
- Cover Crops
- Insectary Plants
- Korean Natural Farming
- Soil Health Management
- Sustainable Pest Management Projects

Banana Pest and Disease Management in the Tropical Pacific: A guidebook for banana growers

Chapter VIII: Tissue Culture of Banana
Tissue culture
Tissue culture is the growth of tissues or cells separate from the organism. This is typically facilitated via the use of a liquid, semi-solid, or solid growth medium, such as broth or agar, in vitro under sterile growing conditions. Banana is typically propagated vegetatively; thus tissue culture as a propagation technique provides a robust means to prepare disease-free planting materials that can provide the first line of defense in developing an integrated disease-management program for banana. Tissue-culture techniques established for banana include shoot and meristem culture, callus culture, somatic embryogenesis, cell suspension, and protoplast cultures. However, commercial tissue-cultured banana seedlings are not always conveniently available. Larger-scale banana farmers may wish to establish a banana tissue-culture facility in-farm to ensure availability of disease-free seedlings for replanting in conjunction with a practice of rogueing (destroying) diseased plants. This book chapter will describe a banana shoot tip culture technique developed by Damasco (2005).
Collection of suckers
- Different stages of banana keikis (peepers, sword, or maiden suckers) about 1–3 ft (40–100 cm) tall that are free of BBTV symptoms can be collected for tissue culture.
- Separate the desired keiki from the main stem without cracking the corm of the keiki. Collect at least two suckers from each plant source, one for micropropagation and the other for a nursery farm for future keiki needs.
- Banana suckers selected are excised to obtain approximately 4 inches (10 cm) of inner pseudostem tissue containing the banana meristem, as described in detail in Fig. 8-2. To ensure the plant is BBTV-free, it is recommended to collect a newly unfolded banana leaf from the keiki and submit it to a plant disease diagnostic laboratory such as the Agriculture Diagnostic Service Center (ADSC) at the College of Tropical of Agriculture and Human Resources (CTAHR) to check for BBTV.
Disinfection of propagule
- Wash the pseudostem collected from the field with running water to remove adhering soil.
- Immerse the excised pseudostem in a container of undiluted household bleach (5.25% NaOCl) for 30–45 minutes.
- Decant the bleach solution and keep the surface-sterilized pseudostem in the container.
Tissue-culture medium for shoot growth
(based on Damasco and Barba’s (1984) recipe.
Inoculation
- Mix the medium according to Table 1. Autoclave medium scalpels, forceps, cutting plates, and Magenta boxes (Fig. 8-4) according to standard autoclaving procedure.
- Work under surface-sterilized laminar flow hood.
- Trim the surface-sterilized banana pseudostem, peeling off the outer leaf sheath that come in contact with the bleach. Transfer to a clean cutting dish and continue cutting until the shoot measures 1×1 cm, with the corm tissue as thin as possible.
- Transfer the shoot tip to a fresh cutting dish and cut the shoot into quarters longitudinally, through the center. Transplant each quarter onto a solid culture medium.
Maintenance of shoot cultures
- Keep shoot cultures in an air-conditioned room under a 16-hour photoperiod 40 µE/m 2 S -1 (provided by two 40-watt fluorescent tubes).
- Observe the cultures for contamination. Discard contaminated cultures as soon as contamination is noted.
- Observe for browning and bulging of corm tissue, greening of leaf tissues, and growth of new shoots during the first month of culture.
- When shoots coming out from the apex of the leaf axis are almost 2 cm tall, the shoot tips are ready for subculture.
Proliferation of shoots (subculture)
- Transfer the shoot or sections of shoot to fresh culture medium in vitro whenever the propagules are about 2 cm tall. Overgrown shoots are less proliferative. If shoots are beyond 2 cm, make a longitudinal cut through the apex of the growing shoot.
- Subculture onto half-strength MS medium supplemented with 5 mg/l BAP and 100 ml/l coconut water. This medium, without auxins, is used to avoid early forming of nubbins at high frequencies. All subculturing needs to be conducted in sterile conditions.
- Subculture about 3–4 weeks until desired number of shoots is obtained.
- Record number of proliferated shoots.
- Repeat the subculture for no more than 5 cycles. A higher number of subculturing cycles will lead to off-type banana mutations such as dwarfism, elongation, or other abnormalities.
- When sufficient shoots have proliferated as nuclear stock, proceed with rooting.
- Prepare rooting medium in Table 2 (Damasco 2005) and use within a week of preparation for best results.
- Let the last cycle of the shoot subculture establish 3–4 week (proliferation period) so as to obtain small plantlets.
- Separate individual shoots from a cluster of shoots and transfer them onto rooting medium.
- Roots will form in 3–4 weeks.
- When the plantlets have 3–4 expanded leaves and are well rooted, they are ready to be planted into soil.
Preparing tissue-cultured banana plantlets for field planting
- Prior to planting tissue-cultured banana plantlets into soil, the seedlings need to be hardened or acclimatized to the external environment. This can be done by transferring them to a liquid medium (without agar), or exposing them to partial sunlight in the tissue-culture vessel under greenhouse conditions for a few days.
- Any agar medium adhering to the tissue-cultured plantlets should be gently washed off, after which they are ready to be planted into potting media in a nursery.
- Choose a potting mix with good moisture-holding and drainage characteristics, for example 2 parts Sunshine Pro mix, 1 part perlite, and 3 parts medium- to coarse-grade vermiculite. Keeping the media moist to maintaining the health of the tissue-cultured seedlings.
- Fertilize with slow-release or liquid fertilizer.
- Place banana seedlings in a partially shaded area (50% shade) for 2 weeks before exposing them to full sunlight.
- Plants should be placed in a BBTV-free and banana aphid-free area. Other aphids, whiteflies, and spider mites are commonly found on banana plants in greenhouses and clustered nurseries, and these should be managed by employing insecticide when populations are high. However, after the plants are transplanted into the field, these pests are typically not problematic.
- The full acclimatization process should take about 2 months, or until seedlings reach about 8 inches or taller, depending on variety, before field planting.
- If using tissue-cultured banana to replace plants in a BBTV-infected field, an aggressive scouting program for BBTV should be in place. This includes inspecting young plants every 5 days, as new leaves unfold every 5 days.
- The length of time to harvest after transplanting tissue-cultured banana depends on the cultivar. In general, ‘Dwarf Apple’ bananas may be harvestable within 9–10 months after transplanting into the field.
Home Gardener’s and Farmer’s Corner
Tissue culture of plants requires a sterile working environment to avoid contamination of the growing medium. Commercial tissue-culture laboratories are generally equipped with laminar flow hoods and autoclaves, and they operate using sterile techniques. Home gardeners can purchase tissue-cultured banana at plant sales if available. Farmers who are interested in propagating tissue-cultured banana but do not have the right facilities to do their own tissue-culture production can contact tissue-culture laboratories that provide these services. For example, Hawaii Agriculture Research Center (HARC) provides micropropagation services upon special order ( http://www.harc-hspa.com/index.php?section=services&page=microprop ).
Web Resources
Sathes, R. 2010. Banana culture. http://www.slideshare.net/sathes32/tissue-culture-techniques-of-banana
Jamale, A.V. 2011. Micropropagation for production of quality banana planting material. http://www.slideshare.net/ajamale7/micro-propagation-of-banana .
Damasco, O.P. 2005. Tissue culture of banana. pp. 59-62. In : F.S. dela Cruz et al. (eds). Towards management of Musa nematodes in Asia and the Pacific. International Plant Genetic Resources Institute (INIBAP), Laguna, Philippines.
Perez, E.A. and C.R.R. Hooks. 2008. Preparing tissue-cultured banana plantlets for field planting. CTAHR Cooperative Extension Service Publication. BIO-8. 3 pp
If you require information in an Alternative format, please contact us at: [email protected]

- Search this journal
- Search all journals
Advertisement

Article Sections
- Materials and Methods
- Results and Discussion
Article Figures
Reversion, rooting and acclimatization of hyperhydric banana ‘Grand Naine’ shoots. ( A ) Normal multiple shoots from the fourth subculture in Murashige and Skoog (MS) medium supplemented with 6-benzylaminopurine (3 mg·L −1 ) and Kinetin (1 mg·L −1 ). ( B ) Hyperhydric shoots obtained from the fourth subculture. ( C ) In vitro reversion and rooting of the hyperhydric shoots in agar (8 g·L −1 )-solidified MS medium supplemented with calcium nitrate (0.5 g·L −1 ) after 3 weeks in culture. ( D ) Reverted plantlets after 4 weeks of acclimatization.
Scanning electron microscopy of stomata in the leaves of banana ‘Grand Naine’ shoots after 3 weeks in culture. ( A and B ) Normal and hyperhydric shoots [fourth subculture in MS medium supplemented with 6-benzylaminopurine (3 mg·L −1 ) and kinetin (1 mg·L −1 )]. ( C ) Reverted shoots [cultured in agar (8 g·L −1 )-solidified MS medium supplemented with calcium nitrate (0.5 g·L −1 )].
Histochemical analysis of reactive oxygen species in the leaves of normal, hyperhydric, and reverted banana ‘Grand Naine’ shoots after 3 weeks in culture. ( A ) Superoxide and ( B ) hydrogen peroxide.
Quantification of reactive oxygen species in the leaves of normal, hyperhydric, and reverted banana ‘Grand Naine’ shoots after 3 weeks in culture. ( A ) Superoxide and ( B ) hydrogen peroxide.
Electrolyte leakage in the leaves of normal, hyperhydric, and reverted banana ‘Grand Naine’ shoots after 3 weeks in culture.
- View raw image
- Download Powerpoint Slide
Related Content
- Previous Article
- Next Article
Micropropagation of Banana: Reversion, Rooting, and Acclimatization of Hyperhydric Shoots
Hyperhydricity is a physiological disorder impacting plant growth and multiplication and acclimatization of regenerated plantlets. We report the use of calcium nitrate for reversion and acclimatization of banana ‘Grand Naine’ hyperhydric shoots cultured on Murashige and Skoog medium containing agar or gellan. Although 100% rooting of hyperhydric shoots occurred at all concentrations of calcium nitrate, only 50% rooting was recorded in the absence of calcium nitrate. Electrolyte leakage decreased significantly in the reverted banana tissues compared with the hyperhydric tissues. Histochemical staining for reactive oxygen species indicated that reverted banana tissues possess lower levels of both hydrogen peroxide (H 2 O 2 ) and superoxide (O 2 - ) than do hyperhydric tissues. Rooting, growth, and survival of the reverted banana plantlets were significantly influenced by calcium nitrate concentrations as well as the type of gelling agent. Reverted banana plantlets in medium containing calcium nitrate (0.5–1 g·L −1 ) were acclimatized with 100% survival in a growing substrate of peatmoss and vermiculite (1:1).
Banana ( Musa spp. AAA; Musaceae) is an economically important fruit crop in tropical and subtropical countries, and it ranks as the largest fruit crop produced worldwide ( FAOSTAT, 2017 ). The plant is vegetatively propagated using corms and suckers, allowing for the spread of diseases. There has been a shift toward cyclic replacement with new plantation because the yield starts to decline after 3 to 5 years and then declines rapidly after 10 to 15 years ( Singh et al., 2011 ). To overcome disease spread through vegetative propagation and to meet the commercial demand, plant tissue culture techniques have been routinely used for propagation. Several reports have reviewed the biotechnological improvements and progress in banana tissue culture, thereby highlighting clonal mass propagation through direct and indirect regeneration pathways ( Deepika et al., 2018 ; Rout et al., 2000 ; Strosse et al., 2004 ). Developing efficient protocols for banana tissue culture is the foundation for producing high-quality and pathogen-free planting materials and reducing production costs.
Hyperhydricity has been described as a physiological disorder of tissue-cultured plants whereby the hyperhydric propagules become translucent due to excessive hydration of tissues and exhibit glassy morphology ( Dewir et al., 2014 ; Kevers et al., 2004 ). The limited aeration and ethylene accumulation and the high relative humidity inside the tissue culture container create an unsuitable environment for plant growth and induce physiological abnormalities such as hyperhydricity ( Chakrabarty et al., 2006 ; Dewir et al., 2005 , 2014 ; Rojas-Martinez et al., 2010 ). Moreover, cyclic subcultures and prolonged exposure to cytokinins, such as 6-benzylaminopurine (BAP) and thidiazuron, induce hyperhydricity ( Dewir et al., 2018 ; Ivanova and Van Staden, 2011 ). As a consequence of the plant response to these in vitro stress conditions, the cell metabolism is altered and the production of reactive oxygen species (ROS) is increased ( Balen et al., 2009 ; Franck et al., 1995 ; Tian et al., 2014 ) due to changes in the activity of antioxidant enzymes in hyperhydric tissues ( Chakrabarty and Datta, 2008 ; Dewir et al., 2006 ; Gao et al., 2017a ). Increasing evidence suggests a close link between oxidation stress and hyperhydricity ( Chakrabarty et al., 2006 ; Dewir et al., 2006 , 2014 ; Tian et al., 2014 ), ultimately resulting in plant malformation and malfunctioning. Several approaches, including modifications to the growth medium and improved aeration in the culture container, have been attempted to alleviate or eradicate hyperhydricity in plant tissue culture ( Dewir et al., 2014 ; Hazarika, 2006 ). Although the majority of studies have focused on the prevention of hyperhydricity, few studies have investigated the reversion of hyperhydric propagules ( Gao et al., 2017a , 2017b ; Hassannejad et al., 2012 ; Reyes-Vera et al., 2008 ; Soundararajan et al., 2017 ).
Hyperhydricity is a common problem in plant tissue culture that hinders growth, multiplication, and acclimatization of regenerated plantlets ( Debergh et al., 1992 ; Pospisilova et al., 2007 ). Losses of up to 60% in cultured shoots or explants have been reported due to hyperhydricity in commercial plant micropropagation ( Piqueras et al., 2002 ; van den Dries et al., 2013 ). Consequently, hyperhydricity can limit the success and efficiency of micropropagation by decreasing the quantity and quality of the tissue-cultured plantlets and increasing the cost of production ( Dewir et al., 2014 ; Gao et al., 2017a ; Hazarika, 2006 ; Kozai et al., 1997 ). In this study, the effects of calcium nitrate on the reversion of hyperhydric banana ‘Grand Naine’, a commercially important dessert banana of the Cavendish subgroup, and the survival and acclimatization of the reverted plantlets under greenhouse conditions were investigated.
Plant material.
Shoot tips of banana ( Musa × paradisiaca L. ‘Grand Naine’) were cyclically subcultured four times (4 weeks per culture cycle) for multiplication on Murashige and Skoog (MS) medium ( Murashige and Skoog, 1962 ) containing 3% sucrose and supplemented with 3 mg·L −1 6-benzylaminopurine (BAP) and 1 mg·L −1 Kinetin ( Fig. 1A ). The medium was gelled with 0.2% gellan (Dephyte, Hannover, Germany). The pH of the medium was adjusted to 5.8 before autoclaving at 121 °C and 118 kPa for 15 min. The cultures were incubated for 4 weeks at 25 ± 1 °C during a 16-h photoperiod at 25 μmol·m −2 ·s −1 photosynthetic photon flux density ( PPFD ) provided by cool white fluorescent tubes. Ten percent of the proliferated shoots showed symptoms of hyperhydricity during the fifth re-culture. These hyperhydric shoots were used as the plant material for the reversion experiments ( Fig. 1B ).

Citation: HortScience horts 54, 8; 10.21273/HORTSCI14036-19
- Download Figure
Reversion of hyperhydric banana shoots.
The hyperhydric shoots were cultured on MS medium supplemented with calcium nitrate [Ca (NO 3 ) 2 ] at different concentrations (0, 0.25, 0.50, 0.75, and 1 g·L −1 ) and 3% (w/v) sucrose. The media were solidified using 0.8% (w/v) agar-agar (Dephyte) or 0.2% (w/v) gellan. There were four replicates per treatment. Each replicate represented a culture of five individual shoots, resulting in a group of 20 shoots per treatment. All other culture conditions were as previously described. After 3 weeks of culture, the following parameters were recorded for each explant: rooting percentage, number of roots, root length (seedlings were washed and the longest root was measured), number of leaves, shoot length, and fresh weight.
Determination of chlorophyll content.
Chlorophyll was extracted overnight at 5 °C with 5 mL of dimethyl-formamide and determined according to the methods of Moran and Porath (1982) using a double-beam ultraviolet/visible spectrophotometer (Libra S80PC; Biochrom, Cambridge, UK) at 663 nm and 647 nm. Chlorophyll concentration is expressed as mg·g −1 fresh weight of leaves.
Microscopic observation of stoma.
For scanning electron microscopy, samples (4 mm 2 ) were obtained from the leaves and fixed in glutaraldehyde (2.5%) for 24 h at 4 °C. Then, they were postfixed in osmium tetraoxide (1% OsO 4 ) for 1 h at room temperature ( Harley and Ferguson, 1990 ). Samples were dehydrated by passing them through increasing concentrations of acetone (30% to 100%). Samples were air-dried until the critical point and sputter-coated with gold. Images were obtained using a JEOL JSM T330A scanning electron microscope (JEOL, Tokyo, Japan).
Histochemical analysis of ROS.
Detection of superoxide (O 2 − ) and hydrogen peroxide (H 2 O 2 ) were visualized as blue coloration of nitroblue tetrazolium (NBT) and red–brown coloration of 3, 3-diaminobenzidine (DAB). Cross and longitudinal leaf discs were vacuum-infiltrated with 10 m m of potassium phosphate buffer (pH 7.8) containing 0.1% (w/v) NBT (Sigma-Aldrich, Steinheim, Germany) according to the methods of Ádám et al. (1989) or 0.1% (w/v) DAB (Fluka, Buchs, Switzerland). NBT-treated and DAB-treated samples were incubated in daylight for 20 min and 2 h, respectively, and subsequently cleared in 0.15% (w/v) trichloroacetic acid in ethanol: chloroform at 4:1 (v/v) for 1 d ( Hückelhoven et al., 1999 ). Cleared samples were washed with water and placed in 50% glycerol before evaluation. Discoloration of stem discs resulting from NBT or DAB staining was quantified using a ChemiImager 4000 digital imaging system (Alpha Innotech Corp., San Leandro, CA).
Electrolyte leakage.
Measurements were performed as described by Szalai et al. (1996) and Whitlow et al. (1992) . Leaf discs of hyperhydric tissues and recovered tissues were placed individually in 25 mL of deionized water (Milli-Q 50; Millipore, Bedford, MA). Flasks were shaken for 20 h at ambient temperature to facilitate electrolyte leakage from injured tissues. Initial electrical conductivity (EC) measurements were recorded for each vial using an Acromet AR20 EC meter (Fisher Scientific, Chicago, IL). Flasks were then immersed in a hot water bath (Fisher Isotemp, Indiana, PA) at 80 °C for 1 h to induce cell rupture. The vials were again placed in an Innova 2100 platform shaker for 20 h at 21 °C. Final conductivity was measured for each flask. The electrolyte leakage percentage was calculated as follows: (initial conductivity/final conductivity) × 100.
Acclimatization.
Banana plantlets at the five-leaf stage were transplanted to culture pots (diameter, 5 cm) filled with a mixture of sterilized peatmoss and perlite (1:2). Each treatment had three replicates, and each replicate was represented by 20 plantlets. The plantlets were covered with a clear plastic film during the first 15 d of culture in a shade-controlled greenhouse and watered with 1 g·L −1 of solution containing 19N–8.3P–15.7K water-soluble fertilizer (Rosasol; Rosier, Moustier, Belgium). The environment of the greenhouse was adjusted to a temperature of 27 ± 2 °C, 60% to 70% relative humidity, and 100 µmol·m −2 ·s −1 PPFD . After 4 weeks of acclimatization, the following parameters were recorded for each plantlet: survival percentage, root length (seedlings were washed and the longest root was measured), shoot length, and fresh weight.
Experimental design and statistical analysis.
All experiments had a completely randomized design. All data were subjected to an analysis of variance and Duncan’s multiple range test using SAS statistical software (version 8.1; SAS Institute, Cary, NC).
Reversion and rooting of hyperhydric banana shoots.
BAP is a commonly used cytokinin for micropropagation of Musa sp. ( Bairu et al., 2008 ; Escalona et al., 2003 ; Hui et al., 2013 ; Vuylsteke, 1989 ). However, in this study, hyperhydricity (10%) was recorded during the fourth subculture ( Fig. 1B ) of ‘Grand Naine’ multiple shoots in MS medium fortified with BAP (3 mg·L −1 ) and Kinetin (1 mg·L −1 ). High BAP concentrations and/or cyclic subcultures on BAP-enriched media have been reported to induce hyperhydricity in Musa sp. (Buah et al., 1999 ; Jafari et al., 2011 ) and other plant species, including Fragaria × ananassa ( Barbosa et al., 2013 ) and Thymus daenensis ( Hassannejad et al., 2012 ). BAP has been associated with the rapid formation of N-glucosides, and its accumulation may enhance severe alterations in in vitro cultures ( Bairu et al., 2007 ; Valero-Aracama et al., 2010 ).
Rooting and growth parameters (root length, number of leaves, shoot length, chlorophyll content, and fresh weight) of the hyperhydric shoots were significantly improved by the addition of calcium nitrate in the culture medium ( Table 1 ; Fig. 1C ). Although 100% rooting occurred at all concentrations of calcium nitrate, only 50% was recorded in the control experiments. The highest values of rooting and growth were obtained on gellan-solidified medium supplemented with 0.75 g·L −1 calcium nitrate. High rooting and growth were also observed in agar-solidified medium supplemented with 0.5 g·L −1 calcium nitrate. The type of gelling agent also significantly affected the number of roots, root length, and number of leaves; however, shoot length, chlorophyll content, and fresh weight were not significantly affected. The interaction effect for the type of gelling agent and calcium nitrate significantly influenced the number of roots and leaves and the chlorophyll content ( Table 1 ). A previous report by Buah et al. (1999) indicated that the type of gelling agent influenced the growth of banana shoots, mainly due to the physical properties of the medium (i.e., water potential). Moreover, the hardness of gellan-solidified medium decreases when calcium is reduced from 80 to 40 mg·L −1 , but it is unaffected in the agar-solidified medium ( Cameron, 2001 ). De Klerk et al. (2017) proposed that chelating compounds excreted by plant tissues liquefy the gellan-solidified medium. Therefore, variations in the growth of banana shoots ( Table 1 ) could be attributed to the enhanced water availability and nutrient uptake in gellan-solidified medium compared with that in agar-solidified medium.
Effects of calcium nitrate on rooting and growth of hyperhydric banana ‘Grand Naine’ shoots after 3 weeks in culture.

Calcium is associated with several attributes, such as membrane structure and function, ion uptake, interactions with growth regulators, and enzymatic activation (via calmodulin) ( Malavolta et al., 1997 ). The structural function of calcium is characterized by its use in the synthesis of new cell wall, particularly the middle lamellae that separate newly divided cells ( Taiz and Zeiger, 2006 ). Calcium deficiency is well-known in the hyperhydric tissues of Dianthus caryophyllus ( Kevers and Gaspar 1986 ) and Petunia hybrida ( Zimmerman et al., 1988 ). Machado et al. (2014) demonstrated that the addition of calcium chloride (1.32 g·L −1 ) to the MS culture medium reduced hyperhydricity in Lavendula angustifolia shoots from 23% and 30% to 6% and 1.3% in the first and second subcultures, respectively. Similar findings in Cydonia oblonga ( Singha et al., 1990 ) and Solanum tuberosum ( Sha et al., 1985 ) indicated that increases in calcium improve plant growth and reduce or eliminate deformities such as hyperhydricity and shoot tip necrosis in cultures.
Supplementation of growth media with calcium nitrate improved the chlorophyll content in the reverted banana shoots ( Table 1 ). A decrease in the intensity of the chlorophyll pigment in the hyperhydric shoots of Fragaria × ananassa ( Barbosa et al., 2013 ), Thymus daenensis ( Hassannejad et al., 2012 ), and Vanilla planifolia ( Sreedhar et al., 2009 ), compared with that in normal shoots has been reported. This decrease in chlorophyll concentration may be due to fewer chloroplasts in the hyperhydric leaves or the damaging effects of hyperhydricity on thylakoid membranes ( Chakrabarty et al., 2006 ; Marschner and Possingham, 1975 ). The malformed nonfunctional stomata is a common abnormality in hyperhydric shoots ( Apóstolo and Llorente, 2000 ; Barbosa et al., 2013 ; Gribble et al., 1996 ; Olmos and Hellin, 1998 ). Our results indicated the presence of widely open deformed stomata in the hyperhydric banana shoots ( Fig. 2B ), thus indicating abnormal functioning of stomata compared with that in normal and reverted shoots ( Fig. 2A and C ). Unlike the typical elliptical-shape cells found in normal and reverted banana shoots, the stomata in hyperhydric shoots are nearly round, with deformed guard cells ( Fig. 2B ). Guard cell deformity could be due to the excessive water absorption leading to turgidity, consequently changing the cell wall structure and elasticity ( Fontes et al., 1999 ).

Histochemical staining for ROS, including O 2 − and H 2 O 2 , were visualized as blue and brown colorations, respectively. NBT or DAB staining and quantification indicated that the recovered banana tissues possess lower levels of both H 2 O 2 and O 2 − compared with those in hyperhydric tissues ( Figs. 3 and 4 ). The excessive water accumulation in plant tissue, which is the most characteristic symptom of hyperhydricity, generates aeration stress that depletes oxygen levels and limits its diffusion in cells. Therefore, it has been proposed that hyperhydric tissues can be under hypoxic stress ( Franck et al., 1998 , 2004 ; Gribble et al., 1996 , 1998 ; Kevers and Gaspar, 1986 ; Kevers et al., 2004 ; Olmos et al., 1997 ). Increased levels of ROS involving the superoxide and hydroxyl free radicals as well as hydrogen peroxide have been observed in hyperhydric tissues of Dianthus chinensis ( Gao et al.,2017a , 2017b ), Malus sp. ( Chakrabarty et al., 2006 ), and Mammillaria gracilis ( Balen et al., 2009 ). Several reports suggested that oxidative stress, an important damaging factor in hyperhydricity induction, may be responsible for many metabolic changes in hyperhydric tissues such as lipid peroxidation and, consequently, membrane injury, protein degradation, enzyme inactivation, and DNA damage ( Chen and Ziv, 2001 ; Dewir, 2005 ; Dewir et al., 2006 ; Franck et al., 1995 , 1998 ; Olmos et al., 1997 ).

Electrolyte leakage was significantly decreased in the reverted banana tissues compared with that in hyperhydric tissues ( Fig. 5 ). Cellular membrane dysfunction due to stress increases permeability and the release of ions, which can be readily measured based on the efflux of electrolytes ( Dewir et al., 2015a , 2015b ). Cell wall properties and composition are considered some of the most important factors affecting the development of anomalous morphology in hyperhydric tissues ( Dewir et al., 2014 ). Different researchers have shown modifications in the cell wall constituents of hyperhydric tissues, mainly cellulose, lignin, and pectins ( Kevers et al., 1987 ; Majada et al., 2000 ; Olmos et al., 1997 ; Saher et al., 2005a , 2005b ) and their mechanical properties ( Kevers et al., 1987 ; Komali et al., 1998 ). Hypolignification has been attributed to the decrease in enzyme activities, as reported for Origanum vulgare ( Andarwulan and Shetty, 1999 ) and Prunus avium ( Phan and Hegedus, 1986 ). Electrolyte leakage has been used to quantify damage to cell membranes in hyperhydric Saintpaulia ionantha ( Dewir et al., 2015b ). Foyer et al. (1994) observed a higher rate of solute leakage in hyperhydric leaves compared with that in the control, indicating marked deterioration of the membrane. Our results indicated that banana shoots cultured on calcium nitrate–perhydric shoots, indicating the protective role of calcium nitrate against oxidative stress.

Acclimatization and survival of the reverted banana plantlets.
Survival and growth of the reverted banana plantlets were significantly influenced by calcium nitrate as well as gelling agents used during in vitro rooting ( Table 2 ). Furthermore, 100% survival was recorded for plantlets reverted in medium containing 0.5 to 1 g·L −1 calcium nitrate regardless of the solidifying agent. The reverted plantlets grown in medium lacking calcium nitrate and solidified with gellan or agar resulted in 43% and 83% survival, respectively. A low calcium nitrate concentration (0.25 g·L −1 ) resulted in 85% and 92% survival of plantlets reverted on gellan and agar, respectively. Therefore, solidifying the MS medium with agar during the reversion of hyperhydric shoots was more efficient than using gellan for the survivability of plantlets. Dehydration and death of hyperhydric plants during the acclimatization stage were mainly due to water loss through epidermal discontinuities and nonfunctional stomata ( Apóstolo and Llorente, 2000 ; Gribble et al., 1996 ; Olmos and Hellin, 1998 ). Hyperhydric Simmondsia chinensis shoots exhibiting malformed nonfunctional stomata fail to survive acclimatization ( Apóstolo and Llorente, 2000 ). Nonfunctional stomata, hypolignification, and epidermal discontinuity resulted in the loss of protection needed for acclimatization. Calcium nitrate proved effective for reversion and acclimatization of ‘Grand Naine’ hyperhydric shoots. All plantlets cultured in a medium containing calcium nitrate (0.5–1 g·L −1 ) were reverted and acclimatized ( Fig. 1D ). Previous studies reported varied percentages of reversion for hyperhydric shoots such as Dianthus chinensis (67% on medium containing 5 mg·L −1 silver nitrate) ( Gao et al., 2017b ) and Atriplex canescens (39.7% on vented Magenta vessels; pore size, 0.22 μm) ( Reyes-Vera et al., 2008 ), indicating that reversion to normal morphology is influenced by the culture conditions and plant genotype.
Effects of calcium nitrate on survival and growth of the reverted banana ‘Grand Naine’ plantlets after 4 weeks of acclimatization in a greenhouse.

