Advanced Organic Chemistry: Infrared spectrum of cyclohexene cyclo C 6 H 10
Interpreting the i nfrared spectrum of cyclohexene
email doc brown Re-edit cyclo C 6 H 10
Links associated with cyclohexene
This is a BIG chemistry website, PLEASE take time to explore it
Infrared spectroscopy - spectra index
Introductory note on the infrared spectrum of cyclohexene
Students and teachers please note my explanation of the infrared spectrum of cyclohexene is designed for advanced, but pre-university, chemistry courses . Based in the infrared spectrum diagram for cyclohexene, only some of the most prominent peaks for particular bond vibrations are discussed, particularly if cyclohexene has a functional group with a particular characteristic wavenumber peak. The infrared spectrum of cyclohexene is unique and the whole, or selected wavenumbers, can be used to fingerprint its identity, sometimes analysing a mixture containing cyclohexene or following its change of concentration in a reaction.
Spectra obtained from a liquid film of cyclohexene. The right-hand part of the of the infrared spectrum of cyclohexene, wavenumbers ~1500 to 400 cm -1 is considered the fingerprint region for the identification of cyclohexene and most organic compounds. It is due to a unique set of complex overlapping vibrations of the atoms of the molecule of cyclohexene.
aliphatic cyclohexene , , ,
Interpretation of the infrared spectrum of cyclohexene The most prominent infrared absorption lines of cyclohexene Multiple C-H stretching vibration absorption lines peaking at ~3100 to 2950 cm -1 . The very characteristic absorption peaking at ~1640 cm -1 for C=C stretching vibrations, typical of an aliphatic alkene molecule. (I have seen the 1440 cm -1 absorption peak attributed to C=C vibrations, but this does not fit in with most spectral data of aliphatic alkenes that I've come across which attributes this to CH 2 bending vibrations). Most of the peaks in the fingerprint region are due to C-H vibrations of some origin e.g. from alkyl CH 2 , C-H and =C-H groupings.
The absence of other specific functional group bands will show that a particular functional group is absent from the cyclohexene molecular structure.
Key words & phrases: isomer of molecular formula C6H10 image and diagram explaining the infrared spectrum of cyclohexene, complete infrared absorption spectrum of cyclohexene, comparative spectra of cyclohexene, prominent peaks/troughs for identifying functional groups in the infrared spectrum of cyclohexene, important wavenumber values in cm-1 for peaks/troughs in the infrared spectrum of cyclohexene, revision of infrared spectroscopy of cyclohexene, fingerprint region analysis of cyclohexene, how to identify cyclohexene from its infrared spectrum, identifying organic compounds like cyclohexene from their infrared spectrum, how to analyse the absorption bands in the infrared spectrum of cyclohexene detection of alkene functional groups in the cyclohexene molecule example of the infrared spectrum of a molecule like cyclohexene with a alkene functional group interpreting interpretation of the infrared spectrum of cyclohexene shows presence of alkene functional group How do you interpret the infrared absorption spectrum of cyclohexene How to interpret the infrared spectrum of cyclohexene Explanatory diagram of the infrared spectrum of the cyclohexene molecule in terms of its molecular structure. Listing data of the prominent main wavenumber peaks troughs in the infrared spectrum of cyclohexene. How to explain the infrared spectrum of cyclohexene. Use of the infrared spectrum of cyclohexene, identification of cyclohexene from its infrared spectrum - fingerprint wavenumber pattern to identify the cyclohexene molecule. The uses of the infrared spectrum of the cyclohexene molecule. The distinctive features of the infrared spectrum of the cyclohexene molecule explained. explaining the peaks-trough of the transmittance of the infrared spectrum of cyclohexene what does the infrared spectrum tell you about the structure and properties of the cyclohexene molecule? How do you interpret the infrared absorption spectrum of cyclohexene How to interpret the infrared spectrum of cyclohexene Explanatory diagram of the infrared spectrum of the cyclohexene molecule in terms of its molecular structure. Listing data of the prominent main wavenumber peaks troughs in the infrared spectrum of cyclohexene. How to explain the infrared spectrum of cyclohexene. Use of the infrared spectrum of cyclohexene, identification of cyclohexene from its infrared spectrum - fingerprint wavenumber pattern to identify the cyclohexene molecule. The uses of the infrared spectrum of the cyclohexene molecule. The distinctive features of the infrared spectrum of the cyclohexene molecule explained interpretation diagram explaining the peaks-trough of the transmittance of the infrared spectrum of cyclohexene what does the infrared spectrum tell you about the structure and properties of the cyclohexene molecule? How is infrared spectrum of cyclohexene used to identify cyclohexene?
The mass spectrum of cyclohexene
The H-1 NMR spectrum of cyclohexene
The C-13 NMR spectrum of cyclohexene
The chemistry of ALKENES revision notes INDEX
Infrared spectroscopy index
ALL SPECTROSCOPY INDEXES
Use My Google search site box
Email doc b: [email protected]
TOP OF PAGE and indexes

12.8 Infrared Spectra of Some Common Functional Groups
12.8 • Infrared Spectra of Some Common Functional Groups
As each functional group is discussed in future chapters, the spectroscopic properties of that group will be described. For the present, we’ll point out some distinguishing features of the hydrocarbon functional groups already studied and briefly preview some other common functional groups. We should also point out, however, that in addition to interpreting absorptions that are present in an IR spectrum, it’s also possible to get structural information by noticing which absorptions are not present. If the spectrum of a compound has no absorptions at 3300 and 2150 cm –1 , the compound is not a terminal alkyne; if the spectrum has no absorption near 3400 cm –1 , the compound is not an alcohol; and so on.
The IR spectrum of an alkane is fairly uninformative because no functional groups are present and all absorptions are due to C–H and C–C bonds. Alkane C–H bonds show a strong absorption from 2850 to 2960 cm –1 , and saturated C–C bonds show a number of bands in the 800 to 1300 cm –1 range. Since most organic compounds contain saturated alkane-like portions, most organic compounds have these characteristic IR absorptions. The C–H and C–C bands are clearly visible in the three spectra shown previously in Figure 12.21 .
