Sorry, it looks like you have JavaScript disabled. Learn how to enable it.

A Virtual Animal Behavior Research Project for an Introductory Biology Course
In December 2019, I was preparing to teach the lab section for the second half of an introductory biology sequence, which includes evolution, form and function, and ecology. I’d taught this course many times in the past, though I hadn’t for a few years before 2019. I knew I wanted to move away from rote learning through memorization or canned laboratory activities, and to create an authentic experience that would allow me the overarching theme of developing students’ scientific skills, as well as their science identity. Therefore, I redesigned the course so that our scheduled lab time was used for knowledge and skill development. The course focused on the research skills in the Vision and Change Biology Core Concepts (AAAS 2011) and supporting literature outlining how to apply the core concepts (Branchaw et al. 2020) so that students developed the necessary skills to conduct the research project at the end of the course.
Unfortunately, my initial plans were sidelined by the ongoing global pandemic, which required a portion of our laboratory activities to be conducted virtually. I ended up developing a multiweek virtual model to develop basic scientific knowledge and skills using BioInteractive resources that culminated in an eight-week-long animal behavior research project.
In developing this course, I focused on skill development because it’s essential for building confidence. When students are more confident in their skills, this confidence generates a sense of belonging in science, contributing to their science identity. This is essential for retention of students identified historically as Persons Excluded because of their Ethnicity or Race (PEERs), who may be marginalized and less comfortable in the science environment (Asai 2020).
Ethogram and Time-Budget Study
In order to move students toward more open-ended experiments, I chose ethograms with a time-budget study as their final research project. Ethograms are used in the field of animal behavior to collect data during observations and require making a series of field observations that result in a catalog of behaviors and activities identified by the observer.
For this research project, students conducted independent ethological research observing the behavior of an animal species of their choice. I asked students to choose between focusing on a group of animals or an individual, since these require different observational techniques. In observing a group of animals via webcam, students needed to understand that they should focus on one individual of the group for set intervals. Students could also choose to focus on an individual animal for longer and more frequent observations, though that comes with its own limitations.
Initial observations of specific behaviors helped students construct their data-collection instruments, which are used to construct a basic ethogram. Students determined how they would collect data, which helped to develop observational skills and rudimentary experimental design. I provided students with some examples of ethogram templates. (Many zoos have a basic version posted for students, such as this one: Virtual Classroom | Animal Ethograms - Denver Zoo .)
Finally, students used the list of behaviors they collected for their ethogram to observe their animal(s) several more times. They were required to create a data-collection tool to record the number of times each behavior was observed during a specified period of time for at least three more observation periods. These data were used to create a time-budget study, which is a study that identifies the activities an animal is performing in order to determine how the animal uses its energy during a specific time period.
Overall, ethograms and time-budget studies ease students into research before they are introduced to experimental variables and more advanced research methodology. Plus, it’s fun because they choose their own study animal, so it allows for an authentic final assessment in which students demonstrate the skills they have learned and take ownership of their project.
Weekly Modules
For context, this course consisted of a three-credit lecture and a one-credit lab. The first six weeks of the 15-week laboratory portion were conducted in a synchronous virtual format, using BioInteractive materials to teach the basic skills necessary to start the ethogram project. (The first six weeks, as well as the culminating project description, are presented here.) Starting in Week 7, we also conducted in-person lab activities that enhanced students’ background knowledge on animal behavior and taxonomy. All work for the ethogram project was submitted through the course learning management system.
Week 1: Science Literacy Part 1 & Evaluating Science in the News
The first week of lab class introduced students to the process of science by having them evaluate scientific news articles to prepare them for the literature review of their animal behavior project. During our synchronous meeting time, I provided a minilecture on scientific literacy, pseudoscience, and understanding logical fallacies, followed by a short quiz using an online polling system. I then assigned students into breakout groups. Each team completed the short handout for the activity “Evaluating Science in the News,” which involves using the CRAP (Currency, Reliability, Authority, and Purpose) test to evaluate a science news source.
Each team evaluated a “science” article about SARS-CoV-2 that was filled with misinformation by filling out the handout. I assigned the extended version of the “ Scientist Role Models” activity as homework because I wanted them to begin creating their science identity so that they considered themselves as scientists.
Week 2: Scientific Literacy Part 2: Reading Scientific Articles
During Week 2, we continued exploring scientific literacy to scaffold skills they learned in Week 1. The synchronous virtual meeting began with a case study activity that provided students with information about experimental design and basic data analysis. This case study also showed an animal observation study in which there is no laboratory experiment, but data were still collected based on a hypothesis.
We discussed the case study as a class, with students responding in the chat or out loud. Once we completed the case study, I created teams for another article analysis activity. We used this activity to become familiar with the structure of a scientific paper and describe what kind of information is provided in each section (abstract, introduction, methods, results, and conclusion). The activity goals were:
- Identify hypotheses in scientific writing.
- Evaluate evidence in support of a claim in scientific and journalistic writing.
- Identify appropriate search terms.
- Effectively search library databases to find relevant peer-reviewed scientific literature.
- Gain experience reviewing peer-reviewed literature.
Here are guiding questions that I asked students to keep in mind when reading a scientific article. (I also provided an optional resource article: “How to (Seriously) Read a Scientific Paper.” )
- What basic research question are the authors trying to answer?
- What makes that research question significant? (That is, why try to answer that question? Why does it matter?)
- What data did the authors collect?
- What is the authors’ interpretation of their data?
- Do you think that the data they collected supports their conclusions? Why or why not?
This activity consisted of two parts:
Part 1: I reviewed how scientists formulate a hypothesis, test it, and share their information with their peers through publication. I briefly introduced a topic using a short video. While students watched the video, I asked them to focus on how an observation, no matter how trivial, could help form a testable scientific question and emphasized that observation is the beginning of all scientific investigations.
I used a video about penguin defecation to maintain the theme of research related to animal observation. It gave students a chuckle, but is related to actual research, which they review in Part 2 of the activity.
Part 2: Students were divided into groups to read an article about penguin defecation ( Meyer-Rochow and Gal 2003 ) related to the research depicted in the video. Students were asked to work as a team to identify various components of the article, including the scientist’s hypothesis, the evidence used to accept or reject the hypothesis, and whether the hypothesis was accepted or rejected. For the activity, students chose one person from their group to be the notetaker and one person to report back to the entire class when we reconvened.
When the groups finished, we reconvened and students shared out. I recommend doing this as a group activity after they watch the video, with a follow-up discussion, because both of my sections found this particularly difficult. The article was a bit complex for them to understand, but as we talked through it, they understood the importance of becoming familiar with primary literature. I also reminded students that they were not expected to fully understand the paper.
Homework for Week 2 consisted of a similar reading assignment that related to the work they would do in Week 4 (Lizard Evolution Lab). Students watched a BioInteractive video on reproductive isolation and speciation in lizards , then read “Rapid evolution of a native species following invasion by a congener” ( Stuart et al. 2014 ).
In the directions for the article analysis, I reminded students that they were working toward a course goal of being able to understand scientific journal articles. I also allayed students’ concerns about the complexity of the article by reassuring them that I would do my best to teach them the background information needed to understand each article before we read it. I also told them to focus their attention on what they wanted to glean from the article.
Week 3: Sampling Distribution Lab
During Week 3, students were introduced to graph analysis and the concept of sample distributions using the Sampling and Normal Distribution Click & Learn and its accompanying worksheet. I converted the worksheet to a Google Form that students could easily fill out and submit online, since they would be working asynchronously. During the synchronous meeting, we did a quick recap of the article that students read for their homework from Week 2. I also showed the annotated summary of the same article entitled “There's a new kid in town” posted on Science in the Classroom .
After the article discussion, I did a minilecture on sampling distribution and how to use the Click & Learn. I then allowed students to work individually or in teams during class time. I stayed online in the virtual classroom so that students could pop in if they had questions for me. This activity proved to be difficult for some students, so I set up individual virtual meetings to go over their questions. No homework was assigned this week as they were working on the activity asynchronously.
Week 4: Lizard Evolution Lab
Week 4 included one of the favorite activities for both of my groups. Like in Week 3, I spent the synchronous meeting time showing students how to use the Lizard Evolution Virtual Lab and its accompanying worksheet. I also showed the related video The Origin of Species: Lizards in an Evolutionary Tree , which helped students understand how the data for the virtual lab were collected. I reminded them that observational skills were key to this research and that this was the research from the article they read in Week 2.
As in Week 3, I converted the worksheet questions into a Google Form. Similarly, no homework was assigned as they worked on this virtual lab asynchronously.
Week 5: Animal Behavior & Communication Part 1
Students were now ready to apply their skills. For Week 5, I used the synchronous time to go over the following topics with students via videos, a minilecture, and exemplars of previous work:
- How to keep a field journal (discussion and examples posted)
- Overview on ethograms and how they are created (videos and examples posted)
- Various types of animal behaviors that can be observed and methods of sampling animal behavior (videos)
I found several good video examples on YouTube and various examples of ethograms, which I also posted in the learning management system.
For homework, students reviewed the materials, then conducted an initial observation of an animal species of their choice. I’ve written about a similar project here: “Teaching Ecology and Animal Behavior in an Online Setting.” These observations helped them decide on the animal species they would like to study.
I also asked them to find at least two peer-reviewed articles about their animal species. I will admit that I was surprised that, at this point, students struggled with understanding what this meant. Many started off with non-peer-reviewed resources, such as encyclopedias and popular websites. I provided feedback on their resources and did not award the points for the assignment until they submitted peer-reviewed articles. In some cases, this took a virtual meeting to discuss this with students.
Week 6: Animal Behavior & Communication Part 2
For Week 6, students were introduced to a more in-depth example of animal observations so they could apply their problem-solving skills, as well as the knowledge we had learned in class thus far. This example really created a deeper understanding of the process of science once students saw how it was done.
For the synchronous class time, we used the How Animals Use Sound to Communicate Click & Learn. Students were provided with a Google Doc version of the accompanying worksheet so they could fill it in as we worked through the Click & Learn. With the class, I clicked through and discussed the “Introduction” slides to provide students with the knowledge base for the activity.
On Slides 3 and 4, students watched a video of the various animal behaviors identified and defined by the researchers. On Slide 3, I played the video and asked students to try to identify which of the auditory signals they observed the animals using. After this, we moved to Slide 4, which has the same video but highlights the auditory signals that students should have observed. This really showed that observational work, especially when there are multiple animals, is difficult.
This example connected with what students should have done during their observations the previous week. The example also assisted them in that week’s homework, which was constructing their data-collection tool. In addition, we discussed how animals use sound to communicate as we continued to watch the videos. This was much more interesting than reading about animal behavior in their textbook!
At this point, students should also have been thinking about the types of behaviors they could have been observing in Week 5. Some opted to redo their initial observation because they realized they did not adequately observe their animal. I loved that this happened because it let them experience the actual process of science in action. In other words, they realized that their original observational skills were not honed and were better able to understand the types of behaviors they should be looking for in their chosen species.
Once we finished the introduction of the Click & Learn, as a class, we worked through the first case study about how elephants communicate across long distances. This case study begins with an introduction to various types of elephant sounds and describes the combination of low- and high-frequency vocalizations used in elephant communication. This is a great thinking exercise that shows students how observational research can be used to develop a quantitative research study.
As homework for this week, students were asked to revise/develop descriptions of the behaviors they had identified. Then, they developed a definition for each behavior and created their data-collection sheet for the time-budget study.
The time-budget study was created by each student based on the list of behaviors they had generated. Once they identified the timeframe (e.g., observing animals for two 15-minute intervals twice per week at a specific time of day) for their observations, they would count how many times the animal presented with each behavior within the time they observed the animal. This is where they would use the ethogram to create a checklist used during the time-budget study observations. They were provided with a detailed instruction sheet for the entire project.
Once students submitted their data-collection table and received feedback from me (either written or via a meeting), they could start collecting data. They were required to collect data on three separate dates.
After Week 6
After working through Weeks 5 and 6, which helped students design their projects, students collected data for the rest of the semester (Weeks 7–14), with at least three separate data-collection periods required for their time-budget study. The final assessment for the course included an oral presentation of their results, as well as a written paper. I created a slide template for them and a sample of a research paper (I used a former student’s paper with permission), as many were not familiar with how to present authentic research.
After Week 6, the students met for in-person lab exercises (we were masked and in full PPE) where we practiced skills they would need to successfully complete their project. For example, they practiced behavioral observation skills via a pill-bug experiment where they made their own hypotheses and tested them.
During these final weeks, I also scheduled time to meet with students in-person to discuss issues with their projects. I tried to highlight the importance of interacting with a mentor (in this case, me) and helped them practice the skills they would use in graduate school or at work.
All but one student out of 30 successfully completed the project. The final presentations were conducted virtually. Students proudly presented their authentic research and clearly showed how they had developed their research skills with this project. I was ecstatic that students were able to accomplish so much during a global pandemic. They were able to get a feel for what it is like to work with a research mentor and develop their own research projects. I really enjoy mentoring students, and this is a perfect way to interact with them and model for them what it is to be mentored and to engage them in the process of science. Through the creation of the student-mentor bond, I was able to help them begin to see themselves as scientists. The seed for the base of their science identity was planted.
American Association for the Advancement of Science. Vision and Change in Undergraduate Biology Education: A Call to Action . Washington, DC: American Association for the Advancement of Science, 2011.
Asai, D. J. “Race Matters.” Cell 181, 4 (2020): 754–757. https://doi.org/10.1016/j.cell.2020.03.044 .
Branchaw, J. L., P. A. Pape-Lindstrom, K. D. Tanner, S. A. Bissonnette, T. L. Cary, B. A. Couch, A. J. Crowe, et al. “Resources for Teaching and Assessing the Vision and Change Biology Core Concepts. CBE—Life Sciences Education 19, 2 (2020): es1. https://doi.org/10.1187/cbe.19-11-0243 .
Meyer-Rochow, V. B., and J. Gal. “Pressures produced when penguins pooh—calculations on avian defaecation.” Polar Biology 27, 1 (2003): 56–58. https://doi.org/10.1007/s00300-003-0563-3 .
Stuart, Y. E., T. S. Campbell, P. A. Hohenlohe, R. G. Reynolds, L. J. Revell, and J. B. Losos. “Rapid evolution of a native species following invasion by a congener.” Science 346, 6208 (2014): 463–466. https://doi.org/10.1126/science.1257008 .
Melissa Haswell is currently the Associate Dean of Science and Mathematics at Delta College in Michigan. Previously, she taught introductory biology and science ethics for a biology majors program, and anatomy and physiology, and pathophysiology for the nursing program at Davenport University, a private university in Michigan. When she’s not focused on working to improve higher education, she enjoys hiking and camping with her husband and Dalmatian, Chloe, as well as reading, cooking, and spending time with their two cats.
Related Articles
Paul Strode introduces the concepts of species richness and diversity through an outdoor data collection activity to help his students ask authentic scientific questions, collect real data, and communicate like scientists.
Video activities can be easily translated into dynamic online learning activities. In this Educator Voices article, Melissa Haswell details a two-week series of video activities for an ecology and animal behavior unit that she's used in both in-person and online classes.
If you're interested in using formative assessments in an online setting, this Educator Voices article from Valerie May details how she uses a variety of online tools to assess her students’ understanding of factors that regulate populations.
Research using animals: an overview
Around half the diseases in the world have no treatment. Understanding how the body works and how diseases progress, and finding cures, vaccines or treatments, can take many years of painstaking work using a wide range of research techniques. There is overwhelming scientific consensus worldwide that some research using animals is still essential for medical progress.
Animal research in the UK is strictly regulated. For more details on the regulations governing research using animals, go to the UK regulations page .

Why is animal research necessary?
There is overwhelming scientific consensus worldwide that some animals are still needed in order to make medical progress.
Where animals are used in research projects, they are used as part of a range of scientific techniques. These might include human trials, computer modelling, cell culture, statistical techniques, and others. Animals are only used for parts of research where no other techniques can deliver the answer.
A living body is an extraordinarily complex system. You cannot reproduce a beating heart in a test tube or a stroke on a computer. While we know a lot about how a living body works, there is an enormous amount we simply don’t know: the interaction between all the different parts of a living system, from molecules to cells to systems like respiration and circulation, is incredibly complex. Even if we knew how every element worked and interacted with every other element, which we are a long way from understanding, a computer hasn’t been invented that has the power to reproduce all of those complex interactions - while clearly you cannot reproduce them all in a test tube.
While humans are used extensively in Oxford research, there are some things which it is ethically unacceptable to use humans for. There are also variables which you can control in a mouse (like diet, housing, clean air, humidity, temperature, and genetic makeup) that you could not control in human subjects.
Is it morally right to use animals for research?
Most people believe that in order to achieve medical progress that will save and improve lives, perhaps millions of lives, limited and very strictly regulated animal use is justified. That belief is reflected in the law, which allows for animal research only under specific circumstances, and which sets out strict regulations on the use and care of animals. It is right that this continues to be something society discusses and debates, but there has to be an understanding that without animals we can only make very limited progress against diseases like cancer, heart attack, stroke, diabetes, and HIV.
It’s worth noting that animal research benefits animals too: more than half the drugs used by vets were developed originally for human medicine.
Aren’t animals too different from humans to tell us anything useful?
No. Just by being very complex living, moving organisms they share a huge amount of similarities with humans. Humans and other animals have much more in common than they have differences. Mice share over 90% of their genes with humans. A mouse has the same organs as a human, in the same places, doing the same things. Most of their basic chemistry, cell structure and bodily organisation are the same as ours. Fish and tadpoles share enough characteristics with humans to make them very useful in research. Even flies and worms are used in research extensively and have led to research breakthroughs (though these species are not regulated by the Home Office and are not in the Biomedical Sciences Building).
What does research using animals actually involve?
The sorts of procedures research animals undergo vary, depending on the research. Breeding a genetically modified mouse counts as a procedure and this represents a large proportion of all procedures carried out. So does having an MRI (magnetic resonance imaging) scan, something which is painless and which humans undergo for health checks. In some circumstances, being trained to go through a maze or being trained at a computer game also counts as a procedure. Taking blood or receiving medication are minor procedures that many species of animal can be trained to do voluntarily for a food reward. Surgery accounts for only a small minority of procedures. All of these are examples of procedures that go on in Oxford's Biomedical Sciences Building.

How many animals are used?
Figures for 2023 show numbers of animals that completed procedures, as declared to the Home Office using their five categories for the severity of the procedure.
# NHPs - Non Human Primates
Oxford also maintains breeding colonies to provide animals for use in experiments, reducing the need for unnecessary transportation of animals.
Figures for 2017 show numbers of animals bred for procedures that were killed or died without being used in procedures:
Why must primates be used?
Primates account for under half of one per cent (0.5%) of all animals housed in the Biomedical Sciences Building. They are only used where no other species can deliver the research answer, and we continually seek ways to replace primates with lower orders of animal, to reduce numbers used, and to refine their housing conditions and research procedures to maximise welfare.
However, there are elements of research that can only be carried out using primates because their brains are closer to human brains than mice or rats. They are used at Oxford in vital research into brain diseases like Alzheimer’s and Parkinson’s. Some are used in studies to develop vaccines for HIV and other major infections.

What is done to primates?
The primates at Oxford spend most of their time in their housing. They are housed in groups with access to play areas where they can groom, forage for food, climb and swing.
Primates at Oxford involved in neuroscience studies would typically spend a couple of hours a day doing behavioural work. This is sitting in front of a computer screen doing learning and memory games for food rewards. No suffering is involved and indeed many of the primates appear to find the games stimulating. They come into the transport cage that takes them to the computer room entirely voluntarily.
After some time (a period of months) demonstrating normal learning and memory through the games, a primate would have surgery to remove a very small amount of brain tissue under anaesthetic. A full course of painkillers is given under veterinary guidance in the same way as any human surgical procedure, and the animals are up and about again within hours, and back with their group within a day. The brain damage is minor and unnoticeable in normal behaviour: the animal interacts normally with its group and exhibits the usual natural behaviours. In order to find out about how a disease affects the brain it is not necessary to induce the equivalent of full-blown disease. Indeed, the more specific and minor the brain area affected, the more focussed and valuable the research findings are.
The primate goes back to behavioural testing with the computers and differences in performance, which become apparent through these carefully designed games, are monitored.
At the end of its life the animal is humanely killed and its brain is studied and compared directly with the brains of deceased human patients.
Primates at Oxford involved in vaccine studies would simply have a vaccination and then have monthly blood samples taken.

How many primates does Oxford hold?
* From 2014 the Home Office changed the way in which animals/ procedures were counted. Figures up to and including 2013 were recorded when procedures began. Figures from 2014 are recorded when procedures end.
What’s the difference between ‘total held’ and ‘on procedure’?
Primates (macaques) at Oxford would typically spend a couple of hours a day doing behavioural work, sitting in front of a computer screen doing learning and memory games for food rewards. This is non-invasive and done voluntarily for food rewards and does not count as a procedure. After some time (a period of months) demonstrating normal learning and memory through the games, a primate would have surgery under anaesthetic to remove a very small amount of brain tissue. The primate quickly returns to behavioural testing with the computers, and differences in performance, which become apparent through these carefully designed puzzles, are monitored. A primate which has had this surgery is counted as ‘on procedure’. Both stages are essential for research into understanding brain function which is necessary to develop treatments for conditions including Alzheimer’s, Parkinson’s and schizophrenia.
Why has the overall number held gone down?
Numbers vary year on year depending on the research that is currently undertaken. In general, the University is committed to reducing, replacing and refining animal research.
You say primates account for under 0.5% of animals, so that means you have at least 16,000 animals in the Biomedical Sciences Building in total - is that right?
Numbers change daily so we cannot give a fixed figure, but it is in that order.
Aren’t there alternative research methods?
There are very many non-animal research methods, all of which are used at the University of Oxford and many of which were pioneered here. These include research using humans; computer models and simulations; cell cultures and other in vitro work; statistical modelling; and large-scale epidemiology. Every research project which uses animals will also use other research methods in addition. Wherever possible non-animal research methods are used. For many projects, of course, this will mean no animals are needed at all. For others, there will be an element of the research which is essential for medical progress and for which there is no alternative means of getting the relevant information.
How have humans benefited from research using animals?
As the Department of Health states, research on animals has contributed to almost every medical advance of the last century.
Without animal research, medicine as we know it today wouldn't exist. It has enabled us to find treatments for cancer, antibiotics for infections (which were developed in Oxford laboratories), vaccines to prevent some of the most deadly and debilitating viruses, and surgery for injuries, illnesses and deformities.
Life expectancy in this country has increased, on average, by almost three months for every year of the past century. Within the living memory of many people diseases such as polio, tuberculosis, leukaemia and diphtheria killed or crippled thousands every year. But now, doctors are able to prevent or treat many more diseases or carry out life-saving operations - all thanks to research which at some stage involved animals.
Each year, millions of people in the UK benefit from treatments that have been developed and tested on animals. Animals have been used for the development of blood transfusions, insulin for diabetes, anaesthetics, anticoagulants, antibiotics, heart and lung machines for open heart surgery, hip replacement surgery, transplantation, high blood pressure medication, replacement heart valves, chemotherapy for leukaemia and life support systems for premature babies. More than 50 million prescriptions are written annually for antibiotics.
We may have used animals in the past to develop medical treatments, but are they really needed in the 21st century?
Yes. While we are committed to reducing, replacing and refining animal research as new techniques make it possible to reduce the number of animals needed, there is overwhelming scientific consensus worldwide that some research using animals is still essential for medical progress. It only forms one element of a whole research programme which will use a range of other techniques to find out whatever possible without animals. Animals would be used for a specific element of the research that cannot be conducted in any alternative way.
How will humans benefit in future?
The development of drugs and medical technologies that help to reduce suffering among humans and animals depends on the carefully regulated use of animals for research. In the 21st century scientists are continuing to work on treatments for cancer, stroke, heart disease, HIV, malaria, tuberculosis, diabetes, neurodegenerative diseases like Alzheimer's and Parkinson’s, and very many more diseases that cause suffering and death. Genetically modified mice play a crucial role in future medical progress as understanding of how genes are involved in illness is constantly increasing.

