- Research article
- Open access
- Published: 27 October 2020

Ethics and the marketing authorization of pharmaceuticals: what happens to ethical issues discovered post-trial and pre-marketing authorization?
- Rosemarie D. L. C. Bernabe ORCID: orcid.org/0000-0002-4999-3117 1 , 2 ,
- Ghislaine J. M. W. van Thiel 3 ,
- Nancy S. Breekveldt 4 ,
- Christine C. Gispen 4 &
- Johannes J. M. van Delden 3
BMC Medical Ethics volume 21 , Article number: 103 ( 2020 ) Cite this article
2725 Accesses
2 Citations
Metrics details
In the EU, clinical assessors, rapporteurs and the Committee for Medicinal Products for Human Use are obliged to assess the ethical aspects of a clinical development program and include major ethical flaws in the marketing authorization deliberation processes. To this date, we know very little about the manner that these regulators put this obligation into action. In this paper, we intend to look into the manner and the extent that ethical issues discovered during inspection have reached the deliberation processes.
To gather data, we used the Dutch Medicines Evaluation Board database and first searched for the inspections, and their accompanying site inspection reports and integrated inspection reports, related to central marketing authorization applications (henceforth, application/s) of drugs submitted to the European Medicines Agency (EMA) from 2011 to 2015. We then extracted inspection findings that were purely of ethical nature, i.e., those that did not affect the benefit/risk balance of the study (issues related to informed consent, research ethics committees, and respect for persons). Only findings graded at least major by the inspectorate were included. Lastly, to identify how many of the ethically relevant findings (ERFs) reach the application deliberation processes, we extracted the relevant joint response assessment reports and reviewed the sections that discussed inspection findings.
From 2011 to 2015, there were 390 processed applications, of which 65 had inspection reports and integrated inspection reports accessible via the database of the Dutch Medicines Evaluation Board. Of the 65, we found ERFs in 37 (56.9%). The majority of the ERFs were graded as major and half of the time it was informed-consent related. A third of these findings were related to research ethics committee processes and requirements. Of the 37 inspections with ERFs, 30 were endorsed in the integrated inspection reports as generally GCP compliant. Day 150 joint response assessment reports and Day 180 list of outstanding issues were reviewed for all 37 inspections, and none of the ERFs were carried over in any of the assessment reports or list of outstanding issues.
None of the ethically relevant findings, all of which were graded as major or critical in integrated inspection reports, were explicitly carried over to the joint assessment reports. This calls for more transparency in EMA application deliberations on how ERFs are considered, if at all, in the decision-making processes.
Peer Review reports
Several documents from the European Medicines Agency (EMA) speak of the place of ethics in the regulatory processes involved in a marketing authorization application (henceforth, application) [ 1 , 2 , 3 , 4 ]. One of these is the document, Points to consider on Good Clinical Practice (GCP) inspection findings and the benefit-risk balance where the mandate of regulators in terms of the place of these ethical issues in the evaluation process is explained as follows:
GCP inspection findings – even if not directly influencing the benefit-risk balance —will still be important if they raise serious questions about the rights, safety and well-being of trial subjects and hence the overall ethical conduct of the study. It is an obligation of clinical assessors, rapporteurs and the CHMP also to assess the ethics of a clinical development programme, and major ethical flaws should have an impact on the final conclusions about approvability of an application. Consequently, ethical misconduct could result in rejection of the application [ 4 ]. ( italics mine ).
In a previous publication, we identified the types of ethical issues that pharmaceutical regulators encounter post-marketing through inspection reports [ 5 ]. In this publication, we discovered that based on 2008–2012 inspection reports comprising of 112 medicinal products and 288 clinical trial sites, inspectors frequently and regularly encounter ethically relevant findings (ERFs). Specifically, "At least major ERFs were present in almost all medicinal products with ERFs. The categories with the highest number of ERFs were protocol issues, patient safety, and professionalism issues." Also, "on average, there were 7.54 major and 2.95 critical ERFs per medicinal product application, although ERFs can increase to 30 major and 12 critical" [ 5 ]. For more information on what inspectors consider as major and critical ERFs, the reader is directed to consult our article entitled, “Ethics in clinical trial regulation: ethically relevant issues from EMA inspection reports” [ 5 ]. Though it is fair to assume that at least some of the ERFs that “directly influence the benefit-risk balance” of an investigational medicinal product submitted for marketing authorization application would be carried over to the succeeding regulatory deliberation processes, we cannot make the same assumption about GCP inspection findings that do “not directly influence the benefit-risk balance.” The latter remains unknown and, as such, we know very little about the manner that “clinical assessors, rapporteurs and the Committee for Medicinal Products for Human Use (CHMP)” fulfill this obligation of “assessing the ethics of a clinical development programme.” To respond to this need, it is the goal of this article to look into the manner and extent that ethical issues that do not affect benefit-risk balanced and were discovered during inspection have reached the deliberation processes, i.e., how “major ethical flaws” have impacted “the final conclusions about (the) approvability of an application.”
Before we elaborate on our methodology, it is imperative that we quickly go through the European centralized procedure for authorizing medicinal products, which we have outlined in Fig. 1 .
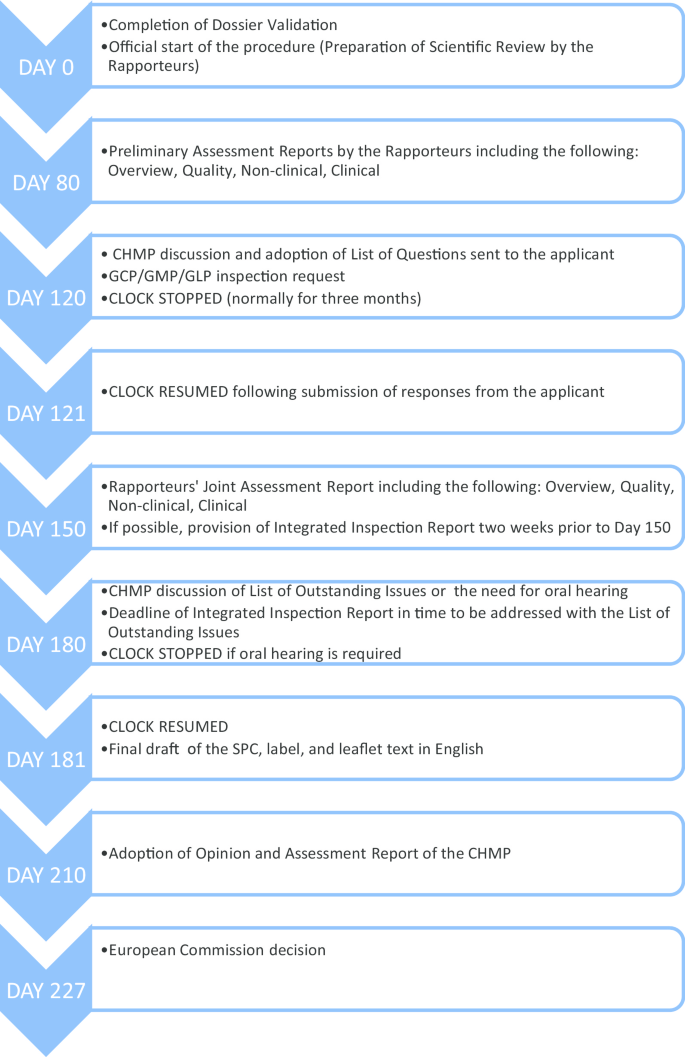
European centralized procedure for authorizing medicinal products [ 6 , 7 , 8 ]
As can be seen from Fig. 1 , the request for GCP inspections and the eventual circulation of the integrated inspection report to the CHMP happens between Day 120 and Day 180. All inspection reports and integrated inspection reports are submitted to the CHMP for the latter’s consideration. Figure 2 provides the details leading to the circulation of the integrated inspection report.
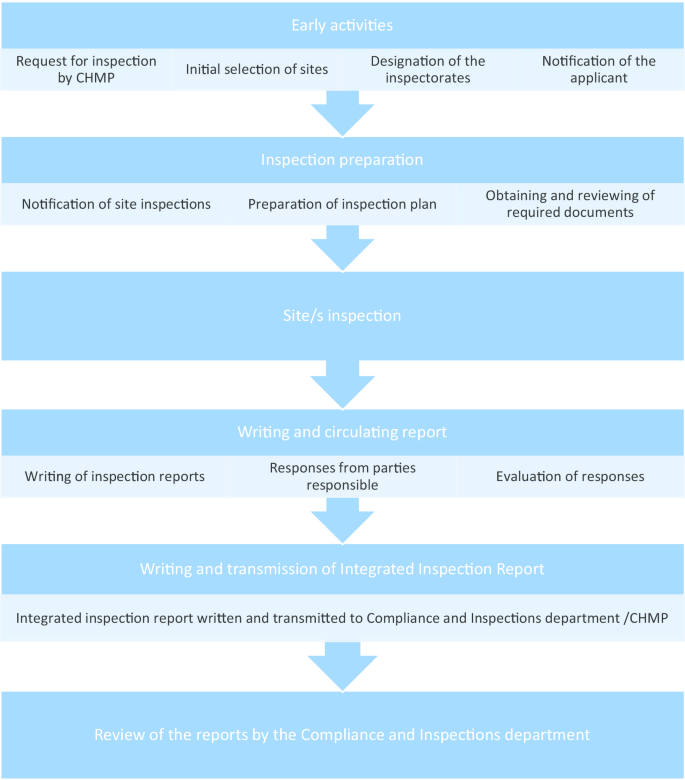
Process of inspection activities related to CHMP request [ 2 , 3 ]
Given the centralized procedure outlined above, to understand the extent to which ethical issues have reached the application deliberation processes, we searched for inspection reports, integrated inspection reports, Day 150 joint assessment reports, and Day 180 List of Outstanding Issues .
To gather data, we used the Dutch Medicines Evaluation Board database and first searched for inspections, and their accompanying site inspection reports and integrated inspection reports, related to central application of drugs submitted to the EMA from 2011 to 2015. For the list of drugs processed for central marketing authorization, we used the European public assessments report database [ 9 ].
Inspection findings include both scientific and ethical issues. To determine which issues to extract, we used the following system. In another publication, we extracted the ethical issues from GCP inspection reports and came up with the following classifications of ethical issues: informed consent, monitoring and oversight, patient safety, professionalism and or qualification issues, protocol compliance or protocol issues, research ethics committees, and respect for persons [ 5 ]. It can be observed that the issues in some of the classifications can both be scientific and ethical. An ethical issue can also be a scientific issue when it could affect the benefit-risk balance of a scientific evaluation of an application [ 4 ]. The following classifications have this dual characteristic: monitoring and oversight, patient safety, professionalism and or qualification issues, protocol compliance or protocol issues. Since we wish to investigate the impact of an ethical issue that is not a scientific issue, we shall look at the issues under the following classifications only: informed consent, research ethics committees, and respect for persons. The former three classifications coincide with the list of ethical issues that may trigger a “for cause” inspection as stated in the document, Points to consider for assessors, inspectors and EMA inspection coordinators [ 1 ]. We used another of our publications [ 10 ] to define the scope of informed consent ( IC ), research ethics committees ( REC ), and respect for persons.
Even within the latter three categories, since we are testing how far purely ethical issues identified in inspections reach the evaluation processes, we excluded inspection findings that may influence the benefit-risk balance evaluation. For example, one of the issues identified as likely to influence benefit-risk evaluation is “inadequate reporting of adverse events and other safety endpoints.” If we look at the definition of respect for persons, patient safety is an aspect of its definition and inadequate reporting of (severe) adverse events a concrete example. Because this finding is likely to affect benefit-risk evaluation, i.e., it is clearly both a scientific and an ethical issue, it was excluded from our analysis.
The GCP deviation findings from inspection reports that were graded by the inspectors as either major or critical and that may be categorized under IC, REC, and/or respect for persons were extracted (henceforth ethically relevant findings, ERFs ). We used the integrated inspection reports to validate if the inspection findings still hold after the evaluation of the responses of the responsible parties on the initial inspection reports (see Fig. 2 ) and if the gravity rating remains the same. In case of a discrepancy, we followed the integrated inspection reports. The conclusion from the integrated inspection reports were extracted.
Next, to identify how many ERFs reach the evaluation of the application, the relevant joint response assessment reports (specifically the documents “overview” and “clinical”) and the list of outstanding issues (see Fig. 1 ) were extracted. We reviewed the sections where these assessment reports discussed the inspection findings and identified if and how these ERFs were considered in the evaluation processes and how the issues ultimately affected the decision on the application.
To avoid privacy and confidentiality issues, the results are on an aggregated format.
From 2011 to 2015, 390 applications were processed, of which 65 had inspection reports and integrated inspection reports accessible via the database of the Dutch Medicines Evaluation Board. Of the 65, we found ERFs in 37 (56.9%). These findings are summarized in Table 1 .
As can be seen from Table 1 , the majority of the ERFs were graded as major and half of the time it was IC-related. A third of these findings were related to research ethics committee processes and requirements.
Of the 37 inspections with ERFs, 30 were endorsed in the integrated inspection reports as generally GCP compliant. Table 2 presents the mean, mode, minimum, and maximum ERF values in all inspections, endorsed inspections (the 30 inspections), and not-fully-endorsed inspections (the remaining 7 inspections).
From Table 2 , we see that there is a difference in terms of the average number of ERFs and the maximum number of ERFs per inspection between the endorsed and the non-endorsed inspections. Non-endorsed inspections have higher values on both counts than endorsed inspections in terms of total number of ERFs, major ERFs, and critical ERFs. This means that the non-endorsed inspections have more and graver ERFs than the endorsed inspections.
In all the 30 endorsed inspections, the gravity ratings were retained and the corrective and preventive action (CAPA) proposals of the sponsors and investigators to address the ERFs were accepted by the inspectors. Note that CAPAs would in most instances be preventive, i.e., changes can be made only for future trials. Seven of the inspections were not fully endorsed as GCP compliant, partly due to ERFs.
Of the seven not-fully-endorsed inspection cases, three were declared non-GCP compliant with the consequence that (part of) the data were not endorsed for use for the assessment of an application. One was declared non-GCP compliant but data were still endorsed for use during assessment. In three cases, data were endorsed for use for assessment, but the inspectors expressed lingering concerns about ERFs and required a better approach from the sponsor in the future.
Day 150 joint response assessment reports and Day 180 list of outstanding issues were reviewed for all 37 inspections, and none of the ERFs were carried over in any of the assessment reports or list of outstanding issues. Table 3 summarizes these results.
In our study, we wanted to see how many of the ethical issues that were not likely to affect the scientific validity of the study and that were discovered during inspection have reached the evaluation processes for centralized applications of drugs. We did this by investigating how many of the ERFs from integrated inspection reports were reflected in Day 150 and Day 180 joint assessment reports. Our results are straightforward: of the 77 ERFs found in 56.9% of all inspections from 2011–2015, none of the ERFs were factored in, i.e., none of them were mentioned at all as factors to consider in either Day 150 joint response assessment reports or Day 180 list of outstanding issues. This means that though these ERFs may have been discussed internally, none of these were explicitly carried over to the joint assessment reports. Whether or not the inspections were endorsed was not a factor in the uptake of ERFs in Day 150 and Day 180 assessments. This is disturbing especially for the seven inspections where the inspectors did not guarantee general GCP compliance of the trial sites, three of which lingering concerns about ERFs were expressed by the inspectors. Overall, and based on inspection and assessment reports, this means that the mandate obliging clinical assessors, rapporteurs and the CHMP to also “assess the ethics of a clinical development programme, and major ethical flaws should have an impact on the final conclusions about approvability of an application” [ 1 ] have yet to be actualized or at least seen as factors explicitly considered during the assessment of an application. With that said, some considerations are worth mentioning.
First, it is unclear what standards inspectors use to declare that the inspected sites were generally GCP compliant in spite of major/critical ERFs. Major/critical issues are defined as “conditions, practices or processes that might adversely/adversely affect the rights, safety or well-being of the subjects and/or the quality and integrity of data” [ 11 ]. If major/critical ERFs at the very least have the possibility of affecting the rights, safety, or well-being of the subjects, how were these weighed and factored in the conclusion that the sites were generally GCP compliant? At the time of writing, we know of no EMA document that speaks about this process. Thus, there is a need for a transparent structure on grading standards as well as guidelines on the place of minor/major/critical findings in application decision-making.