Alkenes show several characteristic stretching absorptions. Vinylic =C–H bonds absorb from 3020 to 3100 cm –1 , and alkene C═C C═C bonds usually absorb near 1650 cm –1 , although in some cases their peaks can be rather small and difficult to see clearly when the alkene is symmetric, or nearly so. Both absorptions are visible in the 1-hexene spectrum in Figure 12.21 b.
Alkenes have characteristic =C–H out-of-plane bending absorptions in the 700 to 1000 cm –1 range, thereby allowing the substitution pattern on a double bond to be determined ( Figure 12.23 ). For example, monosubstituted alkenes such as 1-hexene show strong characteristic bands at 910 and 990 cm –1 , and 1,1-disubstituted alkenes ( R 2 C═ CH 2 R 2 C═ CH 2 ) have an intense band at 890 cm –1 .
Alkynes show a C≡C C≡C stretching absorption at 2100 to 2260 cm –1 , an absorption that is much more intense for terminal alkynes than for internal alkynes. Terminal alkynes such as 1-hexyne also have a characteristic ≡C–H ≡C–H stretching absorption at 3300 cm –1 ( Figure 12.21 c). This band is diagnostic for terminal alkynes because it is fairly intense and quite sharp.
Aromatic Compounds
Aromatic compounds, such as benzene, have a weak C–H stretching absorption at 3030 cm –1 , just to the left of a typical saturated C–H band. In addition, they have a series of weak absorptions in the 1660 to 2000 cm –1 range and a series of medium-intensity absorptions in the 1450 to 1600 cm –1 region. These latter absorptions are due to complex molecular motions of the entire ring. The C–H out-of-plane bending region for benzene derivatives, between 650 to 1000 cm –1 , gives valuable information about the ring’s substitution pattern, as it does for the substitution pattern of alkenes ( Figure 12.24 ).
The IR spectrum of phenylacetylene, shown in Figure 12.29 at the end of this section, gives an example, clearly showing the following absorbances: ≡C–H ≡C–H stretch at 3300 cm –1 , C–H stretches from the benzene ring at 3000 to 3100 cm –1 , C═C C═C stretches of the benzene ring between 1450 and 1600 cm –1 , and out-of-plane bending of the ring’s C–H groups, indicating monosubstitution at 750 cm –1 .
The O–H functional group of alcohols is easy to spot. Alcohols have a characteristic band in the range 3400 to 3650 cm –1 that is usually broad and intense. Hydrogen bonding between O–H groups is responsible for making the absorbance so broad. If an O–H stretch is present, it’s hard to miss this band or to confuse it with anything else.
Cyclohexanol ( Figure 12.25 ) is a good example.
The N–H functional group of amines is also easy to spot in the IR, with a characteristic absorption in the 3300 to 3500 cm –1 range. Although alcohols absorb in the same range, an N–H absorption band is much sharper and less intense than an O–H band.
Primary amines (R–NH 2 ) have two absorbances—one for the symmetric stretching mode and one for the asymmetric mode ( Figure 12.26 ). Secondary amines (R 2 N–H) only have one N–H stretching absorbance in this region.
Carbonyl Compounds
Carbonyl functional groups are the easiest to identify of all IR absorptions because of their sharp, intense peak in the range 1670 to 1780 cm –1 . Most important, the exact position of absorption within this range can often be used to identify the exact kind of carbonyl functional group—aldehyde, ketone, ester, and so forth.
Saturated aldehydes absorb at 1730 cm –1 ; aldehydes next to either a double bond or an aromatic ring absorb at 1705 cm –1 .
The C–H group attached to the carbonyl is responsible for the characteristic IR absorbance for aldehydes at 2750 and 2850 cm –1 ( Figure 12.27 ). Although these are not very intense, the absorbance at 2750 cm –1 is helpful when trying to distinguish between an aldehyde and a ketone.
Saturated open-chain ketones and six-membered cyclic ketones absorb at 1715 cm –1 . Ring strain stiffens the C═O C═O bond, making five-membered cyclic ketones absorb at 1750 cm –1 and four-membered cyclic ketones absorb at 1780 cm –1 , about 20 to 30 cm –1 lower than the corresponding saturated ketone.
Saturated esters have a C═O C═O absorbance at 1735 cm –1 and two strong absorbances in the 1300 to 1000 cm –1 range from the C–O portion of the functional group. Like other carbonyl functional groups, esters next to either an aromatic ring or a double bond absorb at 1715 cm –1 , about 20 to 30 cm –1 lower than a saturated ester.
Worked Example 12.5
Predicting ir absorptions of compounds.
Where might the following compounds have IR absorptions?
(b) Absorptions: 3300 cm –1 ( ≡C–H ≡C–H ), 2100 to 2260 cm –1 ( C≡C C≡C ), 1735 cm –1 ( C═O C═O ). This molecule has a terminal alkyne triple bond and a saturated ester carbonyl group.
Worked Example 12.6
Identifying functional groups from an ir spectrum.
The IR spectrum of an unknown compound is shown in Figure 12.28 . What functional groups does the compound contain?
The IR spectrum of phenylacetylene is shown in Figure 12.29. What absorption bands can you identify?
Where might the following compound have IR absorptions?
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution-NonCommercial-ShareAlike License and you must attribute OpenStax.
Access for free at https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Authors: John McMurry, Professor Emeritus
- Publisher/website: OpenStax
- Book title: Organic Chemistry
- Publication date: Sep 20, 2023
- Location: Houston, Texas
- Book URL: https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Section URL: https://openstax.org/books/organic-chemistry/pages/12-8-infrared-spectra-of-some-common-functional-groups
© Jan 9, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
JavaScript is required...
Please enable Javascript in order to use PubChem website.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Organic chemistry
Course: organic chemistry > unit 14.
