- Search Menu
- Advance articles
- Author Guidelines
- Submission Site
- Open Access
- Reasons to publish
- About Human Molecular Genetics
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Introduction, recent advances in understanding phenotypes associated with ds, recent advances in therapy and future prospects, acknowledgements.
- < Previous
Down syndrome—recent progress and future prospects
- Article contents
- Figures & tables
- Supplementary Data
Frances K. Wiseman, Kate A. Alford, Victor L.J. Tybulewicz, Elizabeth M.C. Fisher, Down syndrome—recent progress and future prospects, Human Molecular Genetics , Volume 18, Issue R1, 15 April 2009, Pages R75–R83, https://doi.org/10.1093/hmg/ddp010
- Permissions Icon Permissions
Down syndrome (DS) is caused by trisomy of chromosome 21 (Hsa21) and is associated with a number of deleterious phenotypes, including learning disability, heart defects, early-onset Alzheimer's disease and childhood leukaemia. Individuals with DS are affected by these phenotypes to a variable extent; understanding the cause of this variation is a key challenge. Here, we review recent research progress in DS, both in patients and relevant animal models. In particular, we highlight exciting advances in therapy to improve cognitive function in people with DS and the significant developments in understanding the gene content of Hsa21. Moreover, we discuss future research directions in light of new technologies. In particular, the use of chromosome engineering to generate new trisomic mouse models and large-scale studies of genotype–phenotype relationships in patients are likely to significantly contribute to the future understanding of DS.
Down syndrome (DS) is caused by trisomy of human chromosome 21 (Hsa21). Approximately 0.45% of human conceptions are trisomic for Hsa21 ( 1 ). The incidence of trisomy is influenced by maternal age and differs between populations (between 1 in 319 and 1 in 1000 live births are trisomic for Hsa21) ( 2 – 6 ). Trisomic fetuses are at an elevated risk of miscarriage, and people with DS have an increased risk of developing several medical conditions ( 7 ). Recent advances in medical treatment and social inclusion have significantly increased the life expectancy of people with DS. In economically developed countries, the average life span of people who are trisomic for Hsa21 is now greater than 55 years ( 8 ). In this review, we will discuss novel findings in the understanding of DS and highlight future important avenues of research.
Mouse models of Hsa21 trisomy and monosomy. Hsa21 (orange) is syntenic with regions of mouse chromosomes 16 (Mmu16, blue), 17 (Mmu 17, green) and 10 (Mmu10, grey). The Tc1 mouse model carries a freely segregating copy of Hsa21, which has two deleted regions, such that the model is trisomic for the majority of genes on Hsa21. The Dp1Yu, Ts65Dn, Ts1Cje and Ts1Rhr mouse models contain an additional copy of regions of mouse chromosome 16 that are syntenic with Hsa21, such that they are trisomic for a proportion of Hsa21 genes. The Ms1Rhr mouse model contains a deletion of a region of Mmu16; the Ms1Yah mouse model contains a deletion of a region of Mmu10. Hence, these models are monosomic for the genes in these deleted Hsa21 syntenic segments.
Box 1: What is a gene?
The definition of a gene has shifted over the past 100 years since it was first coined by Wilhelm Johannsen in 1909, based on the ideas of Mendel, de Vries, Correns and Tschermak. Their original theoretical definition of the gene being ‘the smallest unit of genetic inheritance’ remains the cornerstone of our understanding; however, the definition has grown with our knowledge of molecular biology. The gene has recently been defined as ‘a union of genomic sequences encoding a coherent set of potentially overlapping functional products’ ( 133 ). Splicing generates multiple transcripts from one gene. Moreover, exons from genes previously considered to be separate may be spliced together to generate novel transcripts ( 9 ). How to classify these fusion transcripts is a significant challenge. In addition, alternative transcription start sites that generate novel 5′ untranslated regions continue to be discovered, even for well-characterized genes ( 134 ). Although many of these novel transcripts are rare and their functional importance is not understood, our definition of a gene must encompass the observed diversity of the genome.
Trisomy of Hsa21 is associated with a small number of conserved features, occurring in all individuals, including mild-to-moderate learning disability, craniofacial abnormalities and hypotonia in early infancy ( 17 ). Although these phenotypes are always found in people with DS, the degree to which an individual is affected varies. Additionally, trisomy of Hsa21 is also associated with variant phenotypes that only affect some people with DS, including atrioventricular septal defects (AVSDs) in the heart, acute megakaryoblastic leukaemia (AMKL) and a decrease in the incidence of some solid tumours. This phenotypic variation is likely to be caused by a combination of environmental and genetic causes. Genetic polymorphisms in both Hsa21 and non-Hsa21 genes may account for much of this variation. Genome-wide association studies to identify these polymorphisms constitute a promising strategy to gain novel insights into the pathology of DS.
A central goal of DS research is to understand which of the genes on Hsa21, when present in three copies, lead to each of the different DS-associated phenotypes, and to elucidate how increased expression leads to the molecular, cellular and physiological changes underlying DS pathology. Two distinct approaches are being taken to address these issues. First, genomic association studies, such as that recently published by Lyle et al ( 18 )., may point to genes that play an important role in pathology. Secondly, a number of animal models of Hsa21 trisomy have been generated. Recent advances in chromosome engineering have led to the establishment of mice trisomic for different sets of mouse genes syntenic to Hsa21, and a mouse strain, Tc(Hsa21)1TybEmcf (Tc1), carrying most of Hsa21, as a freely segregating chromosome (Fig. 1 ) ( 19 – 27 ). These strains are being used both to map dosage-sensitive genes on Hsa21 and to understand pathological mechanisms. Here, we review recent advances in the understanding of DS-associated phenotypes and the development of therapeutic strategies to treat them.
Development
Trisomy of Hsa21 has a significant impact on the development of many tissues, most notably the heart and the brain. A recent paper has suggested that trisomy of the Hsa21 genes, dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase 1A ( DYRK1A ) and regulator of calcineurin 1 ( RCAN1 ), may have an impact on the development of multiple tissues ( 28 ). DYRK1A is a priming kinase that facilitates the further phosphorylation of numerous proteins by other kinases (Fig. 2 ) ( 29 – 38 ). It is up-regulated in a number of tissues from people with DS ( 39 , 40 ). RCAN1 is a regulator of the protein phosphatase calcineurin ( 41 ). Crabtree and colleagues hypothesized that trisomy of these two genes may act synergistically to alter signalling via the NFAT family of transcription factors ( 28 ). In an independent study, increased DYRK1A gene dosage was shown to decrease the expression level of RE1-silencing transcription factor ( REST ) ( 42 ). As REST is required both to maintain pluripotency and to facilitate neuronal differentiation, a perturbation in REST expression may alter the development of many cell types. Indeed, over-expression of DYRK1A in some animal models is associated with a number of phenotypes, including heart defects and abnormal learning and memory ( 28 , 33 , 43 – 45 ). However, not all animal models that over-express DYRK1A exhibit these defects, suggesting that polymorphisms or differences in the expression of other genes influence the outcome of DYRK1A trisomy ( 24 ).
Phosphorylation targets of DYRK1A. The Hsa21-encoded kinase DYRK1A has been shown to phosphorylate a multitude of targets, which have been implicated in a number of biological processes and DS-associated phenotypes, including endocytosis and AD.
Trisomy of Hsa21 is associated with a reduction in brain volume, the size of the hippocampus and cerebellum being particularly affected ( 46 – 49 ). A similar phenotype is also observed in the Ts65Dn model ( 50 ). Recent studies have started to elucidate the developmental mechanisms underlying these important phenotypes. Trisomic granule cell precursors from the cerebellum have a reduced mitogenic response to the morphogen sonic hedgehog ( 51 ). This was shown to underlie the reduced number of cerebellar granular cells observed in the Ts65Dn mouse model of DS. Hypocellularity in the hippocampus also has a developmental origin ( 52 , 53 ). Abnormalities in cell-cycle length, apoptosis and neocortical neurogenesis have been shown to contribute to this phenotype ( 53 – 55 ). The reduced level of neurogenesis in Ts65Dn adult hippocampus can be ameliorated by treatment with the anti-depressant fluoxetine, which is a serotonin reuptake inhibitor ( 56 ). Fluoxetine may promote neurogenesis via a number of potential mechanisms, including a direct effect on serotonin levels or via an indirect effect on behaviour. Whether this drug has similar effect during embryonic development has yet to be determined.
Ts65Dn pups exhibit a delay in attaining several developmental milestones, such as forelimb grip and the righting reflex, mimicking the developmental delay observed in babies with DS ( 57 ). A recent report has demonstrated that treatment of Ts65Dn embryos with two neuroprotective peptides reduced the delay in achieving a number of sensory and motor developmental milestones during early post-natal development ( 58 ).
People with DS exhibit craniofacial dysmorphology, including a mandible of reduced size. This phenotype is also observed in the Ts65Dn and Tc1 models ( 26 , 59 ). In the Ts65Dn model, craniofacial dysmorphology is present from early post-natal development and may be related to specific changes in bone development ( 60 , 61 ). The small mandible in people with DS may be caused by migration and proliferation defects in mandible precursor (neural crest) cells in the developing embryo, related to an altered response to sonic hedgehog ( 62 ).
Learning and memory
All people with DS have a mild-to-moderate learning disability. Over-expression of a number of Hsa21 genes, including DYRK1A, synaptojanin 1 and single-minded homologue 2 (SIM2), results in learning and memory defects in mouse models, suggesting that trisomy of these genes may contribute to learning disability in people with DS ( 43 , 45 , 63 , 64 ). In addition, trisomy of neuronal channel proteins, such as G-protein-coupled inward-rectifying potassium channel subunit 2 ( GIRK2 ), may also influence learning in people with DS ( 65 – 67 ). Recent work has demonstrated that trisomy of a segment of mouse chromosome 16 ( Mmu16 ) containing 33 genes including DYRK1A , GIRK2 and SIM2 was necessary, but not sufficient for the hippocampal-based learning deficits in the Ts65Dn mouse model ( 68 ). These data indicate that trisomy of multiple Hsa21 genes is required for the deficits in learning associated with DS. Moreover, Hsa21 trisomy may independently impact on multiple learning pathways.
Recent work on the Tc1 transchromosomic mouse model of DS has examined in detail the learning pathways affected by trisomy of Hsa21 ( 26 , 69 ). The Tc1 transchromosomic model exhibits abnormalities in short-term but not in long-term hippocampal-dependent learning. The learning deficits are correlated with specific abnormalities in long-term potentiation (LTP) in the dentate gyrus of the hippocampus. LTP is an electrophysiological process proposed to be the cellular basis of learning and memory ( 70 ). These data provide insight into which learning mechanisms may be affected by Hsa21 trisomy and can be used to further understand their genetic cause. Structural abnormalities may contribute to these deficits in learning and memory. Indeed, a correlation between specific synaptic abnormalities in the hippocampus of the Ts(16C-tel)1Cje (Ts1Cje) mouse and a defect in LTP has been reported ( 71 ). Moreover, a recent paper has demonstrated an alteration in the amounts of a number of synaptic components in the hippocampus of the Ts65Dn mouse ( 72 ).
Alzheimer's disease
People with DS have a greatly increased risk of early-onset Alzheimer's disease (AD). By the age of 60, between 50 and 70% of the people with DS develop dementia ( 73 – 77 ). The known AD risk factor amyloid precursor protein ( APP ) is encoded on Hsa21. Trisomy of APP is likely to make a significant contribution to the increased frequency of dementia in people with DS. Indeed, triplication of a short segment of Hsa21 that includes APP in people without DS has been recently shown to be associated with early-onset AD. A number of features of neurodegeneration have been observed in mouse models of DS ( 78 – 86 ). Loss of basal forebrain cholinergic neurons (BFCNs) occurs early in AD and also is observed in the Ts65Dn mouse model ( 87 ). Degeneration of BFCNs in Ts65Dn mice is dependent on trisomy of APP and is mediated by the effect of increased APP expression of retrograde axonal transport ( 83 ).
Hsa21 genes other than APP may also contribute to the early onset of AD in people with DS ( 33 , 34 , 40 , 88 – 97 ). Indeed, the Ts1Cje mouse model, which is not trisomic for APP , exhibits tau hyperphosphorylation, an early sign of AD ( 98 ). Recent evidence suggests that trisomy of DYRK1A may contribute to the development of AD in people with DS. DYRK1A can phosphorylate Tau at a key priming site that permits its hyperphosphorylation ( 33 , 36 , 40 , 95 ). DYRK1A may also influence the alternative splicing of Tau and the phosphorylation of APP ( 34 , 99 ). A reduction in the level of protein phosphatase 2A and a decrease in the activity of α-secretase in the brains of people with DS have also been reported, both of which may contribute to AD in this population ( 94 , 100 ). Further studies are required to determine the identity of the trisomic genes that contribute to these phenotypes.
Heart defects
Trisomy of Hsa21 is associated with a number of congenital heart defects, the most common being AVSD that occurs in ∼20% of the people with DS ( 101 ). Mutations in the non-Hsa21 CRELD1 gene may contribute to the development of AVSD in DS ( 102 ). CRELD1 has also been linked to AVSDs by mapping the deletion breakpoints, on chromosome 3, in people with 3p-syndrome. Further studies are required to determine the identity of other genes that are important for heart development in people with DS. A number of Hsa21 trisomy mouse models exhibit heart defects similar to those observed in DS, suggesting that trisomy of one or more of the approximately 100 genes common to these models influences development of the heart ( 22 , 26 , 103 , 104 ).
Leukaemia and cancer
DS increases the risk of developing AMKL and acute lymphoblastic leukaemia (ALL). Approximately 10% of the DS newborns present with a transient myeloproliferative disorder (TMD), characterized by a clonal population of megakaryoblasts in the blood. This transient disease usually spontaneously resolves; however, 10–20% of the DS patients with TMD develop AMKL before 4 years of age (reviewed in 105 ). The development of TMD requires both trisomy 21 and mutations in the transcription factor GATA1 ( 106 , 107 ). It is likely that further mutations are required for TMD to develop into AMKL. The GATA1 mutations found in TMD and AMKL always have the same effect, causing translation to initiate at the second ATG of the coding region, leading to the production of a shorter protein, termed GATA1s. Trisomy of Hsa21 on its own, even in the absence of GATA1s, leads to an expansion of the megakaryocyte-erythroid progenitor population in fetal livers from human DS abortuses ( 108 , 109 ). These data suggest that trisomy of Hsa21 perturbs hematopoiesis, making megakaryocyte-erythroid progenitors susceptible to the effects of GATA1s, thereby promoting development of TMD. Several groups have reported the presence of mutations in Janus Kinase 3 ( JAK3 ) in a small proportion of TMD/AMKL patients ( 110 – 115 ). It was suggested that JAK3 inhibitors could be used as a therapy ( 111 , 114 ). However, both loss- and gain-of-function mutations have been found, so this may not be a viable treatment. Stem cell factor/KIT signalling has recently been demonstrated to stimulate TMD blast cell proliferation, and inhibitors of this pathway may be a treatment for severe TMD ( 116 ).
Attempts have been made to model these disorders in mice with a view to establishing which genes on Hsa21 need to be present in three copies in order to induce disease. A study of the Ts65Dn mouse model showed that it developed a late-onset myeloproliferative disorder, but did not develop leukaemia ( 117 ). It may be that the Ts65Dn model is not trisomic for the relevant dosage-sensitive genes required for the development of AMKL or that the expression of a mutant form of GATA1 will be required to increase the frequency of leukaemogenesis in this mouse model of DS.
The genetic events involved in DS-ALL are less well understood than those in DS-AMKL. A number of studies have reported DS-ALL cases with chromosomal abnormalities, gain-of-function mutations in JAK2 and submicroscopic deletions of genes including ETV6 , CDKN2A and PAX5 ( 118 – 121 ).
Although the incidence of leukaemia and cancer of the testis are increased in DS, the risk of developing most solid tumours is reduced ( 122 , 123 ). Crossing mouse models of DS with mice heterozygous for the Apc min mutation reduced the number of tumours, which would normally accumulate in this model of colon cancer ( 124 ). Protection against the development of tumours required three copies of the Hsa21 ‘proto-oncogene’ Ets2 , suggesting that in this context, Ets2 may be acting as a tumour suppressor ( 124 ).
Hypertension
People with DS have been reported to have a reduced incidence of hypertension ( 125 , 126 ). Trisomy of the Hsa21 microRNA hsa-miR-155 may contribute to this ( 12 ). Hsa-miR-155 is proposed to specifically target one allele of the type-1 angiotensin II receptor ( AGTR1 ) gene, resulting in its under-expression, which may contribute to a reduced risk of hypertension. Further studies are required to validate this hypothesis and determine whether other genes may also protect people with DS against hypertension.
Recent interest in therapy for people with DS has focused on pharmacological treatment to enhance cognition. A number of compounds have been shown to improve learning in the Ts65Dn mouse model. Chronic treatment with picrotoxin or pentylenetetrazole improved hippocampal-based learning and LTP deficits in Ts65Dn mice, even after treatment had ceased ( 127 ). These compounds reduce gamma-aminobutyric acid-mediated inhibition in the hippocampus and are proposed to improve cognition by releasing normal learning from excess inhibition. Learning in Ts65Dn mice is also improved by the non-competitive N-methyl-D-aspartic acid receptor (NMDAR) antagonist, memantine ( 128 ). Memantine partially inhibits the opening of the NMDAR and is proposed to counter the effect of trisomy of RCAN1 on the function of the receptor. Further studies and clinical trials are required to further investigate the potential of these drugs to improve cognition in people who have DS.
To develop new therapeutic targets, it is necessary to determine the identity of genes that contribute to DS phenotypes. This requires a precise and standardized definition of phenotype. Ideally, these measurements should be formulated into a standardized protocol that can be applied at multiple centres, to permit sufficiently large numbers of samples for meaningful analysis to be collected. This can be facilitated by a carefully designed and curated biobank of detailed phenotypic data alongside DNA and tissue samples from participating individuals. These collections can then be used for both candidate gene and genome-wide analyses, by different investigators, permitting the identification of both dosage-sensitive trisomic Hsa21 and non-Hsa21 genes that contribute to DS phenotypes. Pooling of large data sets has led to recent important findings in the study of schizophrenia, diabetes and obesity, illustrating the importance of large-scale collaboration ( 129 – 132 ). The careful collection of additional patient data will add much to our current understanding of DS.