Second, though the grading of critical/major/minor is used by the inspectors, it is not clear in EMA documents if the assessors should use the same grading system. Whether inspectors and assessors should and in fact use the same grading system is an area for future research.
Third, ERFs are best addressed early, and not during application deliberations when “damage” has already been done. This may mean encouraging preventive measures at the design stage of clinical trials, widening the capacity of research ethics committees to monitor approved clinical trials, reviewing sponsor responsibility in actively pursuing ethically compliant trials, and/or more active collaboration between RECs and drug regulators in terms of approving and monitoring clinical trials, among others.
Fourth, inspection reports provide a lot of insight on ethical and scientific matters such as the ethical acceptability of the elements of a pharmaceutical clinical trial which eventually becomes a basis for an application, integrity of the clinical trial data based on which pharmaceutical products are provided marketing authorization, among others. This should be sufficient reason for drug regulatory agencies to make them more accessible, if not public. This is a concern that was earlier made by Dal-Re, Kesselheim, and Bourgeois [ 12 ] in an opinion piece calling for the publication of inspection reports by drug regulatory bodies. Dal-re and colleagues correctly point out that doing so is part of these regulatory bodies’ public health mandate. It also allows for (a) individual assessment of “trial quality in publication decisions”; (b) provides more inputs for systematic reviews; and (c) provide means for clinical trial sponsors to correct mistakes and ensure participant safety [ 12 ].
Fifth, we saw above the EMA position that GCP issues, even those that do not affect the benefit-risk balance so long as these issues raise “serious questions about the rights, safety, and well-being of trial subjects” should have an “impact on the final conclusions about approvability of an application” [ 4 ]. In our study, we found that this is not (yet) the case. Unfortunately, we found no EMA document that elaborates on how ethical issues should affect application evaluation processes and no other publication to our knowledge engaged these issues, except ours. In an earlier publication [ 13 ], we proposed a 4-step procedure in evaluating ERFs, with sanctions depending on the evaluation of the gravity and magnitude of the ERF. However, it still remains to be seen how ERFs that do not affect the risk–benefit balance of an application such as the ones we dealt with in this manuscript should be evaluated by assessors and how such an assessment should impact the assessment process. This is work for future research.
None of the ethically relevant findings, all of which were graded as major or critical in integrated inspection reports, were explicitly carried over to the joint assessment reports. This means that from the vantage of these joint assessment reports , none of the ethically relevant findings seemed to have reached or impacted the application deliberation processes. This calls for more transparency in EMA application deliberations, specifically on how ERFs are considered in the decision-making processes.
Availability of data and material
The inspection reports, integrated inspection reports, Day 150, and Day 180 data that support the findings of this study are available from the database of the Dutch Medicines Evaluation Board (MEB). Because of the sensitivity of the sources, the data may only be accessed with the permission of the MEB or similar European regulatory body.
Abbreviations
Committee for Medicinal Products for Human Use
Corrective and preventive action
Ethically relevant findings
European Medicines Agency
Good Clinical Practice
Informed consent
Research ethics committees
European Medicines Agency. Points to consider for assessors, inspectors and EMA inspection coordinators on the identification of triggers for the selection of applications for “routine” and/or “for cause” inspections, their investigation and scope of such inspections. 2013. No description about the ethical conduct of the study (e.g. inclusion of vulnerable patients, high.
EMA. Procedure for reporting of GCP inspections requested by the Committee for Medicinal Products for Human Use (CHMP). 2013. https://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004479.pdf .
European Medicines Agency. Procedure for coordinating GCP inspections requested by the CHMP. 2014. https://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004446.pdf .
European Medicines Agency. Points to consider on GCP inspection findings and the benefit-risk balance. 2012. https://www.ema.europa.eu/docs/en_GB/document_library/Other/2013/01/WC500137945.pdf .
Bernabe RDLC, van Thiel GJMW, Breekveldt NS, Gispen-de Wied CC, van Delden JJM. Ethics in clinical trial regulation: ethically relevant issues from EMA inspection reports. Curr Med Res Opin. 2019;35(4):637–45.
Article Google Scholar
Tobin JJ, Walsh G. Medical product regulatory affairs: pharmaceuticals, diagnostics, medical devices. Weinheim: Wiley-VCH; 2008.
Book Google Scholar
European Commission. Authorisation procedures—the centralised procedure. 2020. https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines#centralised-authorisation-procedure-section .
Schneider CK, Schäffner-Dallmann G. Typical pitfalls in applications for marketing authorization of biotechnological products in Europe. Nat Rev Drug Discov. 2008;7:893–9.
EMA. European public assessment reports: background and context, 2020. https://www.ema.europa.eu/en/medicines/what-we-publish-when/european-public-assessment-reports-background-context .
Bernabe RDLC, Van Thiel GJMW, Van Delden JJM. What do international ethics guidelines say in terms of the scope of medical research ethics? BMC Med Ethics. 2016;17:23.
EMA. Classification and analysis of the GCP inspection findings of GCP inspections conducted at the request of the CHMP. 2014. https://www.ema.europa.eu/docs/en_GB/document_library/Other/2014/12/WC500178525.pdf .
Dal-Ré R, Kesselheim AS, Bourgeois FT. Increasing access to FDA Inspection reports on irregularities and misconduct in clinical trials. J Am Med Assoc: JAMA. 2020;19:1903–4.
Bernabe RDLC, van Thiel GJMW, Breekveldt NS, Gispen CC, van Delden JJM. Regulatory sanctions for ethically relevant GCP violations. Drug Discov Today. 2019;24:2116–9.
Download references
Acknowledgements
The views expressed by the authors are their personal views and may not be understood or quoted as being made on behalf of or reflecting the position of the Dutch Medicines Evaluation Board.
This project was funded by the Dutch Medicines Evaluation Board. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and affiliations.
Faculty of Health and Social Sciences, University of South-Eastern Norway, Kongsberg, Norway
Rosemarie D. L. C. Bernabe
Centre for Medical Ethics, Institute of Health and Society, University of Oslo, Oslo, Norway
Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands
Ghislaine J. M. W. van Thiel & Johannes J. M. van Delden
Dutch Medicines Evaluation Board, Utrecht, The Netherlands
Nancy S. Breekveldt & Christine C. Gispen
You can also search for this author in PubMed Google Scholar
Contributions
RDLCB downloaded the data, processed, and interpreted the data. She wrote all the drafts as well. GJMWT, NSB, CCG, and JJMD discussed the interpretation results and contributed to the various drafts. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Rosemarie D. L. C. Bernabe .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval was waived according to Dutch Medical Research Involving Human Subjects Act.
Consent for publication
Not applicable.
Competing interests
RDLC Bernabe received funds from the Dutch Medicines Evaluation Board for this project. CC Gispen and NS Breekveldt worked/works for the Dutch Medicines Evaluation Board. GJMW van Thiel and JJM van Delden have no competing interests to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bernabe, R.D.L.C., van Thiel, G.J.M.W., Breekveldt, N.S. et al. Ethics and the marketing authorization of pharmaceuticals: what happens to ethical issues discovered post-trial and pre-marketing authorization?. BMC Med Ethics 21 , 103 (2020). https://doi.org/10.1186/s12910-020-00543-w
Download citation
Received : 24 April 2019
Accepted : 13 October 2020
Published : 27 October 2020
DOI : https://doi.org/10.1186/s12910-020-00543-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]
- Research article
- Open access
- Published: 30 January 2019
Pharmaceutical marketing strategies’ influence on physicians' prescribing pattern in Lebanon: ethics, gifts, and samples
- Micheline Khazzaka ORCID: orcid.org/0000-0002-9764-7243 1
BMC Health Services Research volume 19 , Article number: 80 ( 2019 ) Cite this article
47k Accesses
35 Citations
68 Altmetric
Metrics details
Drug companies rely on their marketing activities to influence physicians. Previous studies showed that pharmaceutical companies succeeded to manage physicians prescribing behavior in developed countries. However, very little studies investigated the impact of pharmaceutical marketing strategies on prescribing pattern in developing countries, middle-eastern countries. The objective of this research was to examine the influence of drug companies’ strategies on physicians’ prescription behavior in the Lebanese market concerning physicians’ demographic variables quantitatively. Moreover, this study tested whether Lebanese physicians considered gifts and samples acceptance as an ethical practice.
Sampling was done by using a non-probability method. An online cross-sectional study was conducted through WhatsApp. A self-administered questionnaire survey was conducted during the months of February and March 2018. Cronbach’s Alpha reliability coefficient was calculated. Data were statistically analyzed by using IBM SPSS statistics version 24 software. Chi-square and Cramer’s v tests were used to finding sign correlation, and Spearman test was used to measure the strength and direction of a relationship between variables.
Results found that pharmaceutical marketing strategies are correlated to physicians’ prescribing behavior. We demonstrated that the majority of the promotional tools tested were mostly or sometimes motivating physicians to prescribe promoted drugs. The major tools that physicians agreed to be mostly motivated by are visits of medical representatives and drug samples while sales calls made by pharmaceutical companies are the less influential tool. Regarding gift acceptance, this study demonstrated that physicians consider gifts’ acceptance as a non-ethical practice. Results showed that most physicians use free samples to treat their patients.
We demonstrated that there is a relationship between physicians’ prescribing pattern and their age, gender and the location of practice.
Conclusions
Findings of this study provided an insightful work, serving as one of the first humble steps in the imminent direction of merging this paper with the previous literature. From a managerial perspective, pharmaceutical marketing managers of drug companies can use the research findings to design better their strategies directed to the Lebanese physicians who can also benefit from the results obtained.
Peer Review reports
Pharmaceutical marketing efforts directed to physicians are getting more and more attention over the years. There are many tactics adopted by pharmaceutical companies [ 1 ] such as physicians-targeted promotions which are free samples, journal advertisements [ 2 ], printed product literature and other gifts that helped them to increase the acceptability of their products [ 3 ]. On average, pharmaceutical companies spent 20% or more of their sales on marketing [ 4 ] which made them a lot of money, and they had little incentive to stop those tactics [ 5 ]. It was estimated that 84% of pharmaceutical marketing efforts are directed toward physicians [ 2 ] because from the manufacturer’s point of view, physicians are the key decision makers [ 6 , 7 , 8 ], the gatekeepers to drug sales [ 9 ]. The structure of pharmaceutical markets differs from country to country because it has a national character. However, the pharmaceutical industry has an international nature [ 4 ]. To the best of our knowledge, few published studies addressed the situation in the developing world, and very few were those in the middle-eastern countries.
In Lebanon, inappropriate prescribing practices for certain prescription drugs are a common problem. One study found that 40% of all prescriptions in seven hospitals in Lebanon contained an error, of which 9% were unnecessary medication prescription [ 10 ]. One of the explanations to this observation might be because of physician-targeted promotions [ 2 ] adopted by pharmaceutical companies to increase the acceptability of their products [ 3 ].
Although many articles contemplated various theories of marketing influence on physicians, there is still a gap to fill. Ethical acceptability of gifts and samples is a comparatively new topic drawing momentous attention. Given the interdisciplinary nature of this research, it was appropriate for us to consider relevant theories from some different disciplines: diffusion and adoption theory, advertising theory, agency theory and role theory that influence the individual and the environment in which new products are adopted (drugs prescribed). Therefore, this study covered physicians’ opinion regarding the ethical acceptability of gifts and samples and highlighted physicians’ profile and demographic parameters influencing their prescribing behavior.
This desk research was an outcome of the authors’ academic and professional interest in the subject of pharmaceutical marketing and its impact on physicians’ prescribing pattern, especially in the mentioned country.
From an academic view, this present study aimed to add knowledge to the existing literature. It was an aggregate account of the connected exploration across disciplines with practical connotations.
Therefore, this investigation subtly attempted to bring substantial academic and managerial implications.
Academically, in searching existing literature, the present study identified some gaps. The majority of researches conducted were in developed countries. Developing countries received quite a bit of attention.
Therefore, a study of drug companies’ impact on Lebanese physicians prescribing behavior would report an original empirical research and would inform practice and future research by providing an insight into which extent pharmaceutical marketing strategies influenced Lebanese physicians prescribing behavior and what was the most influential strategy. No scholarly article merged in one study pharmaceutical marketing strategies in Lebanon, their impact on physicians prescribing pattern and physicians opinion regarding ethical acceptability of gifts and samples to find the correlation between them. This paper was virtually the first to advocate such a reform, and it served as one of the first humble steps in the imminent direction of merging this paper with the previous literature.
From a managerial perspective, a good understanding of drug companies influence on physicians provided pharmaceutical managers a framework to optimize promotion activities by firstly deciding where to focus their efforts to increase their benefits and secondly by choosing the best promotional approach and tool to persuade physicians best and thus avoiding any wasteful expenditure [ 8 ]. Additionally, the observance of ethical theories for medicinal drug promotion by doctors contributed to a more rational prescription of drugs. From another hand, when drug companies had more influence on some physicians and not on others, companies’ managers began to address the question regarding physicians opinion about the ethicality of receiving gifts and samples as a factor preventing these doctors from being influenced. If there was a difference in physicians’ opinion, managers then began to address possible reasons for the differences in answers observed such as demographic factors and physicians’ profile (location of practice, age or year of graduation and its influence on rates of adoption, gender, and nature of practice).
Conducting such a scholarly study from a managerial perspective aimed at highlighting functional implications and future research possibilities was of equal interest to academia and professionals.
The primary focus of the pharmaceutical industry was on profitability [ 11 ]. Thus, it was trying to leave a lasting impression on the prescribers (physicians) mind [ 12 ]. Normative principles of justice and fidelity required that physicians stay free from outside influence with regards to decisions about patient care [ 11 ].
Thus, it would be helpful for both, physicians and pharmaceutical industries to be aware of ethical theories and ethical principles [ 13 ]. The four fundamental ethical theories that are among the most frequently discussed in the business ethics literature are egoism [ 14 ], utilitarianism, deontology [ 15 ], and social justice.
The application of ethical theories during interaction with the pharmaceutical industry
The purpose of these theories was to help doctors and pharmaceutical companies’ managers acquire insight into their beliefs about the many criticisms that were made against marketing and, especially, insight about where they stood on the morally difficult situations that confronted them and what actions they would take in response to them. In practical ethics, two concepts existed while making decisions: utilitarian and deontological. In utilitarian ethics, consequences justified the ways to achieve it, but in deontological ethics, duties were of significant importance and outcomes may not justify the means [ 13 ].
Pharmaceutical marketing personalized to physicians such as the provision of samples and gifts raised such ethical issues [ 16 ]. To make effective decisions, the key was to think about different choices regarding their ability to accomplish one of the physicians’ most important goals that were ethical prescribing of drugs. Five ethical principles were identified as the cornerstone of the ethical guidelines [ 17 ], they helped to explain and to clarify the issues involved in a specific dilemma, and they were globally valuable to approach ethical and appropriate decision-making: beneficence, nonmaleficence, respect for autonomy, justice, and fidelity [ 18 ].
Marketing and promotion practices regarding the Lebanese code of ethics
In Lebanon, in 2016, the Lebanese Ministry of Public Health undertook the initiative to stipulate a Code of Ethics in partnership with the concerned parties to set regulatory frameworks. These frameworks ensured respect of legal, ethical, and scientific principles in the medicinal market as well as enhancing the rational use of drugs to prevent practices that do not comply with ethics by acting as a reliable reference to marketing practices. It was established in accordance with the Lebanese cultural context leading to the particular need for this research in Lebanon since no well-documented studies were done previously. The Lebanese Code of Ethics was divided into three main components which are: Marketing and Promotion Practices, Implementation Procedures, and the Pledge and signature [ 19 ].
While we were concerned with the process of prescription (adoption of drugs), we must also take into account many reasons why prescribing a specific medication may be unaccepted by some physicians and not by others [ 20 , 21 ]. Variables such as the degree of socialization, proximity to peers, rural vs. urban local of practice, do exist between product message and the act of prescribing.