- News/Events
- Arts and Sciences
- Design and the Arts
- Engineering
- Global Futures
- Health Solutions
- Nursing and Health Innovation
- Public Service and Community Solutions
- University College
- Thunderbird School of Global Management
- Polytechnic
- Downtown Phoenix
- Online and Extended
- Lake Havasu
- Research Park
- Washington D.C.
- Biology Bits
- Bird Finder
- Coloring Pages
- Experiments and Activities
- Games and Simulations
- Quizzes in Other Languages
- Virtual Reality (VR)
- World of Biology
- Meet Our Biologists
- Listen and Watch
- PLOSable Biology
- All About Autism
- Xs and Ys: How Our Sex Is Decided
- When Blood Types Shouldn’t Mix: Rh and Pregnancy
- What Is the Menstrual Cycle?
- Understanding Intersex
- The Mysterious Case of the Missing Periods
- Summarizing Sex Traits
- Shedding Light on Endometriosis
- Periods: What Should You Expect?
- Menstruation Matters
- Investigating In Vitro Fertilization
- Introducing the IUD
- How Fast Do Embryos Grow?
- Helpful Sex Hormones
- Getting to Know the Germ Layers
- Gender versus Biological Sex: What’s the Difference?
- Gender Identities and Expression
- Focusing on Female Infertility
- Fetal Alcohol Syndrome and Pregnancy
- Ectopic Pregnancy: An Unexpected Path
- Creating Chimeras
- Confronting Human Chimerism
- Cells, Frozen in Time
- EvMed Edits
- Stories in Other Languages
- Virtual Reality
- Zoom Gallery
- Ugly Bug Galleries
- Ask a Question
- Top Questions
- Question Guidelines
- Permissions
- Information Collected
- Author and Artist Notes
- Share Ask A Biologist
- Articles & News
- Our Volunteers
- Teacher Toolbox

show/hide words to know
Computer model: a computer program designed to predict what might happen based off of collected data.
Ethical: relating to a person's moral principles.
Morals: a person's beliefs concerning what is right and wrong.
Zoologist: a person who studies animals.

Scientists learn a lot about snakes and other animals through basic research. Image by the Virginia State Park staff.
“Don’t worry, they aren’t dangerous” you hear the zoologist say as she leads you and a group of others toward an area with a number of different snakes. She removes a long snake from a larger glass enclosure and asks who would like to hold it. You take a step back, certain that holding a snake is the last thing you’d like to do.
"But how do you know they aren’t dangerous?” you ask. The zoologist looks up and smiles. She explains that scientists have studied this type of snake, and so we actually know quite a bit about it. This type of snake rarely bites and does not produce venom, so it isn’t dangerous to people. You nod along as she talks about the snakes, their natural habitats, and other details like what they eat.
Animals in the Research Process
How do we know so much about snakes or other animals? Animals are all unique, and scientists study them to learn more about them. For example, by studying snakes we have learned that they stick their tongues out because they are trying to pick up odors around them. This helps them sense food, predators, and other things that may be nearby. When research is performed to expand our understanding of something, like an animal, we call it basic research .
Scientists study animals for other reasons too. What we learn about animals can actually help us find solutions to other problems or to help people. For example, studying snakes helps us understand which ones are venomous so that humans know what kinds of snakes they shouldn't touch. Scientists also study animals to find new treatments to diseases and other ailments that affect both people and animals. If we learn what is in snake venom, we can create a medicine to give to people that have been bitten as a treatment to help them feel better. Using what we know about an animal or thing to help us solve problems or treat disease is called applied research .
Scientists use many other tools, such as computer models, in addition to animals to study different topics. Image by Andreas Horn.
No matter what type of research is being performed, scientists must consider many things when they study animals.
Do Scientists Need to Study Animals?
Of course we can learn a lot from using animals for research, but are there alternative options? Sometimes there are. For example, scientists could use some other method, like cells or computer models, to study a particular topic instead of using animals. However, for a number of reasons , scientists have found that using animals is sometimes the best way to study certain topics.
What If Scientists Harm Animals for Research?
Some research using animals only requires scientists to watch behavior or to take a few samples (like blood or saliva) from the animal. These activities may cause the animals some stress, but they are unlikely to harm the animals in any long-term way. Studies of the behavior or physiology of an animal in its natural environment is an example of such research.
In other cases, scientists may need to harm or kill an animal in order to answer a research question. For example, a study could involve removing a brain to study it more closely or giving an animal a treatment without knowing what effects it may have. While the intention is never to purposely harm animals, harm can be necessary to answer a research question.
How Do Scientists Decide When It’s OK to Study Animals?

Many animals are used in research. But there is still debate on whether they should be used for this purpose. Image by the United States Department of Agriculture.
There are many guidelines for when it’s ok to use animals in research. Scientists must write a detailed plan of why and how they plan to use animals for a research project. This information is then reviewed by other scientists and members of the public to make sure that the research animals will be used for has an important purpose. Whatever the animals are used for, the scientists also make sure to take care of animal research subjects as best as they can.
Even with rules in place about using animals for research, many people (both scientists and non-scientists) continue to debate whether animals should be used in research. This is an ethical question, or one that depends on a person's morals. Because the way each person feels about both research and animals may be different, there is a range of views on this matter.
- Some people argue that it doesn’t matter that there are rules in place to protect animals. Animals should never be used for research at all, for any reason.
- Others say we should be able to use animals for any kind of research because moving science forward is more important than the rights or well-being of animals.
- Lastly, there are people whose opinions sit somewhere in the middle. They might argue that it’s ok to use animals for research, but only in some cases. For example, if the results of the research are very likely to help treat something that affects people, then it may be okay to use animals.
Along with this debate, there are many advantages and disadvantages of doing animal research . Scientists must weigh these options when performing their research.
Additional Images via Wikimedia Commons. White rat image by Alexandroff Pogrebnoj.
Read more about: Using Animals in Research
View citation, bibliographic details:.
- Article: Using Animals in Research
- Author(s): Patrick McGurrin and Christian Ross
- Publisher: Arizona State University School of Life Sciences Ask A Biologist
- Site name: ASU - Ask A Biologist
- Date published: December 4, 2016
- Date accessed: May 21, 2024
- Link: https://askabiologist.asu.edu/explore/Animal-use-in-Research
Patrick McGurrin and Christian Ross. (2016, December 04). Using Animals in Research. ASU - Ask A Biologist. Retrieved May 21, 2024 from https://askabiologist.asu.edu/explore/Animal-use-in-Research
Chicago Manual of Style
Patrick McGurrin and Christian Ross. "Using Animals in Research". ASU - Ask A Biologist. 04 December, 2016. https://askabiologist.asu.edu/explore/Animal-use-in-Research
MLA 2017 Style
Patrick McGurrin and Christian Ross. "Using Animals in Research". ASU - Ask A Biologist. 04 Dec 2016. ASU - Ask A Biologist, Web. 21 May 2024. https://askabiologist.asu.edu/explore/Animal-use-in-Research

Animals are an important part of research. But many argue about whether it's ethical to use animals to help advance scientific progress.
Using Animals in Research
Be part of ask a biologist.
By volunteering, or simply sending us feedback on the site. Scientists, teachers, writers, illustrators, and translators are all important to the program. If you are interested in helping with the website we have a Volunteers page to get the process started.
Share to Google Classroom
Loading metrics
Open Access
Essays articulate a specific perspective on a topic of broad interest to scientists.
See all article types »
A guide to open science practices for animal research
Contributed equally to this work with: Kai Diederich, Kathrin Schmitt
Affiliation German Federal Institute for Risk Assessment, German Centre for the Protection of Laboratory Animals (Bf3R), Berlin, Germany
* E-mail: [email protected]
- Kai Diederich,
- Kathrin Schmitt,
- Philipp Schwedhelm,
- Bettina Bert,
- Céline Heinl

