- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Diagnosis and...
Diagnosis and management of bipolar disorders
- Related content
- Peer review
- Fernando S Goes , associate professor of psychiatry and behavioral sciences 1 2
- 1 Precision Medicine Center of Excellence in Mood Disorders, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
- 2 Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA
- Correspondence to: F S Goes fgoes1{at}jhmi.edu
Bipolar disorders (BDs) are recurrent and sometimes chronic disorders of mood that affect around 2% of the world’s population and encompass a spectrum between severe elevated and excitable mood states (mania) to the dysphoria, low energy, and despondency of depressive episodes. The illness commonly starts in young adults and is a leading cause of disability and premature mortality. The clinical manifestations of bipolar disorder can be markedly varied between and within individuals across their lifespan. Early diagnosis is challenging and misdiagnoses are frequent, potentially resulting in missed early intervention and increasing the risk of iatrogenic harm. Over 15 approved treatments exist for the various phases of bipolar disorder, but outcomes are often suboptimal owing to insufficient efficacy, side effects, or lack of availability. Lithium, the first approved treatment for bipolar disorder, continues to be the most effective drug overall, although full remission is only seen in a subset of patients. Newer atypical antipsychotics are increasingly being found to be effective in the treatment of bipolar depression; however, their long term tolerability and safety are uncertain. For many with bipolar disorder, combination therapy and adjunctive psychotherapy might be necessary to treat symptoms across different phases of illness. Several classes of medications exist for treating bipolar disorder but predicting which medication is likely to be most effective or tolerable is not yet possible. As pathophysiological insights into the causes of bipolar disorders are revealed, a new era of targeted treatments aimed at causal mechanisms, be they pharmacological or psychosocial, will hopefully be developed. For the time being, however, clinical judgment, shared decision making, and empirical follow-up remain essential elements of clinical care. This review provides an overview of the clinical features, diagnostic subtypes, and major treatment modalities available to treat people with bipolar disorder, highlighting recent advances and ongoing therapeutic challenges.
Introduction
Abnormal states of mood, ranging from excesses of despondency, psychic slowness, diminished motivation, and impaired cognitive functioning on the one hand, and exhilaration, heightened energy, and increased cognitive and motoric activity on the other, have been described since antiquity. 1 However, the syndrome in which both these pathological states occur in a single individual was first described in the medical literature in 1854, 2 although its fullest description was made by the German psychiatrist Emil Kraepelin at the turn of the 19th century. 3 Kraepelin emphasized the periodicity of the illness and proposed an underlying trivariate model of mood, thought (cognition), and volition (activity) to account for the classic forms of mania and depression and the various admixed presentations subsequently know as mixed states. 3 These initial descriptions of manic depressive illness encompassed most recurrent mood syndromes with relapsing remitting course, minimal interepisode morbidity, and a wide spectrum of “colorings of mood” that pass “without a sharp boundary” from the “rudiment of more severe disorders…into the domain of personal predisposition.” 3 Although Kraepelin’s clinical description of bipolar disorder (BD) remains the cornerstone of today’s clinical description, more modern conceptions of bipolar disorder have differentiated manic depressive illness from recurrent depression, 4 partly based on differences in family history and the relative specificity of lithium carbonate and mood stabilizing anticonvulsants as anti-manic and prophylactic agents in bipolar disorder. While the boundaries of bipolar disorder remain a matter of controversy, 5 this review will focus on modern clinical conceptions of bipolar disorder, highlighting what is known about its causes, prognosis, and treatments, while also exploring novel areas of inquiry.
Sources and selection criteria
PubMed and Embase were searched for articles published from January 2000 to February 2023 using the search terms “bipolar disorder”, “bipolar type I”, “bipolar type II”, and “bipolar spectrum”, each with an additional search term related to each major section of the review article (“definition”, “diagnosis”, “nosology”, “prevalence”, “epidemiology”, “comorbid”, “precursor”, “prodrome”, “treatment”, “screening”, “disparity/ies”, “outcome”, “course”, “genetics”, “imaging”, “treatment”, “pharmacotherapy”, “psychotherapy”, “neurostimulation”, “convulsive therapy”, “transmagnetic”, “direct current stimulation”, “suicide/suicidal”, and “precision”). Searches were prioritized for systematic reviews and meta-analyses, followed by randomized controlled trials. For topics where randomized trials were not relevant, searches also included narrative reviews and key observational studies. Case reports and small observations studies or randomized controlled trials of fewer than 50 patients were excluded.
Modern definitions of bipolar disorder
In the 1970s, the International Classification of Diseases and the Diagnostic and Statistical Manual of Mental Disorders reflected the prototypes of mania initially described by Kraepelin, following the “neo-Kraepelinian” model in psychiatric nosology. To meet the primary requirement for a manic episode, an individual must experience elevated or excessively irritable mood for at least a week, accompanied by at least three other typical syndromic features of mania, such as increased activity, increased speed of thoughts, rapid speech, changes in esteem, decreased need for sleep, or excessive engagement in impulsive or pleasurable activities. Psychotic symptoms and admission to hospital can be part of the diagnostic picture but are not essential to the diagnosis. In 1994, Diagnostic and Statistical Manual of Mental Disorders , fourth edition (DSM-IV) carved out bipolar disorder type II (BD-II) as a separate diagnosis comprising milder presentations of mania called hypomania. The diagnostic criteria for BD-II are similar to those for bipolar disorder type I (BD-I), except for a shorter minimal duration of symptoms (four days) and the lack of need for significant role impairment during hypomania, which might be associated with enhanced functioning in some individuals. While the duration criteria for hypomania remain controversial, BD-II has been widely accepted and shown to be as common as (if not more common than) BD-I. 6 The ICD-11 (international classification of diseases, 11th revision) included BD-II as a diagnostic category in 2019, allowing greater flexibility in its requirement of hypomania needing to last several days.
The other significant difference between the two major diagnostic systems has been their consideration of mixed symptoms. Mixed states, initially described by Kraepelin as many potential concurrent combinations of manic and depressive symptoms, were more strictly defined by DSM as a week or more with full syndromic criteria for both manic and depressive episodes. In DSM-5, this highly restrictive criterion was changed to encompass a broader conception of subsyndromal mixed symptoms (consisting of at least three contrapolar symptoms) in either manic, hypomanic, or depressive episodes. In ICD-11, mixed symptoms are still considered to be an episode, with the requirement of several prominent symptoms of the countervailing mood state, a less stringent requirement that more closely aligns with Kraepelin's broader conception of mixed states. 7
Epidemiology
Using DSM-IV criteria, the National Comorbidity Study replication 6 found similar lifetime prevalence rates for BD-I (1.0%) and BD-II (1.1%) among men and women. Subthreshold symptoms of hypomania (bipolar spectrum disorder) were more common, with prevalence rate estimates of 2.4%. 6 Incidence rates, which largely focus on BD-I, have been estimated at approximately 6.1 per 100 000 person years (95% confidence interval 4.7 to 8.1). 8 Estimates of the incidence and lifetime prevalence of bipolar disorder show moderate variations according to the method of diagnosis (performed by lay interviewers in a research context v clinically trained interviews) and the racial, ethnic, and demographic context. 9 Higher income, westernized countries have slightly higher rates of bipolar disorder, 10 which might reflect a combination of westernized centricity in the specific idioms used to understand and elicit symptoms, as well as a greater knowledge, acceptance, and conceptualization of emotional symptoms as psychiatric disorders.
Causes of bipolar disorder
Like other common psychiatric disorders, bipolar disorder is likely caused by a complex interplay of multiple factors, both at the population level and within individuals, 11 which can be best conceptualized at various levels of analysis, including genetics, brain networks, psychological functioning, social support, and other biological and environmental factors. Because knowledge about the causes of bipolar disorder remains in its infancy, for pragmatic purposes, most research has followed a reductionistic model that will ultimately need to be synthesized for a more coherent view of the pathophysiology that underlies the condition.
Insights from genetics
From its earliest descriptions, bipolar disorder has been observed to run in families. Indeed, family history is the strongest individual risk factor for developing the disorder, with first degree relatives having an approximately eightfold higher risk of developing bipolar disorder compared with the baseline population rates of ~1%. 12 While family studies cannot separate the effects of genetics from behavioral or cultural transmission, twin and adoption studies have been used to confirm that the majority of the familial risk is genetic in origin, with heritability estimates of approximately 60-80%. 13 14 There have been fewer studies of BD-II, but its heritability has been found to be smaller (~46%) 15 and closer to that of more common disorders such as major depressive disorder or generalized anxiety. 15 16 Nevertheless, significant heritability does not necessarily imply the presence of genes of large effect, since the genetic risk for bipolar disorder appears likely to be spread across many common variants of small effect sizes. 16 17 Ongoing studies of rare variations have found preliminary evidence for variants of slightly higher effect sizes, with initial evidence of convergence with common variations in genes associated with the synapse and the postsynaptic density. 18 19
While the likelihood that the testing of single variants or genes will be useful for diagnostic purposes is low, analyses known as polygenic risk studies can sum across all the risk loci and have some ability to discriminate cases from controls, albeit at the group level rather than the individual level. 20 These polygenic risk scores can also be used to identify shared genetic risk factors across other medical and psychiatric disorders. Bipolar disorder has strong evidence for common variant based coheritability with schizophrenia (genetic correlation (r g ) 0.69) and major depressive disorder (r g 0.48). BD-I has stronger coheritability with schizophrenia compared with BD-II, which is more strongly genetically correlated with major depressive disorder (r g 0.66). 16 Lower coheritability was observed with attention deficit hyperactivity disorder (r g 0.21), anorexia nervosa (0.20), and autism spectrum disorder (r g 0.21). 16 These correlations provide evidence for shared genetic risk factors between bipolar disorder and other major psychiatric syndromes, a pattern also corroborated by recent nationwide registry based family studies. 12 14 Nevertheless, despite their potential usefulness, polygenic risk scores must currently be interpreted with caution given their lack of populational representation and lingering concerns of residual confounds such as gene-environment correlations. 21
Insights from neuroimaging
Similarly to the early genetic studies, small initial studies had limited replication, leading to the formation of large worldwide consortiums such as ENIGMA (enhancing neuroimaging genetics through meta-analysis) which led to substantially larger sample sizes and improved reproducibility. In its volumetric analyses of subcortical structures from MRI (magnetic resonance imaging) of patients with bipolar disorder, the ENIGMA consortium found modest decreases in the volume of the thalamus (Cohen’s d −0.15), the hippocampus (−0.23), and the amygdala (−0.11), with an increased volume seen only in the lateral ventricles (+0.26). 22 Meta-analyses of cortical regions similarly found small reductions in cortical thickness broadly across the parietal, temporal, and frontal cortices (Cohen’s d −0.11 to −0.29) but no changes in cortical surface area. 23 In more recent meta-analyses of white matter tracts using diffuse tension imaging, widespread but modest decreases in white matter integrity were found throughout the brain in bipolar disorder, most notably in the corpus callosum and bilateral cinguli (Cohen’s d −0.39 to −0.46). 24 While these findings are likely to be highly replicable, they do not, as yet, have clinical application. This is because they reflect differences at a group level rather than an individual level, 25 and because many of these patterns are also seen across other psychiatric disorders 26 and could be either shared risk factors or the effects of confounding factors such as medical comorbidities, medications, co-occurring substance misuse, or the consequences (rather than causes) of living with mental illness. 27 Efforts to collate and meta-analyze large samples utilizing longitudinal designs 28 task based, resting state functional MRI measurents, 29 as well as other measures of molecular imaging (magnetic resonance spectroscopy and positron emission tomography) are ongoing but not as yet synthesized in large scale meta-analyses.
Environmental risk factors
Because of the difficulty in measuring and studying the relevant and often common environmental risk factors for a complex illness like bipolar disorder, there has been less research on how environmental risk factors could cause or modify bipolar disorder. Evidence for intrauterine risk factors is mixed and less compelling than such evidence in disorders like schizophrenia. 30 Preliminary evidence suggests that prominent seasonal changes in solar radiation, potentially through its effects on circadian rhythm, can be associated with an earlier onset of bipolar disorder 31 and a higher likelihood of experiencing a depressive episode at onset. 31 However, the major focus of environmental studies in bipolar disorder has been on traumatic and stressful life events in early childhood 32 and in adulthood. 33 The effects of such adverse events are complex, but on a broad level have been associated with earlier onset of bipolar disorder, a worse illness course, greater prevalence of psychotic symptoms, 34 substance misuse and psychiatric comorbidities, and a higher risk of suicide attempts. 32 35 Perhaps uniquely in bipolar disorder, evidence also indicates that positive life events associated with goal attainment can also increase the risk of developing elevated states. 36
Comorbidity
Bipolar disorder rarely manifests in isolation, with comorbidity rates indicating elevated lifetime risk of several co-occurring symptoms and comorbid disorders, particularly anxiety, attentional disorders, substance misuse disorders, and personality disorders. 37 38 The causes of such comorbidity can be varied and complex: they could reflect a mixed presentation artifactually separated by current diagnostic criteria; they might also reflect independent illnesses; or they might represent the downstream effects of one disorder increasing the risk of developing another disorder. 39 Anxiety disorders tend to occur before the frank onset of manic or hypomanic symptoms, suggesting that they could in part reflect prodromal symptoms that manifest early in the lifespan. 37 Similarly, subthreshold and syndromic symptoms of attention deficit/hyperactivity disorder are also observed across the lifespan of people with bipolar disorder, but particularly in early onset bipolar disorder. 40 On the other hand, alcohol and substance misuse disorders occur more evenly before and after the onset of bipolar disorder, consistent with a more bidirectional causal association. 41
The association between bipolar disorder and comorbid personality disorders is similarly complex. Milder manifestations of persistent mood instability (cyclothymia) or low mood (dysthymia) have previously been considered to be temperamental variants of bipolar disorder, 42 but are now classified as related but separate disorders. In people with persistent emotional dysregulation, making the diagnosis of bipolar disorder can be particularly challenging, 43 since the boundaries between longstanding mood instability and phasic changes in mood state can be difficult to distinguish. While symptom overlap can lead to artificially inflated prevalence rates of personality disorders in bipolar disorder, 44 the elevated rates of most personality disorders in bipolar disorder, particularly those related to emotional instability, are likely reflective of an important clinical phenomenon that is understudied, particularly with regard to treatment implications. 45 In general, people with comorbidities tend to have greater symptom burden and functional impairment and have lower response rates to treatment. 46 47 Data on approaches to treat specific comorbid disorders in bipolar disorder are limited, 48 49 and clinicians are often left to rely on their clinical judgment. The most parsimonious approach is to treat primary illness as fully as possible before considering additional treatment options for remaining comorbid symptoms. For certain comorbidities, such as anxiety symptoms and disorders of attention, first line pharmacological treatment—namely, antidepressants and stimulants, should be used with caution, since they might increase the long term risks of mood switching or overall mood instability. 50 51
Like other major mental illnesses, bipolar disorder is also associated with an increased prevalence of common medical disorders such as obesity, hyperlipidemia, coronary artery disease, chronic obstructive pulmonary disease, and thyroid dysfunction. 52 These have been attributed to increase risk factors such as physical inactivity, poor nutrition, smoking, and increased use of addictive substances, 53 but some could also be consequences of specific treatments, such as the atypical antipsychotics and mood stabilizers. 54 Along with poor access to care, this medical burden likely accounts for much of the increased standardized mortality (approximately 2.6 times higher) in people with bipolar disorder, 55 highlighting the need to utilize treatments with better long term side effect profiles, and the need for better integration with medical care.
Precursors and prodromes: who develops bipolar disorder?
While more widespread screening and better accessibility to mental health providers should in principle shorten the time to diagnosis and treatment, early manifestation of symptoms in those who ultimately go on to be diagnosed with bipolar disorder is generally non-specific. 56 In particular, high risk offspring studies of adolescents with a parent with bipolar disorder have found symptoms of anxiety and attentional/disruptive disorders to be frequent in early adolescence, followed by higher rates of depression and sleep disturbance in later teenage years. 56 57 Subthreshold symptoms of mania, such as prolonged increases in energy, elated mood, racing thoughts, and mood lability are also more commonly found in children with prodromal symptoms (meta-analytic prevalence estimates ranging from 30-50%). 58 59 Still, when considered individually, none of these symptoms or disorders are sensitive or specific enough to accurately identify individuals who will transition to bipolar disorder. Ongoing approaches to consider these clinical factors together to improve accuracy have a promising but modest ability to identify people who will develop bipolar disorder, 60 emphasizing the need for further studies before implementation.
Screening for bipolar disorder
Manic episodes can vary from easily identifiable prototypical presentations to milder or less typical symptoms that can be challenging to diagnose. Ideally, a full diagnostic evaluation with access to close informants is performed on patients presenting to clinical care; however, evaluations can be hurried in routine clinical care, and the ability to recall previous episodes might be limited. In this context, the use of screening scales can be a helpful addition to clinical care, although screening scales must be regarded as an impetus for a confirmatory clinical interview rather than a diagnostic instrument by themselves. The two most widely used and openly available screening scales are the mood disorders questionnaire (based on the DSM-IV criteria for hypomania) 61 and the hypomania check list (HCL-32), 62 that represent a broader overview of symptoms proposed to be part of a broader bipolar spectrum.
Racial/ethnic disparities
Although community surveys using structured or semi-structured diagnostic instruments, have provided little evidence for variation across ethnic groups, 63 64 observational studies based on clinical diagnoses in healthcare settings have found a disproportionately higher rate of diagnosis of schizophrenia relative to bipolar disorder in black people. 65 Consistent with similar disparities seen across medicine, these differences in clinical diagnoses are likely influenced by a complex mix of varying clinical presentations, differing rates of comorbid conditions, poorer access to care, greater social and economic burden, as well as the potential effect of subtle biases of healthcare professionals. 65 While further research is necessary to identify driving factors responsible for diagnostic disparities, clinicians should be wary of making a rudimentary diagnosis in patients from marginalized backgrounds, ensuring comprehensive data gathering and a careful diagnostic formulation that incorporates shared decision making between patient and provider.
Bipolar disorder is a recurrent illness, but its longitudinal course is heterogeneous and difficult to predict. 46 66 The few available long term studies of BD-I and BD-II have found a consistent average rate of recurrence of 0.40 mood episodes per year in historical studies 67 and 0.44 mood episodes per year in more recent studies. 68 The median time to relapse is estimated to be 1.44 years, with higher relapse rates seen in BD-I (0.81 years) than in BD-II (1.63 years) and no differences observed with respect to age or sex. 1 2 In addition to focusing on episodes, an important development in research and clinical care of bipolar disorder has been the recognition of the burden of subsyndromal symptoms. Although milder in severity, these symptoms can be long lasting, functionally impairing, and can themselves be a risk factor for episode relapse. 69 Recent cohort studies have also found that a substantial proportion of patients with bipolar disorder (20-30%) continue to have poor outcomes even after receiving guideline based care. 46 70 Risk factors that contribute to this poor outcome include transdiagnostic indicators of adversity such as substance misuse, low educational attainment, socioeconomic hardship, and comorbid disorders. As expected, those with more severe past illness activity, including those with rapid cycling, were also more likely to remain symptomatically and psychosocially impaired. 46 71 72
The primary focus of treating bipolar disorder has been to manage the manic, mixed, or depressive episodes that present to clinical care and to subsequently prevent recurrence of future episodes. Owing to the relapse remitting nature of the illness, randomized controlled trials are essential to determine treatment efficacy, as the observation of clinical improvement could just represent the ebbs and flows of the natural history of the illness. In the United States, the FDA (Food and Drug Administration) requires at least two large scale placebo controlled trials (phase 3) to show significant evidence of efficacy before approving a treatment. Phase 3 studies of bipolar disorder are generally separated into short term studies of mania (3-4 weeks), short term studies for bipolar depression (4-6 weeks), and longer term maintenance studies to evaluate prophylactic activity against future mood episodes (usually lasting one year). Although the most rigorous evaluation of phase 3 studies would be to require two broadly representative and independent randomized controlled trials, the FDA permits consideration of so called enriched design trials that follow participants after an initial response and tolerability has been shown to an investigational drug. Because of this initial selection, such trials can be biased against comparator agents, and could be less generalizable to patients seen in clinical practice.
A summary of the agents approved by the FDA for treatment of bipolar disorder is in table 1 , which references the key clinical trials demonstrating efficacy. Figure 1 and supplementary table 1 are a comparison of treatments for mania, depression, and maintenance. Effect sizes reflect the odds ratios or relative risks of obtaining response (defined as ≥50% improvement from baseline) in cases versus controls and were extracted from meta-analyses of randomized controlled trials for bipolar depression 86 and maintenance, 94 as well as a network meta-analysis of randomized controlled trials in bipolar mania. 73 Effect sizes are likely to be comparable for each phase of treatment, but not across the different phases, since methodological differences exist between the three meta-analytic studies.
FDA approved medications for bipolar disorder
- View inline

Summary of treatment response rates (defined as ≥50% improvement from baseline) of modern clinical trials for acute mania, acute bipolar depression, and long term recurrence. Meta-analytic estimates were extracted from recent meta-analyses or network meta-analyses of acute mania, 73 acute bipolar depression, 86 and bipolar maintenance studies 94
- Download figure
- Open in new tab
- Download powerpoint
Acute treatment of mania
As mania is characterized by impaired judgment, individuals can be at risk for engaging in high risk, potentially dangerous behaviors that can have substantial personal, occupational, and financial consequences. Therefore, treatment of mania is often considered a psychiatric emergency and is, when possible, best performed in the safety of an inpatient unit. While the primary treatment for mania is pharmacological, diminished insight can impede patients' willingness to accept treatment, emphasizing the significance of a balanced therapeutic approach that incorporates shared decision making frameworks as much as possible to promote treatment adherence.
The three main classes of anti-manic treatments are lithium, mood stabilizing anticonvulsants (divalproate and carbamazepine), and antipsychotic medications. Almost all antipsychotics are effective in treating mania, with the more potent dopamine D2 receptor antagonists such as risperidone and haloperidol demonstrating slightly higher efficacy ( fig 1 ). 73 In the United States, the FDA has approved the use of all second generation antipsychotics for treating mania except for lurasidone and brexpriprazole. Compared with mood stabilizing medications, second generation antipsychotics have a faster onset of action, making them a first line treatment for more severe manic symptoms that require rapid treatment. 99 The choice of which specific second generation antipsychotic to use depends on a balance of efficacy, tolerability concerns, and cost considerations (see table 1 ). Notably, the FDA has placed a black box warning on all antipsychotics for increasing the risk of cerebral vascular accidents in the elderly. 100 While this was primarily focused on the use of antipsychotics in dementia, this likely class effect should be taken into account when considering the use of antipsychotics in the elderly.
Traditional mood stabilizers, such as lithium, divalproate, and carbamazepine are also effective in the treatment of active mania ( fig 1 ). Since lithium also has a robust prophylactic effect (see section on prevention of mood episodes below) it is often recommended as first line treatment and can be considered as monotherapy when rapid symptom reduction is not clinically indicated. On the other hand, other anticonvulsants such as lamotrigine, gabapentin, topiramate, and oxcarbazepine have not been found to be effective for the treatment of mania or mixed episodes. 101 Although the empirical evidence for polypharmacy is limited, 102 combination treatment in acute mania, usually consisting of a mood stabilizer and a second generation antipsychotic, is commonly used in clinical practice despite the higher burden of side effects. Following resolution of an acute mania, consideration should be given to transitioning to monotherapy with an agent with proven prophylactic activity.
Pharmacological approaches to bipolar depression
Depressed episodes are usually more common than mania or hypomania, 103 104 and often represent the primary reason for individuals with bipolar disorder to seek treatment. Nevertheless, because early antidepressant randomized controlled trials did not distinguish between unipolar and bipolar depressive episodes, it has only been in the past two decades that large scale randomized controlled trials have been conducted specifically for bipolar depression. As such trials are almost exclusively funded by pharmaceutical companies, they have focused on the second generation antipsychotics and newer anticonvulsants still under patent. These trials have shown moderate but robust effects for most recent second generation antipsychotics, five of which have received FDA approval for treating bipolar depression ( table 1 ). No head-to-head trials have been conducted among these agents, so the choice of medication depends on expected side effects and cost considerations. For example, quetiapine has robust antidepressant efficacy data but is associated with sedation, weight gain, and adverse cardiovascular outcomes. 105 Other recently approved medications such as lurasidone, cariprazine, and lumateperone have better side effect profiles but show more modest antidepressant activity. 106
Among the mood stabilizing anticonvulsants, lamotrigine has limited evidence for acute antidepressant activity, 107 possibly owing to the need for an 8 week titration to reach the full dose of 200 mg. However, as discussed below, lamotrigine can still be considered for mild to moderate acute symptoms owing to its generally tolerable side effect profile and proven effectiveness in preventing the recurrence of depressive episodes. Divalproate and carbamazepine have some evidence of being effective antidepressants in small studies, but as there has been no large scale confirmatory study, they should be considered second or third line options. 86 Lithium has been studied for the treatment of bipolar depression as a comparator to quetiapine and was not found to have a significant acute antidepressant effect. 88
Antidepressants
Owing to the limited options of FDA approved medications for bipolar depression and concerns of metabolic side effects from long term second generation antipsychotic use, clinicians often resort to the use of traditional antidepressants for the treatment of bipolar depression 108 despite the lack of FDA approval for such agents. Indeed, recent randomized clinical trials of antidepressants in bipolar depression have not shown an effect for paroxetine, 89 109 bupropion, 109 or agomelatine. 110 Beyond the question of efficacy, another concern regarding antidepressants in bipolar disorder is their potential to worsen the course of illness by either promoting mixed or manic symptoms or inducing more subtle degrees of mood instability and cycle acceleration. 111 However, the risk of switching to full mania while being treated with mood stabilizers appears to be modest, with a meta-analysis of randomized clinical trials and clinical cohort studies showing the rates of mood switching over an average follow-up of five months to be approximately 15.3% in people with bipolar disorder treated on antidepressants compared with 13.8% in those without antidepressant treatment. 111 The risk of switching appears to be higher in the first 1-2 years of treatment in people with BD-I, and in those treated with a tricyclic antidepressant 112 or the dual reuptake inhibitor venlafaxine. 113 Overall, while the available data have methodological limitations, most guidelines do not recommend the use of antidepressants in bipolar disorder, or recommend them only after agents with more robust evidence have been tried. That they remain so widely used despite the equivocal evidence base reflects the unmet need for treatment of depression, concerns about the long term side effects of second generation antipsychotics, and the challenges of changing longstanding prescribing patterns.
Pharmacological approaches to prevention of recurrent episodes
Following treatment of the acute depressive or manic syndrome, the major focus of treatment is to prevent future episodes and minimize interepisodic subsyndromal symptoms. Most often, the medication that has been helpful in controlling the acute episode can be continued for prevention, particularly if clinical trial evidence exists for a maintenance effect. To show efficacy for prevention, studies must be sufficiently long to allow the accumulation of future episodes to occur and be potentially prevented by a therapeutic intervention. However, few long term treatment studies exist and most have utilized enriched designs that likely favor the drug seeking regulatory approval. As shown in figure 1 , meta-analyses 94 show prophylactic effect for most (olanzapine, risperidone, quetiapine, aripiprazole, asenapine) but not all (lurasidone, paliperidone) recently approved second generation antipsychotics. The effect sizes are generally comparable with monotherapy (odds ratio 0.42, 95% confidence interval 0.34 to 0.5) or as adjunctive therapy (odds ratio 0.37, 95% confidence interval 0.25 to 0.55). 94 Recent studies of lithium, which have generally used it as a (non-enriched) comparator drug, show a comparable protective effect (odds ratio 0.46, 95% confidence interval 0.28 to 0.75). 94 Among the mood stabilizing anticonvulsant drugs, a prophylactic effect has also been found for both divalproate and lamotrigine ( fig 1 and supplementary table 1), although only the latter has been granted regulatory approval for maintenance treatment. While there are subtle differences in effect sizes in drugs approved for maintenance ( fig 1 and table 1 ), the overlapping confidence intervals and methodological differences between studies prevent a strict comparison of the effect measures.
Guidelines often recommend lithium as a first line agent given its consistent evidence of prophylaxis, even when tested as the disadvantaged comparator drug in enriched drug designs. Like other medications, lithium has a unique set of side effects and ultimately the decision about which drug to use among those which are efficacious should be a decision carefully weighed and shared between patient and provider. The decision might be re-evaluated after substantial experience with the medication or at different stages in the long term treatment of bipolar disorder (see table 1 ).
Psychotherapeutic approaches
The frequent presence of residual symptoms, often associated with psychosocial and occupational dysfunction, has led to renewed interest in psychotherapeutic and psychosocial approaches to bipolar disorder. Given the impairment of judgment seen in mania, psychotherapy has more of a supportive and educational role in the treatment of mania, whereas it can be more of a primary focus in the treatment of depressive states. On a broad level, psychotherapeutic approaches effective for acute depression, such as cognitive behavioral therapy, interpersonal therapy, behavioral activation, and mindfulness based strategies, can also be recommended for acute depressive states in individuals with bipolar disorder. 114 Evidence for more targeted psychotherapy trials for bipolar disorder is more limited, but meta-analyses have found evidence for decreased recurrence (odds ratio 0.56; 95% confidence interval 0.43 to 0.74) 115 and improvement of subthreshold interepisodic depressive and manic symptoms with cognitive behavioral therapy, family based therapy, interpersonal and social rhythm therapy, and psychoeducation. 115 Recent investigations have also focused on targeted forms of psychotherapy to improve cognition 116 117 118 as well as psychosocial and occupational functioning. 119 120 Although these studies show evidence of a moderate effect, they remain preliminary, methodologically diverse, and require replication on a larger scale. 121
The implementation of evidence based psychotherapy as a treatment faces several challenges, including clinical training, fidelity monitoring, and adequate reimbursement. Novel approaches, leveraging the greater tractability of digital tools 122 and allied healthcare workers, 123 are promising means of lessening the implementation gap; however, these approaches require validation and evidence of clinical utility similar to traditional methods.
Neurostimulation approaches
For individuals with bipolar disorder who cannot tolerate or do not respond well to standard pharmacotherapy or psychotherapeutic approaches, neurostimulation techniques such as repetitive transcranial magnetic stimulation or electric convulsive therapy should be considered as second or third line treatments. Electric convulsive therapy has shown response rates of approximately 60-80% in severe acute depressions 124 125 and 50-60% in cases with treatment resistant depression. 126 These response rates compare favorably with those of pharmacological treatment, which are likely to be closer to ~50% and ~30% in subjects with moderate to severe depression and treatment resistant depression, respectively. 127 Although the safety of electric convulsive therapy is well established, relatively few medical centers have it available, and its acceptability is limited by cognitive side effects, which are usually short term, but which can be more significant with longer courses and with bilateral electrode placement. 128 While there have been fewer studies of electric convulsive therapy for bipolar depression compared with major depressive disorder, it appears to be similarly effective and might show earlier response. 129 Anecdotal evidence also suggests electric convulsive therapy that is useful in refractory mania. 130
Compared with electric convulsive therapy, repetitive transcranial magnetic stimulation has no cognitive side effects and is generally well tolerated. Repetitive transcranial magnetic stimulation acts by generating a magnetic field to depolarize local neural tissue and induce excitatory or inhibitory effects depending on the frequency of stimulation. The most studied FDA approved form of repetitive transcranial magnetic stimulation applies high frequency (10 Hz) excitatory pulses to the left prefrontal cortex for 30-40 minutes a day for six weeks. 131 Like electric convulsive therapy, repetitive transcranial magnetic stimulation has been primarily studied in treatment resistant depression and has been found to have moderate effect, with about one third of patients having a significant treatment response compared with those treated with pharmacotherapy. 131 Recent innovations in transcranial magnetic stimulation have included the use of a novel, larger coil to stimulate a larger degree of the prefrontal cortex (deep transcranial magnetic stimulation), 132 and a shortened (three minutes), higher frequency intermittent means of stimulation known as theta burst stimulation that appears to be comparable to conventional (10 Hz) repetitive transcranial magnetic stimulation. 133 A preliminary trial has recently assessed a new accelerated protocol of theta burst stimulation marked by 10 sessions a day for five days. It found that theta burst stimulation had a greater effect on people with treatment resistant depression compared with treatment as usual, although larger studies are needed to confirm these findings. 134
Conventional repetitive transcranial magnetic stimulation (10 Hz) studies in bipolar disorder have been limited by small sample sizes but have generally shown similar effects compared with major depressive disorder. 135 However, a proof of concept study of single session theta burst stimulation did not show efficacy in bipolar depression, 136 reiterating the need for specific trials for bipolar depression. Given the lack of such trials in bipolar disorder, repetitive transcranial magnetic stimulation should be considered a potentially promising but as yet unproven treatment for bipolar depression.