Research by Tamblyn, McLeod, Hanley, Girard, and Hurley (2003) [ 22 ] indicated that new drug utilization was lower among generalists and specialists practicing in rural. In consideration of the influence of age, sociologists, psychologists, and marketers did much to document the resistance to change common among the elderly [ 23 ]. The combined observations from the literature were best summarized with the recognition that the older the consumer, the more negative the view toward technology, and the lower the use of various technologies (including new prescription drugs).
While there was very little in the literature that considered the influence of gender on prescribing, there were also relatively few studies that were conducted in the area of customer (physicians) behavior on gender differences [ 24 ]. A review of the consumer behavior literature [ 25 , 26 ], identified men as being more independent, confident, competitive, extremely motivated and more willing to take risks. The results of Inman and Pearce (1993) [ 27 ] suggested that female gender was strongly associated with the likelihood of not prescribing, or prescribing less than their male colleagues.
Adoption and diffusion theory
Physician prescribing pattern is a very wide concept including various dimensions. In this research, the focus was on the adoption of drugs. The process of adoption often was referred to as the process of diffusion by which new ideas and products became adopted by society [ 28 ]. An undeniable fact is that marketing efforts have a significant impact on physicians’ decision to adopt [ 29 ] and can initiate the process of diffusion [ 30 ].
Advertising theory
One of the main purposes of advertising was to entice the consumer to purchase the product [ 31 ], that was to prescribe as this is ultimately the metric against which pharmaceutical manufacturers measure success or failure.
Regardless of the role the physician occupied, in an environment in which the chooser is not the user, he is still the target of extensive pharmaceutical marketing within the context of advertising theory [ 32 ]. Therefore, the focus of this study was mainly on pharmaceutical marketing strategies as a factor influencing doctors’ decision. In their role as consumers, physicians created and store a set of preferred brands against which they simplify routine decision making.
In their role as consumers, physicians created and store a set of preferred brands against which they simplify routine decision making [ 33 ].
Agency theory
Agency theory served this research well, as in an agency relationship, the principal delegates the decision-making authority to an agent to perform some action on the principal’s behalf [ 34 ].
In the context of the above definition, the manufacturer as principal depended on the physician as the agent to select drugs from their specific offering. The patient, in their role as principal, depended on the physician, acting as the agent, to choose or prescribe the appropriate drug. Saying that the theory of planned behavior [ 35 ] was primordial to understand physicians’ behavior as it relates to prescribing, and oft considering when attempting to modify or influence physician prescribing [ 36 ].
Role theory
It served as a link between agency theory and the theory of planned behavior. It considered the foundations of the theory of planned behavior which are based upon an individual’s attitude toward the behavior, a perceived behavioral control and subjective norms [ 35 ] and agency theory, which focused on principal-agent relationships [ 37 ]. In this predefined context, role theory thus permitted better management/understanding of the dynamic aspects of the provider-client (agent-principal/physician-manufacturer) interface and centers on role performance and the interpersonal dimensions of service quality [ 38 ].
Physicians as customers and relationship marketing theory
Pharmaceutical sales and pharmaceutical marketing analysts realized that the success of a brand depended mostly on the prescribing behavior (change to another brand) of the physician [ 39 ] who is the most crucial target customer for the pharmaceutical enterprises.
To remain profitable, monitoring the prescribing practice of each physician needed a suitable and successful relationship marketing program. The overall purpose of relationship marketing was to improve marketing productivity and enhance mutual value for the parties involved in the relationship. Consequently, instead of manipulating the customers (physicians) they were involved in the relationship [ 40 ].
As shown in Table 1 above, very few studies covered and studied the impact of pharmaceutical marketing strategies on the prescribing behavior of physicians in the developing countries of the middle-east. A report was published in “executive magazine” in 2015 by El-Jardali and Fadlallah [ 10 ] where it was found that 40% of all prescriptions in seven hospitals in Lebanon contained an error, of which 9% were unnecessary medication prescription.
The author set up a search alert on Google Scholar to be updated when new items that match his topic are published. In 2017, Hajjar, Bassatne, Cheaito, Naser El Dine, Traboulsy, Haddadin, Honein-AbouHaidar & Akl [ 41 ] studied the impact of only pharmaceutical representatives on drug prescription qualitatively and dispensing practices which were a negative impact. No later articles investigated other managing strategies in the Lebanese market regarding the topic of interest, leading to the particular need of this study. The next chapter formulated the hypothesis to be tested.
Research philosophy
The epistemological orientation of this research was the positivism philosophy. The ontological orientation was objectivism. To test theories, we adopted a deductive research analysis method. We opted a quantitative method to measure the impact of pharmaceutical marketing strategies on the prescribing behavior of physicians and physicians’ opinion regarding ethical acceptability of gifts and samples along with any other characteristics that interested us. Therefore, in our cross-sectional study, we measured the impact of 10 pharmaceutical promotional tools across two age groups of physicians, under- 43 and over 43. We compared the impact of these tools among the two genders (male and female) and among physicians practicing in a hospital located in a Lebanese urban country and physicians practicing in another hospital located in a Lebanese rural country. The participants were asked to mention any additional promotional tool that they considered as a motivator for their prescription behavior.
Data collection, study site, sampling method
Sampling was done by using a non-probability, quota, and convenience method. We chose this method because it is not feasible to draw a random probability-based sample of the population due to time considerations. The limitation of this method is that proportion of the entire population is not included in the sample group i.e. lack of representation of the entire population. Thus, the level of generalization of research findings compared to probability sampling is lower. The questionnaire format (Additional file 1 ) was written in english and was given to 364 Lebanese practicing physicians who are working in Lebanon at one of the two hospitals chosen by the author: a hospital in a Lebanese rural region and another one in a Lebanese urban region. However, 282 (response rate 77.47%) participated in the study and filled in the questionnaire completely. For this purpose, and to test the research hypotheses, a survey in a well- structured and self- administrated questionnaire format was developed and carried out by the researcher among the two hospitals of interest from March till April 2018. The questionnaire was piloted before final data collection to get at the thinking behind the answers so that we could accurately assess whether the questionnaire was filled out properly, whether respondents understood the questions, and whether the questions asked what we thought they were asking. Pre-testing also helped assess whether respondents were able and willing to provide the needed information. Twenty nine physicians undertook the pre-testing questionnaire, and their answers were excluded from the final analysis. No changes in the questions were recommended since the questions were clear and easy to understand. Thus, the link of the questionnaire was sent to each physician via “WhatsApp” after obtaining the approval from the Ethical Review Committee of each hospital. A reminder follow up notification was sent out after 7 days. The questionnaire included a covering letter questions to obtain demographic information about physicians, the influence of a list of 10 promotional tools used by most of the Lebanese pharmaceutical companies, questions regarding guidelines and physicians’ opinion regarding ethical acceptability of gifts and drug samples. Statistical Analysis was done using SPSS version 24 software for windows throughout the study to analyze the data.
Chi-square and Cramer’s V tests were carried out for the ten promotional tools identified through literature with the prescribing behavior of physicians that were respectively, shifting physicians’ drug prescription from one company to another if both drugs were generic and changing clinical practice after attending conferences and meetings.
Then the same test was applied to demographic parameters (gender and practice address) and physicians’ prescribing behavior. P values ≤0.05 were considered significant. Spearman’s Rho test was used to measure the strength of association between ordinal variables (age range, receiving of the Lebanese code of ethics and physicians’ prescribing behavior).
Doctors’ prescription behavior of drugs was taken as a dependent variable.
As independent variables, 10 main promotional tools used by pharmaceutical firms were taken into consideration, i.e. visits of medical representatives, sales calls made by pharmaceutical companies, drug samples, promotional drug brochures, medical equipment as gifts, low-cost gifts, sponsorship for travel or personal tour or expenses in conferences, direct mails, subscription of journals and participation by company in continuing medical education conferences. Also, physicians’ demographic parameters and their ethical opinion were considered as mediating variables. Reliability test and validating data were done using IBM SPSS statistics version 24 software, and the results showed that no rules were violated.
Hypotheses development
As mentioned above, this study examined the impact of pharmaceutical marketing strategies on Lebanese physicians prescribing pattern concerning their demographic variables. Moreover, this study tested whether physicians consider gifts and samples acceptance as an ethical practice or an unethical one. For this purpose, the author addressed some questions that marketing managers of the drug companies are interested in: what is the most effective promotional tool in motivating Lebanese physicians to prescribe drugs? Do physicians’ demographic variables affect their perception towards different pharmaceutical marketing strategies? Do ethical codes affect physicians’ acceptance of gifts and samples? To answers these questions, this study formulated hypotheses based on theoretical background and summarized them in Table 2 below.
H1: Pharmaceutical marketing strategies change the prescribing behavior of physicians.
H2: Accepting gifts by physicians is unethical.
H3: Physicians with practices in rural settings are less likely to prescribe new drugs than are physicians operating in urban environments.
H4: Younger physicians are less likely to prescribe from new product categories.
H5: The probability of prescribing from a new drug category is greater among male than female physicians.
Two hundred eighty-two (response rate 77.47%) participated in the study and filled in the questionnaire completely. The majority of male practicing physicians liked to participate in the survey questionnaire (71.63%).
Results (Table 3 ) showed that 8 out of 10 dimensions taken as pharmaceutical marketing strategies were correlated to physicians prescribing behavior that is shifting drug prescription from one company to another if both were generic while seven dimensions out of 10 were correlated to this prescribing behavior that is changing clinical practice after attending conferences and meetings.
According to this study, the strategies used by pharmaceutical companies to promote their drugs demonstrated a correlation and a substantial relationship with physicians’ prescribing behavior. Therefore, the first hypothesis (there’s a relationship between pharmaceutical marketing strategies and the prescribing behavior of physicians) was accepted. Physicians declared that different promotional tools influenced them.
As shown in Table 4 , the majority of these promotional tools were mostly or sometimes motivating physicians to prescribe promoted drugs. The two first tools that the majority of physicians agreed to be motivated mainly by were visits of medical representatives (34.8%) and drug samples (34.8%). Most physicians were sometimes influenced by promotional drug brochures (44.7%), medical equipment as gifts (41.5%). More than half of physicians (54.3%) agreed to be never influenced by sales calls made by pharmaceutical companies, and scarce were physicians who declared to be always affected by a particular pharmaceutical marketing strategy.
The results showed that pharmaceutical companies’ promotional tools moderately motivated physicians. Visits of medical representatives (34.8%), drug samples (34.8%), participation by the company in continuing medical education conferences (31.6%), sponsorship for travel/ expenses in conferences/ sponsorship for a personal tour (28%) could be considered as the most influential tools.
These results showed that practicing physicians didn’t add any new promotional tool other than those we suggested in the questionnaire. However, their answers revealed a need for more scientific evidence provided by continual medical education and by well-educated medical representatives. This scientific evidence will help them to make the decision and to prescribe the best drug among the others.
Moreover, results shown in Table 5 revealed that physicians considered gifts’ acceptance as a non-ethical practice and 53% of them used free samples offered by pharmaceutical companies to treat their patients. Therefore, the second hypothesis was rejected.
H 3 was moderately accepted showing that there is a relationship between physicians prescribing pattern and the location of practice where changing in prescribing practices was more observable among physicians practicing in a hospital located in a rural region. H 4 was moderately accepted showing that there was a relationship between physician’s ages and prescribing pattern which is shifting generic drug prescription from one company to another. Regarding the last hypothesis, there was a relationship between physician’s gender and shifting drug prescription from one company to another, and H 5 was accepted (Table 6 ).
Chi-square and Cramer’s v tests reveal that physicians’ prescribing behaviors are correlated with pharmaceutical promotional tools used in the Lebanese market. It was found that physicians are motivated by pharmaceutical companies’ promotional tools to prescribe promoted drugs. Similarly, some other studies found a direct correlation between physicians’ prescribing patterns and pharmaceutical promotional tools [ 20 , 29 , 30 ]. This can easily be explained by the fact that the persuasive effect of pharmaceutical marketing strategies put extra pressure on physicians to prescribe onerous, expensive drugs even when a cheaper generic drug would be appropriate [ 4 ].
This study found that physicians consider the following promotional tools as the most influential tools among the ten tools tested: visits of medical representatives, drug samples, participation by the company in continuing medical education conferences and sponsorship for traveling and personal tours. Thus, visits to medical representatives been perceived to be the most important and the most influential tool. Researchers suggest that physicians rely heavily on commercial sources of information such as detailer and that the more doctors rely on commercial sources of information, the less likely they are to prescribe drugs in a manner consistent with patient needs. Information provided by detailers is often biased and sometimes dangerously misleading [ 42 , 43 ].
Regarding gift acceptance, the results of this study showed that there is no statistically significant difference among physicians who accept low and/or high-cost gifts and those who don’t. Moreover, statistically, there is no significance among physicians who consider the continuous supply of gifts (low and/or high-cost) at every visit of the medical representative as not justifiable and those who consider it as justifiable. However, the results showed that more physicians consider low cost-gift acceptance as an ethical practice than those who don’t (37.6%), but their continuous supply at every visit of the medical representative is more considered as not ethical (63.1%). This high percentage of physicians who do not accept the continuous supply of low-cost gifts means that some of the participated physicians in the study are not ashamed of the behavior of moderately accepting low-cost gifts. However, they know that gift acceptance is unethical, and they don’t accept it continuously. This could be explained by the fact that the Lebanese physicians are aware and are more considering the acceptance of small, low-cost gifts permissible than non-permissible even if the majority of them didn’t receive a copy of the 2016 code of ethics for medicinal products [ 19 ]. While in other studies, such as in Austria and Saudi Arabia, larger percent (66–80%) of physicians accept gifts from drug companies [ 44 , 45 ].
For high-cost gifts, this study showed that most Lebanese physicians consider their acceptance as unethical (74.8%). The same is for the continuous supply of high cost-gifts (71.6%). While in Iraq and the United States of America, respectively 59 and 74.6% of physicians consider the acceptance of high-cost gifts inappropriate and unethical [ 44 , 45 ]. Those high percentages can be explained by that physicians feel that small gifts do not significantly alter or influence their prescribing pattern but expensive gifts might do so [ 46 ]. Again, these results may give us a hope in that physicians are conscious of unethical expensive gifts’ acceptance.
However, social scientists demonstrated that the tendency to reciprocate for gifts, even the small ones, is a powerful influence on people’s behavior. Individuals who receive gifts are often unable to remain objective. Whenever a physician accepts an award, an implicit relationship is established between the doctor and the company resulting in a prescription [ 3 ]. Physicians not acknowledging the power of receiving small gifts are more likely to be influenced because their defenses are down.
Regarding free samples, it was found in this study that most physicians use free samples to treat their patients (53%). These free samples could be provided for a poor patient who cannot afford to buy it and therefore who manages drug costs in the long term or in some cases to initiate treatment for a new patient.
This study showed that Lebanese physicians significantly shift drug prescription from one company to another if both drugs were generic and they change their clinical practice. This means that the prescribing behavior of Lebanese physicians is highly influenced by pharmaceutical marketing strategies. Similarly, there are some other studies where it was showed that physicians who are interested in drug promotion and who have a positive attitude toward pharmaceutical companies’ promotions adopt rapidly prescription sponsored medications [ 47 ]. From another hand, previous studies showed that most physicians don’t feel that drug companies influence their prescribing pattern [ 48 ]. The difference in this study may result from the indirect way of questioning, which may provide a more realistic result than other studies.
It was found that the perception of practicing physicians towards some promotional tools’ influence on their prescribing behavior is dependent on demographic factors that are practice address, age, and gender of physicians. Practicing physicians in the hospital located in the urban region assign a greater shift in prescribing drugs as compared to physicians practicing in the hospital located in the rural area. Usually, physicians practicing in the rural areas do not get high access to new information unlike physicians practicing in urban regions; this may be one of the reasons for assigning more significant influence to promotional tools by physicians practicing in the urban area. These findings are in accordance with the findings in previous researches by Tamblyn, McLeod, Hanley, Girard, and Hurley (2003) [ 22 ] and Cutts and Tett (2003) [ 49 ].
This empirical investigation confirmed that older physicians assign to be less influenced by pharmaceutical promotional tools as compared to younger physicians. As older the consumers (physicians) are, the more the view is negative towards technology, and the lower the use of various technologies (including the prescription of new drugs). In other words, older physicians are less modern than younger ones. These results are similar to the results found by Peay and Peay’s work in 1994 [ 50 ]. They suggested that older doctors were less innovative than younger ones.