Published: September 15, 2022
- https://doi.org/10.1371/journal.pbio.3001810
- Reader Comments
Translational biomedical research relies on animal experiments and provides the underlying proof of practice for clinical trials, which places an increased duty of care on translational researchers to derive the maximum possible output from every experiment performed. The implementation of open science practices has the potential to initiate a change in research culture that could improve the transparency and quality of translational research in general, as well as increasing the audience and scientific reach of published research. However, open science has become a buzzword in the scientific community that can often miss mark when it comes to practical implementation. In this Essay, we provide a guide to open science practices that can be applied throughout the research process, from study design, through data collection and analysis, to publication and dissemination, to help scientists improve the transparency and quality of their work. As open science practices continue to evolve, we also provide an online toolbox of resources that we will update continually.
Citation: Diederich K, Schmitt K, Schwedhelm P, Bert B, Heinl C (2022) A guide to open science practices for animal research. PLoS Biol 20(9): e3001810. https://doi.org/10.1371/journal.pbio.3001810
Copyright: © 2022 Diederich et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The authors received no specific funding for this work.
Competing interests: I have read the journal’s policy and the authors of this manuscript have the following competing interests: All authors are employed at the German Federal Institute for Risk Assessment and part of the German Centre for the Protection of Laboratory Animals (Bf3R) which developed and hosts animalstudyregistry.org , a preregistration platform for animal studies and animaltestinfo.de, a database for non-technical project summaries (NTS) of approved animal study protocols within Germany.
Abbreviations: CC, Creative Commons; CIRS-LAS, critical incident reporting system in laboratory animal science; COVID-19, Coronavirus Disease 2019; DOAJ, Directory of Open Access Journals; DOI, digital object identifier; EDA, Experimental Design Assistant; ELN, electronic laboratory notebook; EU, European Union; IMSR, International Mouse Strain Resource; JISC, Joint Information Systems Committee; LIMS, laboratory information management system; MGI, Mouse Genome Informatics; NC3Rs, National Centre for the Replacement, Refinement and Reduction of Animals in Research; NTS, non-technical summary; RRID, Research Resource Identifier
Introduction
Over the past decade, the quality of published scientific literature has been repeatedly called into question by the failure of large replication studies or meta-analyses to demonstrate sufficient translation from experimental research into clinical successes [ 1 – 5 ]. At the same time, the open science movement has gained more and more advocates across various research areas. By sharing all of the information collected during the research process with colleagues and with the public, scientists can improve collaborations within their field and increase the reproducibility and trustworthiness of their work [ 6 ]. Thus, the International Reproducibility Networks have called for more open research [ 7 ].
However, open science practices have not been adopted to the same degree in all research areas. In psychology, which was strongly affected by the so-called reproducibility crisis, the open science movement initiated real practical changes leading to a broad implementation of practices such as preregistration or sharing of data and material [ 8 – 10 ]. By contrast, biomedical research is still lagging behind. Open science might be of high value for research in general, but in translational biomedical research, it is an ethical obligation. It is the responsibility of the scientist to transparently share all data collected to ensure that clinical research can adequately evaluate the risks and benefits of a potential treatment. When Russell and Burch published “The Principles of Humane Experimental Technique” in 1959, scientists started to implement their 3Rs principle to answer the ethical dilemma of animal welfare in the face of scientific progress [ 11 ]. By replacing animal experiments wherever possible, reducing the number of animals to a strict minimum, and refining the procedures where animals have still to be used, this ethical dilemma was addressed. However, in recent years, whether the 3Rs principle is sufficient to fully address ethical concerns about animal experiments has been questioned [ 12 ].
Most people tolerate the use of animals for scientific purposes only under the basic assumption that the knowledge gained will advance research in crucial areas. This implies that performed experiments are reported in a way that enables peers to benefit from the collected data. However, recent studies suggest that a large proportion of animal experiments are never actually published. For example, scientists working within the European Union (EU) have to write an animal study protocol for approval by the competent authorities of the respective country before performing an animal experiment [ 13 ]. In these protocols, scientists have to describe the planned study and justify every animal required for the project. By searching for publications resulting from approved animal study protocols from 2 German University Medical Centers, Wieschowski and colleagues found that only 53% of approved protocols led to a publication after 6 years [ 14 ]. Using a similar approach, Van der Naald and colleagues determined a publication rate of 60% at the Utrecht Medical Center [ 15 ]. In a follow-up survey, the respective researchers named so-called “negative” or null-hypothesis results as the main cause for not publishing outcomes [ 15 ]. The current scientific system is shaped by publishers, funders, and institutions and motivates scientists to publish novel, surprising, and positive results, revealing one of the many structural problems that the numerous efforts towards open science initiatives are targeting. Non-publication not only strongly contradicts ethical values, but also it compromises the quality of published literature by leading to overestimation of effect sizes [ 16 , 17 ]. Furthermore, publications of animal studies too often show poor reporting that strongly impairs the reproducibility, validity, and usefulness of the results [ 18 ]. Unfortunately, the idea that negative or equivocal findings can also contribute to the gain of scientific knowledge is frequently neglected.
So far, the scientific community using animals has shown limited resonance to the open science movement. Due to the strong controversy surrounding animal experiments, scientists have been reluctant to share information on the topic. Additionally, translational research is highly competitive and researchers tend to be secretive about their ideas until they are ready for publication or patent [ 19 , 20 ]. However, this missing openness could also point to a lack of knowledge and training on the many open science options that are available and suitable for animal research. Researchers have to be convinced of the benefits of open science practices, not only for science in general, but also for the individual researcher and each single animal. Yet, the key players in the research system are already starting to value open science practices. An increasing number of journals request open sharing of data, funders pay for open access publications and institutions consider open science practices in hiring decisions. Open science practices can improve the quality of work by enabling valuable scientific input from peers at the early stages of research projects. Furthermore, the extended communication that open science practices offer can draw attention to research and help to expand networks of collaborators and lead to new project opportunities or follow-up positions. Thus, open science practices can be a driver for careers in academia, particularly those of early career researchers.
Beyond these personal benefits, improving transparency in translational biomedical research can boost scientific progress in general. By bringing to light all the recorded research outputs that until now have remained hidden, the publication bias and the overestimation of effect sizes can be reduced [ 17 ]. Large-scale sharing of data can help to synthesize research outputs in preclinical research that will enable better decision-making for clinical research. Disclosing the whole research process will help to uncover systematic problems and support scientists in thoroughly planning their studies. In the long run, we predict that the implementation of open science practices will lead to the use of fewer animals in unintentionally repeated experiments that previously showed unreported negative results or in the establishment of methods by avoiding experimental dead ends that are often not published. More collaborations and sharing of materials and methods can further reduce the number of animal experiments used for the implementation of new techniques.
Open science can and should be implemented at each step of the research process ( Fig 1 ). A vast number of tools are already provided that were either directly conceptualized for animal research or can be adapted easily. In this Essay, we provide an overview of open science tools that improve transparency, reliability, and animal welfare in translational in vivo biomedical research by supporting scientists to clearly communicate their research and by supporting collaborative working. Table 1 lists the most prominent open science tools we discuss, together with their respective links. We have structured this Essay to guide you through which tools can be used at each stage of the research process, from planning and conducting experiments, through to analyzing data and communicating the results. However, many of these tools can be used at many different steps. Table 1 has been deposited on Zenodo and will be updated continuously [ 21 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Application of open science practices at each step of the research process can maximize the impact of performed animal experiments. The implementation of these practices will lead to less time pressure at the end of a project. Due to the connection of most of these open science practices, spending more time in the planning phase and during the conduction of experiments will save time during the data analysis and publication of the study. Indeed, consulting reporting guidelines early on, preregistering a statistical plan, and writing down crucial experimental details in an electronic lab notebook, will strongly accelerate the writing of a manuscript. If protocols or even electronic lab notebooks were made public, just citing these would simplify the writing of publications. Similarly, if a data management plan is well designed before starting data collection, analyzing, and depositing data in a public repository, as is increasingly required, will be fast. NTS, non-technical summary.
https://doi.org/10.1371/journal.pbio.3001810.g001
https://doi.org/10.1371/journal.pbio.3001810.t001
Planning the study
Transparent practices can be adopted at every stage of the research process. However, to ensure full effectivity, it is highly recommended to engage in detailed planning before the start of the experiment. This can prevent valuable time from being lost at the end of the study due to careless decisions being made at the beginning. Clarifying data management at the start of a project can help avoiding filing chaos that can be very time consuming to untangle. Keeping clear track of a project and study design will also help if new colleagues are included later on in the project or if entire project parts are handed over. In addition, all texts written on the rationale and hypothesis of the study or method descriptions, or design schemes created during the planning phase can be used in the final publications ( Fig 1 ). Similarly, information required for preregistration of animal studies or for reporting according to the ARRIVE guidelines are an extension of the details required for ethical approval [ 22 , 23 ]. Thus, the time burden within the planning phase is often overestimated. Furthermore, the thorough planning of experiments can avoid the unnecessary use of animals by preventing wrong avenues from being pursued.
Implementing open scientific practices at the beginning of a project does not mean that the idea and study plan must be shared immediately, but rather is critical for making the entire workflow transparent at the end of the project. However, optional early sharing of information can enable peers to give feedback on the study plan. Studies potentially benefit more from this a priori input than they would from the classical a posteriori peer-review process.
Most people perceive guidelines as advice that instructs on how to do something. However, it is sometimes useful to consider the term in its original meaning; “the line that guides us”. In this sense, following guidelines is not simply fulfilling a duty, but is a process that can help to design a sound research study and, as such, guidelines should be consulted at the planning stage of a project. The PREPARE guidelines are a list of important points that should be thought-out before starting a study involving animal experiments in order to reduce the waste of animals, promote alternatives, and increase the reproducibility of research and testing [ 24 ]. The PREPARE checklist helps to thoroughly plan a study and focuses on improving the communication and collaboration between all involved participants of the study (i.e., animal caretakers and scientists). Indeed, open science begins with the communication within a research facility. It is currently available in 33 languages and the responsible team from Norecopa, Norway’s 3R-center, takes requests for translations into further languages.
The UK Reproducibility Network has also published several guiding documents (primers) on important topics for open and reproducible science. These address issues such as data sharing [ 25 ], open access [ 26 ], open code and software [ 27 ], and preprints [ 28 ], as well as preregistration and registered reports [ 27 ]. Consultation of these primers is not only helpful in the relevant phases of the experiment but is also encouraged in the planning phase.
Although the ARRIVE guidelines are primarily a reporting guideline specifically designed for preparing a publication containing animal data, they can also support researchers when planning their experiments [ 22 , 23 ]. Going through the ARRIVE website, researchers will find tools and explanations that can support them in planning their experiments [ 29 ]. Consulting the ARRIVE checklist at the beginning of a project can help in deciding what details need to be documented during conduction of the experiments. This is particularly advisable, given that compliance to ARRIVE is still poor [ 18 ].
Experimental design
To maximize the validity of performed experiments and the knowledge gained, designing the study well is crucial. It is important that the chosen animal species reflects the investigated disease well and that basic characteristics of the animal, such as sex or age, are considered carefully [ 30 ]. The Canadian Institutes of Health Research provides a collection of resources on the integration of sex and gender in biomedical research with animals, including tips and tools for researchers and reviewers [ 31 ]. Additionally, it is advisable to avoid unnecessary standardization of biological and environmental factors that can reduce the external validity of results [ 32 ]. Meticulous statistical planning can further optimize the use of animals. Free to use online tools for calculating sample sizes such as G*Power or the inVivo software package for R can further support animal researchers in designing their statistical plan [ 33 , 34 ]. Randomization for the allocation of groups can be supported with specific tools for scientists like Research Randomizer, but also by simple online random number generators [ 35 ]. Furthermore, it might be advisable when designing the study to incorporate pathological analyses into the experimental plan. Optimal planning of tissue collection, performance of pathological procedures according to accepted best practices, and use of optimal pathological analysis and reporting methods can add some extra knowledge that would otherwise be lost. This can improve the reproducibility and quality of translational biomedicine, especially, but not exclusively, in animal studies with morphological endpoints. In all animal studies, unexpected deaths in experimental animals can occur and be the cause of lost data or missed opportunities to identify health problems [ 36 , 37 ].
To support researchers in designing their animal research, the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) has also developed the Experimental Design Assistant (EDA) [ 38 , 39 ]. This online tool helps researchers to better structure in vivo research by creating detailed schemes of the study design. It provides feedback on the entered design, drawing researcher’s attention to crucial decisions in the project. The resulting schemes can be used to transparently share the study design by uploading it into a study preregistration, enclosing it in a grant application, or submitting it with a final manuscript. The EDA can be used for different study designs in diverse scenarios and helps to communicate researcher plans to others [ 40 ]. The EDA might be particularly of interest to clarify very complex study designs involving multiple experimental groups. Working with the EDA might appear rather complex in the beginning, but the NC3R provides regular webinars that can help to answer any questions that arise.
Preregistration
Preregistration is an effective tool to improve the quality and transparency of research. To preregister their work, scientists must determine crucial details of the study before starting any experiment. Changes occurring during a study can be outlined at the end. A preregistered study plan should include at least the hypothesis and determine all the parameters that are known in advance. A description of the planned study design and statistical analysis will enable reviewers and peers to better retrace the workflow. It can prevent the intentional use of the flexibility of analysis to reach p -values under a certain significance level (e.g., p-hacking or HARKing (Hypothesizing After Results are Known)). With preregistration, scientists can also claim their idea at an early stage of their research with a citable individual identifier that labels the idea as their own. Some open preregistration platforms also provide a digital object identifier (DOI), which makes the registered study citable. Three public registries actively encourage the preregistration of animal studies conducted around the world: OSF registry, preclinicaltrials.eu, and animalstudyregistry.org [ 41 – 45 ]. Scientists can choose the registry according to their needs. Preregistering a study in a public registry supports scientists in planning their study and later to critically reevaluate their own work and assess its limitations and potentials.
As an alternative to public registries, researchers can also submit their study plan to one of hundreds of journals already publishing registered reports, including many journals open to animal research [ 8 ]. A submitted registered report passes 2 steps of peer review. In the first step, reviewers comment on the idea and the study design. After an “in-principle-acceptance,” researchers can conduct their study as planned. If the authors conduct the experiments as described in the accepted study protocol, the journal will publish the final study regardless of the outcome. This might be an attractive option, especially for early career researchers, as a manuscript is published at the beginning of a project with the guarantee of a future final publication.
The benefits of preregistration can already be observed in clinical research, where registration has been mandatory for most trials for more than 20 years. Preregistration in clinical research has helped to make known what has been tested and not just what worked and was published, and the implementation of trial registration has strongly reduced the number of publications reporting significant treatment effects [ 46 ]. In animal research, with its unrealistically high percentage of positive results, preregistration seems to be particularly worthwhile.
Research data management
To get the most out of performed animal experiments, effective sharing of data at the end of the study is essential. Sharing research data optimally is complex and needs to be prepared in advance. Thus, data management can be seen as one part of planning a study thoroughly. Many funders have recognized the value of the original research data and request a data management plan from applicants in advance [ 25 , 47 ]. Various freely available tools such as DMPTool or DMPonline already exist to design a research data management plan that complies to the requirements of different funders [ 48 , 49 ]. The data management plan defines the types of data collected and describes the handling and names responsible persons throughout the data lifecycle. This includes collecting the data, analyzing, archiving, and sharing it. Finally, a data management plan enables long-term access and the possibility for reuse by peers. Developing such a plan, whether it is required by funders or not, will later simplify the application of the FAIR data principle (see section on the FAIR data principle). The Longwood Medical Area Research Data Management Working Group from the Harvard Medical School developed a checklist to assist researchers in optimally managing their data throughout the data lifecycle [ 50 ]. Similarly, the Joint Information Systems Committee (JISC) provides a great research data management toolkit including a checklist for researchers planning their project [ 51 ]. Consulting this checklist in the planning phase of a project can prevent common errors in research data management.
Non-technical project summary
One instrument specifically conceived to create transparency on animal research for the general public is the so-called non-technical project summary (NTS). All animal protocols approved within the EU must be accompanied by these comprehensible summaries. NTSs are intended to inform the public about ongoing animal experiments. They are anonymous and include information on the objectives and potential benefits of the project, the expected harm, the number of animals, the species, and a statement of compliance with the requirements of the 3Rs principle. However, beyond simply informing the public, NTSs can also be used for meta-research to help identify new research areas with an increased need for new 3R technologies [ 52 , 53 ]. NTSs become an excellent tool to appropriately communicate the scientific value of the approved protocol and for meta-scientists to generate added value by systematically analyzing theses summaries if they fulfill a minimum quality threshold [ 54 , 55 ]. In 2021, the EU launched the ALURES platform ( Table 1 ), where NTSs from all member states are published together, opening the opportunities for EU-wide meta-research. NTSs are, in contrast to other open science practices, mandatory in the EU. However, instead of thinking of them as an annoying duty, it might be worth thoroughly drafting the NTS to support the goals of more transparency towards the public, enabling an open dialogue and reducing extreme opinions.
Conducting the experiments
Once the experiments begin, documentation of all necessary details is essential to ensure the transparency of the workflow. This includes methodological details that are crucial for replicating experiments, but also failed attempts that could help peers to avoid experiments that do not work in the future. All information should be stored in such a way that it can be found easily and shared later. In this area, many new tools have emerged in recent years ( Table 1 ). These tools will not only make research transparent for colleagues, but also help to keep track of one’s own research and improve internal collaboration.
Electronic laboratory notebooks
Electronic laboratory notebooks (ELNs) are an important pillar of research data management and open science. ELNs facilitate the structured and harmonized documentation of the data generation workflow, ensure data integrity, and keep track of all modifications made to the original data based on an audit trail option. Moreover, ELNs simplify the sharing of data and support collaborations within and outside the research group. Methodological details and research data become searchable and traceable. There is an extensive amount of literature providing advice on the selection and the implementation process of an ELN depending on the specific needs and research area and its discussion would be beyond the scope of this Essay [ 56 – 58 ]. Some ELNs are connected to a laboratory information management system (LIMS) that provides an animal module supporting the tracking of animal details [ 59 ]. But as research involving animals is highly heterogeneous, this might not be the only decision point and we cannot recommend a specific ELN that is suitable for all animal research.
ELNs are already established in the pharmaceutical industry and their use is on the rise among academics as well. However, due to concerns around costs for licenses, data security, and loss of flexibility, many research institutions still fear the expenses that the introduction of such a system would incur [ 56 ]. Nevertheless, an increasing number of academic institutions are implementing ELNs and appreciating the associated benefits [ 60 ]. If your institution already has an ELN, it might be easiest to just use the option available in the research environment. If not, the Harvard Medical School provides an extensive and updated overview of various features of different ELNs that can support scientists in choosing the appropriate one for their research [ 61 ]. There are many commercial ELN products, which may be preferred when the administrative workload should be outsourced to a large extent. However, open-source products such as eLabFTW or open BIS provide a greater opportunity for customization to meet specific needs of individual research institutions [ 62 – 64 ]. A huge number of options are available depending on the resources and the features required. Some scientists might prefer generic note taking tools such as Evernote or just a simple Word document that offers infinite flexibility, but specific ELNs can further support good record keeping practice by providing immutability, automated backups, standardized methods, and protocols to follow. Clearly defining the specific requirements expected might help to choose an adequate system that would improve the quality of the record compared to classical paper laboratory notebooks.
Sharing protocols
Adequate sharing of methods in translational biomedical sciences is key to reproducibility. Several repositories exist that simplify the publication and exchange of protocols. Writing down methods at the end of the project bears the risk that crucial details might be missing [ 65 ]. On protocols.io, scientists can note all methodological details of a procedure, complete them with uploaded documents, and keep them for personal use or share them with collaborators [ 66 ]. Authors can also decide at any point in time to make their protocol public. Protocols published on protocols.io receive a DOI and become citable; they can be commented on by peers and adapted according to the needs of the individual researcher. Protocol.io files from established protocols can also be submitted together with some context and sample datasets to PLOS ONE , where it can be peer-reviewed and potentially published [ 67 , 68 ]. Depending on the affiliation of the researchers to academia or industry and on an internal or public sharing of files, protocols.io can be free of charge or come with costs. Other journals also encourage their authors to deposit their protocols in a freely accessible repository, such as protocol exchange from Nature portfolio [ 69 ]. Another option might be to separately submit a protocol that was validated by its use in an already published research article to an online and peer-reviewed journal specific for research protocols, such as Bio-Protocol. A multitude of journals, including eLife and Science already collaborate with Bio-Protocol and recommend authors to publish the method in Bio-Protocol [ 70 ]. Bio-Protocol has no submission fees and is freely available to all readers. Both protocols.io and Bio-Protocol allow the illustration of complex scientific methods by uploading videos to published protocols. In addition, protocols can be deposited in a general research repository such as the Open Science Framework (OSF repository) and referenced in appropriate publications.
Sharing critical incidents
Sharing critical or even adverse events that occur in the context of animal experimentation can help other scientists to avoid committing the same mistakes. The system of sharing critical incidents is already established in clinical practice and helps to improve medical care [ 71 , 72 ]. The online platform critical incident reporting system in laboratory animal science (CIRS-LAS) represents the first preclinical equivalent to these clinical systems [ 73 ]. With this web-based tool, critical incidents in animal research can be reported anonymously without registration. An expert panel helps to analyze the incident to encourage an open dialogue. Critical incident reporting is still very marginal in animal research and performed procedures are very variable. These factors make a systemic analysis and a targeted search of incidence difficult. However, it may be of special interest for methods that are broadly used in animal research such as anesthesia. Indeed, a broad feed of this system with data on errors occurring in standard procedures today could help avoid critical incidences in the future and refine animal experiments.
Sharing animals, organs, and tissue
When we think about open science, sharing results and data are often in focus. However, sharing material is also part of a collaborative and open research culture that could help to greatly reduce the number of experimental animals used. When an animal is killed to obtain specific tissue or organs, the remainder is mostly discarded. This may constitute a wasteful practice, as surplus tissue can be used by other researchers for different analyses. More animals are currently killed as surplus than are used in experiments, demonstrating the potential for sharing these animals [ 74 , 75 ].
Sharing information on generated surplus is therefore not only economical, but also an effective way to reduce the number of animals used for scientific purposes. The open-source software Anishare is a straightforward way for breeders of genetically modified lines to promote their surplus offspring or organs within an institution [ 76 ]. The database AniMatch ( Table 1 ) connects scientists within Europe who are offering tissue or organs with scientists seeking this material. Scientists already sharing animal organs can support this process by describing it in publications and making peers aware of this possibility [ 77 ]. Specialized research communities also allow sharing of animal tissue or animal-derived products worldwide that are typically used in these fields on a collaborative basis via the SEARCH-framework [ 78 , 79 ]. Depositing transgenic mice lines into one of several repositories for mouse strains can help to further minimize efforts in producing new transgenic lines and most importantly reduce the number of surplus animals by supporting the cryoconservation of mouse lines. The International Mouse Strain Resource (IMSR) can be used to help find an adequate repository or to help scientists seeking a specific transgenic line find a match [ 80 ].
Analyzing the data
Animal researchers have to handle increasingly complex data. Imaging, electrophysiological recording, or automated behavioral tracking, for example, produce huge datasets. Data can be shared as raw numerical output but also as images, videos, sounds, or other forms from which raw numerical data can be generated. As the heterogeneity and the complexity of research data increases, infinite possibilities for analysis emerge. Transparently reporting how the data were processed will enable peers to better interpret reported results. To get the most out of performed animal experiments, it is crucial to allow other scientists to replicate the analysis and adapt it to their research questions. It is therefore highly recommended to use formats and tools during the analysis that allow a straightforward exchange of code and data later on.
Transparent coding
The use of non-transparent analysis codes have led to a lack of reproducibility of results [ 81 ]. Sharing code is essential for complex analysis and enables other researchers to reproduce results and perform follow-up studies, and citable code gives credit for the development of new algorithms ( Table 1 ). Jupyter Notebooks are a convenient way to share data science pipelines that may use a variety of coding languages, including like Python, R or Matlab, and also share the results of analyses in the form of tables, diagrams, images, and videos. Notebooks contain source code and can be published or collaboratively shared on platforms like GitHub or GitLab, where version control of source code is implemented. The data-archiving tool Zenodo can be used to archive a repository on GitHub and create a DOI for the archive. Thereby contents become citable. Using free and open-source programming language like R or Python will increase the number of potential researchers that can work with the published code. Best practice for research software is to publish the source code with a license that allows modification and redistribution.
Choice of data visualization
Choosing the right format for the visualization of data can increase its accessibility to a broad scientific audience and enable peers to better judge the validity of the results. Studies based on animal research often work with very small sample sizes. Visualizing these data in histograms may lead to an overestimation of the outcomes. Choosing the right dot plots that makes all recorded points visible and at the same time focusses on the summary instead of the individual points can further improve the intuitive understanding of a result. If the sample size is too low, it might not be meaningful to visualize error bars. A variety of freely available tools already exists that can support scientists in creating the most appropriate graphs for their data [ 82 ]. In particular, when representing microscopy results or heat maps, it should be kept in mind that a large part of the population cannot perceive the classical red and green representation [ 83 ]. Opting for the color-blind safe color maps and checking images with free tools such as color oracle ( Table 1 ) can increase the accessibility of graphs. Multiple journals have already addressed flaws in data visualization and have introduced new policies that will accelerate the uptake of transparent representation of results.
Publication of all study outcomes
Open science practices have received much attention in the past few years when it comes to publication of the results. However, it is important to emphasize that although open science tools have their greatest impact at the end of the project, good study preparation and sharing of the study plan and data early on can greatly increase the transparency at the end.
The FAIR data principle
To maximize the impact and outcome of a study, and to make the best long-term use of data generated through animal experiments, researchers should publish all data collected during their research according to the FAIR data principle. That means the data should be findable, accessible, interoperable, and reusable. The FAIR principle is thus an extension of open access publishing. Data should not only be published without paywalls or other access restrictions, but also in such a manner that they can be reused and further processed by others. For this, legal as well as technical requirements must be met by the data. To achieve this, the GoFAIR initiative has developed a set of principles that should be taken into account as early as at the data collection stage [ 49 , 84 ]. In addition to extensively described and machine-readable metadata, these principles include, for example, the application of globally persistent identifiers, the use of open file formats, and standardized communication protocols to ensure that humans and machines can easily download the data. A well-chosen repository to upload the data is then just the final step to publish FAIR data.
FAIR data can strongly increase the knowledge gained from performed animal experiments. Thus, the same data can be analyzed by different researchers and could be combined to obtain larger sample sizes, as already occurs in the neuroimaging community, which works with comparable datasets [ 85 ]. Furthermore, the sharing of data enables other researchers to analyze published datasets and estimate measurement reliabilities to optimize their own data collection [ 86 , 87 ]. This will help to improve the translation from animal research into clinics and simultaneously reduce the number of animal experiment in future.
Reporting guidelines
In preclinical research, the ARRIVE guidelines are the current state of the art when it comes to reporting data based on animal experiments [ 22 , 23 ]. The ARRIVE guidelines have been endorsed by more than 1,000 journals who ask that scientists comply with them when reporting their outcomes. Since the ARRIVE guidelines have not had the expected impact on the transparency of reporting in animal research publications, a more rigorous update has been developed to facilitate their application in practice (ARRIVE 2.0 [ 23 ]). We believe that the ARRIVE guidelines can be more effective if they are implemented at a very early stage of the project (see section on guidelines). Some more specialized reporting guidelines have also emerged for individual research fields that rely on animal studies, such as endodontology [ 88 ]. The equator network collects all guidelines and makes them easily findable with their search tool on their website ( Table 1 ). MERIDIAN also offers a 1-stop shop for all reporting guidelines involving the use of animals across all research sectors [ 89 ]. It is thus worth checking for new reporting guidelines before preparing a manuscript to maximize the transparency of described experiments.
Identifiers
Persistent identifiers for published work, authors, or resources are key for making public data findable by search engines and are thus a prerequisite for compliance to FAIR data principles. The most common identifier for publications will be a DOI, which makes the work citable. A DOI is a globally unique string assigned by the International DOI Foundation to identify content permanently and provide a persistent link to its location on the Internet. An ORCID ID is used as a personal persistent identifier and is recommendable to unmistakably identify an author ( Table 1 ). This will avoid confusions between authors with the same name or in the case of name changes or changes of affiliation. Research Resource Identifiers (RRID) are unique ID numbers that help to transparently report research resources. RRID also apply to animals to clearly identify the species used. RRID help avoid confusion between different names or changing names of genetic lines and, importantly, make them machine findable. The RRID Portal helps scientists find a specific RRID or create one if necessary ( Table 1 ). In the context of genetically altered animal lines, correct naming is key. The Mouse Genome Informatics (MGI) Database is the authoritative source of official names for mouse genes, alleles, and strains ([ 90 ]).
Preprint publication
Preprints have undergone unprecedented success, particularly during the height of the Coronavirus Disease 2019 (COVID-19) pandemic when the need for rapid dissemination of scientific knowledge was critical. The publication process for scientific manuscripts in peer-reviewed journals usually requires a considerable amount of time, ranging from a few months to several years, mainly due to the lengthy review process and inefficient editorial procedures [ 91 , 92 ]. Preprints typically precede formal publication in scientific journals and, thus, do not go through a peer review process, thus, facilitating the prompt open dissemination of important scientific findings within the scientific community. However, submitted papers are usually screened and checked for plagiarism. Preprints are assigned a DOI so they can be cited. Once a preprint is published in a journal, its status is automatically updated on the preprint server. The preprint is linked to the publication via CrossRef and mentioned accordingly on the website of the respective preprint platform.
After initial skepticism, most publishers now allow papers to be posted on preprint servers prior to submission. An increasing number of journals even allow direct submission of a preprint to their peer review process. The US National Institutes of Health and the Wellcome Trust, among other funders, also encourage prepublication and permit researchers to cite preprints in their grant applications. There are now numerous preprint repositories for different scientific disciplines. BioASAP provides a searchable database for preprint servers that can help in identifying the one that best matches an individual’s needs [ 93 ]. The most popular repository for animal research is bioRxiv, which is hosted by the Cold Spring Harbor Laboratory ( Table 1 ).
The early exchange of scientific results is particularly important for animal research. This acceleration of the publication process can help other scientists to adapt their research or could even prevent animal experiments if other scientists become aware that an experiment has already been done before starting their own. In addition, preprints can help to increase the visibility of research. Journal articles that have a corresponding preprint publication have higher citation and Altmetric counts than articles without preprint [ 94 ]. In addition, the publication of preprints can help to combat publication bias, which represents a major problem in animal research [ 16 ]. Since journals and readers prioritize cutting-edge studies with positive results over inconclusive or negative results, researchers are reluctant to invest time and money in a manuscript that is unlikely to be accepted in a high-impact journal.
In addition to the option of publishing as preprint, other alternative publication formats have recently been introduced to facilitate the publication of research results that are hard to publish in traditional peer-reviewed journals. These include micro publications, data repositories, data journals, publication platforms, and journals that focus on negative or inconclusive results. The tool fiddle can support scientists in choosing the right publication format [ 95 , 96 ].
Open access publication
Publishing open access is one of the most established open science strategies. In contrast to the FAIR data principle, the term open access publication refers usually to the publication of a manuscript on a platform that is accessible free of charge—in translational biomedical research, this is mostly in the form of a scientific journal article. Originally, publications accessible free of charge were the answer to the paywalls established by renowned publishing houses, which led to social inequalities within and outside the research system. In translational biomedical research, the ethical aspect of urgently needed transparency is another argument in favor of open access publication, as these studies will not only be findable, but also internationally readable.
There are different ways of open access publishing; the 2 main routes are gold open access and green open access. Numerous journals offer now gold open access. It refers to the immediate and fully accessible publication of an article. The Directory of Open Access Journals (DOAJ) provides a complete and updated list for high-quality, open access, and peer-reviewed journals [ 97 ]. Charité–Universitätsmedizin Berlin offers a specific tool for biomedical open access journals that supports animal researchers to choose an appropriate journal [ 49 ]. In addition, the Sherpa Romeo platform is a straightforward way to identify publisher open access policies on a journal-by-journal basis, including information on preprints, but also on licensing of articles [ 51 ]. Hybrid open access refers to openly accessible articles in otherwise paywalled journals. By contrast, green open access refers to the publication of a manuscript or article in a repository that is mostly operated by institutions and/or universities. The publication can be exclusively on the repository or in combination with a publisher. In the quality-assured, global Directory of Open Access Repositories (openDOAR), scientists can find thousands of indexed open access repositories [ 49 ]. The publisher often sets an embargo during which the authors cannot make the publication available in the repository, which can restrict the combined model. It is worth mentioning that gold open access is usually more expensive for the authors, as they have to pay an article processing charge. However, the article’s outreach is usually much higher than the outreach of an article in a repository or available exclusively as subscription content [ 98 ]. Diamond open access refers to publications and publication platforms that can be read free of charge by anyone interested and for which no costs are incurred by the authors either. It is the simplest and fairest form of open access for all parties involved, as no one is prevented from participating in scientific discourse by payment barriers. For now, it is not as widespread as the other forms because publishers have to find alternative sources of revenue to cover their costs.
As social media and the researcher’s individual public outreach are becoming increasingly important, it should be remembered that the accessibility of a publication should not be confused with the licensing under which the publication is made available. In order to be able to share and reuse one’s own work in the future, we recommend looking for journals that allow publications under the Creative Commons licenses CC BY or CC BY-NC. This also allows the immediate combination of gold and green open access.
Creative commons licenses
Attributing Creative Commons (CC) licenses to scientific content can make research broadly available and clearly specifies the terms and conditions under which people can reuse and redistribute the intellectual property, namely publications and data, while giving the credit to whom it deserves [ 49 ]. As the laws on copyright vary from country to country and law texts are difficult to understand for outsiders, the CC licenses are designed to be easily understandable and are available in 41 languages. This way, users can easily avoid accidental misuse. The CC initiative developed a tool that enables researchers to find the license that best fits their interests [ 49 ]. Since the licenses are based on a modular concept ranging from relatively unrestricted licenses (CC BY, free to use, credit must be given) to more restricted licenses (CC BY-NC-ND, only free to share for non-commercial purposes, credit must be given), one can find an appropriate license even for the most sensitive content. Publishing under an open CC license will not only make the publication easy to access but can also help to increase its reach. It can stimulate other researchers and the interested public to share this article within their network and to make the best future use of it. Bear in mind that datasets published independently from an article may receive a different CC license. In terms of intellectual property, data are not protected in the same way as articles, which is why the CC initiative in the United Kingdom recommends publishing them under a CC0 (“no rights reserved”) license or the Public Domain Mark. This gives everybody the right to use the data freely. In an animal ethics sense, this is especially important in order to get the most out of data derived from animal experiments.
Data and code repositories
Sharing research data is essential to ensure reproducibility and to facilitate scientific progress. This is particularly true in animal research and the scientific community increasingly recognizes the value of sharing research data. However, even though there is increasing support for the sharing of data, researchers still perceive barriers when it comes to doing so in practice [ 99 – 101 ]. Many universities and research institutions have established research data repositories that provide continuous access to datasets in a trusted environment. Many of these data repositories are tied to specific research areas, geographic regions, or scientific institutions. Due to the growing number and overall heterogeneity of these repositories, it can be difficult for researchers, funding agencies, publishers, and academic institutions to identify appropriate repositories for storing and searching research data.
Recently, several web-based tools have been developed to help in the selection of a suitable repository. One example is Re3data, a global registry of research data repositories that includes repositories from various scientific disciplines. The extensive database can be searched by country, content (e.g., raw data, source code), and scientific discipline [ 49 ]. A similar tool to help find a data archive specific to the field is FAIRsharing, based at Oxford University [ 102 ]. If there is no appropriate subject-specific data repository or one seems unsuitable for the data, there are general data repositories, such as Open Science Framework, figshare, Dryad, or Zenodo. To ensure that data stored in a repository can be found, a DOI is assigned to the data. Choosing the right license for the deposited code and data ensures that authors get credit for their work.
Publication and connection of all outcomes
If scientists have used all available open science tools during the research process, then publishing and linking all outcomes represents the well-deserved harvest ( Fig 2 ). At the end of a research process, researchers will not just have 1 publication in a journal. Instead, they might have a preregistration, a preprint, a publication in a journal, a dataset, and a protocol. Connecting these outcomes in a way that enables other scientists to better assess the results that link these publications will be key. There are many examples of good open science practices in laboratory animal science, but we want to highlight one of them to show how this could be achieved. Blenkuš and colleagues investigated how mild stress-induced hyperthermia can be assessed non-invasively by thermography in mice [ 103 ]. The study was preregistered with animalstudyregistry.org , which is referred to in their publication [ 104 ]. A deviation from the originally preregistered hypothesis was explained in the manuscript and the supplementary material was uploaded to figshare [ 105 ].
Application of open science practices can increase the reproducibility and visibility of a research project at the same time. By publishing different research outputs with more detailed information than can be included in a journal article, researchers enable peers to replicate their work. Reporting according to guidelines and using transparent visualization will further improve this reproducibility. The more research products that are generated, the more credit can be attributed. By communicating on social media or additionally publishing slides from delivered talks or posters, more attention can be raised. Additionally, publishing open access and making the work machine-findable makes it accessible to an even broader number of peers.
https://doi.org/10.1371/journal.pbio.3001810.g002
It might also be helpful to provide all resources from a project in a single repository such as Open Science Framework, which also implements other, different tools that might have been used, like GitHub or protocols.io.
Communicating your research
Once all outcomes of the project are shared, it is time to address the targeted peers. Social media is an important instrument to connect research communities [ 106 ]. In particular, Twitter is an effective way to communicate research findings or related events to peers [ 107 ]. In addition, specialized platforms like ResearchGate can support the exchange of practical experiences ( Table 1 ). When all resources related to a project are kept in one place, sharing this link is a straightforward way to reach out to fellow scientists.
With the increasing number of publications, science communication has become more important in recent years. Transparent science that communicates openly with the public contributes to strengthening society’s trust in research.
Conclusions
Plenty of open science tools are already available and the number of tools is constantly growing. Translational biomedical researchers should seize this opportunity, as it could contribute to a significant improvement in the transparency of research and fulfil their ethical responsibility to maximize the impact of knowledge gained from animal experiments. Over and above this, open science practices also bear important direct benefits for the scientists themselves. Indeed, the implementation of these tools can increase the visibility of research and becomes increasingly important when applying for grants or in recruitment decisions. Already, more and more journals and funders require activities such as data sharing. Several institutions have established open science practices as evaluation criteria alongside publication lists, impact factor, and h-index for panels deciding on hiring or tenure [ 108 ]. For new adopters, it is not necessary to apply all available practices at once. Implementing single tools can be a safe approach to slowly improve the outreach and reproducibility of one’s own research. The more open science products that are generated, the more reproducible the work becomes, but also the more the visibility of a study increases ( Fig 2 ).
As other research fields, such as social sciences, are already a step ahead in the implementation of open science practices, translational biomedicine can profit from their experiences [ 109 ]. We should thus keep in mind that open science comes with some risks that should be minimized early on. Indeed, the more open science practices become incentivized, the more researchers could be tempted to get a transparency quality label that might not be justified. When a study is based on a bad hypothesis or poor statistical planning, this cannot be fixed by preregistration, as prediction alone is not sufficient to validate an interpretation [ 110 ]. Furthermore, a boom of data sharing could disconnect data collectors and analysts, bearing the risk that researchers performing the analysis lack understanding of the data. The publication of datasets could also promote a “parasitic” use of a researcher’s data and lead to scooping of outcomes [ 111 ]. Stakeholders could counteract such a risk by promoting collaboration instead of competition.
During the COVID-19 pandemic, we have seen an explosion of preprint publications. This unseen acceleration of science might be the adequate response to a pandemic; however, the speeding up science in combination with the “publish or perish” culture could come at the expense of the quality of the publication. Nevertheless, a meta-analysis comparing the quality of reporting between preprints and peer-reviewed articles showed that the quality of reporting in preprints in the life sciences is at most slightly lower on average compared to peer-reviewed articles [ 112 ]. Additionally, preprints and social media have shown during this pandemic that a premature and overconfident communication of research results can be overinterpreted by journalists and raise unfounded hopes or fears in patients and relatives [ 113 ]. By being honest and open about the scope and limitations of the study and choosing communication channels carefully, researchers can avoid misinterpretation. It should be noted, however, that by releasing all methodological details and data in research fields such as viral engineering, where a dual use cannot be excluded, open science could increase biosecurity risk. Implementing access-controlled repositories, application programming interfaces, and a biosecurity risk assessment in the planning phase (i.e., by preregistration) could mitigate this threat [ 114 ].
Publishing in open access journals often involves higher publication costs, which makes it more difficult for institutes and universities from low-income countries to publish there [ 115 ]. Equity has been identified as a key aim of open science [ 116 ]. It is vital, therefore, that existing structural inequities in the scientific system are not unintentionally reinforced by open science practices. Early career researchers have been the main drivers of the open science movement in other fields even though they are often in vulnerable positions due to short contracts and hierarchical and strongly networked research environments. Supporting these early career researchers in adopting open science tools could significantly advance this change in research culture [ 117 ]. However, early career researchers can already benefit by publishing registered reports or preprints that can provide a publication much faster than conventional journal publications. Communication in social media can help them establish a network enabling new collaborations or follow-up positions.
Even though open science comes with some risks, the benefits easily overweigh these caveats. If a change towards more transparency is accompanied by the implementation of open science in the teaching curricula of the universities, most of the risks can be minimized [ 118 ]. Interestingly, we have observed that open science tools and infrastructure that are specific to animal research seem to mostly come from Europe. This may be because of strict regulations within Europe for animal experiments or because of a strong research focus in laboratory animal science along with targeted research funding in this region. Whatever the reason might be, it demonstrates the important role of research policy in accelerating the development towards 3Rs and open science.
Overall, it seems inevitable that open science will eventually prevail in translational biomedical research. Scientists should not wait for the slow-moving incentive framework to change their research habits, but should take pioneering roles in adopting open science tools and working towards more collaboration, transparency, and reproducibility.
Acknowledgments
The authors gratefully acknowledge the valuable input and comments from Sebastian Dunst, Daniel Butzke, and Nils Körber that have improved the content of this work.
- View Article
- PubMed/NCBI
- Google Scholar
- 6. Cary Funk MH, Brian Kennedy, Courtney Johnson. Americans say open access to data and independent review inspire more trust in research findings. Pew Research Center Website: Pew Research Center; 2019. Available from: https://www.pewresearch.org/science/2019/08/02/americans-say-open-access-to-data-and-independent-review-inspire-more-trust-in-research-findings/ .
- 7. International Reproducibility Networks. International Networks Statement UK Reproducibility Network Website: UK Reproducibility Network. 2021. Available from: https://cpb-eu-w2.wpmucdn.com/blogs.bristol.ac.uk/dist/b/631/files/2021/09/International-Networks-Statement-v1.0.pdf .
- 13. Article 36 of Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 amended by Regilation (EU) 2019/1010 of the European Parliament and of the Council of 5 June 2019. OJEU. 2010;L276:36.
- 19. American Association for Cancer Research. Editorial Policies. 2021. Available from: https://aacrjournals.org/content/authors/editorial-policies .
- 21. Diederich K, Schmitt K, Schwedhelm P, Bert B, Heinl C. Open Science Toolbox for Animal Research. Zenodo. 2022. Available from: https://zenodo.org/record/6497560 .
- 29. NC3R. ARRIVE guidelines. NC3R Website. Available from: https://arriveguidelines.org/ .
- 31. Canadian Institutes of Health Research. How to integrate sex and gender into research. Website of the Canadian Institutes of Health Research: Canadian Institutes of Health Research. 2019 [cited 2019 Aug 21]. Available from: https://cihr-irsc.gc.ca/e/50836.html .
- 33. Simon T, Bate RAC. InVivoStat. Available from: https://invivostat.co.uk/ .
- 35. Urbaniak G, Plous S. Research randomizer (version 4.0) [computer software]. 2013.
- 47. Medical Research Council’s. Data sharing policy. UK Research and Innovation Website 2021. Available from: https://www.ukri.org/publications/mrc-data-sharing-policy/ .
- 48. University of California Curation Center. DMPTool. 2021. Available from: https://dmptool.org/ .
- 49. Digital Curation Centre. DMPOnline. Available from: https://dmponline.dcc.ac.uk/ . Digital Curation Centre; 2021.
- 50. Harvard Longwood Medical Area Research Data Management Working Group. Biomedical Data Lifecycle. Harvard Medical School Website: Harvard Medical School; 2021. Available from: https://datamanagement.hms.harvard.edu/about/what-research-data-management/biomedical-data-lifecycle .
- 51. Joint Information Systems Committee. Research data management toolkit JISC Website: JISC; 2018. Available from: https://www.jisc.ac.uk/guides/rdm-toolkit .
- 54. German Centre for the Protection of Laboratory Animals (Bf3R). NTPs—Nicht Technische Projektzusammenfassungen 3R-SMART; 2020. Available from: https://www.3r-smart.de/index.php?id=6895 .
- 55. Understanding Animal Research. Guide to writing non-technical summaries concordat on openness on animal research in the UK2018. Available from: https://concordatopenness.org.uk/guide-to-writing-non-technical-summaries .
- 56. Gerlach B, Untucht C, Stefan A. Electronic Lab Notebooks and Experimental Design Assistants. In: Bespalov A, Michel MC, Steckler T, editors. Good Research Practice in Non-Clinical Pharmacology and Biomedicine. Cham: Springer International Publishing; 2020. p. 257–75.
- 58. Adam BL, Birte L. ELN Guide: electronic laboratory notebooks in the context of research data management and good research practice–a guide for the life sciences. Cologne, Germany: ZB MED–Information Centre for Life Sciences; 2021.
- 59. AgileBio. LabCollector Website https://labcollector.com/labcollector-lims/features/modules/animals-module/2022 . Available from: https://labcollector.com/labcollector-lims/features/modules/animals-module/ .
- 61. Harvard Longwood Medical Area Research Data Management Working Group. Electronic Lab Notebook Comparison Matrix. Zenodo. 2021.
- 70. Bio-protocol. Collaborating Journals bio-protocol website2021. Available from: https://bio-protocol.org/default.aspx?dw=Collaborating .
- 76. Dinkel H. anishare: GitHub; [updated June 2018]. Available from: https://github.com/hdinkel/anishare .
- 89. O’Connor AM. MERIDIAN: Menagerie of Reporting guidelines Involving Animals. Iowa State University; 2022. Available from: https://meridian.cvm.iastate.edu/ .
- 90. The Jackson Laboratory. Mouse Nomenclature Home Page at the Mouse Genome Informatics website World Wide Web: The Jackson Laboratory,Bar Harbor, Maine. Available from: http://www.informatics.jax.org/mgihome/nomen/index.shtml .
- 97. Directory of Open Access Journals. Find open access journals & articles. Available from: https://doaj.org/ . Directory of Open Access Journals, [DOAJ]; 2021.
- 98. Gold Open Access research has greater societal impact as used more outside of academia [press release]. Springer Nature Website: Springer. Nature. 2020;30:2020.
- 104. Franco NH. Can we use infrared thermography for assessing emotional states in mice?—A comparison between handling-induced stress by different techniques. Available from: animalstudyregistry.org . German Federal Institute for Risk Assessment (BfR); 2020. https://doi.org/10.17590/asr.0000224

