

About the Journal
Diabetes publishes original research about the physiology and pathophysiology of diabetes mellitus. Submitted manuscripts can report any aspect of laboratory, animal, or human research. More About Diabetes
Editor in Chief
David A. D'Alessio, MD Duke University
Impact Factor
Journal Statistics
Current Issue Highlights

An Insulin-Chromogranin A Hybrid Peptide Activates DR11-Restricted T Cells in Human Type 1 Diabetes

Cardiovascular Autonomic Neuropathy and Risk of Kidney Function Decline in Type 1 and Type 2 Diabetes: Findings From the PERL and ACCORD Cohorts

GRP78 Contributes to the Beneficial Effects of SGLT2 Inhibitor on Proximal Tubular Cells in DKD

Cell-Surface ZnT8 Antibody Prevents and Reverses Autoimmune Diabetes in Mice
Access the complete Table of Contents .

Follow Diabetes on X
Join us on X @Diabetes_ADA to connect with the diabetes research community and share ideas with fellow professionals. Find the newest published articles, latest podcasts, new professional books, and up-to-date diabetes news. You can also follow ADA Publications on Facebook @adaPublications .

New Podcast: DiabetesBio
DiabetesBio, the American Diabetes Association's monthly podcast, features Drs. Kirk Habegger, Darleen Sandoval, and Kevin Williams interviewing authors of key articles from the journal Diabetes .

Paper of the Month
Each month, the Editors of Diabetes select an original research article to designate Paper of the Month. View these exclusive articles in a freely accessible special article collection.