As recent progress demonstrates, mouse models can be used in parallel with data collected from people with DS to test genetic associations, to explore biological mechanisms and to trial therapies. In addition to the long-standing Ts65Dn and Ts1Cje models, the newly developed mouse strains such as Tc1, Dp1Yu and Ts1Rhr have generated a range of models with distinct sets of trisomic genes (Fig. 1 ) ( 19 – 27 ). Furthermore, the crossing of these strains with mice-bearing deletions of chromosomal segments syntenic to Hsa21, such as Ms1Yah and Ms1Rhr (Fig. 1 ), will allow systematic mapping and eventually identification of the dosage-sensitive genes causing DS-associated pathology.
DS was once thought to be an intractable condition because of the genetic complexity underlying it. Here, we have described recently reported breakthroughs in the understanding of Hsa21 trisomy, illustrating that research efforts in this field are making significant strides to understand and to develop treatments for the debilitating aspects of the syndrome. Many issues vital to the health and well-being of people with DS remain to be studied, making this an important and exciting time for Hsa21 trisomy research.
V.L.J.T. and K.A.A. are funded by the UK Medical Research Council, the EU, the Leukaemia Research Fund and the Wellcome Trust; F.K.W. and E.M.C.F. are funded by the UK Medical Research Council, the Wellcome Trust and the Fidelity Foundation.
We thank Roger Reeves, Dalia Kasperaviciute, Olivia Sheppard and Matilda Haas for advice on the manuscript and we thank Ray Young for help with preparation of the figures. We apologize to the many authors whose work we were unable to cite owing to space limitations.
Conflict of Interest statement . None declared.
Hassold T. Abruzzo M. Adkins K. Griffin D. Merrill M. Millie E. Saker D. Shen J. Zaragoza M. Human aneuploidy: incidence, origin, and etiology Environ. Mol. Mutagen. 1996 28 167 175
Google Scholar
O'Nuallain S. Flanagan O. Raffat I. Avalos G. Dineen B. The prevalence of Down syndrome in County Galway Ir. Med. J. 2007 100 329 331
Carothers A.D. Hecht C.A. Hook E.B. International variation in reported livebirth prevalence rates of Down syndrome, adjusted for maternal age J. Med. Genet. 1999 36 386 393
Canfield M.A. Honein M.A. Yuskiv N. Xing J. Mai C.T. Collins J.S. Devine O. Petrini J. Ramadhani T.A. Hobbs C.A. et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001 Birth Defects Res. A Clin. Mol. Teratol. 2006 76 747 756
Murthy S.K. Malhotra A.K. Mani S. Shara M.E. Al Rowaished E.E. Naveed S. Alkhayat A.I. Alali M.T. Incidence of Down syndrome in Dubai, UAE Med. Princ. Pract. 2007 16 25 28
Wahab A.A. Bener A. Teebi A.S. The incidence patterns of Down syndrome in Qatar Clin. Genet. 2006 69 360 362
Morris J.K. Wald N.J. Watt H.C. Fetal loss in Down syndrome pregnancies Prenat. Diagn. 1999 19 142 145
Glasson E.J. Sullivan S.G. Hussain R. Petterson B.A. Montgomery P.D. Bittles A.H. The changing survival profile of people with Down's syndrome: implications for genetic counselling Clin. Genet. 2002 62 390 393
Birney E. Stamatoyannopoulos J.A. Dutta A. Guigo R. Gingeras T.R. Margulies E.H. Weng Z. Snyder M. Dermitzakis E.T. Thurman R.E. et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project Nature 2007 447 799 816
Gardiner K. Costa A.C. The proteins of human chromosome 21 Am. J. Med. Genet. C Semin. Med. Genet. 2006 142C 196 205
Kuhn D.E. Nuovo G.J. Martin M.M. Malana G.E. Pleister A.P. Jiang J. Schmittgen T.D. Terry A.V. Jr Gardiner K. Head E. et al. Human chromosome 21-derived miRNAs are overexpressed in Down syndrome brains and hearts Biochem. Biophys. Res. Commun. 2008 370 473 477
Sethupathy P. Borel C. Gagnebin M. Grant G.R. Deutsch S. Elton T.S. Hatzigeorgiou A.G. Antonarakis S.E. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3’ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes Am. J. Hum. Genet. 2007 81 405 413
Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function Cell 2004 116 281 297
Prandini P. Deutsch S. Lyle R. Gagnebin M. Delucinge V.C. Delorenzi M. Gehrig C. Descombes P. Sherman S. Dagna B.F. et al. Natural gene-expression variation in Down syndrome modulates the outcome of gene–dosage imbalance Am. J. Hum. Genet. 2007 81 252 263
Ait Yahya-Graison E. Aubert J. Dauphinot L. Rivals I. Prieur M. Golfier G. Rossier J. Personnaz L. Creau N. Blehaut H. et al. Classification of human chromosome 21 gene-expression variations in Down syndrome: impact on disease phenotypes Am. J. Hum. Genet. 2007 81 475 491
Sultan M. Piccini I. Balzereit D. Herwig R. Saran N.G. Lehrach H. Reeves R.H. Yaspo M.L. Gene expression variation in Down's syndrome mice allows prioritization of candidate genes Genome Biol. 2007 8 R91
Antonarakis S.E. Lyle R. Dermitzakis E.T. Reymond A. Deutsch S. Chromosome 21 and Down syndrome: from genomics to pathophysiology Nat. Rev. Genet. 2004 5 725 738
Lyle R. Bena F. Gagos S. Gehrig C. Lopez G. Schinzel A. Lespinasse J. Bottani A. Dahoun S. Taine L. et al. Genotype–phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21 Eur. J. Hum. Genet. 2008 advance online publication 12 November 2008; doi: 10.1038/ejhg.2008.214
Adams D.J. Biggs P.J. Cox T. Davies R. van der W.L. Jonkers J. Smith J. Plumb B. Taylor R. Nishijima I. et al. Mutagenic insertion and chromosome engineering resource (MICER) Nat. Genet. 2004 36 867 871
Brault V. Besson V. Magnol L. Duchon A. Herault Y. Cre/loxP-mediated chromosome engineering of the mouse genome Handb. Exp. Pharmacol. 2007 178 29 48
Duchon A. Besson V. Pereira P.L. Magnol L. Herault Y. Inducing segmental aneuploid mosaicism in the mouse through targeted asymmetric sister chromatid event of recombination Genetics 2008 180 51 59
Li Z. Yu T. Morishima M. Pao A. LaDuca J. Conroy J. Nowak N. Matsui S. Shiraishi I. Yu Y.E. Duplication of the entire 22.9 Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal abnormalities Hum. Mol. Genet. 2007 16 1359 1366
Tybulewicz V.L. Fisher E.M. New techniques to understand chromosome dosage: mouse models of aneuploidy Hum. Mol. Genet. 2006 15 Spec no. 2 R103 R109
Olson L.E. Richtsmeier J.T. Leszl J. Reeves R.H. A chromosome 21 critical region does not cause specific down syndrome phenotypes Science 2004 306 687 690
Brault V. Pereira P. Duchon A. Herault Y. Modeling chromosomes in mouse to explore the function of genes, genomic disorders, and chromosomal organization PLoS Genet. 2006 2 e86
O'Doherty A. Ruf S. Mulligan C. Hildreth V. Errington M.L. Cooke S. Sesay A. Modino S. Vanes L. Hernandez D. et al. An aneuploid mouse strain carrying human chromosome 21 with down syndrome phenotypes Science 2005 309 2033 2037
Besson V. Brault V. Duchon A. Togbe D. Bizot J.C. Quesniaux V.F. Ryffel B. Herault Y. Modeling the monosomy for the telomeric part of human chromosome 21 reveals haploinsufficient genes modulating the inflammatory and airway responses Hum. Mol. Genet. 2007 16 2040 2052
Arron J.R. Winslow M.M. Polleri A. Chang C.P. Wu H. Gao X. Neilson J.R. Chen L. Heit J.J. Kim S.K. et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21 Nature 2006 441 595 600
de Graaf K. Hekerman P. Spelten O. Herrmann A. Packman L.C. Bussow K. Muller-Newen G. Becker W. Characterization of cyclin L2, a novel cyclin with an arginine/serine-rich domain: phosphorylation by DYRK1A and colocalization with splicing factors J. Biol. Chem. 2004 279 4612 4624
de Graaf K. Czajkowska H. Rottmann S. Packman L.C. Lilischkis R. Luscher B. Becker W. The protein kinase DYRK1A phosphorylates the splicing factor SF3b1/SAP155 at Thr434, a novel in vivo phosphorylation site BMC Biochem. 2006 7 7
Adayev T. Chen-Hwang M.C. Murakami N. Wang R. Hwang Y.W. MNB/DYRK1A phosphorylation regulates the interactions of synaptojanin 1 with endocytic accessory proteins Biochem. Biophys. Res. Commun. 2006 351 1060 1065
Kim E.J. Sung J.Y. Lee H.J. Rhim H. Hasegawa M. Iwatsubo T. Min d.S. Kim J. Paik S.R. Chung K.C. Dyrk1A phosphorylates alpha-synuclein and enhances intracellular inclusion formation J. Biol. Chem. 2006 281 33250 33257
Ryoo S.R. Jeong H.K. Radnaabazar C. Yoo J.J. Cho H.J. Lee H.W. Kim I.S. Cheon Y.H. Ahn Y.S. Chung S.H. et al. DYRK1A-mediated hyperphosphorylation of Tau. A functional link between Down syndrome and Alzheimer disease J. Biol. Chem. 2007 282 34850 34857
Ryoo S.R. Cho H.J. Lee H.W. Jeong H.K. Radnaabazar C. Kim Y.S. Kim M.J. Son M.Y. Seo H. Chung S.H. et al. Dual-specificity tyrosine(Y)-phosphorylation regulated kinase 1A-mediated phosphorylation of amyloid precursor protein: evidence for a functional link between Down syndrome and Alzheimer's disease J. Neurochem. 2008 104 1333 1344
Huang Y. Chen-Hwang M.C. Dolios G. Murakami N. Padovan J.C. Wang R. Hwang Y.W. Mnb/Dyrk1A phosphorylation regulates the interaction of dynamin 1 with SH3 domain-containing proteins Biochemistry 2004 43 10173 10185
Woods Y.L. Cohen P. Becker W. Jakes R. Goedert M. Wang X. Proud C.G. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase Biochem. J. 2001 355 609 615
Aranda S. Alvarez M. Turro S. Laguna A. de la L.S. Sprouty2-mediated inhibition of fibroblast growth factor signaling is modulated by the protein kinase DYRK1A Mol. Cell. Biol. 2008 28 5899 5911
Gwack Y. Sharma S. Nardone J. Tanasa B. Iuga A. Srikanth S. Okamura H. Bolton D. Feske S. Hogan P.G. et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT Nature 2006 441 646 650
Dowjat W.K. Adayev T. Kuchna I. Nowicki K. Palminiello S. Hwang Y.W. Wegiel J. Trisomy-driven overexpression of DYRK1A kinase in the brain of subjects with Down syndrome Neurosci. Lett. 2007 413 77 81
Liu F. Liang Z. Wegiel J. Hwang Y.W. Iqbal K. Grundke-Iqbal I. Ramakrishna N. Gong C.X. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome FASEB J. 2008 22 3224 3233
Fuentes J.J. Genesca L. Kingsbury T.J. Cunningham K.W. Perez-Riba M. Estivill X. de la L.S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways Hum. Mol. Genet. 2000 9 1681 1690
Canzonetta C. Mulligan C. Deutsch S. Ruf S. O'Doherty A. Lyle R. Borel C. Lin-Marq N. Delom F. Groet J. et al. DYRK1A-dosage imbalance perturbs NRSF/REST levels, deregulating pluripotency and embryonic stem cell fate in Down syndrome Am. J. Hum. Genet. 2008 83 388 400
Altafaj X. Dierssen M. Baamonde C. Marti E. Visa J. Guimera J. Oset M. Gonzalez J.R. Florez J. Fillat C. et al. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome Hum. Mol. Genet. 2001 10 1915 1923
Martinez D.L. Altafaj X. Gallego X. Marti E. Estivill X. Sahun I. Fillat C. Dierssen M. Motor phenotypic alterations in TgDyrk1a transgenic mice implicate DYRK1A in Down syndrome motor dysfunction Neurobiol. Dis. 2004 15 132 142
Ahn K.J. Jeong H.K. Choi H.S. Ryoo S.R. Kim Y.J. Goo J.S. Choi S.Y. Han J.S. Ha I. Song W.J. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects Neurobiol. Dis. 2006 22 463 472
Weis S. Weber G. Neuhold A. Rett A. Down syndrome: MR quantification of brain structures and comparison with normal control subjects AJNR Am. J. Neuroradiol. 1991 12 1207 1211
Aylward E.H. Habbak R. Warren A.C. Pulsifer M.B. Barta P.E. Jerram M. Pearlson G.D. Cerebellar volume in adults with Down syndrome Arch. Neurol. 1997 54 209 212
Pearlson G.D. Breiter S.N. Aylward E.H. Warren A.C. Grygorcewicz M. Frangou S. Barta P.E. Pulsifer M.B. MRI brain changes in subjects with Down syndrome with and without dementia Dev. Med. Child Neurol. 1998 40 326 334
Aylward E.H. Li Q. Honeycutt N.A. Warren A.C. Pulsifer M.B. Barta P.E. Chan M.D. Smith P.D. Jerram M. Pearlson G.D. MRI volumes of the hippocampus and amygdala in adults with Down's syndrome with and without dementia Am. J. Psychiatry 1999 156 564 568
Aldridge K. Reeves R.H. Olson L.E. Richtsmeier J.T. Differential effects of trisomy on brain shape and volume in related aneuploid mouse models Am. J. Med. Genet. A 2007 143A 1060 1070
Roper R.J. Baxter L.L. Saran N.G. Klinedinst D.K. Beachy P.A. Reeves R.H. Defective cerebellar response to mitogenic Hedgehog signaling in Down syndrome mice Proc. Natl Acad. Sci. USA 2006 103 1452 1456
Lorenzi H.A. Reeves R.H. Hippocampal hypocellularity in the Ts65Dn mouse originates early in development Brain Res. 2006 1104 153 159
Guidi S. Bonasoni P. Ceccarelli C. Santini D. Gualtieri F. Ciani E. Bartesaghi R. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome Brain Pathol. 2008 18 180 197
Contestabile A. Fila T. Ceccarelli C. Bonasoni P. Bonapace L. Santini D. Bartesaghi R. Ciani E. Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice Hippocampus 2007 17 665 678
Clark S. Schwalbe J. Stasko M.R. Yarowsky P.J. Costa A.C. Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome Exp. Neurol. 2006 200 256 261
Holtzman D.M. Santucci D. Kilbridge J. Chua-Couzens J. Fontana D.J. Daniels S.E. Johnson R.M. Chen K. Sun Y. Carlson E. et al. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome Proc. Natl Acad. Sci. USA 1996 93 13333 13338
Toso L. Cameroni I. Roberson R. Abebe D. Bissell S. Spong C.Y. Prevention of developmental delays in a Down syndrome mouse model Obstet. Gynecol. 2008 112 1242 1251
Richtsmeier J.T. Baxter L.L. Reeves R.H. Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice Dev. Dyn. 2000 217 137 145
Hill C.A. Reeves R.H. Richtsmeier J.T. Effects of aneuploidy on skull growth in a mouse model of Down syndrome J Anat. 2007 210 394 405
Parsons T. Ryan T.M. Reeves R.H. Richtsmeier J.T. Microstructure of trabecular bone in a mouse model for Down syndrome Anat. Rec. (Hoboken.) 2007 290 414 421
Roper R.J. Vanhorn J.F. Cain C.C. Reeves R.H. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog Mech. Dev 2008 Published online 21 November, doi: 10.1016/j.mod.2008.11.002
Voronov S.V. Frere S.G. Giovedi S. Pollina E.A. Borel C. Zhang H. Schmidt C. Akeson E.C. Wenk M.R. Cimasoni L. et al. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome Proc. Natl Acad. Sci. USA 2008 105 9415 9420
Meng X. Peng B. Shi J. Zheng Y. Chen H. Zhang J. Li L. Zhang C. Effects of overexpression of Sim2 on spatial memory and expression of synapsin I in rat hippocampus Cell Biol. Int. 2006 30 841 847
Best T.K. Cho-Clark M. Siarey R.J. Galdzicki Z. Speeding of miniature excitatory post-synaptic currents in Ts65Dn cultured hippocampal neurons Neurosci. Lett. 2008 438 356 361
Best T.K. Siarey R.J. Galdzicki Z. Ts65Dn, a mouse model of Down syndrome, exhibits increased GABAB-induced potassium current J. Neurophysiol. 2007 97 892 900
Harashima C. Jacobowitz D.M. Witta J. Borke R.C. Best T.K. Siarey R.J. Galdzicki Z. Abnormal expression of the G-protein-activated inwardly rectifying potassium channel 2 (GIRK2) in hippocampus, frontal cortex, and substantia nigra of Ts65Dn mouse: a model of Down syndrome J. Comp. Neurol. 2006 494 815 833
Olson L.E. Roper R.J. Sengstaken C.L. Peterson E.A. Aquino V. Galdzicki Z. Siarey R. Pletnikov M. Moran T.H. Reeves R.H. Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice Hum. Mol. Genet. 2007 16 774 782
Morice E. Andreae L.C. Cooke S.F. Vanes L. Fisher E.M. Tybulewicz V.L. Bliss T.V. Preservation of long-term memory and synaptic plasticity despite short-term impairments in the Tc1 mouse model of Down syndrome Learn. Mem. 2008 15 492 500
Bliss T.V. Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path J. Physiol. 1973 232 331 356
Belichenko P.V. Kleschevnikov A.M. Salehi A. Epstein C.J. Mobley W.C. Synaptic and cognitive abnormalities in mouse models of Down syndrome: exploring genotype–phenotype relationships J. Comp. Neurol. 2007 504 329 345
Belichenko P.V. Kleschevnikov A.M. Masliah E. Wu C. Takimoto-Kimura R. Salehi A. Mobley W.C. Excitatory–inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of down syndrome J. Comp. Neurol. 2008 512 453 466
Holland A.J. Hon J. Huppert F.A. Stevens F. Incidence and course of dementia in people with Down's syndrome: findings from a population-based study J. Intellect. Disabil. Res. 2000 44 138 146
Holland A.J. Hon J. Huppert F.A. Stevens F. Watson P. Population-based study of the prevalence and presentation of dementia in adults with Down's syndrome Br. J. Psychiatry 1998 172 493 498
Janicki M.P. Dalton A.J. Prevalence of dementia and impact on intellectual disability services Ment. Retard. 2000 38 276 288
Johannsen P. Christensen J.E. Mai J. The prevalence of dementia in Down syndrome Dementia 1996 7 221 225
Lai F. Williams R.S. A prospective study of Alzheimer disease in Down syndrome Arch. Neurol. 1989 46 849 853
Granholm A.C. Sanders L.A. Crnic L.S. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down's syndrome Exp. Neurol. 2000 161 647 663
Granholm A.C. Ford K.A. Hyde L.A. Bimonte H.A. Hunter C.L. Nelson M. Albeck D. Sanders L.A. Mufson E.J. Crnic L.S. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome Physiol. Behav. 2002 77 371 385
