: The COVID-19 pandemic was characterized by mild-to-moderate disease in children and adolescents, with low incidences of severe cases and mortality. Most of the information on drug therapy in COVID-19-positive children was derived from research in adult patients. Remdesivir, an inhibitor of viral RNA polymerase, was shown to be effective in COVID-19 patients with moderate-to-severe ...
ISARIC, Global Support Centre, COVID-19 Clinical Research Resources, Epidemic diseases Research Group, Oxford (ERGO), University of Oxford, Oxford, UK James Lee & Daniel Plotkin
Further research should review the effectiveness of specific interventions during the COVID-19 pandemic for collation in a subsequent update to this current paper. Fourthly, the current review synthesises findings from countries with individualistic and collectivistic cultures, which may account for some variation in the findings.
Worldwide surveys done in 2020 and 2021 found higher than typical levels of stress, insomnia, anxiety and depression. By 2022, levels had lowered but were still higher than before 2020. Though feelings of distress about COVID-19 may come and go, they are still an issue for many people. You aren't alone if you feel distress due to COVID-19.
The virus spreads when other people breathe in infected droplets or when the droplets land in their eyes, noses or mouths. The COVID-19 virus also can spread if people touch their eyes, noses or mouths after touching a surface with the virus on it. Without cleaning and disinfection, the COVID-19 virus may stay on surfaces from hours to days.