We concluded that 58% and 88% of the hyperhydric banana ‘Grand Naine’ shoots cultured in media lacking calcium nitrate and solidified gellan or agar, respectively, were estimated as losses because these shoots failed to either root or survive past the acclimatization stage. Moreover, 100% rooting of hyperhydric shoots occurred at all concentrations of calcium nitrate. Growth and survival of the reverted banana plantlets were significantly influenced by calcium nitrate concentrations as well as the type of gelling agent used. Reverted banana plantlets in medium containing calcium nitrate (0.5–1 g·L −1 ) were acclimatized with 100% survival.
Literature Cited
Ádám, A. , Farkas, T. , Somlyai, G. , Hevesi, M. & Király, Z. 1989 Consequence of O 2 − generation during a bacterially induced hypersensitive reaction in tobacco: Deterioration of membrane lipids Physiol. Mol. Plant Pathol. 34 13 26
- Search Google Scholar
- Export Citation
Andarwulan, N. & Shetty, K. 1999 Influence of acetyl salicylic acid in combination with fish protein hydrolysates on hyperhydricity reduction and phenolic synthesis in oregano ( Origanum v ulgare ) tissue cultures J. Food Biochem. 23 619 635
Apóstolo, N.M. & Llorente, B.E. 2000 Anatomy of normal and hyperhydric leaves and shoots of in vitro grown Simmondsia chinensis (Link) Schn In Vitro Cell. Dev. Biol. Plant 36 243 249
Bairu, M.W. , Stirk, W.A. , Dolezal, K. & Van Staden, J. 2007 Optimizing the micropropagation protocol for the endangered Aloe polyphylla : Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tissue Organ Cult. 90 15 23
Bairu, M.W. , Stirk, W.A. , Dolezal, K. & Van Staden, J. 2008 The role of topolins in micropropagation and somaclonal variation of banana cultivars ‘Williams’ and ‘Grand Naine’ ( Musa spp. AAA) Plant Cell Tissue Organ Cult. 95 373 379
Balen, B. , Tkalec, M. , Pavokovic, D. , Pevalek-Kozlina, B. & Krsnik-Rasol, M. 2009 Growth conditions in in vitro culture can induce oxidative stress in Mammillaria gracilis tissues J. Plant Growth Regul. 28 36 45
Barbosa, L.M.P. , de Paiva Neto, V.B. , Dias, L.L.C. , Festucci-Buselli, R.A. , Alexandre, R.S. , Iarema, L. , Finger, F.L. & Otoni, W.C. 2013 Biochemical and morpho-anatomical analyses of strawberry vitroplants hyperhydric tissues affected by BA and gelling agents Rev. Ceres 60 152 160
Buah, J.N. , Kawamitsu, Y. , Sato, S. & Murayama, S. 1999 Effects of different types and concentrations of gelling agents on the physical and chemical properties of media and the growth of banana ( Musa spp.) in vitro Plant Prod. Sci. 2 138 145
Cameron, S.I. 2001 Use of a prototype gel hardness tester to demonstrate the effect of variable calcium concentration of gel rigidity In Vitro Cell. Dev. Biol. Plant 37 419 424
Chakrabarty, D. & Datta, S.K. 2008 Micropropagation of gerbera: Lipid peroxidation and antioxidant enzymes activities during acclimatization process Acta Physiol. Plant. 30 325 331
Chakrabarty, D. , Park, S.Y. , Ali, M.B. , Shin, K.S. & Paek, K.Y. 2006 Hyperhydricity in apple: Ultrastuctural and physiological aspects Tree Physiol. 26 377 388
Chen, J. & Ziv, M. 2001 The effect of ancymidol on hyperhydricity, regeneration, starch and antioxidant enzymatic activities in liquid-cultured Narcissus Plant Cell Rep. 20 22 27
De Klerk, G.-J.M. , van den Dries, N. & Krens, F.A. 2017 Hyperhydricity: Underlying mechanisms Acta Hort. 1155 269 276
Debergh, P. , Aitkencrhistie, J. , Cohen, D. , Grout, B. , Von Arnold, S. , Zimmerman, R. & Ziv, M. 1992 Reconsideration of the term vitrification as used in micropropagation Plant Cell Tissue Organ Cult. 30 135 140
Deepika, C. , Basanti, B. , Singh, D.J. , Subhash, K. & Anil, P. 2018 An insight into in vitro micropropagation studies for banana- Review Intl. J. Agr. Sci. 10 5346 5349
Dewir, Y.H. 2005 Ornamental Euphorbia and Spathiphyllum : Application of bioreactor system and microponics for large-scale production, in vitro flowering and its physiology. Ph.D. dissertation, Chungbuk National University, South Korea
Dewir, Y.H. , Chakrabarty, D. , Hahn, E.J. & Paek, K.Y. 2005 Reversion of inflorescence in Euphorbia milii and its application to large scale micropropagation in an air-lift bioreactor J. Hort. Sci. Biotechnol. 80 581 587
Dewir, Y.H. , Chakrabarty, D. , Ali, M.B. , Hahn, E.J. & Paek, K.Y. 2006 Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots Environ. Exp. Bot. 58 93 99
Dewir, Y.H. , El-Mahrouk, M.E. , Hafez, Y.M. , Rihan, H.Z. , Sáez, C.A. & Fuller, M.P. 2015a Antioxidative capacity and electrolyte leakage in healthy versus phytoplasma infected tissues of Euphorbia coerulescens and Orbea gigantea J. Plant Physiol. Pathol. 3 1 doi: 10.4172/2329-955X.1000139
Dewir, Y.H. , El-Mahrouk, M.E. , Hafez, Y.M. , Teixeira da Silva, J.A. & Naidoo, Y. 2015b Hyperhydricity in African violet ( Saintpaulia ionantha H. Wendl)-biochemical aspects of normal versus hyperhydric shoots regenerated via direct adventitious shoots formation Propag. Ornam. Plants 15 53 62
Dewir, Y.H. , Nurmansyah, , Naidoo, Y. & Teixeira da Silva, J.A. 2018 Thidiazuron-induced abnormalities in plant tissue cultures Plant Cell Rep 37 1451 1470
Dewir, Y.H. , Indoliya, Y. , Chakrabarty, D. & Paek, K.Y. 2014 Biochemical and physiological aspects of hyperhydricity in liquid culture system, p. 693–703. In: K.Y. Paek, H.N. Murthy, and J.J. Zhong (eds.). Production of biomass and bioactive compounds using bioreactor technology. Springer, Berlin
Escalona, M. , Cejas, I. , Gonzalez-Olmedo, J. , Capote, I. , Roels, S. , Canal, M.J. , Rodriguez, R. , Sandoval, J. & Debergh, P. 2003 The effect of meta-topolin on plantain propagation using a temporary immersion bioreactor InfoMusa 12 28 30
FAOSTAT 2017 < http://www.fao.org/faostat/en/#data/QC >
Fontes, M.A. , Otoni, W.C. , Carolino, S.M.B. , Brommonschenkel, S.H. , Fontes, E.P.B. , Fari, M. & Louro, R.P. 1999 Hyperhydricity in pepper plants regenerative in vitro : Involvement of BiP (Binding Protein) and ultrastructural aspects Plant Cell Rep. 19 81 87
Foyer, C.H. , Lelandais, M. & Kunert, K.J. 1994 Photooxidative stress in shoots Physiol. Plant. 92 696 717
Franck, T. , Kevers, C. & Gaspar, T. 1995 Protective enzymatic systems against activated oxygen species compared in normal and vitrified shoots of Prunus avium L. raised in vitro Plant Growth Regulat. 16 253 256
Franck, T. , Kevers, C. , Penel, C. , Greppin, H. , Hausman, J.F. & Gaspar, T. 1998 Reducing properties, and markers of lipid peroxidation in normal and hyperhydrating shoots of Prunus avium L J. Plant Physiol. 153 339 346
Franck, T. , Kevers, C. , Gaspar, T. , Dommes, J. , Deby, C. , Greimers, R. , Serteyn, D. & Deby-Dupont, G. 2004 Hyperhydricity of Prunus avium shoots cultured on gelrite: A controlled stress response Plant Physiol. Biochem. 42 519 527
Gao, H. , Xu, P. , Li, J. , Ji, H. , An, L. & Xia, X. 2017a AgNO 3 prevents the occurrence of hyperhydricity in Dianthus chinensis L. by enhancing water loss and antioxidant capacity In Vitro Cell. Dev. Biol. Plant 53 561 570
Gao, H. , Xia, X. , An, L. , Xin, X. & Liang, Y. 2017b Reversion of hyperhydricity in pink ( Dianthus chinensis L.) plantlets by AgNO 3 and its associated mechanism during in vitro culture Plant Sci. 254 1 11
Gribble, K. , Sarafis, V. , Nailon, J. , Holford, P. & Uwins, P. 1996 Environmental scanning electron microscopy of the surface of normal and vitrified leaves of Gypsophila paniculata (Babies Breath) cultured in vitro Plant Cell Rep. 15 771 776
Gribble, K. , Tingle, J. , Sarafis, V. , Heaton, A. & Holford, P. 1998 Position of water in vitrified plants visualized by NMR imaging Protoplasma 201 110 114
Harley, M.M. & Ferguson, I.K. 1990 The role of the SEM in pollen morphology and plant systematics, p. 45–68. In: D. Claugher (ed.). Scanning electron microscopy in taxonomy and functional morphology. Sys. Assoc. Spe., Clarendon Press, Oxford
Hassannejad, S. , Bernard, F. , Mirzajani, F. & Gholami, M. 2012 SA improvement of hyperhydricity reversion in Thymus daenensis shoots culture may be associated with polyamines changes Plant Physiol. Biochem. 51 40 46
Hazarika, B.N. 2006 Morpho-physiological disorders in in vitro culture of plants Scientia Hort. 108 105 120
Hückelhoven, R. , Fodor, J. , Preis, C. & Kogel, K.H. 1999 Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation Plant Physiol. 119 1251 1260
Hui, A.V. , Bhatt, A. , Sreeramanan, S. & Keng, C.L. 2013 Establishment of a shoot proliferation protocol for banana (ABB Group) Cv. ‘Pisang Awak’ via temporary immersion system J. Plant Nutr. 36 529 538
Ivanova, M. & Van Staden, J. 2011 Influence of gelling agent and cytokinins on the control of hyperhydricity in Aloe polyphylla Plant Cell Tissue Organ Cult. 104 13 21
Jafari, N. , Othman, R.Y. & Khalid, N. 2011 Effect of benzylaminopurine (BAP) pulsing on in vitro shoot multiplication of Musa acuminata (banana) cv Berangan. Afr. J. Biotechnol. 10 2446 2450
Kevers, C. & Gaspar, T. 1986 Vitrification of carnation in vitro : Changes in water content, intracellular space, air volume and ion levels Physiol. Veg. 24 647 653
Kevers, C. , Prat, R. & Gaspar, T. 1987 Vitrification of carnation in vitro : Changes in cell wall mechanical properties, cellulose and lignin content Plant Growth Regulat. 5 59 66
Kevers, C. , Franck, T. , Strasser, R.J. , Dommes, J. & Gaspar, T. 2004 Hyperhydricity of micropropagated shoots: A typically stress-induced change of physiological state Plant Cell Tissue Organ Cult. 77 181 191
Komali, A.S. , Peleg, M. , Gerhards, C. & Shetty, K. 1998 A study of the cell wall mechanical properties in unhyperhydrated shoots of oregano ( Origanum vulgare ) inoculated with Pseudomonas sp. by load deformation analysis Food Biotechnol. 12 209 220
Kozai, T. , Kubota, C. & Ryoung Jeong, B. 1997 Environmental control for the large-scale production of plants through in vitro techniques Plant Cell Tissue Organ Cult. 51 49 56
Machado, M.P. , da Silva, A.L.L. , Biasi, L.A. , Deschamps, C. , Filho, J.C.B. & Zanette, F. 2014 Influence of calcium content of tissue on hyperhydricity and shoot-tip necrosis of in vitro regenerated shoots of Lavandula angustifolia Mill. Braz. Arch. Biol. Technol. 57 636 643
Majada, J.P. , Tadeo, F. , Fal, M.A. & Sánchez Tamés, R. 2000 Impact of culture vessel ventilation on the anatomy and morphology of micropropagated carnation Plant Cell Tissue Organ Cult. 63 207 214
Malavolta, E. , Vitti, G.C. & de. Oliveira, S.A. 1997 Avaliacao do estado nutricional das plantas: Principios e aplicacoes. 2nd ed. Piracicaba: Associacao Brasileira para Pesquisa da Potassa e do Fosfato
Marschner, H. & Possingham, J.V. 1975 Effects of K and Na on the growth of leaf discs of sugar beet and spinach Z. Pflanzenphysiol. 75 6 16
Moran, R. & Porath, D. 1982 Chlorophyll determination in intact tissues using N,N-Dimethyl formamide Plant Physiol. 69 1370 1381
Murashige, T. & Skoog, F.A. 1962 A revised medium for rapid growth and bioassays with tobacco tissue cultures Physiol. Plant. 15 473 479
Olmos, E. & Hellin, E. 1998 Ultrastructural differences of hyperhydric and normal leaves from regenerated carnation plants Scientia Hort. 75 91 101
Olmos, E. , Piqueras, A. , Martinez-Solano, J.R. & Hellin, E. 1997 The subcellular localization of peroxidase and the implication of oxidative stress in hyperhydrated leaves of regenerated carnation shoots Plant Sci. 130 97 105
Phan, C.T. & Hegedus, P. 1986 Possible metabolic basis for the developmental anomaly observed in in vitro culture, called ‘vitreous plants’ Plant Cell Tissue Organ Cult. 6 83 94
Piqueras, A. , Cortina, M. , Serna, M.D. & Casas, J.L. 2002 Polyamines and hyperhydricity in micropropagated carnation plants Plant Sci. 162 671 678
Pospisilova, J. , Synkova, H. , Haisel, D. & Semoradova, S. 2007 Acclimation of plantlets to ex vitro conditions: Effects of air humidity, irradiance, CO 2 concentration and abscisic acid (a review) Acta Hort. 748 29 38
Reyes-Vera, I. , Potenza, C. & Barrow, J. 2008 Hyperhydricity reversal and clonal propagation of four-wing saltbush ( Atriplex canescens , Chenopodiaceae) cultivated in vitro Austral. J. Bot. 56 358 362
Rojas-Martinez, L. , Visser, R.G.F. & de Klerk, G. 2010 The hyperhydricity syndrome: Waterlogging of plant tissues as a major cause Propag. Ornam. Plants 10 169 175
Rout, G.R. , Samantaray, S. & Das, P. 2000 Biotechnology of the banana: A review of recent progress Plant Biol. 2 512 524
Saher, S. , Piqueras, A. , Hellin, E. & Olmos, E. 2005a Prevention of hyperhydricity in micropropagated carnation shoots by bottom cooling: Implications of oxidative stress Plant Cell Tissue Organ Cult. 81 149 158
Saher, S. , Fernández-García, N. , Piqueras, A. , Hellín, E. & Olmos, E. 2005b Reducing properties, energy efficiency and carbohydrate metabolism in hyperhydric and normal carnation shoots cultured in vitro : A hypoxia stress? Plant Physiol. Biochem. 43 573 582
Sha, L. , McCown, B.H. & Peterson, L.A. 1985 Occurrence and cause of shoot-tip necrosis in shoot cultures J. Amer. Soc. Hort. Sci. 110 631 634
Singh, H.P. , Uma, S. , Selvarajan, R. & Karihaloo, J.L. 2011 Micropropagation for production of quality banana planting material in Asia-Pacific. Asia-Pacific Consortium on Agricultural Biotechnology (APCoAB), New Delhi, India
Singha, S. , Townsend, E.C. & Oberly, G.E. 1990 Relationship between calcium and agar on vitrification and shoot-tip necrosis of quince (Cydonia oblonga Mill.) shoots in vitro Plant Cell Tissue Organ Cult. 23 135 142
Soundararajan, P. , Manivannan, A. , Cho, Y.S. & Jeong, B.R. 2017 Exogenous supplementation of silicon improved the recovery of hyperhydric shoots in Dianthus caryophyllus L. by stabilizing the physiology and protein expression Front. Plant Sci. 8 738
Sreedhar, R.V. , Venkatachalam, L. & Neelwarne, B. 2009 Hyperhydricity-related morphologic and biochemical changes in Vanilla ( Vanilla planifolia ) J. Plant Growth Regul. 28 46 57
Strosse, H. , Houwe, I. , Panis, B. , Jain, S. & Swennen, R. 2004 Banana cell and tissue culture—Review. Banana improvement: Cellular, molecular biology, and induced mutations. Proc. Meeting, Leuven, 24–28 Sept. 2001, 1–12
Szalai, G. , Janda, T. , Paldi, E. & Szigeti, Z. 1996 Role of light in post-chilling symptoms in maize J. Plant Physiol. 148 378 383
Taiz, L. & Zeiger, E. 2006 Plant physiology. 3rd ed. Sinauer Associates Inc., Sunderland, MA
Tian, J. , Jiang, F. & Wu, Z. 2014 The apoplastic oxidative burst as a key factor of hyperhydricity in garlic plantlet in vitro Plant Cell Tissue Organ Cult. 120 571 584
Valero-Aracama, C. , Kane, M. , Wilson, S. & Philman, N. 2010 Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult and easy-to-acclimatize sea oats ( Uniola paniculata L.) genotypes Plant Growth Regulat. 60 43 49
van den Dries, N. , Gianní, S. , Czerednik, A. , Krens, F.A. & De Klerk, G.-J.M. 2013 Flooding of the apoplast is a key factor in the development of hyperhydricity J. Expt. Bot. 64 5221 5230
Vuylsteke, D. 1989 Shoot-tip culture for the propagation, conservation and exchange of Musa germplasm. IBPGR, Rome
Whitlow, T.H. , Bassuk, N.L. , Ranney, T.G. & Reichert, D.L. 1992 An improved method for using electrolyte leakage to assess membrane competence in plant tissues Plant Physiol. 98 198 205
Zimmerman, T.W. , Rogers, S.M.D. & Cobb, B.G. 1988 Physiological differences between normal and vitreous petunias grown in vitro. ASHS/CSHS 1988 Ann Mtg, East Lansing, Mich, Prog & Abstr HortScience 23 780 (abstr.)
Contributor Notes
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group NO (RGP-1438-012), and the Research Support & Services Unit (RSSU) for their technical support.
1 Corresponding author. E-mail: [email protected] or [email protected] .
Headquarters:
1018 Duke Street
Alexandria, VA 22314
Phone : 703.836.4606
Email : [email protected]
© 2018-2024 American Society for Horticultural Science
- [66.249.64.20|185.80.150.64]
- 185.80.150.64
Character limit 500 /500

Horticulture — New Technologies and Applications pp 219–223 Cite as
Tissue Culture Strategies for Banana
- R. Dore Swamy 4 &
- Leela Sahijram 4
Part of the book series: Current Plant Science and Biotechnology in Agriculture ((PSBA,volume 12))
The importance of banana as an international horticultural commodity needs no emphasis. Being a monocotyledonous crop it was thought to be intractable to in vitro techniques. Mohan Ram and Steward in 1964 demonstrated the possibility of raising callus cultures from the fruit tissue of various genomes. In recent years there has been a spurt of activity in developing tissue culture protocols for this crop (Cronauer and Krikorian,1986). However, most of the protocols have been addressed to developing rnicropropagation methods which have now been exploited commercially. This paper describes results of our efforts in developing a multipronged tissue culture based biotechnology for amelioration of this important fruit crop.
- micropropagation
- embryo rescue
- ahoot tip culture
- floral apex culture
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Unable to display preview. Download preview PDF.
Bannerjee N. Vuylsteke D. and De Langhe E.A. (1986). Meristem tip culture of Musa : histomorphoìogical studies of shoot bud proliferation. In: Bajaj Y.P.S. ed. Biotechnology in forestry and agriculture. Vol.1:233–252 Springer-Verlag Berlin F.R.G.
Google Scholar
Cronauer S.J. and Krikorian A.D. (1985). Reinitiation of vegetative growth from aseptically cultured terminal floral apex of banana. Amer.J.Bot. 72:1598–1601.
Article CAS Google Scholar
Dore Swamy and R. Leela Sahijram (1989). Micropropagation of banana from male floral apices cultured in vitro. Scientia Hortic.40:181–188.
Article Google Scholar
Dore Swamy R. Srinivasa Rao N.K. and Chacko E.K. (1983). Tissue culture propagation of banana. Scientia Hortic.18:247–252.
George E.F. and Sherrington P.D. (1984). Plant propagation by tissue culture.Exegetics, Basingstoke, England.
Menendez T. and Shepherd K. (1984). Breeding new bananas. World Crops 27:104–112.
Mohan Ram H.Y. and Steward F.C. (1964). The induction of growth in explanted tissue of the banana fruit. Can.J.Bot.42:1569–1579.
Murashige T. (1974). Plant propagation through tissue cultures. Ann.Rev.Plant Physio1.25:135–166.
Murashige T. and Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol.Flant.15:473–497.
Simmonds N.W. (1986). Bananas. Longman, London, England. 512pp.
Vuylsteke D. and De Langhe E.A. (1985). Feasibility of in vitro propagation of bananas and plantains. Trop.Agric.(Trinidad),62:323–328.
Vuylsteke D. Swennen R. Wilson G.F. and De Langhe E.A. (1988). Phenotypic variation among in vitro propagated plantain (Musa sp.cv.AAB). Scientia Hortic.36:70–88.
Download references
Author information
Authors and affiliations.
Tissue Culture Laboratory, Division of Plant Physiology and Biochemistry, Indian institute of Horticultural Research, Hessaraghatta, Bangalore, 560089, India
R. Dore Swamy & Leela Sahijram
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Indo-American Hybrid Seeds, P.O. Box 7099, Bangalore, 560 070, India
Department of Horticulture, Agricultural University, P.O. Box 30, 6700 AA, Wageningen, The Netherlands
R. L. M. Pierik
Rights and permissions
Reprints and permissions
Copyright information
© 1991 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter.
Swamy, R.D., Sahijram, L. (1991). Tissue Culture Strategies for Banana. In: Prakash, J., Pierik, R.L.M. (eds) Horticulture — New Technologies and Applications. Current Plant Science and Biotechnology in Agriculture, vol 12. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-3176-6_35
Download citation
DOI : https://doi.org/10.1007/978-94-011-3176-6_35
Publisher Name : Springer, Dordrecht
Print ISBN : 978-94-010-5401-0
Online ISBN : 978-94-011-3176-6
eBook Packages : Springer Book Archive
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
ORIGINAL RESEARCH article
Bio-priming of banana tissue culture plantlets with endophytic bacillus velezensis eb1 to improve fusarium wilt resistance.