The other major form of neurostimulation studied in both unipolar and bipolar depression is transcranial direct current stimulation, an easily implemented method of delivering a low amplitude electrical current to the prefrontal area of the brain that could lead to local changes in neuronal excitability. 137 Like repetitive transcranial magnetic stimulation, transcranial direct current stimulation is well tolerated and has been mostly studied in unipolar depression, but has not yet generated sufficient evidence to be approved by a regulatory agency. 138 Small studies have been performed in bipolar depression, but the results have been mixed and require further research before use in clinical settings. 137 138 139 Finally, the evidence for more invasive neurostimulation studies such as vagal nerve stimulation and deep brain stimulation remains extremely limited and is currently insufficient for clinical use. 140 141
Treatment resistance in bipolar disorder
As in major depressive disorder, the use of term treatment resistance in bipolar disorder is controversial since differentiating whether persistent symptoms are caused by low treatment adherence, poor tolerability, the presence of comorbid disorders, or are the result of true treatment resistance, is an essential but often challenging clinical task. Treatment resistance should only be considered after two or three trials of evidence based monotherapy, adjunctive therapy, or both. 142 In difficult-to-treat mania, two or more medications from different mechanistic classes are typically used, with electric convulsive therapy 143 and clozapine 144 being considered if more conventional anti-manic treatments fail. In bipolar depression, it is common to combine antidepressants with anti-manic agents, despite limited evidence for efficacy. 145 Adjunctive therapies such as bright light therapy, 146 the dopamine D2/3 receptor agonist pramipexole, 147 and ketamine 148 149 have shown promising results in small open label trials that require further study.
Treatment considerations to reduce suicide in bipolar disorder
The risk of completed suicide is high across the subtypes of bipolar disorder, with estimated rates of 10-15% across the lifespan. 150 151 152 Lifetime rates of suicide attempts are much higher, with almost half of all individuals with bipolar disorder reporting at least one attempt. 153 Across a population and, often within individuals, the causes of suicide attempts and completed suicides are likely to be multifactorial, 154 affected by various risk factors, such as symptomatic illness, environmental stressors, comorbidities (particularly substance misuse), trait impulsivity, interpersonal conflict, loneliness, or socioeconomic distress. 155 156 Risk is highest in depressive and dysphoric/mixed episodes 157 158 and is particularly high in the transitional period following an acute admission to hospital. 159 Among the available treatments, lithium has potential antisuicidal properties. 160 However, since suicide is a rare event, with very few to zero suicides within a typical clinical trial, moderate evidence for this effect emerges only in the setting of meta-analyses of clinical trials. 160 Several observational studies have shown lower mortality in patients on lithium treatment, 161 but such associations might not be causal, since lithium is potentially fatal in overdose and is often avoided by clinicians in patients at high risk of suicide.
The challenge of studying scarce events has led most studies to focus on the reduction of the more common phenomena of suicidal ideation and behavior as a proxy for actual suicides. A recent such multisite study of the Veterans Affairs medical system included a mixture of unipolar and bipolar disorder and was stopped prematurely for futility, indicating no overall effect of moderate dose lithium. 162 Appropriate limitations of this study have been noted, 163 164 including difficulties in recruitment, few patients with bipolar disorder (rather than major depressive disorder), low levels of compliance with lithium therapy, high rates of comorbidity, and a follow-up of only one year. Nevertheless, while the body of evidence suggests that lithium has a modest antisuicidal effect, its degree of protection and utility in complex patients with comorbidities and multiple risk factors remain matters for further study. Treatment of specific suicidal risk in patients with bipolar disorder must therefore also incorporate broader interventions based on the individual’s specific risk factors. 165 Such an approach would include societal interventions like means restriction 166 and a number of empirically tested suicide focused psychotherapy treatments. 167 168 Unfortunately, the availability of appropriate training, expertise, and care models for such treatments remains limited, even in higher income countries. 169
More scalable solutions, such as the deployment of shortened interventions via digital means could help to overcome this implementation gap; however, the effectiveness of such approaches cannot be assumed and requires empirical testing. For example, a recent large scale randomized controlled trial of an abbreviated online dialectical behavioral therapy skills training program was paradoxically associated with slightly increased risk of self-harm. 170
Treatment consideration in BD-II and bipolar spectrum conditions
Because people with BD-II primarily experience depressive symptoms and appear less likely to switch mood states compared with individuals with BD-I, 50 171 there has been a greater acceptance of the use of antidepressants in BD-II depression, including as monotherapy. 172 However, caution should be exercised when considering the use of antidepressants without a mood stabilizer in patients with BD-II who might also experience high rates of mood instability and rapid cycling. Such individuals can instead respond better to newer second generation antipsychotic agents such as quetiapine 173 and lumateperone, 93 which are supported by post hoc analyses of these more recent clinical trials with more BD-II patients. In addition, despite the absence of randomized controlled trials, open label studies have suggested that lithium and other mood stabilizers can have similar efficacy in BD-II, especially in the case of lamotrigine. 174
Psychotherapeutic approaches such as psychoeducation, cognitive behavioral therapy, and interpersonal and social rhythm therapy have been found to be helpful 115 and can be considered as the primary form of treatment for BD-II in some patients, although in most clinical scenarios BD-II is likely to occur in conjunction with psychopharmacology. While it can be tempting to consider BD-II a milder variant of BD-I, high rates of comorbid disorders, rapid cycling, and adverse consequences such as suicide attempts 175 176 highlight the need for clinical caution and the provision of multimodal treatment, focusing on mood improvement, emotional regulation, and better psychosocial functioning.
Precision medicine: can it be applied to improve the care of bipolar disorder?
The recent focus on precision medicine approaches to psychiatric disorders seeks to identify clinically relevant heterogeneity and identify characteristics at the level of the individual or subgroup that can be leveraged to identify and target more efficacious treatments. 1 177 178
The utility of such an approach was originally shown in oncology, where a subset of tumors had gene expression or DNA mutation signatures that could predict response to treatments specifically designed to target the aberrant molecular pathway. 179 While much of the emphasis of precision medicine has been on the eventual identification of biomarkers utilizing high throughput approaches (genetics and other “omics” based measurements), the concept of precision medicine is arguably much broader, encompassing improvements in measurement, potentially through the deployment of digital tools, as well as better conceptualization of contextual, cultural, and socioeconomic mechanisms associated with psychopathology. 180 181 Ultimately, the goal of precision psychiatry is to identify and target driving mechanisms, be they molecular, physiological, or psychosocial in nature. As such, precision psychiatry seeks what researchers and clinicians have often sought: to identify clinically relevant heterogeneity to improve prediction of outcomes and increase the likelihood of therapeutic success. The novelty being not so much the goals of the overarching approach, but the increasing availability of large samples, novel digital tools, analytical advances, and an increasing armamentarium of biological measurements that can be deployed at scale. 177
Although not unique to bipolar disorder, several clinical decision points along the life course of bipolar disorder would benefit from a precision medicine approach. For example, making an early diagnosis is often not possible based on clinical symptoms alone, since such symptoms are usually non-specific. A precision medicine approach could also be particularly relevant in helping to identify subsets of patients for whom the use of antidepressants could be beneficial or harmful. Admittedly, precision medicine approaches to bipolar disorder are still in their infancy, and larger, clinically relevant, longitudinal, and reliable phenotypes are needed to provide the infrastructure for precision medicine approaches. Such data remain challenging to obtain at scale, leading to renewed efforts to utilize the extant clinical infrastructure and electronic medical records to help emulate traditional longitudinal analyses. Electronic medical records can help provide such data, but challenges such as missingness, limited quality control, and potential biases in care 182 need to be resolved with carefully considered analytical designs. 183
Emerging treatments
Two novel atypical antipsychotics, amilsupride and bifeprunox, are currently being tested in phase 3 trials ( NCT05169710 and NCT00134459 ) and could gain approval for bipolar depression in the near future if these pivotal trials show a significant antidepressant effect. These drugs could offer advantages such as greater antidepressant effects, fewer side effects, and better long term tolerability, but these assumptions must be tested empirically. Other near term possibilities include novel rapid antidepressant treatments, such as (es)ketamine that putatively targets the glutamatergic system, and has been recently approved for treatment resistant depression, but which have not yet been tested in phase 3 studies in bipolar depression. Small studies have shown comparable effects of intravenous ketamine, 149 184 in bipolar depression with no short term evidence of increased mood switching or mood instability. Larger phase 2 studies ( NCT05004896 ) are being conducted which will need to be followed by larger phase 3 studies. Other therapies targeting the glutamatergic system have generally failed phase 3 trials in treatment resistant depression, making them unlikely to be tested in bipolar depression. One exception could be the combination of dextromethorphan and its pharmacokinetic (CYP2D6) inhibitor bupropion, which was recently approved for treatment resistant depression but has yet to be tested in bipolar depression. Similarly, the novel GABAergic compound zuranolone is currently being evaluated by the FDA for the treatment of major depressive disorder and could also be subsequently studied in bipolar depression.
Unfortunately, given the general efficacy for most patients of available treatments, few scientific and financial incentives exist to perform large scale studies of novel treatment in mania. Encouraging results have been seen in small studies of mania with the selective estrogen receptor modulator 185 tamoxifen and its active metabolite endoxifen, both of which are hypothesized to inhibit protein kinase C, a potential mechanistic target of lithium treatment. These studies remain small, however, and anti-estrogenic side effects have potentially dulled interest in performing larger studies.
Finally, several compounds targeting alternative pathophysiological mechanisms implicated in bipolar disorder have been trialed in phase 2 academic studies. The most studied has been N -acetylcysteine, a putative mitochondrial modulator, which initially showed promising results only to be followed by null findings in larger more recent studies. 186 Similarly, although small initial studies of anti-inflammatory agents provided impetus for further study, subsequent phase 2 studies of the non-steroidal agent celecoxib, 187 the anti-inflammatory antibiotic minocycline, 187 and the antibody infliximab (a tumor necrosis factor antagonist) 188 have not shown efficacy for bipolar depression. Secondary analyses have suggested that specific anti-inflammatory agents might be effective only for a subset of patients, such as those with elevated markers of inflammation or a history of childhood adversity 189 ; however, such hypotheses must be confirmed in adequately powered independent studies.
Several international guidelines for the treatment of bipolar disorder have been published in the past decade, 102 190 191 192 providing a list of recommended treatments with efficacy in at least one large randomized controlled trial. Since effect sizes tend to be moderate and broadly comparable across classes, all guidelines allow for significant choice among first line agents, acknowledging that clinical characteristics, such as history of response or tolerability, severity of symptoms, presence of mixed features, or rapid cycling can sometimes over-ride guideline recommendations. For acute mania requiring rapid treatment, all guidelines prioritize the use of second generation antipsychotics such as aripiprazole, quetiapine, risperidone, asenapine, and cariprazine. 102 192 193 Combination treatment is considered based on symptom severity, tolerability, and patient choice, with most guidelines recommending lithium or divalproate along with a second generation antipsychotic for mania with psychosis, severe agitation, or prominent mixed symptoms. While effective, haloperidol is usually considered a second choice option owing to its propensity to cause extrapyramidal symptoms. 102 192 193 Uniformly, all guidelines agree on the need to taper antidepressants in manic or mixed episodes.
For maintenance treatment, guidelines are generally consistent in recommending lithium if tolerated and without relative contraindications, such as baseline renal disease. 194 The second most recommended maintenance treatment is quetiapine, followed by aripiprazole for patients with prominent manic episodes and lamotrigine for patients with predominant depressive episodes. 194 Most guidelines recommend considering prophylactic properties when initially choosing treatment for acute manic episodes, although others suggests that acute maintenance treatments can be cross tapered with maintenance medications after several months of full reponse. 193
For bipolar depression, recent guidelines recommend specific second generation antipsychotics such as quetiapine, lurasidone, and cariprazine 102 192 193 For more moderate symptoms, consideration is given to first using lamotrigine and lithium. Guidelines remain cautious about the use of antidepressants (selective serotonin reuptake inhibitors, venlafaxine, or bupropion) in patients with BP-I, restricting them to second or third line treatments and always in the context of an anti-manic agent. However, for patients with BP-II and no rapid cycling, several guidelines allow for the use of carefully monitored antidepressant monotherapy.
Bipolar disorder is a highly recognizable syndrome with many effective treatment options, including the longstanding gold standard therapy lithium. However, a significant proportion of patients do not respond well to current treatments, leading to negative consequences, poor quality of life, and potentially shortened lifespan. Several novel treatments are being developed but limited knowledge of the biology of bipolar disorder remains a major challenge for novel drug discovery. Hope remains that the insights of genetics, neuroimaging, and other investigative modalities could soon be able to inform the development of rational treatments aimed to mitigate the underlying pathophysiology associated with bipolar disorder. At the same time, however, efforts are needed to bridge the implementation gap and provide truly innovative and integrative care for patients with bipolar disorder. 195 Owing to the complexity of bipolar disorder, few patients can be said to be receiving optimized care across the various domains of mental health that are affected in those with bipolar disorder. Fortunately, the need for improvement is now well documented, 196 and concerted efforts at the scale necessary to be truly innovative and integrative are now on the horizon.
Questions for future research
Among adolescents and young adults who manifest common mental disorders such as anxiety or depressive or attentional disorders, who will be at high risk for developing bipolar disorder?
Can we predict the outcomes for patients following a first manic or hypomanic episode? This will help to inform who will require lifelong treatment and who can be tapered off medications after sustained recovery.
Are there reliable clinical features and biomarkers that can sufficiently predict response to specific medications or classes of medication?
What are the long term consequences of lifelong treatments with the major classes of medications used in bipolar disorder? Can we predict and prevent medical morbidity caused by medications?
Can we understand in a mechanistic manner the pathophysiological processes that lead to abnormal mood states in bipolar disorder?
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: FSG performed the planning, conduct, and reporting of the work described in the article. FSG accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Competing interests: I have read and understood the BMJ policy on declaration of interests and declare no conflicts of interest.
Patient involvement: FSG discussed of the manuscript, its main points, and potential missing points with three patients in his practice who have lived with longstanding bipolar disorder. These additional viewpoints were incorporated during the drafting of the manuscript.
Provenance and peer review: Commissioned; externally peer reviewed.
- ↵ . Falret’s discovery: the origin of the concept of bipolar affective illness. Translated by M. J. Sedler and Eric C. Dessain. Am J Psychiatry 1983;140:1127-33. doi: 10.1176/ajp.140.9.1127 OpenUrl CrossRef PubMed Web of Science
- ↵ Kraepelin E. Manic-depressive Insanity and Paranoia. Translated by R. Mary Barclay from the Eighth German. Edition of the ‘Textbook of Psychiatry.’ 1921.
- Merikangas KR ,
- Akiskal HS ,
- Koukopoulos A ,
- Jongsma HE ,
- Kirkbride JB ,
- Rowland TA ,
- Kessler RC ,
- Kazdin AE ,
- Aguilar-Gaxiola S ,
- WHO World Mental Health Survey collaborators
- Bergen SE ,
- Kuja-Halkola R ,
- Larsson H ,
- Lichtenstein P
- Smoller JW ,
- Lichtenstein P ,
- Sjölander A ,
- Mullins N ,
- Forstner AJ ,
- O’Connell KS ,
- Palmer DS ,
- Howrigan DP ,
- Chapman SB ,
- Pirooznia M ,
- Murray GK ,
- McGrath JJ ,
- Hickie IB ,
- ↵ Mostafavi H, Harpak A, Agarwal I, Conley D, Pritchard JK, Przeworski M. Variable prediction accuracy of polygenic scores within an ancestry group. Loos R, Eisen MB, O’Reilly P, eds. eLife 2020;9:e48376. doi: 10.7554/eLife.48376 OpenUrl CrossRef PubMed
- Westlye LT ,
- van Erp TGM ,
- Costa Rica/Colombia Consortium for Genetic Investigation of Bipolar Endophenotypes
- Pauling M ,
- ENIGMA Bipolar Disorder Working Group
- Schnack HG ,
- Ching CRK ,
- ENIGMA Bipolar Disorders Working Group
- Goltermann J ,
- Hermesdorf M ,
- Dannlowski U
- Gurholt TP ,
- Suckling J ,
- Lennox BR ,
- Bullmore ET
- Marangoni C ,
- Hernandez M ,
- Achtyes ED ,
- Agnew-Blais J ,
- Gilman SE ,
- Upthegrove R ,
- ↵ Etain B, Aas M. Childhood Maltreatment in Bipolar Disorders. In: Young AH, Juruena MF, eds. Bipolar Disorder: From Neuroscience to Treatment . Vol 48. Current Topics in Behavioral Neurosciences. Springer International Publishing; 2020:277-301. doi: 10.1007/7854_2020_149
- Johnson SL ,
- Weinberg BZS
- Stinson FS ,
- Costello CG
- Klein & Riso LP DN
- Sandstrom A ,
- Perroud N ,
- de Jonge P ,
- Bunting B ,
- Nierenberg AA
- Hantouche E ,
- Vannucchi G
- Zimmerman M ,
- Ruggero CJ ,
- Chelminski I ,
- Leverich GS ,
- McElroy S ,
- Mignogna KM ,
- Balling C ,
- Dalrymple K
- Kappelmann N ,
- Stokes PRA ,
- Jokinen T ,
- Baldessarini RJ ,
- Faedda GL ,
- Offidani E ,
- Viktorin A ,
- Launders N ,
- Osborn DPJ ,
- Roshanaei-Moghaddam B ,
- De Hert M ,
- Detraux J ,
- van Winkel R ,
- Lomholt LH ,
- Andersen DV ,
- Sejrsgaard-Jacobsen C ,
- Skjelstad DV ,
- Gregersen M ,
- Søndergaard A ,
- Brandt JM ,
- Van Meter AR ,
- Youngstrom EA ,
- Taylor RH ,
- Ulrichsen A ,
- Strawbridge R
- Hafeman DM ,
- Merranko J ,
- Hirschfeld RM ,
- Williams JB ,
- Spitzer RL ,
- Adolfsson R ,
- Benazzi F ,
- Regier DA ,
- Johnson KR ,
- Akinhanmi MO ,
- Biernacka JM ,
- Strakowski SM ,
- Goldberg JF ,
- Schettler PJ ,
- Coryell W ,
- Scheftner W ,
- Endicott J ,
- Zarate CA Jr . ,
- Matsuda Y ,
- Fountoulakis KN ,
- Zarate CA Jr .
- Bowden CL ,
- Brugger AM ,
- The Depakote Mania Study Group
- Calabrese JR ,
- Depakote ER Mania Study Group
- Weisler RH ,
- Kalali AH ,
- Ketter TA ,
- SPD417 Study Group
- Keck PE Jr . ,
- Cutler AJ ,
- Caffey EM Jr . ,
- Grossman F ,
- Eerdekens M ,
- Jacobs TG ,
- Grundy SL ,
- The Olanzipine HGGW Study Group
- Versiani M ,
- Ziprasidone in Mania Study Group
- Sanchez R ,
- Aripiprazole Study Group
- McIntyre RS ,
- Panagides J
- Calabrese J ,
- McElroy SL ,
- EMBOLDEN I (Trial 001) Investigators
- EMBOLDEN II (Trial D1447C00134) Investigators
- Lamictal 606 Study Group
- Lamictal 605 Study Group
- Keramatian K ,
- Chakrabarty T ,
- Nestsiarovich A ,
- Gaudiot CES ,
- Neijber A ,
- Hellqvist A ,
- Paulsson B ,
- Trial 144 Study Investigators
- Schwartz JH ,
- Szegedi A ,
- Cipriani A ,
- Salanti G ,
- Dorsey ER ,
- Rabbani A ,
- Gallagher SA ,
- Alexander GC
- Cerqueira RO ,
- Yatham LN ,
- Kennedy SH ,
- Parikh SV ,
- Højlund M ,
- Andersen K ,
- Correll CU ,
- Ostacher M ,
- Schlueter M ,
- Geddes JR ,
- Mojtabai R ,
- Nierenberg AA ,
- Goodwin GM ,
- Agomelatine Study Group
- Vázquez G ,
- Baldessarini RJ
- Altshuler LL ,
- Cuijpers P ,
- Miklowitz DJ ,
- Efthimiou O ,
- Furukawa TA ,
- Strawbridge R ,
- Tsapekos D ,
- Hodsoll J ,
- Vinberg M ,
- Kessing LV ,
- Forman JL ,
- Miskowiak KW
- Lewandowski KE ,
- Sperry SH ,
- Torrent C ,
- Bonnin C del M ,
- Martínez-Arán A ,
- Bonnín CM ,
- Tamura JK ,
- Carvalho IP ,
- Leanna LMW ,
- Karyotaki E ,
- Individual Patient Data Meta-Analyses for Depression (IPDMA-DE) Collaboration
- Vipulananthan V ,
- Hurlemann R ,
- UK ECT Review Group
- Haskett RF ,
- Mulsant B ,
- Trivedi MH ,
- Wisniewski SR ,
- Espinoza RT ,
- Vazquez GH ,
- McClintock SM ,
- Carpenter LL ,
- National Network of Depression Centers rTMS Task Group ,
- American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments
- Levkovitz Y ,
- Isserles M ,
- Padberg F ,
- Blumberger DM ,
- Vila-Rodriguez F ,
- Thorpe KE ,
- Williams NR ,
- Sudheimer KD ,
- Bentzley BS ,
- Konstantinou G ,
- Toscano E ,
- Husain MM ,
- McDonald WM ,
- International Consortium of Research in tDCS (ICRT)
- Sampaio-Junior B ,
- Tortella G ,
- Borrione L ,
- McAllister-Williams RH ,
- Gippert SM ,
- Switala C ,
- Bewernick BH ,
- Hidalgo-Mazzei D ,
- Mariani MG ,
- Fagiolini A ,
- Swartz HA ,
- Benedetti F ,
- Barbini B ,
- Fulgosi MC ,
- Burdick KE ,
- Diazgranados N ,
- Ibrahim L ,
- Brutsche NE ,
- Sinclair J ,
- Gerber-Werder R ,
- Miller JN ,
- Vázquez GH ,
- Franklin JC ,
- Ribeiro JD ,
- Turecki G ,
- Gunnell D ,
- Hansson C ,
- Pålsson E ,
- Runeson B ,
- Pallaskorpi S ,
- Suominen K ,
- Ketokivi M ,
- Hadzi-Pavlovic D ,
- Stanton C ,
- Lewitzka U ,
- Severus E ,
- Müller-Oerlinghausen B ,
- Rogers MP ,
- Li+ plus Investigators
- Manchia M ,
- Michel CA ,
- Auerbach RP
- Altavini CS ,
- Asciutti APR ,
- Solis ACO ,
- Casañas I Comabella C ,
- Riblet NBV ,
- Young-Xu Y ,
- Shortreed SM ,
- Rossom RC ,
- Amsterdam JD ,
- Brunswick DJ
- Gustafsson U ,
- Marangell LB ,
- Bernstein IH ,
- Karanti A ,
- Kardell M ,
- Collins FS ,
- Armstrong K ,
- Concato J ,
- Singer BH ,
- Ziegelstein RC
- ↵ Holmes JH, Beinlich J, Boland MR, et al. Why Is the Electronic Health Record So Challenging for Research and Clinical Care? Methods Inf Med 2021;60(1-02):32-48. doi: 10.1055/s-0041-1731784
- García Rodríguez LA ,
- Cantero OF ,
- Martinotti G ,
- Dell’Osso B ,
- Di Lorenzo G ,
- REAL-ESK Study Group
- Palacios J ,
- DelBello MP ,
- Husain MI ,
- Chaudhry IB ,
- Subramaniapillai M ,
- Jones BDM ,
- Daskalakis ZJ ,
- Carvalho AF ,
- ↵ Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition Recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2016;30:495-553. doi: 10.1177/0269881116636545 OpenUrl CrossRef PubMed
- Verdolini N ,
- Del Matto L ,
- Regeer EJ ,
- Hoogendoorn AW ,
- Harris MG ,
- WHO World Mental Health Survey Collaborators
Bipolar disorders
Affiliations.
- 1 Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada; Department of Pharmacology, University of Toronto, Toronto, ON, Canada; Brain and Cognition Discovery Foundation, Toronto, ON, Canada. Electronic address: [email protected].
- 2 Institute for Mental and Physical Health and Clinical Translation Strategic Research Centre, School of Medicine, Deakin University, Melbourne, VIC, Australia; Mental Health Drug and Alcohol Services, Barwon Health, Geelong, VIC, Australia; Orygen, The National Centre of Excellence in Youth Mental Health, Melbourne, VIC, Australia; Centre for Youth Mental Health, Florey Institute for Neuroscience and Mental Health, Melbourne, VIC, Australia; Department of Psychiatry, The University of Melbourne, Melbourne, VIC, Australia.
- 3 Department of Psychiatry, Adult Division, Kingston General Hospital, Kingston, ON, Canada; Department of Psychiatry, Queen's University School of Medicine, Queen's University, Kingston, ON, Canada; Centre for Neuroscience Studies, Queen's University, Kingston, ON, Canada.
- 4 Department of Psychiatry, University of Toronto, Toronto, ON, Canada; Centre for Youth Bipolar Disorder, Sunnybrook Health Sciences Centre, Toronto, ON, Canada.
- 5 Department of Psychiatry, Faculty of Medicine, University of Antioquia, Medellín, Colombia; Mood Disorders Program, Hospital Universitario San Vicente Fundación, Medellín, Colombia.
- 6 Copenhagen Affective Disorders Research Centre, Psychiatric Center Copenhagen, Rigshospitalet, Copenhagen, Denmark; Department of Psychiatry, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
- 7 Discipline of Psychiatry, Northern Clinical School, University of Sydney, Sydney, NSW, Australia; Department of Academic Psychiatry, Northern Sydney Local Health District, Sydney, Australia.
- 8 Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
- 9 Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada; Dauten Family Center for Bipolar Treatment Innovation, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
- 10 Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, ON, Canada.
- 11 Hospital Clinic, Institute of Neuroscience, University of Barcelona, IDIBAPS, CIBERSAM, Barcelona, Spain.
- 12 Department of Psychiatry, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Psychiatric Research Unit, Psychiatric Centre North Zealand, Hillerød, Denmark.
- 13 Department of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience, King's College London and South London and Maudsley National Health Service Foundation Trust, Bethlem Royal Hospital, London, UK.
- 14 Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
- PMID: 33278937
- DOI: 10.1016/S0140-6736(20)31544-0
Bipolar disorders are a complex group of severe and chronic disorders that includes bipolar I disorder, defined by the presence of a syndromal, manic episode, and bipolar II disorder, defined by the presence of a syndromal, hypomanic episode and a major depressive episode. Bipolar disorders substantially reduce psychosocial functioning and are associated with a loss of approximately 10-20 potential years of life. The mortality gap between populations with bipolar disorders and the general population is principally a result of excess deaths from cardiovascular disease and suicide. Bipolar disorder has a high heritability (approximately 70%). Bipolar disorders share genetic risk alleles with other mental and medical disorders. Bipolar I has a closer genetic association with schizophrenia relative to bipolar II, which has a closer genetic association with major depressive disorder. Although the pathogenesis of bipolar disorders is unknown, implicated processes include disturbances in neuronal-glial plasticity, monoaminergic signalling, inflammatory homoeostasis, cellular metabolic pathways, and mitochondrial function. The high prevalence of childhood maltreatment in people with bipolar disorders and the association between childhood maltreatment and a more complex presentation of bipolar disorder (eg, one including suicidality) highlight the role of adverse environmental exposures on the presentation of bipolar disorders. Although mania defines bipolar I disorder, depressive episodes and symptoms dominate the longitudinal course of, and disproportionately account for morbidity and mortality in, bipolar disorders. Lithium is the gold standard mood-stabilising agent for the treatment of people with bipolar disorders, and has antimanic, antidepressant, and anti-suicide effects. Although antipsychotics are effective in treating mania, few antipsychotics have proven to be effective in bipolar depression. Divalproex and carbamazepine are effective in the treatment of acute mania and lamotrigine is effective at treating and preventing bipolar depression. Antidepressants are widely prescribed for bipolar disorders despite a paucity of compelling evidence for their short-term or long-term efficacy. Moreover, antidepressant prescription in bipolar disorder is associated, in many cases, with mood destabilisation, especially during maintenance treatment. Unfortunately, effective pharmacological treatments for bipolar disorders are not universally available, particularly in low-income and middle-income countries. Targeting medical and psychiatric comorbidity, integrating adjunctive psychosocial treatments, and involving caregivers have been shown to improve health outcomes for people with bipolar disorders. The aim of this Seminar, which is intended mainly for primary care physicians, is to provide an overview of diagnostic, pathogenetic, and treatment considerations in bipolar disorders. Towards the foregoing aim, we review and synthesise evidence on the epidemiology, mechanisms, screening, and treatment of bipolar disorders.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Anticonvulsants / therapeutic use
- Antidepressive Agents / therapeutic use
- Antimanic Agents / therapeutic use
- Antipsychotic Agents / therapeutic use
- Bipolar Disorder / classification*
- Bipolar Disorder / drug therapy*
- Bipolar Disorder / genetics
- Bipolar Disorder / psychology
- Carbamazepine / therapeutic use
- Cardiovascular Diseases / complications
- Cardiovascular Diseases / mortality
- Child Abuse / psychology
- Comorbidity
- Depressive Disorder, Major / drug therapy*
- Depressive Disorder, Major / genetics
- Depressive Disorder, Major / psychology
- Environmental Exposure / adverse effects
- Lamotrigine / therapeutic use
- Lithium / therapeutic use
- Mania / drug therapy
- Mania / psychology
- Suicide / psychology
- Suicide Prevention*
- Valproic Acid / therapeutic use
- Young Adult
- Anticonvulsants
- Antidepressive Agents
- Antimanic Agents
- Antipsychotic Agents
- Carbamazepine
- Valproic Acid
- Lamotrigine
- Open access
- Published: 06 November 2018
The challenges of living with bipolar disorder: a qualitative study of the implications for health care and research
- Eva F. Maassen ORCID: orcid.org/0000-0003-0211-0994 1 , 2 ,
- Barbara J. Regeer 1 ,
- Eline J. Regeer 2 ,
- Joske F. G. Bunders 1 &
- Ralph W. Kupka 2 , 3
International Journal of Bipolar Disorders volume 6 , Article number: 23 ( 2018 ) Cite this article
38k Accesses
18 Citations
21 Altmetric
Metrics details
In mental health care, clinical practice is often based on the best available research evidence. However, research findings are difficult to apply to clinical practice, resulting in an implementation gap. To bridge the gap between research and clinical practice, patients’ perspectives should be used in health care and research. This study aimed to understand the challenges people with bipolar disorder (BD) experience and examine what these challenges imply for health care and research needs.