Moreover, it was found in this study that the probability of prescribing from a new drug category (prescribing behavior) is greater among female than male physicians.
The general questions which were asked to practicing physicians to get more insights regarding the improvement of regulations framing the relationship between physicians and pharmaceutical companies confirm the previous results obtained in this study. The answers showed that physicians investigated are conscious and aware of the need for more strengthening the ethical norms regulating their interaction with pharmaceutical industries. This interaction should be with medical representatives holding a certificate of professional and ethical capability to execute a given profession, and the number of visits should be regulated. Besides, physicians appreciate that pharmaceutical companies invite them to international congresses which means that they are interested in acquiring scientific knowledge.
Contributions of this research (theoretical, managerial)
This present study adds to the previous researches that conceptualize the fact that clinical practice decision making is a dynamic process which is affected by some pharmaceutical marketing strategies. Although the doctors generally use the pharmacological criteria in deciding which drug to prescribe, the findings of the study show that the demographic influences are also rated essential factors in the doctor’s decision to prescribe. The present study identified and analyzed the demographic variables like the gender of the doctors, the age of doctors and practice location of the doctors and the results reveal that they have an impact on prescribing behavior.
Our empirical investigation of physicians’ prescription behavior in Lebanon and its findings contribute to an improvement in the marketing practices of the pharmaceutical industry. Knowing that when prescribing habits are once learned, it may be difficult to change them, pharmaceutical managers should use appropriate promotional tools and target the category of physicians that are more influenced than the others. Therefore, the pharmaceutical company optimizes its marketing expenditures better than competitors and increases its sales with lower promotional budgets.
Therefore, the results imply that public policymakers should take prescription behavior seriously and conduct more prescription behavior studies at regular intervals, to precisely understand the impact of tangible rewards on physicians’ prescribing patterns and control unethical practices by both pharmaceutical companies and physicians. So, it is recommended to establish guidelines and to be sure that all physicians are aware of them and always to keep physicians updated regarding new guidelines and to control guidelines’ implementation to limit unethical promotion practices and unethical prescription patterns.
These results, although not surprising, add to the existing body of information because they come from direct observations of behavior in a non-probability, quota and convenience sample.
Limitations and suggestions for future research
Although this study is limited by maturation that is quite simply, people change, and the ways in which they change may have implications for the dependent variable, but it rings a bell of danger by the non-ethical interaction between practicing physicians and drug companies in Lebanon, which negatively affect physicians prescribing behavior and in turn affect patients’ health negatively. It is suggested for future research to replicate this study and include pharmaceutical companies and pharmacists since they play a role in the pharmaceutical market. Also, the same research can be replicated by including more demographic factors of the physicians.
Most of the investigated physicians change their prescribing behavior, and it can simply be concluded that prescribing pattern of Lebanese physicians is negatively affected by promotion tactics.
It can be concluded that the pharmaceutical marketers have to understand the real needs, beliefs, and behaviors of physicians towards their marketing and promotional tools while taking into account physicians’ demographic factors and physicians’ opinion regarding ethical acceptability of gifts and samples. Physicians are the most substantial determinants in pharmaceutical sales by deciding which drug will be used by patients. Influencing the physician is a key to pharmaceutical sales. The marketing efforts of drug companies must target female, young physicians practicing in rural regions. This might generally be the most influenced category of practicing physicians.
Chiu, H. (2005). Selling drugs: marketing strategies in the pharmaceutical industry and their effect on healthcare and research. Explorations.
Marco CA, Moskop JC, Solomon RC, Geiderman JM, Larkin GL. Gifts to physicians from the pharmaceutical industry: an ethical analysis. Ann Emerg Med. 2006;48(5):513–21.
Article PubMed Google Scholar
Goyal R, Pareek P. A review article on prescription behavior of doctors, influenced by the medical representative in Rajasthan, India. IOSR J Bus Manag. 2013;8(1):56–60.
Article Google Scholar
De Laat E, Windmeijer F, Douven RCMH. How does pharmaceutical marketing influence doctors’ prescribing behaviour? Den Haag: CPB; 2002.
Google Scholar
Seaman M. New pharma ethics rules eliminate gifts and meals. USA Today. 2008.
Gönül FF, Carter F, Petrova E, Srinivasan K. Promotion of prescription drugs and its impact on physicians’ choice behavior. J Mark. 2001;65(3):79–90.
Al-Areefi MA, Hassali MA. Physicians’ perceptions of medical representative visits in Yemen: a qualitative study. BMC Health Serv Res. 2013;13(1):331.
Article PubMed PubMed Central Google Scholar
Tahmasebi N, Kebriaeezadeh A. Evaluation of factors affecting prescribing behaviors, in Iran pharmaceutical market by econometric methods. Iran J Pharm Res. 2015;14(2):651.
PubMed PubMed Central Google Scholar
Buckley J. Pharmaceutical marketing-time for change. Electron J Bus Ethics Org Stud. 2004;9(2).
El-Jardali, F., & Fadlallah, R. (2015). retrieved in, http://www.executive-magazine.com/opinion/irrational-drug-prescription-in-lebanon .
Lamont J, Favor C. Distributive justice. In: Handbook of political theory; 2004. p. 1.
Sen A, Siebert H. Markets and the Freedom to Choose. In: The ethical foundations of the market economy. Tubingen: J.C.B. Mohr; 1994.
Mack P. Utilitarian ethics in healthcare. Int J Comput Integr Manuf. 2004;12(3):63–72.
Lantos GP. In Defense of advertising: arguments from reason, ethical egoism, and laissez-faire capitalism; 1995.
Kirkpatrick J. Ethical Theory in Marketing. In: Marketing Education: Challenges, Opportunities and Solutions: Proceedings of the Western Marketing Educators’ Association Conference; 1989. p. 50–3.
Brett AS, Burr W, Moloo J. Are gifts from pharmaceutical companies ethically problematic?: a survey of physicians. Arch Intern Med. 2003;163(18):2213–8.
Kitchener KS. Intuition, critical evaluation and ethical principles: the foundation for ethical decisions in counseling psychology. Couns Psychol. 1984;12(3):43–55.
Denman RB, Purow B, Rubenstein R, Miller DL. Hammerhead ribozyme cleavage of hamster prion pre-mRNA in complex cell-free model systems. Biochem Biophys Res Commun. 1992;186(2):1171–7.
Article CAS PubMed Google Scholar
The Lebanese Ministry of Public Health (MOPH), 2016, The Lebanese Code of Ethics: a New Milestone.
Van den Bulte C, Lilien GL. Medical innovation revisited: social contagion versus marketing effort. Am J Sociol. 2001;106(5):1409–35.
Berndt ER, Pindyck RS, Azoulay P. Consumption externalities and diffusion in pharmaceutical markets: antiulcer drugs. J Ind Econ. 2003;51(2):243–70.
Tamblyn R, McLeod P, Hanley JA, Girard N, Hurley J. Physician and practice characteristics associated with the early utilization of new prescription drugs. Med Care. 2003;41(8):895–908.
Gilly MC, Zeithaml VA. The elderly consumer and adoption of technologies. J Consum Res. 1985;12(3):353–7.
Mitchell VW, Walsh G. Gender differences in German consumer decision-making styles. J Consum Behav. 2004;3(4):331–46.
Chang J, Samuel N. Internet shopper demographics and buying behaviour in Australia. J Am Acad Bus. 2004;5(1/2):171–6.
Fischer E, Arnold SJ. Sex, gender identity, gender role attitudes, and consumer behavior. Psychol Mark. 1994;11(2):163–82.
Inman W, Pearce G. Prescriber profile and post-marketing surveillance. Lancet. 1993;342(8872):658–61.
Rogers EM. Diffusion of innovations: Simon and Schuster; 2010.
Narayanan S, Manchanda P, Chintagunta PK. Temporal differences in the role of marketing communication in new product categories. J Mark Res. 2005;42(3):278–90.
Hahn M, Park S, Krishnamurthi L, Zoltners AA. Analysis of new product diffusion using a four-segment trial-repeat model. Mark Sci. 1994;13(3):224–47.
Vakratsas D, Ambler T. How advertising works: what do we really know? J Mark. 1999;63(1):26–43.
Dorfman R, Steiner PO. Optimal advertising and optimal quality. Am Econ Rev. 1954:826–36.
Turley LW, LeBlanc RP. Evoked sets: a dynamic process model. J Mark Theory Pract. 1995;3(2):28–36.
Mott DA, Schommer JC, Doucette WR, Kreling DH. Agency theory, drug formularies, and drug product selection: implications for public policy. J Public Policy Mark. 1998:287–95.
Netemeyer R, Ryn MV, Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179–211.
Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362(9391):1225–30.
Eisenhardt KM. Agency theory: an assessment and review. Acad Manag Rev. 1989;14(1):57–74.
Broderick A. Role theory and the management of service encounters. Serv Ind J. 1999;19(2):117–31.
Barnes ML. Marketing to a segment of one. Pharm Exec. 2003;23(3):110.
Sheth JN, Parvatiyar A. Relationship marketing in consumer markets: antecedents and consequences. J Acad Mark Sci. 1995;23(4):255–71.
Hajjar R, Bassatne A, Cheaito MA, Naser El Dine R, Traboulsy S, Haddadin F, Honein-AbouHaidar G, Akl EA. Characterizing the interaction between physicians, pharmacists and pharmaceutical representatives in a middle-income country: a qualitative study. PLoS ONE. 2017;12(9).
Avorn J, Chren M, Hartley R. Scientific versus commercial sources of influence on the prescribing behavior of physicians. Am J Med. 1982;73(1):4–8.
Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273(16):1296–8.
McNeill PM, Kerridge IH, Henry DA, Stokes B, Hill SR, Newby D, et al. Giving and receiving of gifts between pharmaceutical companies and medical specialists in Australia. Intern Med J. 2006;36(9):571–8.
Alosaimi F, Alkaabba A, Qadi M, Albahlal A, Alabdulkarim Y, Alabduljabbar M, et al. Acceptance of pharmaceutical gifts. Variability by specialty and job rank in a Saudi healthcare setting. Saudi Med J. 2013;34(8):854–60.
PubMed Google Scholar
Brennan TA, Rothman DJ, Blank L, Blumenthal D, Chimonas SC, Cohen JJ, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295(4):429–33.
Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283(3):373–80.
Steunman M, Shilpak M, McPhee S. Of principles and pens: attitudes and practices of medicine house staff toward pharmaceutical industry promotions. Am J Med. 2001;110(7):551–7.
Cutts C, Tett SE. Influences on doctors’ prescribing: is geographical remoteness a factor? Aust J Rural Health. 2003;11(3):124–30.
Peay MY, Peay ER. Innovation in high risk drug therapy. Soc Sci Med. 1994;39(1):39–52.
Alssageer MA, Kowalski SR. What do Libyan doctors perceive as the benefits, ethical issues and influences of their interactions with pharmaceutical company representatives? Pan Afr Med J. 2013;14:132.
Ghaith A, Aldmour H, Alabbadi H. Investigating the effect of pharmaceutical companies’ gifts on doctors’ prescribing behavior in Jordan. Eur J Soc Sci. 2012;36(4):528–36.
Mikhael EM, Alhilali DN. Gift acceptance and its effect on prescribing behavior among Iraqi specialist physicians. Pharmacol Pharm. 2014;5(7):705–15.
Download references
Acknowledgments
No Acknowledgements
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due the large amount of data analyzed and because it contains very sensitive and private information but is available from the corresponding author on reasonable request.
Author information
Authors and affiliations.
Independent Researcher, Zahle, Lebanon
Micheline Khazzaka
You can also search for this author in PubMed Google Scholar
Contributions
MK contributed alone to all what was needed to accomplish this article. The author read and approved the final manuscript.
Corresponding author
Correspondence to Micheline Khazzaka .
Ethics declarations
Ethics approval and consent to participate.
The ethical approvals were received from the ethical review committees of “Tel Chiha Hospital” and from “Saint George Hospital University Medical Center” where the survey was run.
The first ethical review committee gave oral approval to send the questionnaire to its physicians as I already know the head of this committee by its person Dr. Raymond Khazzaka and he accepted that the physicians of the hospital participate in the survey.
Regarding the second hospital, I received approval that was sent to me by email from the head of the ethical review committee.
In both cases, physicians received a link via “Whatsapp” directing them to participate in the questionnaire built via a software “QuestionPro” and to fill it if they want to, they had the choice to participate or not in the survey when the questionnaire was sent to them. The results of the completed questionnaires sent back by the physicians were then recorded in this software with all the information answered.
Consent for publication
Competing interests.
The author declares that she has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:.
Questionnaire format. (DOCX 220 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions

About this article
Cite this article.
Khazzaka, M. Pharmaceutical marketing strategies’ influence on physicians' prescribing pattern in Lebanon: ethics, gifts, and samples. BMC Health Serv Res 19 , 80 (2019). https://doi.org/10.1186/s12913-019-3887-6
Download citation
Received : 19 September 2018
Accepted : 08 January 2019
Published : 30 January 2019
DOI : https://doi.org/10.1186/s12913-019-3887-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pharmaceutical marketing
- Prescribing behavior
- Physicians’ profile
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Med Ethics

Considerations for applying bioethics norms to a biopharmaceutical industry setting
Luann e. van campen.
1 Ethics Matters, LLC, 5868 E. 71st Street, E-125, Indianapolis, IN 46220 USA
2 Eli Lilly and Company, Indianapolis, IN USA
Tatjana Poplazarova
3 GlaxoSmithKline Vaccines, Wavre, Belgium
Donald G. Therasse
Michael turik.
4 Indiana University Institutional Review Board, Indianapolis, IN USA
Associated Data
Not applicable.
The biopharmaceutical industry operates at the intersection of life sciences, clinical research, clinical care, public health, and business, which presents distinct operational and ethical challenges. This setting merits focused bioethics consideration to complement legal compliance and business ethics efforts. However, bioethics as applied to a biopharmaceutical industry setting often is construed either too broadly or too narrowly with little examination of its proper scope.
Any institution with a scientific or healthcare mission should engage bioethics norms to navigate ethical issues that arise from the conduct of biomedical research, delivery of clinical care, or implementation of public health programs. It is reasonable to assume that while bioethics norms must remain constant, their application will vary depending on the characteristics of a given setting. Context “specification” substantively refines ethics norms for a particular discipline or setting and is an expected, needed and progressive ethical activity. In order for this activity to be meaningful, the scope for bioethics application and the relevant contextual factors of the setting need to be delineated and appreciated. This paper defines biopharmaceutical bioethics as: the application of bioethics norms (concepts, principles, and rules) to the research, development, supply, commercialization, and clinical use of biopharmaceutical healthcare products . It provides commentary on this definition, and presents five contextual factors that need to be considered when applying bioethics norms to a biopharmaceutical industry setting: (1) dual missions; (2) timely and pragmatic guidance; (3) resource stewardship; (4) multiple stakeholders; and (5) operational complexity.
Understanding the scope of the biopharmaceutical enterprise and contextual factors of a biopharmaceutical industry setting is foundational for the application of bioethics norms. Establishing a common language and approach for biopharmaceutical bioethics will facilitate breadth and depth of discussion and subsequent implementation to benefit patients, the healthcare system and society.
Research-based biopharmaceutical companies deliver medicines and vaccines to patients by means of innovative research that occurs in a dynamic and complex ecosystem. Operating at the intersection of life sciences, clinical research, clinical care, public health, and business presents distinct operational and ethical challenges. Ethical integrity in the research, development, manufacturing, and commercialization processes is foundational to the delivery of safe and effective products and provision of reliable and credible information to support their appropriate use. Therefore, companies have a responsibility to patients and other stakeholders to carry out their activities in accordance with applicable public policy (i.e., laws, statutes, regulations), industry guidelines [ 1 ], and ethics standards.
As life sciences research and clinical care become increasingly complex, the biopharmaceutical enterprise frequently faces issues with important ethical implications that may not be fully addressed by public policy or industry guidelines. Current examples include access to medicines, drug shortages; use of human biological samples; genomics; use of “big data”; personalized medicine; quality of life and regenerative medicines; data transparency; research in low resource settings; clinical trial diversity; and most recently, the conduct of clinical trials during a pandemic. Systematic analysis and application of ethics standards provides needed guidance for ‘gray areas’ like these topics that lack specific policy or guidelines.