- 1 Key Laboratory of South Subtropical Fruit Biology and Genetic Resource Utilization, Ministry of Agriculture and Rural Affairs, Guangdong Provincial Key Laboratory of Tropical and Subtropical Fruit Tree Research, Institute of Fruit Tree Research, Guangdong Academy of Agricultural Sciences, Guangzhou, China
- 2 College of Horticulture, Sichuan Agricultural University, Chengdu, China
Tissue culture techniques have been routinely used for banana propagation and offered rapid production of planting materials with favorable genotypes and free of pathogenic microorganisms in the banana industry. Meanwhile, extensive scientific work suggests that micropropagated plantlets are more susceptible to Fusarium oxysporum f. sp. cubense ( Foc ), the deadly strain that causes Fusarium wilt of bananas than conventional planting material due to the loss of indigenous endophytes. In this study, an endophytic bacterium Bacillus velezensis EB1 was isolated and characterized. EB1 shows remarkable in vitro antagonistic activity against Foc with an inhibition rate of 75.43% and induces significant morphological and ultrastructural changes and alterations in the hyphae of Foc . Colony-forming unit (c.f.u.) counting and scanning electron microscopy (SEM) revealed that EB1 could colonize both the surface and inner tissues of banana tissue culture plantlets. Banana tissue culture plantlets of late rooting stage bioprimed with EB1 could efficiently ward off the invasive of Foc . The bio-priming effect could maintain in the acclimatized banana plants and significantly decrease the disease severity of Fusarium wilt and induce strong disease resistance by manipulating plant defense signaling pathways in a pot experiment. Our results provide the adaptability and potential of native endophyte EB1 in protecting plants from pathogens and infer that banana tissue culture plantlets bio-priming with endophytic microbiota could be a promising biological solution in the fight against the Fusarium wilt of banana.
Introduction
As the most important fruit in the world and the major staple crop in more than 130 countries across the tropical belt, banana ( Musa spp.) production contributes significantly to income and food security ( Kema et al., 2021 ). However, the banana industry is under severe threat from Fusarium wilt, the most destructive disease of banana in history whose causal agent is Fusarium oxysporum f. sp. cubense ( Foc ). Foc is composed of different evolutionary lineages and at least 24 vegetative compatibility groups (VCGs). Foc race 1 wiped out the highly susceptible Gros Michel ( Musa AAA) variety in Central America in the mid-twentieth century ( O'Donnell et al., 2009 ; Staver et al., 2020 ). The plague caused by Foc race 1 was mitigated by gradually adopting a resistant cultivar Cavendish ( Musa AAA) as a replacement for Gros Michel ( Dita et al., 2018 ). The recent emergence of Foc tropical race 4 ( Foc TR4), the most destructive and uncontrollable pathogen of banana, to which Cavendish and many other cultivars are highly susceptible, has created havoc on banana production worldwide again ( Dita et al., 2018 ). Ever since it was first reported to destroy the Cavendish-based banana industry in the 1960s in Taiwan, Foc TR4 has expanded across Southeast Asia, the Middle East, Africa, and most recently has been reported in Colombia and is present in 27 countries where thousands of hectares have been affected in the past several years ( Ordonez et al., 2015 ; Galvis, 2019 ). A recent projection by the Food and Agriculture Organization of the United Nations (FAO) estimated that the inexorable spread of Foc TR4 would lead to a 2.0% drop in global output, 240,000 direct jobs loss, and induce a 9.2% rise in the global reference price for bananas by 2028 ( Altendorf, 2019 ).
The management of Fusarium wilt is particularly challenging due to several conspiring factors. First, as a soil-borne fungus, Foc can survive in the soil in the form of chlamydospore for up to 30 years even in the absence of host plants and be dispersed through diverse ways (i.e., infecting plant material, soil, water, and others) ( Cook et al., 2015 ; Dita et al., 2018 ). Second, the only effective measure to manage this disease is stated frequently as planting resistant cultivars, but resistant cultivars might not meet the current market demand and may be overcome by continually emerging pathogens ( Ploetz, 2015 ). The latter cultivated banana is almost exclusively of the Foc TR4 susceptible Cavendish for its export properties, which facilitates the dispersion of the disease worldwide. Third, as a typical vascular wilt disease, Foc can escape from contacting with non-contact fungicides, non-endophytic biological control agents (BCAs), and other control measures once it penetrates the host plant ( Bubici et al., 2019 ). Thus, it is almost impossible to eliminate the disease incidence once the field gets contaminated with Foc . Therefore, highly efficient and sustainable strategies should be implemented to alleviate the influences of Fusarium wilt on susceptible varieties and to improve the durability of available resistant varieties.
In recent years, the use of BCAs for the control of many plant diseases including the Fusarium wilt of banana has gained great interest as an alternative to chemical application. Among the BCAs, Among the BCAs, the pivotal role of endophyte in the health and fitness of their host plants has become evident only in recent years ( Papik et al., 2020 ; Matsumoto et al., 2021 ). Endophytes refer to microbes that colonize internally in different plant tissues and perform mutualistic symbiotic associations with their hosts ( Papik et al., 2020 ). Their unique ecological niches similar to that of vascular wilt pathogens make them better targets for biocontrol agents against wilt disease than their rhizospheric counterparts ( Strobel and Daisy, 2003 ; Eljounaidi et al., 2016 ). As the second microbiological layer of plant defense, endophytes can defend plants from biotic stresses either by showing direct antagonistic activity such as parasitism, antibiosis, and competition or by inducing indirect antagonism effects (induced systemic resistance, ISR) in host plants to an array of phytopathogens ( Dini-Andreote, 2020 ; Dubey et al., 2020 ). Several studies have shown that endophytic microbes may serve as environmentally safe measures to combat Fusarium wilt of banana ( Cao et al., 2005 ; Bubici et al., 2019 ; Gómez-Lama Cabanás et al., 2021 ; Savani et al., 2021 ; Zhang et al., 2022 ). Applications of endophytic Trichoderma asperellum Prr2 ( Thangavelu and Gopi, 2015 ), Pseudomonas aeruginosa ( Yu et al., 2010 ), Pseudomonas sp. UPMP3, and Burkholderia sp. UPMB3 ( Mohd Fishal et al., 2010 ) have reduced the disease incidence of Fusarium wilt in banana significantly under greenhouse and field conditions.
Nowadays, the most common application strategies of endophytes in agricultural systems are adding them directly into the soil and preparing them as seed-coating agents, which are rather inefficient in practice. Thus, it is imperative to explore alternative strategies for endophyte application ( Dubey et al., 2020 ). Even more significant is the fact that, unlike most other seed plants, the propagation of banana is mainly dependent on tissue culture with all microorganisms eliminated during the micropropagation process under strict aseptic conditions. The regenerated plants are, therefore, particularly vulnerable when transferred directly to natural conditions with multiple environmental stresses ( Lian et al., 2009 ; Soumare et al., 2021 ). In this sense, the establishment of beneficial interactions between explants and beneficial microbes to offer protection for young host plantlets against environmental stress in field conditions might represent a valuable approach to efficiently solve those restrictions ( Soumare et al., 2021 ). Unfortunately, only few studies have reported inoculation with endophytes in banana tissue culture plantlets during the rooting or acclimatization stages ( Guez-Romero et al., 2008 ; Lian et al., 2009 ; Kavino and Manoranjitham, 2018 ). As the key components for achieving sustainable agriculture, the interactions between plants, fungi, and endophytes in tissue culture plantlets have not been sufficiently studied. In the present study, a bacterial endophytic strain EB1 was isolated from a healthy banana plant in a wilt-diseased banana field in Dongguan, Guangdong Province, China (23.045315° N, 113.546177° E). We critically aimed to decipher (i) the phylogenetic, genomic, and antagonistic effect of EB1 against Foc by in vitro test and (ii) how EB1 modulates the resistance of banana plants against Foc by using a banana plant–EB1system created by inoculating banana tissue culture plantlets with EB1 at the end of rooting stage. Our study was designed to lend new insights into the sophisticated mechanisms of host plants–endophytes interactions for coping with environmental stresses and to provide potential strategies to control the Fusarium wilt of banana.
Materials and methods
Strain, media, and cultural conditions.
Wild-type Foc TR4 strain II5 (VCG01213) was cultivated on a potato dextrose agar (PDA) plate at 28°C and used in this study. Isolated endophytic bacteria were inoculated in Luria-Bertani (LB) agar (Sangong Co., Ltd., Shanghai, China) plates. Basal Murashige and Skoog (MS) medium was used for tissue culture experiments.
Isolation and selection of endophytic bacteria against Foc TR4 from healthy banana plant
The healthy banana plants used in this study were collected from a wilt-diseased banana field in Dongguan, Guangdong Province, China (23.045315° N, 113.546177° E). Banana plant samples were washed with tap water thoroughly to remove the airborne counterparts and soaked in 75% (v/v) ethanol for 1 min, 0.1% (v/v) NaClO for 15 min, followed by being rinsed 5 times with sterile water to deplete epiphytic microorganisms in aseptic conditions. Ten grams of plant tissue was weighed and ground with 20 ml sterilized distilled water premixed with sterilized quartz sand using a sterilized mortar and pestle for 5 min. Aliquots of 1 ml of the resulting suspension were diluted using a serial dilution method and spread evenly on an LB agar plate and incubated at 28°C for 5 days. All culturable bacterial colonies were purified and selected based on their morphological characteristics. The antagonistic efficacies of these endophytic bacterial isolates were evaluated against Foc TR4 by a dual-culture experiment ( Fan et al., 2019 ). One actively growing agar plug (5 mm diameter) of Foc TR4 was placed on the center of a fresh PDA plate. Then, 10 μL-drop of each isolate from an overnight culture (OD 600 = 1.0) was uniformly inoculated 2.0 cm away from the fungal inoculum. Plates inoculated only with Foc TR4 plug were served as control. Plates were incubated at 28°C for 5 days and recorded with a Canon EOS 77D camera (Canon, Tokyo, Japan) with the same parameters, and the surface area of the mycelia was measured using the Image J software (Image J, NIH, USA). The inhibitory effect was evaluated by calculating the percentage of area inhibition using the following formula: (Sc - St)/Sc × 100, where Sc and St represent the growth area of Foc TR4 in the control and treated plates, respectively. The experiment was repeated 3 times, with 4 replicates each time. Strain EB1 was isolated through the above screening and stored at −80°C with glycerol (50%, v/v). The antifungal efficiency of EB1 against Foc TR4 was further measured by observing the morphology and ultrastructure characteristics of Foc TR4 in the dual-culture experiment by applying a scanning electron microscope (SEM, Hitachi Model S-3400N, Hitachi, Tokyo, Japan) and a transmission electron microscope (TEM, Hitachi HT7700, Hitachi, Tokyo, Japan).
Whole-genome sequencing of EB1
Overnight bacterial cultures of EB1 in LB broth were collected, centrifuged at 3,000 rpm for 15 min, and washed two times with sterile PBS buffer (50 μM, pH = 7.4). Whole-Genome Sequencing of EB1 was performed using a combination of the Oxford Nanopore Technologies (ONT) GridION platform (Oxford Nanopore Technologies Ltd, Oxford, UK) and Illumina MiSeq platform (Illumina MiSeq PE300, Illumina, USA) by Gene Denovo Biotechnology Co. (Guangzhou, China). DNA was extracted from Qiagen's DNeasy UltraClean Microbial Kit (Qiagen GmbH, Hilden, Germany) and its quality and concentrations were determined using a Nanodrop spectrophotometer (NanoDrop, Wilmington, DE, USA) and Qubit Fluorometer (Thermo Fisher Scientific, MA, USA). For ONT sequencing, library preparation was conducted according to the manufacturer's protocol of the SQK-LSK109 sequencing kit (Oxford Nanopore Technologies Ltd., Oxford, UK). For Illumina sequencing, genomic DNA (gDNA) was fragmented and a paired-end library with an average DNA insert size of 300–400 bp was constructed using Illumina TruSeq Nano DNA Library Prep Kit (Illumina). The assembled sequences were deposited in the NCBI (BioProject ID: PRJNA807456). The components of coding genes, noncoding RNA (ncRNA), and functional annotation were analyzed using a range of databases including the non-redundant protein database (Nr), SwissProt, Cluster of Orthologous Groups (COGs), and Kyoto Encyclopedia of Genes and Genomes (KEGG). Gene clusters for the biosynthesis of secondary metabolites were identified by using antiSMASH.
Phylogenetic analysis of EB1
The 16S rDNA sequence of strain EB1 derived from the EB1 genome was aligned with an NCBI 16S ribosomal RNA sequences database by Nucleotide BLAST ( https://blast.ncbi.nlm.nih.gov/Blast.cgi ), and 16S rRNA gene sequences closest to the isolates (98% sequence homology) were recovered for further phylogenetic analysis. Strain EB1 was subjected to phylogenetic analysis using MEGA version 7 (University, Pennsylvania, PA, USA) based on a full-length 16S ribosomal RNA (16S rRNA) sequence, and a phylogenetic tree was constructed using the neighbor-joining method. The reliability of this resulting tree was evaluated by the bootstrap method with 1,000 replications.
Colonization capacity of EB1 on banana tissue culture plantlets
Uniformly grown banana tissue culture plantlets [“Cavendish” banana (AAA) cv. “Brazilian”] of rooting stage were surface sterilized in 75% (v/v) ethanol for 1 min and 0.1% (v/v) NaClO for 15 min, rinsed with sterile water for 5 times, air-dried, and then transferred and grown vertically in tissue culture flasks containing 100 ml of cooled-down MS. Four plantlets were transferred to each flask. For EB1 inoculation, overnight culture of EB1 in LB broth was harvested, centrifuged, and washed in liquid MS twice, and resuspended in MS to a final optical density (OD 600 ) = 0.2. Each flask was inoculated with 0.1 ml of the bacterial suspension (~10 6 colony-forming unit, c.f.u.) or 0.1 ml MS by pipetting to the rhizosphere of banana tissue culture plantlets and cultured at 22°C and 16/8 h light/dark cycle. The colonization and reproduction of EB1 on the plantlets were quantified each day over a period of 7 days. Total c.f.u. values of EB1 were quantified per the programs described previously ( de Zélicourt et al., 2018 ; Berlanga-Clavero et al., 2022 ). Briefly, 1.0 g root and pseudostem tissues of the banana tissue culture plantlets were sampled and gently washed by dipping in the distilled water to remove non-attached bacteria cells at the same time each day (±2 h). Each sample was transferred to a 2-ml microcentrifuge tube with 1 ml PBS buffer, sonicated on ice for 1 min, and vortexed for 10 min, and 100 μl of the resulting suspensions were spread on LB agar plates after a 10-time dilution. The c.f.u. was counted after overnight incubation at 28°C, and the total c.f.u. was normalized per gram of root or pseudostem. To explore the interactions between strain EB1 and Foc in planta , an additional experiment was conducted in banana tissue culture plantlets by pipetting 0.1 ml Foc spore suspension (1 × 10 8 spores/L) or 0.1 ml MS to the rhizosphere of banana tissue culture plantlets after prior inoculation with EB1 for 3 days and cultured at 22°C and 16:8 h light/dark cycle for another 4 days. The experiment was conducted in triplicate, with at least eight plants per treatment. After 7 days of successive culture, SEM was used to observe the distributions of EB1 and the interactions between EB1 and Foc on the banana tissue plantlets.
Biocontrol efficacy of EB1 on banana plantlets
The above banana tissue culture plantlets and symbionts (banana tissue culture plantlets colonized with EB1) after 7 days of successive culture were subjected to hardening for 10 days by transferring into pots with sterilized planting soil (40 × 19 × 15 cm pots, ca. 2.0 kg soil each). Then the biocontrol efficacy of EB1 on banana plantlets was investigated in greenhouse experiments with the acclimatized banana plants ( Supplementary Figure S1 ). Three treatments including EB1 only, TR4 only, EB1+ TR4, and control were applied in the pot. The banana plants were inoculated with or without Foc TR4 isolates at the concentration of 1,000 conidia/g soil, with a temperature ranging from 25 to 35°C. Three plantlets were grown in each pot and at least 20 plantlets were included in each treatment. In addition, due to the lethality of plantlets in the TR4 only, EB1 + TR4 groups, 60 plantlets were employed in each group to ensure sufficient plant material. Plant survival rates were recorded every 10 days, and observations on morphological characters such as plant height (cm) and fresh weight of shoot and root (g) were conducted after 60 days of planting. The disease index of each plantlet was assessed according to the rating scale of 0–4: 0 = no symptom; 1 = some brown spots in the inner rhizome; 2 = < 25% of the inner rhizome show browning; 3 = up to 75% of the inner rhizome show browning; and 4 = entire inner rhizome and pseudostem show dark brown, dead ( Liu S. et al., 2020 ). Harvested banana plant tissues were stored at −80°C pending for further analysis of defense-related enzymes and genes.
RNA extraction and gene expression analysis by RT-qPCR
Total RNA was extracted from the frozen banana plant using SteadyPure Plant RNA Extraction Kit (Accurate Biotechnology Co., Ltd., Hunan, China ) following the manufacturer's instructions. HiScript II One Step qRT-PCR SYBR Green kit (Vazyme Biotech, Nanjing, China) was employed for qRT-PCR assays according to the manufacturer's instructions. First-strand cDNA was prepared by reverse transcription from 1 μg of DNA-free total RNA in a final reaction volume of 20 μl. RT-qPCR was conducted using a QuantStudio 5 Real-Time PCR System (Applied Biosystems, CA, USA) in four replicates. The qTUB gene (banana) was used as a reference for data normalization, and the target genes were amplified using the primer sets listed in Supplementary Table S1 . The relative transcript abundance of each gene was estimated using the 2 −ΔΔ Ct method ( Livak and Schmittgen, 2001 ).
Statistical analyses
All statistical analyses were performed using the SPSS 20.0 statistical software package (SPSS, Chicago, IL, USA). Data were analyzed using Student's t -test and one-way ANOVA test after being verified for normality and homogeneity of variance with Kolmogorov-Smirnov and Levene's tests. Cases with p -values of < 0.05 were considered statistically significant.
EB1 shows strong inhibitory efficiency against Foc TR4
The morphological observation was preliminarily carried out for strain EB1. It was found that the colony of EB1 on LB medium was dry and round with irregular protrusions at the margin, showing the typical characteristics of Bacillus species ( Supplementary Figure S2A ). The cells were short rod-shaped structures and ~1.2–1.6 μm in length, 0.6–0.7 μm in width, as revealed by SEM ( Supplementary Figure S2B ). EB1 showed strong inhibitory activities with the inhibition rates of mycelium growth area 75.43% against Foc TR4 ( Figure 1A ) and other Fusarium pathogens ( Supplementary Figure S3 ) during co-cultivation compared to control. To confirm the antagonistic activity of EB1, the morphological and ultrastructural changes of Foc TR4 after a confrontation with EB1 were scrutinized by SEM ( Figure 1B ) and TEM ( Figure 1C ). The untreated hyphae of Foc TR4 appeared straight, uniform, and well-developed tube-like structure in shape under SEM. Conversely, phenotypes of abnormalities were noted in fungal hyphae co-culture with EB1. Severe forms of abnormalities, including highly deformed, irregular distorted, inflated were observed in fungal hyphae. TEM micrographs of untreated fungal hyphae had a well-defined cell wall (CW), intact plasma membrane (PM), and normal cytoplasm containing an intact nucleus and all organelles. In reverse, noticeably disruptions such as loss of cellular integrity, thickened CW, evident plasmolysis, serious vacuolation, invaginated PM, abnormal architecture of the nucleus and degenerated organelles were observed in EB1 treated hyphae. The results indicated that the morphology and structural integrity of the treated fungal were dramatically affected during co-culture with EB1.
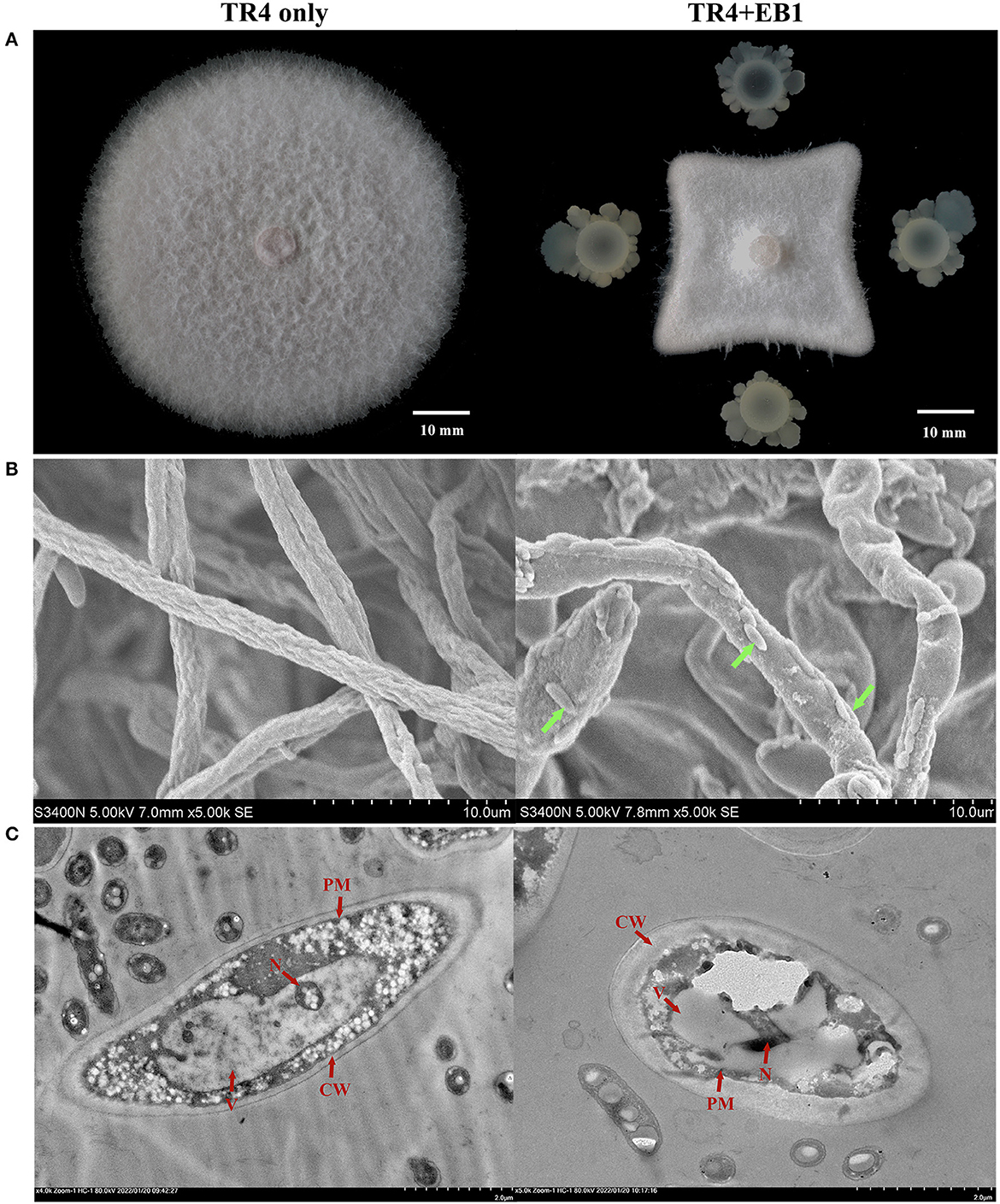
Figure 1 . The antagonistic potential of strain EB1 against Fusarium oxysporum f. sp. cubense tropical race 4 ( Foc TR4). (A) The antagonistic potential of EB1 against TR4 in vitro with a dual-culture experiment. Morphological (marked by green arrow heads) and ultrastructural changes (marked by red arrow heads) of TR4 after the confrontation with EB1 were scrutinized by scanning electron microscopy (SEM) (B) and transmission electron microscope (TEM) (C) , respectively.