The global resource for scientific evidence in animal research
Where you can find detailed scientific information about the use of animals...

Venom-derived drugs
A guide to the venomous creatures that help in imagining and creating drugs
The developments in medicine since the end of the 19th...
Top 20 prescribed medicines
How do they work and what animals were used during their discovery?
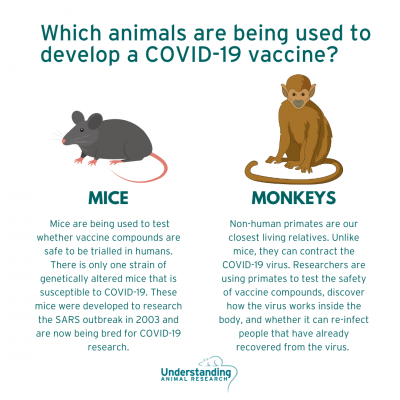
Animal research & COVID-19
Animal research and COVID-19 / SARS-COV-2 coronavirus

Nobel Prizes
The animal research behind a century of Nobel Prizes

Paget Lectures
Annual lectures given by prominent scientists.

Animals used in research
A-Z of animals used in research
Quotes database
Quotes about animal research from scientists, politicians and scientific organisations.
Read some of the most common questions we get asked.
Animal research articles
Browse articles about the role that animal research plays in science.
If you have questions or a research paper you would like to include please contact us.
- Understanding Animal Research
- Animal Rights Extremism
© 2024 Understanding Animal Research

Writing Unit of Study: Animal Research Project

This free animal research project will provide you with a writing unit of study that will help you build excitement about writing informational text in your classroom.
You can download this free animal research project to help your writers develop their research and writing skills.
This project will be a great fit for your first, second or third grade writing workshop.
This is another free resource for teachers and homeschool families from The Curriculum Corner.

Why should I introduce my students to research through animal study?
Animal research can be a great topic for writing informational text because students tend to be curious about animals.
Nothing seems to spark interest in most kids like learning about animals in our world. Turn their enthusiasm into an engaging animal research writing project.
They can take the time to learn about different habitats and diets.
You can also encourage students to expand their vocabulary by having them create a glossary to accompany their writing.

About this animal research project
Within this post you will find over 30 pages of anchor charts, mini-lesson ideas, writing planners and graphic organizers.
The unit will help guide your students through the complete process. In the end, you will be helping to teach your students how to write their own pieces of informational text.
The intended end product for students is an animal booklet that they can staple together to share with others.
Students who are ready for more advanced work, can create a larger project with less direction.
A description of the mini-lessons
Lesson 1: introduction.
- Begin the unit by having the students brainstorm a list of animals that they might see everyday.
- Then, have them brainstorm a list of animals they see when they visit the zoo or walk in the forest. You can do this on the blank anchor chart provided or on cart paper.
- Another option is to place students in groups. They could work to create a list together.
- You might assign each group a continent and have them find animals that live there.
- Pull the class together and have each group share what animals they found that live on their continent.
Lesson 2: Noticings
- Next you might want to get your students familiar with common characteristics about informational texts that teach about animals.
- Have them work in pairs or small groups to go through some books and record their “noticings” about the writing.
- Then come together in a community circle to discuss those noticings and create a class anchor chart.

Lesson 3: Opinion vs. Facts
- Before getting truly into this unit, you might need to conduct a lesson on opinions vs. facts.
- After a brief discussion you can use the giraffe paragraph provided in our resources to give your students some practice differentiating between the two. This paragraph contains both opinions and facts.
- With your class read through the paragraph and record facts and opinions on the T-chart.
- Discuss both sides and how they are different from each other.
- A black & white copy of this giraffe paragraph has also been provided. You can have them work in pairs or groups to distinguish between the facts and opinions.
- If you need more resources for your students surrounding fact & opinion check out our Fact & Opinion Sort .
Lesson 4: Choosing a Topic for the Animal Research Project
- We want to help students to narrow their topic choices by giving them some guidance.
- Gather students and begin a discussion about choosing an animal research topic.
- For this lesson we have provided two pages where students can individually brainstorm the animals they are interested in.
- You might have students work in groups or independently to make their choice. Conference with students as needed to help.
- Don’t shy away from letting more than one student research about the same animal. This can be a great way to promote group work. It might also help out with some of your literacy center choices throughout this unit.
Lesson 5: Good Places to Find Information about an Animal
- At this age we want students to begin to understand that all they read online about animals isn’t always true. Sometimes writing might sound true without being filled with facts.
- Show students two possible places to find information online about their animal. One should be a trusted site with reliable and accurate information. Another should be a site that perhaps a child has created. (There are many that you can find if you search.)
- Pose these questions: Is everything on the internet true? Why? How can you tell? Why is it important for your research writing to contain accurate information?

Lesson 6: Taking Notes
- Sometimes giving students resources and a blank sheet of notebook paper can be too overwhelming for them. Some students will copy word for word. Others might feel overwhelmed. We need to guide them to read and pull out facts & relevant information to use later in their writing.
- For this lesson we have provided four templates for note-taking that you might choose to use for your students.
- You might need to provide different organizers to students depending on their needs.
- You will want to model the organizers your students are use. Show them how to take notes as they read.
- After initial teaching, you may find that you need to pull small groups for extra practice. Others might benefit from a conference as you take a look at the notes they are taking.
Lesson 7: Word Choice in Research Writing
- To help students think about making their writing more interesting, have them brainstorm words about their animal.
- Together brainstorm words that would be appropriate for animals. They might add words about what they look like, their movement, their habitats, their life cycles, their diets, etc. You can create a class anchor chart on the page provided. You might even think about using the real life picture of the wolf in the download. This can get the students to begin thinking of more interesting words for animals (fierce, mighty, strong, etc).
- Then, pass out the individual brainstorm pages. Students can use the anchor chart as a guide to begin their own word choice pages about their animal. This might be a good partner activity as well.
Lesson 8: Writing Sketch for the Animal Research Project
- Next, you can model the writing sketch planner for your class.
- One idea to help your students narrow down all of the information they have learned about their animals is to give them a specific number of animals facts that they can focus on.
- Each of these facts can serve as the actual text that they will put on each page of their animal research book. Or the facts could serve as a focus for each paragraph in their writing.
- You might find that this would be a good mini-lesson to do with smaller groups of children.
Lesson 9: Creating a Table of Contents
- Another idea that can be a writing planner AND a page in their animal research book is the table of contents. Pull out one of the Table of Contents pages from the resources provided and model how to fill in the blanks on each page.
- This page will then serve as their Table of Contents (with a focus discussion on what that is and the purpose it serves) and also their writing planner so they know what they will put in the pages of their booklet.
Lesson 10: Creating a Glossary
- There are two pages provided in the resources that might help your students to learn to pull out topic specific words to put into a glossary for the end of their animal research book.
- Be sure to model how you would like for your students to use these organizers (keeping in mind that you may need to copy more than one page if there are more words than the page provides for).
- If your students need a refresher on ABC order check out these links for some added practice/review: ABC Order Task Cards & Fry Word ABC Order Task Cards
Lesson 11: Writing Your Animal Research
- You will decide on the best method for your students to showcase their published animal research.
- You may want your students to use their own creativity in the texts that they write and share. If you’d like a first experience to provide a bit more guidance, we have provided two different sets of pages for booklets.
- One is more guided and the other has less structure and smaller lines for more writing. 15 pages are provided so that you or students can pick what fits their needs.
- This “lesson” may actually become a series of lessons if you choose to model how each page can be used. (We have also included a page with simple writing lines in case students need less guidance than the booklet pages provided.)

Lesson 12: Labeling Pictures
- One final lesson idea that pairs well with writing informational text is to teach your students how to label pictures.
- Since most nonfiction writing has real photographs, students can find some pictures online to print out and label for their booklet. Hand-drawn pictures are also great if you would rather encourage some or all of your students in that direction.
- Whatever you choose, show your class how to effectively label a picture so that it teaches the reader more. You can use the picture of the polar bear provided to model how to add words or even short facts as labels. (For example if the simple label “fur” wouldn’t add additional information to the book, you might teach them to label it with a short fact such as “dense fur protects the animal’s skin from the weather”.
- To make this idea more user friendly, you might want them to use the page of blank white boxes provided to write their labels for their pictures. Then all they need to do is cut them out and glue them to a printed picture.
Lesson 13: Writing Celebration
As always, find a way to celebrate your students’ writing.
Invite guests (younger students or special adults) to read the books with your young authors. You might simply want to pair or group them, or some students might choose to present their book to everyone.
Provide some light snacks if possible to give it a party atmosphere and pass out the author certificates to each child for his/her hard work.
You can download this free writing unit of study here:
Writing Download
As with all of our resources, The Curriculum Corner creates these for free classroom use. Our products may not be sold. You may print and copy for your personal classroom use. These are also great for home school families!
You may not modify and resell in any form. Please let us know if you have any questions.
Christine E.
Saturday 8th of May 2021
Thank you so much for this resource and the many pages that I can use in my homeschooling. It is exactly what I've been looking for to help me get my kids to write about our animal units! You are doing a great job, keep up the amazing work you do. I appreciate the hard work you put into putting these together.
Planning a Dynamic Writing Workshop - The Curriculum Corner 123
Saturday 14th of July 2018
[…] Animal Research […]
Editable Writing Management Binder - The Curriculum Corner 123
Friday 3rd of March 2017
[…] Writing Unit of Study: Animal Research […]

- ELEMENTARY TEACHING , INTEGRATED CURRICULUM ACTIVITIES
Animal Research Project for Kids at the Elementary Level in 2024
Whether you are doing a simple animal study or a fully integrated science, reading, and writing unit, this animal research project for kids includes everything you need. From the graphic organizer worksheets and guided note templates to the writing stationary, printable activities, projects, and rubrics.
Thousands of teachers have used this 5-star resource to have students complete self-guided animal research projects to learn about any animal they choose. The best part is, the resource can be used over and over again all year long by just picking a new animal! Learn all about this animal research project for kids at the elementary level below!

What is the Animal Research Project?
The animal research project is a resource that is packed with printable and digital activities and projects to choose from. It is perfect for elementary teachers doing a simple animal study or a month-long, fully integrated unit. It’s open-ended nature allows it to be used over and over again throughout the school year. In addition, it includes tons of differentiated materials so you can continue to use it even if you change grade levels. Learn about what’s included in it below!

What is Included in the Animal Research Project
The following resources are included in the animal research project :
Teacher’s Guide
The teacher’s guide includes tips and instructions to support you with your lesson planning and delivery.
Parent Letter
The parent communication letter promotes family involvement.
Graphic Organizers
There are graphic organizers for brainstorming a topic, activating schema, taking notes, and drafting writing.
Research Report
There are research report publishing printables including a cover, writing templates, and resource pages.
There is a grading rubric so expectations are clear for students and grading is quick and easy for you.
Research Activities
The research activities include a KWL chart, can have are chart, compare and contrast venn diagram, habitat map, vocabulary pages, illustration page, and life cycle charts.
Animal Flip Book Project
There are animal flip book project printables to give an additional choice of how students can demonstrate their understanding.
Animal Flap Book Project
There is an animal flap book project printables that offers students yet another way to demonstrate their learning.
Animal Research Poster
The animal research poster serves as an additional way to demonstrate student understanding.
Poetry Activities
The resource includes poetry activities to offer students an alternative way to demonstrate their learning.
Digital Versions
There is a digital version of the resource so your students can access this resource in school or at home.
Why Teachers love the Animal Research Project
Teachers love this animal research project because of the following reasons:
- This resource guides students through the research and writing process, so they can confidently work their way through this project.
- It is a great value because it can be used over and over again throughout the school year because the pages can be used to learn about any animal.
- It offers several ways students can demonstrate their learning.
- It includes a ton of resources, so you can pick and choose which ones work best for you and your students.
- It is printable and digital so it can be used for in-class and at-home learning.
This animal research packet is great because it can be used over and over again using absolutely any animal at all. The printables in this packet are ideal to use with your entire class in school, as an at-home learning extension project or as a purposeful, open-ended, independent choice for your students who often finish early and need an enrichment activity that is so much more than “busy work.”
The Research Report Process
This animal research project packet was designed in a manner that allows you to use all of the components when studying any animal. Because the printables can be used over and over, I will often work through the entire researching and writing process with the whole class focusing on one animal together, This allows me to model the procedure and provide them with support as they “get their feet wet” as researchers. Afterwards I then have them work through the process with an animal of choice. You may find it helpful to have them select from a specific category (i.e. ocean animals, rainforest animals, etc) as this will help to streamline the resources you’ll need to obtain.
Step 1: Brainstorm a list of animals to research. Select one animal.
During this stage you may want to provide the students with a collection of books and magazines to explore and help them narrow down their choice.

