- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Editorial Commentaries
- Perspectives
- Cover Archive
- IDSA Journals
- Clinical Infectious Diseases
- Open Forum Infectious Diseases
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About The Journal of Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Conclusions.
- < Previous
The Extended Impact of Human Immunodeficiency Virus/AIDS Research
- Article contents
- Figures & tables
- Supplementary Data
Tara A Schwetz, Anthony S Fauci, The Extended Impact of Human Immunodeficiency Virus/AIDS Research, The Journal of Infectious Diseases , Volume 219, Issue 1, 1 January 2019, Pages 6–9, https://doi.org/10.1093/infdis/jiy441
- Permissions Icon Permissions
Human immunodeficiency virus (HIV) is one of the most extensively studied viruses in history, and numerous extraordinary scientific advances, including an in-depth understanding of viral biology, pathogenesis, and life-saving antiretroviral therapies, have resulted from investments in HIV/AIDS research. While the substantial investments in HIV/AIDS research are validated solely on these advances, the collateral broader scientific progress resulting from the support of HIV/AIDS research over the past 30 years is extraordinary as well. The positive impact has ranged from innovations in basic immunology and structural biology to treatments for immune-mediated diseases and cancer and has had an enormous effect on the research and public and global health communities well beyond the field of HIV/AIDS. This article highlights a few select examples of the unanticipated and substantial positive spin-offs of HIV/AIDS research on other scientific areas.
The first cases of AIDS were reported in the United States 37 years ago. Since then, >77 million people have been infected worldwide, resulting in over 35 million deaths. Currently, there are 36.9 million people living with human immunodeficiency virus (HIV), 1.8 million new infections, and nearly 1 million AIDS-related deaths annually [ 1 ]. Billions of research dollars have been invested toward understanding, treating, and preventing HIV infection. The largest funder of HIV/AIDS research is the National Institutes of Health (NIH), investing nearly $69 billion in AIDS research from fiscal years 1982–2018. Despite the staggering disease burden, the scientific advances directly resulting from investments in AIDS research have been extraordinary. HIV is one of the most intensively studied viruses in history, leading to an in-depth understanding of viral biology and pathogenesis. However, the most impressive advances in HIV/AIDS research have come in the arena of antiretroviral therapy. Before the development of these life-saving drugs, AIDS was an almost universally fatal disease. Since the demonstration in 1987 that a single drug, zidovudine, better known as azidothymidine or AZT, could partially and temporarily suppress virus replication [ 2 ], the lives of people living with HIV have been transformed by the current availability of >30 antiretroviral drugs that, when administered in combinations of 3 drugs, now in a single daily pill, suppress the virus to undetectable levels. Today, if a person in their 20s is infected and given a combination of antiretroviral drugs that almost invariably will durably suppress virus to below detectable levels, they can anticipate living an additional 50 years, allowing them almost a normal life expectancy [ 3 ]. In addition, a person receiving antiretroviral therapy with an undetectable viral load will not transmit virus to their uninfected sexual partner. This strategy is referred to as “treatment as prevention” [ 4 ]. Also, administration of a single pill containing 2 antiretroviral drugs taken daily by an at-risk uninfected person decreases the chance of acquiring HIV by >95%. Finally, major strides are being made in the quest for a safe and effective HIV vaccine [ 5 ].
The enormous investment in HIV research is clearly justified and validated purely on the basis of advances specifically related to HIV/AIDS. However, the collateral advantages of this investment above and beyond HIV/AIDS have been profound, leading to insights and concrete advances in separate, diverse, and unrelated fields of biomedical research and medicine. In the current Perspective, we discuss a few select examples of the positive spin-offs of HIV/AIDS research on other scientific areas ( Table 1 ).
Positive Spin-offs of Human Immuno deficiency Virus/AIDS Research on Other Areas of Medicine
Abbreviation: HIV, human immunodeficiency virus.
Regulation of the Human Immune System
Congenital immunodeficiencies have been described as “experiments of nature,” whereby a specific defect in a single component of the complex immune system sheds light on the entire system. Such is the case with AIDS, an acquired defect in the immune system whereby HIV specifically and selectively infects and destroys the CD4 + subset of T lymphocytes [ 6 ]. In this respect, HIV infection functions as a natural experiment that elucidates the complexity of the human immune system. The selectivity of this defect and its resulting catastrophic effect on host defense mechanisms, as manifested by the wide range of opportunistic infections and neoplasms, underscore the critical role this cell type plays in the overall regulation of the human immune system. This has provided substantial insights into the pathogenesis of an array of other diseases characterized by aberrancies of immune regulation. Additionally, the in-depth study of immune dysfunction in HIV disease has shed light on the role of the immune system in surveillance against a variety of neoplastic diseases, such as non-Hodgkin lymphoma and Kaposi sarcoma. As a result of its association with HIV/AIDS, Kaposi sarcoma was discovered to be caused by human herpesvirus 8 [ 7 ].
Targeted Antiviral Drug Development
Targeted antiviral drug development did not begin with HIV infection. However, the enormous investments in biomedical research supported by the NIH and in drug development supported by pharmaceutical companies led to highly effective antiretroviral drugs targeting the enzymes reverse transcriptase, protease, and integrase, among other vulnerable points in the HIV replication cycle, and have transformed the field of targeted drug development, bringing it to an unprecedented level of sophistication. Building on 3 decades of experience, this HIV model has been applied in the successful development of antiviral drugs for other viral diseases, including the highly effective and curative direct-acting antivirals for hepatitis C [ 8 ].
Probing the B-Cell Repertoire
The past decade has witnessed extraordinary advances in probing the human B-cell lineage resulting from the availability of highly sophisticated technologies in cellular cloning and genomic sequencing [ 9 ]. AIDS research aimed at developing broadly reactive neutralizing antibodies against HIV and an HIV vaccine that could induce broadly neutralizing antibodies has greatly advanced the field of interrogation of human B-cell lineages, leading to greater insights into the humoral response to other infectious diseases, including Ebola [ 10 ], Zika [ 11 ], and influenza [ 12 ], as well as a range of autoimmune, neoplastic, and other noncommunicable diseases [ 13 ].
Structure-Based Vaccine Design
Although a safe and effective HIV vaccine has not yet been developed, the discipline of structure-based vaccine design using protein X-ray crystallography and cryoelectron microscopy has matured greatly in the context of HIV vaccine research. The design of immunogens based on the precise conformation of epitopes in the viral envelope as they bind to neutralizing antibodies has been perfected within the arena of HIV vaccine immunogen design. This has had immediate positive spinoffs in the design of vaccines for other viruses, such as respiratory syncytial virus, in which the prefusion glycoprotein was identified as the important immunogen for a vaccine using structure-based approaches [ 14 ].
Advances in HIV/AIDS-Related Technologies
Insights into the basic immunology of HIV drove the development and optimization of several broadly applicable technologies. Using inactivated HIV as a means of altering T lymphocytes to modulate the immune response, safe lentiviral gene therapy vectors are now US Food and Drug Administration–approved to treat certain cancers (eg, acute lymphoblastic leukemia) [ 15 ]. Additionally, it was discovered early in the epidemic that HIV is associated with the loss of CD4 + T lymphocytes [ 16 ]. While much of the initial research on CD4 + T lymphocytes was possible due to existing flow cytometry technologies, probing the complexities of immune dysregulation in HIV infection spurred the development of multicolor cytofluorometric technologies that have proven extremely useful for studying a variety of other diseases characterized by immune dysfunction [ 17 ]. The reality of utilizing these technologies in resource-poor areas accelerated the advancement of new simplified, automated, affordable, and portable point-of-care devices with broader implications for clinical medicine [ 18 ].
Role of Immune Activation in Disease Pathogenesis
Studying the pathogenesis of HIV disease has clearly demonstrated that aberrant immune activation stimulated by virus replication is the driving force of HIV replication [ 19 ]. In essence, the somewhat paradoxical situation exists whereby the very immune activation triggered by the virus in an attempt to control virus replication creates the microenvironment where the virus efficiently replicates. Even when the virus is effectively suppressed by antiretroviral drugs, a low degree of immune activation persists [ 20 ]. In this regard, the flagrant immune activation associated with uncontrolled virus replication, as well as the subtle immune activation associated with control of virus replication, are important pathogenic triggers of the increased cardiovascular and other organ system diseases associated with HIV infection. This direct association of even subtle levels of immune activation seen in HIV infection with a variety of systemic diseases has led to considerable insight into the role of immune activation and inflammation in human disease [ 21 ]. For example, recognition of the increased incidence of heart disease in the HIV population that is associated with chronic inflammation has stimulated interdisciplinary advances in understanding and treating coronary heart disease apart from HIV infection [ 22 ].
Comorbidities in HIV Disease
Antiretroviral therapy, which has transformed HIV treatment, is shifting the incidence of certain diseases in people living with HIV. Even when well-controlled by antiretrovirals, HIV disease is associated with an increased incidence of diseases, such as cardiovascular disease, kidney and liver disease, the premature appearance of pathophysiologic processes associated with aging, and several cancers [ 21–24 ]. This is especially true for non-AIDS-defining cancers, whose incidence rates are increasing while AIDS-defining cancer rates are decreasing [ 24 ]. In lower-income countries, tuberculosis is a common coinfection with HIV, and HIV coinfection was shown to be a key risk factor for progression of latent Mycobacterium tuberculosis infection to active disease [ 25 ]. There are a variety of ongoing studies [ 21 ] investigating the pathogenic bases of these conditions to shed greater insight into their causes and potential interventions that might impact these diseases apart from HIV infection and immunodeficiency.
The collateral advantages resulting from the substantial resources devoted to HIV/AIDS research over the past 30 years are extraordinary. From innovations in basic immunology and structural biology to treatments for immune-mediated diseases and cancer, the conceptual and technological advances resulting from HIV/AIDS research have had an enormous impact on the research and public and global health communities over and above the field of HIV/AIDS. The HIV/AIDS research model has proven that cross-fertilization of ideas, innovation, and research progress can lead to unforeseen and substantial advantages for a variety of other diseases.
Acknowledgments. The authors thank Carl Dieffenbach, Daniel Rotrosen, Charles Hackett, and Robert Eisinger for their helpful input in preparation of the manuscript.
Potential conflicts of interest. Both authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Joint United Nations Progamme on HIV/AIDS . Fact sheet: latest statistics on the status of the AIDS epidemic . http://www.unaids.org/en/resources/fact-sheet . Accessed 23 July 2018.
Fischl MA , Richman DD , Grieco MH , et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial . N Engl J Med 1987 ; 317 : 185 – 91 .
Google Scholar
Samji H , Cescon A , Hogg RS , et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA . Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada . PLoS One 2013 ; 8 : e81355 .
Lundgren JD , Babiker AG , Gordin F , et al. INSIGHT START Study Group . Initiation of antiretroviral therapy in early asymptomatic HIV infection . N Engl J Med 2015 ; 373 : 795 – 807 .
Trovato M , D’Apice L , Prisco A , De Berardinis P . HIV vaccination: a roadmap among advancements and concerns . Int J Mol Sci 2018 ; 19 . doi: 10.3390/ijms19041241 .
Dalgleish AG , Beverley PC , Clapham PR , Crawford DH , Greaves MF , Weiss RA . The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus . Nature 1984 ; 312 : 763 – 7 .
Schulz TF , Boshoff CH , Weiss RA . HIV infection and neoplasia . Lancet 1996 ; 348 : 587 – 91 .
Wyles DL . Antiviral resistance and the future landscape of hepatitis C virus infection therapy . J Infect Dis 2013 ; 207 ( Suppl 1 ): S33 – 9 .
Boyd SD , Crowe JE Jr . Deep sequencing and human antibody repertoire analysis . Curr Opin Immunol 2016 ; 40 : 103 – 9 .
Flyak AI , Kuzmina N , Murin CD , et al. Broadly neutralizing antibodies from human survivors target a conserved site in the Ebola virus glycoprotein HR2-MPER region . Nat Microbiol 2018 ; 3 : 670 – 7 .
Sapparapu G , Fernandez E , Kose N , et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice . Nature 2016 ; 540 : 443 – 7 .
Raymond DD , Bajic G , Ferdman J , et al. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody . Proc Natl Acad Sci U S A 2018 ; 115 : 168 – 73 .
Röhn TA , Bachmann MF . Vaccines against non-communicable diseases . Curr Opin Immunol 2010 ; 22 : 391 – 6 .
Tian D , Battles MB , Moin SM , et al. Structural basis of respiratory syncytial virus subtype-dependent neutralization by an antibody targeting the fusion glycoprotein . Nat Commun 2017 ; 8 : 1877 .
US Food and Drug Administration . FDA approval brings first gene therapy to the United States . Silver Spring, MD : FDA , 2017 .
Google Preview
Gottlieb MS , Schroff R , Schanker HM , et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency . N Engl J Med 1981 ; 305 : 1425 – 31 .
Chattopadhyay PK , Roederer M . Cytometry: today’s technology and tomorrow’s horizons . Methods 2012 ; 57 : 251 – 8 .
Kestens L , Mandy F . Thirty-five years of CD4 T-cell counting in HIV infection: from flow cytometry in the lab to point-of-care testing in the field . Cytometry B Clin Cytom 2017 ; 92 : 437 – 44 .
Moir S , Fauci AS . B-cell exhaustion in HIV infection: the role of immune activation . Curr Opin HIV AIDS 2014 ; 9 : 472 – 7 .
Paiardini M , Müller-Trutwin M . HIV-associated chronic immune activation . Immunol Rev 2013 ; 254 : 78 – 101 .
Lucas S , Nelson AM . HIV and the spectrum of human disease . J Pathol 2015 ; 235 : 229 – 41 .
Boccara F , Lang S , Meuleman C , et al. HIV and coronary heart disease: time for a better understanding . J Am Coll Cardiol 2013 ; 61 : 511 – 23 .
Torres RA , Lewis W . Aging and HIV/AIDS: pathogenetic role of therapeutic side effects . Lab Invest 2014 ; 94 : 120 – 8 .
Thrift AP , Chiao EY . Are non-HIV malignancies increased in the HIV-infected population ? Curr Infect Dis Rep 2018 ; 20 : 22 .
Getahun H , Gunneberg C , Granich R , Nunn P . HIV infection-associated tuberculosis: the epidemiology and the response . Clin Infect Dis 2010 ; 50 ( Suppl 3 ): S201 – 7 .
- acquired immunodeficiency syndrome
Email alerts
Related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6613
- Print ISSN 0022-1899
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Research & training, advances in hiv/aids research.
For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV .
Over the past several decades, researchers have learned a lot about the human immunodeficiency virus (HIV) and the disease it causes, acquired immunodeficiency syndrome (AIDS). But still more research is needed to help the millions of people whose health continues to be threatened by the global HIV/AIDS pandemic.
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as well as to better understand the virus with the goal of finding a cure. For example, in recent months, NIAID and its partners made progress toward finding a vaccine to prevent HIV infection. Check out other promising areas of NIAID-funded research on HIV/AIDS at http://www.niaid.nih.gov/topics/hivaids/Pages/Default.aspx .
Other NIH institutes, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute on Alcohol Abuse and Alcoholism, also support research to better control and ultimately end the HIV/AIDS pandemic. Some of these researchers have found a simple, cost-effective way to cut HIV transmission from infected mothers to their breastfed infants. Others have developed an index to help measure the role of alcohol consumption in illness and death of people with HIV/AIDS.
Find out more about these discoveries and what they mean for improving the health of people in the United States and all around the globe.
This page last reviewed on August 20, 2015
Connect with Us
- More Social Media from NIH
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Emily Mullin
There’s New Hope for an HIV Vaccine
Since it was first identified in 1983, HIV has infected more than 85 million people and caused some 40 million deaths worldwide.
While medication known as pre-exposure prophylaxis , or PrEP, can significantly reduce the risk of getting HIV, it has to be taken every day to be effective. A vaccine to provide lasting protection has eluded researchers for decades. Now, there may finally be a viable strategy for making one.
An experimental vaccine developed at Duke University triggered an elusive type of broadly neutralizing antibody in a small group of people enrolled in a 2019 clinical trial. The findings were published today in the scientific journal Cell .
“This is one of the most pivotal studies in the HIV vaccine field to date,” says Glenda Gray, an HIV expert and the president and CEO of the South African Medical Research Council, who was not involved in the study.
A few years ago, a team from Scripps Research and the International AIDS Vaccine Initiative (IAVI) showed that it was possible to stimulate the precursor cells needed to make these rare antibodies in people. The Duke study goes a step further to generate these antibodies, albeit at low levels.
“This is a scientific feat and gives the field great hope that one can construct an HIV vaccine regimen that directs the immune response along a path that is required for protection,” Gray says.
Vaccines work by training the immune system to recognize a virus or other pathogen. They introduce something that looks like the virus—a piece of it, for example, or a weakened version of it—and by doing so, spur the body’s B cells into producing protective antibodies against it. Those antibodies stick around so that when a person later encounters the real virus, the immune system remembers and is poised to attack.
While researchers were able to produce Covid-19 vaccines in a matter of months, creating a vaccine against HIV has proven much more challenging. The problem is the unique nature of the virus. HIV mutates rapidly, meaning it can quickly outmaneuver immune defenses. It also integrates into the human genome within a few days of exposure, hiding out from the immune system.
“Parts of the virus look like our own cells, and we don’t like to make antibodies against our own selves,” says Barton Haynes, director of the Duke Human Vaccine Institute and one of the authors on the paper.
The particular antibodies that researchers are interested in are known as broadly neutralizing antibodies, which can recognize and block different versions of the virus. Because of HIV’s shape-shifting nature, there are two main types of HIV and each has several strains. An effective vaccine will need to target many of them.
Some HIV-infected individuals generate broadly neutralizing antibodies, although it often takes years of living with HIV to do so, Haynes says. Even then, people don’t make enough of them to fight off the virus. These special antibodies are made by unusual B cells that are loaded with mutations they’ve acquired over time in reaction to the virus changing inside the body. “These are weird antibodies,” Haynes says. “The body doesn’t make them easily.”

By Carlton Reid

By Emily Mullin

By Steven Levy

By Andy Greenberg
Haynes and his colleagues aimed to speed up that process in healthy, HIV-negative people. Their vaccine uses synthetic molecules that mimic a part of HIV’s outer coat, or envelope, called the membrane proximal external region. This area remains stable even as the virus mutates. Antibodies against this region can block many circulating strains of HIV.
The trial enrolled 20 healthy participants who were HIV-negative. Of those, 15 people received two of four planned doses of the investigational vaccine, and five received three doses. The trial was halted when one participant experienced an allergic reaction that was not life-threatening. The team found that the reaction was likely due to an additive in the vaccine, which they plan to remove in future testing.
Still, they found that two doses of the vaccine were enough to induce low levels of broadly neutralizing antibodies within a few weeks. Notably, B cells seemed to remain in a state of development to allow them to continue acquiring mutations, so they could evolve along with the virus. Researchers tested the antibodies on HIV samples in the lab and found that they were able to neutralize between 15 and 35 percent of them.
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but that challenges remain. “It outlines a path for vaccine development, but there’s a lot of work that needs to be done,” he says.
For one, he says, a vaccine would need to generate antibody levels that are significantly higher and able to neutralize with greater efficacy. He also says a one-dose vaccine would be ideal. “If you’re ever going to have a vaccine that’s helpful to the world, you’re going to need one dose,” he says.
Targeting more regions of the virus envelope could produce a more robust response. Haynes says the next step is designing a vaccine with at least three components, all aimed at distinct regions of the virus. The goal is to guide the B cells to become much stronger neutralizers, Haynes says. “We’re going to move forward and build on what we have learned.”
You Might Also Like …
In your inbox: Will Knight's Fast Forward explores advances in AI
Indian voters are being bombarded with millions of deepfakes
They bought tablets in prison —and found a broken promise
The one thing that’s holding back the heat pump
It's always sunny: Here are the best sunglasses for every adventure

Max G. Levy

Beth Mole, Ars Technica
Carl Zimmer
Matt Reynolds
- Open access
- Published: 17 September 2020
HIV/AIDS research in Africa and the Middle East: participation and equity in North-South collaborations and relationships
- Gregorio González-Alcaide ORCID: orcid.org/0000-0003-3853-5222 1 na1 ,
- Marouane Menchi-Elanzi 2 na1 ,
- Edy Nacarapa 3 , 4 &
- José-Manuel Ramos-Rincón 2 , 5
Globalization and Health volume 16 , Article number: 83 ( 2020 ) Cite this article
10k Accesses
18 Citations
3 Altmetric
Metrics details
HIV/AIDS has attracted considerable research attention since the 1980s. In the current context of globalization and the predominance of cooperative work, it is crucial to analyze the participation of the countries and regions where the infection is most prevalent. This study assesses the participation of African countries in publications on the topic, as well as the degree of equity or influence existing in North-South relations.
We identified all articles and reviews of HIV/AIDS indexed in the Web of Science Core Collection. We analyzed the scientific production, collaboration, and contributions from African and Middle Eastern countries to scientific activity in the region. The concept of leadership, measured through the participation as the first author of documents in collaboration was used to determine the equity in research produced through international collaboration.
A total of 68,808 documents published from 2010 to 2017 were analyzed. Researchers from North America and Europe participated in 82.14% of the global scientific production on HIV/AIDS, compared to just 21.61% from Africa and the Middle East. Furthermore, the publications that did come out of these regions was concentrated in a small number of countries, led by South Africa (41% of the documents). Other features associated with HIV/AIDS publications from Africa include the importance of international collaboration from the USA, the UK, and other European countries (75–93% of the documents) and the limited participation as first authors that is evident (30 to 36% of the documents). Finally, the publications to which African countries contributed had a notably different disciplinary orientation, with a predominance of research on public health, epidemiology, and drug therapy.
Conclusions
It is essential to foster more balance in research output, avoid the concentration of resources that reproduces the global North-South model on the African continent, and focus the research agenda on local priorities. To accomplish this, the global North should strengthen the transfer of research skills and seek equity in cooperative ties, favoring the empowerment of African countries. These efforts should be concentrated in countries with low scientific activity and high incidence and prevalence of the disease. It is also essential to foster intraregional collaborations between African countries.
HIV infection and its clinical manifestation, AIDS, are considered a pre-eminent challenge for global public health [ 1 ], affecting populations worldwide since the 1980s. Despite the progress made in prevention and treatment programs, the disease is still pandemic, with the African continent being the hardest hit [ 2 ]. An estimated 37.9 million people were living with HIV in 2018, of whom 20.6 million lived in Eastern and Southern Africa, 5 million in Western and Central Africa, and 240,000 in the Middle East and North Africa. The same year saw about 770,000 deaths from this disease and 1.7 million new infections, 61% of which occurred in sub-Saharan Africa. Over half of the new cases in Eastern and Southern Africa were concentrated in Mozambique, South Africa, and Tanzania, while 71% of new infections in Western and Central Africa were in Cameroon, the Côte d’Ivoire, and Nigeria. In the Middle East and North Africa, two-thirds of new cases were registered in Egypt, Iran and Sudan [ 3 ]. In response to this challenge, researchers worldwide have worked to produce evidence on HIV/AIDS across a wide range of biomedical disciplines, including epidemiology, virology, immunology, and pharmacology, as well as in non-biomedical fields such as social sciences and the humanities. This body of work has situated HIV/AIDS among the most studied infectious diseases today [ 4 ].
Bibliometrics is a method that enables the quantitative and qualitative assessment of scientific research in any area of knowledge, at an individual, institutional, or national level [ 5 ]. In that sense, ample literature has been published on bibliometric analyses of HIV/AIDS research since the 1980s [ 6 , 7 ], including some papers that focus specifically on the regions most affected by the virus and the infection, like Central Africa [ 8 ]; sub-Saharan Africa [ 9 ]; or on countries like Kenya, Uganda, Nigeria, or Lesotho [ 10 , 11 , 12 ]. However, many of these papers were published more than a decade ago and investigated the scientific production in the geographical areas analyzed. In the current context of globalization and predominance of cooperative work, Africans are under-represented in terms of authorship in collaborative research publications. This situation has led some investigators to call for studies that quantify authorship equity [ 13 ] and explore North-South relationships in research collaboration [ 8 ].
The overarching objective of the present study is to provide an up-to-date description of participation from Africa and the Middle East in the literature on HIV/AIDS published in high-visibility journals, and of the role played by researchers from African countries in publications produced in international collaboration. Our specific research questions were: (1) What was the contribution from Africa and the Middle East, both overall and by country, to the global scientific research output on HIV/AIDS? (2) Is North-South participation balanced international collaboration papers? and (3) Are there differences in the subject-area orientation between publications produced with or without participation from African and Middle Eastern authors on HIV/AIDS research?
The methodological process was as follows.
Identification of global scientific research production on HIV/AIDS
To identify the scientific literature on HIV/AIDs, we used the Medical Subject Headings (MeSH) thesaurus of the National Library of Medicine, selecting all of the descriptors related to HIV, human immunodeficiency related to HIV infection, and the development of vaccines for preventing or clinically treating the immunodeficiency. The final MeSH (plus their variants and synonyms) were: HIV, HIV Infections, Acquired Immunodeficiency Syndrome, and AIDS Vaccines.
Although the MeSH thesaurus is linked to the MEDLINE database, which is freely available through the PubMed platform, we performed a second search of the documents identified in MEDLINE and which were also indexed in the Web of Science Core Collection (WoS-CC) databases. Although this database does not cover all of the documents indexed in MEDLINE/PubMed, it does include all of the institutional affiliations (which MEDLINE started listing only in 2014), making it an ideal source for characterizing scientific production by country and the collaboration from Africa and the Middle East in HIV/AIDS publications during the study period.
The collection of journals in the WoS-CC, moreover, represents the information sources with the highest visibility at an international level. Thus, using that source to calculate our bibliometric study indicators allows a vision of the development of the most relevant and impactful research worldwide.
Definition of the document sample analyzed
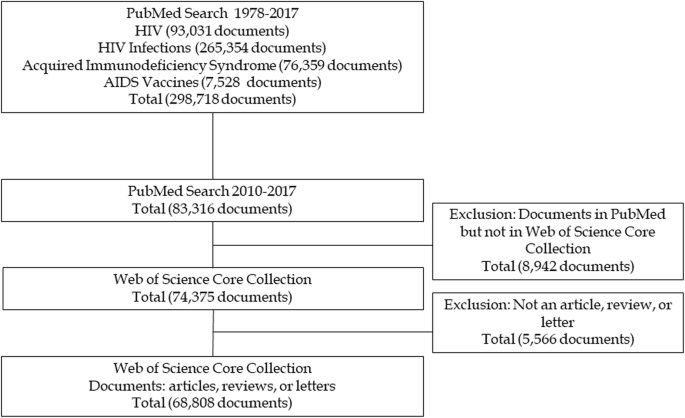
Our literature search yielded 93,031 documents on HIV, 256,354 on HIV Infections, 76,359 on Acquired Immunodeficiency Syndrome, and 7528 on AIDS Vaccines. After removing duplicate descriptors, there were 298,718 unique documents. We then restricted the results to those published from 2010 to 2017 ( n = 83,316) in order to focus the analysis on the most recent research. We ruled out the inclusion of documents from 2018 to avoid delays related to indexation, as at least a year is needed to ensure updated information related to the assignment of MeSH terms. We subsequently identified the documents that were also included in the WoS-CC databases by searching for all of the documents from the initial sample using their PMIDs (the PubMed identifier used as a reference in MEDLINE and included as a bibliographic field in WoS-CC). In total, 89.29% ( n = 74,375) of the MEDLINE documents were also in the WoS-CC. This set of papers was further restricted to three document types: articles, reviews, and letters ( n = 68,808), chosen because they are the most prominent papers for transmitting the results of original research (articles); situating and evaluating the development of research in a highly relevant way for other researchers (reviews); and contributing critical viewpoints, comments, relevant information, and perspectives on published studies (letters). The searches took place in November 2018. Figure 1 presents a flow chart showing the selection process for the sample of documents analyzed in the study.
Flow chart for the selection of included documents
Download of bibliographic information and review of the standardization of data
Following the bibliographic search and document selection, we downloaded the bibliographic information from the selected records ( n = 68,808), generating a relational database in Microsoft Access in order to enumerate and individualize the multiple entries contained in certain bibliographic fields. This is the case of institutional affiliations, as a single field collates the data for all co-authors’ institutions and countries. Likewise, the subject area field for the journal of publication may also have several assigned topics, and various MeSH and other text words are assigned to different documents to describe their content.
We also reviewed the standardization and quality of the data. For example, we looked at the years of publication, as the date of some documents’ public dissemination on the journal website differed from the definitive date of publication in the journal (the latter was taken as the reference). Likewise, we consolidated all the information on geographic origins from England, Scotland, Wales, and North Ireland—presented individually in the WoS-CC—under the UK.
Identification of participation from Africa and the Middle East in HIV/AIDS publications
To analyze the participation from Africa and the Middle East in HIV/AIDS publications, we took as a reference the UNAIDS (2018) definitions of geographical regions, assigning each country to its respective region as defined in that source. The regions were: North America, Western and Central Europe, Asia and Pacific, Eastern and Southern Africa, Latin America and the Caribbean, West and Central Africa, the Middle East and North Africa, and Eastern Europe and Central Asia.
Indicators obtained and analyses performed
The indicators and analyses applied in our study are structured in three blocks.
Analysis of the scientific production, research collaboration and leadership, by geographical region
As an introductory step to understanding global HIV/AIDS research, we quantified absolute scientific production by UNAIDS regions, calculating the number of documents authored by researchers from these areas. Moreover, we assessed inter-regional and international collaboration along with research leadership. The concepts used in the present study are defined as follows:
- International collaboration: joint participation in the authorship of a document by researchers from two or more countries.
- Inter-regional collaboration: joint participation in the authorship of a document by researchers from countries in two or more regions.
- Leadership: the degree of participation as the first author of documents in collaboration (number or % with respect to the total documents produced in collaboration).
Geographical affiliations were based, therefore, on authors’ institutional affiliations. The section on limitations includes an in-depth discussion on the shortcomings of this procedure, which should be considered when interpreting the results.
Analysis of research production, collaboration and leadership from countries in Africa and the Middle East
To specifically analyze HIV/AIDS research publications from African and Middle Eastern countries, we determined the number of documents authored by researchers from these countries as well as the proportion of total publications with their participation. With regard to research collaboration and leadership, the absolute and relative values on international collaboration are complemented by a specific analysis of research leadership in the top 10 most productive countries in Africa. Furthermore, a directed collaboration network was generated, representing the main African countries collaborating in global HIV/AIDS research. The nodes represent countries, and the links represent countries’ participation in the first positions of authorship. This visual representation clarifies the position that different countries occupy in the network and the collaborative links that they have established.
Subject areas and research fields in global HIV/AIDS research production
We analyzed the research subject areas and fields according to the disciplines that contributed most to scientific production on HIV/AIDS, as identified by means of the subject area classification of scientific journals in the WoS-CC as well as the MeSH descriptors and qualifiers assigned to the documents. To compare research orientations, we present data for global research output, for publications produced solely by researchers from African countries, and publications produced through collaborations between researchers from African countries and others (Africa+global collaboration). Pearson’s correlation coefficient was estimated for these three groupings to determine the affinity between African and global research production.
Finally, a co-occurrence network of MeSH terms was generated to analyze the relationships between them and to identify the specific subject areas or research orientations on HIV/AIDS in Africa and the Middle East.
Pajek and VoSViewer (Version 1.6.8, Center for Science and Technology, Leiden University) software were used to perform all processes (analysis, network generation) and obtain all descriptive indicators.
Scientific production by region and degree of international collaboration
Scientific production on HIV/AIDS is dominated by North America (which participated in 55.60% of all documents analyzed) and by Western and Central Europe (35.79%). Together, these regions participated in 82.13% of global scientific research production on HIV/AIDS that was indexed in the sources consulted. For their part, the three regions of Africa and the Middle East participated in 21.61% of the documents, albeit contributions from Eastern and Southern Africa (17.80%) were much higher than those from Western and Central Africa (3.34%) and Middle East and North Africa (1.18%) (Table 1 ). This limited scientific production contrasts with the high percentages of collaboration observed in these regions; in Eastern and Southern Africa, 82.42% of the papers were published in collaboration with authors from countries in other regions, and in Western and Central Africa, 78.39%. In contrast, 43.22% of the documents from North America were produced in inter-regional collaboration, and 47.99% from Western and Central Europe. Looking only at documents produced with inter-regional collaboration, authors from Africa and the Middle East occupied the first position on just 30 to 36% of the papers, compared to 45% for Western and Central Europe and 54% for North America (Table 1 ).
Scientific production by country and degree of international collaboration
Research production in Africa and the Middle East is concentrated in South Africa, whose researchers participated in 40.94% of the documents from these regions. At some distance are several other countries from Eastern and Southern Africa: Uganda (12.97%), Kenya (10.71%), Malawi (6.19%) and Tanzania (6.03%). Thirteen other countries show values ranging from 1.32 to 4.73%. Nigeria is the most prominent producer in Western and Central Africa, at 4.59%, while Iran leads production in the Middle East and North Africa (2.02%). Another 45 countries in Africa and the Middle East contributed to less than 1 % of the total research output (Table 2 ). Among the most productive countries (> 100 documents), Iran, Ethiopia, Nigeria, and South Africa present the lowest degree of international collaboration and the highest participation as first authors. Many of these show values of international collaboration that exceed 90%, with participation as first author under 30%. This situation is similar or even more pronounced in most low-producing countries (Table 2 ).
Generally speaking, African research output on HIV/AIDS is characterized by its cooperative links, particularly with the USA, UK, and other European countries (75 to 93% of the collaborations). However, South Africa also stands out for its intraregional ties, and it has become the main reference for research collaboration on HIV/AIDS, both in Eastern and Southern Africa and among the top 10 most productive African countries. It has collaborated with 34 different countries, led 41.44% of the collaborations, and participated in 35.76% of the papers led by other African countries. Uganda ranks second in terms of collaborative leadership within Africa, albeit with values that are much more modest, having led 14.06% of its collaborative research and participated in 11.11% of papers led by other African countries. The rest of the countries contribute less than 10% to the total collaborative links established. Except for South Africa, Uganda, and a few other countries like Zimbabwe, the collaborative links between different countries in Africa are few and far between, constituting weak and sporadic ties (Table 3 ).
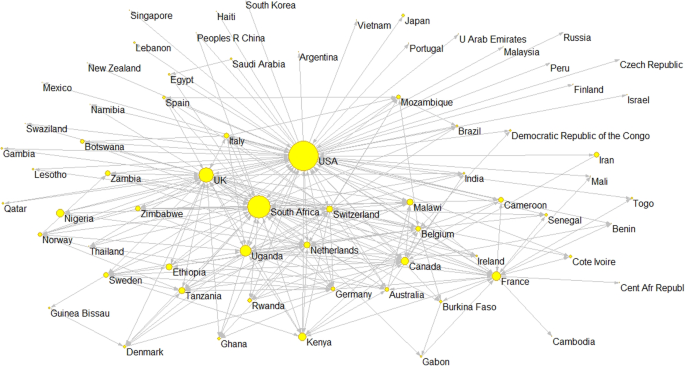
Figure 2 shows a graphic representation of the collaboration network. The USA is in the center as the main reference for international collaboration on scientific output on HIV/AIDS, while the UK, Canada, and other European countries like France, Switzerland, the Netherlands, and Belgium also occupy prominent locations. South Africa is the main African reference for HIV/AIDS publications, reflecting not only its collaborations with the USA, Canada and the European countries but also its prominent role in intraregional collaborations.
International collaboration network of research papers on HIV/AIDS with African and Middle Eastern countries (2010–2017)
Subject areas addressed in publications on HIV/AIDS in Africa and the Middle East
The correlation analysis on scientific HIV/AIDS output, produced by all countries worldwide, by African countries alone, and through Africa+global collaborations, shows differences in disciplinary orientations and research topics. In terms of disciplines involved, the lowest degree of correlation pertains to global publications versus solely African publications (k = 0.73; Table 4 ). There is also certain discordance between solely African publications and Africa+global collaborations (k = 0.79). In contrast, there is great affinity between global research output and output from Africa+global collaborations (k = 0.97). Of note, HIV/AIDS publications from Africa alone was dominated by papers in the field of “Public, Environmental & Occupational Health,” while the disciplines of “Infectious Diseases” and “Immunology” occupy the first rankings both globally and in African+global collaborations. The disciplines of “Medicine, General & Internal” and “Health Policy & Services” were also of great relevance in the publications from African countries alone (Table 4 ).
Our comparison of the MeSH qualifiers revealed similar disparities (Table 5 ). The lowest degrees of correlation were between global versus solely African research output (k = 0.68) and between global versus Africa+global collaborations (k = 0.69). However, there was a high degree of correlation between solely African publications and Africa+global collaborations (k = 0.97). With regard to the most prominent MeSH qualifiers, epidemiological studies occupy the top spot in both global and solely African publications. However, “Drug therapy” and “Therapeutic use” are more popular orientations in solely African publications than “Inmmunology,” “Genetics,” and “Metabolism” (Table 5 ).
Finally, with regard to MeSH descriptors, publications from Africa and the Middle East reflects the high prioritization of terms related to prevalence and treatment approaches (Table 6 ). Furthermore, global scientific production on HIV/AIDS suggests gender parity in terms of the research focus (both the “Male” and “Female” terms were assigned to 55% of the documents). However, for publications produced by researchers from solely African countries, the “Female” term is present in 73.38% of the documents, and for publications produced by Africa+global collaborations, this MeSH appeared in 76.71% of the documents.
Figure 3 presents a visualization of the main MeSH terms used to represent Africa and Middle East HIV/AIDS research topics and the links between them. Overall, studies that analyze anti-HIV agents, prevalence, and risk factors constitute the main subject areas that articulate the research. Incidence and its relation to sexual behaviors and health education (knowledge, prevention, acceptance of treatment for the disease) is also an important topic, as is research on pregnancy, maternal health, and prenatal care. Other relevant areas focus on co-infection (with tuberculosis, hepatitis B, hepatitis C, meningitis), resistance to anti-viral agents, and the use of certain medicines to treat the infection (lamivudine, tenofovir etc.).
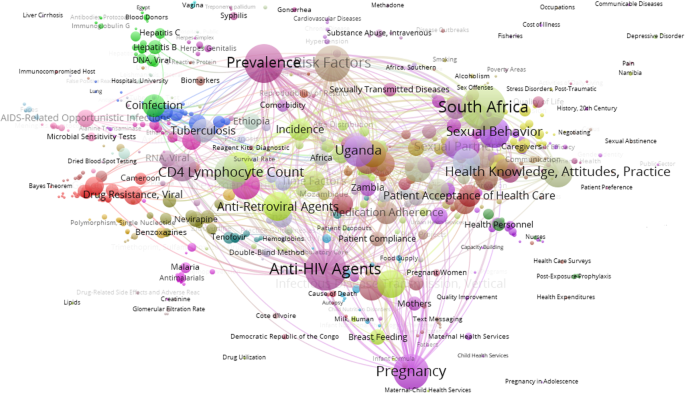
MeSH co-occurrence network on HIV/AIDS research papers from African and Middle Eastern countries (2010–2017)
Growth, visibility, and concentration of scientific production
Our analysis shows that scientific production on HIV/AIDS is still dominated by researchers from North America and Western and Central Europe, which together participated in 82% of the documents analyzed, although just 6% of people with HIV live in these regions. In contrast, researchers from countries in Africa and the Middle East participated in less than a quarter of the research papers on HIV/AIDS published between 2010 and 2017 (22%), although two-thirds of all people who are infected with the virus live there. Nevertheless, in relation to previous studies analyzing HIV/AIDS publications produced by researchers from African countries, our results indicate two highly relevant trends: (a) the notable growth in scientific production on HIV/AIDS in this region and (b) the elevated participation in scientific publications with greater visibility and international impact. In absolute terms, the number of documents we identified are double those reported by Macías-Chapula & Mijangos-Nolasco [ 8 ], based on their analysis of HIV/AIDS literature from sub-Saharan Africa included in the National Library of Medicine from 1980 to 2000, and by Uthman [ 9 ] analyzing scientific production on HIV/AIDS from sub-Saharan Africa and indexed in PubMed from 1981 to 2009.