Hunter C.L. Bimonte H.A. Granholm A.C. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory Behav. Brain Res. 2003 138 121 131
Hunter C.L. Bachman D. Granholm A.C. Minocycline prevents cholinergic loss in a mouse model of Down's syndrome Ann. Neurol. 2004 56 675 688
Necchi D. Lomoio S. Scherini E. Axonal abnormalities in cerebellar Purkinje cells of the Ts65Dn mouse Brain Res. 2008 1238 181 188
Salehi A. Delcroix J.D. Belichenko P.V. Zhan K. Wu C. Valletta J.S. Takimoto-Kimura R. Kleschevnikov A.M. Sambamurti K. Chung P.P. et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration Neuron 2006 51 29 42
Cooper J.D. Salehi A. Delcroix J.D. Howe C.L. Belichenko P.V. Chua-Couzens J. Kilbridge J.F. Carlson E.J. Epstein C.J. Mobley W.C. Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion Proc. Natl Acad. Sci. USA 2001 98 10439 10444
Seo H. Isacson O. Abnormal APP, cholinergic and cognitive function in Ts65Dn Down's model mice Exp. Neurol. 2005 193 469 480
Holtzman D.M. Li Y. Chen K. Gage F.H. Epstein C.J. Mobley W.C. Nerve growth factor reverses neuronal atrophy in a Down syndrome model of age-related neurodegeneration Neurology 1993 43 2668 2673
Mann D.M. Yates P.O. Marcyniuk B. Ravindra C.R. Pathological evidence for neurotransmitter deficits in Down's syndrome of middle age J. Ment. Defic. Res. 1985 29 125 135
Porta S. Serra S.A. Huch M. Valverde M.A. Llorens F. Estivill X. Arbones M.L. Marti E. RCAN1 (DSCR1) increases neuronal susceptibility to oxidative stress: a potential pathogenic process in neurodegeneration Hum. Mol. Genet. 2007 16 1039 1050
Ermak G. Morgan T.E. Davies K.J. Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer's disease J. Biol. Chem. 2001 276 38787 38794
Ermak G. Davies K.J. DSCR1(Adapt78)—a Janus gene providing stress protection but causing Alzheimer's disease? IUBMB Life 2003 55 29 31
Ermak G. Harris C.D. Battocchio D. Davies K.J. RCAN1 (DSCR1 or Adapt78) stimulates expression of GSK-3beta FEBS J. 2006 273 2100 2109
Lee J.H. Chulikavit M. Pang D. Zigman W.B. Silverman W. Schupf N. Association between genetic variants in sortilin-related receptor 1 (SORL1) and Alzheimer's disease in adults with Down syndrome Neurosci. Lett. 2007 425 105 109
Kimura R. Kamino K. Yamamoto M. Nuripa A. Kida T. Kazui H. Hashimoto R. Tanaka T. Kudo T. Yamagata H. et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease Hum. Mol. Genet. 2007 16 15 23
Liang Z. Liu F. Iqbal K. Grundke-Iqbal I. Wegiel J. Gong C.X. Decrease of protein phosphatase 2A and its association with accumulation and hyperphosphorylation of tau in Down syndrome J. Alzheimers Dis. 2008 13 295 302
Park J. Yang E.J. Yoon J.H. Chung K.C. Dyrk1A overexpression in immortalized hippocampal cells produces the neuropathological features of Down syndrome Mol. Cell. Neurosci. 2007 36 270 279
Wegiel J. Dowjat K. Kaczmarski W. Kuchna I. Nowicki K. Frackowiak J. Mazur K.B. Wegiel J. Silverman W.P. Reisberg B. et al. The role of overexpressed DYRK1A protein in the early onset of neurofibrillary degeneration in Down syndrome Acta Neuropathol. 2008 116 391 407
Shi J. Zhang T. Zhou C. Chohan M.O. Gu X. Wegiel J. Zhou J. Hwang Y.W. Iqbal K. Grundke-Iqbal I. et al. Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of Tau in Down syndrome J. Biol. Chem. 2008 283 28660 28669
Shukkur E.A. Shimohata A. Akagi T. Yu W. Yamaguchi M. Murayama M. Chui D. Takeuchi T. Amano K. Subramhanya K.H. et al. Mitochondrial dysfunction and tau hyperphosphorylation in Ts1Cje, a mouse model for Down syndrome Hum. Mol. Genet. 2006 15 2752 2762
Nistor M. Don M. Parekh M. Sarsoza F. Goodus M. Lopez G.E. Kawas C. Leverenz J. Doran E. Lott I.T. et al. Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain Neurobiol. Aging 2007 28 1493 1506
Freeman S.B. Bean L.H. Allen E.G. Tinker S.W. Locke A.E. Druschel C. Hobbs C.A. Romitti P.A. Royle M.H. Torfs C.P. et al. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project Genet. Med. 2008 10 173 180
Maslen C.L. Babcock D. Robinson S.W. Bean L.J. Dooley K.J. Willour V.L. Sherman S.L. CRELD1 mutations contribute to the occurrence of cardiac atrioventricular septal defects in Down syndrome Am. J. Med. Genet. A 2006 140 2501 2505
Moore C.S. Postnatal lethality and cardiac anomalies in the Ts65Dn Down syndrome mouse model Mamm. Genome 2006 17 1005 1012
Williams A.D. Mjaatvedt C.H. Moore C.S. Characterization of the cardiac phenotype in neonatal Ts65Dn mice Dev. Dyn. 2008 237 426 435
Izraeli S. Rainis L. Hertzberg L. Smooha G. Birger Y. Trisomy of chromosome 21 in leukemogenesis Blood Cells Mol. Dis. 2007 39 156 159
Groet J. McElwaine S. Spinelli M. Rinaldi A. Burtscher I. Mulligan C. Mensah A. Cavani S. Dagna-Bricarelli F. Basso G. et al. Acquired mutations in GATA1 in neonates with Down's syndrome with transient myeloid disorder Lancet 2003 361 1617 1620
Wechsler J. Greene M. McDevitt M.A. Anastasi J. Karp J.E. Le Beau M.M. Crispino J.D. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome Nat. Genet. 2002 32 148 152
Chou S.T. Opalinska J.B. Yao Y. Fernandes M.A. Kalota A. Brooks J.S. Choi J.K. Gewirtz A.M. Danet-Desnoyers G.A. Nemiroff R.L. et al. Trisomy 21 enhances human fetal erythro-megakaryocytic development Blood 2008 112 4503 4506
Tunstall-Pedoe O. Roy A. Karadimitris A. de la F.J. Fisk N.M. Bennett P. Norton A. Vyas P. Roberts I. Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations Blood 2008 112 4507 4511
Malinge S. Ragu C. Della-Valle V. Pisani D. Constantinescu S.N. Perez C. Villeval J.L. Reinhardt D. Landman-Parker J. Michaux L. et al. Activating mutations in human acute megakaryoblastic leukemia Blood 2008 112 4220 4226
Sato T. Toki T. Kanezaki R. Xu G. Terui K. Kanegane H. Miura M. Adachi S. Migita M. Morinaga S. et al. Functional analysis of JAK3 mutations in transient myeloproliferative disorder and acute megakaryoblastic leukaemia accompanying Down syndrome Br. J. Haematol. 2008 141 681 688
Klusmann J.H. Reinhardt D. Hasle H. Kaspers G.J. Creutzig U. Hahlen K. van den Heuvel-Eibrink M.M. Zwaan C.M. Janus kinase mutations in the development of acute megakaryoblastic leukemia in children with and without Down's syndrome Leukemia 2007 21 1584 1587
Kiyoi H. Yamaji S. Kojima S. Naoe T. JAK3 mutations occur in acute megakaryoblastic leukemia both in Down syndrome children and non-Down syndrome adults Leukemia 2007 21 574 576
Walters D.K. Mercher T. Gu T.L. O'Hare T. Tyner J.W. Loriaux M. Goss V.L. Lee K.A. Eide C.A. Wong M.J. et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia Cancer Cell 2006 10 65 75
De Vita S. Mulligan C. McElwaine S. Dagna-Bricarelli F. Spinelli M. Basso G. Nizetic D. Groet J. Loss-of-function JAK3 mutations in TMD and AMKL of Down syndrome Br. J. Haematol. 2007 137 337 341
Toki T. Kanezaki R. Adachi S. Fujino H. Xu G. Sato T. Suzuki K. Tauchi H. Endo M. Ito E. The key role of stem cell factor/KIT signaling in the proliferation of blast cells from Down syndrome-related leukemia Leukemia 2008 advance online publication 2 October 2008; doi: 10.1038/leu.2008.267
Kirsammer G. Jilani S. Liu H. Davis E. Gurbuxani S. Le Beau M.M. Crispino J.D. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome Blood 2008 111 767 775
Forestier E. Izraeli S. Beverloo B. Haas O. Pession A. Michalova K. Stark B. Harrison C.J. Teigler-Schlegel A. Johansson B. Cytogenetic features of acute lymphoblastic and myeloid leukemias in pediatric patients with Down syndrome: an iBFM-SG study Blood 2008 111 1575 1583
Malinge S. Ben Abdelali R. Settegrana C. Radford-Weiss I. Debre M. Beldjord K. Macintyre E.A. Villeval J.L. Vainchenker W. Berger R. et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia Blood 2007 109 2202 2204
Kearney L. Gonzalez D.C. Yeung J. Procter J. Horsley S.W. Eguchi-Ishimae M. Bateman C.M. Anderson K. Chaplin T. Young B.D. et al. A specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukaemia Blood 2008 prepublished online 16 October 2008, doi:10.1182/blood-2008-08-170928
Bercovich D. Ganmore I. Scott L.M. Wainreb G. Birger Y. Elimelech A. Shochat C. Cazzaniga G. Biondi A. Basso G. et al. Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome Lancet 2008 372 1484 1492
Hasle H. Pattern of malignant disorders in individuals with Down's syndrome Lancet Oncol. 2001 2 429 436
Yang Q. Rasmussen S.A. Friedman J.M. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study Lancet 2002 359 1019 1025
Sussan T.E. Yang A. Li F. Ostrowski M.C. Reeves R.H. Trisomy represses Apc(Min)-mediated tumours in mouse models of Down's syndrome Nature 2008 451 73 75
Morrison R.A. McGrath A. Davidson G. Brown J.J. Murray G.D. Lever A.F. Low blood pressure in Down's syndrome, a link with Alzheimer's disease? Hypertension 1996 28 569 575
Draheim C.C. McCubbin J.A. Williams D.P. Differences in cardiovascular disease risk between nondiabetic adults with mental retardation with and without Down syndrome Am. J. Ment. Retard. 2002 107 201 211
Fernandez F. Morishita W. Zuniga E. Nguyen J. Blank M. Malenka R.C. Garner C.C. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome Nat. Neurosci. 2007 10 411 413
Costa A.C. Scott-McKean J.J. Stasko M.R. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test Neuropsychopharmacology 2008 33 1624 1632
Saxena R. Voight B.F. Lyssenko V. Burtt N.P. de Bakker P.I. Chen H. Roix J.J. Kathiresan S. Hirschhorn J.N. Daly M.J. et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels Science 2007 316 1331 1336
Loos R.J. Lindgren C.M. Li S. Wheeler E. Zhao J.H. Prokopenko I. Inouye M. Freathy R.M. Attwood A.P. Beckmann J.S. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity Nat. Genet. 2008 40 768 775
O'Donovan M.C. Craddock N. Norton N. Williams H. Peirce T. Moskvina V. Nikolov I. Hamshere M. Carroll L. Georgieva L. et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up Nat. Genet. 2008 40 1053 1055
Stefansson H. Rujescu D. Cichon S. Pietilainen O.P. Ingason A. Steinberg S. Fossdal R. Sigurdsson E. Sigmundsson T. Buizer-Voskamp J.E. et al. Large recurrent microdeletions associated with schizophrenia Nature 2008 455 232 236
Gerstein M.B. Bruce C. Rozowsky J.S. Zheng D. Du J. Korbel J.O. Emanuelsson O. Zhang Z.D. Weissman S. Snyder M. What is a gene, post-ENCODE? History and updated definition Genome Res. 2007 17 669 681
Denoeud F. Kapranov P. Ucla C. Frankish A. Castelo R. Drenkow J. Lagarde J. Alioto T. Manzano C. Chrast J. et al. Prominent use of distal 5’ transcription start sites and discovery of a large number of additional exons in ENCODE regions Genome Res. 2007 17 746 759
- down syndrome
- chromosomes
- chromosomes, human, pair 21
- engineering
- mental processes
- animal model
- learning disabilities
- cognitive ability
- childhood leukemia
- alzheimer disease, early onset
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2083
- Print ISSN 0964-6906
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Your Privacy on DSE sites
We use cookies to provide essential functionality and to analyse how our sites are used.
Down Syndrome Research and Practice
Down Syndrome Research and Practice is a peer-reviewed journal focused on Down syndrome research. It was published by Down Syndrome Education International in partnership with the University of Portsmouth from 1992 to 2009.
- Research and Practice
- News and Update
Presentations
- Research Forum
- Online courses
- See and Learn
Legal information
- Cookies Policy
- Privacy Policy
- Terms of Use
- Terms of sale
- DSE Client ID
- Access RLI Online
- Subscriptions
- English (Australia)
- English (Canada)
- English (India)
- English (Ireland)
- English (Malaysia)
- English (New Zealand)
- English (Philippines)
- English (Singapore)
- English (South Africa)
- English (United Kingdom)
- English (United States)
Behavioral Challenges in Young Children with Down Syndrome
- First Online: 07 May 2024
Cite this chapter

- Galina Yu Odinokova ORCID: orcid.org/0000-0001-7549-3530 4
Behavioral challenges in children with Down syndrome can seriously impact their socialization and development. The precursors of behavioral challenges have been found to emerge early in life. This survey involves families from different federal districts of Russia, including the Central, Southern, North Caucasian, Volga, Siberian, and Far Eastern Federal Districts of the Russian Federation. Of all survey participants, 80% of parents who raise children from 1 to 4 years old with Down syndrome reported that their children have behavioral difficulties. They reported that the child is aggressive, refuses to play and interact, does not respond to adult suggestions, and may engage in behavior that the parent does not understand, including provoking dangerous situations. The analysis of mother-child interactions in early childhood through video recordings showed that children with Down syndrome exhibit undesirable behaviors similar to children with normal development. Behavioral problems are combined with the child’s specific characteristics of his or her interactions, indicating low motivation to interact with the mother. The child does not make requests and offers to his or her mother in socially accepted ways. The child often expresses their desires through provocative actions because they have limited traditional interaction means. The mother does not support the child’s activity in interaction. A disturbed parity in the interaction between the mother and the child is expressed in the child’s preference to engage in toys alone while trying different ways of acting that force the parent to pay attention to the child, consider their will, and make changes. Looking at a child’s problem behavior through an analysis of his or her reactive and initiatory behaviors offers a different perspective on the root causes of these difficulties and requires that professionals consider this when working with families.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Bakli, S. (2009). Development of children with Down syndrome. In S. J. Skallerup (Eds.), Babies with Down syndrome: A new parent’s guide (O. K. Vasilyeva, & M. D. Shikhireva Transl. from English) (pp. 191–223). Moscow, Russia: Downside Up Charitable Fund. (Original work published 2008)
Google Scholar
Dykens, E. M. (1995). Measuring behavioral phenotypes: Provocations from the “new genetics”. American Journal on Mental Retardation , 99 (5), 522–532.
Dykens, E. M., & Kasari, C. (1997). Maladaptive behavior in children with Prader-Willi syndrome, Down syndrome, and nonspecific mental retardation. American Journal on Mental Retardation , 102 (3), 228–237.
Article Google Scholar
Feeley, K. M., & Jones, E. A. (2010). Overcoming challenging behavior in children with Down syndrome. Down Syndrome. XXI Century , 2 (5), 26–33.
Fidler, D. J. (2005). The emerging Down syndrome behavioral phenotype in early childhood: Implications for practice. Infants & Young Children , 18 (2), 86–103. https://doi.org/10.1097/00001163-200504000-00003
Lisina, M. I. (2009). Problems of the ontogeny of communication. In M. I. Lisina (Ed.), Formation of the child’s personality in communication (pp. 21–129). St. Petersburg, Russia: Piter.
Murphy, J. (2004). The secret lives of toddlers: A parent’s guide to the wonderful, terrible, fascinating behavior of children ages 1 to 3. New York, NY: TarcherPerigee.
Odinokova, G. Yu. (2016). Communication between a mother and a young child with Down syndrome. Moscow, Russia: Polygraph Service.
Pantley, E. (2007). The no-cry discipline solution: Gentle ways to encourage good behavior without whining, tantrums, and tears. New York, NY: McGraw-Hill.
Razenkova, Yu. A. (2017). Difficulties in the development of communication in young children with health limitations: Identification, prevention, intervention. Moscow, Russia: Polygraph Service.
Razenkova, Yu. A., Odinokova, G. Yu., & Ayvazyan, E. B. (2018). Maternal communicative behavior as a factor in the development of communication in children with Down Syndrome. Psychology in Russia: State of the Art , 11 (3), 111–127.
Razenkova, Yu. A., Orlova, E. V., Odinokova, G. Yu., Kudrina, T. P., & Ajvazyan, E. B. (2018). The study of adult–infant communication in the early years, with a methodological toolbox. Almanac of the Institute of Special Education , 32 , 17–33. Retrieved from https://alldef.ru/ru/articles/almanac-32/a-study-of-adult-child-communication-the-first-years-of-life-with-disabilities-a-methodological-toolkit (Accessed 12 March 2023)
Smirnova, E. O., Galiguzova, L. N., Yermolova, T. V., & Meshcheryakova, S. Yu. (2003). Diagnosing mental development in children with Down syndrome from birth to 3 years. Moscow, Russia: Moscow State University of Psychology & Education.