Pathway to Stop Diabetes
The ADA vision: find a new generation of scientists at the peak of their creativity and provide them the freedom, autonomy, and financial and professional resources for breakthrough discoveries.
- Online ISSN 1939-327X
- Print ISSN 0012-1797
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 08 May 2024
Advances and challenges of the cell-based therapies among diabetic patients
- Ramin Raoufinia 1 , 2 ,
- Hamid Reza Rahimi 2 ,
- Ehsan Saburi 2 &
- Meysam Moghbeli ORCID: orcid.org/0000-0001-9680-0309 2
Journal of Translational Medicine volume 22 , Article number: 435 ( 2024 ) Cite this article
77 Accesses
Metrics details
Diabetes mellitus is a significant global public health challenge, with a rising prevalence and associated morbidity and mortality. Cell therapy has evolved over time and holds great potential in diabetes treatment. In the present review, we discussed the recent progresses in cell-based therapies for diabetes that provides an overview of islet and stem cell transplantation technologies used in clinical settings, highlighting their strengths and limitations. We also discussed immunomodulatory strategies employed in cell therapies. Therefore, this review highlights key progresses that pave the way to design transformative treatments to improve the life quality among diabetic patients.
Diabetes mellitus poses a formidable global public health challenge due to its rapid growing prevalence and associated morbidity, disability, and mortality [ 1 ]. According to the International Diabetes Federation, over 537 million adults aged 20–79 had diabetes worldwide in 2021 that is expected to rise to around 783 million cases by 2045 [ 2 ]. Obesity, unhealthy diets, physical inactivity as well as genetic and epigenetic predispositions are important risk factors of diabetes [ 3 , 4 , 5 ]. Diabetes is typically classified into type 1 diabetes mellitus (T1DM), gestational diabetes mellitus (GDM), and type 2 diabetes mellitus (T2DM) [ 2 ]. T1DM primarily arises from autoimmune-related damage of insulin-secreting beta cells, resulting in severe hyperglycemia and ketoacidosis [ 6 ]. In contrast, T2DM generally has a more gradual onset characterized by insulin resistance along with diminished compensatory insulin secretion from pancreatic beta cell dysfunction [ 7 ]. Diabetes is associated with macrovascular complications such as heart disease and stroke, as well as microvascular issues in eyes, kidneys, and nervous system [ 8 ]. Cancer is also a leading cause of diabetes-related death, and dementia-associated mortality has risen in recent decades [ 9 , 10 , 11 , 12 ]. Cell therapy involves transferring autologous or allogenic cellular material into patients [ 13 ]. The global market size of cell therapy is estimated to grow from $9.5 billion in 2021 to $23 billion by 2028 [ 14 ]. It combines stem and non-stem cell therapies consisting of unicellular or multicellular preparations. Cell therapies typically use autologous or allogenic cells via injection and infusion [ 15 ]. In the present review, we discussed the recent advances in cell-based therapy of diabetes, from foundational islet transplantation to regenerative strategies to highlight key developments that improve the effective treatments for diabetic patients.
Cell replacement therapy for diabetes
Pancreatic transplantation was firstly used in 1966 to treat type 1 diabetes using whole organ transplants. During the 1970s–80s, segmental pancreatic grafts were combined with techniques to divert digestive secretions away from transplanted cells. Three main techniques emerged; simultaneous pancreas-kidney transplants, pancreas transplants following kidney transplants, and pancreatic transplants. International collaboration on tracking outcomes began in 1980 with the formation of several pancreatic transplant registries and associations. However, whole organ transplantation was faced with several challenges including organ rejection, vascular complications, limited organ availability, and the effects of lifelong immunosuppression [ 16 , 17 ]. Islet cell transplantation was explored as an alternative, however isolating and transplanting pancreatic islets proved difficult due to donor availability, rejection, and immunosuppression side effects. Recent research has focused on stem cell sources that could reconstitute immune tolerance and preserve beta cell function such as mesenchymal stem cells, bone marrow cells, and embryonic stem cells [ 18 ]. A novel stem cell therapy called VX-880 was developed using proprietary technology to grow insulin-producing beta cells from allogeneic stem cells. Clinical trials began in 2021 after FDA approval to deliver the cells intrahepatically under immune suppression. A second approach called VX-264 encapsulates the same cells, avoiding immunosuppression but requiring surgical implantation [ 17 ]. In 2023, FDA approved the first allogeneic pancreatic islet cell therapy called Lantidra for adults with type 1 diabetes experiencing severe hypoglycemia. Approval was based on two studies where 21–30% of participants no longer required insulin one year post-treatment, with benefits lasting over five years in some cases. However, this treatment have mild and serious adverse events that are associated with treatment dose and the methods of islet cell infusion [ 19 , 20 ].
Emerging strategies for cell delivery via microencapsulation and biological devices in clinical trials
Alginate capsules as cell delivery systems.
A seminal investigation conducted in 1994 demonstrated the successful transplantation of alginate-encapsulated islets into the peritoneum of kidney transplant patients who were receiving immunosuppression therapy. Remarkably, these patients achieved insulin independence for up to nine months [ 21 ]. However, subsequent trials conducted without immunosuppression yielded inconsistent outcomes. In a study conducted in 2006, islets were encapsulated in triple-layer alginate capsules and implanted intraperitoneally in type 1 diabetes (T1D) patients. There was a positive correlation between the encapsulation and insulin production that reduced exogenous insulin requirements during one year. Despite this progress, the entry of cytokines remained a potential concern [ 22 ]. Another study employed the single-layer barium-alginate capsules that sustained insulin production for up to 2.5 years [ 23 ]. It has been reported that the microneedle, comprising a calcium alginate frame with polydopamine-coated poly-lactic-co-glycolic acid microspheres encapsulating insulin, enables light-triggered insulin release. Microneedle provided a suitable insulin dose to maintain blood glucose levels in line with daily fluctuations. These results established the efficacy and safety of the developed microneedle for diabetes treatment [ 24 ]. Another therapeutic approach explored the encapsulation of pancreatic islets with mesenchymal stem cells (MSCs) and decellularized pancreatic extracellular matrix (ECM). ECM derived from the pancreas supported islet cell growth and maintenance to enhance insulin expression [ 25 ]. Sodium alginate and hyaluronic acid were incorporated due to their roles in collagen production, wound healing, and physical crosslinking. The 3D porous membranes allowed optimal water and oxygen transfer while diverting excess exudate from diabetic wounds. Hydrogel accelerated re-epithelization, while decreased inflammation, indicating potential as the diabetic wound dressings [ 26 ]. Additionally, the incorporation of specific ECM components, such as collagen IV and RGD, into alginate-based microcapsules significantly improved the survival, insulin secretion, and longevity of microencapsulated islets [ 27 ].
Encaptra® device from ViaCyte
In contrast to microencapsulation techniques, ViaCyte developed a semipermeable pouch method named Encaptra, which contains pancreatic precursor cells derived from the embryonic stem cells [ 28 ]. In the initial trial conducted in 2014, the “VC-01” device was implanted in T1D individuals without the use of immunosuppression [ 29 ]. The trial confirmed the safety of the device; however, the occurrence of hypoxia induced cellular necrosis [ 30 ]. The device was modified as “VC-02” with larger pores, and two trials (NCT03162926, NCT03163511) demonstrated promising outcomes, including increased fasting C-peptide levels and a 20% reduction in insulin requirements during one year in the majority of participants [ 31 ]. In order to eliminate the necessity for immunosuppressants, ViaCyte collaborated with Gore to develop an expanded polytetrafluoroethylene (ePTFE) device with both immuno-isolating and pro-angiogenic properties [ 32 ]. This device (NCT04678557) aimed to prevent immune cell attachment and T-cell activation [ 33 ]. Additionally, ViaCyte is exploring the integration of CRISPR technology to modify stem cells, specifically by eliminating β2-microglobulin expression and PD-L1 up regulation. It is hypothesized that these genetic modifications will further hinder immune cell attachment and T-cell activation [ 30 , 34 ].
Semipermeable device from Semma therapeutics
Semma Therapeutics, which has been acquired by Vertex, pioneered the utilization of differentiated stem cell-derived islet cell clusters in clinical trials. Semma houses these cells between two semipermeable polyvinylidene fluoride membranes and is designed for subcutaneous implantation (NCT04786262) [ 31 , 35 ]. Vertex reported a significant breakthrough by infusing differentiated beta cells via the portal vein in a participant who was receiving immunosuppressants. This approach led to substantial C-peptide production and improved glycemic control during 90 days [ 36 ].
βAir device from Beta O2
Beta O2’s innovative βAir device utilizes an alginate-PTFE membrane complex to encapsulate islets, providing partial immunoisolation while ensuring a continuous supply of oxygen, which is crucial for optimal islet function [ 37 , 38 ]. The βAir device that was seeded with human islets was subcutaneously implanted in T1D individuals (NCT02064309). Although, low insulin levels were produced for up to eight weeks, there was not any reduction in the required exogenous insulin [ 37 ]. While, increasing the number of islets could potentially enhance their function, it is important to note that the continuous reliance on oxygen poses a risk of infection, despite efforts to optimize the survival of encapsulated islets [ 39 , 40 ].
Cell pouch™ device from Sernova
Sernova has developed the Cell Pouch device, which offers pre-vascularized polypropylene chambers for islet transplantation without the need for immunoprotection. The device consists of multiple cylindrical chambers that are prefilled with PTFE plugs, which are then removed after implantation to create the empty space [ 41 ]. In a 2012 trial (NCT01652911), islets were placed in the vascularized pouches of three recipients who were also receiving immunosuppression that resulted in a transient increase in C-peptide levels [ 41 ]. In a 2018 trial (NCT03513939), immunosuppression was administered after implantation and islet introduction. This trial reported sustained C-peptide production for up to nine months in two recipients, along with improved glycemic control [ 42 ]. Regarding the limitations of immunosuppression, Sernova is exploring the possibility of encapsulating islets in hydrogel as an alternative approach [ 43 ].
Shielded living therapeutics™ from Sigilon Therapeutics
Sigilon has developed the Shielded Living Therapeutics sphere, which consists of cell clusters enclosed within an alginate-TMTD coating [ 44 ]. Preclinical studies demonstrated that murine islet transplants encapsulated within these spheres maintained normoglycemia for a period of six months [ 45 ]. In a 2020 trial conducted for hemophilia (NCT04541628), the spheres were evaluated for their ability to express Factor VIII [ 46 ]. However, the trial was paused due to the development of antibodies in the third recipient receiving the highest cell doses. While, preclinical studies have shown promising efficacy, there are safety concerns regarding the TMTD coating that need to be addressed before these spheres can be used for human islet transplantation as a treatment for diabetes [ 31 ]. Emerging technologies have been investigated in clinical trials for delivering insulin-producing islets or stem cell-derived beta cells via microencapsulation or use of implantable biological devices (Table 1). Optimizing encapsulation and developing alternative implantable devices moves the field toward delivering safe and effective islet replacement without chronic immunosuppression dependency that represented an important new frontier for the cell-based treatment of diabetes. However, continued refining will be required to fully realize this promising vision and using these preclinical concepts in clinic.
Immunoengineering strategies: biomaterials for modulating immune responses
Islet encapsulation aims to prevent immune responses toward transplant antigens. However, foreign body response (FBR) against biomaterials induces inflammation around encapsulated islets that obstructs oxygen/nutrient access and causes graft failure [ 31 ]. Extensive research revealed biomaterial properties profoundly influence FBR severity, with high purity/biocompatibility moderating inflammation [ 47 ]. Deeper understanding of biomaterial immunobiology enabled developing immune-modulating constructs to steer host interactions. By altering topology/chemistry to hinder nonspecific binding and cell adhesion, these “immune-evasive biomaterials” intended to attenuate xenograft rejection at inception [ 44 ]. Both innate and adaptive immune responses have crucial roles in the context of pancreatic islet transplantation. These responses encompass the activation of tissue macrophages and neutrophils following injury, leading to the release of inflammatory cytokines that subsequently activate antigen-presenting cells (APCs), CD8 + T cells, CD4 + T cells, and cytotoxic T lymphocytes (Fig. 1 ). Zwitterionic polymers conferred anti-fouling attributes but crosslinking limitations constrained their application [ 48 ]. Novel mild zwitterionization introduced alginate modifications that prolonged prevention of fibrotic overgrowth by mitigating initial responses [ 49 , 50 , 51 ]. The prevention of graft rejection following islet cell transplantation necessitates the systemic administration of immunosuppressive agents. While, these agents effectively suppress immune responses, their continuous use exposes patients to an increased risk of infection and cancer. To mitigate these concerns, an alternative approach involving the localized delivery of immunosuppressants at the transplantation site has emerged. This localized delivery system offers several advantages, including targeted drug delivery, reduced systemic exposure, and potentially reduces the immunosuppressants doses [ 52 ]. Polymeric carriers dispersed cyclosporine A continuously at the graft site to dynamically tamp down proinflammatory cascades and T-cell activation [ 53 , 54 ]. TGF-β/IL-10 co-delivery at the microencapsulation interface hindered innate antigen presentation, obstructing adaptive response priming [ 55 , 56 ]. Regulatory T-cells emerged as the potent immunomodulators when coated on islets to improve insulin production in vitro [ 57 ]. Similarly, recombinant Jagged-1 surface patterning increased regulatory lymphocytes in vitro while enhancing glycemic oversight in vivo [ 58 ]. Targeting proinflammatory effector T-cells or presenting their Fas ligand death receptor improved long-term viability when combined with rapamycin prophylaxis [ 52 , 59 ]. Immobilizing thrombomodulin or urokinase mitigated local inflammation, with the latter conferring lifelong xenotransplant survival [ 60 ]. Peptides recognizing IL-1 receptors provided robust protection from destabilizing proinflammatory cytokines [ 61 ]. Leukemia inhibiting factor improved islet performance over polyethylene glycol encapsulation alone by inducing regulatory T-cell lineages [ 62 ]. Silk scaffolds facilitated IL-4/dexamethasone emancipation that meaningfully decreased immune reactions to grafts [ 63 ]. Therefore, the localized delivery of immunosuppressants at the transplantation site represents a promising strategy for islet cell transplantation. Compared to systemic administration, local delivery can achieve targeted immune modulation only at the graft location while reducing drug exposure throughout the body. This localized approach aims to sufficiently suppress the immune response to prevent rejection, while limiting negative side effects that may occur from systemic immunosuppression. A variety of biomaterials and surface modification strategies have been developed and investigated for the local delivery of immunosuppressive agents and immunomodulatory cytokines [ 64 , 65 , 66 ]. Understanding how biomaterial properties influence the immune response is critical to design biomaterials that can modulate inflammation and improve islet graft survival through localized immunomodulation.
Cell-based therapy through the integration of additive manufacturing techniques
Additive manufacturing utilizes computer modeling to fabricate complex 3D structures on-site with minimal post-processing. Common methods for the biomedical application are fused filament fabrication (FFF), stereolithography (SLA), and bioprinting [ 67 ]. FFF is a layer-by-layer technique that extrudes heated thermoplastics [ 68 ]. Commonly used feedstocks include acrylonitrile butadiene styrene (ABS) and polylactic acid (PLA). Other thermoplastics that have been utilized with FDM include thermoplastic polyurethane (TPU), polycarbonate (PC), polystyrene (PS), polyetherimide (PEI), polycaprolactone (PCL), polyaryletherketone (PAEK), and polyetheretherketone (PEEK), with the latter demonstrating high strength and heat tolerance. A major advantage of FDM is its ability to fabricate multi-material objects through continuous printing and alteration of the build material. In addition to typical polymers like PC and polystyrene (PS), FDM can print composites reinforced with glass, metals, ceramics, and bioresorbable polymers via integration of the constituent powders with a binding matrix. This enables enhanced control over the experimental component fabrication. While, ceramic and metal filaments traditionally contain the corresponding powder mixed with a binder, FDM provides versatility in the functional prototype construction from a wide range of thermoplastic feedstocks using precise and additive layer manufacture [ 68 , 69 , 70 , 71 , 72 ]. It provides geometric reproducibility and reduced variability compared to traditional techniques. FFF prints served as scaffolds for the transplanted cells [ 67 ]. However, minimum feature size is limited to ? ∼ 250 μm by nozzle diameter [ 68 ]. SLA employs light-curable liquid resins and achieves higher 50–150 μm resolution than FFF but with restricted material choices. Bone grafts and surgical guides are common applications [ 67 ]. Incorporating biomaterials like hydroxyapatite has expanded utility, though processing is required to mitigate cytotoxicity. Additive manufacturing can address limitations in oxygen transport, cell/material placement control and vasculature formation, and clinically translatable insulin-secreting implants [ 67 ]. Therefore, additive manufacturing technologies have the potential to enhance various aspects of the cell-based transplant design, from improving nutrient transport through optimized implant geometry to achieving precision integration of therapeutic agents (Table 2).
Enhancing nutrient transport through optimization of implant geometry
Tissue engineering for the islet transplantation requires maximizing nutrient transport [ 73 , 74 ]. Traditional scaffold fabrication introduces macroporosity but lacks precision that results in inflammation [ 67 ]. Cell encapsulation provides immunoprotection by limiting interactions between transplanted cells and the host immune system. However, this protective barrier also poses challenges for the efficient transport of essential nutrients, including oxygen, to the encapsulated cells. Modifying the geometries of encapsulation devices using conventional methods to enhance oxygen delivery has proven to be inconsistently challenging [ 67 ], so that novel approaches are required to address these challenges. Additive manufacturing allows customizing biomaterial scaffolds with defined geometries and micropore sizes to improve transport [ 75 , 76 , 77 , 78 , 79 ]. The 3D printed PLA scaffolds with islets have successful vascularization and cellular survival after subcutaneous transplantation [ 80 , 81 ]. Interlocking toroidal hydrogel-elastomer constructs also increased surface area and cell viability [ 82 , 83 , 84 ].
Enhancing vascularization and engraftment
Rich host vascularization of transplant devices is essential to support long-term islet survival through efficient nutrient delivery and insulin kinetics. Early platforms modified bulk material properties to promote vessel infiltration and anastomoses [ 85 , 86 , 87 , 88 , 89 ]. Additive manufacturing can further optimize microscale geometry to both accelerate host vessel connections and control intra-device vasculature homogeneity beyond traditional fabrication. Initial work reproduced macroscale vessels but scales were diverged from cell-based therapies [ 73 , 90 , 91 , 92 ]. Leveraging Additive manufacturing designed structures guided vessel formation in vitro and in vivo [ 80 , 89 , 93 ]. Shifting to bioprinting complex branching conduits in supportive hydrogels facilitated clinical translation for diverse cell therapies [ 94 , 95 , 96 , 97 , 98 ]. Researchers focused on developing a 3D scaffold platform to improve the transplantation outcomes of islet cells in T1D. The scaffold featured a heparinized surface and immobilized vascular endothelial growth factor (VEGF) to enhance vascularization. Scaffold effectively promoted angiogenesis and facilitated the growth of new blood vessels. Additionally, encapsulated islets within the scaffold had functional responses to glucose stimuli. These findings suggested that the developed scaffold platform holds potential for successful extra-hepatic islet transplantation, offering new possibilities for T1D treatment [ 99 ]. Research on vascularization of islets via additive manufacturing techniques has primarily focused on the fundamental discoveries. In one study, engineered pseudo islets (EPIs) were created by combining the mouse insulin-secreting beta cells with rat heart microvascular endothelial cells. EPIs demonstrated extensive outgrowth of capillaries into the surrounding matrix. Although, EPIs containing both cell types that underwent capillarization maintained viability and function over time in culture, non-vascularized EPIs lacking endothelial cells could not sustain viability or functionality long-term. This supported the potential for inducing angiogenesis within bioengineered islet constructs. Future work may combine patient-specific stem cell-derived human beta cells with endothelial cells using this approach to promote long-term graft survival for treating type 1 diabetes [ 98 ]. While, large-scale 3D printed vascularized structures are currently limited for the islet transplantation, advancements in leveraging additive manufacturing for the optimization vascularization conditions through the pore sizes and material choices, may facilitate translation to β-cell therapy in type 1 diabetes.
Precision placement of cells and matrix for enhanced control
Beyond distributing biomaterials, additive manufacturing enables micro-level cell and protein control. For islet transplantation, optimal cellular distribution and supportive extracellular matrix niche reduce rapid dysfunction and apoptosis [ 100 , 101 , 102 ]. Traditional techniques heterogeneously load cells after fabrication or struggle with incomplete encapsulation [ 103 , 104 ]. Bioprinting allows in situ encapsulation and printing of multiple cell types and matrix components while dictating 3D placement and dimensions [ 105 , 106 ]. Islet transplant research prints hydrogel-encapsulated clusters surrounded by supportive cells and doped with immune modulators to improve the transplant environment [ 107 ]. Progress in bioprinting offers consistency and defines physical/chemical graft properties beyond traditional fabrication.
Achieving controlled integration of therapeutic agents for enhanced efficacy
In addition to the cell and matrix placement, additive manufacturing enables precision therapeutic integration. Incorporating therapeutics aims to recapitulate the in vivo environment through angiogenesis, islet health promotion, and immunomodulation [ 67 , 108 ]. Growth factors promote vessel formation and insulin secretion while decrease apoptosis [ 108 , 109 , 110 , 111 ]. Local immunomodulators regulate the immune system in a specific site of the body. They decrease inflammation and promote the successful integration of transplanted cells or tissues by minimizing the need for widespread immune suppression in whole body [ 67 ]. Traditional homogeneous delivery methods restrict the ability to customize the spatial distribution of substances and pose a risk of harmful effects on transplants or hosts [ 112 ]. The use of discreet gradients in bioprinting can offer precise physiological signals. By combining traditional drug release methods with AM, it becomes possible to create tissues that exhibit distinct therapeutic localization. Bioprinted composites have the ability to release factors with gradients throughout the entire construct that enables a more comprehensive and targeted approach in tissue engineering [ 112 , 113 , 114 ].
Cell based gene therapy
Gene therapy holds great promise for diabetes management, offering innovative approaches to deliver and manipulate the insulin gene in various tissues. Viral methods, such as lentivirus, adenovirus, and adeno-associated virus (AAV), along with non-viral techniques like liposomes and naked DNA, have been utilized to deliver the insulin gene to target tissues [ 115 ]. This section aims to provide an overview of important studies in the field of gene therapy for diabetes management, emphasizing advancements in insulin gene delivery and manipulation (Table 3).
Enteroendocrine K-cells and pancreatic β-cells
Enteroendocrine K-cells in the intestines and pancreatic β-cells share similarities in their production of glucose-dependent insulinotropic polypeptide (GIP) and their regulatory mechanisms. Understanding these similarities offers insights into T2D management and improving glucose homeostasis. However, attempts to reverse diabetes effectively through K-cell transplantation have been unsuccessful. Nevertheless, research on gene editing techniques has shown promising results in management of the diabetes mellitus [ 116 , 117 ]. AAV vectors have been employed to co-express insulin and glucokinase genes in skeletal muscles, demonstrating long-term effectiveness in achieving normo-glycemia without exogenous insulin [ 118 , 119 ].
Gene editing techniques
Gene editing techniques using AAV vectors effectively improved normo-glycemia in animal models. Co-expression of insulin and glucokinase in transgenic mice increased glucose absorption and regulated insulin production. Duodenal homeobox 1 (PDX1) gene transfer via AAV2 in a humanized liver mouse model also led to insulin secretion and glycemic control [ 120 ]. Adenovirus-mediated transfection of hepatic cells with neurogenin 3 (NGN3) resulted in insulin production and trans-differentiation of oval cell populations [ 121 , 122 ]. Targeting specific promoters in liver cells such as phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase), albumin, and insulin-like growth factor binding protein-1 (IGFBP-1) enhanced hepatic insulin gene therapy [ 123 , 124 ]. AAV-mediated overexpression of SIRT1 reduced inflammation, hypoxia, apoptosis and improved neural function in the retina of diabetic db/db mice [ 125 ]. Another study developed a plasmid expressing a single-strand insulin analogue for intramuscular injection using a specialized gene delivery technique. A single administration provided sustained insulin expression for 1.5 months and effectively regulated blood glucose levels without immune responses or tissue damage in diabetic mice.
Non-viral gene delivery methods
Non-viral approaches have also key roles in achieving glycemic control. The combination of insulin fragments with DNA plasmid, administered via intravenous injection improved normo-glycemia for extended periods. DNA transposon facilitated gene integration into the host chromosome that addressed the short-term liver expression. Additionally, the co-injection of DNA plasmid containing insulin with furin significantly enhanced insulin production within muscles [ 126 ]. Non-viral plasmids were engineered to carry proinsulin and pancreatic regenerating genes to ameliorate streptozotocin-induced T1DM [ 127 ]. The pVAX plasmid vectors prolonged therapeutic effects in achieving normo-glycemia without the need for further treatment [ 127 ]. Bioreducible cationic polymers, such as poly-(cystamine bisacrylamide-diamino hexane) (p(CBA-DAH)), have been employed to deliver RAE-1 to pancreatic islets, resulting in improved insulin levels [ 128 ]. Furthermore, ex vivo gene transfer and autologous grafts have shown promising outcomes in animal models. The introduction of the human insulin gene into pancreatic or liver cells followed by autologous grafts improved insulin secretion, glycemic control, and alleviated the diabetic complications in pigs. However, gene silencing eventually occurred, necessitating a deeper understanding of the underlying mechanisms [ 128 , 129 ].
Stem cell based therapy in diabetes
Efforts are ongoing to develop standardized processes for donor and recipient selection/allocation to increase pancreas utilization [ 130 , 131 , 132 , 133 ]. Techniques for isolating pancreatic islets are being optimized to become more standardized and consistent. Noninvasive imaging technologies allow the monitoring of the transplanted islets without surgery [ 134 , 135 ]. Biomarkers could also evaluate how immunomodulation strategies are working [ 136 , 137 , 138 ]. Researchers are also exploring alternative transplant sites in the body beyond just the liver, to see if the other locations may better support islet graft survival and function. Together, these areas of refinement aim to improve the safety and reliability of islet transplantation procedures as a potential therapy for diabetes [ 139 ]. Bioengineering approaches are being developed to optimize the islet transplantation microenvironment using biomaterials which enhance islet engraftment and function through engineered extracellular niches [ 140 , 141 ]. For example, encapsulation techniques aim to protect pancreatic islets against immune reponse by enclosing them within semipermeable hydrogel polymer capsules [ 142 , 143 ]. This localized immunoisolation strategy utilizes biomaterials like alginate to create a physical barrier preventing immune cell contact while still allowing nutrient and oxygen diffusion. Researchers concurrently seek alternative unlimited cellular sources to address limited islet availability. Mesenchymal stem cells possess immunomodulatory properties and their adjuvant delivery, either early in disease onset or simultaneously with islet transplantation, has shown promising signs of improving outcomes in preclinical investigations. By dampening inflammatory responses and favoring regenerative processes, stem cells may help to establish a more tolerogenic transplant environment. These bioengineering and cell therapy approaches offer potential pathways towards eliminating the exogenous insulin requirement [ 144 , 145 ]. A variety of stem cell types have therapeutic potential for diabetes (Fig. 2 ). Pluripotent stem cells possess immense promise for overcoming the limitations of islet transplantation. Human embryonic stem cells and induced pluripotent stem cells are especially attractive candidates due to their unique ability to both self-renew indefinitely and differentiate into any cell type. This makes them an ideal source of replacement pancreatic beta cells. Significant research effort across academic and industrial laboratories has led to advancement in differentiation protocols that can convert pluripotent stem cells into functional beta-like cells in vitro. However, establishing consistent, well-characterized cellular production methods that comply with stringent safety and efficacy standards remains a priority for clinical translation. Ongoing work aims to generate therapeutic stem cell-derived beta cell replacements exhibiting stable, glucose-responsive insulin secretion comparable to primary islets. Although, technological and regulatory hurdles still must be cleared, pluripotent stem cells have the greatest potential to finally solve the problem of limited cell availability and provide an unlimited source of transplantable tissue suitable for widespread treatment of diabetes [ 145 , 146 , 147 , 148 ]. There are currently six registered clinical trials evaluating the use of human pluripotent stem cells for the T1D treatment. All trials except one use PEC-01 cells, which consist of a mixture of pancreatic endoderm and polyhormonal cell population derived from CyT49 stem cells that are fully committed to endocrine differentiation upon implantation [ 149 ]. The initial trial implanted PEC-01 cells within an encapsulation device, hypothesizing no need for immunosuppression. While, well-tolerated with minor adverse effects, insufficient engraftment occurred due to foreign body responses that eliminated the cells [ 150 ]. The trial transitioned in 2017 to use an open encapsulation device that required immunosuppression. Subcutaneous engraftment, differentiation of cells into islet-like clusters, and glucose-responsive insulin production provided the first evidence that pancreatic progenitor cells can survive, mature, and function as the endocrine cells in humans. Potential benefits on stimulated C-peptide levels and glycemic control were observed in one patient [ 151 , 152 ]. Two reports in late 2021 described results in 17 patients receiving PEC-01 cells in an open device. Engraftment and insulin expression occurred in the majority, glucose-responsive secretion in over one-third, and various glycemic improvements were observed at six months. Explanted tissues contained heterogeneous pancreatic compositions including mature beta cells, with no teratoma formation and mild adverse effects related to surgery/immunosuppression. VX-880 uses fully differentiated insulin-producing stem cell-derived islet cells in phase 1/2 trial evaluating portal infusion and different doses requiring immunosuppression. Preliminary results suggest early engraftment and insulin secretion. The manin challenge was controlling immune rejection without systemic immunosuppression [ 149 ]. Several strategies are being explored to address the challenges of immune rejection in stem cell therapies for diabetes. They include generating stem cell lines that are universally compatible through HLA silencing, developing milder regimens of immunosuppression, and refining encapsulation and containment approaches to protect transplanted cells toward immune response. Establishing standardized stem cell banks is also an area of investigation [ 153 , 154 ]. Xenotransplantation using gene-edited porcine islets remains an exciting avenue of research given advances to improve engraftment and reduce immunogenicity in preclinical studies [ 155 ]. Novel approaches continue to emerge as well, such as decellularization techniques, 3D bioprinting of tissue constructs, and creating interspecies chimeras. Rapid evolution of cell-based therapies across both academic and commercial sectors is promising to restore normoglycemic control in diabetic cases. Refinement of existing methods and development of new strategies hold potential to perform a safe and effective cell replacement without reliance on systemic immunosuppression. Stem cell and regenerative therapies may ultimately manage diabetes through restored endogenous insulin production [ 156 ]. Recently a meta analysis evaluated the safety and efficacy of MSC-based therapy for diabetes in humans. This comprehensive analysis was conducted on 262 patients across six trials that met the inclusion criteria within the last five years. The results reveal that treatment with MSCs significantly reduced the dosage of anti-diabetic drugs over a 12-months. Following treatment, HbAc1 levels decreased by an average of 32%, fasting blood glucose levels decreased by an average of 45%, and C-peptide levels showed a decrease of 38% in two trials and an increase of 36% in four trials. Notably, no severe adverse events were reported across all trials. Therefore, it can be concluded that MSC therapy for type 2 diabetes is safe and effective [ 157 ].
Advances in islet transplantation and stem cell-derived Beta cells