Step 2: Set a purpose and activate schema.
Students share why they selected the animal and tell what they already know about it. Next, they generate a list of things they are wondering about the animal. This will help to guide their research.
Step 3: Send home the family letter.
To save you time, involve families, and communicate what is happening in the classroom, you may want to send home a copy of the family letter. It’s so helpful when they send in additional research materials for the students.
Step 4: Research and take notes.
The two-column notes template is a research-based tool that helps the kids organize their notes. I added bulleted prompts to guide the students in finding specific information within each category. This method has proven to be highly effective with all students, but is especially useful with writers who need extra support.
I have included two versions of the organizers (with and without lines). I print a copy of the organizer for each student. I also copy the lined paper back to back so it is available to students who need more space.
Step 5: Write a draft.
Using the information gathered through the research process, the students next compose drafts. The draft papers were designed to guide the students through their writing by providing prompts in the form of questions. Answering these questions in complete sentences will result in strong paragraphs. It may be helpful to give them only one page at a time instead of a packet as it make the task more manageable.
Step 6: Edit the draft.
Editing can be done in many ways, but it is most effective when a qualified editor sits 1:1 with a student to provides effective feedback to them while editing.
Step 7: Publish.
Print several copies of the publishing pages. I like to have all my students start with the page that has a large space for an illustration, but then let them pick the pages they want to use in the order they prefer after that. I have them complete all the writing first and then add the illustrations.
Finally, have the children design a cover for the report. Add that to the front and add the resources citation page to the back. Use the criteria for success scoring rubric to assign a grade. The rubric was designed using a 20 point total so you can simply multiply their score by 5 to obtain a percentage grade. The end result is a beautiful product that showcases their new learning as well as documents their reading and writing skills.
In closing, we hope you found this animal research project for kids helpful! If you did, then you may also be interested in these posts:
- How to Teach Research Skills to Elementary Students
- 15 Animals in Winter Picture Books for Elementary Teachers
- How to Teach Informative Writing at the Elementary Level
You might also like...

Project Based Learning Activities for Elementary Students

Me on the Map Activities and Printables for Elementary Teachers – 2024

Student-Made Board Games Ideas for Elementary Teachers in 2024
Join the newsletter.

- CLUTTER-FREE TEACHER CLUB
- FACEBOOK GROUPS
- EMAIL COMMUNITY
- OUR TEACHER STORE
- ALL-ACCESS MEMBERSHIPS
- OUR TPT SHOP
- JODI & COMPANY
- TERMS OF USE
- Privacy Policy
Animal Studies and School Project Ideas
From Science Fair Project Ideas on Mammals to Experiments About Insects
David Williams / EyeEm / Getty Images
- Cell Biology
- Weather & Climate
- B.A., Biology, Emory University
- A.S., Nursing, Chattahoochee Technical College
Animal research is important for understanding various biological processes in animals , humans included. Scientists study animals in order to learn ways for improving their agricultural health, our methods of wildlife preservation, and even the potential for human companionship. These studies also take advantage of certain animal and human similarities to discover new methods for improving human health.
Learning From Animals
Researching animals to improve human health is possible because animal behavior experiments study disease development and transmission as well as animal viruses . Both of these fields of study help researchers to understand how disease interacts between and within animals.
We can also learn about humans by observing normal and abnormal behavior in non-human animals, or behavioral studies. The following animal project ideas help to introduce animal behavioral study in many different species. Be sure to get permission from your instructor before beginning any animal science projects or behavioral experiments, as some science fairs prohibit these. Select a single species of animal to study from each subset, if not specified, for best results.
Amphibian and Fish Project Ideas
- Does temperature affect tadpole growth?
- Do water pH levels affect tadpole growth?
- Does water temperature affect amphibian respiration?
- Does magnetism affect limb regeneration in newts?
- Does water temperature affect fish color?
- Does the size of a population of fish affect individual growth?
- Does music affect fish activity?
- Does the amount of light affect fish activity?
Bird Project Ideas
- What species of plants attract hummingbirds?
- How does temperature affect bird migration patterns?
- What factors increase egg production?
- Do different bird species prefer different colors of birdseed?
- Do birds prefer to eat in a group or alone?
- Do birds prefer one type of habitat over another?
- How does deforestation affect bird nesting?
- How do birds interact with manmade structures?
- Can birds be taught to sing a certain tune?
Insect Project Ideas
- How does temperature affect the growth of butterflies?
- How does light affect ants?
- Do different colors attract or repel insects?
- How does air pollution affect insects?
- How do insects adapt to pesticides?
- Do magnetic fields affect insects?
- Does soil acidity affect insects?
- Do insects prefer the food of a certain color?
- Do insects behave differently in populations of different sizes?
- What factors cause crickets to chirp more often?
- What substances do mosquitoes find attractive or repellent?
Mammal Project Ideas
- Does light variation affect mammal sleep habits?
- Do cats or dogs have better night vision?
- Does music affect an animal's mood?
- Do bird sounds affect cat behavior?
- Which mammal sense has the greatest effect on short-term memory?
- Does dog saliva have antimicrobial properties?
- Does colored water affect mammal drinking habits?
- What factors influence how many hours a cat sleeps in a day?
Science Experiments and Models
Performing science experiments and constructing models are fun and exciting ways to learn about science and supplement studies. Try making a model of the lungs or a DNA model using candy for these animal experiments.
- Biology Science Fair Project Ideas
- 23 Ideas for Science Experiments Using Plants
- 8th Grade Science Fair Project Ideas
- Human Body Project Ideas
- High School Science Fair Projects
- Household Product Testing Science Fair Projects
- Chemistry Science Fair Project Ideas
- Plastics & Polymers Science Fair Project Ideas
- Middle School Science Fair Project Ideas
- 9th Grade Science Fair Projects
- Animal Science Fair Project Ideas
- Elementary School Science Fair Projects
- Science Fair Experiment Ideas: Food and Cooking Chemistry
- Sports Science Fair Project Ideas
- Grade School Science Fair Project Ideas
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 September 2021
On the past, present, and future of in vivo science
- Ellen P. Neff 1
Lab Animal volume 50 , pages 273–276 ( 2021 ) Cite this article
269 Accesses
2 Citations
6 Altmetric
Metrics details
Lab Animal asked a group of experts in industry and academia about how the field has changed over their careers and how they think animal research can be improved in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Garner, J. P. et al. Lab. Anim. (NY) 46 , 103–113 (2017).
Article Google Scholar
Voelkl, B. et al. Nat. Rev. Neurosc. 21 , 384–393 (2020).
Article CAS Google Scholar
Kafkafi, N. et al. Neurosci. Biobehav. Rev. 87 , 218–232 (2018).
Agassi, J. Francis Bacon, Robert Boyle (eds), Boston Studies in the Philosophy and History of Science 298 (Springer Science + Business Media, Dordrecht, 2013).
Macleod, M. R. et al. PLoS Biol. 13 , e1002273 (2015).
Nuzzo, R. Nature 506 , 150–152 (2014).
Kilkenny, C. et al. PLoS ONE 4 , e7824 (2009).
van der Worp, H. B. et al. PLoS Med. 7 , e1000245 (2010).
Kilkenny, C. et al. PLoS Biol. 8 , e1000412 (2010).
Percie du Sert, N. et al. PLOS Biol. 18 , e3000410 (2020).
Percie du Sert, N. et al. PLOS Biol. 18 , e3000411 (2020).
Karp, N. A. & Fry, D. BMJ Open Sci. 5 , e100126 (2021).
Smith, A. J. Lab. Anim. Res. 36 , 21 (2020).
Bertelsen, T. & Øvlisen, K. Lab. Anim. https://doi.org/10.1177/00236772211014433 (2021).
Garner, J. P. ILAR J. 55 , 438–56 (2014).
Genzel, L. et al. Curr. Biol. 30 , R1014–R1018 (2020).
Eggel, M. & Wurbel, H. Lab. Anim. 55 , 233–24 (2021).
Voelkl, B. et al. PLoS Biol. 16 , e2003693 (2018).
Karp, N. A. et al. Sci. Rep. 10 , 6178 (2020).
Download references
Author information
Authors and affiliations.
Lab Animal http://www.nature.com/laban/
Ellen P. Neff
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ellen P. Neff .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Neff, E.P. On the past, present, and future of in vivo science. Lab Anim 50 , 273–276 (2021). https://doi.org/10.1038/s41684-021-00848-2
Download citation
Published : 24 September 2021
Issue Date : October 2021
DOI : https://doi.org/10.1038/s41684-021-00848-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Turning fifty.
Lab Animal (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

How to Explode Student Engagement with this Habitat Research Project

One HUGE 2nd grade standard is researching and learning about animals and their adaptations. Students LOVE this unit, but teachers can be intimidated by the overwhelming pressure involved in guiding student research at such a young age. I love doing this 2nd grade animal research project with my students every March! This project has been reworked for a digital platform as well .

I love to start by playing a Brain Pop Jr, Flocabulary or YouTube video for my kids on all of the different habitats that exist. Typically, we have previously researched habitats during our social studies unit before starting this writing project, so they already have the background knowledge.
Then, I let students pick the habitat they are most interested in studying. From there, they pick 3-4 animals that live in the habitat that they would like to research more about. We use National Geographic Kids , Epic! Books and library books [all free resources] to learn about our animals.
2. RESEARCH/PLANNING

The next day, I model my own notes for students. Then, I give students lots of time to research their animals and take notes. It is really important that you are walking around the room and guiding students during this time.
If you have a struggling group of writers, I like to work with them at the back table during this time. We all research the same animals and take notes together. This helps them build confidence and feel sure about their writing in future days.
3. DRAFTING
I break drafting days up into 2 days so that students can really focus on the craft of what they are writing. I also always model before releasing students to write on their own.

Depending on what we have covered so far in the year, I encourage students to be sure to add:
- embedded definitions
- transition words
- conjunctions
- adjectives, adverbs and prepositions where appropriate
- 3-4 details per fact
4. PUBLISHING/GRADING

On the last day for each animal (typically Friday), I give students time to publish. While they publish, I model then ask them to add a map and diagram to their writing. I also show them how to grade themselves on the rubric, so they can double check that they are not missing anything.
After they finish, I give them free time to explore other animals in their habitat while I grade their writing. I find grading at the end of each animal rather than at the end of the entire project saves me a TON of time.
We repeat steps 2-4 for either 3 or 4 animals. Some students may work faster, while some may take a bit more time on each step. I try to adjust the project to be appropriate for the majority of the class.

When the project is done, I try to find a special way for us to share our work. This can include sharing to younger buddies, parents or doing an author’s chair.
Since they work so hard on this project, we make a BIG DEAL out of the finished project, and I typically send it home with parents during conferences. It makes a great writing portfolio and talking piece with parents.

Teaching digitally or wanting to add a digital component to your writing block? This project can also be completed in a digital format . Students will go through the same process, completing all of their work on Google Slides rather than writing using paper and pencil.
Grab the resources pictured above here:

Do you teach about a 2nd grade animal research project each year? Drop your ideas in the comments below!
Some other posts you might find helpful are:
- Teaching Animal Habitats During Science Ideas
- Animal Adaptations Writing Project
- Life Science Unit: Animal Adaptation
Emily - The Mountain Teacher
Share your thoughts... cancel reply.
Your email address will not be published. Required fields are marked *
DON'T MISS THE LATEST FREEBIES, RESOURCES, IDEAS & MORE!
Quick links.
- The Mountain Teacher 2024
- Site Design by Laine Sutherland Designs
Library Management
Lesson Plans
Reading Motivation

Library Skills
Reading & Literacy

5 Animal Research Websites for Students
- stayingcoolinthelibrary
- February 24, 2019
- Lesson Plans , Research

Ready to do some animal research? Finding trustworthy and appropriate animal websites for students to use can be a challenge. Below are my go-to websites that you can feel confident having your students go to. Most of these sites also have videos, games and other educational activities as well.
National Geographic for Kids

DK Find Out
San Diego Zoo Kids
These websites below do have some advertisements on them.

Animal Fact Guide
Ready to research and use these animal websites for students?

Thank you for subscribing! A confirmation message has been sent to you with a link you MUST click on in order to begin receiving emails and gain access to the free resource library. Open your inbox and look for a message from " [email protected] ".
There was an error submitting your subscription. Please try again.
QUICK LINKS
RESOURCE LIBRARY
BESTSELLING PRODUCTS
DIGITAL LITERACY SKILLS
RESEARCH SKILLS
NOPREP PRINTABLES
PICTURE BOOK ACTIVITIES
LEGAL STUFF
DISCLOSURE POLICY
PRIVACY POLICY
TERMS AND CONDITIONS
© 2024 STAYING COOL IN THE LIBRARY | All Rights Reserved
Privacy Overview

- About Grants
- Forms & Deadlines
- Grants Policy
- News & Events

- Updated NIH Animals In Research Webpage Collection
Updated NIH Animals in Research Webpage Collection
NIH has updated and expanded its content and resources discussing the involvement of Animals in NIH Funding. The website is designed for a general audience, providing a plain language explanation of the following:
- Why Animals are Used in Research
- How Animals Have Helped Improve Public Health
- Why Properly Designed Experiments Are Critical for Animal Research, and Advancing Public Health
- Ensuring the Care of Research Animals
- Adoption of Laboratory Animals after Research (new)
- When Are Alternatives to Animals Used in Research?
Additional resources on the topics highlight webinars, blog posts, press releases, podcasts, links to other webpages, and more based in part from prior feedback received.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- Rumor Control
Facts about FDA and Animal Welfare, Testing & Research
Medical and veterinary products save lives every day in the U.S. FDA-regulated products like blood pressure medicine, chemotherapy, and MRI machines help people and animals live longer and healthier. The FDA regulates human and animal medical products to ensure they are safe and effective.
Products undergo different types of testing to determine their safety and effectiveness. These tests may include animal testing, and almost always include other types of tests. Here are some facts about animal testing of FDA-regulated medical products and alternatives to animal testing.
Fact: The FDA encourages and accepts scientifically valid alternatives to animal testing. However, validated alternatives to animal testing are not available yet for many medical products.
- Laboratory tests in a petri dish or test tube, which may include tests with human or animal cells and tissues.
- Computer modeling.
- Animal testing.
Fact: The FDA supports the development of alternatives to animal testing.
Fact: federal laws regulate the treatment of test or research animals. .
- The Animal Welfare Act and Animal Welfare Act Regulations from the U.S. Department of Agriculture (USDA).
- The Public Health Service Policy of Humane Care and Use of Laboratory Animals from the National Institutes of Health Office of Laboratory Animal Welfare (OLAW).
- The Guide for the Care and Use of Laboratory Animals from the National Research Council.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.20(9); 2022 Sep

A guide to open science practices for animal research
Kai diederich.
German Federal Institute for Risk Assessment, German Centre for the Protection of Laboratory Animals (Bf3R), Berlin, Germany
Kathrin Schmitt
Philipp schwedhelm, bettina bert, céline heinl.
Translational biomedical research relies on animal experiments and provides the underlying proof of practice for clinical trials, which places an increased duty of care on translational researchers to derive the maximum possible output from every experiment performed. The implementation of open science practices has the potential to initiate a change in research culture that could improve the transparency and quality of translational research in general, as well as increasing the audience and scientific reach of published research. However, open science has become a buzzword in the scientific community that can often miss mark when it comes to practical implementation. In this Essay, we provide a guide to open science practices that can be applied throughout the research process, from study design, through data collection and analysis, to publication and dissemination, to help scientists improve the transparency and quality of their work. As open science practices continue to evolve, we also provide an online toolbox of resources that we will update continually.
Open science has become a buzzword in the scientific community that too often misses the practical application for individual researchers. This Essay, provides a guide to choosing the most appropriate tools to make animal research more transparent.
Introduction
Over the past decade, the quality of published scientific literature has been repeatedly called into question by the failure of large replication studies or meta-analyses to demonstrate sufficient translation from experimental research into clinical successes [ 1 – 5 ]. At the same time, the open science movement has gained more and more advocates across various research areas. By sharing all of the information collected during the research process with colleagues and with the public, scientists can improve collaborations within their field and increase the reproducibility and trustworthiness of their work [ 6 ]. Thus, the International Reproducibility Networks have called for more open research [ 7 ].
However, open science practices have not been adopted to the same degree in all research areas. In psychology, which was strongly affected by the so-called reproducibility crisis, the open science movement initiated real practical changes leading to a broad implementation of practices such as preregistration or sharing of data and material [ 8 – 10 ]. By contrast, biomedical research is still lagging behind. Open science might be of high value for research in general, but in translational biomedical research, it is an ethical obligation. It is the responsibility of the scientist to transparently share all data collected to ensure that clinical research can adequately evaluate the risks and benefits of a potential treatment. When Russell and Burch published “The Principles of Humane Experimental Technique” in 1959, scientists started to implement their 3Rs principle to answer the ethical dilemma of animal welfare in the face of scientific progress [ 11 ]. By replacing animal experiments wherever possible, reducing the number of animals to a strict minimum, and refining the procedures where animals have still to be used, this ethical dilemma was addressed. However, in recent years, whether the 3Rs principle is sufficient to fully address ethical concerns about animal experiments has been questioned [ 12 ].
Most people tolerate the use of animals for scientific purposes only under the basic assumption that the knowledge gained will advance research in crucial areas. This implies that performed experiments are reported in a way that enables peers to benefit from the collected data. However, recent studies suggest that a large proportion of animal experiments are never actually published. For example, scientists working within the European Union (EU) have to write an animal study protocol for approval by the competent authorities of the respective country before performing an animal experiment [ 13 ]. In these protocols, scientists have to describe the planned study and justify every animal required for the project. By searching for publications resulting from approved animal study protocols from 2 German University Medical Centers, Wieschowski and colleagues found that only 53% of approved protocols led to a publication after 6 years [ 14 ]. Using a similar approach, Van der Naald and colleagues determined a publication rate of 60% at the Utrecht Medical Center [ 15 ]. In a follow-up survey, the respective researchers named so-called “negative” or null-hypothesis results as the main cause for not publishing outcomes [ 15 ]. The current scientific system is shaped by publishers, funders, and institutions and motivates scientists to publish novel, surprising, and positive results, revealing one of the many structural problems that the numerous efforts towards open science initiatives are targeting. Non-publication not only strongly contradicts ethical values, but also it compromises the quality of published literature by leading to overestimation of effect sizes [ 16 , 17 ]. Furthermore, publications of animal studies too often show poor reporting that strongly impairs the reproducibility, validity, and usefulness of the results [ 18 ]. Unfortunately, the idea that negative or equivocal findings can also contribute to the gain of scientific knowledge is frequently neglected.
So far, the scientific community using animals has shown limited resonance to the open science movement. Due to the strong controversy surrounding animal experiments, scientists have been reluctant to share information on the topic. Additionally, translational research is highly competitive and researchers tend to be secretive about their ideas until they are ready for publication or patent [ 19 , 20 ]. However, this missing openness could also point to a lack of knowledge and training on the many open science options that are available and suitable for animal research. Researchers have to be convinced of the benefits of open science practices, not only for science in general, but also for the individual researcher and each single animal. Yet, the key players in the research system are already starting to value open science practices. An increasing number of journals request open sharing of data, funders pay for open access publications and institutions consider open science practices in hiring decisions. Open science practices can improve the quality of work by enabling valuable scientific input from peers at the early stages of research projects. Furthermore, the extended communication that open science practices offer can draw attention to research and help to expand networks of collaborators and lead to new project opportunities or follow-up positions. Thus, open science practices can be a driver for careers in academia, particularly those of early career researchers.
Beyond these personal benefits, improving transparency in translational biomedical research can boost scientific progress in general. By bringing to light all the recorded research outputs that until now have remained hidden, the publication bias and the overestimation of effect sizes can be reduced [ 17 ]. Large-scale sharing of data can help to synthesize research outputs in preclinical research that will enable better decision-making for clinical research. Disclosing the whole research process will help to uncover systematic problems and support scientists in thoroughly planning their studies. In the long run, we predict that the implementation of open science practices will lead to the use of fewer animals in unintentionally repeated experiments that previously showed unreported negative results or in the establishment of methods by avoiding experimental dead ends that are often not published. More collaborations and sharing of materials and methods can further reduce the number of animal experiments used for the implementation of new techniques.
Open science can and should be implemented at each step of the research process ( Fig 1 ). A vast number of tools are already provided that were either directly conceptualized for animal research or can be adapted easily. In this Essay, we provide an overview of open science tools that improve transparency, reliability, and animal welfare in translational in vivo biomedical research by supporting scientists to clearly communicate their research and by supporting collaborative working. Table 1 lists the most prominent open science tools we discuss, together with their respective links. We have structured this Essay to guide you through which tools can be used at each stage of the research process, from planning and conducting experiments, through to analyzing data and communicating the results. However, many of these tools can be used at many different steps. Table 1 has been deposited on Zenodo and will be updated continuously [ 21 ].