- Introduction to infrared spectroscopy
- Bonds as springs
- Signal characteristics - wavenumber
- IR spectra for hydrocarbons
- Signal characteristics - intensity
- Signal characteristics - shape
- Symmetric and asymmetric stretching
- IR signals for carbonyl compounds
IR spectra practice
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
- Share full article
Advertisement
Supported by
Solar Storm Intensifies, Filling Skies With Northern Lights
Officials warned of potential blackouts or interference with navigation and communication systems this weekend, as well as auroras as far south as Southern California or Texas.

By Katrina Miller and Judson Jones
Katrina Miller reports on space and astronomy and Judson Jones is a meteorologist.
A dramatic blast from the sun set off the highest-level geomagnetic storm in Earth’s atmosphere on Friday that is expected to make the northern lights visible as far south as Florida and Southern California and could interfere with power grids, communications and navigations system.
It is the strongest such storm to reach Earth since Halloween of 2003. That one was strong enough to create power outages in Sweden and damage transformers in South Africa.
The effects could continue through the weekend as a steady stream of emissions from the sun continues to bombard the planet’s magnetic field.
The solar activity is so powerful that the National Oceanic and Atmospheric Administration, which monitors space weather, issued an unusual storm watch for the first time in 19 years, which was then upgraded to a warning. The agency began observing outbursts on the sun’s surface on Wednesday, with at least five heading in the direction of Earth.
“What we’re expecting over the next couple of days should be more significant than what we’ve seen certainly so far,” Mike Bettwy, the operations chief at NOAA’s Space Weather Prediction Center, said at a news conference on Friday morning.
For people in many places, the most visible part of the storm will be the northern lights, known also as auroras. But authorities and companies will also be on the lookout for the event’s effects on infrastructure, like global positioning systems, radio communications and even electrical power.
While the northern lights are most often seen in higher latitudes closer to the North Pole, people in many more parts of the world are already getting a show this weekend that could last through the early part of next week.

As Friday turned to Saturday in Europe, people across the continent described skies hued in a mottling of colors.
Alfredo Carpineti , an astrophysicist, journalist and author in North London, saw them with his husband from the rooftop of their apartment building.
“It is incredible to be able to see the aurora directly from one’s own backyard,” he said. “I was hoping to maybe catch a glimpse of green on the horizon, but it was all across the sky in both green and purple.”
Here’s what you need to know about this weekend’s solar event.
How will the storm affect people on Earth?
A geomagnetic storm watch or warning indicates that space weather may affect critical infrastructure on or orbiting near Earth. It may introduce additional current into systems, which could damage pipelines, railroad tracks and power lines.
According to Joe Llama, an astronomer at Lowell Observatory, communications that rely on high frequency radio waves, such as ham radio and commercial aviation , are most likely to suffer. That means it is unlikely that your cellphone or car radio, which depend on much higher frequency radio waves, will conk out.
Still, it is possible for blackouts to occur. As with any power outage, you can prepare by keeping your devices charged and having access to backup batteries, generators and radio.
The most notable solar storm recorded in history occurred in 1859. Known as the Carrington Event, it lasted for nearly a week, creating aurora that stretched down to Hawaii and Central America and impacting hundreds of thousands of miles of telegraph lines.
But that was technology of the 19th century, used before scientists fully understood how solar activity disrupted Earth’s atmosphere and communication systems.
“That was an extreme level event,” said Shawn Dahl, a forecaster at NOAA’s Space Weather Prediction Center. “We are not anticipating that.”
Unlike tornado watches and warnings, the target audience for NOAA’s announcements is not the public.
“For most people here on planet Earth, they won’t have to do anything,” said Rob Steenburgh, a space scientist at NOAA’s Space Weather Prediction Center.
The goal of the announcements is to give agencies and companies that operate this infrastructure time to put protection measures in place to mitigate any effects.
“If everything is working like it should, the grid will be stable and they’ll be able to go about their daily lives,” Mr. Steenburgh said.

Will I be able to see the northern lights?
It is possible that the northern lights may grace the skies this week over places that don’t usually see them. The best visibility is outside the bright lights of cities.
Clouds or stormy weather could pose a problem in some places. But if the skies are clear, even well south of where the aurora is forecast to take place, snap a picture or record a video with your cellphone. The sensor on the camera is more sensitive to the wavelengths produced by the aurora and may produce an image you can’t see with the naked eye.
Another opportunity could be viewing sunspots during the daytime, if your skies are clear. As always, do not look directly at the sun without protection. But if you still have your eclipse glasses lying around from the April 8 event, you may try to use them to try to spot the cluster of sunspots causing the activity.
How strong is the current geomagnetic storm?
Giant explosions on the surface of the sun, known as coronal mass ejections, send streams of energetic particles into space. But the sun is large, and such outbursts may not cross our planet as it travels around the star. But when these particles create a disturbance in Earth’s magnetic field, it is known as a geomagnetic storm.
NOAA classifies these storms on a “G” scale of 1 to 5, with G1 being minor and G5 being extreme. The most extreme storms can cause widespread blackouts and damage to infrastructure on Earth. Satellites may also have trouble orienting themselves or sending or receiving information during these events.
The current storm is classified as G5, or “extreme.” It is caused by a cluster of sunspots — dark, cool regions on the solar surface — that is about 16 times the diameter of Earth. The cluster is flaring and ejecting material every six to 12 hours.
“We anticipate that we’re going to get one shock after another through the weekend,” said Brent Gordon, chief of the space weather services branch at NOAA’s Space Weather Prediction Center.
Why is this happening now?
The sun’s activity ebbs and flows on an 11-year cycle, and right now, it is approaching a solar maximum. Three other severe geomagnetic storms have been observed so far in the current activity cycle, which began in December 2019, but none were predicted to cause effects strong enough on Earth to warrant a watch or warning announcement.