Limited number of the islet transplantation donors highlights the importance of cell therapy in diabetes. Although, higher islet numbers from multiple donors increase the success, limited pancreas availability restricts widespread use [ 158 ]. Using multiple donors also increases rejection risk, while isolation of the islets can cause tissue damage [ 159 ]. To overcome these challenges, researchers have explored the differentiation of stem cells into beta cells in vitro to generate an unlimited supply of insulin-producing cells with standardized and characterized products. Genetic engineering techniques have also been investigated to confer advantages such as stress resistance or immune evasion [ 158 ]. ViaCyte has developed a stem cell-derived pancreatic progenitor called PEC-01, which has the ability to mature into endocrine cells in rodent models. To protect the transplanted cells from immune response, retrieval encapsulation devices were also created [ 160 , 161 , 162 ]. In an initial human clinical trial conducted in 2014 (NCT02239354), the Encaptra device was utilized with the aim of providing complete immunoprotection of transplanted cells through the use of a cell-impermeable membrane. Although, the PEC-Encap product showed reliable tolerance and minimal adverse effects, the trial was stopped due to the inadequate engraftment of functional products. While, a few endocrine cells were observed, fibrosis around the capsule led to graft loss and supression of the insulin secretion. To address this challenge, a more recent development called the PEC-Direct device was introduced, which featured openings in the membrane to facilitate vascularization, thereby improving nutrient exchange and supporting cell viability. However, since host cells could infiltrate the device, immunosuppression was necessary following the transplantation [ 163 , 164 , 165 ]. Protocols were developed to generate clusters of stem cell-derived beta cells that secreted glucose-responsive insulin. These clusters, referred to SC-islets, also contained other endocrine cells, including glucagon-producing cells. SC-islets improved glycemic control in diabetic mice and nonhuman primates [ 146 , 166 , 167 , 168 ]. In a trial conducted in 2017 (NCT03163511), the transplantation of progenitor cells resulted in the maturation of endocrine cells, and glucose-responsive C-peptide secretion was observed 6–9 months post-transplantation. Notably, the majority of these mature endocrine cells exhibited glucagon-positive characteristics. The porous regions housing the endocrine cells allowed for the infiltration of host vessels to facilitate vascularization. However, non-cellular regions were isolated by the presence of fibrosis [ 164 , 165 ]. Although, there was not a sufficient levels of circulating C-peptide in these trials, the findings underscored the significance of promoting vascularization and minimizing fibrotic reactions [ 164 , 169 ]. Vertex conducted a human trial in 2021 (NCT04786262) involving the transplantation of half-dose VX-880 cells (SC-islets) without a device to avoid previous problems, which necessitated immunosuppression. Preliminary results reported improved glycemic control, although it took longer to achieve the same outcome compared to rodent models [ 158 ]. Overall, progresses in islet transplantation and stem cell-derived beta cells pave the way for overcoming the limitations of traditional approaches. Further research and refinements are also required to achieve consistent and clinically significant outcomes in the treatment of diabetes.
Chalenges and limitations
Cell-based therapies have been significantly progressed for diabetes; however, there are still several challenges that need to be overcome. Clinical trials investigating encapsulation devices and islet transplantation techniques have provided valuable insights but face several obstacles including oxygenation, host immune responses, and insufficient long-term engraftment success. Immunoengineering of biomaterials and additive manufacturing for the development of 3D islet structures aim to modulate inflammation and promote graft revascularization. Nevertheless, achieving consistent normalization of blood glucose levels without exogenous insulin remains a challenge in human studies. In the field of gene therapy and stem cell differentiation, research focuses on genetically-modified or progenitor-derived insulin-secreting β-like cells to optimize protocols that ensure safety and functionality. The main challenge is to establish stable and functional cells capable of permanently restoring normoglycemia without the need for external intervention. One major barrier is the immune response, which targets allogeneic and xenogeneic islet grafts. Although, local immunotherapy minimizes the systemic effects, evading graft destruction through biomaterials without the requirement of immune suppression remains a significant challenge. The translation of precision 3D islet constructs and genetically reprogrammed cells also necessitates scalable manufacturing processes to ensure consistent function and long-term safety across batches. When critically appraising progress in the field of cell-based diabetes treatments, it is imperative to consider the regulatory, ethical, economic, and safety factors that shape translational applications. At the regulatory level, oversight bodies play a pivotal role in establishing standards to ensure patient welfare while enabling therapeutic innovation. FDA oversees clinical trials and product approvals in the United States (US), while in Europe the EMA provides parallel regulatory guidance. Within the US, organizations like the United Network for Organ Sharing (UNOS) and Organ Procurement and Transplantation Network (OPTN) govern organ and cell allocation protocols [ 17 , 170 ]. However, as regenerative approaches diverge from traditional organ transplantation, regulatory pathways require ongoing harmonization between the agencies and jurisdictions. Continual dialogue between researchers, oversight boards, and policymakers will be crucial to streamline guidelines in a patient-centric manner that balances safety, efficacy, and timely access to cutting-edge therapies. For instance, as stem cell-derived beta cells and 3D bioprinted tissue constructs emerge, traditional drug and device frameworks may not adequately address product characterization and manufacturing complexities for these advanced therapeutic products [ 67 ]. Within clinics, maintaining compliance with evolving regulations impacts research directives and ultimately patients’ access to the novel treatments. Addressing informed consent, clinical trial design, and privacy protections for sensitive health data are also paramount from an ethical perspective [ 128 , 129 ]. Autonomy and agency of research participants in decision-making related to experimental therapies demand prudency. Equitable accessibility of new treatment options also warrants attention to avoid certain populations facing undue barriers. Cell sourcing presents ethical issues depending on derivation from embryonic, fetal or adult tissues. Logistical matters like shipping and processing stem cell-derived islets prior to transplantation necessitate scrutiny. Tumorigenic potential of the undifferentiated pluripotent stem cells should be optimized through rigorous preclinical testing. Transitioning therapies between animal and early human investigations necessitates well-characterized cellular products showing consistent safety and glucose-responsive insulin secretion profiles comparable to pancreatic islets. Long-term animal model data substantiating lack of malignant transformation following transplantation aids allaying ethical safety concerns as the therapies progress clinically. Researchers carefully screen new concepts to prevent side effects in participants while pursuing curative goals. In terms of economic costs, islet and stem cell transplant procedures remain prohibitively expensive for broad applicability despite promising clinical signals. The field requires sustained study to validate techniques, track long-term outcomes, assess healthcare costs offsets from mitigating diabetes’ debilitating complications, and establish cost-benefit ratios for national reimbursement paradigms. Public-private partnerships may accelerate large, interventional trials and longitudinal research to precisely quantify the cellular therapies’ safety profiles and real-world efficacies compared to intensive management versus costs of intensive diabetes care. Ongoing developments like 3D bioprinting offer catalytic manufacturing potential fundamentally recalibrating economics by enhancing yields, standardizing procedures, and reducing costs through scale. By thoroughly and sensitively examining regulatory frameworks, informed consent processes, risks and benefits, as well as financial considerations at both micro and macro levels, researchers, oversight boards and broader stakeholder networks can advance cell-based therapies towards delivering life-changing benefits for all communities. A multidisciplinary, conscientious approach balances progress against patient welfare. A combination of multiple strategies may help to overcome these limitations. For instance, gene-modified islets integrated within vascularized biomaterial implants or sequenced therapies have promising results to prime grafts in pro-regenerative environments before transplantation. Collaboration across disciplines offers hope that refined individualized therapies may eventually achieve durable insulin independence through functional pancreatic cell or tissue engraftment, not only for diabetes but also for chronic pancreatitis. Regarding, ongoing progresses in unraveling these barriers, cell replacement approaches have the potential to improve diabetes management.
Conclusions
This review provides a comprehensive overview of the advances, challenges, and future directions in various cell-based therapeutic approaches for the treatment of diabetes. Significant progresses have been achieved in microencapsulation design, immunomodulation, tissue constructs, genetic and cellular reprogramming techniques, as well as initial clinical translation. However, the complete restoration of normoglycemia without the need for lifelong immunosuppression is still considered as a significant therapeutic challenge. Therefore, addressing the transplant environment of the hostile nature, developing minimally invasive delivery methods, and overcoming limitations in engraftment efficiency and longevity are crucial issues for the future researches. Through the sustained multidisciplinary efforts for the improvement of existing strategies and establishing novel paradigms, achieving durable insulin independence can be a realistic goal for all diabetic cases through the personalized cell replacement or regeneration.
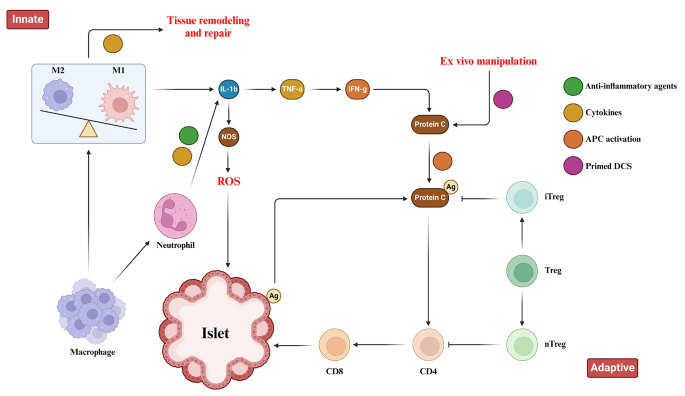
Immune Responses toward pancreatic islets following transplantation. This figure illustrates the immune responses, including the innate and adaptive immunity that are triggered upon pancreatic islet transplantation. Immune response begins with the activation of tissue macrophages and neutrophils in response to injury. Subsequent, release of inflammatory cytokines stimulates antigen-presenting cells (APCs), CD4 + T cells, CD8 + T cells, and cytotoxic T lymphocytes to orchestrate the immune response
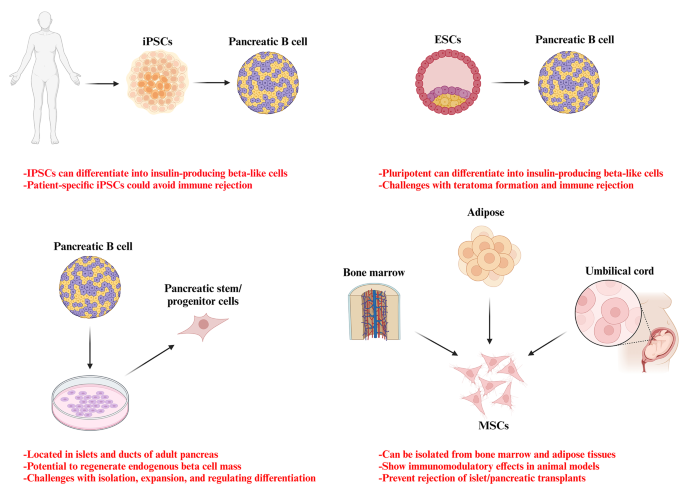
Potential stem cell sources for the treatment of diabetes
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Acrylonitrile butadiene styrene
Activate antigen-presenting cells
Adeno-associated virus
Duodenal homeobox 1
Engineered pseudo islets
Expanded polytetrafluoroethylene
Extracellular matrix
Foreign body response
Fused filament fabrication
Gestational diabetes mellitus
Glucose 6-phosphatase
Insulin-like growth factor binding protein-1
Mesenchymal stem cells
Neurogenin 3
Organ Procurement and Transplantation Network
Phosphoenolpyruvate carboxykinase
Polyaryletherketone
Polycaprolactone
Polycarbonate
Polyetheretherketone
Polyetherimide
Poly-lactic acid
Polystyrene
Stereolithography
Thermoplastic polyurethane
Type 1 diabetes
Type 1 diabetes mellitus
Type 2 diabetes mellitus
United Network for Organ Sharing
United States
Vascular endothelial growth factor
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Article Google Scholar
Cho NH, Shaw J, Karuranga S, Huang Y, da Rocha Fernandes J, Ohlrogge A, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Article CAS PubMed Google Scholar
Moghbeli M, Naghibzadeh B, Ghahraman M, Fatemi S, Taghavi M, Vakili R, et al. Mutations in HNF1A gene are not a Common cause of familial young-onset diabetes in Iran. Indian J Clin Biochem. 2018;33(1):91–5.
Akhlaghipour I, Bina AR, Mogharrabi MR, Fanoodi A, Ebrahimian AR, Khojasteh Kaffash S, et al. Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population. Hum Genomics. 2022;16(1):11.
Article CAS PubMed PubMed Central Google Scholar
Moghbeli M, Khedmatgozar H, Yadegari M, Avan A, Ferns GA, Ghayour Mobarhan M. Cytokines and the immune response in obesity-related disorders. Adv Clin Chem. 2021;101:135–68.
Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Reviews Endocrinol. 2020;16(7):349–62.
Article CAS Google Scholar
Siqueira ISLd, Alves Guimarães R, Mamed SN, Santos TAP, Rocha SD, Pagotto V, et al. Prevalence and risk factors for self-report diabetes mellitus: a population-based study. Int J Environ Res Public Health. 2020;17(18):6497.
Free radical research.
Zhu B, Qu S. The relationship between diabetes mellitus and cancers and its underlying mechanisms. Front Endocrinol. 2022;13:800995.
Mojarrad M, Moghbeli M. Genetic and molecular biology of bladder cancer among Iranian patients. Mol Genet Genomic Med. 2020;8(6):e1233.
Article PubMed PubMed Central Google Scholar
Moghbeli M. Genetic and molecular biology of breast cancer among Iranian patients. J Transl Med. 2019;17(1):218.
Abbaszadegan MR, Moghbeli M. Genetic and molecular origins of colorectal Cancer among the iranians: an update. Diagn Pathol. 2018;13(1):97.
Kim I. A brief overview of cell therapy and its product. J Korean Association Oral Maxillofacial Surg. 2013;39(5):201.
Mount NM, Ward SJ, Kefalas P, Hyllner J. Cell-based therapy technology classifications and translational challenges. Philosophical Trans Royal Soc B: Biol Sci. 2015;370(1680):20150017.
El-Kadiry AE-H, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Front Med. 2021;8:756029.
Squifflet J-P, Gruessner R, Sutherland D. The history of pancreas transplantation: past, present and future. Acta Chir Belg. 2008;108(3):367–78.
Article PubMed Google Scholar
Parums DV. First Regulatory approval for allogeneic pancreatic islet Beta cell infusion for adult patients with type 1 diabetes Mellitus. Med Sci Monitor: Int Med J Experimental Clin Res. 2023;29:e941918–1.
Yang L, Hu Z-M, Jiang F-X, Wang W. Stem cell therapy for insulin-dependent diabetes: are we still on the road? World J Stem Cells. 2022;14(7):503.
Affan M, Dar MS. Donislecel-the first approved pancreatic islet cell therapy medication for type 1 diabetes: a letter to the editor. Ir J Med Sci (1971-). 2023:1–2.
Harris E. FDA greenlights first cell therapy for adults with type 1 diabetes. JAMA. 2023.
Soon-Shiong P, Heintz R, Merideth N, Yao Q, Yao Z, Zheng T, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet (London England). 1994;343(8903):950–1.
Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29(1):137–8.
Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32(10):1887–9.
Weng L, Wang X, Liu H, Yu Z, Liu S. Light-responsive microneedle array with tunable insulin release function for painless and on-demand anti-diabetic therapy. Mater Lett. 2023:135684.
Okcu A, Yazir Y, Şimşek T, Mert S, Duruksu G, Öztürk A, et al. Investigation of the effect of pancreatic decellularized matrix on encapsulated islets of Langerhans with mesenchymal stem cells. Tissue Cell. 2023;82:102110.
Khaliq T, Sohail M, Minhas MU, Mahmood A, Munir A, Qalawlus AHM, et al. Hyaluronic acid/alginate-based biomimetic hydrogel membranes for accelerated diabetic wound repair. Int J Pharm. 2023;643:123244.
Kuwabara R, Qin T, Llacua LA, Hu S, Boekschoten MV, de Haan BJ, et al. Extracellular matrix inclusion in immunoisolating alginate-based microcapsules promotes longevity, reduces fibrosis, and supports function of islet allografts in vivo. Acta Biomater. 2023;158:151–62.
Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem cell Res. 2014;12(3):807–14.
Dufrane D, van Steenberghe M, Goebbels R-M, Saliez A, Guiot Y, Gianello P. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials. 2006;27(17):3201–8.
Pullen LC. Stem cell–derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Wiley Online Library; 2018. pp. 1581–2.
Dang HP, Chen H, Dargaville TR, Tuch BE. Cell delivery systems: toward the next generation of cell therapies for type 1 diabetes. J Cell Mol Med. 2022;26(18):4756–67.
Viacyte. ViaCyte and gore enter clinical phase agreement based on novel membrane technology for PEC-encap product candidate. 2020.
Viacyte. viacyte announces initiation of phase 2 study of encapsulated cell therapy for type 1 diabetes patients 2021 2021. https://viacyte.com/press-releases/viacyte‐announces‐initiation‐of‐phase‐2‐study‐of‐encapsulated‐cell‐ther‐apy‐for‐type‐1‐diabetes‐patients/ .
Hodgson J. Drug pipeline 3Q23—ERT, bispecifics and CRISPR in sickle cell disease. Nat Biotechnol. 2023;41(11):1498–500.
Pagliuca F. Pre-clinical proof-of-Concept in two lead programs in type 1 diabetes. International Socety for Stem Cell Research; 2019.
Jones PM, Persaud SJ. β-cell replacement therapy for type 1 diabetes: closer and closer. Diabet Med. 2022;39(6).
Carlsson P-O, Espes D, Sedigh A, Rotem A, Zimerman B, Grinberg H, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018;18(7):1735–44.
Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010;42(13):918–22.
Evron Y, Colton CK, Ludwig B, Weir GC, Zimermann B, Maimon S, et al. Long-term viability and function of transplanted islets macroencapsulated at high density are achieved by enhanced oxygen supply. Sci Rep. 2018;8(1):6508.
Cao R, Avgoustiniatos E, Papas K, de Vos P, Lakey JR. Mathematical predictions of oxygen availability in micro-and macro‐encapsulated human and porcine pancreatic islets. J Biomedical Mater Res Part B: Appl Biomaterials. 2020;108(2):343–52.
Gala-Lopez B, Pepper A, Dinyari P, Malcolm A, Kin T, Pawlick L, et al. Subcutaneous clinical islet transplantation in a prevascularized subcutaneous pouch–preliminary experience. CellR4. 2016;4(5):e2132.
Google Scholar
Sernova Corp Presents Positive Preliminary. Safety and Efficacy Data in its Phase I/II Clinical Trial for Type-1 Diabetes: Biospace. https://www.biospace.com/article/sernova‐corp‐presents‐positive‐preliminary‐safety‐and‐efficacy‐data‐in‐its‐phase‐i‐ii‐clinical‐trial‐for‐type‐1‐diabetes/ .
Bachul PJ, Perez-Gutierrez A, Juengel B, Golab K, Basto L, Perea L et al. 306-OR: modified approach for improved isllotransplantation into prevascularized sernova cell pouch device: preliminary results of the phase i/ii clinical trial at University of Chicago. Diabetes. 2022;71(Supplement_1).
Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34(3):345–52.
Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, et al. Long-term glycemic control using polymer-encapsulated human stem cell–derived beta cells in immune-competent mice. Nat Med. 2016;22(3):306–11.
Shapiro AD, Konkle BA, Croteau SE, Miesbach WA, Hay CRM, Kazmi R, et al. First-in-human phase 1/2 clinical trial of SIG-001, an innovative shielded cell therapy platform, for hemophilia Α. Blood. 2020;136:8.
Taraballi F, Sushnitha M, Tsao C, Bauza G, Liverani C, Shi A, et al. Biomimetic tissue engineering: tuning the immune and inflammatory response to implantable biomaterials. Adv Healthc Mater. 2018;7(17):1800490.
Yesilyurt V, Veiseh O, Doloff JC, Li J, Bose S, Xie X, et al. A facile and versatile method to endow biomaterial devices with zwitterionic surface coatings. Adv Healthc Mater. 2017;6(4):1601091.
Liu Q, Chiu A, Wang L-H, An D, Zhong M, Smink AM, et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat Commun. 2019;10(1):5262.
Noverraz F, Montanari E, Pimenta J, Szabó L, Ortiz D, Gonelle-Gispert C, et al. Antifibrotic effect of ketoprofen-grafted alginate microcapsules in the transplantation of insulin producing cells. Bioconjug Chem. 2018;29(6):1932–41.
Jeon SI, Jeong J-H, Kim JE, Haque MR, Kim J, Byun Y, et al. Synthesis of PEG-dendron for surface modification of pancreatic islets and suppression of the immune response. J Mater Chem B. 2021;9(11):2631–40.
Derakhshankhah H, Sajadimajd S, Jahanshahi F, Samsonchi Z, Karimi H, Hajizadeh-Saffar E, et al. Immunoengineering Biomaterials in Cell-based therapy for type 1 diabetes. Tissue Eng Part B: Reviews. 2022;28(5):1053–66.
Piemonti L, Maffi P, Nano R, Bertuzzi F, Melzi R, Mercalli A, et al. Treating diabetes with islet transplantation: lessons from the Milan experience. Transplantation, Bioengineering, and regeneration of the endocrine pancreas. Elsevier; 2020. pp. 645–58.
Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, et al. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB J. 2010;24(10):3927.
Chen X, Liu H, Li H, Cheng Y, Yang L, Liu Y. In vitro expansion and differentiation of rat pancreatic duct-derived stem cells into insulin secreting cells using a dynamic three-dimensional cell culture system. Genet Mol Res. 2016;15(2).
Becker MW, Simonovich JA, Phelps EA. Engineered microenvironments and microdevices for modeling the pathophysiology of type 1 diabetes. Biomaterials. 2019;198:49–62.
Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, et al. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng Part A. 2013;19(11–12):1465–75.
Izadi Z, Hajizadeh-Saffar E, Hadjati J, Habibi-Anbouhi M, Ghanian MH, Sadeghi-Abandansari H, et al. Tolerance induction by surface immobilization of Jagged-1 for immunoprotection of pancreatic islets. Biomaterials. 2018;182:191–201.
McHugh MD, Park J, Uhrich R, Gao W, Horwitz DA, Fahmy TM. Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials. 2015;59:172–81.
Chen H, Teramura Y, Iwata H. Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly (ethylene glycol)-conjugated phospholipid. J Controlled Release. 2011;150(2):229–34.
Su J, Hu B-H, Lowe WL Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31(2):308–14.
Dong H, Fahmy TM, Metcalfe SM, Morton SL, Dong X, Inverardi L, et al. Immuno-isolation of pancreatic islet allografts using pegylated nanotherapy leads to long-term normoglycemia in full MHC mismatch recipient mice. PLoS ONE. 2012;7(12):e50265.
Kumar M, Nandi SK, Kaplan DL, Mandal BB. Localized immunomodulatory silk macrocapsules for islet-like spheroid formation and sustained insulin production. ACS Biomaterials Sci Eng. 2017;3(10):2443–56.
Hotaling NA, Tang L, Irvine DJ, Babensee JE. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng. 2015;17:317–49.
Shi Y, Zhao YZ, Jiang Z, Wang Z, Wang Q, Kou L, et al. Immune-Protective formulations and process strategies for improved survival and function of transplanted islets. Front Immunol. 2022;13:923241.
Zhang S, Yang H, Wang M, Mantovani D, Yang K, Witte F, et al. Immunomodulatory biomaterials against bacterial infections: Progress, challenges, and future perspectives. Innovation. 2023;4(6):100503.
CAS PubMed PubMed Central Google Scholar
Accolla RP, Simmons AM, Stabler CL. Integrating Additive Manufacturing techniques to improve cell-based implants for the treatment of type 1 diabetes. Adv Healthc Mater. 2022;11(13):e2200243.
Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. ACS; 2014.
Bol RJ, Šavija B. Micromechanical models for FDM 3D-Printed polymers: a review. Polymers. 2023;15(23):4497.
Paul S. Finite element analysis in fused deposition modeling research: a literature review. Measurement. 2021;178:109320.
Monaldo E, Ricci M, Marfia S. Mechanical properties of 3D printed polylactic acid elements: experimental and numerical insights. Mech Mater. 2023;177:104551.
Anoop M, Senthil P. Microscale representative volume element based numerical analysis on mechanical properties of fused deposition modelling components. Materials Today: Proceedings. 2021;39:563 – 71.
McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proceedings of the National Academy of Sciences. 2006;103(31):11461-6.
Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences. 2012;109(11):4245-50.
Espona-Noguera A, Ciriza J, Cañibano-Hernández A, Orive G, Hernández RM, del Saenz L, et al. Review of advanced hydrogel-based cell encapsulation systems for insulin delivery in type 1 diabetes mellitus. Pharmaceutics. 2019;11(11):597.
Dimitrioglou N, Kanelli M, Papageorgiou E, Karatzas T, Hatziavramidis D. Paving the way for successful islet encapsulation. Drug Discovery Today. 2019;24(3):737–48.
Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79(1):52–8.
Song S, Roy S. Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: cells, biomaterials, and devices. Biotechnol Bioeng. 2016;113(7):1381–402.
Zhi ZL, Kerby A, King AJF, Jones PM, Pickup JC. Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes. Diabetologia. 2012;55(4):1081–90.
Farina M, Chua CYX, Ballerini A, Thekkedath U, Alexander JF, Rhudy JR, et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials. 2018;177:125–38.
Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D et al. 3D printed vascularized device for Subcutaneous Transplantation of Human islets. Biotechnol J. 2017;12(9).
Lei D, Yang Y, Liu Z, Yang B, Gong W, Chen S, et al. 3D printing of biomimetic vasculature for tissue regeneration. Mater Horiz. 2019;6(6):1197–206.
Melchels FP, Domingos MA, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012;37(8):1079–104.
Ernst AU, Wang LH, Ma M. Interconnected toroidal hydrogels for islet encapsulation. Adv Healthc Mater. 2019;8(12):1900423.
Liang J-P, Accolla RP, Jiang K, Li Y, Stabler CL. Controlled release of anti-inflammatory and proangiogenic factors from macroporous scaffolds. Tissue Eng Part A. 2021;27(19–20):1275–89.
Pedraza E, Brady A-C, Fraker CA, Molano RD, Sukert S, Berman DM, et al. Macroporous three-dimensional PDMS scaffolds for extrahepatic islet transplantation. Cell Transplant. 2013;22(7):1123–35.
Chiu Y-C, Cheng M-H, Engel H, Kao S-W, Larson JC, Gupta S, et al. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials. 2011;32(26):6045–51.
Kuss MA, Wu S, Wang Y, Untrauer JB, Li W, Lim JY, et al. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J Biomedical Mater Res Part B: Appl Biomaterials. 2018;106(5):1788–98.
Liu X, Jakus AE, Kural M, Qian H, Engler A, Ghaedi M, et al. Vascularization of natural and synthetic bone scaffolds. Cell Transplant. 2018;27(8):1269–80.
Costa-Almeida R, Gomez-Lazaro M, Ramalho C, Granja PL, Soares R, Guerreiro SG. Fibroblast-endothelial partners for vascularization strategies in tissue engineering. Tissue Eng Part A. 2015;21(5–6):1055–65.
Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell. 2011;22(20):3791–800.
Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proceedings of the National Academy of Sciences. 2017;114(35):9337-42.
Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D, et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol J. 2017;12(9):1700169.
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14(13):2202–11.
Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68.
Gao Q, Liu Z, Lin Z, Qiu J, Liu Y, Liu A, et al. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomaterials Sci Eng. 2017;3(3):399–408.
Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci. 2019;6(11):1900344.
Hospodiuk M, Dey M, Ayan B, Sosnoski D, Moncal KK, Wu Y, et al. Sprouting angiogenesis in engineered pseudo islets. Biofabrication. 2018;10(3):035003.
Marchioli G, Luca AD, de Koning E, Engelse M, Van Blitterswijk CA, Karperien M, et al. Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de novo vessel formation for the transplantation of islets of Langerhans. Adv Healthc Mater. 2016;5(13):1606–16.
Dionne KE, Colton CK, Lyarmush M. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21.
de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, Van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12(8):867–75.
Thomas F, Wu J, Contreras JL, Smyth C, Bilbao G, He J, et al. A tripartite anoikis-like mechanism causes early isolated islet apoptosis. Surgery. 2001;130(2):333–8.
Barkai U, Rotem A, de Vos P. Survival of encapsulated islets: more than a membrane story. World J Transplantation. 2016;6(1):69.
Jiang K, Chaimov D, Patel SN, Liang JP, Wiggins SC, Samojlik MM, et al. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials. 2019;198:37–48.
Pati F, Jang J, Ha D, Won Kim S, Rhie J, Shim J, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
Kim BS, Kwon YW, Kong J-S, Park GT, Gao G, Han W, et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53.
Hu S, Martinez-Garcia FD, Moeun BN, Burgess JK, Harmsen MC, Hoesli C, et al. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater Sci Engineering: C. 2021;123:112009.
Phelps EA, Templeman KL, Thulé PM, García AJ. Engineered VEGF-releasing PEG–MAL hydrogel for pancreatic islet vascularization. Drug Delivery Translational Res. 2015;5:125–36.
Kooptiwut S, Kaewin S, Semprasert N, Sujjitjoon J, Junking M, Suksri K, et al. Estradiol prevents high glucose-induced β-cell apoptosis by decreased BTG2 expression. Sci Rep. 2018;8(1):12256.
Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials. 2013;34(23):5792–801.
Wang Y, He D, Ni C, Zhou H, Wu S, Xue Z, et al. Vitamin D induces autophagy of pancreatic β-cells and enhances insulin secretion. Mol Med Rep. 2016;14(3):2644–50.
Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016;8(2):025003.
Liu YY, Yu HC, Liu Y, Liang G, Zhang T, Hu QX. Dual drug spatiotemporal release from functional gradient scaffolds prepared using 3 D bioprinting and electrospinning. Polym Eng Sci. 2016;56(2):170–7.
Freeman FE, Pitacco P, van Dommelen LH, Nulty J, Browe DC, Shin J-Y, et al. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci Adv. 2020;6(33):eabb5093.
Wong MS, Hawthorne WJ, Manolios N. Gene therapy in diabetes. Self Nonself. 2010;1(3):165.
Ahmad Z, Rasouli M, Azman AZF, Omar AR. Evaluation of insulin expression and secretion in genetically engineered gut K and L-cells. BMC Biotechnol. 2012;12:1–9.
Tudurí E, Bruin JE, Kieffer TJ. Restoring insulin production for type 1 diabetes. J Diabetes. 2012;4(4):319–31.
Romer AI, Sussel L. Pancreatic islet cell development and regeneration. Current opinion in endocrinology, diabetes, and obesity. 2015;22(4):255.
Jaén ML, Vilà L, Elias I, Jimenez V, Rodó J, Maggioni L, et al. Long-term efficacy and safety of insulin and glucokinase gene therapy for diabetes: 8-year follow-up in dogs. Mol therapy-methods Clin Dev. 2017;6:1–7.
Li H, Li X, Lam KS, Tam S, Xiao W, Xu R. Adeno-associated virus-mediated pancreatic and duodenal homeobox gene-1 expression enhanced differentiation of hepatic oval stem cells to insulin-producing cells in diabetic rats. J Biomed Sci. 2008;15:487–97.
Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127(16):3533–42.
Abed A, Critchlow C, Flatt PR, McClenaghan NH, Kelly C. Directed differentiation of progenitor cells towards an islet-cell phenotype. Am J Stem Cells. 2012;1(3):196.
PubMed PubMed Central Google Scholar
Zhao M, Amiel SA, Ajami S, Jiang J, Rela M, Heaton N, et al. Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS ONE. 2008;3(7):e2666.
Handorf AM, Sollinger HW, Alam T. Genetic engineering of surrogate β cells for treatment of type 1 diabetes mellitus. J Diabetes Mellitus. 2015;5(04):295–312.
Grant MB, Adu-Agyeiwaah Y, Vieira CP, Asare-Bediako B, Hammer SS, Calzi SL, et al. Intravitreal administration of AAV2-SIRT1 reverses diabetic retinopathy (DR) in a murine model of type 2 diabetes (T2D). Investig Ophthalmol Vis Sci. 2022;63(7):2310.
Yoon J-W, Jun H-S. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med. 2002;8(2):62–8.
Hou W-R, Xie S-N, Wang H-J, Su Y-Y, Lu J-L, Li L-L, et al. Intramuscular delivery of a naked DNA plasmid encoding proinsulin and pancreatic regenerating III protein ameliorates type 1 diabetes mellitus. Pharmacol Res. 2011;63(4):320–7.
Joo WS, Jeong JH, Nam K, Blevins KS, Salama ME, Kim SW. Polymeric delivery of therapeutic RAE-1 plasmid to the pancreatic islets for the prevention of type 1 diabetes. J Controlled Release. 2012;162(3):606–11.
Dezashibi HM, Shabani A. A Mini-review of Current Treatment approaches and Gene Therapy as potential interventions for diabetes Mellitus types 1. Adv Biomed Res. 2023;12:219.
Vantyghem M-C, de Koning EJ, Pattou F, Rickels MR. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet. 2019;394(10205):1274–85.
Hudson A, Bradbury L, Johnson R, Fuggle S, Shaw J, Casey J, et al. The UK pancreas allocation scheme for whole organ and islet transplantation. Am J Transplant. 2015;15(9):2443–55.
Cornateanu SM, O’Neill S, Dholakia S, Counter CJ, Sherif AE, Casey JJ, et al. Pancreas utilization rates in the UK–an 11-year analysis. Transpl Int. 2021;34(7):1306–18.
Nordheim E, Lindahl JP, Carlsen RK, Åsberg A, Birkeland KI, Horneland R, et al. Patient selection for islet or solid organ pancreas transplantation: experiences from a multidisciplinary outpatient-clinic approach. Endocr Connections. 2021;10(2):230–9.
Arifin DR, Bulte JW. In vivo imaging of pancreatic islet grafts in diabetes treatment. Front Endocrinol. 2021;12:640117.
Murakami T, Fujimoto H, Inagaki N. Non-invasive beta-cell imaging: visualization, quantification, and beyond. Front Endocrinol. 2021;12:714348.
Piemonti L, Everly MJ, Maffi P, Scavini M, Poli F, Nano R, et al. Alloantibody and autoantibody monitoring predicts islet transplantation outcome in human type 1 diabetes. Diabetes. 2013;62(5):1656–64.
Anteby R, Lucander A, Bachul PJ, Pyda J, Grybowski D, Basto L, et al. Evaluating the prognostic value of islet autoantibody monitoring in islet transplant recipients with long-standing type 1 diabetes mellitus. J Clin Med. 2021;10(12):2708.
Buron F, Reffet S, Badet L, Morelon E, Thaunat O. Immunological monitoring in beta cell replacement: towards a pathophysiology-guided implementation of biomarkers. Curr Diab Rep. 2021;21:1–11.
Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep. 2011;11:364–74.
Tremmel DM, Odorico JS. Rebuilding a better home for transplanted islets. Organogenesis. 2018;14(4):163–8.
Citro A, Moser PT, Dugnani E, Rajab TK, Ren X, Evangelista-Leite D, et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials. 2019;199:40–51.
Basta G, Montanucci P, Calafiore R. Microencapsulation of cells and molecular therapy of type 1 diabetes mellitus: the actual state and future perspectives between promise and progress. J Diabetes Invest. 2021;12(3):301–9.
Samojlik MM, Stabler CL. Designing biomaterials for the modulation of allogeneic and autoimmune responses to cellular implants in type 1 diabetes. Acta Biomater. 2021;133:87–101.
Carlsson P-O, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–92.
Madani S, Setudeh A, Aghayan HR, Alavi-Moghadam S, Rouhifard M, Rezaei N, et al. Placenta derived mesenchymal stem cells transplantation in type 1 diabetes: preliminary report of phase 1 clinical trial. J Diabetes Metabolic Disorders. 2021;20:1179–89.
Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–39.
Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34(13):1759–72.
Sambathkumar R, Migliorini A, Nostro MC. Pluripotent stem cell-derived pancreatic progenitors and β-like cells for type 1 diabetes treatment. Physiology. 2018;33(6):394–402.
Sordi V, Monaco L, Piemonti L. Cell therapy for type 1 diabetes: from islet transplantation to stem cells. Hormone Res Paediatrics. 2022;96(6):658–69.
Henry RR, Pettus J, Wilensky J, SHAPIRO AJ, Senior PA, Roep B et al. Initial clinical evaluation of VC-01TM combination product—a stem cell–derived islet replacement for type 1 diabetes (T1D). Diabetes. 2018;67(Supplement_1).
Shapiro A, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus JH et al. Insulin expression and glucose-responsive circulating C-peptide in type 1 diabetes patients implanted subcutaneously with pluripotent stem cell-derived pancreatic endoderm cells in a macro-device. David and Donner, Thomas W and Bellin, Melena D and Hsueh, Willa and Pettus, Jeremy H and Wilensky, Jon S and Daniels, Mark and Wang, Richard M and Kroon, Evert J and Brandon, Eugene Paul and D’Amour, Kevin A and Foyt, Howard, Insulin Expression and Glucose-Responsive Circulating C-Peptide in Type. 2019;1.
Keymeulen B, Jacobs-Tulleneers-Thevissen D, Kroon EJ, Jaiman MS, Daniels M, Wang R et al. 196-LB: stem cell–derived islet replacement therapy (VC-02) demonstrates production of C-peptide in patients with type 1 diabetes (T1D) and hypoglycemia unawareness. Diabetes. 2021;70(Supplement_1).
Piemonti L. Felix dies natalis, insulin… ceterum autem censeo beta is better. Acta Diabetol. 2021;58(10):1287–306.
Sordi V, Pellegrini S, Piemonti L. Immunological issues after stem cell-based β cell replacement. Curr Diab Rep. 2017;17:1–8.
Coe TM, Markmann JF, Rickert CG. Current status of porcine islet xenotransplantation. Curr Opin Organ Transpl. 2020;25(5):449–56.
Edgar L, Pu T, Porter B, Aziz J, La Pointe C, Asthana A, et al. Regenerative medicine, organ bioengineering and transplantation. J Br Surg. 2020;107(7):793–800.
Mathur A, Taurin S, Alshammary S. The safety and efficacy of mesenchymal stem cells in the treatment of type 2 Diabetes- A literature review. Diabetes Metab Syndr Obes. 2023;16:769–77.
Hogrebe NJ, Ishahak M, Millman JR. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell. 2023;30(5):530–48.
Paraskevas S, Maysinger D, Wang R, Duguid WP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–6.
Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29(8):750–6.
Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, et al. Maturation of human embryonic stem cell–derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Translational Med. 2015;4(10):1214–22.
Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell. 2021;28(12):2047–61. e5.
Dolgin E, Diabetes. Encapsulating the problem. Nature. 2016;540(7632):S60–2.
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33.
Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol. 2020;38(4):460–70.
Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol. 2019;21(2):263–74.
Shapiro AJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2(12).
Witkowski P, Anteby R, Olaitan OK, Forbes RC, Niederhaus S, Ricordi C, et al. Pancreatic islets Quality and Potency cannot be verified as required for drugs: reflection on the FDA Review of a biological license application for human islets. Transplantation. 2021;105(12):e409–10.
Download references
Acknowledgements
Author information, authors and affiliations.
Noncommunicable Diseases Research Center, Neyshabur University of Medical Sciences, Neyshabur, Iran
Ramin Raoufinia
Department of Medical Genetics and Molecular Medicine, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Ramin Raoufinia, Hamid Reza Rahimi, Ehsan Saburi & Meysam Moghbeli
You can also search for this author in PubMed Google Scholar
Contributions
RR, HRR, and ES were involved in search strategy and drafting. MM revised, designed, and supervised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Meysam Moghbeli .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Raoufinia, R., Rahimi, H.R., Saburi, E. et al. Advances and challenges of the cell-based therapies among diabetic patients. J Transl Med 22 , 435 (2024). https://doi.org/10.1186/s12967-024-05226-3
Download citation
Received : 14 February 2024
Accepted : 22 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1186/s12967-024-05226-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cell therapy
- Translplantation
- Immunosuppression
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Logo Left Content