Speck, O. (2003). People with intellectual disability: Learning and education (A. P. Golubeva Transl. from German) . Moscow, Russia: Academia.
Stores, R., & Stores, G. (1996). Research on sleep problems and psychological function in children with Down syndrome: Implications for clinical practice and everyday care. Down Syndrome Research and Practice , 4 (3), 110–112.
Vygotsky, L. S. (1983). Volume 3: Problems of mental development (by Ed. A. M. Matyushkin). In A. V. Zaporozhets (Ed.), Collected Works: In 6 vols. Moscow, USSR: Pedagogika.
White, P. (2014). Practical approaches to behaviors that drive you crazy . Moscow, Russia: Downside Up Charitable Fund.
Download references
Acknowledgments
This work was performed within the framework of the state assignment of the Ministry of Education of the Russian Federation, The Federal State Budget Scientific Institution “Institute of Special Education of the Russian Academy of Education,” No. 073-00063-23-01
Author information
Authors and affiliations.
Institute of Special Education, Moscow, Russia
Galina Yu Odinokova
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Galina Yu Odinokova .
Editor information
Editors and affiliations.
Tatiana A. Solovyova
Faculty of Educational Studies, Lomonosov Moscow State University, Moscow, Russia
Anna A. Arinushkina
Ekaterina A. Kochetova
Rights and permissions
Reprints and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Odinokova, G.Y. (2024). Behavioral Challenges in Young Children with Down Syndrome. In: Solovyova, T.A., Arinushkina, A.A., Kochetova, E.A. (eds) Educational Management and Special Educational Needs. Springer, Cham. https://doi.org/10.1007/978-3-031-57970-7_2
Download citation
DOI : https://doi.org/10.1007/978-3-031-57970-7_2
Published : 07 May 2024
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-57969-1
Online ISBN : 978-3-031-57970-7
eBook Packages : Education Education (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
MINI REVIEW article
Development of down syndrome research over the last decades–what healthcare and education professionals need to know.

- 1 Division of Obstetrics and Feto-Maternal Medicine, Department of Obstetrics and Gynecology, Medical University of Vienna, Vienna, Austria
- 2 Research Unit Developmental Psychology, Department of Developmental and Educational Psychology, Faculty of Psychology, University of Vienna, Vienna, Austria
Down syndrome (DS) is the most prevalent neurodevelopmental disorder, with a known genetic cause. Besides facial dysmorphologies and congenital and/or acquired medical conditions, the syndrome is characterized by intellectual disability, accelerated aging, and an increased likelihood of an early onset Alzheimer's disease in adulthood. These common patterns of DS are derived from the long-held standard in the field of DS research, that describes individuals with DS as a homogeneous group and compares phenotypic outcomes with either neurotypical controls or other neurodevelopmental disorders. This traditional view has changed, as modern research pinpoints a broad variability in both the occurrence and severity of symptoms across DS, arguing for DS heterogeneity and against a single “DS profile.” Nevertheless, prenatal counseling does not often prioritize the awareness of potential within-group variations of DS, portraying only a vague picture of the developmental outcomes of children with DS to expectant parents. This mini-review provides a concise update on existent information about the heterogeneity of DS from a full-spectrum developmental perspective, within an interdisciplinary context. Knowledge on DS heterogeneity will not only enable professionals to enhance the quality of prenatal counseling, but also help parents to set targeted early interventions, to further optimize daily functions and the quality of life of their children.
Introduction
Down syndrome (DS) is the most common neurodevelopmental disorder with known genetic causes, and an incidence of 1 in 691 live births ( 1 ). This suggests that ~417,000 people with DS live in Europe ( 2 ). Currently, an expansive menu of prenatal diagnostic methods for DS is spreading worldwide, advancing the diagnosis of DS from postnatal to prenatal ( 3 ). Giving an expectant parent a fetal diagnosis of DS provides them with 2 options: keeping or terminating their pregnancy, following the lack of a cure ( 4 ).
Prenatal counseling is crucial for providing parents with an accurate picture of DS so that informed decisions can be made in the context of their own beliefs and values ( 3 ). Although studies are still examining the nature of DS, portraying the expected neurodevelopmental outcomes of affected children remains challenging. Indeed, retrospective studies indicate that parents felt that the information received during prenatal counseling was inaccurate, outdated, and unbalanced, and either too negative or too optimistic ( 5 – 7 ). Without appropriate professional training or updated professional development regarding the individual variability in outcomes associated with DS, prenatal counselors might present expectant parents with inaccurate information or impressions. Therefore, expectant parents may not receive the level of information needed. Accordingly, all professionals working with families affected by DS must be aware of the most current scientific research regarding the heterogeneity of phenotypic outcomes ( 8 ).
This mini-review closes an existent literature gap by providing a concise update on the available information on within-group variations in the DS phenotype of infants, children, and adolescents for professionals. First, a gross outline of DS research is given, focusing on the significant paradigm shift from a group- to an individual-level approach. Second, the current knowledge on significant within-group variations of DS in cognitive, behavioral, emotional, and olfactory functioning is summarized. Finally, the review concludes by arguing that only an interdisciplinary approach allows for the description of realistic individual DS profiles. The scope of this review is to further increase the awareness on DS heterogeneity concerning developmental outcomes.
A Paradigm Shift in DS Research: From a Group- to Individual-Level Approach
DS research dates back to 1866, when the English physician John Langdon Down systematically described the syndrome for the first time ( 9 , 10 ). In addition to intellectual disability (ID), he chronicled a distinct physical phenotype of individuals with DS, conjecturing that they were “born to the same family” (page 9) ( 10 , 11 ). The century following his pioneering work was filled with publications of diverse medical case studies documenting a range of physical traits and medical comorbidities, leading to various etiologies ( 10 , 11 ).
Almost 100 years later, the French pediatrician and cytogeneticist, Jérôme Lejeune, identified the genetic basis of DS in 1959 as an extra copy of all or part of chromosome 21 ( 10 , 12 ). The discovery of “trisomy 21” paved the way for further research, to elucidate genotype-phenotype-relationships ( 13 , 14 ). Since its original description, classical DS research has analyzed the syndrome's phenotypes relative to neurotypicals and/or other neurodevelopmental disorders, hence providing group-level data that have advanced our basic knowledge of DS ( 8 ). It is characterized by both typical physical features that make the syndrome “instantly recognizable” (page 8) and ID ( 11 ). Common appearance includes craniofacial dysmorphologies, short stature, low muscle tone, and a proportionally large tongue. Additionally, medical comorbidities, such as sleep apnea, visual and/or hearing problems, congenital heart defects, and altered behavioral, hematopoietic, endocrine, gastrointestinal, neurological, and musculoskeletal conditions, are linked to DS ( 10 ).
Most of these medical problems are treatable with pharmacotherapy and/or surgical interventions. Therefore, among the key focuses in recent DS research is the widespread field of neurocognition, associating DS with weaknesses in motor ability, auditory processing, verbal short-term memory, and expressive language. However, relative strengths in visuospatial processing, receptive language, and some aspects of social functioning have been reported ( 15 – 18 ). Further, DS is associated with accelerated aging and an increased likelihood of the early onset of Alzheimer's disease (AD) ( 18 ).
Although the generalizability of the characteristics of DS has been questioned repeatedly in the history of DS research, the group-level approach is a long-held standard ( 19 , 20 ). However, this traditional view has changed, following a growing number of studies, which pinpoint significant within-group variations across individuals with DS at many levels of description. Pioneer studies have launched this paradigm shift, from a group to an individual-level approach, by highlighting significant individual differences in genetics, cell biology, brain research, and subsequently, parts of cognitive research on DS [see ( 8 )]. These studies suggest that this heterogeneity may be continued in DS phenotypes ( 8 ). The following review aims to supplement the prevailing knowledge about the variability of the developmental outcomes of DS by addressing this issue from an interdisciplinary and applied science perspective, as this practical information may be the most useful for professionals to pass to expectant parents.
Infants, Children, and Adolescents With DS: Variability in Developmental Outcomes
Acquisition of developmental milestones.
Generally, it was assumed that infants and children with DS reached developmental milestones in the same linear fashion as their non-DS peers, but at later chronological ages. This view is too simplistic, as the age of acquiring milestones among infants and children with DS is reported to vary significantly ( 21 , 22 ). For example, the mean age at the onset of babbling is ~15 months, with an interindividual variability of 10 months. Similarly, sphincter control is acquired by DS children at an approximate age of 44 months, with 22 months of interindividual variability ( 22 ). Notably, Locatelli et al. suggested that the age at which developmental milestones are reached influences the subsequent development of diverse cognitive domains significantly ( 21 , 22 ).
Intellectual Disability (ID)
ID, defined by an intelligence quotient (IQ) score of <70, is reported to be universal in the DS population. However, this construct presents in DS with large interindividual variability ( 23 ). The majority of individuals with DS fall within the severe (IQ 20–35) to mild (IQ 50–69) range of ID. However, some cases reach IQ scores equivalent to children without ID ( 14 , 24 ). Research on the developmental trajectories of cognitive function in neurotypicals shows that IQ is a construct that remains relatively stable and consistent across ages. A slight decline was observed only in older adults ( 14 ). Conversely, DS research has identified a linear decline in IQ scores as development progresses, starting in the first year of life (i.e., cognitive gains do not keep pace with chronological age). Notably, single IQ levels and the degree of cognitive decline vary across the DS group ( 14 ).
Language is another cognitive domain that generates significant differences among individuals with DS. DS is associated with weaknesses in expressive language and a relative strength in the receptive language ( 18 ). The available literature reports developmental delays in both language domains, becoming apparent no later than age five, yet with wide individual differences ( 25 , 26 ). Regarding vocabulary acquisition and growth, longitudinal studies reported an existing continuum, ranging from non-verbal children to those with a vocabulary close to the normal range ( 27 , 28 ). Children with DS use gestures as a means of communication, which has been positively associated with the development of spoken vocabulary ( 29 ). Nevertheless, significant individual variability in the extent to which this “gestural advantage” is used has been demonstrated by empirical data ( 30 ). All within-group differences in language development persist into adulthood ( 26 ).
Memory and learning deficits are universal characteristics of DS and are known to become more pronounced as development progresses ( 14 ). In classical DS research, the findings of affected memory domains are mixed, suggesting underlying variability ( 18 ). Indeed, scientific data demonstrate that there are individual differences in both implicit and explicit memory ( 8 , 31 ). Regarding the latter, significant within-group variations are described for short-term verbal and long-term visual memory ( 8 ). Individuals with DS often show deficits in processing local detail. Therefore, classical DS literature claims that individuals with DS were “global processors.” However, this preference for global over local processing does not always occur in the DS population. Therefore, individuals with DS cannot be simply categorized into one of these processing styles ( 32 ).
Executive Function (EF)
EF encompasses a range of cognitive processes involved in goal-oriented behavior, and is a domain in which individuals with DS are shown to have pronounced difficulties ( 33 ). The areas of working memory, attention, planning, and inhibition are considered particularly challenging for individuals with DS; emotional control is considered a relative strength ( 34 , 35 ). However, significant individual differences in EF across the DS group have become evident ( 33 , 36 ). Within-group variations in auditory attention have been identified via electrophysiological measurement among toddlers with DS, data that also predict differences in language abilities as development progresses ( 37 ). Patterns of executive dysfunction appear to be relatively consistent across development until adulthood ( 23 , 34 ).
Adaptive Behavior (AB)
Children and adolescents with DS are known to be severely impaired in AB, which subsumes behavioral skills that enable them to function independently in their everyday life ( 23 , 38 ). Generally, AB encompasses 4 domains: socialization, communication, daily living, and motor skills ( 23 ). Significant within-group variations were apparent for all the 4 domains. For example, DS has been associated with sociability, friendliness, affection, empathy, good competence in forming relationships, and high tendency to smile ( 39 ). Yet, children and adolescents with DS are also considered stubborn, to show little accommodation to social partners, and approach strangers inappropriately ( 40 ). Some individuals with DS have even deficits in socialization to the extent of a comorbid diagnosis of autism ( 41 ).
Maladaptive Behavior (MB) and Psychiatric Comorbidities
MB encompasses a range of behaviors that impede an individual's activities of daily living or the ability to adjust to and participate in particular settings ( 23 ). Approximately 1/4 to 1/3 of individuals with DS exhibit clinically significant levels of maladaptive behavioral concerns ( 42 – 44 ). This behavioral construct is another domain that yields significant within-group differences ( 21 , 23 , 45 ). More difficulties with “anxious-depressed” symptoms are observed among adolescents than younger children with DS ( 23 ). Children with DS often exhibit externalizing behavior ( 46 ). The manifestation of MB is significantly higher when neurobehavioral disorders are concomitant ( 47 – 49 ). According to the available literature, the manifestation of psychiatric features, including autism, depression, and the attention-deficit/hyperactivity disorder, vary significantly, between 6 and >50% ( 42 , 44 , 50 , 51 ). Channell et al. underscored within-group differences in the behavioral domain by subtyping a >300-person DS group, hence identifying a separate “behavioral” class as described in Table 1 ( 23 ).
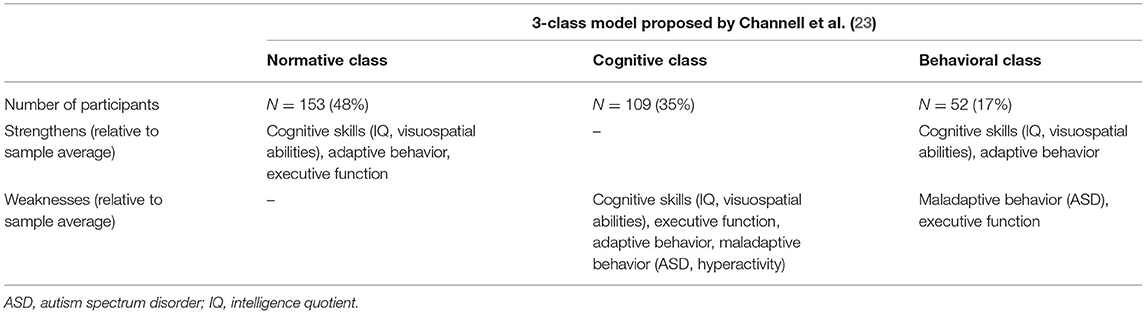
Table 1 . Characterization of the 3-class model of individuals with DS ( N = 314; 6–25 years) based on the variability observed in cognitive and behavioral measures, identified by Channell et al. ( 23 ) using a latent profile analysis.
Emotional Functioning
The emotional profiles of individuals with DS have remained underexplored, which could be attributed to the assumed stereotype of high sociability in this population ( 52 , 53 ). Available literature provides variable data about whether children and adolescents have difficulties in emotional functioning ( 52 ). Whereas, some studies negate differences in identifying basic emotion in faces between DS and non-DS groups, other scientific reports indicate that children and adolescents with DS have impairments in this emotional skill [see Roch et al. ( 52 )] ( 54 – 57 ). Deficits in recognizing facial expressions were not generalized to all emotions, but mostly to fear ( 52 , 58 ). Other studies report impairments in determining feelings, including surprise, anger, and neutral expression ( 40 , 58 – 61 ). Some studies pinpoint problems in ascertaining negative emotions ( 40 ). Moreover, an inability to distinguish between fear and sadness is another atypical pattern that has been reported among some individuals ( 58 ). Most of these deficits are identified during infancy and childhood. Therefore, a negative impact on the subsequent development of interpersonal relationships is discussed ( 52 ). As previously mentioned, studies have exclusively gathered data at the group level. Moreover, further research should examine whether inconsistencies in findings across studies can be attributed to underlying within-group variations.

Olfactory Functioning
The number of studies on olfactory function among patients with DS is limited and relatively out of date ( 62 – 69 ). Historical studies have described olfactory deficits in the DS population for many years ( 62 , 63 , 65 , 70 ). Because rhinologic pathologies have been ruled out by studies showing nasal function in DS as comparable to controls, central-nervous causes are suggested ( 64 ). More recently, Cecchini et al. described olfactory function as severely impaired among adults with DS ( 71 ). They found a positive correlation between odor identification and cognition ( 71 ). To date, the largest study, which included people with DS and under 18 years, described a minimal impairment of olfactory functioning among children and adolescents (9–17 years), which became pronounced in young adulthood (18–29 years) and was the lowest in adulthood (30–50 years) ( 72 ). Of the three groups, DS, IQ, and age-matched controls, significant within-group differences were evident only in the DS group ( 72 ). However, large and detailed analyses of olfactory function in light of within-group variations among children and adolescents with DS are still lacking. Odor identification deficits are considered a valid non-invasive early marker of AD. Therefore, future research on whether olfactory dysfunction can help to ascertain the subset of children and adolescents with DS that will later develop AD is warranted.
Alzheimer's Disease (AD)
Although the issue of AD appears outside the scope of this review, the following considerations must be made when the heterogeneity of DS is discussed with expectant parents from a full-spectrum developmental perspective. Owing to a shared genetic predisposition, individuals with DS have an increased likelihood of developing early onset AD in adulthood ( 18 ). Prevalence rates of dementia among the DS population vary significantly in the literature, from 8 to 100% ( 18 , 73 ). Recent brain research has identified Alzheimer's plaques among some children with DS, that is, as early as 8 years of age, whereas some DS brains show no plaques until early adulthood ( 14 , 26 ). Although AD neuropathology occurs in virtually all individuals with DS over the age of 30, only a subset of people develop clinical symptoms of dementia ( 26 , 74 , 75 ). Hence, it is apparent that the widespread interindividual variability, typical for DS, is a pivotal feature not only during development, but also during aging ( 26 ). Aging is part of the continuous lifespan development. Accordingly, some authors argue that AD should be considered a disease that occurs during development, rather than aging ( 76 ).
Extrinsic Influencing Factors of Developmental Outcomes of Infants, Children, and Adolescents With DS
Medical comorbidities.