The cluster of sunspots generating the current storm is the largest seen in this solar cycle, NOAA officials said. They added that the activity in this cycle has outperformed initial predictions .
More flares and expulsions from this cluster are expected, but because of the sun’s rotation the cluster will be oriented in a position less likely to affect Earth. In the coming weeks, the sunspots may appear again on the left side of the sun, but it is difficult for scientists to predict whether this will cause another bout of activity.
“Usually, these don’t come around packing as much of a punch as they did originally,” Mr. Dahl said. “But time will tell on that.”
Jonathan O’Callaghan contributed reporting from London.
An earlier version of this article misstated the radio frequencies used by cellphones and car radios. They are higher frequencies, not low.
How we handle corrections
Katrina Miller is a science reporting fellow for The Times. She recently earned her Ph.D. in particle physics from the University of Chicago. More about Katrina Miller
Judson Jones is a meteorologist and reporter for The Times who forecasts and covers extreme weather. More about Judson Jones
What’s Up in Space and Astronomy
Keep track of things going on in our solar system and all around the universe..
Never miss an eclipse, a meteor shower, a rocket launch or any other 2024 event that’s out of this world with our space and astronomy calendar .
A dramatic blast from the sun set off the highest-level geomagnetic storm in Earth’s atmosphere, making the northern lights visible around the world .
With the help of Google Cloud, scientists who hunt killer asteroids churned through hundreds of thousands of images of the night sky to reveal 27,500 overlooked space rocks in the solar system .
A celestial image, an Impressionistic swirl of color in the center of the Milky Way, represents a first step toward understanding the role of magnetic fields in the cycle of stellar death and rebirth.
Scientists may have discovered a major flaw in their understanding of dark energy, a mysterious cosmic force . That could be good news for the fate of the universe.
Is Pluto a planet? And what is a planet, anyway? Test your knowledge here .

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

11.5: Infrared Spectra of Some Common Functional Groups
- Last updated
- Save as PDF
- Page ID 45261
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Common Group Frequencies Summary
When analyzing an IR spectrum, it is helpful to overlay the diagram below onto the spectrum with our mind to help recognize functional groups.
The region of the infrared spectrum from 1200 to 700 cm -1 is called the fingerprint region. This region is notable for the large number of infrared bands that are found there. Many different vibrations, including C-O, C-C and C-N single bond stretches, C-H bending vibrations, and some bands due to benzene rings are found in this region. The fingerprint region is often the most complex and confusing region to interpret, and is usually the last section of a spectrum to be interpreted. However, the utility of the fingerprint region is that the many bands there provide a fingerprint for a molecule.
Group Frequencies - a closer look
Detailed information about the infrared absorptions observed for various bonded atoms and groups is usually presented in tabular form. The following table provides a collection of such data for the most common functional groups. Following the color scheme of the chart, stretching absorptions are listed in the blue-shaded section and bending absorptions in the green shaded part. More detailed descriptions for certain groups (e.g. alkenes, arenes, alcohols, amines & carbonyl compounds) may be viewed by clicking on the functional class name . Since most organic compounds have C-H bonds, a useful rule is that absorption in the 2850 to 3000 cm -1 is due to sp 3 C-H stretching; whereas, absorption above 3000 cm -1 is from sp 2 C-H stretching or sp C-H stretching if it is near 3300 cm -1 .
Recognizing Group Frequencies in IR Spectra - a very close look
Hydrocarbons.
Hydrocarbons compounds contain only C-H and C-C bonds, but there is plenty of information to be obtained from the infrared spectra arising from C-H stretching and C-H bending.
In alkanes, which have very few bands, each band in the spectrum can be assigned:
- C–H stretch from 3000–2850 cm -1
- C–H bend or scissoring from 1470-1450 cm -1
- C–H rock, methyl from 1370-1350 cm -1
- C–H rock, methyl, seen only in long chain alkanes, from 725-720 cm -1
Figure 3. shows the IR spectrum of octane. Since most organic compounds have these features, these C-H vibrations are usually not noted when interpreting a routine IR spectrum. Note that the change in dipole moment with respect to distance for the C-H stretching is greater than that for others shown, which is why the C-H stretch band is the more intense.
.png?revision=1&size=bestfit&width=548&height=282)
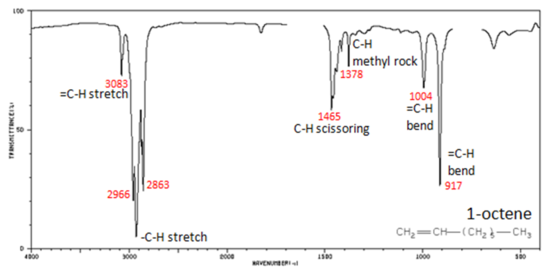
In alkenes compounds, each band in the spectrum can be assigned:
- C=C stretch from 1680-1640 cm -1
- =C–H stretch from 3100-3000 cm -1
- =C–H bend from 1000-650 cm -1
Figure 4. shows the IR spectrum of 1-octene. As alkanes compounds, these bands are not specific and are generally not noted because they are present in almost all organic molecules.
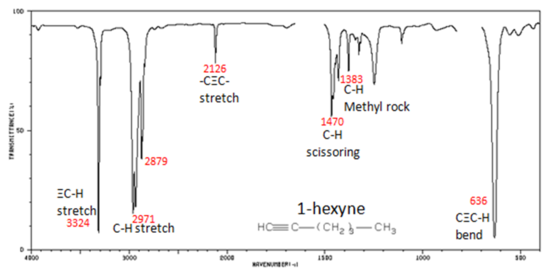
In alkynes, each band in the spectrum can be assigned:
- –C?C– stretch from 2260-2100 cm -1
- –C?C–H: C–H stretch from 3330-3270 cm -1
- –C?C–H: C–H bend from 700-610 cm -1
The spectrum of 1-hexyne, a terminal alkyne, is shown below.