Genome sequence assembly and general features of EB1
The whole genome of EB1 was sequenced and analyzed, through which the 16S rRNA region was extracted, and which was 1,404 bp in length. EB1 was identified as Bacillus velezensis based on a phylogenetic tree constructed from the 16S rRNA gene ( Figure 2A ). The complete genome sequence of EB1 was deposited in GenBank under accession number CP093218. Accordingly, the genome of EB1 consists of a single circular chromosome of 3,929,912 bp, with an average of 46.5% GC content and a clear GC skew transition ( Figure 2B ). All predicted 3,622 open reading frames (ORFs) with a maximum E -value of 1.0 E-5 were subjected to annotation analysis by comparing with Nr, SwissProt, COG, and KEGG databases, and a total of 3,606 candidate genes had annotation information. The overall functional annotation is depicted in Supplementary Figure S4 . A total of 2,756 genes were categorized into 21 functional groups using COG analysis ( Figure 2C ). Three main functional gene classes were revealed in the results: amino acid transport and metabolism (329 genes), transcription (267 genes), and carbohydrate transport and metabolism (240 genes), representing 30.33% of the predicted genes in the COG analysis. Other clusters of represented genes involved in inorganic ions transport and metabolism (200 genes), energy production and conversion (188 genes), cell wall/membrane/envelope biogenesis (182 genes), signal transduction (167 genes), and translation, ribosomal structure and biogenesis (161 genes) account for 32.58% of predicted genes. In addition, a high proportion of predicted genes (26.78%) involved in general function prediction only and function unknown is poorly characterized. A total of 2,250 genes were mapped to 5 KEGG branches, including metabolism, genetic information processing, environmental information processing, cellular processes, and organismal systems, and among which, a high proportion of the annotated genes were assigned to metabolism, especially the pathways belonging to global and overview maps (682 genes), carbohydrate metabolism (240 genes), and amino acid metabolism (201 genes) ( Figure 2D ). Twelve biosynthetic gene clusters (BGCs) involved in the biosynthesis of secondary metabolites including non-ribosomal peptides synthase (NRPS), bacteriocin-NRPS, trans-AT polyketide synthase (transatpks), type III polyketides synthase (t3pks), terpene, transatpks-nrps, lantipeptide, and other types of polyketide synthases (OtherKS) were identified in EB1 using AntiSMASH.
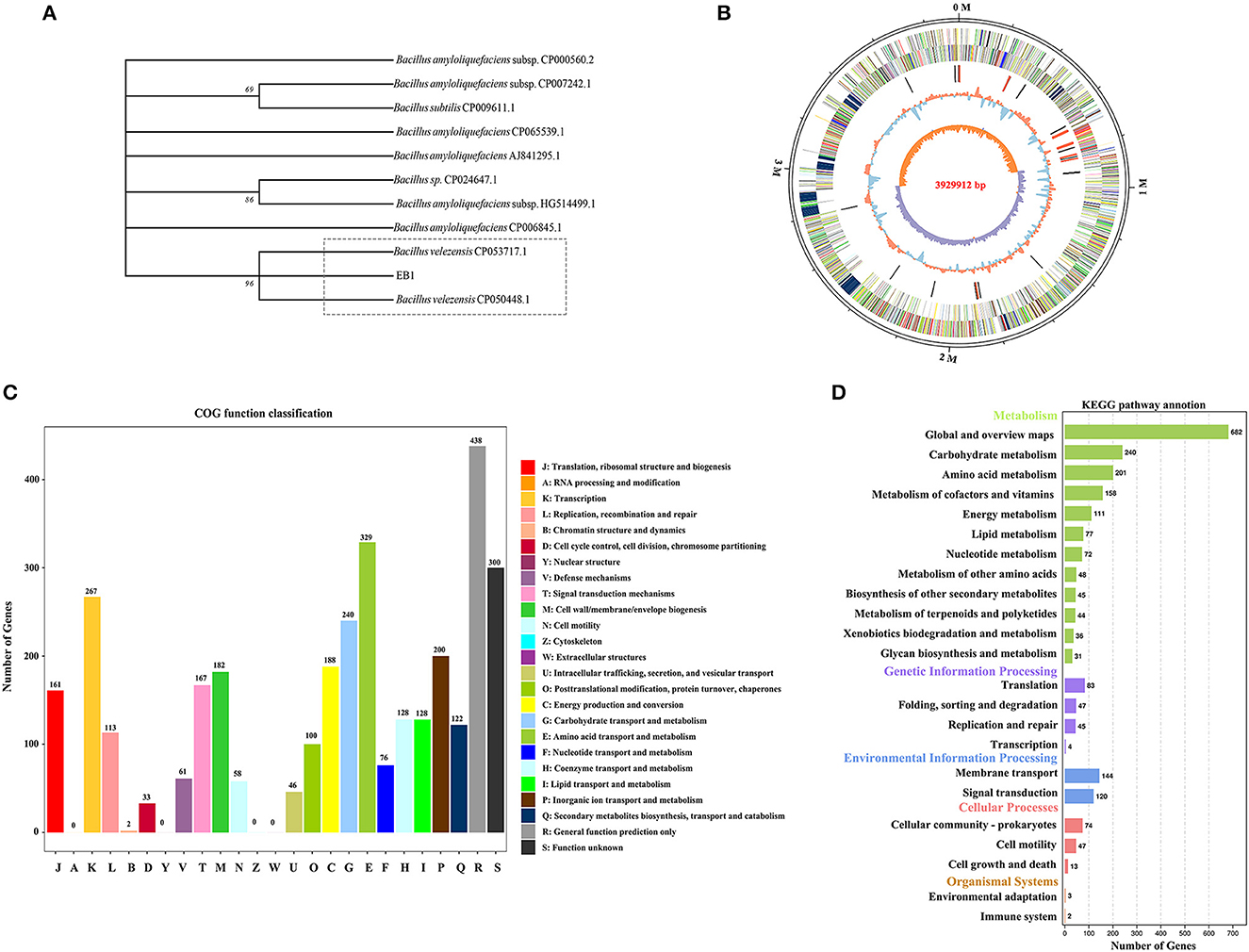
Figure 2 . Phylogenetic and genomic analyses of strain EB1. (A) Phylogenetic trees of strain EB1 based on 16S rRNA gene. The tree was constructed using the MEGA software. The level of bootstrap support (1,000 repetitions) was indicated at all nodes. (B) Graphical circular map of EB1 genome. The distribution of the circle from the outermost to the center is (i) scale marks of the genome; (ii) protein-coding genes on the forward strand; (iii) protein-coding genes on the reverse strand; (iv) tRNA (black) and rRNA (red); (v) GC content; (vi) GC skew. (C) The COG annotation of strain EB1 genome. (D) The KEGG pathway annotation of strainEB1 genome.
EB1 showed strong colonization ability on the banana plantlets
The growth dynamics of the EB1 population during the 7 days after banana tissue plantlets treatment in tissue culture flasks were investigated in the root and shoot by c.f.u. counts and SEM ( Figure 3 ). In the root, the colonization of EB1 numbered at 357 c.f.u./g on day 1 and increased to 1.20 × 10 8 c.f.u./g following an algorithm by day 5 and remained at 1.53 × 10 8 c.f.u./g by day 7. In contrast, in the case of shoot, no EB1 was detected on day 1, after which the colonization level slowly started to increase from 9.73 × 10 3 c.f.u./g on day 2 to 1.96 × 10 7 c.f.u./g by day 7 ( Figure 3A ). Using SEM, no colony could be observed in the root or shoot and the pant epidermal cells were integral and smooth in the control group ( Figure 3B ). In the EB1-inoculated group, small rod-shaped EB1 can be seen in grooves between epidermal cells and intercellular space in root cells ( Figure 3B ). To explore whether EB1 inhibits Foc in planta , an additional experiment was conducted in banana tissue culture plantlets by inoculating with Foc after prior inoculation with EB1 for 7 days. The SEM observation revealed that infection of Foc seriously destroyed the surface structures of the root of banana tissue culture plantlets ( Figure 3C ). However, inoculation of EB1 prior to Foc treatment led to serious morphological deformities of Foc and the damaging effects of pathogen infection were alleviated ( Figure 3C ).
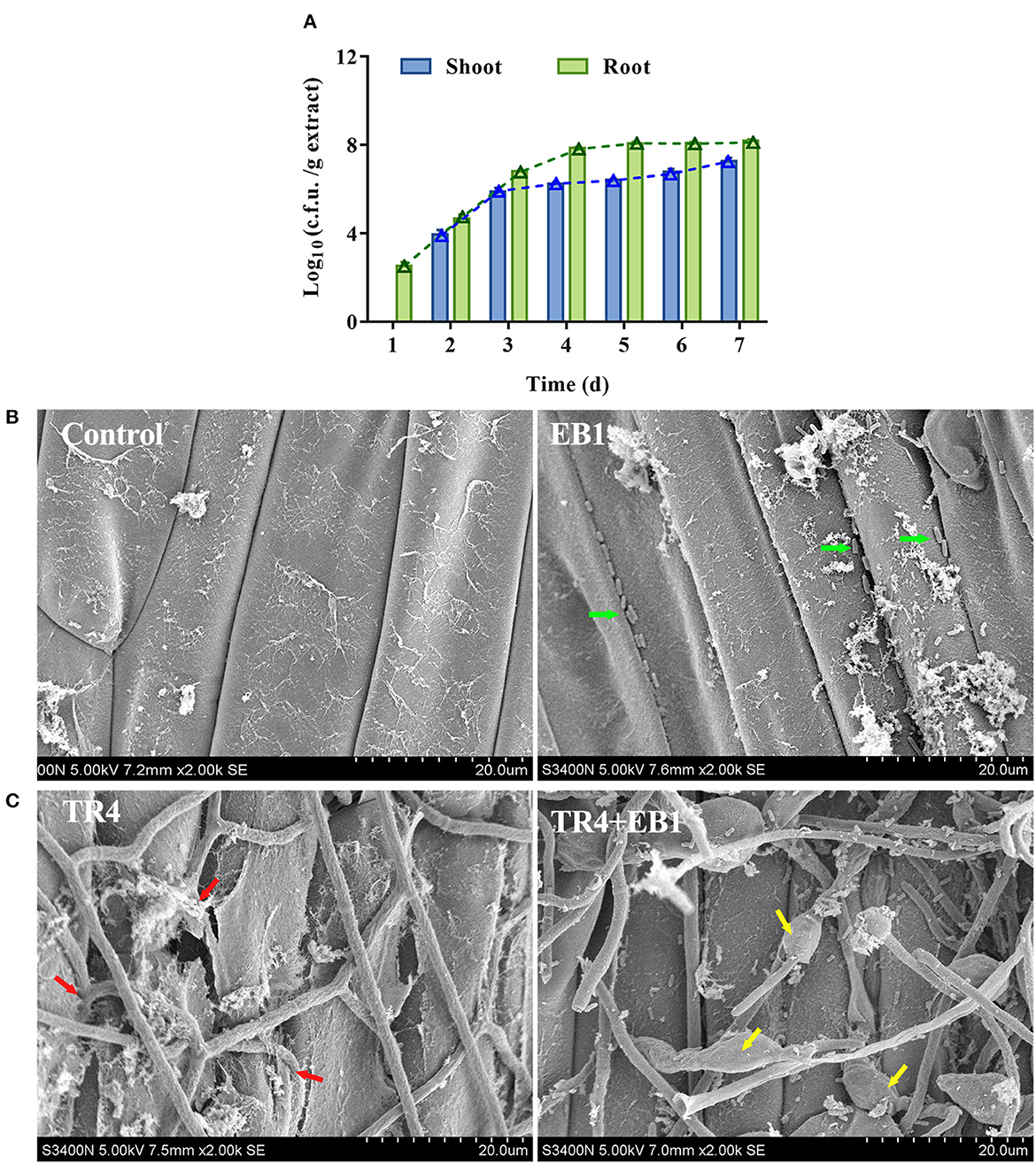
Figure 3 . Strain EB1 showed strong colonization ability on the banana plantlets and provided protection against the infection of TR4. (A) EB1 dynamics (c.f.u. counts) in shoot and root extracts during the first 7 days after banana tissue culture plantlets bacterized. Bars represent average values ± SD of total c.f.u. in shoot and root extracts. Green and blue lines represent c.f.u. corresponding to the number of spores in shoot and root extracts, respectively. (B) Root colonization of strain EB1 visualized by scanning electron microscopy (SEM). Strain EB1 colonies occur in grooves between epidermal cells and intercellular space in root cells (marked by green arrow heads). (C) Strain EB1 inhibits the infection of TR4 in planta . TR4 seriously destroyed the surface structures of the root of banana tissue culture plantlets (marked by red arrow heads) and inoculation of EB1 prior to Foc treatment led to serious morphological deformities of Foc and the damaging effects by pathogen infection were alleviated (marked by yellow arrow heads).
EB1 significantly promoted plant growth and conferred protection against Foc TR4
As EB1 was pre-inoculated on the culture plantlets at the rooting stage, to evaluate whether the acclimatized banana plants have been primed by EB1, the plant growth, survival, and disease severity were inspected in banana plants. Overall, pre-inoculation with EB1 at the rooting stage significantly promoted plant growth and conferred protection against Foc TR4 compared with the non-inoculation groups ( Figure 4 ). No differences were detected in the height of banana plants between the control and EB1 groups. By contrast, the heights of banana plants were significantly reduced when subjected to Foc TR4 infections ( p < 0.001), and this inhibitory effect was dramatically reversed upon co-inoculation with EB1 (TR4 + EB1) ( Figures 4A , B ). Compared with control, EB1 inoculation (EB1) significantly increased the plant biomass in both above-ground (shoot) and below-ground (roots) by 1.22- ( p = 0.002) and 1.49-fold ( p = 0.007), respectively. Plants in the EB1 + TR4 treatment group also had increased biomass compared with those treated by Foc TR4 only (shoot: 1.35-fold, p = 0.002 and root: 1.94-fold, p = 0.004). Moreover, EB1 did not cause any mortality or disease symptoms in banana plants when inoculated alone and greatly enhanced the survival rates and reduced the disease severity caused by Foc TR4 in banana plants who had been pre-inoculated with EB1 prior to inoculation with Foc TR4 (EB1 + TR4) compared with those inoculated only with Foc TR4 ( Figures 4D , E ; Supplementary Figure S5 ). Overall, these findings reveal that EB1 is a bacterial endophyte of banana plants that efficiently suppresses Fusarium wilt caused by Foc TR4.
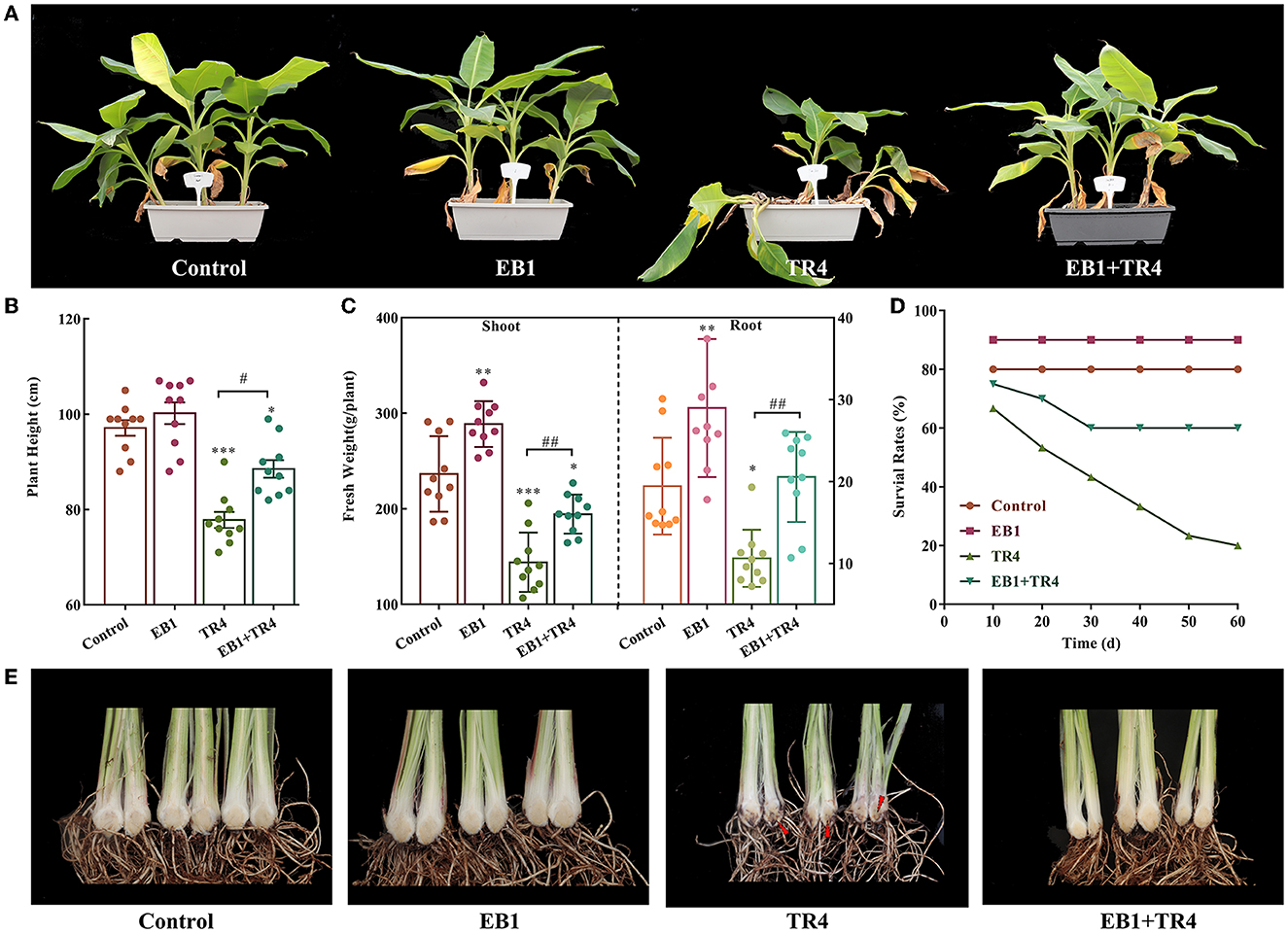
Figure 4 . Strain EB1 enhances Fusarium wilt resistance and promoted banana growth in the pot experiment. Control, no inoculation; EB1, inoculation with EB1 only, TR4, inoculation with TR4 only; EB1 + TR4, inoculation of EB1 prior to Foc treatment. (A) Phenotypes of acclimatized banana plants after being primed with EB1 at the rooting stage of tissue culture plantlets. (B–E) Plant height (B) , biomass of shoots and roots (C) , survival rates (D) , and severity of Fusarium wilt of acclimatized banana plants under different treatments (marked by red arrow heads) (E) . All data are expressed as the mean ± SD of at least 10 replicate samples. * p < 0.05, ** p < 0.01, and *** p < 0.001 indicate significant differences between the treatment groups and control group. # p < 0.05 and ## p < 0.01 indicate significant differences between treatment groups.
EB1 manipulated the SA and JA pathways in banana plants
To further examine whether the EB1 could activate defense signaling in the banana plant, the expression patterns of the defense-related marker genes involved in SA and JA pathways including NPR1, PR1, LOX2 , and MYC2 were analyzed ( Figure 5 ). Compared with the control, EB1 inoculation showed no induction in the expression of NPR1 and PR1 genes of the SA signaling pathway but showed an increase in the expression of the MYC2 gene of the JA signaling pathway by 1.67-fold ( p = 0.016). Whereas, the degree of expression changes in the Foc TR4 treatment group varied from gene to gene. Foc TR4 downregulated the expressions of NPR1 and LOX2 by 0.46- ( p = 0.0024) and 0.63-fold ( p = 0.016) and upregulated the expressions of PR1 and MYC2 by 16.62- ( p < 0.001) and 2.23-fold ( p < 0.001), respectively. Notably, both SA and JA signaling pathways were significantly upregulated by 1.40- ( p = 0.015), 35.80- ( p < 0.001), 1.50- ( p = 0.0028), and 2.44-fold ( p < 0.001) for NPR1, PR1, LOX2 , and MYC2 , respectively. The above results have indicated that EB1 primes the plants for enhanced immunity following a subsequent attack by Foc TR4.
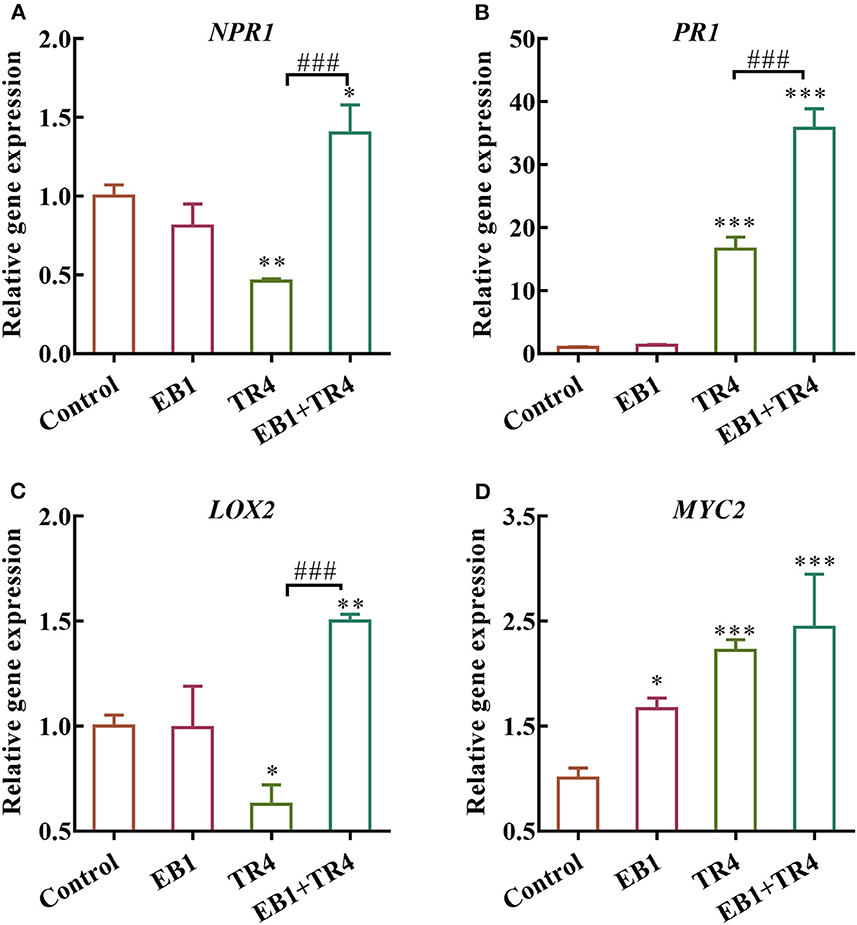
Figure 5 . Effects of strain EB1 and TR4 inoculations on the expression of defense genes. The expression patterns of (A) NPR1 , (B) PR1 , (C) LOX2 , and (D) MYC2 were analyzed by RT-qPCR. All data are expressed as the mean ± SD ( n = 4). * p < 0.05, ** p < 0.01, and *** p < 0.001 indicate significant differences between treatment groups and control group. ### p < 0.001 indicate significant differences between treatment groups.
Besides resistant cultivar breeding, BCA comprising endophytic bacteria has been considered another promising control strategy against Fusarium wilt that reached the field-testing stage ( Bubici et al., 2019 ). However, their control efficacy has been always unstable due to the varying environmental conditions, accentuating the need to develop a new efficient strategy for endophytes to harness their maximum benefits ( Dubey et al., 2020 ; Papik et al., 2020 ; Savani et al., 2021 ; Jana et al., 2022 ). Plant tissue culture is the main strategy for banana propagation with the advantages of high multiplication rates and the production of disease-free planting materials with high genetic fidelity and high standards of hygiene ( Pegg et al., 2019 ). More recently, tissue-cultured banana plantlets are questioned to be more susceptible to Fusarium wilt than vegetative planting materials because the first three stages (Stage I: establishment of explants, Stage II: elongation and multiplication, and Stage III: rooting) of the plant tissue culture process are taken place in aseptic conditions without the possibility of interaction with beneficial microbes ( Orlikowska et al., 2017 ). In this context, co-cultivation of the plant tissue culture plantlets with beneficial microorganisms may be indispensable to improve the adaptive ability of plantlets in the acclimation stage, which has accentuated the urgent to develop new technologies based on endophytes as microbial inoculants in tissue culture banana plantlets. Therefore, in the current study, the endophytic bacterial strain B. velezensis EB1 was isolated and selected for in-depth analysis based on its antifungal activity against Foc TR4 and strong colonization ability in banana plants to support a new approach using EB1 to control Fusarium wilt of banana by introducing into banana tissue culture plantlets at the end of rooting stage.
As one of the largest bacterial genera, Bacillus strains coexist with plants and are among the most studied microorganisms in the biological control of various plant diseases ( Shafi et al., 2017 ; Fira et al., 2018 ). For instance, endophytic bacteria B. mojavensis and B. cereus exhibited potent inhibition activities against various rice Fusarium pathogens such as F. proliferum, F. verticillioides , and F. fujikuroi ( Etesami and Alikhani, 2017 ). Consistent with these findings, the in vitro dual-culture experiment demonstrated that the growth of Foc TR4 could be significantly inhibited by EB1. Interestingly, considerable ultra-structural alterations such as wizened, flattened, thickened CW and plasmolysis that were observed in Foc TR4 cells at confronting with EB1 indicated that EB1 might be capable of producing antagonistic metabolites, penetrating into Foc TR4 cell, and leading to leakage of cytoplasm and disruption of internal organelles of Foc TR4. Accordingly, genes related to the biosynthesis of antifungal compounds such as lipopeptides and ketones, which were proven to inhibit hyphal extension and spore formation of phytopathogens, were identified in EB1 genome by the antiSMASH tool ( Arrebola et al., 2010 ; Li et al., 2015 ).
Plants can act as a filter of microbial communities and select the right endophytes to maintain their normal growth and development ( Dubey et al., 2020 ; Liu H. et al., 2020 ). Therefore, stable root colonization and persistence of BCAs in the plant is a key factor for their application in the biological management of microbial diseases ( Shafi et al., 2017 ). Detections of EB1 inside both roots and shoots of banana tissue culture plantlets in our study supporting EB1 is capable of entering through the root system and migrating upwards into the pseudostem. EB1 is an endophyte isolated from banana pseudostem; thus, it is conceivable that it has evolved strategies for efficient adaptation to this niche. To determine whether EB1 inhibits Fo c TR4 in planta , banana tissue culture plantlets were infected with Foc TR4 but only after a prior bacterization with an EB1. Using SEM, EB1 colonies were observed in grooves between root epidermal cells, indicating that the mechanism of entry of EB1 into roots occurs most probably via cracks, which also represent the major routes for phytopathogen to enter into plants ( Compant et al., 2010 ; de Zélicourt et al., 2018 ). Correspondingly, the deformed hyphae of Foc TR4 and alleviated host damage were observed in planta due to the inoculation of EB1, suggesting that penetration of Foc TR4 through cellophane membranes and invasion of banana tissue were impaired upon co-inoculation with EB1. Therefore, EB1 might occupy the ecological niches and nutrition rapidly and act as an extracellular barrier for the host plant for blocking the pathogen invasion ( Gao et al., 2010 ; Shafi et al., 2017 ; Dubey et al., 2020 ). It is noteworthy that the response of banana plants toward EB1 bacterization in the rooting stage was maintained and further amplified in the pot experiment. Plants whose roots had been pre-inoculated with EB1 at the rooting stage showed significantly higher survival rates and better growth states compared with those inoculated only with Foc TR4. Using antiSMASH, 12 BGCs responsible for the synthesis of 8 secondary metabolites including surfactin, bacilysin, bacillibactin, difficidin, fengycin, bacillaene, macrolactin, and butirosin have been identified in the genome of EB1. Surfactin and fengycin have been widely characterized to mediate biofilm formation and root colonization processes, which are suggested to have a role in plant development and growth promotion ( Aleti et al., 2016 ; Berlanga-Clavero et al., 2022 ). In addition, putative genes involved in the production of indole-3-acetic acid (IAA), spermidine, and polyamine, which are related to plant growth-prompting activity, have also been discovered in the genome of EB1 ( Xie et al., 2014 ; Zaid et al., 2022 ). Thus, our results have demonstrated the promising application of endophytic antifungal strains in agriculture to breed “microbe-optimized crops”.
Different from the fighting to the death in pathogen and host relationship, recent conceptual and experimental framework has indicated that beneficial endophytes usually can evade plant defense and reach a stable harmonious commensalism with the plant ( Sessitsch et al., 2012 ; Deng et al., 2019 ; Yu et al., 2019 ). To figure out the role EB1 plays in the three-way interactions with the host plant immunity and the fungal pathogen, expressions of genes known to be markers of plant defense signaling pathways including SA-mediated NPR1 and PR1 as well as JA-mediated LOX and MYC2 were analyzed ( Mhamdi, 2019 ). It is found that the expressions of these genes were stronger in plants with EB1 pretreatment and Foc TR4 infection than that in plants with pathogen infection only which is in accord with previous research ( Chandrasekaran and Chun, 2016 ; Nie et al., 2017 ). Similarly, inoculation of wheat with endophytic bacterium Stenotrophomonas rhizophila SR80 increased the expressions of a range of genes in SA and JA signaling pathways, but only when the F. pseudograminearum , the causal agent of Crown rot disease, was present ( Liu H. et al., 2020 ). Our findings suggested that EB1 plays a key role in the interactions with the host plant immunity and the fungal pathogen via a mechanism that enhances plant defense and growth ( Khare et al., 2018 ). A few studies mentioned that beneficial microbes can quench plant immune responses by downregulating the expression of the microbial-associated molecular patterns (MAMPs) ( Bardoel et al., 2011 ; Zamioudis and Pieterse, 2012 ), producing the MAMPs with a low-elicit ability ( Trda et al., 2014 ), or minimizing the stimulation of plant defensive response ( Liu et al., 2018 ; Deng et al., 2019 ). Accordingly, the whole-genome annotation data suggest that EB1 contains multiple genes that encode key components that function by these mechanisms. Combined with the localization and in vitro data, these observations suggest that EB1 forms a symbiotic relationship with banana plants and efficiently wards off the invasive of Foc TR4 in planta inferring the adaptability and potential of the banana tissue culture plantlets bio-primed with EB1 could be a promising biological solution for the management of Fusarium wilt of banana.
Our current study focused on providing a comprehensive understanding of the endophytic strain Bacillus velezensis EB1 isolated from a healthy banana plant in a wilt-diseased banana field and exploring its potential application in tissue culture plant of banana for the environmental sustainability management of Fusarium wilt based on its strong antagonistic effects against the devastating fungal pathogen Foc and mutualistic functional roles with banana plants. To realize large-scale implementation of microbial strains in agricultural practice, new strategies for successful delivery of BCAs into plant under field conditions are needed. Therefore, in the future, we intend to (1) understand the underlying molecular mechanisms of the beneficial effect of EB1 on the growth and stress tolerance of banana plants, (2) isolate more efficient, multifunctional, stress tolerant microbes and design an artificial disease suppressive synthetic community (SynCom) which comprised by multiple microbial strains rather than mono-strain inoculums to take advantage of functional complementarity to mimic a natural disease-suppressive community in plants, and (3) develop bioformulations for sustainable application of endophytic microbes in plant tissue culture. New strategies for the successful delivery of BCAs into the plant under field conditions are needed to realize the large-scale implementation of microbial strains in agricultural practice. The introduction of endophytic microbes, as a probiotic material that enhances plant growth as well as induces defense responses of plants to cope with stress, into tissue-cultured banana plantlets, could be a novel and stable biological control method to protect bananas from Foc infection.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary material .
Author contributions
DX and SL: conceptualization. DX, XY, BL, and YC: experimentation. DX, XY, and CL: review and drafting. SL and CL: validation and statistical analysis. All authors contributed to the article and approved the submitted version.
This study was supported by Grants from R&D Projects in Key Areas of Guangdong Province (Grant No. 2019B020216001), the Science and Technology Planning Project of Guangzhou Municipal Science and Technology Bureau, China (Grant No. 202102020567), and Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (Grant No. 2022KJ109).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1146331/full#supplementary-material
Aleti, G., Lehner, S., Bacher, M., Compant, S., Nikolic, B., Plesko, M., et al. (2016). Surfactin variants mediate species-specific biofilm formation and root colonization in Bacillus . Environ. Microbiol. 18, 2634–2645. doi: 10.1111/1462-2920.13405
PubMed Abstract | CrossRef Full Text | Google Scholar
Altendorf, S. (2019). Banana fusarium wilt tropical race 4: a mounting threat to global banana markets? Food outlook 11, 13–20.
Google Scholar
Arrebola, E., Sivakumar, D., and Korsten, L. (2010). Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol. Control 53, 122–128. doi: 10.1016/j.biocontrol.2009.11.010
CrossRef Full Text | Google Scholar
Bardoel, B. W., van der Ent, S., Pel, M. J., Tommassen, J., Pieterse, C. M., van Kessel, K. P., et al. (2011). Pseudomonas evades immune recognition of flagellin in both mammals and plants. PLoS Pathogens 7, e1002206. doi: 10.1371/journal.ppat.1002206
Berlanga-Clavero, M. V., Molina-Santiago, C., Caraballo-Rodríguez, A. M., Petras, D., Díaz-Martínez, L., Pérez-García, A., et al. (2022). Bacillus subtilis biofilm matrix components target seed oil bodies to promote growth and anti-fungal resistance in melon. Nat. Microbiol. 7, 1001–1005. doi: 10.1038/s41564-022-01134-8
Bubici, G., Kaushal, M., Prigigallo, M. I., Gómez-Lama Cabanás, C., and Mercado-Blanco, J. (2019). Biological control agents against Fusarium wilt of banana. Front. Microbiol. 10, 616. doi: 10.3389/fmicb.2019.00616
Cao, L., Qiu, Z., You, J., Tan, H., and Zhou, S. (2005). Isolation and characterization of endophytic streptomycete antagonists of Fusarium wilt pathogen from surface-sterilized banana roots. Fems Microbiol. Letters 247, 147–52. doi: 10.1016/j.femsle.2005.05.006
Chandrasekaran, M., and Chun, S. C. (2016). Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato ( Solanum lycopersicum ) plants challenged with Erwinia carotovora subsp. carotovora. Biosci. Biotechnol. Biochem. 80, 2277–2283. doi: 10.1080/09168451.2016.1206811
Compant, S., Clément, C., and Sessitsch, A. (2010). Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 42, 669–678. doi: 10.1016/j.soilbio.2009.11.024
Cook, D. C., Taylor, A. S., Meldrum, R. A., and Drenth, A. (2015). Potential economic impact of Panama disease (tropical race 4) on the Australian banana industry. J. Plant Dis. Protect. 122, 229–237. doi: 10.1007/BF03356557
de Zélicourt, A., Synek, L., Saad, M. M., Alzubaidy, H., Jalal, R., Xie, Y., et al. (2018). Ethylene induced plant stress tolerance by Enterobacter sp. SA187 is mediated by 2-keto-4-methylthiobutyric acid production. PLoS Genetics , 14, e1007273. doi: 10.1371/journal.pgen.1007273
Deng, Y., Chen, H., Li, C., Xu, J., Qi, Q., Xu, Y., et al. (2019). Endophyte Bacillus subtilis evade plant defense by producing lantibiotic subtilomycin to mask self-produced flagellin. Commun. Biol. 2, 368. doi: 10.1038/s42003-019-0614-0
Dini-Andreote, F. (2020). Endophytes: the second layer of plant defense. Trends Plant Sci. 25, 319–322. doi: 10.1016/j.tplants.2020.01.007
Dita, M., Barquero, M., Heck, D., Mizubuti, E. S. G., and Staver, C. P. (2018). Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 9, 1468. doi: 10.3389/fpls.2018.01468
Dubey, A., Malla, M. A., Kumar, A., Dayanandan, S., and Khan, M. L. (2020). Plants endophytes: unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 40, 1210–1321. doi: 10.1080/07388551.2020.1808584
Eljounaidi, K., Lee, S. K., and Bae, H. (2016). Bacterial endophytes as potential biocontrol agents of vascular wilt diseases-review and future prospects. Biol. Control 103, 62–68. doi: 10.1016/j.biocontrol.2016.07.013
Etesami, H., and Alikhani, H. A. (2017). Evaluation of gram-positive rhizosphere and endophytic bacteria for biological control of fungal rice ( Oryzia sativa L.) pathogens. Eur. J. Plant Pathol. 147, 7–14. doi: 10.1007/s10658-016-0981-z
Fan, X., Matsumoto, H., Wang, Y., Hu, Y., Liu, Y., Fang, H., et al. (2019). Microenvironmental interplay predominated by beneficial Aspergillus abates fungal pathogen incidence in paddy environment. Environ. Sci. Technol. 53, 13042–13052. doi: 10.1021/acs.est.9b04616
Fira, D., Dimkić, I., Berić, T., Lozo, J., and Stanković, S. (2018). Biological control of plant pathogens by Bacillus species. J. Biotechnol. 285, 44–55. doi: 10.1016/j.jbiotec.2018.07.044
Galvis, S. (2019). Colombia confirms that dreaded fungus has hit its banana plantations. Science , 1–5. doi: 10.1126/science.aaz1033
CrossRef Full Text
Gao, F., Dai, C., and Liu, X. (2010). Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 4, 1346–1351. doi: 10.5897/AJMR.9000480
Gómez-Lama Cabanás, C., Fernández-González, A. J., Cardoni, M., Valverde-Corredor, A., López-Cepero, J., Fernández-López, M., et al. (2021). The Banana root endophytome: differences between mother plants and suckers and evaluation of selected bacteria to control Fusarium oxysporum f. sp. cubense. J. Fungi 7, 194. doi: 10.3390/jof7030194
Guez-Romero, A. S. R., Badosa, E., Montesinos, E., and Jaizme-Vega, M. C. (2008). Growth promotion and biological control of root-knot nematodes in micropropagated banana during the nursery stage by treatment with specific bacterial strains. Ann. Appl. Biol. 152, 41–48. doi: 10.1111/j.1744-7348.2007.00189.x
Jana, S. K., Islam, M. M., and Mandal, S. (2022). Endophytic microbiota of rice and their collective impact on host fitness. Curr. Microbiol. 79, 37. doi: 10.1007/s00284-021-02737-w
Kavino, M., and Manoranjitham, S. K. (2018). In vitro bacterization of banana ( Musa spp.) with native endophytic and rhizospheric bacterial isolates: novel ways to combat Fusarium wilt. Eur. J. Plant Pathol. 151, 371–387. doi: 10.1007/s10658-017-1379-2
Kema, G. H. J., Drenth, A., Dita, M., Jansen, K., Vellema, S., and Stoorvogel, J. J. (2021). Fusarium wilt of banana, a recurring threat to global banana production. Front. Plant Sci. 11, 628888. doi: 10.3389/fpls.2020.628888
Khare, E., Mishra, J., and Arora, N. K. (2018). Multifaceted interactions between endophytes and plant: developments and prospects. Front. Microbiol. 9, 2732. doi: 10.3389/fmicb.2018.02732
Li, X., Mao, Z., Wu, Y., Ho, H., and He, Y. (2015). Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocontrol Sci. Technol. 25, 132–143. doi: 10.1080/09583157.2014.960809
Lian, J., Wang, Z. F., Cao, L. X., Tan, H. M., Patrik, I., Jiang, Z., et al. (2009). Artificial inoculation of banana tissue culture plantlets with indigenous endophytes originally derived from native banana plants. Biol. Control 51, 427–434. doi: 10.1016/j.biocontrol.2009.08.002
Liu, H., Li, J., Carvalhais, L. C., Percy, C. D., Prakash Verma, J., Schenk, P. M., et al. (2020). Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 229, 2873–2885. doi: 10.1111/nph.17057
Liu, S., Li, J., Zhang, Y., Liu, N., Viljoen, A., Mostert, D., et al. (2020). Fusaric acid instigates the invasion of banana by Fusarium oxysporum f. sp. cubense TR4. New Phytol . 225, 913–929. doi: 10.1111/nph.16193
Liu, Z., Beskrovnaya, P., Melnyk, R. A., Hossain, S. S., Khorasani, S., O'Sullivan, L. R., et al. (2018). A genome-wide screen identifies genes in rhizosphere-associated Pseudomonas required to evade plant defenses. MBio , 9, 18. doi: 10.1128/mBio.00433-18
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ CT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Matsumoto, H., Fan, X., Wang, Y., Kusstatscher, P., Duan, J., Wu, S., et al. (2021). Bacterial seed endophyte shapes disease resistance in rice. Nature Plants 7, 60–72. doi: 10.1038/s41477-020-00826-5
Mhamdi, A. (2019). NPR1 has everything under control. Plant Physiol. 181, 6–7. doi: 10.1104/pp.19.00890
Mohd Fishal, E. M., Meon, S., and Yun, W. M. (2010). Induction of tolerance to Fusarium wilt and defense-related mechanisms in the plantlets of susceptible berangan banana pre-inoculated with Pseudomonas sp. (UPMP3) and Burkholderia sp. (UPMB3). Agric. Sci. China 9, 1140–1149. doi: 10.1016/S1671-2927(09)60201-7
Nie, P., Li, X., Wang, S., Guo, J., Zhao, H., and Niu, D. (2017). Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis . Front. Plant Sci. 8, 238. doi: 10.3389/fpls.2017.00238
O'Donnell, K., Gueidan, C., Sink, S., Johnston, P. R., Crous, P. W., et al. (2009). A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. 46, 936–948. doi: 10.1016/j.fgb.2009.08.006
PubMed Abstract | CrossRef Full Text
Ordonez, N., Seidl, M. F., Waalwijk, C., Drenth, A., Kilian, A., Thomma, B. P. H. J., et al. (2015). Worse comes to worst: bananas and Panama disease-when plant and pathogen clones meet. PLoS Pathogens 11, e1005197. doi: 10.1371/journal.ppat.1005197
Orlikowska, T., Nowak, K., and Reed, B. (2017). Bacteria in the plant tissue culture environment. Plant Cell Tissue Organ Culture. 128, 487–508. doi: 10.1007/s11240-016-1144-9
Papik, J., Folkmanova, M., Polivkova-Majorova, M., Suman, J., and Uhlik, O. (2020). The invisible life inside plants: deciphering the riddles of endophytic bacterial diversity. Biotechnol. Adv. 44, 107614. doi: 10.1016/j.biotechadv.2020.107614
Pegg, K. G., Coates, L. M. O., Neill, W. T., and Turner, D. W. (2019). The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 10, 1395. doi: 10.3389/fpls.2019.01395
Ploetz, R. C. (2015). Management of Fusarium wilt of banana: a review with special reference to tropical race 4. Crop Prot. 73, 7–15. doi: 10.1016/j.cropro.2015.01.007
Savani, A. K., Bhattacharyya, A., Boro, R. C., Dinesh, K., and JC, N. S. (2021). Exemplifying endophytes of banana ( Musa paradisiaca ) for their potential role in growth stimulation and management of Fusarium oxysporum f. sp cubense causing panama wilt. Folia Microbiol. 66, 317–330. doi: 10.1007/s12223-021-00853-5
Sessitsch, A., Hardoim, P., Doring, J., Weilharter, A., Krause, A., Woyke, T., et al. (2012). Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant-Microbe Interact. 25, 28–36. doi: 10.1094/MPMI-08-11-0204
Shafi, J., Tian, H., and Ji, M. (2017). Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol. Biotechnol. Equip. 31, 446–459. doi: 10.1080/13102818.2017.1286950
Soumare, A., Diédhiou, A. G., Arora, N. K., Tawfeeq Al-Ani, L. K., Ngom, M., Fall, S., et al. (2021). Potential role and utilization of plant growth promoting microbes in plant tissue culture. Front. Microbiol. 12, 649878. doi: 10.3389/fmicb.2021.649878
Staver, C., Pemsl, D. E., Scheerer, L., Perez Vicente, L., and Dita, M. (2020). Ex ante assessment of returns on research investments to address the impact of Fusarium wilt tropical race 4 on global banana production. Front. Plant Sci. 11, 844. doi: 10.3389/fpls.2020.00844
Strobel, G., and Daisy, B. (2003). Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 67, 491–502. doi: 10.1128/MMBR.67.4.491-502.2003
Thangavelu, R., and Gopi, M. (2015). Combined application of native Trichoderma i solates possessing multiple functions for the control of Fusarium wilt disease in banana cv. Grand Naine. Biocontr. Sci. Technol. 25, 1147–1164. doi: 10.1080/09583157.2015.1036727
Trda, L., Fernandez, O., Boutrot, F., Heloir, M. C., Kelloniemi, J., Daire, X., et al. (2014). The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin-derived epitopes from the endophytic growth-promoting bacterium Burkholderia phytofirmans and plant pathogenic bacteria. New Phytol. 201, 1371–1384. doi: 10.1111/nph.12592
Xie, S., Wu, H., Zang, H., Wu, L., Zhu, Q., and Gao, X. (2014). Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol. Plant Microbe Interact . 27, 655–663. doi: 10.1094/MPMI-01-14-0010-R
Yu, C., Xiao, R., Liu, B., Lin, N., and Chen, L. (2010). Endophytic colonization of biocontrol bacterium FJAT-346-PA and its efficiency against Banana Fusarium Wilt. Acta Phytophylacica Sin. 37, 493–498. doi: 10.13802/j.cnki.zwbhxb.2010.06.020
Yu, K., Liu, Y., Tichelaar, R., Savant, N., Lagendijk, E., van Kuijk, S. J. L., et al. (2019). Rhizosphere-associated pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr. Biol. 29, 3913–3920. doi: 10.1016/j.cub.2019.09.015
Zaid, D. S., Cai, S., Hu, C., Li, Z., and Li, Y. (2022). Comparative genome analysis reveals phylogenetic identity of Bacillus velezensis HNA3 and genomic insights into its plant growth promotion and biocontrol effects. Microbiol. Spectr. 10, e216921. doi: 10.1128/spectrum.02169-21
Zamioudis, C., and Pieterse, C. M. J. (2012). Modulation of host immunity by beneficial microbes. Mol. Plant Microbe Interact. 25, 139. doi: 10.1094/MPMI-06-11-0179
Zhang, L., Liu, Z., Wang, Y., Zhang, J., Wan, S., Huang, Y., et al. (2022). Biocontrol potential of endophytic Streptomyces malaysiensis 8ZJF-21 from medicinal plant against banana Fusarium wilt caused by Fusarium oxysporum f. sp. cubense tropical race 4. Front. Plant Sci. 13, 874819. doi: 10.3389/fpls.2022.874819
Keywords: endophyte, Bacillus , Fusarium wilt of banana, biological control, banana tissue culture plantlets
Citation: Xiang D, Yang X, Liu B, Chu Y, Liu S and Li C (2023) Bio-priming of banana tissue culture plantlets with endophytic Bacillus velezensis EB1 to improve Fusarium wilt resistance. Front. Microbiol. 14:1146331. doi: 10.3389/fmicb.2023.1146331
Received: 17 January 2023; Accepted: 20 February 2023; Published: 16 March 2023.
Reviewed by:
Copyright © 2023 Xiang, Yang, Liu, Chu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyu Li, lichunyu@gdaas.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.8(11); 2022 Nov