In addition to cognitive limitations, parents must be informed that there is a list of medical comorbidities associated with DS. Some of them, including congenital heart defects (CHD), seizures, visual and/or hearing impairments, autism, and sleep disruptions, are known to moderate cognitive functioning ( 18 ). Analogous to neurodevelopmental outcomes, both the occurrence and expression of congenital and/or acquired medical complications are variable ( 18 ). For example, 41–56% of infants with DS are born with a CHD, with an atrioventricular septal defect that occurs between 31 and 61% being the most common form ( 77 , 78 ). Cognition, gross motor skills, and language are significantly worse among infants with DS and CHD, relative to peers without CHD, in some, but not in all related studies ( 79 – 81 ). For example, Alsaied et al. showed that children with DS and CHD, who undergo cardiac surgery during their first year, have no significant differences in neurodevelopmental outcomes at preschool and school age. However, as infants and toddlers, they were prone to poorer outcomes in receptive, expressive, and composite language compared to children with DS without CHD, suggesting that deleterious effects may be dependent on clinical management ( 82 ).
Home Environment
Another variable that affects the observed variability of DS phenotypes, which is influenced by the expectant parents, is the home environment. According to Karmiloff-Smith et al., the genetic syndrome changes the family context in terms of parent-child-interactions ( 8 ). D'Souza et al. demonstrated that parental depression, a disease linked to difficulties in responding to the child in a sensitive and consistent manner, explained deficits in expressive language development among children between 8 and 48 months of age with DS ( 83 ). Similarly, there is evidence that vocabulary development among children with DS is influenced by how parents respond to their children's communication. Deckers et al. argued that mothers with a higher level of education had a better ability to fine-tune their communication with their children with DS ( 28 ). Further demographic factors, including socioeconomic status, neighborhood demographics, and the availability of therapeutic resources, modulate the developmental outcomes of DS effectively ( 84 , 85 ). These data demonstrate that only an interdisciplinary approach that considers psychological, physical, and social parameters will enable professionals to accurately inform expectant parents on how the DS phenotype will be expressed in each individual.
Although DS has been examined for a long time, that is 155 years, it is still one of the least understood genetic ID syndromes. The most significant reason for this is the high degree of phenotypic variability observed in the DS population, an issue that professionals are often unaware of when discussing the diagnosis with expectant parents. However, DS research has advanced from a group to an individual-level approach, attempting to acknowledge within-group differences at many levels of basic science ( 8 ). To expand on this wealth of data, this mini-review has shed light on the available information on individual variability in the developmental outcomes of infants, children, and adolescents with DS from an applied science perspective, which will enhance the quality of prenatal counseling. Diverse developmental domains, including cognition, behavior, and emotional and olfactory functioning, have been discussed.
The evaluation of developmental outcomes from a full-spectrum perspective, however, must not only address different developmental domains, but also the change of phenotypes over time ( 86 ). Outcome variables are not completely intact or impaired uniformly throughout development, but manifest as variations at an early state, that may be magnified with age, ending up as either a strength or a weakness. Therefore, parents should be made aware that early development can be considered a critical window of opportunity to set adequate phenotype-specific interventions before deficits become severely pronounced ( 87 ). Thus, the maximization of individual potential is possible. In addition to psychological factors, other influencing variables must be considered by parents when the variability of DS phenotypes is discussed. According to Karmiloff-Smith who states that having a neurodevelopmental disorder changes both the social environment and physical status, only an interdisciplinary research approach can successfully describe valid profiles of individuals with DS ( 8 ).
The most convincing argument for emphasizing individual variability among DS groups and discussing them with expectant parents are both an average life expectancy of 60 years combined with an early onset of Alzheimer's disease in the DS population ( 18 ). Focusing on individual differences in the development of DS may be the best approach for exploring the risk and protective factors of AD ( 88 , 89 ).
Modern DS research shows that developmental heterogeneity has become increasingly validated ( 23 ). Moving forward, these up-to-date data must be disseminated under the supervision of professionals so that prenatal counseling can be optimized in quality, hence allowing parents to gain realistic expectations about the future of their children. Thus, more targeted treatments and interventions can be set to improve the daily function and quality of life.
Author Contributions
KW and SH designed the paper. KW did the literature research and wrote the manuscript. SH provided intellectual input and critically revised the manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. (2010) 88:1008–16. doi: 10.1002/bdra.20735
PubMed Abstract | CrossRef Full Text | Google Scholar
2. de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in Europe. Eur J Hum Genet. (2021) 29:402–10. doi: 10.1038/s41431-020-00748-y
3. Minear MA, Alessi S, Allyse M, Michie M, Chandrasekharan S. Noninvasive prenatal genetic testing: current and emerging ethical, legal, and social issues. Annu Rev Genomics Hum Genet. (2015) 16:369–98. doi: 10.1146/annurev-genom-090314-050000
4. Guedj F, Bianchi DW. Noninvasive prenatal testing creates an opportunity for antenatal treatment of Down syndrome. Prenat Diagn. (2013) 33:614–8. doi: 10.1002/pd.4134
5. Skotko BG, Kishnani PS, Capone GT, Group DSDS. Prenatal diagnosis of Down syndrome: how best to deliver the news. Am J Med Genet A . (2009) 149A:2361–7. doi: 10.1002/ajmg.a.33082
6. Zerres K, Rudnik-Schöneborn S, Holzgreve W. Do non-invasive prenatal tests promote discrimination against people with Down syndrome? What should be done? J Perinat Med. (2021) 49:965–71. doi: 10.1515/jpm-2021-0204
7. Skotko BG. With new prenatal testing, will babies with Down syndrome slowly disappear? Arch Dis Child. (2009) 94:823–6. doi: 10.1136/adc.2009.166017
8. Karmiloff-Smith A, Al-Janabi T, D'Souza H, Groet J, Massand E, Mok K, et al. The importance of understanding individual differences in Down syndrome. F1000Res . (2016) 5:389. doi: 10.12688/f1000research.7506.1
9. Down JL. Observations on an ethnic classification of idiots. Ment Retard . (1995) 33:54–6.
Google Scholar
10. Hickey F, Hickey E, Summar KL. Medical update for children with Down syndrome for the pediatrician and family practitioner. Adv Pediatr. (2012) 59:137–57. doi: 10.1016/j.yapd.2012.04.006
11. D W. Downs: The History of a Disability. Oxford: Oxford University Press (2011).
12. Lejeune J, Gautier M, Turpin R. Study of somatic chromosomes from 9 mongoloid children. C R Hebd Seances Acad Sci. (1959) 248:1721–2.
PubMed Abstract | Google Scholar
13. Patterson D, Costa AC. Down syndrome and genetics - a case of linked histories. Nat Rev Genet. (2005) 6:137–47. doi: 10.1038/nrg1525
14. Lukowski AF, Milojevich HM, Eales L. Cognitive functioning in children with down syndrome: current knowledge and future directions. Adv Child Dev Behav. (2019) 56:257–89. doi: 10.1016/bs.acdb.2019.01.002
15. Daunhauer LA, Fidler DJ. The down syndrome behavioral phenotype: implications for practice and research in occupational therapy. Occup Ther Health Care. (2011) 25:7–25. doi: 10.3109/07380577.2010.535601
16. Jarrold C, Baddeley AD. Short-term memory for verbal and visuospatial information in down's syndrome. Cogn Neuropsychiatry. (1997) 2:101–22. doi: 10.1080/135468097396351
17. Jarrold C, Baddeley AD, Phillips C. Down syndrome and the phonological loop: the evidence for, and importance of, a specific verbal short-term memory deficit. Downs Syndr Res Pract. (1999) 6:61–75. doi: 10.3104/reviews.97
18. Grieco J, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: cognitive and behavioral functioning across the lifespan. Am J Med Genet C Semin Med Genet. (2015) 169:135–49. doi: 10.1002/ajmg.c.31439
19. Desai SS. Down syndrome: a review of the literature. Oral Surg Oral Med Oral. (1997) 84:279–85. doi: 10.1016/S1079-2104(97)90343-7
20. Hodapp RM. Studying interactions, reactions, and perceptions: can genetic disorders serve as behavioral proxies? J Autism Dev Disord. (2004) 34:29–34. doi: 10.1023/B:JADD.0000018071.02942.00
21. van Gameren-Oosterom HB, Fekkes M, Buitendijk SE, Mohangoo AD, Bruil J, Van Wouwe JP. Development, problem behavior, and quality of life in a population based sample of eight-year-old children with Down syndrome. PLoS ONE. (2011) 6:e21879. doi: 10.1371/journal.pone.0021879
22. Locatelli C, Onnivello S, Antonaros F, Feliciello A, Filoni S, Rossi S, et al. Is the age of developmental milestones a predictor for future development in down syndrome? Brain Sci. (2021) 11:655. doi: 10.3390/brainsci11050655
23. Channell MM, Mattie LJ, Hamilton DR, Capone GT, Mahone EM, Sherman SL, et al. Capturing cognitive and behavioral variability among individuals with Down syndrome: a latent profile analysis. J Neurodev Disord. (2021) 13:16. doi: 10.1186/s11689-021-09365-2
24. Edgin JO. Cognition in Down syndrome: a developmental cognitive neuroscience perspective. Wiley Interdiscip Rev Cogn Sci. (2013) 4:307–17. doi: 10.1002/wcs.1221
25. Guralnick MJ. Involvement with peers: comparisons between young children with and without Down's syndrome. J Intellect Disabil Res. (2002) 46(Pt5):379–93. doi: 10.1046/j.1365-2788.2002.00405.x
26. Thomas MSC, Ojinaga Alfageme O, D'Souza H, Patkee PA, Rutherford MA, Mok KY, et al. A multi-level developmental approach to exploring individual differences in Down syndrome: genes, brain, behaviour, and environment. Res Dev Disabil. (2020) 104:103638. doi: 10.1016/j.ridd.2020.103638
27. Zampini L, D'Odorico L. Communicative gestures and vocabulary development in 36-month-old children with Down's syndrome. Int J Lang Commun Disord. (2009) 44:1063–73. doi: 10.1080/13682820802398288
28. Deckers SRJM, Van Zaalen Y, Van Balkom H, Verhoeven L. Predictors of receptive and expressive vocabulary development in children with Down syndrome. Int J Speech Lang Pathol. (2019) 21:10–22. doi: 10.1080/17549507.2017.1363290
29. Özçalişkan S, Adamson LB, Dimitrova N, Baumann S. Early gesture provides a helping hand to spoken vocabulary development for children with autism, Down syndrome and typical development. J Cogn Dev. (2017) 18:325–37. doi: 10.1080/15248372.2017.1329735
30. Kaat-van den Os D, Volman C, Jongmans M, Lauteslager P. Expressive vocabulary development in children with down syndrome: a longitudinal study. J Policy Pract Intellect Disabil. (2016) 14:311–8. doi: 10.1111/jppi.12212
CrossRef Full Text | Google Scholar
31. Vicari S. Implicit versus explicit memory function in children with Down and Williams syndrome. Downs Syndr Res Pract. (2001) 7:35–40. doi: 10.3104/reports.112
32. D'Souza D, Booth R, Connolly M, Happé F, Karmiloff-Smith A. Rethinking the concepts of 'local or global processors': evidence from Williams syndrome, Down syndrome, and Autism Spectrum Disorders. Dev Sci. (2016) 19:452–68. doi: 10.1111/desc.12312
33. Tungate AS, Conners FA. Executive function in Down syndrome: a meta-analysis. Res Dev Disabil. (2021) 108:103802. doi: 10.1016/j.ridd.2020.103802
34. Loveall SJ, Conners FA, Tungate AS, Hahn LJ, Osso TD. A cross-sectional analysis of executive function in Down syndrome from 2 to 35 years. J Intellect Disabil Res. (2017) 61:877–87. doi: 10.1111/jir.12396
35. Lee NR, Anand P, Will E, Adeyemi EI, Clasen LS, Blumenthal JD, et al. Everyday executive functions in Down syndrome from early childhood to young adulthood: evidence for both unique and shared characteristics compared to youth with sex chromosome trisomy (XXX and XXY). Front Behav Neurosci. (2015) 9:264. doi: 10.3389/fnbeh.2015.00264
36. Grealish KG, Price AM, Stein DS. Systematic review of recent pediatric down syndrome neuropsychology literature: considerations for regression assessment and monitoring. J Dev Behav Pediatr. (2020) 41:486–95. doi: 10.1097/DBP.0000000000000800
37. D'Souza D, D'Souza H, Johnson MH, Gliga T, Kushnerenko E, Scerif G, et al. Are early neurophysiological markers of ASD syndrome-specific? A cross-syndrome comparison. In: Paper presented at the XIX Biennial International Conference on Infant Studies. Berlin: International Society on Infant Studies (2014).
38. de Weger C, Boonstra FN, Goossens J. Differences between children with Down syndrome and typically developing children in adaptive behaviour, executive functions and visual acuity. Sci Rep. (2021) 11:7602. doi: 10.1038/s41598-021-85037-4
39. Fidler DJ. The emerging Down syndrome behavioral phenotype in early childhood. Infants Young Child. (2005) 18:86–103. doi: 10.1097/00001163-200504000-00003
40. Porter MA, Coltheart M, Langdon R. The neuropsychological basis of hypersociability in Williams and Down syndrome. Neuropsychologia. (2007) 45:2839–49. doi: 10.1016/j.neuropsychologia.2007.05.006
41. Glennon JM, Karmiloff-Smith A, Thomas MSC. Syndromic autism: progressing beyond current levels of description. Rev J Autism Dev Disord. (2017) 4:321–7. doi: 10.1007/s40489-017-0116-2
42. Dykens EM. Psychiatric and behavioral disorders in persons with Down syndrome. Ment Retard Dev Disabil Res Rev. (2007) 13:272–8. doi: 10.1002/mrdd.20159
43. Coe DA, Matson JL, Russell DW, Slifer KJ, Capone GT, Baglio C, et al. Behavior problems of children with Down syndrome and life events. J Autism Dev Disord. (1999) 29:149–56. doi: 10.1023/A:1023044711293
44. Roizen NJ, Patterson D. Down's syndrome. Lancet. (2003) 361:1281–9. doi: 10.1016/S0140-6736(03)12987-X
45. van Gameren-Oosterom HB, Fekkes M, van Wouwe JP, Detmar SB, Oudesluys-Murphy AM, Verkerk PH. Problem behavior of individuals with Down syndrome in a nationwide cohort assessed in late adolescence. J Pediatr. (2013) 163:1396–401. doi: 10.1016/j.jpeds.2013.06.054
46. Dykens EM, Shah B, Sagun J, Beck T, King BH. Maladaptive behaviour in children and adolescents with Down's syndrome. J Intellect Disabil Res. (2002) 46(Pt6):484–92. doi: 10.1046/j.1365-2788.2002.00431.x
47. Capone GT, Grados MA, Kaufmann WE, Bernad-Ripoll S, Jewell A. Down syndrome and comorbid autism-spectrum disorder: characterization using the aberrant behavior checklist. Am J Med Genet A. (2005) 134:373–80. doi: 10.1002/ajmg.a.30622
48. Carter JC, Capone GT, Gray RM, Cox CS, Kaufmann WE. Autistic-spectrum disorders in Down syndrome: further delineation and distinction from other behavioral abnormalities. Am J Med Genet B Neuropsychiatr Genet. (2007) 144B:87–94. doi: 10.1002/ajmg.b.30407
49. Ji NY, Capone GT, Kaufmann WE. Autism spectrum disorder in Down syndrome: cluster analysis of Aberrant Behaviour Checklist data supports diagnosis. J Intellect Disabil Res. (2011) 55:1064–77. doi: 10.1111/j.1365-2788.2011.01465.x
50. Siegel MS, Smith WE. Psychiatric features in children with genetic syndromes: toward functional phenotypes. Pediatr Clin North Am. (2011) 58:833–64. doi: 10.1016/j.pcl.2011.06.010
51. Capone G, Goyal P, Ares W, Lannigan E. Neurobehavioral disorders in children, adolescents, and young adults with Down syndrome. Am J Med Genet C Semin Med Genet. (2006) 142C:158–72. doi: 10.1002/ajmg.c.30097
52. Roch M, Pesciarelli F, Leo I. How individuals with down syndrome process faces and words conveying emotions? Evidence From a Priming Paradigm. Front Psychol. (2020) 11:692. doi: 10.3389/fpsyg.2020.00692
53. Pitcairn TK, Wishart JG. Reactions of young children with Down's syndrome to an impossible task. Br J Dev Psychol. (1994) 12:485–9. doi: 10.1111/j.2044-835X.1994.tb00649
54. Turk J, Cornish K. Face recognition and emotion perception in boys with fragile-X syndrome. J Intellect Disabil Res. (1998) 42:490–9. doi: 10.1046/j.1365-2788.1998.4260490.x
55. Celani G, Battacchi MW, Arcidiacono L. The understanding of the emotional meaning of facial expressions in people with autism. J Autism Dev Disord. (1999) 29:57–66. doi: 10.1023/A:1025970600181
56. Goldman KJ, Shulman C, Bar-Haim Y, Abend R, Burack JA. Attention allocation to facial expressions of emotion among persons with Williams and Down syndromes. Dev Psychopathol. (2017) 29:1189–97. doi: 10.1017/S0954579416001231
57. Martínez-Castilla P, Burt M, Borgatti R, Gagliardi C. Facial emotion recognition in Williams syndrome and Down syndrome: a matching and developmental study. Child Neuropsychol. (2015) 21:668–92. doi: 10.1080/09297049.2014.945408
58. Wishart JG, Cebula KR, Willis DS, Pitcairn TK. Understanding of facial expressions of emotion by children with intellectual disabilities of differing aetiology. J Intellect Disabil Res. (2007) 51(Pt7):551–63. doi: 10.1111/j.1365-2788.2006.00947.x
59. Hippolyte L, Barisnikov K, Van der Linden M. Face processing and facial emotion recognition in adults with Down syndrome. Am J Ment Retard. (2008) 113:292–306. doi: 10.1352/0895-8017(2008)113[292:FPAFER]2.0.CO;2
60. Kasari C, Freeman SF, Hughes MA. Emotion recognition by children with Down syndrome. Am J Ment Retard. (2001) 106:59–72. doi: 10.1352/0895-8017(2001)106<0059:ERBCWD>2.0.CO;2
61. Wishart JG, Pitcairn TK. Recognition of identity and expression in faces by children with Down syndrome. Am J Ment Retard. (2000) 105:466–79. doi: 10.1352/0895-8017(2000)105 <0466:ROIAEI>2.0.CO;2
62. Murphy C, Jinich S. Olfactory dysfunction in Down's Syndrome. Neurobiol Aging. (1996) 17:631–7. doi: 10.1016/0197-4580(96)00008-5
63. Hemdal P, Corwin J, Oster H. Olfactory identification deficits in Down's syndrome and idiopathic mental retardation. Neuropsychologia. (1993) 31:977–84. doi: 10.1016/0028-3932(93)90152-P
64. Chen MA, Lander TR, Murphy C. Nasal health in Down syndrome: a cross-sectional study. Otolaryngol Head Neck Surg. (2006) 134:741–5. doi: 10.1016/j.otohns.2005.12.035
65. Warner MD, Peabody CA, Berger PA. Olfactory deficits and Down's syndrome. Biol Psychiatry. (1988) 23:836–9. doi: 10.1016/0006-3223(88)90073-X
66. Zucco GM, Negrin NS. Olfactory deficits in Down subjects: a link with Alzheimer disease. Percept Mot Skills. (1994) 78:627–31. doi: 10.2466/pms.1994.78.2.627
67. McKeown DA, Doty RL, Perl DP, Frye RE, Simms I, Mester A. Olfactory function in young adolescents with Down's syndrome. J Neurol Neurosurg Psychiatry. (1996) 61:412–4. doi: 10.1136/jnnp.61.4.412
68. Wetter S, Murphy C. Individuals with Down's syndrome demonstrate abnormal olfactory event-related potentials. Clin Neurophysiol. (1999) 110:1563–9. doi: 10.1016/S1388-2457(99)00086-3
69. Sliger M, Lander T, Murphy C. Effects of the ApoE epsilon4 allele on olfactory function in Down syndrome. J Alzheimers Dis. (2004) 6:397–402; discussion 43–9. doi: 10.3233/JAD-2004-6407
70. Brousseau K, Brainerd MG. A Study of the Physical and Mental Characteristics of Mongoloid Imbeciles. Baltimore: Williams and Wilkins (1928). doi: 10.1097/00000441-192808000-00020
71. Cecchini MP, Viviani D, Sandri M, Hähner A, Hummel T, Zancanaro C. Olfaction in people with down syndrome: a comprehensive assessment across four decades of age. PLoS ONE. (2016) 11:e0146486. doi: 10.1371/journal.pone.0146486
72. Nijjar RK, Murphy C. Olfactory impairment increases as a function of age in persons with Down syndrome. Neurobiol Aging. (2002) 23:65–73. doi: 10.1016/S0197-4580(01)00263-9
73. Zigman WB, Lott IT. Alzheimer's disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev. (2007) 13:237–46. doi: 10.1002/mrdd.20163
74. Head E, Silverman W, Patterson D, Lott IT. Aging and down syndrome. Curr Gerontol Geriatr Res. (2012) 2012:412536. doi: 10.1155/2012/412536
75. Head E, Powell D, Gold BT, Schmitt FA. Alzheimer's disease in down syndrome. Eur J Neurodegener Dis. (2012) 1:353–64.