At a country level, the advances made in research are even more significant. In their study on HIV/AIDS literature included in the National Library of Medicine, Onyancha & Ocholla [ 10 ] reported negligible contributions from Uganda and Kenya in the form of journal articles published from 1989 to 1998 ( n = 11 and n = 16, respectively). Our results show that these two countries have now become the second and third most productive on the continent, with a high number of contributions to journals indexed in the WoS-CC ( n = 1921 documents from Uganda and n = 1586 in Kenya). Uthman [ 11 ] studied HIV/AIDS research production from Nigeria between 1987 and 2006, identifying 254 articles in the WoS databases. Our findings, of 679 documents, nearly triple that number, even though the study period is substantially shorter. In South Africa, the production we identified from 2010 to 2017 ( n = 6063) is close to that reported by Uthman [ 9 ] for the entire period from 1981 to 2009 ( n = 8361).
Our results also show a trend toward greater research concentration, with an increase in the relative weight of high-producing countries (particularly South Africa, Uganda, and Kenya), which stand out as the main references for African scientific production on HIV/AIDS. Indeed, these countries now account for over half of all publications from Africa, and their relative contributions are trending upward. Thus, while Uthman [ 9 ] reported that South Africa participated in 34% of the HIV/AIDS publications produced by sub-Saharan Africa, in our results this figure stands at 43%. Similarly, the relative weight of Uganda and Kenya (the second and third most productive countries) has risen from 8 and 7% of the total contributions, respectively, to 14 and 11%. Similar observations have been made in other research fields [ 14 ] and particularly in the biomedical area [ 15 , 16 ], demonstrating that economic development and investments in research constitute key factors explaining the rise in scientific productivity [ 17 ].
The trend toward a greater concentration of research production in a few countries indicates the need to develop policies that facilitate a greater integration of lower-producing and less-developed countries in research activities. The literature describes some measures to stimulate research in these countries that go beyond economic investments, including training and retaining experienced researchers and fostering long-term partnerships based on equitable collaborative research ties. These strategies can enable researchers from these countries to acquire the methodological skills they need and can favor their leadership in spearheading or directing the research [ 13 ].
More specifically to the field of HIV/AIDS, Uthman [ 9 ] analyzed the factors associated with scientific productivity on HIV/AIDS in sub-Saharan Africa. His results showed that the number of people living with HIV and the number of indexed journals published in the country were predictive of an increase in publications. Other relevant factors include national scientific policies related to countries’ research agendas for this area, plus the adequate integration and participation in the system for publication and dissemination of scientific knowledge. These variables are more closely associated with scientific productivity on HIV/AIDS than others like the number of higher institutions or the number of physicians. The fact that South Africa is the country with the highest number of HIV-positive people and that this subject area has become a priority on the national research agenda [ 18 ] is clearly related to the country’s high research productivity in the field. Its economic growth has complemented this boost; together with other BRICS countries, especially China and Brazil, South Africa has laid the groundwork for development by strengthening its educational, healthcare, and social systems [ 19 , 20 ]. Increased investments in research go hand in hand with this strategy, including through establishing collaborative links with the most advanced economies at a scientific level [ 21 , 22 ]. However, as Adams et al. [ 23 ] signaled in their study, a myriad of factors affect scientific productivity and collaboration in African countries apart from structural factors like the level of economic growth or population size. For example, countries in the Commonwealth sphere, mostly situated in Eastern and Southern Africa and using English as a second language, generally present a higher level of scientific production and collaborative research than other African countries, like those in the Francophone community [ 16 ]. Our results are consistent with this trend: 10 of the 12 most productive countries are linked with the Commonwealth.
Although some countries like Nigeria or Ethiopia have made important research efforts, with corresponding increases in their scientific productivity, different studies have highlighted the need for increasing ties with neighboring countries. This would enable a more fluid exchange of knowledge and experience and foster research in key areas like detection and treatment [ 11 , 24 ].
High degree of international collaboration, low level of leadership
The two main bibliometric features we observed to be associated with HIV/AIDS research activity in Africa were: (a) a high degree of international collaboration with countries from other geographical regions, dominated by the USA and Europe (81% of the documents) and (b) a low level of research leadership, as seen through the low participation of African investigators as the first authors of documents produced in collaboration (20 to 38% among the top 10 most productive countries).
These two features may reflect a certain scientific dependence and subordination among African countries in relation to more developed countries. Moreover, the same situation has been observed in other biomedical research fields that are of special importance to the global South, like tropical diseases, infectious diseases, and pediatrics [ 22 , 25 , 26 ]. More specifically, Kelaher et al. [ 27 ] analyzed randomized controlled trials in the fields of HIV/AIDS, malaria, and tuberculosis that were undertaken in low- and middle-income countries (LMICs) from 1990 to 2013, identifying three relevant features associated with research leadership. First, there was a much higher proportion of first authors from LMICs in studies funded by LMICs (90%) than in studies funded by the USA (32%). Second, participation as first authors from LMICs was sensibly lower in the field of HIV/AIDS (33%) than for other diseases like malaria (67%). Finally, among first authors from all LMICs worldwide, those from Africa authored fewer papers than those from other regions like Latin America or Asia.
The literature describes different barriers that hinder researchers in LMICs from assuming leadership roles. Some of these are related to the absence of infrastructures or adequate financing [ 28 ]. Without an established institutional framework, stable research groups cannot be created or sustained; researchers cannot access the technical and financial support they need to submit research tenders; and coordination and monitoring of research priorities in relation to local research agendas is inadequate [ 13 , 29 , 30 , 31 ]. Other barriers have to do with deficits in methodological skills (like research design and statistical interpretation) or language (composition of articles or fluency in English). All of these factors can affect researchers’ capacity to lead studies and authorship [ 32 , 33 , 34 ].
At the same time, there are structural factors related to the hub-and-spoke model that favor the increased recognition and success of countries conducting mainstream research. Economic and human resources are concentrated in North America and Europe, and these regions also establish priority research topics. Editorial bias and the Matthew effect of accumulated advantage cement the structural forces perpetuating the under-representation of researchers from the global South from assuming positions of leadership in scientific publications [ 26 , 32 ].
The two countries constituting the axis of the collaborative research network on HIV/AIDS are the USA and South Africa. The former stands out for the high number of collaborative links it has established, with its researchers co-authoring papers with most African and Middle Eastern countries (52 countries). In total, 7693 collaborative ties (co-authored papers) were established in the study period, 70% of which were led by researchers in American institutions. Other bibliometric studies have also described the relevance of the USA in collaborative HIV/AIDS research output in Africa [ 11 ], Latin America and the Caribbean [ 35 ], and Asia [ 36 ]. Our own group have highlighted this role in other biomedical research fields [ 37 ].
For its part, South Africa is clearly the country of reference for HIV/AIDS research activity on the African continent, with a quantitative weight that is well above that observed in other biomedical areas in which it also exercises leadership. Nachega et al. [ 16 ] assessed the participation of African countries in publications on epidemiology and public health in the WoS databases, reporting that South Africa was represented in 22% of the documents, Kenya in 10%, and Nigeria in 9%. In our study, 41% of the documents on HIV/AIDS were authored by researchers in South Africa. This country, along with Ethiopia, is also notable for its leadership, figuring in the affiliations of 38% of the first authors. A similar phenomenon has also been observed in other fields of the health sciences, such as infectious diseases [ 15 , 38 ].
In addition to maintaining important collaborative ties with the USA and different European countries [ 39 , 40 ], South Africa has also emerged as a hub for intraregional collaborations within Africa. It has established links with 35 countries—far more than other African countries. Indeed, it is the main collaborator for all the other African countries in the top 10 for HIV/AIDS research productivity, even though these collaborations represent just 12% of the total collaborations in which South Africa participates. In that sense, some papers have called for BRICS countries, including South Africa, to increase their efforts to tackle the challenges primarily affecting the developing world [ 19 ]. In the case of South Africa, this could be done by promoting intraregional collaborations in sub-Saharan Africa, as research undertaken at a local level has the most potential to produce benefits, both for population health and socioeconomic development [ 20 , 41 ]. Hernandez-Villafuerte, Li & Hofman [ 42 ] analyzed collaborations among sub-Saharan countries conducting economic evaluations of healthcare interventions, reporting results consistent with ours: researchers in this region tend to collaborate more with Europeans and North Americans than with each other.
The literature highlights specific barriers impeding equitable research collaboration for African researchers, for example the paper by Okeke [ 43 ], who pointed to the limited duration of research programs, which should be longer in order to nurture stable collaborations that build hard and leadership capacities. In addition to infrastructure, other aspects mentioned include managerial expertise, administrative capabilities, and the capacity to improvise at African partner institutions. In the same line, Boum II [ 44 ] and Boum II et al. [ 45 ] discuss the difficulties in harmonizing conflicting interests between Western and African countries, making it essential to prioritize financing for equitable initiatives that lay out specific goals and expectations for partnerships, or which promote initiatives like mentorship programs and investment in Africa-based researchers that strengthen institutional capacity.
Some examples of successful collaborations for promoting equitable research partnerships and African leadership in HIV research include initiatives like the Academic Model Providing Access to Healthcare (AMPATH) in Kenya, the International Epidemiology Databases to Evaluate AIDS (IeDEA) consortium, and different initiatives coordinated and driven by the Africa Centres for Disease Control and Prevention (Africa CDC) or the Joint United Nations Programme on HIV and AIDS (UNAIDS), among others.
Research interests in public health, epidemiology, and treatment approaches
HIV/AIDS research produced by solely African countries differed from global research in terms of disciplinary and subject area orientations, with a greater focus on public health, epidemiology, and treatment. This finding indicates the need to consider regional, national, and local specificities and interests when determining research priorities. In fact, numerous studies have already signaled the poor alignment between the priorities laid out in African countries’ national research agendas and the research topics that are actually financed [ 12 , 16 , 46 , 47 , 48 , 49 ].
From a public health perspective, for example, Uthman [ 11 ] pointed out the need for further research evidence to inform HIV prevention and control programs. In this field, some countries perform better than others: South Africa is particularly strong in public health research [ 50 ], while other African countries and regions, such as French Africa, have made limited contributions [ 51 ].
Studies on epidemiology and treatment approaches for HIV/AIDS are very relevant for research produced in Africa, in contrast to what occurs on a global scale, where these orientations have a relatively limited weight. Nachega et al. [ 16 ] pointed out that research on HIV/AIDS, tuberculosis, and malaria have become the main research topics addressed in epidemiological and public health publications in African countries. However, these authors argued for moving epidemiology and public health research beyond the limited sphere of communicable disease control in order to address the regional impact of non-communicable diseases, for example in maternal and child health. This is especially relevant in the case of sub-Saharan Africa, where epidemiologists are overwhelmingly deployed to control infectious diseases, especially HIV/AIDS, tuberculosis, and malaria. The study also calls for strengthening regional expertise in epidemiology in order to shed light on the underlying causes of ill health, rather than to merely control infections and outbreaks [ 16 ].
In addition to epidemiological studies, African research also reflects an intense interest in drug therapies for HIV/AIDS, illustrating that control of the infection is a priority for research agendas and policies in African countries [ 12 ].
More specifically, previous literature on HIV/AIDS research has shown a greater focus on women in studies carried out with the participation of African researchers [ 10 ]. Our study confirms this finding: 73 to 77% of the documents investigated women, compared to 55% in the global literature. One possible explanation for this includes the fact that women are more biologically, economically, socially, and culturally vulnerable to infection. Indeed, for every 10 African men who are HIV-positive, there are 12 to 13 infected women; moreover, 55% of adults who acquire HIV are women, with profound implications for mother-to-child transmission [ 10 ]. In consonance with this fact, a greater number of women participate and work on HIV care programs in Africa, and a large proportion of the clinicoepidemiological investigations in these settings are based on care program data [ 52 ].
The different epidemiological patterns of HIV/AIDS transmission in North America and Western and Central Europe must also be taken into account, that motivate a greater interest of research in these regions on sexual transmission between men and intravenous drug users. These epidemiological patterns are less important in Africa [ 53 ]. The presence in the MeSH co-occurrence network of the descriptors “pregnancy” and “sexual behavior” are noteworthy, reflecting how African researchers are investigating aspects like maternal-fetal transmission of HIV [ 54 ] or knowledge and prevention of sexual risk, and changing the preconceptions that still persist about the social determinants of transmission [ 47 ]. The prominence of topics related to preventing mother-to-child transmission stands in contrast to the near absence of topics related to children and young people. These groups are especially sensitive to the physical and psychosocial impacts of HIV and AIDS, indicating the need for increased research on young people who are at risk of or living with HIV [ 55 ].
The greater research attention to topics related to public health, epidemiology, and treatment may also respond to limited laboratory capacity, which is needed for virologic, immunological, and basic research. In that sense, it is essential to promote initiatives that strengthen these research structures and capacities in African countries, rather than only supporting programs and projects on preventive and clinical approaches.
Limitations and future lines of research
Limitations of the present study include the fact that a considerable portion of HIV/AIDS research in African countries is disseminated using document types and media that we did not consider, such as meeting abstracts and journals that are not indexed in the WoS-CC. Moreover, using the MeSH thesaurus from the field of health sciences could have resulted in an underestimation of research spheres related to our subject area, such as research in the social sciences. In that sense, some papers have indicated that stigma and discrimination still constitute the main barriers to controlling HIV/AIDS [ 56 ]. The process used to assign geographic place variables to the papers included in the sample was based on authors’ stated institutional affiliation; this method has the inherent limitation of not being able to measure the author’s origin, nationality, or identification with the country, but rather the institution’s (and the country’s) capacity to generate outputs in the form of scientific publications. Thus, many researchers of African origin who work at institutions in the USA and Europe would be coded as US/European researchers. Furthermore, the use of first author status as a proxy for African leadership may be misleading, as an African senior author may be the last author on a publication or may have played a leadership role in some aspects other than the manuscript preparation.
Our study focused on obtaining macro indicators on scientific collaboration and output by regions and countries. Future lines of research could conduct meso- or microlevel analyses, for example focusing on the participation of institutions or authors in African HIV/AIDS research or on the impact of the publications. It would also be of great interest to identify the organisms or programs that have funded the research inspiring the publications about HIV, measuring resource contributions according to domestic versus international as well as public versus private origins.
The main conclusions of our study are as follows.
1. Our results reflect significant progress in African-produced HIV/AIDS research, at both a quantitative level (with notable increases in the number of publications) and qualitative level (through participation in journals indexed in a bibliographic database that brings together the most high-impact and high-visibility international publications). Despite these advances, however, scientific output is still concentrated in a small number of countries, chief among them South Africa, while other countries in Africa and the Middle East make only negligible contributions, despite the high burden of HIV infections.
2. The participation of African countries conducting HIV/AIDS research is characterized by a dependence on and subordination to the USA and European countries. Collaborations between these regions reflect limited leadership by African countries, as measured by the participation of African researchers as the first authors of published studies.
3. HIV/AIDS research conducted with participation from African countries shows appreciably different disciplinary and subject-area interests than global HIV/AIDS research, with a stronger focus on public health, epidemiology, and drug treatments.
It is essential to promote balanced North-South research that properly addresses the most acute needs and gaps in the places where HIV/AIDS has the largest impact. To achieve this balance, it is necessary to transfer research skills to African partners, promote equitable collaborative ties, and empower African countries, especially those with less scientific activity and more disease prevalence. In the same way, the lack of investment in research infrastructure by African governments likely makes it more difficult for African investigators to lead their own research. Intraregional collaborations among African countries can also help to avoid the further concentration of research capacity, reproducing the global North-South model on the African continent.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Harvard Dataverse repository, https://doi.org/10.7910/DVN/RJMAY5 .
GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the global burden of disease study 2015. Lancet HIV. 2016;3:e361–87.
Galvani AP, Pandey A, Fitzpatrick MC, Medlock J, Gray GE. Defining control of HIV epidemics. Lancet HIV. 2018;5:e667–70.
Article PubMed Google Scholar
UNAIDS Data 2018. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf . .
Fajardo-Ortiz D, López-Cervantes M, Duran L, Dumontier M, Lara M, Ochoa H, et al. The emergence and evolution of the research fronts in HIV/AIDS research. PLoS One. 2017;12:e0178293.
Article PubMed PubMed Central CAS Google Scholar
González-Alcaide G, Salinas A, Ramos JM. Scientometrics analysis of research activity and collaboration patterns in Chagas cardiomyopathy. PLoS Negl Trop Dis. 2018;12:e0006602.
Article PubMed PubMed Central Google Scholar
Macias-Chapula CA, Rodeo-Castro IP, Narvaez-Berthelemot N. Bibliometric analysis of AIDS literature in Latin America and the Caribbean. Scientometrics. 1998;41:41–9.
Article Google Scholar
Sengupta IN, Kumari L. Bibliometric analysis of AIDS literature. Scientometrics. 1991;20:297–315.
Macías-Chapula CA, Mijangos-Nolasco A. Bibliometric analysis of AIDS literature in Central Africa. Scientometrics. 2002;54:309–17.
Uthman OA. Pattern and determinants of HIV research productivity in sub-Saharan Africa: bibliometric analysis of 1981 to 2009 Pubmed papers. BMC Infect Dis. 2010;10:47.
Onyancha OB, Ocholla DN. A comparative study if the literature on HIV/AIDS in Kenya and Uganda: a bibliometric study. Libr Inf Sci. 2004;26:434–47.
Uthman OA. HIV/AIDS in Nigeria: a bibliometric analysis. BMC Infect Dis. 2008;8:19.
Mugomeri E, Bekele BS, Mafaesa M, Maibvise C, Tarirai C, Aiyuk SE. A 30-year bibliometric analysis of research coverage on HIV and AIDS in Lesotho. Health Res Policy Syst. 2017;15:21.
Chu KM, Jayaraman S, Kyamanywa P, Ntakiyiruta G. Building research capacity in Africa: equity and global health collaborations. PLoS Med. 2014;11:e1001612.
Tijssen R. Africa’s contribution to the worldwide research literature: new analytical perspectives, trends, and performance indicators. Scientometrics. 2007;71:303–27.
Uthman OA, Uthman MB. Geography of Africa biomedical publications: an analysis of 1996–2005 PubMed papers. Int J Health Geogr. 2007;6:46.
Nachega JB, Uthman OA, Ho Y-S, Lo M, Anude C, Kayembe P. Current status and future prospects of epidemiology and public health training and research in the WHO African region. Int J Epidemiol. 2012;41:1829–46.
Rahman M, Fukui T. Biomedical research productivity: factors across the countries. Int J Technol Assess Health Care. 2003;19:249–52.
Dwyer-Lindgren L, Cork MA, Sligar A, Steuben KM, Wilson KF, Provost NR, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570:189–93.
Article CAS PubMed PubMed Central Google Scholar
Bai J, Li W, Huang YM, Guo Y. Bibliometric study of research and development for neglected diseases in the BRICS. Infect Dis Poverty. 2016;5:89.
Sun J, Boing AC, Silveira MPT, Bertoldi AD, Ziganshina LE, Khaziakhmetova VN, et al. Efforts to secure universal access to HIV/AIDS treatment: a comparison of BRICS countries. J Evid Based Med. 2014;7:2–21.
Bornmann L, Wagner C, Leydesdorff L. BRICS countries and scientific excellence: a bibliometric analysis of most frequently cited papers. J Assoc Inf Sci Technol. 2015;66:1507–13.
Article CAS Google Scholar
González-Alcaide G, Park J, Huamaní C, Ramos JM. Dominance and leadership in research activities: collaboration between countries of differing human development is reflected through authorship order and designation as corresponding authors in scientific publications. PLoS One. 2017;12:e0182513.
Adams J, Gurney K, Hook D, Leydesdorff L. International collaboration clusters in Africa. Scientometrics. 2014;98:547–56.
Deribew A, Biadgilign S, Deribe K, Dejene T, Tessema GA, Melaku YA, et al. The burden of HIV/AIDS in Ethiopia from 1990 to 2016: evidence from the global burden of diseases 2016 study. Ethiop J Health Sci. 2019;29:859–68.
PubMed PubMed Central Google Scholar
Keiser J, Utzinger J. Trends in the core literature on tropical medicine: a bibliometric analysis from 1952-2002. Scientometrics. 2005;62:351–65.
Keiser J, Utzinger J, Tanner M, Singer BH. Representation of authors and editors from countries with different human development indexes in the leading literature on tropical medicine: survey of current evidence. BMJ. 2004;328:1229–32.
Kelaher M, Ng L, Knight K, Rahadi A. Equity in global health research in the new millennium: trends in first-authorship for randomized controlled trials among low- and middle-income country researchers 1990-2013. Int J Epidemiol. 2016;45:2174–83.
Zakumumpa H, Bennett S, Ssengooba F. Leveraring the lessons learned from financing HIV programs to advance the universal health coverage (UHC) agenda in the east African community. Glob Health Res Policy. 2019;4:27.
Feldacker C, Pintye J, Jacob S, Chung MH, Middleton L, Iliffe J, et al. Continuing professional development for medical, nursing, and midwifery cadres in Malawi, Tanzania and South Africa: a qualitative evaluation. PLoS One. 2017;12(10):e0186074.
Nchinda TC. Research capacity strengthening in the south. Soc Sci Med. 2002;54:1699–711.
Wight D, Ahikireb J, Kwesigac JC. Consultancy research as a barrier to strengthening social science research capacity in Uganda. Soc Sci Med. 2014;116:32–40.
Langer A, Díaz-Olavarrieta C, Berdichevsky K, Villar J. Why is research from developing countries underrepresented in international health literature, and what can be done about it? Bull World Health Organ. 2004;82:802–3.
Smith E, Hunt M, Master Z. Authorship ethics in global health research partnerships between researchers from low or middle income countries and high income countries. BMC Med Ethics. 2014;15:42.
Yousefi-Nooraie R, Shakiba B, Mortaz-Hejri S. Country development and manuscript selection bias: a review of published studies. BMC Med Res Methodol. 2006;6:37.
Macias-Chapula CA. AIDS in Haiti: a bibliometric analysis. Bull Med Libr Assoc. 2000;88:56–61.
CAS PubMed PubMed Central Google Scholar
Chen TJ, Chen YC, Hwang SJ, Chou LF. International collaboration of clinical medicine research in Taiwan, 1990–2004: a bibliometric analysis. J Chin Med Assoc. 2007;70:110–6.
Ramos-Rincón JM, Pinargote-Celorio H, Belinchón-Romero I, et al. A snapshot of pneumonia research activity and collaboration patterns (2001–2015): a global bibliometric analysis. BMC Med Res Methodol. 2019;19:184.
Badenhorst A, Mansoori P, Chan KY. Assessing global, regional, national and sub-national capacity for public health research: a bibliometric analysis of the web of science in 1996-2010. J Glob Health. 2016;6:010504.
Falagas ME, Bliziotis IA, Soteriades ES. Eighteen years of research on AIDS: contribution of and collaborastion between different world regions. AIDS Res Hum Retrovir. 2006;22:1199–205.
Breugelmans JG, Makanga MM, Cardoso AL, Mathewson SB, Sheridan-Jones BR, Gurney KA, et al. Bibliometric assessment of European and sub-Saharan African research output on poverty-related and neglected infectious diseases from 2003 to 2011. PLoS Negl Trop Dis. 2015;9:e0003997.
Ettarh R. Patterns of international collaboration in cardiovascular research in sub-Saharan Africa. Cardiovasc J Afr. 2016;27:194–200.
Hernandez-Villafuerte K, Li R, Hofman KJ. Bibliometric trends of health economic evaluation in sub-Saharan Africa. Glob Health. 2016;12:50.
Okeke IN. Partnerships for now? Temporality, capacities, and the durability of outcomes from global health ‘partnerships’. Med Anthropol Theory. 2018;5(2):7–24.
Google Scholar
Boum Y II. Is Africa part of the partnership? Med Anthropol Theory. 2018;5(2):25–34.
Boum Y II, Burns BF, Siedner M, Mburu Y, Bukusi E, Haberer JE. Advancing equitable global health research partnerships in Africa. BMJ Glob Health. 2018;3:e000868.
Mayosi BM, Lawn JE, van Niekerk A, Bradshaw D, Abdool Karim SS, Coovadia HM, et al. Health in South Africa: changes and challenges since 2009. Lancet. 2012;380:2029–43.
Hodes R, Morrell R. Incursions from the epicentre: southern theory, social science, and the global HIV research domain. Afr J AIDS Res. 2018;17:22–31.
Esser DE, Bench KK. Does global health funding respond to recipients’ needs? Comparing public and private donors’ allocations in 2005–2007. World Dev. 2011;39:1271–80.
Swingler GH, Pillay V, Pienaar ED, Ioannidis JP. International collaboration, funding and association with burden of disease in randomized controlled trials in Africa. Bull World Health Organ. 2005;83:511–7.
Wright CY, Dominick F, Kunene Z, Kapwata T, Street RA. Bibliometric trends of south African environmental health articles between 1998 and 2015: making local research visible and retrievable. S Afr Med J. 2017;107:915–24.
Article CAS PubMed Google Scholar
Benie-Bi J, Cambon L, Grimaud O, Kivits J, Alla F. Health needs and public health functions addressed in scientific publications in francophone sub-Saharan Africa. Public Health. 2013;127:860–6.
Nnko S, Nyato D, Kuringe E, Casalini C, Shao A, Komba A, et al. Female sex workers perspectives and concerns regarding HIV self-testing: an exploratory study in Tanzania. BMC Public Health. 2020;20(1):959.
Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–77.
Poreau B. Prenatal diagnosis, care and management in Africa: bibliometric analysis. Pan Afr Med J. 2018;29:146.
Tran BX, Nathan KI, Phan HT, Hall BJ, Vu GT, Vu LG, et al. A global bibliometric analysis of services for children affected by HIV/acquired immune deficiency syndrome: implications for impact mitigation programs (GAPRESEARCH). AIDS Rev. 2019;21.
Sweileh WM. Bibliometric analysis of literature in AIDS-related stigma and discrimination. Transl Behav Med. 2019;9:617–28.
Download references
Acknowledgements
We gratefully acknowledge the assistance of Meggan Harris in translating our manuscript from Spanish.
Open access funding provided by University of Valencia, Special Research Actions Program (UV-19-INV-AE19).
Author information
Gregorio González-Alcaide and Marouane Menchi-Elanzi contributed equally to this work.
Authors and Affiliations
Department of History of Science and Documentation, University of Valencia, Valencia, Spain
Gregorio González-Alcaide
Department of Internal Medicine, General University Hospital of Alicante, Alicante, Spain
Marouane Menchi-Elanzi & José-Manuel Ramos-Rincón
Infectious Disease Division, Carmelo Hospital of Chókwè – Daughters of Charity, Saint Vincent of Paul, Chókwè, Gaza Province, Mozambique
Edy Nacarapa
Tinpswalo Association, Research Unit, Vincentian Association to Fight AIDS and TB, Chókwè, Gaza Province, Mozambique
Department of Clinical Medicine, Miguel Hernandez University of Elche, Alicante, Spain
José-Manuel Ramos-Rincón
You can also search for this author in PubMed Google Scholar
Contributions
GGA: study conception, data collection, data analysis, manuscript writing and final manuscript approval; MME: study conception, data collection, data analysis, manuscript writing and final manuscript approval; NE: data analysis, manuscript writing and final manuscript approval; JMRR: study conception, manuscript writing and final manuscript approval.
Authors’ information
Two of the four authors of the present study are European (GGA and JMRR), including the first author of the study. In addition, a third author (MME) working at a European institution has signed with this affiliation. As we were aware of this imbalance, these three authors sought the collaboration of an African researcher (EN), who is from one of the most affected countries by HIV/AIDS and has extensive knowledge of the subject matter at a clinical and epidemiological level. His participation facilitated the acquisition of the methodological skills necessary for leading a study like the present one, and it strengthened our commitment to seek future collaborators enabling more balanced representation among our author teams. However, African research teams wishing to perform similar studies would likely face a range of problems, such as the lack of recognition for their research activities; time constraints due to clinical workload; and economic barriers impeding access to bibliographic databases, full-text articles for many scientific journals, translation services, and publishing costs. Indeed, although BMC “offers waivers and discounts for article processing charges (APCs) for papers whose corresponding authors are based in low-income countries,” the publication itself is only the culmination of the research process, which includes numerous processes and activities requiring economic resources or financing.
Corresponding author
Correspondence to José-Manuel Ramos-Rincón .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
González-Alcaide, G., Menchi-Elanzi, M., Nacarapa, E. et al. HIV/AIDS research in Africa and the Middle East: participation and equity in North-South collaborations and relationships. Global Health 16 , 83 (2020). https://doi.org/10.1186/s12992-020-00609-9
Download citation
Received : 31 December 2019
Accepted : 11 August 2020
Published : 17 September 2020
DOI : https://doi.org/10.1186/s12992-020-00609-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Scientific research
- Human immunodeficiency virus infection
- Acquired immune deficiency syndrome
- African countries
- Bibliometrics
- International collaboration
Globalization and Health
ISSN: 1744-8603
- Submission enquiries: [email protected]
- General enquiries: [email protected]
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- How It Spreads
- Living with HIV
- HIV Awareness Days
- HIV in the United States
- Resource Library
- Occupational Exposure
- Connect With Us
- HIV Nexus Home
- HIV Public Health Partners
- Ending the HIV Epidemic in the U.S. (EHE)
- Let's Stop HIV Together
Fast Facts: HIV in the United States
At a glance.
HIV remains a persistent problem in the United States. In the United States, estimated HIV infections decreased 12% overall from 2017 to 2021. Learn more about HIV trends in the United States.
HIV affects some groups more than others. Social and structural issues—such as HIV stigma, homophobia, discrimination, poverty, and limited access to high-quality health care—influence health outcomes and continue to drive inequities.
HIV incidence
HIV incidence refers to the estimated number of new HIV infections in a given year.
Estimated HIV infections in the US by transmission category, 2021*
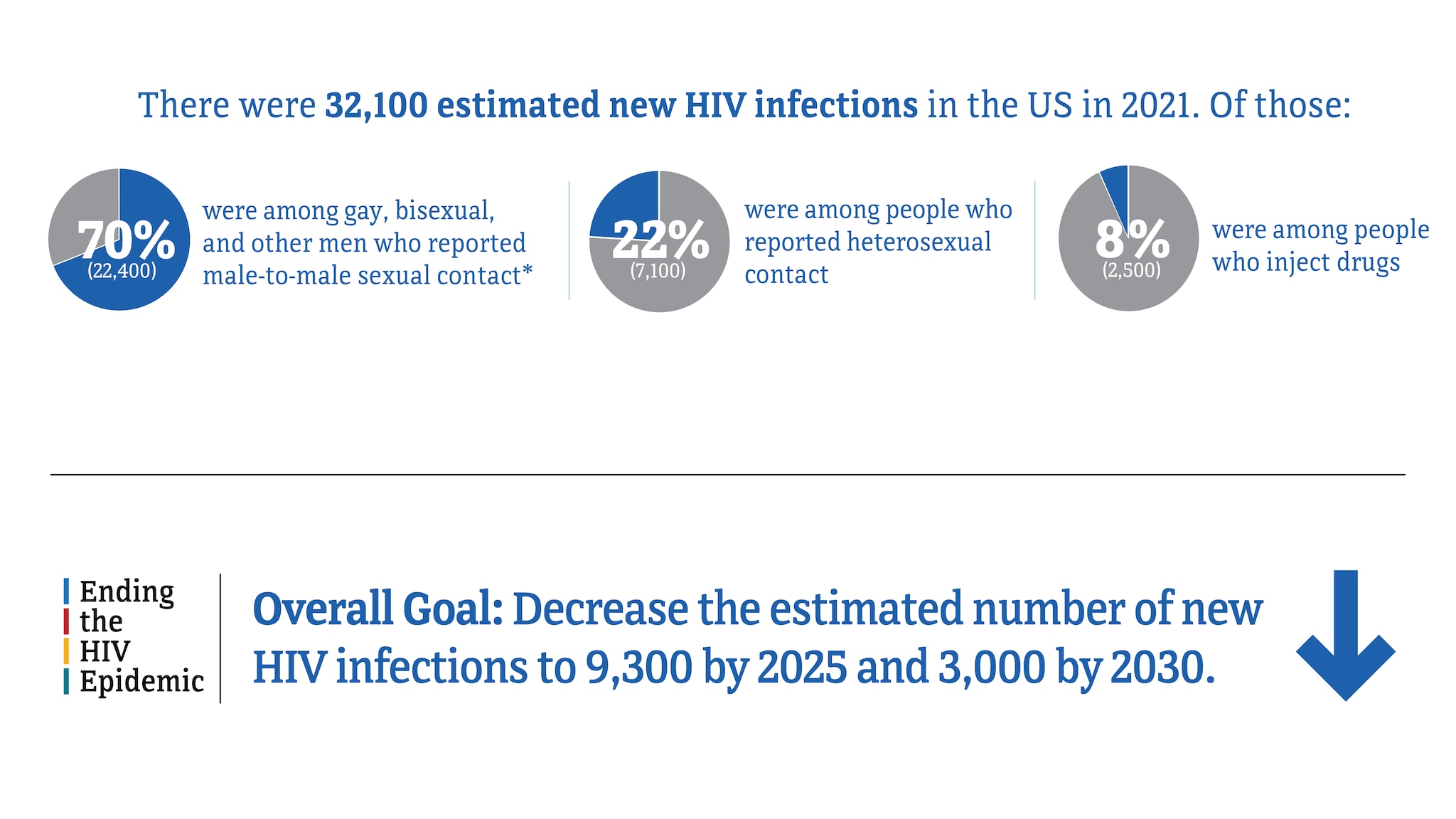
* Includes infections attributed to male-to-male sexual contact and injection drug use (men who reported both risk factors).
Source: CDC. Estimated HIV incidence and prevalence in the United States, 2017–2021. HIV Surveillance Supplemental Report , 2023; 28(3).
HIV infections among gay, bisexual, and other men who reported male-to-male sexual contact
In 2021, gay, bisexual, and other men who reported male-to-male sexual contact accounted for 70% (22,400) of the 32,100 estimated new HIV infections and 86% of estimated infections among all men.
Estimated HIV infections among gay and bisexual men in the US, 2017-2021*
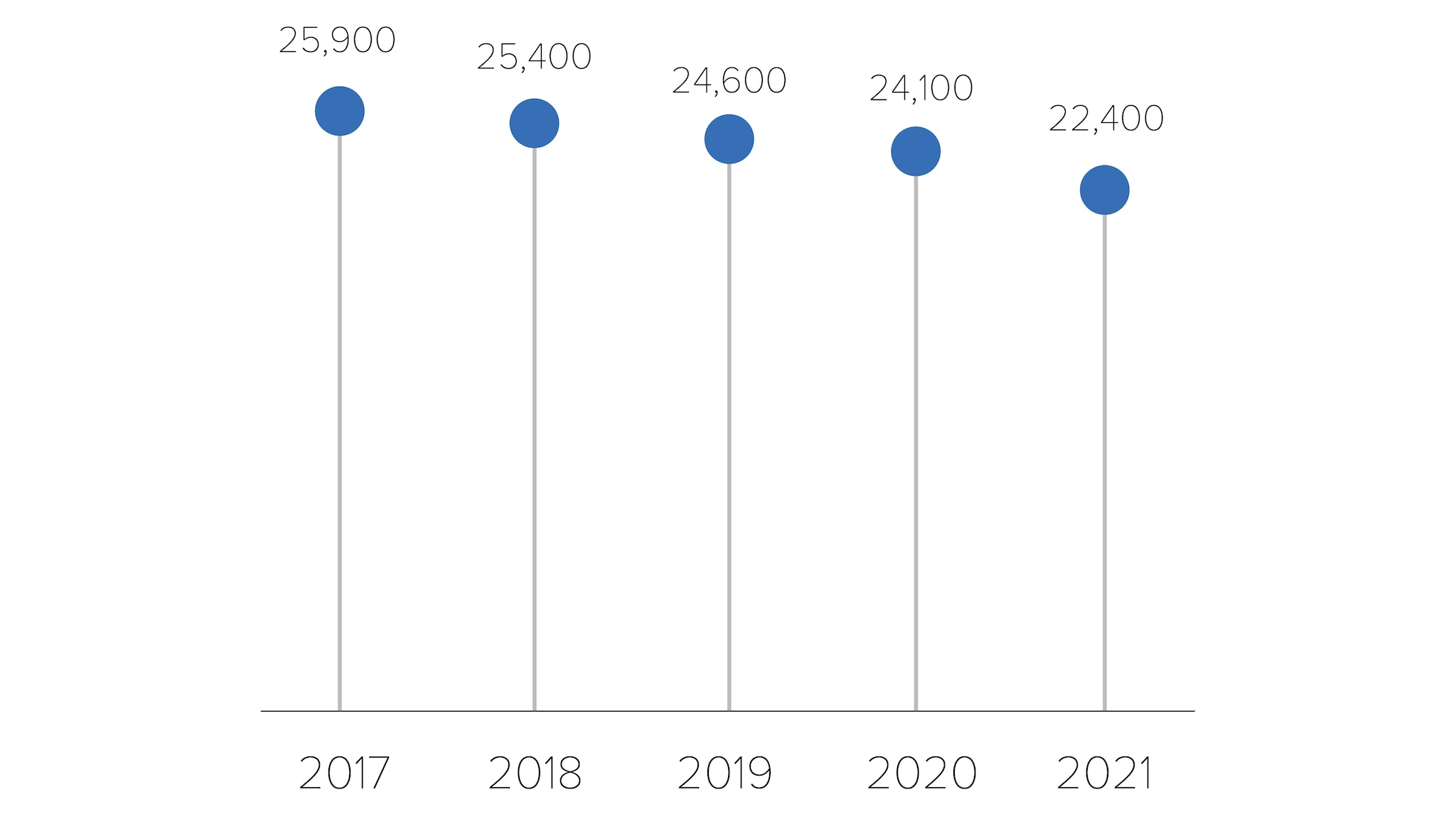
* Among people aged 13 and older.
Source: CDC. Estimated HIV incidence and prevalence in the United States, 2017–2021. HIV Surveillance Supplemental Report , 2023; 28(3).
HIV infections among people who reported heterosexual contact
In 2021, people reporting heterosexual contact accounted for 22% (7,100) of the 32,100 estimated new HIV infections.
- Men reporting heterosexual contact accounted for 6% (2,000) of estimated new HIV infections.
- Women reporting heterosexual contact accounted for 16% (5,100) of estimated new HIV infections.