Biotechnological interventions in banana: current knowledge and future prospects
Angima kibari justine.
a Department of Biotechnology, Guru Nanak Dev University, Amritsar, 143005, Punjab, India
Navdeep Kaur
b Centre for Agricultural Research and Innovation, Guru Nanak Dev University, Amritsar, 143005, Punjab, India
c Department of Botany, Hansraj College, University of Delhi, 110007, Delhi, India
Pratap Kumar Pati
d Department of Agriculture, Guru Nanak Dev University, Amritsar, 143005, Punjab, India
Associated Data
Data included in article/supp. material/referenced in article.
Banana is an important food crop responsible for ensuring food security, nutrition, and employment for a significant portion of the world population. It has fairly broad genetic diversity and is distributed widely across the globe. Due to its socio-economic importance, there has been growing demand for healthy and improved planting materials of banana. In recent years many companies and organizations are working hard to narrow down the gap between demand and supply of quality planting materials. The other challenges includes its susceptibility to adverse environmental conditions, attack of various pests/pathogens and improvement of nutritional quality of bananas. To address these issues, refinement of existing techniques and introduction of new experimental tools are required. However, the genetic improvement of bananas to a large extent is limited by using conventional methods due to polyploidy, heterozygosity, and sterility of this plant. For rapid multiplication and obtaining disease free and healthy plants, efficient in vitro propagation techniques and fine tuning of the existing protocols are being tried in many laboratories across the globe. Besides, for developing a successful protocol for propagation of different cultivars of bananas, a deeper understanding of the factors associated with various steps of its multiplication till transfer to the land is immensely critical. Similarly, developing biotic and abiotic stress tolerant banana and enhancing its commercial value through biotechnological interventions could be very useful. The key intent of this review is to highlight the research endeavor in this direction, associated challenges and future prospects.
Banana; Micropropagation; Regeneration; Genetic improvement; Stress tolerance.
1. Introduction
Banana (Musa spp . L.) is one of the most important cash crops and contributes immensely to global food security ( Esan et al., 2022 ; Tripathi et al., 2020 ). It belongs to the family Musaceae and is distributed all around the world. With an annual estimated production of 153 million tonnes ( FAOSTAT, 2019 ), the banana is the most popular fruit in terms of international trade ( Tripathi et al., 2020 ). The major producers of bananas include India, China, Indonesia, Brazil, Ecuador, Philippines, Guatemala, Colombia, and Angola, where India is the largest producer with 30.808 million metric tons of production ( FAO, 2020 ). Banana fruit is very popular especially in developing nations due to its low price and high nutritive value. It is a rich source of carbohydrates, proteins, minerals (such as potassium calcium, magnesium, and manganese), and vitamins including vitamins A, C, and B6 ( Ranjha et al., 2020 ). Besides the fruit, the other parts obtained from the banana plant such as peels, pseudo-stem, rhizome, leaves, etc. are utilized in various industries such as agro, food, textiles, etc ( Adeniyi et al., 2019 , 2021 ; Ighalo and Adeniyi, 2019 ; Akatwijuk et al., 2022 ; Mathew et al., 2021 ).
The genus Musa is divided into 5 sections viz. Australimusa, Callimusa, Rhodochlamys, Ingentimusa, Eumusa ( Lamare et al., 2017 ). Section Australimusa having basic chromosome number 10 is distributed in Queensland, New Caledonia Philippines, and Australia and is mainly used in the form of fiber, fruit, and vegetables. It includes 5–6 species ( M. textilis, M. maclayi, M. lolodensis, M. peekelii, and M. fehi ). Section Callimusa with chromosome number 10 is distributed in Indo-China and Indonesia and is mainly used for ornamental purposes. It consists of three species ( M. coccinea, M. violascens, and M. gracilis ). Section Eumusa having chromosome number 11 is distributed in India and is mainly used in the form of fiber, fruit, vegetables and have medicinal applications. It consists of the following species : M. acuminata, M. balbisiana, M. cheesmani, M. flaviflora, M. itinerans, M. schizocarpa, M. sikkimensis, M. nagensium, M. halabanensis and, M. ochracea. Section Rhodochlamis possessing chromosome number 11 mainly consists of ornamental species (M. aurantiaca, M. laterita, M. sanguinea, M. ornate, M. velutina, M. mannii, M. rosea and, M. rubra ) which are distributed throughout India, Indo-China, Philippines, Thailand, and Malaysia ( Debnath et al., 2019 ). There are more than 1000 varieties of banana cultivated all over the world, however, the Cavendish varieties of banana are considered as one of the most important commercial varieties ( Tripathi et al., 2019 ; Thangavelu et al., 2021 ).
Banana cultivation largely regulates the agri-based global bioeconomy. Hence, understanding the associated challenges in its production and developing appropriate strategies for addressing these concerns are of paramount importance. In recent years, there has been increasing interest to grow healthy and commercially important cultivars of bananas. However, there exists a huge gap between the demand and supply of healthy planting materials ( Jacobsen et al., 2019 ; Nkengla-Asi et al., 2021 ). Further, in the last few decades, banana production is under threat due to different climatic factors and pathogenic agents such as bacteria, viruses, fungi, and nematodes ( Tripathi et al., 2019 ). To circumvent these challenges and to produce agronomically superior disease-resistant banana crop, various traditional breeding methods have been employed such as diploid breeding ( Rowe and Rosales, 1994 ), 3x/2x strategy (pollination of susceptible triploids with male fertile diploids that are resistant), and 4x/2x strategy which involves the production of a tetraploid parent by chromosome doubling of an ancestral diploid with good agronomic trait followed by the production of triploid hybrids through hybridization of a diploid parent with the tetraploid ( Menon, 2016 ). However, polyploidy in Musa is the biggest challenge in banana breeding which ranges from diploid to tetraploidy ( Nansamba et al., 2020 ). Thus, the development of a new banana cultivar is very exhaustive via breeding because the selection of desirable characters can take more than 12 years ( Menon, 2016 ). A high level of heterozygosity and the requirement of a large population for the selection of individual clones with desirable agronomic traits, make it more cumbersome. Moreover, introgression of desired gene loci from diploid wild cultivars of banana also carries certain undesirable traits such as non-parthenocarpy and low yield ( Menon, 2016 ). Thus, to sustain banana cultivation in the era of changing global climate, the development of new elite banana varieties and preparation of disease-free planting material for its commercial plantations using non-conventional approaches is much warranted. Biotechnology has largely provided solutions to many of these existing problems. The present review focuses on biotechnological interventions in bananas for their mass multiplications and improvement.
2. Micropropagation of banana
Banana plants are traditionally propagated through vegetative means using suckers ( Nkengla-Asi et al., 2021 ). However, plants produced through suckers have their own limitations as it leads to disease transmission, low productivity, and poor preservation of original plant genetic material ( Hussein, 2012 ). Moreover, there is a huge demand for quality planting materials to narrow the gap between demand and supply. In this scenario, micropropagation techniques have been used in many parts of the world to produce healthy, disease-free banana plants throughout the year that perform better under field conditions ( Abdalla et al., 2022 ). There are several reports available on micropropagation of different cultivars of banana (Table S1). These reports suggest that for successful micropropagation of bananas, optimization of micropropagation protocol is critical. In the present review, we have divided the protocol of micropropagation of bananas into four distinct stages such as (i) initiation of aseptic cultures (ii) shoot multiplication (iii) in vitro rooting of microshoots (iv) hardening of rooted microshoots ( Figure 1 ) and tried to understand the factors that influence each of these stages. A better understanding of these factors will lead to the development of robust micropropagation protocols for large-scale propagation of different cultivars of bananas.

Different steps involved in the in vitro propagation of banana.
2.1. Factors that influence micropropagation of banana
2.1.1. stage i: initiation of aseptic cultures.
Initiation of aseptic culture is one of the most important steps for the development of a robust micropropagation protocol ( Kaur et al., 2022 ). It involves the identification and selection of the desired explants, and the development of an effective explant sterilization process ( Loyola-Vargas and Ochoa-Alejo, 2018 ) (Figures 2 a, 2b). Further, initial troubleshooting involving the browning of explants is critical for the initial culture establishment of banana.

An overview of micropropagation in banana. (a) Sword sucker from mother plant; (b) Primary inoculation; (c) Shoot induction; (d) Shoot multiplication; (e) Rooting; (f) Primary hardening; (g) Secondary hardening.
2.1.1.1. Choice of explants
Shoot cultures of bananas can be initiated conventionally from mother sword suckers ( Hasan et al., 2020 ) ( Figure 2 a). However, explant material from mature individuals with known responses to environmental factors and with proclaimed quality traits is preferred. Moreover, larger explants are advantageous because they contain a shoot apex with more lateral buds that develop into shoots, but they are more susceptible to contamination and blackening ( Strosse et al., 2004 ; Kannahi and Buvaneswari, 2019 ). Among different plant parts of banana, suckers are considered the most suitable explant for micropropagation as they have a vascular connection with the mother plant, are easy to isolate and culture, and produce true to mother type plantlets ( Heslop-Harrison and Schwarzacher, 2007 ).
2.1.1.2. Sterilization
Surface sterilization of explants is a process in which explants are immersed into the appropriate concentration of chemical sterilant to establish contamination-free cultures ( Bello et al., 2018 ). The type of sterilant used primarily depends on the size and type of explants along with the procedure of disinfection ( Strosse et al., 2004 ; Mihaljević et al., 2013 ). Sodium hypochlorite is the most commonly used agent for surface sterilization. It is very effective against all types of microbial contaminations. It causes biosynthetic alterations in cellular metabolism through phospholipids destruction, fatty acid degradation, formation of chloramines that interfere in cellular metabolism, oxidative damage, and irreversible enzymatic inactivation in bacteria ( Estrela et al., 2002 ). Mercuric chloride (HgCl 2 ) has antimicrobial activity against both fungi and bacteria. It may be used at 0.05–0.1% for 1–5 min according to the type of explant of different plants. It has been observed that a longer duration of 0.1% concentration of mercuric chloride treatment is effective to decrease bacterial contamination in cultures. However, higher concentrations of mercuric chloride can be toxic to plant cells and tissues ( Kang et al., 2020 ; Kapadia and Patel, 2021 ). Besides, bavistin (0.1%) and 70% (v/v) ethanol also act as a powerful sterilizing agent for banana explants ( Bhutani et al., 2021 ; Prakasha et al., 2019 ; Yadav et al., 2021 ). In addition, some antibiotics such as penicillin, ampicillin, and ticarcillin have also been used to curb bacterial contaminants in banana tissue cultures ( El-Banna et al., 2021 ). Liquid cultures containing rifampicin for up to 30 days were able to inhibit the growth of Gram-positive bacteria in banana shoot tips with no effect on plant growth ( Van Den Houwe and Swennen, 1999 ).
2.1.1.3. Browning of the medium
Browning of the medium occurs due to the oxidation of phenolic compounds produced by banana explants resulting in reduced cell division and plant growth ( Rodrigues et al., 2022 ). These exudates form a barrier around the tissue which prevents nutrient uptake ( Tabiyeh et al., 2005 ; Krishna et al., 2008 ). They further leach into the culture medium and result in the browning of the media. It is for this reason that fresh shoots need to be transferred to new media every now and then ( Ahmad et al., 2013 ). It has been observed that when banana explants were treated with citric acid and ascorbic acid for 30 min, the browning ceased ( Ko et al., 2009 ; De Anicezio, 2012 ). Cessation of media browning could be attributed to the ascorbic acid's activity to scavenge oxygen radicals produced when the plant is wounded which prevents the cells from further damage. Washing of explants with antioxidant solution {0.125% potassium citrate: citrate (K–C: C) in a ratio of 4:1 w/w} was useful to eliminate browning in Musa spp. cv. Kanthali ( Titov et al., 2006 ). A similar response was observed when explants of banana were presoaked/pre-treated with 0.1–0.5 mg/mL of potassium citrate and citrate (K–C: C) ( Onuoha et al., 2011 ). Pre-soaking of explants in 1.2 g/l of ascorbic acid solution prevented lethal browning in local Musa spp. cv. Mzuzu ( Ngomuo et al., 2014 ). For genotypes like Musa spp . ABB and BB groups, which produce compact proliferating masses of buds, the addition of activated charcoal to the medium minimized the phenol exudation from explants ( Strosse et al., 2004 ). Other anti-browning agents that have been used in controlling phenolics in bananas include L-cysteine, polyvinylpyrrolidone ( Onuoha et al., 2011 ; Oliveira et al., 2011 ).
2.1.2. Stage II: shoot multiplication
Shoot multiplication is the most crucial step in the development of an efficient micropropagation protocol that directly affects the success of the given protocol ( Pati et al., 2006 ; Nowakowska et al., 2022 ) ( Figures 2 c, 2d). Various factors that greatly influence the shoot multiplication in bananas are highlighted in this section.
2.1.2.1. Genotype
Genotype of the given cultivar is one of the key factors that influence the shoot proliferation rate. In comparison to other banana genotypes, the B genotype exhibits a higher shoot proliferation rate under in vitro conditions ( Vuylsteke et al., 1996 ). This could be due to the variation in the activity of cytokinins in different genotypes that can be explained by their different uptake rates ( Blakesley, 1991 ), varied translocation rates to meristematic regions, and metabolic processes, in which the cytokinin may be degraded or conjugated with amino acids or sugars to form biologically inactive compounds ( Tran Thanh Van and Trinh, 1990 ; Kaminek, 1992 ). The presence of less endogenous cytokinin prompts the requirement of a higher concentration of exogenous cytokinin for multiplication in recalcitrant cultivars ( Makara et al., 2010 ). Diploid and triploid banana cultivars showed different micropropagation responses to exogenous cytokinin treatment concerning their genotype and ploidy ( Resmi and Nair, 2011 ).
2.1.2.2. Media
Tissue culture media plays a vital role in the growth and development of shoot tips. The choice of nutrient media, chemical composition, and concentration of the salt largely determine the success of micropropagation ( Suman, 2017 ; Park et al., 2020 ) (Table S1). Several media have been used for shoot multiplication of bananas including Murashige and Skoog (MS) media, SH ( Schenk and Hildebrant, 1972 ), Linsmaier and Skoog (LS) ( Linsmaier and Skoog, 1965 ), N6 ( Chu et al., 1975 ) and B5 ( Gamborg et al., 1968 ) media (Table S1). Among all the different types of media, MS medium (supplemented with specific growth regulators) was reported to be the most efficient for shoot multiplication ( Shirani et al., 2009 ; Hui et al., 2012 ; Ferdous et al., 2015 ; Hossain et al., 2016 ). Modified MS mediums have also been used to study the effect of media substitution with foliar fertilizers and coconut water. Three different types of media were prepared by modification of MS media such as full MS media, ½ MS, ½ MS + ½ foliar fertilizer, and fully foliar fertilizer each containing different concentrations of coconut. However, these substitute media could not compete with the full MS media supplemented with coconut water (50–100 ml l −1 ) water ( Mardhikasari et al., 2020 ).
2.1.2.3. Growth regulators
For the shoot multiplication of banana, various plant growth regulators (PGRs) such as abscisic acid, auxins, cytokinins, and gibberellins are used (Table S1). Cytokinins such as BAP, zeatin, thiadizuron (TDZ), and kinetin (KN) are used for the growth of axillary buds, and shoot multiplication, while auxins such as indole acetic acid (IAA), indole butyric acid (IBA) and naphthalene acetic acid (NAA) promote root development ( Gupta et al., 2020 ; Kaur et al., 2022 ). BAP is the most preferred cytokinin to enhance shoot multiplication in Musa spp. due to its high cytokinin activity, accessibility, and low cost ( Shirani et al., 2009 ; Singh et al., 2017 ). It has been reported to be the optimum cytokinin for shoot multiplication in bananas either alone or in combination with different auxins such as IAA and NAA ( Huq et al., 2012 ; Shirani et al., 2009 ; Ahmed et al., 2014 ; Mahdi et al., 2014 ; Shankar et al., 2014 ; Ferdous et al., 2015 ; Qamar et al., 2015 ; Suman and Kumar 2015 ; Uzaribara et al., 2015 ; Hossain et al., 2016 ; Devi et al., 2017 ; Khatun et al., 2017 ; Safarpour et al., 2017 ; Khatab et al., 2017 ; Hoque et al., 2018 ; Rajoriya et al., 2018 ; Selvakumar and Parasurama, 2020 ) (Table S1). MS medium fortified with BAP (20.0 μM) in combination with NAA (1.0 μM) was reported to give the best proliferation for banana cultivar Grand Nain ( Safarpour et al., 2017 ). Moreover, a higher multiplication rate in Dwarf Cavendish bananas on media supplemented with BAP (13.31 μM) and IAA (2.28 μM) was observed in the five subcultures which then declined after the 5 th cycle ( Dagnew et al., 2012 ). However, the proliferation of shoots was lesser at lower concentrations of BAP (10 μM) compared to a higher concentration of BAP (30.0 μM). For the banana cultivars ‘SH3362’, “-‘Basrai’, ‘William’, ‘GN60A’ and ‘High gate', MS + BAP (10.0 μM) + IAA (5.0 μM) + 40 mg/l cysteine HCl + 4% sucrose was used for initiation of cultures, and MS + BAP (20.0 μM) + 40 mg/l cystein HCl + 4% sucrose for the shoot proliferation ( Khatri et al., 1997 ). In addition, other cytokinins like TDZ and KN have been used for in vitro shoot proliferation in bananas ( Gubbuk and Pekmezci, 2009 ; Farahani et al., 2008 ; Gubbuk, and Pekmezci 2009 ; Roy et al., 2010 ; Hrahsel et al., 2014 ). TDZ at a lower concentration (2.0 μM) promoted shoot proliferation ( Shirani et al., 2009 ; Manjula et al., 2015 ), however, a higher concentration of TDZ (5.0 μM) was reported to cause a high abnormality index of shoots in bananas ( Shirani et al., 2009 ). Besides, a new compound with cytokinin-like activity, named meta-Topolin (N6-(3-hydroxy-benzyladenine) (mT) has been used in place of BAP for the micropropagation of certain banana genotypes ( Aremu et al., 2012 ; Escalona et al., 2003 ; Bairu et al., 2008 ).
2.1.2.4. Status of the medium
Besides media composition and plant growth regulators, the type of gelling agent used in a tissue culture medium is also critical ( Thorpe et al., 2008 ). Gelling agents such as agar and phytagel are commonly used in plant tissue culture because of their higher gelling capabilities. Agar is routinely used because of its inertness, stability, and clarity ( Palanyandy et al., 2020 ). However, due to the high cost of tissue-culture-grade agar, the use of alternative cost-effective gelling agents including phytagel, gelrite, isabagol, etc is being explored ( Ayenew et al., 2021 ; Dhawale et al., 2021 ). During the in vitro propagation of banana Grand Nain, when the gelrite in the medium was replaced with a mixture of gelrite/starch, the micropropagation rates were found to be relatively equal ( Kodym and Zapata-Arias, 2001 ). When isabgol was used as an alternate gelling agent to phytagel and agar for in vitro propagation of banana cv. Karpura Chakkarakeli (AAB; Mysore subgroup), there was no significant difference in the number of shoots produced, although the survival rate of the shoots was higher with a slower multiplication rate in isagbol media than in phytagel and agar supplemented media. This could be ascribed to the low availability of water and a hence slower rate of absorption of nutrients from the isabgol matrix to the plantlets than that of other media tested ( Agrawal et al., 2010 ). Use of phytagel in micropropagation of banana cultivar Dwarf Cavendish resulted in a higher shoot number and shoot weight than agar-agar, agargel, and plant agar ( Kacar et al., 2010 ). Besides, a higher shoot multiplication rate and fresh weight were also observed in medium solidified with 0.9 g/l gelrite in comparison to medium gelled with 2.6 g/l gellan gum and 4–8 g/l agar in Shima Banana (AAA) ( Buah et al., 1999 ). In addition, when sago + Isabgol were used, the maximum number of shoots was observed in ‘Udhayam’ and ‘Rasthali’ cultivars compared to the control ( Saraswathi et al., 2016 ). Nevertheless, till now, there are only a fewer reports on the use of low-cost gelling agents for banana micropropagation and thus more research is necessary to optimize their use in banana in vitro propagation.
Total elimination of gelling agents (liquid medium) has also been tried for banana micro propagation by different researchers ( Alvard et al., 1993 ). It has been observed that the rate of shoot multiplication and dry weight of shoots was higher on liquid media when compared with gelled media ( Alvard et al., 1993 ). However, these experiments required specialized culture vessels for highly controlled intermittent submergence of cultures in the medium. A simple polypropylene container with cotton fiber support was found more effective than that of an agar-gelled medium for micropropagation in banana ( Musa acuminata ) cv. Grand Nain ( Prabhuling and Sathyanarayana, 2017 ). The plantlets produced were also sturdier and were of better quality in comparison with the agar-gelled medium. Liquid medium not only reduces the cost of propagation but also facilitates better availability of nutrients and plant growth regulators leading to higher shoot multiplication. Moreover, the liquid medium promotes proper aeration of cultures and dilutes any exude from the explant which might inhibit the growth of the culture ( Ziv and Halevy, 1983 ; Abdulmalik et al., 2020 . However, in general, many in vitro propagated plants respond poorly to liquid culture medium due to hyperhydricity that is a result of prolonged contact between the explants and liquid culture ( Ziv, 2005 ; Snyman et al., 2011 ). To circumvent this challenge, a partial immersion system is utilized to make sure explants are properly aerated. Materials such as rockwool, coconut coir, filter paper, luffa sponge, cotton fiber, glass wool, polystyrene foam, nylon cloth, polyester screen raft, and polypropylene membrane raft are utilized to ensure there is contact between the lower portion of the explant and the culture medium ( Gupta and Prasad, 2006 ). Filter sterilized air can also be bubbled through the medium for micropropagation ( Preil, 1991 ). In bananas, four different liquid medium systems (solid medium (A), liquid medium with the immersion of plants (B), liquid medium with cotton culture support (C), and liquid medium aerated by bubbling (D) were compared for micropropagation. In this study, maximum shoot number in a temporary immersion bioreactor was observed in banana cultivar Musa cv. Dwarf Cavendish and hyperhydricity were observed in shoots that were cultured in a continuously aerated liquid media setup ( Farahani and Majd, 2012 ). Thus, temporary immersion bioreactors can help to overcome the hyperhydricity deformities through better aeration, and intermittent or partial submergence of the cultures in the medium ( Sajid and Parvaiz, 2008 ; Farahani and Majd, 2012 ).
2.1.2.5. Physical factors
Temperature plays a critical role in the shoot multiplication of bananas. For banana shoot proliferation, a temperature in the range of 24 °C–26 °C has been reported by various workers to be optimum ( Safarpour et al., 2017 ; Bello-Bello et al., 2019 ). However, there are few reports where a temperature up to 28 ± 2 °C is recommended for banana micropropagation ( Alvard et al., 1993 ; Gebeyehu 2015 ; Bohra et al., 2016 ). Along with temperature, light intensity and photoperiod also depict an important role in plant tissue culture ( Kaur et al., 2021 ). In bananas, most workers have reportedly used a light intensity in the range of 30 and 100 μmol/m 2 /s ( El-Mahdy and Youssef 2019 ; Mekonen et al., 2021 ; Subrahmanyeswari and Gantait, 2022 ). However, optimum banana shoot proliferation was found at 40 μmol/m 2 /s ( Wilken et al., 2014 ). Exposure to higher light intensity during later stages led to the improved survival rate of banana plantlets upon subsequent transfer to soil ( Suman, 2017 ). Fluorescent lamps are currently the most common light for photosynthesis; however they are expensive and produce unnecessary wavelengths and radiations ( Yeh et al., 2009 ; Sonthisut et al., 2022 ). Thus, the use of light-emitting diodes (LEDs) (combining LEDs emitting in far red, red, and blue colors) is encouraged these days for better plant growth under in vitro conditions ( Nhut and Nam, 2010 ). It was reported that blue and red LED lights ( B:R = 1:1) are more suitable for higher fresh and dry weight in bananas ( Duong et al., 2003 ). Moreover, a maximum number of shoots was observed under the LED lamps as compared to the leaves under the white fluorescent lamps ( Bhaya and Al-RazzaqSalim, 2019 ). Besides, high chlorophyll content was also observed in the case of two ornamental banana varieties using LED illumination ( Vendrame et al., 2022 ).
2.1.3. Stage III: rooting of microshoots
For an efficient micropropagation protocol, rooting of microshoots is necessary for the successful transfer of in vitro propagated shoots to the field ( Waman et al., 2015 ) ( Figure 2 e). MS basal medium was found to be the best suitable medium for inducing rooting from shoot tips in Musa spp . Cv. Sirumalai ( Mahadev et al., 2011 )). 1/2 MS, MS0 media alone or supplemented with activated charcoal and other additives have also been reported to promote rooting of in vitro banana microshoots ( Mahadev et al., 2011 ; Hrahsel et al., 2014 ; Thanakronpaisan et al., 2019 ; Selvakumar and Parasurama, 2020 ). Moreover, rooting in micropropagated shoots was successfully induced in the banana cultivar Elaki using ½ MS medium augmented with 20 mg/l adenine sulfate + 200 mg/l activated charcoal (AC) + 3% sucrose ( Selvakumar and Parasurama, 2020 ). Further, MS medium supplemented with chitosan (20 mg l −1 ) showed maximum rooting in shoots obtained from shoot tips ( Thanakronpaisan et al., 2019 ).
MS media augmented with different auxins such as IBA, IAA and NAA had also been used for in vitro rooting of banana microshoots (Table S1). Auxins stimulate lateral root initiation and primordium growth by stimulating cell division, differentiation, and expansion ( Kaur et al., 2021 ). Among different auxins, IBA has been reported by most workers as the most ideal auxin for rooting of in vitro developed banana shoots ( Suman et al., 2013 ; Safarpour et al., 2017 ; Kavitha et al., 2021 ; Quiñonez et al., 2021 ). MS medium with 3% sucrose supplemented with IBA (1.47 μM) + 1 g/l activated charcoal was able to elicit rooting in vitro propagated shoots of Musa (AAB) Curare ( Quiñonez et al., 2021 ). It was also observed that MS medium fortified with BAP (8.87 μM) + IAA (11.42 μM) + 0.1% activated charcoal is suitable for root induction in shoot tip culture ( Vani et al., 1999 ). Further, MS media supplemented with NAA (2.65 μM) + 0.2% activated charcoal successfully induced rooting in bananas ( Shashikumar et al., 2017 ). Besides, IAA (2.8 μM) in combination with BAP (22.19 μM) has also been widely used by different groups for in vitro rooting of banana shoots ( Khan et al., 2021 ; Quiñonez et al., 2021 ).
2.1.4. Stage IV: hardening of rooted microshoots
Successful hardening of in vitro propagated plantlets is a prerequisite for an efficient lab-to-land transfer protocol ( Khatik and Joshi, 2016 ) (Figures 2 f, 2g). It is dependent on factors such as the composition of potting mixture, genotype of the plant, temperature, and humidity, etc ( Twaij et al., 2020 ; Waman et al., 2015 ). Soil mixtures containing different ratios of vermiculite, peat moss, perlite, sand, and vermicompost have been used for the successful hardening of banana cultivars ( Robinson and Sauco, 2009 ; Safarpour et al., 2017 ; Chamling et al., 2021 ). Besides combinations of bio-fertilizers (including VAM), peat fortified with nitrogen fixing and phosphate solubilizing microbes (each 1 g/plantlet) has been used for hardening in banana plantlets ( Vasane et al., 2008 ). For hardening of banana cultivar Grand Nain (cocopeat: red soil: sand (1:1:1) was found to be the optimum potting mix for vigorous growth with a 96.5% survival rate ( Selvakumar and Parasurama, 2020 ). In a similar study for Grand Nain banana, cocopeat (temperature 25 ± 2 °C) was used for hardening in the green house and further acclimatization was carried out using soil and vermicompost (1:1) ( Manokari et al., 2022 ). Sand and farmyard manure (FYM) have also been used for acclimatization and hardening of banana cv. Malbhog and B. B. Battisa, with a survival rate of 95 % and 80%, respectively ( Suman et al., 2013 ; Suman and Kumar, 2015 ).
3. Somatic embryogenesis system
The establishment of a high-frequency regeneration protocol is an important prerequisite for direct regeneration and genetic transformation ( Rajput et al., 2022 ). It relies on the utilization of PGRs to induce tissue differentiation thus forming embryogenic callus (EC). Embryogenic callus has been found to have a high competence for embryogenesis ( Rustagi et al., 2019 ). Moreover, the embryogenic callus also provides the starting material for the formation of embryogenic cell suspensions (ECS). From these cell suspensions, somatic embryos are produced and plants are regenerated. The first successful protocol on somatic embryogenesis in bananas was reported by Cronauer-Mitra and Krikorian (1988) followed by Escalant Teision (1989) . However, despite their high regeneration potential, they are not widely used for propagation because of the frequent occurrence of somaclonal variations ( Kavitha et al., 2021 ). Banana regeneration has been successfully achieved using different types of explants as discussed in the following sections (Table S2).
3.1. Embryogenesis from leaf sheath and rhizome