Two qualitative studies were used, one to formulate research needs and another to formulate healthcare needs. In both studies focus group discussions were conducted with patients to explore their challenges in living with BD and associated needs, focusing on the themes diagnosis, treatment and recovery.
Patients’ needs are clustered in ‘disorder-specific’ and ‘generic’ needs. Specific needs concern preventing late or incorrect diagnosis, support in search for individualized treatment and supporting clinical, functional, social and personal recovery. Generic needs concern health professionals, communication and the healthcare system.
Patients with BD address disorder-specific and generic healthcare and research needs. This indicates that disorder-specific treatment guidelines address only in part the needs of patients in everyday clinical practice.
Bipolar disorder (BD) is a major mood disorder characterized by recurrent episodes of depression and (hypo)mania (Goodwin and Jamison 2007 ). According to the Diagnostic and Statistical Manual 5 (DSM-5), the two main subtypes are BD-I (manic episodes, often combined with depression) and BD-II (hypomanic episodes, combined with depression) (APA 2014 ). The estimated lifetime prevalence of BD is 1.3% in the Dutch adult population (de Graaf et al. 2012 ), and BD is associated with high direct (health expenditure) and indirect (e.g. unemployment) costs (Fajutrao et al. 2009 ; Michalak et al. 2012 ), making it an important public health issue. In addition to the economic impact on society, BD has a tremendous impact on patients and their caregivers (Granek et al. 2016 ; Rusner et al. 2009 ). Even between mood episodes, BD is often associated with functional impairment (Van Der Voort et al. 2015 ; Strejilevich et al. 2013 ), such as occupational or psychosocial impairment (Huxley and Baldessarini 2007 ; MacQueen et al. 2001 ; Yasuyama et al. 2017 ). Apart from symptomatic recovery, treatment can help to overcome these impairments and so improve the person’s quality of life (IsHak et al. 2012 ).
Evidence Based Medicine (EBM), introduced in the early 1990s, is a prominent paradigm in modern (mental) health care. It strives to deliver health care based on the best available research evidence, integrated with individual clinical expertise (Sackett et al. 1996 ). EBM was introduced as a new paradigm to ‘de - emphasize intuition’ and ‘ unsystematic clinical experience’ (Guyatt et al. 1992 ) (p. 2420). Despite its popularity in principle (Barratt 2008 ), EBM has also been criticized. One such criticism is the ignorance of patients’ preferences and healthcare needs (Bensing 2000 ). A second criticism relates to the difficulty of adopting evidence-based treatment options in clinical practice (Bensing 2000 ), due to the fact that research outcomes measured in ‘the gold standard’ randomized-controlled trials (RCTs) seldom correspond to the outcomes clinical practice seeks and are not responsive to patients’ needs (Newnham and Page 2010 ). Moreover, EBM provides an overview on population level instead of individual level (Darlenski et al. 2010 ). Thus, adopting research evidence in clinical practice entails difficulties, resulting in an implementation gap.
To bridge the gap between research and clinical practice, it is argued that patients’ perspectives should be used in both health care and research. Patients have experiential knowledge about their illness, living with it in their personal context and their care needs (Tait 2005 ). This is valuable for both clinical practice and research as their knowledge complements that of health professionals and researchers (Tait 2005 ; Broerse et al. 2010 ; Caron-Flinterman et al. 2005 ). This source of knowledge can be used in the process of translating evidence into clinical practice (Schrevel 2015 ). Moreover, patient participation can enhance the clinical relevance of and support for research and the outcomes in practice (Abma and Broerse 2010 ). Hence, it is argued that these perspectives should be explicated and integrated into clinical guidelines, clinical practice, and research (Misak 2010 ; Rycroft-Malone et al. 2004 ).
Given the advantages of including patients’ perspectives, patients are increasingly involved in healthcare services (Bagchus et al. 2014 ; Larsson et al. 2007 ), healthcare quality (e.g. guideline development) (Pittens et al. 2013 ) and health-related research (e.g. agenda setting, research design) (Broerse et al. 2010 ; Boote et al. 2010 ; Elberse et al. 2012 ; Teunissen et al. 2011 ). However, patients’ perspectives on health care and on research are often studied separately. We argue that to be able to provide care focused on the patients and their needs, care and research must closely interact.
We hypothesize that the challenges BD patients experience and the associated care and research needs are interwoven, and that combining them would provide a more comprehensive understanding. We hypothesize that this more comprehensive understanding would help to close the gap between clinical practice and research. For this reason, this study aims to understand the challenges people with BD experience and examine what these challenges imply for healthcare and research needs.
To understand the challenges and needs of people with BD, we undertook two qualitative studies. The first aimed to formulate a research agenda for BD from a patient’s perspective, by gaining insights into their challenges and research needs. A second study yielded an understanding of the care needs from a patient’s perspective. In this article, the results of these two studies are combined in order to investigate the relationship between research needs and care needs. Challenges are defined as ‘difficulties patients face, due to having BD’. Care needs are defined as that what patients ‘desire to receive from healthcare services to improve overall health’ (Asadi-Lari et al. 2004 ) (p. 2). Research needs are defined as that what patients ‘desire to receive from research to improve overall health’.
Study on research needs
In this study, mixed-methods were used to formulate research needs from a patient’s perspective. First six focus group discussions (FGDs) with 35 patients were conducted to formulate challenges in living with BD and hopes for the future, and to formulate research needs arising from these difficulties and aspirations. These research needs were validated in a larger sample (n = 219) by means of a questionnaire. We have reported this study in detail elsewhere (Maassen et al. 2018 ).
Study on care needs
This study was part of a nationwide Dutch project to generate a practical guideline for BD: a translation of the existing clinical guideline to clinical practice, resulting in a standard of care that patients with BD could expect. The practical guideline (Netwerk Kwaliteitsontwikkeling GGZ 2017 ) was written by a taskforce comprising health professionals, patients. In addition to the involvement of three BD patients in the taskforce, a systematic qualitative study was conducted to gain insight into the needs of a broader group of patients.
Participants and data collection
To formulate the care needs of people with BD, seven FGDs were conducted, with a total of 56 participants, including patients (n = 49) and caregivers (n = 9); some participants were both patient and caregiver. The inclusion criteria for patients were having been diagnosed with BD, aged 18 years or older and euthymic at time of the FGDs. Inclusion criteria for caregivers were caring for someone with BD and aged 18 years or older. To recruit participants, a maximum variation sampling strategy was used to collect a broad range of care needs (Kuper et al. 2008 ). First, all outpatient clinics specialized in BD affiliated with the Dutch Foundation for Bipolar Disorder (Dutch: Kenniscentrum Bipolaire Stoornissen) were contacted by means of an announcement at regular meetings and by email if they were interested to participate. From these outpatient clinics, patients were recruited by means of flyers and posters. Second, patients were recruited at a quarterly meeting of the Dutch patient and caregiver association for bipolar disorder. The FGDs were conducted between March and May 2016.
The FGDs were designed to address challenges experienced in BD health care and areas of improvement for health care for people with BD. The FGDs were structured by means of a guide and each session was facilitated by two moderators. The leading moderator was either BJR or EFM, having both extensive experience with FGD’s from previous studies. The first FGD explored a broad range of needs. The subsequent six FGDs aimed to gain a deeper understanding of these care needs, and were structured according to the outline of the practical guideline (Netwerk Kwaliteitsontwikkeling GGZ 2017 ). Three chapters were of particular interest: diagnosis, treatment and recovery. These themes were discussed in the FGDs, two in each session, all themes three times in total. Moreover, questions on specific aspects of care formulated by the members of the workgroup were posed. The sessions took 90–120 min. The FGDs were audiotaped and transcribed verbatim. A summary of the FGDs was sent to the participants for a member check.
Data analysis
To analyze the data on challenges and needs, a framework for thematic analysis to identify, analyze and report patterns (themes) in qualitative data sets by Braun and Clarke ( 2006 ) was used. First, we familiarized ourselves with the data by carefully reading the transcripts. Second, open coding was used to derive initial codes from the data. These codes were provided to quotes that reflected a certain challenge or care need. Third, we searched for patterns within the codes reflecting challenges and within those reflecting needs. For both challenges and needs, similar or overlapping codes were clustered into themes. Subsequently, all needs were categorized as ‘specific’ or ‘generic’. The former are specific to BD and the latter are relevant for a broad range of psychiatric illnesses. Finally, a causal analysis provided a clear understanding of how challenges related to each other and how they related to the described needs.
To analyze the data on needs regarding recovery, four domains were distinguished, namely clinical, functional, social and personal recovery (Lloyd et al. 2008 ; van der Stel 2015 ). Clinical recovery refers to symptomatic remission; functional recovery concerns recovery of functioning that is impaired due to the disorder, particularly in the domain of executive functions; social recovery concerns the improvement of the patient’s position in society; personal recovery concerns the ability of the patient to give meaning to what had happened and to get a grip on their own life. The analyses were discussed between BR and EM. The qualitative software program MAX QDA 11.1.2 was used (MaxQDA).
Ethical considerations
According to the Medical Ethical Committee of VU University Medical Center, the Medical Research Involving Human Subjects Act does not apply to the current study. All participants gave written or verbal informed consent regarding the aim of the study and for audiotaping and its use for analysis and scientific publications. Participation was voluntary and participants could withdraw from the study at any time. Anonymity was ensured.
This section is in three parts. The first presents the participants’ characteristics. The second presents the challenges BD patients face, derived from both studies, and the disorder-specific care and research needs associated with these challenges. The third part describes the generic care needs that patients formulated.
Characteristics of the participants
In the study on care needs, 56 patients and caregivers participated. The mean age of the participants was 52 years (24–75), of whom 67.8% were women. The groups varied from four to sixteen participants, and all groups included men and women. Of all participants 87.5% was diagnosed with BD, of whom 48.9% was diagnosed with BD I. 3.5% was both caregivers and diagnosed with BD. Of 4 patients the age was missing, and from 6 patients the bipolar subtype.
Despite the fact that participants acknowledge the inevitable diagnostic difficulties of a complex disorder like BD, in both studies they describe a range of challenges in different phases of the diagnostic process (Fig. 1 ). Patients explained that the general practitioner (GP) and society in general did not recognize early-warning signs and mood swings were not well interpreted, resulting in late or incorrect diagnosis. Patients formulated a need for more research on what early-warning signs could be and on how to improve GPs’ knowledge about BD. Formulated care needs were associated with GPs using this knowledge to recognize early-warning signs in individual patients. One participant explained that certain symptoms must be noticed and placed in the right context:
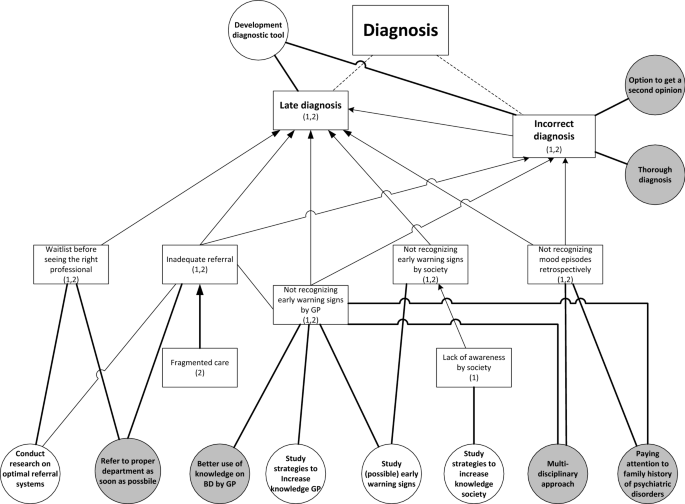
Challenges with diagnosis (squares) including relating research needs (white circles) and care needs (grey circles). (1): mentioned in study on research needs; (2): mentioned in study on care needs. Dotted lines: division of challenges into sub challenges. Arrows: causal relation between challenges
I call it, ‘testing overflow of ideas’. [….] When it happens for the first time you yourself do not recognize it. Someone else close to you or the health professional, who is often not involved yet, must signal it. (FG6)
Moreover, these challenges are associated with the need to pay attention to family history and to use a multidisciplinary approach to diagnosis to benefit from multiple perspectives. The untimely recognition of early symptoms also results in another challenge: inadequate referral to the right specialized health professional. After referral, people often face a waiting list, again causing delay in the diagnostic process. These challenges result in the need for research on optimal referral systems and the care need for timely referral. One participant described her process after the GP decided to refer her:
But, yes, at that moment the communication wasn’t good at all. Because the general practitioner said: ‘she urgently has to be seen by someone’. Subsequently, three weeks went by, until I finally arrived at depression [department]. And at that department they said: ‘well, you are in the wrong place, you need to go to bipolar [department ]’. (FG1)
The challenge of being misdiagnosed is associated with the need to be able to ask for a second opinion and to have a timely and thorough diagnosis. On the one hand, it is important for patients that health professionals quickly understand what is going on, on the other hand that health professionals take the time to thoroughly investigate the symptoms by making several appointments.
From both studies, two main challenges related to the treatment of BD were derived (Fig. 2 ). The first is finding appropriate and satisfactory treatment. Participants explained that it is difficult to find the right medication and dosage that is effective and has acceptable side-effects. One participant illustrates:
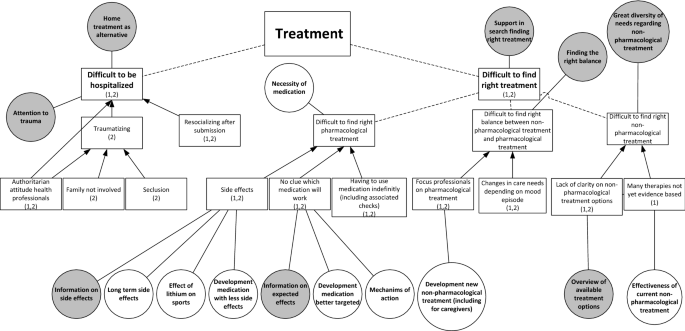
Challenges with treatment (squares) including relating research needs (white circles) and care needs (grey circles). (1): mentioned in study on research needs; (2): mentioned in study on care needs. Dotted lines: division of challenges into sub challenges. Arrows: causal relation between challenges
I think, at one point, we have to choose, either overweight or depressed. (FG1)
Some participants said that they struggle with having to use medication indefinitely, including the associated medical checks. The difficult search for the right pharmacological treatment results in the need for research on long-term side-effects, on the mechanism of action of medicine and on the development of better targeted medication with fewer adverse side-effects. In care, patients would appreciate all the known information on the side-effects and intended effects. One participant explained the importance of being properly informed about medication:
I don’t read anything [about medication], because then I wouldn’t dare taking it. But I do think, when you explain it well, the advantages, the disadvantages, the treatment, the idea behind it, that would help a lot in compliance. (FG1)
A second aspect is the challenge of finding non-pharmacological therapies that fit patients’ needs. They said they and the health professionals often do not know which non-pharmacological therapies are available and effective:
But we found the carefarm ourselves Footnote 1 [….]. You have to search for yourself completely. Yes, I actually hoped that that would be presented to you, like: ‘this would be something for you’. (FG3)
Participants mentioned a variety of non-pharmacological therapies they found useful, namely cognitive behavior therapy (CBT), EMDR, running therapy, social-rhythm training, light therapy, mindfulness, psychotherapy, psychoeducation, and training in living with mood swings. They formulated the care need to receive an overview of all available treatment options in order to find a treatment best suited to their needs. They would appreciate research on the effectiveness of non-pharmacological treatments.
A third aspect within this challenge is finding the right balance between non-pharmacological and pharmacological treatment. Participants differed in their opinion about the need for medication. Whereas some participants stated that they need medication to function, others pointed out that they found non-pharmacological treatments effective, resulting in less or no medication use. They explained that the preferred balance can also change over time, depending on their mood. However, they experience a dominant focus on pharmacological treatment by the health professionals. To address this challenge, patients need support in searching for an appropriate balance.
Next to the challenge of finding appropriate and satisfactory treatment, a second treatment-related challenge is hospitalization. Participants often had a traumatic experience, due to seclusion, the authoritarian attitudes of clinical staff, and not involving their family. Patients therefore found it important to try preventing being hospitalized, for example by means of home treatment, which some participants experienced positively. Despite the challenges relating to hospitalization, participants did acknowledge that in some cases it cannot be avoided, in which case they urged for close family involvement, open communication and being treated by their own psychiatrist. Still, in the study on research needs, hospitalization did not emerge as an important research theme.
In both studies, participants described challenges in all four domains of recovery: clinical, functional, social and personal (Fig. 3 ). In relation to clinical recovery, participants struggled with the symptoms of mood episodes, the psychosis and the fear of a future episode. In contrast, some participants mentioned that they sometimes miss the hypomanic state they had experienced previously due to effective medical treatment. In the domain of functional recovery, participants contended with having to function below their educational level due to residual symptoms, such as cognitive problems, due to the importance of preventing stress in order to reduce the risk of a new episode, and because of low energy levels. This leads to the care need that health professionals should pay attention to the level of functioning of their patients.
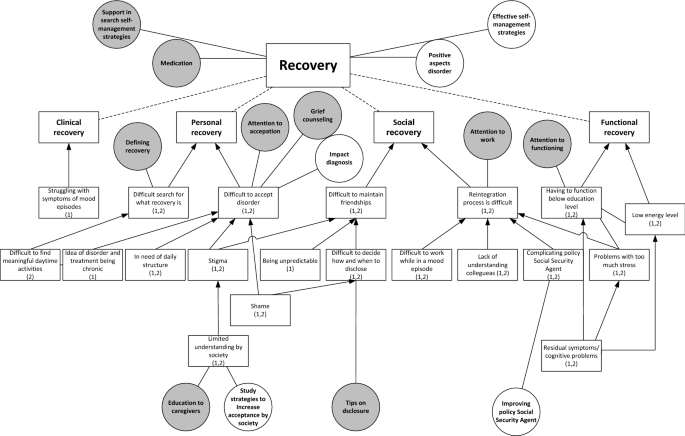
Challenges with recovery (squares) including relating research needs (white circles) and care needs (grey circles). (1): mentioned in study on research needs; (2): mentioned in study on care needs. Dotted lines: division of challenges into sub challenges. Arrows: causal relation between challenges
In the domain of social recovery, participants described challenges with maintaining friendships, due to stigma, being unpredictable and with deciding when to disclose the disorder. The latter resulted in the care need for tips on disclosure. Moreover, patients experienced challenges with reintegration to work, due to colleagues’ lack of understanding, problems with functioning during an episode, the complicating policy of the (Dutch) Employee Insurance Agency Footnote 2 in relation to the fluctuating course of BD and the negative impact of stress. These challenges are associated with the care need that health professionals should pay attention to work and the need for research on how to improve the Social Security Agency’s policy.
For their personal recovery, participants struggled with acceptance of the disorder, due to shame, stigma, having to live by structured rules and disciplines, and the chronic nature of BD. This results in care needs for grief counselling and attention to acceptance and the need for research on the impact of being diagnosed with BD. Limited understanding within society also causes problems with acceptance, corresponding with the care need for education for caregivers and for research on how to increase social acceptance. Another challenge in personal recovery was discovering what recovery means and what constitute meaningful daily activities. Patients appreciated the support of health professionals in this area. One participant described the difficult search for the meaning of recovery:
I have been looking to recover towards the situation [before diagnosis] for a long time; that I could do what I always did and what I liked. But then I was confronted with the fact that I shouldn’t expect that to happen, or only with a lot of effort. (…) Then you start thinking, now what? A compromise. I don’t want to call that recovery, but it is a recovered, partly accepted, situation. But it is not recovery as I expected it to be. (FG5)
In general, participants considered frequent contact with a nurse or psychiatrist supportive, to help them monitor their mood and help them find (efficient) self-management strategies. Most participants appreciated the involvement of caregivers in the treatment and contact with peers.
Generic care needs
We have described BD-specific needs, but patients mentioned also mentioned several generic care needs. The latter are clustered into three categories. The first concerns the health professionals . Participants stressed the importance of a good health professional, who carefully listens, takes time, and makes them feel understood, resulting in a sense of connection. Furthermore, a good health professional treats beyond the guideline, and focuses on the needs of the individual patient. When there is no sense of connection, it should be possible to change to another health professional. The second category concerns communication between the patient and the health professional . Health professionals should communicate in an open, honest and clear way both in the early diagnostic phase and during treatment. Open communication facilitates individualized care, in which the patient is involved in decision making. In addition, participants wanted to be treated as a person, not as a patient, and according to a strength-based approach. The third category concerns needs at the level of the healthcare system . Participants struggled with the availability of the health professionals and preferred access to good care 24/7 and being able to contact their health professional quickly when necessary. Currently, according to the participants, the care system is not geared to the mood swings of BD, because patients often faced waiting lists before they could see a health professional.
Is adequate treatment also having a number from a mental health institution you can always call when you are in need, that you can go there? And not that you can go in three weeks, but on a really short notice. So at least a phone call. (FG3)
Participants were often frustrated by the limited collaboration between health professionals, within their own team, between departments of the organization, and between different organizations, including complementary health professionals. They would appreciate being able to merge their conventional and complementary treatment, with greater collaboration among the different health professionals. Furthermore, they would like continuity of health professionals as this improves both the diagnostic phase and treatment, and because that health professional gets to know the patient.
We hypothesized that research and care needs of patients are closely intertwined and that understanding these, by explicating patients’ perspectives, could contribute to closing the gap between research and care. Therefore, this study aimed to understand the challenges patients with BD face and examine what these imply for both healthcare and research. In the study on needs for research and in the study on care needs, patients formulated challenges relating to receiving the correct diagnosis, finding the right treatment, including the proper balance between non-pharmacological and pharmacological treatment, and to their individual search for clinical, functional, social and personal recovery. The formulated needs in both studies clearly reflected these challenges, leading to closely corresponding needs. Another important finding of our study is that patients not only formulate disorder-specific needs, but also many generic needs.
The needs found in our study are in line with the current literature on the needs of patients with BD, namely for more non-pharmacological treatment (Malmström et al. 2016 ; Nestsiarovich et al. 2017 ), timely recognition of early-warning signs and self-management strategies to prevent a new episode (Goossens et al. 2014 ), better information on treatment and treatment alternatives (Malmström et al. 2016 ; Neogi et al. 2016 ) and coping with grief (Goossens et al. 2014 ). Moreover, the need for frequent contact with health professionals, being listened to, receiving enough time, shared decision-making on pharmacological treatment, involving caregivers (Malmström et al. 2016 ; Fisher et al. 2017 ; Skelly et al. 2013 ), and the urge for better access to health care and continuity of health professionals (Nestsiarovich et al. 2017 ; Skelly et al. 2013 ) are confirmed by the literature. Our study added to this set of literature by providing insights in patients’ needs in the diagnostic process and illustrating the interrelation between research needs and care needs from a patient’s perspective.
The generic healthcare needs patients addressed in this study are clustered into three categories: the health professional , communication between the patient and the health professional and the health system. These categories all fit in a model of patient-centered care (PCC) by Maassen et al. ( 2016 ) In their review, patients’ perspectives on good care are compared with academic perspectives of PCC and a model of PCC is created comprising four dimensions: patient, health professional, patient – professional interaction and healthcare organization. All the generic needs formulated in this study fit into these four dimensions. The need to be treated as a person with strengths fits the dimension ‘patient’, and the need for a good health professional who carefully listens, takes time and makes them feel understood, resulting in a good connection with the professional, fits the dimension ‘health professional’ of this model. Furthermore, patients in this study stressed the importance of open communication in order to provide individualized care, which fits the dimension of ‘patient–professional interaction’. The urge for better access to health care, geared to patients’ mood swings and the need for better collaboration between health professionals and continuity of health professionals fits the dimension of ‘health care organization’ of the model. This study confirms the findings from the review and contributes to the literature stressing the importance of a patient-centered care approach (Mills et al. 2014 ; Scholl et al. 2014 ).
In the prevailing healthcare paradigm, EBM, the best available evidence should guide treatment of patients (Sackett et al. 1996 ; Darlenski et al. 2010 ). This evidence is translated into clinical and practical guidelines, which thus facilitate EBM and could be used as a decision-making tool in clinical practice (Skelly et al. 2013 ). For many psychiatric disorders, treatment is based on such disorder - specific clinical and practical guidelines. However, this disease-focused healthcare system has contributed to its fragmented nature Stange ( 2009 ) argues that this fragmented care system has expanded without the corresponding ability to integrate and personalize accordingly. We argue that acknowledging that disorder - specific clinical and practical guidelines address only parts of the care needs is of major importance, since otherwise important aspects of the patients’ needs will be ignored. Because there is an increasing acknowledgement that health care should be responsive to the needs of patients and should change from being disease-focused towards being patient-focused (Mead and Bower 2000 ; Sidani and Fox 2014 ), currently in the Netherlands generic practical guidelines are written on specific care themes (e.g. co-morbidity, side-effects, daily activity and participation). These generic practical guidelines address some of the generic needs formulated by the patients in our study. We argue that in addition to disorder-specific guidelines, these generic practical guidelines should increasingly be integrated into clinical practice, while health professionals should continuously be sensitive to other emerging needs. We believe that an integration of a disorder-centered and a patient-centered focus is essential to address all needs a patient.
Strengths, limitations and future research
This study has several strengths. First, it contributes to the literature on the challenges and needs of patients with BD. Second, the study is conducted from a patient’s perspective. Moreover, addressing this aim by conducting two separate studies enabled us to triangulate the data.
This study also has several limitations. First, this study reflects the challenges, care needs and research needs of Dutch patient with BD and caregivers. Despite the fact that a maximum variation sampling strategy was used to derive a broad range of challenges and needs throughout the Netherlands, the Dutch setting of the study may limit the transferability to other countries. To understand the overlap and differences between countries, similar research should be conducted in other contexts. Second, given the design of the study, we could not differentiate between patients and caregivers since they participated together in the FGDs. More patients than caregivers participated in the study. For a more in-depth understanding of the challenges and needs faced by caregivers, in future research separate FGDs should be conducted. Third, due to the fixed outline of the practical guideline used to conduct the FGDs, only the healthcare needs for diagnosis, treatment and recovery of BD are studied. Despite the fact that these themes might cover a broad range of health care, it could have resulted in overlooking certain needs in related areas of well-being. Therefore, future research should focus on needs outside of these themes in order to provide a complete set of healthcare needs.
Patients and their caregivers face many challenges in living with BD. Our study contributes to the literature on care and research needs from a patient perspective. Needs specific for BD are preventing late or incorrect diagnosis, support in search for individualized treatment, and supporting clinical, functional, social and personal recovery. Generic healthcare needs concern health professionals, communication and the healthcare system. This explication of both disorder-specific and generic needs indicates that clinical practice guidelines should address and integrate both in order to be responsive to the needs of patients and their caregivers.
Care farm: farms that combine agriculture and services for people with disabilities (Iancu 2013 ). These farms are used as interventions in mental care throughout Europe and the USA to facilitate recovery (Iancu et al. 2014 ).
A government agency involved in the implementation of employee insurance and providing labor market and data services.
Abma T, Broerse J. Patient participation as dialogue: setting research agendas. Health Expect. 2010;13(2):160–73.
Article Google Scholar
APA. Beknopt overzicht van de criteria (DSM-5). Nederlands vertaling van de Desk Reference to the Diagnostic Criteria from DSM-5. Amsterdam: Boom; 2014.
Google Scholar
Asadi-Lari M, Tamburini M, Gray D. Patients’ needs, satisfaction, and health related quality of life: towards a comprehensive model. Health Qual Life Outcomes. 2004;2:1–15.
Bagchus C, Dedding C, Bunders JFG. “I”m happy that I can still walk’—participation of the elderly in home care as a specific group with specific needs and wishes. Health Expect. 2014;18(6):1–9.
Barratt A. Evidence based medicine and shared decision making: the challenge of getting both evidence and preferences into health care. Patient Educ Couns. 2008;73(3):407–12.
Bensing J. Bridging the gap. The separate worlds of evidence-based medicine and patient-centered medicine. Patient Educ Couns. 2000;39:17–25.
Article CAS Google Scholar
Boote J, Baird W, Beecroft C. Public involvement at the design stage of primary health research: a narrative review of case examples. Health Policy. 2010;95(1):10–23.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Broerse J, Zweekhorst M, van Rensen A, de Haan M. Involving burn survivors in agenda setting on burn research: an added value? Burns. 2010;36(2):217–31.
Caron-Flinterman JF, Broerse JEW, Bunders JFG. The experiential knowledge of patients: a new resource for biomedical research? Soc Sci Med. 2005;60(11):2575–84.
Darlenski RB, Neykov NV, Vlahov VD, Tsankov NK. Evidence-based medicine: facts and controversies. Clin Dermatol. 2010;28(5):553–7.
de Graaf R, ten Have M, van Gool C, van Dorsselaer S. Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study-2. Soc Psychiatry Psychiatr Epidemiol. 2012;47(2):203–13.
Elberse J, Pittens C, de Cock Buning T, Broerse J. Patient involvement in a scientific advisory process: setting the research agenda for medical products. Health Policy. 2012;107(2–3):231–42.
Fajutrao L, Locklear J, Priaulx J, Heyes A. A systematic review of the evidence of the burden of bipolar disorder in Europe. Clin Pract Epidemiol Ment Health. 2009;5(1):3.
Fisher A, Manicavasagar V, Sharpe L, Laidsaar-Powell R, Juraskova I. A qualitative exploration of patient and family views and experiences of treatment decision-making in bipolar II disorder. J Ment Health. 2017;27(1):66–79.
Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorder and recurrent depression. 2nd ed. New York: Oxford University Press; 2007.
Goossens P, Knoopert-van der Klein E, Kroon H, Achterberg T. Self reported care needs of outpatients with a bipolar disorder in the Netherlands: a quantitative study. J Psychiatr Ment Health Nurs. 2014;14:549–57.
Granek L, Danan D, Bersudsky Y, Osher Y. Living with bipolar disorder: the impact on patients, spouses, and their marital relationship. Bipolar Disord. 2016;18(2):192–9.
Guyatt G, Cairns J, Churchill D, Cook D, Haynes B, Hirsh J, Irvine J, Levine M, Levine M, Nishikawa J, Sackett D, Brill-Edwards P, Gerstein H, Gibson J, Jaeschke R, Kerigan A, Neville A, Panju A, Detsky A, Enkin M, Frid P, Gerrity M, Laupacis A, Lawrence V, Menard J, Moyer V, Mulrow C, Links P, Oxman A, Sinclair J, Tugwell P. Evidence-based medicine: a new approach to teaching the practice of medicine. JAMA. 1992;268(17):2420–5.
Huxley N, Baldessarini R. Disability and its treatment in bipolar disorder patients. Bipolar Disord. 2007;9(1–2):183–96.
Iancu SC. New dynamics in mental health recovery and rehabilitation. Amsterdam: Vu University; 2013.
Iancu SC, Zweekhorst MBM, Veltman DJ, Van Balkom AJLM, Bunders JFG. Mental health recovery on care farms and day centres: a qualitative comparative study of users’ perspectives. Disabil Rehabil. 2014;36(7):573–83.
IsHak WW, Brown K, Aye SS, Kahloon M, Mobaraki S, Hanna R. Health-related quality of life in bipolar disorder. Bipolar Disord. 2012;14(1):6–18.
Kuper A, Lingard L, Levinson W. Critically appraising qualitative research. BMJ. 2008;337(7671):687–9.
Larsson IE, Sahlsten MJM, Sjöström B, Lindencrona CSC, Plos KAE. Patient participation in nursing care from a patient perspective: a grounded theory study. Scand J Caring Sci. 2007;21(3):313–20.