Logo Right Content
Stanford University School of Medicine blog
These are the tools for providing top-notch diabetes care to everyone
For 30 years, doctors have known that maintaining near-normal blood sugar has huge benefits for people with Type 1 diabetes.
A 1993 clinical trial found that participants who were taught methods for tightly managing their disease -- checking their blood sugar many times each day, making adjustments to insulin doses and receiving frequent help from their medical caregivers -- reduced their risk for long-term complications, including blindness, kidney failure and peripheral nerve damage, by 50% to 70%.
Yet since that trial, physicians have struggled to roll out intensive diabetes management programs to all patients. On average, patients across the U.S. still don't achieve the level of diabetes control that would minimize their long-term risks.

"It's not because people haven't been trying," said David Maahs , MD, PhD, a pediatric endocrinologist at Stanford Medicine Children's Health. "It's a complicated condition to take care of, and for the individual with diabetes and their family, it's constant work."
Stanford Medicine experts are making headway on the problem. A new scientific study published recently in Nature Medicine describes how the research team, led by Maahs and Priya Prahalad , MD, PhD, have implemented major advances in intensive diabetes management.
First, they tackled equity issues to ensure the latest diabetes technology got into the hands of every patient as soon as they were diagnosed. They also built artificial intelligence tools that gave diabetes caregivers the ability to quickly identify which patients most needed their help. Ultimately these steps enabled adolescent Type 1 diabetes patients to maintain better control of their blood sugar levels.
It's not because people haven't been trying. It's a complicated condition to take care of, and for the individual with diabetes and their family, it's constant work. David Maahs
Maahs talked about the methods used and the long-term ramifications. This interview was edited for length and clarity.
Your study built on recent technological advances that automate many tasks involved in living with diabetes. What are the advantages of the newer devices?
Patients can now wear continuous glucose monitors, which have a sensor inserted under the skin that reads a glucose value every 5 to 15 minutes. This is really helpful because you don't have to poke your finger six to 10 times a day to measure glucose levels, and the monitor can warn you if you're going low or high. If you're the parent of a child with Type 1 diabetes, you can get their glucose data from the cloud and onto your phone.
Another recent improvement in diabetes technology is that continuous glucose monitors can now communicate with an insulin pump. An algorithm helps control dosing to reduce or stop insulin if it predicts your blood sugar is going to go low, and it adds a bit more insulin if you're going high. You still have to give an insulin dose before you eat, but it really takes a lot of the burden out of managing Type 1 diabetes.
It's been a big challenge to get these improved diabetes devices into the hands of every U.S. patient; your earlier work shows that disadvantaged groups tend to be left behind. How did your new study tackle equity concerns?
We were testing the benefits of starting pediatric Type 1 diabetes patients on continuous glucose monitors as soon as possible after they were diagnosed, which we were usually able to do in the first week after diagnosis. Although insulin pumps were not a focus of this study, about half of our patients began using an insulin pump within a year of their diagnosis.

We all agreed that we wanted every new patient we saw to be included. If you look at earlier studies of diabetes technology, it tended to be tested in college-educated, white, privately insured people and not in other populations. We had to figure out how to meet challenges faced by less-advantaged patients. We learned that this went beyond the most obvious barriers we addressed, such as providing care in multiple languages.
For instance, at first, it seemed like some groups of patients were wearing their continuous glucose monitors less than we asked them to. But in fact, the transmission of their data to our system was incomplete because they had poor Wi-Fi access at home. That's an equity issue. We've been giving out devices to those who need them as part of the research so that everyone has enough internet connectivity to upload their data.
Also, at diagnosis, we sometimes can't tell whether someone has Type 1 or Type 2 diabetes. This happens more in minoritized populations, in youth who have an elevated body mass index, and these children are more likely to be publicly insured or non-English speakers. We made a conscious decision to include all these patients while we waited to learn the details of their diagnosis, so as not to miss anyone who might be eligible for our study.
We made a conscious decision to include (a diverse mix of) patients while we waited to learn the details of their diagnosis, so as not to miss anyone who might be eligible for our study. David Maahs
Close to 90% of our new Type 1 diabetes patients participated in this study, and a lot of those who chose not to participate enrolled instead in a different study of artificial pancreas technology, so we did quite a good job of including everyone. It was a very diverse population: About 35% of patients were publicly insured, and only about 40% were non-Hispanic white.
Getting the new technology to every patient was important. What else was needed to make sure all patients could succeed in managing their blood sugar levels?
The 1993 trial showed that it was really useful for patients to have frequent communication with their diabetes team. That can be hard to do with the resources of a typical diabetes clinic, where each diabetes educator has many patients to track.
To address this problem, we built an AI-powered data dashboard, which filters our patients' continuous glucose monitor data and puts it into a format that helps our team identify who is struggling. Instead of spending a lot of time manually evaluating their data, we can automatically rank which patients most need our help.
We look at the percentage of time the continuous glucose monitor has been worn, and if it's below a certain threshold, that's our first starting point. Sometimes people have lost their prescription for their CGM, continuous glucose monitor, and need a new one. We're able to reach out and help them.
If a patient is having too many low blood sugar readings, which are dangerous, that's another reason for them to go to the top of the list. Our diabetes educators can contact them to help adjust their insulin dosing. Likewise, if their average glucose is out of the target range less than 70% of the time, we can flag that they need some extra attention between their visits to the clinic.
On the other hand, if someone is wearing their monitor, they're not having low blood sugar readings and they are in the right blood sugar range most of the time, they're doing well. We'll check in with them at their quarterly clinic visit, but they don't need outreach in between. That knowledge helps our team shift its attention to the patients who most need it.
How did you build the algorithm that powers this dashboard?
The platform was developed at Stanford with systems design expert David Scheinker , PhD, and his SURF team ; they use tools such as machine learning and statistics to improve how health care is delivered. He teaches a class in which engineering undergraduates and grad students solve technical problems in health care.
We presented our concept to his class. Our problem was that each brand of diabetes equipment has a different system to share data. A diabetes educator with 10 patients might have had five or six different places to go look at their data, log in and so on. This made it extremely time-consuming to figure out which patients needed help.
More on diabetes
- NIH grant establishes Stanford Medicine as center of national diabetes training program
- 'Smart speaker' shows potential for better self-management of Type 2 diabetes
- Improved blood sugar control helps normalize diabetic teens' brains, Stanford-led study finds
- Managing type 1 diabetes: Voices of the underserved
Instead, our system has all the data in one place. It was built through an iterative process between our diabetes educators and the engineering team, so that ultimately the data is presented in the way that is most useful to the diabetes educators.
We published a study showing that the dashboard has shifted the diabetes educators' workload in a helpful way. They spend less time sifting through troves of data - something an algorithm can do perfectly - and more time talking to patients who need extra help between clinic visits.
How do you know that your approach worked?
Compared with our past patients who were diagnosed with Type 1 diabetes before this research began, our newer patients were more likely to reach their glucose targets after a year of living with the disease.
One treatment target for our patients, after a year with diabetes, was a glycosylated hemoglobin A1c measurement below 7%. This laboratory test assesses patients' blood sugar control over the prior three months, and a reading below 7% is the target for optimum health.
In our earlier data, 28% of patients met this target 12 months after diagnosis; now we have 64% of our patients meeting this goal. We also looked at how many people had very high A1c measurements, with values above 9%, and that measure has reduced dramatically. Similarly, by one year after diagnosis, our patients' blood sugar was in the target range 68% of the time.
We also have data showing that compared with our historic cohort, everyone received a similar benefit from our intensive approach to treatment, meaning that if you looked at people who had public versus private insurance, or were or were not English speakers, every group had similar improvements when we implemented our study. There are still some gaps between more- and less-privileged patients, so we still have work to do, but everyone benefited a similar amount. Often, when new medical technology becomes available, more privileged people get more benefit; it is very encouraging that we could buck that trend.
Image: habrovich ( stock.adobe.com )
Related posts
Kids fare better with early use of diabetes technology