Modified Schenk and Hildebrandt medium fortified with TDZ (5.0 μM) and 20.0 μM Dicamba (3,6-dichloro-2- methoxy benzoic acid) was used for inducing callus from rhizome slices and leaf bases of cooking banana (ABB) and dessert banana (AAA). Later, somatic embryos were obtained from the induced calli in cell suspension after one month in media augmented with zeatin (5.0 μM) in dessert (AA and AAA) and cooking (ABB) bananas ( Musa spp. ) ( Novak et al., 1989 ). Leaf sheath disks of 'Nanico' banana ( Musa sp ., AÁA group, Cavendish subgroup) were used to induce embryogenic calli on MS medium supplemented with activated charcoal (0.2 %), MES (2 [N-morpholino]ethanesulfonic acid) (15.3 mM), Picloram (414.0 μM), 2-iP (492.0 μM) and arginine (300 mM) (DA- Silva et al., 1998). Besides, MS medium supplemented with picloram (16.56 μM) induced embryogenic callus in Musa acuminata cv. Njalipoovan (AB). Further, subculturing of the callus in dark on MS basal media resulted in the development of shoots and roots ( Smitha and Nair, 2011 ).
3.2. Embryogenesis from zygotic embryos
Somatic embryogenesis was induced in ornamental banana ( Musa ornata Roxb .) using zygotic embryos propagated on semi-solid MS medium supplemented with 2,4–D (2.25 μM, 4.52 μM, 9.04 μM) + coconut water (5%) + 3% sucrose ( Cronauer and Krikorian, 1988 ). Embryo germination and growth were achieved through the elimination of 2,4-D and subsequent transfer of the somatic embryos to Schenk and Hildebrandt media. Similarly, somatic embryos were obtained in Musa balbisiana (BB) and Musa acuminata (AA) from zygotic embryos using MS medium containing picloram (7.5 μM) or NAA (5.5 μM). Plant regeneration was achieved on MS media augmented with NAA (5.3 μM) ( Eschalant and Teisson, 1989 ). Immature zygotic embryos of Musa acuminata ssp. burmannicoides and Musa acuminata ssp. malaccensis were used to induce embryogenic calli on MS medium containing picloram (7.5 μM). These calli were used to prepare embryogenic cell suspensions (ECS) in a liquid MS medium. ECS produced somatic embryos that germinated on an MS medium supplemented with BAP (0.22 μM) + IAA (1.14 μM) (Morroquin et al., 1993). However, regeneration of plantlets from immature embryos was higher than mature embryos ( Uma et al., 2021 ).
3.3. Embryogenesis from immature male/female flowers
Immature floral tissues have been reported to have excellent embryogenic potential and are thus widely used in in vitro cultures ( Ammirato, 1983 ). MS medium supplemented with 2,4-D (1.0 μM) + NAA (5.7 μM) + IAA (5.4 μM) + 1 mg/l biotin + 100 mg/l glutamine + 100 mg/l malt extract + 3% sucrose and gelled with 2.6 g/l phytagel was used for induction of embryogenic callus and regeneration of Musa acuminata cv. Mas (AA) using immature male flowers as the starting material ( Jalil et al., 2003 ). Callus was obtained in MS medium supplemented with 2,4-D (4.52 μM). However, embryo development occurred on MS medium supplemented with 2,4-D (0.23 μM). For embryo-to-plant retrieval, MS medium with BAP (9.76 μM) was used. 2,4-D has been used in many studies to induce somatic embryos from immature male flower buds of different cultivars of banana (Wei et al., 2005; Kulkarni et al., 2006; Sidha et al., 2006; Ali et al., 2013). Besides, embryogenic calli of Musa acuminata cv. Matti was obtained through inoculation of bract explants on MS medium supplemented with TDZ (0.45 μM) + 3% sucrose ( Divakaran and Nair, 2011 ). Embryo development was attained on MS + 8.18 μM biotin. Similar results were obtained in Musa acuminata cv. Njalipoovan using MS medium with TDZ (4.5 μM) + 3% sucrose for initiation of embryogenic callus and MS medium supplemented with 16.37 μM biotin for embryo development. In addition, embryogenic callus in Musa spp . Rasthali (AAB) was established using shoot tips as explants on MS medium augmented with 2, 4-D (9.05 μM) + zeatin (1.0 μM) and 1 mg/1 D-biotin and MS medium supplemented with 2,4-D (4.5 μM) + 1 mg/l D biotin +100 mg/l glutamine + 100 mg/l malt extract for induction of embryogenic cell suspension. Embryo development was finally achieved on ½ MS medium supplemented with Zeatin (10.0 μM) + 3% sucrose ( Ganapathi et al., 2001 ). In a recent study, somatic embryos were induced in three ornamental bananas using immature male flower buds as the starting material. In this study, it was observed that embryogenic calli desiccated up to 2 h at 25 ± 1 °C resulted in higher frequencies of embryo induction and maturation in comparison with non-desiccated embryos ( Natarajan et al., 2020 ).
3.4. Embryogenesis from scalps
Liquid ½ MS medium supplemented with zeatin (1.0 μM) and 2, 4-D (5.0 μM) was used to induce embryogenic cell suspensions of cooking banana cv. `Bluggoes' ( Musa spp. ABB group) using scalps as the explant. Embryo maturation was achieved in MS basal medium and further plant regeneration in ½ MS medium supplemented with BAP (10.0 μM) ( Dhed'a et al., 1991 ). Shoot tips of Musa sp. cavendish were inoculated in ½ MS medium supplemented with BAP (22.19 μM) and IAA (1.14 μM). Explants were subsequently transferred to a medium composed of ½ MS salts augmented with BAP (1.02 μM) and NAA (1.08 μM). The explants were further transferred to MS medium supplemented with BAP (0.88 μM) + 2,4-D (9.04 μM) + 1 mg/l biotin,whereby somatic embryos were observed after two weeks. Moreover, somatic embryos of hybrid banana FHIA-18 were obtained using the liquid medium in a bioreactor ( Koskyet al., 2002 ).
3.5. Embryogenesis from shoot tips obtained from in vitro cultures
Direct somatic embryos in banana ( Musa acuminata AAA cv. Grand Nain) were induced from split shoot tips obtained from 4 weeks old in vitro multiple shoot cultures on MS medium supplemented with picloram (4.14 μM) and BAP (0.22 μM). These somatic embryos germinated into plantlets on MS medium supplemented with NAA (0.53–2.68 μM) together with BAP (2.22–44.39 μM), or thidiazuron (4.54 μM) plus glutamine (200 mg/l) ( Remakanthan et al., 2014 ).
3.6. Plant regeneration from protoplasts
The isolation of protoplasts in a banana for the very first time was reported in 1984 from inflorescence–derived callus of Cavendish ( Musa AAA) ( Bakry, 1984 ). However, no regeneration was obtained from the protoplasts. Later, successful plant regeneration from protoplasts in wild banana Long Tavoy (AA) was obtained using immature seeds ( Megia et al., 1993 ). Embryogenic cell suspensions were established from the upper meristematic part of proliferating shoot-tips in cv. 'Bluggoe' ( Musa spp., ABB subgroup). Protoplasts were isolated from ECS and they directly formed somatic embryos without undergoing any callus phase on ½ MS salt solution, mannitol (5%), 2,4-D (5.0 μM), and agarose (0.8%) or Gelrite (0.2%) (Panis et al., 1993). Moreover, protoplast regeneration through somatic embryogenesis using immature male flowers of seven banana cultivars viz. Dominico and curare Enano (subgroup plantain AAB), IRFA 903, SF 265 and Col 49 (AA); Grand Nain and Gros Michel (subgroup Cavendish AAA) were achieved ( Assani et al., 2001 ). The conversion rate of protoplast into somatic embryos was 2% on MS medium supplemented with BAP (2.2 μM) and IAA (11.4 μM), vitamins of Morel + 3% sucrose, and solidified with 0.75% agarose. Of all the embryos, 43% were able to germinate and develop into plantlets ( Assani et al., 2001 ).
4. Genetic transformation of banana
In the current scenario of global climate change and threatened food security, genetic improvement of bananas using biotechnological tools has fascinated the researchers ( Ganapathi et al., 2021b , Ganapathi et al., 2021a ; Subrahmanyeswari and Gantait, 2022 ). There are several methods available for the genetic transformation of bananas including electroporation of protoplasts, Agrobacterium-mediated transformation, and particle bombardment using embryogenic cells ( Ganapathi et al., 2021a , Ganapathi et al., 2021b ; Tripathi et al., 2015 ). Nevertheless, Agrobacterium-mediated transformation is the most sought-after approach for the genetic improvement of bananas with the advent of biotechnology. This method has an edge over other techniques as it relies on the utilization of differentiated tissues that can be regenerated into the complete plant using routine protocols ( Tripathi et al., 2015 ). Besides, it offers integration of the transgene in low copy number and can transform larger stretches of DNA as compared to other direct methods of transformation ( Tripathi et al., 2015 ). In recent decades, banana has been successfully transformed using several genes that have resulted in improved stress endurance, introduction of novel attributes, and value addition into the Musa spp. as discussed below:
4.1. Genetic transformation for production of transgenic banana resistant to biotic and abiotic stresses
Banana plants experience different biotic and abiotic stresses through their production cycle that significantly hampers their overall productivity ( Li et al., 2021 ; Wang et al., 2021 ). Among biotic factors, the major diseases that are causing serious concerns and losses to banana yield currently include black Sigatoka, Fusarium wilt tropical race 4 (TR4), banana Xanthomonas wilt (BXW), and banana bunchy top disease ( De souza-pollo and de Goes , 2020 ). Among these, Fusarium wilt (Panama disease) caused by Fusarium oxysproum f. sp. cubense (Foc) was the first major calamity of banana (Gros Michel). There are four different races of Foc fungus, and all of these except race 3 are pathogenic ( Ganapathi et al., 2021 ). Fusarium wilt tropical race-4 (TR-4) imposes the sternest threat on the cultivation of bananas as it can destroy the banana plantations and result in global pandemics ( Rocha et al., 2021 ). As control of Foc using different physical, chemical, or biological methods is practically impossible, thus, in the past few decades, sufficient attention has been given to the development of Foc-resistant genetically engineered banana varieties ( Wang et al., 2021 ). Foc-resistant banana varieties have been generated by overexpressing the genes encoding for antifungal proteins like ferredoxin-like proteins, defensins, chitinase, lysozyme, anti-apoptosis proteins, etc from other organisms (Table S3). Another yield-limiting fungal pathogen that affects banana production is Mycosphaerella fijiensis which results in black Sigatoka disease. This disease can be managed by the use of certain chemicals, however, the arbitrarily use of such chemicals imposes a serious hazard on mankind and the environment ( Sowmya et al., 2016 ; Soares et al., 2021 ). Thus, the development of Sigatoka-resistant banana varieties by employing genetic engineering approach has provided a most suitable option to protect the plants from this harmful fungus ( Sowmya et al., 2016 ). Sigatoka-resistant banana has been developed by overexpressing antifungal proteins including chitinase, glucanase, RCC2, RCG3, etc (Table. S3) ( Kosky et al., 2010 ; Vishnevetsky et al., 2011 ; Kovács et al., 2013 ). Apart from fungal pathogens, the transgenics approach has been effectively used for the management of the bacterial disease Xanthomonas wilt which can devastate banana production ( Ocimati et al., 2020 ). The transgenic banana plants developed using sweet pepper Pflp or, Hrap genes showed high resistance to Xanthomonas campestris pv. Musa cearum that causes Xanthomonas wilt disease ( Namukwaya et al., 2012 ; Tripathi et al., 2014a , Tripathi et al., 2010 ). Similarly, transgenic banana plants expressing the rice Xa21 gene showed increased resistance towards the Xanthomonas wilt pathogen ( Tripathi et al., 2014b ).
Apart from bacterial and fungal pathogens, infestation by nematodes and weevils is another persistent challenge for banana cultivation. Nematodes account for almost 20% loss in global banana production that may rise to even 40% in tropical storms-prone areas ( Roderick et al., 2016 ). Nematodes are conventionally controlled with the use of harmful pesticides that contaminate the environment ( Tripathi et al., 2019 ). Thus, to circumvent this problem, nematode-resistant transgenic banana cultivars were developed using cysteine proteinase inhibitors (cystatins) and nicotinic acetylcholine receptors (naCHR) inhibiting peptides encoding genes. Cystatins inhibit the dietary protein's intestinal digestion, whereas naCHR inhibiting peptides interfere with the nervous system of pests ( Roderick et al., 2016 ) (Table S3). Rice cystatin ( OcIΔD86 ) transformed banana plants have been developed that show almost 69–70% resistance towards Radopholus similis ( Atkinson et al., 2004 ). In another attempt, genetic transformation of banana using maize naCHR inhibiting peptide or cystatin or both of these used together conferred resistance against Helicotylenchus multicinctus, and Radopholus similis under field trials conducted in a confined area in Uganda ( Roderick et al., 2012 ). Moreover, transgenic banana ‘Sukali Ndiizi’ (ABB) has been developed using the cystatin ( CpCYS-Mut89 ) gene from papaya for their potential resistance against common pests that infest banana. However, a precise evaluation of such plants for resistance against nematodes and weevils is yet to be conducted ( Namuddu et al., 2013 ).
Besides different biotic agents, abiotic stresses including salinity, sub-optimum temperatures, and drought also act as a major impediment to banana cultivation ( Ganapathi et al., 2021 ). Thus, researchers have undertaken profound efforts to improve the abiotic stress tolerance window of banana plants using a transgenic approach. Genetically modified banana plants with enhanced tolerance against salinity and drought stress were generated by overexpression of genes that belong to different families including WRKY transcription factors, late embryogenesis abundant (LEA) proteins, aquaporin coding genes, pathogenesis-related (PR) proteins, MYB transcription factors, NAC transcription factors, and several other stress-associated proteins, etc (Table S4) ( Sreedharan et al., 2012 , 2013 , 2015 ; Shekhawat and Ganapathi 2013 ; Rustagi et al., 2015 ; Dou et al., 2016 ). However, limited attention has been given to the generation of genetically engineered banana cultivars that can resist changes in their optimum growth temperature.
Although, appreciable efforts have been undertaken by the researchers to develop stress-tolerant genetically modified banana cultivars, yet there is no report so far on the commercialization of such varieties. A major portion of such studies are greenhouse restricted and are not extended to field conditions. However, recent field trials conducted for over 3 years using the RGA2 gene ( resistance gene analogue2 ) expressing TR4-resistant Cavendish transgenic plants showed promising results of resistance towards TR4 Foc strain ( Dale et al., 2017 ). Similarly, field trials are underway for the transgenic banana plants resistant to Xanthomonas wilt disease and nematodes. Moreover, researchers have identified several candidate genes that are associated with the stress-induced senescence and decreased productivity of banana plants. The manipulation of such genes using modern genomics approaches can enhance the stress endurance and production of banana plants in the coming years ( Lira et al., 2017 ; Tak et al., 2018 ; Ma et al., 2018 ).
4.2. Genetic transformation of banana for crop improvement
The improvement of banana cultivars for better agronomic traits to enhance their growth, yield and nutritional value are one of the prime agendas of researchers for long time ( Sipen et al., 2011 ; Ganapathi et al., 2021 ; Wang et al., 2021 ). A Global Programme for Musa Improvement named ‘PROMUSA’ has been initiated by the World Bank and INIBAP since 1997. The major aim of this program is to develop novel banana varieties using conventional breeding approaches in conjunction with genetic engineering to address the needs and challenges faced by banana farmers in different regions of the world ( Frison et al., 1998 ; Eksoy, 2018). It has been realized that semi-dwarf banana varieties with thick/sturdy stems and root system that shows better hydrotropism are ideal for increasing the production of banana ( Wang et al., 2021 ). However, limited genetic engineering efforts have been made to impart these traits in banana varieties. In one such investigation, the overexpression of the NAC domain-containing MaVND1 , MaVND2 , and MaVND3 genes in bananas has been observed to modulate the secondary wall deposition that may influence the stem thickness ( Negi et al., 2015 , 2016 ). In addition, the biofortification of bananas is one of the key components of the global mission of banana improvement, especially in the African continent ( Paul et al., 2018 ). Banana is one of the staple fruits for African populations; however, it has a lower content of iron, vitamin A, and protein ( Paul et al., 2018 ). As people in African nations especially younger women and children are mostly deficient in micronutrients; thus reasonable efforts have been undertaken for the biofortification of bananas to enhance the content of iron and vitamin A ( Amah et al., 2019 ; Ganapathi et al., 2021 ; Yadav et al., 2017 ). Iron fortification of bananas has been done using IRT1 , FRO2 , SFER , NAS1 , NAS2 , FER1 , and YSL2 genes from different sources ( Kumar et al., 2011 ; Moses et al., 2016 ; Yadav et al., 2017 ). Similarly, PSY2a , PSY1 , and CRTL genes have been used to generate pro-vitamin A-rich transgenic banana plants ( Paul et al., 2017 ). Moreover, a banana project named Banana-21 has been initiated by the National Banana Research Program of the National Agricultural Research Organisation (NARO) of Uganda in collaboration with the Centre for Tropical Crops and Biocommodities at Queensland University of Technology (QUT) in Australia since 2005 for the development of vitamin A-rich transgenic banana by stacking and overexpressing various genes that participate in the pro-vitamin A synthesis ( Paul et al., 2017 , 2018 ).
Along with biofortification, the genetic transformation of banana plants for ‘molecular farming’ has come into the limelight in recent years ( Tak et al., 2016 ). Molecular farming involves the production of pharmaceutically important products including antibodies, hormones, enzymes, and vaccines using plants ( Schillberg and Finnern, 2021 ). Bananas can serve as the ideal system for molecular farming owing to their year-round availability, large-scale cultivation, easy digestibility especially by infants, and availability of an efficient genetic transformation protocol, etc ( Tak et al., 2016 ). Earlier, embryogenic cells of bananas have been successfully transformed with hepatitis B surface antigen (HBsAg) for the generation of edible banana vaccines against hepatitis B ( Kumar et al., 2005 ). Thus, in the future banana can provide a suitable platform for the large-scale production of a range of pharmaceuticals.
5. Genome editing system
Genome editing is the latest approach that is in limelight for improving the different traits in crop plants ( Alvarez et al., 2021 ; Hüdig et al., 2022 ; Kaur et al., 2019 , 2022 ). Although, there are not many reports on exploring the potential of genome editing for the genetic improvement of bananas, yet, in the forthcoming era, genome editing may act as a saviour for sustaining banana cultivation. The initial experiments of genome editing in bananas were conducted on the Phytoene desaturase ( PDS ) gene. The PDS enzyme participates in the carotene biosynthetic pathway and its silencing results in the albino phenotype ( Tripathi et al., 2019 ). The mutations induced in this gene using single gRNA resulted in the silencing of the PDS gene with 59% efficiency in the “Rasthali” cultivar ( Kaur et al., 2018 ). Later, the same gene was mutated in the “Cavendish Williams” banana cultivar with the advent of polycistronic gRNAs ( Naim et al., 2018 ). In the year 2020, the PDS gene was silenced in “Sukali Ndiizi” and “Gonja Manjaya” cultivars by employing multiple gRNAs with an efficiency of almost 100% ( Ntui et al., 2020 ). All these efforts paved the way for the successful generation of genome-edited banana plants with specific elite traits. In 2019, endogenous banana streak virus (eBSV) that integrates into the B genome of Musa balbisiana was mutated using CRISPR/Cas. eBSV can reactivate upon exposure to stress conditions and due to this reason, the banana cultivars that comprise of atleast one B genome are not selected as a parent for genetic improvement of banana. Upon disruption of eBSV sequence in the B genome of banana using multiple gRNAs, approximately 75% of the mutated plants were observed to be asymptomatic in response to water stress thus affirming the successful deactivation of eBSV ( Tripathi et al., 2019 ). Moreover, the transgenic banana plants that carried the small interfering RNA (siRNA) designed to target the Fusarium transcription factor 1 ( ftf1 ) and the Foc velvet genes showed increased resistance towards Foc Race 1 ( Ghag et al., 2014 ). Similarly, silencing of ERG6 and ERG11 genes involved in ergosterol biosynthesis genes enhanced the resistance of banana plants towards Foc Race 4 ( Dou et al., 2020 ). The introduction of RNAi targets designed against replicase-associated protein ( Rep ) gene of viral origin was found to successfully resist BBTV infection in banana plants (Table S3) ( Shekhawat et al., 2012 ; Elayabalan et al., 2013 ). In another report, semi-dwarf plants of “Gros Michel” have been developed by mutating the gibberellin 20ox2 ( MaGA20ox2 ) gene involved in gibberellin biosynthesis ( Shao et al., 2020 ). Besides, the shelf life of banana fruits has been improved through the modulation of the MaAC O 1 gene that plays a key role in the ripening of banana fruits using CRISPR/Cas ( Hu et al., 2021 ). In a recent report, the role of the carotenoid cleavage dioxygenases4 ( CCDs4 ) gene in the regulation of carotenoid accumulation was unravelled using CRISPR/Cas. In this investigation, transgene-free editing of the banana genome was successfully done by transfecting the embryogenic cells and protoplasts of the banana ( Awasthi et al., 2022 ).
Till now, in the majority of the reports, the agrobacterium-mediated transformation method has been used for transforming the genome editing cassette to the different banana cultivars. But, as the banana is a sterile plant, the elimination of foreign DNA sequences derived from plasmids including the selection marker is a major hurdle ( Tripathi et al., 2021 ). To overcome this issue, researchers have suggested that preassembled Cas9 protein-gRNA ribonucleoproteins (RNPs) may be designed with specific gene targets ( Awasthi et al., 2022 ; Ntui et al., 2020 ). These RNPs could be coated on gold particles and can be transferred to either embryonic cells or protoplast through particle bombardment, etc. thus escaping the GM legislation and to further easing the commercialization of genome-edited bananas ( Awasthi et al., 2022 ; Ntui et al., 2020 ).
6. Integration of omics studies for banana improvement
An in-depth understanding of the structure and evolution of the banana genome is critical in overcoming the challenges faced by the banana breeders ( Sampangi and Ravishanker, 2016 ). Further, the precise identification and elucidation of the mode of action of key genes regulating different features of the banana plant are much warranted for the implementation of modern genomics approaches including genetic engineering and genome editing for banana crop improvement ( Tripathi et al., 2019 ). In this scenario, different omics-based methods involving metabolomics, proteomics, transcriptomics, and genomics have opened new avenues to improve banana varieties for ensuring their sustainable and eco-friendly cultivation ( Mohanty et al., 2017 ; Kissel and Carpentier, 2016 ). The size of the banana haploid genome was estimated to be 600 Mbp in M. acuminata and 550 Mbp in M. balbisiana using flow cytometry ( Dolezel et al. , 1994 ). The genome sequence of bananas was decoded by researchers working in association with the Global Musa Genomics Consortium in 2012 ( D'Hont et al., 2012 ). This unravelling of the blueprint of the banana genome provided the necessary impetus to the research efforts focused on the genetic improvement of bananas. The draft sequence of the banana genome suggests that there are almost 36000 protein-coding regions, 37 Micro RNA (MIR) families and around half of the genome is comprised of transposable elements ( Sampangi and Ravishanker, 2016 ). Besides, transcriptomics studies conducted using different parts of the plant including the flower, rhizome root, leaf, etc. aided in the precise functional interpretation of the critical elements of the banana genome ( Ibrahim and Thangjam, 2015 ; Singh et al., 2021 ). The comparative transcriptome analysis of different banana varieties assisted in the identification of key genes involved in the regulation of vital processes of the banana life cycle including growth, development, and disease resistance, etc ( Hu et al., 2017 ; Li et al., 2013 ; Sun et al., 2019 ; Dong et al., 2020 ; Kaushal et al., 2021 ; Yumbya et al., 2021 ). Moreover, transcriptomics studies generated useful resources to identify the critical genes that were not annotated in the banana draft genome sequence ( Li et al., 2013 ). The deep sequencing of the transcriptome of Foc 1 and Foc race 4 infected banana plants using Illumina unravelled almost 842 genes that were not annotated in the banana draft genome sequence ( Li et al., 2013 ). In addition to transcriptomics, several studies have been conducted to study the proteome of banana plants for the characterization and analysis of genetic variations observed in various banana varieties. Proteome analysis provided insights into the molecular dynamics of key proteins and their modulations at the post-transcriptional and post-translational levels during the vital processes of Foc resistance, drought tolerance, cold stress tolerance, fruit ripening, identification of banana allergens, etc ( Vanhove et al., 2012 ; Li et al., 2015 ; Nikolic et al., 2018 ; Dong et al., 2019 ; Bhuyian et al., 2020 ). Thus, understanding banana genetics through an integrative approach that involves comparative genomics in conjunction with transcriptomics and proteomics may provide better access to the genes and their cis-regulatory elements for the domestication of banana ‘new generations’ through breeding and for the development of genetically modified banana with elite traits.
Apart from the analysis of DNA, RNA, and proteins, the investigation of plant metabolomis has emerged as a powerful technology in recent years ( Price et al., 2020 ; Li et al., 2021 ). The metabolic profiling of bananas holds promise for the precise interpretation of biochemical diversity that exists within banana germplasm ( Drapal et al., 2016 ). Researchers have generated a database of metabolic fingerprinting of 20 different accessions of bananas using their vegetative parts. The varieties included in this collection comprised both diploid and triploid along with Musa acuminata , Musa balbisiana , and distant wild species ( Musa ornata ). These varieties were then categorized based on their differential metabolites and genotypes ( Drapal et al., 2016 ). As banana is a nutritionally rich plant, metabolic profiling in combination with other omics approaches can help the researchers to choose the banana varieties as per the consumer demands and elite agronomic traits for future crop improvement programs ( Price et al., 2020 ).
7. Nano-biotechnology for banana
The use of nano-biotechnology in the field plant science is emerging rapidly ( Arab et al., 2014 ). Lately, the exploration of the potential of nanoparticles (NPs) in improving the different traits of plants has grasped the attention of researchers ( Sanzari et al., 2019 ; Zahedi et al., 2020 ). For in vitro propagation and regeneration of bananas, the addition of NPs in the routine media has given promising preliminary results. The zinc (Zn) and zinc oxide (ZnO) NPs were effective in eliminating the 9 different types of bacterial and four types of fungal contaminants from in vitro grown cultures of bananas. They further positively influenced the rate of regeneration and rooting of plantlets ( Helaly et al., 2014 ). Moreover, augmentation of Ag NPs promoted the shoot proliferation rate in bananas. They also increased the shoot length, the number of roots per explant, root length, fresh weight, and the number of leaves ( Do et al., 2018 ). In addition, Ag NPs promoted callus induction, shoot regeneration, rooting, and secondary hardening during in vitro propagation of bananas ( Huong et al., 2021 ). Besides, the treatment of AgNPs was also effective in combating the spread of banana bunchy top virus (BBTV) in the field-grown Grand Nain banana. The treated plants showed an increase in leaf area, and dry weight compared to the control plants ( Mahfouze et al., 2020 ). The foliar application of chitosan nanoparticles (CH-NPs) increased the fresh weight and dry weight of hardened Musa acuminata var. Baxi. Further, the application of CH-NPs resulted in a decrease in reactive oxygen species (ROS) levels and malondialdehyde (MDA) content in response to cold stress ( Wang et al., 2021 ). Silicon oxide (SiO2-NPs) promoted shoot growth and proliferation, and also improved the photosynthesis in banana plants exposed to drought stress conditions. Moreover, a significant enhancement in phenolic secretion and a reduction in lipid peroxidation rate were observed at low concentrations of SiO2 (50 mg/l) ( Subrahmanyeswari and Gantait 2022 ). Further, silicon NPs were helpful in the mitigation of the salinity and drought stress-induced oxidative stress in banana plants. The treated plants also exhibited improved chlorophyll levels, and Na + /K + homeostasis under stress conditions ( Mahmoud et al., 2020 ).
8. Conclusion and future prospects
Banana is one of the most loved food crops across the globe owing to its taste, easy digestibility, and nutritional value. The use of biotechnology has provided ample opportunities to solve the different problems associated with the conventional cultivation of banana ( Figure 3 ). Traditionally, banana is cultivated using suckers as the planting material. However, these suckers often act as the reservoirs for different pests/pathogens that limit the productivity of the crop. Moreover, the age, size, and uneven maturity of suckers extend the crop duration. The establishment of various protocols for in vitro propagation of bananas has assured the availability of disease-free planting material throughout the year. However, still, an efficient and cost-effective protocol to propagate various nutritionally rich, recalcitrant banana varieties that can thrive in the changing global climate needs to be developed. In addition to micropropagation, the genetic transformations, biofortification and use of the banana in the production of edible vaccines have resulted in value addition to the crop. Although, many success stories have emerged in the past where biotechnology has served as a saviour for banana cultivation, still many hurdles limit the use of this approach to its full potential in banana improvement. The most important among these are the strict guidelines that restrict the field trials and commercialization of genetically modified banana plants. Moreover, the lack of public acceptance of genetically modified crops is another censorious issue. In this scenario, the recent breakthrough biotechnological tool CRISPR/Cas seems to be the samaritan option that can help in sustaining banana cultivation in near future. Researchers have identified several key candidate genes that regulate the agronomic and stress ameliorative traits in bananas. The exploitation of such genes using genome editing can assist in designing next-generation banana varieties that will be more palatable to the regulatory authorities and the public. Besides, considering the public's concern about genetically modified crops, the development of transgene-free or DNA-free genetics transformation and genome editing technology like the use of RNPs should be considered in the future.