Bioethics is a field of study that examines ethical issues in life sciences, medicine and healthcare. It provides direction to navigate ethical uncertainty associated with these disciplines, as well as rationale for related public policies [ 2 ] and discipline-specific guidelines. Any institution with a scientific or healthcare mission should engage with bioethics—whether it be a hospital, research center, clinic, or biopharmaceutical company. Although bioethics norms must remain constant across settings, it is reasonable to assume their application will vary depending on the characteristics of a given setting.
In their seminal book, Principles of Biomedical Ethics , Beauchamp and Childress explain that although ethics norms (concepts, principles and rules) are foundational to a common morality, they lack specificity [ 3 ]. ‘Practical’ or ‘applied’ ethics, such as bioethics or business ethics, interpret ethics norms for the purpose of generating action-guiding content specific to a context. However, even within a particular applied ethics domain, ethics norms can and should be further specified to provide discipline- or setting-specific guidance. Bioethics ‘specialties’ such as research ethics, clinical ethics and public health ethics are examples of progressive specification [ 3 ].
The concept of specification is evident with the bioethics principle of “respect for persons” or “autonomy”, from which the procedural requirement of informed consent was derived [ 4 ]. Although this principle is generalizable, without specification it lacks practicality. To be more pragmatic, informed consent requirements have been specified for different settings. Clinical informed consent focuses on the risks and benefits of individual patient treatment. Clinical research informed consent focuses on an individual’s well-being within the setting of a clinical trial and its associated risks and benefits. Public health informed consent focuses on an individual’s well-being in the context of a societal intervention and its associated risks and benefits.
As Beauchamp and Childress state, specification “does not merely analyze meaning; it adds content” [ 3 ] (p. 17). In order for this additional content to be meaningful, it is important to understand the scope and context to which bioethics norms are to be applied. Bioethics as applied to a biopharmaceutical industry setting often is construed either too broadly (merged into a general discussion of ethics) or too narrowly (limited to research ethics) with little examination of its proper scope. Given the influence of the biopharmaceutical industry and the ongoing scrutiny of its ethics, it is necessary to consider how bioethics as a discipline (distinct from but complementary to legal compliance and business ethics) relates to this influential sector of the global healthcare system. As MacDonald states, “It is highly unlikely that an adequate understanding of complex issues in health policy will be possible in the absence of an adequate understanding of one of the major players” [ 5 ] (p. W38).
There are scores of articles and a number of books [ 6 – 11 ] that examine and discuss industry ‘ethics’ broadly (including a mix of business ethics, corporate responsibility, legal compliance, and bioethics), and there is substantive bioethics literature on topics that relate to industry activities (for example, those listed in the introduction). However, there are a limited number of articles [ 12 – 18 ] and one book [ 19 ] that have explored how and why bioethics as a discipline is being applied within a biopharmaceutical industry context. Even with these efforts, none has defined the scope of biopharmaceutical bioethics nor described bioethically relevant characteristics of an industry setting.
Using Beauchamp and Childress’ perspective on ethics specification as a springboard, this paper presents a definition and scope for biopharmaceutical bioethics , and describes five bioethically relevant contextual factors that are generalizable to a biopharmaceutical industry setting. We propose that the scope of the biopharmaceutical enterprise and the distinct contextual factors of a biopharmaceutical industry setting necessitate focused bioethics consideration, and that an understanding of this setting is foundational for application of bioethics norms.
The paper is written specifically through an industry lens because all authors have industry experience, but we acknowledge there are other institutions that also engage in biopharmaceutical research and development (R&D) and commercialization endeavors. These organizations may be subject to many of the same bioethical challenges as the industry and may find the presented concepts relevant. We also acknowledge the industry has obligations to both humans and animals, and that these obligations have unique considerations that deserve their own discussion. This paper addresses human biopharmaceutical bioethics.
Definition and scope of biopharmaceutical bioethics
We define biopharmaceutical bioethics as: “ the application of bioethics norms (concepts, principles, and rules) to the research, development, supply, commercialization, and clinical use of biopharmaceutical healthcare products. ”
Upon first reading, this definition may seem fairly obvious. However, bioethics discussions often focus on one particular aspect of the biopharmaceutical enterprise without considering the whole, so commentary is warranted. The scope of this definition is explained phrase by phrase in the following sections. First, however, “ biopharmaceutical healthcare products ” needs to be clarified. “Biopharmaceutical” can be specific to biologically derived medicinal treatments or vaccines (“biologics”) or can refer to both biologics and traditional chemically derived medicinal treatments [ 20 ]. In this paper, “biopharmaceutical” has the broader meaning. “ Healthcare products ” can have broad meaning, but in this paper it refers to medicines, vaccines and diagnostics.
“ The application of bioethics norms (concepts, principles, and rules) ”
Many company codes of conduct were written for business purposes and were required by the U.S. Sarbanes-Oxley Act of 2002 [ 21 ]. Company values and principles play an important direction-setting role for establishing standard operating procedures to protect the interests, rights and well-being of research participants and patients, and protect the integrity of the scientific process [ 16 , 17 , 22 ]. Specific guidance, however, is derived from regulations and from bioethics concepts (e.g., character and virtues, values, moral ideals and moral emotions), principles (e.g., autonomy, beneficence, nonmaleficence and justice), and rules (e.g., informed consent, confidentiality and privacy).
Application of bioethics norms should be undertaken at two levels. The first is at a company guidance level, such as a company’s position or policy on a topic like clinical development of biopharmaceutical healthcare products for pediatric use. The second is at a case-specific level, such as a clinical development team’s decision whether to use an outcome-adaptive clinical trial design for a specific pediatric study. Both levels are asking the same fundamental questions: (a) Whether it is ethical to do X?; and (b) If ‘yes’, then how can X be done ethically? Sometimes the “how” question will determine if the “whether” question can be answered affirmatively. For example, it is considered ethically permissible to conduct clinical research with children (“whether”), provided the research is conducted with special protections (“how”). To answer these two questions at a company guidance level bioethics norms are specified, while at a case-specific level they are balanced. To continue with Beauchamp & Childress (p. 18):
Specification entails a substantive refinement of the range and scope of norms, whereas, balancing consists of deliberation and judgment about the relative weights or strengths of norms. Balancing is especially important for reaching judgments in individual cases, and specification is especially useful for policy development [ 23 ].
The multidimensional nature of the biopharmaceutical enterprise necessitates application of guidelines from several bioethics specialties—research, clinical and public health ethics (see Fig. 1 ). This engagement is highlighted throughout the paper. Although not the focus of this paper, the biopharmaceutical enterprise also intersects with business ethics [ 7 , 24 – 26 ] and organization ethics [ 27 ]. The former addresses general business conduct and the latter addresses corporate culture and processes. Applied ethics domains are not necessarily mutually exclusive and should be integrated when appropriate, including integration with professional codes of conduct (e.g., medicine, nursing, science, etc.). As explained by Drews [ 25 ], the obligations derived from medical, scientific or corporate codes of ethics “are not incompatible a priori” (p. 27), but they can conflict with each other in important ways. A physician or scientist in a corporate setting can find it challenging to be bound by all three. Facilitating such integration takes intentional, system-wide effort that acknowledges the challenges.

Bioethics specialties. Biopharmaceutical bioethics utilizes ethics norms from the bioethics specialties of research ethics, clinical ethics and public health ethics, and specifies them for the biopharmaceutical industry context
“ related to the research, development, ”
‘ Research’ refers to scientific activities that precede or support human clinical trials of a novel investigational biopharmaceutical product or line extension (i.e., new use) for an already-approved healthcare product. This includes basic research, drug discovery and preclinical testing [ 28 ]. The focus of research is to identify, evaluate and optimize molecular or biologic entities. Once an entity demonstrates sufficient pharmacological activity and is deemed safe enough to test in humans, then it becomes a ‘candidate’ product and moves to clinical development.
‘ Development’ refers to activities associated with planning and conducting human clinical trials (Phases 1–3) designed to demonstrate safety and efficacy of a new healthcare product or new indication. Development also encompasses activities requisite for submitting an application to regulators and introducing a new healthcare product or new indication into the market [ 28 ].
During R&D, research ethics guidance [ 1 , 2 , 4 , 29 , 30 ] is of obvious importance because it addresses ethical aspects of the design and conduct of research studies; collection, analysis, reporting, publication and sharing of study data; treatment and protection of research participants; and aspects of scientific misconduct. However, R&D activities and directional decisions made at this time can affect not only research participants, but also future patients and prescribing patterns. Therefore, limiting discussions of biopharmaceutical bioethics to research ethics is not sufficient. Engaging guidance from clinical [ 3 , 31 ] and public health ethics [ 32 , 33 ] also is important. The former is important because it addresses individual healthcare delivery and the latter because it addresses public health action, which is particularly relevant for infectious diseases vaccines, treatments and diagnostic tests.
“ supply , commercialization, and clinical use of biopharmaceutical healthcare products.”
Industry activities are more extensive than R&D. They also include supply and commercialization of biopharmaceutical healthcare products. Commercialization involves both availability of and accessibility to biopharmaceutical healthcare products. The term ‘availability’ refers to whether a product can reasonably be offered to a specific market, which requires review and approval by local regulators as well as adequate manufacturing capacity and supply. ‘Accessibility’ refers to whether patients, healthcare practitioners and healthcare systems can reasonably acquire biopharmaceutical healthcare products when needed, which is dependent upon adequate supply chains and distribution networks and is influenced by local formulary decisions (considering multiple clinical and economic factors), price, and reimbursement.
Biopharmaceutical companies have a direct responsibility to work towards availability, but cannot control regulatory decisions. Responsibilities for accessibility are shared among manufacturers and healthcare delivery and reimbursement systems, which vary globally. In addition to approved biopharmaceutical healthcare products, access also can relate to investigational products still in development. Access to investigational products is referred to by many names, such as early access, expanded access, “right-to-try”, and compassionate use, among others.
Supply and commercialization activities primarily relate to business ethics [ 34 ], but bioethics can help guide decisions that could impact clinical use of the product. Biopharmaceutical bioethics draws upon guidance from clinical ethics to address anticipated individual patient needs—such as disease burden, formulation safety and tolerability, medication adherence, when to start/stop/switch treatment, long-term effects, treatment regimens, and diagnostic test interpretations. It also draws upon public health ethics to address anticipated societal healthcare needs—such as disease burden and prevention, pandemic interventions, societal uses or abuses of prescription drugs, drug shortages, and treatment burdens. By engaging with clinical ethics and public health ethics, biopharmaceutical companies can provide accurate and balanced information to healthcare practitioners and patients to enable the appropriate clinical use of healthcare products. This engagement also can facilitate consistency and fairness for multiple stakeholders.
The commercialization phase of a biopharmaceutical healthcare product also involves post-approval activities; including product marketing, safety surveillance, expansion of approved uses (i.e., new indications), and commitments made to regulatory bodies or payers to conduct further studies of safety, efficacy, health outcomes or real-world evidence. All of these activities can have issues that relate to the bioethics specialties of research, clinical or public health ethics.
Definition and scope summary
The application of bioethics norms is necessary at both a company and case-specific level. The multidimensional nature of the biopharmaceutical healthcare product enterprise connects many aspects of contemporary bioethics—including research, clinical and public health ethics. Guidance from each of these specialties is the foundation for specifying and balancing bioethical responsibilities to multiple industry stakeholders. These responsibilities must be considered at both an individual and societal level and extend throughout the product lifecycle—from research to regulatory approval to post-approval activities. In all deliberations, bioethics guidance and company values and principles should be discussed in parallel to ensure consistent institutional guidance.
The biopharmaceutical industry context
Bioethics provides needed guidance for issues not directly or incompletely addressed by public or private policy. Therefore, it is a vital component of any healthcare organization’s approach to ethics and a necessary complement to compliance efforts. In order to fulfill this role, what we refer to as “contextual factors” must be taken into consideration. Contextual factors are operating requirements and characteristics specific to a healthcare setting. These factors do not change bioethics norms, but rather provide a frame within which the norms should be interpreted and applied. In other words, contextual factors are critical considerations when developing guidance and deliberating cases.
We present five contextual factors for the biopharmaceutical industry: (1) dual missions; (2) timely and pragmatic guidance; (3) resource stewardship; (4) multiple stakeholders; and (5) operational complexity. These factors were identified as generalizable, inter-related and bioethically relevant based upon the shared industry and bioethics experience of the authors and working group members of this paper. The factors routinely present themselves during company position/policy development and case consultations. The purpose of this discussion is to highlight the five factors rather than to fully delineate how one or more of them can or should influence deliberation. In isolation, these factors are not necessarily unique to the biopharmaceutical industry, but when considered as a whole they comprise a distinct context for application of bioethics norms.
Dual missions
All business sectors have dual missions. They have a products/services mission (to produce trustworthy deliverables for the benefit of customers), and they have a commercial mission (to make a profit for the benefit of business owners/investors, whether public or private) [ 35 , 36 ]. Dual missions are symbiotic in that they are mutually beneficial and one would cease to exist without the other. However, the products/services mission is the foundation for existence, and therefore core. Both of these missions fall under the applied ethics domain of business ethics (and legal compliance), but when the products/services mission relates to healthcare, bioethics norms also must be applied to business conduct.
With regard to the biopharmaceutical industry, the core mission of the enterprise is to innovate and deliver new healthcare products for unmet medical needs (spanning the spectrum from quality-of-life to lifesaving, life-extending or disease-preventing products). The pace of scientific, technical and medical innovation is rapid and companies must stay apprised of developments to be relevant and ethical. Sometimes innovation advances can outpace ethical discussions, making a proactive approach to bioethics essential to guide R&D decisions. Two such examples are stem cell research and genomic editing. Biotechnologies used in both innovations have been advancing quickly and have accompanying ethical implications. Anticipating such issues is an important biopharmaceutical bioethics responsibility.
Regarding the industry’s commercial mission, many argue this is an inherent conflict of interest with the industry’s innovative healthcare products mission. The argument presumes that a biopharmaceutical company’s real motive is to make a profit rather than to deliver on its business purpose to improve patients’ lives. A related argument is that a profit motive at least should be tempered—because healthcare products are inherently different from other products due to their consumable nature, and because patients have potential vulnerabilities that reduce their ability to make autonomous decisions (e.g., vulnerabilities due to the disease, treatment, emotions, relationship power differentials, or lack of choice or access) [ 37 , 38 ]. Alternatively, some have proposed that (a) profit is a virtue because it fuels an enterprise from which many benefit [ 11 ]; (b) a commercially competitive biopharmaceutical industry is the best option for developing safe and effective healthcare products [ 39 ]; (c) there is no morally relevant distinction between biopharmaceutical companies and businesses that produce other commodities [ 40 ]; and (d) profitability is acceptable as long as the societal benefits are deemed substantial enough to balance the monetary gain. Society’s willingness for an industry to make a profit from the misfortune of ill-health has been referred to as “the grand bargain” [ 41 , 42 ].
Regardless of the arguments, the industry’s commercial mission is a characteristic that must be acknowledged when deliberating bioethics issues. The challenge is how to ethically manage dual responsibilities to make a profit and contribute to society’s common good. The literature is replete with discussion on the topic of balancing these dual missions. This paper does not aim to resolve the issue, nevertheless, we share several pragmatic ways it can be addressed. First, there needs to be a corporate commitment to bioethics. Operationalizing this commitment falls under the applied ethics domain of organization ethics. There are a number of operational models that can be adapted for an industry setting [ 12 , 13 , 27 , 43 – 47 ], depending on available resources. Whichever model is chosen, a concerted educational effort to make bioethics part of daily discussion facilitates the incorporation of norms into business decisions—serving as an important ballast to commercial factors. It is prudent to keep the bioethics effort organizationally separate from R&D or business units to manage internal bias for specific research efforts or product decisions [ 18 ]. Soliciting external expert input is another important exercise for navigating challenging issues; although the professional ethics of this practice is debated due to conflicts of interest and potential corporate pressure to tailor advice to commercial desires [ 5 , 48 – 51 ]. Some, however, think the legitimate need for advice can be managed with methodology to mitigate these risks [ 5 , 52 , 53 ].