Lloyd C, Waghorn G, Williams PL. Conceptualising recovery in mental health. Br J Occup Ther. 2008;71:321–8.
Maassen EF, Schrevel SJC, Dedding CWM, Broerse JEW, Regeer BJ. Comparing patients’ perspectives of “good care” in Dutch outpatient psychiatric services with academic perspectives of patient-centred care. J Ment Health. 2016;26(1):1–11.
Maassen EF, Regeer BJ, Bunders JGF, Regeer EJ, Kupka RW. A research agenda for bipolar disorder developed from a patient’s perspective. J Affect Disord. 2018;239:11–17. https://doi.org/10.1016/j.jad.2018.05.061 .
Article PubMed Google Scholar
MacQueen GM, Young LT, Joffe RT. A review of psychosocial outcome in patients with bipolar disorder. Acta Psychiatr Scand. 2001;103(3):163–70.
Malmström E, Hörberg N, Kouros I, Haglund K, Ramklint M. Young patients’ views about provided psychiatric care. Nord J Psychiatry. 2016;70(7):521–7.
MaxQDA [Internet]. Available from: https://www.maxqda.com/ . Accessed 2 Aug 2018.
Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51(7):1087–110.
Michalak EE, Hole R, Livingston JD, Murray G, Parikh SV, Lapsley S, et al. Improving care and wellness in bipolar disorder: origins, evolution and future directions of a collaborative knowledge exchange network. Int J Ment Health Syst. 2012;6:16.
Mills I, Frost J, Cooper C, Moles DR, Kay E. Patient-centred care in general dental practice—a systematic review of the literature. BMC Oral Health. 2014;14(1):64.
Misak CJ. Narrative evidence and evidence-based medicine. J Eval Clin Pract. 2010;16(2):392–7.
Neogi R, Chakrabarti S, Grover S. Health-care needs of remitted patients with bipolar disorder: a comparison with schizophrenia. World J Psychiatry. 2016;6(4):431–41.
Nestsiarovich A, Hurwitz NG, Nelson SJ, Crisanti AS, Kerner B, Kuntz MJ, et al. Systemic challenges in bipolar disorder management: a patient-centered approach. Bipolar Disord. 2017;19(8):676–88.
Netwerk Kwaliteitsontwikkeling GGZ. Zorgstandaard Bipolaire stoornissen. 2017;1–54.
Newnham EA, Page AC. Bridging the gap between best evidence and best practice in mental health. Clin Psychol Rev. 2010;30(1):127–42.
Pittens C, Noordegraaf A, van Veen S, Broerse J. The involvement of gynaecological patients in the development of a clinical guideline for resumption of (work) activities in the Netherlands. Health Expect. 2013;18:1397–412.
Rusner M, Carlsson G, Brunt D, Nyström M. Extra dimensions in all aspects of life—the meaning of life with bipolar disorder. Int J Qual Stud Health Well-being. 2009;4(3):159–69.
Rycroft-Malone J, Seers K, Titchen A, Harvey G, Kitson A, McCormack B. What counts as evidence in evidence-based practice? J Adv Nurs. 2004;47(1):81–90.
Sackett D, Rosenberg W, Gray J, Haynes R, Richardson W. Evidence based medicine: what it is and what it isn’t. Br Med J. 1996;312(7023):71–2.
Scholl I, Zill JM, Härter M, Dirmaier J. An integrative model of patient-centeredness—a systematic review and concept analysis. PLoS ONE. 2014;9(9):e107828.
Schrevel SJC. Surrounded by controversy: perspectives of adults with ADHD and health professionals on mental healthcare. Amsterdam: VU University; 2015.
Sidani S, Fox M. Patient-centered care: clarification of its specific elements to facilitate interprofessional care. J Interprof Care. 2014;28(2):134–41.
Skelly N, Schnittger RI, Butterly L, Frorath C, Morgan C, McLoughlin DM, et al. Quality of care in psychosis and bipolar disorder from the service user perspective. Qual Health Res. 2013;23(12):1672–85.
Stange KC. The problem of fragmentation and the need for integrative solutions. Ann Fam Med. 2009;7(2):100–3.
Strejilevich SA, Martino DJ, Murru A, Teitelbaum J, Fassi G, Marengo E, et al. Mood instability and functional recovery in bipolar disorders. Acta Psychiatr Scand. 2013;128(3):194–202.
Tait L. Encouraging user involvement in mental health services. Adv Psychiatr Treat. 2005;11(3):168–75.
Teunissen T, Visse M, De Boer P, Abma TA. Patient issues in health research and quality of care: an inventory and data synthesis. Health Expect. 2011;16:308–22.
van der Stel. Het begrip herstel in de psychische gezondheidzorg, Leiden; 2015. p. 1–3.
Van Der Voort TYG, Van Meijel B, Hoogendoorn AW, Goossens PJJ, Beekman ATF, Kupka RW. Collaborative care for patients with bipolar disorder: effects on functioning and quality of life. J Affect Disord. 2015;179:14–22.
Yasuyama T, Ohi K, Shimada T, Uehara T, Kawasaki Y. Differences in social functioning among patients with major psychiatric disorders: interpersonal communication is impaired in patients with schizophrenia and correlates with an increase in schizotypal traits. Psychiatry Res. 2017;249:30–4.
Download references
Authors’ contributions
EFM designed the study, contributed to the data collection, managed the analysis and wrote the first draft of the manuscript. BJR designed the study and contributed to the data collection, data analysis, and writing of the manuscript. JFGB contributed to the study design and critical revision of the manuscript. EJR contributed to the study conception and critical revision of the manuscript. RWK contributed to the study design, acquisition of data, and critical revision of the manuscript. All authors contributed to the final manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
The authors received no financial support for the research.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Athena Institute, Faculty of Earth and Life Sciences, VU University Amsterdam, Boelelaan 1085, 1081HV, Amsterdam, Netherlands
Eva F. Maassen, Barbara J. Regeer & Joske F. G. Bunders
Altrecht Institute for Mental Health Care, Nieuwe Houtenseweg 12, 3524 SH, Utrecht, Netherlands
Eva F. Maassen, Eline J. Regeer & Ralph W. Kupka
Amsterdam Public Health Research Institute, Amsterdam UMC, Vrije Universiteit Amsterdam, Psychiatry, De Boelelaan 1117, Amsterdam, Netherlands
Ralph W. Kupka
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Eva F. Maassen .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Maassen, E.F., Regeer, B.J., Regeer, E.J. et al. The challenges of living with bipolar disorder: a qualitative study of the implications for health care and research. Int J Bipolar Disord 6 , 23 (2018). https://doi.org/10.1186/s40345-018-0131-y
Download citation
Received : 06 June 2018
Accepted : 22 August 2018
Published : 06 November 2018
DOI : https://doi.org/10.1186/s40345-018-0131-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- General Healthcare
- Personal Recovery
- Focus Group Discussions
- Professional-patient Interaction
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
The Dynamics of Mood and Coping in Bipolar Disorder: Longitudinal Investigations of the Inter-Relationship between Affect, Self-Esteem and Response Styles
* E-mail: [email protected]
Affiliation School of Psychology, Bangor University, Bangor, United Kingdom
Affiliation School of Psychological Sciences, University of Manchester, Manchester, United Kingdom
Affiliation Greater Manchester West NHS Trust, Manchester, United Kingdom
Affiliation Department of Psychology and Neuropsychology, University of Maastricht, Maastricht, The Netherlands
Affiliation Centre for Biostatistics, Institute of Population Health, University of Manchester, Manchester, United Kingdom
Affiliation Institute of Psychology, Health and Society, University of Liverpool, Liverpool, United Kingdom
- Hana Pavlickova,
- Filippo Varese,
- Angela Smith,
- Inez Myin-Germeys,
- Oliver H. Turnbull,
- Richard Emsley,
- Richard P. Bentall

- Published: April 26, 2013
- https://doi.org/10.1371/journal.pone.0062514
- Reader Comments
Previous research has suggested that the way bipolar patients respond to depressive mood impacts on the future course of the illness, with rumination prolonging depression and risk-taking possibly triggering hypomania. However, the relationship over time between variables such as mood, self-esteem, and response style to negative affect is complex and has not been directly examined in any previous study – an important limitation, which the present study seeks to address.
In order to maximize ecological validity, individuals diagnosed with bipolar disorder (N = 48) reported mood, self-esteem and response styles to depression, together with contextual information, up to 60 times over a period of six days, using experience sampling diaries. Entries were cued by quasi-random bleeps from digital watches. Longitudinal multilevel models were estimated, with mood and self-esteem as predictors of subsequent response styles. Similar models were then estimated with response styles as predictors of subsequent mood and self-esteem. Cross-sectional associations of daily-life correlates with symptoms were also examined.
Cross-sectionally, symptoms of depression as well as mania were significantly related to low mood and self-esteem, and their increased fluctuations. Longitudinally, low mood significantly predicted rumination, and engaging in rumination dampened mood at the subsequent time point. Furthermore, high positive mood (marginally) instigated high risk-taking, and in turn engaging in risk-taking resulted in increased positive mood. Adaptive coping (i.e. problem-solving and distraction) was found to be an effective coping style in improving mood and self-esteem.
Conclusions
This study is the first to directly test the relevance of response style theory, originally developed to explain unipolar depression, to understand symptom changes in bipolar disorder patients. The findings show that response styles significantly impact on subsequent mood but some of these effects are modulated by current mood state. Theoretical and clinical implications are discussed.
Citation: Pavlickova H, Varese F, Smith A, Myin-Germeys I, Turnbull OH, Emsley R, et al. (2013) The Dynamics of Mood and Coping in Bipolar Disorder: Longitudinal Investigations of the Inter-Relationship between Affect, Self-Esteem and Response Styles. PLoS ONE 8(4): e62514. https://doi.org/10.1371/journal.pone.0062514
Editor: Xiang Yang Zhang, Baylor College of Medicine, United States of America
Received: November 22, 2012; Accepted: March 21, 2013; Published: April 26, 2013
Copyright: © 2013 Pavlickova et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This research was supported by a studentship for A. Smith from the Economic and Social Research Council ( www.esrc.ac.uk ) and a studentship for H. Pavlickova from the National Institute for Social Care and Health Research, the Welsh Assembly Government ( www.wales.gov.uk/nischr ; Project ref.: HS/09/004), and Betsi Cadwaladr University Health Board (BCUHB) Charitable Funds. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Attempts to understand the psychological mechanisms underlying bipolar disorder are made difficult by the multidimensional, dynamic and fluctuating nature of the symptoms experienced by patients. For example, although the term ‘bipolar disorder’ implies that depression and mania lie at opposite ends on a spectrum of affect, cross-sectional comparisons indicate that these two groups of symptoms lie on separate dimensions of psychopathology, so that patients can be simultaneously depressed and manic [1] , explaining why patients sometimes present with mixed episodes [2] . It has been reported that mood in bipolar patients can fluctuate chaotically over short periods of time [3] , and longitudinal studies have shown that, within individuals, manic and depressive symptoms vary relatively independently with each other, although with a small but statistically significant positive correlation between them [4] , again explaining why mixed episodes are sometimes observed. The implication of these observations is that psychological studies of bipolar patients should ideally be conducted with sophisticated designs that take into account the complex cross-sectional and longitudinal structure of symptoms, so that covariations between symptoms and psychological processes can be adequately detected.
Problems of self-esteem and related processes seem to be particularly evident in bipolar disorder; almost a century ago, Kreapelin [5] described in detail how manic grandiosity sharply contrasts with low self-esteem and withdrawal during periods of depression. More recent research on the psychological mechanisms in bipolar disorder has focused on self-related cognitive processes already implicated in unipolar depression, for example as proposed in theories by Beck [6] and by Abramson et al. [7] . These studies have shown that individuals with bipolar disorder often present with a negative attributional (explanatory) style [8] , a negative self-concept, and dysfunctional attitudes towards the self [9] , [10] , [11] , [12] . In contrast to Kraepelin’s earlier observations, cross-sectional comparisons suggest that these pessimistic cognitive biases may be evident across all phases of bipolar disorder [13] .
However, a somewhat different picture has emerged from studies employing longitudinal designs or studies examining symptoms rather than episodes. These studies have indicated that bipolar disorder is associated with substantial instability in affective and self-related processes. Pronounced daily fluctuations in self-esteem have been observed in studies of remitted patients [14] , those in depressive episode [13] , and also in studies of individuals assessed by questionnaire measures to be at high-risk of bipolar disorder [15] . Further, low self-esteem in persons with bipolar disorder prospectively predicts worsening of affective, particularly depressive, symptoms [10] , [16] , [17] . In a longitudinal study [18] , where patients were assessed every 6 months, although self-esteem correlated positively with current mania and negatively with current depression, negative self-esteem predicted both future depressive and future manic symptoms. Other self-related cognitive measures administered in the study, although correlating with current symptoms, did not predict future symptoms.
In a similar vein, pronounced fluctuations of affect in bipolar disorder have been indicated by studies of high-risk student samples [15] , [19] , subsyndromal individuals [20] , remitted bipolar patients [14] , and those currently in manic and depressive episode [13] . Notably, affect and self-esteem appear to fluctuate in concert and hence to be tightly linked [21] , [22] .
One way of examining shifts in mood and self-esteem is in the context of the coping mechanisms or response styles individuals employ as a response to low, or elevated, mood. In her work on unipolar depression, Nolen-Hoeksema [23] argued that these mechanisms include rumination, problem solving, distraction activities and risk-taking. In a factor-analytic study by Knowles et al. [24] , problem-solving and distraction loaded on a single factor they labeled active coping.
A number of studies have found that rumination predicts onset and severity of depression in unipolar patients [25] , [26] , [27] . Expanding on the original theory, Thomas and Bentall [28] hypothesized that, whilst at times rumination may exacerbate depressive mood in bipolar patients, at other times it may instigate vigorous attempts to avoid negative mood by engaging in high-risk activities resulting, in turn, in hypomania or full-blown mania. Thomas et al. [29] found high levels of rumination in remitted bipolar patients compared to controls, and high levels of self-reported active coping (problem solving and distraction activities) and risk-taking in manic patients compared to controls. Van der Gucht et al. [13] found high levels of rumination in patients in all phases of bipolar disorder, including remission, but again that self-reported risk-taking was elevated only in currently manic patients. Only one study has examined response styles in relation to daily life experiences and fluctuations in mood and self-esteem [15] . In this experience sampling study of high-risk sample of students selected by questionnaire, higher levels of rumination were associated with lower self-esteem, even though no differences in rumination between the low-risk and high-risk groups were identified.
Insight into the temporal dynamics of response styles in relation to other variable psychological processes such as mood and self-esteem has been precluded by the cross-sectional designs employed in most previous studies of bipolar disorder.
Therefore, the aim of the present study was to examine processes specific to bipolar disorder. First, we investigated cross-sectional associations between symptoms of depression and mania with daily life correlates (i.e. affect and self-esteem) and coping styles (rumination, risk-taking and adaptive coping). We predicted that symptoms of depression would be associated with low mood and self-esteem, and more pronounced fluctuations of both. In addition, we expected depressive symptoms to be related to increased levels of rumination. As to symptoms of mania, we predicted associations with increased mood, self-esteem, and their fluctuations. Furhtermore, mania was expected to be associated with risk-taking.
Second, this study sought to examine prospective associations between mood, self-esteem and response styles in two ways: a) whether mood and self-esteem at time T−1 predict engagement in response styles at the subsequent time point. We expected that low mood and self-esteem at time T−1 would predict increased levels of rumination at time T. In turn, high mood and self-esteem would predict increased risk-taking at time T; b) whether engaging in coping styles at time T−1 influences mood and self-esteem at time T. We expected that engaging in rumination would lead to decreased mood and self-esteem, whilst engaging in risk-taking would improve mood and self-esteem.
Materials and Methods
Ethical approval was obtained from the Leeds (East) Research Ethics Committee and the University of Manchester Senate Ethics Committee. Inclusion criteria for inception into the study were a) diagnosis of bipolar affective disorder, b) currently receiving outpatient care, c) ability to speak/read English, and d) ability to complete the self-report measures independently. Participants were excluded from the study if they met diagnostic criteria for schizophrenia, schizoaffective disorder, primary substance misuse disorder, or had a history of post-natal depression with no hypomania/mania according to DSM-IV [30] . Potential participants were approached via secondary care and self-help groups: 129 covering letters were posted by consultant psychiatrists, resulting in 40 responses, out of which 7 individuals withdrew prior to interview, 5 after receiving further information. Out of the 28 participants commencing the study, 5 dropped out, and 23 completed the study. In addition, consultant psychiatrists approached prospective participants during clinics (N unknown), out of which 3 withdrew after gaining further information, and 24 completed the study. Only one participant was recruited via self-help groups. A total of 48 participants diagnosed with bipolar disorder provided written informed consent and were included into the study: 28 were in a remission, 12 were currently depressed, and 8 currently hypomanic. Participants’ characteristics are described in Table 1 . All participants completed the Structured Clinical Interview for Axis I DSM-IV Disorders [31] .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0062514.t001
Instruments
1. clinical measures..
To assess symptom levels at the beginning of the study, participants completed two clinical measures in a face-to-face interview.
The Hamilton rating scale for depression [HRSD, 32] consists of 17 items rated by the interviewer on a 0–4 scale with higher scores indicating more sever depressive symptomatology. The HRSD shows inter-rater reliability coefficients up to 0.90 [32] , and good validity and reliability [33] .
The Bech-Refaelson Mania Scale, Modified Version [MAS, 34] is widely used to assess symptoms of mania and designed to be administered alongside the HRSD. Each of its 11 items is rated on a five-point scale, resulting in a total score ranging between 0–44. The scale shows a high inter-observer reliability and an acceptable level of consistency across items [34] .
2. Psychological measures.
All variables pertaining to the psychological processes of concern in this study were derived from experience sampling method (ESM) diaries that participants were asked to complete over a six-day period.
Experience sampling method. The experience sampling method (ESM, [35] ) is a repeated self-assessment procedure completed in participants’ natural environments and thus advantageous over classically administered self-report questionnaires for its high ecological validity [36] . Its validity, reliability and feasibility have been demonstrated in a number of clinical populations, such as in samples of individuals with diagnosis of schizophrenia [37] , [38] , depression [39] , [40] , panic disorder [41] and bipolar disorder [42] , [43] , [44] .
Participants received a pre-programmed digital wristwatch emitting 10 bleeps a day in quasi-random intervals (between 7.30 a.m. and 10.30 p.m.) and six pocketsize diaries to be completed over the period of six days (i.e. one dairy to be completed per each study day). The diary booklet consisted of 10 self-report forms (one per beep), and each comprised scales assessing mood, self-esteem, and styles of coping with depressive mood. Participants received a thorough explanation of the method during a briefing session. To ensure that participants understood the method, they were asked to fill in one form in a trial booklet during the briefing. During the 6-day study period, participants were contacted by telephone to ascertain that they had managed to comply with the procedure, and were thoroughly debriefed after completion of the study. Only participants who completed more than 20 valid responses (i.e. an entry between 5 minutes prior and 15 minutes after the beep) were included in the analyses [45] . This resulted in exclusion of two participants (both females, mean age 59, with depression ratings of 0, 0 and mania ratings of 1 and 2.
Experience Sampling Method Variables
The items included in the ESM self-assessment forms were all rated on 7-point Likert scales and used to define the following variables:
Momentary self-esteem and self-esteem fluctuations.
Four items in the self-report form assessed momentary self-esteem (i.e. “I am a failure”, “I am ashamed of myself”, “I like myself”, and “I am a good person”). Using the Kaiser criterion, principal component analysis (PCA) on the raw within-participant scores revealed one factor accounting for 63% of the total variance. Both negative and positive items showed a strong loading on the factor (positive items<−.68; negative items >.80) and high internal consistency after reversing the two negative items scores (Cronbach’s α = .79). The momentary self-esteem score was defined as the mean score of the four items. Each fluctuation in self-esteem was defined as the absolute difference in the ratings of self-esteem between consecutive time points, with higher scores reflecting more intense fluctuations.
Positive and negative affect, and mood fluctuations.
Nine items assessing momentary positive (e.g. “I feel cheerful”) and negative (e.g. “I feel sad”) affect were used. PCA confirmed two separate factors (eigenvalues >1) together accounting for 66% of variance. The positive affect (PA) factor consisted of four items (“cheerful”, “excited”, “relaxed” and “satisfied”; Cronbach’s α = .82) and the negative affect (NA) factor incorporated five items (“lonely”, “anxious”, “sad”, “irritated” and “guilty”; Cronbach’s α = .86). Fluctuation in mood was defined as the absolute moment-to-moment change in ratings of a) positive mood, and b) negative mood; that is, at each time point two variables were obtained, fluctuation in positive mood and fluctuation in negative mood; higher values reflected more pronounced fluctuations.
Assessment of responses to depression.
Based on the revised version of Nolen-Hoeksema’s Response Style Questionnaire [23] , [24] , the self-assessment forms contained eight items evaluating participants’ coping and response strategies for depression (e.g. “Since the last bleep I have thought about the bad things that have happened to me.”) rated on a 7-point Likert scale ranging from −3 (Disagree) to +3 (Agree). Due to bimodal distribution of the scores suggesting that a portion of participants misunderstood the scale as 0 indicating ‘no engagement’, we have recoded all responses rated negatively (i.e. −3, −2, and −1) as 0. Consistent with previous studies [13] , [24] , PCA confirmed three independent factors accounting for 72% of the variance: rumination (2 items with loadings >.90; Cronbach’s α = .82), adaptive coping (4 distraction and problem-solving items with loadings >.59; Cronbach’s α = .72) and risk-taking (2 items with loadings >.91; Cronbach’s α = .84).
Data Analyses
The structure of ESM data allows for the investigation of longitudinal associations between ESM variables using regression methods, i.e. testing whether ESM variables at a given beep (i.e. T) are predicted by responses at the previous beep (T−1). The longitudinal nature of these data implies that ESM data have a hierarchical structure (i.e. ESM entries at each beep are clustered within participants); therefore the assumption of the independence of residuals required for linear models is violated. Multilevel modeling adequately account for this type of violations [46] , [47] , [48] . Data were analyzed with the XTREG module of STATA version 12.0 using maximum likelihood estimation. As a number of variables (i.e. symptoms of depression and mania, and all response styles) were severely positively skewed, bootstrapping (1000 iterations) was utilized, the recommended procedure when the assumptions of normality are violated [49] .
Multilevel regression models were employed as follows:
- We investigated the daily life correlates of depressive and manic symptoms measured at baseline. Separate multilevel regression models were estimated for the following dependent variables: PA, NA, SE, fluctuations of PA, fluctuations of NA, fluctuations of SE, rumination, active-copying and risk-taking. For each model, symptoms of depression and mania were entered as independent variables.
- We examined whether PA, NA and SE predicted subsequent response style behaviors. Response style items were phrased “Since the last bleep…” in the diary booklets and as such, assessed coping behaviours between successive time points T−1 and T. For the purpose of the present analyses they were treated as time T items. Separate multilevel regression models were estimated for each independent variable (i.e. PA, NA and SE) as measured at T−1 and response styles (i.e. rumination, active copying and risk-taking) at time T were entered into the models as dependent variables. We controlled for the confounding effect of response style at the previous time point (T−1), as well as for the baseline symptoms of depression and mania.
- We tested whether response styles predicted subsequent levels of PA, NA, and SE. Separate multilevel regression models were estimated for each dependent variable (i.e. PA, NA and SE) at time T with response styles (rumination, adaptive copying, and risk-taking) at time T−1 as predictors. We controlled for the confounding effect of PA, NA and SE at the previous beep, and symptoms of depression and mania measured at a baseline.
Are Symptoms of Depression (HRSD) and Mania (MAS) Associated?
In preliminary analyses, we first examined the distributions of depression (HRSD) and mania (MAS) scores, and their associations. As previous studies found a weak, but significant correlation between symptoms of depression and mania [4] , [50] , we first examined the relatedness of the two scores. Correlation analyses in the present study did not reach statistical significance, r s = 0.18, p = .23. Nevertheless, in the following analyses both symptoms were controlled for simultaneously.
i. Are symptoms of depression and mania associated with daily life correlates?
Although our main goal was to investigate the longitudinal relationship between variables, the cross-sectional associations were examined first, see Table 2 . First, we investigated whether positive and negative mood, and self-esteem were related to symptom ratings. Statistical analyses were carried out for momentary level of each variable (i.e. PA, NA, SE) as well as their fluctuations. We found that both depression and mania were associated with higher momentary negative affect (p<.001), lower momentary positive affect (p<.001), and lower momentary self-esteem (p<.01), as well as with more pronounced fluctuations of all variables (all p s <.001).
https://doi.org/10.1371/journal.pone.0062514.t002
We also examined the associations between symptom ratings and response style scores (i.e. rumination, adaptive coping, and risk-taking). Depression was significantly associated with higher levels of rumination, adaptive coping and risk-taking (all ps <.001), whilst mania was significantly associated only with increased levels of risk-taking (p<.001; Table 2 ).
ii. Does affect and self-esteem at time T-1 predict response styles at time T?
The main aim of the present study was to examine associations between affect, self-esteem, and response styles over time. We first examined how affect and self-esteem influenced the way individuals engaged in response styles, and then (in the next section), how response styles affected subsequent mood and self-esteem.
First, the predictive properties of each affect and self-esteem variable at each time point (T−1) on rumination at the subsequent time point (T) was investigated ( Table 3 , upper rows). Multilevel regression analyses revealed that negative affect was associated with increased rumination (p<.001), whereas positive affect (p<.001) and self-esteem (p<.001) were associated with decreases in ruminative thinking at the subsequent time point. When all predictors were entered into the model simultaneously, only affect remained a significant predictor of subsequent rumination: positive affect was associated with a decrease (p<.01), whilst negative affect with an increase (p<.001) of rumination ( Table 3 lower rows).
https://doi.org/10.1371/journal.pone.0062514.t003
None of the independent variables was significantly associated with adaptive coping (all p s = ns; Table 3 ).
Finally, we examined whether affect and self-esteem at time T−1 predicted risk-taking at time T ( Table 3 , upper rows). Risk-taking was significantly predicted by high positive (p<.01), and low negative mood (p<.01) at the previous time point, but only positive affect (p = .071) remained marginally associated with risk-taking when all predictors were entered into the model simultaneously ( Table 3 , lower rows).
iii. Do response styles assessed at T-1 predict affect and self-esteem at T?
Multilevel regression models were estimated to examine whether response styles to depression predicted changes in positive affect, negative affect and self-esteem at subsequent time points. When separate models were estimated for a model with positive affect as the dependent variable, adaptive coping (p<.05), and risk taking (p<.01) at the previous time point significantly predicted an increase in positive affect (both p s <.05), whilst rumination significantly predicted a decrease in self-esteem, and only marginally in positive affect (p = .05). All predictors were significantly associated with positive affect when entered into the model simultaneously (all p s <.05, Table 4 ).
https://doi.org/10.1371/journal.pone.0062514.t004
When separate models were estimated with negative affect as the outcome variable, no significant associations were revealed. Nevertheless, in a model with all response styles entered into the model simultaneously, a marginally significant relationship between rumination at time T−1 and negative affect at the subsequent time point was found (p = .079).
In a model with self-esteem as the dependent variable, no significant associations with response styles at the previous time point were revealed. When all predictors were entered into the model simultaneously, adaptive coping at time T-1 significantly predicted an increase in self-esteem at time T (p<.05).
iv. Follow-up analyses.
In order to examine whether any of the identified relationships were moderated by symptoms of depression or mania, an interaction term between each predictor and symptoms was added into each of the models described in ii) and iii) above with all relevant predictors entered simultaneously. Each model was calculated twice, first with interactions between symptoms of depression and the predictors, followed by a similar model with interactions between symptoms of mania and the predictors. For example, in the case of the model with positive affect as a dependent variable and all three response styles as predictors, three interaction terms were added (between each response style and ratings of depression). A similar model was then calculated with interaction terms between each response style and ratings of mania.
Only one model yielded a significant baseline symptom × predictor interaction. A significant interaction term between symptoms of mania and levels of rumination (β = 0.02, SE = 0.01, p<.01, CI [.01.04]), was found when positive affect was the dependent variable. Additional analyses indicated that rumination led to a decrease in positive affect in individuals with low symptoms of mania at baseline (β = −.27, SE = .04, p<.001, CI [−.35 −.19]) but not in those with high symptoms of mania at baseline. No other significant interaction terms were identified (all p s>.05).
The present study is a novel investigation of the prospective relationships between affect, self-esteem and response styles in individuals diagnosed with bipolar disorder. It tests Nolen-Hoeksema’s [23] response style theory and its later adaptations [24] , [28] , originally formulated to explain the course of unipolar depression using longitudinal data from bipolar patients to examine the impact of psychological variables on response styles and, subsequently, the effect of response styles on psychological variables. The experience sampling method employed in this study allowed the capture of these dynamic relationships, which cannot be assessed using more conventional cross-sectional designs.
Before reviewing the main results, we will comment first on the observed cross-sectional relationships between mood and self-esteem in daily life and baseline symptoms of depression and mania. It was expected that low self-esteem and high negative affect would be associated with symptoms of depression, whereas high positive affect and self-esteem would relate to symptoms of mania. Further, we predicted that increased fluctuations of these processes would be related to both symptoms. Our expectations regarding associations with depression were confirmed, and in line with previous literature. Here, associations between depression and negative mood, as well as its instability, have been consistently reported in studies of high risk students [19] , [24] , [51] , subclinical samples [20] and bipolar patients [13] , [52] . Similarly, previous findings have indicated an association between depression and self-esteem [16] , as well as instability of self-esteem in high risk student [15] and patient studies [14] .
Contrary to our expectations, symptoms of mania showed similar associations with mood and self-esteem as depression (i.e. mania was associated with low mood and self-esteem, and their increased instability), although the effect found was smaller. In contrast to our findings, previous studies have found mania to be related to high mood [51] , and self-esteem comparable to that of controls [13] . Yet, our findings are not the first of its kind. An earlier factor analytic study suggested dysphoria to be the strongest component of mania [53] , and underlying negativity of affect and self-concept during mania have been suggested by studies employing implicit assessments [14] , [54] .
The discrepancy between the present study and previous reports, both employing explicit assessments, might be related to methodological differences. For example, a number of studies employed comparisons of different phases of bipolar disorder, rather than investigating associations of psychological measures with symptoms (e.g. [13] ), an approach complicated by frequent co-existence of depressive and manic symptoms. Another explanation might be related to age differences between examined populations. Several previous studies employed high-risk student populations, and it is likely that personal context of students is considerably different to that of adults with a history of severe mental illness. Although both kinds of studies may be tapping the same underlying vulnerabilities, their expression might be changing across the course of life. The present study is methodologically advantageous in that it has employed patients, representative of bipolar phenomenology, and utilized a longitudinal and ecologically valid assessment and robust statistical methods controlling for covariation of symptoms and non-normality of data.
The increased fluctuations in affect and self-esteem seen in relation to symptoms of depression and mania in the present study suggests that the fluctuations we have observed in remitted patients in previous studies [13] , [24] may have been the consequence of subsyndromal symptoms.
In respect of associations between symptoms and response styles, we expected that rumination would be associated with depression, and risk-taking with mania. Indeed, symptoms of depression were related to increased rumination, an observation that is consistent with Nolen-Hoeksema’s [23] original response style theory, and with findings from bipolar high-risk [24] , [28] , [55] , and patient studies [13] , [29] . The association observed between depressive symptoms and adaptive coping was unexpected, as an earlier patient study found adaptive coping to be related to mania rather than depression [29] . The disparity might reflect the differences between the retrospective questionnaire assessments employed by Thomas et al. [29] and the more ecologically valid experience sampling method utilized in the current study. Finally, risk-taking was positively associated with symptoms of depression as well as mania. Although we did not predict an association between depression and risk-taking, similar cross-sectional relationships have been reported previously [14] , [24] , [29] .