Biotechnological interventions for the improvement of banana.
Declarations
Author contribution statement.
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Declaration of interest's statement.
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge the support from the Rashtriya Uchchattar Shiksha Abhiyan (RUSA-III) Program, Ministry of Human Resource Development (MHRD), Government of India, New Delhi; the Department of Biotechnology, Government of India, New Delhi (DBT-NER/AGRI/33/2016); and Centre for Agricultural Research and Innovation (CARI), Guru Nanak Dev University, Amritsar, Punjab, India.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
- Abdalla N., El-Ramady H., Seliem M.K., El-Mahrouk M.E., Taha N., Bayoumi Y., Dobránszki J. An academic and technical overview on plant micropropagation challenges. Horticulturae. 2022; 8 (8):677. [ Google Scholar ]
- Abdulmalik M.M., Usman I.S., Nasir A.U., Sani L.A. Micropropagation of banana (Musa spp) using temporary immersion bioreactor system. Bayero j. pure appl. sci. 2020; 12 (2):197–200. [ Google Scholar ]
- Adeniyi A.G., Adeoye A.S., Ighalo J.O., Onifade D.V. FEA of effective elastic properties of banana fiber-reinforced polystyrene composite. Mech. Adv. Mater. Struct. 2021; 28 (18):1869–1877. [ Google Scholar ]
- Adeniyi A.G., Ighalo J.O., Onifade D.V. Banana and plantain fiber-reinforced polymer composites. J. Polym. Eng. 2019; 39 (7):597–611. [ Google Scholar ]
- Agrawal A., Sanayaima R., Tandon R., Tyagi R.K. Cost-effective in vitro conservation of banana using alternatives of gelling agent (isabgol) and carbon source (market sugar) Acta Physiol. Plant. 2010; 32 (4):703–711. [ Google Scholar ]
- Ahmad I., Hussain T., Ashraf I., Nafees M., Maryam R.M., Iqbal M. Lethal effects of secondary metabolites on plant tissue culture. Am.-Eurasian J. Agric. Environ. Sci. 2013; 13 (4):539–547. [ Google Scholar ]
- Ahmed S., Sharma A., Bhushan B., Wali V.K., Bakshi P., Singh A.K. Studies on hardening and acclimatization of micropropagated plantlets of banana cv. Grand Naine. Bioscan. 2014; 9 (3):965–967. [ Google Scholar ]
- Akatwijuka O., Gepreel M.A.H., Abdel-Mawgood A., Yamamoto M., Saito Y., Hassanin A.H. Overview of banana cellulosic fibers: agro-biomass potential, fiber extraction, properties, and sustainable applications. Biomass Convers. Biorefin. 2022:1–17. [ Google Scholar ]
- Alvard D., Cote F., Teisson C. Comparison of methods of liquid medium culture for banana micropropagation: effects of temporary immersion of explants. PCTOC. 1993; 32 :55–60. [ Google Scholar ]
- Alvarez D., Cerda-Bennasser P., Stowe E., Ramirez-Torres F., Capell T., Dhingra A., Christou P. Fruit crops in the era of genome editing: closing the regulatory gap. Plant Cell Rep. 2021; 40 (6):915–930. [ PubMed ] [ Google Scholar ]
- Amah D., Van Biljon A., Brown A., Perkins-Veazie P., Swennen R., Labuschagne M. Recent advances in banana (Musa spp.) biofortification to alleviate vitamin A deficiency. Crit. Rev. Food Sci. Nutr. 2019; 59 (21):3498–3510. [ PubMed ] [ Google Scholar ]
- Ammirato P.V. The regulation of somatic embryo development in plant cell cultures: suspension culture techniques and hormone requirements. Biotechnol. 1983; 1 (1):68–73. [ Google Scholar ]
- Arab M.M., Yadollahi A., Hosseini-Mazinani M., Bagheri S. Effects of antimicrobial activity of silver nanoparticles on in vitro establishment of G× N15 (hybrid of almond× peach) rootstock. J. Genet Eng. Biotechnol. 2014; 12 (2):103–110. [ Google Scholar ]
- Aremu A.O., Bairu M.W., Szüčová l., Doležal K., Finnie J.F., Staden J.V. Shoot and root proliferation in ‘Williams’ banana: are the topolins better cytokinins. PCTOC. 2012; 111.2 :209–218. [ Google Scholar ]
- Assani A., Haicour R., Wenzel G., Cote F., Bakry F., Foroughi-Wehr B., Grapin A. Plant regeneration from protoplasts of dessert banana cv. Grande Naine (Musa spp., Cavendish sub-group AAA) via somatic embryogenesis. Plant Cell Rep. 2001; 20 (6):482–488. [ Google Scholar ]
- Atkinson H.J., Grimwood S., Johnston K., Green J. Prototype demonstration of transgenic resistance to the nematode Radopholussimilis conferred on banana by a cystatin. Transgenic Res. 2004; 13 (2):135–142. [ PubMed ] [ Google Scholar ]
- Awasthi P., Khan S., Lakhani H., Chaturvedi S., Kaur N., Singh J., Tiwari S. Transgene-free genome editing supports the role of carotenoid cleavage dioxygenase 4 as a negative regulator of β--carotene in banana. J. Exp. Bot. 2022; 73 (11):3401–3416. [ PubMed ] [ Google Scholar ]
- Ayenew B., Mengesha A., Tadesse T., GebreMariam E. Ensete ventricosum (Welw.) Cheesman: a cheap and alternative gelling agent for pineapple (Ananas comosus var. smooth cayenne) in vitro propagation. J. Microbiol. Biotechnol. Food Sci. 2021:640–652. [ Google Scholar ]
- Bairu M.W., Stirk W.A., Doležal K., Van Staden J. The role of topolins in micropropagation and somaclonal variation of banana cultivars ‘Williams’ and ‘Grand Naine’(Musa spp. AAA) PCTOC. 2008; 95 (3):373–379. [ Google Scholar ]
- Bakry F. Choix du mateáriel a` utiliser pour I’solement de protoplasts de bananier (Musa sp.) Fruits. 1984; 39 :449–452. [ Google Scholar ]
- Bello O.A., Esan E.B., Obembe O.O. Establishing surface sterilization protocol for nodal culture of Solanecio biafrae. IOP Conf. Ser. Earth Environ. Sci. 2018; 210 (1) [ Google Scholar ]
- Bello-Bello J.J., Cruz-Cruz C.A., Pérez-Guerra J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). In Vitro Cell. Dev. Biol. 2019; 55 (3):313–320. [ Google Scholar ]
- Bhaya M.H.M., Al-Razzaq Salim S.A. Impacts of plant growth regulators and light quality on banana (Musa spp) micropropagation. Plant Arch. 2019; 19 (1):1379–1385. [ Google Scholar ]
- Bhuiyan F., Campos N.A., Swennen R., Carpentier S. Characterizing fruit ripening in plantain and Cavendish bananas: a proteomics approach. J. Proteonomics. 2020; 214 [ PubMed ] [ Google Scholar ]
- Bhutani R., Shukla S., Shukla S. Impact of sterilants on culture establishment of indigenous Musa L. varieties: a step forward for conservation. Environ. Sci. Pollut. Res. 2021; 28 (4):3913–3919. [ PubMed ] [ Google Scholar ]
- Blakesley D. Uptake and metabolism of 6-benzyladenine in shoot proliferation of Musa and Rhododendron. PCTOC. 1991; 25 :69–74. [ Google Scholar ]
- Bohra P., Waman A.A., Sathyanarayana B.N., Umesha K., Gowda B. Influence of different growth regulators on in vitro multiplication of mixed diploid banana (Musa AB) Proc. Natl. Acad. Sci. India B Biol. Sci. 2016; 86 (1):179–185. [ Google Scholar ]
- Buah J.N., Kawamitsu Y., Sato S., Murayama S. Effects of different types and concentrations of gelling agents on the physical and chemical properties of media and the growth of banana (Musa spp.) in vitro. Plant Prod. Sci. 1999; 2 (2):138–145. [ Google Scholar ]
- Chamling N., Bhowmick N. Effect of secondary hardening media on the performance of in-vitro raised banana plantlets cv. Grand Naine. J. crop weed. 2021; 17 (1):93–98. [ Google Scholar ]
- Chu C.C., Wang C.C., Sun C.S., Hsu C., Yin K.C., Chu C.Y., Bi F.Y. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci. Sin. 1975; 18 :659–668. [ Google Scholar ]
- Cronauer-Mitra S.S., Krikorian A.D. Plant regeneration via somatic embryogenesis in the seeded diploid banana Musa ornata Roxb. Plant Cell Rep. 1988; 7 (1):23–25. [ PubMed ] [ Google Scholar ]
- Dagnew A., Shibru S., Debebe A., Lemma A., Dessalegn L., Berhanu B., Sierra Y.M. Micropropagation of banana varieties (Musa spp.) using shoot-tip culture. Ethiop. J. Agric. Sci. 2012; 22 (1):14–25. [ Google Scholar ]
- Dale J., Paul J.Y., Dugdale B., Harding R. Modifying bananas: from transgenics to organics. Sustainabilit y . 2017; 9 (3):333. [ Google Scholar ]
- De Anicezio L.C. Efeito de Antioxidantes e Descontaminantes no Estabelecimento de Explantes de Bananeira (Musa spp) in vitro. Uniciencia. 2012; 16 (1) [ Google Scholar ]
- De Souza-Pollo A., De Goes A. Handbook of Banana Production, Postharvest Science, Processing Technology, and Nutrition. 2020. Banana pathology and diseases; pp. 45–59. [ Google Scholar ]
- Debnath S., Khan A.A., Das A., Murmu I., Khan A., Mandal K.K. Genetic Diversity in Horticultural Plants. 2019. Genetic diversity in banana; pp. 217–241. [ Google Scholar ]
- Devi K., Gogoi M.B., Singh S., Sarmah B.K., Modi M.K., Sen P. In vitro regeneration of banana and assessment of genetic fidelity in the regenerated plantlets through RAPD. Annu. res. rev. 2017:1–11. [ Google Scholar ]
- Dhawale R., Patwari L., Sharma K., Bharose A. A mixture of Isubgol Husk together with agar as gelling agent for sugarcane callus induction. J. Pharm. Innov. 2021; 10 :657–680. [ Google Scholar ]
- Dhed'a D.B., Dumortier F., Panis B., Vuylsteke D. Plant regeneration in cell suspension cultures of the cooking banana cv. ‘Bluggoes’ (Musa spp. ABB group) Fruits. 1991; 46 (2):125–135. [ Google Scholar ]
- D'Hont A., Wincker P. 2012. The Sequence of the Banana (Musa Acuminata) Genome; p. W068. [ Google Scholar ]
- Divakaran S.P., Nair A.S. Somatic embryogenesis from bract cultures in diploid Musa acuminata cultivars from South India. Sci. Hortic. 2011; 131 :99–102. [ Google Scholar ]
- Do D.G., Dang T.K.T., Nguyen T.H.T., Nguyen T.D., Tran T.T., Hieu D.D. Effects of nano silver on the growth of banana (Musa spp.) cultured in vitro. J. Vietnam. environ. 2018; 10 (2):92–98. [ Google Scholar ]
- Doležel J., Doleželová M., Novák F.J. Flow cytometric estimation of nuclear DNAamountin diploid bananas (Musa acuminata and M. balbisiana) Biol. Plant. (Prague) 1994; 36 (3):351–357. [ Google Scholar ]
- Dong H., Li Y., Fan H., Zhou D., Li H. Quantitative proteomics analysis reveals resistance differences of banana cultivar ‘Brazilian’ to Fusarium oxysporum f. sp. cubense races 1 and 4. J. Proteonomics. 2019; 203 [ PubMed ] [ Google Scholar ]
- Dong H., Ye Y., Guo Y., Li H. Comparative transcriptome analysis revealed resistance differences of Cavendish bananas to Fusarium oxysporum f. sp. cubense race 1 and race 4. BMC Genet. 2020; 21 (1):1–17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dou T.X., Hu C.H., Sun X.X., Shao X.H., Wu J.H., Ding L.J., Yi G.J. MpMYBS3 as a crucial transcription factor of cold signaling confers the cold tolerance of banana. PCTOC. 2016; 125 (1):93–106. [ Google Scholar ]
- Dou T., Shao X., Hu C., Liu S., Sheng O., Bi F., Yi G. Host-induced gene silencing of Foc TR4 ERG6/11 genes exhibits superior resistance to Fusarium wilt of banana. Plant Biotechnol. J. 2020; 18 (1):11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Drapal M., Carvalho E., Houwe I., Rouard M., Sardos J., Amah D., Fraser P. XXIV Plant and Animal Genome Conference. San Diego, CA (USA) 2016. Metabolomics Approach to the Assessment of Banana Diversity and Traits. [ Google Scholar ]
- Duong T.N., Hong L.T.A., Watanabe H., Goi M., Tanaka M. Efficiency of a novel culture system by using light-emitting diode (LED) on in vitro and subsequent growth of micropropagated banana plantlets. Acta Hortic. 2003; 616 :121–127. [ Google Scholar ]
- Elayabalan S., Kalaiponmani K., Subramaniam S., Selvarajan R., Panchanathan R., Muthuvelayoutham R., Balasubramanian P. Developmentof Agrobacterium-mediated transformation of highly valued hill banana cultivar Virupakshi(AAB) for resistance to BBTV disease. World J. Microbiol. Biotechnol. 2013; 29 (4):589–596. [ PubMed ] [ Google Scholar ]
- El-Banna A.N., El-Mahrouk M.E., Dewir Y.H., Farid M.A., Abou Elyazid D.M., Schumacher H.M. Endophytic bacteria in banana in vitro cultures: molecular identification, antibiotic susceptibility, and plant survival. Horticulturae. 2021; 7 (12):526. [ Google Scholar ]
- El-Mahdy M.T., Youssef M. Genetic homogeneity and high shoot proliferation in banana (Musa acuminata Colla) by altering medium thiamine level and sugar typeIn Vitro Cell. Dev. Biol. Plant . 2019; 55 (6):668–677. [ Google Scholar ]
- Esan A.M., Akeredolu O.E., Adeniji A.O., Olaiya C.O. Comparative effects of gibberellic acid, salicylic acid and Bacillus subtilis on oxidative stress marker and antioxidant potential of Musa sapientum Linn. Arch. Phytopathol. Pflanzenschutz. 2022; 55 (5):549–563. [ Google Scholar ]
- Escalant J.V., Teisson C. Somatic embryogenesis and plants from immature zygotic embryos of the species Musa acuminata and Musa balbisiana. Plant Cell Rep. 1989; 7 (8):665–668. [ PubMed ] [ Google Scholar ]
- Escalona M., Cejas I., Gonzalez-Olmedo J., Capote I., Roels S., Canal M.J., Rodreguez R., Sandoval J., Debergh P. The effect of meta-topolin on plantain propagation using a temporary immersion bioreactor. Info. 2003; 12 :28–30. [ Google Scholar ]
- Estrela C., Estrela C.R., Barbin E.L., Spanó J.C.E., Marchesan M.A., Pécora J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002; 13 :113–117. [ PubMed ] [ Google Scholar ]
- FAOSTAT. 2020. www.fao.org [ Google Scholar ]
- Farahani F., Aminpoor H., Sheidai M., Noormohammad i Z., Mazinani M.H. An improved system for in vitro propagation of banana (Musa acuminate L.) cultivars. Asian J. Plant Sci. 2008; 7 (1):116–118. [ Google Scholar ]
- Farahani F., Majd A. Comparison of liquid culture methods and effect of temporary immersion bioreactor on growth and multiplication of banana (Musa, cv. Dwarf Cavendish) Afr. J. Biotechnol. 2012; 11 (33):8302–8308. [ Google Scholar ]
- Ferdous M.H., Billah A.A.M., Mehraj H., Taufique T., Uddin A.F.M.J. BAP and IBA pulsing for in vitro multiplication of banana cultivars through shoot-tip culture. J. biosci. agric. res. 2015; 3 (2):87–95. [ Google Scholar ]
- Food and Agriculture Organization of the United Nations . 2019. FAOSTAT Crops. http://www.fao.org/faostat/en/#data/QC Accessed on 13 November 2019. [ Google Scholar ]
- Frison E.A., Orjeda G., Sharrock S. 1998. Pro Musa: the Global Programme for Musa Improvement; pp. 6–8. [ Google Scholar ]
- Gamborg O.L., Miller R.A., Ojima K. Plant cell cultures. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968; 50 :151–158. [ PubMed ] [ Google Scholar ]
- Ganapathi T.R., Negi S., Tak H., Bapat V.A. Transgenic banana: current status, opportunities and challenges. Genetically Modified Crops. 2021:111–128. [ Google Scholar ]
- Ganapathi T.R., Higgs N.S., Balint-Kurti P.J., Arntzen C.J., May G.D., Van Eck J.M. Agrobacterium-mediated transformation of embryogenic cell suspensions of the banana cultivar Rasthali (AAB) Plant Cell Rep. 2001; 20 (2):157–162. [ PubMed ] [ Google Scholar ]
- Ganapathi T.R., Srinivas L., Suprasanna P., Bapat V.A. Regeneration of plants from alginate-encapsulated somatic embryos of banana cv. Rasthali (Musa spp. AAB group) Vitro Cell Dev. Biol. 2001; 37 (2):178–181. [ Google Scholar ]
- Ganapathi T.R., Negi S., Tak H., Bapat V.A. TransgenicBanana: current status, opportunities and challenges. Genetically Modified Crops. 2021:111–128. [ Google Scholar ]
- Gebeyehu A. Effects of different concentrations of BAP (6-Benzyl amino purine) and NAA (Naphthalene acetic acid) on Banana (Musa spp.) cv. giant Cavendish shoot proliferation. International Journal of Plant Science and Ecology. 2015; 1 (2):36–43. [ Google Scholar ]
- Ghag S.B., Shekhawat U.K., Ganapathi T.R. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol. J. 2014; 12 (5):541–553. [ PubMed ] [ Google Scholar ]
- Gubbuk H., Pekmezci M. The effect of different thidiazuron and indole butyric acid concentrations on in vitro propagation of 'grand nain' and 'basrai' banana cultivars. Acta Hortic. 2009; 829 :295–298. [ Google Scholar ]
- Gupta S.D., Prasad V.S.S. Vol. 2. 2006. Matrix-supported liquid culture systems for efficient micropropagation of floricultural plants; pp. 488–495. (Floriculture, ornamental and plant biotechnology: advances and topical issues). [ Google Scholar ]
- Gupta V., Guleri R., Gupta M., Kaur N., Kaur K., Kumar P., Anand M., Kaur G., Pati P.K. Anti-neuroinflammatory potential of Tylophora indica (Burm.f) Merrill and development of an efficient in vitro propagation system for its clinical use. PLoS One. 2020; 15 (3):e0230142. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hasan A.S., Khasim S.M., Ramudu J. Medicinal Plants: Biodiversity, Sustainable Utilization and Conservation. Springer; Singapore: 2020. Development of standard protocols for in vitro regeneration of some selected banana cultivars (Musa spp.) from India; pp. 743–759. [ Google Scholar ]
- Helaly M.N., El-Metwally M.A., El-Hoseiny H., Omar S.A., El-Sheery N.I. Effect of nanoparticles on biological contamination of'in vitro cultures and organogenic regeneration of banana. Aust. J. Crop. Sci. 2014; 8 (4):612–624. [ Google Scholar ]
- Heslop-Harrison J.S., Schwarzacher T. Domestication, genomics and the future for banana. Ann. Bot. 2007; 100 (5):1073–1084. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hoque F., Robbani M., Hasan M.F., Parvin J. Standardization of protocol for in vitro propagation of banana (Musa sapientum) J. Bangladesh Agric. Univ. 2018; 16 (1):27–30. [ Google Scholar ]
- Hossain M.A., Rubel M.H., Nasiruddin K.M., Evamoni F.Z. Influence of BAP and NAA on in vitro plantlet regeneration of local and exotic banana cultivars. J. Biosci. Agric. Res. 2016; 6 (2):553–564. [ Google Scholar ]
- Hrahsel L., Basu A., Sahoo L., Thangjam R. In vitro propagation and assessment of the genetic fidelity of Musa acuminata (AAA) cv. Vaibalhla derived from immature male flowers. Appl. Biochem. Biotechnol. 2014; 172 (3):1530–1539. [ PubMed ] [ Google Scholar ]
- Hu C., Sheng O., Deng G., He W., Dong T., Yang Q., Bi F. CRISPR/Cas9-mediated genome editing of MaACO1 (aminocyclopropane-1-carboxylate oxidase 1) promotes the shelf life of banana fruit. Plant Biotechnol. J. 2021; 19 (4):654. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hu W., Ding Z., Tie W., Yan Y., Liu Y., Wu C., Jin Z. Comparative physiological and transcriptomic analyses provide integrated insight into osmotic, cold, and salt stress tolerance mechanisms in banana. Sci. Rep. 2017; 7 (1):1–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hüdig M., Laibach N., Hein A.C. Genome editing in crop plant research— alignment of expectations and current developments. Plants. 2022; 11 (2):212. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hui A.V., Bhatt A., Keng C.L. Micropropagation of Musa acuminata x M. balbisiana cv. Pisang Awak (ABB genome) and three other cultivars. Pakistan J. Bot. 2012; 44 (2):777–780. [ Google Scholar ]
- Huong B.T.T., Xuan T.D., Trung K.H., Ha T.T.T., Duong V.X., Khanh T.D., Gioi D.H. Influences of silver nanoparticles in vitro morphogenesis of specialty king banana (Musa ssp.) in Vietnam. Plant Cell Biotechnol. Mol. Biol. 2021; 22 :163–175. [ Google Scholar ]
- Huq A., Akter S., Islam S., Khan S. In vitro plant regeneration in Banana (Musa sp.)cv. Sabri.Bangladesh J. Sci.Ind. Res. 2012; 47 (2):143–146. [ Google Scholar ]
- Hussein N. Effects of nutrient media constituents on growth and development of banana (Musa spp.) shoot tips cultured in vitro. Afr. J. Biotechnol. 2012; 11 (37):9001–9006. [ Google Scholar ]
- Ibrahim K.S., Kumar N.S., Thangjam R. Transcriptome Analysis in Banana. Transcriptomics 3: 117, 2 cultivars from northeast India as revealed by ITS and IRAP markers. Appl. Biochem. Biotechnol. 2015; 172 :3939–3948. [ PubMed ] [ Google Scholar ]
- Ighalo J.O., Adeniyi A.G. Thermodynamic modelling and temperature sensitivity analysis of banana (Musa spp.) waste pyrolysis. SN Appl. Sci. 2019; 1 (9):1–9. [ Google Scholar ]
- Jacobsen K., Omondi B.A., Almekinders C., Alvarez E., Blomme G., Dita M., Staver C. Seed degeneration of banana planting materials: strategies for improved farmer access to healthy seed. Plant Pathol. 2019; 68 (2):207–228. [ Google Scholar ]
- Jalil M., Khalid N., Yasmin O.R. Plant regeneration from embryogenic suspension cultures of Musa acuminata cv. Mas (AA) PCTOC. 2003; 75 (3):209–214. [ Google Scholar ]
- Kacar Y.A., Biçen B., Varol I., Mendi Y.Y., Serçe S., Çetiner S. Gelling agents and culture vessels affect in vitro multiplication of banana plantlets. Genet. Mol. Res. 2010; 9 (1):416–424. [ PubMed ] [ Google Scholar ]
- Kaminek M. Progress in cytokinin research. TIBTECH. 1992; 10 :159–162. [ Google Scholar ]
- Kang L., Zheng K., Xie Y., Deng Y., Yu Y., Zhu M., Deng X. Efficient tissue culture protocol for Magnolia lucida (Magnoliaceae) and confirmation of genetic stability of the regenerated plants. Plants. 2020; 9 (8):997. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kannahi M., Buvaneswari R. Review on micropropagation of musa accuminata l. AJMR (Am. J. Ment. Retard.) 2019; 8 (3):147–163. [ Google Scholar ]
- Kapadia C., Patel N. Sequential sterilization of banana (musa spp.) sucker tip reducing microbial contamination with highest establishment percentage. Bangladesh J. Bot. 2021; 50 (4):1151–1158. [ Google Scholar ]
- Kaur K., Dolker D., Behera S., Pati P.K. Critical factors influencing in vitro propagation and modulation of important secondary metabolites in Withania somnifera (L.) dunal. PCTOC. 2022:1–20. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kaur K., Singh P., Kaur K., Bhandawat A., Nogia P., Pati P.K. Development of robust in vitro culture protocol for the propagation of genetically and phytochemically stable plants of Withania somnifera (L.) Dunal (Ashwagandha) Ind. Crop. Prod. 2021; 166 [ Google Scholar ]
- Kaur N., Alok A., Kaur N., Pandey P., Awasthi P., Tiwari S. CRISPR/Cas9-mediated efficient editing in phytoene desaturase (PDS) demonstrates precise manipulation in banana cv. Rasthali genome. Funct. Integr. Genomics. 2018; 18 (1):89–99. [ PubMed ] [ Google Scholar ]
- Kaur N., Kaur G., Pati P.K. Advances in Rice Research for Abiotic Stress Tolerance. Woodhead Publishing; 2019. Deciphering strategies for salt stress tolerance in rice in the context of climate change; pp. 113–132. [ Google Scholar ]
- Kaushal M., Mahuku G., Swennen R. Comparative transcriptome and expression profiling of resistant and susceptible banana cultivars during infection by Fusarium oxysporum. Int. J. Mol. Sci. 2021; 22 (6):3002. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kavitha N., Saraswathi M.S., Kannan G., Bathrinath M., Backiyarani S., Uma S. Development of direct regeneration protocol for mass multiplication of Musa spp. variety Udhayam (Pisang Awak, ABB) using different explants. Sci. Hortic. 2021; 290 [ Google Scholar ]
- Khan A., Bashir A., Erum S., Khatak J.Z.K., Muhammad A. Effects of 6-Benzylaminopurine and Indole-3-acetic acid on growth and root development of banana explants in micropropagation. Sarhad J. Agric. 2021; 37 (1):9–13. [ Google Scholar ]
- Khatik N., Joshi R. In vitro adventitious shoot regeneration from cotyledon and hypocotyl explants of Murraya koenigii (l) spreng. J. Phytol. Res. 2016; 29 (1):7–16. [ Google Scholar ]
- Khatri A., Khan I.A., Siddiqui S.H., Ahmad M., Siddiqui K.A. In vitro culture of indigenous and exotic banana clones for maximising multiplication. Pakistan J. Bot. 1997; 29 :143–150. [ Google Scholar ]
- Khatun F., Hoque M.E., Huq H., Adil M., Ashraf-Uz-Zaman K., Rabin M.H. Effect of BAP and IBA on in vitro regeneration of local banana variety of sabri. Biotechnol. J. Int. 2017; 18 (1):1–10. [ Google Scholar ]
- Kissel E., Carpentier S.C. Banana. Genomics And Transgenic Approaches For Genetic Improvement. Springer; Singapore: 2016. Abiotic stress tolerance research using-omics approaches; pp. 77–91. [ Google Scholar ]
- Ko W.H., Su C.C., Chen C.L., Chao C.P. Control of lethal browning of tissue culture plantlets of Cavendish banana cv. Formosana with ascorbic acid. PCTOC. 2009; 96 (2):137–141. [ Google Scholar ]
- Kodym A., Zapata-Arias F.J. Low-cost alternatives for the micropropagation of banana. PCTOC. 2001; 66 (1):67–71. [ Google Scholar ]
- Kosky R.G., Chong-Pérez B., López-Torres J., Reyes M., Bermúdez-Caraballoso I., Martín N.M., Hernández L. Plantain (Musa spp. cv.‘Navolean’AAB) transgenic plants from Agrobacterium tumefaciens-mediated transformation of embryogenic cell suspensions. Biotecnol. Veg. 2010; 10 (4):209–218. [ Google Scholar ]
- Kosky R.G., Silva M.deF.., Perez L.P., Gilliard T., Martínez F.,B., Vega M.R., Milian M.C., Mendoza E Q. Somatic embryogenesis of the banana hybrid cultivar FHIA-18 (AAAB) in liquid medium and scaled-up in a bioreactor. PCTOC. 2002; 68 (1):21–26. [ Google Scholar ]
- Kovács G., Sági L., Jacon G., Arinaitwe G., Busogoro J.P., Thiry E., Remy S. Expression of a rice chitinase gene in transgenic banana (‘Gros Michel’, AAA genome group) confers resistance to black leaf streak disease. Transgenic Res. 2013; 22 (1):117–130. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Krishna H., Sairam R.K., Singh S.K., Patel V.B., Sharma R.R., Grover M.N.L., Sachdev A. Mango explants browning: effect of ontogenic age, mycorrhization and pre- treatments. Sci. Hortic. 2008; 118 (2):132–138. [ Google Scholar ]
- Kumar G.B.S., Srinivas L., Ganapathi T.R. Iron fortification of banana by the expression of soybean ferritin. Biol. Trace Elem. Res. 2011; 142 (2):232–241. [ PubMed ] [ Google Scholar ]
- Kumar G.S., Ganapathi T.R., Revathi C.J., Srinivas L., Bapat V.A. Expression of hepatitis B surface antigen in transgenic banana plants. Planta. 2005; 222 (3):484–493. [ PubMed ] [ Google Scholar ]
- Lamare A., Otaghvari A.M., Rao S.R. Phylogenetic implications of the internal transcribed spacers of nrDNA and chloroplast DNA fragments of Musa in deciphering the ambiguities related to the sectional classification of the genus. Genet. Resour. Crop Evol. 2017; 64 (6):1241–1251. [ Google Scholar ]
- Li C., Shao J., Wang Y., Li W., Guo D., Yan B., Peng M. Analysis of banana transcriptome and global gene expression profiles in banana roots in response to infection by race 1 and tropical race 4 of Fusarium oxysporum f. sp. cubense. BMC Genet. 2013; 14 (1):1–16. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li T., Li M., Jiang Y., Duan X. Genome-wide identification, characterization and expression profile of glutaredoxin gene family in relation to fruit ripening and response to abiotic and biotic stresses in banana (Musaacuminata) Int. J. Biol. Macromol. 2021; 170 :636–651. [ PubMed ] [ Google Scholar ]
- Li T., Yun Z., Zhang D., Yang C., Zhu H., Jiang Y., Duan X. Proteomicanalysisof differentially expressed proteins involved in ethylene-induced chilling tolerance in harvested banana fruit. Front. Plant Sci. 2015; 6 :845. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Linsmaier E.M., Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plantarum. 1965; 18 :100–127. [ Google Scholar ]
- Lira B.S., Gramegna G., Trench B.A., Alves F.R., Silva E.M., Silva G.F., Rossi M. Manipulation of a senescence-associated gene improves fleshy fruit yield. Plant Physiol. 2017; 175 (1):77–91. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Loyola-Vargas V.M., Ochoa-Alejo N. An introduction to plant tissue culture: advances and perspectives. Plant cell culture protocols. 2018:3–13. [ PubMed ] [ Google Scholar ]
- Ma X., Zhang Y., Turečková V., Xue G.P., Fernie A.R., Mueller-Roeber B., Balazadeh S. The NAC transcription factor SlNAP2 regulates leaf senescence and fruit yield in tomato. Plant Physiol. 2018; 177 (3):1286–1302. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mahadev S.R., Kathithachalam A., Marimuthu M. An efficient protocol for large-scale plantlet production from male floral meristems of Musa spp. cultivars Virupakshi and Sirumalai. In Vitro Cell. Dev. Biol. 2011; 47 (5):611–617. [ Google Scholar ]
- Mahdi R., Islam M.J., Rahman M.A., Biswas A., Azam F.S., Rahmatullah M. In vitro regeneration protocol for anupam and chini champa: two banana (Musa sapientum) cultivars of Bangladesh. Am.-Eurasian J. Sustain. Agric. (AEJSA) 2014; 8 :28–33. [ Google Scholar ]
- Mahfouze H.A., El-Dougdoug N.K., Mahfouze S.A. Virucidal activity of silver nanoparticles against Banana bunchy top virus (BBTV) in banana plants. Bull. Natl. Res. Cent. 2020; 44 (1):1–11. [ Google Scholar ]
- Mahmoud L.M., Dutt M., Shalan A.M., El-Kady M.E., El-Boray M.S., Shabana Y.M., Grosser J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. South Afr. J. Bot. 2020; 132 :155–163. [ Google Scholar ]
- Makara A.M., Rubaihayo P.R., Magambo M.J.S. Carry-over effect of Thidiazuron on banana in vitro proliferation at different culture cycles and light incubation conditions. Afr. J. Biotechnol. 2010; 9 (21):3079–3085. [ Google Scholar ]
- Manjula R., Praveen J., Sunnaiah K.V., Swamy G.S.K., Prabhuling G. Enhancement of in vitro shoot multiplication in banana cv. Rajapuri (AAB) using TDZ. Res. J. Biotechnol. 2015; 10 (5):5–10. [ Google Scholar ]
- Manokari M., Badhepuri M.K., Cokulraj M., Rajput B.S., Dey A., Faisal M., Shekhawat M.S. High-throughput in vitro propagation and evaluation of foliar micro- morpho-anatomical stability in Musa acuminata cv.‘Grand Nain’using 6-benzoyladenine (BOA) in the nutrient medium. Sci. Hortic. 2022; 304 [ Google Scholar ]
- Mardhikasari S., Yunus A. Modification of media for banana in vitro propagation with foliar fertilizer and coconut water in cv. Rajabulu. Caraka Tani: J. Sustain. Agric. 2020; 35 (1):23–32. [ Google Scholar ]
- Mathew N.S., Kurrey N.K., Bettadaiah B.K., Negi P.S. Anti-proliferative activity of Ensete superbum Roxb. Cheesman extract and its active principles on human colorectal cancer cell lines. J. Food Sci. 2021; 86 (11):5026–5040. [ PubMed ] [ Google Scholar ]
- Megia R., Haicour R., Tizroutine S., Trang V.B., Rossignol L., Sihachakr D., Schwendiman J. Plant regeneration from cultured protoplasts of the cooking banana cv. Bluggoe (Musa spp., ABB group) Plant Cell Rep. 1993; 13 (1):41–44. [ PubMed ] [ Google Scholar ]
- Mekonen G., Egigu M.C., Muthsuwamy M. In vitro propagation of banana (Musa paradisiaca L.) plant using shoot tip explant. TURJAF) 2021; 9 (12):2339–2346. [ Google Scholar ]
- Menon R. Banana: Genomics and Transgenic Approaches for Genetic Improvement. Springer; Singapore: 2016. Banana breeding; pp. 13–34. [ Google Scholar ]
- Mihaljević I., Dugalić K., Tomaš V., Viljevac M., Pranjić A., Čmelik Z., Jurković Z. In vitro sterilization procedures for micropropagation of ‘Oblačinska’sour cherry. J. Agric. Sci. 2013; 58 (2):117–126. [ Google Scholar ]
- Mohanty S., Das M.P., Surabhi G.K. ‘OMICS’-approach to regulate ripening and enhance fruit shelf-life in banana: an important fruit crop for food security. Can J Biotech. 2017; 1 :289. [ Google Scholar ]
- Moses M. Queensland University of Technology at Brisbane; Queensland, Australia ) : 2016. Molecular and Biochemical Characterisation of Transgenic Banana Lines Containing Iron Uptake and Storage Enhancing Genes (Doctoral Dissertation, PhD Thesis. [ Google Scholar ]
- Naim F., Dugdale B., Kleidon J., Brinin A., Shand K., Waterhouse P., Dale J. Gene editing the phytoene desaturase alleles of Cavendish banana using CRISPR/Cas9. Transgenic Res. 2018; 27 (5):451–460. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Namuddu A., Kiggundu A., Mukasa S.B., Kurnet K., Karamura E., Tushemereirwe W. Agrobacterium mediated transformation of banana (Musa sp.) cv. Sukali Ndiizi (ABB) with a modified Carica papaya cystatin (CpCYS) gene. Afr. J. Biotechnol. 2013; 12 (15) [ Google Scholar ]
- Namukwaya B., Tripathi L., Tripathi J.N., Arinaitwe G., Mukasa S.B., Tushemereirwe W.K. Transgenic banana expressing Pflp gene confers enhanced resistance to Xanthomonas wilt disease. Transgenic Res. 2012; 21 (4):855–865. [ PubMed ] [ Google Scholar ]