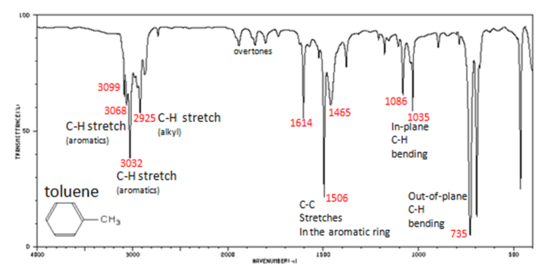
In aromatic compounds, each band in the spectrum can be assigned:
- C–H stretch from 3100-3000 cm -1
- overtones, weak, from 2000-1665 cm -1
- C–C stretch (in-ring) from 1600-1585 cm -1
- C–C stretch (in-ring) from 1500-1400 cm -1
- C–H "oop" from 900-675 cm -1
Note that this is at slightly higher frequency than is the –C–H stretch in alkanes. This is a very useful tool for interpreting IR spectra. Only alkenes and aromatics show a C–H stretch slightly higher than 3000 cm -1 .
Figure 6. shows the spectrum of toluene.
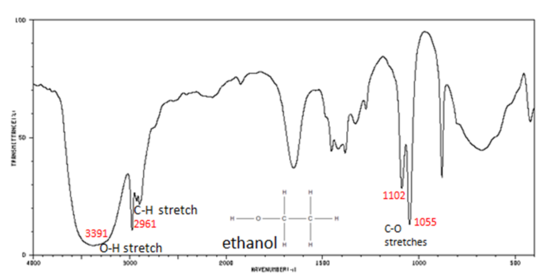
Functional Groups Containing the C-O Bond
Alcohols have IR absorptions associated with both the O-H and the C-O stretching vibrations.
- O–H stretch, hydrogen bonded 3500-3200 cm -1
- C–O stretch 1260-1050 cm -1 (s)
Figure 7. shows the spectrum of ethanol. Note the very broad, strong band of the O–H stretch.
The carbonyl stretching vibration band C=O of saturated aliphatic ketones appears:
- C=O stretch - aliphatic ketones 1715 cm -1
- ?, ?-unsaturated ketones 1685-1666 cm -1
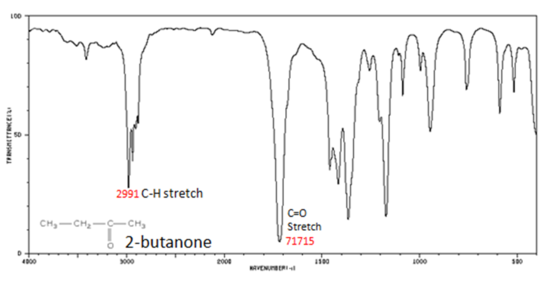
Figure 8. shows the spectrum of 2-butanone. This is a saturated ketone, and the C=O band appears at 1715.
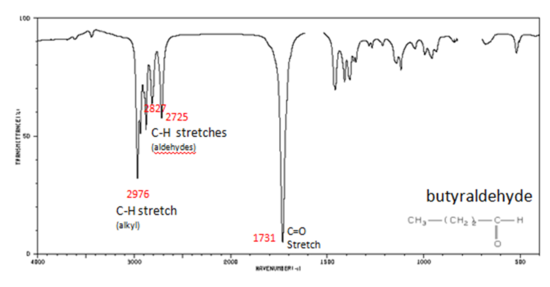
If a compound is suspected to be an aldehyde, a peak always appears around 2720 cm -1 which often appears as a shoulder-type peak just to the right of the alkyl C–H stretches.
- H–C=O stretch 2830-2695 cm -1
- aliphatic aldehydes 1740-1720 cm -1
- alpha, beta-unsaturated aldehydes 1710-1685 cm -1
Figure 9. shows the spectrum of butyraldehyde.
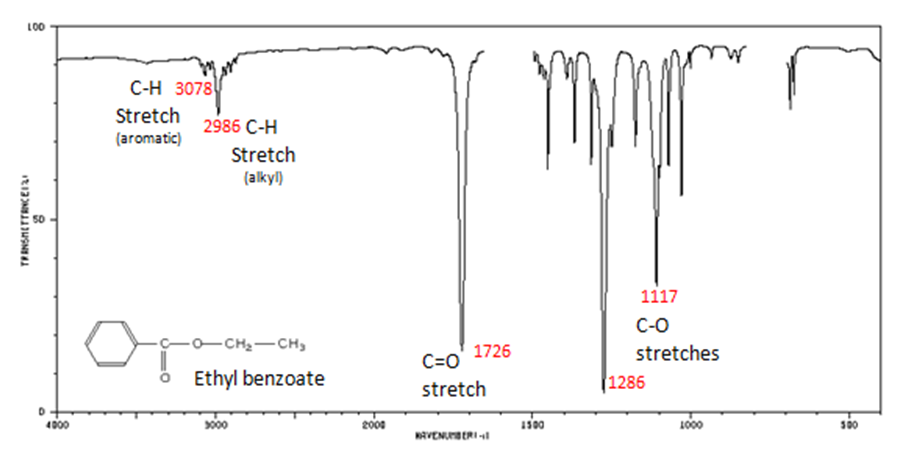
The carbonyl stretch C=O of esters appears:
- aliphatic from 1750-1735 cm -1
- ?, ?-unsaturated from 1730-1715 cm -1
- C–O stretch from 1300-1000 cm -1
Figure 10. shows the spectrum of ethyl benzoate.