Estimated HIV infections among people who reported heterosexual contact in the US, 2017-2021*
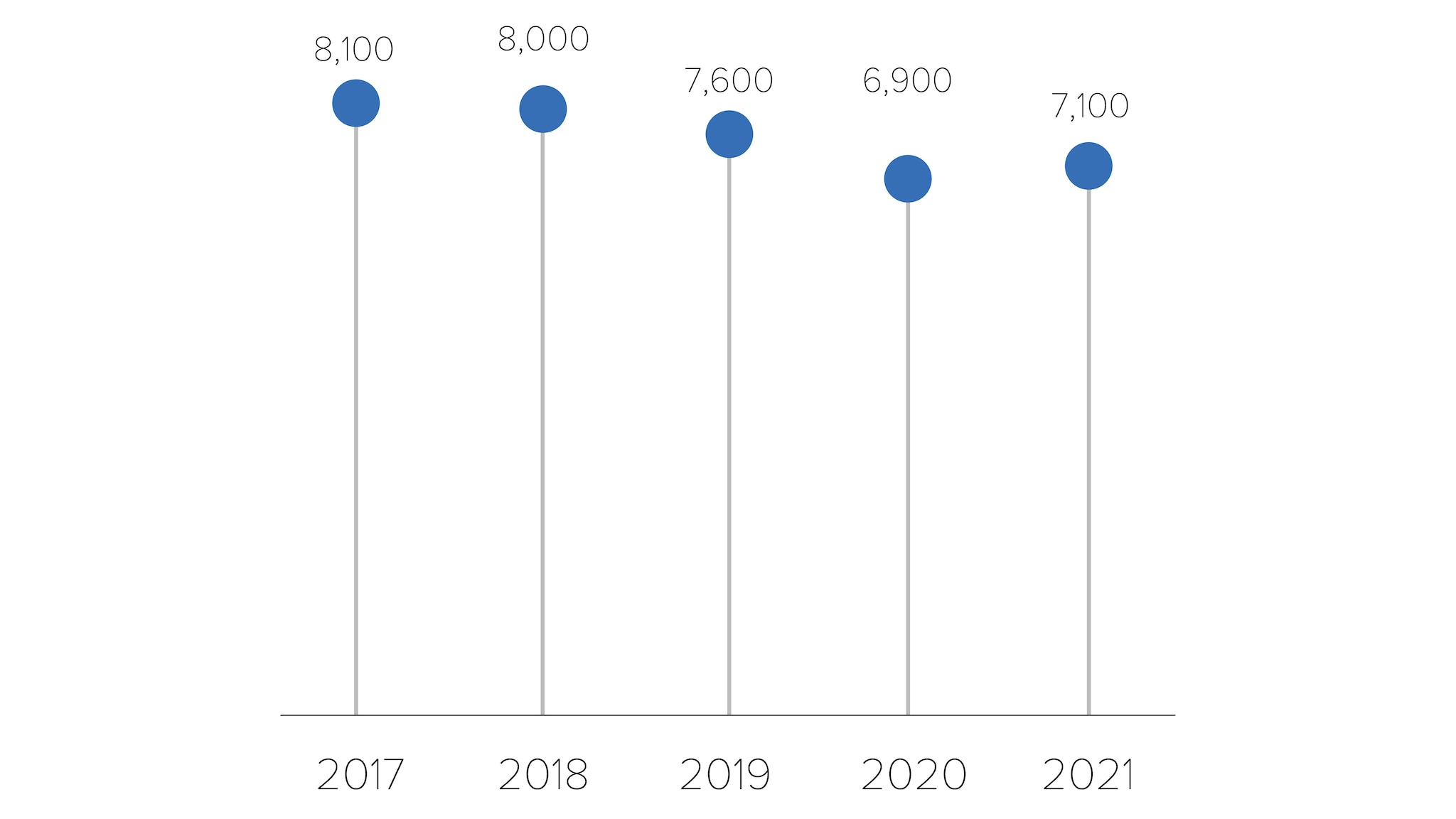
HIV infections among people who inject drugs (PWID)
In 2021, PWID accounted for 8% (2,500) of the 32,100 estimated new HIV infections.
- Men who inject drugs accounted for 4% (1,400) of estimated new HIV infections.
- Women who inject drugs accounted for 3% (1,100) of estimated new HIV infections.
Estimated HIV infections among people who inject drugs (PWID) in the US, 2017-2021*
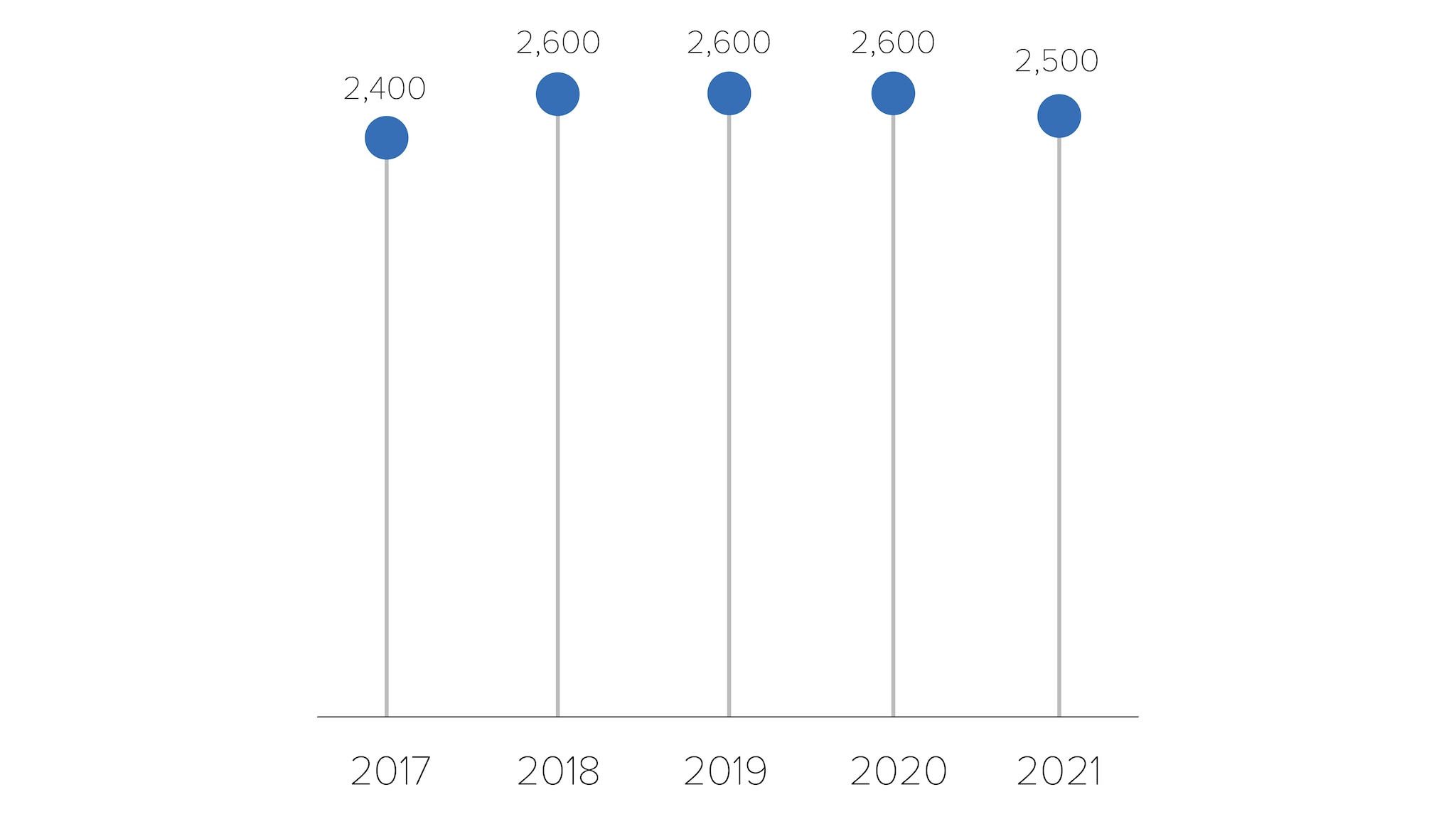
HIV infections by region
In 2021, the South accounted for more than half (52%) of the 32,100 estimated new HIV infections.
Estimated HIV infections in the US by region, 2021*
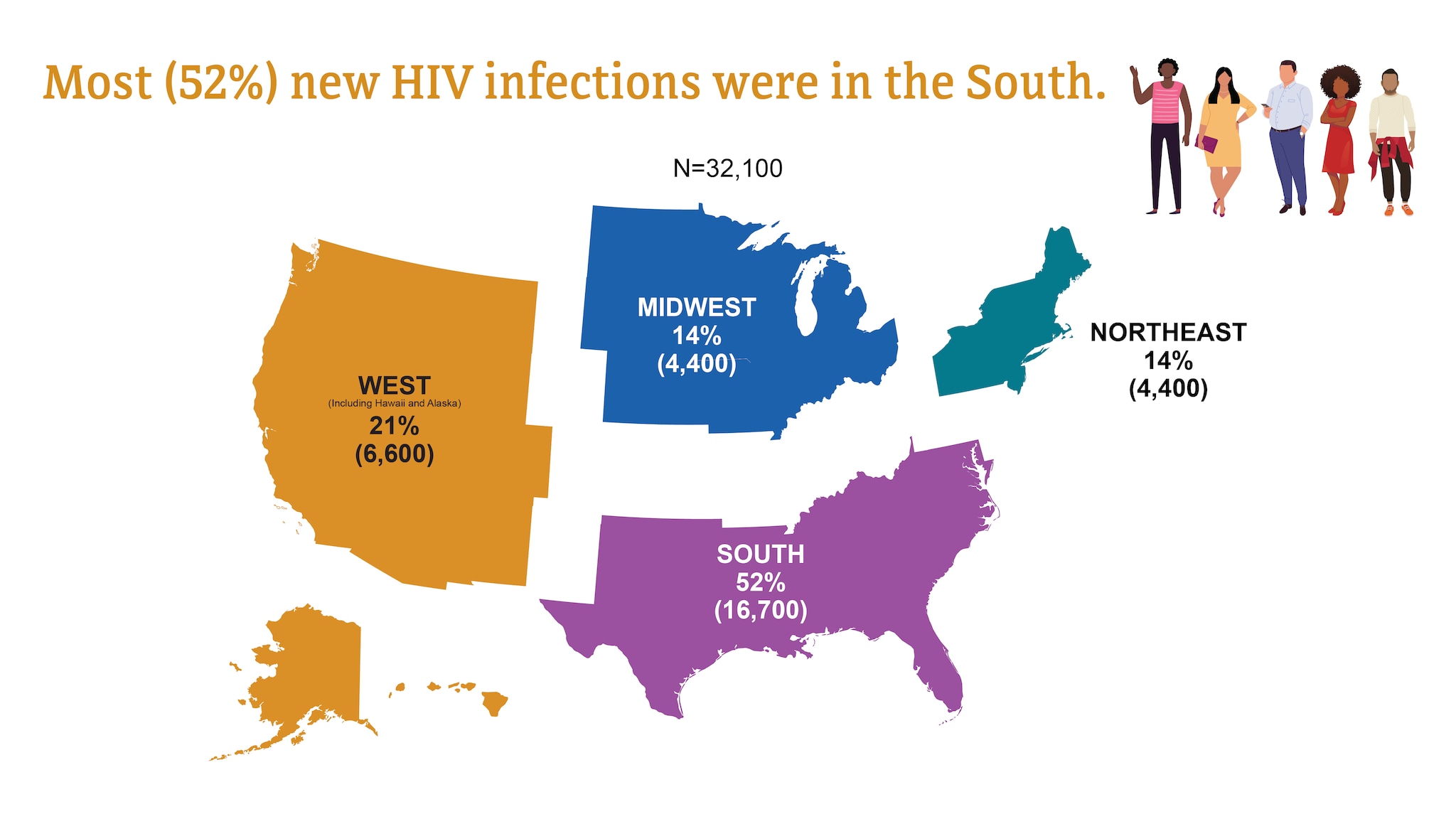
HIV diagnoses
HIV diagnoses refers to the number of people who received an HIV diagnosis during a given year.
HIV diagnoses in the US and dependent areas by transmission category, 2021*
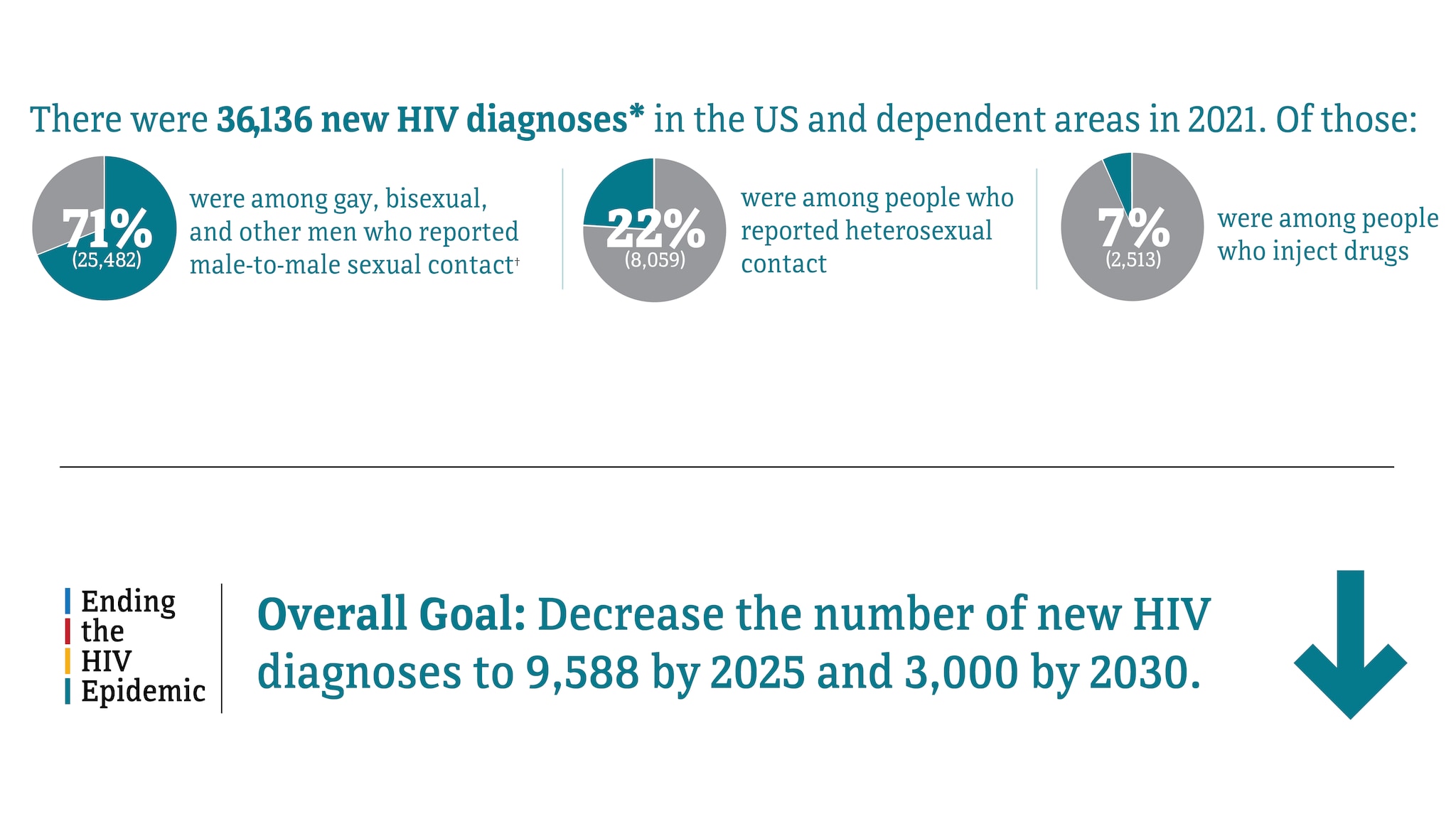
† Includes infections attributed to male-to-male sexual contact and injection drug use (men who reported both risk factors).
Source: CDC. Diagnoses of HIV infection in the United States and dependent areas, 2021. HIV Surveillance Report 2023;34.
HIV diagnoses in the US and Dependent areas for the most-affected subpopulations, 2021*†
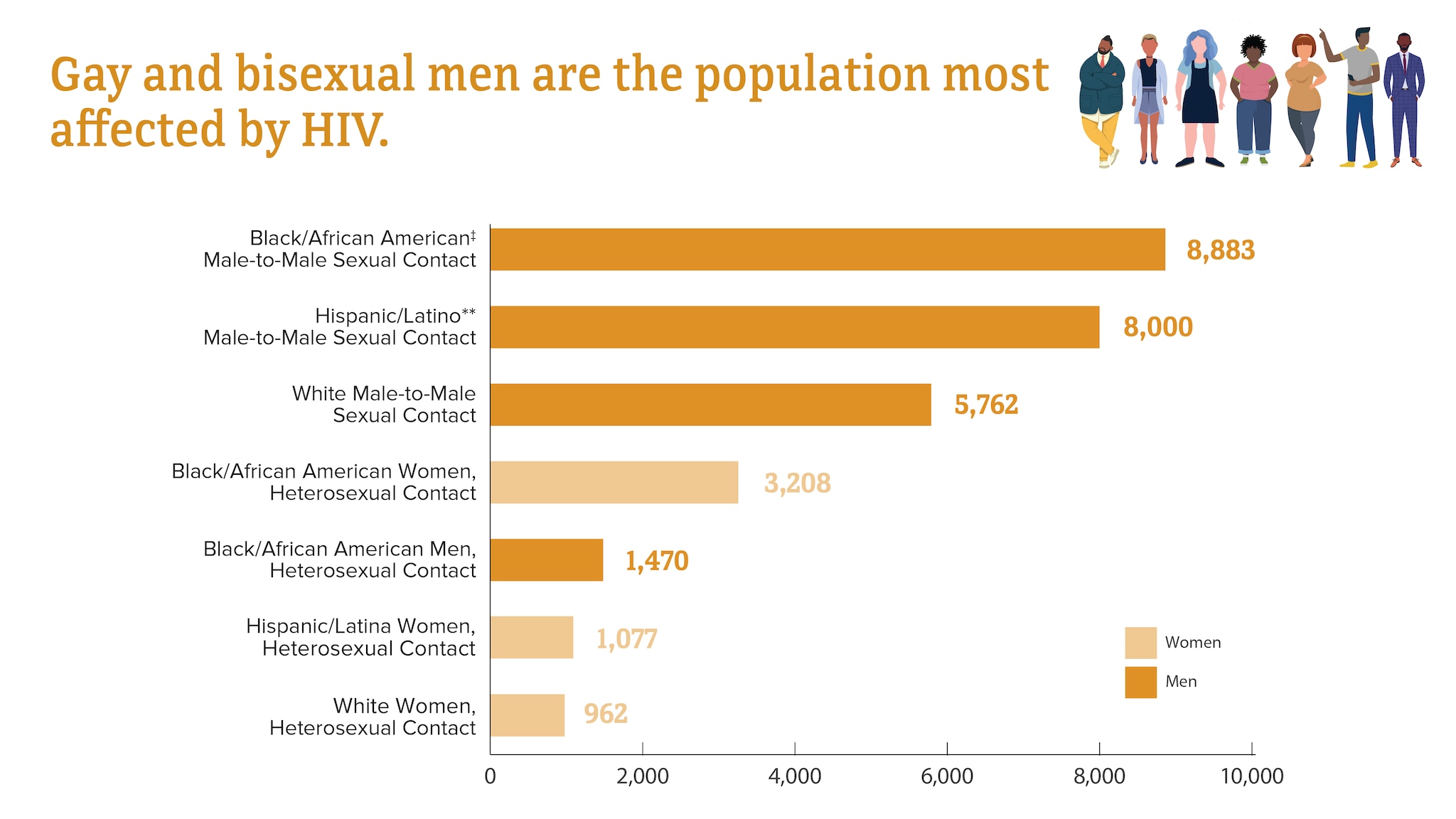
Subpopulations representing 2% or less of all people who received an HIV diagnosis in 2021 are not represented in this chart.
† Transmission category is classified based on a hierarchy of risk factors most likely responsible for HIV transmission. Classification is determined based on the person’s assigned sex at birth. Data have been statistically adjusted to account for missing transmission category.
‡ Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
** Hispanic/Latino people can be of any race.
Source: CDC. Diagnoses of HIV infection in the United States and dependent areas, 2021. HIV Surveillance Report 2023;34.
HIV diagnoses among transgender people
In 2021, transgender people accounted for 2% (868) of the 36,136 new HIV diagnoses.
- Transgender women accounted for 2% (812) of new HIV diagnoses.
- Transgender men accounted for less than 1% (56) of new HIV diagnoses.
HIV diagnoses among transgender people in the US and dependent areas by race and ethnicity, 2021*
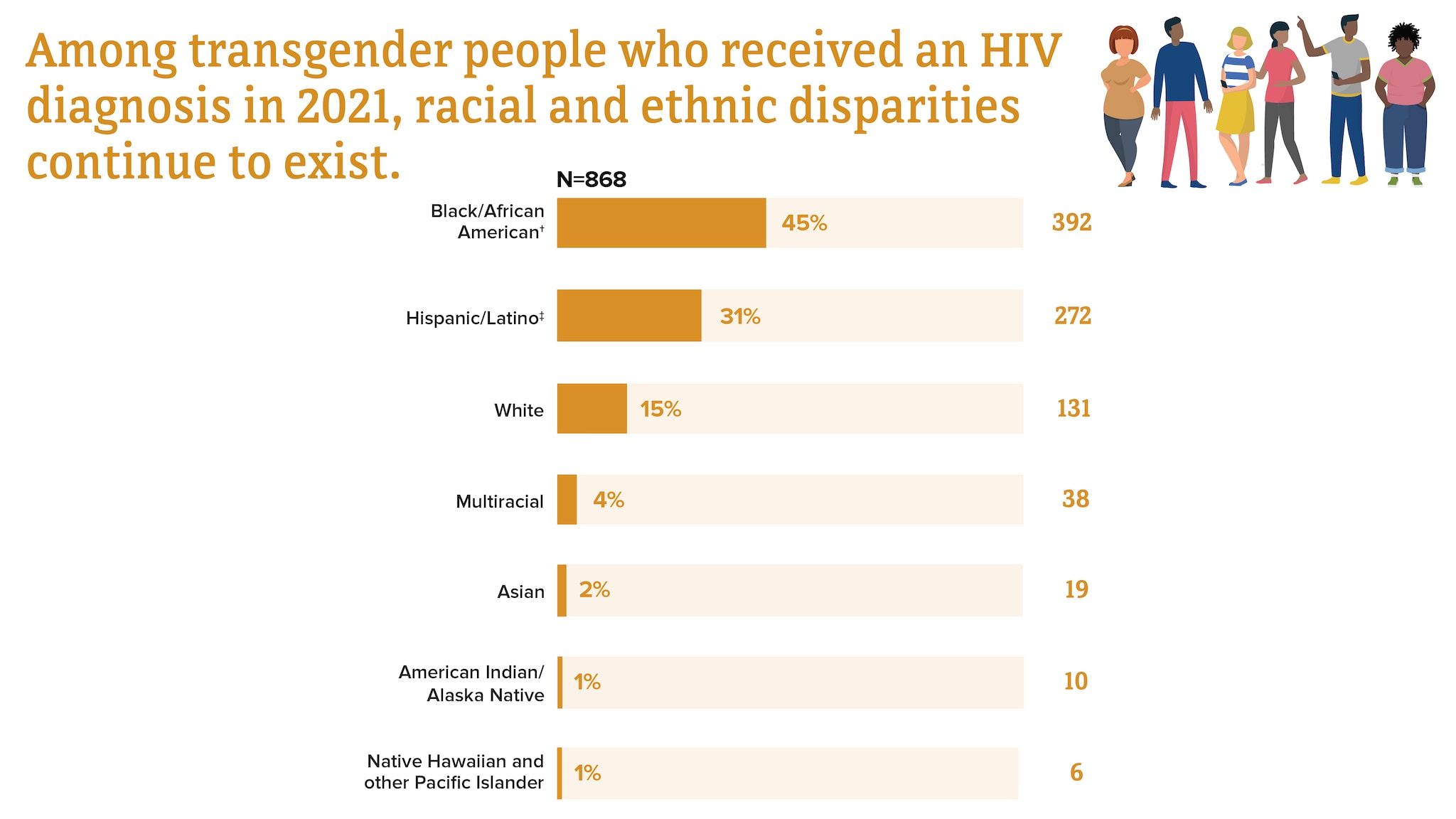
† Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
‡ Hispanic/Latino people can be of any race.
Trends in HIV diagnoses among transgender people in the US and dependent areas by race and ethnicity, 2017-2021*
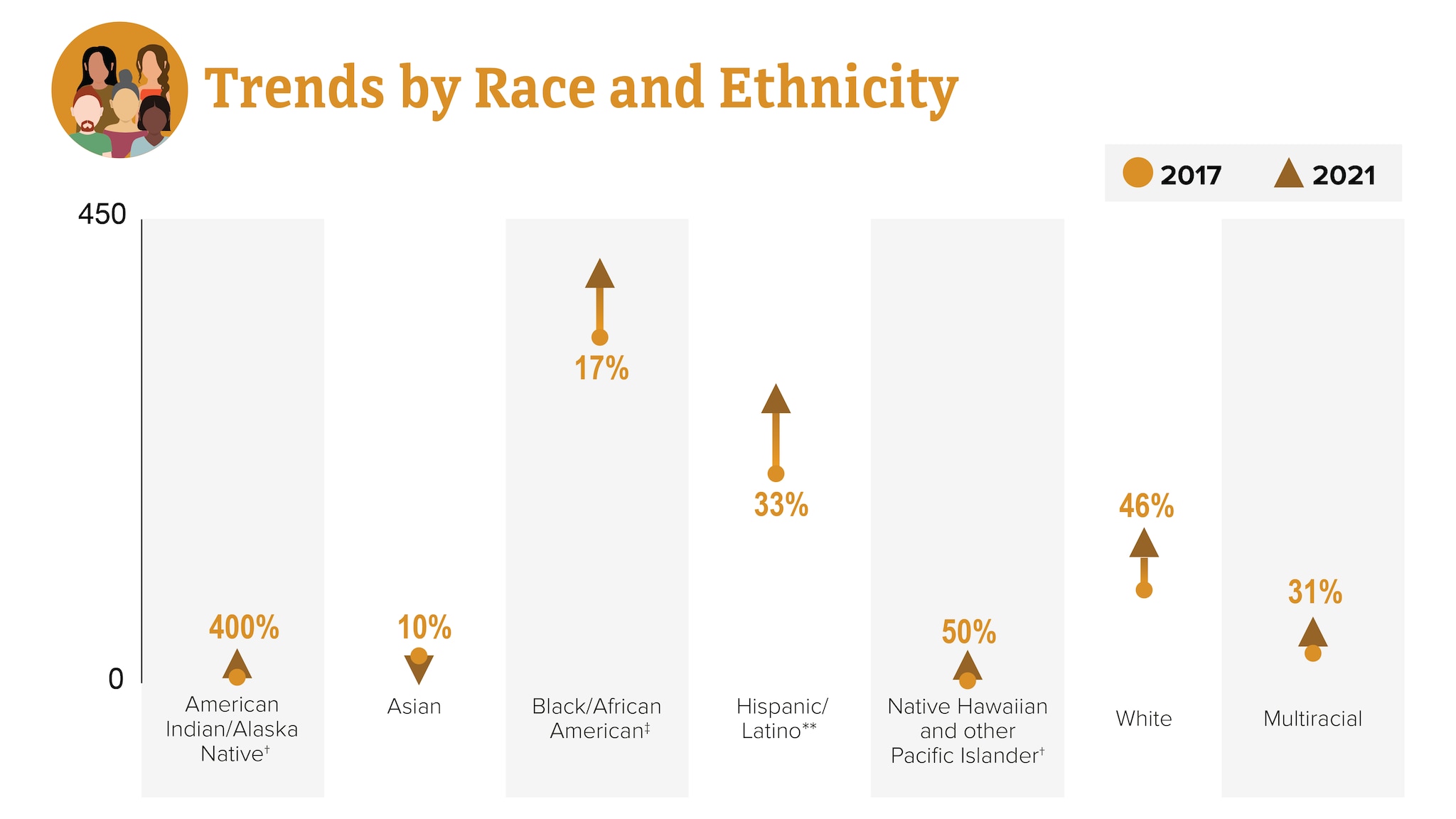
† Changes in subpopulations with fewer HIV diagnoses can lead to a large percentage increase or decrease.
‡ Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
** Hispanic/Latino people can be of any race.
HIV diagnoses among gay and bisexual men
Gay, bisexual, and other men who reported male-to-male sexual contact are the population most affected by HIV. In 2021, gay and bisexual men accounted for 71% (25,482) of the 36,136 new HIV diagnoses and 86% of diagnoses among all men.
HIV diagnoses among gay and bisexual men in the US and dependent areas by race and ethnicity, 2021*†
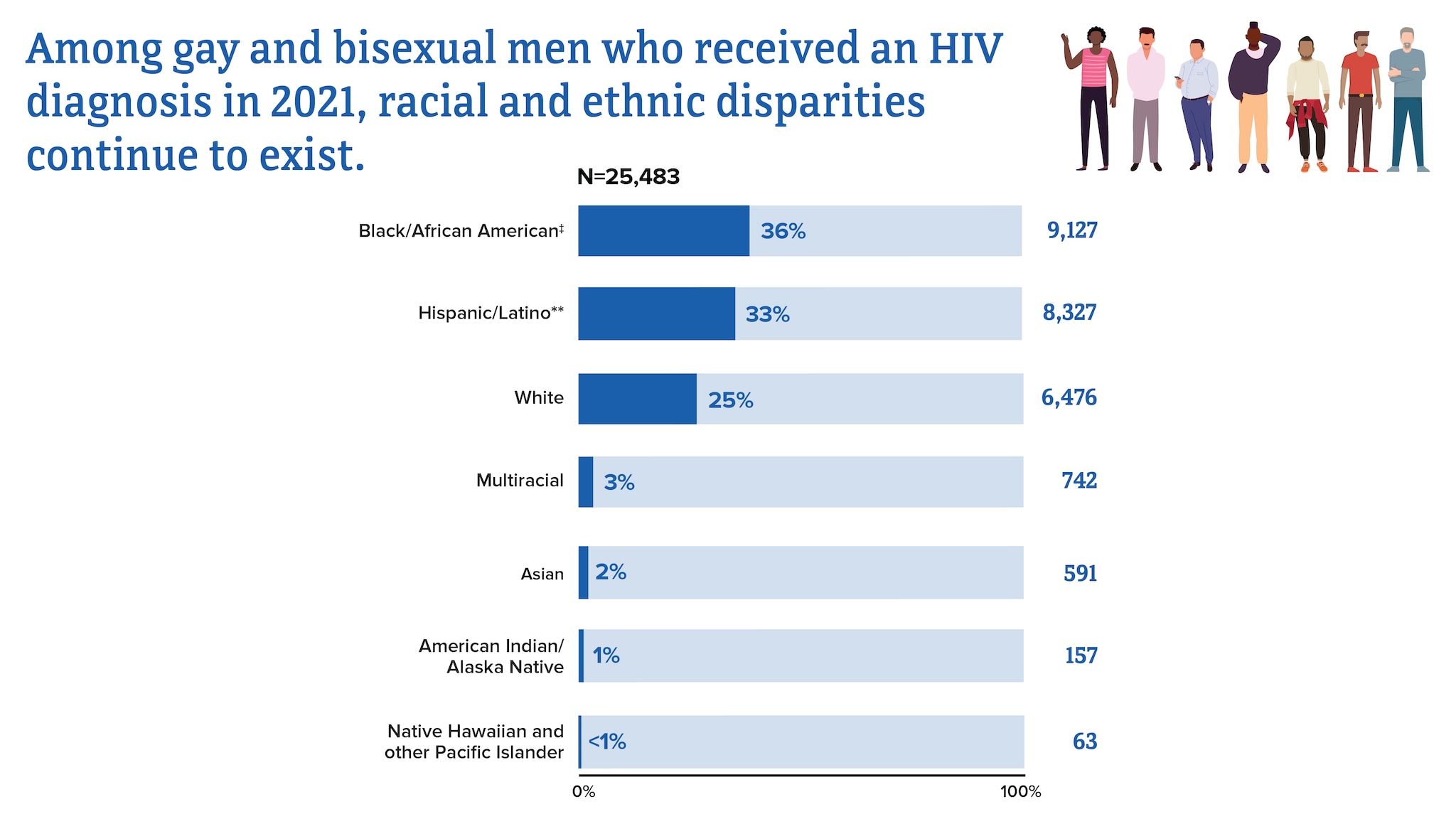
* Among people aged 13 and older
†Includes infections attributed to male-to-male sexual contact and injection drug use (men who reported both risk factors).
‡ Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
From 2017 to 2021, HIV diagnoses decreased 6% among gay and bisexual men overall. But trends varied for different groups of gay and bisexual men.
Trends in HIV diagnoses among gay and bisexual men in the US and dependent areas by race and ethnicity, 2017-2021*
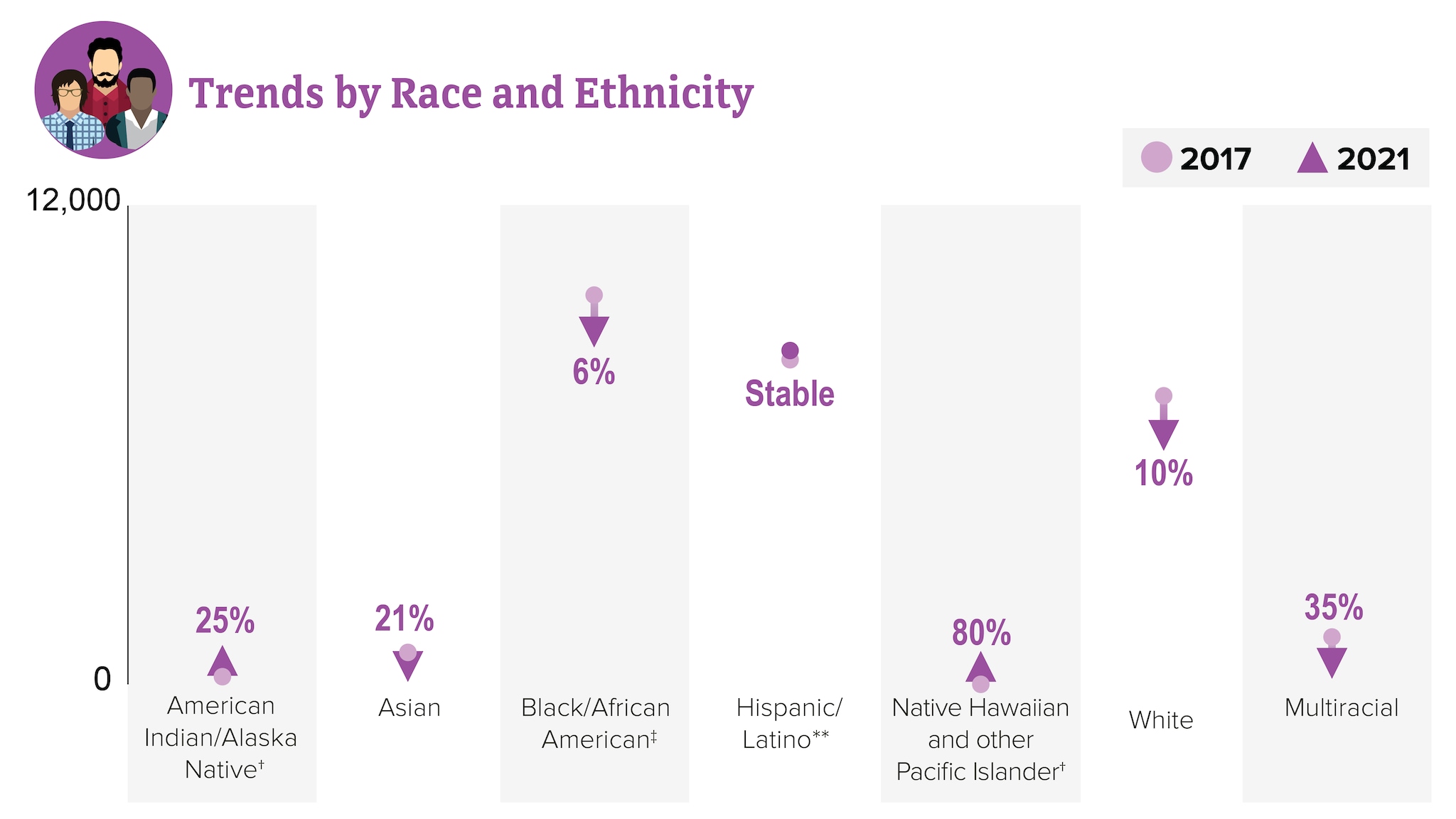
HIV diagnoses among people who reported heterosexual contact
Men and women who reported heterosexual contact continue to be affected by HIV. In 2021, people reporting heterosexual contact accounted for 22% (8,059) of the 36,136 new HIV diagnoses.
- Men reporting heterosexual contact accounted for 7% (2,523) of new HIV diagnoses.
- Women reporting heterosexual contact accounted for 15% (5,536) of new HIV diagnoses.
HIV diagnoses among people who reported heterosexual contact in the US and dependent areas by race and ethnicity, 2021*
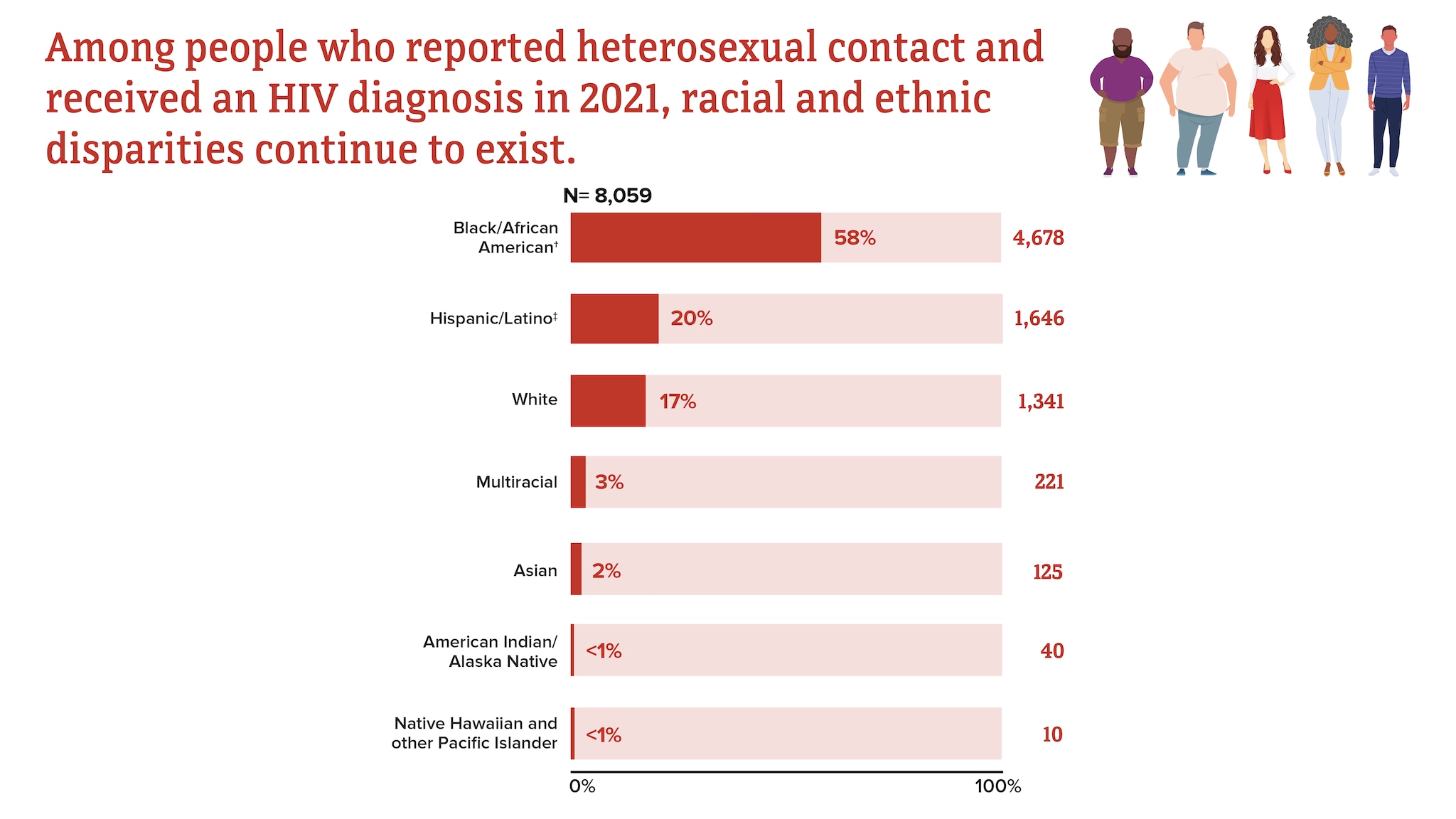
† Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
‡ Hispanic/Latino people can be of any race.
From 2017 to 2021, HIV diagnoses from heterosexual contact decreased 12% overall.