Second, an approach that integrates business ethics and bioethics is warranted, and some biopharmaceutical companies have begun to speak out on the topic of balancing business issues with ethical responsibilities [ 54 ]. Third, a balanced perspective must recognize the needs of multiple stakeholders while prioritizing patient well-being [ 10 , 25 , 41 , 42 ]. This, however, is “easier said than done”—not just because of varied business stakeholders but also because of varied patient stakeholders (discussed below).
Finally, it is helpful to acknowledge that financial conflict of interest is not unique to the industry. Dual responsibilities and competing interests also are present in other settings, such as academic and healthcare institutions [ 24 , 55 ]. The industry can look for opportunities to learn from and collaborate with others in the healthcare system on this issue [ 5 ]. There is additional value in public–private partnerships in that they take advantage of the strengths of both types of institutions and can serve as a check and support for one another.
Timely and pragmatic guidance
The industry’s need for timely and pragmatic guidance is analogous to a clinical care (‘bedside’) need. In both settings there is an expectation for well-timed and effective healthcare interventions to prevent or cure disease, extend life, or alleviate or mitigate symptoms. In both settings, bioethics discussion must be thoughtful and thorough, but theoretical and lengthy discourse on a pressing issue is not tenable. Additionally, in both settings, the outcome of deliberation needs to be sensible, defined and action oriented.
The settings differ in the speed with which decisions impact patient care. Unlike a clinical setting, the patient impact of a biopharmaceutical industry decision generally is delayed because of the inherently long timeline to develop a healthcare product (on average 10 years [ 28 ]). Sometimes the impact can be rather immediate, for example with the recall of an approved healthcare product, but this is not the norm. Delayed impact, however, does not negate the necessity of timely and pragmatic decisions. Deciding whether and when to initiate, pause or terminate an endeavor, or to stay or change the course will affect resource utilization, which ultimately impacts patients and multiple stakeholders of the biopharmaceutical enterprise.
Timely and pragmatic guidance is especially relevant during epidemics or pandemics, as in the case of the 2014 Ebola virus disease (EVD) outbreak and the 2019 coronavirus disease (COVID-19) global pandemic. These types of public health crises can place high demands on the biopharmaceutical industry in two ways. First, there is an urgent need for new healthcare products to address the crisis. Second, there is a critical need for continuing development and commercialization of current products to address prevailing medical conditions.
The first demand compels swift development, manufacturing, and distribution of innovative products without sacrificing quality, safety, and ethics [ 56 ]. From a bioethics perspective, there is utmost concern for ethical study designs and protocols, as well as equitable distribution of a successful product—especially for marginalized and vulnerable patient populations. The second demand compels skillful management of ongoing product development, manufacturing, and distribution commitments in light of an unanticipated public health crisis. This involves overseeing internal and external resources and relationships. With regard to clinical trials, the US Food and Drug Administration (FDA) states, “Challenges may arise, for example, from self-isolation, site closures, travel limitations, interruptions to the supply chain for the investigational product, or other considerations if site personnel or trial subjects become infected….” [ 57 ]. Timely and pragmatic bioethics advice can assist with making difficult prioritization and operational decisions (i.e., resource stewardship decisions).
Because of time constraints and the need for broad cooperation (both within a company and among industry, academic, and government collaborators) during a public health crisis, bioethics expertise should be proactively included in planning, implementation, and after-action reviews. In other words, bioethics advice should be as much a part of public health crisis management as technical, scientific, clinical, regulatory, etc. advice.
Resource stewardship
Resources (human, financial, other) for any institution are finite—whether they be modest or considerable. As such, resource stewardship is critical for institutional sustainability and ultimately for promoting the common good in an ethical way. Upholding stewardship or fiduciary responsibilities is a fundamental tenet of business ethics, but it takes on bioethical overtones when resource decisions relate to patient well-being and protection of scientific integrity.
Appropriately managing failure is one aspect of stewardship that is characteristic of the biopharmaceutical industry. R&D and commercial disappointments are commonplace [ 58 – 60 ] because biomedical research and the practice of medicine are complex and results are difficult to predict. Therefore, as resources are allocated, the potential for or the reality of failure needs to be factored into bioethics deliberations. For example, should a medically important trial be initiated when completion feasibility is uncertain due to slow patient enrollment? Or should R&D programmatic efforts be terminated quickly and resources redirected because of a failed trial(s)? Are the medical/scientific/societal needs great enough to consider additional clinical trials despite initially failed attempts (e.g., Alzheimer’s disease treatment research)? Appropriately managing failure encompasses balancing bioethics responsibilities to multiple stakeholders.
Multiple stakeholders
As well-stated by Santoro, “Perhaps no business engages the worlds of science, medicine, economics, health, human rights, government, and social welfare as much as the pharmaceutical industry” [ 42 ] (p. 1). The industry has an inherently complex network of varied stakeholders—both internal and external to its operations. A possible (but not exhaustive) list includes patients, patient advocates, research participants and collaborators, independent researchers, business partners, vendors, healthcare practitioners, payers, regulators, healthcare authorities, local communities, policy makers, employees, and investors. Because the industry is but one of many members in a modern healthcare system, a company may have limited ability to directly affect an issue involving multiple parties. Nevertheless, it is a key participant and stakeholders have high expectations that it should uphold its responsibilities across its broad scope of influence. Therefore, discernment of industry-specific roles and responsibilities and how to address them is a fundamental part of bioethics deliberation.
One of the more challenging aspects of having multiple stakeholders is the variety of patient groups that must be considered. It is appropriate and good to prioritize patient well-being when making decisions involving multiple stakeholders, but often it is not clear which type of patient should receive top priority [ 22 ]. Figure 2 depicts a framework to appreciate categories of patients from a clinical trial perspective that may be impacted by a bioethics issue. There can be up to six categories of patients whose medical needs and contributions to research must be considered and prioritized. Identifying patient categories relevant to an issue is an effective way to ensure comprehensive bioethics deliberations. Appreciating the stories of individual patients within these categories is an effective method for avoiding group generalizations [ 61 , 62 ]. The needs of each relevant group can be evaluated in light of clinical trials factors, such as phase of development, severity of the indication under study, and availability of treatment options.

Patient categories. There are two patient categories that must be considered when assessing bioethical responsibilities of the biopharmaceutical industry—current and future patients. Within the category of current patients, there are two subcategories—those for which there is not a clinical trial and those for which there is a clinical trial. Within the latter category, there are four additional subcategories. Most biopharmaceutical bioethics deliberations require consideration of at least two or more categories or subcategories
An illustration of how this framework plays into the assessment of patient-prioritization is that of drug shortages. Drug shortages of a marketed healthcare product present significant challenges as to whether available drug should be diverted to clinical patients or to clinical trial participants. If clinical trial activity is halted or slowed in favor of clinical patients, future patients with a high unmet medical need can suffer. If, however, clinical trial activity is prioritized, current clinical patients with verified medical needs can suffer.
Another illustration is that of early access to investigational biopharmaceutical healthcare products. This topic increasingly is generating bioethical debate in the public and private policy arena, most notably in the United States [ 63 – 67 ]. The debate centers on whether a biopharmaceutical company has an ethical obligation to provide investigational product to patients with a serious or life-threatening disease who have exhausted treatment options. The ethical tension is that company resources are focused on active R&D efforts designed to help future patients with specific indications, but current patients with serious conditions may not be able to wait for a new product approval. Diverting effort away from clinical trials may delay product access for current and future patients waiting for innovative treatment. However, not providing immediate access to an individual could adversely affect the well-being of that patient. The number of stakeholders with interests in this issue is extensive and highlights the need to delineate industry’s role and responsibility(s) to provide treatment outside of clinical trials [ 68 ].
Both of these examples underscore an inherent strain between research and public health ethics and clinical ethics. The former two operate in the interest of a patient population and the latter in the interest of one patient. Whether invoking research, public health or clinical ethics, a company has a responsibility to adequately characterize and manage product benefits and risks and give thoughtful consideration to product availability and access for the patient categories represented in Fig. 2 .
When issues have multiple stakeholders, it is essential to respect each stakeholder’s values and perspectives on the endeavor(s) to be undertaken. However, it’s important to acknowledge that not all stakeholder views necessarily should have equal weight. As just discussed, patient interests are given greater weight. It’s also important to acknowledge that the likelihood of finding mutually agreeable solutions decreases as the number of stakeholders increases. This can be a difficult reality for decision makers and is why balancing bioethics norms is so important.
Operational complexity
To one degree or another operational complexity is a given for the biopharmaceutical enterprise due to global regulatory requirements. The complexity increases for larger companies with an extensive portfolio of products, and international offices and manufacturing facilities. Acknowledging the impact of operational complexity necessitates acknowledging the ripple effect of individual decisions. Bioethics deliberations cannot be conducted in the vacuum of a specific, local situation. Rather, there must be an accounting for broader-scale implications as well as possible future scenarios and outcomes.
Multilayered structures and simultaneous activities. A larger operation necessitates multilayered structures to research, develop, manufacture and commercialize new products, and conduct post marketing pharmacovigilance and research. It is common to manage simultaneous preclinical and clinical research efforts in multiple therapeutic areas, which may include codevelopment agreements with other biopharmaceutical entities. For a specific product, research may be conducted at a variety of sites engaging a variety of employees and third parties to assess the safety and efficacy for one or more indications. Additionally, product manufacturing capacity and supply logistics must be developed and maintained, accounting for research needs and anticipated and realized market demand. The multidimensional character of industry activities requires multifactorial consideration during bioethics deliberations.
Globalization . A global effort requires comprehensive understanding of large-scale multinational research program logistics and worldwide regulatory, commercialization and distribution requirements. It also requires comprehensive understanding of local health needs, customs, beliefs, expectations, and mores [ 69 ]. For example, when deciding whether to conduct clinical trials in resource-poor settings, it is essential to consider how these factors pertain to the appropriateness of the study, volunteer recruitment and incentives, informed consent, and continued access to investigational products. A global effort also necessitates an appreciation of bioethical issues associated with unregulated or inconsistently regulated scientific methodologies. For example, there are no global requirements for gene editing. To be innovative and proactive, companies must discuss the known and anticipated issues of a biotechnology like gene editing [ 70 , 71 ] as they could affect both public welfare and diverse components of the global biopharmaceutical enterprise (e.g., lab, animal and human research, cell procurement).
Contextual factors summary
Contextual factors are operating requirements and characteristics specific to a given setting. They provide a frame within which bioethics norms are refined and applied. This paper identifies five contextual factors for the biopharmaceutical industry. For a given issue, one or more of them may be in play at either a company guidance or case-specific level.
Application
There are a number of examples of how bioethics is being applied to the biopharmaceutical industry context. With regard to specification, bioethics position statements and policies are being developed by various companies and posted on their websites. A bioethics framework [ 16 , 17 ] and a values-based decision-making model [ 22 ] have been developed for industry biomedical research. Industry-academic collaborations have generated informed guidance on topics like early access to investigational products [ 72 ], clinical trial protocol content [ 73 ] and clinical trial conduct in vulnerable populations [ 74 ]. With regard to case-specific balancing, in-house consultation services are being incorporated into organizational processes [ 18 ], and academic evaluations of industry bioethics decision-making have been conducted [ 15 , 72 ]. Although these efforts are notable, they have been accomplished with an implicit understanding rather than an explicit articulation of the biopharmaceutical industry context. It is our hope there will be continuing collaborative development of biopharmaceutical bioethics because of the definition and contextual scope and factors presented in this paper.
Conclusions
Bioethics as applied to a biopharmaceutical industry setting often is construed either too broadly or too narrowly. The purpose of this paper is to articulate its proper scope because understanding the characteristics of a setting is foundational for the application of bioethics norms. In this paper, biopharmaceutical bioethics is defined as: the application of bioethics norms (concepts, principles, and rules) to the research, development, supply, commercialization, and clinical use of biopharmaceutical healthcare products . Commentary on the scope of this definition explains that companies have ethical responsibilities to both individuals and society, which extend throughout the product lifecycle. To address these responsibilities, biopharmaceutical bioethics engages with research, clinical and public health ethics. Application of bioethics norms can occur at a company guidance level and at a case-specific level. Deliberations should incorporate both bioethics and company values and principles. Finally, five generalizable, inter-related and bioethically relevant contextual factors need to be acknowledged to frame bioethics deliberations: (1) dual missions; (2) timely and pragmatic guidance; (3) resource stewardship; (4) multiple stakeholders; and (5) operational complexity.
We recognize this characterization will require further refinement. Nevertheless, we conclude that the scope of the biopharmaceutical enterprise and the distinct contextual factors of a biopharmaceutical industry setting necessitate focused bioethics consideration to complement legal compliance and business ethics efforts. The stakes for health and well-being are too high for anything less than substantive discourse on how bioethics can be applied to this influential sector of the healthcare system. Establishing a common language and approach for biopharmaceutical bioethics will facilitate breadth and depth of collaborative discussion and subsequent implementation to benefit patients, the healthcare system and society.
Acknowledgements
The authors and the Biopharmaceutical Bioethics Working Group members are founding members of the Biopharmaceutical Industry Bioethics Forum. In addition to the primary authors, the working group members who served as collaborating authors for this article include: Ariella Kelman (Genentech—a member of the Roche Group, South San Francisco, California); Angela Rossetti (Albert Einstein College of Medicine, Bronx, New York); Curtis Chang (Takeda Pharmaceutical Company, Boston, Massachusetts); Kathleen Novak Stern (Takeda Pharmaceutical Company, Bannockburn, Illinois); and Wendell Fortson (Factor, Chicago, Illinois). The authors and working group members thank the participants of the 2016 Pharmaceutical Bioethics Summit, and the 2017 Biopharmaceutical Industry Bioethics Summit for thoughtful discussions. We thank Susan B. Watson, Mitchell Klopfenstein, Karla Childers, Albert J. Allen and Sheldon Sloan for contributions to earlier versions of the paper, and thank Charles Weijer for helpful comments on several drafts. We also thank Eli Lilly and Company (Lilly) for initiating the biopharmaceutical industry collaborative conversation about bioethics.
Abbreviations
Authors' contributions.
LVC was the primary writer and primary contributor to the conception, design, literature review, and analysis of the ideas presented. TP was the secondary writer and secondary contributor to the conception, design, literature review, and analysis of the ideas presented. DGT was the teritiary writer and contributed to the conception, design, and analysis of the ideas presented. MT contributed to the conception, design, and analysis of the ideas presented, and made substantive edits of the content. The Biopharmaceutical Bioethics Working Group members all contributed to the conception and analysis of the ideas presented, provided literature, and made substantive edits of the content. All authors read and approved the final manuscript.
Lilly sponsored the 2016 Pharmaceutical Bioethics Summit, Indianapolis, IN, USA, and the 2017 Biopharmaceutical Industry Bioethics Summit, Indianapolis, IN, USA; which led to the formation of a collaborative industry group named the “Biopharmaceutical Industry Bioethics Forum”. Lilly played no financial role with the project represented by this manuscript, and was not involved in project design, data collection, analysis, or interpretation, or manuscript writing.
Availability of data and materials
Declarations.
All authors and members of the Biopharmaceutical Bioethics Working Group have been or are employees and equity holders of biopharmaceutical companies. LVC, DGT, MAT, and some members of the working group have been or are consultants for biopharmaceutical companies. The views expressed in this paper are those of the authors and working group members and do not represent their respective employers, past or present.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ariella kelman.
5 Genentech, a member of the Roche Group, South San Francisco, CA USA
Angela Rossetti
6 Albert Einstein College of Medicine, Bronx, NY USA
Curtis Chang
7 Takeda Pharmaceutical Company, Boston, MA USA
Kathleen Novak Stern
8 Takeda Pharmaceutical Company, Bannockburn, IL USA
Wendell Fortson
9 Factor, Chicago, IL USA
Pharmaceutical Marketing Ethics
A review of literature.