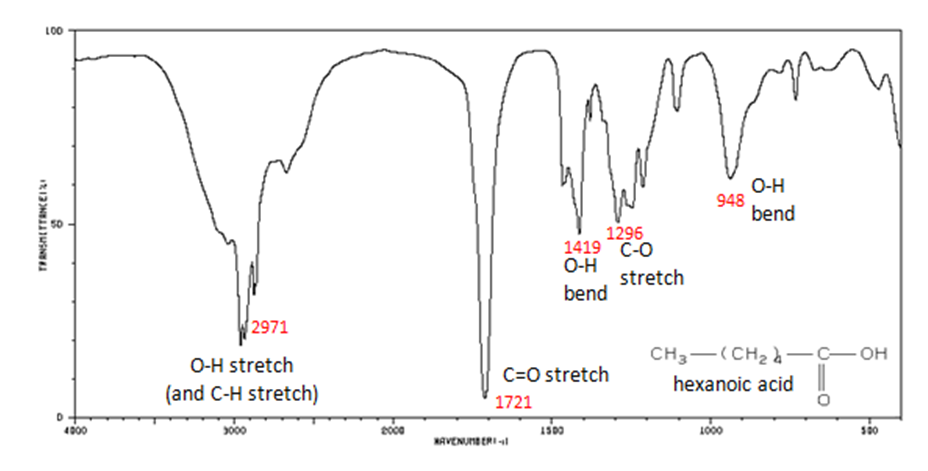
The carbonyl stretch C=O of a carboxylic acid appears as an intense band from 1760-1690 cm -1 . The exact position of this broad band depends on whether the carboxylic acid is saturated or unsaturated, dimerized, or has internal hydrogen bonding.
- O–H stretch from 3300-2500 cm -1
- C=O stretch from 1760-1690 cm -1
- C–O stretch from 1320-1210 cm -1
- O–H bend from 1440-1395 and 950-910 cm -1
Figure 11. shows the spectrum of hexanoic acid.
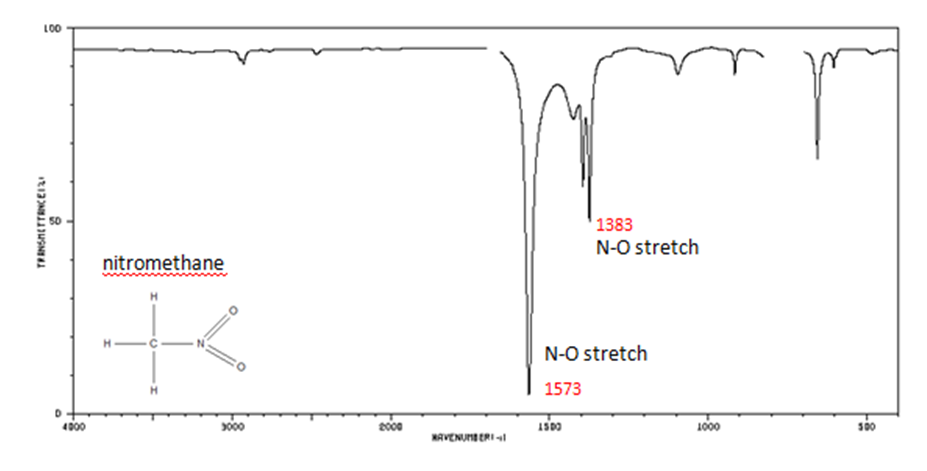
Organic Nitrogen Compounds
- N–O asymmetric stretch from 1550-1475 cm -1
- N–O symmetric stretch from 1360-1290 cm -1
Organic Compounds Containing Halogens
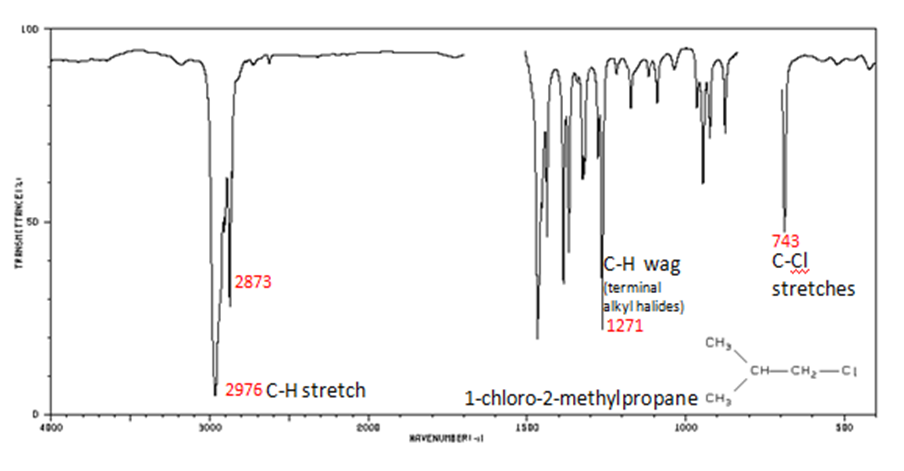
Alkyl halides are compounds that have a C–X bond, where X is a halogen: bromine, chlorine, fluorene, or iodine.
- C–H wag (-CH 2 X) from 1300-1150 cm -1
- C–Cl stretch 850-550 cm -1
- C–Br stretch 690-515 cm -1
The spectrum of 1-chloro-2-methylpropane are shown below.
For more Infrared spectra Spectral database of organic molecules is introduced to use free database. Also, the infrared spectroscopy correlation table is linked on bottom of page to find other assigned IR peaks.
1. What functional groups give the following signals in an IR spectrum?
A) 1700 cm -1
B) 1550 cm -1
C) 1700 cm -1 and 2510-3000 cm -1
2. How can you distinguish the following pairs of compounds through IR analysis?
A) CH 3 OH (Methanol) and CH 3 CH 2 OCH 2 CH 3 (Diethylether)
B) Cyclopentane and 1-pentene.
3. The following spectra is for the accompanying compound. What are the peaks that you can I identify in the spectrum?
Source: SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology, 2 December 2016)
4. What absorptions would the following compounds have in an IR spectra?
A) A OH peak will be present around 3300 cm -1 for methanol and will be absent in the ether.
B) 1-pentene will have a alkene peak around 1650 cm -1 for the C=C and there will be another peak around 3100 cm -1 for the sp 2 C-H group on the alkene
C) Cannot distinguish these two isomers. They both have the same functional groups and therefore would have the same peaks on an IR spectra.