Trends in HIV diagnoses among people who reported heterosexual contact in the US and dependent areas, 2017-2021*†
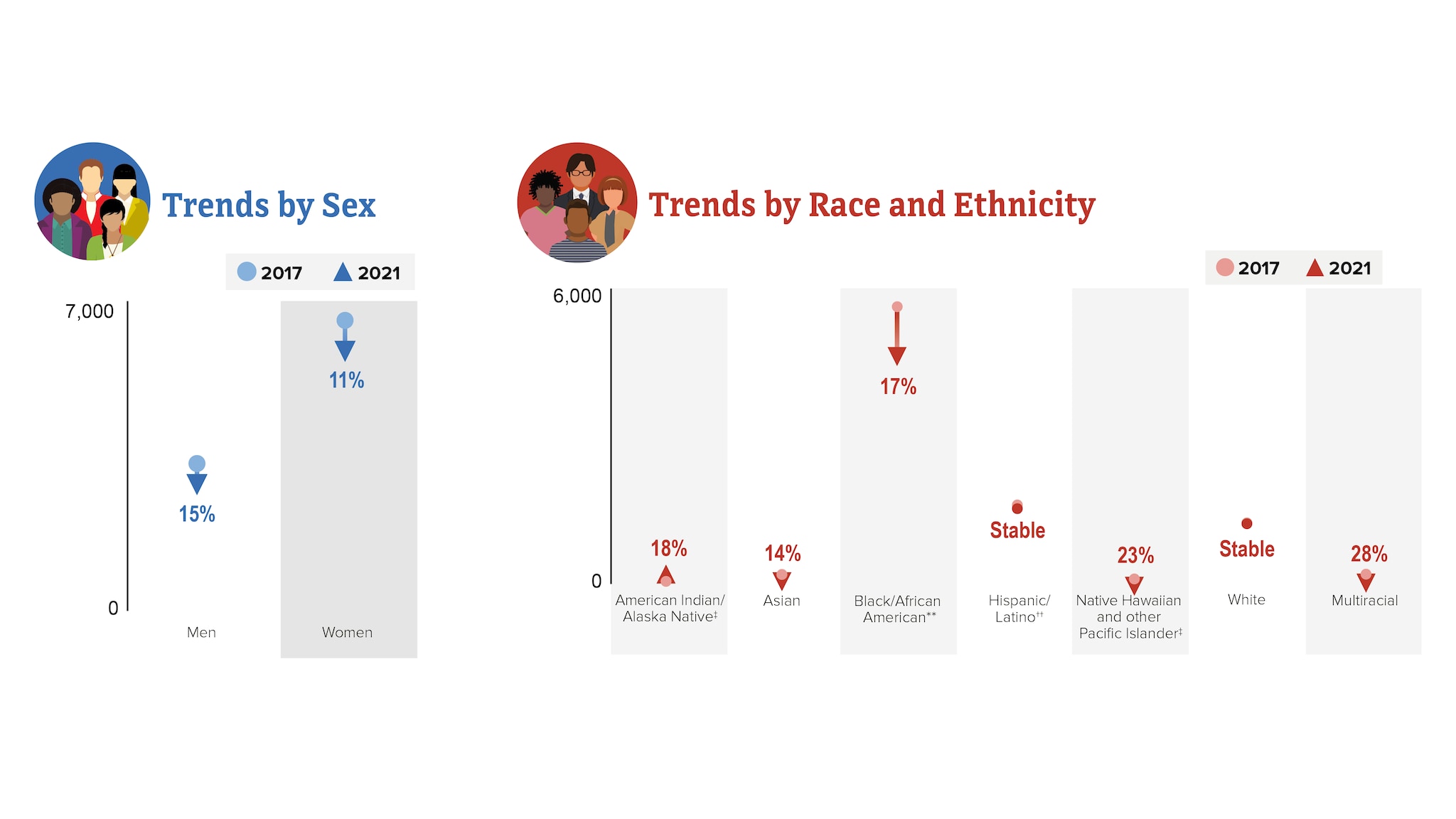
† Based on assigned sex at birth and includes transgender people.
‡ Changes in subpopulations with fewer HIV diagnoses can lead to a large percentage increase or decrease.
** Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
†† Hispanic/Latino people can be of any race.
HIV diagnoses among people who inject drugs (PWID)
In 2021, PWID accounted for 7% (2,512) of the 36,136 new HIV diagnoses.
- Men who inject drugs accounted for 4% (1,436) of new HIV diagnoses.
- Women who inject drugs accounted for 3% (1,076) of new HIV diagnoses.
HIV diagnoses among people who inject drugs in the US and dependent areas by race and ethnicity, 2021*
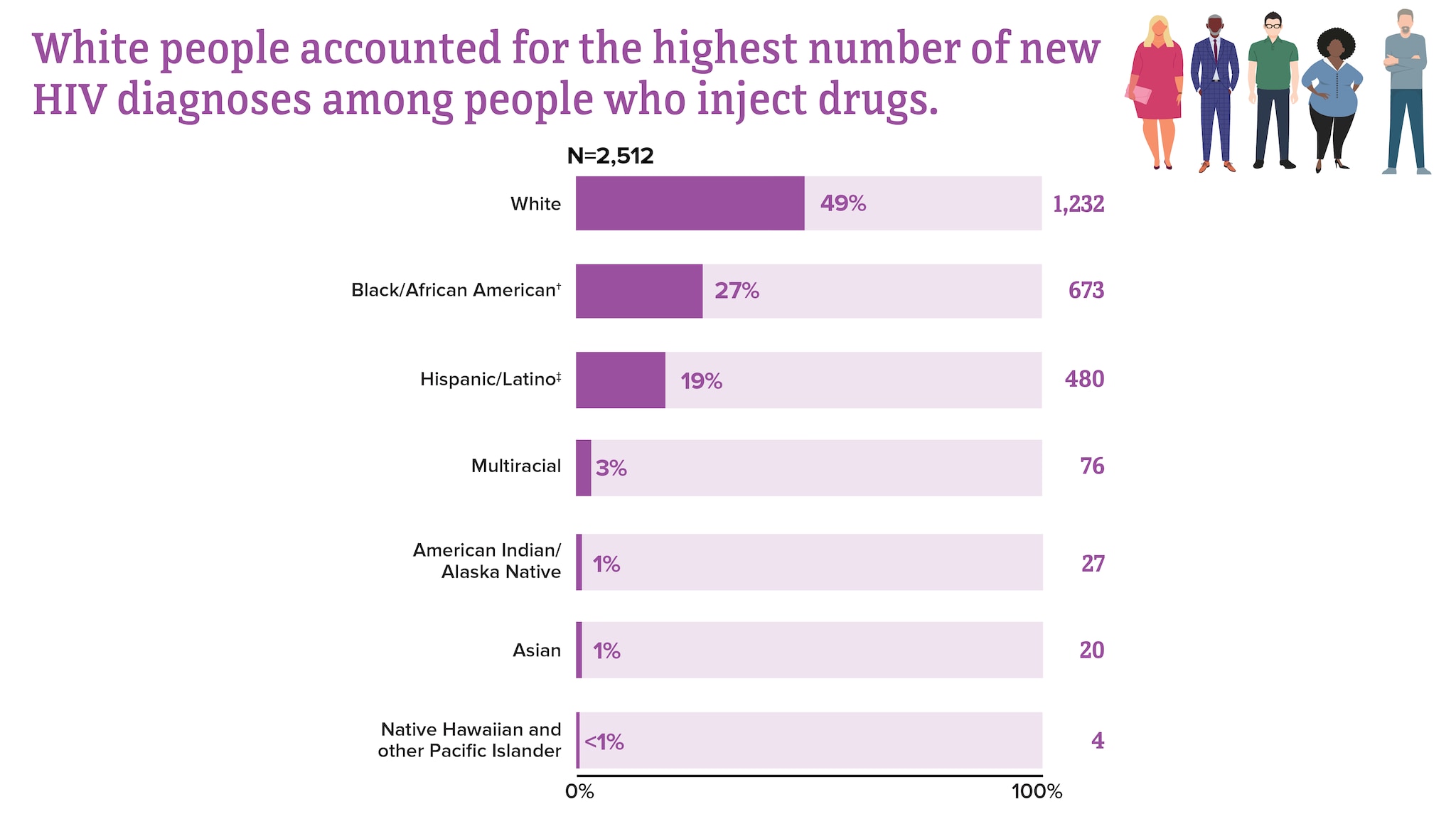
† Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
From 2017 to 2021, HIV diagnoses remained stable among PWID overall.
Trends in HIV diagnoses among people who inject drugs in the US and dependent areas, 2017-2021*†‡
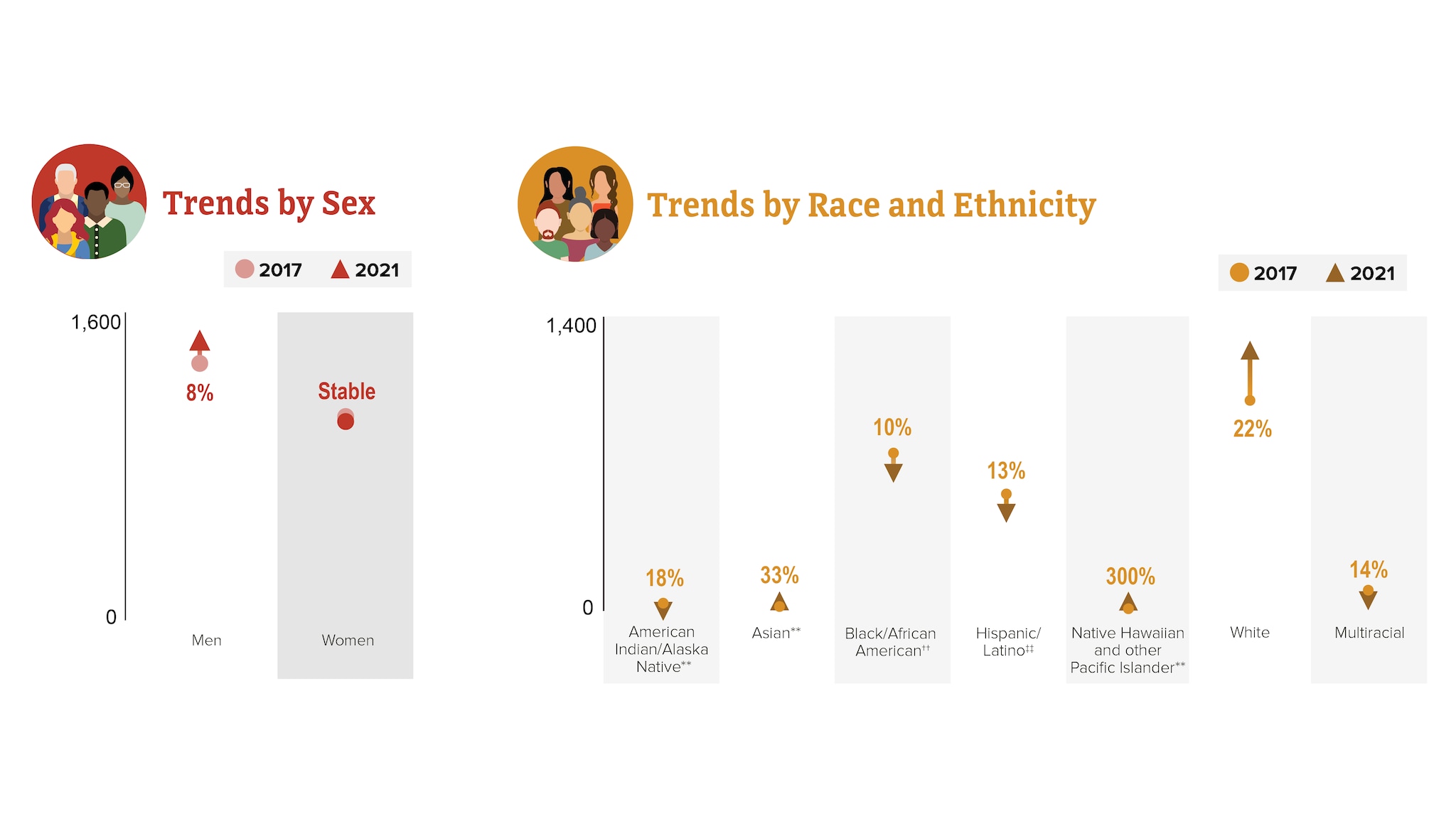
† Includes infections attributed to male-to-male sexual contact and injection drug use (men who reported both risk factors).
‡ Based on assigned sex at birth and includes transgender people.
** Changes in subpopulations with fewer HIV diagnoses can lead to a large percentage increase or decrease.
†† Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
‡‡ Hispanic/Latino people can be of any race.
HIV diagnoses by region
HIV diagnoses are not evenly distributed regionally in the US and dependent areas.
Rates of HIV diagnoses in the US and dependent areas by region, 2021*†
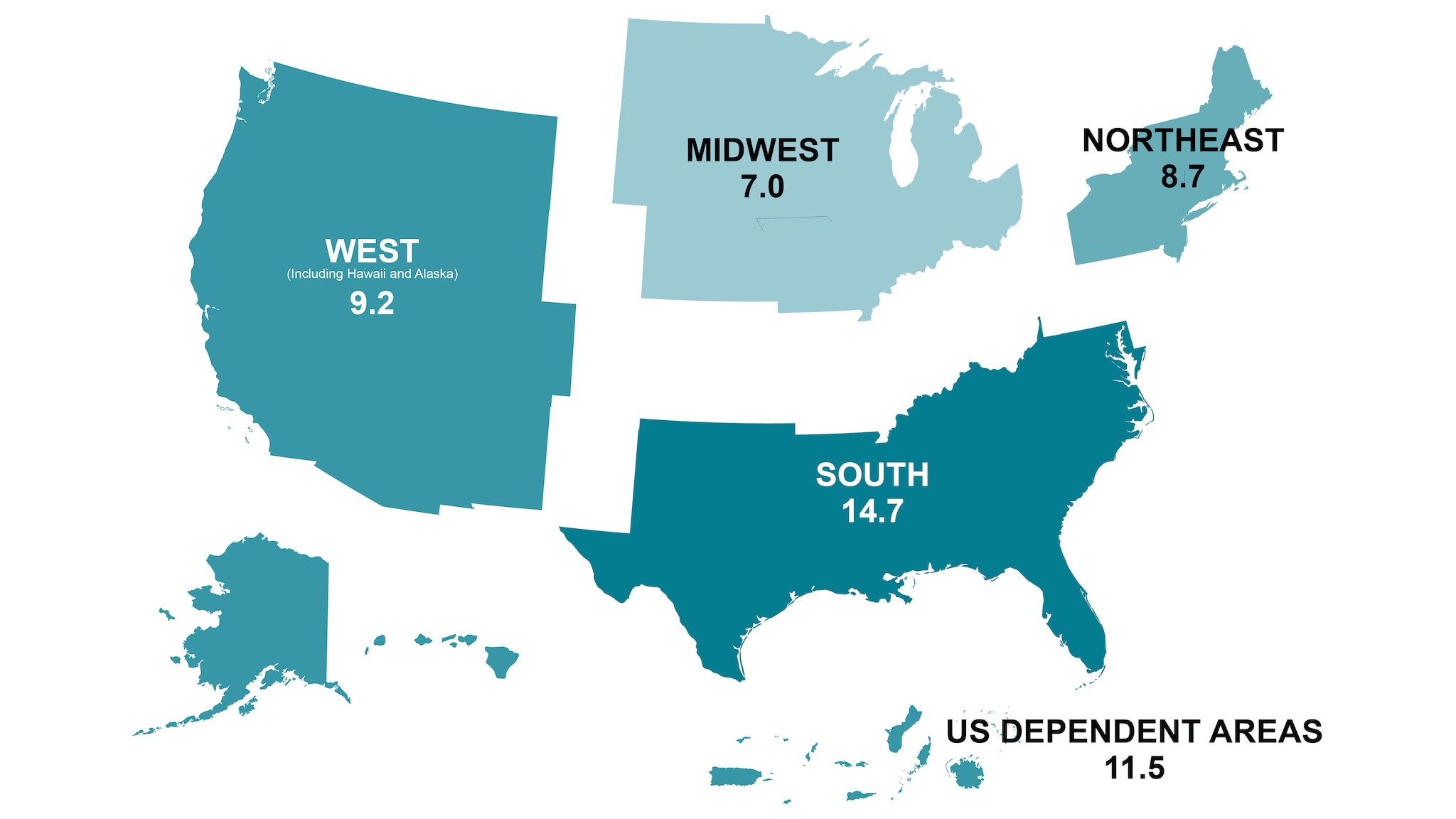
*Rates are per 100,000 people.
† Among adults, adolescents, and children under the age of 13.

Knowledge of status
Knowledge of status refers to the estimated percentage of people with HIV who have received an HIV diagnosis.
Knowledge of HIV status in the US, 2021*
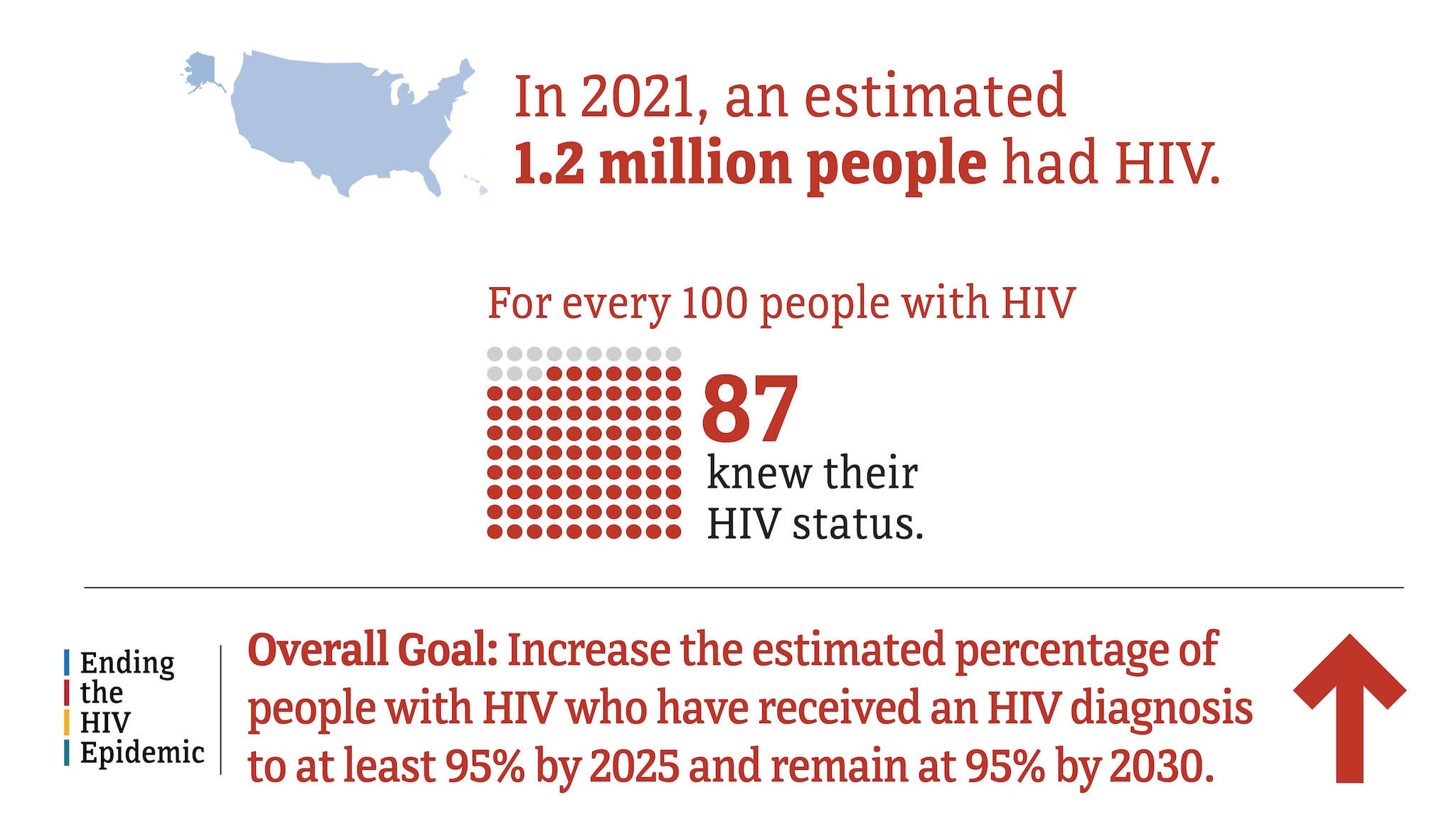
Knowledge of HIV status in the US by transmission category, 2021*
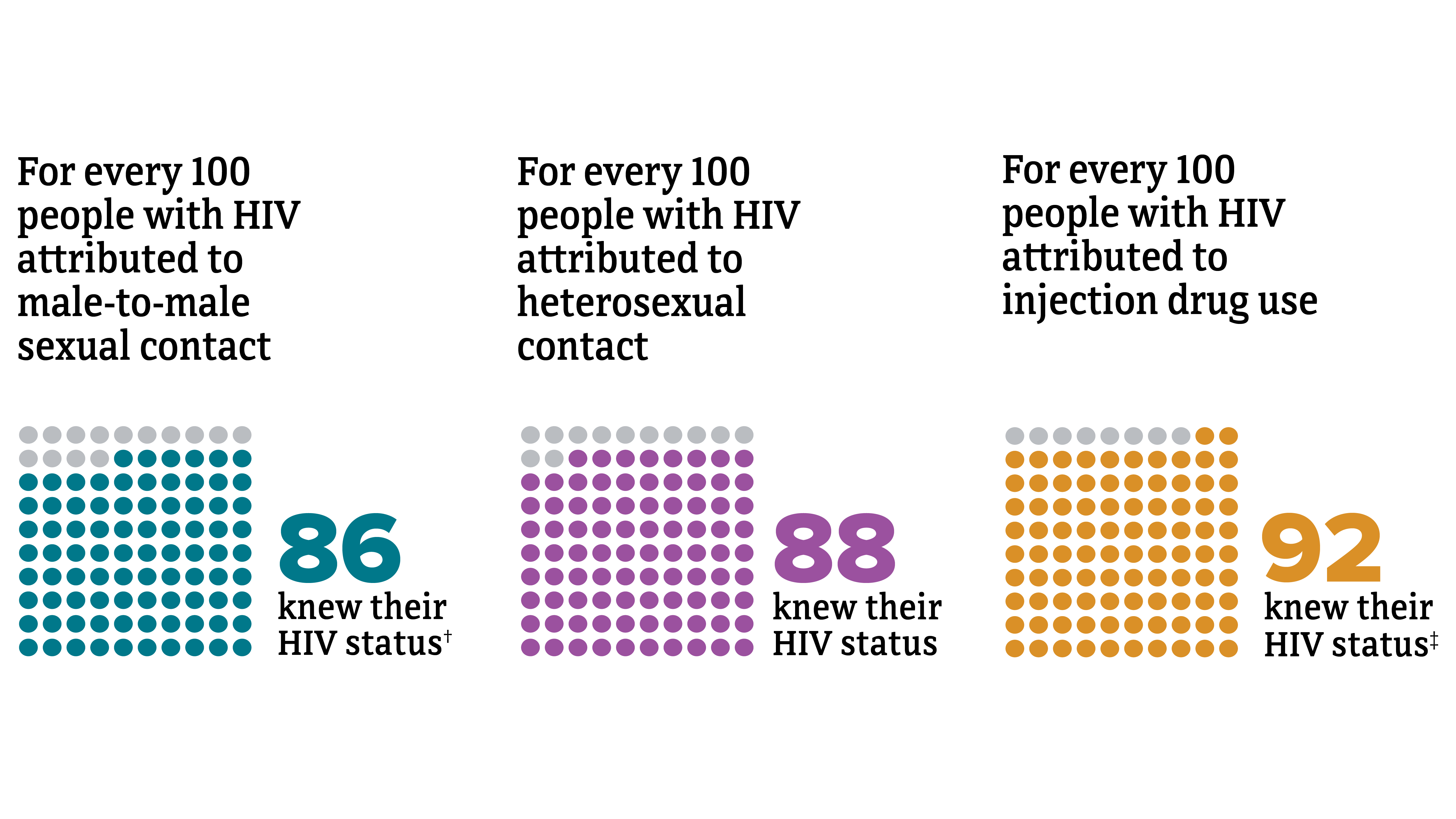
† Includes infections attributed to male-to-male sexual contact only .
‡ Includes infections attributed to injection drug use only. Among men with HIV attributed to male-to-male sexual contact and injection drug use, 92% knew they had HIV.
Knowledge of HIV status in the US by region, 2021*
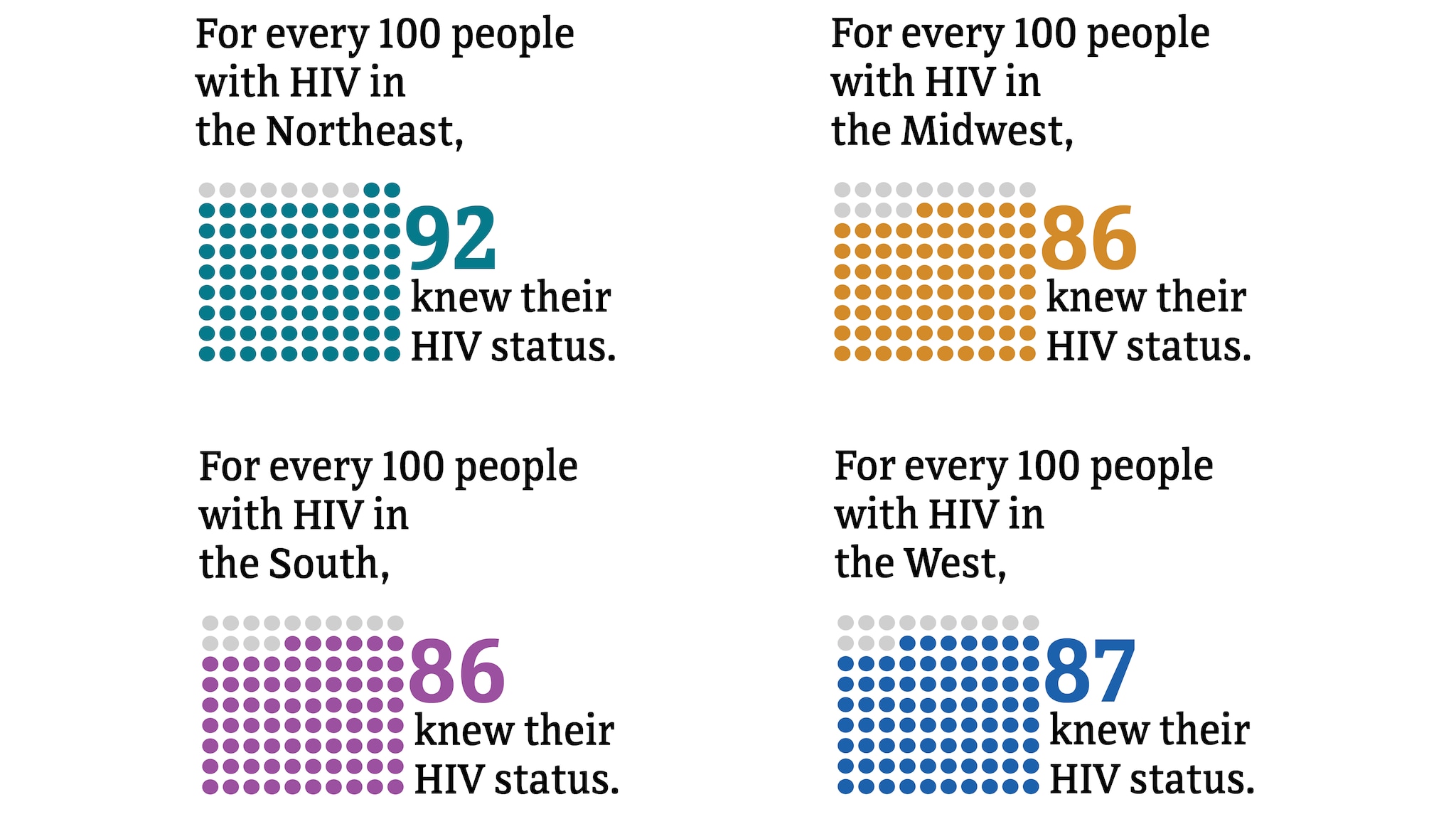
PrEP coverage
PrEP (pre-exposure prophylaxis) coverage refers to the estimated percentage of people with indications for PrEP classified as having been prescribed PrEP.
PrEP coverage in the US and Puerto Rico, 2021

Source: CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2021 . HIV Surveillance Supplemental Report 2023;28(4).
PrEP coverage in the US and Puerto Rico by area of residence, 2021*
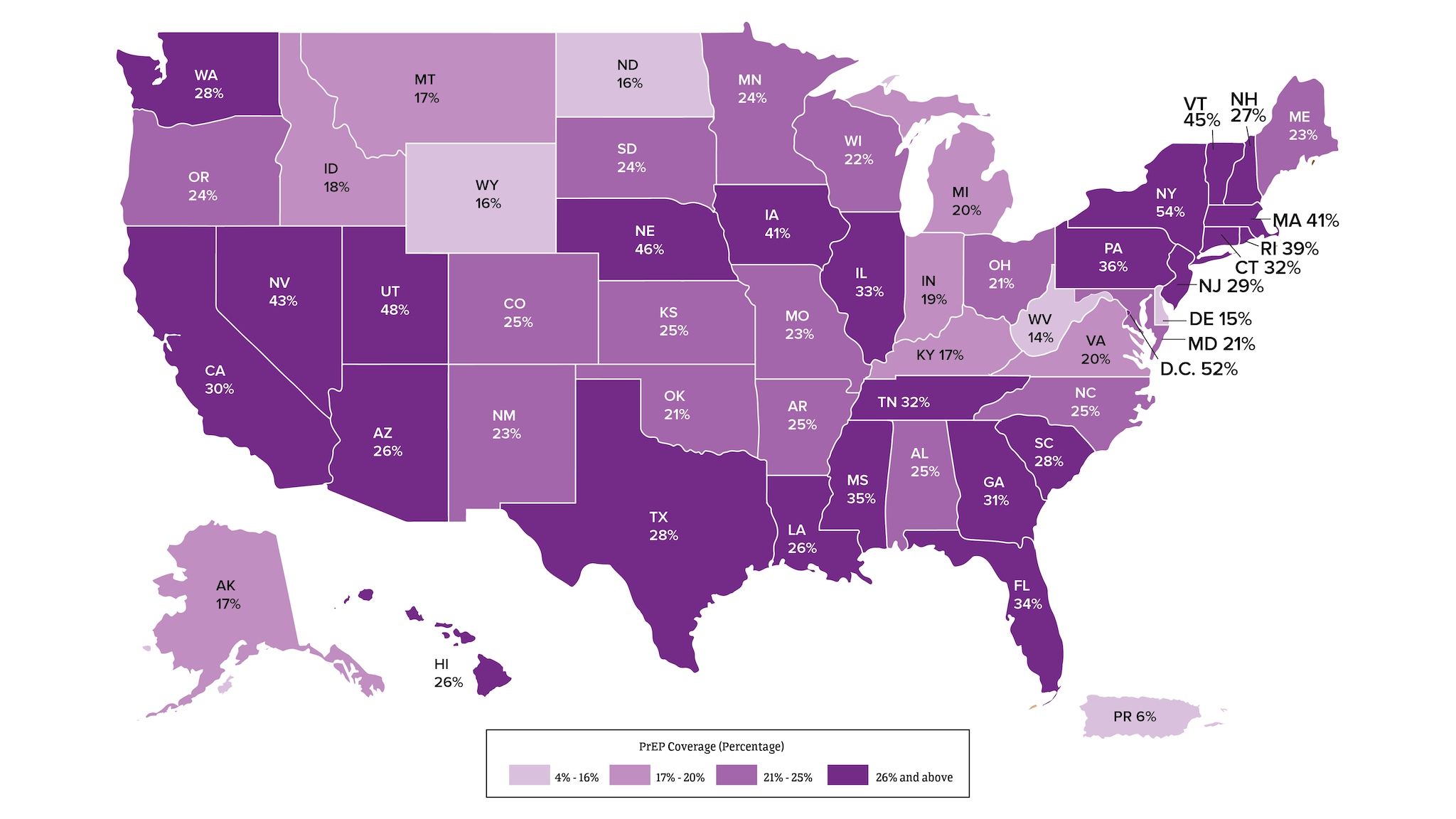
*Among people aged 16 and older.
Viral suppression and barriers to care
Viral suppression refers to the percentage of people with diagnosed HIV who have less than 200 copies of HIV per milliliter of blood.
HIV care continuum among people with diagnosed HIV in 47 states and the District of Columbia, 2021*
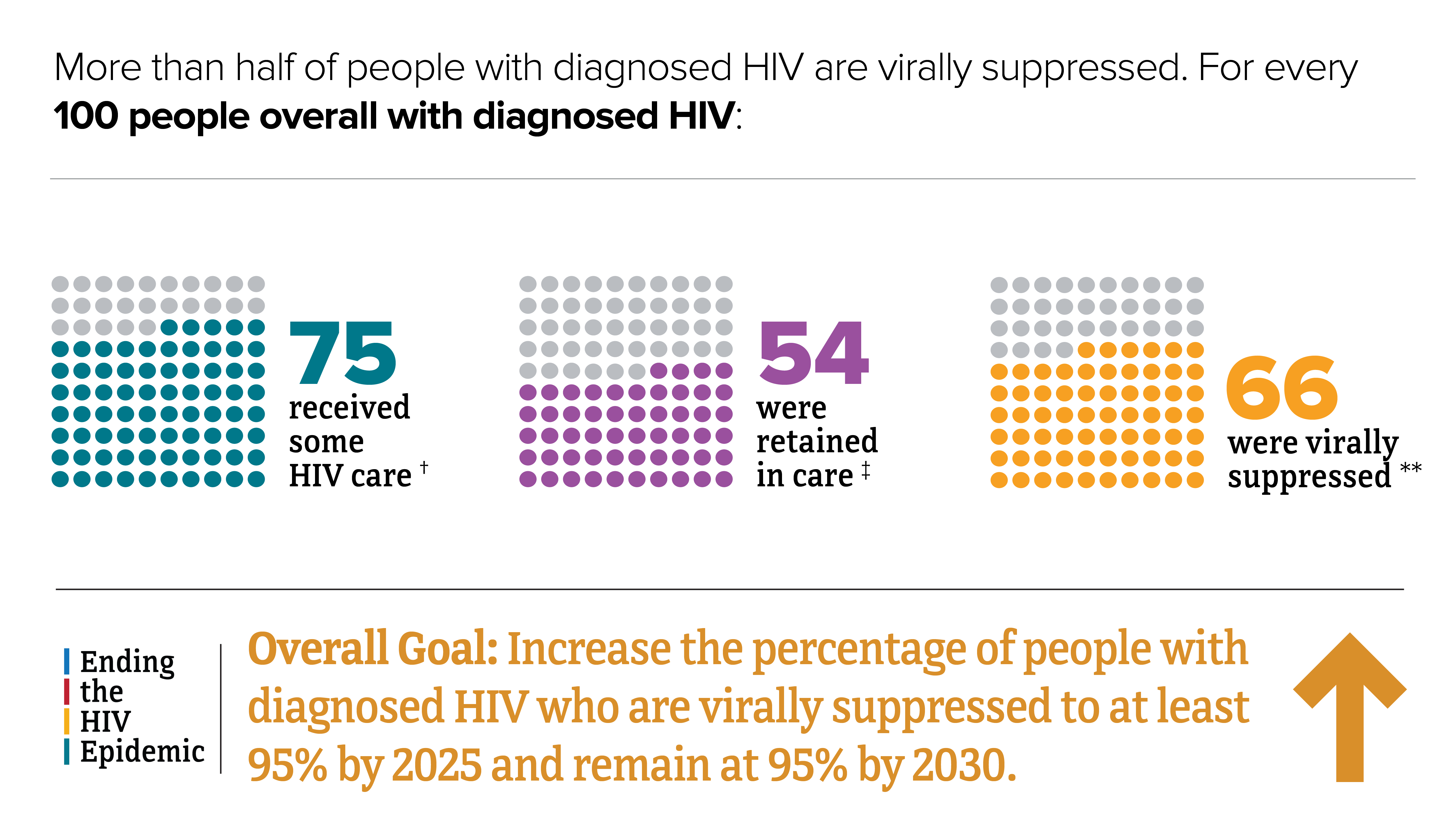
*Among people aged 13 and older.
†At least 1 viral load or CD4 test.
‡Had 2 viral load or CD4 tests at least 3 months apart in a year.
**Based on most recent viral load test.
HIV care continuum among transgender people with diagnosed HIV in 47 states and the District of Columbia, 2021*
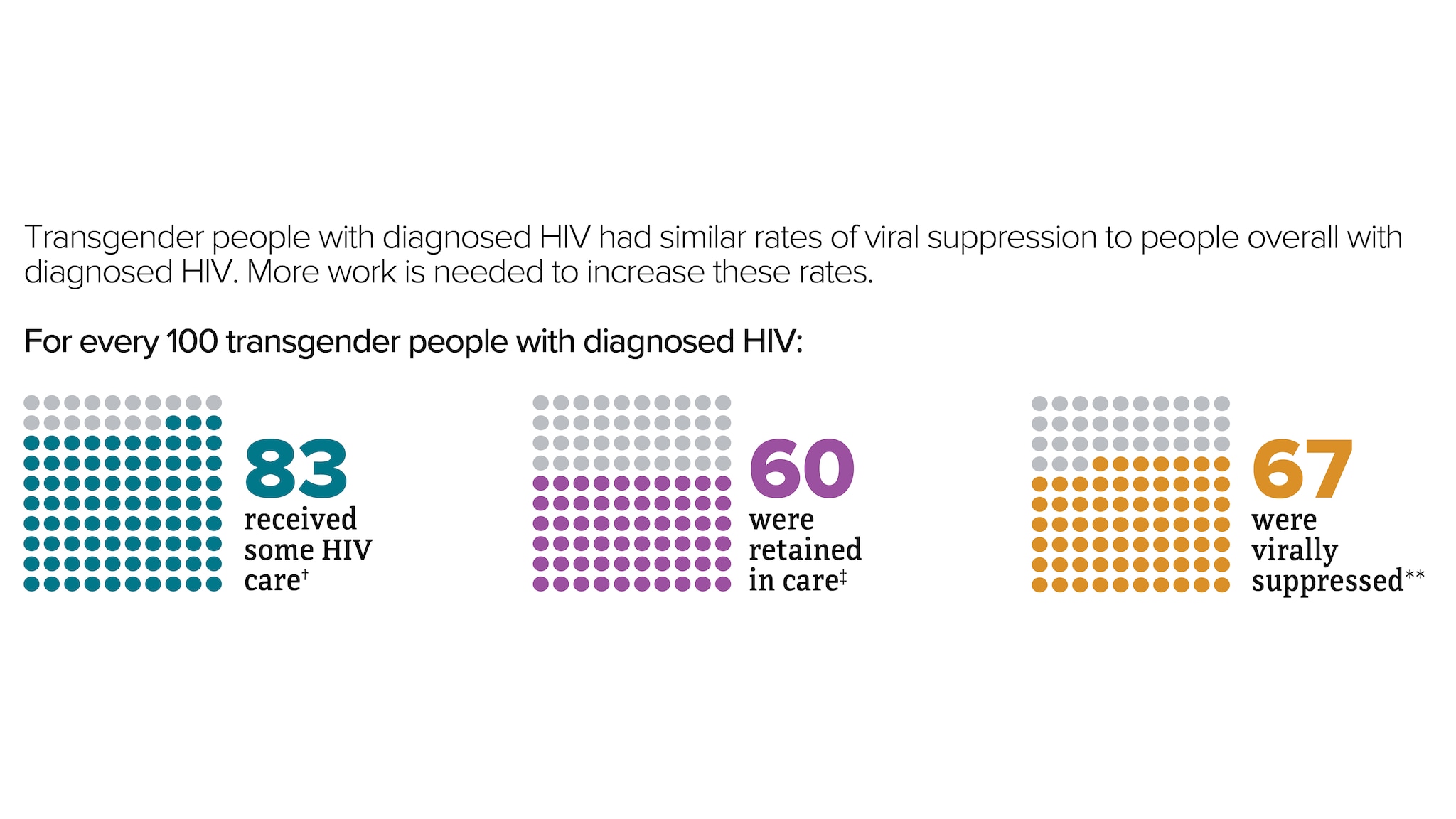
*Among people aged 13 and older.
‡Had 2 viral load or CD4 tests at least 3 months apart in a year.