Application of open science practices at each step of the research process can maximize the impact of performed animal experiments. The implementation of these practices will lead to less time pressure at the end of a project. Due to the connection of most of these open science practices, spending more time in the planning phase and during the conduction of experiments will save time during the data analysis and publication of the study. Indeed, consulting reporting guidelines early on, preregistering a statistical plan, and writing down crucial experimental details in an electronic lab notebook, will strongly accelerate the writing of a manuscript. If protocols or even electronic lab notebooks were made public, just citing these would simplify the writing of publications. Similarly, if a data management plan is well designed before starting data collection, analyzing, and depositing data in a public repository, as is increasingly required, will be fast. NTS, non-technical summary.
A copy of this table has been deposited at Zenodo and will be updated continuously 10.5281/zenodo.6497559 .
DOAJ, Directory of Open Access Journals; DOI, digital object identifier; EDA, Experimental Design Assistant; MGI, Mouse Genome Informatics; RRID, Research Resource Identifier.
Planning the study
Transparent practices can be adopted at every stage of the research process. However, to ensure full effectivity, it is highly recommended to engage in detailed planning before the start of the experiment. This can prevent valuable time from being lost at the end of the study due to careless decisions being made at the beginning. Clarifying data management at the start of a project can help avoiding filing chaos that can be very time consuming to untangle. Keeping clear track of a project and study design will also help if new colleagues are included later on in the project or if entire project parts are handed over. In addition, all texts written on the rationale and hypothesis of the study or method descriptions, or design schemes created during the planning phase can be used in the final publications ( Fig 1 ). Similarly, information required for preregistration of animal studies or for reporting according to the ARRIVE guidelines are an extension of the details required for ethical approval [ 22 , 23 ]. Thus, the time burden within the planning phase is often overestimated. Furthermore, the thorough planning of experiments can avoid the unnecessary use of animals by preventing wrong avenues from being pursued.
Implementing open scientific practices at the beginning of a project does not mean that the idea and study plan must be shared immediately, but rather is critical for making the entire workflow transparent at the end of the project. However, optional early sharing of information can enable peers to give feedback on the study plan. Studies potentially benefit more from this a priori input than they would from the classical a posteriori peer-review process.
Most people perceive guidelines as advice that instructs on how to do something. However, it is sometimes useful to consider the term in its original meaning; “the line that guides us”. In this sense, following guidelines is not simply fulfilling a duty, but is a process that can help to design a sound research study and, as such, guidelines should be consulted at the planning stage of a project. The PREPARE guidelines are a list of important points that should be thought-out before starting a study involving animal experiments in order to reduce the waste of animals, promote alternatives, and increase the reproducibility of research and testing [ 24 ]. The PREPARE checklist helps to thoroughly plan a study and focuses on improving the communication and collaboration between all involved participants of the study (i.e., animal caretakers and scientists). Indeed, open science begins with the communication within a research facility. It is currently available in 33 languages and the responsible team from Norecopa, Norway’s 3R-center, takes requests for translations into further languages.
The UK Reproducibility Network has also published several guiding documents (primers) on important topics for open and reproducible science. These address issues such as data sharing [ 25 ], open access [ 26 ], open code and software [ 27 ], and preprints [ 28 ], as well as preregistration and registered reports [ 27 ]. Consultation of these primers is not only helpful in the relevant phases of the experiment but is also encouraged in the planning phase.
Although the ARRIVE guidelines are primarily a reporting guideline specifically designed for preparing a publication containing animal data, they can also support researchers when planning their experiments [ 22 , 23 ]. Going through the ARRIVE website, researchers will find tools and explanations that can support them in planning their experiments [ 29 ]. Consulting the ARRIVE checklist at the beginning of a project can help in deciding what details need to be documented during conduction of the experiments. This is particularly advisable, given that compliance to ARRIVE is still poor [ 18 ].
Experimental design
To maximize the validity of performed experiments and the knowledge gained, designing the study well is crucial. It is important that the chosen animal species reflects the investigated disease well and that basic characteristics of the animal, such as sex or age, are considered carefully [ 30 ]. The Canadian Institutes of Health Research provides a collection of resources on the integration of sex and gender in biomedical research with animals, including tips and tools for researchers and reviewers [ 31 ]. Additionally, it is advisable to avoid unnecessary standardization of biological and environmental factors that can reduce the external validity of results [ 32 ]. Meticulous statistical planning can further optimize the use of animals. Free to use online tools for calculating sample sizes such as G*Power or the inVivo software package for R can further support animal researchers in designing their statistical plan [ 33 , 34 ]. Randomization for the allocation of groups can be supported with specific tools for scientists like Research Randomizer, but also by simple online random number generators [ 35 ]. Furthermore, it might be advisable when designing the study to incorporate pathological analyses into the experimental plan. Optimal planning of tissue collection, performance of pathological procedures according to accepted best practices, and use of optimal pathological analysis and reporting methods can add some extra knowledge that would otherwise be lost. This can improve the reproducibility and quality of translational biomedicine, especially, but not exclusively, in animal studies with morphological endpoints. In all animal studies, unexpected deaths in experimental animals can occur and be the cause of lost data or missed opportunities to identify health problems [ 36 , 37 ].
To support researchers in designing their animal research, the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) has also developed the Experimental Design Assistant (EDA) [ 38 , 39 ]. This online tool helps researchers to better structure in vivo research by creating detailed schemes of the study design. It provides feedback on the entered design, drawing researcher’s attention to crucial decisions in the project. The resulting schemes can be used to transparently share the study design by uploading it into a study preregistration, enclosing it in a grant application, or submitting it with a final manuscript. The EDA can be used for different study designs in diverse scenarios and helps to communicate researcher plans to others [ 40 ]. The EDA might be particularly of interest to clarify very complex study designs involving multiple experimental groups. Working with the EDA might appear rather complex in the beginning, but the NC3R provides regular webinars that can help to answer any questions that arise.
Preregistration
Preregistration is an effective tool to improve the quality and transparency of research. To preregister their work, scientists must determine crucial details of the study before starting any experiment. Changes occurring during a study can be outlined at the end. A preregistered study plan should include at least the hypothesis and determine all the parameters that are known in advance. A description of the planned study design and statistical analysis will enable reviewers and peers to better retrace the workflow. It can prevent the intentional use of the flexibility of analysis to reach p -values under a certain significance level (e.g., p-hacking or HARKing (Hypothesizing After Results are Known)). With preregistration, scientists can also claim their idea at an early stage of their research with a citable individual identifier that labels the idea as their own. Some open preregistration platforms also provide a digital object identifier (DOI), which makes the registered study citable. Three public registries actively encourage the preregistration of animal studies conducted around the world: OSF registry, preclinicaltrials.eu, and animalstudyregistry.org [ 41 – 45 ]. Scientists can choose the registry according to their needs. Preregistering a study in a public registry supports scientists in planning their study and later to critically reevaluate their own work and assess its limitations and potentials.
As an alternative to public registries, researchers can also submit their study plan to one of hundreds of journals already publishing registered reports, including many journals open to animal research [ 8 ]. A submitted registered report passes 2 steps of peer review. In the first step, reviewers comment on the idea and the study design. After an “in-principle-acceptance,” researchers can conduct their study as planned. If the authors conduct the experiments as described in the accepted study protocol, the journal will publish the final study regardless of the outcome. This might be an attractive option, especially for early career researchers, as a manuscript is published at the beginning of a project with the guarantee of a future final publication.
The benefits of preregistration can already be observed in clinical research, where registration has been mandatory for most trials for more than 20 years. Preregistration in clinical research has helped to make known what has been tested and not just what worked and was published, and the implementation of trial registration has strongly reduced the number of publications reporting significant treatment effects [ 46 ]. In animal research, with its unrealistically high percentage of positive results, preregistration seems to be particularly worthwhile.
Research data management
To get the most out of performed animal experiments, effective sharing of data at the end of the study is essential. Sharing research data optimally is complex and needs to be prepared in advance. Thus, data management can be seen as one part of planning a study thoroughly. Many funders have recognized the value of the original research data and request a data management plan from applicants in advance [ 25 , 47 ]. Various freely available tools such as DMPTool or DMPonline already exist to design a research data management plan that complies to the requirements of different funders [ 48 , 49 ]. The data management plan defines the types of data collected and describes the handling and names responsible persons throughout the data lifecycle. This includes collecting the data, analyzing, archiving, and sharing it. Finally, a data management plan enables long-term access and the possibility for reuse by peers. Developing such a plan, whether it is required by funders or not, will later simplify the application of the FAIR data principle (see section on the FAIR data principle). The Longwood Medical Area Research Data Management Working Group from the Harvard Medical School developed a checklist to assist researchers in optimally managing their data throughout the data lifecycle [ 50 ]. Similarly, the Joint Information Systems Committee (JISC) provides a great research data management toolkit including a checklist for researchers planning their project [ 51 ]. Consulting this checklist in the planning phase of a project can prevent common errors in research data management.
Non-technical project summary
One instrument specifically conceived to create transparency on animal research for the general public is the so-called non-technical project summary (NTS). All animal protocols approved within the EU must be accompanied by these comprehensible summaries. NTSs are intended to inform the public about ongoing animal experiments. They are anonymous and include information on the objectives and potential benefits of the project, the expected harm, the number of animals, the species, and a statement of compliance with the requirements of the 3Rs principle. However, beyond simply informing the public, NTSs can also be used for meta-research to help identify new research areas with an increased need for new 3R technologies [ 52 , 53 ]. NTSs become an excellent tool to appropriately communicate the scientific value of the approved protocol and for meta-scientists to generate added value by systematically analyzing theses summaries if they fulfill a minimum quality threshold [ 54 , 55 ]. In 2021, the EU launched the ALURES platform ( Table 1 ), where NTSs from all member states are published together, opening the opportunities for EU-wide meta-research. NTSs are, in contrast to other open science practices, mandatory in the EU. However, instead of thinking of them as an annoying duty, it might be worth thoroughly drafting the NTS to support the goals of more transparency towards the public, enabling an open dialogue and reducing extreme opinions.
Conducting the experiments
Once the experiments begin, documentation of all necessary details is essential to ensure the transparency of the workflow. This includes methodological details that are crucial for replicating experiments, but also failed attempts that could help peers to avoid experiments that do not work in the future. All information should be stored in such a way that it can be found easily and shared later. In this area, many new tools have emerged in recent years ( Table 1 ). These tools will not only make research transparent for colleagues, but also help to keep track of one’s own research and improve internal collaboration.
Electronic laboratory notebooks
Electronic laboratory notebooks (ELNs) are an important pillar of research data management and open science. ELNs facilitate the structured and harmonized documentation of the data generation workflow, ensure data integrity, and keep track of all modifications made to the original data based on an audit trail option. Moreover, ELNs simplify the sharing of data and support collaborations within and outside the research group. Methodological details and research data become searchable and traceable. There is an extensive amount of literature providing advice on the selection and the implementation process of an ELN depending on the specific needs and research area and its discussion would be beyond the scope of this Essay [ 56 – 58 ]. Some ELNs are connected to a laboratory information management system (LIMS) that provides an animal module supporting the tracking of animal details [ 59 ]. But as research involving animals is highly heterogeneous, this might not be the only decision point and we cannot recommend a specific ELN that is suitable for all animal research.
ELNs are already established in the pharmaceutical industry and their use is on the rise among academics as well. However, due to concerns around costs for licenses, data security, and loss of flexibility, many research institutions still fear the expenses that the introduction of such a system would incur [ 56 ]. Nevertheless, an increasing number of academic institutions are implementing ELNs and appreciating the associated benefits [ 60 ]. If your institution already has an ELN, it might be easiest to just use the option available in the research environment. If not, the Harvard Medical School provides an extensive and updated overview of various features of different ELNs that can support scientists in choosing the appropriate one for their research [ 61 ]. There are many commercial ELN products, which may be preferred when the administrative workload should be outsourced to a large extent. However, open-source products such as eLabFTW or open BIS provide a greater opportunity for customization to meet specific needs of individual research institutions [ 62 – 64 ]. A huge number of options are available depending on the resources and the features required. Some scientists might prefer generic note taking tools such as Evernote or just a simple Word document that offers infinite flexibility, but specific ELNs can further support good record keeping practice by providing immutability, automated backups, standardized methods, and protocols to follow. Clearly defining the specific requirements expected might help to choose an adequate system that would improve the quality of the record compared to classical paper laboratory notebooks.
Sharing protocols
Adequate sharing of methods in translational biomedical sciences is key to reproducibility. Several repositories exist that simplify the publication and exchange of protocols. Writing down methods at the end of the project bears the risk that crucial details might be missing [ 65 ]. On protocols.io, scientists can note all methodological details of a procedure, complete them with uploaded documents, and keep them for personal use or share them with collaborators [ 66 ]. Authors can also decide at any point in time to make their protocol public. Protocols published on protocols.io receive a DOI and become citable; they can be commented on by peers and adapted according to the needs of the individual researcher. Protocol.io files from established protocols can also be submitted together with some context and sample datasets to PLOS ONE , where it can be peer-reviewed and potentially published [ 67 , 68 ]. Depending on the affiliation of the researchers to academia or industry and on an internal or public sharing of files, protocols.io can be free of charge or come with costs. Other journals also encourage their authors to deposit their protocols in a freely accessible repository, such as protocol exchange from Nature portfolio [ 69 ]. Another option might be to separately submit a protocol that was validated by its use in an already published research article to an online and peer-reviewed journal specific for research protocols, such as Bio-Protocol. A multitude of journals, including eLife and Science already collaborate with Bio-Protocol and recommend authors to publish the method in Bio-Protocol [ 70 ]. Bio-Protocol has no submission fees and is freely available to all readers. Both protocols.io and Bio-Protocol allow the illustration of complex scientific methods by uploading videos to published protocols. In addition, protocols can be deposited in a general research repository such as the Open Science Framework (OSF repository) and referenced in appropriate publications.
Sharing critical incidents
Sharing critical or even adverse events that occur in the context of animal experimentation can help other scientists to avoid committing the same mistakes. The system of sharing critical incidents is already established in clinical practice and helps to improve medical care [ 71 , 72 ]. The online platform critical incident reporting system in laboratory animal science (CIRS-LAS) represents the first preclinical equivalent to these clinical systems [ 73 ]. With this web-based tool, critical incidents in animal research can be reported anonymously without registration. An expert panel helps to analyze the incident to encourage an open dialogue. Critical incident reporting is still very marginal in animal research and performed procedures are very variable. These factors make a systemic analysis and a targeted search of incidence difficult. However, it may be of special interest for methods that are broadly used in animal research such as anesthesia. Indeed, a broad feed of this system with data on errors occurring in standard procedures today could help avoid critical incidences in the future and refine animal experiments.
Sharing animals, organs, and tissue
When we think about open science, sharing results and data are often in focus. However, sharing material is also part of a collaborative and open research culture that could help to greatly reduce the number of experimental animals used. When an animal is killed to obtain specific tissue or organs, the remainder is mostly discarded. This may constitute a wasteful practice, as surplus tissue can be used by other researchers for different analyses. More animals are currently killed as surplus than are used in experiments, demonstrating the potential for sharing these animals [ 74 , 75 ].
Sharing information on generated surplus is therefore not only economical, but also an effective way to reduce the number of animals used for scientific purposes. The open-source software Anishare is a straightforward way for breeders of genetically modified lines to promote their surplus offspring or organs within an institution [ 76 ]. The database AniMatch ( Table 1 ) connects scientists within Europe who are offering tissue or organs with scientists seeking this material. Scientists already sharing animal organs can support this process by describing it in publications and making peers aware of this possibility [ 77 ]. Specialized research communities also allow sharing of animal tissue or animal-derived products worldwide that are typically used in these fields on a collaborative basis via the SEARCH-framework [ 78 , 79 ]. Depositing transgenic mice lines into one of several repositories for mouse strains can help to further minimize efforts in producing new transgenic lines and most importantly reduce the number of surplus animals by supporting the cryoconservation of mouse lines. The International Mouse Strain Resource (IMSR) can be used to help find an adequate repository or to help scientists seeking a specific transgenic line find a match [ 80 ].
Analyzing the data
Animal researchers have to handle increasingly complex data. Imaging, electrophysiological recording, or automated behavioral tracking, for example, produce huge datasets. Data can be shared as raw numerical output but also as images, videos, sounds, or other forms from which raw numerical data can be generated. As the heterogeneity and the complexity of research data increases, infinite possibilities for analysis emerge. Transparently reporting how the data were processed will enable peers to better interpret reported results. To get the most out of performed animal experiments, it is crucial to allow other scientists to replicate the analysis and adapt it to their research questions. It is therefore highly recommended to use formats and tools during the analysis that allow a straightforward exchange of code and data later on.
Transparent coding
The use of non-transparent analysis codes have led to a lack of reproducibility of results [ 81 ]. Sharing code is essential for complex analysis and enables other researchers to reproduce results and perform follow-up studies, and citable code gives credit for the development of new algorithms ( Table 1 ). Jupyter Notebooks are a convenient way to share data science pipelines that may use a variety of coding languages, including like Python, R or Matlab, and also share the results of analyses in the form of tables, diagrams, images, and videos. Notebooks contain source code and can be published or collaboratively shared on platforms like GitHub or GitLab, where version control of source code is implemented. The data-archiving tool Zenodo can be used to archive a repository on GitHub and create a DOI for the archive. Thereby contents become citable. Using free and open-source programming language like R or Python will increase the number of potential researchers that can work with the published code. Best practice for research software is to publish the source code with a license that allows modification and redistribution.
Choice of data visualization
Choosing the right format for the visualization of data can increase its accessibility to a broad scientific audience and enable peers to better judge the validity of the results. Studies based on animal research often work with very small sample sizes. Visualizing these data in histograms may lead to an overestimation of the outcomes. Choosing the right dot plots that makes all recorded points visible and at the same time focusses on the summary instead of the individual points can further improve the intuitive understanding of a result. If the sample size is too low, it might not be meaningful to visualize error bars. A variety of freely available tools already exists that can support scientists in creating the most appropriate graphs for their data [ 82 ]. In particular, when representing microscopy results or heat maps, it should be kept in mind that a large part of the population cannot perceive the classical red and green representation [ 83 ]. Opting for the color-blind safe color maps and checking images with free tools such as color oracle ( Table 1 ) can increase the accessibility of graphs. Multiple journals have already addressed flaws in data visualization and have introduced new policies that will accelerate the uptake of transparent representation of results.
Publication of all study outcomes
Open science practices have received much attention in the past few years when it comes to publication of the results. However, it is important to emphasize that although open science tools have their greatest impact at the end of the project, good study preparation and sharing of the study plan and data early on can greatly increase the transparency at the end.
The FAIR data principle
To maximize the impact and outcome of a study, and to make the best long-term use of data generated through animal experiments, researchers should publish all data collected during their research according to the FAIR data principle. That means the data should be findable, accessible, interoperable, and reusable. The FAIR principle is thus an extension of open access publishing. Data should not only be published without paywalls or other access restrictions, but also in such a manner that they can be reused and further processed by others. For this, legal as well as technical requirements must be met by the data. To achieve this, the GoFAIR initiative has developed a set of principles that should be taken into account as early as at the data collection stage [ 49 , 84 ]. In addition to extensively described and machine-readable metadata, these principles include, for example, the application of globally persistent identifiers, the use of open file formats, and standardized communication protocols to ensure that humans and machines can easily download the data. A well-chosen repository to upload the data is then just the final step to publish FAIR data.
FAIR data can strongly increase the knowledge gained from performed animal experiments. Thus, the same data can be analyzed by different researchers and could be combined to obtain larger sample sizes, as already occurs in the neuroimaging community, which works with comparable datasets [ 85 ]. Furthermore, the sharing of data enables other researchers to analyze published datasets and estimate measurement reliabilities to optimize their own data collection [ 86 , 87 ]. This will help to improve the translation from animal research into clinics and simultaneously reduce the number of animal experiment in future.
Reporting guidelines
In preclinical research, the ARRIVE guidelines are the current state of the art when it comes to reporting data based on animal experiments [ 22 , 23 ]. The ARRIVE guidelines have been endorsed by more than 1,000 journals who ask that scientists comply with them when reporting their outcomes. Since the ARRIVE guidelines have not had the expected impact on the transparency of reporting in animal research publications, a more rigorous update has been developed to facilitate their application in practice (ARRIVE 2.0 [ 23 ]). We believe that the ARRIVE guidelines can be more effective if they are implemented at a very early stage of the project (see section on guidelines). Some more specialized reporting guidelines have also emerged for individual research fields that rely on animal studies, such as endodontology [ 88 ]. The equator network collects all guidelines and makes them easily findable with their search tool on their website ( Table 1 ). MERIDIAN also offers a 1-stop shop for all reporting guidelines involving the use of animals across all research sectors [ 89 ]. It is thus worth checking for new reporting guidelines before preparing a manuscript to maximize the transparency of described experiments.
Identifiers
Persistent identifiers for published work, authors, or resources are key for making public data findable by search engines and are thus a prerequisite for compliance to FAIR data principles. The most common identifier for publications will be a DOI, which makes the work citable. A DOI is a globally unique string assigned by the International DOI Foundation to identify content permanently and provide a persistent link to its location on the Internet. An ORCID ID is used as a personal persistent identifier and is recommendable to unmistakably identify an author ( Table 1 ). This will avoid confusions between authors with the same name or in the case of name changes or changes of affiliation. Research Resource Identifiers (RRID) are unique ID numbers that help to transparently report research resources. RRID also apply to animals to clearly identify the species used. RRID help avoid confusion between different names or changing names of genetic lines and, importantly, make them machine findable. The RRID Portal helps scientists find a specific RRID or create one if necessary ( Table 1 ). In the context of genetically altered animal lines, correct naming is key. The Mouse Genome Informatics (MGI) Database is the authoritative source of official names for mouse genes, alleles, and strains ([ 90 ]).
Preprint publication
Preprints have undergone unprecedented success, particularly during the height of the Coronavirus Disease 2019 (COVID-19) pandemic when the need for rapid dissemination of scientific knowledge was critical. The publication process for scientific manuscripts in peer-reviewed journals usually requires a considerable amount of time, ranging from a few months to several years, mainly due to the lengthy review process and inefficient editorial procedures [ 91 , 92 ]. Preprints typically precede formal publication in scientific journals and, thus, do not go through a peer review process, thus, facilitating the prompt open dissemination of important scientific findings within the scientific community. However, submitted papers are usually screened and checked for plagiarism. Preprints are assigned a DOI so they can be cited. Once a preprint is published in a journal, its status is automatically updated on the preprint server. The preprint is linked to the publication via CrossRef and mentioned accordingly on the website of the respective preprint platform.
After initial skepticism, most publishers now allow papers to be posted on preprint servers prior to submission. An increasing number of journals even allow direct submission of a preprint to their peer review process. The US National Institutes of Health and the Wellcome Trust, among other funders, also encourage prepublication and permit researchers to cite preprints in their grant applications. There are now numerous preprint repositories for different scientific disciplines. BioASAP provides a searchable database for preprint servers that can help in identifying the one that best matches an individual’s needs [ 93 ]. The most popular repository for animal research is bioRxiv, which is hosted by the Cold Spring Harbor Laboratory ( Table 1 ).
The early exchange of scientific results is particularly important for animal research. This acceleration of the publication process can help other scientists to adapt their research or could even prevent animal experiments if other scientists become aware that an experiment has already been done before starting their own. In addition, preprints can help to increase the visibility of research. Journal articles that have a corresponding preprint publication have higher citation and Altmetric counts than articles without preprint [ 94 ]. In addition, the publication of preprints can help to combat publication bias, which represents a major problem in animal research [ 16 ]. Since journals and readers prioritize cutting-edge studies with positive results over inconclusive or negative results, researchers are reluctant to invest time and money in a manuscript that is unlikely to be accepted in a high-impact journal.
In addition to the option of publishing as preprint, other alternative publication formats have recently been introduced to facilitate the publication of research results that are hard to publish in traditional peer-reviewed journals. These include micro publications, data repositories, data journals, publication platforms, and journals that focus on negative or inconclusive results. The tool fiddle can support scientists in choosing the right publication format [ 95 , 96 ].
Open access publication
Publishing open access is one of the most established open science strategies. In contrast to the FAIR data principle, the term open access publication refers usually to the publication of a manuscript on a platform that is accessible free of charge—in translational biomedical research, this is mostly in the form of a scientific journal article. Originally, publications accessible free of charge were the answer to the paywalls established by renowned publishing houses, which led to social inequalities within and outside the research system. In translational biomedical research, the ethical aspect of urgently needed transparency is another argument in favor of open access publication, as these studies will not only be findable, but also internationally readable.
There are different ways of open access publishing; the 2 main routes are gold open access and green open access. Numerous journals offer now gold open access. It refers to the immediate and fully accessible publication of an article. The Directory of Open Access Journals (DOAJ) provides a complete and updated list for high-quality, open access, and peer-reviewed journals [ 97 ]. Charité–Universitätsmedizin Berlin offers a specific tool for biomedical open access journals that supports animal researchers to choose an appropriate journal [ 49 ]. In addition, the Sherpa Romeo platform is a straightforward way to identify publisher open access policies on a journal-by-journal basis, including information on preprints, but also on licensing of articles [ 51 ]. Hybrid open access refers to openly accessible articles in otherwise paywalled journals. By contrast, green open access refers to the publication of a manuscript or article in a repository that is mostly operated by institutions and/or universities. The publication can be exclusively on the repository or in combination with a publisher. In the quality-assured, global Directory of Open Access Repositories (openDOAR), scientists can find thousands of indexed open access repositories [ 49 ]. The publisher often sets an embargo during which the authors cannot make the publication available in the repository, which can restrict the combined model. It is worth mentioning that gold open access is usually more expensive for the authors, as they have to pay an article processing charge. However, the article’s outreach is usually much higher than the outreach of an article in a repository or available exclusively as subscription content [ 98 ]. Diamond open access refers to publications and publication platforms that can be read free of charge by anyone interested and for which no costs are incurred by the authors either. It is the simplest and fairest form of open access for all parties involved, as no one is prevented from participating in scientific discourse by payment barriers. For now, it is not as widespread as the other forms because publishers have to find alternative sources of revenue to cover their costs.
As social media and the researcher’s individual public outreach are becoming increasingly important, it should be remembered that the accessibility of a publication should not be confused with the licensing under which the publication is made available. In order to be able to share and reuse one’s own work in the future, we recommend looking for journals that allow publications under the Creative Commons licenses CC BY or CC BY-NC. This also allows the immediate combination of gold and green open access.
Creative commons licenses
Attributing Creative Commons (CC) licenses to scientific content can make research broadly available and clearly specifies the terms and conditions under which people can reuse and redistribute the intellectual property, namely publications and data, while giving the credit to whom it deserves [ 49 ]. As the laws on copyright vary from country to country and law texts are difficult to understand for outsiders, the CC licenses are designed to be easily understandable and are available in 41 languages. This way, users can easily avoid accidental misuse. The CC initiative developed a tool that enables researchers to find the license that best fits their interests [ 49 ]. Since the licenses are based on a modular concept ranging from relatively unrestricted licenses (CC BY, free to use, credit must be given) to more restricted licenses (CC BY-NC-ND, only free to share for non-commercial purposes, credit must be given), one can find an appropriate license even for the most sensitive content. Publishing under an open CC license will not only make the publication easy to access but can also help to increase its reach. It can stimulate other researchers and the interested public to share this article within their network and to make the best future use of it. Bear in mind that datasets published independently from an article may receive a different CC license. In terms of intellectual property, data are not protected in the same way as articles, which is why the CC initiative in the United Kingdom recommends publishing them under a CC0 (“no rights reserved”) license or the Public Domain Mark. This gives everybody the right to use the data freely. In an animal ethics sense, this is especially important in order to get the most out of data derived from animal experiments.
Data and code repositories
Sharing research data is essential to ensure reproducibility and to facilitate scientific progress. This is particularly true in animal research and the scientific community increasingly recognizes the value of sharing research data. However, even though there is increasing support for the sharing of data, researchers still perceive barriers when it comes to doing so in practice [ 99 – 101 ]. Many universities and research institutions have established research data repositories that provide continuous access to datasets in a trusted environment. Many of these data repositories are tied to specific research areas, geographic regions, or scientific institutions. Due to the growing number and overall heterogeneity of these repositories, it can be difficult for researchers, funding agencies, publishers, and academic institutions to identify appropriate repositories for storing and searching research data.
Recently, several web-based tools have been developed to help in the selection of a suitable repository. One example is Re3data, a global registry of research data repositories that includes repositories from various scientific disciplines. The extensive database can be searched by country, content (e.g., raw data, source code), and scientific discipline [ 49 ]. A similar tool to help find a data archive specific to the field is FAIRsharing, based at Oxford University [ 102 ]. If there is no appropriate subject-specific data repository or one seems unsuitable for the data, there are general data repositories, such as Open Science Framework, figshare, Dryad, or Zenodo. To ensure that data stored in a repository can be found, a DOI is assigned to the data. Choosing the right license for the deposited code and data ensures that authors get credit for their work.
Publication and connection of all outcomes
If scientists have used all available open science tools during the research process, then publishing and linking all outcomes represents the well-deserved harvest ( Fig 2 ). At the end of a research process, researchers will not just have 1 publication in a journal. Instead, they might have a preregistration, a preprint, a publication in a journal, a dataset, and a protocol. Connecting these outcomes in a way that enables other scientists to better assess the results that link these publications will be key. There are many examples of good open science practices in laboratory animal science, but we want to highlight one of them to show how this could be achieved. Blenkuš and colleagues investigated how mild stress-induced hyperthermia can be assessed non-invasively by thermography in mice [ 103 ]. The study was preregistered with animalstudyregistry.org , which is referred to in their publication [ 104 ]. A deviation from the originally preregistered hypothesis was explained in the manuscript and the supplementary material was uploaded to figshare [ 105 ].