76. Karmiloff-Smith A. What Can Studying Babies With Down Syndrome Possibly Tell Us About Alzheimer's Disease in Adults? UCSD Dart Neuroscience Seminar. Retrieved from: https://tdlcucsdedu/research/DNS/videos/Karmiloff-Smithmp4 (accessed August 28, 2019).
77. Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. (1998) 77:431–8. doi: 10.1002/(SICI)1096-8628(19980605)77:5<431::AID-AJMG15>3.0.CO;2-J
78. Freeman SB, Bean LH, Allen EG, Tinker SW, Locke AE, Druschel C, et al. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project. Genet Med. (2008) 10:173–80. doi: 10.1097/GIM.0b013e3181634867
79. Visootsak J, Mahle WT, Kirshbom PM, Huddleston L, Caron-Besch M, Ransom A, et al. Neurodevelopmental outcomes in children with Down syndrome and congenital heart defects. Am J Med Genet A. (2011) 155A:2688–91. doi: 10.1002/ajmg.a.34252
80. Visootsak J, Huddleston L, Buterbaugh A, Perkins A, Sherman S, Hunter J. Influence of CHDs on psycho-social and neurodevelopmental outcomes in children with Down syndrome. Cardiol Young. (2016) 26:250–6. doi: 10.1017/S1047951115000062
81. Startin CM, D'Souza H, Ball G, Hamburg S, Hithersay R, Hughes KMO, et al. Health comorbidities and cognitive abilities across the lifespan in Down syndrome. J Neurodev Disord. (2020) 12:4. doi: 10.1186/s11689-019-9306-9
82. Alsaied T, Marino BS, Esbensen AJ, Anixt JS, Epstein JN, Cnota JF. Does congenital heart disease affect neurodevelopmental outcomes in children with down syndrome? Congenit Heart Dis. (2016) 11:26–33. doi: 10.1111/chd.12322
83. D'Souza H, Lathan A, Karmiloff-Smith A, Mareschal D. Down syndrome and parental depression: A double hit on early expressive language development. Res Dev Disabil. (2020) 100:103613. doi: 10.1016/j.ridd.2020.103613
84. Cebula KR, Moore DG, Wishart JG. Social cognition in children with Down's syndrome: challenges to research and theory building. J Intellect Disabil Res. (2010) 54:113–34. doi: 10.1111/j.1365-2788.2009.01215.x
85. Moore DG, Oates JM, Hobson RP, Goodwin J. Cognitive and social factors in the development of infants with Down syndrome. Downs Syndr Res Pract. (2002) 8:43–52. doi: 10.3104/reviews.129
86. Karmiloff-Smith A, Grant J, Berthoud I, Davies M, Howlin P, Udwin O. Language and Williams syndrome: how intact is “intact”? Child Dev. (1997) 68:246–62. doi: 10.1111/j.1467-8624.1997.tb01938.x
87. Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends Cogn Sci. (1998) 2:389–98. doi: 10.1016/S1364-6613(98)01230-3
88. Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. (2019) 15:135–47. doi: 10.1038/s41582-018-0132-6
89. Hithersay R, Startin CM, Hamburg S, Mok KY, Hardy J, Fisher EMC, et al. Association of Dementia With Mortality Among Adults With Down Syndrome Older Than 35 Years. JAMA Neurol. (2019) 76:152–60. doi: 10.1001/jamaneurol.2018.3616
Keywords: Down syndrome, trisomy 21, developmental outcome, phenotypic heterogeneity, Alzheimer's disease, medical comorbidities, social environment, prenatal counseling
Citation: Windsperger K and Hoehl S (2021) Development of Down Syndrome Research Over the Last Decades–What Healthcare and Education Professionals Need to Know. Front. Psychiatry 12:749046. doi: 10.3389/fpsyt.2021.749046
Received: 28 July 2021; Accepted: 22 November 2021; Published: 14 December 2021.
Reviewed by:
Copyright © 2021 Windsperger and Hoehl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefanie Hoehl, stefanie.hoehl@univie.ac.at
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Mental Development in Children With Down Syndrome Research Paper
Down syndrome is a chromosomal disorder resulting from the existence of an extra copy chromosome 21. The condition got its name from John Land Down; the doctor who first described it. Down syndrome is associated with symptoms that impair cognitive ability, physical development and often alter facial appearance.
Down syndrome patients are also prone to various health complications including heart disease, hearing problems, dementia, gastroesophageal reflux disease, recurrent ear infections, obstructive sleep apnea and complications with their intestines, eyes, skeleton, and thyroid. Research has shown that the odds of having a baby with Down syndrome grow as the woman ages.
People with Down syndrome have largely varying levels of mental developmental disability. A few of these individuals have notable to extreme mental disability while others show little or no mental problem symptoms. Downs syndrome occurrence is estimated at about 1 in 800-1000 births.
A number of factors affect this statistic but the most profound influencing factor has been found to be the age of the mother. It is not unusual for people with the proper set of chromosomes to share some physical features associated with Down syndrome. Some of these shared features may include an unusually small chin, an unusually round face, a large protruding tongue, Simian crease across palms, uneven toe spacing and poorly toned muscles (Kumin, 113).
The health and overall development of children with Down syndrome can be greatly improved by early intervention, regular screening for any complications, vocational training, and the existence of a caring and supportive social environment. The physical implications of Down syndrome caused by the chromosomal disorders can however not be overcome. Ironically, Down syndrome has some positive health implications; Down syndrome patients have been observed to have greatly reduced incidences of cancer.
Mental development in children with Down syndrome varies greatly and at birth, it is not possible to predict the extent to which the child will be affected in terms of physical symptoms and cognitive development. Intervention methods for these children are normally unique depending on the individual and are developed soon after birth to ensure that the child gets the best chance at leading a normal life (Dykens, 250).
Speech delay is common among individuals with Down syndrome and the individuals need to be taken through speech therapy to help them develop speech. Walking in children could also be impaired by Down syndrome. Some children will not walk up to age 4, while others are able to walk at age 2.
Language learning can be enhanced by screening for ear problems and hearing loss, employing hearing aids (as necessary) and fostering timely communication intervention. The use of augmentative and alternative communication methods is common to aid in communication. Some of these methods include body language, pointing, signs, objects, and specially designed graphics.
Down syndrome does not have a cure or standard management program due to the diversity in its manifestation. Some individuals may need intensive surgery and therapy while others have minimal health complications and can lead normal lives without the need for any therapy. Parents of children with Down syndrome have come together to try and find alternative therapies to improve mental growth and physical appearance. Suggested methods are plastic surgery and nutritional supplements (Roizen, 150).
Ethically, there have been concerns about the number of abortions associated with Down syndrome. In the year 2002, 91-92% of pregnancies in the US diagnosed with Down syndrome were terminated. In the UK, the figure remains relatively constant at about 92%.
Strides have been made to ensure that individuals with Down syndrome are accepted more in society to facilitate their leading normal lives. Parents, teachers and other stakeholders have in recent years advocated the inclusion of these individuals in society rather than exclude them in isolated institutions as was the case before.
Works Cited
Dykens, Elisabeth M. “Psychiatric and behavioral disorders in persons with Down syndrome .” Mental Retardation and Developmental Disabilities Research Reviews 13(2007):272-278
Kumin, Libby.”Speech and language skills in children with Down syndrome.”Mental Retardation and Developmental Disabilities Research Reviews 2(1996):109-115
Roizen, Nancy J. “Complementary and alternative therapies for Down syndrome.”Mental Retardation and Developmental Disabilities Research Reviews 11(2005):149-155.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2020, April 10). Mental Development in Children With Down Syndrome. https://ivypanda.com/essays/down-syndrome-research-paper/
"Mental Development in Children With Down Syndrome." IvyPanda , 10 Apr. 2020, ivypanda.com/essays/down-syndrome-research-paper/.
IvyPanda . (2020) 'Mental Development in Children With Down Syndrome'. 10 April.
IvyPanda . 2020. "Mental Development in Children With Down Syndrome." April 10, 2020. https://ivypanda.com/essays/down-syndrome-research-paper/.
1. IvyPanda . "Mental Development in Children With Down Syndrome." April 10, 2020. https://ivypanda.com/essays/down-syndrome-research-paper/.
Bibliography
IvyPanda . "Mental Development in Children With Down Syndrome." April 10, 2020. https://ivypanda.com/essays/down-syndrome-research-paper/.
- Childhood Ear Infection and Determinants of Health
- Trisomy 21: Characteristics of the Syndrome
- Acute Lymphoblastic Leukemia: Causes, Origin, and Gene Mutation
- Suicidal Behavior: Triggers and Solutions
- Treatment, Symptoms, and Prevention of Bipolar Disorder
- Medical Procedures of Psychiatric Diagnosis
- Various Anxiety Disorders' Comparison
- Psychiatric Issues: Schizophrenia's Demystify
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 06 May 2024
APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease
- Juan Fortea ORCID: orcid.org/0000-0002-1340-638X 1 , 2 , 3 na1 ,
- Jordi Pegueroles ORCID: orcid.org/0000-0002-3554-2446 1 , 2 ,
- Daniel Alcolea ORCID: orcid.org/0000-0002-3819-3245 1 , 2 ,
- Olivia Belbin ORCID: orcid.org/0000-0002-6109-6371 1 , 2 ,
- Oriol Dols-Icardo ORCID: orcid.org/0000-0003-2656-8748 1 , 2 ,
- Lídia Vaqué-Alcázar 1 , 4 ,
- Laura Videla ORCID: orcid.org/0000-0002-9748-8465 1 , 2 , 3 ,
- Juan Domingo Gispert 5 , 6 , 7 , 8 , 9 ,
- Marc Suárez-Calvet ORCID: orcid.org/0000-0002-2993-569X 5 , 6 , 7 , 8 , 9 ,
- Sterling C. Johnson ORCID: orcid.org/0000-0002-8501-545X 10 ,
- Reisa Sperling ORCID: orcid.org/0000-0003-1535-6133 11 ,
- Alexandre Bejanin ORCID: orcid.org/0000-0002-9958-0951 1 , 2 ,
- Alberto Lleó ORCID: orcid.org/0000-0002-2568-5478 1 , 2 &
- Víctor Montal ORCID: orcid.org/0000-0002-5714-9282 1 , 2 , 12 na1
Nature Medicine ( 2024 ) Cite this article
11k Accesses
4199 Altmetric
Metrics details
- Alzheimer's disease
- Predictive markers
This study aimed to evaluate the impact of APOE4 homozygosity on Alzheimer’s disease (AD) by examining its clinical, pathological and biomarker changes to see whether APOE4 homozygotes constitute a distinct, genetically determined form of AD. Data from the National Alzheimer’s Coordinating Center and five large cohorts with AD biomarkers were analyzed. The analysis included 3,297 individuals for the pathological study and 10,039 for the clinical study. Findings revealed that almost all APOE4 homozygotes exhibited AD pathology and had significantly higher levels of AD biomarkers from age 55 compared to APOE3 homozygotes. By age 65, nearly all had abnormal amyloid levels in cerebrospinal fluid, and 75% had positive amyloid scans, with the prevalence of these markers increasing with age, indicating near-full penetrance of AD biology in APOE4 homozygotes. The age of symptom onset was earlier in APOE4 homozygotes at 65.1, with a narrower 95% prediction interval than APOE3 homozygotes. The predictability of symptom onset and the sequence of biomarker changes in APOE4 homozygotes mirrored those in autosomal dominant AD and Down syndrome. However, in the dementia stage, there were no differences in amyloid or tau positron emission tomography across haplotypes, despite earlier clinical and biomarker changes. The study concludes that APOE4 homozygotes represent a genetic form of AD, suggesting the need for individualized prevention strategies, clinical trials and treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study
Association of APOE e2 genotype with Alzheimer’s and non-Alzheimer’s neurodegenerative pathologies
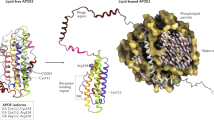
Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies
Data availability.
Access to tabular data from ADNI ( https://adni.loni.usc.edu/ ), OASIS ( https://oasis-brains.org/ ), A4 ( https://ida.loni.usc.edu/collaboration/access/appLicense.jsp ) and NACC ( https://naccdata.org/ ) can be requested online, as publicly available databases. All requests will be reviewed by each studyʼs scientific board. Concrete inquiries to access the WRAP ( https://wrap.wisc.edu/data-requests-2/ ) and ALFA + ( https://www.barcelonabeta.org/en/alfa-study/about-the-alfa-study ) cohort data can be directed to each study team for concept approval and feasibility consultation. Requests will be reviewed to verify whether the request is subject to any intellectual property.
Code availability
All statistical analyses and raw figures were generated using R (v.4.2.2). We used the open-sourced R packages of ggplot2 (v.3.4.3), dplyr (v.1.1.3), ggstream (v.0.1.0), ggpubr (v.0.6), ggstatsplot (v.0.12), Rmisc (v.1.5.1), survival (v.3.5), survminer (v.0.4.9), gtsummary (v.1.7), epitools (v.0.5) and statsExpression (v.1.5.1). Rscripts to replicate our findings can be found at https://gitlab.com/vmontalb/apoe4-asdad (ref. 32 ). For neuroimaging analyses, we used Free Surfer (v.6.0) and ANTs (v.2.4.0).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54 , 412–436 (2022).
Article CAS PubMed PubMed Central Google Scholar
Frisoni, G. B. et al. The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat. Rev. Neurosci. 23 , 53–66 (2022).
Article CAS PubMed Google Scholar
Bateman R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367 , 795–804 (2012).
Genin, E. et al. APOE and Alzheimer disease: a major gene with semidominant inheritance. Mol. Psychiatry 16 , 903–907 (2011).
Fortea, J. et al. Alzheimer’s disease associated with Down syndrome: a genetic form of dementia. Lancet Neurol. 20 , 930–942 (2021).
Fortea, J. et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet 395 , 1988–1997 (2020).
Jansen, W. J. et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313 , 1924–1938 (2015).
Article PubMed PubMed Central Google Scholar
Saddiki H. et al. Age and the association between apolipoprotein E genotype and Alzheimer disease: a cerebrospinal fluid biomarker-based case-control study. PLoS Med. https://doi.org/10.1371/JOURNAL.PMED.1003289 (2020).
Jack, C. R. et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 14 , 535–562 (2018).
Article Google Scholar
Beekly, D. L. et al. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis. Assoc. Disord. 18 , 270–277 (2004).
PubMed Google Scholar
Montine, T. J. et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 123 , 1–11 (2012).
Reiman, E. M. et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat. Commun. 11 , 1–11 (2020).
Iulita M. F. et al. Association of Alzheimer disease with life expectancy in people with Down syndrome. JAMA Netw. Open https://doi.org/10.1001/JAMANETWORKOPEN.2022.12910 (2022).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261 , 921–923 (1993).
Fortea, J., Quiroz, Y. T. & Ryan, N. S. Lessons from Down syndrome and autosomal dominant Alzheimer’s disease. Lancet Neurol. 22 , 5–6 (2023).
Therriault, J. et al. Frequency of biologically defined Alzheimer’s disease in relation to age, sex, APOE ε4, and cognitive impairment. Neurology 96 , e975–e985 (2021).
Betthauser, T. J. et al. Multi-method investigation of factors influencing amyloid onset and impairment in three cohorts. Brain 145 , 4065–4079 (2022).
Snellman, A. et al. APOE ε4 gene dose effect on imaging and blood biomarkers of neuroinflammation and beta-amyloid in cognitively unimpaired elderly. Alzheimers Res. Ther. 15 , 71 (2023).