The main aim of the present study was to examine the unique associations between momentary mood, self-esteem and coping styles, and vice versa, whilst controlling for symptoms of depression and mania. To our knowledge, this is the first study to prospectively investigate Nolen-Hoeksema’s [23] response style hypothesis, utilizing measures of response styles in daily life. It was predicted that both low mood and low self-esteem would prompt rumination at a subsequent time point, whilst positive mood and high self-esteem might trigger risky behaviors. The hypotheses were mostly confirmed, with a number of implications requiring comment. As noted, previous cross-sectional studies reported an association between rumination and symptoms of depression. The present findings suggest that high levels of negative, and low levels of positive affect instigate the subsequent engagement in rumination and that, in turn, rumination impacts most robustly via the dampening of positive mood. Furthermore, rumination led to decrease in positive affect only in individuals with few symptoms of mania, whilst no effect was found in those with manic symptoms. These findings are in line with Nolen-Hoeksema’s notion that rumination as such does not cause depression, but rather moderates already depressive mood [56] . The null finding regarding the causal role of self-esteem potentially points to the precedence of affect over cognitive psychological processes in affective disorders, but further investigations are warranted, and this conjecture should be viewed with caution.
The findings regarding risk-taking have both theoretical and clinical implications. Although risk-taking have been found to be related to symptoms of depression and mania cross-sectionally, in a prospective design, positive, rather than negative, mood led to greater risk taking when controlling for the effect of symptoms (although the association reached only marginal significance). In turn, engaging in risk-taking resulted in improvements of mood. In a similar vein, Thomas et al. [29] and Van der Gucht [13] reported higher levels of risk-taking, as measured by questionnaire, in manic participants compared to controls. The failure to detect an association between risk-taking and negative affect, then, implies that this response style might not necessarily act as a defense against low mood as proposed previously [28] , but rather is associated with an increased emotional and behavioral reactivity to reward stimuli as proposed by the behavioural activation theory of mania [57] , [58] , [59] . This account is consistent with recent neuroimaging studies, which have pointed to the abnormal processing of reward stimuli in bipolar patients and at-risk samples [60] , [61] , [62] .
In her original theory, Nolen-Hoeksama (1991) suggested that engaging in distraction (which, along with problem-solving, was incorporated into adaptive coping in this and some previous studies) ameliorates depressive symptoms. Moreover, Nolen-Hoeksema argued that employing healthy coping strategies such as problem solving may be prevented by rumination. Our findings support these hypotheses only partially. Although in the current study neither mood, nor self-esteem instigated subsequent engagement in adaptive coping, employing this coping style led to substantial improvements in mood and self-esteem at the following time point. Furthermore, adaptive coping was found to be an effective strategy even when controlling for other coping strategies. Hence, adaptive coping appears to be a top-down strategy, that can be deliberately employed to improve one’s affective state, an observation that is consistent with earlier studies showing its effectiveness in natural and laboratory conditions [25] , [56] .
A number of limitations should be acknowledged. Despite methodological advantages of experience sampling method over classical self-report assessments [45] , some authors have raised concerns regarding participants’ compliance with, and hence reliability of, the pencil-and-paper protocol of experience sampling, favoring the use of electronic diaries [63] , [64] , [65] . Whilst this might be an important limitation in studies employing predetermined entries, previous studies have demonstrated comparable, and relatively high, compliance in electronic and paper diary studies, when using a random-entry design [66] , [67] , [68] , also employed in the present study. Further, it is possible that utilizing different time lags in the predictive analyses would have led to different results.
The findings have a number of clinical implications. Various psychotherapies operate by means of modifying coping strategies – though often using different methods (for review, see [69] ); the response style theory has been found to provide a useful framework for understanding the utility of coping styles. Our findings highlight the importance of therapeutic strategies to ameliorate rumination in bipolar patients, and also the potential value of psychoeducational methods of reducing risk taking in response to incipient manic symptoms. The observation that risk-taking prompted by positive affect leads to a further escalation of affect points to the need to interrupt this cycle during the earliest phase of a hypomanic episode. Existing cognitive behavior therapy strategies which have been shown to be effective already address these issues to some degree [70] . The results regarding adaptive coping are promising as they imply that individuals with severe illness retain some ability to effectively regulate their mood.
Author Contributions
Contributed to revising manuscript critically for important intellectual content: IM-G AS FV OT RPB RE HP. Conceived and designed the experiments: AS IM-G RPB. Performed the experiments: AS. Analyzed the data: FV HP RE. Wrote the paper: HP RPB OT IM-G FV RE.
- View Article
- Google Scholar
- 5. Kraepelin E (1921) Manic-depressive insanity and paranoia. Barclay RM, translator. Edinburgh: Livingstone.
- 7. Abramson LY, Alloy LB, Metalsky GI (1988) The cognitive diathesis-stress theories of depression: Towards and evaluation of the theories’ validities. In: Alloy LB, editor. Cognitive Processes in Depression. New York: Guilford.
- 30. American Psychiatric Association (1994) Diagnostic and statistical manual for mental disorders, 4th edition. Washington DC: Author.
- 31. First M, Spitzer R, Gibbon M, Williams J (1995) Structured clinical interview for Axis I DSM-IV disorders. Washington DC: American Psychiatric Association Press.
- 33. Rehm LP (1988) Assessment of depression. In: Bellack AS, Hersen M, editors. Behavioral Assessment : A Practical Handbook 3rd Edition. Oxford: Permagon. 313–364.
- 35. Csikszentmihalyi M, Larson R (1987) Validity and reliability of the Experience Sampling Method. Journal of Nervous and Mental Disease 175.
- 38. Myin-Germeys I, van Os J (2007) Stress-reactivity in psychosis: Evidence for an affective pathway to psychosis. Clinical Psychology Review: 409–424.
- 43. Kwapil TR, Barrantes-Vidal N, Armistead MS, Hope GA, Brown LH, et al.. (2011) The expression of bipolar spectrum psychopathology in daily life. Journal of Affective Disorders 130.
- 44. Walsh MA, Royal A, Brown LH, Barrantes-Vidal N, Kwapil TR (in press) Looking for bipolar spectrum psychopathology: identification and expression in daily life. Comprehensive Psychiatry.
- 45. Delespaul P (1995) Assessing schizophrenia in daily life: The Experience Sampling Method. Maastricht, the Netherlands: Universitaire Pers Maastricht.
- 47. Hox JJ (2010) Multilevel analysis: Techniques and applications. Hove: Routledge.
- 48. Twisk JWR (2006) Applied Multilevel Analysis. New York: Cambridge University Press.
- 49. Mooney CZ, Duval RD (1993) Bootstrapping: A non-parametric approach to statistical inference. Newbury Park, CA: Sage Publications.
- 50. Pavlickova H, Varese F, Turnbull O, Scott J, Morriss R, et al. (under review) Symptom-specific self-referential cognitive processes in bipolar disorder: A longitudinal analysis. Psychological Medicine.
- 69. Roth A, Fonagy P (2005) What works for whom: A critical review of psychotherapy research. London: Guildford Press.
Advertisement
Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications
- Open access
- Published: 13 February 2021
- Volume 37 , pages 863–880, ( 2021 )
Cite this article
You have full access to this open access article

- Zezhi Li 1 , 2 ,
- Meihua Ruan 3 ,
- Jun Chen 1 , 5 &
- Yiru Fang ORCID: orcid.org/0000-0002-8748-9085 1 , 4 , 5
46k Accesses
109 Citations
16 Altmetric
Explore all metrics
A Correction to this article was published on 17 May 2021
This article has been updated
Major depressive disorder (MDD), also referred to as depression, is one of the most common psychiatric disorders with a high economic burden. The etiology of depression is still not clear, but it is generally believed that MDD is a multifactorial disease caused by the interaction of social, psychological, and biological aspects. Therefore, there is no exact pathological theory that can independently explain its pathogenesis, involving genetics, neurobiology, and neuroimaging. At present, there are many treatment measures for patients with depression, including drug therapy, psychotherapy, and neuromodulation technology. In recent years, great progress has been made in the development of new antidepressants, some of which have been applied in the clinic. This article mainly reviews the research progress, pathogenesis, and treatment of MDD.
Similar content being viewed by others

Dopamine: Functions, Signaling, and Association with Neurological Diseases

Molecular Mechanisms of Psilocybin and Implications for the Treatment of Depression

Lavender oil preparation Silexan is effective in mild-to-moderate major depression: a randomized, placebo- and reference-controlled trial
Avoid common mistakes on your manuscript.
Major depressive disorder (MDD) also referred to as depression, is one of the most severe and common psychiatric disorders across the world. It is characterized by persistent sadness, loss of interest or pleasure, low energy, worse appetite and sleep, and even suicide, disrupting daily activities and psychosocial functions. Depression has an extreme global economic burden and has been listed as the third largest cause of disease burden by the World Health Organization since 2008, and is expected to rank the first by 2030 [ 1 , 2 ]. In 2016, the Global Burden of Diseases, Injuries, and Risk Factors Study demonstrated that depression caused 34.1 million of the total years lived with disability (YLDs), ranking as the fifth largest cause of YLD [ 3 ]. Therefore, the research progress and the clinical application of new discoveries or new technologies are imminent. In this review, we mainly discuss the current situation of research, developments in pathogenesis, and the management of depression.
Current Situation of Research on Depression
Analysis of published papers.
In the past decade, the total number of papers on depression published worldwide has increased year by year as shown in Fig. 1 A. Searching the Web of Science database, we found a total of 43,863 papers published in the field of depression from 2009 to 2019 (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009 – 2019), Articles). The top 10 countries that published papers on the topic of depression are shown in Fig. 1 B. Among them, researchers in the USA published the most papers, followed by China. Compared with the USA, the gap in the total number of papers published in China is gradually narrowing (Fig. 1 C), but the quality gap reflected by the index (the total number of citations and the number of citations per paper) is still large, and is lower than the global average (Fig. 1 D). As shown in Fig. 1 E, the hot research topics in depression are as follows: depression management in primary care, interventions to prevent depression, the pathogenesis of depression, comorbidity of depression and other diseases, the risks of depression, neuroimaging studies of depression, and antidepressant treatment.
Analysis of published papers around the world from 2009 to 2019 in depressive disorder. A The total number of papers [from a search of the Web of Science database (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009 – 2019), Articles)]. B The top 10 countries publishing on the topic. C Comparison of papers in China and the USA. D Citations for the top 10 countries and comparison with the global average. E Hot topics.
Analysis of Patented Technology Application
There were 16,228 patent applications in the field of depression between 2009 and 2019, according to the Derwent Innovation Patent database. The annual number and trend of these patents are shown in Fig. 2 A. The top 10 countries applying for patents related to depression are shown in Fig. 2 B. The USA ranks first in the number of depression-related patent applications, followed by China. The largest number of patents related to depression is the development of antidepressants, and drugs for neurodegenerative diseases such as dementia comorbid with depression. The top 10 technological areas of patents related to depression are shown in Fig. 2 C, and the trend in these areas have been stable over the past decade (Fig. 2 D).
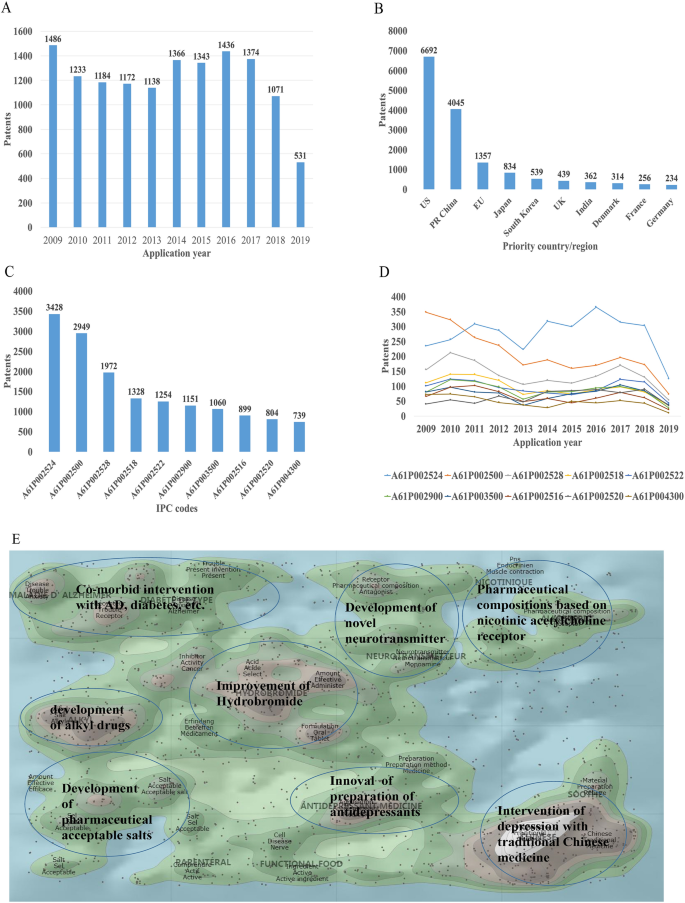
Analysis of patented technology applications from 2009 to 2019 in the field of depressive disorder. A Annual numbers and trends of patents (the Derwent Innovation patent database). B The top 10 countries/regions applying for patents. C The top 10 technological areas of patents. D The trend of patent assignees. E Global hot topic areas of patents.
Analysis of technical hotspots based on keyword clustering was conducted from the Derwent Innovation database using the "ThemeScape" tool. This demonstrated that the hot topic areas are as follows (Fig. 2 E): (1) improvement for formulation and the efficiency of hydrobromide, as well as optimization of the dosage; intervention for depression comorbid with AD, diabetes, and others; (3) development of alkyl drugs; (4) development of pharmaceutical acceptable salts as antidepressants; (5) innovation of the preparation of antidepressants; (6) development of novel antidepressants based on neurotransmitters; (7) development of compositions based on nicotinic acetylcholine receptors; and (8) intervention for depression with traditional Chinese medicine.
Analysis of Clinical Trial
There are 6,516 clinical trials in the field of depression in the ClinicalTrials.gov database, and among them, 1,737 valid trials include the ongoing recruitment of subjects, upcoming recruitment of subjects, and ongoing clinical trials. These clinical trials are mainly distributed in the USA (802 trials), Canada (155), China (114), France (93), Germany (66), UK (62), Spain (58), Denmark (41), Sweden (39), and Switzerland (23). The indications for clinical trials include various types of depression, such as minor depression, depression, severe depression, perinatal depression, postpartum depression, and depression comorbid with other psychiatric disorders or physical diseases, such as schizophrenia, epilepsy, stroke, cancer, diabetes, cardiovascular disease, and Parkinson's disease.
Based on the database of the Chinese Clinical Trial Registry website, a total of 143 clinical trials for depression have been carried out in China. According to the type of research, they are mainly interventional and observational studies, as well as a small number of related factor studies, epidemiological studies, and diagnostic trials. The research content involves postpartum, perinatal, senile, and other age groups with clinical diagnosis (imaging diagnosis) and intervention studies (drugs, acupuncture, electrical stimulation, transcranial magnetic stimulation). It also includes intervention studies on depression comorbid with coronary heart disease, diabetes, and heart failure.
New Medicine Development
According to the Cortellis database, 828 antidepressants were under development by the end of 2019, but only 292 of these are effective and active (Fig. 3 A). Large number of them have been discontinued or made no progress, indicating that the development of new drugs in the field of depression is extremely urgent.
New medicine development from 2009 to 2019 in depressive disorder. A Development status of new candidate drugs. B Top target-based actions.
From the perspective of target-based actions, the most common new drugs are NMDA receptor antagonists, followed by 5-HT targets, as well as dopamine receptor agonists, opioid receptor antagonists and agonists, AMPA receptor modulators, glucocorticoid receptor antagonists, NK1 receptor antagonists, and serotonin transporter inhibitors (Fig. 3 B).
Epidemiology of Depression
The prevalence of depression varies greatly across cultures and countries. Previous surveys have demonstrated that the 12-month prevalence of depression was 0.3% in the Czech Republic, 10% in the USA, 4.5% in Mexico, and 5.2% in West Germany, and the lifetime prevalence of depression was 1.0% in the Czech Republic, 16.9% in the USA, 8.3% in Canada, and 9.0% in Chile [ 4 , 5 ]. A recent meta-analysis including 30 Countries showed that lifetime and 12-month prevalence depression were 10.8% and 7.2%, respectively [ 6 ]. In China, the lifetime prevalence of depression ranged from 1.6% to 5.5% [ 7 , 8 , 9 ]. An epidemiological study demonstrated that depression was the most common mood disorder with a life prevalence of 3.4% and a 12-month prevalence of 2.1% in China [ 10 ].
Some studies have also reported the prevalence in specific populations. The National Comorbidity Survey-Adolescent Supplement (NCS-A) survey in the USA showed that the lifetime and 12-month prevalence of depression in adolescents aged 13 to 18 were 11.0% and 7.5%, respectively [ 11 ]. A recent meta-analysis demonstrated that lifetime prevalence and 12-month prevalence were 2.8% and 2.3%, respectively, among the elderly population in China [ 12 ].
Neurobiological Pathogenesis of Depressive Disorder
The early hypothesis of monoamines in the pathophysiology of depression has been accepted by the scientific community. The evidence that monoamine oxidase inhibitors and tricyclic antidepressants promote monoamine neurotransmission supports this theory of depression [ 13 ]. So far, selective serotonin reuptake inhibitors and norepinephrine reuptake inhibitors are still the first-line antidepressants. However, there remain 1/3 to 2/3 of depressed patients who do not respond satisfactorily to initial antidepressant treatment, and even as many as 15%–40% do not respond to several pharmacological medicines [ 14 , 15 ]. Therefore, the underlying pathogenesis of depression is far beyond the simple monoamine mechanism.
Other hypotheses of depression have gradually received increasing attention because of biomarkers for depression and the effects pharmacological treatments, such as the stress-responsive hypothalamic pituitary adrenal (HPA) axis, neuroendocrine systems, the neurotrophic family of growth factors, and neuroinflammation.
Stress-Responsive HPA Axis
Stress is causative or a contributing factor to depression. Particularly, long-term or chronic stress can lead to dysfunction of the HPA axis and promote the secretion of hormones, including cortisol, adrenocorticotropic hormone, corticotropin-releasing hormone, arginine vasopressin, and vasopressin. About 40%–60% of patients with depression display a disturbed HPA axis, including hypercortisolemia, decreased rhythmicity, and elevated cortisol levels [ 16 , 17 ]. Mounting evidence has shown that stress-induced abnormality of the HPA axis is associated with depression and cognitive impairment, which is due to the increased secretion of cortisol and the insufficient inhibition of glucocorticoid receptor regulatory feedback [ 18 , 19 ]. In addition, it has been reported that the increase in cortisol levels is related to the severity of depression, especially in melancholic depression [ 20 , 21 ]. Further, patients with depression whose HPA axis was not normalized after treatment had a worse clinical response and prognosis [ 22 , 23 ]. Despite the above promising insights, unfortunately previous studies have shown that treatments regulating the HPA axis, such as glucocorticoid receptor antagonists, do not attenuate the symptoms of depressed patients [ 24 , 25 ].
Glutamate Signaling Pathway
Glutamate is the main excitatory neurotransmitter released by synapses in the brain; it is involved in synaptic plasticity, cognitive processes, and reward and emotional processes. Stress can induce presynaptic glutamate secretion by neurons and glutamate strongly binds to ionotropic glutamate receptors (iGluRs) including N-methyl-D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) [ 26 ] on the postsynaptic membrane to activate downstream signal pathways [ 27 ]. Accumulating evidence has suggested that the glutamate system is associated with the incidence of depression. Early studies have shown increased levels of glutamate in the peripheral blood, cerebrospinal fluid, and brain of depressed patients [ 28 , 29 ], as well as NMDAR subunit disturbance in the brain [ 30 , 31 ]. Blocking the function of NMDARs has an antidepressant effect and protects hippocampal neurons from morphological abnormalities induced by stress, while antidepressants reduce glutamate secretion and NMDARs [ 32 ]. Most importantly, NMDAR antagonists such as ketamine have been reported to have profound and rapid antidepressant effects on both animal models and the core symptoms of depressive patients [ 33 ]. On the other hand, ketamine can also increase the AMPAR pathway in hippocampal neurons by up-regulating the AMPA glutamate receptor 1 subunit [ 34 ]. Further, the AMPAR pathway may be involved in the mechanism of antidepressant effects. For example, preclinical studies have indicated that AMPAR antagonists might attenuate lithium-induced depressive behavior by increasing the levels of glutamate receptors 1 and 2 in the mouse hippocampus [ 35 ].

Gamma-Aminobutyric Acid (GABA)
Contrary to glutamate, GABA is the main inhibitory neurotransmitter. Although GABA neurons account for only a small proportion compared to glutamate, inhibitory neurotransmission is essential for brain function by balancing excitatory transmission [ 36 ]. Number of studies have shown that patients with depression have neurotransmission or functional defects of GABA [ 37 , 38 ]. Schür et al ., conducted a meta-analysis of magnetic resonance spectroscopy studies, which showed that the brain GABA level in depressive patients was lower than that in healthy controls, but no difference was found in depressive patients in remission [ 39 ]. Several postmortem studies have shown decreased levels of the GABA synthase glutamic acid decarboxylase in the prefrontal cortex of patients with depression [ 40 , 41 ]. It has been suggested that a functional imbalance of the GABA and glutamate systems contributes to the pathophysiology of depression, and activation of the GABA system might induce antidepressant activity, by which GABA A receptor mediators α2/α3 are considered potential antidepressant candidates [ 42 , 43 ]. Genetic mouse models, such as the GABA A receptor mutant mouse and conditional the Gad1-knockout mouse (GABA in hippocampus and cerebral cortex decreased by 50%) and optogenetic methods have verified that depression-like behavior is induced by changing the level of GABA [ 44 , 45 ].
Neurotrophin Family
The neurotrophin family plays a key role in neuroplasticity and neurogenesis. The neurotrophic hypothesis of depression postulates that a deficit of neurotrophic support leads to neuronal atrophy, the reduction of neurogenesis, and the destruction of glia support, while antidepressants attenuate or reverse these pathophysiological processes [ 46 ]. Among them, the most widely accepted hypothesis involves brain-derived neurotrophic factor (BDNF). This was initially triggered by evidence that stress reduces the BDNF levels in the animal brain, while antidepressants rescue or attenuate this reduction [ 47 , 48 ], and agents involved in the BDNF system have been reported to exert antidepressant-like effects [ 49 , 50 ]. In addition, mounting studies have reported that the BDNF level is decreased in the peripheral blood and at post-mortem in depressive patients, and some have reported that antidepressant treatment normalizes it [ 51 , 52 ]. Furthermore, some evidence also showed that the interaction of BDNF and its receptor gene is associated with treatment-resistant depression [ 15 ].
Recent studies reported that depressed patients have a lower level of the pro-domain of BDNF (BDNF pro-peptide) than controls. This is located presynaptically and promotes long-term depression in the hippocampus, suggesting that it is a promising synaptic regulator [ 53 ].
Neuroinflammation
The immune-inflammation hypothesis has attracted much attention, suggesting that the interactions between inflammatory pathways and neural circuits and neurotransmitters are involved in the pathogenesis and pathophysiological processes of depression. Early evidence found that patients with autoimmune or infectious diseases are more likely to develop depression than the general population [ 54 ]. In addition, individuals without depression may display depressive symptoms after treatment with cytokines or cytokine inducers, while antidepressants relieve these symptoms [ 55 , 56 ]. There is a complex interaction between the peripheral and central immune systems. Previous evidence suggested that peripheral inflammation/infection may spread to the central nervous system in some way and cause a neuroimmune response [ 55 , 57 ]: (1) Some cytokines produced in the peripheral immune response, such as IL-6 and IL-1 β, can leak into the brain through the blood-brain barrier (BBB). (2) Cytokines entering the central nervous system act directly on astrocytes, small stromal cells, and neurons. (3) Some peripheral immune cells can cross the BBB through specific transporters, such as monocytes. (4) Cytokines and chemokines in the circulation activate the central nervous system by regulating the surface receptors of astrocytes and endothelial cells at the BBB. (5) As an intermediary pathway, the immune inflammatory response transmits peripheral danger signals to the center, amplifies the signals, and shows the external phenotype of depressive behavior associated with stress/trauma/infection. (6) Cytokines and chemokines may act directly on neurons, change their plasticity and promote depression-like behavior.
Patients with depression show the core feature of the immune-inflammatory response, that is, increased concentrations of pro-inflammatory cytokines and their receptors, chemokines, and soluble adhesion molecules in peripheral blood and cerebrospinal fluid [ 58 , 59 , 60 ]. Peripheral immune-inflammatory response markers not only change the immune activation state in the brain that affects explicit behavior, but also can be used as an evaluation index or biological index of antidepressant therapy [ 61 , 62 ]. Li et al . showed that the level of TNF-α in patients with depression prior to treatment was higher than that in healthy controls. After treatment with venlafaxine, the level of TNF-α in patients with depression decreased significantly, and the level of TNF-α in the effective group decreased more [ 63 ]. A recent meta-analysis of 1,517 patients found that antidepressants significantly reduced peripheral IL-6, TNF-α, IL-10, and CCL-2, suggesting that antidepressants reduce markers of peripheral inflammatory factors [ 64 ]. Recently, Syed et al . also confirmed that untreated patients with depression had higher levels of inflammatory markers and increased levels of anti-inflammatory cytokines after antidepressant treatment, while increased levels of pro-inflammatory cytokines were found in non-responders [ 62 ]. Clinical studies have also found that anti-inflammatory cytokines, such as monoclonal antibodies and other cytokine inhibitors, may play an antidepressant role by blocking cytokines. The imbalance of pro-inflammatory and anti-inflammatory cytokines may be involved in the pathophysiological process of depression.
In addition, a recent study showed that microglia contribute to neuronal plasticity and neuroimmune interaction that are involved in the pathophysiology of depression [ 65 ]. When activated microglia promote inflammation, especially the excessive production of pro-inflammatory factors and cytotoxins in the central nervous system, depression-like behavior can gradually develop [ 65 , 66 ]. However, microglia change polarization as two types under different inflammatory states, regulating the balance of pro- and anti-inflammatory factors. These two types are M1 and M2 microglia; the former produces large number of pro-inflammatory cytokines after activation, and the latter produces anti-inflammatory cytokines. An imbalance of M1/M2 polarization of microglia may contribute to the pathophysiology of depression [ 67 ].
Microbiome-Gut-Brain Axis
The microbiota-gut-brain axis has recently gained more attention because of its ability to regulate brain activity. Many studies have shown that the microbiota-gut-brain axis plays an important role in regulating mood, behavior, and neuronal transmission in the brain [ 68 , 69 ]. It is well established that comorbidity of depression and gastrointestinal diseases is common [ 70 , 71 ]. Some antidepressants can attenuate the symptoms of patients with irritable bowel syndrome and eating disorders [ 72 ]. It has been reported that gut microbiome alterations are associated with depressive-like behaviors [ 73 , 74 ], and brain function [ 75 ]. Early animal studies have shown that stress can lead to long-term changes in the diversity and composition of intestinal microflora, and is accompanied by depressive behavior [ 76 , 77 ]. Interestingly, some evidence indicates that rodents exhibit depressive behavior after fecal transplants from patients with depression [ 74 ]. On the other hand, some probiotics attenuated depressive-like behavior in animal studies, [ 78 ] and had antidepressant effects on patients with depression in several double-blind, placebo-controlled clinical trials [ 79 , 80 ].
The potential mechanism may be that gut microbiota can interact with the brain through a variety of pathways or systems, including the HPA axis, and the neuroendocrine, autonomic, and neuroimmune systems [ 81 ]. For example, recent evidence demonstrated that gut microbiota can affect the levels of neurotransmitters in the gut and brain, including serotonin, dopamine, noradrenalin, glutamate, and GABA [ 82 ]. In addition, recent studies showed that changes in gut microbiota can also impair the gut barrier and promote higher levels of peripheral inflammatory cytokines [ 83 , 84 ]. Although recent research in this area has made significant progress, more clinical trials are needed to determine whether probiotics have any effect on the treatment of depression and what the potential underlying mechanisms are.
Other Systems and Pathways
There is no doubt that several other systems or pathways are also involved in the pathophysiology of depression, such as oxidant-antioxidant imbalance [ 85 ], mitochondrial dysfunction [ 86 , 87 ], and circadian rhythm-related genes [ 88 ], especially their critical interactions ( e.g. interaction between the HPA and mitochondrial metabolism [ 89 , 90 ], and the reciprocal interaction between oxidative stress and inflammation [ 2 , 85 ]). The pathogenesis of depression is complex and all the hypotheses should be integrated to consider the many interactions between various systems and pathways.
Advances in Various Kinds of Research on Depressive Disorder
Genetic, molecular, and neuroimaging studies continue to increase our understanding of the neurobiological basis of depression. However, it is still not clear to what extent the results of neurobiological studies can help improve the clinical and functional prognosis of patients. Therefore, over the past 10 years, the neurobiological study of depression has become an important measure to understand the pathophysiological mechanism and guide the treatment of depression.
Genetic Studies
Previous twin and adoption studies have indicated that depression has relatively low rate of heritability at 37% [ 91 ]. In addition, environmental factors such as stressful events are also involved in the pathogenesis of depression. Furthermore, complex psychiatric disorders, especially depression, are considered to be polygenic effects that interact with environmental factors [ 13 ]. Therefore, reliable identification of single causative genes for depression has proved to be challenging. The first genome-wide association studies (GWAS) for depression was published in 2009, and included 1,738 patients and 1,802 controls [ 92 , 93 ]. Although many subsequent GWASs have determined susceptible genes in the past decade, the impact of individual genes is so small that few results can be replicated [ 94 , 95 ]. So far, it is widely accepted that specific single genetic mutations may play minor and marginal roles in complex polygenic depression. Another major recognition in GWASs over the past decade is that prevalent candidate genes are usually not associated with depression. Further, the inconsistent results may also be due to the heterogeneity and polygenic nature of genetic and non-genetic risk factors for depression as well as the heterogeneity of depression subtypes [ 95 , 96 ]. Therefore, to date, the quality of research has been improved in two aspects: (1) the sample size has been maximized by combining the data of different evaluation models; and (2) more homogenous subtypes of depression have been selected to reduce phenotypic heterogeneity [ 97 ]. Levinson et al . pointed out that more than 75,000 to 100,000 cases should be considered to detect multiple depression associations [ 95 ]. Subsequently, several recent GWASs with larger sample sizes have been conducted. For example, Okbay et al . identified two loci associated with depression and replicated them in separate depression samples [ 98 ]. Wray et al . also found 44 risk loci associated with depression based on 135,458 cases and 344,901 controls [ 99 ]. A recent GWAS of 807,553 individuals with depression reported that 102 independent variants were associated with depression; these were involved in synaptic structure and neural transmission, and were verified in a further 1,507,153 individuals [ 100 ]. However, even with enough samples, GWASs still face severe challenges. A GWAS only marks the region of the genome and is not directly related to the potential biological function. In addition, a genetic association with the indicative phenotype of depression may only be part of many pathogenic pathways, or due to the indirect influence of intermediate traits in the causal pathway on the final result [ 101 ].