Frequency (cm-1) Functional Group
3200 C≡C-H
2900-3000 C-C-H, C=C-H
2100 C≡C
(There is also an aromatic undertone region between 2000-1600 which describes the substitution on the phenyl ring.)
3300 (broad) O-H
2000-1800 Aromatic Overtones
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University )
Prof. Steven Farmer ( Sonoma State University )
William Reusch, Professor Emeritus ( Michigan State U. ), Virtual Textbook of Organic Chemistry
Jump to content
NIST Chemistry WebBook , SRD 69
- IUPAC identifier
- More options
- SRD Program
- Science Data Portal
- Office of Data and Informatics
- More documentation
Cyclohexane
- Formula : C 6 H 12
- Molecular weight : 84.1595

- IUPAC Standard InChIKey: XDTMQSROBMDMFD-UHFFFAOYSA-N Copy
- CAS Registry Number: 110-82-7
- Cyclohexane, d12
- Cyclohexane-d12
- Cyclohexane-1,2,3-d6
- Hephane-d16
- Other names: Benzene, hexahydro-; Hexahydrobenzene; Hexamethylene; Hexanaphthene; Cicloesano; Cykloheksan; Rcra waste number U056; UN 1145; NSC 406835
- Permanent link for this species. Use this link for bookmarking this species for future reference.
Infrared Spectrum
- Gas phase thermochemistry data
- Condensed phase thermochemistry data
- Phase change data
- Reaction thermochemistry data
- Henry's Law data
- Gas phase ion energetics data
- Ion clustering data
- IR Spectrum
- Mass spectrum (electron ionization)
- UV/Visible spectrum
- Vibrational and/or electronic energy levels
- Gas Chromatography
- Fluid Properties
- Computational Chemistry Comparison and Benchmark Database
- Gas Phase Kinetics Database
- Switch to calorie-based units
Data at NIST subscription sites:
- NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data)
- NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data)
NIST subscription sites provide data under the NIST Standard Reference Data Program , but require an annual fee to access. The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Go To: Top , References , Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: Coblentz Society, Inc.
Condensed Phase Spectrum
Notice: This spectrum may be better viewed with a Javascript and HTML 5 enabled browser.
- Help / Software credits
The interactive spectrum display requires a browser with JavaScript and HTML 5 canvas support.
Select a region with data to zoom. Select a region with no data or click the mouse on the plot to revert to the orginal display.
The following components were used in generating the plot:
- Resize (distributed with Flot)
- Selection (distributed with Flot)
- Axis labels
- Labels ( Modified by NIST for use in this application )
Additonal code used was developed at NIST: jcamp-dx.js and jcamp-plot.js .
Use or mention of technologies or programs in this web site is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that these items are necessarily the best available for the purpose.
Notice: Except where noted, spectra from this collection were measured on dispersive instruments, often in carefully selected solvents, and hence may differ in detail from measurements on FTIR instruments or in other chemical environments. More information on the manner in which spectra in this collection were collected can be found here.
Notice: Concentration information is not available for this spectrum and, therefore, molar absorptivity values cannot be derived.
Additional Data
View scan of original (hardcopy) spectrum .
View image of digitized spectrum (can be printed in landscape orientation).
View spectrum image in SVG format .
Download spectrum in JCAMP-DX format.
This IR spectrum is from the Coblentz Society's evaluated infrared reference spectra collection .
Go To: Top , Infrared Spectrum , Notes
No reference data available.
Go To: Top , Infrared Spectrum , References
- Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.
- If you believe that this page may contain an error, please fill out the error report form for this page.

IMAGES
VIDEO
COMMENTS
Introductory note on the infrared spectrum of cyclohexene. Students and teachers please note my explanation of the infrared spectrum of cyclohexene is designed for advanced, but pre-university, chemistry courses.. Based in the infrared spectrum diagram for cyclohexene, only some of the most prominent peaks for particular bond vibrations are discussed, particularly if cyclohexene has a ...
1-cyclohexene: State: LIQUID (NEAT) 99.9% PURE: Instrument: BECKMAN IR-12 (GRATING) Instrument parameters: FILTERS AT 303, 473, 790, 1036, 1880, 2930 CM-1. ... This IR spectrum is from the Coblentz Society's evaluated infrared reference spectra collection. References. Go To: Top, Infrared Spectrum, Notes.
IR Spectrum. Go To: Top, Condensed phase thermochemistry data, Henry's Law data, Mass spectrum (electron ionization), UV/Visible spectrum, Gas Chromatography, References, Notes Data compiled by: Coblentz Society, Inc. GAS (60 mmHg, N2 ADDED, TOTAL PRESSURE 600 mmHg); DOW KBr FOREPRISM-GRATING; DIGITIZED BY COBLENTZ SOCIETY (BATCH II) FROM HARD COPY; 2 cm-1 resolution
This condition can be summarized in equation (2) form as follows: (2) Vibrations that satisfy this equation are said to be infrared active. The H-Cl stretch of hydrogen chloride and the asymmetric stretch of CO 2 are examples of infrared active vibrations. Infrared active vibrations cause the bands seen in an infrared spectrum.
• IR spectrum of cyclohexene (Fig 12.5) 3000. Organic Lecture Series 21 Alkynes • IR spectrum of 1-octyne (Fig 12.6) 3000 Organic Lecture Series 22 ... peaks in this area indicate C=O and this is often the strongest peak in the spectrum. 9The area from 1250 to 1000 cm-1 are the C—O stretches of ethers, esters, acids.
Check the warning message from your browser and/or enable Java applets in. your web browser preferences, or install the Java Runtime Environment from www.java.com. The vibrational mode corresponding to this peak is: Toggle Grid Toggle Coordinates Reverse Plot. spin On/Off bg black/white. spacefill/ball_and_stick wireframe/stick. vibration big ...