Why precision medicine leads to better diabetes care
Popular posts.
Is an increase in penile length cause for concern?
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 12, Issue 2
- The case for a ketogenic diet in the management of kidney disease
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-5427-6748 Shaminie J Athinarayanan 1 ,
- Caroline G P Roberts 1 ,
- Chandan Vangala 2 ,
- Greeshma K Shetty 1 ,
- Amy L McKenzie 3 ,
- Thomas Weimbs 4 ,
- Jeff S Volek 5
- 1 Virta Health , Denver , Colorado , USA
- 2 BCM , Houston , Texas , USA
- 3 Abbott , Wiesbaden , Germany
- 4 Department of Molecular Cellular & Developmental Biology , University of California Santa Barbara , Santa Barbara , California , USA
- 5 Department of Human Sciences , The Ohio State University , Columbus , Ohio , USA
- Correspondence to Dr Shaminie J Athinarayanan; sarassam52{at}gmail.com
Ketogenic diets have been widely used for weight loss and are increasingly used in the management of type 2 diabetes. Despite evidence that ketones have multiple positive effects on kidney function, common misconceptions about ketogenic diets, such as high protein content and acid load, have prevented their widespread use in individuals with impaired kidney function. Clinical trial evidence focusing on major adverse kidney events is sparse. The aim of this review is to explore the effects of a ketogenic diet, with an emphasis on the pleiotropic actions of ketones, on kidney health. Given the minimal concerns in relation to the potential renoprotective effects of a ketogenic diet, future studies should evaluate the safety and efficacy of ketogenic interventions in kidney disease.
- Kidney Diseases
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjdrc-2024-004101
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Low carbohydrate eating patterns including a very low carbohydrate or ketogenic diet have been successfully used for weight loss and remitting type 2 diabetes (T2D). Among patients with T2D, the prevalence of chronic kidney disease (CKD), whether characterized as a reduced estimated glomerular filtration rate (eGFR) function or albuminuria is almost 40%. 1 Yet, a ketogenic diet is cautioned against in individuals with impaired kidney function, 2 in part, due to concerns about increased protein intake. The effect of protein intake in CKD is controversial, but high protein intake has been associated with hyperfiltration, increased acid excretion, and potentially, a decline in kidney function. 3 4 However, protein intake on a well-formulated ketogenic diet (WFKD) is moderate to effectively permit nutritional ketosis. Dietary analysis of very low carbohydrate studies usually reports daily protein intake ranging from 0.6 g/kg to 1.4 g/kg, 5–7 which is similar to that in the standard American diet and below the high protein threshold (≥2.0 g/kg) believed to be of concern. 8 The Kidney Disease Outcomes Quality Initiative clinical practice guideline for nutrition in CKD not dependent on dialysis recommends a “low-protein diet providing 0.55–0.6 g of dietary protein/kg body weight/day, or a very low-protein diet providing 0.28–0.43 g of dietary protein/kg of body weight/day with additional keto acid/amino acid analogs to meet protein requirements (0.55–0.60 g/kg/day).” 9 In contrast, Kidney Disease Improving Global Outcomes (KDIGO) 2022 CKD guideline recommended a slightly higher daily protein allowance of 0.8 g/kg/day for individuals with advanced CKD with or without T2D. 10 The Modification of Diet in Renal Disease (MDRD) study, a landmark trial examining the effect of protein restriction among 585 patients with non-diabetic CKD, did not demonstrate a significantly slower progression of disease, 11 and in fact a very low protein diet (0.28 g/kg/day) was associated with increased risk of death at a median follow-up of 3.2 years. 12 The null findings from MDRD are one of numerous inconsistent results studying protein restriction in patients with CKD. Taken altogether, systematic reviews have suggested—at best—a modest benefit for patients on a low protein diet 13 14 and given the aforementioned long-term data noting increased risk of death with very low protein diets, most nephrology experts are more comfortable with moderate protein restriction to the degree of 0.8 g/kg/day as recommended by the KDIGO 2022 guideline.
The impact of carbohydrate restriction interventions on kidney function is poorly understood. Existing studies consistently reported improvements in glycemic control, blood pressure, weight, and insulin resistance, all of which have favorable downstream implications for slowing kidney disease progression ( figure 1 ). In addition, ketone bodies themselves have a myriad of physiologic and signaling effects that could elicit renoprotective effects. For example, the renoprotective effect of sodium-glucose cotransporter 2 inhibitors (SGLT2i) has been postulated to be partially mediated by the modest medication-induced ketosis. 15–17 This low-grade ketosis induced by SGLT2i may directly or indirectly benefit the kidney by serving as an energy source during stress and kidney injury, and through its anti-inflammatory, antifibrotic, and antioxidant effects ( figure 1 ). 15 16 Given that SGLT2i-induced ketosis may be beneficial for the kidney, endogenously produced ketones resulting from a WFKD may prove to be another therapeutic option for diabetic nephropathy or kidney disease. 18 19
- Download figure
- Open in new tab
- Download powerpoint
Summary of pleiotropic renal protective effect of ketones and carbohydrate restriction. BHB, β-hydroxybutyrate; mTORC1, mammalian target of rapamycin complex 1; NLRP3, NOD-, LRR-, and pyrin domain-containing 3; PGC-1ɑ, peroxisome proliferator-activator receptor γ (PPARγ) coactivator-1-alpha; FFAR-3, free fatty acid receptor-3; HCAR, hydroxycarboxylic acid receptor-2; HDAC-3, Histone deacetylase-3; NRF2, nuclear factor erythroid 2-related factor 2; SIRT1, silent information regulator transcript-1.
In this review, we explore the pleiotropic roles and signaling effects of ketones on kidney physiology, address potential concerns of ketogenic therapy, summarize the available literature on the effect of low carbohydrate diets on kidney function, and discuss future studies that could help address the gaps in knowledge and discrepancies in the literature.
Potential roles of ketones on kidney pathophysiology and disease
Ketones as an alternative energy-efficient fuel.
The human body naturally produces ketones, mostly in the liver, at varying rates that result in circulating ketones that span more than four orders of magnitude (<0.01 to >10 mM) 20 21 depending primarily on a person’s carbohydrate intake and insulin level. Increased lipolysis and ketogenesis are upregulated in response to a low insulin-to-glucagon ratio, which occurs during calorie restriction/fasting, prolonged exercise, consumption of a ketogenic diet, or pathologic insulin deficiency. In the liver, when production of fatty acyl coenzyme A (CoA) increases during low insulin secretion and increased lipolysis, fatty acyl CoA is transported to the mitochondrial matrix, where it is then β-oxidized to produce acetyl-CoA. 22 23 Acetyl-CoA is either converted to malonyl CoA or to acetoacetyl CoA ( figure 2 ). Acetoacetyl CoA and acetyl-CoA are further condensed by a rate-limiting enzyme, 3-hydroxy 3-methylglutaryl-CoA synthase 2 (HMGCS2) to generate hydroxymethylglutaryl CoA (HMG-CoA). HMG-CoA is then converted into acetoacetate (AcAc) by hydroxymethylglutaryl coenzyme A lyase (HMGCL). 22 23 Finally, AcAc is reduced to β-hydroxybutyrate (BHB) by BHB dehydrogenase (BDH) ( figure 2 ). Both AcAc and BHB are released from the liver and transported in the blood circulation to extrahepatic tissues where they can have signaling effects and be metabolized and released or oxidized to produce energy. BHB is a vital energy source for the brain with uptake occurring in proportion to circulating levels. As such, during prolonged starvation, ketones can provide over half the brain’s energy requirements. 24 Generally, glucose is considered the most efficient fuel since it produces more ATP per oxygen consumed with a phosphate/oxygen (P/O) ratio of 2.58. 24 25 However, in a state of insulin resistance where glucose uptake and oxidation are impaired, BHB is an effective alternative dense energy molecule with a P/O ratio of 2.50. In contrast to free fatty acids (FFAs), another form of energy-dense fuel, 25 26 BHB gives better ATP yield per oxygen consumed, is water soluble, and generates fewer reactive oxygen species (ROS).
Ketogenesis pathway. AcAc, acetoacetate; BDH, BHB dehydrogenase; CoA, coenzyme A; HMGCL, hydroxymethylglutaryl coenzyme A lyase; HMG-CoA, hydroxymethylglutaryl CoA; HMGCS2, 3-hydroxy 3-methylglutaryl-CoA synthase 2; CPT1/2, carnitine palmitoyltransferase 1/2; MCT, moncarboxylate transporter.
The kidney is among the most metabolically active organs, with very high oxygen demand and the second-highest mitochondrial density after the myocardium. 27 While oxidative metabolism is the principal source of energy in the kidney, the fuel substrates for metabolism differ across regions of the kidney. In the healthy kidney, both fatty acid oxidation (FAO) in the proximal tubules and glycolysis in the distal tubules support its metabolism. The renal cortex, especially the S1/S2 tubule segments, generates energy primarily from FFAs, lactate, and glutamate versus glucose. 28 The outer medulla uses glucose, lactate, FFAs, and ketones for energy. However, in diseased kidneys, mitochondrial dysfunction has been reported as a key pathologic feature that contributes to disease initiation and progression. 29 30 For example, in diabetic nephropathy (DN), hyperglycemia-induced flux of glycolysis increases oxygen demand with the by-product of amplified ROS. 30 31 Excess glucose use in the kidney shifts the energy reliance from fatty acid metabolism to glycolysis, even in the proximal tubules. 30 31
The renoprotective effect of SGLT2i in diabetic kidney disease is driven by amelioration of the pathologic metabolic shift from FAO to glycolysis. SGLT2i decreases reabsorption of excessive glucose, reduces energy production from glucose in the kidney, and increases fatty acid utilization in the kidney. 32 Furthermore, the glycosuric effect of SGLT2i also augments the BHB level in the kidney mainly through increased production of ketones rather than reduced kidney clearance. 33 The kidney is indeed an avid consumer of ketones. 34 BHB serves as an important alternative source of energy for the kidney during metabolic imbalance. It can be effectively metabolized in all nephron regions, except the S1/S2 proximal tubule segments. 35 During starvation or fasting, the BHB level in the kidney increases 20-fold and is used as a substrate for mitochondrial energy production. 20 In the diabetic kidney disease mouse model, both SGLT2i and exogenous ketone treatment normalized the renal ATP levels by restoring its production and this intervention was also associated with kidney function improvement. 36
Anti-inflammatory effect
Inflammation is critical in both acute kidney disease and CKD, especially through activation of inflammasomes such as NOD-, LRR-, and pyrin domain-containing 3 (NLRP3). 37 Numerous studies highlight the link between DN and NLRP3 inflammasome activation, which negatively impacts podocyte function, escalates the expression of inflammatory markers like IL-1β, and is also linked with albuminuria and tubulointerstitial injury. 38 39 Consequently, targeting NLRP3 inflammasome inhibition emerges as a promising approach for kidney disease treatment, despite concerns over the safety of current experimental drugs. 40 BHB stands out for its wide-ranging anti-inflammatory actions, including its effect on inhibiting NLRP3 inflammasome activation. 41 BHB successfully suppresses NLRP3 inflammasome activation in human monocytes and murine neutrophils in vitro and in animal models of NLRP3-mediated diseases. 42 Likewise, the anti-inflammatory effect of SGLT2i in diabetic rats, characterized by subdued NLRP3 inflammasome activation and lower interleukin (IL)-1β and tumor necrosis factor (TNF)-ɑ levels, correlates with elevated BHB and reduced insulin levels in the bloodstream. 43 BHB’s primary receptor is GPR109A (HCAR2), a G protein coupled receptor (GPCR) that acts by suppressing cyclic adenosine monophosphate (cAMP). 44 Beyond NLRP3 inflammasome suppression, animal studies reveal BHB diminishes other proinflammatory cytokines, including IL-6, chemokine (C–C motif) ligand 2, and monocyte chemoattractant protein-1, through activation of GPR109A, partially influenced by BHB’s effect on nuclear factor kappa B translocation. 45–47 In humans, ketogenic diets consistently reduce inflammation indicators. Individuals with T2D on a ketogenic diet show decreased serum C reactive protein and white cell counts, 48 along with significant reductions in 15 out of 16 inflammatory/immune modulators after 1 and 2 years. 49 This anti-inflammatory benefit aligns with prior findings that observed a greater reduction in 7 out of 14 inflammation/immune modulators with a ketogenic diet compared with a low-fat diet after 12 weeks. 50
Antifibrotic effects
The antifibrotic effect of BHB is mainly mediated through the mammalian target of rapamycin complex 1 (mTORC1) pathway. In diabetic kidney disease, mTORC1 hyperactivation is associated with kidney dysfunction and increased fibrosis. 43 In a mouse model of non-proteinuric diabetic kidney disease, SGLT2i, particularly empagliflozin conferred renal protection by increasing endogenous ketones and suppressing mTORC1 activation in the kidneys. 36 The treatment with empagliflozin mirrored the effect of exogenous ketone supplementation, where both treatments reduced kidney damage as evident through lower plasma cystatin-C levels and decreased interstitial fibrosis. 36 The renoprotective mechanism of SGLT2i hinges on the ketogenesis rate-limiting enzyme HMGCS2 highlighting ketone production’s central role in its antifibrotic effects. 36 51
Antioxidative effects
Ketones, specifically BHB, act as an important signaling molecule influencing gene expression through various regulatory pathways. BHB notably inhibits class I histone deacetylase enzyme activity in kidney tissue, enhancing the expression of genes that respond to oxidative stress, including Foxo3a and Mt2 . 52 This confers protection against oxidative stress in human kidney cells and various animal models. Studies also show that a ketogenic diet or BHB treatment can activate the major detoxification and oxidative stress nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. 53 In a spontaneous mouse model of T2D (db/db mice) treated with dapagliflozin, there was a noticeable reduction in the expression of genes related to oxidative stress compared with those treated with a standard vehicle or glimepiride. 54 This reduction is associated with increased levels of BHB and NRF2 protein expression. 54 Similarly, BHB treatment in human proximal tubular cells (HK-2) led to increased NRF2 expression and induced NRF2 nuclear translocation. 55 Furthermore, a ketogenic diet has been reported to increase the expression of other antioxidants such as NAD(P)H dehydrogenase quinone I (NQO1) and superoxide dismutase (SOD1/2), 56 and ameliorated paraquat (PQ)-induced elevated lipid peroxidation, toxicity, reduced antioxidant activity and decreased Nrf2 expression, 57 highlighting its potential therapeutic role in combating oxidative stress and tissue hypoxia.
Mitochondrial dysfunction
Mitochondrial dysfunction is another key feature of both acute kidney failure and CKD. 58 Ketogenic diet activates the expression of peroxisome proliferator-activator receptor γ (PPARγ) coactivator-1-alpha (PGC-1ɑ) 59 and silent information regulator transcript-1 (SIRT1). 60 61 PGC-1ɑ is the main transcription factor that controls the expression of genes involved in mitochondrial biogenesis and function, while SIRT1 activation protects organelle damage including the mitochondria and reduces oxidative stress. In a recent study on diabetic mice, a ketogenic diet improved mitochondrial function and capacity through its activation of PGC-1ɑ and SIRT1. 62 Further, administration of exogenous BHB was found to increase PGC-1ɑ and mitochondrial copy number in rat kidneys. 63 Human data on mitochondrial function are lacking, but we obtained skeletal muscle biopsies from physically active adults before and after a 12-week ketogenic diet and demonstrated that mitochondrial function and efficiency shifted towards fat oxidation while improving insulin sensitivity. 64
Traditional concerns of a ketogenic diet on kidney function
Common misconceptions about ketogenic diets related to kidney health include potential adverse effects on acid–base and electrolyte balance and risk for kidney stones. The next section briefly discusses the typical renal metabolic response to a ketogenic diet that maintains pH and electrolyte status. Most work in this space has been done in the context of normal kidney function, so we mention how the situation in CKD may differ.
Electrolyte and acid–base imbalance
Ketogenic diets promote a natriuretic and diuretic effect similar to that demonstrated during starvation. 65 66 This in part accounts for the typical rapid weight loss that occurs during the initiation of a ketogenic diet. If sodium intake is not commensurate with the additional loss of sodium, two deleterious outcomes are more likely to manifest: (1) Individuals may develop common signs and symptoms of hypovolemia, colloquially referred to as “Keto-Flu,” which include dizziness when standing, lethargy, and muscle spasms/cramps. (2) Counter-regulatory mechanisms are activated that include sympathetic and aldosterone stimulation that act to preserve plasma volume by increasing sodium reabsorption and a concomitant excretion of potassium and magnesium. These side effects can be eliminated with attention to proper electrolyte intake. For most individuals with normal kidney function consuming a ketogenic diet, it should be emphasized to ingest an additional 1–2 g sodium/day (4–5 g sodium/day total), a maintenance of 3–4 g/day potassium, and sufficient fluid intake.
In CKD, a decrease in viable nephrons and reduction in glomerular filtration rate (GFR) change the kidney’s normal physiology and sodium balance. 67 68 Even though an adaptive fractional increase in sodium excretion per individual nephron unit compensates for the reduced number of working nephrons, the kidney’s inability to excrete sufficient amounts of sodium results in sodium retention, extracellular fluid expansion, and blood pressure increase. 68 69 Likewise, the renin–angiotensin–aldosterone system is activated in CKD, further exacerbating sodium retention and causing vasoconstriction which could significantly raise blood pressure. 69 Sodium retention and its association with blood pressure in CKD are often referred to as “sodium-sensitive hypertension.” 70 Therefore, reducing salt intake is recommended to manage hypertension in patients with CKD. 68 The natriuretic and diuretic effect of the ketogenic diet may help alleviate sodium retention and improve systemic and glomerular blood pressure. Low carbohydrate and ketogenic diet studies often report a reduction in systolic and diastolic blood pressure 71 72 and blood pressure medication requirements. 73 However, the current recommendation of sodium intake in a WFKD is based on individuals with normal kidney function. 74 Recommendations for sodium and electrolyte intake for patients with CKD following a ketogenic diet should be individualized by a healthcare professional based on the patient’s renal function and electrolyte status. Future studies should assess the relationship between ketosis, sodium balance, and blood pressure.
Another misconception associated with ketogenic diets relates to promotion of acidosis owing to specific food items and the weakly acidifying effects of ketones, which could worsen kidney function, bone health, and kidney disease-associated endocrinopathies. 75 76 In healthy subjects provided a carefully prepared ketogenic diet with mean BHB levels >2 mM, serum bicarbonate was modestly reduced but well within normal ranges. 77 A ketogenic diet with mild ketosis (~1 mM) in individuals with normal kidney function has no significant impact on blood pH, serum bicarbonate level, and anion gap over 21 days 78 and 4 months. 79
When faced with an increased acid load, normal kidney function affords compensatory increases in ammonium excretion. In a somewhat mirrored perspective where the acid load is stable and the kidney function is reduced, the fewer working nephrons compensate with increased ammoniagenesis and excretion. This adaptation results in high intrarenal ammonia, which is thought to activate the alternative complement pathway eventually leading to tubulointerstitial fibrosis. Decreases in GFR to levels below 40–50 mL/min diminish the kidney’s ability to excrete more ammonium and overall acid 80 ; hence, metabolic acidosis is more commonly encountered at this level of disease. In patients with kidney disease, clinicians commonly monitor steady-state serum bicarbonate levels to assess overall acid load. However, decreases in serum bicarbonate are often reported at a later stage of the disease and it is considered inadequate to reflect the overall acid load. Eubicarbonatemic hydrogen ion retention among patients with earlier CKD is increasingly an area of focus 81 ; thus, studying ketogenic diet in all stages of CKD requires longer term study of acid excretion and the rate of kidney function decline.
Urinary acid excretion is favored as the gold standard for estimating acid load, and the prevailing wisdom was that an increased dietary acid load would burden the kidneys further and lead to more dysfunction. However, recent observations from the rich data collected in the Chronic Renal Insufficiency Cohort Study have demonstrated pitfalls to that simplistic view. 82 83 Among patients with diabetes, higher levels of net acid excretion were associated with a lower risk of CKD progression. These studies suggested that the changes in acid excretion were diet-independent and may be elicited by changes in energy metabolism and endogenous acid production from insulin resistance. 82 83 Currently, the effect of ketogenic diet on net acid excretion is unknown and this would be worthwhile exploring in patients with T2D and varying stages of CKD.
Kidney stones
Kidney stones, especially genetically driven stones, are associated with an increased risk of CKD. 84 A recent meta-analysis reported a pooled kidney stone incidence of 5.9% among patients on a ketogenic diet followed for a median of 3.7 years, 85 compared with a historical incidence rate of <0.3% per year in the general population. 86 Most studies reporting risk of kidney stones were in children receiving a ketogenic diet therapy for epilepsy 85 87–91 with higher incidence during long-term exposure (ie, 25% over 6 years, 91 which is complicated by concurrent use of antiseizure medications (eg, carbonic anhydrase inhibitors) and other risk factors in this population. In adults with obesity, who are at higher kidney stone risk based on their higher adiposity, 92 consuming a ketogenic diet over 2 years revealed no harmful effects on GFR, albuminuria, or fluid and electrolyte balance compared with a low-fat diet 93 ; and there was one possible, but not confirmed, case of kidney stones out of 153 subjects. 94
Uric acid stones are the most frequently reported by individuals on a ketogenic diet, followed by calcium oxalate stones or mixed stones with calcium and uric acid. 85 A ketogenic diet transiently increases uric acid concentration 25%–50%, which usually peaks at 2–4 weeks, and gradually returns to prediet levels by 8 weeks. 95–97 The initial rise in uric acid is concomitant with the rise in ketones, and it was postulated that the reason for this may be competition between uric acid and ketones for the same organic acid transporters, which are required for renal excretion. 98–101 After several weeks, the kidney conserves ketones, 102 presumably allowing for return of normal renal uric acid excretion and serum levels.
There may be effective strategies to mitigate the kidney stone risk in patients following a ketogenic diet. Increasing fluid intake to maintain dilute urine limits the possibility of mineral crystallization. 103 Urine alkalinization, particularly addressing hypocitraturia, may inhibit supersaturation of calcium salts and aggregation. 104 105 Moreover, studies of kidney stones have largely precluded patients with CKD where their urine parameters change alongside diminishing kidney function. A retrospective study of 811 patients with kidney stones noted that advancing kidney disease afforded reduced calcium stone formation, presumably due to reduced calciuria 106 and increased uric acid stone formation. 107 Metabolic acidosis resulting in acidic urine pH is common among individuals with CKD. 108 Low urine pH is a well-known risk factor for forming uric acid kidney stones due to the low solubility of uric acidic in acidic conditions. 109 110 At the same time, low urine pH leads to hypocitraturia which increases the risk of forming calcium oxalate kidney stones. 111 Hence, future examination of how a ketogenic diet impacts the incidence of kidney stones among patients with T2D and CKD is paramount. Being aware of and addressing the potential kidney stone risk with well-established measures—such as urine alkalization, correcting hypocitraturia, and increasing fluid intake—is prudent. Additionally, understanding that diet-imposed change in risk through modulation of ammonia excretion, uricosuria, calciuria, citraturia, and other urinary parameters will assist with future guidance.
Current evidence on very low or low carbohydrate diet intervention and its effect on kidney function
Evidence from animal studies.
Several rodent studies have specifically investigated the effects of a ketogenic diet on kidney function and disease. Two mouse studies reported benefit of ketogenic diet on DN, even reversing some of the key molecular features of DN. Poplawski et al assessed the effect of ketogenic diet on DN using both type 1 (Akita) and type 2 (db/db) murine diabetes models. In both models, the mice initially developed albuminuria on chow diet, and after transitioning to the ketogenic diet reversed and normalized urinary albumin/creatinine ratio (UACR) within 8 weeks. 112 Furthermore, the expression of several stress-induced genes involved in oxidative stress and toxicity was completely normalized by ketogenic diet in both models, with an observed effect that was more consistently robust in the type 1 mouse model. Likewise, histopathologic features of glomerular sclerosis were also partially reversed by the ketogenic diet in the T2D mouse model. 112 Jung et al examined db/db DN mice fed normal chow diet (dbNCD), high-fat diet (dbHFD), or ketogenic diet (dbKETO). dbKETO animals had lower UACR and blood urea nitrogen to creatinine ratio levels after 5 weeks compared with the dbNCD and dbHFD mice. 55 Histologic analysis of the kidney showed that dbKETO mice had less fibrotic changes than the dbNCD and dbHCD mice suggesting that the dbKETO mice delayed progression of DN histologic phenotypes. Furthermore, in the same report, treatment of the human proximal tubular cell line (HK-2) with BHB led to activated autophagy by increasing the LC3 I to LC3 II ratio, phosphorylation of adenosine 5 monophosphate-activated protein kinase (AMPK), beclin, p62 degradation, NRF2 expression, and decreased glucose-induced ROS levels. 55 Studies in a rat model of a genetic form of CKD, polycystic kidney disease, showed that a ketogenic diet not only slowed disease progression and preserved renal function in young animals but even partially reversed existing renal cystic disease in older animals. 18 The treatment resulted in improvement of renal fibrosis and inhibition of mTORC1 and epithelial proliferation. Remarkably, the effects could be replicated by administering BHB in the drinking water in a dose-dependent manner, without any food changes. 18 63 These results suggest that the actions of BHB may underlie most of the renoprotective mechanisms of nutritional ketosis, and that exogenous BHB can be effectively supplemented.
Evidence from clinical and observational studies
Clinical and observational studies that examined kidney function in response to low-carbohydrate diets ranging from <20 g/day to 30%–40% of energy expenditure are presented in online supplemental table 1 . Three of the six randomized controlled trials (RCTs) reported no significant changes in kidney function in the low carbohydrate arm compared with the comparison diet group. Two of the three studies followed the participants with normal baseline eGFR for 52 weeks 113 114 and the third study followed subjects with slightly lower baseline eGFR (<80) for 12 weeks. 115 Another two RCTs reported renal benefit in the low carbohydrate arm with improvements in serum creatinine, cystatin C, eGFR, and albumin. 93 116 The study by Tirosh et al reported greater eGFR improvement in those following a low-carbohydrate diet versus both a Mediterranean and low-fat diet. 116
Supplemental material
The use of surrogate markers, especially serum creatinine-derived estimates of kidney function, is less accurate at higher eGFRs and may be mischaracterized amidst dietary intervention, highlighting the importance of studying major adverse kidney events and assessing cystatin C-derived kidney function estimates. Thus far, only one RCT has reported hard kidney endpoints including all-cause mortality that compared a carbohydrate-restricted, low-iron, polyphenol enriched diet (CR-LIPE) with a standard protein restriction diet (SPRD). 117 The 191 participants in this study were followed for approximately 4 years. In this study, CR-LIPE significantly decreased doubling of serum creatinine (relative risk, 0.53, 95% CI 0.33 to 0.86, p<0.01), all-cause mortality (relative risk, 0.5, 95% CI 0.2 to 1.12) and also delayed end-stage renal disease and renal replacement therapy when compared with SPRD. 117 However, the CR-LIPE intervention was a multimodal dietary intervention that included carbohydrate restriction (35% of the energy intake) as one of the dietary modifications along with low-iron availability and polyphenol enrichment in the diet. Future study involving major adverse kidney endpoints is warranted to confirm if a ketogenic diet has beneficial impact on kidney disease.
Presumably because eGFR is less accurate at healthier function (eGFR >80 mL/min), some of these studies have shown that the beneficial effect of low carbohydrate diet is greater in those with lower starting baseline eGFR. For example, the study by Tirosh et al reported that the increase in eGFR was greater in those with CKD stage 3 (a 7.1-point; 10% eGFR increase from baseline) than the whole cohort (+5.3% increase from baseline) in the low carbohydrate arm. 116 While other studies included a range of baseline eGFRs, the subset of patients with more significant kidney dysfunction (eGFR <60 mL/min) exhibited a slower decline in function, and no deterioration was evident in participants with normal baseline eGFR. 118 119 Furthermore, caution is warranted when interpreting creatinine-derived eGFR measurements because any change in skeletal muscle mass during a nutritional study may affect the endogenous production of creatinine independent of actual changes in renal function. Hence, corroboration with cystatin-C measurements would strengthen these observations. The single-arm prospective 12 weeks study on individuals with relatively advanced diabetes nephropathy (eGFR <40 mL/min) reported statistically significant improvements in eGFR, serum creatinine, and cystatin C. 118 Three additional retrospective observational studies reported improvements in kidney function in individuals following a low carbohydrate diet 120–122 ( online supplemental table 1 ). One of these studies reported improvement in eGFR and decrease in UACR at an average follow-up of 30 months 119 while the other two studies reported eGFR improvement in individuals with reduced kidney function at baseline (eGFR <90 in one study and eGFR <70 in the other study). 121 122
In contrast, there were only two observational studies frequently cited when suggesting that a low carbohydrate diet is associated with adverse kidney outcomes. These studies did not focus on individuals adhering to a ketogenic diet or on those limiting their carbohydrate intake. For instance, Farhadnejad et al ’s 2018 study, which was a population-based prospective analysis, investigated the association between different tertiles of low carbohydrate high protein (LCHP) scores and the incidence of CKD. 123 Notably, none of the LCHP score tertiles in the study indicated a carbohydrate-restricted diet. Even in the tertile with the lowest LCHP score, carbohydrates contributed to 51.0% of the total energy, resembling the carbohydrate profile of a standard Western diet where 40%–60% of energy typically comes from carbohydrates. The other retrospective observational study by Li et al reported an association between elevated fasting ketone level with abnormal renal function 124 in people with T2D who were admitted to the hospital, and who were not specifically eating a ketogenic diet. The association of ketones and renal function in this study is not relevant to dietary carbohydrate restriction in an ambulatory population.
Altogether, these clinical and observational studies show no harm from low or very low carbohydrate diets for people with diabetes in the setting of normal renal function, and a possible beneficial effect in the setting of moderately reduced renal function. The kidney function improvement observed in these studies may be an ancillary outcome associated with other improvements seen in these interventions including weight loss, glycemic control, or blood pressure improvement. Interestingly, Unwin et al reported no association between observed kidney function improvement with the magnitude of weight loss, improvement in blood pressure and HbA1c, 120 while another study reported that the increase in eGFR was significantly associated with a decrease in fasting insulin and systolic blood pressure but not with the level of weight loss and protein intake in the intervention. 116 In our previous study on patients with T2D following a very low carbohydrate intervention, there was a marginally significant increase in eGFR at 1 year. 72 A post hoc analysis of these data revealed that a higher mean BHB at 1 year (β=5.04, p=0.005) was significantly associated with a greater increase in mean eGFR (unpublished data). Furthermore, in a subgroup analysis of 22 trial participants with an eGFR <60 mL/min/1.73 m 2 at baseline who remained in the study for 2 years, 72 the mean eGFR progressively increased from 51 mL/min/1.73 m 2 to 60, 63, and 68 mL/min/1.73 m 2 at 10 weeks, 1 year, and 2 years (unpublished data). Notably, the majority of the 22 participants reverted to stage 2 and no one progressed to stage 4 CKD. Evidently, a dose-dependent association exists between ketosis trajectory classes and the increase in total eGFR slope at 2 years. 125 Participants with higher endogenous ketone concentration and longer duration of ketosis maintenance exhibited the greatest rise in the 2-year eGFR slope compared with those with lower endogenous ketone concentration and unsustainable ketosis maintenance. 125 Hence, available evidence suggests that carbohydrate restriction and ketosis afford benefits to kidney function. It will be important to determine in future trials whether the improvement in kidney function translates to a sustained long-term reduction, or even reversal, in the progression of kidney disease.