Given the diversity of findings, epigenetic factors are now being investigated. Recent studies indicated that epigenetic mechanisms may be the potential causes of "loss of heritability" in GWASs of depression. Over the past decade, a promising discovery has been that the effects of genetic information can be directly influenced by environment factors, and several specific genes are activated by environmental aspects. This process is described as interactions between genes and the environment, which is identified by the epigenetic mechanism. Environmental stressors cause alterations in gene expression in the brain, which may cause abnormal neuronal plasticity in areas related to the pathogenesis of the disease. Epigenetic events alter the structure of chromatin, thereby regulating gene expression involved in neuronal plasticity, stress behavior, depressive behavior, and antidepressant responses, including DNA methylation, histone acetylation, and the role of non-coding RNA. These new mechanisms of trans-generational transmission of epigenetic markers are considered a supplement to orthodox genetic heredity, providing the possibility for the discovery of new treatments for depression [ 102 , 103 ]. Recent studies imply that life experiences, including stress and enrichment, may affect cellular and molecular signaling pathways in sperm and influence the behavioral and physiological phenotypes of offspring in gender-specific patterns, which may also play an important role in the development of depression [ 103 ].
Brain Imaging and Neuroimaging Studies
Neuroimaging, including magnetic resonance imaging (MRI) and molecular imaging, provides a non-invasive technique for determining the underlying etiology and individualized treatment for depression. MRI can provide important data on brain structure, function, networks, and metabolism in patients with depression; it includes structural MRI (sMRI), functional MRI (fMRI), diffusion tensor imaging, and magnetic resonance spectroscopy.
Previous sMRI studies have found damaged gray matter in depression-associated brain areas, including the frontal lobe, anterior cingulate gyrus, hippocampus, putamen, thalamus, and amygdala. sMRI focuses on the thickness of gray matter and brain morphology [ 104 , 105 ]. A recent meta-analysis of 2,702 elderly patients with depression and 11,165 controls demonstrated that the volumes of the whole brain and hippocampus of patients with depression were lower than those of the control group [ 106 ]. Some evidence also showed that the hippocampal volume in depressive patients was lower than that of controls, and increased after treatment with antidepressants [ 107 ] and electroconvulsive therapy (ECT) [ 108 ], suggesting that the hippocampal volume plays a critical role in the development, treatment response, and clinical prognosis of depression. A recent study also reported that ECT increased the volume of the right hippocampus, amygdala, and putamen in patients with treatment-resistant depression [ 109 ]. In addition, postmortem research supported the MRI study showing that dentate gyrus volume was decreased in drug-naive patients with depression compared to healthy controls, and was potentially reversed by treatment with antidepressants [ 110 ].
Diffusion tensor imaging detects the microstructure of the white matter, which has been reported impaired in patients with depression [ 111 ]. A recent meta-analysis that included first-episode and drug-naïve depressive patients showed that the decrease in fractional anisotropy was negatively associated with illness duration and clinical severity [ 112 ].
fMRI, including resting-state and task-based fMRI, can divide the brain into self-related regions, such as the anterior cingulate cortex, posterior cingulate cortex, medial prefrontal cortex, precuneus, and dorsomedial thalamus. Many previous studies have shown the disturbance of several brain areas and intrinsic neural networks in patients with depression which could be rescued by antidepressants [ 113 , 114 , 115 , 116 ]. Further, some evidence also showed an association between brain network dysfunction and the clinical correlates of patients with depression, including clinical symptoms [ 117 ] and the response to antidepressants [ 118 , 119 ], ECT [ 120 , 121 ], and repetitive transcranial magnetic stimulation [ 122 ].
It is worth noting that brain imaging provides new insights into the large-scale brain circuits that underlie the pathophysiology of depressive disorder. In such studies, large-scale circuits are often referred to as “networks”. There is evidence that a variety of circuits are involved in the mechanisms of depressive disorder, including disruption of the default mode, salience, affective, reward, attention, and cognitive control circuits [ 123 ]. Over the past decade, the study of intra-circuit and inter-circuit connectivity dysfunctions in depression has escalated, in part due to advances in precision imaging and analysis techniques [ 124 ]. Circuit dysfunction is a potential biomarker to guide psychopharmacological treatment. For example, Williams et al . found that hyper-activation of the amygdala is associated with a negative phenotype that can predict the response to antidepressants [ 125 ]. Hou et al . showed that the baseline characteristics of the reward circuit predict early antidepressant responses [ 126 ].
Molecular imaging studies, including single photon emission computed tomography and positron emission tomography, focus on metabolic aspects such as amino-acids, neurotransmitters, glucose, and lipids at the cellular level in patients with depression. A recent meta-analysis examined glucose metabolism and found that glucose uptake dysfunction in different brain regions predicts the treatment response [ 127 ].
The most important and promising studies were conducted by the ENIGMA (Enhancing NeuroImaging Genetics through Meta Analysis) Consortium, which investigated the human brain across 43 countries. The ENIGMA-MDD Working Group was launched in 2012 to detect the structural and functional changes associated with MDD reliably and replicate them in various samples around the world [ 128 ]. So far, the ENIGMA-MDD Working Group has collected data from 4,372 MDD patients and 9,788 healthy controls across 14 countries, including 45 cohorts [ 128 ]. Their findings to date are shown in Table 1 [ 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 ].
Objective Index for Diagnosis of MDD
To date, the clinical diagnosis of depression is subjectively based on interviews according to diagnostic criteria ( e.g. International Classification of Diseases and Diagnostic and Statistical Manual diagnostic systems) and the severity of clinical symptoms are assessed by questionnaires, although patients may experience considerable differences in symptoms and subtypes [ 138 ]. Meanwhile, biomarkers including genetics, epigenetics, peripheral gene and protein expression, and neuroimaging markers may provide a promising supplement for the development of the objective diagnosis of MDD, [ 139 , 140 , 141 ]. However, the development of reliable diagnosis for MDD using biomarkers is still difficult and elusive, and all methods based on a single marker are insufficiently specific and sensitive for clinical use [ 142 ]. Papakostas et al . showed that a multi-assay, serum-based test including nine peripheral biomarkers (soluble tumor necrosis factor alpha receptor type II, resistin, prolactin, myeloperoxidase, epidermal growth factor, BDNF, alpha1 antitrypsin, apolipoprotein CIII, brain-derived neurotrophic factor, and cortisol) yielded a specificity of 81.3% and a sensitivity of 91.7% [ 142 ]. However, the sample size was relatively small and no other studies have yet validated their results. Therefore, further studies are needed to identify biomarker models that integrate all biological variables and clinical features to improve the specificity and sensitivity of diagnosis for MDD.
Management of Depression
The treatment strategies for depression consist of pharmacological treatment and non-pharmacological treatments including psychotherapy, ECT [ 98 ], and transcranial magnetic stimulation. As psychotherapy has been shown to have effects on depression including attenuating depressive symptoms and improving the quality of life [ 143 , 144 ]; several practice guidelines are increasingly recommending psychotherapy as a monotherapy or in combination with antidepressants [ 145 , 146 ].
Current Antidepressant Treatment
Antidepressants approved by the US Food and Drug Administration (FDA) are shown in Table 2 . Due to the relatively limited understanding of the etiology and pathophysiology of depression, almost all the previous antidepressants were discovered by accident a few decades ago. Although most antidepressants are usually safe and effective, there are still some limitations, including delayed efficacy (usually 2 weeks) and side-effects that affect the treatment compliance [ 147 ]. In addition, <50% of all patients with depression show complete remission through optimized treatment, including trials of multiple drugs with and without simultaneous psychotherapy. In the past few decades, most antidepressant discoveries focused on finding faster, safer, and more selective serotonin or norepinephrine receptor targets. In addition, there is an urgent need to develop new approaches to obtain more effective, safer, and faster antidepressants. In 2019, the FDA approved two new antidepressants: Esketamine for refractory depression and Bresanolone for postpartum depression. Esmolamine, a derivative of the anesthetic drug ketamine, was approved by the FDA for the treatment of refractory depression, based on a large number of preliminary clinical studies [ 148 ]. For example, several randomized controlled trials and meta-analysis studies showed the efficacy and safety of Esketamine in depression or treatment-resistant depression [ 26 , 149 , 150 ]. Although both are groundbreaking new interventions for these debilitating diseases and both are approved for use only under medical supervision, there are still concerns about potential misuse and problems in the evaluation of mental disorders [ 151 ].
To date, although several potential drugs have not yet been approved by the FDA, they are key milestones in the development of antidepressants that may be modified and used clinically in the future, such as compounds containing dextromethorphan (a non-selective NMDAR antago–nist), sarcosine (N-methylglycine, a glycine reuptake inhibitor), AMPAR modulators, and mGluR modulators [ 152 ].
Neuromodulation Therapy
Neuromodulation therapy acts through magnetic pulse, micro-current, or neural feedback technology within the treatment dose, acting on the central or peripheral nervous system to regulate the excitatory/inhibitory activity to reduce or attenuate the symptoms of the disease.
ECT is one of most effective treatments for depression, with the implementation of safer equipment and advancement of techniques such as modified ECT [ 153 ]. Mounting evidence from randomized controlled trial (RCT) and meta-analysis studies has shown that rTMS can treat depressive patients with safety [ 154 ]. Other promising treatments for depression have emerged, such as transcranial direct current stimulation (tDCS) [ 155 ], transcranial alternating current stimulation (tACS)[ 156 ], vagal nerve stimulation [ 157 ], deep brain stimulation [ 158 ] , and light therapy [ 159 ], but some of them are still experimental to some extent and have not been widely used. For example, compared to tDCS, tACS displays less sensory experience and adverse reactions with weak electrical current in a sine-wave pattern, but the evidence for the efficacy of tACS in the treatment of depression is still limited [ 160 ]. Alexander et al . recently demonstrated that there was no difference in efficacy among different treatments (sham, 10-Hz and 40-Hz tACS). However, only the 10-Hz tACS group had more responders than the sham and 40-Hz tACS groups at week 2 [ 156 ]. Further RCT studies are needed to verify the efficacy of tACS. In addition, the mechanism of the effect of neuromodulation therapy on depression needs to be further investigated.
Precision Medicine for Depression
Optimizing the treatment strategy is an effective way to improve the therapeutic effect on depression. However, each individual with depression may react very differently to different treatments. Therefore, this raises the question of personalized treatment, that is, which patients are suitable for which treatment. Over the past decade, psychiatrists and psychologists have focused on individual biomarkers and clinical characteristics to predict the efficiency of antidepressants and psychotherapies, including genetics, peripheral protein expression, electrophysiology, neuroimaging, neurocognitive performance, developmental trauma, and personality [ 161 ]. For example, Bradley et al . recently conducted a 12-week RCT, which demonstrated that the response rate and remission rates of the pharmacogenetic guidance group were significantly higher than those of the non-pharmacogenetic guidance group [ 162 ].
Subsequently, Greden et al . conducted an 8-week RCT of Genomics Used to Improve Depression Decisions (GUIDED) on 1,167 MDD patients and demonstrated that although there was no difference in symptom improvement between the pharmacogenomics-guided and non- pharmacogenomics-guided groups, the response rate and remission rate of the pharmacogenomics-guided group increased significantly [ 163 ].
A recent meta-analysis has shown that the baseline default mode network connectivity in patients with depression can predict the clinical responses to treatments including cognitive behavioral therapy, pharmacotherapy, ECT, rTMS, and transcutaneous vagus nerve stimulation [ 164 ]. However, so far, the biomarkers that predict treatment response at the individual level have not been well applied in the clinic, and there is still a lot of work to be conducted in the future.
Future Perspectives
Although considerable progress has been made in the study of depression during a past decade, the heterogeneity of the disease, the effectiveness of treatment, and the gap in translational medicine are critical challenges. The main dilemma is that our understanding of the etiology and pathophysiology of depression is inadequate, so our understanding of depression is not deep enough to develop more effective treatment. Animal models still cannot fully simulate this heterogeneous and complex mental disorder. Therefore, how to effectively match the indicators measured in animals with those measured in genetic research or the development of new antidepressants is another important challenge.
Change history
17 may 2021.
A Correction to this paper has been published: https://doi.org/10.1007/s12264-021-00694-9
Dadi AF, Miller ER, Bisetegn TA, Mwanri L. Global burden of antenatal depression and its association with adverse birth outcomes: an umbrella review. BMC Public Health 2020, 20: 173
Article PubMed PubMed Central Google Scholar
Zhu S, Zhao L, Fan Y, Lv Q, Wu K, Lang X. Interaction between TNF-alpha and oxidative stress status in first-episode drug-naive schizophrenia. Psychoneuroendocrinology 2020, 114: 104595
Article CAS PubMed Google Scholar
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390: 1211–1259
Article Google Scholar
Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res 2003, 12: 3–21
Article PubMed Google Scholar
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health 2013, 34: 119–138
Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep 2018, 8: 2861
Liu J, Yan F, Ma X, Guo HL, Tang YL, Rakofsky JJ, et al. Prevalence of major depressive disorder and socio-demographic correlates: Results of a representative household epidemiological survey in Beijing, China. J Affect Disord 2015, 179: 74–81
Zhang YS, Rao WW, Cui LJ, Li JF, Li L, Ng CH, et al. Prevalence of major depressive disorder and its socio-demographic correlates in the general adult population in Hebei province, China. J Affect Disord 2019, 252: 92–98
Ma X, Xiang YT, Cai ZJ, Li SR, Xiang YQ, Guo HL, et al. Prevalence and socio-demographic correlates of major depressive episode in rural and urban areas of Beijing, China. J Affect Disord 2009, 115: 323–330
Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 2019, 6: 211–224
Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 2015, 54(37–44): e32
Google Scholar
Wang F, Zhang QE, Zhang L, Ng CH, Ungvari GS, Yuan Z, et al. Prevalence of major depressive disorder in older adults in China: A systematic review and meta-analysis. J Affect Disord 2018, 241: 297–304
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature 2008, 455: 894–902
Article CAS PubMed PubMed Central Google Scholar
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006, 163: 1905–1917
Li Z, Zhang Y, Wang Z, Chen J, Fan J, Guan Y, et al. The role of BDNF, NTRK2 gene and their interaction in development of treatment-resistant depression: data from multicenter, prospective, longitudinal clinic practice. J Psychiatr Res 2013, 47: 8–14
Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol 1991, 38: 537–559
Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci 2008, 31: 464–468
Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry 2017, 22: 527–536
Gomez RG, Fleming SH, Keller J, Flores B, Kenna H, DeBattista C, et al. The neuropsychological profile of psychotic major depression and its relation to cortisol. Biol Psychiatry 2006, 60: 472–478
Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013, 18: 692–699
Nandam LS, Brazel M, Zhou M, Jhaveri DJ. Cortisol and major depressive disorder-translating findings from humans to animal models and back. Front Psychiatry 2019, 10: 974
Owashi T, Otsubo T, Oshima A, Nakagome K, Higuchi T, Kamijima K. Longitudinal neuroendocrine changes assessed by dexamethasone/CRH and growth hormone releasing hormone tests in psychotic depression. Psychoneuroendocrinology 2008, 33: 152–161
Mickey BJ, Ginsburg Y, Sitzmann AF, Grayhack C, Sen S, Kirschbaum C, et al. Cortisol trajectory, melancholia, and response to electroconvulsive therapy. J Psychiatr Res 2018, 103: 46–53
Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011, 73: 114–126
Aubry JM. CRF system and mood disorders. J Chem Neuroanat 2013, 54: 20–24
Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: A randomized, double-blind, non-inferiority study. J Affect Disord 2020, 264: 527–534
Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 2012, 35: 47–56
Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 2008, 7: 426–437
Hashimoto K. The role of glutamate on the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 2011, 35: 1558–1568
Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 2015, 20: 1057–1068
Chandley MJ, Szebeni A, Szebeni K, Crawford JD, Stockmeier CA, Turecki G, et al. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int J Neuropsychopharmacol 2014, 17: 1569–1578
Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry 2013, 73: 1180–1188
Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr. Glutamatergic Neurotransmission: Pathway to Developing Novel Rapid-Acting Antidepressant Treatments. Int J Neuropsychopharmacol 2019, 22: 119–135
Beurel E, Grieco SF, Amadei C, Downey K, Jope RS. Ketamine-induced inhibition of glycogen synthase kinase-3 contributes to the augmentation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor signaling. Bipolar Disord 2016, 18: 473–480
Gould TD, O’Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology 2008, 54: 577–587
Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102: 75–90
Ghosal S, Hare B, Duman RS. Prefrontal Cortex GABAergic Deficits and Circuit Dysfunction in the Pathophysiology and Treatment of Chronic Stress and Depression. Curr Opin Behav Sci 2017, 14: 1–8
Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: Cortical microcircuit and therapeutic perspectives. Biol Psychiatry 2017, 82: 549–559
Schur RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joels M, et al. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp 2016, 37: 3337–3352
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 2012, 17: 1130–1142
Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 2010, 13: 411–420
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry 2017, 81: 886–897
Chen X, van Gerven J, Cohen A, Jacobs G. Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety. Acta Pharmacol Sin 2019, 40: 571–582
Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry 2016, 80: 457–468
Kolata SM, Nakao K, Jeevakumar V, Farmer-Alroth EL, Fujita Y, Bartley AF, et al. Neuropsychiatric phenotypes produced by GABA reduction in mouse cortex and hippocampus. Neuropsychopharmacology 2018, 43: 1445–1456
Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 2012, 367: 2475–2484
Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos Trans R Soc Lond B Biol Sci 2012, 367: 2378–2381
Li K, Shen S, Ji YT, Li XY, Zhang LS, Wang XD. Melatonin augments the effects of fluoxetine on depression-like behavior and hippocampal BDNF-TrkB signaling. Neurosci Bull 2018, 34: 303–311
Zhang JJ, Gao TT, Wang Y, Wang JL, Guan W, Wang YJ, et al. Andrographolide exerts significant antidepressant-like effects involving the hippocampal BDNF system in mice. Int J Neuropsychopharmacol 2019, 22: 585–600
Wang JQ, Mao L. The ERK pathway: Molecular mechanisms and treatment of depression. Mol Neurobiol 2019, 56: 6197–6205
Chiou YJ, Huang TL. Serum brain-derived neurotrophic factors in taiwanese patients with drug-naive first-episode major depressive disorder: Effects of antidepressants. Int J Neuropsychopharmacol 2017, 20: 213–218
CAS PubMed Google Scholar
Youssef MM, Underwood MD, Huang YY, Hsiung SC, Liu Y, Simpson NR, et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int J Neuropsychopharmacol 2018, 21: 528–538
Kojima M, Matsui K, Mizui T. BDNF pro-peptide: physiological mechanisms and implications for depression. Cell Tissue Res 2019, 377: 73–79
Jeon SW, Kim YK. Inflammation-induced depression: Its pathophysiology and therapeutic implications. J Neuroimmunol 2017, 313: 92–98
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016, 16: 22–34
Zhao G, Liu X. Neuroimmune advance in depressive disorder. Adv Exp Med Biol 2019, 1180: 85–98
Li Z, Wang Z, Zhang C, Chen J, Su Y, Huang J, et al. Reduced ENA78 levels as novel biomarker for major depressive disorder and venlafaxine efficiency: Result from a prospective longitudinal study. Psychoneuroendocrinology 2017, 81: 113–121
Ambrosio G, Kaufmann FN, Manosso L, Platt N, Ghisleni G, Rodrigues ALS, et al. Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology 2018, 91: 132–141
Mao R, Zhang C, Chen J, Zhao G, Zhou R, Wang F, et al. Different levels of pro- and anti-inflammatory cytokines in patients with unipolar and bipolar depression. J Affect Disord 2018, 237: 65–72
Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 2019, 81: 24–40
Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 2018, 95: 43–49
Syed SA, Beurel E, Loewenstein DA, Lowell JA, Craighead WE, Dunlop BW, et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron 2018, 99(914–924): e913
Li Z, Qi D, Chen J, Zhang C, Yi Z, Yuan C, et al. Venlafaxine inhibits the upregulation of plasma tumor necrosis factor-alpha (TNF-alpha) in the Chinese patients with major depressive disorder: a prospective longitudinal study. Psychoneuroendocrinology 2013, 38: 107–114
Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol 2018, 55: 4195–4206
Innes S, Pariante CM, Borsini A. Microglial-driven changes in synaptic plasticity: A possible role in major depressive disorder. Psychoneuroendocrinology 2019, 102: 236–247
Sochocka M, Diniz BS, Leszek J. Inflammatory response in the CNS: Friend or foe? Mol Neurobiol 2017, 54: 8071–8089
Zhang L, Zhang J, You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci 2018, 12: 306
Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 2017, 179: 223–244
Gonzalez-Arancibia C, Urrutia-Pinones J, Illanes-Gonzalez J, Martinez-Pinto J, Sotomayor-Zarate R, Julio-Pieper M, et al. Do your gut microbes affect your brain dopamine? Psychopharmacology (Berl) 2019, 236: 1611–1622
Article CAS Google Scholar
Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol 2014, 20: 14105–14125
Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002, 122: 1140–1156
Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2011: CD003460.
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep 2017, 7: 43859
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016, 21: 786–796
Curtis K, Stewart CJ, Robinson M, Molfese DL, Gosnell SN, Kosten TR, et al. Insular resting state functional connectivity is associated with gut microbiota diversity. Eur J Neurosci 2019, 50: 2446–2452
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 2009, 65: 263–267
Garcia-Rodenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, et al. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr 2006, 43: 16–24
Hao Z, Wang W, Guo R, Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 2019, 104: 132–142
Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2: 256–261
Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100: 213–222
Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013, 36: 305–312
Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J Cell Physiol 2017, 232: 2359–2372
Diviccaro S, Giatti S, Borgo F, Barcella M, Borghi E, Trejo JL, et al. Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology 2019, 99: 206–215
Kiecolt-Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, et al. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98: 52–60
Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76: 197–205
Czarny P, Wigner P, Galecki P, Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry 2018, 80: 309–321
Xie X, Yu C, Zhou J, Xiao Q, Shen Q, Xiong Z, et al. Nicotinamide mononucleotide ameliorates the depression-like behaviors and is associated with attenuating the disruption of mitochondrial bioenergetics in depressed mice. J Affect Disord 2020, 263: 166–174
Wang XL, Yuan K, Zhang W, Li SX, Gao GF, Lu L. Regulation of circadian genes by the MAPK pathway: Implications for rapid antidepressant action. Neurosci Bull 2020, 36: 66–76
Xie X, Shen Q, Yu C, Xiao Q, Zhou J, Xiong Z, et al. Depression-like behaviors are accompanied by disrupted mitochondrial energy metabolism in chronic corticosterone-induced mice. J Steroid Biochem Mol Biol 2020, 200: 105607
Zhang LF, Shi L, Liu H, Meng FT, Liu YJ, Wu HM, et al. Increased hippocampal tau phosphorylation and axonal mitochondrial transport in a mouse model of chronic stress. Int J Neuropsychopharmacol 2012, 15: 337–348
Flint J, Kendler KS. The genetics of major depression. Neuron 2014, 81: 1214
Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry 2009, 14: 359–375
Dunn EC, Brown RC, Dai Y, Rosand J, Nugent NR, Amstadter AB, et al. Genetic determinants of depression: recent findings and future directions. Harv Rev Psychiatry 2015, 23: 1–18
Major Depressive Disorder Working Group of the Psychiatric GC, Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry 2013, 18: 497–511.
Levinson DF, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, Wray NR, et al. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol Psychiatry 2014, 76: 510–512
Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Borglum AD, Breen G, et al. Psychiatric genomics: An update and an agenda. Am J Psychiatry 2018, 175: 15–27
Schwabe I, Milaneschi Y, Gerring Z, Sullivan PF, Schulte E, Suppli NP, et al. Unraveling the genetic architecture of major depressive disorder: merits and pitfalls of the approaches used in genome-wide association studies. Psychol Med 2019, 49: 2646–2656
Okbay A, Baselmans BM, De Neve JE, Turley P, Nivard MG, Fontana MA, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 2016, 48: 624–633
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018, 50: 668–681
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019, 22: 343–352
Ormel J, Hartman CA, Snieder H. The genetics of depression: successful genome-wide association studies introduce new challenges. Transl Psychiatry 2019, 9: 114
Uchida S, Yamagata H, Seki T, Watanabe Y. Epigenetic mechanisms of major depression: Targeting neuronal plasticity. Psychiatry Clin Neurosci 2018, 72: 212–227
Yeshurun S, Hannan AJ. Transgenerational epigenetic influences of paternal environmental exposures on brain function and predisposition to psychiatric disorders. Mol Psychiatry 2019, 24: 536–548
van Eijndhoven P, van Wingen G, Katzenbauer M, Groen W, Tepest R, Fernandez G, et al. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am J Psychiatry 2013, 170: 1477–1486
Peng W, Chen Z, Yin L, Jia Z, Gong Q. Essential brain structural alterations in major depressive disorder: A voxel-wise meta-analysis on first episode, medication-naive patients. J Affect Disord 2016, 199: 114–123
Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: A systematic review and meta-analysis. Biol Psychiatry 2017, 82: 339–350
Maller JJ, Broadhouse K, Rush AJ, Gordon E, Koslow S, Grieve SM. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Mol Psychiatry 2018, 23: 1737–1744
Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry 2016, 79: 282–292
Gryglewski G, Baldinger-Melich P, Seiger R, Godbersen GM, Michenthaler P, Klobl M, et al. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br J Psychiatry 2019, 214: 159–167
Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 2013, 38: 1068–1077
Liang S, Wang Q, Kong X, Deng W, Yang X, Li X, et al. White matter abnormalities in major depression biotypes identified by diffusion tensor imaging. Neurosci Bull 2019, 35: 867–876
Chen G, Guo Y, Zhu H, Kuang W, Bi F, Ai H, et al. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: A voxel-based meta-analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry 2017, 76: 179–187
Brakowski J, Spinelli S, Dorig N, Bosch OG, Manoliu A, Holtforth MG, et al. Resting state brain network function in major depression - Depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res 2017, 92: 147–159
Dutta A, McKie S, Downey D, Thomas E, Juhasz G, Arnone D, et al. Regional default mode network connectivity in major depressive disorder: modulation by acute intravenous citalopram. Transl Psychiatry 2019, 9: 116
Meyer BM, Rabl U, Huemer J, Bartova L, Kalcher K, Provenzano J, et al. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl Psychiatry 2019, 9: 64
Muller VI, Cieslik EC, Serbanescu I, Laird AR, Fox PT, Eickhoff SB. Altered brain activity in unipolar depression revisited: Meta-analyses of neuroimaging studies. JAMA Psychiatry 2017, 74: 47–55
Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, et al. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord 2017, 207: 86–94
Godlewska BR, Browning M, Norbury R, Igoumenou A, Cowen PJ, Harmer CJ. Predicting treatment response in depression: The role of anterior cingulate cortex. Int J Neuropsychopharmacol 2018, 21: 988–996
Goldstein-Piekarski AN, Staveland BR, Ball TM, Yesavage J, Korgaonkar MS, Williams LM. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl Psychiatry 2018, 8: 57
Leaver AM, Vasavada M, Joshi SH, Wade B, Woods RP, Espinoza R, et al. Mechanisms of antidepressant response to electroconvulsive therapy studied with perfusion magnetic resonance imaging. Biol Psychiatry 2019, 85: 466–476
Miskowiak KW, Macoveanu J, Jorgensen MB, Stottrup MM, Ott CV, Jensen HM, et al. Neural response after a single ECT session during retrieval of emotional self-referent words in depression: A randomized, sham-controlled fMRI study. Int J Neuropsychopharmacol 2018, 21: 226–235
Du L, Liu H, Du W, Chao F, Zhang L, Wang K, et al. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry 2018, 7: 3
Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety 2017, 34: 9–24
Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016, 3: 472–480
Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology 2015, 40: 2398–2408
Hou Z, Gong L, Zhi M, Yin Y, Zhang Y, Xie C, et al. Distinctive pretreatment features of bilateral nucleus accumbens networks predict early response to antidepressants in major depressive disorder. Brain Imaging Behav 2018, 12: 1042–1052
De Crescenzo F, Ciliberto M, Menghini D, Treglia G, Ebmeier KP, Janiri L. Is (18)F-FDG-PET suitable to predict clinical response to the treatment of geriatric depression? A systematic review of PET studies. Aging Ment Health 2017, 21: 889–894
Schmaal L, Pozzi E, T CH, van Velzen LS, Veer IM, Opel N, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry 2020, 10: 172.
Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016, 21: 806–812
Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017, 22: 900–909
Frodl T, Janowitz D, Schmaal L, Tozzi L, Dobrowolny H, Stein DJ, et al. Childhood adversity impacts on brain subcortical structures relevant to depression. J Psychiatr Res 2017, 86: 58–65
Renteria ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry 2017, 7: e1116
Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med 2020, 50: 1020–1031
de Kovel CGF, Aftanas L, Aleman A, Alexander-Bloch AF, Baune BT, Brack I, et al. No alterations of brain structural asymmetry in major depressive disorder: An ENIGMA consortium analysis. Am J Psychiatry 2019, 176: 1039–1049
Ho TC, Gutman B, Pozzi E, Grabe HJ, Hosten N, Wittfeld K, et al. Subcortical shape alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Hum Brain Mapp 2020.
Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry 2020.
van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry 2020, 25: 1511–1525
Lv X, Si T, Wang G, Wang H, Liu Q, Hu C, et al. The establishment of the objective diagnostic markers and personalized medical intervention in patients with major depressive disorder: rationale and protocol. BMC Psychiatry 2016, 16: 240
Fabbri C, Hosak L, Mossner R, Giegling I, Mandelli L, Bellivier F, et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J Biol Psychiatry 2017, 18: 5–28
Li Z, Zhang C, Fan J, Yuan C, Huang J, Chen J, et al. Brain-derived neurotrophic factor levels and bipolar disorder in patients in their first depressive episode: 3-year prospective longitudinal study. Br J Psychiatry 2014, 205: 29–35
Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry 2015, 77: 223–235
Papakostas GI, Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, et al. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: a pilot and replication study. Mol Psychiatry 2013, 18: 332–339
Kolovos S, Kleiboer A, Cuijpers P. Effect of psychotherapy for depression on quality of life: meta-analysis. Br J Psychiatry 2016, 209: 460–468
Park LT, Zarate CA Jr. Depression in the primary care setting. N Engl J Med 2019, 380: 559–568
Health N C C F M . Depression: The treatment and management of depression in adults (updated edition) 2010.
Qaseem A, Barry MJ, Kansagara D. Clinical Guidelines Committee of the American College of P. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: A clinical practice guideline from the american college of physicians. Ann Intern Med 2016, 164: 350–359
Dodd S, Mitchell PB, Bauer M, Yatham L, Young AH, Kennedy SH, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: An international consensus statement. World J Biol Psychiatry 2018, 19: 330–348
Cristea IA, Naudet F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 2019, 6: 975–977
Zheng W, Cai DB, Xiang YQ, Zheng W, Jiang WL, Sim K, et al. Adjunctive intranasal esketamine for major depressive disorder: A systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 2020, 265: 63–70
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A Randomized Double-Blind Active-Controlled Study. Am J Psychiatry 2019, 176: 428–438
Turner EH. Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry 2019, 6: 977–979
Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 2017, 16: 472–486
Gill SP, Kellner CH. Clinical practice recommendations for continuation and maintenance electroconvulsive therapy for depression: Outcomes from a review of the evidence and a consensus workshop held in Australia in May 2017. J ECT 2019, 35: 14–20
McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry 2018, 79.