Application of open science practices can increase the reproducibility and visibility of a research project at the same time. By publishing different research outputs with more detailed information than can be included in a journal article, researchers enable peers to replicate their work. Reporting according to guidelines and using transparent visualization will further improve this reproducibility. The more research products that are generated, the more credit can be attributed. By communicating on social media or additionally publishing slides from delivered talks or posters, more attention can be raised. Additionally, publishing open access and making the work machine-findable makes it accessible to an even broader number of peers.
It might also be helpful to provide all resources from a project in a single repository such as Open Science Framework, which also implements other, different tools that might have been used, like GitHub or protocols.io.
Communicating your research
Once all outcomes of the project are shared, it is time to address the targeted peers. Social media is an important instrument to connect research communities [ 106 ]. In particular, Twitter is an effective way to communicate research findings or related events to peers [ 107 ]. In addition, specialized platforms like ResearchGate can support the exchange of practical experiences ( Table 1 ). When all resources related to a project are kept in one place, sharing this link is a straightforward way to reach out to fellow scientists.
With the increasing number of publications, science communication has become more important in recent years. Transparent science that communicates openly with the public contributes to strengthening society’s trust in research.
Conclusions
Plenty of open science tools are already available and the number of tools is constantly growing. Translational biomedical researchers should seize this opportunity, as it could contribute to a significant improvement in the transparency of research and fulfil their ethical responsibility to maximize the impact of knowledge gained from animal experiments. Over and above this, open science practices also bear important direct benefits for the scientists themselves. Indeed, the implementation of these tools can increase the visibility of research and becomes increasingly important when applying for grants or in recruitment decisions. Already, more and more journals and funders require activities such as data sharing. Several institutions have established open science practices as evaluation criteria alongside publication lists, impact factor, and h-index for panels deciding on hiring or tenure [ 108 ]. For new adopters, it is not necessary to apply all available practices at once. Implementing single tools can be a safe approach to slowly improve the outreach and reproducibility of one’s own research. The more open science products that are generated, the more reproducible the work becomes, but also the more the visibility of a study increases ( Fig 2 ).
As other research fields, such as social sciences, are already a step ahead in the implementation of open science practices, translational biomedicine can profit from their experiences [ 109 ]. We should thus keep in mind that open science comes with some risks that should be minimized early on. Indeed, the more open science practices become incentivized, the more researchers could be tempted to get a transparency quality label that might not be justified. When a study is based on a bad hypothesis or poor statistical planning, this cannot be fixed by preregistration, as prediction alone is not sufficient to validate an interpretation [ 110 ]. Furthermore, a boom of data sharing could disconnect data collectors and analysts, bearing the risk that researchers performing the analysis lack understanding of the data. The publication of datasets could also promote a “parasitic” use of a researcher’s data and lead to scooping of outcomes [ 111 ]. Stakeholders could counteract such a risk by promoting collaboration instead of competition.
During the COVID-19 pandemic, we have seen an explosion of preprint publications. This unseen acceleration of science might be the adequate response to a pandemic; however, the speeding up science in combination with the “publish or perish” culture could come at the expense of the quality of the publication. Nevertheless, a meta-analysis comparing the quality of reporting between preprints and peer-reviewed articles showed that the quality of reporting in preprints in the life sciences is at most slightly lower on average compared to peer-reviewed articles [ 112 ]. Additionally, preprints and social media have shown during this pandemic that a premature and overconfident communication of research results can be overinterpreted by journalists and raise unfounded hopes or fears in patients and relatives [ 113 ]. By being honest and open about the scope and limitations of the study and choosing communication channels carefully, researchers can avoid misinterpretation. It should be noted, however, that by releasing all methodological details and data in research fields such as viral engineering, where a dual use cannot be excluded, open science could increase biosecurity risk. Implementing access-controlled repositories, application programming interfaces, and a biosecurity risk assessment in the planning phase (i.e., by preregistration) could mitigate this threat [ 114 ].
Publishing in open access journals often involves higher publication costs, which makes it more difficult for institutes and universities from low-income countries to publish there [ 115 ]. Equity has been identified as a key aim of open science [ 116 ]. It is vital, therefore, that existing structural inequities in the scientific system are not unintentionally reinforced by open science practices. Early career researchers have been the main drivers of the open science movement in other fields even though they are often in vulnerable positions due to short contracts and hierarchical and strongly networked research environments. Supporting these early career researchers in adopting open science tools could significantly advance this change in research culture [ 117 ]. However, early career researchers can already benefit by publishing registered reports or preprints that can provide a publication much faster than conventional journal publications. Communication in social media can help them establish a network enabling new collaborations or follow-up positions.
Even though open science comes with some risks, the benefits easily overweigh these caveats. If a change towards more transparency is accompanied by the implementation of open science in the teaching curricula of the universities, most of the risks can be minimized [ 118 ]. Interestingly, we have observed that open science tools and infrastructure that are specific to animal research seem to mostly come from Europe. This may be because of strict regulations within Europe for animal experiments or because of a strong research focus in laboratory animal science along with targeted research funding in this region. Whatever the reason might be, it demonstrates the important role of research policy in accelerating the development towards 3Rs and open science.
Overall, it seems inevitable that open science will eventually prevail in translational biomedical research. Scientists should not wait for the slow-moving incentive framework to change their research habits, but should take pioneering roles in adopting open science tools and working towards more collaboration, transparency, and reproducibility.
Acknowledgments
The authors gratefully acknowledge the valuable input and comments from Sebastian Dunst, Daniel Butzke, and Nils Körber that have improved the content of this work.
Abbreviations
Funding statement.
The authors received no specific funding for this work.
Germ-Free Animals
Germ-free, or GF, animals are laboratory animals that completely lack microbes, making them useful tools for microbiome research. Researchers create GF animals in laboratories by delivering the newborn animals in a way that protects them from microbes, which are microscopic organisms such as bacteria and viruses. They then house the GF animals in sterile conditions to ensure that the animals stay germ free. The creation of GF animals began in the late nineteenth century. Prior to that, scientists had no way to study the effects of the microbiome on overall health. The creation of GF animals allowed researchers to examine the microbiome under controlled conditions. They could colonize the animal with specific microbes and study their effects on the animal’s health without the confounding presence of other microbes. Researchers have used GF animals as a living model to study the microbiome, which has provided evidence for a relationship between the microbiome and health, including a role for the microbiome in shaping the development of multiple body systems.
Researchers use GF animals to study the microbiome and its effects on health. The microbiome is a community of microbes living on and in the human body. Those microbes often colonize mucosal membranes, which are surfaces in the body that line organs and cavities, such as the gastrointestinal tract, respiratory tract, and reproductive tract. Microbes can be harmful or commensal, which means that they do not hurt the host, though most microbial colonization is commensal. Daily environmental factors, such as diet and germ exposure, contribute to the microbiome composition, making each individual’s microbiome unique. The diversity of microbial colonization during infancy affects development of multiple body systems, such as the immune system and nervous system. Without a microbiome, humans would not develop into adulthood normally. Purposely creating GF humans would therefore be unethical.
Research to develop GF animals began in the late nineteenth century. In 1885, Louis Pasteur , who studied chemistry and microbiology in France, was one of the first to conceptualize the idea of animals living without microorganisms. However, he also noted that their development would likely be impossible due to the vital role microbes play in the host’s health. Then in 1895, George H. F. Nuttall and Hans Thierfelder, who at the time conducted research together in Germany, disproved Pasteur’s theory and created the first GF animals. The pair carried out experiments to determine whether having a microbiome was necessary for normal life. They delivered guinea pigs via sterile Cesarean section, which is a procedure where a surgeon cuts into the mother’s abdomen to deliver the neonate. Delivery by Cesarean section ensured that the neonates did not encounter the mother’s vaginal microbes as they would have during vaginal delivery. Nuttall and Thierfelder sterilized a glass chamber using high temperature steam to kill all bacteria, and then raised the guinea pigs in the sterile chamber. However, since the researchers stored the sterile food for the germ-free animal in the chamber, when the food ran out, the researchers moved the animals out, and the animals were no longer GF animals.
In 1915, Ernst Küster, a professor and surgeon who worked in Germany, designed a sterile chamber where he housed a GF goat for thirty-four days. For sterile food delivery, Küster devised a smaller compartment that led into the main compartment. Materials were sterilized with steam in the small compartment before being passed into the main compartment, so they did not contain any bacteria. Even after Küster found a way to feed GF animals without exposing them to bacteria, the goat only lived for thirty-four days. Researchers attempted similar experiments but could ensure that their animals remained germ-free for only a short period of time.
In 1932, James Reyniers, who taught bacteriology at the University of Notre Dame, in Notre Dame, Indiana, and his colleagues generated one of the first colonies of GF animals. Reyniers’s group researched microbes and therefore investigated creating GF animals so that they could introduce a single microbe species in the absence of all other potentially confounding microbes. The isolator they used to house the GF animals was similar to the one Küster designed. The isolator was a stainless-steel drum that could undergo steam sterilization and could also be attached to other isolators. The apparatus also included long rubber gloves to handle the animals and materials without introducing bacteria. In the laboratory of the Notre Dame group, a pair of GF rats mated inside the sterilized chamber, which was one of the first times a GF animal reproduced and gave birth to GF newborns. That occurrence demonstrated that GF animals could mate, though not as readily as animals with microbes.
Researchers used stainless steel chambers to house GF animals until 1957, when the researchers at Notre Dame conducting germ-free research developed a chamber with walls that consisted of transparent, flexible vinyl film. Switching to flexible film allowed researchers to introduce food and other materials more conveniently into the isolator. In previous steel chambers, researchers had to wait for the steam to sterilize the chamber before adding materials. The new chambers allowed researchers to use heat to quickly seal packages containing materials like food into the flexible-film chamber and then use a hot wire inside the chamber to cut into the package and release the contents. Researchers at that time also began using peracetic acid, which is an acid that kills most bacteria within minutes, for sterilization, as peracetic acid could sterilize surfaces more quickly than steam.
During the mid-1900s, researchers in the farming industry also began using GF animals to aid in livestock production. Initially, they found that administering antibiotics to farm animals grown for human consumption aided in physical growth, while also reducing the amount of food the animals needed. That growth effect was due to the antibiotic’s killing of intestinal bacteria that hindered growth. However, at the time, researchers’ use of antibiotics in agriculture incited public debate because many people feared that the antibiotics would cause bacteria to become resistant to it and could ultimately harm humans. Therefore, using animals raised in completely sterile conditions to ensure the absence of the growth inhibiting bacteria was an alternative option for creating farm animals that had increased physical growth.
In the 1970s, researchers also began to extend the sterile isolators used to house GF animals towards applications in humans. In 1971, a boy named David Vetter in Houston, Texas, was born with severe combined immune deficiency, which is a genetic disorder that affects the immune system, making the body highly susceptible to infection, and exposure to microbes unsafe. Because his sister was not a match for a bone marrow transplant, which is a treatment that could have replaced Vetter’s dysfunctional immune cells with healthy cells, instead researchers helped create a sterile, germ-free environment for him to grow up in. From birth, Vetter stayed in a sterile isolator, and NASA even provided him with a custom-built space suit so he could potentially travel around outside of his bubble, without encountering microbes. Ultimately, he did receive a bone marrow transplant but died in 1984. Vetter became known as Houston’s bubble boy, and was one of the first GF humans to grow up in a GF environment.
Researchers in 2023 typically follow a general protocol to create GF animals, but the details of the protocol differ across species. The general protocol usually involves delivering GF animals in a sterile manner, raising them in a sterile environment, and feeding them a sterile diet. However, because animals can have different ways of producing offspring, the methods of generating the GF animal differ slightly. For example, chickens lay eggs and thus procedures such as Cesarean sections are more complex and uncommon for them. To generate GF chickens, researchers usually collect eggs and sterilize them using a solution of peracetic acid. The eggs then stay in a sterile hatching isolator that maintains a warm temperature optimal for hatching. Researchers use an egg candler, which is a light that allows for visualization of the inside of the egg, to verify embryo development in fertilized eggs. Those chicks then hatch in a separate sterile hatching isolators. One day after the chicks hatch, researchers confirm that they are germ free by analyzing stool samples and seeing if any bacterial cultures arise from them. The GF chicks grow up in sterile isolators and consume sterile food and water until the end of the experiment.
Unlike for chickens, when generating GF mice, researchers usually utilize sterile Cesarean section. Sterile Cesarean section typically entails removing the uterus of the animal which contains the fetus, which is then placed in a sterile isolator where the fetus is delivered. Researcher often use mice for GF research due to their biological similarity to humans, as well as ease in handling. Humans and mice share approximately ninety-nine percent similarity in their genes, and most of their internal organ systems also operate similarly. As the primary method to create GF mice, the sterile Cesarean delivery method has generally remained the same since the late nineteenth century. However, as of 2023, techniques to create GF animals have become more cost-effective with improvements in technology such as isolators and feeding systems.
Another method that researchers have devised to create GF mice is through embryo transfer. In 1999, Masanori Okamoto and Tsuneya Matsumoto, who both conducted research in Japan, published a paper that detailed the embryo transfer method. In the paper, they note that sterile Cesarean section presented challenges such as improper delivery timing. As an alternative, they propose the embryo transfer method, which involves harvesting embryos from female mice and then aseptically transferring them to the uterus of a GF female. Because the GF female does not possess any vaginal microbes, the birth would then be sterile. The researchers state the embryo transfer method was effective as the researchers did not have to intervene in the birthing process, though it required a GF animal to already be present to carry the fetus to term.
Researchers use GF animals to study health effects when the animal either does not have any microbes or possesses a known set of microbes. In the latter case, researchers introduce specific microbes they are interested in studying into the GF animal. The microbes introduced may either consist of a single strain or a mixture of different microbes. Using GF animals to study the microbiome allows researchers to look at the colonization effect of the specific, known set of microbes they introduce without worrying about the impact of other microbes. Additionally, researchers can use GF animals to examine the role of the microbiome at different time points in development. Colonizing the GF animal at various critical stages of development allows for research on the temporal relationship between microbial colonization and development.
However, one limitation of GF animals is that their germ-free nature begins from birth. Therefore, they cannot model certain cases of microbiome status, such as when the microbiome composition becomes altered later in life. For example, such alterations include infections by harmful bacteria that cause irregular changes in the microbiome composition. An alternative that researchers use in those cases is antibiotic treatment, which kills specific microbes in the animal. Using broad-spectrum antibiotics, which act on most bacterial groups, can effectively deplete an animal’s microbiome. Researchers can administer antibiotics to the animal at any time, which allows for flexibility in experimental design. A pitfall of antibiotic treatment, however, is the possibility that the drug may not kill the microbes consistently, as well as possibly create harmful side effects.
Researchers have specifically used GF animals to establish the effect of the microbiome in immune system responses. In a 2010 study, Siegfried Hapfelmeier and colleagues, a group of researchers from Switzerland, Canada, and the US, demonstrated the link between microbial colonization and immune response in the gut. They looked at B cells, which are a type of immune cell that produces antibodies. Antibodies are molecules that recognize and destroy foreign proteins. In the gut, certain B cells produce specific antibodies called IgA that target microbes on mucosal surfaces. Hapfelmeier introduced microbes into GF animals and found a dose-dependent relationship where higher loads of bacterial colonization led to higher levels of IgA in response to the introduced bacteria. Therefore, using GF animals as an experimental model, Hapfelmeier concluded that there is a link between gut microbiome composition and B cell production of IgA.
The use of GF animals also contributed to confirming the presence of the gut-brain axis, which is the connection between the nervous system and the gut. A 2010 study by Karen-Anne M. Neufeld and colleagues, a group of researchers from McMaster University in Ontario, Canada, studying medicine, psychiatry, and neuroscience, found that GF mice presented with reduced anxiety when compared to mice with some form of a microbiome. The researchers observed brain-derived neurotrophic factor, or BDNF, a molecule in the brain that promotes the survival of nerve cells and influences stress-related behaviors. The authors observed that the absence of microbes in GF mice correlated with high levels of BDNF in certain areas of the brain, indicating that the lack of microbiome had some sort of impact on the reduced anxiety in the GF mice. Heather Hulme and colleagues, a group of researchers from the United Kingdom primarily studying immunology, published an article in 2022 examining the use of mass spectrometry imaging, or MSI, which can picture the distribution of molecules, to track molecular changes in relation to the gut-brain axis. The researchers used GF mice and examined the molecular changes through MSI and promoted further research on gut-brain axis using MSI.
The development of GF animals in microbiome research has allowed for more experimental possibilities when studying the microbiome. Studies that utilize GF animals contributed to establishing the link between the microbiome and the development of various body systems such as the immune system and nervous system. The technology that GF animal creation employs also applies to other areas such as industrial farming and even human treatments.
- Bryda, Elizabeth C. "The Mighty Mouse: The Impact of Rodents on Advances in Biomedical Research." Missouri Medicine 110 (2013): 207–11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3987984/ (Accessed March 30, 2023).
- Gensollen, Thomas, Shankar S. Iyer, Dennis L. Kasper, and Richard S. Blumberg. “How Colonization by Microbiota in Early Life Shapes the Immune System.” Science 352 (2016): 539–44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5050524/ (Accessed March 30, 2023).
- Gilbert, Jack A., and Josh D. Neufeld. “Life in a World without Microbes.” PLoS Biology 12 (2014). https://doi.org/10.1371/journal.pbio.1002020 (Accessed March 30, 2023).
- Graham-Smith, George S. "George Henry Falkiner Nuttall:(5 July 1862—16 December 1937)." Epidemiology & Infection 38 (1938): 129–40. https://doi.org/10.1017/S0022172400010986 (Accessed March 30, 2023).
- Guitton, Edouard, Arnaud Faurie, Sebastien Lavillatte, Thierry Chaumeil, Pauline Gaboriaud, Francoise Bussiere, Fabrice Laurent, Sonia Lacroix-Lamande, Rodrigo Guabiraba, and Catherine Schouler. "Production of Germ-free Fast-growing Broilers from a Commercial Line for Microbiota Studies." JoVE (Journal of Visualized Experiments) 160 (2020): e61148.
- Hapfelmeier, Siegfried, Melissa A.E. Lawson, Emma Slack, Jorum K. Kirundi, Maaike Stoel, Mathias Heikenwalder, Julia Cahenzli, Yuliya Velykoredko, Maria L. Balmer, Kathrin Endt, Markus B. Geuking, Roy Curtiss III, Kathy D. McCoy, and Andrew J. Macpherson. "Reversible Microbial Colonization of Germ-Free Mice Reveals the Dynamics of IgA Immune Responses." Science 328 (2010): 1705–9.
- Huang, Pan, Shanrong Yi, Leilei Yu, Fengwei Tian, Jianxin Zhao, Hao Zhang, and Wei Chen, and Qixiao Zhai. “Integrative Analysis of the Metabolome and Transcriptome Reveals the Influence of Lactobacillus Plantarum CCFM8610 on Germ-free Mice.” Food & Function (2022): 388–98. https://pubs.rsc.org/en/content/articlehtml/2022/fo/d2fo03117e (Accessed March 30, 2023).
- Hulme, Heather, Lynsey M. Meikle, Nicole Strittmatter, John Swales, Gregory Hamm, Sheila L. Brown, Simon Milling, Andrew S. MacDonald, Richard J.A. Goodwin, Richard Burchmore, and Daniel M. Wall. “Mapping the Influence of the Gut Microbiota on Small Molecules Across the Microbiome Gut Brain Axis.” Journal of the American Society for Mass Spectrometry 33 (2022): 649–59.
- Kennedy, Elizabeth A., Katherine Y. King, and Megan T. Baldridge. "Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria." Frontiers in Physiology 9 (2018). https://www.frontiersin.org/articles/10.3389/fphys.2018.01534/full (Accessed March 30, 2023).
- Kirchhelle, Claas. "Swann Song: Antibiotic Regulation in British Livestock Production (1953–2006)." Bulletin of the History of Medicine 92 (2018): 317–50.
- Kirk, Robert G.W. “’Life in a Germ-Free World’: Isolating Life from the Laboratory Animal to the Bubble Boy." Bulletin of the History of Medicine 86 (2012): 237–75. https://muse.jhu.edu/article/485805 (Accessed March 30, 2023).
- Luczynski, Pauline, Karen-Anne McVey Neufeld, Clara Seira Oriach, Gerard Clarke, Timothy G. Dinan, and John F. Cryan. "Growing Up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior." International Journal of Neuropsychopharmacology 19 (2016). https://academic.oup.com/ijnp/article/19/8/pyw020/2910071?login=false (Accessed March 30, 2023).
- Luczynski, Pauline, Karen-Anne McVey Neufeld, Gerard Clarke, Timothy G. Dinan, and John F. Cryan. “Chapter 7 - Germ-Free Animals: A Key Tool in Unraveling How the Microbiota Affects the Brain and Behavior.” In The Gut-Brain Axis: Dietary, Probiotic and Prebiotic Intervention on the Microbiota, eds . Niall Hyland, and Catherine Stanton , 109–140. Academic Press, 2006.
- Miao, Zhuang, Wang, Yan, and Sun, Zhongsheng. “The Relationships Between Stress, Mental Disorders, and Epigenetic Regulation of BDNF.” International Journal of Molecular Sciences 21 (2020). https://doi.org/10.3390/ijms21041375 (Accessed March 30, 2023).
- Neufeld, Karen Anne McVey, Nancy Kang, John Bienenstock, and Jane A. Foster. "Reduced Anxiety‐Like Behavior and Central Neurochemical Change in Germ‐Free Mice." Neurogastroenterology & Motility 23 (2010): 255–e119. https://doi.org/10.1111/j.1365-2982.2010.01620.x (Accessed March 30, 2023).
- Okamoto, Masanori, and Tsuneya Matsumoto. "Production of Germfree Mice by Embryo Transfer." Experimental Animals 48 (1999): 59–62. https://www.jstage.jst.go.jp/article/expanim/48/1/48_1_59/_article (Accessed March 30, 2023).
- Qv, Lingling, Zhenggang Yang, Mingfei Yao, Sunbing Mao, Yongjun Li, Jia Zhang, and Lanjuan Li. “Methods for Establishment and Maintence of Germ–Free Rat Models.” Frontiers in Microbiology (2020). 10.3389/fmicb.2020.01148 (Accessed March 30, 2023).
- Rodríguez, Juan Miguel, Kiera Murphy, Catherine Stanton, R. Paul Ross, Olivia I. Kober, Nathalie Juge, Ekaterina Avershina, Knut Rudi, Arjan Narbad, Maria C. Jenmalm, Julian R. Marchesi, and Maria Carmen Collado. “The Composition of the Gut Microbiota Throughout Life, with an Emphasis on Early Life.” Microbial Ecology in Health and Disease 26 (2015). 10.3402/mehd.v26.26050 (Accessed March 30, 2023).
- Rosenthal, Nadia, and Steve Brown. "The Mouse Ascending: Perspectives For Human-Disease Models." Nature cell biology 9 (2007): 993–9.
- Science History Institute. “Louis Pasteur.” Science History Institute. Last Modified December 17, 2014. https://www.sciencehistory.org/historical-profile/louis-pasteur (Accessed March 30, 2023).
- Thursby, Elizabeth and Nathalie Juge. “Introduction to the Human Gut Microbiota.” Biochemical Journal 474 (2017): 1823–36. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5433529/ (Accessed March 30, 2023).
- Trexler, Philip C. "Germ-free Isolators." Scientific American 211 (1964): 78–88. https://www.scientificamerican.com/index.cfm/_api/render/file/?method=inline&fileID=E77718A5-5005-4CE6-BE7709AC60A365CE (Accessed March 30, 2023).
- Uzbay, Tayfun. "Germ-free Animal Experiments in the Gut Microbiota Studies." Current Opinion in Pharmacology 49 (2019): 6–10.
How to cite
Articles rights and graphics.
Copyright Arizona Board of Regents Licensed as Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported (CC BY-NC-SA 3.0)
Last modified
Share this page.
St. Vincent National Wildlife Refuge