Evidence from meta-analyses, systematic and narrative review
A recent review discussing the potential negative effect of purported ketogenic diets on kidney health focused on observational studies that compared low protein versus high protein diets that were not ketogenic or low carbohydrate diets, 126 and raised concern about the association of albuminuria with high animal fat but only referred to observational studies that assessed high animal fat intake in the context of a Western diet 126 negating the relevance of the studies cited for concern.
In contrast, systematic reviews and meta-analyses that assessed pooled effects of RCTs reported beneficial effects of low-carbohydrate diets. The meta-analysis by Oyabu et al evaluated nine RCTs with 861 participants in the low carbohydrate arm and 826 participants in the control group. 127 Despite a large variation in the proportion of carbohydrate intake from 4% to 45% in the low carbohydrate arm of the nine studies with a study duration ranging from 6 to 24 months, the review revealed that there was a significant increase in eGFR in the low carbohydrate group versus control group. 127 Another meta-analysis with 12 RCTs that only included patients with T2D reported no significant difference in the pooled eGFR and creatinine mean estimate between the lower carbohydrate diets (14%–45% of calories from carbohydrate) versus control diets over 5 weeks to 24 months. 128 Similarly, another meta-analysis that included five RCTs with the low carbohydrate arm had carbohydrate intake <45%, and the studies ranging from 5 weeks to 24 months reported no difference in the pooled eGFR estimate between the control and low carbohydrate diets. 129 The current evidence from systematic reviews and meta-analyses with a range of carbohydrate intake suggests that carbohydrate restriction is not associated with adverse effects on kidney function, or in some cases might be beneficial.
Evidence from genetically driven kidney disease
Individuals with autosomal dominant polycystic kidney disease (ADPKD) may benefit from calorie restriction or ketogenic diet. 19 130 131 This chronic progressive condition is characterized by hyperproliferation, inflammation, fibrosis, and cyst growth, leading to deterioration of kidney function over time. 19 132 mTOR is one of the main signaling pathways activated in ADPKD. 132 A study of various polycystic kidney disease animal models showed that time-restricted feeding, administration of a ketogenic diet, or supplementation with exogenous BHB prevented kidney cyst disease progression by inhibiting cell proliferation, fibrosis, and cyst growth. 18 63 Furthermore, the mTOR activity was inhibited in these animal models suggesting that blunting the signaling pathway inhibits cell proliferation, growth, and fibrosis in ADPKD. 18 63 133 In humans, a retrospective observational study of ADPKD patients who self-initiated ketosis either using ketogenic or time-restricted diets reported improvement in eGFR after 6 months. 134 A pilot study on 24 patients with ADPKD demonstrated the feasibility of the ketogenic diet, reporting high adherence rates and improvements in blood pressure, eGFR, and kidney pain. 130 In another exploratory RCT, 66 participants with ADPKD were randomized to ketogenic, water fasting, or control diets. The study confirmed the feasibility of the therapy in the ketogenic arm (KD) and revealed significant improvements in eGFR, including both creatinine and cystatin C-derived eGFR in the KD group but no improvements were observed in the water fasting and control diets. 131 Additionally, there were no significant differences in UACR and blood pressure among the three diets. 131
Perspectives and future direction
There is a considerable body of research suggesting that a very low carbohydrate ketogenic diet is safe in individuals with moderately diminished kidney function, even in studies that had higher protein intake than what is recommended for kidney disease and diets that are not plant-based. The diet can be safely prescribed in patients with T2D for treating and remitting diabetes even if they have underlying stage 2 or 3 CKD or reduced kidney function. Beyond safety, mechanistic plausibility, preclinical data, and even some RCT studies suggest that carbohydrate-restricted diets may be beneficial in improving moderate kidney dysfunction and in reducing progression of CKD. The preliminary proof of concept from small and short duration studies in humans and animals suggests a very low carbohydrate diet could be an effective dietary intervention for patients with CKD. Furthermore, there are predeveloped ketogenic nutritional options to consider when we plan a future trial to assess the impact of ketogenic diet on patients with CKD, such as the recently developed program for treating ADPKD known as Ren.Nu. This program is a plant-focused ketogenic medical nutrition therapy, designed to avoid renal stressors like oxalate, inorganic phosphate, and purines/uric acid. It includes a medical food formulation, KetoCitra, containing BHB with alkaline citrate which helps antagonize kidney stone formation. 129 130 Based on the findings from these different studies and currently available ketogenic medical therapy specific for kidney disease, there is a need for future larger and longer follow-up randomized controlled clinical trials on very low carbohydrate diet, including nutritional ketosis in patients with CKD with or without T2D on kidney hard endpoints including major adverse kidney events (a composite event of death, persistent renal decline >25% decline in eGFR, and a new initiation of dialysis) and other kidney-related outcomes to firmly establish the long-term effectiveness. For example, a head-to-head comparison of the safety and efficacy of ketogenic nutritional therapy versus SGLT2i pharmacologic intervention (that involves the same mechanism of raising ketone levels) could be of high interest. Weight loss from the diet can improve filtration and albuminuria. Thus, including other surrogate endpoints like eGFR slope and microalbuminuria in these studies have the potential to elucidate the degree to which weight loss and blood pressure improvement from the diet affects kidney function markers and also to explore if ketone levels independently have an impact on these markers and endpoints. Furthermore, these studies should also assess the diet’s overall safety in patients with T2D and CKD, specifically exploring its effect on net acid excretion, kidney stone formation, and maybe its beneficial effect on sodium retention hypertension. Finally, another important consideration in the clinical trial design for evaluating the efficacy of a very low carbohydrate diet in patients with CKD is understanding the diet’s additive role, especially how the diet interacts with currently available treatment drugs for patients with CKD including renin–angiotensin system blockade (angiotensin-converting enzyme inhibitor, ACEi and angiotensin receptor blockers, ARBs), SGLT2i, glucagon-like peptide-1 receptor agonists (GLP1-RA), and the non-steroidal mineralocorticoid receptor antagonists (finerenone).
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- Dean BB , et al
- ElSayed NA ,
- Aroda VR , et al
- Park S , et al
- Esmeijer K ,
- Geleijnse JM ,
- de Fijter JW , et al
- Guyton JR , et al
- Seshadri P , et al
- Westman EC ,
- Mavropoulos JC , et al
- Cuenca-Sánchez M ,
- Navas-Carrillo D ,
- Orenes-Piñero E
- Ikizler TA ,
- Burrowes JD ,
- Byham-Gray LD , et al
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group
- Beck GJ , et al
- Kopple JD ,
- Wang X , et al
- Laville M ,
- Boissel JP , et al
- Wen Y , et al
- Sugahara S , et al
- Torres JA ,
- Kruger SL ,
- Broderick C , et al
- Saville J ,
- Kalantar-Zadeh K
- Balasse EO ,
- Puchalska P ,
- Crawford PA
- Röhling M , et al
- Mudaliar S ,
- Bosy-Westphal A , et al
- Baverel G ,
- Ferrier B ,
- Forbes JM ,
- Thorburn DR
- Qiao Y , et al
- Audzeyenka I ,
- Bierżyńska A ,
- Fang Y , et al
- Ferrannini E ,
- Frascerra S , et al
- Cuenoud B ,
- Hartweg M ,
- Godin J-P , et al
- McDonough AA ,
- Shahzad K ,
- Dong W , et al
- Qiu J , et al
- Liu C , et al
- Nguyen KY ,
- Grant RW , et al
- Goldberg EL ,
- Molony RD , et al
- Kim SH , et al
- Spigoni V ,
- Cinquegrani G ,
- Iannozzi NT , et al
- Gambhir D ,
- Veeranan-Karmegam R , et al
- Wang J-F , et al
- Wang JF , et al
- Bhanpuri NH ,
- Hallberg SJ ,
- Williams PT , et al
- Phinney S ,
- Athinarayanan S , et al
- Forsythe CE ,
- Phinney SD ,
- Fernandez ML , et al
- Kogot-Levin A ,
- Riahi Y , et al
- Shimazu T ,
- Hirschey MD ,
- Newman J , et al
- Milder JB ,
- Kim YJ , et al
- Hovda DA , et al
- Liu F , et al
- Pathak SJ ,
- McCarty MF ,
- DiNicolantonio JJ ,
- Scheibye-Knudsen M ,
- Mitchell SJ ,
- Fang EF , et al
- Roberts MN ,
- Wallace MA ,
- Tomilov AA , et al
- Holznecht N ,
- Asplund DA , et al
- Miller VJ ,
- LaFountain RA ,
- Barnhart E , et al
- Palmer BF ,
- Saudek CD ,
- Boulter PR ,
- Borrelli S ,
- Provenzano M ,
- Gagliardi I , et al
- Cuevas CA ,
- Zietse R , et al
- Murray SW , et al
- McKenzie AL ,
- Athinarayanan SJ ,
- McKenzie AL , et al
- Dhondup T ,
- Bistrian BR ,
- Wolfe RR , et al
- Sharma AP ,
- Ross ML , et al
- Gomez-Arbelaez D ,
- Crujeiras AB ,
- Castro AI , et al
- Pourafshar N ,
- Pourafshar S ,
- Soleimani M
- Scialla JJ ,
- Dobre M , et al
- Luciano A ,
- Pendergast J , et al
- Bergstralh EJ ,
- Melton LJ , et al
- Acharya P ,
- Acharya C ,
- Thongprayoon C , et al
- Pyzik PL , et al
- Kossoff EH ,
- Furth SL , et al
- Herzberg GZ ,
- Fivush BA ,
- Kinsman SL , et al
- Sampath A ,
- Groesbeck DK ,
- Taylor EN ,
- Stampfer MJ ,
- Friedman AN ,
- Foster GD , et al
- Foster GD ,
- Hill JO , et al
- Horton ES ,
- Sims EAH , et al
- García-Nieto VM ,
- Claverie-Martín F ,
- Moraleda-Mesa T , et al
- Goldfinger S ,
- Klinenberg E ,
- Seegmiller JE
- Lecocq FR ,
- Agarwal N ,
- Farooq O , et al
- McNally MA ,
- Rubenstein JE , et al
- Zupec-Kania BA ,
- Amark PE , et al
- Cirillo M ,
- Bilancio G ,
- Cavallo P , et al
- Simmons K ,
- Phadke M , et al
- Reaven NL , et al
- Pazos Pérez F
- Haghighatdoost F ,
- Sadeghian R ,
- Clark CCT , et al
- Poplawski MM ,
- Mastaitis JW ,
- Isoda F , et al
- Thompson CH ,
- Luscombe-Marsh ND , et al
- Brinkworth GD ,
- Buckley JD ,
- Noakes M , et al
- Zainordin NA ,
- Eddy Warman NA ,
- Mohamad AF , et al
- Harman-Boehm I , et al
- Facchini FS ,
- Chambers M ,
- Kamendulis LM , et al
- Tuccinardi D ,
- Tozzi R , et al
- Crocombe D , et al
- Mitchell NS ,
- Wilmsen N ,
- Geerlings W , et al
- Farhadnejad H ,
- Asghari G ,
- Emamat H , et al
- Shen X , et al
- Roberts CGP ,
- Adams RN , et al
- Joshi S , et al
- Hashimoto Y ,
- Fukuda T , et al
- Kingaard JJ ,
- Munits M , et al
- Cukoski S ,
- Lindemann CH ,
- Arjune S , et al
- Shillingford JM ,
- Murcia NS ,
- Larson CH , et al
- Torres JA , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Contributors SJA, JV, and CGPR conceptualized the review topic and formulated the objectives; SJA conducted the comprehensive literature search, synthesized and interpreted the data from the collected literature; SJA drafted the original manuscript; JSV, TW, CGPR, and CV provided critical revisions and edits to the manuscript; ALM and GKS reviewed and edited the manuscript; All authors have read and agreed to the final version of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests TW is an inventor on issued and pending patents filed by the University of California, Santa Barbara related to the topic of this article. TW is a founder and shareholder of Santa Barbara Nutrients, Inc., holds a managerial position, and has contributed to the development of the Ren.Nu ketogenic dietary program and the medical food KetoCitra. TW received speaker fees from Otsuka, was a scientific advisor of Chinook Therapeutics, and received research funding from Chinook Therapeutics. JSV is a cofounder and shareholder of Virta Health, serves as a science advisor for Simply Good Foods and Nutrishus Brands, and has authored books on ketogenic diets. SJA, CGPR, and GKS are employees and shareholders of Virta Health. ALM is a shareholder of Virta Health.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 06 March 2024
Type 1 diabetes: from the dream of automated insulin delivery to a fully artificial pancreas
- Moshe Phillip 0 ,
- Aaron Kowalski 1 &
- Tadej Battelino 2
The Institute for Endocrinology and Diabetes, Schneider Children's Medical Center, Petah Tikva, and Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
You can also search for this author in PubMed Google Scholar
JDRF International, New York, NY, USA.
Faculty of Medicine, University of Ljubljana, and Department of Endocrinology, Diabetes and Metabolism, University Children's Hospital, University Medical Centre Ljubljana, Ljubljana, Slovenia.
The journey from pioneering concept to practical clinical application of automated insulin delivery (AID) systems traces back to 1963, when Arnold Kadish described the first wearable system for delivering insulin, calibrated by an in vivo continuous glucose monitoring (CGM) system (Fig. 1). In effect, this was the first automated closed-loop device, also able to infuse glucagon to counter hypoglycemia. Dubbed ‘The Backpack’, it was an impractical proof-of-concept system that ignited the development of genuinely wearable insulin pumps and CGM systems. By 1974, a portable closed-loop artificial pancreas, which used algorithms on a computer consol to deliver intravenous insulin, was used successfully to treat diabetic ketoacidosis and coma in five adult patients 22–89 years of age 1 . By 1977, this bulky ‘Biostator’ was in limited use in hospitals. From that point on, the development of diabetes technology focused separately on insulin delivery and CGM systems, rather than on an integrated artificial pancreas. Commercial, wearable, continuous subcutaneous insulin infusion (CSII) pumps were introduced from 1978 onward, and the first ‘smart insulin pump’ was launched in 2002, with a bolus calculator and missed-meal-bolus alarms.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41591-024-00013-5
Competing interests
M.P. has participated on advisory boards for AstraZeneca, Eli Lilly, Insulet, Mannkind, Medtronic Diabetes, Pfizer, Sanofi and Dompé, has received consulting fees from Eli Lilly, Medtronic Diabetes, Novo Nordisk, Pfizer, Sanofi and Qulab Medical, has received, through his institute, research grants from Dexcom, Eli Lilly, Insulet, Medtronic Diabetes, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, DreaMed Diabetes, NG Solutions, Dompe, Lumos, GWAVE and OPKO, and owns stocks in DreaMed-Diabetes and NG Solutions. T.B. has participated on advisory boards for Novo Nordisk, Sanofi, Eli Lilly, Boehringer-Ingelheim, Medtronic and Indigo Diabete, and as a speaker for AstraZeneca, Eli Lilly, Novo Nordisk, Medtronic, Sanofi and Roche, and owns stocks of DreaMed Diabetes, and his institution has received research grant support and travel expenses from Abbott Diabetes Care, Medtronic, Novo Nordisk, Sanofi, Sandoz and Novartis. A.K. declares no competing interests.
Pfeiffer, E., Thum, Ch. & Clemens, A. Horm. Metab. Res. 6 , 339–342 (1974).
Google Scholar
Bergenstal, R.M. et al. N. Engl. J. Med. 369 , 224–232 (2013).
Battelino, T. & Phillip, M. J. Pediatr. Endocrinol. Metab. 17 , 375–376 (2004).
Kowalski, A.J. Diabetes Technol. Ther. 11 S1, S113–S119 (2009).
Bergenstal, R.M. et al. N. Engl. J. Med. 363 , 311–320 (2010).
Battelino, T., Nimri, R., Dovc, K., Phillip, M. & Bratina, N. Diabetes Care 40 , 764–770 (2017).
Hovorka, R. et al. Lancet 375 , 743–751 (2010).
Phillip, M. et al. N. Engl. J. Med. 368 , 824–833 (2013).
Tauschmann, M. et al. Lancet 392 , 1321–1329 (2018).
Armiger, R., Reddy, M., Oliver, N.S., Georgiou, P. & Herrero, P. J. Diabetes Sci. Technol. 16 , 29–39 (2022).
Biester, T. et al. Diabetes Obes. Metab. 23 , 599–608 (2021).
Alva, S., Castorino, K., Cho, H. & Ou, J. J. Diabetes Sci. Technol. 15 , 768–774 (2021).
Nwokolo, M. et al. Diabetes Technol. Ther. 25 , 856–863 (2023).
Blauw, H., Onvlee, A.J., Klaassen, M., van Bon, A.C. & DeVries, J.H. Diabetes Care 44 , 836–838 (2021).
Tsoukas, M.A. et al. Lancet Digital Heal 3, e723–e732 (2021).
Download references
Reprints and permissions
Southeast University Future Technology Institute Recruitment Notice
Professor openings in mechanical engineering, control science and engineering, and integrating emerging interdisciplinary majors
Nanjing, Jiangsu (CN)
Southeast University
Staff Scientist
A Staff Scientist position is available in the laboratory of Drs. Elliot and Glassberg to study translational aspects of lung injury, repair and fibro
Maywood, Illinois
Loyola University Chicago - Department of Medicine
W3-Professorship (with tenure) in Inorganic Chemistry
The Institute of Inorganic Chemistry in the Faculty of Mathematics and Natural Sciences at the University of Bonn invites applications for a W3-Pro...
53113, Zentrum (DE)
Rheinische Friedrich-Wilhelms-Universität
Principal Investigator Positions at the Chinese Institutes for Medical Research, Beijing
Studies of mechanisms of human diseases, drug discovery, biomedical engineering, public health and relevant interdisciplinary fields.
Beijing, China
The Chinese Institutes for Medical Research (CIMR), Beijing
Research Associate - Neural Development Disorders
Houston, Texas (US)
Baylor College of Medicine (BCM)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Association of ultra...
Association of ultra-processed food consumption with all cause and cause specific mortality: population based cohort study
Linked editorial.
Ultra-processed foods linked to higher mortality
- Related content
- Peer review
- Zhe Fang , doctoral student 1 ,
- Sinara Laurini Rossato , adjunct professor 2 3 ,
- Dong Hang , associate professor 3 4 ,
- Neha Khandpur , assistant professor 3 5 6 ,
- Kai Wang , research associate 1 ,
- Chun-Han Lo , resident physician 7 ,
- Walter C Willett , professor 1 3 8 ,
- Edward L Giovannucci , professor 1 3 ,
- Mingyang Song , associate professor 1 3 9
- 1 Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA
- 2 Laboratory of Research and Extension in Epidemiology (Lapex-Epi), Institute of Geography, Universidade Federal de Uberlândia, Uberlândia, MG, Brazil
- 3 Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA
- 4 Department of Epidemiology, Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, School of Public Health, Gusu School, Nanjing Medical University, Nanjing, China
- 5 Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands
- 6 Department of Nutrition, School of Public Health, University of São Paulo, São Paulo, Brazil
- 7 Department of Internal Medicine, Kirk Kerkorian School of Medicine, University of Nevada, Las Vegas, NV, USA
- 8 Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
- 9 Clinical and Translational Epidemiology Unit and Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
- Correspondence to: M Song msong{at}hsph.harvard.edu (or @MingyangSong3 on X/Twitter)
- Accepted 13 March 2024
Objective To examine the association of ultra-processed food consumption with all cause mortality and cause specific mortality.
Design Population based cohort study.
Setting Female registered nurses from 11 US states in the Nurses’ Health Study (1984-2018) and male health professionals from all 50 US states in the Health Professionals Follow-up Study (1986-2018).
Participants 74 563 women and 39 501 men with no history of cancer, cardiovascular diseases, or diabetes at baseline.
Main outcome measures Multivariable Cox proportional hazard models were used to estimate hazard ratios and 95% confidence intervals for the association of ultra-processed food intake measured by semiquantitative food frequency questionnaire every four years with all cause mortality and cause specific mortality due to cancer, cardiovascular, and other causes (including respiratory and neurodegenerative causes).
Results 30 188 deaths of women and 18 005 deaths of men were documented during a median of 34 and 31 years of follow-up, respectively. Compared with those in the lowest quarter of ultra-processed food consumption, participants in the highest quarter had a 4% higher all cause mortality (hazard ratio 1.04, 95% confidence interval 1.01 to 1.07) and 9% higher mortality from causes other than cancer or cardiovascular diseases (1.09, 1.05 to 1.13). The all cause mortality rate among participants in the lowest and highest quarter was 1472 and 1536 per 100 000 person years, respectively. No associations were found for cancer or cardiovascular mortality. Meat/poultry/seafood based ready-to-eat products (for example, processed meat) consistently showed strong associations with mortality outcomes (hazard ratios ranged from 1.06 to 1.43). Sugar sweetened and artificially sweetened beverages (1.09, 1.07 to 1.12), dairy based desserts (1.07, 1.04 to 1.10), and ultra-processed breakfast food (1.04, 1.02 to 1.07) were also associated with higher all cause mortality. No consistent associations between ultra-processed foods and mortality were observed within each quarter of dietary quality assessed by the Alternative Healthy Eating Index-2010 score, whereas better dietary quality showed an inverse association with mortality within each quarter of ultra-processed foods.
Conclusions This study found that a higher intake of ultra-processed foods was associated with slightly higher all cause mortality, driven by causes other than cancer and cardiovascular diseases. The associations varied across subgroups of ultra-processed foods, with meat/poultry/seafood based ready-to-eat products showing particularly strong associations with mortality.
Introduction
Ultra-processed foods are ready-to-eat/heat industrial formulations made mostly or entirely from substances derived from foods, including flavors, colors, texturizers, and other additives, with little if any intact whole food. 1 Ultra-processed foods, which are typically of low nutritional quality and high energy density, have been dominating the food supply of high income countries, and their consumption is markedly increasing in middle income countries. 2 Ultra-processed food consumption accounts for 57% of daily energy intake among adults and 67% among youths in the US according to the National Health and Nutrition Examination Survey (NHANES). 3 4
Ultra-processed foods usually disproportionately contribute added sugars, sodium, saturated fats and trans fats, and refined carbohydrates to the diet together with low fiber. 5 6 As well as having low nutritional quality, ultra-processed foods may contain harmful substances, such as additives and contaminants formed during the processing. 7 8 9 10 Growing evidence from large prospective cohorts show that ultra-processed food is associated with adverse health outcomes, such as overweight/obesity, cardiovascular diseases, type 2 diabetes, and colorectal cancer. 11 12 13 14 A systematic review showed that high ultra-processed food consumption was associated with increased risk of all cause mortality, cardiovascular diseases, metabolic syndrome, depression, and postmenopausal breast cancer. 15 However, few prospective cohort studies with a follow-up longer than 20 years have examined the association for all cause mortality or cause specific mortality, especially mortality due to cancer. High quality evidence from cohorts with a long follow-up is critical to inform dietary recommendations and food policies.
Leveraging the rich data obtained through repeated assessments for more than 30 years in two large US prospective cohorts, we examined the associations of total ultra-processed food and subgroups of ultra-processed food with mortality from all causes and major individual causes.
Study population
We used data from two large prospective cohorts in the US: the Nurses’ Health Study (NHS) began in 1976 and included 121 700 female registered nurses aged 30-55 years from 11 states; the Health Professionals Follow-up Study (HPFS) began in 1986 and enrolled 51 529 male health professionals aged 40-75 years from all 50 states. Every two years participants completed a mailed questionnaire enquiring about medical and lifestyle information. The baseline of this study was set to 1984 for the NHS and 1986 for the HPFS when the ultra-processed food data were first available. We excluded participants at baseline if they had reported a history of cancer, cardiovascular diseases, or diabetes; left more than 70 food items blank in the food frequency questionnaire or had implausible caloric intakes (<800 or >4200 kcal/d for men; <600 or >3500 kcal/d for women); or had missing data on ultra-processed food intakes. After exclusions, we included 74 563 women from the NHS and 39 501 men from the HPFS (supplementary figure A).
Assessment of ultra-processed food intake
Diet was assessed using a validated semiquantitative food frequency questionnaire administered every four years. 16 We grouped all foods into four categories of the Nova classification: unprocessed or minimally processed foods, processed culinary ingredients, processed foods, and ultra-processed foods, which has been described in detail elsewhere. 17 we further categorized ultra-processed foods into nine mutually exclusive subgroups (supplementary table B; supplementary figure B): ultra-processed breads and breakfast foods; fats, condiments, and sauces; packaged sweet snacks and desserts; sugar sweetened and artificially sweetened beverages; ready-to-eat/heat mixed dishes; meat/poultry/seafood based ready-to-eat products (for example, processed meat); packaged savory snacks; dairy based desserts; and other. Because alcohol is a well studied risk factor for premature death and a distinct factor in diet, we did not consider alcohol in ultra-processed foods in the primary analysis. Moreover, as wholegrain foods have established benefit for lowering all cause mortality, 18 we removed whole grains from ultra-processed foods in the primary analysis. We measured ultra-processed food intake as servings per day and adjusted it for total energy intake by using the residual method. 19
Ascertainment of outcomes
Death of a cohort member was notified by the next of kin via the post office when questionnaires or newsletters were returned or was identified through searches of the vital records of states and of the National Death Index. Study investigators blinded to the exposure status reviewed death certificates and extracted information from medical records to confirm the cause of death according to ICD-8 (international classification of diseases, 8th revision). The primary outcome of this study was all cause mortality. The secondary outcomes included deaths from cancer (ICD-8 codes 140-207), cardiovascular diseases (ICD-8 codes 390-459), and other causes (including respiratory diseases (ICD-8 codes 460-519) and neurodegenerative diseases (ICD-8 codes 290, 332, 340, 342, and 348)).
Assessment of covariates
Biennial follow-up questionnaires were used to collect self-reported information on body weight, marital status, smoking status and pack years, physical activity, family history of cancer/cardiovascular diseases/diabetes, and physical examination for screening purposes, as well as menopausal status and postmenopausal hormone use for women. We calculated body mass index as weight in kilograms divided by height squared in meters. Physical activity was assessed with a validated questionnaire and converted into metabolic equivalent task hours. 20 Alcohol drinking was measured by food frequency questionnaires as the number of drinks per week (considering one drink as one glass, bottle, or can of beer; one 4 ounce glass of wine; or one shot of liquor) and then converted into grams per day. We assessed overall dietary quality by using the Alternative Healthy Eating Index-2010 (AHEI) score. 21
Statistical analysis
Follow-up time accrued from the date of return of the baseline questionnaire to the date of death or the end of follow-up (30 June 2018 for NHS; 31 January 2018 for HPFS), whichever came first. To better represent long term dietary habits and to minimize within person variation, we calculated cumulative averages of ultra-processed food consumption as the primary exposure. We did primary analyses in pooled cohorts and a secondary analysis in each cohort separately. We used time varying Cox proportional hazards models stratified by age (months), questionnaire cycle (two year interval), and cohort (in pooled analyses) with the counting process data structure to estimate the hazard ratios and 95% confidence intervals according to quarters of ultra-processed food consumption. We calculated P for trend on the basis of the Wald test by assigning the median intake to each quarter and modeling it as a continuous variable. In the multivariable model, we adjusted for race/ethnicity, marital status, physical activity, body mass index, smoking status and pack years, alcohol consumption, physical examination performed for screening purposes, family history of diabetes mellitus, myocardial infarction, or cancer, and menopausal status and hormone use (women only). We carried forward non-missing values from the previous survey cycle to replace missing data. If the value remained missing, we created missing indicators. The percentage of missing data is shown in supplementary table A. We also tested for the dose-response relation by using the restricted cubic spline regression. 22
In secondary analyses, we further categorized ultra-processed foods into mutually exclusive subgroups (supplementary tables B and C) to investigate whether the associations were driven by specific food groups. 13 Furthermore, to assess the independent and combined association of ultra-processed food consumption and overall dietary quality with mortality, we categorized individuals jointly according to quarters of AHEI score and quarters of ultra-processed food intake and estimated the hazard ratios by using participants with the highest quarter of AHEI score and lowest quarter of ultra-processed food intake as the reference.
We did several sensitivity analyses to test the robustness of the results. Firstly, given that people are likely to change their dietary habits after the diagnosis of certain chronic diseases, we stopped updating ultra-processed food consumption after the diagnosis of cardiovascular diseases, cancer, or diabetes during follow-up. Secondly, because of the uncertainty of the etiological time window, we introduced an eight to 12 year lag period between assessment of ultra-processed food intake and each follow-up period (for example, we used ultra-processed food intake from the 1986 questionnaire to assess the mortality risk in the period of 1994 to 1998). Thirdly, we added back to total ultra-processed food whole grains and distilled alcohol individually and in combination (that is, using the standard Nova definition) and repeated the analysis. Finally, we removed from the multivariable model pack years of smoking, which was not adjusted for in most previous studies, and further adjusted for AHEI score, to assess the confounding by smoking and dietary quality, respectively. We also removed from the multivariable model body mass index, which might be a mediator. Furthermore, we did the stratified analysis by major risk factors and repeated the primary analysis with ultra-processed food intake measured by percentage of energy.
We used SAS statistical package (version 9.4) for all the statistical analyses. We considered a P value <0.05 (two sided) to be statistically significant unless otherwise specified.
Patient and public involvement
The public was concerned about the health effects of ultra-processed foods, and their concerns informed our research question. Although participants were not involved in the study design, they played a central role in the conduct of the study by completing the biennial questionnaires in our cohorts, and we appreciate their contributions. We could not directly involve members of the public in this study, as no funding was available or set aside for patient and public involvement and our study team was not trained to work directly with the public.
During a median of 34 years of follow-up, we documented 48 193 deaths (30 188 deaths of women and 18 005 deaths of men), including 13 557 deaths due to cancer, 11 416 deaths due to cardiovascular diseases, 3926 deaths due to respiratory diseases, and 6343 deaths due to neurodegenerative diseases. Table 1 shows the characteristics of participants according to quarters of energy adjusted ultra-processed food consumption throughout follow-up. Participants with higher ultra-processed food consumption were younger, more physically inactive, and more likely to smoke and had higher body mass index, lower consumption of alcohol, whole fruits and vegetables, and whole grains, and lower AHEI score.
Age standardized characteristics of study participants according to quarters of ultra-processed food (UPF) consumption across entire follow-up period. Values are number (percentage) of person years unless stated otherwise
- View inline
Table 2 shows the hazard ratios of mortality according to quarters of ultra-processed food consumption. In the age, sex, and total calorie adjusted analysis, we observed strong positive associations between ultra-processed food and mortality outcomes. The associations became substantially attenuated in the multivariable analysis ( table 2 ; supplementary figure C). Compared with participants in the lowest quarter (median 3.0 servings/day), those in the highest quarter (median 7.4 servings/day) had a 4% higher risk of total deaths (multivariable adjusted hazard ratio 1.04, 95% confidence interval 1.01 to 1.07; P for trend=0.005) and a 9% higher risk of other deaths (1.09, 1.05 to 1.13; P for trend<0.001), including an 8% higher risk of neurodegenerative deaths (1.08, 1.01 to 1.17; P for trend=0.1). We found no associations for deaths due to cardiovascular diseases, cancer, or respiratory diseases. The all cause mortality rate among participants in the lowest and highest quarter of ultra-processed food consumption was 1472 and 1536 per 100 000 person years, respectively.
Hazard ratios and 95% confidence intervals for mortality according to quarters of ultra-processed food (UPF) consumption
Table 3 shows the associations for nine subgroups of ultra-processed foods. Meat/poultry/seafood based ready-to-eat products (for example, processed meat) showed the strongest association with higher all cause mortality (hazard ratio 1.13 (1.10 to 1.16) comparing highest versus lowest quarter) and mortality due to individual causes other than cardiovascular diseases and neurodegenerative diseases (hazard ratios ranged from 1.06 to 1.43). Other subgroups also showed an association with higher all cause mortality, including sugar sweetened and artificially sweetened beverages (1.09, 1.07 to 1.12), other ultra-processed foods (mainly composed of artificial sweeteners) (1.08, 1.05 to 1.11), dairy based desserts (1.07, 1.04 to 1.10), and ultra-processed breakfast foods excluding whole grains (1.04, 1.02 to 1.07). When further separating sugar sweetened and artificially sweetened beverages, we found a generally stronger association for sugar sweetened than artificially sweetened beverages; we present these results and those for other selected individual ultra-processed food categories in supplementary table D.
Multivariable hazard ratios and 95% confidence intervals for mortality according to quarters of subgroups of ultra-processed food consumption *
When we examined ultra-processed food intake and AHEI score together ( fig 1 ), we did not observe a consistent association of ultra-processed foods with mortality within each quarter of the AHEI score, whereas AHEI score generally showed an inverse association with mortality within each of the quarters of ultra-processed food consumption.