Chase HW, Boudewyn MA, Carter CS, Phillips ML. Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry 2020, 25: 397–407
Alexander ML, Alagapan S, Lugo CE, Mellin JM, Lustenberger C, Rubinow DR, et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl Psychiatry 2019, 9: 106
Akhtar H, Bukhari F, Nazir M, Anwar MN, Shahzad A. Therapeutic efficacy of neurostimulation for depression: Techniques, current modalities, and future challenges. Neurosci Bull 2016, 32: 115–126
Zhou C, Zhang H, Qin Y, Tian T, Xu B, Chen J, et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 2018, 82: 224–232
Li X, Li X. The antidepressant effect of light therapy from retinal projections. Neurosci Bull 2018, 34: 359–368
Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia: A systematic review. Ann Intern Med 2018, 168: 414–421
Kessler RC. The potential of predictive analytics to provide clinical decision support in depression treatment planning. Curr Opin Psychiatry 2018, 31: 32–39
Bradley P, Shiekh M, Mehra V, Vrbicky K, Layle S, Olson MC, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: A randomized clinical trial demonstrating clinical utility. J Psychiatr Res 2018, 96: 100–107
Greden JF, Parikh SV, Rothschild AJ, Thase ME, Dunlop BW, DeBattista C, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res 2019, 111: 59–67
Long Z, Du L, Zhao J, Wu S, Zheng Q, Lei X. Prediction on treatment improvement in depression with resting state connectivity: A coordinate-based meta-analysis. J Affect Disord 2020, 276: 62–68
Download references
Acknowledgments
This review was supported by the National Basic Research Development Program of China (2016YFC1307100), the National Natural Science Foundation of China (81930033 and 81771465; 81401127), Shanghai Key Project of Science & Technology (2018SHZDZX05), Shanghai Jiao Tong University Medical Engineering Foundation (YG2016MS48), Shanghai Jiao Tong University School of Medicine (19XJ11006), the Sanming Project of Medicine in Shenzhen Municipality (SZSM201612006), the National Key Technologies R&D Program of China (2012BAI01B04), and the Innovative Research Team of High-level Local Universities in Shanghai.
Author information
Authors and affiliations.
Clinical Research Center and Division of Mood Disorders of Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, 200030, China
Zezhi Li, Jun Chen & Yiru Fang
Department of Neurology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
Shanghai Institute of Nutrition and Health, Shanghai Information Center for Life Sciences, Chinese Academy of Science, Shanghai, 200031, China
Meihua Ruan
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Science, Shanghai, 200031, China
Shanghai Key Laboratory of Psychotic Disorders, Shanghai, 201108, China
Jun Chen & Yiru Fang
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yiru Fang .
Ethics declarations
Conflicts of interest.
The authors declare no conflicts of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Li, Z., Ruan, M., Chen, J. et al. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 37 , 863–880 (2021). https://doi.org/10.1007/s12264-021-00638-3
Download citation
Received : 30 May 2020
Accepted : 30 September 2020
Published : 13 February 2021
Issue Date : June 2021
DOI : https://doi.org/10.1007/s12264-021-00638-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Major depressive disorder
- Pathogenesis
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 May 2024
The role of bipolar disorder and family wealth in choosing creative occupations
- Barbara Biasi 1 , 2 ,
- Michael S. Dahl 3 , 4 , 5 &
- Petra Moser 2 , 6
Scientific Reports volume 14 , Article number: 10703 ( 2024 ) Cite this article
8 Altmetric
Metrics details
- Human behaviour
- Risk factors
Research in psychology and medicine has linked mental health disorders, and particularly bipolar disorder (BD), to employment in creative professions. Little is known, however, about the mechanisms for this link, which could be due to biology (primarily through a person’s genes) or environmental (through socioeconomic status). Using administrative data on mental health diagnoses and occupations for the population of Denmark, we find that people with BD are more likely to be musicians than the population, but less likely to hold other creative jobs. Yet, we also show that healthy siblings of people with BD are significantly more likely to work in creative professions. Notably, people from wealthy families are consistently more likely to work in creative professions, and access to family wealth amplifies the likelihood that siblings of people with BD pursue creative occupations. Nevertheless, family wealth explains only a small share of the correlation between BD and creative employment.
Research in psychology and medicine has pointed to a link between mental health disorders and creative pursuits, such as employment in creative professions. For instance, studies of Swedish population data have found that writers (but not people in other creative professions) face an elevated risk of bipolar disorder (BD), schizophrenia, and depression 1 , 2 . Similarly, polygenic risk scores for individuals in Iceland indicate that people with an elevated genetic predisposition for BD and schizophrenia are more likely to work in creative professions 3 . [Polygenic risk scores measure a person’s genetic predisposition for a trait or disorder, abstracting from environmental factors ( https://www.genome.gov/Health/Genomics-and-Medicine/Polygenic-risk-scores ).]
Despite this evidence, less is known about the mechanisms that link creativity with mental health. One set of possible causes is neurobiological. Existing studies point to the importance of dopamine, a neurotransmitter that regulates our perception of pleasure and the ability to think and plan. Dopamine regulation is affected in people with mood disorders, such as BD 4 . At the same time, dopamine is related to divergent thinking 3 , 5 , which allows for greater freedom to pursue high-risk projects and fresh ideas that are essential for creative work 6 , 7 .
Another set of possible causes is environmental. There could be aspects of a person’s background—such as socioeconomic status—which influence both their likelihood of being in a creative profession and their mental health. For example children from families with income in the top percentile have been shown to be 10 times as likely to become inventors as those from below-median income families 8 . Similarly, 9 have emphasized the importance of exposure to role models and parental income 9 . Conversely, financial distress has been shown to negatively impact mental health 10 , 11 and increase the incidence of BD 12 , 13 , 14 and other mental health disorders. Access to specialized mental health care has also been shown to depend, at least in part, on a person’s socio-economic status 9 , 15 .
Connecting these insights from psychology and economics, we study the channels behind the observed link between mental health and creativity, with a focus on the role of socioeconomic factors such as parental wealth. We focus on BD, a prevalent “brain disorder that causes unusual shifts in mood, energy, activity levels, and the ability to carry out day-to-day tasks.”[ https://www.nimh.nih.gov/health/topics/bipolar-disorder/index.shtml , accessed November 22, 2019.] BD affects 1 in 11 people in the US population and 40 million people world-wide, creating major career costs for affected individuals 16 , 17 BD, however, has also been strongly and consistently associated with creative employment 2 , 3 , 18 . For instance, biographical evidence suggests that many exceptionally creative individuals were affected by BD, including visual artists such as Vincent van Gogh, writers such as Virginia Woolf, and composers such as Robert Schumann 18 . Our goal is to investigate this tension and explore a potential role of familial wealth in the connection between creativity and BD.
To perform this analysis, we use registry data on mental health diagnoses, creative employment, and parental wealth for the population of Denmark. These data include individual-level administrative records of mental health diagnoses and occupations for all 2,524,325 people who were active in Denmark’s labor force between 1995 and 2015. Family identifiers, available for 71 percent of the population, allow us to identify siblings of people with a mental health condition and observe family wealth. To define employment in creative professions, we implement definitions from psychology which include designers, university teachers (academics), visual artists, architects, display artists, performing artists, musicians, and photographers 2 , 3 , 19 .
We begin by revisiting the association between mental health and creativity. We compare the likelihood of creative employment among people with BD, their siblings, and the population. We find that while people with BD are 20 percent less likely to be active in any creative occupation than the population, they are 50 percent more likely to be composers and musicians. Occupations that people with BD are most likely to pursue, instead, include clerks, librarians, archivists and curators, as well as waiters and bartenders.
Notably, we also find that healthy siblings of people with BD are 11 percent more likely to work in creative professions, confirming the results for Sweden 1 . This finding is consistent with a biological explanation for the link between mental health and creativity. Siblings of people with BD may be affected by a milder (subthreshold) form of BD that eludes diagnosis and experience a greater penchant for divergent thinking, without suffering the adverse health effects of BD, which could lead them to successfully pursue creative employment 20 .
An increased likelihood of creative employment for siblings of people with BD, however, is also consistent with an alternative, socioeconomic explanation: people who grew up in wealthy families have better access to both medical care (including diagnoses for mental health disorders) and employment in creative professions (for instance, because they can afford specialized training required for these jobs). To investigate this alternative channel, we first examine whether people from wealthy families are more likely to hold creative jobs. Then, we test whether the link between mental health and creativity can be explained by differences in parental wealth.
Our results indicate a strong link between parental wealth and employment in creative professions. People from the top decile of the parental wealth distribution are 7 times more likely to work in creative occupations compared with people from the bottom decile. These findings extend existing results for patentees 1 , 2 to a broader set of creative occupations.
Nevertheless, the correlation between wealth and creative employment explains only a small portion of the correlation between BD and creativity. Differences in parental wealth can account for at most 8 percent of the correlation between creativity and mental health. Notably, the gradient between parental wealth and creative employment is stronger for siblings of people with BD than for the population. This suggests that environmental factors can reinforce the biological channel, even though they cannot fully explain the link between mental health and creativity.
Our paper contributes to two main strands of literature. The first has explored the link between mental health and creativity. The channels behind the BD-creativity link are still still poorly understood. Polygenic risk scores for BD and schizophrenia are correlated with elevated odds of creative employment in a sample of 86,000 people in Iceland 3 . Yet, polygenic risk scores explain less than 1 percent of these odds, suggesting a statistically significant but small role for biology. In Swedish administrative data, people who have been hospitalized with BD have been found to be over-represented in creative professions, while those with depression are not 1 . In a similar setting, people in creative professions—and particularly writers—are more likely to have BD, but not other psychiatric disorders 2 . Our study complements these works by analyzing the entire population of Denmark and by considering all people with a BD diagnosis, including those with less severe symptoms, not requiring hospitalization. In addition, the richness of our data and a broader classification of creative professions (which combines definitions previously used in the literature) allow us to explore the link between this condition and each creative occupation individually. Lastly, we investigate the role of what is perhaps the most powerful alternative explanation—socioeconomic status, which has been linked to creativity for inventors 1 , 2 .
Our paper also relates to research on the relationship between health and work, and occupational choice more specifically 21 , 22 . Most of this research considers occupational choice as a factor influencing health (for example through differences in the intensity of work between manual and white-collar jobs). Our research complements these analyses by investigating the link between a particular dimension of health—mental health—and the way it relates to occupational choice through biology and socioeconomic status.
BD and creative employment
People with bd are less likely to work in creative jobs.
First, we test whether people with BD are more likely to work in creative occupations compared with the population. Figure 1 reports occupation-specific estimates and 95-percent confidence intervals for the difference in the likelihood of holding a creative job between people with BD and the population, controlling for variation across calendar years, birth cohorts, and gender. We divide this difference by the population share of each occupation, so that estimates can be interpreted as percent differences (panel A of Table 1 reports the unscaled differences). These estimates indicate that people with BD are − 0.293 percentage points less likely to work in any creative occupation (significant at 1 percent, Table 1 , panel A, column 1). Compared with a population share of 1.474 percent, this implies that people with BD are 20 percent less likely to work in a creative occupation (Fig. 1 ).
Share in creative occupations: people with BD and their siblings, compared with the population. Note OLS estimates (and 95-percent confidence intervals) of β in the equation creative it = β X i + γ F i + θ c(i) + τ t + ε it where creative it equals one if person i is employed in a creative profession in year t . In the BD series, X i is an indicator for people who have received at least one diagnosis of BD at least once. For siblings of BD , X i indicates siblings of people with BD . The variable F i is an indicator for females. A vector of cohort fixed effects θ c(i) controls for systematic differences in the propensity to hold a creative job across cohorts; a vector of year fixed effects τ t controls for differences over time. We report coefficients and confidence intervals as the share of the mean of the dependent variable. Standard errors are clustered at the individual level. Creative professions are listed in Appendix Table A2 , together with ISCO-4 codes.
Occupation-specific estimates reveal moderate heterogeneity in the share of people with BD across occupations. People with BD are 15 percent less likely to be writers, 39 percent less likely to be academics, 23 percent less likely to be architects, and 14 percent less likely to be designers (significant at 5, 1, 5, and 5 percent, respectively, Fig. 1 and Table 1 , panel A). People with BD are also 14 percent less likely to be photographers and 8 percent less likely to be visual artists, although these estimates are not statistically significant (with p-values of 0.55 and 0.53, respectively).
Notably, people with BD are 50 percent more likely to be musicians and composers (significant at 5 percent). This result is consistent with biographical evidence suggesting that prominent composers (including Berlioz, Brahms, Cherubini, Gluck, Mahler, Mendelssohn, Schubert, and Schumann) may have been affected by bipolar disorder 23 . Interestingly, people with BD are also slightly more likely to be performing artists (actors, dancers, choreographers, and directors), though this estimate is smaller and not statistically significant (11 percent, with a p-value of 0.47). [In contrast to our results and those of others 24 , people who are diagnosed with BD in Sweden are more likely to be writers, but not musicians.]
Healthy siblings of people with BD are more likely to be creative
If BD is associated with creativity through a genetic link, siblings may have a milder “subthreshold” form of BD that allows them to be more creative, without experiencing debilitating symptoms. Models of BD in molecular neuropsychiatry have proposed an inverted U-shaped relationship between the genetic risk for BD and creativity. Greenwood (2016, p. 200) 25 conjectures that “some aspects of the bipolar spectrum may confer advantages, while more severe expressions of symptoms negatively influence creative accomplishment.”
Our data indicate that siblings of people with BD are 11 percent more likely to work in creative professions compared with the population (Fig. 1 and Table 1 , panel B, column 1). Occupation-specific estimates imply that siblings of people with BD are 35 percent more likely to be visual artists, 28 percent more likely to be architects, and 16 percent more likely to be writers (Table 1 , panel B, significant at the 5, 5, and 10 percent level, respectively). Siblings are also 23 percent more likely to be musicians and composers than the population, even though this estimate is imprecise due to the small number of observations (with a p value of 0.27). These findings confirm the higher share of creative employment among siblings of people who have received in-patient treatment for BD in Sweden 1 .
Most frequent occupations for people with BD
Looking beyond creative professions, we investigate what type of jobs people with BD are instead most likely to pursue. Formally, we estimate multinomial models of occupational choice, using the broader 3-digit ISCO08 codes to classify occupations and an indicator for BD as the explanatory variable, together with an indicator for women to account for gender differences. [To define occupations consistently over time, we restrict the analysis to 2010–2015, when ISCO08 codes are available. Following the psychology literature, we use 4-digit codes to examine employment in creative professions in the main specifications. To estimate multinomial choice models of an individual’s choice across all occupations, we use 3-digit ISCO codes. An earlier study 26 estimates a multinomial model of a choice between five occupations, using data from a total of 20,861 interviews in 5 university towns. They find that people with BD and mania are most likely to work in services and show, using a measure of creativity, that services are a creative occupation.]
Multinomial logit estimates also confirm that people with BD are less likely to work in creative professions. For example, people with BD are 34 percent less likely to be “architects, planners, and surveyor designers” and 38 percent less likely to be “artistic, cultural, and culinary associate professionals,” including designers. The largest positive coefficient among creative professions is again for composers, musicians, and performing artists (here included in “creative and performing artists”). People with BD are 27 percent more likely to be employed in this category (significant at 5 percent).
Interestingly, three of the five occupations that people with BD are least likely to pursue relate to management. This is in contrast with the popular idea that BD is a “CEO’s disease” 27 , 28 because entrepreneurs share certain traits that are associated with BD, including overconfidence and an excessive tolerance for risk. [Medical studies document excessive risk tolerance and impulsive behavior in people with BD. In experiments with a balloon analogue risk task (BART) people with BD score higher on self-reported tests of impulsiveness 29 . Overconfidence and tolerance for risk are also consistent with narcissistic personality 30 . Impulsivity—the tendency to pursue rewards without considering negative consequences—has also been shown to be elevated in people who experience mania 31 .] Rejecting the hypothesis that BD is a CEO’s disease, we find that people with BD are 82 percent less likely to be sales, marketing, and development managers, 81 percent less likely to be construction and distribution managers, and 80 percent less likely to be business and administration managers.
Instead, people with BD are 177 percent more likely to be clerks; 50 percent more likely to be librarians, archivists, and curators; and 43 percent more likely to be waiters and bartenders (Fig. 2 ).
Multinomial logit estimates of occupation: people with BD vs. population. Note Multinomial logit estimates and 95 percent confidence intervals of the parameters β j in equation Pr(Y it = j) = exp(β j BD i + θ j F i )/ Σ k exp(β k BD i + θ k F i ), where Y it is the occupation of person i in year t , BD i equals one for people with BD, and F i is an indicator for females. This figure shows the five largest and the five smallest estimates of β j , along with estimates of β j for creative occupations, defined by 3-digit ISCO08 codes. Standard errors are clustered at the individual level.
Can parental wealth explain the link between BD and creativity?
Our analysis of population data for Denmark indicates a link between mental health and creativity. In this final section we test whether this link can be explained by differences in family backgrounds, and specifically parental wealth.
Children of wealthier parents are more likely to be employed in creative professions
We first test whether the finding that people with wealthy parents are more likely to become inventors extends to other creative occupations. Specifically, we plot the share (and 95-percent confidence interval) of people employed in creative professions by their decile of parental wealth.
We find that people in the top decile of parental wealth are 7 times more likely to work in creative professions (with 2.9 percent, Fig. 3 ) compared with people in the bottom decile (just 0.4 percent). This suggests that earlier findings based on inventors (a profession where financial resources are needed to be able to patent 1 , 2 ) hold more broadly across creative professions: differences in parental income and wealth help shape the link between innate creativity and professional outcomes.
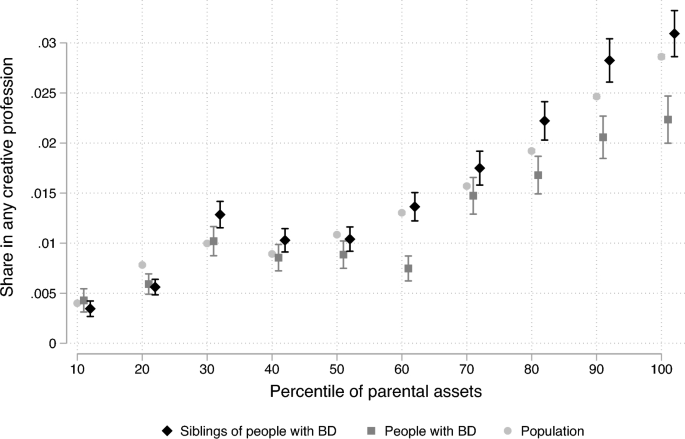
Creative professions and parental wealth: population, people with BD, and their siblings. Note Share of people employed in any of the eight creative professions in Fig. 2 , separately by the median decile of parental asset for people with BD, their siblings, and the population (with 95-percent confidence intervals). Implementing definitions from psychology, we define creative professions to include academics, architects, designers and display artists, musicians, performance artists, photographers, visual artists, and writers (Appendix Table A2 ).
Employment in creative professions for people with BD and their siblings
Higher rates of creative employment for healthy siblings are consistent with a biological link between BD and creativity. Yet, analyses of polygenic risk scores have found that only 1.2 percent of the variance in creative employment can be explained by genes that are associated with BD 3 . Indeed, an increased chance of creative employment for BD siblings is also consistent with an environmental explanation for the link between BD and creativity, as siblings share family backgrounds.
To better quantify the role of environmental factors, we examine whether parental wealth can account for the observed relationship between mental health and creative employment. We have shown above that people with wealthier parents are more likely to hold creative jobs. Differences in income and wealth can also directly impact mental health 10 , 11 . For instance, recipients of large and unconditional cash transfers in rural Kenya experienced significant increases in psychological well-being 10 .
To test this hypothesis, we use information on parental assets and investigate how differences in wealth affect the link between mental health and creativity. We formally test for the influence of parental wealth by re-estimating the difference in the likelihood of holding a creative job between people with BD and the population, controlling for indicators for low (below median) and high (above median) parental wealth.
This exercise indicates that the link between BD and creative employment is robust to controlling for, and cannot be explained by, parental wealth: estimates of the BD-population difference are essentially unchanged when we control for wealth. In these specifications, people with BD are 61 percent more likely to be musicians (Table 2 , panel A, column 5), while they are 50 percent more likely when we do not control for wealth (Fig. 3 , panel A). Across all occupations, people with BD are 19 percent less likely to have a creative job (Table 2 , panel A, column 1, significant at 1 percent), while they are 20 percent less likely not controlling for wealth. These results indicate that only a small share—approximately 6 percent—of the overall association between creativity and mental health can be explained by differences in wealth. All results are robust to alternative definitions of wealth, using of terciles, quartiles or other quantiles of parental assets.
Estimates looking at siblings of people with BD paint a similar picture: controlling for parental wealth in Eq. ( 2 ) leaves the estimates virtually unchanged. Controlling for wealth, siblings of people with BD are 12 percent more likely to work in any creative profession (with an estimated coefficient for BD Sibling equal to 0.00169 and a share of people in creative professions equal to 0.0147, Table 2 , panel B, column 1). Compared with estimates that do not control for wealth, this implies that differences in wealth explain 8 percent of the observed association between creativity and BD among siblings.
Interestingly, we find that the gradient between wealth and creative employment is weaker for people with BD than for the general population (Fig. 2 ). Among people with BD, 2.2 percent of those with the wealthiest parents work in creative professions (compared with 2.9 percent in the population). By comparison, only 0.4 percent of people with BD with the least wealthy parents work in creative professions, the same rate as the population.
At the same time, the gradient between wealth and creative employment is slightly stronger for healthy siblings of people with BD than for the population. Among siblings of people with BD, 3.0 percent of those with the wealthiest parents and only 0.4 percent of those with the least wealthy parents work in creative professions. This finding suggests that differences in wealth may amplify the biological links between mental health and creativity.
Using individual-level data on mental health diagnoses and occupations, we revisit the link between creativity and mental health disorders and study the channels behind this relationship, with a specific focus on biological and environmental forces. We show that people with BD are 50 percent more likely to be musicians, but less likely to be employed in other creative professions. Notably, healthy siblings of people with BD are consistently more likely to work in creative professions. For instance, siblings of people with BD are 16 percent more likely to be writers, 28 percent more likely to be architects, and 28 percent more likely to be visual artists. These findings indicate that family-level traits, either through genes or socioeconomic status, link mental health disorders with creativity.
Consistent with a strong influence of socioeconomic status, we find that people with parents in the top quartile of parental wealth are over 7 times as likely to hold a creative job compared with those in the bottom quartile. This striking correlation, however, explains only a small share—no more than 8 percent—of the observed correlation between mental health and creative employment. Interestingly, we also find that the creativity-wealth gradient is stronger for siblings of people with BD than for the population.
Taken together, our findings indicate that neurobiological factors may be the primary link between mental health and creativity, and that socioeconomic status amplifies this biological link. Siblings people with BD from high-income families may be additionally more likely to pursue creative jobs because their occupational choices are less constrained by financial needs, or because high-income people are more likely to experience positive moods. While comprehensive, our analysis faces a few limitations. First, our measure of creativity is limited to the choice of creative occupations and may therefore miss other expressions of creativity that do not result in creative employment. We may be missing expressions of Big Creativity 32 , 33 , which have been linked to mental health conditions 34 . Empirically, expressions of Big Creativity can be proxied through creative output, e.g., through inventions 8 , publications 31 , or musical creations 37 future analysis could establish such links by linking health records with these measures. Second, it is possible for the set of occupations that have a creative component to change over time, due to changes in the nature of work. Our time-invariant classification of creative occupations makes it possible to compare our results to findings in previous literature 1 , 2 but is not able to explicitly incorporate such changes. Third, our analysis of the relationship between BD, creative employment, and wealth is not causal and there is the possibility of reverse causality. For example, it is possible that BD leads to certain choices of occupation, but it is also possible that the choice of a certain job creates stress, e.g., over financial uncertainty, which may trigger latent pre-dispositions to develop BD and other mood and anxiety disorders 35 .
Data and sample
We use registry data on the population of Denmark, obtained from Statistics Denmark. Our data comprise mental health diagnoses and occupations for 2,524,325 people born between 1946 and 1975. Family identifiers, which we use to link people to their siblings and measure differences in parental wealth, are available for 71 percent of the population. Appendix Table A1 summarizes the variables used in our analysis. While we are not allowed to share the data ourselves, researchers will be able to access it through an application with Statistics Denmark. [ https://sundhedsdatastyrelsen.dk/da/english/health_data_and_registers/research_services/apply/data_statistics_dk ].
Mental health diagnoses
Information on diagnoses is taken from the Central Psychiatric Register ( Landspatientregistret for Psykiatri Diagnoser ), which records all mental health diagnoses in Denmark between January 1, 1995, and December 31, 2015. The Register classifies mental health disorders according to the World Health Organization International Statistical Classification of Diseases and Related Health Problems (ICD-10; see http://apps.who.int/classifications/icd10/browse/2016/en#/F30-F39 ).
Implementing this classification, our variable BD identifies 18,729 people who have received at least one diagnosis of bipolar disorder (ICD-10: F31) or mania (ICD-10: F30). BD is defined as “A disorder characterized by […] some occasions of an elevation of mood and increased energy and activity (hypomania or mania) and on others of a lowering of mood and decreased energy and activity (depression).” Mania is described as “A disorder […] which varies from carefree joviality to almost uncontrollable excitement, […] accompanied by increased energy, resulting in overactivity, pressure of speech, and a decreased need for sleep.”
Creative occupations
In this analysis, we measure a person’s tendency for creativity through employment in creativity professions. Existing economic analyses have measured creativity through output, such as patents 8 , 9 , publications 36 , and musical compositions 37 . By contrast, studies of mental health disorders and creativity in psychology and medicine have treated creativity as an individual-level characteristic, proxied by a person’s choice of occupation 1 , 2 , 19 .
We follow the psychology literature in defining creative occupations. Previous studies have classified creative professions to include designers, writers, academics, visual artists, architects, display artists, performing artists, composers, and musicians 19 . Others have excluded architects but included photographers 1 . We include both architects and photographers among the creative occupations and report results separately by occupation.
Denmark’s registries follow the International Standard Classification of Occupations (ISCO) to classify occupations. Years 1995–2009 use the 1988 classification and years 2010–2015 use the 2008 classification. Using 4-digit ISCO codes we distinguish academics (ISCO code 2310), photographers (3131), visual artists (2452 in 1988; 2651 and 2166 in 2008), designers (3471 in 1988; 3432, 3435, 2163, and 2166 in 2008), performing artists (2454 and 2455 in 1988; 2654 and 2655 in 2008), composers and musicians (2453 in 1988, 2652 in 2008), writers (2451 in 1988; 2431, 2432, 2641, and 2642 in 2008), and architects (2141 in 1998; 2161 and 2162 in 2008; Appendix Table A2 ). In multinomial logit regressions we aggregate ISCO codes to the 3-digit level to reduce the number of choices.
Family identifiers and parental wealth
To match people with their siblings, we use their mother’s or father’s social security number as a family identifier. Family identifiers are available for 1,788,166 people (71 percent of the population); 75 percent of them have one or more siblings. Family identifiers allow us to identify siblings of people with BD.
Data on parental wealth are available for people whose mother or father reported assets for at least one year between 1980 and 2015. We set assets to zero for people whose parents are listed but do not have any financial assets. Assets are reported by banks and other financial institutions and not by the individuals themselves. All results are robust to excluding individuals without information on parental assets from the analyses. To define a person’s position in the distribution of parental wealth, we calculate the percentile of parental assets for each year (from 1980 to 2015) and assign each person to their parents’ median percentile across all years.
Empirical framework
First, we test whether people with BD are more likely to work in creative occupations compared with the population. To avoid picking up differences in labor force participation between people with BD and the population, we restrict attention to people with positive earnings in any given year. We estimate the following equation separately for eight creative occupations, including writers, academics, architects, designers, musicians, photographers, visual artists, and performing artists:
where the variable creative it equals one if person i is employed in any or in a specific creative profession in year t , and BD i equals one if the person has been diagnosed with BD at least once. An indicator for women F i controls for possible gender differences in occupational choices. A vector of cohort fixed effects θ c(i) controls for systematic differences in the propensity to hold a creative job across cohorts. A vector of year fixed effects τ t controls for differences in the same propensity over time. We cluster standard errors at the individual level. The coefficient β estimates the difference in the likelihood of holding a creative job between people with BD and the population, controlling for variation in creative employment across calendar years, birth cohorts, and gender.
Creative employment among siblings of people With BD
If BD is associated with creativity through a genetic link, siblings may have a milder “subthreshold” form of BD that allows them to be more creative, without experiencing debilitating symptoms. Models of BD in molecular neuropsychiatry have proposed an inverted U-shaped relationship between the genetic risk for BD and creativity 25 . Conjectures that “some aspects of the bipolar spectrum may confer advantages, while more severe expressions of symptoms negatively influence creative accomplishment.”
To test whether healthy siblings of people with BD are more likely to pursue creative jobs, we estimate Eq. ( 1 ) with an indicator for BD siblings instead of the indicator for BD:
In this modified equation, the coefficient β S estimates the difference in the likelihood of holding a creative job between siblings of people with BD and other people of the same gender, in the same birth cohort and calendar year.
Most frequent occupations for people with BD: multinomial logit
Looking beyond creative professions, we investigate what type of jobs people with BD are instead most likely to pursue. Formally, we estimate multinomial models of occupational choice, using the broader 3-digit ISCO08 codes to classify occupations. To define occupations consistently over time, we restrict the analysis to 2010–2015, when ISCO08 codes are available. Following the psychology literature, we use 4-digit codes to examine employment in creative professions in the main specifications. To estimate multinomial choice models of an individual’s choice across all occupations, we use 3-digit ISCO codes. [An earlier study estimates a multinomial model of a choice between five occupations, using data from a total of 20,861 interviews in 5 university towns. They find that people with BD and mania are most likely to work in services and show, using a measure of creativity, that services are a creative occupation 28 .]We model the probability that a person works in occupation j at time t as:
where Y it is the occupation of person i in year t and F i is an indicator for women. First, we estimate β j and γ j for 129 occupations via maximum likelihood. We normalize both parameters to zero for “Primary Schools and Early Childhood Teachers” (ISCO08 code 234), the most common occupation (with 6.8 percent of workers). We then compute the excess probability of occupation j for people with BD as exp( β j )− 1.
Data availability
We obtained our data from Statistics Denmark. While we are not allowed to share the data ourselves, researchers will be able to access it through an application with Statistics Denmark. [ https://sundhedsdatastyrelsen.dk/da/english/health_data_and_registers/research_services/apply/data_statistics_dk ].
Kyaga, S. et al. Creativity and mental disorder: Family study of 300 000 people with severe mental disorder. Br. J. Psychiatry 199 (5), 373–379 (2011).
Article PubMed Google Scholar
Kyaga, S. et al. Mental illness, suicide and creativity: 40-year prospective total population study. J. Psychiatr. Res. 47 (1), 84–90 (2013).
Article Google Scholar
Power, R. A. et al. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat. Neurosci. 18 (7), 953–955 (2015).
Article CAS PubMed Google Scholar
Miklowitz, D. J. & Johnson, S. L. The psychopathology and treatment of bipolar disorder. Annu. Rev. Clin. Psychol. 2 , 199–235 (2006).
Article PubMed PubMed Central Google Scholar
Takeuchi, H. et al. Regional gray matter volume of dopaminergic system associate with creativity: Evidence from voxel-based morphometry. Neuroimage 51 (2), 578–585 (2010).
MacCabe, J. H. et al. Excellent school performance at age 16 and risk of adult bipolar disorder: National cohort study. Br. J. Psychiatry 196 (2), 109–115 (2010).
Runco, M. A. Creativity: Theories and themes: Research, development, and practice (Elsevier, Amsterdam, 2007).
Google Scholar
Bell, A., Chetty, R., Jaravel, X., Petkova, N. & van Reenen, J. Who becomes an inventor in America? The importance of exposure to innovation. Q. J. Econ. 134 (2), 647–713 (2019).
Aghion, Phillipe, Ufuk Akcigit, A. Hyytinen, and Otto Toivanen. 2017. “The social origins of inventors.” NBER Working Paper No. 24110.