The key absorption peak in this spectrum is that from the carbonyl double bond, at 1716 cm-1 (corresponding to a wavelength of 5.86 mm, ... Alkynes have characteristic IR absorbance peaks in the range of 2100-2250 cm-1 due to stretching of the carbon-carbon triple bond, ...
Look at the IR spectra of hexane, 1-hexene, and 1-hexyne in Figure 12.21 to see an example of how IR spectroscopy can be used. Although all three IR spectra contain many peaks, there are characteristic absorptions of C═C C═C and C≡C C≡C functional groups that allow the three compounds to be distinguished.
An infrared spectroscopy correlation table (or table of infrared absorption frequencies) is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups. In physical and analytical chemistry, infrared spectroscopy (IR spectroscopy) is a technique used to identify chemical compounds based on the way infrared radiation is ...
The wavenumber is defined as the reciprocal of wavelength ( Formula 6.3 ), and the wavenumbers of infrared radiation are normally in the range of 4000 cm -1 to 600 cm -1 (approximate corresponds the wavelength range of 2.5 μm to 17 μm of IR radiation). Formula 6.3 Wavenumber. Please note the direction of the horizontal axis (wavenumber) in IR ...
1-cyclohexene: State: LIQUID (60 mmHg DILUTED TO A TOTAL PRESSURE OF 600 mmHg WITH NITROGEN) Instrument: DOW KBr FOREPRISM: Instrument parameters: GRATING BLAZED AT 3.5, 12.0, 20.0 MICRONS AND CHANGED AT 5.0, 7.5, 14.9 MICRONS: Path length: 5 CM: Resolution: 4: Sampling procedure: TRANSMISSION: Data processing: DIGITIZED BY NIST FROM HARD COPY ...
The IR spectrum of an alkane is fairly uninformative because no functional groups are present and all absorptions are due to C-H and C-C bonds. Alkane C-H bonds show a strong absorption from 2850 to 2960 cm -1, and saturated C-C bonds show a number of bands in the 800 to 1300 cm -1 range. Since most organic compounds contain ...
C p,gas (J/mol*K) Temperature (K) Reference Comment; 35.12: 50. Dorofeeva O.V., 1986: Recommended S(298.15 K) value agrees well with experimental one [ Beckett C.W., 1948], however calculated Cp(T) values are about 5 J/mol*K lower than those obtained from experimental measurements [ Montgomery J.B., 1942].To fit calculated Cp(T) values to experiment, [ Beckett C.W., 1948] suggested existence ...
Cyclohexene was qualitatively detected in roadside ambient air samples (2), and air samples taken in the Allegheny Mountain Tunnel of the Pennsylvania Turnpike, 1979 (3), and in roadway samples in Raleigh, NC, May 1983 (4). Cyclohexene was detected at a concentration of 0.19 mg/cu m in the vicinity of an oil fire (5).
1.you are correct, each H that is different and a different length from the C=O will show up as a peak. 2. you would see 4 spikes like the 3 above, they may be smashed together in a broad peak from 2900-3100cm-1 so you may or may not be able to tell there are 4 peaks. Hydrogen can be pretty wild in IR spectra.
Go To: Top, IR Spectrum, References Data from NIST Standard Reference Database 69: NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment.
IR SPECTRUM OF AMIDES. The amide functional group combines the features of amines and ketones because it has both the N-H bond and the C=O bond. Therefore amides show a very strong, somewhat broad band at the left end of the spectrum, in the range between 3100 and 3500 cm-1 for the N-H stretch. At the same time they also show the stake-shaped ...
The CH2 scissoring peak at 1450 cm-1 is also strong and sharp, reflecting the six equivalent CH2 groups in the ring. The CH2 rocking peak at 1375 cm-1 is weaker and broader, due to the coupling with the ring deformation mode. The C-O stretch peak at 1260 cm-1 is medium and broad, indicating a single bond between carbon and oxygen.
Once the mull has been prepared, add a drop to one IR plate (Figure 4.2.4 4.2. 4 ), place the second plate on top of the drop and give it a quarter turn in order to evenly coat the plate surface as seen in Figure 4.2.5 4.2. 5. Place it into the spectrometer and acquire the desired data.
LOS ANGELES, May 14, 2024 (GLOBE NEWSWIRE) - FAT (Fresh. Authentic. Tasty.) Brands Inc. , (NASDAQ: FAT), a leading global franchising company and parent company of 18 iconic brands, is pleased to announce that the operating unit for its Twin Peaks and Smokey Bones restaurant brands has confidentially submitted a registration statement to the Securities and Exchange Commission to become a ...
Officials warned of potential blackouts or interference with navigation and communication systems this weekend, as well as auroras as far south as Southern California or Texas.
B) 1-pentene will have a alkene peak around 1650 cm-1 for the C=C and there will be another peak around 3100 cm-1 for the sp 2 C-H group on the alkene. C) Cannot distinguish these two isomers. They both have the same functional groups and therefore would have the same peaks on an IR spectra. 3. Frequency (cm-1) Functional Group. 3200 C≡C-H
IR Spectrum. Go To: Top, References, Notes Data compiled by: Coblentz Society, Inc. GAS (25 mmHg, N2 ADDED, TOTAL PRESSURE 600 mmHg); PERKIN-ELMER 180; DIGITIZED BY NIST FROM HARD COPY (FROM TWO SEGMENTS); 2 cm-1 resolution; LIQUID (NEAT); DOW KBr FOREPRISM-GRATING; DIGITIZED BY COBLENTZ SOCIETY (BATCH I) FROM HARD COPY; 2 cm-1 resolution; SOLUTION (10% CCl4 FOR 3800-1350, AND 10% CS2 FOR 1350 ...
Notice: Except where noted, spectra from this collection were measured on dispersive instruments, often in carefully selected solvents, and hence may differ in detail from measurements on FTIR instruments or in other chemical environments. More information on the manner in which spectra in this collection were collected can be found here. Notice: Concentration information is not available for ...