Several literatures have been devoted to pharmaceutical marketing practices used by manufacturers in their global operations. Authors have been interested to find out the benefits of pharmaceutical marketing to the end-users. It is suggested that its activities ultimately shape supply chain management strategies and practises. The objective of the review is to find out whether prescribers are affected by the marketing practices of drug companies. The focus of the review will be on detailing practices, cost of promotions, and outlook of the industry. US pharmaceutical practices have been taken into account as it is one of the biggest sources of pharmaceutical drugs in the world. It is suggested that its activities ultimately shape supply chain management strategies and practises.
In the pharmaceutical industry, pharmaceutical marketing has been described as any operation involving advertisement, detailing, freebies, and the funding by a drug maker of conferences and symposia designed to encourage the selling of its drugs. (Dictionary of Medicine). Pharmaceutical marketing is regulated by a code of ethics which sets “standard for the ethical marketing and promotion of prescription products directed to the healthcare professions” (Kintanar and Teehankee) The pharmaceutical advertisement culture should subscribe to the freedom of health care practitioners, which ensures that in medical assessment they should exercise impartiality. This suggests that there is no conflict of interest, whether possible, real or evident.
[sociallocker id=”568″]
Marketing Practices
Three marketing strategies that affect the prescribing actions of physicians were outlined in Cousin’s (2009) research that used interviews. Firstly, by their targeted marketing and detailing, physicians were found to be impaired by marketing officials. Physicians, for example, are affected by advertising executives visiting them to sell an items. Second, as previously mentioned, that is by funding or preparation as a means of ongoing medical activities. Fourth, the impact on patients of direct-to-customer ad
As described by Hasbey, pharmaceutical promotion has been segmented into many activities (2009). Accordingly, 28% of advertisers using sample delivery, 36% comprehensive practises, 7% direct to consumer approach (DTCA), conference keeping, 3%, magazines, 25% unmonitored and 0% e-commerce.
Chart 1. Segmentation of Marketing Practices
In his research, Ashley detailed that samples represent a major cost in pharmaceutical marketing. Samples go hand and hand with information, since the sales force utilises them as they meet their consumers. Chart 1 demonstrates the segmentation as prepared by Hashley of marketing practises in the sector and its related operations.
In pharmacy groups, these activities appear to be the standard and are considered conventional marketing. The Braun Community observed that conventional pharmaceutical marketing techniques supply physicians with medications as free trials, offering information about their products by journal papers or political makers. Gifts with a brand emblem or descriptions of the drugs are often customary for pharmaceutical firms. Via funding conferences, pharmaceutical firms frequently participate in continuing patient research. In his assessment, Braun saw the need for modern prescription solutions owing to behavioural shifts and customer behaviour, such as online use and interactive media use.
Holding of Meetings
The “continuing medical education” was addressed in the Cousins research (2003) in which he described the platform as an immoral practise, conducting meetings or in pharmaceutical parlance. Cousins claimed that CME is one of the advertisement tactics at question when it acts as a promotional promotion of their drugs rather than a help for patient education while the pharmaceutical industry funds a case. In this, he said that what is meant to be an informative forum is a pharmaceutical publicity fair in which doctors are named to join. In these conferences, pharmaceuticals foot the bills of the physicians, food and travel expenses plus the medicine samples they give to the doctors. It is possible, therefore, to infer, as Cousin did, that there is a potential conflict of interest. As stated in this study that for every $1.00 invested by pharmaceutical companies in this type of event, they reap $3.56 in increased sales. For instance, Purdue, a drug company that promoted Oxycontin, sponsored about 40 conferences that were attended by 50,000 prescribers. Trainings of this sort, according to research, are one way of influencing prescribers (Chen . 23 September 2009).
The above views are contradicted by the manufacturing consortia such as the Association of the British Pharmaceutical Industry, US PhRMA Association and the Irish Pharmaceutical Association that it is an acceptable practice to pay for the expenses of the delegate in attending conferences and should not be extended beyond health professionals. These organizations further justified the need for further training of doctors in the uses and techniques of new medicines. They rationalized that doctors and health practitioners will find it difficult to keep up to date with scientific advances without these kind of initiatives from pharmaceuticals.
To polish the ranks in terms of gift-giving, the US, through the PhRMA, issued a directive toning down extremes of gift-giving and inducements to doctors in 2002. However, Bucks said that it is not clear whether this rule is being followed because the FDA lack resources for monitoring.
Detailing, as part of marketing strategy, refers to “ the practice of pharmaceutical companies sending representatives – essentially lobbyists for their drugs – into doctors’ offices” (Consumers Union 27 Jan. 2005). According to Consumers Union, the pharmaceutical industry in the US employed 90,000 drug company detailers – a ratio of 1 salesperson for every 4.7 office-based physicians. During its peak in 2007, according to Reilley (2009) pharmaceuticals fielded 102,000 medical representatives. This figure has declined as drug makers shift to on line marketing and layoffs due to hard times. It has been projected that this number will be reduced to 75,000 by 2010 and apparently saved the industry $3.5 billion.
Some studies showed the importance of marketing representatives to prescribers. In a study conducted by Fischer et al, prescribers that participated in the survey believe that pharmaceutical representatives interactions with prescribers tend to benefit patient care and practice health. Prescribers, in this study, trust the information given to them by the pharmaceutical representatives and “that they are equipped to evaluate it independently. However, while others view marketing representatives importantly, they are facing the problem of time squeeze wherein reps are required to have an appointment before seeing a doctor. This has been the practice recommended by American Medical Congress that started in 2008. Time restraints are also the reason why doctors close doors to reps while reps struggle to get a slice of their time (Reilley 2009)
Samples and Gifts
Companies spend a lot to samples and gifts as shown in the above chart. Gift giving has been an accepted norm of detailing, but medical students who are just starting their medical profession are divided in their opinion as to the ethics in pharmaceutical marketing. Some believe that it is ethically permissible and justified to accept gifts from drug manufacturers. Mizik and Robert (2004) concluded in their study that detailing and free gifts have significant effects on the number of prescriptions done by a physician. With the use of pooled time-series cross-sectional data involving three drugs, 24 monthly observations, and 74,075 individual physicians (more than 2 million observations in total) results showed that detailing and free drug samples have positive and statistically significant effects on the number of new prescriptions issued by a physician.
This practice however, does not conform to a study in Science Daily (25 May 2011) that believes it will make the medical students immune to bias induced by promotion, gifts, or interactions with sales representative. Student opinions were divided on whether this practice should be regulated by medical schools or the government, study said.
Despite of impending policy trends on pharmaceutical practice, many prescribers still maintain an affirmative view on receiving gifts and another form of marketing interactions with pharmaceutical manufacturers, according to an article in Science Daily (03 June 2010). Concerns on this practice have received scrutiny because of potential conflict of interest that they pose, such that there are recommendations from individuals and organizations to improve transparency and independent regulation, the report said.
Aside from prescribers, pharmaceuticals eye the nursing influence in prescribing (Science Daily, 09 February 2008). Pharmaceuticals, according to this article, have been persuading nurses for a long time such that it appears they are also receiving gifts from representatives. From among the articles reviewed by authors in this article, a conclusion is arrived that there are undue influence upon nurses and that they should be made aware of the problem to avoid the complexity of unethical drug promotions. Authors suggest that nurses should be encouraged to re-evaluate the educational benefits of promotional information that accordingly, “is carefully selected, prone to bias and hardly likely to be as beneficial as many believe.” This article detailed the review of 32 published nursing literatures wherein 13 have serious concerns on the role of the pharmaceutical industry in influencing nursing behavior; 14 articles were “industry-friendly; while 14 put across “mild” concern about pharmaceutical industry. They either “viewed support of the industry as generally favorable, or identified the harms and benefits of the industry’s involvement in health care” (Science Daily, 09 February 2008).
Direct to customer advertising. “Direct-to-Consumer Advertising” refers to any marketing or advertising of prescription drugs that is targeted specifically to consumers, rather than to physicians, pharmacists, or other health professionals. (PAL)
The impact of DCTA has been viewed by US FDA, through a survey, the result of which showed relative awareness of the patients on the advertised drugs. Accordingly, survey findings revealed that patients inquire about the advertised product when they visit their doctors; ads make patients aware of treatment; and physicians believe that patients are now more concerned about health care. However, physicians, in the survey, expressed concerns that ads do not convey information correctly. Patients have different understanding of the risks and benefits, and at times pressure the physicians to prescribe the advertised drugs. In all of these apprehensions, doctors still believe that patients understand the rule that they know better what is best for them.
Ads that do not convey information correctly is a form of misrepresentation which illegal. The case of Purdue (Chen 2009) is an example of a misrepresentation on their ads while promoting Oxycontin. Company raked in sales and profits but their improper prescription resulted to a huge drug problem and to some extent death of some patients. Purdue plead guilty of misbranding and was made to pay $646 million dollars in fines.
A similar example of misleading advertising is the promotion of Viagra by Pfizer in 2000, wherein product claims made were inappropriate and did not offer clarity. The ad for Viagra draw unrealistic expectations, but what is considered as more serious is the intended downplaying of risks that resulted in some deaths. (Buckley)
This incident became a wake up call to Food and Drug Administration to make policies to ensure that henceforth all advertisements on prescription drugs should be honest and truthful. FDA however admitted that they are understaffed to do all the duties assigned to their office, such as watching misrepresentations on ads (Chen, 2009). Before 1997 FDA rules were strict on this, but after 1997, rules were relaxed such that drug advertisements has to make a general statements on the risks and side effects of the products, and are asked to call another source for full information (Prescription Access Litigation)
Conflict of Interest
As the issue of unethical practices continues to plague the strategies of pharmaceutical marketing, the cases of bribery and corruption in the procurement of medicine came to light. Transparency International (n.d.) discusses this corruption that goes in buying medical equipment and medicine (typically involving drug manufacturers as payers and government hospital administrators and staff as recipients). In another stance, corruption is suspected between doctors and drug companies. Doctors are offered commissions for prescribing a particular drug from a particular company. This is of course illegal, but Transparency International sensed that companies used other schemes to cover up.
In another scope, Transparency International noted that conflict of interest may become a source of bias in presenting evidences in clinical trials. Doctors are paid by pharmaceuticals to recruit patients in their clinical trials and experiments and are on the payroll of the company. For ethical consideration, conflict of interest may arise, and the question that lies in what will be the sanctions to doctors who fail to reveal information because of conflict of interest.
The issue of too much or heavily regulated system of medical control is also questioned as again this encourages bribery and corruption of those companies who wanted to be favored. For instance, spending on political campaigns is one way to corrupt public official, Transparency International observed.
The World Health Organization found conclusive evidence from their studies that doctors who consider promotion highly and rely on it as a source of information about drugs prescribe more drugs and less rationally; they were also shown to prescribe new drugs earlier than other doctors. WHO did not find any circumstantial evidence for a causal link between promotion and individual prescribing.
Pharmaceutical Marketing Outlook
Pharmaceutical Drug Manufacturers (PDM) presents a global scenario of pharmaceutical industry forecasting growth of 4 to 6 percent in 2010 exceeding $825 million. According to PDM, the global pharmaceutical market sales are expected to grow at a compound annual growth of 4 percent to 7 percent through 2013. This growth is determined by the strong growth in the US market, the global macroeconomy, a combination of innovative and mature products, and growing interest in healthcare. Global market, PDM, estimates to expand to $975 + billion by 2013. PDM reports that Asia Pacific Region emerges as the fastest growing pharmaceutical market in the recent past. This growth is seen in China, India, Malaysia, South Korea, and Indonesia because of their reported rising disposable income, several health insurance schemes, and intense competition among top pharmaceuticals. One ethical consideration seen in health insurance companies, as shown in PDM, is that these companies ensure the sale of branded drugs.
PDM said that diabetic and cardiovascular patients are expected to increase, particularly in the US, because of changes in demographics and lifestyles.
As the pharmaceutical industry turns to global distribution, countries that do not manufacture their own drugs and medicine, turn to importation. For instance, South Africa, one of the emerging economies, depends on importation and is thus subject to price fluctuations due to inflation. Prices of drugs in South Africa is also regulated by its government that had recently increased its cost to 13% as a move to combat margin pressures due to importation as against a weakening economy. (EPISCOM)
Outlook of the Middle East market, combined with African Pharmaceutical markets according to Pharmaceutical Drug Manufacturers estimated the trend to be growing at the rate of Compound Annual Growth Rate (CAGR) around 11% during 2010-2012. This trend is caused by the development of infrastructure and the change of the regulations in the region. It has been noted also that the Middle East pharmaceutical market relies on importation of therapeutics and pharmaceutical drugs. In this instance, governments of these countries are taking their turns in developing their own pharmaceutical industry to become a pharmaceutical production center, the report said. EPISCOM reports that generics medicines have overtaken branded in terms of market volume, and this trend is expected to continue because of the demand for cheaper medicine.
With regards to pharmaceutical outlook in 2020, PWC projects changes in the supply chain such that pharmaceutical supply will undergo radical modification. (PWC refers to the network of firm members of Pricewaterhouse Coopers International Ltd.). By 2010, PWC sees more diverse product types and therapies that have shorter lifecycles to appear; there will new ways of assessing, approving and monitoring of medicine that emphasized outcomes. By this year also, PWC foretells of new modes of delivering healthcare where the care is directed into the community and access to information on patients becomes equally important as the products themselves. It is also foreseen that growing emerging markets will be given due attention, and that the public will be more concerned on the ability of compliance and risks; and a tougher environmental controls.
PWC sees changes in the marketing system in 2020, suggesting that the current role of marketing sales and workforce in pharmaceutical agency will shift to a new model of a marketing system. PWC sees the industry shifting from a mass-market to a target-market approach to increase revenue.
According to a report of Environment & Health, the pharmaceutical market of South Africa is worth billions in 2008. About 75% of its pharmaceutical requirements are imported, and the market is expected to rise by a double digit growth in local currency terms. The growth of the South African pharmaceutical market, according to said report is due to the overhaul of the pharmaceutical regulatory system and adoption of the National Health Insurance. The establishment of a universal healthcare buyer and provider promises increased drug delivery and utilization in the country. Nonetheless, implication in pharmaceutical pricing threatens profit margins because it is expected that the bargaining power of a central financier will be substantially higher than the existing fragmented consumer base. Outlook of South African pharmaceuticals to 2014 according to the website of Articlesbase (21 July 2010) will focus on changes in government policies, market structure, competitive landscape, and growth opportunities. Significant changes in government policy in South Africa, such as the replacement of the Medicines Control Council to South African Health Products Regulatory Agency has been viewed positively by the organizations in the industry. The consensus of opinion that prevails is that the change in the regulatory system will expedite drug approvals, faster commercialization, and faster return on investments.
As a universal healthcare buyer and provider, Southern Africa supplies all medical support to its neighboring territories. One of these is Namibia wherein, according to report of the High Commission of India (HCI) is entirely controlled by South African based pharmaceutical manufacturers and middlemen such as Pfizer and Glaxo Smith. HCI observed that the concentration of local distribution rests in the hands of few family-run firms of European descent that prevents other international firms to enter the market.
Namibia is one of the neighboring territories of South Africa and is also a member of the South African Countries Union (SACU) wherein there is a common external tariff to all its members. Therefore, in order to understand the pharmaceutical industry in South Africa, the High Commission of India contends that the context of 48 million SACU populations should be taken into account. This is so because once a good or service is exported to Namibia and to other SACU territories, it can move freely without tariff restrictions in all SACU member states.
Cost of Pharmaceutical Promotions
Buckley, citing authors in his report, said that expenditures for pharmaceutical marketing have been higher than the gross national product of the European Countries and that of the United States. Buckley said that these governments have made efforts to control these expenditures that include cost control by price-fixing and drug budgeting. In some instances, according to Buckley, the government will now allow reimbursements for drugs. For example, payment for Viagra is not allowed by the California Health Maintenance Organization in the US and the NHS in Britain.