Haushofer, J. & Shapiro, J. The short-term impact of unconditional cash transfers to the poor: Experimental evidence from Kenya. Q. J. Econ. 131 (4), 1973–2042 (2016).
Ridley, Matthew W., Gautam Rao, Frank Schilbach and Vikram H. Patel. 2020. “Poverty, Depression, and Anxiety: Causal Evidence and Mechanisms.” NBER Working Paper 27157.
Bauer, Michael, Tasha Glenn, Natalie Rasgon, Wendy Marsh, Kemal Sagduyu, Rodrigo Munoz, Rita Schmid, Sara Haack, and Peter C. Whybrow. 2011. “Association between median family income and self-reported mood symptoms in bipolar disorder.” Comprehensive psychiatry 52, no. 1: 17–25. -treated patients with bipolar disorder,” Biological Psychiatry , 62 (1): 7–16.
Sareen, J., Afifi, T. O., McMillan, K. A. & Asmundson, G. J. G. Relationship between household income and mental disorders: findings from a population-based longitudinal study. Arch. Gen. Psychiatry 68 (4), 419–427 (2011).
Hakulinen, C., Musliner, K. L. & Agerbo, E. Bipolar disorder and depression in early adulthood and long-term employment, income, and educational attainment: A nationwide cohort study of 2,390,127 individuals. Depress. Anxiety 36 (11), 1080–1088 (2019).
Alegría, M., Bijl, R. V., Lin, E., Walters, E. E. & Kessler, R. C. Income differences in persons seeking outpatient treatment for mental disorders: a comparison of the United States with Ontario and The Netherlands. Arch. Gen. Psychiatry 57 (4), 383–391 (2000).
Simpson, S. G. & Jamison, K. R. The risk of suicide in patients with bipolar disorders. J. Clin. Psychiatry 60 (2), 53–56 (1999).
PubMed Google Scholar
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. The Lancet 396 (10258), 1204–1222 (2000).
Jamison, K. R. Touched with fire manic depressive illness and the artistic temperament (Free Press, New York, 1993).
Ludwig, & Arnold, M. Creative achievement and psychopathology: Comparison among professions. Am. J. Psychiatry 46 (3), 330–356 (1992).
MathSciNet CAS Google Scholar
Richards, R., Kinney, D. K., Lunde, I., Benet, M. & Merzel, A. P. C. Creativity in manic-depressives, cyclothymes, their normal relatives, and control subjects. J. Abnormal Psychol. 97 (3), 281 (1988).
Article CAS Google Scholar
Grossman, M. On the concept of health capital and the demand for health. J. Political Econ. 80 (2), 223–255 (1972).
Strulik, H. A health economic theory of occupational choice, aging, and longevity. J. Health Econ. 82 , 102599 (2022).
Mula, M. & Trimble, M. R. Music and madness: Neuropsychiatric aspects of music. Clin. Med. 9 (1), 83 (2009).
Borowiecki, K. How are you, my dearest Mozart? Well-being and creativity of three famous composers based on their letters. Rev. Econ. Stat. 99 (4), 591–605 (2017).
Greenwood, T. A. Positive traits in the bipolar spectrum. The space between madness and genius. Molecular Neuropsychiatry 2 (4), 198–212 (2016).
MathSciNet PubMed PubMed Central Google Scholar
Tremblay, C. H., Grosskopf, S. & Yang, Ke. Brainstorm: Occupational choice, bipolar illness and creativity. Econ. Human Biol. 8 (2), 233–241 (2020).
Elsberry, Richard. 1998. “Bipolar Disorder: Why Are They Calling It the CEO Disease?” Electrical Apparatus.
Gartner, J. D. The hypomanic edge: The link between (A Little) craziness and (A Lot of) success in America (Simon & Schuster, New York, 2011).
Reddy, L. F. et al. Impulsivity and risk taking in bipolar disorder and schizophrenia. Neuropsychopharmacology 39 , 456–463 (2014).
Larcker, David F., Charles A. O'Reilly, Brian Tayan, and Anastasia A. Zakolyukina. 2021. "Are Narcissistic CEOs All That Bad?" Rock Center for Corporate Governance at Stanford University Working Paper.
Swann, A. C. D. M., Dougherty, P. J. P., Pham, M. & Moeller, F. G. Impulsivity: A link between bipolar disorder and substance abuse. Bipolar Disorder 6 , 204–2012 (2004).
Simonton, D. K. What is a creative idea? Little-c versus Big-C creativity. In Handbook of research on creativity (eds Thomas, K. & Chan, J.) (Edward Elgar Publishing, Camberley, 2013). https://doi.org/10.4337/9780857939814.00015 .
Chapter Google Scholar
Simonton, D. K. The mad (creative) genius: What do we know after a century of historiometric research? In Creativity and mental illness (ed. Kaufman, J. C.) 25–41 (Cambridge University Press, Cambridge, 2014).
Simonton, D. K. The mad-genius paradox: Can creative people be more mentally healthy but highly creative people more mentally ill?. Persp. Psychol. Sci. 9 , 470–480 (2014).
Weisberg, R. W. Creativity: Understanding innovation in problem solving, science, invention, and the arts (Wiley, Hoboken, 2006).
Biasi, B. & Moser, P. Effects of copyrights on science – evidence from the wwii book republication program. Am. Econ. J. Microecon. 13 (4), 218–260 (2021).
Giorcelli, M. & Moser, P. Copyright and creativity. Evidence from Italian operas in the neapoleonic age. J. Political Econ. 128 (11), 4163–4210 (2020).
Download references
Author information
Authors and affiliations.
Yale School of Management, New Haven, USA
Barbara Biasi
National Bureau of Economic Research, Cambridge, USA
Barbara Biasi & Petra Moser
Aalborg University, Aalborg, Denmark
Michael S. Dahl
NHH Norwegian School of Economics, Bergen, Norway
Aarhus University, Aarhus, Denmark
New York University Stern School of Business, New York, USA
Petra Moser
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed equally.
Corresponding author
Correspondence to Barbara Biasi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Biasi, B., Dahl, M.S. & Moser, P. The role of bipolar disorder and family wealth in choosing creative occupations. Sci Rep 14 , 10703 (2024). https://doi.org/10.1038/s41598-024-61320-y
Download citation
Received : 05 July 2023
Accepted : 03 May 2024
Published : 10 May 2024
DOI : https://doi.org/10.1038/s41598-024-61320-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mental health
- Occupational choice
- Parental wealth
- Bipolar disorder
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
- Bipolar Disorder Symptoms and Traits
What Causes Bipolar Disorder?
- Is Bipolar Hereditary?
- How to Diagnose Bipolar Disorder
- Bipolar Disorder Treatments to Manage Symptoms
- When Your Loved One Has Bipolar Disorder
Bipolar Disorder Explained: Everything You Need to Know
What is bipolar disorder, living with bipolar disorder.
- Next in Bipolar Disorder Guide Bipolar Disorder Symptoms and Traits
Bipolar disorder refers to a group of mental health conditions characterized by sudden, dramatic changes in mood, energy, and behavior. Formerly known as manic depression, this condition causes mood episodes lasting days or weeks at a time and hinder day-to-day functioning, school or work performance, and relationships.
This article describes the symptoms , causes, and treatments for bipolar disorder and discusses how to cope if you’re diagnosed with this mental health condition.
FG Trade / Getty Images
Estimated to affect 4.4% of U.S. adults at some point in their lives, bipolar disorder causes distinct periods of extreme emotional states or episodes that can last for days or weeks. Episodes are characterized by manic or depressive behavior.
Manic Episodes
A manic episode is a phase of a week or more during which you have an elevated mood and energy most of the time for most days. In this phase, you may feel abnormally happy, agitated, restless, and don’t need much sleep.
In rare and severe cases, people experience hallucinations and delusions during manic episodes. In addition, some people experience hypomanic episodes—less severe manic episodes lasting four or more days.
Everything You Should Know About Bipolar Disorder
Major depressive episodes.
A major depressive episode is a period of two or more weeks of depressive symptoms, such as sadness, hopelessness, lethargy (lack of energy), and apathy. These episodes can become severe, leading to suicidal thoughts. As with manic episodes, severe depressive episodes can lead to hallucinations or delusions.
What Are the Types of Bipolar Disorder?
Healthcare providers break down bipolar disorder into four primary types : bipolar 1, bipolar 2, cyclothymic disorder, and unspecified bipolar disorder.
Bipolar 1 Disorder
With bipolar 1 disorder, manic episodes last a week or become so severe that you require hospitalization. In most cases, bipolar 1 also causes depressive episodes. Some people have “mixed” episodes that feature both manic and depressive symptoms at the same time. Neutral periods—neither manic nor depressive—are also common with this type.
Bipolar 2 Disorder
Bipolar 2 disorder occurs when you experience one depressive episode and at least one hypomanic episode (milder manic episodes that last four or more days). In between these are symptom-free periods. Those with bipolar 2 disorder often have other mental health conditions, such as anxiety or depression.
Cyclothymic Disorder (Cyclothymia)
Cyclothymic disorder is a milder type of bipolar disorder that causes regular mood swings. Ranging between those of mild depression and hypomania, the symptoms aren’t severe enough to be considered clinically depressive or hypomanic episodes.
Unspecified Bipolar Disorder
Unspecified bipolar disorder is when you have extreme mood fluctuations, but the symptoms aren’t as bad as those of bipolar 1 or 2. Still, with this type, the symptoms are significant enough to affect daily functioning, relationships, and work or school.
Bipolar Disorder Symptoms
Dramatic and intense changes in your mood, emotions, behaviors, and activity level are the primary signs of bipolar disorder. These shifts tend to be noticeable to others and impact your relationships, performance at work or school, or daily functioning.
The symptoms you experience depend on whether you’re having a manic or depressive episode.
Manic Episode Symptoms
During manic episodes, emotion and activity levels are elevated. Manic episode symptoms include the following:
- Abnormal giddiness or happiness
- Changing topics when speaking
- Distractibility
- Feeling energetic despite insufficient sleep
- Increased irritability or agitation
- Racing, uncontrollable thoughts
- Recklessness or risky, impulsive behaviors
- Restlessness, increased activity
- Talking faster or more often
Major Depressive Episode Symptoms
In contrast to manic episodes, during a depressive episode, you feel “low” in terms of energy, mood, and emotion. Symptoms of this type include combinations of the following:
- Despair, thoughts about death or suicide
- Difficulty falling or staying asleep or sleeping excessively
- Difficulty with routine tasks
- Feeling sad, hopeless, or anxious
- Forgetfulness, slowed speech, not knowing what to say
- Loss of energy or motivation
- Loss of interest in activities
- Restlessness
When to Call 911
If you have bipolar disorder, go to an emergency room (ER) if you experience:
- Suicidal thoughts
- Thoughts about hurting yourself or others
- Hallucinations or delusions
- Lithium toxicity symptoms: nausea, vomiting, dizziness, changes in vision, and slurred speech
Researchers don’t know what exactly causes bipolar disorder. The consensus is that genetic factors, brain chemistry and structure, and environmental factors all play a role in this condition.
Genetic Factors
Though more work is needed, researchers have linked genetics with an increased risk of developing bipolar disorder. This condition is heritable, making family history a risk factor; people with a parent or sibling with the condition are more likely to have it.
Brain Chemistry and Structure
Using imaging techniques, researchers have found differences between the brains of those with and without bipolar disorder. Some research shows that people with bipolar disorder have smaller subcortical structures (associated with mood and cognition) and a thinner cortex (the outer layer of the brain).
In addition, researchers have linked imbalances in certain neurotransmitters (brain chemicals), particularly dopamine and serotonin, to bipolar disorder.
Environmental Factors
Stressful or traumatic life events and certain behaviors can also raise your risk of developing bipolar disorder. Examples of traumatic events found to trigger attacks include childbirth, losing a job or a loved one, divorce, misusing or overusing drugs or alcohol, or traumatic head injuries.
How Is Bipolar Disorder Diagnosed?
To diagnose bipolar disorder, a healthcare provider will ask about your medical history, current medications, symptoms, and your family’s mental health history. You’ll also undergo a physical exam and, in some cases, blood tests to rule out other potential causes of bipolar disorder symptoms, such as hypothyroidism, stroke, and substance use disorder.
A healthcare provider or a mental health specialist, like a psychiatrist or psychologist , will perform a mental health evaluation. They will diagnose bipolar disorder and identify the type based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).
Diagnosing Bipolar 1 Disorder
According to the DSM-5, to be diagnosed with bipolar 1 disorder, you must have had at least one manic episode. This may be followed or preceded by a hypomanic or major depressive episode. While hypomanic or major depressive episodes can occur in bipolar 1, they are not required for a diagnosis.
In bipolar 1 disorder, manic episodes last at least one week or are severe enough to require hospitalization. A healthcare provider will look for at least three (or four if you experience irritability) of the following to diagnose you with bipolar 1 disorder:
- An inflated self-esteem or sense of grandiosity
- Difficulty concentrating; being easily distracted
- Increased activities, agitation, toe-tapping, pacing, or other unnecessary movements
- Increased engagement in unusually risky or self-destructive activities
- Racing thoughts; thoughts in flight
- Reduced need for sleep
Diagnosing Bipolar 2 Disorder
A diagnosis of bipolar 2 disorder is made based on four criteria:
- A current or past episode of hypomania and at least one major depressive episode
- Never having a manic episode
- No other psychological or neurological issues can explain the symptoms
- The mood changes cause impairments in social, personal, and professional life and daily functioning
"Hypomania" is defined as at least four days of manic symptoms that aren’t as severe or numerous as with a full manic episode. Major depressive episodes are defined as having daily or nearly daily symptoms for at least two weeks. According to the DSM-5, these are diagnosed when you display five of the following criteria:
- Agitation, toe-tapping, or pacing
- A lack of interest or enjoyment in life
- Decreased ability to concentrate
- Depression, sadness
- Fatigue, insufficient energy
- Inappropriate guilt or lack of self-worth
- Thinking about suicide without making a concrete plan (suicidal ideation)
- Weight loss without dieting, weight gain, decrease or increase in appetite
Diagnostic Criteria for Cyclothymic Disorder
In the DSM-5, among the criteria for cyclothymic disorder are the following:
- You have neutral, asymptomatic periods for no more than three months at a time.
- Symptoms arise independent of substance use disorder.
- Symptoms hinder your ability to function and impact your work, school, home, or social life.
- Symptoms are inconsistent with bipolar 1 or 2 or another mental health condition.
- You experience two or more years of hypomania and depressive episodes if an adult and at least one year of symptoms if a child.
Bipolar Disorder Treatment
Treating bipolar disorder typically involves adopting multiple strategies, including medications, counseling, and lifestyle changes.
Medications
Antidepressants, mood stabilizers, and atypical antipsychotics are medication types that healthcare providers consider. A healthcare provider may prescribe selective serotonin reuptake inhibitors (SSRIs), a class of antidepressants, for depression associated with bipolar disorder. However, these can cause what is known as cycling—rapid mood shifts—so healthcare providers prescribe them with caution.
Mood-stabilizing drugs, such as Lithobid (lithium) and Depacon (valproate), are indicated alongside SSRIs and help ease or shorten the length of mood episodes. A provider may also prescribe medications to address insomnia (sleep problems) and anxiety, which often accompany bipolar disorder.
Medication Side Effects
The side effects of medications depend on the type you’re taking. For bipolar disorder, the most common of these are unintended weight gain, sedation, restlessness, and changes in metabolism.
Psychotherapy and Counseling
Psychotherapy and counseling involve talk therapy with a psychiatrist, therapist, or trained counselor. This work aims to identify and change problematic behaviors, thoughts, or emotions that set off episodes. Another alternative is cognitive behavioral therapy (CBT), which focuses on changing thought patterns.
Lifestyle Changes
Alongside medical treatments or therapy, lifestyle changes can help you manage bipolar disorder, including:
- Relax : Activities like yoga or meditation may help ease anxiety and help with symptoms.
- Stay active : Regular exercise improves sleep and helps with stress, among other benefits.
- Dietary changes : Poor diet is associated with an increased risk for bipolar disorder and a reduced risk of co-occurring conditions.
- Avoid substances : Drinking alcohol, smoking tobacco, or using recreational drugs can all increase the risk of bipolar symptoms.
- Education : Understand the symptoms of bipolar disorder and keep track of events or things that trigger symptoms; know your medications and their side effects.
Living with bipolar disorder means finding a support system , developing coping mechanisms, and managing the shifts in your mood and behaviors. Strategies that can help include:
- Adding structure to your daily activities
- Enlisting loved ones and/or family members in your care
- Ensuring you’re getting regular exercise and enough sleep
- Making sure to take part in enjoyable activities, staying connected to friends, family, and the local community
- Seeking out social support from online or in-person support groups, social media, or message boards
- Seeking treatment, developing a treatment plan with your healthcare provider
- Tracking and logging your symptoms, medications, and triggers
Bipolar disorder causes dramatic and lasting mood and behavior shifts. People with the condition go through high-energy manic episodes and often also experience depressive episodes. Because of its effects on behavior, bipolar disorder can significantly impact your professional, academic, and/or personal life. If you suspect you or someone you care for has this condition, talk to a healthcare provider for an accurate diagnosis and treatment.
American Psychiatric Association. What are bipolar disorders?
MedlinePlus. Bipolar disorder .
National Institute of Mental Health. Bipolar disorder: definition .
National Institute of Mental Health. Bipolar disorder: overview .
National Alliance on Mental Illness (NAMI). Bipolar disorder .
MedlinePlus. Lithium toxicity .
Rowland TA, Marwaha S. Epidemiology and risk factors for bipolar disorder . Ther Adv Psychopharmacol . 2018 26;8(9):251-269. doi:10.1177/2045125318769235.
Abé C, Liberg B, Klahn AL, Petrovic P, Landén M. Mania-related effects on structural brain changes in bipolar disorder – a narrative review of the evidence. Mol Psychiatry . 2023;28(7):2674-2682. doi:10.1038/s41380-023-02073-4
Lee JG, Woo YS, Park SW, Seog DH, Seo MK, Bahk WM. Neuromolecular etiology of bipolar disorder: possible therapeutic targets of mood stabilizers . Clin Psychopharmacol Neurosci . 2022;20(2):228-239. doi:10.9758/cpn.2022.20.2.228
Substance Abuse and Mental Health Services Administration. DSM-5 changes: implications for child serious emotional disturbance . Substance Abuse and Mental Health Services Administration; 2016. Table 12, DSM-IV to DSM-5 Bipolar I Disorder Comparison.
Perugi G, Hantouche E, Vannucchi G. Diagnosis and treatment of cyclothymia: the "primacy" of temperament . Curr Neuropharmacol . 2017;15(3):372-379. doi:10.2174/1570159X14666160616120157
Marzani G, Neff AP. Bipolar disorders: evaluation and treatment . Am Fam Physician. 2021;103(4):227-239
Bauer IE, Gálvez JF, Hamilton JE, et al. Lifestyle interventions targeting dietary habits and exercise in bipolar disorder: A systematic review . J Psychiatr Res . 2016;74:1-7. doi:10.1016/j.jpsychires.2015.12.006
By Mark Gurarie Gurarie is a freelance writer and editor. He is a writing composition adjunct lecturer at George Washington University.
- Virtual Tour
- Ask the Brain
- Message from the Director
- The McGoverns
- Administration
- Explore the Brain
- Polina Anikeeva
- Emilio Bizzi
- Martha Constantine-Paton
- Robert Desimone
- James DiCarlo
- Ev Fedorenko
- Michale Fee
- Guoping Feng
- John Gabrieli
- Ann Graybiel
- Mark Harnett
- H. Robert Horvitz
- Alan Jasanoff
- Mehrdad Jazayeri
- Nancy Kanwisher
- Josh McDermott
- Tomaso Poggio
- Rebecca Saxe
- Nidhi Seethapathi
- Guangyu Robert Yang
- Satrajit Ghosh
- Dimitrios Pantazis
- Ian Wickersham
- Brain Imaging
- Cellular & Molecular Neuroscience
- Cognitive Neuroscience
- Computational Neuroscience
- Genome Engineering
- Neurotechnology
- Systems Neuroscience
- Alzheimer’s Disease
- Autism Spectrum Disorder
- Bipolar Disorder
- Huntington’s Disease
- Obsessive-Compulsive Disorder
- Parkinson’s Disease
- Schizophrenia
- Participate in a Study
- Community Resources
- Athinoula A. Martinos Imaging Center
- Poitras Center for Psychiatric Disorders Research
- Yang Tan Collective
- Join Our Mailing List
- Newsletter Archive
- Sponsored Researchers
- Meet Our Supporters
- Events Calendar
- McGovern Institute Annual Symposium
- Edward M. Scolnick Prize in Neuroscience
- Phillip A. Sharp Lecture in Neural Circuits
The new technique could enable detailed studies of how brain cells develop and communicate with each other.
Using MRI, engineers have found a way to detect light deep in the brain
Anne Trafton | May 10, 2024 May 10, 2024
Categories: Cellular & Molecular Neuroscience , Brain Imaging , Alan Jasanoff
Scientists often label cells with proteins that glow, allowing them to track the growth of a tumor, or measure changes in gene expression that occur as cells differentiate.
While this technique works well in cells and some tissues of the body, it has been difficult to apply this technique to image structures deep within the brain, because the light scatters too much before it can be detected.
MIT engineers have now come up with a novel way to detect this type of light, known as bioluminescence, in the brain: They engineered blood vessels of the brain to express a protein that causes them to dilate in the presence of light. That dilation can then be observed with magnetic resonance imaging (MRI), allowing researchers to pinpoint the source of light.
“A well-known problem that we face in neuroscience, as well as other fields, is that it’s very difficult to use optical tools in deep tissue. One of the core objectives of our study was to come up with a way to image bioluminescent molecules in deep tissue with reasonably high resolution,” says Alan Jasanoff, an MIT professor of biological engineering, brain and cognitive sciences, and nuclear science and engineering.
The new technique developed by Jasanoff and his colleagues could enable researchers to explore the inner workings of the brain in more detail than has previously been possible.
Jasanoff, who is also an associate investigator at MIT’s McGovern Institute for Brain Research, is the senior author of the study, which appears today in Nature Biomedical Engineering . Former MIT postdocs Robert Ohlendorf and Nan Li are the lead authors of the paper.
Detecting light
Bioluminescent proteins are found in many organisms, including jellyfish and fireflies. Scientists use these proteins to label specific proteins or cells, whose glow can be detected by a luminometer. One of the proteins often used for this purpose is luciferase, which comes in a variety of forms that glow in different colors.
Jasanoff’s lab, which specializes in developing new ways to image the brain using MRI, wanted to find a way to detect luciferase deep within the brain. To achieve that, they came up with a method for transforming the blood vessels of the brain into light detectors. A popular form of MRI works by imaging changes in blood flow in the brain, so the researchers engineered the blood vessels themselves to respond to light by dilating.
“Blood vessels are a dominant source of imaging contrast in functional MRI and other non-invasive imaging techniques, so we thought we could convert the intrinsic ability of these techniques to image blood vessels into a means for imaging light, by photosensitizing the blood vessels themselves,” Jasanoff says.
To make the blood vessels sensitive to light, the researcher engineered them to express a bacterial protein called Beggiatoa photoactivated adenylate cyclase (bPAC). When exposed to light, this enzyme produces a molecule called cAMP, which causes blood vessels to dilate. When blood vessels dilate, it alters the balance of oxygenated and deoxygenated hemoglobin, which have different magnetic properties. This shift in magnetic properties can be detected by MRI.
BPAC responds specifically to blue light, which has a short wavelength, so it detects light generated within close range. The researchers used a viral vector to deliver the gene for bPAC specifically to the smooth muscle cells that make up blood vessels. When this vector was injected in rats, blood vessels throughout a large area of the brain became light-sensitive.
“Blood vessels form a network in the brain that is extremely dense. Every cell in the brain is within a couple dozen microns of a blood vessel,” Jasanoff says. “The way I like to describe our approach is that we essentially turn the vasculature of the brain into a three-dimensional camera.”
Once the blood vessels were sensitized to light, the researchers implanted cells that had been engineered to express luciferase if a substrate called CZT is present. In the rats, the researchers were able to detect luciferase by imaging the brain with MRI, which revealed dilated blood vessels.
Tracking changes in the brain
The researchers then tested whether their technique could detect light produced by the brain’s own cells, if they were engineered to express luciferase. They delivered the gene for a type of luciferase called GLuc to cells in a deep brain region known as the striatum. When the CZT substrate was injected into the animals, MRI imaging revealed the sites where light had been emitted.
This technique, which the researchers dubbed bioluminescence imaging using hemodynamics, or BLUsH, could be used in a variety of ways to help scientists learn more about the brain, Jasanoff says.
For one, it could be used to map changes in gene expression, by linking the expression of luciferase to a specific gene. This could help researchers observe how gene expression changes during embryonic development and cell differentiation, or when new memories form. Luciferase could also be used to map anatomical connections between cells or to reveal how cells communicate with each other.
The researchers now plan to explore some of those applications, as well as adapting the technique for use in mice and other animal models.
The research was funded by the U.S. National Institutes of Health, the G. Harold and Leila Y. Mathers Foundation, Lore Harp McGovern, Gardner Hendrie, a fellowship from the German Research Foundation, a Marie Sklodowska-Curie Fellowship from the European Union, and a Y. Eva Tan Fellowship and a J. Douglas Tan Fellowship, both from the McGovern Institute for Brain Research.

Women in STEM — A celebration of excellence and curiosity
A new computational technique could make it easier to engineer useful proteins
Reevaluating an approach to functional brain imaging

IMAGES
VIDEO
COMMENTS
Current status of depression in bipolar disorder. Depression in bipolar disorder (BD) is the major residual psychiatric morbidity with available treatments, accounting for three-quarters of the 40-50% long-term time-ill. Unresolved morbidity, and especially depression, is associated with excess medical morbidity, including metabolic syndrome ...
Overview of bipolar disorder. Bipolar disorder is a chronic and complex mood disorder that is characterized by an admixture of manic (bipolar mania), hypomanic and depressive (bipolar depression) episodes, with significant subsyndromal symptoms that commonly present between major mood episodes Citation 1.Ranked among the leading causes of worldwide disability Citation 2, bipolar I disorder has ...
Depression in bipolar disorder (BD) patients presents major clinical challenges. As the predominant psychopathology even in treated BD, depression is associated not only with excess morbidity, but also mortality from co-occurring general-medical disorders and high suicide risk. In BD, risks for medical disorders including diabetes or metabolic syndrome, and cardiovascular disorders, and ...
Major depression is a common and often disabling illness with significant morbidity and mortality. In 2019, the 12-month prevalence of major depression in U.S. adults was estimated to be 7.8%, and in adolescents 15.7% ().While children can also suffer from major depression, the peak prevalence of major depression occurs during adolescence and early adulthood.
Bipolar disorder (BD) is defined by the occurrence of active episodes of mania and depression, which show opposite constellations of disturbances in psychomotricity, affectivity, and thought ...
Bipolar II disorder is characterized mainly by episodes of depression but alternat-ing with hypomania rather than mania. The presence of at least one hypomanic episode in a life trajectory is ...
Bipolar disorders (BDs) are recurrent and sometimes chronic disorders of mood that affect around 2% of the world's population and encompass a spectrum between severe elevated and excitable mood states (mania) to the dysphoria, low energy, and despondency of depressive episodes. The illness commonly starts in young adults and is a leading cause of disability and premature mortality.
Abstract. Bipolar disorders are a complex group of severe and chronic disorders that includes bipolar I disorder, defined by the presence of a syndromal, manic episode, and bipolar II disorder, defined by the presence of a syndromal, hypomanic episode and a major depressive episode. Bipolar disorders substantially reduce psychosocial ...
Bipolar disorder is a recurrent disorder that affects more than 1% of the world population and usually has its onset during youth. Its chronic course is associated with high rates of morbidity and mortality, making bipolar disorder one of the main causes of disability among young and working-age people. The implementation of early intervention strategies may help to change the outcome of the ...
Bipolar disorder (BD) is a major mood disorder characterized by recurrent episodes of depression and (hypo)mania (Goodwin and Jamison 2007).According to the Diagnostic and Statistical Manual 5 (DSM-5), the two main subtypes are BD-I (manic episodes, often combined with depression) and BD-II (hypomanic episodes, combined with depression) (APA 2014).
Bipolar disorder (BD) is a severe psychiatric illness with an increasing prevalence worldwide. Although the pathological mechanism of and pharmacological interventions for BD have been extensively investigated in preclinical and clinical studies, a scientometric analysis of the developmental trends, interdisciplinary frontiers, and research hotspots in this field has not yet been conducted.
Background Previous research has suggested that the way bipolar patients respond to depressive mood impacts on the future course of the illness, with rumination prolonging depression and risk-taking possibly triggering hypomania. However, the relationship over time between variables such as mood, self-esteem, and response style to negative affect is complex and has not been directly examined ...
Abstract. Bipolar disorder is a severe, complicated, and often misunderstood disorder that can have serious impacts on a person's quality of life, sense of self-worth, and overall health. This ...
ObjectiveThe treatment of bipolar depression remains challenging due to the limited effective and safe therapeutic options available; thus, developing newer treatments that are effective and well t...
In line with the Research Domain Criteria Project launched by the National Institute of Mental Health (Insel, 2014; Insel et al., 2010), a distinguished aim in developing an integrated neuroscientific model of depression therefore has to be the separation of distinct aetiological and pathophysiological trajectories which, although eventually ...
BD II diagnosis requires at least one lifetime hypomanic episode and one major depressive episode. Despite clarity of BD II diagnostic criteria, clinicians struggle to accurately identify it in practice. BD II is often either missed or incorrectly diagnosed, resulting in an over 10-year delay in diagnosis.
Analysis of Published Papers. In the past decade, the total number of papers on depression published worldwide has increased year by year as shown in Fig. 1A. Searching the Web of Science database, we found a total of 43,863 papers published in the field of depression from 2009 to 2019 (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009-2019), Articles).
Our paper also relates to research on the relationship between health and work, ... Musliner, K. L. & Agerbo, E. Bipolar disorder and depression in early adulthood and long-term employment, income ...
When our clinical research group developed the Psychiatric Diagnostic Screening Questionnaire (PDSQ), we intended it as a diagnostic aid to be used in clinical practice to reduce underdiagnosis of disorders comorbid with the principal diagnosis and improve clinicians' efficiency in conducting the initial diagnostic evaluation 8. Consequently ...
Add-on ketamine seems to be a good choice for the treatment of anhedonia in treatment resistant bipolar depression and showed a good effect in reducing symptoms of anxiety in this group of patients. BACKGROUND Bipolar depression is the major cause of morbidity in patients with bipolar disorder. It affects psychosocial functioning and markedly impairs occupational productivity.
Formerly known as manic depression, this condition causes mood episodes lasting days or weeks at a time and hinder day-to-day functioning, school or work performance, and relationships. ... Some research shows that people with bipolar disorder have smaller subcortical structures (associated with mood and cognition) and a thinner cortex (the ...
The research was funded by the U.S. National Institutes of Health, the G. Harold and Leila Y. Mathers Foundation, Lore Harp McGovern, Gardner Hendrie, a fellowship from the German Research Foundation, a Marie Sklodowska-Curie Fellowship from the European Union, and a Y. Eva Tan Fellowship and a J. Douglas Tan Fellowship, both from the McGovern ...
Results. Nearly 77 % of participants consumed milk tea at least 6-11 cups in the last year. The confirmatory factor analysis (CFA) supported the one-factor structure of the milk tea addiction scale, developed according to DSM-5 substance use guidelines. Moreover, we found that a higher level of milk tea addiction was significantly associated with a higher risk of depression (b = 0.24, p < 0. ...