Joint analysis for mortality according to quarters of ultra-processed food (UPF) consumption and quarters of Alternative Healthy Eating Index-2010 (AHEI) score. Alcohol was removed from calculation of AHEI score. Each participant was categorized according to their quarter of UPF intake and their quarter of AHEI score, resulting in 16 distinct groups. Using this combined variable as exposure, its association with mortality outcomes was assessed, with reference group being participants in highest quarter of AHEI score (Q4) and lowest quarter of UPF intake (Q1). Results were from multivariable Cox proportional hazards model stratified by age (months), questionnaire cycle (two year interval), and cohort and adjusted for total energy intake, race, marital status, physical activity, body mass index, smoking status and pack years, alcohol consumption, physical examination performed for screening purposes, and family history of diabetes mellitus, myocardial infarction, or cancer; for women, also menopausal status and hormone use. Markers denote point estimates of hazard ratios and error bars indicate 95% confidence intervals
- Download figure
- Open in new tab
- Download powerpoint
We found similar results in men and women (supplementary table E). The results of sensitivity analyses are summarized in supplementary table F. The lagged analysis showed similar results to the primary analysis. The associations were attenuated when we stopped updating the information on ultra-processed food intake at a diagnosis of chronic disease, likely owing to the increased intake of ultra-processed foods over time (supplementary figures D and E). Unsurprisingly, including wholegrain products in ultra-processed foods weakened the associations, whereas including distilled alcohol strengthened the associations. Removing pack years of smoking from the multivariable model led to a much stronger positive association, whereas adjusting for the AHEI score attenuated the association toward null.
In the stratified analysis by major risk factors, the associations between ultra-processed food intake and all cause mortality seemed to be stronger in participants consuming less alcohol (P for interaction=0.005) and not currently smoking (P for interaction<0.001), but we found no interaction by body mass index or physical activity (supplementary table G). We repeated the primary analysis using percentage of energy to measure ultra-processed food intake and observed similar results (supplementary table H).
In two large prospective cohorts with up to 34 years of follow-up, we found that higher consumption of ultra-processed foods was associated with modestly higher all cause mortality. We found no associations for mortality due to cancer or cardiovascular diseases. The associations varied across subgroups of ultra-processed foods, with meat/poultry/seafood based ready-to-eat products consistently showing associations with higher all cause mortality and cause specific mortality. The associations between ultra-processed food consumption and mortality were attenuated after we accounted for overall dietary quality.
Comparison with other studies and possible explanations
Existing evidence suggests a relation between ultra-processed food consumption and mortality. A meta-analysis of prospective cohorts reported that the highest ultra-processed food consumption was associated with higher all cause mortality compared with the lowest consumption (hazard ratio 1.21, 1.13 to 1.30). 23 Two studies were conducted in the US, 24 25 whereas the other six were conducted in Spain, 26 27 28 France, 29 Italy, 30 and the UK. 31 Unlike our study, which excluded alcohol from ultra-processed foods and carefully controlled for smoking status and pack years, all the above studies included alcohol in ultra-processed foods and adjusted for smoking status (never, former, and current) only. As noted in our sensitivity analysis, pack years of smoking strongly confounded the association—additionally adjusting for smoking pack years remarkably attenuated the hazard ratios toward the null. That may partly explain why the associations found in our study were weaker than those in previous studies. Another possible reason could be tighter control for socioeconomic status because our participants were all health professionals and had similar levels of education.
The evidence on mortality due to cancer is relatively sparse. Consistently, the Moli-sani Study did not observe a statistically significant association but reported a positive association with other mortality. 30 An analysis of three cohorts including the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO), NHANES (1999-2018), and UK Biobank reported null findings for mortality due to cancer in the PLCO and NHANES (1999-2018). 32 By contrast, the UK Biobank study found that every 10% increment in ultra-processed food consumption was associated with a 6% higher cancer mortality. 33 Diet was assessed in the UK Biobank through multiple 24 hour recalls between 2009 and 2012, and 40% of the participants had only one 24 hour recall, thus limiting the ability to capture long term dietary intake.
In agreement with our study, the Prospective Urban and Rural Epidemiology study from 25 high income, middle income, and low income countries in America, Europe, Africa, and Asia observed a null association with mortality due to cardiovascular diseases but a positive association with non-cardiovascular disease mortality. 34 Our findings on the relation between ultra-processed foods and mortality due to cardiovascular diseases are inconsistent with previous evidence from Europe but consistent with the null finding in the US NHANES III (1988-94). 24 25 30 Moreover, a much stronger positive association was reported in the UK Biobank (1.28, 1.13 to 1.45) compared with the two US cohorts (1.12, 1.05 to 1.09; 1.11, 0.92 to 1.34). 32 In addition to the methodological differences mentioned above, different study populations, ultra-processed food compositions, and eating patterns may also contribute. Ultra-processed food intake in our two US cohorts is mainly contributed by “sauces, spreads, and condiments” and “sweet snacks and desserts,” which together accounted for nearly 50% (supplementary figure B), but neither of the two subgroups was associated with increased mortality due to cardiovascular diseases. On the other hand, compelling evidence shows that nuts and (dark) chocolate, common constituents of “sweet snacks and desserts,” are inversely associated with cardiovascular diseases. 35 36 We observed that dark chocolate in the subgroup “packaged sweet snacks and desserts” was associated with decreased mortality (supplementary table D). Therefore, the diverse array of constituents contained in ultra-processed foods with heterogeneous health effects may have contributed to the discrepant findings. Our findings suggest that meat/poultry/seafood based ready-to-eat products and sugar sweetened and artificially sweetened beverages are major factors contributing to the harmful influence of ultra-processed foods on mortality, which is in accordance with previous studies. 13 37 38 39
Few studies have investigated the relation with cause specific mortality other than that due to cancer and cardiovascular diseases. We found that ultra-processed food intake was associated with higher neurodegenerative mortality. Increasing evidence suggests that ultra-processed food is linked to higher risk of central nervous system demyelination (a precursor of multiple sclerosis), 40 lower cognitive function, 41 and dementia. 42 Studies have shown that a diet rich in ultra-processed foods may drive neuroinflammation and impairment of the blood-brain barrier, leading to neurodegeneration. 43 44 Of note, among ultra-processed food subgroups, diary based desserts showed the strongest association with neurodegenerative mortality. Earlier finding from the HPFS and NHS cohorts showed that intake of sherbet/frozen yogurt was associated with an increased risk of Parkinson’s disease. 45 Furthermore, we found a positive association between ultra-processed food intake measured by percentage of energy and respiratory mortality. Emerging evidence suggests that higher ultra-processed food intake is associated with increased risk of respiratory multimorbidity. 46 The increased respiratory mortality associated with processed red meat may be partly due to heme iron and nitrate/nitrite. 47
An important question not answered by previous studies is whether and how food processing level and nutritional quality jointly influence health. We observed that in the joint analysis, the AHEI score but not ultra-processed food intake showed a consistent association with mortality and that further adjustment for the AHEI score attenuated the association of ultra-processed food intake with mortality. Although including AHEI in the multivariable model for ultra-processed food may represent an overadjustment because common foods are included in both the AHEI and ultra-processed food, our data together suggest that dietary quality has a predominant influence on long term health, whereas the additional effect of food processing is likely to be limited. Furthermore, foods may have dual attributes according to their processing level and nutritional quality, and these two features may have quantitatively and even qualitatively different effects on health. Another added value of our study is the exclusion of wholegrain products that fall in the ultra-processed foods from the primary exposure, based on the well established health benefits associated with whole grains. By taking this approach, we aim to rectify the potential misperception that all ultra-processed food products should be universally restricted and to avoid oversimplification when formulating dietary recommendations.
Besides neglecting overall nutritional quality, the ultra-processed food classification system has other limitations. The Nova classification is based on broad categories that do not capture the full complexity of food processing, 48 leading to potential misclassification. Further work is needed to improve the assessment and categorization of ultra-processed foods. On the other hand, dietary guidelines should provide clear and sound food selections that are available, actionable, attainable, and affordable for the largest proportion of the population. Thus, careful deliberation is necessary when considering incorporation of ultra-processed foods into dietary guidelines. 49 50 Again, on the basis of our data, limiting total ultra-processed food consumption may not have a substantial influence on premature death, whereas reducing consumption of certain ultra-processed food subgroups (for example, processed meat) can be beneficial.
We note that mortality is a more complicated endpoint than disease incidence and is also influenced by several factors including early detection, treatment, and individuals’ overall health status. The findings for mortality should not be regarded as synonymous with those pertaining to disease incidence but rather considered as more comprehensive assessment of the health impact of risk factors.
Strengths and limitations of study
The strengths of the study include the prospective study design, large sample size, long follow-up, and detailed, validated, and repeated measurements. In addition, we rigorously controlled for confounding, did thorough sensitivity analyses, explored major specific causes of mortality, and examined individual ultra-processed food subgroups. Several limitations should also be noted. Firstly, we cannot rule out unmeasured and residual confounding due to the nature of the observational study. Secondly, our participants are health professionals and predominantly non-Hispanic white, limiting the generalizability of our findings. Thirdly, as the food frequency questionnaires collected intake of only a limited number of pre-defined items representing the primary source of energy and nutrients in the US population and were not designed to classify foods by processing level, they may not capture the full spectrum of ultra-processed foods. Although the food frequency questionnaires used in our cohorts have been validated for foods and nutrients, they were not specifically validated for ultra-processed foods. Moreover, we classified ultra-processed foods by using the same algorithm throughout follow-up that did not account for changes in the grade of food processing over time. These factors may have introduced non-differential misclassification, likely biasing our results toward the null.
Conclusions
Higher ultra-processed food intake was associated with slightly increased all cause mortality. The mortality associations for ultra-processed food consumption were more modest than those for dietary quality and varied across ultra-processed food subgroups, with meat/poultry/seafood based ready-to-eat products generally showing the strongest and most consistent associations with mortality. The findings provide support for limiting consumption of certain types of ultra-processed food for long term health. Future studies are warranted to improve the classification of ultra-processed foods and confirm our findings in other populations.
What is already known on this topic
Ultra-processed foods have been suggested to have adverse health effects
Evidence is limited on the influence of ultra-processed food consumption on mortality outcomes in large cohorts with long term follow-up and repeated dietary assessment
What this study adds
A higher intake of ultra-processed foods was associated with slightly higher all cause mortality, driven by causes other than cancer and cardiovascular diseases
The positive associations were mainly driven by meat/poultry/seafood based ready-to-eat products, sugar and artificially sweetened beverages, dairy based desserts, and ultra-processed breakfast foods
Dietary quality was observed to have a more predominant influence on mortality outcomes than ultra-processed food consumption
Ethics statements
Ethical approval.
The Nurses’ Health Study I and the Health Professionals Follow-up Study were approved by the Institutional Review Board at the Brigham and Women’s Hospital, the Harvard T.H. Chan School of Public Health (IRB protocol number: 1999-P-011114 and 10162). The completion of the self-administered questionnaire was considered to imply informed consent.
Data availability statement
Data can be shared through mechanisms detailed at https://www.nurseshealthstudy.org and https://www.hsph.harvard.edu/hpfs/ .
Acknowledgments
We thank the participants of the Nurses’ Health Study and the Health Professionals Follow-up Study and the staff of the Channing Division of Network Medicine for their valuable contributions. We acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, and Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
Contributors: ZF did the statistical analysis and drafted the manuscript. SLR and NK made a substantial contribution to the concept of the article. DH, WK, CHL, WCW, and ELG were involved in the acquisition and interpretation of data. MS was responsible for the study design. All authors critically assessed, edited, and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. MS is the guarantor.
Funding: This work was supported by the US National Institutes of Health grants (UM1 CA186107; P01 CA87969; U01 CA167552; U01 CA261961; R01 CA263776; and K99 CA283146). The funders had no role in considering the study design or in the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the National Institutes of Health for the submitted work; NK received a consulting fee from the Pan American Health Organization for three months on the topic of nutrition disclosure initiatives and nutrient profiling models; no other relationships or activities that could appear to have influenced the submitted work.
Transparency: The manuscript’s guarantor affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The research findings are disseminated to participants through periodic newsletters and study websites at https://www.nurseshealthstudy.org and https://www.hsph.harvard.edu/hpfs/ . The manuscript will be disseminated to the general public through press releases.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Monteiro CA ,
- Moubarac JC ,
- Martinez-Steele E ,
- Martínez Steele E ,
- Mendez MA ,
- Louzada MLC ,
- Buckley JP ,
- Monteiro CA
- Kesse-Guyot E ,
- Khandpur N ,
- Desjardins C ,
- Giovannucci EL ,
- Stampfer MJ ,
- Colditz GA ,
- Rossato S ,
- Drouin-Chartier JP ,
- Giovannucci E ,
- Chasan-Taber S ,
- McCullough ML ,
- Gauthier J ,
- Suksatan W ,
- Rico-Campà A ,
- Martínez-González MA ,
- Alvarez-Alvarez I ,
- Blanco-Rojo R ,
- Sandoval-Insausti H ,
- López-Garcia E ,
- Romero Ferreiro C ,
- Martín-Arriscado Arroba C ,
- Cancelas Navia P ,
- Lora Pablos D ,
- Gómez de la Cámara A
- Schnabel L ,
- Bonaccio M ,
- Di Castelnuovo A ,
- Costanzo S ,
- Gunter MJ ,
- Dehghan M ,
- Rangarajan S ,
- Prospective Urban Rural Epidemiology (PURE) study investigators
- Guasch-Ferré M ,
- Schwedhelm C ,
- Canhada SL ,
- Cordova R ,
- Viallon V ,
- Fontvieille E ,
- Mannino A ,
- Ausimmune Investigator Group
- R Cardoso B ,
- Machado P ,
- Więckowska-Gacek A ,
- Mietelska-Porowska A ,
- Wydrych M ,
- Martínez Leo EE ,
- Segura Campos MR
- Hughes KC ,
- Etemadi A ,
- Petrus RR ,
- do Amaral Sobral PJ ,
- Tadini CC ,
- Vadiveloo MK ,
- Comeau ME ,
- Casperson S ,