Several studies showed that pharmaceutical companies spend more on promotions rather than on research and development. In an article by Science Daily (07 January 2008) cited a study done by two York University researchers wherein data shows pharmaceutical industry in the U.S. as a percentage of US domestic revenue of US$235.4 billion, 24.4 percent of sales dollars is invested on marketing thus devoting just 13.4 percent on research and growth. Canada, according to the same report, said companies are estimated to be allocating a budget of 2.4 and $4.75 billion annually on promotion. The promotional expenses reduce the budget of other priorities such as research and development. Gaglin and Lexchin (2008) estimates that the average expenditure on direct to customer advertising is $61,000 in promotion per physician. PAL reports DOTC costs to the industry in 2005 as $4.65 billion. Results of the review on costs coincides with the conclusion arrived at by Medscape Today (n.d.) that promotion costs predominates R & D and that this conclusion should serve as a basis to form a petition to transform the industry to become more research-oriented. It seems from this recent report that pharmaceutical firms invest about twice as much on promotion as they do on research and growth. These figures clearly illustrate how, counter to the industry’s contention, marketing predominates over R&D in the pharmaceutical industry. While the sum expended on ads is not in itself a validation of Kefauver’s representation of the pharmaceutical sector, it reinforces the public perception of a marketing-driven industry and offers a significant point in favour of transforming the industry’s activities in the direction of more testing and fewer advertising.
Legislation
Various studies point to the overbearing criticisms on marketing practices of pharmaceutical companies. Suggestions to this effect have been found in several studies, but still state laws in the US contend this is unconstitutional. Sommers (2008), in his article, said that the attempts of the United States District Court of New Hampshire and of Maine were denied, but subsequently appealed. These laws, if approved, will shape the direction of the manner of pharmaceutical marketing.
In the compendium of state laws and bills filed that concerns advertising and marketing of pharmaceuticals, the National Conference of State Legislatures (NCSL) stated that none of the proposals mentioned direct-to-consumer advertising (DTCA) as a source of problem, nor do they prescribe to change DTCA practices. What concerns the legislators is the rising cost of advertising that contributes to the rising cost of medicine. NCSL likewise noted that with the huge amount spent on advertising, pharmaceuticals have managed to make their brands become a household name that work to the company’s advantage.
Despite appeals from developing countries (South Africa as one) and poorer countries to produce cheaper brands of medicine to make it affordable, the multinationals were adamant because of the billions of dollars spent on these medicines and therefore want a patent to protect their investment. Under the international rules, generic medicines are allowed.(Shah, Anup. October 2010). Shah stated that for poorer countries, patents and rights are significant that mean “life and death”. For instance, an incident is quoted: “At the end of 1990, the pharmaceutical industry in U.S. lobbied the government to threaten sanctions on South Africa to produce generic drugs to fight its growing AIDS problem. It took huge public outcry to get the case dropped two years later”. Meanwhile, according to Shah, the World Trade Organization succeeded in imposing similar impositions to countries around the world in order to reduce access to generic drugs. Moreover, Shah commented that developing countries got nothing in return because multi-nationals spent more on advertising rather than on research and development; they developed lifestyle drugs rather than on disease curing drugs. As the chief executive of Novartis, a well known drug company, said: “We have no model which would meet the need for new drugs in a sustainable way…You can’t expect for-profit organizations to do this on a large scale” (Shah)
References;
- Articlesbase. 21 July 2010. South African Market Outlook to 2014: Policy environment, market structure, competitive landscape, growth opportunity. Viewed 18 August 2011 http://www.articlesbase.com/health-articles/south-african-pharmaceutical-market- outlook-to-2014-policy-environment-market-structure-competitive-landscape-growth- opportunities-2871883.html
- Braun Group. Need of new pharmaceutical strategies. Retrieved 17 August 2011 from http://www.pharmaceutical-drug-manufacturers.com/articles/marketing-strategy.html-
- Buckley, Joan. Pharmaceutical Marketing: Time for a Change. Economic Journal of Business Ethics and Organization Studies. Vol. 9. No. 2. Pages 4-11
- Consumers Union 27 January 2005. Requiring Drug Companies to Disclose Marketing Expenditures to Physicians. Viewed 17 August 2011 from http://www.consumersunion.org/campaigns/learn_more/001813indiv.html
- Cousins, C. 2009. Pharmaceutical Marketing: The unethical reform of an industry. Gatton Student Research Publication, Volume 1, Number 2, Gatton College of Business & Economics, University of Kentucky
- Environment & Health.28 July 2011. South African Pharmaceutical Market Outlook to 2014: Policy environment, market structure, competitive landscape, growth opportunities. Viewed 16 August 2011 http://www.environmentandhealth.org/south-african-pharmaceutical-market-outlook-to-2014-policy-environment-market-structure-competitive-landscape-growth-opportunities.htm
- EPISCOM. 30 June 2011. The Pharmaceutical Market: South Africa. Opportunities and challenges. Retrieved 18 August 2011 from http://www.espicom.com/prodcat2.nsf/Product_ID_Lookup/00000372?OpenDocument
- Fischer, Melissa A, Keough, Mary Ellen, Baril, Joann L, Saccoccio, Laura, Mazor, Kathleen,Ladd, Elissa, Worley, Ann Von and Gurwitz Jerry H. Prescribers and Pharmaceutical Representatives: Why Are We Still Meeting? Retrieved 18 August 2011 from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2695530/
- Haxby, Dean. January 2009. Pharmaceutical Industry. Marketing and Influence. Retrieved 17 August 2011 from pharmacy.oregonstate.edu/drug…/AG…/Marketing/Marketing_081216.ppt
- High Commission of India. March 2006. Market Survey: Export of Indian Pharmaceuticals to Namibia. Final Report.
- Gagnon, Marc Andre and Lexchin, Joel (2008) The Cost of Pushing Pills: A new estimate of pharmaceutical promotion expenditures in the United States, Pharmaceutical Policy. Viewed 16 August 2011 from http://www.pharmaceuticalpolicy.ca/research/cost- pushing-pills-new-estimate-pharmaceutical-promotion-expenditures-united-states
- Jutel A, Menkes DB (2008) Soft targets: Nurses and the pharmaceutical industry. Journal citation: PLoS Med 5(2): e5. doi:10.1371/journal.pmed.0050005. Retrieved 17 August 2011 from http://medicine.plosjournals.org/perlserv/?request=get- document&doi=10.1371/journal.pmed.0050005
- Kintanar, Quintin and Teehankee, Benito. PHAP Code of PharmaceuticalMarketing Practice. Ethics Committee, Pharmaceutical and Healthcare Association of the Philippines. Viewed 18 August 2011 from www.pna.org.ph/files/phap.pp
- Medscape Today. (n.d.) Pharmaceutical Expenditures in the United States: Conclusion. Viewed 16 August 2011 from http://www.medscape.com/viewarticle/571493_6
- Mizik, Natalie and Jacobson, Robert. 08 March 2004. Our Physicians “Easy Marks”? Quantifying the Effects of Detailing and Sampling on New Prescriptions. Management Science.December 2004 vol. 50 no. 12 1704-1715. Viewed 18 August 2011 from http://mansci.journal.informs.org/content/50/12/1704.abstract
- National Conference of State Legislatures Marketing and Direct-to-Consumer Advertising (DTCA) of Pharmaceuticals, June 2009. Viewed 18 August, 2011 from http://www.ncsl.org/default.aspx?tabid=14461
- O’Reilley, Kevin B. 23 March 2009. Doctors increasingly close doors to drug reps, while pharma cuts ranks. Amednews.com. Retrieved 17 August 2011 from http://www.ama-assn.org/amednews/2009/03/23/prl10323.htm
- PWC n.d. Pharma 2020. Supplying the future. Which path will you take? Network of Pricewaters Coopers International Ltd. Viewed 18 August 2010 from http://www.pwc.com/en_GX/gx/pharma-life-sciences/pharma-2020/pharma-2020- supplying-the-future.jhtml
- PWC. Nd. Pharma 2020. Marketing the future. Which path will you take? Network of Pricewaters Coopers International Ltd. Viewed 18 August 2010 from http://www.pwc.com/gx/en/pharma-life-sciences/pharma-2020/pharma-2020- marketing-the-future-which-path-will-you-take.jhtml
- Pharmaceutical Drug Manufacturers. 2010. Pharmaceutical Market Trends 2010. Retrieved 17 August 2010 from http://www.pharmaceutical-drug-manufacturers.com/articles/pharmaceutical-market-trends-2010.html
- Pharmaceutical marketing definition. Retrieved 17 August 2011 from http://medical- dictionary.thefreedictionary.com/Pharmaceutical+marketing
- Prescription Access Litigation. Direct-to-Consumer-Advertising of Prescription Drugs. http://www.prescriptionaccess.org/learnmore?id=0003
- Science Daily (May 25, 2011) —Medical Students Have Substantial Exposure to Pharmaceutical Industry Marketing Public Library of Science. Retrieved August 16, 2011 from http://www.ScienceDaily.com /releases/2011/05/110524171245.htm
- Science Daily. 06 May 2010. Many Clinicians Maintain Positive Attitudes Toward Industry Marketing Activities. AMA and Archives Journals Retrieved 16 August, 2011, from http://www.ScienceDaily.com /releases/2010/06/100621173729.htm
- Science Daily 09 February 2008. Nurses As ‘Soft Targets’ Of Drug Company Promotion Public Library of Science Retrieved 16 August 2011 from http://www.ScienceDaily.com /releases/2008/02/080204223759.htm
- Science Daily. 07 January 2008. (2008, January 7). Big Pharma Spends More On Advertising Than Research And Development, Study Finds. York University. Retrieved August 16, 2011, from http://www.ScienceDaily.com /releases/2008/01/080105140107.htm
- Shah, Anup. 02 October 2010. Pharmaceutical Corporations and Medical Research. Global Issues. Viewed 18 August 2010 from http://www.globalissues.org/article/52/pharmaceutical-corporations-and-medical-research
- Transparency International. Corruption in the pharmaceutical industry. The global coalition against corruption. Viewed 18 August 2011 from http://www.transparency.org/global_priorities/other_thematic_issues/health/pharmaceutic al industry
- USDA Food and Drug Administration. The impact to direct to consumer advertising. Viewed 18 August, 2011 http://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm143562.htm
- World Health Organization, 2004. Drug Promotion – What We Know, What We Have Yet to Learn – Reviews of Materials in the WHO/HAI Database on Drug Promotion – EDM Research Series No. 032 Viewed 18 August 2011 fromhttp://apps.who.int/medicinedocs/en/d/Js8109e/6.3.html
[/sociallocker]
Hi I am Jason. My passion is about writing SEO Optimized Content following google standards and make high-quality content for users. I would love to provide Quality Articles here. Thanks
Related Content
Medical marijuana dispensaries in america, gap 360 situational analysis, negotiation research proposal example, design comparison of the boeing 787 and airbus..., sherwood elementary school health problems assessment and diagnosis, dream inn marketing strategy, nextgen faa technology’s implementation and transformation benefits, analysis of trade partnership between china and latin..., how united states corporate tax rates affect global..., hershey ethical business activity and its corporate social....

IMAGES
VIDEO
COMMENTS
This systematic review of cross-disciplinary peer-reviewed quantitative research into pharmaceutical marketing ethical issues from 1990 to 2021 reveals a focus on direct-to-consumer advertising and physician-directed promotion. This review documents inconsistent findings across studies due to discrepancies and limitations in research designs ...
These various codes are outlined in this paper along with relevant historical perspectives and interrelationship with concepts of professionalism. ... Revised CIOMS research ethics ... Whitney P. (2016). Ethics and compliance in global pharmaceutical industry marketing and promotion: The role of the IFPMA and self-regulation. PPL. 27 18, 199 ...
This systematic review of cross-disciplinary peer-reviewed quantitative research into pharmaceutical marketing ethical issues from 1990 to 2021 reveals a focus on direct-to-consumer advertising ...
Background In the EU, clinical assessors, rapporteurs and the Committee for Medicinal Products for Human Use are obliged to assess the ethical aspects of a clinical development program and include major ethical flaws in the marketing authorization deliberation processes. To this date, we know very little about the manner that these regulators put this obligation into action. In this paper, we ...
A focus on direct-to-consumer advertising and physician-directed promotion is revealed and a comprehensive taxonomy of ethical issues from the systematic review, additional scholarly and industry publications, and expert interviews is presented. Abstract This systematic review of cross-disciplinary peer-reviewed quantitative research into pharmaceutical marketing ethical issues from 1990 to ...
Abstract. This paper analyses the major ethical issues raised by direct-to-consumer communications (DTCC) of pharmaceutical drugs, with a view to proposing ethical standards of practice for the ...
Pharmaceutical marketing personalized to physicians such as the provision of samples and gifts raised such ethical issues . To make effective decisions, the key was to think about different choices regarding their ability to accomplish one of the physicians' most important goals that were ethical prescribing of drugs.
Fourth, inspection reports provide a lot of insight on ethical and scientific matters such as the ethical acceptability of the elements of a pharmaceutical clinical trial which eventually becomes a basis for an application, integrity of the clinical trial data based on which pharmaceutical products are provided marketing authorization, among ...
This article offers marketing and public policy researchers and professionals a peek into pharmaceutical marketing from the practitioner's perspective. Through an interview process with eight active pharmaceutical marketing managers and medical doctors, the authors highlight some of the most pressing challenges facing pharmaceutical marketing ...
To synthesize the essential ethical imperatives related to DTCC, an oath for marketing professionals is offered as a guide to ethical conduct. Abstract This paper analyses the major ethical issues raised by direct-to-consumer communications (DTCC) of pharmaceutical drugs, with a view to proposing ethical standards of practice for the marketing profession. A case-based analysis of four types of ...
Pharmaceutical marketing is also blameworthy of over marketing and not take in to considerations the vulnerability of other stakeholders like patients. Journal of Management Research Vol 7, Issue 1, Journal of Management Research, ISSN, 2617-0361 , Jan-June 202 1 This preprint research paper has not been peer reviewed.
From a managerial perspective, pharmaceutical marketing managers of drug companies can use the research findings to design better their strategies directed to the Lebanese physicians who can also benefit from the results obtained. Keywords: Pharmaceutical marketing, Prescribing behavior, Ethics, Physicians' profile
This systematic review of cross-disciplinary peer-reviewed quantitative research into pharmaceutical marketing ethical issues from 1990 to 2021 reveals a focus on direct-to-consumer advertising and physician-directed promotion. This review documents inconsistent findings across studies due to discrepancies and limitations in research designs, study populations, sampling procedures, and ...
ethics and compliance initiatives has led to an international framework of self-regulation for the global pharmaceutical industry's marketing and promotion activi-ties. For example, many national codes have been updated and expanded over the last 10 to 20 years, both in developed and emerging markets. Code revisions at the na-
Pharmaceutical Research and Manufacturers of America ... (2022), "Practitioner Perspectives on Key Challenges in Pharmaceutical Marketing and Future Research Opportunities," Journal of Public Policy & Marketing, 41 (4), 368-82. Crossref. ... Drug Company Ethics. 2003. SAGE Knowledge. Book chapter . Social Marketing.
Pharmaceutical marketing efforts directed to physicians are getting more and more attention over the years. There are many tactics adopted by pharmaceutical companies [] such as physicians-targeted promotions which are free samples, journal advertisements [], printed product literature and other gifts that helped them to increase the acceptability of their products [].
The current state of affairs can be improved if the key stakeholders ensure ethical behaviors through self-regulation and adherence to international and national codes of ethics. Reforms in prevalent DRAP laws for industry and pharmacies can play a vital to tackle this issue. The theme of the thesis is ethical marketing.
The pharmaceutical industry stands at the intersection of science, business, and healthcare, where innovation in marketing and sales strategies plays a pivotal role in driving growth and ensuring access to life-saving medications. With advancements in technology, changes in regulatory landscapes, and evolving consumer preferences ...
This paper defines biopharmaceutical bioethics as: the application of bioethics norms (concepts, principles, and rules) to the research, development, supply, commercialization, and clinical use of biopharmaceutical healthcare products. It provides commentary on this definition, and presents five contextual factors that need to be considered ...
Pharmaceutical marketing is regulated by a code of ethics which sets "standard for the ethical marketing and promotion of prescription products directed to the healthcare professions" (Kintanar and Teehankee) The pharmaceutical advertisement culture should subscribe to the freedom of health care practitioners, which ensures that in medical ...
Education, especially development education (DE), and a number of socially focused disciplines, including corporate social responsibility (CSR) and social marketing (SM), have long been targeted by policy makers for deriving advice on the 'wisdom' of levelling up differences and addressing sources of disadvantages at individual, group and/or regional levels. Additionally, the combined ...