Ghisays, V. et al. Brain imaging measurements of fibrillar amyloid-β burden, paired helical filament tau burden, and atrophy in cognitively unimpaired persons with two, one, and no copies of the APOE ε4 allele. Alzheimers Dement. 16 , 598–609 (2020).
Mehta, R. I. & Schneider, J. A. What is ‘Alzheimer’s disease’? The neuropathological heterogeneity of clinically defined Alzheimer’s dementia. Curr. Opin. Neurol. 34 , 237–245 (2021).
van der Lee, S. J. et al. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 17 , 434–444 (2018).
Belloy, M. E., Napolioni, V. & Greicius, M. D. A quarter century of APOE and Alzheimera’s disease: progress to date and the path forward. Neuron 101 , 820–838 (2019).
Belloy, M. E. et al. APOE genotype and Alzheimer disease risk across age, sex, and population ancestry. JAMA Neurol. 80 , 1284–1294 (2023).
Jack, C. R. et al. Long-term associations between amyloid positron emission tomography, sex, apolipoprotein E and incident dementia and mortality among individuals without dementia: hazard ratios and absolute risk. Brain Commun. 4 , fcac017 (2022).
Morris, J. C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43 , 2412–2414 (1993).
Weiner, M. W. et al. The Alzheimer’s Disease Neuroimaging Initiative 3: continued innovation for clinical trial improvement. Alzheimer’s Dement. 13 , 561–571 (2017).
Sperling R. A. et al. The A4 Study: stopping AD before symptoms begin? Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.3007941 (2014).
Molinuevo, J. L. et al. The ALFA project: a research platform to identify early pathophysiological features of Alzheimer’s disease. Alzheimer’s Dement.: Transl. Res. Clin. Interventions 2 , 82–92 (2016).
Johnson, S. C. et al. The Wisconsin Registry for Alzheimer’s Prevention: a review of findings and current directions. Alzheimer’s Dement.: Diagnosis, Assess. Dis. Monit. 10 , 130–142 (2018).
Google Scholar
LaMontagne P. J. et al. OASIS-3: longitudinal neuroimaging, clinical and cognitive dataset for normal aging and Alzheimer disease. Preprint at MedRxiv https://doi.org/10.1101/2019.12.13.19014902 (2019).
La Joie, R. et al. Multisite study of the relationships between antemortem [ 11 C]PIB-PET Centiloid values and postmortem measures of Alzheimer’s disease neuropathology. Alzheimers Dement. 15 , 205–216 (2019).
Montal, V. APOE4-ASDAD. GitLab https://gitlab.com/vmontalb/apoe4-asdad (2024).
Download references
Acknowledgements
We acknowledge the contributions of several consortia that provided data for this study. We extend our appreciation to the NACC, the Alzheimer’s Disease Neuroimaging Initiative, The A4 Study, the ALFA Study, the Wisconsin Register for Alzheimer’s Prevention and the OASIS3 Project. Without their dedication to advancing Alzheimer’s disease research and their commitment to data sharing, this study would not have been possible. We also thank all the participants and investigators involved in these consortia for their tireless efforts and invaluable contributions to the field. We also thank the institutions that funded this study, the Fondo de Investigaciones Sanitario, Carlos III Health Institute, the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas and the Generalitat de Catalunya and La Caixa Foundation, as well as the NIH, Horizon 2020 and the Alzheimer’s Association, which was crucial for this research. Funding: National Institute on Aging. This study was supported by the Fondo de Investigaciones Sanitario, Carlos III Health Institute (INT21/00073, PI20/01473 and PI23/01786 to J.F., CP20/00038, PI22/00307 to A.B., PI22/00456 to M.S.-C., PI18/00435 to D.A., PI20/01330 to A.L.) and the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas Program 1, partly jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, Una Manera de Hacer Europa. This work was also supported by the National Institutes of Health grants (R01 AG056850; R21 AG056974, R01 AG061566, R01 AG081394 and R61AG066543 to J.F., S10 OD025245, P30 AG062715, U54 HD090256, UL1 TR002373, P01 AG036694 and P50 AG005134 to R.S.; R01 AG027161, R01 AG021155, R01 AG037639, R01 AG054059; P50 AG033514 and P30 AG062715 to S.J.) and ADNI (U01 AG024904), the Department de Salut de la Generalitat de Catalunya, Pla Estratègic de Recerca I Innovació en Salut (SLT006/17/00119 to J.F.; SLT002/16/00408 to A.L.) and the A4 Study (R01 AG063689, U24 AG057437 to R.A.S). It was also supported by Fundación Tatiana Pérez de Guzmán el Bueno (IIBSP-DOW-2020-151 o J.F.) and Horizon 2020–Research and Innovation Framework Programme from the European Union (H2020-SC1-BHC-2018-2020 to J.F.; 948677 and 847648 to M.S.-C.). La Caixa Foundation (LCF/PR/GN17/50300004 to M.S.-C.) and EIT Digital (Grant 2021 to J.D.G.) also supported this work. The Alzheimer Association also participated in the funding of this work (AARG-22-923680 to A.B.) and A4/LEARN Study AA15-338729 to R.A.S.). O.D.-I. receives funding from the Alzheimer’s Association (AARF-22-924456) and the Jerome Lejeune Foundation postdoctoral fellowship.
Author information
These authors contributed equally: Juan Fortea, Víctor Montal.
Authors and Affiliations
Sant Pau Memory Unit, Hospital de la Santa Creu i Sant Pau - Biomedical Research Institute Sant Pau, Barcelona, Spain
Juan Fortea, Jordi Pegueroles, Daniel Alcolea, Olivia Belbin, Oriol Dols-Icardo, Lídia Vaqué-Alcázar, Laura Videla, Alexandre Bejanin, Alberto Lleó & Víctor Montal
Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas. CIBERNED, Barcelona, Spain
Juan Fortea, Jordi Pegueroles, Daniel Alcolea, Olivia Belbin, Oriol Dols-Icardo, Laura Videla, Alexandre Bejanin, Alberto Lleó & Víctor Montal
Barcelona Down Medical Center, Fundació Catalana Síndrome de Down, Barcelona, Spain
Juan Fortea & Laura Videla
Department of Medicine, Faculty of Medicine and Health Sciences, Institute of Neurosciences, University of Barcelona, Barcelona, Spain
Lídia Vaqué-Alcázar
Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain
Juan Domingo Gispert & Marc Suárez-Calvet
Neurosciences Programme, IMIM - Hospital del Mar Medical Research Institute, Barcelona, Spain
Department of Medicine and Life Sciences, Universitat Pompeu Fabra, Barcelona, Spain
Centro de Investigación Biomédica en Red Bioingeniería, Biomateriales y Nanomedicina. Instituto de Salud carlos III, Madrid, Spain
Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain
Wisconsin Alzheimer’s Disease Research Center, University of Wisconsin-Madison School of Medicine and Public Health, Madison, WI, USA
Sterling C. Johnson
Brigham and Women’s Hospital Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Reisa Sperling
Barcelona Supercomputing Center, Barcelona, Spain
Víctor Montal
You can also search for this author in PubMed Google Scholar
Contributions
J.F. and V.M. conceptualized the research project and drafted the initial manuscript. V.M., J.P. and J.F. conducted data analysis, interpreted statistical findings and created visual representations of the data. O.B. and O.D.-I. provided valuable insights into the genetics of APOE. L.V., A.B. and L.V.-A. meticulously reviewed and edited the manuscript for clarity, accuracy and coherence. J.D.G., M.S.-C., S.J. and R.S. played pivotal roles in data acquisition and securing funding. A.L. and D.A. contributed to the study design, offering guidance and feedback on statistical analyses, and provided critical review of the paper. All authors carefully reviewed the manuscript, offering pertinent feedback that enhanced the study’s quality, and ultimately approved the final version.
Corresponding authors
Correspondence to Juan Fortea or Víctor Montal .
Ethics declarations
Competing interests.
S.C.J. has served at scientific advisory boards for ALZPath, Enigma and Roche Diagnostics. M.S.-C. has given lectures in symposia sponsored by Almirall, Eli Lilly, Novo Nordisk, Roche Diagnostics and Roche Farma, received consultancy fees (paid to the institution) from Roche Diagnostics and served on advisory boards of Roche Diagnostics and Grifols. He was granted a project and is a site investigator of a clinical trial (funded to the institution) by Roche Diagnostics. In-kind support for research (to the institution) was received from ADx Neurosciences, Alamar Biosciences, Avid Radiopharmaceuticals, Eli Lilly, Fujirebio, Janssen Research & Development and Roche Diagnostics. J.D.G. has served as consultant for Roche Diagnostics, receives research funding from Hoffmann–La Roche, Roche Diagnostics and GE Healthcare, has given lectures in symposia sponsored by Biogen, Philips Nederlands, Esteve and Life Molecular Imaging and serves on an advisory board for Prothena Biosciences. R.S. has received personal consulting fees from Abbvie, AC Immune, Acumen, Alector, Bristol Myers Squibb, Janssen, Genentech, Ionis and Vaxxinity outside the submitted work. O.B. reported receiving personal fees from Adx NeuroSciences outside the submitted work. D.A. reported receiving personal fees for advisory board services and/or speaker honoraria from Fujirebio-Europe, Roche, Nutricia, Krka Farmacéutica and Esteve, outside the submitted work. A.L. has served as a consultant or on advisory boards for Almirall, Fujirebio-Europe, Grifols, Eisai, Lilly, Novartis, Roche, Biogen and Nutricia, outside the submitted work. J.F. reported receiving personal fees for service on the advisory boards, adjudication committees or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Fujirebio, Ionis, Laboratorios Carnot, Life Molecular Imaging, Lilly, Lundbeck, Perha, Roche and outside the submitted work. O.B., D.A., A.L. and J.F. report holding a patent for markers of synaptopathy in neurodegenerative disease (licensed to Adx, EPI8382175.0). The remaining authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Naoyuki Sato, Yadong Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary Methods, Results, Bibliography, Figs. 1–7 and Tables 1–3.
Reporting Summary
Supplementary code.
This code is also available in the GitLab repository.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Fortea, J., Pegueroles, J., Alcolea, D. et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nat Med (2024). https://doi.org/10.1038/s41591-024-02931-w
Download citation
Received : 03 November 2023
Accepted : 19 March 2024
Published : 06 May 2024
DOI : https://doi.org/10.1038/s41591-024-02931-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Down syndrome.
Faisal Akhtar ; Syed Rizwan A. Bokhari .
Affiliations
Last Update: August 8, 2023 .
- Continuing Education Activity
Down syndrome (trisomy 21) is a genetic disorder caused by the presence of all or a portion of a third chromosome 21. Patients typically present with mild to moderate intellectual disability, growth retardation, and characteristic facial features. This activity reviews the evaluation and management of Down syndrome and explains the role of the interprofessional team in improving care for patients with this condition.
- Describe the etiology of Down syndrome.
- Identify atrial septal defects as the most common cardiac abnormalities in patients with Down syndrome.
- Summarize the use of ultrasound, amniocentesis, and chorionic villus sampling in the prenatal diagnosis of Down syndrome.
- Outline the importance of collaboration and communication among the interprofessional team to enhance the delivery of care and improve outcomes for patients affected by Down syndrome.
- Introduction
Down syndrome was first described by an English physician, John Langdon Down, in 1866, but its association with chromosome 21 was established almost 100 years later by Dr. Jerome Lejeune in Paris. It is the presence of all or part of the third copy of chromosome 21 that causes Down syndrome, the most common chromosomal abnormality occurring in humans. [1] It is also found that the most frequently occurring live-born aneuploidy is trisomy 21, which causes this syndrome. [2]
The majority of patients with Down syndrome have an extra copy of chromosome 21. There are different hypotheses related to the genetic basis of Down syndrome and the association of different genotypes with the phenotypes. Among them is gene dosage imbalance, in which there is an increased dosage or number of genes of Hsa21, which results in increased gene expansion. [3] . It further includes the possibility of association of different genes with different phenotypes of Down syndrome. The other popular hypothesis is the amplified development instability hypothesis, according to which the genetic imbalance created by a number of trisomic genes results in a greater impact on the expression and regulation of many genes. [3]
The critical region hypothesis is also well-known in this list. Down syndrome critical regions (DSCR) are a few chromosomal regions that are associated with partial trisomy for Has21. DSCR on 21q21.22 is responsible for many clinical features of Down syndrome. [3] [4] After a thorough study of different analyses, it became clear that a single critical region gene cannot cause all the phenotypical features associated with trisomy 21, rather it is more evident that multiple critical regions or critical genes have a role to play in this phenomenon. [5]
- Epidemiology
The incidence of Down syndrome increases with maternal age, and its occurrence varies in different populations (1 in 319 to 1 in 1000 live births) [6] [7] . It is also known that the frequency of Down syndrome fetuses is quite high at the time of conception, but about 50% to 75% of these fetuses are lost before term. The occurrence of other autosomal trisomy is much more common than the 21, but the postnatal survival is very poor as compared to Down syndrome. This high percentage of survival of patients with trisomy 21 is thought to be a function of a small number of genes on chromosome 21 called Hsa21, which is the smallest and least dense of the autosomes. [8]
- Pathophysiology
An extra copy of chromosome 21 is associated with Down syndrome, which occurs due to the failure of chromosome 21 to separate during gametogenesis, resulting in an extra chromosome in all the body cells. Robertsonian translocation and isochromosome or ring chromosome are the other 2 possible causes of trisomy 21. Isochromosome is a condition when 2 long arms separate together instead of the long and short arms while in Robertsonian translocation. This occurs in 2% to 4% of the patients. The long arm of chromosome 21 is attached to another chromosome, mostly chromosome 14. In mosaicism, there are 2 different cell lines because of the error of division after fertilization. [6]
- History and Physical
Clinical Features
Different clinical conditions are associated with Down syndrome as different systems are affected by it. These patients have a wide array of signs and symptoms like intellectual and developmental disabilities or neurological features, congenital heart defects, gastrointestinal (GI) abnormalities, characteristic facial features, and abnormalities. [9]
Congenital Cardiac Defects (CHD)
Congenital cardiac defects are by far the most common and leading cause associated with morbidity and mortality in patients with Down syndrome, especially in the first 2 years of life. Though different suggestions have been made about the geographical as well as seasonal variation in the occurrence of different types of congenital cardiac defects in trisomy 21, so far none of the results have been conclusive. [10]
The incidence of CHD in babies born with Down syndrome is up to 50%. The most common cardiac defect associated with Down syndrome is an atrioventricular septal defect (AVSD), and this defect makes up to 40% of the congenital cardiac defects in Down syndrome. [6] It is said to be associated with the mutation of the non-Hsa21 CRELD1 gene [6] [11] The second most common cardiac defect in Down syndrome is a ventricular septal defect (VSD), which is seen in about 32% of the patients with Down syndrome. Together with AVSD, these account for more than 50% of congenital cardiac defects in patients with Down syndrome. [6] [11]
The other cardiac defects associated with trisomy 21 are secundum atrial defect (10%), tetralogy of Fallot (6%), and isolated PDA (4%), while about 30% of the patients have more than one cardiac defect. There is geographical variation in the prevalence of the cardiac defect in Down syndrome, with VSD being the most common in Asia and secundum type ASD in Latin America. The reason behind this difference in the prevalence of different types of CHD in different regions is still unclear, and many factors such as regional proximity have been found to contribute. [6]
Because of such a high prevalence of CHD in patients with Down syndrome, it has been recommended that all patients get an echocardiogram within the first few weeks of life.
Gastrointestinal (GI) Tract Abnormalities
Patients with trisomy 21 have many structural and functional disorders related to the GI tract. Structural defects can occur anywhere from the mouth to anus, and it has been found that certain defects like duodenal and small bowel atresia or stenosis, annular pancreas, imperforate anus, and Hirschsprung disease occur more commonly in these patients as compared to the general population. [1]
About 2% of patients with Down syndrome have Hirschsprung disease while 12% of patients with Hirschsprung disease have Down syndrome. [1] [6] Hirschsprung disease is a form of functional lower intestinal obstruction in which the neural cells fail to migrate to the distal segment of the rectum resulting in an aganglionic segment which does not have normal peristalsis resulting in failure of normal defecation reflex causing a functional obstruction. [12] The infant usually presents with signs and symptoms related to intestinal obstruction. Duodenal atresia and imperforate anus usually present in the neonatal period.