**Based on most recent viral load test.
HIV care continuum among people with diagnosed HIV in 47 states and the District of Columbia by transmission category, 2021*
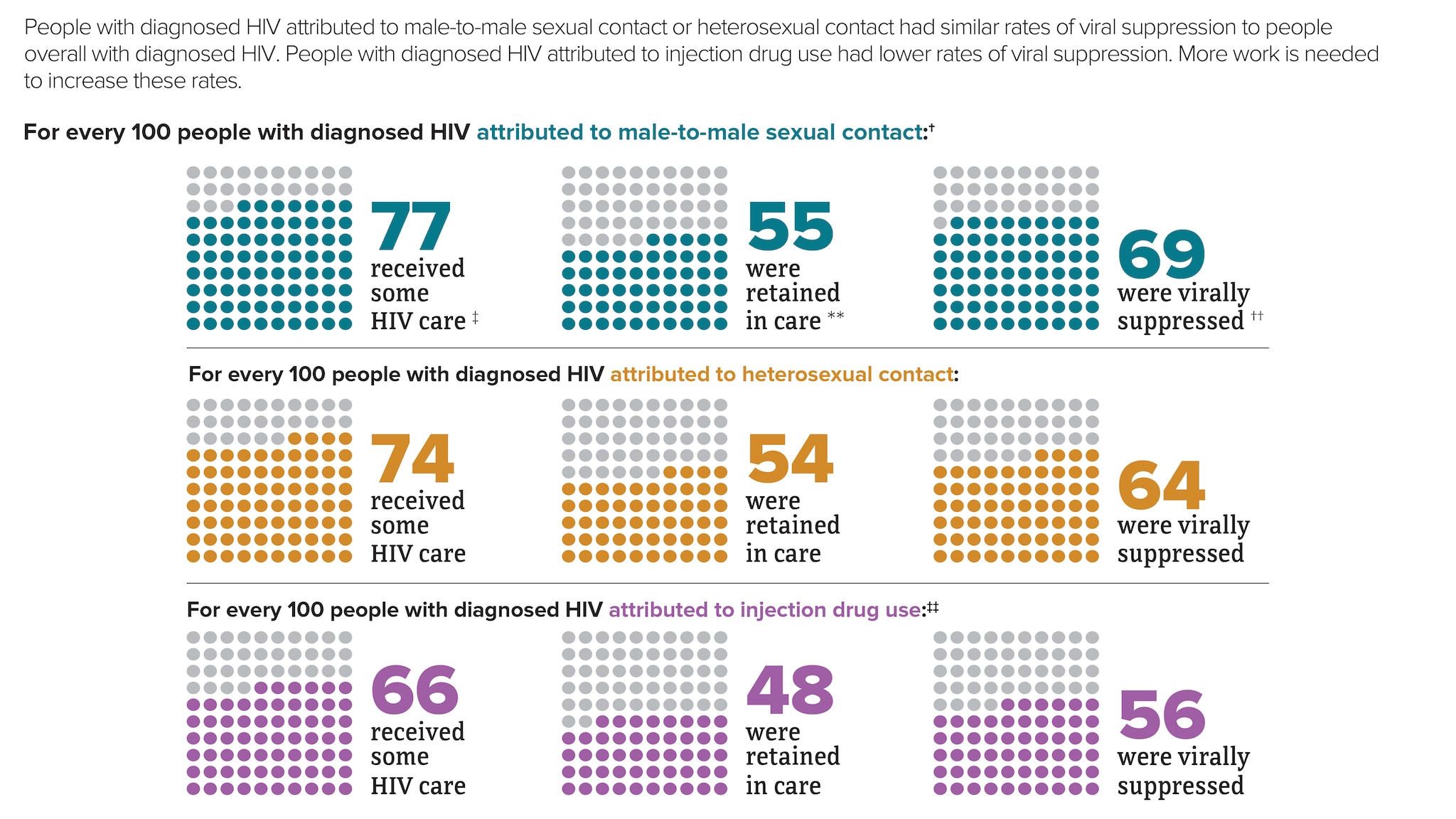
† Includes infections attributed to male-to-male sexual contact only.
‡ At least 1 viral load or CD4 test.
** Had 2 viral load or CD4 tests at least 3 months apart in a year.
†† Based on most recent viral load test.
‡‡ Includes infections attributed to injection drug use only. For every 100 men with HIV attributed to male-to-male sexual contact and injection drug use, 78 received some HIV care, 56 were retained in care, and 65 were virally suppressed.
HIV care continuum among people with diagnosed HIV in 47 states and the District of Columbia by region, 2021*
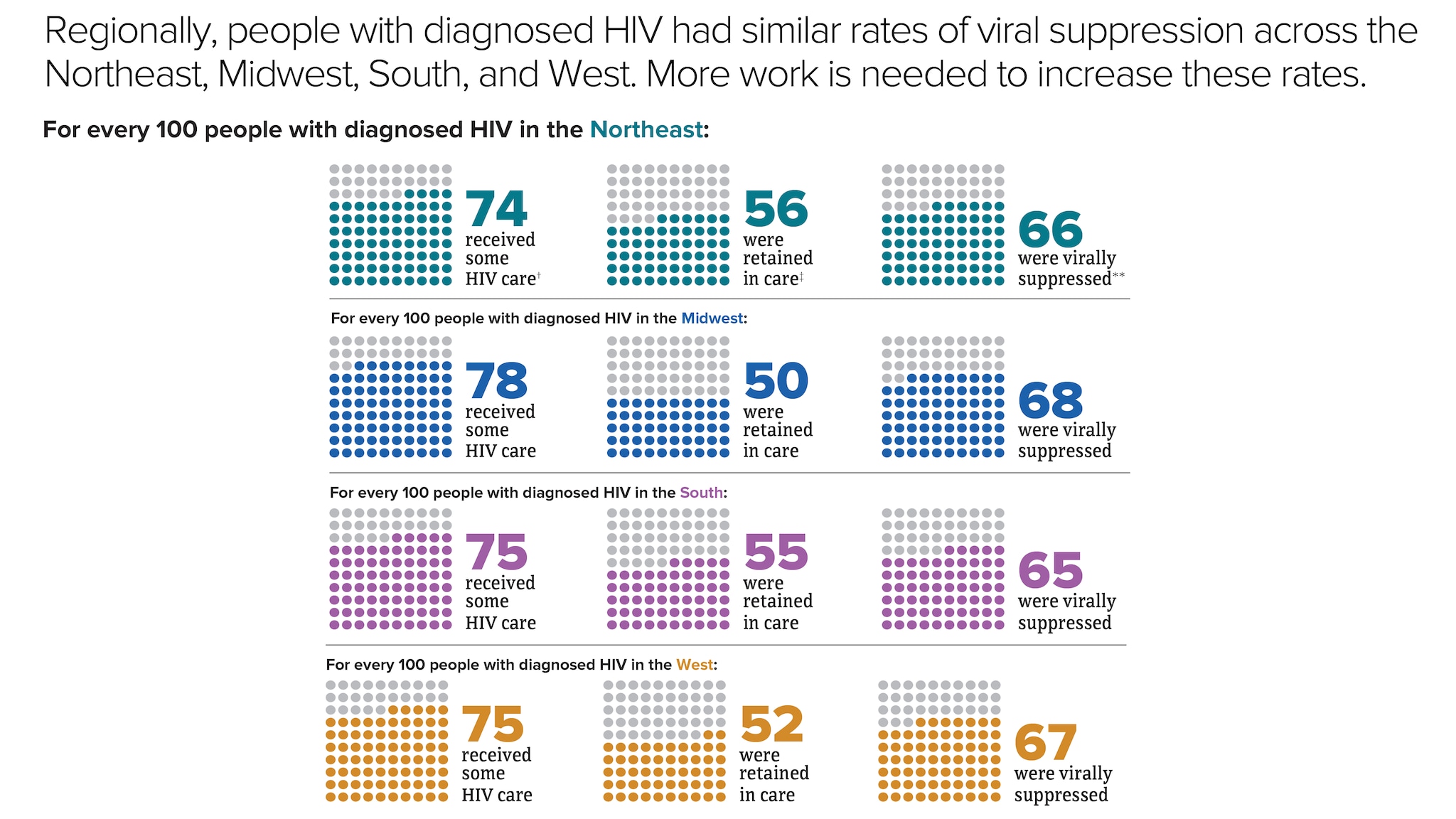
Source: CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2021 . HIV Surveillance Supplemental Report 2023;28(4).
People with diagnosed HIV in the US and dependent areas by age, 2021
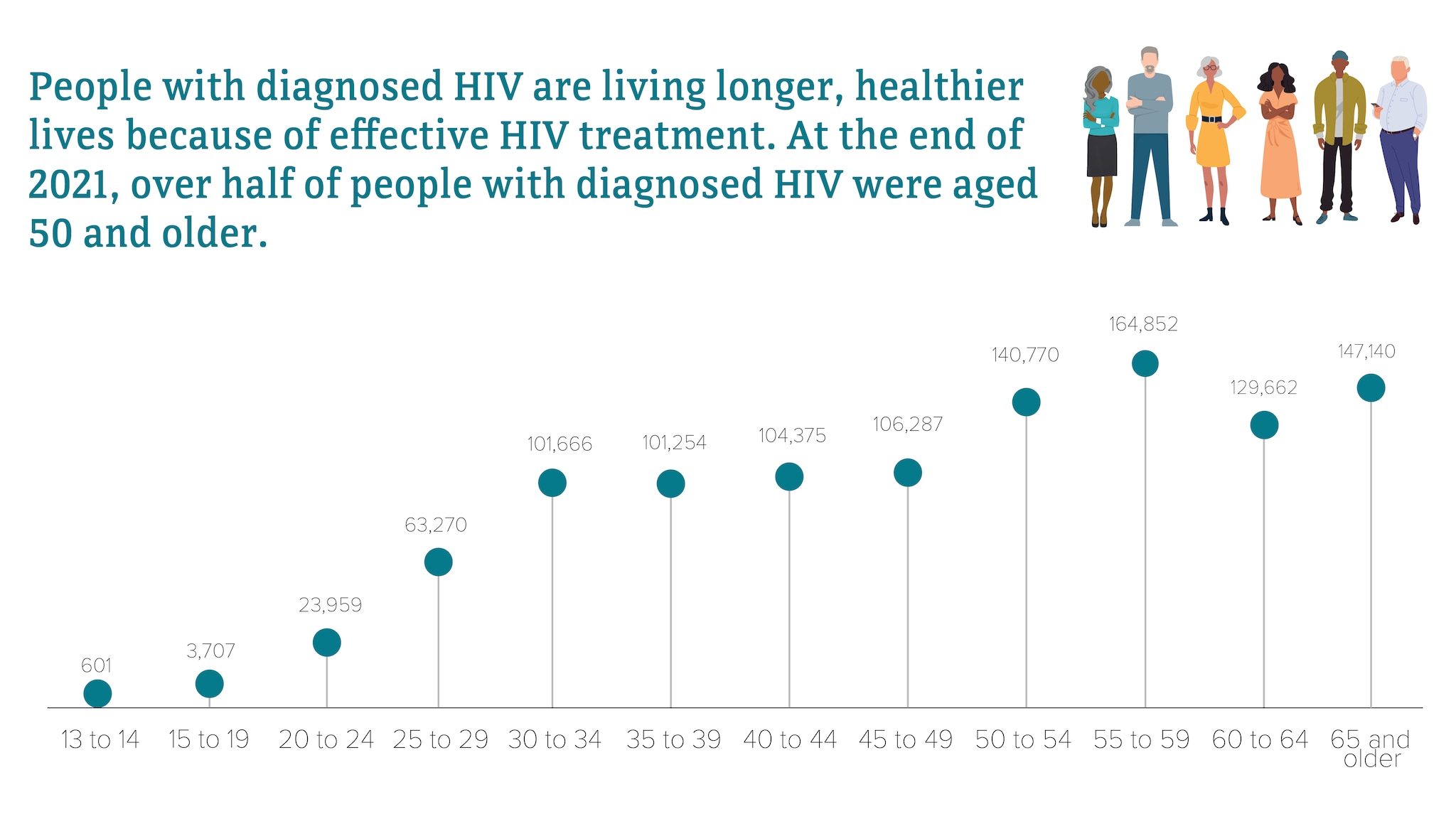
Most HIV cases occur in metropolitan areas with 500,000 or more people. The South has the highest number of people living with HIV, but if population size is taken into account, the Northeast has the highest rate of people living with HIV.
Rates of people with diagnosed HIV in the US and dependent areas by region of residence, 2021*†
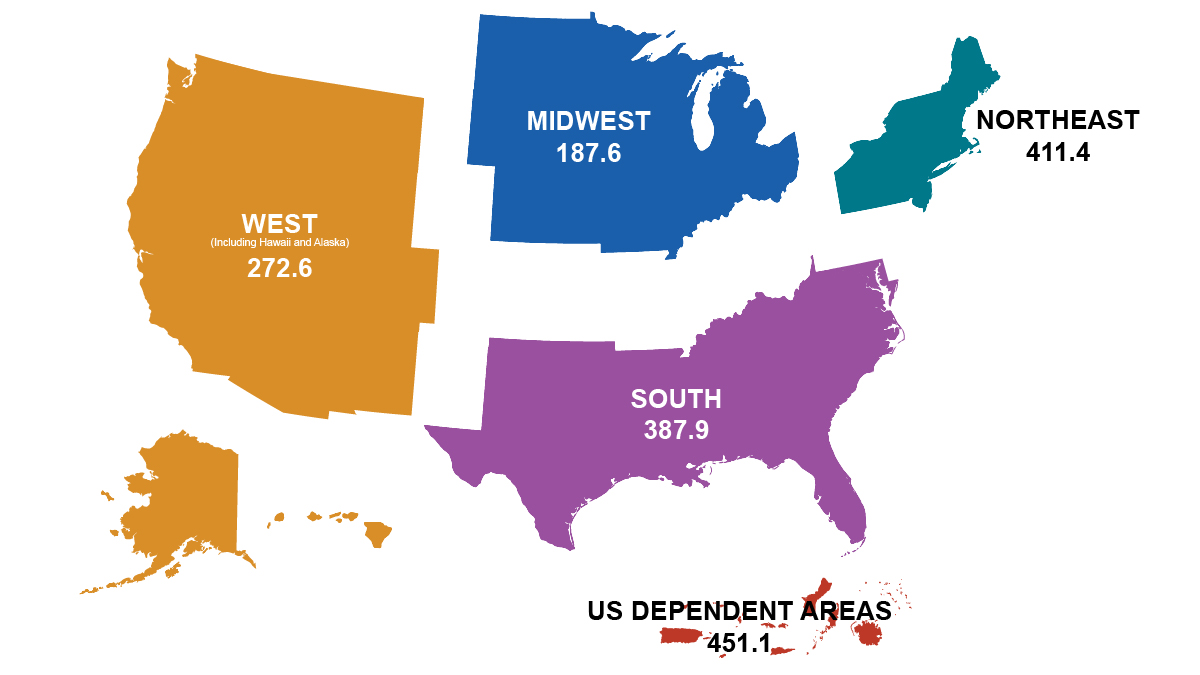
* Rates per 100,000 people.
† Includes adults, adolescents, and children under the age of 13.
Although many people taking HIV medicine are virally suppressed, some people with HIV are currently not virally suppressed or do not maintain viral suppression over time. Some challenges with achieving and maintaining viral suppression include HIV stigma, physical health, mental health, and structural issues—such as food insecurity, unemployment, and unstable housing or homelessness.
Median HIV stigma score among people with diagnosed HIV in the US, 2020*
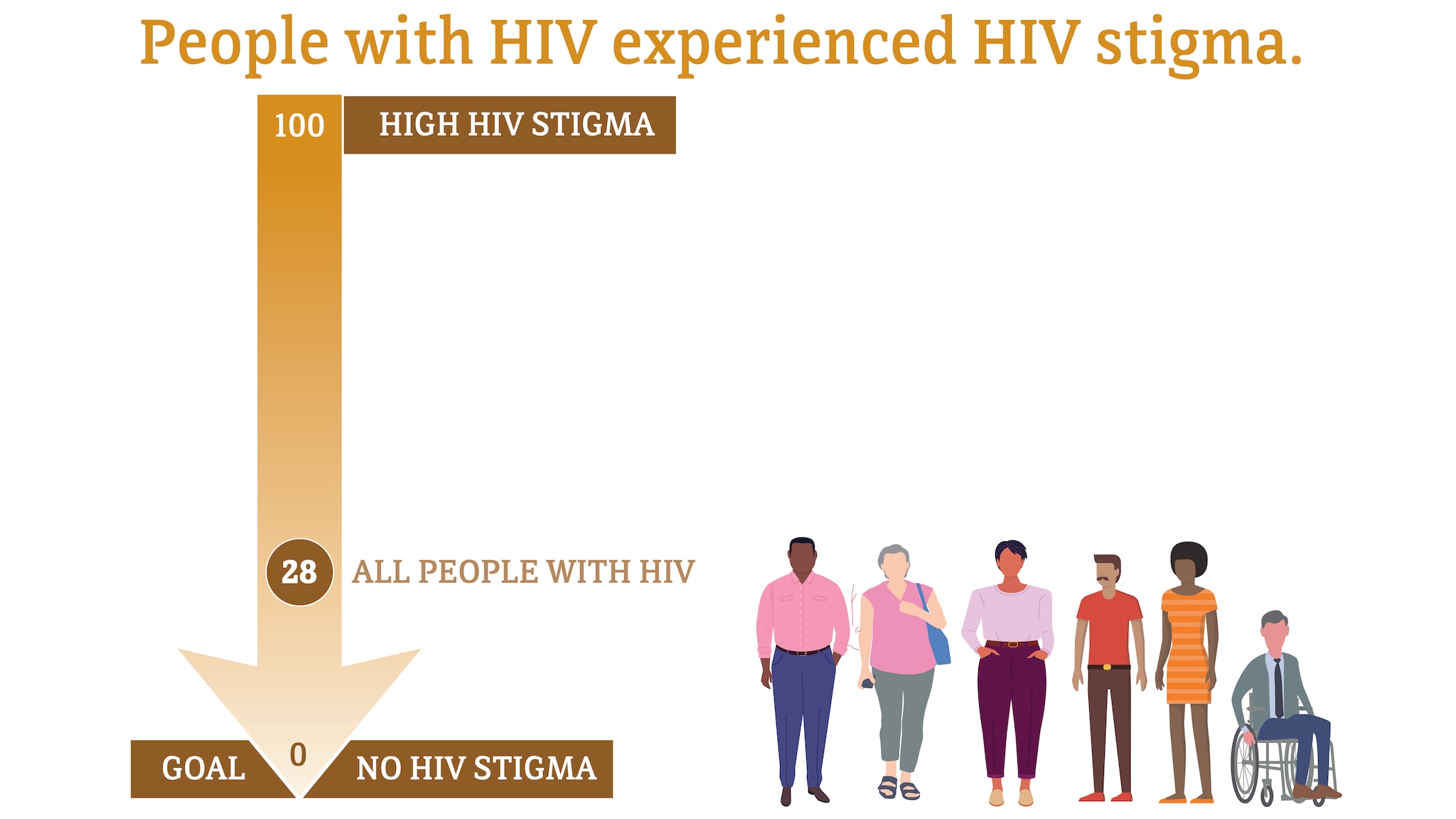
Median HIV stigma scores are presented based on a ten-item scale ranging from 0 (no stigma) to 100 (high stigma) that measures personalized stigma during the past 12 months, current disclosure concerns, current negative self-image, and current perceived public attitudes about people with HIV.
* Among people aged 18 and older.
Source: CDC. Behavioral and clinical characteristics of persons with diagnosed HIV infection—Medical Monitoring Project, United States 2020 cycle (June 2020–May 2021) . HIV Surveillance Special Report 2022;29.
Self-rated health among people with diagnosed HIV in the US, 2020*
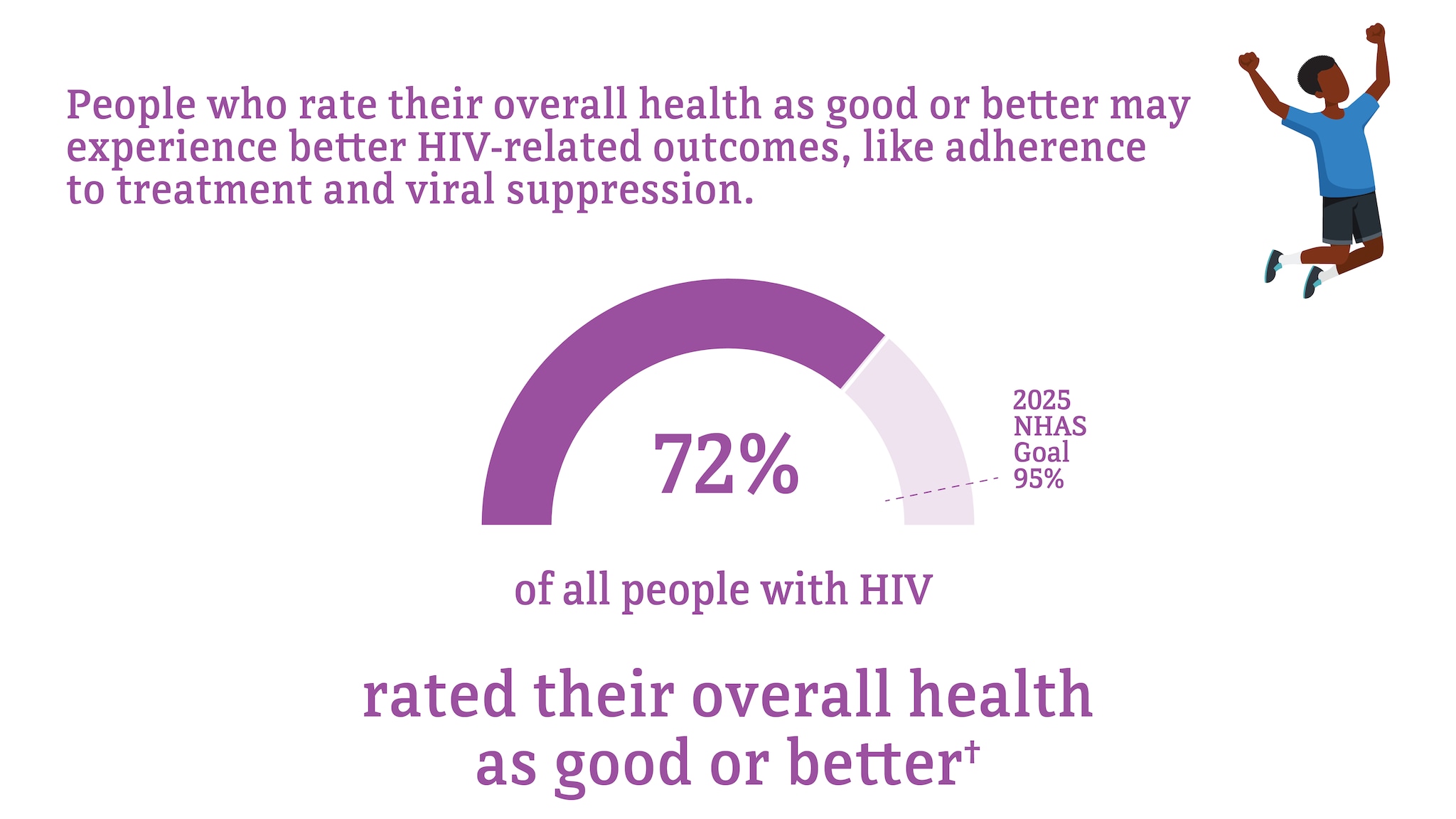
* Among people aged 18 and older.
† Good or better self-rated health is defined as rating one’s health as good, very good, or excellent (as opposed to poor or fair) at the time of interview.
Source: CDC. Quality of life and HIV stigma—Indicators for the National HIV/AIDS Strategy, 2022–2025, CDC Medical Monitoring Project, 2017–2020 cycles . HIV Surveillance Special Report 2022;30.
Unmet need for services from a mental health professional among people with diagnosed HIV in the US, 2020*†
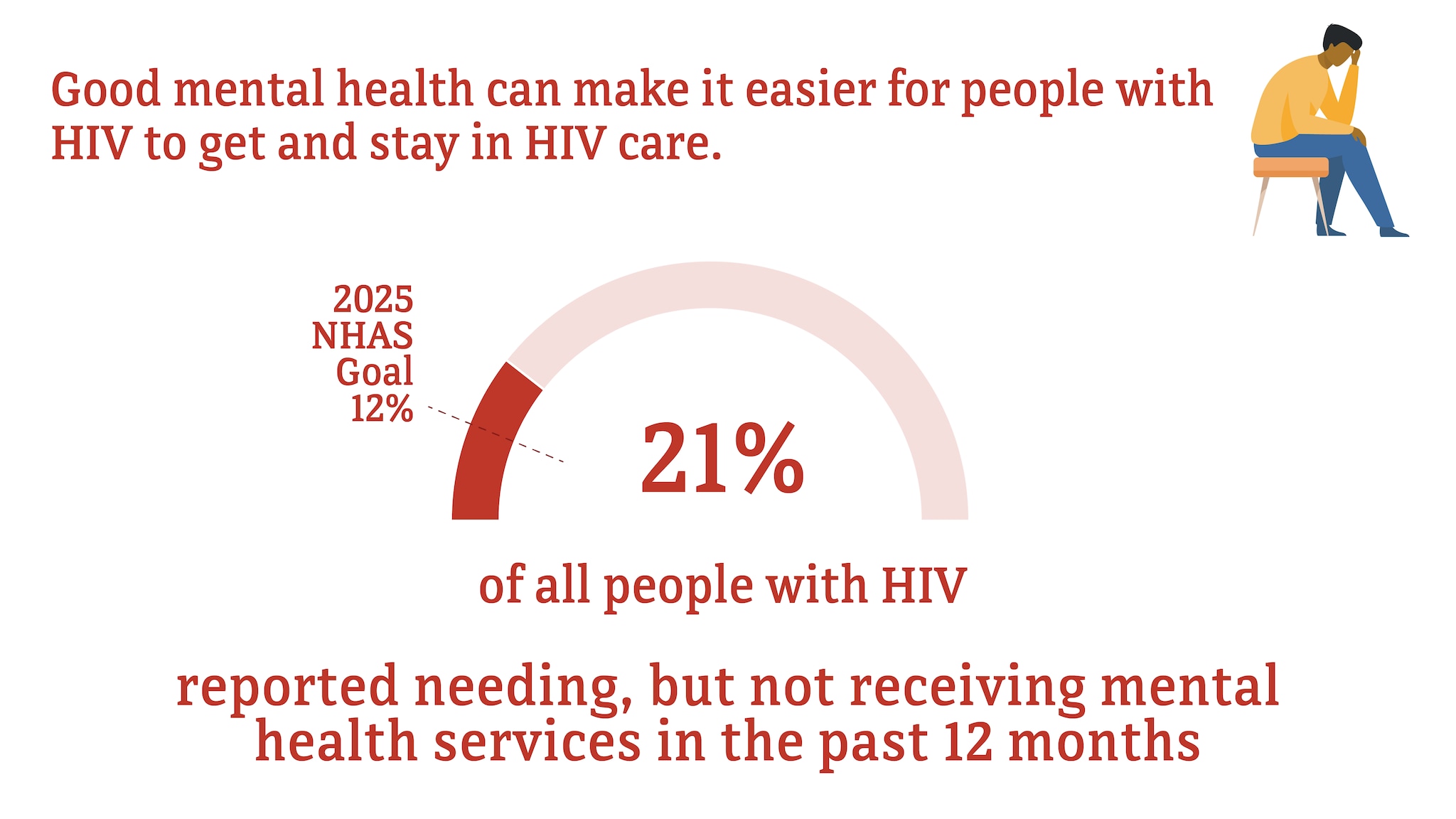
† Among people with diagnosed HIV who reported an unmet need for mental health services in the past 12 months.
Source: CDC. Quality of life and HIV stigma—Indicators for the National HIV/AIDS Strategy, 2022–2025, CDC Medical Monitoring Project, 2017–2020 cycles . HIV Surveillance Special Report 2022;30.
Food insecurity, unemployment, and unstable housing among people with diagnosed HIV in the US, 2020*
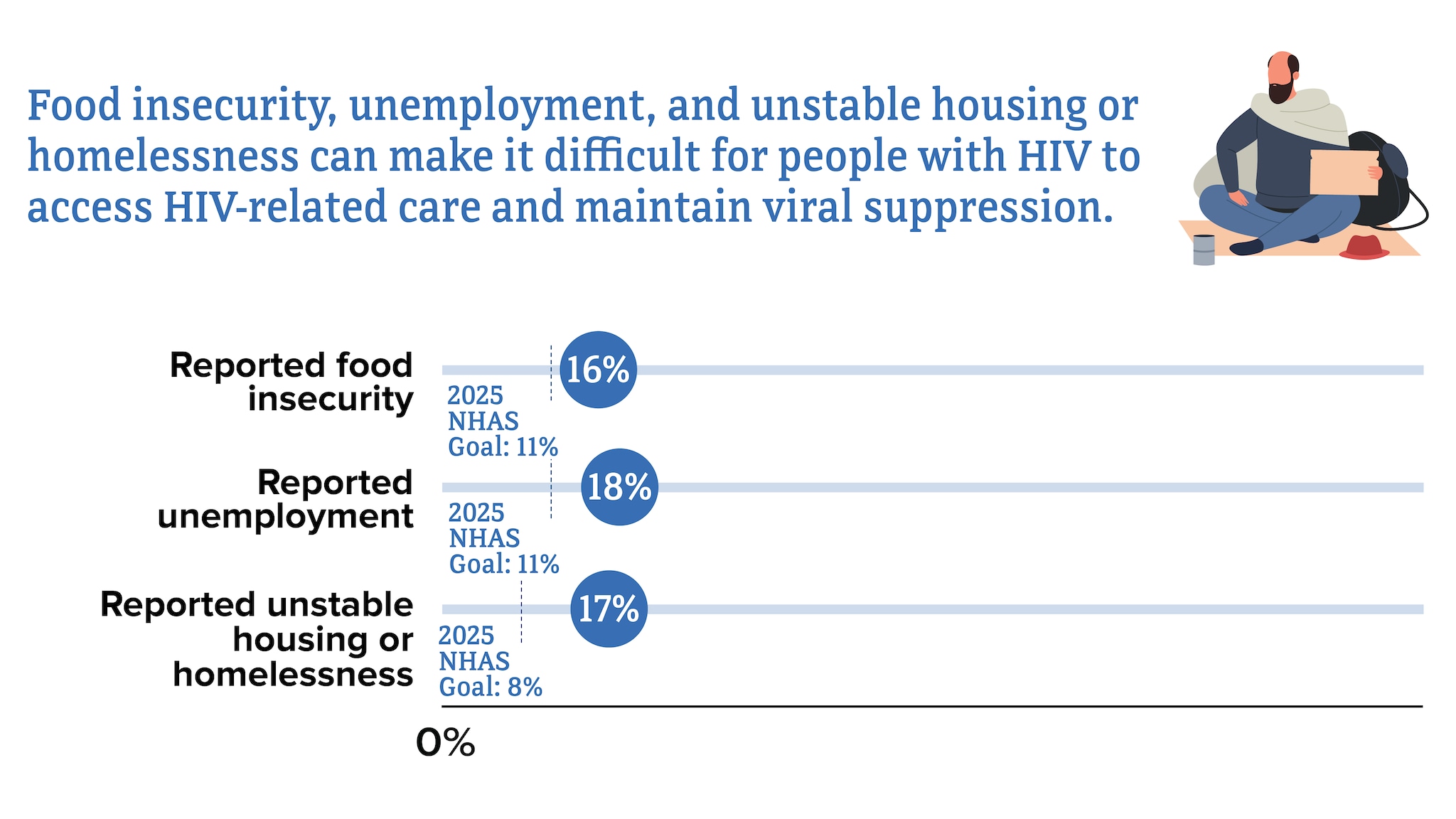
* Among people with HIV aged 18 and older.
Learn about HIV and how the virus is transmitted, how to protect yourself and others, and how to live well with HIV. Also learn how HIV impact the American people.
- Open access
- Published: 22 May 2024
Barriers to viral load suppression among adolescents living with HIV on anti-retroviral therapy: a retrospective study in Tanga, Tanzania
- Stella Emmanuel Mushy 1 ,
- Expeditho Mtisi 2 ,
- Simon Mkawe 3 ,
- Eric Mboggo 3 ,
- John Ndega 3 ,
- Khadija I. Yahya-Malima 1 ,
- Denice Kamugunya 4 ,
- Edwin Samuel Kilimba 3 ,
- Boniface S. Mlay 5 ,
- Aisa Muya 3 &
- Frida Ngalesoni 3
AIDS Research and Therapy volume 21 , Article number: 35 ( 2024 ) Cite this article
Metrics details
Despite the decreased incidence of the human immunodeficiency virus (HIV) in Tanzania, the number of adolescents living with HIV is increasing. This study aimed to describe factors independently associated with viral load non-suppression among adolescents living with HIV (ALHIV) on ART in the Tanga region.
We conducted a retrospective study of routinely collected data from ALHIV on ART from October 2018 to April 2022. We extracted data from the Care and Treatment Clinics form number 2 (CTC2) database that included age, sex, BMI, World Health Organization HIV clinical disease stage, marital status, ART duration, viral load suppression, facility level, and Dolutegravir (DTG)-based regimen. We did descriptive analysis using frequencies to describe the study participants’ socio-demographic and clinical characteristics. The Cox proportional hazard regression model was used to identify factors associated with viral load non-suppression (VLS). Viral load non-suppression was defined as viral load ≥ 1000 copies/ml. A total of 4735 ALHIV on ART were extracted from CTC2, then 2485 were excluded (2186 missed viral load results, 246 were lost to follow-up, and 53 deaths).
2250 ALHIV on ART were tested for viral load, of whom 2216 (98.62%) adolescents were on first-line ART, and 2024 (89.96%) participants were virally suppressed, while 226 (10.04%) were virally non-suppressed. In addition, 2131 (94.71%) of participants were using a DTG-based regimen; of them, 1969 (92.40%) were virally suppressed. Not using a DTG-based regimen (HR: 9.36, 95% CI 3.41–15.31) and dispensary facility level (HR: 3.61, 95% CI 1.44–7.03) were independently associated with increased hazard for viral load non-suppression. In addition, adolescents aged between 15 and 19 years are less likely to be virally suppressed (HR: 0.55, 95% CI 0.30–0.99).
Conclusions
The dispensary facility level and not using a DTG-based regimen were significantly associated with viral load non-suppression. HIV intervention strategies should ensure a DTG-based regimen utilization in all adolescents living with HIV, and techniques used by higher-level health facilities should be disseminated to lower-level facilities.
Globally, as of 2021, the number of people living with human immunodeficiency virus (HIV) was estimated at 38.4 million, with 1.7 million being children aged 0 to 14 years [ 1 ]. Within the Sustainable Development Goals (SDGs), Goal 3 focuses on ensuring healthy lives and promoting well-being for all at every stage of life, including targeting an end to the AIDS epidemic by 2030. This goal encompasses reducing new HIV infections, ensuring universal access to prevention, treatment, care, and support services for HIV, and achieving viral suppression for individuals living with the virus [ 2 ]. In 2021, 85% of people living with HIV globally knew their status, 75% were accessing treatment, and 68% had achieved a low viral load [ 1 ]. However, these achievements vary significantly across different population groups, with adolescents in sub-Saharan Africa (SSA) notably falling behind in viral load suppression (VLS) [ 1 ]. The World Health Organization (WHO) defines adolescents as individuals aged 10 to 19 years [ 3 ].
In SSA, three in every four new HIV infections are young people, and six in every seven new infections among adolescents aged 15–19 years were among women, accounting for 63% of all new HIV infections [ 1 ]. Adolescents in SSA continue to be disproportionately affected, contributing the most significant part of the overall HIV suppression failure prevalence of 47% [ 4 ]. Information, especially regarding the prevalence of VLS among adolescents (10–19 years) living with HIV on ART, remains scarce in Tanzania.
Viral load suppression is a crucial marker of therapy efficacy in PLHIV. To be termed that the virus is suppressed, the VLS is thought to be < 1000 copies/ml of plasma and unsuppressed when ≥ 1000 copies/ml of plasma [ 5 ]. The fundamental goal of HIV infection treatment is to suppress HIV, which will ultimately increase survival, improve quality of life, and reduce HIV transmission [ 5 ]. In addition, follow-up of the VLS status among adolescents is crucial for early identification of treatment failure for patients needing intensive adherence counselling and prevents the occurrence of drug resistance [ 6 ]. Therefore, UNAIDS launched the 95–95–95 targets in 2014, the “Third 95” target aiming to increase viral load suppression to 95% of all HIV-infected individuals on ART by 2030 [ 4 ]. The “Third 95” target focused on defeating HIV/AIDS because HIV patients with VLS have low chances of transmitting infections to others [ 4 ].
Several efforts have been undertaken to address the issue of viral non-suppression among people living with HIV (PLHIV) in Tanzania. These efforts encompass enhanced treatment adherence programs offering education, counseling, and support services, alongside increased availability and accessibility of viral load (VL) testing services to monitor antiretroviral therapy (ART) effectiveness and detect non-suppression early. Healthcare providers have been provided with training on the importance of VL monitoring, result interpretation, and appropriate actions in case of non-suppression. Additionally, newer, more potent ART medications like DTGs are being promoted, while communities affected by HIV/AIDS are being engaged to raise awareness about the importance of ART adherence. Advocacy for policies supporting comprehensive HIV/AIDS care and treatment, including strategies to improve viral load suppression rates, is also ongoing [ 7 , 8 , 9 , 10 ].
In Tanzania, among people living with HIV (PLHIV) aged 15 years and older who are receiving antiretroviral therapy (ART), only 37.9% achieved viral load suppression (VLS) among adolescents and young adults aged 15–24 years [ 9 ]. Despite the concerning treatment outcomes regarding VLS among adolescents receiving ART in Tanzania, their circumstances are often overlooked. This oversight may stem from how age is categorized in Tanzania, where adolescents between 10 and 14 years are grouped with children aged 0–14, while those between 15 and 19 years are considered adults [ 9 , 10 ]. This classification system limits the availability of specific HIV treatment outcome data for adolescents aged 10–19 years. Furthermore, while adolescents constitute a significant proportion of individuals with virally non-suppressed HIV in Tanzania, there is limited published data on treatment outcomes for adolescents aged 10 -19 years regarding VLS and associated factors.
Adolescents are in the transition phase from childhood to adulthood, so the physiological changes resulting from puberty put them at different risks, including behavioural changes that might interrupt ART adherence and delay achieving the “Third 95” target. Lack of special attention to this population segment puts them at high risk of HIV-related morbidity and mortality. Understanding HIV treatment outcomes for adolescents (10–19 years) and associated factors is critical to urgently identify and inform program interventions to optimize ART outcomes to help achieve the “Third 95” target of suppressing viral load to 95%.
Study design and setting
We conducted a retrospective review of routinely collected data from ALHIV on ART from October 2018 to April 2022 from the Tanga region, Tanzania. The current HIV prevalence in Tanga is 5% (3.7 men: 6.2 females). Administratively, Tanga has eight districts (Tanga, Muheza, Korogwe, Lushoto, Handeni, Pangani, and Kilindi). The study sites were all HIV/AIDS care and treatment districts' health care services facilities in Tanga supported by Amref Health Africa Tanzania. Tanzania's healthcare system blends public and private providers, with significant variations in access and quality of care across different regions of the country. The Ministry of Health, oversees policy, regulation, and service coordination. Primary care, offered by dispensaries, health centers, and district hospitals, delivers basic preventive and curative services. Regional and national referral hospitals provide specialized care. CTC services are integrated into the primary healthcare system, which includes dispensaries, health centers, and district hospitals. This integration ensures that HIV/AIDS care is accessible at the community level and provides a continuum of care for individuals living with HIV/AIDS [ 11 ].
Study population and sampling
We included adolescents aged 10–19 years on antiretroviral therapy (ART) in the selected care and treatment districts’ health services facilities in the Tanga region supported by Amref Tanzania and on ART for at least six months. We included ALHIV on ART for at least six months because the first VL test is done in the sixth month after initiating ART. From the CTC2 database, 4735 ALHIV were on ART, then excluded 2485 adolescents (2186 missed viral load results, 246 lost to follow-up, and 53 deaths). After data cleaning, 2250 adolescents were included in the final analysis (Fig. 1 ). The viral load suppression reported for ALHIV relied on the participants on ART for at least six months, who were followed up over a particular time until they had non-viral load suppression or a competing event (attrition and death).