- Nansamba M., Sibiya J., Tumuhimbise R., Karamura D., Kubiriba J., Karamura E. Breeding banana (Musa spp.) for drought tolerance: a review. Plant Breed. 2020; 139 (4):685–696. [ Google Scholar ]
- Natarajan N., Sundararajan S., Ramalingam S., Chellakan P.S. Efficient and rapid in- vitro plantlet regeneration via somatic embryogenesis in ornamental bananas (Musa spp.) Biologia. 2020; 75 (2):317–326. [ Google Scholar ]
- Negi S., Tak H., Ganapathi T.R. Cloning and functional characterization of Musa VND1 using transgenic banana plants. Transgenic Res. 2015; 24 (3):571–585. [ PubMed ] [ Google Scholar ]
- Negi S., Tak H., Ganapathi T.R. Functional characterization of secondary wall deposition regulating transcription factors MusaVND2 and MusaVND3 in transgenic banana plants. Protoplasma. 2016; 253 (2):431–446. [ PubMed ] [ Google Scholar ]
- Ngomuo M., Mneney E., Ndakidemi P. Control of lethal browning by using ascorbic acid on shoot tip cultures of a local Musa spp.(Banana) cv. Mzuzu in Tanzania. Afr. J. Biotechnol.1. 2014; 3 (16) [ Google Scholar ]
- Nhut D.T., Nam N.B. The Third International Conference on the Development of Biomedical Engineering in Vietnam. Springer; Berlin, Heidelberg: 2010. Light-emitting diodes (LEDs): an artificial lighting source for biological studies; pp. 134–139. [ Google Scholar ]
- Nikolić J., Nešić A.N., Kull S., Schocker F., Jappe U., Gavrović-Jankulović M., Supplementary Material for the Article. Nikolić J., Nešić A., Kull S., Schocker F., Jappe U., Gavrović-Jankulović M. Employment of proteomic and immunological based methods for the identification of catalase as novel allergen from banana. J. Proteonomics. 2018; 175 :87–94. [ PubMed ] [ Google Scholar ]
- Nkengla-Asi L., Eforuoku F., Olaosebikan O., Adejoju Ladigbolu T., Amah D., Hanna R., Kumar P.L. Gender roles in sourcing and sharing of banana planting material in communities with and without banana bunchy top disease in Nigeria. Sustainability. 2021; 13 (6):3310. [ Google Scholar ]
- Novak F.J., Afza R., Van Duren M., Perea-Dallos M., Conger B.V., Xiaolang T. Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA and AAA) and cooking (ABB) bananas (Musa spp.) Biotechnol. 1989; 7 (2):154–159. [ Google Scholar ]
- Nowakowska K., Pińkowska A., Siedlecka E., Pacholczak A. The effect of cytokinins on shoot proliferation, biochemical changes and genetic stability of Rhododendron ‘Kazimierz Odnowiciel’in the in vitro cultures. PCTOC. 2022; 149 (3):675–684. [ Google Scholar ]
- Ntui V.O., Tripathi J.N., Tripathi L. Robust CRISPR/Cas9 mediated genome editing tool for banana and plantain (Musa spp.) Curr. Plant Biol. 2020; 21 [ Google Scholar ]
- Ocimati W., Groot J.J., Tittonell P., Taulya G., Ntamwira J., Amato S., Blomme G. Xanthomonas wilt of banana drives changes in land-use and ecosystem services across infected landscapes. Sustainability. 2020; 12 (8):3178. of banana using alternatives of gelling agent (isabgol) and carbon source (market sugar). Acta. [ Google Scholar ]
- Oliveira H.S.D., Lemos O.F.D., Miranda V.S., Moura H.C.D.P., Campelo M.F., Santos L.R.R.D. Estabelecimento e multiplicação in vitro de brotos no processo de micropropagação de cultivares de bananeira (Musa spp.) Acta Amazonica. 2011;(41):369–376. [ Google Scholar ]
- Onuoha I.C., Eze C.J., Unamba C.I.N. In vitro prevention of browning in plantain culture. Online J. Biol. Sci. 2011; 11 (1):13–17. [ Google Scholar ]
- Palanyandy S.R., Gantait S., Sinniah U.R. Effects of some gelling agents and their concentrations on conversion of oil palm polyembryoids into plantlets. J. Genet. Eng. Biotechnol. 2020; 18 (1):1–5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Park S.Y., Ho T.T., Paek K.Y. Orchid Biology: Recent Trends & Challenges. Springer; Singapore: 2020. Medicinal orchids: production of bioactive compounds and biomass; pp. 439–450. [ Google Scholar ]
- Pati P.K., Rath S.P., Sharma M., Sood A., Ahuja P.S. In vitro propagation of rose- a review. Biotechnol. Adv. 2006; 24 (1):94–114. [ PubMed ] [ Google Scholar ]
- Paul J.Y., Harding R., Tushemereirwe W., Dale J. Banana 21: from gene discovery to deregulated golden bananas. Front. Plant Sci. 2018; 9 :558. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paul J.Y., Khanna H., Kleidon J., Hoang P., Geijskes J., Daniells J., Dale J. Golden bananas in the field: elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant Biotechnol. J. 2017; 15 (4):520–532. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Prabhuling G., Sathyanarayana B.N. Liquid medium culture method for rapid multiplication of banana (Musa acuminata) cv.‘Grand Naine’through tissue culture. Int. J. Plant Sci. 2017; 12 (1):85–89. [ Google Scholar ]
- Prakasha D., Ramya G., Shivayogi R., Gajanana K., Kattimani K. Kamalapur red (AAA); 2019. In Vitro Mass Propagation System in Banana Cv. [ Google Scholar ]
- Preil W. Micropropagation. Springer; Dordrecht: 1991. Application of bioreactors in plant propagation; pp. 425–445. [ Google Scholar ]
- Price E.J., Drapal M., Perez-Fons L., Amah D., Bhattacharjee R., Heider B., Fraser P.D. Metabolite database for root, tuber, and banana crops to facilitate modern breeding in understudied crops. Plant J. 2020; 101 (6):1258–1268. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Qamar M., Qureshi S.T., Khan I.A., Raza S. Optimization of in vitro multiplication for exotic banana (Musa spp.) in Pakistan. Afr. J. Biotechnol. 2015; 14 (24):1989–1995. [ Google Scholar ]
- Quiñonez L., Ponce F.M., Moreira M.D.V., Mieles A.M., Gavilanes F.Z., Alcívar F.A., Cedeño-García G., Vélez-Olmedo J., Jaimez R.E. Effect of auxins, cytokinin and activated charcoal on in vitro propagation of plantains barraganete and curare (musa AAB). Proc.Natl. Acad. Sci. India B: biological sciences. Proc. Biol. Sci. 2021; 91 (2):431–440. [ Google Scholar ]
- Rajoriya P., Singh V.K., Lall N.J.R. Optimizing the effect of plant growth regulators on in vitro micro propagation of Indian red banana (Musa acuminata) J. Pharmacogn. Phytochem. 2018; 8 :628–634. [ Google Scholar ]
- Rajput P., Agarwal P., Gangapur D.R., Agarwal P.K. Development of a high-frequency adventitious shoot regeneration using cotyledon explants of an important oilseed crop Sesamum indicum L. In Vitro Cell. Dev. Biol. 2022:1–9. [ Google Scholar ]
- Ranjha M.M.A.N., Irfan S., Nadeem M., Mahmood S. A comprehensive review on nutritional value, medicinal uses, and processing of banana. Food Rev. Int. 2020:1–27. [ Google Scholar ]
- Remakanthan A., Menon T.G., Soniya E.V. Somatic embryogenesis in banana (Musa acuminata AAA cv. Grand Naine): effect of explant and culture conditions. In Vitro Cell. Dev. Biol. 2014; 50 (1):127–136. [ Google Scholar ]
- Resmi L., Nair A.S. Differential effect of cytokinins in the micropropagation of diploid and triploid Musa cultivars. Int. J. Integr. Biol. 2011; 11 (1):35–38. [ Google Scholar ]
- Robinson J.C., Sáuco V.G. Weaning (acclimatization) of in vitro-produced banana plants. Fruits. 2009; 64 (5):325–332. [ Google Scholar ]
- Rocha A.D.J., Soares J.M.D.S., Nascimento F.D.S., Santos A.S., Amorim V.B.D.O., Ferreira C., Haddad F., Santos-Serejo J.A.D.F., Amorim E.P. Improvements in the resistance of the banana species to Fusarium wilt: a systematic review of methods and perspectives. J. Fungi. 2021; 7 (4):249. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Roderick H., Tripathi L., Babirye A., Wang D., Tripathi J., Urwin P.E., Atkinson H.J. Generation of transgenic plantain (Musa spp.) with resistance to plant pathogenic nematodes. Mol. Plant Pathol. 2012; 13 (8):842–851. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Roderick H., Tripathi L., Poovarasan S. Banana: Genomics and Transgenic Approaches for Genetic Improvement. Springer; Singapore: 2016. Transgenic approaches to improve resistance to nematodes and weevils; pp. 247–260. [ Google Scholar ]
- Rodrigues P.H.V., Oliveira E.L., Demetrio C.A., Ambrosano G.B., Piedade S.M.S. Effects of different light spectra on the slow-grown in vitro storage and quality of banana plantlets cv. Prata Catarina (AAB) PCTOC. 2022:1–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rowe P., Rosales F. INIBAP; Montpellier, France: 1994. Musa Breeding at FHIA. The Improvement and Testing of Musa: a Global Partnership ; pp. 117–129. [ Google Scholar ]
- Roy O.S., Bantawa P., Ghosh S.K., da Silva J.A.T., Ghosh P.D., Mondal T.K. Micropropagation and field performance of ‘Malbhog’(Musa paradisiaca, AAB group): a popular banana cultivar with high keeping quality of North East India. Tree For. Sci. Biotech. 2010; 4 (1):52–58. [ Google Scholar ]
- Rustagi A., Jain S., Kumar D., Shekhar S., Jain M., Bhat V., Sarin N.B. High efficiency transformation of banana [Musa acuminata L.cv. Matti (AA)] for enhanced tolerance to salt and drought stress through overexpression of a peanut salinity-induced pathogenesis-related class 10 protein. Mol. Biotechnol. 2015; 57 (1):27–35. [ PubMed ] [ Google Scholar ]
- Rustagi A., Shekhar S., Kumar D., Lawrence K., Bhat V., Sarin N.B. High speed regeneration via somatic embryogenesis in elite Indian banana cv. Somrani monthan (ABB) Vegetos. 2019; 32 (1):39–47. [ Google Scholar ]
- Safarpour M., Sinniah U.R., Subramaniam S., Swamy M.K. A novel technique for Musa acuminata Colla ‘Grand Naine’(AAA) micropropagation through transverse sectioning of the shoot apex. In Vitro Cell. Dev. Biol. 2017; 53 (3):226–238. [ Google Scholar ]
- Sajid G.M., Pervaiz S. Bioreactor mediated growth, culture ventilation, stationary and shake culture effects on in vitro growth of sugarcane. Pakistan J. Bot. 2008; 40 (5):1949–1956. [ Google Scholar ]
- Sampangi-Ramaiah M.H., Ravishankar K.V. Banana: Genomics and Transgenic Approaches for Genetic Improvement. Springer; Singapore: 2016. Current status of banana genome in the age of next generation sequencing; pp. 51–59. [ Google Scholar ]
- Sanzari I., Leone A., Ambrosone A. Nanotechnology in plant science: to make a long story short. Front. Bioeng. Biotechnol. 2019; 7 :120. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Saraswathi M.S., Uma S., Kannan G., Selvasumathi M., Mustaffa M.M., Backiyarani S. Cost-effective tissue culture media for large-scale propagation of three commercial banana (Musa spp.) varieties. J. Hortic. Sci. Biotechnol. 2016; 91 (1):23–29. [ Google Scholar ]
- Schenk R.U., Hildebrand t H.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell culture. Can. J. Bot. 1972; 50 :199–204. [ Google Scholar ]
- Schillberg S., Finnern R. Plant molecular farming for the production of valuable proteins–Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021; 258 [ PubMed ] [ Google Scholar ]
- Selvakumar S., Parasurama D.S. Maximization of micropropagule production in banana cultivars Grand naine (AAA) and Elakki (AB). In Vitro Cell. Dev. Biol. 2020; 56 (4):515–525. [ Google Scholar ]
- Shankar C.S., Balaji P., Sekar D.S. Mass propagation of banana (Musa sp.) cv. grand naine through direct organogenesis by benzyl adenine purine and kinetin. J. Acad. ind. res. 2014; 3 (2):92–97. [ Google Scholar ]
- Shao X., Wu S., Dou T., Zhu H., Hu C., Huo H., Yi G. Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana. Plant Biotechnol. J. 2020; 18 (1):17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shashikumar R., Krishna V., Lokesha S. Analysis of genetic variations in Musa paradisiaca cv Karibale-Monthan using AFLP markers. Indian J. Agric. Res. 2017; 51 (6):543–549. [ Google Scholar ]
- Shekhawat U.K., Ganapathi T.R. MusaWRKY71 overexpression in banana plants leads to altered abiotic and biotic stress responses. PLoS One. 2013; 8 (10) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shekhawat U.K., Ganapathi T.R., Hadapad A.B. Transgenic banana plants expressing small interfering RNAs targeted against viral replication initiation genedisplay high-level resistance to banana bunchy top virus infection. J. Gen. Virol. 2012; 93 (8):1804–1813. [ PubMed ] [ Google Scholar ]
- Shirani S., Mahdavi F., Maziah M. Morphological abnormality among regenerated shoots of banana and plantain (Musa spp.) after in vitro multiplication with TDZ and BAP from excised shoot tips. Afr. J. Biotechnol. 2009; 8 (21) [ Google Scholar ]
- Singh P., Guleri R., Angurala A., Kaur K., Kaur K., Kaul S.C., Wadhwa R., Pati P.K. Addressing challenges to enhance the bioactives of Withania somnifera through organ, tissue, and cell culture based approaches. BioMed Res. Int. 2017; 2017 :1–15. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Singh S., Thangjam R., Harish G.D., Singh H., Kumar R., Meena D.P.S., Agrawal A. Conservation protocols for Ensete glaucum, a crop wild relative of banana, using plant tissue culture and cryopreservation techniques on seeds and zygotic embryos. PCTOC. 2021; 144 (1):195–209. [ Google Scholar ]
- Sipen P., Chubo J.K., King P.J., Huat O.K., Davey M.R. Genetic improvementof banana using conventional and in vitro technologies. J. Crop Improv. 2011; 25 (6):697–727. [ Google Scholar ]
- Smitha P.D., Nair A.S. Effect of picloram on somatic embryogenesis from leaf-sheath explants in diploid Musa acuminata cv. Njalipoovan. Plant Tissue Cult. Biotechnol. 2011; 21 (1):83–87. [ Google Scholar ]
- Snyman S.J., Nkwanyana P.D., Watt M.P. Alleviation of hyperhydricity of sugarcane plantlets produced in RITA® vessels and genotypic and phenotypic characterization of acclimated plants. South Afr. J. Bot. 2011; 77 (3):685–692. [ Google Scholar ]
- Soares J.M., Rocha A.J., Nascimento F.S., Santos A.S., Miller R.N., Ferreira C.F., Amorim E.P. Genetic improvement for resistance to Black Sigatoka in bananas: a systematic review. Front. Plant Sci. 2021; 12 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sonthisut M., Wongpanya R., Phonphoem A., Phonphoem W.P. Enhancement of 1-deoxynojirimycin production in mulberry (Morus spp.) using LED irradiation. PCTOC. 2022; 148 (1):167–176. [ Google Scholar ]
- Sowmya H.D., Usharani T.R., Sunisha C., Mohandas S. Banana:Genomics and Transgenic Approaches for Genetic Improvement. Springer; Singapore: 2016. Engineering resistance to Sigatoka; pp. 227–236. [ Google Scholar ]
- Sreedharan S., Shekhawat U.K.S., Ganapathi T.R. Constitutive and stress -inducible overexpression of a native aquaporin gene (MusaPIP2; 6) in transgenic banana plants signals its pivotal role in salt tolerance. Plant Mol. Biol. 2015; 88 (1-2):41–52. [ PubMed ] [ Google Scholar ]
- Sreedharan S., Shekhawat U.K.S., Ganapathi T.R. MusaSAP1, a A20/AN1 zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Mol. Biol. 2012; 80 (4-5):503–517. [ PubMed ] [ Google Scholar ]
- Sreedharan S., Shekhawat U.K., Ganapathi T.R. Transgenic banana plants overexpressing a native plasma membrane aquaporin M USA PIP 1; 2 display high tolerance levels to different abiotic stresses. Plant Biotechnol. J. 2013; 11 (8):942–952. [ PubMed ] [ Google Scholar ]
- Strosse H., Van den Houwe I., Panis B. 2004. Banana Cell and Tissue Culture-Review. Banana Improvement: Cellular, Molecular Biology, and Induced Mutations; pp. 1–12. [ Google Scholar ]
- Subrahmanyeswari T., Gantait S. Biotechnology of banana (Musa spp.): multi-dimensional progress and prospect of in vitro–mediated system. Appl. Microbiol. Biotechnol. 2022:1–25. [ PubMed ] [ Google Scholar ]
- Suman S. Plant tissue culture: a promising tool of quality material production with special reference to micropropagation of banana. Biochem. Cell. Arch. 2017; 17 (1):1–26. [ Google Scholar ]
- Suman S., Rajak K.K., Kumar H. Micropropagation of banana cv. BB Battisa. Biochem. Cell. Arch. 2013; 13 :249–254. [ Google Scholar ]
- Suman ., Kumar H. Micropropagation of banana cv. Malbhog. Bioscan . 2015; 10 (2):647–650. [ Google Scholar ]
- Sun J., Zhang J., Fang H., Peng L., Wei S., Li C., Lu J. Comparative transcriptome analysis reveals resistance-related genes and pathways in Musa acuminate banana'Guijiao 9' in response to Fusarium wilt. Plant Physiol. Biochem. 2019; 141 :83–94. [ PubMed ] [ Google Scholar ]
- Tabiyeh D., Bernard F., Shacker H. Investigation of glutathione, salicylic acid and GA3 effects on browning in Pistacia vera shoot tips culture. Acta Hortic. 2005; 726 :201–204. [ Google Scholar ]
- Tak H., Negi S., Ganapathi T.R., Bapat V.A. 2016. Molecular Farming: Prospects and Limitation. Banana: Genomics and Transgenic Approaches for Genetic Improvement; pp. 261–275. [ Google Scholar ]
- Tak H., Negi S., Gupta A., Ganapathi T.R. A stress associated NAC transcription factor MpSNAC67 from banana (Musa x paradisiaca) is involved in regulation of chlorophyll catabolic pathway. Plant Physiol. Biochem. 2018; 132 :61–71. [ PubMed ] [ Google Scholar ]
- Thanakronpaisan K., Kongbangkerd A., Premjet S. Effect of BA and chitosan on in vitro growth of musa (ABB Group)‘Kluai namwa Mali-ong’. Asia-pac. J. Sci. Technol. 2019; 24 (1) [ Google Scholar ]
- Thangavelu R., Saraswathi M.S., Uma S., Loganathan M., Backiyarani S., Durai P., Swennen R. Identification of sources resistant to a virulent Fusarium wilt strain (VCG 0124) infecting Cavendish bananas. Sci. Rep. 2021; 11 (1):1–14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thorpe T., Stasolla C., Yeung E.C., de Klerk G.J., Roberts A., George E.F. In: Plant Propagation by Tissue Culture. third ed. George E.F., Hall M.A., de Klerk G.J., editors. Springer; Dordrecht: 2008. The components of plant tissue culture media II: organic additions, osmotic and pH effects, and support systems; pp. 115–173. [ Google Scholar ]
- Titov S., Bhowmik S.K., Mandal A., Alam M.S., Udin S.N. Control of phenolic compound secretion and effect of growth regulators for organ formation from Musa spp. cv. kanthali floral bud explants. Am. J. Biochem. Biotechnol. 2006; 2 (3):97–104. [ Google Scholar ]
- Tran Thanh Van K., Trinh . In: Plant Tissue Culture, Application and Limitations. Bhojwani S.S., editor. Elservier; Amsterdam: 1990. Organogenic differentiation. [ Google Scholar ]
- Tripathi J.N., Ntui V.O., Ron M., Muiruri S.K., Britt A., Tripathi L. CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun. Biol. 2019; 2 (1):1–11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tripathi J.N., Oduor R.O., Tripathi L. A high-throughput regeneration and transformation platform for production of genetically modified banana. Front. Plant Sci. 2015; 6 :1025. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tripathi L., Tripathi J.N., Kiggundu A., Korie S., Shotkoski F., Tushemereirwe W.K. Field trial of Xanthomonas wilt disease-resistant bananas in East Africa. Nat. Biotechnol. 2014; 32 (9):868–870. [ PubMed ] [ Google Scholar ]
- Tripathi L., Mwaka H., Tripathi J.N., Tushemereirwe W.K. Expression of sweet pepper Hrap gene in banana enhances resistance to Xanthomonas campestris pv. musacearum. Mol. Plant Pathol. 2010; 11 (6):721–731. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tripathi L., Ntui V.O., Tripathi J.N. CRISPR/Cas9-based genome editing of banana for disease resistance. Curr. Opin. Plant Biol. 2020; 56 :118–126. [ PubMed ] [ Google Scholar ]
- Tripathi L., Ntui V.O., Tripathi J.N., Kumar P. Application of CRISPR/Cas for diagnosis and management of viral diseases of banana. Front. Microbiol. 2021:3622. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Twaij B.M., Jazar Z.H., Hasan M. Trends in the use of tissue culture, applications and future aspects. Int. J. Plant Biol. 2020; 11 (1):8385. [ Google Scholar ]
- Uma S., KumaraveL M., Backiyarani S., Saraswathi M.S., Durai P., Karthic R. Somatic embryogenesis as a tool for reproduction of genetically stable plants in banana and confirmatory field trials. PCTOC. 2021; 147 (1):181–188. [ Google Scholar ]
- Uzaribara E., Nachegowda V., Ansar H., Sathyanarayana B.N., Taj A. In vitro propagation of red banana (Musa acuminata) Bioscan. 2015; 10 :125–129. [ Google Scholar ]
- Van den Houwe I., Swennen R. Vol. 530. 1999. Characterization and control of bacterial contaminants in in vitro cultures of banana (Musa spp.) pp. 69–79. (International Symposium on Methods and Markers for Quality Assurance in Micropropagation). [ Google Scholar ]
- Vanhove A.C., Vermaelen W., Panis B., Swennen R., Carpentier S. Screening the banana biodiversity for drought tolerance: can an in vitro growth model and proteomics be used as a tool to discover tolerant varieties and understand homeostasis. Front. Plant Sci. 2012; 3 :176. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vani A.K.S., Reddy G.M. Novel techniques in efficient micropropagation of certain popular banana cultivars. Indian J. Genet. Plant Breed. 1999; 53 :247–250. [ Google Scholar ]
- Vasane S.R., Patil A.B., Kothari R.M. Vol. 865. 2008. Bio-Acclimatization of in vitro propagated banana plantlets'GRAND NAIN'; pp. 217–224. (IV International Symposium on Acclimatization and Establishment of Micropropagated Plants). [ Google Scholar ]
- Vendrame W.A., Feuille C., Beleski D., Bueno P.M.C. In vitro growth responses of ornamental bananas (musa sp.) as affected by light sources. Horticulturae. 2022; 8 (2):92. [ Google Scholar ]
- Vishnevetsky J., White T.L., Palmateer A.J., Flaishman M., Cohen Y., Elad Y., Perl A. Improved tolerance toward fungal diseases in transgenic Cavendish banana (Musa spp. AAA group) cv. Grand Nain. Transgenic Res. 2011; 20 (1):61–72. [ PubMed ] [ Google Scholar ]
- Vuylsteke D., Ortiz R., Ferris R.S.B., Crouch J.H. Vol. 14. John Wiley & Sons; New York, NY: 1996. Plantain improvement; pp. 267–273. (Plant Breeding Reviews). [ Google Scholar ]
- Waman A.A., Bohra P., Sathyanarayana B.N., Umesha K., Mukunda G.K., Ashok T.H., Gowda B. Optimization of factors affecting in vitro establishment, ex vitro rooting and hardening for commercial scale multiplication of silk banana (Musa AAB) Erwerbsobstbau. 2015; 57 (3):153–164. [ Google Scholar ]
- Wang A., Li J., Al-Huqail A.A., Al-Harbi M.S., Ali E.F., Wang J., Eissa M.A. Mechanisms of chitosan nanoparticles in the regulation of cold stress resistance in banana plants. Nanomaterials. 2021; 11 (10):2670. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilken D., Jiménez Gonzalez E., Gerth A., Gómez-Kosky R., Schumann A., Claus D. Effect of immersion systems, lighting, and TIS designs on biomass increase in micropropagating banana (Musa spp. cv.'Grande naine'AAA). In Vitro Cell. Dev. Biol. Plant. 2014; 50 (5):582–589. [ Google Scholar ]
- Yadav A., Prasad Y., Kumar M., Pandey S., Maurya R., Pandey P. Effects of Mercuric chloride and Ethanol for surface sterilization under in vitro plant growth in banana (Musa paradisiaca L.) variety “Udhayam” J. Pharmacogn. Phytochem. 2021; 10 (1):2281–2283. [ Google Scholar ]
- Yadav K., Patel P., Srivastava A.K., Ganapathi T.R. Overexpression of native ferritin gene MusaFer1 enhances iron content and oxidative stress tolerance in transgenic banana plants. PLoS One. 2017; 12 (11) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yeh N., Chung J.P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 2009; 13 (8):2175–2180. [ Google Scholar ]
- Yumbya P., Ambuko J., Hutchinson M., Owino W., Juma J., Machuka E., Mutuku J.M. Transcriptome analysis to elucidate hexanal's mode of action in preserving the post-harvest shelf life and quality of banana fruits (Musa acuminata) J. Agric. Food Inf. 2021; 3 [ Google Scholar ]
- Zahedi S.M., Karimi M., Teixeira da Silva J.A. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2020; 100 (1):25–31. [ PubMed ] [ Google Scholar ]
- Ziv M., Halevy A.H. Control of oxidative browning and in vitro propagation of Strelitzia reginae. Hortic. Sci. (Stuttg.) 1983; 18 (4):434–436. [ Google Scholar ]
- Ziv M. Liquid Culture Systems for in Vitro Plant Propagation. Springer; Dordrecht: 2005. Simple bioreactors for mass propagation of plants; pp. 79–93. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Micropropagation (or in vitro propagation) is the most common term used for clonal, true‐to‐. type propagation of plants produced into large number by using a variety of tissue, cell, and ...
Comparing adopters of tissue culture banana technology to non-adopters in Kisii County, this suggests that the former had a better grasp of the advantages in tissue culture banana production. Consequently, there is a need to raise farmers' understanding of the general challenges surrounding technology in the area in order to improve on the ...
Tissue culture media plays a vital role in the growth and development of shoot tips. ... Materials such as rockwool, coconut coir, filter paper, luffa sponge, cotton fiber, glass wool, polystyrene foam, nylon ... a banana project named Banana-21 has been initiated by the National Banana Research Program of the National Agricultural Research ...
The tissue culture of banana is particularly important in virus indexing, production of clean planting material, and genetic transformation procedures [9]. However, contamination of in-vitro ...
The use of tissue culture (TC) banana (Musa spp.) planting material is an effective method of providing pest and disease-free plants. Although there are many added benefits to using TC plants, the ...
Tissue culture. Tissue culture is the growth of tissues or cells separate from the organism. This is typically facilitated via the use of a liquid, semi-solid, or solid growth medium, such as broth or agar, in vitro under sterile growing conditions. Banana is typically propagated vegetatively; thus tissue culture as a propagation technique ...
Hyperhydricity is a physiological disorder impacting plant growth and multiplication and acclimatization of regenerated plantlets. We report the use of calcium nitrate for reversion and acclimatization of banana 'Grand Naine' hyperhydric shoots cultured on Murashige and Skoog medium containing agar or gellan. Although 100% rooting of hyperhydric shoots occurred at all concentrations of ...
This paper describes results of our efforts in developing a multipronged tissue culture based biotechnology for amelioration of this important fruit crop. ... Tissue culture propagation of banana. Scientia Hortic.18:247-252. Article ... Division of Plant Physiology and Biochemistry, Indian institute of Horticultural Research, Hessaraghatta ...
Research Full Length Research Paper Farmer-based dynamics in tissue culture banana technology adoption: a socio-economic perspective among small holder farmers in Uganda Murongo M. Flarian1, 2*, Ayuke O. Frederick3, Mwine. T. Julius1,2 and Wangai K. John4 1Faculty of Agriculture, Uganda Martyrs University P. O. Box, 5498, Kampala, Uganda.
2022. TLDR. This work demonstrates that reliable SNV markers of tissue culture-derived and propagated banana cultivars with a triploid genome can be developed through RNA-seq data analysis, and the analysis of sequence heterozygosity can uncover chromosomal deletions and chimerism in banana somaclonal variants. Expand.
The project intent to help the owner to automatically control and monitor temperature and humidity in banana tissue culture laboratory to provide appropriate temperature and relative humidity for banana which is the basic requirements to maintain the fast growth and resistant to diseases and infection. Expand. 1. 1 Excerpt.
The above banana tissue culture plantlets and symbionts (banana tissue culture plantlets colonized with EB1) after 7 days of successive culture were subjected to hardening for 10 days by transferring into pots with sterilized planting soil (40 × 19 × 15 cm pots, ca. 2.0 kg soil each). ... The authors declare that the research was conducted in ...
In this study, the solid culture method, and Plantform™ and SETIS™ temporary immersion bioreactor systems were used comparatively to propagate, root, and acclimatize 'Grande Naine' and 'Azman' banana varieties for rapid, cheap, and mass production in in vitro conditions. Micropropagation rate, plant height, number of leaves, and fresh and dry weight parameters were investigated in ...
Shooting advice Banana culture is a simple approach for achieving a 10-fold increase in yield. After each succeeding culture, the rate of multiplication (Swamy and Sahijram, 1989). Apart from that, in vitro, plants are a valuable source of disease-free plant material. from bacteria and viruses (Crouch et al., 1998) [15]. Banana tissue culture
Tissue culture is a technique for immunization and separation of tissues in manufactured medium under in vitrocondition. It is a gathering of test strategies by utilizing organs, tissues and cell in a simulated medium under in vitroaseptic environment. Banana is real natural product crop in India as Maharashtra stands first in banana creation ...
Hardening is an important step in tissue culture of any species as it involves acclimatization of plants for the shift from relatively comfortable laboratory conditions to the field conditions ...
The banana tissue culture project imagined because of the fast decrease in banana Production realized by the invasion of Banana bunchy best infection (BBTV), Panama sicknesses, sigatoka, nematode edifices and general natural corruption that had been recognized and reported amid the most recent two decades.
Developing a successful protocol for banana in vitro culture is a guarantee for the mass propagation of pathogen-free, high-quality, true-to-type planting materials with low production costs. ... Optimizing the concentration of CuSO 4 in banana tissue culture media could be a more efficient way to improve the growth and development of ...
Research and development efforts on banana have been ongoing through the years. These have led into significant contributions to the industry namely: mass propagation through tissue culture, use of improved cultivars, better pest control, good soil management, improved postharvest handling practices and development of diversified
Developing a successful protocol for banana in vitro culture is a guarantee for the mass propagation of pathogen-free, high-quality, true-to-type planting materials with low production costs. The current work aimed to investigate the influence of increasing copper levels in an MS medium on endophytic bacterial contamination; shoot multiplication; rooting and the acclimatization of in vitro ...
2. Micropropagation of banana. Banana plants are traditionally propagated through vegetative means using suckers (Nkengla-Asi et al., 2021).However, plants produced through suckers have their own limitations as it leads to disease transmission, low productivity, and poor preservation of original plant genetic material (Hussein, 2012).Moreover, there is a huge demand for quality planting ...
The objective of this paper is to decide the folklore contrasts by especially applying arrangement of two procedures for the proliferation of banana plant for ordinary and tissue culture ...
Banana Tissue Culture Research Papers - Free download as PDF File (.pdf), Text File (.txt) or read online for free. banana tissue culture research papers
Abstract. Enzymatic browning poses a significant challenge that limits in vitro propagation and genetic transformation of plant tissues. This research focuses on investigating how adding antioxidant substances can suppress browning, leading to improved efficiency in transforming plant tissues using Agrobacterium and subsequent plant regeneration from rough lemon (Citrus × jambhiri).