IMAGES
COMMENTS
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance ...
Diabetes remains a substantial public health issue. Type 2 diabetes, which makes up the bulk of diabetes cases, is largely preventable and, in some cases, potentially reversible if identified and managed early in the disease course. However, all evidence indicates that diabetes prevalence is increasing worldwide, primarily due to a rise in obesity caused by multiple factors. Preventing and ...
Type 2 diabetes affects more than 34 million U.S. adults and significantly increases the risk of cardiovascular events, microvascular disease, and premature death. 1 Tight glycemic, blood-pressure ...
New estimates published this week in The Lancet indicate that more than 1·31 billion people could be living with diabetes by 2050 worldwide. That's 1·31 billion people living with a disease that causes life-altering morbidity, high rates of mortality, and interacts with and exacerbates many other diseases. The increase in prevalence (up from 529 million in 2021) is expected to be driven by ...
The best evidence for a link between diabetes mellitus and breast cancer comes from a systematic review of six prospective cohort studies and more than 150,000 women, in which the hazard ratio (HR ...
A paper published in 2014 provided the first demonstration of the use of a bihormonal closed-loop system under free-living conditions in adults and adolescents with type 1 diabetes. Read more.
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Nanotechnology in diabetes ...
Prevention of Cardiovascular Disease in Type 1 Diabetes. C. Manrique-Acevedo, I.B. Hirsch, and R.H. EckelN Engl J Med 2024;390:1207-1217. More than half of newly diagnosed cases of type 1 diabetes ...
About the journal. Diabetes Research and Clinical Practice is an international journal for health-care providers and clinically oriented researchers that publishes high-quality original research articles and expert reviews in diabetes and related areas. The role Diabetes Research and Clinical Practice is to provide …. View full aims & scope.
Diabetes publishes original research about the physiology and pathophysiology of diabetes mellitus. Submitted manuscripts can report any aspect of laboratory, animal, or human research. ... Each month, the Editors of Diabetes select an original research article to designate Paper of the Month. View these exclusive articles in a freely ...
PDF | The systematic review widely focused on diabetes and the developments in nutrition and diets required to prevent or control all types of diabetes.... | Find, read and cite all the research ...
In a year marked by the centenary of the discovery of insulin 1 and outstanding scientific progress in the therapeutic management of diabetes mellitus 2,3, these advances have been contrasted by ...
29 Apr 2024. 29 Apr 2024. 28 Mar 2024. 28 Mar 2024. Journal of Diabetes Research publishes articles related to type 1 and type 2 diabetes. Topics include etiology, pathogenesis, management, and prevention of diabetes, as well as associated complications such as nephropathy.
The research could lead to a better availability of beta cells for future research purposes. Type 1 and type 2 diabetes results when beta cells in the pancreas fail to produce enough insulin, the ...
Journal of Diabetes is an international, imminently open access platform for diabetes research, therapeutics, and education. Uniting East and West, researchers and practitioners are invited to submit papers spanning all aspects of epidemiology, etiology, pathogenesis, management, complications, and prevention of diabetes including the molecular ...
Diabetes mellitus (DM) also known as simply diabetes, is a group of metabolic. diseases in which there are high blood sugar levels over a prolonged period This high. blood sugar produces the ...
Diabetes mellitus is a significant global public health challenge, with a rising prevalence and associated morbidity and mortality. Cell therapy has evolved over time and holds great potential in diabetes treatment. In the present review, we discussed the recent progresses in cell-based therapies for diabetes that provides an overview of islet and stem cell transplantation technologies used in ...
For 30 years, doctors have known that maintaining near-normal blood sugar has huge benefits for people with Type 1 diabetes. A 1993 clinical trial found that participants who were taught methods for tightly managing their disease -- checking their blood sugar many times each day, making adjustments to insulin doses and receiving frequent help from their medical caregivers -- reduced their risk ...
Ketogenic diets have been widely used for weight loss and are increasingly used in the management of type 2 diabetes. Despite evidence that ketones have multiple positive effects on kidney function, common misconceptions about ketogenic diets, such as high protein content and acid load, have prevented their widespread use in individuals with impaired kidney function. Clinical trial evidence ...
Type 2 Diabetes Mellitus (T2DM) is one of the most common metabolic disorders worldwide and its development is primarily caused by a combination of two main factors: defective insulin secretion by pancreatic β-cells and the inability of insulin-sensitive tissues to respond to insulin [ 1 ]. Insulin release and action have to precisely meet the ...
Automated insulin delivery systems have transformed the care of people living with type 1 diabetes and continue to move closer to the ultimate goal of a fully autonomous artificial pancreas.
Objective To examine the association of ultra-processed food consumption with all cause mortality and cause specific mortality. Design Population based cohort study. Setting Female registered nurses from 11 US states in the Nurses' Health Study (1984-2018) and male health professionals from all 50 US states in the Health Professionals Follow-up Study (1986-2018). Participants 74 563 women ...