St. Vincent Island is 9 miles southwest of Apalachicola and is only accessable by boat and there is no visitor center, no potable water, nor public phones on the island. The closest public boat ramp to the island is located 22 miles west of Apalachicola at the end of County Road 30-B. From that boat ramp it is one-quarter of a mile across to the island. Boaters should be sensitive to winds, tide fluctuations, currents, storms, and oyster bars. Visitor use is only during daylight hours however the refuge is closed for general use during hunts, fires, and storms. Bring insect repellent, drinking water, sunscreen, and a hat.
Location and Contact Information
The Refuge is comprised of two islands and two mainland tracts totaling approximately 12,492 acres. Access to the island is by boat only. The Refuge office is in Apalachicola.
No tours are scheduled at this time.
Our Species
Our library.

Get Involved
Volunteers are an integral part of St. Vincent National Wildlife Refuge. They assist with numerous projects ranging from wildlife inventories to presenting interpretive programs, and their help has been instrumental in the accomplishment of a number of refuge projects and activities.
Projects and Research
You are exiting the u.s. fish and wildlife service website.
You are being directed to
We do not guarantee that the websites we link to comply with Section 508 (Accessibility Requirements) of the Rehabilitation Act. Links also do not constitute endorsement, recommendation, or favoring by the U.S. Fish and Wildlife Service.
Archaeologists Found 5 Mysterious Bodies Under the Floors of Hitler's Secret Lair
A grim discovery sheds new light on the dark history of a Nazi fortress.

Amateur archaeologists were recently searching for stray artifacts in an abandoned building in Poland when they made a sinister discovery beneath the floorboards: five decaying bodies with their hands and feet missing.
The site was an infamous villa that belonged to Nazi Party leader Hermann Göring within Adolf Hitler ’s secret military headquarters, the Wolfsschanze ( Wolf’s Lair), which was deserted after a 1944 assassination attempt on Hitler and later partially destroyed by retreating German forces near the end of World War II.
Reuters reports that the Latebra Foundation , which has scoured the site of the Wolf’s Lair “with official permission for several years,” discovered the bodies of three adults, an older child, and a baby buried just below what would have been the floor of Göring’s villa. In 1939, Hitler designated Göring, at the time the commander of the German air force, as his successor.
The discovery at the site raises a host of haunting questions, including about what transpired beneath the villa’s ruins in the decades since the fall of the Third Reich.
“Everyone wondered what might have happened there ... We tried to think of something, but nothing reasonable comes to mind,” Latebra member Dominik Markiewicz told Reuters . “We didn’t know what we were dealing with at all. Were they some occult rituals of Third Reich fanatics? We have no idea.”
Latebra called the police, prompting an investigation and a forensic examination of the remains. Although the case has since been closed, and there are no conclusive answers, a new report has provided additional clues into the potential origins of the bodies.

How did it take so long for the bodies to be discovered? While the Latebra Foundation has been searching the Wolf’s Lair site for years, mostly finding “uniform buttons, tools, and machine parts,” per Reuters , the archaeologists only recently focused on Göring’s villa due to the sheer amount of ground they had to cover. The villa was just one of approximately 200 buildings scattered across the huge compound, which spans an area of over 617 acres of land.
The Wolf’s Lair was off limits to the public for nearly 50 years after World War II. It sustained significant damage at the hands of the Nazis themselves, who didn’t want it to fall into the hands of the advancing Soviet forces. Hitler ordered its destruction shortly after his last visit in November 1944, with the actual demolition occurring in late January 1945, after the Soviets began their Vistula–Oder offensive.
On the same day the Soviets liberated the Auschwitz concentration camp, they captured the Wolf’s Lair without firing a single shot. Under Soviet control, the site fell into disrepair and was closed off to any exploration or excavation. The public couldn’t access the site until the collapse of communism in Poland in 1990, which may explain how the corpses remained hidden for so long.
Besides, archaeologists wouldn’t have necessarily looked to find remains at the Wolf’s Lair, as it wasn’t a death camp. Designed as Hitler’s Eastern Front headquarters, this highly secretive complex served as the strategic center for the Nazi leadership to orchestrate their military campaign against the Soviet Union.
In fact, the most famous act of violence to take place at the Wolf’s Lair during the Third Reich was directed at Hitler himself. On July 20, 1944, Colonel Claus von Stauffenberg carried out an assassination attempt on Hitler in a Wolf’s Lair conference room, by placing a bomb concealed in a briefcase. Although the explosion resulted in the death of four Nazi officers and injured others, Hitler narrowly escaped with his life, largely due to the fortuitous shielding provided by a sturdy table leg that absorbed the brunt of the blast.

Who were the five people buried where Nazi leaders once plotted? A new report in National Geographic pieces together some clues from the initial investigation:
“According to the medical examiner, the skeletons appear from their age to be from the ‘interwar’ years between 1918 and 1939, and the poor condition of the remains means it is now impossible to determine the causes of their deaths.”
So, it’s possible the bodies were buried on the site before the Wolf’s Lair was even built. (Construction concluded on the compound in June of 1941.)
Some experts have tried to connect the corpses to ritual sacrifice, as leading Nazi figures like Heinrich Himmler reportedly engaged in occult practices, according to NatGeo . They point to the presence of “dart-shaped stones called belemnites” near the bodies, which were reportedly sometimes placed at pagan burial sites as a “good luck charm.” But belemnite also naturally occurs in that area, so it’s tough to definitively interpret the stones’ proximity to the graves as evidence of ritualistic practices.
Since the corpses date back to a period between 1918 and 1939, the Latebra Foundation plans to proceed with radiocarbon dating. This analysis aims to pinpoint the bodies’ time of death more precisely, potentially narrowing it down to a span of just a few years. But, as NatGeo notes, even if these three adults and two children were buried before the Nazis ever set foot within the Wolf’s Lair, that also carries a disturbing implication:
“The skeletons were buried in the floor just a few inches below the surface and right next to 1940s-era plumbing pipes for the house, meaning that if they were indeed buried before Göring moved in, construction workers would have found the remains—and must have left them where they were.”
Michael Natale is the news editor for Best Products , covering a wide range of topics like gifting, lifestyle, pop culture, and more. He has covered pop culture and commerce professionally for over a decade. His past journalistic writing can be found on sites such as Yahoo! and Comic Book Resources , his podcast appearances can be found wherever you get your podcasts, and his fiction can’t be found anywhere, because it’s not particularly good.

.css-cuqpxl:before{padding-right:0.3125rem;content:'//';display:inline;} Pop Mech Pro .css-xtujxj:before{padding-left:0.3125rem;content:'//';display:inline;}

What We Know About Russia’s Mystery Radio Signal

A Battery-Powered Race Car Takes On Pikes Peak
Dark Matter Could Unlock a Limitless Energy Source

Navy Remove Stealth Destroyer’s Big Guns

The Long and Messy Search for Al Capone's Treasure

Skunk Works Unveils Stealthy Tanker Design

Rebuilding the Maze

A Groundbreaking Discovery For Light-Speed Travel

Russia Is Now Using Golf Carts in Combat

The Bradley Gets a New Iron Fist

South Africa Wins Fight for 'Indian Titanic' Loot
Project Controller
Would you like to work as a project controller on the greenest campus in the Netherlands and contribute to important social issues of our time? Are you financially well-grounded, analytical, and precise? Are you looking for a challenging job where you can grow? Then read on and apply!
The Wageningen Environmental Sciences Group (ESG) is looking for a Project Controller to strengthen the Finance & Control (F&C) team.
As a Project Controller, you will be part of an enthusiastic F&C department with approximately 40 FTE. There is a pleasant, driven, and open working atmosphere. Besides the Project Control team, the F&C department also consists of the Financial Administration, Business Control, and Chair Administration teams. In this role, you will work closely with your colleagues on various topics in the field of project control and safeguard processes to minimize risks.
As a Project Controller:
- you are responsible for the financial management of various projects;
- you are involved in all phases of the project cycle, from acquisition to financial completion;
- you are the financial point of contact for project leaders, providing both solicited and unsolicited advice;
- you are responsible for the determination of project results for monthly and annual figures for internal monitoring and external reporting (e.g., annual accounts);
- you contribute to identifying and monitoring project risks and assist in implementing internal control measures;
- you work independently with a high degree of responsibility and collaborate intensively with direct colleagues from F&C.
As a Project Controller at ESG, you will have the opportunity to develop your skills and make a valuable contribution to the most important social issues of our time.
Your qualities
You are/have:
- an HBO degree, preferably in business economics, business administration, accountancy, or a similar field;
- proven affinity with finance and a good understanding of basic administration;
- relevant work experience in a complex (project) organization is a plus;
- analytical skills, working carefully and systematically to ensure good results;
- good communication skills, easily bridging the gap between project leaders and F&C;
- someone who enjoys working both independently and as part of a team;
Furthermore, you recognize the following skills in yourself: you take initiative, are honest, customer-oriented, and always look for opportunities to develop yourself.
We offer you
Wageningen University & Research offers excellent terms of employment . A few highlights from our Collective Labour Agreement include:
- working hours that can be discussed and arranged so that they allow for the best possible work-life balance;
- the option to accrue additional compensation hours by working more;
- sabbatical leave, study leave, and partially paid parental leave;
- there is a strong focus on vitality and you can make use of the sports facilities available on campus for a small fee;
- a fixed December bonus of 8,3%;
- excellent pension scheme.
Do you want more information?
For more information about this position, please contact Wilma van der Straten, team leader projectcontrol, [email protected] . For more information about the procedure, please contactJeanine van ‘t Veer, corporate recruiter, [email protected]. Do you want to apply? You can apply directly using the apply button on the vacancy page on our website which will allow us to process your personal information with your approval. This vacancy will be listed up to and including June 17th, 2024. We review applications on an ongoing basis and will schedule a meeting with you if appropriate. The vacancy will close once we have found a suitable candidate, so don't hesitate to apply. Procedure As part of our selection process, an assessment may be incorporated within the procedure Equal opportunities Wageningen University & Research (WUR) employs a large number of people with very different backgrounds and qualities, who inspire and motivate each other. We want every talent to feel at home in our organisation and be offered the same career opportunities. We therefore especially welcome applications from people who are underrepresented at WUR. A good example of how WUR deals with inclusiveness can be read on the page working at WUR with a functional impairment .

IMAGES
COMMENTS
Learn about animal behavior, anatomy, and adaptations with these free STEM activities for K-12 students. Explore habitats, body structures, camouflage, mimicry, and more with hands-on projects and games.
From classifying animals in the Serengeti to discovering new exoplanets using the Kepler space telescope, researchers of all backgrounds have used the free project builder to create engaging, accessible citizen science projects. ... The contributions of Zooniverse volunteers produce real research. Our projects have led to hundreds of peer ...
Assess the reproducibility and translatability of the project. 2. Legal issues Consider how the research is affected by relevant legislation for animal research and other areas, e.g. animal transport, occupational health and safety. Locate relevant guidance documents (e.g. EU guidance on project evaluation). 3.
Learn how to design and implement a virtual ethogram and time-budget study project for students in an introductory biology course. This project develops scientific skills and confidence in observing and analyzing animal behavior.
Learn why and how animals are used in medical research at Oxford, and what regulations and ethical considerations apply. Find out the types of procedures, numbers and species of animals involved, and the benefits and limitations of animal models.
Find out how to do zoology science projects with animals, such as dinoflagellates, water fleas, slime molds, planaria, ants, and birds. Learn about their bioluminescence, regeneration, intelligence, behavior, and more.
Scientists must write a detailed plan of why and how they plan to use animals for a research project. This information is then reviewed by other scientists and members of the public to make sure that the research animals will be used for has an important purpose. Whatever the animals are used for, the scientists also make sure to take care of ...
This article provides a guide to open science practices that can improve the transparency and quality of animal research. It covers topics such as study design, data sharing, preregistration, and publication of animal studies.
Animal research & COVID-19. Animal research and COVID-19 / SARS-COV-2 coronavirus. Nobel Prizes. The animal research behind a century of Nobel Prizes. Paget Lectures. Annual lectures given by prominent scientists. Animals used in research. A-Z of animals used in research. Quotes database.
Animals provide a way to study the fundamental workings of the human body and explore how its basic building blocks—molecules and cells—work in health and disease. In doing so, researchers can unravel the most basic mechanisms that fuel illness. Animal models help researchers understand how the normal processes in molecules, cells, and ...
The ICARE Project is a collaboration of federal agencies involved in animal research, teaching, and testing in the U.S. It offers online and in-person events to improve animal welfare and compliance with federal standards.
Consider how the research is affected by relevant legislation for animal research and other areas, e.g. animal transport, occupational health and safety. Locate relevant guidance documents (e.g. EU guidance on project evaluation). 3. Ethical issues, harm-benefit assessment and humane endpoints Construct a lay summary.
Learn how to teach your students to write informational text about animals with this free unit of study. It includes anchor charts, mini-lessons, writing planners and graphic organizers to guide your students through the research and writing process.
The short answer is: It depends. Which animal model is most suitable depends on multiple factors, including the type of research performed, the physiologic or biologic phenomenon and/or the disease process under investigation, as well as the degree of similarity between a particular animal organism and humans.
A printable and digital resource for elementary teachers to guide students through animal research and writing. Includes graphic organizers, research activities, flip book, flap book, poster, and poetry projects.
Learn how to conduct animal research projects on various species and their behaviors. Find out how to design experiments and construct models for science fairs or school assignments.
Experiment with Animal Behavior Science Projects. (14 results) Investigate a question about animal ethology, their behavior. Discover what safely repels ants, how animals prefer to eat, what environments animals prefer, or how animals journey. How to Design a Great Cat Toy. Add Favorite. Remove Favorite.
The first part of this research will be learning about our animal: environment, appearance, survival, features, behaviors, traits, food chain, and more…. You will need to read and complete EACH SLIDE in ORDER. Do NOT rush through this project, it is for several grades! Every day or so, a new part of the powerpoint will be added so that you ...
VV: "Using animals in research is a privilege granted by society to the research community with the expectation that such use will provide either significant new knowledge or lead to improvement ...
5. REPEAT. We repeat steps 2-4 for either 3 or 4 animals. Some students may work faster, while some may take a bit more time on each step. I try to adjust the project to be appropriate for the majority of the class. 6. SHARE. When the project is done, I try to find a special way for us to share our work.
5 Animal Research Websites for Students. Ready to do some animal research? Finding trustworthy and appropriate animal websites for students to use can be a challenge. Below are my go-to websites that you can feel confident having your students go to. Most of these sites also have videos, games and other educational activities as well.
Learn how to plan and guide animal research projects in kindergarten using a six-step roadmap. Find resources, tips, and examples for creating informational books, models, and presentations about animals.
05/20/2024. NIH has updated and expanded its content and resources discussing the involvement of Animals in NIH Funding. The website is designed for a general audience, providing a plain language explanation of the following: Why Animals are Used in Research. How Animals Have Helped Improve Public Health.
More Information: Scientists who conduct research with animals must follow the applicable laws, regulations and standards regarding the treatment and care of animals used in research and testing ...
Fig 1. Using open science practices throughout translational research studies. Application of open science practices at each step of the research process can maximize the impact of performed animal experiments. The implementation of these practices will lead to less time pressure at the end of a project.
Morris Animal Foundation's mission is to bridge science and resources to advance the health of animals. Founded in 1948 and headquartered in Denver, it is one of the largest nonprofit animal health research organizations in the world, funding nearly $160 million in more than 3,000 critical animal health studies to date across a broad range of ...
Published: 2024-05-24. Germ-free, or GF, animals are laboratory animals that completely lack microbes, making them useful tools for microbiome research. Researchers create GF animals in laboratories by delivering the newborn animals in a way that protects them from microbes, which are microscopic organisms such as bacteria and viruses.
Established as a National Wildlife Refuge in 1968 for the protection and conservation of migratory birds, St. Vincent NWR is managed to preserve, in as natural a state as possible, it's highly varied plant and animal communities. The Refuge is comprised of two islands and two mainland tracts totaling approximately 12,492 acres. Popular recreational opportunities include fishing, hunting ...
Amateur archaeologists were recently searching for stray artifacts in an abandoned building in Poland when they made a sinister discovery beneath the floorboards: five decaying bodies with their ...
As a Project Controller: you are responsible for the financial management of various projects; you are involved in all phases of the project cycle, from acquisition to financial completion; you are the financial point of contact for project leaders, providing both solicited and unsolicited advice; you are responsible for the determination of ...