Apart from the structural defects patients with Down syndrome, patients are also prone to many other GI disorders like gastroesophageal reflux (GERD), chronic constipation, intermittent diarrhea, and celiac disease. Since there is a strong association of celiac disease with Down syndrome being present in about 5% of these patients, it is recommended to do yearly screening of celiac disease. Once diagnosed, these patients will have to remain on a gluten-free diet for the rest of life. [13]
Hematologic Disorders
There are several hematological disorders associated with Down syndrome. The hematological abnormalities in a newborn with Down syndrome (HANDS) constitute neutrophilia, thrombocytopenia, and polycythemia, which are seen in 80%, 66% and 34% of Down syndrome babies respectively. [14] [15] [16] HANDS is usually mild and resolves within the first thr3e weeks of life. [14] [15] [16]
The other disorder that is quite specific to Down syndrome is a transient myeloproliferative disorder, which is defined as detection of blast cells in younger than 3 month old babies with Down syndrome. It is characterized by the clonal proliferation of megakaryocytes and is detected during the first week of life and is resolved by 3 months of life. It is also known as transient abnormal myelopoiesis or transient leukemia and is known to be present in about 10% of patients with Down syndrome. If this occurs in the fetus, it can cause spontaneous abortion. [17] [18]
Patients with Down syndrome are 10-times more at risk of developing leukemia, [19] which constitute about 2% of all pediatric acute lymphoblastic leukemia and 10% of all pediatric acute myeloid leukemia. Thirty percent of Down syndrome patients with acute lymphoblastic leukemia have an association with function mutation in Janus Kinase 2 gene. [20]
About 10% of patients with chronic myeloid leukemia (TML) develop leukemogenesis of acute megakaryoblastic leukemia (AMKL) before the age of 4 years. AMKL is associated with GATA1 gene which is an X-linked transcriptor factor leading to an uncontrolled proliferation of immature megakaryocytes. [21]
Neurologic Disorders
Trisomy of Hsa21 has associated with reduced brain volume especially hippocampus and cerebellum. [22] Hypotonia is the hallmark of babies with Down syndrome and is present in almost all of them. It is defined as decreased resistance to passive muscle stretch and is responsible for delayed motor development in these patients. [23] . Because of hypotonia Down syndrome patients have joint laxity that causes decreased gait stability and increased energy requirement for physical exertion. [24] . These patients are prone to decreased bone mass and increased risk of fractures due to the low level of physical activity [25] , while the ligamentous laxity predisposes these patients to atlantoaxial subluxation. [26]
Five percent to 13% of children with Down syndrome have seizures [27] , out of that, 40% will have seizures before their first birthday, and in these cases, the seizures are usually infantile spasms. [28] Down syndrome children with infantile spasm do respond better to antiepileptics as compared to other kids with the same, and therefore, early intervention and treatment improve the developmental outcome. [27]
Lennox-Gestaut syndrome is also seen to be more prevalent in children with Down syndrome when it does occur, has a late onset, and is associated with reflex seizures along with an increased rate of EEG abnormalities. [29]
Forty percent of patients with Down syndrome develop tonic-clonic or myoclonic seizures in their first 3 decades. [28] Dementia occurs more commonly in patients older than 45 years of age with Down syndrome [30] , and about 84% are more prone to develop seizures. [31] The seizures in these patients are related to the rapid decline in their cognitive functions. [32]
The risk of developing early-onset Alzheimer disease is significantly high in patients with Down syndrome with 50% to 70% of patients developing dementia by the age of 60 years. [33] Amyloid precursor protein (APP), which is known to be associated with increased risk for the Alzheimer disease is found to be encoded on Hsa21, and trisomy of this protein is likely to be responsible for increased frequency of dementia in people with Down syndrome. Recent studies have shown that triplication of APP is associated with increased risk of early-onset Alzheimer disease even in the normal population. [34]
Nearly all the patients with Down syndrome have mild to moderate learning disability. Trisomy of multiple genes including DYRK1A, synaptojanin 1, and single-minded homolog 2 (SIM2) have been found to cause learning and memory defects in mice, which suggests the possibility that the overexpression of these genes may likely be causing the learning disability in people with Down syndrome. [35]
Endocrinological Disorders
Thyroid gland dysfunction is most commonly associated with Down syndrome. Hypothyroidism can be congenital or acquired at any time during life. [25] The newborn screening program in New York has reported an increased incidence of congenital hypothyroidism in babies with Down syndrome as compared to the others. [36] The anti-thyroid autoantibodies were found in 13% to 34% of patients with Down syndrome who had acquired hypothyroidism, and the concentration of these antibodies increased after 8 years of life. [25] . About half of the patients with Down syndrome have been shown to have subclinical hypothyroidism with elevated TSH and normal thyroxine levels. [37] Hyperthyroidism is much less frequent in patients with Down syndrome as compared to hypothyroidism, although the rate of it still exceeds the incidence of hyperthyroidism in the general pediatric population. [38]
Abnormalities in sexual development are also noted to be significant with delayed puberty in both genders. In girls, primary hypogonadism presents as delay in menarche or adrenarche, while in boys it can manifest as cryptorchidism, ambiguous genitalia, micropenis, small testes, low sperm count, and scanty growth of axillary and pubic hair. [25]
The insulin-like growth factor is also said to be responsible for the delay in skeletal maturation and short stature in patients with Down syndrome. [25]
Musculoskeletal Disorders
Children with Down syndrome are at an increased risk of reduced muscle mass because of hypotonia increased ligamentous laxity which causes retardation of gross motor skills and can result in joint dislocation. [39] These patients also have vitamin D deficiency due to several factors like inadequate exposure to sunlight, inadequate intake of vitamin D, malabsorption secondary to celiac disease, increased breakdown because of anticonvulsant therapy, among other factors. These factors increase the risk of decreased bone mass in children with Down syndrome and predispose them to recurrent fractures. [40]
Refractive Errors and Visual Abnormalities
Ocular and orbital anomalies are common in children with Down syndrome. These include blepharitis (2-7%), keratoconus (5-8%), cataract (25% to 85%), retinal anomalies (0% to 38%), strabismus (23% to 44%), amblyopia (10% to 26%), nystagmus (5% to 30%), refractive errors (18% to 58%), glaucoma (less than 1%), iris anomalies (38% to 90%) and optic nerve anomalies (very few cases).
The ocular anomalies, if left untreated, can significantly affect the lives of these patients. Therefore, all the patients with Down syndrome should have an eye exam is done during the first 6 months of life and then annually. [41]
Otorhinolaryngological ( ENT) Disorders
Ear, nose, and throat problems are also quite common in patients with Down syndrome. The anatomical structure of the ear in Down syndrome patients predisposes them to hearing deficits. Hearing loss is usually conductive because of impaction of cerumen and middle ear pathologies, including chronic middle ear effusion due to the small Eustachian tube, acute otitis media, and eardrum perforation. These patients usually require pressure equalization tubes for the treatment.
The sensorineural hearing loss has also been associated with Down syndrome because of the structural abnormalities in the inner ears such as narrow internal auditory canals. [42]
There are different methods used for the prenatal diagnosis of Down syndrome. Ultrasound, between 14 and 24 weeks of gestation, can be used as a tool for diagnosis based on soft markers like increased nuchal fold thickness, small or no nasal bone, and large ventricles. [43] Nuchal translucency (NT) is detected by ultrasound and is caused by a collection of fluid under the skin behind the fetal neck. It is done between 11 and 14 weeks of gestation. Other causes of this finding include Other causes are trisomy 13 (Patau syndrome), trisomy 18 (Edwards syndrome), and Turner syndrome. Amniocentesis and chorionic villus sampling have widely been used for the diagnosis, but there is a small risk of miscarriages between 0.5% to 1%. [44]
Several other methods have also been developed and are used for the rapid detection of trisomy 21 both during fetal life and after birth. The FISH of interphase nuclei is most commonly used by either using Hsa21-specific probes or the whole of the Hsa21. [45] Another method that is currently being used is QF-PCR, in which the presence of 3 different alleles is determined by using DNA polymorphic markers. [46] The success of this method depends upon the informative markers and the presence of DNA. It has been found that up to 86.67% of cases of Down syndrome can be identified by using the STR marker method. [47]
A relatively new method called paralogue sequence quantification (PSQ) uses the paralogue sequence on the Hsa21 copy number. It is a PCR-based method that uses the paralogue genes to detect the targeted chromosome number abnormalities, which is known as paralogue sequence quantification. [48]
There are non-invasive prenatal diagnostic methods that are being studied to be used for the diagnosis of Down syndrome prenatally. These are based on the presence of fetal cells in the maternal blood and the presence of cell-free fetal DNA in the maternal serum. [49]
Cell-free fetal DNA makes up 5% to 10% of the maternal plasma, and it increases during pregnancy and clears after delivery. Though this method has been used to determine fetal Rh status in Rhive women [50] , sex in sex-linked disorders [51] , and for the detection of paternally inherited autosomal recessive and dominant traits, [52] its use for the detection of chromosomal aneuploidy, especially the trisomy is still a challenge.
Few other recent methods like digital PCR and next-generation sequencing (NGS) are also being developed for the diagnosis of Down syndrome. [53]
- Treatment / Management
The management of patients with Down syndrome is multidisciplinary. Newborns with suspicion of Down syndrome should have a karyotyping done to confirm the diagnosis. The family needs to be referred to the clinical geneticist for the genetic testing and counseling of both parents.
Parental education is one of the foremost aspects regarding the management of Down syndrome, as parents need to be aware of the different possible conditions associated with it so that they can be diagnosed and treated appropriately. Treatment is basically symptomatic, and complete recovery is not possible.
These patients should have their hearing and vision assessed, and as they are more prone to have cataracts, timely surgery is required. Thyroid function tests should be done on a yearly basis and, if deranged, should be managed accordingly.
A balanced diet, regular exercise, and physical therapy are needed for optimum growth and weight gain, although feeding problems improve after cardiac surgery.
Cardiac referral should arranged for all the patients regardless of the clinical signs of congenital heart disease. If present, this should be corrected within the first 6 months of life to ensure optimum growth and development of the child.
Other specialties involved include a developmental pediatrician, pediatric pulmonologist, gastroenterologist, neurologist, neurosurgeon, orthopedic specialist, child psychiatrist, physical and occupational therapist, speech and language therapist, and audiologist.
- Differential Diagnosis
- Congenital hypothyroidism
- Mosaic trisomy 21 syndrome
- Partial trisomy 21(or 21q duplication)
- Robertsonian trisomy 21
- Zellweger syndrome or other peroxisomal disorders
With the recent advances in the medical practice, development of surgical techniques for the correction of congenital disabilities, and improvement in general care, there has been a tremendous increase in the survival of infants and life expectancy of patients with Down syndrome. A Birmingham (United Kingdom) study done almost 60 years ago showed that 45% of infants survived the first year of life, and only 40% would be alive at 5 years. [54] A later study conducted about 50 years after that showed that 78% of patients with Down syndrome plus a congenital heart defect survived for 1 year, while the number went up to 96% in patients without the anomalies. [55] This rise in the life expectancy of these patients should continue to rise significantly because of the developments in medical science. Healthcare facilities aim to provide proper and timely management to these patients and to help them to have a fulfilled and productive life. [56]
- Enhancing Healthcare Team Outcomes
The management of patients with Down syndrome is an interprofessional endeavor. Newborns with suspicion of Down syndrome should have a karyotyping done to confirm the diagnosis. The family needs to be referred to the clinical geneticist for the genetic testing and counseling of both parents.
Because almost every organ system is involved, the child needs to be seen by the ophthalmologist, orthopedic surgeon, cardiologist, dermatologist, gastroenterologist, physical therapist, mental health nurse, ENT surgeon, and behavior specialist.
While life span has increased over the past 3 decades, these individuals still have a shorter life expectancy compared to healthy individuals.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
A cropped photo of the eyes of a baby with Down Syndrome. Brushfield spots are visible between the inner and outer circle of the iris. Contributed by Wikimedia Commons, Szymon Tomczak (Public Domain)
"This photograph depicts a newborn with the genetic disorder Down Syndrome, due to the presence of an extra 21st chromosome." Contributed by The Centers for Disease Control and Prevention -- ID# 2634/Dr. Godfrey P. Oakley (Public Domain)
Karyotype for trisomy Down syndrome: Notice the three copies of chromosome 21 Contributed by The National Human Genome Research Institute, Human Genome Project
A drawing of the facial features of Down syndrome Contributed by the Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities (Public Domain)
Disclosure: Faisal Akhtar declares no relevant financial relationships with ineligible companies.
Disclosure: Syed Rizwan Bokhari declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Akhtar F, Bokhari SRA. Down Syndrome. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. [Genet Med. 2009] Review The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Mégarbané A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethoré MO, Delabar JM, Mobley WC. Genet Med. 2009 Sep; 11(9):611-6.
- Review Down syndrome-associated periodontitis: a critical review of the literature. [Compend Contin Educ Dent. 2012] Review Down syndrome-associated periodontitis: a critical review of the literature. Frydman A, Nowzari H. Compend Contin Educ Dent. 2012 May; 33(5):356-61.
- Review Cognitive and medical features of chromosomal aneuploidy. [Handb Clin Neurol. 2013] Review Cognitive and medical features of chromosomal aneuploidy. Hutaff-Lee C, Cordeiro L, Tartaglia N. Handb Clin Neurol. 2013; 111:273-9.
- Down syndrome and the molecular pathogenesis resulting from trisomy of human chromosome 21. [J Biomed Res. 2010] Down syndrome and the molecular pathogenesis resulting from trisomy of human chromosome 21. Ruparelia A, Wiseman F, Sheppard O, Tybulewicz VL, Fisher EM. J Biomed Res. 2010 Mar; 24(2):87-99.
- Review Chances and Challenges of New Genetic Screening Technologies (NIPT) in Prenatal Medicine from a Clinical Perspective: A Narrative Review. [Genes (Basel). 2021] Review Chances and Challenges of New Genetic Screening Technologies (NIPT) in Prenatal Medicine from a Clinical Perspective: A Narrative Review. Bedei I, Wolter A, Weber A, Signore F, Axt-Fliedner R. Genes (Basel). 2021 Mar 29; 12(4). Epub 2021 Mar 29.
Recent Activity
- Down Syndrome - StatPearls Down Syndrome - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

COMMENTS
Abstract. Down syndrome (DS) is a birth defect with huge medical and social costs, caused by trisomy of whole or part of chromosome 21. It is the most prevalent genetic disease worldwide and the common genetic cause of intellectual disabilities appearing in about 1 in 400-1500 newborns. Although the syndrome had been described thousands of ...
Down's syndrome is the most common autosomal abnormality worldwide, affecting around 1 in 1000 live births (World Health Organization, 2018).In 2011, it was estimated that there were 37 000 people with the condition in England and Wales, with a population prevalence of 0.66 per 1000 (Wu and Morris, 2013).Down's syndrome accounts for one-third of cases of severe learning disability.
A Paradigm Shift in DS Research: From a Group- to Individual-Level Approach. DS research dates back to 1866, when the English physician John Langdon Down systematically described the syndrome for the first time (9, 10).In addition to intellectual disability (ID), he chronicled a distinct physical phenotype of individuals with DS, conjecturing that they were "born to the same family" (page ...
This paper is dedicated to the memory of Dr. Angelika Amon who was a leader in Down syndrome research and who made significant contributions to this review. References [1] Mai CT CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, et al., National population-based estimates for major birth defects, 2010-2014 , Birth Defects Res 111 ...
Down syndrome (DS) is one of the commonest disorders with huge medical and social cost. DS is associated with number of phenotypes including congenital heart defects, leukemia, Alzeihmer's disease, Hirschsprung disease etc. DS individuals are affected by these phenotypes to a variable extent thus understanding the cause of this variation is a key challenge. In the present review article, we ...
Down syndrome (DS) is a genetic disorder caused by trisomy 21, the presence of a supernumerary chromosome 21, which results in physical and neurocognitive alterations. ... A landmark paper on the ...
INTRODUCTION. Down syndrome (DS) is caused by trisomy of human chromosome 21 (Hsa21). Approximately 0.45% of human conceptions are trisomic for Hsa21 ().The incidence of trisomy is influenced by maternal age and differs between populations (between 1 in 319 and 1 in 1000 live births are trisomic for Hsa21) (2- 6).Trisomic fetuses are at an elevated risk of miscarriage, and people with DS ...
Down syndrome, the most common chromosomal condition (approximately 1 in 700 births), is associated with an increased risk of common autoimmune diseases, including rheumatic diseases 1. For ...
Seborrheic dermatitis may occur in up to 30% of persons. with Down syndrome (general population 2-5%), with red. cheeks being common. With time, the skin has a tendency to. become dry and rough ...
Abstract. Down's syndrome is caused by trisomy of chromosome 21; it is one of the best known chromosomal disorders in humans. It has effects on most body systems, giving rise to a variety of characteristic clinical features including intellectual impairment, short stature, flat face, flat nasal bridge, prominent epicanthic folds, up slanting ...
We are pleased to present this Special Research Topic on advancements in modeling both developmental and age-related changes in Down Syndrome, including Alzheimer's disease (AD) in Down syndrome (DS-AD). AD is characterized by a progressive deterioration of memory and other neural functions, resulting in impairments to decision-making, behavior ...
Down Syndrome Research and Practice. Down Syndrome Research and Practice is a peer-reviewed journal focused on Down syndrome research. It was published by Down Syndrome Education International in partnership with the University of Portsmouth from 1992 to 2009. Volume 1. Issue 1.
Provocative new findings suggest a surprising cause of Down syndrome: cells linked to aging. Neural progenitor cells derived from stem cells of a person with Down syndrome. Courtesy Hiruy Meharena ...
Overall, the analysis presents an everyday practice aimed at a desirable future for the child with Down syndrome and at a management of everyday life on the family's own terms. In conclusion, this study provides specific knowledge on parents' everyday practice, which may inform genetic counseling about Down syndrome and be of value to service ...
Down syndrome (DS) is the most common genomic disorder of intellectual disability and is caused by trisomy of Homo sapiens chromosome 21 (HSA21). The eponym of the syndrome is from Down, who described the clinical aspects of the syndrome in 1866 (REF. 1).The DS phenotype involves manifestations that affect multiple bodily systems, in particular the musculoskeletal, neurological and ...
Down syndrome is one of the most leading causes of in-tellectual disability and millions of these patients face various health issues including learning and memory, congenital heart diseases(CHD), Alzheimer's diseases (AD), leukemia, cancers and Hirschprung disease(HD). The incidence of trisomy is influenced by maternal age and differs in ...
The research also involved cross-group comparisons of mother-child interactions, which supported the hypothesis that behavioral difficulties are observed in young children with typical development and Down syndrome, with some manifestations of challenging behavior occurring equally in both groups.
In Hendrix et al.'s (2021) paper, the Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study reported early findings from a natural history study of adults with DS in the USA. The LIFE-DSR study consists of 11 sites, who are collectively recruiting 270 individuals with DS over the age of 25.
KW and SH designed the paper. KW did the literature research and wrote the manuscript. SH provided intellectual input and critically revised the manuscript. ... Development of Down Syndrome Research Over the Last Decades-What Healthcare and Education Professionals Need to Know. Front. Psychiatry 12:749046. doi: 10.3389/fpsyt.2021.749046 ...
Exclusively available on IvyPanda. Down syndrome is a chromosomal disorder resulting from the existence of an extra copy chromosome 21. The condition got its name from John Land Down; the doctor who first described it. Down syndrome is associated with symptoms that impair cognitive ability, physical development and often alter facial appearance.
"I would expect life expectancy to continue to expand," says Dr. Skotko. "Research is continuing to uncover new answers to the mysteries that we still have for people with Down syndrome." Research breakthroughs in Down syndrome. One promising breakthrough is a surgically implanted device often referred to as a "pacemaker for the ...
Introduction. Down syndrome is one of the most leading causes of intellectual disability and millions of these patients face various health issues including learning and memory, congenital heart diseases (CHD), Alzheimer's diseases (AD), leukemia, cancers and Hirschprung disease (HD). The incidence of trisomy is influenced by maternal age and ...
We would like to note that Down syndrome underwent a similar recent reappraisal 5 based on the demonstration of universal AD pathology, the predictable sequence of biomarkers and clinical changes ...
Down syndrome was first described by an English physician, John Langdon Down, in 1866, but its association with chromosome 21 was established almost 100 years later by Dr. Jerome Lejeune in Paris. It is the presence of all or part of the third copy of chromosome 21 that causes Down syndrome, the most common chromosomal abnormality occurring in humans.[1] It is also found that the most ...