Flow chart of CTC2 data for adolescence from 1st October 2018 to April 2022 in Tanga region, Tanzania
Study variables and analysis
After a thorough review of similar studies that assessed barriers to viral load suppression (VLS) among ALHIV on ART, we considered the following variables as possible determinants of VLS; age (10–14; 15–19); sex (female, male); body mass index (BMI) (< 18.5, 18.5–< 25, 25–< 30, 30 +); WHO HIV clinical disease stage (I, II, III, IV); marital status (cohabiting, divorced, married, single and widow); ART duration (< = 1, > 1 years); ART regimen (first-line, second-line). First, we did a descriptive analysis to provide basic information about the variables of interest in our study to highlight potential relationships between the variables. Numbers and frequencies were used to describe categorical variables, while means/medians and interquartile ranges were used to describe continuous variables. Next, we did a bivariate analysis to ascertain the degree of association between each independent and dependent variable.
We used Cox proportional hazard regression model to describe the hazard ratio of participants with viral load non-suppression from the date of ART initiation. We used Kaplan–Meier curves to estimate the cumulative incidence of viral load non-suppression. The death date and attrition marked the end time of viral load non-suppression. We included the variables in bivariate analysis with a p ≤ 0.2 in the Cox hazard regression model as potential confounders on a two-sided test with a significant p-value less than or equal to 0.05. We used the missing indicator method to handle missing data. We used the SAS statistical software package version 9.3 to perform data analysis (Cary, NC, USA).
Study sample characteristics
A total of 2250 ALHIV on ART for at least six months, with results of the last viral load, were included for analysis. The median age of the participants was 15.2 years (Interquartile Range [IQR] = 12.8–17.2). Table 1 shows that half of the participants (51.56%) were aged 15–19 years and females (51.33%). More than half (58.58%) of the participants attended the health centre facility level. Results also showed that most participants were on a first-line drug regimen (98.62%), and the majority (95.29%) were on ART for over one year. A great proportion of participants (89.96%) were virally suppressed, and 94.71% of ALHIV on ART were using a DTG-based regimen; of the DTG-based regimen users, 92.40% were virally suppressed. Most participants reported being single (75.34%), and 76.67% had a BMI of 18.5 – < 25 kg/m 2 . Nearly two-thirds of the participants were in WHO clinical stage III (60.35%).
Socio-demographic and clinical characteristics of the participants with viral load non-suppression
As illustrated in Table 1 , more males (11.60%) compared to females (8.57%) were virally non-suppressed. The age group 15–19 years accounted for 10.52% viral load non-suppression compared to the age group 10–14 years (9.54%). Furthermore, adolescents on the first-line regimen accounted for 9.61% viral load non-suppression compared to 32.26% on the second-line regimen. More virally non-suppressed participants (15.86%) were observed from the dispensary than at the health center and hospital facility levels.
Covariates-adjusted Kaplan Meir plots for the overall viral load non-suppression by the facility and DTG-based regimen suppression categories are presented in Figs. 2 and 3

Survival probability among adolescents by facility level

Survival probability among adolescents by DTG-based regimen
Factors independently associated with viral load non-suppression
Table 2 shows that the likelihood of being virally non-suppressed for participants not on a DTG-based regimen was more than nine times at increased hazard (HR: 9.36, 95% CI 3.41–15.31) than those on a DTG-based regimen, p < 0.0001. Additionally, participants in care and treatment at the dispensary level (HR: 3.61, 95% CI 1.44–7.03) had 3.6 times increased hazard of being virally non-suppressed than participants in the health center and the hospital, p = 0.007. Furthermore, the age of 15–19 years (HR:0.55, 95% CI 0.30–0.99) is less likely to be virally non-suppressed than the age of 10–14 years, p = 0.04376.
This study investigated the factors associated with viral load non-suppression among adolescents living with HIV (ALHIV) on ART in care and treatment health services facilities in Tanga. Anti-retroviral therapy (ART) aims to suppress viral replication to reduce the patient’s viral load (VL) to undetectable levels [ 5 ]. This reduction of viral load prevents further damage to the body’s immune system and restores and maintains the quality of life [ 5 ]. Therefore, early enrolment into care, retention, and good adherence to ART are vital components of AIDS and HIV transmission prevention.
The current study found that 226 participants (10.04%) were virally non-suppressed. Factors independently associated with viral load non-suppression included not using a DTG-based regimen and receiving services at the dispensary level. Additionally, adolescents aged 15–19 years were less likely to achieve viral suppression.
The viral load non-suppression prevalence among adolescents in this study was lower than the national prevalence of 17.7% reported in 2016 [69. This rate is lower than the studies in Uganda, Swaziland, and South Africa, at 31.4%, 16%, and 15%, respectively [ 12 , 13 , 14 ]. However, this observed viral load non-suppression rate falls short of the UNAIDS’ 95-95-95 targets. The observed lower viral load non-suppression rate in the current study may be related to the rollout of a DTG-based regimen for all PLHIV that could have helped to influence viral load suppression. The present study showed that ALHIV on a DTG-based regimen was significantly virally suppressed. In addition, PLHIV on ART, once detected to have a high viral load, receive three consecutive sessions of enhanced adherence counselling that might have contributed to the improved adherence and eventually reduced viral load suppression. Our findings suggest that the WHO target of 95% viral suppression by 2025 is possible for ALHIV in Tanzania if HIV services are improved.
The DTG-based regimen is more effective in suppressing viral load than the non-DTG-based regimen [ 15 , 16 , 17 ]. However, in the present study, most virally non-suppressed did not use a DTG-based regimen. In Tanzania, the use of ART with a DTG-based regimen was rolled out in 2019. Unfortunately, to the best of our review, limited published studies looked at the association between viral load suppression and the use of a DGT-based regimen.
The ALHIV accessing care and treatment at the dispensary facility level had a higher risk of being virally non-suppressed than those who attended HIV care and treatment at the hospital and health center facilities. The possible reason for this observation could be limited strategies to follow up with patients at the lower health facility levels than higher facilities. However, limited studies examined the association between facility-level attendance and viral load suppression.
Evidence points to a connection between sex and the suppression of viral load. For instance, one study indicated that males were more likely than females to be virally suppressed [ 18 ]. In contrast, another study found that females were more likely than males to be virally suppressed [ 19 ]. However, our study found that males were less likely to be virally suppressed than females. Implying that implemented efforts to improve VLS should put more efforts on reaching men. The outcome may be related to their reported lower treatment adherence rates. It is difficult to link and keep males in ART therapy [ 20 ] and have them tested for HIV since they also exhibit poor treatment-seeking behavior [ 19 ]. This finding has been evidenced in the first studies that analysed ART treatment after five years of ART initiation in Tanzania. Excess mortality in men was rooted in poor health-seeking behavior and non-compliance with medication [ 21 ]. Males are also discouraged from obtaining healthcare services due to a shortage of male-friendly services [ 20 , 22 , 23 ].
The study limitations and recommendations
The longitudinal nature of this study gave room to calculate the rate and barriers that independently hindered viral load suppression among ALHIV in Tanga. However, this study used secondary data that limited information on important variables like pregnant status, ART adherence, support from significant others, and disclosure status, which are reported to delay viral load suppression. Using secondary data presented challenges in the quality, completeness, and missing data that we mitigated by employing statistical and data management techniques to ensure data quality. In addition, the VLS success observed in this study was only for those whose viral load testing was possible, which might have underrepresented adolescents in the Tanga region.
Viral load non‐suppression among ALHIV was almost lower than the national average. Not using a DTG-based regimen, attending care and treatment at the dispensary facility level, and age group of 15–19 years were independently associated with viral load-non-suppression among ALHIV. Therefore, adolescent-friendly strategies are essential, especially at the dispensary facility level, to help improve viral load suppression to foster the attainment of the UNAIDS's third 95 target. In addition, program implementers should improve the accessibility of a DTG-based regimen to all PLHIV on ART in the country. Furthermore, we recommend innovative interventions to ensure ART adherence and retention that would ultimately improve VLS.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
Acquired immunodeficiency syndrome
All people living with HIV
- Antiretroviral therapy
Care and treatment clinics form number two
- Dolutegravir
Human immunodeficiency virus
People living with HIV
Sub-Saharan Africa
World Health Organization
UNAIDS. Global HIV & AIDS statistics—2022 fact sheet. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf . Accessed 14 Sept 2022.
Joint United Nations Programme on HIV/AIDS (UNAIDS). (2020). Global AIDS Update 2020: Seizing the moment—tackling entrenched inequalities to end epidemics. https://www.unaids.org/en/resources/documents/2020/global-aids-report . Accessed 10 April 2024
World Health Organization. Global Fund, US centers for disease control and prevention. HIV drug resistance report 2017. Geneva: World Health Organization. 2017. P.82. https://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf . Accessed 15 Sept 2022.
Global AIDS Monitoring. 2022. Geneva: Joint United Nations Programme on HIV/AIDS; [2022]. License: CC BY-NC-SA 3.0 IGO. https://www.unaids.org/sites/default/files/media_asset/global-aids-monitoring_en.pdf . Accessed 13 Sept 2022.
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization. 2016. https://www.who.int/hiv/pub/arv/arv-2016/en/ . Accessed 14 Sept 2022.
Katumba A. Sub-optimal HIV viral suppression and its determinants among adolescents in the post-viral load monitoring scale-up era in Mulago Iss Clinic and Bwizibwera HIV Clinic in Uganda [thesis]. Makerere University. 2019. http://makir.mak.ac.ug/handle/10570/7762 . Accessed 14 Sept 2022.
UNAIDS. Country factsheets: Tanzania. UNAIDS Tanzania. 2020. https://www.unaids.org/sites/default/files/country/documents/TZA_2020_countryreport.pdf . Accessed 8 April 2024.
MoHCDGEC Ministry of Health CD, Gender, Elderly, and Children—MoHCDGEC/Tanzania Mainland, MOH Ministry of Health—MoH/Zanzibar, NBS National Bureau of Statistics—NBS/Tanzania, OCGS Office of Chief Government Statistician—OCGS/Zanzibar, ICF. Tanzania Demographic and Health Survey and Malaria Indicator Survey 2015—2016. Dar es Salaam, Tanzania. 2016. https://dhsprogram.com/pubs/pdf/fr321/fr321.pdf . Accessed 2 Oct 2022.
Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC). Tanzania HIV impact survey (THIS) 2016–2017: final report. Dar es Salaam, Tanzania. december 2018. https://phia.icap.columbia.edu/wp-content/uploads/2019/06/FINAL_THIS-2016-2017_Final-Report__06.21.19_for-web_TS.pdf . Accessed 12 Sept 2022.
Tanzania Ministry of Health, Community Development, Gender, Elderly, and Children. National guidelines for the management of HIV and AIDS (6th Edition). 2017.
Tanzania Ministry of Health, Community Development, Gender, Elderly and Children. National guidelines for the management of HIV and AIDS. 2019. https://differentiatedservicedelivery.org/wp-content/uploads/national_guidelines_for_the_management_of_hiv_and_aids_2019.pdf
Maena J, Banke-Thomas A, Mukiza N, Kuteesa CN, Kakumba RM, Kataike H, et al. Determinants of viral load non-suppression among adolescents in Mbale district, Eastern Rural Uganda. AIDS Res Ther. 2021. https://doi.org/10.1186/s12981-021-00408-1 .
Article PubMed PubMed Central Google Scholar
Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents, and adults on antiretroviral therapy for HIV in Swaziland. PLoS ONE. 2015;10(2): e0116144. https://doi.org/10.1371/journal.pone.0116144 .
Article CAS PubMed PubMed Central Google Scholar
Joseph DD, Abrahams Z, Feinberg M, Prins M, Serrao C, Medeossi B, et al. Factors associated with recent unsuppressed viral load in HIV-1-infected patients receiving first-line antiretroviral therapy in South Africa. Int J STD AIDS. 2018;29(6):603–10. https://doi.org/10.1177/0956462417748859 .
Article Google Scholar
Orrell C, Hagins DP, Belonosova E, Porteiro N, Walmsley S, Falcó V, et al. Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomized, open-label, non-inferiority, phase 3b study. Lancet HIV. 2017;4(12):e536–46. https://doi.org/10.1016/S2352-3018(17)30095-4 .
Article PubMed Google Scholar
Van Lunzen J, Maggiolo F, Arribas JR, Rakhmanova A, Yeni P, Young B, et al. Once-daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48-week results from SPRING-1, a dose-ranging, randomized, phase 2b trial. Lancet Infect Dis. 2012;12:111–8.
Calmy A, Tovar Sanchez T, Kouanfack C, Mpoudi-Etame M, Leroy S, Perrineau S, New Antiretroviral and Monitoring Strategies in HIV-infected Adults in Low-Income Countries (NAMSAL) ANRS 12313 Study Group, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicenter, randomized, open-label, phase 3 non-inferiority trial in Cameroon. Lancet HIV. 2020;7(10):677–87. https://doi.org/10.1016/S2352-3018(20)30238-1 .
Van Wyk BE, Kriel E, Mukumbang F. Two-year viral load suppression among adolescents receiving antiretroviral therapy in the Cape Metropole, South Africa, 2013–2015: a retrospective cohort analysis. South Afr Med J. 2020;110(12):1213.
Umar E, Levy JA, Bailey RC, Donenberg G, Hershow RC, Mackesy-Amiti ME. Virological non-suppression and its correlates among adolescents and young people living with HIV in Southern Malawi. AIDS Behav. 2019;23(2):513–22. https://doi.org/10.1007/s10461-018-2255-6 .
Colvin CJ. Strategies for engaging men in HIV services. Lancet HIV. 2019;6(3):e191–200. https://doi.org/10.1016/S2352-3018(19)30032-3 .
Somi G, Matee M, Makene J, Van den Hombergh BK, Yahya-Malima KI, Masako P, et al. Three years of HIV/AIDS care and treatment services in Tanzania: achievements and challenges. Tanzania J Health Res. 2009. https://doi.org/10.4314/thrb.v1i3.47700 .
Mukumbang F. Leaving no man behind: how differentiated service delivery models increase men’s engagement in HIV care. Int J Health Policy Manage. 2021;10(3):129–40. https://doi.org/10.34172/ijhpm.2020.32 .
Schneider H, Govender V, Harris B, Cleary S, Moshabela M, Birch S. Gender differences in experiences of ART services in South Africa: a mixed methods study. Trop Med Int Health. 2012;17(7):820–6. https://doi.org/10.1111/j.1365-3156.2012.03009.x .
Download references
Acknowledgements
Not applicable
This work was supported by Amref Health Africa Tanzania, headquarters office in Dar es Salaam.
Author information
Authors and affiliations.
Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
Stella Emmanuel Mushy & Khadija I. Yahya-Malima
Dar Es Salaam Institute of Technology, Dar es Salaam, Tanzania
Expeditho Mtisi
Amref Health Africa, Ali Hassan Mwinyi Road, Dar es Salaam, Tanzania
Simon Mkawe, Eric Mboggo, John Ndega, Edwin Samuel Kilimba, Aisa Muya & Frida Ngalesoni
Center of Disease Control and Prevention, Dar es Salaam, Tanzania
Denice Kamugunya
National Aids, Sexually Transmitted Diseases and Hepatitis Control Progralmme, Ministry of Health, Dodoma, Tanzania
Boniface S. Mlay
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualisation: SEM, FN, AM, EM, EM. Formal analysis: EM, SEM, JN, ESK, DK. Funding acquisition: AM, FN. Investigation: FN, AM, EM, SM. Methodology: EM, FN, AM, EM, EM, SM, KIYM, ESK, DK. Project administration, Validation, Supervision, and Resources: FN, AM. Visualisation: FN, AM, EM. Writing—original draft: SEM. Writing—review and editing: SEM, EM, FN, AM, EM, SM, KIYM, ESK, DK. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Stella Emmanuel Mushy .
Ethics declarations
Ethics approval and consent to participate.
The Muhimbili University of Health and Allied Sciences Institutional Review Board granted ethical clearance for this study. Data access permits from the database were obtained from the Ministry of Health through the National Aids Control Program department. Study data with patients’ identities were first de-identified before analysis, and data was stored in a password-protected computer. The Muhimbili University of Health and Allied Sciences Institutional Review Board waived the need for informed consent with clearance number MUHAS-REC-12-2022-1467. In addition, all experiments were performed per relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mushy, S.E., Mtisi, E., Mkawe, S. et al. Barriers to viral load suppression among adolescents living with HIV on anti-retroviral therapy: a retrospective study in Tanga, Tanzania. AIDS Res Ther 21 , 35 (2024). https://doi.org/10.1186/s12981-024-00622-7
Download citation
Received : 18 November 2023
Accepted : 17 May 2024
Published : 22 May 2024
DOI : https://doi.org/10.1186/s12981-024-00622-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adolescents
- Determinants
- Viral load suppression
AIDS Research and Therapy
ISSN: 1742-6405
- Submission enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Hiv and aids.
Helena M. Swinkels ; Angel A. Justiz Vaillant ; Andrew D. Nguyen ; Peter G. Gulick .
Affiliations
Last Update: May 6, 2024 .
- Continuing Education Activity
Clinical prevention of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) is the cornerstone of controlling the global HIV pandemic that has now killed more than 40.4 million people globally, including 1.5 million children. While a cure remains out of reach, HIV infection is now seen as a chronic illness due to the effectiveness of antiretroviral therapy. Combined with significant advancements in prevention, the goal of halting the global HIV pandemic is now feasible.
This activity comprehensively reviews HIV transmission, pathophysiology, clinical presentations, evaluation, up-to-date treatment, reporting, and program implementation considerations for specific population groups. Clinicians can expect to increase their knowledge, skills, and competence in HIV care, improving patient outcomes, reducing transmissions, and contributing to global efforts against the disease. The role of the interprofessional team is highlighted throughout the course, emphasizing collaboration among clinical, public health, and other interdisciplinary team members. This approach acknowledges the complexity of clinical HIV management, considering socio-economic factors, the need for patient-centered care and continuous quality improvement, and the importance of social and regulatory environments to achieve optimal patient and population outcomes. By providing up-to-date information on HIV management and fostering collaboration among healthcare professionals, this activity's goal is to empower clinicians to navigate the evolving challenges posed by HIV, ultimately contributing to better patient care.
- Determine the stage of HIV infection and related comorbidities to ensure accurate and timely diagnosis and appropriate treatment planning for individual patients.
- Implement current guidelines and evidence-based practices in the treatment of HIV to optimize patient outcomes, including the initiation and management of antiretroviral therapy, prophylaxis for opportunistic infections, and prevention and management of adverse effects.
- Interpret HIV-related information effectively to patients, ensuring a clear understanding of the diagnosis, treatment options, and the importance of adherence while demonstrating cultural sensitivity and maintaining confidentiality.
- Collaborate with interprofessional team members, including nurses, pharmacists, social workers, nutritionists, public health professionals, community health workers, peer support workers, researchers, and others, to ensure continuity of care and improve individual and population outcomes for human immunodeficiency virus prevention and treatment.
- Introduction
The human immunodeficiency virus (HIV) was first identified in 1983 and has since claimed approximately 40.4 million lives worldwide (as of 2022). This number is staggering, and if left unchecked, HIV could become a global health crisis. However, the research, development, and widespread availability of highly active antiretroviral therapies (ARTs) have helped contain the HIV pandemic. Likewise, advances in the treatment of HIV and opportunistic infections have rendered the disease a manageable chronic illness. People living with HIV can live long and healthy lives, with chronic disease prevention being a health priority in this population.
Along with adequate resources and will, advances in prevention, treatment, and implementation science make the United Nations General Assembly's 95-95-95 goals attainable. By 2025, these aim for 95% of people living with HIV to have a diagnosis, 95% with a diagnosis to be taking ART, and 95% of those taking ART to have a suppressed viral load.[WHO. Global Health Sector HIV Strategies. 2022] Globally, HIV infection and mortality rates show a steady decrease. However, some countries report an uptrend in the rate of infections, mostly where political or other turmoil is occurring or where HIV is highly stigmatized.[UNAIDS. Global Report. 2023] With improvements in treatment, the number of people living with HIV is also increasing, with approximately 37.7 million people living with HIV in 2020 and 39 million people in 2022—two-thirds of whom live in Africa. [1]
HIV still comes with high costs to the patient and healthcare system. Infection with HIV increases the risk of chronic disease, particularly cardiac and neurological. Despite the effectiveness of ART in delaying disease progress, treatment does not cure HIV, comes with adverse effects, and requires consistent, prolonged connection to the healthcare system. Barriers to universal treatment exist, including public- and self-stigma, lack of adequate access to care, inappropriate care, and costs.
Utilizing current local clinical guidelines for managing HIV improves patient outcomes and prevents HIV transmission. Implementation recommendations within clinical guidelines promote quality programming for prompt diagnosis, treatment, and connection to care for everyone living with or at risk of acquiring HIV. Increasing the involvement of community-led organizations in HIV testing, treatment, and integrating HIV services with those for other related health issues extends the reach of HIV services, improves linkage to care, and contributes to improved overall health. Supportive social and policy environments regarding access to services, screening, reporting test results, and discrimination can safeguard patients and the community.
Focusing primarily on HIV-1, this clinical reference is designed to review the pathophysiology, clinical manifestations, and recommended treatment options for patients with HIV to help clinicians obtain concise and up-to-date guidance for managing HIV. The optimal social and policy environments to support the HIV response, as recommended by the World Health Organization (WHO), the Joint UN Programme for HIV/AIDS (UNAIDS), the United States Centers for Disease Control (CDC), and state legislatures, with Florida legislation provided as an example, are discussed.
See StatPearls' companion topic, " HIV-2 Infection ," for further information on HIV-2 infection.
HIV belongs to the Retroviridae family in the Lentivirus genus. The virus mainly targets CD4+ T-lymphocyte helper cells, leading to an extreme form of immune subversion with a continuous loss of these cells. This weakens the immune system and causes many clinical manifestations of this disease. Untreated HIV infection eventually progresses to acquired immunodeficiency syndrome (AIDS). At this stage, the immune system cannot prevent infections, resulting in the individual's death due to opportunistic infections. [1]
Two main types of HIV exist: HIV type 1 (HIV-1) and HIV type 2 (HIV-2). Although HIV-1 and HIV-2 genomes are structurally similar, they have significant divergence at the amino acid level. The 2 viruses result from 2 different zoonotic transmissions of simian immunodeficiency viruses and, as a result, have substantial differences in their severity, transmissibility, and prognosis. Note that HIV-1 and HIV-2 are only 60% identical at the amino acid level and have a mere 48% identity at the nucleotide level. [2]
HIV-1 and HIV-2 particles comprise a lipid membrane surrounding a protein capsid. The capsid, in turn, holds a nucleoprotein complex or core consisting of 2 identical copies of viral genomic ribonucleic acid (RNA) along with nucleocapsid, integrase, and reverse transcriptase proteins. The capsid protein organizes into a lattice structure, giving the capsid a characteristic conical shape. [3]
HIV is transmitted via various body fluids (ie, blood, amniotic fluid, breast milk, semen, pre-ejaculate, rectal fluids, and vaginal fluids). HIV can be transmitted via sexual contact, during pregnancy and delivery, and via fomites, such as reusable medical equipment or injection drug equipment.[WHO. Consolidated HIV Guidelines. 2021]
See StatPearls' companion topic, " HIV Prevention ," for further information on HIV transmission.
- Epidemiology
HIV remains a significant public health issue worldwide. The HIV-1 virus causes most HIV infections, with HIV-2 infections accounting for only 1 to 2 million HIV infections. The prevalence of HIV-2 exceeds 1% of infections only in West Africa, although infections occur less commonly on all continents, particularly those with colonial or other ties to the area. According to the WHO HIV factsheet, 39 million people were living with HIV at the end of 2022, with the majority (25.6 million) living in Sub-Saharan Africa.[WHO. HIV Data and Statistics. 2023] In 2022, 1.3 million new cases of HIV were reported worldwide, with 630,000 deaths related to HIV in the same year.
While some countries report an increase in new infections, the overall global trend in HIV incidence is down. In particular, significant gains have been made in preventing and treating HIV infection in eastern and southern Africa, where the virus is most prevalent. From 2010 to 2022, there were 57% fewer new infections and 58% fewer AIDS-related deaths in the region.[UNAIDS. Global Report. 2023]
Progress is slower in other areas, mainly where marked inequities and low prioritization of the HIV response exist. A study of global trends in HIV among adolescents and young adults showed a decrease in HIV incidence from 34.5 per 100,000 population in 1990 to 22.7 per 100,000 population in 2019. [4] However, between 2010 and 2022 in Asia and the Pacific, a quarter of new infections affected people (in those between 15 and 24) and their partners, with some countries reporting nearly half of all new infections in this population. In total, new HIV infections have decreased by only 14% in the region.[UNAIDS. Global Report. 2023]
Most concerningly, HIV incidence is increasing in some regions. The WHO Middle East and North African regions have seen a 61% increase in the incidence of HIV between 2010 and 2022, the largest in the world.[UNAIDS. Global Report. 2023] Due to the low prevalence of HIV in this region, the number of people infected (about 16,000 people in 2022) is small relative to the 160,000 people estimated to be infected in Eastern Europe and Central Asia in 2022. New HIV infections in this region increased by 49% between 2010 and 2022. Challenging legal environments, human rights violations, and military conflicts have hindered the HIV response.
HIV infection, diagnosis, treatment, and viral suppression rates vary across and within countries and regions. Globally, women accounted for 65.8% of new HIV cases in 2019. [4] However, diagnosis, treatment, and viral suppression rates are lower among adolescent and adult men. For example, a phylogenetic study in Uganda estimated that men are 1.5 to 1 times less likely to be virally suppressed than women. Interventions that increase viral suppression rates among men to the same level as those in women would close the gender disparity in incident HIV infections.
UNAIDS and WHO identify 5 key populations who are disproportionately affected by HIV and warrant specific care and support to reduce global transmissions: men who have sex with men (MSM), sex workers, people in prisons and other closed settings, people who inject drugs (PWID), and transgender and gender-diverse people. [5] [UNAIDS. Global Report. 2023]
The vast majority of new HIV infections worldwide occur from sexual contact. Most of these infections are transmitted via heterosexual contact due to the high number of infections in Africa, where this mode of transmission is dominant. [6] Most new HIV diagnoses in most other regions of the world occur in MSM.[WHO. HIV Data and Statistics. 2023] In the United States (US), in 2021, 70% of all new infections were in MSM, while only 22% occurred via heterosexual contact.[CDC. Basic Statistics. 2023]
Injection drug use is another significant risk factor for HIV infection. According to a meta-analysis from 2008, approximately 3.0 million PWID are living with HIV worldwide. [7] According to the CDC HIV Factsheet, 1 in 10 new HIV infections in the US can be attributed to injection drug use either alone or in MSM who report injection drug use. A systematic review evaluating the effectiveness of needle and syringe exchange programs reported that adequate needle and syringe exchange programs were effective in reducing the sharing and reuse of needles and syringes and HIV transmission among PWID. [8]
See StatPearls' companion topic, " HIV Prevention ," for more details on the epidemiology of HIV, including the populations most impacted by HIV.
- Pathophysiology
The Retroviridae family is unique among viruses, with the RNA viral genome reverse-transcribed into deoxyribonucleic acid (DNA) before being integrated into the host DNA, resulting in lifelong infection. The pathophysiologic process is best known for HIV-1, with more recent research contributing to the understanding of differences in HIV-2 and HTLV viral replication and biology.
As the primary host cellular receptor for HIV-1 and HIV-2 is the CD4 molecule, T cells and macrophages that express the CD4 molecule are the primary viral targets. [2] Envelope glycoprotein on the surface of the viral particle helps facilitate viral entry into the host cell. Chemokine coreceptors 5 (CCR5) and 4 (CXCR4) on the host cell surface trigger a conformational change in the envelop protein, which results in the fusion of the viral and host cellular membranes. Once membrane fusion occurs, the viral capsid is released into the host cell. [2]
The HIV-1 viral capsid remains intact or nearly intact until reaching the nuclear pore complexes on the nuclear envelope of the host cell. Previously thought to occur in the cytoplasm, reverse transcription is now believed to occur during or shortly after the capsid is imported into the nucleus; the nuclear capsid is essential in the efficiency of reverse transcription. The precise location and mechanism of reverse transcription and the role of the capsid in this have not been fully elucidated. [2] Some molecular studies suggest uncoating and reverse transcription begins in the nucleus. In contrast, others suggest a partial uncoating of the viral capsid at the nuclear pore complexes, with early stages of viral DNA production occurring near the nuclear envelope of the host cell. [2]
The viral reverse transcriptase enzyme initiates reverse transcription using host transfer RNA as primers, which bind at the 5' ends of the 2 identical RNA strands and progress in a 5' to 3' direction. Negative-sense single-stranded DNA is initially produced, with doubling of the strand beginning part way along the viral genome. Elongation continues to the end of the genome, after which each strand's synthesis is completed using the other as a template. [2] The viral integrase protein then somewhat randomly integrates the dsDNA into the host DNA.
The process of viral formation is beyond the scope of this review. Once viral particles are formed, they can infect other host cells to propagate the infection. Within 2 days of the initial mucosal exposure, HIV can be detected in the regional lymph node tissue. From here, the virus only requires an additional 3 days to be detectable in the plasma. [4]
Genomic diversification is an important aspect of the pathogenesis of HIV infection, leading to changes in the severity of the disease and its response to ART. One of the primary driving factors for HIV-1 mutagenesis is the error rate of the reverse transcriptase encoded by the virus. According to results from several studies, the error rate of HIV-1 group M reverse transcriptase (subtype B) is 100 to 1000 times higher than that of the cellular DNA polymerases. These errors are incorporated into the viral genome and contribute to viral diversification. [2] Other intrinsic and extrinsic factors, such as recombination errors, host restriction factors, and depletion of host deoxynucleoside triphosphates, can lead to viral mutagenesis and diversification, leading to ART failure. [2]
In the initial phase of the infection, viral replication is rampant, with an exponential increase in the plasma HIV RNA level due to the large population of susceptible CD4+ T cells without any host immune response. Subsequently, a significant decline from the peak viremia level occurs due to the HIV-specific immune response from the cytotoxic CD8+ T cells. [4] After this decline, the HIV replication settles at a point where ongoing replication and infection continue, but the initial intense immune response with its associated symptoms resolves.
The exact mechanisms involving the failure of humoral immunity are not fully understood. T cells within B cell follicles, particularly the follicular T helper cells and follicular T regulatory cells, are thought to be involved in poor humoral immunity and HIV persistence in patients treated with ART. In addition, follicular CD8+ cytotoxic T cells are relatively less abundant than their extrafollicular counterparts in people with HIV, likewise thought to contribute to disrupted immunogenesis. [5]
- Histopathology
Lymphadenopathy exhibiting distinctive morphological changes is evident in HIV patients. In untreated cases, pronounced follicular hyperplasia emerges initially, marked by enlarged, irregularly shaped follicles that occupy a substantial portion of the lymph node's cross-sectional area. The mantle cell zones are absent or dramatically decreased. Centroblasts are the dominant cell types; the germinal centers have a "starry-sky" appearance. Follicle lysis or fragmentation is present when small lymphocytes infiltrate the follicle. Sinusoidal monocytoid B-cell hyperplasia is also present. [6] Mixed follicular hyperplasia is the intermediate stage between florid and follicular involution. Here, the interfollicular area is relatively large compared to florid follicular hyperplasia, and the follicles and interfollicular area are more cellular than those in follicular involution. [6]
In follicular involution, small, atrophic, and hypocellular follicles are seen. The germinal centers contain hyalinized follicular dendritic cell meshworks with few germinal center B-cells. Hyalinized blood vessels are seen penetrating the follicles. Due to the scarcity of lymphocytes, the interfollicular area is expanded with a washed-out appearance. Histiocytes and polytypic plasma cells are abundantly present. [6] Eventually, the lymph nodes enter the lymphocyte depletion stage, characterized by the loss of germinal centers and the near absence of lymphocytes. These lymph nodes contain medullary cords and sinusoids. The interfollicular area primarily consists of histiocytes, plasma cells, and a few immunoblasts. Focal hyaline deposits may be present with subcapsular and sinusoidal fibrosis. [6]
- History and Physical
A thorough medical history and physical exam, including a review of systems, are indicated for patients with confirmed or suspected HIV infections. Attention should be paid to signs and symptoms that help rule out other conditions in the differential diagnosis or that signal opportunistic infections or other HIV-associated sequelae. These aid in the staging of HIV infection and ensure any concurrent conditions are addressed. [7]
In addition to characterizing symptoms, the history should seek risk factors for HIV transmission in a nonjudgmental manner; this includes sexual contact and behavior, drug use, and receipt of blood transfusions. The sexual history should elucidate information regarding the number of partners, sexual practices, frequency and type of barrier protection used, and previous history of sexually transmitted infections (STIs). The HIV status of current and past partners should additionally be obtained. [7] Drug use history should include the type and frequency of substances used, means of administration, and source and sharing of equipment. A mental health history identifies depression, other mental illnesses, or substance use that may result in barriers to care or contribute to the development of chronic diseases. Gatheingr an immunization history to determine which vaccines could offer future protection against vaccine-preventable illnesses is crucial. For instance, Streptococcus pneumoniae, associated with adverse outcomes in HIV-positive individuals, and hepatitis B, linked to a heightened risk of hepatocellular carcinoma progression in those with HIV, underscore the importance of such proactive measures. [8] [9]
Social history is an integral part of any medical evaluation. In the context of HIV and AIDS, this history provides insights into the patient’s perceptions of and ability to adhere to treatment, potential barriers to ongoing connection to healthcare services, and identifying needed supports, and should include, among others, the individual’s living situation, income, insurance, family or other support, experiences of stigma, coping strategies, and exposure to sexual or other violence.
The US National Institutes of Health (NIH) describes 3 broad stages of HIV infection that develop over time: acute HIV infection, chronic or asymptomatic HIV infection, and AIDS.[NIH. Stages of HIV Fact Sheet. 2021] The CDC maintains a staging system based on CD4 and AIDS-defining illnesses intended for surveillance purposes [10] . The WHO has a staging system based on clinical presentation that can be used for clinical or surveillance purposes in areas with limited or no availability of CD4 testing.[WHO. HIV Case Definitions. 2007]
Acute HIV Infection
While 90% of persons with acute HIV infection have at least 1 symptom in the first 4 weeks after primary HIV infection, these are usually mild, nonspecific, self-limited, and never brought to clinical attention. [11] Some patients present to care with more severe symptoms, known as acute retroviral syndrome or seroconversion illness . These symptoms are listed below in order of decreasing frequency. [11]
- Muscle pain
- Sore throat
- Swollen lymph nodes
- Night sweats
Symptom onset occurs acutely around 2 to 4 weeks (range 4 days to 8 weeks) after viral infection, just before peak viremia. [11] Lasting an average 18 days, the resolution of symptoms coincides with the setting of a viral replication set-point around 30 days after the initial viremia. [12] Without treatment, higher viral load and increased severity and duration of conversion illness are early predictors of a poorer prognosis. [13] Mucocutaneous ulceration is a characteristic feature of acute HIV characterized by shallow, sharply demarcated ulcers that have a white base surrounded by a thin area of erythema. Depending on the mode of transmission, ulcers may be located on the oral, anal, penile, or esophageal mucosa. [14] Acute aseptic meningoencephalitis has also been reported as a clinical presentation of acute HIV-1 infection. [4]
Chronic HIV Infection
After HIV acquisition and subsequent setting of the viral set point, patients enter the chronic phase of the infection. Most patients with chronic HIV infection remain asymptomatic before developing AIDS. However, nonspecific fatigue may be present, and persistent generalized lymphadenopathy is usual. Generalized lymphadenopathy is characterized by at least 2 noncontiguous sites other than inguinal nodes exhibiting enlarged lymph nodes for more than 3 to 6 months, not explained by other lymphoproliferative or infectious causes. [6] [15] Patients with chronic HIV infection without AIDS can develop oropharyngeal candidiasis, [16] recurrent vulvovaginal candidiasis, [17] oral hairy leukoplakia, [18] disseminated cutaneous herpes simplex virus, [19] and cervical dysplasia or cervical carcinoma in situ. [20] Cutaneous manifestations such as seborrheic dermatitis, bacillary angiomatosis, varicella-zoster virus reactivations, and molluscum contagiosum infections are common and tend to be severe in patients with HIV. [21]
Eventually, HIV progresses to advanced disease, particularly if un- or inadequately treated. AIDS can be diagnosed when specific AIDS-defining conditions are noted, regardless of the CD4 count. These AIDS-defining conditions, as outlined by the CDC, include:
- Candidiasis of the digestive tract (other than thrush)
- Candidiasis of the pulmonary tract
- Invasive cervical cancer
- Extrapulmonary or disseminated coccidioidomycosis, histoplasmosis, or cryptococcosis, including cryptococcal meningitis
- Chronic intestinal cryptosporidiosis or isosporiasis
- Cytomegalovirus retinitis
- Kaposi sarcoma
- HIV encephalitis and HIV-associated neurocognitive disorder
- Tuberculosis
- Primary lymphoma of the brain
- Non-Hogdkin lymphoma
- Burkitt lymphoma
- Mycobacterial infections
- Pneumocystis jirovecii pneumonia
- Progressive multifocal leukoencephalopathy
- Salmonella septicemia
- HIV-associated wasting syndrome [10]
These AIDS-defining illnesses tend to occur most frequently with low CD4 counts <200 cells/mm 3 , which correlates with untreated advanced HIV disease as the total lymphocyte count depletes over time. [22] [23]
(Refer to the Staging section below for further information on WHO and CDC staging systems).
Testing is required to confirm a diagnosis of HIV. Antibody tests, antigen-antibody tests, and nucleic acid amplification tests (NAATs) are available for screening or confirmation of HIV infection in symptomatic illness. No currently available testing technology can detect HIV infection during the initial aviremic phase of the infection, known as the window or eclipse period . The following tests are used to detect viral proteins:
- NAAT: detects HIV RNA in the blood around 6 to 8 days after infection
- Antigen tests: detect viral proteins such as p24 antigen as early as days 13 to 20 after infection
- Antigen-antibody tests: detect viral proteins as with other antigen tests, plus anti-HIV immunoglobulin (Ig) IgM and IgG antibodies around days 20 and 30, respectively, after infection [10]
In clinical settings, combination antigen/antibody (Ag/Ab) tests are recommended to identify patients with HIV. [11] . These tests are available in most commercial laboratories and hospitals in developed nations and are increasingly available worldwide. Ideally, the Ag/Ab test should be fourth-generation, which can detect HIV-1 and HIV-2 antibodies and the HIV-1 p24 antigen. A supplemental antibody immunoassay that differentiates between HIV-1 and -2 is required if the initial positive test cannot make this distinction. All initial positive test results are followed by a second, different HIV test, preferably one that is laboratory-based, to confirm a diagnosis of HIV. The false positive rate of third- and fourth-generation tests is very low.
If the combination assay result is negative, no further testing is indicated unless HIV exposure was too recent for detectable p24 antigen levels to have developed. If the initial Ag/Ab test result is negative and early HIV infection is suspected, an HIV-1 NAAT to detect HIV RNA should be performed. [10] Specimens indeterminate on the initial Ag/Ab test or nonreactive or indeterminate with the HIV-1–specific and HIV-2–specific antibody assay are also followed up with an HIV-1 NAAT.
An acute HIV infection is diagnosed in the context of a positive NAAT test result in the setting of:
- A recent negative screening immunoassay result
- A positive antigen-antibody immunoassay result, with a negative antibody-only immunoassay result
- A nonreactive or indeterminate HIV-1–specific and HIV-2–specific antibody assay result following a positive screening assay result
A negative HIV-1 NAAT result in the last settings indicates a false-positive HIV-1 test result. [10] [12] When clinical suspicion for HIV is high and initial test results are negative, testing should be repeated in 1 to 3 weeks. Home-based, point-of-care, or rapid tests are essential to increase testing frequency or extend testing into populations that may otherwise not get tested. As with other HIV tests, positive results from point-of-care testing should be followed with standard laboratory, instrument-based immunoassays to confirm the diagnosis. [11]
Once the diagnosis of HIV is established, a baseline laboratory evaluation facilitates the staging of HIV progression, selection of ART, and identification of comorbidities. [11] [NIH. Guidelines for HIV. 2023][WHO. Consolidated HIV Guidelines. 2021]
- Quantitative CD4+ T-lymphocyte cell count
- Quantitative plasma HIV-1 RNA (viral load)
- Complete blood count, glucose, blood urea nitrogen, creatinine, liver enzymes, bilirubin, urinalysis, serum lipids, and serology for Hepatitis A, B, and C
- HLAb*5701 test (if abacavir is being considered)
- Genotypic drug-resistance assessment focusing on genes for reverse transcriptase and protease in treatment-naive people, with the addition of integrase strand transfer inhibitor (INSTI) for those who have previously taken ARTs
- Other tests, as indicated from the history and physical exam, such as testing for STIs, opportunistic infections, or cancer
Viral load is the most important indicator of initial and sustained response to ART. As some treatment regimes are ineffective in patients with high baseline viral loads, viral load also aids in ART selection. Viral load should be monitored at entry into care, initiation of therapy, and periodically afterward. CD4 is the best indicator of immune function, disease progression, and survival and determines the need to start prophylaxis for opportunistic infections. Blood must be drawn for CD4 testing before ART starts, but ART should not be delayed pending results. If CD4 is unavailable, the WHO staging system should be used. Monitoring of other CD subsets is not recommended due to costs and lack of clinical utility. (Refer to the Staging section below for more information on the WHO staging system). Routine testing for herpes simplex IgG, cytomegalovirus IgG, and toxoplasma IgG is not recommended, given that these do not delineate active disease versus previous exposure. Testing for serum cryptococcal antigen should be considered in patients with a CD4 count of 100 cells/mm 3 or less.
See StatPearls' companion topic, " HIV Testing ," for further information on HIV testing.
- Treatment / Management
The goal of HIV-1 therapy with antiretroviral medications is to achieve sustained virologic suppression. According to current WHO and CDC guidelines, ART should begin after the diagnosis is confirmed and an initial assessment is completed for all persons with HIV, regardless of their immune status (CD4 count) or clinical stage, unless a severe opportunistic infection is present. [11] [NIH. HIV Guidelines. 2023][WHO. Global HIV Strategies. 2022] Management of HIV must include identifying specific support required by the patient to maintain medication adherence, particularly for those who are marginalized by their socioeconomic circumstances. The choice of treatment must consider client preferences.
Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), integrase strand transfer inhibitors (INSTIs), and protease inhibitors (PIs) are used in treatment-naive patients. NRTIs inhibit viral replication by binding the viral reverse transcriptase, effectively terminating DNA prolongation in HIV-1 and HIV-2 infections. NNRTIs likewise block DNA prolongation by binding the viral reverse transcriptase at a separate site but are only active against HIV-1 infections. INSTIs inhibit the transcribed viral DNA from integrating into the host genome. PIs block the last step in the viral maturation process, rendering assembled viral particles immature and noninfectious. The most common drugs within each class used in the initial treatment of HIV infection are listed below.[NIH. HIV Guidelines. 2023]
Nucleoside/nucleotide reverse transcriptase inhibitors
- Abacavir (contraindicated for patients who are HLA-B*5701 positive)
- Emtricitabine
- Tenofovir (tenofovir alafenamide or tenofovir disoproxil fumarate)
Non-nucleoside reverse transcriptase inhibitors
- Rilpivirine
Integrase strand transfer inhibitors
- Raltegravir
- Elvitegravir with pharmacologic boosting agent cobicistat
- Dolutegravir
- Bictegravir
- Cabotegravir
Protease inhibitors
- Atazanavir
- Fosamprenavir
- Ritonavir boosted lopinavir
Other drug classes, such as CCR5 antagonists, fusion inhibitors, attachment inhibitors, capsid inhibitors, and post-attachment inhibitors, are reserved for patients with multidrug-resistant HIV infections.
Recommended Therapy for Treatment-Naive Patients
Current guidelines recommend that INSTI-based therapy with dual NRTI-backbone be started before receipt of further laboratory testing results for most persons with HIV.[NIH. HIV Guidelines. 2023] Tenofovir plus lamivudine or emtricitabine are the preferred NRTIs, and bictegravir and dolutegravir are the preferred INSTIs due to their effectiveness, side-effect profiles and lower propensity to develop resistance. [10] INSTI-based regimens achieve faster viral suppression than PI- or NNRTI-containing regimens. [13] The addition of other agents, even in the setting of advanced HIV on presentation, does not improve clinical outcomes at the onset of treatment and is therefore not recommended. [14] Abacavir is no longer recommended as initial therapy due to its association with cardiovascular disease, the risk of hypersensitivity, and the need for HLA B*5701 testing. [13]
Comorbid conditions affect HIV ART selection due to contraindications or co-benefits of treatment, including the following:
- Hepatitis B virus (HBV) coinfection: Tenofovir-containing ART regimens are preferred as tenofovir suppresses HBV replication. ART regimens that use lamivudine or emtricitabine without tenofovir should be avoided as they can result in the rapid emergence of resistant HBV.
- Renal dysfunction: Tenofovir disoproxil fumarate (TDF) is associated with proximal tubular dysfunction and should be avoided in patients with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m 2 . Tenofovir alafenamide (TAF) can be used unless the patient has severely reduced renal function (eGFR <30 mL/min/1.73 m 2 ) and is not on dialysis, in which case no tenofovir formulation should be given. The preferred regimen is dolutegravir plus lamivudine, adjusted for renal function. Atazanavir should also be avoided in patients with reduced kidney function.
- Osteoporosis: TAF is associated with less bone loss and is preferred over TDF-containing regimens.
- Pregnancy: Bictegravir, doravirine, cabotegravir, dolutegravir/lamivudine combination, and dolutegravir/rilpivirine combination should not be initiated during pregnancy as there is limited data to support their safety. Cobicistat-containing regimens should not be used during pregnancy due to inadequate drug levels. [13] [NIH. HIV Guidelines. 2023]
Once laboratory test results are available, the initial regime may be adjusted. If the patient’s HIV RNA level is less than 500,000 copies/mL, and no evidence of HBV coinfection or lamivudine resistance exists, switching to a 2-drug regimen using dolutegravir and lamivudine combination can be considered. [13] A dual NRTI coformulation plus an antiretroviral from another class is used for those unable to take an INSTI-based regimen. For example, the PI darunavir is boosted with cobicistat, ritonavir, or an NNRTI, either efavirenz or rilpivirine. [15]
Recommended Therapy for Patients on Preexposure Prophylaxis
Patients who are on preexposure prophylaxis (PrEP) with TAF or TDF with emtricitabine who subsequently acquire HIV require resistance testing before initiating therapy. They can be initiated on INSTI-containing regimens outlined above while genotype/resistance testing is pending. [13] Patients who acquire HIV after receiving cabotegravir for PrEP require INSTI genotyping before beginning therapy with an INSTI-based regimen. While the results are pending, a boosted PI regimen containing darunavir and a tenofovir-based dual NRTI coformulation should be used. [13]
Therapy Considerations for Patients With HIV-2 Infections
Current recommendations for treating HIV-2 are primarily based on single-arm or observational studies, as most research and treatment efforts have focused on HIV-1. The initial regimen for managing HIV-2 should include 2 NRTIs plus a second-generation INSTI or a ritonavir-boosted PI. [16] [NIH. HIV Guidelines. 2023] TDF and emtricitabine are the preferred NRTIs. Dolutegravir is recommended as the preferred INSTI. Darunavir and lopinavir are more active against HIV-2 than other PIs and are the preferred agents. Two-drug regimens that are used to treat HIV-1 and any regimen containing NNRTIs are ineffective against HIV-2 and should not be used in these infections.
Drug resistance assays for HIV-2 are limited to research laboratories, complicating the management of these infections. Current data suggests that HIV-2 is susceptible to NRTIs; however, HIV-2 is more likely to develop resistance than HIV-1. Patients with HIV-2 mutations may exhibit complex patterns of PI cross-resistance, making sequential PI regimens ineffective for patients with this infection. [17] In vitro studies report strong efficacy for INSTIs against HIV-2. Clinical data regarding mutations and efficacy of the most commonly used INSTI (eg, dolutegravir) is limited, especially in those previously exposed to other INSTIs. [18] Further research is needed to understand resistance patterns for antiretrovirals in patients with HIV-2 infections.
Opportunistic Infections
In addition to rapid ART initiation, prophylaxis for opportunistic infections should be started based on the level of immunosuppression.
- Pneumocystis jirovecii (previous P carinii ): Prophylaxis is indicated for patients with thrush on presentation or a CD4 count of less than 200 cells/mm 3 .
- Cryptococcus neoformans and Cryptococcus gatti: Patients with a CD4 count of less than 100 cells/mm3 and a positive serum cryptococcal antigen result require prophylaxis.
- Histoplasma capsulatum: Prophylaxis is recommended in areas where histoplasmosis is endemic, and the patient's CD4 count is less than 150 cells/mm 3 .
- Mycobacterium avium complex (MAC) infection: If patients with HIV are rapidly initiated on ART, prophylaxis for MAC is not required. [10] Patients with a CD4 count of less than 50 cells/mm 3 without ART should receive prophylaxis.
- Toxoplasma gondii: Patients with CD4 counts less than 100 cells/mm 3 who have positive test results for toxoplasma antibodies require chemoprophylaxis . [11]
In patients with a severe opportunistic infection, rapid initiation of ART can result in immune reconstitution inflammatory syndrome. [11] To decrease the risk, the opportunistic infection should be treated before initiating ART. Initiate ART within 2 weeks of initiating treatment for most acute opportunistic infections, except acute cryptococcal meningitis. [15] Patients with cryptococcal meningitis may be initiated on HIV therapy within 2 to 4 weeks of antifungal therapy. HIV treatment should be initiated within 2 weeks of tuberculosis treatment in patients who have active tuberculosis, especially if they have severe immunosuppression (CD4 count <50 cells/mm 3 ); however, if there is evidence of tuberculous meningitis, ART should be given with high-dose corticosteroid treatment. [13]
Monitoring Following Initiation of Treatment
Once treatment is initiated, the patient’s HIV viral load should be evaluated in 2 to 4 weeks and no later than 8 weeks. The viral load can be rechecked every 4 to 8 weeks after that to ensure it continues to decline. Virologic suppression to undetectable levels (defined as an HIV RNA level of <200 copies/mL) may take up to 24 weeks of continuous therapy. If the HIV RNA level has not declined by 2 log10 copies/mL within 12 weeks and adherence is confirmed, evaluation for resistance with genotype testing for the patient’s regimen is recommended. [13] Genotype resistance testing should also be obtained if virologic suppression is not achieved. [13] Once viral suppression is established, the viral load should be monitored every 3 to 4 months. [7] An HIV RNA level that remains persistently below this lower limit of detection demonstrates sustained virologic suppression—the primary goal of HIV therapy.
See StatPearls’ companion topic, “ HIV Antiretroviral Therapy, ” for further information on the adverse effects, contraindications, monitoring, and toxicity of ART. See StatPearls’ companion topic, “HIV-2 Infection,” for further information on the treatment of HIV-2 Infection.
- Differential Diagnosis
HIV infection should be considered in any patient with recurrent serious infections. Other conditions that may have similar effects on the patient's immune system include:
- Severe malnutrition
- Severe combined immune deficiency syndrome
- Chemotherapy-induced immunosuppression
The differential diagnosis in patients who present with acute HIV infection includes:
- Mononucleosis
- Toxoplasmosis
- Viral hepatitis
- Systemic lupus erythematosus
- Toxicity and Adverse Effect Management
When prescribing antiretrovirals, a wide variety of potential drug-drug interactions, toxicities, and other adverse effects must be considered. Consultation with a pharmacologist is recommended.[NIH. HIV Guidelines. 2023] Some common reactions are provided below.
Nucleoside/Nucleotide Reverse Transcriptase Inhibitors
- Abacavir is contraindicated for patients who are positive for the HLA-B*5701 allele due to the risk of hypersensitivity reactions. Pretesting is required before prescribing any regimen containing abacavir. Some studies have also demonstrated increased cardiovascular risk with abacavir.
- TAF is associated with higher lipid levels and weight gain.
- TDF is associated with renal toxicity, proximal tubulopathy, and acute or chronic renal insufficiency, particularly when combined with boosters. Tubulopathy can cause osteomalacia. TDF can also cause decreased bone density.
- Didanosine and stavudine are no longer used due to severe adverse reactions and toxicity.
Non-Nucleoside Reverse Transcriptase Inhibitors
- Doravirine, efavirenz, and rilpivirine have the potential for cytochrome (CYP) P450 enzyme drug interactions.
- Efavirenz can also cause dyslipidemia, rash, and QTc interval prolongations. This therapy has the potential for short- and long-term psychiatric complications, suicidality, catatonia, and late-onset ataxia and encephalopathy.
- Rilpivirine also causes QTc interval prolongation. This medicine is less commonly associated with depression, suicidality, and rash than efavirenz.
Integrase Strand Transfer Inhibitors
- All of these types of inhibitors can cause weight gain.
- Several inhibit creatinine excretion without affecting glomerular filtration, including bictegravir, dolutegravir, and cobicistat.
- Bictegravir has the potential for drug-drug interactions as it is a substrate for the enzymes CYP3A4 and UGT1A1 (uridine diphosphate glucuronosyltransferase 1A1).
- Dolutegravir exposure during conception may be associated with neural tube defects. Clinicians should discuss this possibility when prescribing medications to people of childbearing potential.
- Raltegravir can increase creatinine kinase and cause myopathy and rhabdomyolysis. This medicine has been reported to cause severe hypersensitivity reactions, including Stevens-Johnson syndrome and toxic epidermal necrosis. As a UGT1A1 substrate, it has the potential for drug-drug interactions. Rarely, raltegravir has been associated with depression and suicidal ideation in those with preexisting psychiatric conditions.
- Cobicistat is an INSTI used exclusively as a pharmacokinetic enhancer of certain PIs and INSTIs. This therapy strongly inhibits CYP3A4, resulting in significant potential for drug-drug interactions. Cobicistat must be discontinued in severe hepatic impairment. Like other INSTIs, it can cause increases in creatinine excretion without impacting glomerular filtration.
Protease Inhibitors
- PIs other than atazanavir are associated with an increased risk of cardiovascular events.
- Atazanavir and darunavir are both CYP3A4 inhibitors and substrates with the potential for many drug interactions; both are co-formulated with cobicistat or ritonavir.
- Atazanavir coformulations can cause indirect hyperbilirubinemia, nephrolithiasis, cholelithiasis, nephrotoxicity, and gastrointestinal adverse effects.
- Darunavir coformulations can cause skin rash, gastrointestinal adverse effects, and hepatotoxicity, particularly in those with preexisting liver disease.
Staging systems can be used for clinical or surveillance purposes to assess the rate of progression to more advanced stages, assist in monitoring the HIV burden at a population level, plan for prevention and care, and evaluate interventions. [13] The CDC published the current definitions of HIV surveillance cases in 2014. The definitions of surveillance cases are not intended for clinical decision-making. [13] HIV testing confirms the diagnosis and determines acute infection (stage 0), and CD4 count determines stages 1 to 3. All surveillance cases are assumed to be HIV-1 unless laboratory evidence indicates HIV-2. The criteria for stage 0 supersede and are independent of the criteria used for other stages.
Confirmed HIV Case
For all adults, adolescents, and children 18 months and older:
- A second, different HIV test confirms a positive result from an initial HIV antibody or antibody-antigen test.
- Clinical criteria can be used where HIV test results have not been recorded, but other presumptive evidence exists, such as receipt of therapy for HIV or an AIDS-defining illness.
This stage is defined by a positive test result within 180 days of:
- A negative or indeterminate test result
- A negative initial immunoassay result followed by a positive NAAT test result done to confirm acute infection
- A positive NAAT test result following a positive antigen or antigen-antibody test result but unconfirmed by a second test
Stages 1 to 3
These stages are determined based on the CD4 count for all people 6 and older (separate criteria exist for infants younger than 1 year and children 1 to 5 years):
- Stage 1: 500 or more cells/μL
- Stage 2: 200 to 499 cells/μL
- Stage 3: less than 200 cells/μL
Globally, case definitions for HIV vary by country based on the testing technologies most appropriate for use in the local context. The WHO recommends test-based confirmation in adults and children 18 months or older to require a positive result from a rapid or laboratory-based HIV antibody, antigen, or virological test, confirmed by a second positive test result relying on different antigens or different test operating characteristics.[WHO. HIV Case Definitions. 2007] Advanced HIV is confirmed by the diagnosis of a condition associated with advanced disease or a CD4 count of less than 200 cells/mm 3 in an adult or child living with HIV.[WHO. Consolidated HIV Guidelines. 2021]
The WHO's clinical staging for HIV can be used for clinical and surveillance purposes in resource-limited areas where CD4 testing may not be available.[WHO. HIV Case Definition. 2007] Advanced disease is defined as stage 3 or 4.
Stage 0 or Primary HIV Infection
- Patients have 1 or more symptoms that are associated with an acute HIV infection or a constellation of symptoms consistent with acute retroviral syndrome.
- In stage 1, patients are asymptomatic or have persistent generalized lymphadenopathy, defined as enlarged lymph nodes (>1 cm) in 2 or more non-contiguous sites (excluding inguinal nodes) that are not better explained by any other cause.
- In this stage, patients have unexplained weight loss (moderate degree, <10% of body weight), recurrent respiratory tract infections, herpes zoster exacerbations (mild to moderate severity), angular cheilitis, recurrent oral ulcerations, papular pruritic eruptions, seborrhoeic dermatitis, or fungal fingernail infections.
- In stage 3, patients have severe weight loss (>10% of body weight), unexplained chronic diarrhea, persistent fever, oral candidiasis, oral hairy leukoplakia, pulmonary tuberculosis, severe invasive bacterial infections (such as pneumonia, empyema, osteomyelitis, meningitis, and bacteremia), acute necrotizing ulcerative stomatitis, gingivitis or periodontitis, or unexplained anemia, neutropenia, or thrombocytopenia for more than 1 month.
Stage 4 or AIDS
- In this stage, patients develop HIV wasting syndrome, pneumocystis pneumonia, chronic herpes simplex infection, esophageal candidiasis, extrapulmonary tuberculosis, Kaposi sarcoma, toxoplasmosis, HIV encephalopathy, extrapulmonary cryptococcosis infections, disseminated non-tuberculous mycobacterial infections, progressive multifocal leukoencephalopathy, pulmonary candidiasis, cryptosporidiosis, isosporiasis, cytomegalovirus retinitis (or in an organ other than liver, spleen or lymph nodes), disseminated mycoses (such as histoplasmosis, coccidiomycosis, penicilliosis), recurrent salmonella septicemia, lymphoma (cerebral or B-cell non-Hodgkin), invasive cervical carcinoma, or visceral leishmaniasis.
The WHO defines HIV wasting syndrome as the presence of unexplained weight loss that is greater than 10% of the body weight, with the presence of either unexplained chronic diarrhea or unexplained fever for 1 month or more. WHO clinical stage 4 and the CDC criteria for AIDS are almost identical. [13] [WHO. HIV Case Definition. 2007]
A wide variety of studies in resource-limited settings have evaluated the utility of the WHO staging system for predicting immunological status as defined by CD4 count. These have found highly variable sensitivity and specificity across various CD4 cut-off levels, questioning its utility for clinical decision-making. [14] [15] [35] [43] [44] This underscores the importance of expanding the availability of CD4 and viral load testing and the WHO and CDC recommendations to offer treatment to all those who are HIV positive.[WHO. Global HIV Strategies. 2022][NIH. HIV Guidelines. 2023] [16]
Without therapy, HIV infections are invariably fatal. Effective ART with sustained virologic suppression dramatically improves the clinical outcomes for persons living with HIV. A meta-analysis from 2017 estimates a life expectancy in high-income countries of 43.3 years after starting ART at the age of 20 years and 32.2 years if ART starts at 35 years of age. [14] Life expectancy in low to middle-income countries is 28.3 and 25.6 years if ART starts at ages 20 and 35, respectively. In all income regions, life expectancy after starting ART has improved, likely reflecting improvements in therapy and management such as improved ART regimens, earlier initiation of ART, and better socioeconomic and adherence support. [14]
Viral suppression is the key determinant of prognosis. Patients who achieve virologic suppression for at least 3 years without full immunologic recovery (CD4 count <200 cells/mm 3 ) have 2.6 times greater all-cause mortality than those who achieve immunologic recovery (CD4 >200 cells/mm 3 ). [15] Treatment at diagnosis is associated with improved outcomes and better immune system recovery. Delaying ART until the patient's CD4 count is less than 200 cells/mm 3 decreases the likelihood of the CD4 count normalizing, even after multiple years of otherwise effective antiretroviral therapy. This increases the patient's risk of AIDS and non-AIDS-related morbidity and mortality. [16] Other factors correlating with poor immunologic recovery include older age, lower nadir CD4 count, and extended ART initiation to viral suppression time. [15]
Hepatitis C infection, hepatitis B infection, and active injection drug use are identified as important factors contributing to higher morbidity and mortality among patients with HIV-1 infections. [17] Clients should be offered treatment. For PWID, ongoing socioeconomic and adherence support should be offered, mainly if addiction treatment is not currently indicated or available.
- Complications
Complications Related to HIV Infection
The advent of ART has dramatically decreased the incidence of opportunistic infections and HIV-associated malignancies. However, progression to AIDS remains a significant complication of HIV infection. Screening and monitoring are warranted for AIDS-defining illnesses as appropriate to the patient's clinical status. In addition, screening and monitoring for specific HIV- and ART-related complications is required.
- HIV-associated neurocognitive disorders and psychiatric complications, with the use of efavirenz and, less frequently, rilpivirine and other INSTIs
- HIV-associated distal symmetric polyneuropathy
- HIV-associated lipodystrophy
- Mitochondrial toxicity of HIV nucleoside reverse transcriptase inhibitors
Detailed pathophysiology, evaluation, and treatment of AIDS-defining illnesses and other HIV- and ART-related conditions are beyond the scope of this review. See StatPearls' companion articles on "AIDS," "HIV Neurocognitive Disorders," and "HIV-Associated Lipodystrophy" for further information on these topics.
ART-Related Complications
The advent of ART has also raised the risk of cardiovascular disease morbidity and mortality. Persons living with HIV now experience age-related comorbidities such as cardiovascular disease much more frequently than in the past, and much of the long-term care for patients with HIV focuses on minimizing cardiovascular risks for these patients. Multiple different factors contribute to increased cardiovascular risk for patients with HIV, including:
- The prevalence of dyslipidemia among patients with HIV, with and without ART, is high.
- Glucose intolerance or diabetes frequently occurs in patients receiving ART; this may be due to specific ART agents, such as earlier-generation protease inhibitors.
- Multiple ART regimens are associated with weight gain.
Weight gain is essentially ubiquitous in patients on ART and is one of the most important contributors to cardiovascular risk for patients with HIV. The exact mechanisms involved are unknown. Some ART agents contribute more to weight gain than others. INSTIs are associated with more weight gain compared to PIs or NNRTIs, and TAF is associated with more weight gain than TDF, abacavir, or zidovudine. [18]
Current guidelines recommend the initiation of lifestyle modification counseling from the onset of ART to mitigate weight gain and metabolic complications. Routine screening for glucose intolerance, diabetes, and hyperlipidemia is recommended throughout care. [7]
- Deterrence and Patient Education
Ongoing education, support, and counseling from the interdisciplinary care team are essential for patients living with HIV. Patients can also be referred to organizations such as the CDC or NIH, which have various resources available to support patients, including those for specific populations.[CDC. HIV Basics. 2022][NIH. Fact-sheets. 2023]
HIV Treatment
Patients should be aware of the need for regular labwork and follow-up with a healthcare professional to ensure optimal HIV control and prevent potential complications. They should also be encouraged to keep all medical appointments and speak freely and openly with their clinician to ensure adverse effects, potential barriers to treatment, and other health concerns can be addressed promptly and effectively.
Patients undergoing HIV treatment require intense education and ongoing counseling to promote medication adherence; this includes the importance of starting ART as soon as possible after diagnosis and the need to take the prescribed medications consistently for life. Medication adherence is essential to achieve HIV viral load suppression. Viral load increases within weeks of stopping HIV medications; this enhances the risk of developing resistant organisms, complications from HIV infections, and transmission. Helping patients understand the challenges often faced with medication adherence can encourage them to seek support with any barriers they may encounter. Patients should be offered home nurse visits, blister packs, or automated reminders to support adherence. (Refer to the Enhancing Healthcare Team Outcomes section for more on the role of the interdisciplinary care team in supporting client adherence to therapy.)
Patients should be aware of signs and symptoms that might indicate toxicity from ART and the recommended actions should these occur. Minor adverse effects, such as nausea upon initiation of therapy, can be managed with over-the-counter medications. At the same time, signs and symptoms of liver or kidney injury should lead patients to seek immediate medical care. Patients should be aware of the potential for drug-drug interactions and the importance of the pharmacist in managing their medications. They should be encouraged to record their medications with them at all times should medical care be required.
People with HIV are at elevated risk of cardiac and metabolic complications, may face complications that affect their nutrition, and may need to avoid certain foods due to immunocompromise. Lifestyle modifications to encourage healthy eating, regular exercise, and the management of other risk factors, such as smoking, should be recommended and supported throughout the person's life. (Refer to the Toxicity and Adverse Effect Management section for further information on toxicity and adverse effect management).
Prevention of HIV Transmission
People living with HIV are often highly motivated to prevent transmission to others, particularly when they have a partner who is not infected with HIV. Recent US data reveals that every 10% increase in viral suppression on a population level is associated with a 4% decline in the incidence of HIV in the subsequent year. [19] Ongoing clinical education and emphasis on "undetectable equals untransmittable" is an essential part of treatment for individuals with HIV, for their health and that of their partner or partners. People of child-bearing age may be concerned about transmission of HIV infection during pregnancy and should be made aware of the options for treatment and the benefits of planning ahead.
People who do not have an undetectable viral load because they are early in treatment or unable to achieve one for any reason have other ways to protect others. The person can correctly and consistently use condoms, choose sexual activities with lower risk, encourage their partners to take PrEP and avoid sharing needles, syringes, and other drug injection equipment. People may be required to disclose their HIV or other communicable disease status, for example, to clinicians in jurisdictions with these requirements or to healthcare regulatory authorities if they provide healthcare services.
Stigma, Discrimination, and Mental Health
Being diagnosed with a chronic illness can be a significant source of stress for anyone due to the need to adhere carefully to treatment and regularly undergo testing and follow-up with healthcare professionals. The diagnosis can challenge one's sense of well-being or complicate existing mental health or other conditions. Individuals may feel sadness, hopelessness, or anger.
In addition to the challenges facing anyone with a new diagnosis of a serious chronic illness, persons living with HIV face additional challenges due to stigma and discrimination, including self-stigma.[CDC. HIV Basics. 2021] This occurs when persons with HIV internalize the negative opinions of others, such as believing only certain kinds of people acquire HIV or that they deserve to get it because of their behaviors. The need to tell sexual or injection partners about an HIV infection before sex or drug use can be uncomfortable for people or can provoke anxiety if punitive laws exist for the failure to disclose. Persons with HIV may have fears associated with protecting others, which limit their interactions with other people and lead to isolation. A referral to a psychologist, social worker, specialized nurse, public health, other interdisciplinary team member, or support group can assist patients in coping with these issues. Likewise, encouraging patients to share their HIV status with certain friends and family can lead to practical and emotional benefits for them.
See StatPearls' companion topics, " HIV Antiretroviral Therapy " and " HIV Prevention ," for further information on toxicity and management of adverse effects of antiretroviral therapy and HIV prevention.
- Pearls and Other Issues
Clinicians must be aware of any rules and regulations regarding HIV screening, testing, reporting, and managing HIV that may exist in the jurisdiction in which they are practicing. These rules and regulations vary widely in their approach. In some jurisdictions, rules and regulations may sit within public health acts and follow a progressive enforcement framework; in others, they may sit within criminal law and carry significant penalties. (Refer to “Social and Legal Environments” in the Enhancing Healthcare Team Outcomes section for more information on policy recommendations).
Relevant State Laws
Most states have defined guidelines in their administrative codes in the US. For example, Florida Administrative Code Rule 64B8-13.005 states that every physician licensee must complete 1 hour of Category I American Medical Association Continuing Medical Education that covers HIV and AIDS every 2 years. This includes information regarding Florida State Law for HIV testing and test result reporting provided in statutes 381.004 and 384.25 mandating the following:
- Before HIV testing, the patient must be notified orally or in writing that the test is planned. The patient must be given the right to refuse testing, which is documented in the medical record.
- If the patient or their legal guardian signs a medical consent form for medical care in a healthcare setting (eg, clinic, emergency setting, or hospital), a separate consent form for an HIV test during the period in which the general consent is in effect is not required.
- In a nonhealthcare setting, such as community outreach programs, informed consent with the option to decline testing and information regarding sites that provide anonymous testing in the community must be obtained.
- In the event of a positive test result, the patient must be provided with appropriate care and medical support services, information on the importance of notifying partners who may have been exposed, and ways to prevent transmission.
- In the event of a negative test result, every effort should be made to notify the patient of the result, and information regarding ways to prevent the acquisition of HIV should be provided.
- In a healthcare setting, if the patient is discharged before the results are available, the county health department must notify the test subject to fulfill the responsibility, even in the event of a positive test result.
- The person tested and clinicians directly responsible for the medical care and decision-making for the tested individual.
- The person tested and clinicians responsible for the care and decision-making of a newborn who may be affected by these results.
- Healthcare personnel subject to a significant exposure from the person whose results were positive.
- Individuals tested using rapid testing technologies in accordance with manufacturers’ instructions approved by the US Food and Drug Administration.
- Unconfirmed test results must not be characterized as an HIV diagnosis to the patient. The rationale for releasing unconfirmed results must be documented in the chart. Confirmatory testing must be obtained, and the results must be communicated to the individual tested.
- Positive HIV test results, after confirmatory testing, may only be released to the tested individual or their designated legally authorized representative.
- Results must be reported to the State Health Department in accordance with rules for reporting and controlling disease. Results may also be shared with clinicians who use semen or body parts from an infected individual, health facility committees for purposes of program monitoring and evaluation, and authorized researchers and epidemiologists, as proscribed under appropriate statutes and procedures.
- The patient must provide written authorization for the release of such testing to any other individual or third-party payor for HIV test results to be released. In this scenario, there must be more than general consent to release medical records. A specific authorization for the release of HIV test results must be provided.
- If HIV testing is conducted due to medical personnel exposure, the occurrence shall be documented and recorded only in the medical personnel’s personnel record. In addition, the cost of the initial HIV test shall be borne by the medical personnel or their employer.
- If the source of the exposure is not available or will not voluntarily present for testing, the medical personnel or the employer may seek a court order for HIV testing from the source individual. The test results should be released to the source and the person who experienced the exposure.
- Clinicians, laboratories, and healthcare facilities that make a diagnosis of or treat a person with HIV/AIDS must report the result as outlined above no later than 2 weeks following the diagnosis or treatment.
- Violation of these rules is subject to penalties, fines, and disciplinary actions.
Impact of Legislation on HIV Testing
Laws and regulations concerning HIV and AIDS are developed considering a variety of parameters that impact patients’ willingness to test for HIV. For example, to provide patients with privacy, confidentiality, and dignity, the Florida legislature considered the need for informed consent and privacy in designing its laws regarding HIV and other sexually transmitted diseases, including laws for reporting.
Informed consent
- Informed consent includes an explanation to the patient regarding confidentiality, mandatory reporting, and the opportunity for anonymous testing.
- This consent maintains that in a healthcare setting, a patient must be notified of a planned HIV test, and they have the right to refuse the test.
- Informed consent also allows a legal guardian to provide informed consent if a person is incompetent, incapacitated, or a legal minor.
Confidentiality
- The patient gives consent.
- The data is provided for statistical purposes and excludes identifying information.
- The clinician or facility must disclose the result for mandatory reporting to medical personnel, state agencies, or mandated court jurisdiction.
- The information needs to be disclosed during a medical emergency; only relevant information for the patient’s care can be disclosed.
- The person commits a misdemeanor of the first degree, which is punishable by a fine of up to $1,000 and up to 1 year in prison.
- A person who spreads information about an individual with HIV or another sexually transmitted disease for monetary gain or with malicious intent commits a felony in the third degree, which is punishable by a fine of up to $5,000 or imprisonment of up to 5 years.
- Reporting ensures results are promptly and confidentially reported to the Florida Department of Health to allow for contact follow-up and other public health activities, such as surveillance. A positive test result or other diagnosis of HIV or AIDS must be reported within 2 weeks using the system developed by the CDC or an equivalent system to ensure confidentiality.
- The Department of Health may fine anyone who fails to report HIV or AIDS up to $500 for each offense, and a regulatory agency will be informed of the violation.
[See Florida State Statutes, Title XXIX Public Health, Chapter 381 Public Health: General Provisions ]
- Enhancing Healthcare Team Outcomes
Almost 21 million lives have been saved with antiretroviral therapy worldwide.[UNAIDS. Global Report. 2023] However, millions of people around the globe are missed or inadequately served by current prevention and treatment services. Key indicators of the quality of HIV care for optimal patient and population health outcomes include the linkage of HIV-positive individuals to care, receipt of treatment by those who are linked to care, and attainment of viral suppression amongst those who are treated.
For example, in the US in 2021, 80% of people diagnosed with HIV were linked to care within 30 days of diagnosis, defined as at least 1 viral load or CD4 test within that time. However, of all people with an HIV diagnosis in 2021, only 54% were retained in care (defined as ≥2 viral load or CD4 tests ≥3 months apart in 2021), and only 66% were virally suppressed (defined as an HIV RNA level of <200 copies/mL).
Within countries, variability in key quality care indicators exists across populations. While the disparities are greatest in low- and middle-income countries, significant inequities continue to exist in HIV prevalence and incidence across population groups, even in countries with well-developed HIV responses. For example, compared with the general population in the United States, HIV disproportionately affects PWID, those who live in the US South, and those who are Black, Hispanic, Latino, transgender, or MSM. [19] Across the European Economic Area, migrants accounted for 44% of new HIV diagnoses in 2019. [19]
A successful HIV response depends on multiple factors, including utilizing a team-based and patient-centered care approach, a focus on prevention in the community and healthcare settings, robust monitoring and surveillance systems, continuous program quality improvement, and supportive social and legal environments. An integrated interprofessional team of physicians, nurses, pharmacists, social workers, public health officials, and community partners is essential to improve clinical outcomes for patients with HIV, improve population health, and ultimately change the course of the global HIV pandemic.
Patient-Centered, Team-Based Care
Putting patients at the center allows the maximum benefit from HIV care and prevention services, leading to improved clinical and public health outcomes.[WHO. People-Centred Health Services. 2016][WHO. Men and HIV Interventions. 2023]. As HIV is an epidemic that disproportionately affects marginalized populations, programming that places the individual at the center of care ensures equality of access to testing and care, minimizes stigma, and overcomes socioeconomic barriers that limit access to care. For example, patients who have HIV or are at risk for HIV acquisition and have underlying socioeconomic disadvantages or substance use disorders are at high risk for medical nonadherence.
Physicians, nurse practitioners, and physician assistants all provide primary care to patients with HIV. Clinician use of current HIV guidelines, genotype resistance testing, and measures to support adherence to therapy ensures patients receive adequate medical treatment throughout their lives. Systematic monitoring for complications minimizes the risk and consequences of the disease and ART.
Nurses play an essential role in achieving care and public health goals by supporting patients in understanding, for example, the risk of transmission, complications, and the need for medication adherence. Home-visiting nurses can help patients stay linked with medical care and obtain laboratory testing in patients with limited transportation resources. According to a study from sub-Saharan Africa, immediate ART at the time of home-based positive test results increased clinical follow-up and ongoing linkage to care. [10] Nurses and nutritionists can monitor weight and promote adequate nutrition and hydration to promote overall health, minimize cardiovascular risks, and minimize the risk of HIV wasting syndrome.
Pharmacists augment the patient's understanding of their treatment by discussing potential adverse effects of therapy, ways to minimize drug interactions, and ensuring patients know when to seek help should any treatment complications occur. Measures such as blister-packed medications can ease the burden of setting up pills, minimize the risk of incorrect dosing, and improve medication adherence.
Public health nurses and physicians are important in connecting and following up with contacts to prevent further transmission. They have a primary role in preventing HIV in the community, including identifying and responding rapidly to HIV outbreaks in the community. They also support the patient care team in unusual instances where patients are persistently unwilling or unable to follow recommendations to prevent transmission. (Refer to "Community HIV Prevention" below for further information on this topic).
Community health, peer support, and social workers are vital in promoting patient well-being and improving linkage to care. Social workers can allocate resources for transportation and childcare support, provide assistance with mental health services, or make referrals to finance medications where this is a barrier. Community health and peer support workers, for example, assist in the development of stigma-free services or increase the availability of home-based services.
See the Statpearls' companion article "HIV Antiretroviral Therapy" for further information on interprofessional team roles in maintaining HIV therapy.
Community HIV Prevention
Primordial, primary, and secondary prevention are all important in the context of improving outcomes for HIV infection. The percentage of new transmissions and the number of HIV-positive people who are aware of their HIV status are key quality indicators for prevention efforts. Worldwide, 86% of the 39 million people estimated to be living with HIV knew their status in 2022.[UNAIDS. Global Report. 2023] In the US, 87% of the estimated 1.2 million people living with HIV knew their status in 2021.[CDC. US HIV Statistics. 2023]
Primordial prevention includes addressing the determinants of health that lead to increased risks for acquiring HIV, including poverty, discrimination, stigma, or other mechanisms that marginalize certain groups of people. Beneficial social and policy environments are essential in primordial prevention and the prevention and care spectrum. (Refer to "Social and Policy Environments" below for more information on measures to improve social determinants of health for HIV prevention).
Primary prevention targets those who have risk factors, including public awareness and education regarding safe sexual practices and ways to reduce the risk of HIV transmission among PWID. The promotion of safer sexual practices, treatment of opioid use disorder, and widespread access to clean syringe services and other harm reduction approaches are effective prevention strategies that should be implemented across the globe. [19] Voluntary medical circumcision for heterosexual males is another effective prevention approach in southern and eastern areas of Africa where HIV is highly prevalent. PrEP is a highly effective strategy for those at high risk, for example, due to having a sexual partner who is HIV positive, having multiple sexual partners without using condoms consistently, or the use of injectable drugs.
Developing cost-effective recommendations and education to screen for HIV is a critical component of HIV prevention. For example, the US Preventive Services Task Force (USPSTF) recommends that clinicians in the US screen all pregnant individuals as well as routine and voluntary HIV screening among all persons 15 to 65 years, with particular emphasis on individuals at high risk of infection. [20] Maternal HIV screening has resulted in a significant decline in mother-to-child HIV transmission, preventing nearly 22,000 perinatal infections between 1994 and 2010. [21]
Treatment as prevention recognizes the fact that HIV is not sexually transmitted if the viral load is undetectable (defined as <200 copies of HIV-1 RNA/mL of plasma). [22] [23] Otherwise known as undetectable=utransmittable or U=U, evidence is increasing of its effectiveness for those who share drug injection equipment. Emphasis on U=U can be a strong motivator for patient adherence to medication. However, even with undetectable viral loads, HIV transmission still occurs perinatally and via breast milk.
See the Statpearls' companion articles on "Prevention of HIV" for further information on PrEP, PEP, and recommendations for screening for HIV, amongst others.
HIV Prevention in Healthcare Settings
In the healthcare context, the use of rigorous infection prevention and control procedures has led to widespread decreases in occupationally and iatrogenically transmitted HIV. The elimination in many countries of the reuse of needles, syringes, and other medical equipment that can transmit HIV has been a global success story. The widespread implementation of standard precautions, previously called universal precautions, is another key preventive strategy. Initially developed to prevent HIV transmission, standard precautions are utilized to avoid the transmission of all infectious agents. The first line of defense to break the chain of transmission of infectious agents in healthcare settings is the application of standard precautions to all patients, irrespective of their known or suspected infection status. The type of infection control practice necessary is based on the level of anticipated contact with the patient and assumes that any patient's blood or body fluids may contain an infectious agent.
Hand hygiene is the single most important measure to prevent transmission of disease. Hand hygiene encompasses washing hands with soap and water for at least 40 to 60 seconds when visibly soiled, after restroom use, or potential exposure to spore-forming organisms. Alcohol-based hand rubs can otherwise be used. Clinicians should wash their hands between patients immediately after gloves are removed and before and after any direct patient contact or contact with invasive devices, blood or body fluids, secretions, mucous membranes, or nonintact skin, even if gloves are worn. Gloves are used when touching blood, body fluids, secretions, mucous membranes, or nonintact skin. A mask, goggles, eye visor, face shield, or gown are used when blood, body fluids, secretions, or excretions may be splashed. Needles and sharps should be discarded immediately in appropriate puncture-resistant containers.
Occupational PEP is essential to prevention in healthcare settings, as eliminating inadvertent exposures to potentially infectious body fluids is difficult to achieve. PEP involves the provision of a minimum of 3 antiretroviral drugs for 28 days for all occupational exposures to HIV, starting as soon as possible after the exposure and within 72 hours. [24] Counseling, HIV testing at baseline and follow-up, and monitoring for toxicity are also provided. Using the PEP consultation service for clinicians is recommended in all cases. A live hotline in the US is available from 9 am to 2 am Eastern time at 1-888-448-4911. [27]
See the Statpearls' companion article on "Universal Precautions" for further information on standard precautions.
Monitoring, Surveillance, and Continuous Quality Improvement
Collecting and using reliable, granular, and timely data is essential to improve team performance and population health. Using data means identifying at-risk clients, setting targets, and developing strategies to reach those targets. UNAIDS first proposed a set of global 90-90-90 HIV prevention targets for HIV in 2014, which were committed to by countries of the UN General Assembly. Although these goals were not reached, many countries made significant progress. Setting 95-95-95 goals for 2025, the goals were updated in 2020 and adopted by the UN General Assembly in 2021 and include:
- 95% of all individuals with HIV will know their status
- 95% of all those who have HIV will receive antiretroviral therapy
- 95% of all people on ART will achieve viral suppression
The projected impact of this will be fewer than 370,000 people acquiring HIV and fewer than 250,000 people dying from HIV worldwide in 2025.
The WHO and country and state disease control and prevention centers, such as the US CDC, are primary data sources for monitoring HIV trends and progress toward goals. Behavioral and clinical characteristics of adults diagnosed with HIV infection are followed in the US by the Medical Monitoring Project. Instituted in 2005, this is a cross-sectional, nationally representative, complex sample survey.[CDC. HIV Surveillance Report 2021 Cycle. 2023] The CDC's AtlasPlus allows practitioners to explore national HIV, hepatitis, STI, and tuberculosis data relevant to their population.[CDC. NCCHHSTP AtlasPlus. 2023] All data from 2020 and 2021 must be interpreted in the context of decreased case surveillance and access to HIV services during the COVID-19 pandemic.[CDC. HIV Basic Statistics. 2023]
In collaboration with local or state public health authorities, clinicians can improve care and contribute to population health improvements by completing reporting forms where required, participating in initiatives to streamline and improve surveillance, and engaging in continuous program quality improvement within their practice setting utilizing public health and program-specific data.
Social and Policy Environments
Local legislative bodies, other policy-making organizations, and institutions can improve patient-centered care and population health outcomes for HIV by basing legislative and policy frameworks that influence the effectiveness of HIV and AIDS programming on recommendations developed by organizations such as the UNAIDS, WHO, and the NIH.[UNAIDS. Global Report. 2023][WHO. Global HIV Strategies. 2022]
The WHO recommends specific HIV policies that promote optimal HIV testing and care and track the country's progression toward these. These recommendations include the use of PrEP, dual HIV and syphilis rapid diagnostic tests, self-testing, optimal first- and second-line treatments, routine viral load testing, and same-day ART initiation, amongst other measures, in national policies and guidelines.[WHO. Global HIV Strategies. 2022] UNAIDS, WHO, and national HIV strategies outline key social, legal, and policy factors that lead to better patient and population health outcomes for HIV prevention and care.[UNAIDS. Global Report. 2023][WHO. Global HIV Strategies. 2022]
Political commitment and adequate resources are fundamental building blocks for an adequate HIV response.[UNAIDS. Global Report. 2023] The setting of targets can be an important component in garnering political commitment. The US National HIV/AIDS Strategy [US Gov. National HIV/AIDS Strategy. 2023] and the Ending the HIV Epidemic in the United States initiative aims to reduce new HIV transmissions in the US by 90% by 2030, prioritizing the reduction of HIV-related health disparities and health inequities and improving well-being for people who live with HIV.[CDC. Ending the HIV epidemic. 2023]
A renewed global commitment to funding is necessary. The gap between actual and required funding is widening in low- and middle-income countries, mainly due to a decrease in the proportion of funding provided by high-income nations.[UNAIDS. Global Report. 2023] Fully funded resilient, integrated, and accessible public and community health systems lead to increased uptake of both HIV and other health services. Social and structural inequalities to HIV-related services, resources, and tools can be addressed through universal health care, shared service delivery models, and measures to improve access to medicines and other health technologies for those experiencing marginalization, including uninsured or underinsured individuals in the US.
The removal, or at a minimum, the nonenforcement of harmful laws must be a priority. These continue to limit access to health care for people living with HIV and increase the risk of acquiring HIV for those already vulnerable. For example, laws criminalizing HIV exposure, non-disclosure, and transmission discourage people from getting tested, increasing transmission and creating a barrier to early treatment. Likewise, the criminalization of particular groups of people undermines an effective HIV response by discouraging sex workers, MSM, and those who are transgender or inject drugs from using prevention and treatment programs due to fear of arrest and prosecution. A 2015 study estimated that 33% to 46% of all new HIV infections over a decade could be avoided by decriminalizing sex work worldwide.[UNAIDS. Global Report. 2023]
Many countries have amended laws to remove barriers to the HIV response, including decriminalizing vertical HIV transmission in Belize in 2023, sex work in Belgium in 2022, and same-sex sexual relations in several others in 2022 and 2023. However, there have been concerning setbacks in many countries, including Indonesia. Strengthening laws, policies, and systems that realize and protect human rights is also necessary. For example, the promotion of gender equity and safe work environments leads to lower vulnerability for women and girls. Modeling studies from Canada and Kenya show that the elimination of violence by police, clients, and strangers could avert 17% to 20% of new HIV infections among women who are sex workers and their clients in a decade.[UNAIDS. Global Report. 2023]
Strengthened policies and laws augment the education and community action necessary to address the stigma and discrimination that impact the health and well-being of people living with HIV. One in 3 of the countries reporting to UNAIDS on HIV policies report that 10% of MSM and more than 50% of sex workers, PWID, and transgender individuals avoid healthcare due to fears of stigma, discrimination, or confidentiality. Rooted in outdated fears of HIV from the 1980s, stigma and discrimination must be addressed through open discussion about HIV using non-stigmatizing language both within and outside of health care.[CDC. HIV Basics. 2022] Discrimination can manifest in healthcare settings as the refusal of service to people with HIV, the use of stigmatizing language, or the absence of appropriate services, resources, or tools.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Helena Swinkels declares no relevant financial relationships with ineligible companies.
Disclosure: Angel Justiz Vaillant declares no relevant financial relationships with ineligible companies.
Disclosure: Andrew Nguyen declares no relevant financial relationships with ineligible companies.
Disclosure: Peter Gulick declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Swinkels HM, Justiz Vaillant AA, Nguyen AD, et al. HIV and AIDS. [Updated 2024 May 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- HIV and AIDS (Nursing). [StatPearls. 2024] HIV and AIDS (Nursing). Swinkels HM, Justiz Vaillant AA, Nguyen AD, Gulick PG, Pinto KM. StatPearls. 2024 Jan
- Florida HIV Safety for Florida Clinical Laboratory Personnel. [StatPearls. 2024] Florida HIV Safety for Florida Clinical Laboratory Personnel. Caruso JR, Swift CJ. StatPearls. 2024 Jan
- Animal models for HIV infection and AIDS: memorandum from a WHO meeting. [Bull World Health Organ. 1988] Animal models for HIV infection and AIDS: memorandum from a WHO meeting. . Bull World Health Organ. 1988; 66(5):561-74.
- Review Human immunodeficiency virus and acquired immunodeficiency syndrome: correlation but not causation. [Proc Natl Acad Sci U S A. 1989] Review Human immunodeficiency virus and acquired immunodeficiency syndrome: correlation but not causation. Duesberg PH. Proc Natl Acad Sci U S A. 1989 Feb; 86(3):755-64.
- Review AIDS: Part II. [Dis Mon. 1992] Review AIDS: Part II. Kessler HA, Bick JA, Pottage JC Jr, Benson CA. Dis Mon. 1992 Oct; 38(10):691-764.
Recent Activity
- HIV and AIDS - StatPearls HIV and AIDS - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Understanding the Stigma Experience of Men Living with HIV in Sub-Saharan Africa: A Qualitative Meta-synthesis
- Substantive Review
- Published: 22 May 2024
Cite this article

- Sarah E. Janek ORCID: orcid.org/0009-0002-1213-2791 1 ,
- Sandy Hatoum ORCID: orcid.org/0009-0002-3618-9733 2 ,
- Leila Ledbetter ORCID: orcid.org/0000-0002-5206-8002 3 &
- Michael V. Relf ORCID: orcid.org/0000-0002-4951-8869 1 , 2
Men living with HIV (MLWH) in sub-Saharan Africa experience poor health outcomes and increased AIDS-related deaths due to stigma influencing testing and treatment uptake and adherence. PRISMA 2020 was used to report a meta-synthesis of the stigma experiences of MLWH in SSA. With the help of an expert librarian, a search of six databases was formulated and performed to examine the available qualitative and mixed method studies with qualitative results relevant to the research question. Studies focused on adult men living with HIV, with five studies specifically examining the HIV experience of men who have sex with men. Study themes were synthesized to describe MLWH’s perceived, internalized, anticipated, enacted, and intersectional stigma experiences. Most studies included masculinity as a key theme that affected both testing and treatment adherence upon diagnosis. Future research is needed to better understand subpopulations, such as men who have sex with men living with HIV, and what interventions may be beneficial to mitigate the disparities among MLWH in SSA.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Kaiser Family Foundation. The global HIV/AIDS epidemic. KFF. 2022. https://www.kff.org/global-health-policy/fact-sheet/the-global-hivaids-epidemic/ . Accessed 13 Nov 2022.
UNAIDS. Fact sheet—latest global and regional statistics on the status of the AIDS epidemic. Joint United Nations Programme on HIV/AIDS (UNAIDS). 2022. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf . Accessed 23 Oct 2022.
West CA, Chang GC, Currie DW, Bray R, Kinchen S, Behel S, McCullough-Sanden R, Low A, Bissek A, Shang JD, Ndongmo CB, Dokubo EK, Balachandra S, Lobognon LR, Dube L, Nuwagaba-Biribonwoha H, Li M, Pasipamire M, Getaneh Y, Lulseged S, Eshetu F, Kingwara L, Zielinski-Gutierrez E, Tlhomola M, Ramphalla P, Kalua T, Auld AF, Williams DB, Remera E, Rwibasira GN, Mugisha V, Malamba SS, Mushi J, Jalloh MF, Mgomella GS, Kirungi WL, Biraro S, Awor AC, Barradas DT, Mugurungi O, Rogers JH, Bronson M, Bodika SM, Ajiboye A, Gaffga N, Moore C, Patel HK, Voetsch AC. Unawareness of HIV infection among men aged 15–59 years in 13 sub-Saharan African countries: findings from the population-based HIV impact assessments, 2015–2019. JAIDS. 2021;87:S97.
PubMed Google Scholar
UNAIDS. Executive summary—in danger: UNAIDS global AIDS update 2022 [Internet]. Joint United Nations Programme on HIV/AIDS (UNAIDS). 2022. https://www.unaids.org/sites/default/files/media_asset/2022-global-aids-update-summary_en.pdf . Accessed 23 Oct 2022.
UNAIDS. Addressing a blind spot in the response to HIV—reaching out to men and boys. Joint United Nations Programme on HIV/AIDS (UNAIDS). 2017. https://www.unaids.org/sites/default/files/media_asset/blind_spot_en.pdf . Accessed 23 Oct 2022.
Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. Am J Public Health. 2013;103:813–21. https://doi.org/10.2105/AJPH.2012.301069 .
Article PubMed PubMed Central Google Scholar
Relf MV, Holzemer WL, Holt L, Nyblade L, Caiola CE. A review of the state of the science of HIV and stigma: context, conceptualization, measurement, interventions, gaps, and future priorities. J Assoc Nurses AIDS Care. 2021;32:392–407. https://doi.org/10.1097/JNC.0000000000000237 .
Herek GM. AIDS and stigma. Am Behav Sci. 1999;42:1106–16. https://doi.org/10.1177/00027649921954787 .
Article Google Scholar
Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV stigma mechanisms and well-being among PLWH: a test of the HIV stigma framework. AIDS Behav. 2013;17:1785–95. https://doi.org/10.1007/s10461-013-0437-9 .
Derlega VJ, Winstead BA, Greene K, Serovich J, Elwood WN. Perceived HIV-related stigma and HIV disclosure to relationship partners after finding out about the seropositive diagnosis. J Health Psychol. 2002;7:415–32. https://doi.org/10.1177/1359105302007004330 .
Article PubMed Google Scholar
Arinaitwe I, Amutuhaire H, Atwongyeire D, Tusingwire E, Kawungezi PC, Rukundo GZ, Ashaba S. Social support, food insecurity, and HIV stigma among men living with HIV in rural southwestern Uganda: a cross-sectional analysis. HIV AIDS (Auckl). 2021;13:657–66. https://doi.org/10.2147/HIV.S316174 .
Mburu G, Ram M, Siu G, Bitira D, Skovdal M, Holland P. Intersectionality of HIV stigma and masculinity in eastern Uganda: implications for involving men in HIV programmes. BMC Public Health. 2014;14:1061. https://doi.org/10.1186/1471-2458-14-1061 .
Sileo KM, Fielding-Miller R, Dworkin SL, Fleming PJ. A scoping review on the role of masculine norms in men’s engagement in the HIV care continuum in sub-Saharan Africa. AIDS Care. 2019;31:1435–46. https://doi.org/10.1080/09540121.2019.1595509 .
Abara WE, Garba I. HIV epidemic and human rights among men who have sex with men in sub-Saharan Africa: implications for HIV prevention, care, and surveillance. Glob Public Health. 2017;12:469–82. https://doi.org/10.1080/17441692.2015.1094107 .
Joshi K, Lessler J, Olawore O, Loevinsohn G, Bushey S, Tobian AAR, Grabowski MK. Declining HIV incidence in sub-Saharan Africa: a systematic review and meta-analysis of empiric data. J Int AIDS Soc. 2021. https://doi.org/10.1002/jia2.25818 .
Nyato D, Kuringe E, Drake M, Casalini C, Nnko S, Shao A, Komba A, Baral SD, Wambura M, Changalucha J. Participants’ accrual and delivery of HIV prevention interventions among men who have sex with men in sub-Saharan Africa: a systematic review. BMC Public Health. 2018. https://doi.org/10.1186/s12889-018-5303-2 .
Hamilton A, Thompson N, Choko AT, Hlongwa M, Jolly P, Korte JE, Conserve DF. HIV self-testing uptake and intervention strategies among men in sub-Saharan Africa: a systematic review. Front Public Health. 2021. https://doi.org/10.3389/fpubh.2021.594298 .
Hlongwa M, Hlongwana K, Makhunga S, Choko AT, Dzinamarira T, Conserve D, Tsai AC. Linkage to HIV care following HIV self-testing among men: systematic review of quantitative and qualitative studies from six countries in sub-Saharan Africa. AIDS Behav. 2022. https://doi.org/10.1007/s10461-022-03800-8 .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. https://doi.org/10.1136/bmj.n71 .
Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM. Framing mechanisms linking HIV-related stigma, adherence to treatment, and health outcomes. Am J Public Health. 2017;107:863–9. https://doi.org/10.2105/AJPH.2017.303744 .
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021 .
UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS (UNAIDS). 2014. https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf . Accessed 10 June 2023.
UNAIDS. AIDS by the numbers: AIDS is not over, but it can be. Joint United Nations Programme on HIV/AIDS (UNAIDS). 2016. https://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-numbers-2016_en.pdf . Accessed 10 June 2023.
Covidence systematic review software. Veritas Health Innovation. 2022. https://www.covidence.org/ . Accessed 8 Mar 2022.
Kmet LM, Lee RC, Cook LS. HTA initiative #13: standard quality assessment criteria for evaluating primary research papers from a variety of fields. Alberta Heritage Foundation for Medical Research. 2004. https://www.ihe.ca/download/standard_quality_assessment_criteria_for_evaluating_primary_research_papers_from_a_variety_of_fields.pdf . Accessed 24 Oct 2022.
Sandelowski M, Barroso J. Handbook for synthesizing qualitative research. New York: Springer Publishing Company; 2007.
Google Scholar
Belay YA, Yitayal M, Atnafu A, Taye FA. Patient experiences and preferences for antiretroviral therapy service provision: implications for differentiated service delivery in Northwest Ethiopia. AIDS Res Ther. 2022;19:1–16. https://doi.org/10.1186/s12981-022-00452-5 .
Hlongwa M, Jama NA, Mehlomakulu V, Pass D, Basera W, Nicol E. Barriers and facilitating factors to HIV treatment among men in a high-HIV-burdened district in KwaZulu-Natal, South Africa: a qualitative study. Am J Mens Health. 2022. https://doi.org/10.1177/15579883221120987 .
Lofgren SM, Tsui S, Atuyambe L, Ankunda L, Komuhendo R, Wamala N, Sadiq A, Kirumira P, Srishyla D, Flynn A, Pastick KA, Meya DB, Nakasujja N, Porta C. Barriers to HIV care in Uganda and implications for universal test-and-treat: a qualitative study. AIDS Care. 2022;34:597–605. https://doi.org/10.1080/09540121.2021.1946000 .
Mandawa MB, Mahiti GR. Factors contributing to loss to follow-up from HIV care among men living with HIV/AIDS in Kibaha District, Tanzania. HIV AIDS (Auckl). 2022;14:503–16. https://doi.org/10.2147/hiv.S381204 .
Mange T, Henderson N, Lukelelo N. ‘After 25 years of democracy we are still stigmatised and discriminated against’ healthcare experiences of HIV-positive older black gay men in a township in South Africa. J Pract Teach Learn. 2022;9:87–100. https://doi.org/10.1921/jpts.v19i1-2.1674 .
Mathenjwa M, Khidir H, Milford C, Mosery N, Rambally Greener L, Pratt MC, O’Neil K, Harrison A, Bangsberg DR, Safren SA, Smit JA, Psaros C, Matthews LT. Acceptability of an intervention to promote viral suppression and serostatus disclosure for men living with HIV in South Africa: qualitative findings. AIDS Behav. 2022;26:1–12. https://doi.org/10.1007/s10461-021-03278-w .
Muwanguzi PA, Nelson LE, Ngabirano TD, Kiwanuka N, Osingada CP, Sewankambo NK. Linkage to care and treatment among men with reactive HIV self-tests after workplace-based testing in Uganda: a qualitative study. Front Public Health. 2022. https://doi.org/10.3389/fpubh.2022.650719 .
Nabikande S, Namutundu J, Nangendo J, Okello T, Agwang W, Tusabe J, Kabwama SN, Katahoire AR. Men’s late presentation for HIV care in Eastern Uganda: the role of masculinity norms. PLoS ONE. 2022. https://doi.org/10.1371/journal.pone.0277534 .
Ndione AG, Procureur F, Senne JN, Cornaglia F, Gueye K, Ndour CT, Lépine A. Sexuality-based stigma and access to care: intersecting perspectives between health care providers and men who have sex with men in HIV care centres in Senegal. Health Policy Plan. 2022;37:587–96. https://doi.org/10.1093/heapol/czac010 .
Rich C, Mavhu W, France NF, Munatsi V, Byrne E, Willis N, Nolan A. Exploring the beliefs, experiences and impacts of HIV-related self-stigma amongst adolescents and young adults living with HIV in Harare, Zimbabwe: a qualitative study. PLoS ONE. 2022. https://doi.org/10.1371/journal.pone.0268498 .
Hendrickson ZM, Naugle DA, Tibbels N, Dosso A, Van Lith LM, Mallalieu EC, Kamara D, Dailly-Ajavon P, Cisse A, Ahanda KS, Thaddeus S, Babalola S, Hoffman CJ. “You take medications, you live normally”: the role of antiretroviral therapy in mitigating men’s perceived threats of HIV in Côte d’Ivoire. AIDS Behav. 2019;23:2600–9. https://doi.org/10.1007/s10461-019-02614-5 .
Naugle DA, Tibbels NJ, Hendrickson ZM, Dosso A, Van Lith LM, Mallalieu EC, Kouadio AM, Kra W, Kamara D, Dailly-Ajavon P, Cissé A, Seifert-Ahanda K, Thaddeus S, Babalola S, Hoffman CJ. Bringing fear into focus: the intersections of HIV and masculine gender norms in Côte d’Ivoire. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0223414 .
Tibbels NJ, Hendrickson ZM, Naugle DA, Dosso A, Van Lith LM, Mallalieu EC, Kouadio AM, Kra W, Kamara D, Dailly-Ajavon P, Cisse A, Seifert-Ahanda K, Thaddeus S, Babalola S, Hoffmann CJ. Men’s perceptions of HIV care engagement at the facility- and provider-levels: experiences in Cote d’Ivoire. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0211385 .
Balogun A, Bissell P, Saddiq M. Negotiating access to the Nigerian healthcare system: the experiences of HIV-positive men who have sex with men. Cult Health Sex. 2020;22:233–46. https://doi.org/10.1080/13691058.2019.1582802 .
Mukumbang FC. Leaving no man behind: how differentiated service delivery models increase men’s engagement in HIV care. Int J Health Policy Manag. 2021;10:129–40. https://doi.org/10.34172/ijhpm.2020.32 .
Ogunbajo A, Kershaw T, Kushwaha S, Boakye F, Wallace-Atiapah N-D, Nelson LE. Barriers, motivators, and facilitators to engagement in HIV care among HIV-infected Ghanaian men who have sex with men (MSM). AIDS Behav. 2018;22:829–39. https://doi.org/10.1007/s10461-017-1806-6 .
Okal J, Lango D, Matheka J, Obare F, Ngunu-Gituathi C, Mugambi M, Avina S. “It is always better for a man to know his HIV status”—a qualitative study exploring the context, barriers and facilitators of HIV testing among men in Nairobi, Kenya. PLoS ONE. 2020. https://doi.org/10.1371/journal.pone.0231645 .
Okoror TA, Falade CO, Walker EM, Olorunlana A, Anaele A. Social context surrounding HIV diagnosis and construction of masculinity: a qualitative study of stigma experiences of heterosexual HIV positive men in southwest Nigeria. BMC Public Health. 2016;16:507. https://doi.org/10.1186/s12889-016-3165-z .
Sileo KM, Reed E, Kizito W, Wagman JA, Stockman JK, Wanyenze RK, Chemusto H, Musoke W, Mukasa B, Kiene SM. Masculinity and engagement in HIV care among male fisherfolk on HIV treatment in Uganda. Cult Health Sex. 2019;21:774–88. https://doi.org/10.1080/13691058.2018.1516299 .
Zissette S, Watt MH, Prose NS, Mntambo N, Moshabela M. “If you don’t take a stand for your life, who will help you?”: men’s engagement in HIV care in KwaZulu-Natal, South Africa. Psychol Men Masc. 2016;17:265–73. https://doi.org/10.1037/men0000025 .
Berner-Rodoreda A, Ngwira E, Alhassan Y, Chione B, Dambe R, Bärnighausen T, Phiri S, Taegtmeyer M, Neuhann F. “Deadly”, “fierce”, “shameful”: notions of antiretroviral therapy, stigma and masculinities intersecting men’s life-course in Blantyre, Malawi. BMC Public Health. 2021. https://doi.org/10.1186/s12889-021-12314-2 .
Mantell JE, Masvawure TB, Mapingure M, Apollo T, Gwanzura C, Block L, Bennett E, Preko P, Musuka G, Rabkin M. Engaging men in HIV programmes: a qualitative study of male engagement in community-based antiretroviral refill groups in Zimbabwe. J Int AIDS Soc. 2019. https://doi.org/10.1002/jia2.25403 .
Misra S, Mehta HT, Eschliman EL, Rampa S, Poku OB, Wang W-Q, Ho-Foster AR, Mosepele M, Becker TD, Entaile P, Arscott-Mills T, Opondo PR, Blank MB, Yang LH. Identifying “what matters most” to men in Botswana to promote resistance to HIV-related stigma. Qual Health Res. 2021;31:1680–96. https://doi.org/10.1177/10497323211001361 .
Mooney AC, Gottert A, Khoza N, Rebombo D, Hove J, Suárez AJ, Twine R, MacPhail C, Treves-Kagan S, Kahn K, Pettifor A, Lippman SA. Men’s perceptions of treatment as prevention in South Africa: implications for engagement in HIV care and treatment. AIDS Educ Prev. 2017;29:274–87. https://doi.org/10.1521/aeap.2017.29.3.274 .
Moyo I, Macherera M, Mavhandu-Mudzusi AH. The lived experiences of men who have sex with men when accessing HIV care services in Zimbabwe. Health SA. 2021. https://doi.org/10.4102/hsag.v26i0.1462 .
Meskele M, Khuzwayo N, Taylor M. Mapping the evidence of intimate partner violence among women living with HIV/AIDS in sub-Saharan Africa: a scoping review. BMJ Open. 2021. https://doi.org/10.1136/bmjopen-2020-041326 .
Tenkorang EY, Asamoah-Boaheng M, Owusu AY. Intimate partner violence (IPV) against HIV-positive women in sub-Saharan Africa: a mixed-method systematic review and meta-analysis. Trauma Violence Abuse. 2021;22:1104–28. https://doi.org/10.1177/1524838020906560 .
Sileo KM, Fielding-Miller R, Dworkin SL, Fleming PJ. What role do masculine norms play in men’s HIV testing in sub-Saharan Africa?: a scoping review. AIDS Behav. 2018;22:2468–79. https://doi.org/10.1007/s10461-018-2160-z .
Naanyu V, Ruff J, Goodrich S, Spira T, Bateganya M, Toroitich-Ruto C, Otieno-Nyunya B, Siika AM, Wools-Kaloustian K. Qualitative exploration of perceived benefits of care and barriers influencing HIV care in trans Nzoia, Kenya. BMC Health Serv Res. 2020. https://doi.org/10.1186/s12913-020-05236-z .
Makoae LN, Seboni NM, Molosiwa K, Moleko M, Human S, Sukati NA, Holzemer WL. The symptom experience of people living with HIV/AIDS in southern Africa. J Assoc Nurses AIDS Care. 2005;16:22–32. https://doi.org/10.1016/j.jana.2005.03.005 .
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020. https://doi.org/10.1002/jrsm.1411 .
Download references
Acknowledgements
The manuscript was supported by the Fogarty International Center/National Institutes of Health through Award Number R21TW011247 (M. Relf, Contact MPI/L. Nyblade, MPI) and the Duke University Center for AIDS Research (CFAR), an NIH funded program (5P30AI064518). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Research reported in this publication was supported by the Fogarty International Center of the National Institutes for Health under award R21TW012007 and by the Duke Center for AIDS Research, a National Institutes of Health funded program under award number 5P30AI064518. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Author information
Authors and affiliations.
School of Nursing, Duke University, 307 Trent Drive, Box 3322, Durham, NC, 27710, USA
Sarah E. Janek & Michael V. Relf
Duke Global Health Institute, Durham, NC, USA
Sandy Hatoum & Michael V. Relf
Medical Center Library, Duke University, Durham, NC, USA
Leila Ledbetter
You can also search for this author in PubMed Google Scholar
Contributions
All authors on this paper meet the four criteria for authorship as identified by the International Committee of Medical Journal Editors; all authors have contributed to the drafting or been involved in revising it, reviewed the final version of this manuscript before submission, and agree to be accountable for all aspects of the work. Specifically, using the CRediT taxonomy, the specific contribution of each author is as follows: Conceptualization & Methodology: S. Janek, L. Ledbetter, M. Relf. Formal Analysis: S. Hatoum, S. Janek, M. Relf. Funding Acquisition: M. Relf. Investigation: S. Hatoum, S. Janek, L. Ledbetter, M. Relf. Methodology: S. Janek, L. Ledbetter, M. Relf. Project administration: M. Relf. Supervision: M. Relf. Verification: S. Hatoum, S. Janek, M. Relf. Writing—manuscript draft: S. Janek. Writing—review & editing: S. Hatoum, S. Janek, L. Ledbetter, M. Relf.
Corresponding authors
Correspondence to Sarah E. Janek or Michael V. Relf .
Ethics declarations
Conflict of interest.
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Registration The protocol that guided this meta-synthesis was prospectively registered with Prospero (registration ID: CRD42022315871).
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 35 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Janek, S.E., Hatoum, S., Ledbetter, L. et al. Understanding the Stigma Experience of Men Living with HIV in Sub-Saharan Africa: A Qualitative Meta-synthesis. AIDS Behav (2024). https://doi.org/10.1007/s10461-024-04329-8
Download citation
Accepted : 20 March 2024
Published : 22 May 2024
DOI : https://doi.org/10.1007/s10461-024-04329-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Meta-synthesis
- People with HIV
- Sub-Saharan Africa
- Qualitative methodology
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
HIV infections articles from across Nature Portfolio
HIV infections (human immunodeficiency virus infections) include the spectrum of infections caused by the virus HIV that range from asymptomatic seropositivity to acquired immunodeficiency syndrome (AIDS)-related complex (ARC) and AIDS.
Latest Research and Reviews
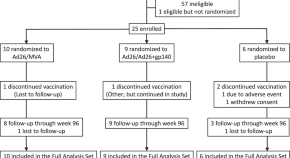
Immunogenicity of 2 therapeutic mosaic HIV-1 vaccine strategies in individuals with HIV-1 on antiretroviral therapy
- Kathryn E. Stephenson
- Dan H. Barouch
HIV-1 RNA in extracellular vesicles is associated with neurocognitive outcomes
Despite effective antiviral drugs, HIV-1 transcripts can be found in extracellular vesicles (EVs) in people living with HIV-1. Comparing EVs from serum and cerebrospinal fluid (CSF), the authors show compartmentalized defective viral transcripts that are enriched in the CSF and corelate with cognitive dysfunction.
- Catherine DeMarino
- Julia Denniss
- Avindra Nath
Human CD4-binding site antibody elicited by polyvalent DNA prime-protein boost vaccine neutralizes cross-clade tier-2-HIV strains
Here the authors isolate monoclonal antibody HmAb64 from a healthy volunteer who received an experimental polyvalent DNA prime-protein boost HIV vaccine, and show that it’s specific for the CD4 binding site and neutralizes cross-subtype HIV isolates including several tier-2 viruses.
- Shixia Wang
- Kun-Wei Chan
Evaluating the spatial accessibility and spatial layout optimization of HIV/AIDS healthcare services in Shandong Province, China
Predictive models-assisted diagnosis of AIDS-associated Pneumocystis jirovecii pneumonia in the emergency room, based on clinical, laboratory, and radiological data
- Oscar José Chagas
- Fabio Augusto Rodrigues Gonçalves

Premature skewing of T cell receptor clonality and delayed memory expansion in HIV-exposed infants
Here, Dzanibe et al show that in utero HIV/ARV exposure sequentially disrupts infant immunologic trajectories, beginning with NK cells that predict vaccine antibody responses and followed by delayed T cell memory maturation linked to skewed TCR clonality.
- Sonwabile Dzanibe
- Aaron J. Wilk
- Clive M. Gray
News and Comment
Combination treatment for immune-mediated HIV remission
In rhesus macaques, treatment with an IL-15 superagonist and broad neutralizing antibodies led to durable suppression of viremia after discontinuation of antiretroviral therapy.
- Karen O’Leary
Affinity maturation of CRISPR-engineered B cell receptors in vivo
CRISPR–Cas12a was used to directly replace mouse antibody variable chain genes with human versions in primary B cells. The edited cells underwent affinity maturation in vivo, improving the potency of HIV-1 and SARS-CoV-2 neutralizing antibodies without loss of bioavailability. Affinity maturation of edited cells also enables new vaccine models and adaptive B cell therapies.
CARD8 kills CD4 + T cells in response to HIV entry
- Alexandra Flemming
Blockbuster obesity drug leads to better health in people with HIV
Semaglutide reduces weight and fat accumulation associated with the antiretroviral regimen that keeps HIV at bay.
- Mariana Lenharo
Why have T cell-inducing vaccines for HIV failed so far?
The failure of T cell-targeted vaccines for HIV in clinical trials is likely due to impaired degranulation of low-avidity CD8 + T cells in the context of low levels of antigen presentation.
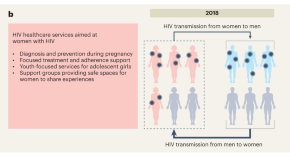
Close the gender gap in Africa’s HIV epidemic
Improving HIV interventions for men could reduce HIV acquisition in women, close the growing gender gap in HIV infections and further reduce HIV incidence in African countries.
- Bryan Tegomoh
- Boghuma K. Titanji
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
R.T. Gandhi and OthersN Engl J Med 2023;389:2468-2476. A 70-year-old woman with advanced HIV infection was evaluated because of cough, shortness of breath, and malaise. Eleven months earlier, she ...
Approximately 1.2 million people in the United States are currently living with HIV, and an estimated 14% are infected, yet unaware of their status (Office of Infectious Disease and HIV/AIDS Policy, 2020).HIV and AIDS continue to have a disproportionate impact on certain populations, including youth—gay, bisexual, and other men who have sex with men (MSM)—racial and ethnic minorities ...
HIV research continues on many fronts that could provide the same results and only some of which rely on the CCR5 delta 32 mutation, which should be explored extensively. There are many strategies which are in the early stages of development. ... This article aims to increase awareness among the society about the current scenario of HIV/AIDS ...
The Integrated Bio-Behavioural Surveillance Survey of 2022-2023 among key populations report from Zanzibar has been released. The prevalence of HIV is estimated to be 21.1%, 11.4%, and 9.3% among Female Sex Wo... Mansour Maulid Mshenga and Weiming Tang. AIDS Research and Therapy 2024 21 :9.
An effective and scalable cure strategy is a top priority for the HIV research field; this Review discusses recent advances, knowledge gaps, and priority research areas for the next 5 years.
While still elusive, cure remains the ultimate long-term goal for Gilead's HIV research and development efforts. ... Aiken C. Current Opinion in HIV and AIDS. 2018; 13(4): 359-365.
Since its inception, PEPFAR, which one of us (J. N.) now leads, has ensured crucial access to life-saving treatment for more than 20 million people in at least 50 countries. Twenty years after the ...
The HIV-1 pandemic is a complex mix of diverse epidemics within and between countries and regions of the world, and is undoubtedly the defining public-health crisis of our time. Research has deepened our understanding of how the virus replicates, manipulates, and hides in an infected person. Although our understanding of pathogenesis and ...
Aims and scope. AIDS Research and Therapy publishes studies of basic, translational, clinical, epidemiological, behavioral and educational science addressing treatment and prevention of HIV/AIDS and associated conditions. This includes studies of disease pathogenesis, novel treatment strategies and the impacts of established treatment regimans.
Discover original research, comment, and correspondence from The Lancet HIV that unifies clinical, epidemiological, and operational disciplines across a single vision of health for people living with HIV ... Reductions of most major causes of death, particularly AIDS-related deaths among people with HIV on ART, were not seen for all subgroups ...
which publishes Confronting AIDS: Directions for Public Health, Health Care, and Research 1987:˘ First FDA approval of a drug to treat HIV (Retrovir [azidothymidine,
The UN global goal to "end AIDS as a public health threat by 2030"1,2 has motivated remarkable progress in eastern and southern African countries most affected by HIV. In these countries since 2010, new HIV infections have decreased by an estimated 57% and AIDS-related deaths by 58%.3 Targeted 90% reductions between 2010 and 2030 are in reach for some countries in eastern and southern ...
The largest funder of HIV/AIDS research is the National Institutes of Health (NIH), investing nearly $69 billion in AIDS research from fiscal years 1982-2018. Despite the staggering disease burden, the scientific advances directly resulting from investments in AIDS research have been extraordinary. HIV is one of the most intensively studied ...
In this Review, Landovitz, Scott and Deeks explore the current state of the art for HIV prevention and treatment, including unmet needs and emerging tools. They describe how researchers are ...
Human immunodeficiency virus (HIV) is an infection that attacks the body's immune system. Acquired immunodeficiency syndrome (AIDS) is the most advanced stage of the disease. HIV targets the body's white blood cells, weakening the immune system. This makes it easier to get sick with diseases like tuberculosis, infections and some cancers.
Advances in HIV/AIDS Research. HIV virions budding and releasing from an infected cell. NIAID, NIH. For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV.
HIV/AIDS is one of the most studied infection diseases with more than 260,000 papers (mentioning the topic) listed in GOPubMed and more than 42,000 papers (mentioning HIV/AIDS in the title) in the Web of Science spanning over thirty year of scientific research. HIV/AIDS is studied by a plurality of biomedical disciplines like epidemiology ...
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but ...
HIV/AIDS has attracted considerable research attention since the 1980s. In the current context of globalization and the predominance of cooperative work, it is crucial to analyze the participation of the countries and regions where the infection is most prevalent. This study assesses the participation of African countries in publications on the topic, as well as the degree of equity or ...
AIDS Research and Treatment publishes original research articles and review articles focused on all aspects of HIV and AIDS, from the molecular basis of the disease to translational and clinical research, prevention and education.
Information and data on HIV in the United States. Fast facts. HIV affects some groups more than others. Social and structural issues—such as HIV stigma, homophobia, discrimination, poverty, and limited access to high-quality health care—influence health outcomes and continue to drive inequities.
From the identification of HIV as the agent that causes AIDS, to the development of effective antiretroviral drugs, the scientific achievements in HIV research in the past 20 years have been ...
Despite the decreased incidence of the human immunodeficiency virus (HIV) in Tanzania, the number of adolescents living with HIV is increasing. This study aimed to describe factors independently associated with viral load non-suppression among adolescents living with HIV (ALHIV) on ART in the Tanga region. We conducted a retrospective study of routinely collected data from ALHIV on ART from ...
The human immunodeficiency virus (HIV) was first identified in 1983 and has since claimed approximately 40.4 million lives worldwide (as of 2022). This number is staggering, and if left unchecked, HIV could become a global health crisis. However, the research, development, and widespread availability of highly active antiretroviral therapies (ARTs) have helped contain the HIV pandemic ...
Men living with HIV (MLWH) in sub-Saharan Africa experience poor health outcomes and increased AIDS-related deaths due to stigma influencing testing and treatment uptake and adherence. PRISMA 2020 was used to report a meta-synthesis of the stigma experiences of MLWH in SSA. With the help of an expert librarian, a search of six databases was formulated and performed to examine the available ...
While a regular vaccine targets about 1 in 1,000 B cells, a vaccine trying to activate a broad HIV response would have to hit cells as rare as 1 in 1 million or more, said Shane Crotty, a ...
In China, non-nucleoside reverse transcriptase inhibitors (NNRTIs) are integral to the antiretroviral therapy (ART) regimen for HIV/AIDS patients, comprising over 80% of such treatments. To broaden treatment options and improve therapeutic effectiveness, Ainuovirine (ANV), a new NNRTI, was authorized for ART in 2021. Nevertheless, the clinical efficacy of ANV and its impact on blood ...
Blockbuster obesity drug leads to better health in people with HIV. Semaglutide reduces weight and fat accumulation associated with the antiretroviral regimen that keeps HIV at bay. Mariana ...
More than 50% of those living with HIV in the United States are older than 50 years of age, and as a result of the virus and related long-term treatment, many experience early onset of age-related conditions such as cardiovascular disease, cancer, metabolic disorders, kidney disease, and cognitive issues. NIA has long been committed to research ...